- WST Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
8-K Filing
West Pharmaceutical Services (WST) 8-KRegulation FD Disclosure
Filed: 13 Mar 08, 12:00am

Investor Day 2008

Don Morel
Chairman and Chief Executive Officer

Agenda and Participants
Welcome and Introduction Don Morel
Market Overview Mike Schaefers
Growth Platforms John Paproski
Daikyo Crystal Zenith Platform Bernie Lahendro
Break
Operations Overview Steve Ellers
Long Term Outlook and Summary Don Morel
Q&A Mike Anderson

Certain statements in the following slides and certain statements that may be made by management of the Company orally during this
presentation contain some forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and
Section 21E of the Securities Exchange Act of 1934, as amended, that are based on management’s plans and assumptions. Such statements
give our current expectations or forecasts of future events; they do not relate strictly to historical or current facts. We have tried, wherever
possible, to identify such statements by using words such as “estimate,” “expect,” “intend,” “believe,” “plan,” “anticipate” and other words
and terms of similar meaning in connection with any discussion of future operating or financial performance or condition. We cannot
guarantee that any forward-looking statement will be realized. If known or unknown risks or uncertainties materialize, or if underlying
assumptions are inaccurate, actual results could differ materially from past results and those expressed or implied in any forward-looking
statement. You should bear this in mind as you consider forward-looking statements. We undertake no obligation to publicly update forward-
looking statements, whether as a result of new information, future events or otherwise.
Important factors that may affect future results include the following: Sales demand and our ability to meet that demand; competition from
other providers in the Company’s businesses, including customers’ in-house operations, and from lower-cost producers in emerging markets,
which can impact unit volume, price and profitability; customers’ changing inventory requirements and manufacturing plans that alter existing
orders or ordering patterns for the products we supply to them; the timing, regulatory approval and commercial success of customer
products that incorporate our products, including relevant third-party reimbursement for prescription products, medical devices and
components and medical procedures in which those products are employed or consumed; average profitability, or mix, of products sold in
any reporting period; maintaining or improving production efficiencies and overhead absorption; the timeliness and effectiveness of capital
investments, particularly capacity expansions, including the effects of delays and cost increases associated with construction, availability
and cost of capital goods, and necessary internal, governmental and customer approvals of planned and completed projects, and the demand
for goods to be produced in new facilities; dependence on third-party suppliers and partners, including our Japanese partner Daikyo Seiko,
Ltd.; the availability and cost of skilled employees required to meet increased production, managerial, research and other needs of the
Company, including professional employees and persons employed under collective bargaining agreements; interruptions or weaknesses in
our supply chain, which could cause delivery delays or restrict the availability of raw materials and key bought-in components and finished
products; raw-material price escalation, particularly petroleum-based raw materials, and our ability to pass raw-material cost increases on to
customers through price increases; claims associated with product quality, including product liability, and the related costs of defending and
obtaining insurance indemnifying the Company for the cost of such claims; the cost and progress of development, regulatory approval and
marketing of new products as a result of the Company’s research and development efforts; the defense of self-developed or in-licensed
intellectual property, including patents, trade and service marks and trade secrets; dependence of normal business operations on information
and communication systems and technologies provided, installed or operated by third parties, including costs and risks associated with
planned upgrades to existing business systems; the relative strength of the U.S. dollar in relation to other currencies, particularly the Euro,
British Pound, and Japanese Yen; changes in tax law or loss of beneficial tax incentives; the conclusion of unresolved tax positions
consistent with currently expected outcomes; the timely execution and realization of savings anticipated by the restructuring plan for certain
operations and functions of The Tech Group, announced in December 2007; and,
Other risks and uncertainties detailed in West’s filings with the Securities and Exchange Commission, including our Registration Statement
on Form 10-K filed with the SEC on February 29, 2008. You should evaluate any statement in light of these important factors.
Safe Harbor Statement: Forward Looking Statements
Trademarks: All trademarks and registered trademarks used in this report are the property of West Pharmaceutical Services,
Inc., in the United States and other jurisdictions, unless otherwise noted.

Founded in 1923
HQ in Lionville, PA
Company Overview

Who we are
World’s premier manufacturer
of components and systems
for injectable drug delivery
Closure systems and prefillable
syringe components
Components for disposable systems
Devices and device sub-assemblies
Safety and administration systems
Record 2007 sales $1.02 billion
Market cap $1.3 billion

Diverse, Stable Customer Base
Company Estimated Market Share: 70% in Pharma; 70% in Device; 95% in
Biotech

West’s Competitive Advantage
World class technical and regulatory support
Global supply chain stability – multiple site sourcing
Protected IP: West’s components and systems
Unmatched experience/expertise: drug - material interface
Regulatory barrier to entry: NDA and ANDA filing must include reference to
all packaging/components in contact with the drug
1.
West Drug Master File (DMF) 1546 is confidential
2.
West DMF includes functionality data (multi-year studies)
3.
All primary package changes require new stability/functionality
studies for new filing
Engineering expertise in high-volume manufacturing and assembly
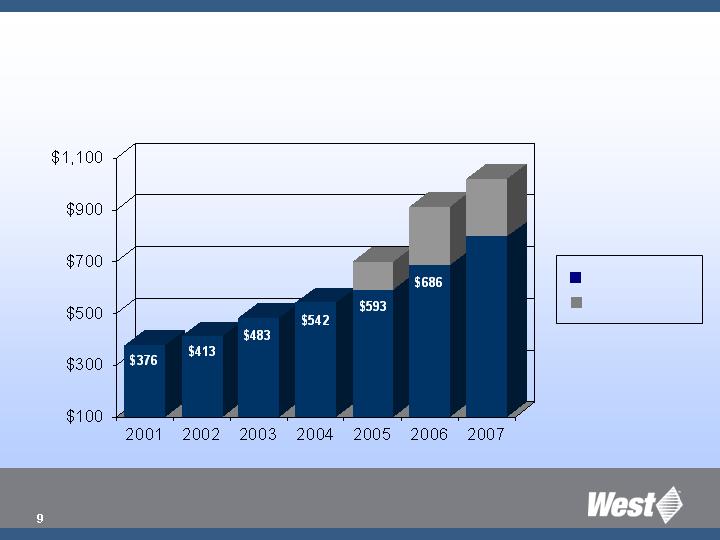
($ millions)
Strong Sales Growth
Existing Business
Acquisitions
$107
$227
$797
$223
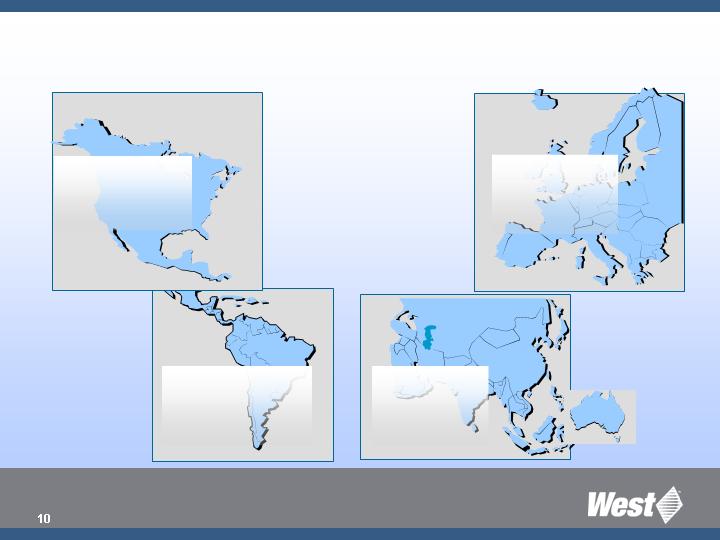
Global
Revenue
Breakdown
Based on 2007 sales
of approx $1 billion
South America:
$55.3 million
(5%)
North America:
$496.4 million
(49%)
Europe:
$430.6 million
(42%)
Asia/Pacific:
$37.8 million
(4%)

Our Long Term Objectives
Build on our global competitive advantages
to create multiple platforms
for sustainable growth

Four Strategic Growth Platforms
Injectable Container Solutions
Prefillable Syringe Systems
Advanced Injection Systems
Safety & Administration Systems

Future Growth Drivers
Aging population creates an increasing number of patients with chronic
illnesses such as diabetes and cancer
Biologic drug growth and a resurgence in vaccine research
Demand for ultra clean silicone free packaging and delivery systems
China, India economic growth and growing demand for advanced
healthcare
Point-of-care shift: Hospital Specialty Clinic Home
Convergence of the primary container and delivery device
West migration from component production to device systems
Tech business model shift to proprietary products
Source: Datamonitor

Convergence of Primary Containers
and Delivery Systems
Disposable
Syringe-based
Auto Injectors
Traditional Injection
System
Components for
Pen System Applications

Our Long-Term Objectives
Build on our global competitive advantages to create
multiple platforms for sustainable growth
Undergo selective geographic expansion
Continued investment in innovation and new manufacturing
technology
Five-year financial goals:
Average annual sales growth 7-9%
Average annual operating profit growth 10-12%

Mike Schaefers, Ph.D.
Vice President of Marketing, Europe

Global Pharmaceutical
Market Update

Global pharmaceutical market predicted to increase by 5-6% in
value in 2008 vs. 6-7% in 2007 driven by:
Aging populations
Strong economies
Introduction of new products
Geographic expansion
Patent expirations ($16 billion in 2007, $12 billion in 2008)
Reimbursement issues
Strong generic competition
IMS Global Insight Nov. 2007, Drug Benefits News, Nov. 2007
Global Pharmaceutical Market Update

Geographic expansion
Growth in emerging markets (China, India, Brazil, Turkey, Russia) accelerated at
12-13%
Trend toward specialty products vs. former mass market approach (i.e.
oncology vs. cardiovascular)
Specialty products expected to grow 14-15% in 2008
80% of the 29 new innovative medicines expected to launch in 2008 will be
specialist products
Global pharmaceutical packaging market growing 6.5% annually to 2011
IMS Global Insight Nov. 2007
Global Pharmaceutical Market Update

Paradigm shift in the pharmaceutical world
Fewer new drugs being launched!
FDA approved 60 new drugs between 2005-2007 (NME, novel biologics)
vs. 60 new drugs approved in 1996 alone
Agencies move towards risk assessment based approval process
Limited claims for newly approved drugs
More black box warnings
More clinical evidence required
FDA, The RPM Report; IMS Global Insights, Nov. 2007
Global Pharmaceutical Market Update

Paradigm shift in the pharmaceutical world
Drug makers cut back costs
Workforce
Inventory
Lean programs
Supply chain programs
Sourcing costs
Companies increase R&D spend and fill pipelines through M&A activities
(mainly biotech)
FDA, The RPM Report; IMS Global Insights, Nov. 2007
Global Pharmaceutical Market Update

Pharma/biotech customer trends
Integration of container/closure system into delivery system
Reduction of drug waste and improved operational throughput
High quality packaging to ensure drug stability
Value-added devices and systems for product/brand differentiation
Secure packaging
Global Pharmaceutical Market Update
Patient/caregiver trends
Point of care shift
Hospital specialty clinic/physician home
Dosage form migration
Increase patient compliance rates through enhanced application systems
i.e. safety systems, pens, auto-injectors
Patient convenience
Ease of use
Minimized pain
Increased focus on healthcare worker, patient safety
Global Pharmaceutical Market Update
Top therapeutic categories
Increasing number of patients with chronic illnesses such as diabetes,
cancer, AIID (RA, MS, etc.)
Resurgence of vaccine market
Emergence of high-value therapeutic vaccines
New technologies and delivery methods
Pandemic concerns
Top therapeutic categories (annual growth 05-10)
Oncology + 15%
Diabetes + 9%
AIID + 10%
Vaccines + 15%
Pharmaceutical & Diagnostic Innovation, 2006 Vo. 4, No. 10,IMS Global Insights, Nov. 2007
Global Pharmaceutical Market Update

Top therapeutic categories
Injectable products currently account for ~15% of global drug delivery
market
Significant growth expected through above mentioned therapeutic
categories
High number of injectable products in pipelines
Biologic drugs represent the fastest growing segment in injectable
pharma market (13% CAGR through 2010)
Arrowhead Publishers; Datamonitor
Global Pharmaceutical Market Update

Strategic Growth Platforms
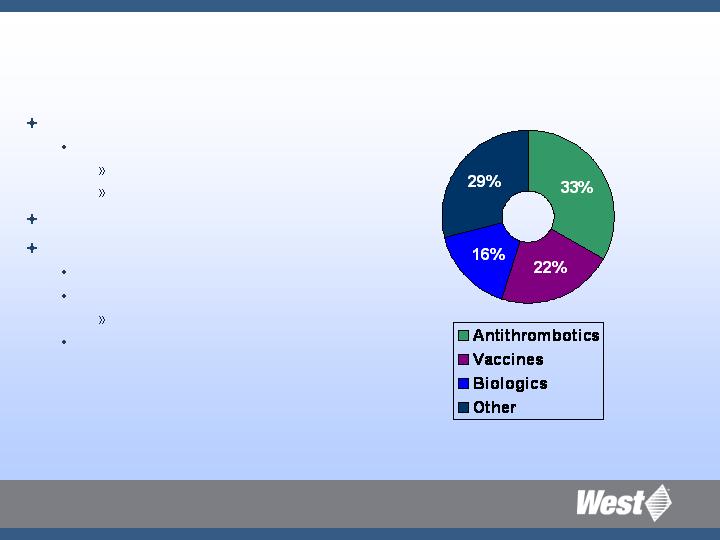
Therapeutic Targets and
West’s Strategic Growth Platforms
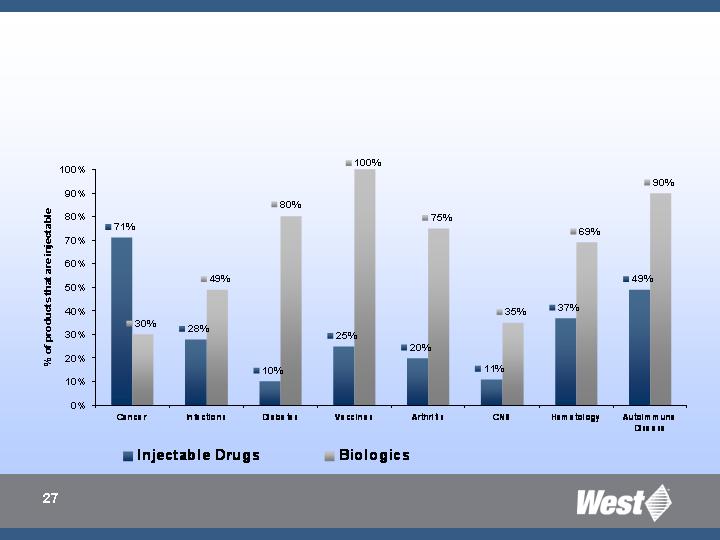
Percentage of injectable products by therapeutic
category and biological injectables
(awaiting approval and in clinical trials in US – all forms)
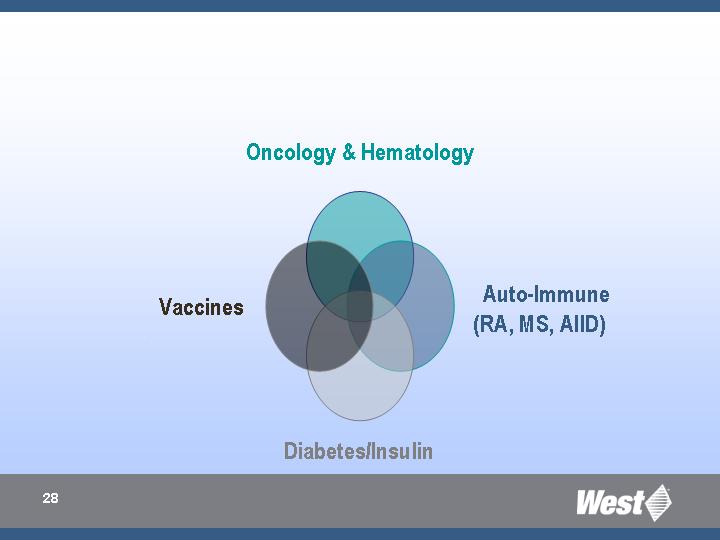
West’s Therapeutic Targets

West’s Therapeutic Targets
Rationale
Current presence in targeted categories/ segments
Future annual growth rates per therapeutic category
Number of products in pipeline per therapeutic category
Percentage of injectable products in pipeline
Need for high-value products and enhanced packaging systems
Growth opportunities through advanced application and administration
systems

Therapeutic Targets and West Strategic Platforms
A Perfect Match
High value products for container
closures systems
Enhanced packaging systems
Advanced application and
administration systems
Needle safety systems
Stability of demanding pharma-
ceuticals/biopharmaceuticals
Need for product/brand differentiation
Secure and tamper evident
packaging (ID, anticounterfeiting)
Healthcare worker/patient safety
Trend towards self-administration
Improved patient compliance
West Strategic Platform Offerings
Therapeutic Target Requirements

Four Strategic Growth Platforms
Injectable Container Solutions
Prefillable Syringe Systems
Advanced Injection Systems
Safety & Administration Systems

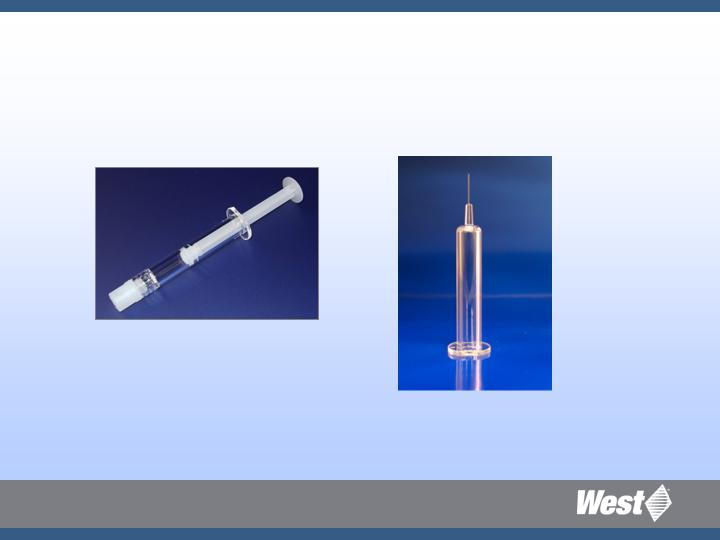
Injectable Container Solutions

Prefillable Syringe Systems
Unique silicone-free syringe systems for demanding
biopharmaceuticals
Daikyo Crystal Zenith RU syringes
Daikyo Crystal Zenith is a registered trademark of Daikyo Seiko, Ltd.
Safety and Administration Systems
West´s reconstitution, administration and safety systems
Project Orion
MixJect

West Advanced Injection Systems
Syringe and cartridge-based autoinjector systems
Customer Account Penetration for
Strategic Platform Products
Secondary

Top Therapeutic Categories
and West’s Position
Westar RS/RU
Westar Lined Seals RS
Envision
VeriSure
Safety & Administration Systems
Advanced Injection Systems
Stability of biotechnologically derived
insulin drugs
High-quality container/closure
systems
Ease of use
Suitability for home administration
Switch from vial/syringe combinations to
pen injectors
West Offerings
Requirements
Diabetes/Insulin

Examples of West
Participation in Insulin Market

Top Therapeutic Categories
and West’s Position
FluroTec stoppers and plungers
Envision
VeriSure
Westar RS/RU
Daikyo Crystal Zenith RU syringe
Safety & Administration Systems
Advanced Injection Systems
High-quality packaging for stability of high
value therapeutic vaccines
Trend towards prefillable syringe
applications
Healthcare worker and patient safety
Minimized pain
Advanced delivery methods
West Offerings
Requirements
Vaccines

Top Therapeutic Categories
and West’s Position
FluroTec stoppers and plungers
Westar RS/RU
Envision
VeriSure
Daikyo Crystal Zenith RU syringe
Safety & Administration Systems
Advanced Injection Systems
Stability of demanding pharmaceutical/
biopharmaceutical compounds
Need for silicone-oil-free packaging
Reduction of drug waste
Break resistant packaging
Trend towards self-administration
Healthcare worker safety
Patient compliance
West Offerings
Requirements
Oncology/Hematology

Top Therapeutic Categories
and West’s Position
FluroTec stoppers and plungers
Westar RS/RU
Westar lined seals RS
Envision
VeriSure
Daikyo Crystal Zenith RU syringe
Safety & Administration Systems
Advanced injection systems
Stability of demanding biopharmaceutical
compounds
Reduction of drug waste
Need for silicone-oil-free packaging
Trend towards prefillable syringe
applications
Trend towards self-administration
Ease of use and patient compliance
Product / brand differentiation
West Offerings
Requirements
Autoimmune (RA, MS, AIID)

Generics
Are generics/biogenerics a threat for West?

West's Generic/Biogeneric Strategy
$12 billion of brand name drugs will go generic in 2008
West addresses challenge through multiple activities
Focus on therapeutic targets (specialty injectable drugs) growing faster than any
other category
Proactive support of lifecycle management activities at pharma/biotech
companies
Product/brand differentiation through innovative packaging, application and
administration systems

West´s Generic/Biogeneric Strategy
West addresses challenge through multiple activities
Growth opportunities through generic/biogeneric companies by
addressing their drug packaging needs
Primarily using originator’s container/closure system
Frequent outsourcing of value-added services to suppliers
Biogenerics forced to offer application and delivery systems

Summary
Major programs directly address market needs
Focus on strongly growing therapeutic categories diabetes/insulin,
vaccines, oncology and AIID
Importance of injectable and biological products within these categories
Growing need for high value container/closure and packaging systems
Growth opportunities through advanced application and administration
systems
Perfect match between requirements of targeted therapeutic categories
and West strategic platforms
Growth opportunities through generic/biogeneric markets

John Paproski
Vice President, Innovation

Growth Platforms

Innovation Focus
Bring unique value added solutions to the growing need
for combining injectable drug containers
with delivery systems
For 2008
Prepare three major products for broad market
introduction
Q2 – Ready-to-Use Daikyo Crystal Zenith luer lock syringe system
Q3 – Disposable auto injector for prefillable syringes
Q4 – Orion prefillable syringe safety system

Injectable Pharma Market Needs
Patient/Caregiver Focused
Increasing patient compliance rates
Patient and clinician convenience
Minimize pain and needle fear
Healthcare worker and patient safety
Sharps exposure
Blood-borne pathogen transmission (HIV, Hepatitis)
Unintended drug exposure
Home and self-administration
Provide cost effective reimbursement

Injectable Pharma Market Needs
Pharma Company Focused
Improve drug stability
Reduce drug waste
Integrate with existing filling operations
Reliable supply
Differentiate brands
Enabling delivery methods
Secure packaging
Risk mitigation
Outsourcing non-core competencies

West Innovation Model
Creating proprietary products that serve
our existing injectable customers
Regulatory hurdles for our customers
Slow time to market
Long product lives
Patent protected
Operate like start-up business units
Manufactured primarily by The Tech Group
or our partner, Daikyo
Provide high-value system solutions

West Sources of Innovation
Entrepreneurs
In-license intellectual property
Acquire small companies
Established products
Knowledgeable people
Intellectual property
Internal idea development
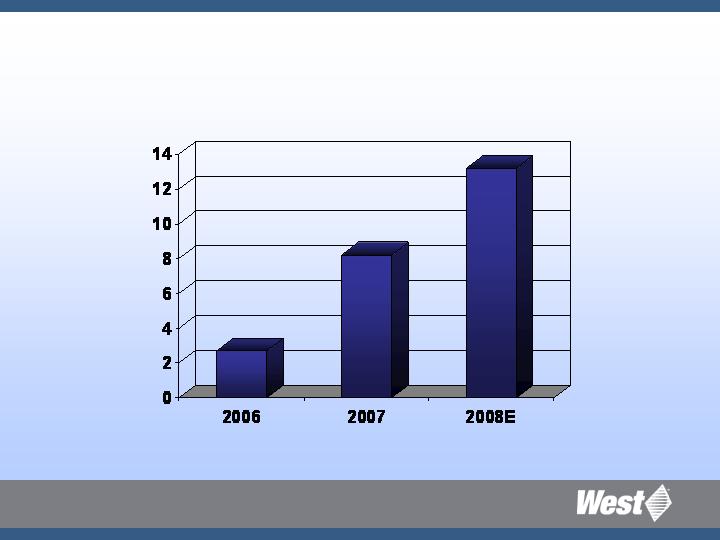
Innovation Spending
$ millions
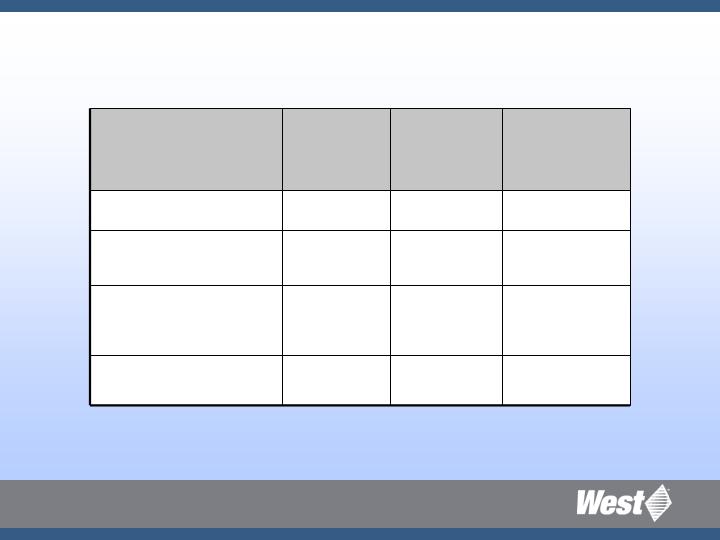
Strategic Platforms: Target Markets
$1.5B
4%
9B
Injectable Containers
$1.5B
11%
5.2B
Safety &
Administration
$210M
8-12%
70M
Advanced Injection
Systems
$900M
8-12%
1.8B
Prefilled Syringe
Total Market
Sales
CAGR
(Unit)
Total
Market
Units
Strategic
Platform

Injectable Container Solutions
Market focus: high-value drugs
Drug stability
Reduced waste
Universal processing and filling
West innovations
Ready-to-Use Daikyo Crystal Zenith vial
system
Ready-to-Use Daikyo Crystal Zenith bulk
bio processing containers

Advanced Injection Systems
Market focus: high-value drugs
Self administration
Home administration
Use standard prefillable syringes
West innovations
Disposable auto-injector: Q3 2008

Advanced Injection Systems
Single Use: Disposable Auto Injector for Staked Needle PFS
Utilizes 1ml long prefilled syringe format
Staked needle with rigid needle shield
Glass or CZ compatible
Full visibility of drug contents
Automatic insertion, delivery and retraction
of the needle
Single push-button activation
Designed for simple assembly of the syringe
into the Auto-Injector
A robust system designed to overcome PFS
variability issues
Auto Injection System Animation
AUTO INJECTION SYSTEM
VIDEO

Safety and Administration Systems
Market focus: entire injectable market
Needle-phobia
Patient and caregiver safety
OSHA and local EU legislation
Patient and caregiver convenience
West innovations
Reconstitution and transfer systems
Vial adaptors
Transfer aids
Safety needles
Project Orion passive safety system Q4
Kitting
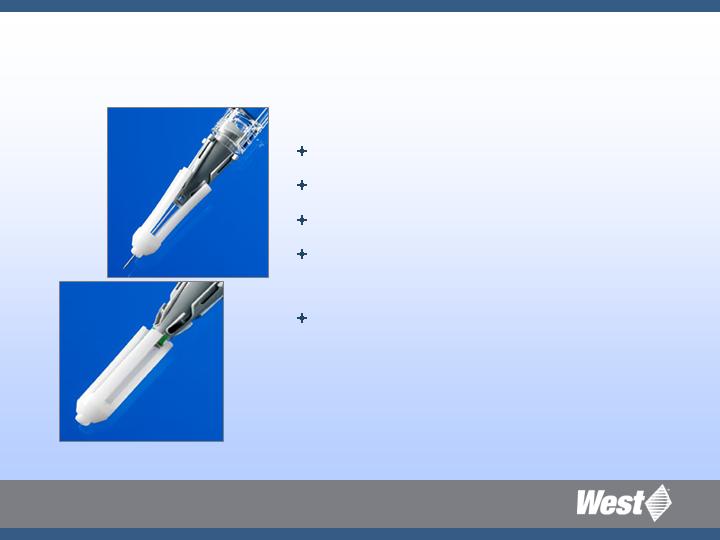
Project Orion: What Does It Offer?
Passive, automatic needle cover
Compact design
Simple and easy to use
Compatible with any standard luer
lock syringe
Minimize patient fear! Minimal amount
of needle exposed
Project Orion
ORION VIDEO
Safety Test
SAFETY VIDEO
Prefillable Syringe Systems
Global PFS units
1.8B units
North America .3M units
Europe (E5) 1.5B units
CAGR 8-12%
Biologic market needs
Drug waste
Drug stability
Silicone, tungsten, adsorption
User convenience and self- administration
Prefillable Syringe Systems
RU Daikyo Crystal Zenith luer lock
syringe: Q2 2008
RU Daikyo Crystal Zenith staked
needle: 2009
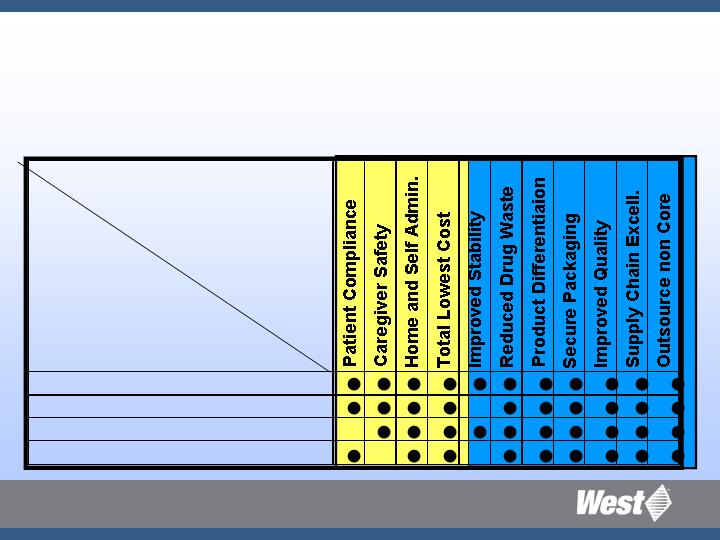
Platform to Market Alignment
STRATEGIC
PLATFORMS
MARKET
NEEDS
Patient or
Healthcare Worker
Pharmaceutical Customer
Injectable Container Systems
Safety and Administration Sys.
Prefillable Syringe Systems
Advanced Injection Systems
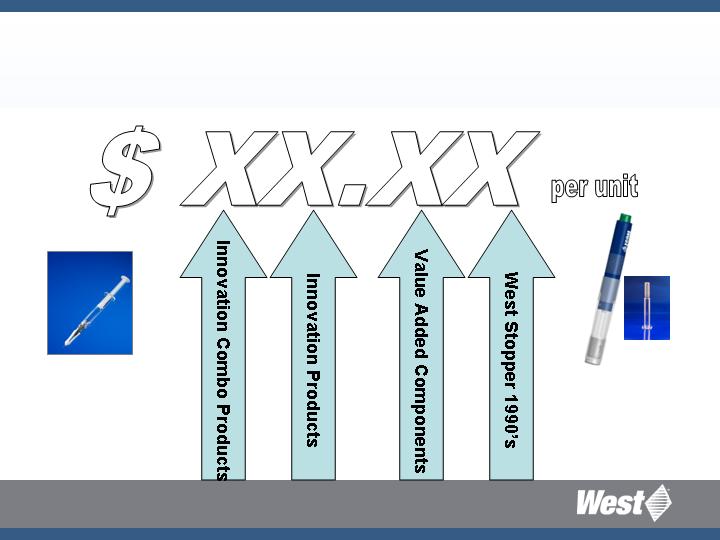
Value of West Products in the
Injectable Drug Package
Daikyo Crystal Zenith
Syringe System with
Orion
West auto injector
with Daikyo
Crystal Zenith
syringe

Bernie Lahendro
Vice President
and General Manager
Daikyo Crystal Zenith® Products

Daikyo Seiko, Ltd.
Daikyo
Family-owned business since 1954
Approximately 500 employees and approximately $110 million U.S.D.
Market leader in Japan for pharmaceutical closures

Daikyo and West Partnership
West purchased 25% of Daikyo Seiko Ltd. in 1973
Technology transfer and marketing agreement signed in 1992 with additional 10
year agreements signed in 1997 & 2007
Daikyo has developed and transferred to West state of the art materials and
processes such as:
B2, Flurotec, Daikyo Crystal Zenith
Flurotec material and B2 process technologies have been transferred to West
Ready-to-Use Crystal Zenith development programs are a cooperative effort of
Daikyo and West

Custom formulated COP resin
developed for pharmaceutical use
Coupled with Flurotec laminated
elastomers for low extractables with
no silicone oil
100% vision inspected
Platform for development of new
innovative products
Daikyo Crystal Zenith

Properties of Daikyo Crystal Zenith
Cyclic Polyolefin
Custom formulated for better break resistance,
scratch resistance and steam sterilizability
Exclusivity for Daikyo/West
High transparency
Colorless

Approved In All Major Markets
Japan
10 syringes: contrast media (5) MRI (2), hyaluronic
acid (2), calcitonin (1 - PEPTIDE)
3 vials: Fluconazole, oncology, anticoagulant
API container (one liter bottle)
US
3 vials: acyclovir and oncology, 1 syringe for
hyaluronic acid; several pending for oncology,
cancer vaccine
Europe
1 syringe: hyaluronic acid
3 vials: oncology; several pending for oncology,
contrast imaging, blood plasma

Daikyo Crystal Zenith Syringe Systems
Sizes available from 0.25 mL to 150 mL
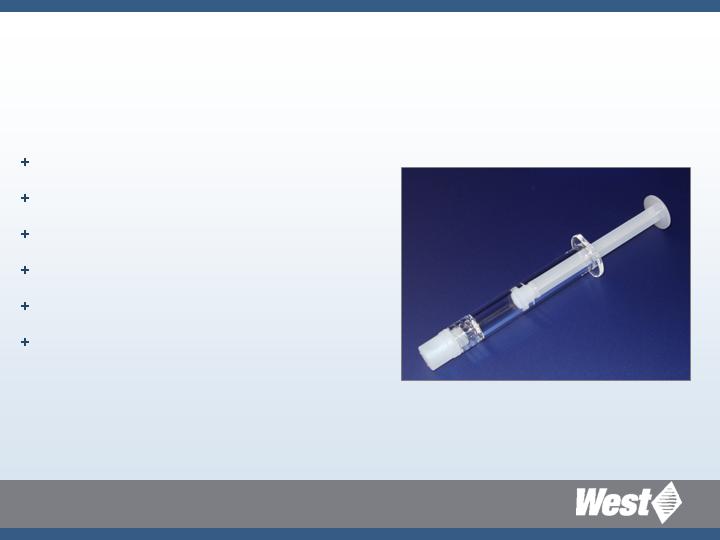
Daikyo Crystal Zenith 1mL-S
RU Luer Lock Syringe System
Daikyo Crystal Zenith barrel
Polypropylene rod
Flurotec laminated piston
Flurotec lamination inside nozzle cap
Silicone oil free
100% vision inspected

Daikyo Crystal Zenith RU Syringe System
Features and Benefits
Provides market differentiation
Less adsorption/absorption
Predictable piston release and travel forces
Suitable for high viscosity products
Ultra low extractables
Reduces aggregation issues
No need for added silicone oil
Tungsten-free
Can be incinerated, eliminating disposal issues
related to blood borne pathogens
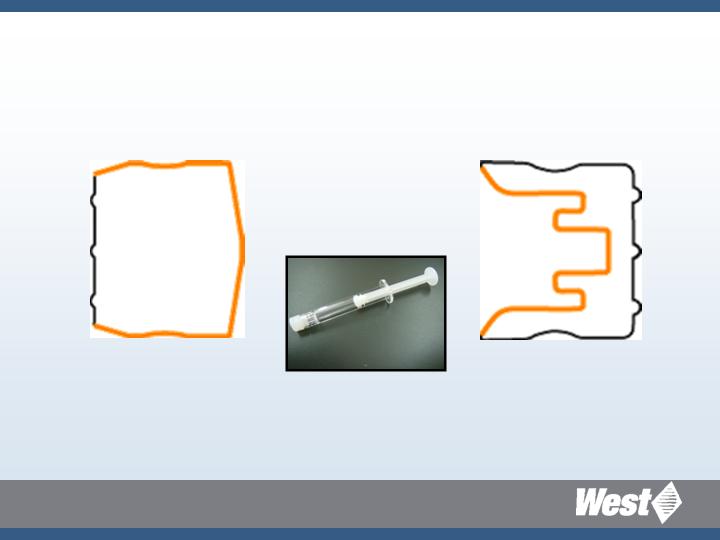
Flurotec Coverage Syringe
Complete coverage of
inert film on drug
contact surfaces
Orange color indicates Flurotec® film coverage
Formula D-21-6-1 Ultra clean chlorobutyl
Plunger
Tip Cap

Daikyo Crystal Zenith 1ml LL-S
RU Syringe Systems
Ready for sampling
Launch April 2008
Sterilization:
Assembled syringes:
E-beam
Pistons: steam
Tubs and nests manufactured in
clean rooms
10 * 10 nest format for standardized
filling machines
Nested or bulk pistons
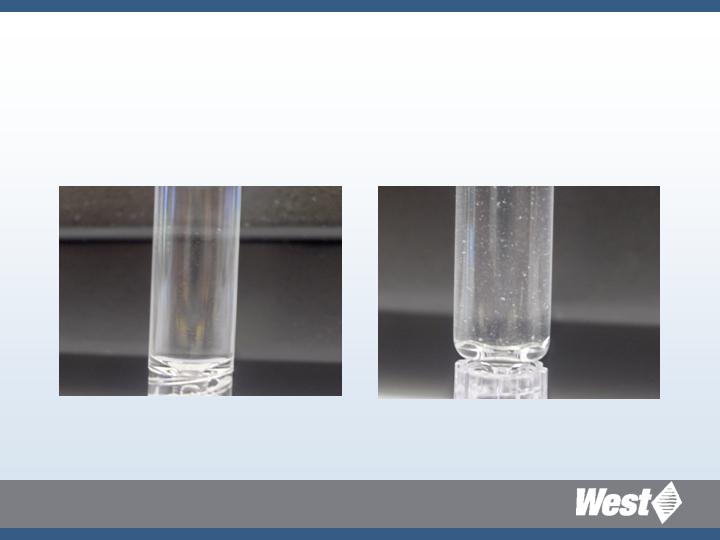
Model Antibody
Higher extent of aggregation in glass
compared to Daikyo Crystal Zenith ®
Crystal Zenith
Glass
With Permission of the University of Kansas
Targeted Therapeutic Categories
Luer Lock Syringes
Large Molecules
Oncology
New Vaccines
Ophthalmic - Macular
Degeneration/Cataract
Surgery
Cosmeceuticals
Anti-Parkinsons
Anti-Alzheimers
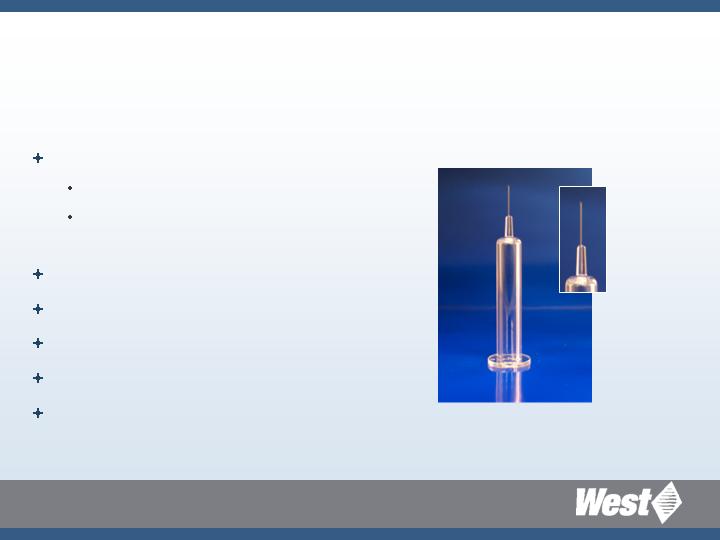
Daikyo Crystal Zenith
Ready-to-Use Staked Needle Syringe
(in development)
Silicone oil-free container
Reduced variability
Consistent piston release and
travel forces
Tungsten free
Glue free
Break resistant
Tight dimensional tolerances
Design flexibility of plastics
Targeted Therapeutic Categories
Staked Needle Syringes
Large Molecules
ESA
Auto-Immune,
MS
Anti-
Rheumatics
Hepatitis C

Daikyo Crystal Zenith® Summary
First in silicone oil free prefillable syringes
Break resistant
Ultra low levels extractables/leachables/particulate
100% vision inspected (all components of syringe)
Zero defects for the high quality markets (Japan)
Protein aggregation - dramatic reductions
Tungsten, glue, silicone oil free staked needle syringes
Reduced need to reformulate
Low temperature storage opportunity

Steve Ellers
President & Chief Operating Officer
Delivering Innovative Solutions

Create profitable and sustainable growth platform
Strengthen market leadership position
Maintain strong financial condition
Strategic Objectives

Business Perspective
Market Position
Profit Margin
Operations
Pipeline
Sales

Business Perspective
Losing share
Market Position
Declining
Profit Margin
Aged & inadequate
factories
Operations
Re-launch old
products
Pipeline
Losing share
Sales
1995 - 2001
Business Perspective
Gaining momentum
Losing share
Market Position
Strong incremental gains
Declining
Profit Margin
Upgrade & catch-up
expansions
Aged & inadequate
factories
Operations
New product launches
Re-launch old
products
Pipeline
Listening and reacting to
customer
Losing share
Sales
2002 - 2006
1995 - 2001
Business Perspective
Leading market in
injectable container
solutions
Gaining momentum
Losing share
Market Position
Funding growth
strategies compress
margins
Strong incremental gains
Declining
Profit Margin
Centers of Excellence
Upgrade & catch-up
expansions
Aged & inadequate
factories
Operations
Innovation & new
growth platforms
New product launches
Re-launch old
products
Pipeline
Creating solutions &
advising customers
Listening and reacting to
customer
Losing share
Sales
2007 - 2012
2002 - 2006
1995 - 2001
Market Knowledge Identifies Market Needs
Manufacturing Strategy
Capital Expenditures
Product Launches

Strengthen Sales and Market Penetration
Market segments
Diabetes, oncology, vaccines and generics
Long-term contracts
BD, Braun, Hospira, Consort, Covidien, Novo
Value-added (revenues 2x unit growth)
FluroTec, Westar, Daikyo, Envision, Spectra
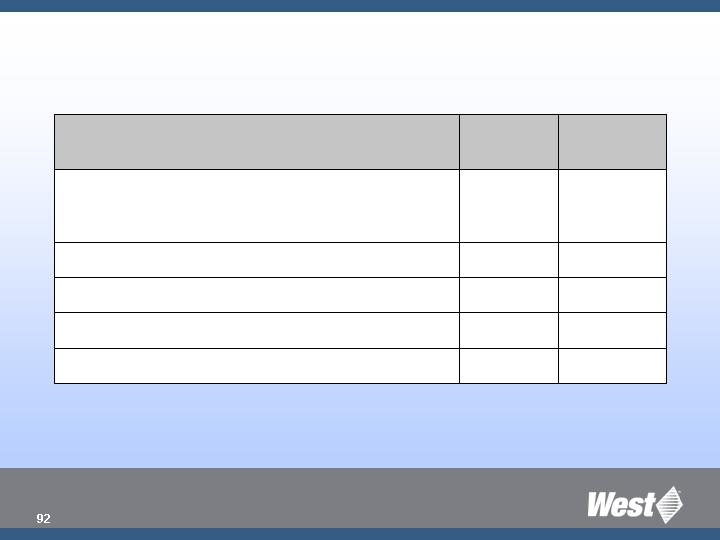
2008 Product Pipeline
Q4
Q4
RSV (Daikyo)
Q4 ‘07
Q4
Envision
- - -
Q2
Inhalation/Nasal Lab Testing
Q2
Q2
RU Crystal Zenith Syringe System (1 mL LL)
Q4
Q2
West RU Ready Pack System
Europe
North
America
Targeted Market Launches
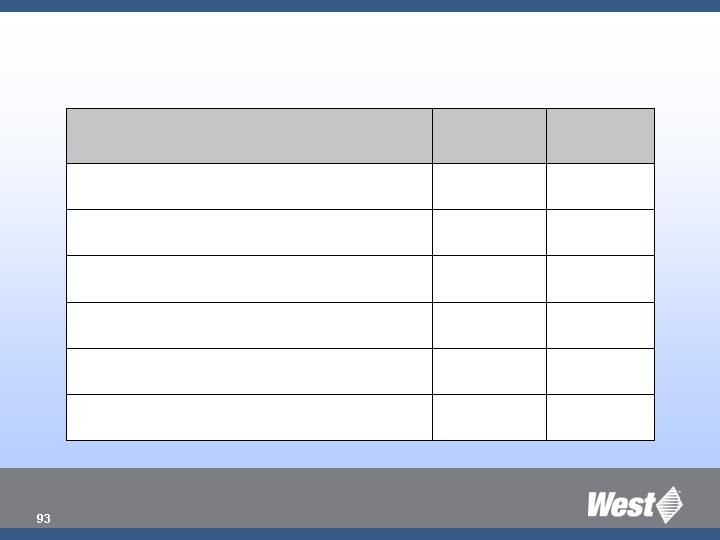
2008 Product Pipeline
Q4
Q4
Orion SQ
Q2
TBD
Westar RS Lined Seals
Q3
Q3
Auto-injector (P2 version)
Q2
Q2
RU PFS Plunger (non-nested)
- - -
Q2
Kitting Capability to Support SAS
Q2
Q2
Vented Vial Adapter
Europe
North
America
Targeted Commercial Introduction
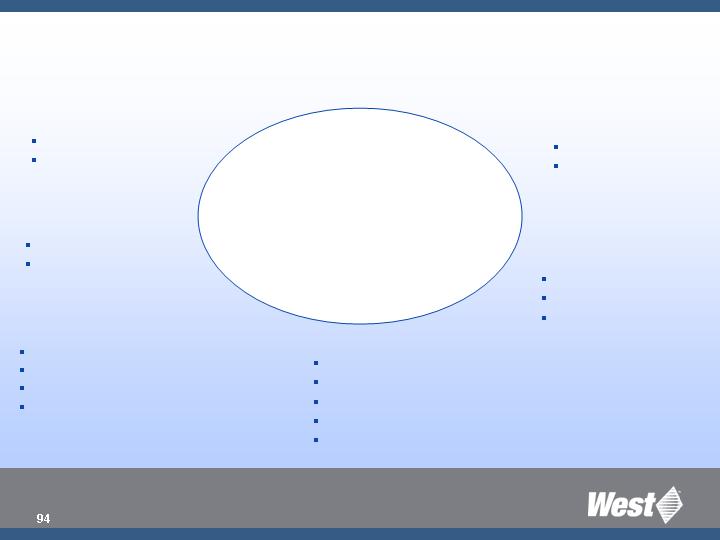
Sales & Pipeline
Core – Growth Platforms
Injectable Container Solutions
Safety & Admin System
Prefillable Syringe System
Advanced Injection System
Safety
Orion
Medimop
Market Needs
Silicone-free
Daikyo Crystal Zenith
PFS
Systems Solution
Westar® - RU
Lined Seals
Ready Pack
Kitting
Reconstitution
Regulatory
Verisure
CCS
TrimTec®
Material Science/drug contact
Quality
Vision Inspection
cGMP
Demographics/Convenience
Auto-injectors
Reconstitution
Close to our Knitting

Creative Access to New
Products and Solutions
Target
Mkt. Launch/ Comm.
Intro
Partners
Action
Market Need
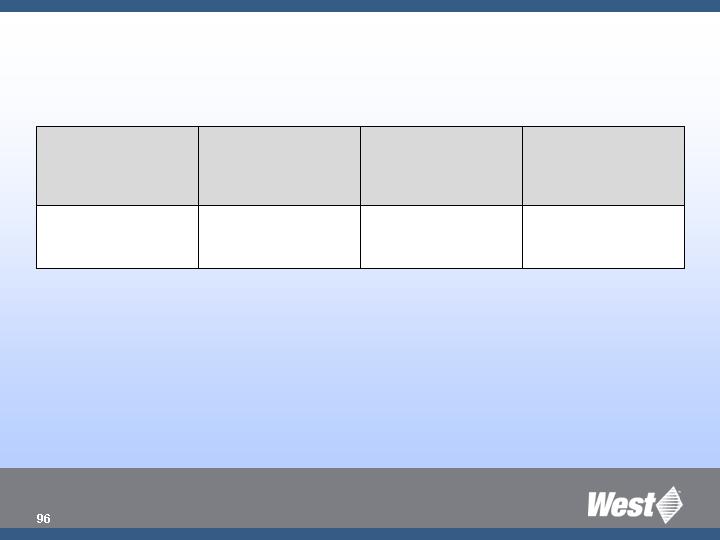
Creative Access to New
Products and Solutions
CAGR 20%
Medimop
Acquire
Reconstitution
Target
Mkt. Launch/ Comm.
Intro
Partners
Action
Market Need
Creative Access to New
Products and Solutions
Q4
Salvus Tech
License
Safety
CAGR 20%
Medimop
Acquire
Reconstitution
Target
Mkt. Launch/ Comm.
Intro
Partners
Action
Market Need

Creative Access to New
Products and Solutions
Q2
Daikyo
Partner
PFS in RU Crystal
Zenith
Q4
Salvus Tech
License
Safety
CAGR 20%
Medimop
Acquire
Reconstitution
Target
Mkt. Launch/ Comm.
Intro
Partners
Action
Market Need
Creative Access to New
Products and Solutions
Q3
Pharma-Pen
Acquire &
in-house Develop
Auto-injector
Q2
Daikyo
Partner
PFS in RU Crystal
Zenith
Q4
Salvus Tech
License
Safety
CAGR 20%
Medimop
Acquire
Reconstitution
Target
Mkt. Launch/ Comm.
Intro
Partners
Action
Market Need
Market Knowledge Identifies Market Needs
Manufacturing Strategy
Capital Expenditures
Product Launches

Manufacturing Strategy
Focused factories – Centers of Excellence
Pharma and device facilities
Products manufactured to a global quality standard
Vision inspection
Six Sigma
Westar RS & RU
cGMP facilities with DMF registrations and FDA audits
Multiple site sourcing
Project Atlas
SAP and MES systems integration
Operate as cost centers
Market Knowledge Identifies Market Needs
Manufacturing Strategy
Capital Expenditures
Product Launches

Investments in Operations
Europe and Asia Expansion Projects
China Greenfield
China plastics in Qingpu
USA Expansions
IT Project Atlas – SAP and MES
Jurong,
Singapore
Bodmin,
England
Kovin,
Serbia
LeNouvion,
France
Eschweiler,
Germany
Jersey Shore,
Pennsylvania
Clearwater,
Florida
Kinston,
North Carolina
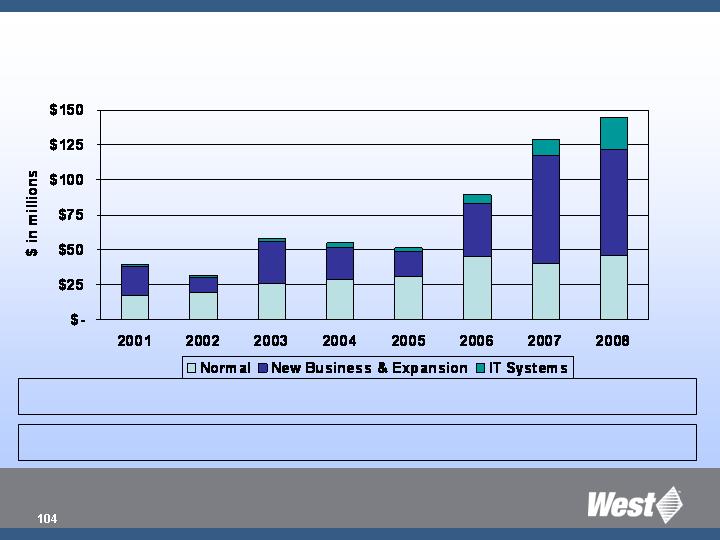
Capital
as % of sales 10% 8% 12% 10% 7% 10% 13% 14%
Depreciation
as % of sales 7%7% 6% 6% 6% 5% 5% 6%
Operations Record Levels of CAPEX
Manufacturing Processes
Rubber
Compounding Molding Trimming Finishing
Specialty products
Metal
TrimTec

Compounding

Molding

Trimming

Washing/Finishing

Vision Inspection
Metals

TrimTec
Market Knowledge Identifies Market Needs
Manufacturing Strategy
Capital Expenditures
Product Launches

Product Launch Process
Match need in market with West product
Target specific customers
Secure development partnerships
Sampling
Machine trials
Stability tests
Regulatory assistance
Analytical Labs

Product Launch Process
Plan – Initiate Launch Campaign
CAPEX investments
Promotion
Training
Sample kits
Broad market - product launch (2 - 3 years later)
Market experience
Market acceptance/regulatory approval
Customer ramp-ups

Product Launches
Westar RS
Elastomer components
Seals
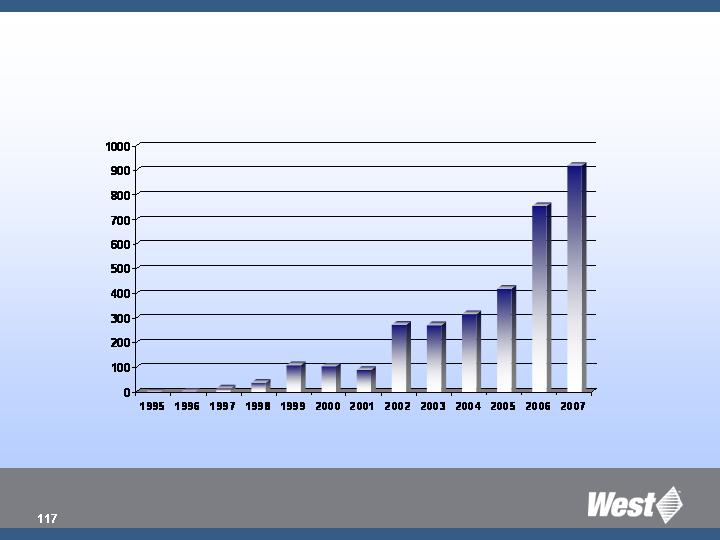
Westar Units Sold in North America
(in millions)

Product Launches
Drive development through superior positioning
FluroTec
Unrivaled
barrier
coating
Westar
State of the art
ready-to-sterilize
elastomer closures
Flip-Off CCS
Prewashed and
sterilized Flip-Off
seals
West Spectra
Covert and overt
security for product
authentication

Branding of Value-Added
Products & Services
West Analytical Services


Tech Restructuring
(December 2007)
Pfizer decision to abandon Exubera
Need to right-size operations
Accelerate transition to IP-based systems supplier

Tech Restructuring Activities
Close Erie, PA tool shop
Right size SG&A and Engineering
Mexico - transfers
Mold moves to create “focused factories”
Grand Rapids turnaround

Strategic Business Units
Organize SBU’s around markets
Consumer
Health Care
Device Systems
General Managers with P&L responsibilities
Improved profitability
Allocation of resources
Provide integrated approach to serve customers
Invest CAPEX based on market/strategic plan
Shed unprofitable business
Tech Group Synergies
Manufactured
by Tech
Product/ Market
Partner
Growth Platform
Tech Group Synergies
Reconstitution
Medimop
Safety & Admin.
Systems
Manufactured
by Tech
Product/ Market
Partner
Growth Platform
Tech Group Synergies
Orion Safety Needle
Salvus Tech
Safety & Admin.
Systems
Reconstitution
Medimop
Safety & Admin.
Systems
Manufactured
by Tech
Product/ Market
Partner
Growth Platform
Tech Group Synergies
Orion Safety Needle
Salvus Tech
Safety & Admin.
Systems
Unique Silicone Oil
Free, Tungsten Free
Syringe System
Daikyo Crystal
Zenith
Prefillable Syringe
Systems
Reconstitution
Medimop
Safety & Admin.
Systems
Manufactured
by Tech
Product/ Market
Partner
Growth Platform
Tech Group Synergies
Orion Safety Needle
Salvus Tech
Safety & Admin.
Systems
Auto Injector
In-house with
Pharma-Pen
Advanced Injection
Unique Silicone Oil
Free, Tungsten Free
Syringe System
Daikyo Crystal
Zenith
Prefillable Syringe
Systems
Reconstitution
Medimop
Safety & Admin.
Systems
Manufactured
by Tech
Product/ Market
Partner
Growth Platform

Tech Direction
Transition to proprietary intellectual property (IP)
Synergies with Innovation and core Pharmaceutical Systems
Conversion from component to systems provider

Summary
Strengthen market leadership position
Grow faster than market
Record number of product introductions
Sufficient cash flow to fund CAPEX and R&D
Accelerate innovation activities:
In-house R&D
Licensing
Strategic partnering
Acquisitions
Attractive opportunities to expand geographically
Synergies of core business with Tech and Innovation create a bright
future

Summary
Delivering Innovative Solutions
Don Morel
Chairman and Chief Executive Officer

Grow revenues and earnings
despite reduced sales of certain products
Organic growth and sales mix will offset lost revenue
associated with Exubera, ESA drugs etc.
Offset cost increases (raw materials, labor, energy) with lean
programs, operating efficiencies and pricing
Generate Tech Group performance improvement
New Grand Rapids facility recovery from 2007 relocation and
expansion
Execute restructuring of operating footprint
Shift product mix: focus on healthcare and proprietary products
Effectively manage global capacity expansion
Monitor relevant changes in demand, lead times
Timely availability of increased capacity
Management Operating Priorities
2008 - 2012

Continue Investing for the future
Innovation programs
Aggressive launch schedule
Geographic expansion plans - China, India
IT Platforms
Management Operating Priorities
2008 - 2012

Investment Considerations
Established foundation for sustainable growth
Significant barriers to entry
Favorable growth drivers in key market segments
Global, diverse customer base
Global manufacturing capability
Strong, experienced management team
Strong corporate governance

2008 Annual Guidance
Revenue estimated between $1.05 and $1.07 billion
Estimated adjusted earnings per diluted share is between $2.40
and $2.50. This estimate:
Excludes anticipated restructuring costs of an approximately $8.6 million
Includes exchange rate assumption of $1.40/1 Euro
Includes between $50 million and $60 million of lower revenue estimates
compared to 2007 and associated with specific customer products,
including Exubera®, ESA drugs and diagnostic device components, which
should adversely impact operating profit by approximately 35% of the
revenue impact

2008 Annual Guidance
Lower revenues from those specified products will have a much
more pronounced impact on periodic comparisons to 2007
during the first half of 2008
Specified products contributed earnings per diluted share of $0.14 in the
first quarter and $0.11 in the second quarter of 2007, as those periods
predated events affecting sales of those products
Impact on comparisons should diminish through the year as other sales
growth is expected to begin to offset and ultimately exceed revenue
losses

Five-Year Financial Goals
Expect average annual sales growth of between 7% and 9%, excluding
acquisitions
Growth in existing products, supported by capacity expansions, in the near- term
New products and geographic expansion contribute significantly beginning in
2010.
Operating profit growth should average between 10% and 12% per year
Sales mix should continue to improve on existing products,
Lean operations, ability to pass on commodity and energy costs, and operating
leverage are expected to protect and improve margins,
New products are expected to further enhance sales mix and profitability.

Five-Year Financial Goals
Continue to manage balance sheet and capital conservatively
Maintain net debt to total capital ratio between 30% and 40%,
excluding acquisitions
Acquisition activity focused on complementary, proprietary
products

Summary
Working through short-term revenue challenges
Long term business drivers remain in place
Strategic Product Platforms create sustainable growth opportunities
West’s competitive advantages uniquely position the Company to
capitalize on these opportunities:
Daikyo partnership
Global manufacturing footprint
Proprietary technology and systems
Strong regulatory barriers
Industry leading knowledge on drug – material interface
Solid balance sheet
Management incentives directly linked to value creation

GAAP and Adjusted EPS
2007
Diluted earnings per share from continuing operations
As reported $2.06
Restructuring, impairment and other charges 0.54
Tax adjustments/settlements (0.23)
As Adjusted $2.37
Our reported 2007 results include the impact of restructuring charges, an impairment loss on our
customer contract intangible asset with Nektar for the Exubera® device, and our provisions for
Brazilian tax issues which collectively totaled $26.4 million pre-tax ($19.4 million net of tax, or $0.54
per diluted share). Our 2007 results also include the recognition of discrete tax benefits totaling $8.2
million ($0.23 per diluted share).
Adjusted results are intended to aid investors in understanding the Company’s results absent non-
recurring or unusual events and are non-GAAP financial measures.

Investor Day 2008