UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended July 31, 2014
☐TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from _______ to _______
Commission file number 000-25169
GENEREX BIOTECHNOLOGY CORPORATION
(Exact name of registrant as specified in its charter)
| Delaware | 98-0178636 | |
| (State or other jurisdiction of | (I.R.S. Employer | |
| incorporation or organization) | Identification No.) | |
| 555 Richmond Street West, Suite 604, Toronto, Canada | M5V 3B1 | |
| (Address of principal executive offices) | (Zip Code) |
(416) 364-2551
(Registrant’s telephone number, including area code)
N/A
(Former name, former address and former fiscal year, if changed since last report)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Name of each exchange on which registered | |
| Common Stock, $.001 par value per share |
Securities registered pursuant to Section 12(g) of the Act:None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§229.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
| i |
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer ☐ | Accelerated filer ☐ | ||
Non-accelerated filer ☐ (Do not check if a smaller reporting company) | Smaller reporting company ☒ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes ☐ No ☒
As of January 31, 2014, the aggregate market value of the registrant’s common stock held by non-affiliates of the registrant was $29,471,312 based on the average bid and asked price at which such stock was last sold as of such date. Generex Biotechnology Corporation has no non-voting common equity. At October 1, 2014, there were 778,512,092 shares of common stock outstanding.
Documents Incorporated by Reference
Portions of the Proxy Statement for the registrant’s 2014 Annual Meeting of Stockholders, or an amendment to this Annual Report on Form 10-K, to be filed within 120 after the end of the fiscal year ended July 31, 2014, are incorporated by reference into Part III of this Annual Report on Form 10-K.
| ii |
Generex Biotechnology Corporation
Form 10-K
July 31, 2014
Index
| Page | ||||
| Forward-Looking Statements | ||||
| Part I | ||||
| Item 1. | Business. | 1 | ||
| Item 1A. | Risk Factors. | 14 | ||
| Item 1B. | Unresolved Staff Comments. | 20 | ||
| Item 2. | Properties. | 20 | ||
| Item 3. | Legal Proceedings. | 21 | ||
| Item 4. | [Not Required for Registrant] | 22 | ||
| Executive Officers of the Registrant | 22 | |||
| Part II | ||||
| Item 5. | Market For Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities. | 23 | ||
| Item 6. | Selected Financial Data. | 26 | ||
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations. | 26 | ||
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk | 37 | ||
| Item 8. | Financial Statements and Supplementary Data. | 38 | ||
| Item 9. | Changes in and Disagreements With Accountants on Accounting and Financial Disclosure. | 63 | ||
| Item 9A. | Controls and Procedures. | 63 | ||
| Item 9B. | Other Information. | 63 | ||
| Part III | ||||
| Item 10. | Directors, Executive Officers and Corporate Governance. | 64 | ||
| Item 11. | Executive Compensation. | 64 | ||
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters. | 64 | ||
| Item 13. | Certain Relationships and Related Transactions, and Director Independence. | 64 | ||
| Item 14. | Principal Accountant Fees and Services. | 64 | ||
| Part IV | ||||
| Item 15. | Exhibits and Financial Statement Schedules. | 64 | ||
| Signatures | 65 | |||
As used herein, the terms the “Company,” “Generex,” “we,” “us,” or “our” refer to Generex Biotechnology Corporation, a Delaware corporation.
| iii |
Forward-Looking Statements
Certain matters in this Annual Report on Form 10-K, including, without limitation, certain matters discussed underItem 1 - Business,Item 1A - Risk Factors,Item 7 - Management’s Discussion and Analysis of Financial Condition and Results of OperationsandItem 7A - Quantitative and Qualitative Disclosures about Market Risk, constitute “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. All statements, other than statements of historical facts, included in this Annual Report that address activities, events or developments that we expect or anticipate will or may occur in the future, including such matters as our projections, future capital expenditures, business strategy, competitive strengths, goals, expansion, market and industry developments and the growth of our businesses and operations, are forward-looking statements. These statements can be identified by introductory words such as "expects," “anticipates,” "plans," "intends," "believes," "will," "estimates," "projects" or words of similar meaning, and by the fact that they do not relate strictly to historical or current facts. Our forward-looking statements address, among other things:
| • | our expectations concerning product candidates for our technologies; | |
| • | our expectations concerning existing or potential development and license agreements for third-party collaborations and joint ventures; | |
| • | our expectations of when different phases of clinical activity may commence and conclude; | |
| • | our expectations of when regulatory submissions may be filed or when regulatory approvals may be received; and | |
| • | our expectations of when commercial sales of our products may commence and when actual revenue from the product sales may be received. |
Any or all of our forward-looking statements may turn out to be wrong. They may be affected by inaccurate assumptions that we might make or by known or unknown risks and uncertainties. Actual outcomes and results may differ materially from what is expressed or implied in our forward-looking statements. Among the factors that could affect future results are:
| • | the inherent uncertainties of product development based on our new and as yet not fully proven technologies; | |
| • | the risks and uncertainties regarding the actual effect on humans of seemingly safe and efficacious formulations and treatments when tested clinically; | |
| • | the inherent uncertainties associated with clinical trials of product candidates; | |
| • | the inherent uncertainties associated with the process of obtaining regulatory approval to market product candidates; | |
| • | the inherent uncertainties associated with commercialization of products that have received regulatory approval; | |
| • | the volatility of, and decline in, our stock price; and | |
| • | our ability to obtain the necessary financing to fund our operations and effect our strategic development plan. |
Additional factors that could affect future results are set forth below underItem 1A. Risk Factors. We caution investors that the forward-looking statements contained in this Report must be interpreted and understood in light of conditions and circumstances that exist as of the date of this Report. We expressly disclaim any obligation or undertaking to update or revise forward-looking statements made in this Annual Report to reflect any changes in management's expectations resulting from future events or changes in the conditions or circumstances upon which such expectations are based.
Part I
Item 1. Business.
Corporate History and Structure
We were incorporated in Delaware in September 1997 for the purpose of acquiring Generex Pharmaceuticals Inc., a Canadian corporation formed in November 1995 to engage in pharmaceutical and biotechnological research and development and other activities. Our acquisition of Generex Pharmaceuticals was completed in October 1997 in a transaction in which the holders of all outstanding shares of Generex Pharmaceuticals exchanged their shares for shares of our common stock.
In January 1998, we participated in a "reverse acquisition" with Green Mt. P. S., Inc., an inactive Idaho corporation formed in 1983. As a result of this transaction, our shareholders (the former shareholders of Generex Pharmaceuticals) acquired a majority (approximately 90%) of the outstanding capital stock of Green Mt., we became a wholly-owned subsidiary of Green Mt., Green Mt. changed its corporate name to Generex Biotechnology Corporation ("Generex Idaho"), and we changed our corporate name to GB Delaware, Inc. Because the reverse acquisition resulted in our shareholders becoming the majority holders of Generex Idaho, we were treated as the acquiring corporation in the transaction for accounting purposes. Thus, our historical financial statements, which essentially represented the historical financial statements of Generex Pharmaceuticals, were deemed to be the historical financial statements of Generex Idaho.
| 1 |
In April 1999, we completed a reorganization in which we merged with Generex Idaho. In this transaction, all outstanding shares of Generex Idaho were converted into our shares, Generex Idaho ceased to exist as a separate entity, and we changed our corporate name back to "Generex Biotechnology Corporation." This reorganization did not result in any material change in our historical financial statements or current financial reporting.
Subsidiaries
Following our reorganization in 1999, Generex Pharmaceuticals Inc., which is incorporated in Ontario, Canada, remained as our wholly-owned subsidiary. All of our Canadian operations are performed by Generex Pharmaceuticals. Generex Pharmaceuticals is the 100% owner of 1097346 Ontario Inc., which is also incorporated in Ontario, Canada. In August 2003, we acquired Antigen Express, Inc., a Delaware incorporated company. Antigen is engaged in the research and development of technologies and immunomedicines for the treatment of malignant, infectious, autoimmune and allergic diseases. Antigen also does business under the names Generex Oncology and Generex Infectious Diseases.
We formed Generex (Bermuda), Inc., which is organized in Bermuda, in January 2001 in connection with a joint venture with Elan International Services, Ltd., a wholly-owned subsidiary of Elan Corporation, plc, to pursue the application of certain of our and Elan's drug delivery technologies, including our platform technology for the buccal delivery of pharmaceutical products. In December 2004, we and Elan agreed to terminate the joint venture. Under the termination agreement, we retained all of our intellectual property rights and obtained full ownership of Generex (Bermuda). Generex (Bermuda) does not currently conduct any business activities. We have additional subsidiaries incorporated in the U.S. and Canada which are dormant and do not carry on any business activities.
Overview of Business
We are engaged primarily in the research and development of drug delivery systems and technologies. Our primary focus at the present time is our proprietary technology for the administration of formulations of large molecule drugs to the oral (buccal) cavity using a hand-held aerosol applicator. Through our wholly-owned subsidiary, Antigen, we have expanded our focus to include immunomedicines incorporating proprietary vaccine formulations.
We believe that our buccal delivery technology is a platform technology that has application to many large molecule drugs and provides a convenient, non-invasive, accurate and cost-effective way to administer such drugs. We have identified several large molecule drugs as possible candidates for development, including estrogen, heparin, monoclonal antibodies, human growth hormone and fertility hormones, but to date have focused our development efforts primarily on one pharmaceutical product, Generex Oral-lyn™, an insulin formulation administered as a fine spray into the oral cavity using our proprietary hand-held aerosol spray applicator known as RapidMist™.
Our wholly-owned subsidiary, Antigen, concentrates on developing proprietary vaccine formulations that work by stimulating the immune system to either attack offending agents (i.e., cancer cells, bacteria, and viruses) or to stop attacking benign elements (i.e., self proteins and allergens). Our immunomedicine products are based on two platform technologies and are in the early stages of development. We continue clinical development of Antigen’s synthetic peptide vaccines designed to stimulate a potent and specific immune response against tumors expressing the HER-2/neu oncogene for patients with HER-2/neu positive breast cancer in a Phase II clinical trial and patients with prostate cancer and against avian influenza in two Phase I clinical trials. We also initiated an additional Phase I clinical trial in patients with either breast or ovarian cancer. The synthetic vaccine technology has certain advantages for pandemic or potentially pandemic viruses, such as the H5N1 avian and H1N1 swine flu. In addition to developing vaccines for pandemic influenza viruses, we have vaccine development efforts underway for seasonal influenza virus, HIV, HPV, melanoma, ovarian cancer, allergy and Type I diabetes mellitus. We have established collaborations with clinical investigators at academic centers to advance these technologies.
To date, we have received regulatory approval in Ecuador, India (subject to regulatory approval of a 2012 in-country study), Lebanon and Algeria for the commercial marketing and sale of Generex Oral-lyn™. We have previously submitted regulatory dossiers for Generex Oral-lyn™ in a number of other countries, including Bangladesh, Kenya, Jordan and Armenia. While we believe these countries will ultimately approve our product for commercial sale, we do not anticipate recognizing revenues in any of these jurisdictions in the next twelve months. No dossier related activities or product shipments have taken place during fiscal 2012 or 2013, nor are any expected to these countries during the remainder of 2014. In March 2008, we initiated Phase III clinical trials for this product in the U.S. with the first patient screening for such trials at a clinical study site in Texas in April 2008. Approximately 450 patients have been enrolled to date at approximately 70 clinical sites around the world, including sites in the United States, Canada, Bulgaria, Poland, Romania, Russia, Ukraine and Ecuador. The final subjects completed the trial in August 2011. After appropriate validation, the data from approximately 450 patients was tabulated, reviewed and analyzed. Those results from the Phase III trial along with a comprehensive review and supplemental analyses of approximately 40 prior Oral-lyn clinical studies were compiled and submitted to the FDA in late December 2011 in a comprehensive package including a composite metanalysis of all safety data. We continue to have discussions with the FDA with respect to the pathway for regulatory approval, including any additional clinical or pharmacological studies that might be required to support regulatory approval or enhance marketing success. We do not currently plan to expend significant resources on additional clinical trials of Oral-lyn™ until after such time that we secure additional financing.
| 2 |
In November 2008 we, together with our marketing partner Shreya Life Sciences Pvt. Ltd., officially launched Generex Oral-lyn™ in India under marketing name of Oral Recosulin™. Each package of Oral Recosulin™ contains two canisters of our product along with one actuator. The product received regulatory price approval in India in January 2009. Per the requirements of the regulatory approval in India, an in-country clinical study must be completed in India with Oral Recosulin™ before commercial sales can commence. The field portion of the study was completed in the third calendar quarter of 2012. Shreya has advised Generex that the dossier was submitted in December of 2012 to the Drugs Controller General (India) (DCGI), Central Drugs Standard Control Organization, Director General of Health Services, Ministry of Health and Family Welfare, Government of India. Generex has provided additional, detailed scientific data to support the Shreya submission. We have not recognized any revenues from the sale of Generex Oral-lyn™ in India through fiscal year ended July 31, 2014.
In December 2008, we, together with our marketing partner Benta S.A., received an approval to market Generex Oral-lyn™ in Lebanon. The official product launch in Lebanon took place in May 2009. In May 2009, the Algerian health authorities granted us permission to import and sell Generex Oral-lyn™ for the treatment of diabetes in Algeria. The official product launch in Algeria took place in October 2009. To date, we have not recognized any revenue from the sales of Generex Oral-lyn™ in Algeria and very minimal revenues in Lebanon. We do not anticipate significant revenues (if any) to be recognized from these jurisdictions in the next twelve months.
In October 2008, we announced the enrollment of subjects in our bioequivalence clinical trial of MetControl™, our proprietary Metformin medicinal chewing gum product, conducted in the United States. The protocol for the study is an open-label, two-treatment, two-period, randomized, crossover study comparing MetControl™ and immediate release Metformin™ tablets in healthy volunteers. The study results that we received and analyzed in December 2008 demonstrated bioequivalence. We have, however, determined that the economics of proceeding with this product do not warrant the expenditure of further resources. We have not expended resources to further develop this product during the fiscal years ended July 31, 2014, 2013 and 2012 and do not currently plan to expend any further resources on this product.
We face competition from other providers of alternate forms of insulin. Some of our most significant competitors, Pfizer, Eli Lilly, and Novo Nordisk, have announced that they will discontinue development and/or sale of their inhalable forms of insulin. Generex Oral-lyn™ is not an inhaled insulin; rather, it is a buccally absorbed formulation with no residual pulmonary deposition. We believe that our buccal delivery technology offers several advantages, including the ease of use, portability, avoidance of pulmonary inhalation and safety profile. Furthermore, insulin administered through the Generex Oral-lyn™ RapidMist™ technology is absorbed directly into the blood stream and not only acts rapidly, but returns to baseline quickly, thereby minimizing the chance of developing hypoglycemia.
Large pharmaceutical companies, such as Merck & Co., Inc., GlaxoSmithKline PLC, Novartis, Inc., MedImmune Inc. (a subsidiary of Astra-Zeneca, Inc.) and others, also compete against us in the oncology, immunomedicine and vaccine markets. These companies have competing experience and expertise in securing government contracts and grants to support research and development efforts, conducting testing and clinical trials, obtaining regulatory approvals to market products, as well as manufacturing and marketing approved products. As such, they are also considered significant competitors in these fields of pharmaceutical products and therapies. There are also many smaller companies which are pursuing similar technologies in these fields who are considered to be competitors of Generex.
We are a development stage company with a limited history of operations, and do not expect sufficient revenues to support our operation in the immediately foreseeable future. To date, we have not been profitable and our accumulated net loss available to shareholders was $369,948,322 at July 31, 2014. As of July 31, 2014, our current cash position is not sufficient to meet our working capital needs for the next twelve months. To continue operations, we will require additional funds to support our working capital requirements and any development activities, or will need to suspend operations. Management is seeking various alternatives to ensure that we can meet some of our operating cash flow requirements through financing activities, such as private placement of our common stock, preferred stock offerings and offerings of debt and convertible debt instruments as well as through merger or acquisition opportunities. In addition, management is actively seeking strategic alternatives, including strategic investments and divestitures. Management has sold its non-essential real estate assets which were classified as Assets Held for Investment to augment its cash position. We cannot provide any assurance that we will obtain the required funding. Our inability to obtain required funding in the near future or our inability to obtain funding on favorable terms will have a material adverse effect on our operations and our strategic development plan for future growth. If we cannot successfully raise additional capital and implement our strategic development plan, our liquidity, financial condition and business prospects will be materially and adversely affected and we may have to cease operations.
We operate in only one segment: the research and development of drug delivery systems and technologies for metabolic and immunological diseases.
| 3 |
Our Business Strategy
Our business model focuses on the research and development of diabetes, oncology and infectious diseases drugs. This business model leverages the expertise of our management team, scientific advisory board and the history of our company. Our goal is to develop next generation drugs for diabetes, oncology and infectious disease by leveraging our buccal delivery technology to administer large and small molecule drugs, including insulin, and proprietary vaccine formulations based upon two Antigen platform technologies to provide innovative biopharmaceutical products that offer the potential for superior efficacy and safety over existing products. To achieve these goals, the key elements of our strategy include:
| • | Completing any additional studies or clinical trials of Generex Oral-lyn™, which may be required in order to obtain regulatory approval in major and other jurisdictions; |
| • | Developing a proprietary portfolio of products for the treatment of diabetes through strategic partnerships licensing and acquisitions; |
| • | A keystone of Generex’s strategy, announced at the annual meeting of stockholders in June 2011 is the proposed spin-out of Antigen Express as a separate company from Generex. Management believes that this action would allow Antigen to establish value for its immunotherapeutic vaccine technologies separate from the Generex buccal drug delivery platform technologies. The spin-out would be accomplished by the issuance of one or more dividends of Antigen Express stock to Generex stockholders; |
| • | Completing the ongoing Phase II clinical trials of Antigen’s synthetic peptide vaccines designed to stimulate a potent and specific immune response against tumors expressing the HER-2/neu oncogene for patients with HER-2/neu positive breast cancer, conducting a Phase II prostate cancer trial and a Phase I trial in patients with breast or ovarian cancer; |
| • | Conducting further clinical trials of Antigen’s synthetic peptide vaccines against avian (H5N1) influenza and initiating clinical trial of such vaccines against swine (H1N1) influenza; and |
| • | Exploring other applications for our RapidMist platform buccal technology; morphine, LWH, fentanyl (all of which have undergone Phase I clinical studies), as well as cell therapy for late stage diabetes. |
Buccal Delivery Technology and Products
Our buccal delivery technology involves the preparation of proprietary formulations in which an active pharmaceutical agent is placed in a solution with a combination of absorption enhancers and other excipients classified “generally recognized as safe” ("GRAS") by the United States Food and Drug Administration (the "FDA") when used in accordance with specified quantities and other limitations. The resulting formulations are aerosolized with a pharmaceutical grade chemical propellant and are administered to patients using our proprietary RapidMist™ brand metered dose inhaler. The device is a small, lightweight, hand-held, easy-to-use aerosol applicator comprised of a container for the formulation, a metered dose valve, an actuator and dust cap. Using the device, patients self-administer the formulations by spraying them into the mouth. The device contains multiple applications, the number being dependent, among other things, on the concentration of the formulation. Absorption of the pharmaceutical agent occurs in the buccal cavity, principally through the inner cheek walls. In clinical studies of our flagship oral insulin product Generex Oral-lyn™, insulin absorption in the buccal cavity has been shown to be efficacious and safe.
Buccal Insulin Product – Generex Oral-Lyn™
Insulin is a hormone that is naturally secreted by the pancreas to regulate the level of glucose, a type of sugar, in the bloodstream. The term “diabetes” refers to a group of disorders that are characterized by the inability of the body to properly regulate blood glucose levels. When glucose is abundant, it is converted into fat and stored for use when food is not available. When glucose is not available from food, these fats are broken down into free fatty acids that stimulate glucose production. Insulin acts by stimulating the use of glucose as fuel and by inhibiting the production of glucose. In a healthy individual, a balance is maintained between insulin secretion and glucose metabolism.
There are two major types of diabetes. Type 1 diabetes (juvenile onset diabetes or insulin dependent diabetes) refers to the condition where the pancreas produces little or no insulin. Type 1 diabetes accounts for 5-10 percent of diabetes cases. It often occurs in children and young adults. Type 1 diabetics must take daily insulin injections, typically three to five times per day, to regulate blood glucose levels. Generex Oral-lyn™ provides a needle-free means of delivering insulin for these patients.
In Type 2 diabetes (adult onset or non-insulin dependent diabetes mellitus), the body does not produce enough insulin, or cannot properly use the insulin produced. Type 2 diabetes is the most common form of the disease and accounts for 90-95 percent of diabetes cases. In addition to insulin therapy, Type 2 diabetics may take oral drugs that stimulate the production of insulin by the pancreas or that help the body to more effectively use insulin. Generex Oral-lyn™ provides a simple means of delivering needed insulin to this major cohort of individuals.
Current studies in diabetes have identified a new condition closely related to diabetes, called impaired glucose tolerance (IGT). People with IGT do not usually meet the criteria for the diagnosis of diabetes mellitus. They have normal fasting glucose levels but two hours after a meal their blood glucose level is far above normal. With the increase use of glucose tolerance tests the number of people diagnosed with this pre-diabetic condition is expanding exponentially. Per the 2012 Diabetes Atlas Update, published by the International Diabetes Federation (IDF), approximately 26 million people in the United States and 280 million people world-wide suffer from IGT. Generex Oral-lyn™ is an ideal solution to providing meal-time insulin to the millions of IGT sufferers. This therapeutic area is currently being investigated.
| 4 |
If not treated, diabetes can lead to blindness, kidney disease, nerve disease, amputations, heart disease and stroke. Each year, between 12,000 and 24,000 people suffer vision impairment or complete blindness because of diabetes. Diabetes is also the leading cause of end-stage renal disease (kidney failure), accounting for about 40 percent of new cases.
In addition, about 60-70 percent of people with diabetes have mild to severe forms of diabetic nerve damage, which, in severe forms, can lead to lower limb amputations. Diabetics are also two to four times more likely to have heart disease, which is present in 75 percent of diabetes-related deaths, and are two to four times more likely to suffer a stroke.
There is no known cure for diabetes. The IDF estimates that there are currently approximately 382 million diabetics worldwide per their 2013 Diabetes Atlas Update and is expected to affect over 592 million people by the year 2035. There are estimated to be over 37 million people suffering from diabetes in North America alone and diabetes is the second largest cause of death by disease in North America.
A substantial number of large molecule drugs (i.e., drugs composed of molecules with a high molecular weight and fairly complex and large spatial orientation) have been approved for sale in the United States or are presently undergoing clinical trials as part of the process to obtain such approval, including various proteins, peptides, monoclonal antibodies, hormones and vaccines. Unlike small molecule drugs, which generally can be administered by various methods, large molecule drugs historically have been administered predominately by injection. The principal reasons for this have been the vulnerability of large molecule drugs to digestion and the relatively large size of the molecule itself, which makes absorption into the blood stream through the skin inefficient or ineffective. The RapidMist technology provides a recognized and proved drug delivery system for the delivery of large molecules directly into the blood stream with the attendant advantages.
In May 2005, we received approval from the Ecuadorian Ministry of Public Health for the commercial marketing and sale of Generex Oral-lyn™ for treatment of Type 1 and Type 2 diabetes. We have successfully completed the delivery and installation of a turnkey Generex Oral-lyn™ production operation at the facilities of PharmaBrand in Quito, Ecuador. The first commercial production run of Generex Oral-lyn™ in Ecuador was completed in May, 2006. While Ecuador production capability may be sufficient to meet the needs of South America, it is believed to be insufficient for worldwide production for future commercial sales and clinical trials.
On the basis of the test results in Ecuador and other pre-clinical data, we made an IND submission to Health Canada (Canada's equivalent to the FDA) in July 1998, and received permission from the Canadian regulators to proceed with clinical trials in September 1998. We filed an Investigational New Drug application with the FDA in October 1998, and received FDA approval to proceed with human trials in November 1998. Annual reports have been filed with the FDA each year since that time.
We began our clinical trial programs in Canada and the United States in January 1999. Between January 1999 and September 2000, we conducted clinical trials of our insulin formulation involving approximately 200 subjects with Type 1 and Type 2 diabetes and healthy volunteers. The study protocols in most trials involved administration of two different doses of our insulin formulation following either a liquid Sustacal meal or a standard meal challenge. The objective of these studies was to evaluate our insulin formulation's efficacy in controlling post-prandial (meal related) glucose levels. These trials demonstrated that our insulin formulation controlled post-prandial hyperglycemia in a manner comparable to injected insulin. In April 2003, a Phase II-B clinical trial protocol was approved in Canada. In September 2006, a Clinical Trial Application relating to our Generex Oral-lyn™ protocol for late-stage trials was approved by Health Canada. The FDA’s review period for the protocol lapsed without objection in July 2007.
In late April 2008, we initiated Phase III clinical trials in North America for Generex Oral-lyn™ with the first subject screening in Texas. Other clinical sites participating in the study are located in the United States (Texas, Maryland, Minnesota and California), Canada (Alberta), European Union (Romania, Poland and Bulgaria), Eastern Europe (Russia and Ukraine),) and Ecuador. At present, approximately 450 subjects have been enrolled in the program at approximately 70 clinical sites around the world. The Phase III protocol called for a six-month trial with a six-month follow-up with the primary objective to compare the efficacy of Generex Oral-lyn™ and the RapidMist™ Diabetes Management System with that of standard regular injectable human insulin therapy as measured by HbA1c, in patients with Type-1 diabetes mellitus. The final subjects completed the trial in August 2011. After appropriate validation, the data from approximately 450 patients was tabulated, reviewed and analyzed. Those results from the Phase III trial along with a comprehensive review and supplemental analyses of approximately 40 prior Oral-lyn clinical studies were compiled and submitted to the FDA in late December 2011 in a comprehensive package including a composite metanalysis of all safety data. We continue to have discussions with the FDA with respect to the pathway for regulatory approval, including any additional clinical or pharmacological studies that might be required to support regulatory approval or enhance marketing success. We do not currently plan to expend significant resources on additional clinical trials of Oral-lyn™ until after such time that we secure additional financing.
We engaged a global clinical research organization to provide many study related site services, including initiation, communication with sites, project management and documentation; a global central lab service company to arrange for the logistics of kits and blood samples shipment and testing; an Internet-based clinical electronic data management company to assist us with global data entry, project management and data storage/processing of the Phase III clinical trial and regulatory processes. We contracted with our third-party manufacturers to produce sufficient quantities of the RapidMist™ components, the insulin, and the raw material excipients required for the production of clinical trial batches of Generex Oral-lyn™.
| 5 |
As described above, we have obtained regulatory approval for the commercial marketing and sale of Generex Oral-lyn™ in Ecuador, India (subject to regulatory approval of a 2012 in-country study), Lebanon and Algeria.
Other Potential Buccal Products
We have had past discussions regarding possible research collaborations with various pharmaceutical companies concerning use of our large molecule drug delivery technology with other compounds, including monoclonal antibodies, human growth hormone, fertility hormone, estrogen and heparin, and a number of vaccines. We have not expended resources to further develop any of these products during the fiscal years ended July 31, 2014, 2013 and 2012 and do not currently have any plans to expend further resources on these products.
Immunomedicine Technology and Products
Our wholly-owned subsidiary Antigen Express is developing proprietary vaccine formulations based upon two platform technologies that were discovered by its founder, the Ii-Key hybrid peptides and Ii-Suppression. These technologies are applicable for either antigen-specific immune stimulation or suppression, depending upon the dosing and formulation of its products. Using active stimulation, we are focusing on major diseases such as breast, prostate and ovarian cancer, melanoma, influenza (including H5N1 avian and H1N1 swine flu) and HIV. Autoimmune diseases such as diabetes and multiple sclerosis are the focus of our antigen-specific immune suppression work.
Antigen’s immunotherapeutic vaccine AE37 is currently in Phase II clinical trials for patients with HER-2/neu positive breast cancer. The trial is being conducted with the United States Military Cancer Institute's (USMCI) Clinical Trials Group and will examine the rate of relapse in patients with node-positive or high-risk node-negative breast cancer after two years. The study is randomized and will compare patients treated with AE37 plus the adjuvant GM-CSF versus GM-CSF alone. The Phase II trial follows a Phase I trial that demonstrated safety, tolerability, and immune stimulation of the AE37 vaccine in breast cancer patients.
Based on positive results in trials of the AE37 vaccine in breast cancer patients, we entered into an agreement in August 2006 with the Euroclinic, a private center in Athens, Greece, to commence clinical trials with the same compound as an immunotherapeutic vaccine for prostate cancer. A Phase I trial involving 29 patients was completed in August 2009, which similarly showed safety, tolerability and induction of a specific immune response. Agreements, as well as a protocol, are in place for initiation of a Phase II clinical trial once additional funding is available.
The same technology used to enhance immunogenicity is being applied in the development of a synthetic peptide vaccine for H5N1 avian influenza and the 2009 H1N1 swine flu. In April 2007, a Phase I clinical trial of Antigen’s proprietary peptides derived from the hemagglutinin protein of the H5N1 avian influenza virus was initiated in healthy volunteers in the Lebanese-Canadian Hospital in Beirut, Lebanon. We have completed the first portion of the Phase I trial. Modified peptide vaccines for avian influenza offer several advantages over traditional egg-based or cell-culture based vaccines. Modified peptide vaccines can be manufactured by an entirely synthetic process which reduces cost and increases both the speed and quantity of vaccine relative to egg- or cell-culture based vaccines. Another advantage is that the peptides are derived from regions of the virus that are similar enough in all H5N1 and H1N1 virus strains such that they would not have to be newly designed for the specific strain to emerge in a pandemic.
A Physician’s Investigational New Drug (“IND”) application for the Phase I and Phase II trials in patients with stage II HER-2/neu positive breast cancer has been filed with the FDA. The Phase I trial was completed at the Walter Reed Army Medical Center in Washington, D.C., and the Phase II trial is taking place at 13 sites, including 11 in the U.S., one in Germany and one in Greece. A Physician’s Investigational New Drug application for a Phase I trial in patients with breast or ovarian cancer also has been filed with the FDA and this Phase I trial is being conducted in Dallas, Texas at the Mary Crowley Cancer Center. Applications were filed and approvals obtained for a Phase I prostate cancer trial using AE37 in Athens, Greece from the Hellenic Organization of Drugs, and this Phase I trial was completed in August 2009. The Ministry of Health in Lebanon gave approval for Phase I trial of our experimental H5N1 prophylactic vaccine in Beirut, Lebanon following submission of an application. All other immunomedicine products are in the pre-clinical stage of development.
Government Regulation
Our research and development activities and the manufacturing and marketing of our pharmaceutical products are subject to extensive regulation by the FDA in the United States, Health Canada in Canada and comparable designated regulatory authorities in other countries. Among other things, extensive regulations require us to satisfy numerous conditions before we can bring products to market. While these regulations apply to all competitors in our industry, having a technology that is unique and novel extends the requisite review period by the various divisions within the FDA and other regulators. Also, other companies in our industry are not limited primarily to products which still need to be approved by government regulators, as we are now.
| 6 |
If requisite regulatory approvals are not obtained and maintained, our business will be substantially harmed. In many cases, we expect that extant and prospective development partners will participate in the regulatory approval process. The following discussion summarizes the principal features of food and drug regulation in the United States and other countries as they affect our business.
United States
All aspects of our research, development and foreseeable commercial activities relating to pharmaceutical products are subject to extensive regulation by the FDA and other regulatory authorities in the United States. United States federal and state statutes and regulations govern, among other things, the testing, manufacturing, safety, efficacy, labeling, storage, record keeping, approval, advertising and promotion of pharmaceutical products. The regulatory approval process, including clinical trials, usually takes several years and requires the expenditure of substantial resources. If regulatory approval of a product is granted, the approval may include significant limitations on the uses for which the product may be marketed.
The steps required before a pharmaceutical product may be marketed in the United States include:
•Conducting appropriate pre-clinical laboratory evaluations, including animal studies, in compliance with the FDA’s Good Laboratory Practice (“GLP”) requirements, to assess the potential safety and efficacy of the product, and to characterize and document the product’s chemistry, manufacturing controls, formulation and stability;
•Submitting the results of these evaluations and tests to the FDA, along with manufacturing information, analytical data, and protocols for clinical studies, in an IND Application, and receiving approval from the FDA that the clinical studies proposed under the IND are allowed to proceed;
• Obtaining approval of Institutional Review Boards (“IRBs”) to administer the product to humans in clinical studies; conducting adequate and well-controlled human clinical trials in compliance with the FDA’s Good Clinical Practice (“GCP”) requirements that establish the safety and efficacy of the product candidate for the intended use;
• Developing manufacturing processes which conform to the FDA’s current Good Manufacturing Practices, or cGMPs, as confirmed by FDA inspection;
• Submitting to the FDA the results of pre-clinical studies, clinical studies, and adequate data on chemistry, manufacturing and control information to ensure reproducible product quality batch after batch, in an NDA or Biologics License Application (“BLA”); and
• Obtaining FDA approval of the NDA, including inspection and approval of the product manufacturing facility as compliant with cGMP requirements, prior to any commercial sale or shipment of the pharmaceutical agent.
Quality and pre-clinical tests and studies include: laboratory evaluation of Drug Substance and Drug Product chemistry, formulation/manufacturing, and stability profiling, as well as a large number of animal studies to assess the potential safety and efficacy of each product. Typically, the pre-clinical studies consist of the following:
Pharmacology
| • | Primary and Secondary Pharmacodynamics |
| • | Safety Pharmacology |
| • | Other Pharmacodynamics |
Pharmacokinetics (“PK”)
| • | Single and Multiple Dose Kinetics |
| • | Tissue Distribution |
| • | Metabolism |
| • | PK Drug Interactions |
| • | Other PK studies |
Toxicology
| • | Single and Multiple Dose Toxicity |
| • | Genotoxicity |
| • | Carcinogenicity |
| • | Reproduction Toxicity |
| • | Other Toxicity |
| 7 |
The results of the quality and pre-clinical tests/studies, in addition to any non-clinical pharmacology, are submitted to the FDA along with the initial clinical study protocol (see descriptive of process below) as part of the initial IND and are reviewed by the FDA before the commencement of human clinical trials. Unless the FDA objects to it, the IND becomes effective 30 days following its receipt by the FDA. The FDA reviews all protocols, protocol amendments, adverse event reports, study reports, and annual reports in connection with a new pharmacological product.
The IND for our oral insulin formulation became effective in November 1998. Amendments are also subsequently filed as new Clinical Studies and their corresponding Study Protocols are proposed. In July 2007, we received a no objection clearance to initiate our Phase III study protocol for our oral insulin product. The Physician’s Investigational New Drug Application for the Phase 1 and Phase II trial of AE37, Antigen’s synthetic peptide vaccine designed to stimulate a potent and specific immune response against tumors expressing the HER-2/neu oncogene, in patients with stage II HER-2/neu positive breast cancer became effective in March 2006.
Clinical trials involve the administration of a new drug to humans under the supervision of qualified investigators. The protocols for the trials must be submitted to the FDA as part of the IND. Also, each clinical trial must be approved and conducted under the auspices of an IRB, which considers, among other things, ethical factors, the safety of human subjects, and the possible liability of the institution conducting the clinical trials.
Clinical trials are typically conducted in three sequential phases (Phase I, Phase II, and Phase III), but the phases may overlap. Phase I clinical trials test the drug on healthy human subjects for safety and other aspects, but usually not effectiveness. Phase II clinical trials are conducted in a limited patient population to gather evidence about the efficacy of the drug for specific purposes, to determine dosage tolerance and optimal dosages, and to identify possible adverse effects and safety risks. When a compound has shown evidence of efficacy and acceptable safety in Phase II evaluations, Phase III clinical trials are undertaken to evaluate and confirm clinical efficacy and to test for safety in an expanded patient population at clinical trial sites in different geographical locations. The FDA and other regulatory authorities require that the safety and efficacy of therapeutic product candidates be supported through at least two adequate and well-controlled Phase III clinical trials (known as “Pivotal Trials”). The successful completion of Phase III clinical trials is a mandatory step in the approval process for the manufacturing, marketing, and sale of products.
In the United States, the results of quality, pre-clinical studies and clinical trials, if successful, are submitted to the FDA in an NDA to seek approval to market and commercialize the drug product for a specified use. The NDA is far more specific than the IND and must also include proposed labeling and detailed technical sections based on the data collected. The FDA is governed by the Prescription Drug User Fee Act (“PDUFA”) regarding response time to the application, which is generally 12 months (and shorter for a priority application). It may deny a NDA if it believes that applicable regulatory criteria are not satisfied. The FDA also may require additional clarifications on the existing application or even additional testing for safety and efficacy of the drug. We cannot be sure that any of our proposed products will receive FDA approval. The multi-tiered approval process means that our products could fail to advance to subsequent steps without the requisite data, studies, and FDA approval along the way. Even if approved by the FDA, our products and the facilities used to manufacture our products will remain subject to review and periodic inspection by the FDA.
To supply drug products for use in the United States, foreign and domestic manufacturing facilities must be registered with, and approved by, the FDA. Manufacturing facilities must also comply with the FDA's cGMPs, and such facilities are subject to periodic inspection by the FDA. Products manufactured outside the United States are inspected by regulatory authorities in those countries under agreements with the FDA. To comply with cGMPs, manufacturers must expend substantial funds, time and effort in the area of production and quality control. The FDA stringently applies its regulatory standards for manufacturing. Discovery of previously unknown problems with respect to a product, manufacturer or facility may result in consequences with commercial significance. These include restrictions on the product, manufacturer or facility, suspensions of regulatory approvals, operating restrictions, delays in obtaining new product approvals, withdrawals of the product from the market, product recalls, fines, injunctions and criminal prosecution.
One final hurdle that is closely associated with the cGMP inspections is the pre-approval inspection that the FDA carries out prior to the issuance of a marketing license. FDA inspectors combine cGMP compliance with a review of research and development documents that were used in the formal NDA. A close inspection of historic data is reviewed to confirm data and to demonstrate that a company has carried out the activities as presented in the NDA. This is generally a long inspection and requires a team of individuals from the company to “host” the FDA inspector(s).
Foreign Countries
Before we are permitted to market any of our products outside of the United States, those products will be subject to regulatory approval by foreign government agencies similar to the FDA. These requirements vary widely from country to country. Generally, however, no action can be taken to market any drug product in a country until an appropriate application has been submitted by a sponsor and approved by the regulatory authorities in that country. Again, similar to the FDA, each country will mandate a specific financial consideration for the Marketing Application dossiers being submitted. Although an important consideration, FDA approval does not assure approval by other regulatory authorities. The current approval process varies from country to country, and the time spent in gaining approval varies from that required for FDA approval. The Canadian regulatory process is substantially similar to that of the United States. To date, we have received the following foreign regulatory approval for our product candidates:
| 8 |
| • | We obtained regulatory approval to begin clinical trials of our oral insulin formulation in Canada in November 1998. In April 2003, we received approval of an Oral-lyn™ Phase II-B clinical trial protocol in Canada. In September 2006 Health Canada approved our Clinical Trial Application in respect of our proposed Generex Oral-lyn™ protocol for late-stage trials; we expect to use the data collected from these trials in the New Drug Submission that will be prepared concurrently with the progression of the late-stage trials. |
| • | We obtained regulatory approval in Canada to begin clinical trials of our buccal morphine product in March 2002 and our fentanyl product in October 2002. |
| • | In May 2005, we received approval from the Ecuadorian Ministry of Public Health for the commercial marketing and sale of Generex Oral-lyn™ for treatment of Type 1 and Type 2 diabetes. To date we have not recognized any revenue from the sale of Generex Oral-lyn™ in Ecuador and we are not currently expending any resources to further commercialization in this country. |
| • | In November 2007, we obtained approval for the importation and commercial marketing and sale in India of Generex Oral-lyn™ under the marketing name of Oral Recosulin™ from the Central Drugs Standard Control Organization (CDSCO), Directorate General of Health Services, Government of India, which is responsible for authorizing marketing approval of all new pharmaceutical products in India. Per the requirements of the approval, an in-country clinical study must be completed in India with Oral Recosulin™ before commercial sales can commence. The field portion of the study was completed in the third calendar quarter of 2012. Shreya has advised Generex that the dossier was submitted in December of 2012 to the Drugs Controller General (India) (DCGI), Central Drugs Standard Control Organization, Director General of Health Services, Ministry of Health and Family Welfare, Government of India. Generex has provided additional, detailed scientific data to support the Shreya submission. |
| • | Applications were filed and approvals obtained in May 2007 for a Phase I prostate cancer trial using AE37 in Athens, Greece from the Hellenic Organization of Drugs. This Phase I trial was completed in August 2009. |
| • | The Ministry of Health in Lebanon gave approval for the Phase I trial of our experimental H5N1 prophylactic vaccine in Beirut, Lebanon following submission of an application. In December 2008, we, together with our marketing partner Benta SA., received an approval to market Generex Oral-lyn™ in Lebanon. The official product launch in Lebanon took place in May 2009. We are not currently expending any resources to further commercialization in this country. |
| • | In May 2009, the Algerian health authorities granted us permission to import and sell Generex Oral-lyn™ for the treatment of diabetes in Algeria. To date we have not recognized any revenue from the sale of Generex Oral-lyn™ in Algeria and we are not currently expending any resources to further commercialization in this country. |
Marketing and Distribution
We market our products through collaborative arrangements with companies that have well-established pharmaceutical marketing and distribution capabilities, including expertise in the regulatory approval processes in their respective jurisdictions.
We have entered into licensing and distribution agreements with a number of multinational distributors to assist us with the process of gaining regulatory approval for the registration, marketing, distribution, and sale of Generex Oral-lyn™ in countries throughout the world, including:
| • | Shreya Life Sciences Pvt. Ltd. for India, Pakistan, Bangladesh, Nepal, Bhutan, Sri Lanka, and Myanmar; |
| • | Adcock Ingram Limited and Adcock Ingram Healthcare (Pty) Ltd. for South Africa, Lesotho, Swaziland, Botswana; Namibia, Mozambique and Zimbabwe; |
| • | E&V Alca Distribution Corp. for Albania, Montenegro, and Kosovo; |
| • | Medrey S.A.L. (formerly MedGen Corp.) and Benta S.A.L. for Lebanon; |
| • | SciGen, Ltd. for China, Hong Kong, Indonesia, Malaysia, the Philippines, Singapore, Thailand and Vietnam; |
| • | Pharmaris Perus S.A.C. for Peru; |
| • | MediPharma SA for Argentina; |
| • | PMG S.A. for Chile; |
| • | Dong Sung Pharm. Co. Ltd. for South Korea; and |
| • | Benta S.A. for Lebanon. |
Under these licensing and distribution agreements excluding the one with Dong Sung Pharm Co., we will not receive an upfront license fee, but the distributor will bear any and all costs associated with the procurement of governmental approvals for the sale of Generex Oral-Lyn™, including any clinical and regulatory costs. We possess the worldwide marketing rights to our oral insulin product. We do not currently plan to expend significant resources on additional clinical trials or to further the commercialization of Generex Oral-lyn™ until after such time that we secure additional financing.
| 9 |
Manufacturing
In December 2000, we completed a pilot manufacturing facility for Generex Oral-lyn™ in Toronto, Canada in the same commercial complex in which our laboratories were located. In the first quarter of fiscal year 2006, we initiated a scale-up commercial production run of several thousand canisters of Generex Oral-lyn™ at this facility. We have sold the property which housed the manufacturing and laboratory facility in July 2012 and expect to engage contract manufacturers in order to manufacture any product in significant quantities for any future commercial sales and clinical trials.
In March 2006, we successfully completed the delivery and installation of a turnkey Generex Oral-lyn™ filling operation at the facilities of PharmaBrand, in Quito, Ecuador for the purposes of commercial supply and sales in Ecuador and other countries that can procure registrations and import licenses. We do not currently have a manufacturing agreement with PharmaBrand and are not currently manufacturing product at this facility.
In anticipation of undertaking late-stage clinical trials of Generex Oral-lyn™ in Canada, we entered into an agreement with Cardinal Health PTS, LLC, now known as Catalent Pharma Solutions (Catalent), in June 2006, pursuant to which Catalent manufactured clinical trial batches of Generex Oral-lyn™. Pursuant to pre-extant supply arrangements, our third-party suppliers had been manufacturing the quantities of the RapidMist™ brand metered dose inhaler components (valves, canisters, actuators, and dust caps), the insulin, and the formulary excipients that were required for the Catalent production. In addition, our Regulatory Affairs, Quality Control and R&D personnel have worked with Catalent to prepare and validate the Catalent production processes. We are not currently manufacturing product under this agreement and we expect that any agreements regarding the manufacturing of Generex Oral-lyn™ for any future trials or commercial sales will need to be renegotiated at such time.
Our subsidiary Antigen leases office and laboratory space in Worcester, Massachusetts, which is sufficient for its present needs. The laboratory has permission to store and use biohazardous (including recombinant DNA materials) and flammable chemicals.
Raw Material Supplies
The excipients used in our formulation are available from numerous sources in sufficient quantities for clinical purposes, and we believe that they will be available in sufficient quantities for commercial purposes when required, although we have not yet attempted to secure a guaranteed commercial supply of any such products. Components suitable for our RapidMist™ brand metered dose inhaler are available from a limited number of potential suppliers, as is the chemical propellant used in the device. The components which now comprise the device are expected to be used in the commercial version of our insulin product in Ecuador, India, Lebanon and Algeria. We have secured supply arrangements with manufacturers for each of the components and the propellant that we presently use in our RapidMist™ brand metered dose inhaler for commercial quantities of such components. All such suppliers are prominent, reputable and reliable suppliers to the pharmaceutical industry. Because we now have a single supplier for many of these, however, we are more vulnerable to supply interruptions than would be the case if we had multiple suppliers for each component. We do not believe that the risk of supply for proprietary raw materials or device components is unusual in the pharmaceutical industry.
Insulin is available worldwide from only a few sources. However, alternative supplies of insulin are under development. On December 7, 2009, we entered into a long-term agreement with sanofi-aventis Deutschland GmbH (“sanofi-aventis”). Under this agreement, sanofi-aventis will manufacture and supply recombinant human insulin to us in the territories specified in the agreement. Through this agreement, we will procure recombinant human insulin crystals for use in the production of Generex Oral-lyn™. The terms of the supply agreement required us to make certain minimum purchases of insulin from sanofi-aventis through the period ending December 31, 2011. As we did not meet the minimum purchase requirements by December 31, 2011, sanofi-aventis may terminate the agreement. Upon termination, we would be obligated to pay sanofi-aventis for all materials and components that it has acquired or ordered to manufacture insulin based on our forecasts or minimum purchase commitments, all related work-in-progress (at cost) and all finished insulin in inventory. We did not provide any forecasts to sanofi-aventis and have not included any accruals related to the purchase commitments in our consolidated financial statements for the fiscal year ended July 31, 2014, nor has sanofi-aventis terminated the agreement.
Intellectual Property
We hold a number of patents in the United States and foreign countries covering our buccal and other delivery technologies. We also have developed brand names and trademarks for products in appropriate areas. We consider the overall protection of our patent, trademark and other intellectual property rights to be of material value and acts to protect these rights from infringement.
Patents are a key determinant of market exclusivity for most branded pharmaceutical products. Protection for individual products or technologies extends for varying periods, in accordance with the expiration dates of patents in the various countries. The protection afforded, which may also vary from country to country, depends upon the type of patent, its scope of coverage and the availability of meaningful legal remedies in the country.
| 10 |
We currently have five issued U.S. patents and one pending U.S. patent applications pertaining to various aspects of drug delivery technology, including oral administration of macromolecular formulations (such as insulin) as well as pain relief medications such as morphine and fentanyl. We currently hold eleven issued Canadian patents and one pending Canadian patent applications also relating to various aspects of drug delivery technology. We also hold eleven issued patents and one pending patent applications covering our drug delivery technology in jurisdictions other than the U.S. and Canada, including Brazil, Argentina, Israel, Australia and Europe.
The expiration dates of the U.S. issued patents range from 2016 to 2022. The expiration dates of the patents issued in Canada range from 2015 to 2021. The expiration dates of the patents issued in other jurisdictions range from 2015 to 2028.
In addition to patents, we hold intellectual property in the form of trademark applications or registrations for GENEREX BIOTECHNOLOGY (Design), GENEREX BIOTECHNOLOGY (Logo), GENEREX ORAL-LYN, ORAL LYN, ORAL-LYN and RAPIDMIST in various jurisdictions in the world. Trademarks have no effect on market exclusivity for a product, but are considered to have marketing value if products bearing the trademark are to be sold commercially. Trademark protection continues in some countries as long as used; in other countries, as long as registered. Registration is for fixed terms and can be renewed indefinitely.
Our subsidiary Antigen Express currently holds ten issued U.S. patents, two allowed U.S. patents and twenty seven other foreign patents. There are also eight pending patent applications worldwide concerning technology for modulating the immune system via activation of antigen-specific helper T lymphocytes, including one in the U.S. and seven in other countries. Some of these patents are held under exclusive licenses from the University of Massachusetts. Dr. Robert Humphreys, a retired officer of Antigen, is the listed inventor or co-inventor on many of these patents and patent applications, including those licensed from the University of Massachusetts.
The expiration dates of the Antigen U.S. issued patents range from 2016 to 2031. The expiration dates of the patents issued in other jurisdictions range from 2017 to 2023.
We possess the worldwide manufacturing and marketing rights to our oral insulin product.
Our long-term success will substantially depend upon our ability to obtain patent protection for our technology and our ability to protect our technology from infringement, misappropriation, discovery and duplication. We cannot be sure that any of our pending patent applications will be granted, or that any patents which we own or obtain in the future will fully protect our position. Our patent rights and the patent rights of biotechnology and pharmaceutical companies in general, are highly uncertain and include complex legal and factual issues. We believe that our existing technology and the patents which we hold or for which we have applied do not infringe anyone else's patent rights. We believe our patent rights will provide meaningful protection against others duplicating our proprietary technologies. We cannot be sure of this, however, because of the complexity of the legal and scientific issues that could arise in litigation over these issues. See the discussion under the caption “Management’s Discussion and Analysis of Financial Condition and Results of Operations” under the heading “Legal Proceedings” in this annual report.
We also rely on trade secrets and other unpatented proprietary information. We seek to protect this information, in part, by confidentiality agreements with our employees, consultants, advisors and collaborators.
Competition
We expect that products based upon our buccal delivery technology and any other products that we may develop will compete directly with products developed by other pharmaceutical and biotechnology companies, universities, government agencies and public and private research organizations.
Products developed by our competitors may use a different active pharmaceutical agent or treatment to treat the same medical condition or indication as our product or may provide for the delivery of substantially the same active pharmaceutical ingredient as our products using different methods of administration. For example, a number of pharmaceutical and biotechnology companies are engaged in various stages of research, development and testing of alternatives to insulin therapy for the treatment of diabetes, as well as new methods of delivering insulin. These methods, including nasal, transdermal, needle-free (high pressure) injection and pulmonary, may ultimately successfully deliver insulin to diabetic patients. Some biotechnology companies also have developed different technologies to enhance the presentation of peptide antigens. Some of our competitors and potential competitors have substantially greater scientific research and product development capabilities, as well as financial, marketing and human resources, than we do.
Where the same or substantially the same active ingredient is available using alternative delivery means or the same or substantially the same result is achievable with a different treatment or technology, we expect that competition among products will be based, among other things, on product safety, efficacy, ease of use, availability, price, marketing and distribution. When different active pharmaceutical ingredients are involved, these same competitive factors will apply to both the active agent and the delivery method.
We consider other drug delivery and biotechnology companies to be direct competitors for the cooperation and support of major drug and biotechnology companies that own or market proprietary pharmaceutical compounds and technologies, as well as for the ultimate patient market. Of primary concern to us are the competitor companies that are known to be developing delivery systems for insulin and other pharmaceutical agents that we have identified as product candidates and technologies to enhance the presentation of peptide antigens.
| 11 |
Large pharmaceutical companies, such as Merck & Co., Inc., GlaxoSmithKline PLC, Novartis, Inc., MedImmune Inc. (a subsidiary of Astra-Zeneca, Inc.) and others, also compete in the oncology, immunomedicine and vaccine markets. These companies have greater experience and expertise in securing government contracts and grants to support research and development efforts, conducting testing and clinical trials, obtaining regulatory approvals to market products, as well as manufacturing and marketing approved products. As such, they are also considered significant competitors in these fields of pharmaceutical products and therapies. There are also many smaller companies which are pursuing similar technologies in these fields and are considered to be competitors of Generex.
The following descriptions of our competitors and their products were obtained from their filings with the Securities and Exchange Commission, information available on their web sites and industry research reports.
Buccal Insulin Product
| • | MannKind Corporation’s product candidates include AFREZZA®, a mealtime insulin therapy being studied for use in adult patients with type 1 and type 2 diabetes. It is a drug-device combination product which administers insulin through inhalation to the lungs. MannKind submitted an NDA to the FDA requesting approval to market AFREZZA® in May 2009. In January 2011, MannKind announced that it had received a complete response letter from the FDA for AFREZZA®. In August 2011, MannKind announced that it has confirmed with the FDA the design of the two additional Phase III studies which are required for AFREZZA®. In August 2013, MannKind announced positive late-stage data on its inhaled insulin AFREZZA® from the two additional Phase III studies on Type 1 and Type 2diabetes and has resubmitted a new drug application to the FDA in October 2013 seeking approval for the marketing of AFREZZA®. Mannkind received FDA approval in June 2014. |
| • | Novo Nordisk A/S, one of the two leading manufacturers of insulin in the world, announced in May 2008 the termination of clinical testing of the pulmonary delivery system for inhaled insulin, the AERx® insulin Diabetes Management System (AERx iDMS), initially developed by Aradigm Corporation. The product was in Phase III clinical trials at the time of Novo Nordisk’s announcement. In December 2010, it was announced that Novo Nordisk had entered into an exclusive Development and License Agreement with Emisphere for its oral insulin formulation. |
| • | Amylin Pharmaceuticals, Inc. received FDA approval in January 2012 for Bydureon, an extended-release injectable formulation, which is the first once-a-week therapy for the treatment of type 2 diabetes. |
| • | CPEX Pharmaceuticals, Inc.’s proprietary permeation enhancer, CPE-215®, provides skin, mouth, nose and eye membrane absorption of a variety of pharmaceuticals. CPEX has applied this technology to Nasulin™, through which insulin is absorbed via nasal mucosa. In April 2010, CPEX announced that it decided not to proceed with any further development activities of Nasulin™, which was currently in Phase II clinical trials. |
| • | There are several companies that are working on developing products which involve the oral delivery of analogs of insulin. Oramed Pharmaceuticals is developing an orally ingestible insulin capsule which is currently in Phase II clinical trials. Biocon Limited has developed IN-105, a tablet for the oral delivery of insulin, which is currently in phase II trials. Diabetology has developed Capsulin IR, an insulin capsule which is currently in Phase II clinical trials. Access Pharmaceuticals has developed Cobalamin, an oral insulin which is currently in pre-clinical trials. Dance Pharmaceuticals is developing an inhaled insulin product based on Aerogen’s proprietary OnQ Aerosol Generator technology. |
There are also a number of companies developing alternative means of delivering insulin in the form of oral pills, transdermal patches, and intranasal methods, which are at early stages of development. In addition to other delivery systems for insulin, there are numerous products, such as sulfonylureas (Amaryl®and Glynase®), biguanides (branded and generic metformin products), thiazolidinediones (Avandia®and Actos®), glucagon-like peptide 1 (Byetta®and Victoza®), and dipeptidyl peptidase IV inhibitors (Januvia® and Onglyza™), which have been approved for use in the treatment of Type 2 diabetics in substitution of, or in addition to, insulin therapy. These products may also be considered to compete with insulin products.
| 12 |
Immunomedicine Technology and Products
| • | Bavarian Nordic, Inc. employs a DNA vector-based technology platform to design and develop immunotherapeutic vaccines for different cancers. Their most advanced compound, PROSTVAC, is in a pivotal Phase III trial in patients with prostate cancer. Additionally they have a HER2 vaccine in a Phase I/II trial in patients with breast cancer. They have recently presented data on studies combining their MVA-BN-HER2 cancer vaccine with different immune checkpoint inhibitors. |
| • | Advaxis, Inc. uses a proprietary technique to bioengineer Listeria bacteria to create a specific antigen that can stimulate an immune response after recognition by the recipient’s immune system. Advaxis’ most advanced product candidate is ADXS-HPV, which is inPhase II trials for HPV-associated CIN (cervical intraepithelial neoplasia) and recurrent cervical cancer. The company has recently partnered with MedImmune to initiate combination studies utilizing their most advanced ADXS-HPV with MedImmune’s anti-PD-L1 immune checkpoint inhibitor in patients with advanced, recurrent or refractory human papillomavirus (HPV)-associated cervical or head and neck cancer. |
| • | Amgen Inc.’s BiTE® technology uses the body’s cell destroying T cells to attack tumor cells. Amgen’s lead product candidate blinatumomab (MT103) has completed a Phase II clinical trial in patients with minimal residual disease positive acute lymphoblastic leukemia. |
| • | Sanofi Pasteur Inc., the vaccine division of sanofi-aventis and one of the largest vaccines companies in the world, has product candidates including inoculations against 20 varieties of infectious diseases. It received FDA approval for an H5N1 avian influenza vaccine in April 2007 and for an H1N1 vaccine in September 2009. |
| • | Dendreon Corporation’s product portfolio includes therapeutic vaccines, monoclonal antibodies and small molecules. Its most advanced product candidate, Provenge® (sipuleucel-T), an investigational autologous (patient-specific) active cellular immunotherapy (ACI) for the treatment of prostate cancer received FDA approval in April 2010. Dendreon is exploring the application of additional active cellular immunotherapy product candidates and small molecules for the potential treatment of a variety of cancers. Among these is DN24-02, a cancer vaccine targeting the HER2 antigen which utilizes the same proprietary antigen-engineering technology as PROVENGE. That compound is currently in a Phase II trial in patients with urothelial carcinoma. |
| • | Galena Biopharma’s (formerly Rxi Pharmaceuticals Corporation) NeuVax™, is currently in Phase III clinical trials to evaluate NeuVax™ for the treatment of early stage, HER2-positive breast cancer. Clinical trials are currently underway to test NeuVax™ as a treatment for prostate cancer, and to use NeuVax™ in combination with Herceptin® to target breast cancer. |
| • | Cell Genesys, Inc. was developing products for the treatment of prostate cancer using the GVAX™ cancer treatments, which are composed of tumor cells that are genetically modified to secrete an immune-stimulating cytokine and are irradiated for safety. Cell Genesys and Takeda Pharmaceutical Co. entered into an exclusive licensing agreement for GVAX in March 2008. In late 2008, Cell Genesys announced it was terminating the Phase III trials for the GVAX™ prostate cancer products. In May 2010, BioSante Pharmaceuticals, Inc. announced that development of the GVAX vaccine for the treatment of prostate cancer has been reinitiated and is in Phase II human clinical trials. In addition to GVAX prostate product, BioSante has several other cancer vaccines which are in Phase II clinical development including vaccines for leukemia, breast cancer and pancreatic cancer and has vaccines in Phase I clinical development including vaccines for colorectal cancer and melanoma. |
| • | CEL-SCI Corporation’s main product is Multikine® an immunotherapeutic agent being developed as a cancer treatment. Multikine®’s goal is to harness the body's natural ability to fight tumors. Multikine® has been cleared in the U.S. and Canada for study in a global Phase III clinical trial in advanced primary (not yet treated) head and neck cancer patients. |
In addition to the companies listed above, there are a number of companies which are pursuing cancer treatments using immunotherapy technologies which have products in various clinical trial stages. Some of these companies are Argos Therapeutics Inc., Celldex Therapeutics Inc., Northwest Therapeutics Inc., Immatics Biotechnology GmbH, Immunocellular Therapeutics Ltd., TVAX Biomedical Inc. and Newlink Genetics Corporation. These companies can also be considered to be competitors.
Environmental Compliance
Our manufacturing, research and development activities involve the controlled use of hazardous materials and chemicals. We believe that our procedures for handling and disposing of these materials comply with all applicable government regulations. However, we cannot eliminate the risk of accidental contamination or injury from these materials. If an accident occurred, we could be held liable for damages, and these damages could severely impact our financial condition. We are also subject to many environmental, health and workplace safety laws and regulations, particularly those governing laboratory procedures, exposure to blood-borne pathogens, and the handling of hazardous biological materials. Violations and the cost of compliance with these laws and regulations could adversely affect us. However, we do not believe that compliance with the United States, Canadian or other environmental laws will have a material effect on us in the foreseeable future.
Research and Development Expenditures
A substantial portion of our activities to date have been in research and development. We expended $1,382,995 in the fiscal year ended July 31, 2014, $2,192,724 in the fiscal year ended July 31, 2013 and $4,987,236 in the year ended July 31, 2012 on research and development. Research and development activities in decreased in 2013 and again in 2014 from 2012, as we have not initiated any new trials after the completion of the global Phase III clinical trial of our oral insulin product due to lack of available funding.
| 13 |
Financial Information About Geographic Areas
The regions in which we had identifiable assets and the amounts of such identifiable assets for each of the last two fiscal years are presented in Note 14 in theNotes to Consolidated Financial Statements in this annual report. Identifiable assets are those that can be directly associated with a geographic area.
Employees
At July 31, 2014, we had eight full-time employees, including our employees at Antigen. Five of our employees are executive and administrative, two are scientific and technical personnel who engage primarily in development activities and in preparing formulations for testing and clinical trials, and one is engaged in corporate and product promotion. We believe our employee relations are good. None of our employees is covered by a collective bargaining agreement.
We will continue to need qualified scientific personnel and personnel with experience in clinical testing, government regulation and manufacturing. We may have difficulty in obtaining qualified scientific and technical personnel as there is strong competition for such personnel from other pharmaceutical and biotechnology companies, as well as universities and research institutions. Our business could be materially harmed if we are unable to recruit and retain qualified scientific, administrative and executive personnel to support our expanding activities, or if one or more members of our limited scientific and management staff were unable or unwilling to continue their association with us. We have fixed-term agreements with only certain members of our key management and scientific staff, Mark Fletcher, President, President and CEO and Eric von Hofe, President of Antigen.
We use non-employee consultants to assist us in formulating research and development strategy, in preparing regulatory submissions, in developing protocols for clinical trials, and in designing, equipping and staffing our manufacturing facilities. We also use non-employee consultants to assist us in business development. These consultants and advisors usually have the right to terminate their relationship with us on short notice. Loss of some of these key advisors could interrupt or delay development of one or more of our products or otherwise adversely affect our business plans.
With respect to all litigation matters, as additional information concerning the estimates used by us becomes known, we reassess each matter’s position both with respect to accrued liabilities and other potential exposures.
Available Information
We were incorporated in the State of Delaware in 1997. Our principal executive offices are located at 555 Richmond Street West, Suite 604, Toronto, Canada, and our telephone number at that address is (416) 364-2551. We maintain an Internet website at www.generex.com. However, information found on, or that can be accessed through, our website is not incorporated by reference into this Annual Report on Form 10-K. We make available free of charge on or through our website our filings with the Securities and Exchange Commission, or SEC, including this annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act, as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Further, a copy of this annual report is located at the SEC’s Public Reference Room at 100 F Street N. E., Washington, D.C. 20549. Information on the operation of the Public Reference Room can be obtained by calling the SEC at 1-800-SEC-0330. The SEC maintains an Internet website that contains reports, proxy and information statements, and other information regarding our filings at www.sec.gov.
Item 1A.Risk Factors
Our business and results of operations are subject to numerous risks, uncertainties and other factors that you should be aware of, some of which are described below. The risks, uncertainties and other factors described below are not the only ones facing our company. Additional risks, uncertainties and other factors not presently known to us or that we currently deem immaterial may also impair our business operations.
Any of the risks, uncertainties and other factors could have a materially adverse effect on our business, financial condition or results of operations and could cause the trading price of our common stock to decline substantially.
Risks Related to Our Financial Condition
We will require additional financing to continue our operations.
As of July 31, 2014, our current cash position is not sufficient to meet our working capital needs for the next twelve months based on the pace of our planned activities. To continue operations, we will require additional funds to support our working capital requirements and any expansion or other activities, or will need to significantly reduce our clinical trials and other planned activities or suspend operations. Management is seeking various alternatives to ensure that we can meet some of our operating cash flow requirements through financing activities, such as private placement of our common stock, preferred stock offerings and offerings of debt and convertible debt instruments as well as through merger or acquisition opportunities. The securities purchase agreements that we entered into on January 14, 2014 and March 27, 2014 with certain investors limits the financing activities that we may undertake in the near future as it prohibits us from (i) issuing additional equity securities until 60 days after the effective date of a registration statement covering the resale of the common stock issuable upon exercise of the warrants and conversion of the preferred stock sold in each transaction and (ii) issuing additional debt or equity securities with a variable conversion or exercise price until January 15, 2015 and March 27, 2015, respectively. In addition, management is actively seeking strategic alternatives, including strategic investments and divestitures. Management has sold non-essential real estate assets which were classified as Assets Held for Investment to augment its cash position.
| 14 |
We cannot provide any assurance that we will obtain the required funding. Our inability to obtain required funding in the near future or our inability to obtain funding on favorable terms will have a material adverse effect on our operations and our strategic development plan for future growth. If we cannot successfully raise additional capital and implement our strategic development plan, our liquidity, financial condition and business prospects will be materially and adversely affected and we may have to cease operations.
We have a history of losses and will incur additional losses.
We are a development stage company with a limited history of operations, and do not expect sufficient revenues to support our operation in the immediately foreseeable future. We do not expect to receive significant revenues in Ecuador, Algeria and Lebanon where we have been approved for commercial sale in the next twelve months. While we have entered into a licensing and distribution agreement with a leading Indian-based pharmaceutical company and insulin distributor, we do not anticipate recognizing revenue from sales of Generex Oral-lyn™ in India in fiscal 2014, as our partner has to finalize the results of an in-country clinical study and receive approval from the Indian regulatory authority before the product can be offered for commercial sale in India.
To date, we have not been profitable and our accumulated deficit was $369,948,322 at July 31, 2014. Our losses have resulted principally from costs incurred in research and development, including clinical trials, and from general and administrative costs associated with our operations. While we seek to attain profitability, we cannot be sure that we will ever achieve product and other revenue sufficient for us to attain this objective.
With the exception of Generex Oral-lyn™, which has received regulatory approval in Ecuador, India (subject to regulatory approval of a 2012 in-country study), Lebanon and Algeria, our product candidates are in research or early stages of pre-clinical and clinical development. We will need to conduct substantial additional research, development and clinical trials. We will also need to receive necessary regulatory clearances both in the United States and foreign countries and obtain meaningful patent protection for and establish freedom to commercialize each of our product candidates. We must also complete further clinical trials and seek regulatory approvals for Generex Oral-lyn™ in countries outside of Ecuador, India, Lebanon and Algeria. We cannot be sure that we will obtain required regulatory approvals, or successfully research, develop, commercialize, manufacture and market any other product candidates. We expect that these activities, together with future general and administrative activities, will result in significant expenses for the foreseeable future.
Our independent auditors have expressed substantial doubt about our ability to continue as a going concern as of July 31, 2014.
To date, we have not been profitable and our accumulated deficit was $369,948,322 at July 31, 2014, and our consolidated balance sheet reflected a stockholders’ deficiency of $6,090,058 at that date. We received a report from our independent auditors for the year ended July 31, 2014 that includes an explanatory paragraph describing an uncertainty as to Generex’s ability to continue as a going concern. We must secure financing to continue our operations.
Due to material weaknesses in our internal controls over financial reporting, our internal controls were determined not to be effective for a prior fiscal year ended July 31, 2012. Our disclosure controls and procedures and internal controls over financial reporting may not be effective in future periods as a result of existing or newly identified material weaknesses in internal controls.
Effective internal controls are necessary for us to provide reasonable assurance with respect to our financial reports and to effectively prevent fraud. If we cannot provide reasonable assurance with respect to our financial reports and effectively prevent fraud, our reputation and operating results could be harmed. Pursuant to the Sarbanes-Oxley Act of 2002, we are required to furnish a report by management on internal control over financial reporting, including management’s assessment of the effectiveness of such control. Internal control over financial reporting may not prevent or detect misstatements because of its inherent limitations, including the possibility of human error, the circumvention or overriding of controls, or fraud. Therefore, even effective internal controls can provide only reasonable assurance with respect to the preparation and fair presentation of financial statements. In addition, projections of any evaluation of effectiveness of internal control over financial reporting to future periods are subject to the risk that the control may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. If we fail to maintain the adequacy of our internal controls, including any failure to implement required new or improved controls, or if we experience difficulties in their implementation, our business and operating results could be adversely impacted, we could fail to meet our reporting obligations, and our business and stock price could be adversely affected.
| 15 |
At July 31, 2012, our Chief Executive Officer and Chief Financial Officer evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) and concluded that, subject to the inherent limitations identified in Item 9A of Part II of the Form 10-K filed on October 15, 2012, our disclosure controls and procedures were not effective due to the existence of material weaknesses in our internal control over financial reporting because of inadequate segregation of duties over authorization, review and recording of transactions, as well as the financial reporting of such transactions. Our independent auditors issued an adverse attestation report regarding the effectiveness of the Company’s internal control over financial reporting at July 31, 2012.
We believe we have taken appropriate and reasonable steps to make the necessary improvements to remediate these deficiencies, however we cannot be certain that our remediation efforts will ensure that our management designs, implements and maintains adequate controls over our financial processes and reporting in the future or that the changes made will be sufficient to address and eliminate the material weaknesses previously identified. Our inability to remedy any additional deficiencies or material weaknesses that may be identified in the future could, among other things, have a material adverse effect on our business, results of operations and financial condition, as well as impair our ability to meet our quarterly, annual and other reporting requirements under the Securities Exchange Act of 1934 in a timely manner, and require us to incur additional costs or to divert management resources.
Our research and development and commercialization efforts may depend on entering into agreements with corporate collaborators.
Because we have limited resources, we have sought to enter into collaboration agreements with other pharmaceutical companies that will assist us in developing, testing, obtaining governmental approval for and commercializing products using our buccal delivery and immunomedicine technologies. We may be unable to achieve commercialization of any of our products until we obtain a large pharmaceutical partner to assist us in such commercialization efforts. To date, we have not entered into any such collaborative arrangements. Any collaborator with whom we may enter into such collaboration agreements may not support fully our research and commercial interests since our program may compete for time, attention and resources with such collaborator's internal programs. Therefore, these collaborators may not commit sufficient resources to our program to move it forward effectively, or that the program will advance as rapidly as it might if we had retained complete control of all research, development, regulatory and commercialization decisions.
Risks Related to Our Technologies
With the exception of Generex Oral-lyn™, our technologies and products are at an early stage of development and we cannot expect significant revenues in respect thereof in the foreseeable future.
We have no products approved for commercial sale at the present time with the exception of Generex Oral-lyn™ in Ecuador, Lebanon, Algeria and India (subject to regulatory approval of a 2012 in-country study). To be profitable, we must not only successfully research, develop and obtain regulatory approval for our products under development, but also manufacture, introduce, market and distribute them once development is completed or find a partner that can perform these activities on our behalf. We have yet to manufacture, market and distribute these products on a large-scale commercial basis, and we do not expect to receive revenues from product sales in calendar year 2014. We may not be successful in one or more of these stages of the development or commercialization of our products, and/or any of the products we develop may not be commercially viable. Until we can establish that they are commercially viable products, we will not receive significant revenues from ongoing operations.
Until we receive regulatory approval to sell our pharmaceutical products in additional countries, our ability to generate revenues from operations may be limited and those revenues may be insufficient to sustain operations. Many factors impact our ability to obtain approvals for commercially viable products.
Our only pharmaceutical product that has been approved for commercial sale by drug regulatory authorities is our oral insulin spray formulation, and that approval was obtained in Ecuador, Lebanon, Algeria and India (subject to regulatory approval of a 2012 in-country study). We have begun the regulatory approval process for our oral insulin, buccal morphine and fentanyl products in other countries, and we have initiated late stage clinical trials of Generex Oral-lyn™ at clinical trial sites in North America and other countries according to the initial Phase III clinical plan. The final subjects completed the trial in August 2011. After appropriate validation, the data from approximately 450 patients was tabulated, reviewed and analyzed. Those results from the Phase III trial along with a comprehensive review and supplemental analyses of approximately 40 prior Oral-lyn clinical studies were compiled and submitted to the FDA in late December 2011 in a comprehensive package including a composite metanalysis of all safety data. We continue to have discussions with the FDA with respect to the pathway for regulatory approval, including any additional clinical or pharmacological studies that might be required to support regulatory approval or enhance marketing success. We do not currently plan to expend significant resources on additional clinical trials of Oral-lyn™ until after such time that we secure additional financing.
| 16 |
Our immunomedicine products are in the pre-clinical stage of development, with the exception of a Phase II trial in human patients with stage II HER-2/neu positive breast cancer (U.S.), a Phase I trial in human patients with prostate cancer (Athens, Greece) completed in August 2009, a Phase I trial in human patients with breast or ovarian cancer (U.S.) and a Phase I trial in human volunteers of a peptide vaccine for use against the H5N1 avian influenza virus (Beirut, Lebanon). Preliminary results from the Phase II breast cancer trial suggest a 46% reduction in breast cancer recurrence in low HER2 expressing tumors, together with an excellent safety profile. While preliminary results are promising, they are not statistically significant and final results could deviate. We announced completion of enrollment in the trial in January 2014 and expect completion of the trial and end-of-trial analysis to occur in the first calendar quarter of 2015.
Pre-clinical and clinical trials of our products, and the manufacturing and marketing of our technologies, are subject to extensive, costly and rigorous regulation by governmental authorities in the United States, Canada and other countries. The process of obtaining required regulatory approvals from the FDA and other regulatory authorities often takes many years, is expensive and can vary significantly based on the type, complexity and novelty of the product candidates. For these reasons, it is possible we will not receive regulatory approval for any prescription pharmaceutical product candidate in any countries other than Ecuador, Lebanon, Algeria and India.
In addition, we cannot be sure when or if we will be permitted by regulatory agencies to undertake additional clinical trials or to commence any particular phase of clinical trials. Because of this, statements in this annual report or our reports filed with the SEC regarding the expected timing of clinical trials cannot be regarded as actual predictions of when we will obtain regulatory approval for any "phase" of clinical trials.
Delays in obtaining United States or other foreign approvals for our oral insulin product could result in substantial additional costs to us, and, therefore, could adversely affect our ability to continue operations. If regulatory approval is ultimately granted in any countries other than Ecuador, Lebanon, Algeria and India, the approval may place limitations on the intended use of the product we wish to commercialize, and may restrict the way in which we are permitted to market the product.
Due to legal and factual uncertainties regarding the scope and protection afforded by patents and other proprietary rights, we may not have meaningful protection from competition.
Our long-term success will substantially depend upon our ability to protect our proprietary technologies from infringement, misappropriation, discovery and duplication and avoid infringing the proprietary rights of others. Our patent rights and the patent rights of biotechnology and pharmaceutical companies in general, are highly uncertain and include complex legal and factual issues. Because of this, our pending patent applications may not be granted. These uncertainties also mean that any patents that we own or will obtain in the future could be subject to challenge, and even if not challenged, may not provide us with meaningful protection from competition. Due to our financial uncertainties, we may not possess the financial resources necessary to enforce our patents. Patents already issued to us or our pending applications may become subject to dispute, and any dispute could be resolved against us.
Because a substantial number of patents have been issued in the field of alternative drug delivery and because patent positions can be highly uncertain and frequently involve complex legal and factual questions, the breadth of claims obtained in any application or the enforceability of our patents cannot be predicted. Consequently, we do not know whether any of our pending or future patent applications will result in the issuance of patents or, to the extent patents have been issued or will be issued, whether these patents will be subject to further proceedings limiting their scope, will provide significant proprietary protection or competitive advantage, or will be circumvented, invalidated or expire.
Also because of these legal and factual uncertainties, and because pending patent applications are held in secrecy for varying periods in the United States and other countries, even after reasonable investigation we may not know with certainty whether any products that we (or a licensee) may develop will infringe upon any patent or other intellectual property right of a third party. For example, we are aware of certain patents owned by third parties that such parties could attempt to use in the future in efforts to affect our freedom to practice some of the patents that we own or have applied for. Based upon the science and scope of these third-party patents, we believe that the patents that we own or have applied for do not infringe any such third-party patents; however, we cannot know for certain whether we could successfully defend our position, if challenged. We may incur substantial costs if we are required to defend our intellectual property in patent suits brought by third parties. These legal actions could seek damages and seek to enjoin testing, manufacturing and marketing of the accused product or process. In addition to potential liability for significant damages, we could be required to obtain a license to continue to manufacture or market the accused product or process.
Risks Related to Marketing of Our Potential Products
We may not become, or stay, profitable even if our pharmaceutical products are approved for sale.
Even if we obtain regulatory approval to market our oral insulin product outside of Ecuador, India, Lebanon and Algeria or to market any other prescription pharmaceutical product candidate, many factors may prevent the product from ever being sold in commercial quantities. Some of these factors are beyond our control, such as:
| • | acceptance of the formulation or treatment by health care professionals and diabetic patients; | |
| • | the availability, effectiveness and relative cost of alternative diabetes or immunomedicine treatments that may be developed by competitors; and | |
| • | the availability of third-party (i.e. insurer and governmental agency) reimbursements. |
| 17 |
We will not receive significant revenues from Generex Oral-lyn™ or any of our other pharmaceuticals products that may receive regulatory approval until we can successfully manufacture, market and distribute them in the relevant markets.
We have to depend upon others for marketing and distribution of our products, and we may be forced to enter into contracts limiting the benefits we may receive and the control we have over our products. We intend to rely on collaborative arrangements with one or more other companies that possess strong marketing and distribution resources to perform these functions for us. We may not be able to enter into beneficial contracts, and we may be forced to enter into contracts for the marketing and distribution of our products that substantially limit the potential benefits to us from commercializing these products. In addition, we will not have the same control over marketing and distribution that we would have if we conducted these functions ourselves.
We may not be able to compete with treatments now being marketed and developed, or which may be developed and marketed in the future by other companies.
Our products will compete with existing and new therapies and treatments. We are aware of a number of companies currently seeking to develop alternative means of delivering insulin, as well as new drugs intended to replace insulin therapy at least in part. We are also aware of a number of companies currently seeking to develop alternative means of enhancing and suppressing peptides. In the longer term, we also face competition from companies that seek to develop cures for diabetes and other malignant, infectious, autoimmune and allergic diseases through techniques for correcting the genetic deficiencies that underlie some of these diseases.
Numerous pharmaceutical, biotechnology and drug delivery companies, hospitals, research organizations, individual scientists and nonprofit organizations are engaged in the development of alternatives to our technologies. Some of these companies have greater research and development capabilities, experience, manufacturing, marketing, financial and managerial resources than we do. Collaborations or mergers between large pharmaceutical or biotechnology companies with competing drug delivery technologies could enhance our competitors’ financial, marketing and other resources. Developments by other drug delivery companies could make our products or technologies uncompetitive or obsolete. Accordingly, our competitors may succeed in developing competing technologies, obtaining FDA approval for products or gaining market acceptance more rapidly than we can.
Some of our most significant competitors, Pfizer, Eli Lilly, and Novo Nordisk, have discontinued development and/or sale of their inhalable forms of insulin. Unlike inhaled insulin formulations, Generex Oral-lyn™ is a buccally absorbed formulation with no residual pulmonary deposition.
If government programs and insurance companies do not agree to pay for or reimburse patients for our pharmaceutical products, our success will be impacted.
Sales of our oral insulin formulation in Ecuador, Lebanon, Algeria and India and our other potential pharmaceutical products in other markets will depend in part on the availability of reimbursement by third-party payers such as government health administration authorities, private health insurers and other organizations. Third-party payers often challenge the price and cost-effectiveness of medical products and services. Governmental approval of health care products does not guarantee that these third-party payers will pay for the products. Even if third-party payers do accept our product, the amounts they pay may not be adequate to enable us to realize a profit. Legislation and regulations affecting the pricing of pharmaceuticals may change before our products are approved for marketing and any such changes could further limit reimbursement.
Risks Related to Potential Liabilities
We face significant product liability risks, which may have a negative effect on our financial condition.
The administration of drugs or treatments to humans, whether in clinical trials or commercially, can result in product liability claims whether or not the drugs or treatments are actually at fault for causing an injury. Furthermore, our pharmaceutical products may cause, or may appear to have caused, serious adverse side effects (including death) or potentially dangerous drug interactions that we may not learn about or understand fully until the drug or treatment has been administered to patients for some time. Product liability claims can be expensive to defend and may result in large judgments or settlements against us, which could have a severe negative effect on our financial condition. We maintain product liability insurance in amounts we believe to be commercially reasonable for our current level of activity and exposure, but claims could exceed our coverage limits. Furthermore, due to factors in the insurance market generally and our own experience, we may not always be able to purchase sufficient insurance at an affordable price. Even if a product liability claim is not successful, the adverse publicity and time and expense of defending such a claim may interfere with our business.
| 18 |
Risks Related to the Market for Our Common Stock
Our stock price is below $5.00 per share and is treated as a “penny stock”, which places restrictions on broker-dealers recommending the stock for purchase.
Our common stock is defined as “penny stock” under the Securities Exchange Act of 1934, as amended, which we refer to as the Exchange Act, and the rules promulgated thereunder. The SEC has adopted regulations that define “penny stock” to include common stock that has a market price of less than $5.00 per share, subject to certain exceptions. These rules include the following requirements:
| • | broker-dealers must deliver, prior to the transaction a disclosure schedule prepared by the SEC relating to the penny stock market; |
| • | broker-dealers must disclose the commissions payable to the broker-dealer and its registered representative; |
| • | broker-dealers must disclose current quotations for the securities; |
| • | if a broker-dealer is the sole market-maker, the broker-dealer must disclose this fact and the broker-dealers presumed control over the market; and |
| • | a broker-dealer must furnish its customers with monthly statements disclosing recent price information for all penny stocks held in the customer’s account and information on the limited market in penny stocks. |
Additional sales practice requirements are imposed on broker-dealers who sell penny stocks to persons other than established customers and accredited investors. For these types of transactions, the broker-dealer must make a special suitability determination for the purchaser and must have received the purchaser’s written consent to the transaction prior to sale. If our common stock remains subject to these penny stock rules these disclosure requirements may have the effect of reducing the level of trading activity in the secondary market for our common stock. As a result, fewer broker-dealers may be willing to make a market in our stock, which could affect a shareholder’s ability to sell their shares.
The price of our common stock may be affected by a limited trading volume, may fluctuate significantly and may not reflect the actual value of our business.
There may be a limited public market for our common stock on the over the counter bulletin board market, and there can be no assurance that an active trading market will continue. An absence of an active trading market could adversely affect our stockholders’ ability to sell our common stock in short time periods, or at all. Our common stock has experienced, and is likely to experience in the future, significant price and volume fluctuations that could adversely affect the market price of our common stock without regard to our operating performance. In addition, we believe that factors, such as our sale of securities in connection with capital raising activities, could cause the price of our common stock to fluctuate substantially. Thus, the price at which shares of our common stock may trade from time to time may not reflect the actual value of our business or the actual value of our common stock.
From time to time, we may hire companies to assist us in pursuing investor relations strategies to generate increased volumes of investment in our common stock. Such activities may result, among other things, in causing the price of our common stock to increase on a short-term basis.
Furthermore, the stock market generally and the market for stocks of companies with lower market capitalizations and small biopharmaceutical companies, like us, have from time to time experienced, and likely will again experience significant price and volume fluctuations that are unrelated to the operating performance of a particular company. During the third calendar quarter of 2008 and continuing to date, we, like many other publicly traded companies, have experienced a sharp decline in the price of our stock attributable to concerns about the current global recession.
Risks Related to Ownership of Our Common Stock
If an exemption under state securities laws is not available for resales of shares of common stock, state securities regulators have the authority to seek rescission of such resales and, in some instances, may seek restitution or disgorgement of amounts received on such resales.
Because the shares of common stock registered under our S-1 registration statements have not been registered or qualified for resale under the securities laws of any state, an exemption from registration or qualification under state law is necessary for compliance with state securities laws. Generex has taken no steps to register or qualify, nor seek an exemption for, the resale of the shares of common stock under the securities laws of any state. The availability of exemptions will depend on the laws of the particular state in which a holder of the shares resides and the circumstances under which such holder seeks to sell the shares. If an exemption is not available but a resale of the shares is effected, state securities laws give state securities regulators authority to seek rescission (or cancellation) of transactions involving sales of securities that are not registered, qualified or exempted and, in some instances, authority to require restitution or disgorgement of profits from the sales of such securities and to impose statutory interest or penalties on disgorged amounts. While we are not aware of any state securities regulator taking action with respect to the resales of shares of our common stock, we cannot provide any assurance that regulators will refrain from taking such action in the future.
Provisions of our Restated Certificate of Incorporation could delay or prevent the acquisition or sale of our business.
Our Restated Certificate of Incorporation permits our Board of Directors to designate new series of preferred stock and issue those shares without any vote or action by our stockholders. Such newly authorized and issued shares of preferred stock could contain terms that grant special voting rights to the holders of such shares that make it more difficult to obtain stockholder approval for an acquisition of our business or increase the cost of any such acquisition.
| 19 |
Provisions of the Delaware General Corporation Law may prohibit us from making required payments with respect to our Series F 9% convertible preferred stock, which default may constitute a violation of our certificate of incorporation or a breach of our contractual obligations to the holders of our preferred stock.
We are incorporated in the State of Delaware and are subject to the provisions of the Delaware General Corporation Law (the “DGCL”). Section 170 of the DGCL provides, among other things, that a Delaware corporation may declare and pay dividends upon shares of its capital stock out of its surplus, as defined in and computed in accordance with Sections 154 and 244 of the DGCL. As of the date hereof, we have 1,225 shares of our Series F 9% convertible preferred stock outstanding. As of the date hereof, we have sufficient surplus to make dividend payments with respect to our outstanding Series F9% convertible preferred stock, as well as sufficient surplus to make the make-whole payments that may be due to the holders of our Series F9% convertible preferred stock, should such make-whole payments be deemed a dividend under the DGCL. However, our surplus will decrease as we spend our capital on operational activities, unless our spending is offset by capital-raising transactions. If our surplus is less than then-due dividend payments, including make-whole payments if they are deemed a dividend under the DGCL, we will be prohibited by the DGCL from making the dividend or make-whole payment, which may constitute a violation of our certificate of incorporation or a breach of our contractual obligations to the holders of our Series F 9% convertible preferred stock.
Our recent equity financing will dilute current stockholders and could prevent the acquisition or sale of our business.
The equity financing transactions into which we have recently entered have and will dilute current stockholders. At July 31, 2014, there were 257,331,254 shares of common stock issuable upon exercise of the warrants that we issued in a private placement in March 2008, in the registered direct offerings conducted in June, August and September 2009, in connection with the sales to Seaside 88, LP in April, May and June 2010 and in the registered direct offerings in February 2012, August 2012, December 2012, June 2013, January 2014 and March 2014. In addition, in connection with the private placements that closed on June 17, 2013 and March 27, 2014, 41,666,667shares of common stock are issuable upon conversion of the remaining Series E and Series F 9% Convertible Preferred Stock at July 31, 2014. Together the shares of common stock issuable upon exercise or conversion of the above-mentioned warrants and preferred stock represent approximately 38% of the shares of common stock currently outstanding. Assuming the holders of the warrants convert and exercise all of the warrants into shares of common stock, the number of shares of issued and outstanding common stock will increase significantly, and current stockholders will own a smaller percentage of the outstanding common stock of Generex. The issuance of shares of common stock pursuant to the warrants will also have a dilutive effect on earnings per share and may adversely affect the market price of the common stock.
In addition, the issuance of shares of common stock upon exercise of the warrants issued in the March 2008 private placement, the registered direct offerings in June, August and September 2009 and in connection with the sales to Seaside in April, May and June 2010, and the private placements in February 2012, August 2012, December 2012, June 2013, January 2014 and March 2014, could have an anti-takeover effect because such issuance will make it more difficult for, or discourage an attempt by, a party to obtain control of Generex by tender offer or other means. The issuance of common stock upon the exercise of the warrants or conversion of convertible preferred stock will increase the number of shares entitled to vote, increase the number of votes required to approve a change of control of the company, and dilute the interest of a party attempting to obtain control of the company.
If we raise funds through one or more additional equity financings in the future, it will have a further dilutive effect on existing holders of our shares by reducing their percentage ownership. The shares may be sold at a time when the market price is low because we are in need of the funds. This will dilute existing holders more than if our stock price was higher. In addition, equity financings normally involve shares sold at a discount to the current market price. Most of our outstanding warrants have price protection provisions, which decrease the exercise price of the warrant and increase the number of shares which may be purchased upon exercise of the warrants, if we sell additional equity at an effective price per common share less than the current exercise price of the warrant. Therefore, equity financings at a low price per share will result in even more dilution to existing shareholders.
Item 1B.Unresolved Staff Comments.
None.
Item 2.Properties.
Our executive and principal administrative offices occupy approximately 2,300 square feet of office space in in downtown Toronto, Ontario, Canada which we rent at an annual rent of approximately $80,000 under a lease that runs to September 2019.
We lease approximately 546 square feet of office space in Worcester, Massachusetts which we rent under a lease agreement which runs on a month-to-month basis, which Antigen uses for its research and development activities at an annual rent of approximately $18,000. This space is sufficient for Antigen’s present activities.
We do not expect to need manufacturing capabilities in Canada related to our insulin product, as it is likely that we will contract out the manufacturing of product requirements for any future clinical trials and commercial sales.
| 20 |
Item 3.Legal Proceedings.
Subash Chandarana et al. v. Generex Biotechnology Corporation. In February 2001, a former business associate of Pankaj Modi ("Modi") (a former officer of Generex) and an entity called Centrum Technologies Inc. ("CTI") commenced an action in the Ontario Superior Court of Justice against us and Modi seeking, among other things, damages for alleged breaches of contract and tortious acts related to a business relationship between this former associate and Modi that ceased in July 1996. The plaintiffs’ statement of claim also seeks to enjoin the use, if any, by us of three patents allegedly owned by CTI. The three patents are entitled Liquid Formulations for Proteinic Pharmaceuticals, Vaccine Delivery System for Immunization, Using Biodegradable Polymer Microspheres, and Controlled Releases of Drugs or Hormones in Biodegradable Polymer Microspheres. It is our position that the buccal drug delivery technologies which are the subject matter of our research, development, and commercialization efforts, including Generex Oral-lyn™ and the RapidMist™ Diabetes Management System, do not make use of, are not derivative of, do not infringe upon, and are entirely different from the intellectual property identified in the plaintiffs’ statement of claim. On July 20, 2001, we filed a preliminary motion to dismiss the action of CTI as a nonexistent entity or, alternatively, to stay such action on the grounds of want of authority of such entity to commence the action. The plaintiffs brought a cross motion to amend the statement of claim to substitute Centrum Biotechnologies, Inc. ("CBI") for CTI. CBI is a corporation of which 50 percent of the shares are owned by the former business associate and the remaining 50 percent are owned by us. Consequently, the shareholders of CBI are in a deadlock. The court granted our motion to dismiss the action of CTI and denied the plaintiffs’ cross motion without prejudice to the former business associate to seek leave to bring a derivative action in the name of or on behalf of CBI. The former business associate subsequently filed an application with the Ontario Superior Court of Justice for an order granting him leave to file an action in the name of and on behalf of CBI against Modi and us. We opposed the application. In September 2003, the Ontario Superior Court of Justice granted the request and issued an order giving the former business associate leave to file an action in the name of and on behalf of CBI against Modi and us. A statement of claim was served in July 2004. Since that time, the plaintiffs have not taken any steps in furtherance of the proceeding. We are not able to predict the ultimate outcome of this legal proceeding at the present time or to estimate an amount or range of potential loss, if any, from this legal proceeding.
In December 2011, a vendor commenced an action against Generex Biotechnology Corporation and its subsidiary, Generex Pharmaceuticals, Inc., in the Ontario Superior Court of Justice claiming damages for unpaid invoices including interest in the amount of $429,000, in addition to costs and further interest. We have responded to this statement of claim and intend to defend this action vigorously. We have also asserted a counterclaim in the proceeding for $200,000 arising from the vendor’s breach of contract and detinue, together with interest and costs. On November 16, 2012, the parties agreed to settle this action and we have agreed to pay the plaintiff $125,000, following the spinout of its subsidiary Antigen, from the proceeds of any public or private financing related to Antigen subsequent to such spinout. Each party agreed to execute mutual releases to the claim and counterclaim to be held in trust by each parties counsel until payment of the settlement amount. Following payment to the plaintiff, the parties agree that a Consent Dismissal Order without costs will be filed with the court. If we fail to make the payment following completion of any post-spinout financing related to Antigen or any other subsidiaries, the Plaintiffs may take out a judgment in the amount of the claim plus interest of 3% per annum and costs fixed at $25,000.
Disputes with Former Officer
In May 2011, Rose C. Perri, our former Chief Operating Officer and Chief Financial Officer, commenced two proceedings against us. On May 11, 2011, Ms. Perri filed a notice of application in the Ontario Superior Court of Justice, Commercial List, against Generex, two of our affiliates (1097346 Ontario, Inc. and Generex Pharmaceuticals Inc.), three of our independent directors (John P. Barratt, Nola Masterson and Brian T. McGee), our President and Chief Executive Officer (Mark A. Fletcher), our Chief Operating Officer (David Brusegard) and our Chief Financial Officer (Stephen Fellows). The application has since been abandoned.
On May 20, 2011, Ms. Perri filed a statement of claim (subsequently amended) in the Ontario Superior Court of Justice, naming the following as defendants: Generex, Mr. Barratt, Ms. Masterson, Mr. McGee, and Mr. Fletcher. In this action, Ms. Perri has alleged that the defendants engaged in discrimination, harassment, bad faith and infliction of mental distress in connection with the termination of her employment with Generex. Ms. Perri is seeking damages in this action in excess of $7,000,000 for, among other things, breach of contract, breach of fiduciary duty, violations of the Ontario Human Rights Code and aggravated and punitive damages. On September 20, 2011, the defendants filed a statement of defense and counterclaim, also naming Time Release Corp., Khazak Group Consulting Corp., and David Khazak, C.A. as defendants by counterclaim, and seeking damages of approximately $2.3 million in funds that the defendants allege Ms. Perri wrongly caused Generex to pay to third parties in varying amounts over several years and an accounting of certain third-party payments, plus interests and costs. The factual basis for the counterclaim involves payments made by Generex to third parties believed to be related to Ms. Perri. For a discussion of certain of these related party transactions, see the disclosures under the caption “Management’s Discussion and Analysis of Financial Condition and Results of Operations” under the heading “Certain Related Party Transactions” in this annual report. We intend to defend this action and pursue our counterclaim vigorously. We are not able to predict the ultimate outcome of this legal proceeding at the present time or to estimate an amount or range of potential loss, if any, from this legal proceeding.
| 21 |
On June 1, 2011, Golden Bull Estates Ltd. filed a claim in the Ontario Superior Court of Justice, naming Generex, 1097346 Ontario, Inc. and Generex Pharmaceuticals Inc. as defendants. The plaintiff, Golden Bull Estates, is controlled by Ms. Perri. The plaintiff alleges damages in the amount of $550,000 for breach of contract and $50,000 for punitive damages, plus interest and costs. The plaintiff’s claims relate to an alleged contract between the plaintiff and Generex for property management services for certain Ontario properties owned by Generex. Generex terminated the plaintiff’s property management services in April 2011. Following the close of pleadings, we served a motion for summary judgment. The plaintiff responded by amending its statement of claim to include a claim to our interest in certain of our real estate holdings. The plaintiff moved for leave to issue and register a Certificate of Pending Litigation in respect of this real estate. The motion was not successful in respect of any current real estate holdings of Generex. We are not able to predict the ultimate outcome of this legal proceeding at the present time or to estimate an amount or range of potential loss, if any, from this legal proceeding.
In August 2011, the estate of Antonio Perri, the late father of Ms. Perri, commenced an action against Generex Pharmaceuticals, Inc., the law firm of Brans, Lehun, Baldwin LLP and William Lehun in the Ontario Superior Court of Justice, claiming that the estate is entitled to the proceeds of sale (approximately $1,730,000) received by Generex on its sale of two properties to Golden Bull Estates, a company controlled by Ms. Perri. The suit alleges that no consideration was received when Generex purchased the two properties from Antonio Perri in 1998. On May 6, 2014, we received a court order which dismissed this action for delay as the plaintiff has not brought this action to conclusion or set it down for trial within the time prescribed by the laws of Ontario.
We are involved in certain other legal proceedings in addition to those specifically described herein. Subject to the uncertainty inherent in all litigation, we do not believe at the present time that the resolution of any of these legal proceedings is likely to have a material adverse effect on our financial position, operations or cash flows.
With respect to all litigation matters, as additional information concerning the estimates used by us becomes known, we reassess each matter’s position both with respect to accrued liabilities and other potential exposures.
Item 4. [Not Required for Registrant]
EXECUTIVE OFFICERS OF THE REGISTRANT
| Name | Age | Position Held with Generex | ||
| Mark A. Fletcher | 48 | President/Chief Executive Officer, General Counsel and Director | ||
| Stephen Fellows | 48 | Chief Financial Officer | ||
| Dr. David Brusegard | 70 | Chief Operating Officer and Secretary | ||
Mark A. Fletcher, Esq. has served as our President and Chief Executive Officer since March 2011. Mr. Fletcher was elected to serve as a member of the Board of Directors at our annual meeting of stockholders held on June 8, 2011. Mr. Fletcher was appointed interim President and Chief Executive Officer on September 29, 2010 to succeed Anna E. Gluskin, who was terminated as President and Chief Executive Officer on that date. On September 29, 2010, Mr. Fletcher was also appointed Secretary and served as such until June 8, 2011. He served as Executive Vice President and General Counsel since April 2003, and he continues in his role as General Counsel. From October 2001 to March 2003, Mr. Fletcher was engaged in the private practice of law as a partner at Goodman and Carr LLP, a leading Toronto law firm. From March 1993 to September 2001, Mr. Fletcher was a partner at Brans, Lehun, Baldwin LLP, a law firm in Toronto. Mr. Fletcher received his LL.B. from the University of Western Ontario in 1989 and was admitted to the Ontario Bar in 1991.
Stephen Fellows hasserved as our Chief Financial Officer since March 2011. Mr. Fellows has served as our Vice President, Finance since June 2009. From August 2005 to December 2008, Mr. Fellows was employed by Sona Mobile Holdings Corporation, a publicly held software company which developed software applications for mobile devices, where he served as Chief Financial Officer. From September 1996 to August 2005, Mr. Fellows worked at 3Com Corporation, where he served in several positions including as the Director of Finance of the corporate accounting group in Marlborough, MA and Director of Finance & Operations of 3Com’s Canadian subsidiary. From January 1992 to August 1996, Mr. Fellows worked at Pennzoil Corporation where he spent time in the international mergers and acquisitions group in Houston, Texas, as well as four years as Controller for Pennzoil Canada. Mr. Fellows received a Bachelor of Business Administration degree from Wilfrid Laurier University in 1988 and earned his Chartered Accountants designation while articling with Arthur Andersen & Company in Toronto in 1990.
David Brusegard, Ph.D. has served as Chief Operating Officer since March 2011 and was appointed Secretary on June 8, 2011. Dr. Brusegard served as a consultant to Generex from March 2010 to March 2011. From 2007 to March 29, 2011, Dr. Brusegard held the position of President of The OSLO Group, his consulting firm. He served as Chief Executive Officer of the Pentius Group from 2004 to 2007. The Pentius Group was a five-company group which designed, sold, and marketed health insurance, and operated a managed care facility staffed with nurses supervised by physician directors. Pentius Group’s company assets were sold in 2007 to Canam Insurance of Windsor, Ontario. Dr. Brusegard has a breadth of experience in several fields, including, medical record design, health informatics, health insurance, digital mapping, database design, global positioning systems applications, business management and strategic planning. He was a senior economist at Statistics Canada for a decade, an adjunct professor at the University of Toronto and taught information ethics and information law at Ryerson University. He has consulted internationally on information management for the World Bank as well as major consumer packaged goods companies, hospitals, municipalities, and all levels of government. Other recent positions of note include; Vice President, Analytics for ICOM Communication and Information, President of Geographic Decision Support Systems, and CEO, Tristar Software. Dr. Brusegard performed his graduate work at The University of North Carolina at Chapel Hill, and the University of Calgary from which he holds a Ph.D. Phil., awarded in 1976.
| 22 |
Other Key Employees and Consultants
Eric von Hofe, Ph.D.Director since June 2011. Dr. von Hofe is currently President of Antigen Express, Inc., a wholly-owned subsidiary of Generex. He has held this position since 2005. Since 2005, he has also been a Vice President of Generex. He has extensive experience with technology development projects, including his previous position at Millennium Pharmaceuticals as Director of Programs & Operations, Discovery Research. Prior to that, Dr. von Hofe was Director, New Targets at Hybridon, Inc., where he coordinated in-house and collaborative research that critically validated gene targets for novel antisense medicines. Dr. von Hofe also held the position of Assistant Professor of Pharmacology at the University of Massachusetts Medical School, where he received a National Cancer Institute Career Development Award for defining mechanisms by which alkylating carcinogens create cancers. He received his Ph.D. from the University of Southern California in Experimental Pathology and was a postdoctoral fellow at both the University of Zurich and Harvard School of Public Health. His work has been published in forty-eight articles in peer-reviewed journals, and he has been an inventor on four patents.
Scientific Advisory Board
Dr. James H. Anderson, Jr. was elected to our Board of Directors on June 8, 2011. Dr. Anderson, a former Lieutenant Colonel in the United States Army's Medical Corps, worked for Eli Lilly and Company from 1985 until 2009. From July 2006 to December 2009, Dr. Anderson served as Eli Lilly's Senior Medical Director, Diabetes and Cardio-metabolic Medicine. Dr. Anderson is presently a Clinical Associate Professor of Medicine in the Division of Endocrinology and Metabolism at Indiana University Medical School, a member of the American Diabetes Association's Community Leadership Board, and a member of the editorial boards of the peer-reviewed journals Diabetes, Technology and Therapeutics and Journal of Diabetes Science and Technology. Dr. Anderson is also an elected Fellow of the Faculty of Pharmaceutical Medicine of the Royal Colleges of Physicians of the United Kingdom and a Fellow of the American College of Endocrinology.
Dr. Gerald Bernstein, M.D., F.A.C.P. graduated from Dartmouth College and Tufts University School of medicine. He is board certified in internal medicine (1966) and endocrinology and metabolism (1973). He entered practice in 1966 after completing a research fellowship. Dr. Bernstein is an associate clinical professor at Albert Einstein College of Medicine in New York. He is an attending physician at Beth Israel Medical Center, Lenox Hill Hospital (1974) and Montefiore Medical Center (1966). He served on the National Board of Directors of the American Diabetes Association, its research foundation and many national committees. Dr. Bernstein is a past president of the American Diabetes Association and was Director of the Beth Israel Health Care Systems Diabetes Management Program. He is currently Director of the Diabetes Management Program of The Friedman Diabetes Institute at Beth Israel Hospital in New York. He has served as Vice President for Medical Affairs at Generex Biotechnology Corp. since 2001 and served as a Director of Generex from October 2002 to May 2008.
Dr. Craig Eagleattended medical school at the University of New South Wales, Sydney, Australia and received his general internist training at Royal North Shore Hospital in Sydney. He completed his hemato-oncology and laboratory hematology training at Royal Prince Alfred Hospital in Sydney. He was granted Fellowship in the Royal Australasian College of Physicians (FRACP) and the Royal College of Pathologists Australasia (FRCPA). After his training he performed basic research at the Royal Prince of Wales hospital to develop a new monoclonal antibody to inhibit platelets. He joined Pfizer Australia in 2001 as part of the medical group. In Australia, his role involved leading and participating in scientific research, regulatory and pricing & re-imbursement negotiations for compounds in therapeutic areas including oncology, anti-infectives, respiratory, arthritis and pain management. In 2003, Pfizer relocated Dr. Eagle to the United States where he was appointed as the world wide lead for development of celecoxib in oncology to oversee the global research program. Since that time he has had increasing responsibility for overseeing the global research plans and teams for irinotecan and dalteparin. In 2007, he became head of the oncology therapeutic area global medical group for Pfizer, including the US oncology business. Dr. Eagle has led, or been directly involved with, teams that resulted in eight new products or indications. As part of his current role at Pfizer, he has led the integration of the Pfizer/Wyeth oncology businesses and portfolio.
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
Our common stock is quoted on the OTC Bulletin Board under the symbol "GNBT.OB." Our common stock was listed on the NASDAQ Capital Market (formerly the NASDAQ SmallCap Market) on June 5, 2003. On October 21, 2010, our common stock was delisted due to our failure to regain compliance with the $1.00 bid price requirement for continued listing set forth in NASDAQ Listing Rule 5550(a)(2). From May 5, 2000 to June 4, 2003, our common stock was listed on the NASDAQ National Market. From February 1998 to May 2000, the "bid" and "asked" prices for our common stock were quoted on the OTC Bulletin Board operated by the National Association of Securities Dealers. Prior to February 1998, there was no public market for our common stock.
| 23 |
The table below sets forth prices for our common stock for the last eight fiscal quarters. The prices below reflect the high and low bid information for the first two quarters of fiscal 2014, the four quarters of fiscal 2013 and the last two quarters of fiscal 2012. The over-the-counter market quotations reflect inter-dealer prices, without retail mark-up, mark-down or commissions and may not represent actual transactions. The table below sets forth prices for our common stock for each fiscal quarter in the prior two years ended July 31, 2014. The prices below reflect the high and low bid information. The over-the-counter market quotations reflect inter-dealer prices, without retail mark-up, mark-down or commissions and may not represent actual transactions.
| Sales/Bid Prices | |||||||
| High | Low | ||||||
| Fiscal 2014 | |||||||
| First Quarter | $ | 0.04 | $ | 0.02 | |||
| Second Quarter | $ | 0.06 | $ | 0.02 | |||
| Third Quarter | $ | 0.05 | $ | 0.03 | |||
| Fourth Quarter | $ | 0.04 | $ | 0.02 | |||
| Fiscal 2013 | |||||||
| First Quarter | $ | 0.10 | $ | 0.05 | |||
| Second Quarter | $ | 0.07 | $ | 0.02 | |||
| Third Quarter | $ | 0.05 | $ | 0.03 | |||
| Fourth Quarter | $ | 0.05 | $ | 0.03 | |||
As of October 1, 2014, there were approximately 587 holders of record of our common stock. Record holders do not include owners whose shares are held in street name by a broker or other nominee.
Dividends
We have not paid dividends on our common stock in the past and have no present intention of paying dividends on our common stock in the foreseeable future. The Certificate of Designations pertaining to our Series F 9% Convertible Preferred Stock imposes certain restrictions on our ability to pay dividends on our common stock. For information about these restrictions and the dividends that we paid on our Series F 9% Convertible Preferred Stock, see the discussion under the caption “Management’s Discussion and Analysis of Financial Condition and Results of Operations” under the heading “Financial Condition, Liquidity and Resources” and the subheadings “Financing – June 2013”, “Financing – January 2014” and “Financing – March 2014” in this Annual Report on Form 10-K.
Stock Performance Graph
The following information under this heading “Stock Performance Graph” in thisPart II, Item 5 of this Annual Report on Form 10-K is not deemed to be “soliciting material” or to be “filed” with the SEC or subject to Regulation 14A or 14C under the Exchange Act, or to the liabilities of Section 18 of the Exchange Act, and will not be deemed to be incorporated by reference into any filing under the Securities Act or the Exchange Act, except to the extent we specifically incorporate it by reference into such a filing.
Set forth below is a line graph comparing the cumulative total return on Generex's common stock with cumulative total returns of the NASDAQ Composite Index and the NASDAQ Biotechnology Index for the period commencing July 31, 2009 and ending on July 31, 2014. The comparison assumes the investment of $100 on July 31, 2009 in our common stock and in each of the indices and, in each case, assumes reinvestment of all dividends. The stock price performance shown below is not necessarily indicative of future performance.
| 24 |
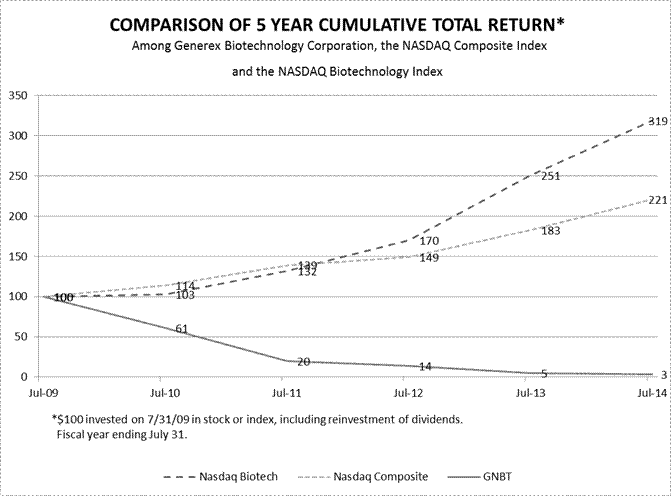
Sales of Unregistered Securities
In addition to our sales of unregistered securities disclosed in our Quarterly Reports on Form 10-Q,we have issued the securities in reliance upon Section 4(2) of the Securities Act as follows in the fiscal quarter ended July 31, 2014.
We have issued shares of our common stock to Joseph Moscato, a consultant, pursuant to an agreement to provide us with investor relation services until September 30, 2014. During the three months ended July 31, 2014, we issued 450,000 shares of common stock to Joseph Moscato pursuant to this agreement. The sale of such shares was exempt from registration under the Securities Act in reliance upon Section 4(2) thereof. We believe that Joseph Moscato is an “accredited investor” as that term is defined in Rule 501(a) of Regulation D under the Securities Act. The certificates issued for the shares of common stock included a legend to indicate that they are restricted. The sales of such securities did not involve the use of underwriters, and no commissions were paid in connection therewith.
Issuer Purchases of Equity Securities
Neither we nor any affiliated purchaser (as defined in Section 240.10 b-18(a)(3) of the Exchange Act) purchased any of our equity securities during the fourth quarter of the fiscal year ending July 31, 2014.
| 25 |
Item 6. Selected Financial Data.
The following selected financial data are derived from and should be read in conjunction with our financial statements and related notes, which appear elsewhere in this Annual Report on Form 10-K. Our financial statements for the years ended July 31, 2014 and 2013 were audited by MNP LLP. Our financial statements for the years ended July 31, 2012, 2011 and 2010 were audited by MSCM LLP, which firm was acquired by MNP LLP on June 1, 2013.
| In thousands (except per share data) | 2014 | 2013 | 2012 | 2011 | 2010 | |||||
| Operating Results: | ||||||||||
| Revenue | $ | — | $ | — | $ | 29 | $ | 292 | $ | 1,173 |
| Net Loss | (1) | (8,554) | (9,490) | (21,676) | (25,280) | |||||
| Net Loss Available to Common Stockholders | (2,060) | (10,277) | (9,867) | (22,442) | (25,280) | |||||
| Cash Dividends per share | — | — | — | — | ||||||
| Loss per Common Share: | ||||||||||
| Basic and Diluted Net Loss Per Common Share | (0.003) | (0.02) | (0.03) | (0.08) | (0.10) | |||||
| Financial Positions: | ||||||||||
| Total Assets | $ | 5,522 | $ | 4,853 | $ | 4,644 | $ | 12,006 | $ | 24,575 |
| Long-Term Debt | $ | — | $ | — | $ | 441 | $ | 1,870 | $ | 1,824 |
| Convertible Debentures | $ | — | $ | — | $ | — | $ | — | $ | — |
| Preferred Stock* | $ | — | $ | — | $ | — | $ | — | $ | — |
| Stockholder's (Deficiency)/Equity | $ | (6,090) | $ | (10,141) | $ | (8,380) | $ | (8,442) | $ | 8,971 |
* At July 31, 2014, there were 1,250 shares of convertible preferred stock outstanding which had a face value of $1,000 per share ($1,250,000 in aggregate), but which have an accounting value of zero. At July 31, 2013, there were 930 shares of convertible preferred stock outstanding which had a face value of $1,000 per share ($930,000 in aggregate), but which have an accounting value of zero. At July 31, 2012, there were 1,490 shares of convertible preferred stock outstanding which had a face value of $1,000 per share ($1,490,000 in aggregate), but which had an accounting value of zero. At July 31, 2011, there were 1,287 shares of convertible preferred stock outstanding which had a face value of $1,000 per share ($1,287,000 in aggregate), but which had an accounting value of zero. See Note 9 to theNotes to Consolidated Financial Statements included elsewhere in this Annual Report. There was no preferred stock outstanding in fiscal year 2010.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion and analysis by management provides information with respect to our financial condition and results of operations for the fiscal years ended July 31, 2014, 2013 and 2012. This discussion should be read in conjunction with the information in the consolidated financial statements and the notes pertaining thereto contained inItem 8 - Financial Statements and Supplementary Data of this Annual Report on Form 10-K for the year ended July 31, 2014 and the information discussed inPart I, Item 1A - Risk Factors.
Overview of Business
We are engaged primarily in the research and development of drug delivery systems and technologies. Our primary focus at the present time is our proprietary technology for the administration of formulations of large molecule drugs to the oral (buccal) cavity using a hand-held aerosol applicator. Through our wholly-owned subsidiary, Antigen, we have expanded our focus to include immunomedicines incorporating proprietary vaccine formulations.
We believe that our buccal delivery technology is a platform technology that has application to many large molecule drugs and provides a convenient, non-invasive, accurate and cost-effective way to administer such drugs. We have identified several large molecule drugs as possible candidates for development, including estrogen, heparin, monoclonal antibodies, human growth hormone and fertility hormones, but to date have focused our development efforts primarily on one pharmaceutical product, Generex Oral-lyn™, an insulin formulation administered as a fine spray into the oral cavity using our proprietary hand-held aerosol spray applicator known as RapidMist™.
Our subsidiary, Antigen Express, concentrates on developing proprietary vaccine formulations that work by stimulating the immune system to either attack offending agents (i.e., cancer cells, bacteria, and viruses) or to stop attacking benign elements (i.e. self proteins and allergens). Our immunomedicine products are based on two platform technologies and are in the early stages of development. We continue clinical development of Antigen’s synthetic peptide vaccines designed to stimulate a potent and specific immune response against tumors expressing the HER-2/neu oncogene for patients with HER-2/neu positive breast cancer in a Phase II clinical trial and patients with prostate cancer and against avian influenza in two Phase I clinical trials. We also initiated an additional Phase I clinical trial in patients with either breast or ovarian cancer. The synthetic vaccine technology has certain advantages for pandemic or potentially pandemic viruses, such as the H5N1 avian and H1N1 swine flu. In addition to developing vaccines for pandemic influenza viruses, we have vaccine development efforts underway for seasonal influenza virus, HIV, HPV, melanoma, ovarian cancer, allergy and Type I diabetes mellitus. We have established collaborations with clinical investigators at academic centers to advance these technologies.
| 26 |
To date, we have received regulatory approval in Ecuador, India (subject to further study), Lebanon and Algeria for the commercial marketing and sale of Generex Oral-lyn™. We have previously submitted regulatory dossiers for Generex Oral-lyn™ in a number of other countries, including Bangladesh, Kenya, Jordan and Armenia. While we believe these countries will ultimately approve our product for commercial sale, we do not anticipate recognizing revenues in any of these jurisdictions in the next twelve months. No dossier related activities or product shipments have taken place during 2013, nor are any expected to these countries during the remainder of 2014.
In March 2008, we initiated Phase III clinical trials for this product in the U.S. with the first patient screening for such trials at a clinical study site in Texas in April 2008. Approximately 450 patients have been enrolled to date at approximately 70 clinical sites around the world, including sites in the United States, Canada, Bulgaria, Poland, Romania, Russia, Ukraine and Ecuador. The final subjects completed the trial in August 2011. After appropriate validation, the data from approximately 450 patients was tabulated, reviewed and analyzed. Those results from the Phase III trial along with a comprehensive review and supplemental analyses of approximately 40 prior Oral-lyn clinical studies were compiled and submitted to the FDA in late December 2011 in a comprehensive package including a composite metanalysis of all safety data. We continue to have discussions with the FDA with respect to the pathway for regulatory approval, including any additional clinical or pharmacological studies that might be required to support regulatory approval or enhance marketing success. We do not currently plan to expend significant resources on additional clinical trials of Oral-lyn™ until after such time that we secure additional financing.
We are a development stage company. From inception through July 31, 2014, we have received only limited revenues from operations. We did not generate any revenue in fiscal 2013 or 2014.
As of July 31, 2014, our current cash position is not sufficient to meet our working capital needs for the next twelve months. To continue operations, we will require additional funds to support our working capital requirements and any development activities, or will need to suspend operations. To continue operations, we will require additional funds to support our working capital requirements and any development activities, or will need to suspend operations. We are seeking various alternatives to ensure that we can meet some of our operating cash flow requirements through financing activities, such as private placement of our common stock, preferred stock offerings and offerings of debt and convertible debt instruments as well as through merger or acquisition opportunities. In addition, we are actively seeking strategic alternatives, including strategic investments and divestitures. We have sold non-essential real estate assets which were classified as Assets Held for Investment to augment its cash position. We cannot provide any assurance that we will obtain the required funding. Our inability to obtain required funding in the near future or our inability to obtain funding on favorable terms will have a material adverse effect on our operations and our strategic development plan for future growth. If we cannot successfully raise additional capital and implement our strategic development plan, our liquidity, financial condition and business prospects will be materially and adversely affected and we may have to cease operations.
We operate in only one segment: the research and development of drug delivery systems and technologies for metabolic and immunological diseases.
Accounting for Research and Development Projects
Our major research and development projects are the refinement of our platform buccal delivery technology, our buccal insulin project (Generex Oral-lyn™) and Antigen’s peptide immunotherapeutic vaccines.
During the fiscal years ended July 31, 2014 and 2013, we expended resources on the clinical testing of our buccal insulin product, Generex Oral-lyn™. The completion of late-stage trials in Canada and the United States may require significantly greater funds than we currently have on hand.
During the fiscal years ended July 31, 2014 and 2013, we expended resources on research and development relating to Antigen’s peptide immunotherapeutic vaccines and related technologies. One Antigen vaccine is currently in Phase II clinical trials in the United States involving patients with HER-2/neu positive breast cancer, and we have completed a Phase I clinical trial for an Antigen vaccine for H5N1 avian influenza which was conducted at the Lebanese-Canadian Hospital in Beirut. Antigen’s prostate cancer vaccine based on AE37 has been tested in a completed (August 2009) Phase I clinical trial in Greece.
Because of various uncertainties, we cannot predict the timing of completion and commercialization of our buccal insulin or Antigen’s peptide immunotherapeutic vaccines or related technologies. These uncertainties include the success of current studies, our ability to obtain the required financing and the time required to obtain regulatory approval even if our research and development efforts are completed and successful, our ability to enter into collaborative marketing and distribution agreements with third-parties, and the success of such marketing and distribution arrangements. For the same reasons, we cannot predict when any products may begin to produce net cash inflows.
| 27 |
Most of our buccal delivery research and development activities to date have involved developing our platform technology for use with insulin. As a result, we have not made significant distinctions in the accounting for research and development expenses among products, as a significant portion of all research has involved improvements to the platform technology in connection with insulin, which may benefit all of our potential buccal products. During the fiscal year ended July 31, 2014, approximately 26% of our $1,382,995 in research and development expenses was attributable to insulin and platform technology development. During the fiscal year ended July 31, 2013, approximately 24% of our $2,192,724 in research and development expenses was attributable to insulin and platform technology development. During the fiscal year ended July 31, 2012, approximately 61% of our $4,987,236 in research expenses was attributable to insulin and platform technology development.
During the fiscal year ended July 31, 2014, approximately 74% of our $1,382,995 in research and development expenses was attributable to Antigen's immunomedicine products. During the fiscal year ended July 31, 2013, approximately 76%, or $1,658,731 of our research and development expenses was attributable to Antigen's immunomedicine products. During the fiscal year ended July 31, 2012, approximately 39% or $1,941,774 in research expenses was attributable to Antigen's immunomedicine products.
Because these products are in initial phases of clinical trials or early, pre-clinical stage of development (with the exception of the Phase II clinical trials of Antigen HER-2/neu positive breast cancer vaccine that are underway), all of the expenses were accounted for as basic research and no distinctions were made as to particular products. Due to the early stage of development, we cannot predict the timing of completion of any products arising from this technology, or when products from this technology might begin producing revenues.
Critical Accounting Policies
Our discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements which have been prepared in conformity with accounting principles generally accepted in the United States of America. It requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
We consider certain accounting policies related to impairment of long-lived assets, intangible assets and accrued liabilities to be critical to our business operations and the understanding of our results of operations:
Going Concern. As shown in the accompanying consolidated financial statements, we have not been profitable and have reported recurring losses from operations. These factors raise substantial doubt about our ability to continue to operate in the normal course of business. The accompanying consolidated financial statements do not include any adjustments that might be necessary should we be unable to continue as a going concern.
Revenue Recognition. Net sales of our over-the-counter consumer products are generally recognized in the period in which the products are delivered. Delivery of the products generally completes the criteria for revenue recognition for us. In the event where the customers have the right of return, sales are deferred until the right of return lapses, the product is sold to a third party or a provision for returns can be reasonably estimated based on historical experience.
Impairment of Long-Lived Assets. Management reviews for impairment whenever events or changes in circumstances indicate that the carrying amount of property and equipment may not be recoverable under the provisions of accounting for the impairment of long-lived assets. If it is determined that an impairment loss has occurred based upon expected future cash flows, the loss is recognized in the Consolidated Statement of Operations. As of July 31, 2014, there were no indications of any impairment of our long-lived assets.
Intangible Assets. We have intangible assets related to patents. The determination of the related estimated useful lives and whether or not these assets are impaired involves significant judgments. In assessing the recoverability of these intangible assets, we use an estimate of undiscounted operating income and related cash flows over the remaining useful life, market conditions and other factors to determine the recoverability of the asset. If these estimates or their related assumptions change in the future, we may be required to record impairment charges against these assets. In the fiscal year ended July 31, 2012, we recorded a write down of $440,780 on certain patents.There were no patent write downs or disposals in the fiscal years ended July 31, 2013 or 2014.
Estimating accrued liabilities, specifically litigation accruals. Management's current estimated range of liabilities related to pending litigation is based on management's best estimate of future costs. While the final resolution of the litigation could result in amounts different than current accruals, and therefore have an impact on our consolidated financial results in a future reporting period, management believes the ultimate outcome will not have a significant effect on our consolidated results of operations, financial position or cash flows.
Share-based compensation. Management determines value of stock-based compensation to employees in accordance with Financial Accounting Standards Board (FASB) Accounting Standards Codification (ASC) 718, Compensation – Stock Compensation. Management determines value of stock-based compensation to non-employees and consultants in accordance with and ASC 505, Equity-Based Payments to Non-Employees.
| 28 |
Derivative warrant liability. FASB ASC 815, Derivatives and Hedging, requires all derivatives to be recorded on the consolidated balance sheet at fair value for fiscal years beginning after December 15, 2008. As a result, certain derivative warrant liabilities (namely those with a price protection feature) are now separately valued as of August 1, 2009 and accounted for on our balance sheet, with any changes in fair value recorded in earnings. On our consolidated balance sheets as of July 31, 2014 and July 31, 2013, we used the binomial lattice model to estimate the fair value of these warrants. Key assumptions of the binomial lattice option-pricing model include the market price of our stock, the exercise price of the warrants, applicable volatility rates, risk-free interest rates, expected dividends and the instrument’s remaining term. These assumptions require significant management judgment. In addition, changes in any of these variables during a period can result in material changes in the fair value (and resultant gains or losses) of this derivative instrument.
Results of Operations
Year Ended July 31, 2014 Compared to Year Ended July 31, 2013
We had a net loss for the fiscal year ended July 31, 2014 (fiscal 2014) of $1,417 versus a net loss of $8,554,322 in the prior fiscal year (fiscal 2013). The loss this year was caused primarily by operating expenses of $4,399,679, offset by a gain due to the change in fair value of the derivative liabilities of $4,525,739, while in the prior year operating expenses were $6,129,359 with a loss due to the change in fair value of the derivative liabilities of $3,140,259. Our operating loss for the fiscal year ended July 31, 2014 decreased to $4,399,679 compared to $6,129,359 in fiscal 2013. The decrease in operating loss resulted from a decrease in research and development expenses (to $1,382,995 from $2,192,724) and a decrease in general and administrative expenses (to $3,016,684 from $3,936,635). We did not have any revenues in either fiscal 2014 or 2013.
The decrease in research and development expenses in the current fiscal year versus the comparative period in the previous fiscal year is primarily due to there being no installments due to the contract research organization for the Antigen Phase II clinical trial in the last three quarters of fiscal 2014. Our efforts to significantly reduce expenses in all categories also contributed to the decrease in this category. The decrease in general and administrative expenses is related to a decrease in professional services expenses, including legal and consulting services of approximately $700,000, in addition to a decrease in payroll related expenses of approximately $56,000 and a decrease in office related expenses of approximately $100,000 in the fiscal year ended July 31, 2014, as compared to fiscal 2013. We also incurred reductions of expenses in most other categories due to our efforts to conserve cash until we complete the strategic development plan announced by management on March 30, 2011.
Our interest expense in fiscal 2014 was $321,670 compared to the previous year’s fiscal period at $530,222 due to the lower fees and costs associated with the November 2012 property refinancing, as well as lower penalties incurred upon the early discharge of the mortgages on the sale of our properties held for investment. We recognized lower income from assets held for investment (net of expense) of $193,607 in fiscal 2014 compared to $1,245,165 in fiscal 2014 due to the almost $1.0 million accounting gain on the aforementioned property sale in the fiscal 2013 period. Change in fair value of derivative liabilities contributed a gain of $4,525,739 in fiscal 2014 compared to a loss of $3,140,259 in fiscal 2013.
Our net income available to shareholders was decreased by $2,058,329 in fiscal 2014 relating to a preferred stock dividend as a result of the accounting treatment of our convertible preferred stock financings in January and March 2014 versus a preferred stock dividend of $1,722,474 in fiscal 2013, also related to financings. These amounts represent deemed dividends to the investors as a result of the accounting treatment for financings in fiscal 2014 (March 2014 and January 2014) and fiscal 2013 (June 2013 and August 2012), as further described in Note 9 to theNotes to Consolidated Financial Statements included elsewhere in this Annual Report.
Year Ended July 31, 2013 Compared to Year Ended July 31, 2012
Our net loss available to shareholders for the fiscal year ended July 31, 2013 (fiscal 2013) was $10,276,796 versus $9,867,024 in the fiscal year ended July 31, 2012 (fiscal 2012). The increase in net loss in fiscal 2013 versus fiscal 2012 is primarily due a larger loss on the revaluation of derivatives, a larger preferred stock dividend and a smaller gain on sale of assets, offset by a decrease in operating expenses by approximately $3.9 million in fiscal 2013. Our operating loss for fiscal 2012 decreased to $6,129,359 compared to $10,024,048 in fiscal 2012. The decrease resulted primarily from a decrease in research and development expenses to $2,192,724 from $4,987,236, a decrease in selling expense to zero from $165,175 and a decrease in general and administrative expenses to $3,936,635 from $4,889,179. Revenue decreased to zero from $28,651 from. The decrease in revenue is attributable to the discontinuation of sales of our consumer/over-the-counter products in early fiscal 2012.
The decrease in general and administrative expenses is primarily related to a decrease in legal and audit expenses of approximately $214,000 in fiscal 2013 versus 2012 due to cost cutting measures, as well as a decrease of approximately $401,000 in payroll related costs due to a reduction in the number of employees which also caused a reduction in travel and office expenses of approximately $84,000 and $151,000, respectively versus fiscal 2012. The decrease in selling expenses to zero from $165,175 for fiscal 2013 versus fiscal 2012 is associated with the elimination in advertising and promotion related to the discontinuation of our consumer/over-the-counter product line. Research and development expenses decreased by almost $2.8 million in fiscal 2013 from fiscal 2012, as we did not have any expenditures relating to the Phase III trials for our Generex Oral-lyn™ product in fiscal 2013.
| 29 |
Our interest expense in fiscal 2013 decreased to $530,222, compared to interest expense of $592,525 in fiscal 2012, due to lower mortgage balances in fiscal 2013 because of the discharge and partial discharge of mortgages upon sale of properties, as well as lower interest penalties related to the discharge of mortgages upon the sale of certain of our properties versus fiscal 2012. We received lower income from assets held for investment (net of expense) in fiscal 2013 of $1,245,165 (which included a $1,081,807 gain on sale of properties) versus $2,206,216 (which included a $1,957,089 gain on sale of properties) in fiscal 2012. In fiscal 2013, we had a larger loss on the revaluation of derivatives of $3,140,259 versus a loss of $1,081,440 in fiscal 2012.
Our net loss available to shareholders was increased by $1,722,474 in fiscal 2013 versus $376,746 in fiscal 2012 relating to preferred stock dividends as a result of the accounting treatment of our convertible preferred stock financings in June 2013, August 2012 and February 2012, respectively. This amount represents a deemed dividend to the investors as a result of these financings, as further described in Note 9 to theNotes to Consolidated Financial Statements included elsewhere in this Annual Report.
Financial Condition, Liquidity and Resources
Sources of Liquidity
To date we have financed our development stage activities primarily through private placements of our common stock and securities convertible into our common stock.
As of July 31, 2014, our current cash position is not sufficient to meet our working capital needs for the next twelve months. Therefore, we will require additional funds to support our working capital requirements and any development or other activities, or will need to curtail our clinical trials and other planned activities or suspend operations.
While we have financed our development stage activities to date primarily through private placements of our common stock and securities convertible into our common stock and raised approximately $5.1 million during fiscal 2014 (including proceeds from warrant exercises and the net proceeds from the sale of our properties held for investment), our cash balances have been low throughout fiscal 2014.
On March 30, 2011, our realigned management team announced its strategic development plan for Generex’s future growth. The plan included the spin-out of Antigen Express, a reverse stock split for Generex and a rights offering to Generex stockholders. As proposed, we would spin out Antigen Express as a separate DTC-eligible company, register its shares with the Securities and Exchange Commission (the “SEC”), and seek to list its shares on a national securities exchange. Management believes that the spin-out would increase value for stockholders and provide Antigen Express with ready access to capital markets to finance its on-going clinical and regulatory initiatives. Management further believes that the spin-out would benefit Generex, by allowing Generex to hold a controlling interest in a publicly-traded company while continuing to focus on maximizing opportunities for its buccal drug delivery platform. The spin-out would be accomplished by the issuance of one or more dividends of Antigen Express stock to Generex stockholders. No determination has been made as to the timing of the proposed spin-out.This annual report on Form 10-K does not constitute an offer of any securities for sale or a solicitation of an offer to buy any securities.
Although stockholders approved a reverse split proposal at our annual general meeting held on May 22, 2014, which approval allows the Board to implement a reverse split in its discretion and is not contingent upon listing our common stock on a national stock exchange. The terms of the securities purchase agreements that we entered into on June 17, 2013, January 14, 2014 and March 27, 2014 prohibit us from undertaking a reverse or forward stock split or reclassification of our common stock except for a reverse stock split made in conjunction with a listing of the common stock on a national securities exchange.
Management may seek to meet all or some of our operating cash flow requirements through financing activities, such as private placement of our common stock, preferred stock offerings and offerings of debt and convertible debt instruments as well as through merger or acquisition opportunities. The securities purchase agreements that we entered into on January 14, 2014 and March 27, 2014 with certain investors prohibits us from (i) issuing additional equity securities until 60 days after the effective date of a registration statement covering the resale of the common stock issuable upon exercise of the warrants and conversion of the preferred stock sold in that transaction and (ii) issuing additional debt or equity securities with a variable conversion or exercise price until January 14, 2015 and March 27, 2015, respectively.
Upon the filing of our Annual Report on Form 10-K on October 14, 2011, we were no longer eligible to use Form S-3 to register shares sold to investors, as the aggregate market value of our outstanding voting and non-voting common equity held by non-affiliates was less than $75 million. As we are required under the registration rights agreements that we entered into in January 2012, August 2012, December 2012, June 2013, January 2014 and March 2014 with certain investors to register shares of our common stock issuable upon conversion or exercise of the securities purchased by the investors, we filed the respective registration statements on Form S-1. We incurred additional legal and accounting fees in connection with the preparation of these Form S-1 registration statements.
| 30 |
In addition, management is actively pursuing financial and strategic alternatives, including strategic investments and divestitures, industry collaboration activities, and potential strategic partners. Management has sold non-essential real estate assets which were classified as Assets Held for Investment to augment its cash position and reduce its long-term debt.
We believe that the successful commercial launch of Oral-lyn™ in countries where we have approval would enhance our ability to access additional sources of funding. We will continue to require substantial funds to continue research and development, including preclinical studies and clinical trials of our product candidates, further clinical trials for Oral-lyn™ and to commence sales and marketing efforts if the FDA or other regulatory approvals are obtained.
Unforeseen problems with the conduct or results of Phase III clinical trials for Oral-lyn™ or further negative developments in general economic conditions could interfere with our ability to raise additional capital as needed, or materially adversely affect the terms upon which such capital is available. We cannot provide any assurance that we will obtain the required funding. Our inability to obtain required funding in the near future or our inability to obtain funding on favorable terms will have a material adverse effect on our operations and our strategic development plan for future growth. If we cannot successfully raise additional capital and implement our strategic development plan, our liquidity, financial condition and business prospects will be materially and adversely affected and we may have to cease operations.
Equity Financings
Following is a summary of the equity financing activities that we have completed in the last twelve months.
Financing – March 2014
Series F 9% Convertible Preferred Stock and Warrants
On March 27, 2014, we entered into a securities purchase agreement with certain investors, pursuant to which we agreed to sell an aggregate of 2,075 shares of our newly designated non-voting Series F 9% Convertible Preferred Stock and warrants to purchase up to an aggregate of 100% of the shares of our common stock issuable upon conversion of the convertible preferred stock. The purchase closed on March 28, 2014. We sold the convertible preferred stock and warrants in units, with each unit consisting of one share of convertible preferred stock and a warrant to purchase 100% of the shares of the Company’s common stock issuable upon conversion of such share of convertible preferred stock. Each unit was sold at a negotiated price of $1,000, for an aggregate purchase price of $2,075,000. An aggregate of 138,333,334 shares of our common stock are issuable upon conversion of, or exercise of, the convertible preferred stock and warrants. We received net proceeds of approximately $2,020,000 from this transaction.
Subject to certain ownership limitations, the Series F convertible preferred stock will be convertible at the option of the holder at any time into shares of our common stock at an effective conversion price of $0.03 per share, and will accrue a 9% dividend until March 27, 2017 and, beginning on March 27, 2017 and on each one year anniversary thereafter, such dividend rate will increase by an additional 3%. The dividend will be payable quarterly on September 30, December 31, March 31 and June 30, beginning on the first such date after the original issue date and on each conversion date in cash, or at our option, in shares of common stock. In the event that the convertible preferred stock is converted prior to March 27, 2017, we will pay the holder of the converted preferred stock an amount equal to $270 per $1,000 of stated value of the convertible preferred stock, less the amount of all prior quarterly dividends paid on such converted preferred stock before the relevant conversion date. Such “make-whole payment” may be made in cash or, at our option, in shares of our common stock. In addition, beginning March 27, 2016, we will pay dividends on shares of preferred stock equal to (on an as-if-converted-to-common-stock basis) and in the same form as dividends (other than dividends in the form of common stock) actually paid on shares of the common stock when, as and if such dividends are paid. We will incur a late fee of 18% per annum on unpaid dividends.
The conversion price of the Series F convertible preferred stock will be subject to adjustment in the case of stock splits, stock dividends, combinations of shares, similar recapitalization transactions and certain pro-rata distributions to common stockholders. The conversion price will also be adjusted if we sell or grant any shares of common stock or securities convertible into, or rights to acquire, common stock at an effective price per share that is lower than the then conversion price, except in the event of certain exempt issuances. In addition, the holders of convertible preferred stock will be entitled to receive any securities or rights to acquire securities or property granted or issued by us pro rata to the holders of our common stock to the same extent as if such holders had converted all of their shares of convertible preferred stock. In the event of a fundamental transaction, such as a merger, consolidation, sale of substantially all assets and similar reorganizations or recapitalizations, the holders of convertible preferred stock will be entitled to receive, upon conversion of their shares, any securities or other consideration received by the holders of our common stock pursuant to the fundamental transaction.
We may become obligated to redeem the Series F convertible preferred stock in cash upon the occurrence of certain triggering events, including, material breach of certain contractual obligations to the holders of the convertible preferred stock, the occurrence of a change in control of Generex, the occurrence of certain insolvency events relating to Generex, or the failure of our common stock to continue to be listed or quoted for trading on one or more specified United States securities exchanges or regulated quotation service. Upon the occurrence of certain triggering events, each holder of convertible preferred stock will have the option to redeem such holder’s shares of convertible preferred stock for a redemption price payable in shares of common stock or receive an increased dividend rate of 18% on all of such holder’s outstanding convertible preferred stock. Late fees will apply on all redemption amounts not paid within five trading days of the payment date.
| 31 |
Subject to certain ownership limitations, the warrants will be exercisable at any time after their date of issuance and on or before the fifth-year anniversary thereafter at an exercise price of $0.03 per share of common stock. The exercise price of the warrants and, in some cases, the number of shares issuable upon exercise, are subject to adjustment in the case of stock splits, stock dividends, combinations of shares, similar recapitalization transactions and certain pro-rata distributions to common stockholders. The exercise price and number of shares of common stock issuable upon exercise will also be adjusted if we sell or grant any shares of common stock or securities convertible into, or rights to acquire, common stock at an effective price per share that is lower than the then exercise price, except in the event of certain exempt issuances. In addition, the warrant holders will be entitled to receive any securities or rights to acquire securities or property granted or issued by us pro rata to the holders of our common stock to the same extent as if such holders had exercised all of their warrants. In the event of a fundamental transaction, such as a merger, consolidation, sale of substantially all assets and similar reorganizations or recapitalizations, the warrant holders will be entitled to receive, upon exercise of their warrants, any securities or other consideration received by the holders of common stock pursuant to the fundamental transaction.
The securities purchase agreement and the certificate of designation authorizing the Series F convertible preferred stock include certain agreements and covenants for the benefit of the holders of the convertible preferred stock, including restrictions on our ability to amend the certificate of incorporation and bylaws, pay cash dividends or distributions with respect to our common stock or other junior securities, repurchase more than ade minimisnumber of shares of our common stock or other junior securities.
| 32 |
With very limited exceptions, the investors will have a pro rata right of first refusal in respect of participation in any private debt or equity financings undertaken by us during the 12 months following the closing of the transaction.
We offered these securities privately pursuant to Rule 506(b) of Regulation D under the Securities Act of 1933. We entered into a registration rights agreement with the investors pursuant to which we agreed to file a registration statement with the SEC covering the public resale of the common stock issuable upon conversion of the preferred stock, issuable as dividends on the preferred stock, issuable upon exercise of the warrants and issued as a finders’ fee.
We agreed to file the registration statement within 25 days of the closing of the transaction and to use our best efforts to have the registration statement declared effective within 75 days after the filing date. The registration statement was declared effective by the SEC on April 16, 2014.
In addition, until the first anniversary date of the securities purchase agreement, each investor may, in its sole determination, elect to purchase, severally and not jointly with the other investors, in one or more purchases, in the ratio of such investor's original subscription amount to the original aggregate subscription amount of all investors, additional units consisting of convertible preferred stock and warrants at a purchase price of $1,000 per unit with an aggregate subscription amount thereof of up to $2,075,000, which units will be identical to the units of convertible preferred stock and warrants issued in connection with the March 2014 closing.
In addition, if, during the six-month period after the issuance of the warrants and continuing until such time that all of the securities may be sold without our meeting the current public information requirement under Securities Act rule 144(c)(1), we fail to meet such requirement, we will pay liquidate damages equal to 2.0% of the purchase price paid by each investor, payable in cash every 30 days until current public information for Generex is available or is no longer required for the investors to rely on Rule 144 to transfer the securities (including underlying securities) acquired under the securities purchase agreement.
Financing – January 2014
Series E 9% Convertible Preferred Stock and Warrants”Greenshoe”
On June 17, 2013, we entered into a securities purchase agreement with certain investors, pursuant to which we agreed to sell an aggregate of 1,225 shares of our newly designated non-voting Series E 9% Convertible Preferred Stock and warrants to purchase up to an aggregate of 100% of the shares of our common stock issuable upon conversion of the convertible preferred stock. Under the June 17, 2013 securities purchase agreement, for a period of up to one year, each investor may, in its sole determination, elect to purchase, in one or more purchases, additional units consisting of convertible preferred stock and warrants at a purchase price in the amount originally purchased by such investor (the “Greenshoe”). The units purchased in the Greenshoe will be identical to the units of convertible preferred stock and warrants originally issued pursuant to the Stock Purchase Agreement.
On January 14, 2014, in connection with the exercise of the Greenshoe by certain investors, we entered into a securities purchase agreement, pursuant to which we agreed to sell an aggregate of 800 shares of our Series E 9% Convertible Preferred Stock and warrants to purchase up to an aggregate of 100% of the shares of our common stock issuable upon conversion of the convertible preferred stock. The purchase closed on January 15, 2014. Each unit was sold at a negotiated price of $1,000, for an aggregate purchase price of $800,000. An aggregate of 53,333,336 shares of the Company’s common stock are initially issuable upon conversion of, or exercise of, the convertible preferred stock and warrants.
With very limited exceptions, the investors will have a pro rata right of first refusal in respect of participation in any private debt or equity financings undertaken by us during the 12 months following the closing of the transaction.
We offered these securities privately pursuant to Rule 506(b) of Regulation D under the Securities Act of 1933. This offering is subject to the registration rights agreement dated June 17, 2013 with the investors pursuant to which we agreed to file a registration statement with the SEC covering the public resale of the common stock issuable upon conversion of the preferred stock, issuable as dividends on the preferred stock, issuable upon exercise of the warrants and issued as a finders’ fee.
We agreed to file the registration statement within 25 days of the closing of the transaction and to use our best efforts to have the registration statement declared effective within 75 days after the filing date. The registration statement was declared effective by the SEC on February 7, 2014.
In addition, if, during the six-month period after the issuance of the warrants and continuing until such time that all of the securities may be sold without our meeting the current public information requirement under Securities Act rule 144(c)(1), we fail to meet such requirement, we will pay liquidate damages equal to 2.0% of the purchase price paid by each investor, payable in cash every 30 days until current public information for Generex is available or is no longer required for the investors to rely on Rule 144 to transfer the securities (including underlying securities) acquired under the securities purchase agreement.
Proceeds from Warrant Exercises
We may receive additional proceeds from the exercise of warrants issued in the registered direct offerings conducted in June, August and September 2009, the sales to Seaside 88, LP in April, May and June 2010 and the warrants issued in February 2012, August 2012, December 2012, June 2013, January 2014 and March 2014 in connection with the issuance of the Series C 9% Convertible Preferred Stock, Series D 9% Convertible Preferred Stock, Series E 9% Convertible Preferred Stock and Series F 9% Convertible Preferred Stock, although some of the warrants include a cashless exercise feature.
| • | In the transaction that closed on June 15, 2009, we sold shares of common stock and warrants exercisable for up to 8,600,000 shares of our common stock to investors and issued Midtown Partners & Co., LLC, our exclusive placement agent for the transaction, a warrant to purchase up to 244,926 shares of our common stock. |
| • | In the August 6, 2009 registered direct offering, we sold shares of common stock and warrants exercisable for up to 2,995,305 shares of our common stock to investors and issued a warrant to purchase 577,666 shares of our common stock to Midtown, which acted as our exclusive placement agent for the August 2009 transaction. |
| • | In the transaction that closed on September 14, 2009, we sold an aggregate of 15,312,500 shares of our common stock and warrants exercisable for up to 5,053,125 shares of our common stock to investors and issued warrants to purchase up to 969,526 shares of our common stock to the two placement agents and a consultant in relation to the transaction. |
| • | In the closings under the common stock purchase agreement that occurred in April, May and June 2010, we sold Seaside 12,000,000 shares of our common stock and issued to Midtown, as placement agent, warrants to purchase an aggregate of 300,000 shares of our common stock. |
| • | In connection with the securities purchase agreement dated August 8, 2012, we sold an aggregate of 750 shares of our Series C 9% Convertible Preferred Stock and issued warrants exercisable for up to 9,375,000 shares of our common stock to investors. |
| • | In connection with the securities purchase agreement dated December 10, 2012, we sold an aggregate of 750 shares of our Series D 9% Convertible Preferred Stock and issued warrants exercisable for up to 24,999,999 shares of our common stock to investors. |
| • | In connection with the securities purchase agreement dated June 17, 2013, we sold an aggregate of 1,225 shares of our Series E 9% Convertible Preferred Stock and issued warrants exercisable for up to 40,833,335 shares of our common stock to investors. |
| • | In connection with the securities purchase agreement dated January 14, 2014, we sold an aggregate of 800 shares of our Series E 9% Convertible Preferred Stock and issued warrants exercisable for up to 26,666,668 shares of our common stock to investors. |
| • | In connection with the securities purchase agreement dated March 27, 2014, we sold an aggregate of 2,075 shares of our Series F 9% Convertible Preferred Stock and issued warrants exercisable for up to 69,166,667 shares of our common stock to investors. |
| 33 |
As of July 31, 2014, all of the warrants issued in the aforementioned registered direct offerings were exercisable. At July 31, 2014, outstanding warrants issued in connection with the June, August and September 2009 registered direct offerings, the April, May and June 2010 sales to Seaside and the January to April 2011, February 2012, August 2012, December 2012, June 2013, January 2014 and March 2014 private placements were as follows (after adjustment for anti-dilution provisions and subsequent exercises):
| Date Issued | Aggregate No. of Shares Unexercised | Exercise Price | Expiration Date | ||||||
| June 15, 2009 | 8,470,661 | 0.76 | December 15, 2014 | ||||||
| August 6, 2009 | 3,413,928 | 0.79 | February 4, 2015 | ||||||
| September 14, 2009 | 5,157,813 | 1.00 | March 15, 2015 | ||||||
| April 8 to June 11, 2010 | 300,000 | 0.395 | February 9, 2015 | ||||||
| February 1, 2012* | 5,675,227 | 0.03 | February 1, 2017 | ||||||
| August 10, 2012* | 4,999,999 | 0.03 | August 10, 2017 | ||||||
| December 10, 2012* | 8,324,144 | 0.03 | December 12, 2017 | ||||||
| June 17, 2013* | 34,166,669 | 0.03 | June 17, 2018 | ||||||
| January 15, 2014* | 25,666,668 | 0.03 | January 15, 2019 | ||||||
| March 27, 2014* | 69,166,667 | 0.03 | March 27, 2019 | ||||||
*Upon issuance of securities at a price per share of common stock less than the then applicable exercise price, the warrants are subject to anti-dilution adjustment of the exercise price and to the number of shares of common stock that may be purchased upon exercise of each warrant such that the aggregate exercise price payable upon exercise of the warrant will be the same as the aggregate exercise price in effect immediately prior to such adjustment. Due to the anti-dilution adjustment provision of these warrants, they have been reclassified on Generex’s balance sheet as a liability under the caption “Derivative Warrant Liability” with any changes in fair value at each reporting period recorded in earnings in accordance with ASC 815.
In addition, we may receive additional proceeds from the exercise of warrants issued in connection with the securities purchase agreement and related documents that we entered into on March 31, 2008 with existing institutional investors relating to a private placement of 8% secured convertible notes (the “Notes”) and warrants (the “Series Warrants”) for aggregate gross proceeds to us of $20,650,000. As of June 1, 2009, the outstanding principal balance and accrued interest on the Notes were satisfied in full.
The Series Warrants issued in connection with the March 2008 securities purchase agreement included:
| (i) | Series A and A-1 Warrants, which are exercisable for a period of 7 years into an aggregate of 75% of the number of shares of our common stock initially issuable upon conversion of the Notes, with the Series A Warrants being exercisable into 5,257,729 shares immediately upon issuance and the Series A-1 warrants being exercisable into 7,541,857 shares as of October 1, 2008; |
| (ii) | Series B Warrants, which became exercisable on October 1, 2008 into 100% of the shares of our common stock initially issuable upon conversion of the Notes (initially 17,066,166 shares) and remain exercisable for a period of 18 months after the registration statement covering the shares of common stock issuable upon conversion or exercise of the Notes and Warrants was declared effective by the SEC; and |
| (iii) | Series C Warrants, which are exercisable for a period of 7 years as of October 1, 2008, but only to the extent that the Series B Warrant are exercised and only in the same percentage that the Series B Warrants are exercised, up to a maximum percentage of 75% of the number of shares of our common stock initially issuable upon conversion of the Notes (initially a maximum of 12,799,580 shares). |
The initial exercise price of each Series Warrant was $1.21. The Series Warrants include a cashless exercise feature. The exercise price of the Series Warrants was subsequently reduced initially to $0.50, then to $0.33, to $0.25, to $0.15, to $0.08 and currently to $0.03 as a result of a price protection provision triggered by our offering of stock in private placements in May 2009, January and July 2011 and February, August 2012 and December 2012. This price protection feature allows for the reduction in the exercise price of the Series Warrants in the event we subsequently issue common stock or securities convertible into or exercisable for common stock, such as options and warrants, at a price per share less than the Series Warrant exercise price then in effect. In addition, with any reduction to the Series Warrant exercise price, the number of shares of common stock that may be purchased upon exercise of each Series Warrant will be increased or decreased proportionately, so that after such adjustment the aggregate Series Warrant exercise price payable for the adjusted number of shares issuable upon exercise will be the same as the aggregate Series Warrant exercise price in effect immediately prior to such adjustment. We account for these warrants with price protection in accordance with ASC 815 as described in Note 10 to theNotes to Consolidated Financial Statements included elsewhere in this annual report.
| 34 |
As of July 31, 2014, outstanding Series Warrants were as follows (after adjustment for anti-dilution provisions and subsequent exercises):
| Date Issued | Aggregate No. of Shares Unexercised | Exercise Price* | Expiration Date | |||||||
| March 31, 2008 | 64,516,758 | $ | 0.03 | March 31, 2016 | ||||||
| March 31, 2008 | 27,272,720 | $ | 0.03 | September 30, 2016 | ||||||
*Upon issuance of securities at a price per share of common stock less than the then applicable exercise price, the warrants are subject to anti-dilution adjustment of the exercise price and to the number of shares of common stock that may be purchased upon exercise of each warrant such that the aggregate exercise price payable upon exercise of the warrant will be the same as the aggregate exercise price in effect immediately prior to such adjustment. Due to the anti-dilution adjustment provision of these warrants, they have been reclassified on Generex’s balance sheet as a liability under the caption “Derivative Warrant Liability” with any changes in fair value at each reporting period recorded in earnings in accordance with ASC 815.
Cash Flows for the Year Ended July 31, 2014
For the fiscal year ended July 31, 2014, we used $3,565,525 in cash to fund our operating activities. The use for operating activities included a net loss of $1,417, changes to working capital including a decrease of $490,352 related to accounts payable and accrued expenses, a decrease of $1,181 related to deferred revenue and a decrease related to other of $104,563.
The use of cash was offset by non-cash expenses of $368,743 related to depreciation and amortization, stock-based compensation to executives, employees, directors and consultants of $679,650, and common stock issued for interest on our convertible preferred stock of $689,850. There was also a year-to-date non-cash gain of $4,525,739 related to the fair valuation of the derivative liabilities at July 31, 2014 and an accounting gain of $188,869 related to the sale of our remaining properties held for investment.
We had net cash provided by investing activities of $794,299 in the fiscal year ended July 31, 2014, representing primarily the net proceeds after real estate commissions of $883,780 related to the sale of the properties held for investment, offset by costs incurred for patents of $89,481.
We had cash provided by financing activities in the fiscal year ended July 31, 2014 of $4,350,664, which pertained primarily to proceeds from cash exercises of warrants of $2,301,944 and net proceeds from the issuance of preferred stock of $2,655,000, offset by the discharge of long-term debt upon the sale of properties of $606,806.
Our net working capital at July 31, 2014 improved to negative $4,786,981 from negative $6,696,473 at July 31, 2013, which was attributed largely to proceeds from cash exercises of warrants and net proceeds from the Series E and Series F convertible preferred stock financings in January and March 2014, offset by our cash used in operations for fiscal 2014.
Conversion of Outstanding Series A, Series B, Series C, Series D, Series E and Series F 9% Convertible Preferred Stock
All outstanding shares of our Series A 9% Convertible Preferred Stock were converted into shares of our common stock prior to the end of our fiscal year ended July 31, 2012. A total of 17,166,666 shares of common stock have been issued upon the conversion of 2,575 shares of Series A convertible preferred stock. Upon conversion, we paid the holders of the Series A convertible preferred stock a “make whole” payment equal to $270 per $1,000 of stated value of the Series A convertible preferred stock, less the amount of all prior quarterly dividends paid on such converted preferred stock before the relevant conversion date. We issued 6,129,666 additional shares of common stock on such conversions of the Series A convertible preferred stock. Dividends paid on the Series A Convertible Preferred Stock were $12,383 during the fiscal year ended July 31, 2012.
As of July 31, 2014, all of the 2,000 shares of our Series B 9% Convertible Preferred Stock had been converted into shares of our common stock. We issued 38,520,832 shares of common stock upon the conversion of the Series B convertible preferred stock and an additional 14,819,679 shares of common stock were issued as “make-whole payments” on such conversions.
As of July 31, 2014, all of the 750 shares of our Series C 9% Convertible Preferred Stock had been converted into shares of our common stock. We issued 22,916,665 shares of common stock upon the conversion of the Series C convertible preferred stock and an additional 6,664,863 shares of common stock were issued as “make-whole payments” on such conversions.
| 35 |
As of July 31, 2014, all of the 750 shares of our Series D 9% Convertible Preferred Stock had been converted into shares of our common stock. We issued 24,999,999 shares of common stock upon the conversion of the Series D convertible preferred stock and an additional 7,825,191 shares of common stock were issued as “make-whole payments” on such conversions.
As of July 31, 2014, 2,000 shares of our Series E 9% Convertible Preferred Stock had been converted into shares of our common stock. We issued 66,666,666 shares of common stock upon the conversion of the Series E convertible preferred stock and an additional 18,585,193 shares of common stock were issued as “make-whole payments” on such conversions.
As of July 31, 2014, 850 shares of our Series F 9% Convertible Preferred Stock had been converted into shares of our common stock. We issued 28,333,331 shares of common stock upon the conversion of the Series F convertible preferred stock and an additional 7,861,623 shares of common stock were issued as “make-whole payments” on such conversions.
Funding Requirements and Commitments
If we obtain necessary financing, we expect to devote substantial resources to obtaining regulatory approval of Generex Oral-lyn™ in the U.S., Canada and Europe and to commercializing Generex Oral-lyn™. We may also devote resources to obtaining approval for the importation, marketing and commercialization of Generex Oral-lyn™ in other countries where we have licensed distributors.
Under the long-term agreement that we signed with sanofi-aventis in December 2009, sanofi-aventis will manufacture and supply recombinant human insulin to us in the territories specified in the agreement. Through this agreement, we will procure recombinant human insulin crystals for use in the production of Generex Oral-lyn™. The terms of the supply agreement required us to make certain minimum purchases of insulin from sanofi-aventis through the period ended December 31, 2011, which minimum purchases we did not satisfy.Sanofi-aventis will be our exclusive supplier in certain countries and a non-exclusive supplier in some other countries. Sanofi-aventis may delete any territory from the agreement in which Generex Oral-lyn™ has not been approved for commercial sale by December 31, 2011. The prices under the supply agreement are subject to adjustment beginning after December 31, 2012. As we did not meet the minimum purchase requirements by December 31, 2011, sanofi-aventis may terminate the agreement. Upon termination, we would be obligated to pay sanofi-aventis for all materials and components that it has acquired or ordered to manufacture insulin based on our forecasts or minimum purchase commitments, all related work-in-progress (at cost) and all finished insulin in inventory. We did not provide any forecasts to sanofi-aventis and have not included any accruals related to the purchase commitments in our financial statements as of July 31, 2014 or July 31, 2013, nor has sanofi-aventis terminated the agreement.
In addition to the resources that we will dedicate to regulatory approval and commercialization of Generex Oral-lyn™, we will expend resources on further clinical development of our immunotherapeutic vaccines.
Our future funding requirements and commitments and our ability to raise additional capital will depend on factors that include:
| • | the timing and amount of expense incurred to complete our clinical trials; | |
| • | the costs and timing of the regulatory process as we seek approval of our products in development; | |
| • | the advancement of our products in development; | |
| • | our ability to generate new relationships with industry partners throughout the world that will provide us with regulatory assistance and long-term commercialization opportunities; | |
| • | the timing, receipt and amount of sales, if any, from Generex Oral-lyn™ in India, Lebanon, Algeria and Ecuador; | |
| • | the cost of manufacturing (paid to third parties) of our licensed products, and the cost of marketing and sales activities of those products; | |
| • | the costs of prosecuting, maintaining, and enforcing patent claims, if any claims are made; | |
| • | our ability to maintain existing collaborative relationships and establish new relationships as we advance our products in development; | |
| • | our ability to obtain the necessary financing to fund our operations and effect our strategic development plan; and | |
| • | the receptivity of the financial market to biopharmaceutical companies. |
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenue or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to investors, and we do not have any non-consolidated special purpose entities.
| 36 |
Contractual Obligations
The following table of contractual obligations as of July 31, 2014 includes interest obligations.
| Contractual Obligations | Total | <1 Year | 1-3 years | 3- 5 years | |||||||||||
| Operating Lease Obligations | 217,628 | 41,750 | 80,818 | 95,060 | |||||||||||
| Purchase Obligations * | - | - | - | - | |||||||||||
| Other Long-Term Liabilities Reflected on the Registrant's Balance Sheet under GAAP | - | - | - | - | |||||||||||
| Total | $ | 217,628 | $ | 41,750 | $ | 80,818 | $ | 95,060 | |||||||
* Although there are no minimum purchase requirements for any of the remaining contract years under Generex’s supply agreement with sanofi-aventis entered into on December 7, 2009, Generex has not fully satisfied the minimum purchase requirements for the contract years ended December 31, 2010 and 2011. To the extent that Generex has any continuing long-term obligations to purchase insulin under this agreement, such obligations are not included in the table above because the quantities and prices relating to Generex’s obligations are subject to confidential treatment.
Recently Adopted Accounting Pronouncements
In June 2014, the FASB issued guidance regarding the elimination of the reporting requirement for development stage entities and removed the definition of development stage entity from the Accounting Standards Codification. We have adopted this guidance effective for our annual fiscal year ended July 31, 2014. The adoption of this new accounting guidance resulted in the elimination of the inception-to-date financial information in the consolidated statements of operations, statements of changes in stockholders’ deficiency and statements of cash flows, as well as the removal of the subheading “A Development Stage Company” from the consolidated financial statements and the notes to the consolidated financial statements.
Recently Issued Accounting Pronouncements
In August 2014, the FASB issued guidance regarding disclosure of uncertainties about an entity’s ability to continue as a going concern. The guidance will be effective for our annual fiscal year ended July 31, 2017 and subsequent interim periods. We are currently evaluating the impact of this accounting standard update on our consolidated financial statements.
Item. 7A. Quantitative and Qualitative Disclosures About Market Risk.
We are exposed to market risks associated with changes in the exchange rates between U.S. and Canadian currencies and with changes in the interest rates related to our fixed rate debt. We do not believe that any of these risks will have a material impact on our financial condition, results of operations and cash flows.
At the present time, we maintain our cash in short-term government or government guaranteed instruments, short-term commercial paper, and interest bearing bank deposits or demand bank deposits which do not earn interest. A substantial majority of these instruments and deposits are denominated in U.S. dollars, with the exception of funds denominated in Canadian dollars on deposit in Canadian banks to meet short-term operating needs in Canada. We do not presently employ any hedging or similar strategy intended to mitigate against losses that could be incurred as a result of fluctuations in the exchange rates between U.S. and Canadian currencies.
As of July 31, 2014, we did not have any fixed rate debt. We have neither issued nor own any long-term debt instruments, or any other financial instruments, for trading purposes to which we would be subject to material market risks.
We have warrants outstanding with price protection provisions that allow for the reduction in the exercise price of the warrants in the event we subsequently issue common stock or securities convertible into or exercisable for common stock, such as options and warrants, at a price per share less than the warrant exercise price then in effect. In addition, with any reduction to the warrant exercise price, the number of shares of common stock that may be purchased upon exercise of each warrant will be increased proportionately, so that after such adjustment the aggregate warrant exercise price payable for the adjusted number of shares issuable upon exercise will be the same as the aggregate warrant exercise price in effect immediately prior to such adjustment. We account for the warrants with price protection in accordance with FASB ASC 815. We recognize the warrants with price protection in our consolidated balance sheet as liabilities. The warrant liability is revalued at each reporting period and changes in fair value are recognized currently in the consolidated statements of operations under the captionChange in fair value of derivative warrant liability. While the change in fair value of the derivative warrant liability has no effect on our cash flows, the gains or losses can have a significant impact on non-operating income and expenses and thus the net income or loss. As of July 31, 2014, there were 239,788,852 warrants outstanding subject to price protection provisions with an estimated fair value of $2,635,643 or $0.011 per warrant. If the estimated fair value of the warrants increases, there will be a corresponding non-operating expense equal to the change in the value of the liability. Likewise, if the estimated fair value of the warrants decreases, there will be a corresponding non-operating gain equal to the change in the value of the liability. There is a directly proportional relationship between the fair value of the warrants and the market price of the stock; therefore increases or decreases in the market price will lead to corresponding increases or decreases in the value of the warrant liability and result in losses or gains, respectively, on our consolidated statements of operations.
| 37 |
Item 8. Financial Statements and Supplementary Data.
GENEREX BIOTECHNOLOGY CORPORATION AND SUBSIDIARIES
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Page | ||
| Report of Independent Registered Public Accounting Firm – October 3, 2014 | 39 | |
| Report of Independent Registered Public Accounting Firm - October 15, 2012 | 40 | |
| Consolidated Balance Sheets at July 31, 2014 and 2013 | 41 | |
| Consolidated Statements of Operations for the Years Ended July 31, 2014, 2013 and 2012 | 42 | |
Consolidated Statements of Changes in Stockholders’ Deficiency for the Years Ended July 31, 2014, 2013 and 2012 | 43 | |
| Consolidated Statements of Cash Flows for the Years Ended July 31, 2014, 2013 and 2012 | 44 | |
| Notes to Consolidated Financial Statements | 45 |
| 38 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board or Directors and Shareholders of Generex Biotechnology Corporation
We have audited the accompanying consolidated balance sheets of Generex Biotechnology Corp. (a Development Stage Company) and subsidiaries as of July 31, 2014 and 2013, and the related consolidated statements of operations, stockholders’ (deficiency)/equity and cash flows for the years ended July 31, 2014 and 2013. Our audit also includes the financial statement schedule listed in the index under Item 15. Generex Biotechnology Corp.’s management is responsible for these consolidated financial statements and the financial statement schedule listed in the index under Item 15. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement. Generex Biotechnology Corp. is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of Generex Biotechnology Corp.’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Generex Biotechnology Corp. as of July 31, 2014 and 2013, and the results of its operations and its cash flows for the years ended July 31, 2014 and 2013 in conformity with accounting principles generally accepted in the United States of America. Also, in our opinion, such financial statement schedule, when considered in relation to the basic consolidated financial statements taken as a whole, presents fairly in all material respects, the information set forth therein.
The accompanying consolidated financial statements have been prepared assuming the Company will continue as a going concern. As discussed in Note 1, the Company’s experience of negative cash flows from operations since inception and its dependency upon future financing, which is uncertain due to the limitations imposed by previous financings on future financings, raise substantial doubt about its ability to continue as a going concern. Management’s plans regarding these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
| MNP LLP | ||
| Chartered Professional Accountants | ||
| Licensed Public Accountants | ||
| Toronto, Canada | ||
| October 3, 2014 |
| 39 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Generex Biotechnology Corporation and Subsidiaries
(A Development Stage Company)
We have audited the accompanying consolidated balance sheets of Generex Biotechnology Corporation (a Development Stage Company) and subsidiaries (the “Company”) as of July 31, 2012 and 2011 and the related consolidated statements of operations, stockholders’ (deficiency)/equity and cash flows for each of the years in the three year period ended July 31, 2012, and for the period November 2, 1995 (date of inception) to July 31, 2012. Our audits also included the financial statement schedule listed in the index under Item 15. These financial statements and financial statement schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements and financial statement schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Generex Biotechnology Corporation and subsidiaries as of July 31, 2012 and 2011 and the results of their operations and their cash flows for each of the years in the three year period ended July 31, 2012, and for the period November 2, 1995 (date of inception) to July 31, 2012 in conformity with accounting principles generally accepted in the United States of America. Also, in our opinion, such financial statement schedule, when considered in relation to the basic consolidated financial statements taken as a whole, presents fairly in all material respects, the information set forth therein.
The accompanying consolidated financial statements have been prepared assuming the Company will continue as a going concern. As discussed in Note 1, the Company’s experience of negative cash flows from operations since inception and its dependency upon future financing, which is uncertain due to the limitations imposed by previous financings on future financings, raise substantial doubt about its ability to continue as a going concern. Management’s plans regarding these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company’s internal control over financial reporting as of July 31, 2012, based on criteria established in Internal Control – Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) and our report dated October 15, 2012 expressed an adverse opinion thereon.
MSCM LLP
Toronto, Canada
October 15, 2012
| 40 |
| GENEREX BIOTECHNOLOGY CORPORATION AND SUBSIDIARIES | |||||||||||||
| CONSOLIDATED BALANCE SHEETS | |||||||||||||
| July 31, 2014 | July 31, 2013 | ||||||||||||
| ASSETS | |||||||||||||
| Current Assets: | |||||||||||||
| Cash and cash equivalents | $ 3,269,489 | $ 1,708,954 | |||||||||||
| Other current assets | 201,314 | 98,315 | |||||||||||
| Total Current Assets | 3,470,803 | 1,807,269 | |||||||||||
| Property and Equipment, Net (Note 3) | 5,293 | 85,406 | |||||||||||
| Assets Held for Investment, Net (Note 3) | -- | 640,772 | |||||||||||
| Patents, Net (Note 4) | 2,046,361 | 2,319,416 | |||||||||||
| TOTAL ASSETS | $ 5,522,457 | $ 4,852,863 | |||||||||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIENCY | |||||||||||||
| Current Liabilities: | |||||||||||||
| Accounts payable and accrued expenses (Note 6) | $ 8,034,122 | $ 7,661,234 | |||||||||||
| Deferred revenue | 223,662 | 224,843 | |||||||||||
| Current maturities of long-term debt (Note 8) | -- | 617,665 | |||||||||||
| Total Current Liabilities | 8,257,784 | 8,503,742 | |||||||||||
| Derivative Warrant Liability (Note 10) | 2,635,643 | 5,234,293 | |||||||||||
| Derivative Additional Investment Rights Liability (Note 10) | 719,088 | 1,256,160 | |||||||||||
| Total Liabilities | 11,612,515 | 14,994,195 | |||||||||||
| Commitments and Contingencies (Note 7) | |||||||||||||
| Stockholders’ Deficiency (Notes 9 and 11): | |||||||||||||
| Series A 9% Convertible Preferred Stock, $1,000 par value; authorized 5,500 | |||||||||||||
| shares, -0- issued shares at July 31, 2014 and July 31, 2013, respectively | -- | -- | |||||||||||
| Series B 9% Convertible Preferred Stock, $1,000 par value; authorized 2,000 | |||||||||||||
| shares, -0- issued shares at July 31, 2014 and July 31, 2013, respectively | -- | -- | |||||||||||
| Series C 9% Convertible Preferred Stock, $1,000 par value; authorized 750 | |||||||||||||
| shares, -0- issued shares at July 31, 2014 and July 31, 2013, respectively | -- | -- | |||||||||||
| Series D 9% Convertible Preferred Stock, $1,000 par value; authorized 750 | |||||||||||||
| shares, -0- issued shares at July 31, 2014 and July 31, 2013, respectively | -- | �� -- | |||||||||||
| Series E 9% Convertible Preferred Stock, $1,000 par value; authorized 2,450 | |||||||||||||
| shares, 25 and 930 issued shares at July 31, 2014 and July 31, 2013, respectively | -- | -- | |||||||||||
| Series F 9% Convertible Preferred Stock, $1,000 par value; authorized 4,150 and -0- | |||||||||||||
| shares at July 31, 2014 and July 31, 2013, respectively; 1,225 and -0- shares | |||||||||||||
| issued and outstanding at July 31, 2014 and July 31, 2013, respectively | -- | -- | |||||||||||
| Common stock, $.001 par value; authorized 1,500,000,000 shares at | |||||||||||||
| July 31, 2014 and July 31, 2013, respectively; 778,512,092 and 580,329,160 | |||||||||||||
| issued and outstanding at July 31, 2014 and July 31, 2013, respectively | 778,512 | 580,329 | |||||||||||
| Additional paid-in capital | 362,307,678 | 356,401,812 | |||||||||||
| Accumulated deficit | (369,948,322) | (367,888,576) | |||||||||||
| Accumulated other comprehensive income | 772,074 | 765,103 | |||||||||||
| Total Stockholders’ Deficiency | (6,090,058) | (10,141,332) | |||||||||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIENCY | $ 5,522,457 | $ 4,852,863 | |||||||||||
| 41 |
| GENEREX BIOTECHNOLOGY CORPORATION AND SUBSIDIARIES | |||||||||||||
| CONSOLIDATED STATEMENTS OF OPERATIONS | |||||||||||||
| For the Years Ended July 31, | |||||||||||||
| 2014 | 2013 | 2012 | |||||||||||
| Revenues, net | $ -- | $ -- | $ 28,651 | ||||||||||
| Cost of Goods Sold | -- | -- | 11,109 | ||||||||||
| Gross profit | -- | -- | 17,542 | ||||||||||
| Operating Expenses: | |||||||||||||
| Research and development | 1,382,995 | 2,192,724 | 4,987,236 | ||||||||||
| Selling and marketing | -- | -- | 165,175 | ||||||||||
| General and administrative | 3,016,684 | 3,936,635 | 4,889,179 | ||||||||||
| Total Operating Expenses | 4,399,679 | 6,129,359 | 10,041,590 | ||||||||||
| Operating Loss | (4,399,679) | (6,129,359) | (10,024,048) | ||||||||||
| Other Income (Expense): | |||||||||||||
| Income from assets held for investment, net (Note 3) | 193,607 | 1,245,165 | 2,206,216 | ||||||||||
| Interest income | 586 | 353 | 1,519 | ||||||||||
| Interest expense | (321,670) | (530,222) | (592,525) | ||||||||||
| Change in fair value of derivative liabilities (Note 10) | 4,525,739 | (3,140,259) | (1,081,440) | ||||||||||
| Net Loss | (1,417) | (8,554,322) | (9,490,278) | ||||||||||
| Preferred Stock Dividend | 2,058,329 | 1,722,474 | 376,746 | ||||||||||
| Net Loss Available to Common Stockholders | $ (2,059,746) | $ (10,276,796) | $ (9,867,024) | ||||||||||
| Basic and Diluted Net Loss Per Common Share (Note 13) | $ (.003) | $ (.023) | $ (.030) | ||||||||||
| Weighted Average Number of Shares of Common Stock | |||||||||||||
| Outstanding - basic and diluted (Note 13) | 679,630,247 | 443,579,247 | 332,333,583 | ||||||||||
| 42 |
| GENEREX BIOTECHNOLOGY CORPORATION AND SUBSIDIARIES | |||||||||||||
| CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS' DEFICIENCY | |||||||||||||
| Accumulated | |||||||||||||
| Preferred | Common | Additional | Other | Total | |||||||||
| Stock | Stock | Paid-In | Accumulated | Comprehensive | Stockholders’ | ||||||||
| Shares | Shares | Amount | Capital | Deficit | Income (Loss) | Deficiency | |||||||
| Balance at July 31, 2011 | 1,287 | 308,519,768 | $308,520 | $ 338,124,525 | $ (347,744,756) | $ 869,575 | $ (8,442,136) | ||||||
| Issuance of common stock as employee compensation | - | 981,353 | 981 | 129,562 | - | - | 130,543 | ||||||
| Issuance of common stock in exchange for services | - | 5,401,722 | 5,402 | 694,043 | - | - | 699,445 | ||||||
| Issuance of preferred stock in financing | 2,000 | - | - | - | - | - | - | ||||||
| Issuance of common stock upon conversion of preferred stock | (1,797) | 11,980,003 | 11,980 | (11,980) | - | - | - | ||||||
| Issuance of common stock for preferred stock make whole payments | - | 5,319,026 | 5,319 | 479,871 | - | - | 485,190 | ||||||
| Exercise of additional investment rights | - | - | - | 841,333 | - | - | 841,333 | ||||||
| Exercise of stock options for cash | - | 1,299,994 | 1,300 | - | - | - | 1,300 | ||||||
| Exercise of warrants for cash | - | 200,000 | 200 | 29,800 | - | - | 30,000 | ||||||
| Cashless exercise of warrants | - | 20,459,431 | 20,459 | 7,210,274 | - | - | 7,230,734 | ||||||
| Stock-based compensation | - | - | - | 602,384 | - | - | 602,384 | ||||||
| Net loss | - | - | - | - | (9,490,278) | - | (9,490,278) | ||||||
| Preferred stock dividend (see Note 9) | - | - | - | - | (376,746) | - | (376,746) | ||||||
| Currency translation adjustment | - | - | - | - | - | (91,960) | (91,960) | ||||||
| Balance at July 31, 2012 | 1,490 | 354,161,297 | $ 354,161 | $ 348,099,813 | $ (357,611,780) | $ 777,615 | $ (8,380,190) | ||||||
| Issuance of common stock in exchange for services | - | 8,251,150 | 8,251 | 384,171 | - | - | 392,422 | ||||||
| Issuance of preferred stock in financing | 2,725 | - | - | - | - | - | - | ||||||
| Issuance of common stock upon conversion of preferred stock | (3,285) | 92,870,829 | 92,871 | 657,129 | - | - | 750,000 | ||||||
| Issuance of common stock for preferred stock make whole payments | - | 30,452,292 | 30,452 | 856,498 | - | - | 886,950 | ||||||
| Cancellation of common stock | - | (1,278,246) | (1,278) | 1,278 | - | - | - | ||||||
| Exercise of stock options for cash | - | 5,180,378 | 5,180 | - | - | - | 5,180 | ||||||
| Exercise of warrants for cash | - | 61,506,785 | 61,507 | 3,231,738 | - | - | 3,293,245 | ||||||
| Cashless exercise of warrants | - | 29,184,675 | 29,185 | 2,333,431 | - | - | 2,362,616 | ||||||
| Stock-based compensation | - | - | - | 837,753 | - | - | 837,753 | ||||||
| Net loss | - | - | - | - | (8,554,322) | - | (8,554,322) | ||||||
| Preferred stock dividend (see Note 9) | - | - | - | - | (1,722,474) | - | (1,722,474) | ||||||
| Currency translation adjustment | - | - | - | - | - | (12,512) | (12,512) | ||||||
| Balance at July 31, 2013 | 930 | 580,329,160 | $580,329 | $ 356,401,812 | $ (367,888,576) | $ 765,103 | $ (10,141,332) | ||||||
| Issuance of common stock as employee compensation | - | 4,333,333 | 4,333 | 125,667 | - | - | 130,000 | ||||||
| Issuance of common stock in exchange for services | - | 7,633,333 | 7,633 | 279,147 | - | - | 286,780 | ||||||
| Issuance of preferred stock in financing | 2,875 | - | - | - | - | - | - | ||||||
| Issuance of common stock upon conversion of preferred stock | (2,555) | 85,166,663 | 85,167 | (85,167) | - | - | - | ||||||
| Issuance of common stock for preferred stock make whole payments | - | 23,791,817 | 23,792 | 666,058 | - | - | 689,850 | ||||||
| Exercise of additional investment rights | - | - | - | 237,566 | - | - | 237,566 | ||||||
| Exercise of stock options for cash | - | 526,306 | 526 | - | - | - | 526 | ||||||
| Exercise of warrants for cash | - | 76,731,480 | 76,731 | 4,419,725 | - | - | 4,496,456 | ||||||
| Stock-based compensation | - | - | - | 262,871 | - | - | 262,871 | ||||||
| Net loss | - | - | - | - | (1,417) | - | (1,417) | ||||||
| Preferred stock dividend (see Note 9) | - | - | - | - | (2,058,329) | - | (2,058,329) | ||||||
| Currency translation adjustment | - | - | - | - | - | 6,971 | 6,971 | ||||||
| Balance at July 31, 2014 | 1,250 | 778,512,092 | $ 778,512 | $ 362,307,678 | $ (369,948,322) | $ 772,074 | $ (6,090,058) | ||||||
| 43 |
| GENEREX BIOTECHNOLOGY CORPORATION AND SUBSIDIARIES | ||||||||||||||||
| CONSOLIDATED STATEMENTS OF CASH FLOWS | ||||||||||||||||
| For the Twelve Months | ||||||||||||||||
| Ended July 31, | ||||||||||||||||
| 2014 | 2013 | 2012 | ||||||||||||||
| Cash Flows From Operating Activities: | ||||||||||||||||
| Net loss | $ (1,417) | $ (8,554,322) | $ (9,490,278) | |||||||||||||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||||||
| Depreciation and amortization | 368,447 | 463,718 | 612,658 | |||||||||||||
| Stock compensation expense | 392,871 | 837,754 | 732,928 | |||||||||||||
| Common stock issued for services rendered | 286,780 | 392,421 | 699,445 | |||||||||||||
| Write-off of abandoned patents | -- | -- | 440,780 | |||||||||||||
| Gain on disposal of property and equipment | (188,869) | (1,037,485) | (2,027,939) | |||||||||||||
| Common stock issued for interest on convertible debentures & preferred stock | 689,850 | 886,950 | 485,190 | |||||||||||||
| Change in fair value of derivative liabilities | (4,525,739) | 3,140,259 | 1,081,440 | |||||||||||||
| Changes in operating assets and liabilities: | ||||||||||||||||
| Accounts payable and accrued expenses | (490,352) | 123,556 | (1,218,616) | |||||||||||||
| Deferred revenue | (1,181) | (38,282) | (105,395) | |||||||||||||
| Other current assets | (104,015) | 124,197 | 745,808 | |||||||||||||
| Net Cash Used in Operating Activities | (3,573,625) | (3,661,234) | (8,043,979) | |||||||||||||
| Cash Flows From Investing Activities: | ||||||||||||||||
| Purchase of property and equipment | -- | -- | (2,416) | |||||||||||||
| Proceeds from sale of property and equipment | 883,780 | 1,764,008 | 4,953,325 | |||||||||||||
| Costs incurred for patents | (89,481) | (78,948) | (173,775) | |||||||||||||
| Net Cash Provided By Investing Activities | 794,299 | 1,685,060 | 4,777,134 | |||||||||||||
| Cash Flows From Financing Activities: | ||||||||||||||||
| Proceeds from issuance of long-term debt | -- | 829,038 | 3,561,688 | |||||||||||||
| Repayment of long-term debt | (606,806) | (1,833,265) | (4,821,511) | |||||||||||||
| Proceeds from exercise of warrants, net | 2,301,944 | 1,845,204 | 30,000 | |||||||||||||
| Proceeds from exercise of stock options | 526 | 5,181 | 1,300 | |||||||||||||
| Proceeds from issuance of preferred stock, net | 2,655,000 | 2,615,000 | 1,975,000 | |||||||||||||
| Net Cash Provided by Financing Activities | 4,350,664 | 3,461,158 | 746,477 | |||||||||||||
| Effect of Exchange Rates on Cash | (10,803) | (22,339) | (32,120) | |||||||||||||
| Net Increase/(Decrease) in Cash and Cash Equivalents | 1,560,535 | 1,462,645 | (2,552,488) | |||||||||||||
| Cash and Cash Equivalents, Beginning ofYear | Y1,708,954 | 246,309 | 2,798,797 | |||||||||||||
| Cash and Cash Equivalents, End ofYear | $ 3,269,489 | $ 1,708,954 | $ 246,309 | |||||||||||||
| Supplemental Disclosure of Cash Flow Information | ||||||||||||||||
| Interest paid in cash | $ 35,541 | $ 327,722 | $ 592,525 | |||||||||||||
| Issuance of stock options to satisfy compensation liabilities | $ 257,505 | $ -- | $ -- | |||||||||||||
| Issuance of stock as financing share issuance costs | $ 115,000 | $ -- | $ -- | |||||||||||||
| Issuance of common stock as payment of dividends on preferred stock | $ 689,850 | $ 886,950 | $ 485,190 | |||||||||||||
| 44 |
Note 1 - Organization and Business and Going Concern:
Generex Biotechnology Corporation (the Company) and its wholly-owned subsidiary Generex Pharmaceuticals, Inc. are engaged in the research and development of drug delivery systems and technology. Since its inception, the Company has devoted its efforts and resources to the development of a platform technology for the oral administration of large molecule drugs, including proteins, peptides, monoclonal antibodies, hormones and vaccines, which historically have been administered by injection, either subcutaneously or intravenously. Oral-lynTMthe first product based on this platform technology, is in various stages of regulatory approval in different jurisdictions around the world.
The Company’s wholly-owned subsidiary, Antigen Express, Inc. (Antigen), is engaged in research and development of technologies and immunomedicines for the treatment of malignant, infectious, autoimmune and allergic diseases. The Company’s immunomedicine products work by stimulating the immune system to either attack offending agents (i.e., cancer cells, bacteria, and viruses) or to stop attacking benign elements (i.e., self proteins and allergens). The immunomedicine products are based on two platform technologies that were discovered by an executive officer of Antigen, the Ii-Key hybrid peptides and Ii-Suppression. These technologies are expected to greatly boost immune cell responses which diagnose and treat the ailments and conditions.
The Company has a limited history of operations and limited revenue to date. The Company has several product candidates that are in various research or early stages of pre-clinical and clinical development. There can be no assurance that the Company will be successful in obtaining regulatory clearance for the sale of existing or any future products or that any of the Company’s products will be commercially viable.
Going Concern
The accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. The Company has experienced negative cash flows from operations since inception and has an accumulated deficit of approximately $370 million and a working capital deficiency of approximately $4.8 million at July 31, 2014. The Company has funded its activities to date almost exclusively from debt and equity financings, as well as the recent sales of non-essential real estate assets in fiscal 2012, fiscal 2013 and the first quarter of fiscal 2014 (see Note 3).
The Company will continue to require substantial funds to continue research and development, including pre-clinical studies and clinical trials of its product candidates, and to commence sales and marketing efforts, if the U.S. Food and Drug Administration or other regulatory approvals are obtained. Management’s plans in order to meet its operating cash flow requirements include financing activities such as private placements of its common stock, preferred stock offerings, issuances of debt and convertible debt instruments. Management will be limited in the financing activities that the Company undertakes in the near future as the securities purchase agreements that the Company entered into on January 14, 2014 and March 27, 2014 with certain investors prohibit the Company from (i) issuing additional equity securities until 60 days after the effective date of a registration statement covering the resale of the common stock issuable upon exercise of the warrants and conversion of the preferred stock sold in those transactions; and (ii) issuing additional debt or equity securities with variable conversion or exercise prices until January 14, 2015 and March 27, 2015, respectively. Management is also actively pursuing financial and strategic alternatives, including strategic investments and divestitures, industry collaboration activities and strategic partners. Management has sold its non-essential real estate assets which were classified as Assets Held for Investment to augment its cash position.
These factors raise substantial doubt regarding the Company’s ability to continue as a going concern. There are no assurances that such additional funding will be achieved and that it will succeed in its future operations. The consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or amounts of liabilities that might be necessary should the Company be unable to continue in existence. The Company’s inability to obtain required funding in the near future or its inability to obtain funding on favorable terms will have a material adverse effect on its operations and strategic development plan for future growth. If the Company cannot successfully raise additional capital and implement its strategic development plan, its liquidity, financial condition and business prospects will be materially and adversely affected, and the Company may have to cease operations.
Note 2 - Summary of Significant Accounting Policies:
Principles of Consolidation
The consolidated financial statements include the accounts of the Company and all of its subsidiaries in which a controlling interest is maintained. For those consolidated subsidiaries where the Company ownership is less than 100 percent, the outside stockholders’ interests are shown as minority interests. Effective December 17, 2004, the Company’s ownership in all consolidated subsidiaries is 100 percent. All significant intercompany transactions and balances have been eliminated.
| 45 |
Cash and Cash Equivalents
The Company considersall highly liquid investments purchased with an original maturity of three months or less to be cash equivalents.
Property and Equipment
Property and equipment are recorded at cost less accumulated depreciation. Depreciation is provided on the straight-line method over the estimated useful lives of the assets, which range from three to thirty years. Gains and losses on depreciable assets retired or sold are recognized in the statement of operations in the year of disposal. Repairs and maintenance expenditures are expensed as incurred.
Assets Held for Investment
Property held for investment is recorded at cost less accumulated depreciation. Depreciation is provided on the straight-line method over the estimated useful lives of the assets of thirty years. Gains and losses on depreciable assets retired or sold are recognized in the statement of operations in the year of disposal. Repairs and maintenance expenditures are expensed as incurred.
Patents
Capitalized patent costs represent legal costs incurred to establish patents and a portion of the acquisition price paid attributed to patents upon the acquisition of Antigen in August 2003. When patents reach a mature stage, any associated legal costs are comprised mostly of maintenance fees and costs of national applications and are expensed as incurred. Capitalized patent costs are amortized on a straight line basis over the remaining life of the patent. As patents are abandoned, the net book value of the patent is written off.In the fiscal year ended July 31, 2012, the Company recorded a write down of $440,780 on certain patents. There were no write downs or disposals in the fiscal years ended July 31, 2014 and 2013.
Impairment or Disposal of Long-Lived Assets and Intangibles
The Company assesses the impairment of long-lived assets under FASB ASC Topic 360 whenever events or changes in circumstances indicate that the carrying value may not be recoverable. For long-lived assets to be held and used, the Company recognizes an impairment loss only if its carrying amount is not recoverable and exceeds its fair value. The carrying amount of the long-lived asset is not recoverable if it exceeds the sum of the undiscounted cash flows expected to result from the use and eventual disposal of the asset. In the fiscal years ended July 31, 2014, 2013 and 2012, the Company sold, wrote off or disposed of certain long-lived assets with net book values of $706,590, $745,172 and $2,945,079, respectively.
Derivative Warrant Liability
The Company’s derivative warrant instruments are measured at fair value using the binomial valuation model which takes into account, as of the valuation date, factors including the current exercise price, the expected life of the warrant, the current price of the underlying stock and its expected volatility, expected dividends on the stock and the risk-free interest rate for the term of the warrant. The liability is revalued at each reporting period and changes in fair value are recognized in the consolidated statements of operations under the caption “Change in fair value of derivative warrant liability.” SeeNote 10 – Derivative Liabilities.
Revenue Recognition and Deferred Revenue
Revenues from the sale of commercial products are recognized at the time title of goods passes to the buyer and the buyer assumes the risks and rewards of ownership. Certain product sales are made to retailers under agreements allowing for a right to return unsold products. In accordance with FASB ASC Topic 605, recognition of revenue on all sales to these retailers is deferred until the right of return expires, the product is sold to a third party or a provision for returns can be reasonably estimated based on historical experience. The cost of inventory under these sales is considered to be consigned inventory until the revenue is recognized. Sales are reported net of estimated returns and allowances, discounts, mail-in rebate redemptions and credit card chargebacks. If actual sales returns, allowances, discounts, mail-in rebate redemptions or credit card chargebacks are greater than estimated by management, additional expense may be incurred. At July 31, 2014, we have $223,662 of deferred revenue for which a provision for returns cannot be reasonably estimated and thus the balance is included in Deferred Revenue on our consolidated balance sheets. The corresponding cost of sales has been previously written off and is not included in inventory as of July 31, 2014 as the timing of the recognition of the revenue cannot be reasonably estimated.
Grant revenue is recognized as the Company provides the services stipulated in the underlying grant based on the time and expenditures incurred. Amounts received in advance of services provided are recorded as deferred revenue and amortized as revenue when the services are provided. There was no grant revenue in fiscal 2014, 2013 or 2012.
Included in miscellaneous income are fees received under licensing agreements. Nonrefundable fees received under licensing agreements are recognized as revenue when received if the Company has no continuing obligations to the other party.
Rental income is recognized as revenue in the period in which the related rental space is occupied.
| 46 |
Research and Development Costs
Expenditures for research and development are expensed as incurred and include, among other costs, those related to the production of experimental drugs, including payroll costs, and amounts incurred for conducting clinical trials. Amounts expected to be received from governments under research and development tax credit arrangements are offset against current research and development expense.
Income Taxes
Income taxes are accounted for under the asset and liability method prescribed by FASB ASC Topic 740. These standards require a company to determine whether it is more likely than not that a tax position will be sustained upon examination based upon the technical merits of the position. If the more likely than not threshold is met, a company must measure the tax position to determine the amount to recognize in the financial statements. Deferred income taxes are recorded for temporary differences between financial statement carrying amounts and the tax basis of assets and liabilities. Deferred tax assets and liabilities reflect the tax rates expected to be in effect for the years in which the differences are expected to reverse. A valuation allowance is provided if it is more likely than not that some or all of the deferred tax asset will not be realized. AtJuly 31, 2014 and 2013, the Company had a full valuation allowance equal to the amount of the net deferred tax asset.
The Company adopted the FASB guidance concerning accounting for uncertainty in income taxes, which clarifies the accounting and disclosure for uncertainty in tax positions, as of August 1, 2007. The guidance requires that the Company determine whether it is more likely than not that a tax position will not be sustained upon examination by the appropriate taxing authority. If a tax position does not meet the more likely than not recognition criterion, the guidance requires that the tax position be measured at the largest amount of benefit greater than 50 percent not likely of being sustained upon ultimate settlement. Based on the Company’s evaluation, management has concluded that there are no significant uncertain tax positions requiring recognition in the consolidated financial statements.
Stock-Based Compensation
The Company follows FASB ASC Topic 718 which requires that new, modified and unvested share-based payment transactions with employees, such as grants of stock options and restricted stock, be recognized in the financial statements based on their fair value at the grant date and recognized as compensation expense over their vesting periods. The Company estimates the fair value of stock options as of the date of grant using the Black-Scholes option pricing model and restricted stock based on the quoted market price or the value of the services provided, whichever is more readily determinable. The Company also follows the guidance in FASB ASC Topic 505 for equity based payments to non-employees for equity instruments issued to consultants and other non-employees.
Net Loss per Common Share
Basic earnings per share is computed by dividing income (loss) available to common stockholders by the weighted average number of common shares outstanding during the period. Diluted earnings per share gives effect to all dilutive potential common shares outstanding during the period. The computation of diluted earnings per share does not assume conversion, exercise or contingent exercise of securities that would have an anti-dilutive effect on earnings. Refer to Note 13 for methodology for determining net loss per share.
Comprehensive Income/(Loss)
Other comprehensive income/(loss), which includes only foreign currency translation adjustments, is shown in the Statement of Changes in Stockholders’ Equity.
Concentration of Credit Risk
The Company maintains cash balances, at times, with financial institutions in excess of amounts insured by the Canada Deposit Insurance Corporation and the U.S. Federal Deposit Insurance Corporation. Management monitors the soundness of these institutions and has not experienced any collection losses with these financial institutions.
Foreign Currency Translation
Foreign denominated assets and liabilities of the Company are translated into U.S. dollars at the prevailing exchange rates in effect at the end of the reporting period. Income statement accounts are translated at a weighted average of exchange rates which were in effect during the period. Translation adjustments that arise from translating the foreign subsidiary’s financial statements from local currency to U.S. currency are recorded in the other comprehensive loss component of stockholders’ equity.
Fair Value of Financial Instruments
Fair value is defined under FASB ASC Topic 820 as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or the most advantageous market for an asset or liability in an orderly transaction between participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The standard describes a fair value hierarchy based on the levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value. The levels are as follows:
| • | Level 1 - Quoted prices in active markets for identical assets or liabilities |
| • | Level 2 - Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or corroborated by observable market data for substantially the full term of the assets or liabilities |
| • | Level 3 - Unobservable inputs that are supported by little or no market activity and that are significant to the value of the assets or liabilities |
| 47 |
The Company’s financial instruments consist of cash and cash equivalents, other current assets, long-term debt, accounts payable and accrued expenses, as well as derivative warrant liabilities and derivative additional investment rights. All of these items, except for the derivative warrant liabilities and derivative additional investment rights, were determined to be Level 1 fair value measurements. The carrying amounts of cash and cash equivalents, other current assets and accounts payable and accrued expenses approximate their respective fair values because of the short maturities of these instruments. Long-term debt balances were determined to approximate their fair value as we believe the borrowing rates reflect the prevailing market rates available for similar debt instruments.
The Company has determined its derivative warrant liability and its derivative additional investment rights liability to be Level 2 fair value measurements and has used the binomial lattice model valuation method to calculate the fair value of the derivative warrant liability and the derivative additional investment rights liability at July 31, 2014, 2013 and 2012. SeeNote 10 – Derivative Liabilities.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the dates of the financial statements and the reported amounts of revenues and expenses during the reporting periods.
The Company evaluates its estimates, including those related to bad debts, long lived assets (including patents) impairment valuations, debt obligations, derivatives, convertible preferred shares, long-term contracts, and contingencies and litigation, on an ongoing basis. The Company bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Critical accounting estimates are reviewed and discussed with the audit committee of the board of directors. The Company considers an accounting estimate to be critical if it requires assumptions to be made that were uncertain at the time the estimate was made, if changes in the estimate or if different estimates that could have been selected would have a material impact on our results of operations or financial condition.
Effects of Recent Accounting Pronouncements
Recently Adopted Accounting Pronouncements
In June 2014, the FASB issued guidance regarding the elimination of the reporting requirement for development stage entities and removed the definition of development stage entity from the Accounting Standards Codification. The Company has adopted this guidance effective for the Company’s annual fiscal year ended July 31, 2014. The adoption of this new accounting guidance resulted in the elimination of the inception-to-date financial information in the consolidated statements of operations, statements of changes in stockholders’ deficiency and statements of cash flows, as well as the removal of the subheading “A Development Stage Company” from the consolidated financial statements and the notes to the consolidated financial statements.
Recently Issued Accounting Pronouncements
In August 2014, the FASB issued guidance regarding disclosure of uncertainties about an entity’s ability to continue as a going concern. The guidance will be effective for the Company’s annual fiscal year ended July 31, 2017 and subsequent interim periods. The Company is currently evaluating the impact of this accounting standard update on its consolidated financial statements.
| 48 |
Note 3 - Long-lived Assets:
Proper and Equipment
The costs and accumulated depreciation of property and equipment are summarized as follows:
| July 31, | |||||
| 2014 | 2013 | ||||
| Buildings and Improvements | $ | -0- | $ | 117,898 | |
| Furniture and Fixtures | 13,078 | 23,452 | |||
| Office Equipment | -0- | 38,690 | |||
| Lab Equipment | -0- | 327,899 | |||
| Total Property and Equipment | 13,078 | 507,939 | |||
| Less: Accumulated Depreciated | 7,785 | 422,533 | |||
| Property and Equipment, Net | $ | 5,293 | $ | 85,406 | |
Depreciation expense related to property and equipment amounted to $10,482, $41,890 and $97,967 for the years ended July 31, 2014, 2013 and 2012, respectively.
Assets Held for Investment, Net
The costs and accumulated depreciation of assets held for investment are summarized as follows:
| July 31, | |||||
| 2014 | 2013 | ||||
| Assets Held For Investment | $ | -0- | $ | 960,726 | |
| Less: Accumulated Depreciation | -0- | 289,954 | |||
| Assets Held for Investment, Net | $ | -0- | $ | 640,772 | |
These assets were held as collateral for long term debt (see Note 8). Depreciation expense on assets held for investment amounted to $0, $30,764 and $74,070 for the years ended July 31, 2014, 2013 and 2012, respectively.
The Company held these properties for investment purposes and collected rental income as described directly below.
Income from Assets Held for Investment, net
In August 2013, the Company sold a property which was held for investment for gross proceeds after real estate commissions of $883,780. This property had a net book value of $694,911, resulting in an accounting gain of $188,869 which is included in income from assets held for investment, net on the consolidated statement of operations. The property was secured by a mortgage which was discharged upon the sale, as described in the last paragraph of this note below. After the discharge of the mortgage ($606,806), as well as legal fees, interest, penalties and other costs ($73,628 in aggregate) the sale resulted in net cash proceeds to the Company of $203,346.
In March 2013, the Company sold a property which was held for investment for gross proceeds after real estate commissions of $256,835. This property had a net book value of $169,566, resulting in an accounting gain of $87,682 which is included in income from assets held for investment, net on the consolidated statement of operations. The property was secured by a mortgage which was partially discharged upon the sale, as described in the last paragraph of this note below. After the partial discharge of the mortgage ($216,810), as well as legal fees, interest, penalties and other costs (approximately $13,000 in aggregate), the sale resulted in net cash proceeds to the Company of $27,025.
In September 2012, the Company sold its head office real estate in Toronto for gross proceeds after real estate commissions of $1,579,189. This property had a net book value of $585,064, resulting in an accounting gain of $994,125 which is included in income from assets held for investment, net on the consolidated statement of operations. The net proceeds after commissions and other expenses were used to discharge or partially discharge the first and second mortgages on the property. The first mortgage on the property, with remaining principal of $480,951, was discharged completely upon sale. The remaining net proceeds of $1,028,780 after expenses and the discharge of the first mortgages was used to partially discharge the second mortgage and the Company did not receive any of the net proceeds from this property sale.
In July 2012, the Company sold a property for gross proceeds after real estate commissions of $342,862. This property had a net book value of $107,203, resulting in an accounting gain of $235,659 which is included in income from assets held for investment, net, on the consolidated statement of operations. The net proceeds after commissions and other expenses were used to partially discharge the first and second mortgages on the property and the Company did not receive any of the net proceeds from this property sale.
| 49 |
In March and April, 2012, the Company sold nine commercial condominium units which were held for investment for gross proceeds after real estate commissions of $2,865,682. These properties had a net book value of $1,783,932, resulting in an accounting gain of $1,081,750 which is included in income from assets held for investment, net on the consolidated statement of operations. The net proceeds after commissions and other expenses were used to discharge or partially discharge the first and second mortgages on the properties. There were two first mortgages on the properties, with combined remaining principals of $571,680, which were discharged completely upon sale. The remaining net proceeds of $2,190,952 after expenses and the discharge of the first mortgages was used to partially discharge the second mortgage and the Company did not receive any of the net proceeds from these property sales.
In August 2011, the Company sold two properties which were held for investment for gross proceeds after real estate commissions of $1,669,115. These two properties had a net book value of $1,029,435, resulting in an accounting gain of $639,680 which is included in income from assets held for investment, net on the consolidated statement of operations. The two properties had mortgages of $659,288 which were discharged upon sale, resulting in net cash proceeds to the Company of $1,009,827.
The total accounting gains in this category from property sales in the fiscal years ended July 31, 2014, 2013 and 2012 were $188,869, $1,081,807 and $1,957,089, respectively.
The remaining income of $4,738, $163,358 and $249,127 in this category in the respective fiscal years ended July 31, 2014, 2013 and 2012, pertains to rental income from properties held for investment, net of carrying and operating expenses. Gross income from rental operations was $7,847, $221,399 and $384,299 and rental expenses were $1,935, $58,041 and $135,172, including the depreciation expense amounts above relating to assets held for investment, for the years ended July 31, 2014, 2013 and 2012, respectively.
Prior to the August 2013 property sale, the properties held for investment had an interest only first mortgage which closed on November 30, 2012 with a principal amount $606,806, an interest rate of 9.75% compounded semi-annually and a maturity date of November 30, 2013. Upon the sale of the property, the mortgage was discharged.
Note 4 - Patents:
The costs and accumulated amortization of patents are summarized as follows:
| July 31, | |||||
| 2014 | 2013 | ||||
| Patents | $ | 5,725,908 | $ | 5,657,455 | |
| Less: Accumulated Amortization | 3,679,547 | 3,338,039 | |||
| Patents, Net | 2,046,361 | 2,319,416 | |||
| Weighted Average Life | 7.8 years | 8.6 years | |||
Amortization expense amounted to $357,965, $391,777 and $441,087 for the years ended July 31, 2014, 2013 and 2012, respectively. Amortization expense is expected to be approximately $315,000 per year for the years ended July 31, 2015 through 2019. During the years ended July 31, 2014 and 2013, the Company did not write off any patents. During the year ended July 31, 2012, the Company wrote off patents with a net book value of $440,780 as the patents had been abandoned or were no longer being used. The charge was included in research and development expenses on our consolidated statements of operations.
Note 5 - Income Taxes:
The Company has incurred losses since inception, which have generated net operating loss (“NOL”) carryforwards. The NOL carryforwards arise from both United States and Canadian sources. Pretax income/(losses) arising from domestic operations (United States) were $1,274,619, $(8,025,582) and $(8,040,033) for the years ended July 31, 2014, 2013 and 2012, respectively. Pretax losses arising from foreign operations (Canada) were $1,276,036, $528,741 and $1,450,244 for the years ended July 31, 2014, 2013 and 2012, respectively. As of July 31, 2014, the Company has NOL carryforwards in Generex Biotechnology Corporation of approximately $202 million, which expire in 2018 through 2034, in Generex Pharmaceuticals Inc. of approximately $44 million, which expire in 2015 through 2034, and in Antigen Express, Inc. of approximately $25 million, which expire in 2016 through 2034. These loss carryforwards are subject to limitation due to the acquisition of Antigen and may be limited in future years due to certain structural ownership changes which have occurred over the last several years, related to the Company’s equity and convertible debenture financing transactions.
| 50 |
For the years ended July 31, 2014, 2013 and 2012, the Company’s effective tax rate differs from the federal statutory rate principally due to net operating losses and other temporary differences for which no benefit was recorded.
Deferred income taxes consist of the following:
| July 31, | |||||
| 2014 | 2013 | ||||
| Net operating loss carryforwards | $ | 90,515,261 | $ | 87,541,552 | |
| Other temporary differences | 299,010 | 800,989 | |||
| Intangible assets | 291,002 | -- | |||
| Total Deferred Tax Assets | 91,105,273 | 88,342,541 | |||
| Valuation Allowance | (91,105,273) | (88,089,860) | |||
| Deferred Tax Liabilities | |||||
| Intangible assets | -- | (386,320) | |||
| Other temporary differences | -- | 142,639 | |||
| Total Deferred Tax Liabilities | -- | (243,681) | |||
| Net Deferred Income Taxes | $ | -- | $ | -- | |
A reconciliation of the United States Federal Statutory rate to the Company’s effective tax rate for the years ended July 31, 2014, 2013 and 2012 is as follows:
| 2014 | 2013 | 2012 | |||||||
| Federal statutory rate | (34.0) | % | (34.0) | % | (34.0) | % | |||
| Increase (decrease) in income taxes resulting from: | |||||||||
| Imputed interest income on intercompany receivables from foreign subsidiaries | 4,037 | 6.0 | 5.0 | ||||||
| Non-deductible or non-taxable items | (111,569) | 13.0 | 4.0 | ||||||
| Other temporary differences | (84,061) | (14.0) | 18.0 | ||||||
| Change in valuation allowance | 191,627 | 29.0 | (3.0) | ||||||
| Effective tax rate | -- | % | -- | % | -- | % |
As of July 31, 2014, the Company had no tax benefits which have not been fully allowed for, and no adjustment to its financial position, results of operations or cash flows was required. The Company does not expect that unrecognized tax benefits will increase within the next twelve months. The Company records interest and penalties related to tax matters within other expense on the accompanying consolidated statement of operations. These amounts are not material to the consolidated financial statements for the periods presented. Generally, tax years 2011 to 2014 remain open to examination by the Internal Revenue Agency or other tax jurisdictions to which the Company is subject. The Company’s Canadian tax returns are subject to examination by federal and provincial taxing authorities in Canada. Generally, tax years 2006 to 2014 remain open to examination by the Canadian Customs and Revenue Agency or other tax jurisdictions to which the Company is subject.
Note 6 - Accounts Payable and Accrued Expenses:
Accounts payable and accrued expenses consist of the following:
| July 31, | |||||
| 2014 | 2013 | ||||
| Accounts Payable and Accruals - General and Administrative | $ | 3,208,069 | $ | 3,447,618 | |
| Accounts Payable and Accruals - Research and Development | 3,955,543 | 3,557,184 | |||
| Accounts Payable and Accruals - Selling and Marketing | 327,067 | 328,629 | |||
| Accrued Make-whole Payments on Covertible Preferred Stock (see Note 9) | 337,500 | 251,100 | |||
| Executive Compensation and Directors’ Fees Payable | 205,943 | 76,703 | |||
| Total | $ | 8,034,122 | $ | 7,661,234 | |
| 51 |
Note 7 - Commitments and Contingent Liabilities:
Leases
The Company has entered into various operating lease agreements for the use of operating space, vehicles and office equipment.
Aggregate minimum annual lease commitments of the Company under non-cancelable operating leases as of July 31, 2014 are as follows:
| Fiscal Year | Amount | ||
| 2015 | $ | 41,750 | |
| 2016 | 39,317 | ||
| 2017 | 41,501 | ||
| 2018 | 41,938 | ||
| 2019 and thereafter | 53,122 | ||
| Total Minimum Lease Payments | $ | 217,628 |
Lease expense amounted to approximately $162,000, $153,000 and $185,000 for the years ended July 31, 2014, 2013 and 2012, respectively.
In June 2014, the Company signed a lease extension for its office space in Toronto, Canada which runs from October 2014 through September 2019 at a monthly gross rent, including taxes and expenses of approximately $6,900 per month.
The preceding data reflects existing leases and does not include replacements upon their expiration. In the normal course of business, operating leases are generally renewed or replaced by other leases.
Supply Agreements and Purchase Obligations
On December 7, 2009, the Company entered into a long-term agreement with sanofi-aventis Deutschland GmbH (“sanofi”). Under this agreement, sanofi will manufacture and supply recombinant human insulin to the Company in the territories specified in the agreement. Through this agreement, the Company will procure recombinant human insulin crystals for use in the production of Generex Oral-lyn™. The terms of the supply agreement required the Company to make certain minimum purchases of insulin from sanofi through the period ended December 31, 2011. To date, the Company has not met the minimum purchase commitments under this agreement. After December 31, 2011, sanofi may terminate the agreement due to the Company’s failure to meet such purchase commitments. Upon termination, the Company would be obligated to pay sanofi for all materials and components that it has acquired or ordered to manufacture insulin based on the Company’s forecasts or minimum purchase commitments, all related work-in-progress (at cost) and all finished insulin in inventory. To date, the Company has not provided forecasts to sanofi for the purchase of insulin and sanofi has not terminated the agreement.
The Company has a supply agreement with Presspart Manufacturing Limited (“Presspart”), whereby the Company will purchase its entire requirements for products to use in the administration of insulin through the buccal mucosa and shall not purchase the products or any metal containers competitive to the products from any other person in exchange for an exclusive non-transferable royalty-free irrevocable license to use the products. The contract shall continue for a minimum period of four contract years from the end of the first contract year in which the total quantity of products purchased by the Company from Presspart exceeds 10,000,000 units, and thereafter, shall continue until terminated by either party by giving twelve months written notice. As of July 31, 2014, the Company has not yet completed a contract year in which the total quantity has exceeded 10,000,000 units and as such the expiration date of this contract cannot be determined.
Pending Litigation
In February 2001, a former business associate of the former Vice President of Research and Development (“VP”) of the Company and an entity known as Centrum Technologies Inc. (“CTI”) commenced an action in the Ontario Superior Court of Justice against the Company and the VP seeking, among other things, damages for alleged breaches of contract and tortious acts related to a business relationship between this former associate and the VP that ceased in July 1996. The plaintiffs’ statement of claim also seeks to enjoin the use, if any, by the Company of three patents allegedly owned by CTI. The three patents are entitledLiquid Formulations for Proteinic Pharmaceuticals,Vaccine Delivery System for Immunization, Using Biodegradable Polymer Microspheres, andControlled Releases of Drugs or Hormones in Biodegradable Polymer Microspheres. It is the Company’s position that the buccal drug delivery technologies which are the subject matter of the Company’s research, development, and commercialization efforts, including Generex Oral-lyn™ and the RapidMist™ Diabetes Management System, do not make use of, are not derivative of, do not infringe upon, and are entirely different from the intellectual property identified in the plaintiffs’ statement of claim. On July 20, 2001, the Company filed a preliminary motion to dismiss the action of CTI as a nonexistent entity or, alternatively, to stay such action on the grounds of want of authority of such entity to commence the action. The plaintiffs brought a cross motion to amend the statement of claim to substitute Centrum Biotechnologies, Inc. (“CBI”) for CTI. CBI is a corporation of which 50 percent of the shares are owned by the former business associate and the remaining 50 percent are owned by the Company. Consequently, the shareholders of CBI are in a deadlock. The court granted the Company’s motion to dismiss the action of CTI and denied the plaintiffs’ cross motion without prejudice to the former business associate to seek leave to bring a derivative action in the name of or on behalf of CBI. The former business associate subsequently filed an application with the Ontario Superior Court of Justice for an order granting him leave to file an action in the name of and on behalf of CBI against the VP and the Company. The Company opposed the application. In September 2003, the Ontario Superior Court of Justice granted the request and issued an order giving the former business associate leave to file an action in the name of and on behalf of CBI against the VP and the Company. A statement of claim was served in July 2004. The Company is not able to predict the ultimate outcome of this legal proceeding at the present time or to estimate an amount or range of potential loss, if any, from this legal proceeding.
| 52 |
On May 20, 2011, Ms. Perri filed a statement of claim (subsequently amended) in the Ontario Superior Court of Justice, naming as defendants the Company and certain directors of the Company, Mr. Barratt, Ms. Masterson, Mr. McGee, and Mr. Fletcher. In this action, Ms. Perri has alleged that defendants engaged in discrimination, harassment, bad faith and infliction of mental distress in connection with the termination of her employment with the Company. Ms. Perri is seeking damages in this action in excess of $7,000,000 for, among other things, breach of contract, breach of fiduciary duty, violations of the Ontario Human Rights Code and aggravated and punitive damages. On September 20, 2011, the defendants filed a statement of defense and counterclaim, also naming Time Release Corp., Khazak Group Consulting Corp., and David Khazak, C.A. as defendants by counterclaim, and seeking damages of approximately $2.3 million in funds that the defendants allege Ms. Perri wrongly caused the Company to pay to third parties in varying amounts over several years and an accounting of certain third-party payments, plus interests and costs. The factual basis for the counterclaim involves payments made by the Company to third parties believed to be related to Ms. Perri. The Company intends to defend this action and pursue its counterclaim vigorously and is not able to predict the ultimate outcome of this legal proceeding at the present time or to estimate an amount or range of potential loss, if any, from this legal proceeding.
On June 1, 2011, Golden Bull Estates Ltd. filed a claim (subsequently amended) in the Ontario Superior Court of Justice, naming the Company, 1097346 Ontario, Inc. and Generex Pharmaceuticals, Inc. as defendants. The plaintiff, Golden Bull Estates, is controlled by Ms. Perri. The plaintiff alleges damages in the amount of $550,000 for breach of contract, $50,000 for punitive damages, plus interest and costs. The plaintiff’s claims relate to an alleged contract between the plaintiff and the Company for property management services for certain Ontario properties owned by the Company. The Company terminated the plaintiff’s property management services in April 2011. Following the close of pleadings, the Company served a motion for summary judgment. The plaintiff responded by amending its statement of claim to include a claim to the Company’s interest in certain of its real estate holdings. The plaintiff moved for leave to issue and register a Certificate of Pending Litigation in respect of this real estate. The motion was not successful in respect of any current real estate holdings of the Company. The Company is not able to predict the ultimate outcome of this legal proceeding at the present time or to estimate an amount or range of potential loss, if any, from this legal proceeding.
In August 2011, the estate of Antonio Perri, the late father of Ms. Perri, commenced an action against Generex Pharmaceuticals, Inc., the law firm of Brans, Lehun, Baldwin LLP and William Lehun in the Ontario Superior Court of Justice claiming that the estate is entitled to the proceeds of sale (approximately $1,730,000) received by the Company on its sale of two properties to Golden Bull Estates Ltd., a company controlled by Ms. Perri. The suit alleges that no consideration was received when the Company purchased the two properties from Antonio Perri in 1998. The Company responded to this statement of claim. On May 6, 2014, the Company received a court order which dismissed this action for delay as the plaintiff has not brought this action to conclusion or set it down for trial within the time prescribed by the laws of Ontario.
In December 2011, a vendor of the Company commenced an action against the Company and its subsidiary, Generex Pharmaceuticals, Inc., in the Ontario Superior Court of Justice claiming damages for unpaid invoices including interest in the amount of $429,000, in addition to costs and further interest. The Company responded to this statement of claim and also asserted a counterclaim in the proceeding for $200,000 arising from the vendor’s breach of contract and detinue, together with interest and costs. On November 16, 2012, the parties agreed to settle this action and the Company has agreed to pay the plaintiff $125,000, following the spinout of its subsidiary Antigen, from the proceeds of any public or private financing related to Antigen subsequent to such spinout. Each party agreed to execute mutual releases to the claim and counterclaim to be held in trust by each party’s counsel until payment of the settlement amount. Following payment to the plaintiff, the parties agree that a Consent Dismissal Order without costs will be filed with the court. If the Company fails to make the payment following completion of any post-spinout financing related to Antigen or any other subsidiaries, the Plaintiffs may take out a judgment in the amount of the claim plus interest of 3% per annum and costs fixed at $25,000.
The Company is involved in certain other legal proceedings in addition to those specifically described herein. Subject to the uncertainty inherent in all litigation, the Company does not believe at the present time that the resolution of any of these legal proceedings is likely to have a material adverse effect on the Company’s consolidated financial position, operations or cash flows.
With respect to all litigation, as additional information concerning the estimates used by the Company becomes known, the Company reassesses its position both with respect to accrued liabilities and other potential exposures.
| 53 |
Employment Agreements
As of July 31, 2014, the Company had an employment arrangement with its President & Chief Executive Officer, whereby the Company is required to pay an annual base salary of $475,000. The term of service for this executive extended through March 16, 2008, which term had not been formally extended as of July 31, 2014. In the event the agreement is terminated, by reason other than cause, death, voluntary retirement or disability, the Company is required to pay the employee in one lump sum twelve months base salary and the average annual bonus.
As of July 31, 2014, the Company has an at will employment agreement with an Antigen employee requiring the Company to pay an annual aggregate salary of $260,480 to the employee. In the event the agreement is terminated by reason other than death, disability, a voluntary termination not for good reason (as defined in the agreement) or a termination for cause, the Company is required to pay the employee severance of six months’ salary, in accordance with the terms of the individual’s employment agreement.
Note 8 - Long-Term Debt:
Long-term debt consists of the following:
| July 31, | |||||
| 2014 | 2013 | ||||
| Mortgage payable - interest at 9.75 percent per annum, monthly interest payments of $6,771, principal due November 2013, secured by secondary rights to real property located at 11 Carlaw Avenue, Toronto, Canada | $ | -0- | $ | 617,665 | |
| Total Debt | -0- | 617,665 | |||
| Less Current Maturities of Long-Term Debt | -0- | 617,665 | |||
| Total Long-Term Debt | -0- | -0- | |||
The first mortgage related to the property at 11 Carlaw was discharged on August 14, 2013, in conjunction with the sale of that property and there is no mortgage debt after that date.
For the years ended July 31, 2014, 2013 and 2012, the Company incurred $4,969, $196,101 and $568,424, respectively in interest expense on its long-term debt.
Note 9 - Series A, B, C, D, E & F 9% Convertible Preferred Stock:
Series A 9% Convertible Preferred Stock
The Company has authorized 5,500 shares of Series A 9% Convertible Preferred Stock with a stated value of one thousand ($1,000) per share. Pursuant to a securities purchase agreement dated July 8, 2011, the Company sold an aggregate of 2,575 shares of convertible preferred stock, as well as accompanying warrants to purchase 17,166,666 shares of common stocks. An aggregate of 17,166,666 shares of the Company’s common stock were issuable upon conversion of the convertible preferred stock which was issued at the initial closing. As of the end of the Company’s fiscal year 2012, all of the issued Series A 9% Convertible Preferred Stock had been converted to common stock. There were 17,166,666 shares of common stock issued upon the conversion of the Series A convertible preferred stock and 6,129,666 shares of common stock issued as “make-whole payments” on such conversions.
Series B 9% Convertible Preferred Stock
The Company has authorized 2,000 shares of Series B 9% Convertible Preferred Stock with a stated value of one thousand ($1,000) per share. Pursuant to a securities purchase agreement dated January 31, 2012, the Company sold an aggregate of 2,000 shares of Series B convertible preferred stock, as well as accompanying warrants to purchase 13,333,333 shares of common stocks. An aggregate of 13,333,333 shares of the Company’s common stock were issuable upon conversion of the Series B convertible preferred stock which was issued at the initial closing. On December 10, 2012, the triggering of the price protection features of the Series B convertible preferred stock resulted in a decrease of the conversion price from $0.08 to $0.03 per share and a corresponding increase in the number of common shares underlying the remaining 792 shares of Series B convertible preferred stock as of December 10, 2012 from 9,897,500 to 26,393,333. As of the end of the Company’s fiscal year 2013, all of the issued Series B 9% Convertible Preferred Stock had been converted to common stock. There were 38,520,832 shares of common stock issued upon the conversion of the Series B convertible preferred stock and 14,819,679 shares of common stock issued as “make-whole payments” on such conversions.
Series C 9% Convertible Preferred Stock
The Company has authorized 750 shares of Series C 9% Convertible Preferred Stock with a stated value of one thousand ($1,000) per share. Pursuant to a securities purchase agreement dated August 8, 2012, the Company sold an aggregate of 750 shares of Series C convertible preferred stock, as well as accompanying warrants to purchase 9,375,000 shares of common stocks. An aggregate of 9,375,000 shares of the Company’s common stock were issuable upon conversion of the Series C convertible preferred stock which was issued at the initial closing. On December 10, 2012, the triggering of the price protection features of the Series C convertible preferred stock resulted in a decrease of the conversion price from $0.08 to $0.03 per share and a corresponding increase in the number of common shares underlying the 650 shares of Series C convertible preferred stock as of December 10, 2012 from 8,125,000 to 21,666,666. As of the end of the Company’s fiscal year 2013, all of the issued Series C 9% Convertible Preferred Stock had been converted to common stock. There were 22,916,665 shares of common stock issued upon the conversion of the Series C convertible preferred stock and 6,664,863 shares of common stock issued as “make-whole payments” on such conversions.
| 54 |
Series D 9% Convertible Preferred Stock
The Company has authorized 750 shares of Series D 9% Convertible Preferred Stock with a stated value of one thousand ($1,000) per share. Pursuant to a securities purchase agreement dated December 10, 2012, the Company sold an aggregate of 750 shares of Series D convertible preferred stock, as well as accompanying warrants to purchase 24,999,999 shares of common stocks. An aggregate of 24,999,999 shares of the Company’s common stock were issuable upon conversion of the Series D convertible preferred stock which was issued at the initial closing. As of the end of the Company’s fiscal year 2013, all of the Series D convertible preferred stock had been converted to common stock. There were 24,999,999 shares of common stock issued upon the conversion of the Series D convertible preferred stock and 7,825,191 shares of common stock issued as “make-whole payments” on such conversions.
Series E and F 9% Covertible Preferred Stock
The Company has authorized 2,450 shares of Series E 9% Convertible Preferred Stock with a stated value of one thousand ($1,000) per share. Pursuant to a securities purchase agreement dated June 17, 2013, the Company sold an aggregate of 1,225 shares of Series E convertible preferred stock, as well as accompanying warrants to purchase 40,833,335 shares of common stocks. An aggregate of 40,833,335 shares of the Company’s common stock are issuable upon conversion of the Series E convertible preferred stock which was issued at the initial closing on June 17, 2013. Pursuant to a securities purchase agreement dated January 14, 2014, the Company sold an aggregate of 800 shares of Series E convertible preferred stock, as well as accompanying warrants to purchase 26,666,668 shares of common stocks. An aggregate of 26,666,668 shares of the Company’s common stock are issuable upon conversion of the Series E convertible preferred stock which was issued at the closing on January 15, 2014.
The Company has authorized 4,150 shares of Series F 9% Convertible Preferred Stock with a stated value of one thousand ($1,000) per share. Pursuant to a securities purchase agreement dated March 27, 2014, the Company sold an aggregate of 2,075 shares of Series F convertible preferred stock, as well as accompanying warrants to purchase 69,166,667 shares of common stocks. An aggregate of 69,166,667 shares of the Company’s common stock are issuable upon conversion of the Series F convertible preferred stock which was issued at the closing on March 27, 2014.
Subject to certain ownership limitations, the convertible preferred stock is convertible at the option of the holder at any time into shares of the Company’s common stock at an effective conversion price of $0.03 per share, and will accrue a 9% dividend until the third year anniversary of the issuances. On each one year anniversary thereafter, such dividend rate will increase by an additional 3%. The dividend is payable quarterly on September 30, December 31, March 31 and June 30, beginning on June 30, 2013 and June 30, 2014, respectively, and on each conversion date in cash, or at the Company’s option, in shares of common stock. In the event that the Series E and F convertible preferred stock is converted prior to June 17, 2016 and March 27, 2017, respectively, the Company will pay the holder of the converted preferred stock an amount equal to $270 per $1,000 of stated value of the convertible preferred stock, less the amount of all prior quarterly dividends paid on such converted preferred stock before the relevant conversion date. Such “make-whole payment” may be made in cash or, at the Company’s option, in shares of its common stock. In addition, beginning on the third anniversary date of the issuances, the Company will pay dividends on shares of preferred stock equal to (on an as-if-converted-to-common-stock basis) and in the same form as dividends (other than dividends in the form of common stock) actually paid on shares of the common stock when, and if such dividends are paid. The Company will incur a late fee of 18% per annum on unpaid dividends.
The conversion price of the convertible preferred stock is subject to adjustment in the case of stock splits, stock dividends, combinations of shares, similar recapitalization transactions and certain pro-rata distributions to common stockholders. The conversion price will also be adjusted if the Company sells or grants any shares of common stock or securities convertible into, or rights to acquire, common stock at an effective price per share that is lower than the then conversion price, except in the event of certain exempt issuances. In addition, the holders of convertible preferred stock will be entitled to receive any securities or rights to acquire securities or property granted or issued by the Company pro rata to the holders of its common stock to the same extent as if such holders had converted all of their shares of convertible preferred stock. In the event of a fundamental transaction, such as a merger, consolidation, sale of substantially all assets and similar reorganizations or recapitalizations, the holders of convertible preferred stock will be entitled to receive, upon conversion of their shares, any securities or other consideration received by the holders of the Company’s common stock pursuant to the fundamental transaction.
| 55 |
In conjunction with the issuance of the Series E convertible preferred stock in June 2013 and January 2014 and the issuance of the Series F convertible preferred stock in June 2014, , the Company also issued 40,833,335, 26,666,668 and 69,166,667 warrants, respectively to the investors. Subject to certain ownership limitations, the warrants will be exercisable at any time after their respective dates of issuance and on or before the fifth-year anniversary thereafter at an exercise price of $0.03 per share of common stock. The exercise price of the warrants and, in some cases, the number of shares issuable upon exercise, are subject to adjustment in the case of stock splits, stock dividends, combinations of shares, similar recapitalization transactions and certain pro-rata distributions to common stockholders. The exercise price and number of shares of common stock issuable upon exercise will also be adjusted if the Company sells or grants any shares of common stock or securities convertible into, or rights to acquire, common stock at an effective price per share that is lower than the then exercise price, except in the event of certain exempt issuances. In addition, the warrant holders will be entitled to receive any securities or rights to acquire securities or property granted or issued by the Company pro rata to the holders of its common stock to the same extent as if such holders had exercised all of their warrants. In the event of a fundamental transaction, such as a merger, consolidation, sale of substantially all assets and similar reorganizations or recapitalizations, the warrant holders will be entitled to receive, upon exercise of their warrants, any securities or other consideration received by the holders of the Company’s common stock pursuant to the fundamental transaction. These warrants have been classified as derivative liabilities and are described further inNote 10 – Derivative Liabilities.
In addition, until the first anniversary date of the June 2013 and March 2014 securities purchase agreements, each investor may, in its sole determination, elect to purchase, severally and not jointly with the other investors, in one or more purchases, in the ratio of such investor's original subscription amount to the original aggregate subscription amount of all investors, additional units consisting of convertible preferred stock and warrants at a purchase price of $1,000 per unit with an aggregate subscription amount thereof of up to $1,225,000 and $2,075,000, respectively, which units will have terms identical to the units of convertible preferred stock and warrants issued in connection with the June 2013 and March 2014 closings. These additional investment rights of the investors have been classified as derivative liabilities and are described further in Note 10 – Derivative Liabilities.On January 15, 2014, certain investors exercised 800 of the 1,225 shares of convertible preferred stock and related warrants available under the additional investment rights. The remaining June 2013 additional investment rights expired on June 17, 2014. The March 2014 additional investment rights expire on March 27, 2015 and none have been exercised to date.
As of July 31, 2014, 2,000 of the Series E convertible preferred stock had been converted to common stock. There were 66,666,666 shares of common stock issued upon the conversion of the Series E convertible preferred stock and 18,585,193 shares of common stock issued as “make-whole payments” on such conversions. As of July 31, 2014, 850 of the Series F convertible preferred stock had been converted to common stock. There were 28,333,331 shares of common stock issued upon the conversion of the Series F convertible preferred stock and 7,861,623 shares of common stock issued as “make-whole payments” on such conversions.
Accounting for proceeds from the Series E convertible preferred stock financing
The net proceeds from the initial Series E convertible preferred stock financing in June 2013 were $1,165,000. The proceeds from the financing were allocated first to the warrants that were issued in the financing, second to the additional investment rights associated with the financing and third to the make whole payments. As the assigned fair values were greater than the net cash proceeds from the transaction, the excess was treated as a “deemed dividend” for accounting purposes and was reported on the Company’s consolidated statement of operations for the year ended July 31, 2013 under the caption “Preferred Stock Dividend”. The calculation methodologies for the fair values of the derivative warrant liability and the derivative additional investment rights liability are described inNote 10 – Derivative Liabilities below. The fair values assigned to each component and the calculation of the amount of the deemed dividend are as follows:
| Accounting allocation of initial proceeds | ||
| Net proceeds | $ | 1,165,000 |
| Derivative warrant liability fair value | (1,189,744) | |
| Derivative additional investment rights fair value | (1,264,683) | |
| Make whole payments liability | (330,750) | |
| Deemed dividend | $ | (1,620,177) |
The initial “make-whole payments” of $330,750 on the Series E convertible preferred stock were accrued as of the date of the financing and the remaining balance of $6,750 (after conversions) is included in Accounts Payable and Accrued Expenses (see Note 6) at July 31, 2014.
The initial net cash proceeds from the Series E convertible preferred stock financing in January 2014 were $750,000. The proceeds from the financing were allocated first to the warrants that were issued in the financing and then to the make whole payments and subsequent issuance costs. As the assigned fair values were greater than the net cash proceeds from the transaction, the excess was treated as a “deemed dividend” for accounting purposes and was reported on the Company’s consolidated statement of operations for the quarter ended January 31, 2014 under the caption “Preferred Stock Dividend”. The calculation methodologies for the fair values of the derivative warrant liability and the derivative additional investment rights liability are described inNote 10 – Derivative Liabilities below. The fair values assigned to each component and the calculation of the amount of the deemed dividend are as follows:
| 56 |
| Accounting allocation of initial proceeds | ||
| Net proceeds | $ | 750,000 |
| Derivative warrant liability fair value | (942,279) | |
| Other issuance costs (Finders’ fee) | (64,000) | |
| Make whole payments liability | (216,000) | |
| Deemed dividend | $ | (472,279) |
The initial “make-whole payments” of $216,000 on the Series E convertible preferred stock were accrued as of the date of the financing, all of which have been converted as of July 31, 2014.
Accounting for proceeds from the Series F convertible preferred stock financing
The initial net cash proceeds from the Series F convertible preferred stock financing in March 2014 were $2,020,000. The proceeds from the financing were allocated first to the warrants that were issued in the financing, second to the additional investment rights associated with the financing and then to the make whole payments and subsequent issuance costs. As the assigned fair values were greater than the net cash proceeds from the transaction, the excess was treated as a “deemed dividend” for accounting purposes and was reported on the Company’s consolidated statement of operations for the fiscal year ended July 31, 2014 under the caption “Preferred Stock Dividend”. The calculation methodologies for the fair values of the derivative warrant liability and the derivative additional investment rights liability are described inNote 10 – Derivative Liabilities below. The fair values assigned to each component and the calculation of the amount of the deemed dividend are as follows:
| Accounting allocation of initial proceeds | ||
| Net proceeds | $ | 2,020,000 |
| Derivative warrant liability fair value | (2,016,064) | |
| Derivative additional investment rights fair value | (863,735) | |
| Other issuance costs (Finders’ fee) | (166,000) | |
| Make whole payments liability | (560,250) | |
| Deemed dividend | $ | (1,586,050) |
The initial “make-whole payments” of $560,250 on the Series F convertible preferred stock were accrued as of the date of the financing and the remaining balance of $330,750 (after conversions) is included in Accounts Payable and Accrued Expenses (see Note 6) at July 31, 2014.
Note 10 - Derivative Liabilities:
Derivative warrant liability
The Company has warrants outstanding with price protection provisions that allow for the reduction in the exercise price of the warrants in the event the Company subsequently issues stock or securities convertible into stock at a price lower than the exercise price of the warrants. Simultaneously with any reduction to the exercise price, the number of shares of common stock that may be purchased upon exercise of each of these warrants shall be increased or decreased proportionately, so that after such adjustment the aggregate exercise price payable for the adjusted number of warrants shall be the same as the aggregate exercise price in effect immediately prior to such adjustment.
Accounting for Derivative Warrant Liability
The Company’s derivative warrant instruments have been measured at fair value at July 31, 2014 and 2013 using the binomial lattice model. The Company recognizes all of its warrants with price protection in its consolidated balance sheets as a liability. The liability is revalued at each reporting period and changes in fair value are recognized currently in the consolidated statements of operations. The initial recognition and subsequent changes in fair value of the derivative warrant liability have no effect on the Company’s consolidated cash flows.
The derivative warrants outstanding at July 31, 2014 are all currently exercisable with a weighted-average remaining life of 3.3 years.
The revaluation of the warrants at each reporting period, as well as the charges associated with issuing additional warrants due to the price protection features, resulted in the recognition of a gain of $3,362,497 within the Company’s consolidated statements of operations for the fiscal year ended July 31, 2014 and a loss of $3,148,782 for the fiscal year ended July 31, 2013, which is included in the consolidated statements of operations under the caption “Change in fair value of derivative liabilities”.
| 57 |
The fair value of the warrants at July 31, 2014 and 2013 was $2,635,643 and $5,234,293, respectively, which is reported on the consolidated balance sheets under the caption “Derivative Warrant Liability”. The following summarizes the changes in the value of the derivative warrant liability from August 1, 2012 until July 31, 2014:
| Value | No. of Warrants | |||||||
| Balance at August 1, 2012 – Derivative warrant liability | $ | 4,081,627 | 55,148,530 | |||||
| Additional warrants issued in August 2012 financing | 624,797 | 9,375,000 | ||||||
| Additional warrants issued in December 2012 financing | 762,355 | 24,999,999 | ||||||
| Additional warrants issued in June 2013 financing | 1,189,744 | 40,833,335 | ||||||
| Additional warrants from price protection features of existing warrants | 7,484,550 | 236,219,094 | ||||||
| Exercise of warrants | (5,629,130 | ) | (145,888,421 | ) | ||||
| Decrease in fair value of derivative warrant liability | (3,279,650 | ) | n/a | |||||
| Balance at July 31, 2013 – Derivative warrant liability | $ | 5,234,293 | 220,687,537 | |||||
| Exercise of warrants | (2,194,496 | ) | (76,732,020 | ) | ||||
| Additional warrants issued in January 2014 financing | 942,279 | 26,666,668 | ||||||
| Additional warrants issued in March 2014 financing | 2,016,064 | 69,166,667 | ||||||
| Decrease in fair value of derivative warrant liability | (3,362,497 | ) | n/a | |||||
| Balance at July 31, 2014 – Derivative warrant liability | $ | 2,635,643 | 239,788,852 |
Fair Value Assumptions Used in Accounting for Derivative Warrant Liability
The Company has determined its derivative warrant liability to be a Level 2 fair value measurement and has used the binominal lattice pricing model to calculate the fair value as of July 31, 2014 and 2013. The binomial lattice model requires six basic data inputs: the exercise or strike price, time to expiration, the risk free interest rate, the current stock price, the estimated volatility of the stock price in the future, and the dividend rate. Because the warrants contain the price protection feature, the probability that the exercise price of the warrants would decrease as the stock price decreased was incorporated into the valuation calculations. The key inputs used in the July 31, 2014 and 2013 fair value calculations were as follows:
| July 31, 2014 | July 31, 2013 | ||||||
| Current exercise price | $ | 0.03 | $ | 0.03 | |||
| Time to expiration | 3.3 years | 3.5 years | |||||
| Risk-free interest rate | 1.02 | % | 0.49 | % | |||
| Estimated volatility | 80 | % | 85 | % | |||
| Dividend | -0- | -0- | |||||
| Stock price at period end date | $ | 0.021 | $ | 0.035 | |||
Fair Value Assumptions Used in Accounting for Derivative Additional Investment Rights Liability
The Company has determined the derivative additional investment rights liability to be a Level 2 fair value measurement and has used the binominal lattice pricing model to measure the fair value. The fair value of the derivative liability associated with the additional investment rights was determined to be $719,088 and $1,256,160 at July 31, 2014 and 2013, respectively. The key inputs used in the fair value calculation at July 31, 2014 were as follows:
| July 31, 2014 | July 31, 2013 | ||||||
| Underlying number of units of convertible preferred stock | 2,075 | 1,225 | |||||
| Underlying number of warrants | 69,166,667 | 40,833,335 | |||||
| Current exercise price of warrants | $ | 0.03 | $ | 0.03 | |||
| Current conversion price of preferred stock | $ | 0.03 | $ | 0.03 | |||
| Time to expiration | 0.65 years | 0.88 years | |||||
| Risk-free interest rate | 0.09 | % | 0.11 | % | |||
| Estimated volatility | 61 | % | 114 | % | |||
| Dividend | -0- | -0- | |||||
| Stock price | $ | 0.021 | $ | 0.035 | |||
The revaluation of the additional investment rights in the fiscal year ended July 31, 2014 and 2013, resulted in the recognition of a gain of $1,163,242 and $8,523, respectively within the Company’s consolidated statements of operations, which is included in the totals under the caption “Change in fair value of derivative liabilities”.
| 58 |
Note 11 - Stockholders’ (Deficiency)/Equity:
Warrants
As of July 31, 2014, the Company has the following warrants to purchase common stock outstanding:
| Number of Shares to be Purchased | Warrant Exercise Price per Share | Warrant Expiration Date | |||
| 8,470,661 | $ | 0.76 | December 15, 2014 | ||
| 3,413,928 | $ | 0.79 | February 4, 2015 | ||
| 300,000 | $ | 0.39 | (average) | February 9, 2015 | |
| 200,000 | $ | 1.25 | March 7, 2015 | ||
| 5,157,813 | $ | 1.00 | March 15, 2015 | ||
| 64,516,758 | $ | 0.03 | March 31, 2016* | ||
| 27,272,720 | $ | 0.03 | September 30, 2016* | ||
| 5,675,227 | $ | 0.03 | February 1, 2017* | ||
| 4,999,999 | $ | 0.03 | August 10, 2017* | ||
| 8,324,144 | $ | 0.03 | December 12, 2017* | ||
| 34,166,669 | $ | 0.03 | June 17, 2018* | ||
| 25,666,668 | $ | 0.03 | January 15, 2019* | ||
| 69,166,667 | $ | 0.03 | March 27, 2019* | ||
| 257,331,254 |
* Subject to price protection provisions as described below.
The outstanding warrants at July 31, 2014 have a weighted average exercise price of $0.085 per share and have a weighted average remaining life of 3.1 years.
As of July 31, 2014, the Company has 239,788,852 warrants with a current exercise price of $0.03 which have price protection provisions that allow for the reduction in the current exercise price upon the occurrence of certain events, including the Company’s issuance of common stock or securities convertible into or exercisable for common stock, such as options and warrants, at a price per share less than the exercise price then in effect. For instance, if the Company issues shares of its common stock or options exercisable for or securities convertible into common stock at an effective price per share of common stock less than the exercise price then in effect, the exercise price will be reduced to the effective price of the new issuance. Simultaneously with any reduction to the exercise price, the number of shares of common stock that may be purchased upon exercise of each of these warrants shall be increased proportionately, so that after such adjustment the aggregate exercise price payable for the adjusted number of warrants shall be the same as the aggregate exercise price in effect immediately prior to such adjustment. There are a limited number of permitted types of stock and equity instrument issuances for each series of warrants which will not invoke the price protection provisions of these warrants.
The Company accounts for the warrants with price protection provisions in accordance with FASB ASC Topic 815 as described inNote 10 - Derivative Liabilitiesabove. As of July 31, 2014, there were a total of 239,788,852 warrants with an estimated fair value of $2,635,643, which are identified on the consolidated balance sheets under the caption “Derivative Warrant Liability”.
Preferred Stock
The Company has authorized 1,000,000 shares of preferred stock with a par value of one-tenth of a cent ($.001) per share. The preferred stock may be issued in various series and shall have preference as to dividends and to liquidation of the Company. The Company’s Board of Directors is authorized to establish the specific rights, preferences, voting privileges and restrictions of such preferred stock, or any series thereof. At July 31, 2014, 25 shares of the Company’s non-voting Series E 9% Convertible Preferred Stock and 1,225 shares of the Company’s non-voting Series F 9% Convertible Preferred Stock were issued and outstanding. At July 31, 2013, 930 shares of the Company’s non-voting Series E 9% Convertible Preferred Stock were issued and outstanding. SeeNote 9 - Series A, B, C, D, E and F 9% Convertible Preferred Stock above.
Equity Instruments Issued for Services Rendered
During the years ended July 31, 2014, 2013 and 2012, the Company issued stock options, warrants and shares of common stock in exchange for services rendered to the Company. The fair value of each stock option and warrant was valued using the Black Scholes pricing model which takes into account as of the grant date the exercise price and expected life of the stock option or warrant, the current price of the underlying stock and its expected volatility, expected dividends on the stock and the risk free interest rate for the term of the stock option or warrant. Shares of common stock are valued at the quoted market price on the date of grant. The fair value of each grant was charged to the related expense in the consolidated statement of operations for the services received (see Note 12).
| 59 |
Note 12 – Stock-Based Compensation:
Stock Option Plans
As of July 31, 2014, the Company had two stockholder-approved stock incentive plans under which shares and options exercisable for shares of common stock have been or may be granted to employees, directors, consultants and advisors. A total of 12,000,000 shares of common stock are reserved for issuance under the 2001 Stock Option Plan (the 2001 Plan) and 135,000,000 shares of common stock are reserved for issuance under the 2006 Stock Plan as amended (the 2006 Plan). At July 31, 2014, there were 1,353,916 and 81,300,576 shares of common stock reserved for future awards under the 2001 Plan and 2006 Plan, respectively. The Company issues new shares of common stock from the shares reserved under the respective Plans upon conversion or exercise of options and issuance of restricted shares.
The 2001 and 2006 Plans (the Plans) are administered by the Board of Directors (the Board). The Board is authorized to select from among eligible employees, directors, advisors and consultants those individuals to whom options are to be granted and to determine the number of shares to be subject to, and the terms and conditions of the options. The Board is also authorized to prescribe, amend and rescind terms relating to options granted under the Plans. Generally, the interpretation and construction of any provision of the Plans or any options granted hereunder is within the discretion of the Board.
The Plans provide that options may or may not be Incentive Stock Options (ISOs) within the meaning of Section 422 of the Internal Revenue Code. Only employees of the Company are eligible to receive ISOs, while employees and non-employee directors, advisors and consultants are eligible to receive options which are not ISOs, i.e. “Non-Qualified Options.” The options granted by the Board in connection with its adoption of the Plans were Non-Qualified Options. In addition, the 2006 Plan also provides for restricted stock grants.
Share-based employee compensation related to stock options for the years ended July 31, 2014, 2013 and 2012 amounted to $262,871, $837,753 and $602,384 for each year and were charged to the consolidated statements of operations. Share-based employee compensation related to common stock grants for the years ended July 31, 2014, 2013 and 2012 amounted to $130,000, $0 and $130,544, respectively, and were charged to the consolidated statements of operations.
The fair value of each option granted is estimated on the grant date using the Black-Scholes option pricing model or the value of the services provided, whichever is more readily determinable. The Black-Scholes option pricing model takes into account, as of the grant date, the exercise price and expected life of the option, the current price of the underlying stock and its expected volatility, expected dividends on the stock and the risk-free interest rate for the term of the option. The Black-Scholes option pricing model was not used to estimate the fair value any option grants in the fiscal years ended July 31, 2014, 2013 and 2012.
The following is a summary of the common stock options granted, forfeited or expired and exercised under the Plan:
| Options | Weighted Average Exercise Price per Share | ||||
| Outstanding - August 1, 2011 | 7,340,182 | $ | 0.46 | ||
| Granted | 5,851,696 | $ | 0.001 | ||
| Forfeited or expired | (912,250) | $ | 0.65 | ||
| Exercised | (1,299,994) | $ | 0.001 | ||
| Outstanding - July 31, 2012 | 10,979,634 | $ | 0.26 | ||
| Granted | 24,532,719 | $ | 0.001 | ||
| Forfeited or expired | (630,778) | $ | 0.75 | ||
| Exercised | (5,180,378) | $ | 0.001 | ||
| Outstanding - July 31, 2013 | 29,701,197 | $ | 0.08 | ||
Granted | 8,879,499 | $ | 0.001 | ||
| Forfeited or expired | (90,000) | $ | 0.53 | ||
| Exercised | (526,306) | $ | 0.001 | ||
| Outstanding - July 31, 2013 | 37,964,390 | $ | 0.06 | ||
| Exercisable - July 31, 2014 | 37,964,390 | $ | 0.06 |
The 37,964,390 outstanding options at July 31, 2014 had a weighted average remaining contractual term of 3.45 years.
Options typically vest over a period of two to four years and have a contractual life of five to ten years.
The following is a summary of the non-vested common stock options granted, vested and forfeited under the Plan:
| Options | Weighted Average Grant Date Fair Value | ||||
| Outstanding - August 1, 2011 | 60,000 | $ | 0.46 | ||
| Granted | 8,879,499 | $ | 0.029 | ||
| Vested | (8,939,499) | $ | 0.031 | ||
| Outstanding - July 31, 2014 | -0- | n/a |
As of July 31, 2014, the Company did not have any unrecognized compensation cost related to non-vested share-based compensation arrangements granted under the Plan.
| 60 |
During the twelve months ended July 31, 2014, the Company granted 8,879,499 options to executives, employees and directors in full and final payment of obligations to pay such individuals deferred salary or director fees. The options were issued in lieu of cash payment of deferred compensation amounts due to such individuals. The number of options granted to each individual was equal to the dollar amount of deferred salary or fees due to such individual divided by the closing price of the Company's common stock on October 31, 2013 ($0.03). The stock options had an exercise price equal to $0.001 per share and were made pursuant to the terms of the Company's 2006 Stock Plan. The options were fully vested at the dates of the grants and expire on the fifth anniversary of the respective dates of grant. The grants were valued at the amount of deferred compensation owed to each such individual.
During the twelve months ended July 31, 2013, the Company granted 24,532,719 options to executives, employees and directors in full and final payment of obligations to pay such individuals deferred salary or director fees. The options were issued in lieu of cash payment of deferred compensation amounts due to such individuals. The number of options granted to each individual was equal to the dollar amount of deferred salary or fees due to such individual divided by the closing price of the Company's common stock on February 5, 2013 ($0.038), March 5, 2013 ($0.034) and June 4, 2013 ($0.0325). The stock options had an exercise price equal to $0.001 per share and were made pursuant to the terms of the Company's 2006 Stock Plan. The options were fully vested at the dates of the grants and expire on the fifth anniversary of the respective dates of grant. The grants were valued at the amount of deferred compensation owed to each such individual.
During the twelve months ended July 31, 2012, the Company granted 5,851,696 options to executives, employees and directors in full and final payment of obligations to pay such individuals deferred salary or director fees. The options were issued in lieu of cash payment of deferred compensation amounts due to such individuals. The number of options granted to each individual was equal to the dollar amount of deferred salary or fees due to such individual divided by the closing price of the Company's common stock on June 6, 2012 ($0.0925). The stock options had an exercise price equal to $0.001 per share and were made pursuant to the terms of the Company's 2006 Stock Plan. The options were fully vested at the date of grant and expire on the fifth anniversary of the date of grant. The grants were valued at the amount of deferred compensation owed to each such individual.
The following table summarizes information on stock options outstanding at July 31, 2014:
| Options Outstanding and Options Exercisable | ||||||||||
| Range of Exercise Price | Number Outstanding at July 31, 2014 | Weighted Average Exercise Price | Weighted Average Remaining Life (Years) | Aggregate Intrinsic Value | ||||||
| $ 0.001 | 32,799,390 | $ | 0.001 | 3.68 | ||||||
| $ 0.19 - $ 0.38 | 3,100,000 | $ | 0.285 | 1.63 | ||||||
| $ 0.39 - $ 0.58 | 200,000 | $ | 0.560 | 0.24 | ||||||
| $ 0.59 - $ 0.77 | 1,470,000 | $ | 0.633 | 3.34 | ||||||
| $ 0.78 - $ 0.96 | 395,000 | $ | 0.940 | 0.24 | ||||||
| 37,964,390 | $ | 0.061 | 3.45 | $ | 655,988 | |||||
| For the Year Ended July 31, | |||||||||
| 2014 | 2013 | 2012 | |||||||
| Weighted Average Grant Date Fair Value of Options Granted | $ | 0.029 | $ | 0.003 | $ | 0.09 | |||
| Aggregate Intrinsic Value of Options Exercised | $ | 21,052 | $ | 178,040 | $ | 119,214 | |||
| Cash Received for Exercise of Stock Options | $ | 526 | $ | 5,180 | $ | 1,299 | |||
The intrinsic value is calculated as the difference between the market value as of July 31, 2014, 2013 and 2012 and the exercise price of the shares on the respective dates. The market values as of July 31, 2014, 2013 and 2012 were $0.021, $0.035 and $0.093, respectively, based on the high and low bid information for July 31, 2014, 2013 and 2012.
| 61 |
Note 13 - Net Loss per Share:
Basic loss per share (“EPS”) and Diluted EPS for the years ended July 31, 2014, 2013 and 2012 have been computed by dividing the net loss available to common stockholders for each respective period by the weighted average shares outstanding during that period. All outstanding options, warrants, non-vested restricted stock and shares to be issued upon conversion of the outstanding convertible preferred stock, representing approximately 336,962,311, 298,931,138 and 94,643,712 incremental shares, have been excluded from the respective 2014, 2013 and 2012 computation of diluted EPS as they are anti-dilutive due to the losses generated during the respective years.
Note 14 - Segment Information:
The Company follows FASB ASC Topic 815 which establishes standards for the way that public business enterprises report information about operating segments in annual financial statements and requires that those enterprises report selected information about operating segments in interim financial reports. This Topic also establishes standards for related disclosures about products and services, geographic areas, and major customers.
This Topic uses a management approach for determining segments. The management approach designates the internal organization that is used by management for making operating decisions and assessing performance as the source of the Company’s reportable segments. The Company’s management reporting structure provides for only one segment: the research, development and commercialization of drug delivery systems and technologies for metabolic and immunological diseases.
The countries in which the Company had identifiable assets are presented in the following table. Identifiable assets are those that can be directly associated with a geographic area.
| 2014 | 2013 | |||||
| Identifiable Assets | ||||||
| Canada | $ | 3,719,760 | $ | 2,825,894 | ||
| United States | 1,802,697 | 2,026,969 | ||||
| Total | $ | 5,522,457 | $ | 4,852,863 |
Note 15 – Quarterly Information (Unaudited):
The following schedule sets forth certain unaudited financial data for the preceding eight quarters ending July 31, 2014. In our opinion, the unaudited information set forth below has been prepared on the same basis as the audited information and includes all adjustments necessary to present fairly the information set forth herein. The operating results for the quarter are not indicative of results for any future period.
| Q1 | Q2 | Q3 | Q4 | |||||||||||||
| Fiscal Year July 31, 2014: | ||||||||||||||||
| Revenues, net | $ | -0- | $ | -0- | $ | -0- | $ | -0- | ||||||||
| Operating Loss | $ | (1,443,051 | ) | $ | (1,010,861 | ) | $ | (1,242,849 | ) | $ | (702,918 | ) | ||||
| Net Income/(Loss) | $ | 530,094 | $ | (3,186,290 | ) | $ | 270,923 | $ | 2,383,856 | |||||||
| Net Loss available to common stockholders | $ | 530,094 | $ | (3,594,569 | ) | $ | (1,149,127 | ) | $ | 2,153,856 | ||||||
| Net Loss per share | $ | 0.001 | $ | (0.010 | ) | $ | (0.002 | ) | $ | 0.003 | ||||||
Fiscal Year July 31, 2013: | ||||||||||||||||
| Revenues, net | $ | -0- | $ | -0- | $ | -0- | $ | -0- | ||||||||
| Operating Loss | $ | (1,940,031 | ) | $ | (1,290,780 | ) | $ | (1,433,640 | ) | $ | (1,464,908 | ) | ||||
| Net Income/(Loss) | $ | (656,199 | ) | $ | (5,640,985 | ) | $ | 1,297,448 | $ | (3,554,586 | ) | |||||
| Net Loss available to common stockholders | $ | (758,496 | ) | $ | (5,640,985 | ) | $ | 1,297,448 | $ | (5,174,763 | ) | |||||
| Net Loss per share | $ | 0.002 | $ | (0.015 | ) | $ | 0.003 | $ | (0.007 | ) |
Note 16 - Subsequent Events:
The Company has evaluated subsequent events occurring after the balance sheet date through the date the consolidated financial statements were issued.
| 62 |
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Prior to the filing of this Report on Form 10-K, an evaluation was performed under the supervision of and with the participation of the Company’s management, including the Chief Executive Officer (“CEO”) and Chief Financial Officer (“CFO”), of the effectiveness of the Company’s disclosure controls and procedures. Based on the evaluation, the CEO and CFO have concluded that, as of July 31, 2014, the Company’s disclosure controls and procedures are effective to ensure that information required to be disclosed by the Company in reports that it files or submits under the Exchange Act, is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms, and is accumulated and communicated to the Company’s management, as appropriate, to allow timely decisions regarding required disclosure.
Changes in Internal Control over Financial Reporting
During the fiscal quarter ended July 31, 2014, there were no changes in the Company’s internal controls over financial reporting that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
MANAGEMENT’S REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
The management of Generex Biotechnology Corporation (the “Company”) is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended. The Company’s internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. The Company’s internal control over financial reporting includes those policies and procedures that:
| (i) | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company; |
| (ii) | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and |
| (iii) | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements.
|
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
The Company’s management assessed the effectiveness of the Company’s internal control over financial reporting as of July 31, 2014. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework. Based on management’s assessment using those criteria, management has concluded that the Company’s internal control over financial reporting was effective as of July 31, 2014.
As of July 31, 2014, the Company is a smaller reporting company. As a smaller reporting company under the SEC rules and regulations, we are currently not subject to the requirements of independent auditor attestation of management’s assessment of our internal controls over financial reporting set forth in Section 404(b) of the Sarbanes Oxley Act of 2002 because the Dodd Frank Wall Street Reform and Consumer Protection Act signed into law on July 21, 2010 permanently exempted companies that are not “accelerated filers” or “large accelerated filers” under the SEC rules from Section 404(b) requirements; therefore, this Annual Report does not include an attestation report of the Company’s registered public accounting firm regarding internal control over financial reporting.
Item 9B. Other Information.
Reference is made to the disclosure set forth under the captionSales of Unregistered Securities in Item 5 of this Annual Report on Form 10-K, which is incorporated by reference herein.
| 63 |
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
The information required by this Item is incorporated by reference from the Proxy Statement, or an amendment to this Annual Report on Form 10-K, to be filed with the Commission not later than 120 days after the end of the fiscal year to which this report relates.
Information with respect to Executive Officers of the Company appears inPart I of this Annual Report on Form 10-K.
Generex has adopted a code of ethics that applies to its directors and the following executive officers: the President, Chief Executive Officer, Chief Financial Officer (principal financial/accounting officer), Chief Operating Officer, any Vice-President, Controller, Secretary, Treasurer and any other personnel performing similar functions. We also expect any consultants or advisors whom we retain to abide by this code of ethics. The Generex Code of Ethics has been posted on Generex's Internet web site - www.generex.com.
Item 11. Executive Compensation.
The information required by this Item is incorporated by reference from the Proxy Statement, or an amendment to this Annual Report on Form 10-K, to be filed with the Commission not later than 120 days after the end of the fiscal year to which this report relates.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The information required by this Item is incorporated by reference from the Proxy Statement, or an amendment to this Annual Report on Form 10-K, to be filed with the Commission not later than 120 days after the end of the fiscal year to which this report relates.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
The information required by this Item is incorporated by reference from the Proxy Statement, or an amendment to this Annual Report on Form 10-K, to be filed with the Commission not later than 120 days after the end of the fiscal year to which this report relates.
Item 14. Principal Accounting Fees and Services.
The information required by this Item is incorporated by reference from the Proxy Statement, or an amendment to this Annual Report on Form 10-K, to be filed with the Commission not later than 120 days after the end of the fiscal year to which this report relates.
PART IV
Item. 15. Exhibits and Financial Statements and Schedules.
| 1. | Financial Statement - SeePart II - Item 8. Financial Statements and Supplementary Data hereof on page 39. |
The financial statements include the following:
Consolidated Balance Sheets as of July 31, 2014 and 2013
Consolidated Statements of Operations for the Years Ended July 31, 2014, 2013 and 2012
Consolidated Statements of Changes in Stockholders’ Deficiency for the for the Years Ended July 31, 2014, 2013 and 2012
Consolidated Statements of Cash Flows for the Years Ended July 31, 2014, 2013 and 2012
| 2. | Financial Statement Schedule and Auditor’s Report |
Schedule I - Condensed financial information of registrant
This schedule is not applicable.
| 64 |
Schedule II - Valuation and qualifying accounts
Balance at Beginning Of Period | Additions Charged To Expenses | Other Additions | Deductions | Balance at End of Period | ||||||||||||
| Year Ended July 31, 2014 Valuation Allowance on Deferred Tax Asset | $ | 88,098,860 | - | 3,006,413 | - | 91,105,273 | ||||||||||
| Year Ended July 31, 2013 Valuation Allowance on Deferred Tax Asset | $ | 85,579,584 | - | 2,519,276 | - | 88,098,860 | ||||||||||
The auditors’ report of MNP LLP with respect to the Financial Statement Schedule information for the years ended July 31, 2014 is included with its report on our financial statements located at page 39.
| 3. | Exhibits |
Exhibits are incorporated herein by reference or are filed with this Annual Report as set forth in the Exhibit Index beginning on page 66 hereof.
All other schedules and exhibits are omitted because they are not applicable, not required, or because the information required has been given as part of this report.
Signatures
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized this 3rd day of October 2014.
| GENEREX BIOTECHNOLOGY CORPORATION | ||
| By: | /s/ Mark A. Fletcher | |
| Name: Mark A. Fletcher | ||
| Title: President and Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Name | Capacity in Which Signed | Date | ||
| /s/ Mark A. Fletcher | President and Chief Executive Officer and General | October 3, 2014 | ||
| Mark A. Fletcher | Counsel and Secretary (Principal Executive Officer) | |||
| /s/ Stephen Fellows | Chief Financial Officer | October 3, 2014 | ||
| Stephen Fellows | (Principal Financial and Accounting Officer) | |||
| /s/ Brian T. McGee | Director | October 3, 2014 | ||
| Brian T. McGee | ||||
| /s/ John P. Barratt | Director | October 3, 2014 | ||
| John P. Barratt | ||||
| /s/ James Anderson | Director | October 3, 2014 | ||
| James Anderson | ||||
| /s/ Eric von Hofe | Director | October 3, 2014 | ||
| Eric von Hofe |
| 65 |
EXHIBIT INDEX
Exhibit Number | Description of Exhibit(1) | |
| 1.1 | Placement Agency Agreement, dated May 5, 2009, by and between Generex Biotechnology Corporation and Rodman & Renshaw (incorporated by reference to Exhibit 1.1 to Generex Biotechnology Corporation’s Current Report on Form 8-K filed on May 18, 2009) | |
| 1.2 | Placement Agency Agreement, dated June 8, 2009, by and between Generex Biotechnology Corporation and Midtown Partners & Co., LLC and amendments dated August 5, August 18, and September 11, 2009 (incorporated by reference to Exhibit 1.1 to Generex Biotechnology Corporation’s Current Report on Form 8-K filed on September 15, 2009) | |
| 1.3 | Amendment dated as of April 7, 2010 to Placement Agent Agreement attached as Exhibit 1.2 hereto (incorporated by reference .reference to Exhibit 1.2 to Generex Biotechnology Corporation’s Current Report on Form 8-K filed on April 8, 2010) | |
| 1.4 | Placement Agency Agreement dated September 11, 2009, by and between Generex Biotechnology Corporation and Maxim Group LLC. (incorporated by reference to Exhibit 1.2 to Generex Biotechnology Corporation’s Current Report on Form 8-K filed on September 15, 2009) | |
| 2 | Agreement and Plan of Merger among Generex Biotechnology Corporation, Antigen Express, Inc. and AGEXP Acquisition Inc. (incorporated by reference to Exhibit 2.1 to Generex Biotechnology Corporation’s Current Report on Form 8-K filed on August 15, 2003) | |
| 3(i)(a) | Restated Certificate of Incorporation of Generex Biotechnology Corporation (incorporated by reference to Exhibit 4.1 to Generex Biotechnology Corporation’s Post-Effective Amendment No. 1 to the Registration Statement on Form S-8 filed on October 26, 2009) | |
3(i)(b)
3(i)(c)
| Certificate of Designation of Preferences, Rights and Limitations of Series A 9% Convertible Preferred Stock (incorporated by reference to Exhibit 3.1 to Generex Biotechnology Corporation’s Current Report on Form 8-K filed on July 11, 2011).
Certificate of Designation of Preferences, Rights and Limitations of Series B 9% Convertible Preferred Stock (incorporated by reference to Exhibit 3.1 to Generex Biotechnology Corporation’s Current Report on form 8-K filed on February 1, 2012).
| |
| 3(i)(d) | Certificate of Designation of Preferences, Rights and Limitations of Series C 9% Convertible Preferred Stock (incorporated by reference to Exhibit 3.1 to Generex Biotechnology Corporation’s Current Report on Form 8-K filed on August 8, 2012).
| |
3(i)(e)
| Certificate of Designation of Preferences, Rights and Limitations of Series D 9% Convertible Preferred Stock (incorporated by reference to Exhibit 3.1 to Generex Biotechnology Corporation’s Current Report on form 8-K filed on December 11, 2012)
| |
3(i)(f)
| Certificate of Amendment to Restated Certificate of Incorporation of Generex Biotechnology Corporation (incorporated by reference to Exhibit 3(i)(f) to Generex Biotechnology Corporation’s Current Report on Registration Statement on Form S-1 (File No. 333-187656) filed on April 1, 2013)
| |
| 3(i)(g) | Certificate of Designation of Preferences, Rights and Limitations of Series E 9% Convertible Preferred Stock (incorporated by reference to Exhibit 3.1 to Generex Biotechnology Corporation’s Current Report on form 8-K filed on June 17, 2013)
| |
| 3(i)(h) | Certificate of Designation of Preferences, Rights and Limitations of Series F 9% Convertible Preferred Stock (incorporated by reference to Exhibit 3.1 to Generex Biotechnology Corporation’s Current Report on form 8-K filed on March 28, 2014) | |
3(ii) |
Amended and Restated By-Laws of Generex Biotechnology Corporation (incorporated by reference to Exhibit 3.2(ii) to Generex Biotechnology Corporation’s Report on Form 8-K filed December 5, 2007) | |
| 4.1 | Form of Common Stock Certificate (incorporated by reference to Exhibit 4.1 to Generex Biotechnology Corporation’s Registration Statement on Form S-1 (File No. 333-82667) filed on July 12, 1999) | |
| 66 |
| 4.2.1 | Form of Securities Purchase Agreement entered into with Cranshire Capital, L.P.; Gryphon Partners, L.P.; Langley Partners, L.P.; Lakeshore Capital, Ltd.; LH Financial; Omicron Capital; Photon Fund, Ltd.; Howard Todd Horberg and Vertical Ventures, LLC dated May 29, 2003 (incorporated by reference to Exhibit 4.1 to Generex Biotechnology Corporation’s Report on Form 10-Q/A for the quarter ended April 30, 2003 filed on August 13, 2003) | |
| 4.2.2 | Form of Registration Rights Agreement entered into with Cranshire Capital, L.P.; Gryphon Partners, L.P.; Langley Partners, L.P.; Lakeshore Capital, Ltd.; LH Financial; Omicron Capital; Photon Fund, Ltd.; Howard Todd Horberg and Vertical Ventures, LLC dated May 29, 2003 (incorporated by reference to Exhibit 4.2 to Generex Biotechnology Corporation’s Report on Form 10-Q/A for the quarter ended April 30, 2003 filed on August 13, 2003) | |
| 4.2.3 | Form of Warrant granted to Cranshire Capital, L.P.; Gryphon Partners, L.P.; Langley Partners, L.P.; Lakeshore Capital, Ltd.; LH Financial; Omicron Capital; Photon Fund, Ltd.; Howard Todd Horberg and Vertical Ventures, LLC dated May 29, 2003 (incorporated by reference to Exhibit 4.3 to Generex Biotechnology Corporation’s Report on Form 10-Q/A for the quarter ended April 30, 2003 filed on August 13, 2003) | |
| 4.3 | Form of replacement Warrant issued to warrant holders exercising at reduced exercise price in May and June 2003 (incorporated by reference to Exhibit 4.13.7 to Generex Biotechnology Corporation’s Report on Form 10-K for the period ended July 31, 2003 filed on October 29, 2003) | |
| 4.4.1 | Securities Purchase Agreement, dated December 19, 2003, by and among Generex Biotechnology Corporation and the investors named therein (incorporated by reference to Exhibit 4.1 to Generex Biotechnology Corporation’s Report on Form 8-K/A filed on March 24, 2004) | |
| 4.4.2 | Registration Rights Agreement, dated December 19, 2003, by and among Generex Biotechnology Corporation and the investors named therein (incorporated by reference to Exhibit 4.2 to Generex Biotechnology Corporation’s Report on Form 8-K/A filed on March 24, 2004) | |
| 4.4.3 | Form of Warrant issued in connection with Exhibit 4.4.1 (incorporated by reference to Exhibit 4.3 to Generex Biotechnology Corporation’s Report on Form 8-K/A filed on March 24, 2004) | |
| 4.4.4 | Form of Additional Investment Right issued in connection with Exhibit 4.4.1 (incorporated by reference to Exhibit 4.4 to Generex Biotechnology Corporation’s Report on Form 8-K/A filed on March 24, 2004) | |
| 4.5.1 | Securities Purchase Agreement, dated January 7, 2004, by and between Generex Biotechnology Corporation and ICN Capital Limited (incorporated by reference to Exhibit 4.1 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 1, 2004) | |
| 4.5.2 | Registration Rights Agreement, dated January 7, 2004, by and between Generex Biotechnology Corporation and ICN Capital Limited (incorporated by reference to Exhibit 4.2 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 1, 2004) | |
| 4.5.3 | Warrant issued in connection with Exhibit 4.5.1 (incorporated by reference to Exhibit 4.3 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 1, 2004) | |
| 4.5.4 | Additional Investment Right issued in connection with Exhibit 4.5.1 (incorporated by reference to Exhibit 4.4 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 1, 2004) | |
| 4.6.1 | Securities Purchase Agreement, dated January 9, 2004, by and between Generex Biotechnology Corporation and Vertical Ventures, LLC (incorporated by reference to Exhibit 4.5 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 1, 2004) | |
| 4.6.2 | Registration Rights Agreement, dated January 9, 2004, by and between Generex Biotechnology Corporation and Vertical Ventures, LLC (incorporated by reference to Exhibit 4.6 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 1, 2004) | |
| 4.6.3 | Warrant issued in connection with Exhibit 4.6.1 (incorporated by reference to Exhibit 4.7 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 1, 2004) | |
| 4.6.4 | Additional Investment Right issued in connection with Exhibit 4.6.1 (incorporated by reference to Exhibit 4.8 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 1, 2004) | |
| 67 |
| 4.7.1 | Securities Purchase Agreement, dated February 6, 2004, by and between Generex Biotechnology Corporation and Alexandra Global Master Fund, Ltd. (incorporated by reference to Exhibit 4.9 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 1, 2004) | |
| 4.7.2 | Registration Rights Agreement, dated February 6, 2004, by and between Generex Biotechnology Corporation and Alexandra Global Master Fund, Ltd. (incorporated by reference to Exhibit 4.10 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 1, 2004) | |
| 4.7.3 | Warrant issued in connection with Exhibit 4.7.1 (incorporated by reference to Exhibit 4.11 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 1, 2004) | |
| 4.7.4 | Additional Investment Right issued in connection with Exhibit 4.7.1 (incorporated by reference to Exhibit 4.12 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 1, 2004) | |
| 4.7.5 | Escrow Agreement, dated February 26, 2004, by and among Generex Biotechnology Corporation, Eckert Seamans Cherin & Mellott, LLC and Alexandra Global Master Fund, Ltd. (incorporated by reference to Exhibit 4.13 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 1, 2004) | |
| 4.8.1 | Securities Purchase Agreement, dated February 11, 2004, by and between Generex Biotechnology Corporation and Michael Sourlis (incorporated by reference to Exhibit 4.14 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 1, 2004) | |
| 4.8.2 | Registration Rights Agreement, dated February 11, 2004, by and between Generex Biotechnology Corporation and Michael Sourlis (incorporated by reference to Exhibit 4.15 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 1, 2004) | |
| 4.8.3 | Additional Investment Right issued in connection with Exhibit 4.8.1 (incorporated by reference to Exhibit 4.17 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 1, 2004) | |
| 4.9.1 | Securities Purchase Agreement, dated February 13, 2004, by and between Generex Biotechnology Corporation and Zapfe Holdings, Inc. (incorporated by reference to Exhibit 4.18 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 1, 2004) | |
| 4.9.2 | Registration Rights Agreement, dated February 13, 2004, by and between Generex Biotechnology Corporation and Zapfe Holdings, Inc. (incorporated by reference to Exhibit 4.19 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 1, 2004) | |
| 4.9.3 | Warrant issued in connection with Exhibit 4.9.1 (incorporated by reference to Exhibit 4.20 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 1, 2004) | |
| 4.9.4 | Additional Investment Right issued in connection with Exhibit 4.9.1 (incorporated by reference to Exhibit 4.21 Generex Biotechnology Corporation’s Report on Form 8-K filed on March 1, 2004) | |
| 4.10.1 | Securities Purchase Agreement, dated June 23, 2004, by and among Generex Biotechnology Corporation and the investors named therein (incorporated by reference to Exhibit 4.1 to Generex Biotechnology Corporation’s Report on Form 8-K filed on July 14, 2004) | |
| 4.10.2 | Registration Rights Agreement, dated June 23, 2004, by and among Generex Biotechnology Corporation and the investors (incorporated by reference to Exhibit 4.2 to Generex Biotechnology Corporation’s Report on Form 8-K filed on July 14, 2004) | |
| 4.10.3 | Form of Warrant issued in connection with Exhibit 4.10.1 (incorporated by reference to Exhibit 4.3 to Generex Biotechnology Corporation’s Report on Form 8-K filed on July 14, 2004) | |
| 4.10.4 | Form of Additional Investment Right issued in connection Exhibit 4.10.1 (incorporated by reference to Exhibit 4.4 to Generex Biotechnology Corporation’s Report on Form 8-K filed on July 14, 2004) | |
| 4.11.1 | Securities Purchase Agreement, dated November 10, 2004, by and among Generex Biotechnology Corporation and the investors named therein (incorporated by reference to Exhibit 4.1 to Generex Biotechnology Corporation’s Report on Form 8-K filed on November 12, 2004) | |
| 4.11.2 | Form of 6% Secured Convertible Debenture issued in connection with Exhibit 4.11.1 (incorporated by reference to Exhibit 4.2 to Generex Biotechnology Corporation’s Report on Form 8-K filed on November 12, 2004) | |
| 68 |
| 4.11.3 | Registration Rights Agreement, dated November 10, 2004, by and among Generex Biotechnology Corporation and the investors named therein (incorporated by reference to Exhibit 4.3 to Generex Biotechnology Corporation’s Report on Form 8-K filed on November 12, 2004) | |
| 4.11.4 | Form of Voting Agreement entered into in connection with Exhibit 4.11.1 (incorporated by reference to Exhibit 4.7 to Generex Biotechnology Corporation’s Report on Form 8-K filed on November 12, 2004) | |
| 4.12 | Warrant issued to The Aethena Group, LLC on April 28, 2005 (incorporated by reference to Exhibit 4.20 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on June 14, 2005) | |
| 4.13.1 | Amendment No. 4 to Securities Purchase Agreement and Registration Rights Agreement entered into by and between Generex Biotechnology Corporation and the Purchasers listed on the signature pages thereto on January 19, 2006 (incorporated by reference herein to Exhibit 4.1 to Generex Biotechnology Corporation’s Report on Form 8-K filed on January 20, 2006) | |
| 4.13.2 | Form of Additional AIRs issued in connection with Exhibit 4.13.1 (incorporated by reference herein to Exhibit 4.4 to Generex Biotechnology Corporation’s Report on Form 8-K filed on January 20, 2006) | |
| 4.14 | Form of Warrant issued by Generex Biotechnology Corporation on January 23, 2006 (incorporated by reference to Exhibit 4.2 to Generex Biotechnology Corporation’s Report on Form 8-K filed on January 24, 2006) | |
| 4.15.1 | Agreement to Amend Warrants between Generex Biotechnology Corporation and Cranshire Capital L.P. dated February 27, 2006 (incorporated by reference to Exhibit 4.1 to Generex Biotechnology Corporation’s Report on Form 8-K filed on February 28, 2006). | |
| 4.15.2 | Agreement to Amend Warrants between Generex Biotechnology Corporation and Omicron Master Trust dated February 27, 2006 (incorporated by reference to Exhibit 4.2 to Generex Biotechnology Corporation’s Report on Form 8-K filed on February 28, 2006) | |
| 4.15.3 | Agreement to Amend Warrants between Generex Biotechnology Corporation and Iroquois Capital L.P. dated February 27, 2006 (incorporated by reference to Exhibit 4.3 to Generex Biotechnology Corporation’s Report on Form 8-K filed on February 28, 2006) | |
| 4.15.4 | Agreement to Amend Warrants between Generex Biotechnology Corporation and Smithfield Fiduciary LLC dated February 27, 2006 (incorporated by reference to Exhibit 4.4 to Generex Biotechnology Corporation’s Report on Form 8-K filed on February 28, 2006) | |
| 4.15.5 | Form of Warrant issued by Generex Biotechnology Corporation on February 27, 2006 (incorporated by reference to Exhibit 4.26 to Generex Biotechnology Corporation’s Report on Form 10-K filed on October 16, 2006) | |
| 4.16.1 | Agreement to Amend Additional Investment Right between Generex Biotechnology Corporation and Cranshire Capital, L.P. dated February 28, 2006 (incorporated by reference to Exhibit 4.1 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 1, 2006) | |
| 4.16.2 | Agreement to Amend Additional Investment Right between Generex Biotechnology Corporation and Omicron Master Trust dated February 28, 2006 (incorporated by reference to Exhibit 4.2 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 1, 2006) | |
| 4.16.3 | Agreement to Amend Additional Investment Right between Generex Biotechnology Corporation and Iroquois Capital LP dated February 28, 2006 (incorporated by reference to Exhibit 4.3 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 1, 2006) | |
| 4.16.4 | Agreement to Amend Additional Investment Right between Generex Biotechnology Corporation and Smithfield Fiduciary LLC dated February 28, 2006 (incorporated by reference to Exhibit 4.4 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 1, 2006) | |
| 4.16.5 | Form of Additional AIR Debenture issued by Generex Biotechnology Corporation on February 28, 2006 (incorporated by reference to Exhibit 4.31 to Generex Biotechnology Corporation’s Report on Form 10-K filed on October 16, 2006) | |
| 4.16.6 | Form of Additional AIR Warrant issued by Generex Biotechnology Corporation on February 28, 2006 (incorporated by reference to Exhibit 4.32 to Generex Biotechnology Corporation’s Report on Form 10-K filed on October 16, 2006) | |
| 69 |
| 4.17.1 | Form of Agreement to Amend Warrants between Generex Biotechnology Corporation and the Investors dated March 6, 2006 (incorporated by reference to Exhibit 4.1 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 7, 2006) | |
| 4.17.2 | Form of Warrant issued by Generex Biotechnology Corporation on March 6, 2006 (incorporated by reference to Exhibit 4.2 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 7, 2006) | |
| 4.18 | Warrant issued by Generex Biotechnology Corporation on April 17, 2006 to Zapfe Holdings, Inc. (incorporated by reference to Exhibit 4.33 to Generex Biotechnology Corporation’s Report on Form 10-Q filed on June 14, 2006) | |
| 4.19 | Form of Warrant issued by Generex Biotechnology Corporation on April 17, 2006 to certain employees (incorporated by reference to Exhibit 4.34 to Generex Biotechnology Corporation’s Report on Form 10-Q filed on June 14, 2006) | |
| 4.20.1 | Securities Purchase Agreement entered into by and between Generex Biotechnology Corporation and four Investors on June 1, 2006 (incorporated by reference to Exhibit 4.1 to Generex Biotechnology Corporation’s Report on Form 8-K filed on June 2, 2006) | |
| 4.20.2 | Form of Warrant issued by Generex Biotechnology Corporation on June 1, 2006 (incorporated by reference to Exhibit 4.2 to Generex Biotechnology Corporation’s Report on Form 8-K filed on June 2, 2006) | |
| 4.21.1 | Form of Amendment to Outstanding Warrants (incorporated by reference to Exhibit 4.3 to Generex Biotechnology Corporation’s Report on Form 8-K filed on June 2, 2006) | |
| 4.21.2 | Form of Warrant issued by Generex Biotechnology Corporation on June 1, 2006 (incorporated by reference to Exhibit 4.4 to Generex Biotechnology Corporation’s Report on Form 8-K filed on June 2, 2006) | |
| 4.22.1 | Securities Purchase Agreement, dated as of March 31, 2008 among the Registrant and each of the purchasers named therein (incorporated by reference to Exhibit 4.1 to Generex Biotechnology Corporation’s Report on Form 8-K filed on April 2, 2008) | |
| 4.22.2 | Form of 8% Secured Convertible Note, as amended (incorporated by reference to Exhibit 4.2 to Generex Biotechnology Corporation’s Registration Statement (333-150562) on Form S-3 filed on April 30, 2008) | |
| 4.22.3 | Form of Series A Warrant, as amended (incorporated by reference to Exhibit 4.3 to Generex Biotechnology Corporation’s Registration Statement on Form S-3 (333-150562) filed on April 30, 2008) | |
| 4.22.4 | Form of Series A-1 Warrant, as amended (incorporated by reference to Exhibit 4.4 to Generex Biotechnology Corporation’s Registration Statement on Form S-3 (333-150562) filed on April 30, 2008) | |
| 4.22.5 | Form of Series B Warrant, as amended (incorporated by reference to Exhibit 4.5 to Generex Biotechnology Corporation’s Registration Statement on Form S-3 (333-150562) filed on April 30, 2008) | |
| 4.22.6 | Form of Series C Warrant, as amended (incorporated by reference to Exhibit 4.6 to Generex Biotechnology Corporation’s Registration Statement on Form S-3 (333-150562) filed on April 30, 2008) | |
| 4.22.7 | Registration Rights Agreement, dated March 31, 2008, among Registrant and each of the purchasers under Securities Purchase Agreement (incorporated by reference to Exhibit 4.7 to Generex Biotechnology Corporation’s Report on Form 8-K filed on April 2, 2008) | |
| 4.22.8 | Security Agreement (incorporated by reference to Exhibit 4.8 to Generex Biotechnology Corporation’s Report on Form 8-K filed on April 2, 2008) | |
| 4.22.9 | Form of Guaranty (incorporated by reference to Exhibit 4.9 to Generex Biotechnology Corporation’s Report on Form 8-K filed on April 2, 2008) | |
| 4.23 | Form of Securities Purchase Agreement, date May 15, 2009, entered into between Generex Biotechnology Corporation and each investor in the offering (incorporated by reference to Exhibit 1.2 to Generex Biotechnology Corporation’s Report on Form 8-K filed on May 18, 2009) | |
| 4.24.1 | Form of Securities Purchase Agreement, dated June 15, 2009, entered into between Generex Biotechnology Corporation and each investor in the offering (incorporated by reference to Exhibit 10.1 to Generex Biotechnology Corporation’s Report on Form 8-K filed on June 16, 2009) | |
| 4.24.2 | Form of Warrant issued in connection with Exhibit 4.24.1 (incorporated by reference to Exhibit 4.1 to Generex Biotechnology Corporation’s Report on Form 8-K filed on June 16, 2009) | |
| 4.24.3 | Form of Warrant issued to Midtown Partners & Co., LLC in connection with Exhibit 4.24.1 (incorporated by reference to Exhibit 4.2 to Generex Biotechnology Corporation’s Report on Form 8-K filed on June 16, 2009)
| |
| 4.25.1 | Form of Securities Purchase Agreement, dated August 6, 2009, entered into between Generex Biotechnology Corporation and each investor in the offering (incorporated by reference to Exhibit 10.1 to Generex Biotechnology Corporation’s Report on Form 8-K filed on August 6, 2009)
| |
| 4.25.2 | Form of Warrant issued in connection with Exhibit 4.25.1 (incorporated by reference to Exhibit 4.1 to Generex Biotechnology Corporation’s Report on Form 8-K filed on August 6, 2009)
| |
| 4.25.3 | Form of Warrant issued to Midtown Partners & Co., LLC in connection with Exhibit 4.25.1 (incorporated by reference to Exhibit 4.28 to Generex Biotechnology Corporation’s Report on Form 8-K filed on August 6, 2009)
| |
| 4.26.1 | Form of Securities Purchase Agreement, dated September 11, 2009, entered into between Generex Biotechnology Corporation and each investor in the offering (incorporated by reference to Exhibit 10.1 to Generex Biotechnology Corporation’s Report on Form 8-K filed on September 15, 2009)
| |
| 4.26.2 | Form of Warrant issued in connection with Exhibit 4.26.1 (incorporated by reference to Exhibit 4.1 to Generex Biotechnology Corporation’s Report on Form 8-K filed on September 15, 2009)
| |
| 4.26.3 | Form of Warrant issued to Midtown Partners & Co., LLC and Maxim Group LLC in connection with Exhibit 4.26.1 (incorporated by reference to Exhibit 4.2 to Generex Biotechnology Corporation’s Report on Form 8-K filed on September 15, 2009)
| |
| 4.27.1 | Common Stock Purchase Agreement dated April 7, 2010 by and between Generex Biotechnology Corporation and Seaside 88, LP. (incorporated by reference to Exhibit 10.1 to Generex Biotechnology Corporation’s Report on Form 8-K filed on April 8, 2010) | |
| 4.27.2 | First Amendment to Common Stock Purchase Agreement dated April 28, 2010 by and between Generex Biotechnology Corporation and Seaside 88, LP. (incorporated by reference to Exhibit 10.1 to Generex Biotechnology Corporation’s Report on Form 8-K filed on April 29, 2010) | |
| 4.27.3 | Form of Warrant issued to Midtown Partners & Co., LLC in connection with Exhibit 4.27.1 hereto (incorporated by reference to Exhibit 4.1 to Generex Biotechnology Corporation’s Report on Form 8-K filed on April 8, 2010) | |
4.28.1
|
|
Form of Securities Purchase Agreement dated January 24, 2011 by and between Generex Biotechnology Corporation and the investors (incorporated by reference to Exhibit 10.1 to Generex Biotechnology Corporation’s Current Report on Form 8-K filed on January 25, 2011).
|
| 4.28.2 | Form of Warrant issued to the investors in connection with Exhibit 4.28.1 (incorporated by reference to Exhibit 4.1 to Generex Biotechnology Corporation’s Current Report on Form 8-K filed on January 25, 2011).
| |
| 4.28.3 | Amendment to Purchase Agreement dated March 25, 2011 (incorporated by reference to Exhibit 10.1 to Generex Biotechnology Corporation’s Current Report on Form 8-K filed on March 30, 2011).
| |
| 4.28.4 | Second Amendment to Purchase Agreement dated April 13, 2011 (incorporated by reference to Exhibit 10.1 to Generex Biotechnology Corporation’s Current Report on Form 8-K filed on April 14, 2011).
| |
| 4.29.1 | Form of Securities Purchase Agreement, dated July 8, 2011, by and among Generex Biotechnology Corporation and the purchaser(s) listed on the signature pages thereto (incorporated by reference to Exhibit 4.1 to Generex Biotechnology Corporation’s Current Report on Form 8-K filed on July 11, 2011).
| |
4.29.2
| Form of Common Stock Warrant issued in connection with Exhibit 4.29.1 (incorporated by reference to Exhibit 4.2 to Generex Biotechnology Corporation’s Current Report on Form 8-K filed on July 11, 2011).
| |
| 4.30.1 | Form of Securities Purchase Agreement, dated January 31, 2012, by and among Generex Biotechnology Corporation and the purchaser(s) listed on the signature pages thereto (incorporated by reference to Exhibit 4.1 to Generex Biotechnology Corporation’s Current Report on Form 8-K filed on February 1, 2012).
| |
| 70 |
| 4.30.2 | Form of Common Stock Warrant issued in connection with Exhibit 4.30.1 (incorporated by reference to Exhibit 4.2 to Generex Biotechnology Corporation’s Report on Form 8-K filed on February 1, 2012).
| |
| 4.30.3 | Form of Registration Rights Agreement by and among Generex Biotechnology Corporation and the purchaser(s) listed on the signature pages thereto (incorporated by reference to Exhibit 4.3 to Generex Biotechnology Corporation’s Report on Form 8-K filed on February 1, 2012)
| |
| 4.31.1 | Form of Securities Purchase Agreement, dated August 8, 2012, by and among Generex Biotechnology Corporation and the purchaser(s) listed on the signature pages thereto (incorporated by reference to Exhibit 4.1 to Generex Biotechnology Corporation’s Current Report on Form 8-K filed on August 8, 2012).
| |
| 4.31.2 | Form of Common Stock Warrant issued in connection with Exhibit 4.31.1 (incorporated by reference to Exhibit 4.2 to Generex Biotechnology Corporation’s Report on Form 8-K filed on August 8, 2012).
| |
| 4.31.3 | Form of Registration Rights Agreement by and among Generex Biotechnology Corporation and the purchaser(s) listed on the signature pages thereto (incorporated by reference to Exhibit 4.3 to Generex Biotechnology Corporation’s Report on Form 8-K filed on August 8, 2012)
| |
| 4.32.1 | Form of Securities Purchase Agreement, dated December 10, 2012, by and among Generex Biotechnology Corporation and the purchaser(s) listed on the signature pages thereto (incorporated by reference to Exhibit 4.1 to Generex Biotechnology Corporation’s Current Report on Form 8-K filed on December 11, 2012).
| |
| 4.32.2 | Form of Common Stock Warrant issued in connection with Exhibit 4.32.1 (incorporated by reference to Exhibit 4.2 to Generex Biotechnology Corporation’s Report on Form 8-K filed on December 11, 2012).
| |
| 4.32.3 | Form of Registration Rights Agreement by and among Generex Biotechnology Corporation and the purchaser(s) listed on the signature pages thereto (incorporated by reference to Exhibit 4.3 to Generex Biotechnology Corporation’s Report on Form 8-K filed on December 10, 2012)
| |
| 4.33.1 | Form of Securities Purchase Agreement, dated June 17, 2013, by and among Generex Biotechnology Corporation and the purchaser(s) listed on the signature pages thereto (incorporated by reference to Exhibit 4.1 to Generex Biotechnology Corporation’s Current Report on Form 8-K filed on June 17, 2013).
| |
| 4.33.2 | Form of Common Stock Warrant issued in connection with Exhibit 4.33.1 (incorporated by reference to Exhibit 4.2 to Generex Biotechnology Corporation’s Report on Form 8-K filed on June 17, 2013).
| |
| 4.33.3 | Form of Registration Rights Agreement by and among Generex Biotechnology Corporation and the purchaser(s) listed on the signature pages thereto (incorporated by reference to Exhibit 4.3 to Generex Biotechnology Corporation’s Report on Form 8-K filed on June 17, 2013)
| |
| 4.34.1 | Form of Securities Purchase Agreement, dated January 14, 2014, by and among Generex Biotechnology Corporation and the purchaser(s) listed on the signature pages thereto (incorporated by reference to Exhibit 4.1 to Generex Biotechnology Corporation’s Current Report on Form 8-K filed on January 15, 2014).
| |
| 4.34.2 | Form of Common Stock Warrant issued in connection with Exhibit 4.34.1 (incorporated by reference to Exhibit 4.2 to Generex Biotechnology Corporation’s Report on Form 8-K filed on January 15, 2014).
| |
| 4.35.1 | Form of Securities Purchase Agreement, dated March 27, 2014, by and among Generex Biotechnology Corporation and the purchaser(s) listed on the signature pages thereto (incorporated by reference to Exhibit 4.1 to Generex Biotechnology Corporation’s Current Report on Form 8-K filed on March 28, 2014).
| |
| 4.35.2 | Form of Common Stock Warrant issued in connection with Exhibit 4.35.1 (incorporated by reference to Exhibit 4.2 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 28, 2014).
| |
| 4.35.3 | Form of Registration Rights Agreement by and among Generex Biotechnology Corporation and the purchaser(s) listed on the signature pages thereto (incorporated by reference to Exhibit 4.3 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 28, 2014)
| |
| 9 | Form of Voting Agreement entered into in connection with Exhibit 4.11.1 (incorporated by reference to Exhibit 4.7 to Generex Biotechnology Corporation’s Current Report on Form 8-K filed on November 12, 2004) | |
| 71 |
| 10.1 | Stock Option Agreement by and between Generex Biotechnology Corporation and Peter G. Amanatides to purchase 100,000 shares of Common Stock at the exercise price of $0.56 per share (incorporated by reference to Exhibit 10.3 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on June 14, 2005)* | |
| 10.2 | Stock Option Agreement by and between Generex Biotechnology Corporation and John P. Barratt to purchase 100,000 shares of Common Stock at the exercise price of $0.56 per share (incorporated by reference to Exhibit 10.4 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on June 14, 2005)* | |
| 10.3 | Stock Option Agreement by and between Generex Biotechnology Corporation and Brian T. McGee to purchase 100,000 shares of Common Stock at the exercise price of $0.56 per share (incorporated by reference to Exhibit 10.5 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on June 14, 2005)* | |
| 10.4 | Stock Option Agreement by and between Generex Biotechnology Corporation and John P. Barratt to purchase 35,714 shares of Common Stock at the exercise price of $0.001 per share (incorporated by reference to Exhibit 10.6 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on June 14, 2005)* | |
| 10.5 | Stock Option Agreement by and between Generex Biotechnology Corporation and Brian T. McGee to purchase 35,714 shares of Common Stock at the exercise price of $0.001 per share (incorporated by reference to Exhibit 10.7 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on June 14, 2005)* | |
| 10.6 | Stock Option Agreement by and between Generex Biotechnology Corporation and Gerald Bernstein, M.D. to purchase 100,000 shares of Common Stock at the exercise price of $0.61 per share (incorporated by reference to Exhibit 10.8 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on June 14, 2005)* | |
| 10.7 | Stock Option Agreement by and between Generex Biotechnology Corporation and Mark Fletcher to purchase 250,000 shares of Common Stock at the exercise price of $0.61 per share (incorporated by reference to Exhibit 10.9 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on June 14, 2005)* | |
| 10.8 | Stock Option Agreement by and between Generex Biotechnology Corporation and Mark A. Fletcher to purchase 470,726 shares of Common Stock at the exercise price of $0.001 per share (incorporated by reference to Exhibit 10.12 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on June 14, 2005)* | |
| 10.9 | Employment Agreement by and between Generex Biotechnology Corporation and Gerald Bernstein M.D. (incorporated by reference to Exhibit 10.16 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on June 14, 2005)* | |
| 10.10 | 1998 Stock Option Plan (incorporated by reference to Exhibit 4.3 to Generex Biotechnology Corporation’s Registration Statement on Form S-1 (File No. 333-82667) filed on July 12, 1999)* | |
| 10.11 | 2000 Stock Option Plan (incorporated by reference to Exhibit 4.3.2 to Generex Biotechnology Corporation’s Annual Report on Form 10-K filed on October 30, 2000)* | |
| 10.12 | Amended 2001 Stock Option Plan (incorporated by reference to Exhibit 4.1 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on December 15, 2003)* | |
| 10.13 | 2006 Stock Plan (incorporated by reference to Annex A to Generex Biotechnology Corporation’s Proxy Statement for the Annual Meeting of Stockholders held on May 30, 2006)* | |
| 10.14 | Stockholders Agreement among Generex Biotechnology Corporation and the former holders of capital stock of Antigen Express, Inc. (incorporated by reference to Exhibit 10.4 to Generex Biotechnology Corporation’s Annual Report on Form 10-K filed on October 29, 2003) | |
| 10.15 | Form of Warrant issued by Generex Biotechnology Corporation on April 17, 2006 to certain employees (incorporated by reference to Exhibit 4.34 to Generex Biotechnology Corporation’s Report on Form 10-Q filed on June 14, 2006)* | |
| 10.16 | Quotation for Contract Manufacturing of Oral-lyn™ entered into between Generex Biotechnology Corporation and Cardinal Health PTS, LLC on June 20, 2006 (subject to confidential treatment) (incorporated by reference to Exhibit 10.25 to Generex Biotechnology Corporation’s Report on Form 10-K/A filed on February 14, 2007) | |
| 10.17 | Quotation Amendment for Contract Manufacturing of Oral-lyn™ entered into between Generex Biotechnology Corporation and Cardinal Health PTS, LLC on August 18, 2006 (subject to confidential treatment) (incorporated by reference to Exhibit 10.26 to Generex Biotechnology Corporation’s Report on Form 10-K filed on October 16, 2006) | |
| 72 |
| 10.18 | Clinical Supply Agreement entered into between Generex Biotechnology Corporation and Cardinal Health PTS, LLC on September 6, 2006 (subject to confidential treatment) (incorporated by reference to Exhibit 10.27 to Generex Biotechnology Corporation’s Report on Form 10-K filed on October 16, 2006) | |
| 10.19 | Form of Restricted Stock Agreement for awards to executive officers of Generex Biotechnology Corporation under the Generex Biotechnology Corporation 2006 Stock Plan (incorporated by reference to Exhibit 10.1 to Generex Biotechnology Corporation’s Report on Form 8-K filed on August 23, 2007)* | |
| 10.20 | Summary of Employment Terms for Anna Gluskin effective as of January 1, 2006 (incorporated by reference to Exhibit 10.28 to Generex Biotechnology Corporation’s Report on Form 10-K/A filed on November 28, 2007)* | |
| 10.21 | Summary of Employment Terms for Rose Perri effective as of January 1, 2006 (incorporated by reference to Exhibit 10.29 to Generex Biotechnology Corporation’s Report on Form 10-K/A filed on November 28, 2007)* | |
| 10.22 | Summary of Employment Terms for Mark A. Fletcher effective as of April 21, 2003 (incorporated by reference to Exhibit 10.30 to Generex Biotechnology Corporation’s Report on Form 10-K/A filed on November 28, 2007)* | |
| 10.23 | Employment Agreement between Generex Biotechnology Corporation and Gerald Bernstein, M.D., effective as of April 1, 2002 (incorporated by reference to Exhibit 10.31 to Generex Biotechnology Corporation’s Report on Form 10-K/A filed on November 28, 2007)* | |
| 10.24 | Form of Consent and Waiver Agreement entered into with Cranshire Capital, L.P., Portside Growth and Opportunity Fund and, Smithfield Fiduciary LLC (incorporated by reference to Exhibit 10.1 to Generex Biotechnology Corporation’s Report on Form 8-K filed on August 1, 2008) | |
| 10.25 | Form of Consent and Waiver Agreement entered into with Rockmore Investment Master Fund Ltd. (incorporated by reference to Exhibit 10.2 to Generex Biotechnology Corporation’s Report on Form 8-K filed on August 1, 2008) | |
| 10.26 | Form of Consent and Waiver Agreement entered into with the Iroquois Funds (incorporated by reference to Exhibit 10.3 to Generex Biotechnology Corporation’s Report on Form 8-K filed on August 1, 2008) | |
| 10.27 | Form of separate Agreements entered into with each of Cranshire Capital, L.P., Portside Growth and Opportunity Fund, Rockmore Investment Master Fund Ltd., Smithfield Fiduciary LLC and Iroquois Capital Opportunity Fund, LP on December 22, 2008 (incorporated by reference to Exhibit 10.1 to Generex Biotechnology Corporation’s Report on Form 8-K filed on December 23, 2008) | |
| 10.28 | Form of Agreement entered into with Iroquois Master Fund Ltd. on December 22, 2008 (incorporated by reference to Exhibit 10.2 to Generex Biotechnology Corporation’s Report on Form 8-K filed on December 23, 2008) | |
| 10.29 | Form of separate Letter Agreements dated as of February 13, 2009 and entered into by and between Generex Biotechnology Corporation and each of Cranshire Capital, L.P., Portside Growth and Opportunity Fund, Rockmore Investment Master Fund Ltd., Smithfield Fiduciary LLC, Iroquois Master Fund Ltd. and Iroquois Capital Opportunity Fund, LP. (incorporated by reference to Exhibit 10.1 to Generex Biotechnology Corporation’s Report on Form 8-K filed on February 17, 2009) | |
| 10.30 | Form of Forbearance and Amendment Agreement dated as of February 27, 2009 and entered into by and between Generex Biotechnology Corporation and each of Cranshire Capital, L.P., Portside Growth and Opportunity Fund, Rockmore Investment Master Fund Ltd., Smithfield Fiduciary LLC, Iroquois Master Fund Ltd. and Iroquois Capital Opportunity Fund, LP. (incorporated by reference to Exhibit 10.1 to Generex Biotechnology Corporation’s Report on Form 8-K filed on March 2, 2009) | |
| 10.31 | At Market Offering Issuance Agreement dated October 14, 2009 entered into between Generex Biotechnology Corporation and Wm Smith & Co, LLC (incorporated by reference to Exhibit 10.1 to Generex Biotechnology Corporation’s Report on Form 8-K filed on October 15, 2009) | |
| 10.32 | Recombinant Human Insulin Active Ingredient Manufacturing and Supply Agreement entered into on December 7, 2009 by and between Generex Biotechnology Corporation and Sanofi-Aventis Deutschland GmbH (subject to confidential treatment) (incorporated by reference to Exhibit 10.2 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on December 11, 2009) | |
| 73 |
| 10.33 | Summary of Compensation Arrangements with Executive Officers and Directors as of March 25, 2011 (incorporated by reference to Exhibit 10.1 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on June 3, 2011). | |
| 10.34 | Incentive Stock Option Grant Agreement dated March 9, 2010 by and between Generex Biotechnology Corporation and Mark A. Fletcher (incorporated by reference to Exhibit 10.4 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on June 11, 2010)* | |
| 10.35 | Nonqualified Stock Option Grant Agreement dated March 9, 2010 by and between Generex Biotechnology Corporation and Brian McGee (incorporated by reference to Exhibit 10.5 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on June 11, 2010)* | |
| 10.36 | Nonqualified Stock Option Grant Agreement dated March 9, 2010 by and between Generex Biotechnology Corporation and John P. Barratt (incorporated by reference to Exhibit 10.6 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on June 11, 2010)* | |
| 10.37 | Nonqualified Stock Option Grant Agreement dated March 9, 2010 by and between Generex Biotechnology Corporation and Nola Masterson (incorporated by reference to Exhibit 10.7 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on June 11, 2010).* | |
| 10.38 | Amendment to the Employment Terms for Mark A. Fletcher, dated September 29, 2010 (incorporated by reference to Exhibit 10.46 to Generex Biotechnology Corporation’s Annual Report on Form 10-K filed on October 14, 2010).* | |
| 10.39 | Limited Liability Company Ownership Interest Purchase Agreement by and among Generex Biotechnology Corporation, Global Medical Direct, LLC and Joseph Corso, Jr., Robert S. Shea and Mark Franz (incorporated by reference to Exhibit 10.1 to Generex Biotechnology Corporation’s Report on Form 8-K filed on October 12, 2010) | |
10.40
|
Nonqualified Stock Option Grant Agreement dated March 25, 2011 by and between Generex Biotechnology Corporation and John P. Barratt (incorporated by reference to Exhibit 10.4 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on June 3, 2011).*
| |
| 10.41 | Nonqualified Stock Option Grant Agreement dated March 25, 2011 by and between Generex Biotechnology Corporation and Mark A. Fletcher (incorporated by reference to Exhibit 10.5 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on June 3, 2011).*
| |
| 10.42 | Nonqualified Stock Option Grant Agreement dated March 25, 2011 by and between Generex Biotechnology Corporation and John P. Barratt (incorporated by reference to Exhibit 10.6 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on June 3, 2011).*
| |
| 10.43 | Nonqualified Stock Option Grant Agreement dated March 25, 2011 by and between Generex Biotechnology Corporation and David Brusegard (incorporated by reference to Exhibit 10.7 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on June 3, 2011).*
| |
| 10.44 | Nonqualified Stock Option Grant Agreement dated March 25, 2011 by and between Generex Biotechnology Corporation and Stephen Fellows (incorporated by reference to Exhibit 10.8 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on June 3, 2011).*
| |
| 10.45 | Nonqualified Stock Option Grant Agreement dated March 25, 2011 by and between Generex Biotechnology Corporation and Mark A. Fletcher (incorporated by reference to Exhibit 10.9 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on June 3, 2011).*
| |
| 10.46 | Nonqualified Stock Option Grant Agreement dated March 25, 2011 by and between Generex Biotechnology Corporation and Nola E. Masterson (incorporated by reference to Exhibit 10.10 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on June 3, 2011).*
| |
| 10.47 | Nonqualified Stock Option Grant Agreement dated March 25, 2011 by and between Generex Biotechnology Corporation and Brian T. McGee (incorporated by reference to Exhibit 10.11 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on June 3, 2011).*
| |
| 10.48 | Nonqualified Stock Option Grant Agreement dated June 19, 2012 by and between Generex Biotechnology Corporation and Mark A. Fletcher (incorporated by reference to Exhibit 10.48 to Generex Biotechnology Corporation’s Registration Statement on Form S-1 filed on September 12, 2012).*
| |
| 74 |
| 10.49 | Nonqualified Stock Option Grant Agreement dated June 19, 2012 by and between Generex Biotechnology Corporation and John P. Barratt (incorporated by reference to Exhibit 10.49 to Generex Biotechnology Corporation’s Registration Statement on Form S-1 filed on September 12, 2012).*
| |
| 10.50 | Nonqualified Stock Option Grant Agreement dated June 19, 2012 by and between Generex Biotechnology Corporation and Brian T. McGee (incorporated by reference to Exhibit 10.50 to Generex Biotechnology Corporation’s Registration Statement on Form S-1 filed on September 12, 2012).*
| |
| 10.51 | Nonqualified Stock Option Grant Agreement dated June 19, 2012 by and between Generex Biotechnology Corporation and Nola E. Masterson (incorporated by reference to Exhibit 10.51 to Generex Biotechnology Corporation’s Registration Statement on Form S-1 filed on September 12, 2012).*
| |
| 10.52 | Nonqualified Stock Option Grant Agreement dated June 19, 2012 by and between Generex Biotechnology Corporation and James Anderson (incorporated by reference to Exhibit 10.52 to Generex Biotechnology Corporation’s Registration Statement on Form S-1 filed on September 12, 2012).*
| |
| 10.53 | Nonqualified Stock Option Grant Agreement dated June 19, 2012 by and between Generex Biotechnology Corporation and Eric von Hofe (incorporated by reference to Exhibit 10.53 to Generex Biotechnology Corporation’s Registration Statement on Form S-1 filed on September 12, 2012).*
| |
| 10.54 | Nonqualified Stock Option Grant Agreement dated June 20, 2012 by and between Generex Biotechnology Corporation and Stephen Fellows (incorporated by reference to Exhibit 10.54 to Generex Biotechnology Corporation’s Registration Statement on Form S-1 filed on September 12, 2012).*
| |
| 10.55 | Nonqualified Stock Option Grant Agreement dated June 20, 2012 by and between Generex Biotechnology Corporation and David Brusegard (incorporated by reference to Exhibit 10.55 to Generex Biotechnology Corporation’s Registration Statement on Form S-1 filed on September 12, 2012).*
| |
| 10.56 | Nonqualified Stock Option Grant Agreement dated April 1, 2013 by and between Generex Biotechnology Corporation and Mark A. Fletcher (incorporated by reference to Exhibit 10.56 to Generex Biotechnology Corporation’s Registration Statement on Form S-1 filed on July 2, 2013).*
| |
| 10.57 | Nonqualified Stock Option Grant Agreement dated April 1, 2013 by and between Generex Biotechnology Corporation and John P. Barratt (incorporated by reference to Exhibit 10.57 to Generex Biotechnology Corporation’s Registration Statement on Form S-1 filed on July 2, 2013).*
| |
| 10.58 | Nonqualified Stock Option Grant Agreement dated April 1, 2013 by and between Generex Biotechnology Corporation and Brian T. McGee (incorporated by reference to Exhibit 10.58 to Generex Biotechnology Corporation’s Registration Statement on Form S-1 filed on July 2, 2013).*
| |
| 10.59 | Nonqualified Stock Option Grant Agreement dated April 1, 2013 by and between Generex Biotechnology Corporation and James Anderson (incorporated by reference to Exhibit 10.59 to Generex Biotechnology Corporation’s Registration Statement on Form S-1 filed on July 2, 2013).*
| |
| 10.60 | Nonqualified Stock Option Grant Agreement dated April 1, 2013 by and between Generex Biotechnology Corporation and Eric von Hofe (incorporated by reference to Exhibit 10.60 to Generex Biotechnology Corporation’s Registration Statement on Form S-1 filed on July 2, 2013).*
| |
| 10.61 | Nonqualified Stock Option Grant Agreement dated April 1, 2013 by and between Generex Biotechnology Corporation and Stephen Fellows (incorporated by reference to Exhibit 10.61 to Generex Biotechnology Corporation’s Registration Statement on Form S-1 filed on July 2, 2013).*
| |
| 10.62 | Nonqualified Stock Option Grant Agreement dated April 1, 2013 by and between Generex Biotechnology Corporation and David Brusegard (incorporated by reference to Exhibit 10.62 to Generex Biotechnology Corporation’s Registration Statement on Form S-1 filed on July 2, 2013).*
| |
| 10.63 | Nonqualified Stock Option Grant Agreement dated June 6, 2013 by and between Generex Biotechnology Corporation and Mark A. Fletcher (incorporated by reference to Exhibit 10.63 to Generex Biotechnology Corporation’s Registration Statement on Form S-1 filed on July 2, 2013).*
| |
| 75 |
| 10.64 | Nonqualified Stock Option Grant Agreement dated June 6, 2013 by and between Generex Biotechnology Corporation and John P. Barratt (incorporated by reference to Exhibit 10.64 to Generex Biotechnology Corporation’s Registration Statement on Form S-1 filed on July 2, 2013).*
| |
| 10.65 | Nonqualified Stock Option Grant Agreement dated June 6, 2013 by and between Generex Biotechnology Corporation and Brian T. McGee (incorporated by reference to Exhibit 10.65 to Generex Biotechnology Corporation’s Registration Statement on Form S-1 filed on July 2, 2013).*
| |
| 10.66 | Nonqualified Stock Option Grant Agreement dated June 6, 2013 by and between Generex Biotechnology Corporation and James Anderson (incorporated by reference to Exhibit 10.66 to Generex Biotechnology Corporation’s Registration Statement on Form S-1 filed on July 2, 2013).*
| |
| 10.67 | Nonqualified Stock Option Grant Agreement dated June 6, 2013 by and between Generex Biotechnology Corporation and Eric von Hofe (incorporated by reference to Exhibit 10.67 to Generex Biotechnology Corporation’s Registration Statement on Form S-1 filed on July 2, 2013).*
| |
| 10.68 | Nonqualified Stock Option Grant Agreement dated June 6, 2013 by and between Generex Biotechnology Corporation and Stephen Fellows (incorporated by reference to Exhibit 10.68 to Generex Biotechnology Corporation’s Registration Statement on Form S-1 filed on July 2, 2013).*
| |
| 10.69 | Nonqualified Stock Option Grant Agreement dated June 6, 2013 by and between Generex Biotechnology Corporation and David Brusegard (incorporated by reference to Exhibit 10.69 to Generex Biotechnology Corporation’s Registration Statement on Form S-1 filed on July 2, 2013).*
| |
| 10.70 | Nonqualified Stock Option Grant Agreement dated October 31, 2013 by and between Generex Biotechnology Corporation and James Anderson (incorporated by reference to Exhibit 10.1 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on December 9, 2013).*
| |
| 10.71 | Nonqualified Stock Option Grant Agreement dated October 31, 2013 by and between Generex Biotechnology Corporation and John Barratt (incorporated by reference to Exhibit 10.2 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on December 9, 2013).*
| |
| 10.72 | Nonqualified Stock Option Grant Agreement dated October 31, 2013 by and between Generex Biotechnology Corporation and David Brusegard (incorporated by reference to Exhibit 10.3 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on December 9, 2013).*
| |
| 10.73 | Nonqualified Stock Option Grant Agreement dated October 31, 2013 by and between Generex Biotechnology Corporation and Stephen Fellows (incorporated by reference to Exhibit 10.4 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on December 9, 2013).*
| |
| 10.74 | Nonqualified Stock Option Grant Agreement dated October 31, 2013 by and between Generex Biotechnology Corporation and Mark Fletcher (incorporated by reference to Exhibit 10.5 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on December 9, 2013).*
| |
| 10.75 | Nonqualified Stock Option Grant Agreement dated October 31, 2013 by and between Generex Biotechnology Corporation and Brian McGee (incorporated by reference to Exhibit 10.6 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on December 9, 2013).*
| |
| 10.76 | Nonqualified Stock Option Grant Agreement dated October 31, 2013 by and between Generex Biotechnology Corporation and Eric von Hofe (incorporated by reference to Exhibit 10.7 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on December 9, 2013).*
| |
| 10.77 | Nonqualified Stock Option Grant Agreement dated October 31, 2013 by and between Generex Biotechnology Corporation and James Anderson (incorporated by reference to Exhibit 10.8 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on December 9, 2013).*
| |
| 10.78 | Nonqualified Stock Option Grant Agreement dated October 31, 2013 by and between Generex Biotechnology Corporation and John Barratt (incorporated by reference to Exhibit 10.9 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on December 9, 2013).*
| |
| 10.79 | Nonqualified Stock Option Grant Agreement dated October 31, 2013 by and between Generex Biotechnology Corporation and David Brusegard (incorporated by reference to Exhibit 10.10 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on December 9, 2013).*
| |
| 76 |
| 10.80 | Nonqualified Stock Option Grant Agreement dated October 31, 2013 by and between Generex Biotechnology Corporation and Stephen Fellows (incorporated by reference to Exhibit 10.11 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on December 9, 2013).*
| |
| 10.81 | Nonqualified Stock Option Grant Agreement dated October 31, 2013 by and between Generex Biotechnology Corporation and Mark Fletcher (incorporated by reference to Exhibit 10.12 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on December 9, 2013).*
| |
| 10.82 | Nonqualified Stock Option Grant Agreement dated October 31, 2013 by and between Generex Biotechnology Corporation and Brian McGee (incorporated by reference to Exhibit 10.13 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on December 9, 2013).*
| |
| 10.83 | Nonqualified Stock Option Grant Agreement dated October 31, 2013 by and between Generex Biotechnology Corporation and Eric von Hofe (incorporated by reference to Exhibit 10.14 to Generex Biotechnology Corporation’s Quarterly Report on Form 10-Q filed on December 9, 2013).*
| |
| 21 | Subsidiaries of the Registrant | |
| 23.1 | Consent of MNP LLP, independent registered public accounting firm
| |
| 23.2 | Consent of MSCM LLP, independent registered public accounting firm | |
| 31.1 | Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| 31.2 | Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| 32 | Certification of Chief Executive Officer and Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| * Management contract or management compensatory plan or arrangement. | ||
| (1) | In the case of incorporation by reference to documents filed by the Registrant under the Exchange Act, the Registrant’s file number under the Exchange Act is 000-25169. | |
| 77 |