Table of Contents
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
(Mark One)
| þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2008
or
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number 001-16391
TASER International, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 86-0741227 | |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification Number) | |
| 17800 N. 85th St. | ||
| Scottsdale, AZ | 85255 | |
| (Address of principal executive offices) | (Zip Code) |
(480) 991-0797
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Common Stock, $0.00001 par value per share (Nasdaq Global Select Market)
Securities registered pursuant to Section 12(g) of the Act: None
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Common Stock, $0.00001 par value per share (Nasdaq Global Select Market)
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yeso Noþ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yeso Noþ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yesþ Noo
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K.o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filero | Accelerated Filerþ | Non-accelerated filer o (Do not check if a smaller reporting company) | Smaller Reporting Companyo |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yeso Noþ
The aggregate market value of the Common Stock held by non-affiliates of the issuer, based on the last sales price of the issuer’s common stock on June 30, 2008, which was the last business day of the registrant’s most recently completed second fiscal quarter, as reported by NASDAQ, was $288,980,107. The number of shares of the registrant’s common stock outstanding as of March 9, 2009, was 61,843,295.
DOCUMENTS INCORPORATED BY REFERENCE
Parts of registrant’s definitive proxy statement to be prepared and filed with the Securities and Exchange Commission not later than 120 days after December 31, 2008 are incorporated by reference into Part III of this Form 10-K.
TASER INTERNATIONAL, INC.
ANNUAL REPORT ON FORM 10-K
Year Ended December 31, 2008
TABLE OF CONTENTS
ANNUAL REPORT ON FORM 10-K
Year Ended December 31, 2008
TABLE OF CONTENTS
| Page | ||||||||
| Item 1. | 4 | |||||||
| Item 1A. | 11 | |||||||
| Item 1B. | 16 | |||||||
| Item 2. | 16 | |||||||
| Item 3. | 16 | |||||||
| Item 4. | 16 | |||||||
| Item 5. | 16 | |||||||
| Item 6. | 18 | |||||||
| Item 7. | 19 | |||||||
| Item 7A. | 29 | |||||||
| Item 8. | 30 | |||||||
| Item 9. | 53 | |||||||
| Item 9A. | 53 | |||||||
| Item 9B. | 54 | |||||||
| Item 10. | 55 | |||||||
| Item 11. | 55 | |||||||
| Item 12. | 55 | |||||||
| Item 13. | 55 | |||||||
| Item 14. | 55 | |||||||
| Item 15. | 55 | |||||||
| 57 | ||||||||
| EX-10.17 | ||||||||
| EX-23.1 | ||||||||
| EX-31.1 | ||||||||
| EX-31.2 | ||||||||
| EX-32 | ||||||||
Table of Contents
PART I
The statements contained in this report that are not historical are “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), including statements regarding our expectations, beliefs, intentions or strategies regarding the future. We intend that such forward-looking statements be subject to the safe-harbor provided by the Private Securities Litigation Reform Act of 1995. Such forward-looking statements relate to, among other things:
| 1. | expected revenue and earnings growth; | ||
| 2. | estimates regarding the size of our target markets; | ||
| 3. | our ability to successfully penetrate the law enforcement market; | ||
| 4. | growth expectations for existing accounts; | ||
| 5. | our ability and strategies to expand product sales to the private security, military, corrections, and private citizen self-defense markets; | ||
| 6. | fluctuations in gross margins; | ||
| 7. | expansion of product capability and the sufficiency of our manufacturing capacity; | ||
| 8. | new product introductions including, but not limited to specific introductions planned for 2009; | ||
| 9. | product safety; | ||
| 10. | our business model and strategy; | ||
| 11. | the automation of our production process; | ||
| 12. | our insulation from competition and our competitive advantage; | ||
| 13. | our focus on future development initiatives; | ||
| 14. | our expectation that research and development costs will continue to increase; | ||
| 15. | our litigation strategy and the importance of recent favorable verdicts; | ||
| 16. | that private citizen sales will continue to grow in 2009; | ||
| 17. | our intention to continue to participate in law enforcement trade shows; | ||
| 18. | our strategy to grow our international presence; | ||
| 19. | that we have readily available alternative materials and components suppliers | ||
| 20. | our ability to emerge from the current economic downturn in a strong position; | ||
| 21. | the sufficiency of our liquid assets and capital resources; | ||
| 22. | trends and expectations relating to certain balance sheet accounts and working capital items; | ||
| 23. | anticipated capital expenditures; and | ||
| 24. | our intention to hold our investments to maturity. |
These statements are qualified by important factors that could cause our actual results to differ materially from those reflected by the forward-looking statements. Such factors include but are not limited to: (1) market acceptance of our products; (2) our ability to establish and expand our direct and indirect distribution channels; (3) our ability to attract and retain the endorsement of key opinion-leaders in the law enforcement community; (4) the level of product technology and price competition for our products; (5) the degree and rate of growth of the markets in which we compete and the accompanying demand for our products; (6) risks associated with rapid technological change and new product introductions; (7) challenges to our intellectual property; (8) competition; (9) litigation including lawsuits alleging product related injuries and death; (10) negative media publicity and reporting concerning our products and their uses; (11) TASER device tests and reports; (12) product quality; (13) implementation of manufacturing automation; (14) potential fluctuations in our quarterly operating results; (15) adverse changes in the U.S. or global economies and other issues that may affect the budgets of our current and prospective customers; (16) order delays; (17) dependence upon sole and limited source suppliers; (18) fluctuations in component pricing; (19) government regulations and inquiries; (20) dependence upon key employees and our ability to retain employees; (21) execution and implementation risks of new technology; (22) aligning manufacturing production with sales demand; (23) medical and safety studies; (24) the outcome of any current or future tax audit of us, and (25) other factors detailed in our filings with the Securities and Exchange Commission, including, without limitation, those factors detailed in ITEM 1A of this annual report entitled “Risk Factors.” The risks included in the foregoing list are not exhaustive. Other sections of this report may include additional factors that could adversely affect our business and financial performance. New risk factors emerge from time to time, and it is not possible for management to predict all such factors, nor can it assess the impact of all such risk factors or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. We undertake no obligation to update or revise any forward looking statements to reflect changed assumptions, the occurrence of unanticipated events or changes to expectations over time.
We own the following trademarks: TASER® and AIR TASERtm, TASER-Wavetm, T-Wavetm, AUTO TASERtm, ADVANCED TASER®, Shaped Pulse Technology tm, X-Rail tm, TASER M18 tm, TASER M26 tm, TASER X26 tm, TASER Cam tm, TASER XREP tm, TASER C2 tm, TASER X26c tm and AFID tm. Each other trademark, trade name or service mark appearing in this report belongs to its respective holder.
3
Table of Contents
Item 1. Business
Overview
TASER International, Inc.’s (the “Company” or “TASER” or “we” or “our”) mission is to protect life by providing safer, more effective force options and technologies. We are a market leader in the development and manufacture of advanced Electronic Control Devices (“ECDs”) designed for use in the law enforcement, military, corrections, private security and personal defense markets. Since our inception in 1993, we have remained committed to providing solutions to violent confrontation by developing devices with proprietary technology to incapacitate dangerous, combative, or high-risk subjects who pose a risk to law enforcement officers, innocent citizens, or themselves in a manner that is generally recognized as a safer alternative to other uses of force.
To that end, we have focused our efforts on the continuous development of our technology for both new and existing products which further our mission. At the same time, we have established industry leading training services to provide our users a comprehensive overview of TASER electronic control devices use-of-force, legal and policy issues, medical information and risk mitigation. We have built a network of distribution channels for selling and marketing our products and services to law enforcement agencies, primarily in North America with ongoing focus and effort placed on expanding these programs in international, military and other markets.
Products
Electronic Control Devices
Our Technology
We make ECDs for two main types of market segments; the law enforcement, military, corrections and professional security markets, and the consumer market. Our products use a replaceable cartridge containing compressed nitrogen to deploy and propel two small probes that are attached to the ECD by insulated conductive wires with lengths ranging from 15 to 35 feet. Our ECDs transmit electrical pulses along the wires and into the body affecting the sensory and motor functions of the peripheral nervous system. The energy can penetrate up to two cumulative inches of clothing, or one inch per probe. The initial effect lasts five seconds for our law enforcement, military and corrections products and up to thirty seconds for our consumer market models. This effect can be extended, if necessary, by the operator.
Law Enforcement, Military, Corrections and Professional Security Products
For the law enforcement, military, corrections and professional security markets we manufacture two product lines. Our primary product is the TASER X26 with Shaped Pulse Technology which we introduced in 2003. Shaped Pulse technology is a refined energy pulse that concentrates a small portion of energy to first penetrate any barriers, while the majority of the energy flows into the target freely after the barrier has been penetrated. The TASER X26 product line consists of the TASER X26, various cartridges (described below), a proprietary battery system, a digital power magazine, download software and equipment, extended warranties, and a number of holstering options and accessories. The TASER X26 product line (excluding individual cartridge sales) accounted for approximately $51.2 million, or 55% of our net sales, for the year ended December 31, 2008 and for approximately $61.0 million, or 60% of our net sales, for the year ended December 31, 2007.
Our second law enforcement product line is the ADVANCED TASER M26 which we originally launched in November 1999. The ADVANCED TASER M26 product line consists of the ADVANCED TASER M26, various cartridges (described below), rechargeable batteries, a battery charging system, data download software and equipment, extended warranties, and a number of holstering options and accessories. The ADVANCED TASER M26 product line (excluding individual cartridge sales) accounted for approximately $2.5 million, or approximately 3% of our net sales, for the year ended December 31, 2008 and for approximately $1.3 million, or 1% of our net sales, for the year ended December 31, 2007.
Consumer Products
For the personal defense market our primary product is the TASER C2 consumer product, which we introduced in 2007. This device is a compact system that provides the same proven Neuro-Muscular Incapacitation (NMI) effectiveness as our market leading TASER X26 but in a less intimidating, more compact form and at a price point more attractive to private citizens. Our sale and marketing of the TASER C2 promotes responsible ownership and aims to prevent misuse by keeping the device inactive until the owner has successfully completed a background check either online or via a toll-free telephone number.
We also manufacture the TASER X26C, ADVANCED TASER M18 and ADVANCED TASER M18L, devices for use by consumers. The X26C was developed in conjunction with the law enforcement TASER X26 version; however, its effect lasts longer allowing the owner more time to escape danger. The ADVANCED TASER M18 and ADVANCED TASER M18L are designed after the law enforcement ADVANCED TASER M26 version; however, the electrical pulse rate is lower. The ADVANCED TASER M18 and ADVANCED TASER M18L are identical except that the ADVANCED TASER M18L has an integrated laser-aiming device. These three product lines consist of the units themselves, air cartridges, batteries and digital power magazines, and a number of holstering options and accessories.
Our total consumer products accounted for approximately $7.6 million, or 8.1% of our net sales, for the year ended December 31, 2008 and for approximately $5.7 million, or 5.7% of our net sales for the year ended December 31, 2007.
4
Table of Contents
Cartridges and Accessories
We manufacture six cartridge types; a 15’ cartridge, a 21’ cartridge, a 25’ XP cartridge, a 35’ cartridge, a 21’ training cartridge and a 15’ cartridge for the C2. The 15’ cartridge is capable of firing a distance of 15’ and is sold primarily to the law enforcement market for training and the consumer market for use in the ADVANCED TASER M18, ADVANCED TASER M18L, and TASER X26C devices. The C2 15’ cartridge is designed specifically for use in the TASER C2. The 21’, 25’ XP, 35’, and 21’ training cartridge are sold only to the law enforcement, military, and corrections markets. The 25’ XP cartridge is different from the 21’ cartridge in that it has a longer range and its probes are longer and heavier, which allows it to penetrate a thicker clothing barrier. The training cartridge contains non-conductive wiring, which allows law enforcement, military, and corrections trainers to use the cartridge during training role-playing scenarios. Individual cartridge sales accounted for approximately $20.5 million, or approximately 22% of our net sales, for the year ended December 31, 2008 and for approximately $25.3 million, or 25% of our net sales, for the year ended December 31, 2007.
All of our cartridges, with the exception of the training cartridge, contain numerous colored, confetti-like tags bearing the cartridge’s serial number. These tags, referred to as Anti-Felon Identification tags, or AFIDs, are scattered when one of our cartridges is fired. We require sellers of our products to participate in the AFID program by registering buyers of our cartridges. In many cases, we can use AFIDs to identify the registered owner of cartridges fired.
In 2006, we launched an accessory to the X26 called the TASER Cam. The TASER Cam is a video recording device that captures both video and audio of potential and actual TASER use incidents. The device captures video and audio before, during and after a TASER deployment, which provides law enforcement with a greater level of accountability to support their use of TASER devices against a resistant subject. The TASER Cam is capable of recording in zero light conditions through the use of an infrared illuminator. A non audio version of the device is also available for agencies operating in states where legislation prohibits the use of audio recordings.
In 2004, we introduced an accessory to the X26 that allows the X26 electronic control device to be attached to military and law enforcement rifles via a Picatinny rail giving the user lethal and non-lethal options on the same weapon.
Product Warranties
We offer a one year limited warranty on all of the TASER X26 and ADVANCED TASER devices. After the warranty expires, if the device fails to operate properly for any reason, we will replace the TASER X26 at a discounted price depending on when the product was placed in service and replace the ADVANCED TASER device for a fee of $75. These fees are intended to cover the handling and repair costs and include a profit. We believe this policy is attractive to our law enforcement, military, and corrections agency customers. In particular, it avoids disputes regarding the source or cause of any defect. Extended warranties which provide additional coverage beyond the limited warranty, ranging from one to four years are also offered for specified fees.
We offer a 90 day limited warranty on the TASER C2 and the X26C devices. Our TASER C2 and the X26C are designed to disable an attacker for up to 30 seconds. We encourage private citizens to leave the units and flee after firing them. As a result, we also provide free replacement units to private citizens who follow this suggested procedure. To qualify for the replacement unit, users must file a police report that describes the incident and confirms the use of the TASER C2 or the X26C.
Markets
Law Enforcement and Corrections
Federal, state and local law enforcement agencies in the United States and throughout the world currently represent the primary target market for our TASER X26 and ADVANCED TASER device products. In the law enforcement market, more than 14,000 law enforcement agencies in 45 countries have made initial purchases of our TASER brand devices for testing or deployment. In addition, approximately 5,000 police departments have purchased or are in the process of purchasing TASER devices to issue to their on duty patrol officers. In 2007, additional federal agencies began or increased deployment of TASER devices. The U.S. Marshal Agency approved the TASER X26 for use and decentralized procurement, which made it significantly easier for the regional U.S. Marshal Offices to make their own purchases. The National Park Service made it mandatory to have TASER devices in 2007 and 145 Park Service locations are now carrying TASER X26 devices.
We continue to place increased focus on educating correctional facility personnel as well as parole and probation field officers in the benefits of using TASER brand products. We hired a sales manager to develop new relationships and cultivate existing relationships within the Corrections community and have developed training programs and command staff demonstrations specific to the Corrections market. We attended several Corrections tradeshows and conferences to expand our reach into the market. As a result of these efforts, we received commitments from several state correctional agencies to start pilot tests. Currently there are 94 correctional facilities deploying or testing our products. Our TASER devices are deployed in county correctional facilities such as those operated by the Los Angeles Custody Division and Maricopa County Sheriff (AZ). State correctional agencies deploying TASER devices include Arizona, Arkansas, Colorado, Kentucky, Louisiana, Montana, Nevada, North Dakota, Oregon, Tennessee, Utah, Washington and Wisconsin. We hope to obtain many new correctional facility sales from our sales and marketing efforts in this market segment in the coming years.
5
Table of Contents
Military Forces, both United States and Foreign Allies
TASER devices continued to be deployed in support of key strategic military operations in locations around the world. We continued our focus initiative on supporting our military customers. We expanded our sales efforts by hiring the former head of the Military Joint Non Lethal Weapons Directorate as our Vice President of Government and Military Programs in 2007. Additionally, during 2008, we met quarterly with our Senior Executive Advisory Group (SEAG) comprised of a team of professionals with extensive military, homeland defense and law enforcement experience with the purpose of advising on business models in support of military users. The business group (Federal Programs) has concentrated on supporting military and other federal use of our existing products as well as developing new technology through contracted support. In 2008, we entered into a science and technology contract with the Joint Non-Lethal Weapons Directorate (JNLWD) of the U.S Department of Defense to develop a 40mm projectile, compatible with already fielded weapons, which allows for extension and improvement of the our existing eXtended Range Electronic Projectile (XREP) technology. The development contract comprises three phases, the first of which commenced in 2008 and is expected to be completed in early 2009, while the second and third phases are optional and contingent upon successful completion of the first phase. In 2007, we received our first long term Indefinite Quantity, Indefinite Delivery, (IDIQ) Military contract to provide up to $22.8 million of product over a five year period through our GSA Distributor.
Private Security
We are still in the early stages of pursuing additional opportunities for sales of TASER devices in private security markets, and have made limited sales to date. Private security officers represent a broad range of individuals, including contract security patrol, healthcare, gaming, retail security employees and many others. In 2008, we continued to focus on pursuing additional possibilities for sales of TASER devices in the private security markets. Similar to our other emerging markets, we developed training programs and demonstrations specific to the industry. We met with several large corporate and private patrol security companies to discover their unique needs. We also attended several private security tradeshows, conferences and industry association meetings to expand our reach into the market space. In addition to our current product offerings, we are developing industry specific products that we believe will help penetrate the market. As of December 31, 2008, we had approximately 300 US private security operations deploying or testing our products.
Private Citizen / Personal Protection
In July 2007, we introduced the TASER C2 personal protector, specifically designed for the private citizen market. This new consumer product contributed approximately 6.6% and 4.0% of our total net sales in 2008 and 2007, respectively. We believe private citizen sales will continue to grow in 2009 as a result of various distribution relationships and marketing strategies we have put in place to increase awareness of the TASER C2 in the consumer market.
Sales and Marketing
Law enforcement, military, corrections and security agencies represent our primary target markets. In each of these markets, the decision to purchase TASER devices is normally made by a group of people, including the agency head, the agency’s training staff, and weapons experts. Depending on the size and cost of the device deployment and local procurement rules and customs, the decision may involve political decision-makers such as city council members or the federal government. The decision-making process can take as little as a few weeks or as long as several years. Although we have focused on three primary markets, we have been able to expand our customer base to thousands of end users within these markets. We currently sell our products to more than 14,000 law enforcement agencies.
Since the introduction of the ADVANCED TASER device in 1999, we have used several types of media to communicate the benefits of acquiring and deploying our products. These campaigns have included the development of personalized CD/DVD packages geared toward law enforcement leaders in the community, advertisements in law enforcement publications, and the use of more than 2,400 training classes conducted around the world. We also target key regional and national law enforcement trade shows where we can demonstrate the TASER devices to leading departments. In 2008, we attended and exhibited at over 120 regional and national law enforcement trade shows. In 2008, we held our annual U.S. Tactical Conference for the trained master instructors, and law enforcement training officers, the continued focus of which was to train the officers in the use of the TASER X26.
We plan to continue investment in the area of law enforcement trade shows and conferences in 2009, as it provides us the ability to market our products to our target audience. We believe these types of activities accelerate penetration of our TASER product lines in each market, which should lead to increased visibility in both the private security and private citizen markets and reinforce the value of non-lethal devices for self-defense.
6
Table of Contents
United States Distribution
With the exception of several accounts to which we sell directly, the vast majority of our law enforcement agency sales in the Unites States are made through our network of law enforcement distributors. In addition, we have one military and government contracting distributor. These distributors were selected based upon their reputation within their respective industries, their contacts, and their distribution network. Our regional managers work closely with the distributors in their territory to inform and educate the law enforcement communities. We continue to monitor our law enforcement distributors closely to help ensure that our service standards are achieved. We also reserve the right to take any large agency order directly to secure the agency’s account balance with us.
Sales in the private citizen market are made through our web site sales and commercial distributors. We have also established relationships selling to retail chains. We have implemented a variety of marketing initiatives to support sales of the TASER C2 personal protector. We produced an infomercial which aired in multiple markets during 2008, hired a professional advertising and public relations company to assist us in media and press events, and editorial placements and attended numerous tradeshows specifically to target the consumer market. We also held press conferences and media events in targeted large cities to further educate the public of the availability for citizens to purchase TASER C2 products for private use. We also contracted a third party company call center to assist with the large volume of citizen calls generated from our advertisements, trade shows and press/media conferences. We continue to sell all other TASER citizen devices and products through web sales and our established commercial distributors.
International Distribution
We market and distribute our products to foreign markets through a network of distributors. For geographical and cultural reasons, our distributors usually have a territory defined by their country’s borders. These distributors market both our law enforcement, military, and corrections products, and our consumer products where allowed by law.
Our distributors work with local police, military, and corrections agencies in the same manner as our domestic market distributors. For example, they perform demonstrations, attend industry tradeshows, maintain country specific web sites, engage in print advertising, and arrange training classes.
In 2008, we concentrated our international marketing on the countries that were furthest along in the testing and purchasing process. These countries included the United Kingdom, France, Australia, Singapore, and South Korea. We also plan to continue growing our international presence by expanding our marketing efforts to a larger number of countries.
We shipped products to approximately 50 countries during fiscal 2008. As a percentage of total sales, sales outside the U.S. increased to approximately 18% in 2008 from 15% in 2007 and 14% in 2006. Reference is made to Note 1(o) in the accompanying financial statements in Part II, Item 8 of this Form 10-K for further information concerning our sales by geographic region.
Training Programs
Most law enforcement, military, security and corrections agencies will not purchase new weapons until a training program is in place to instruct and certify personnel in their proper use. We offer a 16 hour class that certifies law enforcement, military, corrections and security agency trainers as instructors in the use of the TASER electronic control devices. As of December 31, 2008, approximately 42,000 law enforcement officers around the world have been trained and certified as instructors in the proper use of TASER brand devices. This includes approximately 38,000 officers in the United States and 4,000 in other countries.
Currently, 825 of our certified instructors have undergone further training and became certified as master instructors. We authorize these individuals to train and certify other law enforcement, military, corrections and professional security agency trainers as TASER instructors, not just end-users within their own organization. The master instructors are independent professional trainers, serve as local area TASER experts, and assist in conducting TASER demonstrations at other police departments within their regions. In addition, 121 of our certified instructors have completed the same training and were certified as advanced instructors. Advanced instructors are authorized to certify others within their own agency as TASER instructors. Military personnel are trained by our Chief Instructor. Approximately 170 of our master instructors have agreed to conduct TASER device training classes on a regular basis. We provide logistical support for the training classes. We charge a fee of $275 for each training attendee. We pay master instructors a per-session training fee for each session they conduct. We conducted 453 training courses in 2008 and, as of December 31, 2008, we have conducted a cumulative 2,478 training courses during which we have trained more than 42,000 individuals as instructors for TASER ECD’s. In 2005, we started a TASER Armorer course to train agencies on proper care and preventative maintenance of TASER devices. We charge a fee of $275 to each attendee. In 2008, we hosted 30 Armorer courses, including 27 in the United States and three internationally; 560 students attended the Armorer course in the United States and 91 attended in other countries. As of December 31, 2008, approximately 1,762 people have been trained and certified as TASER Armorers. In 2008, we started offering a TASER Forensics course to teach investigators how to collect and analyze TASER device related evidence at a crime scene and we conducted eight such Forensic courses in the United States and trained 89 people. The fee for the Forensics course is $150 per student. We have also designed a training course for private citizen customers. Customers who purchase an X26C device receive a certificate good for a one hour, one-on-one training session with an X26C certified instructor. We have 843 instructors certified to give the X26C training.
7
Table of Contents
In order to coordinate the growing demands of our training programs, we created a Training Advisory Board. This board annually reviews the qualifications of the master instructors, and provides retraining or certification as required. In addition, the Training Advisory Board oversees the trainers and curriculum to ensure that new information is properly communicated and implemented. The Training Advisory Board also gives input into new product development. We also created the position of Senior Master Instructor. Twenty four experienced Master Instructors have been promoted to this position based on their exemplary performance as Master Instructors. Their primary duties are to perform quality control checks on Master Instructors during an instructor course and to help instruct at the Master Instructor School. Additionally, we created the position of Staff Instructor who is a full time employee responsible for coordinating course delivery and development. In 2008, we hired two more Staff Instructors and created the position of Training Manager. The Training Manager oversees the law enforcement instructor course and supervises the clerical staff.
Manufacturing
We perform light manufacturing and final assembly operations at our headquarters in Scottsdale, Arizona and own substantially all of the equipment required to develop, prototype, manufacture and assemble our finished products. This includes critical injection molds, schematics, test equipment and prototypes utilized by our supply chain for the production of required raw materials and sub-assemblies. TASER’s dedication to manufacturing excellence in 2008 led to implementation of lean/six sigma methodologies to optimize all direct and indirect resources within the organization. This implementation is expected to boost capacity for existing products, as well as augment production of the new TASER products scheduled for 2009 market release. Our results for 2008 showed improved utilization of operational resources and a significant reduction in inventory, with increased capacity. However, other capacity options will be considered should we experience higher demand resulting from large orders of legacy or new product releases. This was all achieved while maintaining our ISO 9001 certification.
In 2004, TASER began investing in automated equipment for the continuous improvement of product quality, and reduction of manufacturing costs. We have since implemented a number of equipment initiatives including the purchase and integration of robotic equipment, computerized laboratory and medical testing equipment, machining and tooling equipment, as well as sophisticated modeling equipment for our Research and Development Department. In 2007, we also contracted with a full scale automation facility to design and manufacture custom equipment which will be used to replace current manual operations within our manufacturing. In total, we expect this project to cost the company approximately $8.4 million and significantly reduce our direct labor expense, while providing continuous improvement for our product lines. We expect the automation equipment will be installed in 2009.
Our supplier base has and will continue to be a focus for us. Presently, TASER purchases finished circuit boards and components primarily from suppliers located in the United States, along with strategic relationships internationally. Although we currently obtain plastic components from an outside supplier base, we own all designs and tooling, with plans of developing redundant tooling and capacity in other facilities. We believe there are readily available alternative suppliers in most cases who can consistently meet our needs for these components. TASER continues to develop and implement supply chain strategies to insure that both short and long term objectives are achieved, while maintaining efficiencies at all levels within the organization.
Competition
Law Enforcement, Corrections and Private Security Markets
The primary competitive factors in the law enforcement and corrections market include a weapon’s accuracy, effectiveness, safety, cost and ease of use. During 2007, a company introduced a new electronic device to compete with the TASER X26. To date, we do not know of any significant sales of any competing electronic control device products. We believe that our strong relationship with customers, our large installed base of products, and the significant amount of medical and safety testing already performed on our products will provide us with a competitive advantage over our competition.
We also believe the ADVANCED TASER and TASER X26 devices compete indirectly with a variety of non-lethal alternatives. These alternatives include, but are not limited to pepper spray and impact weapons sold by companies such as Armor Holdings, Inc., and Pepperball. We believe our TASER brand device’s advanced technology; versatility, effectiveness, and low injury rate enable it to compete effectively against these non-lethal alternatives.
Military Market
In the military markets, both in the United States and abroad, a wide variety of weapon systems are utilized to accomplish the mission at hand. Conducted energy devices have gained increased acceptance as a result of the policing role of military personnel in the conflicts in both Iraq and Afghanistan. There has also been an increased awareness of the use of non-lethal weapons to preserve human intelligence. TASER devices give our armed forces one means to capture or immobilize targets without using lethal force. We are the only supplier providing electronic control devices to these military agencies. There is indirect competition from pepper spray and impact weapons sold by companies such as Armor Holdings, Inc., and Pepperball.
8
Table of Contents
Private Citizen Market
Electronic control devices have gained limited acceptance in the private citizen market for non-lethal weapons. These weapons compete with other non-lethal weapons such as batons, clubs, and chemical sprays. The primary competitive factors in the private citizen market include a weapon’s cost, effectiveness, safety and ease of use. We believe the widespread adoption of our TASER devices by prominent law enforcement agencies will help us to further penetrate the private citizen market.
Regulation
United States Regulation
The TASER X26, ADVANCED TASER, TASER C2 and AIR TASER devices, as well as the cartridges used by these devices, are subject to regulations. None of our devices are considered to be a “firearm” by the U.S. Bureau of Alcohol, Tobacco, Firearms and Explosives. Therefore, no Federal firearms-related regulations specifically apply to the sale and distribution of our devices within the United States. In the 1980s however, many states introduced regulations restricting the sale and use of stun guns, inexpensive hand-held shock devices and electronic weapons. We believe existing stun gun laws and regulations also apply to our devices.
In 2002 through 2004, we worked with several law enforcement agencies, government agencies and distributors to overturn prior legislation preventing the sale of TASER devices to law enforcement agencies in certain regions of the U.S. These combined efforts were successful in changing the legislation in the states of Hawaii, Massachusetts and Michigan. We considered this to be an important change in regulations. For example, prior to the amendment to the Michigan Penal Code, the possession of a TASER or electronic weapon of any kind in Michigan could result in a felony conviction. Currently, New Jersey is the only remaining state in the U.S. in which TASER technology is prohibited for law enforcement use.
In many cases, the law enforcement and corrections market is subject to different regulations than the private citizen market. Where different regulations exist, we assume the regulations affecting the private citizen market also apply to the private security markets except as the applicable regulations otherwise specifically provide.
As of December 31, 2008, state and local codes prohibit the possession of stun guns, including TASER electronic control devices, by the general public in Hawaii, Wisconsin, Michigan, Massachusetts, Rhode Island, New York, New Jersey and the District of Columbia as well as a number of counties, cities and towns.
We are also subject to environmental laws and regulations, including restrictions on the presence of certain substances in electronic products. Reference is made to Section 1A, Risk Factors, under the heading “Environmental laws and regulations subject us to a number of risks and could result in significant liabilities and costs”.
United States Export Regulation
Our devices are considered a crime control product by the U.S. Government. Accordingly, the export of our devices is regulated under export administration regulations. As a result, we must obtain export licenses from the Department of Commerce for all shipments to foreign countries other than Canada. Most of our requests for export licenses have been granted, and the need to obtain these licenses has not caused a material delay in our shipments. The need to obtain licenses, however, has limited or impeded our ability to ship to certain foreign markets. Export regulations also prohibit the further shipment of our products from foreign markets in which we hold a valid export license to foreign markets in which we do not hold an export license for our products.
In addition, in 2000, the Department of Commerce adopted regulations restricting the export of technology used in our devices. These regulations apply to both the technology incorporated in our device systems and in the processes used to produce them. The technology export regulations do not apply to production that takes place within the United States, but is applicable to all sub-assemblies and controlled items manufactured outside the United States.
Foreign Regulation
Foreign regulations, which may affect our devices, are numerous and often unclear. We prefer to work with a distributor who is familiar with the applicable import regulations in each of our foreign markets. Experience with foreign distributors in the past indicates that restrictions may prohibit certain sales of our products in a number of countries. The vast majority of countries permit TASER devices to be sold and used by Law Enforcement. We rely on our distributors to inform us of those countries where the TASER device is prohibited or restricted.
9
Table of Contents
Previously, the United Kingdom was among the countries where TASER technologies were prohibited. However, in January 2003, the British Police announced that the national government would be backing a TASER pilot program for five police forces within the UK. This decision came after the completion of two years of testing by the Police Scientific Development Branch of the Home Office in England, during which the product was reviewed for operational effectiveness and medical safety. Following a detailed evaluation of a 12-month operational trial of the ADVANCED TASER device, which was carried out by the five police forces, the then Home Secretary David Blunkett agreed that firearms officers in forces nationwide could use the hand-held electrical device as of September 2004. Currently, all 43 police forces in England, Wales and Scotland deploy TASER technology within their firearms teams. During 2007, a further trial across 10 police forces was authorized where TASERs are being deployed by specially trained non-firearms officers. The protocol for deployment has also changed to allow officers to deploy a TASER in incidents involving serious violence that they consider cannot be contained by other means. In 2008, the British Home Office announced its intension to authorize the deployment of electronic control devices by all British police, including non-firearms officers. Additionally, the Police Service of Northern Ireland has now deployed TASER to its Special Operations Branch — a highly trained firearms unit, for an initial trial period.
Intellectual Property
We protect our intellectual property with U.S. and foreign patents and trademarks. Our patents and pending patent applications relate to technology used by us in connection with our products. We also rely on international treaties, organizations and foreign laws to protect our intellectual property. As of December 31, 2008, we held 30 United States patents and 40 foreign patents and also have numerous patents and trademarks pending. Our patents expire at varying dates ranging between 2010 and 2026. Our earliest expiring United States patent generally covers projectile propellant devices having a container of compressed gas in place of gunpowder as a propellant. We use this technology in our cartridges. This patent expires in 2010. We continuously assess whether and where to seek formal protection for particular innovations and technologies based on such factors as: the commercial significance of our operations and our competitors’ operations in particular countries and regions; our strategic technology or product directions in different countries; and the degree to which intellectual property laws exist and are meaningfully enforced in different jurisdictions.
Confidentiality agreements are used with employees, consultants and key suppliers to help ensure the confidentiality of our trade secrets.
TASER has the exclusive rights to many internet domain names primarily including ‘taser.com’ and ‘evidence.com’.
Research and Development
Our research and development initiatives are conducted in two separate categories. The first is internally funded research and development, and the second is research externally funded by customers having requirements for specific capabilities. Both categories focus on next generation technology, yet are differentiated by the anticipated breadth of the market opportunity, the time to project completion and accounting treatment. Internally funded research has been primarily focused on improvements to existing TASER products, or the development of new applications for TASER technology that we believe generally will have broad market appeal. Externally funded work focuses on specific packaging or delivery requirements of existing TASER technology that is of high value to particular customers but may not be viable product solutions to other customers. These projects generally represent product developments which are long-term in nature and require outside resources, team member companies or expert consulting.
Research and development initiatives include bio-medical research and electrical, mechanical and software engineering. We expect that future development projects will focus on extending the range, improving the functionality and developing new delivery options for our ECD products. In addition, we are developing technology for the audio-video recording of an incident from the point of view of the officer with pre-event video capture (Autonomous eXtended on-Officer Network, or AXON). In conjunction with the AXON device we are developing a new integrated digital multi-media evidence storage and management platform — EVIDENCE.com,
Our investment in internally funded research and development totaled approximately $12.9 million, $4.4 million and $2.7 million in 2008, 2007 and 2006, respectively. This allowed our R&D department to expand to 58 engineers, technicians and specialists at the end of 2008. Our investment in research and development staff and equipment continues to represent a significant increase from previous years and reflects our commitment to maintaining and extending our current technology. Our return on that investment is intended to be realized over the long term, although new systems and technologies often have a more immediate impact on our business.
Employees
As of December 31, 2008, we had 353 full-time employees and two temporary employees. The breakdown of our full time employees by department is as follows: 149 direct manufacturing employees and 206 administrative and manufacturing support employees. Of the 206 administrative and manufacturing support employees; 55 were involved in sales, marketing, communication and training; 58 were employed in research, development and engineering; 28 were employed in administrative functions inclusive of executive management, legal, finance and accounting; 11 were employed in information systems technologies; 17 were employed in quality control and 37 were employed in manufacturing support functions. Our employees are not covered by any collective bargaining agreement, and we have never experienced a work stoppage. We believe that our relations with our employees are good.
10
Table of Contents
Available Information
We were incorporated in Arizona in September 1993 as ICER Corporation. We changed our name to AIR TASER, Inc. in December 1993 and to TASER International, Incorporated in April 1998. In January 2001, we reincorporated in Delaware as TASER International, Inc. Our website is located at www.TASER.com. Our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to section 13(a) or 15(d) of the Securities Exchange Act of 1934 are available on our website as soon as reasonably practicable after we electronically file such material with, or furnish such material to, the SEC. Other information that is not part of this Annual Report on Form 10-K can be accessed through our website at www.TASER.com.
Item 1A.Risk Factors
Because of the following factors, as well as other variables affecting our operating results, our past financial performance may not be a reliable indicator of our future performance and historical trends should not be used to anticipate our results or trends in future periods.
We are materially dependent on acceptance of our products by the law enforcement and corrections market, and if law enforcement and corrections agencies do not purchase our products, our revenues will be adversely affected and we may not be able to expand into other markets.
A substantial number of law enforcement and corrections agencies may not purchase our electronic control devices. In addition, if our products are not widely accepted by the law enforcement and corrections market, we may not be able to expand sales of our products into other markets such as the military and private security markets. Law enforcement and corrections agencies may be influenced by claims or perceptions that conducted energy weapons such as our products are unsafe or may be used in an abusive manner. In addition, earlier generation conducted energy devices may have been perceived as ineffective. Sales of our products to these agencies may also be delayed or limited by these claims or perceptions.
Most of our end-user customers are subject to budgetary and political constraints that may delay or prevent sales.
Most of our end-user customers are government agencies. These agencies often do not set their own budgets and therefore have little control over the amount of money they can spend. In addition, these agencies experience political pressure that may dictate the manner in which they spend money. As a result, even if an agency wants to acquire our products, it may be unable to purchase them due to budgetary or political constraints. Currently, many governmental agencies are experiencing severe budgetary stresses as a result of the ongoing worldwide recession. There can be no assurance that the economic and budgeting issues will not worsen and adversely impact sales of our products. Some government agency orders may also be canceled or substantially delayed due to budgetary, political or other scheduling delays which frequently occur in connection with the acquisition of products by such agencies and such cancellations may accelerate or be more severe than we have experienced historically as a result of the current economic environment.
We may face personal injury, wrongful death and other liability claims that harm our reputation and adversely affect our sales and financial condition.
Our products are often used in aggressive confrontations that may result in serious, permanent bodily injury or death to those involved. Our products may be associated with these injuries. A person injured in a confrontation or otherwise in connection with the use of our products may bring legal action against us to recover damages on the basis of theories including personal injury, wrongful death, negligent design, defective product or inadequate warning. We are currently subject to a number of such lawsuits. We may also be subject to lawsuits involving allegations of misuse of our products. If successful, personal injury, misuse and other claims could have a material adverse effect on our operating results and financial condition and could result in negative publicity about our products. Although we carry product liability insurance, we do incur large legal expenses within our self insured retention in defending these lawsuits and significant litigation could also result in a diversion of management’s attention and resources, negative publicity and a potential award of monetary damages in excess of our insurance coverage. The outcome of any litigation is inherently uncertain and there can be no assurance that our existing or any future litigation will not have a material adverse effect on our revenues, our financial condition or financial results.
We substantially depend on sales of our TASER X26 products, and if these products are not widely accepted, our growth prospects will be diminished.
In the years ended December 31, 2008, 2007 and 2006, we derived our revenues predominantly from sales of the TASER X26 brand devices and related cartridges, and expect to depend on sales of these products for the foreseeable future. A decrease in the prices of or demand for these products, or their failure to achieve broad market acceptance, would significantly harm our growth prospects, operating results and financial condition.
If we are unable to manage our growth, our prospects may be limited and our future profitability may be adversely affected.
We intend to expand our sales and marketing programs and our manufacturing capacity as needed to meet future demand. Any significant expansion may strain our managerial, financial and other resources. If we are unable to manage our growth, our business, operating results and financial condition could be adversely affected. We will need to continually improve our operations, financial and other internal systems to manage our growth effectively, and any failure to do so may lead to inefficiencies and redundancies, and result in reduced growth prospects and profitability.
11
Table of Contents
To the extent demand for our products increases, our future success will be dependent upon our ability to ramp manufacturing production capacity which will be accomplished by the implementation of customized manufacturing automation equipment.
To the extent demand for our products increases significantly in future periods, one of our key challenges will be to ramp our production capacity to meet sales demand, while maintaining product quality. Our primary strategies to accomplish this include increasing the physical size of our assembly facilities, the hiring of additional production staff, and the implementation of customized automation equipment. The investments we made in this equipment may not yield the anticipated labor and material efficiencies. Our inability to meet any future increase in sales demand or effectively manage our expansion could have a material adverse affect on our revenues, financial results and financial condition.
Pending litigation may subject us to significant litigation costs, judgments, fines and penalties in excess of insurance coverage, and divert management attention from our business.
We are involved in numerous litigation matters relating to our products or the use of such products, litigation against persons who we believe have defamed our products, litigation against medical examiners who made errors in their autopsy reports, litigation against a competitor and litigation against former employees. Such matters have resulted and are expected to continue to result in substantial costs to us and some diversion of our management’s attention, which could adversely affect our business, financial condition or operating results.
Our future success is dependent on our ability to expand sales through distributors and our inability to recruit new distributors would negatively affect our sales.
Our distribution strategy is to pursue sales through multiple channels with an emphasis on independent distributors. Our inability to establish relationships with and retain police equipment distributors who can successfully sell our products would adversely affect our sales. In addition, our arrangements with our distributors are generally short-term. If we do not competitively price our products, meet the requirements of our distributors or end-users, provide adequate marketing support, or comply with the terms of our distribution arrangements, our distributors may fail to aggressively market our products or may terminate their relationships with us. These developments would likely have a material adverse effect on our sales. Our reliance on the sales of our products by others also makes it more difficult to predict our revenues, cash flow and operating results.
If we are unable to design, introduce and sell new products or new product features successfully, our business and financial results could be adversely affected.
Our future success will depend on our ability to develop new products or new product features that achieve market acceptance in a timely and cost-effective manner. The development of new products and new product features is complex, time consuming and expensive, and we may experience delays in completing the development and introduction of new products. We cannot provide any assurance that products that we may develop in the future will achieve market acceptance. If we fail to develop new products or new product features on a timely basis that achieve market acceptance, our business, financial results and competitive position could be adversely affected.
We expend significant resources in anticipation of a sale due to our lengthy sales cycle and may receive no revenue in return.
Generally, law enforcement and corrections agencies consider a wide range of issues before committing to purchase our products, including product benefits, training costs, the cost to use our products in addition to or in place of other non-lethal products, budget constraints and product reliability, safety and efficacy. The length of our sales cycle may range from a few weeks to as long as several years. Adverse publicity surrounding our products or the safety of such products has in the past and could in the future lengthen our sales cycle with customers. In the past, we believe we have experienced revenue decreases in part as the result of adverse effects on our customers and potential customers of negative publicity surrounding our products or use of our products. We may incur substantial selling costs and expend significant effort in connection with the evaluation of our products by potential customers before they place an order. If these potential customers do not purchase our products, we will have expended significant resources and received no revenue in return.
Government regulation of our products may adversely affect sales.
Federal regulation of sales in the United States:Our devices are not firearms regulated by the U.S. Bureau of Alcohol, Tobacco, Firearms and Explosives, but are consumer products regulated by the U.S. Consumer Product Safety Commission. Although there are currently no federal laws restricting sales of our devices in the United States, future federal regulation could adversely affect sales of our products.
Federal regulation of international sales:Our devices are controlled as a “crime control” product by the U.S. Department of Commerce, or DOC, for export directly from the United States. Consequently, we must obtain an export license from the DOC for the export of our devices from the United States other than to Canada. Our inability to obtain DOC export licenses on a timely basis for sales of our devices to our international customers could significantly and adversely affect our international sales.
State and local regulation:Our devices are controlled, restricted or their use prohibited by a number of state and local governments. Our devices are banned from private citizen sale or use in seven states: New York, New Jersey, Rhode Island, Michigan, Wisconsin, Massachusetts and Hawaii. Law enforcement use of our products is also prohibited in New Jersey. Some municipalities, including Omaha, Nebraska and Washington, D.C., also prohibit private citizen use of our products. Other jurisdictions may ban or restrict the sale of our products and our product sales may be significantly affected by additional state, county and city governmental regulation.
12
Table of Contents
Foreign regulation:Certain foreign jurisdictions prohibit the sale of conducted energy devices such as our products, limiting our international sales opportunities.
Environmental laws and regulations subject us to a number of risks and could result in significant liabilities and costs.
We may be subject to various state, federal and international laws and regulations governing the environment, including restricting the presence of certain substances in electronic products and making producers of those products financially responsible for the collection, treatment, recycling and disposal of those products. Environmental legislation within the European Union (EU) may increase our cost of doing business internationally and impact our revenues from EU countries as we comply with and implement these requirements.
The EU has published Directives on the restriction of certain hazardous substances in electronic and electrical equipment (the RoHS Directive) which became effective in July 2006, and on electronic and electrical waste management (the WEEE Directive). The RoHS Directive restricts the use of a number of substances, including lead. The WEEE Directive directs members of the European Union to enact laws, regulations, and administrative provisions to ensure that producers of electric and electronic equipment are financially responsible for the collection, recycling, treatment and environmentally responsible disposal of certain products sold into the market after August 15, 2005 and from products in use prior to that date that are being replaced. In addition, similar environmental legislation has been or may be enacted in other jurisdictions, including the U.S. (under federal and state laws) and other countries, the cumulative impact of which could be significant.
We continue to monitor the impact of specific registration and compliance activities required by the RoHS and WEEE Directives. We endeavor to comply with applicable environmental laws, yet compliance with such laws could increase our operations and product costs; increase the complexities of product design, procurement, and manufacturing; limit our ability to manage excess and obsolete non-compliant inventory; limit our sales activities; and impact our future financial results. Any violation of these laws can subject us to significant liability, including fines, penalties, and prohibiting sales of our products into one or more states or countries, and result in a material adverse effect on our financial condition.
If we are unable to protect our intellectual property, we may lose a competitive advantage or incur substantial litigation costs to protect our rights.
Our future success depends upon our proprietary technology. Our protective measures, including patents, trademarks and trade secret protection, may prove inadequate to protect our proprietary rights. The right to stop others from misusing our trademarks and service marks in commerce depends to some extent on our ability to show evidence of enforcement of our rights against such misuse in commerce. Our efforts to stop improper use, if insufficient, may lead to loss of trademark and service mark rights, brand loyalty and notoriety among our customers and prospective customers. Our earliest expiring United States patent generally covers projectile propellant devices having a container of compressed gas in place of gunpowder as a propellant. We use this technology in our cartridges. This patent expires in 2010. The scope of any patent to which we have or may obtain rights may not prevent others from developing and selling competing products. The validity and breadth of claims covered in technology patents involve complex legal and factual questions, and the resolution of such claims may be highly uncertain, lengthy and expensive. In addition, our patents may be held invalid upon challenge, or others may claim rights in or ownership of our patents.
We have a pending lawsuit in Federal District Court against Stinger Systems that alleges infringement of three of our U.S. patents: 6,999,295; 7,102,870; and 7,234,262. In an infringement case, the judge in a Markman hearing before trial begins, can resolve disagreements on the meaning of some of the terminology of the patent claims. In the pending case, the holding from the Markman hearing largely adopted the meanings proposed by TASER. Nevertheless, Stinger Systems is expected to challenge the validity of the patents at trial. If at trial the patents are upheld, the extent of relief to TASER including whether Stinger is enjoined and/or forced to pay damages, cannot be predicted.
We may be subject to intellectual property infringement claims, which could cause us to incur litigation costs and divert management attention from our business.
Any intellectual property infringement claims against us, with or without merit, could be costly and time-consuming to defend and divert our management’s attention from our business. If our products were found to infringe a third party’s proprietary rights, we could be required to enter into costly royalty or licensing agreements in order to be able to sell our products. Royalty and licensing agreements, if required, may not be available on terms acceptable to us or at all.
If we face competition in foreign countries, we can enforce patent rights only in the jurisdictions in which our patent applications have been granted.
Our U.S. patents only protect us from imported infringing products coming into the U.S. from abroad. Applications for patents in a few foreign countries have been made; however, these may be inadequate to protect markets for our products in other foreign countries. Each foreign patent is examined and granted according to the law of the country where it was filed independent of whether a U.S. patent on similar technology was granted.
13
Table of Contents
Our efforts to avoid the patent, trademark, and copyright rights of others may not provide notice to us of potential infringements in time to avoid investing in product development and promotion that must later be abandoned if suitable license terms cannot be reached.
There is no guarantee that our use of conventional technology searching and brand clearance searching will identify all potential rights holders. Rights holders may demand payment for past infringements and/or force us to accept costly license terms or discontinue use of protected technology and/or works of authorship that may include for example photos, videos, and software. Our current research and development focus on developing software-based products increases this risk.
Government regulations applied to our products may affect our markets for these products.
We rely on the opinions of The Bureau of Alcohol Tobacco and Firearms, including the determination that a device that has projectiles propelled by the release of compressed gas, in place of the expanding gases from ignited gunpowder, are not classified as firearms. Changes in statutes, regulations, and interpretation outside of our control may result in our products being classified or reclassified as firearms. Our private citizen market could be substantially reduced if consumers are required to obtain registration to own a firearm prior to purchasing our products.
Competition in the law enforcement and corrections market could reduce our sales and prevent us from achieving profitability.
The law enforcement and corrections market is highly competitive. We face competition from numerous larger, better capitalized and more widely known companies that make other non-lethal devices and products. Increased competition may result in greater pricing pressure, lower gross margins and reduced sales. In this regard, two different competitors announced plans to introduce new products in 2005. During 2007, one of those companies introduced a new device to compete with the TASER X26. We are unable to predict the impact such products will have on our sales or our sales cycle, but existing or potential customers may choose to evaluate such products which could lengthen our sales cycle and potentially reduce our future sales.
Defects in our products could reduce demand for our products and result in a loss of sales, delay in market acceptance and injury to our reputation.
Complex components and assemblies used in our products may contain undetected defects that are subsequently discovered at any point in the life of the product. Defects in our products may result in a loss of sales, delay in market acceptance and injury to our reputation and increased warranty costs.
Our dependence on third party suppliers for key components of our devices could delay shipment of our products and reduce our sales.
We depend on certain domestic and foreign suppliers for the delivery of components used in the assembly of our products. Our reliance on third-party suppliers creates risks related to our potential inability to obtain an adequate supply of components or sub-assemblies and reduced control over pricing and timing of delivery of components and sub-assemblies. Specifically, we depend on suppliers of sub-assemblies, machined parts, injection molded plastic parts, printed circuit boards, custom wire fabrications and other miscellaneous customer parts for our products. We also do not have long-term agreements with any of our suppliers and there is no guarantee that supply will not be interrupted. Any interruption of supply for any material components of our products could significantly delay the shipment of our products and have a material adverse effect on our revenues, profitability and financial condition.
Component shortages could result in our inability to produce volume to adequately meet customer demand. This could result in a loss of sales, delay in deliveries and injury to our reputation.
Single source components used in the manufacture of our products may become unavailable or discontinued. Delays caused by industry allocations, or obsolescence may take weeks or months to resolve. In some cases, parts obsolescence may require a product re-design to ensure quality replacement components. These delays could cause significant delays in manufacturing and loss of sales, leading to adverse effects significantly impacting our financial condition or results of operations.
Our dependence on foreign suppliers for key components of our products could delay shipment of our finished products and reduce our sales.
We depend on foreign suppliers for the delivery of certain components used in the assembly of our products. Due to changes imposed for imports of foreign products into the United States, as well as potential port closures and delays created by terrorist threats, public health issues or national disasters, we are exposed to risk of delays caused by freight carriers or customs clearance issues for our imported parts. Delays caused by our inability to obtain components for assembly could have a material adverse effect on our revenues, profitability and financial condition.
We may experience a decline in gross margins due to rising raw material and transportation costs associated with a future increase in petroleum prices.
A significant number of our raw materials are comprised of petroleum based products, or incur some form of landed cost associated with transporting the raw materials or components to our facility. A significant rise in oil prices could adversely impact our ability to sustain current gross margins, by increasing component pricing.
14
Table of Contents
Our revenues and operating results may fluctuate unexpectedly from quarter to quarter, which may cause our stock price to decline.
Our revenues and operating results have varied significantly in the past and may vary significantly in the future due to various factors, including, but not limited to:
| • | budgetary cycles of municipal, state and federal law enforcement and corrections agencies | ||
| • | market acceptance of our products and services | ||
| • | the timing of large orders | ||
| • | the outcome of any existing or future litigation | ||
| • | adverse publicity surrounding our products, the safety of our products, or the use of our products | ||
| • | changes in our sales mix | ||
| • | new product introduction costs | ||
| • | increased raw material expenses | ||
| • | changes in our operating expenses | ||
| • | regulatory changes that may affect the marketability of our products |
As a result of these and other factors, we believe that period- to-period comparisons of our operating results may not be meaningful in the short term, and our performance in a particular period may not be indicative of our performance in any future period.
We may experience difficulties in the future in complying with Sarbanes-Oxley Section 404.
We are required to evaluate our internal controls under Section 404 of the Sarbanes-Oxley Act of 2002. Beginning with our annual report on Form 10-K for the fiscal year ending December 31, 2005, we have been required to furnish a report by our management on our internal control over financial reporting. Such report contains among other matters, an assessment of the effectiveness of our internal control over financial reporting as of the end of our fiscal year, including a statement as to whether or not our internal control over financial reporting is effective. Such report also contains a statement that our independent registered public accounting firm has issued an attestation report on management’s assessment of such internal controls. If we fail to maintain proper and effective internal controls in future periods, it could adversely affect our operating results, financial condition and our ability to run our business effectively and could cause investors to lose confidence in our financial reporting. We expect to continue to incur expense and to devote management resources to Section 404 compliance. In the event that our chief executive officer, chief financial officer or our independent registered public accounting firm determine that our internal control over financial reporting is not effective as defined under Section 404, investor confidence in us may be adversely affected and could cause a decline in the market price of our stock.
Foreign currency fluctuations may affect our competitiveness and sales in foreign markets.
The relative change in currency values creates fluctuations in our product pricing for potential international customers. These changes in foreign end-user costs may result in lost orders and reduce the competitiveness of our products in certain foreign markets. These changes may also negatively affect the financial condition of some existing or potential foreign customers and reduce or eliminate their future orders of our products.
We maintain all of our cash, cash equivalent and short-term investment balances, some of which are not insured, at one depository institution.
We maintain all of our cash, cash equivalent and short-term investment accounts at one depository institution. As of December 31, 2008, our aggregate balances in such accounts were $49.4 million. Of such amount, $250,000 was covered by Federal Deposit Insurance Corporation (FDIC) insurance, and approximately $29.5 million was supported by the U.S. Treasury Department under its Temporary Guarantee Program for Money Market Funds. The remaining amounts were not insured as of the end of fiscal 2008.
Although we believe that the risk of loss associated with our uninsured deposit and investment accounts is low given the financial strength and reputation of our depository institution, we could suffer losses with respect to the uninsured balances if the depositary institution failed and the institution’s assets were insufficient to cover its deposits and/or the Federal government did not take actions to support deposits in excess of existing FDIC insurance limits. Any such losses could have a material adverse effect on our liquidity, financial condition and results of operations.
Use of estimates may cause our financial results to differ from expectations.
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates.
We face risks associated with rapid technological change and new competing products.
The technology associated with non-lethal devices is receiving significant attention and is rapidly evolving. While we have patent protection in key areas of electro-muscular disruption technology, it is possible that new non-lethal technology may result in competing products that operate outside our patents and could present significant competition for our products.
We depend on our ability to attract and retain our key management and technical personnel.
Our success depends upon the continued service of our key management personnel. Our success also depends on our ability to continue to attract, retain and motivate qualified technical personnel. Although we have employment agreements with certain of our officers, the employment of such persons is “at-will” and either we or the employee can terminate the employment relationship at any time, subject to the applicable terms of the employment agreements. The competition for our key employees is intense. The loss of the service of one or more of our key personnel could harm our business.
15
Table of Contents
Item 1B.Unresolved Staff Comments
None.
Item 2.Properties
Our corporate headquarters and manufacturing facilities are based in a 100,000 square foot facility in Scottsdale, Arizona, which we own. We also lease premises in Santa Barbara, California and Washington D.C. We believe our existing facilities are well maintained and in good operating condition. We also believe we have adequate manufacturing capacity for our existing product lines for the foreseeable future. To the extent that we introduce new products in the future, we will likely need to acquire additional facilities to locate the associated production lines. However, we believe we can acquire or lease such facilities on reasonable terms. The Company continues to make investments in capital equipment as needed to meet anticipated demand for its products.
Item 3.Legal Proceedings
See discussion of Legal Proceedings in Note 7(c) to the financial statements included in Part II, Item 8 of this annual report.
Item 4.Submission of Matters to a Vote of Security Holders
No matters were submitted to a vote of security holders during the fourth quarter of 2008.
PART II
Item 5.Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information
Our common stock is quoted under the symbol “TASR” on The NASDAQ Global Select Market. The closing price of our common stock on March 9, 2009 was $3.69 per share.
The following table sets forth the high and low closing sales prices per share for our common stock as reported by NASDAQ for each quarter of the last two fiscal years.
Common Stock “TASR”
| Fiscal Quarters Ended | High | Low | ||||||
| March 31, 2007 | $ | 8.56 | $ | 7.44 | ||||
| June 30, 2007 | $ | 13.96 | $ | 7.85 | ||||
| September 30, 2007 | $ | 17.41 | $ | 13.34 | ||||
| December 31, 2007 | $ | 18.81 | $ | 12.68 | ||||
| March 31, 2008 | $ | 14.06 | $ | 8.90 | ||||
| June 30, 2008 | $ | 10.27 | $ | 4.99 | ||||
| September 30, 2008 | $ | 7.48 | $ | 5.01 | ||||
| December 31, 2008 | $ | 6.94 | $ | 2.68 | ||||
Holders
As of March 9, 2009, there were approximately 362 holders of record of our common stock.
Dividends
To date, we have not declared or paid cash dividends on our common stock. We do not intend to pay cash dividends in the foreseeable future and our revolving line of credit prohibits the payment of cash dividends.
Issuer Purchases of Equity Securities
We did not repurchase any shares of our common stock in the fourth quarter of 2008.
Recent Sales of Unregistered Securities
No unregistered securities were sold by us in 2008.
Stock Performance Graph
The following stock performance graph compares the performance of our common stock to the NASDAQ Stock Market (U.S.) and the Russell 3000 Index. The graph covers the period from December 31, 2003 to December 31, 2008. The graph assumes that the value of the investment in our stock and in each index was $100 at December 31, 2003 and that all dividends were reinvested. We do not pay dividends on our common stock.
16
Table of Contents
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN*
Among TASER International, Inc., The NASDAQ Composite Index
And The Russell 3000 Index
Among TASER International, Inc., The NASDAQ Composite Index
And The Russell 3000 Index
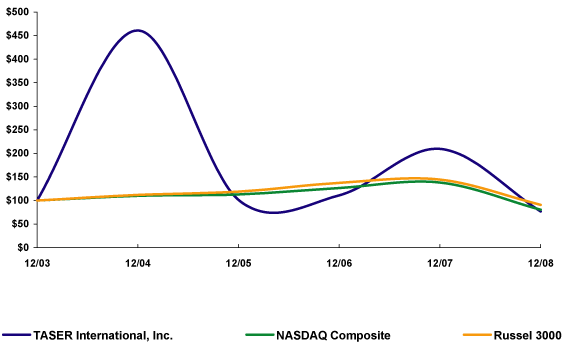
*$100 invested on 12/31/03 in stock & index-including reinvestment of dividends.
Fiscal year ending December 31.
Fiscal year ending December 31.
| 12/03 | 12/04 | 12/05 | 12/06 | 12/07 | 12/08 | |||||||||||||||||||
TASER International, Inc. | 100.00 | 461.09 | 101.40 | 110.87 | 209.64 | 76.92 | ||||||||||||||||||
NASDAQ Composite | 100.00 | 110.08 | 112.88 | 126.51 | 138.13 | 80.47 | ||||||||||||||||||
Russell 3000 | 100.00 | 111.95 | 118.80 | 137.47 | 144.54 | 90.61 | ||||||||||||||||||
17
Table of Contents
Item 6.Selected Financial Data
The following selected financial data should be read in conjunction with our financial statements and the notes thereto, and with Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” The statement of operations data for the years ended December 31, 2008, 2007 and 2006 and the balance sheet data as of December 31, 2008 and 2007 have been derived from and should be read in conjunction with our audited financial statements and the notes thereto included herein. The statement of operations data for the years ended December 31, 2005 and 2004 and the balance sheet data as of December 31, 2006, 2005 and 2004 is derived from audited financial statements and the notes thereto which are not included in this Annual Report on Form 10-K.
| For the Year Ended December 31, | ||||||||||||||||||||
| 2008 | 2007 | 2006 | 2005 | 2004 | ||||||||||||||||
| Statement of Operations Data | ||||||||||||||||||||
| Net sales | $ | 92,845,490 | $ | 100,727,191 | $ | 67,717,851 | $ | 47,694,181 | $ | 67,639,879 | ||||||||||
| Gross margin | 57,004,227 | 57,559,819 | 43,179,061 | 29,597,895 | 45,184,383 | |||||||||||||||
| Sales, general and administrative expenses | 38,860,729 | 32,814,170 | 29,680,764 | 26,483,485 | 13,880,322 | |||||||||||||||
| Research and development expenses | 12,918,161 | 4,421,596 | 2,704,521 | 1,574,048 | 823,593 | |||||||||||||||
| Shareholder litigation settlement expense (a) | — | — | 17,650,000 | — | — | |||||||||||||||
| Income (loss) from operations | 5,225,337 | 20,324,053 | (6,856,224 | ) | 1,540,362 | 30,480,468 | ||||||||||||||
| Net income (loss) | 3,637,041 | 15,026,476 | (4,087,679 | ) | 1,056,516 | 18,881,742 | ||||||||||||||
| Income (loss) per common and common equivalent shares | ||||||||||||||||||||
| Basic | $ | 0.06 | $ | 0.24 | $ | (0.07 | ) | $ | 0.02 | $ | 0.33 | |||||||||
| Diluted | $ | 0.06 | $ | 0.23 | $ | (0.07 | ) | $ | 0.02 | $ | 0.30 | |||||||||
| Weighted average number of common and common equivalent shares outstanding | ||||||||||||||||||||
| Basic | 62,371,004 | 62,621,174 | 61,984,240 | 61,303,939 | 57,232,329 | |||||||||||||||
| Diluted | 64,070,869 | 65,685,667 | 61,984,240 | 63,556,246 | 62,319,590 | |||||||||||||||
| As of December 31, | ||||||||||||||||||||
| 2008 | 2007 | 2006 | 2005 | 2004 | ||||||||||||||||
| Balance Sheet Data | ||||||||||||||||||||
| Working capital | $ | 80,642,516 | $ | 83,953,166 | $ | 37,813,576 | $ | 34,663,101 | $ | 51,100,989 | ||||||||||
| Total assets | 130,015,506 | 137,763,401 | 119,837,689 | 112,241,247 | 109,452,578 | |||||||||||||||
| Total current liabilities | 10,956,199 | 12,473,616 | 18,302,688 | 7,586,701 | 8,933,939 | |||||||||||||||
| Total long term obligations | — | 11,695 | 230,973 | 76,188 | — | |||||||||||||||
| Total stockholders equity | $ | 112,526,262 | $ | 120,636,750 | $ | 99,328,539 | $ | 103,738,375 | $ | 99,910,783 | ||||||||||
| a) | In 2006 we reached an agreement to settle our securities class action and shareholder derivative lawsuits. |
18
Table of Contents
Item 7.Management’s Discussion and Analysis of Financial Condition and Results of Operations
Management’s Discussion and Analysis of Financial Condition and Results of Operations (MD&A) is designed to provide a reader of our financial statements with a narrative from the perspective of our management on our financial condition, results of operations, liquidity and certain other factors that may affect our future results. Our MD&A is presented in eight sections:
| • | Executive Overview and Key Strategic Initiatives | |
| • | 2008 Overview | |
| • | 2009 Outlook | |
| • | Results of Operations | |
| • | Liquidity and Capital Resources | |
| • | Contractual Obligations | |
| • | Off-Balance-Sheet Arrangements | |
| • | Critical Accounting Estimates |
Our MD&A should be read in conjunction with the other sections of this annual report on Form 10-K, including Part I, “Item 1A: Risk Factors”; Part II, “Item 6: Selected Financial Data”; and Part II, “Item 8: Financial Statements and Supplementary Data.” The various sections of this MD&A contain a number of forward-looking statements, all of which are based on our current expectations and could be affected by the uncertainties and risk factors described throughout this filing.
Executive Overview and Key Strategic Initiatives
Our mission is to protect life by providing safer, more effective use of force options and technologies. We are a market leader in the development and manufacture of advanced electronic control devices (ECD’s) designed for use in the law enforcement, military, corrections, private security and personal defense markets. We continue to focus our efforts on the development of our technology for both new and existing products that further our mission. At the same time we have established industry leading training services to provide our users a comprehensive overview of TASER ECDs use-of-force, legal and policy issues, medical information and risk mitigation. We have built a network of distribution channels for selling and marketing our products and services to law enforcement agencies, primarily in North America, with ongoing focus and effort placed on expanding these programs in international, military and other markets. Over 14,000 law enforcement agencies in over 45 countries have made initial purchases of our TASER brand devices for testing or deployment. To date, we do not know of any significant sales of any competing ECD products.
Our key strategies include:
| • | Increase market penetration in both the United States and international law enforcement, military and corrections markets. We believe that a large portion of these markets that do not currently use our products presents an opportunity for our future growth, particularly with respect to international law enforcement agencies. | ||
| • | Grow our presence in the private citizen market. Having demonstrated the effectiveness of our technology in the professional law enforcement community, we aim to leverage this experience to increase our presence in the private citizen market. At the forefront of this initiative is the TASER C2 personal protector, which we launched in the third quarter of 2007. As our flagship consumer product we intend to increase consumer awareness of the TASER C2 by expanding the marketing and distribution of this product in 2009. | ||
| • | Further develop our presence in government and military markets. We intend to continue to place a strong emphasis on supporting our military customers through our Government and Military Programs business group and our Senior Executive Advisory Group (SEAG) comprised of a team of professionals with extensive military, homeland defense and law enforcement experience with the purpose of advising on business development in support of military users. The primary focus of these groups is placed on supporting military use for our existing hardware as well as increasing technology development through contracted support. In 2008, we entered into a multiphase science and technology contract with the Joint Non-Lethal Weapons Directorate (JNLWD) of the U.S Department of Defense to develop a 40mm projectile version, compatible with already fielded weapons, which allows for extension and improvement of our existing eXtended Range Electronic Projectile (XREP) technology. In 2007, we received a five-year indefinite delivery, indefinite quantity contract with the United States Military with the possibility of future orders up to a maximum value of $22.8 million. |
19
Table of Contents
| • | Continued investment in development of innovative new products, which both complement and add to our existing platforms. These development efforts include the following products which we expect to be available for sale during 2009: |
| 1. | The TASER AXON is an on officer tactical networkable computer that initially combines advanced video and audio recording capabilities that integrates with existing communication platforms. The technology allows officers to record video and audio of critical incidents from the visual perspective of the officer. The AXON is designed to be an information multiplier for the officer, providing an unbiased view of situations that the officer chooses to record. We expect the AXON will be available for sale in the third quarter of 2009. | ||
| 2. | EVIDENCE.com is a new integrated digital multi-media evidence storage and management platform which works in conjunction with the TASER AXON device. We consider this is a major platform-computing initiative, utilizing advanced ‘Web 3.0’ technology to create an integrated end-to-end solution to seamlessly capture, store securely, and analyze digital evidence and information that enables tactical and strategic decision making by law enforcement, as well as for legal evidentiary use. We anticipate that EVIDENCE.com will be available for sale in the third quarter of 2009. | ||
| 3. | Our wireless eXtended Range Electromuscular Projectile (XREP) was initially developed in 2007 with a focus on design for manufacturability. A production projectile is expected to be available for sale in the second quarter of 2009 following field trials throughout 2008. | ||
| 4. | Our TASER Shockwave is the first generation of products from the TASER Remote Area Denial (T-RAD) platform. Shockwave is a command activated area denial system consisting of a modular 6-shot TASER ECD that covers a 22-degree arc area and a range of 25 feet. The modular design allows the end user flexibility to configure the units in numerous combinations to facilitate an optimized response for every deployment. Initial engineering prototypes of Shockwave were developed and tested in 2007. Advanced prototypes were made available for customer test and evaluation in 2008 and we expect to have production units available for sale in the second quarter of 2009. |
| • | Continued application for patents and intellectual property rights to protect key technology in our products and further attempt to protect our competitive position. | ||
| • | Continued aggressive litigation defense to protect our brand equity. We have assembled a team of world class medical experts and hired additional internal legal resources to provide an efficient means of defending the Company against numerous product liability claims. Through March 2009, we have had a total of 82 cases dismissed or defense judgments in our favor. We view a continued record of successful litigation defense as a key factor for our long term growth and success. |
2008 Overview
Management believes that its ability to achieve a balance between growing our core business and building the foundations for future growth is the key to increasing long-term shareholder value. Our 2008 performance and the initiatives we have put in place reflect our continuing commitment to achieving this balance. While we experienced a noticeable decline in domestic municipal spending during 2008 as a result of the economic downturn, we were encouraged by the increasing momentum in international acceptance of our products. We remained focused on sustaining an efficient operating environment to enhance gross margin levels and made significant investments in our future by expanding our research and development programs for both hardware and software.
Some 2008 highlights include the following:
| • | Although the majority of our sales occur in North American markets, which were negatively impacted by adverse economic conditions, we continued to focus our efforts on markets outside the U.S. and, during 2008, our International sales continued to grow in significance, accounting for approximately 18% of our total sales compared to 15% in 2007 and 14% in 2006. In particular, 2008 international sales included some significant advances in the United Kingdom, which, following extensive trial and evaluation of our products, announced plans to fund 10,000 TASER ECD’s for police officers in England and Wales. We shipped our products to more than 50 countries during 2008, the more significant of which included the U.K, France, Canada, Korea, Australia and Brazil. |
20
Table of Contents
| • | The TASER C2 personal protector and accessories, generated revenues of approximately $6.1 million, or 7% of our net sales, for the year. | ||
| • | We continue to remain focused on improving our gross margins. Our gross margin improved to 61.4% in 2008 compared to 57.1% in 2007, primarily as a result of concerted efforts to control costs and improve manufacturing efficiency and quality. | ||
| • | We invested $12.9 million in research and development programs during 2008 with a concerted effort made to bring AXON, XREP and Shockwave products to market in 2009, in addition to our ongoing development of other products in the pipeline. | ||
| • | Our strategy of vigorously defending against product liability lawsuits continues to be successful. Courts dismissed 18 lawsuits against us in 2008. We believe these dismissals serve to highlight the extensive medical and scientific evidence confirming the general safety of TASER technology. | ||
| • | We generated $8.1 million in cash from operations in 2008 and ended the year with $49.4 million in cash and short term investments with zero debt. In times of economic uncertainty, we feel our strong balance sheet allows us the flexibility to invest in our key strategic and growth initiatives. |
2009 Outlook
Despite the challenging global economic environment we face in 2009, our view is that this presents us with an opportunity to extend our technological and market leadership. Our goals are to continue making investments towards future growth initiatives including investment in ongoing research and development programs, recruit highly skilled personnel to drive new product development and increase our sales and marketing efforts. We intend to pursue increased market penetration in our primary target markets with continued focus on increasing our international presence. We believe that our planned new product introductions in 2009 and other key initiatives identified above will position us to emerge from the economic downturn in a strong position to drive future revenue growth.
Results of Operations
The following table presents data from our statements of operations as well as the percentage relationship to total net revenues of items included in our statements of operations (dollars in thousands):
| Year ended December 31, | ||||||||||||||||||||||||
| 2008 | 2007 | 2006 | ||||||||||||||||||||||
Net sales | $ | 92,845 | 100 | % | $ | 100,727 | 100 | % | $ | 67,718 | 100 | % | ||||||||||||
| Cost of products sold | 35,841 | 39 | % | 43,167 | 43 | % | 24,539 | 36 | % | |||||||||||||||
Gross margin | 57,004 | 61 | % | 57,560 | 57 | % | 43,179 | 64 | % | |||||||||||||||
| Sales, general and administrative expenses | 38,861 | 42 | % | 32,814 | 33 | % | 29,681 | 44 | % | |||||||||||||||
| Research and development expenses | 12,918 | 14 | % | 4,422 | 4 | % | 2,705 | 4 | % | |||||||||||||||
| Shareholder litigation settlement expense | — | — | — | — | 17,650 | 26 | % | |||||||||||||||||
Income (loss) from operations | 5,225 | 6 | % | 20,324 | 20 | % | (6,857 | ) | -10 | % | ||||||||||||||
| Interest and other income, net | 1,718 | 2 | % | 2,202 | 2 | % | 1,873 | 3 | % | |||||||||||||||
Income (loss) before income taxes | 6,943 | 7 | % | 22,526 | 22 | % | (4,984 | ) | -7 | % | ||||||||||||||
| Provision (benefit) for income taxes | 3,306 | 4 | % | 7,500 | 7 | % | (896 | ) | -1 | % | ||||||||||||||
Net income (loss) | $ | 3,637 | 4 | % | $ | 15,026 | 15 | % | $ | (4,088 | ) | -6 | % | |||||||||||
Net Sales
For the years ended December 31, 2008, 2007 and 2006, sales by product line and by geography were as follows (dollars in thousands):
| 2008 | 2007 | 2006 | ||||||||||||||||||||||
Sales by Product Line | ||||||||||||||||||||||||
| TASER X26 | $ | 51,733 | 56 | % | $ | 61,638 | 61 | % | $ | 45,241 | 67 | % | ||||||||||||
| TASER C2 | 6,127 | 7 | % | 3,983 | 4 | % | — | — | ||||||||||||||||
| TASER Cam | 3,304 | 3 | % | 4,012 | 4 | % | 2,289 | 3 | % | |||||||||||||||
| ADVANCED TASER | 3,422 | 4 | % | 2,412 | 2 | % | 2,578 | 4 | % | |||||||||||||||
| Single Cartridges | 20,526 | 22 | % | 25,250 | 25 | % | 15,269 | 23 | % | |||||||||||||||
| Other | 7,733 | 8 | % | 3,432 | 4 | % | 2,341 | 3 | % | |||||||||||||||
| Total | $ | 92,845 | 100 | % | $ | 100,727 | 100 | % | $ | 67,718 | 100 | % | ||||||||||||
| Sales by Geographic Area | 2008 | 2007 | 2006 | |||||||||||||||||||||
| United States | 82 | % | 85 | % | 86 | % | ||||||||||||||||||
| Other Countries | 18 | % | 15 | % | 14 | % | ||||||||||||||||||
| Total | 100 | % | 100 | % | 100 | % | ||||||||||||||||||
21
Table of Contents
Net sales for the year ended December 31, 2008 were $92.8 million, a decrease of $7.9 million, or 8%, compared to $100.7 million in 2007. The decrease in 2008 was primarily driven by a decline in sales of our core X26 product line and single cartridges which we believe reflects lower municipal spending in the U.S. as agencies experienced constrained budgets due to prevailing adverse economic conditions. This resulted in reduced sales of the TASER X26 product line which decreased by $9.9 million, or 16%, to $51.7 million in 2008 compared to $61.6 million in 2007. Single cartridge sales also decreased by $4.7 million, or 19% to $20.5 million in 2008 compared to $25.2 million in 2007. Partially offsetting these decreases was a full year’s sales in 2008 of the TASER C2 Personal Protector product which began shipping in July 2007. Sales of the TASER C2 were $6.1 million in 2008, an increase of $2.1 million over the same period in 2007. Additionally, sales of the Advanced TASER increased by $1.0 million mainly due to a large purchase made by an international customer in the first quarter of 2008 and other sales grew $4.3 million due to a $2.0 million increase in out of warranty replacement and extended warranty sales as well as $2.1 million reduction of cash and distributor discounts in 2008. Other sales also include government grant, training and shipping revenues.
Net sales increased $33.0 million, or 49%, to $100.7 million in 2007 compared to $67.7 million in 2006. The growth in 2007 was primarily the result of increased sales to our core law enforcement market with new agencies deploying TASER technology following test and evaluation periods and from agencies continuing to expand the use of TASER devices. This resulted in higher sales of the TASER X26 product line which increased $16.4 million, or 36%, to $61.6 million in 2007 compared to $45.2 million in 2006. Single cartridge sales increased $10.0 million, or 65%, to $25.3 million in 2007 compared to $15.3 million in 2006 which is a function of the growing installed base of units in the field. We began shipping our TASER C2 Personal Protector product in July 2007 which contributed $4.0 million of sales in 2007. Also contributing to the growth in net sales for the year ended December 31, 2007 was a full year’s sales of the TASER Cam product which was introduced at the end of the second quarter of 2006. Sales of the TASER Cam were $4.0 million for the year ended December 31, 2007, an increase of $1.7 million, or 75%, over 2006. Other sales include extended warranty, out of warranty replacements, training, shipping and research funding revenues net of cash and distributor discounts.
International sales for 2008 and 2007 represented approximately $17.0 million, or 18% of total net sales, and $15.3 million or 15% of total net sales, respectively. International sales represented approximately $9.3 million or 14% of total net sales in 2006. The growth in international sales in both 2008 and 2007 reflects our continued commitment to marketing efforts in countries outside the United States. In particular, 2008 international sales included some significant advances in the United Kingdom, which following extensive trial and evaluation of our products, announced plans to fund 10,000 TASER ECD’s for police officers in England and Wales. We shipped the first 5,000 of these units in the fourth quarter of 2008.
Cost of Products Sold
Cost of products sold decreased by $7.3 million, or 17%, to $35.8 million in 2008 compared to $43.1 million in 2007. As a percentage of net sales, cost of products sold decreased to 38.6% in 2008 compared to 42.8% in 2007. The 420 basis point improvement in 2008 compared to 2007 was the result of a combination of factors. The $2.1 million reduction in our cash and distributor sales discounts and the $2.0 million growth in extended warranty and out of warranty replacement revenue contributed to the reduction in cost of products sold as a percentage of net sales. Total direct manufacturing costs in 2008 decreased primarily as the result of a $2.5 million reduction in temporary labor and overtime costs while product materials costs decreased due to improved supplier pricing negotiated on various raw material components. Indirect manufacturing costs declined as a percentage of net sales resulting from lower variable manufacturing costs including scrap expense, freight and engineering supplies, a function of improved product quality and operating efficiencies as well as reduced levels of production. In addition, our allocation of manufacturing overhead to inventory increased due to a reduction in direct production hours during 2008 combined with an increase in labor hours in finished goods inventory at December 31, 2008 compared to December 31, 2007.
Cost of products sold increased by $18.6 million, or 75.9%, to $43.1 million in 2007 compared to $24.5 million in 2006. As a percentage of net sales, cost of products sold increased to 42.8% in 2007 compared to 36.2% in 2006. The increase in cost of products sold as a percentage of net sales for 2007 compared to 2006 was driven by the following combination of factors. We experienced a change in sales mix with growth as percentage of net sales in lower margin cartridge sales and TASER Cam’s and the introduction of our lower margin C2 product line. We also experienced a rise in raw material costs due to higher prices for plastics and printed circuit board assemblies. In combination, these factors contributed to a 220 basis point increase in the cost of products sold as a percentage of revenue in 2007 compared to 2006. Also in the second half of 2007, production of our new TASER C2 created a number of production challenges related to the integration of new production lines and personnel which, in combination, initially generated significant line inefficiencies and product scrap. To address the initial low production yield issues, some engineering modifications were made to the injection molding tooling and printed circuit board design to establish a more efficient assembly process. These modifications added significant incremental time to the assembly of each C2 produced and also resulted in inventory rework. The related increases in direct labor, scrap expense and engineering supplies accounted for 200 basis points, 150 basis points and 40 basis points toward the 660 basis point increase in cost of products sold as a percentage of net sales for 2007 compared to 2006. An increase in warranty and obsolescence reserves also contributed 50 basis points to the increase in cost of products sold as a percentage of net sales. The increase in warranty reserves was primarily driven by higher sales of the X26 and the introduction of the C2, while reserves for excess and obsolete inventory increased related to some slow moving parts. These increases were partially offset by improved leverage of our indirect labor and other manufacturing expenses over larger product volumes.
22
Table of Contents
Gross Margin
Gross margin decreased $0.6 million to $57.0 million in 2008 compared to $57.6 million in 2007. As a percentage of net sales, gross margins increased to 61.4% in 2008 compared to 57.1% in 2007. The 430 basis point improvement in gross margin in 2008 was attributable to the decrease in direct and indirect manufacturing costs as a percentage of net sales for the reasons noted above under the discussion of cost of products sold.
Gross margin increased $14.4 million, or 33.3%, to $57.6 million in 2007 compared to $43.2 million in 2006. As a percentage of net sales, gross margins decreased to 57.1% in 2007 compared to 63.8% for 2006. This decrease was attributable to the increased percentage of direct and indirect costs as a percentage of net sales for the reasons noted above under the discussion of cost of products sold.
Sales, General and Administrative Expenses
For the years ended December 31, 2008, 2007 and 2006, sales, general and administrative expenses were comprised as follows(dollars in thousands):
| Year Ended December 31, | Year Ended December 31, | |||||||||||||||||||||||||||||||
| $ | % | $ | % | |||||||||||||||||||||||||||||
| 2008 | 2007 | Change | Change | 2007 | 2006 | Change | Change | |||||||||||||||||||||||||
| Salaries and benefits | $ | 9,004 | $ | 7,009 | $ | 1,995 | 28.5 | % | $ | 7,009 | $ | 5,565 | $ | 1,444 | 25.9 | % | ||||||||||||||||
| Legal, professional and accounting | 5,899 | 5,813 | 86 | 1.5 | % | 5,813 | 7,024 | (1,211 | ) | -17.2 | % | |||||||||||||||||||||
| Consulting and lobbying services | 3,478 | 2,455 | 1,023 | 41.7 | % | 2,455 | 2,274 | 181 | 8.0 | % | ||||||||||||||||||||||
| Travel and meals | 3,739 | 3,762 | (23 | ) | -0.6 | % | 3,762 | 3,312 | 450 | 13.6 | % | |||||||||||||||||||||
| D&O and liability insurance | 2,191 | 2,027 | 164 | 8.1 | % | 2,027 | 2,121 | (94 | ) | -4.4 | % | |||||||||||||||||||||
| Advertising | 2,085 | 931 | 1,154 | 124.0 | % | 931 | 437 | 494 | 113.0 | % | ||||||||||||||||||||||
| Depreciation and amortization | 1,635 | 1,557 | 78 | 5.0 | % | 1,557 | 1,342 | 215 | 16.0 | % | ||||||||||||||||||||||
| Stock based compensation | 1,552 | 987 | 565 | 57.2 | % | 987 | 820 | 167 | 20.4 | % | ||||||||||||||||||||||
| Bonuses | 345 | 1,138 | (793 | ) | -69.7 | % | 1,138 | 546 | 592 | 108.4 | % | |||||||||||||||||||||
| Other | 8,933 | 7,135 | 1,798 | 25.2 | % | 7,135 | 6,240 | 895 | 14.3 | % | ||||||||||||||||||||||
| Total | $ | 38,861 | $ | 32,814 | $ | 6,047 | 18.4 | % | $ | 32,814 | $ | 29,681 | $ | 3,133 | 10.6 | % | ||||||||||||||||
| Sales, general and administrative as percentage of net sales | 41.9 | % | 32.6 | % | 32.6 | % | 43.8 | % | ||||||||||||||||||||||||
Sales, general and administrative expenses were $38.9 million and $32.8 million in 2008 and 2007, respectively, an increase of $6.0 million, or 18.4%. As a percentage of total net sales, sales, general and administrative expenses increased to 41.9% during 2008 compared to 32.6% in 2007. The dollar increase during 2008 over 2007 is attributable to a combination of factors. Specifically, salaries and benefits grew $2.0 million related to the addition of personnel to support the expansion of our business infrastructure combined with an annual salary increase effective January 1, 2008 as well as higher cost of benefits. Advertising expense increased $1.2 million primarily due to expensing of $550,000 in production costs of the TASER C2 infomercial as well as ongoing promotion and infomercial airing costs. Consulting and lobbying services increased $1.0 million primarily attributable to strategic selling and marketing, advertising and process improvement related efforts. In addition, stock based compensation increased $565,000 related to stock options granted in 2008 and D&O and liability insurance costs are up $164,000 from increased annual premiums. The $1.8 million increase in other expense is primarily attributable to a $500,000 increase in recruiting and relocation expenses driven by hiring of new vice presidents of sales, marketing, IT and HR, a $401,000 increase in trade show expenses and a $278,000 increase in computer licensing and maintenance fees. These increases were partially offset by a $793,000 decrease in bonuses due to the lower pre-tax income in 2008 as well as a program which allowed employees to opt out of the cash based bonus program for two years in exchange for additional stock options.
Sales, general and administrative expenses were $32.8 million and $29.7 million in 2007 and 2006, respectively, an increase of $3.1 million, or 10.6% in 2007 compared to 2006. As a percentage of total net sales, sales, general and administrative expenses decreased to 32.6% during 2007 compared to 43.8% in 2006. The dollar increase in 2007 over 2006 is substantially attributable to $1.4 million of growth in salaries and benefits related to an increase in support personnel, annual salary adjustments and higher benefit costs. Bonuses increased $592,000 due to the improved operating results in 2007, travel and meals increased $450,000 mainly as a result of the higher sales related activity, depreciation and amortization increased $215,000 related to acquisitions of computer and office equipment, stock based compensation increased $167,000 associated with stock options granted in 2007 and consulting and lobbying costs increased $181,000 primarily due to higher expert witness fees. Advertising expense increased $494,000 primarily related to the TASER C2 launch and sales commissions increased in line with the increase in total sales. Offsetting these increases was a $1.2 million decrease in legal, professional and accounting costs primarily due to a reduction in legal fees attributable to the timing of proceedings of our outstanding litigation as well as four cases where we exceeded our insurance deductible, subsequent to which we are reimbursed for expenses incurred.
23
Table of Contents
Research and Development Expenses
Research and development expenses increased $8.5 million, or 192%, to $12.9 million in 2008 compared to $4.4 million in 2007. The increase is driven by a $4.2 million increase in third party consulting costs primarily associated with the development of AXON. In addition, there was $1.4 million growth in salary and benefit costs attributable to increased headcount combined with an annual salary increase effective January 1, 2008, and a $1.3 million increase in indirect supplies to support our continuing efforts to develop new products including AXON, XREP (Extended Range Electro-Muscular Projectile) and Shockwave. We expect to further increase research and development spending in 2009 as we continue development of new products in the pipeline.
Research and development expenses for 2007 were $4.4 million, an increase of $1.7 million, or 63% compared to 2006. The increase is predominantly related to salary related costs and production materials in the development of new products such as the TASER C2, XREP and Shockwave.
Shareholder Litigation Settlement Expense
Litigation settlement expenses for the 2006 represented $17.65 million recorded in the second quarter of 2006 as a result of the settlement of our shareholder class action litigation and derivative lawsuits.
Interest and Other Income, Net
Interest and other income decreased by $484,000, or 22%, to $1.7 million in 2008 compared to $2.2 million in 2007. This was attributable to a $710,000 decrease in interest income due to lower average yields on our investments, partially offset by an increase in total average funds invested during 2008 compared to 2007. Our cash and investment accounts earned interest at an average rate of approximately 2.5% during 2008 compared to 4.2% in 2007. The decrease in interest income was partially offset by other income of $405,000 related to the unused deferred insurance settlement proceeds recognized in the second quarter of 2008 upon the dismissal of all final appeals in a personal injury case.
Interest and other income increased $330,000, or 18%, to $2.2 million for 2007 compared to $1.9 million for 2006. The increase is mainly attributable to the increase in average cash and investment balances on hand as well as higher average yields on our cash and investments, 4.2% in 2007 compared to 3.9% in 2006. Our average outstanding cash, cash equivalent and investment balance was approximately $49.4 million in 2007 compared to $47.3 million in 2006.
Provision for Income Taxes
The provision for income taxes decreased by $4.2 million to $3.3 million in 2008 compared to $7.5 million in 2007. The effective income tax rate for 2008 was 47.6% compared to 33.3% for 2007. Contributing to the increase in effective tax rate is the higher impact of certain non-deductible items such as lobbying expenses against a lower taxable income for the year ended December 31, 2008. In addition, the 2008 effective tax rate is reduced by a reduced amount of research and development tax credits ($608,000 in 2008 vs. $2.0 million in 2007), which was partially offset by an increase in the liability for unrecognized tax benefits.
The provision for income taxes increased by $8.4 million to a provision of $7.5 million for 2007 compared to a benefit for income taxes of $0.9 million for 2006. The change in tax position is due to the net income before taxes of $22.5 million in 2007 compared to the net loss before income taxes of $5.0 million in 2006, which primarily resulted from the shareholder litigation expense of $17.65 million recorded in the second quarter of 2006. The effective income tax rate for 2007 was 33.3% compared to (18.0) % for 2006. The effective rate in 2007 reflects the benefit of a research and development tax credit study completed during 2007 which resulted in a $2.0 million reduction in the provision for income taxes. The effective rate in 2006 reflects the recording of non tax deductible items such as lobbying expenses and an impairment of the Arizona State NOL carryforward of $250,000 in the second quarter of 2006, which reduced our effective tax benefit rate by 13.9% and 5.0%, respectively.
Net Income (Loss)
Net income decreased by $11.4 million to $3.6 million in 2008 compared to $15.0 million in 2007. Income per basic and diluted share was $0.06 for 2008. This compares to income per basic and diluted share of $0.24 and $0.23, respectively, in 2007.
Net income increased by $19.1 million to $15.0 million for 2007 compared to a net loss of $4.1 million for 2006. Income per basic and diluted share was $0.24 and $0.23, respectively, for 2007. This compares to a loss per basic and diluted share of $0.07 for 2006.
24
Table of Contents
Liquidity and Capital Resources
The following table presents selected financial information at the end of the last three fiscal years(dollars in thousands):
| 2008 | 2007 | 2006 | ||||||||||
| Cash, cash equivalents and short term investments | $ | 49,379 | $ | 51,301 | $ | 22,331 | ||||||
| Accounts receivable, net | 16,794 | 11,692 | 10,068 | |||||||||
| Inventory | 13,467 | 13,507 | 9,258 | |||||||||
| Working capital | 80,643 | 83,953 | 37,814 | |||||||||
| Net cash provided by operating activities | 8,118 | 13,923 | 7,482 | |||||||||
| Net cash provided by (used in) investing activities | 8,117 | 7,006 | (3,556 | ) | ||||||||
| Net cash (used in) provided by financing activities | $ | (12,156 | ) | $ | 3,099 | $ | (1,504 | ) | ||||
Liquidity
Our most significant sources of liquidity continue to be funds generated by operating activities and available cash and cash equivalents. We believe funds generated from our expected results of operations, available cash and cash equivalents, and short-term investments will be sufficient to finance our operations and strategic initiatives for 2009. In addition, our revolving credit facility is available for additional working capital needs or investment opportunities. There can be no assurance, however, that we will continue to generate cash flows at or above current levels or that we will be able to maintain our ability to borrow under our revolving credit facility.
As of December 31, 2008, we had $49.4 million in cash, cash equivalents and short term investments, a decrease of $1.9 million from the end of 2007 which is primarily attributable to our use of $12.5 million to repurchase our common stock as well as investments in property and equipment and intangible assets, partially offset by net proceeds from the maturities of investment holdings. Net cash provided by operating activities was $8.1 million during 2008. We expect that cash used / generated from accounts receivable, inventory and accounts payable in 2009 will remain relatively consistent with 2008; however, we intend to manage these closely to align with forecasted and actual sales and production levels. Accounts receivable at December 31, 2008 increased by $5.1 million compared to December 31, 2007, primarily as the result of a large individual sale made to the U.K. Government in December 2008, which was paid in full in February 2009. Additionally, we expect to invest a further $6.0 to $10.0 million in capital expenditures in 2009, including $3.9 million in manufacturing automation equipment in the first half of 2009 and anticipate continuing to invest in research and development in excess of 2008 levels as we accelerate development of new products in the pipeline.
Net cash provided by operating activities was $8.1 million in 2008, compared with $13.9 million in 2007 and $7.5 million in 2006. Net cash provided by operating activities during 2008 reflects non-cash changes to net income including, depreciation and amortization expense of $2.6 million, stock-based compensation expense of $2.4 million, provision for warranty expense and excess and obsolete inventory of $1.0 million and the $2.1 million utilization of deferred tax assets. In addition, prepaid and other assets decreased $1.9 million due to i) the net receipt of insurance reimbursements of legal fees incurred in excess of policy retention limits; ii) a decrease in prepaid advertising due to the expensing of TASER C2 infomercial production costs and iii) amortization of prepaid liability and D&O insurance premiums. Deferred revenue also increased $2.1 million driven by extended warranty sales in 2008. Offsetting these items was a $5.1 million increase in accounts receivable as noted above as well as a decrease in accounts payable and accrued liabilities of $2.3 million, which reflects timing differences combined with a reduced rate of material purchasing as well as the payment in January 2008 of $1.2 million for the second installment for automation equipment which was accrued at December 31, 2007.
Net cash provided by operating activities for 2007 of $13.9 million was mainly attributable to our net income for the period of $15.0 million, the $5.8 million utilization of deferred tax assets and total other non cash adjustments to net income of $5.2 million including depreciation and amortization expense of $2.5 million, stock-based compensation expense of $1.4 million and provision for warranty expense of $1.0 million. In addition, deferred revenue related to the growth in sales of extended warranties increased by $2.2 million. These cash sources were partially offset by the final $8.0 million payment for the shareholder litigation settlement, a $4.5 million increase in inventory primarily related to our building of TASER C2 and X26 inventory to satisfy anticipated demand, and a $1.7 million increase in accounts receivable due to the higher sales levels in December 2007 compared to December 2006. While the accounts receivable balance has increased, our days sales outstanding ratio decreased; a function of both improved collections and more customers taking advantage of cash discount offerings. In addition, prepaid and other assets increased $2.2 million primarily related to insurance reimbursement receivables for four legal cases where we have incurred costs in excess of our deductible, deferred infomercial production costs and increased deferred liability insurance premiums.
25
Table of Contents
Net cash provided by investing activities was $8.1 million during 2008 which was comprised of $15.0 million in net proceeds from maturing investments over investment purchases partially offset by the use of $6.1 million to purchase property and equipment mainly related to new automation equipment and computer storage solutions. In addition, we invested $746,000 in intangible assets, primarily consisting of patent application costs.
Our investing activities provided $7.0 million for the 2007 which was comprised of a $11.5 million net decrease in our total investments caused by the maturity of some long term investments partially offset by $4.1 million in acquisitions of property and equipment, which mainly related to new automation equipment, production equipment for the TASER C2 manufacturing line and capitalized website development costs. In addition, we invested $454,000 in intangible assets, primarily consisting of patent applications.
During 2008, we utilized $12.2 million in financing activities, a function of the $12.5 million to repurchase 1.8 million shares of our common stock partially offset by $343,000 of proceeds attributable to stock options exercised in the year.
During the year ended December 31, 2007, we generated $3.1 million from financing activities attributable to stock options exercised in the year.
Capital Resources
On December 31, 2008, we had total cash and cash equivalents and short term investments of $49.4 million.
We have a revolving line of credit with a domestic bank with a total availability of $10.0 million. The line is secured by substantially all of our assets, other than intellectual property, and bears interest at varying rates, ranging from LIBOR plus 1.5% to prime. The line of credit matures on June 30, 2010 and requires monthly payments of interest only. At December 31, 2008, there were no borrowings under the line and all of the line was available based on the defined borrowing base, which is calculated on our eligible accounts receivable and inventory. Our agreement with the bank requires us to comply with certain financial and other covenants including maintenance of minimum tangible net worth and fixed charge coverage. At December 31, 2008 we were in compliance with all covenants.
We believe that our balance of total cash, cash equivalents and short term investments of $49.4 million as of December 31, 2008, together with cash expected to be generated from operations and our existing credit facility will be adequate to fund our operations for at least the next 12 months. We may require additional resources to expedite manufacturing of new and existing technologies in order to meet possible demand for our products. Based on our strong balance sheet and the fact we had no outstanding debt at December 31, 2008 we believe financing will be available, both through our existing credit line and possible additional financing. However, there is no assurance that such funding will be available, or on terms acceptable to us. Capital markets in the United States and throughout the world remain disrupted and under stress. This disruption and stress is evidenced by a lack of liquidity in the debt capital markets, the re-pricing of credit risk in the syndicated credit market and the failure of certain major financial institutions. This stress is compounded by the ongoing severe worldwide recession. Despite actions of the U.S. federal government, these events have contributed to worsening general economic conditions that are materially and adversely impacting the broader financial and credit markets and reduced the availability of debt capital for the market as a whole. Reflecting this concern, many lenders and capital providers have reduced, and in some cases ceased to provide, debt funding to borrowers. The resulting lack of available credit, lack of confidence in the financial sector, increased volatility in the financial markets and reduced business activity could materially and adversely affect our ability to obtain additional or alternative financing.
Contractual Obligations
The following table outlines our future contractual financial obligations by period in which payment is expected, in thousands, as of December 31, 2008:
| Less than | After | |||||||||||||||||||
| Total | 1 year | 1-3 years | 4-5 years | 5 years | ||||||||||||||||
| Non-cancelable operating leases | $ | 773 | $ | 232 | $ | 470 | $ | 71 | $ | — | ||||||||||
| Purchase obligations | $ | 3,884 | 3,884 | — | — | — | ||||||||||||||
| Total contractual cash obligations | $ | 4,657 | $ | 4,116 | $ | 470 | $ | 71 | $ | — | ||||||||||
Purchase obligations consist of $3.9 million of payments for the installation and delivery of equipment. On July 2, 2007, we entered into a contract with ATS Automation Tooling Systems Inc. for the purchase of equipment at a cost of approximately $8.4 million which includes $0.7 million of change orders made in the first quarter of 2008 for additional equipment. Following some construction delays, the equipment is expected to be delivered to and installed at our Scottsdale facility in the second quarter of 2009. Payments are to be made in installments, with an initial $1.5 million deposit paid in 2007, $3.0 million paid during 2008 and the balance of $3.9 million is expected to be paid in 2009.
26
Table of Contents
We are subject to U.S. federal income tax as well as income tax of multiple-state jurisdictions. As of December 31, 2008, we had $1.5 million of gross unrecognized tax benefits related to uncertain tax positions. The settlement period for our long-term income tax liabilities cannot be determined, however, the liabilities are not expected to become due within the next twelve months.
Off Balance Sheet Arrangements
We had no off balance sheet arrangements as of December 31, 2008.
Critical Accounting Estimates
We have identified the following accounting estimates as critical to our business operations and the understanding of our results of operations. The preparation of this annual report on Form 10-K requires us to make estimates and assumptions that affect the reported amount of assets and liabilities, disclosure of contingent assets and liabilities at the date of our financial statements, and the reported amounts of revenue and expenses during the reporting period. There can be no assurance that our actual results will not differ from those estimates. The effect of these policies on our business operations is discussed below.
Standard Product Warranty Reserves
We warrant our law enforcement ECD’s from manufacturing defects on a limited basis for a period of one year after purchase, and thereafter will replace any defective TASER unit for a fee. We warrant our new TASER C2 product for 90 days. We track historical data related to returns and warranty costs on a quarterly basis, and estimate future warranty claims by applying our weighted average rolling four quarter return rate to our product sales for the period. In the fourth quarter of 2007, we made a revision to the basis of calculating the four quarter return rate as the result of being able to more accurately capture data relating to the number of units replaced under standard warranty versus extended warranty terms. In addition, given the trend of sales growth experienced in 2007, particularly in the second half of the year, the estimated four quarter return rate was weighted to account for the higher return rate experienced in those periods. We have also historically increased our reserve amount if we become aware of a component failure that could result in larger than anticipated returns from our customers. As of December 31, 2008, our reserve for warranty returns was $615,000 compared to a $919,000 reserve at December 31, 2007. Our reserve for warranty returns decreased at December 31, 2008 as the result of a reduced returns experience, particularly in our X26 product line which we believe is a function of continuing improvements made in the manufacturing and quality processes. In the event that product returns under warranty differ from our estimates, changes to warranty reserves might become necessary.
Inventory
Inventories are stated at the lower of cost or market, with cost determined using the weighted average cost of raw materials, which approximates the first-in, first-out (FIFO) method, and an allocation of manufacturing labor and overhead costs. The allocation of manufacturing labor and overhead costs includes management judgements of what constitutes normal capacity of our production facilities, and a determination of what costs are considered to be abnormal fixed production costs which are expensed as current period charges in accordance with SFAS 151. Provisions are made to reduce potentially excess, obsolete or slow-moving inventories to their net realizable value. These provisions are based on our best estimates after considering historical demand, projected future demand, inventory purchase commitments, industry and market trends and conditions and other factors. Our reserve for excess and obsolete inventory decreased to $130,000 at December 31, 2008 compared to $321,000 at December 31, 2007 due to the net write off of slow moving raw material components. In the event that actual excess, obsolete or slow-moving inventories differ from these estimates, changes to inventory reserves might become necessary.
Accounts Receivable
Sales are typically made on credit and we generally do not require collateral. We perform ongoing credit evaluations of our customers’ financial condition and maintain an allowance for estimated potential losses. Uncollectible accounts are written off when deemed uncollectible, and accounts receivable are presented net of an allowance for doubtful accounts. This allowance represents our best estimate and is based on our judgment after considering a number of factors including third-party credit reports, actual payment history, customer-specific financial information and broader market and economic trends and conditions. Our allowance for doubtful accounts was $200,000 at December 31, 2008 compared to $190,000 at December 31, 2007. In the event that actual uncollectible amounts differ from these estimates, changes in allowances for doubtful accounts might become necessary.
27
Table of Contents
Valuation of Long-lived Assets
We review long-lived assets, such as property and equipment and intangible assets subject to amortization, whenever events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable. We utilize a two-step approach to testing long-lived assets for impairment. The first step tests for possible impairment indicators. If an impairment indicator is present, the second step measures whether the asset is recoverable based on a comparison of the carrying amount of the asset to the estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated future cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds the fair value of the asset. Our review requires the use of judgment and estimates. Management believes that no such impairments have occurred to date. However, future events or circumstances may result in a charge to earnings if we determine that the carrying value of a long-lived asset is not recoverable.
Income Taxes
Statement of Financial Accounting Standards No. 109, or SFAS No. 109, Accounting for Income Taxes, establishes financial accounting and reporting standards for the effect of income taxes. In accordance with SFAS No. 109, we recognize federal, state and foreign current tax liabilities or assets based on our estimate of taxes payable or refundable in the current fiscal year by tax jurisdiction. We also recognize federal, state and foreign deferred tax assets or liabilities, as appropriate, for our estimate of future tax effects attributable to temporary differences and carryforwards.
In July 2006, the FASB issued Interpretation 48, “Accounting for Uncertainty in Income Taxes” (“FIN 48”), which we adopted effective January 1, 2007. FIN 48 addresses the determination of how tax benefits claimed or expected to be claimed on a tax return should be recorded in the financial statements. Under FIN 48, we recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position are measured based on the largest benefit that has a greater than fifty percent likelihood of being realized upon ultimate resolution. Under FIN 48, management must also assess whether uncertain tax positions as filed could result in the recognition of a liability for possible interest and penalties if any. Interest and penalties are recorded in the provision for income taxes. In 2007, we completed a research and development tax credit study which identified $3.1 million in tax credits for Federal and Arizona income tax purposes related to the 2003 through 2006 tax years and an estimate for the 2007 tax year, and as a result, we recognized $2.0 million in 2007 as a reduction in income tax expense. Additionally, we have estimated another $869,000 of tax credits is available for Federal and Arizona purposes for the 2008 tax year. We made the determination that it was not more likely than not that the full benefit of these research and development tax credits would be sustained on examination and have increased the liability for unrecognized tax benefits by $592,000 to $1.7 million as of December 31, 2008. Also included as part of the $1.7 million liability for unrecognized tax benefits is a management estimate of $106,000 related to uncertain tax positions for certain state income tax liabilities. Our estimates are based on the information available to us at the time we prepare the income tax provisions. Our income tax returns are subject to audit by federal, state, and local governments, generally years after the returns are filed. These returns could be subject to material adjustments or differing interpretations of the tax laws.
Our calculation of current and deferred tax assets and liabilities is based on certain estimates and judgments and involves dealing with uncertainties in the application of complex tax laws. Our estimates of current and deferred tax assets and liabilities may change based, in part, on added certainty or finality to an anticipated outcome, changes in accounting or tax laws in the United States, or changes in other facts or circumstances. In addition, we recognize liabilities for potential United States tax contingencies based on our estimate of whether, and the extent to which, additional taxes may be due. If we determine that payment of these amounts is unnecessary or if the recorded tax liability is less than our current assessment, we may be required to recognize an income tax benefit or additional income tax expense in our financial statements.
In preparing our financial statements, we assess the likelihood that our deferred tax assets will be realized from future taxable income. In evaluating our ability to recover our deferred income tax assets we consider all available positive and negative evidence, including our operating results, ongoing tax planning and forecasts of future taxable income. We establish a valuation allowance if we determine that it is more likely than not that some portion or all of the net deferred tax assets will not be realized. We exercise significant judgment in determining our provisions for income taxes, our deferred tax assets and liabilities and our future taxable income for purposes of assessing our ability to utilize any future tax benefit from our deferred tax assets. Although we believe that our tax estimates are reasonable, the ultimate tax determination involves significant judgments that could become subject to audit by tax authorities in the ordinary course of business as well as the ultimate realization of sufficient future taxable income. As a result of the shareholder litigation settlement expense recorded in the second quarter of 2006, we recorded a valuation allowance of $250,000 in 2006 against our deferred tax assets for Arizona Net Operating Losses (“NOL’s”). In the second quarter of 2008, we recorded an additional $250,000 valuation allowance against the same deferred tax assets. In the fourth quarter of 2008, management updated its analysis regarding its ability to utilize the Arizona NOL to reflect the results of current operations, including the reversal of the punitive damages accrual of $5,200,000 relating to the Heston litigation in the fourth quarter of 2008, as well as its future business plans. Based on this updated analysis, management reduced its valuation allowance against the deferred tax assets for Arizona NOL’s from $500,000 to $200,000 at December 31, 2008. We believe that, other than as previously described, as of December 31, 2008, based on our evaluation, no additional valuation allowance was deemed necessary as it is more likely than not that our net deferred tax assets will be realized. However, the deferred tax asset could be reduced in the near term if estimates of taxable income during the carryforward period are reduced.
28
Table of Contents
Stock Based Compensation
We account for stock-based compensation in accordance with the fair value recognition provisions of SFAS No. 123R. We use the Black-Scholes-Merton option pricing model which requires the input of highly subjective assumptions. These assumptions include estimating the length of time employees will retain their stock options before exercising them (“expected term”), the estimated volatility of our common stock price over the expected term and the number of options that will ultimately not vest (“forfeitures”). We granted 811,000 of performance-based stock options in 2008, the exercise of which is contingent upon the completion of certain performance criteria including the successful development and market acceptance of future product introductions as well as our future operating performance. These options will vest and compensation expense will be recognized based on management’s best estimate of the probability of the performance criteria being satisfied using the most currently available projections of future product adoption and operating performance, adjusted at each balance sheet date. Changes in the subjective and probability based assumptions can materially affect the estimate of fair value of stock-based compensation and consequently, the related amount recognized on our statements of operations. Refer to Note 1(p) to our financial statements for further discussion of how we determined our valuation assumptions.
Contingencies
We are subject to the possibility of various loss contingencies including product related litigation, arising in the ordinary course of business. We consider the likelihood of loss or impairment of an asset or the incurrence of a liability, as well as our ability to reasonably estimate the amount of loss in determining loss contingencies. An estimated loss contingency is accrued when it is probable that an asset has been impaired or a liability has been incurred and the amount of loss can be reasonably estimated. We regularly evaluate current information available to us to determine whether such accruals should be adjusted and whether new accruals are required.
Item 7A.Quantitative and Qualitative Disclosures About Market Risk
Interest Rate Risk
We invest in a limited number of financial instruments, consisting principally of investments in high credit quality debt securities, denominated in United States dollars.
We account for our investment instruments in accordance with Statement of Financial Accounting Standards No. 115,“Accounting for Certain Investments in Debt and Equity Securities,”(SFAS No. 115). All of our cash equivalents and marketable securities are treated as “held-to-maturity” under SFAS No. 115. Investments in fixed rate interest earning instruments carry a degree of interest rate risk as their market value may be adversely impacted due to a rise in interest rates. As a result, we may suffer losses in principal if we sell securities that have declined in market value due to changes in interest rates. However, because we classify our debt securities as “held-to-maturity,” no gains or losses are recognized due to changes in interest rates. These securities are reported at amortized cost, which approximates fair value. As of December 31, 2008, we performed a sensitivity analysis on our fixed rate financial investments. According to our analysis, a change in interest rates of 50 basis points would result in a change in the fair market values for these investments of approximately $2,000. This investment was called at par value by the issuer in February 2009.
Additionally, we have access to a line of credit borrowing facility which bears interest at varying rates, ranging from LIBOR plus 1.5% to prime. At December 31, 2008, there was no amount outstanding under the line of credit and the available borrowing under the line of credit was $10.0 million. We have not borrowed any funds under the line of credit since its inception, however, should we need to do so in the future, such borrowings could be subject to adverse or favorable changes in the underlying interest rate.
Exchange Rate Risk
We consider our direct exposure to foreign exchange rate fluctuations to be minimal. Currently, sales to customers provide for pricing and payment in United States dollars, and therefore are not subject to exchange rate fluctuations. To date, we have not engaged in any currency hedging activities, although we may do so in the future. Fluctuations in currency exchange rates could harm our business in the future.
29
Table of Contents
Item 8.Financial Statements and Supplementary Data
TASER INTERNATIONAL, INC.
BALANCE SHEETS
December 31,
BALANCE SHEETS
December 31,
| 2008 | 2007 | |||||||
Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 46,880,435 | $ | 42,801,461 | ||||
| Short-term investments | 2,498,998 | 8,499,978 | ||||||
| Accounts receivable, net of allowance of $200,000 and $190,000 in 2008 and 2007, respectively | 16,793,553 | 11,691,553 | ||||||
| Inventory | 13,467,117 | 13,506,804 | ||||||
| Prepaids and other assets | 2,528,539 | 4,318,661 | ||||||
| Deferred income tax assets, net | 9,430,073 | 15,608,325 | ||||||
| Total current assets | 91,598,715 | 96,426,782 | ||||||
| Long-term investments | — | 9,006,493 | ||||||
| Property and equipment, net | 27,128,032 | 23,599,680 | ||||||
| Deferred income tax assets, net | 8,826,778 | 6,724,104 | ||||||
| Intangible assets | 2,447,011 | 1,925,139 | ||||||
| Other long-term assets | 14,970 | 81,203 | ||||||
Total assets | $ | 130,015,506 | $ | 137,763,401 | ||||
Liabilities and stockholders’ equity | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 3,856,961 | $ | 7,304,112 | ||||
| Accrued liabilities | 4,275,907 | 2,784,027 | ||||||
| Current portion of deferred revenue | 2,510,645 | 1,694,644 | ||||||
| Customer deposits | 312,686 | 266,728 | ||||||
| Deferred insurance settlement proceeds | — | 404,848 | ||||||
| Current portion of capital lease obligations | — | 19,257 | ||||||
| Total current liabilities | 10,956,199 | 12,473,616 | ||||||
| Deferred revenue, net of current portion | 4,840,965 | 3,541,267 | ||||||
| Liability for unrecorded tax benefits | 1,692,080 | 1,100,073 | ||||||
| Capital lease obligations, net of current portion | — | 11,695 | ||||||
Total liabilities | 17,489,244 | 17,126,651 | ||||||
| Commitments and contingencies | ||||||||
| Stockholders’ equity | ||||||||
| Preferred stock, $0.00001 par value per share; 25 million shares authorized; no shares issued and outstanding at December 31, 2008 and 2007 | — | — | ||||||
| Common stock, $0.00001 par value per share; 200 million shares authorized; 61,795,712 and 63,263,903 shares issued and outstanding at December 31, 2008 and 2007, respectively | 638 | 635 | ||||||
| Additional paid-in capital | 87,663,129 | 86,911,381 | ||||||
| Treasury stock, 2,091,600 and 300,000 shares at December 31, 2008 and 2007, respectively | (14,708,237 | ) | (2,208,957 | ) | ||||
| Retained earnings | 39,570,732 | 35,933,691 | ||||||
Total stockholders’ equity | 112,526,262 | 120,636,750 | ||||||
Total liabilities and stockholders’ equity | $ | 130,015,506 | $ | 137,763,401 | ||||
The accompanying notes are an integral part of these financial statements.
30
Table of Contents
TASER INTERNATIONAL, INC.
STATEMENTS OF OPERATIONS
For The Years Ended December 31,
STATEMENTS OF OPERATIONS
For The Years Ended December 31,
| 2008 | 2007 | 2006 | ||||||||||
| Net sales | $ | 92,845,490 | $ | 100,727,191 | $ | 67,717,851 | ||||||
| Cost of products sold: | ||||||||||||
| Direct manufacturing expense | 26,756,080 | 31,507,727 | 18,296,039 | |||||||||
| Indirect manufacturing expense | 9,085,183 | 11,659,645 | 6,242,751 | |||||||||
| Total cost of products sold | 35,841,263 | 43,167,372 | 24,538,790 | |||||||||
| Gross margin | 57,004,227 | 57,559,819 | 43,179,061 | |||||||||
| Sales, general and administrative expenses | 38,860,729 | 32,814,170 | 29,680,764 | |||||||||
| Research and development expenses | 12,918,161 | 4,421,596 | 2,704,521 | |||||||||
| Shareholder litigation settlement expense | — | — | 17,650,000 | |||||||||
| Income (loss) from operations | 5,225,337 | 20,324,053 | (6,856,224 | ) | ||||||||
| Interest and other income, net | 1,717,967 | 2,202,187 | 1,872,645 | |||||||||
| Income (loss) before provision (benefit) for income taxes | 6,943,304 | 22,526,240 | (4,983,579 | ) | ||||||||
| Provision (benefit) for income taxes | 3,306,263 | 7,499,764 | (895,900 | ) | ||||||||
| Net income (loss) | $ | 3,637,041 | $ | 15,026,476 | $ | (4,087,679 | ) | |||||
| Income (loss) per common and common equivalent shares | ||||||||||||
| Basic | $ | 0.06 | $ | 0.24 | $ | (0.07 | ) | |||||
| Diluted | $ | 0.06 | $ | 0.23 | $ | (0.07 | ) | |||||
| Weighted average number of common and common equivalent shares outstanding | ||||||||||||
| Basic | 62,371,004 | 62,621,174 | 61,984,240 | |||||||||
| Diluted | 64,070,869 | 65,685,667 | 61,984,240 | |||||||||
The accompanying notes are an integral part of these financial statements.
31
Table of Contents
TASER INTERNATIONAL, INC.
STATEMENTS OF STOCKHOLDERS’ EQUITY
For the Years Ended December 31, 2008, 2007 and 2006
STATEMENTS OF STOCKHOLDERS’ EQUITY
For the Years Ended December 31, 2008, 2007 and 2006
| Additional | Total | |||||||||||||||||||||||||||
| Common Stock | Paid-in | Treasury Stock | Retained | Stockholders’ | ||||||||||||||||||||||||
| Shares | Amount | Capital | Shares | Amount | Earnings | Equity | ||||||||||||||||||||||
| Balance, December 31, 2005 | 61,938,654 | $ | 619 | $ | 78,742,862 | — | $ | — | $ | 24,994,894 | $ | 103,738,375 | ||||||||||||||||
| Exercise of stock options | 301,320 | 3 | 747,952 | — | — | — | 747,955 | |||||||||||||||||||||
| Stock-based compensation expense | — | — | 1,138,845 | — | — | — | 1,138,845 | |||||||||||||||||||||
| Purchase of treasury stock | (300,000 | ) | — | — | 300,000 | (2,208,957 | ) | — | (2,208,957 | ) | ||||||||||||||||||
| Net loss | — | — | — | — | — | (4,087,679 | ) | (4,087,679 | ) | |||||||||||||||||||
| Balance, December 31, 2006 | 61,939,974 | 622 | 80,629,659 | 300,000 | (2,208,957 | ) | 20,907,215 | 99,328,539 | ||||||||||||||||||||
| Exercise of stock options | 1,107,574 | 11 | 3,143,758 | — | — | — | 3,143,769 | |||||||||||||||||||||
| Shareholder derivate settlement | 216,355 | 2 | 1,749,998 | — | — | — | 1,750,000 | |||||||||||||||||||||
| Stock-based compensation expense | — | — | 1,387,966 | — | — | — | 1,387,966 | |||||||||||||||||||||
| Net income | — | — | — | — | — | 15,026,476 | 15,026,476 | |||||||||||||||||||||
| Balance, December 31, 2007 | 63,263,903 | 635 | 86,911,381 | 300,000 | (2,208,957 | ) | 35,933,691 | 120,636,750 | ||||||||||||||||||||
| Exercise of stock options | 323,409 | 3 | 342,818 | 342,821 | ||||||||||||||||||||||||
| Deferred tax asset correction (see footnote 8) | (2,014,955 | ) | (2,014,955 | ) | ||||||||||||||||||||||||
| Stock-based compensation expense | 2,423,885 | 2,423,885 | ||||||||||||||||||||||||||
| Purchase of treasury stock | (1,791,600 | ) | 1,791,600 | (12,499,280 | ) | (12,499,280 | ) | |||||||||||||||||||||
| Net income | 3,637,041 | 3,637,041 | ||||||||||||||||||||||||||
| Balance, December 31, 2008 | 61,795,712 | $ | 638 | $ | 87,663,129 | 2,091,600 | $ | (14,708,237 | ) | $ | 39,570,732 | $ | 112,526,262 | |||||||||||||||
The accompanying notes are an integral part of these financial statements.
32
Table of Contents
TASER INTERNATIONAL, INC.
STATEMENTS OF CASH FLOWS
For the Years Ended December 31,
STATEMENTS OF CASH FLOWS
For the Years Ended December 31,
| 2008 | 2007 | 2006 | ||||||||||
| Cash Flows from Operating Activities: | ||||||||||||
| Net income (loss) | $ | 3,637,041 | $ | 15,026,476 | $ | (4,087,679 | ) | |||||
| Adjustments to reconcile net income (loss) to net cash provided by operating activities: | ||||||||||||
| Depreciation and amortization | 2,637,773 | 2,521,237 | 2,096,595 | |||||||||
| Loss on disposal of assets | 171,098 | 49,949 | — | |||||||||
| Provision for doubtful accounts | 78,010 | 80,835 | (830 | ) | ||||||||
| Provision for excess and obsolete inventory | 640,655 | 206,335 | 85,329 | |||||||||
| Provision for warranty | 368,521 | 1,030,291 | 442,195 | |||||||||
| Stock-based compensation expense | 2,423,885 | 1,387,966 | 1,138,845 | |||||||||
| Deferred insurance settlement proceeds | (404,848 | ) | (104,219 | ) | (192,448 | ) | ||||||
| Deferred income taxes | 2,060,623 | 5,831,783 | (1,167,924 | ) | ||||||||
| Provision for unrecognized tax benefits | 592,007 | 1,100,073 | — | |||||||||
| Change in assets and liabilities: | ||||||||||||
| Accounts receivable | (5,180,010 | ) | (1,704,339 | ) | (4,645,192 | ) | ||||||
| Inventory | (600,968 | ) | (4,455,393 | ) | 762,261 | |||||||
| Prepaids and other assets | 1,856,355 | (2,235,862 | ) | 676,028 | ||||||||
| Insurance settlement proceeds receivable | — | — | 575,000 | |||||||||
| Accounts payable and accrued liabilities | (2,323,792 | ) | 1,069,483 | 256,625 | ||||||||
| Deferred revenue | 2,115,699 | 2,222,981 | 1,611,782 | |||||||||
| Accrued litigation settlement | — | (8,000,000 | ) | 9,750,000 | ||||||||
| Other liabilities | — | (199,999 | ) | 199,999 | ||||||||
| Customer deposits | 45,958 | 95,236 | (18,764 | ) | ||||||||
| Net cash provided by operating activities | 8,118,007 | 13,922,833 | 7,481,822 | |||||||||
| Cash Flows from Investing Activities: | ||||||||||||
| Purchases of investments | (43,887,640 | ) | (138,203,034 | ) | (82,610,518 | ) | ||||||
| Proceeds from investments | 58,895,113 | 149,731,426 | 81,123,775 | |||||||||
| Purchases of property and equipment | (6,144,425 | ) | (4,067,579 | ) | (1,839,815 | ) | ||||||
| Purchases of intangible assets | (745,622 | ) | (454,403 | ) | (229,375 | ) | ||||||
| Net cash provided by (used in) investing activities | 8,117,426 | 7,006,410 | (3,555,933 | ) | ||||||||
| Cash Flows from Financing Activities: | ||||||||||||
| Repurchase of common stock | (12,499,280 | ) | — | (2,208,957 | ) | |||||||
| Proceeds from options exercised | 342,821 | 3,143,769 | 747,955 | |||||||||
| Payments under capital leases | — | (45,236 | ) | (43,111 | ) | |||||||
| Net cash provided by (used in) financing activities | (12,156,459 | ) | 3,098,533 | (1,504,113 | ) | |||||||
| Net increase in cash and cash equivalents | 4,078,974 | 24,027,776 | 2,421,776 | |||||||||
| Cash and cash equivalents, beginning of period | 42,801,461 | 18,773,685 | 16,351,909 | |||||||||
| Cash and cash equivalents, end of period | $ | 46,880,435 | $ | 42,801,461 | $ | 18,773,685 | ||||||
| Supplemental Disclosure: | ||||||||||||
| Cash paid for income taxes — net | $ | 523,950 | $ | 475,000 | $ | 229,424 | ||||||
| Cash paid for interest | $ | — | $ | 5,186 | $ | 7,281 | ||||||
| Non Cash Transactions- | ||||||||||||
| Deferred tax asset correction (see footnote 8) | $ | 2,014,955 | $ | — | $ | — | ||||||
| Common stock issued for shareholder derivative lawsuit settlement | $ | — | $ | 1,750,000 | $ | — | ||||||
| Property and equipment purchases in accounts payable | $ | — | $ | 1,198,891 | $ | — | ||||||
The accompanying notes are an integral part of these financial statements.
33
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO FINANCIAL STATEMENTS
NOTES TO FINANCIAL STATEMENTS
1. Organization and Summary of Significant Accounting Policies
TASER International, Inc. (“TASER” or the “Company”) is a developer and manufacturer of advanced electronic control devices (“ECDs”) designed for use in the law enforcement, military, corrections, private security and personal defense markets. The Company sells its products worldwide through its direct sales force, distribution partners, online store and third party resellers. The Company was incorporated in Arizona in September 1993 and reincorporated in Delaware in January 2001. The Company’s headquarters and manufacturing facilities are located in Scottsdale, Arizona.
a. Basis of Presentation and Use of Estimates
The accompanying financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America. The preparation of these financial statements requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Significant estimates in these financial statements include allowances for doubtful accounts receivable, inventory valuation reserves, product warranty reserves, valuation of long lived assets, deferred income taxes, stock-based compensation and contingencies. Actual results could differ from those estimates.
b. Cash, Cash Equivalents and Investments
Cash and cash equivalents include cash and money market funds on hand and short-term investments with original maturities of three months or less. Short-term investments include securities having maturities of 90 days to one year. Long-term investments include securities having maturities of more than one year. The Company’s long-term investments were invested in government sponsored mortgage-backed securities and were classified as held to maturity. In February 2009, the Company’s remaining long term investment of $2,498,998 was called at par value by the issuer and as such was reclassified from long-term to short term investments on the balance sheet at December 31, 2008.These investments are recorded at amortized cost. See Note 2.
The Company’s cash and investment accounts earned interest at an approximate rate of 2.5% during 2008, down from 4.2% in 2007. Included in the Company’s cash balances are deposits with a single bank of $8.8 million, which is in excess of the FDIC insurance coverage limit of $250,000. Approximately $29.5 million of the Company’s cash equivalent investments held in money market funds at December 31, 2008 are supported by the Federal Government as part of the Temporary Guarantee Program for Money Market Funds.
c. Inventory
Inventories are stated at the lower of cost or market. Cost is determined using the weighted average cost of raw materials which approximates the first-in, first-out (FIFO) method and includes allocations of manufacturing labor and overhead. Provisions are made to reduce potentially excess, obsolete or slow-moving inventories to their net realizable value. These provisions are based on management’s best estimate after considering historical demand, projected future demand, inventory purchase commitments, industry and market trends and conditions and other factors.
d. Property and Equipment
Property and equipment are stated at cost net of accumulated depreciation. Additions and improvements are capitalized while ordinary maintenance and repair expenditures are charged to expense as incurred. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets.
e. Impairment of Long-Lived Assets
The Company evaluates whether events and circumstances have occurred that indicate the remaining estimated useful life of long-lived assets and identifiable intangible assets may warrant revision or that the remaining balance of these assets may not be recoverable. In performing the review for recoverability, management estimates the future undiscounted cash flows expected to result from the use of the assets and their eventual disposition. The amount of the impairment loss, if impairment exists, would be calculated based on the excess of the carrying amounts of the assets over their estimated fair value. No impairment losses were recorded in 2008, 2007 or 2006.
f. Customer Deposits
The Company requires certain deposits in advance of shipment for certain customer sales orders. Customer deposits are recorded as a current liability on the accompanying balance sheets.
34
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
NOTES TO FINANCIAL STATEMENTS — (Continued)
g. Revenue Recognition and Accounts Receivable
The Company recognizes revenues when persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, title has transferred, the price is fixed and collectability is reasonably assured. In most instances, sales of the Company’s products are final and its customers do not have a right to return the product. The Company’s consumer product, the TASER C2, has a 30 day right of return for inactivated units purchased direct from the Company. As the Company currently has insufficient history upon which to make a reasonable and reliable estimate of the rate of such product returns, the revenue and related costs are deferred until the 30 day period has expired.
During 2008, the Company began selling the TASER C2 product through certain retailers who do not assume title, risk of loss to the inventory and credit risk. The Company therefore recognizes revenue from such retailers on a sell-through method using information provided by the retailer. The revenue and related costs are deferred until the product has been sold by the retailer.
The Company offers customers the right to purchase extended warranties that include additional services and coverage beyond the limited warranty on the TASER X26 and ADVANCED TASER products. Revenue for extended warranty purchases is deferred at the time of sale, and recognized over the warranty period commencing on the date of sale. The extended warranties range from one to four years. At December 31, 2008 and 2007, $7,349,000 and $5,217,000 was deferred under this program, respectively. The current portion of deferred revenue represents the extended warranty revenue which will be recognized in 2009. Also included in deferred revenue at December 31, 2008 and 2007 is $3,000 and $19,000, respectively, of deferrals related to free training certificates on sales of the TASER X26C consumer device. The Company is deferring the revenue associated with these training sessions until such time as either the training has occurred or the certificate expires, which is 90 days after purchase by the end user. The Company has valued these one-on-one training sessions at their estimated fair value, which is the amount that the Company will pay the independent third party conducting the training.
Sales are typically made on credit and the Company generally does not require collateral. The Company performs ongoing credit evaluations of its customers’ financial condition and maintains an allowance for estimated potential losses. Uncollectible accounts are written off when deemed uncollectible, and accounts receivable are presented net of an allowance for doubtful accounts. This allowance represents our best estimate and is based on our judgment after considering a number of factors, including third-party credit reports, actual payment history, cash discounts, customer-specific financial information and broader market and economic trends and conditions.
Included as a component of revenue is development funding provided by the Joint Non-Lethal Weapons Directorate (JNLWD) of the U.S Department of Defense under a cost-plus fixed fee contract. Periodically, an invoice summarizing the reimbursable expenses is submitted to JNLWD for payment. The payment request submitted by the Company to the JNLWD details the costs expensed in the period and adds a nominal contracted profit margin. The total amount of revenue recognized for this work in the year ended December 31, 2008 was $885,000, of which $204,000 was outstanding at December 31, 2008.
Certain of the Company’s customers are charged shipping fees, which are recorded as a component of net sales. Sales tax collected on sales is netted against government remittances and thus recorded on a net basis. Training revenue is recorded as the service is provided.
h. Cost of Products Sold
Cost of products sold represents manufacturing costs, consisting of materials, labor and overhead related to finished goods and components. Shipping costs incurred related to product delivery are also included in cost of products sold.
i. Advertising Costs
The Company expenses advertising costs in the period in which they are incurred with the exception of commercial advertising production costs which are expensed at the time the first commercial is shown on television. The Company incurred advertising costs of $2,085,000, $931,000 and $437,000 in 2008, 2007 and 2006, respectively. At December 31, 2007, the Company had $523,000 of deferred advertising costs related to the production costs of an infomercial for the TASER C2. These deferred advertising costs were included in prepaid and other assets on the accompanying balance sheets and were expensed in the first quarter of 2008 when the infomercial first aired. There were no deferred advertising costs at December 31, 2008. Advertising costs are included in sales, general and administrative expenses in the accompanying statements of operations.
35
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
NOTES TO FINANCIAL STATEMENTS — (Continued)
j. Warranty Costs
The Company warrants its X26 products from manufacturing defects on a limited basis for a period of one year after purchase, and thereafter will replace any defective unit for a fee. The C2 product is warranted for a period of 90 days after purchase. The Company also sells extended warranties for periods of up to four years after the expiration of the limited one year warranty. Management tracks historical data related to returns and warranty costs on a quarterly basis, and estimates future warranty claims by applying the estimated weighted average return rate to the product sales for the period. If management becomes aware of a component failure that could result in larger than anticipated returns from its customers, the reserve would be increased. After the one year warranty expires, if the device fails to operate properly for any reason, the Company will replace the TASER X26 for a prorated discounted price depending on when the product was placed into service and replace the ADVANCED TASER device for a fee of $75. These fees are intended to cover the handling and repair costs and include a profit. The following table summarizes the changes in the estimated product warranty liabilities for the years ended December 31, 2008, 2007 and 2006:
| 2008 | 2007 | 2006 | ||||||||||
| Balance at Beginning of Period | $ | 919,254 | $ | 713,135 | $ | 851,920 | ||||||
| Utilization of Accrual | (672,744 | ) | (824,172 | ) | (580,980 | ) | ||||||
| Warranty Expense | 368,521 | 1,030,291 | 442,195 | |||||||||
| Balance at End of the Period | $ | 615,031 | $ | 919,254 | $ | 713,135 | ||||||
k. Research and Development Expenses
The Company expenses research and development costs as incurred. The Company incurred product development expense of approximately $12,918,000, $4,422,000 and $2,705,000 in 2008, 2007 and 2006, respectively.
l. Income Taxes
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement amounts of assets and liabilities and their respective tax bases and operating loss and tax credit carry forwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in future years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rate is recognized in income in the period that includes the enactment date. Deferred tax assets are reduced through the establishment of a valuation allowance at the time, based upon available evidence, it becomes more likely than not that the deferred tax assets will not be realized. Income tax-related interest and penalties are recorded as a component of the provision for income taxes.
The FASB issued Interpretation 48, “Accounting for Uncertainty in Income Taxes” (“FIN 48”), which the Company adopted effective January 1, 2007. FIN 48 addresses the determination of how tax benefits claimed or expected to be claimed on a tax return should be recorded in the financial statements. Under FIN 48, the Company recognizes the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position are measured based on the largest benefit that has a greater than fifty percent likelihood of being realized upon ultimate resolution. Under FIN 48, management must also assess whether uncertain tax positions as filed could result in the recognition of a liability for possible interest and penalties. The Company’s policy is to include interest and penalties related to unrecognized tax benefits as a component of income tax expense. Refer to Note 8 for additional information regarding the change in unrecognized tax benefits.
m. Concentration of Credit Risk and Major Customers / Suppliers
Financial instruments that potentially subject the Company to concentrations of credit risk consist of accounts receivable. Sales are typically made on credit and the Company generally does not require collateral. The Company performs ongoing credit evaluations of its customers’ financial condition and maintains an allowance for estimated losses. Uncollectible accounts are written off when deemed uncollectible, and accounts receivable are presented net of an allowance for doubtful accounts. The allowance for bad debts totaled $200,000 and $190,000 as of December 31, 2008 and 2007, respectively. Historically, the Company has experienced a low level of write offs related to doubtful accounts.
36
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
NOTES TO FINANCIAL STATEMENTS — (Continued)
The Company sells its products primarily through a network of unaffiliated distributors. The Company also reserves the right to sell directly to the end user to secure the customer’s account balance. In 2008, one distributor represented approximately 11% of total sales. No other customer exceeded 10% of total sales in 2008. In 2007, one distributor represented approximately 16% of total sales. No other customer exceeded 10% of total sales in 2007. In 2006, one distributor represented 12% of total sales. No other customer exceeded 10% of total sales in 2006.
At December 31, 2008, the Company had receivables from two customers comprising 30% and 12% of the aggregate accounts receivable balance. The customer comprising the 30% balance is the result of a large individual sale made to the U.K. Government in December 2008, which was paid in full in February 2009. At December 31, 2007, the Company had receivables from two customers comprising 20% and 10% of the aggregate accounts receivable balance. These customers are unaffiliated distributors of the Company’s products.
The Company currently purchases finished circuit boards and injection-molded plastic components from suppliers located in the United States. Although the Company currently obtains many of these components from single source suppliers, the Company owns the injection molded component tooling used in their production. As a result, management believes it could obtain alternative suppliers in most cases without incurring significant production delays. The Company also purchases small, machined parts from a vendor in Taiwan, custom cartridge assemblies from a proprietary vendor in the United States, and electronic components from a variety of foreign and domestic distributors. Management believes that there are readily available alternative suppliers in most cases who can consistently meet its needs for these components. The Company acquires most of its components on a purchase order basis and does not have long-term contracts with suppliers.
n. Fair Value of Financial Instruments
On January 1, 2008 the Company adopted Statement of Financial Accounting Standards (“SFAS”) No. 157,Fair Value Measurements (“SFAS 157”). SFAS 157 defines fair value, establishes a framework for measuring fair value in accordance with generally accepted accounting principles and expands financial statement disclosure requirements for fair value measurements. The Company’s adoption of SFAS 157 was limited to its financial assets and financial liabilities, as permitted by FSP 157-2. The Company does not have any nonfinancial assets or nonfinancial liabilities that it recognizes or discloses at fair value in its financial statements on a recurring basis. The implementation of the fair value measurement guidance of SFAS 157 did not result in any material changes to the carrying values of the Company’s financial instruments on its opening balance sheet.
SFAS 157 defines fair value as the price that would be received from the sale of an asset or paid to transfer a liability (an exit price) on the measurement date in an orderly transaction between market participants in the principal or most advantageous market for the asset or liability. SFAS 157 specifies a hierarchy of valuation techniques, which is based on whether the inputs into the valuation technique are observable or unobservable. The hierarchy is as follows:
| • | Level 1 — Valuation techniques in which all significant inputs are unadjusted quoted prices from active markets for assets or liabilities that are identical to the assets or liabilities being measured. | ||
| • | Level 2 — Valuation techniques in which significant inputs include quoted prices from active markets for assets or liabilities that are similar to the assets or liabilities being measured and/or quoted prices for assets or liabilities that are identical or similar to the assets or liabilities being measured from markets that are not active. Also, model-derived valuations in which all significant inputs and significant value drivers are observable in active markets are Level 2 valuation techniques. | ||
| • | Level 3 — Valuation techniques in which one or more significant inputs or significant value drivers are unobservable. Unobservable inputs are valuation technique inputs that reflect our own assumptions about the assumptions that market participants would use in pricing an asset or liability. |
Following is a description of the valuation techniques that the Company uses to measure the fair value of assets and liabilities that it measures and reports on its balance sheet at fair value on a recurring basis:
| • | Cash Equivalents.As of December 31, 2008, the Company’s cash equivalents consisted of money market mutual funds. The Company valued its cash equivalents using observable inputs that reflect quoted prices for securities with identical characteristics, and accordingly, the Company classifies the valuation techniques that use these inputs as Level 1. | ||
| • | Investments.As of December 31, 2008, the Company’s investments consisted of a federal government sponsored entity security. The Company’s investments are held to maturity and stated at amortized cost, which approximates fair value. Information about the fair value of the Company’s investments is included in Note 2. |
The Company’s financial instruments include also include cash, accounts receivable, accounts payable and accrued liabilities. Due to the immediate or short-term nature of these instruments, their fair value approximates their carrying value on the balance sheet as of December 31, 2008.
37
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
NOTES TO FINANCIAL STATEMENTS — (Continued)
o. Segment Information
Management has determined that its operations are comprised of one reportable segment — the sale of advanced electronic control devices and accessories. For the years ended December 31, 2008, 2007 and 2006, sales by geographic area were as follows:
| Sales by Geographic Area | 2008 | 2007 | 2006 | |||||||||
| United States | 82 | % | 85 | % | 86 | % | ||||||
| Other Countries | 18 | % | 15 | % | 14 | % | ||||||
| Total | 100 | % | 100 | % | 100 | % | ||||||
Sales to customers outside of the United States are denominated in U.S. dollars and are attributed to each country based on the billing address of the distributor or customer. To date, no individual country outside the U.S. has represented a material amount of total net revenue. Substantially all assets of the Company are located in the United States.
p. Stock-Based Compensation
The Company applies the fair value recognition provisions of Statement of Financial Accounting Standards No. 123(R), “Share Based Payment” (“SFAS No. 123(R)”) using the modified prospective transition method. Under this transition method, compensation cost recognized includes: (a) compensation cost for all stock-based payments granted prior to, but not yet vested as of January 1, 2006, based on the grant date fair value calculated in accordance with the original provisions of SFAS No. 123, and (b) compensation cost for all stock-based payments granted subsequent to January 1, 2006, based on the grant date fair value calculated in accordance with the provisions of SFAS No. 123(R).
SFAS No. 123(R) requires the use of a valuation model to calculate the fair value of share-based awards. The Company has elected to use the Black-Scholes-Merton option valuation model, which incorporates various assumptions including volatility, expected life forfeiture rate and risk-free interest rates. The assumptions used for the years ended December 31, 2008, 2007 and 2006 and the resulting estimates of weighted-average fair value per share of options granted during those periods are as follows:
| 2008 | 2007 | 2006 | ||||||||||
| Weighted average / range of volatility | 70 | % | 60 | % | 68 | % | ||||||
| Risk-free interest rate | 2.2 | % | 4.7 | % | 4.8 | % | ||||||
| Dividend rate | 0.0 | % | 0.0 | % | 0.0 | % | ||||||
| Expected life of options | 4.0 years | 4.0 years | 3.5 years | |||||||||
| Weighted average fair value of options granted | $ | 2.89 | $ | 5.22 | $ | 4.49 | ||||||
The expected life of the options represents the estimated period of time until exercise and is based on historical experience of similar awards, giving consideration to the contractual terms, vesting schedules and expectations of future employee behavior. Expected stock price volatility is based on a combination of historical volatility of the Company’s stock and the one-year implied volatility of its publicly traded options for the related vesting periods. The risk-free interest rate is based on the implied yield available on U.S. Treasury zero-coupon issues with an equivalent remaining term. The Company has not paid dividends in the past and does not plan to pay any dividends in the near future. The estimated fair value of stock-based compensation awards and other options is amortized to expense on a straight line basis over the relevant vesting period. As share-based compensation expense recognized is based on awards ultimately expected to vest, it is reduced for estimated forfeitures. SFAS No. 123(R) requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The Company forfeiture rate was calculated based on its historical experience of awards which ultimately vested. See Note 10 for further discussion of the Company’s share-based compensation.
q. Income (Loss) Per Common Share
The Company accounts for income (loss) per share in accordance with SFAS No. 128,Earnings per Share. Basic income per share is computed by dividing net income (loss) by the weighted average number of common shares outstanding during the periods presented. Diluted income per share reflects the potential dilution that could occur if outstanding stock options were exercised utilizing the treasury stock method. The calculation of the weighted average number of shares outstanding and earnings per share are as follows:
38
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
NOTES TO FINANCIAL STATEMENTS — (Continued)
| For the Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| Numerator for basic and diluted earnings per share | ||||||||||||
| Net income (loss) | $ | 3,637,041 | $ | 15,026,476 | $ | (4,087,679 | ) | |||||
| Denominator for basic earnings per share — weighted average shares outstanding | 62,371,004 | 62,621,174 | 61,984,240 | |||||||||
| Dilutive effect of shares issuable under stock options outstanding | 1,699,865 | 3,064,493 | — | |||||||||
| Denominator for diluted earnings per share — adjusted weighted average shares outstanding | 64,070,869 | 65,685,667 | 61,984,240 | |||||||||
| Net income (loss) per common share | ||||||||||||
| Basic | $ | 0.06 | $ | 0.24 | $ | (0.07 | ) | |||||
| Diluted | $ | 0.06 | $ | 0.23 | $ | (0.07 | ) | |||||
Basic income (loss) per share is based upon the weighted average number of common shares outstanding during the period. Diluted income (loss) per share includes the dilutive effect of potential stock option exercises, calculated using the treasury stock method. For the years ended December 31, 2008 and 2007, the effects of 2,205,861 and 315,764 stock options, respectively, were excluded from the calculation of diluted income per share as their effect would have been anti-dilutive and increased the net income per share. As a result of the net loss for the year ended December 31, 2006, 3,512,248 shares of potential dilutive securities were considered anti-dilutive and excluded from the calculation as their effect would have been to reduce the net loss per share.
r. Recent Accounting Pronouncements
In October 2008, the FASB issued Staff Position (“FSP”) No. FAS 157-3,Determining the Fair Value of a Financial Asset When the Market for That Asset Is Not Active,which clarifies the application of Statement No. 157 in an inactive market and illustrates how an entity would determine fair value when the market for a financial asset is not active. The Staff Position is effective immediately and applies to prior periods for which financial statements have not been issued, including interim or annual periods ending on or before December 30, 2008. The implementation of FAS 157-3 did not have a material impact on the Company’s consolidated financial statements.
In April 2008, the FASB issued FSP FAS 142-3,Determination of the Useful Life of Intangible Assets(“FSP 142-3”). FSP 142-3 amends the factors that should be considered in developing renewal or extension assumptions used to determine the useful life of a recognized intangible asset under SFAS No. 142, “Goodwill and Other Intangible Assets” (“SFAS 142”). This change is intended to improve the consistency between the useful life of a recognized intangible asset under SFAS 142 and the period of expected cash flows used to measure the fair value of the asset under SFAS 141R and other GAAP standards. The requirement for determining useful lives must be applied prospectively to intangible assets acquired after the effective date and the disclosure requirements must be applied prospectively to all intangible assets recognized as of, and subsequent to, the effective date. FSP 142-3 is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those fiscal years, which will require the Company to adopt prospectively these provisions in the first quarter of fiscal 2009.
In February 2008, the FASB issued FSP 157-2, which delays the effective date of SFAS 157 for all nonfinancial assets and nonfinancial liabilities, except those that are recognized or disclosed at fair value in the financial statements on a recurring basis (at least annually). SFAS 157 establishes a framework for measuring fair value and expands disclosures about fair value measurements. FSP 157-2 partially defers the effective date of SFAS 157 to fiscal years beginning after November 15, 2008, and interim periods within those fiscal years for items within the scope of this FSP. The adoption of SFAS 157 for all nonfinancial assets and nonfinancial liabilities is effective for us beginning January 1, 2009. We do not expect the impact of this adoption to be material.
In December 2007, the FASB issued Statement of Financial Accounting Standards No. 141 (revised) (“SFAS 141(R)”),Business Combinations. The standard changes the accounting for business combinations by requiring that an acquiring entity measure and recognize identifiable assets acquired and liabilities assumed at the acquisition date fair value with limited exceptions. SFAS 141(R) is effective for fiscal years beginning after December 15, 2008, with early adoption prohibited. Management does not expect the adoption of SFAS 141(R) will have an impact on the Company’s financial statements when effective, but the nature and magnitude of the specific effects will depend upon the nature, terms and size of acquisitions, if any, the Company consummates after the effective date.
s. Reclassification
Certain amounts shown in the prior periods’ financial statements have been reclassified to conform to the current year financial statement presentation.
39
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
NOTES TO FINANCIAL STATEMENTS — (Continued)
2. Cash, cash equivalents and investments
Cash and cash equivalents include funds on hand and short-term investments with maturities of three months or less. Short-term investments include securities having maturities of 90 days to one year. Long-term investments include securities having maturities of more than one year. The Company reclassified $5,998,826 at December 31, 2007 from cash equivalents to short term investments based on maturity at date of acquisition. The Company’s long-term investments are invested in federal agency mortgage-backed securities, and are classified as held to maturity. These investments are recorded at amortized cost. Management intends to hold these securities until maturity. Approximately $29.5 million of the Company’s cash equivalent investments held in money market funds as of December 31, 2008 are supported by the Federal Government as part of the Temporary Guarantee Program for Money Market Funds.
The following is a summary of cash, cash equivalents and held-to-maturity securities as distributed by type at December 31:
| 2008 | 2007 | |||||||||||||||||||||||||||||||
| Gross | Gross | |||||||||||||||||||||||||||||||
| Gross Unrealized | Gross Unrealized | Unrealized | Unrealized | |||||||||||||||||||||||||||||
| Cost | Gains | Losses | Fair Value | Cost | Gains | Losses | Fair Value | |||||||||||||||||||||||||
| Cash and money market funds | $ | 46,880,435 | $ | — | $ | — | $ | 46,880,435 | $ | 29,687,138 | $ | — | $ | — | $ | 29,687,138 | ||||||||||||||||
| Government sponsored entity securities | 2,498,998 | 12,876 | — | 2,511,874 | 17,506,471 | 14,238 | (12,022 | ) | 17,508,687 | |||||||||||||||||||||||
| Commercial paper | — | — | — | — | 13,114,323 | — | (37,838 | ) | 13,076,485 | |||||||||||||||||||||||
| $ | 49,379,433 | $ | 12,876 | $ | — | $ | 49,392,309 | $ | 60,307,932 | $ | 14,238 | $ | (49,860 | ) | $ | 60,272,310 | ||||||||||||||||
The following table summarizes the classification of cash, cash equivalents and investments in the accompanying balance sheet.
| December 31, | ||||||||
| 2008 | 2007 | |||||||
| Cash and money market funds | $ | 46,880,435 | $ | 29,687,138 | ||||
| Cash equivalents | — | 13,114,323 | ||||||
| Total cash and cash equivalents | 46,880,435 | 42,801,461 | ||||||
| Short term investments | 2,498,998 | 8,499,978 | ||||||
| Long term investments | — | 9,006,493 | ||||||
| $ | 49,379,433 | $ | 60,307,932 | |||||
In February 2009, the Company’s long term investment in a government sponsored entity was called at par value by the issuing agency. As such, $2.5 million was reclassified from long term to short term investments at December 31, 2008. During 2008, $6.5 million of long term investments held in government sponsored entity securities were also called at par value. The following table summarizes the contractual maturities of commercial paper and government sponsored entity securities, identified above as cash equivalents, short term and long term investments, at December 31, 2008 and 2007.
| 2008 | 2007 | |||||||
| Less than 1 year | $ | 2,498,998 | $ | 21,614,301 | ||||
| 1-3 years | — | 9,006,493 | ||||||
| $ | 2,498,998 | $ | 30,620,794 | |||||
The Company had no unrealized losses on held-to-maturity investments at December 31, 2008.
3. Inventory
Inventories consisted of the following at December 31:
| December 31, 2008 | December 31, 2007 | |||||||
| Raw materials and work-in-process | $ | 7,371,608 | $ | 8,475,055 | ||||
| Finished goods | 6,225,409 | 5,352,304 | ||||||
| Reserve for excess and obsolete inventory | (129,900 | ) | (320,555 | ) | ||||
| Total Inventory | $ | 13,467,117 | $ | 13,506,804 | ||||
40
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
NOTES TO FINANCIAL STATEMENTS — (Continued)
4. Property and Equipment
Property and equipment consisted of the following at December 31:
| Estimated | ||||||||||||
| Useful Life | 2008 | 2007 | ||||||||||
| Land | $ | 2,899,962 | $ | 2,899,962 | ||||||||
| Building | 39 Years | 13,764,555 | 13,611,785 | |||||||||
| Production equipment | 3-7 Years | 3,957,227 | 3,971,829 | |||||||||
| Telephone equipment | 5 Years | — | 297,618 | |||||||||
| Computer equipment | 3-5 Years | 5,400,861 | 4,103,958 | |||||||||
| Furniture and office equipment | 5-7 Years | 2,714,562 | 2,188,056 | |||||||||
| Vehicles | 5 Years | 503,872 | 284,242 | |||||||||
| Website development costs | 3 Years | 605,581 | 348,939 | |||||||||
| Construction in process | n/a | 6,324,612 | 2,741,454 | |||||||||
| Total Cost | 36,171,232 | 30,447,843 | ||||||||||
| Less: Accumulated depreciation | (9,043,200 | ) | (6,848,163 | ) | ||||||||
| Net Property and Equipment | $ | 27,128,032 | $ | 23,599,680 | ||||||||
Construction in process includes $4.5 million of deposits paid on automation equipment (see note 7(b)) and new product production equipment which was not in service at December 31, 2008. Depreciation and amortization expense for the years ended December 31, 2008, 2007 and 2006 was $2,557,000, $2,459,000 and $2,059,000, respectively.
5. Intangible Assets
Intangible assets consisted of the following at December 31:
| 2008 | 2007 | |||||||||||||||||||||||||||
| Gross Carrying | Accumulated | Net Carrying | Gross Carrying | Accumulated | Net Carrying | |||||||||||||||||||||||
| Useful Life | Amount | Amortization | Amount | Amount | Amortization | Amount | ||||||||||||||||||||||
| Amortized intangible assets | ||||||||||||||||||||||||||||
| TASER.com domain names | 5 Years | $ | 117,756 | $ | 60,000 | $ | 57,756 | $ | 60,000 | $ | 60,000 | $ | — | |||||||||||||||
| Issued patents | 4 to 15 Years | 677,808 | 156,297 | 521,511 | 402,058 | 115,863 | 286,195 | |||||||||||||||||||||
| Issued trademarks | 9 to 11 Years | 46,283 | 9,888 | 36,395 | 36,466 | 5,206 | 31,260 | |||||||||||||||||||||
| Non compete agreement | 5 to 7 Years | 150,000 | 79,286 | 70,714 | 150,000 | 52,143 | 97,857 | |||||||||||||||||||||
| 991,847 | 305,471 | 686,376 | 648,524 | 233,212 | 415,312 | |||||||||||||||||||||||
| Unamortized intangible assets | ||||||||||||||||||||||||||||
| TASER Trademark | 900,000 | 900,000 | 900,000 | 900,000 | ||||||||||||||||||||||||
| Patents and trademarks pending | 860,635 | 860,635 | 609,827 | 609,827 | ||||||||||||||||||||||||
| 1,760,635 | 1,760,635 | 1,509,827 | 1,509,827 | |||||||||||||||||||||||||
| Total intangible assets | $ | 2,752,482 | $ | 305,471 | $ | 2,447,011 | $ | 2,158,351 | $ | 233,212 | $ | 1,925,139 | ||||||||||||||||
Amortization expense included in costs of product sold for the years ended December 31, 2008, 2007 and 2006 was $80,301, $61,764 and $37,658, respectively. Estimated amortization for intangible assets with definite lives for the next five years ended December 31, and thereafter is as follows:
| 2009 | $ | 75,817 | ||
| 2010 | 67,317 | |||
| 2011 | 59,605 | |||
| 2012 | 39,605 | |||
| 2013 | 39,606 | |||
| Thereafter | 404,426 | |||
| $ | 686,376 | |||
41
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
NOTES TO FINANCIAL STATEMENTS — (Continued)
6. Accrued Liabilities
Accrued liabilities were comprised as follows at December 31:
| 2008 | 2007 | |||||||
| Accrued salaries and benefits | $ | 1,145,634 | $ | 1,046,534 | ||||
| Accrued expenses | 2,249,193 | 637,114 | ||||||
| Accrued warranty expense | 615,031 | 919,254 | ||||||
| Accrued income tax | 266,049 | 181,125 | ||||||
| Total | $ | 4,275,907 | $ | 2,784,027 | ||||
7. Commitments and Contingencies
a. Lease Obligations
The Company has entered into operating leases for various office space, storage facilities and equipment. Rent expense under all operating leases, including both cancelable and non-cancelable leases, was $223,000, $136,000 and $76,000 for the years ended December 31, 2008, 2007, and 2006, respectively. Future minimum lease payments under non-cancelable operating leases having remaining terms in excess of one year as of December 31, 2008, are as follows:
| Year ending December 31, | ||||
| 2009 | $ | 232,020 | ||
| 2010 | 232,020 | |||
| 2011 | 153,532 | |||
| 2012 | 84,900 | |||
| 2013 | 70,750 | |||
| Thereafter | — | |||
| Total | $ | 773,222 | ||
b. Purchase Commitments
On July 2, 2007, the Company entered into a contract with Automation Tooling Systems Inc. for the purchase of equipment at a total cost of approximately $8.4 million. The equipment is expected to be delivered to and installed at the Company’s facility in 2009. Payments will be made in installments, with an initial $1.5 million deposit paid in 2007, $3.0 paid in 2008, and the balance of $3.9 million will be paid in 2009 upon delivery and installation.
c. Litigation
Product Litigation
The Company is currently named as a defendant in 44 lawsuits in which the plaintiffs allege either wrongful death or personal injury in situations in which the TASER device was used (or present) by law enforcement officers or during training exercises. Companion cases arising from the same incident have been combined into one for reporting purposes. In addition, 82 other lawsuits have been dismissed or judgments entered in favor of the Company and are not included in this number. An appeal was filed by the plaintiff in both the Mann (GA) litigation and the Neal-Lomax (NV) litigation where judgment was entered in favor of the Company.
Also not included in the number of pending lawsuits is the Heston lawsuit in which a jury verdict was entered against the Company on June 6, 2008, and judgment was entered against the Company on January 30, 2009 in the amount of $153,150 as compensatory damages, and $1,423,127 as attorney fees. Costs in the amount of $50,000 were also awarded. These damages, fees and costs are covered by the Company’s insurance policies. The jury found that Mr. Heston’s own actions were 85 percent responsible for his death. The jury assigned 15 percent of the responsibility to TASER for a “negligent failure to warn” that extended or multiple TASER ECD applications could cause muscle contractions that could potentially contribute to acidosis to a degree that could cause cardiac arrest. The jury inappropriately awarded $5,200,000 in punitive damages against TASER, which were subsequently disallowed by the Court on October 24, 2008. As a result the $5,200,000 million in punitive damages recorded by the Company in the second quarter of 2008 were reversed in the fourth quarter of 2008. The Court denied the balance of the Company’s motion for judgment as a matter of law on all other grounds. The Company has filed a notice of appeal with respect to the judgment and plaintiffs have filed a notice of cross appeal.
With respect to each of the pending 44 lawsuits, the following table lists the name of plaintiff, the date the Company was served with process, the jurisdiction in which the case is pending, the type of claim and the status of the matter. This table also lists those cases that were dismissed or judgment entered during the most recent fiscal quarter. Cases that were dismissed or judgment entered in prior fiscal quarters are not included in this table. In each of the pending lawsuits, the plaintiff is seeking monetary damages from the Company. The claims and in some instances, the defense of each of these lawsuits has been submitted to our insurance carriers that maintained insurance coverage during these applicable periods and we continue to maintain product liability insurance coverage with varying limits and deductibles. Our product liability insurance coverage during these periods ranged from $5,000,000 to $10,000,000 in coverage limits and from $10,000 to $1,000,000 in per incident deductibles. We are defending each of these lawsuits vigorously.
42
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
NOTES TO FINANCIAL STATEMENTS — (Continued)
| Month | ||||||||
| Plaintiff | Served | Jurisdiction | Claim Type | Status | ||||
| Glowczenski | Oct-04 | US District Court, ED NY | Wrongful Death | Discovery Phase | ||||
| Washington | May-05 | US District Court, ED CA | Wrongful Death | Discovery Phase | ||||
| Sanders | May-05 | US District Court ED CA | Wrongful Death | Case Stayed | ||||
| Graff | Sep-05 | US District Court, AZ | Wrongful Death | Discovery Phase | ||||
| Heston | Nov-05 | US District Court, ND CA | Wrongful Death | Plaintiff Jury Verdict, punitive damages thrown out, judgment entered against TASER for $153,150 compensatory damages, $1,423,127 attorney fees and $50,000 costs | ||||
| Rosa | Nov-05 | US District Court, ND CA | Wrongful Death | Trial Scheduled July — 09 | ||||
| Yeagley | Nov-05 | Hillsborough County Circuit County, FL | Wrongful Death | Discovery Phase | ||||
| Neal-Lomax | Dec-05 | US District Court, NV | Wrongful Death | Dismissed, Appeal Pending | ||||
| Mann | Dec-05 | US District Court, ND GA, Rome Div | Wrongful Death | Dismissed, Appeal Pending | ||||
| Lee | Jan-06 | Davidson County, TN Circuit Court | Wrongful Death | Dismissed | ||||
| Zaragoza | Feb-06 | CA Superior Court, Sacramento County | Wrongful Death | Trial Scheduled Dec — 09 | ||||
| Bagnell | Jul-06 | Supreme Court for British Columbia, Canada | Wrongful Death | Discovery Phase | ||||
| Hollman | Aug-06 | US District Court, ED NY | Wrongful Death | Discovery Phase | ||||
| Oliver | Sep-06 | US District Court, MD FL, Orlando | Wrongful Death | Trial Stayed | ||||
| Teran/LiSaola | Oct-06 | US District Court, ND CA | Wrongful Death | Taken off Trial Calendar | ||||
| Augustine | Jan-07 | 11th Judicial Circuit Court, Miami-Dade | Wrongful Death | Discovery Phase | ||||
| Bolander | Aug-07 | 17th Circuit Court Broward County, FL | Wrongful Death | Trial Scheduled April — 09, Summary judgment motion pending | ||||
| Wendy Wilson, Estate of Ryan Wilson | Aug-07 | District Court Boulder County, CO | Wrongful Death | Discovery Phase | ||||
| Crawford, Estate of Russell Walker | Oct-07 | District Court Clark County, NV | Wrongful Death | Discovery Phase | ||||
| Walker, Estate of Russell Walker (Companion to Crawford) | Oct-07 | US District Court District of NV | Wrongful Death | Discovery Phase | ||||
| Jack Wilson, Estate of Ryan Wilson (Companion to Wendy Wilson) | Nov-07 | District Court Boulder County, CO | Wrongful Death | Discovery Phase | ||||
| Kasilyan | Feb-08 | District Court Clark County, NV | Wrongful Death | Dismissed | ||||
| Gilliam | Apr-08 | US District Court, MD, AL | Wrongful Death | Trial Scheduled Nov — 09 | ||||
| Romero | May-08 | Dallas County District Court, TX | Wrongful Death | Discovery Phase | ||||
| Guerrero | Jun-08 | US District Court, Central District CA | Wrongful Death | Trial Scheduled Aug — 09 | ||||
| Marquez | Jun-08 | US District Court, Arizona | Wrongful Death | Discovery Phase | ||||
| Preyer | Jul-08 | US District Court, Middle District, FL | Wrongful Death | Trial Scheduled Feb — 2010 | ||||
| Salinas | Aug-08 | US District Court, Northern District CA | Wrongful Death | Trial Scheduled April — 2010 | ||||
| Wells | Sep-08 | US District Court, Northern District CA | Wrongful Death | Dismissed | ||||
| R. Wilson | Oct-08 | SC Court Common Pleas, Charleston County | Wrongful Death | Dismissed w/o Prejudice | ||||
| Thomas (Pike) | Oct-08 | US District Court, WD Louisiana, Alexandria | Wrongful Death | Discovery Phase | ||||
| Haake | Nov-08 | US District Court, Kansas | Wrongful Death | Complaint Served | ||||
| Dwyer | Nov-08 | US District Court, EDD TX, Marshall Division | Wrongful Death | Complaint Served | ||||
| Nykiel | Dec-08 | Common Pleas Court, Allegheny County, PA | Wrongful Death | Complaint Served | ||||
| Starr | Dec-08 | Common Pleas Court, 12th Judicial Circuit, Florence County, SC | Wrongful Death | Trial Scheduled Dec — 09 | ||||
| Carroll | Mar-09 | US District Court, Southern District TX | Wrongful Death | Complaint Served | ||||
| Silva | Mar-09 | US District Court, Northren District CA | Wrongful Death | Complaint Served | ||||
| Stewart | Oct-05 | Circuit Court for Broward County, FL | Training Injury | Discovery Phase | ||||
| Lewandowski | Jan-06 | US District Court, NV | Training Injury | Partial Motion to Dismiss Granted | ||||
| Peterson | Jan-06 | US District Court, NV | Training Injury | Discovery Phase | ||||
| Husband | Mar-06 | British Columbia Supreme Court, Canada | Training Injury | Discovery Phase | ||||
| Wilson | Aug-06 | US District Court, ND GA | Training Injury | Dismissed; Appeal Filed, Appelate Court Affirmed Dismissal | ||||
| Perry | Jul-08 | US District Court CO | Training Injury | Dismissed | ||||
| Grable | Aug-08 | FL 6th Judicial Circuit Court, Pinellas County | Training Injury | Discovery Phase | ||||
| Koon | Dec-08 | 17th Judicial Circuit Court, Broward County, FL | Training Injury | Complaint Served | ||||
| Bickle | Mar-09 | MT 18th Judicial District Court, Gallatin County | Training Injury | Complaint Served | ||||
| Foley | Mar-09 | US District Court, MA | Training Injury | Complaint Served | ||||
| Bynum | Oct-05 | US District Court SD NY | Injury During Arrest | Discovery Phase | ||||
| Wieffenbach | Jun-06 | Circuit Court of 12thJudicial District, Will County, Il | Injury During Arrest | Discovery Phase | ||||
| Payne | Oct-06 | Circuit Court of Cook County, Illinois | Injury During Arrest | Discovery Phase | ||||
| Gomez | May-07 | Circuit Court 11th Judicial Dist. FL | Injury During Arrest | Complaint Served | ||||
| Kern / Banda | Feb-08 | District Court, Tarrant County, TX | Injuty During Admittance | Complaint Served | ||||
| Butler | Sep-08 | CA Superior Court, Santa Cruz County | Injury During Arrest | Discovery Phase | ||||
| Scott | Dec-08 | US District Court, Northern District, WVA | Injury During Arrest | Trial Scheduled April — 09 | ||||
43
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
NOTES TO FINANCIAL STATEMENTS — (Continued)
Other Litigation
In December 2005, we filed a lawsuit in Vigo County, Indiana, Superior Court against Roland M. Kohr for defamation, product disparagement, Lanham Act violations, tortuously affecting the fairness and integrity of litigation as an adverse third-party witness, and intentional interference with a business relationship. The lawsuit seeks monetary damages and injunctive relief against Dr. Kohr. Dr. Kohr was the medical examiner and expert witness in the James Borden wrongful death litigation, which litigation was dismissed with prejudice. This case is in the discovery phase and no trial date has been set.
In June 2006, we filed a lawsuit in U.S. District Court for the Central District of California against Bestex Company, Inc. for patent infringement, false patent marking, unfair competition and breach of written contract. Bestex filed a counterclaim for unfair competition and false advertising. Both parties filed motions for summary judgment to dismiss the other parties’ claims, both of which motions were granted by the Court and the matter was resolved on those motions before the Court in January 2007. An appeal was filed by Bestex and on November 26, 2008, the United States Court of Appeals for the Ninth Circuit entered an order affirming the ruling of the District Court.
In January 2007, we filed a lawsuit in the U.S. District Court for Arizona against Stinger Systems, Inc. alleging patent infringement, patent false marking, and false advertising. Defendant filed an answer and counterclaim for false advertising and punitive damages. Discovery has begun and no trial date has been set. On February 2, 2009 the Court issued an order based on a Markman hearing held on May 7, 2008, in which the Court adopted the Company’s claim construction on the disputed patent claim term in TASER’s U.S. patent number 7,102,870 and all of the Company’s claim construction on all of the disputed patent claim terms in TASER’s U.S. patent number 7,234,262. In addition, the Court adopted the Company’s claim construction on one of the disputed patent claim terms in TASER’s U.S. patent number 6,999,295 and rejected both parties’ claim construction in the other disputed claim term in this patent.
In October 2007, we filed a lawsuit in Arizona Superior Court for Maricopa County against Steve Ward and Mark Johnson, both former employees of the Company, and VIEVU Corporation, et. al. for breach of duty of loyalty, breach of contract, breach of fiduciary duty, and conversion. This lawsuit does not involve our core electronic control device business and we do not expect this litigation to have a material impact on our financial results. Defendants Ward and VIEVU Corporation filed an answer and counterclaim for declaration of non-infringement, tortious interference with contractual relations, tortious interference with business expectancy, and abuse of process. The lawsuit seeks compensatory damages, constructive trust, exemplary damages, injunctive relief, attorneys’ fees and costs. Discovery has begun and no trial date has been set. Cross motions for summary judgment are pending.
In June 2008, we filed an amended complaint in the State Court of Fulton County, Georgia joining as a plaintiff in an existing lawsuit previously filed by certain current and former stockholders of the Company against Morgan Stanley & Co., Inc., and ten other brokerage firms alleging a conspiracy to unlawfully, deceptively, and fraudulently manipulate the price of the Company’s common stock in the context of illegal naked shorting. Specifically, the amended complaint alleges that the defendants committed a conspiracy and endeavor to violate the Georgia Racketeer Influenced and Corrupt Organization Act; Securities Fraud; Theft By Taking; Theft By Deception; Violation of The Georgia Computer Systems Protection Act; Violation of the Georgia Securities Act; Violation of the Georgia Computer Systems Protection Act; and Conversion. The lawsuit seeks compensatory and punitive damages as well as expenses of litigation including attorneys’ fees and costs. Defendants have filed motions to dismiss and discovery has not yet begun.
In July 2008, we were served with a summons and complaint in the lawsuit entitled Proformance Vend USA vs. TASER International, Inc. which was filed in Arizona Superior Court for Maricopa County alleging breach of contract of a vending machine contract and seeking money damages, including tort damages, attorney’s fees and costs. We have filed an answer to this complaint. Discovery has begun and no trial date has been set.
In February 2009, we filed a complaint in the United States District Court for the District of Nevada against James F. McNulty, Jr., Robert Gruder, and Stinger Systems, Inc. alleging securities fraud trade libel, unfair competition under the Lanham Act, abuse of process, and deceptive trade practices; seeking compensatory damages, punitive damages, injunctive relief, attorneys’ fees and costs. The complaint is in the process of being served on the defendants.
44
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
NOTES TO FINANCIAL STATEMENTS — (Continued)
General
From time to time, the Company is notified that it may be a party to a lawsuit or that a claim is being made against it. It is the Company’s policy to not disclose the specifics of any claim or threatened lawsuit until the summons and complaint are actually served on the Company. We intend to defend and pursue any lawsuit filed against or by the Company vigorously. Although we do not expect the outcome in any individual case to be material, the outcome of any litigation is inherently uncertain and there can be no assurance that any expense, liability or damages that may ultimately result from the resolution of these matters will be covered by our insurance or will not be in excess of amounts provided by insurance coverage and will not have a material adverse effect on our business, operating results or financial condition. In addition, the Company has six lawsuits where the costs of legal defense incurred are in excess of its liability insurance deductibles. As of December 31, 2008, the Company has recorded approximately $210,000 in other assets related to the receivable from its insurance company for reimbursement of these legal costs. The Company may settle a lawsuit in situations where a settlement can be obtained for nuisance value and for an amount that is expected to be less than the cost of defending a lawsuit. The number of product liability lawsuits dismissed includes a small number of police officer training injury lawsuits that were settled by the Company and dismissed in cases where the settlement economics to the Company were significantly less than the cost of litigation. One of the training injury lawsuits brought by a law enforcement officer was settled in June 2007 for an amount in excess of nuisance value by our insurance company. Our insurance coverage at that time did not cover our costs of defense if we won at trial. However, our insurance coverage at that time provided for a pro-rata reimbursement of our costs of defense if the lawsuit was settled. Upon final settlement of this case, the Company was paid $241,000 by our insurance company as reimbursement of the Company’s costs of defense. Due to the confidentiality of our litigation strategy and the confidentiality agreements that are executed in the event of a settlement, the Company does not identify or comment on which specific lawsuits have been settled or the amount of any settlement.
d. Employment Agreements
The Company has employment agreements with its Chairman, Chief Executive Officer, President, Chief Financial Officer, Vice President of Research and Development, Vice President Operations and Vice President and General Counsel. The Company may terminate the agreements with or without cause. Should the Company terminate the agreements without cause, or upon a change of control of the Company or death of the employee, the employees are entitled to additional compensation. Under these circumstances, these officers and employees may receive the amounts remaining under their contracts upon termination, which would total $1,861,000 in the aggregate at December 31, 2008.
8. Income Taxes
Significant components of the Company’s deferred income tax assets and liabilities are as follows:
| December 31, | ||||||||
| 2008 | 2007 | |||||||
Deferred income tax assets | ||||||||
| Net operating loss carryforward | $ | 4,114,090 | $ | 13,805,961 | ||||
| Reserves and accruals | 2,512,059 | 1,703,061 | ||||||
| Non-employee stock option expense | 312,218 | 313,474 | ||||||
| Non-qualified stock option expense | 587,795 | 214,003 | ||||||
| Capitalized R&D | 6,718,001 | 2,790,909 | ||||||
| Charitable contributions | — | 466,346 | ||||||
| Alternative minimum tax carryforward | 1,111,541 | 752,247 | ||||||
| R&D tax credit | 4,361,353 | 3,141,781 | ||||||
| Deferred income tax assets | 19,717,057 | 23,187,782 | ||||||
Deferred income tax liabilities | ||||||||
| Depreciation | (1,183,910 | ) | (539,806 | ) | ||||
| Amortization | (76,296 | ) | (65,547 | ) | ||||
| Deferred income tax liabilities | (1,260,206 | ) | (605,353 | ) | ||||
| Net deferred income tax assets before valuation allowance | 18,456,851 | 22,582,429 | ||||||
| Less: Valuation allowance | (200,000 | ) | (250,000 | ) | ||||
Net deferred income tax assets | $ | 18,256,851 | $ | 22,332,429 | ||||
| Reported as: | ||||||||
| Current deferred tax assets | $ | 9,430,073 | $ | 15,608,325 | ||||
| Long-term deferred tax assets | 8,826,778 | 6,724,104 | ||||||
| $ | 18,256,851 | $ | 22,332,429 | |||||
45
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
NOTES TO FINANCIAL STATEMENTS — (Continued)
In July 2000, the Company granted 136,364 warrants to acquire Company stock at an exercise price of $0.55 per share to a member of its Board of Directors as additional consideration for a $1.5 million loan. In October 2004, the stock warrants were exercised with an intrinsic value of $5,233,650. The Board member was incorrectly provided tax forms that indicated the award was taxable income to him. The Company included the $5,233,650 as stock compensation expense in its 2004 tax return and, because the Company had a net operating loss carryforward, recorded a $2,014,955 deferred tax asset on the balance sheet and a corresponding increase to additional paid in capital. The Company’s 2004 tax return was audited by the IRS in 2007 with no adjustment made to the stock compensation expense deduction recorded by the Company. During a tax examination of the Board member’s tax return it was determined that the exercise of the warrant should not have created taxable income and that the inclusion of the intrinsic value of the warrant in the director’s 1099 was in fact, an error. Accordingly, in the second quarter of 2008, the Company reduced its deferred tax asset balance and additional paid in capital by $2,014,955. The adjusting entry was a balance sheet only adjustment and had no impact on retained earnings and was not considered material to the associated account balances or the balance sheet as a whole.
The Company recognizes the income tax benefits associated with certain stock compensation deductions only when such deductions produce a reduction to the company’s actual tax liability. Accordingly, the deferred tax asset reported above for net operating losses carrying forward includes a federal NOL of approximately $10.0 million at December 31, 2008, after utilization of approximately $20.6 million during 2008. When stock compensation deductions of $11.6 million are considered prior to utilization, the federal NOL is approximately $21.6 million at December 31, 2008 after utilization of approximately $19.1 million during 2008. The Company’s federal NOL carryforward expires in 2024. The Company’s state NOL expires at various dates beginning in 2009 through 2024.
In preparing the Company’s financial statements, management has assessed the likelihood that its deferred income tax assets will be realized from future taxable income. In evaluating the ability to recover its deferred income tax assets, management considers all available evidence, positive and negative; including the Company’s operating results, ongoing tax planning and forecasts of future taxable income on a jurisdiction by jurisdiction basis. A valuation allowance is established if it is determined that it is more likely than not that some portion or all of the net deferred income tax assets will not be realized. Management exercises significant judgment in determining the Company’s provisions for income taxes, its deferred income tax assets and liabilities and its future taxable income for purposes of assessing its ability to utilize any future tax benefit from its deferred income tax assets. Although management believes that its tax estimates are reasonable, the ultimate tax determination involves significant judgments that could become subject to audit by tax authorities in the ordinary course of business. As a result of the shareholder litigation settlement expense recorded in the second quarter of 2006, management has recorded a valuation allowance of $250,000 against its deferred income tax assets for Arizona NOL’s. In the second quarter of 2008, an additional $250,000 valuation allowance was recorded against the same deferred tax assets. In the fourth quarter of 2008, management updated its analysis regarding its ability to utilize the Arizona NOL to reflect the results of current operations, including the reversal of the punitive damages accrual of $5,200,000 relating to the Heston litigation in the fourth quarter of 2008, as well as its future business plans. Based on this updated analysis, management reduced its valuation allowance against the deferred tax assets for Arizona NOL’s from $500,000 to $200,000 at December 31, 2008. Management believes that, other than as previously described, as of December 31, 2008, based on an evaluation and projections of future sales and profitability, no other valuation allowance was deemed necessary as management concluded that it is more likely than not that the Company’s net deferred income tax assets will be realized. However, the deferred tax asset could be reduced in the near-term if estimates of future taxable income during the carryforward period are reduced.
Significant components of the provision (benefit) for income taxes are as follows:
| For the Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
Current | ||||||||||||
| Federal | $ | 414,094 | $ | 518,682 | $ | 235,863 | ||||||
| State | 239,539 | 49,226 | 36,161 | |||||||||
| Total Current | 653,633 | 567,908 | 272,024 | |||||||||
Deferred | ||||||||||||
| Federal | 2,520,677 | 6,014,663 | (1,292,900 | ) | ||||||||
| State | (460,054 | ) | (182,880 | ) | 124,976 | |||||||
| Total Deferred | 2,060,623 | 5,831,783 | (1,167,924 | ) | ||||||||
| Tax provision (benefit) recorded as an increase (decrease) in liability for unrecorded tax benefits | 592,007 | 1,100,073 | — | |||||||||
Provision (benefit) for Income Taxes | $ | 3,306,263 | $ | 7,499,764 | $ | (895,900 | ) | |||||
46
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
NOTES TO FINANCIAL STATEMENTS — (Continued)
A reconciliation of the Company’s effective income tax rate to the federal statutory rate follows:
| For the Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| Federal statutory rate | 35.0 | % | 35.0 | % | (35.0 | )% | ||||||
| State tax, net of federal benefit | 4.8 | % | 3.6 | % | (3.4 | )% | ||||||
| Permanent differences a) | 12.3 | % | 3.6 | % | 13.9 | % | ||||||
| Research and development | (10.5 | )% | (13.8 | )% | 0.0 | % | ||||||
| Change in liability for unrecognized tax benefits | 8.5 | % | 4.9 | % | 0.0 | % | ||||||
| Change in valuation allowance | (0.7 | )% | 0.0 | % | 5.0 | % | ||||||
| Other | (1.8 | )% | 0.0 | % | 1.5 | % | ||||||
| Effective income tax rate | 47.6 | % | 33.3 | % | (18.0 | )% | ||||||
| a) | Permanent differences include certain expenses which are not deductible for tax purposes including lobbying fees and stock-based compensation expense related to ISO’s. |
In 2007, the Company completed a research and development tax credit study which identified $3.1 million in tax credits for Federal and Arizona income tax purposes related to the 2003 through 2007 tax years net of the federal benefit on the AZ research and development tax credits. As a result, the Company recognized $2.0 million in 2007 as a reduction in income tax expense. Management made the determination that it was more likely than not that the full benefit of the research and development tax credit would not be sustained on examination and recorded a liability for unrecognized tax benefits of $1.1 million as of December 31, 2007. Management has estimated that an additional $869,000 of tax credits are available for Federal and state income tax purposes for the 2008 tax year net of the federal benefit on the AZ research and development tax credits. In addition, during 2008 management accrued approximately $106,000 for estimated uncertain tax positions related to certain state income tax liabilities. As of December 31, 2008, management does not expect the amount of the unrecognized tax benefit liability to increase or decrease within the next 12 months. Should the unrecognized tax benefit of $1.7 million be recognized, our effective tax rate would be favorably impacted.
The following presents a rollforward of our liability for unrecognized tax benefits as of December 31:
| 2008 | 2007 | |||||||
| Balance at January 1, | $ | 1,100,073 | $ | — | ||||
| Increase in prior year tax positions | — | 1,100,073 | ||||||
| Increase in current year tax positions | 640,850 | — | ||||||
| Decrease related to adjustment of previous estimates of activity | (48,843 | ) | — | |||||
| Decrease related to settlements with taxing authorities | — | — | ||||||
| Decrease related to lapse in statute of limitations | — | — | ||||||
| Balance at December 31, | $ | 1,692,080 | $ | 1,100,073 | ||||
An examination by the United States Internal Revenue Service (the “IRS”) for 2006 was concluded in the third quarter of 2008 with no significant adjustment required by the IRS. Federal income tax returns for 2007 remain open to examination by the IRS, while state and local income tax returns for 2002 through 2007 also remain open to examination.
As part of the examination by the IRS for the Company’s 2004 fiscal year, the IRS notified the Company that it intended to propose an assessment for failure to timely deposit employment taxes with respect to stock option exercises. Although management believed that it had meritorious defenses against a proposed assessment, the matter was settled for $116,000 in October 2007, which was recorded in sales, general and administrative expense for the year ended December 31, 2007.
9. Line of Credit
The Company entered into a line of credit agreement on July 13, 2004. The agreement has a total availability of $10 million. The line is secured primarily by the Company’s accounts receivable and inventory and bears interest at varying rates, ranging from LIBOR plus 1.5% to prime. The availability under this line is computed on a monthly borrowing base, which is based on the Company’s eligible accounts receivable and inventory. The line of credit matures on June 30, 2010 and requires monthly payments of interest only. At December 31, 2008, there was no amount outstanding under the line of credit and the available borrowing under the existing line of credit was $10.0 million. There were no borrowings under the line during the year ended December 31, 2008.
The Company’s agreement with the bank requires the Company to comply with certain financial and other covenants including maintenance of minimum tangible net worth and fixed charge coverage. At December 31, 2008, the Company was in compliance with the covenants.
47
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
NOTES TO FINANCIAL STATEMENTS — (Continued)
10. Stockholders’ Equity
a. Common Stock and Preferred Stock
Concurrent with its re-incorporation in Delaware in January 2001, the Company adopted a certificate of incorporation and authorized the issuance of two classes of stock designated as “common stock” and “preferred stock,” each having a par value of $0.00001 per share. The Company is authorized to issue is 200 million shares of common stock and 25 million shares of preferred stock.
b. Stock Repurchase
In April 2008, TASER’s Board of Directors authorized a stock repurchase program to acquire up to $12.5 million of the Company’s outstanding common stock subject to stock market conditions and corporate considerations. During 2008, the Company repurchased 1.79 million shares at a weighted average cost of $6.98 per share and a total cost of $12.5 million.
In August 2006, TASER’s Board of Directors authorized a stock repurchase program to acquire up to $10 million of the Company’s outstanding common stock. During the third quarter of 2006, the Company repurchased 300,000 shares at a weighted average cost of $7.36 per share and a total cost of approximately $2.2 million. The Company’s ability to repurchase stock under this program terminated upon final payment of the shareholder class action and derivative settlement in the first quarter of 2007.
c. Stock Option Plans
The Company has historically issued stock options to various equity owners and key employees as a means of attracting and retaining quality personnel. The option holders have the right to purchase a stated number of shares at the market value on the grant date. The options issued under the Company’s 1999 Stock Option Plan (the “1999 Plan”) generally vest over a three-year period and have a contractual maturity of ten years. The options issued under the Company’s 2001 Stock Option Plan (the “2001 Plan”) generally vest over a three-year period and have a contractual maturity of ten years. The options issued under the Company’s 2004 Stock Option Plan (the “2004 Plan”) generally vest over a three-year period and have a contractual maturity of ten years, however the majority of options issued under this plan within fiscal 2005 had vesting terms of one year. The shares issuable under each of the plans were registered on Form S-8 with the United States Securities and Exchange Commission. The total number of shares registered under these plans was as follows: 9,952,500 under the 1999 Plan, 6,600,000 under the 2001 Plan, and 6,800,000 under the 2004 Plan. These plans provide for officers, key employees and consultants to receive nontransferable stock options to purchase an aggregate of 23,352,500 shares of the Company’s common stock. As of December 31, 2008, 702,680 options remain available for future grants.
Stock Option Activity
A summary of the Company’s stock options at December 31, 2008, 2007 and 2006 and for the years then ended is presented in the table below:
| 2008 | 2007 | 2006 | ||||||||||||||||||||||
| Weighted Average | Weighted Average | Weighted Average | ||||||||||||||||||||||
| Options | Exercise Price | Options | Exercise Price | Options | Exercise Price | |||||||||||||||||||
| Options outstanding, beginning of year | 5,234,072 | $ | 6.06 | 5,902,182 | $ | 5.13 | 6,161,933 | $ | 4.92 | |||||||||||||||
| Granted | 4,285,671 | $ | 5.38 | 533,404 | $ | 10.42 | 192,038 | $ | 8.66 | |||||||||||||||
| Exercised | (323,409 | ) | $ | 1.06 | (1,107,574 | ) | $ | 2.84 | (301,320 | ) | $ | 2.48 | ||||||||||||
| Expired/terminated | (87,404 | ) | $ | 11.29 | (93,940 | ) | $ | 10.52 | (150,469 | ) | $ | 6.29 | ||||||||||||
| Options outstanding, end of year | 9,108,930 | $ | 5.87 | 5,234,072 | $ | 6.06 | 5,902,182 | $ | 5.13 | |||||||||||||||
| Exercisable at end of year | 4,901,483 | $ | 6.02 | 4,683,066 | $ | 5.58 | 5,608,322 | $ | 4.88 | |||||||||||||||
| Options available for grant at end of year | 702,680 | 4,900,947 | 5,340,411 | |||||||||||||||||||||
| Weighted average fair value of options granted during the year | $ | 2.89 | $ | 5.22 | $ | 4.49 | ||||||||||||||||||
48
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
NOTES TO FINANCIAL STATEMENTS — (Continued)
The following table summarizes information about stock options outstanding and exercisable as of December 31, 2008:
| Options Outstanding | Options Exercisable | |||||||||||||||||||
| Weighted | ||||||||||||||||||||
| Average | ||||||||||||||||||||
| Weighted | Remaining | Weighted | ||||||||||||||||||
| Number | Average | Contractual | Number | Average | ||||||||||||||||
| Range of Exercise Price | Outstanding | Exercise Price | Life Life | Exercisable | Exercise Price | |||||||||||||||
| $0.28 — $0.99 | 954,886 | $ | 0.36 | 3.8 | 954,886 | $ | 0.36 | |||||||||||||
| $1.03 — $2.41 | 858,875 | $ | 1.56 | 3.7 | 858,875 | $ | 1.56 | |||||||||||||
| $3.53 — $9.93 | 6,348,602 | $ | 6.22 | 8.4 | 2,400,744 | $ | 7.67 | |||||||||||||
| $10.07 — $19.76 | 884,567 | $ | 12.17 | 7.3 | 624,978 | $ | 12.71 | |||||||||||||
| $20.12 — $29.98 | 62,000 | $ | 23.91 | 5.5 | 62,000 | $ | 23.91 | |||||||||||||
| $0.28 — $29.98 | 9,108,930 | $ | 5.87 | 7.4 | 4,901,483 | $ | 6.02 | |||||||||||||
The total fair value of options exercisable was approximately $15.4 million, $13.6 million and $14.1 million at December 31, 2008, 2007 and 2006, respectively. The aggregate intrinsic value of options outstanding and options exercisable at December 31, 2008 was $9.4 million and $7.9 million, respectively. The aggregate intrinsic value of unvested options at December 31, 2008 was $1.4 million. Aggregate intrinsic value represents the difference between the Company’s closing stock price on the last trading day of the fiscal period, which was $5.28 as of December 31, 2008, and the exercise price of the option multiplied by the number of options outstanding. Total intrinsic value of options exercised was $2.4 million, $9.7 million and $1.9 million for the years ended December 31, 2008, 2007 and 2006, respectively.
At December 31, 2008, the Company had 4,207,447 unvested options outstanding with a weighted average exercise price of $5.64 per share, weighted average fair value of $3.01 per share and weighted average remaining contractual life of 9.7 years. Of the unvested options outstanding at December 31, 2008, the Company expects that 4,030,734 options will ultimately vest based on its historical experience.
Stock-based Compensation Expense
The Company accounts for share-based compensation under SFAS No. 123(R), using the fair-value method. Reported share-based compensation was classified as follows for the years ended December 31:
| 2008 | 2007 | 2006 | ||||||||||
| Indirect manufacturing expense | $ | 257,964 | $ | 187,585 | $ | 131,086 | ||||||
| Sales, general and administrative expenses | 1,552,411 | 986,616 | 808,341 | |||||||||
| Research and development expenses | 613,510 | 213,765 | 199,418 | |||||||||
| $ | 2,423,885 | $ | 1,387,966 | $ | 1,138,845 | |||||||
As of December 31, 2008, total unrecognized stock-based compensation expense related to non-vested stock options was approximately $11.7 million, which is expected to be recognized over a weighted average period of approximately 16 months.
Total share-based compensation expense recognized in the income statement for the years ended December 31, 2008, 2007 and 2006 includes $1,438,000, $1,094,000 and $874,000, respectively, related to Incentive Stock Options (“ISO“s) for which no tax benefit is recognized. The total deferred tax benefits related to non-qualified stock options were approximately $588,000 and $214,000 for the years ended December 31, 2008 and 2007, respectively. As a result of the adoption of SFAS No. 123(R), the Company did not tax effect the share-based compensation expense for tax purposes related to the non-qualified disposition of ISOs exercised and sold. The benefit will be recorded when the Company is in a position to realize the benefit with an offset to taxes payable in future periods. The total unrecognized tax benefit related to the non-qualified disposition of stock options in 2008, 2007 and 2006 was approximately $1.2 million, $3.0 million and $617,000, respectively.
The Company granted 811,000 performance-based stock options in 2008, the vesting of which is contingent upon the completion of certain performance criteria including the successful development and market acceptance of future product introductions as well as the future operating performance of the Company. Compensation expense is recognized over the implicit service period (the date the performance condition is required to be achieved) based on management’s estimate of the probability of the performance criteria being satisfied, adjusted at each balance sheet date. At December 31, 2008, the fair value of the performance-based options was estimated to be $1.96 million, and the Company recognized related compensation expense of $119,000 during 2008.
49
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
NOTES TO FINANCIAL STATEMENTS — (Continued)
11. Related Party Transactions
Aircraft charter
The Company reimburses Thomas P. Smith, Chairman of the Company’s Board of Directors, and Patrick W. Smith, Chief Executive Officer, for business use of their personal aircraft. For the years ended December 31, 2008, 2007 and 2006, the Company incurred expenses of approximately $197,000, $394,000 and $487,000, respectively, to Thomas P. Smith. For the years ended December 31, 2008 and 2007, the Company incurred expenses of approximately $107,000 and $54,000, respectively, to Patrick W. Smith. No amounts were reimbursed to Patrick W. Smith for the year ended December 31, 2006. At December 31, 2008 and 2007, the Company had outstanding payables of approximately $0 and $27,000, respectively, to Thomas P. Smith. At December 31, 2008 and 2007, the Company had no outstanding payables due to Patrick W. Smith. Management believes that the rates charged by Thomas P. Smith and Patrick W. Smith are equal to or below commercial rates the Company would pay to charter similar aircraft from independent charter companies.
During the first quarter of 2007, Thomas P. Smith chartered an aircraft from Thundervolt, LLC, which is 50% owned by Patrick W. Smith, Chief Executive Officer of the Company, for business related travel. For the year ended December 31, 2007, the Company incurred expenses of $30,000 to reimburse Thomas P. Smith for such travel. Management believes that the rates charged by Thundervolt, LLC to Thomas P. Smith were equal to or below commercial rates the Company would pay to charter similar aircraft from independent charter companies. No expenses were incurred for 2008 or 2006.
Management performed a review of the above relationship with Thundervolt, LLC, in accordance with the provisions of Financial Accounting Standards Board Interpretation No. 46, “Consolidation of Variable Interest Entities, an Interpretation of ARB No. 51” (FIN 46R). The relationships were determined to not meet the definition of a variable interest entity (VIE) as defined by FIN 46R as Thundervolt, LLC is adequately capitalized, their owners possess all of the essential characteristics of a controlling financial interest, and the Company does not have any voting rights in the entity. Therefore, the entity is not required to be consolidated into the Company’s results.
TASER Foundation
In November 2004, the Company established the TASER Foundation. The TASER Foundation is an Internal Revenue Code Section 501(c)(3) non-profit corporation and has been granted tax exempt status by the IRS. The TASER Foundation’s mission is to honor the service and sacrifice of local and federal law enforcement officers in the United States and Canada lost in the line of duty by providing financial support to their families. Daniel M. Behrendt, an officer of the Company, serves on the Board of Directors of the TASER Foundation. Over half of the initial $1 million endowment was contributed directly by the Company’s employees. The Company bears all administrative costs of the TASER Foundation in order to ensure 100% of all donations are distributed to the families of fallen officers. For the years ended December 31, 2008, 2007 and 2006, the Company incurred approximately $233,000, $179,000 and $228,000, respectively, in such administrative costs. For the years ended December 31, 2008, 2007 and 2006, the Company contributed $25,000, $300,000 and $275,000, respectively, to the TASER Foundation. At December 31, 2008 and 2007, the Company had no outstanding payable amounts to the Foundation.
Consulting services
The Company engages Mark Kroll, a member of the Board of Directors, to provide consulting services. The expenses related to these services for the years ended December 31, 2008, 2007 and 2006 were approximately $293,000, $227,000 and $197,000, respectively. At December 31, 2008 and 2007, the Company had accrued liabilities of approximately $23,000 and $20,000, respectively, related to these services.
12. Employee Benefit Plan
In January 2006, the Company established a defined contribution profit sharing 401(k) plan (the “Plan”) for eligible employees, which is qualified under Sections 401(a) and 401(k) of the Internal Revenue Code of 1986, as amended. Employees are entitled to make tax-deferred contributions of up to the maximum allowed by law of their eligible compensation, but not exceeding $15,500 in 2008. The Company matches 100% of the first 3% of eligible compensation contributed to the Plan by each participant and 50% of the next 2% of eligible compensation contributed to the Plan by each participant. Beginning January 1, 2008, the Company’s matching contributions are immediately vested. During 2007 and 2006, the Company’s matching contributions cliff vested at 20% per annum and are fully vested after five years of service, at age 59 1/2 regardless of service, upon the death or permanent disability of the employee, or upon termination of the Plan. The Company’s matching contributions to the Plan for the year ended December 31, 2008, 2007 and 2006 were approximately $398,000, $250,000 and $201,000. Future matching or profit sharing contributions to the Plan are at the Company’s sole discretion.
50
Table of Contents
TASER INTERNATIONAL, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
NOTES TO FINANCIAL STATEMENTS — (Continued)
13. Selected Quarterly Financial Data (unaudited)
Selected quarterly financial data for years ended December 31, 2008 and 2007 follows (in thousands except for per share data):
| Quarter Ended | ||||||||||||||||
| Mar.31, 2008 | Jun.30, 2008 | Sep.30, 2008 | Dec. 31, 2008 | |||||||||||||
| Net sales | $ | 22,486,504 | $ | 21,101,309 | $ | 22,859,459 | $ | 26,398,218 | ||||||||
| Gross margin | $ | 12,763,318 | $ | 13,605,023 | $ | 13,894,793 | $ | 16,741,093 | ||||||||
| Net income (loss) — Note a) | $ | 1,216,587 | $ | (2,015,736 | ) | $ | 650,377 | $ | 3,785,813 | |||||||
| Basic net income (loss) per share | $ | 0.02 | $ | (0.03 | ) | $ | 0.01 | $ | 0.06 | |||||||
| Diluted net income (loss) per share | $ | 0.02 | $ | (0.03 | ) | $ | 0.01 | $ | 0.06 | |||||||
| Quarter Ended | ||||||||||||||||
| Mar.31, 2007 | Jun.30, 2007 | Sep.30, 2007 | Dec. 31, 2007 | |||||||||||||
| Net sales | $ | 15,301,815 | $ | 25,863,376 | $ | 28,533,419 | $ | 31,028,581 | ||||||||
| Gross margin | $ | 8,889,029 | $ | 15,531,259 | $ | 16,006,772 | $ | 17,132,759 | ||||||||
| Net income — Note b) | $ | 494,554 | $ | 3,699,208 | $ | 6,154,038 | $ | 4,678,676 | ||||||||
| Basic net income per share | $ | 0.01 | $ | 0.06 | $ | 0.10 | $ | 0.07 | ||||||||
| Diluted net income per share | $ | 0.01 | $ | 0.06 | $ | 0.09 | $ | 0.07 | ||||||||
| a) | For the quarter ended June 30, 2008, the Company recorded a $5.2 million non-cash charge for punitive damages following an adverse jury verdict in a litigation trial. The $5.2 million was reversed in the fourth quarter of 2008 following a court order that all punitive damages be disregarded. Refer to Note 7c. | |
| b) | In September 2007, the Company completed a research and development tax credit study which identified $3.1 million in tax credits for Federal and Arizona income tax purposes related to the 2003 through 2006 tax years. The Company recognized $1.8 million in the third quarter of 2007 as a reduction in income tax expense. Refer to Note 8. |
51
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders
TASER International, Inc.
TASER International, Inc.
We have audited the accompanying balance sheets of TASER International, Inc. (the Company) as of December 31, 2008 and 2007, and the related statements of operations, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2008. Our audits of the basic financial statements included the financial statement schedule listed in the index appearing under Item 15(a)(2). These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of TASER International, Inc. as of December 31, 2008 and 2007, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2008, in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly, in all material respects, the information set forth therein.
As discussed in Note 1(l) to the financial statements, effective January 1, 2007 Taser International, Inc. adopted FASB Interpretation No. 48, Accounting for Uncertainty in Income Taxes—An interpretation of FASB Statement No. 109.
As discussed in Note 1(p) to the financial statements, effective January 1, 2006 Taser International, Inc. adopted the provisions of Statement of Financial Accounting Standards No. 123(R), Share-Based Payment, applying the modified-prospective method.
We also have audited, in accordance with the standards of the Public Company Oversight Board (United States), TASER International Inc.’s internal control over financial reporting as of December 31, 2008, based on criteria established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) and our report dated March 16, 2009, expressed an unqualified opinion.
/s/ GRANT THORNTON LLP
Phoenix, Arizona
March 16, 2009
March 16, 2009
52
Table of Contents
Item 9.Changes in and Disagreements With Accountants on Accounting and Financial Disclosure
None.
Item 9A.Controls and Procedures
Attached as exhibits to this Form 10-K are certifications of the Company’s Chief Executive Officer (“CEO”) and Chief Financial Officer (“CFO”), which are required in accordance with Rule 13a-14 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). This “Controls and Procedures” section includes information concerning the controls and controls evaluation referred to in the certifications. The report of Grant Thornton LLP, our independent registered public accounting firm, regarding its audit of the Company’s internal control over financial reporting and of management’s assessment of internal control over financial reporting is included herein. This section should be read in conjunction with the certifications and the Grant Thornton attestation report for a more complete understanding of the topics presented.
Evaluation of disclosure controls and procedures
As of the end of the period covered by this Annual Report on Form 10-K, as required by paragraph (b) of Rule 13a-15 or Rule 15d-15 under the Exchange Act, we evaluated under the supervision of our CEO and our CFO, the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) or 15d-15(e) of the Exchange Act. Based on this evaluation, our CEO and our CFO have concluded that as of December 31, 2008 our disclosure controls and procedures are effective to ensure that information we are required to disclose in reports that we file or submit under the Exchange Act (i) is recorded, processed, summarized and reported within the time periods specified in Securities and Exchange Commission rules and forms, and (ii) is accumulated and communicated to our management, including our CEO and our CFO, as appropriate to allow timely decisions regarding required disclosure. Our disclosure controls and procedures are designed to provide reasonable assurance that such information is accumulated and communicated to our management.
Our disclosure controls and procedures include components of our internal control over financial reporting. Management’s assessment of the effectiveness of our internal control over financial reporting is expressed at the level of reasonable assurance because a control system, no matter how well designed and operated, can provide only reasonable, but not absolute, assurance that the control system’s objectives will be met.
Management report on internal control over financial reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with U.S. generally accepted accounting principles. Internal control over financial reporting includes those policies and procedures that:
| (i) | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the company; | ||
| (ii) | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and | ||
| (iii) | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements. |
Management assessed our internal control over financial reporting as of December 31, 2008, the end of our fiscal year. Management based its assessment on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Management’s assessment included evaluation of such elements as the design and operating effectiveness of key financial reporting controls, process documentation, accounting policies and our overall control environment. This assessment is supported by testing and monitoring performed by our Internal Audit organization.
Based on our assessment, management has concluded that our internal control over financial reporting was effective as of the end of the fiscal year to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external reporting purposes in accordance with generally accepted accounting principles. We reviewed the results of management’s assessment with the Audit Committee of our Board of Directors.
Our independent registered public accounting firm, Grant Thornton LLP, who also audited our financial statements, assessed the effectiveness of our internal control over financial reporting. Grant Thornton LLP has issued their attestation report, which is included herein.
53
Table of Contents
Changes in internal control over financial reporting
During the three months ended December 31, 2008, there was no change in our internal control over financial reporting identified in connection with the evaluation required by paragraph (d) of Rule 13a-15 or Rule 15d-15 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders
TASER International, Inc.
TASER International, Inc.
We have audited TASER International, Inc.,’s (the Company) internal control over financial reporting as of December 31, 2008, based on criteria established inInternal Control-Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). TASER International Inc.’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying “management report on internal control over financial reporting” in Item 9A, Controls and Procedures. Our responsibility is to express an opinion on TASER International Inc.’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, TASER International, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2008, based on the criteria established inInternal Control—Integrated Frameworkissued by COSO.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the balance sheets of TASER International, Inc. as of December 31, 2008 and 2007, and the related statements of operations, stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2008. Our report dated March 16, 2009, expressed an unqualified opinion.
/s/ GRANT THORNTON LLP
Phoenix, Arizona
March 16, 2009
March 16, 2009
Item 9B.Other Information
None.
54
Table of Contents
PART III
Item 10.Directors, Executive Officers and Corporate Governance
The information required to be disclosed by this item is incorporated herein by reference to our definitive proxy statement for the 2009 Annual Meeting of Stockholders (the “Proxy Statement”) which proxy statement we expect to file with the Securities and Exchange Commission within 120 days after the end of our fiscal year ended December 31, 2008.
Item 11.Executive Compensation
The information required to be disclosed by this item is incorporated herein by reference to our Proxy Statement.
Item 12.Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required to be disclosed by this item concerning is incorporated herein by reference to our Proxy Statement.
Equity Compensation Plan Information
The following table provides details of our equity compensation plans at December 31, 2008:
| Number of | ||||||||||||||||
| Number of | Securities | |||||||||||||||
| Securities | to be Issued upon | Number of | ||||||||||||||
| Authorized for | Exercise of | Weighted Average | Securities | |||||||||||||
| Issuance | Outstanding | Exercise Price of | Remaining | |||||||||||||
| Under the | Options, | Outstanding | Available for | |||||||||||||
| Plan Category | Plan | Warrants or Rights | Options | Future Issuance | ||||||||||||
| Equity compensation plans approved by security holders | 23,352,500 | 9,108,930 | $ | 5.87 | 702,680 | |||||||||||
| Equity compensation plans not approved by security holders | — | — | $ | — | — | |||||||||||
| Total | 23,352,500 | 9,108,930 | $ | 5.87 | 702,680 | |||||||||||
Refer to note 10(c) to the financial statements in Part II, Item 8 of this annual report for more information on the Company’s equity compensation plans.
Item 13.Certain Relationships and Related Transactions, and Director Independence
The information required to be disclosed by this item is incorporated herein by reference to our Proxy Statement.
Item 14.Principal Accountant Fees and Services
The information required to be disclosed by this item is incorporated by reference to our Proxy Statement.
PART IV
Item 15.Exhibits and Financial Statement Schedules
| (a) | The following documents are filed as part of this report: | |
| 1. | Financial Statements: | |
| All financial statements as set forth under Item 8 of this report. | ||
| 2. | Supplementary Financial Statement Schedules: | |
| Schedule II — Valuation and Qualifying Accounts |
Other schedules have not been included because they are not applicable or because the information is included elsewhere in this report.
| 3. | Exhibits: |
55
Table of Contents
| Exhibit | ||
| Number | Description | |
| 3.1 | Certificate of Incorporation, as amended (incorporated by reference to Exhibit 3.1 to Registration Statement on Form SB-2, effective May 11, 2001 (Registration No. 333-55658), as amended) | |
| 3.2 | Bylaws, as amended (incorporated by reference to Exhibit 3.2 to Registration Statement on Form SB-2, effective May 11, 2001 (Registration No. 333-55658), as amended) | |
| 3.3 | Certificate of Amendment to Certificate of Incorporation dated September 1, 2004 (incorporated by reference to Exhibit 3.3 to the Annual Report on Form 10-KSB, filed March 31, 2005) | |
| 4.1 | Form of Common Stock Certificate (incorporated by reference to Exhibit 4.2 to Registration Statement on Form SB-2, effective May 11, 2001 (Registration No. 333-55658), as amended) | |
| 10.1* | Executive Employment Agreement with Patrick W. Smith, dated July 1, 1998 (incorporated by reference to Exhibit 10.1 to Registration Statement on Form SB-2 effective May 11, 2001 (Registration No. 333-55658), as amended) | |
| 10.2* | Executive Employment Agreement with Thomas P. Smith, dated November 15, 2000 (incorporated by reference to Exhibit 10.2 to Registration Statement on Form SB-2 effective May 11, 2001 (Registration No. 333-55658), as amended) | |
| 10.3* | Executive Employment Agreement with Kathleen C. Hanrahan, dated November 15, 2000 (incorporated by reference to Exhibit 10.3 to Registration Statement on Form SB-2 effective May 11, 2001 (Registration No. 333-55658), as amended) | |
| 10.4* | Form of Indemnification Agreement between the Company and its directors (incorporated by reference to Exhibit 10.4 to Registration Statement on Form SB-2 effective May 11, 2001 (Registration No. 333-55658), as amended) | |
| 10.5* | Form of Indemnification Agreement between the Company and its officers (incorporated by reference to Exhibit 10.5 to Registration Statement on Form SB-2 effective May 11, 2001 (Registration No. 333-55658), as amended) | |
| 10.6* | 1999 Stock Option Plan (incorporated by reference to Exhibit 10.6 to Registration Statement on Form SB-2, effective May 11, 2001 (Registration No. 333-55658), as amended) | |
| 10.7* | 2001 Stock Option Plan (incorporated by reference to Exhibit 10.7 to Registration Statement on Form SB-2, effective May 11, 2001 (Registration No. 333-55658), as amended) | |
| 10.8 | Form of Sales Representative Agreement with respect to services by and between the Company and Sales Representatives (incorporated by reference to Exhibit 10.15 to the Annual Report on Form 10-KSB, filed March 18, 2002) | |
| 10.9* | Executive Employment Agreement with Douglas E. Klint, dated December 15, 2002 (incorporated by referenced to Exhibit 10.17 to the Annual Report on Form 10-KSB, filed March 14, 2003) | |
| 10.10 | Credit Agreement dated June 22, 2004, between the Company and Bank One (incorporated by reference to Exhibit 10.13 to the Annual Report on Form 10-KSB, filed March 31, 2005) | |
| 10.11* | Executive Employment Agreement with Daniel Behrendt, dated April 28, 2004 (incorporated by reference to Exhibit 10.14 to the Annual Report on Form 10-KSB, filed March 31, 2005) | |
| 10.12* | 2004 Stock Option Plan (incorporated by reference to Exhibit 10.15 to the Annual Report on Form 10-KSB, filed March 31, 2005) | |
| 10.13* | 2004 Outside Director Stock Option Plan, as amended. (incorporated by reference to exhibit 10.16 to the Annual Report on Form 10-KSB, filed March 31, 2005) | |
| 10.14 | Amendment to Credit Agreement dated as of October 31, 2006 between the Company and JP Morgan Chase Bank, N.A. (incorporated by reference to Exhibit 10.17 to Form 8-K, filed November 1, 2006) | |
| 10.15 | Agreement with Automation Tooling Systems Inc. for purchase of equipment (incorporated by reference to Exhibit 10.18 to the Quarterly Report on Form 10-Q, filed August 9, 2007) | |
| 10.16* | Executive Employment Agreement with Steven Mercier, dated February 11, 2008. (incorporated by reference to Exhibit 10.16 to Annual Report on Form 10-K, filed February 29, 2008) | |
| 10.17* | Executive Employment Agreement with Jas Dhillon, dated August 1, 2008 | |
| 14.1 | Code of Business Conduct and Ethics, as adopted by the Company’s Board of Directors (incorporated by reference to Exhibit 14.1 to the Annual Report on Form 10-KSB, filed March 31, 2005) | |
| 23.1 | Consent of Grant Thornton, LLP, independent registered public accounting firm | |
| 31.1 | Principal Executive Officer Certification pursuant to Rule 13a-14(a) or Rule 15d-14(a) | |
| 31.2 | Principal Financial Officer Certification pursuant to Rule 13a-14(a) or Rule 15d-14(a) | |
| 32 | Principal Executive Officer and Principal Financial Officer Certification pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| * | Management contract or compensatory plan or arrangement |
56
Table of Contents
SIGNATURES
In accordance with the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized.
TASER INTERNATIONAL, INC.
Date: March 16, 2009
| By: | /s/ PATRICK W. SMITH | |||
| Chief Executive Officer | ||||
Date: March 16 , 2009
| By: | /s/ DANIEL M. BEHRENDT | |||
| Chief Financial Officer (Principal Financial and Accounting Officer) | ||||
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Patrick W. Smith his or her true and lawful attorney-in-fact and agent, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with all exhibits thereto and other documents in connection therewith the Securities and Exchange Commission, granting unto said attorney-in-fact and agent full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully and to all intents and purposes as he or she might or could do in person hereby ratifying and confirming all that said attorney-in-fact and agent, or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
In accordance with the Exchange Act, this report has been signed by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Date | ||||
| /s/ PATRICK W. SMITH | Director | March 16, 2009 | ||
| Patrick W. Smith | ||||
| /s/ THOMAS P. SMITH | Director | March 16, 2009 | ||
| Thomas P. Smith | ||||
| /s/ MATTHEW R. MCBRADY | Director | March 16, 2009 | ||
| Matthew R. McBrady | ||||
| /s/ BRUCE R. CULVER | Director | March 16, 2009 | ||
| Bruce R. Culver | ||||
| /s/ JUDY MARTZ | Director | March 16, 2009 | ||
| Judy Martz | ||||
| /s/ MARK W. KROLL | Director | March 16, 2009 | ||
| Mark W. Kroll | ||||
| /s/ MICHAEL GARNREITER | Director | March 16, 2009 | ||
| Michael Garnreiter | ||||
| /s/ JOHN S. CALDWELL | Director | March 16, 2009 | ||
| John S. Caldwell | ||||
| /s/ RICHARD H. CARMONA | Director | March 16, 2009 | ||
| Richard H. Carmona |
57
Table of Contents
SCHEDULE II — VALUATION AND QUALIFYING ACCOUNTS
| Balance at | Charged to | Charged to | Balance at | |||||||||||||||||
| beginning of | costs and | other | end of | |||||||||||||||||
| Description | period | expenses | accounts | Deductions | period | |||||||||||||||
Allowance for doubtful accounts | ||||||||||||||||||||
| Year ended December 31, 2008 | $ | 189,977 | $ | 78,010 | $ | $ | (67,987 | ) | $ | 200,000 | ||||||||||
| Year ended December 31, 2007 | $ | 110,052 | $ | 80,835 | $ | — | $ | (910 | ) | $ | 189,977 | |||||||||
| Year ended December 31, 2006 | $ | 110,882 | $ | (830 | ) | $ | — | $ | — | $ | 110,052 | |||||||||
Allowance for excess and obsolete inventory | ||||||||||||||||||||
| Year ended December 31, 2008 | $ | 320,555 | $ | 640,655 | $ | — | $ | (831,310 | ) | $ | 129,900 | |||||||||
| Year ended December 31, 2007 | $ | 223,201 | $ | 206,335 | $ | — | $ | (108,981 | ) | $ | 320,555 | |||||||||
| Year ended December 31, 2006 | $ | 260,750 | $ | 85,329 | $ | — | $ | (122,878 | ) | $ | 223,201 | |||||||||
58
Table of Contents
| Exhibit | ||
| Number | Description | |
| 3.1 | Certificate of Incorporation, as amended (incorporated by reference to Exhibit 3.1 to Registration Statement on Form SB-2, effective May 11, 2001 (Registration No. 333-55658), as amended) | |
| 3.2 | Bylaws, as amended (incorporated by reference to Exhibit 3.2 to Registration Statement on Form SB-2, effective May 11, 2001 (Registration No. 333-55658), as amended) | |
| 3.3 | Certificate of Amendment to Certificate of Incorporation dated September 1, 2004 (incorporated by reference to Exhibit 3.3 to the Annual Report on Form 10-KSB, filed March 31, 2005) | |
| 4.1 | Form of Common Stock Certificate (incorporated by reference to Exhibit 4.2 to Registration Statement on Form SB-2, effective May 11, 2001 (Registration No. 333-55658), as amended) | |
| 10.1* | Executive Employment Agreement with Patrick W. Smith, dated July 1, 1998 (incorporated by reference to Exhibit 10.1 to Registration Statement on Form SB-2 effective May 11, 2001 (Registration No. 333-55658), as amended) | |
| 10.2* | Executive Employment Agreement with Thomas P. Smith, dated November 15, 2000 (incorporated by reference to Exhibit 10.2 to Registration Statement on Form SB-2 effective May 11, 2001 (Registration No. 333-55658), as amended) | |
| 10.3* | Executive Employment Agreement with Kathleen C. Hanrahan, dated November 15, 2000 (incorporated by reference to Exhibit 10.3 to Registration Statement on Form SB-2 effective May 11, 2001 (Registration No. 333-55658), as amended) | |
| 10.4* | Form of Indemnification Agreement between the Company and its directors (incorporated by reference to Exhibit 10.4 to Registration Statement on Form SB-2 effective May 11, 2001 (Registration No. 333-55658), as amended) | |
| 10.5* | Form of Indemnification Agreement between the Company and its officers (incorporated by reference to Exhibit 10.5 to Registration Statement on Form SB-2 effective May 11, 2001 (Registration No. 333-55658), as amended) | |
| 10.6* | 1999 Stock Option Plan (incorporated by reference to Exhibit 10.6 to Registration Statement on Form SB-2, effective May 11, 2001 (Registration No. 333-55658), as amended) | |
| 10.7* | 2001 Stock Option Plan (incorporated by reference to Exhibit 10.7 to Registration Statement on Form SB-2, effective May 11, 2001 (Registration No. 333-55658), as amended) | |
| 10.8 | Form of Sales Representative Agreement with respect to services by and between the Company and Sales Representatives (incorporated by reference to Exhibit 10.15 to the Annual Report on Form 10-KSB, filed March 18, 2002) | |
| 10.9* | Executive Employment Agreement with Douglas E. Klint, dated December 15, 2002 (incorporated by referenced to Exhibit 10.17 to the Annual Report on Form 10-KSB, filed March 14, 2003) | |
| 10.10 | Credit Agreement dated June 22, 2004, between the Company and Bank One (incorporated by reference to Exhibit 10.13 to the Annual Report on Form 10-KSB, filed March 31, 2005) | |
| 10.11* | Executive Employment Agreement with Daniel Behrendt, dated April 28, 2004 (incorporated by reference to Exhibit 10.14 to the Annual Report on Form 10-KSB, filed March 31, 2005) | |
| 10.12* | 2004 Stock Option Plan (incorporated by reference to Exhibit 10.15 to the Annual Report on Form 10-KSB, filed March 31, 2005) | |
| 10.13* | 2004 Outside Director Stock Option Plan, as amended. (incorporated by reference to exhibit 10.16 to the Annual Report on Form 10-KSB, filed March 31, 2005) | |
| 10.14 | Amendment to Credit Agreement dated as of October 31, 2006 between the Company and JP Morgan Chase Bank, N.A. (incorporated by reference to Exhibit 10.17 to Form 8-K, filed November 1, 2006) | |
| 10.15 | Agreement with Automation Tooling Systems Inc. for purchase of equipment (incorporated by reference to Exhibit 10.18 to the Quarterly Report on Form 10-Q, filed August 9, 2007) | |
| 10.16* | Executive Employment Agreement with Steven Mercier, dated February 11, 2008. (incorporated by reference to Exhibit 10.16 to Annual Report on Form 10-K, filed February 29, 2008) | |
| 10.17* | Executive Employment Agreement with Jas Dhillon, dated August 1, 2008 | |
| 14.1 | Code of Business Conduct and Ethics, as adopted by the Company’s Board of Directors (incorporated by reference to Exhibit 14.1 to the Annual Report on Form 10-KSB, filed March 31, 2005) | |
| 23.1 | Consent of Grant Thornton, LLP, independent registered public accounting firm | |
| 31.1 | Principal Executive Officer Certification pursuant to Rule 13a-14(a) or Rule 15d-14(a) | |
| 31.2 | Principal Financial Officer Certification pursuant to Rule 13a-14(a) or Rule 15d-14(a) | |
| 32 | Principal Executive Officer and Principal Financial Officer Certification pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
59