- WST Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
8-K Filing
West Pharmaceutical Services (WST) 8-KRegulation FD Disclosure
Filed: 18 Mar 08, 12:00am

Donald E. Morel, Jr., Ph.D.
Chairman and Chief Executive Officer
William J. Federici
Vice President and Chief Financial Officer
Investor Relations Contact:
Michael A. Anderson
Vice President and Treasurer
mike.anderson@westpharma.com
Lehman Brothers 11th Annual Global Healthcare Conference
Miami Beach, Florida
March 18, 2008
NYSE: WST
westpharma.com
1

Certain statements in the following slides and certain statements that may be made by management of the Company orally during this
presentation contain some forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section
21E of the Securities Exchange Act of 1934, as amended, that are based on management’s plans and assumptions. Such statements give our
current expectations or forecasts of future events; they do not relate strictly to historical or current facts. We have tried, wherever possible, to
identify such statements by using words such as “estimate,” “expect,” “intend,” “believe,” “plan,” “anticipate” and other words and terms of
similar meaning in connection with any discussion of future operating or financial performance or condition. We cannot guarantee that any
forward-looking statement will be realized. If known or unknown risks or uncertainties materialize, or if underlying assumptions are inaccurate,
actual results could differ materially from past results and those expressed or implied in any forward-looking statement. You should bear this in
mind as you consider forward-looking statements. We undertake no obligation to publicly update forward-looking statements, whether as a result
of new information, future events or otherwise.
Important factors that may affect future results include the following: Sales demand and our ability to meet that demand; competition from other
providers in the Company’s businesses, including customers’ in-house operations, and from lower-cost producers in emerging markets, which
can impact unit volume, price and profitability; customers’ changing inventory requirements and manufacturing plans that alter existing orders or
ordering patterns for the products we supply to them; the timing, regulatory approval and commercial success of customer products that
incorporate our products, including relevant third-party reimbursement for prescription products, medical devices and components and medical
procedures in which those products are employed or consumed; average profitability, or mix, of products sold in any reporting period;
maintaining or improving production efficiencies and overhead absorption; the timeliness and effectiveness of capital investments, particularly
capacity expansions, including the effects of delays and cost increases associated with construction, availability and cost of capital goods, and
necessary internal, governmental and customer approvals of planned and completed projects, and the demand for goods to be produced in new
facilities; dependence on third-party suppliers and partners, including our Japanese partner Daikyo Seiko, Ltd.; the availability and cost of skilled
employees required to meet increased production, managerial, research and other needs of the Company, including professional employees and
persons employed under collective bargaining agreements; interruptions or weaknesses in our supply chain, which could cause delivery delays
or restrict the availability of raw materials and key bought-in components and finished products; raw-material price escalation, particularly
petroleum-based raw materials, and our ability to pass raw-material cost increases on to customers through price increases; claims associated
with product quality, including product liability, and the related costs of defending and obtaining insurance indemnifying the Company for the
cost of such claims; the cost and progress of development, regulatory approval and marketing of new products as a result of the Company’s
research and development efforts; the defense of self-developed or in-licensed intellectual property, including patents, trade and service marks
and trade secrets; dependence of normal business operations on information and communication systems and technologies provided, installed or
operated by third parties, including costs and risks associated with planned upgrades to existing business systems; the relative strength of the
U.S. dollar in relation to other currencies, particularly the Euro, British Pound, and Japanese Yen; changes in tax law or loss of beneficial tax
incentives; the conclusion of unresolved tax positions consistent with currently expected outcomes; the timely execution and realization of
savings anticipated by the restructuring plan for certain operations and functions of The Tech Group, announced in December 2007; and,
Other risks and uncertainties detailed in West’s filings with the Securities and Exchange Commission, including our Statement on Form 10-K filed
with the SEC on February 29, 2008. You should evaluate any statement in light of these important factors.
Safe Harbor Statement: Forward Looking Statements
Trademarks: All trademarks and registered trademarks used in this report are the property of West Pharmaceutical Services,
Inc., in the United States and other jurisdictions, unless otherwise noted.
2

Founded in 1923
HQ in Lionville, PA
Company Overview
3

Who we are
World’s premier manufacturer
of components and systems
for injectable drug delivery
Closure systems and prefillable
syringe components
Components for disposable systems
Devices and device sub-assemblies
Safety and administration systems
Record 2007 sales - $1.02 billion
Market cap at 12/31/07 $1.3 billion
4

Diverse, Stable Customer Base
Company Estimated Market Share: 70% in Pharma; 70% in Device; 95% in
Biotech
5
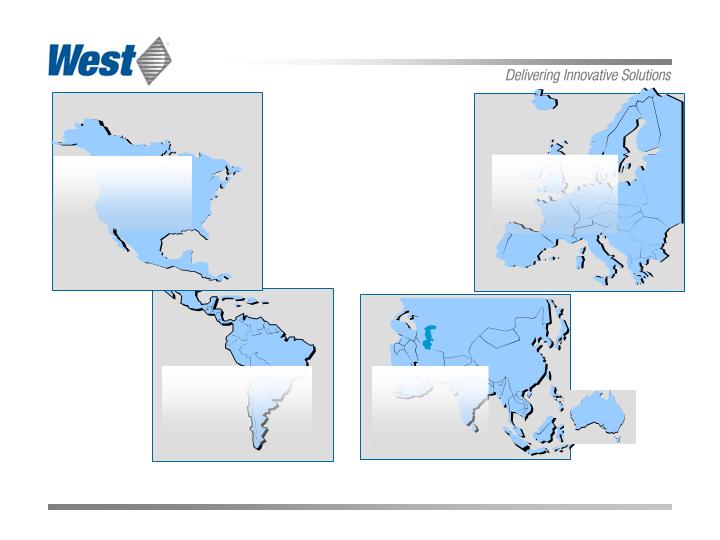
Global
Revenue
Breakdown
Based on 2007 sales
South America:
$55.3 million
(5%)
North America:
$496.4 million
(49%)
Europe:
$430.6 million
(42%)
Asia/Pacific:
$37.8 million
(4%)
6

2007 Overview – A Year of Contrasts
Achieved record sales and earnings
Extended Daikyo sales and technology agreements to 2017
Acquired Pharma Pen Technology
Raised $161.5 million, 4% convertible junior sub debenture
Repurchased 1 million shares
China land use application approval and groundbreaking
Pfizer Exubera® exit
CMS reimbursement guidelines changed for ESA drugs
Initiated Tech Group restructuring
* Exubera is a registered trademark of Pfizer, Inc.
7

Four Strategic Growth Platforms
Advanced Injection Systems
Injectable Container Solutions
Prefillable Syringe Systems
Safety + Administration Systems
8

West’s Competitive Advantage
Unmatched experience/expertise: drug - material interface
Ability to source components globally from multiple locations
Protected IP: West’s components and systems
Regulatory barrier to entry: NDA and ANDA filing must include reference to all
packaging/components in contact with the drug
1.
West’s Drug Master File (DMF) is confidential
2.
West’s DMF includes functionality data (multi-year studies)
3.
All primary package changes require new stability/functionality
studies for new filing
Engineering expertise in high-volume manufacturing and assembly
9

Growth Platform 1 – Injectable Container Solutions
West FluroTec®
Components
Seal - Stopper - Vial
Daikyo Crystal Zenith® Vials
Estimated Market Size – $1.5 BN
CAGR – 4%
Source: Company estimate for vial systems only
West Spectra™ Seals
10

Growth Platform 2 – Safety and Administration Systems
Vial2Bag™
Mix2Vial™
MixJect™
Total Market – $1.5 BN
CAGR – 11%
Source: Greystone Associates and Company estimate
Project Orion
11

Future Growth Drivers
Aging population creates an increasing number of patients
with chronic illnesses such as diabetes and cancer
Biologic drug growth and a resurgence in vaccine research
12
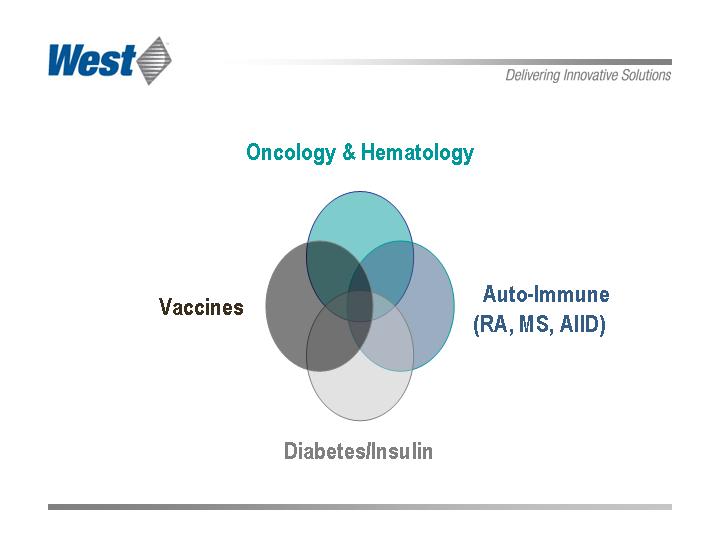
West’s Therapeutic Targets
13

Future Growth Drivers
Demand for ultra clean silicone free packaging and delivery
systems
14

Pharma/biotech Customer Trends
Integration of container/closure system into delivery system
Reduction of drug waste and improved operational
throughput
High quality packaging to ensure drug stability
No extractables or leachables
No silicone oil
Value-added devices and systems for product/brand
differentiation
Secure packaging
15

Growth Platform 3 - Prefillable Syringe Systems
FluroTec® Plungers
Needle Shields – Tip Caps
Daikyo Crystal Zenith®
Staked Needle Syringe
Estimated Market Size – $900 MM
CAGR – 8%
Source: Company estimates
RU Daikyo Crystal Zenith®
luer lock syringe
16

Future Growth Drivers
Build the right capacity in the right areas: Europe, North
America, China, India
17

Investments in Operations
Europe and Asia Expansion Projects
China Greenfield
China plastics in Qingpu
USA Expansions
IT Platforms – SAP and Shop Floor MES
Jurong,
Singapore
Bodmin,
England
Kovin,
Serbia
LeNouvion,
France
Eschweiler,
Germany
Jersey Shore,
Pennsylvania
Clearwater,
Florida
Kinston,
North Carolina
18

Future Growth Drivers
Point-of-care shift: Hospital Specialty Clinic Home
Safety; ease of use; dosing accuracy; compliance
Convergence of the primary container and delivery device
West migration from component production to device systems
19

Convergence of Primary Containers and Delivery Systems
Disposable
Syringe-based
Auto Injectors
Traditional Injection
System
Components for
Pen System Applications
20

Growth Platform 4 - Advanced Injection Systems
Estimated Market Size - $210 million
CAGR – 8%
Source: Greystone Associates and Company estimates
Pre-filled Syringe
Pre-filled Syringe (PFS) as
Primary Drug Container with
elastomeric components (plunger
and needle shield)
21

Future Growth Drivers
Shift Tech Group business model to proprietary products
22
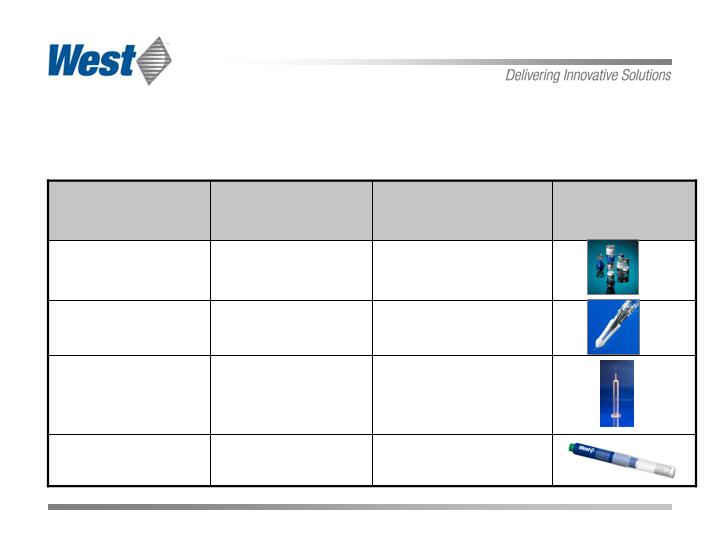
Tech Group Synergies
Orion Safety Needle
Salvus Tech
Safety & Admin.
Systems
Auto Injector
In-house with
Pharma-Pen
Advanced Injection
Unique Silicone Oil
Free, Tungsten Free
Syringe System
Daikyo Crystal
Zenith
Prefillable Syringe
Systems
Reconstitution
Medimop
Safety & Admin.
Systems
Manufactured
by Tech
Product/ Market
Partner
Growth Platform
23

Full Year Comparisons
($ in millions, except per share data)
$1.83
$2.06
EPS from Continuing Operations - GAAP
11.1
16.1
R&D
147.8
152.5
SG&A
$1.93
$2.37
EPS from Continuing Operations - Non-GAAP
61.5
71.2
Income from Continuing Operations
29.0%
28.6%
Gross Margin %
$913.3
$1,020.1
Net Sales
2006
2007
Full year 2006 Non-GAAP EPS from continuing operations excludes a $0.12 charge related to the
refinancing of senior notes and a $0.02 favorable tax benefit related to the settlement of a prior year
tax claim.
Full year 2007 Non-GAAP EPS from continuing operations excludes $0.31 of discrete tax benefits,
restructuring & impairment charges and an unfavorable impact for Brazilian social security, excise and
other tax compliance issues.
24

Capital Management
$129.4
$90.3
Full Year Spending
Capital Expenditures:
31.1%
36.9%
Net Debt to Total Capital
$419.3
$490.9
Total Equity & Minority Interests
$236.3
$395.1
Total Debt
12/31/06
12/31/07
($millions)
25

Grow revenues and earnings despite reduced sales of certain products
Organic growth and sales mix will offset lost revenue associated with Exubera, ESA drugs etc.
Offset cost increases (RM, labor, energy) with lean programs, operating efficiencies and pricing
Generate Tech Group performance improvement
New Grand Rapids facility recovery from 2007 relocation and expansion
Execute restructuring of operating footprint
Shift product mix: focus on healthcare and proprietary products
Effectively manage global capacity expansion
Monitor relevant changes in demand, lead times
Timely availability of increased capacity
Continue Investing for the future
Innovation programs
Aggressive launch schedule
Geographic expansion plans - China, India
IT Platforms
2008 - 2012 Management Operating Priorities
26

2008 Annual Guidance
Revenue estimated between $1.05 and $1.07 billion
Estimated adjusted earnings per diluted share is between $2.40 and $2.50. This
estimate:
Excludes anticipated restructuring costs of an approximately $8.6 million
Includes exchange rate assumption of $1.40/1 Euro
Includes between $50 million and $60 million of lower revenue estimates compared to
2007 and associated with specific customer products, including Exubera®, ESA drugs
and diagnostic device components, which should adversely impact operating profit by
approximately 35% of the revenue impact
27

Summary
Working through short-term revenue challenges
Long term business drivers remain in place
Strategic Product Platforms create sustainable growth opportunities
West’s competitive advantages uniquely position the Company to capitalize
on these opportunities:
Daikyo partnership
Global manufacturing footprint
Proprietary technology and systems
Strong regulatory barriers
Industry leading knowledge on drug – material interface
Strong capital position
Management incentives directly linked to value creation
28

Donald E. Morel, Jr., Ph.D.
Chairman and Chief Executive Officer
William J. Federici
Vice President and Chief Financial Officer
Investor Relations Contact:
Michael A. Anderson
Vice President and Treasurer
mike.anderson@westpharma.com
Lehman Brothers 11th Annual Global Healthcare Conference
Miami Beach, Florida
March 18, 2008
NYSE: WST
westpharma.com
29