UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form10-K
(Mark One)
| | ☒ | | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2017
OR
| | ☐ | | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number:0-32405

Seattle Genetics, Inc.
(Exact name of registrant as specified in its charter)
| | |
| Delaware | | 91-1874389 |
(State or other Jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
21823 30th Drive SE
Bothell, WA 98021
(Address of principal executive offices, including zip code)
Registrant’s telephone number, including area code:(425) 527-4000
Securities registered pursuant to Section 12(b) of the Act:
| | |
Title of class | | Name of each exchange on which registered |
| Common Stock, par value $0.001 | | The Nasdaq Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. YES ☒ NO ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. YES ☐ NO ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. YES ☒ NO ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of RegulationS-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). YES ☒ NO ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of RegulationS-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of thisForm 10-K or any amendment to this Form10-K. ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, anon-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule12b-2 of the Exchange Act.
| | | | | | | | | | |
| Large accelerated filer | | ☒ | | Accelerated filer | | ☐ | | Emerging growth company | | ☐ |
| | | | | |
| Non-accelerated filer | | ☐ (Do not check if smaller reporting company) | | Smaller reporting company | | ☐ | | | | |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule12b-2 of the Exchange Act). YES ☐ NO ☒
The aggregate market value of the voting andnon-voting common equity held bynon-affiliates of the registrant was approximately $4,949,017,768 as of the last business day of the registrant’s most recently completed second fiscal quarter, based upon the closing sale price on The Nasdaq Global Select Market reported for such date. Excludes an aggregate of 47,329,018 shares of the registrant’s common stock held as of such date by officers, directors and stockholders that the registrant has concluded are or were affiliates of the registrant. Exclusion of such shares should not be construed to indicate that the holder of any such shares possesses the power, direct or indirect, to direct or cause the direction of the management or policies of the registrant or that such person is controlled by or under common control with the registrant.
There were 157,951,354 shares of the registrant’s Common Stock issued and outstanding as of February 8, 2018.
DOCUMENTS INCORPORATED BY REFERENCE
Part III incorporates information by reference from the registrant’s definitive proxy statement to be filed with the Securities and Exchange Commission pursuant to Regulation 14A, not later than 120 days after the end of the fiscal year covered by this Annual Report on Form10-K, in connection with the Registrant’s 2018 Annual Meeting of Stockholders.
SEATTLE GENETICS, INC.
FORM10-K
FOR THE YEAR ENDED DECEMBER 31, 2017
TABLE OF CONTENTS
i
This Annual Report on Form10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements are based on our management’s beliefs and assumptions and on information currently available to our management. All statements other than statements of historical facts are “forward-looking statements” for purposes of these provisions, including those relating to future events or our future financial performance and financial guidance. In some cases, you can identify forward-looking statements by terminology such as “may,” “might,” “will,” “should,” “expect,” “plan,” “anticipate,” “project,” “believe,” “estimate,” “predict,” “potential,” “intend” or “continue,” the negative of terms like these or other comparable terminology, and other words or terms of similar meaning in connection with any discussion of future operating or financial performance. These statements are only predictions. All forward-looking statements included in this Annual Report on Form10-K are based on information available to us on the date hereof, and we assume no obligation to update any such forward-looking statements, except as required by law. Any or all of our forward-looking statements in this document may turn out to be incorrect. Actual events or results may differ materially. Our forward-looking statements can be affected by inaccurate assumptions we might make or by known or unknown risks, uncertainties and other factors. We discuss many of these risks, uncertainties and other factors in this Annual Report on Form10-K in greater detail under the heading “Item 1A—Risk Factors.” We caution investors that our business and financial performance are subject to substantial risks and uncertainties.
PART I
Item 1. Business
Overview
Seattle Genetics is a biotechnology company focused on the development and commercialization of targeted therapies for the treatment of cancer. Our marketed product ADCETRIS®, or brentuximab vedotin, is approved by the United States Food and Drug Administration, or FDA, and the European Commission for four indications, encompassing several settings for the treatment of relapsed Hodgkin lymphoma, for relapsed systemic anaplastic large cell lymphoma, or sALCL, and for certain types of cutaneousT-cell lymphoma, or CTCL. ADCETRIS is commercially available in 70 countries, including in the United States, Canada, members of the European Union and Japan. We are collaborating with Takeda Pharmaceutical Company Limited, or Takeda, to develop and commercialize ADCETRIS on a global basis. Under this collaboration, Seattle Genetics has retained commercial rights for ADCETRIS in the United States and its territories and in Canada, and Takeda has commercial rights in the rest of the world.
Beyond our current labeled indications, we have a broad development strategy for ADCETRIS, including to evaluate its therapeutic potential in newly diagnosed patients with Hodgkin lymphoma or matureT-cell lymphoma, or MTCL, also known as peripheralT-Cell lymphoma, or PTCL, including sALCL. We are also evaluating ADCETRIS in combination with a checkpoint inhibitor, or CPI. We and our partners are currently conducting these phase 3 clinical trials of ADCETRIS as described below:
| | • | | ECHELON-1: In collaboration with Takeda, we are investigating ADCETRIS plus AVD (adriamycin, vinblastine, dacarbazine) versus ABVD (adriamycin, bleomycin, vinblastine, dacarbazine) as frontline combination therapy in patients with previously untreated advanced classical Hodgkin lymphoma. TheECHELON-1 trial met its primary endpoint, demonstrating that treatment with ADCETRIS plus AVD resulted in a statistically significant improvement in modified progression-free survival. Interim analysis of overall survival, the key secondary endpoint, also trended in favor of the ADCETRIS plus AVD arm. The FDA granted Breakthrough Therapy Designation, or BTD, to ADCETRIS in combination with chemotherapy for the frontline treatment of patients with advanced classical Hodgkin lymphoma. In December 2017, the FDA granted Priority Review for the supplemental Biologics License Application, or sBLA, we submitted in November 2017 seeking approval of ADCETRIS as part of a frontline |
1
| | combination chemotherapy regimen in patients with previously untreated advanced classical Hodgkin lymphoma, and the Prescription Drug User Fee Act, or PDUFA, target action date is May 1, 2018. |
| | • | | ECHELON-2: In collaboration with Takeda, we are evaluating ADCETRIS in combination with CHP versus CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) for the treatment of newly-diagnosed MTCL patients. In November 2016, we and Takeda completed enrollment of 452 patients in theECHELON-2 trial, and we expect to reporttop-line data in 2018. |
| | • | | CHECKMATE 812: In collaboration with Bristol-Myers Squibb Company, or BMS, we are evaluating the combination of BMS’s immunotherapy nivolumab (Opdivo) with ADCETRIS for the treatment of relapsed or refractory, or transplant-ineligible, advanced classical Hodgkin lymphoma. |
ECHELON-1 andECHELON-2 are both being conducted under Special Protocol Assessment, or SPA, agreements with the FDA and pursuant to scientific advice from the European Medicines Agency, or EMA. A SPA is an agreement with the FDA regarding the design of the clinical trial, including size and clinical endpoints, to support an efficacy claim in a new drug application or a Biologics License Application, or BLA, submission to the FDA if the trial achieves its primary endpoints.
Our clinical-stage pipeline includes two antibody-drug conjugates, or ADCs, for solid tumors with potential accelerated approval pathways. In collaboration with Astellas Pharma, Inc., or Astellas, we are developing enfortumab vedotin, formerly known asASG-22ME. We and Astellas are conducting a pivotal phase 2 clinical trial for patients with locally advanced or metastatic urothelial cancer who have been previously treated with CPI therapy. We and Astellas also initiated a phase 1b trial of enfortumab vedotin in combination with CPI therapy for patients with first- or second-line locally advanced or metastatic urothelial cancer.
In collaboration with Genmab A/S, or Genmab, we are developing tisotumab vedotin. We and Genmab are planning to conduct a pivotal phase 2 clinical trial for patients with recurrent and/or metastatic cervical cancer. In addition, we and Genmab plan to evaluate tisotumab vedotin as part of combination therapy for first-line cervical cancer as well as in other types of solid tumors.
Our earlier-stage clinical pipeline includes six other ADC programs consisting of ladiratuzumab vedotin, orSGN-LIV1A, denintuzumab mafodotin, orSGN-CD19A,SGN-CD19B,SGN-CD123A, SGN-CD33A andSGN-CD352A, as well as two immuno-oncology agents,SEA-CD40, which is based on our sugar-engineered antibody, or SEA, technology, andSGN-2FF, which is a novel small molecule. In addition, we have multiple preclinical and research-stage programs that employ our proprietary technologies, includingSGN-CD48A.
We have collaborations for our ADC technology with a number of biotechnology and pharmaceutical companies, including AbbVie Biotechnology Ltd., or AbbVie; Bayer Pharma AG, or Bayer; Celldex Therapeutics, Inc., or Celldex; Genentech, Inc., a member of the Roche Group, or Genentech; GlaxoSmithKline LLC, or GSK; Pfizer, Inc., or Pfizer; and PSMA Development Company LLC, a subsidiary of Progenics Pharmaceuticals Inc., or Progenics. In addition, we have a collaboration with Unum Therapeutics, Inc., or Unum, to develop and commercialize novel antibody-coupledT-cell receptor, or ACTR, therapies incorporating our antibodies for the treatment of cancer, and a collaboration agreement with Pieris Pharmaceuticals, Inc. and Pieris Pharmaceuticals AG, or together, Pieris, to develop multiple targeted bispecific immuno-oncology treatments for solid tumors and blood cancers.
Proposed Acquisition of Cascadian Therapeutics
On January 30, 2018, we and our wholly owned subsidiary, Valley Acquisition Sub, Inc., or Purchaser, entered into a definitive Agreement and Plan of Merger, or the Merger Agreement, with Cascadian Therapeutics, Inc., or Cascadian, a clinical-stage biopharmaceutical company based in Seattle, Washington, pursuant to which Purchaser has commenced an offer, or the Tender Offer, to acquire all of the outstanding shares of Cascadian common stock at a price of $10.00 per share net to the seller in cash, without interest, less any applicable
2
withholding taxes. As soon as practicable following the consummation of the Tender Offer, and subject to the satisfaction or waiver of certain conditions set forth in the Merger Agreement, Purchaser will merge with and into Cascadian, or the Merger, and Cascadian will survive as our subsidiary. We refer to the Tender Offer and Merger together in this Annual Report on Form10-K as the Cascadian Acquisition. We estimate that the aggregate cash amount we will pay for shares of Cascadian common stock in the Cascadian Acquisition is approximately $614.1 million. The obligations of us and Purchaser to complete the Cascadian Acquisition are subject to customary closing conditions. We expect to consummate the Cascadian Acquisition in the first quarter of 2018. Cascadian’s most advanced program is tucatinib, an investigational oral, small molecule tyrosine kinase inhibitor, or TKI, that is highly selective for HER2, a growth factor receptor that is overexpressed in multiple cancers, including breast, colorectal, ovarian, and gastric. Tucatinib is currently being evaluated in a randomized global pivotal trial called HER2CLIMB for patients with HER2-positive, or HER2+, metastatic breast cancer, including patients with or without brain metastases. Tucatinib has been evaluated as a single agent and in combination with both chemotherapy and other HER2-directed agents including trastuzumab (Herceptin) and trastuzumab emtansine (Kadcyla).
Our Antibody-Drug Conjugate (ADC) Technologies
ADCETRIS and many product candidates in our pipeline of clinical-stage monoclonal antibody-based product candidates utilize our ADC technology. ADCs are monoclonal antibodies that are linked to cytotoxic or cell-killing agents. Our ADCs utilize monoclonal antibodies that internalize within target cells after binding to a specified cell-surface receptor. Enzymes present inside the cell catalyze the release of the cytotoxic agent from the monoclonal antibody, which then results in the desired activity, specific killing of the target cell.
A key component of our ADCs are the linkers that attach the cell-killing agent to the monoclonal antibody, which are designed to hold the cytotoxic agent to the monoclonal antibody until it binds to the cell surface receptor on the target cell and then to release the cytotoxic agent upon internalization within the target cell. This targeted delivery of the cell-killing agent is intended to maximize delivery of the cytotoxic agent to targeted cells while minimizing toxicity to normal tissues. Our ADCs use proprietary auristatins, which are microtubule disrupting agents, or pyrrolobenzodiazepine, or PBD, dimers, which are DNA cross-linkers, as cell-killing agents. In contrast to natural products that are often more difficult to produce and link to antibodies, the cytotoxic drugs used in our ADC’s are synthetically produced and easier to scale for manufacturing. ADCETRIS, enfortumab vedotin(ASG-22ME), tisotumab vedotin, ladiratuzumab vedotin, denintuzumab mafodotin,SGN-CD19B,SGN-CD123A, SGN-CD33A andSGN-CD352A each utilize our proprietary ADC technologies. These technologies are also the basis of our corporate collaborations. In addition, we are advancing a preclinical product candidate,SGN-CD48A, which utilizes a novel linker technology,PEG-Glucuronide linker, attached to an auristatin. We own or hold exclusive or partially-exclusive licenses to multiple issued patents and patent applications covering our ADC technology. We continue to evaluate new linkers, antibody formats, and cell-killing agents for use in our ADC programs.
Our Sugar-Engineered Antibody (SEA) Technology
Our proprietary SEA technology is a method to selectively reduce fucose incorporation in monoclonal antibodies as they are produced in cell line-based manufacturing. We believe that this may result in increased effector function and antitumor activity. Our SEA technology is a novel approach to modify the activity of monoclonal antibodies that is complementary to our ADC technology.
A key feature of our SEA technology is that no genetic modification of the antibody-producing cell line is necessary and standard cell culture conditions can be used while maintaining the underlying manufacturing processes, yields and product quality. We believe the SEA approach may be simpler and more cost-effective to implement as compared to existing technologies for enhancing antibody effector function, most of which require development of new cell lines.
SEA-CD40 is a clinical-stagenon-fucosylated monoclonal antibody developed using SEA technology. Enhanced binding to effector cells results in crosslinking and activation of CD40 signaling in cells of the immune
3
system. We hypothesize that this increased stimulation of the patient’s own immune cells may result in meaningful antitumor activity. We are developingSEA-CD40 as a novel immuno-oncology agent. A phase 1 clinical trial ofSEA-CD40 for solid tumors and hematologic malignancies is ongoing.
Other Technologies
In addition, we utilize other technologies designed to maximize antitumor activity and reduce toxicity of antibody-based therapies. Genetic engineering enables us to produce antibodies that are optimized for their intended uses. For ADCs, we screen and select antibodies that bind to antigens that are differentially expressed on tumor cells versus vital normal tissues, rapidly internalized within target cells and utilize native or engineered conjugation sites to optimize drug attachment. In some cases, we evaluate the use of our monoclonal antibodies and ADCs in combination with conventional chemotherapy and other anticancer agents, which may result in increased antitumor activity.
Our Strategy
Our strategy is to become a leading developer and marketer of targeted therapies for cancer. Key elements of our strategy are to:
| | • | | Successfully Execute our ADCETRIS Commercial Plan. An important near-term objective is to continue to execute our ADCETRIS commercial plan by driving market penetration and duration of therapy consistent with the current ADCETRIS label. We continue to focus our efforts on commercializing ADCETRIS in the United States and Canada through the coordinated efforts of our sales, marketing, reimbursement and market planning groups. ADCETRIS is approved by the FDA and the European Commission for four indications, encompassing several settings for the treatment of relapsed Hodgkin lymphoma, relapsed sALCL and for certain types of CTCL. In addition, as of January 2018, ADCETRIS had received marketing authorizations in relapsed Hodgkin lymphoma and sALCL from regulatory authorities in 70 countries, and we are continuing to support Takeda’s efforts to obtain regulatory approvals and conduct commercial launches in additional countries worldwide. |
| | • | | Expand the Therapeutic Potential of ADCETRIS. We believe ADCETRIS may have applications in earlier lines of therapy for Hodgkin lymphoma and MTCL and in other types of CD30-expressing lymphomas. In this regard, during 2017 we reported data from the phase 3ECHELON-1 trial, received BTD from the FDA and submitted a sBLA to the FDA in November 2017 seeking approval for a new indication of ADCETRIS in combination with chemotherapy for the frontline treatment of patients with advanced classical Hodgkin lymphoma. The FDA has granted Priority Review for the sBLA, and the PDUFA target action date is May 1, 2018. The phase 3ECHELON-2 trial evaluating ADCETRIS in frontline therapy for MTCL, also known as PTCL, and the phase 3 CHECKMATE 812 trial evaluating ADCETRIS in relapsed Hodgkin lymphoma in combination with a CPI are also ongoing. Clinical trials are also being conducted by us, by our collaborators and as investigator-sponsored trials in different CD30-expressing indications, including multiple stages of Hodgkin lymphoma, novel combinations of ADCETRIS plus immuno-oncology or other anticancer agents and in other areas of medical and scientific interest. |
| | • | | Advance our Clinical Pipeline of Oncology Drugs. We are employing our clinical, development, regulatory and manufacturing expertise with the goal of advancing our clinical-stage product candidates towards regulatory approval and commercialization on a global basis. Our key efforts in this regard include: |
| | • | | Advance Enfortumab Vedotin in a Pivotal Trial for Urothelial Cancer. We and Astellas are conducting a pivotal phase 2 clinical trial of enfortumab vedotin for patients with locally advanced or metastatic urothelial cancer who have been previously treated with CPI therapy. In addition, to evaluate its potential in earlier lines of metastatic urothelial cancer, we and Astellas initiated a phase 1b trial of enfortumab vedotin in combination with CPI therapy for patients with first- or second-line locally advanced or metastatic urothelial cancer. We also believe enfortumab vedotin |
4
| | may have application in other types of solid tumors based on the expression ofNectin-4 in cancers, such as ovarian andnon-small cell lung, and are considering potential future clinical trials. |
| | • | | Advance Tisotumab Vedotin into a Pivotal Trial for Cervical Cancer. We and Genmab plan to initiate in the first half of 2018 a pivotal phase 2 clinical trial of tisotumab vedotin for patients with recurrent and/or metastatic cervical cancer. In addition, as part of our strategy to broadly investigate tisotumab vedotin for cancer, we and Genmab plan to evaluate tisotumab vedotin as part of combination therapy for first-line cervical cancer as well as in other types of solid tumors. |
| | • | | Continue to Develop our Other Pipeline Programs. We believe that it is important to maintain a diverse pipeline of product candidates to sustain our future growth. To accomplish this, we are continuing to advance the development of our other clinical product candidates as well as other preclinical and research-stage programs that employ our proprietary technologies. We are evaluating our programs as monotherapy, and in some cases in combination with other anti-cancer agents such as CPIs to broadly assess the potential of our pipeline as part of existing and emerging therapeutic regimens. In addition, we areco-developing immuno-oncology programs with each of Unum and Pieris. |
| | • | | Support Growth of our Pipeline through Internal Research Efforts and Strategic Transactions. We have internal research programs directed toward identifying novel antigen targets, monoclonal antibodies and other targeting molecules, creating new antibody engineering techniques and developing new classes of stable linkers and cell-killing agents for our ADC technology. In addition, we supplement these internal efforts through ongoing initiatives to identify product candidates, products and technologies to acquire orin-license from biotechnology and pharmaceutical companies and academic institutions. For example, in January 2018, we announced the Cascadian Acquisition. If we are successful in consummating the Cascadian Acquisition, we plan to advance Cascadian’s most advanced program, tucatinib, in its current pivotal trial, HER2CLIMB, for patients with HER2-positive, or HER2+, metastatic breast cancer, including patients with or without brain metastases, and also to evaluate other potential development opportunities for tucatinib. |
| | • | | Expand Globally. We have established operations in Zug, Switzerland to support clinical trials, regulatory, medical affairs, manufacturing, and future potential commercial activities for our pipeline. In 2018, we will continue to develop our European presence in support of our global expansion. |
| | • | | Continue to Leverage our Industry-Leading ADC Technology. We have developed proprietary ADC technology designed to empower monoclonal antibodies. We are currently developing multiple product candidates that employ our ADC technology and we have also licensed this technology to biotechnology and pharmaceutical companies to generate collaboration revenues and funding, as well as potential milestones and potential future royalties. Presently, we have active ADC collaborations with AbbVie, Bayer, Celldex, Genentech, GSK, Pfizer, and Progenics, as well as ADCco-development agreements with Agensys (which subsequently became an affiliate of Astellas) and Genmab. These ADC collaboration andco-development agreements have generated over $375 million as of December 31, 2017 through a combination of upfront payments, research support, and other fees, milestone payments and equity purchases. Several of these collaborators are advancing ADCs using our technology in late-stage clinical development across a range of cancer types, illustrating our leadership in the field. |
| | • | | Enter into Strategic Product Collaborations to Supplement our Internal Resources. We have entered into collaborations to broaden and accelerate clinical trial development and potential commercialization of our product candidates. Collaborations can generate significant capital, supplement our own internal expertise in key areas such as manufacturing, regulatory affairs and clinical development, and provide us with access to our collaborators’ marketing, sales and distribution capabilities in specific territories. |
5
ADCETRIS and Lead Product Candidate Development Pipeline
The following table summarizes our ADCETRIS and lead product candidate development pipeline:
| | | | | | |
Name of Product or Product Candidate | | Description | | Commercial Rights | | Status |
| | | |
ADCETRIS® (brentuximab vedotin) | | Anti-CD30 ADC | | Seattle Genetics in United States and Canada; Takeda in rest of world | | ADCETRIS has received regular approval in the United States for the treatment of adult patients with (i) relapsed classical Hodgkin lymphoma, (ii) classical Hodgkin lymphoma at high risk of relapse or progression as post-autologous hematopoietic stem cell transplantation, or post-auto-HSCT consolidation and (iii) primary cutaneous anaplastic large cell lymphoma, or pcALCL, or CD30-expressing mycosis fungoides, or MF, who have received prior systemic therapy. ADCETRIS also has accelerated approval in the United States for the treatment of patients with relapsed sALCL. In addition, ADCETRIS has approval with conditions in Canada for the treatment of patients with relapsed or refractory Hodgkin lymphoma or sALCL, andnon-conditional approval for post-autologous stem cell transplant, or ASCT, consolidation treatment of Hodgkin lymphoma patients at increased risk of relapse or progression. As of January 2018, ADCETRIS had received marketing authorizations in relapsed Hodgkin lymphoma or relapsed sALCL from regulatory authorities in 70 countries. In particular, ADCETRIS has conditional marketing authorization in the European Union for the treatment of adult patients with (i) relapsed or refractory CD30-positive Hodgkin lymphoma, (ii) relapsed or refractory sALCL, (iii) CD30-positive Hodgkin lymphoma at increased risk of relapse or progression following ASCT and (iv) CD30-positive CTCL after at least one prior systemic therapy. Ongoing trials of ADCETRIS include: |
| | | |
| | | | | | TheECHELON-1 phase 3 randomized trial ongoing for patients with newly diagnosed advanced stage classical Hodgkin lymphoma comparing adriamycin, bleomycin, vinblastine and dacarbazine, or ABVD, versus AVD plus ADCETRIS. In 2017, we reported that theECHELON-1 phase 3 trial met its primary endpoint. Based on the |
6
| | | | | | |
| | | | | | results of the trial, in September 2017 the FDA granted BTD to ADCETRIS in combination with chemotherapy for the frontline treatment of patients with advanced classical Hodgkin lymphoma. We submitted a sBLA to the FDA in November 2017 for approval of a new indication for ADCETRIS as part of a frontline combination chemotherapy regimen in patients with previously untreated advanced classical Hodgkin lymphoma. In December 2017, the FDA granted Priority Review for the sBLA, and the PDUFA target action date is May 1, 2018. |
| | | |
| | | | | | TheECHELON-2 phase 3 randomized trial ongoing for patients with newly diagnosed CD30-expressing MTCL, including sALCL, comparing cyclophosphamide, doxorubicin, vincristine and prednisone, or CHOP, versus CHP plus ADCETRIS. We and Takeda completed enrollment of 452 patients in November 2016, and we expect to reporttop-line data in 2018. |
| | | |
| | | | | | The CHECKMATE 812 phase 3 trial ongoing evaluating ADCETRIS in combination with nivolumab for patients with relapsed or refractory, or transplant-ineligible, advanced classical Hodgkin lymphoma. Phase 2 trial ongoing for patients age 60 or older with newly diagnosed Hodgkin lymphoma evaluating ADCETRIS in combination with bendamustine, dacarbazine or nivolumab. Phase 1/2 trial ongoing for patients with relapsed or refractory Hodgkin lymphoma after failure of frontline therapy evaluating ADCETRIS in combination with nivolumab. Phase 1/2 trial ongoing for patients with relapsed or refractoryB-cell andT-cellnon-Hodgkin lymphomas, including DLBCL and other rareB-cell lymphomas, evaluating ADCETRIS in combination with nivolumab. |
| | | |
Enfortumab vedotin (ASG-22ME) | | Anti-Nectin-4 ADC | | 50: 50 co-development and commercialization with Astellas | | Pivotal phase 2 trial ongoing for patients with locally advanced or metastatic urothelial cancer who have been previously treated with CPI therapy. |
7
| | | | | | |
| | | | | | Phase 1b trial for patients with first- or second-line locally advanced or metastatic urothelial cancer evaluating enfortumab vedotin in combination with CPI therapy. Phase 1 trial forNectin-4-positive solid tumors, including urothelial cancers such as bladder cancer. |
| | | |
| Tisotumab vedotin | | Anti-Tissue Factor ADC | | 50: 50 co-development and commercialization with Genmab | | Planned pivotal phase 2 clinical trial for patients with recurrent and/or metastatic cervical cancer. Planned phase 2 trial to evaluate tisotumab vedotin as part of combination therapy for first-line cervical cancer. Planned phase 2 trial in solid tumors. Phase 1/2 trial ongoing in solid tumors. |
| | | |
Ladiratuzumab vedotin (SGN-LIV1A) | | Anti-LIV-1 ADC | | Seattle Genetics | | Phase 1 trial ongoing for patients withLIV-1-positive metastatic breast cancer, in particular triple negative disease. Phase 2 trial ongoing evaluating ladiratuzumab vedotin as part ofneo-adjuvant therapy in patients with breast cancer (theI-SPY2 trial). Planned phase 1b/2 trial of ladiratuzumab vedotin in combination with pembrolizumab for first-line metastatic triple negative breast cancer. Planned phase 1b/2 trial of ladiratuzumab vedotin in combination with atezolizumab for second-line metastatic triple negative breast cancer (the MORPHEUS trial). |
ADCETRIS
ADCETRIS is an ADC comprised of an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a proprietary microtubule disrupting agent, monomethyl auristatin E, or MMAE. ADCETRIS employs a linker system that is designed to be stable in the bloodstream and to release MMAE upon internalization into CD30-expressing cells. We believe that the CD30 antigen is an attractive target for cancer therapy because it is expressed on multiple types of cancer, but has limited expression on normal tissues. We are collaborating with Takeda on the global development and commercialization of ADCETRIS. Under this collaboration, we have rights to commercialize ADCETRIS in the United States and Canada. Takeda has exclusive rights to commercialize ADCETRIS in the rest of the world. ADCETRIS has received regulatory approvals as follows:
| | • | | United States. ADCETRIS® (brentuximab vedotin) injection for intravenous infusion has received approval from the FDA for four indications: (1) regular approval for the treatment of adult patients with classical Hodgkin lymphoma after failure of auto-HSCT or after failure of at least two prior multi-agent chemotherapy regimens in patients who are not auto-HSCT candidates, (2) regular approval for the treatment of classical Hodgkin lymphoma adult patients at high risk of relapse or progression as post-auto-HSCT consolidation, (3) accelerated approval for the treatment of adult patients with sALCL after |
8
| | failure of at least one prior multi-agent chemotherapy regimen, and (4) regular approval for the treatment of adult patients with pcALCL and CD30-expressing MF who have received prior systemic therapy. The sALCL indication is approved under accelerated approval based on overall response rate. Continued approval for the sALCL indication is contingent upon verification and description of clinical benefit in a confirmatory phase 3 trial. |
| | • | | Canada. Health Canada has issued a Notice of Compliance with conditions, authorizing marketing of ADCETRIS for two lymphoma indications: (1) the treatment of patients with Hodgkin lymphoma after failure of ASCT, or after failure of at least two multi-agent chemotherapy regimens in patients who are not ASCT candidates, and (2) the treatment of patients with sALCL after failure of at least one multi-agent chemotherapy regimen. In addition, Health Canada grantednon-conditional approval for post-ASCT consolidation treatment of Hodgkin lymphoma patients at increased risk of relapse or progression. |
| | • | | European Union. ADCETRIS was granted conditional marketing authorization by the European Commission in October 2012 for two indications: (1) for the treatment of adult patients with relapsed or refractory CD30-positive Hodgkin lymphoma following ASCT, or following at least two prior therapies when ASCT or multi-agent chemotherapy is not a treatment option, and (2) the treatment of adult patients with relapsed or refractory sALCL. The European Commission extended the current conditional approval of ADCETRIS and approved ADCETRIS for the treatment of adult patients with CD30-positive Hodgkin lymphoma at increased risk of relapse or progression following ASCT. In addition, in January 2018, the European Commission further extended the marketing authorization for ADCETRIS for the treatment of adult patients with CD30-positive cutaneousT-cell lymphoma (CTCL) after at least one prior systemic therapy. |
| | • | | Worldwide. As of January 2018, ADCETRIS is commercially available in 70 countries for relapsed or refractory Hodgkin lymphoma and relapsed or refractory sALCL. |
ADCETRIS was granted approval for the treatment of patients with sALCL after failure of at least one prior multi-agent chemotherapy regimen under the FDA’s accelerated approval regulations, which allows the FDA to approve products for cancer or other serious or life-threatening illnesses based on surrogate endpoints or on a clinical endpoint other than survival or irreversible morbidity. Under the FDA’s accelerated approval regulations, we are subject to certain post-approval requirements that require an additional confirmatory phase 3 trial to verify and describe the clinical benefit of ADCETRIS. In addition, we are subject to extensive ongoing obligations and continued regulatory review from the FDA and other applicable regulatory agencies, such as continued adverse event reporting requirements and the requirement to have our promotional materialspre-cleared by the FDA.
In the United States, whileECHELON-2 is a required post-approval study in connection with the accelerated approval of the relapsed sALCL indication, results from either theECHELON-1 or theECHELON-2 trial may be sufficient to confirm the clinical benefit of ADCETRIS in relapsed sALCL and convert the approval of ADCETRIS from accelerated approval to regular approval in its currently approved relapsed sALCL indication. In Canada, theECHELON-2 trial is a required post-approval study to remove conditions on the approval of ADCETRIS in the relapsed sALCL indication. In Europe, there are separate post-approval requirements to convert the conditional marketing authorization of ADCETRIS to a standard marketing authorization in the relapsed sALCL indication.
In addition, with respect to the accelerated approval of ADCETRIS for relapsed Hodgkin lymphoma in Canada and Europe,ECHELON-1 is a required post-approval study to remove conditions on the approval of ADCETRIS in relapsed Hodgkin lymphoma in Canada, and in Europe, there are separate post-approval requirements to convert the conditional marketing authorization of ADCETRIS to a standard marketing authorization in the relapsed Hodgkin lymphoma indication.
9
Market Opportunities
According to the American Cancer Society, more than 8,200 cases of Hodgkin lymphoma were expected to be diagnosed in the United States during 2017, and an estimated 1,070 people were expected to die of the disease. Approximately 4,000 patients are diagnosed annually in the United States with a type of CD30-expressing MTCL, including sALCL. The use of combination chemotherapy as frontline therapy for malignant lymphomas has resulted in high remission rates; however, these frontline chemotherapy regimens have substantial associated toxicities and a significant number of lymphoma patients relapse and require additional treatments including other chemotherapy regimens and ASCT. For the reasons discussed in “Item 1A—Risk Factors”, we may not be able to obtain regulatory approvals to market ADCETRIS for frontline Hodgkin lymphoma or MTCL, or otherwise continue to expand its labeled indications of use. An estimated 1,000 people annually have CD30-expressing mycosis fungoides or primary cutaneous ALCL requiring systemic therapy.
ADCETRIS Clinical Development Status and Plan
In collaboration with our partners, we are pursuing a broad development strategy for ADCETRIS that includes clinical trials of ADCETRIS evaluating its therapeutic potential in newly diagnosed patients with Hodgkin lymphoma, or MTCL, also known as PTCL, including sALCL. We are also evaluating ADCETRIS in combination with a CPI. These ongoing clinical trials include:
Phase 3 Frontline Hodgkin Lymphoma(ECHELON-1). In June 2017, we and Takeda announced positive top line data from theECHELON-1 trial, a randomized, open-label, phase 3 trial investigating ADCETRIS plus AVD versus ABVD as frontline combination therapy in 1,334 patients with previously untreated advanced classical Hodgkin lymphoma. Additional data were reported at the 59th American Society of Hematology (ASH) annual meeting. TheECHELON-1 trial met its primary endpoint, demonstrating that treatment with ADCETRIS plus AVD resulted in a statistically significant improvement in modified progression-free survival, or PFS, versus the control arm as assessed by an independent review facility (hazard ratio=0.770;p-value=0.035). Thetwo-year modified PFS rate per independent review for patients in the ADCETRIS plus AVD arm was 82.1 percent compared to 77.2 percent in the control arm. Per investigator assessment, thetwo-year modified PFS rate for patients in the ADCETRIS plus AVD arm was 81.0 percent compared to 74.4 percent in the control arm. All secondary endpoints trended in favor of the ADCETRIS plus AVD arm, including interim analysis of overall survival (hazard ratio=0.72;p-value=0.19), the key secondary endpoint. The safety profile of ADCETRIS plus AVD in theECHELON-1 trial was generally consistent with that known for the single-agent components of the regimen. The most common clinically relevant adverse events of any grade that occurred in at least 15 percent of patients in the ADCETRIS plus AVD and ABVD arms were: neutropenia (58 and 45 percent, respectively), constipation (42 and 37 percent, respectively), vomiting (33 and 28 percent, respectively), fatigue (both 32 percent), peripheral sensory neuropathy (29 and 17 percent, respectively), diarrhea (27 and 18 percent, respectively), pyrexia (27 and 22 percent, respectively), peripheral neuropathy (26 and 13 percent, respectively), abdominal pain (21 and 10 percent, respectively) and stomatitis (21 and 16 percent, respectively). In both the ADCETRIS plus AVD and ABVD arms, the most common Grade 3 or 4 events were neutropenia, febrile neutropenia and neutrophil count decrease. Febrile neutropenia was reduced through the use of prophylactic growth factors(G-CSF) in a subset of patients. In the ADCETRIS plus AVD arm of the study, the rate of febrile neutropenia without the use ofG-CSF was 21 percent and with the use ofG-CSF was reduced to 11 percent.G-CSF primary prophylaxis with ADCETRIS plus AVD resulted in an overall comparable safety profile to ABVD, decreasing the incidence of febrile neutropenia, neutropenia and serious adverse events. Primary prophylaxis withG-CSF was used in a subset of patients enrolled in the study. In the ADCETRIS plus AVD arm, peripheral neuropathy events were observed in 67 percent of patients compared to 43 percent on the ABVD arm. In the ADCETRIS plus AVD arm, the majority of peripheral neuropathy events were Grade 1 or 2. Grade³3 events were reported in 11 percent of patients and Grade 4 events were reported in less than 1 percent of patients. In the ABVD arm, Grade³3 events were reported in 2 percent of patients and there were no Grade 4 events.Two-thirds of the patients with peripheral neuropathy in the ADCETRIS plus AVD arm reported resolution or improvement at lastfollow-up. Pulmonary toxicity, defined as events related to interstitial lung disease, was reported in 2 percent of patients in the ADCETRIS plus AVD arm versus 7 percent of patients in the ABVD arm;
10
Grade³3 events were reported in less than 1 percent versus 3 percent, in the ADCETRIS plus AVD arm and the ABVD arm, respectively. 9 on study deaths occurred in the ADCETRIS plus AVD arm, of which 7 were due to neutropenia or associated complications (all occurred in patients who had not received primary prophylaxis withG-CSF with the exception of 1 patient who entered the trial withpre-existing neutropenia). The remaining 2 deaths were due to myocardial infarction. In the ABVD arm, there were 13 on study deaths, of which 11 were due to or associated with pulmonary-related toxicity, 1 was due to cardiopulmonary failure and 1 death had unknown cause.ECHELON-1 is being conducted under a SPA agreement with the FDA and pursuant to scientific advice from the EMA.
In September 2017, the FDA granted BTD to ADCETRIS in combination with chemotherapy for the frontline treatment of patients with advanced classical Hodgkin lymphoma. In November 2017, we submitted a sBLA to the FDA seeking approval of ADCETRIS as part of a frontline combination chemotherapy regimen in patients with previously untreated advanced classical Hodgkin lymphoma. In December 2017, the FDA granted Priority Review for the sBLA, and the PDUFA target action date is May 1, 2018.
Phase 3 Frontline MatureT-Cell Lymphoma(ECHELON-2). We and Takeda have completed patient enrollment of 452 patients in a global randomized, double-blind, placebo-controlled multi-center phase 3 clinical trial known asECHELON-2. This trial is evaluating ADCETRIS in combination with CHP versus CHOP for the treatment of newly diagnosed CD30-expressing MTCL patients, including patients with sALCL and other types of peripheralT-cell lymphomas. The primary endpoint of the trial is PFS per independent review facility assessment. Secondary endpoints include overall survival, complete remission rate and safety. Based on reviews of pooled, blinded data, we have observed a lower rate of reported PFS events than anticipated in theECHELON-2 trial. We plan to discuss with the FDA the potential to unblind the trial prior to achieving the target number of PFS events specified in our SPA agreement. We cannot predict the outcome of those discussions or whether we would be able to reach agreement with the FDA. See “Item 1A—Risk Factors—Risks Related to Our Business—Our near-term prospects are substantially dependent on ADCETRIS. If we and/or Takeda are unable to effectively commercialize ADCETRIS for the treatment of patients in its approved indications and to continue to expand its labeled indications of use, our ability to generate significant revenue and our prospects for profitability will be adversely affected” and“—Clinical trials are expensive and time consuming, may take longer than we expect or may not be completed at all, and their outcome is uncertain.” Based on the length offollow-up and the slow rate at which PFS events are occurring, we believe the primary endpoint data will be mature and expect to reporttop-line data in 2018. A companion diagnostic test is being used in this trial to assess CD30-expression. We expect that concurrent approval of a CD30 companion diagnostic will be required for any approval of ADCETRIS in the frontline MTCL indication. We are developing a companion diagnostic under a collaboration agreement with Ventana Medical Systems, or Ventana, and Takeda. TheECHELON-2 trial is being conducted under a SPA agreement with the FDA and also received scientific advice from the EMA. We are required to conduct this trial as part of our ADCETRIS post-marketing requirement for the relapsed sALCL indication, and the trial is designed to be confirmatory in the United States and Canada.
Data from a phase 1 trial that evaluated ADCETRIS plus chemotherapy for frontline sALCL, which was subsequently amended to include patients with any CD30-expressing MTCL, supported our decision to initiate theECHELON-2 trial. Among the 26 patients who received the combination regimen of ADCETRIS plus CHP, 88 percent achieved a complete remission. At the December 2017 ASH annual meeting,follow-up data were reported showing that the estimated five-year PFS rate was 52 percent, with no patients receiving a consolidative stem cell transplant in first remission. The estimated five-year overall survival rate was 80 percent. There were no progression events or deaths in the trial since the three-year follow up. 73 percent of patients (19 of 26) experienced peripheral neuropathy, the majority of which was Grade 1 or 2. 95 percent of these patients had complete resolution or some improvement of their symptoms at lastfollow-up with a median time to resolution of 4.2 months and a median time to improvement of symptoms of 2.6 months.
Phase 3 Relapsed/Refractory Hodgkin Lymphoma (CHECKMATE 812). We and BMS are conducting a pivotal phase 3 clinical trial, or the CHECKMATE 812 trial, to evaluate the combination of BMS’s
11
immunotherapy nivolumab (Opdivo) with ADCETRIS for the treatment of relapsed or refractory, or transplant-ineligible, advanced classical Hodgkin lymphoma. Nivolumab is a programmeddeath-1, orPD-1, immune checkpoint inhibitor that is designed to harness the body’s own immune system to help restore antitumor immune response. The primary endpoint for the CHECKMATE 812 trial is PFS and targeted enrollment is 340 patients.
The CHECKMATE 812 trial is supported by interim data from a phase 1/2 trial in second-line Hodgkin lymphoma, which is one of three trials being conducted under a clinical trial collaboration agreement between us and BMS to evaluate the investigational combination of ADCETRIS and nivolumab.
Updated interim data from the phase 1/2 trial evaluating the combination of ADCETRIS and nivolumab for patients with second line Hodgkin lymphoma were presented at the 2017 ASH annual meeting. Data were reported from 62 patients with relapsed or refractory Hodgkin lymphoma who received the combination regimen of ADCETRIS plus nivolumab after failure of frontline therapy. After completion of the fourth cycle of treatment, patients were eligible to undergo an ASCT. Of 60 response-evaluable patients, 83 percent had an objective response, including 62 percent with a complete response. The estimatedsix-month PFS rate was 89 percent. The most common adverse events of any grade occurring prior to ASCT or subsequent salvage therapy in at least 20 percent of patients were nausea, fatigue, infusion-related reaction, or IRR, pruritus, diarrhea, headache, cough, vomiting, dyspnea, nasal congestion, pyrexia and rash. IRRs were observed in 44 percent of patients, of which the majority (41 percent) were Grade 1 or 2. No patients discontinued treatment due to an IRR.
The third ongoing trial under our clinical collaboration with BMS is evaluating the combination of ADCETRIS and nivolumab in patients with relapsed or refractoryB-cell andT-cellnon-Hodgkin lymphomas, including DLBCL and rareB-cell lymphomas, including gray zone and mediastinalB-cell lymphomas.
Frontline Therapy for Hodgkin Lymphoma Patients Age 60 and Over. In October 2012, we initiated a phase 2 clinical trial evaluating ADCETRIS monotherapy as a frontline therapy for patients age 60 or older with newly diagnosed Hodgkin lymphoma. The trial was subsequently amended to include the administration of ADCETRIS in combination with bendamustine or dacarbazine. In 2015, the bendamustine arm was closed because the tolerability of the combination did not meet study goals for this fragile patient population. Subsequently, the study was further expanded to evaluate the combination of ADCETRIS and nivolumab. ADCETRIS monotherapy is included in National Comprehensive Cancer Network, or NCCN, guidelines for older patients with relapsed or refractory Hodgkin lymphoma as a palliative therapy option.
Investigator-Sponsored Trials. In addition to our corporate-sponsored trials, as of December 31, 2017, there were more than 40 reported investigator-sponsored trials of ADCETRIS in the United States. In addition, we and Takeda are reviewing proposals from multiple clinical investigators and cooperative groups in the United States, Canada and Europe about potential investigator-sponsored trials of ADCETRIS. The investigator-sponsored trials to date include the use of ADCETRIS in a number of malignant hematologic indications such as CTCL, DLBCL, untreated limited stage Hodgkin lymphoma, salvage therapy for patients with Hodgkin lymphoma prior to auto-HSCT and graft versus host disease. There are also numerous other investigator-sponsored trials for the use of ADCETRIS in other CD30-expressing and select CD30-undetectable settings, and in solid tumors such as mesothelioma and testicular germ cell tumors. Several investigator-sponsored trials are currently evaluating ADCETRIS with immuno-oncology compounds in Hodgkin lymphoma, and we expect additional investigator-sponsored trials might evaluate ADCETRIS in novel combination regimens.
Enfortumab Vedotin(ASG-22ME)
Enfortumab vedotin is an ADC composed of ananti-Nectin-4 monoclonal antibody linked to a potent auristatin compound using our proprietary ADC technology.Nectin-4 is a novel target expressed in multiple cancers including urothelial cancers, such as bladder cancer, as well as ovarian and lung cancers. We are developing enfortumab vedotin as a potential treatment for solid tumors under ourco-development collaboration with Astellas, and we share all costs and, if commercialized, profits for the product candidate with Astellas on a 50:50 basis.
12
Approximately 15,000 people are diagnosed annually in the United States with metastatic urothelial cancer. Several CPIs have been approved for urothelial cancer in the past several years and are improving outcomes for some patients, yet the vast majority of patients do not benefit, or relapse, and require additional treatment options. There are no approved agents in thepost-CPI setting, representing an unmet medical need and potential rapid development pathway.
In October 2017, we and Astellas initiated a pivotal,single-arm phase 2 clinical trial of single-agent enfortumab vedotin for locally advanced or metastatic urothelial cancer patients who have been previously treated with CPI therapy. The primary endpoint of the trial is confirmed objective response rate per independent review. The trial will also assess overall survival, PFS, safety and tolerability. The study is designed to enroll approximately 120 patients at multiple centers globally.
Data from a phase 1 trial that evaluated enfortumab vedotin in solid tumors, primarily urothelial cancer, supported our decision to initiate the pivotal phase 2 trial. In June 2017, we and Astellas reported updated data from the phase 1, open-label, dose-escalation, multi-center clinical trial of enfortumab vedotin at the American Society of Clinical Oncology, or ASCO, annual meeting. Of the 71 patients with metastatic urothelial cancer evaluated for response, 41 percent had an objective response, including 4 percent who achieved a complete response. The preliminary estimate of median duration of response for all patients was 24 weeks. In 30 patients treated at the recommended phase 2 dose of 1.25 mg/kg, 53 percent had an objective response, including 3 percent who achieved a complete response. Of the 32 patients previously treated with CPIs and evaluated for response, 44 percent had an objective response, including three percent with complete response. Among the 17CPI-treated patients treated at the recommended phase 2 dose, 47 percent achieved a partial response. The most common treatment-related adverse events of any grade occurring in 10 percent or more of patients were nausea (36 percent), pruritus (31 percent), fatigue (30 percent) and diarrhea (28 percent). In February 2018, we and Astellas reported updated data on this trial in a poster presentation the American Society of Clinical Oncology 2018 Genitourinary Cancers Symposium. As of October 2, 2017, the data cut date for the poster, a total of 67 patients with metastatic urothelial carcinoma whose disease progressed after treatment with CPIs received the recommended phase 2 dose of 1.25 mg/kg of enfortumab vedotin once a week for three of every four-week cycle. For the 55 patients with evaluable data, the confirmed response rate was reported as 31 percent (N=17). Since October 2, 2017, an additional 6 patients have achieved a confirmed response. Updated data from these and additional patients, who continue to be enrolled in the trial, are expected to be reported at an upcoming medical meeting in 2018. In data presented, the most common treatment-emergent adverse event(s), or TEAE, of any grade for all patients were fatigue (55 percent), nausea (48 percent), decreased appetite (45 percent), and diarrhea and alopecia (43 percent each). In the presentation, 4 fatalities were reported possibly related to enfortumab vedotin treatment, two reported prior to October 2, 2017 due to respiratory failure and urinary tract obstruction and two cases after October 2, 2017 due to hyperglycemia. Since October 2, 2017, the study protocol has been amended to address the hyperglycemia finding. Hyponatremia (six percent), or low sodium in the blood, was the only Grade 3 or 4 TEAE occurring in greater than five percent of the cohort population.
As part of our effort to evaluate enfortumab vedotin in earlier lines of therapy, we and Astellas initiated in November 2017 a phase 1b trial evaluating the safety and tolerability of enfortumab vedotin in combination with pembrolizumab for first- or second-line treatment of patients with locally advanced or metastatic urothelial cancer. The single arm multi-center trial is designed to enroll up to 85 patients who are ineligible for first-line cisplatin-based chemotherapy or have progressed following treatment with a regimen containing platinum-based chemotherapy. The primary objective of the trial is to assess the safety and tolerability of enfortumab vedotin in combination with CPI therapy.
Tisotumab Vedotin
Tisotumab vedotin is an ADC composed of a human antibody that binds to tissue factor linked to a potent auristatin compound using our proprietary ADC technology. Tissue factor is expressed on many solid tumors, including cervical, ovarian, prostate and bladder. In August 2017, we exercised our option toco-develop
13
tisotumab vedotin with Genmab, sharing all future costs and, if commercialized, profits for the product candidate with Genmab on a 50:50 basis.
According to the American Cancer Society, approximately 13,000 women are diagnosed annually in the United States with cervical cancer and 4,000 are expected to die. Despite improvements in detecting and preventing metastatic cervical cancer, this remains a substantial unmet medical need.
In the first half of 2018, we and Genmab plan to initiate a pivotal phase 2 clinical trial of tisotumab vedotin in patients with recurrent and/or metastatic cervical cancer. Thesingle-arm trial is expected to enroll approximately 100 patients who have relapsed or progressed on or after platinum-containing chemotherapy and who have received or are ineligible for bevacizumab (Avastin). The primary endpoint of the study will be overall response rate as assessed by independent review. The planned trial will also assess duration of response and safety.
Data from a phase 1/2 trial that evaluated tisotumab vedotin in solid tumors, including cervical cancer, supported our decision to initiate the pivotal phase 2 trial. In September 2017, we and Genmab reported data from part 2 of the phase 1/2 trial at the European Society for Medical Oncology, or ESMO, Congress. In an expansion cohort of 34 patients with relapsed, recurrent and/or metastatic cervical cancer, 32 percent achieved a response. Median duration of confirmed responses was 8.3 months. The most common adverse events of any grade were conjunctivitis (50 percent), epistaxis, fatigue and alopecia (47 percent each) and nausea (44 percent).
Beyond recurrent and/or metastatic cervical cancer, we believe there may be opportunities for tisotumab vedotin in earlier lines of cervical cancer and in other solid tumors that express tissue factor. In 2018, we and Genmab also plan to initiate at least two additional clinical trials of tisotumab vedotin. One trial will evaluate tisotumab vedotin as part of a combination regimen for first-line cervical cancer. The second trial will evaluate tisotumab vedotin in other types of solid tumors.
Ladiratuzumab Vedotin(SGN-LIV1A)
Ladiratuzumab vedotin is an ADC composed of ananti-LIV-1 monoclonal antibody linked to a potent auristatin compound using our proprietary ADC technology, and is being developed as a potential treatment of metastatic breast cancer.
In October 2013 we initiated a phase 1, open-label, dose-escalation clinical trial to evaluate the safety and antitumor activity of ladiratuzumab vedotin in patients withLIV-1-positive metastatic breast cancer. At the December 2017 San Antonio Breast Cancer Symposium annual meeting, updated interim data were reported showing that among the 60 efficacy-evaluable patients with metastatic triple negative breast cancer, 25 percent achieved a partial response. At the recommended dose, 29 percent of patients achieved a partial response. The median PFS and median duration of response for patients treated across all dose levels were 11 weeks and 13.3 weeks, respectively. In 19 patients treated at the recommended dose, the median PFS was 12.1 weeks, and the median duration of response was 17.4 weeks. Of the 81 patients treated in the study, peripheral neuropathy events occurred in 20 percent and were generally low grade (Grades 1/2) and manageable. Grades 3/4 adverse events included neutropenia and anemia. Enrollment continues for patients with metastatic triple negative breast cancer at the recommended dose of 2.5 mg/kg, with a maximum dose of 200 mg per cycle.
Ladiratuzumab vedotin is also being evaluated in several other settings for metastatic breast cancer. Inmid-2018, we plan to initiate a phase 1b/2 clinical trial in combination with pembrolizumab (Keytruda) in patients with locally advanced or metastatic triple negative breast cancer. This single arm, open label multicenter study will be conducted under a collaboration agreement with Merck and is anticipated to enroll up to 72 patients.
Ladiratuzumab vedotin is also being evaluated in theI-SPY 2 trial, a phase 2 trial being conducted by a consortium that includes major cancer research centers and receives support from multiple industry partners. In
14
this trial, ladiratuzumab vedotin followed by standard chemotherapy as aneo-adjuvant treatment (prior to surgery) is being evaluated for women with newly diagnosed, locally advanced Stage 2 or 3 HER2-negative breast cancer. This trial is anticipated to enroll up to 75 patients in the ladiratuzumab vedotin treatment arm.
Under a clinical collaboration with Genentech, ladiratuzumab vedotin will be evaluated in combination with atezolizumab (Tecentriq) as part of the MORPHEUS trial. The planned phase 1b/2 MORPHEUS trial will evaluate the combination as second-line therapy in patients with metastatic triple negative breast cancer who have not been previously treated with immunotherapy. Thismulti-arm study is anticipated to enroll up to 45 patients in the ladiratuzumab vedotin arm.
Additional Product Candidates
Our earlier stage clinical pipeline includes five other ADC programs consisting of denintuzumab mafodotin, orSGN-CD19A,SGN-CD19B,SGN-CD123A,SGN-CD352A and SGN-CD33A as well as two immuno-oncology agents,SEA-CD40 andSGN-2FF. We continue to evaluate these product candidates and will advance them into further development as we determine appropriate based on clinical data and resource prioritization.
In June 2017, we announced that we were discontinuing the phase 3 CASCADE clinical trial of SGN-CD33A, or vadastuximab talirine, in frontline older AML patients, and suspending patient enrollment and treatment in all other SGN-CD33A trials. We took this action following consultation with the Independent Data Monitoring Committee, or IDMC, and after reviewing unblinded data from the CASCADE trail which indicated a higher rate of deaths, including fatal infections, in the SGN-CD33A-containing arm versus the control arm of the trial. In addition, the Investigational New Drug application, or IND, for SGN-CD33A has been placed on hold, and no clinical trials may resume under the IND until the FDA lifts the clinical hold. We are continuing to review data for the SGN-CD33A program; however, at this time we have no plans to initiate additional clinical trials of SGN-CD33A.
Research Programs
In addition to our pipeline of product candidates and antibody-based and SEA technologies, we have internal research programs directed toward developing new classes of potent, cell-killing agents and stable linkers, identifying novel antigen targets, monoclonal antibodies and other targeting molecules, and advancing our antibody engineering initiatives.
New Cell-Killing Agents. We continue to study new cell-killing agents that can be linked to antibodies, such as the auristatins and PBDs that we currently use in our ADC technology, and new classes of cell-killing agents.
New Stable Linkers. We are conducting research with the intent to develop new linker systems that are more stable in the bloodstream and more effective at releasing the cell-killing agent once inside targeted cancer cells.
Novel Monoclonal Antibodies and Antigen Targets. We are actively engaged in internal efforts to identify and develop monoclonal antibodies and other targeting molecules and ADCs with novel specificities and activities against selected antigen targets. We focus on antigen targets that are highly expressed on the surface of cancer cells that may serve as targets for monoclonal antibodies or ADCs. We may then create and screen panels of cancer-reactive monoclonal antibodies in our laboratories to identify those with the desired specificity. We supplement these internal efforts by evaluating opportunities toin-license targets and antibodies from academic groups and other biotechnology and pharmaceutical companies, such as our ongoingco-development collaborations with Astellas and Genmab.
Antibody Engineering. We have substantial internal expertise in antibody engineering, both for antibody humanization andnon-fucosylation, as well as engineering of antibodies to improve drug linkage sites for use
15
with our ADC technology. By modifying the number and type of drug-linkage sites found on our antibodies, we believe that we can improve the robustness and cost-effectiveness of our manufacturing processes for conjugation of ADCs.
Research and Development Expense
Since inception, we have devoted a significant amount of resources to develop ADCETRIS, our product candidates and our antibody-based technologies. For the years ended December 31, 2017, 2016, and 2015, we recorded $456.7 million, $379.3 million, and $294.5 million, respectively, in research and development expenses.
Corporate Collaborations
We enter into collaborations with biotechnology and pharmaceutical companies to advance the development and commercialization of our product candidates and to supplement our internal pipeline. We seek collaborations that will allow us to retain significant future participation in product sales through either profit-sharing or royalties paid on net sales. We also have licensed our ADC technology to collaborators to be developed with their own antibodies. These ADC collaborations benefit us in many ways, including generating cash flow and revenues that partially offset expenditures on our internal research and development programs, expanding our knowledge base regarding ADCs across multiple targets and antibodies provided by our collaborators and providing us with future pipeline opportunities throughco-development oropt-in rights to new ADC product candidates.
Takeda ADCETRIS Collaboration
In 2009, we entered into a collaboration agreement with Takeda to develop and commercialize ADCETRIS, under which Seattle Genetics retains commercial rights in the United States and its territories and in Canada, and Takeda and its affiliates have commercial rights in the rest of the world. As of December 31, 2017, we had received an upfront payment of $60 million and had achieved milestone payments totaling $70 million related to regulatory and commercial progress by Takeda. As of December 31, 2017, we were entitled to receive additional progress- and sales-dependent milestone payments of up to $165 million based on Takeda’s achievement of significant events under the collaboration in addition to tiered royalties with percentages ranging from themid-teens and to themid-twenties based on net sales of ADCETRIS within Takeda’s licensed territories. Takeda also bears a portion of third-party royalty costs owed on sales of ADCETRIS in its territory. We and Takeda equallyco-fund the cost of selected development activities conducted under the collaboration. Although we are funding half of the development activities conducted under the collaboration, Takeda is responsible for the achievement of the progress- and sales-dependent milestone payments that we may receive. Either party may terminate the collaboration agreement if the other party materially breaches the agreement and such breach remains uncured. Takeda may terminate the collaboration agreement for any reason upon prior written notice to us and we may terminate the collaboration agreement in certain circumstances. The collaboration agreement can also be terminated by mutual written consent of the parties. If neither party terminates the collaboration agreement, then the agreement automatically terminates on the expiration of all payment obligations.
AstellasCo-Development Collaboration
In 2007, we entered into an agreement with Agensys, which subsequently became an affiliate of Astellas, to jointly research, develop and commercialize ADCs for the treatment of several types of cancer. The collaboration encompasses combinations of our ADC technology with fully-human antibodies developed by Astellas to proprietary cancer targets.
Under this collaboration, we and Astellas areco-funding all development and commercialization costs for enfortumab vedotin and will share on a 50:50 basis in any profits that may come from this product candidate if successfully commercialized. Costs associated withco-development activities are included in research and development expense. Either party may opt out ofco-development and profit-sharing in return for receiving milestones and royalties from the continuing party. The agreement contemplates that the parties will enter into supplemental agreements pursuant to which they will allocate responsibilities.
16
Either party may terminate the collaboration agreement if the other party becomes insolvent or the other party materially breaches the agreement and such breach remains uncured. Subject to certain restrictions, either party may terminate the collaboration agreement for any reason upon prior written notice to the other party. The collaboration agreement can also be terminated by mutual written consent of the parties. If neither party exercises its option to terminate the collaboration agreement, then the agreement will automatically terminate on the later of (a) the expiration of all payment obligations pursuant to the collaboration agreement, or (b) the day upon which we and Astellas cease to develop and commercialize products under the agreement.
GenmabCo-Development Collaboration
In 2011, we entered into an ADC research collaboration agreement with Genmab. Under the agreement, Genmab has rights to utilize our ADC technology with itsHuMax-TF antibody targeting the Tissue Factor, or TF, antigen, which is expressed on numerous types of solid tumors.
Under this agreement, we exercised aco-development option for tisotumab vedotin in August 2017. We and Genmab will share all future costs and profits for development and commercialization of tisotumab vedotin on a 50:50 basis. Costs associated withco-development activities are included in research and development expense. We will be responsible for tisotumab vedotin commercialization activities in the U.S., Canada, and Mexico, while Genmab will be responsible for commercialization activities in all other territories. Each party has the option toco-promote up to a specified percentage of the sales effort in the other party’s territories. Either party may opt out ofco-development and profit-sharing in return for receiving milestones and royalties from the continuing party.
Either party may terminate the collaboration agreement if the other party becomes insolvent or materially breaches the agreement and such breach remains uncured. In addition, either party may terminate the collaboration agreement if such party’s patent rights subject to the agreement are challenged by the other party or its sublicensees.
Unum Therapeutics Collaboration
In June 2015, we entered into a collaboration agreement with Unum to develop and commercialize novel ACTR therapies incorporating our antibodies for the treatment of cancer. Unum’s proprietary ACTR technology enables programming of a patient’sT-cells to attack tumor cells whenco-administered with tumor-specific therapeutic antibodies. Under the terms of the agreement, we and Unum are developing two ACTR product candidates that combine Unum’s ACTR technology with our antibodies. Unum is obligated to conduct preclinical research and clinical development activities through phase 1 clinical trials, and we are obligated to provide funding for these activities. The agreement calls for us to work together toco-develop and jointly fund programs after phase 1 clinical trials unless either company opts out. We and Unum wouldco-commercialize any successfully developed product candidates and share any profits 50:50 on anyco-developed programs in the United States. We retain exclusive commercial rights outside of the United States, paying Unum a royalty that is a high single digit tomid-teens percentage ofex-U.S. sales. The potential future licensing and progress-dependent milestone payments to Unum under the collaboration may total up to $400 million between the two ACTR programs, payment of which is triggered by the achievement of development, regulatory and commercial milestones.
Pieris Pharmaceuticals Collaboration
In February 2018, we entered into a license and collaboration agreement and related platform technology license agreement with Pieris to develop novel bispecifics incorporating our antibodies and Pieris’ proprietary Anticalin proteins for the treatment of cancer. The agreements provide for an upfront payment totaling $30 million to Pieris. Under the terms of the license and collaboration agreement, Pieris is obligated to conduct preclinical research, and we are obligated to provide funding for these activities. Following this initial research phase, we will have the option to select up to three product candidates for further development. We would then develop the product candidates independently, subject to a limited opt in right held by Pieris. Prior to the initiation of pivotal trials with respect to the first product candidate developed,
17
we may in our discretion provide Pieris the option to co-develop that product candidate. Unless Pieris elects to co-develop the first product candidate, we are required to provide Pieris the option to co-develop the second product candidate. Regardless of any prior elections made by Pieris, we have no obligation to provide Pieris with a right to opt in to the development of the third product candidate. In the event Pieris does elect to opt in to the development of the first or second product candidate, Pieris would be required to reimburse us 100% of milestone payments received as of the date of exercise and 50% of post-GLP toxicology development costs. We and Pieris would share costs and profits associated with the co-developed product candidate on a 50:50 basis. Pieris would be responsible for commercialization in the U.S. and we would be responsible for commercialization activities in all other territories. With respect to the other two product candidates, or all three if Pieris does not exercise its right to opt-in, we would have sole responsibility for development, funding and commercialization and would owe Pieris development and sales milestones and royalties on sales in the mid-single digits to low double digits. The potential future licensing and progress-dependent milestone payments to Pieris under the collaboration for the three product candidates total up to $1.2 billion based on the achievement of development, regulatory and commercial milestones. We also have the right to select additional candidates for further development subject to the payment of additional fees, milestone payments and royalties.
Other ADC Collaborations
We have other active collaborations with a number of companies to allow them to use our proprietary ADC technology. Under these ADC collaborations, which we have entered into in the ordinary course of business, we typically receive or are entitled to receive upfront cash payments, progress- and sales-dependent milestones and royalties on net sales of products incorporating our ADC technology, as well as annual maintenance fees and support fees for research and development services and materials provided under the agreements. Our ADC collaborators are solely responsible for research, product development, manufacturing and commercialization of any product candidates under these collaborations, which includes the achievement of the potential milestones.
Our current ADC collaborations are at various stages of clinical and preclinical development. Our ability to generate significant future revenues from our current ADC collaboration agreements will largely depend on a product that incorporates our ADC technology entering late-stage clinical development and receiving marketing approval from the FDA at which point the milestone payments, royalties or other rights and benefits would become more substantial and material to our company.
License Agreements
We havein-licensed antibodies, targets and enabling technologies from pharmaceutical and biotechnology companies and academic institutions for use in our pipeline programs and ADC technology, including the following:
Bristol-Myers Squibb License. In March 1998, we obtained rights to some of our technologies and product candidates, portions of which are exclusive, through a license agreement with Bristol-Myers Squibb. Through this license, we secured rights to use various targeting technologies. Under the terms of the license agreement, we are required to pay royalties in the low single digits on net sales of products, including ADCETRIS, which incorporate various technologies owned by Bristol-Myers Squibb.
University of Miami License. In September 1999, we entered into an exclusive license agreement with the University of Miami, Florida, covering an anti-CD30 monoclonal antibody that is the basis for the antibody component of ADCETRIS. Under the terms of this license, we made an upfront payment and progress-dependent milestone payments. We are required to pay annual maintenance fees and royalties in the low single digits on net sales of products, including ADCETRIS, incorporating technology licensed from the University of Miami.
Patents and Proprietary Technology
Our owned and licensed patents and patent applications are directed to ADCETRIS, our product candidates, monoclonal antibodies, our ADC and SEA technologies and other antibody-based and/or enabling technologies. We commonly seek patent claims directed to compositions of matter, including antibodies, ADCs, and drug-
18
linkers containing highly potent cell-killing agents, as well as methods of using such compositions. When appropriate, we also seek claims to related technologies, such as methods of using certain sugar analogs utilized in our SEA technology. For ADCETRIS and each of our product candidates, we have filed or expect to file multiple patent applications. We maintain patents and prosecute applications worldwide for technologies that we haveout-licensed, such as our ADC technology. Similarly, for partnered products and product candidates, such as ADCETRIS, enfortumab vedotin and tisotumab vedotin, we seek to work closely with our development partners to coordinate patent efforts, including patent application filings, prosecution, term extension, defense and enforcement. As ADCETRIS and our development product candidates advance through research and development, we seek to diligently identify and protect new inventions, such as combination therapies, improvements to methods of manufacturing, and methods of treatment. We also work closely with our scientific personnel to identify and protect new inventions that could eventually add to our development pipeline.
We have the following patents relating to ADCETRIS and our pipeline:
| | • | | For ADCETRIS and our related ADC technology, we own ten patents in the United States and Europe that will expire between 2020 and 2031. |
| | • | | For enfortumab vedotin and our related ADC technology, we own,co-own or have licensed rights to ten patents in the United States and Europe that will expire between 2022 and 2031. Of these patents, we own orco-own eight patents and have licensed rights to two patents. |
| | • | | For tisotumab vedotin and our related ADC technology, we own,co-own or have licensed rights to ten patents in the United States and Europe that will expire between 2022 and 2032. Of these patents, we own orco-own five patents and have licensed rights to five patents. |
| | • | | For ladiratuzumab vedotin and our related ADC technology, we own,co-own or have licensed rights to nine patents in the United States and Europe that will expire between 2020 and 2032. Of these patents, we own orco-own rights to seven patents and have licensed rights to two patents. |
| | • | | For denintuzumab mafodotin and our related ADC technology, we own orco-own eleven patents in the United States and Europe that will expire between 2024 and 2029. |
| | • | | ForSEA-CD40 and our related SEA technology, we own,co-own or have licensed rights to twelve patents in the United States and Europe that will expire between 2019 and 2030. Of these patents, we own orco-own nine patents and have licensed rights to three patents. |
The actual protection afforded by a patent, which can vary from country to country, depends on the type of patent, the scope of its coverage as determined by the patent office or courts in the country, and the availability of legal remedies in the country. This list above does not identify all patents that may be related to ADCETRIS and our product candidates. For example, in addition to the listed patents, we have patents on platform technologies (that relate to certain general classes of products or methods), as well as patents that relate to methods of using, manufacturing or administering a product or product candidate, that may confer additional patent protection. We also have pending patent applications that may give rise to new patents related to one or more of these agents.
The information in the above list is based on our current assessment of patents that we own or control or have exclusively licensed. The information is subject to revision, for example, in the event of changes in the law or legal rulings affecting our patents or if we become aware of new information. Significant legal issues remain unresolved as to the extent and scope of available patent protection for biotechnology products and processes in the U.S. and other important markets outside the U.S. We expect that litigation will likely be necessary to determine the term, validity, enforceability, and/or scope of certain of our patents and other proprietary rights. An adverse decision or ruling with respect to one or more of our patents could result in the loss of patent protection for a product and, in turn, the introduction of competitor products orfollow-on biologics to the market earlier than anticipated, and could force us to either obtain third-party licenses at a material cost or cease using a technology or commercializing a product.
19
Patents expire, on a country by country basis, at various times depending on various factors, including the filing date of the corresponding patent application(s), the availability of patent term extension and supplemental protection certificates and requirements for terminal disclaimers. Although we believe our owned and licensed patents and patent applications provide us with a competitive advantage, the patent positions of biotechnology and pharmaceutical companies can be uncertain and involve complex legal and factual questions. We and our corporate collaborators may not be able to develop patentable products or processes or obtain patents from pending patent applications. Even if patent claims are allowed, the claims may not issue. In the event of issuance, the patents may not be sufficient to protect the proprietary technology owned by or licensed to us or our corporate collaborators. Our or our collaborators’ current patents, or patents that issue on pending applications, may be challenged, invalidated, infringed or circumvented. In addition, changes to patent laws in the United States or in other countries may limit our ability to defend or enforce our patents, or may apply retroactively to affect the term and/or scope of our patents. Our patents have been and may in the future be challenged by third parties in post-issuance administrative proceedings or in litigation as invalid, not infringed or unenforceable under U.S. or foreign laws, or they may be infringed by third parties. As a result, we are or may be from time to time involved in the defense and enforcement of our patent or other intellectual property rights in a court of law and administrative tribunals, such as in U.S. Patent and Trademark Office inter partes review or reexamination proceedings, foreign opposition proceedings or related legal and administrative proceedings in the United States and elsewhere. The costs of defending our patents or enforcing our proprietary rights in post-issuance administrative proceedings or litigation may be substantial and the outcome can be uncertain. An adverse outcome may allow third parties to use our proprietary technologies without a license from us or our collaborators. Our and our collaborators’ patents may also be circumvented, which may allow third parties to use similar technologies without a license from us or our collaborators.
Our commercial success depends significantly on our ability to operate without infringing patents and proprietary rights of third parties. Organizations such as pharmaceutical and biotechnology companies, universities and research institutions may have filed patent applications or may have been granted patents that cover technologies similar to the technologies owned or licensed to us or to our collaborators. In addition, we are monitoring the progress of multiple pending patent applications of other organizations that, if granted, may require us to license or challenge their validity or enforceability in order to continue commercializing ADCETRIS or to commercialize our product candidates. Our challenges to patents of other organizations may not be successful, which may affect our ability to commercialize ADCETRIS or our product candidates. We cannot determine with certainty whether patents or patent applications of other parties may materially affect our or our collaborators’ ability to make, use or sell ADCETRIS or any other products or product candidates.
We require our scientific personnel to maintain laboratory notebooks and other research records in accordance with our policies, which are designed to strengthen and support our intellectual property protection. In addition to our patented intellectual property, we also rely on trade secrets and other proprietary information, especially when we do not believe that patent protection is appropriate or can be obtained. Our policy is to require each of our employees, consultants and advisors to execute a proprietary information and inventions assignment agreement before beginning their employment, consulting or advisory relationship with us. These agreements provide that the individual must keep confidential and not disclose to other parties any confidential information developed or learned by the individual during the course of their relationship with us except in limited circumstances. These agreements also provide that we will own all inventions conceived or reduced to practice by the individual in the course of rendering services to us. Our agreements with collaborators require them to have a similar policy and agreements with their employees, consultants and advisors. Our policy and agreements and those of our collaborators may not sufficiently protect our confidential information, or third parties may independently develop equivalent information.
Government Regulation
The FDA and comparable regulatory agencies in state and local jurisdictions and in foreign countries impose substantial requirements upon the clinical development,pre-market approval, manufacture, marketing
20
and distribution of biopharmaceutical products. These agencies and other regulatory agencies regulate research and development activities and the testing, approval, manufacture, quality control, safety, efficacy, labeling, storage, distribution, import, export, recordkeeping, advertising and promotion of products and product candidates. Failure to comply with applicable FDA or other requirements may result in Warning Letters, civil or criminal penalties, suspension or delays in clinical development, recall or seizure of products, partial or total suspension of production or withdrawal of a product from the market. The development and approval process requires substantial time, effort and financial resources, and we cannot be certain that any approvals for our product candidates will be granted on a timely basis, if at all. We must obtain approval of our product candidates from the FDA before we can begin marketing them in the United States. Similar approvals are also required in other countries.
Product development and approval within this regulatory framework is uncertain, can take many years and requires the expenditure of substantial resources. The nature and extent of the governmental review process for our product candidates will vary, depending on the regulatory categorization of particular product candidates and various other factors.
The necessary steps before a new biopharmaceutical product may be sold in the United States ordinarily include:
| | • | | preclinical in vitro andin vivotests, some of which must comply with Good Laboratory Practices, or GLP; |
| | • | | submission to the FDA of an IND which must become effective before clinical trials may commence, and which must be updated at least annually with a report on development; |
| | • | | development of a drug formulation and manufacture of the drug product for clinical trials, and commercial sale, if approved; |
| | • | | completion of adequate and well controlled human clinical trials to establish the safety and efficacy of the product candidate for its intended use; |
| | • | | submission to the FDA of a marketing authorization application in the form of a BLA, which must be accompanied by a substantial user fee unless the fee is waived; |
| | • | | FDApre-approval inspection of manufacturing facilities for current Good Manufacturing Practices, or GMP, compliance and FDA inspection of select clinical trial sites for Good Clinical Practice, or GCP, compliance; and |
| | • | | FDA review and approval of the marketing authorization application and product prescribing information prior to any commercial sale. |
The results of preclinical tests (which include laboratory evaluation as well as preclinical GLP studies to evaluate toxicity) for a particular product candidate, together with related manufacturing information and analytical data, and a clinical protocol are submitted as part of an IND to the FDA. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30 day time period, raises concerns or questions about the conduct of the clinical trial, including concerns that human research subjects will be exposed to unreasonable health risks. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. IND submissions may be authorized by the FDA, for example, to commence a clinical trial. A separate submission to an existing IND must also be made for each successive clinical trial conducted during product development. Further, an independent institutional review board, or IRB, for each medical center proposing to conduct the clinical trial must review and approve the plan for any clinical trial before it commences at that center and it must monitor the study until completed. The FDA, the IRB or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk. Clinical testing also must satisfy extensive GCP regulations and regulations for informed consent and privacy of individually-identifiable information.
21
Clinical trials generally are conducted in three sequential phases that may overlap or in some instances, be skipped. In phase 1, the initial introduction of the product into humans, the product candidate is tested to assess safety, metabolism, pharmacokinetics and pharmacological actions associated with increasing doses. Phase 2 usually involves trials in a limited patient population to evaluate the efficacy of the potential product for specific, targeted indications, determine dosage tolerance and optimum dosage and further identify possible adverse reactions and safety risks. Phase 3 and pivotal trials are undertaken to evaluate further clinical efficacy and safety often in comparison to standard therapies within a broader patient population, generally at geographically dispersed clinical sites. Phase 4, or post-marketing, trials may be required as a condition of commercial approval by the FDA and may also be voluntarily initiated by us or our collaborators. Since we received accelerated approval for ADCETRIS from the FDA for the relapsed sALCL indication, we are subject to certain post-approval requirements pursuant to which we are conducting an additional confirmatory phase 3 trial, theECHELON-2 trial, to verify and describe the clinical benefit of ADCETRIS in the relapsed sALCL indication. Results from either theECHELON-1 trial or theECHELON-2 trial could fulfill this requirement in the United States. Phase 1, phase 2 or phase 3 testing may not be completed successfully within any specific period of time, if at all, with respect to any of our product candidates. Similarly, suggestions of safety, tolerability or efficacy in earlier stage trials do not necessarily predict findings of safety and efficacy in subsequent trials. Furthermore, the FDA, an IRB or we may suspend a clinical trial at any time for various reasons, including a finding that the subjects or patients are being exposed to an unacceptable health risk. Clinical trials are subject to central registration and results reporting requirements, such as on www.clinicaltrials.gov.
The results of preclinical studies, pharmaceutical development and clinical trials, together with information on a product’s chemistry, manufacturing, and controls, are submitted to the FDA in the form of a BLA, for approval of the manufacture, marketing and commercial shipment of the pharmaceutical product. Data from clinical trials are not always conclusive and the FDA may interpret data differently than we or our collaborators interpret data. The FDA may also convene an Advisory Committee of external advisors to answer questions regarding the approvability and labeling of an application. The FDA is not obligated to follow the Advisory Committee’s recommendation. The submission of a BLA is required to be accompanied by a substantial user fee, with few exceptions or waivers. The user fee is administered under PDUFA, which sets goals for the timeliness of the FDA’s review. A standard review period is twelve months from submission of the application, while priority review is eight months from submission of the application. The testing and approval process is likely to require substantial time, effort and resources, and there can be no assurance that any approval will be granted on a timely basis, if at all. The FDA may deny review of an application by refusing to file the application or not approve an application by issuance of a complete response letter if applicable regulatory criteria are not satisfied, require additional testing or information, or require post-market testing and surveillance to monitor the safety or efficacy of the product. Approval may occur with significant Risk Evaluation and Mitigation Strategies, or REMS, that limit the clinical use in the prescribing information, distribution or promotion of a product. Once an approval is issued, the FDA may require safety-related labeling changes or withdraw product approval if ongoing regulatory requirements are not met or if safety problems occur after the product reaches the market. In addition, the FDA may require further testing of ADCETRIS, including phase 4 clinical trials, and surveillance programs to monitor the safety of ADCETRIS, and the FDA has the power to prevent or limit further marketing of ADCETRIS based on the results of these post-marketing programs or other information.
Products manufactured or distributed pursuant to FDA approvals are subject to continuing regulation by the FDA, including manufacture, labeling, distribution, advertising, promotion, recordkeeping, annual product quality review and reporting requirements. Adverse event experience with the product must be reported to the FDA in a timely fashion and pharmacovigilance programs to proactively look for these adverse events are mandated by the FDA. Manufacturers and their subcontractors are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with ongoing regulatory requirements, including cGMPs, which impose certain procedural and documentation requirements upon us and our third-party manufacturers. Following such inspections, the FDA may issue notices on Form FDA 483 and Warning Letters that could cause us to modify certain activities. A Form FDA 483 notice, if issued at the conclusion of an FDA inspection, can list conditions
22
the FDA investigators believe may have violated cGMP or other FDA regulations or guidance. Failure to adequately and promptly correct the observations(s) can result in further regulatory enforcement action. In addition to Form FDA 483 notices and Warning Letters, failure to comply with the statutory and regulatory requirements can subject a manufacturer to possible legal or regulatory action, such as suspension of manufacturing, seizure of product, injunctive action or possible civil penalties. We cannot be certain that we or our present or future third-party manufacturers or suppliers will be able to comply with the cGMP regulations and other ongoing FDA regulatory requirements. If we or our present or future third-party manufacturers or suppliers are not able to comply with these requirements, the FDA may halt our clinical trials, not approve our products, require us to recall a product from distribution or withdraw approval of the BLA for that product. Failure to comply with ongoing regulatory obligations can result in delay of approval or Warning Letters, product seizures, criminal penalties, and withdrawal of approved products, among other enforcement remedies.
The FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. These regulations include standards and restrictions fordirect-to-consumer advertising, industry-sponsored scientific and educational activities, promotional activities involving the internet, andoff-label promotion. While physicians may prescribe products for off label uses, manufacturers may only promote products for the approved indications and in accordance with the provisions of the approved label. The FDA has very broad enforcement authority under the Federal Food, Drug and Cosmetic Act, and failure to abide by these regulations can result in penalties, including the issuance of a Warning Letter directing entities to correct deviations from FDA standards, and state and federal civil and criminal investigations and prosecutions.
FDA Regulation of Companion Diagnostics
ADCETRIS and certain of our product candidates may rely upon in vitro companion diagnostics for use in selecting the patients that we believe will respond to our therapeutics. If safe and effective use of a therapeutic product depends on an in vitro diagnostic, the FDA generally will require approval or clearance of a reproducible, validated diagnostic test to be used with our therapeutic product at the same time that FDA approves the therapeutic product. This policy is described in an August 2014 FDA guidance document. The review of these in vitro companion diagnostics in conjunction with the review of our cancer treatments involves coordination of review by FDA’s Center for Drug Evaluation and Research and by FDA’s Center for Devices and Radiological Health. The FDA’s premarket approval, or PMA, process is costly, lengthy, and uncertain. The receipt and timing of PMA approval may have a significant effect on the receipt and timing of commercial approval for ADCETRIS or our product candidates. Human diagnostic products are subject to pervasive and ongoing regulatory obligations, including the submission of medical device reports, adherence to the Quality Systems Regulation, recordkeeping and product labeling, as enforced by the FDA and comparable state authorities. We and Takeda have formed a collaboration with Ventana under which Ventana is working to develop, manufacture and commercialize a companion diagnostic test with the goal of identifying patients who might respond to treatment with ADCETRIS based on CD30 expression levels in their tissue specimens. In this regard, we expect that concurrent approval of a CD30 companion diagnostic will be required for any approval of ADCETRIS in the frontline MTCL indication.
Regulation Outside of the United States
In addition to regulations in the U.S., we and our collaborators are and will be subject to regulations of other countries governing clinical trials and commercial sales, manufacturing and distribution of our products. We must obtain approval by the regulatory authorities of countries outside of the U.S. before we can commence clinical trials in such countries and approval of the regulators of such countries or economic areas, such as Canada, before we may market products in those countries or areas. The approval process and requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from place to place, and the time may be longer or shorter than that required for FDA approval.
23
Healthcare Regulation
Federal and state healthcare laws and regulations, including fraud and abuse and health information privacy and security laws and regulations, may also be applicable to our business. If we fail to comply with those laws, we could face substantial penalties and our business, results of operations, financial condition and prospects could be adversely affected. The healthcare laws and regulations that may affect our ability to operate include, without limitation, anti-kickback and false claims laws, data privacy and security laws, as well as transparency laws regarding payments or other items of value provided to healthcare providers.
The federal Anti-Kickback Statute prohibits, among other things, knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, to induce, or in return for, purchasing, leasing, ordering, or arranging for or recommending the purchase, lease, or order of any good, facility, item, or service reimbursable, in whole or in part, under a federal healthcare program, such as the Medicare and Medicaid programs. The term “remuneration” has been broadly interpreted to include anything of value. Although there are a number of statutory exceptions and regulatory safe harbors protecting some common activities from prosecution, the exceptions and safe harbors are drawn narrowly. Practices that involve remuneration that may be alleged to be intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not qualify for an exception or safe harbor. Failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal under the Anti-Kickback Statute. Instead, the legality of the arrangement will be evaluated on acase-by-case basis based on a cumulative review of all its facts and circumstances. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the Anti-Kickback Statute has been violated. Additionally, the intent standard under the Anti-Kickback Statute was amended by the Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act of 2010, collectively PPACA, to a stricter standard such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. In addition, PPACA codified case law that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act.
The federal civil and criminal false claims laws, including the federal civil False Claims Act, prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment to or approval by the federal government, including the Medicare, and Medicaid programs, or knowingly making, using, or causing to be made or used a false record or statement material to a false or fraudulent claim or to avoid, decrease, or conceal an obligation to pay money to the federal government.
The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, created additional federal criminal statutes that prohibit, among other actions, knowingly and willfully executing or attempting to execute a scheme to defraud any healthcare benefit program, including private third party payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing, or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items, or services. Like the Anti-Kickback Statute, PPACA amended the intent standard for certain healthcare fraud under HIPAA such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
The civil monetary penalties statute imposes penalties against any person or entity that, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent.
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and their implementing regulations, imposes certain requirements on certain types of individuals and
24
entities relating to the privacy and security of individually identifiable health information. Among other things, HITECH makes HIPAA’s security standards directly applicable to business associates, independent contractors or agents of covered entities that receive or obtain protected health information in connection with providing a service for or on behalf of a covered entity. HITECH also created four new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions.
The federal Physician Payments Sunshine Act, created under PPACA and its implementing regulations, requires certain manufacturers of drugs, devices, biologicals and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program to annually report information related to certain payments or other transfers of value provided to physicians and teaching hospitals, or to entities or individuals at the request of, or designated on behalf of, the physicians and teaching hospitals, and to report annually certain ownership and investment interests held by physicians and their immediate family members. Failure to submit timely, accurately and completely the required information for all payments, transfers of value and ownership or investment interests may result in civil monetary penalties of up to an aggregate of $150,000 per year and up to an aggregate of $1 million per year for “knowing failures.” Covered manufacturers are required to submit reports on aggregate payment data to the Secretary of the U.S. Department of Health and Human Services on an annual basis.
Many states have similar statutes or regulations to the above federal laws and regulations that may be broader in scope than the aforementioned federal versions and apply regardless of payor, and many of which differ from each other in significant ways and may not have the same effect, further complicating compliance efforts. Additionally, our business operations in foreign countries and jurisdictions, including Canada and the European Union, may subject us to additional regulation.
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available under such laws, it is possible that some of our business activities could be subject to challenge under one or more of such laws. If our operations are found to be in violation of any of the health regulatory laws described above or any other laws that apply to us, we may be subject to penalties, including potentially significant criminal and civil and/or administrative penalties, damages, fines, disgorgement, imprisonment, exclusion from participation in government healthcare programs, contractual damages, reputational harm, administrative burdens, diminished profits and future earnings additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations ofnon-compliance with these laws, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
Coverage and Reimbursement
Sales of ADCETRIS and any future products depend, in significant part, on the extent to which the costs of our products will be covered by third-party payors, such as government health programs, commercial insurance and managed healthcare organizations. Patients who are prescribed treatment for their conditions and providers performing the prescribed services generally rely on third-party payors to reimburse all or part of the associated healthcare costs. Patients and providers are unlikely to use our products unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our products. Pharmaceutical products are typically reimbursed based on FDA labeled indications, recognized compendia listings, available medical literature, evidence of favorable clinical outcomes, determination of medical necessity and cost effectiveness.
Additionally, a third-party payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be approved. In the United States, no uniform policy of coverage and reimbursement for products exists among third-party payors. Therefore, coverage and reimbursement for products can differ significantly from payor to payor. Decisions regarding the extent of coverage and amount of
25
reimbursement to be provided for each of our product candidates is individual to each insurer, can vary based on provider contract, and will be affected by state and federal laws providing for reimbursement formulas based on acquisition cost. Third party payors continue to work diligently to control their spending on prescription drugs and medical service. The containment of healthcare costs has become a priority of the U.S. government and abroad, and the prices of drugs have been a focus in this effort. The U.S. government, state legislatures and foreign governments have shown significant interest in implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net sales and negatively impact our operating results. Payors, commercial and public in the U.S. and abroad, must review the therapeutics value of our products before extending coverage under their plans to reimburse our products. If third-party payors do not find a product to be of therapeutic value, they may not cover it or, if they do, the level of payment may not be sufficient to allow us to sell our products on a profitable basis.
Many of the patients in the U.S. who seek treatment with ADCETRIS may be eligible for Medicare or Medicaid benefits. The Medicare and Medicaid programs are administered by the Centers for Medicare and Medicaid Services, or CMS, and coverage and reimbursement for products and services under these programs are subject to changes in CMS regulations and interpretive policy determinations, in addition to statutory changes made by Congress. For example, PPACA increased the mandated Medicaid rebate from 15.1% to 23.1%, expanded the rebate to Medicaid managed care utilization and increased the types of entities eligible for the federal 340B drug discount program. In January 2017, the White House Office of Management and Budget withdrew the draft August 2015 Omnibus Guidance document that was issued by the Department of Health and Human Services Health Resources and Services Administration, or HRSA, that addressed a broad range of topics including, among other items, the definition of a patient’s eligibility for 340B drug pricing. Federal budget decisions have and may result in reduced Medicare payment rates. Federal budget decisions have and may result in reduced Medicare payment rates. In addition, as a condition of federal funds being made available to cover our products under Medicaid, we are required to participate in the Medicaid drug rebate program. The rebate amount under this program varies by quarter, and is based on pricing data we report to CMS. In addition, because we participate in the Medicaid drug rebate program, we must make ADCETRIS available to authorized users of the Federal Supply Schedule of the General Services Administration. This requires compliance with additional laws and requirements, including offering ADCETRIS at a reduced price to federal agencies including the United States Department of Veterans Affairs and United States Department of Defense, the Public Health Service and the Indian Health Service. We are also required to offer discounted pricing to certain eligible not for profit entities that are eligible for 340B pricing under the Public Health Services Act. Participation in these programs requires submission of pricing data and calculation of discounts and rebates pursuant to complex statutory formulas, as well as the entry into government procurement contracts governed by the Federal Acquisition Regulations and the guidance governing such calculations is not always clear. Compliance with such requirements can require significant investment in personnel, systems and resources, but failure to properly calculate our prices, or offer required discounts or rebates could subject us to substantial criminal, civil and/or administrative penalties, as well as, administrative burdens and exclusion from or contract termination regarding these programs. The terms of these government programs could change in the future which may increase the discounts or rebates we are required to offer, possibly reducing the revenue derived from sales of ADCETRIS to these entities.
The requirements governing drug pricing vary widely from country to country. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products.
Healthcare Reform
PPACA substantially changes the way healthcare is financed by both governmental and private insurers and significantly affects the pharmaceutical industry. PPACA aims to, among other things, expand coverage for the
26
uninsured while at the same time containing overall healthcare costs. With regard to biopharmaceutical products, PPACA is expected to, among other things, expand and increase industry rebates for products covered under Medicaid programs and make changes to the coverage requirements under the Medicare Part D program. We cannot yet predict the full impact of PPACA at this time for many reasons including that many of its provisions require the promulgation of detailed implementing regulations, which are subject to review and revision.
Many provisions of PPACA may impact the biopharmaceutical industry, including that in order for a biopharmaceutical product to receive federal reimbursement under the Medicare Part B and Medicaid programs or to be sold directly to U.S. government agencies, the manufacturer must extend discounts to entities eligible to participate in the drug pricing program under the Public Health Services Act, or PHS. The required PHS discount on a given product is calculated based on the Average Manufacturers Price, or AMP, and Medicaid rebate amounts reported by the manufacturer. PPACA expanded the types of entities eligible to receive discounted PHS pricing, although, under the current state of the law, with the exception of children’s hospitals, these newly eligible entities will not be eligible to receive discounted PHS pricing on orphan drugs when used for the orphan indication. In addition, as PHS drug pricing is determined based on AMP and Medicaid rebate data, revisions, including the recently published AMP rule, to the Medicaid rebate formula and AMP definition described above could cause the required PHS discount to increase.
Since its enactment, there have been judicial and Congressional challenges to certain aspects of PPACA. In January 2017, Congress voted to adopt a budget resolution for fiscal year 2017, or the Budget Resolution, that authorizes the implementation of legislation that would repeal portions of PPACA. The Budget Resolution is not a law; however, it was widely viewed as the first step toward the passage of legislation that would repeal certain aspects of PPACA. Further, on January 20, 2017, President Trump signed an Executive Order directing federal agencies with authorities and responsibilities under PPACA to waive, defer, grant exemptions from, or delay the implementation of any provision of PPACA that would impose a fiscal or regulatory burden on states, individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices. The potential impact of these efforts to repeal or defer and delay enforcement of PPACA on our business remains unclear. Congress also could consider subsequent legislation to replace elements of PPACA that are repealed. While Congress has not passed repeal or replace legislation, the tax reform legislation signed into law on December 22, 2017 includes a provision repealing, effective January 1, 2019, thetax-based shared responsibility payment imposed by the ACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate.” Because of the continued uncertainty about the implementation of the PPACA, including the potential for further legal challenges or repeal of PPACA, we cannot quantify or predict with any certainty the likely impact of the PPACA or its repeal on our business, prospects, financial condition or results of operations.
In addition, other legislative changes have been proposed and adopted since PPACA was enacted. The Budget Control Act of 2011, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee did not achieve a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This includes reductions to Medicare payments to providers, which went into effect in April 2013 and, following passage of the Bipartisan Budget Act of 2015, will remain in effect through 2025 unless additional congressional action is taken. The American Taxpayer Relief Act of 2012, among other things, reduced Medicare payments to several providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
Further, the recently enacted Drug Supply Chain Security Act imposes on manufacturers of certain pharmaceutical products new obligations related to product tracking and tracing, among others, which will be phased in over several years beginning in 2015. Among the requirements of this legislation, manufacturers subject to this federal law will be required to provide certain information regarding the drug product to individuals and entities to which product ownership is transferred, label drug product with a product identifier, and keep certain records regarding the drug product. The transfer of information to subsequent product owners by
27
manufacturers will eventually be required to be done electronically. Covered manufacturers will also be required to verify that purchasers of the manufacturers’ products are appropriately licensed. Further, under this new legislation, covered manufacturers will have drug product investigation, quarantine, disposition, and notification responsibilities related to counterfeit, diverted, stolen, and intentionally adulterated products, as well as products that are the subject of fraudulent transactions or which are otherwise unfit for distribution such that they would be reasonably likely to result in serious health consequences or death.
Additionally, there has been increasing legislative and enforcement interest in the United States with respect to specialty drug pricing practices. Specifically, there have been several recent U.S. Congressional inquiries and proposed bills designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs.
We cannot predict what healthcare reform initiatives may be adopted in the future. However, we anticipate that Congress, state legislatures, and third-party payors may continue to review and assess alternative healthcare delivery and payment systems and may in the future propose and adopt legislation or policy changes or implementations effecting additional fundamental changes in the healthcare delivery system. We also expect ongoing legislative and regulatory initiatives to increase pressure on drug pricing. We cannot assure you as to the ultimate content, timing, or effect of changes, nor is it possible at this time to estimate the impact of any such potential legislation; however, such changes or the ultimate impact of changes could negatively affect our revenue or sales of ADCETRIS or any future approved products.
Competition
The biotechnology and biopharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. Many third parties compete with us in developing various approaches to treating cancer. They include pharmaceutical companies, biotechnology companies, academic institutions and other research organizations.
Many of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approval and marketing than we do. In addition, many of these competitors are active in seeking patent protection and licensing arrangements in anticipation of collecting royalties for use of technology that they have developed. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific and management personnel, as well as in acquiring technologies complementary to our programs.
With respect to ADCETRIS, there are several otherFDA-approved drugs for its approved indications. Bristol-Myers Squibb’s nivolumab (Opdivo) and Merck’s pembrolizumab (Keytruda) are approved for the treatment of certain patients with relapsed or refractory classical Hodgkin lymphoma, and Celgene’s romidepsin (Istodax) and Spectrum Pharmaceuticals’ pralatrexate (Folotyn) and belinostat (Beleodaq) are approved for relapsed or refractory sALCL among otherT-cell lymphomas. The competition ADCETRIS faces from these and other therapies is intensifying. Additionally, Merck is conducting a phase 3 clinical trial in relapsed or refractory classical Hodgkin lymphoma comparing pembrolizumab (Keytruda) with ADCETRIS. If this clinical trial demonstrates that pembrolizumab is more effective than ADCETRIS in that treatment setting, our sales of ADCETRIS would be negatively impacted. We are also aware of multiple investigational agents that are currently being studied, including Roche’s atezolizumab, Pfizer’s avelumab, and Kyowa’s mogamulizumab, which, if successful, may compete with ADCETRIS in the future. Data have also been presented on several developing technologies, including bispecific antibodies and CAR modifiedT-cell therapies that may compete with ADCETRIS in the future. Further, there are many competing approaches used in the treatment of patients in ADCETRIS’ four approved indications, including auto-HSCT, allogeneic stem cell transplant, combination chemotherapy, clinical trials with experimental agents and single-agent regimens.
28
With respect to enfortumab vedotin, treatment in second line metastatic urothelial cancer is limited to CPI monotherapy or generic chemotherapy. There are other investigational agents that, if approved, could be competitive with enfortumab vedotin, including Immunomedics’ sacituzumab govitecan and Lilly’s ramucirumab.
With respect to tisotumab vedotin, we are aware of other companies that currently have products in development for the treatment of late-stage cervical cancer which could be competitive with tisotumab vedotin, including Agenus, Astrazeneca, Bristol-Myers Squibb, Immunomedics, Innovent Biologics, Merck, and Roche. In addition, several CPIs that areFDA-approved in other treatment settings are being explored for the treatment of late-stage cervical cancer in ongoing phase 2 clinical trials.
Many other pharmaceutical and biotechnology companies are developing and/or marketing therapies for the same types of cancer that our product candidates are designed and being developed to treat. For example, we believe that companies including AbbVie, ADC Therapeutics, Affimed, Agios, Amgen, Astellas, Bayer, Biogen, Bristol-Myers Squibb, Celgene, Eisai, Genentech, GSK, Gilead, ImmunoGen, Immunomedics, Infinity, Karyopharm, MedImmune, MEI Pharma, Merck, Novartis, Pfizer, Sanofi-Aventis, Spectrum Pharmaceuticals, Takeda, Teva, and Xencor are developing and/or marketing products or technologies that may compete with ours. In addition, our ADC collaborators may develop compounds utilizing our technology that may compete with product candidates that we are developing.
We are aware of other companies that have technologies that may be competitive with ours, including Astellas, AstraZeneca, Bristol-Myers Squibb, ImmunoGen, Immunomedics, MedImmune, Mersana and Pfizer, all of which have ADC technology. ImmunoGen has several ADCs in development that may compete with our product candidates. ImmunoGen has also established partnerships with other pharmaceutical and biotechnology companies to allow those other companies to utilize ImmunoGen’s technology, including Sanofi-Aventis, Genentech, Novartis, Takeda and Lilly. We are also aware of a number of companies developing monoclonal antibodies directed at the same antigen targets or for the treatment of the same diseases as our product candidates. For example, we believe Amgen and Xencor have anti-CD19 programs that may be competitive with our product candidates.
In addition, in the United States, the Biologics Price Competition and Innovation Act of 2009 created an abbreviated approval pathway for biological products that are demonstrated to be “highly similar” or “biosimilar” to or “interchangeable” with anFDA-approved biological product. This pathway allows competitors to reference the FDA’s prior approvals regarding innovative biological products and data submitted with a BLA to obtain approval of a biosimilar application 12 years after the time of approval of the innovative biological product. The12-year exclusivity period runs from the initial approval of the innovator product and not from approval of a new indication. In addition, the12-year exclusivity period does not prevent another company from independently developing a product that is highly similar to the innovative product, generating all the data necessary for a full BLA and seeking approval. Exclusivity only assures that another company cannot rely on the FDA’s prior approvals in approving a BLA for an innovator’s biological product to support the biosimilar product’s approval. Further, under the FDA’s current interpretation, it is possible that a biosimilar applicant could obtain approval for one or more of the indications approved for the innovator product by extrapolating clinical data from one indication to support approval for other indications. The FDA approved the first biosimilar product in the United States in May 2015. In the European Union, the European Commission has granted marketing authorizations for several biosimilars pursuant to a set of general and product class-specific guidelines for biosimilar approvals issued since 2005. We are aware of many pharmaceutical and biotechnology and other companies that are actively engaged in research and development of biosimilars or interchangeable products.
It is possible that our competitors will succeed in developing technologies that are more effective than ADCETRIS, enfortumab vedotin, tisotumab vedotin or our other product candidates or that would render our technology obsolete or noncompetitive, or will succeed in developing biosimilar or interchangeable products for ADCETRIS, enfortumab vedotin, tisotumab vedotin or our other product candidates. We anticipate that we will
29
continue to face increasing competition in the future as new companies enter our market and scientific developments surrounding biosimilars and other cancer therapies continue to accelerate. We cannot predict to what extent the entry of biosimilars or other competing products will impact potential future sales of ADCETRIS, enfortumab vedotin, tisotumab vedotin or our other product candidates.
With respect to our current and potential future product candidates, we believe that our ability to compete effectively and develop products that can be manufactured cost-effectively and marketed successfully will depend on our ability to:
| | • | | advance our technology platforms; |
| | • | | license additional technology; |
| | • | | complete clinical trials which position our products for regulatory and commercial success; |
| | • | | maintain a proprietary position in our technologies and products; |
| | • | | obtain required government and other public and private approvals on a timely basis; |
| | • | | attract and retain key personnel; |
| | • | | commercialize effectively; |
| | • | | obtain reimbursement for our products in approved indications; |
| | • | | comply with applicable laws, regulations and regulatory requirements and restrictions with respect to the commercialization of our products, including with respect to any changed or increased regulatory restrictions; and |
| | • | | enter into additional collaborations to advance the development and commercialization of our product candidates. |
Manufacturing
We do not currently manufacture the drug products that we sell or need to conduct our clinical trials, and we therefore rely on corporate collaborators and contract manufacturing organizations to supply drug product for commercial supply and ourIND-enabling studies and clinical trials. For the monoclonal antibody used in ADCETRIS, we have contracted with AbbVie for clinical and commercial supplies. For the drug linker used in ADCETRIS, we have contracted with Sigma Aldrich Fine Chemicals, or SAFC, for clinical and commercial supplies. We have multiple contract manufacturers for conjugating the drug linker to the antibody and producing the ADCETRIS product. For our ADC product candidates, multiple contract manufacturers, including AbbVie and SAFC, perform antibody and drug-linker manufacturing and several other contract manufacturers perform conjugation of the drug-linker to the antibody and fill/finish of the drug product. In addition, we rely on other third parties to perform additional steps in the manufacturing process, including shipping and storage of ADCETRIS and our product candidates.
We established our commercial scale supply chain for ADCETRIS prior to commercial launch. For the foreseeable future, we expect to continue to rely on contract manufacturers and other third parties to produce, vial and store sufficient quantities of ADCETRIS for use in our clinical trials and for commercial sale. In addition, we depend on outside vendors for the supply of raw materials used to produce ADCETRIS. For our pipeline programs, we believe that our existing supplies of drug product and our contract manufacturing relationships will be sufficient to accommodate clinical trials through phase 3. However, we may need to obtain additional manufacturing arrangements or increase our own manufacturing capability to meet our future commercial needs, both of which could require significant capital investment. In addition, we have committed to provide Takeda with their needs of certain parts of the ADCETRIS supply chain for a limited period of time, which may require us to arrange for additional manufacturing supply. We may also enter into collaborations with pharmaceutical or larger biotechnology companies to enhance the manufacturing capabilities for our product candidates.
30
AbbVie Biotechnology. In 2004, we entered into a development and supply agreement with AbbVie (formerly a part of Abbott Laboratories) to manufacture developmental, clinical and commercial quantities of anti-CD30 monoclonal antibody, which is a component of ADCETRIS. The agreement generally provides for the supply by AbbVie and the purchase by us of such anti-CD30 monoclonal antibody. Under terms of the supply agreement, we may purchase a portion of our required anti-CD30 monoclonal antibody from a second source third-party supplier. We are required to make a minimum annual purchase. The anti-CD30 monoclonal antibody is purchased by us based upon a rolling forecast. The supply agreement will continue until 2025 with an automaticone-year term extension unless either party provides written termination notice to the other party. Either party has the right to terminate the supply agreement if the other party materially breaches its obligations thereunder.
SAFC. In 2010, we entered into a commercial supply agreement with SAFC to manufacture commercial quantities of drug linker that is a component of ADCETRIS. The agreement generally provides for the supply by SAFC and the purchase by us of drug linker. Under terms of the supply agreement, we may purchase a portion of our required drug linker from a second source third-party supplier. We are required to make a minimum annual purchase. The drug linker is purchased by us based upon a rolling forecast. The supply agreement will continue until the completion of the tenth contract year following the initial commercial order with automatic term extension unless either party provides written termination notice to the other party. Either party has the right to terminate the supply agreement if the other party materially breaches its obligations thereunder.
In October 2017, we acquired a biologics manufacturing facility located in Bothell, Washington. At that time, we also entered into a clinical manufacturing services agreement with Bristol Myers Squibb Company, or BMS, under which we agreed to manufacture certain BMS clinical product candidates in accordance with prescribed production schedules and quantities through the later of December 31, 2018 or when certain technical transfer activities have been completed, and to maintain personnel, equipment and expertise sufficient to perform the agreed upon services. BMS compensates us for services rendered under the clinical manufacturing services agreement based on an agreed upon rate for use of the facility and employees. Following the completion of the clinical manufacturing services agreement, we plan to use the facility to support our clinical supply needs. However, we nonetheless expect to continue to rely on corporate collaborators and contract manufacturing organizations to supply drug product and intermediates for commercial supply and ourIND-enabling studies and clinical trials.
Commercial Operations and Information about Geographic Areas
We have allocated commercial resources, including sales, marketing, supply chain management and reimbursement capabilities, to commercialize ADCETRIS in the United States and Canada. We believe the U.S. and Canadian markets for ADCETRIS in the approved indications are addressable with a targeted sales and marketing organization, and we intend to continue promoting ADCETRIS ourselves in the United States and Canada for these and any additional indications we may obtain in the future. Takeda has commercial rights in the rest of the world. As of January 2018, we and Takeda had received marketing authorizations by regulatory authorities in 70 countries, and Takeda continues to pursue marketing authorizations in multiple other countries.
We sell ADCETRIS through a limited number of pharmaceutical distributors. Health care providers order ADCETRIS through these distributors. We receive orders from distributors and generally ship product directly to the health care provider. Three of our major distributors, together with entities under their common control—AmerisourceBergen Corporation, Cardinal Health, Inc., and McKesson Corporation—each accounted for 10% or more of our total revenue in 2017, 2016 and 2015. Our net product sales of ADCETRIS in 2017, 2016, and 2015 were $307.6 million, $265.8 million, and $226.1 million, respectively. Revenues generated outside the United States as determined by customer location were less than 10% of total revenues in 2017, 2016 and 2015. Substantially all of our long-lived assets are located in the United States.
31
Employees
As of December 31, 2017, we had 1,100 employees. Of these employees, 783 were engaged in or support research, development and clinical activities, 169 were in administrative and business related positions, and 148 were in sales and marketing. Each of our employees has signed confidentiality and inventions assignment agreements and none are covered by a collective bargaining agreement. We have never experienced employment-related work stoppages and consider our employee relations to be good.
Corporate Information
We were incorporated in Delaware on July 15, 1997. Our principal executive offices are located at 21823 30thDrive SE, Bothell, Washington 98021. Our telephone number is(425) 527-4000. Seattle Genetics®, ADCETRIS®and are our registered trademarks in the United States. All other trademarks, tradenames and service marks included in this Annual Report on Form10-K are the property of their respective owners.
are our registered trademarks in the United States. All other trademarks, tradenames and service marks included in this Annual Report on Form10-K are the property of their respective owners.
We file electronically with the Securities and Exchange Commission our Annual Reports on Form10-K, Quarterly Reports on Form10-Q, Current Reports on Form8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934. We make available on our website at www.seattlegenetics.com, free of charge, through a hyperlink on our website, copies of these reports, as soon as reasonably practicable after electronically filing such reports with, or furnishing them to, the Securities and Exchange Commission. Information found on, or accessible through, our website is not part of, and is not incorporated into, this Annual Report on Form10-K.
32
Item 1A. Risk Factors
You should carefully consider the following risk factors, in addition to the other information contained in this Annual Report onForm 10-K, including our consolidated financial statements and related notes. If any of the events described in the following risk factors occur, our business, operating results and financial condition could be seriously harmed. This Annual Report on Form10-K also contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in the forward-looking statements as a result of factors that are described below and elsewhere in this Annual Report on Form10-K.
Risks Related to the Cascadian Acquisition
The completion of the Cascadian Acquisition is subject to conditions and if these conditions are not satisfied or waived, the Cascadian Acquisition will not be completed. Failure to consummate the Cascadian Acquisition could negatively impact our stock price and our future business and financial results.
The obligations of us and Purchaser to complete the Tender Offer are subject to customary closing conditions, including (i) there being validly tendered and not validly withdrawn prior to the expiration date of the Tender Offer, at least a majority of the outstanding shares of Cascadian common stock on a fully-diluted basis, (ii) the expiration or termination of the applicable waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976 as amended, or the HSR Act, (iii) the absence of any legal restraint or prohibition that prevents or prohibits the consummation of the Tender Offer or the Merger, (iv) the accuracy of Cascadian’s representations and warranties under the Merger Agreement subject to the materiality standards set forth in the Merger Agreement, (v) the performance by Cascadian of its obligations under the Merger Agreement in all material respects and (vi) since the date of the Merger Agreement, that there will not have occurred (and be continuing) a Company Material Adverse Effect. Completion of the Merger is conditioned on the absence of any legal restraint or prohibition that prevents or prohibits the consummation of the Merger and that Purchaser (or we on Purchaser’s behalf) have accepted for payment and paid for all shares of Cascadian common stock validly tendered (and not validly withdrawn) pursuant to the Tender Offer. Neither the Tender Offer nor the Merger is subject to a financing condition. We and Cascadian may terminate the Merger Agreement upon mutual consent, and either we or Cascadian may, subject to certain exceptions set forth in the Merger Agreement, terminate the Merger Agreement if the Tender Offer has not been consummated on or before June 30, 2018, the date agreed by us and Cascadian to be the last permissible date of acceptance of the Tender Offer.
The failure of one or more of the required conditions to be satisfied could delay the completion of the Cascadian Acquisition for a significant period of time or prevent it from occurring, and we cannot otherwise guarantee that we will be able to complete the Cascadian Acquisition. In addition, on February 13, 2018, a securities class action lawsuit was filed against Cascadian and its board of directors in the United States District Court for the District of Delaware. Among other things, the complaint seeks to enjoin the closing of the Tender Offer and consummation of the Merger as well as compensatory damages of an undisclosed amount. It is possible that additional lawsuits will be filed, or allegations received from Cascadian stockholders, with respect to these same matters. We cannot predict the timing or outcome of this lawsuit or potential similar lawsuits, or the impact they may have on the closing of the Tender Offer and consummation of the Merger. If the Cascadian Acquisition is not completed for any reason, our ongoing business may be adversely affected and, without realizing any of the benefits of having completed the Cascadian Acquisition, we will be subject to a number of risks, including the following:
| | • | | the price of our common stock may reflect a market assumption that the Cascadian Acquisition will occur, meaning that a failure to complete the Cascadian Acquisition could result in a decline in the price of our common stock; |
| | • | | time and resources, financial and other, committed by our management to matters relating to the Cascadian Acquisition could otherwise have been devoted to pursuing other potentially beneficial opportunities for our company; |
| | • | | we may experience negative reactions from the financial markets or from our customers or employees; and |
33
| | • | | we will be required to pay our respective costs relating to the Cascadian Acquisition, including legal, accounting, financial advisory, financing and printing fees, whether or not the Cascadian Acquisition is completed subject to our rights to receive certain payments in the event the Merger Agreement is terminated under certain circumstances. |
We also could be subject to litigation related to any failure to complete the Cascadian Acquisition or to perform our obligations under the Merger Agreement, or related to any enforcement proceeding commenced against us. If the Cascadian Acquisition is not consummated, these risks may materialize and may adversely affect our business, financial results and stock price.
Obtaining required regulatory approvals may prevent or delay consummation of the Tender Offer or reduce the anticipated benefits of the Cascadian Acquisition or may require changes to the structure or terms of the Cascadian Acquisition.
Consummation of the Tender Offer is conditioned upon, among other things, the expiration or termination of the waiting period (and any extensions thereof) applicable to the Tender Offer under the HSR Act. At any time before or after the Tender Offer is consummated, governmental authorities, including the Department of Justice, the Federal Trade Commission or U.S. state Attorneys General, could take action under the antitrust laws in opposition to the Cascadian Acquisition, including seeking to enjoin completion of the Cascadian Acquisition, imposing additional requirements, limitations or costs on the Cascadian Acquisition, condition completion of the Cascadian Acquisition upon the divestiture of assets of Seattle Genetics, Cascadian, our or its subsidiaries or impose restrictions on our post-acquisition operations. If any such requirements, limitations or costs are imposed and the Cascadian Acquisition is completed, then these could negatively affect our results of operations and financial condition following completion of the Cascadian Acquisition. Any such requirements or restrictions may delay or prevent consummation of the Tender Offer or may reduce the anticipated benefits of the Cascadian Acquisition, which could also have an adverse effect on our business, financial condition and results of operations. No assurance can be given that the required regulatory approvals will be obtained or that the required conditions to closing will be satisfied, and, even if all such approvals are obtained and the conditions are satisfied, no assurance can be given as to the terms, conditions and timing of the approvals.
Cascadian will be subject to business uncertainties and contractual restrictions while the Cascadian Acquisition is pending.
Uncertainty about the effect of the Cascadian Acquisition on employees and counterparties may have an adverse effect on Cascadian. These uncertainties may impair Cascadian’s ability to retain and motivate key personnel and could cause entities dealing with Cascadian to defer entering into contracts with Cascadian or making other decisions concerning Cascadian or seek to change existing business relationships with Cascadian. If the Cascadian Acquisition is completed, such changes could negatively affect our results of operations and financing condition and adversely affect our ability to realize benefits from the Cascadian Acquisition. In addition, if key employees of Cascadian or the Company depart because of uncertainty about their future roles or otherwise, our business could be harmed. These risks may be exacerbated by delays or other adverse developments with respect to the completion of the Cascadian Acquisition.
We and Cascadian will incur substantial direct and indirect costs as a result of the Cascadian Acquisition.
We and Cascadian will incur substantial expenses in connection with and as a result of completing the Cascadian Acquisition and, over a period of time following the completion of the Cascadian Acquisition, we expect to incur substantial additional expenses in connection with coordinating the businesses, operations, policies and procedures of the combined company. While we have assumed that a certain level of transaction expenses will be incurred, factors beyond our control could affect the total amount or the timing of these expenses. Many of the expenses that will be incurred, by their nature, are difficult to estimate accurately.
Combining the two companies may be more difficult, costly or time consuming than we anticipate and we may not realize the intended benefits of the Cascadian Acquisition.
Cascadian has operated, and until the completion of the Cascadian Acquisition, will continue to operate independently of us, with its own business, corporate culture, location, employees and systems. The success of
34
the Cascadian Acquisition, including anticipated benefits, will depend, in part, on our ability to successfully combine and integrate our business with the business of Cascadian. As a result of the Cascadian Acquisition, we will operate our existing business, along with the business of Cascadian, as one combined organization utilizing common information and communication systems, operating procedures, financial controls and human resources practices. There may be substantial difficulties, costs and delays involved in the integration of our business with Cascadian, including as a result of challenges relating to the diversion of management’s attention from our ongoing business, the possibility of faulty assumptions underlying expectations regarding the integration process, retaining and attracting business and operational relationships, eliminating duplicative operations and inconsistent standards and procedures and increased or unforeseen liabilities or costs relating to the Cascadian Acquisition or the Cascadian business. If we experience difficulties with the integration process, the anticipated benefits of the Cascadian Acquisition may not be realized fully or at all, or may take longer to realize than expected, which could materially and adversely affect our business, financial condition and results of operations.
If goodwill or other intangible assets that we record in connection with the Cascadian Acquisition become impaired, our financial position in future periods could be negatively impacted.
In connection with the accounting for the Cascadian Acquisition, it is expected that we will record a significant amount of intangible assets and may also record goodwill. Under GAAP, we must assess, at least annually and potentially more frequently, whether the value of goodwill and other indefinite-lived intangible assets has been impaired. Amortizing intangible assets will be assessed for impairment in the event of an impairment indicator. Events giving rise to impairment are an inherent risk in the pharmaceutical industry and cannot be predicted. Our results of operations and financial position in future periods could be negatively impacted should future impairments of intangible assets or goodwill occur.
Our and Cascadian’s actual financial positions and results of operations may differ materially from the unaudited pro forma financial information that we filed as exhibit 99.2 to our current report onForm 8-K, filed with the SEC on January 31, 2018, or the JanuaryForm 8-K.
The pro forma financial information that we filed as exhibit 99.2 to the JanuaryForm 8-K was presented for illustrative purposes only and may not be an indication of what our financial position or results of operations would have been had the transactions been completed on the dates indicated. The pro forma financial information has been derived from our and Cascadian’s historical financial statements and certain adjustments and assumptions have been made regarding the combined company after giving effect to the indicated transactions. The assets and liabilities of Cascadian have been measured at fair value based on various preliminary estimates using assumptions that our management believes are reasonable utilizing information currently available. The process for estimating the fair value of acquired assets and assumed liabilities requires the use of judgment in determining the appropriate assumptions and estimates. These estimates may be revised as additional information becomes available and as additional analyses are performed. In particular, the pro forma financial information that we filed as exhibit 99.2 to the JanuaryForm 8-K assumes that we utilize a senior secured bridge loan facility, or the Bridge Facility, to finance a portion of the costs of the Cascadian Acquisition; however, we intend to use the net proceeds from our public offering of our common stock that we completed in February 2018 to fund a portion of the costs of the Cascadian Acquisition in lieu of any borrowing pursuant to the Bridge Facility. Accordingly, the pro forma financial information does not reflect the actual financing of the Cascadian Acquisition. Differences between preliminary estimates in the pro forma financial information and the final acquisition accounting, as well as between the assumed and actual financing sources and terms, will occur and could have a material impact on the pro forma financial information and the combined company’s financial position and future results of operations.
Other assumptions used in preparing the pro forma financial information may not prove to be accurate, and other factors may affect our financial condition or results of operations following the closing of the Cascadian Acquisition and related transactions. Any potential decline in our financial condition or results of operations may cause significant variations in the price of our common stock.
35
Cascadian has a limited operating history and no history of commercializing drug products, and risks and uncertainties related to its business may cause the combined company to underperform relative to expectations.
Cascadian is a clinical-stage biopharmaceutical company with a limited operating history and does not have any products approved for commercial sale, which makes it difficult to evaluate the success of its current business and assess the combined company’s future viability. In addition, Cascadian has incurred significant research and development and other expenses related to its ongoing operations resulting in net losses in every year since its inception other than the year ended December 31, 2008. We anticipate that Cascadian will continue to incur net losses in the future as a result of continued expenditures related to the development and commercialization of its lead product candidate, tucatinib, and additional research and development expenditures related to the development and regulatory approval of its other existing and future product candidates. Because Cascadian does not generate any revenue from product sales, following the consummation of the Cascadian Acquisition, we expect to invest significant time, resources and capital to support the expenditures andon-going operations of the acquired Cascadian business. Such investments would reduce our cash available for our existing operations and other uses and divert significant attention of management that may otherwise be focused on development of our existing business. If we are unable to obtain regulatory approval for Cascadian’s product candidates and effectively commercialize its product candidates, we may not realize any benefit from the Cascadian Acquisition, resulting in possible impairments or other charges or losses which may materially and adversely affect our results of operations and financial condition. Additionally, the business operations of Cascadian differ from our business operations, and the combined business will have a different business mix than our business prior to the Cascadian Acquisition, presenting different operational risks and challenges. We expect to rely on the experience and expertise of Cascadian’s existing management team and other key personnel in the development and commercialization of Cascadian’s product candidates. If we were to lose the services of a significant portion or key individuals of this team, such development and commercialization and our financial results could be adversely affected.
The Cascadian business may also face additional risks, including risks relating to (i) the ability to advance the development of tucatinib and Cascadian’s other product candidates through regulatory approval, (ii) competition with companies with more experience and resources in the oncology space and with companies developing other novel targeted therapies for cancers and (iii) maintaining and obtaining intellectual property protection for Cascadian’s product candidates. In particular, clinical data from the pivotal HER2CLIMB clinical trial may fail to establish that tucatinib is effective in treating HER2+ breast cancer or associated brain metastases or may indicate safety profile concerns not indicated by earlier clinical data, in which case, we may not realize any benefit from the Cascadian Acquisition.
Moreover, Cascadian relies on agreements with third parties for its product candidate technology development, manufacture, packaging, supply, and clinical trials. The termination of any of these agreements by the third parties would have an adverse impact on the combined company’s ability to develop and manufacture Cascadian’s product candidates. For example, Cascadian has entered into an exclusive license agreement with Array BioPharma, Inc. for its tucatinib technology. If Array BioPharma were to terminate the license agreement or if the combined company is unable to maintain the exclusivity of that license agreement, the combined company may be unable to continue to develop tucatinib. Additionally, an adverse result in potential future disputes with Cascadian’s licensors and partners, including Array BioPharma, may require the combined company to enter into additional licenses or to incur additional costs in litigation or settlement. Finally, continued development and commercialization of Cascadian’s product candidates may require the combined company to secure licenses to additional technologies, which it may not be able to do on commercially reasonable terms, if at all.
36
Risks Related to Our Business
Our near-term prospects are substantially dependent on ADCETRIS. If we and/or Takeda are unable to effectively commercialize ADCETRIS for the treatment of patients in its approved indications and to continue to expand its labeled indications of use, our ability to generate significant revenue and our prospects for profitability will be adversely affected.
ADCETRISis now approved by the FDA and the European Commission for four indications, encompassing several settings for the treatment of relapsed Hodgkin lymphoma, for relapsed sALCL, and for certain types of CTCL. ADCETRIS is our only product approved for marketing and our ability to generate revenue from product sales and our prospects for profitability are substantially dependent on our continued ability to effectively commercialize ADCETRIS for the treatment of patients in its approved indications and our ability to continue to expand its labeled indications of use. We may not be able to fully realize the commercial potential of ADCETRIS for a number of reasons, including:
| | • | | we and/or Takeda may not be able to obtain and maintain regulatory approvals to market ADCETRIS for any additional indications in our respective territories, including for frontline Hodgkin lymphoma or frontline MTCL, or to otherwise continue to expand its labeled indications of use; |
| | • | | we and/or Takeda may fail to obtain regulatory approvals for ADCETRIS in theECHELON-1 treatment setting in our respective territories, notwithstanding the positive data we reported from theECHELON-1 trial, and even if approved, we and/or Takeda may fail to commercialize ADCETRIS in theECHELON-1 treatment setting, which would limit our sales of, and the commercial potential of, ADCETRIS; |
| | • | | negative or inconclusive results in, or delays in, ourECHELON-2 trial, which would negatively impact, or preclude altogether, our and Takeda’s ability to obtain regulatory approvals and commercialize ADCETRIS in the frontline MTCL indication in our respective territories and which would also limit our sales of, and the commercial potential of, ADCETRIS; |
| | • | | results from theECHELON-1 trial or theECHELON-2 trial, either of which could be considered confirmatory by the FDA for the relapsed sALCL indication, may fail to sufficiently confirm the clinical benefit of ADCETRIS in relapsed sALCL, which could result in the withdrawal of approval of ADCETRIS in the relapsed sALCL indication and negatively impact our potential future product sales for the relapsed sALCL indication; |
| | • | | new competitive therapies, including immuno-oncology agents such asPD-1 inhibitors (e.g., nivolumab and pembrolizumab), have been approved by regulatory authorities or may be submitted in the near term to regulatory authorities for approval in ADCETRIS’ labeled indications, and these competitive products could negatively impact our commercial sales of ADCETRIS; |
| | • | | our commercial sales of ADCETRIS could be lower than our projections due to a lower market penetration rate, increased competition by alternative products or biosimilars, or a shorter duration of therapy in patients in ADCETRIS’ approved indications; |
| | • | | we may be unable to effectively commercialize ADCETRIS in any new indications for which we receive marketing approval, including in the pcALCL and CD30-expressing MF indication that was approved in November 2017; |
| | • | | there may be additional changes to the label for ADCETRIS, including ADCETRIS’ boxed warning, that further restrict how we market and sell ADCETRIS, including as a result of data collected from our required post-approval study, or as the result of adverse events observed in that study or in other studies, including investigator-sponsored studies and in the post-approval confirmatory studies that Takeda is required to conduct as a condition to the conditional marketing authorization of ADCETRIS granted by the European Commission; |
37
| | • | | we may not be able to establish or demonstrate in the medical community the safety, efficacy, or value of ADCETRIS and its potential advantages compared to existing and future therapeutics in the frontline Hodgkin lymphoma setting and other settings; |
| | • | | physicians may be reluctant to prescribe ADCETRIS due to side effects associated with its use or until results from our required post-approval study are available or other long term efficacy and safety data exist; |
| | • | | the estimated incidence rate of new patients in ADCETRIS’ approved indications may be lower than our projections; |
| | • | | there may be adverse results or events reported in any of the clinical trials that we and/or Takeda are conducting or may in the future conduct for ADCETRIS; |
| | • | | we may be unable to continue to effectively market, sell and distribute ADCETRIS; |
| | • | | ADCETRIS may be impacted by adverse reimbursement and coverage policies from government and private payers such as Medicare, Medicaid, insurance companies, health maintenance organizations and other plan administrators, or may be subject to pricing pressures enacted by industry organizations or state and federal governments, including as a result of increased scrutiny over pharmaceutical pricing or otherwise; |
| | • | | the relative price of ADCETRIS may be higher than alternative treatment options, and therefore its reimbursement may be limited by private and governmental insurers; |
| | • | | there may be changed or increased regulatory restrictions; |
| | • | | we may not have adequate financial or other resources to effectively commercialize ADCETRIS; and |
| | • | | we may not be able to obtain adequate commercial supplies of ADCETRIS to meet demand or at an acceptable cost. |
In 2009, we entered into an agreement with Takeda to develop and commercialize ADCETRIS, under which we have commercial rights in the United States and its territories and Canada, and Takeda has commercial rights in the rest of the world. The success of this collaboration and the activities of Takeda will significantly impact the commercialization of ADCETRIS in countries other than the United States and in Canada. In October 2012, Takeda announced that it had received conditional marketing authorization for ADCETRIS from the European Commission for patients with relapsed Hodgkin lymphoma or relapsed sALCL, and has since obtained marketing approvals for ADCETRIS in many other countries. Conditional marketing authorization by the European Commission includes obligations to provide additional clinical data at a later stage to confirm the positive benefit-risk balance. In July 2016, Takeda announced that it had received marketing authorization for ADCETRIS from the European Commission for the treatment of adult patients with CD30-positive Hodgkin lymphoma at increased risk of relapse or progression following autologous stem cell transplant, and in January 2018, Takeda announced that it had received marketing authorization for ADCETRIS from the European Commission for the treatment of adult patients with CD30-positive CTCL after at least one prior systemic therapy. We cannot control the amount and timing of resources that Takeda dedicates to the commercialization of ADCETRIS, or to its marketing and distribution, and our ability to generate revenues from ADCETRIS product sales by Takeda depends on Takeda’s ability to achieve market acceptance of, and to otherwise effectively market, ADCETRIS for its approved indications in Takeda’s territory.
While ADCETRIS product sales have grown over time, and our future plans assume that sales of ADCETRIS will increase, we cannot assure you that, even with the recent expansion to the prescribing label for ADCETRIS in the United States, which now includes the treatment of adult patients with pcALCL and CD30-expressing MF who have received prior systemic therapy, ADCETRIS sales will continue to grow or that we can maintain sales of ADCETRIS at or near current levels. We believe that the level of our ongoing ADCETRIS sales in the United States is largely attributable to the incidence flow of patients eligible for treatment with
38
ADCETRIS. We also believe that the incidence rate of new patients in ADCETRIS’ approved indications is relatively low, particularly when compared to many other oncology indications. In addition, we expect only modest sales growth in the near term as a result of the November 2017 FDA approval of ADCETRIS for the treatment of adult patients with pcALCL and CD30-expressing MF who have received prior systemic therapy, subject to our ability to effectively commercialize ADCETRIS in this indication. For these and other reasons, we expect that our ability to accelerate ADCETRIS sales growth, if at all, will depend primarily on our ability to continue to expand ADCETRIS’ labeled indications of use, particularly with respect to the frontline Hodgkin lymphoma and frontline MTCL indications. Accordingly, we are exploring the use of ADCETRIS as a single agent and in combination therapy regimens earlier in the treatment of Hodgkin lymphoma and MTCL, including sALCL, and in a range of CD30-expressing hematologic lymphomas. This will continue to require additional time and investment in clinical trials, and there can be no assurance that we and/or Takeda will obtain and maintain the necessary regulatory approvals to market ADCETRIS for any additional indications.
In particular, although we reported positive top line data in theECHELON-1 trial in June 2017, there can be no assurance that either we or Takeda will ultimately obtain regulatory approvals of ADCETRIS in theECHELON-1 treatment setting in our respective territories, which would limit our sales of, and the commercial potential of, ADCETRIS. Likewise, we may fail to commercialize ADCETRIS in pcALCL and CD30-expressing MF patients or in theECHELON-1 treatment setting if our sBLA that we submitted in November 2017 is approved by the FDA, either of which would limit our sales of, and the commercial potential of, ADCETRIS. In addition, negative or inconclusive results in ourECHELON-2 trial would negatively impact, or preclude altogether, our and Takeda’s ability to obtain regulatory approvals in the frontline MTCL indication in our respective territories, which would also limit our sales of, and the commercial potential of, ADCETRIS. Moreover, the SPA agreement for theECHELON-2 trial requires that the trial continue until a specified number of PFS events designated for the trial occurs. Based on reviews of pooled, blinded data, we have observed a lower rate of reported PFS events in theECHELON-2 trial than anticipated. We plan to discuss with the FDA the potential to unblind the trial prior to achieving the target number of PFS events specified in the SPA agreement. We cannot predict the outcome of those discussions or whether we would be able to reach agreement with the FDA. If we are unable to reach agreement with the FDA and determine to unblind the trial prior to achieving the target number of PFS events as specified in the SPA agreement, the FDA could treat the SPA agreement forECHELON-2 trial as rescinded. In that event, we would no longer have commitments from the FDA regarding the appropriate design, size and endpoints of the study for regulatory approval, making our ability to obtain regulatory approval of ADCETRIS in theECHELON-2 treatment setting more uncertain. In addition, earlier unblinding in theECHELON-2 trial could also negatively impact the likelihood of achieving positive results in the trial sufficient to support regulatory approval. Alternatively, if we are unable to reach agreement with the FDA, we could determine to continue theECHELON-2 trial until the target number of PFS events specified in the SPA agreement is achieved, which could result in a substantial delay in our ability to conduct the final data analysis from theECHELON-2 trial.
We and Takeda have formed a collaboration with Ventana under which Ventana is working to develop, manufacture and commercialize a companion diagnostic test with the goal of identifying patients who might respond to treatment with ADCETRIS based on CD30 expression levels in their tissue specimens. The FDA and similar regulatory authorities outside the United States regulate companion diagnostics. Companion diagnostics require separate or coordinated regulatory approval prior to commercialization of the related therapeutic product. In this regard, we expect that concurrent approval of a CD30 companion diagnostic will be required for any approval of ADCETRIS in the frontline MTCL indication. However, Ventana may not be able to successfully develop and obtain regulatory approval for a companion diagnostic to support regulatory approval of ADCETRIS in the frontline MTCL indication in a timely manner or at all. If Ventana is unable to successfully develop a companion diagnostic, or experiences delays in doing so, the development of ADCETRIS in the frontline MTCL indication may be adversely affected, we may fail to receive regulatory approval for ADCETRIS in the frontline MTCL indication and we may not realize the full commercial potential of ADCETRIS. Further, if a companion diagnostic requirement were included in the ADCETRIS label, such a requirement may limit our ability to
39
commercialize ADCETRIS in the applicable setting due to potential label requirements, prescriber practices, constraints on availability of the diagnostic, or other factors.
Even if we and Takeda receive the required regulatory approvals to market ADCETRIS for any additional indications or in additional jurisdictions, we and Takeda may not be able to effectively commercialize ADCETRIS, including for the reasons set forth above. Our ability to grow ADCETRIS product sales in future periods is also dependent on price increases and we periodically increase the price of ADCETRIS. Price increases on ADCETRIS and negative publicity regarding drug pricing and price increases generally, whether on ADCETRIS or products distributed by other pharmaceutical companies, could negatively affect market acceptance of, and sales of, ADCETRIS. In any event, we cannot assure you that price increases we have taken or may take in the future will not in the future negatively affect ADCETRIS sales.
Reports of adverse events or safety concerns involving ADCETRIS or our product candidates could delay or prevent us from obtaining or maintaining regulatory approvals, or could negatively impact sales of ADCETRIS or the prospects for our product candidates.
Reports of adverse events or safety concerns involving ADCETRIS could interrupt, delay or halt clinical trials of ADCETRIS, including the ongoingFDA-required ADCETRIS post-approval confirmatory study as well as the post-approval confirmatory studies that Takeda is required to conduct as a condition to the conditional marketing authorization of ADCETRIS by the European Commission. For example, during 2013 concerns regarding pancreatitis caused an investigator conducting an independent study involving ADCETRIS to temporarily halt enrollment in the trial and to amend the eligibility criteria and monitoring for the trial. Subsequently, we have revised our prescribing information to add pancreatitis as a known adverse event. In addition, reports of adverse events or safety concerns involving ADCETRIS could result in regulatory authorities limiting, denying or withdrawing approval of ADCETRIS for any or all indications, including the use of ADCETRIS for the treatment of patients in its approved indications. For example, there was an increased incidence of febrile neutropenia and peripheral neuropathy in the ADCETRIS plus AVD arm of theECHELON-1 trial, which could limit, narrow or preclude any approval by the FDA, or could limit prescribing of ADCETRIS in theECHELON-1 treatment setting if approved by the FDA, both of which could negatively impact sales of ADCETRIS or adversely affect ADCETRIS’ acceptance in the market. There are no assurances that patients receiving ADCETRIS will not experience serious adverse events in the future. Further, there are no assurances that patients receiving ADCETRIS withco-morbid diseases not previously studied, such as autoimmune diseases, will not experience new or different serious adverse events in the future.
Adverse events may negatively impact the sales of ADCETRIS. We may be required to further update the ADCETRIS prescribing information, including boxed warnings, based on reports of adverse events or safety concerns or implement a Risk Evaluation and Mitigation Strategy, or REMS, which could adversely affect ADCETRIS’ acceptance in the market, make competition easier or make it more difficult or expensive for us to distribute ADCETRIS. For example, the prescribing information for ADCETRIS includes pancreatitis, impaired hepatic function, impaired renal function, pulmonary toxicity, and gastrointestinal complications as known adverse events as well as a boxed warning related to the risk that JC virus infection resulting in progressive multifocal leukoencephalopathy, or PML, and death can occur in patients receiving ADCETRIS. Further, based on the identification of future adverse events, we may be required to further revise the prescribing information, including ADCETRIS’ boxed warning, which could negatively impact sales of ADCETRIS or adversely affect ADCETRIS’ acceptance in the market.
Likewise, reports of adverse events or safety concerns involving ADCETRIS or our product candidates could interrupt, delay or halt clinical trials of such product candidates, or could result in our inability to obtain regulatory approvals for any of our product candidates. For example, in June 2017, we discontinued the phase 3 CASCADE clinical trial ofSGN-CD33A based on unexpected adverse events following a higher rate of deaths in theSGN-CD33A containing arm versus the control arm of this trial, and the Investigational New Drug application, or IND, forSGN-CD33A was subsequently placed on hold by the FDA. At this time, we have no
40
plans to initiate additional clinical trials ofSGN-CD33A. In the future, we may determine to discontinue ourSGN-CD33A program altogether, in which case we will not receive any return on our investment inSGN-CD33A. In addition, we are planning or conducting pivotal trials for enfortumab vedotin and tisotumab vedotin based on only limited phase 1 clinical data. There may be important facts about the safety, efficacy, and risk versus benefit of these product candidates that are not known to us at this time which may negatively impact our ability to develop and commercialize these product candidates. In addition, in response to safety events observed in our ongoing clinical trials of enfortumab vedotin and tisotumab vedotin, including patient deaths, we have in the past, and may in the future, institute additional precautionary safety measures such as dosing caps and delays, enhanced monitoring for side effects, and modified patient inclusion and exclusion criteria. Additional and/or unexpected safety events could be observed in these pivotal or other later stage trials that could delay or prevent us from advancing the clinical development of either enfortumab vedotin or tisotumab vedotin and may adversely affect our business, results of operations and prospects.
Concerns regarding the safety of ADCETRIS or our product candidates as a result of undesirable side effects identified during clinical testing or otherwise could cause the FDA to order us to cease further development or commercialization of ADCETRIS or the applicable product candidate. Undesirable side effects caused by ADCETRIS or our product candidates could also result in denial of regulatory approval by the FDA or other regulatory authorities for any or all targeted indications, the requirement of additional trials or the inclusion of unfavorable information in our product labeling, and in turn delay or prevent us from commercializing ADCETRIS or the applicable product candidate. In addition, actual or potential drug-related side effects could affect patient recruitment or the ability of enrolled patients to complete a trial for ADCETRIS or our product candidates or result in potential product liability claims. Any of these events could prevent us from developing or commercializing ADCETRIS or the particular product candidate, and could significantly harm our business, results of operations and prospects.
Even though we and Takeda have obtained regulatory approvals to market ADCETRIS, we and Takeda are subject to extensive ongoing regulatory obligations and review, including post-approval requirements that could result in the withdrawal of ADCETRIS from certain geographic markets in certain indications if such requirements are not met.
ADCETRIS is approved for treating patients in the relapsed sALCL indication under accelerated approval regulations in the U.S., approved with conditions in relapsed Hodgkin lymphoma and sALCL in Canada, and approved under conditional marketing authorization in relapsed Hodgkin lymphoma and sALCL in Europe, in each case under regulations which allow for approval of products for cancer or other serious or life threatening illnesses based on a surrogate endpoint or on a clinical endpoint other than survival or irreversible morbidity. Under these types of approvals, we are subject to certain post-approval requirements, including the requirement to conduct clinical trials to confirm clinical benefit. In the U.S., either theECHELON-1 trial or theECHELON-2 trial results may be sufficient to confirm the clinical benefit of ADCETRIS in relapsed sALCL and thereby convert the relapsed sALCL accelerated approval to regular approval. In Canada, theECHELON-1 results may be sufficient to confirm the clinical benefit of ADCETRIS in relapsed Hodgkin lymphoma, and theECHELON-2 results may be sufficient to confirm the clinical benefit of ADCETRIS in relapsed sALCL. In Europe, there are other post approval requirements to convert the conditional marketing authorization for ADCETRIS in relapsed Hodgkin lymphoma and relapsed sALCL into a standard marketing authorization. Our failure to complete a required post-approval study, including theECHELON-2 trial, or to confirm a clinical benefit could result in the withdrawal of approval of ADCETRIS in the indications for which approval is conditional which would seriously harm our business. Similarly, Takeda’s failure to provide these additional clinical data from confirmatory studies could result in the European Commission withdrawing approval of ADCETRIS in the European Union for certain indications, which would negatively impact anticipated royalty revenue from ADCETRIS sales by Takeda in the European Union and could adversely affect our results of operations.
In addition, we are subject to extensive ongoing obligations and continued regulatory review from applicable regulatory agencies with respect to any product for which we have obtained regulatory approval,
41
including ADCETRIS in each of its approved indications, such as continued adverse event reporting requirements and the requirement to have some of our promotional materialspre-cleared by the FDA. There may also be additional post-marketing obligations, all of which may result in significant expense and limit our ability to commercialize ADCETRIS in the United States, Canada or potentially other jurisdictions.
We and the manufacturers of ADCETRIS are also required to comply with current Good Manufacturing Practices, or cGMP, regulations, which include requirements relating to quality control and quality assurance as well as the corresponding maintenance of records and documentation. Further, regulatory agencies must approve these manufacturing facilities before they can be used to manufacture ADCETRIS, and these facilities are subject to ongoing regulatory inspections. In addition, regulatory agencies subject an approved product, its manufacturer and the manufacturer’s facilities to continual review and inspections, including periodic unannounced inspections. The subsequent discovery of previously unknown problems with ADCETRIS, including adverse events of unanticipated severity or frequency, or problems with the facilities where ADCETRIS is manufactured, may result in restrictions on the marketing of ADCETRIS, up to and including withdrawal of ADCETRIS from the market. If our manufacturing facilities or those of our suppliers fail to comply with applicable regulatory requirements, such noncompliance could result in regulatory action and additional costs to us.
Failure to comply with applicable FDA and other regulatory requirements may subject us to administrative or judicially imposed sanctions, including:
| | • | | issuance of Form FDA 483 notices or Warning Letters by the FDA or other regulatory agencies; |
| | • | | imposition of fines and other civil penalties; |
| | • | | injunctions, suspensions or revocations of regulatory approvals; |
| | • | | suspension of any ongoing clinical trials; |
| | • | | total or partial suspension of manufacturing; |
| | • | | delays in commercialization; |
| | • | | refusal by the FDA to approve pending applications or supplements to approved applications submitted by us; |
| | • | | refusals to permit drugs to be imported into or exported from the United States; |
| | • | | restrictions on operations, including costly new manufacturing requirements; and |
| | • | | product recalls or seizures. |
The policies of the FDA and other regulatory agencies may change and additional government regulations may be enacted that could prevent or delay regulatory approval of ADCETRIS in any additional indications or further restrict or regulate post-approval activities. We cannot predict the likelihood, nature or extent of adverse government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are not able to maintain regulatory compliance, we or Takeda might not be permitted to market ADCETRIS and our business would suffer.
If we or our collaborators are not able to obtain or maintain required regulatory approvals, we or our collaborators will not be able to successfully commercialize ADCETRIS or our product candidates.
The research, testing, manufacturing, labeling, approval, selling, marketing and distribution of drug products are subject to extensive regulation by the FDA and other regulatory authorities in the United States and other countries, which regulations differ from country to country. Neither we nor our collaborators are permitted to market our product candidates in the United States or foreign countries until we obtain marketing approval from the FDA or other foreign regulatory authorities, and we or our collaborators may never receive regulatory
42
approval for the commercial sale of any of our product candidates. In addition, part of our strategy is to continue to explore the use of ADCETRIS earlier in the treatment of Hodgkin lymphoma and MTCL and in other CD30-expressing lymphomas, and we are currently conducting multiple clinical trials for ADCETRIS. However, we and/or Takeda may be unable to obtain or maintain any regulatory approvals for the commercial sale of ADCETRIS for any additional indications. Obtaining marketing approval is a lengthy, expensive and uncertain process and approval is never assured, and we have only limited experience in preparing and submitting the applications necessary to gain regulatory approvals. Further, the FDA and other foreign regulatory agencies have substantial discretion in the approval process, and determining when or whether regulatory approval will be obtained for any product candidate we develop, including any regulatory approvals for the potential commercial sale of ADCETRIS in additional indications or in any additional territories. In this regard, even if we believe the data collected from clinical trials of ADCETRIS and our product candidates are promising, such data may not be sufficient to support approval by the FDA or any other foreign regulatory authority. In addition, the FDA or their advisors may disagree with our interpretations of data from preclinical studies and clinical trials. For example, based on the positive data we reported from theECHELON-1 trial, we have submitted an sBLA to the FDA for approval of ADCETRIS as part of a frontline combination chemotherapy regimen in patients with previously untreated advanced classical Hodgkin lymphoma. However, even though our sBLA was accepted by the FDA for Priority Review, the FDA may disagree with our interpretations of the data from theECHELON-1 trial and/or may otherwise determine not to approve our sBLA submission in a timely manner or at all. Moreover, even though ourECHELON-1 andECHELON-2 trials are being conducted under SPA agreements with the FDA, this is not a guarantee or indication of approval, and we cannot be certain that the design of, or data collected from, any of our current or potential future clinical trials that were or are being conducted under SPA agreements with the FDA will be sufficient to support FDA approval. Further, a SPA agreement is not binding on the FDA if public health concerns unrecognized at the time the SPA agreement is entered into become evident, other new scientific concerns regarding product safety or efficacy arise, new drugs are approved in the same indication, or if we have failed to comply with the agreed upon trial protocols, including as a result of completing a clinical trial with fewer events than planned. In addition, a SPA agreement may be changed by us or the FDA on written agreement of both parties, and the FDA retains significant latitude and discretion in interpreting the terms of a SPA agreement and the data and results from the applicable clinical trial. For example, even though we believe that the data from theECHELON-1 trial are supportive of approval of ADCETRIS in theECHELON-1 treatment setting, our SPA agreement with the FDA covering theECHELON-1 trial is not a guarantee or indication of approval of ADCETRIS in theECHELON-1 treatment setting or in any other indications. Regulatory agencies also may approve a product candidate for fewer indications than requested or may grant approval subject to the performance of post-approval studies or REMS for a product candidate. Similarly, regulatory agencies may not approve the labeling claims that are necessary or desirable for the successful commercialization of ADCETRIS in additional indications, including any indications in theECHELON-1 treatment setting. For example, there was an increased incidence of febrile neutropenia and peripheral neuropathy in the ADCETRIS plus AVD arm of theECHELON-1 trial, which could limit, narrow or preclude any approval by the FDA, or could limit prescribing of ADCETRIS in theECHELON-1 treatment setting if approved by the FDA, both of which could negatively impact sales of ADCETRIS or adversely affect ADCETRIS’ acceptance in the market.
In addition, changes in regulatory requirements and guidance may occur and we may need to amend clinical trial protocols and/or related SPA agreements to reflect these changes. Amendments may require us to resubmit our clinical trial protocols to institutional review boards, or IRBs, for reexamination, which may impact the costs, timing or successful completion of a clinical trial. In addition, as part of the U.S. Prescription Drug User Fee Act, or PDUFA, the FDA has a goal to review and act on a percentage of all regulatory submissions in a given time frame. In this regard, the sBLA that we submitted to the FDA in November 2017 to seek approval of ADCETRIS as part of a frontline combination chemotherapy regimen in patients with previously untreated advanced classical Hodgkin lymphoma was accepted for filing and designated for priority review with a PDUFA targeted action date of May 1, 2018. However, the FDA does not always meet its PDUFA targeted action dates and if the FDA were to fail to meet the PDUFA targeted action date for our November 2017 sBLA submission or fail to meet future PDUFA targeted action dates established for ADCETRIS or any of our product candidates, if any, the commercialization of the affected product candidate or of ADCETRIS in any additional indications could be
43
delayed or impaired. Due to these and other factors, ADCETRIS and our product candidates could take a significantly longer time to gain regulatory approvals than we expect or may never gain new regulatory approvals, which could delay or eliminate any potential product revenue from sales of our product candidates or of ADCETRIS in any additional indications, which could significantly delay or prevent us from achieving profitability.
The successful commercialization of ADCETRIS and our product candidates will depend in part on the extent to which governmental authorities and health insurers establish adequate coverage and reimbursement levels and pricing policies.
Successful sales of ADCETRIS and any future products will depend, in part, on the extent to which coverage and reimbursement for our products will be available from government and health administration authorities, private health insurers and other third-party payors. To manage healthcare costs, many governments and third-party payors increasingly scrutinize the pricing of new products and require greater levels of evidence of favorable clinical outcomes and cost-effectiveness before extending coverage. In light of such challenges to prices, we cannot be sure that we will achieve and continue to have coverage available for ADCETRIS and any other product candidate that we commercialize and, if available, that the reimbursement rates will be adequate. If we are unable to obtain adequate levels of coverage and reimbursement for our product candidates, their marketability will be negatively and materially impacted. For example, even if we are able to obtain approval of our sBLA submission to the FDA to expand the labeled indications of use for ADCETRIS to the frontline advanced Hodgkin lymphoma setting based on ourECHELON-1 trial data, we cannot be certain that third-party payors will provide reimbursement for ADCETRIS in that indication based on the relative price or perceived benefit of ADCETRIS as compared to alternative treatment options, which may materially harm our ability to maintain or increase sales of ADCETRIS or may otherwise negatively affect future ADCETRIS sales.
Moreover, eligibility for coverage and reimbursement does not imply that a drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. In addition, obtaining and maintaining adequate coverage and reimbursement status is time-consuming and costly. Third-party payors may deny coverage and reimbursement status altogether of a given drug product, or cover the product but may also establish prices at levels that are too low to enable us to realize an appropriate return on our investment in product development. Further, one payor’s determination to provide coverage for a product does not assure that other payors will also provide coverage for the product. Because the rules and regulations regarding coverage and reimbursement change frequently, in some cases at short notice, even when there is favorable coverage and reimbursement, future changes may occur that adversely impact the favorable status.
The unavailability or inadequacy of third-party coverage and reimbursement could have a material adverse effect on the market acceptance of ADCETRIS and any of our future products and the future revenues we may expect to receive from those products. In addition, we are unable to predict what additional legislation or regulation relating to the healthcare industry or third-party coverage and reimbursement may be enacted in the future, or what effect such legislation or regulation would have on our business. Continuing negative publicity regarding pharmaceutical pricing practices and ongoing presidential and congressional focus on this issue create significant uncertainty regarding regulation of the healthcare industry and third-party coverage and reimbursement. If healthcare policies or reforms intended to curb healthcare costs are adopted or if we experience negative publicity with respect to pricing of ADCETRIS or the pricing of pharmaceutical products generally, the prices that we charge for ADCETRIS and any future approved products may be limited, our commercial opportunity may be limited and/or our revenues from sales of ADCETRIS and any future approved products may be negatively impacted.
We do not have sole control of the development and commercialization of enfortumab vedotin and tisotumab vedotin, and we have limited data on the safety and efficacy of these drug candidates
We and our collaborators, Astellas and Genmab respectively, have elected to pursue accelerated development and approval pathways forenfortumab vedotin and tisotumab vedotin. We have initiated a pivotal
44
clinical trial for enfortumab vedotin and intend to initiate a pivotal clinical trial for tisotumab vedotin, in each case based on only limited phase 1 clinical data. There may be important facts about the safety, efficacy, and risk versus benefit of these product candidates that are not known to us at this time which may negatively impact our ability to develop and commercialize these product candidates. In response to safety events observed in our ongoing clinical trials of enfortumab vedotin and tisotumab vedotin, including patient deaths, we have in the past, and may in the future, institute additional precautionary safety measures such as dosing caps and delays, enhanced monitoring for side effects, and modified patient inclusion and exclusion criteria. In addition, enfortumab vedotin and tisotumab vedotin may fail to demonstrate sufficient efficacy in our pivotal trials despite the results observed in previous trials. Additional and/or unexpected safety events or our failure to generate additional efficacy data in our clinical trials that support registration could significantly impact the value of enfortumab vedotin and tisotumab vedotin to our business. Moreover, because control of development and commercialization is shared with our collaborators, we do not have sole discretion and control over the development and commercialization of these product candidates.
Healthcare law and policy changes may have a material adverse effect on us
In March 2010, the Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act of 2010, or collectively PPACA, became law in the United States. PPACA substantially changed the way healthcare is financed by both governmental and private insurers and significantly affects the pharmaceutical industry. The provisions of PPACA of greatest importance to the pharmaceutical industry include increased Medicaid rebates, expanded Medicaid eligibility, extension of Public Health Service eligibility, annual fees payable by manufacturers and importers of branded prescription drugs, annual reporting of financial relationships with physicians and teaching hospitals, and a new Patient-Centered Outcomes Research Institute. Many of these provisions have had the effect of reducing the revenue generated by our sales of ADCETRIS and will have the effect of reducing any revenue generated by sales of any future commercial products we may have.
Certain provisions of the PPACA have been subject to judicial and Congressional challenges, as well as efforts by the Trump administration to repeal or replace certain aspects of the PPACA. For example, on January 20, 2017, President Trump signed an Executive Order directing federal agencies with authorities and responsibilities under the PPACA to waive, defer, grant exemptions from, or delay the implementation of any provision of the PPACA that would impose a fiscal or regulatory burden on states, individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices. In Congress, the U.S. House of Representatives passed PPACA replacement legislation known as the American Health Care Act of 2017 in May 2017, which was not introduced in the Senate. More recently, the Senate Republicans have proposed multiple bills to repeal or repeal and replace portions of the PPACA. Although none of these measures have been enacted, Congress may consider other legislation to repeal or replace certain elements of the PPACA. While Congress has not passed repeal or replace legislation, the tax reform legislation signed into law on December 22, 2017 includes a provision repealing, effective January 1, 2019, thetax-based shared responsibility payment imposed by the ACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate.” On October 12, 2017, President Trump signed another Executive Order directing certain federal agencies to propose regulations or guidelines to permit small businesses to form association health plans, expand the availability of short-term, limited duration insurance, and expand the use of health reimbursement arrangements, which may circumvent some of the requirements for health insurance mandated by the PPACA. In addition, citing legal guidance from the U.S. Department of Justice, the U.S. Department of Health and Human Services, has concluded that cost-sharing reduction, or CSR, payments to insurance companies required under the PPACA have not received necessary appropriations from Congress and announced that it will discontinue these payments immediately until such appropriations are made. The loss of the CSR payments is expected to increase premiums on certain policies issued by qualified health plans under the PPACA. While Congress is considering legislation to appropriate funds for CSR payments the future of that legislation is uncertain. We continue to evaluate the effect that the PPCCA and its possible repeal and replacement has on our business.
45
In addition, we anticipate that the PPACA, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and an additional downward pressure on the price that we receive for ADCETRIS or any future approved product, which may harm our business. For example, increased discounts, rebates or chargebacks may be mandated by governmental or private insurers or fee caps and pricing pressures could be enacted by industry organizations or state and federal governments, any of which could significantly affect the revenue generated by sales of our products, including ADCETRIS. In addition, drug-pricing by pharmaceutical companies has come under increased scrutiny. Specifically, there have been several recent U.S. Congressional inquiries and proposed federal and state legislation designed to, among other things, bring more transparency to drug pricing by requiring drug companies to notify insurers and government regulators of price increases and to provide an explanation as to the reasons for the increase, reduce theout-of-pocket cost of prescription drugs, review the relationship between pricing and manufacturer patient programs and reform government program reimbursement methodologies for drugs. We expect further federal and state legislation and healthcare reforms to continue to be proposed to control increasing healthcare costs and to control the rising cost of prescription drugs. These proposals, if implemented, could limit the price for ADCETRIS or any future approved products. Commercial opportunity could be negatively impacted by legislative action that controls pricing, mandates price negotiations, or increases government discounts and rebates.
Also, price increases on ADCETRIS and negative publicity regarding drug pricing and price increases generally, whether on ADCETRIS or products distributed by other pharmaceutical companies, could negatively affect market acceptance of, and sales of, ADCETRIS. In addition, although ADCETRIS is approved in the European Union, Japan and other countries outside of the United States, government austerity measures or further healthcare reform measures and pricing pressures in other countries could adversely affect demand and pricing for ADCETRIS, which would negatively impact anticipated royalty revenue from ADCETRIS sales by Takeda.
Other legislative changes have also been proposed and adopted since PPACA was enacted. The Budget Control Act of 2011, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee did not achieve a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This includes a 2% reduction in Medicare provider payments paid under Medicare Part B to physicians for physician-administered drugs, such as certain oral oncology drugs, which went into effect in April 2013 and, following passage of the Bipartisan Budget Act of 2015, will remain in effect through 2025 unless additional congressional action is taken. The American Taxpayer Relief Act of 2012, among other things, reduced Medicare payments to several providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. In addition, legislation has been proposed to shorten the period of biologic data and market exclusivity granted by the FDA. If such legislation is enacted, we may face competition from biosimilars of ADCETRIS or any future approved products earlier than otherwise would have occurred. Increased competition may negatively impact coverage and pricing of ADCETRIS, which could negatively affect our financial condition or results of operations.
We expect to experience pricing pressures in connection with the sale of ADCETRIS due to the trend toward managed healthcare, and additional legislative proposals. For example, the PPACA increased the mandated Medicaid rebate from 15.1% to 23.1%, expanded the rebate to Medicaid managed care utilization and increased the types of entities eligible for the federal 340B drug discount program. On January 30, 2017, the White House Office of Management and Budget withdrew the draft August 2015 Omnibus Guidance document that was issued by the Department of Health and Human Services Health Resources and Services Administration, or HRSA, that addressed a broad range of topics including, among other items, the definition of a patient’s eligibility for 340B drug pricing. However, as concerns continue to grow over the need for tighter oversight, there remains the possibility that HRSA or other agency under the Department of Health and Human Services, or HHS, will propose a similar regulation or that Congress will explore changes to the 340B program through legislation. For example, the Centers for Medicare & Medicaid Services has issued a proposed rule that would
46
revise the Medicare hospital outpatient prospective payment system, including a new reimbursement methodology for drugs purchased under the 340B program for Medicare patients. In addition, HHS has currently set July 1, 2018 for implementation of the final rule setting forth the calculation of the ceiling price and application of civil monetary penalties under the 340B program. A significant portion of ADCETRIS purchases are eligible for 340B drug pricing, and therefore an expansion of the 340B program or reduction in 340B pricing, whether in the form of the final rule or otherwise, would likely have a negative impact on our net sales of ADCETRIS.
We cannot predict what healthcare reform initiatives may be adopted in the future. However, we anticipate that Congress, state legislatures, and third-party payors may continue to review and assess alternative healthcare delivery and payment systems and may in the future propose and adopt legislation or policy changes or implementations effecting additional fundamental changes in the healthcare delivery system. We also expect ongoing initiatives to increase pressure on drug pricing. We cannot assure you as to the ultimate content, timing, or effect of changes, nor is it possible at this time to estimate the impact of any such potential legislation; however, such changes or the ultimate impact of changes could negatively affect our revenue or sales of ADCETRIS or any potential future approved products.
Enhanced governmental and private scrutiny over, or investigations or litigation involving, pharmaceutical manufacturer donations to patient assistance programs offered by charitable foundations may require us to modify our programs and could negatively impact our business practices, harm our reputation, divert the attention of management and increase our expenses.
To help patients afford our products, we have a patient assistance program and also occasionally make donations to independent charitable foundations that help financially needy patients. These types of programs designed to assist patients in affording pharmaceuticals have become the subject of scrutiny. In recent years, some pharmaceutical manufacturers were named in class action lawsuits challenging the legality of their patient assistance programs and support of independent charitable patient support foundations under a variety of federal and state laws. At least one insurer also has directed its network pharmacies to no longer accept manufacturerco-payment coupons for certain specialty drugs the insurer identified. Our patient assistance program and support of independent charitable foundations could become the target of similar litigation.
In addition, there has been regulatory review and enhanced government scrutiny of donations by pharmaceutical companies to patient assistance programs operated by charitable foundations. For example, the Office of Inspector General of the U.S. Department of Health & Human Services, or OIG, has established specific guidelines permitting pharmaceutical manufacturers to make donations to charitable organizations who provideco-pay assistance to Medicare patients, provided that such organizations are bona fide charities, are entirely independent of and not controlled by the manufacturer, provide aid to applicants on a first-come basis according to consistent financial criteria, and do not link aid to use of a donor’s product. If we or our vendors or donation recipients are deemed to fail to comply with laws or regulations in the operation of these programs, we could be subject to damages, fines, penalties or other criminal, civil or administrative sanctions or enforcement actions. Further, numerous organizations, including pharmaceutical manufacturers, have received subpoenas from the OIG and other enforcement authorities seeking information related to their patient assistance programs and support. We cannot ensure that our compliance controls, policies and procedures will be sufficient to protect against acts of our employees, business partners or vendors that may violate the laws or regulations of the jurisdictions in which we operate. Regardless of whether we have complied with the law, a government investigation could negatively impact our business practices, harm our reputation, divert the attention of management and increase our expenses.
Clinical trials are expensive and time consuming, may take longer than we expect or may not be completed at all, and their outcome is uncertain.
We are currently conducting multiple clinical trials for ADCETRIS and our product candidates and we plan to commence additional trials of ADCETRIS and our product candidates in the future. We are also conducting a
47
pivotal phase 2 trial of enfortumab vedotin with Astellas for locally advanced or metastatic urothelial cancer patients who have been previously treated with checkpoint inhibitor therapy, and are planning to conduct a pivotal phase 2 trial of tisotumab vedotin with Genmab in patients with recurrent and/or metastatic cervical cancer, in each case based on only limited phase 1 clinical data. Neither enfortumab vedotin nor tisotumab vedotin have previously been evaluated in later stage clinical trials and we cannot be certain that the design of, or data collected from, these trials will be adequate to demonstrate the safety and efficacy of enfortumab vedotin or tisotumab vedotin, or will otherwise be sufficient to support FDA or any foreign regulatory approvals.
Each of our clinical trials requires the investment of substantial expense and time and the timing of the commencement, continuation and completion of these clinical trials may be subject to significant delays relating to various causes, including scheduling conflicts with participating clinicians and clinical institutions, difficulties in identifying and enrolling patients who meet trial eligibility criteria, failure of patients to complete the clinical trial, delays in accumulating the required number of clinical events for data analyses, delay or failure to obtain IRB approval to conduct a clinical trial at a prospective site, and shortages of available drug supply. For example, the SPA agreement for theECHELON-2 trial requires that the trial continue until a specified number of PFS events designated for the trial occurs. Based on reviews of pooled, blinded data, we have observed a lower rate of reported PFS events than anticipated. We plan to discuss with the FDA the potential to unblind the trial prior to achieving the target number of PFS events specified in the SPA agreement. We cannot predict the outcome of those discussions or whether we would be able to reach agreement with the FDA. If we are unable to reach agreement with the FDA and determine to unblind the trial prior to achieving the target number of PFS events as specified in the SPA agreement, the FDA could treat the SPA agreement forECHELON-2 trial as rescinded. In that event, we would no longer have commitments from the FDA regarding the appropriate design, size and endpoints of the study for regulatory approval, making our ability to obtain regulatory approval of ADCETRIS in theECHELON-2 treatment setting more uncertain. In addition, earlier unblinding in theECHELON-2 trial could also negatively impact the likelihood of achieving positive results in the trial sufficient to support regulatory approval. Alternatively, if we are unable to reach agreement with the FDA, we could determine to continue theECHELON-2 trial until the target number of PFS events specified in the SPA agreement is achieved, which could result in a substantial delay in our ability to conduct the final data analysis from theECHELON-2 trial.
Additionally, patient enrollment is a function of many factors, including the size of the patient population, the proximity of patients to clinical sites, the eligibility criteria for the trial, the existence of competing clinical trials, perceived side effects and the availability of alternative or new treatments. Many of our future and ongoing clinical trials are being or will be coordinated or conducted with Takeda, Astellas, Genmab and other collaborators, which may delay the commencement or affect the continuation or completion of these trials. From time to time, we have experienced enrollment-related delays in clinical trials and we will likely continue to experience similar delays in our current and future trials. We depend on medical institutions and clinical research organizations, or CROs, to conduct some of our clinical trials in compliance with Good Clinical Practice, or GCP, and to the extent they fail to enroll patients for our clinical trials, fail to conduct our trials in accordance with GCP, or are delayed for a significant time in achieving full enrollment, we may be affected by increased costs, program delays or both, which may harm our business. In addition, we conduct clinical trials in foreign countries which may subject us to further delays and expenses as a result of increased drug shipment costs, additional regulatory requirements and the engagement of foreign CROs, as well as expose us to risks associated with less experienced clinical investigators who are unknown to the FDA, different standards of medical care, and foreign currency transactions insofar as changes in the relative value of the U.S. dollar to the foreign currency where the trial is being conducted may impact our actual costs.
Clinical trials must be conducted in accordance with FDA or other applicable foreign government guidelines and are subject to oversight by the FDA, other foreign governmental agencies, the data safety monitoring boards for such trials and the IRBs or Ethics Committees for the institutions in which such trials are being conducted. In addition, clinical trials must be conducted with supplies of ADCETRIS or our product candidates produced under cGMP and other requirements in foreign countries, and may require large numbers of test patients. We or our collaborators, the FDA, other foreign governmental agencies or the applicable data safety monitoring boards,
48
IRBs and Ethics Committees could delay, suspend, halt or modify our clinical trials of ADCETRIS or any of our product candidates, and we, our collaborators and/or the FDA could terminate or modify any related SPA agreements, for numerous reasons, including:
| | • | | ADCETRIS or the applicable product candidate may have unforeseen safety issues or adverse side effects, including fatalities, or a determination may be made that a clinical trial presents unacceptable health risks; |
| | • | | deficiencies in the conduct of the clinical trial, including failure to conduct the clinical trial in accordance with regulatory requirements, GCP or clinical protocols; |
| | • | | problems, errors or other deficiencies with respect to data collection, data processing and analysis; |
| | • | | deficiencies in the clinical trial operations or trial sites resulting in the imposition of a clinical hold; |
| | • | | the time required to determine whether ADCETRIS or the applicable product candidate is effective may be longer than expected; |
| | • | | fatalities or other adverse events arising during a clinical trial due to medical problems that may not be related to clinical trial treatments; |
| | • | | ADCETRIS or the applicable product candidate may not appear to be more effective than current therapies; |
| | • | | the quality or stability of ADCETRIS or the applicable product candidate may fall below acceptable standards; |
| | • | | our inability and the inability of our collaborators to produce or obtain sufficient quantities of ADCETRIS or the applicable product candidate to complete the trials; |
| | • | | our inability and the inability of our collaborators to reach agreement on acceptable terms with prospective CROs and trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| | • | | our inability and the inability of our collaborators to obtain IRB or Ethics Committee approval to conduct a clinical trial at a prospective site; |
| | • | | changes in governmental regulations or administrative actions that adversely affect our ability and the ability of our collaborators to continue to conduct or to complete clinical trials; |
| | • | | lack of adequate funding to continue the clinical trial, including the incurrence of unforeseen costs due to enrollment delays, requirements to conduct additional trials and studies and increased expenses associated with the services of our CROs and other third parties; |
| | • | | our inability and the inability of our collaborators to recruit and enroll patients to participate in clinical trials for reasons including competition from other clinical trial programs for the same or similar indications; |
| | • | | our inability and the inability of our collaborators to retain patients who have initiated a clinical trial but may be prone to withdraw due to side effects from the therapy, lack of efficacy or personal issues, or who are lost to furtherfollow-up; or |
| | • | | our inability and the inability of our collaborators to ensure adequate statistical power to detect statistically significant treatment effects, whether through our inability to enroll or retain patients in trials or because the specified number of events designated for a completed trial have not occurred. |
In addition, we or our collaborators may experience significant setbacks in advanced clinical trials, even after promising results in earlier trials, including unexpected adverse events that may occur when our product candidates are combined with other therapies. For example, in June 2017, we suspended patient enrollment and treatment in allSGN-CD33A trials and discontinued the phase 3 CASCADE clinical trial ofSGN-CD33A in
49
frontline older acute myeloid leukemia, or AML, patients, following a higher rate of deaths in theSGN-CD33A containing arm versus the control arm of this trial, and the IND forSGN-CD33A was subsequently placed on hold by the FDA. At this time, we have no plans to initiate additional clinical trials ofSGN-CD33A. In the future, we may determine to discontinue ourSGN-CD33A program altogether, in which case we will not receive any return on our investment inSGN-CD33A.
Negative or inconclusive clinical trial results could adversely affect our ability and the ability of our collaborators to obtain regulatory approvals of our product candidates or to market ADCETRIS and/or expand ADCETRIS into additional indications. In particular, negative or inconclusive results in ourECHELON-2 trial would negatively impact or preclude altogether, our and Takeda’s ability to obtain regulatory approvals in the frontline MTCL indication in our respective territories, which would limit our sales of, and the commercial potential of, ADCETRIS. In addition, clinical trial results are frequently susceptible to varying interpretations that may delay, limit or prevent regulatory approvals. For example, although we reported positive top line data in ourECHELON-1 trial, regulatory agencies, including the FDA, or their advisors, may disagree with our interpretations of data from theECHELON-1 trial and may not approve the expansion of ADCETRIS’ labeled indications of use based on the results of theECHELON-1 trial or any other of our clinical trials. Adverse medical events during a clinical trial, including patient fatalities, could cause a trial to be redone or terminated, require us to cease development of a product candidate or the further development or commercialization of ADCETRIS, result in our failure to expand ADCETRIS into additional indications, adversely affect our ability to market ADCETRIS, and may result in other negative consequences to us, including the inclusion of unfavorable information in our product labeling. Further, some of our clinical trials are overseen by an IDMC, and an IDMC may determine to delay or suspend one or more of these trials due to safety or futility findings based on events occurring during a clinical trial. In addition, we may be required to implement additional risk mitigation measures that could require us to suspend our clinical trials if certain safety events occur.
We depend on collaborative relationships with other companies to assist in the research and development of ADCETRIS and for the development and commercialization of product candidates utilizing or incorporating our technologies. If we are not able to locate suitable collaborators or if our collaborators do not perform as expected, this may negatively affect our ability to commercialize ADCETRIS, develop other product candidates and/or generate revenues through technology licensing, or may otherwise negatively affect our business.
We have established collaborations with third parties to develop and market ADCETRIS and some of our current and future product candidates. For example, we entered into a collaboration agreement with Takeda in December 2009 that granted Takeda rights to develop and commercialize ADCETRIS outside of the United States and Canada. In addition, we have entered into 50:50co-development collaborations with Astellas for the development of enfortumab vedotin, and with Genmab for the development of tisotumab vedotin. We are also collaborating with BMS with respect to the CHECKMATE 812 pivotal phase 3 clinical trial evaluating the combination of Opdivo (nivolumab) with ADCETRIS for the treatment of relapsed or refractory, or transplant-ineligible, advanced classical Hodgkin lymphoma. In addition, we have ADC collaborations with AbbVie, Bayer, Celldex, Genentech, GSK, Pfizer and Progenics, and we have entered into a collaboration agreement with Unum to develop and commercialize novel ACTR therapies incorporating our antibodies for the treatment of cancer and with Pieris to develop targeted bispecific immuno-oncology therapies for the treatment of cancer. Our dependence on collaborative arrangements to assist in the development and commercialization of ADCETRIS and for the development and commercialization of product candidates utilizing or incorporating our technologies subjects us to a number of risks, including:
| | • | | we are not able to control the amount and timing of resources that our collaborators devote to the development or commercialization of products and product candidates utilizing or incorporating our technologies, or to their marketing and distribution; |
| | • | | disputes may arise between us and our collaborators that result in the delay or termination of the research, development or commercialization of the applicable products and product candidates or that result in costly litigation or arbitration that diverts management’s attention and resources; |
50
| | • | | with respect to collaborations under which we have an active role, such as our ADCETRIS collaboration and our 50:50co-development agreements with Astellas and Genmab, we may have differing opinions or priorities than our collaborators, or we may encounter challenges in joint decision making, which may result in the delay or termination of the research, development or commercialization of the applicable products and product candidates, including ADCETRIS, enfortumab vedotin and tisotumab vedotin; |
| | • | | our current and potential future collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing; |
| | • | | significant delays in the development of product candidates by current and potential collaborators could allow competitors to bring products to market before product candidates utilizing or incorporating our technologies are approved and impair the ability of current and potential future collaborators to effectively commercialize these product candidates; |
| | • | | our relationships with our collaborators may divert significant time and effort of our scientific staff and management team and require the effective allocation of our resources to multiple internal collaborative projects; |
| | • | | our current and potential future collaborators may not be successful in their efforts to obtain regulatory approvals in a timely manner, or at all; |
| | • | | our current and potential future collaborators may receive regulatory sanctions relating to other aspects of their business that could adversely affect the development, approval or commercialization of the applicable products or product candidates; |
| | • | | our current and potential future collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our proprietary information or expose us to potential litigation; |
| | • | | business combinations or significant changes in a collaborator’s business strategy may adversely affect such party’s willingness or ability to complete its obligations under any arrangement; |
| | • | | a collaborator could independently move forward with competing products, therapeutic approaches or technologies to develop treatments for the diseases targeted by us or our collaborators that are developed by such collaborator either independently or in collaboration with others, including our competitors; |
| | • | | our current and potential collaborators may experience financial difficulties; and |
| | • | | our collaborations may be terminated, breached or allowed to expire, or our collaborators may reduce the scope of our agreements with them, which could have a material adverse effect on our financial position by reducing or eliminating the potential for us to receive technology access and license fees, milestones and royalties, and/or reimbursement of development costs, and which could require us to devote additional efforts and to incur the additional costs associated with pursuing internal development and commercialization of the applicable products and product candidates. |
If our collaborative arrangements are not successful as a result of any of the above factors, or any other factors, then our ability to advance the development and commercialization of the applicable products and product candidates and to otherwise generate revenue from these arrangements and to become profitable will be adversely affected, and our business and business prospects may be materially harmed. In particular, if Takeda were to terminate the ADCETRIS collaboration, which it may do for any reason upon prior written notice to us, we would not receive milestone payments,co-funded development payments or royalties for the sale of ADCETRIS outside the United States and Canada. As a result of such termination, we may have to engage another collaborator to complete the ADCETRIS development process and to commercialize ADCETRIS outside the United States and Canada, or to complete the development process and undertake commercializing ADCETRIS outside the United States and Canada ourselves, either of which could significantly delay the
51
continued development and commercialization of ADCETRIS and increase our costs. Similarly, both Astellas and Genmab have the right toopt-out of theirco-development obligations relating to enfortumab vedotin and tisotumab vedotin, respectively. If either Astellas or Genmab were toopt-out of theirco-development collaborations with us, this would significantly delay the development of the impacted product candidate and increase our costs. Any of these events could significantly harm our financial position, adversely affect our stock price and require us to incur all the costs of developing and commercializing ADCETRIS, enfortumab vedotin or tisotumab vedotin, which are now beingco-funded by our collaboration partners. In the future, we may not be able to locate third-party collaborators to develop and market products and product candidates utilizing or incorporating our technologies, and we may lack the capital and resources necessary to develop and market these products and product candidates alone.
We face intense competition and rapid technological change, which may result in others discovering, developing or commercializing competing products before or more successfully than we do.
The biotechnology and biopharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. Many third parties compete with us in developing various approaches to treating cancer. They include pharmaceutical companies, biotechnology companies, academic institutions and other research organizations.
Many of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approval and marketing than we do. In addition, many of these competitors are active in seeking patent protection and licensing arrangements in anticipation of collecting royalties for use of technology that they have developed. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific and management personnel, as well as in acquiring technologies complementary to our programs.
With respect to ADCETRIS, there are several otherFDA-approved drugs for its approved indications. Bristol-Myers Squibb’s nivolumab (Opdivo) and Merck’s pembrolizumab (Keytruda) are approved for the treatment of certain patients with relapsed or refractory classical Hodgkin lymphoma, and Celgene’s romidepsin (Istodax) and Spectrum Pharmaceuticals’ pralatrexate (Folotyn) and belinostat (Beleodaq) are approved for relapsed or refractory sALCL among otherT-cell lymphomas. The competition ADCETRIS faces from these and other therapies is intensifying. Additionally, Merck is conducting a phase 3 clinical trial in relapsed or refractory classical Hodgkin lymphoma comparing pembrolizumab (Keytruda) with ADCETRIS. If this clinical trial demonstrates that pembrolizumab is more effective than ADCETRIS in that treatment setting, our sales of ADCETRIS would be negatively impacted. We are also aware of multiple investigational agents that are currently being studied, including Roche’s atezolizumab, Pfizer’s avelumab, and Kyowa’s mogamulizumab, which, if successful, may compete with ADCETRIS in the future. Data have also been presented on several developing technologies, including bispecific antibodies and CAR modifiedT-cell therapies that may compete with ADCETRIS in the future. Further, there are many competing approaches used in the treatment of patients in ADCETRIS’ four approved indications, including autologous hematopoietic stem cell transplant, allogeneic stem cell transplant, combination chemotherapy, clinical trials with experimental agents and single-agent regimens.
With respect to enfortumab vedotin, treatment in second line metastatic urothelial cancer is limited to CPI monotherapy or generic chemotherapy. There are other investigational agents that, if approved, could be competitive with enfortumab vedotin, including Immunomedics’ sacituzumab govitecan and Lilly’s ramucirumab.
With respect to tisotumab vedotin, we are aware of other companies that currently have products in development for the treatment of late-stage cervical cancer which could be competitive with tisotumab vedotin, including Agenus, Astrazeneca, Bristol-Myers Squibb, Immunomedics, Innovent Biologics, Merck, and Roche.
52
In addition, several CPIs that areFDA-approved in other treatment settings are being explored for the treatment of late-stage cervical cancer in ongoing phase 2 clinical trials.
Many other pharmaceutical and biotechnology companies are developing and/or marketing therapies for the same types of cancer that our product candidates are designed and being developed to treat. For example, we believe that companies including AbbVie, ADC Therapeutics, Affimed, Agios, Amgen, Astellas, Bayer, Biogen, Bristol-Myers Squibb, Celgene, Eisai, Genentech, GSK, Gilead, ImmunoGen, Immunomedics, Infinity, Karyopharm, MedImmune, MEI Pharma, Merck, Novartis, Pfizer, Sanofi-Aventis, Spectrum Pharmaceuticals, Takeda, Teva, and Xencor are developing and/or marketing products or technologies that may compete with ours. In addition, our ADC collaborators may develop compounds utilizing our technology that may compete with product candidates that we are developing.
We are aware of other companies that have technologies that may be competitive with ours, including Astellas, AstraZeneca, Bristol-Myers Squibb, ImmunoGen, Immunomedics, MedImmune, Mersana and Pfizer, all of which have ADC technology. ImmunoGen has several ADCs in development that may compete with our product candidates. ImmunoGen has also established partnerships with other pharmaceutical and biotechnology companies to allow those other companies to utilize ImmunoGen’s technology, including Sanofi-Aventis, Genentech, Novartis, Takeda and Lilly. We are also aware of a number of companies developing monoclonal antibodies directed at the same antigen targets or for the treatment of the same diseases as our product candidates. For example, we believe Amgen and Xencor have anti-CD19 programs that may be competitive with our product candidates.
In addition, in the United States, the Biologics Price Competition and Innovation Act of 2009 created an abbreviated approval pathway for biological products that are demonstrated to be “highly similar” or “biosimilar” to or “interchangeable” with anFDA-approved biological product. This pathway allows competitors to reference the FDA’s prior approvals regarding innovative biological products and data submitted with a BLA to obtain approval of a biosimilar application 12 years after the time of approval of the innovative biological product. The12-year exclusivity period runs from the initial approval of the innovator product and not from approval of a new indication. In addition, the12-year exclusivity period does not prevent another company from independently developing a product that is highly similar to the innovative product, generating all the data necessary for a full BLA and seeking approval. Exclusivity only assures that another company cannot rely on the FDA’s prior approvals in approving a BLA for an innovator’s biological product to support the biosimilar product’s approval. Further, under the FDA’s current interpretation, it is possible that a biosimilar applicant could obtain approval for one or more of the indications approved for the innovator product by extrapolating clinical data from one indication to support approval for other indications. The FDA approved the first biosimilar product in the United States in May 2015. In the European Union, the European Commission has granted marketing authorizations for several biosimilars pursuant to a set of general and product class-specific guidelines for biosimilar approvals issued since 2005. We are aware of many pharmaceutical and biotechnology and other companies that are actively engaged in research and development of biosimilars or interchangeable products.
It is possible that our competitors will succeed in developing technologies that are more effective than ADCETRIS, enfortumab vedotin, tisotumab vedotin or our other product candidates or that would render our technology obsolete or noncompetitive, or will succeed in developing biosimilar or interchangeable products for ADCETRIS, enfortumab vedotin, tisotumab vedotin or our other product candidates. We anticipate that we will continue to face increasing competition in the future as new companies enter our market and scientific developments surrounding biosimilars and other cancer therapies continue to accelerate. We cannot predict to what extent the entry of biosimilars or other competing products will impact potential future sales of ADCETRIS, enfortumab vedotin, tisotumab vedotin or our other product candidates.
53
Our operating results are difficult to predict and may fluctuate. If our operating results are below the expectations of securities analysts or investors, the trading price of our stock could decline.
Our operating results are difficult to predict and may fluctuate significantly from quarter to quarter and year to year. In addition, although we provide sales guidance for ADCETRIS from time to time, you should not rely on ADCETRIS sales results in any period as being indicative of future performance. Such guidance is based on assumptions that may be incorrect or that may change from quarter to quarter. Sales of ADCETRIS have, on occasion, been below the expectations of securities analysts and investors and have been below prior period sales, and sales of ADCETRIS in the future may also be below prior period sales, our own guidance and/or the expectations of securities analysts and investors. To the extent that we do not meet our guidance or the expectations of analysts or investors, our stock price may be adversely impacted, perhaps significantly. We believe that our quarterly and annual results of operations may be affected by a variety of factors, including:
| | • | | customer ordering patterns for ADCETRIS, which may vary significantly from period to period; |
| | • | | the overall level of demand for ADCETRIS, including the impact of any competitive or biosimilar products and the duration of therapy for patients receiving ADCETRIS; |
| | • | | the extent to which coverage and reimbursement for ADCETRIS is available from government and health administration authorities, private health insurers, managed care programs and other third-party payers; |
| | • | | changes in the amount of deductions from gross sales, including government-mandated rebates, chargebacks and discounts that can vary because of changes to the government discount percentage, including increases in the government discount percentage resulting from price increases we have taken or may take in the future, or due to different levels of utilization by entities entitled to government rebates and discounts and changes in patient demographics; |
| | • | | increases in the scope of eligibility for customers to purchase ADCETRIS at the discounted government price or to obtain government-mandated rebates on purchases of ADCETRIS; |
| | • | | changes in our cost of sales; |
| | • | | the incidence rate of new patients in ADCETRIS’ approved indications; |
| | • | | the timing, cost and level of investment in our sales and marketing efforts to support ADCETRIS sales; |
| | • | | the timing, cost and level of investment in our research and development and other activities involving ADCETRIS, enfortumab vedotin, tisotumab vedotin and our product candidates by us or our collaborators; |
| | • | | changes in the price of the common stock of Immunomedics that affect the valuation of the Immunomedics common stock that we hold; and |
| | • | | expenditures we will or may incur to develop and/or commercialize any additional products, product candidates, or technologies that we may develop,in-license, or acquire. |
In addition, we have entered into licensing and collaboration agreements with other companies that include development funding and milestone payments to us, and we expect that amounts earned from our collaboration agreements will continue to be an important source of our revenues. Accordingly, our revenues will also depend on development funding and the achievement of development and clinical milestones under our existing collaboration and license agreements, including, in particular, our ADCETRIS collaboration with Takeda, as well as entering into potential new collaboration and license agreements. These upfront and milestone payments may vary significantly from quarter to quarter and any such variance could cause a significant fluctuation in our operating results from one quarter to the next.
Further, changes in our operations, such as increased development, manufacturing and clinical trial expenses in connection with our expanding pipeline programs, or our undertaking of additional programs, business
54
activities, the anticipated completion of the Cascadian Acquisition and the integration of Cascadian’s business into our existing operations, or entry into strategic transactions, including potential future acquisitions of products, technologies or businesses may also cause significant fluctuations in our expenses. In addition, we measure compensation cost for stock-based awards made to employees at the grant date of the award, based on the fair value of the award, and recognize the cost as an expense over the employee’s requisite service period. As the variables that we use as a basis for valuing these awards change over time, including our underlying stock price, the magnitude of the expense that we must recognize may vary significantly. Additionally, we have implemented long-term incentive plans for our employees, and the incentives provided under these plans are contingent upon the achievement of certain regulatory milestones. Costs of performance-based compensation under our long-term incentive plans are not recorded as an expense until the achievement of the applicable milestones is deemed probable of being met, which may result in large fluctuations to the expense we must recognize in any particular period.
Additionally, as of December 31, 2017, we held 11.7 million shares of Immunomedics common stock. Beginning on January 1, 2018, we adopted ASU2016-01 “Financial Instruments: Overall,” and as a result, we will record changes in the fair value of equity securities, including the Immunomedics common stock, in net income or loss, which is expected to increase the volatility of net income or loss to the extent that we continue to hold Immunomedics common stock or other equity securities.
For these and other reasons, it is difficult for us to accurately forecast future sales of ADCETRIS, collaboration and license agreement revenues, royalty revenues, operating expenses or future profits or losses. As a result, our operating results in future periods could be below our guidance or the expectations of securities analysts or investors, which could cause the trading price of our common stock to decline, perhaps substantially.
We have a history of net losses. We expect to continue to incur net losses and may not achieve future profitability for some time, if at all.
We have incurred substantial net losses in each of our years of operation. We have incurred these losses principally from costs incurred in our research and development programs and from our selling, general and administrative expenses. We expect to continue to spend substantial amounts on research and development, including amounts for conducting required post-approval and other clinical trials of, and seeking additional regulatory approvals for, ADCETRIS as well as commercializing ADCETRIS for the treatment of patients in its four approved indications. In addition, we expect to make substantial expenditures to further develop and potentially commercialize enfortumab vedotin, tisotumab vedotin and our product candidates. Likewise, in connection with the anticipated consummation of the Cascadian Acquisition, we have incurred and expect to incur substantial expenses, including to further develop and potentially commercialize tucatinib. Accordingly, we expect to continue to incur net losses and may not achieve profitability in the future for some time, if at all. Although we recognize revenue from ADCETRIS product sales and we continue to earn amounts under our collaboration agreements, our revenue and profit potential is unproven and our limited commercialization history makes our future operating results difficult to predict. Even if we do achieve profitability in the future, we may not be able to sustain or increase profitability on a quarterly or annual basis. If we are unable to achieve and sustain profitability, the market value of our common stock will likely decline.
We have engaged in, and may in the future engage in strategic transactions that increase our capital requirements, dilute our stockholders, cause us to incur debt or assume contingent liabilities and subject us to other risks.
We actively evaluate various strategic transactions on an ongoing basis, including licensing or otherwise acquiring complementary products, technologies or businesses, and we recently announced the Cascadian Acquisition. Any potential acquisitions orin-licensing transactions, including the Cascadian Acquisition, may entail numerous risks, including but not limited to:
| | • | | risks associated with satisfying the closing conditions relating to such transactions and realizing their anticipated benefits; |
55
| | • | | increased operating expenses and cash requirements; |
| | • | | difficulty integrating acquired technologies, products, operations, and personnel with our existing business; |
| | • | | diversion of management’s attention in connection with both negotiating the acquisition or license and integrating the business, technology or product; |
| | • | | retention of key employees; |
| | • | | uncertainties in our ability to maintain key business relationships of any acquired entities; |
| | • | | strain on managerial and operational resources; |
| | • | | difficulty implementing and maintaining effective internal control over financial reporting at businesses that we acquire, particularly if they are not located near our existing operations; |
| | • | | exposure to unforeseen liabilities of acquired companies or companies in which we invest; and |
| | • | | potential costly and time-consuming litigation, including stockholder lawsuits. |
As a result of these or other problems and risks, businesses, technologies or products we acquire or invest in or obtain licenses to may not produce the revenues, earnings or business synergies that we anticipated, acquired or licensed technologies may not result in regulatory approvals, and acquired or licensed products may not perform as expected. As a result, we may incur higher costs and realize lower revenues than we had anticipated. We cannot assure you that any acquisitions or investments we have made or may make in the future, including the Cascadian Acquisition, will be completed or that, if completed, the acquired business, licenses, investments, products, or technologies will generate sufficient revenue to offset the negative costs or other negative effects on our business. Failure to manage effectively our growth through acquisition orin-licensing transactions could adversely affect our growth prospects, business, results of operations, financial condition, and cash flow.
In addition, we may spend significant amounts, issue dilutive securities, assume or incur significant debt obligations, incur largeone-time expenses and acquire intangible assets in connection with acquisitions andin-licensing transactions that could result in significant future amortization expense and write-offs. Moreover, we may not be able to locate suitable acquisition opportunities and this inability could impair our ability to grow or obtain access to technology or products that may be important to the development of our business. Other pharmaceutical companies, many of which may have substantially greater financial, marketing and sales resources, compete with us for these opportunities. Even if appropriate opportunities are available, we may not be able to successfully identify them or we may not have the financial resources necessary to pursue them, and if pursued, we may be unable to structure and execute transactions in the anticipated timeframe, or at all.
Even if we are able to successfully identify and acquire complementary products, technologies or businesses, we cannot assure you that we will be able to successfully manage the risks associated with integrating acquired products, technologies or businesses or the risks arising from anticipated and unanticipated problems in connection with an acquisition orin-licensing transaction. Further, while we seek to mitigate risks and liabilities of potential acquisitions andin-licensing transactions through, among other things, due diligence, there may be risks and liabilities that such due diligence efforts fail to discover, that are not disclosed to us, or that we inadequately assess. Any failure in identifying and managing these risks and uncertainties effectively, including in connection with the Cascadian Acquisition, would have a material adverse effect on our business. Additionally, we may not realize the anticipated benefits of such transactions, including the possibility that expected synergies and accretion will not be realized or will not be realized within the expected time frame.
Our current product candidates are in various stages of development, and it is possible that none of our product candidates will ever become commercial products.
Our clinical-stage product candidates include eight ADC programs, which consist of enfortumab vedotin, tisotumab vedotin, ladiratuzumab vedotin, orSGN-LIV1A, denintuzumab mafodotin, orSGN-CD19A,
56
SGN-CD19B,SGN-CD123A, SGN-CD33A andSGN-CD352A, as well as two immuno-oncology agents,SEA-CD40, which is based on our sugar-engineered antibody, or SEA, technology, andSGN-2FF, which is a novel small molecule. Other than enfortumab vedotin and tisotumab vedotin, which are in or expected to enter pivotal trials based on only limited phase 1 clinical data, our current product candidates are in relatively early stages of development. All of our product candidates will require significant further development, financial resources and personnel to obtain regulatory approval and develop into commercially viable products, if at all.
If a product candidate fails at any stage of development or we or our collaborators otherwise determine to discontinue development of that product candidate, we will not have the anticipated revenues from that product candidate to fund our operations, and we may not receive any return on our investment in that product candidate. Moreover, we still have only limited data from our early trials of our product candidates. In this regard, preclinical studies and any encouraging or positive preliminary and interim data from our clinical trials of our product candidates may not be predictive of the results of ongoing or later clinical trials. Even if we or our collaborators are able to complete our planned clinical trials of our product candidates according to our current development timeline, the encouraging or positive results from clinical trials of our product candidates in earlier stage trials may not be replicated in subsequent clinical trial results. As a result, we and our collaborators may conduct lengthy and expensive clinical trials of our product candidates only to learn that a product candidate is not an effective treatment or is not superior to existing approved therapies, or has an unacceptable safety profile, which could prevent or significantly delay regulatory approval for such product candidate or could cause us to discontinue the development of such product candidate. Also, later-stage clinical trials could differ in significant ways from earlier stage clinical trials, which could cause the outcome of the later-stage trials to differ from earlier stage clinical trials. For example, we are conducting a pivotal phase 2 trial of enfortumab vedotin with Astellas for locally advanced or metastatic urothelial cancer patients who have been previously treated with checkpoint inhibitor therapy, and are planning to conduct a pivotal phase 2 trial of tisotumab vedotin with Genmab in patients with recurrent and/or metastatic cervical cancer, in each case based on only limited phase 1 clinical data. Neither enfortumab vedotin nor tisotumab vedotin have previously been evaluated in later stage clinical trials and we cannot be certain that the design of, or data collected from, these trials will be adequate to demonstrate the safety and efficacy of enfortumab vedotin or tisotumab vedotin, or will otherwise be sufficient to support FDA or any foreign regulatory approvals. Differences in earlier and later stage clinical trials may include changes to inclusion and exclusion criteria, efficacy endpoints and statistical design. Many companies in the pharmaceutical and biotechnology industries, including us, have suffered significant setbacks in late-stage clinical trials after achieving encouraging or positive results in early-stage development. We cannot be certain that we will not face similar setbacks in our ongoing or planned clinical trials, including in the ongoing and planned pivotal phase 2 trials for enfortumab vedotin and tisotumab vedotin. We have not yet completed any late-stage clinical trials for our current product candidates, and if we or our collaborators fail to produce positive results in our ongoing or planned clinical trials of any of our product candidates, the development timeline and regulatory approval and commercialization prospects for our product candidates, and, correspondingly, our business and financial prospects, would be materially adversely affected.
Due to the uncertain and time-consuming clinical development and regulatory approval process, we may not successfully develop any of our product candidates, or we may choose to discontinue the development of product candidates for a variety of reasons such as due to safety, risk versus benefit profile, exclusivity, competitive landscape, or prioritization of our resources. It is possible that none of our current product candidates will ever become commercial products. In addition, we expect that much of our effort and many of our expenditures over the next few years will be devoted to the additional clinical development of and commercialization activities associated with ADCETRIS, which may restrict or delay our ability to develop our clinical and preclinical product candidates. Likewise, we have to make decisions about which clinical stage andpre-clinical product candidates to develop and advance, and we may not have the resources to invest in all of our current product candidates, particularly if we are successful in completing the Cascadian Acquisition, or clinical data and other development considerations may not support the advancement of one or more product candidates. Decision-making about which product candidates to prioritize involves inherent uncertainty, and our development program decision-making and resource prioritization decisions may not improve our results of operations or prospects or
57
enhance the value of our common stock. Our failure to effectively advance our development programs could have a material adverse effect on our business and prospects, and cause the price of our common stock to decline.
To date, we have depended on a small number of collaborators for a substantial portion of our revenue. The loss of any one of these collaborators or changes in their product development or business strategy could result in a material decline in our revenue.
We have collaborations with a limited number of companies. To date, a substantial portion of our revenue has resulted from payments made under agreements with our corporate collaborators, and although ADCETRIS sales currently comprise a greater proportion of our revenue, we expect that a portion of our revenue will continue to come from corporate collaborations. Even though we market ADCETRIS in the United States and Canada, our revenues still depend in part on Takeda’s ability and willingness to market ADCETRIS outside of the United States and Canada. The loss of our collaborators, especially Takeda, changes in product development or business strategies of our collaborators, or the failure of our collaborators to perform their obligations under their agreements with us for any reason, including paying license or technology fees, milestone payments, royalties or reimbursements, could have a material adverse effect on our financial performance. Payments under our existing and potential future collaboration agreements are also subject to significant fluctuations in both timing and amount, which could cause our revenue to fall below the expectations of securities analysts and investors and cause a decrease in our stock price.
We are dependent upon a small number of distributors for a significant portion of our net sales, and the loss of, or significant reduction or cancellation in sales to, any one of these distributors could adversely affect our operations and financial condition.
In the United States and Canada, we sell ADCETRIS through a limited number of pharmaceutical distributors. Customers order ADCETRIS through these distributors. We generally receive orders from distributors and ship product directly to the customer. We do not promote ADCETRIS to these distributors and they do not set or determine demand for ADCETRIS; however, our ability to effectively commercialize ADCETRIS will depend, in part, on the performance of these distributors. Although we believe we can find alternative distributors on relatively short notice, the loss of a major distributor could materially and adversely affect our results of operations and financial condition.
We currently rely on third-party manufacturers and other third parties for production of our drug products and our dependence on these manufacturers may impair the continued development and commercialization of ADCETRIS and our product candidates.
Although we recently acquired a biologics manufacturing facility located in Bothell, Washington, we rely and expect to continue to rely on corporate collaborators and contract manufacturing organizations to supply drug product or intermediates for commercial supply and ourIND-enabling studies and clinical trials. For the monoclonal antibody used in ADCETRIS, we have contracted with AbbVie for clinical and commercial supplies. For the drug linker used in ADCETRIS, we have contracted with Sigma Aldrich Fine Chemicals, or SAFC, for clinical and commercial supplies. We have multiple contract manufacturers for conjugating the drug linker to the antibody and producing the ADCETRIS product. For our ADC product candidates, multiple contract manufacturers, including AbbVie and SAFC, perform antibody and drug-linker manufacturing and several other contract manufacturers perform conjugation of the drug-linker to the antibody and fill/finish of the drug product. In addition, we rely on other third parties to perform additional steps in the manufacturing process, including shipping and storage of ADCETRIS and our product candidates. For the foreseeable future, we expect to continue to rely on contract manufacturers and other third parties to produce, vial and store sufficient quantities of ADCETRIS for use in our clinical trials and for commercial sale. If our contract manufacturers or other third parties fail to deliver ADCETRIS for clinical use or sale on a timely basis, with sufficient quality, and at commercially reasonable prices, and we fail to find replacement manufacturers or to develop our own manufacturing capabilities, we may be required to delay or suspend clinical trials or otherwise discontinue development, production and sale of ADCETRIS. Moreover, contract manufacturers have a limited number of
58
facilities in which ADCETRIS can be produced and any interruption of the operation of those facilities due to events such as equipment malfunction or failure or damage to the facility by natural disasters or as the result of regulatory actions could result in the cancellation of shipments, loss of product in the manufacturing process, a shortfall in ADCETRIS supply, or the inability to sell our products in the U.S. or abroad. In addition, we have committed to provide Takeda with their needs of certain parts of the ADCETRIS supply chain for a limited period of time, which may require us to arrange for additional manufacturing supply. Moreover, we depend on outside vendors for the supply of raw materials used to produce ADCETRIS. If the third-party suppliers were to cease production or otherwise fail to supply us with quality raw materials and we were unable to contract on acceptable terms for these raw materials with alternative suppliers, our ability to have ADCETRIS manufactured to meet commercial and clinical requirements would be adversely affected.
We are planning to use our own manufacturing facility to support our growing pipeline. As an organization, we have no prior experience operating a manufacturing facility.
In October 2017, we acquired a biologics manufacturing facility located in Bothell, Washington, which facility we intend to use to support our clinical supply needs. Under the terms of this acquisition, we are required to operate the facility and produce certain clinical drug product components for BMS under a transitional services agreement for a period of time. As an organization, we have no prior experience manufacturing for ourselves or other parties, and operating this facility requires us to comply with complex regulations and to continue to hire and retain experienced scientific, quality control, quality assurance and manufacturing personnel. We could encounter challenges in operating the manufacturing facility in compliance with cGMP, regulatory or other applicable requirements, resulting in potential negative consequences, including regulatory actions, which could undermine our ability to utilize this facility for our own manufacturing needs and/or result in a breach of our contractual manufacturing obligations to BMS. Any of these risks, if actualized, could materially and adversely affect our business and financial position. In addition, despite the acquisition of this facility, we nonetheless expect to continue to rely on corporate collaborators and contract manufacturing organizations to supply drug product and intermediates for commercial supply and ourIND-enabling studies and clinical trials. Our continuing dependence on these manufacturers may impair the continued development and commercialization of ADCETRIS and our product candidates.
We are subject to various state and federal laws and regulations, including healthcare laws and regulations, that may impact our business and could subject us to significant fines and penalties or other negative consequences.
Our operations may be directly or indirectly subject to various state and federal healthcare laws, including, without limitation, the federal Anti-Kickback Statute, federal civil and criminal false claims laws, HIPAA/HITECH, the federal civil monetary penalties statute, and the federal transparency requirements under the PPACA. These laws may impact, among other things, the sales, marketing and education programs for ADCETRIS.
The federal Anti-Kickback Statute prohibits persons and entities from knowingly and willingly soliciting, offering, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing or arranging for a good or service, for which payment may be made under a federal healthcare program such as the Medicare and Medicaid programs. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the statute has been violated. Additionally, PPACA amended the intent requirement of the federal Anti-Kickback Statute such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it. The Anti-Kickback Statute is broad and prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. Penalties for violations of the federal Anti-Kickback Statute include criminal penalties and civil sanctions such as fines, imprisonment and possible exclusion from Medicare, Medicaid and other federal healthcare programs.
The federal civil and criminal false claims laws, including the civil False Claims Act, prohibit, among other things, persons or entities from knowingly presenting, or causing to be presented, a false claim to, or the knowing
59
use of false statements to obtain payment from or approval by the federal government, including the Medicare and Medicaid programs, or knowingly making, using, or causing to be made or used a false record or statement material to a false or fraudulent claim or to avoid, decrease, or conceal an obligation to pay money to the federal government. PPACA provides that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act. Suits filed under the civil False Claims Act, known as “qui tam” actions, can be brought by any individual on behalf of the government and such individuals, commonly known as “whistleblowers,” may share in any amounts paid by the entity to the government in fines or settlement. Many pharmaceutical and other healthcare companies have recently been investigated or subject to lawsuits by whistleblowers and have reached substantial financial settlements with the federal government under the False Claims Act for a variety of alleged improper marketing or other activities, including providing free product to customers with the expectation that the customers would bill federal programs for the product; providing consulting fees, grants, free travel, and other benefits to physicians to induce them to prescribe the company’s products; and inflating prices reported to private price publication services, which are used to set drug reimbursement rates under government healthcare programs.
The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, created additional federal criminal statutes that prohibit, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing, or covering up a material fact or making any materially false, fictitious, or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items, or services. Similar to the Anti-Kickback Statute, PPACA amended the intent requirement of the criminal healthcare fraud statutes such that a person or entity no longer needs to have actual knowledge of the statute or intent to violate it.
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and its implementing regulations, governs certain types of individuals and entities with respect to the conduct of certain electronic healthcare transactions and imposes certain obligations with respect to the security and privacy of protected health information.
The federal civil monetary penalties statute imposes penalties against any person or entity that, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent.
The federal transparency requirements under PPACA, the Physician Payments Sunshine Act, require certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program to annually report to the U.S. Department of Health and Human Services’ Centers for Medicare & Medicaid Services information related to payments and other transfers of value to physicians and teaching hospitals, and physician ownership and investment interests.
There are foreign and state law equivalents of these laws and regulations, such as anti-kickback, false claims, and data privacy and security laws, to which we are currently and/or may in the future, be subject. We may also be subject to state laws that require manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures. Many of these state laws differ from each other in significant ways, thus complicating compliance efforts.
The FDA and other governmental authorities also actively investigate allegations ofoff-label promotion activities in order to enforce regulations prohibiting these types of activities. In recent years, private whistleblowers have also pursued False Claims Act cases against a number of pharmaceutical companies for causing false claims to be submitted as a result ofoff-label promotion. If we are found to have promoted an approved product, including ADCETRIS, foroff-label uses we may be subject to significant liability, including
60
civil and administrative financial penalties and other remedies as well as criminal financial penalties and other sanctions. Even when a company is not determined to have engaged inoff-label promotion, the allegation from government authorities or market participants that a company has engaged in such activities could have a significant impact on the company’s sales, business and financial condition. The U.S. government has also required companies to enter into complex corporate integrity agreements and/ornon-prosecution agreements that impose significant reporting and other burdens on the affected companies.
We are also subject to numerous other laws and regulations that are not specific to the healthcare industry. For instance, the U.S. Foreign Corrupt Practices Act, or FCPA, prohibits companies and individuals from engaging in specified activities to obtain or retain business or to influence a person working in an official capacity. Under the FCPA, it is illegal to pay, offer to pay, or authorize the payment of anything of value to any foreign government official, governmental staff members, political party or political candidate in an attempt to obtain or retain business or to otherwise influence a person working in an official capacity. The FCPA also requires public companies to make and keep books and records that accurately and fairly reflect the transactions of the corporation and to devise and maintain an adequate system of internal accounting controls.
The number and complexity of both U.S. federal and state laws continue to increase. In addition to enforcement by governmental agencies, we also expect a continuation of the trend of private plaintiff lawsuits against pharmaceutical manufacturers under the whistleblower provisions of the False Claims Act and state equivalents or other laws and regulations such as securities rules and the evolution of new theories of liability under those statutes. Government agencies will likely continue to intervene in such private whistleblower lawsuits and such intervention typically raises the company’s cost significantly. For example, federal enforcement agencies have recently scrutinized product and patient assistance programs, including manufacturer reimbursement support services as well as relationships with specialty pharmacies. Several investigations have resulted in government enforcement authorities intervening in related whistleblower lawsuits and obtaining significant civil and criminal settlements.
In order to comply with these laws, we have implemented a compliance program to actively identify, prevent and mitigate risk through the implementation of compliance policies and systems and by promoting a culture of compliance. Although we take our obligation to maintain our compliance with these various laws and regulations seriously and our compliance program is designed to prevent the violation of these laws and regulations, we cannot guarantee that our compliance program will be sufficient or effective, that our employees will comply with our policies and that our employees will notify us of any violation of our policies, that we will have the ability to take appropriate and timely corrective action in response to any such violation, or that we will make decisions and take actions that will necessarily limit or avoid liability for whistleblower claims that individuals, such as employees or former employees, may bring against us or that governmental authorities may prosecute against us based on information provided by individuals. If we are found to be in violation of any of the laws and regulations described above or other applicable state and federal healthcare laws, we may be subject to penalties, including civil and criminal penalties, damages, fines, disgorgement, contractual damages, reputational harm, imprisonment, diminished profits and future earnings, exclusion from government healthcare reimbursement programs, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations ofnon-compliance with these laws, and/or the curtailment or restructuring of our operations, any of which could have a material adverse effect on our business, results of operations and growth prospects. Any action against us for violation of these laws or regulations, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. Moreover, achieving and sustaining compliance with applicable federal, state and foreign healthcare laws is costly and time-consuming for our management.
61
As we expand our operations internationally, we are subject to an increased risk of conducting activities in a manner that violates applicable anti-bribery or anti-corruption laws. We are also subject to foreign laws and regulations covering data privacy and the protection of health-related and other personal information. These laws and regulations could create liability for us or increase our cost of doing business, any of which could have a material adverse effect on our business, results of operations and growth prospects.
We are expanding our operations internationally, and we currently have subsidiaries in the U.K., Switzerland and Canada. Though we are at an early stage with our international expansion, our business activities outside of the United States are subject to the FCPA, which is described above, and similar anti-bribery or anti-corruption laws, regulations or rules of other countries in which we currently and may in the future operate, including the U.K. Bribery Act. The U.K. Bribery Act prohibits giving, offering, or promising bribes to any person, includingnon-U.K. government officials and private persons, as well as requesting, agreeing to receive, or accepting bribes from any person. In addition, under the U.K. Bribery Act, companies which carry on a business or part of a business in the U.K. may be held liable for bribes given, offered or promised to any person, includingnon-U.K. government officials and private persons, by employees and persons associated with such company in order to obtain or retain business or a business advantage for such company. In the course of expanding our operations internationally, we will need to establish and expand business relationships with various third parties, such as independent contractors, distributors, vendors, advocacy groups and physicians, and we will interact more frequently with foreign officials, including regulatory authorities and physicians employed bystate-run healthcare institutions who may be deemed to be foreign officials under the FCPA, U.K. Bribery Act or similar laws of other countries that may govern our activities. Any interactions with any such parties or individuals where compensation is provided that are found to be in violation of such laws could result in substantial fines and penalties and could materially harm our business. Furthermore, any finding of a violation under one country’s laws may increase the likelihood that we will be prosecuted and be found to have violated another country’s laws. If our business practices outside the United States are found to be in violation of the FCPA, U.K. Bribery Act or other similar laws, we may be subject to significant civil and criminal penalties which could have a material adverse effect on our business, results of operations and growth prospects. We are also subject to foreign laws and regulations covering data privacy and the protection of health-related and other personal information. In this regard, European Union, or EU, member states and other foreign jurisdictions, including Switzerland, have adopted data protection laws and regulations which impose significant compliance obligations. Failure to comply with these laws could lead to government enforcement actions and significant penalties against us, which could have a material adverse effect on our business, results of operations and growth prospects. In December 2015, a proposal for an EU General Data Protection Regulation, intended to replace the current EU Data Protection Directive, was agreed between the European Parliament, the Council of the European Union and the European Commission. The EU General Data Protection Regulation, which was officially adopted in April 2016 and will be applicable in May 2018, will introduce new data protection requirements in the EU, as well as substantial fines for breaches of the data protection rules. The EU General Data Protection Regulation will increase our responsibility and liability in relation to personal data that we process, including in clinical trials, and we may be required to put in place additional mechanisms to ensure compliance with the new EU data protection rules, which could divert management’s attention and increase our cost of doing business.
Any failures or further setbacks in our ADC development program would negatively affect our business and financial position.
ADCETRIS and our enfortumab vedotin, tisotumab vedotin, ladiratuzumab vedotin, denintuzumab mafodotin,SGN-CD19B,SGN-CD123A, SGN-CD33A andSGN-CD352A product candidates are all based on our ADC technology, which utilizes proprietary stable linkers and potent cell-killing synthetic agents. Our ADC technology is also the basis of our collaborations with AbbVie, Astellas, Bayer, Celldex, Genentech, GSK, Pfizer, and Progenics, and our collaboration agreements with Takeda, Astellas, and Genmab. Although ADCETRIS has received marketing approval in the United States, Canada, the European Union, Japan and other countries, ADCETRIS is our first and only ADC product that has been approved for commercial sale in any jurisdiction. In addition, certain of our ADC product candidates include additional proprietary technologies that have not yet been proven in late stage clinical development. Any failures or further setbacks in our ADC
62
development program or with respect to our additional proprietary technologies, including adverse effects resulting from the use of this technology in human clinical trials and/or the imposition of additional clinical holds on our trials of any of our other product candidates, could have a detrimental impact on the continued commercialization of ADCETRIS in its current or any potential future approved indications and on our internal product candidate pipeline, as well as our ability to maintain and/or enter into new corporate collaborations regarding our ADC technology, which would negatively affect our business and financial position.
We have been named a defendant in a purported securities class action lawsuit and a stockholder derivative lawsuit. These, and potential similar or related lawsuits, could result in substantial damages and may divert management’s time and attention from our business.
On January 10, 2017, a purported securities class action lawsuit was commenced in the United States District Court for the Western District of Washington, naming as defendants us and certain of our officers. The lawsuit alleges material misrepresentations and omissions in public statements regarding our business, operational and compliance policies, violations by all named defendants of Section 10(b) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and Rule10b-5 thereunder, as well as violations of Section 20(a) of the Exchange Act. The complaint seeks compensatory damages of an undisclosed amount. The plaintiff alleges, among other things, that we made false and/or misleading statements and/or failed to disclose thatSGN-CD33A presents a significant risk of fatal hepatotoxicity and that we had therefore overstated the viability ofSGN-CD33A as a treatment for AML. We filed a motion to dismiss this complaint on July 28, 2017. On October 18, 2017, the Court granted our motion to dismiss with leave for plaintiff to file a second consolidated amended complaint. Plaintiff filed a second consolidated amended complaint on November 17, 2017 and we filed a motion to dismiss this new complaint on January 5, 2018.It is possible that additional suits will be filed, or allegations received from stockholders, with respect to these same matters and also naming us and/or our officers and directors as defendants.
On March 29, 2017, a stockholder derivative lawsuit was filed in Washington Superior Court for the County of Snohomish. The complaint names as defendants certain of our current and former executives and members of our board of directors. We are named as a nominal defendant. The complaint generally makes the same allegations as the securities class action, claiming that the individual defendants breached their duties to us. The complaint seeks unspecified damages, disgorgement of compensation, corporate governance changes, and attorneys’ fees and costs. Because the complaint is derivative in nature, it does not seek monetary damages from us. On June 8, 2017, the Snohomish County Superior Court entered an order staying this derivative action until resolution of the motion to dismiss the class action suit above. On October 18, 2017, in light of the granting of our motion to dismiss the first class action complaint, the parties in the derivative action filed a joint status report with the Snohomish County Superior Court stipulating to continue to stay the derivative action pending a ruling on a motion to dismiss the second consolidated amended class action complaint.
These lawsuits and any other related lawsuits are subject to inherent uncertainties, and the actual costs to be incurred relating to the lawsuits will depend upon many unknown factors. The outcome of these lawsuits is necessarily uncertain, and we could be forced to expend significant resources in the defense of these lawsuits, and we may not prevail. Monitoring and defending against legal actions is time-consuming for our management and detracts from our ability to fully focus our internal resources on our business activities, which could result in delays of our clinical trials or our development and commercialization efforts. In addition, we may incur substantial legal fees and costs in connection with these lawsuits. We are also generally obligated, to the extent permitted by law, to indemnify our current and former directors and officers who are named as defendants in these and similar lawsuits. We are not currently able to estimate the possible cost to us from these matters, as these lawsuits are currently at an early stage and we cannot be certain how long it may take to resolve these matters or the possible amount of any damages that we may be required to pay. We have not established any reserves for any potential liability relating to these lawsuits. It is possible that we could, in the future, incur judgments or enter into settlements of claims for monetary damages. Decisions adverse to our interests in these lawsuits could result in the payment of substantial damages, or possibly fines, and could have a material adverse
63
effect on our cash flow, results of operations and financial position. In addition, the uncertainty of the currently pending litigation could lead to increased volatility in our stock price.
We may need to raise significant amounts of additional capital that may not be available to us.
We expect to make additional capital outlays and to increase operating expenditures over the next several years as we hire additional employees, support our preclinical development, manufacturing and clinical trial activities for ADCETRIS and our other pipeline programs, and expand internationally, as well as commercialize ADCETRIS and position ADCETRIS for potential additional regulatory approvals. In addition, we anticipate committing substantial capital resources to the transactions contemplated by the Merger Agreement and the anticipated integration and development activities related to the acquired Cascadian business and Cascadian’s product candidates, including tucatinib. Our commitment of resources to the continuing development, regulatory and commercialization activities for ADCETRIS, and the research, continued development and manufacturing of our product candidates will likely require us to raise substantial amounts of additional capital. Further, we actively evaluate various strategic transactions on an ongoing basis, including licensing or otherwise acquiring complementary products, technologies or businesses, and we may require significant additional capital in order to complete or otherwise provide funding for any additional acquisitions. We may seek additional funding through some or all of the following methods: corporate collaborations, licensing arrangements and public or private debt or equity financings. We do not know whether additional capital will be available when needed, or that, if available, we will obtain financing on terms favorable to us or our stockholders. If we are unable to raise additional funds when we need them, we may be required to delay, reduce the scope of, or eliminate one or more of our development programs, which may adversely affect our business and operations. Our future capital requirements will depend upon a number of factors, including:
| | • | | the level of sales and market acceptance of ADCETRIS; |
| | • | | the rate of progress and cost of the confirmatory post-approval study that we are required to conduct as a condition to the FDA’s accelerated approval of ADCETRIS in the relapsed sALCL indication; |
| | • | | the time and costs involved in obtaining regulatory approvals of ADCETRIS in additional indications, if any; |
| | • | | the size, complexity, timing, progress and number of our clinical programs and our collaborations; |
| | • | | the timing, receipt and amount of milestone-based payments or other revenue from our collaborations or license arrangements, including royalty revenue generated from commercial sales of ADCETRIS by Takeda; |
| | • | | the cost of establishing and maintaining clinical and commercial supplies of ADCETRIS; |
| | • | | the costs associated with acquisitions or licenses of additional technologies, products, or companies, including the Cascadian Acquisition, as well as licenses we may need to commercialize our products; |
| | • | | the terms and timing of any future collaborative, licensing and other arrangements that we may establish; |
| | • | | expenses associated with the pending and potential additional related purported securities class action or derivative lawsuits, as well as any other potential litigation; |
| | • | | the potential costs associated with international, state and federal taxes; and |
| | • | | competing technological and market developments. |
In addition, changes in our spending rate may occur that would consume available capital resources sooner, such as increased development, manufacturing and clinical trial expenses in connection with our expanding pipeline programs, and the Cascadian Acquisition, or our undertaking of additional programs, business activities or entry into additional strategic transactions, including potential future acquisitions of products, technologies or businesses. To the extent that we raise additional capital by issuing equity securities, our stockholders may
64
experience substantial dilution. To the extent that we raise additional funds through collaboration and licensing arrangements, we may be required to relinquish some rights to our technologies or product candidates, or grant licenses on terms that are not favorable to us.
During the past several years, domestic and international financial markets have experienced extreme disruption from time to time, including, among other things, high volatility and significant declines in stock prices and severely diminished liquidity and credit availability for both borrowers and investors. Such adverse capital and credit market conditions could make it more difficult to obtain additional capital on favorable terms, or at all, which could have a material adverse effect on our business and growth prospects.
We rely on license agreements for certain aspects of ADCETRIS, our product candidates and technologies such as our ADC technology. Failure to maintain these license agreements or to secure any required new licenses could prevent us from continuing to develop and commercialize ADCETRIS and our product candidates.
We have entered into agreements with third-party commercial and academic institutions to license technology for use in ADCETRIS and our ADC technology. Currently, we have license agreements with BMS and the University of Miami, among others. In addition to royalty provisions, some of these license agreements contain diligence and milestone-based termination provisions, in which case our failure to meet any agreed upon royalty or diligence requirements or milestones may allow the licensor to terminate the agreement. Many of our license agreements grant us exclusive licenses to the underlying technologies. If our licensors terminate our license agreements or if we are unable to maintain the exclusivity of our exclusive license agreements, we may be unable to continue to develop and commercialize ADCETRIS or our product candidates. Further, we have had in the past, and may in the future have, disputes with our licensors, which may impact our ability to develop and commercialize ADCETRIS or our product candidates or require us to enter into additional licenses. An adverse result in potential future disputes with our licensors may impact our ability to develop and commercialize ADCETRIS and our product candidates, or may require us to enter into additional licenses or to incur additional costs in litigation or settlement. In addition, continued development and commercialization of ADCETRIS and our product candidates will likely require us to secure licenses to additional technologies. We may not be able to secure these licenses on commercially reasonable terms, if at all.
If we are unable to enforce our intellectual property rights or if we fail to sustain and further build our intellectual property rights, we may not be able to successfully commercialize ADCETRIS or future products and competitors may be able to develop competing therapies.
Our success depends, in part, on obtaining and maintaining patent protection and successfully enforcing these patents and defending them against third-party challenges in the United States and other countries. We own multiple U.S. and foreign patents and pending patent applications for our technologies. We also have rights to issued U.S. patents, patent applications, and their foreign counterparts, relating to our monoclonal antibody, linker and drug-based technologies. Our rights to these patents and patent applications are derived in part from worldwide licenses from third parties. In addition, we have licensed certain of our U.S. and foreign patents and patent applications to third parties.
The standards that the U.S. Patent and Trademark Office, or USPTO, and foreign patent offices use to grant patents are not always applied predictably or uniformly and can change. Consequently, our pending patent applications may not be allowed and, if allowed, may not contain the type and extent of patent claims that will be adequate to conduct our business as planned. Additionally, any issued patents we currently own or obtain in the future may have a shorter patent term than expected or may not contain claims that will permit us to stop competitors from using our technology or similar technology or from copying our products. Similarly, the standards that courts use to interpret patents are not always applied predictably or uniformly and may evolve, particularly as new technologies develop. In addition, changes to patent laws in the United States or other countries may be applied retroactively to affect the validity, enforceability, or term of our patent. For example, the U.S. Supreme Court has modified some legal standards applied by the USPTO in examination of U.S. patent
65
applications, which may decrease the likelihood that we will be able to obtain patents and may increase the likelihood of challenges to patents we obtain or license. In addition, changes to the U.S. patent system have come into force under the Leahy-Smith America Invents Act, or the America Invents Act, including changes from a“first-to-invent” system to a “first to file” system, changes to examination of U.S. patent applications and changes to the processes for challenging issued patents. These changes include provisions that affect the way patent applications are being filed, prosecuted and litigated. For example, the America Invents Act enacted proceedings involving post-issuance patent review procedures, such as inter partes review, or IPR, and post-grant review and covered business methods. These proceedings are conducted before the Patent Trial and Appeal Board, or PTAB, of the USPTO. Each proceeding has different eligibility criteria and different patentability challenges that can be raised. In this regard, the IPR process permits any person (except a party who has been litigating the patent for more than a year) to challenge the validity of some patents on the grounds that it was anticipated or made obvious by prior art. As a result,non-practicing entities associated with hedge funds, pharmaceutical companies who may be our competitors and others have challenged certain valuable pharmaceutical U.S. patents based on prior art through the IPR process. A decision in such a proceeding adverse to our interests could result in the loss of valuable patent rights which would have a material adverse effect on our business, financial condition, results of operations and growth prospects. In any event, the America Invents Act and any other potential future changes to the U.S. patent system could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
We rely on trade secrets and other proprietary information where we believe patent protection is not appropriate or obtainable. However, trade secrets and other proprietary information are difficult to protect. We have taken measures to protect our unpatented trade secrets andknow-how, including the use of confidentiality and assignment of inventions agreements with our employees, consultants and certain contractors. It is possible, however, that these persons may breach the agreements or that our competitors may independently develop or otherwise discover our trade secrets or other proprietary information. Our research collaborators may publish confidential data or other restricted information to which we have rights. If we cannot maintain the confidentiality of our technology and other confidential information in connection with our collaborations, then our ability to receive patent protection or protect our proprietary information may be impaired.
We may incur substantial costs and lose important rights or may not be able to continue to commercialize ADCETRIS or to commercialize any of our product candidates that may be approved for commercial sale as a result of litigation or other proceedings relating to patent and other intellectual property rights, and we may be required to obtain patent and other intellectual property rights from others.
We may face potential lawsuits by companies, academic institutions or others alleging infringement of their intellectual property. Because patent applications can take a few years to publish, there may be currently pending applications of which we are unaware that may later result in issued patents that adversely affect the continued commercialization of ADCETRIS or future commercialization of our product candidates in development. In addition, we are monitoring the progress of multiple pending patent applications of other organizations that, if granted, may require us to license or challenge their enforceability in order to continue commercializing ADCETRIS or to commercialize our product candidates that may be approved for commercial sale. Our challenges to patents of other organizations may not be successful, which may affect our ability to commercialize ADCETRIS or our product candidates. As a result of the patent infringement lawsuits that have been filed or may be filed against us in the future by third parties alleging infringement by us of patent or other intellectual property rights, we may be required to pay substantial damages, including lost profits, royalties, treble damages, attorneys’ fees and costs, for past infringement if it is ultimately determined that our products infringe a third party’s intellectual property rights. Even if infringement claims against us are without merit, the results may be unpredictable. In addition, defending lawsuits takes significant time, may be expensive and may divert management’s attention from other business concerns. Further, we may be stopped from developing, manufacturing or selling our products until we obtain a license from the owner of the relevant technology or
66
other intellectual property rights, or be forced to undertake costly design-arounds, if feasible. If such a license is available at all, it may require us to pay substantial royalties or other fees.
We are or may be from time to time involved in the defense and enforcement of our patent or other intellectual property rights in a court of law, USPTO interference, IPR, post-grant review or reexamination proceeding, foreign opposition proceeding or related legal and administrative proceeding in the United States and elsewhere. In addition, if we choose to go to court to stop a third party from infringing our patents, that third party has the right to ask the court to rule that these patents are invalid, not infringed and/or should not be enforced. Under the America Invents Act, a third party may also have the option to challenge the validity of certain patents at the PTAB, whether they are accused of infringing our patents or not, and certain entities associated with hedge funds, pharmaceutical companies and other entities have challenged valuable pharmaceutical patents through the IPR process. These lawsuits and administrative proceedings are expensive and consume time and other resources, and we may not be successful in these proceedings or in stopping infringement. In addition, there is a risk that a court will decide that these patents are not valid or not infringed or otherwise not enforceable, or that the PTAB will decide that certain patents are not valid, and that we do not have the right to stop a third party from using the patented subject matter. Successful challenges to our patent or other intellectual property rights through these proceedings could result in a loss of rights in the relevant jurisdiction and may allow third parties to use our proprietary technologies without a license from us or our collaborators, which may also result in loss of future royalty payments. Furthermore, if such challenges to our rights are not resolved promptly in our favor, our existing business relationships may be jeopardized and we could be delayed or prevented from entering into new collaborations or from commercializing potential products, which could adversely affect our business and results of operations. In addition, we may challenge the patent or other intellectual property rights of third parties and if we are unsuccessful in actions we bring against the rights of such parties, through litigation or otherwise, and it is determined that we infringe the intellectual property rights of such parties, we may be prevented from commercializing potential products in the relevant jurisdiction, or may be required to obtain licenses to those rights or develop or obtain alternative technologies, any of which could harm our business.
If we lose our key personnel or are unable to attract and retain additional qualified personnel, our future growth and ability to compete would suffer.
We are highly dependent on the efforts and abilities of the principal members of our senior management. Additionally, we have scientific personnel with significant and unique expertise in monoclonal antibodies, ADCs and related technologies. The loss of the services of any one of the principal members of our managerial or scientific staff may prevent us from achieving our business objectives.
In addition, the competition for qualified personnel in the biotechnology field is intense, and our future success depends upon our ability to attract, retain and motivate highly skilled scientific, technical and managerial employees. In order to continue to commercialize ADCETRIS and advance our pipeline, we have been required to expand our workforce, particularly in the areas of manufacturing, clinical trials management, regulatory affairs, business development, sales and marketing. We continue to face intense competition for qualified individuals from numerous pharmaceutical and biotechnology companies, as well as academic and other research institutions. To the extent we are not able to retain these individuals on favorable terms or attract any additional personnel that may be required, our business may be harmed. For example, we may not be successful in attracting or retaining key personnel necessary to support our strategy to develop and commercialize ADCETRIS in earlier lines of therapy, including potentially in theECHELON-1 treatment setting.
If we are unable to manage our growth, our business, financial condition, results of operations and prospects may be adversely affected.
We have experienced and expect to continue to experience significant growth in the number of our employees and in the scope of our operations, including in connection with the Cascadian Acquisition and our recent acquisition of, and planned operation of, a manufacturing facility. This growth places significant demands
67
on our management, operational and financial resources, and our current and planned personnel, systems, procedures and controls may not be adequate to support our growth. To effectively manage our growth, we must continue to improve existing, and implement new, operational and financial systems, procedures and controls and must expand, train and manage our growing employee base, and there can be no assurance that we will effectively manage our growth without experiencing operating inefficiencies or control deficiencies. We expect that we may need to increase our management personnel to oversee our expanding operations, and recruiting and retaining qualified individuals is difficult. In addition, the physical expansion of our operations may lead to significant costs and may divert our management and capital resources. If we are unable to manage our growth effectively, or are unsuccessful in recruiting qualified management personnel, our business, financial condition, results of operations and prospects may be adversely affected.
Product liability and product recalls could harm our business, and we may not be able to obtain adequate insurance to protect us against product liability losses.
The current and future use of ADCETRIS by us and our corporate collaborators in clinical trials and the sale of ADCETRIS, expose us to product liability claims. These claims have and may in the future be made directly by patients or healthcare providers or indirectly by pharmaceutical companies, our corporate collaborators or others selling such products. Additionally, in connection with our acquisition of the manufacturing facility from BMS, we have agreed to enter into certain transitional services agreements under which we expect to manufacture certain clinical drug product components for BMS for a period of time. As a result, it is possible that we may be named as a defendant in product liability suits that may allege that drug products we manufacture for BMS have resulted in injury to patients. We may experience substantial financial losses in the future due to product liability claims. We have obtained product liability coverage, including coverage for human clinical trials and product sold commercially. However, such insurance is subject to coverage limits and exclusions, as well as significant deductibles. However, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against all losses. If a successful product liability claim or series of claims is brought against us for uninsured liabilities or in excess of insured amounts, our assets may not be sufficient to cover such claims and our business operations could be impaired.
Product recalls may be issued at our discretion, or at the discretion of government agencies and other entities that have regulatory authority for pharmaceutical sales. Any recall of ADCETRIS could materially adversely affect our business by rendering us unable to sell ADCETRIS for some time and by adversely affecting our reputation.
Risks associated with operating in foreign countries could materially adversely affect our business.
We are expanding our operations internationally, and we currently have subsidiaries in the U.K., Switzerland and Canada. Consequently, we are, and will continue to be, subject to risks related to operating in foreign countries. Risks associated with conducting operations in foreign countries include:
| | • | | diverse regulatory, financial and legal requirements, and any future changes to such requirements, in one or more countries where we are located or do business; |
| | • | | adverse tax consequences, including changes in applicable tax laws and regulations; |
| | • | | applicable trade laws, tariffs, export quotas, custom duties or other trade restrictions and any changes to them; |
| | • | | economic weakness, including inflation, or political or economic instability in particular foreign economies and markets; |
| | • | | compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; |
| | • | | foreign currency fluctuations, which could result in increased operating expenses or reduced revenues, and other obligations incident to doing business or operating in another country; |
| | • | | liabilities for activities of, or related to, our international operations; |
68
| | • | | workforce uncertainty in countries where labor unrest is more common than in the United States; and |
| | • | | laws and regulations relating to data security and the unauthorized use of, or access to, commercial and personal information. |
For example, since a significant proportion of the regulatory framework in the U.K. is derived from European Union directives and regulations, Brexit could materially change the regulatory regime applicable to our operations and those of our collaborators, including with respect to marketing authorizations for ADCETRIS and our product candidates. We may also face new regulatory costs and challenges as result of Brexit that could have a material adverse effect on our operations. Depending on the terms of Brexit, the U.K. could lose the benefits of global trade agreements negotiated by the European Union on behalf of its members, which may result in increased trade barriers which could make our doing business in Europe more difficult. In addition, currency exchange rates for the British Pound and the Euro with respect to each other and the U.S. dollar have already been affected by Brexit. Should this foreign exchange volatility continue, it could cause volatility in our quarterly financial results. In any event, we cannot predict to what extent these changes will impact our business or results of operations, or our ability to conduct operations in Europe.
These and other risks described elsewhere in these risk factors associated with expanding our international operations could materially adversely affect our business.
Our operations involve hazardous materials and are subject to environmental, health and safety controls and regulations.
We are subject to environmental, health and safety laws and regulations, including those governing the use of hazardous materials, and we spend considerable time complying with such laws and regulations. Our business activities involve the controlled use of hazardous materials and although we take precautions to prevent accidental contamination or injury from these materials, we cannot completely eliminate the risk of using these materials. In addition, with respect to our recently-acquired manufacturing facility, we may incur substantial costs to comply with environmental laws and regulations and may become subject to the risk of accidental contamination or injury from the use of hazardous materials in our manufacturing process. It is also possible that our recently-acquired manufacturing facility may expose us to environmental liabilities associated with historical site conditions that we are not currently aware of and did not cause. In this regard, some environmental laws impose liability for contamination on current owners and operators of affected sites, regardless of fault. In the event of an accident or environmental discharge, or new or previously unknown contamination is discovered or new cleanup obligations are otherwise imposed in connection with any of our currently or previously owned or operated facilities, we may be held liable for any resulting damages, which may materially harm our business, financial condition and results of operations.
If any of our facilities are damaged or our clinical, research and development or other business processes are interrupted, our business could be seriously harmed.
We conduct most of our business in a limited number of facilities in a single geographical location in Bothell, Washington. Damage or extended periods of interruption to our corporate, development or research facilities due to fire, natural disaster, power loss, communications failure, unauthorized entry or other events could cause us to cease or delay development of some or all of our product candidates or interrupt the sales process for ADCETRIS. Although we maintain property damage and business interruption insurance coverage on these facilities, our insurance might not cover all losses under such circumstances and our business may be seriously harmed by such delays and interruption.
If we experience a significant disruption in our information technology systems or breaches of data security, our business could be adversely affected.
We rely on information technology systems to keep financial records, capture laboratory data, maintain clinical trial data and corporate records, communicate with staff and external parties and operate other critical
69
functions. Our information technology systems are potentially vulnerable to disruption due to breakdown, malicious intrusion and computer viruses or other disruptive events including but not limited to natural disaster. If we were to experience a prolonged system disruption in our information technology systems or those of certain of our vendors, it could delay or negatively impact the development and commercialization of ADCETRIS and our product candidates, which could adversely impact our business. Although we maintain offsiteback-ups of our data, if operations at our facilities were disrupted, it may cause a material disruption in our business if we are not capable of restoring function on an acceptable timeframe. In addition, our information technology systems are potentially vulnerable to data security breaches—whether by employees or others—which may expose sensitive data to unauthorized persons. Such data security breaches could lead to the loss of trade secrets or other intellectual property, or could lead to the public exposure of personal information (including sensitive personal information) of our employees, customers and others, any of which could have a material adverse effect on our business, financial condition and results of operations. Moreover, a security breach or privacy violation that leads to disclosure or modification of, personally identifiable information, could harm our reputation, compel us to comply with federal and/or state breach notification laws and foreign law equivalents, subject us to mandatory corrective action, require us to verify the correctness of database contents and otherwise subject us to liability under laws and regulations that protect personal data, which could disrupt our business, result in increased costs or loss of revenue, and/or result in significant legal and financial exposure. In addition, a data security breach could result in loss of clinical trial data or damage to the integrity of that data. If we are unable to prevent such security breaches or privacy violations or implement satisfactory remedial measures, our operations could be disrupted, and we may suffer loss of reputation, financial loss and other negative consequences because of lost or misappropriated information. In addition, these breaches and other inappropriate access can be difficult to detect, and any delay in identifying them may lead to increased harm of the type described above.
Increasing use of social media could give rise to liability.
We are increasingly relying on social media tools as a means of communications. To the extent that we continue to use these tools as a means to communicate about ADCETRIS and our product candidates or about the diseases that ADCETRIS and our product candidates are intended to treat, there are significant uncertainties as to either the rules that apply to such communications, or as to the interpretations that health authorities will apply to the rules that exist. As a result, despite our efforts to comply with applicable rules, there is a significant risk that our use of social media for such purposes may cause us to nonetheless be found in violation of them. Such uses of social media could have a material adverse effect on our business, financial condition and results of operations.
Legislative actions and new accounting pronouncements are likely to impact our future financial position or results of operations.
Future changes in financial accounting standards may cause adverse, unexpected revenue fluctuations and affect our financial position or results of operations. New pronouncements and varying interpretations of pronouncements have occurred with frequency in the past and are expected to occur again in the future and as a result we may be required to make changes in our accounting policies. Those changes could adversely affect our reported revenues and expenses, future profitability or financial position. Compliance with new regulations regarding corporate governance and public disclosure may result in additional expenses.
For example, in May 2014, the Financial Accounting Standards Board, or FASB, issued an Accounting Standards Update entitled “ASU2014-09, Revenue from Contracts with Customers” which replaced previous revenue recognition guidance under U.S. GAAP when it became effective for us on January 1, 2018. We do not expect that the new standard will generally change the way in which we recognize product revenue from sales of ADCETRIS. However, we expect that sales-based royalties and commercial sales-based milestones will be recorded in the period of the related sale based on estimates, rather than recording them as reported by the customer. In addition, the achievement of development milestones under our collaborations will be recorded in the period their achievement becomes probable, which may result in their recognition earlier than under current accounting principles. Additionally, on January 1, 2018, we adopted ASU2016-01 “Financial Instruments:
70
Overall,” and as a result, we will record changes in the fair value of equity securities, including our investment in Immunomedics common stock, in net income or loss, which is expected to increase the volatility of net income or loss to the extent that we continue to hold Immunomedics common stock or other equity securities. In any event, the application of existing or future financial accounting standards, particularly those relating to the way we account for revenues and costs, could have a significant impact on our reported results. In addition, compliance with new regulations regarding corporate governance and public disclosure may result in additional expenses. As a result, we intend to invest all reasonably necessary resources to comply with evolving standards, and this investment may result in increased general and administrative expenses and a diversion of management time and attention from science and business activities to compliance activities.
Risks Related to Our Common Stock
Our stock price is volatile and our shares may suffer a decline in value.
The market price of our stock has in the past been, and is likely to continue in the future to be, very volatile. During the year ended December 31, 2017, our closing stock price fluctuated between $45.92 and $68.91 per share. As a result of fluctuations in the price of our common stock, you may be unable to sell your shares at or above the price you paid for them. The market price of our common stock may be subject to substantial volatility in response to many risk factors listed in this section, and others beyond our control, including:
| | • | | the level of ADCETRIS sales in the United States, Canada, the European Union, Japan and other countries in which Takeda has received approval by relevant regulatory authorities; |
| | • | | announcements regarding the results of discovery efforts and preclinical, clinical and commercial activities by us, or those of our competitors; |
| | • | | announcements of FDA or foreign regulatory approval ornon-approval of ADCETRIS, or specific label indications for or restrictions, warnings or limitations in its use, or delays in the regulatory review or approval process, including in connection with our sBLA submission to the FDA to seek approval of ADCETRIS in theECHELON-1 treatment setting; |
| | • | | announcements regarding the results of the clinical trials we, Takeda and/or BMS are conducting or may in the future conduct for ADCETRIS, including theECHELON-2 trial and the CHECKMATE 812 trial; |
| | • | | announcements regarding the results of the clinical trials we and our collaborators are conducting for enfortumab vedotin and tisotumab vedotin; |
| | • | | announcements regarding, or negative publicity concerning, adverse events or safety concerns associated with the use of ADCETRIS or our product candidates; |
| | • | | issuance of new or changed analysts’ reports and recommendations regarding us or our competitors; |
| | • | | termination of or changes in our existing collaborations or licensing arrangements, especially our ADCETRIS collaboration with Takeda, our enfortumab vedotinco-development collaboration with Astellas, and our tisotumab vedotinco-development collaboration with Genmab, or establishment of new collaborations or licensing arrangements; |
| | • | | our ability to complete the Cascadian Acquisition on the anticipated terms or at all; |
| | • | | our entry into additional material strategic transactions including licensing or acquisition of products, businesses or technologies; |
| | • | | actions taken by regulatory authorities with respect to our product candidates, our clinical trials or our regulatory filings; |
| | • | | our raising of additional capital and the terms upon which we may raise any additional capital; |
| | • | | market conditions for equity investments in general, or the biotechnology or pharmaceutical industries in particular; |
71
| | • | | developments or disputes concerning our proprietary rights; |
| | • | | developments regarding the pending and potential additional related purported securities class action lawsuits, as well as any other potential litigation; |
| | • | | share price and volume fluctuations attributable to inconsistent trading volume levels of our shares; |
| | • | | changes in government regulations; and |
| | • | | economic or other external factors. |
The stock markets in general, and the markets for biotechnology and pharmaceutical stocks in particular, have historically experienced significant volatility that has often been unrelated or disproportionate to the operating performance of particular companies. For example, negative publicity regarding drug pricing and price increases by pharmaceutical companies has negatively impacted, and may continue to negatively impact, the markets for biotechnology and pharmaceutical stocks. Likewise, as a result of Brexit and/or significant changes in U.S. social, political, regulatory and economic conditions or in laws and policies governing foreign trade and health care spending and delivery, including the possible repeal and/or replacement of all or portions of PPACA or greater restrictions on free trade stemming from Trump Administration policies, the financial markets could experience significant volatility that could also negatively impact the markets for biotechnology and pharmaceutical stocks. These broad market fluctuations have adversely affected and may in the future adversely affect the trading price of our common stock.
In the past, class action or derivative litigation has often been instituted against companies whose securities have experienced periods of volatility in market price. In this regard, we have become, and may in the future again become, subject to claims and litigation alleging violations of the securities laws or other related claims, which could harm our business and require us to incur significant costs. The pending purported securities class action lawsuit and any additional lawsuits brought against us could result in substantial costs, which would hurt our financial condition and results of operations and divert management’s attention and resources, which could result in delays of our clinical trials or our development and commercialization efforts.
Substantial future sales of shares of our common stock or equity-related securities could cause the market price of our common stock to decline.
Sales of a substantial number of shares of our common stock into the public market, including sales by members of our management or board of directors or entities affiliated with such members, could occur at any time. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our common stock and could impair our ability to raise capital through the sale of additional equity or equity-related securities. We are unable to predict the effect that such sales may have on the prevailing market price of our common stock. As of December 31, 2017, we had 144,395,049 shares of common stock outstanding, all of which shares are eligible for sale in the public market, subject in some cases to the volume limitations and manner of sale and other requirements under Rule 144. In addition, we may issue a substantial number of shares of our common stock or equity-related securities, including convertible debt, to meet our capital needs, including in connection with funding potential future acquisition or licensing opportunities, capital expenditures or product development costs, which issuances could be substantially dilutive and could adversely affect the market price of our common stock. Likewise, future issuances by us of our common stock upon the exercise, conversion or settlement of equity-based awards or other equity-related securities would dilute existing stockholders’ ownership interest in our company and any sales in the public market of these shares, or the perception that these sales might occur, could also adversely affect the market price of our common stock.
Moreover, we have in the past and may in the future grant rights to some of our stockholders that require us to register the resale of our common stock or other securities on behalf of these stockholders and/or facilitate public offerings of our securities held by these stockholders, including in connection with potential future
72
acquisition or capital-raising transactions. For example, in connection with our September 2015 public offering of common stock, we entered into a registration rights agreement with entities affiliated with Baker Bros. Advisors LP, or the Baker Entities, that together, based on information available to us, collectively beneficially owned approximately 32.0% of our common stock as of January 26, 2018. Under the registration rights agreement, if at any time and from time to time the Baker Entities demand that we register their shares of our common stock for resale under the Securities Act of 1933, as amended, or the Securities Act, we would be obligated to effect such registration. On October 12, 2016, pursuant to the registration rights agreement, we registered for resale, from time to time, up to 44,059,594 shares of our common stock held by the Baker Entities. Our registration obligations under the registration rights agreement cover all shares now held or hereafter acquired by the Baker Entities, will continue in effect for up to ten years, and include our obligation to facilitate certain underwritten public offerings of our common stock by the Baker Entities in the future. If the Baker Entities, by its exercise of these registration and/or underwriting rights in the future, or otherwise, sell a large number of our shares, or the market perceives that the Baker Entities intend to sell a large number of our shares, including in connection with our October 2016 registration of shares held by the Baker Entities for resale, this could adversely affect the market price of our common stock. We have also filed registration statements to register the sale of our common stock reserved for issuance under our equity incentive and employee stock purchase plans. Accordingly, these shares will be able to be freely sold in the public market upon issuance as permitted by any applicable vesting requirements.
Our existing stockholders have significant control of our management and affairs.
Our executive officers and directors and holders of greater than five percent of our outstanding voting stock, together with entities that may be deemed affiliates of, or related to, such persons or entities, beneficially owned approximately 67.4% of our voting power as of January 26, 2018. As a result, these stockholders, acting together, are able to control our management and affairs and matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions, such as mergers, consolidations or the sale of substantially all of our assets. Consequently, this concentration of ownership may have the effect of delaying, deferring or preventing a change in control, including a merger, consolidation, takeover or other business combination involving us or discourage a potential acquirer from making a tender offer or otherwise attempting to obtain control, which might affect the market price of our common stock.
The recently passed comprehensive tax reform bill could adversely affect our business and financial condition.
On December 22, 2017, President Trump signed into law new legislation that significantly revises the Internal Revenue Code of 1986, as amended. The newly enacted federal income tax law, among other things, contains significant changes to corporate taxation, including reduction of the corporate tax rate from a top marginal rate of 35% to a flat rate of 21%, limitation of the tax deduction for interest expense to 30% of adjusted earnings (except for certain small businesses), limitation of the deduction for net operating losses to 80% of current year taxable income and elimination of net operating loss carrybacks, one time taxation of offshore earnings at reduced rates regardless of whether they are repatriated, immediate deductions for certain new investments instead of deductions for depreciation expense over time, and modifying or repealing many business deductions and credits (including reducing the business tax credit for certain clinical testing expenses incurred in the testing of certain drugs for rare diseases or conditions). Notwithstanding the reduction in the corporate income tax rate, the overall impact of the new federal tax law is uncertain and our business and financial condition could be adversely affected. In addition, it is uncertain if and to what extent various states will conform to the newly enacted federal tax law. The impact of this tax reform on holders of our common stock is also uncertain and could be adverse. We urge our stockholders to consult with their legal and tax advisors with respect to this legislation and the potential tax consequences of investing in or holding our common stock.
Anti-takeover provisions could make it more difficult for a third party to acquire us.
Our Board of Directors has the authority to issue up to 5,000,000 shares of preferred stock and to determine the price, rights, preferences, privileges and restrictions, including voting rights, of those shares without any
73
further vote or action by the stockholders, which authority could be used to adopt a “poison pill” that could act to prevent a change of control of Seattle Genetics that has not been approved by our Board of Directors. The rights of the holders of common stock may be subject to, and may be adversely affected by, the rights of the holders of any preferred stock that may be issued in the future. The issuance of preferred stock may have the effect of delaying, deferring or preventing a change of control of Seattle Genetics without further action by the stockholders and may adversely affect the voting and other rights of the holders of common stock. Further, certain provisions of our charter documents, including provisions eliminating the ability of stockholders to take action by written consent and limiting the ability of stockholders to raise matters at a meeting of stockholders without giving advance notice, may have the effect of delaying or preventing changes in control or management of Seattle Genetics, which could have an adverse effect on the market price of our stock. In addition, our charter documents provide for a classified board, which may make it more difficult for a third party to gain control of our Board of Directors. Similarly, state anti-takeover laws in Delaware and Washington related to corporate takeovers may prevent or delay a change of control of Seattle Genetics.
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
Our headquarters are in Bothell, Washington. We lease seven buildings of office space that we use for laboratory, discovery, research and development and general and administrative purposes. All of our leases include renewal options. We also own a biologics manufacturing facility located in Bothell, Washington, which we acquired in 2017. We believe that our facilities are currently adequate to meet our needs. As we continue to expand our operations, we may need to lease additional or alternative facilities.
Item 3. Legal Proceedings
Stockholder Class Action. On January 10, 2017, a purported securities class action lawsuit was commenced in the United States District Court for the Western District of Washington, naming as defendants us and certain of our officers. A consolidated amended complaint was filed on June 6, 2017, following the court’s appointment of a lead plaintiff and its approval of lead plaintiff’s counsel. The lawsuit alleges material misrepresentations and omissions in public statements regarding our business, operational and compliance policies, violations by all named defendants of Section 10(b) of the Exchange Act, and Rule10b-5 thereunder, as well as violations of Section 20(a) of the Exchange Act. The complaint seeks compensatory damages of an undisclosed amount. The plaintiff alleges, among other things, that we made false and/or misleading statements and/or failed to disclose thatSGN-CD33A presents a significant risk of fatal hepatotoxicity and that we had therefore overstated the viability ofSGN-CD33A as a treatment for AML. It is possible that additional suits will be filed, or allegations received from stockholders, with respect to these same matters and also naming us and/or our officers and directors as defendants. We filed a motion to dismiss this complaint on July 28, 2017. On October 18, 2017, the Court granted our motion to dismiss with leave for plaintiff to file a second consolidated amended complaint. The plaintiff filed a second consolidated amended complaint on November 17, 2017, and we filed a motion to dismiss this new complaint on January 5, 2018. It is possible that additional suits will be filed, or allegations received from stockholders, with respect to these same matters and also naming us and/or our officers and directors as defendants. We do not believe it is feasible to predict or determine the ultimate outcome or resolution of this litigation, or to estimate the amount of, or potential range of, loss with respect to this proceeding. In addition, the timing of the final resolution of this proceeding is uncertain. As a result of the lawsuit, we will incur litigation expenses and may incur indemnification expenses, and potential resolutions of the lawsuit could include a settlement requiring payments. Those expenses could have a material impact on our financial position, results of operations, and cash flows.
Stockholder Derivative Action. On March 29, 2017, a stockholder derivative lawsuit was filed in Washington Superior Court for the County of Snohomish, or the Snohomish County Superior Court. The
74
complaint names as defendants certain of our current and former executives and members of our board of directors. We are named as a nominal defendant. The complaint generally makes the same allegations as the Stockholder Class Action, claiming that the individual defendants breached their duties to us. The complaint seeks unspecified damages, disgorgement of compensation, corporate governance changes, and attorneys’ fees and costs. Because the complaint is derivative in nature, it does not seek monetary damages from us. On June 8, 2017, the Snohomish County Superior Court entered an order staying the Stockholder Derivative Action until resolution of the motion to dismiss the Stockholder Class Action. On October 18, 2017, in light of the granting of our motion to dismiss in the Stockholder Class Action, the parties in the Stockholder Derivative Action filed a joint status report with the Snohomish County Superior Court stipulating to continue to stay the Stockholder Derivative Action pending a ruling on a motion to dismiss the second consolidated amended complaint in the Stockholder Class Action. As a result of the lawsuit, we may incur litigation and indemnification expenses.
In addition, from time to time in the ordinary course of business we become involved in various lawsuits, claims and proceedings relating to the conduct of our business, including those pertaining to the defense and enforcement of our patent or other intellectual property rights. These proceedings are costly and time consuming. Successful challenges to our patent or other intellectual property rights through these proceedings could result in a loss of rights in the relevant jurisdiction and may allow third parties to use our proprietary technologies without a license from us or our collaborators.
Item 4. Mine Safety Disclosures
Not applicable.
75
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Price Range of Our Common Stock
Our common stock is traded on the Nasdaq Global Select Market under the symbol “SGEN.” As of February 8, 2018, there were 157,951,354 shares of our common stock outstanding, which were held by approximately 65 holders of record of our common stock. On February 8, 2018, the closing price of our common stock as reported on the Nasdaq Global Select Market was $50.54 per share.
The following table sets forth, for the periods indicated, the reported high and low sales prices per share of our common stock as reported on the Nasdaq Global Select Market, as applicable:
| | | | | | | | |
| | | High | | | Low | |
2016 | | | | | | | | |
First Quarter | | $ | 44.45 | | | $ | 26.02 | |
Second Quarter | | | 44.07 | | | | 32.40 | |
Third Quarter | | | 57.23 | | | | 39.38 | |
Fourth Quarter | | | 75.36 | | | | 47.29 | |
2017 | | | | | | | | |
First Quarter | | $ | 68.91 | | | $ | 53.00 | |
Second Quarter | | | 68.30 | | | | 51.39 | |
Third Quarter | | | 55.02 | | | | 45.92 | |
Fourth Quarter | | | 64.08 | | | | 51.82 | |
Dividend Policy
We have not paid any cash dividends on our common stock since our inception. We do not intend to pay any cash dividends in the foreseeable future, but intend to retain all earnings, if any, for use in our business operations.
Sales of Unregistered Securities and Issuer Repurchases of Securities
There were no unregistered sales of equity securities by us during the year ended December 31, 2017. In addition, we did not repurchase any of our equity securities during 2017.
76
Stock Performance Graph
We show below the cumulative total return to our stockholders during the period from December 31, 2012 through December 31, 2017 in comparison to the cumulative return on the Nasdaq Pharmaceutical Index, the Nasdaq Composite Index and the Nasdaq Biotechnology Index during that same period. The results assume that $100 was invested on December 31, 2012 in our common stock and each of the indexes listed above, including reinvestment of dividends, if any.
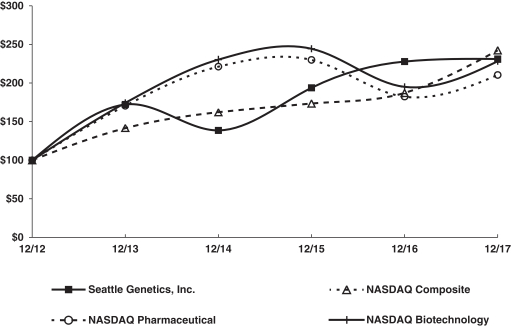
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Years ended | |
| | 12/12 | | | 12/13 | | | 12/14 | | | 12/15 | | | 12/16 | | | 12/17 | |
Seattle Genetics, Inc. | | | 100.00 | | | | 172.16 | | | | 138.67 | | | | 193.70 | | | | 227.75 | | | | 230.90 | |
Nasdaq Composite | | | 100.00 | | | | 141.63 | | | | 162.09 | | | | 173.33 | | | | 187.19 | | | | 242.29 | |
Nasdaq Pharmaceutical | | | 100.00 | | | | 170.57 | | | | 221.26 | | | | 229.97 | | | | 182.33 | | | | 210.44 | |
Nasdaq Biotechnology | | | 100.00 | | | | 174.05 | | | | 230.33 | | | | 244.29 | | | | 194.95 | | | | 228.29 | |
This information under “Stock Performance Graph” is not deemed filed with the Securities and Exchange Commission and is not to be incorporated by reference in any filing of Seattle Genetics, Inc. under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this Annual Report on Form10-K and irrespective of any general incorporation language in those filings.
77
Item 6. Selected Financial Data
The following selected financial data should be read in conjunction with our consolidated financial statements and notes to our consolidated financial statements and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” contained elsewhere in this Annual Report on Form10-K. The selected Consolidated Statements of Comprehensive Loss data for the years ended December 31, 2017, 2016, and 2015, and Consolidated Balance Sheet data as of December 31, 2017 and 2016 have been derived from our audited financial statements appearing elsewhere in this Annual Report on Form10-K. The selected Consolidated Statements of Comprehensive Loss data for the years ended December 31, 2014 and 2013 and Consolidated Balance Sheet data as of December 31, 2015, 2014, and 2013 have been derived from our audited financial statements that are not included in this Annual Report on Form10-K. Historical results are not necessarily indicative of future results.
| | | | | | | | | | | | | | | | | | | | |
| | | Years ended December 31, | |
| | | 2017 | | | 2016 | | | 2015 | | | 2014 | | | 2013 | |
| | | (in thousands, except for per share amounts) | |
Consolidated Statements of Comprehensive Loss Data: | | | | | | | | | | | | |
Revenues: | | | | | | | | | | | | |
Net product sales | | $ | 307,562 | | | $ | 265,766 | | | $ | 226,052 | | | $ | 178,198 | | | $ | 144,665 | |
Collaboration and license agreement revenues | | | 108,632 | | | | 84,926 | | | | 69,770 | | | | 68,556 | | | | 106,781 | |
Royalty revenues | | | 66,056 | | | | 67,455 | | | | 40,980 | | | | 40,004 | | | | 17,818 | |
| | | | | | | | | | | | | | | | | | | | |
Total revenues | | | 482,250 | | | | 418,147 | | | | 336,802 | | | | 286,758 | | | | 269,264 | |
| | | | | | | | | | | | | | | | | | | | |
Costs and expenses: | | | | | | | | | | | | |
Cost of sales | | | 34,768 | | | | 28,168 | | | | 24,476 | | | | 17,513 | | | | 13,759 | |
Cost of royalty revenues | | | 19,350 | | | | 14,149 | | | | 12,964 | | | | 11,545 | | | | 7,385 | |
Research and development | | | 456,700 | | | | 379,308 | | | | 294,529 | | | | 230,743 | | | | 218,627 | |
Selling, general and administrative | | | 167,233 | | | | 139,247 | | | | 125,783 | | | | 104,320 | | | | 92,354 | |
| | | | | | | | | | | | | | | | | | | | |
Total costs and expenses | | | 678,051 | | | | 560,872 | | | | 457,752 | | | | 364,121 | | | | 332,125 | |
| | | | | | | | | | | | | | | | | | | | |
Loss from operations | | | (195,801 | ) | | | (142,725 | ) | | | (120,950 | ) | | | (77,363 | ) | | | (62,861 | ) |
Investment and other income, net | | | 36,914 | | | | 2,614 | | | | 464 | | | | 1,222 | | | | 341 | |
| | | | | | | | | | | | | | | | | | | | |
Loss before income taxes | | | (158,887 | ) | | | (140,111 | ) | | | (120,486 | ) | | | (76,141 | ) | | | (62,520 | ) |
Income tax benefit | | | 33,357 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | |
| | | | | | | | | | | | | | | | | | | | |
Net loss | | $ | (125,530 | ) | | $ | (140,111 | ) | | $ | (120,486 | ) | | $ | (76,141 | ) | | $ | (62,520 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net loss per share—basic and diluted | | $ | (0.88 | ) | | $ | (1.00 | ) | | $ | (0.93 | ) | | $ | (0.62 | ) | | $ | (0.51 | ) |
| | | | | | | | | | | | | | | | | | | | |
Shares used in computation of net loss per share—basic and diluted | | | 143,174 | | | | 140,746 | | | | 129,184 | | | | 123,408 | | | | 121,575 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | December 31, | |
| | | 2017 | | | 2016 | | | 2015 | | | 2014 | | | 2013 | |
| | | (in thousands) | |
Consolidated Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | |
Cash, cash equivalents and investment securities | | $ | 413,171 | | | $ | 618,974 | | | $ | 712,711 | | | $ | 313,413 | | | $ | 374,267 | |
Working capital | | | 409,932 | | | | 586,132 | | | | 636,793 | | | | 282,093 | | | | 338,058 | |
Total assets | | | 877,949 | | | | 838,396 | | | | 895,095 | | | | 458,965 | | | | 483,898 | |
Stockholders’ equity | | | 677,569 | | | | 634,087 | | | | 685,911 | | | | 210,834 | | | | 230,185 | |
78
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion of our financial condition and results of operations contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements are based on our management’s beliefs and assumptions and on information currently available to our management. All statements other than statements of historical facts are “forward-looking statements” for purposes of these provisions, including those relating to future events or our future financial performance and financial guidance. In some cases, you can identify forward-looking statements by terminology such as “may,” “might,” “will,” “should,” “expect,” “plan,” “anticipate,” “project,” “believe,” “estimate,” “predict,” “potential,” “intend” or “continue,” the negative of terms like these or other comparable terminology, and other words or terms of similar meaning in connection with any discussion of future operating or financial performance. These statements are only predictions. All forward-looking statements included in this Annual Report on Form10-K are based on information available to us on the date hereof, and we assume no obligation to update any such forward-looking statements. Any or all of our forward-looking statements in this document may turn out to be wrong. Actual events or results may differ materially. Our forward-looking statements can be affected by inaccurate assumptions we might make or by known or unknown risks, uncertainties and other factors. We discuss many of these risks, uncertainties and other factors in this Annual Report on Form10-K in greater detail under the heading “Item 1A—Risk Factors.” We caution investors that our business and financial performance are subject to substantial risks and uncertainties.
You should read the following discussion and analysis in conjunction with the Selected Financial Data and our consolidated financial statements and notes thereto included elsewhere in this Annual Report on Form10-K.
Overview
Seattle Genetics is a biotechnology company focused on the development and commercialization of targeted therapies for the treatment of cancer. Our antibody-drug conjugate, or ADC, technology utilizes the targeting ability of monoclonal antibodies to deliver cell-killing agents directly to cancer cells. We are commercializing ADCETRIS®, or brentuximab vedotin, for the treatment of several types of lymphomas. We are also advancing a pipeline of novel therapies for solid tumors and blood-related cancers designed to address unmet medical needs and improve treatment outcomes for patients.
ADCETRIS® (brentuximab vedotin)
Our marketed product ADCETRIS® is approved by the United States Food and Drug Administration, or FDA, and the European Commission for four indications, encompassing several settings for the treatment of relapsed Hodgkin lymphoma, for relapsed systemic anaplastic large cell lymphoma, or sALCL, and for certain types of cutaneousT-cell lymphoma, or CTCL. ADCETRIS is commercially available in 70 countries, including in the United States, Canada, members of the European Union and Japan. We are collaborating with Takeda Pharmaceutical Company Limited, or Takeda, to develop and commercialize ADCETRIS on a global basis. Under this collaboration, Seattle Genetics has retained commercial rights for ADCETRIS in the United States and its territories and in Canada, and Takeda has commercial rights in the rest of the world. Beyond our current labeled indications, we have a broad development strategy for ADCETRIS evaluating its therapeutic potential in earlier lines of therapy for patients with Hodgkin lymphoma, matureT-cell lymphoma, or MTCL, also known as peripheralT-cell lymphoma, or PTCL, including sALCL. We are also evaluating ADCETRIS in combination with a checkpoint inhibitor, or CPI. We and our partners are currently conducting these phase 3 clinical trials of ADCETRIS, all of which are described in more detail under the heading“—ADCETRIS and Lead Product Candidate Development Pipeline” in Part I Item 1 of this Annual Report on Form10-K:
| | • | | ECHELON-1: In collaboration with Takeda, we are investigating ADCETRIS plus AVD (adriamycin, vinblastine, dacarbazine) versus ABVD (adriamycin, bleomycin, vinblastine, dacarbazine) as frontline combination therapy in patients with previously untreated advanced classical Hodgkin lymphoma. TheECHELON-1 trial met its primary endpoint, demonstrating that treatment with ADCETRIS plus AVD resulted in a statistically significant improvement in modified progression-free survival. Interim analysis |
79
| | of overall survival, the key secondary endpoint, also trended in favor of the ADCETRIS plus AVD arm. The FDA granted BTD to ADCETRIS in combination with chemotherapy for the frontline treatment of patients with advanced classical Hodgkin lymphoma. In December 2017, the FDA granted Priority Review for the supplemental Biologics License Application, or sBLA, we submitted in November 2017 seeking approval of ADCETRIS as part of a frontline combination chemotherapy regimen in patients with previously untreated advanced classical Hodgkin lymphoma, and the Prescription Drug User Fee Act, or PDUFA, target action date is May 1, 2018. |
| | • | | ECHELON-2: In collaboration with Takeda, we are evaluating ADCETRIS in combination with CHP versus CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) for the treatment of newly-diagnosed MTCL patients. In November 2016, we and Takeda completed enrollment of 452 patients in theECHELON-2 trial, and we expect to reporttop-line data in 2018. |
| | • | | CHECKMATE 812: In collaboration with Bristol-Myers Squibb Company, or BMS, we are evaluating the combination of BMS’s immunotherapy nivolumab (Opdivo) with ADCETRIS for the treatment of relapsed or refractory, or transplant-ineligible, advanced classical Hodgkin lymphoma. |
Clinical-stage product candidates
In collaboration with Astellas Pharma, Inc., or Astellas, we are developing enfortumab vedotin, formerly known asASG-22ME. We and Astellas are conducting a pivotal phase 2 clinical trial for patients with locally advanced or metastatic urothelial cancer who have been previously treated with CPI therapy. We and Astellas also initiated a phase 1b trial of enfortumab vedotin in combination with CPI therapy for patients with first- or second-line locally advanced or metastatic urothelial cancer.
In collaboration with Genmab A/S, or Genmab, we are developing tisotumab vedotin. We and Genmab plan to initiate a pivotal phase 2 clinical trial for patients with recurrent and/or metastatic cervical cancer.
We have a collaboration with Unum Therapeutics, Inc., or Unum, to develop and commercialize novel antibody-coupledT-cell receptor, or ACTR, therapies incorporating our antibodies for the treatment of cancer.
Our earlier-stage clinical pipeline includes other ADC programs consisting of ladiratuzumab vedotin, denintuzumab mafodotin,SGN-CD19B,SGN-CD123A, SGN-CD33A andSGN-CD352A, as well as immuno-oncology agents,SEA-CD40, which is based on our sugar-engineered antibody, or SEA, technology, andSGN-2FF, which is a novel small molecule. In addition, we have multiple preclinical and research-stage programs that employ our proprietary technologies, includingSGN-CD48A.
We have collaborations for our ADC technology with a number of other biotechnology and pharmaceutical companies, including AbbVie Biotechnology Ltd., or AbbVie; Bayer Pharma AG, or Bayer; Celldex Therapeutics, Inc., or Celldex; Genentech, Inc., a member of the Roche Group, or Genentech; GlaxoSmithKline LLC, or GSK; Pfizer, Inc., or Pfizer; and PSMA Development Company LLC, a subsidiary of Progenics Pharmaceuticals Inc., or Progenics.
Recent developments
On January 30, 2018, we entered into a definitive Agreement and Plan of Merger, or the Merger Agreement, with Cascadian Therapeutics, Inc., or Cascadian, a clinical-stage biopharmaceutical company based in Seattle, Washington, pursuant to which we agreed to acquire all of the outstanding shares of common stock of Cascadian for $10.00 per share net to the seller in cash, without interest, less any applicable withholding taxes, which we estimate is approximately $614.1 million in the aggregate. Valley Acquisition Sub, Inc., our wholly owned subsidiary, has commenced the offer contemplated by the Merger Agreement to acquire all of the outstanding shares of Cascadian common stock, or the Tender Offer, and as soon as practicable following the consummation of the Tender Offer, and subject to the satisfaction or waiver of certain conditions set forth in the Merger Agreement, our wholly owned subsidiary will merge with and into Cascadian, or the Merger, and Cascadian will
80
survive as our subsidiary. Our obligation to complete the Tender Offer and the Merger is subject to customary closing conditions. We refer to the Tender Offer and Merger together in this Annual Report on Form10-K as the Cascadian Acquisition. We expect to consummate the Cascadian Acquisition in first quarter of 2018. For additional details about the Cascadian Acquisition, see “Item 1. Business—Proposed Acquisition of Cascadian Therapeutics” above.
On February 5, 2018, we completed an underwritten public offering of 13,269,230 shares of our common stock at a public offering price of $52.00 per share, resulting in gross proceeds to us of approximately $690.0 million, before deducting the underwriting discounts and commissions and offering expenses payable by us. We intend to use the proceeds from the recently-completed public offering to fund a portion of the costs of the Cascadian Acquisition. In addition, as a result of the net proceeds to us from the public offering exceeding $400.0 million, the senior secured bridge loan facility various banks committed to provide to us on January 30, 2018 in connection with the Cascadian Acquisition is no longer available to us in accordance with the terms of the related commitment letter.
On February 8, 2018, we entered into a license and collaboration agreement and related platform technology license agreement with Pieris Pharmaceuticals, Inc. and Pieris Pharmaceuticals AG, or together, Pieris, to develop novel bispecifics incorporating our antibodies and Pieris’ proprietary Anticalin proteins for the treatment of cancer. For additional details about this agreement, see“Item 1. Business—Corporate Collaborations” above.
Outlook
Our ongoing research, development, manufacturing and commercial activities, together with the anticipated consummation of the Cascadian Acquisition and the anticipated integration and development activities related to the acquired Cascadian business and Cascadian’s product candidates, including tucatinib, will require substantial amounts of capital and may not ultimately be successful. In addition, we may encounter unexpected difficulties during our anticipated integration and development activities related to the acquired Cascadian business and Cascadian’s product candidates, any of which may cause us to expend greater funds and efforts or may slow, delay or limit development progress with respect to Cascadian’s product candidates that we may acquire in the Cascadian Acquisition. Over the next several years, we expect that we will incur substantial expenses, primarily as a result of activities related to the commercialization of ADCETRIS, the continued development of ADCETRIS, enfortumab vedotin, and tisotumab vedotin, and, if the Cascadian Acquisition is completed, tucatinib. Our other product candidates are in relatively early stages of development. Enfortumab vedotin, tisotumab vedotin and our other product candidates will require significant further development, financial resources and personnel to pursue and obtain regulatory approval and develop into commercially viable products, if at all. Our commitment of resources to the continuing development, regulatory and commercialization activities for ADCETRIS, the research, continued development and manufacturing of our product candidates and expansion of our pipeline, the transactions contemplated by the Merger Agreement, and the anticipated integration and development activities related to the acquired Cascadian business and Cascadian’s product candidates will likely require us to raise substantial amounts of additional capital and our operating expenses may fluctuate as a result of such activities. We may also incur significant milestone payment obligations to certain of our licensors as our product candidates progress through clinical trials towards potential commercialization.
In addition, we may be unable to complete the Cascadian Acquisition. Even if completed, the success of the Cascadian Acquisition will depend, in part, on our ability to successfully combine and integrate our business with the business of Cascadian and to advance the development of Cascadian’s product candidates. For additional details on these risks, see“Item 1A. Risk Factors—Risks Related to the Cascadian Acquisition” above.
We recognize revenue from ADCETRIS product sales in the United States and Canada. Our future ADCETRIS product sales are difficult to accurately predict from period to period. In this regard, our product sales have varied, and may continue to vary, significantly from period to period and may be affected by a variety of factors. Such factors include the extent to which coverage and reimbursement for ADCETRIS is available
81
from government and other third-party payers, competition, the incidence rate of new patients in ADCETRIS’ approved indications, customer ordering patterns, the overall level of demand for ADCETRIS, and the duration of therapy for patients receiving ADCETRIS. In particular:
| | • | | Obtaining and maintaining appropriate coverage and reimbursement for ADCETRIS is increasingly challenging due to, among other things, the attention being paid to healthcare cost containment and other austerity measures in the U.S. and worldwide, as well as increasing legislative and enforcement interest in the United States with respect to pharmaceutical drug pricing practices. We anticipate that healthcare reform measures that may be adopted in the future may result in more rigorous coverage criteria and an additional downward pressure on the price that we receive for ADCETRIS. We also anticipate that Congress, state legislatures, and third-party payors may continue to review and assess alternative healthcare delivery and payment systems and may in the future propose and adopt legislation or policy changes or implementations effecting additional fundamental changes in the healthcare delivery system, any of which could negatively affect our revenue or sales of ADCETRIS (or any future approved products). |
| | • | | The competition ADCETRIS faces from competing therapies is intensifying, and we anticipate that we will continue to face increasing competition in the future as new companies enter our market and scientific developments surrounding biosimilars and other cancer therapies continue to accelerate. |
| | • | | We believe that the level of our current ADCETRIS sales in the United States has been attributable to the incidence flow of patients eligible for treatment with ADCETRIS, which can vary significantly from period to period. Moreover, we believe that the incidence rate in ADCETRIS’ approved indications is relatively low, particularly when compared to many other oncology indications. In addition, we expect only modest sales growth in the near term as a result of the November 2017 FDA approval of ADCETRIS for the treatment of patients with primary cutaneous anaplastic large cell lymphoma, or pcALCL, and CD30-expressing mycosis fungoides, or MF, who have received prior systemic therapy, subject to our ability to effectively commercialize ADCETRIS in this indication. |
For these and other reasons, we expect that our ability to accelerate ADCETRIS sales growth, if at all, will depend primarily on our ability to continue to expand ADCETRIS’ labeled indications of use, particularly with respect to the frontline Hodgkin lymphoma and frontline MTCL indications. Our efforts to continue to expand ADCETRIS’ labeled indications of use will continue to require additional time and investment in clinical trials to complete and may not be successful. Our ability to successfully commercialize ADCETRIS and to continue to expand its labeled indications of use are subject to a number of risks and uncertainties, including those discussed in Part I, Item 1A of this Annual Report on Form10-K. In particular, although we reported positive top line data in ourECHELON-1 trial in June 2017, there can be no assurance that either we or Takeda will ultimately obtain regulatory approvals of ADCETRIS in theECHELON-1 treatment setting in our respective territories, which would limit our sales of, and the commercial potential of, ADCETRIS. Likewise, we may fail to commercialize ADCETRIS in pcALCL and CD30-expressing MF patients or in theECHELON-1 treatment setting if our sBLA that we submitted in November 2017 is approved by the FDA, either of which would limit our sales of, and the commercial potential of ADCETRIS. In addition, negative or inconclusive results in ourECHELON-2 trial would negatively impact, or preclude altogether, our and Takeda’s ability to obtain regulatory approvals in the frontline MTCL indication in our respective territories, which would also limit our sales of, and the commercial potential of, ADCETRIS.
We also expect that amounts earned from our collaboration agreements, including royalties, will continue to be an important source of our revenues and cash flows. These revenues will be impacted by future development funding and the achievement of development, clinical and commercial success by our collaborators under our existing collaboration and license agreements, including our ADCETRIS collaboration with Takeda, as well as by entering into potential new collaboration and license agreements. Our results of operations may vary substantially from year to year and from quarter to quarter and, as a result, we believe that period to period comparisons of our operating results may not be meaningful and should not be relied upon as being indicative of our future performance.
82
Financial summary
Total revenues increased 15% to $482.3 million in 2017, compared to $418.1 million in 2016. This increase was primarily driven by ADCETRIS net product sales that increased 16% to $307.6 million in 2017 as compared to $265.8 million in 2016, and by collaboration and license agreement revenues that increased 28% to $108.6 million in 2017 as compared to $84.9 million in 2016. Total costs and expenses increased 21% to $678.1 million in 2017 as compared to $560.9 million in 2016. This primarily reflects increased investment in our pipeline of preclinical and clinical-stage programs, increased drug product supply to Takeda, and higher staffing costs to support our continued growth. Net losses were $125.5 million and $140.1 million for the years ended December 31, 2017 and 2016, respectively. Net loss for the year ended December 31, 2017 included a gain of $33.8 million resulting from the change in the fair value of the Immunomedics warrant derivative as discussed below, and an income tax benefit of $33.4 million related to the change in the fair value of our Immunomedics common stock investment.
As of December 31, 2017, we had $413.2 million in cash, cash equivalents and investments, and $677.6 million in total stockholders’ equity. See “—Recent developments” above for a discussion on the underwritten public offering that was completed in February 2018.
Critical Accounting Policies
The preparation of financial statements in accordance with generally accepted accounting principles, or GAAP, requires us to make estimates, assumptions and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosures of contingent assets and liabilities. We believe the following critical accounting policies describe the more significant judgments and estimates used in the preparation of our financial statements.
We evaluate our estimates on an ongoing basis. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form our basis for making judgments about the carrying values of assets and liabilities and the reported amounts of revenues and expenses that are not readily apparent from other sources. Actual results may differ from those estimates under different assumptions and conditions.
Revenue Recognition. Our revenues are comprised of ADCETRIS net product sales, amounts earned under our collaboration and licensing agreements and royalties. Under the accounting guidance that was in effect through December 31, 2017, revenue recognition was predicated upon persuasive evidence of an agreement existing, delivery of products or services being rendered, amounts payable being fixed or determinable, and collectibility being reasonably assured.
We adopted the Accounting Standards Update entitled “ASU2014-09, Revenue from Contracts with Customers” on January 1, 2018 using the modified retrospective method of adoption. For additional information, see the section “Recent accounting pronouncements” in Note 2 to the Notes to Consolidated Financial Statements in Part II Item 8 of this Annual Report onForm 10-K. This “Management’s Discussion and Analysis of Financial Condition and Results of Operations”is based on accounting guidance in effect through December 31, 2017.
Net product sales
We sell ADCETRIS through a limited number of pharmaceutical distributors. Customers order ADCETRIS through these distributors and we typically ship product directly to the customer. We record product sales when title and risk of loss pass, which generally occurs upon delivery of the product to the customer. Product sales are recorded net of estimated government-mandated rebates and chargebacks, distribution fees, estimated product returns and other deductions. These are generally referred to asgross-to-net deductions. Accruals are established for these deductions and actual amounts incurred are offset against applicable accruals. We reflect these accruals as either a reduction in the related account receivable from the distributor, or as an accrued liability depending on the nature of the sales deduction. Sales deductions are based on our estimates that consider payer mix in target markets and our experience to date. These estimates involve a substantial degree of judgment.
83
Government-mandated rebates and chargebacks: We have entered into a Medicaid Drug Rebate Agreement with the Centers for Medicare & Medicaid Services. This agreement provides for a rebate based on covered purchases of ADCETRIS. Medicaid rebates are invoiced to us by the various state Medicaid programs. We estimate Medicaid rebates based on a variety of factors, including our experience to date. We also have completed our Federal Supply Schedule, or FSS, agreement under which certain U.S. government purchasers receive a discount on eligible purchases of ADCETRIS. We have entered into a Pharmaceutical Pricing Agreement with the Secretary of Health and Human Services, which enables certain entities that qualify for government pricing under the Public Health Services Act, or PHS, to receive discounts on their qualified purchases of ADCETRIS. Under these agreements, distributors process a chargeback to us for the difference between wholesale acquisition cost and the applicable discounted price. As a result of our direct-ship distribution model, we can identify the entities purchasing ADCETRIS and this information enables us to estimate expected chargebacks for FSS and PHS purchases based on each entity’s eligibility for the FSS and PHS programs. We also review actual rebate and chargeback information to further refine these estimates.
Distribution fees, product returns and other deductions: Our distributors charge a volume-based fee for distribution services that they perform for us. We allow for the return of product that is within 30 days of its expiration date or that is damaged. We estimate product returns based on our experience to date. In addition, we consider our direct-ship distribution model, our belief that product is not typically held in the distribution channel, and the expected rapid use of the product by healthcare providers. We provide financial assistance to qualifying patients that are underinsured or cannot cover the cost of commercial coinsurance amounts through SeaGen Secure. SeaGen Secure is available to patients in the U.S. and its territories who meet various financial and treatment need criteria. Estimated contributions for commercial coinsurance under SeaGen Secure are deducted from gross sales and are based on an analysis of expected plan utilization. These estimates are adjusted as necessary to reflect our actual experience.
Collaboration and license agreement revenues
We have collaboration and license agreements with a number of biotechnology and pharmaceutical companies. Our proprietary ADC technologies are the basis of many of our collaboration and license agreements, including our ADC collaborations that we have entered into in the ordinary course of our business under which we grant our collaborators research and commercial licenses to our technology and typically provide technology transfer services, technical advice, supplies and services for a period of time.
If there are continuing performance obligations, we use a time-based proportional performance model to recognize revenue over our performance period for the related agreement when no other discernible pattern exists. Collaboration and license agreements are evaluated to determine whether the multiple elements and associated deliverables can be considered separate units of accounting. To date, thepre-commercial deliverables under our collaboration and license agreements have not qualified as separate units of accounting. The assessment of multiple element arrangements requires judgment in order to determine the appropriate point in time, or period of time, that revenue should be recognized. We believe that the development period in each agreement is a reasonable estimate of the performance obligation period of such agreement. Accordingly, all amounts received or due, including any upfront payments, maintenance fees, development and regulatory milestone payments and reimbursement payments, are recognized as revenue over the performance obligation periods of each agreement. These performance obligation periods typically range from one to three years. The agreements with Takeda Pharmaceutical Company Limited, or Takeda, and Genentech, Inc., a member of the Roche Group, or Genentech, have performance obligation periods of ten and seventeen years, respectively. All of the remaining performance obligation periods for our active collaborations are currently expected to be completed in three years or less. When no performance obligations are required of us, or following the completion of the performance obligation period, such amounts are recognized as revenue when collectibility is reasonably assured. Generally, all amounts received or due other than sales-based milestones and royalties are classified as collaboration and license agreement revenues as they are earned. Sales-based milestones and royalties are recognized as royalty revenue as they are reported to us.
84
Our collaboration and license agreements include contractual milestones. Generally, the milestone events contained in our collaboration and license agreements coincide with the progression of the collaborators’ product candidates from development to regulatory approval and then to commercialization.
Development milestones in our collaborations may include the following types of events:
| | • | | Designation of a product candidate or initiation ofpre-clinical studies. Our collaborators must undertake significantpre-clinical research and studies to make a determination of the suitability of a product candidate and the time from those studies or designation to initiation of a clinical trial may take several years. |
| | • | | Initiation of a phase 1 clinical trial. Generally, phase 1 clinical trials may take one to two years to complete. |
| | • | | Initiation of a phase 2 clinical trial. Generally, phase 2 clinical trials may take one to three years to complete. |
| | • | | Initiation of a phase 3 clinical trial. Generally, phase 3 clinical trials may take two to six years to complete. |
Regulatory milestones in our collaborations may include the following types of events:
| | • | | Filing of regulatory applications for marketing approval such as a BLA in the United States or a Marketing Authorization Application in Europe. Generally, it may take up to twelve months to prepare and submit regulatory filings. |
| | • | | Receiving marketing approval in a major market, such as in the United States, Europe, Japan or other significant countries. Generally it may take up to three years after a marketing application is submitted to obtain approval for marketing and pricing from the applicable regulatory agency. |
Commercialization milestones in our collaborations may include the following types of events:
| | • | | First commercial sale in a particular market, such as in the United States, Europe, Japan or other significant countries. |
| | • | | Product sales in excess of apre-specified threshold. The amount of time to achieve this type of milestone depends on several factors, including, but not limited to, the dollar amount of the threshold, the pricing of the product, market penetration of the product and the rate at which customers begin using the product. |
Our ADC collaborators are solely responsible for the development of their product candidates and the achievement of milestones in any of the categories identified above is based solely on the collaborators’ efforts.
In the case of our ADCETRIS collaboration with Takeda, we may be involved in certain development activities; however, the achievement of milestone events under the agreement is primarily based on activities undertaken by Takeda.
The process of successfully developing a product candidate, obtaining regulatory approval and ultimately commercializing a product candidate is highly uncertain and the attainment of any milestones is therefore uncertain and difficult to predict. In addition, since we do not take a substantive role or control the research, development or commercialization of any products generated by our ADC collaborators, we are not able to reasonably estimate when, if at all, any milestone payments or royalties may be payable to us by our ADC collaborators. As such, the milestone payments associated with our ADC collaborations involve a substantial degree of uncertainty and risk that they may never be received. Similarly, even in those collaborations where we may have an active role in the development of the product candidate, such as our ADCETRIS collaboration with Takeda, the attainment of a milestone is based on the collaborator’s activities and is generally outside our direction and control.
85
We generally invoice our collaborators and licensees on a monthly or quarterly basis, or upon the completion of the effort or achievement of a milestone, based on the terms of each agreement. Any deferred revenue arising from amounts received in advance of the culmination of the earnings process is recognized as revenue in future periods when the applicable revenue recognition criteria have been met.
Royalty revenues and cost of royalty revenues
Royalty revenues primarily reflect amounts earned under the ADCETRIS collaboration with Takeda. These royalties include commercial sales-based milestones and sales royalties. Sales royalties are based on a percentage of Takeda’s net sales of ADCETRIS, with rates that range from themid-teens to themid-twenties depending on sales volumes and reset annually. Takeda bears a portion of third-party royalty costs owed on its sales of ADCETRIS. These amounts are included in our royalty revenues. Cost of royalty revenues reflect amounts owed to our third-party licensors related to Takeda’s sales of ADCETRIS. These amounts are recognized in the quarter in which Takeda reports its sales activity to us, which is the quarter following the related sales. Royalty revenues also include certain amounts earned in connection with our ADC collaborations.
Investments. We have investments in debt and equity securities. We classify our investments asavailable-for-sale, which are reported at estimated fair value with the changes in fair value included in accumulated other comprehensive income and loss in stockholders’ equity. Upon our adoption of the Accounting Standards Update entitled “ASU2016-01, Financial Instruments: Overall” on January 1, 2018, we are required to record changes in the fair value of equity securities in net income or loss. The fair value of our investments is subject to volatility and could adversely affect our future operating results.
Accrued Liabilities. As part of the process of preparing financial statements, we are required to estimate accrued liabilities. This process involves identifying services that have been performed on our behalf and estimating the level of services performed and the associated costs incurred for such services where we have not yet been invoiced or otherwise notified of actual cost. We record these estimates in our consolidated financial statements as of each balance sheet date. Examples of estimated accrued liabilities include amounts due to contract research organizations and other costs in conjunction with clinical trials, amounts due in conjunction with manufacturing ADCETRIS and our product candidates, third-party royalties that accrue on our sales of ADCETRIS and professional service fees, among other items.
In accruing service fees, we estimate the time period over which services will be provided and the level of effort in each period. If the actual timing of the provision of services or the level of effort varies from the estimate, we will adjust the accrual accordingly. In the event that we do not identify costs that have been incurred or we under or overestimate the level of services performed or the costs of such services, our actual liabilities would differ from such estimates. The date on which some services commence, the level of services performed on or before a given date and the cost of such services are often subjective determinations. We make judgments based upon the facts and circumstances known to us at the time and in accordance with GAAP.
Research and Development. Research and development expenses consist of salaries, benefits and other headcount-related costs of our research and development staff, preclinical activities, clinical trials and related manufacturing costs, lab supplies, contract and outside service fees, and facilities and overhead expenses. Research and development activities are expensed as incurred.
Clinical trial expenses are a significant component of research and development expenses, and we outsource a significant portion of these costs to third parties. Third-party clinical trial expenses include investigator fees, site costs, clinical research organization costs, and costs for central laboratory testing and data management. Costs associated with activities performed under research and developmentco-development collaborations are reflected in research and development expense.In-licensing fees, milestones, maintenance fees, and other costs to acquire technologies utilized in research and development for product candidates that have not yet received regulatory approval and that are not expected to have alternative future use are expensed when incurred.
86
Non-refundable advance payments for goods or services that will be used or rendered for future research and development activities are capitalized and recognized as expense as the related goods are delivered or the related services are performed.
Share-based Compensation. Share-based compensation cost is based on the fair value of the award on the date of grant. We use the Black-Scholes option pricing model to determine the fair value of options on the date of grant which requires certain estimates to be made by management, including the expected forfeiture rate and expected term of the options. We also make decisions regarding the method of calculating the expected stock price volatility and the risk free interest rate used in the model. Fluctuations that affect these estimates could have an impact on the resulting compensation cost. We recognize this estimated fair value over the vesting period of the arrangement using the graded-vesting attribution method for stock options which vest ratably over the vesting period. For performance-based stock options, we recognized this estimated fair value over the service period of the award when we believe vesting of the performance-based stock options is considered probable. Once vesting of performance-based stock options is considered probable, we record compensation expense based on the portion of the service period elapsed to date, with a cumulativecatch-up, net of estimated forfeitures, and recognize remaining compensation expense, if any, over the remaining estimated service period.
The fair value of each restricted stock unit, or RSU, equals the closing price of our common stock on the date of grant. RSUs granted to date vest 100% at a single point in time. We therefore amortize the value of RSUs, net of estimated forfeitures, to expense on a straight-line basis over the vesting period of the award.
Long-term Incentive Plans. We have long term incentive plans which provide eligible employees with the opportunity to receive performance-based incentive compensation, which may be comprised of cash, stock options, and/or restricted stock units. The payment of cash and the grant or vesting of equity are contingent upon the achievement ofpre-determined regulatory milestones. We record compensation expense over the estimated service period for each milestone when we believe the milestone is considered probable, which we assess at each reporting date. Once a milestone is considered probable, we record compensation expense based on the portion of the service period elapsed to date with respect to that milestone, with a cumulativecatch-up, net of estimated forfeitures, and recognize any remaining compensation expense, if any, over the remaining estimated service period.
Income Taxes. We have net deferred tax assets which are fully offset by a valuation allowance due to our determination that it is more likely than not that the deferred tax assets will not be realized. We believe that a full valuation allowance is appropriate as we have a history of net operating losses. In the event we were to determine that we would be able to realize our net deferred tax assets in the future, an adjustment to the valuation allowance would be made, a portion of which would increase income (or decrease losses) in the period in which such a determination was made. We follow the guidance related to accounting for uncertainty in income taxes, which requires the recognition of an uncertain tax position when it is more likely than not to be sustainable upon audit by the applicable taxing authority.
Inventories. We consider regulatory approval of product candidates to be uncertain. Accordingly, we charge manufacturing costs to research and development expense until such time as a product has received regulatory approval for commercial sale. We began capitalizing ADCETRIS production costs into inventory following its accelerated approval by the FDA in 2011. ADCETRIS inventory that is deployed into clinical, research or development use is charged to research and development expense when it is no longer available for commercial sales. Production costs for our other product candidates continue to be charged to research and development expense.
We value our inventories at the lower of cost or market value. Cost is determined on a specific identification basis. Inventory includes the cost of materials, third-party contract manufacturing and overhead associated with the production of ADCETRIS. We would write-down inventory cost to net realizable value if we were to determine that we had any excess, obsolete or unsalable inventory.
87
Loss Contingencies. We are involved in various legal proceedings in the normal course of our business. A loss contingency is recorded if it is probable that an asset has been impaired or a liability has been incurred and the amount of the loss can be reasonably estimated. We evaluate, among other factors, the probability of an unfavorable outcome and our ability to make a reasonable estimate of the amount of the ultimate loss. Loss contingencies that we determine to be reasonably possible, but not probable, are disclosed but not recorded. Changes in these estimates could materially affect our financial position and results of operations. Legal fees incurred as a result of our involvement in legal proceedings are expensed as incurred.
Results of Operations—Years Ended December 31, 2017, 2016, and 2015
Net product sales
We sell ADCETRIS in the U.S. and Canada.
| | | | | | | | | | | | | | | | | | | | |
| | | 2017 | | | 2016 | | | 2015 | | | Percentage change | |
(dollars in thousands) | | | | | 2017/2016 | | | 2016/2015 | |
Net product sales | | $ | 307,562 | | | $ | 265,766 | | | $ | 226,052 | | | | 16 | % | | | 18 | % |
Net product sales increased in 2017 and 2016 as compared to prior years primarily due to an increase in sales volume and, to a lesser extent, price increases. The increases in sales volumes in both periods were driven primarily by increased use of ADCETRIS across multiple lines of therapy in Hodgkin lymphoma and for the treatment of other malignancies.
In November 2017, the FDA approved ADCETRIS for the treatment of adult patients with pcALCL and CD30-expressing MF who have received prior systemic therapy. While we expect continued growth in ADCETRIS sales in 2018 as compared to 2017, we expect only modest sales growth in the near term as a result of the FDA approval of ADCETRIS for the treatment of patients with pcALCL and CD30-expressing MF who have received prior systemic therapy, subject to our ability to effectively commercialize ADCETRIS in this indication. Our ability to accelerate the rate of ADCETRIS sales growth in future periods, if at all, will be primarily dependent on our ability to continue to expand ADCETRIS’ labeled indications of use. Our efforts to expand ADCETRIS’ labeled indications of use will continue to require additional time and investment in clinical trials to complete and may not be successful.
We record product sales net of estimated government-mandated rebates and chargebacks, distribution fees, product returns and other deductions. These are generally referred to asgross-to-net deductions.Gross-to-net deductions, net of related payments and credits, were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | December 31, 2017 | | | December 31, 2016 | | | December 31, 2015 | |
(in thousands) | | Rebates &
chargebacks | | | Distribution fees,
product returns
and other | | | Total | | | Rebates &
chargebacks | | | Distribution fees,
product returns
and other | | | Total | | | Rebates &
chargebacks | | | Distribution fees,
product returns
and other | | | Total | |
Balance, beginning of
year | | $ | 9,500 | | | $ | 3,198 | | | $ | 12,698 | | | $ | 7,111 | | | $ | 2,359 | | | $ | 9,470 | | | $ | 5,268 | | | $ | 1,618 | | | $ | 6,886 | |
Provision related to current year sales | | | 105,764 | | | | 7,778 | | | | 113,542 | | | | 74,075 | | | | 6,522 | | | | 80,597 | | | | 48,214 | | | | 5,391 | | | | 53,605 | |
Adjustments for prior period sales | | | 1,558 | | | | (294 | ) | | | 1,264 | | | | (1,043 | ) | | | (141 | ) | | | (1,184 | ) | | | (1,065 | ) | | | 34 | | | | (1,031 | ) |
Payments/credits for current year sales | | | (92,947 | ) | | | (5,939 | ) | | | (98,886 | ) | | | (65,598 | ) | | | (4,733 | ) | | | (70,331 | ) | | | (42,656 | ) | | | (4,070 | ) | | | (46,726 | ) |
Payments/credits for prior year sales | | | (9,501 | ) | | | (1,222 | ) | | | (10,723 | ) | | | (5,045 | ) | | | (809 | ) | | | (5,854 | ) | | | (2,650 | ) | | | (614 | ) | | | (3,264 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, end of year | | $ | 14,374 | | | $ | 3,521 | | | $ | 17,895 | | | $ | 9,500 | | | $ | 3,198 | | | $ | 12,698 | | | $ | 7,111 | | | $ | 2,359 | | | $ | 9,470 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Mandatory government discounts are the most significant component of our totalgross-to-net deductions and the discount percentage has been increasing. These discount percentages increased during 2017 and 2016 as
88
a result of price increases we instituted that exceeded the rate of inflation, and to a lesser extent in 2017, as a result of an increase in the proportion of our sales eligible for government mandated rebates or chargebacks. Generally, the change in government prices is limited to the rate of inflation. Distribution fees, product returns and othergross-to-net deductions were relatively stable as a percentage of our gross sales during 2017, 2016 and 2015. We expect futuregross-to-net deductions to fluctuate based on the volume of purchases eligible for government mandated discounts and rebates, as well as changes in the discount percentage which is impacted by potential future price increases, the rate of inflation, and other factors.
As a result of price increases and a continued increase in the percentage of our gross sales that are eligible for government mandated rebates and chargebacks, we expectgross-to-net deductions to increase in 2018 as compared to 2017. There has been extensive discussion in the United States about expanding government discount programs, including allowing Medicare to negotiate drug prices, and pressure on pharmaceutical drug pricing is increasing. For example, the Bipartisan Budget Act, which was enacted on February 9, 2018, contains measures targeting the cost of drugs under federal health care programs, including, among others, an increase from 50% to 70% in the point-of-sale discount that is owed by pharmaceutical manufacturers who participate in Medicare Part D, effective January 1, 2019. Further, the Trump administration’s budget proposal for fiscal year 2019 contains additional drug pricing initiatives that could be enacted during the 2019 budget process or in other future legislation, including, for example, a measure to allow some states to negotiate drug prices under Medicaid. If government discount programs are expanded or discounts are increased as a result of changes in regulations in the United States, our gross-to-net deductions will increase and our net sales will be negatively impacted.
Collaboration and license agreement revenues
Collaboration and license agreement revenues reflect amounts earned under product, ADC andco-development collaborations. These revenues reflect the earned portion of payments received by us for technology access and maintenance fees, milestone payments and reimbursement payments for research and development support that we provide to our collaborators. Collaboration and license agreement revenues by collaborator were as follows:
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | Percentage change | |
(dollars in thousands) | | 2017 | | | 2016 | | | 2015 | | | 2017/2016 | | | 2016/2015 | |
Takeda | | $ | 74,872 | | | $ | 44,384 | | | $ | 17,234 | | | | 69 | % | | | 158 | % |
AbbVie | | | 23,260 | | | | 25,676 | | | | 31,055 | | | | (9 | %) | | | (17 | %) |
Other | | | 10,500 | | | | 14,866 | | | | 21,481 | | | | (29 | %) | | | (31 | %) |
| | | | | | | | | | | | | | | | | | | | |
Total | | $ | 108,632 | | | $ | 84,926 | | | $ | 69,770 | | | | 28 | % | | | 22 | % |
| | | | | | | | | | | | | | | | | | | | |
Collaboration revenues from Takeda fluctuate based on changes in the earned portion of reimbursement funding under the ADCETRIS collaboration, which are influenced by the activities each party is performing under the collaboration agreement at a given time. For example, when Takeda’s level of spending on clinical collaboration activities increases above our own, our earned portion of reimbursement funding generally decreases. Additionally, we receive reimbursement for the cost of drug product supplied to Takeda for its use, the timing of which fluctuates based on Takeda’s product supply needs. The earned portion of reimbursement funding fluctuates based upon how much drug product Takeda has purchased from us in a given period.
Collaboration revenues from Takeda increased in 2017 as compared to 2016 primarily due to an increase in drug product supply activities to Takeda. The increase in collaboration revenues from Takeda in 2016 as compared to 2015 primarily reflected a decrease in clinical trial costs related to activity performed by Takeda as theECHELON-1 and other studies advanced and an increase in drug product supply activities to Takeda.
The decrease in revenues from AbbVie in 2017 as compared to 2016 reflected the timing differences of development milestones and licensing fees as well as a decrease in the earned portion of milestone payments
89
achieved in prior years. Revenues from AbbVie decreased in 2016 as compared to 2015 primarily due to a decrease in the earned portion of milestone payments achieved in prior years.
Changes in revenues recognized from our other collaboration agreements, which include our ADC collaborations and ourco-development collaborations, reflected the timing and the earned portion of development milestones and licensing fees.
Our collaboration and license agreement revenues are impacted by the term and duration of those agreements and by progress-dependent milestones, annual maintenance fees and reimbursement of materials and support services. Collaboration and license agreement revenues may vary substantially from year to year and quarter to quarter depending on the progress made by our collaborators with their product candidates, the level of support we provide to our collaborators, specifically to Takeda under our ADCETRIS collaboration, the timing of milestones achieved and our ability to enter into additional collaborations. We expect our collaboration and license agreement revenues in 2018 to be lower than 2017, driven by the lower expected volume of drug to be supplied to Takeda. We have a significant balance of deferred revenue, representing prior payments from our collaborators that have not yet been recognized as revenue. This deferred revenue will be recognized as revenue in future periods using a time-based approach as we fulfill our performance obligations.
Collaboration Agreements
Takeda
Our ADCETRIS collaboration with Takeda provides for the globalco-development of ADCETRIS and the commercialization of ADCETRIS by Takeda in its territory. We received an upfront payment and have received and are entitled to receive progress- and sales-dependent milestone payments based on Takeda’s achievement of significant events under the collaboration, in addition to tiered royalties with percentages ranging from themid-teens to themid-twenties based on net sales of ADCETRIS within Takeda’s licensed territories. Additionally, the companies equallyco-fund the cost of selected development activities conducted under the collaboration. We recognize as collaboration revenue the upfront payment, progress-dependent development and regulatory milestone payments, and net development cost reimbursement payments from Takeda over theten-year development period of the collaboration, which began in 2009. When the performance of development activities under the collaboration results in us making a reimbursement payment to Takeda, the effect is to reduce the amount of collaboration revenue that we record. We also receive reimbursement for the cost of drug product supplied to Takeda for its use and, in some cases, pay Takeda for drug product they supply to us. The earned portion of net collaboration payments is reflected in collaboration and license agreement revenues.
As of December 31, 2017, total future potential milestone payments to us under the ADCETRIS collaboration could total approximately $165 million. Of the remaining amount, up to approximately $7 million relates to the achievement of development milestones, up to approximately $118 million relates to the achievement of regulatory milestones and up to approximately $40 million relates to the achievement of commercial milestones. As of December 31, 2017, $70 million in milestones had been achieved as a result of regulatory and commercial progress by Takeda.
Astellas
We have an agreement with Agensys, which subsequently became an affiliate of Astellas, to jointly research, develop and commercialize ADCs for the treatment of several types of cancer. The collaboration encompasses combinations of our ADC technology with fully-human antibodies developed by Astellas to proprietary cancer targets.
Under this collaboration, we and Astellas areco-funding all development and commercialization costs for enfortumab vedotin and will share equally in any profits that may come from this product candidate if successfully commercialized. Costs associated withco-development activities are included in research and development expense.
90
Genmab
We have an agreement with Genmab for the development and commercialization of ADCs for the treatment of several types of cancer.
Under this agreement, we exercised aco-development option for tisotumab vedotin in August 2017. We and Genmab will share all future costs and profits for development and commercialization of tisotumab vedotin on an equal basis. Costs associated withco-development activities are included in research and development expense. We will be responsible for tisotumab vedotin commercialization activities in the U.S., Canada, and Mexico, while Genmab will be responsible for commercialization activities in all other territories. Each party has the option toco-promote up to a specified percentage of the sales effort in the other party’s territories.
Unum Therapeutics
We have a collaboration agreement with Unum to develop and commercialize novel ACTR therapies for cancer. We and Unum are developing two ACTR product candidates that combine Unum’s ACTR technology with our antibodies. Unum is conducting preclinical research and clinical development activities through phase 1 clinical trials, and we are providing funding for these activities. The agreement calls for us to work together toco-develop and jointly fund programs after phase 1 clinical trials unless either company opts out. Costs associated withco-development activities are included in research and development expense.
We and Unum wouldco-commercialize any successfully developed product candidates and share any profits equally on anyco-developed programs in the United States. We retain exclusive commercial rights outside of the United States, paying Unum a royalty that is a high single digit tomid-teens percentage ofex-U.S. sales. The potential future licensing and progress-dependent milestone payments to Unum under the collaboration may total up to $400 million between the two ACTR programs, payment of which is triggered by the achievement of development, regulatory and commercial milestones.
ADC Collaboration Agreements
We have other active collaborations with a number of companies to allow them to use our proprietary ADC technology. Under these ADC collaborations, which we have entered into in the ordinary course of business, we typically receive or are entitled to receive upfront cash payments, progress- and sales-dependent milestones and royalties on net sales of products incorporating our ADC technology, as well as annual maintenance fees and support fees for research and development services and materials provided under the agreements. These amounts are recognized as revenue as they are realized, or over the performance obligation period of the agreements during which we provide limited support to the collaborator. Our ADC collaborators are solely responsible for research, product development, manufacturing and commercialization of any product candidates under these collaborations, which includes achievement of the potential milestones.
As of December 31, 2017, our ADC collaborations had generated more than $375 million, primarily in the form of upfront and milestone payments. Total milestone payments to us under our current ADC collaborations could total up to approximately $2.9 billion if all potential product candidates achieved all of their milestone events. Of this amount, approximately $0.4 billion relates to the achievement of development milestones, approximately $1.1 billion relates to the achievement of regulatory milestones and approximately $1.4 billion relates to the achievement of commercial milestones. Since we do not control the research, development or commercialization of any of the products that would generate these milestones, we are not able to reasonably estimate when, if at all, any milestone payments or royalties may be payable by our collaborators. Successfully developing a product candidate, obtaining regulatory approval and ultimately commercializing it is a significantly lengthy and highly uncertain process which entails a significant risk of failure. In addition, business combinations, changes in a collaborator’s business strategy and financial difficulties or other factors could result and have resulted in a collaborator abandoning or delaying development of its product candidates. As such, the milestone payments associated with our ADC collaborations andco-development agreements involve a
91
substantial degree of risk and may never be received. Accordingly, we do not expect, and investors should not assume, that we will receive all of the potential milestone payments described above, and it is possible that we may never receive any significant milestone payments under these agreements.
Royalty revenues and cost of royalty revenues
Royalty revenues primarily reflect royalties paid to us by Takeda under the ADCETRIS collaboration. These royalties include commercial sales-based milestones and sales royalties. The royalty rate paid by Takeda is calculated as a percentage of Takeda’s net sales of ADCETRIS, ranges from themid-teens to themid-twenties depending on sales volumes, and resets annually. Takeda bears a portion of third-party royalty costs owed on sales of ADCETRIS in its territory. This amount is included in our royalty revenues. Cost of royalty revenues reflect amounts owed to our third-party licensors related to the sale of ADCETRIS in Takeda’s territory.
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | Percentage change | |
(dollars in thousands) | | 2017 | | | 2016 | | | 2015 | | | 2017/2016 | | | 2016/2015 | |
Royalty revenues | | $ | 66,056 | | | $ | 67,455 | | | $ | 40,980 | | | | (2 | %) | | | 65 | % |
Cost of royalty revenues | | | 19,350 | | | | 14,149 | | | | 12,964 | | | | 37 | % | | | 9 | % |
Royalty revenues decreased in 2017 as compared to 2016, as 2016 included aone-time $20.0 million sales-based milestone triggered by Takeda. This decrease was partially offset by Takeda’s higher sales volume and higher royalty rate in 2017. Royalty revenues increased in 2016 as compared to 2015 primarily due to theone-time $20.0 million sales-based milestone triggered by Takeda, as well as Takeda’s higher sales volume in 2016.
Cost of royalty revenues fluctuates based on the amount of net sales of ADCETRIS by Takeda in its territories.
We expect that royalty revenues will increase in 2018, as compared to 2017, primarily due to higher sales volume by Takeda. We expect cost of royalty revenues to increase in 2018 primarily due to anticipated increases in sales volumes in Takeda’s territory and, to a lesser extent, increases in the applicable royalty rate.
Cost of sales
ADCETRIS cost of sales includes manufacturing costs of product sold, third-party royalty costs, amortization of technology license costs, and distribution and other costs.
| | | | | | | | | | | | | | | | | | | | |
| | | 2017 | | | 2016 | | | 2015 | | | Percentage change | |
(dollars in thousands) | | | | | 2017/2016 | | | 2016/2015 | |
Cost of sales | | $ | 34,768 | | | $ | 28,168 | | | $ | 24,476 | | | | 23 | % | | | 15 | % |
Cost of sales increased in 2017 and 2016 as compared to prior periods, primarily due to higher third-party royalty costs that were driven by increased sales volumes. We expect cost of sales to increase in 2018, primarily due to anticipated increases in sales volumes.
Research and development
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | Percentage change | |
(dollars in thousands) | | 2017 | | | 2016 | | | 2015 | | | 2017/2016 | | | 2016/2015 | |
Research | | $ | 78,152 | | | $ | 62,071 | | | $ | 77,215 | | | | 26 | % | | | (20 | %) |
Development and contract manufacturing | | | 172,297 | | | | 143,920 | | | | 93,734 | | | | 20 | % | | | 54 | % |
Clinical | | | 206,251 | | | | 173,317 | | | | 123,580 | | | | 19 | % | | | 40 | % |
| | | | | | | | | | | | | | | | | | | | |
Total research and development expenses | | $ | 456,700 | | | $ | 379,308 | | | $ | 294,529 | | | | 20 | % | | | 29 | % |
| | | | | | | | | | | | | | | | | | | | |
92
Research expenses include, among other things, personnel, occupancy and laboratory expenses, technology access fees associated with the discovery and identification of new monoclonal antibodies and related technologies, and the development of novel classes of stable linkers and cell-killing agents for our ADC technology. Research expenses also include research activities associated with our product candidates, such as preclinical translational biology andin vitroandin vivostudies, andIND-enabling pharmacology and toxicology studies. The increase in 2017 as compared to 2016 primarily reflected increases in both internal andco-development costs to support our growing pipeline of product candidates and, to a lesser extent, increases in staffing and related occupancy costs. The decrease in 2016 as compared to 2015 was primarily the result of a $25.0 million technology access fee related to the initiation of our collaboration agreement with Unum in June of 2015, offset partially by increased staffing, facilities and other costs to support our growing pipeline of product candidates, as well as increases in technology access fees paid and cost reimbursements to Unum under our collaboration agreement.
Development and contract manufacturing expenses include personnel and occupancy expenses, external contract manufacturing costs for thescale-up andpre-approval manufacturing of drug product used in research and our clinical trials, and costs for drug product supplied to our collaborators. Development and contract manufacturing expenses also include quality control and assurance activities, and storage and shipment of our product candidates. The increases in 2017 and 2016 as compared to prior periods primarily reflected increased drug product supplied to Takeda and, to a lesser extent, increases in staffing and other costs to support our growing pipeline of product candidates. The increase in 2016 as compared to 2015 also included cost reimbursements to Astellas under our collaboration.
Clinical expenses include personnel, travel, occupancy costs, and external clinical trial costs including costs for clinical sites, clinical research organizations, contractors and regulatory activities associated with conducting human clinical trials. The increases in 2017 and 2016 as compared to prior periods reflected increased clinical trial activity related to our product candidates, primarily enfortumab vedotin and ladiratuzumab vedotin, and related increases in personnel costs, offset in part by a decline inSGN-CD33A costs.
93
We utilize our employee and infrastructure resources across multiple research and development projects. We track human resource efforts expended on many of our programs for purposes of billing our collaborators for time incurred at agreed upon rates and for resource planning. We do not account for actual costs on a project basis as it relates to our infrastructure, facility, employee and other indirect costs; however, we do separately track significant third-party costs including clinical trial costs, manufacturing costs and other contracted service costs on a project basis. To that end, the following table shows third-party costs incurred for research, contract manufacturing of our product candidates and clinical and regulatory services, as well aspre-commercial milestone payments forin-licensed technology for ADCETRIS and certain of our clinical-stage product candidates. The table also presents other costs and overhead consisting of third-party costs for our preclinical stage programs, personnel, facilities and other indirect costs not directly charged to development programs.
| | | | | | | | | | | | | | | | | | | | | | | | |
(dollars in thousands) | | | | | | | | | | | Percentage change | | | (5 years)
January 1, 2013 to
December 31, 2017 | |
| | 2017 | | | 2016 | | | 2015 | | | 2017/2016 | | | 2016/2015 | | |
ADCETRIS (brentuximab vedotin) | | $ | 79,343 | | | $ | 73,623 | | | $ | 50,965 | | | | 8 | % | | | 44 | % | | $ | 328,571 | |
SGN-CD33A (vadastuximab
talirine) | | | 34,151 | | | | 49,387 | | | | 15,769 | | | | (31 | %) | | | 213 | % | | | 109,762 | |
ASG-22ME (enfortumab vedotin) | | | 20,834 | | | | 5,607 | | | | 2,618 | | | | 272 | % | | | 114 | % | | | 33,569 | |
SGN-LIV1A (ladiratuzumab vedotin) | | | 18,483 | | | | 4,721 | | | | 2,607 | | | | 292 | % | | | 81 | % | | | 30,770 | |
Other clinical stage programs | | | 24,892 | | | | 26,135 | | | | 24,732 | | | | (5 | %) | | | 6 | % | | | 108,404 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total third-party costs for clinical stage programs | | | 177,703 | | | | 159,473 | | | | 96,691 | | | | 11 | % | | | 65 | % | | | 611,076 | |
Other costs and overhead | | | 278,997 | | | | 219,835 | | | | 197,838 | | | | 27 | % | | | 11 | % | | | 968,831 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total research and development | | $ | 456,700 | | | $ | 379,308 | | | $ | 294,529 | | | | 20 | % | | | 29 | % | | $ | 1,579,907 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Third-party costs for ADCETRIS increased in 2017 as compared to 2016 primarily due to an increase in drug product supplied to Takeda, offset partially by a decrease in clinical trial activities. Third-party costs for ADCETRIS increased in 2016 as compared to 2015 primarily due to an increase in drug product supplied to Takeda and, to a lesser extent, third-party clinical trial costs as we evaluated the use of ADCETRIS in other lines of therapy. The cost of drug product supplied to Takeda is charged to research and development expense. We are reimbursed for the drug product, which is included in collaboration and license agreement revenues.
Third-party costs forSGN-CD33A decreased in 2017 as compared to 2016 due to the discontinuation of our phase 3 CASCADE and otherSGN-CD33A clinical trials. Third-party costs forSGN-CD33A increased in 2016 as compared to 2015 due to increases in clinical trial costs and drug supply activities for then ongoing and anticipated additional clinical trials. At this time, we have no plans to initiate additional clinical trials ofSGN-CD33A.
Third-party costs for enfortumab vedotin and ladiratuzumab vedotin increased in 2017 and 2016 as compared to prior periods primarily due to increases in drug supply activities and, to a lesser extent, clinical trial costs as we initiated additional clinical trials in 2017.
Third-party costs for our other clinical stage programs were related to multiple earlier-stage development programs and were relatively consistent across 2017, 2016 and 2015.
Other costs and overhead include third-party costs of our preclinical programs, including our strategic collaborations with Genmab and Unum, and costs associated with personnel and facilities. These costs increased
94
in 2017 and 2016 as compared to prior periods due to increased development activities to expand our product pipeline, including increases in staffing levels and the expansion of our facilities to accommodate our growth.
Our expenditures on our ADCETRIS clinical development program and on our current and future preclinical and clinical development programs are subject to numerous uncertainties in timing and cost to completion. In order to advance our product candidates toward commercialization, the product candidates are tested in numerous preclinical safety, toxicology and efficacy studies. We then conduct clinical trials for those product candidates that take several years or more to complete. The length of time varies substantially based upon the type, complexity, novelty and intended use of a product candidate. Likewise, in order to expand ADCETRIS’ labeled indications of use, we are required to conduct additional extensive clinical trials. The cost of clinical trials may vary significantly over the life of a project as a result of a variety of factors, including:
| | • | | the number of patients required in our clinical trials; |
| | • | | the length of time required to enroll trial participants; |
| | • | | the number and location of sites included in the trials; |
| | • | | the costs of producing supplies of the product candidates needed for clinical trials and regulatory submissions; |
| | • | | the safety and efficacy profile of the product candidate; |
| | • | | the use of clinical research organizations to assist with the management of the trials; and |
| | • | | the costs and timing of, and the ability to secure, regulatory approvals. |
We anticipate that our total research and development expenses in 2018 will increase compared to 2017 due primarily to higher costs for the development of our product candidates, primarily enfortumab vedotin and ladiratuzumab vedotin, as well as the operation of the biologics manufacturing facility that we acquired in October 2017 and Cascadian research and development activities, if the Cascadian Acquisition closes. Certain ADCETRIS development activities, including some clinical studies, will be conducted by Takeda, the costs of which are not reflected in our research and development expenses. Because of these and other factors, expenses will fluctuate based upon many factors, including the degree of collaborative activities, timing of manufacturing campaigns, numbers of patients enrolled in our clinical trials and the outcome of each clinical trial event.
The risks and uncertainties associated with our research and development projects are discussed more fully in Part I, Item 1A—Risk Factors. As a result of the uncertainties discussed above, we are unable to determine with any degree of certainty the duration and completion costs of our research and development projects, anticipated completion dates or when and to what extent we will receive cash inflows from the commercialization and sale of ADCETRIS in any additional approved indications or of any of our product candidates.
Selling, general and administrative
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | Percentage change | |
(dollars in thousands) | | 2017 | | | 2016 | | | 2015 | | | 2017/2016 | | | 2016/2015 | |
Selling, general and administrative | | $ | 167,233 | | | $ | 139,247 | | | $ | 125,783 | | | | 20 | % | | | 11 | % |
Selling, general and administrative expenses increased in 2017 and 2016 as compared to prior periods primarily due to higher costs for staffing to support our continued growth. Selling, general and administrative expenses also increased in 2017 as compared to 2016 due to higher expenses for legal matters.
We anticipate that selling, general and administrative expenses will increase in 2018 as we continue our activities in support of the commercialization of ADCETRIS, as well as our support of general operations and Cascadian selling, general and administrative activities, if the Cascadian Acquisition closes.
95
Investment and other income, net
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | Percentage change | |
(dollars in thousands) | | 2017 | | | 2016 | | | 2015 | | | 2017/2016 | | | 2016/2015 | |
Net gain on Immunomedics warrant derivative | | $ | 33,777 | | | $ | 0 | | | $ | 0 | | | | N/A | | | | N/A | |
Investment income, net | | | 3,137 | | | | 2,614 | | | | 464 | | | | 20 | % | | | N/A | |
| | | | | | | | | | | | | | | | | | | | |
Investment and other income, net | | $ | 36,914 | | | $ | 2,614 | | | $ | 464 | | | | N/A | | | | N/A | |
| | | | | | | | | | | | | | | | | | | | |
N/A: No amount in comparable period or not a meaningful comparison.
The net gain on Immunomedics warrant derivative primarily was due to the increase in the fair value of the warrant derivative prior to it being exercised in December 2017. We acquired the warrant in February 2017 and received 8.7 million shares of Immunomedics common stock upon exercise in December 2017. The resulting shares of common stock are classified asavailable-for-sale securities and carried at estimated fair value with unrealized gains and losses included in accumulated other comprehensive income and loss in stockholders’ equity. The fair value of the Immunomedics common stock fluctuates based on changes in the stock price of Immunomedics. Upon our adoption of the Accounting Standards Update entitled “ASU2016-01, Financial Instruments: Overall” on January 1, 2018, we will record changes in the fair value of equity securities in net income or loss. To the extent that we continue to hold Immunomedics common stock or other equity securities, our operating results may fluctuate significantly. For additional information on our adoption of this ASU, see the section “Recent accounting pronouncements” in Note 2 to the Notes to Consolidated Financial Statements in Part II Item 8 of this Annual Report onForm 10-K.
Investment income reflects amounts earned on our investments in U.S. Treasury securities. Investment income increased in 2017 compared to 2016 due to a higher effective yield of our portfolio. Investment income increased in 2016 as compared to 2015 due to higher average investment balances following our public offering in September 2015, which resulted in net proceeds to us of approximately $526.6 million.
Income taxes
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | Percentage change | |
(dollars in thousands) | | 2017 | | | 2016 | | | 2015 | | | 2017/2016 | | | 2016/2015 | |
Income tax benefit | | $ | 33,357 | | | $ | 0 | | | $ | 0 | | | | N/A | | | | N/A | |
N/A: No amount in comparable period or not a meaningful comparison.
The income tax benefit was due to unrealized gains on our common stock investment in Immunomedics, which was offset by an income tax provision for the same amount in other comprehensive income in stockholders’ equity.
Liquidity and capital resources
| | | | | | | | | | | | |
| | | December 31, | |
(dollars in thousands) | | 2017 | | | 2016 | | | 2015 | |
Cash, cash equivalents and investments | | $ | 413,171 | | | $ | 618,974 | | | $ | 712,711 | |
Working capital | | | 409,932 | | | | 586,132 | | | | 636,793 | |
Stockholders’ equity | | | 677,569 | | | | 634,087 | | | | 685,911 | |
96
| | | | | | | | | | | | |
| | | Years ended December 31, | |
(dollars in thousands) | | 2017 | | | 2016 | | | 2015 | |
Cash provided by (used in): | | | | | | | | | | | | |
Operating activities | | $ | (118,900 | ) | | $ | (96,971 | ) | | $ | (133,203 | ) |
Investing activities | | | 129,861 | | | | 68,193 | | | | (375,850 | ) |
Financing activities | | | 41,311 | | | | 35,196 | | | | 554,381 | |
The changes in net cash from operating activities primarily are related to our net loss, working capital fluctuations and changes in ournon-cash expenses, all of which are highly variable. The changes in cash from investing activities reflect differences between the proceeds received from sale and maturity of our investments and amounts reinvested, and for 2017 included $55.1 million paid for the Immunomedics common stock investment (as part of purchases ofavailable-for-sale securities) and $41.7 million paid for the acquisition of a biologics manufacturing facility in October 2017. Cash from financing activities includes proceeds from stock option exercises and our employee stock purchase plan for all years presented, and for 2015 included $526.6 million in net proceeds from our public offering in September 2015.
We primarily have financed our operations through the issuance of equity securities, collections from commercial sales of ADCETRIS, and amounts received pursuant to product collaborations and our ADC collaborations. To a lesser degree, we have also financed our operations through royalty revenues and interest earned on cash, cash equivalents and investment securities. These financing and revenue sources have allowed us to maintain adequate levels of cash and investments. See “—Recent developments” above for a discussion on the Cascadian Acquisition, as well as the underwritten public offering that was completed in February 2018.
Our cash, cash equivalents, and investments are held in a variety ofnon-interest bearing bank accounts and interest-bearing instruments subject to investment guidelines allowing for holdings in U.S. government and agency securities, corporate securities, taxable municipal bonds, commercial paper and money market accounts. Our investment portfolio is structured to provide for investment maturities and access to cash to fund our anticipated working capital needs. However, if our liquidity needs should be accelerated for any reason in the near term, or investments do not pay at maturity, we may be required to sell investment securities in our portfolio prior to their scheduled maturities, which may result in a loss. As of December 31, 2017, we had $413.2 million held in cash or investments scheduled to mature within the next twelve months.
At our currently planned spending rates, we believe that our financial resources, including the proceeds from our February 2018 public offering of common stock, together with product and royalty revenues from sales of ADCETRIS and the fees, milestone payments and reimbursements we expect to receive under our existing collaboration and license agreements, will be sufficient to fund the costs of the Cascadian Acquisition and to fund our operations for at least the next twelve months. Changes in our spending rate may occur that would consume available capital resources sooner, such as increased development, manufacturing and clinical trial expenses in connection with our expanding pipeline programs, and the Cascadian Acquisition, our undertaking of additional programs, business activities, or entry into additional strategic transactions, including potential additional acquisitions of products, technologies or businesses.
Accordingly, we may be required to, or may otherwise determine to, raise additional capital to fund those obligations. Further, in the event of a termination of the ADCETRIS collaboration agreement with Takeda, we would not receive development cost sharing payments or milestone payments or royalties for the development or sale of ADCETRIS in Takeda’s territory, and we would be required to fund all ADCETRIS development and commercial activities. Any of these factors could lead to a need for us to raise additional capital.
We expect to make additional capital outlays and to increase operating expenditures over the next several years as we hire additional employees, support our preclinical development, manufacturing and clinical trial activities for ADCETRIS and our other pipeline programs, and expand internationally, as well as commercialize ADCETRIS and position ADCETRIS for potential additional regulatory approvals. In addition, we anticipate
97
committing substantial capital resources to the transactions contemplated by the Merger Agreement and the anticipated integration and development activities related to the acquired Cascadian business and Cascadian’s product candidates, including tucatinib. Our commitment of resources to the continuing development, regulatory and commercialization activities for ADCETRIS, and the research, continued development and manufacturing of our product candidates will likely require us to raise substantial amounts of additional capital. Further, we actively evaluate various strategic transactions on an ongoing basis, including licensing or otherwise acquiring complementary products, technologies or businesses, and we may require significant additional capital in order to complete or otherwise provide funding for any additional acquisitions. We may seek additional funding through some or all of the following methods: corporate collaborations, licensing arrangements and public or private debt or equity financings. We do not know whether additional capital will be available when needed, or that, if available, we will obtain financing on terms favorable to us or our stockholders. If we are unable to raise additional funds when we need them, we may be required to delay, reduce the scope of, or eliminate one or more of our development programs, which may adversely affect our business and operations.
Commitments
The following table reflects our future minimum contractual commitments as of December 31, 2017:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
(dollars in thousands) | | Total | | | 2018 | | | 2019 | | | 2020 | | | 2021 | | | 2022 | | | Thereafter | |
Operating leases | | $ | 43,661 | | | $ | 7,537 | | | $ | 6,982 | | | $ | 6,844 | | | $ | 6,486 | | | $ | 6,277 | | | $ | 9,535 | |
Tenant improvements | | | 3,939 | | | | 3,939 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | | 0 | |
Manufacturing, license & collaboration agreements | | | 256,761 | | | | 67,159 | | | | 33,569 | | | | 31,643 | | | | 30,760 | | | | 24,720 | | | | 68,910 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total | | $ | 304,361 | | | $ | 78,635 | | | $ | 40,551 | | | $ | 38,487 | | | $ | 37,246 | | | $ | 30,997 | | | $ | 78,445 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
We have entered into leases for our office and laboratory facilities expiring in 2018 through 2024 that contain rate escalations and options for us to extend the leases. Operating lease obligations in the table above do not assume the exercise by us of any extension options.
Manufacturing, license and collaboration agreement commitments includenon-cancelable obligations under our manufacturing, license and collaboration agreements. A substantial portion of the minimum payments under manufacturing, license and collaboration agreements represents contractual obligations related to manufacturing our product candidates for use in our clinical trials and for commercial operations in the case of ADCETRIS.
Some of our manufacturing, license and collaboration agreements provide for periodic maintenance fees over specified time periods, as well as payments by us upon the achievement of development and regulatory milestones. Some of our licensing agreements obligate us to pay royalties from the low single digit tomid-teens based on net sales of products utilizing licensed technology. Such royalties are dependent on future product sales and are not provided for in the table above as they are dependent on events that have not yet occurred. Future milestone payments for research andpre-clinical stage development programs have not been included in the above table as the event triggering such payment or obligation has not yet occurred. The above table also excludes up to $400.0 million in potential future milestone payments to Unum under our collaboration agreement with Unum and up to approximately $98 million in potential future milestone payments to other third parties under license agreements for our clinical-stage development programs. These milestone payments generally become due and payable only upon the achievement of certain developmental, clinical, regulatory and/or commercial milestones. These contingent payments have not been included in the above table as the event triggering such payment or obligation has not yet occurred.
We entered into a Merger Agreement with Cascadian in January 2018, and we entered into a license and collaboration agreement and related platform technology license agreement with Pieris in February 2018. We are obligated to make payments under these agreements, and those payments have not been included in the above
98
table as these agreements were executed after December 31, 2017. For additional details about these agreements, see“—Recent developments” above.
Recent accounting pronouncements
See the section “Recent accounting pronouncements” in Note 2 to the Notes to Consolidated Financial Statements in Part II Item 8 of this Annual Report onForm 10-K.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
Interest Rate Risk
Our exposure to market risk for changes in interest rates relates primarily to our investment portfolio. We currently have holdings in U.S. Treasury securities. We do not have any outstanding derivative financial instruments in our investment portfolio. A summary of our investment securities follows:
| | | | | | | | |
(dollars in thousands) | | December 31, | |
| | 2017 | | | 2016 | |
Short-term investments | | $ | 252,226 | | | $ | 480,313 | |
Long-term investments | | | 0 | | | | 29,988 | |
| | | | | | | | |
Total | | $ | 252,226 | | | $ | 510,301 | |
| | | | | | | | |
We have estimated the effect on our investment portfolio of a hypothetical increase in interest rates by one percent to be a reduction of $0.9 million in the fair value of our investments as of December 31, 2017. In addition, a hypothetical decrease of 10% in the effective yield of our investments would reduce our expected investment income by $0.3 million over the next twelve months based on our investment balance at December 31, 2017.
Equity Price Risk
As of December 31, 2017, we held 11.7 million shares of Immunomedics common stock. The fair value of the common stock fluctuates based on changes in the stock price of Immunomedics.
Upon our adoption of the Accounting Standards Update entitled “ASU2016-01, Financial Instruments: Overall” on January 1, 2018, we will record changes in the fair value of equity securities in net income or loss. To the extent that we continue to hold Immunomedics common stock or other equity securities, our operating results may fluctuate significantly. Based on our shares of Immunomedics common stock held as of December 31, 2017, under the new standard, a hypothetical decrease of 10% in the price of Immunomedics common stock would reduce the fair value of the investment and, accordingly, our net income by approximately $18.8 million.
Foreign Currency Risk
Most of our revenues and expenses are denominated in U.S. dollars and as a result, we have not experienced significant foreign currency transaction gains and losses to date. Our commercial sales in Canada are denominated in Canadian Dollars. We also had other transactions denominated in foreign currencies during the year ended December 31, 2017, primarily related to contract manufacturing andex-U.S. clinical trial activities, and we expect to continue to do so. Our royalties from Takeda are derived from their sales of ADCETRIS in multiple countries and in multiple currencies that are converted into U.S. dollars for purposes of determining the royalty owed to us. Our primary exposure is to fluctuations in the Euro, British Pound, Canadian Dollar and Swiss Franc. We do not anticipate that foreign currency transaction gains or losses will be significant at our current level of operations. However, transaction gains or losses may become significant in the future as we continue to expand our operations internationally. We have not engaged in foreign currency hedging to date; however, we may do so in the future.
99
Item 8. Financial Statements and Supplementary Data
Seattle Genetics, Inc.
Index to Financial Statements
100
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Seattle Genetics, Inc.
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of Seattle Genetics, Inc. and its subsidiaries as of December 31, 2017 and 2016 and the related consolidated statements of comprehensive loss, stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2017, including the related notes (collectively referred to as the “consolidated financial statements”). We also have audited the Company’s internal control over financial reporting as of December 31, 2017, based on criteria established inInternal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2017 and 2016, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2017 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2017, based on criteria established inInternal Control - Integrated Framework (2013) issued by the COSO.
Basis for Opinions
The Company’s management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in Management’s Annual Report on Internal Control over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company’s internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
101
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP
Seattle, Washington
February 14, 2018
We have served as the Company’s auditor since 1998.
102
Seattle Genetics, Inc.
Consolidated Balance Sheets
(In thousands, except par value)
| | | | | | | | |
| | | December 31, | |
| | | 2017 | | | 2016 | |
Assets | | | | | | | | |
Current assets | | | | | | | | |
Cash and cash equivalents | | $ | 160,945 | | | $ | 108,673 | |
Short-term investments | | | 252,226 | | | | 480,313 | |
Accounts receivable, net | | | 84,774 | | | | 61,928 | |
Inventories | | | 59,978 | | | | 68,124 | |
Prepaid expenses and other current assets | | | 19,138 | | | | 15,610 | |
| | | | | | | | |
Total current assets | | | 577,061 | | | | 734,648 | |
Property and equipment, net | | | 103,756 | | | | 62,870 | |
Long-term investments | | | 0 | | | | 29,988 | |
Othernon-current assets | | | 197,132 | | | | 10,890 | |
| | | | | | | | |
Total assets | | $ | 877,949 | | | $ | 838,396 | |
| | | | | | | | |
Liabilities and Stockholders’ Equity | | | | | | | | |
Current liabilities | | | | | | | | |
Accounts payable and accrued liabilities | | $ | 132,672 | | | $ | 120,669 | |
Current portion of deferred revenue | | | 34,457 | | | | 27,847 | |
| | | | | | | | |
Total current liabilities | | | 167,129 | | | | 148,516 | |
| | | | | | | | |
Long-term liabilities | | | | | | | | |
Deferred revenue, less current portion | | | 30,618 | | | | 53,006 | |
Deferred rent and other long-term liabilities | | | 2,633 | | | | 2,787 | |
| | | | | | | | |
Total long-term liabilities | | | 33,251 | | | | 55,793 | |
| | | | | | | | |
Commitments and contingencies | | | | | | | | |
Stockholders’ equity | | | | | | | | |
Preferred stock, $0.001 par value, 5,000 shares authorized; none issued | | | 0 | | | | 0 | |
Common stock, $0.001 par value, 250,000 shares authorized; 144,395 shares issued and outstanding at December 31, 2017 and 142,193 shares issued and outstanding at December 31, 2016 | | | 144 | | | | 142 | |
Additionalpaid-in capital | | | 1,806,159 | | | | 1,701,048 | |
Accumulated other comprehensive income (loss) | | | 63,836 | | | | (63 | ) |
Accumulated deficit | | | (1,192,570 | ) | | | (1,067,040 | ) |
| | | | | | | | |
Total stockholders’ equity | | | 677,569 | | | | 634,087 | |
| | | | | | | | |
Total liabilities and stockholders’ equity | | $ | 877,949 | | | $ | 838,396 | |
| | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
103
Seattle Genetics, Inc.
Consolidated Statements of Comprehensive Loss
(In thousands, except per share amounts)
| | | | | | | | | | | | |
| | | Years ended December 31, | |
| | | 2017 | | | 2016 | | | 2015 | |
Revenues | | | | | | | | | | | | |
Net product sales | | $ | 307,562 | | | $ | 265,766 | | | $ | 226,052 | |
Collaboration and license agreement revenues | | | 108,632 | | | | 84,926 | | | | 69,770 | |
Royalty revenues | | | 66,056 | | | | 67,455 | | | | 40,980 | |
| | | | | | | | | | | | |
Total revenues | | | 482,250 | | | | 418,147 | | | | 336,802 | |
| | | | | | | | | | | | |
Costs and expenses | | | | | | | | | | | | |
Cost of sales | | | 34,768 | | | | 28,168 | | | | 24,476 | |
Cost of royalty revenues | | | 19,350 | | | | 14,149 | | | | 12,964 | |
Research and development | | | 456,700 | | | | 379,308 | | | | 294,529 | |
Selling, general and administrative | | | 167,233 | | | | 139,247 | | | | 125,783 | |
| | | | | | | | | | | | |
Total costs and expenses | | | 678,051 | | | | 560,872 | | | | 457,752 | |
| | | | | | | | | | | | |
Loss from operations | | | (195,801 | ) | | | (142,725 | ) | | | (120,950 | ) |
Investment and other income, net | | | 36,914 | | | | 2,614 | | | | 464 | |
| | | | | | | | | | | | |
Loss before income taxes | | | (158,887 | ) | | | (140,111 | ) | | | (120,486 | ) |
Income tax benefit | | | 33,357 | | | | 0 | | | | 0 | |
| | | | | | | | | | | | |
Net loss | | $ | (125,530 | ) | | $ | (140,111 | ) | | $ | (120,486 | ) |
| | | | | | | | | | | | |
Net loss per share—basic and diluted | | $ | (0.88 | ) | | $ | (1.00 | ) | | $ | (0.93 | ) |
| | | | | | | | | | | | |
Shares used in computation of per share amount—basic and diluted | | | 143,174 | | | | 140,746 | | | | 129,184 | |
| | | | | | | | | | | | |
Comprehensive loss: | | | | | | | | | | | | |
Net loss | | $ | (125,530 | ) | | $ | (140,111 | ) | | $ | (120,486 | ) |
Other comprehensive income (loss): | | | | | | | | | | | | |
Unrealized gain (loss) on securitiesavailable-for-sale net of tax provision of $33,357, $0, and $0, respectively | | | 63,888 | | | | 616 | | | | (642 | ) |
Foreign currency translation gain (loss) | | | 11 | | | | 4 | | | | (12 | ) |
| | | | | | | | | | | | |
Total other comprehensive income (loss) | | | 63,899 | | | | 620 | | | | (654 | ) |
| | | | | | | | | | | | |
Comprehensive loss | | $ | (61,631 | ) | | $ | (139,491 | ) | | $ | (121,140 | ) |
| | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
104
Seattle Genetics, Inc.
Consolidated Statements of Stockholders’ Equity
(In thousands)
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Common stock | | | Additional
paid-in
capital | | | Accumulated
other
comprehensive
income (loss) | | | Accumulated
deficit | | | Total
stockholders’
equity | |
| | | Shares | | | Amount | | | | | |
Balances at December 31, 2014 | | | 123,973 | | | $ | 124 | | | $ | 1,017,182 | | | $ | (29 | ) | | $ | (806,443 | ) | | $ | 210,834 | |
Net loss | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | | (120,486 | ) | | | (120,486 | ) |
Other comprehensive loss | | | 0 | | | | 0 | | | | 0 | | | | (654 | ) | | | 0 | | | | (654 | ) |
Issuance of common stock for employee stock purchase plan | | | 201 | | | | 0 | | | | 5,317 | | | | 0 | | | | 0 | | | | 5,317 | |
Stock option exercises | | | 1,502 | | | | 2 | | | | 22,444 | | | | 0 | | | | 0 | | | | 22,446 | |
Restricted stock vested during the period, net | | | 535 | | | | 1 | | | | (1 | ) | | | 0 | | | | 0 | | | | 0 | |
Issuance of common stock | | | 13,463 | | | | 13 | | | | 526,605 | | | | 0 | | | | 0 | | | | 526,618 | |
Share-based compensation | | | 0 | | | | 0 | | | | 41,836 | | | | 0 | | | | 0 | | | | 41,836 | |
Balances at December 31, 2015 | | | 139,674 | | | | 140 | | | | 1,613,383 | | | | (683 | ) | | | (926,929 | ) | | | 685,911 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Net loss | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | | (140,111 | ) | | | (140,111 | ) |
Other comprehensive income | | | 0 | | | | 0 | | | | 0 | | | | 620 | | | | 0 | | | | 620 | |
Issuance of common stock for employee stock purchase plan | | | 203 | | | | 0 | | | | 5,686 | | | | 0 | | | | 0 | | | | 5,686 | |
Stock option exercises | | | 1,778 | | | | 1 | | | | 29,509 | | | | 0 | | | | 0 | | | | 29,510 | |
Restricted stock vested during the period, net | | | 538 | | | | 1 | | | | (1 | ) | | | 0 | | | | 0 | | | | 0 | |
Share-based compensation | | | 0 | | | | 0 | | | | 52,471 | | | | 0 | | | | 0 | | | | 52,471 | |
Balances at December 31, 2016 | | | 142,193 | | | | 142 | | | | 1,701,048 | | | | (63 | ) | | | (1,067,040 | ) | | | 634,087 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Net loss | | | 0 | | | | 0 | | | | 0 | | | | 0 | | | | (125,530 | ) | | | (125,530 | ) |
Other comprehensive income | | | 0 | | | | 0 | | | | 0 | | | | 63,899 | | | | 0 | | | | 63,899 | |
Issuance of common stock for employee stock purchase plan | | | 172 | | | | 0 | | | | 7,303 | | | | 0 | | | | 0 | | | | 7,303 | |
Stock option exercises | | | 1,494 | | | | 1 | | | | 34,007 | | | | 0 | | | | 0 | | | | 34,008 | |
Restricted stock vested during the period, net | | | 536 | | | | 1 | | | | (1 | ) | | | 0 | | | | 0 | | | | 0 | |
Share-based compensation | | | 0 | | | | 0 | | | | 63,802 | | | | 0 | | | | 0 | | | | 63,802 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balances at December 31, 2017 | | | 144,395 | | | $ | 144 | | | $ | 1,806,159 | | | $ | 63,836 | | | $ | (1,192,570 | ) | | $ | 677,569 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
105
Seattle Genetics, Inc.
Consolidated Statements of Cash Flows
(In thousands)
| | | | | | | | | | | | |
| | | Years ended December 31, | |
| | | 2017 | | | 2016 | | | 2015 | |
Operating activities | | | | | | | | | | | | |
Net loss | | $ | (125,530 | ) | | $ | (140,111 | ) | | $ | (120,486 | ) |
Adjustments to reconcile net loss to net cash used in operating activities | | | | | | | | | | | | |
Share-based compensation | | | 63,802 | | | | 52,471 | | | | 41,836 | |
Depreciation and amortization | | | 24,269 | | | | 18,034 | | | | 14,505 | |
Amortization of premiums, accretion of discounts and (gain) loss on
investments | | | 497 | | | | 4,746 | | | | 2,846 | |
Net gain on Immunomedics warrant derivative | | | (33,777 | ) | | | 0 | | | | 0 | |
Income tax benefit on unrealized gain onavailable-for-sale securities | | | (33,357 | ) | | | 0 | | | | 0 | |
Deferred rent and other long-term liabilities | | | (154 | ) | | | (882 | ) | | | (921 | ) |
Changes in operating assets and liabilities | | | | | | | | | | | | |
Accounts receivable, net | | | (22,846 | ) | | | (8,998 | ) | | | (13,682 | ) |
Inventories | | | 8,146 | | | | (11,161 | ) | | | (13,512 | ) |
Prepaid expenses and other assets | | | (2,170 | ) | | | (4,378 | ) | | | (1,952 | ) |
Accounts payable and accrued liabilities | | | 19,098 | | | | 29,939 | | | | 6,539 | |
Deferred revenue | | | (16,878 | ) | | | (36,631 | ) | | | (48,376 | ) |
| | | | | | | | | | | | |
Net cash used in operating activities | | | (118,900 | ) | | | (96,971 | ) | | | (133,203 | ) |
| | | | | | | | | | | | |
Investing activities | | | | | | | | | | | | |
Purchases ofavailable-for-sale securities | | | (513,016 | ) | | | (603,772 | ) | | | (754,663 | ) |
Proceeds from maturities ofavailable-for-sale securities | | | 653,200 | | | | 699,800 | | | | 367,200 | |
Proceeds from sales ofavailable-for-sale securities | | | 60,056 | | | | 0 | | | | 30,005 | |
Purchases of property and equipment | | | (28,722 | ) | | | (27,835 | ) | | | (13,392 | ) |
Acquisition of manufacturing facility | | | (41,657 | ) | | | 0 | | | | 0 | |
Purchase of cost-method investment | | | 0 | | | | 0 | | | | (5,000 | ) |
| | | | | | | | | | | | |
Net cash provided by (used in) investing activities | | | 129,861 | | | | 68,193 | | | | (375,850 | ) |
| | | | | | | | | | | | |
Financing activities | | | | | | | | | | | | |
Net proceeds from issuance of common stock | | | 0 | | | | 0 | | | | 526,618 | |
Proceeds from exercise of stock options and employee stock purchase plan | | | 41,311 | | | | 35,196 | | | | 27,763 | |
| | | | | | | | | | | | |
Net cash provided by financing activities | | | 41,311 | | | | 35,196 | | | | 554,381 | |
| | | | | | | | | | | | |
Net increase in cash and cash equivalents | | | 52,272 | | | | 6,418 | | | | 45,328 | |
Cash and cash equivalents at beginning of year | | | 108,673 | | | | 102,255 | | | | 56,927 | |
| | | | | | | | | | | | |
Cash and cash equivalents at end of year | | $ | 160,945 | | | $ | 108,673 | | | $ | 102,255 | |
| | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
106
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements
1. Organization and Business
Organization
The Company is a biotechnology company focused on the development and commercialization of targeted therapies for the treatment of cancer. The Company’s marketed product ADCETRIS®, or brentuximab vedotin, is approved for four indications encompassing several settings for the treatment of relapsed Hodgkin lymphoma, for relapsed systemic anaplastic large cell lymphoma, or sALCL, and for certain types of cutaneousT-cell lymphoma, or CTCL. ADCETRIS is commercially available in 70 countries, including in the United States, Canada, members of the European Union and Japan. The Company is also advancing a pipeline of novel therapies for solid tumors and blood-related cancers designed to address unmet medical needs and improve treatment outcomes for patients.
Capital requirements
To execute the Company’s growth plans, it may need to seek additional funding through public or private financings, including debt or equity financings, and through other means, including collaborations and license agreements. If the Company cannot maintain adequate funds, it may be required to borrow funds, delay, reduce the scope of or eliminate one or more of its development programs. Additional financing may not be available when needed, or if available, the Company may not be able to obtain financing on favorable terms.
2. Summary of Significant Accounting Policies
Basis of presentation
The accompanying consolidated financial statements reflect the accounts of Seattle Genetics, Inc. and its wholly-owned subsidiaries (collectively “Seattle Genetics” or the “Company”). The consolidated financial statements have been prepared in accordance with U.S. generally accepted accounting principles, or GAAP. All significant intercompany transactions and balances have been eliminated. Management has determined that the Company operates in one segment: the development and sale of pharmaceutical products on its own behalf or in collaboration with others. Substantially all of the Company’s assets and revenues are related to operations in the United States; however, the Company also has subsidiaries in Canada, Switzerland, and the United Kingdom.
Use of estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts in the financial statements and accompanying notes. Actual results could differ from those estimates. Estimates include those used for revenue recognition, valuation of investments, inventory valuation, accrued liabilities (including those related to the long-term incentive plans, clinical trials and contingencies), stock option valuation, and valuation allowance for deferred tax assets.
Cash and cash equivalents
The Company considers all highly liquid investments with maturities of three months or less at the date of acquisition to be cash equivalents.
Non-cash investing activities
The Company had $1.0 million and $8.1 million of accrued capital expenditures as of December 31, 2017 and 2016, respectively. Accrued capital expenditures have been treated as anon-cash investing activity and, accordingly, have not been included in the statement of cash flows until such amounts have been paid in cash.
As further described in Note 8, the Company exercised a warrant to purchase additional shares of common stock in Immunomedics, Inc., or Immunomedics, in 2017. The fair value of the warrant derivative on the exercise date represented anon-cash investing activity and, accordingly, has not been included in the statement of cash flows.
107
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
Investments
The Company invests primarily in debt securities. In addition, as of December 31, 2017, the Company held an equity investment in the common stock of Immunomedics, as further described in Note 8. These debt and equity securities are classified asavailable-for-sale, which are reported at estimated fair value with unrealized gains and losses included in accumulated other comprehensive income and loss in stockholders’ equity. Realized gains, realized losses and declines in the value of investments judged to be other-than-temporary, are included in investment and other income, net. The cost of investments for purposes of computing realized and unrealized gains and losses is based on the specific identification method. Amortization of premiums and accretion of discounts on debt securities are included in investment and other income, net. Interest and dividends earned on all securities are included in investment and other income, net. The Company classifies investments in debt securities maturing within one year of the reporting date, or where management’s intent is to use the investments to fund current operations or to make them available for current operations, as short-term investments. The Company classifies its equity investment in Immunomedics in othernon-current assets.
If the estimated fair value of a security is below its carrying value, the Company evaluates whether it is more likely than not that it will sell the security before its anticipated recovery in market value and whether evidence indicating that the cost of the investment is recoverable within a reasonable period of time outweighs evidence to the contrary. The Company also evaluates whether or not it intends to sell the investment. If the impairment is considered to be other-than-temporary, the security is written down to its estimated fair value. In addition, the Company considers whether credit losses exist for any securities. A credit loss exists if the present value of cash flows expected to be collected is less than the amortized cost basis of the security. Other-than-temporary declines in estimated fair value and credit losses are charged against investment and other income, net.
Derivative financial instruments
The Company accounts for financial instruments as derivatives when the instrument includes an underlying and notional amount or payment provision, an initial net investment, and a net settlement. Derivative financial instruments are measured at fair value on the issuance date and are revalued on each subsequent balance sheet date. The Company uses the Black-Scholes model using observable market inputs to estimate the fair value of derivatives. The changes in estimated fair value are recognized as current period income or loss. The Company does not hold derivative instruments for trading or speculative purposes and had no derivative instruments outstanding as of December 31, 2017 or 2016.
Fair value of financial instruments
The recorded amounts of certain financial instruments, including cash and cash equivalents, interest receivable, accounts receivable, accounts payable and accrued liabilities approximate fair value due to their relatively short maturities. Investments that are classified asavailable-for-sale are recorded at estimated fair value. The estimated fair value for securities held is determined using quoted market prices, broker or dealer quotations, or alternative pricing sources with reasonable levels of price transparency.
Inventories
The Company considers regulatory approval of product candidates to be uncertain. Accordingly, it charges manufacturing costs to research and development expense until such time as a product has received regulatory approval for commercial sale. Production costs for the Company’s marketed product, ADCETRIS, are capitalized into inventory. ADCETRIS inventory that is deployed for clinical, research or development use is charged to research and development expense when it is no longer available for commercial sales. Production costs for the Company’s other product candidates continue to be charged to research and development expense.
108
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
The Company values its inventories at the lower of cost or market value. Cost is determined on a specific identification basis. Inventory includes the cost of materials, third-party contract manufacturing and overhead associated with the production of ADCETRIS. In the event that the Company identifies excess, obsolete or unsalable inventory, its value is written down to net realizable value.
Property and equipment
Property and equipment are stated at cost. Land is not depreciated, while all other property and equipment are depreciated using the straight-line method over the estimated useful lives of the assets, which are generally as follows:
| | | | |
| | | Years | |
Building | | | 30 | |
Laboratory and manufacturing equipment | | | 5-15 | |
Furniture and fixtures | | | 5 | |
Computers, software and office equipment | | | 3 | |
Leasehold improvements are amortized over the shorter of the remaining term of the applicable lease or the useful life of the asset. Gains and losses from the disposal of property and equipment are reflected in income or loss at the time of disposition and have not been significant. Expenditures for additions and improvements to the Company’s facilities are capitalized and expenditures for maintenance and repairs are charged to expense as incurred. Concessions received by the Company in connection with leases, including tenant improvement allowances and prorated rent, are included in deferred rent and other long-term liabilities and recognized as a reduction in rent expense over the term of the applicable lease.
Othernon-current assets
Othernon-current assets included:
| | • | | A $188.4 million investment in the common stock of Immunomedics as of December 31, 2017. See Notes 3, 4 and 8 for additional information. |
| | • | | A $5.0 millionnon-controlling investment in a privately-held company that is accounted for under the cost method of accounting as of December 31, 2017 and 2016. The Company periodically evaluates the carrying value of the investment if significant adverse events or circumstances indicate an impairment in value. |
| | • | | Intangible assets resulting from milestone payments that became due upon the approval of ADCETRIS related to certainin-licensed technology. Intangible assets are amortized to cost of sales over the estimated life of the related licenses, which range from six to ten years. |
| | | | | | | | |
| | | December 31, | |
| | | 2017 | | | 2016 | |
Intangible assets | | $ | 5,650 | | | $ | 5,650 | |
Less: accumulated amortization | | | (4,886 | ) | | | (4,115 | ) |
| | | | | | | | |
Total | | $ | 764 | | | $ | 1,535 | |
| | | | | | | | |
Amortization expense on intangible assets was $0.8 million for each of the years ended December 31, 2017, 2016, and 2015, respectively. Assuming no changes in the cost basis of intangible assets, the estimated aggregate amortization for the next five years will total $0.8 million.
109
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
Impairment of long-lived assets
The Company assesses the impairment of long-lived assets, primarily property and equipment and intangible assets, whenever events or changes in business circumstances indicate that the carrying amounts of the assets may not be fully recoverable. When such events occur, management determines whether there has been an impairment in value by comparing the asset’s carrying value with its fair value, as measured by the anticipated undiscounted net cash flows of the asset. If an impairment in value exists, the asset is written down to its estimated fair value. The Company has not recognized any impairment losses through December 31, 2017 as there have been no events warranting an impairment analysis. The Company’s long-lived assets are primarily located in the United States.
Revenue recognition
The Company’s revenues are comprised of ADCETRIS net product sales, amounts earned under its collaboration and licensing agreements and royalties. Revenue recognition is predicated upon persuasive evidence of an agreement existing, delivery of products or services being rendered, amounts payable being fixed or determinable, and collectibility being reasonably assured.
Net product sales
The Company sells ADCETRIS through a limited number of pharmaceutical distributors in the U.S. and Canada. Customers order ADCETRIS through these distributors and the Company typically ships product directly to the customer. The Company records product sales when title and risk of loss pass, which generally occurs upon delivery of the product to the customer. Product sales are recorded net of estimated government-mandated rebates and chargebacks, distribution fees, estimated product returns and other deductions. Accruals are established for these deductions and actual amounts incurred are offset against applicable accruals. The Company reflects these accruals as either a reduction in the related account receivable from the distributor, or as an accrued liability depending on the nature of the sales deduction. Sales deductions are based on management’s estimates that consider payer mix in target markets and experience to date. These estimates involve a substantial degree of judgment.
Government-mandated rebates and chargebacks: The Company has entered into a Medicaid Drug Rebate Agreement, or MDRA, with the Centers for Medicare & Medicaid Services. This agreement provides for a rebate based on covered purchases of ADCETRIS. Medicaid rebates are invoiced to the Company by the various state Medicaid programs. The Company estimates Medicaid rebates based on a variety of factors, including its experience to date. The Company has also completed a Federal Supply Schedule, or FSS, agreement under which certain U.S. government purchasers receive a discount on eligible purchases of ADCETRIS. The Company has entered into a Pharmaceutical Pricing Agreement with the Secretary of Health and Human Services, which enables certain entities that qualify for government pricing under the Public Health Services Act, or PHS, to receive discounts on their qualified purchases of ADCETRIS. Under these agreements, distributors process a chargeback to the Company for the difference between wholesale acquisition cost and the applicable discounted price. As a result of the Company’s direct-ship distribution model, it can identify the entities purchasing ADCETRIS and this information enables the Company to estimate expected chargebacks for FSS and PHS purchases based on each entity’s eligibility for the FSS and PHS programs. The Company also reviews historical rebate and chargeback information to further refine these estimates.
Distribution fees, product returns and other deductions: The Company’s distributors charge a volume-based fee for distribution services that they perform for the Company. The Company allows for the return of product that is within 30 days of its expiration date or that is damaged. The Company estimates product returns based on its experience to date. In addition, the Company considers its direct-ship distribution model, its belief
110
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
that product is not typically held in the distribution channel, and the expected rapid use of the product by healthcare providers. The Company provides financial assistance to qualifying patients that are underinsured or cannot cover the cost of commercial coinsurance amounts through SeaGen Secure. SeaGen Secure is available to patients in the U.S. and its territories who meet various financial and treatment need criteria. Estimated contributions for commercial coinsurance under SeaGen Secure are deducted from gross sales and are based on an analysis of expected plan utilization. These estimates are adjusted as necessary to reflect the Company’s actual experience.
Collaboration and license agreement revenues
The Company has collaboration and license agreements with a number of biotechnology and pharmaceutical companies. The Company’s proprietary technology for linking cytotoxic agents to monoclonal antibodies called antibody-drug conjugates, or ADCs, is the basis for many of these collaboration and license agreements, including the ADC collaborations that the Company has entered into in the ordinary course of business, under which the Company grants its collaborators research and commercial licenses to the Company’s technology and typically provides technology transfer services, technical advice, supplies and services for a period of time.
If there are continuing performance obligations, the Company uses a time-based proportional performance model to recognize revenue over the Company’s performance period for the related agreement when no other discernible pattern exists. Collaboration and license agreements are evaluated to determine whether the multiple elements and associated deliverables can be considered separate units of accounting. To date, thepre-commercial deliverables under the Company’s collaboration and license agreements have not qualified as separate units of accounting. The assessment of multiple element arrangements requires judgment in order to determine the appropriate point in time, or period of time, that revenue should be recognized. The Company believes that the development period in each agreement is a reasonable estimate of the performance obligation period of such agreement. Accordingly, all amounts received or due, including any upfront payments, maintenance fees, development and regulatory milestone payments and reimbursement payments, are recognized as revenue over the performance obligation periods of each agreement. These performance obligation periods typically range from one to three years. The agreements with Takeda Pharmaceutical Company Limited, or Takeda, and Genentech, Inc., a member of the Roche Group, or Genentech, have performance obligation periods of ten and seventeen years, respectively. All of the remaining performance obligation periods for active collaborations are currently expected to be completed in three years or less. When no performance obligations are required of the Company, or following the completion of the performance obligation period, such amounts are recognized as revenue when collectibility is reasonably assured. Generally, all amounts received or due other than sales-based milestones and royalties are classified as collaboration and license agreement revenues as they are earned. Sales-based milestones and royalties are recognized as royalty revenue as they are reported to the Company.
The Company’s collaboration and license agreements include contractual milestones. Generally, the milestone events contained in the Company’s collaboration and license agreements coincide with the progression of the collaborators’ product candidates from development, to regulatory approval and then to commercialization and fall into the following categories.
Development milestones in the Company’s collaborations may include the following types of events:
| | • | | Designation of a product candidate or initiation of preclinical studies. The Company’s collaborators must undertake significant preclinical research and studies to make a determination of the suitability of a product candidate and the time from those studies or designation to initiation of a clinical trial may take several years. |
| | • | | Initiation of a phase 1 clinical trial. Generally, phase 1 clinical trials may take one to two years to complete. |
111
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
| | • | | Initiation of a phase 2 clinical trial. Generally, phase 2 clinical trials may take one to three years to complete. |
| | • | | Initiation of a phase 3 clinical trial. Generally, phase 3 clinical trials may take two to six years to complete. |
Regulatory milestones in the Company’s collaborations may include the following types of events:
| | • | | Filing of regulatory applications for marketing approval such as a Biologics License Application in the United States or a Marketing Authorization Application in Europe. Generally, it may take up to twelve months to prepare and submit regulatory filings. |
| | • | | Receiving marketing approval in a major market, such as in the United States, Europe, Japan or other significant countries. Generally, it may take up to three years after a marketing application is submitted to obtain approval for marketing and pricing from the applicable regulatory agency. |
Commercialization milestones in the Company’s collaborations may include the following types of events:
| | • | | First commercial sale in a particular market, such as in the United States, Europe, Japan or other significant countries. |
| | • | | Product sales in excess of apre-specified threshold. The amount of time to achieve this type of milestone depends on several factors, including, but not limited to, the dollar amount of the threshold, the pricing of the product, market penetration of the product and the rate at which customers begin using the product. |
The Company’s ADC collaborators are solely responsible for the development of their product candidates and the achievement of milestones in any of the categories identified above is based solely on the collaborators’ efforts.
In the case of the Company’s ADCETRIS collaboration with Takeda, the Company may be involved in certain development activities; however, the achievement of milestone events under the agreement is primarily based on activities undertaken by Takeda.
The process of successfully developing a product candidate, obtaining regulatory approval and ultimately commercializing a product candidate is highly uncertain and the attainment of any milestones is therefore uncertain and difficult to predict. In addition, since the Company does not take a substantive role or control the research, development or commercialization of any products generated by its ADC collaborators, the Company is not able to reasonably estimate when, if at all, any milestone payments or royalties may be payable to the Company by its ADC collaborators. As such, the milestone payments associated with its ADC collaborations involve a substantial degree of uncertainty and risk that they may never be received. Similarly, even in those collaborations where the Company may have an active role in the development of the product candidate, such as the Company’s ADCETRIS collaboration with Takeda, the attainment of a milestone is based on the collaborator’s activities and is generally outside the direction and control of the Company.
The Company generally invoices its collaborators and licensees on a monthly or quarterly basis, or upon the completion of the effort or achievement of a milestone, based on the terms of each agreement. Deferred revenue arises from amounts received in advance of the culmination of the earnings process and is recognized as revenue in future periods when the applicable revenue recognition criteria have been met. Deferred revenue expected to be recognized within the next twelve months is classified as a current liability.
112
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
Royalty revenues and cost of royalty revenues
Royalty revenues primarily reflect amounts earned under the ADCETRIS collaboration with Takeda. These royalties include commercial sales-based milestones and sales royalties. Sales royalties are based on a percentage of Takeda’s net sales of ADCETRIS, with rates that range from themid-teens to themid-twenties based on sales volume. Takeda bears a portion of third-party royalty costs owed on its sales of ADCETRIS. This amount is included in royalty revenues. Cost of royalty revenues reflects amounts owed to the Company’s third-party licensors related to Takeda’s sales of ADCETRIS. These amounts are recognized in the quarter in which Takeda reports its sales activity to the Company, which is the quarter following the related sales. Royalty revenues also include amounts earned in connection with the Company’s ADC collaborations.
Research and development expenses
Research and development, or R&D, expenses consist of salaries, benefits and other headcount-related costs of the Company’s R&D staff, preclinical activities, clinical trials and related manufacturing costs, lab supplies, contract and outside service fees and facilities and overhead expenses for research, development and preclinical studies focused on drug discovery, development and testing. R&D activities are expensed as incurred.
Clinical trial expenses are a significant component of research and development expenses, and the Company outsources a significant portion of these costs to third parties. Third party clinical trial expenses include investigator fees, site costs, clinical research organization costs, and costs for central laboratory testing and data management. Costs associated with activities performed underco-development collaborations are reflected in R&D expense.In-licensing fees, milestones, maintenance fees and other costs to acquire technologies utilized in R&D for product candidates that have not yet received regulatory approval and that are not expected to have alternative future use are expensed when incurred.Non-refundable advance payments for goods or services that will be used or rendered for future R&D activities are capitalized and recognized as expense as the related goods are delivered or the related services are performed. This results in the temporary deferral of recording expense for amounts incurred for research and development activities from the time payments are made until the time goods or services are provided.
Advertising
Advertising costs are expensed as incurred. The Company incurred $13.8 million, $12.9 million, and $16.4 million in advertising expense during 2017, 2016, and 2015, respectively.
Concentration of credit risk
Cash, cash equivalents and investments are invested in accordance with the Company’s investment policy. The policy includes guidelines for the investment of cash reserves and is reviewed periodically to minimize credit risk. Most of the Company’s investments are not federally insured. The Company has accounts receivable from the sale of ADCETRIS from a small number of distributors. The Company does not require collateral on amounts due from its distributors or its collaborators and is therefore subject to credit risk. The Company has not experienced any significant credit losses to date as a result of credit risk concentration and does not consider an allowance for doubtful accounts to be necessary.
Major customers
The Company sells ADCETRIS through a limited number of distributors. Certain of these distributors, together with entities under their common control, each individually accounted for greater than 10% of total revenues and greater than 10% of accounts receivable as noted below. In addition, one of the Company’s collaborators accounted for greater than 10% of total revenues as noted below. Revenues generated outside the United States, as determined by customer location, were less than 10% of total revenues for all years presented.
113
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
The following table presents each major distributor or collaborator that comprised more than 10% of total revenue:
| | | | | | | | | | | | |
| | | Years ended December 31, | |
| | | 2017 | | | 2016 | | | 2015 | |
Distributor A | | | 23 | % | | | 22 | % | | | 24 | % |
Distributor B | | | 19 | % | | | 19 | % | | | 21 | % |
Distributor C | | | 18 | % | | | 17 | % | | | 18 | % |
Collaborator A | | | 29 | % | | | 27 | % | | | 17 | % |
The following table presents each major distributor that accounted for more than 10% of accounts receivable:
| | | | | | | | |
| | | December 31, | |
| | | 2017 | | | 2016 | |
Distributor A | | | 32 | % | | | 34 | % |
Distributor B | | | 26 | % | | | 26 | % |
Distributor C | | | 29 | % | | | 26 | % |
Major suppliers
The use of a relatively small number of contract manufacturers to supply drug product necessary for the Company’s commercial operations and clinical trials creates a concentration of risk for the Company. While primarily one source of supply is utilized for certain components of ADCETRIS and each of the Company’s product candidates, other sources are available should the Company need to change suppliers. The Company also endeavors to maintain reasonable levels of drug supply for its use. A change in suppliers, however, could cause a delay in delivery of drug product which could result in the interruption of commercial operations or clinical trials. Such an event would adversely affect the Company’s business.
Income taxes
The Company recognizes deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Deferred tax assets and liabilities are determined based on the differences between the financial statement and tax bases of assets and liabilities using tax rates in effect for the year in which the differences are expected to reverse. The Company has provided a full valuation allowance against its deferred tax assets for all periods presented. A valuation allowance is recorded when it is more likely than not that the net deferred tax asset will not be realized. The Company follows the guidance related to accounting for uncertainty in income taxes, which requires the recognition of an uncertain tax position when it is more likely than not to be sustainable upon audit by the applicable taxing authority.
Share-based compensation
The Company uses the graded-vesting attribution method for recognizing compensation expense for its stock options and the straight-line method for recognizing compensation expense for its restricted stock units (“RSUs”). Compensation expense is recognized over the requisite service periods on awards ultimately expected to vest and reduced for forfeitures that are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. For performance-based stock options and awards, the Company records compensation expense over the estimated service period once the achievement of the performance-based milestone is considered probable. At each reporting date, the Company assesses whether achievement of a milestone is considered probable, and if so, records compensation expense based on the portion
114
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
of the service period elapsed to date with respect to that milestone, with a cumulativecatch-up, net of estimated forfeitures. The Company will recognize remaining compensation expense with respect to a milestone, if any, over the remaining estimated service period.
Long-term incentive plans
The Company has established Long-Term Incentive Plans, or LTIPs. The LTIPs provide eligible employees with the opportunity to receive performance-based incentive compensation, which may be comprised of cash, stock options, and/or restricted stock units. The payment of cash and the grant or vesting of equity are contingent upon the achievement ofpre-determined regulatory milestones. The Company records compensation expense over the estimated service period for each milestone subject to the achievement of the milestone being considered probable in accordance with the provisions of ASC 450, Contingencies. At each reporting date, the Company assesses whether achievement of a milestone is considered probable, and if so, records compensation expense based on the portion of the service period elapsed to date with respect to that milestone, with a cumulativecatch-up, net of estimated forfeitures. The Company recognizes compensation expense with respect to a milestone over the remaining estimated service period.
The total estimate of unrecognized compensation expense is expected to change in the future for several reasons, including the addition of more eligible employees, or the addition, termination, or modification of an LTIP.
Comprehensive loss
Comprehensive loss is the change in stockholders’ equity from transactions and other events and circumstances other than those resulting from investments by stockholders and distributions to stockholders. The Company’s comprehensive loss is comprised of net loss, unrealized gains and losses onavailable-for-sale investments, and foreign currency translation adjustments, net of any applicable income taxes.
Loss contingencies
The Company is involved in various legal proceedings in the normal course of its business. A loss contingency is recorded if it is probable that an asset has been impaired or a liability has been incurred and the amount of the loss can be reasonably estimated. The Company evaluates, among other factors, the probability of an unfavorable outcome and its ability to make a reasonable estimate and the amount of the ultimate loss. Loss contingencies that are determined to be reasonably possible, but not probable, are disclosed but not recorded. Legal fees incurred as a result of the Company’s involvement in legal procedures are expensed as incurred.
Certain risks and uncertainties
The Company’s revenues are derived from ADCETRIS sales and royalties and from collaboration and license agreements. ADCETRIS is the Company’s only product available for sale and is subject to regulation by the FDA in the United States and other regulatory agencies outside the United States as well as competition by other pharmaceutical companies. The Company’s collaboration and license agreement revenues are derived from a relatively small number of agreements. Each of these agreements can be terminated by the Company’s collaborators at their discretion. The Company is also subject to risks common to companies in the pharmaceutical industry, including risks and uncertainties related to commercial success and acceptance of ADCETRIS and the Company’s potential future products by patients, physicians and payers, competition from other products, regulatory approvals, regulatory requirements, business combinations and product or product candidate acquisition andin-licensing transactions, and protection of intellectual property. Also, drug development is a lengthy process characterized by a relatively low rate of success. The Company may commit
115
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
substantial resources toward developing product candidates that never result in further development, achieve regulatory approvals or achieve commercial success. Likewise, the Company has committed and expects to continue to commit substantial resources towards additional clinical development of ADCETRIS in an effort to continue to expand ADCETRIS’ labeled indications of use, and there can be no assurance that the Company and/or Takeda will obtain and maintain the necessary regulatory approvals to market ADCETRIS for any additional indications.
Guarantees
In the normal course of business, the Company indemnifies its directors, certain employees and other parties, including distributors, collaboration partners, lessors and other parties that perform certain work on behalf of, or for the Company or take licenses to the Company’s technologies. The Company has agreed to hold these parties harmless against losses arising from the Company’s breach of representations or covenants, intellectual property infringement or other claims made against these parties in performance of their work with the Company. These agreements typically limit the time within which the party may seek indemnification by the Company and the amount of the claim. It is not possible to prospectively determine the maximum potential amount of liability under these indemnification agreements. Further, each potential claim would be based on the unique facts and circumstances of the claim and the particular provisions of each agreement.
Net loss per share
Basic and diluted net loss per share is computed by dividing net loss by the weighted average number of common shares outstanding during the period. The Company excluded all RSUs and options to purchase common stock from the calculation of basic and diluted net loss per share as such securities are anti-dilutive for all periods presented.
The following table presents the weighted-average shares that have been excluded from the number of shares used to calculate basic and diluted net loss per share as their effect is anti-dilutive (in thousands):
| | | | | | | | | | | | |
| | | Years ended December 31, | |
| | | 2017 | | | 2016 | | | 2015 | |
Stock options and RSUs | | | 13,592 | | | | 12,987 | | | | 11,953 | |
Recent accounting pronouncements
In May 2014, the Financial Accounting Standards Board, or FASB, issued an Accounting Standards Update entitled “ASU2014-09, Revenue from Contracts with Customers,” which was followed by additional related updates on various revenue recognition topics. The standard requires entities to recognize revenue through an evaluation that includes identification of the contract, identification of the performance obligations, determination of the transaction price, allocation of the transaction price to the performance obligations, and recognition of revenue as the entity satisfies the performance obligations. The Company has completed its assessment and concluded that this standard will generally not change the way in which the Company recognizes product revenue from sales of ADCETRIS. However, sales-based royalties and commercial sales-based milestones will be recorded in the period of the related sale based on estimates, rather than recording them as reported by the customer. In addition, the achievement of development milestones under the Company’s collaborations will be recorded in the period their achievement becomes probable, which will result in their recognition earlier than under current accounting principles. The new standard also requires more extensive disclosures related to revenue recognition, particularly in quarterly financial statements. The Company adopted the standard on January 1, 2018 using the modified retrospective method of adoption, which resulted in a cumulative effect adjustment to the opening balance of retained earnings. Based on the Company’s current
116
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
assessment, the Company expects a cumulative effect adjustment of approximately $27 million as an increase to retained earnings.
In January 2016, FASB issued an Accounting Standards Update entitled “ASU2016-01, Financial Instruments: Overall.” The standard addresses certain aspects of recognition, measurement, presentation and disclosure of financial instruments, including that changes in the fair value of equity securities be recorded in income or loss rather than accumulated other comprehensive income or loss in stockholders’ equity. The Company adopted the standard on January 1, 2018 using the modified retrospective approach, which resulted in a cumulative effect adjustment to the opening balance of retained earnings. Based on the Company’s current assessment, the Company expects a cumulative effect adjustment of approximately $64 million as an increase to retained earnings. The implementation of this standard is expected to increase the volatility of net income or loss to the extent that the Company continues to hold equity securities.
In February 2016, FASB issued an Accounting Standards Update entitled “ASU2016-02, Leases.” The standard requires entities to recognize in the consolidated balance sheet a liability to make lease payments and aright-of-use asset representing its right to use the underlying asset for the lease term. The standard will become effective for the Company beginning January 1, 2019, with early adoption permitted. The Company is currently evaluating the guidance to determine the potential impact on its financial condition, results of operations and cash flows, and financial statement disclosures, and expects that the adoption of the standard will increase assets and liabilities related to the Company’s operating leases in the consolidated balance sheets.
In March 2016, FASB issued an Accounting Standards Update entitled “ASU2016-09, Compensation – Stock Compensation.” The standard is intended to simplify certain elements of accounting for share-based payment transactions, including the income tax impact, classification of awards as either equity or liabilities, and classification on the statement of cash flows. In addition, the standard allows an entity-wide accounting policy election to either estimate the number of awards that are expected to vest, as currently required, or account for forfeitures when they occur. The Company has elected to continue estimating the number of awards that are expected to vest. The Company adopted the standard as of January 1, 2017. Since the Company has incurred annual net losses since its inception and maintains a full valuation allowance on its net deferred tax assets, the adoption did not have a material impact on the Company’s financial condition, results of operations and cash flows.
In June 2016, FASB issued an Accounting Standards Update entitled “ASU2016-13, Financial Instruments: Credit Losses.” The objective of the standard is to provide information about expected credit losses on financial instruments at each reporting date, and to change how other-than-temporary impairments on investments securities are recorded. The standard will become effective for the Company beginning on January 1, 2020, with early adoption permitted. The Company is currently evaluating the guidance to determine the potential impact on its financial condition, results of operations and cash flows, and financial statement disclosures.
117
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
3. Investments
Investments consisted ofavailable-for-sale securities as follows (in thousands):
| | | | | | | | | | | | | | | | |
| | | Amortized
cost | | | Gross
unrealized
gains | | | Gross
unrealized
losses | | | Fair
value | |
December 31, 2017 | | | | | | | | | | | | | | | | |
U.S. Treasury securities (debt securities) | | $ | 252,511 | | | $ | 0 | | | $ | (285 | ) | | $ | 252,226 | |
Common stock investment in Immunomedics (equity securities) | | | 90,882 | | | | 97,476 | | | | 0 | | | | 188,358 | |
| | | | | | | | | | | | | | | | |
Total | | $ | 343,393 | | | $ | 97,476 | | | $ | (285 | ) | | $ | 440,584 | |
| | | | | | | | | | | | | | | | |
Contractual maturities of debt securities (at date of purchase) | | | | | | | | | | | | | | | | |
Due in one year or less | | $ | 252,511 | | | | | | | | | | | $ | 252,226 | |
Due in one to two years | | | 0 | | | | | | | | | | | | 0 | |
| | | | | | | | | | | | | | | | |
Total | | $ | 252,511 | | | | | | | | | | | $ | 252,226 | |
| | | | | | | | | | | | | | | | |
December 31, 2016 | | | | | | | | | | | | | | | | |
U.S. Treasury securities (debt securities) | | $ | 510,356 | | | $ | 68 | | | $ | (123 | ) | | $ | 510,301 | |
| | | | | | | | | | | | | | | | |
Contractual maturities of debt securities (at date of purchase) | | | | | | | | | | | | | | | | |
Due in one year or less | | $ | 229,856 | | | | | | | | | | | $ | 229,864 | |
Due in one to two years | | | 280,500 | | | | | | | | | | | | 280,437 | |
| | | | | | | | | | | | | | | | |
Total | | $ | 510,356 | | | | | | | | | | | $ | 510,301 | |
| | | | | | | | | | | | | | | | |
4. Fair Value
The Company has certain assets that are measured at fair value on a recurring basis according to a fair value hierarchy that prioritizes the inputs, assumptions and valuation techniques used to measure fair value. The three levels of the fair value hierarchy are:
| | |
Level 1: | | Unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities. |
Level 2: | | Quoted prices in markets that are not active or financial instruments for which all significant inputs are observable, either directly or indirectly. |
Level 3: | | Prices or valuations that require inputs that are both significant to the fair value measurement and unobservable. |
The determination of a financial instrument’s level within the fair value hierarchy is based on an assessment of the lowest level of any input that is significant to the fair value measurement. The Company considers observable data to be market data which is readily available, regularly distributed or updated, reliable and verifiable, not proprietary, and provided by independent sources that are actively involved in the relevant market.
118
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
The fair value hierarchy of the Company’s assets carried at fair value and measured on a recurring basis was as follows (in thousands):
| | | | | | | | | | | | | | | | |
| | | Fair value measurement using: | |
| | | Quoted prices
in active
markets for
identical
assets
(Level 1) | | | Other
observable
inputs
(Level 2) | | | Significant
unobservable
inputs
(Level 3) | | | Total | |
December 31, 2017 | | | | | | | | | | | | | | | | |
Short-term investments—U.S. Treasury securities | | $ | 252,226 | | | $ | 0 | | | $ | 0 | | | $ | 252,226 | |
Othernon-current assets—Common stock investment in Immunomedics | | | 188,358 | | | | 0 | | | | 0 | | | | 188,358 | |
| | | | | | | | | | | | | | | | |
Total | | $ | 440,584 | | | $ | 0 | | | $ | 0 | | | $ | 440,584 | |
| | | | | | | | | | | | | | | | |
December 31, 2016 | | | | | | | | | | | | | | | | |
Short-term investments—U.S. Treasury securities | | $ | 480,313 | | | $ | 0 | | | $ | 0 | | | $ | 480,313 | |
Long-term investments—U.S. Treasury securities | | | 29,988 | | | | 0 | | | | 0 | | | | 29,988 | |
| | | | | | | | | | | | | | | | |
Total | | $ | 510,301 | | | $ | 0 | | | $ | 0 | | | $ | 510,301 | |
| | | | | | | | | | | | | | | | |
5. Inventories
The following table presents the Company’s inventories of ADCETRIS (in thousands):
| | | | | | | | |
| | | December 31, | |
| | | 2017 | | | 2016 | |
Raw materials | | $ | 52,398 | | | $ | 62,516 | |
Work in process | | | 0 | | | | 8 | |
Finished goods | | | 7,580 | | | | 5,600 | |
| | | | | | | | |
Total | | $ | 59,978 | | | $ | 68,124 | |
| | | | | | | | |
6. Property and equipment
Property and equipment consisted of the following (in thousands):
| | | | | | | | |
| | | December 31, | |
| | | 2017 | | | 2016 | |
Leasehold improvements | | $ | 86,778 | | | $ | 77,133 | |
Laboratory and manufacturing equipment | | | 57,800 | | | | 37,639 | |
Building | | | 23,448 | | | | 0 | |
Computers, software and office equipment | | | 20,928 | | | | 15,076 | |
Furniture and fixtures | | | 6,627 | | | | 6,598 | |
Land | | | 4,771 | | | | 0 | |
| | | | | | | | |
| | | 200,352 | | | | 136,446 | |
Less: accumulated depreciation and amortization | | | (96,596 | ) | | | (73,576 | ) |
| | | | | | | | |
Total | | $ | 103,756 | | | $ | 62,870 | |
| | | | | | | | |
119
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
Depreciation and amortization expenses on property and equipment totaled $23.5 million, $17.3 million, and $13.7 million for the years ended December 31, 2017, 2016, and 2015, respectively. Leasehold improvements included $3.5 million and $17.8 million of construction in process at December 31, 2017 and 2016, respectively.
7. Manufacturing facility acquisition
Under a series of agreements among Bristol Myers Squibb Company, or BMS, and its landlord, the Company completed the acquisition of a biologics manufacturing facility and certain related equipment and improvements located in Bothell, Washington in October 2017. The purchase price was paid for in cash. The acquisition of the manufacturing facility and the related assets were accounted for as a business combination using the acquisition method. The results of operations of the manufacturing facility and the estimated fair values of the assets acquired and liability assumed have been included in the Company’s consolidated financial statements as of the closing date of the acquisition. Acquisition-related costs were not significant.
The Company also entered into a clinical manufacturing services agreement in October 2017 with BMS, under which the Company agreed to manufacture certain BMS clinical product candidates in accordance with prescribed production schedules and quantities through the later of December 31, 2018 or when certain technical transfer activities have been completed. The Company records revenue under the clinical manufacturing services agreement within collaboration and license agreement revenues. This revenue was not significant during the year ended December 31, 2017.
The purchase price was allocated to the assets acquired and liability assumed based on their estimated fair values as follows (in thousands):
| | | | |
Building | | $ | 23,448 | |
Land | | | 4,771 | |
Other property and equipment | | | 14,538 | |
Current portion of deferred revenue | | | (1,100 | ) |
| | | | |
Total purchase price | | $ | 41,657 | |
| | | | |
Pro forma results of operations have not been presented because the effects of this acquisition were not significant to the Company’s consolidated results of operations.
8. Immunomedics stock purchase agreement
In February 2017, the Company paid Immunomedics $14.7 million for 3.0 million shares of Immunomedics common stock and a warrant to purchase an additional 8.7 million shares of Immunomedics common stock at an exercise price of $4.90 per share, pursuant to a stock purchase agreement. The consideration was primarily allocated to the common stock based on the relative fair values as of the purchase date. The shares of common stock were classified asavailable-for-sale securities and carried at estimated fair value with unrealized gains and losses included in accumulated other comprehensive income and loss in stockholders’ equity.
In September 2017, Immunomedics registered the resale of the shares of its common stock underlying the warrant under the Securities Act of 1933, as amended, and as a result, the warrant met the definition of a derivative as of that date and was recorded at fair value. The Company recorded anon-cash net gain of $33.8 million in investment and other income, primarily resulting from the change in the fair value of the warrant derivative, during 2017. This amount approximated the fair value of the warrant derivative upon exercise in December 2017, when the Company exercised the warrant in its entirety for cash of $42.4 million and received 8.7 million shares of Immunomedics common stock upon exercise. The shares of
120
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
common stock that are held by the Company as a result of the warrant exercise, similar to the shares purchased in February 2017, were classified asavailable-for-sale securities and carried at estimated fair value with unrealized gains and losses included in accumulated other comprehensive income and loss in stockholders’ equity.
The common stock investment was included in othernon-current assets as of December 31, 2017.
9. Accounts payable and accrued liabilities
Accounts payable and accrued liabilities consisted of the following (in thousands):
| | | | | | | | |
| | | December 31, | |
| | | 2017 | | | 2016 | |
Employee compensation and benefits | | $ | 38,476 | | | $ | 29,670 | |
Trade accounts payable | | | 29,357 | | | | 29,005 | |
Clinical trial and related costs | | | 23,595 | | | | 21,006 | |
Contract manufacturing | | | 14,401 | | | | 22,008 | |
Third-party royalties and government rebates | | | 19,966 | | | | 12,351 | |
Other | | | 6,877 | | | | 6,629 | |
| | | | | | | | |
Total | | $ | 132,672 | | | $ | 120,669 | |
| | | | | | | | |
10. Income taxes
The Company’spre-tax loss by jurisdiction consisted of the following (in thousands):
| | | | | | | | | | | | |
| | | December 31, | |
| | | 2017 | | | 2016 | | | 2015 | |
U.S. | | $ | (71,698 | ) | | $ | (66,215 | ) | | $ | (95,860 | ) |
Foreign | | | (87,189 | ) | | | (73,896 | ) | | | (24,626 | ) |
| | | | | | | | | | | | |
Total | | $ | (158,887 | ) | | $ | (140,111 | ) | | $ | (120,486 | ) |
| | | | | | | | | | | | |
The Tax Cuts and Jobs Act, or the Act, was enacted on December 22, 2017, which reduced the U.S. federal corporate tax rate from 35% to 21%, among other changes. The Company’s accounting for the elements of the Act is complete and resulted in a $114.8 million reduction in its net deferred tax assets as of December 31, 2017 to reflect the new statutory rate. The rate adjustment to the deferred tax assets was fully offset by a decrease in the valuation allowance, resulting in no rate impact to the Company.
121
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
A reconciliation of the federal statutory income tax rate to the effective income tax rate is as follows:
| | | | | | | | | | | | |
| | | Years ended December 31, | |
| | | 2017 | | | 2016 | | | 2015 | |
Statutory federal income tax rate | | | (35 | %) | | | (35 | %) | | | (35 | %) |
Tax credits | | | (11 | ) | | | (25 | ) | | | (13 | ) |
Foreign rate differential | | | 14 | | | | 16 | | | | 5 | |
State income taxes and other | | | (6 | ) | | | 4 | | | | 2 | |
Valuation allowance | | | (55 | ) | | | 40 | | | | 41 | |
Impact of the Act | | | 72 | | | | — | | | | — | |
| | | | | | | | | | | | |
Effective tax rate, before impact in other comprehensive income | | | (21 | ) | | | 0 | | | | 0 | |
Impact in other comprehensive income | | | 21 | | | | — | | | | — | |
| | | | | | | | | | | | |
Effective tax rate, after impact in other comprehensive income | | | 0 | % | | | 0 | % | | | 0 | % |
| | | | | | | | | | | | |
The Company recorded an income tax benefit of $33.4 million due to unrealized gains on the Company’s common stock investment in Immunomedics, which was offset by an income tax provision for the same amount in other comprehensive income. The deferred tax liability related to the unrealized gain on the common stock investment in Immunomedics was recorded through other comprehensive income, which enabled the Company to recognize a benefit for the U.S. during 2017.
The foreign rate differential in the table above reflects the effect of operations in jurisdictions with tax rates that differ from the rate in the United States. This primarily resulted from the Company’s operations in Switzerland, which began in 2015. At December 31, 2017, unremitted earnings of the Company’s foreign subsidiaries, which were insignificant, will be retained indefinitely by the foreign subsidiaries for continuing investment. If foreign earnings were to be repatriated to the United States, the Company could be subject to additional state income and withholding taxes.
The Company’s net deferred tax assets consisted of the following (in thousands):
| | | | | | | | |
| | | December 31, | |
| | | 2017 | | | 2016 | |
Deferred tax assets | | | | | | | | |
Net operating loss carryforwards | | $ | 158,951 | | | $ | 150,465 | |
Foreign net operating loss carryforwards | | | 2,651 | | | | 1,702 | |
Tax credit carryforwards | | | 148,027 | | | | 124,396 | |
Deferred revenue | | | 13,779 | | | | 30,731 | |
Share-based compensation | | | 26,454 | | | | 33,041 | |
Capitalized research and development | | | 17,150 | | | | 12,578 | |
Depreciation and amortization | | | 7,606 | | | | 8,970 | |
Other | | | 15,178 | | | | 19,468 | |
| | | | | | | | |
Total deferred tax assets | | | 389,796 | | | | 381,351 | |
Less: valuation allowance | | | (364,538 | ) | | | (381,351 | ) |
| | | | | | | | |
Total deferred tax assets, net of valuation allowance | | | 25,258 | | | | — | |
Deferred tax liability | | | | | | | | |
Unrealized gain onavailable-for-sale securities | | | (25,258 | ) | | | — | |
| | | | | | | | |
Net deferred tax assets (liability) | | $ | — | | | $ | — | |
| | | | | | | | |
122
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
The Company’s deferred tax assets primarily consist of net operating loss, or NOL, carryforwards, tax credit carryforwards, share-based compensation, capitalized research and development expense and deferred revenue. Realization of deferred tax assets is dependent upon a number of factors, including future earnings, the timing and amount of which is uncertain. Accordingly, the deferred tax assets have been fully offset by a valuation allowance. At December 31, 2017, the Company had gross federal NOL carryforwards of $666.3 million expiring from 2021 to 2037 if not utilized, gross state NOL carryforwards of $287.9 million, gross foreign NOL carryforwards of $29.7 million and tax credit carryforwards of $166.2 million expiring from 2021 to 2037.
Utilization of the NOL and tax credit carryforwards may be subject to a substantial annual limitation in the event of a change in ownership as set forth in Section 382 of the Internal Revenue Code of 1986, as amended. The Company has evaluated ownership changes through the year ended December 31, 2016 and believes that it is likely that utilization of its NOLs would not be limited under Section 382 as of December 31, 2016. It is possible that there has been or may be a change in ownership after this date, which would limit the Company’s ability to utilize its NOLs. Any limitation may result in the expiration of the NOLs and tax credit carryforwards before utilization.
In 2017, the Company adopted Accounting Standards Update entitled “ASU2016-09, Compensation – Stock Compensation.” As a result, the net operating loss deferred tax asset increased by $70.9 million as a result of the inclusion of the net operating losses related to excess tax benefits. The increase in the deferred tax asset has been offset by a full valuation allowance.
The valuation allowance decreased by $16.8 million in 2017, and increased by $59.2 million in 2016 and $4.5 million in 2015, all related to the changes in the Company’s deferred tax asset balances. The decrease in the valuation allowance in 2017 included the $114.8 million decrease to reflect the new statutory rate and the $33.4 million decrease related to the unrealized gain on the Immunomedics common stock investment recorded through other comprehensive income, offset by the $70.9 million increase in connection with the adoption of ASU2016-09 and a $60.5 million increase for the current year loss, tax credits and other activity.
The financial statement recognition of the benefit for a tax position is dependent upon the benefit being more likely than not to be sustainable upon audit by the applicable taxing authority. If this threshold is met, the tax benefit is then measured and recognized at the largest amount that is greater than 50% likely of being realized upon ultimate settlement. A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows (in thousands):
| | | | | | | | | | | | |
| | | Years ended December 31, | |
| | | 2017 | | | 2016 | | | 2015 | |
Balance at January 1 | | $ | 16,023 | | | $ | 0 | | | $ | 0 | |
Increase (decrease) related to prior year tax positions | | | (1,292 | ) | | | 12,631 | | | | 0 | |
Increase related to current year tax positions | | | 3,441 | | | | 3,392 | | | | 0 | |
Lapses of statute of limitations | | | 0 | | | | 0 | | | | 0 | |
| | | | | | | | | | | | |
Balance at December 31 | | $ | 18,172 | | | $ | 16,023 | | | $ | 0 | |
| | | | | | | | | | | | |
The Company does not anticipate any significant changes to its unrecognized tax positions or benefits during the next twelve months. Interest and penalties related to the settlement of uncertain tax positions, if any, will be reflected in income tax expense. Tax years 2001 to 2017 remain subject to future examination for federal income taxes.
123
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
11. Collaboration and license agreements
The Company has entered into various product, collaboration and license agreements with pharmaceutical and biotechnology companies. Revenues recognized under these agreements were as follows (in thousands):
| | | | | | | | | | | | |
| | | Years ended December 31, | |
| | | 2017 | | | 2016 | | | 2015 | |
Takeda | | $ | 74,872 | | | $ | 44,384 | | | $ | 17,234 | |
AbbVie | | | 23,260 | | | | 25,676 | | | | 31,055 | |
Other | | | 10,500 | | | | 14,866 | | | | 21,481 | |
| | | | | | | | | | | | |
Total | | $ | 108,632 | | | $ | 84,926 | | | $ | 69,770 | |
| | | | | | | | | | | | |
Takeda ADCETRIS Collaboration
The ADCETRIS collaboration provides for the globalco-development of ADCETRIS and the commercialization of ADCETRIS by Takeda in its territory. Under this collaboration, the Company has retained commercial rights for ADCETRIS in the United States and its territories and in Canada, and Takeda has commercial rights in the rest of the world. Additionally, the companies equallyco-fund the cost of selected development activities conducted under the collaboration. In Japan, Takeda is solely responsible for development costs. Costs incurred by the Company associated withco-development activities performed under this collaboration are included in research and development expense in the accompanying consolidated statements of comprehensive loss.
The Company recognizes as collaboration revenue the upfront payment, progress-dependent development and regulatory milestone payments, and net development cost reimbursement payments from Takeda over theten-year development period of the collaboration, which began in 2009. When the performance of development activities under the collaboration results in the Company making a reimbursement payment to Takeda, the effect is to reduce the amount of collaboration revenue recorded by the Company. The Company also receives reimbursement for the cost of drug product supplied to Takeda for its use and, in some cases, pays Takeda for drug product they supply to the Company. The earned portion of net collaboration payments is reflected in collaboration and license agreement revenues.
The Company receives royalties based on a percentage of Takeda’s net sales of ADCETRIS in its licensed territories ranging from themid-teens to themid-twenties based on sales volume, as well as sales-based milestones. Takeda also bears a portion of third-party royalty costs owed on its sales of ADCETRIS which is included as a component of the Company’s royalty revenue. Either party may terminate the collaboration agreement if the other party materially breaches the agreement and such breach remains uncured. Takeda may terminate the collaboration agreement for any reason upon prior written notice to the Company. The collaboration agreement can also be terminated by mutual written consent of the parties. If neither party terminates the collaboration agreement, then the agreement automatically terminates on the expiration of all payment obligations.
AstellasCo-Development Collaboration
The Company has an agreement with Agensys, which subsequently became an affiliate of Astellas, to jointly research, develop and commercialize ADCs for the treatment of certain types of cancer. The agreement encompasses combinations of the Company’s ADC technology with fully-human antibodies developed by Astellas to proprietary cancer targets.
124
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
The Company and Astellas areco-funding all development and commercialization costs for enfortumab vedotin and will share equally in any profits that may come from this product candidate if successfully commercialized. Either party may opt out ofco-development and profit-sharing in return for receiving milestones and royalties from the continuing party. Either party may terminate the collaboration agreement if the other party becomes insolvent or materially breaches the agreement and such breach remains uncured. Subject to certain restrictions, either party may terminate the collaboration agreement for any reason upon prior written notice to the other party. The collaboration agreement can also be terminated by mutual written consent of the parties. If neither party terminates the collaboration agreement, then the agreement automatically terminates on the expiration of all payment obligations or the day upon which the parties cease to develop and commercialize products under the collaboration agreement.
Costs associated withco-development activities performed under this collaboration are included in research and development expense in the accompanying consolidated statements of comprehensive loss. The Astellas collaboration defines a mechanism for calculating the costs ofco-development activities and for reimbursing the other party in order to maintain an equal sharing of development costs. Third-party costs are billed at actual cost and internal labor and support costs are billed at a contractual rate. The following table summarizes research and development expenses incurred by the Company and funding provided to Astellas under the collaboration to achieve equal cost sharing (in thousands):
| | | | | | | | | | | | |
| | | Years ended December 31, | |
| | | 2017 | | | 2016 | | | 2015 | |
Research and development expense using contractual rates | | $ | 16,884 | | | $ | 2,947 | | | $ | 539 | |
Co-development funding paid to Astellas | | | 19,409 | | | | 12,043 | | | | 5,545 | |
| | | | | | | | | | | | |
Total | | $ | 36,293 | | | $ | 14,990 | | | $ | 6,084 | |
| | | | | | | | | | | | |
GenmabCo-Development Collaboration
The Company has an agreement with Genmab for the development and commercialization of ADCs for the treatment of several types of cancer.
Under the agreement, the Company exercised aco-development option for tisotumab vedotin in August 2017. The Company and Genmab will share all future costs and profits for development and commercialization of tisotumab vedotin on an equal basis. The Company will be responsible for tisotumab vedotin commercialization activities in the U.S., Canada, and Mexico, while Genmab will be responsible for commercialization activities in all other territories. Each party has the option toco-promote up to a specified percentage of the sales effort in the other party’s territories. Either party may opt out ofco-development and profit-sharing in return for receiving milestones and royalties from the continuing party. Either party may terminate the collaboration agreement if the other party becomes insolvent or materially breaches the agreement and such breach remains uncured. Either party may terminate the collaboration agreement if such party’s patent rights subject to the agreement are challenged by the other party or its sublicensees.
Costs associated withco-development activities are included in research and development expense in the accompanying consolidated statements of comprehensive loss. In 2017, the Company incurred $0.7 million of third-party research and development expenses and provided $6.0 million ofco-development funding to Genmab under the collaboration.
125
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
Unum Therapeutics Collaboration
In June 2015, the Company entered into a collaboration agreement with Unum Therapeutics, Inc., or Unum, to develop and commercialize novel antibody-coupledT-cell receptor, or ACTR, therapies for cancer. Under the terms of the agreement, the Company made an upfront payment of $25.0 million and an equity investment of $5.0 million in Unum. The agreement provides for the Company and Unum to initially develop two ACTR products combining Unum’s ACTR technology with the Company’s antibodies, and the Company has an option to expand the collaboration to include a third ACTR product upon payment of an additional fee. Unum is conducting preclinical research and clinical development activities through phase 1 clinical trials, and the Company is providing funding for these activities. The agreement calls for the Company and Unum to work together toco-develop and jointly fund programs after phase 1 clinical trials unless either company opts out.
The Company and Unum wouldco-commercialize any successfully developed product candidates and share any profits equally on anyco-developed programs in the United States. The Company retains exclusive commercial rights outside of the United States, paying Unum a royalty that is a high single digit tomid-teens percentage ofex-U.S. sales. The potential future licensing and progress-dependent milestone payments to Unum under the collaboration may total up to $400 million for the ACTR programs, payment of which is triggered by the achievement of development, regulatory and commercial milestones.
Costs associated withco-development activities performed under this collaboration are included in research and development expense in the accompanying consolidated statements of comprehensive loss. The following table summarizes third-party research and development expenses incurred by the Company and funding provided to Unum under the collaboration (in thousands):
| | | | | | | | | | | | |
| | | Years ended December 31, | |
| | | 2017 | | | 2016 | | | 2015 | |
Co-development funding paid to Unum | | $ | 4,512 | | | $ | 3,243 | | | $ | 569 | |
Third-party research and development expenses incurred | | | 4,017 | | | | 2,086 | | | | 0 | |
| | | | | | | | | | | | |
Total | | $ | 8,529 | | | $ | 5,329 | | | $ | 569 | |
| | | | | | | | | | | | |
ADC collaboration agreements
The Company has other active collaborations with a number of biotechnology and pharmaceutical companies to allow them to use its proprietary ADC technology. Under these ADC collaborations, which the Company has entered into in the ordinary course of business, the Company has granted research and commercial licenses to use its technology in conjunction with the collaborator’s technology. The Company also has agreed to conduct limited development activities and to provide other materials, supplies and services to its ADC collaborators during the performance obligation period of the collaboration. The Company receives upfront cash payments, progress- and sales-dependent milestones for the achievement by its collaborators of certain events, annual maintenance fees and support fees for research and development services and materials provided under the agreements. The Company also is entitled to receive royalties on net sales of any resulting products incorporating its ADC technology. The Company’s ADC collaborators are solely responsible for research, product development, manufacturing and commercialization of any product candidates under these collaborations, which includes the achievement of the potential milestones.
126
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
12. License agreements
The Company hasin-licensed antibodies, targets and enabling technologies from pharmaceutical and biotechnology companies and academic institutions for use in ADCETRIS, its pipeline programs and ADC technology, including the following:
Bristol-Myers Squibb.The Company has obtained rights to some of its technologies and product candidates, portions of which are exclusive, through a license agreement with Bristol-Myers Squibb. Through this license, the Company secured rights to use various targeting technologies. Under the terms of the license agreement, the Company is required to pay royalties in the low single digits on net sales of products, including ADCETRIS, which incorporate various technologies owned by Bristol-Myers Squibb.
University of Miami.The Company has entered into an exclusive license agreement with the University of Miami, Florida, covering an anti-CD30 monoclonal antibody that is the basis for the antibody component of ADCETRIS. Under the terms of this license, the Company made an upfront payment and progress-dependent milestone payments. The Company is required to pay annual maintenance fees and royalties in the low single digits on net sales of products, including ADCETRIS, incorporating technology licensed from the University of Miami.
Other Licenses. The Company has othernon-exclusive licenses to other technology used in ADCETRIS that require the Company to pay a low single-digit royalty on net sales of ADCETRIS.
13. Commitments and contingencies
Commitments. The Company is obligated to make future minimum payments under operating leases for building space used for general office and research and development purposes. The leases expire between 2018 through 2024 and include options to renew at the then fair market rental for the facilities. The lease agreements typically contain scheduled rent increases and provide for tenant improvement allowances. Accordingly, the Company has recorded a deferred rent liability of $1.4 million and $2.2 million at December 31, 2017 and 2016, respectively. This deferred rent liability is amortized over the terms of the related leases. Assuming the Company does not exercise any extensions, future minimum lease payments under allnon-cancelable operating leases are set forth below.
In addition, the Company has certainnon-cancelable obligations under other agreements, including supply agreements relating to the manufacture of ADCETRIS and the Company’s product candidates which contain annual minimum purchase commitments and other firm commitments when a binding forecast is provided.
As of December 31, 2017, the Company’s future obligations related to its leases and supply and other agreements are as follows (in thousands):
| | | | | | | | |
| | | Leases | | | Supply and
Other
Agreements | |
Years ending December 31, | | | | | | | | |
2018 | | $ | 7,537 | | | $ | 71,098 | |
2019 | | | 6,982 | | | | 33,569 | |
2020 | | | 6,844 | | | | 31,643 | |
2021 | | | 6,486 | | | | 30,760 | |
2022 | | | 6,277 | | | | 24,720 | |
Thereafter | | | 9,535 | | | | 68,910 | |
| | | | | | | | |
| | $ | 43,661 | | | $ | 260,700 | |
| | | | | | | | |
127
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
Rent expense attributable tonon-cancelable operating leases totaled approximately $6.6 million, $5.6 million, and $4.1 million for the years ended December 31, 2017, 2016, and 2015, respectively.
Non-cancelable obligations under other agreements do not include payments that are contingent upon achievement of certain progress-dependent milestones, as well as the payment of royalties based on net sales of commercial products. These amounts have been excluded from the table because the events triggering the obligations have not yet occurred.
Contingencies. On January 10, 2017, a purported securities class action lawsuit was commenced in the United States District Court for the Western District of Washington, naming as defendants the Company and certain of its officers. A consolidated amended complaint was filed on June 6, 2017, following the court’s appointment of a lead plaintiff and its approval of lead plaintiff’s counsel. The lawsuit alleges material misrepresentations and omissions in public statements regarding the Company’s business, operational and compliance policies, violations by all named defendants of Section 10(b) of the Exchange Act, and Rule10b-5 thereunder, as well as violations of Section 20(a) of the Exchange Act. The complaint seeks compensatory damages of an undisclosed amount. The plaintiff alleges, among other things, that the Company made false and/or misleading statements and/or failed to disclose thatSGN-CD33A presents a significant risk of fatal hepatotoxicity and that the Company had therefore overstated the viability ofSGN-CD33A as a treatment for AML. It is possible that additional suits will be filed, or allegations received from stockholders, with respect to these same matters and also naming the Company and/or its officers and directors as defendants. The Company filed a motion to dismiss this complaint on July 28, 2017. On October 18, 2017, the Court granted the Company’s motion to dismiss with leave for plaintiff to file a second consolidated amended complaint. The plaintiff filed a second consolidated amended complaint on November 17, 2017, and the Company filed a motion to dismiss this new complaint on January 5, 2018. It is possible that additional suits will be filed, or allegations received from stockholders, with respect to these same matters and also naming the Company and/or its officers and directors as defendants. The Company does not believe it is feasible to predict or determine the ultimate outcome or resolution of this litigation, or to estimate the amount of, or potential range of, loss with respect to this proceeding. In addition, the timing of the final resolution of this proceeding is uncertain. As a result of the lawsuit, the Company will incur litigation expenses and may incur indemnification expenses, and potential resolutions of the lawsuit could include a settlement requiring payments. Those expenses could have a material impact on its financial position, results of operations, and cash flows.
On March 29, 2017, a stockholder derivative lawsuit was filed in Washington Superior Court for the County of Snohomish, or the Snohomish County Superior Court. The complaint names as defendants certain of the Company’s current and former executives and members of its board of directors. The Company is named as a nominal defendant. The complaint generally makes the same allegations as the securities class action, claiming that the individual defendants breached their duties to the Company. The complaint seeks unspecified damages, disgorgement of compensation, corporate governance changes, and attorneys’ fees and costs. Because the complaint is derivative in nature, it does not seek monetary damages from the Company. On June 8, 2017, the Snohomish County Superior Court entered an order staying the Stockholder Derivative Action until resolution of the motion to dismiss the Stockholder Class Action. On October 18, 2017, in light of the granting of the Company’s motion to dismiss in the Stockholder Class Action, the parties in the Stockholder Derivative Action filed a joint status report with the Snohomish County Superior Court stipulating to continue to stay the Stockholder Derivative Action pending a ruling on a motion to dismiss the second consolidated amended complaint in the Stockholder Class Action. As a result of the lawsuit, the Company may incur litigation and indemnification expenses.
On February 13, 2017, the Company was named aco-defendant in a lawsuit filed by venBio Select Advisors LLC, or venBio, in the Delaware Chancery Court, against the members of the board of directors of
128
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
Immunomedics, Inc., or Immunomedics. The lawsuit, or the venBio lawsuit, alleged that the members of the Immunomedics board breached their fiduciary duties toward their stockholders by hastily licensingIMMU-132 to the Company. The Company was alleged to have aided and abetted the breach of fiduciary duties. Among other things, venBio sought to enjoin the closing of the transactions contemplated by the development and license agreement, or the Immunomedics License, the Company entered into with Immunomedics in February 2017 that provided for the grant to the Company of exclusive worldwide rights toIMMU-132, and a related stock purchase agreement. On May 4, 2017, the Company and Immunomedics agreed to terminate the Immunomedics License and to amend the term of the warrant Immunomedics issued to the Company under the stock purchase agreement to be exercisable by the Company only until December 31, 2017. In connection therewith, Immunomedics and venBio agreed to fully settle, resolve and release the Company, and the Company agreed to fully settle, resolve and release Immunomedics and venBio, from all disputes, claims and liabilities arising from the Immunomedics License, the stock purchase agreement and the transactions contemplated thereby, subject of the terms of the related termination agreement and settlement agreement. The termination agreement between Immunomedics and the Company and the settlement of the venBio lawsuit were effective August 25, 2017.
In addition, from time to time in the ordinary course of business the Company becomes involved in various lawsuits, claims and proceedings relating to the conduct of its business, including those pertaining to the defense and enforcement of its patent or other intellectual property rights. These proceedings are costly and time consuming. Successful challenges to the Company’s patent or other intellectual property rights through these proceedings could result in a loss of rights in the relevant jurisdiction and may allow third parties to use the Company’s proprietary technologies without a license from the Company or its collaborators.
14. Stockholders’ equity
Common stock
In September 2015, the Company completed an underwritten public offering of 13,463,415 shares of its common stock at a public offering price of $41.00 per share. The offering resulted in net proceeds to the Company of $526.6 million, after deducting underwriting discounts and commissions and other offering expenses.
At December 31, 2017, shares of common stock reserved for future issuance are as follows (in thousands):
| | | | |
Stock options and RSUs outstanding | | | 13,702 | |
Shares available for future grant under the 2007 Equity Incentive Plan | | | 3,261 | |
Employee stock purchase plan shares available for future issuance | | | 588 | |
| | | | |
Total | | | 17,551 | |
| | | | |
15. Share-based compensation
2007 Equity Incentive Plan
In 2007, the Company adopted the 2007 Equity Incentive Plan, or the 2007 Plan, that provides for the issuance of the Company’s common stock to employees, including officers, directors and consultants of the Company and its affiliates. The 2007 Plan was amended and restated in May 2016 to reserve an additional 6,000,000 shares thereunder, such that an aggregate of 27,000,000 shares of the Company’s common stock were authorized for issuance under the 2007 Plan at December 31, 2017. Under the 2007 Plan, the Company may issue stock options (including incentive stock options and nonstatutory stock options), restricted stock, RSUs, stock appreciation rights and other similar types of awards (including awards, such as RSUs, that do not require the awardee to pay any amount in connection with receiving the shares or that have an exercise or purchase price that
129
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
is less than the grant date fair market value of the Company’s stock). No awardee may be granted, in any calendar year under the 2007 Plan, options or stock awards covering more than 1,000,000 shares. The 2007 Plan was also amended and restated in May 2014 to extend its term through May 2024 unless it is terminated earlier pursuant to its terms.
Restricted stock grants are awards of a specific number of shares of the Company’s common stock. RSUs represent a promise to deliver shares of the Company’s common stock, or an amount of cash or property equal to the value of the underlying shares, at a future date. Stock appreciation rights are rights to receive cash and/or shares of the Company’s common stock based on the amount by which the exercise date fair market value of a specific number of shares exceeds the grant date fair market value of the exercised portion of the stock appreciation right. The Company has only issued options to purchase shares of common stock and RSUs under the 2007 Plan.
Incentive stock options under the 2007 Plan may be granted only to employees of the Company or its subsidiaries. The exercise price of an incentive stock option or a nonstatutory stock option may not be less than 100% of the fair market value of the common stock on the date the option is granted and the options have a maximum term of ten years from the date of grant. In the case of options granted to holders of more than 10% of the voting power of the Company, the exercise price may not be less than 110% of the fair market value of the common stock on the date the option is granted and the term of the option may not exceed five years. The Company may grant options with exercise prices lower than the fair market value of its common stock on the date of grant in connection with an acquisition by the Company of another company. Options become exercisable in whole or in part from time to time as determined by the Board of Directors, which administers the 2007 Plan.
Generally, options granted to employees under the 2007 Plan vest 25% one year after the beginning of the vesting period and thereafter ratably each month over the followingthirty-six months. RSUs granted to employees vest 100% on the third anniversary of the beginning of the vesting period. Option and RSU grants to independent members of the Company’s board of directors vest over one year. Performance-based options granted under the 2007 Plan pursuant to the LTIP commence vesting upon the achievement of a regulatory milestone and vest 25% each year over four years beginning one year after the achievement of the milestone. The Equity Plan provides for (i) the full acceleration of vesting of equity awards, including stock options and RSUs, upon a change in control (as defined in the 2007 Plan) if the successor company does not assume, substitute or otherwise replace the stock awards upon the change in control; and (ii) the full acceleration of vesting of any equity awards, including stock options and RSUs, if at the time of, immediately prior to or within twelve months after a change in control of the Company, the holder of such equity awards is involuntarily terminated without cause or is constructively terminated by the successor company that assumed, substituted or otherwise replaced such stock awards in connection with the change in control.
Share-based compensation cost
The Company recorded total share based compensation cost of $63.8 million, $52.5 million, and $41.8 million for the years ended December 31, 2017, 2016, and 2015, respectively. No tax benefit was recognized related to share-based compensation cost since the Company has not reported taxable income to date and has established a full valuation allowance to offset all of the potential tax benefits associated with its deferred tax assets. During 2017, 2016, and 2015, $1.3 million, $1.0 million, and $1.4 million of share based compensation costs were included in production overhead used in the determination of inventory cost, respectively.
130
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
Valuation assumptions
The Company calculates the fair value of each option award on the date of grant using the Black-Scholes option pricing model. The following weighted-average assumptions were used for the periods indicated:
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | 2007 Plan
Years ended December 31, | | | Employee Stock Purchase Plan
Years ended December 31, | |
| | | 2017 | | | 2016 | | | 2015 | | | 2017 | | | 2016 | | | 2015 | |
Risk-free interest rate | | | 1.8 | % | | | 1.3 | % | | | 1.5 | % | | | 0.76 | % | | | 0.35 | % | | | 0.1 | % |
Expected lives in years | | | 5.7 | | | | 6.5 | | | | 5.5 | | | | 0.5 | | | | 0.5 | | | | 0.5 | |
Expected dividends | | | 0 | % | | | 0 | % | | | 0 | % | | | 0 | % | | | 0 | % | | | 0 | % |
Expected volatility | | | 42 | % | | | 44 | % | | | 42 | % | | | 46 | % | | | 46 | % | | | 42 | % |
The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant for the expected life of the award. The Company’s computation of expected life was determined based on its historical experience with similar awards, giving consideration to the contractual terms of the share-based awards, vesting schedules and expectations of future employee behavior. A forfeiture rate is estimated at the time of grant to reflect the amount of awards that are granted but are expected to be forfeited by the award holder prior to vesting. The estimated forfeiture rate applied to these amounts is derived from historical stock award forfeiture behavior. The Company has never paid cash dividends and does not currently intend to pay cash dividends. The Company’s computation of expected volatility is based on the historical volatility of the Company’s stock price. Determination of all of these assumptions involves management’s best estimates at the time, which impact the fair value of the awards calculated under the Black-Scholes methodology and ultimately the expense that will be recognized over the life of the award.
The fair value of RSUs is determined based on the closing price of the Company’s common stock on the date of grant.
Stock option activity
A summary of stock option activity, excluding performance-based stock options, is as follows:
| | | | | | | | | | | | | | | | |
| | | Shares | | | Weighted-
average
exercise
price per share | | | Weighted-average
remaining contractual
term
(in years) | | | Aggregate
intrinsic value
(in thousands) | |
Balance at December 31, 2016 | | | 10,596,320 | | | $ | 30.14 | | | | | | | | | |
Granted | | | 2,102,207 | | | $ | 49.01 | | | | | | | | | |
Exercised | | | (1,494,263 | ) | | $ | 22.76 | | | | | | | | | |
Forfeited/expired | | | (446,804 | ) | | $ | 44.22 | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Balance at December 31, 2017 | | | 10,757,460 | | | $ | 34.26 | | | | 6.20 | | | $ | 209,865 | |
| | | | | | | | | | | | | | | | |
Expected to vest | | | 10,405,744 | | | $ | 33.84 | | | | 6.10 | | | $ | 207,275 | |
Options exercisable | | | 6,971,836 | | | $ | 27.71 | | | | 4.73 | | | $ | 179,898 | |
The weighted average grant-date fair values of options granted with exercise prices equal to market were $20.34, $18.20, and $15.84 for the years ended December 31, 2017, 2016, and 2015, respectively.
The aggregate intrinsic value in the table above is calculated as the difference between the exercise price of the underlying options and the quoted price of the Company’s common stock for all options that werein-the-money at December 31, 2017. The aggregate intrinsic value of options exercised was $52.9 million during
131
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
2017, $61.4 million during 2016, and $36.2 million during 2015, determined as of the date of option exercise. As of December 31, 2017, there was approximately $37.7 million of total unrecognized compensation cost related to unvested option arrangements, as adjusted for expected forfeitures. That cost is expected to be recognized over a weighted-average period of 1.42 years. The Company utilizes newly issued shares to satisfy option exercises.
A summary of performance-based stock option activity pursuant to the LTIP plans is as follows:
| | | | | | | | | | | | | | | | |
| | | Shares | | | Weighted-
average
exercise
price per share | | | Weighted-average
remaining contractual
term
(in years) | | | Aggregate
intrinsic value
(in thousands) | |
Balance at December 31, 2016 | | | 804,401 | | | $ | 34.20 | | | | | | | | | |
Granted | | | 36,223 | | | $ | 53.00 | | | | | | | | | |
Exercised | | | 0 | | | $ | 0 | | | | | | | | | |
Forfeited/expired | | | (87,919 | ) | | $ | 34.78 | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Balance at December 31, 2017 | | | 752,705 | | | $ | 35.04 | | | | 8.38 | | | $ | 13,897 | |
| | | | | | | | | | | | | | | | |
The weighted average grant-date fair values of performance-based options granted with exercise prices equal to market was $27.26 and $18.54 for the years ended December 31, 2017 and 2016, respectively. As of December 31, 2017, the estimated unrecognized compensation expense related to the LTIPs was $36.2 million.
RSU activity
A summary of RSU activity is as follows:
| | | | | | | | |
| | | Share
equivalent | | | Weighted-
average
grant date
fair value | |
Non-vested at December 31, 2016 | | | 2,078,939 | | | $ | 42.58 | |
Granted | | | 911,015 | | | $ | 50.12 | |
Vested | | | (535,299 | ) | | $ | 43.20 | |
Forfeited | | | (263,305 | ) | | $ | 43.49 | |
| | | | | | | | |
Non-vested at December 31, 2017 | | | 2,191,350 | | | $ | 45.46 | |
| | | | | | | | |
The weighted average grant-date fair values of RSUs granted were $50.12, $44.72, and $39.17 for the years ended December 31, 2017, 2016, and 2015, respectively. The total fair value of RSUs that vested during 2017, 2016, and 2015 (measured on the date of vesting) was $27.5 million, $23.3 million, and $22.3 million, respectively. As of December 31, 2017, there was approximately $50.9 million of total unrecognized compensation cost related tonon-vested RSU awards that will be recognized as expense over a weighted-average period of 1.69 years. The Company will utilize newly issued shares for RSUs that vest.
132
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
Employee Stock Purchase Plan
Activity under the Amended and Restated 2000 Employee Stock Purchase Plan for the years ended December 31 was as follows:
| | | | | | | | |
| | | Shares
Purchased | | | Weighted-
average
purchase price
per share | |
2017 | | | 172,421 | | | $ | 42.35 | |
2016 | | | 203,225 | | | $ | 27.98 | |
2015 | | | 201,103 | | | $ | 26.44 | |
Under the current terms of the Stock Purchase Plan, shares are purchased at the lower of 85 percent of the fair market value of the Company’s common stock on either the first day or the last day of eachsix-month offering period.
16. Employee benefit plan
The Company has a 401(k) Plan for all of its employees. The 401(k) Plan allows eligible employees to defer, at the employee’s discretion, up to 75% of their pretax compensation up to the IRS annual limit. The Company has a 401(k) matching program whereby the Company may, at its discretion, match a portion of an employee’s contributions, not to exceed a prescribed annual limit. The Company’s matching contribution vests over four years from the start of employment. Under this matching program, the Company contributed $5.7 million in 2017, $4.7 million in 2016, and $2.6 million in 2015.
17. Quarterly financial data (unaudited)
The unaudited quarterly financial information should be read in conjunction with the Company’s financial statements and related notes included elsewhere in this report. The Company believes that the following unaudited information reflects all normal recurring adjustments necessary for a fair presentation of the information for the periods presented. The operating results for any quarter are not necessarily indicative of results for any future period. The following table contains selected unaudited financial data for each of the indicated periods (in thousands, except per share data):
| | | | | | | | | | | | | | | | |
| | | Three months ended | |
| | | March 31, | | | June 30, | | | September 30, | | | December 31, | |
2017 | | | | | | | | | | | | | | | | |
Total revenues | | $ | 109,131 | | | $ | 108,223 | | | $ | 135,291 | | | $ | 129,605 | |
Net income (loss) | | $ | (59,990 | ) | | $ | (56,360 | ) | | $ | 50,021 | | | $ | (59,201 | ) |
Net income (loss) per share—basic | | $ | (0.42 | ) | | $ | (0.39 | ) | | $ | 0.35 | | | $ | (0.41 | ) |
Net income (loss) per share—diluted | | $ | (0.42 | ) | | $ | (0.39 | ) | | $ | 0.34 | | | $ | (0.41 | ) |
2016 | | | | | | | | | | | | | | | | |
Total revenues | | $ | 111,155 | | | $ | 95,402 | | | $ | 106,315 | | | $ | 105,275 | |
Net loss | | $ | (20,478 | ) | | $ | (32,743 | ) | | $ | (31,752 | ) | | $ | (55,138 | ) |
Net loss per share—basic and diluted | | $ | (0.15 | ) | | $ | (0.23 | ) | | $ | (0.23 | ) | | $ | (0.39 | ) |
18. Subsequent events
In January 2018, the Company signed a definitive merger agreement with Cascadian Therapeutics, Inc., or Cascadian, a clinical-stage biopharmaceutical company based in Seattle, Washington, under which the Company
133
Seattle Genetics, Inc.
Notes to Consolidated Financial Statements (Continued)
agreed to acquire all of the outstanding shares of common stock of Cascadian for $10.00 per share in cash, or approximately $614.1 million (referred to as the Cascadian Acquisition). The Cascadian Acquisition is expected to close in the first quarter of 2018 and, if consummated, will be accounted for as a business combination.
In February 2018, the Company completed an underwritten public offering of 13,269,230 shares of its common stock at a public offering price of $52.00 per share, resulting in gross proceeds of approximately $690.0 million, before deducting underwriting discounts, commissions, and other offering expenses. The Company intends to use the proceeds from the recently-completed public offering to fund a portion of the costs of the Cascadian Acquisition. In addition, as a result of the proceeds to the Company from the public offering exceeding $400.0 million, the senior secured bridge loan facility that various banks committed to provide to the Company in connection with the Cascadian Acquisition is no longer available to the Company in accordance with the terms of the related commitment letter.
In February 2018, the Company entered into a license and collaboration agreement and related platform technology license agreement with Pieris Pharmaceuticals, Inc., or Pieris, to develop novel bispecifics incorporating the Company’s antibodies and Pieris’ proprietary proteins for the treatment of cancer. The agreement provides for an upfront payment totaling $30.0 million to Pieris. Under the terms of the license and collaboration agreement, Pieris is obligated to conduct preclinical research, and the Company is obligated to provide funding for these activities. Following this initial research phase, the Company will have the option to select up to three product candidates for further development. The Company would then develop the product candidates independently, subject to a limited opt in right held by Pieris as described in the following sentences. Prior to the initiation of pivotal trials with respect to the first product candidate developed, the Company may in its discretion provide Pieris the option to co-develop that product candidate. Unless Pieris elects to co-develop the first product candidate, the Company is required to provide Pieris the option to co-develop the second product candidate. Regardless of any prior elections made by Pieris, the Company has no obligation to provide Pieris with a right to opt in to the development of the third product candidate. In the event Pieris does elect to opt in to the development of the first or second product candidate, Pieris would be required to reimburse the Company for all milestone payments received as of the date of exercise and 50% of post-toxicology development costs. The Company and Pieris would share equally in costs and profits associated with theco-developed product candidate. Pieris would be responsible for commercialization in the U.S., and the Company would be responsible for commercialization activities in all other territories. With respect to the other two product candidates (or all three if Pieris does not exercise its right to opt-in), the Company would have sole responsibility for development, funding and commercialization and would owe Pieris development and sales milestones and royalties in the mid-single to low-double digits. The potential future licensing and progress-dependent milestone payments to Pieris under the collaboration for the three product candidates total up to $1.2 billion based on the achievement of development, regulatory and commercial milestones. The Company also has the right to select additional candidates for further development subject to the payment of additional fees, milestone payments and royalties.
134
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
(a)Evaluation of disclosure controls and procedures. Our Chief Executive Officer and our Chief Financial Officer have evaluated our disclosure controls and procedures (as defined in Rule13a-15(e) under the Securities Exchange Act of 1934, as amended) prior to the filing of this annual report. Based on that evaluation, they have concluded that, as of the end of the period covered by this annual report, our disclosure controls and procedures were, in design and operation, effective.
(b) Changes in internal control over financial reporting. There have not been any changes in our internal control over financial reporting during the quarter ended December 31, 2017 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
(c) Management’s Annual Report on Internal Control over Financial Reporting. Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule13a-15(f) under the Securities Exchange Act of 1934, as amended. Our management conducted an evaluation of the effectiveness of our internal control over financial reporting based on the 2013 framework inInternal Control—Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on its evaluation under the framework inInternal Control—Integrated Framework,our management concluded that our internal control over financial reporting was effective as of December 31, 2017.
The effectiveness of our internal control over financial reporting as of December 31, 2017 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report which is included in Item 8 in this Annual Report on Form10-K.
Item 9B. Other Information
On February 9, 2018, the Compensation Committee of our Board of Directors, or the Compensation Committee, terminated a performance-based Long Term Incentive Plan originally approved by the Compensation Committee on July 13, 2016, or the Plan, that was designed to incentivize our employees to potentially achieve regulatory approvals of vadastuximab talirine(SGN-CD33A). The Compensation Committee terminated the Plan as a result of our decision to discontinue the phase 3 CASCADE clinical trial ofSGN-CD33A in frontline older acute myeloid leukemia patients. Because of the termination of the Plan, no participant in the Plan is eligible to be paid or granted any amount or award under the Plan, including cash awards or restricted stock unit awards. To date, no awards have been granted or paid under the Plan.
135
PART III
The information required by Part III is omitted from this report because we will file a definitive proxy statement within 120 days after the end of our 2017 fiscal year pursuant to Regulation 14A for our 2018 Annual Meeting of Stockholders, or the 2018 Proxy Statement, and the information to be included in the 2018 Proxy Statement is incorporated herein by reference.
Item 10. Directors, Executive Officers and Corporate Governance
(1) The information required by this Item concerning our executive officers and our directors and nominees for director, including information with respect to our audit committee and audit committee financial expert, may be found under the section entitled “Proposal No. 1—Election of Directors” appearing in the 2018 Proxy Statement. Such information is incorporated herein by reference.
(2) The information required by this Item concerning our code of ethics may be found under the section entitled “Proposal No. 1—Election of Directors—Certain Other Corporate Governance Matters—Code of Ethics” appearing in the 2018 Proxy Statement. Such information is incorporated herein by reference.
(3) The information required by this Item concerning compliance with Section 16(a) of the Securities Exchange Act of 1934 may be found in the section entitled “Section 16(a) Beneficial Ownership Reporting Compliance” appearing in the 2018 Proxy Statement. Such information is incorporated herein by reference.
Item 11. Executive Compensation
The information required by this Item may be found under the sections entitled “Proposal No. 1—Election of Directors—Director Compensation” and “Compensation of Executive Officers” appearing in the 2018 Proxy Statement. Such information is incorporated herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
(1) The information required by this Item with respect to security ownership of certain beneficial owners and management may be found under the section entitled “Security Ownership of Certain Beneficial Owners and Management” appearing in the 2018 Proxy Statement. Such information is incorporated herein by reference.
(2) The information required by this Item with respect to securities authorized for issuance under our equity compensation plans may be found under the sections entitled “Equity Compensation Plan Information” appearing in the 2018 Proxy Statement. Such information is incorporated herein by reference.
Item 13. Certain Relationships and Related Transactions, and Director Independence
(1) The information required by this Item concerning related party transactions may be found under the section entitled “Certain Relationships and Related Party Transactions” appearing in the 2018 Proxy Statement. Such information is incorporated herein by reference.
(2) The information required by this Item concerning director independence may be found under the section entitled “Proposal No. 1—Election of Directors” appearing in the 2018 Proxy Statement. Such information is incorporated herein by reference.
Item 14. Principal Accounting Fees and Services
The information required by this Item may be found under the section entitled “Proposal No. 2—Ratification of Appointment of Independent Registered Public Accounting Firm” appearing in the 2018 Proxy Statement. Such information is incorporated herein by reference.
136
PART IV
Item 15. Exhibits, Financial Statement Schedules
| (a) | The following documents are filed as part of this report: |
| | (1) | | Financial Statements and Report of Independent Registered Public Accounting Firm |
| | (2) | | Financial Statement Schedules |
Financial Statement Schedules have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto.
| | (3) | | Exhibits are incorporated herein by reference or are filed with this report as indicated below (numbered in accordance with Item 601 of RegulationS-K). |
| | | | | | | | | | |
Exhibit
Number | | | | Incorporation By Reference |
| | Exhibit Description | | Form | | SEC File No. | | Exhibit | | Filing Date |
| | | | | |
| 2.1†** | | Asset Purchase Agreement, dated July 31, 2017, between Bristol-Myers Squibb Company and Seattle Genetics, Inc | | 10-Q | | 000-32405 | | 2.1 | | 11/6/2017 |
| | | | | |
| 2.2** | | Agreement and Plan of Merger, dated January 30, 2018, among Seattle Genetics, Inc., Valley Acquisition Sub, Inc. and Cascadian Therapeutics, Inc. | | 8-K | | 000-32405 | | 2.1 | | 1/31/2018 |
| | | | | |
| 3.1 | | Fourth Amended and Restated Certificate of Incorporation of Seattle Genetics, Inc. | | 10-Q | | 000-32405 | | 3.1 | | 11/7/2008 |
| | | | | |
| 3.2 | | Certificate of Amendment of Fourth Amended and Restated Certificate of Incorporation of Seattle Genetics, Inc. | | 8-K | | 000-32405 | | 3.3 | | 5/26/2011 |
| | | | | |
| 3.3 | | Amended and Restated Bylaws of Seattle Genetics, Inc | | 8-K | | 000-32405 | | 3.1 | | 11/25/2015 |
| | | | | |
| 4.1 | | Specimen Stock Certificate. | | S-1/A | | 333-50266 | | 4.1 | | 2/8/2001 |
| | | | | |
| 4.2 | | Investor Rights Agreement dated July 8, 2003 among Seattle Genetics, Inc. and certain of its stockholders. | | 10-Q | | 000-32405 | | 4.3 | | 11/7/2008 |
| | | | | |
| 4.3 | | Registration Rights Agreement, dated September 10, 2015, by and between Seattle Genetics, Inc. and the persons listed on Schedule A attached thereto. | | 8-K | | 000-32405 | | 10.1 | | 9/11/2015 |
| | | | | |
| 10.1 | | License Agreement dated March 30, 1998 between Seattle Genetics, Inc. and Bristol-Myers Squibb Company. | | 10-K/A | | 000-32405 | | 10.1 | | 11/26/2010 |
| | | | | |
| 10.2 | | Amendment Letter to the Bristol-Myers Squibb Company License Agreement dated July 29, 1999 between Seattle Genetics, Inc. and Bristol-Myers Squibb Company. | | 10-K/A | | 000-32405 | | 10.2 | | 11/26/2010 |
| | | | | |
| 10.3 | | Amendment Agreement to the Bristol-Myers Squibb Company License Agreement dated July 26, 2000 between Seattle Genetics, Inc. and Bristol-Myers Squibb Company. | | S-1/A | | 333-50266 | | 10.7 | | 12/5/2000 |
137
| | | | | | | | | | |
Exhibit
Number | | | | Incorporation By Reference |
| | Exhibit Description | | Form | | SEC File No. | | Exhibit | | Filing Date |
| | | | | |
| 10.4† | | Amendment to License Agreement to the Bristol-Myers Squibb Company License Agreement dated December 18, 2015 between Seattle Genetics, Inc. and Bristol-Myers Squibb Company. | | 10-K | | 000-32405 | | 10.4 | | 2/19/2016 |
| 10.5 | | License Agreement dated September 20, 1999 between Seattle Genetics, Inc. and the University of Miami. | | 10-K/A | | 000-32405 | | 10.6 | | 11/26/2010 |
| 10.6 | | Amendment No. 1 to the University of Miami License Agreement dated August 4, 2000 between Seattle Genetics, Inc. and the University of Miami. | | 10-K/A | | 000-32405 | | 10.7 | | 11/26/2010 |
| 10.7† | | Letter Agreement Regarding Royalty between the University of Miami and Seattle Genetics, Inc. dated April 11, 2016 | | 10-Q | | 000-32405 | | 10.1 | | 7/26/2016 |
| 10.8 | | Lease Agreement dated December 1, 2000 between Seattle Genetics, Inc. and WCM132-302, LLC. | | S-1/A | | 333-50266 | | 10.21 | | 1/4/2001 |
| 10.9 | | First Amendment to Lease dated May 28, 2003 between Seattle Genetics, Inc. andB&N 141-302, LLC. | | 10-Q | | 333-50266 | | 10.1 | | 8/12/2003 |
| 10.10† | | Second Amendment to Lease dated July 1, 2008 between Seattle Genetics, Inc. andB&N 141-302, LLC. | | 10-Q | | 000-32405 | | 10.1 | | 11/7/2008 |
| 10.11† | | Third Amendment to Lease dated May 9, 2011 between Seattle Genetics, Inc. and B&N141-302, LLC. | | 10-Q | | 000-32405 | | 10.2 | | 8/5/2011 |
| 10.12†+ | | Fourth Amendment to Lease dated October 24, 2017 between Seattle Genetics, Inc. and SNH Medical Office Properties Trust, as successor in interest to B&N141-302, LLC | | — | | — | | — | | — |
| 10.13† | | Office Lease dated May 9, 2011 between Seattle Genetics, Inc. and WCM Highlands II, LLC. | | 10-Q | | 000-32405 | | 10.1 | | 8/5/2011 |
| 10.14†+ | | First Amendment to Office Lease dated October 24, 2017 between Seattle Genetics, Inc. and SNH Medical Office Properties Trust, as successor in interest to WCM Highlands II, LLC | | — | | — | | — | | — |
| 10.15† | | Collaboration and License Agreement dated January 7, 2007 between Seattle Genetics, Inc. and Agensys, Inc. | | 10-Q | | 000-32405 | | 10.1 | | 5/8/2007 |
| 10.16† | | Amendment to the Collaboration and License Agreement between Seattle Genetics, Inc. and Agensys, Inc. dated effective November 20, 2009. | | 10-K | | 000-32405 | | 10.49 | | 3/12/2010 |
| 10.17† | | Collaboration Agreement between Seattle Genetics, Inc. and Millennium Pharmaceuticals, Inc. (a wholly-owned subsidiary of Takeda Pharmaceutical Company Limited) dated December 14, 2009. | | 10-K | | 000-32405 | | 10.50 | | 3/12/2010 |
| 10.18† | | Commercial Supply Agreement dated December 1, 2010 between Seattle Genetics, Inc. and SAFC, an operating division of Sigma-Aldrich, Inc. | | 10-Q | | 000-32405 | | 10.1 | | 11/4/2011 |
| 10.19† | | First Amendment to Commercial Supply Agreement effective as of January 20, 2014 between Seattle Genetics, Inc. and SAFC, an operating division of Sigma-Aldrich, Inc. | | 10-K | | 000-32405 | | 10.17 | | 2/21/2017 |
138
| | | | | | | | | | |
Exhibit
Number | | | | Incorporation By Reference |
| | Exhibit Description | | Form | | SEC File No. | | Exhibit | | Filing Date |
| 10.20† | | Second Amendment to Commercial Supply Agreement effective as of December 2, 2016 between Seattle Genetics, Inc. and SAFC, an operating division of Sigma-Aldrich, Inc. | | 10-K | | 000-32405 | | 10.18 | | 2/21/2017 |
| | | | | |
| 10.21 | | Development and Supply Agreement dated February 23, 2004 between Seattle Genetics, Inc. and Abbott Laboratories. | | 10-K | | 000-32405 | | 10.15 | | 2/27/2015 |
| | | | | |
| 10.22† | | First Amendment to Development and Supply Agreement dated April 17, 2008 between Seattle Genetics, Inc. and Abbott Laboratories, Inc. | | 10-Q | | 000-32405 | | 10.1 | | 8/8/2008 |
| | | | | |
| 10.23† | | Second Amendment to Development and Supply Agreement dated June 15, 2009 between Seattle Genetics, Inc. and Abbott Laboratories, Inc. | | 10-Q | | 000-32405 | | 10.4 | | 11/4/2011 |
| | | | | |
| 10.24† | | Third Amendment to Development and Supply Agreement dated November 5, 2009 between Seattle Genetics, Inc. and Abbott Laboratories, Inc. | | 10-Q | | 000-32405 | | 10.5 | | 11/4/2011 |
| | | | | |
| 10.25† | | Fourth Amendment to Development and Supply Agreement dated April 18, 2010 between Seattle Genetics, Inc. and Abbott Laboratories, Inc. | | 10-Q | | 000-32405 | | 10.6 | | 11/4/2011 |
| | | | | |
| 10.26† | | Fifth Amendment to Development and Supply Agreement dated August 24, 2010 between Seattle Genetics, Inc. and Abbott Laboratories, Inc. | | 10-Q | | 000-32405 | | 10.7 | | 11/4/2011 |
| | | | | |
| 10.27† | | Sixth Amendment to Development and Supply Agreement dated November 18, 2010 between Seattle Genetics, Inc. and Abbott Laboratories, Inc. | | 10-Q | | 000-32405 | | 10.8 | | 11/4/2011 |
| | | | | |
| 10.28† | | Seventh Amendment to Development and Supply Agreement dated January 2, 2013 between Seattle Genetics, Inc. and Abbott Laboratories, Inc. | | 10-K | | 000-32405 | | 10.42 | | 2/27/2013 |
| | | | | |
| 10.29† | | Eighth Amendment to Development and Supply Agreement dated July 7, 2015 between Seattle Genetics, Inc. and AbbVie Inc. (formerly part of Abbott Laboratories, Inc.). | | 10-Q | | 000-32405 | | 10.2 | | 7/30/2015 |
| | | | | |
| 10.30† | | Ninth Amendment to Development and Supply Agreement, effective as of August 23, 2016 between Seattle Genetics, Inc. and AbbVie Inc. (formerly part of Abbott Laboratories, Inc.). | | 10-Q | | 000-32405 | | 10.1 | | 10/27/2016 |
| | | | | |
| 10.31† | | Tenth Amendment to Development and Supply Agreement, effective as of December 26, 2016 between Seattle Genetics, Inc. and AbbVie, Inc. (formerly part of Abbott Laboratories, Inc.). | | 10-K | | 000-32405 | | 10.29 | | 2/21/2017 |
| | | | | |
| 10.32† | | Development and License Agreement dated February 10, 2017 between Seattle Genetics, Inc. and Immunomedics, Inc | | 10-Q/A | | 000-32405 | | 10.3 | | 9/15/2017 |
| | | | | |
| 10.33 | | Stock Purchase Agreement, dated February 10, 2017 between Seattle Genetics, Inc. and Immunomedics, Inc | | 10-Q | | 000-32405 | | 10.4 | | 5/1/2017 |
139
| | | | | | | | | | |
Exhibit
Number | | | | Incorporation By Reference |
| | Exhibit Description | | Form | | SEC File No. | | Exhibit | | Filing Date |
| | | | | |
| 10.34 | | Registration Rights Agreement, dated February 10, 2017 between Seattle Genetics, Inc. and Immunomedics, Inc | | 10-Q | | 000-32405 | | 10.5 | | 5/1/2017 |
| | | | | |
| 10.35 | | Immunomedics, Inc. Common Stock Purchase Warrant, dated February 16, 2017 | | 10-Q | | 000-32405 | | 10.6 | | 5/1/2017 |
| | | | | |
| 10.36 | | Warrant Agreement, dated February 16, 2017, between Immunomedics, Inc. and Broadridge Corporate Issuer Solutions, Inc., as Warrant Agent | | 10-Q | | 000-32405 | | 10.7 | | 5/1/2017 |
| | | | | |
| 10.37 | | Termination Agreement, dated May 4, 2017, between Immunomedics, Inc. and Seattle Genetics, Inc | | 10-Q/A | | 000-32405 | | 10.1 | | 9/15/2017 |
| | | | | |
| 10.38† | | Purchase Agreement, dated June 16, 2017, betweenBMR-3450 Monte Villa Parkway, LLC and ZymoGenetics, Inc | | 10-Q | | 000-32405 | | 10.1 | | 11/6/2017 |
| | | | | |
| 10.39 | | Assignment and Assumption of Purchase Agreement, dated July 30, 2017, between ZymoGenetics, Inc. and Seattle Genetics, Inc. | | 10-Q | | 000-32405 | | 10.2 | | 11/6/2017 |
| | | | | |
| 10.40† | | License and Collaboration Agreement, effective October 7, 2011, between Genmab A/S and Seattle Genetics, Inc | | 10-Q | | 000-32405 | | 10.3 | | 11/6/2017 |
| | | | | |
| 10.41* | | Form of Indemnification Agreement between Seattle Genetics, Inc. and each of its officers and directors. | | S-1/A | | 333-50266 | | 10.29 | | 1/4/2001 |
| | | | | |
| 10.42* | | Amended and Restated 1998 Stock Option Plan, effective as of August 5, 2009. | | 10-Q | | 000-32405 | | 10.1 | | 8/10/2009 |
| | | | | |
| 10.43* | | Form Notice of Grant and Stock Option Agreement under Seattle Genetics, Inc. Amended and Restated 1998 Stock Option Plan. | | 10-K | | 000-32405 | | 10.11 | | 3/15/2005 |
| | | | | |
| 10.44* | | 2000 Directors’ Stock Option Plan, as amended February 5, 2010. | | 10-K | | 000-32405 | | 10.13 | | 3/12/2010 |
| | | | | |
| 10.45* | | Form Notice of Grant and Stock Option Agreement under Seattle Genetics, Inc. 2000 Directors’ Stock Option Plan. | | 10-K | | 000-32405 | | 10.12 | | 3/15/2005 |
| | | | | |
| 10.46* | | Amended and Restated 2000 Employee Stock Purchase Plan, effective May 20, 2011. | | 10-K | | 000-32405 | | 10.35 | | 2/21/2017 |
| | | | | |
| 10.47* | | Seattle Genetics, Inc. Amended and Restated 2007 Equity Incentive Plan, effective as of May 18, 2012. | | 10-Q | | 000-32405 | | 10.1 | | 8/8/2012 |
| | | | | |
| 10.48 | | Seattle Genetics, Inc. Amended and Restated 2007 Equity Incentive Plan, effective as of May 16, 2014. | | 10-Q | | 000-32405 | | 10.1 | | 8/8/2014 |
| | | | | |
| 10.49* | | Seattle Genetics, Inc. Amended and Restated 2007 Equity Incentive Plan, effective as of May 20, 2016. | | 10-Q | | 000-32405 | | 10.4 | | 7/26/2016 |
| | | | | |
| 10.50* | | Form Stock Option Agreement for employees under Seattle Genetics, Inc. 2007 Equity Incentive Plan. | | 10-K | | 000-32405 | | 10.44 | | 3/13/2009 |
| | | | | |
| 10.51* | | Form of Stock Unit Grant Notice and Stock Unit Agreement for employees under Seattle Genetics, Inc. Amended and Restated 2007 Equity Incentive Plan. | | 8-K | | 000-32405 | | 10.1 | | 8/30/2011 |
140
| | | | | | | | | | |
Exhibit
Number | | | | Incorporation By Reference |
| | Exhibit Description | | Form | | SEC File No. | | Exhibit | | Filing Date |
| | | | | |
| 10.52* | | Form of Notice of Stock Option Grant and Stock Option Agreement fornon-employee directors under the Amended and Restjated 2007 Equity Incentive Plan. | | 10-Q | | 000-32405 | | 10.4 | | 8/5/2011 |
| | | | | |
| 10.53* | | Form of Stock Unit Grant Notice and Stock Unit Agreement fornon-employee directors under the Amended and Restated 2007 Equity Incentive Plan. | | 10-K | | 000-32405 | | 10.33 | | 2/28/2014 |
| | | | | |
| 10.54* | | Amended and Restated Employment Agreement, dated October 26, 2016, between Seattle Genetics, Inc. and Clay B. Siegall. | | 10-Q | | 000-32405 | | 10.4 | | 10/27/2016 |
| | | | | |
| 10.55* | | Amended and Restated Employment Agreement, dated October 26, 2016, between Seattle Genetics, Inc. and Todd E. Simpson. | | 10-Q | | 000-32405 | | 10.5 | | 10/27/2016 |
| | | | | |
| 10.56* | | Amended and Restated Employment Agreement, dated October 26, 2016, between Seattle Genetics, Inc. and Eric L. Dobmeier. | | 10-Q | | 000-32405 | | 10.6 | | 10/27/2016 |
| | | | | |
| 10.57* | | Amended and Restated Employment Agreement, dated October 26, 2016, between Seattle Genetics, Inc. and Jonathan Drachman. | | 10-Q | | 000-32405 | | 10.7 | | 10/27/2016 |
| | | | | |
| 10.58* | | Amended and Restated Employment Agreement, dated October 26, 2016, between Seattle Genetics, Inc. and Vaughn Himes. | | 10-Q | | 000-32405 | | 10.8 | | 10/27/2016 |
| | | | | |
| 10.59* | | Amended and Restated Employment Agreement, dated October 26, 2016, between Seattle Genetics, Inc. and Jean Liu. | | 10-Q | | 000-32405 | | 10.9 | | 10/27/2016 |
| | | | | |
| 10.60* | | Amended and Restated Employment Agreement, dated October 26, 2016, between Seattle Genetics, Inc. and Darren Cline. | | 10-Q | | 000-32405 | | 10.10 | | 10/27/2016 |
| | | | | |
| 10.61* | | Seattle Genetics, Inc. Senior Executive Annual Bonus Plan | | 8-K | | 000-32405 | | 10.1 | | 2/1/2017 |
| | | | | |
| 10.62* | | Seattle Genetics, Inc. Long Term Incentive Plan forECHELON-1. | | 10-Q | | 000-32405 | | 10.2 | | 7/26/2016 |
| | | | | |
| 10.63* | | Form of Stock Option Agreement for Long Term Incentive Plan forECHELON-1 under the Seattle Genetics, Inc. Amended and Restated 2007 Equity Incentive Plan. | | 10-Q | | 000-32405 | | 10.3 | | 7/26/2016 |
| | | | | |
| 10.64* | | Seattle Genetics, Inc. Long Term Incentive Plan forSGN-CD33A. | | 10-Q | | 000-32405 | | 10.2 | | 10/27/2016 |
| | | | | |
| 10.65* | | Form of Stock Option Agreement for Long Term Incentive Plan forSGN-CD33A under the Seattle Genetics, Inc. Amended and Restated 2007 Equity Incentive Plan. | | 10-Q | | 000-32405 | | 10.3 | | 10/27/2016 |
| | | | | |
| 10.66* | | Seattle Genetics, Inc. Long Term Incentive Plan for TV and EV | | 10-Q | | 000-32405 | | 10.4 | | 11/6/2017 |
| | | | | |
| 10.67* | | Form of Stock Unit Grant Notice for Long Term Incentive Plan for TV and EV | | 10-Q | | 000-32405 | | 10.5 | | 11/6/2017 |
141
| † | | Pursuant to a request for confidential treatment, portions of this Exhibit have been redacted from the publicly filed document and have been furnished separately to the Securities and Exchange Commission as required by Rule24b-2 under the Securities Exchange Act of 1934. |
| * | | Indicates a management contract or compensatory plan or arrangement. |
| ** | | Schedules have been omitted pursuant to Item 601(b)(2) of RegulationsS-K. The registrant will furnish copies of any such schedules to the Securities and Exchange Commission upon request. |
Item 16. Form10-K Summary
Not applicable.
142
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | |
| Date: February 14, 2018 | | SEATTLE GENETICS, INC. |
| | |
| | By: | | /S/ CLAY B. SIEGALL |
| | | | Clay B. Siegall President & Chief Executive Officer (Principal Executive Officer) |
| | |
| Date: February 14, 2018 | | By: | | /S/ TODD E. SIMPSON |
| | | | Todd E. Simpson Chief Financial Officer (Principal Financial and Accounting Officer) |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | |
Signature | | Title | | Date |
| | |
/S/ CLAY B. SIEGALL Clay B. Siegall | | Director, President & CEO (Principal Executive Officer) | | February 14, 2018 |
| | |
/S/ TODD E. SIMPSON Todd E. Simpson | | Chief Financial Officer (Principal Financial and Accounting Officer) | | February 14, 2018 |
| | |
/S/ SRINIVAS AKKARAJU Srinivas Akkaraju | | Director | | February 14, 2018 |
| | |
/S/ FELIX BAKER Felix Baker | | Director | | February 14, 2018 |
| | |
/S/ DAVID W. GRYSKA David W. Gryska | | Director | | February 14, 2018 |
| | |
/S/ MARC E. LIPPMAN Marc E. Lippman | | Director | | February 14, 2018 |
| | |
/S/ JOHN A. ORWIN John A. Orwin | | Director | | February 14, 2018 |
| | |
/S/ NANCY A. SIMONIAN Nancy A. Simonian | | Director | | February 14, 2018 |
| | |
/S/ DANIEL G. WELCH Daniel G. Welch | | Director | | February 14, 2018 |
143

 are our registered trademarks in the United States. All other trademarks, tradenames and service marks included in this Annual Report on Form10-K are the property of their respective owners.
are our registered trademarks in the United States. All other trademarks, tradenames and service marks included in this Annual Report on Form10-K are the property of their respective owners.