
Bicifadine SR
Stuart Apfel, M.D.
Senior Director of Clinical Research, DOV
Associate Professor of Neurology,
Albert Einstein College of Medicine

Safe Harbor Statement
This document contains forward-looking statements that involve significant
risks and uncertainties, including those discussed below and described more
fully in our annual report on Form 10-K filed with the Securities and Exchange
Commission on March 15, 2006 and subsequent 10-Q filings. These
statements are made as aids to a verbal presentation. They reflect our
current expectations concerning future events, and thus our actual results
could differ materially from those anticipated in these forward-looking
statements as a result of many factors. These factors include our ability to
achieve and maintain profitability, the extent to which we collaborate with third
parties on drug discovery and development activities, the ability of our
collaborators and of DOV to meet drug development objectives tied to
milestones and royalties and our ability to attract and retain experienced
scientists and management. We undertake no duty and have no intention to
update any forward-looking statements to reflect new facts that come to light.
2

Bicifadine: Molecular Pharmacology Compared to
Other Analgesic Uptake Inhibitors
Bicifadine is relatively “balanced” inhibitor of NE, 5-HT uptake
>100,000
68
151
Milnacipran1
3070
1420
145
Venlafaxine1
439
20
3.7
Duloxetine1
872a
46.3a
105a
Bicifadine
DA, (Ki nM)
NE, (Ki nM)
5-HT, (Ki nM)
Drug
Human transporters
a: Ki estimated using Km values of: 170 for 5-HT; 110 for NE; 460 for DA
Vaishnavi et al., Biol Psych 55:320, 20
3

How Relevant Is Reuptake Blockade to MOA?
K. Rodgers, Wyeth
4

Bicifadine Efficacy in Pre-Clinical Tests
Effective analgesic
Acute Pain:
Yeast inflamed-paw
Formalin test (both phases)
PPQ-induced writhing
Colonic distention
Chronic Pain:
Chung SNL model
Diabetic neuropathy
Ineffective in hot plate, tail flick
Antidepressant profile
Behavioral despair
Tetrabenazine-induced ptosis, sedation
FST
TST
5

Bicifadine Suppresses Thermal Hypersensitivity
in the Chung Model of Neuropathic Pain
6

Bicifadine SR:
Clinical Overview

Clinical Pharmacology Program
Mean Plasma Concentrations of Bicifadine in Healthy Male Volunteers
Following a Single Oral Dose of 200 mg and 400 mg on Day 1
8
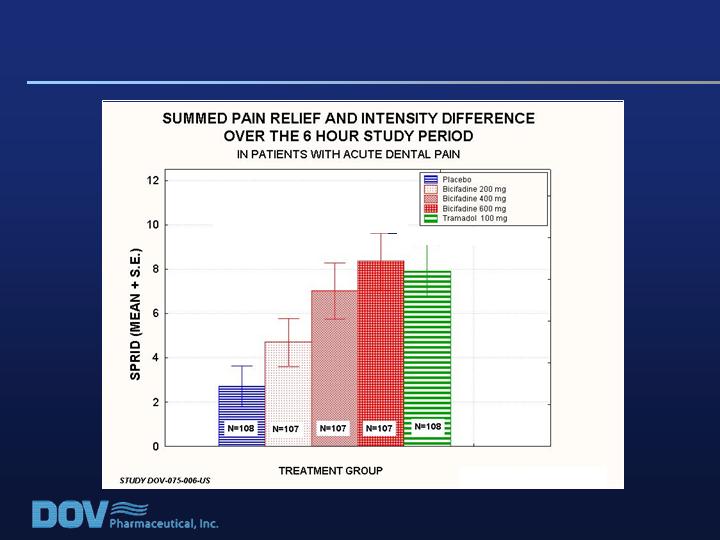
Dental Pain Efficacy Study DOV-075-006-US
p<.0001 dose response
9
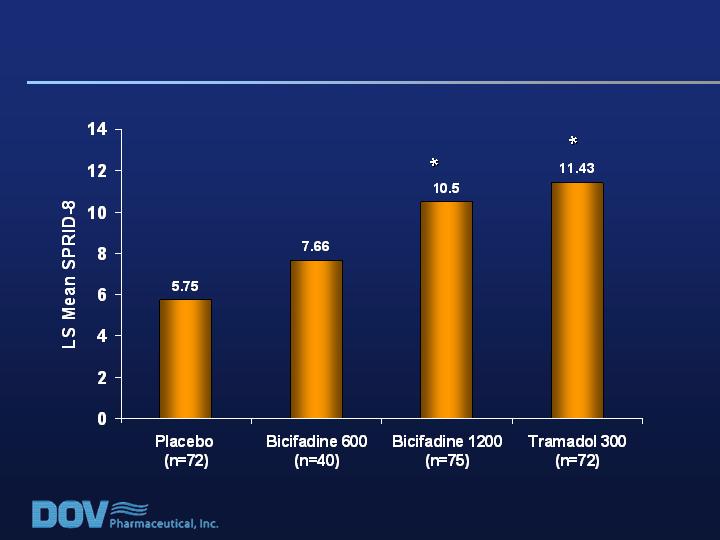
* P = 0.01 vs placebo
Analgesia Scores During First 8 Hours
10

Chronic Low Back Pain (CLBP) Trials
Ongoing:
Study 020: three-month double-blind placebo
controlled dose-response trial
Bicifadine doses: 400, 600, 800 mg/day
Study recently completed
Study 021: three-month replication trial using
overlapping dose levels
Bicifadine doses: 400, 800 mg/day
Study 022: long term safety and efficacy trial
One year, 800 mg/day
11

Study 020: Design in CLBP
Double-blind comparison of 0, 400, 600, 800 mg/day
bicifadine given for up to 3 months
54 US centers, n=636 randomized
Assessments at weeks 1, 2, 4, 8 and 12
Primary endpoint:
Change from baseline to termination in VAS pain score
Secondary endpoints:
Time course of changes in VAS, RDQ, MPQ, global, SF-36
Safety measures:
AEs, labs, ECG, physical, withdrawal effects
Completers can rollover to one-year open label study
(022), bicifadine 800 mg/day
12

Study 020: Main Entry Criteria
CLBP treated pharmacologically for at least the
prior 3 months
Baseline VAS =>40 mm
Back pain not due to cancer, severe congenital
malformation
Quebec Class 1-3
Classes 2 & 3 = radicular pain in the leg
No recent treatment with steroids, epidurals,
back pain surgery
No depression; BECK <17
13

606 Randomized*
151 Randomized to
Placebo
154 Randomized to
Bicifadine 400mg daily
153 Randomized to
Bicifadine 800mg daily
148 Randomized to
Bicifadine 600mg daily
29% Discontinued
9% Lack of efficacy
7% Adverse Event
13% All Other Reasons†
29% Discontinued
8% Lack of efficacy
9% Adverse Event
12% All Other Reasons†
47% Discontinued
7% Lack of efficacy
20% Adverse Event
20% All Other Reasons†
45% Discontinued
6% Lack of efficacy
27% Adverse Event
12% All Other Reasons†
72%
Completed Study
71%
Completed Study
53%
Completed Study
55%
Completed Study
Figure 1. Subject disposition. This figure presents the flow of patients through the study from randomization to completion.
† All other reasons include: Non-compliance, lost to follow-up, patient request, administrative, other.
* 28 Additional patients from 3 related sites were randomized, but not shown due to data integrity issues.
Study 020: Patient Flow & Disposition
14
14
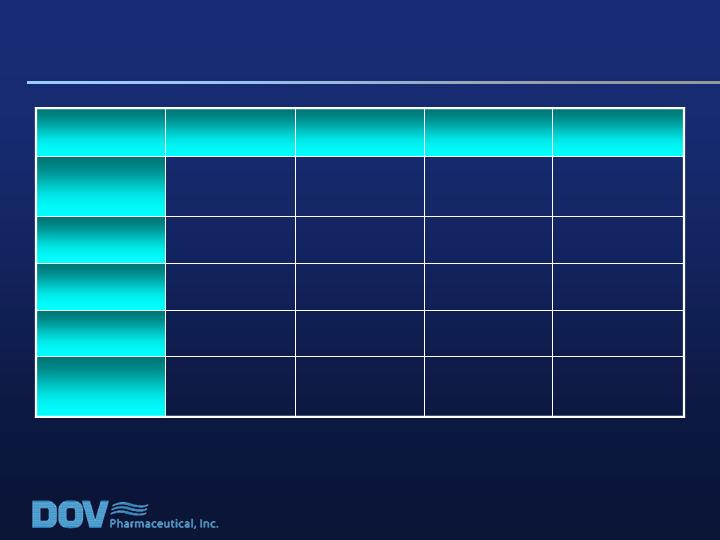
Study 020: Patient Demography
48.2
(20-73)
47.4
(19-67)
48.8
(19-72)
47.5
(21-71)
Age
(range)
186
197
187
190
Weight
(lbs)
11%
15%
16%
15%
% Black
66%
69%
66%
68%
% White
58%
55%
61%
56%
% Female
800 mg/d
600 mg/d
400 mg/d
Placebo
15
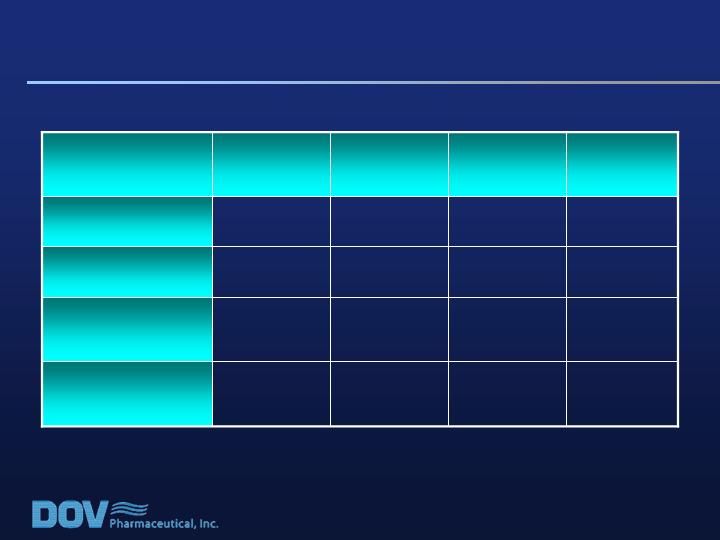
Study 020: Change from Baseline in VAS Pain
Scores at End of Treatment (LOCF) in ITT Pop.
0.50
0.50
0.29
p-Value
2.31
2.43
2.30
2.30
±SE
-0.5
0.1
-3.4
Effect Size (P-B)
25.0
24.4
27.9
24.5
LS Mean
800mg/d
(n=153)
600mg/d
(n=148)
400mg/d
(n=154)
Placebo
(n=151)
16
Study 020: Change from Baseline in VAS Pain
Scores at Week 12 (Completers) in ITT Pop.
0.40
0.22
0.04
p-Value
2.99
3.13
2.60
2.63
±SE
-3.3
-4.9
-7.2
Effect Size (P-B)
29.6
31.2
33.5
26.3
LS Mean
800mg/d
(n=85)
600mg/d
(n=81)
400mg/d
(n=112)
Placebo
(n=111)
17

Bicifadine SR Study 020:
Analyses of
Identified Key Baseline Variables
18

Study 020:
Disability Effect on Bicifadine Effect Size (Pain)
RDQ at Baseline: Impact on VAS Score Difference by Dosea
a – Change in score from Baseline to Termination; bicifadine minus placebo
Mean placebo response: RDQ>17 = 10.8; RDQ=17 = 27.7
Min Effect Size
Detectable
19

Placebo Bicifadine (mg b.i.d)
200 300 400
n=117 n=113 n=103 n=120
Placebo Bicifadine (mg b.i.d.)
200 300 400
n=28 n=34 n=28 n=26
Baseline Disability Scores:
Effect on Pain Score Improvement
20

Study 020: Impact of Baseline RDQ Disability
Score on Bicifadine and Placebo Pain Scores
Bicifadine n = 50 375 318 269 240 195 163 123 88
Placebo n = 18 127 114 101 83 69 57 43 28
21
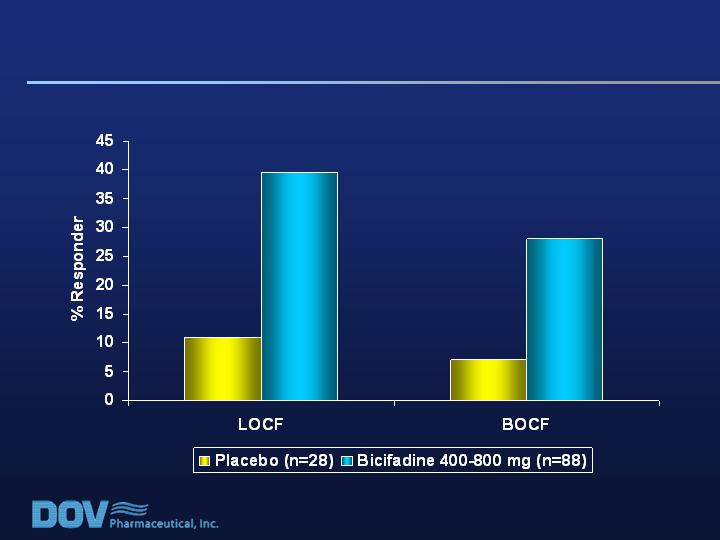
Pain Responder Analysis (50% Improvement)
for RDQ > 17 Baseline
P = 0.004
P = 0.02
22
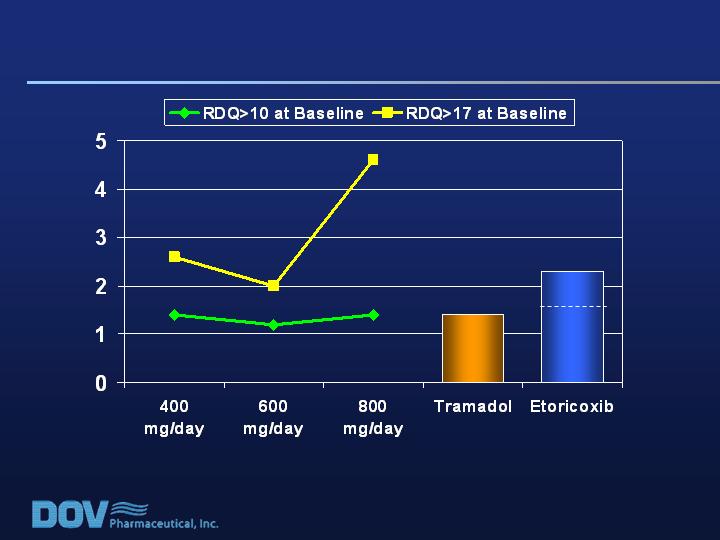
RDQ Effect Size (LOCF)
RDQ >10 n = 132 111 133
RDQ >17 n = 35 28 27
n = 132 n = 103-107
23

The Quebec Task Force Classification
for Spinal Disorders
Class 1: Pain without radiation to an extremity and without
neurological signs
Class 2: Pain with radiation to a proximal extremity (not below the
knee) and without neurological signs
Class 3: Pain with radiation to a distal extremity (below the knee)
and without neurological signs
Class 4: Pain with radiation and neurological finding
Class 5: Spinal instability or fracture
Class 6: Spinal nerve root compression
Class 7: Spinal stenosis
Class 8: Post-surgical status, 1-6 months after intervention
Class 9: Post-surgical status, >6 months after intervention
Class 10: Chronic pain syndrome
Class 11: Other diagnoses
Included in Study 020 Excluded in Study 020
24
Placebo Bicifadine (mg b.i.d)
200 300 400
n=84 n=82 n=67 n=79
Placebo Bicifadine (mg b.i.d.)
200 300 400
n=30 n=36 n=38 n=40
Quebec Classification (1 vs. 2-3): Predictor of
Bicifadine Efficacy on VAS Pain Score (LOCF)
25
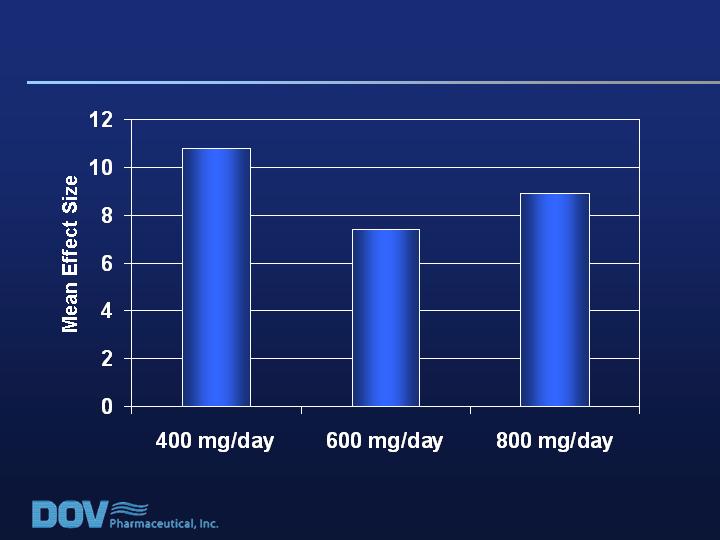
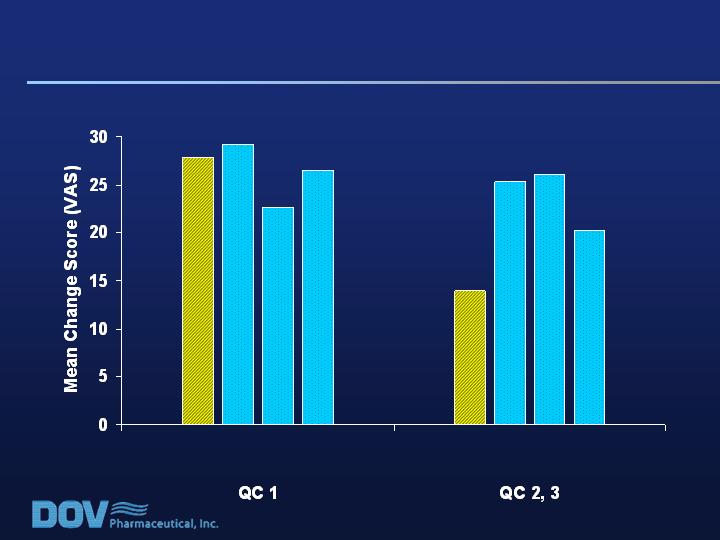
Mean Placebo change score = 14.2
* p<0.05
(n=58)
(n=60)
(n=64)
*
Key Patient Population (RDQ >17; QC 2-3):
Bicifadine Effect Size on VAS Pain Scores
26

Bicifadine SR:
Safety
27
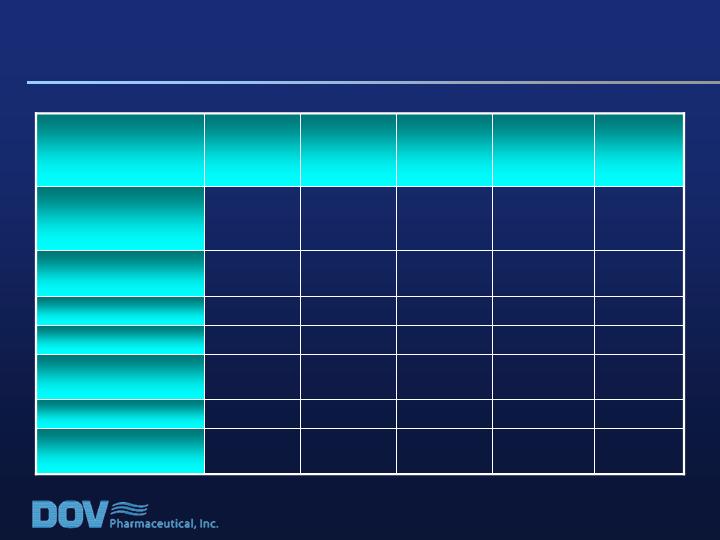
Incidents of Serious Adverse Events (SAEs)
1
0
0
1
0
Cerebrovascular
Accident
1
1
0
0
0
Dehydration
1
0
1
0
0
Pharyngitis
Streptococcal
1
1
0
0
0
Gastroenteritis
1
0
1
0
0
Tachycardia
1
0
0
0
1
Myocardial
Infarction
5
1
2
1
1
Total # of Patients
with at Least One
SAE
Total
(n=606)
Bicifadine
800mg/d
(n=153)
Bicifadine
600mg/d
(n=148)
Bicifadine
400mg/d
(n=154)
Placebo
(n=151)
28
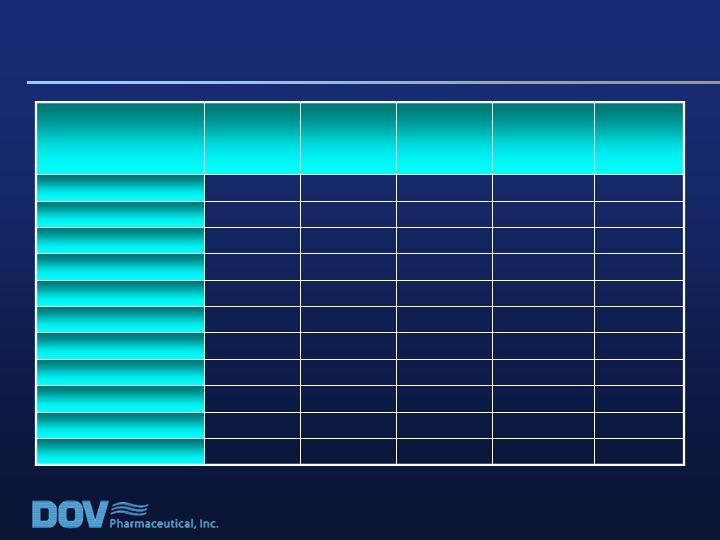
Common Adverse Events (>5%)*
2.8%
5.9%
0.7%
3.2%
1.3%
Dyspepsia
3.1%
6.5%
2.7%
2.6%
0.7%
Somnolence
3.3%
4.6%
6.1%
1.9%
0.7%
Vomiting
3.5%
3.3%
4.7%
1.9%
4.0%
Nasopharyngitis
3.5%
1.3%
6.1%
3.9%
2.6%
Abdominal Pain
3.8%
3.9%
2.7%
5.2%
3.3%
URI
4.1%
3.3%
4.1%
3.9%
5.3%
Diarrhea
4.8%
7.2%
6.1%
5.2%
0.7%
Insomnia
7.6%
15.0%
8.1%
5.2%
2.0%
Dizziness
12.7%
13.1%
10.8%
16.2%
10.6%
Headache
13.2%
17.6%
16.2%
12.3%
6.6%
Nausea
Total
(n=606)
Bicifadine
800mg/d
(n=153)
Bicifadine
600mg/d
(n=148)
Bicifadine
400mg/d
(n=154)
Placebo
(n=151)
* Note: Any individual patient might appear in more than one category
29
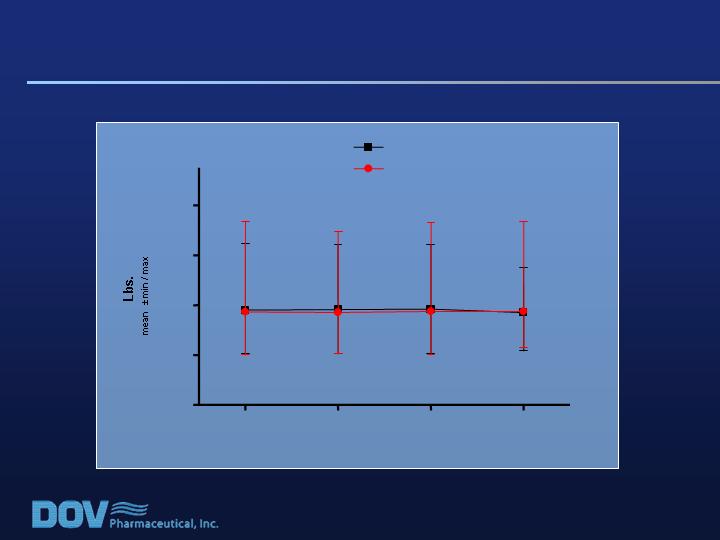
0
8
12
F/U
0
100
200
300
400
Placebo (n=150)
Weeks Post Baseline
Bicifadine 800 mg/day (n=152)
Study 020: No Weight Change
30

0
1
2
4
8
12
F/U
0
20
40
60
80
100
120
140
Placebo (n=151)
Weeks Post Baseline
Bicifadine 800 mg/day (n=153)
Study 020: No Change in Vital Signs / Pulse
31
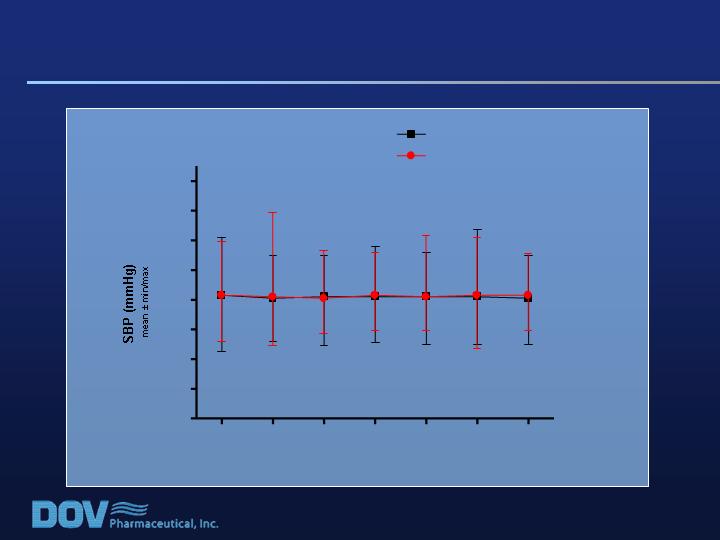
0
1
2
4
8
12
F/U
40
60
80
100
120
140
160
180
200
Placebo (n=151)
Weeks Post Baseline
Bicifadine 800 mg/day (n=153)
Study 020: No Change in Vital Signs / SBP
32

Overall Conclusions
The first phase III trial of bicifadine for the treatment of
CLBP failed to demonstrate efficacy against placebo in
the full ITT population
Key Populations:
RDQ > 17 and QC 2-3 are strong and independent predictors
of bicifadine-placebo differences
Patients with QC1 who do not have moderate to strong
functional impairment show a marked placebo response (mean
change score > 25mm on VAS)
VAS pain scores and RDQ scores can serve as primary
endpoints of bicifadine efficacy
Dose Response:
Bicifadine dose response for CLBP patients within the range of
200-400 mg BID is relatively flat
Placebo-controlled acute pain trial showed doses of 400 mg/day
were minimally effective
33

Overall Conclusions
Bicifadine at doses up to 400 mg BID in
CLBP patients is well-tolerated
200 mg BID has an adverse event profile
similar to placebo
Bicifadine will likely prove to be a useful
analgesic for the treatment of CLBP
Particularly in patients with radicular pain
and/or substantial functional impairment
34

































