QuickLinks -- Click here to rapidly navigate through this documentAs filed with the Securities and Exchange Commission on August 30, 2001
Registration No. 333-49858
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
POST-EFFECTIVE
AMENDMENT NO. 1
to
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
ABGENIX, INC.
(Exact Name of Registrant as Specified in Its Charter)
| Delaware | 2836 | 94-3248826 |
(State or Other Jurisdiction of
Incorporation or Organization) | (Primary Standard Industrial
Classification Code Number) | (I.R.S. Employer
Identification No.) |
6701 Kaiser Drive
Fremont, CA 94555
(510) 608-6500
(Address, Including Zip Code, and Telephone Number, Including Area Code,
of Registrant's Principal Executive Offices)
R. Scott Greer
Chief Executive Officer
Abgenix, Inc.
6701 Kaiser Drive
Fremont, CA 94555
(510) 608-6500
Susan L. Thorner
Vice President, General Counsel and Secretary
Abgenix, Inc.
6701 Kaiser Drive
Fremont, CA 94555
(510) 608-6500
(Name, Address, Including Zip Code, and Telephone Number, Including Area Code, of Agent For Service)
With a copy to:
William H. Hinman, Esq.
Simpson Thacher & Bartlett
3330 Hillview Avenue
Palo Alto, CA 94304
(650) 251-5000
Approximate date of commencement of proposed sale to the public: As soon as practicable after this Registration Statement becomes effective.
If the securities being registered on this form are being offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. /x/
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. / /
If this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. / /
If this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. / /
If delivery of the prospectus is expected to be made pursuant to Rule 434, please check the following box. / /
The registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act or until this Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
1,418,705 Shares
Common Stock
The holders of our common stock who are identified as selling shareholders in this prospectus may offer and sell from time to time up to 1,418,705 shares of our common stock by using this prospectus. We and one of our shareholders sold 4,050,000 shares of our common stock to the selling shareholders in a private placement transaction on November 6, 2000.
The offering price for our common stock may be the market price for our common stock prevailing at the time of sale, a price related to the prevailing market price, a negotiated price or such other price as the selling shareholders determine from time to time. We will not receive any of the proceeds from the sales of the shares.
Our common stock is traded on the Nasdaq National Market under the ticker symbol "ABGX." On August 27, 2001, the closing sale price of our common stock, as reported by Nasdaq, was $30.08 per share.
An investment in our common stock involves a high degree of risk. See "Risk Factors" beginning on page 1 of this prospectus.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR PASSED UPON THE ADEQUACY OR ACCURACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The date of this prospectus is August 30, 2001.
You should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with information different from that contained in this prospectus. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of our common stock.
TABLE OF CONTENTS
| RISK FACTORS | | 1 |
| CERTAIN INFORMATION | | 17 |
| USE OF PROCEEDS | | 17 |
| PRICE RANGE OF COMMON STOCK | | 18 |
| DIVIDEND POLICY | | 18 |
| SELECTED CONSOLIDATED FINANCIAL DATA | | 19 |
| MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | | 20 |
| BUSINESS | | 33 |
| MANAGEMENT | | 61 |
| CERTAIN TRANSACTIONS | | 75 |
| DESCRIPTION OF CAPITAL STOCK | | 76 |
| SHARES ELIGIBLE FOR FUTURE SALE | | 79 |
| PRINCIPAL SHAREHOLDERS | | 79 |
| SELLING SHAREHOLDERS | | 81 |
| PLAN OF DISTRIBUTION | | 82 |
| WHERE YOU CAN FIND MORE INFORMATION | | 83 |
| LEGAL MATTERS | | 83 |
| EXPERTS | | 83 |
| INDEX TO CONSOLIDATED FINANCIAL STATEMENTS | | F-1 |
Abgenix and the Abgenix logo are trademarks of Abgenix. XenoMouse® is a registered trademark of Xenotech, Inc., a wholly-owned subsidiary of Abgenix. XenoMax™ is a trademark of Abgenix. SLAM™ is a trademark of Abgenix Biopharma, Inc. registered in Canada. This prospectus also contains trademarks of third parties.
FORWARD-LOOKING STATEMENTS
This prospectus includes forward-looking statements based largely on our current expectations and projections about future events and financial trends affecting the financial condition of our business. The words "believe," "may," "will," "estimate," "continue," "anticipate," "intend," "expect" and similar expressions identify these forward-looking statements. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described below under the caption "Risk Factors." In light of these risks and uncertainties, the forward-looking events and circumstances discussed in this prospectus may not occur and actual results could differ materially from those anticipated in the forward-looking statements. We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
RISK FACTORS
Investing in our common stock involves a high degree of risk. In addition to the other information in this prospectus, you should carefully consider the risks described below before purchasing our common stock. If any of the following risks actually occurs, our business could be materially harmed, and our financial condition and results of operations could be materially harmed. As a result, the trading price of our common stock could decline, and you might lose all or part of your investment.
Risks Related to the Development and Commercialization of our Products
Our XenoMouse and XenoMax technologies may not produce safe, efficacious or commercially viable products.
Our XenoMouse and XenoMax technologies are new approaches to the generation of antibody therapeutic products. We have not commercialized any antibody products based on our technologies. Moreover, we are not aware of any commercialized, fully human antibody therapeutic products that have been generated from any technologies similar to ours. Our antibody product candidates are still at an early stage of development. Clinical trials have begun with respect to only four fully human antibody product candidates generated by XenoMouse technology. We cannot be certain that either XenoMouse technology or XenoMax technology will generate antibodies against every antigen to which they are exposed in an efficient and timely manner, if at all. Furthermore, XenoMouse technology and XenoMax technology may not result in any meaningful benefits to our current or potential customers or in product candidates that are safe and efficacious for patients. If our technologies fail to generate antibody product candidates that lead to the successful development and commercialization of products, our business, financial condition and results of operations will be materially harmed.
Successful development of our products is uncertain.
Our development of current and future product candidates, either alone or in conjunction with collaborators, is subject to the risks of failure inherent in the development of new pharmaceutical products and products based on new technologies. These risks include:
- •
- delays in product development, clinical testing or manufacturing;
- •
- unplanned expenditures in product development, clinical testing or manufacturing;
- •
- failure in clinical trials or failure to receive regulatory approvals;
- •
- emergence of superior or equivalent products;
- •
- inability to manufacture on our own, or through others, product candidates on a commercial scale;
- •
- inability to market products due to third-party proprietary rights;
- •
- election by our customers not to pursue product development;
- •
- failure by our customers to develop products successfully; and
- •
- failure to achieve market acceptance.
Because of these risks, our research and development efforts and those of our customers and collaborators may not result in any commercially viable products. If a significant portion of these development efforts is not successfully completed, required regulatory approvals are not obtained or any approved products are not commercially successful, our business, financial condition and results of operations will be materially harmed.
1
Clinical trials for our product candidates will be expensive and their outcome is uncertain.
Conducting clinical trials is a lengthy, time-consuming and expensive process. Before obtaining regulatory approvals for the commercial sale of any products, we must demonstrate through pre-clinical testing and clinical trials that our product candidates are safe and effective for use in humans. We will incur substantial expense for, and devote a significant amount of time to, pre-clinical testing and clinical trials.
Historically, the results from pre-clinical testing and early clinical trials have often not been predictive of results obtained in later clinical trials. A number of new drugs and biologics have shown promising results in clinical trials, but subsequently failed to establish sufficient safety and efficacy data to obtain necessary regulatory approvals. Data obtained from pre-clinical and clinical activities are susceptible to varying interpretations, which may delay, limit or prevent regulatory approval. In addition, regulatory delays or rejections may be encountered as a result of many factors, including changes in regulatory policy during the period of product development.
As of June 30, 2001 three of our proprietary product candidates, ABX-CBL, ABX-IL8 and ABX-EGF, were in clinical trials. Patient follow-up for these clinical trials has been limited. To date, data obtained from these clinical trials has been insufficient to demonstrate safety and efficacy under applicable Federal Drug Administration, or FDA, guidelines. As a result, this data will not support an application for regulatory approval without further clinical trials. Clinical trials conducted by us or by third parties on our behalf may not demonstrate sufficient safety and efficacy to obtain the requisite regulatory approvals for ABX-CBL, ABX-IL8, ABX-EGF or any other potential product candidates. Regulatory authorities may not permit us to undertake any additional clinical trials for our product candidates.
In addition, our other product candidates are in pre-clinical development, but we have not submitted investigational new drug applications nor begun clinical trials for these product candidates. Our pre-clinical or clinical development efforts may not be successfully completed, we may not file further investigational new drug applications and clinical trials may not commence as planned.
Completion of clinical trials may take several years or more. The length of time generally varies substantially according to the type, complexity, novelty and intended use of the product candidate. Our commencement and rate of completion of clinical trials may be delayed by many factors, including:
- •
- inability to manufacture sufficient quantities of materials for use in clinical trials;
- •
- slower than expected rate of patient recruitment;
- •
- inability to adequately follow patients after treatment;
- •
- unforeseen safety issues;
- •
- lack of efficacy during the clinical trials; or
- •
- government or regulatory delays.
We have limited experience in conducting and managing clinical trials. We rely on third parties, including our customers, to assist us in managing and monitoring clinical trials. Our reliance on these third parties may result in delays in completing, or in failure to complete, these trials if the third parties fail to perform under our agreements with them.
Our product candidates may fail to demonstrate safety and efficacy in clinical trials. This failure may delay development of other product candidates and hinder our ability to conduct related pre-clinical testing and clinical trials. As a result of these failures, we may also be unable to obtain additional financing. Any delays in, or termination of, our clinical trials could materially harm our business, financial condition and results of operations.
2
We currently rely on a sole source third-party manufacturer.
We currently rely, and will continue to rely for at least the next five years, on a single contract manufacturer, Lonza Biologics (Lonza), to produce ABX-CBL, ABX-IL8 and ABX-EGF under good manufacturing practice regulations, for use in our clinical trials. In December 2000, we entered into a manufacturing supply agreement with Lonza, under which Lonza will make available exclusively to us, for a period of five years, a cell culture production suite, with associated purification capacity, within Lonza's facility. As a result of this agreement, we expect to gain access to production capacity and scheduling flexibility similar to owning the production capability, while Lonza retains responsibility for staffing and operating the facility. The term of the agreement is five years with an option to extend the term. The dedicated cell culture production suite is being refurbished and is expected to be operational and available to us in the third or fourth quarter of 2001. In July 2001, we entered into an agreement giving us the right to enter into exclusive negotiations with Lonza for an additional manufacturing supply agreement under which Lonza will make available to us, for a period of up to five years, extendable for an additional two years, one third of a cell culture production suite for large-scale manufacturing of products. The exclusive negotiation period will expire on December 31, 2001, subject to a limited further extension. We currently anticipate that construction of the facility will be completed in the fourth quarter of 2004.
Lonza has a limited number of facilities in which our product candidates can be produced and has limited experience in manufacturing ABX-CBL, ABX-IL8 and ABX-EGF in quantities sufficient for conducting clinical trials or for commercialization. We currently rely on Lonza to produce our product candidates under good manufacturing practice regulations, which meet acceptable standards for our clinical trials.
Third-party manufacturers may encounter difficulties in scaling up production, including problems involving production yields, quality control and assurance, shortage of qualified personnel, compliance with FDA regulations, production costs, and development of advanced manufacturing techniques and process controls. Our third-party manufacturer may not perform as agreed or may not remain in the contract manufacturing business for the time required by us to successfully produce and market our product candidates. If our third-party manufacturer fails to deliver the required quantities of our product candidates for clinical use on a timely basis and at commercially reasonable prices, and we fail to find a replacement manufacturer or develop our own manufacturing capabilities, our business, financial condition and results of operations will be materially harmed.
Our own ability to manufacture is uncertain.
We are building our own manufacturing facility for the manufacture of products for clinical trials and early commercial launch, in compliance with FDA good manufacturing practices. In May 2000, we signed a long-term lease for a building to contain this manufacturing facility. Construction has started and this facility is expected to be operational by year-end 2002. The costs of the facility, including design, leasehold improvements and equipment, will approximate $140 million. Construction of this facility may take longer than expected, and the planned and actual construction costs of building and qualifying the facility for regulatory compliance may be higher than expected. The process of manufacturing antibody products is complex. We have no experience in the clinical or commercial scale manufacturing of ABX-CBL, ABX-IL8 and ABX-EGF, or any other antibody products. Such antibody products will also need to be manufactured in a facility and by a process that comply with FDA and other regulations. It may take a substantial period of time to begin producing antibodies in compliance with such regulations. Our manufacturing operations will be subject to ongoing, periodic unannounced inspection by the FDA and state agencies to ensure compliance with good manufacturing practices. If we are unable to establish and maintain a manufacturing facility within our planned time and cost parameters, the development and sales of our products and our financial performance may be materially harmed.
3
We also may encounter problems with the following:
- •
- production yields;
- •
- quality control and assurance;
- •
- shortages of qualified personnel;
- •
- on-going compliance with FDA regulations;
- •
- production costs; and
- •
- development of advanced manufacturing techniques and process controls.
We continually evaluate our options for commercial production of our antibody products, which include use of third-party manufacturers, establishing our own commercial scale manufacturing facility or entering into a manufacturing joint venture relationship with a third party. We are aware of only a limited number of companies on a worldwide basis who operate manufacturing facilities in which our product candidates can be manufactured under good manufacturing practice regulations, a requirement for all pharmaceutical products. It would take a substantial period of time for a contract manufacturing facility that has not been producing antibodies to begin producing antibodies under good manufacturing practice regulations. We may not be able to contract with any of these companies on acceptable terms, if at all.
In addition, we and any third-party manufacturer will be required to register with the FDA and other regulatory authorities any manufacturing facilities in which our antibody products are manufactured. The facilities will then be subject to inspections confirming compliance with FDA good manufacturing practice or other regulations. If we or any of our third-party manufacturers fail to maintain regulatory compliance, our business, financial condition and results of operations will be materially harmed.
We will need to find third parties to license and develop many of our product candidates.
Our strategy for the development and commercialization of antibody therapeutic products depends, in large part, upon the formation of collaboration agreements with third parties. Potential third parties include pharmaceutical and biotechnology companies, academic institutions and other entities. We must enter into these agreements to successfully develop and commercialize product candidates. These agreements are necessary in order for us to:
- •
- access proprietary antigens for which we can generate fully human antibody products;
- •
- fund our research and development activities;
- •
- fund pre-clinical development, clinical trials and manufacturing;
- •
- seek and obtain regulatory approvals; and
- •
- successfully commercialize existing and future product candidates.
Only a limited number of fully human antibody product candidates have been generated pursuant to our collaboration agreements, and only four antibody product candidates generated with XenoMouse technology have entered clinical testing. These product candidates may not result in commercially successful products. Current or future collaboration agreements may not be successful. If we fail to maintain our existing collaboration agreements or to enter into additional agreements, our business, financial condition and results of operations could be materially harmed.
Our dependence on licensing and other agreements with third parties subjects us to a number of risks. These agreements may not be on terms that prove favorable to us, and collaborators typically are afforded significant discretion in electing whether to pursue any of the planned activities. Licensing and
4
other contractual agreements may require us to relinquish our rights to certain of our technologies, products or marketing territories. We cannot control the amount or timing of resources our collaborators may devote to the product candidates, and collaborators may not perform their obligations as expected. Additionally, business combinations or significant changes in a collaborator's business strategy may adversely affect a collaborator's willingness or ability to complete its obligations under the arrangement. Even if we fulfill our obligations under an agreement, typically our collaborators can terminate the agreement at any time following proper written notice. If any of our collaborators were to terminate or breach our agreement, or otherwise fail to complete its obligations in a timely manner, our business, financial condition and results of operations may be materially harmed. If we are not able to establish further collaboration agreements or any or all of our existing agreements are terminated, we may be required to seek new collaborators or to undertake product development and commercialization at our own expense. Such an undertaking may:
- •
- limit the number of product candidates that we will be able to develop and commercialize;
- •
- reduce the likelihood of successful product introduction;
- •
- significantly increase our capital requirements; and
- •
- place additional strain on our management's time.
Existing or future collaborators may pursue alternative technologies, including those of our competitors. Disputes may arise with respect to the ownership of rights to any technology or products developed with any current or future collaborator. Lengthy negotiations with potential new collaborators or disagreements between us and our collaborators may lead to delays or termination in the research, development or commercialization of product candidates or result in time-consuming and expensive litigation or arbitration. If any of our collaborators pursue alternative technologies or fail to develop or commercialize successfully any product candidate to which they have obtained rights from us, our business, financial condition and results of operations may be materially harmed.
We do not have marketing and sales experience.
We do not have marketing, sales or distribution experience or capability. For certain products, we may establish an internal marketing and sales force. We intend to enter into arrangements with third parties to market and sell most of our products. We may not be able to enter into marketing and sales arrangements with others on acceptable terms, if at all. To the extent that we enter into marketing and sales arrangements with other companies, our revenues, if any, will depend on the efforts of others. These efforts may not be successful. If we are unable to enter into third-party arrangements, then we must develop a marketing and sales force, which may need to be substantial in size, in order to achieve commercial success for any product candidate approved by the FDA. We may not successfully develop marketing and sales capabilities or have sufficient resources to do so. If we do develop such capabilities, we will compete with other companies that have experienced and well-funded marketing and sales operations. If we fail to establish successful marketing and sales capabilities or fail to enter into successful marketing arrangements with third parties, our business, financial condition and results of operations will be materially harmed.
We are subject to extensive government regulation and we may not be able to obtain regulatory approvals.
Our product candidates under development are subject to extensive and rigorous domestic government regulation. The FDA regulates, among other things, the development, testing, manufacture, safety, efficacy, record-keeping, labeling, storage, approval, advertising, promotion, sale and distribution of biopharmaceutical products. If our products are marketed abroad, they also are subject to extensive regulation by foreign governments. None of our product candidates has been approved for sale in the United States or any foreign market. The regulatory review and approval process, which includes
5
pre-clinical studies and clinical trials of each product candidate, is lengthy, expensive and uncertain. Securing FDA approval requires the submission of extensive pre-clinical and clinical data and supporting information to the FDA for each indication to establish the product candidates' safety and efficacy. The approval process takes many years, requires the expenditure of substantial resources, involves post-marketing surveillance, and may involve ongoing requirements for post-marketing studies. Regulatory requirements are subject to frequent change. Delays in obtaining regulatory approvals may:
- •
- adversely affect the successful commercialization of any drugs that we or our customers develop;
- •
- impose costly procedures on us or our customers;
- •
- diminish any competitive advantages that we or our customers may attain; and
- •
- adversely affect our receipt of revenues or royalties.
Certain material changes to an approved product such as manufacturing changes or additional labeling claims are subject to further FDA review and approval. Any required approvals, once obtained, may be withdrawn. Compliance with other regulatory requirements may not be maintained. Further, if we fail to comply with applicable FDA and other regulatory requirements at any stage during the regulatory process, we or our third-party manufacturers may be subject to sanctions, including:
- •
- delays;
- •
- warning letters;
- •
- fines;
- •
- product recalls or seizures;
- •
- injunctions;
- •
- refusal of the FDA to review pending market approval applications or supplements to approval applications;
- •
- total or partial suspension of production;
- •
- civil penalties;
- •
- withdrawals of previously approved marketing applications; and
- •
- criminal prosecutions.
We expect to rely on our customers to file investigational new drug applications and generally direct the regulatory approval process for many of our products. Our customers may not be able to conduct clinical testing or obtain necessary approvals from the FDA or other regulatory authorities for any product candidates. If we fail to obtain required governmental approvals, our customers will experience delays in or be precluded from marketing products developed through our research. In addition, the commercial use of our products will be precluded. Delays and limitations may materially harm our business, financial condition and results of operations.
We and our third-party manufacturers also are required to comply with the applicable FDA current good manufacturing practice regulations. Good manufacturing practice regulations include requirements relating to quality control and quality assurance as well as the corresponding maintenance of records and documentation. Manufacturing facilities are subject to inspection by the FDA. These facilities must be approved before we can use them in commercial manufacturing of our products. We or our third-party manufacturers may not be able to comply with the applicable good manufacturing practice requirements and other FDA regulatory requirements. If we or our third-party manufacturers fail to comply, our business, financial condition and results of operations will be materially harmed.
6
Market acceptance of our products is uncertain.
Our product candidates may not gain market acceptance among physicians, patients, healthcare payors and the medical community. We may not achieve market acceptance even if clinical trials demonstrate safety and efficacy, and the necessary regulatory and reimbursement approvals are obtained. The degree of market acceptance of any product candidates that we develop will depend on a number of factors, including:
- •
- establishment and demonstration of clinical efficacy and safety;
- •
- cost-effectiveness of our product candidates;
- •
- their potential advantage over alternative treatment methods;
- •
- reimbursement policies of government and third-party payors; and
- •
- marketing and distribution support for our product candidates, including the efforts of our collaborators where they have marketing and distribution responsibilities.
Physicians will not recommend therapies using our products until such time as clinical data or other factors demonstrate the safety and efficacy of such procedures as compared to conventional drug and other treatments. Even if the clinical safety and efficacy of therapies using our antibody products is established, physicians may elect not to recommend the therapies for any number of other reasons, including whether the mode of administration of our antibody products is effective for certain indications. For example, antibody products are typically administered by infusion or injection, which requires substantial cost and inconvenience to patients. Our product candidates, if successfully developed, will compete with a number of drugs and therapies manufactured and marketed by major pharmaceutical and other biotechnology companies. Our products may also compete with new products currently under development by others. Physicians, patients, third-party payors and the medical community may not accept and utilize any product candidates that we or our customers develop. If our products do not achieve significant market acceptance, our business, financial condition and results of operations will be materially harmed.
Risks Related to our Finances
We are an early stage company.
You must evaluate us in light of the uncertainties and complexities present in an early stage biopharmaceutical company. Our product candidates are in early stages of development. We will require significant additional investment in research and development, pre-clinical testing and clinical trials, and regulatory and sales and marketing activities to commercialize current and future product candidates. Our product candidates, if successfully developed, may not generate sufficient or sustainable revenues to enable us to be profitable.
We have a history of losses.
We have incurred net losses in each of the last five years of operation, including net losses of $7.1 million in 1996, $35.9 million in 1997, $16.8 million in 1998, $20.5 million in 1999, $8.8 million in 2000 and $22.4 million in the six months ended June 30, 2001. As of June 30, 2001, our accumulated deficit was $121.0 million. Our losses to date have resulted principally from:
- •
- research and development costs relating to the development of our XenoMouse technology and antibody product candidates;
- •
- costs associated with certain agreements with Japan Tobacco and certain 1997 settlement and cross-licensing agreements with GenPharm International, Inc.;
7
- •
- in-process research and development costs and amortization of intangible assets associated with our acquisitions of Abgenix Biopharma Inc. (formerly known as ImmGenics Pharmaceuticals, Inc.), IntraImmune Therapies, Inc. and Xenotech;
- •
- general and administrative costs relating to our operations.
We expect to incur additional losses for the foreseeable future as a result of increases in our research and development costs, including costs associated with conducting pre-clinical development and clinical trials, and charges related to purchases of technology or other assets. We intend to invest significantly in our products prior to entering into licensing agreements. This will increase our need for capital and will result in losses for several years. We expect that the amount of operating losses will fluctuate significantly from quarter to quarter as a result of increases or decreases in our research and development efforts, the execution or termination of licensing and contractual agreements, and the initiation, success or failure of clinical trials.
Our future profitability is uncertain.
Prior to June 1996, our business was owned by Cell Genesys, Inc. and operated as a business unit of Cell Genesys. Since that time, we have funded our research and development activities primarily from private placements and public offerings of our securities and from revenues generated by our licensing and contractual agreements.
We expect that substantially all of our revenues for the foreseeable future will result from payments under licensing and other contractual arrangements and from interest income. To date, payments under licensing and other agreements have been in the form of option fees, reimbursement for research and development expenses, license fees and milestone payments. Payments under our existing and any future customer agreements will be subject to significant fluctuation in both timing and amount. Our revenues may not be indicative of our future performance or of our ability to continue to achieve such milestones. Our revenues and results of operations for any period may also not be comparable to the revenues or results of operations for any other period. We may not be able to:
- •
- enter into further licensing and other agreements;
- •
- successfully complete pre-clinical development or clinical trials;
- •
- obtain required regulatory approvals;
- •
- successfully develop, manufacture and market product candidates; or
- •
- generate additional revenues or profitability.
If we fail to achieve any of the above goals, our business, financial condition and results of operations will be materially harmed.
We may require additional financing.
We will continue to expend substantial resources for the expansion of research and development, including costs associated with conducting pre-clinical development and clinical trials. We will be required to expend substantial funds in the course of completing required additional development, pre-clinical testing and clinical trials of and regulatory approval for product candidates. Our future liquidity and capital requirements will depend on many factors, including:
- •
- the scope and results of pre-clinical development and clinical trials;
- •
- the retention of existing and establishment of further licensing and other agreements, if any;
- •
- continued scientific progress in our research and development programs;
8
- •
- the size and complexity of these programs;
- •
- the cost of establishing manufacturing capabilities and conducting commercialization activities and arrangements;
- •
- the time and expense involved in seeking regulatory approvals;
- •
- competing technological and market developments;
- •
- the time and expense of filing and prosecuting patent applications and enforcing patent claims;
- •
- our investment in, or acquisition of, other companies;
- •
- the amount of product in-licensing in which we engage; and
- •
- other factors not within our control.
We believe that our current cash balances, cash equivalents, marketable securities, and the cash generated from our licensing and contractual agreements will be sufficient to meet our operating and capital requirements for at least one year. We may choose to obtain additional financing from time to time. We may choose to raise additional funds through public or private financing, licensing and contractual agreements or other arrangements. We cannot be sure that any additional funding, if needed, will be available on terms favorable to us. Furthermore, any additional equity financing may be dilutive to our shareholders, and debt financing, if available, may involve restrictive covenants. We may also choose to obtain funding through licensing and other contractual agreements. Such agreements may require us to relinquish our rights to certain of our technologies, products or marketing territories. Our failure to raise capital when needed would harm our business, financial condition and results of operations.
Risks Related to Our Intellectual Property
Our patent position is uncertain and our success depends on our proprietary rights.
Our success depends in part on our ability to:
- •
- obtain patents;
- •
- protect trade secrets;
- •
- operate without infringing the proprietary rights of others; and
- •
- prevent others from infringing our proprietary rights.
We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our proprietary rights are covered by valid and enforceable patents or are effectively maintained as trade secrets. We own six issued patents in the United States, one granted patent in Europe, and three granted patents in Japan, and we have several pending patent applications in the United States and abroad, each of which relates to XenoMouse technology and products generated by it. Our wholly owned subsidiary, Xenotech, owns two issued U.S. patents, one Australian patent, and several pending U.S. and foreign patent applications related to methods of treatment of bone disease in cancer patients. Our wholly owned subsidiary, Abgenix Biopharma, owns one issued U.S. patent and has one pending patent application in Canada and Europe relating to the Selected Lymphocyte Antibody Method, or SLAM, technology. Our wholly owned subsidiary, IntraImmune Therapies, is licensed under patents and pending applications in the United States and in Europe related to intrabody technology. In addition, we have seven issued U.S. patents and several pending patent applications in the United States and abroad that are jointly owned with Japan Tobacco relating to antibody technology or genetic manipulation. We attempt to protect our proprietary position by filing U.S. and foreign patent applications related to our proprietary technology, inventions and
9
improvements that are important to the development of our business. However, the patent position of biopharmaceutical companies involves complex legal and factual questions, and, therefore, enforceability cannot be predicted with certainty. Patents, if issued, may be challenged, invalidated or circumvented. Thus, any patents that we own or license from third parties may not provide any protection against competitors. Our pending patent applications, those we may file in the future, or those we may license from third parties, may not result in patents being issued. Also, patent rights may not provide us with adequate proprietary protection or competitive advantages against competitors with similar technologies. The laws of certain foreign countries do not protect our intellectual property rights to the same extent as do the laws of the United States.
In addition to patents, we rely on trade secrets and proprietary know-how. We seek protection, in part, through confidentiality and proprietary information agreements. These agreements may not provide meaningful protection or adequate remedies for our technology in the event of unauthorized use or disclosure of confidential and proprietary information, and, in addition, the parties may breach such agreements. Also, our trade secrets may otherwise become known to, or be independently developed by, our competitors. Furthermore, others may independently develop similar technologies or duplicate any technology that we have developed.
We may face challenges from third parties regarding the validity of our patents and proprietary rights.
Research has been conducted for many years in the antibody and transgenic animal fields. This has resulted in a substantial number of issued patents and an even larger number of pending patent applications. The publication of discoveries in the scientific or patent literature frequently occurs substantially later than the date on which the underlying discoveries were made. Our commercial success depends significantly on our ability to operate without infringing the patents and other proprietary rights of third parties. Our technologies may unintentionally infringe the patents or violate other proprietary rights of third parties. In the event of such infringement or violation, we and our customers may be prevented from pursuing product development or commercialization. Such a result could materially harm our business, financial condition and results of operations.
In March 1997, we entered into a cross-license and settlement agreement with GenPharm International, Inc. to avoid protracted litigation. Under the cross-license, we licensed on a non-exclusive basis certain patents, patent applications, third-party licenses and inventions pertaining to the development and use of certain transgenic rodents, including mice, that produce fully human antibodies that are integral to our products and business. Our business, financial condition and results of operations could be materially harmed if any of the parties breaches the cross-license agreement.
We have one granted European patent relating to XenoMouse technology that is currently undergoing opposition proceedings within the European Patent Office and the outcome of this opposition is uncertain.
GlaxoSmithKline, plc, or Glaxo, has a family of patents relating to certain methods for generating monoclonal antibodies that Glaxo is asserting against Genentech, Inc. in litigation that was commenced in 1999. On May 4, 2001, Genentech announced that a jury had determined that Genentech had not infringed Glaxo's patents and that all of the patent claims asserted against Genentech are invalid. We understand that Glaxo has filed a notice of appeal with the Court of Appeals for the Federal Circuit. If any of the claims of these patents are finally determined in the litigation to be valid, and if we were to use manufacturing processes covered by the patents to make our products, we may then need to obtain a license should one be available. Should a license be denied or unavailable on commercially reasonable terms, commercialization of one or more of our products could be impeded in any territories in which these claims were in force.
Genentech, Inc. owns a U.S. patent that issued in June 1998 relating to inhibiting the growth of tumor cells that involves an anti-EGF receptor antibody in combination with a cytotoxic factor.
10
ImClone Systems, Inc. owns or is licensed under a U.S. patent that issued in April 2001, relating to inhibiting the growth of tumor cells that involves an anti-EGF receptor antibody in combination with an anti-neoplastic agent. We believe there are strong arguments that all claims of both the Genentech patent and the ImClone patent are invalid. We are continuing to analyze the scope of these patents. We believe that currently all of the Company's activities relating to anti-EGFr monoclonal antibodies are within the exemption provided by the U.S. patent laws for uses reasonably related to obtaining FDA approval of a drug. Based on our product development plans, we do not expect the scope of our activities in this regard to change in the future prior to filing an application for a biologic license with the FDA. If the claims of either the Genentech patent or the ImClone patent are judicially determined to cover our activities with ABX-EGF and are held valid, we may be required to obtain a license to Genentech's or ImClone's patent, as the case may be, to label and sell ABX-EGF for some or all such combination indications. Should a license be denied or unavailable on commercially reasonable terms, our commercialization of ABX-EGF could be impeded in the United States.
In 2000, the Japanese Patent Office granted a patent to Kirin Beer Kabushiki Kaisha, one of our competitors, relating to non-human transgenic mammals. Kirin has filed corresponding patent applications in Europe and Australia. Kirin may also have filed a corresponding patent application in the United States. Our licensee, Japan Tobacco, has filed opposition proceedings against the Kirin patent. We cannot predict the outcome of those opposition proceedings, which may take years to be resolved. We are analyzing the patent to determine its relevance to our business and if appropriate will analyze the scope and validity of its claims.
The biotechnology and pharmaceutical industries have been characterized by extensive litigation regarding patents and other intellectual property rights. The defense and prosecution of intellectual property suits, United States Patent and Trademark Office interference proceedings, and related legal and administrative proceedings in the United States and internationally involve complex legal and factual questions. As a result, such proceedings are costly and time-consuming to pursue and their outcome is uncertain. Litigation may be necessary to:
- •
- enforce patents that we own or license;
- •
- protect trade secrets or know-how that we own or license; or
- •
- determine the enforceability, scope and validity of the proprietary rights of others.
If we become involved in any litigation, interference or other administrative proceedings, we could incur substantial expense and the efforts of our technical and management personnel could be significantly diverted. An adverse determination may subject us to loss of our proprietary position or to significant liabilities, or require us to seek licenses that may not be available from third parties. We may be restricted or prevented from manufacturing and selling our products, if any, in the event of an adverse determination in a judicial or administrative proceeding or if we fail to obtain necessary licenses. Costs associated with these arrangements may be substantial and may include ongoing royalties. Furthermore, we may not be able to obtain the necessary licenses on satisfactory terms, if at all. These outcomes could materially harm our business, financial condition and results of operations.
Risks Related to Our Industry
We face intense competition and rapid technological change.
The biotechnology and pharmaceutical industries are highly competitive and subject to significant and rapid technological change. We are aware of several pharmaceutical and biotechnology companies that are actively engaged in research and development in areas related to antibody therapy. These companies have commenced clinical trials of antibody product candidates or have successfully commercialized antibody products. Many of these companies are addressing the same diseases and disease indications as us or our customers. Also, we compete with companies that offer antibody
11
generation services to companies that have antigens. These competitors have specific expertise or technology related to antibody development and introduce new or modified technologies from time to time. These companies include GenPharm International, Inc., a wholly-owned subsidiary of Medarex, Inc.; Medarex's joint venture partner, Kirin Brewing Co., Ltd.; Cambridge Antibody Technology Group plc; Protein Design Labs, Inc.; and MorphoSys AG.
Some of our competitors have received regulatory approval of or are developing or testing product candidates that may compete directly with our product candidates. For example, SangStat Medical Corp., Novartis, Pharmacia Corporation and Roche market organ transplant rejection products that may compete with ABX-CBL, which is in clinical trials. In addition, MedImmune, Inc. has a potential antibody product candidate in clinical trials for graft versus host disease that may compete with ABX-CBL. We are also aware that several companies, including Genentech, Inc., Biogen, Inc. and Immunex Corporation have potential product candidates for the treatment of psoriasis that may compete with ABX-IL8, which is in clinical trials. Furthermore, we are aware that ImClone Systems, Inc., AstraZeneca PLC, GlaxoSmithKline and a collaboration of OSI Pharmaceuticals, Inc., Genentech, Inc. and Roche have potential antibody and small molecule product candidates in clinical development that may compete with ABX-EGF, which is also in clinical trials.
Many of these companies and institutions, either alone or together with their customers, have substantially greater financial resources and larger research and development staffs than we do. In addition, many of these competitors, either alone or together with their customers, have significantly greater experience than we do in:
- •
- developing products;
- •
- undertaking pre-clinical testing and human clinical trials;
- •
- obtaining FDA and other regulatory approvals of products; and
- •
- manufacturing and marketing products.
Accordingly, our competitors may succeed in obtaining patent protection, receiving FDA approval or commercializing products before we do. If we commence commercial product sales, we will be competing against companies with greater marketing and manufacturing capabilities, areas in which we have limited or no experience.
We also face, and will continue to face, competition from academic institutions, government agencies and research institutions. There are numerous competitors working on products to treat each of the diseases for which we are seeking to develop therapeutic products. In addition, any product candidate that we successfully develop may compete with existing therapies that have long histories of safe and effective use. Competition may also arise from:
- •
- other drug development technologies and methods of preventing or reducing the incidence of disease;
- •
- new small molecules; or
- •
- other classes of therapeutic agents.
Developments by competitors may render our product candidates or technologies obsolete or non-competitive. We face and will continue to face intense competition from other companies for agreements with pharmaceutical and biotechnology companies for establishing relationships with academic and research institutions, and for licenses to proprietary technology. These competitors, either alone or with their customers, may succeed in developing technologies or products that are more effective than ours.
12
We face uncertainty over reimbursement and healthcare reform.
In both domestic and foreign markets, sales of our product candidates will depend in part upon the availability of reimbursement from third-party payors. Such third-party payors include government health administration authorities, managed care providers, private health insurers and other organizations. These third-party payors are increasingly challenging the price and examining the cost effectiveness of medical products and services. In addition, significant uncertainty exists as to the reimbursement status of newly approved healthcare products. We may need to conduct post-marketing studies in order to demonstrate the cost-effectiveness of our products. Such studies may require us to provide a significant amount of resources. Our product candidates may not be considered cost-effective. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development. Domestic and foreign governments continue to propose and pass legislation designed to reduce the cost of healthcare. Accordingly, legislation and regulations affecting the pricing of pharmaceuticals may change before our proposed products are approved for marketing. Adoption of such legislation could further limit reimbursement for pharmaceuticals. If the government and third-party payors fail to provide adequate coverage and reimbursement rates for our product candidates, the market acceptance of our products may be adversely affected. If our products do not receive market acceptance, our business, financial condition and results of operations will be materially harmed.
Other Risks Related to Our Company
We acquired Abgenix Biopharma, a Vancouver-based biotechnology company, in November 2000. We may experience difficulty in the integration of this acquisition, or any future acquisition, with the operations of our business.
In November 2000, we acquired all of the voting stock of Abgenix Biopharma, a Canadian biotechnology company that develops and intends to commercialize antibody-based therapeutic and diagnostic products for the treatment and diagnosis of a variety of diseases, for an aggregate consideration of approximately $77.2 million.
We have a limited history of operating the business of our company and Abgenix Biopharma on a consolidated basis, and we have no prior experience operating a business outside of the United States. We may have difficulty integrating Abgenix Biopharma's research and development operations with our own. Difficulty managing the integration of Abgenix Biopharma could result from many factors, some of which are beyond our control, including the following:
- •
- the geographic distance between our Fremont, California headquarters and our acquired Vancouver, British Columbia subsidiary;
- •
- potential differences in research and development protocols between Abgenix Biopharma and ourselves; and
- •
- the potential loss of personnel from our acquired operations.
In the future, we may from time to time seek to expand our business through additional corporate acquisitions. Our acquisition of companies and businesses and expansion of operations, involve risks such as the following:
- •
- the potential inability to identify target companies best suited to our business plan;
- •
- the potential inability to successfully integrate acquired operations and businesses and to realize anticipated synergies, economies of scale or other expected value;
- •
- incurrence of expenses attendant to transactions that may or may not be consummated; and
13
- •
- difficulties in managing and coordinating operations at multiple venues, which, among other things, could divert our management's attention from other important business matters.
In addition, our acquisition of companies and businesses and expansion of operations, including the recent acquisition of Abgenix Biopharma, may result in dilutive issuances of equity securities, the incurrence of additional debt, large one-time write-offs and the creation of goodwill or other intangible assets that could result in amortization expense.
We depend on key personnel and must continue to attract and retain key employees and consultants.
We are highly dependent on the principal members of our scientific and management staff. For us to pursue product development, marketing and commercialization plans, we will need to hire additional qualified scientific personnel to perform research and development. We will also need to hire personnel with expertise in clinical testing, government regulation, manufacturing, marketing and finance. Attracting and retaining qualified personnel will be critical to our success. We may not be able to attract and retain personnel on acceptable terms given the competition for such personnel among biotechnology, pharmaceutical and healthcare companies, universities and non-profit research institutions. If we lose any of these persons, or are unable to attract and retain qualified personnel, our business, financial condition and results of operations may be materially harmed.
In addition, we rely on members of our Scientific Advisory Board and other consultants to assist us in formulating our research and development strategy. All of our consultants and the members of our Scientific Advisory Board are employed by other entities. They may have commitments to, or advisory or consulting agreements with, other entities that may limit their availability to us. If we lose the services of these advisors, the achievement of our development objectives may be impeded. Such impediments may materially harm our business, financial condition and results of operations.
We have implemented a stockholder rights plan and are subject to other anti-takeover provisions.
In June 1999, our board of directors adopted a stockholder rights plan, which was amended in November 1999. The stockholder rights plan and certain provisions of our amended and restated certificate of incorporation and amended and restated bylaws may have the effect of making it more difficult for a third party to acquire, or of discouraging a third party from attempting to acquire, control of us. This could limit the price that certain investors might be willing to pay in the future for our common stock. Certain provisions of our amended and restated certificate of incorporation and amended and restated bylaws allow us to:
- •
- issue preferred stock without any vote or further action by the stockholders;
- •
- eliminate the right of stockholders to act by written consent without a meeting;
- •
- specify procedures for director nominations by stockholders and submission of other proposals for consideration at stockholder meetings; and
- •
- eliminate cumulative voting in the election of directors.
We are subject to certain provisions of Delaware law which could also delay or make more difficult a merger, tender offer or proxy contest involving us. In particular, Section 203 of the Delaware General Corporation Law prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years unless certain conditions are met. The stockholder rights plan, the possible issuance of preferred stock, the procedures required for director nominations and stockholder proposals and Delaware law could have the effect of delaying, deferring or preventing a change in control of us, including, without limitation, discouraging a proxy contest or making more difficult the acquisition of a substantial block of our common stock. The provisions could also limit the price that investors might be willing to pay in the future for shares of our common stock.
14
We face product liability risks and may not be able to obtain adequate insurance.
The use of any of our product candidates in clinical trials, and the sale of any approved products, may expose us to liability claims resulting from such use or sale of our products. These claims might be made directly by consumers, healthcare providers or by pharmaceutical companies or others selling such products. We may experience financial losses in the future due to product liability claims. We have obtained limited product liability insurance coverage for our clinical trials, under which the coverage limits are $5.0 million per occurrence and $5.0 million in the aggregate. We intend to expand our insurance coverage to include the sale of commercial products if marketing approval is obtained for product candidates in development. We may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses. If a successful product liability claim or series of claims is brought against us for uninsured liabilities or in excess of insured liabilities, our business, financial condition and results of operations may be materially harmed.
Our operations involve hazardous materials.
Our research and manufacturing activities involve the controlled use of hazardous materials. We cannot eliminate the risk of accidental contamination or injury from these materials. In the event of an accident or environmental discharge, we may be held liable for any resulting damages, which may exceed our financial resources and may materially harm our business, financial condition and results of operations.
We do not intend to pay cash dividends on our common stock.
We intend to retain any future earnings to finance the growth and development of our business and we do not plan to pay cash dividends on our common stock in the foreseeable future.
Our stock price is highly volatile.
The market price and trading volume of our common stock are volatile, and we expect such volatility to continue for the foreseeable future. For example, during the period between June 30, 2000 and June 30, 2001, our common stock closed as high as $93.1875 per share and as low as $16.75 per share. This may impact your decision to buy or sell our common stock. Factors affecting our stock price include:
- •
- our financial results;
- •
- fluctuations in our operating results;
- •
- announcements of technological innovations or new commercial therapeutic products by us or our competitors;
- •
- published reports by securities analysts;
- •
- progress with clinical trials;
- •
- government regulation;
- •
- changes in reimbursement policies;
- •
- developments in patent or other proprietary rights;
- •
- developments in our relationship with customers;
- •
- public concern as to the safety and efficacy of our products; and
- •
- general market conditions.
15
The state of California is currently experiencing a shortage of electrical energy that may cause certain of our operations to be suspended temporarily.
Substantially all of our operations in Fremont, California are run by electrical energy purchased from a local utility. We have not experienced energy shortages and do not anticipate any significant difficulties in the foreseeable future. We have limited back-up generating capacity. Extended shortages of energy could slow our research efforts and increase our operating costs.
16
CERTAIN INFORMATION
We were incorporated on June 24, 1996, and subsequently on July 15, 1996, were organized pursuant to a stock purchase and transfer agreement with Cell Genesys. Our business and operations were started in 1989 by Cell Genesys and prior to our organization were conducted within Cell Genesys. In 1991, Cell Genesys and JT Immunotech USA, Inc., the predecessor company to JT America and a medical subsidiary of Japan Tobacco, formed Xenotech, an equally owned joint venture, to develop genetically modified strains of mice known as XenoMouse animals or mice that can produce fully human monoclonal antibodies and to commercialize products generated from these mice. At the time of our organization, Cell Genesys assigned to us substantially all of its rights in Xenotech. On December 31, 1999, we became the sole owner of Xenotech by buying JT America's interest therein. As used in this prospectus, Japan Tobacco refers to either or both of Japan Tobacco or its wholly-owned subsidiary, JT America. Our principal executive offices are located at 6701 Kaiser Drive, Fremont, California 94555, and our telephone number is (510) 608-6500.
Unless otherwise indicated, the information in this prospectus is based on 86,156,183 shares outstanding on July 31, 2001 and:
- •
- excludes 12,656,057 shares of common stock issuable upon exercise of options outstanding as of July 31, 2001 under our various stock incentive plans; and
- •
- excludes 100,000 shares of common stock issuable pursuant to the terms of a license agreement.
USE OF PROCEEDS
We will not receive any proceeds from the sale of the shares of common stock offered by the selling shareholders pursuant to this prospectus.
17
PRICE RANGE OF COMMON STOCK
Our common stock began trading publicly on the Nasdaq National Market on July 2, 1998, under the symbol "ABGX." The following table lists quarterly information on the price range of our common stock based on the high and low reported closing prices for our common stock as reported on the Nasdaq National Market for the periods indicated below, as adjusted to reflect a two-for-one common stock split effective on April 6, 2000 and a two-for-one common stock split effective on July 7, 2000. These prices do not include retail markups, markdowns or commissions.
| | High
| | Low
|
|---|
| Fiscal 1999: | | | | | | |
| | First Quarter | | $ | 4.53 | | $ | 3.31 |
| | Second Quarter | | | 4.97 | | | 3.31 |
| | Third Quarter | | | 11.91 | | | 4.72 |
| | Fourth Quarter | | | 33.13 | | | 9.32 |
| Fiscal 2000: | | | | | | |
| | First Quarter | | $ | 99.75 | | $ | 29.03 |
| | Second Quarter | | | 69.02 | | | 32.31 |
| | Third Quarter | | | 85.81 | | | 50.13 |
| | Fourth Quarter | | | 93.19 | | | 46.81 |
| Fiscal 2001: | | | | | | |
| | First Quarter | | $ | 52.31 | | $ | 16.75 |
| | Second Quarter | | | 46.15 | | | 18.06 |
| | Third Quarter (through August 27, 2001) | | | 44.10 | | | 23.51 |
As of July 31, 2001, there were approximately 229 holders of record of our common stock. On August 27, 2001, the closing price on the Nasdaq National Market for our common stock was $30.08.
DIVIDEND POLICY
We have never declared or paid cash dividends on our common stock. We currently expect to retain our future earnings, if any, for use in the operation and expansion of our business and do not anticipate paying any cash dividends on our common stock in the foreseeable future.
18
SELECTED CONSOLIDATED FINANCIAL DATA
The consolidated statement of operations data for the years ended December 31, 1998, 1999 and 2000 and the consolidated balance sheet data as of December 31, 1999 and 2000 are derived from our audited consolidated financial statements. These financial statements are included elsewhere in this prospectus. The balance sheet data at December 31, 1996, 1997 and 1998, and the statement of operations data for the years ended December 31, 1996 and 1997, are derived from our audited financial statements. These financial statements are not included in this prospectus. The selected data as of June 30, 2001 and for each of the six-month periods ended June 30, 2000 and 2001 have been derived from our unaudited consolidated financial statements included in this prospectus, which reflect, in our management's judgment, all adjustments, consisting only of normal recurring adjustments, necessary for a fair presentation of the results of these periods. The results for the six-month period ended June 30, 2001 are not necessarily indicative of results for the full year.
You should read the following selected consolidated financial data in conjunction with our financial statements and notes that are included elsewhere in this prospectus and "Management's Discussion and Analysis of Financial Condition and Results of Operations."
| | Years Ended December 31,
| | Six Months Ended June 30,
| |
|---|
| | 2000
| | 1999
| | 1998
| | 1997
| | 1996
| | 2001
| | 2000
| |
|---|
| | (In thousands, except share and per share data)
| |
|---|
| Consolidated Statement of Operations Data: | | | | | | | | | | | | | | | | | | | | | | |
| Revenues | | | | | | | | | | | | | | | | | | | | | | |
| Contract revenue | | $ | 26,601 | | $ | 12,285 | | $ | 2,498 | | $ | 611 | | $ | — | | $ | 12,530 | | $ | 5,443 | |
| Revenue under collaborative agreements from related parties | | | — | | | — | | | 1,344 | | | 1,343 | | | 4,719 | | | — | | | — | |
| Interest income | | | 32,848 | | | 3,045 | | | 961 | | | 307 | | | 203 | | | 17,971 | | | 13,122 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| Total revenues | | | 59,449 | | | 15,330 | | | 4,803 | | | 2,261 | | | 4,922 | | | 30,501 | | | 18,565 | |
| Costs and expenses: | | | | | | | | | | | | | | | | | | | | | | |
| Research and development | | | 51,329 | | | 21,106 | | | 17,588 | | | 11,405 | | | 9,433 | | | 42,383 | | | 19,126 | |
| Amortization of intangible assets, related to research and development | | | 3,992 | | | — | | | — | | | — | | | — | | | 4,093 | | | 1,553 | |
| General and administrative | | | 7,667 | | | 5,164 | | | 3,405 | | | 3,525 | | | 2,565 | | | 6,200 | | | 3,390 | |
| In process research and development charge | | | 5,215 | | | — | | | — | | | — | | | — | | | — | | | — | |
| Charge for cross-license and settlement amount allocated from Cell Genesys | | | — | | | — | | | — | | | 11,250 | | | — | | | — | | | — | |
| Equity in (income) losses from the Xenotech joint venture | | | — | | | (546 | ) | | 107 | | | 11,250 | | | — | | | — | | | — | |
| Non-recurring termination fee | | | — | | | 8,667 | | | — | | | — | | | — | | | — | | | — | |
| Interest expense | | | 39 | | | 438 | | | 530 | | | 711 | | | 24 | | | 255 | | | 300 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| Total costs and expenses | | | 68,242 | | | 34,829 | | | 21,630 | | | 38,141 | | | 12,022 | | | 52,931 | | | 24,369 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| Loss before income taxes | | | (8,793 | ) | | (19,499 | ) | | (16,827 | ) | | (35,880 | ) | | (7,100 | ) | | (22,430 | ) | | (5,804 | ) |
| Foreign income tax expense | | | — | | | 1,000 | | | — | | | — | | | — | | | — | | | — | |
| | |
| |
| |
| |
| |
| |
| |
| |
| Net loss | | $ | (8,793 | ) | $ | (20,499 | ) | $ | (16,827 | ) | $ | (35,880 | ) | $ | (7,100 | ) | $ | (22,430 | ) | $ | (5,804 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| Net loss per share | | $ | (0.11 | ) | $ | (0.35 | ) | $ | (0.75 | ) | $ | (258.13 | ) | $ | (11,677.63 | ) | $ | (0.26 | ) | $ | (0.07 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| Shares used in computing net loss per share | | | 80,076 | | | 58,148 | | | 22,412 | | | 139 | | | 0.61 | | | 85,805 | | | 77,522 | |
| | December 31,
| |
| |
|---|
| | June 30,
2001
| |
|---|
| | 2000
| | 1999
| | 1998
| | 1997
| | 1996
| |
|---|
| | (In thousands)
| |
|---|
| Consolidated Balance Sheet Data: | | | | | | | | | | | | | | | | | | | |
| Cash, cash equivalents, marketable securities and interest receivable | | $ | 702,676 | | $ | 58,012 | | $ | 16,744 | | $ | 15,321 | | $ | 10,172 | | $ | 581,430 | |
| Working capital | | | 621,480 | | | 56,113 | | | 13,101 | | | 6,637 | | | 5,564 | | | 572,642 | |
| Total assets | | | 936,800 | | | 148,541 | | | 24,220 | | | 22,084 | | | 14,357 | | | 869,101 | |
| Long term debt, less current portion | | | — | | | 421 | | | 2,180 | | | 3,979 | | | 1,757 | | | — | |
| Redeemable convertible stock | | | — | | | — | | | — | | | 31,189 | | | 10,150 | | | — | |
| Accumulated deficit | | | (98,593 | ) | | (89,800 | ) | | (69,301 | ) | | (52,474 | ) | | (16,594 | ) | | (121,022 | ) |
| Total stockholders' equity | | | 839,675 | | | 137,060 | | | 16,959 | | | (22,318 | ) | | (2,316 | ) | | 843,394 | |
19
MANAGEMENT'S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
"Management's Discussion and Analysis of Financial Condition and Results of Operations" contains forward-looking statements based upon current expectations that involve risks and uncertainties. When used in this prospectus, the words "intend," "anticipate," "believe," "estimate," "plan" and "expect" and similar expressions as they relate to us are included to identify forward-looking statements. Our actual results and the timing of certain events could differ materially from those anticipated in these forward-looking statements as a result of certain factors, including those set forth under "Risk Factors" and elsewhere in this prospectus.
Overview
We are a biopharmaceutical company that develops and intends to commercialize antibody therapeutic products for the treatment of a variety of disease conditions, including transplant-related diseases, inflammatory and autoimmune disorders, cardiovascular disease, infectious diseases and cancer. We have proprietary technologies that facilitate rapid generation of highly specific, fully human antibody product candidates that bind to disease targets appropriate for antibody therapy. We developed our XenoMouse® technology, a technology utilizing genetically modified mice. We also own a technology that enables the rapid identification of antibodies with desired function and characteristics, referred to as SLAM™ technology. In our newly developed XenoMax™ technology, we use SLAM technology to select and isolate antibodies with particular function and characteristics from antibody-producing cells generated by XenoMouse animals. We believe XenoMax technology enhances our capabilities in product development and flexibility in manufacturing. We intend to use our technologies to build a large and diversified product portfolio that we expect to develop and commercialize through licensing arrangements with pharmaceutical companies and others, through joint development and through internal product development programs. We have entered into a variety of contractual arrangements with multiple pharmaceutical, biotechnology and genomics companies involving our XenoMouse technology. Two of our customers, Pfizer, Inc. and Amgen, Inc., have initiated clinical trials with fully human antibodies generated from XenoMouse animals. In addition, we have three proprietary antibody product candidates currently in clinical trials, two of which we have agreed to co-develop and commercialize with others.
As of August 27, 2001, we had entered into contracts covering numerous antigen targets with twenty-nine customers to use our XenoMouse technology or SLAM technology to produce and/or develop the resulting fully human antibody product candidates. Pursuant to these contracts, we and our customers intend to generate antibody product candidates for the treatment of cancer, inflammation, autoimmune diseases, transplant rejection, cardiovascular disease, growth factor modulation, neurological diseases and infectious diseases. We expect that substantially all of our revenues for the foreseeable future will result from payments under these and other contracts. The terms of these arrangements vary, but can generally be categorized as follows:
- •
- Antigen Target Sourcing Contracts—We have entered into several target sourcing contracts with genomics and biopharmaceutical companies that may enable us to generate a pipeline of proprietary fully human antibody product candidates. Typically, these contracts provide for Abgenix to make fully human antibodies to the antigen targets provided or identified by the contract counterparty. The contracts typically contain provisions that allow either Abgenix or the other contract party to evaluate and select particular antibodies from the pool of generated antibodies for further development and commercialization. The party selecting a product candidate for further development or commercialization will generally pay to the other party license fees, milestone payments and royalty payments on any eventual product sales, in exchange for rights to develop and commercialize the product.
20
- •
- Proprietary Product Development—In July and August 2000, we entered into two joint development and commercialization agreements. The first is with Immunex Corporation for ABX-EGF, a fully human antibody created by us. Under the agreement, Immunex paid us an initial license fee at signing and a second license fee in May 2001. Development costs will be shared equally, as would any profits from sales of collaboration products. We and Immunex share responsibility for product development. We will be responsible for completing the Phase I trial and any additional Phase I trials that are initiated, and both companies share responsibility for the execution of Phase II trials across a variety of indications. Immunex will have primary responsibility for Phase III clinical trials and will market any potential product, while we will retain co-promotion rights. The second agreement is with SangStat Medical Corporation for ABX-CBL, an antibody developed by us. Under that agreement, SangStat paid us an initial license fee in 2000 and a second milestone payment in June 2001, and has agreed to make a final milestone payment in 2002. Development costs will be shared equally, as would any profits from sales of collaboration products. We and SangStat share responsibility for product development, including the ongoing Phase II/III clinical trials. SangStat will market any potential product and we will be responsible for manufacturing ABX-CBL.
- •
- Technology Licensing—We also license our XenoMouse technology to third parties for the purpose of generating antibody product candidates to one or more specific antigen targets provided by the customer. In most cases, we provide our mice to the customers who then carry out immunizations with their specific antigen targets. In other cases, we immunize the mice with the customers' antigen targets for additional compensation. The customer generally has an option for a period of time to acquire a product license for any antibody product identified using XenoMouse technology that the customer wishes to develop and commercialize. The financial terms of these agreements may include license fees, option fees and milestone payments paid to us by the customers. Based on our agreements, these payments and fees would average $8.0 to $10.0 million per antigen target if our customer takes the antibody product candidate into development and ultimately to commercialization. Additionally, our license agreements entitle us to receive royalties on any future product sales by the customer.
Our dependence on contracts with third parties subjects us to a number of risks and uncertainties. Agreements with licensees typically allow the licensees significant discretion in electing whether to pursue any of the planned activities. We cannot control the amount or timing of resources our licensees may devote to the product candidates. Even if we fulfill our obligations under an agreement, the licensee can terminate the agreement at any time following proper written notice. If any licensee were to terminate or breach its agreement with us, or otherwise fail to complete its obligations in a timely manner, our business, financial condition and results of operations may be materially harmed.
We have three proprietary antibody product candidates that are currently in clinical trials, two of which are now being co-developed with our collaborators, as follows:
- •
- ABX-IL8—Generated using XenoMouse technology, ABX-IL8 is our fully human antibody candidate for the treatment of inflammatory diseases, including psoriasis and rheumatoid arthritis.
- •
- The status of clinical trials for ABX-IL8 is as follows:
- •
- Psoriasis—We have completed Phase I, Phase I/II and Phase IIa clinical trials. We initiated a Phase IIb clinical trial in February 2001 and enrollment is ongoing.
- •
- Rheumatoid arthritis—We initiated a Phase IIa clinical trial in December 2000. Enrollment is complete and patient treatment is ongoing.
- •
- ABX-EGF—Generated using XenoMouse technology, ABX-EGF is our fully human antibody candidate for the treatment of a variety of cancers. In July 2000, we entered into the joint
21
- •
- ABX-CBL—We developed ABX-CBL, an in-licensed mouse antibody, for the treatment of a transplant-related disease known as graft versus host disease, or GVHD. We have completed a multi-center Phase II clinical trial for ABX-CBL and initiated a Phase II/III clinical trial in December 1999 in which enrollment is ongoing. In August 2000, we entered into the joint development and commercialization agreement with SangStat Medical Corporation for ABX-CBL described above.
We will expend significant capital to conduct clinical trials for our proprietary product candidates, including several Phase II clinical trials we have initiated or plan to initiate in 2001 and 2002. We believe that more extensive clinical data will enable us to enter into additional contractual arrangements related to those proprietary product candidates. We expect that this will substantially increase our capital needs over the next few years and increase our operating losses. However, we believe that we will be able to receive more favorable proceeds from our contract parties if we have completed significant development of these products.
In addition to our proprietary antibody product candidates in clinical trials, there are two customer-developed antibodies generated with XenoMouse technology in clinical trials as follows:
- •
- Pfizer, Inc.—We generated a XenoMouse-derived antibody for treating cancer, which Pfizer has advanced into clinical trials.
- •
- Amgen, Inc.—We generated a XenoMouse-derived antibody that binds to an undisclosed antigen target, which Amgen has advanced into clinical trials.
Internal Development Activities
We intend to build our product portfolio by using our XenoMouse and XenoMax technologies to generate antibodies to antigen targets that we source, self-funding clinical activities to determine preliminary safety and efficacy, and entering into more development and commercialization agreements with pharmaceutical and biotechnology companies. We plan to enter into agreements to use our XenoMax technology to assist our licensees and collaborators in isolating antibodies with desired functions and characteristics. These arrangements may or may not involve joint sharing of costs and profits.
22
Results of Operations
Three Months and Six Months Ended June 30, 2001 and 2000
Contract revenue totaled $8.4 million and $12.5 million in the three and six-month periods ended June 30, 2001 compared to $3.5 million and $5.4 million, respectively, in the comparable 2000 periods. The primary components for both periods were as follows:
- •
- Proprietary Product Development
A total of $6.4 and $9.0 million was recognized in the three and six-month periods ended June 30, 2001, including license fees, reimbursement of development costs and milestone fees under joint development and commercialization agreements with Immunex Corporation and SangStat Medical Corporation for the development of ABX-EGF and ABX-CBL, respectively. As the agreements were executed in the third quarter of 2000, no revenue was recognized related to these agreements in the comparable three and six-month periods ended June 30, 2000. Under these agreements, we recognize license fees ratably over the minimum periods we are obligated to share in development costs. Under the Immunex agreement, this is the 17-month period ending December 31, 2001. Under the SangStat agreement, this was the 6-month period ended January 31, 2001.
- •
- Technology Licensing
A total of $2.0 and $3.5 million was recognized in the three and six-month periods ended June 30, 2001, including license fees, research fees, option fees and milestone fees primarily from Amgen Inc., Pfizer, Inc., Centocor, Inc., CuraGen Corporation and Chiron Corporation. Included in these amounts was a milestone fee from Amgen Inc. for the advancement of a XenoMouse derived antibody into clinical trials.
A total of $3.5 and $5.4 was recognized in the three and six-month periods ended June 30, 2000, including license fees, research fees and option fees primarily from Millennium Pharmaceutical Inc., Pfizer, Inc., Japan Tobacco Inc. and CuraGen Corporation. The primary component of this revenue was research and license fees related to an agreement with Millennium Pharmaceuticals Inc. in which we granted several licenses to make, use and sell antibodies generated with our XenoMouse technology. Payments totaling $10.0 million were received in the first quarter of 2000 representing a research license fee, product license fees and service fees to establish XenoMouse technology at Millennium. We recognized these fees ratably each month over the period ended December 31, 2000, during which we fulfilled our obligation to assist in establishing XenoMouse technology at Millennium, enabling Millennium to practice the research license and product licenses. No such fees were recognized in the three and six-month periods ended June 30, 2001.
Interest and other income consist primarily of interest from cash, cash equivalents and marketable securities. Interest and other income decreased to $7.7 million in the three months ended June 30, 2001 compared to $8.8 million in the same period in 2000. Interest and other income increased to $18.0 million in the six-month period ended June 30, 2001 compared to $13.1 million in the same period in 2000. Interest and other income in the second quarter of 2001 decreased $2.6 million compared to the first quarter of 2001. The decrease in interest and other income for the three months ended June 30, 2001 compared to the same period one year ago and the decrease in interest and other income sequentially from the first quarter of 2001 was primarily due to lower cash, marketable securities and cash equivalent balances as well as lower interest rates. The increase in interest and other income for the six months ended June 30, 2001 compared to the same period in 2000 is the result of higher average balances of our marketable securities and cash equivalents as a result of the investment of $717.1 million of net proceeds from a follow-on offering in February 2000 and a private placement in November 2000.
23
Research and development expenses consist primarily of compensation and other expenses related to research and development personnel; costs associated with pre-clinical testing and clinical trials of our product candidates, including the costs of manufacturing the product candidates; and facilities expenses. Research and development expenses increased to $25.6 million in the three months ended June 30, 2001 from $11.9 million in the comparable period in 2000 and to $42.4 million in the six months ended June 30, 2001 from $19.1 million in the comparable period in 2000. The increase is primarily due to costs associated with the following:
- •
- Increased Personnel—Staffing at June 30, 2001 increased by approximately 141% from June 30, 2000. The increase in staff is to support the increased level of product development activities, including new target validation, process sciences, manufacturing and clinical activities. Also, the increase includes additional employees resulting from our acquisition of our Canadian subsidiary, Abgenix Biopharma Inc., in November 2000. Included in the increase are salary, related fringe benefits, and recruiting and relocation costs. We expect personnel costs to increase further as we continue to build our organization.
- •
- Facility Costs—Related to the increased staffing, we acquired new facilities and related leasehold improvements, furniture and fixtures. As a result, rent, depreciation and utilities have increased in the three and six-month periods ended June 30, 2001 as compared to the same periods in 2000. We expect facility costs to increase in future periods as a result of our capital expansion plans.
- •
- Research Costs—In the first quarter of 2001, we entered into an agreement with Impath Inc. under which Impath will evaluate target expression by performing certain tissue studies, enabling us to better identify potential diagnostic and therapeutic products. The agreement includes a monthly fee payable over 13 months. Also, research costs and lab supplies costs have increased in the first quarter of 2001 in comparison to the first quarter of 2000 as a result of the acquisition of Abgenix Biopharma as well as increased research activity and staff in the United States.
- •
- Clinical Costs—Clinical costs have increased in 2001 as we have initiated new clinical trials and progressed to later stage clinical trials for our three product candidates, ABX-CBL, ABX-IL8 and ABX-EGF. The costs of such trials include the clinical investigator site fees, monitoring costs and data management costs. Additionally, such costs include the costs of manufacturing the antibody used in clinical trials. In July and August 2000, we entered into separate agreements with Immunex and SangStat to share equally in the costs of developing and commercializing ABX-EGF and ABX-CBL, respectively. However, we expect clinical costs will increase in the future as we enter additional clinical trials for both new and existing product candidates.
- •
- Toxicology Costs—Related to our increased clinical trial activity, we incurred higher costs for toxicology studies in the three and six-month periods ended June 30, 2001 in comparison to the comparable periods in 2000.
Amortization of intangible assets relates to existing technology (including patents and certain royalty rights), goodwill and assembled workforce acquired through the acquisitions of Abgenix Biopharma and IntraImmune Therapies Inc. in November 2000 and Xenotech in December 1999. As a result of the acquisitions in 2000, amortization increased to $2.0 million in the three months ended June 30, 2001 from $0.8 million in the comparable period in 2000 and to $4.1 million in the six months ended June 30, 2001 from $1.6 million in the comparable period in 2000.
General and administrative expenses include compensation and other expenses related to finance and administrative personnel, professional services and facilities. General and administrative expenses
24
increased to $3.1 million in the three months ended June 30, 2001 from $1.8 million in the comparable period in 2000 and to $6.2 million in the six months ended June 30, 2001 from $3.4 million in the comparable period in 2000. The increase reflects increased personnel costs, including recruiting costs and incentive compensation and additional consulting costs associated with our Information Services group. The increase is also related to the acquisition of Abgenix Biopharma. We expect personnel costs to increase further as we continue to build our organization, including consulting costs associated with new information systems.
Years Ended December 31, 2000, 1999 and 1998
Contract revenues increased to $26.6 million in 2000 from $12.3 million in 1999. Contract revenue in 2000 included the following: Research and license fees of $10.0 million were recognized under an agreement with Millennium Pharmaceuticals in which we granted several licenses to make, use and sell antibodies generated with XenoMouse. Payments totaling $10.0 million were received in the first quarter of 2000 representing a research license fee, product license fees and service fees to establish the technology at Millennium Pharmaceuticals Inc. We recognized these fees ratably each month over the period ended December 31, 2000 during which Abgenix fulfilled its obligation to assist in establishing the technology at Millennium, enabling Millennium to practice the research license and product licenses. A total of $6.4 million was recognized under agreements related to the respective joint development and commercialization agreements with Immunex and SangStat, for the development of ABX-EGF and ABX-CBL, respectively. License fees of $7.0 million were received in 2000 and we are recognizing these fees ratably over the minimum periods we are obligated to share in development costs. For Immunex this is the 17-month period ended December 31, 2001 and the amount recognized in 2000 was $1.5 million. For SangStat this is the six-month period ending January 31, 2001, and the amount recognized in 2000 was $1.7 million. Additionally, in 2000, we recognized in total $3.2 million as revenue from both Immunex and SangStat, which represents 50% of the development costs of ABX-EGF and ABX-CBL we incurred and recorded as expense in 2000, net of 50% of the development costs incurred by Immunex and SangStat. License fees of $2.7 million were recognized in 2000 related to the license of certain technologies to Japan Tobacco in 1999. This technology was completed and delivered in 2000. Additionally in 2000, the following fees were recognized: a technology transfer fee was recognized from Pfizer; a milestone fee was recognized from Pfizer related to Pfizer's filing of an Investigational New Drug application with the FDA for an antibody product candidate for the treatment of cancer; licensing fees for four antigen targets under existing collaborations; option fees; research funding; and fees for research milestones and certain research work.
In comparison, contract revenue in 1999 and 1998 included the following: In 1999, a total of $8.3 million was recognized under agreements with Japan Tobacco Inc. as follows: $6.0 million for the license of certain technology in December 1999; and $2.3 million in fees for the reimbursement of clinical trial costs and certain joint interest rights in the data from the ABX-IL8 clinical trials. Additionally in 1999, contract revenues included licensing fees for three antigen targets under existing collaborations, option fees, and fees for research milestones and certain research work. In 1998, approximately $1.3 million was recognized for performing research for Xenotech, which, up until December 1999 when we acquired 100% of Xenotech, was an equally owned joint venture with JT America (a wholly owned subsidiary of Japan Tobacco Inc.). Additionally in 1998, contract revenues included option fees, and fees for research milestones and certain research work.
Interest income consists primarily of interest from cash, cash equivalents and short-term investments. Interest income increased to $32.8 million in 2000 compared to $3.0 million in 1999 and $1.0 million in 1998. These increases are due to higher average balances of marketable securities and cash equivalents as a result of our follow-on offerings and private placements in 2000, in which we raised $717.1 million, and in 1999, in which we raised $123.6 million.
25
Research and development expenses consist primarily of compensation and other expenses related to research and development personnel, costs associated with pre-clinical testing and clinical trials of our product candidates, including the costs of manufacturing the product candidates, and facilities expenses. Research and development expenses increased to $51.3 million in 2000 from $21.1 million in 1999 and from $17.6 million in 1998. The increases reflect primarily costs associated with the following:
- •
- Increased personnel—Staffing increased 103% and 25% in 2000 and 1999, respectively. The increase is to support the increased level of product development activities, including new target validation, process sciences, manufacturing and increased clinical activities. Additionally, the increase in personnel is related to increased licensing activity. Included in the increase are salary and related fringe benefits, recruiting and relocation costs.
- •
- Product Supply Agreement—Included in 2000 is a charge of approximately $3.8 million to reserve manufacturing capacity by acquiring an option to negotiate a supply agreement with a contract manufacturer. In December 2000, the option expired and the five-year manufacturing supply agreement was executed.
- •
- Technology-in-licensing—Included in 2000, are fees of $5.0 million paid to ImmunoGen for providing the Company with non-exclusive access to ImmunoGen's maytansinoid Tumor-Activated Prodrug technology. Under the agreement with ImmunoGen, future payments are due if certain milestones are met and royalties are due on net sales of any resulting products. Additionally in 2000 a fee was paid to Genzyme Transgenics Corporation related to research they are performing in which they agreed to produce our antibody product candidate, ABX-IL8 using Genzyme's manufacturing system. Under this agreement, for undisclosed fees and milestone payments, Genzyme will develop transgenic goats that express ABX-IL8 in their milk. Additionally, several future payments are required if certain milestones are met.
- •
- Clinical Costs—Clinical costs increased from 1999 to 2000 and from 1998 to 1999 as we have initiated new clinical trials and progressed to later stage clinical trials for our three product candidates, ABX-CBL, ABX-IL8 and ABX-EGF. During 2000, we had clinical trials in progress during the entire year for ABX-CBL, ABX-IL8 and ABX-EGF. During 1999 we had clinical trials in progress for the entire year for ABX-CBL and ABX-IL8, and for the last 6 months of the year for ABX-EGF. During 1998 we initiated clinical trials for ABX-CBL and ABX-IL8. Costs of such trials include the clinical investigator site fees, monitoring costs and data management costs. Additionally, such costs include the costs of manufacturing the antibody used in clinical trials. In July and August 2000, we entered into separate
agreements with Immunex and SangStat to share equally in the costs of developing and commercializing ABX-EGF and ABX-CBL, respectively.
- •
- Other—The increase in 1999, is also due to the increased compensation expense resulting from valuation of non-employee stock options.
Amortization of intangible assets of $4.0 million in 2000 relates to existing technology (including patents and certain royalty rights), goodwill and assembled workforce, which were acquired through the Abgenix Biopharma and IntraImmune in November 2000 and Xenotech in December 1999. The existing technology and goodwill are being amortized over 15 years and the assembled workforce over 2 years, their expected useful lives.
General and administrative expenses include compensation and other expenses related to finance and administrative personnel, professional services and facilities. General and administrative expenses increased to $7.7 million in 2000 from $5.2 million in 1999 and $3.4 million in 1998. The increases reflect increased personnel costs, including recruiting costs and incentive compensation, and additional investor relations costs.
26
The in-process research and development charge of $5.2 million relates to our acquisition of Abgenix Biopharma. This acquisition was accounted for using the purchase method of accounting. The total purchase price was $77.2 million, including transaction costs.
A valuation was performed in which the total purchase price of Abgenix Biopharma was allocated among the acquired assets. The income approach was used to develop the value for the existing technology and the in-process research and development, as applicable. The income approach incorporates the calculation of the present value of future economic benefits such as cash earnings, cost savings, and tax deductions. The rate utilized to discount the net cash flows to their present value was 40%. The cost approach was utilized to value the assembled workforce. The cost approach measures the benefits related to an asset by the cost to reconstruct or replace it with another of like utility.
Abgenix Biopharma's SLAM technology is patented in the United States and patent applications are pending in Canada and Europe. SLAM is technologically feasible and we have licensed the technology to a customer. At the time of our acquisition of Abgenix Biopharma, its assembled workforce was comprised of 27 employees, primarily scientists, with specific experience and knowledge of the Abgenix Biopharma SLAM technology and other technologies in process. The combined allocated value of these two intangible assets is $36.0 million.
Abgenix Biopharma' primary in-process research and development activities focused on two efforts: agonist antibodies and antibodies that induce apoptosis. Agonist antibodies are antibodies that trigger a biological process, rather than simply block a biological pathway. Antibodies that induce apoptosis are antibodies that trigger a process that cause the death of the cell they bind to, for use in treating diseases like cancer. These two efforts were estimated to be 31% and 57% complete, based on the estimated costs to complete of approximately $120,000 and $250,000, respectively. Remaining efforts on these projects are significant and include most phases of product design, development and testing. As a result of the developmental work and additional testing required to produce these products in accordance with all clinical, technical and functional specifications of the FDA and other governmental authorities, technological feasibility of these products has not yet been achieved. As such, at the date of the acquisition the in-process technology had no alternative future use and did not otherwise qualify for capitalization. The value assigned to each acquired in-process research and development project were as follows (in thousands): Agonist antibodies—$3,259; Antibodies that induce apoptosis—$1,956; and the total of $5,215.
The estimates used in valuing in-process research and development were based upon assumptions believed to be reasonable but which are inherently uncertain and unpredictable. Assumptions may be incomplete or inaccurate, and unanticipated events and circumstances may occur. Accordingly, actual results may vary from the projected results. Any such variance may result in a material adverse effect on our financial condition and results of operations.
Equity in income from the Xenotech joint venture in 1999 reflects our percentage ownership in the net income from the joint venture, prior to our acquisition of 100% of the joint venture in December 1999. In 1999, prior to our acquisition, the joint venture recorded net income primarily from the sale of licenses to Abgenix and our partner, JT America, Inc. In 1998, the joint venture incurred losses and our equity in those losses was in part netted against our revenues from the joint venture.
The non-recurring termination fee in 1999 is a one-time net charge of $8.7 million related to the termination of certain rights licensed by Japan Tobacco from Xenotech.
Interest expense declined in 2000, 1999 and 1998 due to the continued pay down of debt on our equipment leaseline financing and loan facility.
Foreign income tax in 1999 reflects the withholding income tax imposed by Japan on certain transactions with Japan Tobacco.
27
Liquidity and Capital Resources
At June 30, 2001, we had cash, cash equivalents and marketable securities of approximately $575.4 million. We invest our cash equivalents and marketable securities in highly liquid, interest bearing, investment grade and government securities in order to preserve principal.
Net cash used in operating activities was $14.1 million for the six months ended June 30, 2001, and net cash provided by operating activities was $1.4 million for the six months ended June 30, 2000. The increased use of cash in operations reflects primarily our increased funding of research and development and manufacturing costs related to the development of new products. Total research and development expenses increased $23.3 million in the six months ended June 30, 2001 from the comparable period in 2000. Also affecting the increased use of cash in operations in the six months ended June 30, 2001, was the timing of customer payments. Customers provided cash of $10.3 million in the six months ended June 30, 2001 in comparison to $17.8 million in the six months ended June 30, 2000, both net of the change in accounts receivable and deferred revenue. Additionally, cash was used in the six months ended June 30, 2001 for a security deposit related to a new facility lease. Partially offsetting the increased use of cash was interest income. Cash provided by interest income was $21.6 million in the six months ended June 30, 2001 in comparison to $8.3 million in the six months ended June 30, 2000, both net of the change in interest receivable.
Net cash used in operating activities was $1.4 million and $21.0 million in 2000 and 1999, respectively. In 2000, cash was provided by interest income of $24.2 million net of the increase in interest receivable of $8.7 million. Additionally in this period, customers provided cash of $30.6 million including $3.2 million recorded as a net increase in deferred revenue and $0.8 million from a net reduction in accounts receivable. Cash was used for operations in both periods primarily to fund research and development expenses and manufacturing costs related to the development of new products. Additionally, cash was used in 2000 for a deposit related to a supply agreement and new technology licensing.
Net cash used in investing activities was $68.7 million and $389.0 million for the six months ended June 30, 2001, and June 30, 2000, respectively. For the six months ended June 30, 2001, we received cash from our investment activities as certain of our marketable securities matured in comparison to the six months ended June 30, 2000, in which cash received from our follow-on public offering in February 2000 was invested in marketable securities. Cash was also used in the six months ended June 30, 2001, as follows: $67.0 million to the holders of Abgenix Biopharma special shares in connection with our acquisition of Abgenix Biopharma; $4.1 million for the buy-out of certain stock options issued in connection with the Abgenix Biopharma acquisition; $20.1 million for the acquisition of property and equipment, primarily leasehold improvements related to our new facility and computer equipment; and $15.0 million for an equity investment in MDS Proteomics Inc. In the six months ended June 30, 2000, $1.7 million was spent on property and equipment, primarily leasehold improvements related to our new facility and computer equipment.
Net cash used in investing activities was $567.9 million and $90.7 million in 2000 and 1999, respectively. The activity reflects the following:
- •
- Net purchases of marketable securities with the funds we received from follow-on public offerings and private placements in 2000 and 1999.
- •
- The acquisition of IntraImmune Therapies, Inc. for $9.3 million in 2000;
- •
- The obligation to former shareholders of Abgenix Biopharma that was paid in cash during the first and second quarters of 2001, as discussed above;
- •
- The acquisition of Xenotech for $47.0 million in 1999.
28
- •
- Investments of $15.0 and $50.0 million in the common stock of Immunogen and CuraGen, respectively in 2000.
- •
- An investment of $15.0 million in the common stock of CuraGen in 1999. An investment of $13.8 and $1.1 million in capital expenditures in 2000 and 1999, respectively. The investment in 2000 reflects primarily investment in leasehold improvements in our new office facility and construction in progress for the new process science laboratory and manufacturing facility.
During the six months ended June 30, 2001, net cash provided by financing activities was $2.8 million, consisting of proceeds from the exercise of stock options. This activity compares to the six months ended June 30, 2000, in which net cash provided by financing activities was $498.8 million, consisting primarily of the proceeds of our follow-on public offering in February 2000, in which we raised $496.5 million. In addition to this funding, in 2000 we received $3.8 million from the exercise of warrants and stock options and proceeds from the issuance of stock under our employee stock purchase plan.
During 2000, net cash provided by financing activities was $723.2 million provided primarily from the sale of 9,936,000 shares of our common stock in a follow-on public offering in February 2000, and from the sale of 3,300,000 shares of our common stock in a private placement in November 2000. Additionally during this period, we received $0.7 million from Cell Genesys for the exercise of warrants and $7.3 million from the exercise of stock options and our employee stock purchase plan. During 1999, net cash provided by financing activities was $123.7 million, received primarily from the sale of our common stock in a follow-on public offering and a private placement.
In March 2000 and February 2001, we obtained stand-by letters of credit for $2.0 and $3.0 million, respectively, from a commercial bank as security for our obligations on the leases on our two new leased facilities. The stand-by letters of credit are secured by an investment account, in which we must maintain a $5.5 million balance. Additionally, in 1997 we leased $2.0 million of our laboratory and office equipment from a financing company. The lease term is 48 months. The lease bears interest at approximately 12.5%, and matures in September 2001. We also had a construction financing line with a bank in the amount of $4.3 million that was used to finance construction of leasehold improvements at our first facility. The line was paid off in May 2000.
We plan to continue to make significant expenditures to establish our own manufacturing facility and expand our research and development activities, including pre-clinical product development and clinical trials. We will also continue to look for new technology suppliers as potential acquisitions or alliance collaborators. Over the next eighteen months, we estimate that we will spend approximately $180.0-$200.0 million on leasehold improvements and equipment for our new manufacturing and research and development facilities. Additionally, we expect to spend approximately $10.0-$20.0 million on new computer hardware and software, including the acquisition of a new enterprise resource planning system. We also plan to spend significant amounts to develop, on a proprietary or co-developed basis, investigational new drug applications (INDs) for up to three product candidates annually, beginning in 2002. We believe that the annual goals of our customers and collaborators for 2002 and beyond include up to five INDs for additional product candidates based on our XenoMouse technology. If unforeseen difficulties arise in the course of developing product candidates, obtaining needed licenses, manufacturing product candidates, performing pre-clinical development and clinical trials of such product candidates, obtaining necessary regulatory approvals, or in other aspects of our business, we may be required to make further substantial expenditures. Our future liquidity and capital requirements will depend on many factors, including:
- •
- the scope and results of pre-clinical testing and clinical trials;
- •
- the retention of existing and establishment of further licensing and other agreements, if any;
- •
- continued scientific progress in our research and development programs;
29
- •
- the size and complexity of these programs;
- •
- the cost of establishing our manufacturing capabilities;
- •
- the cost of conducting commercialization activities and arrangements;
- •
- the time and expense involved in seeking regulatory approvals;
- •
- competing technological and market developments;
- •
- the time and expense of filing and prosecuting patent applications and enforcing patent claims;
- •
- our investment in, or acquisition of, other companies;
- •
- the amount of product in-licensing in which we engage; and
- •
- other factors not within our control.
We believe that our current cash balances, cash equivalents, marketable securities, and the cash generated from our licensing and contractual agreements will be sufficient to meet our operating and capital requirements for at least one year. We may choose to obtain additional financing from time to time. We may choose to raise additional funds through public or private financing, licensing and contractual agreements or other arrangements. We cannot be sure that any additional funding, if needed, will be available on terms favorable to us. Furthermore, any additional equity financing may be dilutive to our shareholders, and debt financing, if available, may involve restrictive covenants. We may also choose to obtain funding through licensing and other contractual agreements. Such agreements may require us to relinquish our rights to certain of our technologies, products or marketing territories. Our failure to raise capital when needed would harm our business, financial condition and results of operations.
We have incurred net losses in each of the last five years of operation, including net losses of $7.1 million in 1996, $35.9 million in 1997, $16.8 million in 1998, $20.5 million in 1999, $8.8 million in 2000 and $22.4 million in the six months ended June 30, 2001. As of June 30, 2001, our accumulated deficit was $121.0 million. Our losses to date have resulted principally from:
- •
- research and development costs relating to the development of our XenoMouse technology and antibody product candidates;
- •
- costs associated with certain agreements with Japan Tobacco and certain 1997 settlement and cross-licensing agreements with GenPharm International, Inc.;
- •
- in-process research and development costs and amortization of intangible assets associated with our acquisitions of Abgenix Biopharma, IntraImmune Therapies and Xenotech;
- •
- general and administrative costs relating to our operations.
We expect to incur additional losses for the foreseeable future as a result of increases in our research and development costs, including costs associated with conducting pre-clinical development and clinical trials, and charges related to purchases of technology or other assets, and costs associated with establishing our manufacturing facilities. We intend to invest significantly in our products prior to entering into licensing agreements. This may increase our need for capital and will result in losses for several years. We expect that the amount of operating losses will fluctuate significantly from quarter to quarter as a result of increases or decreases in our research and development efforts, the execution or termination of licensing and contractual agreements, and the initiation, success or failure of clinical trials.
As of December 31, 2000, we had federal net operating loss carryforwards of approximately $148.0 million. Our net operating loss carryforwards exclude losses incurred prior to our formation in July 1996. Further, the amounts associated with the 1997 cross-license and settlement that have been
30
expensed for financial statement accounting purposes have been capitalized and are being amortized over a period of approximately 15 years for tax purposes. The net operating loss and credit carryforwards will expire in the years 2011 through 2020, if not utilized. Utilization of the net operating losses and credits may be subject to a substantial annual limitation due to the "change in ownership" provisions of the Internal Revenue Code of 1986 and similar state provisions. The annual limitation may result in the expiration of net operating losses and credits before utilization.
Selected Quarterly Financial Data
| | Quarter Ended
| |
|---|
| | Mar 31,
| | June 30,
| | Sept 30,
| | Dec 31,
| |
|---|
| | (in thousands, except per share data)
| |
|---|
| 1999 | | | | | | | | | | | | | |
| Contract revenues | | $ | — | | $ | 1,720 | | $ | 3,670 | | $ | 6,895 | |
| Interest income | | $ | 395 | | $ | 797 | | $ | 762 | | $ | 1,091 | |
| Total revenues | | $ | 395 | | $ | 2,517 | | $ | 4,432 | | $ | 7,986 | |
| Net income (loss) | | $ | (5,397 | ) | $ | (3,568 | ) | $ | (1,279 | ) | $ | (10,255 | ) |
| Basic and diluted net loss per share | | $ | (0.11 | ) | $ | (0.06 | ) | $ | (0.02 | ) | $ | (0.16 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2000 | | | | | | | | | | | | | |
| Contract revenues | | $ | 1,965 | | $ | 3,478 | | $ | 7,634 | | $ | 13,524 | |
| Interest income | | $ | 4,341 | | $ | 8,781 | | $ | 9,213 | | $ | 10,513 | |
| Total revenues | | $ | 6,306 | | $ | 12,259 | | $ | 16,847 | | $ | 24,037 | |
| Net income (loss) | | $ | (3,383 | ) | $ | (2,420 | ) | $ | 1,507 | | $ | (4,497 | ) |
| Basic and diluted net income (loss) per share | | $ | (0.05 | ) | $ | (0.03 | ) | $ | 0.02 | | $ | (0.05 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2001 | | | | | | | | | | | | | |
| Contract revenues | | $ | 4,176 | | $ | 8,354 | | | | | | | |
| Interest income | | $ | 10,306 | | $ | 7,664 | | | | | | | |
| Total revenues | | $ | 14,482 | | $ | 16,018 | | | | | | | |
| Net income (loss) | | $ | (7,644 | ) | $ | (14,787 | ) | | | | | | |
| Basic and diluted net income (loss) per share | | $ | (0.09 | ) | $ | (0.17 | ) | | | | | | |
Quantitative and Qualitative Disclosures About Market Risk
Interest Rate Risk. The objective of our investment activities is to preserve principal, while at the same time maximizing yields without significantly increasing risk. To achieve this objective, we invest in highly liquid and high quality debt securities. Our investments in debt securities are subject to interest rate risk. To minimize the exposure due to an adverse shift in interest rates, we invest in short-term securities and our goal is to maintain an average maturity of approximately one year. A hypothetical 1.0% per annum increase in interest rates would result in a decrease in the fair market value of our debt securities of approximately $1.0 million, at June 30, 2001 and approximately $1.1 million, at December 31, 2000.
Equity Price Risk. We are exposed to equity price risk on strategic investments in CuraGen Corporation and Immunogen, Inc. We typically do not attempt to reduce or eliminate our market exposure on these securities. With respect to CuraGen and Immunogen, each of whose common stock trades on public exchanges, assuming an adverse change of 30% in the market price of their stock, the fair value of our equity investments would decrease in value by approximately $29.6 million and $23.8 million as of June 30, 2001 and December 31, 2000, respectively. This estimate is not necessarily indicative of future performance and actual results may differ materially.
31
Recent Accounting Pronouncements
In December 1999, the Securities and Exchange Commission issued Staff Accounting Bulletin No. 101—Revenue Recognition in Financial Statements (SAB 101), which provides guidance on the accounting for revenue recognition. As amended, SAB 101 was required to be implemented no later than the quarter ended December 2000. We have evaluated the applicability of SAB 101 to our existing agreements. We believe that our policy and approach to revenue recognition is consistent with the guidance provided by SAB 101. We have followed the following principles in recognizing revenue:
- •
- Research license fees: Fees to license the use of XenoMouse technology in research performed by the customer are generally recognized when both the inception of the license period and delivery of the technology have occurred. If Abgenix is obligated to provide significant assistance to enable the customer to practice the license, then the revenue is recognized over the period of such obligation.
- •
- Product license fees: Fees to license the production, use and sale of an antibody generated by XenoMouse technology are generally recognized when both the inception of the license period and delivery of the technology have occurred. If Abgenix is obligated to provide significant assistance to enable the customer to practice the license, then the revenue is recognized over the period of such obligation.
- •
- Option fees: Fees for granting options to obtain product licenses are recognized as revenue when the option is exercised or when the option period expires, whichever occurs first.
- •
- Payments for research services performed by Abgenix are recognized ratably over the period during which these services are performed. However, fees for research services received under co-development arrangements are recorded as contract revenues in the period rendered, net of fees payable by Abgenix to such co-developers for reimbursements of research and development costs.
- •
- Milestone payments are recognized as revenue when the milestone is achieved.
32
BUSINESS
The following description of our business should be read in conjunction with the information included elsewhere in this prospectus. The description contains certain forward-looking statements that involve risks and uncertainties. When used in this prospectus, the words "intend," "anticipate," "believe," "estimate," "plan," and "expect" and similar expressions as they relate to us are included to identify forward-looking statements. Our actual results could differ materially from the results discussed in the forward-looking statements as a result of certain of the risk factors set forth in this prospectus.
Abgenix
We are a biopharmaceutical company that develops and intends to commercialize antibody therapeutic products for the treatment of a variety of disease conditions, including transplant-related diseases, inflammatory and autoimmune disorders, cardiovascular disease, infectious diseases and cancer. We have proprietary technologies that facilitate rapid generation of highly specific, fully human antibody product candidates that bind to disease targets appropriate for antibody therapy. We developed our XenoMouse technology, a technology utilizing genetically modified mice. We also own a technology that enables the rapid identification of antibodies with desired function and characteristics, referred to as SLAM technology. In our newly developed XenoMax technology, we use SLAM technology to select and isolate antibodies with particular function and characteristics from antibody-producing cells generated by XenoMouse animals. We believe XenoMax technology enhances our capabilities in product development and flexibility in manufacturing. We intend to use our technologies to build a large and diversified product portfolio that we expect to develop and commercialize through licensing arrangements with pharmaceutical companies and others, through joint development and through internal product development programs. We have entered into a variety of contractual arrangements with multiple pharmaceutical, biotechnology and genomics companies involving our XenoMouse technology. Two of our customers, Pfizer, Inc. and Amgen, Inc., have initiated clinical trials with fully human antibodies generated from XenoMouse animals. In addition, we have three proprietary antibody product candidates currently in clinical trials, two of which we have agreed to co-develop and commercialize with others.
Overview of Contractual Arrangements
As of August 27, 2001 we had entered into contracts covering numerous antigen targets with twenty-nine customers to use our XenoMouse technology or SLAM technology to generate and/or develop the resulting fully human antibody product candidates. Pursuant to these contracts, we and our customers intend to generate antibody project candidates for the treatment of cancer, inflammation, autoimmune diseases, transplant rejection, cardiovascular disease, growth factor modulation, neurological diseases and infectious diseases. We expect that substantially all of our revenues for the foreseeable future will result from payments under these and other contracts. The terms of the arrangements vary, but can generally be categorized as follows:
- •
- Antigen Target Sourcing Contracts—We have entered into several target sourcing contracts with genomics and biopharmaceutical companies that may enable us to generate a pipeline of proprietary fully human antibody product candidates. Typically, these contracts provide for Abgenix to make fully human antibodies to the antigen targets provided or identified by the contract counterparty. The contracts typically contain provisions that allow either Abgenix or the other contract party to evaluate and select particular antibodies from the pool of generated antibodies for further development and commercialization. The party selecting a product candidate for further development or commercialization will generally pay to the other party license fees, milestone payments and royalty payments on any eventual product sales, in exchange for rights to develop and commercialize the product.
33
- •
- Proprietary Product Development—In July and August 2000, we entered into two joint development and commercialization agreements. The first is with Immunex Corporation for ABX-EGF, a fully human antibody created by us. Under the agreement, Immunex paid us an initial license fee at signing and a second license fee in May 2001. Development costs will be shared equally, as would any profits from sales of collaboration products. We and Immunex share responsibility for product development. We will be responsible for completing the Phase I trial and any additional Phase I trials that are initiated, and both companies share responsibility for the execution of Phase II trials across a variety of indications. Immunex will have primary responsibility for Phase III clinical trials and will market any potential product, while we will retain co-promotion rights. The second agreement is with SangStat Medical Corporation for ABX-CBL, an antibody developed by us. Under that agreement, SangStat paid us an initial license fee in 2000 and a second milestone payment in June 2001, and has agreed to make a final milestone payment in 2002. Development costs will be shared equally, as would any profits from sales of collaboration products. We and SangStat share responsibility for product development, including the ongoing Phase II/III clinical trials. SangStat will market any potential product and we will be responsible for manufacturing ABX-CBL.
We intend to build our product portfolio by using our XenoMouse and XenoMax technologies to generate antibodies to antigen targets that we source, self-funding clinical activities to determine preliminary safety and efficacy, and entering into more development and commercialization agreements with pharmaceutical and biotechnology companies. We plan to enter into agreements to use our XenoMax technology to assist our licensees and collaborators in isolating antibodies with desired functions and characteristics. These arrangements may or may not involve joint sharing of costs and profits.
- •
- Technology Licensing—We also license our XenoMouse technology to third parties for the purpose of generating antibody product candidates to one or more specific antigen targets provided by the
customer. In most cases, we provide our mice to the customers who then carry out immunizations with their specific antigen targets. In other cases, we immunize the mice with the customers' antigen targets for additional compensation. The customer generally has an option for a period of time to acquire a product license for any antibody product identified using XenoMouse technology that the customer wishes to develop and commercialize. The financial terms of these agreements may include license fees, option fees and milestone payments paid to us by the customers. Based on our agreements, these payments and fees would average $8.0 to $10.0 million per antigen target if our customer takes the antibody product candidate into development and ultimately to commercialization. Additionally, our license agreements entitle us to receive royalties on any future product sales by the customer.
Our dependence on contracts with third parties subjects us to a number of risks. Agreements with licensees typically allow such licensees significant discretion in electing whether to pursue any of the planned activities. We cannot control the amount and timing of resources our licensees may devote to the product candidates. Even if we fulfill our obligations under an agreement, the licensee can terminate the agreement at any time following proper written notice. If any licensee were to terminate or breach its agreement with us, or otherwise fail to complete its obligations in a timely manner, our business, financial condition and results of operations may be materially harmed.
34
Overview of Proprietary Products
We have three proprietary antibody product candidates that are currently in clinical trials, two of which are now being co-developed with our collaborators, as follows:
- •
- ABX-IL8—Generated using XenoMouse technology, ABX-IL8 is our fully human antibody candidate for the treatment of inflammatory diseases, including psoriasis and rheumatoid arthritis. The status of clinical trials for ABX-IL8 is as follows:
- •
- Psoriasis—We have completed Phase I, Phase I/II and Phase IIa clinical trials. We initiated a Phase IIb clinical trial in February 2001 and enrollment is ongoing.
- •
- Rheumatoid arthritis—We initiated a Phase IIa clinical trial in December 2000. Enrollment is complete and patient treatment is ongoing.
- •
- ABX-EGF—Generated using XenoMouse technology, ABX-EGF is our fully human antibody candidate for the treatment of a variety of cancers. In July 2000, we entered into the joint development and
commercialization agreement with Immunex Corporation for ABX-EGF described above. The status of clinical trials for ABX-EGF is as follows:
- •
- Various cancers—We initiated a Phase I clinical trial for ABX-EGF in cancer in July 1999 and enrollment is ongoing.
- •
- Renal cell cancer—We initiated a Phase II clinical trial evaluating the effect of ABX-EGF monotherapy in patients with renal cell cancer in April 2001 and enrollment is ongoing. In May 2001, we received the second milestone payment from Immunex contemplated by the agreement described above.
- •
- Non-small cell lung cancer—Immunex initiated a Phase II clinical trial for ABX-EGF in non-small cell lung cancer in combination with standard chemotherapy, compared to standard chemotherapy alone, in July 2001 and enrollment is ongoing.
- •
- ABX-CBL—We developed ABX-CBL, an in-licensed mouse antibody, for the treatment of a transplant-related disease known as graft versus host disease, or GVHD. We have completed a multi-center Phase II clinical trial for ABX-CBL and initiated a Phase II/III clinical trial in December 1999 in which enrollment is ongoing. In August 2000, we entered into the joint development and commercialization agreement with SangStat Medical Corporation for ABX-CBL described above.
We will expend significant capital to conduct clinical trials for our proprietary product candidates, including several Phase II clinical trials we have initiated or plan to initiate in 2001 and 2002. We believe that more extensive clinical data will enable us to enter into additional contractual arrangements related to those proprietary product candidates. We expect that this will substantially increase our capital needs over the next few years and increase our operating losses. However, we believe that we will be able to receive more favorable proceeds from our contract parties if we have completed significant development of these products.
In addition to our proprietary antibody product candidates in clinical trials, there are two customer-developed antibodies generated with XenoMouse technology in clinical trials as follows:
- •
- Pfizer, Inc.—We generated a XenoMouse-derived antibody for treating cancer, which Pfizer has advanced into clinical trials.
- •
- Amgen, Inc.—We generated a XenoMouse-derived antibody that binds to an undisclosed antigen target, which Amgen has advanced into clinical trials.
35
Background
The Normal Antibody Response
The human immune system protects the body against a variety of infections and other illnesses. Specialized cells, which include B cells and T cells, work in concert with the other components of the immune system to recognize, neutralize and eliminate from the body numerous foreign substances, infectious organisms and malignant cells. In particular, B cells generally produce protein molecules, known as antibodies, which are capable of recognizing substances potentially harmful to the human body. Such substances are called antigens. Upon being bound by an antibody, antigens can be neutralized and blocked from interacting with and causing damage to normal cells. In order to effectively neutralize or eliminate an antigen without harming normal cells, the immune system must be able to generate antibodies that bind tightly (i.e., with high affinity) to one specific antigen (i.e., with specificity).
All antibodies have a common core structure composed of four subunits, two identical light (L) chains and two identical heavy (H) chains, named according to their relative size. The heavy and light chains are assembled within the B cell to form an antibody molecule that consists of a constant region and a variable region. As shown in the diagram below, an antibody molecule may be represented schematically in the form of a "Y" structure.
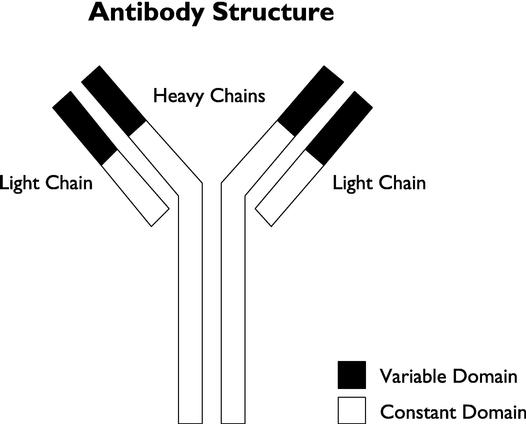
The base of the "Y," together with the part of each arm immediately next to the base, is called the constant region because its structure tends to be very similar across all antibodies. In contrast, the variable regions are at the end of the two arms and are unique to each antibody with respect to their three-dimensional structures and protein sequences. Because variable regions define the specific binding sites for a variety of antigens, there is a need for significant structural diversity in this portion of the antibody molecule. Such diversity is achieved in the body primarily through a unique mode of assembly
36
involving a complex series of recombination steps for various gene segments of the variable region, including the V, D and J segments (see the diagram below).
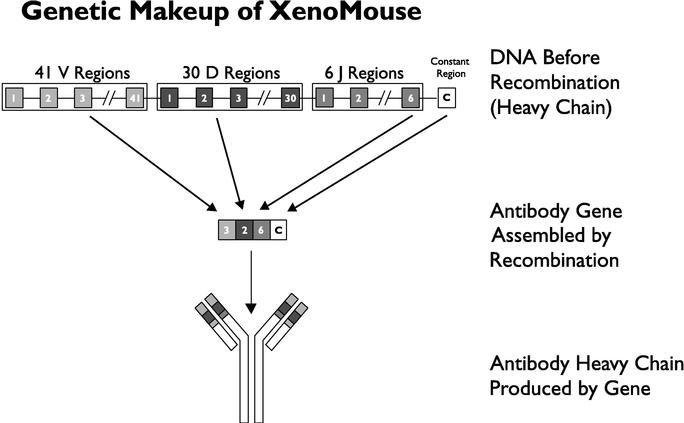
The human body is repeatedly exposed to a variety of different antigens. Accordingly, the immune system must be able to generate a diverse repertoire of antibodies that are capable of recognizing these multiple antigen structures with a high degree of specificity. The immune system has evolved a two-step mechanism in order to accomplish this objective. The first step, immune surveillance, is achieved through the generation of diverse circulating B cells, each of which assembles different antibody gene segments in a semi-random fashion to produce and display on its surface a specific antibody. As a result, a large number of distinct, albeit lower affinity, antibodies are generated in the circulation so as to recognize essentially any foreign antigen that enters the body. While capable of recognizing the antigens as foreign, these lower affinity antibodies are generally incapable of effectively neutralizing them.
This limitation of the immune surveillance process is generally overcome by the normal immune system in a second step called "affinity maturation." Triggered by the initial binding to a specific antigen, the small fraction of B cells that recognize this antigen is then primed by the immune system to progressively generate antibodies with higher and higher affinity through a process of repeated mutation and selection. As a result, the reactive antibodies develop increasingly higher specificity and affinity with the latter being potentially a hundred to a thousand times higher than those generated in the immune surveillance process. These more specific, higher affinity antibodies have a greater likelihood of effectively neutralizing or eliminating the antigen while minimizing the potential of damaging healthy cells.
37
Antibodies as Products
Recent advances in the technologies for creating and producing antibody products coupled with a better understanding of how antibodies and the immune system function in key disease states have led to renewed interest in the commercial development of antibodies as therapeutic products. According to a recent survey by the Pharmaceutical Research and Manufacturers of America, antibodies account for over 20% of all biopharmaceutical products in clinical development. As of August 27, 2001 we were aware of ten antibody therapeutic products approved for marketing in the United States. These products are Orthoclone, ReoPro, Rituxan, Zenapax, Herceptin, Synagis, Remicade, Simulect, Mylotarg and Campath. These products are currently being marketed for a wide range of medical disorders such as transplant rejection, cardiovascular disease, cancer and infectious diseases.
We believe that, as products, antibodies have several potential clinical and commercial advantages over traditional therapies. These advantages include the following:
- •
- faster product development;
- •
- fewer unwanted side effects as a result of high specificity for the disease target;
- •
- greater patient compliance and higher efficacy as a result of favorable pharmacokinetics;
- •
- delivery of various payloads, including drugs, radiation and toxins, to specific disease sites; and
- •
- ability to elicit a desired immune response.
Limitations of Current Approaches to Development of Antibody Products
Despite the early recognition of antibodies as promising therapeutic agents, most approaches thus far to develop them as products have been met with a number of commercial and technical limitations. Initial efforts were aimed at the development of hybridoma cells from mice. Such hybridoma cells are immortalized mouse antibody-secreting B cells. These hybridoma cells are derived from normal mouse B cells that have been fused with a perpetually-growing cell so that they are capable of reproducing over an indefinite period of time. They are then cloned to produce a homogeneous population of identical cells that produce one single type of mouse antibody capable of recognizing one specific antigen ("monoclonal antibody").
While mouse monoclonal antibodies can be generated to bind to a number of antigens, they contain mouse protein sequences and tend to be recognized as foreign by the human immune system. As a result, they are quickly eliminated by the human body and have to be administered frequently. When patients are repeatedly treated with mouse antibodies, they will begin to produce antibodies that effectively neutralize the mouse antibody, a reaction referred to as a Human Anti-Mouse Antibody, or HAMA, response. In many cases, the HAMA response prevents the mouse antibodies from having the desired therapeutic effect and may cause the patient to have an allergic reaction. The potential use of mouse antibodies is thus best suited to situations where the patient's immune system is compromised or where only short-term therapy is required. In such settings, the patient is often incapable of producing antibodies that neutralize the mouse antibodies or has insufficient time to do so.
Recognizing the limitations of mouse monoclonal antibodies, researchers have developed a number of approaches to make them appear more human-like to a patient's immune system. For example, improved forms of mouse antibodies, referred to as "chimeric" and "humanized" antibodies, are genetically engineered and assembled from portions of mouse and human antibody gene fragments. While these chimeric and humanized antibodies are more human-like, they still retain a varying amount of the mouse antibody protein sequence, and accordingly may continue to trigger the HAMA response.
Additionally, the humanization process can be expensive and time consuming, requiring at least two months and sometimes over a year of secondary manipulation after the initial generation of the
38
mouse antibody. Once the humanization process is complete, the remodeled antibody gene must then be expressed in a recombinant cell line appropriate for antibody manufacturing, adding additional time before the production of pre-clinical and clinical material can be initiated. In addition, the combination of mouse and human antibody gene fragments can result in a final antibody product which is sufficiently different in structure from the original mouse antibody that a decrease in specificity or a loss of affinity results.
Human Antibodies
The HAMA response can potentially be avoided through the generation of antibody products with fully human protein sequences. Such fully human antibodies may increase the market acceptance and expand the use of antibody therapeutics. Several antibody technologies have been developed to produce antibodies with 100% human protein sequences (see the diagram above). One approach to generating human antibodies, called "phage display" technology, involves the cloning of human antibody genes into bacteriophages, viruses that infect bacteria, in order to display antibody fragments on the surfaces of bacteriophage particles. This approach attempts to mimic in vitro the immune surveillance and affinity maturation processes that occur in the body. Because phage display technology cannot take advantage of the naturally occurring in vivo affinity maturation process, the antibody fragments initially isolated by this approach are typically of moderate affinity. In addition, further genetic engineering is required to convert the antibody fragments into fully assembled antibodies and significant manipulation, taking from several months to a year, may be required to increase their affinities to a level appropriate for human therapy. Before pre-clinical or clinical material can be produced, the gene encoding the antibody derived from phage display technology must, as with a humanized antibody, be introduced into a recombinant cell line.
Two additional approaches involving the isolation of human immune cells have been developed to generate human antibodies. One such approach is the utilization of immunodeficient mice that lack both B and T cells. Human B cells and other immune tissue are transplanted into these mice which are
39
then subsequently immunized with target antigens to stimulate the production of human antibodies. However, this process is generally limited to generating antibodies only to nonhuman antigens or antigens to which the human B cell donor had previously responded. Accordingly, this approach may not be suitable for targeting many key diseases such as cancer, and inflammatory and autoimmune disorders where antibodies to human antigens may be required for appropriate therapy. The other approach involves collecting human B cells that have been producing desired antibodies from patients exposed to a specific virus or pathogen. As with the previous approach, this process may not be suitable for targeting diseases where antibodies to human antigens are required, and therefore is generally limited to infectious disease targets which will be recognized as foreign by the human immune system.
The Abgenix Solution—XenoMouse and XenoMax Technologies
Our approach to generating human antibodies with fully human protein sequences is to use genetically engineered strains of mice in which mouse antibody gene expression is suppressed and functionally replaced with human antibody gene expression, while leaving intact the rest of the mouse immune system. Rather than engineering each antibody product candidate, these transgenic mice capitalize on the natural power of the mouse immune system in surveillance and affinity maturation to produce a broad repertoire of high affinity antibodies. By introducing human antibody genes into the mouse genome, transgenic mice with such traits can be bred indefinitely. Importantly, these transgenic mice are capable of generating human antibodies to human antigens because the only human products expressed in the mice (and therefore recognized as "self") are the antibodies themselves. Any other human tissue or protein is thus recognized as a foreign antigen by the mouse and an immune response will be mounted. Abnormal production of certain human proteins, such as cytokines and growth factors or their receptors has been implicated in various human diseases. Neutralization or elimination of these abnormally produced or regulated human proteins with the use of human antibodies could ameliorate or suppress the target disease. Therefore, the ability of these transgenic mice to generate human antibodies against human antigens could offer an advantage to drug developers compared with some of the other approaches described previously. A challenge with this approach, however, has been to introduce enough of the human antibody genes in appropriate configuration into the mouse genome to ensure that these mice are capable of recognizing the broad diversity of antigens relevant for human therapies.
To make our transgenic mice a robust tool capable of consistently generating high affinity antibodies which can recognize a broad range of antigens, we equipped the XenoMouse with approximately 80% of the human heavy chain antibody genes and a significant amount of the human light chain genes. We believe that the complex assembly of these genes together with their semi-random pairing allows XenoMouse animals to recognize a diverse repertoire of antigen structures. XenoMouse technology further capitalizes on the natural in vivo affinity maturation process to generate high affinity, fully human antibodies. In addition, we have developed multiple strains of XenoMouse animals, each of which is capable of producing a different class of antibody to perform different therapeutic functions. We believe that our various XenoMouse strains will provide maximum flexibility for drug developers in generating antibodies of the specific type best suited for a given disease indication.
The antibodies generated by XenoMouse animals are obtained by extracting the antibody-producing B cells. These B cells can be transformed into hybridomas to generate the quantities of antibodies needed for standard methods of assaying and selecting antibodies for further development. Hybridoma technology captures only about 1% of the antibodies originally generated by the mouse. Alternatively, the B cells can be submitted to our proprietary Selected Lymphocyte Antibody Method (SLAM) technology, which cultures the B-cells directly and rapidly assays them over a period of several days using a microplate-based, high throughput system. Using SLAM, the number of different antigen-reactive monoclonal antibodies identified in a single experiment is typically increased by 100 to
40
1000-fold compared to hybridoma technology. The use of SLAM technology together with XenoMouse technology is called XenoMax technology.
Our XenoMax technology incorporates XenoMouse technology and SLAM technology to enhance the speed and capability of generating fully human, high affinity antibodies. XenoMax technology allows researchers to rapidly scan the majority of the immune repertoire of an immunized XenoMouse animal, and to identify B-cells that produce antibodies with the desired functional properties and the optimal affinities. Using rapid microplate-based assays to measure and rank antibodies according to design goals (e.g., potency, affinity, specificity), individual B-cells producing extremely high-quality antibodies can be identified and the antibody encoding genes recovered. Within three to five weeks after immunizing XenoMouse animals, XenoMax technology can produce a ranked set of recombinant antibody candidates resulting from the harvested B-cells. We believe XenoMax technology can speed product development timelines by allowing researchers to move directly into pre-clinical assessment of panels of suitable recombinant candidate antibody products, each ready for manufacturing scale-up. XenoMax technology samples up to 2.5 X106 B-cells per immunized XenoMouse animal, dramatically increasing the number of antibodies from which to choose optimal therapeutic product candidates. In contrast to phage display technology, antibodies derived from XenoMax technology retain their native pairing of heavy and light chains, and do not requirein vitro affinity and/or potency maturation.
Other approaches to generating fully human antibodies from mice that we understand are being pursued by competitors include: (i) transgenic mice containing heavy human chain and human light chain genes on a "minilocus" (which are mice that possess a relatively small number of representative human heavy and light chain genes in their genome), (ii) "transchromosomic" mice that contain large numbers of human heavy chain and light chain genes on one or more separate, or extra, chromosomes, and (iii) mice that are generated as a result of breeding "minilocus" containing mice with "transchromosomic" mice. It is our understanding that "minilocus" containing mice are used by GenPharm International, Inc. and Medarex, Inc. "Transchromosomic" mice (developed by Kirin Brewing Co., Ltd.) and mice generated as a result of breeding "transchromosomic" mice and "minilocus" mice (developed by Medarex and its joint venture partner, Kirin Brewing Co.) are relatively new and it is not yet known how useful the technology will be.
In addition to the generation of human antibodies from mice, we understand that competitors such as Cambridge Antibody Technology Group plc and MorphoSys AG utilize phage display technology for the generation of human antibodies from phage display libraries derived from human samples.
Our Technology Advantages
We believe that our technologies offer the following advantages:
Producing antibodies with fully human protein sequences. Our XenoMouse technology, unlike chimeric and humanization technologies, allows the generation of antibodies with 100% human protein sequences. Antibodies created using XenoMouse technology are not expected to cause a HAMA response even when administered repeatedly to patients without compromised immune systems. For this reason, antibodies produced using XenoMouse technology are expected to offer a better safety profile and to be eliminated less quickly from the human body, reducing the frequency of dosing.
Generating a diverse antibody response to essentially any disease target appropriate for antibody therapy. Because a substantial majority of human antibody genes has been introduced into XenoMouse animals, the technology has the potential to generate high affinity antibodies that recognize more antigen structures than some other transgenic technologies. In addition, through immune surveillance, XenoMouse technology is expected to be capable of generating antibodies to almost any medically relevant antigen, human or otherwise. For a given antigen target, having multiple antibodies to choose from could be important in selecting the optimal antibody product.
41
Generating high affinity antibodies that do not require further engineering. XenoMouse technology uses the natural in vivo affinity maturation process to generate antibody product candidates usually in two to four months. These antibody product candidates may have affinities as much as a hundred to a thousand times higher than those seen in phage display. In contrast to antibodies generated using humanization and phage display technology, XenoMouse antibodies are produced without the need for any subsequent engineering, a process that at times has proven to be challenging and time consuming. By avoiding the need to further engineer antibodies, we reduce the risk that an antibody's structure and therefore functionality will be altered between the initial antibody selected and the final antibody placed into production.
Enabling more efficient product development. XenoMouse technology can potentially produce multiple product candidates more quickly than humanization and phage display technology and pre-clinical testing can be conducted on several antibodies in parallel to identify the optimal product candidate that will be tested in clinical trials.
Providing flexibility in choosing manufacturing processes. Once an antibody with the desired characteristics has been identified, pre-clinical material can be produced either directly from hybridomas or from recombinant cell lines. Humanized and phage display antibodies, having been engineered, cannot be produced in hybridomas. In addition to potential timesaving, production in hybridomas avoids the need to license certain third party intellectual property rights covering certain processes for production of antibodies in recombinant cell lines.
Enhancing the speed and capability of generating fully human, high affinity antibodies. Our XenoMax technology allows researchers to rapidly scan the majority of the immune repertoire of an immunized XenoMouse animal to identify B-cells that produce antibodies with the desired functional properties and the optimal affinities. We believe XenoMax technology can speed product development timelines by allowing researchers to move directly into pre-clinical assessment of panels of suitable recombinant candidate antibody products, each ready for manufacturing scale-up.
Abgenix Strategy
Our objective is to be a leader in the generation, development and commercialization of novel antibody-based biopharmaceutical products. Key elements of our strategy to accomplish this objective include the following:
Building a large and diversified product portfolio. Utilizing our XenoMouse and XenoMax technologies, we intend to build a large and diversified product portfolio, including a mix of out-licensed and internally developed product candidates. We and our collaborators are targeting serious medical conditions, including cancer, inflammation, autoimmune diseases, transplant rejection, cardiovascular disease, growth factor modulation, neurological diseases and infectious diseases. For our internal programs, we intend to enter into contractual agreements with leading academic researchers and companies involved in the identification and development of novel antigens. We believe the speed and cost advantages of our technology will enable us to make cost-effective use of available human and capital resources. We can thus pursue multiple product candidates in parallel as far as completion of the Phase II clinical stage before entering into a contractual agreement to complete clinical and developmental stages and to bring the product candidate to market. Thus, we believe we can create a package that includes antigen rights, human antibodies, and pre-clinical and clinical data for use by us or for marketing to potential contract parties.
Leveraging XenoMouse technology through licensing. We intend to diversify our product portfolio and generate revenues by licensing XenoMouse technology to numerous pharmaceutical and biotechnology companies interested in developing antibody-based products. We expect to enter into multiple XenoMouse technology licenses each year. These agreements typically allow our licensee to
42
generate fully human antibodies to one or more specific antigen targets provided by the licensee. In most cases, we provide our mice to licensees who then carry out immunizations with their specific antigen target. In other cases, we immunize the mice with the licensee's antigen target for additional compensation. Our licensees will also need to obtain product licenses for any antibody product they wish to develop and commercialize.
The financial terms of our XenoMouse technology licenses often include upfront payments, potential license fees and milestone payments plus royalties on any future product sales. We have established technology licenses with twenty-eight customers covering numerous antigen targets. To date, many of these licensees have each entered into new or expanded licenses that allow them to specify additional antigens for XenoMouse antibody development.
Establishing collaborations for proprietary product candidates. We also intend to build our product portfolio and generate revenues by licensing proprietary product candidates. These proprietary product collaborations would involve antibodies made to antigen targets that we source. After generating antibody product candidates and self-funding clinical activities to determine preliminary safety and efficacy, we intend to enter into development and commercialization agreements with contract parties for these proprietary product candidates that we created. For most of our products, we may enter into proprietary product contracts before entering the Phase III clinical development stage allowing the contract parties to complete development and to market the product. For other products, we may develop the product through clinical trials and license the product candidate to a contract party for marketing.
The financial terms of these product contracts could include license fees upon signing, milestone payments, and reimbursement for research and development activities that we perform, plus profit-sharing or royalties on future product sales, if any. Given our greater investment in creating a proprietary product candidate, we expect that an arrangement for these product candidates could afford higher payments and royalty rates than a typical XenoMouse technology contract. We have entered into two such product contracts: with Immunex for ABX-EGF; and with SangStat for ABX-CBL.
Proprietary Product Development Programs
We are currently developing antibody therapeutics for a variety of indications. The table below sets forth the development status of our proprietary product candidates:
Proprietary Product Candidate
| | Indication
| | Status(1)
|
|---|
| ABX-CBL | | GVHD | | Phase II/III |
| ABX-IL8 | | Psoriasis | | Phase IIb |
| | | Rheumatoid Arthritis | | Phase IIa |
| ABX-EGF | | Various cancers | | Phase I |
| | | Renal cell cancer | | Phase II |
| | | Non-small cell lung cancer | | Phase II |
- (1)
- "Phase I" indicates safety and proof of concept testing in a limited patient population and toxicology testing in animal models. "Phase II" indicates safety, dosing and efficacy testing in a limited patient population. "Phase III" indicates safety and efficacy testing with a larger patient population.
ABX-CBL
The CBL antigen is selectively over-expressed on activated immune cells including T cells, B cells and certain microphages. We obtained an exclusive license to ABX-CBL in February 1997. We believe
43
that a mouse antibody can be utilized to treat GVHD patients because their immune system is either non-functioning or severely suppressed and, therefore, no HAMA responses should be generated. We believe ABX-CBL has the ability to destroy activated immune cells without affecting the rest of the immune system.
Graft Versus Host Disease. We are co-developing ABX-CBL with Sangstat. The goal for the development program is to reduce unwanted immune responses that occur in GVHD. GVHD is a life-threatening complication that frequently occurs following an allogeneic bone marrow transplant, or BMT. BMTs are used in the treatment of patients with end-stage leukemia, certain other serious cancers and immune system disorders. An allogeneic BMT procedure involves transferring marrow, the graft, from a healthy person into an immunosuppressed patient, the host. The transplant is intended to restore normal circulating immune cells to a patient whose own immune system is functionally deficient or has been damaged by the treatment of an underlying disease such as cancer and, therefore, does not have the ability to mount a sufficient immune response. Often a portion of the graft recognizes the host's own cells as foreign, becomes activated and attacks them, resulting in GVHD. It typically involves damage to multiple organ systems, including the skin, liver and intestines. GVHD causes extreme suffering and is the primary cause of death in allogeneic BMT patients. It is estimated that approximately 12,000 allogeneic BMTs were performed worldwide in 1998, and this number has been growing at about 15% per year. GVHD occurs in approximately 50% of allogeneic BMTs and the treatment costs for GVHD in the United States are estimated to be about $80,000 per patient. Based on a published clinical study, it is estimated that roughly 50% of patients with GVHD fail to respond to current treatments, which consist of corticosteroids and other drug treatments to suppress the grafted immune cells. Less than 15% of steroid-resistant GVHD sufferers survive for more than one year. We believe that a safer and more effective treatment for GVHD could result in increased use of BMTs.
Clinical Status. We completed a multi-center Phase II clinical trial for ABX-CBL for the treatment of steroid-resistant, grade II to IV GVHD. We submitted data from 27 patients included in the Phase II Study to the FDA. As an extension to the original Phase II trial protocol, we have enrolled an additional 32 patients. The trial studied four escalating intravenous dose regimens. It was conducted at nine sites and involved 59 patients evaluated for safety, 51 of which were evaluable for response of GVHD. A clinical response was defined as a two-grade improvement in the International Bone Marrow Transplant Registry GVHD Severity Scale. GVHD is graded based on clinical symptoms from grade I, which is the mildest form, to grade IV, which is the most severe form. Three of eight patients responded in the lowest dose cohort. Twenty-three of 43 patients responded among the three highest doses.
In December 1999, we reported additional data from this trial regarding survival. Among patients in the three higher dose cohorts (0.1-0.3 mg/kg), 52% (26 of 50) survived at least 100 days from the start of treatment with ABX-CBL. This compared to a 22% (2 of 9) survival rate in the presumed no effect dose cohort (0.01 mg/kg).
In December 2000, we reported the following results of longer term follow up: 22% of patients receiving the presumed no effect dose (0.01 mg/kg) of ABX-CBL were alive at 180 days following treatment initiation, while 48% of patients receiving the higher dose were alive at this time point.
Thirteen of the 59 patients treated in the Phase II study received additional treatment with ABX-CBL in a separate retreatment study. Six of these patients (46%) were alive at least 180 days after their first dose of ABX-CBL.
In addition, 20 patients received treatment with ABX-CBL in a compassionate use study for treatment of steroid resistant acute GVHD and for chronic GVHD. Forty percent of the patients with acute GVHD and 25% of patients with chronic GVHD responded to treatment with ABX-CBL.
44
In December 1999, we initiated a Phase II/III clinical trial with ABX-CBL. The results of the Phase II/III trial may not be favorable or may not extend the findings of the original Phase II study. The FDA may view the results of our Phase II/III trial as insufficient and may require additional clinical trials. There are several issues that could adversely affect the clinical trial results, including the lack of a standard therapy for GVHD patients in the control group, unforeseen side effects, variability in the number and types of patients in the study, and response rates required to achieve statistical significance in the study. In addition, our clinical trials are being conducted with patients who have failed conventional treatments and who are in the most advanced stages of GVHD. During the course of treatment, these patients can die or suffer adverse medical effects for reasons that may not be related to ABX-CBL. These adverse effects may affect the interpretation of clinical trial results. There can be no assurance that the FDA will accept the results of the Phase II/III study or other elements of the product license application as being sufficient for approval to market. Additional clinical trials will be extensive, expensive and time-consuming.
In four separate clinical studies conducted prior to our obtaining an exclusive license to ABX-CBL, a total of 25 patients with GVHD were treated with the antibody. One such trial, which has been published, was conducted on eleven patients at St. Jude Hospital in Memphis, Tennessee. In this trial, ten patients with steroid-resistant, Grade III to IV GVHD were treated with daily doses of ABX-CBL for up to six weeks. The publication reported that five of ten patients had a complete remission of GVHD, while four of ten had at least a two-grade improvement in their GVHD score. Only one patient did not respond to the therapy. Another patient who was treated at St. Jude Hospital after publication of the study experienced a two-grade improvement in the patient's GVHD score without adverse side effects. Six additional patients with GVHD were treated at the University of Wisconsin and Cook-Ft. Worth Hospital. The reports from these sites indicated that these patients showed similar results to those described in the published trial conducted at St. Jude Hospital, with four of the six patients showing at least a two-grade improvement in their GVHD score. In addition, eight other GVHD patients received treatment at Stanford University and four of the patients were noted to have some improvement in their GVHD score, despite using a dose of less than one-tenth of that employed at the other sites. Immune reaction to the mouse antibody was assessed in several patients and no HAMA response was detected clinically. Furthermore, no adverse clinical responses consistent with an antibody-induced allergic reaction were observed. In addition, a number of patients were followed after the conclusion of the study for as long as one year and no adverse ABX-CBL events were observed. We face the risk that the results of our ABX-CBL clinical trials may not demonstrate the same levels of safety and efficacy as those shown by the clinical trials completed prior to our obtaining an exclusive license to ABX-CBL.
In November 2000, the FDA granted orphan drug status to ABX-CBL. Orphan drug status provides certain marketing advantages to the seller of a drug having that status.
ABX-IL8
IL-8, an inflammatory cytokine produced at sites of inflammation, attracts and activates white blood cells that mediate the inflammation process. A number of pre-clinical studies suggest that excess IL-8 may contribute to the pathology and clinical symptoms associated with some inflammatory disorders. Clinical studies have demonstrated significantly increased levels of IL-8 in tissues or body fluids of patients with certain inflammatory diseases, including psoriasis, rheumatoid arthritis, reperfusion injury and inflammatory bowel disease. Antibodies to IL-8 have been shown to block immune cell infiltration and the associated pathology in animal models of several of these diseases. Using our XenoMouse technology, we have generated ABX-IL8, a proprietary fully human monoclonal antibody that binds to IL-8 with high affinity. We are evaluating ABX-IL8 for possible use in the treatment of psoriasis and rheumatoid arthritis.
45
Psoriasis. Psoriasis is a chronic disease that results in plaques, a thickening and scaling of the skin accompanied by local inflammation. The disease effects approximately four to five million patients in the United States and can be debilitating in its most severe form. Approximately 500,000 psoriasis patients suffer from a severe enough form of the disease to require systemic therapy with immune suppressants and ultraviolet phototherapy. The risk of serious adverse side effects associated with these therapies often requires the patients to alternate these various therapeutic modalities as a precautionary measure.
Scientific studies have shown that IL-8 concentrations can be elevated by a factor of 150 in psoriatic plaques when compared to normal tissue. We believe that IL-8 may promote psoriasis by contributing to three distinct disease-associated processes. First, IL-8 is produced by a type of skin cell called keratinocytes, and is a potent growth factor for these skin cells. It may therefore contribute to the abnormal keratinocyte proliferation in psoriatic plaques. Second, IL-8 attracts and activates immune cells that contribute to the inflammation of the psoriatic plaque. Finally, IL-8 promotes angiogenesis that augments the blood supply necessary for growth of the psoriatic plaque.
Clinical Status. In February 1999, we completed a Phase I dose-escalating human clinical trial examining the safety of administering a single intravenous infusion of five different doses of ABX-IL8 to patients with moderate to severe psoriasis. In October 1999, we completed a Phase I/II multi-center, multi-dose, dose escalating, placebo-controlled clinical trial with ABX-IL8 including 45 patients with moderate to severe psoriasis. In March 2001, we completed a Phase IIa double-blind, placebo-controlled study, which included 94 moderate-to-severe psoriasis patients at 18 sites in the United States. Two doses of ABX-IL8 were assessed, 3mg/kg and 6mg/kg. The primary objective of the Phase IIa study was to evaluate the safety of ABX-IL8 at the doses administered. Based on the results of the Phase IIa study it was concluded that ABX-IL8 administered intravenously at doses ranging up to 6 mg/kg appears to be safe and well tolerated and that ABX-IL8 at 3mg/kg was associated with a statistically significant improvement in plaque psoriasis. On the basis of the Phase IIa data, Abgenix has initiated a Phase IIb study of ABX-IL8, which is designed to confirm the efficacy of ABX-IL8. This study, which is expected to enroll 228 patients with moderate-to-severe psoriasis, will evaluate two doses of ABX-IL8 and a placebo. Having seen no incremental benefit to the 6mg/kg dose over the 3mg/kg in the Phase IIa trial, Abgenix now plans to evaluate more convenient fixed doses of 200mg and 300mg (approximating 1mg/kg and 2mg/kg as weight-adjusted doses) administered every three weeks in the Phase IIb study.
Rheumatoid Arthritis. Rheumatoid arthritis is a chronic disease marked by inflammation and pain in joints throughout the body. The disease affects over two million people in the United States. Elevated levels of IL-8 in the synovial fluid of rheumatoid arthritis patients have been reported to correlate with the number of infiltrating immune cells. Third-party published studies have reported that the injection of non-human antibodies to IL-8 into a rabbit model of rheumatoid arthritis blocked immune cell infiltration and synovial membrane damage.
Clinical Status. In December 2000, we initiated a Phase IIa double-blind, placebo-controlled study designed to evaluate the efficacy and safety of ABX-IL8 in rheumatoid arthritis. A total of 132 patients across 20 clinical sites in the U.S. will participate in the study. The primary efficacy analysis will be measured by percentage of patients who achieve the American College of Rheumatology 20% responder criteria (ACR20), defined as a minimum improvement of 20% in patients' rheumatoid arthritis.
ABX-EGF
Tumor cells that overexpress epidermal growth factor receptors, or EGFr, on their surface often depend on EGFr's activation for growth. EGFr is overexpressed in a variety of cancers including lung, breast, ovarian, bladder, prostate, colorectal, kidney and head and neck. This activation is triggered by
46
the binding to EGFr by epidermal growth factor, or EGF, or Transforming Growth Factor alpha, or TGFa, both of which are expressed by the tumor or by neighboring cells. We believe that blocking the ability of EGF and TGFa to bind with EGFr may offer a treatment for certain cancers. ABX-EGF, a fully human monoclonal antibody generated using XenoMouse technology, binds to EGFr with high affinity and has been shown to inhibit tumor cell proliferation in vivo and cause eradication of EGF dependent human tumors established in mouse models. We are conducting pre-clinical studies and assessing which tumor types to pursue as possible targets for treatment with ABX-EGF. Published studies have shown that ABX-EGF can inhibit growth of EGF-dependent human tumors cells in mouse models. ABX-EGF has also demonstrated the ability to reverse cancer cell growth and cause eradication of established tumors in mice even when administered after significant tumor growth has occurred. Furthermore, in these models where tumors were eradicated, no relapse of the tumor was observed after discontinuation of the antibody treatment.
Clinical Status. In July 1999, we initiated a Phase I dose-escalating human clinical trial examining the safety, pharmacokinetics and biological activity of multiple doses ABX-EGF in patients with a variety of advanced cancers. Data from this ongoing phase I clinical study were reported at the annual meeting of the American Society of Clinical Oncology in May 2001. Twenty-eight patients had been recruited to this study at that time. ABX-EGF appeared be well tolerated at weekly doses ranging up to 0.75 mg/kg preceded by a loading dose of 1.5 mg/kg. No allergic reactions, clinically significant infusion-related reactions or human anti-human antibody formation were observed. Typical EGF receptor mediated skin rashes were seen at a dose of 1 mg/kg when preceded by a 2 mg/kg loading dose. Two patients who had received low dose levels of ABX-EGF as monotherapy (0.1 or 0.75 mg/kg) achieved disease stabilization.
On the basis of this data we have initiated two phase II studies in April and July of 2001. The first phase II study is evaluating the effect of ABX-EGF monotherapy in patients with renal cell cancer. The second phase II study is conducted in patients with non small cell lung cancer receiving either standard chemotherapy with carboplatin and paclitaxel alone or in combination with ABX-EGF. We plan to initiate further Phase II studies in 2001 and 2002.
Contractual Arrangements
The following table lists parties to technology licensing arrangements as of August 27, 2001:
Contract Party
| | Type
| | Date
|
|---|
| Abbott Laboratories | | XenoMouse License | | May 2000 |
| Agensys | | XenoMouse License | | Aug 2001 |
| Amgen | | XenoMouse License
XenoMouse License | | Dec 2000
Apr 1999 |
| AVI | | XenoMouse License | | Jan 1999 |
| BASF | | XenoMouse License | | Mar 1999 |
| Biogen | | XenoMouse License | | June 2001 |
| Cell Genesys | | XenoMouse License | | Nov 1997 |
| Celltech | | SLAM License | | Jan 2001 |
| Centocor/Johnson and Johnson | | XenoMouse License | | June 2001
Dec 1998 |
| Chiron | | XenoMouse License | | Mar 2001
Dec 1999 |
| Corixa | | Target Sourcing Contract | | Mar 2000 |
| CuraGen | | Target Sourcing Contract | | Nov 2000 |
| | | Target Sourcing Contract | | Dec 1999 |
| Diabetogen | | XenoMouse License | | Mar 2001 |
| Dyax | | In-License | | Jan 2001 |
| Elan | | XenoMouse License | | Jan 2000 |
47
| Genentech | | XenoMouse License | | Jan 1999 |
| Genzyme Transgenics | | In-License | | May 2000 |
| Gliatech | | XenoMouse License | | Jan 2000 |
| Human Genome Sciences | | Target Sourcing Contract | | Jul 2001 |
| Immunex | | Target Sourcing Contract | | Nov 2000 |
| | | Product-Co Development | | Jul 2000 |
| ImmunoGen | | In-License | | Sep 2000 |
| Japan Tobacco | | XenoMouse License | | Dec 1999 |
| Lexicon | | Target Sourcing Contract | | Jul 2000 |
| MDS Proteomics | | Target Sourcing Contract | | June 2001 |
| Millennium | | XenoMouse License | | Mar 2000 |
| | | XenoMouse License | | Oct 1998 |
| | | XenoMouse License | | Sep 1998 |
| Pfizer | | XenoMouse License | | Nov 2000 |
| | | XenoMouse License | | Nov 1999 |
| | | XenoMouse License | | Oct 1998 |
| | | XenoMouse License | | Dec 1997 |
| PSMA Development | | XenoMouse License | | Feb 2001 |
| Research Corporation Technologies | | XenoMouse License and
Co-Development | | Dec 1998 |
| SangStat | | Product-Co Development | | Aug 2000 |
| Schering-Plough | | XenoMouse License | | Jan 1998 |
| SmithKline Beecham | | XenoMouse License | | May 2000 |
| U.S. Army | | XenoMouse License | | Oct 1999 |
| | | XenoMouse License | | Jul 1999 |
Abbott: In May 2000, we entered into a research collaboration and license agreement with Abbott Laboratories under which we will generate fully human antibodies to several undisclosed disease targets for Abbott.
Agensys: In August 2001, we entered into a research collaboration, option and license agreement with Agensys, Inc. to discover and develop fully human antibodies to up to 25 selected cancer antigens, for a term of five years.
Amgen: In April 1999, we entered into a research collaboration agreement with Amgen Inc., to generate fully human antibodies to an undisclosed antigen target. In 2000, Amgen exercised its option to acquire a product license. In December 2000, we extended our agreement to generate fully human antibodies to multiple antigen targets to be supplied by Amgen, during a five-year term. Also provided in the April 1999 agreement, Abgenix will perform the immunizations and certain research activities. In June 2001, Amgen entered into a Phase I clinical trial, triggering a milestone fee to us.
AVI: In January 1999, we entered into a research license and option agreement with AVI BioPharma to generate fully human antibodies to human chorionic gonadotropin, or HCG, for the treatment of various cancers. In 1999, AVI exercised its option to acquire a product license.
BASF: In March 1999, we entered into a research collaboration agreement with BASF Bioresearch Corporation to generate fully human antibodies to an undisclosed antigen target.
Biogen: In June 2001, we entered into a research collaboration, option and license agreement with Biogen to generate fully human monoclonal antibodies to up to 15 undisclosed antigen targets.
Cell Genesys: In November 1997, we entered into the gene therapy rights agreement, with Cell Genesys, Inc. Cell Genesys received certain rights to commercialize products based on antibodies generated with XenoMouse technology in the field of gene therapy.
48
Celltech: In January 2001, we agreed to license our Selected Lymphocyte Antibody Method (SLAM) technology with Celltech. This will be our first license of the SLAM technology platform we obtained when we acquired Abgenix Biopharma in November 2000.
Centocor/Johnson and Johnson: In December 1998, we entered into a research collaboration agreement with Centocor to generate fully human antibodies to an undisclosed Centocor antigen in the cardiovascular field. In June 2001, the agreement was extended to multiple antigen targets in various disease indications.
Chiron: In December 1999, we entered into a research license and option agreement with Chiron Corporation to generate fully human antibodies to an undisclosed antigen in the field of autoimmune diseases. In 2000, Chiron exercised its option to acquire a product license. Under a separate research collaboration agreement, Chiron may use XenoMouse technology to generate fully human antibodies to up to four cancer targets. In March 2001, we entered into a research and license agreement with Chiron to generate fully human antibodies to multiple cancer-specific antigen targets supplied by Chiron.
Corixa: In March 2000, we entered into a collaboration agreement with Corixa to discover and develop human monoclonal antibodies against selected targets from Corixa's library of autoimmune disease, cancer and infectious disease antigens.
Curagen: In December 1999, we entered into a broad collaboration agreement with CuraGen Corporation to make fully human antibodies to genomics-based antigen targets. Under the agreement, CuraGen will supply a large number of antigen targets to us and we will be responsible for generating fully human antibodies to them. We will share with CuraGen responsibility for evaluation of the antibodies product candidates generated. Each of us will be able to select antibody product candidates from the pool generated in the course of the agreement. The party selecting a product candidate will pay to the other, for rights to develop and commercialize any such product, license fees, milestone payments and royalty payments on any eventual product sales. In November 2000, we expanded the collaboration to include 250 antigen targets over a five-year period. In connection with the November 2000 agreement, we also agreed to purchase $50.0 million of CuraGen's common stock.
Diabetogen: In March 2001, we entered into a research collaboration to generate fully human monoclonal antibodies to the antigen target CD28 for the treatment of Type 1 diabetes, and potentially other autoimmune diseases.
Dyax: In January 2001 we entered a collaboration agreement to develop new technology for discovering and developing human antibody therapeutics. Under the collaboration, we and Dyax will combine our XenoMouse technology with Dyax's proprietary phage display technology to create libraries of human antibody sequences. With the new libraries, the companies expect to be able to rapidly generate novel antibody sequences for multiple types of therapeutic targets. We and Dyax will share equally in the costs of creating the new antibody libraries. Both companies have the right to use antibodies discovered from the libraries for in-house research and are entitled to select a number of therapeutic product candidates from the libraries. The agreement additionally provides that for any antibody product developed, each company will pay reciprocal commercialization fees to the other party.
Elan: In January 2000, we entered into a research license and option agreement with a subsidiary of Elan Corporation, plc. to generate fully human antibodies to an undisclosed antigen in the field of neurological diseases.
Genentech: In April 1998, we entered into a research license and option agreement with Genentech, Inc. to produce fully human antibodies to an antigen target in the field of growth factor
49
modulation. In June 1998, Genentech expanded its research collaboration with us to include a second antigen target in the field of cardiovascular disease.
In January 1999, we entered into a multi-antigen research license and option agreement with Genentech. Under the agreement, we granted Genentech a license to utilize XenoMouse technology in its antibody product research efforts and an option to obtain product licenses for up to ten antigen targets, but not more than two in any one year, over the agreement's six-year term. Included in the ten are the two previously identified antigen targets under the now superseded 1998 research license and option agreement at the new option, license fee and milestone payment levels. The agreement can be renewed by Genentech for up to an additional four targets over a subsequent three-year period. Genentech acquired 1,981,424 shares of our common stock for an aggregate purchase price of $8.0 million. To renew the agreement at the end of the sixth year, Genentech must purchase an additional $2.5 million of our common stock at a 50% premium to the then current market price.
Genzyme Transgenics: In May 2000, we entered into an agreement to produce commercial quantities of our fully human antibody, ABX-IL8, using Genzyme Transgenics Corporation's manufacturing system. Under the terms of the agreement, in exchange for fees and milestone payments, Genzyme Transgenics agreed to develop transgenic goats that express ABX-IL8 in their milk.
Gliatech: In January 2000, we entered into a research license and option agreement with Gliatech Inc. to generate fully human antibodies to the complement protein properdin for use in the fields of cardiovascular and inflammatory diseases.
Human Genome Sciences: In December 1999, we entered into a broad collaboration agreement with Human Genome Sciences, Inc. to generate fully human antibodies to genomics-based antigen targets. Under the agreement as amended and restated in July 2001, Human Genome Sciences has the right to use XenoMouse technology for research purposes and to take out options and/or licenses on a pre-set number of antigen targets. We also may collaborate with Human Genome Sciences on a pre-set number of antigen targets to which we will generate fully human antibodies. The companies will then jointly develop and commercialize any such products. We also are able to select antigen targets from the Human Genome Sciences database to make antibodies against and will have an option to license a pre-set number of such antigen targets for our in-house development and commercialization. If we enter into a license regarding Human Genome Science's antigen target, we would pay license fees, milestone payments and royalties equivalent to what Human Genome Science pays us for licenses regarding XenoMouse technology.
Immunex: In July 2000, we entered into a joint development and commercialization agreement with Immunex Corporation for ABX-EGF, a fully human antibody created by us. Initiation of the first Phase I clinical trial in April 2001 triggered a milestone fee to us.
Additionally, in November 2000, Abgenix and Immunex entered a multi-year collaboration agreement designed to discover, develop, and potentially commercialize fully human antibody therapies for treatment of various forms of cancer. Each party will provide five cancer-specific antigen targets during the first five years of the collaboration, as well as contributing proprietary technologies and development capabilities. Each company will have an option, exercisable at various stages of development of each antibody, to continue or discontinue participating in the development of the antibody. If both companies decide to continue development of an antibody, the development and commercialization costs will be shared equally, as would any potential profits from the sale of the antibody. If only one company decides to continue development of an antibody, it may do so at its own expense and would then be required to pay the other company a royalty on product sales.
ImmunoGen: In September 2000, we entered into a collaboration with ImmunoGen providing us with access to ImmunoGen's maytansinoid Tumor-Activated Prodrug (TAP) technology. for use with Abgenix's fully human antibodies generated with XenoMouse technology. Under the agreement, we
50
paid ImmunoGen $5 million in technology access fees, and are obligated to pay potential milestone payments, and royalties on net sales of any resulting products. In addition, we purchased $15 million of ImmunoGen common stock at $19.00 per share.
Japan Tobacco: In December 1999, we entered into a collaboration agreement with Japan Tobacco allowing it to use XenoMouse technology for research purposes and to obtain options and/or product licenses for a limited number of specific antigen targets each year. In 2000, JT exercised two options to acquire product licenses.
Lexicon: In July 2000, we entered into a drug discovery alliance with Lexicon Genetics Incorporated in which Lexicon will contribute drug targets for which we will generate fully human antibodies using XenoMouse technology.
MDS Proteomics: In June 2001, we entered into a collaboration with MDS Proteomics to generate fully human antibodies to up to 150 proteomics-based antigen targets. Once targets are identified by MDS Proteomics, we independently or jointly with MDS Proteomics, will develop and commercialize the proteomics-derived product candidates. Both companies will receive reciprocal milestone and royalty payments resulting from the alliance. In addition, we purchased $15 million of MDS Proteomics common stock in June 2001.
Millennium: In July 1998, we entered into a research license and option agreement with Millennium BioTherapeutics, or Mbio, a majority owned subsidiary of Millenium Pharmaceuticals, Inc., which was extended in October 1998 to a research, license and option agreement to generate fully human antibodies to an antigen target in the field of inflammation. In September 1998, we entered into a second research collaboration agreement with Mbio covering a second antigen target in the field of inflammation. In March 2000, we entered into a broad collaboration agreement with Millenium Pharmaceuticals Inc. Under the agreement, Millenium receives a license to use XenoMouse technology for research purposes and a group of product licenses for a certain number of antigens. For additional future payments to us, Millenium may renew its research license and buy additional groups of product licenses.
Pfizer: In December 1997, we entered into a research collaboration agreement with Pfizer to generate fully human antibodies to an antigen target in the cancer field. Subsequently, in 1998, 1999 and 2000 Pfizer exercised its options to include three more antigen targets and had one option remaining. Pfizer is paying us to perform the immunizations and to undertake certain research activities. In 2000, Pfizer exercised its option for a product license. Additionally, Pfizer filed an investigational new drug application (IND) for its first product candidate, triggering a milestone fee. In November 2000, Pfizer amended the original agreement to provide for up to ten additional antigen targets over an 8-year period.
PSMA Development: In February 2001, we entered into a research collaboration with PSMA Development Company LLC, to generate human antibodies to an antigen target for the treatment of prostate cancer.
Research Corporation Technologies: In December 1998, we entered into a binding memorandum of understanding for a research collaboration agreement with RCT to generate fully human antibodies to CD45rb. Resultant antibody product candidates could potentially be used in treating organ transplant rejection and autoimmune disorders. The terms of the memorandum of understanding were implemented and expanded in a limited liability agreement in February 2001, creating the Alimmune LLC to generate antibodies to CD45.
SangStat: In August 2000, we entered into a co-development, supply and license agreement with SangStat Medical Corporation for ABX-CBL, an antibody in-licensed and further developed by us. SangStat will have an exclusive worldwide license for the marketing and sale of ABX-CBL and, subject
51
to the terms and conditions of the agreement, the right to commercialize other CD-147 antibodies, including ABX-RB2, the next generation fully human antibody to CD-147, generated using XenoMouse technology.
Schering-Plough: In January 1998, we entered into a research collaboration agreement with Schering-Plough Research Institute to generate fully human antibodies to an antigen target in the field of inflammation. Under this agreement, Schering-Plough is paying us to perform the immunizations and certain research activities. In 1999, Schering-Plough exercised its option for a product license.
SmithKline Beecham: In May 2000, we entered into a license agreement under which SmithKline Beecham Pharmaceuticals Inc. will use XenoMouse technology to generate fully human antibodies to an undisclosed antigen target.
U.S. Army: In July 1999, we entered into a collaboration agreement with the U.S. Army to generate fully human antibodies to filoviruses. In October 1999, the U.S. Army expanded the agreement to include poxviruses.
Joint Venture with Japan Tobacco
Xenotech prior to our acquisition
In June 1991, Cell Genesys entered into several agreements with Japan Tobacco for the purpose of forming an equally owned limited partnership named Xenotech. In connection with the formation of Xenotech, both Cell Genesys and Japan Tobacco contributed cash, and Cell Genesys contributed the exclusive right to certain of its technology for the research and development of genetically modified strains of mice that can produce fully human antibodies. Cell Genesys assigned its rights in Xenotech to us in connection with our formation. We provided research and development on behalf of Xenotech in exchange for cash payments. As of December 31, 1998, we had made capital contributions to Xenotech of approximately $18.6 million and had received approximately $42.9 million in funding for research related to the development of XenoMouse technology.
XenoMouse Technology
On December 20, 1999, we executed several agreements with Japan Tobacco that became effective December 31, 1999 under which we acquired Japan Tobacco's interest in the Xenotech joint venture. Under the agreements, we paid $47.0 million in cash to Japan Tobacco for its 50% interest in the Xenotech joint venture under which the XenoMouse technology was developed; and we also made a non-recurring payment of $10.0 million to Japan Tobacco to terminate its then applicable rights to the current XenoMouse technology. Additionally, Japan Tobacco paid $4.0 million to us for a license to use the existing XenoMouse technology on a more limited basis than previously, and to use future XenoMouse technology that we develop. Japan Tobacco will also make royalty payments on any future sales of antibody products generated using XenoMouse. Lastly, under the December agreements, we granted to Japan Tobacco a license for certain new technology related to the generation of mouse models of certain human diseases. In return for this license, Japan Tobacco paid us $6.0 million, which was recorded in contract revenue.
Gene Therapy Rights Agreement with Cell Genesys
In connection with the formation of Abgenix by Cell Genesys, Abgenix entered into the Gene Therapy Rights Agreement (GTRA), which provides Cell Genesys with certain rights to commercialize products based on antibodies generated with XenoMouse technology in the field of gene therapy. Under the GTRA, Cell Genesys has certain rights to direct us to make antibodies to two antigens per year. In addition, Cell Genesys has an option to enter into a license to commercialize antibodies binding to such antigens in the field of gene therapy. Cell Genesys is obligated to make certain
52
payments to us for these rights, including reimbursement of license fees and royalties on future product sales. The GTRA also prohibits us from granting any third-party licenses for antibody products based on antigens nominated by us for our own purposes where the primary field of use is gene therapy. In the case of third-party licenses granted by us where gene therapy is a secondary field, we are obligated to share with Cell Genesys a portion of the cash milestone payments and royalties resulting from any products in the field of gene therapy.
Intellectual Property
We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our proprietary rights are covered by valid and enforceable patents or are effectively maintained as trade secrets. We own six issued patents in the United States, one granted patent in Europe, three granted patents in Japan and have several pending patent applications in the United States and abroad relating to XenoMouse technology. Our wholly-owned subsidiary, Xenotech, owns two issued U.S. patents, one Australian patent and several pending U.S. and foreign patent applications related to methods of treatment of bone disease in cancer patients. Our wholly owned subsidiary Abgenix Biopharma owns one issued U.S. patent and has one pending patent in Canada and Europe relating to the SLAM technology. Our wholly owned subsidiary IntraImmune owns several patents and pending applications in the United States and in Europe related to intrabody technology, which may give antibodies access to intracellular targets. In addition, we have six issued U.S. patents and several pending patent applications in the United States and abroad that are jointly owned with Japan Tobacco relating to antibody technology or genetic manipulation. We attempt to protect our proprietary position by filing U.S. and foreign patent applications related to our proprietary technology, inventions and improvements that are important to the development of our business. The patent position of biopharmaceutical companies involves complex legal and factual questions and, therefore, enforceability cannot be predicted with certainty. Patents, if issued, may be challenged, invalidated or circumvented. Thus, any patents that we own or license from third parties may not provide any protection against competitors. Our pending patent applications, those we may file in the future, or those we may license from third parties, may not result in patents being issued. Also, patent rights may not provide us with adequate proprietary protection or competitive advantages against competitors with similar technology. The laws of certain foreign countries do not protect our intellectual property rights to the same extent as do the laws of the United States.
In addition to patents, we rely on trade secrets and proprietary know-how. We seek protection of our trade secrets and proprietary know-how, in part, through confidentiality and proprietary information agreements. These agreements may not provide meaningful protection or adequate remedies for our technology in the event of unauthorized use or disclosure of such information. The parties to these agreements may breach them. Also, our trade secrets may otherwise become known to, or be independently developed by, our competitors. Furthermore, others may independently develop similar technologies or duplicate any technology that we have developed.
Research has been conducted for many years in the antibody and transgenic animal fields. This has resulted in a substantial number of issued patents and an even larger number of pending patent applications. Patent applications in the United States are, in most cases, maintained in secrecy until patents are issued. The publication of discoveries in the scientific or patent literature frequently occurs substantially later than the date on which the underlying discoveries were made. Our commercial success depends significantly on our ability to operate without infringing the patents and other proprietary rights of third parties. Our technologies may unintentionally infringe the patents or violate other proprietary rights of third parties. In the event of such infringement or violation, we and our contract parties may be prevented from pursuing product development or commercialization. Such a result will materially harm our business, financial condition and results of operations.
53
We have one granted European patent relating to XenoMouse technology that is currently undergoing opposition proceedings within the European Patent Office and the outcome of this opposition is uncertain.
Glaxo has a family of patents relating to certain methods for generating monoclonal antibodies that Glaxo is asserting against Genentech, Inc. in litigation that was commenced in 1999. On May 4, 2001, Genentech announced that a jury had determined that Genentech had not infringed Glaxo's patents and that all of the patent claims asserted against Genentech are invalid. We understand that Glaxo has filed a notice of appeal with the Court of Appeals for the Federal Circuit. If any of the claims of these patents are finally determined in the litigation to be valid, and if we were to use manufacturing processes covered by the patents to make our products, we may then need to obtain a license should one be available. Should a license be denied or unavailable on commercially reasonable terms, commercialization of one or more of our products could be impeded in any territories in which these claims were in force.
Genentech, Inc. owns a U.S. patent that issued in June 1998 relating to inhibiting the growth of tumor cells that involves an anti-EGF receptor antibody in combination with a cytotoxic factor. ImClone Systems, Inc. owns or is licensed under a U.S. patent that issued in April 2001, relating to inhibiting the growth of tumor cells that involves an anti-EGF receptor antibody in combination with an anti-neoplastic agent. We believe there are strong arguments that all claims of both the Genentech patent and the ImClone patent are invalid. We are continuing to analyze the scope of these patents. We believe that currently all of the Company's activities relating to anti-EGFr monoclonal antibodies are within the exemption provided by the U.S. patent laws for uses reasonably related to obtaining FDA approval of a drug. Based on our product development plans, we do not expect the scope of our activities in this regard to change in the future prior to filing an application for a biologic license with the FDA. If the claims of either the Genentech patent or the ImClone patent are judicially determined to cover our activities with ABX-EGF and are held valid, we may be required to obtain a license to Genentech's or ImClone's patent, as the case may be, to label and sell ABX-EGF for some or all such combination indications. Should a license be denied or unavailable on commercially reasonable terms, our commercialization of ABX-EGF could be impeded in the United States.
In 2000, the Japanese Patent Office granted a patent to Kirin Beer Kabushiki Kaisha, one of our competitors, relating to non-human transgenic mammals. Kirin has filed corresponding patent applications in Europe and Australia. Kirin may also have filed a corresponding patent application in the United States. Our licensee, Japan Tobacco, has filed opposition proceedings against the Kirin patent. We cannot predict the outcome of those opposition proceedings, which may take years to be resolved. We are analyzing the patent to determine its relevance to our business and if appropriate will analyze the scope and validity of its claims.
The biotechnology and pharmaceutical industries have been characterized by extensive litigation regarding patents and other intellectual property rights. The defense and prosecution of intellectual property suits, United States Patent and Trademark Office interference proceedings and related legal and administrative proceedings in the United States and internationally involve complex legal and factual questions. As a result, such proceedings are costly and time-consuming to pursue and their outcome is uncertain. Litigation may be necessary to:
- •
- enforce patents that we own or license;
- •
- protect trade secrets or know-how that we own or license; or
- •
- determine the enforceability, scope and validity of the proprietary rights of others.
If we become involved in any litigation, interference or other administrative proceedings, we will incur substantial expense and the efforts of our technical and management personnel will be significantly diverted. An adverse determination may subject us to loss of our proprietary position or to
54
significant liabilities, or require us to seek licenses that may not be available from third parties. We may be restricted or prevented from manufacturing and selling our products, if any, in the event of an adverse determination in a judicial or administrative proceeding or if we fail to obtain necessary licenses. Costs associated with such arrangements may be substantial and may include ongoing royalties. Furthermore, we may not be able to obtain the necessary licenses on satisfactory terms, if at all. These outcomes will materially harm our business, financial condition and results of operations.
Patent Cross-License and Settlement Agreement with GenPharm
In 1994, Cell Genesys and GenPharm and, beginning in 1996, Abgenix became involved in litigation primarily related to intellectual property rights associated with a method for inactivating a mouse's antibody genes and technology pertaining to transgenic mice capable of producing fully human antibodies. Rather than endure the cost and business interruption of protracted litigation, in March 1997, Cell Genesys, along with us, Xenotech and Japan Tobacco, signed a comprehensive patent cross-license and settlement agreement with GenPharm that resolved all related litigation and claims between the parties. Under the cross-license and settlement agreement, we have licensed on a non-exclusive basis certain patents, patent applications, third-party licenses and inventions pertaining to the development and use of certain transgenic rodents, including mice that produce fully human antibodies. We use our XenoMouse technology to generate fully human antibody products and have not licensed the use of, and do not use, any transgenic rodents developed or used by GenPharm. As initial consideration for the cross-license and settlement agreement, Cell Genesys issued a note to GenPharm for $15 million, which was paid in full on September 30, 1998. Of this note, approximately $3.8 million thereof satisfied certain of Xenotech's obligations under the agreement. Japan Tobacco also made an initial payment. During 1997, GenPharm achieved two patent milestones, and Xenotech was obligated to pay $7.5 million for each milestone. No additional payments will accrue under this agreement. In 1997, we recognized, as a non-recurring charge for the cross-license and settlement, a total of $22.5 million. We do not have any future financial obligations under the cross-license and settlement agreement.
Government Regulation
Our product candidates under development are subject to extensive and rigorous domestic government regulation. The FDA regulates, among other things, the development, testing, manufacture, safety, efficacy, record keeping, labeling, storage, approval, advertising, promotion, sale and distribution of biopharmaceutical products. If our products are marketed abroad, they also are subject to extensive regulation by foreign governments. Non-compliance with applicable requirements can result in fines, warning letters, recall or seizure of products, clinical study holds, total or partial suspension of production, refusal of the government to grant approvals, withdrawal of approval, and civil and criminal penalties.
We believe our antibody products will be classified by the FDA as "biologic products" as opposed to "drug products." The steps ordinarily required before a biological product may be marketed in the United States include:
- •
- pre-clinical testing;
- •
- the submission to the FDA of an investigational new drug application, or IND, which must become effective before clinical trials may commence;
- •
- adequate and well-controlled clinical trials to establish the safety and efficacy of the biologic;
- •
- the submission to the FDA of a Biologics License Application; and
- •
- FDA approval of the application, including approval of all product labeling.
55
Pre-clinical testing includes laboratory evaluation of product chemistry, formulation and stability, as well as animal studies to assess the potential safety and efficacy of each product. Pre-clinical safety tests must be conducted by laboratories that comply with FDA regulations regarding good laboratory practices. The results of the pre-clinical tests, together with manufacturing information and analytical data, are submitted to the FDA as part of the IND and are reviewed by the FDA before the commencement of clinical trials. Unless the FDA objects to an IND, the IND will become effective 30 days following its receipt by the FDA. If we submit an IND, our submission may not result in FDA authorization to commence clinical trials. Also, the lack of an objection by the FDA does not mean it will ultimately approve an application for marketing approval. Furthermore, we may encounter problems in clinical trials that cause us or the FDA to delay, suspend or terminate our trials.
Clinical trials involve the administration of the investigational product to humans under the supervision of a qualified principal investigator. Clinical trials must be conducted in accordance with Good Clinical Practices under protocols submitted to the FDA as part of the IND. In addition, each clinical trial must be approved and conducted under the auspices of an Institutional Review Board and with patient informed consent. The Institutional Review Board will consider, among other things, ethical factors, the safety of human subjects and the possibility of liability of the institution conducting the trial.
Clinical trials are conducted in three sequential phases that may overlap. Phase I clinical trials may be performed in healthy human subjects or, depending on the disease, in patients. The goal of a Phase I clinical trial is to establish initial data about safety and tolerance of the biologic agent in humans. In Phase II clinical trials, evidence is sought about the desired therapeutic efficacy of a biologic agent in limited studies of patients with the target disease. Efforts are made to evaluate the effects of various dosages and to establish an optimal dosage level and dosage schedule. Additional safety data are also gathered from these studies. The Phase III clinical trial program consists of expanded, large-scale, multi-center studies of persons who are susceptible to or have developed the disease. The goal of these studies is to obtain definitive statistical evidence of the efficacy and safety of the proposed product and dosage regimen.
Historically, the results from pre-clinical testing and early clinical trials have often not been predictive of results obtained in later clinical trials. A number of new drugs and biologics have shown promising results in clinical trials, but subsequently failed to establish sufficient safety and efficacy data to obtain necessary regulatory approvals. Data obtained from pre-clinical and clinical activities are susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. In addition, delays or rejections by regulatory authorities may be encountered as a result of many factors, including changes in regulatory policy during the period of product development.
Only three of our product candidates, ABX-CBL, ABX-IL8 and ABX-EGF, are currently in clinical trials. Patient follow-up for these clinical trials has been limited. To date, we have not obtained enough data from these clinical trials to demonstrate safety and efficacy under applicable FDA guidelines. As a result, such data will not support an application for regulatory approval without further clinical trials. Clinical trials conducted by us or by third parties on our behalf may not demonstrate sufficient safety and efficacy to obtain the requisite regulatory approvals for ABX-CBL, ABX-IL8, ABX-EGF or any other potential product candidates. Regulatory authorities may not permit us to undertake any additional clinical trials for our product candidates.
Our other product candidates are still in pre-clinical development, and we have not submitted INDs or begun clinical trials for these product candidates. Our pre-clinical or clinical development efforts may not be successfully completed. Further INDs may not be filed. Clinical trials may not commence as planned.
56
Completion of clinical trials may take several years or more. The length of time generally varies substantially according to the type, complexity, novelty and intended use of the product candidate. Our commencement and rate of completion of clinical trials may be delayed by many factors, including:
- •
- inability to manufacture sufficient quantities of materials for use in clinical trials;
- •
- slower than expected rate of patient recruitment;
- •
- inability to adequately follow patients after treatment;
- •
- unforeseen safety issues;
- •
- lack of efficacy during the clinical trials; or
- •
- government or regulatory delays.
We have limited experience in conducting and managing clinical trials. We rely on third parties, including our contract parties, to assist us in managing and monitoring clinical trials. Our reliance on third parties may result in delays in completing, or failing to complete, clinical trials if they fail to perform under our agreements with them.
Our product candidates may fail to demonstrate safety and efficacy in clinical trials. Such failure may delay development of other product candidates, and hinder our ability to conduct related pre-clinical testing and clinical trials. As a result of such failures, we may also be unable to obtain additional financing. Our business, financial condition and results of operations will be materially harmed by any delays in, or termination of, our clinical trials.
We and our third-party manufacturer also are required to comply with the applicable FDA current good manufacturing practice. Good manufacturing practice regulations include requirements relating to quality control and quality assurance as well as the corresponding maintenance of records and documentation. Manufacturing facilities are subject to inspection by the FDA. The facilities must be approved before they can be used in commercial manufacturing of our products. We or our third-party manufacturer may not be able to comply with the applicable good manufacturing practice requirements and other FDA regulatory requirements. If we or our third-party manufacturer fails to comply, our business, financial condition and results of operations will be materially harmed.
For clinical investigation and marketing outside the United States, we may be subject to the regulatory requirements of other countries, which vary from country to country. The regulatory approval process in other countries includes requirements similar to those associated with FDA approval set forth above.
Competition
The biotechnology and pharmaceutical industries are highly competitive and subject to significant and rapid technological change. We are aware of several pharmaceutical and biotechnology companies that are actively engaged in research and development in areas related to antibody therapy. These companies have commenced clinical trials of antibody product candidates or have successfully commercialized antibody products. Many of these companies are addressing the same diseases and disease indications as us or our customers. Also, we compete with companies that offer antibody generation services to companies that have antigens. These competitors have specific expertise or technology related to antibody development and introduce new or modified technologies from time to time. These companies include GenPharm International, Inc., a wholly-owned subsidiary of Medarex, Inc.; Medarex's joint venture partner, Kirin Brewing Co., Ltd.; Cambridge Antibody Technology Group plc; Protein Design Labs, Inc.; and MorphoSys AG.
Some of our competitors have received regulatory approval of or are developing or testing product candidates that may compete directly with our product candidates. For example, SangStat Medical
57
Corp., Novartis, Pharmacia Corporation and Roche market organ transplant rejection products that may compete with ABX-CBL, which is in clinical trials. In addition, MedImmune, Inc. has a potential antibody product candidate in clinical trials for graft versus host disease that may compete with ABX-CBL. We are also aware that several companies, including Genentech, Inc., Biogen, Inc. and Immunex Corporation, have potential product candidates for the treatment of psoriasis that may compete with ABX-IL8, which is in clinical trials. Furthermore, we are aware that ImClone Systems, Inc., AstraZeneca PLC, GlaxoSmithKline and a collaboration of OSI Pharmaceuticals, Inc., Genentech, Inc., Roche have potential antibody and small molecule product candidates in clinical development that may compete with ABX-EGF, which is also in clinical trials.
Many of these companies and institutions, either alone or together with their customers, have substantially greater financial resources and larger research and development staffs than we do. In addition, many of these competitors, either alone or together with their customers, have significantly greater experience than we do in:
- •
- developing products;
- •
- undertaking pre-clinical testing and human clinical trials;
- •
- obtaining FDA and other regulatory approvals of products; and
- •
- manufacturing and marketing products.
Accordingly, our competitors may succeed in obtaining patent protection, receiving FDA approval or commercializing products before we do. If we commence commercial product sales, we will be competing against companies with greater marketing and manufacturing capabilities, areas in which we have limited or no experience.
We also face, and will continue to face, competition from academic institutions, government agencies and research institutions. There are numerous competitors working on products to treat each of the diseases for which we are seeking to develop therapeutic products. In addition, any product candidate that we successfully develop may compete with existing therapies that have long histories of safe and effective use. Competition may also arise from:
- •
- other drug development technologies and methods of preventing or reducing the incidence of disease;
- •
- new small molecules; or
- •
- other classes of therapeutic agents.
Developments by competitors may render our product candidates or technologies obsolete or non-competitive. We face and will continue to face intense competition from other companies for agreements with pharmaceutical and biotechnology companies for establishing relationships with academic and research institutions, and for licenses to proprietary technology. These competitors, either alone or with their customers, may succeed in developing technologies or products that are more effective than ours.
Pharmaceutical Pricing and Reimbursement
In both domestic and foreign markets, sales of our product candidates will depend in part upon the availability of reimbursement from third-party payors. Third-party payors include government health administration authorities, managed care providers, private health insurers and other organizations. These third-party payors are increasingly challenging the price and examining the cost-effectiveness of medical products and services. In addition, significant uncertainty exists as to the reimbursement status of newly approved healthcare products. We may need to conduct post-marketing studies in order to demonstrate the cost-effectiveness of our products. These studies may require us to incur significant
58
costs. Our product candidates may not be considered cost-effective. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development. Domestic and foreign governments continue to propose and pass legislation designed to reduce the cost of healthcare. Accordingly, legislation and regulations affecting the pricing of pharmaceuticals may change before our proposed products are approved for marketing. Adoption of such legislation could further limit reimbursement for pharmaceuticals. If the government and third party payors fail to provide adequate coverage and reimbursement rates for our product candidates, the market acceptance of our products may be adversely affected. If our products do not receive market acceptance, our business, financial condition and results of operations will be materially harmed.
Manufacturing
We are building our own manufacturing facility for the manufacture of products for clinical trials and early commercial launch, in compliance with FDA good manufacturing practices. In May 2000, we signed a long-term lease for a building to contain this manufacturing facility. Construction has started and this facility is expected to be operational by year-end 2002. The costs of the facility, including design, leasehold improvements and equipment, will approximate $140 million. Construction of this facility may take longer than expected, and the planned and actual construction costs of building and qualifying the facility for regulatory compliance may be higher than expected. The process of manufacturing antibody products is complex. We have no experience in the clinical or commercial scale manufacturing of ABX-CBL, ABX-IL8 and ABX-EGF, or any other antibody products. Such antibody products will also need to be manufactured in a facility and by a process that comply with FDA and other regulations. It may take a substantial period of time to begin producing antibodies in compliance with such regulations. Our manufacturing operations will be subject to ongoing, periodic unannounced inspection by the FDA and state agencies to ensure compliance with good manufacturing practices. If we are unable to establish and maintain a manufacturing facility within our planned time and cost parameters, the development and sales of our products and our financial performance may be materially harmed.
We currently rely, and will continue to rely for at least the next five years, on a single contract manufacturer, Lonza Biologics (Lonza), to produce ABX-CBL, ABX-IL8 and ABX-EGF under good manufacturing practice regulations, for use in our clinical trials. In December 2000, we entered into a manufacturing supply agreement with Lonza, under which Lonza will make available exclusively to us, for a period of five years, a cell culture production suite, with associated purification capacity, within Lonza's facility. As a result of this agreement, we expect to gain access to production capacity and scheduling flexibility similar to owning the production capability, while Lonza retains responsibility for staffing and operating the facility. The term of the agreement is five years with an option to extend the term. The dedicated cell culture production suite is being refurbished and is expected to be operational and available to us in the third or fourth quarter of 2001. In July 2001, we entered into an agreement giving us the right to enter into exclusive negotiations with Lonza for an additional manufacturing supply agreement under which Lonza will make available to us, for a period of up to five years, extendable for an additional two years, one third of a cell culture production suite for large-scale manufacturing of products. The exclusive negotiation period will expire on December 31, 2001, subject to a limited further extension. We currently anticipate that construction of the facility will be completed in the fourth quarter of 2004.
Contract manufacturers often encounter difficulties in scaling up production, including problems involving production yields, quality control and assurance, shortage of qualified personnel, compliance with FDA regulations, production costs and development of advanced manufacturing techniques and process controls. Our third-party manufacturer may not perform as agreed or may not remain in the contract manufacturing business for the time required by us to successfully produce and market our
59
product candidates. If our third-party manufacturer fails to deliver the required quantities of our product candidates for clinical use on a timely basis and at commercially reasonable prices, and we fail to find a replacement manufacturer or develop our own manufacturing capabilities, our business, financial condition and results of operations will be materially harmed.
Recent Developments
On August 27, 2001, the University of British Columbia assigned the SLAM technology to our subsidiary Abgenix Biopharma, effective as of August 2, 2001. We had previously exclusively licensed the SLAM technology from the University.
Employees
As of June 30, 2001, we employed 243 persons. Approximately 207 employees were engaged in research and development, and 36 supported administration, finance, management information systems and human resources.
Our success will depend in large part upon our ability to attract and retain employees. We face competition in this regard from other companies, research and academic institutions, government entities and other organizations. We believe that we maintain good relations with our employees.
Facilities
We are currently leasing approximately 339,000 square feet of office, laboratory and pilot scale manufacturing facilities in Fremont, California and Vancouver, Canada. Our leases expire in the years 2002 through 2015 and each includes an option to extend, other than our facility in Vancouver. We believe that our current facilities are adequate for our needs for the foreseeable future and that, should it be needed, suitable additional space will be available to accommodate expansion of our operations on commercially reasonable terms.
Legal Proceedings
We are not a party to any material legal proceedings.
Scientific Advisory Board
We have established a Scientific Advisory Board to provide specific expertise in areas of research and development relevant to our business. The Scientific Advisory Board meets periodically with our scientific and development personnel and management to discuss our present and long-term research and development activities. Scientific Advisory Board members include:
| Anthony DeFranco, M.D., Ph.D. | | Professor, Biochemistry and Biophysics, University of California, San Francisco |
John Gallin, M.D. |
|
Director, Warren Grant Magnusen Clinical Center, National Institute of Health |
Raju S. Kucherlapati, Ph.D. |
|
Professor and Chair, Molecular Genetics, Albert Einstein College of Medicine |
Michel Nussenzweig, M.D., Ph.D. |
|
Professor, Molecular Immunology, Rockefeller University |
Greg T. Went |
|
President and Chief Executive Officer, DNA Sciences Inc. |
Philip Hieter |
|
Professor, Medical Genetics, University of British Columbia |
60
MANAGEMENT
Executive Officers and Directors
The names and ages of our executive officers and directors as of June 30, 2001 are as follows:
Name
| | Age
| | Position(s)
|
|---|
R. Scott Greer |
|
42 |
|
Chairman and Chief Executive Officer |
Raymond M. Withy, Ph.D. |
|
46 |
|
President and Chief Operating Officer |
C. Geoffrey Davis, Ph.D. |
|
50 |
|
Chief Scientific Officer |
Kurt W. Leutzinger |
|
50 |
|
Chief Financial Officer |
Steve M. Chamow, Ph.D. |
|
48 |
|
Vice President, Process Sciences |
Gregory M. Landes, Ph.D. |
|
50 |
|
Vice President, Product Discovery |
John C. Meyer |
|
57 |
|
Vice President, Human Resources |
Gayle M. Mills |
|
46 |
|
Vice President, Business Development |
Patrick M. Murphy |
|
47 |
|
Vice President, Manufacturing |
Gisela M. Schwab, M.D. |
|
45 |
|
Vice President, Clinical Development |
Susan L. Thorner |
|
52 |
|
Vice President, General Counsel and Secretary |
M. Kathleen Behrens, Ph.D.(2) |
|
48 |
|
Director |
Raju S. Kucherlapati, Ph.D. |
|
58 |
|
Director |
Mark B. Logan(1)(2) |
|
62 |
|
Director |
Joseph E. Maroun |
|
72 |
|
Director |
Stephen A. Sherwin, M.D.(1)(2) |
|
52 |
|
Director |
- (1)
- Member of the Compensation Committee
- (2)
- Member of the Audit Committee
R. Scott Greer, has served as our Chairman of the Board since May 2000, and as our Chief Executive Officer and director since June 1996. From June 1996 until December 2000, he served as our President. He also serves as a director of CV Therapeutics, Inc. and Illumina, Inc. From July 1994 to July 1996, Mr. Greer was Senior Vice President of Corporate Development at Cell Genesys. From April 1991 to July 1994, Mr. Greer was Vice President of Corporate Development and from April 1991 to September 1993 was Chief Financial Officer of Cell Genesys. From 1986 to 1991, Mr. Greer held various positions at Genetics Institute, Inc., a biotechnology company, including Director, Corporate Development. Mr. Greer received a BA degree in Economics from Whitman College and an MBA degree from Harvard University and is a certified public accountant.
Raymond W. Withy, Ph.D., has served as our President and Chief Operating Officer since January 2001. From January 2000 to December 2000 he served as our Chief Business Officer and from June 1996 to January 2000 as our Vice President, Corporate Development. He also serves as a director of Xenotech. From May 1993 to June 1996, Dr. Withy served in various positions at Cell Genesys, most recently as Director of Business Development. From 1991 to May 1993, Dr. Withy was a private consultant to the biotechnology industry in areas of strategic planning, business development and licensing. From 1984 to 1991, Dr. Withy was an Associate Director and Senior Scientist at Genzyme
61
Corporation, a biotechnology company. Dr. Withy received a BS degree in Chemistry and Biochemistry and a Ph.D. degree in Biochemistry, both from the University of Nottingham.
C. Geoffrey Davis, Ph.D., has served as our Chief Scientific Officer since January 2000 and from June 1996 until December 2000 as our Vice President, Research. From January 1995 to June 1996, Dr. Davis was Director of Immunology at the Xenotech Division of Cell Genesys. From November 1991 to December 1994, he served at Repligen Corporation, a biotechnology company, first as Principal Investigator and then as Director of Immunology. Dr. Davis received a BA degree in Biology from Swarthmore College and a Ph.D. degree in Immunology from the University of California, San Francisco.
Kurt W. Leutzinger, has served as our Chief Financial Officer since July 1997. From June 1987 to July 1997, Mr. Leutzinger was a Vice-President of General Electric Investments and a portfolio manager of the General Electric Pension Fund. At General Electric, he was responsible for private equity investments with a focus on medical technology. Mr. Leutzinger received a BA degree in Economics from Fairleigh Dickinson University and an MBA degree in Finance from New York University and is a certified public accountant.
Steven M. Chamow, Ph.D., has served as our Vice President, Process Sciences since April 2000. From 1998 to April 2000, Dr. Chamow was Director, Biopharmaceutical Development at Scios, Inc., a biotechnology company. From 1987 to 1998, he held various positions at Genentech, a biotechnology company, including Senior Scientist, Recovery Sciences. Dr. Chamow received a BA degree in Biology from the University of California, Santa Cruz and a Ph.D. degree in Biochemistry from the University of California, Davis.
Gregory M. Landes, Ph.D., has served as our Vice President, Product Discovery since May 2000. From 1982 to May 2000, Dr. Landes held various positions at Genzyme, a biotechnology company, most recently as Vice President, Genetics and Genomics. From 1978 to 1981, Dr. Landes was a Postdoctoral Fellow at the Department of Chemistry and Biochemistry at the University of California, Los Angeles. Dr. Landes received a BA degree in Chemistry and a Ph.D. degree in Biochemistry from the University of Kansas.
John C. Meyer, has served as our Vice President, Human Resources since September 2000. Mr. Meyer was Vice President, Human Resources at various high technology and biotechnology companies, including Somnus Medical Technologies from 1999 to September 2000, Vivus Inc. from 1998 to 1999, Target Therapeutics from 1996 to 1997 and Chipcom Corporation from 1991 to 1995. Mr. Meyer received a BS degree in Business Administration from Colorado State University.
Gayle M. Mills, has served as our Vice President, Business Development since September 2000. From 1998 to September 2000, Ms. Mills was Vice President, Business Development at EOS Biotechnology. From 1995 to 1998, Ms. Mills was Vice President, Business Development and Strategic Marketing for the Neurobiology Unit at Roche Bioscience, a biopharmaceutical company. Ms. Mills served as Director, Business Development both at Affymax Technologies from 1993 to 1995 and at Syntex Corp. from 1991 to 1993. Ms. Mills received a BS degree in Business Administration from the College of Notre Dame and an MBA degree from Santa Clara University.
Patrick M. Murphy, has served as our Vice President, Manufacturing since May 2000. From 1981 to May 2000, Mr. Murphy held various positions at Genentech, a biotechnology company, most recently as Director, Strategic Operations. During his 18 years at Genentech, Mr. Murphy guided seven new products through the manufacturing, approval and facility licensing processes. Mr. Murphy received a BS degree in Biochemistry from the State University of New York.
Gisela M. Schwab, M.D., joined us as our Vice President, Clinical Development in November 1999. From September 1992 to October 1999, Dr. Schwab held various positions at
62
Amgen Inc., a biotechnology company, most recently as Director, Clinical Research and Therapeutic Area Team Leader for Oncology/Hematology. Dr. Schwab received an M.D. degree from the University of Heidelberg in Germany. She is board certified in Hematology and Oncology and has performed research in molecular biology at the National Cancer Institute in Bethesda, Maryland, and at the French National Institute for Health and Research in Paris.
Susan L. Thorner, joined us as our Vice President, General Counsel and Secretary in February 2001. From August 1999 to February 2001, Ms. Thorner was Special Counsel at the law firm of Farella Braun & Martel. From August 1998 to August 1999, Ms. Thorner was Director of Legal Affairs at Ross Stores, Inc. and prior to this from August 1994 to August 1998 held various positions, most recently Director of Corporate Law, at Apple Computer, Inc. Ms. Thorner was previously a partner at two law firms, Morrison & Foerster in San Francisco and Hughes Hubbard & Reed in New York City. Ms. Thorner received her JD degree from Harvard Law School.
M. Kathleen Behrens, Ph.D., has served as one of our directors since December 1997. Dr. Behrens joined Robertson Stephens Investment Management Co. in 1983 and became a general partner in 1986 and a managing director in 1993. In 1988, Dr. Behrens joined the venture capital group of Robertson Stephens Investment Management Co. and has helped in the founding of the following three biotechnology companies: Mercator Genetics, Inc.; Protein Design Laboratories, Inc.; and COR Therapeutics, Inc. Dr. Behrens is currently president and a director of the National Venture Capital Association. Dr. Behrens received a Ph.D. degree in Microbiology from the University of California, Davis, where she performed genetic research for six years.
Raju S. Kucherlapati, Ph.D., has served as one of our directors since June 1996. Dr. Kucherlapati was a founder of Cell Genesys and served as a director of Cell Genesys from 1988 to 1999. Since July 1989, he has been the Saul and Lola Kramer Professor and the Chairman of the Department of Molecular Genetics at the Albert Einstein College of Medicine. Dr. Kucherlapati also serves as a director of Valentis Corp. and Millennium Pharmaceuticals, Inc. Dr. Kucherlapati received a BS degree in Biology from Andhra University in India and a Ph.D. degree in Genetics from the University of Illinois, Urbana.
Mark B. Logan, has served as one of our directors since August 1997. Mr. Logan has served as Chairman of the Board, President and Chief Executive Officer of VISX, Incorporated, a medical device company, since November 1994. From January 1992 to October 1994, he was Chairman of the Board and Chief Executive Officer of INSMED Pharmaceuticals, Inc., a pharmaceutical company. Previously, Mr. Logan held several senior management positions at Bausch & Lomb, Inc., a medical products company, including Senior Vice President, Healthcare and Consumer Group and also served as a member of its board of directors. Mr. Logan currently serves as a director of Somnus Medical Technologies, Inc. and Vivus, Inc. Mr. Logan received a BA degree from Hiram College and a PMD degree from Harvard Business School.
Joseph E. Maroun, has served as one of our directors since July 1996 and served as a director of Cell Genesys from June 1995 to June 2000. Mr. Maroun spent 30 years with Bristol-Myers Squibb, a pharmaceuticals company, serving until his retirement in 1990, at which time he was President of the International Group, Senior Vice President of the corporation, and a member of its Policy Committee. He also headed the U.S.-Japan Pharmaceutical Advisory Group. Mr. Maroun received a BA degree from the University of Witwaterrand, Johannesburg.
Stephen A. Sherwin, M.D., has served as one of our directors since June 1996, and Dr. Sherwin served as Chairman of the Board from June 1996 to May 2000. Since March 1990, Dr. Sherwin has served as President, Chief Executive Officer and a director of Cell Genesys. Since March 1994, he has served as Chairman of the Board of Cell Genesys. From 1983 to 1990, Dr. Sherwin held various positions at Genentech, Inc., a biotechnology company, most recently as Vice President, Clinical
63
Research. Dr. Sherwin currently serves as a Director of the California Healthcare Institute and Neurocrine Biosciences, Inc.. Dr. Sherwin received a BA degree in Biology from Yale University and an M.D. degree from Harvard Medical School.
Board Composition
Our amended and restated bylaws provide that the number of members of our board of directors shall be determined by the board of directors. The number of directors is currently set at seven. All members of our board of directors hold office until the next annual meeting of shareholders or until their successors are duly elected and qualified. There are no family relationships among any of our directors, officers or key employees.
Board Committees
Our compensation committee consists of Dr. Sherwin and Mr. Logan. The compensation committee makes recommendations regarding our various incentive compensation and benefit plans and determines salaries for our executive officers and incentive compensation for our employees and consultants.
Our audit committee consists of Dr. Sherwin, Mr. Logan and Dr. Behrens, who serves as Chairman of the committee. The audit committee makes recommendations to the board of directors regarding the selection of our independent auditors, reviews the results and scope of the audit and other services provided by our independent auditors and reviews and evaluates our control functions.
Compensation Committee Interlocks and Insider Participation
None of the members of our compensation committee was, at any time since our formation, an officer or employee of ours. None of our executive officers serves as a member of the board of directors or compensation committee of any entity that has one or more executive officers serving as a member of our board of directors or compensation committee.
Director Compensation
Each non-employee director of the Company receives a yearly retainer of $5,000 and a per meeting fee of $1,000 (plus $500 for each committee meeting attended by committee members or for special assignments of the Board of Directors). The members of the Board of Directors are also eligible for reimbursement for their expenses incurred in connection with attendance at Board meetings in accordance with Company policy.
Each non-employee director of the Company also receives stock option grants under the 1998 Director Option Plan, as amended effective April 26, 2001, or the Directors' Plan. Only non-employee directors of the Company are eligible to receive options under the Directors' Plan. Options granted under the Directors' Plan do not qualify as incentive stock options under the Internal Revenue Code. We have reserved 1,000,000 shares of our common stock for issuance under the Directors' Plan. As of June 30, 2001, options to purchase an aggregate of 337,500 shares had been granted under the Directors' Plan.
Under the Directors' Plan, each new non-employee director receives a stock option grant on the date such person first becomes a non-employee director, and each non-employee director receives an annual stock option grant. The size of the initial grant for each new director is determined by our board of directors at the time such director joins our board. The size of each annual grant to directors is determined on an annual basis by resolution of the Board of Directors. The annual grants are made on the date of the Company's Annual Meeting of Stockholders to each non-employee director who has served on the board for at least six months. The annual and initial grants do not require action by the
64
Company's stockholders. No other options may be granted at any time under the Directors' Plan. The exercise price of options granted under the Directors' Plan is 100% of the fair market value of our common stock on the date of grant.
All options granted on or after June 1999 are fully vested upon grant. The term of options granted under the Directors' Plan is ten years. In the event of a merger of the Company with or into another corporation or a consolidation, acquisition of assets or other change-in-control transaction involving the Company, each option either will continue in effect, if the Company is the surviving entity, or will be assumed or an equivalent option will be substituted by the successor corporation, if the Company is not the surviving entity. If the successor corporation does not assume an outstanding option or substitute it for an equivalent option, then the option shall become fully vested and exercisable. In addition, following such assumption or substitution, if the optionee's status as a director is terminated other than upon a voluntary resignation by the optionee, the option shall become fully vested and exercisable. The Directors' Plan will terminate in 2008, unless terminated earlier in accordance with its terms.
At the time of our 2000 annual meeting, Stephen A. Sherwin, M. Kathleen Behrens, Raju S. Kucherlapati, Mark B. Logan and Joseph E. Maroun each received options to purchase 30,000 shares of our common stock at an exercise price of $48.06. At the time of our 2001 annual meeting, Stephen A. Sherwin, M. Kathleen Behrens, Raju S. Kucherlapati, Mark B. Logan and Joseph E. Maroun each received options to purchase 7,500 shares of our common stock at an exercise price of $40.09.
Executive Compensation
The following table sets forth the compensation paid by us during the years ended December 31, 2000, 1999 and 1998 to our Chief Executive Officer and the four other most highly compensated executive officers at December 31, 2000, each of whose aggregate compensation during our last fiscal year exceeded $100,000. We refer to these individuals as the "named executive officers" elsewhere in this prospectus.
65
Summary Compensation Table
| |
| |
| |
| | Long-Term
Compensation Awards
| |
| |
|---|
| |
| | Annual Compensation
| |
| |
|---|
Name and Principal Position
| | Fiscal
Year
| | Securities
Underlying
Options (#)
| | All Other
Compensation($)
| |
|---|
| | Salary ($)
| | Bonus ($)
| |
|---|
| R. Scott Greer | | 2000 | | $ | 366,000 | | $ | 250,000 | | 405,000 | | $ | — | |
| | Chief Executive Officer | | 1999 | | | 283,147 | | | 200,000 | | 540,000 | | | — | |
| | | 1998 | | | 267,120 | | | — | | 160,000 | | | — | |
| Raymond M. Withy, Ph.D. | | 2000 | | | 241,500 | | | 90,563 | | 153,000 | | | — | |
| | President | | 1999 | | | 184,547 | | | 100,000 | | 153,000 | | | — | |
| | | 1998 | | | 165,350 | | | — | | 40,000 | | | — | |
| C. Geoffrey Davis, Ph.D. | | 2000 | | | 237,110 | | | 88,916 | | 153,000 | | | 1,011(1 | ) |
| | Chief Scientific Officer | | 1999 | | | 184,546 | | | 100,000 | | 153,000 | | | 1,482(1 | ) |
| | | 1998 | | | 165,350 | | | — | | 40,000 | | | — | |
| Kurt W. Leutzinger | | 2000 | | | 240,750 | | | 90,281 | | 153,000 | | | 5,630(2 | ) |
| | Chief Financial Officer | | 1999 | | | 187,922 | | | 100,000 | | 153,000 | | | 4,940(2 | ) |
| | | 1998 | | | 179,830 | | | — | | 51,000 | | | 19,623(3 | ) |
| Gisela M. Schwab, M.D.(4) | | 2000 | | | 220,000 | | | 144,788 | | — | | | 121,932(4 | ) |
| | Vice President, Clinical Development | | 1999 | | | 36,667 | | | 100,000 | | 400,000 | | | — | |
- (1)
- Consists of imputed interest income on a loan from us to Dr. Davis.
- (2)
- Consists of imputed interest income on a loan from us to Mr. Leutzinger.
- (3)
- Consists of $18,714 for reimbursement of relocation expenses and $909 for imputed interest income on a loan from us to Mr. Leutzinger.
- (4)
- Dr. Schwab has been our Vice President, Clinical Development since November 1999. Her 1999 annualized salary was $220,000. Other compensation consists of $117,465 for reimbursement of relocation expenses and $4,467 of imputed interest.
66
Option Grants in Last Fiscal Year
The following table provides information relating to stock options awarded to each of the named executive officers during the year ended December 31, 2000. All these options were awarded under our 1996 Incentive Stock Plan.
| | Individual Grants
| |
| |
|
|---|
| | Potential Realizable Value
at Assumed Annual Rates of
Stock Price Appreciation
For Options Term(4)
|
|---|
| | Number of
Securities
Underlying
Options
Granted(1)
| | Percent of
Total Options
Granted to
Employees in
Fiscal Year(2)
| |
| |
|
|---|
Name
| | Exercise or
Base Price
($/Share)(3)
| | Expiration
Date
|
|---|
| | 5%
| | 10%
|
|---|
| R. Scott Greer | | 405,000 | | 7.3 | % | $ | 31.8125 | | 2/02/10 | | $ | 8,102,710 | | $ | 20,533,877 |
| Raymond M. Withy, Ph.D. | | 153,000 | | 2.7 | % | | 31.8125 | | 2/02/10 | | | 3,061,027 | | | 7,757,243 |
| C. Geoffrey Davis, Ph.D. | | 153,000 | | 2.7 | % | | 31.8125 | | 2/02/10 | | | 3,061,027 | | | 7,757,243 |
| Kurt W. Leutzinger | | 153,000 | | 2.7 | % | | 31.8125 | | 2/02/10 | | | 3,061,027 | | | 7,757,243 |
| Gisela M. Schwab, M.D. | | — | | — | | | — | | — | | | — | | | — |
- (1)
- All of the options granted became exercisable as to 1/48th of the option shares on the date of grant and an additional 1/48th of the option shares become exercisable on the first day of each calendar month thereafter, with full vesting occurring four years after the date of grant. In each case, vesting is subject to the optionee's continued relationship with us. These options expire ten years from the date of grant, or earlier upon termination of employment.
- (2)
- Based on an aggregate of 5,585,930 options granted by us in the year ended December 31, 2000 to our employees, non-employee directors of and consultants, including the named executive officers.
- (3)
- Options were granted at an exercise price equal to the fair market value of our common stock, as determined by our board of directors on the date of grant.
- (4)
- The 5% and 10% assumed annual rates of compounded stock price appreciation are mandated by rules of the Securities and Exchange Commission. We cannot provide any assurance to any executive officer or any other holder of our securities that the actual stock price appreciation over the option term will be at the assumed 5% and 10% levels or at any other defined level. Unless the market price of our common stock appreciates over the option term, no value will be realized from the option grants made to the executive officers. The potential realizable value is calculated by assuming that the fair value of our common stock on the date of grant appreciates at the indicated rate for the entire term of the option and that the option is exercised at the exercise price and sold on the last day of its term at the appreciated price. The potential realizable value computation is net of the applicable exercise price, but does not take into account applicable federal or state income tax consequences and other expenses of option exercises or sales of appreciated stock.
67
Option Exercises and Holdings
The following table sets forth for each of the named executive officers the number of shares of our common stock acquired and the dollar value realized upon exercise of options during the year ended December 31, 2000 and the number and value of securities underlying unexercised options held at December 31, 2000:
Aggregate Option Exercises in Last Fiscal Year and Fiscal Year-End Option Values
| |
| |
| | Number of Securities
Underlying Unexercised
Options at Fiscal Year-End
| | Value of Unexercised
In-the-Money Options
at Fiscal Year-End(2)
|
|---|
Name
| | # Shares
Acquired on
Exercise
| | Value
Realized(1)
|
|---|
| | Exercisable
| | Unexercisable
| | Exercisable
| | Unexercisable
|
|---|
| R. Scott Greer | | 575,000 | | $ | 27,377,920 | | 621,693 | | 661,557 | | $ | 33,351,309 | | $ | 28,010,568 |
| Raymond M. Withy, Ph.D. | | 364,918 | | | 15,029,485 | | 123,160 | | 218,258 | | | 5,870,995 | | | 8,818,411 |
| C. Geoffrey Davis, Ph.D. | | 275,000 | | | 12,645,250 | | 122,742 | | 218,258 | | | 6,021,889 | | | 8,818,411 |
| Kurt W. Leutzinger | | 50,000 | | | 2,976,250 | | 158,284 | | 268,716 | | | 7,956,165 | | | 11,764,644 |
| Gisela M. Schwab, M.D. | | 15,000 | | | 801,250 | | 93,333 | | 291,667 | | | 4,602,484 | | | 14,382,829 |
- (1)
- Value realized reflects the aggregate fair market value of our common stock underlying the option on the date of exercise minus the aggregate exercise price of the option.
- (2)
- Value of unexercised in-the-money options are based on a value of $59.0625 per share, the closing price of our common stock on December 31, 2000. Amounts reflected are based on the value of $59.0625 per share, minus the per share exercise price, multiplied by the number of shares underlying the option.
Stock Plans
1996 Incentive Stock Plan. As of June 30, 2001, a total of 12,765,000 shares of common stock have been authorized for issuance under our 1996 Incentive Stock Plan, or the Incentive Plan. Under the Incentive Plan, as of June 30, 2001, options to purchase an aggregate of 5,092,734 shares were outstanding, 6,773,078 shares of common stock had been purchased pursuant to exercises of stock options and stock purchase rights and 899,188 shares were available for future grant.
The Incentive Plan provides for the grant of incentive stock options within the meaning of Section 422 of the Internal Revenue Code of 1986, nonqualified stock options and stock purchase rights to our employees, consultants and nonemployee directors. Incentive stock options may be granted only to employees. The Incentive Plan is administered by the board of directors or a committee appointed by the board of directors, which determines the terms of awards granted, including the exercise price, the number of shares subject to the award and the exercisability. The exercise price of incentive stock options granted under the Incentive Plan must be at least equal to the fair market value of our common stock on the date of grant. However, for any employee holding more than 10% of the voting power of all classes of our stock, the exercise price will be no less than 110% of the fair market value. The exercise price of nonqualified stock options is set by the administrator of the Incentive Plan. However, for any person holding more than 10% of the voting power of all classes of our stock, the exercise price will be no less than 110% of the fair market value. The maximum term of options granted under the Incentive Plan is ten years.
An optionee whose relationship with us or any related corporation ceases for any reason, other than death or total and permanent disability, may exercise options in the three-month period following such cessation, or such other period of time as determined by the administrator, unless the options terminate or expire sooner by their terms. The three-month period is extended to twelve months for terminations due to death or total and permanent disability. In the event of a merger of us with or into another corporation, any outstanding options may either by assumed or an equivalent option may be
68
substituted by the surviving entity or, if the options are not assumed or substituted, such options shall become exercisable as to all of the shares subject to the options, including shares as to which they would not otherwise be exercisable. In the event that options become exercisable in lieu of assumption or substitution, the board of directors shall notify optionees that all options shall be fully exercisable for a period of 30 days, after which the options shall terminate.
None of our employees may be granted, in any fiscal year, options to purchase more than 3,000,000 shares, 6,000,000 shares in the case of a new employee's initial employment with us. The Incentive Plan will terminate in June 2006, unless sooner terminated by the board of directors.
The board of directors may also grant stock purchase rights to employees and consultants under the Incentive Plan. These grants are made pursuant to a restricted stock purchase agreement, and the price to be paid for the shares granted thereunder is determined by the administrator. We are generally granted a repurchase option exercisable on the voluntary or involuntary termination of the purchaser's employment with us for any reason, including death or disability. The repurchase price shall be the original purchase price paid by the purchaser. The repurchase option shall lapse at a rate determined by the administrator. Once the stock purchase right has been exercised, the purchaser shall have the rights equivalent to those of a shareholder.
1998 Employee Stock Purchase Plan. We have adopted the 1998 Employee Stock Purchase Plan, or the Purchase Plan, and have reserved a total of 1,000,000 shares of common stock for issuance under the Purchase Plan. The Purchase Plan also provides for an annual increase, commencing in 1999, in the number of shares reserved for issuance under the Purchase Plan equal to the lesser of 1,000,000, 1% of our outstanding capitalization or a lesser amount determined by the board, such that the maximum number of shares which could be reserved under the Purchase Plan over its term would be 10,000,000 shares. Under the Purchase Plan, as of June 30, 2001, 1,596,092 shares had been authorized, 500,146 shares had been issued and 1,095,946 shares were available for future grant. The Purchase Plan, which is intended to qualify under Section 423 of the Code, is administered by our board of directors or by a committee appointed by the board of directors.
Under the Purchase Plan, we withhold a specified percentage, not to exceed 15%, of each salary payment to participating employees over the offering periods. Any employee who is currently employed for at least 20 hours per week and for at least five consecutive months in a calendar year, either by us or by one of our majority-owned subsidiaries, is eligible to participate in the Purchase Plan. Unless the board of directors or the committee determines otherwise, each offering period will run for 24 months and will be divided into consecutive purchase periods of approximately six months. The first offering period and the first purchase period commenced on July 2, 1998. New 24-month offering periods commence every six months on each November 1 and May 1. In the event of a change in our control, including a merger with or into another corporation, or the sale of all or substantially all of our assets, the offering and purchase periods then in progress will be shortened.
The price of common stock purchased under the Purchase Plan is equal to 85% of the fair market value of the common stock on the first day of the applicable offering period or the last day of the applicable purchase period, whichever is lower. Employees may end their participation in the offering at any time during the offering period, and participation ends automatically on termination of employment with us. The maximum number of shares that a participant may purchase on the last day of any offering period is determined by dividing the payroll deductions accumulated during the purchase period by the purchase price. However, no person may purchase shares under the Purchase Plan to the extent such person would own 5% or more of the total combined value or voting power of all classes of our capital stock or of any of our subsidiaries, or to the extent that such person's rights to purchase stock under all employee stock purchase plans would exceed $25,000 for any calendar year. The board of directors may amend the Purchase Plan at any time. The Purchase Plan will terminate in March 2008, unless terminated earlier in accordance with the provisions of the Purchase Plan.
69
1998 Director Option Plan. We have granted shares of our common stock to our non-employee directors pursuant to our Directors' Plan, as amended, as described above under "Director Compensation".
1999 Nonstatutory Stock Option Plan. We have adopted the 1999 Nonstatutory Stock Option Plan, or Nonstatutory Plan, and authorized a total of 8,600,000 shares of common stock for issuance to employees and consultants under the Nonstatutory Plan. As of June 30, 2001, options to purchase an aggregate of 7,075,715 shares were outstanding and 1,207,800 shares were available for future grants under the Nonstatutory Plan. The Nonstatutory Plan provides for the grant of stock options at no less than the public market closing price of the underlying common stock on the date of grant. Options granted under the Nonstatutory Plan generally have a term of ten years and vest over four years at the rate of 25% one year from the date of hire and 1/48th per month after that.
An optionee whose relationship with us or any related corporation ceases for any reason, other than death or total and permanent disability, may exercise options within the time period specified by the terms of the option, to the extent it is vested on the date of the cessation, or, in the absence of a specified time, in the three-month period following such cessation. The three-month period is extended to twelve months for terminations due to death or total and permanent disability. In the event we or into another corporation, any outstanding options may either by assumed or an equivalent option may be substituted by the surviving entity or, if the options are not assumed or substituted, the options shall become exercisable as to all of the shares subject to the options, including shares as to which they would not otherwise be exercisable. In the event that options become exercisable in lieu of assumption or substitution, the board of directors shall notify optionees that all options shall be fully exercisable for a period of 15 days, after which the options shall terminate.
401(k) Plan
All of our employees who are located in the United States and who work a minimum of 30 hours per week are eligible to participate in our 401(k) Retirement Plan. Pursuant to the 401(k) Plan, employees may elect to reduce their current compensation by up to the lesser of 15% of their annual compensation or the statutorily prescribed annual limit allowable under Internal Revenue Service Regulations and to have the amount of this reduction contributed to the 401(k) Plan. The 401(k) Plan permits us, but does not require us, to make additional matching contributions on behalf of all participants in the 401(k) Plan. We have not made any matching contributions to the 401(k) Plan. The 401(k) Plan is intended to qualify under Section 401(k) of the Code so that contributions to the 401(k) Plan by employees or by us, and the investment earnings on these contributions, are not taxable to employees until withdrawn from the 401(k) Plan, and that our contributions, if any, will be deductible by us when made.
Change of Control Arrangements
We have entered into change of control severance agreements with Messrs. Greer, Davis, Leutzinger and Withy, and Ms. Schwab. These agreements provide in pertinent part that if any of the following events occur within 24 months following a change of control, then the Company, or the company with which we merge, must pay the affected officer such officer's salary and bonus, at the rate in effect just prior to the change of control, for one year or, in Mr. Greer's case, two years: (i) a termination of the officer's employment without good cause; (ii) a material reduction in the officer's salary or benefits or a substantial reduction of the officer's perquisites, such as office space, without such officer's consent or good business reason; (iii) a significant reduction in the officer's duties, position or responsibilities without such officer's consent; or (iv) a relocation of the officer's employment by more than 35 miles without such officer's consent.
70
These agreements further provide for "gross up" payments to the officers in the event that they are subject to the tax code's excise tax on so-called "excess parachute payments." For purposes of these agreements, a change in control includes (1) a person becoming the beneficial owner of more than 50% of the total voting power represented by the Company's securities; (2) a merger or consolidation in which Abgenix stockholders immediately before the transaction own less than 50% of the total voting power represented by our securities; (3) liquidation or sale of all or substantially all of the Company's assets; or (4) certain changes in the composition of the Board such that the incumbent directors before the change are less than a majority of the Board after the change.
Our board of directors has approved a plan which provides that in the event of a change in control of Abgenix, the options of each Abgenix employee whose employment is terminated without cause within 24 months of the change in control will become exercisable in full. For these purposes, a change in control includes: (1) a person becoming the beneficial owner of 50% or more of our outstanding voting securities; (2) certain changes in the composition of our board of directors occurring within a two-year period; or (3) a merger or consolidation in which Abgenix stockholders immediately before the transaction own less than a majority of the outstanding voting securities of the surviving entity, or its parent, immediately after the transaction.
Report of the Compensation Committee of The Board of Directors on Executive Compensation
Our compensation committee is responsible for making recommendations to our board of directors concerning salaries and incentive compensation for employees of and consultants to Abgenix. The compensation committee also has the authority and power to grant stock options to our employees and consultants.
The goal of our compensation policies is to align executive compensation with business objectives, corporate performance, and to attract and retain executives who contribute to the long-term success and value of Abgenix. We endeavor to achieve our compensation goals through the implementation of policies that are based on the following principles:
- •
- THE COMPANY PAYS COMPETITIVELY FOR EXPERIENCED, HIGHLY SKILLED EXECUTIVES:
The Company operates in a competitive and rapidly changing biopharmaceutical industry. Executive base compensation is targeted to the median salary paid to comparable executives in companies of similar size and location, and with comparable responsibilities. The individual executive's salary is adjusted annually based on individual performance, corporate performance, and the relative compensation of the individual compared to the comparable medians.
- •
- THE COMPANY REWARDS EXECUTIVES FOR SUPERIOR PERFORMANCE:
The Compensation Committee believes that a substantial portion of each executive's compensation should be in the form of bonuses. Executive bonuses are based on a combination of individual performance and the attainment of corporate goals. Individual performance goals are based on specific objectives which must be met in order for the Company to achieve its corporate goals. In order to attract and retain executives who are qualified to excel in the biopharmaceutical industry, the Company awards higher bonuses based on performance in excess of the corporate goals.
- •
- THE COMPANY STRIVES TO ALIGN LONG-TERM STOCKHOLDER AND EXECUTIVE INTERESTS:
In order to align the long-term interests of executives with those of stockholders, the Company grants all employees, and particularly executives, options to purchase stock. Options are granted at the closing price of one share of the Company's common stock on the date of grant and will provide value only when the price of the common stock increases above the exercise price. Options are subject to vesting provisions designed to encourage executives to remain employed
71
Compensation of R. Scott Greer, Chief Executive Officer and Chairman of the Board
Mr. Greer's salary and stock option grant for fiscal 2000 are consistent with the criteria described above and with the Compensation Committee's evaluation of his overall leadership and management of Abgenix. 2000 was a year of significant accomplishments for us. We made significant progress in advancing our product pipeline, expanding our list of XenoMouse™ technology collaborations, entering into product development partnerships, acquiring new, complementary technologies through acquisitions, and building our financial strength. A Phase II trial of one product candidate was completed, and Phase II trial of another was initiated. Three product candidates are presently undergoing clinical trials, in indications including psoriasis, graft-versus-host disease, cancer and rheumatoid arthritis. In addition, we completed two equity offerings in 2000, raising approximately $717.1 million. Mr. Greer has continued to provide strategic direction to us and to build our organization. Mr. Greer's compensation for 2000 is set forth in the Summary Compensation Table appearing on page 66.
Compliance with Internal Revenue Code Section 162(m)
Section 162(m) of the Internal Revenue Code provides that compensation paid to a public company's chief executive officer and its four other highest paid executive officers in tax years 1994 and thereafter in excess of $1 million is not deductible unless such compensation is paid only upon the achievement of objective performance goals where certain procedural requirements have been satisfied. Alternatively, such compensation may be deferred until the executive officer is no longer a covered person under Section 162(m). Based on fiscal year 2000 compensation levels, no such limits on the deductibility of compensation applied to any officer of Abgenix.
Summary
The Compensation Committee believes that our compensation policy as practiced to date by the Compensation Committee and the Board has been successful in attracting and retaining qualified employees and in tying compensation directly to corporate performance relative to corporate goals. Our compensation policy will evolve over time as we attempt to achieve the many short-term goals we face while maintaining our focus on building long-term stockholder value through technological leadership and development and expansion of the market for our products.
| |
|
|---|
| | | Respectfully submitted, |
|
|
/s/ Stephen A. Sherwin, M.D.
/s/ Mark B. Logan |
72
COMPARISON OF CUMULATIVE TOTAL RETURN ON INVESTMENT*
The stock price performance depicted in the following graph is not necessarily indicative of future price performance.
The following graph shows a comparison of total stockholder return for holders of our common stock from July 2, 1998, the date our common stock first traded on the Nasdaq National Market, through December 31, 2000 compared with the Nasdaq Composite Index and the Pharmaceutical Index. This graph is presented pursuant to the Securities and Exchange Commission rules. We believe that while total stockholder return can be an important indicator of corporate performance, the prices of biopharmaceutical stocks like that of Abgenix are subject to a number of market-related factors other than company performance, such as competitive announcements, drug discovery and commercialization, mergers and acquisitions in the industry, the general state of the economy, and the performance of other biopharmaceutical stocks.
- *
- $100 invested on 7/2/98 in stock or on 6/30/98 in the indices—including reinvestment of dividends. Fiscal year ending December 31.
Limitations of Liability and Indemnification Matters
Our amended and restated certificate of incorporation limits the liability of directors to the maximum extent permitted by Delaware law. Delaware law provides that directors of a corporation will not be personally liable for monetary damages for breach of their fiduciary duties as directors, except liability for (1) any breach of their duty of loyalty to the corporation or its shareholders, (2) acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (3) unlawful payments of dividends or unlawful stock repurchases or redemptions or (4) any transaction from which the director derived an improper personal benefit. This limitation of liability does not apply to liabilities arising under the federal securities laws and does not affect the availability of equitable remedies such as injunctive relief or rescission.
Our amended and restated bylaws provide that we shall indemnify our directors and executive officers and may indemnify our other officers and employees and other agents to the fullest extent permitted by law. We believe that indemnification under our amended and restated bylaws covers at least negligence and gross negligence on the part of indemnified parties. Our amended and restated
73
bylaws also permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in such capacity, regardless of whether the amended and restated bylaws would permit indemnification.
We have entered into agreements to indemnify our directors and executive officers, in addition to indemnification provided for in our amended and restated bylaws. These agreements, among other things, indemnify our directors and executive officers for certain expenses, including attorneys' fees, judgments, fines and settlement amounts incurred by any such person in any action or proceeding, including any action by us arising out of such person's services as our director or executive officer, any of our subsidiaries or any other company or enterprise to which the person provides services at our request. We believe that these provisions and agreements are necessary to attract and retain qualified persons as directors and executive officers.
74
CERTAIN TRANSACTIONS
Relationship with Cell Genesys
As of July 31, 2001, Cell Genesys beneficially owned approximately 10.39% of our outstanding capital stock. As a result, Cell Genesys has significant influence over all matters requiring the approval of our shareholders.
One of our directors, Stephen A. Sherwin, M.D., is also the Chairman of the Board and Chief Executive Officer of Cell Genesys.
Transactions with Employees
On February 27, 1998, Kurt Leutzinger, our Chief Financial Officer, entered into a relocation loan agreement with us pursuant to which we loaned $100,000 to Mr. Leutzinger in exchange for a promissory note secured by a deed of trust. No interest accrues on the loan until June 30, 2003. As of December 31, 2000, the outstanding principal balance of the promissory note was $100,000.
On May 5, 2000, Gisela Schwab entered into a relocation loan agreement with us pursuant to which we loaned $100,000 to Ms. Schwab in exchange for a promissory note secured by a deed of trust. No interest accrues on the loan until May 5, 2005. As of December 31, 2000, the outstanding principal balance of the promissory note was $100,000.
On October 11, 2000, Gayle Mills entered into a loan agreement with us pursuant to which we loaned $100,000 to Ms. Mills in exchange for a promissory note secured by her options to buy our stock. No interest accrues on the loan until October 11, 2005.
On October 18, 2000, Gregory Landes entered into a relocation loan agreement with us pursuant to which we loaned $100,000 to Dr. Landes in exchange for a promissory note secured by a deed of trust. No interest accrues on the loan until October 18, 2005.
We have entered into indemnification agreements with each of our directors and executive officers which provide, among other things, that we will indemnify such officer or director, under the circumstances and to the extent provided for therein, for expenses, damages, judgments, fines and settlements he or she may be required to pay in actions or proceedings which he or she is or may be made a party by reason of his or her position as a director, officer or other agent of Abgenix, and otherwise to the full extent permitted under Delaware law and our Bylaws.
All future transactions, including any loans from us to our officers, directors, principal shareholders or affiliates, will be approved by a majority of the board of directors, including a majority of the independent and disinterested members of the board of directors or, if required by law, a majority of disinterested shareholders, and will be on terms no less favorable to us than could be obtained from unaffiliated third parties.
75
DESCRIPTION OF CAPITAL STOCK
General
Our amended and restated certificate of incorporation, as amended, authorizes the issuance of up to 220,000,000 shares of common stock, $0.0001 par value per share and authorizes the issuance of 5,000,000 shares of preferred stock, $0.0001 par value per share, the rights and preferences of which may be established from time to time by our board of directors. As of July 31, 2001, 86,156,183 shares of common stock were issued and outstanding and held by approximately 229 stockholders of record and no shares of preferred stock were issued and outstanding.
Common Stock
Each holder of common stock is entitled to one vote for each share held on all matters to be voted upon by the stockholders and there are no cumulative voting rights. Subject to preferences that may be applicable to any outstanding preferred stock, holders of common stock are entitled to receive ratably such dividends as may be declared by the board of directors out of funds legally available therefor. In the event of our liquidation, dissolution or winding up, holders of common stock would be entitled to share in our assets remaining after the payment of liabilities and the satisfaction of any liquidation preference granted to the holders of any outstanding shares of preferred stock. Holders of common stock have no preemptive or conversion rights or other subscription rights. There are no redemption or sinking fund provisions applicable to the common stock. All outstanding shares of common stock are fully paid and nonassessable. The rights, preferences and privileges of the holders of common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of preferred stock which we may designate in the future.
Preferred Stock
Our board of directors is authorized, without any further action by the stockholders, subject to any limitations prescribed by law, from time to time to issue up to an aggregate of 5,000,000 shares of preferred stock, $0.0001 par value per share, in one or more series, each of such series to have such rights and preferences, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences, as shall be determined by our board of directors. Issuance of preferred stock, while providing desirable flexibility in connection with possible acquisitions and other corporate purposes, could have the effect of making it more difficult for a third party to acquire, or of discouraging a third party from attempting to acquire, a majority of our outstanding voting stock.
As of June 30, 2001, there was one series of preferred stock, Series A Participating Preferred Stock. The Series A Participating Preferred Stock has a par value of $0.0001 per share, and the number of shares constituting such series is 50,000, of which none is issued and outstanding. The Series A Participating Preferred Stock is entitled to quarterly dividends payable in cash in an amount per share equal to 1,000 times the aggregate per share amount of all dividends declared on our common stock. Each share of Series A Participating Preferred Stock is entitled to 1,000 votes on all matters submitted to a vote of our stockholders. In the event of our liquidation, dissolution or winding up, the holders of shares of Series A Participating Preferred Stock are entitled to receive an aggregate amount per share equal to 1000 times the aggregate amount to be distributed per share to holders of our common stock plus an amount equal to any accrued and unpaid dividends on such shares of Series A Participating Preferred Stock. The shares of Series A Participating Preferred Stock are not redeemable. The Series A Participating Preferred Stock ranks junior to all other series of our Preferred Stock as to the payment of dividends and the distribution of assets, unless the terms of any such other series provide otherwise.
76
Other Obligations to Issue Capital Stock
We are obligated to issue up to 100,000 shares of our common stock upon the occurrence of certain milestones pursuant to the terms of a license agreement.
Registration Rights of Certain Holders
The holders of certain shares of our common stock, the registrable securities, or their transferees, are entitled to certain rights with respect to the registration of such shares under the Securities Act. These rights are provided under the terms of an agreement, the amended and restated stockholder rights agreement, between us and the holders of the registrable securities. The holders of at least 50% of the registrable securities may require, subject to certain limitations in the amended and restated stockholder rights agreement, on two occasions, that we use our best efforts to register the registrable securities for public resale. If we register any of our common stock either for our own account or for the account of other security holders with certain exceptions, the holders of registrable securities are entitled to include their shares of common stock in the registration. A holder's right to include shares in an underwritten registration statement is subject to the right of the underwriters to limit the number of shares included in the offering, subject to certain limitations. The holders of registrable securities may also require us, on no more than two occasions during any 12-month period, to register all or a portion of their registrable securities on Form S-3, provided, among other limitations, that the proposed aggregate selling price, net of underwriting discounts and commissions, is at least $500,000. We will bear all registration expenses (subject to certain limitations) and all selling expenses relating to registrable securities must be borne by the holders of the securities being requested. If such holders, by exercising their demand registration rights, cause a large number of securities to be registered and sold in the public market, the market price for our common stock could be adversely affected. If we were to initiate a registration and include registrable securities pursuant to the exercise of piggyback registration rights, the sale of such registrable securities may have an adverse effect on our ability to raise capital.
In November 1999, we entered into a common stock purchase agreement with certain individuals and entities pursuant to which we sold 7,112,000 shares of our common stock. Pursuant to that sale, we agreed to register the shares under the Securities Act for resale to the public. Under the registration agreement, we must use reasonable efforts to keep that registration statement, or a replacement, continuously effective under the Securities Act until the earlier of (1) November 19, 2001 or (2) such time as the selling shareholders have sold all shares offered under that registration statement.
In November 2000, we consummated a private placement of our common stock, selling 3,300,000 shares of our common stock to certain institutional investors. The filing of the registration statement of which this prospectus forms a part relates to our undertaking to register the shares sold in the November 2000 private placement. We have also undertaken to use our best efforts to keep that registration statement, or a replacement, continuously effective under the Securities Act until the earliest of (1) two years after the closing of the private placement; (2) the date on which a selling shareholder may sell all shares that were bought in the private placement then held by such selling shareholder without restriction by the volume limitations of Rule 144(e) under the Securities Act or (3) such time as all shares purchased by such selling shareholder in the private placement have been sold pursuant to a registration statement.
Stockholder Rights Plan and Certain Charter and Bylaw Provisions and Delaware Law
In June 1999, our board of directors adopted a stockholder rights plan, which was amended in November 1999. Pursuant to the stockholder rights plan, we made a dividend distribution of one preferred share purchase right on each outstanding share of our common stock. Preferred share purchase rights will also accompany all future common stock issuances during the life of the plan,
77
including the shares issued in this offering. The purchase rights will trade together with the common shares until they become exercisable. Each right entitles stockholders to buy 1/1000th of a share of our Series A participating preferred stock at an exercise price of $120.00. Each right will become exercisable following the tenth day after a person or group, other than Cell Genesys or its affiliates, successors or assigns, announces an acquisition of 15% or more of our common stock, or announces commencement of a tender offer, the consummation of which would result in ownership by the person or group of 15% or more of our common stock. In the case of Cell Genesys or its affiliates, successors or assigns, which beneficially owned approximately 10.39% of our outstanding common stock as of July 31, 2001, each right will become exercisable following the tenth day after it announces the acquisition of more than 25% of our common stock, or announces commencement of a tender offer, the consummation of which would result in ownership by Cell Genesys or its affiliates, successors or assigns of more than 25% of our common stock. We will be entitled to redeem the rights at $0.01 per right at any time on or before the close of business on the tenth day following acquisition by a person or group of 15% or more (or in the case of Cell Genesys or its affiliates, successors or assigns, more than 25%) of our common stock.
The stockholder rights plan and some provisions of our amended and restated certificate of incorporation and amended and restated bylaws may have the effect of making it more difficult for a third party to acquire, or of discouraging a third party from attempting to acquire control of us. This could limit the price that certain investors might be willing to pay in the future for our shares of common stock. Certain provisions of our amended and restated certificate of incorporation and amended and restated bylaws allow us to:
- •
- issue preferred stock without any vote or further action by the stockholders;
- •
- eliminate the right of stockholders to act by written consent without a meeting;
- •
- specify procedures for director nominations by stockholders and submission of other proposals for consideration at stockholder meetings; and
- •
- eliminate cumulative voting in the election of directors.
We are subject to certain provisions of Delaware law which could also delay or make more difficult a merger, tender offer or proxy contest involving us. In particular, Section 203 of the Delaware General Corporation Law prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years unless certain conditions are met. The stockholder rights plan, the possible issuance of preferred stock, the procedures required for director nominations and stockholder proposals and Delaware law could have the effect of delaying, deferring or preventing a change in our control, including without limitation, discouraging a proxy contest or making more difficult the acquisition of a substantial block of our common stock. The provisions could also limit the price that investors might be willing to pay in the future for shares of our common stock.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is ChaseMellon Shareholder Services L.L.C.
78
SHARES ELIGIBLE FOR FUTURE SALE
As of July 31, 2001, we had outstanding 86,156,183 shares of common stock, including the shares covered by this prospectus. On that date, we also had outstanding employee and director stock options to purchase 12,505,949 common shares and other obligations to issue up to 100,000 common shares. All of our currently outstanding shares and, we expect that upon issuance, all of these other shares, may be immediately resold by their holders, either pursuant to Rule 144 under the Securities Act or pursuant to registration statements we have filed under the Securities Act.
PRINCIPAL SHAREHOLDERS
The following table sets forth certain information with respect to the beneficial ownership of our common stock as of July 31, 2001 by (1) each person, or group of affiliated persons, who is known by us to own beneficially more than 5% of our common stock, (2) each of our directors, (3) each of our named executive officers and (4) all of our directors and executive officers as a group.
| | Beneficial Ownership
| |
|---|
| | Number of
Shares(2)
| | Percent of
Total(3)
| |
|---|
| Beneficial Owner(1) | | | | | |
Cell Genesys(4)
342 Lakeside Drive
Foster City, CA 94404 | | 8,954,136 | | 10.39 | % |
FMR Corp.(5)
82 Devonshire Street
Boston, MA 02109 |
|
8,821,044 |
|
10.24 |
|
R. Scott Greer(6) |
|
906,935 |
|
1.04 |
|
M. Kathleen Behrens, Ph.D.(7) |
|
319,217 |
|
* |
|
Raju S. Kucherlapati, Ph.D.(8) |
|
339,000 |
|
* |
|
Mark B. Logan(9) |
|
139,500 |
|
* |
|
Joseph E. Maroun(10) |
|
843,384 |
|
* |
|
Stephen A. Sherwin, M.D.(11) |
|
362,125 |
|
* |
|
Raymond M. Withy, Ph.D.(12) |
|
269,675 |
|
* |
|
C. Geoffrey Davis, Ph.D.(13) |
|
256,576 |
|
* |
|
Kurt W. Leutzinger(14) |
|
324,813 |
|
* |
|
Gisela M. Schwab(15) |
|
174,623 |
|
* |
|
All directors and executive officers as a group (16 persons)(16) |
|
4,362,417 |
|
4.89 |
% |
- *
- Represents beneficial ownership of less than one percent of the Common Stock.
- (1)
- Unless otherwise indicated in these footnotes, the mailing address for each individual is c/o Abgenix, Inc., 6701 Kaiser Drive, Fremont, California 94555.
- (2)
- This table is based upon information supplied by officers, directors and principal stockholders and Schedule 13Gs filed with the Securities and Exchange Commission. Unless otherwise indicated in the footnotes to this table and subject to community property laws where applicable, each of the stockholders named in this table has sole voting and investment power with respect to the shares indicated as beneficially owned.
79
- (3)
- Applicable percentages are based on 86,156,183 shares outstanding on July 31, 2001.
- (4)
- Based on a Schedule 13G dated February 13, 2001.
- (5)
- In a filing on Schedule 13G, dated February 13, 2001, Fidelity Management & Research Company ("Fidelity"), a wholly-owned subsidiary of FMR Corp. and a registered investment adviser, reported that it is the beneficial owner of 8,577,562 shares as a result of acting as investment adviser to various registered investment companies (each a "Fund" or the "Funds"). According to the disclosure contained in Fidelity's Schedule 13G, (a) each of Edward C. Johnson 3d (Chairman of FMR Corp.), FMR Corp. (through its control of Fidelity) and each of the Funds, has sole power to dispose of the 8,577,562 shares owned by the Funds, (b) neither FMR Corp. nor Edward C. Johnson 3d has the sole power to vote or direct the voting of the shares owned directly by the Funds, which power resides with the Funds' Boards of Trustees, (c) Fidelity Management Trust Company, a wholly-owned subsidiary of FMR Corp. and a bank, is the beneficial owner of 213,100 shares as a result of its service as investment manager to certain institutional accounts, (d) Edward C. Johnson 3d and FMR Corp., through its control of Fidelity Management Trust Company, each has sole
dispositive power over 213,100 shares and sole power to vote or to direct the voting of 182,630 shares owned by such institutional accounts, and (e) Fidelity International Limited is the beneficial owner of 30,382 shares.
- (6)
- Includes 690,617 shares issuable upon exercise of options exercisable within 60 days of July 31, 2001.
- (7)
- Includes 180,000 shares issuable upon exercise of options exercisable within 60 days of July 31, 2001.
- (8)
- Includes 309,000 shares issuable upon exercise of options exercisable within 60 days of July 31, 2001.
- (9)
- Includes 139,500 shares issuable upon exercise of options exercisable within 60 days of July 31, 2001.
- (10)
- Includes 5,602 shares owned by Mr. Maroun's wife and 603,782 shares owned by the Maroun Family Limited Partnership. Mr. Maroun disclaims beneficial ownership of such shares. Also includes 234,000 shares issuable upon exercise of options exercisable within 60 days of July 31, 2001.
- (11)
- Includes 362,125 shares issuable upon exercisable of options within 60 days of July 31, 2001. Does not include 8,954,136 shares beneficially owned by Cell Genesys, Inc. (the "CG Shares"). Dr. Sherwin is an officer, director and beneficial stockholder of Cell Genesys, Inc. As such, he may be deemed to have voting and dispositive power over the CG Shares. However, Dr. Sherwin disclaims beneficial ownership of the CG Shares for purposes of Section 13 of the Exchange Act and this Post-Effective Amendment No. 1 to Form S-1.
- (12)
- Includes 215,327 shares issuable upon exercise of options exercisable within 60 days of July 31, 2001.
- (13)
- Includes 206,576 shares issuable upon exercise of options exercisable within 60 days of July 31, 2001.
- (14)
- Includes 183,727 shares issuable upon exercise of options exercisable within 60 days of July 31, 2001.
- (15)
- Includes 167,083 shares issuable upon exercise of options exercisable within 60 days of July 31, 2001.
- (16)
- Includes 3,111,699 shares issuable upon exercise of options exercisable within 60 days of July 31, 2001.
80
SELLING SHAREHOLDERS
All 1,418,705 shares of our common stock covered by this prospectus are part of 4,050,000 shares of our common stock that were sold to certain selling shareholders (or their assignees) in a private placement completed on November 6, 2000 pursuant to an exemption from registration contained in Regulation D promulgated under Section 4(2) of the Securities Act. Of the 4,050,000 shares sold, 3,300,000 were newly issued and sold by us and 750,000 were sold by Cell Genesys.
The following table sets forth certain information with respect to the beneficial ownership of shares of our common stock by the selling shareholders as of August 27, 2001 and the number of shares which may be offered pursuant to this prospectus for the account of each of the selling shareholders (or their transferees) from time to time. Except as described in the footnotes to the table, to the best of our knowledge, none of the selling shareholders has had any position, office or other material relationship with us or any of our affiliates.
Selling Shareholder
| | Number of
Shares
Beneficially
Owned Prior to
Offering
| | Maximum
Number of
Shares Which
May Be Sold in
This Offering
| | Number of
Shares
Beneficially
Owned After
the Offering(1)
| | Percentage of
Shares
Beneficially
Owned After the
Offering(1)
| |
|---|
| T. Rowe Price Health Sciences Fund, Inc. | | 180,000 | | 40,000 | | 140,000 | | * | |
| Alliance Health Care Fund | | 34,000 | | 34,000 | | 0 | | * | |
| Alliance Select Investor Series Biotechnology | | 272,000 | | 272,000 | | 0 | | * | |
| ACM International Healthcare | | 34,000 | | 34,000 | | 0 | | * | |
| DCF Partners L.P. | | 25,000 | | 25,000 | | 0 | | * | |
| Oppenheimer Enterprise Fund | | 100,000 | | 100,000 | | 0 | | * | |
| MFS SERIES TRUST I, on behalf of MFS New Discovery Fund(2) | | 132,205 | | 132,205 | | 0 | | * | |
| MFS/SUN LIFE SERIES TRUST, on behalf of MFS New Discoveries Series(2) | | 18,100 | | 18,100 | | 0 | | * | |
| MFS VARIABLE INSURANCE TRUST, on behalf of MFS Emerging Growth Series (2) | | 48,300 | | 48,300 | | 0 | | * | |
| Sealion & Co. | | 789,860 | | 231,400 | | 558,460 | | * | |
| Pirate Ship & Co. | | 322,700 | | 84,700 | | 238,000 | | * | |
| Above anchor & Co. | | 109,240 | | 34,200 | | 75,040 | | * | |
| Cudd & Co. | | 251,500 | | 110,900 | | 140,600 | | * | |
| Covegrass & Co. | | 149,960 | | 32,100 | | 117,860 | | * | |
| Canal Reef & Co. | | 323,500 | | 81,800 | | 241,700 | | * | |
| JP Morgan Investment Management | | 1,727,586 | | 100,000 | | 1,627,586 | | 1.89 | % |
| Lombard Odier & Cie | | 40,000 | | 40,000 | | 0 | | * | |
- *
- less than one percent.
- (1)
- Assumes that each selling shareholder will sell all shares of common stock offered pursuant to this prospectus, but not any other shares of common stock beneficially owned by such shareholder. Percentages are based on 86,156,183 shares outstanding on July 31, 2001.
- (2)
- The selling shareholder has appointed Massachusetts Financial Services Company (MFS) as its investment adviser with respect to the shares. As investment adviser, MFS has been given discretionary voting and dispositive control over the shares.
81
PLAN OF DISTRIBUTION
The selling shareholders may sell the shares from time to time. The selling shareholders will act independently of us in making decisions regarding the timing, manner and size of each sale. The sales may be made on one or more exchanges or in the over-the-counter market or otherwise, at prices and at terms then prevailing or at prices related to the then current market price, or in privately negotiated transactions. The selling shareholders may effect these transactions by selling the shares to or through broker-dealers. The selling shareholders may sell their shares in one or more of, or a combination of:
- •
- a block trade in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction;
- •
- purchases by a broker-dealer as principal and resale by a broker-dealer for their account under this prospectus;
- •
- an exchange distribution in accordance with the rules of an exchange;
- •
- ordinary brokerage transactions and transactions in which the broker solicits purchasers; and
- •
- privately negotiated transactions.
To the extent required, this prospectus may be amended or supplemented from time to time to describe a specific plan of distribution.
From time to time, a selling shareholder may transfer, pledge, donate or assign our shares of common stock to lenders or others and each of such persons will be deemed to be a "selling shareholder" for purposes of this prospectus. The number of shares of common stock beneficially owned by the selling shareholder will decrease as and when it takes such actions. The plan of distribution for the selling shareholders' shares of common stock sold under this prospectus will otherwise remain unchanged, except that the transferees, pledgees, donees or other successors will be selling shareholders hereunder.
The selling shareholders may enter into hedging transactions with broker-dealers in connection with distributions of the shares or otherwise. In these transactions, broker-dealers may engage in short sales of the shares in the course of hedging the positions they assume with selling shareholders. The selling shareholders also may sell shares short and redeliver the shares to close out short positions. The selling shareholders may enter into option or other transactions with broker-dealers which require the delivery to the broker-dealer of the shares. The broker-dealer may then resell or otherwise transfer the shares under this prospectus. The selling shareholders also may loan or pledge the shares to a broker-dealer. The broker-dealer may sell the loaned shares, or upon a default the broker-dealer may sell the pledged shares under this prospectus.
In effecting sales, broker-dealers engaged by the selling shareholders may arrange for other broker-dealers to participate in the resales. Broker-dealers or agents may receive compensation in the form of commissions, discounts or concessions from selling shareholders. Broker-dealers or agents may also receive compensation from the purchasers of the shares for whom they act as agents or to whom they sell as principals, or both. Compensation as to a particular broker-dealer might be in excess of customary commissions and will be in amounts to be negotiated in connection with the sale. Broker-dealers or agents and any other participating broker-dealers or the selling shareholders may be deemed to be "underwriters" within the meaning of Section 2(11) of the Securities Act, in connection with sales of the shares. Accordingly, any commission, discount or concession received by them and any profit on the resale of the shares purchased by them may be deemed to be underwriting discounts or commissions under the Securities Act. Because selling shareholders may be deemed to be "underwriters" within the meaning of Section 2(11) of the Securities Act, the selling shareholders will be subject to the prospectus delivery requirements of the Securities Act.
82
In addition, any securities covered by this prospectus that qualify for sale under Rule 144 promulgated under the Securities Act may be sold under Rule 144 rather than under this prospectus. There is no underwriter or coordinating broker acting in connection with the proposed sale of shares by the selling shareholders. The shares will be sold only through registered or licensed brokers or dealers if required under applicable state securities laws. In addition, in some states the shares may not be sold unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
WHERE YOU CAN FIND MORE INFORMATION
A registration statement on Form S-1, including amendments thereto, relating to the common stock offered by this prospectus has been filed by us with the Securities and Exchange Commission. This prospectus, which constitutes a part of the Registration Statement, does not contain all of the information set forth in the Registration Statement and the exhibits and schedules thereto. For further information with respect to us and the common stock offered by this prospectus, reference is made to the registration statement, exhibits and schedules. A copy of the registration statement may be inspected by anyone without charge at the public reference facilities maintained by the Securities and Exchange Commission at 450 Fifth Street, NW, Judiciary Plaza, Washington, D.C. 20549, and copies of all or any part thereof may be obtained from the Securities and Exchange Commission upon payment of certain fees. The public may obtain information on the operation of the public reference room by calling the Securities and Exchange Commission at 1-800-SEC-0330. The Securities and Exchange Commission maintains a World Wide Web site that contains reports, proxy and information statements and other information filed electronically with it. The address of the site ishttp://www.sec.gov.
LEGAL MATTERS
O'Melveny & Myers LLP, San Francisco, California has passed upon legal matters for us regarding the validity of the securities intended to be sold pursuant to this prospectus.
EXPERTS
Our financial statements at December 31, 2000 and 1999, and for each of the three years in the period ended December 31, 2000, appearing in this prospectus and the registration statement have been audited by Ernst & Young LLP, independent auditors, as set forth in their report thereon appearing elsewhere herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
83
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| | Page
|
|---|
| Report of Independent Auditors | | F-2 |
Consolidated Balance Sheets as at December 31, 2000 and 1999 |
|
F-3 |
Consolidated Statements of Operations for the Years Ended
December 31, 2000, 1999 and 1998 |
|
F-4 |
Consolidated Statement of Changes in Redeemable Convertible
Preferred Stock and Stockholders' Equity for the Years Ended
December 31, 2000, 1999 and 1998 |
|
F-5 |
Consolidated Statements of Cash Flows for the Years Ended
December 31, 2000, 1999 and 1998 |
|
F-7 |
Notes to Consolidated Financial Statements |
|
F-8 |
Condensed Consolidated Balance Sheets as at June 30, 2001
and December 31, 2000 |
|
F-22 |
Condensed Consolidated Statements of Operations for Three Months and
Six Months ended June 30, 2001 and 2000 |
|
F-23 |
Condensed Consolidated Statements of Cash Flows for Three Months and
Six Months ended June 30, 2001 and 2000 |
|
F-24 |
Notes to Condensed Consolidated Financial Statements |
|
F-25 |
F–1
REPORT OF ERNST & YOUNG LLP, INDEPENDENT AUDITORS
The Board of Directors and Stockholders
Abgenix, Inc.
We have audited the accompanying consolidated balance sheets of Abgenix, Inc. as of December 31, 2000 and 1999, and the related consolidated statements of operations, changes in redeemable convertible preferred stock and stockholders' equity and cash flows for each of the three years in the period ended December 31, 2000. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with auditing standards generally accepted in the United States. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the accounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Abgenix, Inc. at December 31, 2000 and 1999, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2000, in conformity with accounting principles generally accepted in the United States.
Palo Alto, California
January 26, 2001
F–2
ABGENIX, INC. CONSOLIDATED BALANCE SHEETS
(in thousands, except share and per share data)
| | December 31,
| |
|---|
| | 2000
| | 1999
| |
|---|
| ASSETS | | | | | | | |
| Current assets: | | | | | | | |
| | Cash and cash equivalents | | $ | 167,242 | | $ | 13,366 | |
| | Marketable securities | | | 525,641 | | | 43,543 | |
| | Interest receivable | | | 9,793 | | | 1,103 | |
| | Accounts receivable | | | 3,397 | | | 4,150 | |
| | Prepaid expenses and other current assets | | | 11,965 | | | 4,861 | |
| | |
| |
| |
| | |
Total current assets |
|
|
718,038 |
|
|
67,023 |
|
| Property and equipment, net | | | 18,374 | | | 5,300 | |
| Long-term investments | | | 79,181 | | | 29,225 | |
| Intangible assets, net of accumulated amortization of $3,992 ($67 in 1999) | | | 117,997 | | | 46,591 | |
| Deposits and other assets | | | 3,210 | | | 402 | |
| | |
| |
| |
|
|
$ |
936,800 |
|
$ |
148,541 |
|
| | |
| |
| |
LIABILITIES AND STOCKHOLDERS' EQUITY |
|
|
|
|
|
|
|
| Current liabilities: | | | | | | | |
| | Accounts payable | | $ | 6,339 | | $ | 1,705 | |
| | Deferred revenue | | | 6,978 | | | 3,767 | |
| | Accrued product development costs | | | 2,338 | | | 1,667 | |
| | Accrued employee benefits | | | 2,034 | | | 1,287 | |
| | Other accrued liabilities | | | 3,124 | | | 725 | |
| | Current portion of long-term debt | | | 316 | | | 1,759 | |
| | Acquisition liability | | | 75,429 | | | — | |
| | |
| |
| |
| | |
Total current liabilities |
|
|
96,558 |
|
|
10,910 |
|
| Deferred rent | | | 567 | | | 150 | |
| Long-term debt | | | — | | | 421 | |
| Commitments | | | | | | | |
| Stockholders' equity: | | | | | | | |
| | Preferred stock, $0.0001 par value; 5,000,000 shares authorized; none issued or outstanding | | | — | | | — | |
| | Common stock, $0.0001 par value; 220,000,000 shares authorized; 85,401,548 and 68,669,092 shares issued and outstanding at December 31, 2000 and 1999, respectively, at amount paid in | | | 906,358 | | | 181,263 | |
| | Additional paid-in capital | | | 32,849 | | | 32,254 | |
| | Deferred compensation | | | (234 | ) | | (670 | ) |
| | Accumulated other comprehensive income/(loss) | | | (705 | ) | | 14,013 | |
| | Accumulated deficit | | | (98,593 | ) | | (89,800 | ) |
| | |
| |
| |
| | |
Total stockholders' equity |
|
|
839,675 |
|
|
137,060 |
|
| | |
| |
| |
|
|
$ |
936,800 |
|
$ |
148,541 |
|
| | |
| |
| |
See Accompanying Notes
F–3
ABGENIX, INC. CONSOLIDATED STATEMENTS OF OPERATIONS
in thousands, except per share data)
| | Year ended December 31,
| |
|---|
| | 2000
| | 1999
| | 1998
| |
|---|
| Revenues: | | | | | | | | | | |
| | Contract revenue | | $ | 26,601 | | $ | 12,285 | | $ | 2,498 | |
| | Revenue under collaborative agreements from related parties | | | — | | | — | | | 1,344 | |
| | Interest income | | | 32,848 | | | 3,045 | | | 961 | |
| | |
| |
| |
| |
| | | Total revenues | | | 59,449 | | | 15,330 | | | 4,803 | |
| Costs and expenses: | | | | | | | | | | |
| | Research and development | | | 51,329 | | | 21,106 | | | 17,588 | |
| | Amortization of intangible assets, related to research and development | | | 3,992 | | | — | | | — | |
| | General and administrative | | | 7,667 | | | 5,164 | | | 3,405 | |
| | In-process research and development charge | | | 5,215 | | | — | | | — | |
| | Equity in (income) losses from the Xenotech joint venture | | | — | | | (546 | ) | | 107 | |
| | Non-recurring termination fee | | | — | | | 8,667 | | | — | |
| | Interest expense | | | 39 | | | 438 | | | 530 | |
| | |
| |
| |
| |
| | | Total costs and expenses | | | 68,242 | | | 34,829 | | | 21,630 | |
| | |
| |
| |
| |
| Loss before income tax | | | (8,793 | ) | | (19,499 | ) | | (16,827 | ) |
| | | Foreign income tax expense | | | — | | | 1,000 | | | — | |
| | |
| |
| |
| |
| Net loss | | $ | (8,793 | ) | $ | (20,499 | ) | $ | (16,827 | ) |
| | |
| |
| |
| |
| Basic and diluted net loss per share | | $ | (0.11 | ) | $ | (0.35 | ) | $ | (0.75 | ) |
| | |
| |
| |
| |
| Shares used in computing basic and diluted net loss per share | | | 80,076 | | | 58,148 | | | 22,412 | |
See Accompanying Notes
F–4
ABGENIX, INC.
CONSOLIDATED STATEMENT OF CHANGES IN REDEEMABLE CONVERTIBLE
PREFERRED STOCK AND STOCKHOLDERS' EQUITY
(in thousands, except share and per share data)
| | Stockholders' Equity
| |
|---|
| | Redeemable
Convertible
Preferred
Stock
| | Common
Stock
| | Additional
Paid-in
Capital
| | Deferred
Compensation
| | Accumulated
Other
Comprehensive
Income (Loss)
| | Accumulated
Deficit
| | Total
Stockholders'
Equity
| |
|---|
| Balance at December 31, 1997 | | $ | 31,189 | | $ | 351 | | $ | 31,053 | | $ | (1,248 | ) | $ | — | | $ | (52,474 | ) | $ | (22,318 | ) |
| | Net loss | | | — | | | — | | | — | | | — | | | — | | | (16,827 | ) | | (16,827 | ) |
| | Issuance of 160,000 shares of series C redeemable convertible preferred stock at $8.00 per share | | | 1,280 | | | — | | | — | | | — | | | — | | | — | | | — | |
| | Issuance of 421,143 shares of series B redeemable convertible preferred stock at $6.5 per share (net of issuance cost of $81) | | | 2,656 | | | — | | | — | | | — | | | — | | | — | | | — | |
| | Conversion of 7,844,352 shares of series A, series B and series C redeemable convertible preferred stock to common stock. | | | (35,125 | ) | | 35,125 | | | — | | | — | | | — | | | — | | | 35,125 | |
| | Issuance of 11,500,000 shares of common stock at $2.00 per share upon initial public offering (net of issuance costs and commissions of $2,860) | | | — | | | 20,140 | | | — | | | — | | | — | | | — | | | 20,140 | |
| | Issuance of 613,076 shares of common stock upon exercise of stock options | | | — | | | 130 | | | — | | | — | | | — | | | — | | | 130 | |
| | Issuance of 56,520 shares of common stock pursuant to the employee stock purchase plan | | | — | | | 96 | | | — | | | — | | | — | | | — | | | 96 | |
| | Deferred compensation related to grant of certain stock below deemed fair value | | | — | | | — | | | 520 | | | (520 | ) | | — | | | — | | | — | |
| | Amortization of deferred compensation | | | — | | | — | | | — | | | 598 | | | — | | | — | | | 598 | |
| | Compensation related to grant of stock options to consultants | | | — | | | — | | | 15 | | | — | | | — | | | — | | | 15 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| Balance at December 31, 1998 | | | — | | | 55,842 | | | 31,588 | | | (1,170 | ) | | — | | | (69,301 | ) | | 16,959 | |
| | Unrealized gains on available for sale securities | | | — | | | — | | | — | | | — | | | 14,013 | | | — | | | 14,013 | |
| | Net loss | | | — | | | — | | | — | | | — | | | — | | | (20,499 | ) | | (20,499 | ) |
| | | | | | | | | | | | | | | | | | | | |
| |
| | Comprehensive loss | | | | | | | | | | | | | | | | | | | | | (6,486 | ) |
| | | | | | | | | | | | | | | | | | | | |
| |
| | Issuance of 12,000,000 shares of common stock at $3.75 per share (net of issuance costs and commissions of $3,364) | | | — | | | 41,636 | | | — | | | — | | | — | | | — | | | 41,636 | |
| | Issuance of 1,981,424 shares of common stock at $4.04 per share to Genentech | | | — | | | 8,000 | | | — | | | — | | | — | | | — | | | 8,000 | |
F–5
| | Issuance of 832,000 shares of common stock at $3.75 per share (net of issuance costs and commissions of $207) | | | — | | | 2,913 | | | — | | | — | | | — | | | — | | | 2,913 | |
| | Issuance of 7,112,000 shares of common stock at $10.50 per share (net of issuance costs and commissions of $3,603) | | | — | | | 71,073 | | | — | | | — | | | — | | | — | | | 71,073 | |
| | Issuance of 2,058,388 shares of common stock upon exercise of stock options | | | — | | | 1,463 | | | — | | | — | | | — | | | — | | | 1,463 | |
| | Issuance of 194,104 shares of common stock pursuant to the employee stock purchase plan | | | — | | | 336 | | | — | | | — | | | — | | | — | | | 336 | |
| | Amortization of deferred compensation | | | — | | | — | | | — | | | 500 | | | — | | | — | | | 500 | |
| | Compensation related to grant of stock options to consultants | | | — | | | — | | | 666 | | | — | | | — | | | — | | | 666 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| Balance at December 31, 1999 | | | — | | | 181,263 | | | 32,254 | | | (670 | ) | | 14,013 | | | (89,800 | ) | | 137,060 | |
| | Change in unrealized gains (losses) on available for sales securities | | | — | | | — | | | — | | | — | | | (14,718 | ) | | — | | | (14,718 | ) |
| | | | | | | | | | | | | | | | | | | | |
| |
| | Net loss | | | — | | | — | | | — | | | — | | | — | | | (8,793 | ) | | (8,793 | ) |
| | | | | | | | | | | | | | | | | | | | |
| |
| | Comprehensive loss | | | | | | | | | | | | | | | | | | | | | (23,511 | ) |
| | Issuance of 486,668 shares of common stock upon exercise of warrants | | | — | | | 730 | | | — | | | — | | | — | | | — | | | 730 | |
| | Issuance of 9,936,000 shares of common stock at $52.50 per share (net of issuance costs and commissions of $25,217) | | | — | | | 496,423 | | | — | | | — | | | — | | | — | | | 496,423 | |
| | Issuance of 3,300,000 shares of common stock at $70.00 per share (net of issuance costs and commissions of $10,310) | | | — | | | 220,690 | | | — | | | — | | | — | | | — | | | 220,690 | |
| | Issuance of 2,799,324 shares of common stock upon exercise of stock options | | | — | | | 6,490 | | | — | | | — | | | — | | | — | | | 6,490 | |
| | Issuance of 210,464 shares of common stock pursuant to the employee stock purchase plan | | | — | | | 762 | | | — | | | — | | | — | | | — | | | 762 | |
| | Amortization of deferred compensation | | | — | | | — | | | — | | | 436 | | | — | | | — | | | 436 | |
| | Compensation related to grant of stock options to consultants | | | — | | | — | | | 595 | | | — | | | — | | | — | | | 595 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| Balance at December 31, 2000 | | $ | — | | $ | 906,358 | | $ | 32,849 | | $ | (234 | ) | $ | (705 | ) | $ | (98,593 | ) | $ | 839,675 | |
| | |
| |
| |
| |
| |
| |
| |
| |
See Accompanying Notes
F–6
ABGENIX, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| | Year ended December 31,
| |
|---|
| | 2000
| | 1999
| | 1998
| |
|---|
| Operating activities | | | | | | | | | | |
| Net loss | | $ | (8,793 | ) | $ | (20,499 | ) | $ | (16,827 | ) |
| Adjustments to reconcile net loss to net cash used by operating activities: | | | | | | | | | | |
| | Equity in (income) losses of Xenotech | | | — | | | (546 | ) | | 411 | |
| | Depreciation and amortization | | | 5,948 | | | 1,763 | | | 1,715 | |
| | Stock options issued to consultants | | | 595 | | | 666 | | | — | |
| | In-process research and development charge | | | 5,215 | | | — | | | — | |
| | Changes for certain assets and liabilities: | | | | | | | | | | |
| | | Interest receivable | | | (8,690 | ) | | (835 | ) | | (268 | ) |
| | | Accounts receivable | | | 753 | | | (3,242 | ) | | — | |
| | | Prepaid expenses and other current assets | | | (6,288 | ) | | (4,337 | ) | | (888 | ) |
| | | Deposits and other assets | | | (1,042 | ) | | 100 | | | (166 | ) |
| | | Payable to Xenotech for cross-license and settlement | | | — | | | — | | | (3,750 | ) |
| | | Accounts payable | | | 3,891 | | | 1,266 | | | (199 | ) |
| | | Deferred revenue | | | 3,211 | | | 3,342 | | | 425 | |
| | | Accrued product development costs | | | 671 | | | 442 | | | 482 | |
| | | Accrued employee benefits | | | 747 | | | 1,028 | | | 39 | |
| | | Other accrued liabilities | | | 1,953 | | | (329 | ) | | (1,203 | ) |
| | | Deferred rent | | | 417 | | | 150 | | | — | |
| | |
| |
| |
| |
| Net cash used in operating activities | | | (1,412 | ) | | (21,031 | ) | | (20,229 | ) |
| | |
| |
| |
| |
| Investing activities | | | | | | | | | | |
| Purchases of marketable securities | | | (1,089,518 | ) | | (60,763 | ) | | (24,600 | ) |
| Maturities of marketable securities | | | 609,637 | | | 32,069 | | | 20,243 | |
| Capital expenditures | | | (13,809 | ) | | (1,108 | ) | | (697 | ) |
| Acquisition of IntraImmune, net of cash acquired | | | (9,253 | ) | | — | | | — | |
| Acquisition of Xenotech, net of cash acquired | | | — | | | (45,938 | ) | | — | |
| Purchases of long-term investments | | | (65,000 | ) | | (15,000 | ) | | — | |
| Contributions to Xenotech | | | — | | | — | | | (475 | ) |
| | |
| |
| |
| |
| Net cash used in investing activities | | | (567,943 | ) | | (90,740 | ) | | (5,529 | ) |
| | |
| |
| |
| |
| Financing activities | | | | | | | | | | |
| Net proceeds from issuances of common stock | | | 725,095 | | | 125,421 | | | 20,366 | |
| Payments on long-term debt | | | (1,864 | ) | | (1,699 | ) | | (1,746 | ) |
| Net proceeds from issuances of redeemable convertible preferred stock | | | — | | | — | | | 3,936 | |
| | |
| |
| |
| |
| Net cash provided in financing activities | | | 723,231 | | | 123,722 | | | 22,556 | |
| | |
| |
| |
| |
Net increase (decrease) in cash and cash equivalents |
|
|
153,876 |
|
|
11,951 |
|
|
(3,202 |
) |
| Cash and cash equivalents at the beginning of the year | | | 13,366 | | | 1,415 | | | 4,617 | |
| | |
| |
| |
| |
| Cash and cash equivalents at the end of the year | | $ | 167,242 | | $ | 13,366 | | $ | 1,415 | |
| | |
| |
| |
| |
| Supplemental disclosures of cash flow information | | | | | | | | | | |
| Cash paid during the year for interest | | $ | 132 | | $ | 438 | | $ | 549 | |
| Cash paid during the year for foreign income tax | | $ | — | | $ | 1,000 | | $ | — | |
| Non-cash investing and financing activities | | | | | | | | | | |
| Acquisition of ImmGenics in exchange for a liability to ImmGenics shareholders | | $ | 75,429 | | $ | — | | $ | — | |
See Accompanying Notes
F–7
1. ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Business and Organization
Abgenix, Inc., ("Abgenix" or the "Company"), is a biopharmaceutical company that develops and intends to commercialize antibody therapeutic products for the treatment of a variety of disease conditions, including transplant-related diseases, inflammatory and autoimmune disorders, cardiovascular disease, infectious diseases and cancer. The Company's antibody technology platform, which includes XenoMouse™ technology, enables the rapid generation and selection of high affinity, fully human antibody product candidates to essentially any disease target appropriate for antibody therapy. Abgenix leverages its leadership position in human antibody technology by building a large and diversified product portfolio through the establishment of licensing arrangements with multiple pharmaceutical, biotechnology and genomics companies and through the development of its own internal proprietary products.
In November 2000, in two separate transactions, the Company acquired ImmGenics Pharmaceuticals, Inc. ("ImmGenics") and IntraImmune Therapies, Inc. ("IntraImmune").
Effective December 31, 1999 the Company acquired Japan Tobacco Inc.'s ("Japan Tobacco"), interest in the Xenotech joint venture ("Xenotech"), increasing the Company's ownership of the joint venture from 50% to 100%. The consolidated financial statements include the accounts of Xenotech as of December 31, 1999. Intercompany accounts have been eliminated in consolidation. Prior to the acquisition, Xenotech was accounted for under the equity method of accounting, accordingly the Company's operations include equity in income and losses from Xenotech for the years 1999 and 1998. (See Note 2.)
Accounts denominated in foreign currency have been remeasured using the U.S. dollar as the functional currency. Significant intercompany accounts and transactions have been eliminated.
Cash Equivalents, Marketable Securities and Long-Term Investments
Cash equivalents—The Company considers all highly liquid investments with a maturity date of three months or less when purchased to be cash equivalents.
Marketable securities—Marketable securities consist of highly liquid investments with a maturity of greater than three months when purchased. The Company's marketable securities have been classified as "available-for-sale," and are carried at market value. Unrealized gains and losses are reported as accumulated other comprehensive income/(loss), which is a separate component of stockholders' equity.
Long-term investments—The Company has purchased certain strategic marketable equity securities. These investments have been classified as "available-for-sale," and are carried at market value. Unrealized gains and losses are reported as accumulated other comprehensive income/(loss), which is a separate component of stockholders' equity.
Depreciation and Amortization
The Company records property and equipment at cost and provides depreciation using the straight-line method over the estimated useful lives of the assets. Leasehold improvements are depreciated over the remaining life of the facility lease, and all other assets are generally depreciated over two to five years. Furniture and equipment leased under capital leases is amortized over the shorter of the useful lives or the lease term. Amortization of leased assets is included in depreciation and amortization expense and is combined with accumulated depreciation and amortization of the Company's owned assets.
F–8
Intangible Assets
The intangible assets consist primarily of acquired existing technology (including patents and royalty rights), goodwill, and assembled workforce. They are being amortized on a straight-line basis over their estimated useful lives of 15 years for the existing technology and 2 years for the assembled workforce.
Long-Lived Assets
The carrying value of our long-lived assets is reviewed for impairment whenever events or changes in circumstances indicate that the asset may not be recoverable. An impairment loss would be recognized when estimated future cash flows expected to result from the use of the asset and its eventual disposition is less than its carrying amount. Long-lived assets include property, plant and equipment, goodwill and other intangible assets.
Revenue Recognition
The Company receives payments from customers for licenses, options and services. These payments are generally non-refundable but are reported as deferred revenue until they are recognizable as revenue. The Company has followed the following principles in recognizing revenue:
- •
- Research license fees: Fees to license the use of XenoMouse in research performed by the customer are generally recognized when both the inception of the license period and delivery of the technology have occurred. If Abgenix is obligated to provide significant assistance to enable the customer to practice the license, then the revenue is recognized over the period of such obligation.
- •
- Product license fees: Fees to license the production, use and sale of an antibody generated by XenoMouse are generally recognized when both the inception of the license period and delivery of the technology have occurred. If Abgenix is obligated to provide significant assistance to enable the customer to practice the license, then the revenue is recognized over the period of such obligation.
- •
- Option fees: Fees for granting options to obtain product licenses are recognized as revenue when the option is exercised or when the option period expires, whichever occurs first.
- •
- Payments for research services performed by Abgenix are recognized ratably over the period during which these services are performed. However, fees for research services received under co-development arrangements are recorded as contract revenues in the period rendered, net of fees payable by Abgenix to such co-developers for reimbursements of research and development costs.
- •
- Milestone payments are recognized as revenue when the milestone is achieved.
Research and Development
Research and development expenses, including direct and allocated expenses, consist of independent research and development costs and costs associated with sponsored research and development.
F–9
Stock Based Compensation
The Company accounts for stock-based awards to employees and directors using the intrinsic value method in accordance with Accounting Principles Board Opinion No. 25, "Accounting for Stock Issued to Employees." Accordingly, the Company does not recognize compensation expense for employee stock options granted at fair market value.
Net Loss Per Share
Basic earnings per share is calculated based on the weighted average number of shares outstanding during the period. The impact of common stock options and warrants was excluded from the computation of diluted earnings per share, as their effect is antidilutive for the periods presented.
Pro forma net loss per share has been computed to give effect to the automatic conversion of redeemable convertible preferred stock into common stock which occurred at the completion of the Company's initial public offering in July 1998, using the as-if-converted method, from the original date of issuance.
A reconciliation of shares used in calculation of basic and diluted and pro forma net loss per share follows:
| | Year ended December 31,
| |
|---|
| | 2000
| | 1999
| | 1998
| |
|---|
| | (In thousands, except per share data)
| |
|---|
| Net loss | | $ | (8,793 | ) | $ | (20,499 | ) | $ | (16,827 | ) |
| | |
| |
| |
| |
| Basic and diluted: | | | | | | | | | | |
| | Weighted-average shares of common stock outstanding used in computing basic and diluted net loss per share | | | 80,076 | | | 58,148 | | | 22,412 | |
| | |
| |
| |
| |
| Basic and diluted net loss per share | | $ | (0.11 | ) | $ | (0.35 | ) | $ | (0.75 | ) |
| | |
| |
| |
| |
| Pro forma (unaudited): | | | | | | | | | | |
| | Shares used in computing basic and diluted net loss per share (from above) | | | | | | | | | 22,412 | |
| Adjusted to reflect the effect of the assumed conversion of preferred stock from the date of issuance | | | | | | | | | 17,204 | |
| | | | | | | | |
| |
| | Weighted-average shares used in computing pro forma net loss per share | | | | | | | | | 39,616 | |
| | | | | | | | |
| |
| Pro forma net loss per share | | | | | | | | $ | (0.42 | ) |
| | | | | | | | |
| |
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
Stock Splits
The accompanying financial statements have been restated to reflect both a two-for-one common stock split effective on April 6, 2000 and a two-for-one common stock split effective on July 7, 2000.
F–10
Reclassifications
Certain prior-year balances have been reclassified to conform to the current-year presentation.
2. ACQUISITIONS
Acquisition of ImmGenics
In November of 2000, the Company acquired ImmGenics, a private biotechnology company with proprietary technology for accelerating antibody product discovery. This acquisition was accounted for using the purchase method of accounting. The total purchase price was $77.2 million, including cash compensation amounts associated with the purchase of employee stock options, and transaction costs. Under the terms of the agreement, ImmGenics special shares were issued to former shareholders of common and preferred shares and debenture holders of ImmGenics. The ImmGenics special shares were convertible into common shares of Abgenix if the common shares were registered and declared effective with the Securities and Exchange Commission (SEC). If the shares were not registered the special shareholders have the right to put them to the Company for cash. Their put rights will be fully vested on May 12, 2001 and will expire March 31, 2002. As of December 31, 2000 the registration was not declared effective and therefore the purchase price was recorded as a current liability at December 31, 2000. See Note 10 for subsequent event.
Acquisition of IntraImmune
In November of 2000, the Company acquired IntraImmune, a private research company with technologies to give antibodies access to intracellular targets. The total cash purchase price was $9.3 million, including transaction costs. This acquisition was accounted for using the purchase method of accounting.
Purchase Price Allocation
The Company performed an allocation of the total purchase price of both ImmGenics and IntraImmune among the acquired assets. The income approach was used to develop the value for the existing technology and the in-process research and development, as applicable. The income approach incorporates the calculation of the present value of future economic benefits such as cash earnings, cost savings, and tax deductions. The cost approach was utilized to value the assembled workforce. The cost approach measures the benefits related to an asset by the cost to reconstruct or replace it with another of like utility.
ImmGenics Selected Lymphocyte Antibody Method (SLAM) technology is patented in the United States with applications outstanding in Canada and Europe. This technology is technologically feasible and the Company has licensed it to a customer. The existing technology of IntraImmune is also patented and has been determined to be technologically feasible.
The in-process development activities of ImmGenics included two distinct research projects. The Company determined the amounts to be allocated to in-process technology based on whether technological feasibility had been achieved and whether there was any alternative future use for the technology. The Company concluded that the in-process technology had no alternative future use after taking into consideration the potential for both usage of the technology in different products and for resale of the technology. The rate utilized to discount the net cash flows to their present value was 40%. For the in-process research and development. IntraImmune had no in-process development activities at the time of acquisition.
F–11
The purchase price allocations for ImmGenics and IntraImmune were as follows:
| | ImmGenics
| | IntraImmune
|
|---|
| | Amount
| | Useful
Lives
| | Amount
| | Useful
Lives
|
|---|
| | (dollars in thousands)
|
|---|
| Purchase price allocation: | | | | | | | | | | |
| | Tangible net assets (liabilities) | | $ | 5,508 | | n/a | | $ | (704 | ) | n/a |
| | Intangible assets acquired: | | | | | | | | | | |
| | | Existing technology | | | 35,851 | | 15 years | | | 2,700 | | 15 years |
| | | Assembled workforce | | | 195 | | 2 years | | | — | | n/a |
| | | Goodwill | | | 29,393 | | 15 years | | | 7,257 | | 15 years |
| | | Deferred compensation | | | 1,052 | | 2 years | | | — | | n/a |
| | In-process research and development | | | 5,215 | | n/a | | | — | | n/a |
| | |
| | | |
| | |
Total purchase price allocation |
|
$ |
77,214 |
|
|
|
$ |
9,253 |
|
|
| | |
| | | |
| | |
Acquisition of Xenotech and Transactions with Japan Tobacco
On December 20, 1999, the Company executed several agreements with Japan Tobacco that became effective December 31, 1999, under which the Company acquired Japan Tobacco's interest in the XenoMouse, a technology for generating fully human antibody drugs used in treating a wide range of diseases. Under the agreements, Abgenix paid $47.0 million in cash to Japan Tobacco for its 50% interest in Xenotech under which the XenoMouse technology was developed. This acquisition brought the Company's ownership of Xenotech to 100% and was accounted for under the purchase method of accounting. The purchase price of $47.2 million, including transaction costs, was allocated $0.6 million to cash and $46.6 million to intangibles consisting primarily of the patents for the XenoMouse technology and the rights to royalties under certain licenses. The intangible asset is being amortized over 15 years, the estimated average life of the patents and licenses, using the straight-line method. Because Xenotech was acquired effective December 31, 1999, and prior to this date was owned 50% by the Company, operations of Xenotech were recorded on the equity method of accounting through December 31,1999, and upon acquisition the accounts were consolidated with the Company.
Under the agreements, the Company also paid $10.0 million as compensation to Japan Tobacco for relinquishment of its existing license rights to the current XenoMouse technology. Additionally, Japan Tobacco paid $4.0 million to the Company for a license to use the existing XenoMouse technology on a more limited basis than previously, and to use future XenoMouse technology in development at Abgenix. One third of the $4.0 million payment, or $1.3 million, was allocated to the current XenoMouse technology and netted with the $10.0 million payment. The remainder of the $4.0 million payment, or $2.7 million, was recorded as deferred revenue at December 31, 1999 and was recognized as revenue in 2000 when the new XenoMouse technologies were delivered. Japan Tobacco will also make royalty payments on any future sales of antibody products generated using XenoMouse.
Lastly, under the December agreements, the Company granted to Japan Tobacco a license for certain technology related to the generation of mouse models of certain human diseases. In return for this license, Japan Tobacco paid Abgenix $6.0 million, which was recorded as revenue in 1999.
In June 1999 the Company entered into a collaboration agreement with Japan Tobacco, Inc. relating to the clinical development of one of the Company's products. Under the agreement, Japan Tobacco made payments totaling $1,280,000 to the Company, which was recorded as revenue in 1999.
F–12
Xenotech Prior to the Acquisition
Prior to the acquisition, the Company and a subsidiary of Japan Tobacco equally owned Xenotech. Research performed by Xenotech, which was generally outsourced to the Company, was funded through capital contributions from the partners. Revenues recognized by the Company for performing the research for Xenotech were $1,344,000 in 1998 and $0 thereafter, net of its cash contributions to Xenotech related to this revenue. The Company acquired options and product licenses for antigen targets developed from the XenoMouse technology from Xenotech, prior to the acquisition, as well. The cost of such options and licenses were expensed as research and development by the Company in the amounts of $645,000 and $453,000 in 1999 and 1998, respectively. The Company accounted for its investment in Xenotech under the equity method and therefore recorded 50% of Xenotech's net income or losses, up to the Company's investment amount.
Proforma Unaudited Financial Information for All Acquisitions
The following unaudited pro forma financial information presents the results of operations of the Company, ImmGenics and IntraImmune for the years ended December 31, 2000 and 1999 and Xenotech for the years ended December 31, 1999 and 1998, as if the acquisitions had been consummated as of the beginning of the periods presented.
| | December 31,
| |
|---|
| | 2000
| | 1999
| | 1998
| |
|---|
| | (in thousands)
| |
|---|
| Total revenues | | $ | 60,301 | | $ | 17,563 | | $ | 2,723 | |
| Net loss | | $ | (16,639 | ) | $ | (29,807 | ) | $ | (20,344 | ) |
| Net loss per share | | $ | (0.21 | ) | $ | (0.51 | ) | $ | (0.91 | ) |
The pro forma financial information includes the effect of the amortization of intangible assets acquired, using a 2-year life for the assembled workforce and a 15-year life for the existing technology and goodwill. Due to their non-recurring nature, the in-process research and development charge attributable to the ImmGenics transaction has been excluded from the pro forma financial information. The pro forma condensed financial information is presented for illustrative purposes only. This information is not necessarily indicative of the Company's financial position or results of operations for future periods or the results that actually would have been realized had the acquisition and certain transactions occurred as of the beginning of the periods presented.
F–13
3. MARKETABLE SECURITIES
The following is a summary of marketable securities at December 31, 2000 and 1999 (in thousands):
| | 2000
| | 1999
|
|---|
| | Amortized
Cost
| | Unrealized
Gain/(Loss)
| | Estimated Fair
Value
| | Amortized
Cost
| | Unrealized
Gain/(Loss)
| | Estimated Fair
Value
|
|---|
| Commercial obligations | | $ | 31,823 | | $ | 13 | | $ | 31,836 | | $ | 22,277 | | $ | (73 | ) | $ | 22,204 |
| Commercial paper | | | 638,735 | | | 106 | | | 638,841 | | | 15,358 | | | 10 | | | 15,368 |
| Obligations of the U.S. government and its agencies | | | 20,000 | | | (6 | ) | | 19,994 | | | 17,271 | | | (149 | ) | | 17,122 |
| Marketable equity securities | | | 79,999 | | | (818 | ) | | 79,181 | | | 15,000 | | | 14,225 | | | 29,225 |
| | |
| |
| |
| |
| |
| |
|
Total |
|
$ |
770,557 |
|
$ |
(705 |
) |
$ |
769,852 |
|
$ |
69,906 |
|
$ |
14,013 |
|
$ |
83,919 |
| | |
| |
| |
| |
| |
| |
|
| Classified as: | | | | | | | | | | | | | | | | | | |
| | Cash equivalents | | | | | | | | $ | 165,030 | | | | | | | | $ | 11,151 |
| | Marketable securities | | | | | | | | | 525,641 | | | | | | | | | 43,543 |
| | Long-term investments | | | | | | | | | 79,181 | | | | | | | | | 29,225 |
| | | | | | | | |
| | | | | | | |
|
| | | | | | | | | $ | 769,852 | | | | | | | | $ | 83,919 |
| | | | | | | | |
| | | | | | | |
|
All of the Company's available for sale debt securities mature in one year or less as of December 31, 2000. Estimated fair values have been determined by the Company using available market information.
The unrealized gains and losses as of December 31, 1999 and 2000 were reported as accumulated other comprehensive income/(loss), which is a separate component of stockholders' equity.
4. COMPREHENSIVE INCOME
Other comprehensive gains/(losses) consist of unrealized gains or losses on available-for-sale securities. The components of comprehensive income(loss), net of tax, were as follows:
| | December 31,
| |
|---|
| | 2000
| | 1999
| |
|---|
| | (in thousands)
| |
|---|
| Net loss | | $ | (8,793 | ) | $ | (20,499 | ) |
| Increase (decrease) in net unrealized gains on available for sale investments | | | (14,718 | ) | | 14,013 | |
| | |
| |
| |
| Comprehensive income (loss) | | $ | (23,511 | ) | $ | (6,486 | ) |
| | |
| |
| |
There were no significant unrealized gains or losses as of December 31, 1998.
F–14
5. PROPERTY AND EQUIPMENT
Property and equipment consists of the following, at cost:
| | December 31,
| |
|---|
| | 2000
| | 1999
| |
|---|
| | (in thousands)
| |
|---|
| Furniture, machinery and equipment | | $ | 9,316 | | $ | 4,163 | |
| Leasehold improvements | | | 10,761 | | | 4,333 | |
| | |
| |
| |
|
|
|
20,077 |
|
|
8,496 |
|
| Less accumulated depreciation and amortization | | | (5,010 | ) | | (3,196 | ) |
| Construction-in-progress | | | 3,307 | | | — | |
| | |
| |
| |
|
|
$ |
18,374 |
|
$ |
5,300 |
|
| | |
| |
| |
Property and equipment financed under capital leases was $1,956,000 at December 31, 2000 and 1999.
6. COMMITMENTS
Facility Lease
The Company has three operating leases for its office, research and development and manufacturing facilities in California and one lease for its facility in British Columbia, Canada. The leases expire in 2007 and 2015, each with options to extend for 10 years. The Company issued a stand-by letter of credit for $2.0 million to one of the lessors for the lease term expiring in 2015, as a condition to the lease. Future minimum payments under noncancelable operating leases at December 31, 2000 are as follows:
| | (in thousands)
|
|---|
| Year ending December 31, | | | |
| 2001 | | $ | 4,927 |
| 2002 | | | 5,774 |
| 2003 | | | 5,973 |
| 2004 | | | 6,174 |
| 2005 | | | 6,386 |
| Thereafter | | | 61,380 |
| | |
|
| | Total lease payments | | $ | 90,614 |
| | |
|
Rent expense, in thousands, was $2,534 and $1,043 for the years ended December 31, 2000 and 1999, respectively.
Property and Equipment
The Company has contracted with developers and designers for the completion of its new office, research and development facility and improvements for its new manufacturing facility. Both facilities were leased in 2000 (see above). As of December 31, 2000, the Company has outstanding purchase order commitments for approximately $4.2 million related to the design and improvements of these new facilities.
F–15
Loan, Capital Lease and Letter of Credit
The Company issued a stand-by letter of credit for $2.0 million to one of its lessors for a term expiring in 2015, as a security deposit for a facility lease. Cash and marketable securities, which secure the letter of credit, have been recorded in long-term deposits and other assets.
On January 24, 1997, the Company secured a loan with a bank in the amount of $4,300,000 in order to finance tenant improvements on its facility in Fremont, California. The loan was paid in full in May 2000. The interest rate at December 31, 1999 was 9.50% and the loan was secured by substantially all tangible and intangible assets of the Company.
On March 28, 1997, the Company entered into a lease agreement with a financing company under which the Company financed approximately $2,000,000 of its laboratory and office equipment. The lease term is 48 months and bears interest at rates ranging from 12.5% to 13.0%. The last lease schedule matures in September 2001.
CBL License Agreement
In 1997, the Company entered into a license agreement for exclusive worldwide rights to commercialize ABX-CBL. The Company paid an initial license fee and is further obligated to pay an annual maintenance fee of $50,000, to commit at least $1,000,000 annually to the development of ABX-CBL until ABX-CBL receives regulatory approval in any country and to pay royalties on potential product sales. The Company is also obligated to issue 100,000 shares of its common stock upon the submission of a Product License Application for the first indication of the product.
Commitment for Product Development
The Company has contracted with a third party, Lonza Biologics ("Lonza") for the manufacture of its product candidates for use in its clinical trials. As of December 31, 2000, the Company has outstanding approximately $10.8 million in non-cancelable orders related to future deliveries of these products over the next 12 months. The Company has not recorded these obligations in its accrued liabilities as no legal liability exits until the products are delivered to and accepted by the Company.
In December 2000, the Company entered into a five-year manufacturing supply agreement with Lonza. Under the agreement, Lonza will provide a cell culture production suite within its facility for the Company's exclusive use. Abgenix and its licensees will use the production suite for the manufacture of product candidates in development. The dedicated cell culture production suite with associated purification capacity is undergoing refurbishment and will be operational in the third quarter of 2001. For use of the production suite payments of approximately $1.0 million plus a 15% raw material charge are due monthly for 5 years and certain performance fees are due annually. In May 2000, prior to the negotiation of this manufacturing supply agreement, the Company paid Lonza $3.8 million for the option to reserve manufacturing capacity.
7. STOCKHOLDERS' EQUITY
Common Stock
Initial Public Offering—In July 1998, the Company completed an initial public offering of 10,000,000 shares of its common stock to the public, at a price of $2.00 per share. On July 27, 1998, the Company's underwriters exercised an option to purchase 1,500,000 additional shares of common stock at a price of $2.00 per share to cover over-allotments. The Company received net proceeds from the offerings of approximately $20.1 million. Upon the closing of the initial public offering, each of the
F–16
outstanding 31,377,408 shares of redeemable convertible preferred stock was automatically converted into one share of common stock.
Genentech—In January 1999, Genentech acquired 1,981,424 shares of our common stock for an aggregate purchase price of $8.0 million.
Follow-on Public Offering—In March 1999, the Company completed a follow-on public offering of 12,000,000 shares of its common stock to the public, at a price of $3.75 per share. On April 7, 1999 the Company's underwriters exercised an option to purchase 832,000 additional shares of common stock at a price of $3.75 per share to cover over-allotments. The Company received net proceeds from the offerings of approximately $44.5 million.
Private Placement—In November 1999, the Company completed a private placement of 7,112,000 shares of its common stock to qualified institutional and other accredited investors at a net price of $10.50 per share The Company received net proceeds of $71.1 million.
Follow-on Public Offering: In February 2000, the Company completed a follow-on public offering in which the Company sold 8,640,000 shares and a stockholder sold 3,360,000 shares of the Company's common stock to the public at a price of $52.50 per share. On February 29, 2000, the Company's underwriters exercised an option to purchase 1,800,000 additional shares, of which 1,296,000 shares were sold by the Company and 504,000 shares were sold by a stockholder at a price of $52.50 per share. The Company received net proceeds from the offerings of $496.5 million after the underwriters' discount and estimated costs of offering.
Private Placement—In November 2000, the Company completed a private placement of 3,300,000 shares of its common stock to qualified institutional and other accredited investors at a net price of $70.00 per share. The Company received net proceeds of $221.0 million.
Stockholder Rights Plan
In June 1999, our Board of Directors adopted a Stockholder Rights Plan. The Stockholder Rights Plan provides for a dividend distribution of one Preferred Shares Purchase Right on each outstanding share of our common stock. Each Right entitles stockholders to buy 1/1000th of a share of our Series A participating preferred stock at an exercise price of $30.00. Each Right will become exercisable following the tenth day after a person or group announces acquisition of 15 percent or more of our common stock, or announces commencement of a tender offer, the consummation of which would result in ownership by the person or group of 15 percent or more of our common stock. In the case of Cell Genesys, which beneficially owned approximately 10.45% of our outstanding common stock as of February 28, 2001, each right will become exercisable following the tenth day after it announces the acquisition of 25 percent or more of our common stock, or announces commencement of a tender offer, the consummation of which would result in ownership by Cell Genesys of 25 percent or more of our common stock. We will be entitled to redeem the Rights at $0.01 per Right at any time on or before the tenth day following acquisition by a person or group of 15 percent or more (or in the case of Cell Genesys, 25 percent or more) of our common stock.
Warrants
In connection with loan guarantees it received in 1997, the Company issued warrants to purchase a total of 486,668 shares of the Company's common stock, at an exercise price of $1.50 per share. The original terms were such that the warrants were exercisable immediately and expired in three years. The fair value of the above warrants was determined at the time to be insignificant for accounting purposes. These warrants were exercised in January 2000.
F–17
8. STOCK OPTION AND BENEFIT PLANS
Incentive Stock Plans
The Company has three stock option plans, which allow for the granting of incentive and non-qualified stock options to employees, outside directors and consultants of the Company. There are 19,365,000 shares of common stock authorized for issuance under the plans. The Company grants shares of common stock for issuance under the plans at no less than the fair value of the stock. Options granted under the plans generally have a term of ten years and vest over four years.
Information with respect to activity under the plans is as follows:
| | Shares
Available
| | Number of
Shares
| | Weighted
Average
Exercise Price
|
|---|
| Balances at December 31, 1997 | | 2,597,128 | | 6,033,704 | | $ | 0.31 |
| | Authorized | | 2,000,000 | | — | | | — |
| | Options granted | | (1,414,204 | ) | 1,414,204 | | $ | 1.71 |
| | Options exercised | | — | | (613,072 | ) | $ | 0.22 |
| | Options canceled | | 266,088 | | (266,088 | ) | $ | 0.53 |
| | |
| |
| | | |
Balances at December 31, 1998 |
|
3,449,012 |
|
6,568,748 |
|
$ |
0.61 |
| | Authorized | | 6,600,000 | | — | | | — |
| | Options granted | | (4,111,500 | ) | 4,111,500 | | $ | 5.56 |
| | Options exercised | | — | | (2,058,388 | ) | $ | 0.70 |
| | Options canceled | | 665,552 | | (665,552 | ) | $ | 1.64 |
| | |
| |
| | | |
Balances at December 31, 1999 |
|
6,603,064 |
|
7,956,308 |
|
$ |
3.06 |
| | Authorized | | 1,200,000 | | — | | | — |
| | Options granted | | (5,585,930 | ) | 5,585,930 | | $ | 48.76 |
| | Options exercised | | — | | (2,799,324 | ) | $ | 54.99 |
| | Options canceled | | 256,551 | | (256,551 | ) | $ | 16.10 |
| | |
| |
| | | |
Balances at December 31, 2000 |
|
2,473,685 |
|
10,486,363 |
|
$ |
27.28 |
| | |
| |
| | | |
The following table summarizes information about options outstanding at December 31, 2000:
| | Outstanding Options
| | Exercisable Options
|
|---|
Range of
Exercise Price
| | Number of Options
| | Weighted Average
Exercise Price
| | Remaining
Contractual Life,
in Years
| | Number
of Options
| | Weighted Average
Exercise Price
|
|---|
| $ | 0.15-$ 2.50 | | 2,148,250 | | $ | 0.76 | | 6.33 | | 1,702,371 | | $ | 0.63 |
| $ | 3.59-$11.00 | | 2,803,778 | | $ | 5.38 | | 8.31 | | 880,238 | | $ | 5.10 |
| $ | 22.13-$39.50 | | 3,208,035 | | $ | 33.46 | | 9.18 | | 412,313 | | $ | 31.18 |
| $ | 44.78-$59.93 | �� | 772,100 | | $ | 50.82 | | 9.63 | | 155,623 | | $ | 48.13 |
| $ | 75.17-$80.81 | | 1,554,200 | | $ | 78.99 | | 9.65 | | 16,000 | | $ | 79.75 |
| | | |
| | | | | | |
| | | |
|
|
|
10,486,363 |
|
$ |
27.28 |
|
8.46 |
|
3,166,545 |
|
$ |
8.59 |
| | | |
| | | | | | |
| | | |
From inception to December 31, 1997, options to purchase a total of 7,446,976 shares of common stock were granted at prices ranging from $0.15 to $1.25 per share. Deferred compensation of $1,776,000 was recorded for these option grants based on the deemed fair value of common stock (ranging from $0.30 to $1.63 per share). In the first quarter of 1998, the Company granted options to purchase 1,040,700 shares of common stock at $1.50 per share for which deferred compensation of
F–18
approximately $520,000 was recorded based on the deemed fair value of common stock at $2.00 per share. During the second, third and fourth quarters of 1998, the Company granted an additional 205,504 options to employees to purchase shares of common stock at prices ranging from $1.25 to $2.50 per share. No deferred compensation expense was recorded as the options were granted at the then current market price of the stock on the date of the grant. The Company amortized $569,000, $500,000 and $598,000 of the deferred compensation balance during the years ended December 31, 2000, 1999, and 1998, respectively.
Additionally, the Company granted 18,000, 6,000 and 168,000 options to purchase shares of common stock in 2000, 1999 and 1998, respectively to independent consultants. The prices of the options range from $2.13 to $79.75 per share. The options granted in 2000 were issued fully vested. The prior options vest ranging from one to two years. Compensation expense of $595,000, $666,000 and $15,000 was recorded in 2000, 1999 and 1998, respectively.
Pro Forma Information
Pro forma information regarding net loss and net loss per share is required by SFAS 123, and has been determined as if the Company had accounted for its employee stock options under the fair value method of that Statement. The fair value for these options was estimated at the date of grant using a Black-Scholes option pricing model with the following assumptions for 2000, 1999 and 1998, respectively: risk-free interest rate of 5.08%, 6.39%, and 4.67%; no dividend yield in 2000, 1999, or 1998; volatility factor of 1.10, 1.03, and 0.78; and an expected life of the option of six years in 2000 and 1999 and five years in 1998. These same assumptions were applied in the determination of the option values related to stock options granted to non-employees, except for the option life for which the term of the consulting contracts, 1 to 5 years, were used. The value has been recorded in the financial statements.
The Black-Scholes option valuation model was developed for use in estimating the fair value of traded options, which have no vesting restrictions and are fully transferable. In addition, option valuation models require the input of highly subjective assumptions, including the expected stock price volatility. Because the Company's employee stock options have characteristics significantly different from those of traded options, and because changes in the subjective input assumptions can materially affect the fair value estimate, in management's opinion, the existing models do not necessarily provide a reliable single measure of the fair value of its employee stock options.
The weighted-average fair values of options granted during the years ended December 31, 2000, 1999 and 1998 were $40.55, $4.52 and $1.71 per share. All options granted in 1997 and 1996 were granted at exercise prices below the deemed fair value of the underlying common stock. All options granted in 2000, 1999 and 1998 were granted at exercise prices at the then current market value of the stock. The following table illustrates what net loss would have been had the Company accounted for its stock-based awards under the provisions of SFAS 123. Pro forma amounts may not be representative of future years.
| | December 31,
| |
|---|
| | 2000
| | 1999
| | 1998
| |
|---|
| | (in thousands, except per share amounts)
| |
|---|
| Pro forma net loss | | $ | (49,345 | ) | $ | (24,064 | ) | $ | (17,160 | ) |
| Pro forma net loss per share | | $ | (0.62 | ) | $ | (0.41 | ) | $ | (0.77 | ) |
F–19
Employee Stock Purchase Plan
The Company's employee stock purchase plan enables eligible employees to purchase common stock at 85% of the average market price on the first or the last day of each 24 month offering period, whichever is lower. Employees may authorize periodic payroll deductions of up to 15% of eligible compensation for common stock purchases, with certain limitations. The number of shares which may be issued under the plan are 1,000,000, plus an annual increase equal to the lesser of 1,000,000, 1% of the Company's outstanding capitalization or a lesser amount determined by the Board. The maximum shares that can be issued over the 10-year term of the plan are 10,000,000. As of December 31, 2000, 1,596,092 shares have been authorized under the plan and 461,088 shares have been issued.
Benefit Plan
The Company has available a 401(k) retirement plan in the United States. Eligible employees may contribute up to 15% of their compensation. The Company does not match contributions and therefore no expense has been recorded. The Company also has available a retirement plan in Canada. Eligible employees may contribute an unlimited amount of their compensation.
9. INCOME TAXES
For the year ended December 31, 1999, the Company recorded a tax provision of $1.0 million which represents foreign withholding taxes on certain payments received from Japan Tobacco during the year.
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company's deferred tax assets for federal and state income taxes as of December 31, 2000 are as follows:
| | December 31,
| |
|---|
| | 2000
| | 1999
| |
|---|
| | (in thousands)
| |
|---|
| Deferred tax assets: | | | | | | | |
| | Net operating loss carryforwards | | $ | 52,000 | | $ | 20,900 | |
| | Capitalized research and development | | | 4,900 | | | 2,900 | |
| | Research credit carryforwards | | | 4,800 | | | 2,500 | |
| | Capitalized license agreements | | | 4,800 | | | 6,300 | |
| | Deferred revenue | | | 2,800 | | | — | |
| | Other | | | 800 | | | — | |
| | |
| |
| |
| | Total deferred tax assets | | | 70,100 | | | 32,600 | |
| | Valuation allowance | | | (51,500 | ) | | (32,600 | ) |
| | |
| |
| |
| | Net deferred tax assets | | | 18,600 | | | — | |
| | Deferred tax liabilities: | | | | | | | |
| | Purchased intangibles | | | (18,600 | ) | | — | |
| | |
| |
| |
| | Net deferred taxes | | $ | — | | $ | — | |
| | |
| |
| |
Realization of deferred tax assets is dependent upon future earnings, if any, the timing and amount of which are uncertain. Accordingly, the net deferred tax assets have been fully offset by a valuation allowance. The net valuation allowance increased by $18.9 and $3.0 million during the year ended December 31, 2000 and 1999, respectively. Approximately $39.5 million of the valuation allowance for
F–20
deferred tax assets relates to benefits of stock options. As of December 31, 2000, the Company had net operating loss carryforwards for federal income tax purposes of approximately $148.0 million, which expire in the years 2010 through 2020, and federal research and development tax credits of approximately $3.2 million, which expire in the years 2001 through 2020. Utilization of the Company's net operating loss may be subject to substantial annual limitation due to the ownership change limitations provided by Internal Revenue Code and similar state provisions. Such an annual limitation could result in the expiration of the net operating loss before utilization.
10. SUBSEQUENT EVENTS (UNAUDITED)
Acquisition of ImmGenics
In February 2001, the Company notified the holders of ImmGenics special shares that the purchase price would be settled in cash. As of March 23, 2001, 6,465,378 ImmGenics special shares have been exchanged for $32,114,825 and 7,250,107 of the shares were still outstanding.
Facility Lease and Letter of Credit
In February 2001, the Company signed an operating lease for an additional facility. The lease expires in the year 2011. The Company issued a stand-by letter of credit for $3.0 million to the lessor for the lease term, as a condition to the lease. The letter of credit is secured by cash and marketable securities. Future minimum payments under this non-cancelable operating lease are as follows (in thousands): 2001—$1,981; 2002—$3,049; 2003—$3,171; 2004—$3,298; 2005—$3,430; and thereafter $20,732.
F–21
ABGENIX, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
(in thousands, except share and per share data)
| | June 30,
2001
| | December 31,
2000
| |
|---|
| | (unaudited)
| |
| |
|---|
| ASSETS | |
| Current assets: | | | | | | | |
| | Cash and cash equivalents | | $ | 87,198 | | $ | 167,242 | |
| | Marketable securities | | | 488,170 | | | 525,641 | |
| | Interest receivable | | | 6,062 | | | 9,793 | |
| | Accounts receivable | | | 3,030 | | | 3,397 | |
| | Prepaid expenses and other current assets | | | 12,740 | | | 11,965 | |
| | |
| |
| |
| | | Total current assets | | | 597,200 | | | 718,038 | |
| Property and equipment, net | | | 36,425 | | | 18,374 | |
| Long-term investments | | | 113,861 | | | 79,181 | |
| Intangible assets, net of accumulated amortization | | | 114,834 | | | 117,997 | |
| Deposits and other assets | | | 6,781 | | | 3,210 | |
| | |
| |
| |
| | | $ | 869,101 | | $ | 936,800 | |
| | |
| |
| |
| LIABILITIES AND STOCKHOLDERS' EQUITY | |
| Current liabilities: | | | | | | | |
| | Accounts payable | | $ | 8,085 | | $ | 6,339 | |
| | Deferred revenue | | | 4,330 | | | 6,978 | |
| | Accrued product development costs | | | 1,294 | | | 2,338 | |
| | Accrued employee benefits | | | 2,334 | | | 2,034 | |
| | Other accrued liabilities | | | 6,534 | | | 3,124 | |
| | Current portion of long-term debt | | | 31 | | | 316 | |
| | Acquisition liability | | | 1,950 | | | 75,429 | |
| | |
| |
| |
| | | Total current liabilities | | | 24,558 | | | 96,558 | |
| Deferred rent | | | 1,149 | | | 567 | |
| Commitments | | | | | | | |
| Stockholders' equity: | | | | | | | |
| | Preferred stock, $0.0001 par value; 5,000,000 shares authorized; none issued or outstanding | | | — | | | — | |
| | Common stock, $0.0001 par value; 220,000,000 shares authorized; 86,125,217 and 85,401,548 shares issued and outstanding at June 30, 2001 and December 31, 2000, respectively, at amount paid in | | | 912,531 | | | 906,358 | |
| Additional paid-in capital | | | 32,849 | | | 32,849 | |
| Deferred compensation | | | (65 | ) | | (234 | ) |
| Accumulated other comprehensive income/(loss) | | | 19,101 | | | (705 | ) |
| Accumulated deficit | | | (121,022 | ) | | (98,593 | ) |
| | |
| �� |
| |
| | | Total stockholders' equity | | | 843,394 | | | 839,675 | |
| | |
| |
| |
| | | $ | 869,101 | | $ | 936,800 | |
| | |
| |
| |
See accompanying notes.
F–22
ABGENIX, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except per share data)
(unaudited)
| | Three Months Ended June 30,
| | Six Months Ended June 30,
| |
|---|
| | 2001
| | 2000
| | 2001
| | 2000
| |
|---|
| Revenues: | | | | | | | | | | | | | |
| | Contract revenue | | $ | 8,354 | | $ | 3,478 | | $ | 12,530 | | $ | 5,443 | |
| | Interest and other income | | | 7,664 | | | 8,781 | | | 17,971 | | | 13,122 | |
| | |
| |
| |
| |
| |
| | Total revenues | | | 16,018 | | | 12,259 | | | 30,501 | | | 18,565 | |
| Costs and expenses: | | | | | | | | | | | | | |
| | Research and development | | | 25,628 | | | 11,912 | | | 42,383 | | | 19,126 | |
| | General and administrative | | | 3,131 | | | 1,805 | | | 6,200 | | | 3,390 | |
| | Amortization of intangible assets, related to research and development | | | 2,046 | | | 777 | | | 4,093 | | | 1,553 | |
| | Interest expense | | | — | | | 185 | | | 255 | | | 300 | |
| | |
| |
| |
| |
| |
| | Total costs and expenses | | | 30,805 | | | 14,679 | | | 52,931 | | | 24,369 | |
| | |
| |
| |
| |
| |
| Net loss | | $ | (14,787 | ) | $ | (2,420 | ) | $ | (22,430 | ) | $ | (5,804 | ) |
| | |
| |
| |
| |
| |
| Basic and diluted net loss per share | | $ | (0.17 | ) | $ | (0.03 | ) | $ | (0.26 | ) | $ | (0.07 | ) |
| | |
| |
| |
| |
| |
| Shares used in computing basic and diluted net loss per share | | | 85,947 | | | 80,545 | | | 85,805 | | | 77,522 | |
See accompanying notes.
F–23
ABGENIX, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
(unaudited)
| | Six Months Ended June 30,
| |
|---|
| | 2001
| | 2000
| |
|---|
| Operating activities | | | | | | | |
| Net loss | | $ | (22,430 | ) | $ | (5,804 | ) |
| Adjustments to reconcile net loss to net cash provided by (used in) operating activities: | | | | | | | |
| | Depreciation and amortization | | | 6,236 | | | 2,511 | |
| | Stock options issued to consultants | | | — | | | 595 | |
| | Changes for certain assets and liabilities: | | | | | | | |
| | Interest receivable | | | 3,731 | | | (4,804 | ) |
| | Accounts receivable | | | 367 | | | 3,550 | |
| | Prepaid expenses and other current assets | | | (775 | ) | | (3,864 | ) |
| | Deposits and other assets | | | (3,581 | ) | | (315 | ) |
| | Accounts payable | | | 1,746 | | | 116 | |
| | Deferred revenue | | | (2,648 | ) | | 8,772 | |
| | Accrued product development costs | | | (1,044 | ) | | 524 | |
| | Accrued employee benefits | | | 300 | | | (269 | ) |
| | Other accrued liabilities | | | 3,410 | | | 310 | |
| | Deferred rent | | | 582 | | | 57 | |
| | |
| |
| |
| Net cash provided by (used in) operating activities | | | (14,106 | ) | | 1,379 | |
| | |
| |
| |
| Investing activities | | | | | | | |
| Purchases of marketable securities | | | (621,174 | ) | | (514,607 | ) |
| Maturities of marketable securities | | | 658,872 | | | 127,214 | |
| Capital expenditures | | | (20,148 | ) | | (1,652 | ) |
| Payments for acquisition liabilities | | | (71,145 | ) | | — | |
| Purchases of long-term investments | | | (15,101 | ) | | — | |
| | |
| |
| |
| Net cash used in investing activities | | | (68,696 | ) | | (389,045 | ) |
| | |
| |
| |
| Financing activities | | | | | | | |
| Net proceeds from issuances of common stock | | | 3,043 | | | 500,452 | |
| Payments on long-term debt | | | (285 | ) | | (1,606 | ) |
| | |
| |
| |
| Net cash provided by financing activities | | | 2,758 | | | 498,846 | |
| | |
| |
| |
| Net increase (decrease) in cash and cash equivalents | | | (80,044 | ) | | 111,180 | |
| Cash and cash equivalents at the beginning of the period | | | 167,242 | | | 13,366 | |
| | |
| |
| |
| Cash and cash equivalents at the end of the period | | $ | 87,198 | | $ | 124,546 | |
| | |
| |
| |
See accompanying notes.
F–24
ABGENIX, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2001
1. Basis of Presentation and Summary of Significant Accounting Policies
Basis of Presentation The unaudited condensed consolidated financial statements of Abgenix, Inc. (the "Company" or "Abgenix") included herein have been prepared by the Company pursuant to the rules and regulations of the Securities and Exchange Commission. Certain information or footnote disclosure normally included in financial statements prepared in accordance with generally accepted accounting principles has been condensed or omitted pursuant to such rules and regulations. In the opinion of the Company, the accompanying unaudited condensed consolidated financial statements contain all adjustments, consisting only of normal recurring adjustments, necessary to present fairly the financial information included therein. These financial statements should be read in conjunction with the audited financial statements for the year ended December 31, 2000 and accompanying notes included in the Company's Annual Report as filed on Form 10-K with the Securities and Exchange Commission. The results of operations for the three and six months ended June 30, 2001 are not necessarily indicative of the results to be expected for the full year or for any other future period. The financial statements as of December 31, 2000 are derived from audited financial statements.
Revenue Recognition The Company receives payments from customers for licenses, options and services. These payments are generally non-refundable but are reported as deferred revenue until they are recognizable as revenue. The Company has followed the following principles in recognizing revenue:
- •
- Research license fees: Fees to license the use of the Company's proprietary XenoMouser technology in research performed by customers of the Company are generally recognized only after the license period has commenced and the technology has been delivered. If Abgenix is obligated to provide significant assistance to enable the customer to utilize the license, the revenue is recognized over the period of such obligation.
- •
- Product license fees: Fees to license the production, use and sale of an antibody generated by the XenoMouse technology are generally recognized only after the license period has commenced and the technology has been delivered. If Abgenix is obligated to provide significant assistance to enable the customer to utilize the license, the revenue is recognized over the period of such obligation.
- •
- Option fees: Fees for granting options to customers to obtain a license to develop a product are recognized as revenue when the option is exercised or when the option period expires, whichever occurs first.
- •
- Payments for research services performed by Abgenix are recognized ratably over the period during which these services are performed. However, fees earned for research services under co-development arrangements are recorded as contract revenues in the period services are rendered, net of fees payable by Abgenix to such co-developers for reimbursements of research and development costs.
- •
- Milestone payments are recognized as revenue when the milestone is achieved.
Earnings per Share Net loss per share is based on the weighted average common shares outstanding. Potentially dilutive securities have been excluded from the computation, as their effect is antidilutive.
F–25
2. Comprehensive Income (Loss)
Other comprehensive income/(loss) consists of unrealized gains or losses on available-for-sale securities. The components of comprehensive income/(loss) were as follows (in thousands):
| | Three Months Ended
June 30,
| | Six Months Ended
June 30,
| |
|---|
| | 2001
| | 2000
| | 2001
| | 2000
| |
|---|
| Net income (loss) | | $ | (14,787 | ) | $ | (2,420 | ) | $ | (22,430 | ) | $ | (5,804 | ) |
| Increase (decrease) in net unrealized gains on available-for-sale investments | | | 34,637 | | | (7,331 | ) | | 19,806 | | | 2,727 | |
| | |
| |
| |
| |
| |
| Comprehensive income (loss) | | $ | 19,850 | | $ | (9,751 | ) | $ | (2,624 | ) | $ | (3,077 | ) |
| | |
| |
| |
| |
| |
3. Acquisition of Abgenix Biopharma Inc. (formerly known as ImmGenics Pharmaceuticals, Inc.)
In November 2000, the Company acquired all of the voting stock of Abgenix Biopharma Inc. (formerly known as ImmGenics Pharmaceuticals Inc.), a Canadian biotechnology company. Under the terms of the agreement, Abgenix Biopharma special shares were issued to former common and preferred shareholders and debenture holders of Abgenix Biopharma. The holders of the Abgenix Biopharma special shares have the right to put their shares to the Company for cash at $4.97 per share. In February 2001, the Company notified the holders of the special shares that the purchase price would be settled in cash. The put rights will expire on March 31, 2002. As of June 30, 2001, approximately 13.5 million Abgenix Biopharma special shares had been exchanged for $67.0 million and approximately 200,000 special shares were still outstanding.
In connection with the acquisition, the Company agreed to exchange Abgenix Biopharma stock options held by employees and directors of Abgenix Biopharma for stock options of the Company, based on an exchange ratio that entitled the holder of each Abgenix Biopharma option to receive a replacement option for Company shares having a total value (less the total exercise price) not exceeding the total value of Abgenix Biopharma shares underlying the Abgenix Biopharma option (less the total exercise price), as fixed in November 2000 when the Abgenix Biopharma options were terminated. Replacement options covering a total of 247,155 shares of common stock of the Company were issued in exchange for the Abgenix Biopharma options. The replacement options were fully vested at the time of the exchange. Pursuant to the Company's stock option plan, the Company also offered the employees and certain former directors of Abgenix Biopharma a cash buy-out election. As of June 30, 2001, option holders had exercised their cash buy-out rights in respect of options for 137,073 Company shares pursuant to which a total of $4.1 million in cash was paid. In addition, options representing 84,009 Company shares had been exercised as of that date. As of August 1, 2001, options for an additional 22,340 shares had been cashed out for $724,286, options covering 3,733 shares had been exercised, and no options remained outstanding.
4. Facility Lease and Letter of Credit
In February 2001, the Company signed an operating lease for an additional facility for research and development activities. The lease expires in April 2011. As a condition to the lease, the Company provided a stand-by letter of credit for $3.0 million to the lessor as security for the Company's obligations under the lease. The letter of credit is secured by $3.3 million of cash and marketable
F–26
securities in an investment account that the Company must maintain for the term of the lease. The investment account is classified as deposits and other assets on the balance sheet. Future minimum payments under this non-cancelable operating lease are as follows (in thousands): 2001—$1,485; 2002—$3,049; 2003—$3,171; 2004—$3,298; 2005—$3,430; and $20,732 over the remaining term of the lease.
5. Segment Information
The operations of the Company and its wholly owned subsidiaries constitute one business segment.
Revenue from three customers represented 62%, 14% and 13% respectively, of contract revenues for the three months ended June 30, 2001, compared with two customers that represented 86% and 11% respectively, in the same period in 2000. Revenue from three customers represented 49%, 23% and 10%, respectively, of contract revenues for the six months ended June 30, 2001, compared with two customers that represented 75% and 14% respectively, in the same period.
F–27
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth the costs and expenses, payable by us in connection with the registration of the securities being registered by this prospectus. All amounts are estimates except the SEC registration fee.
| SEC registration fee | | $ | 86,905 |
| Printing and engraving expenses | | | 150,000 |
| Legal fees and expenses | | | 100,000 |
| Accounting fees and expenses | | | 50,000 |
| Transfer Agent and Registrar fees | | | 10,000 |
| Miscellaneous fees and expenses | | | 103,095 |
| | |
|
| | Total | | $ | 500,000 |
| | |
|
Item 14. Indemnification of Directors and Officers
Section 145 of the Delaware General Corporation Law allows for the indemnification of officers, directors and any corporate agents in terms sufficiently broad to indemnify such persons under certain circumstances for liabilities (including reimbursement for expenses incurred) arising under the Securities Act. Our amended and restated certificate of incorporation and our amended and restated bylaws provide for indemnification of our directors, officers, employees and other agents to the extent and under the circumstances permitted by the Delaware General Corporation Law. We have also entered into agreements with our directors and executive officers that require us among other things to indemnify them against certain liabilities that may arise by reason of their status or service as directors and executive officers to the fullest extent permitted by Delaware law. We have also purchased directors and officers liability insurance, which provides coverage against certain liabilities including liabilities under the Securities Act.
Item 15. Recent Sales of Unregistered Securities
(a) Since our incorporation on June 24, 1996, we have issued and sold the following unregistered securities:
(1) On July 15, 1996, we issued 1,691,667 shares of series A senior convertible preferred stock to Cell Genesys in exchange for $10.0 million.
(2) On July 15, 1996, we issued 2,058,333 shares of series 1 subordinated convertible preferred stock to Cell Genesys, and in exchange, Cell Genesys contributed research, development and manufacturing technology, as well as patents and other intellectual property specific to the antibody therapy programs to be pursued by us, including Cell Genesys' interest in its joint venture with Japan Tobacco.
(3) On July 15, 1996, we, in exchange for a loan in the principal amount of up to $4,000,000, issued a convertible promissory note to Cell Genesys convertible at an exercise price per share of $6.00 into up to 666,667 shares of series A convertible preferred stock.
(4) From July 15, 1996 to October 22, 1998, we granted options to purchase 2,156,295 shares of common stock to employees, directors and consultants under the 1996 Incentive Stock Plan at exercise prices ranging from $0.60 to $10.00 per share.
(5) On January 23, 1997 and March 27, 1997, we issued two warrants to purchase an aggregate of 121,667 shares of series A senior convertible preferred stock, convertible into 121,667
II–1
shares of common stock, to Cell Genesys with a weighted average exercise price per share of $6.00.
(6) On December 23, 1997, we issued 3,267,685 shares of series B preferred stock to 29 accredited or institutional purchasers at a purchase price per share of $6.50. In connection with and contemporaneous to this transaction the 1,691,667 shares of series A senior convertible preferred stock, the 2,058,333 shares of series 1 subordinated convertible preferred stock and the $4,000,000 convertible promissory note issued to Cell Genesys, described above, were all converted into an aggregate 4,416,667 shares of series A convertible preferred stock.
(7) On January 12, 1998, we issued 160,000 shares of series C preferred stock to Pfizer at a per share purchase price of $8.00. This issuance was in connection with a contractual arrangement entered into between Abgenix and Pfizer.
(8) On January 27, 1999, we issued 495,356 shares of common stock to Genentech at a per share purchase price of $16.15. This issuance was in connection with a multi-antigen research license and option agreement entered into between us and Genentech.
(9) On November 19, 1999, we issued 1,778,000 shares of common stock in a private placement to certain individuals and entities at a per share purchase price of $42.00.
(10) On November 6, 2000, we issued 3,300,000 shares of common stock in a private placement to certain individuals and entities at a per share purchase price of $70.
The sales and issuances of securities in the transactions described above were deemed to be exempt from registration under the Securities Act in reliance upon Section 4(2) of the Securities Act, Regulation D promulgated thereunder, or Rule 701 promulgated under Section 3(b) of the Securities Act, as transactions by an issuer not involving any public offering or transactions pursuant to compensatory benefit plans and contracts relating to compensation as provided under Rule 701. The recipients of securities in each transaction represented their intentions to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof and appropriate legends were affixed to the securities issued in such transactions. All recipients had adequate access, through their relationship with us, to information about us.
(b) There were no underwritten offerings employed in connection with any of the transactions set forth in Item 15(a).
Item 16. Exhibits and Financial Statement Schedules
Number
| | Description
|
|---|
| 3.1(1) | | Amended and Restated Certificate of Incorporation of Abgenix, as currently in effect. |
| 3.2(28) | | Amended and Restated Bylaws of Abgenix, as currently in effect. |
| 4.1(1) | | Specimen Common Stock Certificate. |
| 5.1* | | Form of Legal Opinion of O'Melveny & Myers LLP. |
| 10.1(1) | | Form of Indemnification Agreement between Abgenix and each of its directors and officers. |
| 10.2(1) | | 1996 Incentive Stock Plan and form of agreement thereunder. |
| 10.3(1) | | 1998 Employee Stock Purchase Plan and form of agreement thereunder. |
| 10.4(1) | | 1998 Director Option Plan and form of agreement thereunder. |
| 10.4.1(28) | | 1998 Director Option Plan, as amended effective April 2, 2001. |
| 10.5(24) | | 1999 Nonstatutory Stock Option Plan and form of agreement thereunder. |
| 10.6(1) | | Warrant dated January 23, 1997 exercisable for shares of Series A Preferred Stock. |
| 10.7(1) | | Warrant dated March 27, 1997 exercisable for shares of Series A Preferred Stock. |
II–2
| 10.8(3) | | Joint Venture Agreement dated June 12, 1991 between Cell Genesys and JT Immunotech USA Inc. |
| 10.8A(6) | | Amendment No. 1 dated January 1, 1994 to Joint Venture Agreement. |
| 10.8B(9) | | Amendment No. 2 dated June 28, 1996 to Joint Venture Agreement. |
| 10.9(3) | | Collaboration Agreement dated June 12, 1991 among Cell Genesys, Xenotech, Inc. and JT Immunotech USA Inc. |
| 10.9A(5) | | Amendment No. 1 dated June 30, 1993 to Collaboration Agreement. |
| 10.9B(13) | | Amendment No. 2 dated January 1, 1994 to Collaboration Agreement. |
| 10.9C(7) | | Amendment No. 3 dated July 1, 1995 to Collaboration Agreement. |
| 10.9D(9) | | Amendment No. 4 dated June 28, 1996 to Collaboration Agreement. |
| 10.9E(2) | | Amendment No. 5 dated November 1997 to Collaboration Agreement. |
| 10.10(3) | | Limited Partnership Agreement dated June 12, 1991 among Cell Genesys, Xenotech, Inc. and JT Immunotech USA Inc. |
| 10.10A(6) | | Amendment No. 2 dated January 1, 1994 to Limited Partnership Agreement. |
| 10.10B(8) | | Amendment No. 3 dated July 1, 1995 to Limited Partnership Agreement. |
| 10.10C(10) | | Amendment No. 4 dated June 28, 1996 to Limited Partnership Agreement. |
| 10.11(4) | | Field License dated June 12, 1991 among Cell Genesys, JT Immunotech USA Inc. and Xenotech, L.P. |
| 10.11A(10) | | Amendment No. 1 dated March 22, 1996 to Field License. |
| 10.11B(10) | | Amendment No. 2 dated June 28, 1996 to Field License. |
| 10.12(3) | | Expanded Field License dated June 12, 1991 among Cell Genesys, JT Immunotech USA Inc. and Xenotech, L.P. |
| 10.12A(10) | | Amendment No. 1 dated June 28, 1996 to Expanded Field License. |
| 10.13(2) | | Amended and Restated Anti-IL-8 License Agreement dated March 19, 1996 among Xenotech, L.P., Cell Genesys and Japan Tobacco Inc. |
| 10.14(9) | | Master Research License and Option Agreement dated June 28, 1996 among Cell Genesys, Japan Tobacco Inc. and Xenotech, L.P. |
| 10.14A(2) | | Amendment No. 1 dated November 1997 to the Master Research License and Option Agreement. |
| 10.15(2) | | Stock Purchase and Transfer Agreement dated July 15, 1996 by and between Cell Genesys and Abgenix. |
| 10.16(1) | | Governance Agreement dated July 15, 1996 between Cell Genesys and Abgenix. |
| 10.16A(1) | | Amendment No. 1 dated October 13, 1997 to the Governance Agreement. |
| 10.16B(1) | | Amendment No. 2 dated December 22, 1997 to the Governance Agreement. |
| 10.17(1) | | Tax Sharing Agreement dated July 15, 1996 between Cell Genesys and Abgenix. |
| 10.18(2) | | Gene Therapy Rights Agreement effective as of November 1, 1997 between Abgenix and Cell Genesys. |
| 10.19(2) | | Patent Assignment Agreement dated July 15, 1996 by Cell Genesys in favor of Abgenix. |
| 10.20(11) | | Lease Agreement dated July 31, 1996 between John Arrillaga, Trustee, or his Successor Trustee, UTA dated 7/20/77 (Arrillaga Family Trust) as amended, and Richard T. Peery, Trustee, or his Successor Trustee, UTA dated 7/20/77 (Richard T. Peery Separate Property Trust) as amended, and Abgenix. |
| 10.21(1) | | Loan and Security Agreement dated January 23, 1997 between Silicon Valley Bank and Abgenix. |
| 10.22(1) | | Master Lease Agreement dated March 27, 1997 between Transamerica Business Credit Corporation and Abgenix. |
| 10.23(2) | | License Agreement dated February 1, 1997 between Ronald J. Billing, Ph.D. and Abgenix. |
II–3
| 10.24(12) | | Release and Settlement Agreement dated March 26, 1997 among Cell Genesys, Abgenix, Xenotech, L.P., Japan Tobacco Inc. and GenPharm International, Inc. |
| 10.25(12) | | Cross License Agreement effective as of March 26, 1997, among Cell Genesys, Abgenix, Xenotech, L.P., Japan Tobacco Inc. and GenPharm International, Inc. |
| 10.26(12) | | Interference Settlement Procedure Agreement, effective as of March 26, 1997, among Cell Genesys, Abgenix, Xenotech, L.P., Japan Tobacco Inc. and GenPharm International, Inc. |
| 10.27(2) | | Agreement dated March 26, 1997 among Xenotech, L.P., Xenotech, Inc., Cell Genesys, Abgenix, Japan Tobacco Inc. and JT Immunotech USA Inc. |
| 10.28(2) | | Contractual Research Agreement dated December 22, 1997 between Pfizer, Inc. and Abgenix. |
| +10.28A(22) | | Amendment No. 1 dated May 26, 1998 to Contractual Research Agreement between Abgenix and Pfizer, Inc. |
| +10.28B(22) | | Amendment No. 2 dated October 22, 1998 to Contractual Research Agreement between Abgenix and Pfizer, Inc. |
| 10.29(1) | | Amended and Restated Stockholder Rights Agreement dated January 12, 1998 among Abgenix and certain holders of Abgenix's capital stock. |
| 10.30(2) | | Contractual Research Agreement effective as of January 28, 1998 between Schering-Plough Research Institute and Abgenix. |
| 10.30A(16) | | Amendment No. 2 effective January 28, 1999 to Contractual Research Agreement between Schering-Plough Research Institute and Abgenix. |
| 10.30B(16) | | Amendment No. 3 effective February 12, 1999 to the Contractual Research Agreement between Schering-Plough Research Institute and Abgenix. |
| 10.31(1) | | Excerpts from the Minutes of a Meeting of the Board of Directors of Abgenix, dated October 23, 1996. |
| 10.32(1) | | Excerpts from the Minutes of a Meeting of the Board of Directors of Abgenix, dated October 22, 1997. |
| 10.33(2) | | Exclusive Worldwide Product License dated November 1997 between Xenotech, L.P. and Abgenix. |
| 10.34(2) | | Research License and Option Agreement effective as of April 6, 1998 between Abgenix and Genentech, Inc. |
| 10.34A(2) | | Amendment No. 1 effective as of June 18, 1998 to Research License and Option Agreement between Abgenix and Genentech, Inc. |
| 10.35(14) | | Research Collaboration Agreement dated July 15, 1998 between Millennium BioTherapeutics, Inc. and Abgenix. |
| +10.36(22) | | Research Collaboration Agreement dated September 29, 1998 between Millennium BioTherapeutics, Inc. and Abgenix. |
| 10.36A(22) | | Amendment No. 1 effective as of November 29, 1998 to the Research Collaboration Agreement between Millennium BioTherapeutics, Inc. and Abgenix. |
| +10.37(22) | | Research License and Option Agreement dated October 30, 1998 between Millennium BioTherapeutics, Inc. and Abgenix. |
| 10.38(16) | | Research Collaboration Agreement dated December 22, 1998 between Centocor, Inc. and Abgenix. |
| +10.39(22) | | Memorandum of Understanding between Research Corporation Technologies, Inc. and Abgenix. |
| 10.40(15) | | Registration Rights Agreement dated November 18, 1998 between the selling stockholders and Abgenix. |
| +10.41(22) | | Research License and Option Agreement dated January 4, 1999 between AVI BioPharma, Inc. and Abgenix. |
II–4
| 10.42(17) | | Registration Rights Agreement dated January 27, 1999 between Genentech and Abgenix. |
| 10.43(16) | | Multi-Antigen Research License and Option Agreement dated January 27, 1999 between Genentech and Abgenix. |
| 10.44(18) | | Preferred Shares Rights Agreement, dated as of June 14, 1999, between Abgenix and ChaseMellon Shareholder Services, L.L.C., including the Certificate of Determinations, the Form of Rights Certificate and the Summary of Rights attached thereto as Exhibits A, B and C, respectively. |
| +10.45(21) | | Multi-Antigen Research License and Option Agreement by and between Abgenix, Inc. and Japan Tobacco Inc. effective December 31, 1999. |
| +10.46(21) | | Amended and Restated Field License by and among Abgenix, Inc., JT America Inc. and Xenotech L.P. effective December 31, 1999. |
| 10.47(21) | | Agreement to Terminate the Collaboration Agreement by and among Abgenix, Inc., JT America Inc., and Xenotech L.P. effective December 31, 1999. |
| +10.48(21) | | Agreement to Terminate the Interest of Japan Tobacco Inc. in the Master Research License and Option Agreement by and among Abgenix, Inc., Japan Tobacco Inc. and Xenotech L.P. effective December 31, 1999. |
| +10.49(21) | | Amendment of the Expanded Field License by and among Abgenix, Inc., JT America Inc. and Xenotech L.P. effective December 31, 1999. |
| 10.50(21) | | Limited Partnership Interest and Stock Purchase Agreement between Abgenix, Inc. and JT America Inc. made December 20, 1999. |
| +10.51(21) | | License Agreement by and between Abgenix, Inc. and Japan Tobacco Inc. effective December 31, 1999. |
| 10.52(18) | | Amended Preferred Shares Rights Agreement, dated as of November 19, 1999, between Abgenix, Inc. and Chase Mellon Shareholder Services, L.L.C., including the Certificate of Determination, the form of Rights Certificate and the Summary of Rights attached thereto as Exhibits A, B and C, respectively. |
| 10.53(19) | | 1999 Nonstatutory Stock Option Plan and form of agreement thereunder. |
| 10.54(20) | | Registration Rights Agreement dated November 19, 1999 by and among Abgenix and the selling stockholders. |
| 10.55(23) | | Lease Agreement dated February 24, 2000 between Ardenwood Corporate Park Associates, a California Limited Partnership and Abgenix, Inc. |
| 10.56(23) | | Lease Agreement dated May 19, 2000 between Ardenwood Corporate Park Associates, a California Limited Partnership and Abgenix, Inc. |
| 10.57(23) | | Amendments to 1996 Incentive Stock Option Plan and 1999 Nonstatutory Stock Option Plan to eliminate the Company's ability to reprice issued and outstanding options. |
| 10.58(25) | | Acquisition Agreement dated as of September 25, 2000 among Abgenix, Inc., Abgenix Canada Corporation and ImmGenics Pharmaceuticals Inc. |
| 10.59(25) | | Voting, Exchange and Cash Put Trust Agreement dated as of November 3, 2000 among Abgenix, Inc., ImmGenics Pharmaceuticals Inc. and CIBC Mellon Trust Company. |
| 10.60(25) | | Support Agreement dated as of November 3, 2000 among Abgenix, Inc., Abgenix Canada Corporation and ImmGenics Pharmaceuticals Inc. |
| 10.61(25) | | Form of Stock Purchase Agreement between Abgenix, Inc. and the purchasers in the private placement in November 2000. |
| +10.62(26) | | License Agreement among BR Centre Limited, Imgenix Biomedical Inc. and Dr. John W. Schrader, dated May 9, 1994. |
| +10.63(26) | | License Agreement Amendment among BR Centre Limited, Imgenix Biomedical Inc. and Dr. John W. Schrader, dated May 9, 1994. |
II–5
| 10.64(26) | | Assignment Agreement among BR Centre Limited and The University of British Columbia Foundation, dated March 10, 1998. |
| 10.65(27) | | Lease Agreement dated February 14, 2001 between AMB Property, L.P., a Delaware limited partnership, and Abgenix, Inc. |
| ++10.66(27) | | Product Supply Agreement by and between Lonza Biologics PLC and Abgenix, Inc. dated November 30, 2000 |
| 21.1* | | List of subsidiaries. |
| 23.1 | | Consent of Ernst & Young LLP, Independent Auditors. |
| 23.2** | | Consent of O'Melveny & Myers LLP (included in Exhibit 5.1 to this Registration Statement). |
| 24.1* | | Power of Attorney. |
- *
- Previously filed.
- **
- Previously filed as Exhibit 23.3 to Abgenix's Registration Statement on Form S-1 (File No. 333- 49858), filed with the Commission on November 13, 2000.
- +
- Confidential treatment granted for portions of these exhibits. Omitted portions have been filed separately with the Commission.
- ++
- Confidential treatment has been requested for portions of this exhibit. Omitted portions have been filed separately with the Commission.
- (1)
- Incorporated by reference to the same exhibit filed with Abgenix's Registration Statement on Form S-1 (File No. 333-49415).
- (2)
- Incorporated by reference to the same exhibit filed with Abgenix 's Registration Statement on Form S-1 (File No. 333-49415), portions of which have been granted confidential treatment.
- (3)
- Incorporated by reference to the same exhibit filed with Cell Genesys' Registration Statement on Form S-1 (File No. 33-46452), portions of which have been granted confidential treatment.
- (4)
- Incorporated by reference to the same exhibit filed with Cell Genesys' Registration Statement on Form S-1 (File No. 33-46452).
- (5)
- Incorporated by reference to the same exhibit filed with Cell Genesys' Quarterly Report on Form 10-Q for the quarter ended June 30, 1993, portions of which have been granted confidential treatment.
- (6)
- Incorporated by reference to the same exhibit filed with Cell Genesys' Annual Report on Form 10-K for the year ended December 31, 1993, portions of which have been granted confidential treatment.
- (7)
- Incorporated by reference to the same exhibit filed with Cell Genesys' Quarterly Report on Form 10-Q for the quarter ended June 30, 1995, portions of which have been granted confidential treatment.
- (8)
- Incorporated by reference to the same exhibit filed with Cell Genesys' Quarterly Report on Form 10-Q for the quarter ended June 30, 1995.
- (9)
- Incorporated by reference to the same exhibit filed with Cell Genesys' Quarterly Report on Form 10-Q for the quarter ended June 30, 1996, portions of which have been granted confidential treatment.
- (10)
- Incorporated by reference to the same exhibit filed with Cell Genesys' Quarterly Report on Form 10-Q for the quarter ended June 30, 1996.
II–6
- (11)
- Incorporated by reference to the same exhibit filed with Cell Genesys' Quarterly report on Form 10-Q for the quarter ended September 30, 1996.
- (12)
- Incorporated by reference to the same exhibit filed with Cell Genesys' Annual Report on Form 10-K for the year ended December 31, 1996, as amended, portions of which have been granted confidential treatment.
- (13)
- Incorporated by reference to the same exhibit filed with Cell Genesys' Annual Report on Form 10-K for the year ended December 31, 1993.
- (14)
- Incorporated by reference to the same exhibit filed with Abgenix's Current Report on Form 8-K filed with the Commission on July 17, 1998, portions of which have been granted confidential treatment.
- (15)
- Incorporated by reference to the same exhibit filed with Abgenix's Current Report on Form 8-K filed with the Commission on November 24, 1998.
- (16)
- Incorporated by reference to the same exhibit filed with Abgenix's Registration Statement on Form S-1 (File No. 333-71289), portions for which Abgenix has requested confidential treatment.
- (17)
- Incorporated by reference to the same exhibit filed with Abgenix's Registration Statement on Form S-1 (File No. 333-71289).
- (18)
- Incorporated by reference to the same exhibit filed with Abgenix's Registration Statement on Form 8-A (File No. 000-24207).
- (19)
- Incorporated by reference to the same exhibit filed with Abgenix's Registration Statement on Form S-8 (File No. 333-90707).
- (20)
- Incorporated by reference to the same exhibit filed with Abgenix's Registration Statement on Form S-3 (File No. 333-91699).
- (21)
- Incorporated by reference to the same exhibits filed with Abgenix's Current Report on Form 8-K filed with the Commission on January 27, 2000.
- (22)
- Incorporated by reference to the same exhibits filed with Abgenix's Registration Statement on Form S-1 (File No. 333-70631).
- (23)
- Incorporated by reference to the same exhibits filed with Abgenix's Quarterly report on Form 10-Q for the quarter ended June 30, 2000.
- (24)
- Incorporated by reference to the same exhibit filed with Abgenix's Registration Statement on Form S-8 (File No. 333-45426).
- (25)
- Incorporated by reference to the same exhibits filed with Abgenix's Quarterly report on Form 10-Q for the quarter ended September 30, 2000.
- (26)
- Incorporated by reference to the same exhibits filed with Abgenix's Annual Report on Form 10-K for the year ended December 31, 2000.
- (27)
- Incorporated by reference to the same exhibits filed with Abgenix's Quarterly Report on Form 10-Q for the quarter ended March 31, 2001.
- (28)
- Incorporated by reference to the same exhibits filed with Abgenix's Quarterly Report on Form 10-Q for the quarter ended June 30, 2001.
- (b)
- Financial Statement Schedules
Schedules not listed above have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto.
II–7
Item 17. Undertakings
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the provisions described in Item 14, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act, and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer, or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post- effective amendment to this registration statement:
- (i)
- To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
- (ii)
- To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20 percent change in the maximum aggregate offering price set forth in the "Calculation of Registration Fee" table in the effective registration statement.
- (iii)
- To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(5) That, for the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II–8
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this post-effective amendment number 1 to this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the city of Fremont, State of California on August 30, 2001.
| | | ABGENIX, INC. |
|
|
By: |
/s/ R. SCOTT GREER |
| | | |
Name:R. Scott Greer
Title:Chief Executive Officer and Chairman of the Board |
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in their capacities and on the dates indicated:
Signature
| | Title
| |
|
|---|
|
|
|
|
|
/s/R. SCOTT GREER
R. Scott Greer |
|
Chief Executive Officer and Chairman of the Board (Principal Executive Officer) |
|
August 30, 2001 |
/s/KURT W. LEUTZINGER
Kurt W. Leutzinger |
|
Chief Financial Officer (Principal Financial and Accounting Officer) |
|
August 30, 2001 |
/s/M. KATHLEEN BEHRENS, PH.D.*
M. Kathleen Behrens, Ph.D. |
|
Director |
|
August 30, 2001 |
/s/RAJU S. KUCHERLAPATI, PH.D.*
Raju S. Kucherlapati, Ph.D. |
|
Director |
|
August 30, 2001 |
/s/MARK B. LOGAN*
Mark B. Logan |
|
Director |
|
August 30, 2001 |
/s/JOSEPH E. MAROUN*
Joseph E. Maroun |
|
Director |
|
August 30, 2001 |
/s/STEPHEN A. SHERWIN, M.D.*
Stephen A. Sherwin, M.D.* |
|
Director |
|
August 30, 2001 |
|
|
|
|
|
|
*By: |
/s/KURT W. LEUTZINGER |
| | | |
Name:Kurt W. Leutzinger
Title:Attorney-in-Fact |
II–9
EXHIBIT INDEX
Number
| | Description
|
|---|
| 3.1(1) | | Amended and Restated Certificate of Incorporation of Abgenix, as currently in effect. |
| 3.2(28) | | Amended and Restated Bylaws of Abgenix, as currently in effect. |
| 4.1(1) | | Specimen Common Stock Certificate. |
| 5.1* | | Form of Legal Opinion of O'Melveny & Myers LLP. |
| 10.1(1) | | Form of Indemnification Agreement between Abgenix and each of its directors and officers. |
| 10.2(1) | | 1996 Incentive Stock Plan and form of agreement thereunder. |
| 10.3(1) | | 1998 Employee Stock Purchase Plan and form of agreement thereunder. |
| 10.4(1) | | 1998 Director Option Plan and form of agreement thereunder. |
| 10.4.1(28) | | 1998 Director Option Plan, as amended effective April 26, 2001. |
| 10.5(24) | | 1999 Nonstatutory Stock Option Plan and form of agreement thereunder. |
| 10.6(1) | | Warrant dated January 23, 1997 exercisable for shares of Series A Preferred Stock. |
| 10.7(1) | | Warrant dated March 27, 1997 exercisable for shares of Series A Preferred Stock. |
| 10.8(3) | | Joint Venture Agreement dated June 12, 1991 between Cell Genesys and JT Immunotech USA Inc. |
| 10.8A(6) | | Amendment No. 1 dated January 1, 1994 to Joint Venture Agreement. |
| 10.8B(9) | | Amendment No. 2 dated June 28, 1996 to Joint Venture Agreement. |
| 10.9(3) | | Collaboration Agreement dated June 12, 1991 among Cell Genesys, Xenotech, Inc. and JT Immunotech USA Inc. |
| 10.9A(5) | | Amendment No. 1 dated June 30, 1993 to Collaboration Agreement. |
| 10.9B(13) | | Amendment No. 2 dated January 1, 1994 to Collaboration Agreement. |
| 10.9C(7) | | Amendment No. 3 dated July 1, 1995 to Collaboration Agreement. |
| 10.9D(9) | | Amendment No. 4 dated June 28, 1996 to Collaboration Agreement. |
| 10.9E(2) | | Amendment No. 5 dated November 1997 to Collaboration Agreement. |
| 10.10(3) | | Limited Partnership Agreement dated June 12, 1991 among Cell Genesys, Xenotech, Inc. and JT Immunotech USA Inc. |
| 10.10A(6) | | Amendment No. 2 dated January 1, 1994 to Limited Partnership Agreement. |
| 10.10B(8) | | Amendment No. 3 dated July 1, 1995 to Limited Partnership Agreement. |
| 10.10C(10) | | Amendment No. 4 dated June 28, 1996 to Limited Partnership Agreement. |
| 10.11(4) | | Field License dated June 12, 1991 among Cell Genesys, JT Immunotech USA Inc. and Xenotech, L.P. |
| 10.11A(10) | | Amendment No. 1 dated March 22, 1996 to Field License. |
| 10.11B(10) | | Amendment No. 2 dated June 28, 1996 to Field License. |
| 10.12(3) | | Expanded Field License dated June 12, 1991 among Cell Genesys, JT Immunotech USA Inc. and Xenotech, L.P. |
| 10.12A(10) | | Amendment No. 1 dated June 28, 1996 to Expanded Field License. |
| 10.13(2) | | Amended and Restated Anti-IL-8 License Agreement dated March 19, 1996 among Xenotech, L.P., Cell Genesys and Japan Tobacco Inc. |
| 10.14(9) | | Master Research License and Option Agreement dated June 28, 1996 among Cell Genesys, Japan Tobacco Inc. and Xenotech, L.P. |
| 10.14A(2) | | Amendment No. 1 dated November 1997 to the Master Research License and Option Agreement. |
| 10.15(2) | | Stock Purchase and Transfer Agreement dated July 15, 1996 by and between Cell Genesys and Abgenix. |
| 10.16(1) | | Governance Agreement dated July 15, 1996 between Cell Genesys and Abgenix. |
| 10.16A(1) | | Amendment No. 1 dated October 13, 1997 to the Governance Agreement. |
| 10.16B(1) | | Amendment No. 2 dated December 22, 1997 to the Governance Agreement. |
| 10.17(1) | | Tax Sharing Agreement dated July 15, 1996 between Cell Genesys and Abgenix. |
| 10.18(2) | | Gene Therapy Rights Agreement effective as of November 1, 1997 between Abgenix and Cell Genesys. |
| 10.19(2) | | Patent Assignment Agreement dated July 15, 1996 by Cell Genesys in favor of Abgenix. |
| 10.20(11) | | Lease Agreement dated July 31, 1996 between John Arrillaga, Trustee, or his Successor Trustee, UTA dated 7/20/77 (Arrillaga Family Trust) as amended, and Richard T. Peery, Trustee, or his Successor Trustee, UTA dated 7/20/77 (Richard T. Peery Separate Property Trust) as amended, and Abgenix. |
| 10.21(1) | | Loan and Security Agreement dated January 23, 1997 between Silicon Valley Bank and Abgenix. |
| 10.22(1) | | Master Lease Agreement dated March 27, 1997 between Transamerica Business Credit Corporation and Abgenix. |
| 10.23(2) | | License Agreement dated February 1, 1997 between Ronald J. Billing, Ph.D. and Abgenix. |
| 10.24(12) | | Release and Settlement Agreement dated March 26, 1997 among Cell Genesys, Abgenix, Xenotech, L.P., Japan Tobacco Inc. and GenPharm International, Inc. |
| 10.25(12) | | Cross License Agreement effective as of March 26, 1997, among Cell Genesys, Abgenix, Xenotech, L.P., Japan Tobacco Inc. and GenPharm International, Inc. |
| 10.26(12) | | Interference Settlement Procedure Agreement, effective as of March 26, 1997, among Cell Genesys, Abgenix, Xenotech, L.P., Japan Tobacco Inc. and GenPharm International, Inc. |
| 10.27(2) | | Agreement dated March 26, 1997 among Xenotech, L.P., Xenotech, Inc., Cell Genesys, Abgenix, Japan Tobacco Inc. and JT Immunotech USA Inc. |
| 10.28(2) | | Contractual Research Agreement dated December 22, 1997 between Pfizer, Inc. and Abgenix. |
| +10.28A(22) | | Amendment No. 1 dated May 26, 1998 to Contractual Research Agreement between Abgenix and Pfizer, Inc. |
| +10.28B(22) | | Amendment No. 2 dated October 22, 1998 to Contractual Research Agreement between Abgenix and Pfizer, Inc. |
| 10.29(1) | | Amended and Restated Stockholder Rights Agreement dated January 12, 1998 among Abgenix and certain holders of Abgenix's capital stock. |
| 10.30(2) | | Contractual Research Agreement effective as of January 28, 1998 between Schering-Plough Research Institute and Abgenix. |
| 10.30A(16) | | Amendment No. 2 effective January 28, 1999 to Contractual Research Agreement between Schering-Plough Research Institute and Abgenix. |
| 10.30B(16) | | Amendment No. 3 effective February 12, 1999 to the Contractual Research Agreement between Schering-Plough Research Institute and Abgenix. |
| 10.31(1) | | Excerpts from the Minutes of a Meeting of the Board of Directors of Abgenix, dated October 23, 1996. |
| 10.32(1) | | Excerpts from the Minutes of a Meeting of the Board of Directors of Abgenix, dated October 22, 1997. |
| 10.33(2) | | Exclusive Worldwide Product License dated November 1997 between Xenotech, L.P. and Abgenix. |
| 10.34(2) | | Research License and Option Agreement effective as of April 6, 1998 between Abgenix and Genentech, Inc. |
| 10.34A(2) | | Amendment No. 1 effective as of June 18, 1998 to Research License and Option Agreement between Abgenix and Genentech, Inc. |
| 10.35(14) | | Research Collaboration Agreement dated July 15, 1998 between Millennium BioTherapeutics, Inc. and Abgenix. |
| +10.36(22) | | Research Collaboration Agreement dated September 29, 1998 between Millennium BioTherapeutics, Inc. and Abgenix. |
| 10.36A(22) | | Amendment No. 1 effective as of November 29, 1998 to the Research Collaboration Agreement between Millennium BioTherapeutics, Inc. and Abgenix. |
| +10.37(22) | | Research License and Option Agreement dated October 30, 1998 between Millennium BioTherapeutics, Inc. and Abgenix. |
| 10.38(16) | | Research Collaboration Agreement dated December 22, 1998 between Centocor, Inc. and Abgenix. |
| +10.39(22) | | Memorandum of Understanding between Research Corporation Technologies, Inc. and Abgenix. |
| 10.40(15) | | Registration Rights Agreement dated November 18, 1998 between the selling stockholders and Abgenix. |
| +10.41(22) | | Research License and Option Agreement dated January 4, 1999 between AVI BioPharma, Inc. and Abgenix. |
| 10.42(17) | | Registration Rights Agreement dated January 27, 1999 between Genentech and Abgenix. |
| 10.43(16) | | Multi-Antigen Research License and Option Agreement dated January 27, 1999 between Genentech and Abgenix. |
| 10.44(18) | | Preferred Shares Rights Agreement, dated as of June 14, 1999, between Abgenix and ChaseMellon Shareholder Services, L.L.C., including the Certificate of Determinations, the Form of Rights Certificate and the Summary of Rights attached thereto as Exhibits A, B and C, respectively. |
| +10.45(21) | | Multi-Antigen Research License and Option Agreement by and between Abgenix, Inc. and Japan Tobacco Inc. effective December 31, 1999. |
| +10.46(21) | | Amended and Restated Field License by and among Abgenix, Inc., JT America Inc. and Xenotech L.P. effective December 31, 1999. |
| 10.47(21) | | Agreement to Terminate the Collaboration Agreement by and among Abgenix, Inc., JT America Inc., and Xenotech L.P. effective December 31, 1999. |
| +10.48(21) | | Agreement to Terminate the Interest of Japan Tobacco Inc. in the Master Research License and Option Agreement by and among Abgenix, Inc., Japan Tobacco Inc. and Xenotech L.P. effective December 31, 1999. |
| +10.49(21) | | Amendment of the Expanded Field License by and among Abgenix, Inc., JT America Inc. and Xenotech L.P. effective December 31, 1999. |
| 10.50(21) | | Limited Partnership Interest and Stock Purchase Agreement between Abgenix, Inc. and JT America Inc. made December 20, 1999. |
| +10.51(21) | | License Agreement by and between Abgenix, Inc. and Japan Tobacco Inc. effective December 31, 1999. |
| 10.52(18) | | Amended Preferred Shares Rights Agreement, dated as of November 19, 1999, between Abgenix, Inc. and Chase Mellon Shareholder Services, L.L.C., including the Certificate of Determination, the form of Rights Certificate and the Summary of Rights attached thereto as Exhibits A, B and C, respectively. |
| 10.53(19) | | 1999 Nonstatutory Stock Option Plan and form of agreement thereunder. |
| 10.54(20) | | Registration Rights Agreement dated November 19, 1999 by and among Abgenix and the selling stockholders. |
| 10.55(23) | | Lease Agreement dated February 24, 2000 between Ardenwood Corporate Park Associates, a California Limited Partnership and Abgenix, Inc. |
| 10.56(23) | | Lease Agreement dated May 19, 2000 between Ardenwood Corporate Park Associates, a California Limited Partnership and Abgenix, Inc. |
| 10.57(23) | | Amendments to 1996 Incentive Stock Option Plan and 1999 Nonstatutory Stock Option Plan to eliminate the Company's ability to reprice issued and outstanding options. |
| 10.58(25) | | Acquisition Agreement dated as of September 25, 2000 among Abgenix, Inc., Abgenix Canada Corporation and ImmGenics Pharmaceuticals Inc. |
| 10.59(25) | | Voting, Exchange and Cash Put Trust Agreement dated as of November 3, 2000 among Abgenix, Inc., ImmGenics Pharmaceuticals Inc. and CIBC Mellon Trust Company. |
| 10.60(25) | | Support Agreement dated as of November 3, 2000 among Abgenix, Inc., Abgenix Canada Corporation and ImmGenics Pharmaceuticals Inc. |
| 10.61(25) | | Form of Stock Purchase Agreement between Abgenix, Inc. and the purchasers in the private placement in November 2000. |
| 10.62(26)+ | | License Agreement among BR Centre Limited, Imgenix Biomedical Inc. and Dr. John W. Schrader, dated May 9, 1994. |
| 10.63(26)+ | | License Agreement Amendment among BR Centre Limited, Imgenix Biomedical Inc. and Dr. John W. Schrader, dated May 9, 1994. |
| 10.64(26) | | Assignment Agreement among BR Centre Limited and The University of British Columbia Foundation, dated March 10, 1998. |
| 10.65(27) | | Lease Agreement dated February 14, 2001 between AMB Property, L.P., a Delaware limited partnership, and Abgenix, Inc. |
| 10.66(27)++ | | Product Supply Agreement by and between Lonza Biologics PLC and Abgenix, Inc. dated November 30, 2000 |
| 21.1* | | List of subsidiaries. |
| 23.1 | | Consent of Ernst & Young LLP, Independent Auditors. |
| 23.2** | | Consent of O'Melveny & Myers LLP (included in Exhibit 5.1 to this Registration Statement). |
| 24.1* | | Power of Attorney. |
- *
- Previously filed.
- **
- Previously filed as Exhibit 23.3 to Abgenix's Registration Statement on Form S-1
(File No. 333- 49858), filed with the Commission on November 13, 2000.
- +
- Confidential treatment granted for portions of these exhibits. Omitted portions have been filed separately with the Commission.
- ++
- Confidential treatment has been requested for portions of this exhibit. Omitted portions have been filed separately with the Commission.
- (1)
- Incorporated by reference to the same exhibit filed with Abgenix's Registration Statement on Form S-1 (File No. 333-49415).
- (2)
- Incorporated by reference to the same exhibit filed with Abgenix's Registration Statement on Form S-1 (File No. 333-49415), portions of which have been granted confidential treatment.
- (3)
- Incorporated by reference to the same exhibit filed with Cell Genesys' Registration Statement on Form S-1 (File No. 33-46452), portions of which have been granted confidential treatment.
- (4)
- Incorporated by reference to the same exhibit filed with Cell Genesys' Registration Statement on Form S-1 (File No. 33-46452).
- (5)
- Incorporated by reference to the same exhibit filed with Cell Genesys' Quarterly Report on Form 10-Q for the quarter ended June 30, 1993, portions of which have been granted confidential treatment.
- (6)
- Incorporated by reference to the same exhibit filed with Cell Genesys' Annual Report on Form 10-K for the year ended December 31, 1993, portions of which have been granted confidential treatment.
- (7)
- Incorporated by reference to the same exhibit filed with Cell Genesys' Quarterly Report on Form 10-Q for the quarter ended June 30, 1995, portions of which have been granted confidential treatment.
- (8)
- Incorporated by reference to the same exhibit filed with Cell Genesys' Quarterly Report on Form 10-Q for the quarter ended June 30, 1995.
- (9)
- Incorporated by reference to the same exhibit filed with Cell Genesys' Quarterly Report on Form 10-Q for the quarter ended June 30, 1996, portions of which have been granted confidential treatment.
- (10)
- Incorporated by reference to the same exhibit filed with Cell Genesys' Quarterly Report on Form 10-Q for the quarter ended June 30, 1996.
- (11)
- Incorporated by reference to the same exhibit filed with Cell Genesys' Quarterly Report on Form 10-Q for the quarter ended September 30, 1996.
- (12)
- Incorporated by reference to the same exhibit filed with Cell Genesys' Annual Report on Form 10-K for the year ended December 31, 1996, as amended, portions of which have been granted confidential treatment.
- (13)
- Incorporated by reference to the same exhibit filed with Cell Genesys' Annual Report on Form 10-K for the year ended December 31, 1993.
- (14)
- Incorporated by reference to the same exhibit filed with Abgenix's Current Report on Form 8-K filed with the Commission on July 17, 1998, portions of which have been granted confidential treatment.
- (15)
- Incorporated by reference to the same exhibit filed with Abgenix's Current Report on Form 8-K filed with the Commission on November 24, 1998.
- (16)
- Incorporated by reference to the same exhibit filed with Abgenix's Registration Statement on Form S-1 (File No. 333-71289), portions for which Abgenix has requested confidential treatment.
- (17)
- Incorporated by reference to the same exhibit filed with Abgenix's Registration Statement on Form S-1 (File No. 333-71289).
- (18)
- Incorporated by reference to the same exhibit filed with Abgenix's Registration Statement on Form 8-A (File No. 000-24207).
- (19)
- Incorporated by reference to the same exhibit filed with Abgenix's Registration Statement on Form S-8 (File No. 333-90707).
- (20)
- Incorporated by reference to the same exhibit filed with Abgenix's Registration Statement on Form S-3 (File No. 333-91699).
- (21)
- Incorporated by reference to the same exhibits filed with Abgenix's Current Report on Form 8-K filed with the Commission on January 27, 2000.
- (22)
- Incorporated by reference to the same exhibits filed with Abgenix's Registration Statement on Form S-1 (File No. 333-70631).
- (23)
- Incorporated by reference to the same exhibits filed with Abgenix's Quarterly Report on Form 10-Q for the quarter ended June 30, 2000.
- (24)
- Incorporated by reference to the same exhibit filed with Abgenix's Registration Statement on Form S-8 (File No. 333-45426).
- (25)
- Incorporated by reference to the same exhibits filed with Abgenix's Quarterly Report on Form 10-Q for the quarter ended September 30, 2000.
- (26)
- Incorporated by reference to the same exhibits filed with Abgenix's Annual Report on Form 10-K for the year ended December 31, 2000.
- (27)
- Incorporated by reference to the same exhibits filed with Abgenix's Quarterly Report on Form 10-Q for the quarter ended March 31, 2001.
- (28)
- Incorporated by reference to the same exhibits filed with Abgenix's Quarterly Report on Form 10-Q for the quarter ended June 30, 2001.
QuickLinks
TABLE OF CONTENTSFORWARD-LOOKING STATEMENTSRISK FACTORSCERTAIN INFORMATIONUSE OF PROCEEDSPRICE RANGE OF COMMON STOCKDIVIDEND POLICYSELECTED CONSOLIDATED FINANCIAL DATAMANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONSBUSINESSMANAGEMENTSummary Compensation TableAggregate Option Exercises in Last Fiscal Year and Fiscal Year-End Option ValuesCOMPARISON OF CUMULATIVE TOTAL RETURN ON INVESTMENTCERTAIN TRANSACTIONSDESCRIPTION OF CAPITAL STOCKSHARES ELIGIBLE FOR FUTURE SALEPRINCIPAL SHAREHOLDERSSELLING SHAREHOLDERSPLAN OF DISTRIBUTIONWHERE YOU CAN FIND MORE INFORMATIONLEGAL MATTERSEXPERTSINDEX TO CONSOLIDATED FINANCIAL STATEMENTSREPORT OF ERNST & YOUNG LLP, INDEPENDENT AUDITORSABGENIX, INC. CONSOLIDATED BALANCE SHEETS (in thousands, except share and per share data)ABGENIX, INC. CONSOLIDATED STATEMENTS OF OPERATIONS in thousands, except per share data)ABGENIX, INC. CONSOLIDATED STATEMENT OF CHANGES IN REDEEMABLE CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS' EQUITY (in thousands, except share and per share data)ABGENIX, INC. CONSOLIDATED STATEMENTS OF CASH FLOWS (in thousands)ABGENIX, INC. CONDENSED CONSOLIDATED BALANCE SHEETS (in thousands, except share and per share data)ABGENIX, INC. CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS (in thousands, except per share data) (unaudited)ABGENIX, INC. CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS (in thousands) (unaudited)ABGENIX, INC. NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS June 30, 2001PART II INFORMATION NOT REQUIRED IN PROSPECTUSSIGNATURESEXHIBIT INDEX