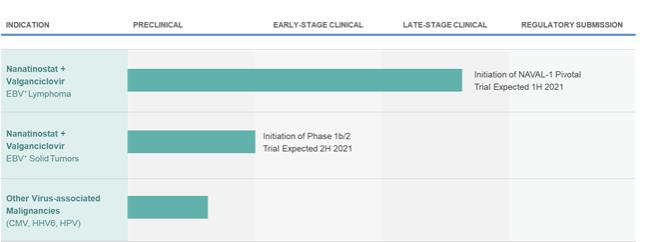
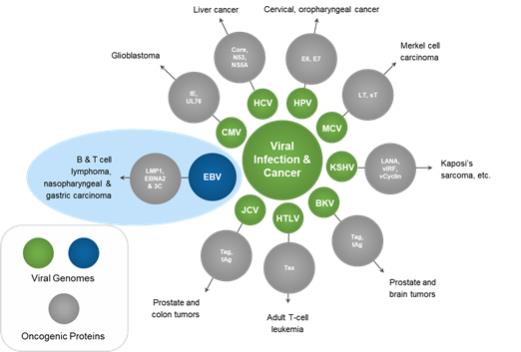
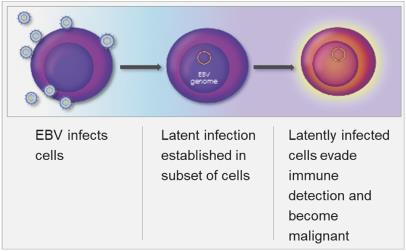
Latent infection and intermittent reactivation are two important characteristics of the EBV lifecycle. The maintenance of latent EBV infection requires the expression of a small subset of genes, and specific expression patterns (Types I – III) of these genes are associated with specific EBV-driven malignancies. EBV has been shown to infect B-cells, T-cells, T/NK-cells and epithelial cells, though its greatest predilection is for B-cells. EBV has been associated with a wide spectrum of human malignancies, with B-cell lymphomas being the most common, and include EBV+ diffuse large B cell lymphoma, not otherwise specified (“EBV+ DLBCL, NOS”), Burkitt’s lymphoma (“BL”), PTLD, and lymphomas associated with congenital and acquired immunodeficiencies, including HIV-related lymphomas.
Mechanism of Action of Nanatinostat in Combination with Valganciclovir
Viracta’s lead product candidate utilizes a combination of Viracta’s oral proprietary epigenetic drug, nanatinostat, in combination with the oral antiviral drug valganciclovir. Nanatinostat targets the EBV genome and induces the expression of certain viral kinase genes. Valganciclovir is converted to ganciclovir in the gut, and these viral genes then activate ganciclovir, which disrupts the DNA replication cycle, leading to DNA chain termination and killing of the tumor cells by inducing apoptosis. This type of killing can be considered a form of synthetic lethality, where neither drug alone would be as effective in killing the tumor cells, but together the two drugs are lethal to the tumor cells.
Epigenetic Re-Programming
EBV gene products can drive aberrant cell proliferation, inhibit programmed cell death and other mechanisms that promote formation of cancer. Viruses and cancers have also evolved mechanisms to evade immune detection by up-regulating immunosuppressive signals, including the induction of PD-L1. Due to these pleiotropic effects, so-called molecularly targeted approaches that target only one oncogenic activity have shown limited ability to impact these cancers.
Epigenetics refers to mechanisms that control which genes or gene programs are turned on or which ones are silenced. Chemical modification of gene promoter regions via methylation, or acetylation or deacetylation of histones around which DNA is coiled, are major mechanisms by which gene expression is controlled.
EBV-Associated Lymphomas
EBV-associated lymphomas are a heterogeneous group of malignancies that harbor latent EBV within the tumor cells. Within a specific histologic subtype of lymphoma, the frequency of EBV positivity may vary considerably. EBV is associated with approximately 5% of DLBCL in Western countries and approximately 10%-15% in Asia and South America, whereas approximately 30% of peripheral T cell lymphoma (“PTCL”) and HL within North America are EBV+, and endemic BL are EBV+ in approximately 95% of the cases. The risk for developing an EBV+ lymphoma has been observed to be higher in the setting of immunodeficiency, including in patients with HIV, congenital immunodeficiencies and post-transplant immunosuppression. EBV-related lymphomas express a limited number of viral genes, with latency expression patterns associated with specific lymphoma subtypes. The limited expression of EBV genes may allow for persistence of the viral DNA in cells by restricting the visibility of the virus to the immune system. The observation that lymphomas in patients with impaired immune function are more likely to express a greater number of viral genes is supportive of this concept. EBV-related lymphoproliferative disease in immunosuppressed patients may respond to therapies that improve immune function, such as reduced immunosuppression (e.g., transplant patients) or adoptive immunotherapy. In contrast, EBV-related lymphomas that arise in immunocompetent patients generally express fewer viral genes and proteins, and as a result are less prone to immune attack.
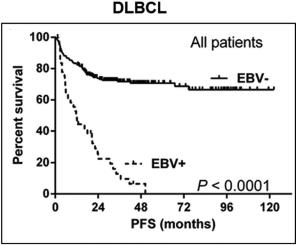
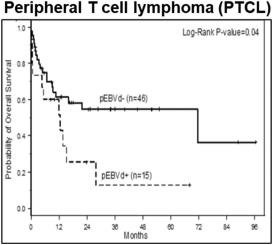
While outcomes vary based on the specific malignancy, EBV-associated lymphomas (e.g., DLBCL, HL, BL, and a number of T-cell lymphomas) often present a treatment challenge as clinical outcomes such as progression free survival and overall survival are worse following standard of care regimens as compared to those for EBV-negative patients. The presence of EBV in lymphomas is therefore generally considered to be a poor prognostic indicator. As shown in the figure below, across a series of patients with DLBCL, those who were EBV+ generally presented with more aggressive disease, had lower response rates to first-line therapy, and poorer progression free survival and overall survival. In a meta-analysis evaluating survival outcomes across several lymphoma types (HL, DLBCL, T/NKCL, PTCL), EBV+ disease was associated with significantly worse overall survival.