Inovio Pharmaceuticals, Inc.
We are a biotechnology company focused on rapidly bringing to market precisely designed DNA medicines to treat, cure, and protect people from diseases associated with human papillomavirus, or HPV, cancer, and infectious diseases. Our DNA medicine pipeline is composed of three types of product candidates, DNA vaccines, DNA immunotherapies and DNA encoded monoclonal antibodies. In clinical trials, we have demonstrated that a DNA medicine can be delivered directly into cells in the body via our proprietary smart device to consistently activate robust and fully functional T cell and antibody responses against targeted cancers and pathogens.
Our novel DNA medicine candidates are made using our proprietary SynCon technology that creates optimized plasmids, which are circular strands of DNA that can produce antigens independently inside a cell to help the person’s immune system recognize and destroy cancerous or virally infected cells.
Our hand-held CELLECTRA smart delivery devices provide optimized uptake of our DNA medicines within the cell, overcoming a key limitation of other DNA-based technology approaches. Human data to date have shown a favorable tolerability profile of our DNA medicines delivered directly into cells in the body using the CELLECTRA smart delivery device in more than 7,000 administrations across approximately 2,500 patients.
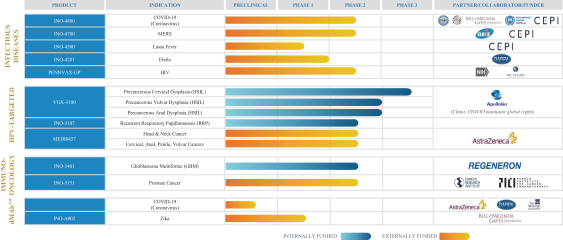
Our corporate strategy is to advance, protect, and provide our novel DNA medicines to meet urgent and emerging global health needs. We continue to advance and validate an array of DNA medicine candidates that target COVID-19, HPV-related diseases, cancer, and infectious diseases. We aim to advance these candidates through commercialization and continue to leverage third-party resources through collaborations and partnerships, including product license agreements.
Our partners and collaborators include ApolloBio Corp., AstraZeneca, Beijing Advaccine, The Bill & Melinda Gates Foundation, Coalition for Epidemic Preparedness Innovations, Defense Advanced Research Projects Agency, The U.S. Department of Defense, GeneOne Life Science, HIV Vaccines Trial Network, the U.S. Defense Threat Reduction Agency’s Medical CBRN Defense Consortium, International Vaccine Institute, National Cancer Institute, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Ology Bioservices, the Parker Institute for Cancer Immunotherapy, Plumbline Life Sciences, Regeneron Pharmaceuticals, Thermo Fisher Scientific, Richter-Helm BioLogics, the University of Pennsylvania, the Walter Reed Army Institute of Research, and The Wistar Institute.
We or our collaborators are currently conducting or planning clinical studies of our DNA medicines for HPV-associated precancers, including cervical, vulvar, and anal dysplasia; HPV-associated cancers, including head & neck, cervical, anal, penile, vulvar, and vaginal; other HPV-associated disorders, such as recurrent respiratory papillomatosis; glioblastoma multiforme; prostate cancer; HIV; Ebola; Middle East Respiratory Syndrome; Lassa fever; Zika virus; and the COVID-19 virus.
All of our product candidates are in the research and development phase. We have not generated any revenues from the sale of any products, and we do not expect to generate any such revenues for at least the next several years. We earn revenue from license fees and milestone revenue and collaborative research and development agreements. Our product candidates will require significant additional research and development efforts, including extensive preclinical and clinical testing. All product candidates that we advance to clinical testing will require regulatory approval prior to commercial use and will require significant costs for commercialization. We may not be successful in our research and development efforts, and we may never generate sufficient product revenue to be profitable.
1