
1 West Pharmaceutical Services, Inc.

2 Safe harbor statement Cautionary Statement Under the Private Securities Litigation Reform Act of 1995 This presentation and any accompanying management commentary contain “forward-looking statements” as that term is defined in the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements about product development and operational performance. Each of these statements is based on preliminary information, and actual results could differ from any preliminary estimates. We caution investors that the risk factors listed under “Cautionary Statement” in our press releases, as well as those set forth under the caption "Risk Factors" in our most recent Annual Report on Form 10-K as filed with the Securities and Exchange Commission and as revised or supplemented by our quarterly reports on Form 10-Q, could cause our actual results to differ materially from those estimated or predicted in the forward-looking statements. You should evaluate any statement in light of these important factors. Except as required by law or regulation, we undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events, or otherwise. Non-U.S. GAAP Financial Measures Certain financial measures included in these presentation materials, or which may be referred to in management’s discussion of the Company’s results and outlook, have not been calculated in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”), and therefore are referred to as non-U.S. GAAP financial measures. Non-U.S. GAAP financial measures should not be considered in isolation or as an alternative to such measures determined in accordance with U.S. GAAP. Trademarks West and the diamond logo and By your side for a healthier world™, NovaPure®, EnvisionTM, Westar®, ConfiDose®, SelfDose®, and FluroTec® are trademarks or registered trademarks of West Pharmaceutical Services, Inc., in the United States and other jurisdictions. SmartDose® is a registered trademark of Medimop Medical Projects Ltd., a subsidiary of West Pharmaceutical Services, Inc. Daikyo Crystal Zenith® is a registered trademark of Daikyo Seiko, Ltd. Daikyo Crystal Zenith® technology is licensed from Daikyo Seiko, Ltd.

3 Who we are By your side… Every day, injectable drugs are administered to improve the lives of millions of patients around the world. West works side-by-side with its pharmaceutical, biopharmaceutical, generic and medical device partners to design and manufacture packaging and delivery systems that will bring their drugs from concept to the patient efficiently and reliably.

4 Proprietary Products Stoppers Seals Caps Prefillable Syringes & Components Self-Injection Devices CZ Vials & Syringes Drug Reconstitution Administration Proprietary Products $1.1B Contract Manufacturing $0.3B West’s role in delivering medicines to patients Contract Manufacturing Engineering Injection Molding Project Management Tooling Chart reflects 2014 relative net sales of $1.4 billion
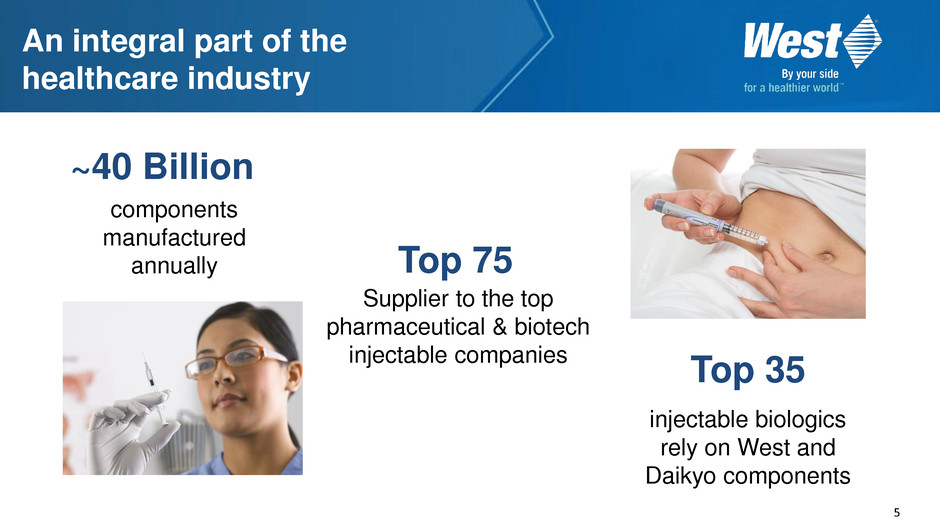
5 An integral part of the healthcare industry Top 35 injectable biologics rely on West and Daikyo components ~40 Billion components manufactured annually Top 75 Supplier to the top pharmaceutical & biotech injectable companies

6 Market trends support growth opportunities West is well-positioned to offer packaging & delivery options to address customer needs Increase in Generics & Biosimilars Biologic Product Pipelines Demand for Alternative Treatment & Delivery Options Aging Population & Developing Economies Increase Demand Increasing Quality Standards in Manufacturing

7 West business—at a glance 2014 Net Sales by Category No customer greater than 8% of net sales Operations in 20 countries & 26 sites >7,000 employees 7% 35% 6% 31% High-Value Components Standard Packaging Disposable Components Proprietary Devices Contract Manufacturing 2014 Net Sales by Geography 49% 44% 7% Americas Europe, Middle East, Africa Asia Pacific Proprietary Products 21%

Sustained, consistent growth 2010 2011 2012 2013 2014 6.1% CAGR $1,104.7 $1,421.4 2010 2011 2012 2013 2014 $0.95 $1.75 2010 2011 2012 2013 2014 + 260 BPS 7.6% 10.2% Net Sales 2010 – 2014 (reported, $ millions) Diluted EPS 2010 – 2014 ROIC 2010 – 2014 8 6.1% CAGR Strong net sales & EPS growth and ROIC expansion 10.5% CAGR +260 BPS

Strong Operating Cash Flow Capital Allocation Priorities: Investing in our business Dividends Inorganic growth Debt repayment Stock repurchase Cash-flow and allocation 9
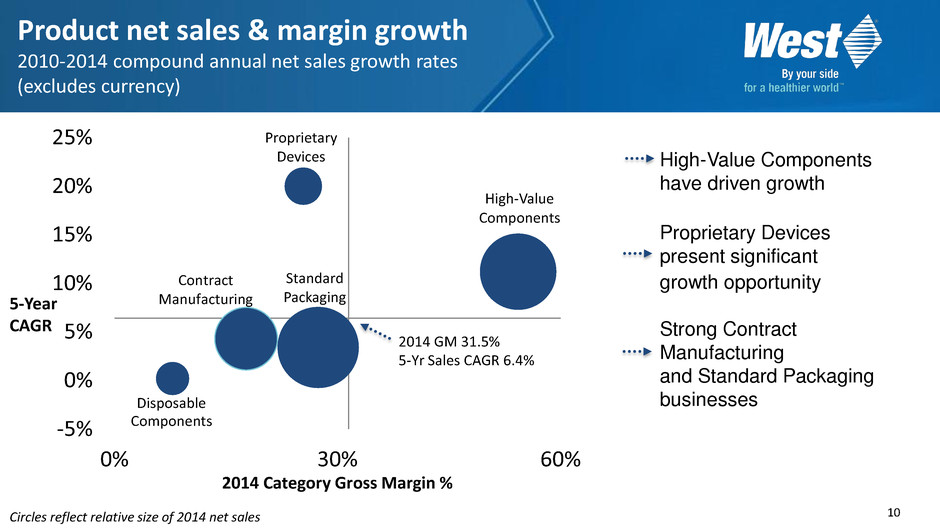
10 Circles reflect relative size of 2014 net sales Disposable Components Standard Packaging High-Value Components -5% 0% 5% 10% 15% 20% 25% 0% 30% 60% 2014 Category Gross Margin % Proprietary Devices Contract Manufacturing 2014 GM 31.5% 5-Yr Sales CAGR 6.4% Product net sales & margin growth 2010-2014 compound annual net sales growth rates (excludes currency) High-Value Components have driven growth Proprietary Devices present significant growth opportunity Strong Contract Manufacturing and Standard Packaging businesses 5-Year CAGR
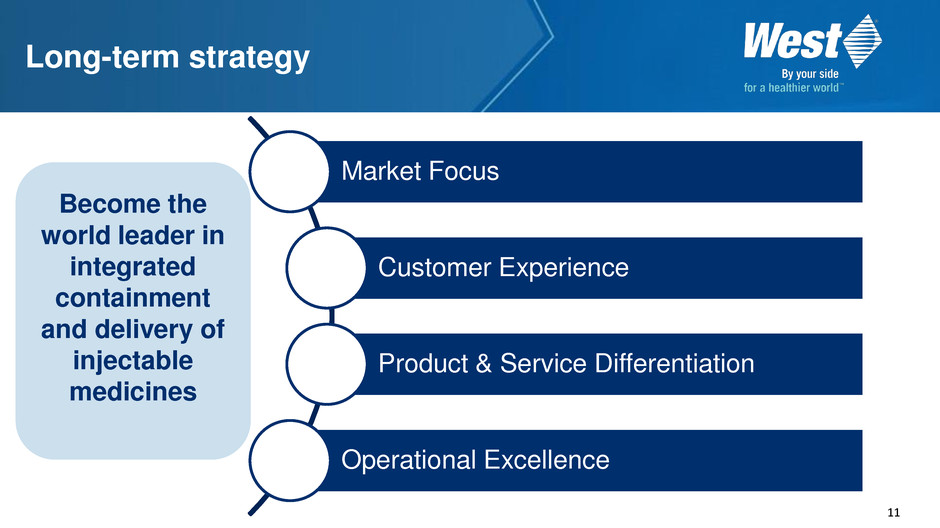
11 Long-term strategy Market Focus Customer Experience Product & Service Differentiation Operational Excellence Become the world leader in integrated containment and delivery of injectable medicines

12 Market focus Biologics are expected to account for 50% of the top 100 selling drugs in 2016. The injectable drug volume market is growing at a 6% CAGR. Generics expected to represent 10.5% of total prescriptions by 2020. West can address these unique market needs with tailored product and service offerings Pharmaceutical Biologics Generics Source: IMS, Patient Safety & Quality Healthcare, EvaluatePharma, team estimates

13 Differentiated products: Increasing levels of quality Coated Washed Sterilized Camera- inspected Top Quality West can deliver increasing levels of quality to suit particular customer and drug requirements
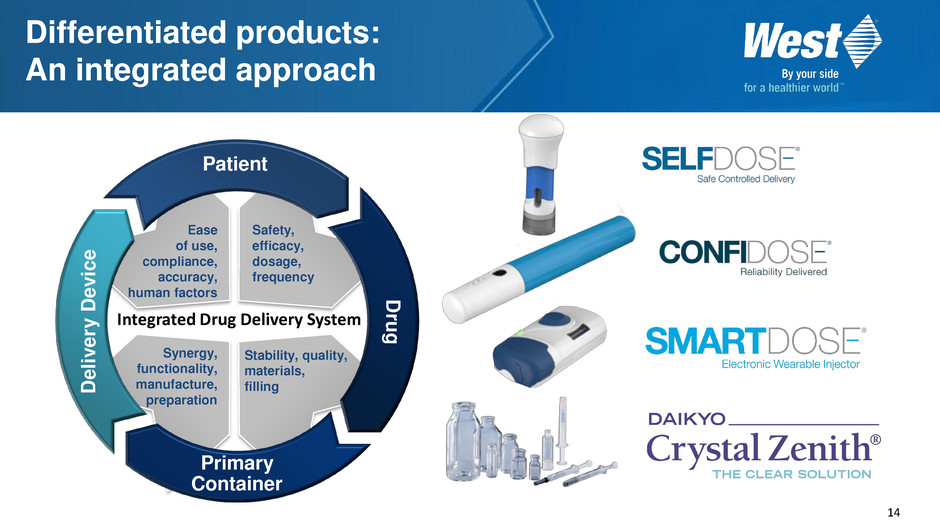
14 Ease of use, compliance, accuracy, human factors Safety, efficacy, dosage, frequency Synergy, functionality, manufacture, preparation Stability, quality, materials, filling Patient Dru g D e li v e ry D e v ic e Primary Container Integrated Drug Delivery System Differentiated products: An integrated approach

15 Focused on pharmaceutical and medical device markets Recognized expertise in design, manufacture and automated assembly of complex delivery devices Key partner in the growing diabetes market Received Frost & Sullivan Manufacturing Leadership Award Excellence in quality control Differentiated services: Contract Manufacturing

16 Operational excellence Leveraging our manufacturing plants Establishing centers of excellence to serve key markets New campus facility in Waterford, Ireland Expanded & upgraded Kinston, NC & Singapore New India plant serving emerging markets Lean and operational excellence initiatives to drive margin and ROIC expansion Commitment to quality continues to drive demand for premium products

17 Key partner to pharmaceutical, biotech, generic and medical device customers Strong competitive position with high barriers to entry Designed into regulated products Substantial investment in global footprint Recognized for scientific and technical expertise Differentiated product offerings Recognized for delivering quality Market drivers support business model Proprietary packaging, containment and delivery products expected to drive net sales growth and margin expansion Financial strength to invest Strong balance sheet and operating cash flow Take-away messages

18 West Pharmaceutical Services, Inc.