
Filed by: OSI Pharmaceuticals, Inc.
Pursuant to Rule 425 Under the Securities Act of 1933
Subject Company: Cell Pathways, Inc.
Commission File No.: 000-24889
The following booth panels and posters were exhibited at the ASCO (American Society of Clinical Oncology) 2003 Annual Meeting held in Chicago, Illinois from May 31 to June 3, 2003.
Information About the Merger and Where to Find It
In connection with the proposed merger of OSI Pharmaceuticals, Inc. and Cell Pathways, Inc., OSI Pharmaceuticals has filed with the Securities and Exchange Commission (SEC) a registration statement on Form S-4. The registration statement has been declared effective, and it contains the proxy statement of Cell Pathways for the meeting of its stockholders scheduled for June 10, 2003 to consider and vote upon the proposed merger. The proxy statement also serves as a prospectus of OSI Pharmaceuticals with respect to the shares of OSI Pharmaceuticals to be distributed to stockholders of Cell Pathways in the proposed transaction. INVESTORS AND SECURITY HOLDERS ARE URGED TO CAREFULLY READ THE PROXY STATEMENT/PROSPECTUS REGARDING THE PROPOSED MERGER TRANSACTION AND ANY OTHER DOCUMENTS FILED WITH THE SEC, BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT OSI, CELL PATHWAYS, THE MERGER AND RELATED MATTERS.
Investors and security holders may obtain a free copy of the proxy statement/prospectus and other documents filed by OSI Pharmaceuticals and Cell Pathways at the SEC’s web site at http://www.sec.gov. In addition, they may obtain such documents from OSI Pharmaceuticals or from Cell Pathways free of charge by requesting them in writing from OSI Pharmaceuticals, Inc., 58 South Service Road, Suite 110, Melville, New York 11747, Attention: Investor Relations, telephone: (631) 962-2000, or from Cell Pathways, Inc., 702 Electronic Drive, Horsham, Pennsylvania 19044, Attention: Investor Relations, telephone: (215) 706-3800.
OSI, Cell Pathways, their respective officers and directors and certain other members of management or employees may be deemed to be participants in the solicitation of proxies from stockholders of Cell Pathways with respect to the transactions contemplated by the merger agreement. A description of any interests that OSI Pharmaceuticals’ or Cell Pathways’ directors and executive officers have in the proposed merger is available in the proxy statement/prospectus.
This booth panels and posters are not offers to sell shares of OSI Pharmaceuticals common stock which may be issued in the proposed merger with Cell Pathways. Such OSI Pharmaceuticals common stock is offered only by means of the proxy statement/prospectus referred to herein.

| For patients suffering from oral mucositis associated with cancer therapy, * |
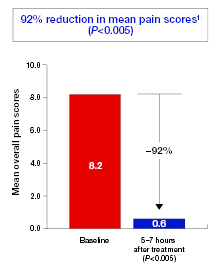
Results from an open-label study of 30 patients with oral mucositis, severe aphthous ulcers, or pain from oral surgery. Pain was scored on a Visual Numerical Scale (VNS) ranging from 0 to 10, with the higher number related to worst pain experienced. Short-term effect VNS total pain scores were recorded at baseline (prior to intervention) and at 5 to 7 hours following intervention.1
Provides dramatic and long-lasting pain relief
| • | | 100% of patients had a significant reduction in oral discomfort within 5–7 hours of initial treatment (P<0.005)1 |
Enables nutrition
| • | | 87% of patients reported overall improvement related to pain on swallowing foods, liquids, and saliva over a 7- to 10-day period compared to baseline (P<0.005)1 |
Gelclair™ has a mechanical action indicated for the management of pain and relief of pain by adhering to the mucosal surface of the mouth, soothing oral lesions of various etiologies, including oral mucositis/stomatitis (may be caused by chemotherapy or radiation therapy), irritation due to oral surgery, traumatic ulcers caused by braces or ill-fitting dentures, or disease. Also indicated for diffuse aphthous ulcers.
| | | | | |
| * | | North American marketing rights held by Cell Pathways, Inc. Proposed merger between OSI Pharmaceuticals, Inc. and Cell Pathways, Inc. subject to Cell Pathways shareholder approval. | | |
| | | | | |
| | | Reference: 1.Data on file, Cell Pathways, Inc. | | |
| | | | | |
| | | Please see a Celgene sales representative for additional information at booth #104. | |  |
| | | | | |
| | | 585-023 GEL000000 05/03 | | |

The Clarion Portfolio
Shields exposed, sensitized nerve endings from overstimulation1
| • | | Non-numbing |
| |
| • | | Non-stinging |
| |
| • | | Non-drying — No alcohol |
Convenient and well tolerated1
| • | | Helps patients to eat and drink more comfortably |
| |
| • | | Pleasant tasting |
| |
| • | | Easy-to-use |
This product is not recommended for use in patients with a known or suspected allergy to any of the product’s ingredients.
| | | | | |
| * | | North American marketing rights held by Cell Pathways, Inc. Proposed merger between OSI Pharmaceuticals, Inc. and Cell Pathways, Inc. subject to Cell Pathways shareholder approval. | | |
| | | | | |
| | | Reference: 1.Data on file, Cell Pathways, Inc. | | |
| | | | | |
| | | Please see a Celgene sales representative for additional information at booth #104. | |  |
| | | | | |
| | | 585-023 GEL000000 05/03 | | |

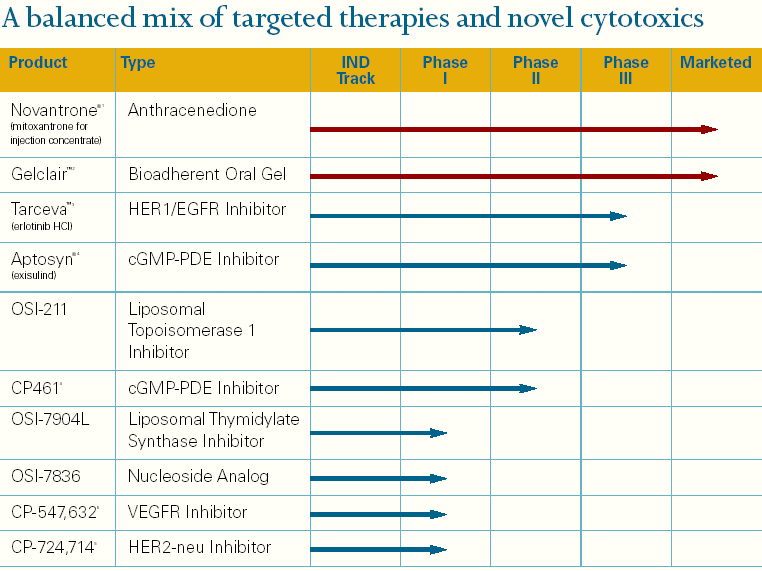
| A balanced mix of targeted therapies and novel cytotoxics |
| Product Type IND Phase Phase Phase Marketed Track I II III |
| Novantrone®1 Anthracenedione (mitoxantrone for injection concentrate) |
| Gelclair™2 Bioadherent Oral Gel |
| Tarceva™3 HER1/EGFR Inhibitor (erlotinib HCl) |
| Aptosyn®4 cGMP-PDE Inhibitor (exisulind) |
| OSI-211 Liposomal Topoisomerase 1 Inhibitor |
| CP4614 cGMP-PDE Inhibitor |
| OSI-7904L Liposomal Thymidylate Synthase Inhibitor OSI-7836 Nucleoside Analog CP-547,6325 VEGFR Inhibitor CP-724,7145 HER2-neu Inhibitor |
1. Oncology indications only.2.North American marketing rights held by Cell Pathways, Inc. Proposed merger between OSI Pharmaceuticals, Inc. and Cell Pathways, Inc. subject to Cell Pathways shareholder approval.3.Developed in collaboration with Genentech, Inc. and F. Hoffmann-La Roche, Ltd.4.Currently owned by Cell Pathways, Inc. Proposed merger between OSI Pharmaceuticals, Inc and Cell Pathways, Inc. subject to Cell Pathways shareholder approval.5. Developed by Pfizer, Inc.
©2003 OSI Pharmaceuticals, Inc. All rights reserved. 585-029 OSIXXXXXX 05/03
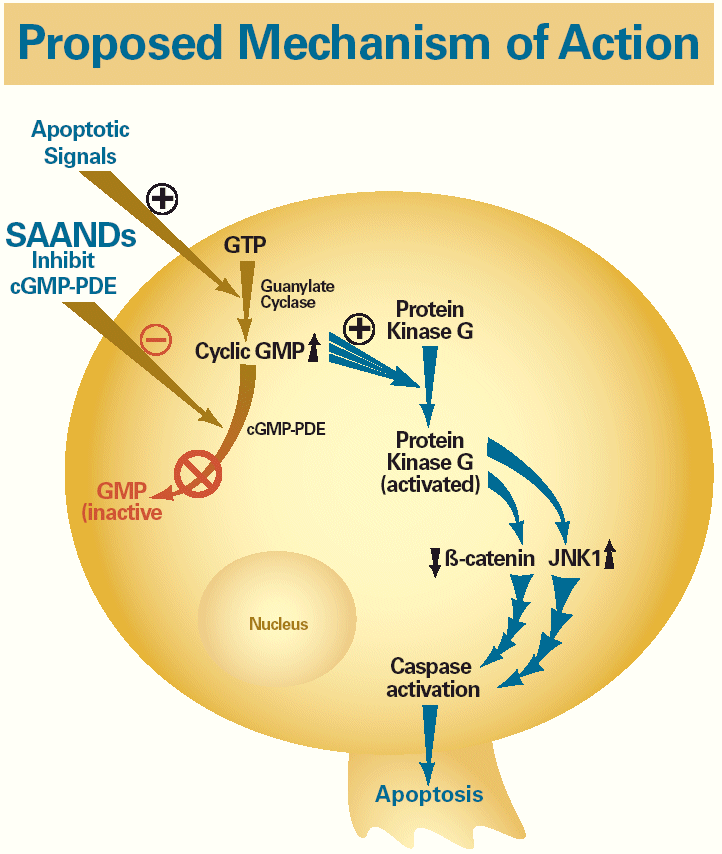
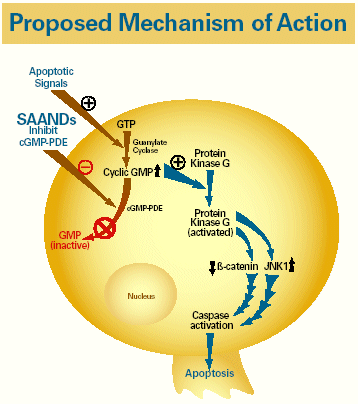
| Proposed Mechanism of Action Apoptotic Signals + SAANDs GTP Inhibit cGMP-PDE Guanylate Cyclase Protein – + Kinase G Cyclic GMP cGMP-PDE Protein Kinase G GMP (activated) (inactive ß-catenin JNK1 Nucleus Caspase activation Apoptosis |

| Proposed Mechanism of Action |
| Apoptotic Signals + SAANDs GTP Inhibit cGMP-PDE Guanylate Cyclase Protein – + Kinase G Cyclic GMP |
| cGMP-PDE Protein Kinase G GMP (activated) (inactive) |
Description
| • | | Selective apoptotic anti-neoplastic drugs (SAANDs) represent a potentially novel anti-cancer mechanism of action. Aptosyn® (exisulind)* and CP461* selectively induce apoptosis in neoplastic tissue via cyclic GMP phosphodiesterase (cGMP-PDE) inhibition and protein kinase G activation, ultimately leading to cell death1,2 |
Rationale for the study of SAANDs
| • | | SAANDs induce apoptosis in precancerous and cancerous cells but not in normal healthy cells and tissues.3 Potential cancer targets include3: |
| | | |
| • Lung (non-small cell) | | • Renal |
| • Prostate | | • Colorectal |
| • Breast | | • Esophagus |
Rationale for the study of SAANDs in combination with chemotherapy
| • | | SAANDs exhibited synergistic or additive activity when administered with chemotherapy in preclinical models3 |
*Currently owned by Cell Pathways, Inc. Proposed merger between OSI Pharmaceuticals, Inc. and Cell Pathways, Inc. subject to Cell Pathways shareholder approval. All compounds listed are investigational drugs. Safety and efficacy have not been established in human testing.
References: 1.Thompson WJ, Piazza GA, Li H, et al. Exisulind induction of apoptosis involves guanosine 3’,5’-cyclic monophosphate phosphodiesterase inhibition, protein kinase G activation, and attenuated beta-catenin.Cancer Res.2000;60:3338-3342.2.Soh J-W, Mao Y, Kim M-G, et al. Cyclic GMP mediates apoptosis induced by sulindac derivatives via activation of c-Jun NH2-terminal kinase 1.Clin Cancer Res.2000;6:4136-4141.3.Data on file, Cell Pathways, Inc.
585-021 TAR000000 05/03
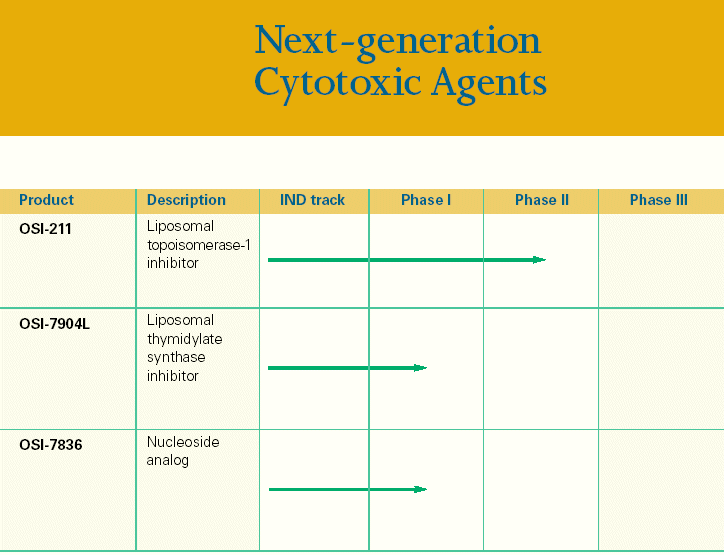
| Next-generation Cytotoxic Agents Product Description IND track Phase I Phase II Phase III OSI-211 Liposomal topoisomerase-1 inhibitor OSI-7904L Liposomal thymidylate synthase inhibitor OSI-7836 Nucleoside analog |
All compounds listed are investigational drugs. Safety and efficacy have not been established in human testing.
©2003 OSI Pharmaceuticals, Inc. All rights reserved. 585-021 TAR000000 05/03
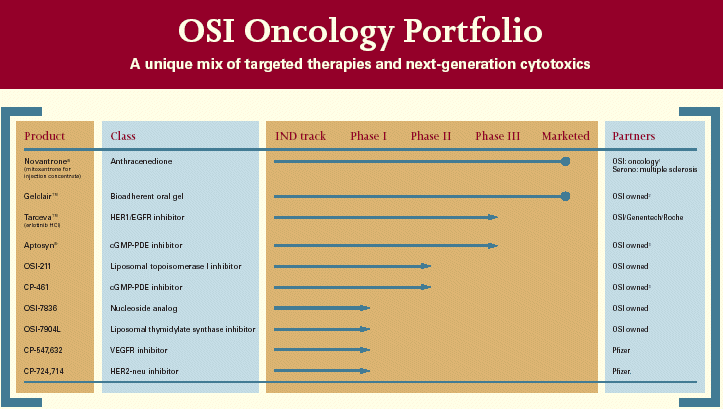
| OSI Oncology Portfolio A unique mix of targeted therapies and next-generation cytotoxics Product Class IND track Phase I Phase II Phase III Marketed Partners Novantrone® Anthracenedione OSI: oncology1 (mitoxantrone for Serono: multiple sclerosis injection concentrate) Gelclair™ Bioadherent oral gel OSI owned 2 Tarceva™ HER1/EGFR inhibitor OSI/Genentech/Roche (erlotinib HCI) Aptosyn® cGMP-PDE inhibitor OSI owned 3 OSI-211 Liposomal topoisomerase I inhibitor OSI owned CP-461 cGMP-PDE inhibitor OSI owned 3 OSI-7836 Nucleoside analog OSI owned OSI-7904L Liposomal thymidylate synthase inhibitor OSI owned CP-547,632 VEGFR inhibitor Pfizer CP-724,714 HER2-neu inhibitor Pfizer |
| | | | | |
| 1 | | Oncology indications only. | | |
| | | | | |
| 2 | | North American marketing rights held by Cell Pathways. Proposed merger between OSI Pharmaceuticals and Cell Pathways subject to Cell Pathways’ shareholder approval. | | (OSI)TMoncology |
| | | | | |
| 3 | | Currently owned by Cell Pathways. Proposed merger between OSI Pharmaceuticals and Cell Pathways subject to Cell Pathways’ shareholder approval. | | |

| Targeted apoptosis: Aptosyn®* and CP461* |
Introduction
| • | | Cancer cells have two specific characteristics in common: rapid cell division (proliferation) and the loss of programmed cell death (apoptosis). The genetic and biochemical components of cell signaling responsible for maintaining the fine balance between proliferation and apoptosis in normal cells become dysregulated in cancer, resulting in cellular ‘immortality’. Both processes offer potential targets for novel, specific anticancer agents. |
| |
| • | | SelectiveApoptoticAntiNeoplasticDrug (SAAND) technology is a novel approach to the treatment and prevention of cancer, via targeted apoptosis, which has significant advantages compared with conventional chemotherapy, including oral bioavailability and the sparing of healthy tissue. |
| |
| • | | Standard chemotherapeutic agents induce apoptosis in rapidly proliferating cells without differentiating between those that are cancerous and those that are normal. This ‘non-specific’ apoptosis is responsible for many of the adverse effects (AEs) associated with conventional cancer treatments. SAANDs differ because they selectively induce apoptosis in cancerous or precancerous cells (Figure 1). |
| |
| • | | Aptosyn® (exisulind) and CP461 are the first two SAAND products in development. Both compounds have shown single-agent in-vitro growth inhibitory activity against a number of human tumor cell lines, and in-vivo antitumor activity in animal models (human tumor xenografts); both have also shown preclinical evidence of synergistic or additive activity with standard chemotherapeutic agents and other novel anticancer agents. |
| |
| • | | Aptosyn® is being evaluated in combination with standard chemotherapy, in a number of clinical trials in patients with various solid tumors (lung, prostate, colon, and breast). CP461 monotherapy is being evaluated in several clinical trials in patients with prostate, renal, and other solid tumors. |
Figure 1. Non-specific versus targeted apoptosis
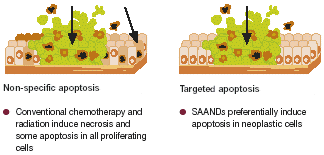
| Non-specific apoptosis - - Conventional chemotherapy and radiation induce necrosis and some apoptosis in all proliferating cellsTargeted apoptosis - - SAANDs preferentially induce apoptosis in neoplastic cells |
Mechanism of action
| • | | SAANDs selectively induce apoptosis in neoplastic cells via inhibition of a key enzyme involved in cell signaling – cyclic GMP phosphodiesterase (cGMP-PDE) – which such cells overexpress. |
| |
| • | | PDE inhibition results in increased cellular cGMP, which in turn initiates a cascade of cellular signaling steps culminating in cell death. This process includes activation of protein kinase G, which results in decreased ε-catenin and activation of JNK1, resulting in cell death (Figure 2).1,2 The SAAND cellular target, cGMP-PDE, has been identified in various solid tumor types, including lung, prostate, colon, and breast. |
| |
| • | | This unique mechanism differs radically from the effects of radiation and many standard chemotherapeutic agents, which act via other cellular proteins to activate apoptosis as a secondary response to cellular insult. In contrast, SAANDs stimulate a fundamental, primary apoptotic pathway to restore signal transmission, and show a high degree of specificity for abnormal cells. |
| |
| • | | There is also evidence to suggest that CP461 may have a secondary anticancer action via microtubule disruption and cell-cycle blockade.3 |
* Currently owned by Cell Pathways Inc. Proposed merger between OSI Pharmaceuticals and Cell Pathways Inc. subject to Cell Pathways’ shareholder approval.
Figure 2. Induction of apoptosis in neoplastic cells by SAANDs (Aptosyn® and CP461)
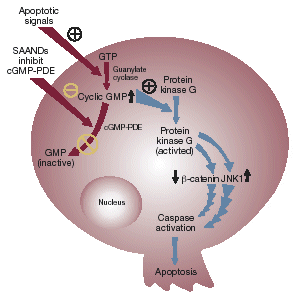
| Apoptotic signals SAANDs GTP inhibit cGMP-PDE Guanylate cyclase Protein kinase G Cyclic GMP cGMP-PDE Protein kinase G GMP(activted) (inactive) (beta)-catenin JNK1 Nucleus Caspase activation Apoptosis |
Preclinical studies
| • | | SAANDs induce apoptosis in numerous human tumor cell lines in vitro (data from selected experiments are shown in Figure 3). |
Figure 3. Effect of SAANDs on tumor cell growth inhibition in a range of human tumor cell linesin vitro
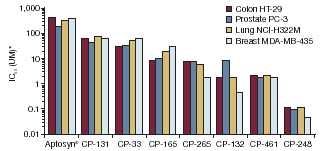
| 1,000 Colon HT-29 Prostate PC-3 Lung NCI-H322M 100 Breast MDA-MB-435 (UM)* 10 50 IC 1 0.1 0.01 Aptosyn® CP-131 CP-33 CP-165 CP-265 CP-132 CP-461 CP-248 |
*IC50 is the concentration of each agent that is required to inhibit cell growth by 50% Data on file at Cell Pathways Inc.
| • | | Aptosyn® inhibits the growth of both cancerous and precancerous cells in cell culture and animal models, restoring normal growth patterns by stimulating apoptosis. Importantly, Aptosyn® induces apoptosis in a significant number of clinically important cancer cell lines without the development of resistance. |
| |
| • | | In both androgen-sensitive, and androgen-insensitive human prostate cancer cell lines, Aptosyn® induces apoptosis in a dose-dependent manner, without affecting normal prostate cells.4 |
| |
| • | | At concentrations that induced apoptosis in androgen-sensitive human prostate cancer cells, Aptosyn® also down-regulated androgen receptor expression. |
| |
| • | | In-vivo studies in rodent models of colon, prostate, lung, breast, and bladder cancer show that Aptosyn®significantly inhibits tumor occurrence or progression (data from a prostate model are shown in Figure 45). |
| |
| • | | Aptosyn® and CP461 have shown synergistic or additive activityin vitro and in animal experiments (Aptosyn® only) in combination with a range of chemotherapeutic agents. |
| |
| • | | In-vitro experiments show |
| | – | | enhancement of the growth inhibitory effects of gemcitabine and vinorelbine in human lung cancer cell lines by Aptosyn ®6 |
| |
| | – | | synergistic effects of Aptosyn® with paclitaxel, cisplatin, and the chemoprevention agent, 13-cis-retinoic acid, in NSCLC and SCLC7 |
Figure 4. Antitumor activity of Aptosyn® in a human prostate cancer xenograft model
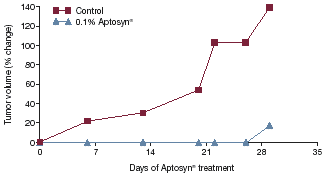
| (p=0.02 versus control) 140 Control 0.1% Aptosyn® 120 100 80 60 Tumor volume (% change) 40 20 0 0 7 14 21 28 35 Days of Aptosyn® treatment |
| | – | | activity of CP461 in combination with gemcitabine, vinorelbine or irinotecan in non-small cell lung cancer (NSCLC) cell lines8 |
| |
| | – | | synergy of both Aptosyn® and CP461 with the monoclonal antibody Herceptin® (trastuzumab) in breast cancer cells. 9 |
| • | | In an animal model of human NSCLC in athymic nude rats, the combined use of Aptosyn® and docetaxel resulted in 100% survival at 80 days (Figure 5).10 |
Figure 5. Effect of Aptosyn® in combination with docetaxel on survival in a human NSCLC xenograft model
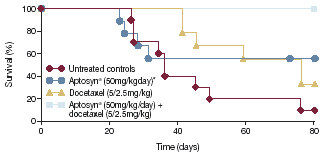
| 100 80 60 Untreated controls 40 Survival (%) Aptosyn® (50mg/kgday)* Docetaxel (5/2.5mg/kg) 20 Aptosyn® (50mg/kg/day) + docetaxel (5/2.5mg/kg) 0 0 20 40 60 80 Time (days) |
*Aptosyn® treament started 1 week after cell implantation
1Docetaxel was injected IP, 5mg/kg/day twice during the first week then 2.5mg/kg/day for the next 4 weeks
Clinical trials
| • | | A program of clinical trials is underway for both Aptosyn® and CP461. Both compounds have shown acceptable toxicity in Phase I studies, and are currently being evaluated for safety and efficacy in several cancer indications. |
Aptosyn®
| • | | In a double-blind, randomized, placebo-controlled Phase II trial of 96 men with increasing prostate-specific antigen (PSA) after radical prostatectomy, Aptosyn® inhibited rise in PSA overall and prolonged PSA doubling time in high-risk patients.11 |
| | – | | Treatment duration was 1 year. |
| |
| | – | | Patients received oral Aptosyn® 250mg twice daily, with dose reduction to 400mg/day, or discontinuation, if AEs occurred; resumption of normal dosing was permitted after demonstrated stability at the lower dose. |
| |
| | – | | Inclusion criteria were increased PSA (0.4–15ng/mL) and an increase in PSA of 10% or more during the 4 months prior to enrollment. Patients were classified as low, intermediate or high risk (for metastatic disease progression). |
| |
| | – | | Aptosyn® significantly suppressed increasing PSA in the overall study population (p=0.017). |
| |
| | – | | In high-risk patients, Aptosyn® significantly increased PSA doubling time (increase from 5.63 to 8.84 months versus decrease from 4.9 to 2.39 months in placebo; p=0.048). |
| |
| | – | | Aptosyn® was well tolerated; AEs occurred in the first 3 months of therapy and were short-lasting and mild to moderate in severity. |
| • | | A Phase III randomized, double-blind, multicenter, placebo-controlled trial evaluating Aptosyn® in combination with docetaxel (Taxotere®) in 600 patients with refractory NSCLC has completed enrollment. The primary endpoint of this study is survival. |
CP461
| • | | CP461 is being evaluated in Phase I/II studies for CLL, renal cell carcinoma, hormone-refractory prostate cancer and a range of solid tumors. |
| |
| • | | In a Phase I study of 21 patients with a range of solid tumors, oral CP461 twice daily for 28 days was well tolerated, with only minimal toxicity at doses up to 800mg/day. The compound showed dose-dependent pharmacokinetics throughout most of the dosing range.12 |
| |
| • | | Interim analysis of data from a Phase II(a) study of 14 patients with hormone-refractory prostate cancer, who received CP461 200mg twice daily, shows one patient with a decrease in liver metastases (with 40% decrease in PSA), one patient with 90% decrease in adenopathy, and one patient with stable disease in the liver at 6 months.13 There were no serious AEs. |
Summary
| • | | Apoptosis is down-regulated/absent in cancer cells; targeting this cellular process represents a strategy for anticancer therapy. |
| |
| • | | SelectiveApoptoticAntiNeoplastic Drug (SAAND) technology is a novel approach to cancer treatment and prevention via targeted apoptosis. |
| |
| • | | Advantages include oral bioavailability and selectivity for abnormal tissues. |
| |
| • | | Both agents have demonstrated in-vitro inhibitory activity against a range of human tumor cell lines, and Aptosyn® showed in-vivo antitumor activity in various animal models of human disease, including cancers of the lung, prostate, colon, and breast. |
| |
| • | | Aptosyn® has numerous completed, ongoing and approved trials in patients with colon, prostate, lung, and breast cancer, and has recently completed enrollment of a Phase III trial evaluating Aptosyn® plus docetaxel versus placebo plus docetaxel in patients with refractory NSCLC. |
| |
| • | | CP461 is being evaluated in Phase I/II studies for CLL, renal cell carcinoma, hormone-refractory prostate cancer and a range of solid tumors, and has shown encouraging preliminary results. |
Conclusions
| • | | SAANDs represent a novel approach to cancer treatment. |
| |
| • | | The first SAANDs, Aptosyn® and CP461, have the potential to complement both conventional chemotherapies and the rapidly developing armamentarium of targeted anticancer therapies becoming available to practicing oncologists. |
| |
| • | | Both compounds are well tolerated, and have shown encouraging results in several clinical trials. |
References
| 1. | | Thompson WJ et al. Cancer Res 2000;60:3338–42. |
| 2. | | Liu L et al. J Pharmacol Exp Ther 2001;299:583–92. |
| 3. | | Whitehead CM et al. Proc Am Assoc Cancer Res 2002;43:410 (Abstract 2043). |
| 4. | | Lim JT et al. Biochem Pharmacol 1999;58:1097–197. |
| 5. | | Goluboff ET et al. Urology 1999;53:440–5. |
| 6. | | Schiller JH et al. Ann Oncol 2000;11:640P, 140. |
| 7. | | Soriano AF et al. Cancer Res 1999;59:6178–84. |
| 8. | | Schiller JH et al. Ann Oncol 2000;11:661P, 144. |
| 9. | | Pegram M et al. Eur J Cancer 2001;37:30. |
| 10. | | Chan DC et al. Clin Cancer Res 2002;8:904–12. |
| 11. | | Goluboff ET et al. J Urol 2001;166:882–6. |
| 12. | | Sun W et al. Clin Cancer Res 2002;8:3100–4. |
| 13. | | Data on file. Cell Pathways Inc. |
(OSI)TMoncology

Introduction
| • | | Oral mucositis, a painful inflammation and ulceration of the surface of the mouth and throat, is a common side effect of cancer chemotherapy and radiotherapy. |
| |
| • | | Reports estimate that of the 1.2 million cancer patients receiving cytotoxic chemotherapy in the USA, approximately 400,000 suffer from oral mucositis.1 |
| |
| • | | Studies indicate that this condition occurs in approximately 40% of patients receiving standard-dose chemotherapy,2 up to 89% of patients receiving higher doses of chemotherapy in bone marrow transplantation,3 and up to 100% of patients receiving radiotherapy for head and neck cancer (Table 1).4 |
| |
| | | Table 1. The main causes of oral mucositis |
| | | |
| Therapy | | Mucositis-related observations |
| |
|
| Standard-dose chemotherapy | | Mucositis is generally grade 1/2 in severity. Symptoms usually last 1-10 days. S-phase specific antineoplastics such as 5-fluorouracil, methotrexate, and cytarabine are more likely to cause mucositis than non cell-cycle phase-specific drugs. |
| | | |
| High-dose chemotherapy | | Mucositis is often grade 3/4 in severity, particularly if chemotherapy is accompanied by total body irradiation. Mucositis is a particularly serious in these patients, causing morbidity and even death.5 |
| | | |
| Radiotherapy and chemotherapy for head and neck cancer | | Approximately 88% of patients may experience grade 3/4 mucositis with particularly aggressive therapy.6 Symptoms begin 1-2 weeks after the first dose of radiation and usually continue for 1-3 weeks after the last dose. |
Oral mucositis: potential impact on therapeutic efficacy
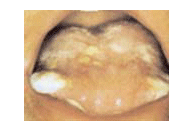
| • | | The symptoms of oral mucositis include painful ulcerations, redness, and swelling in the mouth (Figure 1). In more severe cases, reduction/interruption of therapy or hospitalization may be necessary; there is an increased risk of infection; and extreme pain may interfere with patient’s ability to eat, necessitating intravenous nutrition. |
| |
| • | | Oral mucositis is now recognized as a dose-limiting toxicity in certain cancer models. Thus, it may have a direct impact on duration of disease, remission, cure rates, and long-term survival.7 |
| |
| • | | Several treatment-related adverse events, such as neutropenia and nausea, are now treated with more success and this provides an opportunity to improve therapeutic outcome. Therefore, the successful management of oral mucositis may be clinically valuable.8 |
| |
| • | | The incidence of oral mucositis is expected to increase as oncologists continue to use more advanced chemotherapeutic agents and add new targeted or cytotoxic agents to standard regimens. |
| |
| | | Figure 1. Oral mucositis (grade 3/4) |
Risk factors for oral mucositis
Several risk factors have been identified for the development of chemotherapy-related oral mucositis
| • | | Type and intensity of therapy (Table 1) |
| * | | North American marketing rights held by Cell Pathways. Proposed merger between OSI Pharmaceuticals and Cell Pathways subject to Cell Pathways’ shareholder approval |
| • | | age — younger patients are at greater risk of developing oral mucositis than older patients receiving the same type of treatment. However, once formed, lesions do heal more quickly in younger patients |
| |
| • | | diagnosis — patients with hematologic malignancies are at greater risk of developing oral mucositis than those with solid tumors |
| |
| • | | oral health — patients with a high standard of oral hygiene during cancer therapy tend to have fewer episodes of mucositis than those with poor oral health. |
The patient’s perception of oral mucositis
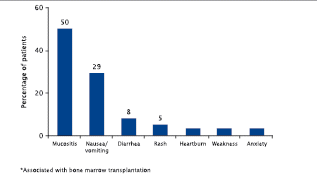
| • | | Oral mucositis is perceived by patients as one of the most debilitating side effects associated with cancer therapy (Figure 2).9 |
| |
| • | | Many patients indicate that commonly prescribed treatments (e.g. ‘Magic Mouthwash’) only provide marginal relief from the symptoms of oral mucositis. |
| |
| • | | Most mild-to-moderate cases are usually left untreated as patients are hesitant to bother their oncologists with complaints, resulting in ‘silent suffering’. |
| |
| • | | Offering susceptible patients an effective treatment is likely to improve their quality of life and could limit any negative effect of this condition on their medical treatment. |
Figure 2. Oral mucositis* — from the patient’s perspective, one of the most debilitating side effects associated with cancer therapy9
Gelclair™
| • | | Gelclair™ is a bioadherent oral gel that provides rapid and durable pain relief. Its protective, hydrating, and lubricating properties allows patients with oral mucositis to function more normally. Its unique characteristics are summarized in Table 2. |
| |
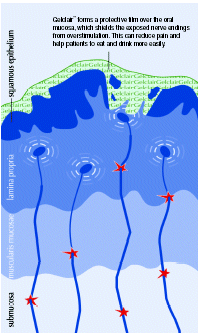
| • | | Gelclair™ forms a protective coating over the surface of the mouth and throat soothing pain from oral lesions and protecting the tissue from further irritation caused by eating, drinking and/or speaking (Figure 3). |
| |
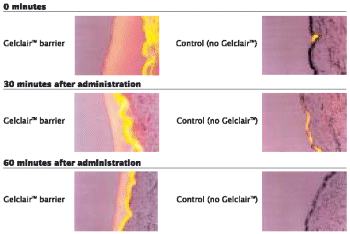
| • | | The protective barrier formed by Gelclair™ is durable and is still intact 60 minutes after application (Figure 4). |
| |
| • | | Gelclair™ contains sodium hyaluronate, polyvinylpyrrolidone, and glycyrrhetinic acid that provide its unique mucoadherent, lubricating, and hydrating properties. |
Table 2. Gelclair™: an innovative product with distinct characteristics
| | | Rapid and dramatic pain relief |
| |
| • | | 92% reduction in oral pain 5-7 hours after treatment; this is significantly longer than other recommended treatments |
| |
| • | | 100% of patients experience substantial pain relief
Durable pain relief that may lead to improved nutrition |
| |
| • | | 50% of patients report overall improvement related to pain and discomfort on swallowing and eating after 7-10 days of Gelclair™
Other characteristics |
| |
| • | | Well tolerated |
| |
| • | | Excellent lubricating, hydrating, and coating properties |
| |
| • | | No numbing, stinging, or drying |
| |
| • | | Pleasant tasting and easily applied |
Sodium hyaluronate
| • | | Hyaluronic acid, a naturally occurring polysaccharide, is found in the extracellular matrix of connective tissue. As a result of its viscosity and elasticity, hyaluronic acid acts in the body as a lubricating and shock absorbing fluid. |
Figure 3. GelclairTM: a bioadherent oral gel that provides rapid and durable pain relief
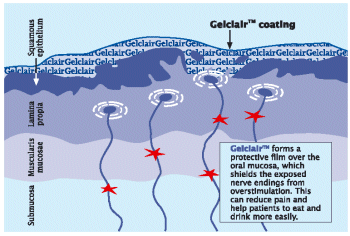
Figure 4. The durability of the GelclairTM barrier compared with control
| • | | In GelclairTM, sodium hyaluronate works as a film-forming, mucoadherent agent to aid in covering the oral mucosa. |
| |
| • | | Sodium hyaluronate may also enhance tissue hydration, mediate tissue repair, and wound healing; it is also thought to have anti-inflammatory properties. |
Polyvinylpyrrolidone
| • | | Polyvinylpyrrolidone is a hydrophilic polymer with mucoadherent and film-forming properties. In GelclairTM, these properties aid in coating the oral mucosa. |
Glycyrrhetinic acid
| • | | Glycyrrhetinic acid is a metabolite of glycyrrhizin, a component of liquorice. Recent reports show glycyrrhizin is an allergic and antihepatitis agent; it has also been used topically because of its anti-inflammatory properties.10 |
| |
| • | | Data from animal models suggest glycyrrhetinic acid may have pain-relief properties. |
| |
| • | | In GelclairTM, glycyrrhetinic acid is used as a sweetening and flavoring agent. |
Symptomatic response to Gelclair™ use
Three studies have examined symptomatic response to GelclairTM
| • | | In an open-label study of 30 patients with oral lesions of varying etiology, pain and functionality level were measured before and after using GelclairTM 11 |
| | - | | 100% of patients reported a substantial reduction in oral discomfort within 5-7 hours of initial application (p<0.005) |
| |
| | - | | mean pain score was reduced by 92%, 5-7 hours after initial application of GelclairTM (p<0.005) (Figure 5) |
| |
| | - | | 50% of patients reported that GelclairTM improved overall pain and discomfort associated with eating and drinking after 7-10 days of use compared with baseline (p<0.005) (Figure 6) |
| |
| | - | | GelclairTM was easy to use and well tolerated, with no reported side effects in this study. |
| • | | In an open-label study, 30 patients with chemotherapy induced oral mucositis were evaluated for pain, functionality, and grade of oral mucositis at baseline, 1, and 3 days after treatment with GelclairTM 12 |
| | - | | 25 patients (83%) reported a reduction in pain, four (13%) had no change, and one (3%) improved then worsened |
| |
| | - | | 25 patients (83%) had a distinct improvement in functionality, two (7%) did not change, and three (10%) became worse |
| |
| | - | | the grade of mucositis improved in 17 patients (57%), 12 (40%) remained the same, and one (3%) became worse. |
Figure 5. Reduction in mean pain score 5-7 hours after initial application of GelclairTM 11
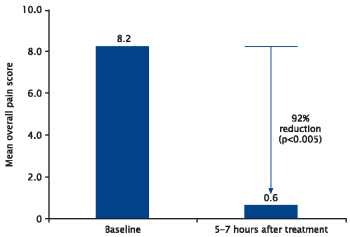
Figure 6. Improvement in overall pain score associated with eating and drinking after 7-10 days of GelclairTM use11
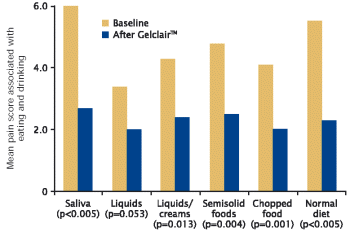
| Mean pain score associated with eating and drinking |
| • | | In the final study, 10 patients evaluated the effect of GelclairTM on their oral mucositis symptoms. All the patients reported that the product was acceptable and had a palliative, soothing effect.13 |
Summary
| • | | GelclairTM provides rapid and durable pain relief, and improves functionality in patients with oral mucositis. |
| |
| • | | GelclairTM contains three key agents that result in its unique mucoadherent, lubricating, and hydrating properties. |
| |
| • | | GelclairTM is well tolerated. |
| |
| • | | GelclairTM provides rapid and durable oral pain relief and may improve a patient’s nutrition. |
References
| 1. | | National Institute of Dental and Craniofacial Research. Accessed 22th April 2003, http://wwwnidr.nih.gov/about/open2002.asp |
| |
| 2. | | Scully C et al. Eur J Cancer Oral Oncol 1996;32:281-92. |
| |
| 3. | | Woo S et al. Cancer 1993;72:1612-17. |
| |
| 4. | | Sonis S et al. Cancer 1999;85:2103-13. |
| |
| 5. | | Sefcick A et al. Bone Marrow Transplant 2001;28:1135-9. |
| |
| 6. | | Lavertu P et al. Arch Otolaryngol Head Neck Surg 1999;125:142-8. |
| |
| 7. | | Peterson D. Curr Opin Oncol 1999;11;261-6. |
| |
| 8. | | Karthaus M et al. Bone Marrow Transplant 1999;24:1095-108. |
| |
| 9. | | Stiff P. Bone Marrow Transplant 2001;27(Suppl. 2):S3-S11. |
| |
| 10. | | Shibata S. Yakugaku Zasshi 2000;120:849-62. |
| |
| 11. | | Innocenti M et al. J Pain Symptom Manage 2002;24:456-7. |
| |
| 12. | | De Cordi D et al. Italian Tumour League III congress for professional oncology nurses, Conegliano, Italy, 10-12 Oct. 2001. |
| |
| 13. | | Berndtson M, Swedish Hospital Dentistry 2001;3:17-21. |
(OSI)TMoncology

























