As filed with the Securities and Exchange Commission on April 10,2006 Registration No. 333-126687
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
___________________
FORM SB-2
POST-EFFECTIVE AMENDMENT NO. 2
TO
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
___________________
PharmaFrontiers Corp.
(Name of small business issuer on its charter)
Texas | 2834 | 76-0333165 |
| (State or Other Jurisdiction of Incorporation or Organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification Number) |
2635 N. Crescent Ridge Drive
The Woodlands, Texas 77381
(281) 272-9331
(Address and telephone number
of principal executive offices and principal place of business)
___________________
C. William Rouse
2635 N. Crescent Ridge Drive
The Woodlands, Texas 77381
(281) 272-9331
(Name, address and telephone number
of agent for service)
___________________
Copy to:
Michael C. Blaney
Vinson & Elkins L.L.P.
1001 Fannin, Suite 2300
Houston, TX 77002
(713) 758-2222
___________________
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box: x
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering: o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering: o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering: o
If delivery of the prospectus is expected to be made pursuant to Rule 434, check the following box: o

PharmaFrontiers Corp.
25,217,237 Shares of Common Stock
This prospectus relates to the resale from time to time by the selling stockholders of up to 25,217,237 shares of our common stock, including 12,723,562 shares of common stock previously issued and 12,493,678 shares of common stock issuable upon the exercise of common stock purchase warrants. A series of warrants underlying 10,411,400 shares of common stock expired on February 17, 2006. The selling stockholders may sell the shares of common stock from time to time in the principal market on which the stock is traded at the prevailing market price or in negotiated transactions.
Shares of our common stock are traded on the NASD OTC Bulletin Board under the symbol “PFTR.OB.” April 6, 2006, the last reported sales price for our common stock on the OTC Bulletin Board was $0.56 per share.
We will not receive any proceeds from the sale of the shares of our common stock covered by this prospectus.
___________________________________
Investing in our common stock involves a high degree of risk. You should read carefully this entire prospectus, including the section captioned “Risk Factors” beginning on page 3, before making a decision to purchase our stock.
___________________________________
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is April 10, 2006.
TABLE OF CONTENTS
Page | |
| PROSPECTUS SUMMARY | 1 |
| RISK FACTORS | 3 |
| FORWARD LOOKING STATEMENTS | 9 |
| USE OF PROCEEDS | 10 |
| PRICE RANGE OF OUR COMMON STOCK AND DIVIDEND POLICY | 10 |
| SELECTED HISTORICAL FINANCIAL DATA | 11 |
| MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITIONS AND RESULTS OF OPERATIONS | 11 |
| OUR BUSINESS | 16 |
| MANAGEMENT | 30 |
| EXECUTIVE COMPENSATION | 34 |
| SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT | 36 |
| SELLING STOCKHOLDERS | 38 |
| CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS | 61 |
| DESCRIPTION OF SECURITIES | 61 |
| PLAN OF DISTRIBUTION | 63 |
| LEGAL MATTERS | 65 |
| EXPERTS | 65 |
| WHERE YOU CAN FIND MORE INFORMATION | 65 |
| INDEX TO FINANCIAL STATEMENTS | 67 |
| INFORMATION NOT REQUIRED IN PROSPECTUS | 69 |
You should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with information that is different. This prospectus may only be used where it is legal to sell these shares of our common stock. The information in this prospectus may only be accurate as of the date of this prospectus.
This prospectus provides you with a general description of the shares of our common stock that the selling stockholders may offer. Each time a selling stockholder sells shares of our common stock, the selling stockholder is required to provide you with a prospectus containing specific information about the selling stockholder and the terms of the shares of our common stock being offered to you.
This prospectus is part of a registration statement that we filed with the Securities and Exchange Commission for a continuous offering. Under this prospectus, the selling stockholders may, from time to time, sell the shares of our common stock described in this prospectus in one or more offerings. This prospectus may be supplemented from time to time to add, update or change information in this prospectus. Any statement contained in this prospectus will be deemed to be modified or superseded for the purposes of this prospectus to the extent that a statement contained in a prospectus supplement modifies such statement. Any statement so modified will be deemed to constitute a part of this prospectus only as so modified, and any statement so modified will be deemed to constitute a part of this prospectus.
The registration statement containing this prospectus, including the exhibits to the registration statement, provides additional information about us, the selling stockholders and the shares of our common stock offered under this prospectus. The registration statement, including the exhibits, can be read on the SEC website or at the SEC offices mentioned under the heading “Where You Can Find More Information.”
-i-
PROSPECTUS SUMMARY
The following summary highlights selected information from this prospectus and does not contain all of the information that you should consider before investing in our common stock. This prospectus contains information regarding our businesses and detailed financial information. You should carefully read this entire prospectus, including the historical financial statements and related notes, before making an investment decision.
In this prospectus, “PharmaFrontiers Corp.,” the “company,” “we,” “us” or “our” refer to PharmaFrontiers Corp., a Texas corporation, and its subsidiaries, except where otherwise indicated or required by context.
Our Business
We are a biopharmaceutical company engaged in developing autologous personalized cell therapies. Our strategy is to develop and commercialize cell therapies to treat several major diseases including multiple sclerosis, cardiovascular diseases, and diabetes. We have an exclusive license to an individualized T cell therapy that is in FDA Phase I/II human dose ranging clinical trials to evaluate its safety and effectiveness in treating multiple sclerosis. We have an exclusive worldwide license for the intellectual property rights and research results of an autologous T cell vaccine for rheumatoid arthritis from the Shanghai Institutes for Biological Sciences (SIBS), Chinese Academy of Sciences of the People's Republic of China. We also have an exclusive license to a stem cell technology in which adult pluripotent stem cells are derived from monocytes obtained from the patient’s own blood. We are initially pursuing indications in heart failure and Type I diabetes with our stem cell therapy.
Autologous therapies use cells or other materials from the patient’s own body to create treatments for the patient, thus preventing rejection complications that result when “foreign” or “non-self” cells are introduced into a patient. Cellular therapies are expected to play a large role in the treatment and cure of a broad spectrum of human diseases. According to independent market researchers, cellular therapies along with their related technologies, such as diagnostics and blood banking, may exceed $30 billion by 2010.
Our multiple sclerosis cell therapy, Tovaxin™, is currently in Phase I/II studies. Tovaxin™ consists of modified autoreactive T cells. Multiple sclerosis is a result of a person’s own T cells attacking the myelin sheath that coats the nerve cells of the central nervous system. These T cells, that attack a person’s own body, are referred to as “autoreactive” T cells. In our treatment the T cells are taken from the patient, modified and returned to the patient. The modified T cells cause an immune response directed at the autoreactive T cells in the patient’s body. This immune response reduces the level of autoreactive T cells and potentially allows the myelin sheath to be repaired. In addition, we are evaluating whether this technology will allow us to diagnose multiple sclerosis and determine the severity of the disease through an analysis of the level of autoreactive T cells in a patient’s blood.
Two clinical studies of Tovaxin™ have reached critical milestones:
| · | The dose escalation study was designed for patients with relapsing-remitting or secondary-progressive MS, intolerant of, or having failed, current therapy. Blood was obtained from each patient from which T cells reactive to two peptides each of three proteins (MBP, PLP, and MOG) were expanded ex vivo and prepared as a trivalent formulation of MRTCs. The MRTCs were attenuated by Cesium137 irradiation prior to patients receiving subcutaneous injections of either 6-9 million cells (Dose 1) or 30-45 million cells (Dose 2) at weeks 0, 4, 12 and 20. MRTC frequencies were performed at baseline and weeks 5, 13, 21, 28 and 52. Patients were evaluated for changes in EDSS, MSIS and exacerbations. |
-1-
Tovaxin is a patient-specific therapeutic vaccination strategy for MS patients. To formulate Tovaxin T cell vaccine, the patient's own myelin peptide-specific activated T cell lines are harvested and attenuated on the day of vaccine administration.
The study's results demonstrated that MRTCs in the peripheral blood were depleted in a dose dependent manner and analyses showed reductions in all three types of MRTCs at all follow-up visits. All patients in the Dose 2 group had a 100% reduction in MRTC counts at the week five follow-up visit. Percentage reductions were greater in the Dose 2 group than in the Dose 1 group at every follow-up visit. Correlation between the reduction in overall MRTC frequencies and the physical component of the MSIS (p=0.0086) was strong. There was a trend to improved EDSS (p=0.0561). The annual relapse rate (ARR) for the patients prior two years before therapy was 1.28 and following therapy the ARR was 0.10 (92 percent reduction) adjusted for the number of months in the study. The treatment appears to be safe and well tolerated with minimal adverse events and no dose-limiting toxicities.
| · | Phase I/II extension study: The analysis of data on ten (10) patients that have been enrolled in a Phase I/II open-label extension study of Tovaxin(TM) T-Cell vaccine in worsening multiple sclerosis indicates that the treatment is safe and well-tolerated. Adverse events were mild or moderate in severity. None of the ten patients reported an MS exacerbation while on study. Analysis of myelin-reactive T-cell (MRTC) counts showed a percentage reduction from baseline at 3, 6, and 9 months, for all three types of MRTC, as well as the Total MRTC. Reductions in disease assessment disability scores were observed at all follow-up visits. No therapy induced lesions were observed on week 52 MRI's for three patients. These results suggest that MRTC vaccination is safe and well tolerated and also suggest that MRTC vaccination reduces MRTC counts, as well as EDSS and MSIS scores. |
In October 2005, the FDA approved the protocol for our Phase IIb clinical trial of Tovaxin. We intend to enroll the first patient in this pivotal Phase IIb in the first half of 2006.
Our Rheumatoid Arthritis (RA) T-cell vaccination (TCV) technology is conceptually similar to Tovaxin. RA is an autoimmune T-cell-mediated disease in which Pathogenic T-cells trigger an inflammatory autoimmune response of the synovial joints of the wrists, shoulders, knees, ankles and feet which causes pain, stiffness, and swelling around the joints. Our RA TCV technology allows the isolation of these pathogenic T-cells from synovial fluid drawn from a patient. We expand and modify these T-cells in our laboratory. The modified T-cells are injected subcutaneously into patients thereby inducing an immune response directed at the Pathogenic T-cells in the patient’s body. This immune response reduces the level of Pathogenic T-cells and potentially allows the reduction of joint swelling in RA patients. Human trials that have been conducted in China show minimal side-effects and promising efficacy measured as a reduction of joint swelling following the T-cell vaccination.
Our stem cell technology allows us to create adult pluripotent stem cells from monocytes isolated from blood drawn from the patient. We believe that these stem cells, if successfully developed, may provide the basis for therapies to treat a variety of diseases and conditions. We anticipate that our stem cell technology will have a significant competitive advantage over many of the other stem cell technologies. The peripheral blood monocytes, used by our technology to produce stem cells, have the advantage of being relatively abundant and easy and cost effective to obtain. Our technology does not have the collection and storage difficulties presented by umbilical cord blood or the controversial ethical and regulatory issues associated with embryonic stem cells. In addition, our technology is less difficult and less risky than collecting adult stem cells from tissues such as bone marrow, spinal fluid or adipose (fat) tissue. Furthermore, our stem cells are pluripotent, whereas adult stem cells used in competitive technologies are not likely to be pluripotent.
-2-
Our stem cell technology will also avoid rejection issues because it is autologous (“self”). This is as opposed to the embryonic, umbilical, and some adult stem cell technologies, which in some cases must be taken from one individual and given to another. Further, we believe our stem cell therapies will be regulated as autologous “manipulated” non-homologous use cell therapies. Thus, we use one’s own stem cells, and we therefore do not expect to encounter the same significant pre-clinical and clinical development regulatory hurdles that embryonic, umbilical, and some adult stem cells therapies are expected to face.
Initially we are conducting pre-clinical research to develop stem cell therapies to treat Type I diabetes and heart failure. We believe that with our stem cell technology plus our additional technology related to the differentiation of stem cells into islet cells, we will be able to create insulin producing islet cells derived from the patient’s own blood. We are currently conducting laboratory research and plan to move expeditiously through pre-clinical development of our diabetes stem cell therapy and, if successful, initiate human testing in 2006.
Our Executive Offices
Our principal executive and administrative office facility is located in The Woodlands, Texas at 2635 N. Crescent Ridge Drive, The Woodlands, Texas 77381 and our telephone number is (281) 272-9331. We maintain a website at www.pharmafrontierscorp.com, however the information on our website is not part of this prospectus, and you should rely only on information contained in this prospectus when making a decision as to whether or not to invest in shares of our common stock.
RISK FACTORS
The shares offered hereby have not been approved or disapproved by the SEC or the securities regulatory authority of any state, nor has any such regulatory body reviewed this memorandum for accuracy or completeness. The shares offered hereby are speculative, involve an unusually high degree of risk and should only be purchased by those who can afford to lose their entire investment. Therefore, prospective investors should carefully consider the following risk factors before purchasing the shares offered hereby.
The following factors affect our business and the industry in which we operate. The risks and uncertainties described below are not the only ones that we face. Additional risks and uncertainties not presently known or that we currently consider immaterial may also have an adverse effect on our business. If any of the matters discussed in the following risk factors were to occur, our business, financial condition, results of operations, cash flows, or prospects could be materially adversely affected.
Risks Related to Our Business
Our business is at an early stage of development.
Our business is at an early stage of development. We do not have any products in late-stage clinical trials or on the market. We are still in the early stages of identifying and conducting research on potential products. Only one of our products has progressed to the stage of being studied in human clinical trials. Our potential products will require significant research and development and preclinical and clinical testing prior to regulatory approval in the United States and other countries. We may not be able to develop any products, to obtain regulatory approvals, to enter clinical trials for any of our product candidates, or to commercialize any products. Our product candidates may prove to have undesirable and unintended side effects or other characteristics adversely
-3-
affecting their safety, efficacy or cost-effectiveness that could prevent or limit their use. Any product using any of our technology may fail to provide the intended therapeutic benefits, or achieve therapeutic benefits equal to or better than the standard of treatment at the time of testing or production.
We have a history of operating losses and do not expect to be profitable in the near future.
We have not generated any profits since our entry into the biotechnology business, have no source of revenues, and have incurred significant operating losses. We expect to incur additional operating losses for the foreseeable future and, as we increase our research and development activities, we expect our operating losses to increase significantly. We do not have any sources of revenues and may not have any in the foreseeable future.
We will need additional capital to conduct our operations and develop our products and our ability to obtain the necessary funding is uncertain.
We need to obtain significant additional capital resources from sources including equity and/or debt financings, license arrangements, grants and/or collaborative research arrangements in order to develop products and continue our business. As of December 31, 2005, we had cash and cash equivalents of approximately $2.5 million. Our current burn rate is approximately $400,000 per month excluding capital expenditures. However, this burn rate is expected to increase to $800,000 per month once the IIb clinical trails begin. We will need to raise additional capital to fund our working capital needs during the second quarter of 2006. We do not have any credit facilities available with financial institutions or any other third parties and as such we must rely upon best efforts third-party funding and we can provide no assurance that we will be successful in any future investment best efforts. The failure to raise such funds will necessitate the curtailment of operations and delay of the start of the clinical trials. The timing and degree of any future capital requirements will depend on many factors, including:
| · | the accuracy of the assumptions underlying our estimates for capital needs in 2005 and beyond; |
| · | scientific progress in our research and development programs; |
| · | the magnitude and scope of our research and development programs; |
| · | our ability to establish, enforce and maintain strategic arrangements for research, development, clinical testing, manufacturing and marketing; |
| · | our progress with preclinical development and clinical trials; |
| · | the time and costs involved in obtaining regulatory approvals; |
| · | the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims; and |
| · | the number and type of product candidates that we pursue. |
We do not have any committed sources of capital, although we have issued and outstanding warrants that, if exercised, would result in an equity capital raising transaction. Additional financing through strategic collaborations, public or private equity financings, capital lease transactions or other financing sources may not be available on acceptable terms, or at all. Additional equity financings could result in significant dilution to our stockholders. Further, if additional funds are obtained through arrangements with collaborative partners, these arrangements may require us to relinquish rights to some of our technologies, product candidates or products that we would otherwise seek to develop and commercialize ourselves. If sufficient capital is not available, we may be required to delay, reduce the scope of or eliminate one or more of our programs, any of which could have a material adverse effect on our financial condition or business prospects.
-4-
Approximately 88% of our total assets are comprised of intangible assets that are subject to review on a periodic basis to determine whether an impairment on these assets is required. An impairment would not only greatly diminish our assets, but would also require us to record a significant non-cash expense charge.
We are required under generally accepted accounting principles to review our intangible assets for impairment when events or changes in circumstances indicate the carrying value may not be recoverable. Goodwill is required to be tested for impairment at least annually. At December 31, 2005, our intangible assets, consisting of the University of Chicago license and acquired intangible assets from the Opexa acquisition that is an inseparable group of patents and licenses that can’t function independently, were approximately $26.1 million. If management determines that impairment exists, we will be required to record a significant charge to expense in our financial statements during the period in which any impairment of our goodwill is determined.
Clinical trials are subject to extensive regulatory requirements, very expensive, time-consuming and difficult to design and implement. Our products may fail to achieve necessary safety and efficacy endpoints during clinical trials.
Human clinical trials are very expensive and difficult to design and implement, in part because they are subject to rigorous regulatory requirements. The clinical trial process is also time consuming. We estimate that clinical trials of our product candidates will take at least several years to complete. Furthermore, failure can occur at any stage of the trials, and we could encounter problems that cause us to abandon or repeat clinical trials. The commencement and completion of clinical trials may be delayed by several factors, including:
| · | unforeseen safety issues; |
| · | determination of dosing issues; |
| · | lack of effectiveness during clinical trials; |
| · | slower than expected rates of patient recruitment; |
| · | inability to monitor patients adequately during or after treatment; and |
| · | inability or unwillingness of medical investigators to follow our clinical protocols. |
In addition, we or the FDA may suspend our clinical trials at any time if it appears that we are exposing participants to unacceptable health risks or if the FDA finds deficiencies in our IND submissions or the conduct of these trials.
We are dependent upon our management team and a small number of employees.
Our business strategy is dependent upon the skills and knowledge of our management team. We believe that the special knowledge of these individuals gives us a competitive advantage. If any critical employee leaves, we may be unable on a timely basis to hire suitable replacements to effectively operate our business. We also operate with a very small number of employees and thus have little or no backup capability for their activities. The loss of the services of any member of our management team or the loss of a number of other employees could have a material adverse effect on our business.
-5-
We are dependent on contract research organizations and other contractors for clinical testing and for certain research and development activities, thus the timing and adequacy of our clinical trials and such research activities are, to a certain extent, beyond our control.
The nature of clinical trials and our business strategy requires us to rely on contract research organizations, independent clinical investigators and other third party service providers to assist us with clinical testing and certain research and development activities. As a result, our success is dependent upon the success of these outside parties in performing their responsibilities. Although we believe our contractors are economically motivated to perform on their contractual obligations, we cannot directly control the adequacy and timeliness of the resources and expertise applied to these activities by our contractors. If our contractors do not perform their activities in an adequate or timely manner, the development and commercialization of our drug candidates could be delayed.
Our current research and manufacturing facility is not large enough to manufacture future stem cell and T-cell therapies.
We conduct our research and development in a 10,000 square foot facility in The Woodlands, Texas, which includes a 1,200 square foot suite of three rooms for the future manufacture of stem cell and T-cell therapies through Phase III trials. Our current facility is not large enough to conduct commercial-scale manufacturing operations. We will need to expand further our manufacturing staff and facility, obtain a new facility or contract with corporate collaborators or other third parties to assist with future drug production.
In the event that we decide to establish a commercial-scale manufacturing facility, we will require substantial additional funds and will be required to hire and train significant numbers of employees and comply with applicable regulations, which are extensive. We do not have funds available for building a manufacturing facility, and we may not be able to build a manufacturing facility that both meets regulatory requirements and is sufficient for our commercial-scale manufacturing.
We may arrange with third parties for the manufacture of our future products. However, our third-party sourcing strategy may not result in a cost-effective means for manufacturing our future products. If we employ third-party manufacturers, we will not control many aspects of the manufacturing process, including compliance by these third parties with the FDA’s current Good Manufacturing Practices and other regulatory requirements. We further may not be able to obtain adequate supplies from third-party manufacturers in a timely fashion for development or commercialization purposes, and commercial quantities of products may not be available from contract manufacturers at acceptable costs.
Patents obtained by other persons may result in infringement claims against us that are costly to defend and which may limit our ability to use the disputed technologies and prevent us from pursuing research and development or commercialization of potential products.
A number of pharmaceutical, biotechnology and other companies, universities and research institutions have filed patent applications or have been issued patents relating to cell therapy, stem cells, T-cells, and other technologies potentially relevant to or required by our expected products. We cannot predict which, if any, of such applications will issue as patents or the claims that might be allowed. We are aware that a number of companies have filed applications relating to stem cells. We are also aware of a number of patent applications and patents claiming use of stem cells and other modified cells to treat disease, disorder or injury.
-6-
If third party patents or patent applications contain claims infringed by either our licensed technology or other technology required to make and use our potential products and such claims are ultimately determined to be valid, there can be no assurance that we would be able to obtain licenses to these patents at a reasonable cost, if at all, or be able to develop or obtain alternative technology. If we are unable to obtain such licenses at a reasonable cost, we may not be able to develop some products commercially. There can be no assurance that we will not be obliged to defend ourselves in court against allegations of infringement of third party patents. Patent litigation is very expensive and could consume substantial resources and create significant uncertainties. An adverse outcome in such a suit could subject us to significant liabilities to third parties, require disputed rights to be licensed from third parties, or require us to cease using such technology.
If we are unable to obtain future patents and other proprietary rights our operations will be significantly harmed.
Our ability to compete effectively is dependent in part upon obtaining patent protection relating to our technologies. The patent positions of pharmaceutical and biotechnology companies, including ours, are uncertain and involve complex and evolving legal and factual questions. The coverage sought in a patent application can be denied or significantly reduced before or after the patent is issued. Consequently, we do not know whether the patent applications for our technology will result in the issuance of patents, or if any future patents will provide significant protection or commercial advantage or will be circumvented by others. Since patent applications are secret until the applications are published (usually eighteen months after the earliest effective filing date), and since publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain that the inventors of our licensed patents were the first to make the inventions covered by the patent applications or that the licensed patent applications were the first to be filed for such inventions. There can be no assurance that patents will issue from the patent applications or, if issued, that such patents will be of commercial benefit to us, afford us adequate protection from competing products, or not be challenged or declared invalid.
Our competition includes fully integrated biopharmaceutical and pharmaceutical companies that have significant advantages over us.
The markets for therapeutic stem cell products, multiple sclerosis products, and rheumatoid arthritis products are highly competitive. We expect that our most significant competitors are fully integrated pharmaceutical companies and more established biotechnology companies. These companies are developing stem cell-based products and they have significantly greater capital resources and expertise in research and development, manufacturing, testing, obtaining regulatory approvals, and marketing than we currently do. Many of these potential competitors are further along in the process of product development and also operate large, company-funded research and development programs. As a result, our competitors may develop more competitive or affordable products, or achieve earlier patent protection or product commercialization than we are able to achieve. Competitive products may render any products or product candidates that we develop obsolete.
If we fail to meet our obligations under our license agreements, we may lose our rights to key technologies on which our business depends.
Our business depends on three licenses from third parties. Additionally, any business relating to a T cell vaccine for rheumatoid arthritis depends upon a license from the Shanghai Institute for Biological Science. These third party license agreements impose obligations on us, such as payment obligations and obligations to diligently pursue development of commercial products under the licensed patents. If a licensor believes that we have failed to meet our obligations under a l icense agreement, the licensor could
-7-
seek to limit or terminate our license rights, which could lead to costly and time-consuming litigation and, potentially, a loss of the licensed rights. During the period of any such litigation, our ability to carry out the development and commercialization of potential products could be significantly and negatively affected. If our license rights were restricted or ultimately lost, our ability to continue our business based on the affected technology platform could be severely adversely affected.
Restrictive and extensive government regulation could slow or hinder our production of a cellular product.
The research and development of stem cell therapies is subject to and restricted by extensive regulation by governmental authorities in the United States and other countries. The process of obtaining U.S. Food and Drug Administration, or FDA, and other necessary regulatory approvals is lengthy, expensive and uncertain. We may fail to obtain the necessary approvals to continue our research and development, which would hinder our ability to manufacture or market any future product.
To be successful, our product candidates must be accepted by the health care community, which can be very slow to adopt or unreceptive to new technologies and products.
Our product candidates, if approved for marketing, may not achieve market acceptance since hospitals, physicians, patients or the medical community in general may decide to not accept and utilize these products. The product candidates that we are attempting to develop represent substantial departures from established treatment methods and will compete with a number of more conventional drugs and therapies manufactured and marketed by major pharmaceutical companies. The degree of market acceptance of any of our developed products will depend on a number of factors, including:
| · | our establishment and demonstration to the medical community of the clinical efficacy and safety of our product candidates; |
| · | our ability to create products that are superior to alternatives currently on the market; |
| · | our ability to establish in the medical community the potential advantage of our treatments over alternative treatment methods; and |
| · | reimbursement policies of government and third-party payers. |
If the health care community does not accept our products for any of the foregoing reasons, or for any other reason, our business would be materially harmed.
Risks Related to Our Common Stock
There is currently a limited market for our common stock, and any trading market that exists in our common stock may be highly illiquid and may not reflect the underlying value of the Company’s net assets or business prospects.
Although our common stock is currently traded on the OTC Bulletin Board, there is currently a limited market for our common stock and there can be no assurance that an improved market will ever develop. Investors are cautioned not to rely on the possibility that an active trading market may develop.
As our share price is volatile, we may be or become the target of securities litigation, which is costly and time-consuming to defend.
-8-
In the past, following periods of market volatility in the price of a company’s securities or the reporting of unfavorable news, security holders have often instituted class action litigation. If the market value of our common stock experiences adverse fluctuations and we become involved in this type of litigation, regardless of the outcome, we could incur substantial legal costs and our management’s attention could be diverted from the operation of our business, causing our business to suffer.
Our "blank check" preferred stock could be issued to prevent a business combination not desired by management or our current majority shareholders.
Our articles of incorporation authorize the issuance of "blank check" preferred stock with such designations, rights and preferences as may be determined by our board of directors without shareholder approval. Our preferred stock could be utilized as a method of discouraging, delaying, or preventing a change in our control and as a method of preventing shareholders from receiving a premium for their shares in connection with a change of control.
Future sales of our common stock in the public market could lower our stock price.
We may sell additional shares of common stock in subsequent public or private offerings. We may also issue additional shares of common stock to finance future acquisitions. We cannot predict the size of future issuances of our common stock or the effect, if any, that future issuances and sales of shares of our common stock will have on the market price of our common stock. Sales of substantial amounts of our common stock (including shares issued in connection with an acquisition), or the perception that such sales could occur, may adversely affect prevailing market prices for our common stock.
We presently do not intend to pay cash dividends on our common stock.
We currently anticipate that no cash dividends will be paid on the common stock in the foreseeable future. While our dividend policy will be based on the operating results and capital needs of the business, it is anticipated that all earnings, if any, will be retained to finance the future expansion of the our business. Therefore, prospective investors who anticipate the need for immediate income by way of cash dividends from their investment should not purchase the shares offered in this offering.
FORWARD LOOKING STATEMENTS
This Prospectus contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933. These statements relate to future events and/or future financial performance and involve known and unknown risks, uncertainties and other factors that may cause the actual results, levels of activity, performance or achievements of the Company or the industry in which it operates to be materially different from any future results, levels of activity, performance or achievements expressed or implied by the forward-looking statements. These risks and other factors include those listed under “Risk Factors” and those described elsewhere in this Memorandum.
In some cases, you can identify forward-looking statements by the Company’s use of terms such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” or the negative of these terms or other comparable terminology. These statements are only predictions. Actual events or results may differ materially. In evaluating these statements, you should specifically consider various factors, including the risks outlined under “Risk Factors.” These factors may cause the Company’s actual results to differ materially from any forward-looking statement.
Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, it cannot guarantee future results, levels of activity, performance, or achievements. Moreover, neither the Company nor any other person assumes responsibility for the accuracy and completeness of these forward-looking statements. The Company does not intend to update any of the forward-looking statements after the date of this Memorandum to conform prior statements to actual results.
-9-
USE OF PROCEEDS
The selling stockholders will receive all of the proceeds from any sales of shares of our common stock. We will not receive any of the proceeds from any such sale by any selling stockholder. See “Selling Stockholders.”
PRICE RANGE OF OUR COMMON STOCK AND DIVIDEND POLICY
Shares of our common stock are traded on the National Association of Securities Dealers Inc. Over the Counter Bulletin Board under the symbol “PFTR.OB”. Our Common Stock trades on a limited, sporadic and volatile basis. As of April 5, 2006, the last reported sales price of our common stock on the OTC Bulletin Board was $0.60. As of March 31, 2006, there were 20,967,035 shares of our common stock outstanding that were held of record by 566 persons.
The following table sets forth, for the periods indicated, the range of high and low bid information for our common stock. These quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission, and may not represent actual transactions.
Price Ranges | ||||||||||
High | Low | |||||||||
Fiscal Year Ended December 31, 2004 | ||||||||||
| First Quarter | 0.03 | 0.01 | ||||||||
| Second Quarter | 14.25 | 0.01 | ||||||||
| Third Quarter | 8.15 | 6.50 | ||||||||
| Fourth Quarter | 9.50 | 5.90 | ||||||||
Fiscal Year Ended December 31, 2005 | ||||||||||
| First Quarter | 8.70 | 4.50 | ||||||||
| Second Quarter | 5.50 | 2.46 | ||||||||
| Third Quarter | 1.41 | 1.25 | ||||||||
| Fourth Quarter | 0.63 | 0.59 | ||||||||
| Fiscal Year Ended December 31, 2006 | ||||||||||
| First Quarter | 0.62 | 0.55 | ||||||||
Holders of shares of common stock will be entitled to receive cash dividends when, as and if declared by our Board of Directors, out of funds legally available for payment thereof. However, if dividends are not declared by our Board of Directors, no dividends shall be paid. We have not paid any dividends on our common stock since our inception.
We do not anticipate that any cash dividends will be paid in the foreseeable future. While our dividend policy will be based on the operating results and capital needs of the business, we anticipate that all earnings, if any, will be retained to finance our future expansion. Therefore, prospective investors who anticipate the need for immediate income by way of cash dividends from their investment should not purchase the shares offered by this prospectus.
-10-
SELECTED HISTORICAL FINANCIAL DATA
The following selected consolidated financial data should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes included elsewhere in this prospectus. The selected consolidated financial data in this section is not intended to replace the consolidated financial statements and accompanying footnotes. The selected consolidated balance sheet data as of December 31, 2005 and 2004 have been derived from our audited consolidated financial statements included elsewhere in this prospectus. Historical results are not necessarily indicative of the results to be expected in the future.
Year Ended December 31, | Year Ended December 31, | ||||||
2005 | 2004 | ||||||
Consolidated Statements of Operations Data: | |||||||
| Revenues | $ | - | $ | - | |||
| Operating Expenses: | |||||||
General and administrative | $ | 550,178 | $ | 572,534 | |||
Depreciation and amortization | 1,735,209 | 264,819 | |||||
Research and development | 9,892,253 | 2,465,634 | |||||
Loss on disposal of assets | 22,810 | 457,122 | |||||
Net operating loss | (12,200,450 | ) | (3,760,109 | ) | |||
Interest Income | 81,930 | 5,992 | |||||
Other Income | 28,174 | 2,379 | |||||
Interest expense | (7,323,851 | ) | (868,926 | ) | |||
Net loss | $ | (19,414,197 | ) | $ | (4,620,664 | ) | |
Net loss per common share, basic and diluted | $ | (1.24 | ) | $ | (0.73 | ) | |
Weighted average number of common shares outstanding, basic and diluted | 15,648,365 | 6,309,145 | |||||
As of December 31, | |||||||
2005 | 2004 | ||||||
Consolidated Balance Sheet Data: | |||||||
Cash and cash equivalents and prepaid expenses | 2,743,190 | 946,329 | |||||
Intangible assets | 26,130, 441 | 26,791,073 | |||||
Fixed Assets | 479,996 | 341,984 | |||||
Other assets | 388,210 | - | |||||
Total assets | 29,741,837 | 28,079,386 | |||||
Current liabilities | 2,429,776 | 4,883,165 | |||||
Common stock | 1,030,977 | 502,992 | |||||
Total stockholders’ equity | 27,312,061 | 23,196,221 | |||||
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITIONS AND RESULTS OF OPERATIONS
The following discussion of our financial condition and results of operations should be read together with the consolidated financial statements and related notes that are included elsewhere in this prospectus. This discussion may contain forward-looking statements based upon current expectations that involve risks and uncertainties. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth under “Risk Factors”, “Disclosure Regarding Forward-Looking Statements” or in other parts of this prospectus. We undertake no obligation to update any information in our forward-looking statements except as required by law.
-11-
Overview
We are a development-stage company and have a limited operating history. Our predecessor company for financial reporting purposes was formed on January 22, 2003 to acquire rights to our adult stem cell technology. In November 2004 we acquired Opexa Pharmaceuticals, Inc. and its multiple sclerosis treatment technology. We are still developing all of our technology, and to date, we have not generated any revenues from our operations. As we continue to execute our operations plan, we expect our development and operating expenses to increase.
Research and development. We have made and expect to continue to make substantial investments in research and development in order to develop and market our technology. Research and development costs consist primarily of general and administrative and operating expenses related to research and development activities. We expense research and development costs as incurred. Property, plant and equipment for research and development that has an alternative future use is capitalized and the related depreciation is expensed as research and development costs. We expect our research and development expense to increase as we continue to invest in the development of our technology.
General and administrative. General and administrative expenses consist primarily of salaries and benefits, office expense, professional services fees, and other corporate overhead costs. We anticipate increases in general and administrative expenses as we continue to develop and prepare for commercialization of our technology
Results of Operations
Comparison of Year Ended December 31, 2005 with the Year Ended December 31, 2004
Net Sales.
We recorded no sales for the twelve months ended December 31, 2005 and 2004.
General and Administrative Expenses
Our general and administrative expenses during the twelve months ended December 31, 2005, was $550,178 as compared to $572,534 for the twelve months ended December 31, 2004. General and administrative expenses consist primarily of salaries and benefits, office expense, professional services fees, and other corporate overhead costs. We anticipate increases in general and administrative expenses as we continue to develop and prepare for commercialization of our technology
Research and Development Expense
Research and development expense was $9,892,253 for the twelve months ended December 31, 2005, as compared to $2,465,634 the twelve months ended December 31, 2004. The increase in expenses was primarily due the acquisition of Opexa and the assumption of its operations and research and development programs as well as our Phase I/II clinical trials for Tovaxin, stem cell development and pre-clinical costs, the hiring of personnel and other expenses associated with the increase in research and development efforts. We have made and expect to continue to make substantial investments in research and development in order to develop and market our technology. Research and development costs consist primarily of general and administrative and operating expenses related to research and development activities. We expense research and development costs as incurred. Property, plant and equipment for research and development that has an alternative future use is capitalized and the related depreciation is expensed as research and development costs. We expect our research and development expense to increase as we continue to invest in the development of our technology.
-12-
Interest Expense
Interest expense was $7,323,851 for the twelve months ended December 31, 2005 compared to $868,926 for the twelve months ended December 31, 2004. The increase is primarily related to the amortization of the remaining discount under the beneficial conversion feature of the 15% exchangeable convertible promissory notes (the “Notes”); in 2005 the accrued interest on the Notes was converted into shares of Common Stock.
Net loss
We had a net loss for the year ended December 31, 2005, of $19,414,197 or ($1.24) per share (basic and diluted), compared with a net loss of $4,620,664 or ($.73) per share (basic and diluted), for the twelve months ended December 31, 2004. The increase in net loss is due primarily to the amortization of the remaining discount under the beneficial conversion feature of the Notes and the accrued interest on the Notes that was converted into shares of common stock, along with start-up of operations which included the hiring of new employees, directors and scientific advisory board members. These individuals have agreements with us that provide for salary payments. The increase in net loss is also attributable to the acquisition of Opexa Pharmaceuticals and the assumption of its operations and research and development programs. Also included are professional fees incurred from legal, accounting, and consulting services to secure and expand our license patent claims. Anticipated future expenses include research and development, professional and consulting fees, and expenses associated with the expansion of the office and laboratory/manufacturing facilities.
Liquidity and Capital Resources
Since our inception, the Company has financed its operations from the sale of its debt and equity securities (including the issuance of its securities in exchange for goods and services) to accredited investors. Between September 2004 and February 2005, the Company privately placed an aggregate principal amount of $6.1 million of Bridge Notes. In June 3, 2005, the Company exchanged its Bridge Notes aggregating approximately $6.7 million in principal and interest for 4,433,598 units at a purchase price of $1.50 per unit; each unit comprised of one share of common stock and three separate types of warrants to purchase a total of 2.75 shares of common stock as follows: a series A warrant for 1.25 shares with an exercise price of $2.00 which expired on February 17, 2006; a series B warrant for one-half of a share with an exercise price of $2.90 which expires on October 17, 2006; and a Series C Warrant for one share with an exercise price of $4.00 that expires on May 25, 2010.
In June 2005, the Company completed a private placement of approximately $5.08 million to accredited investors by issuing 3,387,217 units at a purchase price of $1.50 per unit; each unit identical to those issued in the Bridge Note exchange. On July 18, 2005 the Company completed a follow-on private placement of approximately $760,000 to accredited investors and issued 507,292 additional units at a purchase price of $1.50 per unit.
As of December 31, 2005, the Company had cash of approximately $2.5 million. Our current burn rate is approximately $400,000 per month excluding capital expenditures. Although our burn rate is expected to increase to $800,000 per month once the Phase IIb clinical trails begin, we do not intend to start the Phase 2b until we have raised additional capital. We will need to raise additional capital to fund our working capital needs during the second quarter of 2006. We do not have any credit facilities available with financial institutions or any other third parties and as such we must rely upon best efforts third-party debt or equity funding and we can provide no assurance that we will be successful in any funding effort. The failure to raise such funds will necessitate the curtailment of operations and delay of the start of the clinical trials.
-13-
Contractual Commitments
In October 2005 the Company leased a facility to house its executive offices and research facilities for a term of ten years with two options for an additional five years each at the then prevailing market rate. The 10,200 sq. ft. facility is located on 3 acres at 2635 N. Crescent Ridge Drive in The Woodlands, TX. This location provides space for pipeline development through R & D; a specialized Flow Cytometry and Microscopy lab; support of clinical trials with GMP manufacturing Suites; Quality Systems management with Quality Control Laboratory, Regulatory Affairs, Quality Assurance; as well as administrative support space. There is 2,500 sq. ft. of space still available for future build-out. The facility including the property is leased for a term of ten years with two options for an additional five years each at the then prevailing market rate.
Off-Balance Sheet Arrangements
As of December 31, 2005, we had no off-balance sheet arrangements.
Related Party Transactions
For more information on these transactions, please read “Certain Relationships and Related Party Transactions,” in this prospectus.
Critical Accounting Policies
Our discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of these consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities and expenses. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. We believe the following critical accounting policies affect our most significant judgments and estimates used in preparation of our consolidated financial statements.
Reverse Acquisition. We treated the merger of PharmaFrontiers Corp. into Sportan as a reverse acquisition. Pursuant to the guidance in Appendix B of SEC Accounting Disclosure Rules and Practices Official Text, the “merger of a private operating company into a non-operating public shell corporation with nominal net assets typically results in the owners and management of the private company having actual or effective operating control of the combined company after the transaction, with the shareholders of the former public shell continuing only as passive investors. These transactions are considered by the staff to be capital transactions in substance, rather than business combinations. That is, the transaction is equivalent to the issuance of stock by the private company for the net monetary assets of the shell corporation, accompanied by a recapitalization.” Accordingly, the reverse acquisition has been accounted for as a recapitalization. For accounting purposes, the original PharmaFrontiers Corp. is considered the acquirer in the reverse acquisition. The historical financial statements are those of the original PharmaFrontiers Corp. Earnings per share for periods prior to the merger are restated to reflect the number of equivalent shares received by the acquiring company.
-14-
Impairment of Long-Lived Assets. We review long-lived assets and certain identifiable assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. If the sum of the expected future undiscounted cash flows is less than the carrying amount of the asset, further impairment analysis is performed. An impairment loss is measured as the amount by which the carrying amount exceeds the fair value of assets.
Stock-Based Compensation. We have adopted FAS No. 123, “Accounting for Stock-Based Compensation” for non-cash stock-based compensation issued to employees, directors and non-employees for goods or services. FAS No. 123 allows companies to continue to measure compensation costs for employees and directors using the intrinsic value method as prescribed by APB Opinion 25; however, the fair value method must be used to measure compensation costs issued to non-employees. FAS No. 123 states that the fair value method of accounting for stock-based compensation is preferable to the intrinsic value method; therefore, we use the fair value method to measure all stock-based compensation, including stock-based compensation to our employees and directors. Under this method, compensation cost is measured at the fair value of the award on the applicable measurement date. See Note 1 of the Notes to Consolidated Financial Statements for the pro forma net income and net income per share amounts, for Fiscal 2004 through Fiscal 2005, as if the Company had used a fair-value-based method similar to the methods required under SFAS 123R to measure compensation expense for employee stock incentive awards. The Company is evaluating the terms and structure of its current share based payments and does not expect the adoption to have a significant, adverse impact on the consolidated statements of income and net income per share as it relates to current granted options and warrants as of the date of the adoption.
Consolidation of Variable Interest Entities. In January 2003, the FASB issued Interpretation No. 46(R) (“FIN 46”), Consolidation of Variable Interest Entities. FIN 46 addresses consolidation by business enterprises of variable interest entities (formerly special purpose entities). In general, a variable interest entity is a corporation, partnership, trust or any other legal structure used for business purposes that either (a) does not have equity investors with voting rights or (b) has equity investors that do not provide sufficient financial resources for the entity to support its activities. The objective of FIN 46 is not to restrict the use of variable interest entities, but to improve financial reporting by companies involved with variable interest entities. FIN 46 requires a variable interest entity to be consolidated by a company if that company is subject to a majority of the risk of loss from the variable interest entity’s activities or entitled to receive a majority of the entity’s residual returns or both. The consolidation requirements are effective for the first period that ends after March 15, 2004; the Company elected to adopt the requirements effective for the reporting period ending December 31, 2005. The adoption of FIN 46 had no effect on the consolidated financial statements.
Research and Development. The costs of materials and equipment or facilities that are acquired or constructed for research and development activities and that have alternative future uses are capitalized as tangible assets when acquired or constructed. The cost of such materials consumed in research and development activities and the depreciation of such equipment or facilities used in those activities are research and development costs. However, the costs of materials, equipment, or facilities acquired or constructed for research and development activities that have no alternative future uses are considered research and development costs and are expensed at the time the costs are incurred.
-15-
OUR BUSINESS
Overview
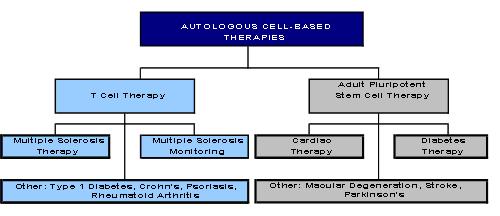
We are a biopharmaceutical company engaged in developing autologous personalized cell therapies. Our strategy is to develop and commercialize cell therapies to treat several major diseases including multiple sclerosis, cardiovascular diseases, and diabetes. We have an exclusive license to an individualized T cell therapy that is in FDA Phase I/II human dose ranging clinical trials to evaluate its safety and effectiveness in treating multiple sclerosis. We also have an exclusive license to a stem cell technology in which adult pluripotent stem cells are derived from monocytes obtained from the patient’s own blood. We are initially pursuing indications in heart failure and Type I diabetes with our stem cell therapy.
Our lead product, Tovaxin™, is a T-cell-based therapeutic vaccine for MS, offering a unique and personalized approach to treating the disease by inducing an immune response against the pathogenic myelin autoreactive T-cells. Tovaxin has just been accepted by the U.S. Food and Drug Administration’s (FDA) Center for Biologics Evaluation and Research (CBER) for a Phase IIb clinical study, entitled “A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study of Subcutaneous Tovaxin™ in Subjects with Clinically Isolated Syndrome or Relapsing Remitting Multiple Sclerosis” following two successful Phase I/II open-label studies. The results from two Tovaxin Phase I/II clinical trials provided safety and effectiveness information. Human trials have shown that the our T-cell vaccination (TCV) safely induces immune responses that deplete and regulate myelin autoreactive T-cells, thus stabilizing the disease and it is the first MS drug to demonstrate sustained improvement in many of the patients and sustained reversal of disability in some of the patients that were treated. Moreover, we are evaluating T-cell assay technology, which can be used to monitor T-cell therapy and may have the potential for early diagnosis of MS.
We also hold the exclusive worldwide license to adult pluripotent stem cells derived from peripheral blood monocytes that allow for the isolation, propagation, and differentiation into cells and tissues for patient-specific cell-based therapies. We are currently pursuing indications for congestive heart failure (CHF) and Type 1 diabetes (T1D) with its stem cell technology. We expect to conduct basic research to determine the potential use of its stem cells in other indications, such as macular degeneration, stroke, and Parkinson’s disease.
Overview of PharmaFrontiers Corp Technologies and Programs
-16-
In the United States, approximately 400,000 people suffer from multiple sclerosis, a chronic progressive autoimmune disease of the central nervous system (CNS) that is caused by myelin autoreactive T-cells progressively eroding the myelin that surrounds and insulates nerve fibers of the brain and spinal cord. Globally, there are approximately 2.5 million MS patients representing a drug market in excess of $4 billion. The US markets accounted for 50 per cent of global MS sales in 2004, at US$2.3 billion. MS remains a challenging autoimmune disease to study because the pathophysiologic mechanisms are diverse, and the chronic, unpredictable course of the disease makes it difficult to determine whether the favorable effects of short-term treatment will be sustained. Therapies that can prevent or stop the progression of disease and allow reversal of the neurological damage and disability caused by the disease represent the greatest unmet need in MS.
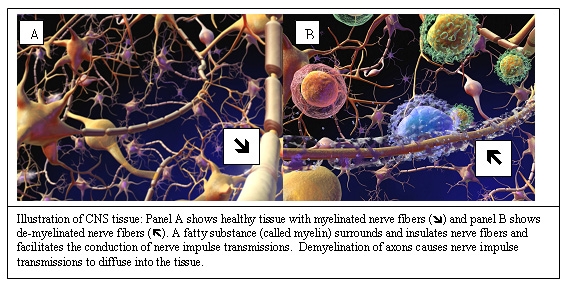
In recent years, the understanding of MS pathogenesis has evolved to comprise an initial, T-cell-mediated inflammatory activity followed by selective demyelination (erosion of the myelin coating of the nerve fibers) and then neurodegeneration. The discovery of disease-relevant immune responses has accelerated the development of targeted therapeutic products for the treatment of the early stages of MS. Healthy individuals have been found to have autoreactive T-cells, which recognize a variety of self-antigens (e.g., myelin basic protein [MBP], proteolipid protein [PLP], and myelin oligodendrocyte glycoprotein [MOG]) as part of the normal T-cell repertoire and circulate naturally in the periphery without causing an autoimmune disease.
Some subjects unfortunately who have the appropriate genetic background have increased susceptibility for the in vivo activation and clonal expansion of myelin autoreactive T-cells. These myelin autoreactive T-cells may remain dormant, but at some point they are activated in the periphery, possibly by molecular mimicry (i.e., recognition of epitopes that are common to autoantigens and microbial antigens as exogenous triggers), thus enabling them to cross the blood-brain barrier (BBB) and infiltrate the healthy tissue of the brain and spinal cord. The cascade of pathogenic events leads to demyelination of axons, which causes nerve impulse transmissions to diffuse into the tissue.
 |
Current Therapy for Multiple Sclerosis
Current MS disease modifying drugs on the market are only palliative and generally work through a mechanism of immunomodulation or immunosuppression. These therapies for MS are dominated by three forms of interferon that require frequent subcutaneous or intramuscular injections. Copaxone is an immunomodulator composed of a random copolymer of amino acids that is administered daily. Novantrone (mitoxanthrone) is an immunosuppressive drug that can only be given four times per year with a life
-17-
time limit of 8 to 12 doses. All of the current therapies only slow the progression of MS and they have significant patient compliance challenges because of the dosing schedule, limited decrease in relapse rate, side effects profile (e.g., the interferon formulations produce severe flu-like symptoms, injection site reactions, infection and neutralizing antibodies (range from 5% to 45%) are developed that limits the efficacy of treatment; copaxone causes significant injection site reactions; while novantrone causes infections, bone marrow suppression, nausea, hair thinning, bladder infections, and mouth sores). These drugs must be administered daily to weekly and they reduce relapses by about 38-75% (as compared to a patient’s prior 2-year history).
Tysabri, a selective adhesion molecule inhibitor (an alpha 4 integrin antagonist), represents another class of MS drugs which works by preventing immune system cells (all leukocytes carrying the alpha 4 integrin glycoprotein on their surface) from crossing the BBB and move into the CNS. Unfortunately, Tysabri blocks the movement of all inflammatory T-cells not just the myelin autoreactive T-cells and leaves patients at a risk of life threatening infections. Tysabri with a reduction in relapse rates of 67% (versus placebo) was still expected to generate $2 to 3 billion in peak annual sales in an existing products market of approximately $4 billion had it not been for the severe side effects.
A number of companies have committed resources to research and development programs to develop novel MS drug therapies. These programs represent incremental improvements over current therapy and their mechanisms of action are similar to current therapy. Tovaxin is the only whole T-cell based vaccination strategy that safely and effectively eliminates the myelin autoreactive T-cells and induces immune tolerance with the potential to prevent the disease or stop disease progression and perhaps to allow the reversal of disease symptoms and progression to severe disability.
In our open-label Phase I/II studies, Tovaxin has shown a reduction in relapses in excess of 90% with virtually no side effects based on only 4 injections annually administered over 3-4 months. Furthermore, approximately 40% of the MS patients treated with Tovaxin demonstrated a reversal of disability as measured by the Kurtzke Expanded Disability Status Scale (EDSS; a method of quantifying disability of MS patients) while the remainder of patients (except for one relapse) experienced no progression of disease. Based on the results of the Phase I/II studies a Phase IIb clinical trial to study Tovaxin therapy in early (clinically isolated syndrome (CIS) and early relapsing remitting (RR)) MS patients has been cleared by the FDA and is scheduled to be initiated the first half of 2006.
Tovaxin™ for Multiple Sclerosis
Tovaxin works selectively on the myelin autoreactive T-cells by harnessing the body’s natural immune defense system and feedback mechanisms to deplete these T-cells and induce favorable immune regulatory responses which rebalance the immune system. Tovaxin induces immune responses that deplete (Appendix C) and regulate the myelin autoreactive pro-inflammatory T-cells that cause the inflammation and erosion of the myelin sheath resulting in MS. Specifically, Tovaxin is manufactured by taking the MRTCs from the blood, expanding them to a therapeutic dose ex-vivo, and attenuating them with gamma irradiation to prevent DNA replication. These attenuated MRTCs are then injected subcutaneously into the body in large quantities. The body recognizes specific T-cell receptor molecules of these MRTCs as foreign and mounts an immune response reaction against them, not only destroying the injected attenuated MRTCs, but also the circulating, myelin autoreactive T-cells carrying the peptide-specific T-cell receptor molecules. In addition, T-cell activation molecules on the surface of the activated MRTCs used as vaccine induce favorable immune regulatory responses, which promote anti-inflammatory responses. Because the therapy uses an individual’s own cells, the only directly identifiable side effect is injection site reaction in a small percentage of the patients. These reactions clear within 24 hours. Clinical studies indicate that following TCV therapy the body does not attack normal T-cells so the patient is not put at peril for side effects and life threatening infections.
-18-
Tovaxin consists of a trivalent formulation (MBP, PLP and MOG) of attenuated myelin-peptide reactive T-cells (MRTCs) which targets only the myelin-specific subset of T-cells. Current therapies on the market and under development do not eliminate the MRTCs and are, therefore, only a palliative treatment to reduce the frequency and occurrence of MS symptoms. The current iteration of Tovaxin is a trivalent formulation, which uses six peptides (two each from three myelin proteins) to select the MRTCs. This formulation is very pharmacologically active with only minimal local and systemic side effects. Tovaxin, a whole T-cell vaccine, is a completely new class of drug for MS that works in concert with the body’s immune regulatory system to suppress the T-cell-mediated inflammatory activity and deplete the MRTCs.
This same technology platform will have application in other T-cell mediated diseases such as Crohn’s disease, psoriasis, rheumatoid arthritis and type 1diabetes.
Tovaxin™ Intellectual Property
Tovaxin is protected by a series of patents and patent applications. There is also substantial know-how surrounding the Tovaxin manufacturing process that should be patentable.
The technology was discovered by Dr. Jingwu Zhang of Baylor College of Medicine in Houston. We have an exclusive, worldwide license from the Baylor College of Medicine to develop and commercialize three technology areas for MS, namely T-cell vaccination, peptides, and diagnostics. Under the License Agreement with the Baylor College of Medicine, we have rights to a total of 7 patents (2 U.S. and 5 foreign) and 69 patent applications (6 U.S., 62 foreign, and 1 Patent Cooperation Treaty [PCT]).
The license was granted to us by Baylor in exchange for common stock in Opexa Pharmaceuticals, Inc. (acquired by us in November 2004). The key terms of the agreement are: exclusive, worldwide, and a 2% royalty on net sales of licensed products. The royalty decreases after the aggregate net sales exceed $500 million. There are no other performance or payment terms in the license. We also have a separate consulting agreement with the inventor, Dr. Jingwu Zhang, which grants us the right of first refusal on all future discoveries made by Dr. Zhang.
Tovaxin™ Manufacturing
We manufacture our TCV therapy in our own GMP facility. The TCV technology is similar to that of traditional microbial vaccine technology, where the pathogen (or the attenuated derivative) is used to derive the protective antigens necessary to induce protective immune responses. In preparing a TCV therapy, the myelin autoreactive T-cells causing the disease are taken from the blood, specifically identified, and expanded ex vivo by incubating these T-cells with MBP, PLP, and MOG-selected peptides in the presence of antigen-presenting cells and growth factors. Myelin-peptide reactive T-cells are grown to therapeutic levels and cryopreserved. Prior to use, the MRTCs are expanded, formulated, and attenuated (by irradiation) to render them incompetent to replicate but viable for therapy. These attenuated T-cells are administered subcutaneously through a series of injections. A single draw of a 500 ml bag of blood is sufficient to provide a full year’s therapeutic regime of Tovaxin.
-19-
We have improved the manufacturing process of Tovaxin. Based on a new process, turnaround (receipt of blood from the patient and return Tovaxin to the patient) is decreased from 12 to 5 weeks and it is anticipated that the material and labor costs of an annual course of therapy will be approximately $4,000. Current therapies are priced at $16,000 to $25,000 annually. The price of newer therapies with better safety and efficacy profiles are anticipated to be priced at $20,000 to $25,000 annually.
We have also validated supply chain logistics improvements that make manufacturing with a regional central facility economical. The viability of blood MRTCs from the time the blood is drawn from the patient to receipt at the processing facility has been established at a minimum of 24 hours, which is sufficient for anywhere in the United States and, likely, Canada. Experiments are underway to determine whether blood MRTCs viability can be extended to at least 72 hours. Stability on the final TCV formulation for injection into patients has already been established at 72 hours. We are actively conducting experiments to improve the stability profile of Tovaxin.
We have developed a supply chain agreement with Lifeblood Biological Services (Memphis, TN) in which Lifeblood will manage blood collection and shipment to our manufacturing facility using the same infrastructure that is used to collect transfusable products. We will manage direct shipment of the TCV to the investigator for administration to patient.
Clinical Development of Tovaxin™
The intent of our clinical development program is to position Tovaxin as first-line therapy for MS. Improvements in efficacy combined with the inherent safety of the treatment make this goal realistic. If successful, Tovaxin would be the first product that specifically targets the “root cause” of MS—the myelin autoreactive T-cells. Compared to other treatments available, this therapy is individualized autologous, easier to tolerate and potentially places the disease into remission. If patients are treated early enough in the disease course, this therapy may prevent progression to more serious forms of MS and possibly allows the reversal of disease. Furthermore, Tovaxin seems to be appropriate to be combined with existing therapies to form a therapeutic cocktail that could be used over the entire life cycle of a patient as other treatments are added or replaced. Remyelination therapies should be easier to develop and implement following the depletion and regulation of the myelin autoreactive T-cells.
The clinical effectiveness of whole T-cell vaccines has evolved from 1990 to 2005 due to formulation improvements. There are three primary myelin proteins (MBP, MOG and PLP) that have been implicated in T-cell pathogenesis of MS. In the early 1990’s, Dr. Zhang used monovalent MBP-reactive T cell formulations to treat patients in an open-label clinical trial, which demonstrated an excellent safety profile and a 40% reduction in Annualized Relapse Rate (ARR; a primary outcome measure for licensing MS therapies) (Table 1). Patients treated in Israel with a divalent (MBP, MOG) formulation had an excellent safety profile and reduced ARR by 55% (Table 1). Most recently, patients treated in our Phase I/II open-label studies with trivalent (MBP, MOG, and PLP) formulations had an excellent safety profile and reduced ARR by 93% (Table 1). In the upcoming Phase IIb trial, a new formulation refinement will be implemented that is expected to further improve efficacy.
-20-
Table 1. Comparison of Annual Relapse Rate Reductions with T-Cell Vaccine Formulations | |||
T-Cell Vaccine Formulation | Peptide Formulation | Annual Relapse Rate Reduction (%)* | Number Patients |
| Monovalent | MBP | 40 | 114 |
| Divalent | MBP, MOB | 55 | 20 |
| Trivalent | MBP, MOG, PLP | 93 | 16 |
| Patient-Specific** | Variable based on patient-specific T-cells | TBD*** | 150 |
* All three clinical trials were open-label. ** The Phase IIb trial will select peptide-specific myelin-reactive T-cells to tailor the T-cell vaccine specifically for the individual patient. *** TBD = to be determined. Over the past 10 years 150 patients have been treated with T-cell vaccine.
Tovaxin™ Phase IIb
The Phase IIb trial, entitled “A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study of Subcutaneous Tovaxin™ in Subjects with Clinically Isolated Syndrome or Relapsing Remitting Multiple Sclerosis”, to be initiated in the first half of 2006, will be a multi-site double-blind, randomized, placebo-controlled 150 (100 treated, 50 placebo) patient trial. The patient population will be those patients with early stage disease where Tovaxin is likely to have its greatest impact. The primary endpoint will be lesion evaluation (the total number of gadolinium-enhancing lesions) via MRI with a secondary endpoint being annual relapse rate. This trial is designed to rigorously demonstrate the safety and efficacy of Tovaxin in the fastest, most economical manner prior to initiating a pivotal Phase III trial approximately the second half of 2007.
A rigorous, blinded study is now required using patient-specific formulation(s) in subjects with early stage disease (where the therapy is likely to work best) to specifically define safety and efficacy parameters prior to entering a Phase III registration study. The Tovaxin vaccine unlike other MS products in development activates regulatory T-cells (Tregs) that (1) induce the depletion of the myelin autoreactive T-cells (anti-idiotypic response of anti-id networks of CD4+ and CD8+ Tregs) that recognize specific MRTCs by their unique TCR CDR3 peptides, and (2) induce immune responses to T-cell activation markers (anti-ergotypic response that recognizes the state of activation of T-cells irrespective of their TCR specificity), and (3) skews the pro-inflammatory Th1 phenotype autoantigen-specific cells that produce IFN-gamma and interleukin [IL]-2 and IL-12 to anti-inflammatory Th2 phenotype autoantigen-specific cells that produce IL-4, IL-10, IL-6 and IL-12. These combined effects of TCV have the potential to allow the body to repair the multiple sclerosis lesions.
Stem Cell Therapy
Stem cells are undifferentiated primary cells that have the potential to become any tissues and organs of the body. They hold enormous therapeutic promise for the development of effective treatments and possibly cure various diseases. Hematopoietic stem cells, present in the bone marrow and precursors to all blood cells, are currently the only type of stem cells commonly used for therapy. Doctors have been transferring HSCs in bone marrow transplants for more than 40 years. Advanced techniques for collecting or “harvesting” HSCs are now used to treat leukemia, lymphoma and several inherited blood disorders.
The clinical potential of stem cells has also been demonstrated in the treatment of other human diseases, including diabetes and advanced kidney cancer. However, these new therapies have been offered only to a very limited number of patients using adult stem cells.
-21-
New clinical applications for stem cells are currently being tested therapeutically for the treatment of liver diseases, coronary diseases, autoimmune and metabolic disorders (amyloidosis), chronic inflammatory diseases (lupus) and other advanced cancers.
Unfortunately stem cell therapies have technical, ethical and legal hurdles to overcome before they will be able to be used to effect tissue and organ repair in disease states that heretofore have only treated the symptoms. A significant hurdle to most uses of stem cells is that scientists do not yet fully understand the signals that turn specific genes on and off to influence the differentiation of the stem cell. Therefore, scientists will have to be able to precisely control the differentiation of stem cells into the specific cell type to be used in therapy and drug testing Current knowledge of the signals controlling differentiation fall well short of being able to mimic these conditions precisely to consistently have identical differentiated cells for each specified use.
To realize the promise of novel cell-based therapies for such pervasive and debilitating diseases, scientists must be able to easily and reproducibly manipulate stem cells so that they possess the necessary characteristics for successful differentiation, transplantation and engraftment. The following is a list of steps in successful cell-based treatments that scientists will have to learn to precisely control to bring such treatments to the clinic. To be useful for transplant purposes, stem cells must be reproducibly made to:
| o | Proliferate extensively and generate sufficient quantities of tissue |
| o | Differentiate into the desired cell type(s) |
| o | Survive in the recipient after transplant |
| o | Integrate into the surrounding tissue after transplant |
| o | Function appropriately for the duration of the recipient's life |
| o | Avoid harming the recipient in any way |
| o | Avoid the problem of immune rejection |
Although there are many ways to access stem cells, our autologous blood monocyte-derived stem cells offer distinct advantages. The press has been focused on embryonic stem cells because, intrinsically, they have the most potential but recent studies have indicated that adult stem cells maybe better clinically. Embryonic stem cells have three major drawbacks: (1) They are very expensive to access, requiring 150 to 250 embryos to develop one cell line; (2) They are very difficult to control; and (3) They are used by administering them into another individual (allogeneic), thus risking rejection. Some of these drawbacks can be overcome if the cord blood stem cells are frozen at birth; but most people have not done this. Therefore, the only practical way to access one’s own stem cells is either through bone marrow, the spinal cord or fat tissue. All of these procedures require expensive, hospital treatment and are fairly traumatic to the patient.
Stem cell transplants have been shown to be successful. Investigators have conducted clinical studies on late stage heart failure patients using stem cells from the patients’ bone marrow injected directly into the damaged parts of the heart. This procedure involved threading a catheter through an artery into the left ventricle (the heart’s main pumping chamber) and “mapping” specific sites of muscle damage. Stem cells were then injected into these areas. After two months, the treated patients had significantly less heart failure and angina and were better able to pump blood than the untreated patients. The treated group also tended to do better on treadmill tests, and after four months, the treated patients had a sustained improvement in pumping power and the ability to supply blood to the entire body. A summary of several studies using stem cells to treat heart failure is provided in Appendix D.
Stem Cell Platform
-22-
Our second exciting opportunity and comprehensive technology platform involves a proprietary process to produce large quantities of monocyte-derived stem cells (MDSC) from blood at a relatively low cost. The importance of these MDSC is that they can be prepared from a patient’s monocytes, expanded ex vivo, and then administered to the same patient. Because this is an autologous therapy, there should be no rejection issues and no need for the use of debilitating anti-rejection drugs. Moreover, this method avoids the controversial ethical issues associated with embryonic stem cells. Lastly, since the source of MDSC is the patient’s own peripheral blood, it has the advantage of being abundant, easy to obtain even on a repetitive basis, and cost effective.
Adult stem cells are unspecialized cells that have potential to develop into many different cell types in the body. Adult stem cells are believed to be a potential autologous renewable replacement source which might be used to treat various diseases, such as heart disease, type 1 diabetes, stroke, heart attack, burn injuries, spinal cord injuries, Parkinson’s and Alzheimer’s diseases, and many others. Adult stem cells are in clinical trials globally and are currently being used therapeutically in Thailand where hematopoietic stem cells (HSCs) are being used to treat cardiac disease (severe angina pectoris). In addition, adult stem cells are in clinical trials in the U.S. (mesenchymal stem cells), Brazil, Uruguay and Europe (bone marrow derived stem cells).
Working either alone or in conjunction with strategic partners, we intend to utilize these MDSC from the same patient to effect autologous tissue or organ repair. The initial internal therapeutic targets are late stage heart failure and type 1 diabetes. Other therapeutic targets would be pursued through early-stage licensing or strategic alliances. This program is currently in pre-clinical development. The value proposition is to confirm that we can make large amounts of inexpensive functional stem cells from easily accessible monocytes and then leverage off the advances being made globally to accelerate clinical trials.
Our technology and solution circumvents many of the problems other stem cell technologies have by being able to access large quantities of pluripotent (able to self replicate and be differentiated into all cell types except germ cells) stem cells from a patients own blood. The proprietary system works by first separating the monocytes (a type of white blood cell) from a patient’s blood. These monocytes are then reversed in their evolution (de-differentiated) by applying specific growth factors to them to yield a unique MDSC. Blood is rich in monocytes and a 500 ml bag of blood will yield over 100 million stem cells.
 |
-23-
Stem Cell Pre-Clinical and Clinical Development
We plan to conduct pre-clinical animal studies on these MDSC in the first quarter of 2006 before filing and IND. The plan is to leverage off of extensive international research and clinical trials and develop these cells internally for late stage heart failure and type 1 diabetes. All other potential applications will be out-licensed to generate upfront cash, milestone payments, and royalties.
Type 1 diabetes and heart failure were chosen for internal development because they are large markets (over 1 million patients in the U.S.) and we have a proprietary method of creating islet cells. Stem cell research using more expensive processes has shown encouraging clinical results for the use of cellular therapy in these indications. The objective will be to develop these programs through proof of principle in human and then out-license them to a major company.
Stem Cell Intellectual Property
We have an exclusive, worldwide license from the University of Chicago, through its prime contractor relationship with Argonne National Laboratory, to the development of pluripotent stem cells from monocytes isolated from human peripheral blood and their use in treating diseases. The technology was discovered at the Argonne National Laboratory, a U.S. Department of Energy Laboratory. Under the License Agreement with the University of Chicago, we have the rights to a total of 8 patent applications (3 US, 2 PCT and 3 Foreign). We hold a license to produce cells that generate insulin using its core stem cell technology as well as a license for the use of some unique growth factors, which have overcome the challenge of differentiating an adult stem cell to produce insulin. A patent application has been filed for the use of MDSC to develop beta-islet cells for treatment of type 1 diabetes. We have filed a patent application for the use of MDSC to treat heart disease based on technology developed by us since acquiring the license from the University of Chicago. Under the terms of this license, the University is entitled to 4% royalty on sales.
Stem Cell Strategy
We will review possible strategic partners for stem cells following the proof-of-principle in animal models and the submission of an IND for diabetes and cardiology. Possible licensing would be for marketing rights in exchange for upfront money, milestone payments, payment of all ongoing clinical costs in licensed territories, and royalties on sales.
Synergy between T-Cell Vaccination and Stem Cell Therapeutics
Insulin dependent type 1 diabetes (T1D; insulin dependent diabetes (IDDM)) mellitus is a T cell-mediated inflammatory autoimmune disease of the pancreas, resulting in a lack of insulin. In a person with T1D, beta cells of Langerhans are damaged by autoimmune inflammation, leading to an insufficiency of insulin. In T1D several autoantigen-specific T cell autoreactivities have been identified. These include islet-specific antigens, like insulin, and other nonislet-specific antigens, like glutamic acid decarboxylase (GAD), carboxypeptidase H (CPH), and tyrosine phosphatase (IA-2) among others. Of all the known autoantigens implicated in the disease process, treatment with only insulin, GAD 65, and the heat shock protein 60 (HSP60) peptide p277 can protect nonobese diabetic (NOD) mice from disease. Insulin and GAD 65 are also the most prominent islet autoantigens shown to be recognized by peripheral T cells from T1D patients. These antigens, among others, may form the basis for a PharmaFrontier’s TCV therapy for T1D.
-24-
Our TCV program has significant synergy with our stem cell program with respect to the treatment modality for T1D. Because autoreactive T-cells are implicated in the destruction of beta cells, a therapeutic strategy that includes T1D TCV followed by new stem cell derived beta cell replacement could be an extraordinary therapy or cure for T1D.
Licenses, Patents and Proprietary Rights
We believe that proprietary protection of our technologies will be critical to the development of our business. We intend to protect our proprietary intellectual property through patents and other appropriate means. We rely upon trade-secret protection for some confidential and proprietary information and take active measures to control access to that information. We currently have non-disclosure agreements will all of our employees.
Our intellectual property strategy includes developing proprietary technology for the sourcing, scale up, manufacturing, and storage of T cells and pluripotent adult stem cells and the use of these cells in multiple therapeutic applications. This strategy will include expanding on technologies in-licensed to us as well as in-licensing additional technologies through collaborations with universities and biotech companies.
We have licenses to certain patents that relate to our T cell technology and our pluripotent adult stem cell technology.
T Cell Therapy IP
We have an exclusive, worldwide license from the Baylor College of Medicine to patent applications claiming rights to the treatment of multiple sclerosis using modified T cells and to the use of the T cell technology as a diagnostic. Under the Baylor license we are obligated to pay a percentage of net sales of products subject to the licensed patents.
We also have an exclusive worldwide license for the intellectual property rights and research results of an autologous T cell vaccine for the treatment of rheumatoid arthritis from the Shanghai Institutes for Biological Sciences (SIBS), Chinese Academy of Sciences of the People's Republic of China. Pursuant to the SIBS license agreement, we made an initial payment to SIBS and we are obligated to pay a percentage of net sales of products subject to the licensed technology. SIBS’ initial human clinical trial results indicate that the T cell vaccination induces immune responses that correlate with clinical improvements measured as reductions in ACR50 (American College Rheumatology (ACR) criteria, which measures joint swelling and tenderness and other factors such as pain and disability) and reductions in rheumatoid arthritis laboratory parameters
Stem Cell Therapy IP
We have an exclusive, worldwide license from the University of Chicago to a patent application claiming rights to the development of adult pluripotent stem cells from monocytes isolated from adult human peripheral blood. The technology was developed at the Argonne National Laboratory, a U.S. Department of Energy Laboratory operated by the University of Chicago.
-25-
Pursuant to the license we have issued a total of 533,064 shares of our common stock to the University of Chicago. We have also agreed to pay the University of Chicago $1.5 million upon the earlier of April 30, 2006 or our raising $10 million or more in any financing. We are also obligated to issue to the University of Chicago sufficient additional shares of common stock so that the University holds a total of 2.6% of our outstanding stock after our raising $10 million or more in any financing. We have agreed to pay a percentage of royalties on sales of products subject to the licensed patents, as well as sublicense fees. In addition, the University of Chicago license requires us to expend on research and development at least $2,000,000 before February 2006 and at least an additional $4,000,000 before February 2008. To date the Company has spent in excess of $2,000,000 and has met our obligations required by the license. The license also requires us to sell a product or method based on the licensed technology by February 2011.
Research Collaborations
We anticipate that from time to time in the future we will enter into collaborative research agreements with other academic and research institutions. We will use such agreements to enhance our research capabilities. Typically, in the industry, such agreements provide the industry partner with rights to license the intellectual property created through the collaboration. We may also enter into collaborative research agreements with other pharmaceutical companies when we believe such collaboration will support the development and commercialization of our technology.
Commercialization Through Third Parties
We anticipate that we will grant sublicenses for certain applications of our technologies. We believe that by sublicensing some of the rights to our technology to pharmaceutical companies and other third parties, we will be able to more efficiently develop some applications of our technologies. We currently do not have any sublicenses.
Competition
The development of therapeutic and diagnostic agents for human disease is intensely competitive. Major pharmaceutical companies currently offer a number of pharmaceutical products to treat heart attack, stroke, Parkinson’s disease, diabetes, liver diseases, arthritis and other diseases for which our technologies may be applicable. Many pharmaceutical and biotechnology companies are investigating new drugs and therapeutic approaches for the same purposes, which may achieve new efficacy profiles, extend the therapeutic window for such products, alter the prognosis of these diseases, or prevent their onset. We believe that our products, when and if successfully developed, will compete with these products principally on the basis of improved and extended efficacy and safety and their overall economic benefit to the health care system. We expect competition to increase. We believe that our most significant competitors will be fully integrated pharmaceutical companies and more established biotechnology companies. Smaller companies may also be significant competitors, particularly through collaborative arrangements with large pharmaceutical or biotechnology companies. Some of our primary competitors in the current treatment of and in the development of treatments for multiple sclerosis include Biogen, Elan, Serono, Aventis, Teva, and Schering AG. Some of our primary competitors in the development of stem cell therapies include Aastrom Biosciences, Geron, Gamida-Cell Ltd, Stem Cells Inc., Cellerant Therapeutics, Viacell, and Osiris Therapeutics. Many of these competitors have significant products in development that could be competitive with our potential products.
Sales and Marketing
We intend to develop a sales force to market our multiple sclerosis cell therapy and diagnostic products in the U.S. Given the concentration of multiple sclerosis among a relatively small number of specialized neurologists, we believe that a modest size sales force would be sufficient to market the multiple sclerosis products. Our plan is to start building the sales force with the launch of the multiple sclerosis diagnostic products.
-26-
We expect to partner with large biotech and pharmaceutical companies for the marketing and sales of our stem cell therapy products.
Description of Property
Our principal executive offices are located at 2635 N. Crescent Ridge Drive, The Woodlands, Texas, and our telephone number is (281) 272-9331. This 10,200 sq. ft. facility is located on 3 acres. This location provides space for research and development laboratories; a specialized flow cytometry and microscopy lab; support of clinical trials with GMP manufacturing suites; quality systems management with a quality control laboratory, regulatory affairs offices, quality assurance space; as well as administrative support space. There is 2500 sq. ft. of space in the facility still available for future build-out. The facility including the property is leased for a term of ten years with two options for an additional five years each at the then prevailing market rate. We believe that our lease is at a competitive or market rate.
Government Regulation
Our research and development activities and the future manufacturing and marketing of our potential products are, and will be, subject to regulation for safety and efficacy by a number of governmental authorities in the United States and other countries.
In the United States, pharmaceuticals, biologicals and medical devices are subject to Food and Drug Administration or FDA, regulation. The Federal Food, Drug and Cosmetic Act, as amended, and the Public Health Service Act, as amended, the regulations promulgated thereunder, and other Federal and state statutes and regulations govern, among other things, the testing, manufacture, safety, efficacy, labeling, storage, export, record keeping, approval, marketing, advertising and promotion of our potential products. Product development and approval within this regulatory framework takes a number of years and involves significant uncertainty combined with the expenditure of substantial resources.
FDA Approval
We will need to obtain FDA approval of any therapeutic product we plan to market and sell. The steps required before our potential products may be marketed in the United States include:
1. Preclinical Laboratory and Animal Tests. Preclinical tests include laboratory evaluation of the product and animal studies in specific disease models to assess the potential safety and efficacy of the product and our formulation as well as the quality and consistency of the manufacturing process.
2. Submission to the FDA of an Application for an Investigational New Drug Exemption, or IND, Which Must Become Effective Before U.S. Human Clinical Trials May Commence. The results of the preclinical tests are submitted to the FDA as part of an IND, and the IND becomes effective 30 days following its receipt by the FDA, as long as there are no questions, requests for delay or objections from the FDA.
3. Adequate and Well-Controlled Human Clinical Trials to Establish the Safety and Efficacy of the Product. Clinical trials involve the evaluation of the product in healthy volunteers or, as may be the case with our potential products, in a small number of patients under the supervision of a qualified physician. Clinical trials are conducted in accordance with protocols that detail the objectives of the study, the parameters to be used to monitor safety and the efficacy criteria to be evaluated. Any product administered in a U.S. clinical trial must be manufactured in accordance with clinical good manufacturing practices, or GMP, determined by FDA. Each protocol is submitted to the FDA as part of the IND.
-27-
The protocol for each clinical study must be approved by an independent Institutional Review Board, or IRB, at the institution at which the study is conducted and the informed consent of all participants must be obtained. The IRB will consider, among other things, the existing information on the product, ethical factors, the safety of human subjects, the potential benefits of the therapy and the possible liability of the institution.
Clinical development is traditionally conducted in three sequential phases, which may overlap:
| · | In Phase I, products are typically introduced into healthy human subjects or into selected patient populations to test for adverse reactions, dosage tolerance, absorption and distribution, metabolism, excretion and clinical pharmacology. |
| · | Phase II involves studies in a limited patient population to (i) determine the efficacy of the product for specific targeted indications and populations, (ii) determine optimal dosage and dosage tolerance and (iii) identify possible adverse effects and safety risks. When a dose is chosen and a candidate product is found to be effective and to have an acceptable safety profile in Phase II evaluations, Phase III trials begin. |
| · | Phase III trials are undertaken to conclusively demonstrate clinical efficacy and to test further for safety within an expanded patient population, generally at multiple study sites. |
The FDA continually reviews the clinical trial plans and results and may suggest changes or may require discontinuance of the trials at any time if significant safety issues arise.
1. Submission to the FDA of Marketing Authorization Applications. The results of the preclinical studies and clinical studies are submitted to the FDA in the form of marketing approval authorization applications.
2. FDA Approval of the Application(S) Prior to Any Commercial Sale or Shipment of the Drug Biologic Product Manufacturing Establishments Located in Certain States Also May be Subject to Separate Regulatory and Licensing Requirement. The testing and approval process will require substantial time, effort and expense. The time for approval is affected by a number of factors, including relative risks and benefits demonstrated in clinical trials, the availability of alternative treatments and the severity of the disease. Additional animal studies or clinical trials may be requested during the FDA review period, which might add to that time.
After FDA approval for the product, the manufacturing and the initial indications, further clinical trials may be required to gain approval for the use of the product for additional indications. The FDA may also require unusual or restrictive post-marketing testing and surveillance to monitor for adverse effects, which could involve significant expense, or may elect to grant only conditional approvals.
FDA Manufacturing Requirements
Among the conditions for product licensure is the requirement that the prospective manufacturer’s quality control and manufacturing procedures conform to the FDA’s current good manufacturing practices, or GMP, requirement. Even after product licensure approval, the manufacturer must comply with GMP on a continuing basis, and what constitutes GMP may change as the state of the art of manufacturing changes. Domestic manufacturing facilities are subject to regular FDA inspections for GMP compliance, which are normally held at least every two years. Foreign manufacturing facilities are subject to periodic FDA inspections or inspections by the foreign regulatory authorities with reciprocal inspection agreements with the FDA. Domestic manufacturing facilities may also be subject to inspection by foreign authorities.
-28-
Fast Track, Priority Review and Accelerated Approval
Fast Track refers to a process for interacting with the FDA during drug development. Priority Review applies to the time frame the FDA targets for reviewing a completed application. Accelerated Approval (Subpart H) applies to the design and content of the studies used to support a marketing claim.
Fast Track is a formal mechanism to interact with the FDA using approaches that are available to all applicants for marketing claims. The Fact Track mechanism is described in the Food and Drug Administration Modernization Act of 1997. The benefits of Fast Track include scheduled meetings to seek FDA input into development plans, the option of submitting a New Drug Application (NDA) in sections rather than all components simultaneously, and the option of requesting evaluation of studies using surrogate endpoints as discussed below. The Fast Track designation is intended for the combination of a product and a claim that addresses an unmet medical need, but is independent of Priority Review and Accelerated Approval. An applicant may use any or all of the components of Fast Track without the formal designation. Fast Track designation does not necessarily lead to a Priority Review or Accelerated Approval.
Priority Review is a designation for an application after it has been submitted to the FDA for review for approval of a marketing claim. Under the Food and Drug Administration Modernization Act of 1997, reviews for New Drug Applications, or NDAs, are designated as either Standard or Priority. A Standard designation sets the target date for completing all aspects of a review and the FDA taking an action on the application (approve or not approve) at 10 months after the date it was filed. A Priority designation sets the target date for the FDA action at 6 months. A Priority designation is intended for those products that address unmet medical needs.
Accelerated Approval or Subpart H Approval is a program described in the NDA regulations that is intended to make promising products for life threatening diseases available on the market on the basis of preliminary evidence prior to formal demonstration of patient benefit. The studies are designed to measure and the FDA evaluation is performed on the basis of a surrogate marker (a measurement intended to substitute for the clinical measurement of interest, usually prolongation of survival) that is considered likely to predict patient benefit. The approval that is granted may be considered a provisional approval with a written commitment to complete clinical studies that formally demonstrate patient benefit. The Federal Register published a discussion of Accelerated Approval with comments. Absent a formal demonstration of patient benefit, a risk benefit assessment cannot be made. Accelerated Approval designation does not necessarily lead to a Priority Review.
Proposed FDA Regulations
The FDA is requiring human cell, tissue, and cellular and tissue-based product (HCT/P) establishments to follow current good tissue practice, which governs the methods used in, and the facilities and controls used for, the manufacture of HCT/Ps; recordkeeping; and the establishment of a quality program. The agency is also issuing new regulations pertaining to labeling, reporting, inspections, and enforcement that will apply to manufacturers of those HCT/Ps regulated solely under the authority of the Public Health Service Act, and not as drugs, devices, and/or biological products.
-29-
As part of this approach, the FDA has published final rules for registration of establishments that engage in the recovery, screening, testing, processing, storage or distribution of human cells, tissues, and cellular and tissue-based products, and for the listing of such products. These products specifically include stem cells that are progenitors of blood cells; however, the FDA makes no explicit statement regarding the inclusion of other types of stem cells. In addition, the FDA has published proposed rules for making suitability determinations for donors of cells and tissue and for current good tissue practice for manufacturers using them. We cannot now determine the full effects of this regulatory initiative, including precisely how it may affect the clarity of regulatory obligations and the extent of regulatory burdens associated with pluripotent adult stem cell research (for stem cells that give rise to various tissue types, including blood), and the manufacture and marketing of adult stem cell products.
Other Regulations
In addition to safety regulations enforced by the FDA, we are also subject to regulations under the Occupational Safety and Health Act, the Environmental Protection Act, the Toxic Substances Control Act and other present and potential future foreign, Federal, state and local regulations.
Outside the United States, we will be subject to regulations that govern the import of drug products from the United States or other manufacturing sites and foreign regulatory requirements governing human clinical trials and marketing approval for our products. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursements vary widely from country to country. In particular, the European Union is revising its regulatory approach to high tech products, and representatives from the United States, Japan and the European Union are in the process of harmonizing and making more uniform the regulations for the registration of pharmaceutical products in these three markets.
Employees
As of December 31, 2005, we had 18 full time employees. We believe that our relations with our employees are good. None of our employees is represented by a union or covered by a collective bargaining agreement.
Legal Proceedings
We are not currently a party to any legal proceedings.
MANAGEMENT
The following table sets forth certain information regarding our current directors and executive officers.
Our executive officers are elected by the board of directors and serve at the discretion of the board. All of the current directors serve until the next annual stockholders’ meeting or until their successors have been duly elected and qualified.
Name | Age | Position |
| David B. McWilliams | 62 | President and Chief Executive Officer, Director |
| Brooks Boveroux | 62 | Chairman of the Board |
| C. W. Rouse | 58 | Chief Financial Officer |
| Jim C. Williams | 62 | Chief Operating Officer |
| Donna R. Rill | 52 | Vice President of Operations |
-30-
| Sandy L. Livney | 50 | Vice President of Administration / Controller |
| Anthony N. Kamin | 45 | Director |
| Paul M. Frison | 69 | Director |
| Terry H. Wesner | 62 | Director |
Biographical information for our directors and executive officers is set forth below:
Brooks Boveroux was elected as the Chairman of the Board since October, 2005. Mr. Boveroux also serves as Chairman of our Audit Committee since that date. Mr. Boveroux had previously served as the senior vice president—finance, chief financial officer and treasurer of IMCOR Pharmaceutical Co. since August 1, 2000 and as that company's secretary since November 12, 2002 until his retirement in September 2004. For the five years prior to joining PharmaFrontiers Corp., Mr. Boveroux served as chief financial officer and vice president of investor relations for The Liposome Company, Inc., chief financial officer of Synergy Pharmaceuticals, Inc. and as a consultant advising corporations in the area of corporate finance. Mr. Boveroux is a graduate of Hamilton College and received his Masters of Business Administration in Finance from the Wharton School of the University of Pennsylvania.
David B. McWilliams was appointed President and Director in August 2004. From December 2004 until August 2004, Mr. McWilliams was a private investor. From June 2003 to December 2003, Mr. McWilliams served as President and CEO of Bacterial Barcodes, Inc., a molecular diagnostics company. From May 2002 to June 2003, Mr. McWilliams served as CEO of Signase, Inc., a cancer therapy company. Mr. McWilliams served as CEO of Encysive Pharmaceuticals Inc., a cardiovascular therapeutics company from June 1992 to March 2002. Prior to June 1992, Mr. McWilliams served as CEO of Zonagen Inc., a human reproductive products company. Prior to that time, Mr. McWilliams was a senior executive with Abbott Laboratories and a management consultant with McKinsey & Co. He currently serves as a director of Novelos Therapeutics, Inc. and Fairway Medical Systems, Inc. He also serves on the boards of the Texas Healthcare and Bioscience Institute and the Houston Technology Center. He received an MBA in finance from the University of Chicago, and a B.A. in chemistry, Phi Beta Kappa, from Washington and Jefferson College.
Jim C. Williams has served as our Chief Operating Officer since November 2004. Dr. Williams served as Vice President of Clinical and Regulatory Affairs and as Chief Operating Officer for Opexa Pharmaceuticals, Inc. from February 2004 to November 2004. From August 2003 to February 2004 he was Senior Vice President, Regulatory Affairs and Operations for OSIRIS Therapeutics, Inc., and from November 2002 to August 2003 Dr. Williams was Vice President US Regulatory Affairs for Powderject Vaccines. From September 2001 to November 2002 Dr. Williams served as Assistant Vice President, Worldwide Regulatory Affairs for Wyeth BioPharma. Prior to this Dr. Williams served as Executive Director Regulatory Affairs for Aventis Pasteur from November 1994 to September 2001. Dr. Williams retired in 1994 as Captain from the U.S. Public Health Service with over twenty years of service in applied research and human-use product development which included assignments with the FDA, Center for Biologics Evaluation and Research as a Director Scientist in the Division of Vaccine and Related Product Applications; the US Army Medical Research Institute of Infectious Diseases as Chief, Intracellular Pathogens branch and the National Institutes of Health, National Institutes of Allergy and Infectious Diseases; and at the U.S. Naval Medical Research Institute as a research microbiologist.
C. William Rouse has served as our Chief Financial Officer since May 2004. Prior to May 2004, Mr. Rouse was Managing Director of Rouse Associates from April 1999 until May 2004. From January 1995 to April 1999 he was Chief Marketing Officer for Futorian Inc. and from December 1990 to January 1995 he was a Division General Manager for Masco Corporation. Prior to 1990 Mr. Rouse was President of BEI, Inc. Mr. Rouse has led several startups and turnarounds and founded several successful companies.
-31-
Donna R. Rill has served as our Vice President of Operations since November 2004. Ms. Rill has nearly 30 years of extensive clinical and research laboratory experience in cell and gene therapy research and clinical application, immunological techniques and assessment, microbiology, diagnostic virology, experimental design, and method development and implementation. She has expertise in the areas of laboratory development and operations, FDA GMP and regulatory compliance, quality control/assurance system development, and clinical Standards of Practice. From April 2003 to November 2004 she was the Director of Quality Systems and Process Development at Opexa Pharmaceuticals, Inc. From November 1997 to April 2003 she was the Director of Translational Research for the Center for Cell & Gene Therapy at Baylor College of Medicine. She has worked to design, and qualify GMP Cell & Gene Therapy Laboratories, GMP Vector Production facilities, and Translational Research Labs at St. Jude Children¹s Research Hospital, Texas Children¹s Hospital, and Baylor College of Medicine. Ms. Rill has held the positions of Laboratory Director of Cell and Gene Therapy, Translational Research Center for Cell and Gene Therapy, Baylor College of Medicine; Associate Scientist/Lab Manager of the Bone Marrow Transplant Research Laboratory, and the GMP Cell & Gene Therapy Laboratories, St. Jude Children¹s Research Hospital; Clinical Infectious Disease Laboratory Manager, Education Coordinator and Clinical Instructor, Department of Clinical Laboratory, LeBonheur Children’s Medical Center and University of Tennessee Center for the Health Sciences..
Sandy L. Livney has served as our Vice President of Administration and Controller since November 2004. Ms. Livney has over 25 years of experience working in private and public enterprises in various accounting and marketing roles. She became certified as a Certified Public Accountant by the State of Texas in 1981. Prior to November 2004, Ms. Livney worked for Systems Management Solutions, Inc. as its Controller beginning in June 2003 and served as Secretary, Treasurer and Chief Financial Officer until joining PharmaFrontiers. From January 2001 until May 2003, Ms. Livney worked for Petro-Valve, Inc., a privately owned enterprise, in a variety of capacities including Vice President of Business Development, Interim Controller and Personal Assistant to the owner. From January 1999 to 2001, Ms. Livney worked for T. Warren Investments, Inc., a privately owned enterprise, in a variety of functions including Controller, Business Manager and Personal Assistant to the Owner.
Anthony N. Kamin has served as a Director since December, 2004. Since Mr. Kamin currently serves as President of two companies. Eastwood Investment Management is a private equity multi-strategy and multi-asset class manager. Interim Medical Management provides a range of management services primarily in the biotechnology and medical device fields. From 1998 to 2003, Kamin was a venture partner with Venture Strategy Partners. He is a co-founder of Nobex Corporation (oral protein and peptide delivery) and Hi Fidelity Entertainment. He is also currently Chairman of the Board of Advisors for Devlab, a center for technology commercialization at Northwestern University. He also serves on boards for Illinois Technology Enterprise Center (ITEC) in Chicago, Real American Restaurants, B2P.com, and The Cove School for children with learning disabilities. Kamin received a Masters Degree from Yale University in International Relations with a concentration in International Law.
Paul M. Frison has served as a Director of the Company since November, 2004. Mr. Frison has been President and CEO of the Houston Technology Center since January 1999. Before helping to found the Houston Technology Center in 1999, Frison spent 24 years as President and/or CEO building three public companies, NYSE-listed LifeMark, NASDAQ-listed ComputerCraft, and LifeCell Corp. (LIFC: NASDAQ-NM). Mr. Frison currently serves on the Board of Directors of the Houston Technology Center, Micromed Technologies, Inc., The Institute of Research and Rehabilitation, The Entrepreneurship Institute, The Houston Entrepreneurs Foundation, Texas Council of AEA, Texchange, and the Advisory Council of the University of Houston - College of Technology. He received his B.A. from Occidental College in Los Angeles, California.
-32-
Terry H. Wesner has served as a Director since August 2005. Mr. Wesner is a biostatistician and a graduate of The University of Memphis with degrees in biology, mathematics, and statistics. His first position after graduate school was an appointment to the faculty of Harvard School of Medicine as Director of Research for the National Diabetes Association. While a faculty member at Harvard School of Medicine, Mr. Wesner researched gestational diabetics. Since then he has been a Professor of Mathematics and Statistics, published a successful series of 30 college level mathematics textbooks with McGraw-Hill Publishers, and for the last five years has been the CEO and owner of Bernard J. Klein Publishing and GetMath Educational Software. Mr. Wesner brings a unique perspective to the Board as one of the founding investors in Opexa Pharmaceuticals, our wholly owned subsidiary; his son has been undergoing treatment with our T-cell based treatment for Multiple Sclerosis. Mr. Wesner also has been elected to the National Council of The Text and Academic Authors Association, his company has formed a partnership with World Vision to bring free educational materials to needy schools throughout the United States, served as Chair of his community's planning commission, founded and is now Vice-Chair of his community's building authority, serves on The Advisory Board for The Ann Arbor Academy a school for children with learning disabilities, and volunteers for various other community and civic organizations.
Committees of the Board of Directors
We currently have an audit committee, a compensation committee, and a nominating and corporate governance committee.
Audit Committee. The audit committee selects, on behalf of our board of directors, an independent public accounting firm to be engaged to audit our financial statements, discuss with the independent auditors their independence, review and discuss the audited financial statements with the independent auditors and management and recommend to our board of directors whether the audited financials should be included in our Annual Reports to be filed with the Securities and Exchange Commission. The Audit Committee operates pursuant to a written charter, which was adopted February 3, 2005. The current members of the audit committee are Messrs. Boveroux and Wesner.
All of the current members of the Audit Committee are non-employee directors who: (1) meet the criteria for independence set forth in Rule 10A-3(b)(1) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”); (2) have not participated in the preparation of our financial statements or the financial statements of Opexa; and (3) are able to read and understand fundamental financial statements, including a balance sheet, income statement and cash flow statement. The board has determined that Mr. Boveroux qualifies as an “Audit Committee Financial Expert” as defined by item 401(e) of Regulation S-B of the Exchange Act.
Compensation Committee. The compensation committee reviews and either approves, on behalf of our board of directors, or recommends to the board of directors for approval (1) the annual salaries and other compensation of our executive officers and (2) individual stock and stock option grants. The compensation committee also provides assistance and recommendations with respect to our compensation policies and practices and assists with the administration of our compensation plans. The members of our Compensation Committee are independent directors as defined in the applicable rules and regulations promulgated by the SEC, and are neither an officer nor employee of us or our subsidiary. The Compensation Committee operates pursuant to a written charter, which was adopted August 11, 2004. The current members of the compensation committee are Messrs. Wesner and Frison.
Nominating and Corporate Governance Committee. The nominating and corporate governance committee assists our board of directors in fulfilling its responsibilities by: identifying and approving individuals qualified to serve as members of our board of directors, selecting director nominees for our annual meetings of shareholders, evaluating the performance of our board of directors, and developing and recommending to our board of directors corporate governance guidelines and oversight with respect to corporate governance and ethical conduct. Messrs. Wesner, Kamin and Frison are the current members of the Nominating and Corporate Governance Committee, all of whom have been found by the Board of Directors to be an “independent director” pursuant to the applicable rules and regulations promulgated by the SEC. This committee operates pursuant to a written charter adopted on February 3, 2005.
-33-
Compensation Committee Interlocks And Insider Participation. Our Compensation Committee is comprised of Messrs. Wesner and Frison. None of the committee members has ever been an employee of PharmaFrontiers Corp. None of our executive officers serve as a member of the board of directors or compensation committee of any entity that has any executive officer serving as a member of our Board of Directors or Compensation Committee.
Compensation of Directors
Mr. Boveroux is compensated $3,000 per month and $5,000 quarterly plus $2,000 for each regular Board meeting attended in person and $1,000 for each regular meeting attended by teleconference. Mr. Boveroux is also compensated $1,000 for each Audit Committee meeting he attends. Mr. Kamin is compensated $3,000 per month and $2,500 quarterly plus $1,000 for each regular Board meeting attended in person and $500 for each regular meeting attended by teleconference. Mr. Kamin is also compensated $500 for each Nominating Committee meeting he attends. Messrs. Wesner and Frison are compensated $2,500 quarterly plus $1,000 for each regular Board meeting attended in person and $500 for each regular meeting attended by teleconference. Mr. Wesner is compensated $500 for each committee meeting he attends and Mr. Frison is compensated $1,000 for each Compensation Committee meeting he attends and $500 for each Nominating Committee meeting he attends. Mr. McWilliams who is a director and an officer does not receive any compensation for his services as a member of our board of directors. We reimburse our directors for travel and lodging expenses in connection with their attendance at board and committee meetings.
EXECUTIVE COMPENSATION
Summary Compensation Table
The following tables set forth certain information regarding our CEO and each of our most highly-compensated officers whose total annual salary and bonus for the fiscal years ending December 31, 2005, 2004 and 2003 exceeded $100,000.
Annual Compensation | Long Term Compensation Awards | |||||||||||||||
Name and Principal Position | Year | Salary ($) | Bonus ($) | Securities Underlying Options (#) | All Other Compensation ($) | |||||||||||
| David B. McWilliams (1) | 2005 | 250,000 | - | 50,000 | - | |||||||||||
| 2004 | 83,000 | - | 370,000 (2 | ) | - | |||||||||||
| 2003 | - | - | - | - | ||||||||||||
| C. William Rouse (3) | 2005 | 180,000 | - | 50,000 | - | |||||||||||
| 2004 | 77,500 | - | 100,000 (2 | ) | - | |||||||||||
| 2003 | - | - | - | - | ||||||||||||
| Warren Lau (4) | 2005 | - | - | - | - | |||||||||||
| 2004 | 98,000 | - | - | - | ||||||||||||
| 2003 | - | - | - | - | ||||||||||||
-34-
| Jason Otteson (5) | 2005 | - | - | - | - | |||||||||||
| 2004 | 42,000 | - | - | - | ||||||||||||
| 2003 | 102,000 | - | 24,000 | - |
___________
(1) Served as chief executive officer since August 2004.
(2) See “Executive Employment Contracts” for a discussion of the option.
(3) Served as chief financial officer since May 2004.
(4) Served as chief executive officer from June 2004 through August 2004.
(5) Served as chief executive officer until June 2004.
Option Grants in Last Fiscal Year
(Individual Grants)
Name | Number of Securities Options Granted | % of Total Options Granted to Fiscal Year | Exercise/Base Price ($/Share) | Expiration Date |
| David B. McWilliams | 50,000 | 2% | 3.00 | 01/21/2010 |
| C. William Rouse | 50,000 | 2% | 3.00 | 01/21/2010 |
| Warren Lau | - | - | - | - |
| Jason Otteson | - | - | - | - |
Options Exercises and Fiscal 2005 Year End Values
Number of Shares Underlying Unexercised Options at December 31 2005 | Value of Unexercised In-the-Money Options at December 31 2005 (1) | |||||||||||||||||
Name | Exercisable | Unexercisable | Exercisable | Unexercisable | ||||||||||||||
| David B. McWilliams | 243,333 | 176,667 | $ | -0- | (1) | $ | -0- | (1) | ||||||||||
| C. William Rouse | 133,333 | 16,667 | -0- | (1) | -0- | (1) | ||||||||||||
| Warren Lau | - | - | - | - | ||||||||||||||
| Jason Otteson | - | - | - | - | ||||||||||||||
| (1) | The value of “in-the-money” stock options represents the difference between the $3.00 exercise price of such options and the fair market value of $0.60 per share of common stock as of December 31, 2005, the closing price of the common stock reported on the OTC Bulletin Board for December 30, 2005. | |||||||||||||||||
| (2) | The value of “in-the-money” stock options represents the difference between the $3.00 exercise price of 83,333 options and the fair market value of $0.60 per share of common stock as of December 31, 2005, the closing price of the common stock reported on the OTC Bulletin Board for December 30, 2005. | |||||||||||||||||
No options were exercised during the fiscal year ended December 31, 2005. No stock appreciation rights were outstanding at the end of the 2005 fiscal year.
Executive Employment Contracts
Mr. David B. McWilliams has an existing employment agreement with us that he entered into effective August 23, 2004. Mr. McWilliams’ current agreement for the position of chief executive officer is at an annual salary of $250,000 and may be terminated by us or Mr. McWilliams at any time for any or no reason. Mr. McWilliams has the right to purchase 370,000 shares of our common stock at a price per share of $3.00, of which 10,000 shares vest on the 1st day of each month of Mr. McWilliams’ employment. In January 2005, Mr. McWilliams was granted an option to purchase 50,000 shares of our common stock at a purchase price of $3.00 per share.
-35-
C. William Rouse entered into an employment agreement, expiring April 2006, providing for an annual salary of $180,000. Mr. Rouse has the right to purchase 100,000 shares of our common stock at a price per share of $3.00. This option will vest in three parts: 33,333 on April 29, 2005, 33,333 on April 29, 2006 and finally 33,334 on April 29, 2007. Any unexercised options will expire on April 29, 2009. In January 2005, Mr. Rouse was granted an option to purchase 50,000 shares of common stock at a purchase price of $3.00 per share.
Mr. Jim C. Williams has an existing employment agreement with us that he entered into effective November 6, 2004. Mr. Williams’ current agreement for the position of chief operating officer is at an annual salary of $215,000 and may be terminated by us or Mr. Williams at any time for any or no reason. In November 2004 Mr. Williams was granted a five year option to purchase 125,000 shares of common stock at a purchase price of $3.00 per share with such option vesting on the one year anniversary of the grant date over a period of three years. In December 2005, Mr. Williams was granted a 10 year option to purchase 125,000 shares of our common stock at a purchase price of $0.70 per share with such option vesting on the one year anniversary of the grant date over a period of four years.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth the number of shares of our common stock beneficially owned as of December 31, 2005 by:
| · | those persons or groups known to beneficially own more than 5% of our common stock; |
| · | each of our executive officers and directors; and |
| · | all of our directors and executive officers as a group. |
For purposes of this table, beneficial ownership is determined in accordance with Rule 13d-3 promulgated under the Securities Exchange Act of 1934. Except as indicated below, the security holders listed possess sole voting and investment power with respect to the shares beneficially owned by that person.
Name and Address of Beneficial Owner(1) | Number of Shares Owned | Percentage of Class(2) |
| George Jarkesy, Jr. (3) | 1,897,887 | 9.10% |
| Top Tier Investments (4) | 1,228,837 | 5.96% |
| Terry Wesner (5) | 458,881 | 2.20% |
| David B. McWilliams (6) | 387,973 | 1.85% |
| C. William Rouse ((7) | 311,507 | 1.50% |
| Anthony N. Kamin (8) | 170,000 | * |
| Jim C. Williams (9) | 108,376 | * |
| Sandy Livney (10) | 63,333 | * |
| Paul M. Frison (11) | 38,333 | * |
| Brooks Boveroux (12) | 16,667 | * |
| Donna R. Rill (13) | 23,617 | * |
| All directors and executive officers as a group (6 persons including the executive officers and directors listed above) | 1,383,360 | 6.44% |
_______
* Less than 1%.
-36-
| (1) | Unless otherwise indicated the address of the beneficial owner is: c/o PharmaFrontiers Corp., 2635 N. Crescent Ridge Drive, The Woodlands, Texas 77381. | |
| (2) | Ownership percentages are based on 20,619,545 shares of common stock issued and outstanding as of December 31, 2005. | |
| (3) | Includes 635,387 shares held by the Jarkesy Foundation, Inc. Mr. Jarkesy’s spouse holds voting and dispositive power with respect to these shares. (3) Address for Mr. Jarkesy and Jarkesy Foundation is 18205 Burkhardt Road, Tomball, TX 77377. | |
| (4) | Address of the beneficial owner is 1103 North Bay Front, Newport Beach, CA 92662-1237. | |
| (5) | Includes 33,332 shares of common stock that Mr. Wesner may purchase upon exercise of stock options that are currently vested or will become vested within 60 days of March 15, 2006. Includes 181,977 shares of common stock that Mr. Wesner may purchase upon exercise of warrants that are currently exercisable and held by Mr. Wesner and his wife Mary Ann Wesner. | |
| (6) | Includes 243,333 shares of common stock that Mr. McWilliams may purchase upon exercise of stock options that are currently vested or will become vested within 60 days of March 15, 2006. Includes 78,383 shares of common stock that Mr. McWilliams may purchase upon exercise of warrants that are currently exercisable. | |
| (7) | Includes 133,333 shares of common stock that Mr. Rouse may purchase upon exercise of stock options that are currently vested or will become vested within 60 days of March 15, 2006. Includes 13,753 shares of common stock that Mr. Rouse may purchase upon exercise of warrants that are currently exercisable. | |
| (8) | Includes 65,000 shares of common stock that Mr. Kamin may purchase upon exercise of stock options that are currently vested or will become vested within 60 days of March 15, 2006. | |
| (9) | Includes 41,667 shares of common stock that Dr. Williams purchase upon exercise of stock options that are currently vested or will become vested within 60 days of March 15, 2006. Includes 30,991 shares of common stock that Dr. Williams may purchase upon exercise of warrants that are currently exercisable. | |
| (10) | Includes 10,000 shares owned by Mrs. Livney’s husband, David. Includes 13,333 shares of common stock that Mrs. Livney may purchase upon exercise of stock options that are currently vested or will become vested within 60 days of March 15, 2006. Includes 15,000 shares of common stock that Mrs. Livney may purchase upon exercise of warrants that are currently exercisable. Includes 15,000 shares of common stock that Mrs. Livney’s husband, David, may purchase upon exercise of warrants that are currently exercisable. | |
| (11) | Includes 38,333 shares of common stock that Mr. Frison may purchase upon exercise of stock options that are currently vested or will become vested within 60 days of March 15, 2006. | |
| (12) | Includes 16,667 shares of common stock that Mr. Boveroux may purchase upon exercise of stock options that are currently vested or will become vested within 60 days of March 15, 2006. | |
| (13) | Includes 20,000 shares of common stock that Mrs. Rill may purchase upon exercise of stock options that are currently vested or will become vested within 60 days of March 15, 2006. |
-37-
SELLING STOCKHOLDERS
No stockholder may offer or sell shares of our common stock under this prospectus unless such stockholder has notified us of his or her intention to sell shares of our common stock and this prospectus has been declared effective by the SEC, and remains effective at the time such selling stockholder offers or sells such shares. We are required to amend this prospectus to reflect material developments in our business, financial position and results of operations. Each time we file an amendment to this prospectus with the SEC, it must first be declared effective prior to the offer or sale of shares of our common stock by the selling stockholders.
The common stock covered by this prospectus is to be offered for the account of the selling stockholders in the following table. The selling stockholders may from time to time sell all, some or none of the shares of common stock offered by this prospectus.
The following table, which we have prepared based on information provided to us by the applicable selling stockholder, sets forth the name, the number of shares of common stock beneficially owned by the selling stockholders intending to sell our common stock and the number of shares of common stock to be offered. Unless set forth below, none of the selling stockholders selling in connection with the prospectus has held any position or office with, been employed by, or otherwise has had a material relationship with us or any of our affiliates during the three years prior to the date of the prospectus.
Name of Selling Stockholder | Footnote Numbers | Number of Shares of Common Stock Beneficially Owned (1) | Number of Shares of Common Stock Offered Hereunder | Number and % of Outstanding Shares of Common Stock Owned After Completion of Offering | ||||||||||||
Number | % (2) | |||||||||||||||
| 682501 Alberta Ltd. | 3. | 41,668 | 41,668 | -0- | * | |||||||||||
| AFDSMSSAS, L.P. | 4. | 35,000 | 35,000 | -0- | * | |||||||||||
| Albert and Margaret Alkek Foundation | 5. | 416,668 | 416,668 | -0- | * | |||||||||||
| Alkek & Williams Ventures | 6. | 341,668 | 341,668 | 0- | * | |||||||||||
| Alpine Atlantic Asset Management AG | 7. | 868,863 | 868,863 | -0- | * | |||||||||||
| Anastasios & Tabitha Belesis | 8. | 1,867 | 1,867 | -0- | * |
-38-
Name of Selling Stockholder | Footnote Numbers | Number of Shares of Common Stock Beneficially Owned (1) | Number of Shares of Common Stock Offered Hereunder | Number and % of Outstanding Shares of Common Stock Owned After Completion of Offering | ||||||||||||
Number | % (2) | |||||||||||||||
| Andrew B. & Shanna Sue Linbeck | 9. | 85,000 | 85,000 | -0- | * | |||||||||||
| Anthony J. Spatacco, Jr. | 10. | 51,547 | 51,547 | -0- | * | |||||||||||
| Anthony M. Sensoli | 11. | 176,263 | 176,263 | -0- | * | |||||||||||
| Anthony M. Sensoli, IRA Charles Schwab & Co., Inc. Custodian | 12. | 31,247 | 31,247 | -0- | * | |||||||||||
| Archie McK Malone | 13. | 93,674 | 93,674 | -0- | * | |||||||||||
| Armand LaSorsa | 14. | 165 | 165 | -0- | * | |||||||||||
| Arthur J. & Phyllis C. Goodwin 2001 Family Trust Dated 4-26-01 | 15. | 20,824 | 20,824 | -0- | * | |||||||||||
Ball Family Trust dtd 03/08/96 Richard K. Ball & Polly Ball Co-TTEEs | 16. | 31 | 31 | -0- | * | |||||||||||
| Beverly B. Arnold | 17. | 166,668 | 166,668 | -0- | * | |||||||||||
| Beverly E. Wrubel | 18. | 20,756 | 20,756 | -0- | * | |||||||||||
| Billie Willmon Jenkin | 19. | 42,237 | 42,237 | -0- | * | |||||||||||
| Bobby D. Perry | 20. | 83,335 | 83,335 | -0- | * | |||||||||||
| Bradley S. Stewart | 21. | 53,961 | 53,961 | -0- | * | |||||||||||
| Brewer & Pritchard, PC | 22. | 365,838 | 365,838 | -0- | * | |||||||||||
| Bruce C. Marek | 23. | 215,571 | 215,571 | -0- | * | |||||||||||
| Bruno Nordberg | 24. | 41,750 | 41,750 | -0- | * | |||||||||||
| Bruno or Joan A. Nordberg, JWROS | 25. | 51,838 | 51,838 | -0- | * | |||||||||||
| C. William Rouse | 26. | 311,505 | 26,672 | 284,833 | * |
-39-
Name of Selling Stockholder | Footnote Numbers | Number of Shares of Common Stock Beneficially Owned (1) | Number of Shares of Common Stock Offered Hereunder | Number and % of Outstanding Shares of Common Stock Owned After Completion of Offering | ||||||||||||
Number | % (2) | |||||||||||||||
| Cameron Living Trust Ltd 8/31/95 | 27. | 20,735 | 20,735 | -0- | * | |||||||||||
| Capital Growth Planning | 28. | 46 | 46 | -0- | * | |||||||||||
| Carmelo Troccoli | 29. | 350 | 350 | -0- | * | |||||||||||
| Centrum Bank AG | 30. | 206,667 | 206,667 | -0- | * | |||||||||||
| Charles L. Bradley | 31. | 83,335 | 83,335 | -0- | * | |||||||||||
| Cimarron Biomedical Equity Master Fund, L.P. | 32. | 416,668 | 416,668 | -0- | * | |||||||||||
Citigroup Global Markets Custodian FBO Mary Ann Wesner, 2004 Roth IRA | 33. | 9,288 | 9,288 | -0- | * | |||||||||||
Citigroup Global Markets Custodian FBO Mary Ann Wesner, 2005 Roth IRA | 34. | 5,064 | 5,064 | -0- | * | |||||||||||
| Citigroup Global Markets Custodian FBO Terry Wesner, 2004 Roth IRA | 35. | 7,224 | 7,224 | -0- | * | |||||||||||
| Citigroup Global Markets Custodian FBO Terry Wesner, 2005 Roth IRA | 36. | 9,288 | 9,288 | -0- | * | |||||||||||
| CKW LLC | 37. | 41,668 | 41,668 | -0- | * | |||||||||||
| Clariden Investments LTD | 38. | 107,785 | 107,785 | -0- | * | |||||||||||
| Clemente Capital Management, LLC | 39. | 3,733 | 3,733 | -0- | * | |||||||||||
| Crestview Capital Master, LLC | 40. | 1,250,000 | 1,250,000 | -0- | * | |||||||||||
| Crutchfield Family 1976 Trust | 41. | 103,402 | 103,402 | -0- | * | |||||||||||
| Dale W. Spradling | 42. | 345,502 | 215,502 | 130,000 | * | |||||||||||
| Daniel L. Zimmerman | 43. | 51,924 | 51,924 | -0- | * |
-40-
Name of Selling Stockholder | Footnote Numbers | Number of Shares of Common Stock Beneficially Owned (1) | Number of Shares of Common Stock Offered Hereunder | Number and % of Outstanding Shares of Common Stock Owned After Completion of Offering | ||||||||||||
Number | % (2) | |||||||||||||||
| David B. McWilliams | 44. | 387,972 | 142,639 | 245,333 | * | |||||||||||
| David Carl Lustig, III | 45. | 33,335 | 33,335 | -0- | * | |||||||||||
| David Livney | 46. | 25,000 | 25,000 | -0- | * | |||||||||||
| David P. Haswell | 47. | 21,180 | 21,180 | -0- | * | |||||||||||
| David R. & Alice M. Evers | 48. | 154 | 154 | -0- | * | |||||||||||
| Davis Investments V LP | 49. | 1,532,089 | 1,474,316 | 57,773 | * | |||||||||||
| Delaware Charter Guaranty & Trust fbo Andre Guay, IRA | 50. | 16,851 | 16,851 | -0- | * | |||||||||||
| Delaware Charter Guaranty & Trust fbo Gisele Guay, IRA | 51. | 25,277 | 25,277 | -0- | * | |||||||||||
| Delaware Charter Guaranty & Trust fbo Ronald Brangwyn, IRA | 52. | 21,256 | 21,256 | -0- | * | |||||||||||
| Dietrich & Rosemarie Riemer | 53. | 50,000 | 50,000 | -0- | * | |||||||||||
| DLD Family Investments, LLC | 54. | 333,335 | 333,335 | -0- | * | |||||||||||
| Donald G. Stewart | 55. | 202,113 | 202,113 | -0- | * | |||||||||||
| Donald J. Zinda | 56. | 31 | 31 | -0- | * | |||||||||||
| Donald M. Ureel Trust dtd 09/24/97; Donald M. Ureel, TTEE | 57. | 240 | 240 | -0- | * | |||||||||||
| Donald Zinman | 58. | 206 | 206 | -0- | * | |||||||||||
| Douglas Alan Jenkin | 59. | 83,050 | 83,050 | -0- | * | |||||||||||
| Douglas J. Cook & Christine Cook | 60. | 31 | 31 | -0- | * | |||||||||||
| Douglas Miller | 61. | 6,548 | 6,548 | -0- | * |
-41-
Name of Selling Stockholder | Footnote Numbers | Number of Shares of Common Stock Beneficially Owned (1) | Number of Shares of Common Stock Offered Hereunder | Number and % of Outstanding Shares of Common Stock Owned After Completion of Offering | ||||||||||||
Number | % (2) | |||||||||||||||
| E. Elaine Schuster | 62. | 41,553 | 41,553 | -0- | * | |||||||||||
| E55 LP | 63. | 41,668 | 41,668 | -0- | * | |||||||||||
| Edward W. Gray and Sharon H. Gray | 64. | 20,694 | 20,694 | -0- | * | |||||||||||
| Elizabeth J. Hanson | 65. | 50,000 | 50,000 | -0- | * | |||||||||||
| Elizabeth J. Hanson, IRA | 66. | 20,783 | 20,783 | -0- | * | |||||||||||
| Enable Growth Partners LP | 67. | 1,322,260 | 1,322,260 | -0- | * | |||||||||||
| Enable Opportunity Partners LP | 68. | 150,000 | 150,000 | -0- | * | |||||||||||
| Ervin Living Trust | 69. | 42,333 | 42,333 | -0- | * | |||||||||||
| Ervin Living Trust Dtd.7/6/95, Robert D. Ervin & Rita Y. Ervin Co-TTEES | 70. | 41,668 | 41,668 | -0- | * | |||||||||||
| First Trust Corporation TTEE FBO: Lynn C. Kalcic | 71. | 9,963 | 9,963 | -0- | * | |||||||||||
| First Trust Corporation TTEE FBO: Mary A. Kalcic | 72. | 27,387 | 27,387 | -0- | * | |||||||||||
| Frank M. Mandola | 73. | 86,228 | 86,228 | -0- | * | |||||||||||
| Fred S. Harper | 74. | 114,241 | 114,241 | -0- | * | |||||||||||
| Gary Hanson & Elizabeth Hanson | 75. | 22,239 | 22,239 | -0- | * | |||||||||||
| Gemini Master Fund, Ltd. | 76. | 207,694 | 207,694 | -0- | * | |||||||||||
| Geoffrey Kopecky | 77. | 556 | 556 | -0- | * | |||||||||||
| George E. Liberato | 78. | 43,114 | 43,114 | -0- | * | |||||||||||
| George Jarkesy, Jr. | 1,212,500 | 1,212,500 | -0- | * |
-42-
Name of Selling Stockholder | Footnote Numbers | Number of Shares of Common Stock Beneficially Owned (1) | Number of Shares of Common Stock Offered Hereunder | Number and % of Outstanding Shares of Common Stock Owned After Completion of Offering | ||||||||||||
Number | % (2) | |||||||||||||||
| George and Linda Boston | 79. | 8,250 | 8,250 | -0- | * | |||||||||||
| Gerald L. King & Sherry J. King | 80. | 31 | 31 | -0- | * | |||||||||||
| Gerald W. Brown | 81. | 31 | 31 | -0- | * | |||||||||||
Greeley Orthodontic Center, P.C. Profit Sharing Trust fbo Gary J. Kloberdanz | 82. | 171 | 171 | -0- | * | |||||||||||
| Gregg Lerman | 83. | 3,733 | 3,733 | -0- | * | |||||||||||
| H. Michael Lambert | 84. | 258,105 | 213,105 | 45,000 | * | |||||||||||
| Harold E. Tellefsen Trust | 85. | 58,269 | 58,269 | -0- | * | |||||||||||
| Harry Groszecki | 86. | 75,000 | 75,000 | -0- | * | |||||||||||
| HRBFA Custo. of the IRA FBO Mary Ann Sharrow | 87. | 75,016 | 75,016 | -0- | * | |||||||||||
| HRBFA Custo. of the IRA FBO Paul G. Sharrow | 88. | 70,849 | 70,849 | -0- | * | |||||||||||
| I. Dwyane Davis | 89. | 82,987 | 82,987 | -0- | * | |||||||||||
| Insiders Trend Fund LP | 90. | 90,541 | 90,541 | -0- | * | |||||||||||
| J. Roy Jones & James M. Jones, Charitable Remainder Unitrust | 91. | 31 | 31 | -0- | * | |||||||||||
| Jack Dulworth | 92. | 83,335 | 83,335 | -0- | * | |||||||||||
| Jack M. Franks Revocable Trust dtd 06/25/91; Jack M. Franks, TTEE) | 93. | 103 | 103 | -0- | * | |||||||||||
| James A. Boston | 94. | 25,335 | 25,335 | -0- | * | |||||||||||
| James E. Striedel | 95. | 107,135 | 107,135 | -0- | * | |||||||||||
| James G. Geistfeld Living Trust | 96. | 20,763 | 20,763 | -0- | * |
-43-
Name of Selling Stockholder | Footnote Numbers | Number of Shares of Common Stock Beneficially Owned (1) | Number of Shares of Common Stock Offered Hereunder | Number and % of Outstanding Shares of Common Stock Owned After Completion of Offering | ||||||||||||
Number | % (2) | |||||||||||||||
| Jarkesy Foundation, Inc. | 97. | 635,387 | 635,387 | -0- | * | |||||||||||
| Jaye S. Venuti, D.D.S. Retirement Plan Trust; Jaye S. Venuti & Michael Yokoyama, TTEES | 98. | 137 | 137 | -0- | * | |||||||||||
| Jerome T. Usalis | 99. | 606,886 | 606,886 | -0- | * | |||||||||||
| Jerry Sexton | 100. | 3,949 | 3,949 | -0- | * | |||||||||||
| Jessica Spradling | 101. | 250,000 | 250,000 | -0- | * | |||||||||||
| Jimmy C. Williams | 102. | 108,376 | 54,652 | 53,724 | * | |||||||||||
| John C. Bult, TTEE | 103. | 31 | 31 | -0- | * | |||||||||||
| John G. Ariko, Jr. | 104. | 343 | 343 | -0- | * | |||||||||||
| John H. Crutchfield | 105. | 206,872 | 206,872 | -0- | * | |||||||||||
| John Parmigiani | 106. | 1,867 | 1,867 | -0- | * | |||||||||||
| John T. Borgese | 107. | 99,053 | 99,053 | -0- | * | |||||||||||
| Jonathan Rich | 108. | 333 | 333 | -0- | * | |||||||||||
| Joseph D. Mandola | 109. | 171,010 | 171,010 | -0- | * | |||||||||||
| Joseph L. Draskovich | 110. | 21,167 | 21,167 | -0- | * | |||||||||||
| Joyce E. Burris | 111. | 21,221 | 21,221 | -0- | * | |||||||||||
| JSM Capital Holdings Corp. | 112. | 1,225 | 1,225 | -0- | * | |||||||||||
| Kalcic Exemption Trust | 113. | 21,208 | 21,208 | -0- | * |
-44-
Name of Selling Stockholder | Footnote Numbers | Number of Shares of Common Stock Beneficially Owned (1) | Number of Shares of Common Stock Offered Hereunder | Number and % of Outstanding Shares of Common Stock Owned After Completion of Offering | ||||||||||||
Number | % (2) | |||||||||||||||
| Kenneth & Jill Flint, TTEES - The Kenneth W. Flint Family Protection Tr. | 114. | 62 | 62 | -0- | * | |||||||||||
| Kenneth O. Stahl/Frederick R. Stahl, Jr. (POA) | 115. | 15 | 15 | -0- | * | |||||||||||
| Kirk Folkerts | 116. | 202,586 | 149,419 | 53,167 | * | |||||||||||
| Lakeview Direct Investments, LP | 117. | 166,668 | 166,668 | -0- | * | |||||||||||
| Larry R. Cramer | 118. | 339 | 339 | -0- | * | |||||||||||
| Lawrence S. Yunker | 119. | 12,371 | 12,371 | -0- | * | |||||||||||
| LB (Swiss) Private Bank LTD | 120. | 53,876 | 53,876 | -0- | * | |||||||||||
| Linda M. Barone/Larry R. Zilli | 121. | 51,941 | 51,941 | -0- | * | |||||||||||
| Liparus, LLC | 122. | 20,742 | 20,742 | -0- | * | |||||||||||
| Lippert Heilshorn & Associates Inc. | 123. | 33,225 | 33,225 | -0- | * | |||||||||||
| Lone Star No. 1, Ltd | 124. | 85,000 | 85,000 | -0- | * | |||||||||||
| Lorie Cook | 125. | 2,619 | 2,619 | -0- | * | |||||||||||
| Louis R. Reif | 126. | 75,534 | 52,934 | 22,600 | * | |||||||||||
| Lynn Rach | 127. | 80 | 80 | -0- | * | |||||||||||
| Marcus F. Wray | 128. | 41,668 | 41,668 | -0- | * | |||||||||||
| Mark A. Stewart | 129. | 244,424 | 191,257 | 53,167 | * | |||||||||||
| Mark S. Boland | 130. | 83,754 | 83,754 | -0- | * |
-45-
Name of Selling Stockholder | Footnote Numbers | Number of Shares of Common Stock Beneficially Owned (1) | Number of Shares of Common Stock Offered Hereunder | Number and % of Outstanding Shares of Common Stock Owned After Completion of Offering | ||||||||||||
Number | % (2) | |||||||||||||||
| Mark Stutzman | 131. | 5,354 | 5,354 | -0- | * | |||||||||||
| Mary A. Kalcic Irrevocable Trust dtd 11/11/87 - Paul Kalcic, TTEE | 132. | 31 | 31 | -0- | * | |||||||||||
| Michael and Kristine Marrale | 133. | 50,000 | 50,000 | -0- | * | |||||||||||
| Michael Hamblett | 134. | 103,402 | 103,402 | -0- | * | |||||||||||
| Michael K. Boudreaux | 135. | 20,728 | 20,728 | -0- | * | |||||||||||
| Michael C. Neumann | 136. | 20,975 | 20,975 | -0- | * | |||||||||||
| Millard B. Ryland, IRA | 137. | 58,333 | 58,333 | -0- | * | |||||||||||
| Mitchell Sassower | 138. | 25,000 | 25,000 | -0- | * | |||||||||||
| Monarch Capital Group, LLC | 139. | 850 | 850 | -0- | * | |||||||||||
| Nancy A. Korpi | 140. | 206 | 206 | -0- | * | |||||||||||
| Nancy R. Greer Linn | 141. | 41,237 | 41,237 | -0- | * | |||||||||||
| Nelson Wooster Living Trust; Nelson Wooster, TTEE | 142. | 15 | 15 | -0- | * | |||||||||||
| NFS LLC/FMTC FBO Richard E Crawford | 143. | 103,847 | 103,847 | -0- | * | |||||||||||
| Nick Lippuner & Marianne Lippuner | 144. | 62,951 | 62,951 | -0- | * | |||||||||||
| Norman R. Morris Living Trust | 145. | 41,668 | 41,668 | -0- | * | |||||||||||
| Oxnard Camarillo Anesthesiologist Group MPP; Vance L. Kalcic TTEE | 146. | 171 | 171 | -0- | * | |||||||||||
| Pamela Dru Sutton | 147. | 51,804 | 51,804 | -0- | * | |||||||||||
| Panacea Fund, LLC | 148. | 417,500 | 417,500 | -0- | * | |||||||||||
| Pankaj A. Patel | 149. | 207,146 | 207,146 | -0- | * |
-46-
Name of Selling Stockholder | Footnote Numbers | Number of Shares of Common Stock Beneficially Owned (1) | Number of Shares of Common Stock Offered Hereunder | Number and % of Outstanding Shares of Common Stock Owned After Completion of Offering | ||||||||||||
Number | % (2) | |||||||||||||||
| Parsifal Investments, L.P. | 150. | 87,500 | 87,500 | -0- | * | |||||||||||
| Patrick Linbeck | 151. | 78,607 | 28,607 | 50,000 | * | |||||||||||
| Paul G. Sharrow | 152. | 20,763 | 20,763 | -0- | * | |||||||||||
| Paul Masters, IRA | 153. | 103,128 | 103,128 | -0- | * | |||||||||||
| Pershing LLC as Custodian FBO Kinnary Patel Rollover IRA | 154. | 82,475 | 82,475 | -0- | * | |||||||||||
| Pershing LLC as Custodian FBO Kinnary Patel Roth IRA | 155. | 82,475 | 82,475 | -0- | * | |||||||||||
| Peter Bischofberger | 156. | 46 | 46 | -0- | * | |||||||||||
| Pinnacle Trust Co., LTA | 157. | 416,668 | 416,668 | -0- | * | |||||||||||
| Plum Glen Partners, L.P.; Jerry Mendelson General Partner | 158. | 171 | 171 | -0- | * | |||||||||||
| Provident Premier Master Fund, Ltd | 159. | 414,292 | 414,292 | -0- | * | |||||||||||
| Ratcliff Investments; Attn: Robert Ratcliff | 160. | 171 | 171 | -0- | * | |||||||||||
| Renaissance Interests, L.P. | 161. | 175,000 | 175,000 | -0- | * | |||||||||||
| Richard N. Ernst | 162. | 207,694 | 207,694 | -0- | * | |||||||||||
| Richard T. Jeleniewski | 163. | 52,026 | 52,026 | -0- | * | |||||||||||
| Robert F. Donathan | 164. | 206,872 | 206,872 | -0- | * | |||||||||||
| Robert H. & Joy D. Caldwell, TTEES - Caldwell Family Trust - U/A dated 7/22/85 | 165. | 15 | 15 | -0- | * | |||||||||||
| Roland Hartman | 166. | 303,881 | 103,881 | 200,000 | * | |||||||||||
| Ronald Brangwyn | 167. | 41,668 | 41,668 | -0- | * |
-47-
Name of Selling Stockholder | Footnote Numbers | Number of Shares of Common Stock Beneficially Owned (1) | Number of Shares of Common Stock Offered Hereunder | Number and % of Outstanding Shares of Common Stock Owned After Completion of Offering | ||||||||||||
Number | % (2) | |||||||||||||||
Rudy Aguirre and Therese Mosqueda Ponce | 168. | 21,256 | 21,256 | -0- | * | |||||||||||
| S. Edmund Resciniti | 169. | 83,335 | 83,335 | -0- | * | |||||||||||
| SAA Trust | 170. | 41,750 | 41,750 | -0- | * | |||||||||||
| SAA Trust, Paul & MaryAnn Mallis TTEES | 171. | 31,113 | 31,113 | -0- | * | |||||||||||
| Salient Partners | 172. | 87,920 | 87,920 | -0- | * | |||||||||||
| Sam Buck | 173. | 50,000 | 50,000 | -0- | * | |||||||||||
| Sanders Morris Harris | 174. | 540,142 | 540,142 | -0- | * | |||||||||||
| Sandra L. Livney | 175. | 38,333 | 25,000 | 13,333 | * | |||||||||||
| Schroder & Co Bank AG | 176. | 366,354 | 366,354 | -0- | * | |||||||||||
| Scott B. Seaman | 177. | 100,000 | 100,000 | -0- | * | |||||||||||
| Scott Shapiro | 178. | 3,733 | 3,733 | -0- | * | |||||||||||
| Shantilal C. Patidar | 179. | 139,944 | 94,944 | 45,000 | * | |||||||||||
| SIBEX Capital Fund, Inc. | 180. | 500,000 | 500,000 | - | * | |||||||||||
| SMI Re Limited | 181. | 2,558,265 | 2,358,265 | 200,000 | * | |||||||||||
| Snehal Patel | 182. | 322,316 | 322,316 | -0- | * | |||||||||||
| Snehal S Patel & Kinnary Patel, Jt. Tenants in Common | 183. | 685,073 | 685,073 | -0- | * | |||||||||||
| Stahl Family Revocable Living Trust dated 8-23-01 | 184. | 21,174 | 21,174 | -0- | * | |||||||||||
| Starboard Capital Markets | 185. | 1,000 | 1,000 | -0- | * |
-48-
Name of Selling Stockholder | Footnote Numbers | Number of Shares of Common Stock Beneficially Owned (1) | Number of Shares of Common Stock Offered Hereunder | Number and % of Outstanding Shares of Common Stock Owned After Completion of Offering | ||||||||||||
Number | % (2) | |||||||||||||||
| Stephen Cox | 186. | 2,711 | 2,711 | -0- | * | |||||||||||
| Sterling Trust Co fbo Carol A. Wynn | 187. | 41,237 | 41,237 | -0- | * | |||||||||||
| Sterling Trust Company, Custodian fbo Harold E. Tellefsen | 188. | 38,421 | 38,421 | -0- | * | |||||||||||
| Steven Sack | 189. | 50,000 | 50,000 | -0- | * | |||||||||||
| Stone & Sutton, P.A. P/S Trust Pam Sutton, Trustee | 190. | 20,721 | 20,721 | -0- | * | |||||||||||
| Suzette Brown Special Needs Trust - Melodie Z. Scott, Trustee | 191. | 46 | 46 | -0- | * | |||||||||||
| Sylvan Associates; Paul A. Kalcic Managing Partner | 192. | 31 | 31 | -0- | * | |||||||||||
| T. William Merrill | 193. | 83,335 | 83,335 | -0- | * | |||||||||||
| TCMP3 Partners, L.P. | 194. | 207,626 | 207,626 | -0- | * | |||||||||||
| Terry H. Wesner | 195. | 302,771 | 215,365 | 87,406 | * | |||||||||||
| Terry Wesner & MaryAnn Wesner | 196. | 106,553 | 106,553 | -0- | * | |||||||||||
| The Barr Asset Family Lt. Partnership, Warren Barr, General Partner | 197. | 31 | 31 | -0- | * | |||||||||||
| The G. W. Sleezer Revocable Trust dtd 12/04/89 | 198. | 309 | 309 | -0- | * | |||||||||||
| The Hazen A. Sandwick & Josephine Sandwick Revocable Living Trust | 199. | 62 | 62 | -0- | * | |||||||||||
| Thomas K. Benedict & Liesbeth L. Benedict | 200. | 199 | 199 | -0- | * | |||||||||||
| Thomas S. Brower | 201. | 15 | 15 | -0- | * | |||||||||||
| Thomas Suppanz | 202. | 3,500 | 3,500 | -0- | * | |||||||||||
| Thomas Thompson | 203. | 4,630 | 4,630 | -0- | * |
-49-
Name of Selling Stockholder | Footnote Numbers | Number of Shares of Common Stock Beneficially Owned (1) | Number of Shares of Common Stock Offered Hereunder | Number and % of Outstanding Shares of Common Stock Owned After Completion of Offering | ||||||||||||
Number | % (2) | |||||||||||||||
| Timothy L. Brawner | 204. | 20,763 | 20,763 | -0- | * | |||||||||||
| Todd R. Allen | 205. | 62,513 | 62,513 | -0- | * | |||||||||||
| Tom Tice | 206. | 2,619 | 2,619 | -0- | * | |||||||||||
| Trappe & Dusseault P.A. Profit Sharing Plan Trust 59-2351454; Owen S. Trappe & Brian Dusseault, TTEES | 207. | 171 | 171 | -0- | * | |||||||||||
| Trevor J. Brown, Inc. DB Pension Plan, Trevor J. Brown &/or Annette Kowalaski, TTEES | 208. | 154 | 154 | -0- | * | |||||||||||
| University of Chicago | 550,397 | 550,397 | -0- | * | ||||||||||||
| vFinance Managed by Jonathan C. Rich | 209. | 8,592 | 8,592 | -0- | * | |||||||||||
| Walter Miller | 210. | 6,814 | 6,814 | -0- | * | |||||||||||
| Walter W. Pollack, Jr. | 211. | 250,000 | 250,000 | -0- | * | |||||||||||
| Yellowstone Equity Partners, Ltd. | 212. | 162,500 | 162,500 | -0- | * |
FOOTNOTES:
| 1. | Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission and generally includes voting or investment power with respect to securities. Shares of common stock subject to options or warrants currently exercisable or exercisable within 60 days of March 16, 2005 are deemed outstanding for computing the percentage of the person holding such option or warrant but are not deemed outstanding for computing the percentage of any other person. |
| 2. | Percentage is based on 20,967,035 shares of common stock outstanding. |
| 3. | Includes 25,001 shares of common stock underlying warrants |
| 4. | Includes 21,000 shares of common stock underlying warrants. Saleh M Shenaq exercises voting and dispositive power over all of the shares beneficially owned by AFDSMSSAS. |
-50-
| 5. | Includes 250,001 shares of common stock underlying warrants. Albert & Margaret Alkek Foundation is a private investment fund. Scott Seaman exercises voting and dispositive power over all of the shares beneficially owned by Albert & Margaret Alkek Foundation. |
| 6. | Includes 205,001 shares of common stock underlying warrants. Alkek & Williams Ventures is a private investment fund. Scott Seaman exercises voting and dispositive power over all of the shares beneficially owned by Alkek & Williams Ventures. |
| 7. | Includes 445,718 shares of common stock underlying warrants. Alpine Atlantic Asset Management AG is a private investment fund. Willy Betschart exercises voting and dispositive power over all of the shares beneficially owned Alpine, a Zurich based private investment fund. |
| 8. | Includes 1,867 shares of common stock underlying warrants. Anastasios and Tabitha Belesis are financial consultants and acquired these securities for a result of the Company’s June/July 2005 funding. |
| 9. | Includes 51,000 shares of common stock underlying warrants. |
| 10. | Includes 26,428 shares of common stock underlying warrants. |
| 11. | Includes 90,458 shares of common stock underlying warrants. |
| 12. | Includes 16,048 shares of common stock underlying warrants. Anthony M. Sensoli exercises voting and dispositive power over all of the shares beneficially owned by Anthony M. Sensoli, IRA Charles Schwab & Co., Inc. Custodian. |
| 13. | Includes 51,704 shares of common stock underlying warrants. |
| 14. | Includes 165 shares of common stock underlying warrants. |
| 15. | Includes 10,695 shares of common stock underlying warrants. Arthur J. Goodwin exercises voting and dispositive power over all of the shares beneficially owned by Arthur J. & Phyllis C. Goodwin 2001 Family Trust Dated 4-26-01. |
| 16. | Includes 31 shares of common stock underlying warrants. |
| 17. | Includes 100,001shares of common stock underlying warrants. |
| 18. | Includes 10,653 shares of common stock underlying warrants. |
| 19. | Includes 21,742 shares of common stock underlying warrants. |
| 20. | Includes 50,001 shares of common stock underlying warrants. |
| 21. | Includes 27,877 shares of common stock underlying warrants. |
| 22. | Includes 20,001 shares of common stock underlying warrants. Brewer and Pritchard, PC is a professional corporation. Thomas Pritchard exercises voting and dispositive power over all of the shares beneficially owned by Brewer and Pritchard PC. |
| 23. | Includes 111,342 shares of common stock underlying warrants. |
-51-
| 24. | Includes 25,050 shares of common stock underlying warrants. |
| 25. | Includes 26,603 shares of common stock underlying warrants. |
| 26. | Includes 13,753 shares of common stock underlying warrants and 133,333 options that are vested with an exercise price of $3.00. |
| 27. | Includes 10,641 shares of common stock underlying warrants. Mr. George R. Cameron exercises voting and dispositive power over all of the shares beneficially owned by Cameron Living Trust. |
| 28. | Includes 46 shares of common stock underlying warrants. |
| 29. | Includes 350 shares of common stock underlying warrants. Carmelo Troccoli is a financial consultant and acquired these securities for a result of the Company’s June/July 2005 funding. |
| 30. | Includes 106,000 shares of common stock underlying warrants. William Pinamonti exercises voting and dispositive power over all of the shares beneficially owned by Centrum Bank AG. |
| 31. | Includes 50,001shares of common stock underlying warrants. |
| 32. | Includes 250,001 shares of common stock underlying warrants. Cimarron Biomedical Equity Master Fund is an investment fund. J. H. Cullum Clark has the power to vote and dispose of PharmaFrontiers Common Stock owned by Cimarron Biomedical Equity Master Fund. |
| 33. | Includes 4,763 shares of common stock underlying warrants. Mary Ann Wesner exercises voting and dispositive power over all of the shares beneficially owned by Mary Ann Wesner, 2004 Roth IRA. |
| 34. | Includes 2,597 shares of common stock underlying warrants. Mary Ann Wesner exercises voting and dispositive power over all of the shares beneficially owned by Mary Ann Wesner, 2005 Roth IRA. |
| 35. | Includes 3,704 shares of common stock underlying warrants. Terry Wesner exercises voting and dispositive power over all of the shares beneficially owned by Terry Wesner, 2004 Roth IRA. |
| 36. | Includes 4,763 shares of common stock underlying warrants. Terry Wesner exercises voting and dispositive power over all of the shares beneficially owned by Terry Wesner, 2005 Roth IRA. |
| 37. | Includes 25,001 shares of common stock underlying warrants. CKW LLC is a private investment fund. David J Kowalick exercises voting and dispositive power over all of the shares beneficially owned by CKW LLC. |
| 38. | Includes 55,671 shares of common stock underlying warrants. Clariden Investments LTD. Is a private investment fund. Ricc-Lee Ingram exercises voting and dispositive power over all of the shares beneficially owned by Clariden Investments LTD. |
| 39. | Includes 3,733 shares of common stock underlying warrants. Clemente Capital Management, LLC is an investment firm that acquired these securities for underwriting activities. Guy Clemente has the power to vote and dispose of PharmaFrontiers Common Stock owned by Clemente Capital Management, LLC. |
-52-
| 40. | Includes 750,000 shares of common stock underlying warrants. Crestview Capital Master, LLC is a private investment fund. Daniel I. Warsh exercises voting and dispositive power over all of the shares beneficially owned by Crestview Capital Master, LLC. |
| 41. | Includes 53,041 shares of common stock underlying warrants. John Crutchfield exercises voting and dispositive power over all of the shares beneficially owned by Crutchfield Family 1976 Trust |
| 42. | Includes 111,301 shares of common stock underlying warrants. |
| 43. | Includes 26,654 shares of common stock underlying warrants. |
| 44. | Includes 78,383 shares of common stock underlying warrants and 243,333 options that are vested with an exercise price of $3.00. |
| 45. | Includes 20,001 shares of common stock underlying warrants. |
| 46. | Includes 15,000 shares of common stock underlying warrants. |
| 47. | Includes 10,908 shares of common stock underlying warrants. |
| 48. | Includes 154 shares of common stock underlying warrants. |
| 49. | Includes 794,590 shares of common stock underlying warrants. Davis Investments V, LP is a private investment fund. Christopher Davis exercises voting and dispositive power over all of the shares beneficially owned by Davis Investments V, LP. |
| 50. | Includes 8,671 shares of common stock underlying warrants. Andre Guay exercises voting and dispositive power over all of the shares beneficially owned by Andre Guay, IRA. |
| 51. | Includes 13,006 shares of common stock underlying warrants. Gisele Guay exercises voting and dispositive power over all of the shares beneficially owned by Gisele Guay, IRA. |
| 52. | Includes 10,953 shares of common stock underlying warrants. Ronald Brangwyn exercises voting and dispositive power over all of the shares beneficially owned by Ronald Brangwyn, IRA. |
| 53. | Includes 30,000 shares of common stock underlying warrants. |
| 54. | Includes 200,001 shares of common stock underlying warrants. Laura Liang exercises voting and dispositive power over all of the shares beneficially owned by DLD Family Investments, LLC. |
| 55. | Includes 107,768 shares of common stock underlying warrants. |
| 56. | Includes 31 shares of common stock underlying warrants. |
| 57. | Includes 240 shares of common stock underlying warrants. |
| 58. | Includes 206 shares of common stock underlying warrants. |
| 59. | Includes 42,630 shares of common stock underlying warrants. |
| 60. | Includes 31 shares of common stock underlying warrants. |
| 61. | Includes 6,548 shares of common stock underlying warrants. |
-53-
| 62. | Includes 21,332 shares of common stock underlying warrants. |
| 63. | Includes 25,001 shares of common stock underlying warrants. Fanny Chan exercises voting and dispositive power over all of the shares beneficially owned by E55LP. |
| 64. | Includes 10,616 shares of common stock underlying warrants. |
| 65. | Includes 30,000 shares of common stock underlying warrants. |
| 66. | Includes 10,670 shares of common stock underlying warrants. Elizabeth J. Hanson exercises voting and dispositive power over all of the shares beneficially owned by Elizabeth J. Hanson, IRA. |
| 67. | Includes 739,356 shares of common stock underlying warrants. Enable Growth Partners LP is a private investment fund. Brendan O’Neil exercises voting and dispositive power over all of the shares beneficially owned by Enable Growth Partners LP. |
| 68. | Includes 90,000 shares of common stock underlying warrants. Enable Opportunity Partners LP is a private investment fund. Brendan O’Neil exercises voting and dispositive power over all of the shares beneficially owned by Enable Opportunity Partners LP. |
| 69. | Includes 21,800 shares of common stock underlying warrants. Robert D. Ervin exercises voting and dispositive power over all of the shares beneficially owned by Ervin Living Trust. |
| 70. | Includes 25,001 shares of common stock underlying warrants. Robert D. Ervin & Rita Y. Ervin Co-TTEES exercise voting and dispositive power over all of the shares beneficially owned by Ervin Living Trust Dated 7/6/95. |
| 71. | Includes 5,117 shares of common stock underlying warrants. Lynn C. Kalcic exercises voting and dispositive power over all of the shares beneficially owned by First Trust Corporation TTEE FBO: Lynn C. Kalcic. |
| 72. | Includes 14,066 shares of common stock underlying warrants. Mary A. Kalcic exercises voting and dispositive power over all of the shares beneficially owned by First Trust Corporation TTEE FBO: Mary A. Kalcic. |
| 73. | Includes 44,537 shares of common stock underlying warrants. |
| 74. | Includes 62,244 shares of common stock underlying warrants. |
| 75. | Includes 12,145 shares of common stock underlying warrants. |
| 76. | Includes 106,616 shares of common stock underlying warrants. Gemini Master Fund, Ltd. is a private investment fund. Steven Winters exercises voting and dispositive power over all of the shares beneficially owned by Gemini Master Fund, Ltd. |
| 77. | Includes 556 shares of common stock underlying warrants. |
| 78. | Includes 22,268 shares of common stock underlying warrants. |
| 79. | Includes 4,950 shares of common stock underlying warrants. |
| 80. | Includes 31 shares of common stock underlying warrants. |
-54-
| 81. | Includes 31 shares of common stock underlying warrants. |
| 82. | Includes 171 shares of common stock underlying warrants. |
| 83. | Includes 3,733 shares of common stock underlying warrants. Gregg Lerman is a financial consultant and acquired these securities for a result of the Company’s June/July 2005 funding. |
| 84. | Includes 109,863 shares of common stock underlying warrants. |
| 85. | Includes 33,521 shares of common stock underlying warrants. Harold E. Tellefsen exercises voting and dispositive power over all of the shares beneficially owned by Harold E. Tellefsen Trust. |
| 86. | Includes 45,000 shares of common stock underlying warrants. |
| 87. | Includes 38,530 shares of common stock underlying warrants. Mary Ann Sharrow exercises voting and dispositive power over all of the shares beneficially owned by HRBFA Custodian of the IRA FBO Mary Ann Sharrow. |
| 88. | Includes 36,389 shares of common stock underlying warrants. Paul G. Sharrow exercises voting and dispositive power over all of the shares beneficially owned by HRBFA Custodian of the IRA FBO Paul G. Sharrow. |
| 89. | Includes 46,192 shares of common stock underlying warrants. |
| 90. | Includes 46,674 shares of common stock underlying warrants. Insiders Trend Fund LP is a private investment fund. Anthony Marchese exercises voting and dispositive power over all of the shares beneficially owned by Insiders Trend Fund LP. |
| 91. | Includes 31 shares of common stock underlying warrants. |
| 92. | Includes 50,001 shares of common stock underlying warrants. |
| 93. | Includes 103 shares of common stock underlying warrants. |
| 94. | Includes 21,301 shares of common stock underlying warrants. |
| 95. | Includes 55,281 shares of common stock underlying warrants. |
| 96. | Includes 10,658 shares of common stock underlying warrants. James G. Geistfeld exercises voting and dispositive power over all of the shares beneficially owned by James G. Geistfeld Living Trust. |
| 97. | Includes 239,632 shares of common stock underlying warrants. George Jarkesy Jr. exercises voting and dispositive power over all of the shares beneficially owned by Jarkesy Foundation. |
| 98. | Includes 137 shares of common stock underlying warrants. |
| 99. | Includes 361,432 shares of common stock underlying warrants. |
| 100. | Includes 3,949 shares of common stock underlying warrants. |
| 101. | Includes 150,000 shares of common stock underlying warrants. |
-55-
| 102. | Includes 30,991 shares of common stock underlying warrants and 41,667 options that are vested with an exercise price of $3.00. |
| 103. | Includes 31 shares of common stock underlying warrants. |
| 104. | Includes 343 shares of common stock underlying warrants. |
| 105. | Includes 106,123 shares of common stock underlying warrants. |
| 106. | Includes 1,867 shares of common stock underlying warrants. John Parmigiani is a financial consultant and acquired these securities for a result of the Company’s June/July 2005 funding. |
| 107. | Includes 55,832 shares of common stock underlying warrants. |
| 108. | Includes 333 shares of common stock underlying warrants. Jonathan Rich is a financial consultant and acquired these securities for a result of the Company’s June/July 2005 funding. |
| 109. | Includes 91,806 shares of common stock underlying warrants. |
| 110. | Includes 10,900 shares of common stock underlying warrants. |
| 111. | Includes 10,933 shares of common stock underlying warrants. |
| 112. | Includes 1,225 shares of common stock underlying warrants. JSM Capital Holdings Corp. is a financial consultant and acquired these securities for a result of the Company’s June/July 2005 funding. |
| 113. | Includes 10,925 shares of common stock underlying warrants. Paul A Kalic exercises voting and dispositive power over all of the shares beneficially owned by Kalic Exemption Trust. |
| 114. | Includes 62 shares of common stock underlying warrants. |
| 115. | Includes 15 shares of common stock underlying warrants. |
| 116. | Includes 80,651 shares of common stock underlying warrants. |
| 117. | Includes 100,001 shares of common stock underlying warrants. Thomas Elden exercises voting and dispositive power over all of the shares beneficially owned by Lakeview Direct Investments, LP. |
| 118. | Includes 339 shares of common stock underlying warrants. |
| 119. | Includes 6,343 shares of common stock underlying warrants. |
| 120. | Includes 27,825 shares of common stock underlying warrants. Olaf Herr exercises voting and dispositive power over all of the shares beneficially owned by LB (Swiss) Private Bank LTD. |
| 121. | Includes 26,664 shares of common stock underlying warrants. |
| 122. | Includes 10,645 shares of common stock underlying warrants. Gregory Mallis exercises voting and dispositive power over all of the shares beneficially owned by Liparus, LLC. |
| 123. | Includes 19,935 shares underlying warrants. Keith L. Lippert has the power to vote and dispose of the shares owned by Lippert Heilshorn & Associates, Inc. |
-56-
| 124. | Includes 51,000 shares of common stock underlying warrants. James H. Glanville exercises voting and dispositive power over all of the shares beneficially owned by Lone Star No. 1, Ltd |
| 125. | Includes 2,619 shares of common stock underlying warrants. |
| 126. | Includes 27,260 shares of common stock underlying warrants. |
| 127. | Includes 80 shares of common stock underlying warrants. |
| 128. | Includes 25,001 shares of common stock underlying warrants. |
| 129. | Includes 105,754 shares of common stock underlying warrants. |
| 130. | Includes 46,653 shares of common stock underlying warrants. |
| 131. | Includes 5,354 shares of common stock underlying warrants. |
| 132. | Includes 31 shares of common stock underlying warrants. |
| 133. | Includes 30,000 shares of common stock underlying warrants. |
| 134. | Includes 53,041shares of common stock underlying warrants. |
| 135. | Includes 10,637 shares of common stock underlying warrants. |
| 136. | Includes 10,785 shares of common stock underlying warrants. |
| 137. | Includes 35,000 shares of common stock underlying warrants. Millard B. Ryland exercises voting and dispositive power over all of the shares beneficially owned by Millard B. Ryland IRA. |
| 138. | Includes 15,000 shares of common stock underlying warrants. |
| 139. | Includes 850 shares of common stock underlying warrants. Monarch Capital Group, LLC is an investment firm that acquired these securities for underwriting activities. Anthony Marchese has the power to vote and dispose of PharmaFrontiers Common Stock owned by Monarch Capital Group, LLC. |
| 140. | Includes 206 shares of common stock underlying warrants. |
| 141. | Includes 21,142 shares of common stock underlying warrants. |
| 142. | Includes 15 shares of common stock underlying warrants. |
| 143. | Includes 53,308 shares of common stock underlying warrants. Richard E. Crawford exercises voting and dispositive power over all of the shares beneficially owned by NFS LLC/FMTC FBO Richard E. Crawford. |
| 144. | Includes 35,971 shares of common stock underlying warrants. |
| 145. | Includes 25,001 shares of common stock underlying warrants. Norman R. Morris exercises voting and dispositive power over all of the shares beneficially owned by Norman R. Morris Living Trust. |
| 146. | Includes 171 shares of common stock underlying warrants. |
-57-
| 147. | Includes 26,582 shares of common stock underlying warrants. |
| 148. | Includes 250,500 shares of common stock underlying warrants. Panacea Fund, LLC is a private investment fund. Charles Polsky exercises voting and dispositive power over all of the shares beneficially owned by Panacea Fund, LLC. |
| 149. | Includes 106,288 shares of common stock underlying warrants. |
| 150. | Includes 52,500 shares of common stock underlying warrants. Parsifal Investments, L.P. is a private investment fund. Alfred L. Deaton III exercises voting and dispositive power over all of the shares beneficially owned by Parsifal Investments, L.P. |
| 151. | Includes 28,607 shares of common stock underlying warrants. Patrick Linbeck is a financial consultant and acquired these securities for a result of the Company’s June/July 2005 funding. |
| 152. | Includes 10,658 shares of common stock underlying warrants. |
| 153. | Includes 52,877 shares of common stock underlying warrants. Paul Masters exercises voting and dispositive power over all of the shares beneficially owned by Paul Masters IRA. |
| 154. | Includes 42,285 shares of common stock underlying warrants. Kinnary Patel exercises voting and dispositive power over all of the shares beneficially owned by Kinnary Patel Rollover IRA. |
| 155. | Includes 42,285 shares of common stock underlying warrants. Kinnary Patel exercises voting and dispositive power over all of the shares beneficially owned by Kinnary Patel Roth IRA. |
| 156. | Includes 46 shares of common stock underlying warrants. |
| 157. | Includes 250,001shares of common stock underlying warrants. Pinnacle Trust Co., LTA is a private investment fund that acquired these securities for underwriting activities. Andrew Linbeck has the power to vote and dispose of PharmaFrontiers Common Stock owned by Pinnacle Trust Co., LTA. |
| 158. | Includes 171 shares of common stock underlying warrants. |
| 159. | Includes 212,575 shares of common stock underlying warrants. Provident Premier Master Fund, Ltd. is a private investment fund. Steven Winters exercises voting and dispositive power over all of the shares beneficially owned by Provident Premier Master Fund, Ltd. |
| 160. | Includes 171 shares of common stock underlying warrants. |
| 161. | Includes 105,000 shares of common stock underlying warrants. Renaissance Interests, L.P. is a private investment fund. Bradley C. Karp exercises voting and dispositive power over all of the shares beneficially owned by Renaissance Interests, L.P. |
| 162. | Includes 106,616 shares of common stock underlying warrants. |
| 163. | Includes 26,716 shares of common stock underlying warrants. |
| 164. | Includes 106,123 shares of common stock underlying warrants. |
| 165. | Includes 15 shares of common stock underlying warrants. |
-58-
| 166. | Includes 53,329 shares of common stock underlying warrants. |
| 167. | Includes 25,001 shares of common stock underlying warrants. |
| 168. | Includes 10,953 shares of common stock underlying warrants. |
| 169. | Includes 50,001 shares of common stock underlying warrants. |
| 170. | Includes 25,050 shares of common stock underlying warrants. Paul and Mary Ann Mallis TTEES exercise voting and dispositive power over all of the shares beneficially owned by SAA Trust. |
| 171. | Includes 15,968 shares of common stock underlying warrants. Paul and Mary Ann Mallis TTEES exercise voting and dispositive power over all of the shares beneficially owned by SAA Trust Paul and Mary Ann Mallis TTEES. |
| 172. | Includes 87,920 shares of common stock underlying warrants. Salient Partners is an investment firm that acquired these securities for underwriting activities. Andrew Linbeck has the power to vote and dispose of PharmaFrontiers Common Stock owned by Salient Partners. |
| 173. | Includes 30,000 shares of common stock underlying warrants. |
| 174. | Includes 304,968 shares of common stock underlying warrants. Sanders Morris Harris is an investment firm that acquired these securities for underwriting activities. Ben Morris has the power to vote and dispose of PharmaFrontiers Common Stock owned by Sanders Morris Harris. |
| 175. | Includes 15,000 shares of common stock underlying warrants and 13,333 options that are vested with an exercise price of $3.00. |
| 176. | Includes 189,212 shares of common stock underlying warrants. Markus Keller exercises voting and dispositive power over all of the shares beneficially owned by Schroder & Co Bank AG |
| 177. | Includes 60,000 shares of common stock underlying warrants. |
| 178. | Includes 3,733 shares of common stock underlying warrants. Scott Shapiro is a financial consultant and acquired these securities for a result of the Company’s June/July 2005 funding. |
| 179. | Includes 52,466 shares of common stock underlying warrants. |
| 180. | Includes 300,000 shares of common stock underlying warrants. SIBEX Capital Fund, Inc. is a private investment fund. Oleg S. Krasnoshchek exercises voting and dispositive power over all of the shares beneficially owned by SIBEX Capital Fund, Inc. |
| 181. | Includes 1,216,,959 shares of common stock underlying warrants. SMI Re Limited is a private investment fund. Dr. Reginal McDaniel exercises voting and dispositive power over all of the shares beneficially owned by SMI Re Limited. |
| 182. | Includes 353,444 shares of common stock underlying warrants. |
| 183. | Includes 103,320 shares of common stock underlying warrants. |
-59-
| 184. | Includes 10,904 shares of common stock underlying warrants. Frederick Stahl Jr. exercises voting and dispositive power over all of the shares beneficially owned by Stahl Family Revocable Living Trust Dated 8-23-01. |
| 185. | Includes 1,000 shares of common stock underlying warrants. Starboard Capital Markets is an investment firm that acquired these securities for underwriting activities. Michael Hamblet has the power to vote and dispose of PharmaFrontiers Common Stock owned by Starboard Capital Markets. |
| 186. | Includes 2,711 shares of common stock underlying warrants. |
| 187. | Includes 21,142 shares of common stock underlying warrants. Carol A Wynn exercises voting and dispositive power over all of the shares beneficially owned by Sterling Trust Company FBO Carol A. Wynn. |
| 188. | Includes 19,812 shares of common stock underlying warrants. Harold E Tellefsen exercises voting and dispositive power over all of the shares beneficially owned by Sterling Trust Company Custodian FBO Harold E Tellefsen. |
| 189. | Includes 30,000 shares of common stock underlying warrants. |
| 190. | Includes 10,633 shares of common stock underlying warrants. Pam Sutton exercises voting and dispositive power over all of the shares beneficially owned by P.A. P/S Trust Pam Sutton Trustee. |
| 191. | Includes 46 shares of common stock underlying warrants. |
| 192. | Includes 31 shares of common stock underlying warrants. |
| 193. | Includes 50,001 shares of common stock underlying warrants. |
| 194. | Includes 106,575 shares of common stock underlying warrants. TCMP3 Partners, LP is a private investment fund. Steve Slawson exercises voting and dispositive power over all of the shares beneficially owned by TCMP3 Partners, LP. |
| 195. | Includes 111,219 shares of common stock underlying warrants, 16,666 options that are vested with an exercise price of $3.00, 6,666 options that are vested with an exercise price of $1.14 and 10,000 options that are vested with an exercise price of $1.15. |
| 196. | Includes 54,932 shares of common stock underlying warrants. |
| 197. | Includes 31 shares of common stock underlying warrants. |
| 198. | Includes 309 shares of common stock underlying warrants. |
| 199. | Includes 62 shares of common stock underlying warrants. |
| 200. | Includes 199 shares of common stock underlying warrants. |
| 201. | Includes 15 shares of common stock underlying warrants. |
| 202. | Includes 3,500 shares of common stock underlying warrants. Thomas Suppanz is a financial consultant and acquired these securities for a result of the Company’s June/July 2005 funding. |
-60-
| 203. | Includes 4,630 shares of common stock underlying warrants. |
| 204. | Includes 10,658 shares of common stock underlying warrants. |
| 205. | Includes 35,708 shares of common stock underlying warrants. |
| 206. | Includes 2,619 shares of common stock underlying warrants. |
| 207. | Includes 171 shares of common stock underlying warrants. |
| 208. | Includes 154 shares of common stock underlying warrants. |
| 209. | Includes 8,592 shares of common stock underlying warrants. VFinance is an investment firm that acquired these securities for underwriting activities. Jonathon C. Rich has the power to vote and dispose of PharmaFrontiers Common Stock owned by vFinance. |
| 210. | Includes 6,814 shares of common stock underlying warrants. |
| 211. | Includes 150,000 shares of common stock underlying warrants. |
| 212. | Includes 97,500 shares of common stock underlying warrants. Yellowstone Equity Partners, Ltd. is a private investment fund. Brenda Lee exercises voting and dispositive power over all of the shares beneficially owned by Yellowstone Equity Partners, Ltd. |
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
There are no related party transactions.
DESCRIPTION OF SECURITIES
Our Capitalization
Common Stock
We are authorized to issue 100,000,000 shares of common stock, par value $0.05 per share. As of March 28, 2006, there were 20,967,035 shares of common stock issued and outstanding. Each share of common stock is entitled to one vote per share for the election of directors and on all other matters submitted to a vote of stockholders. There are no cumulative voting rights. Common stockholders do not have preemptive rights or other rights to subscribe for additional shares, and the common stock is not subject to conversion or redemption. In the event of liquidation, the holders of common stock will share equally in any balance of corporate assets available for distribution to them. Subject to the rights of holders of the any other securities subsequently issued, holders of the common stock are entitled to receive dividends when and as declared by our Board of Directors out of funds legally available. We have not paid any dividends since its inception and has no intention to pay any dividends in the foreseeable future. Any future dividends would be subject to the discretion of the Board of Directors and would depend on, among other things, our future earnings, the operating and financial condition, our capital requirements, and general business conditions.
-61-
Preferred Stock
We are authorized to issue 10,000,000 shares of preferred stock, no par value per share. As of December 31, 2005, no shares of preferred stock are issued and outstanding. Our Board of Directors can, without approval of our stockholders, issue one or more series of preferred stock. If we offer preferred stock, our Board of Directors will determine the number of shares and the rights, preferences and limitations of each series. These rights, preferences and limitations may include specific designations, number of shares, liquidation value, dividend rights, liquidation and redemption rights, voting rights, other rights, including conversion or exchange rights, if any, and any other specific terms.
Promissory Notes
During the six months ended February 14, 2005, we issued 15% Convertible Exchangeable Notes with an aggregate principal amount of $6.1 million. On June 30, 2005, all of the 15% Convertible Exchangeable Notes and associated rights to warrants were exchanged for common stock and Series A Warrants, Series B Warrants and Series C Warrants. None of the notes or rights to warrants remain outstanding.
Warrants and Options
2004 Stock Incentive Plan. Pursuant to our 2004 Stock Incentive Plan we may issue to our officers, directors, employees and consultants incentive stock options, non-qualified stock options and shares of restricted stock. The plan provides for us to issue up to 2,000,000 shares of its common stock pursuant to awards under the plan. The maximum number of shares in the Plan was increased to 3,000,000 by shareholder vote at our annual meeting in June 2005. As of December 31, 2005, we had outstanding options, granted pursuant to the plan, to purchase 2,316,933 shares of common stock. The plan is designed to qualify under the Internal Revenue Code as an incentive stock option plan.
Series A Warrants, Series B Warrants and Series C Warrants. As of December 31, 2005 we have issued and outstanding (i) Series A Warrants to purchase an aggregate of 10,411,400 of our common shares, (ii) Series B Warrants to purchase an aggregate of 4,164,567 of our common shares, and (iii) Series C Warrants to purchase an aggregate of 8,329,108 of our common shares.
(i) The Series A Warrant expired on February 17, 2006.
(ii) The Series B Warrant is exercisable at any time and has an exercise price of $2.90 per share and expires on the later of (i) October 17, 2006 or (ii) 12 months after the registration statement for the re-sale of the warrant shares becomes effective; if we issue common stock or common stock equivalents for a price less than $1.50, the exercise price will be revised to $2.00 and the number of shares subject to the warrant shall increase proportionately.
(iii) The Series C Warrant is exercisable at any time and has an exercise price of $4.00 per share and expires June 17, 2010; if prior to the third anniversary of the closing of the offering we issue common stock or common stock equivalents for a price less than $1.50, the exercise price will be revised to $3.00 and the number of shares subject to the warrant shall increase proportionately; if prior to the third anniversary of the closing of the offering we issue common stock or common stock equivalents for a price less than the then current exercise price but more than $1.50, the exercise price will be revised to equal the weighted average price of the outstanding shares and the newly issued shares; if after the third anniversary of the closing of the offering we issue common stock or common stock equivalents for a price less than the then current exercise price, the exercise price will be revised to equal the weighted average price of the outstanding shares and the newly issued shares; whenever any such adjustments are made to the exercise price, the number of shares subject to the Series C Warrant shall increase proportionately; provided, the exercise price will not be reduced below $3.00 and any increase in the number of shares shall be proportionately limited.
-62-
All of the warrants are exercisable immediately and are only exercisable by “accredited investors” as defined in Regulation D under the Securities Act of 1933.
Placement Agent Warrants. In connection with the 15% Convertible Exchangeable Note offering and the common stock offerings closed June 17, 2005 we have issued to the placement agent and other brokerage firms in those offerings warrants to purchase an aggregate of 460,847 shares of common stock at an exercise price of $1.50. These warrants are exercisable immediately and will expire June 17, 2010.
Other Obligations To Issue Shares. Our license agreement with the University of Chicago obligates us, subsequent to the later of April 30, 2006 or a financing, raising $10 million or more, to issue sufficient additional shares to the University of Chicago so that it holds 2.6% of our common stock.
Restrictions on Sales By Certain Existing Shareholders
A total of 3,941,248 shares of our common stock held by Warren C. Lau, George Jarkesy, Jr., R. Wayne Fritzsche, Robert H. Gow, David M. Klausmeyer, Bruce Mackler and David R. Strawn are subject to a lock-up agreement that precluded any sales prior to June 4, 2005 and, thereafter, limits sales of up to an aggregate of 492,656 shares of our common stock per 90-day period. This lock-up will terminate if the last sales price of our common stock is at or above $10.00 per share for 10 out of 20 consecutive days, or upon a "change of control" transaction.
A total of 891,820 shares of our common stock held by George Jarkesy, Jr. and Brewer & Prichard, P.C. are subject to another lock-up agreement effective May 2004 that limits sales of up to an aggregate of 74,319 shares of our common stock per 90-day period. This lock-up agreement restriction will terminate if the last sales price of the our common stock is at or above $10.00 per share for 10 out of 20 consecutive days, or upon a "change of control" transaction.
A total of 2,500,000 shares held by Top Tier Investment, LLC and various other shareholders are subject to another lock-up agreement effective November 5, 2004 that precludes any sales prior to November 5, 2005 and, thereafter, limits sales of up to an aggregate of 312,500 shares of our common stock per 90-day period. There is no termination provision in this lock-up agreement.
PLAN OF DISTRIBUTION
We are registering shares of our common stock on behalf of the selling stockholders. As used in this prospectus, “selling stockholders” includes donees, transferees, pledgees and other successors in interest (other than purchasers pursuant to this prospectus) selling shares received from a named selling stockholder after the date of this prospectus. We will pay for all costs, expenses and fees in connection with the registration of the shares. The selling stockholders will pay for all selling discounts and commissions, if any. The selling stockholders may offer and sell their shares from time to time in one or more of the following types of transactions (including block transactions):
| · | on any national exchange on which the shares are listed or any automatic quotation system through which the shares are quoted, |
| · | in the over-the-counter market, |
-63-
| · | in privately negotiated transactions, |
| · | through put and call transactions, |
| · | through short sales, and |
| · | a combination of such methods of sale. |
The selling stockholders may sell their shares at prevailing market prices or at privately negotiated prices. The selling stockholders may use brokers, dealers or agents to sell their shares. The persons acting as agents may receive compensation in the form of commissions, discounts or concessions. This compensation may be paid by the selling stockholders or the purchasers of the shares for whom such persons may act as agent, or to whom they may sell as a principal, or both.
The selling stockholders may enter into hedging transactions with broker-dealers or other financial institutions. In connection with these transactions, broker-dealers or other financial institutions may engage in short sales of the shares in the course of hedging positions they assume with selling stockholders. The selling stockholders may also enter into options or other transactions with broker-dealers or other financial institutions which require the delivery to these broker-dealers or other financial institutions of shares, which such broker-dealer or other financial institution may resell pursuant to this prospectus (as amended or supplemented to reflect such transaction). The selling stockholders may also engage in short sales of shares and, in those instances, this prospectus may be delivered in connection with the short sales and the shares offered under this prospectus may be used to cover the short sales.
The selling stockholders and any agents or broker-dealers that participate with the selling stockholders in the offer and sale of the shares may be deemed to be “underwriters” within the meaning of Section 2(11) of the Securities Act of 1933. Any commissions they receive and any profit they realize on the resale of the shares by them may be deemed to be underwriting discounts and commissions under the Securities Act of 1933. Neither we nor any selling stockholder can presently estimate the amount of such compensation. Because a selling stockholder may be deemed to be an “underwriter” within the meaning of the Securities Act of 1933, the selling stockholders will be subject to the prospectus delivery requirements of the Securities Act of 1933, which may include delivery through the facilities of the applicable exchange or automated quotation system pursuant to Rule 153 under the Securities Act of 1933. The selling stockholders may agree to indemnify any agent, dealer or broker-dealer that participates in transactions involving shares against certain liabilities, including liabilities arising under the Securities Act of 1933.
The selling stockholders and any other person participating in a distribution of the securities covered by this prospectus will be subject to applicable provisions of the Securities Exchange Act of 1934 and the rules and regulations under the Securities Exchange Act of 1934, including Regulation M, which may limit the timing of purchases and sales of any of the securities by the selling stockholders and any other such person. Furthermore, under Regulation M, any person engaged in the distribution of the securities may not simultaneously engage in market-making activities with respect to the particular securities being distributed for certain periods prior to the commencement of or during such distribution. Regulation M’s prohibition on purchases may include purchases to cover short positions by the selling stockholders, and a selling stockholder’s failure to cover a short position at a lender’s request and subsequent purchases by the lender in the open market of shares to cover such short positions, may be deemed to constitute an inducement to buy shares, which is prohibited by Regulation M. All of the above may affect the marketability of the securities and the ability of any person or entity to engage in market-making activities with respect to the securities.
-64-
We are not aware of whether the selling stockholders have entered into any agreements, understanding or arrangements with any broker-dealers regarding the sale of their shares, nor as we aware that there is an underwriter or coordinating broker acting in connection with the proposed sale of shares by the selling stockholders.
Selling stockholders also may resell all or a portion of the shares in open market transactions in reliance upon Rule 144 under the Securities Act of 1933, provided they meet the criteria and conform to the requirements of that rule.
Following notification by a selling stockholder that it has entered into any material arrangement with a broker-dealer for the sale of shares through a block trade, special offering, exchange distribution or secondary distribution or a purchase by a broker or dealer, a supplement to this prospectus will be filed, if required, pursuant to Rule 424(b) under the Securities Act, disclosing:
| · | the name of each such selling stockholder and of the participating broker-dealer(s); |
| · | the number of shares involved; |
| · | the initial price at which these shares were sold; |
| · | the commissions paid or discounts or concessions allowed to such broker-dealer(s), where applicable; |
| · | that such broker-dealer(s) did not conduct any investigation to verify the information set out or incorporated by reference in this prospectus; and |
| · | any other facts material to the transactions. |
In addition, following notification by a selling stockholder that a donee, pledgee, transferee or other successor-in-interest of such selling stockholder intends to sell more than 500 shares, we will file a supplement to this prospectus.
LEGAL MATTERS
The validity of the common stock offered by this prospectus was passed upon for us by Vinson & Elkins L.L.P., Houston, Texas.
EXPERTS
The consolidated financial statements for the year ended December 31, 2005 and for the period from January 22, 2003 (date of inception) to December 31, 2005 included in this prospectus have been audited by Malone & Bailey PC, independent registered public accounting firm, as stated in their report appearing herein.
WHERE YOU CAN FIND MORE INFORMATION
This prospectus is part of a registration statement we file with the Securities and Exchange Commission. This prospectus does not contain all of the information contained in the registration statement and all of the exhibits and schedules thereto. For further information about PharmaFrontiers Corp., please see the complete registration statement. Summaries of agreements or other documents in this prospectus are not necessarily complete. Please refer to the exhibits to the registration statement for complete copies of such documents.
-65-
We file annual, quarterly and special reports, proxy statements and other information with the Securities and Exchange Commission under the Securities Exchange Act of 1934. You may read and copy the registration statement, including exhibits and schedules filed with it, at the SEC’s public reference facilities in Room 1024, Judiciary Plaza, 450 Fifth Street, N.W., Washington, D.C. 20549. You may obtain information on the operation of the public reference room in Washington, D.C. by calling the Securities and Exchange Commission at 1-800-SEC-0330.
We file information electronically with the Securities and Exchange Commission. Our Securities and Exchange Commission filings also are available from the Securities and Exchange Commission’s Internet site at http://www.sec.gov, which contains reports, proxy and information statements and other information regarding issuers that file electronically.
-66-
INDEX TO FINANCIAL STATEMENTS
Report of Independent Registered Public Accounting Firm | 68 | |
Consolidated Balance Sheet as of December 31, 2005 | F-1 | |
Consolidated Statements of Expenses for the year ended December 31, 2005 and the period from January 22, 2003 (Inception) through December 31, 2004 and 2005 | F-2 | |
Consolidated Statement of Changes in Stockholders Equity from January 22, 2003 (Inception) through December 31, 2005 | F-3 | |
Consolidated Statements of Cash Flows for the year ended December 31, 2005 and the period from January 22, 2003 (Inception) through December 31, 2004 and 2005 | F-4 | |
Notes to Consolidated Financial Statements | F-6 |
-67-
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors
PharmaFrontiers Corp.
(a development stage company)
The Woodlands, Texas
We have audited the accompanying consolidated balance sheet of PharmaFrontiers Corp., (“Pharma”) (a development stage company), as of December 31, 2005 and the related consolidated statements of expenses, changes in stockholders’ equity and cash flows for the two years ended December 31, 2005 and the period from January 22, 2003 (Inception) through December 31, 2005. These consolidated financial statements are the responsibility of Pharma’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatements. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall consolidated financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of Pharma as of December 31, 2005 and the consolidated results of its operations and its cash flows for the periods described in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial statements have been prepared assuming that Pharma will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, Pharma has suffered recurring losses from operations, which raises substantial doubt about its ability to continue as a going concern. Management’s plans regarding those matters are described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
MALONE & BAILEY, PC
www.malone-bailey.com
Houston, Texas
February 7, 2006
-68-
PHARMAFRONTIERS CORP.
(a development stage company)
CONSOLIDATED BALANCE SHEET
December 31, 2005
| ASSETS | ||||
| Current assets | ||||
Cash | $ | 2,560,666 | ||
Prepaid expenses | 182,524 | |||
Total current assets | 2,743,190 | |||
| Intangible assets, net of $1,888,891 of accumulated amortization | 26,130,441 | |||
| Property & equipment, net of $256,082 of accumulated depreciation | 479,996 | |||
| Other assets | 388,210 | |||
Total assets | $ | 29,741,837 | ||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||
| Current liabilities | ||||
Accounts payable | $ | 689,467 | ||
Accrued expenses | 240,309 | |||
Note payable | 1,500,000 | |||
Total current liabilities | 2,429,776 | |||
| Commitments and contingencies | – | |||
| Stockholders’ equity | ||||
Convertible preferred stock, no par value, 10,000,000 shares authorized, none issued and outstanding | – | |||
Common stock, $.05 par value, 100,000,000 shares authorized, 20,619,545 shares issued and outstanding | 1,030,977 | |||
Additional paid in capital | 50,441,948 | |||
Deficit accumulated during the development stage | (24,160,864 | ) | ||
Total stockholders’ equity | 27,312,061 | |||
| Total liabilities and stockholders’ equity | $ | 29,741,837 |
See accompanying summary of accounting policies
and notes to consolidated financial statements
F-1
PHARMAFRONTIERS CORP.
(A Development Stage Company)
CONSOLIDATED STATEMENTS OF EXPENSES
Years ended December 31, 2005 and 2004 and the
Period from January 22, 2003 (Inception) to December 31, 2005
2005 | 2004 | Inception through 2005 | ||||||||
| General and administrative | $ | 550,178 | $ | 572,534 | $ | 1,203,513 | ||||
| Depreciation and amortization | 1,735,209 | 264,819 | 2,000,028 | |||||||
| Research and development | 9,892,253 | 2,465,634 | 12,357,887 | |||||||
| Loss on disposal of assets | 22,810 | 457,122 | 479,932 | |||||||
Operating loss | (12,200,450 | ) | (3,760,109 | ) | (16,041,360 | ) | ||||
| Interest income | 81,930 | 5,992 | 87,922 | |||||||
| Other income | 28,174 | 2,379 | 30,553 | |||||||
| Interest expense | (7,323,851 | ) | (868,926 | ) | (8,237,979 | ) | ||||
| NET LOSS | $ | (19,414,197 | ) | $ | (4,620,664 | ) | $ | (24,160,864 | ) | |
| Basic and diluted loss per share | $ | (1.24 | ) | $ | (0.73 | ) | N/A | |||
| Weighted average shares outstanding | 15,648,365 | 6,309,145 | N/A | |||||||
See accompanying summary of accounting policies
and notes to consolidated financial statements
F-2
PHARMAFRONTIERS CORP.
(A Development Stage Company)
CONSOLIDATED STATEMENT OF CHANGES IN STOCKHOLDERS’ EQUITY
January 22, 2003 (Inception) through December 31, 2005
Common Stock | Additional Paid in | Accumulated | ||||||||||||||
Shares | Par | Capital | Deficit | Total | ||||||||||||
| Shares issued for cash | 5,250,000 | $ | 262,500 | $ | (261,500 | ) | $ | – | $ | 1,000 | ||||||
| Shares repurchased and cancelled | (1,706,250 | ) | (85,313 | ) | 84,988 | – | (325 | ) | ||||||||
| Discount relating to: | ||||||||||||||||
| - beneficial conversion feature | – | – | 28,180 | – | 28,180 | |||||||||||
| - warrants attached to debt | – | – | 28,180 | – | 28,180 | |||||||||||
| Net loss | – | – | – | (126,003 | ) | (126,003 | ) | |||||||||
| Balances at December 31, 2003 | 3,543,750 | 177,187 | (120,152 | ) | (126,003 | ) | (68,968 | ) | ||||||||
| Shares issued for: | ||||||||||||||||
| - cash | 22,500 | 1,125 | 7,875 | – | 9,000 | |||||||||||
| - services | 2,065,000 | 103,250 | 745,750 | – | 849,000 | |||||||||||
| - license | 242,688 | 12,135 | 414,940 | – | 427,075 | |||||||||||
| - reverse merger with Sportan | 997,399 | 49,870 | (197,603 | ) | – | (147,733 | ) | |||||||||
| - acquisition of Opexa | 2,500,000 | 125,000 | 23,625,000 | – | 23,750,000 | |||||||||||
| - additional shares attached to | ||||||||||||||||
| convertible debt | 161,000 | 8,050 | 280,316 | – | 288,366 | |||||||||||
| - conversion of convertible notes | 607,501 | 30,375 | 217,995 | – | 248,370 | |||||||||||
| Shares cancelled | (80,000 | ) | (4,000 | ) | 4,000 | – | – | |||||||||
| Discount relating to: | ||||||||||||||||
| - beneficial conversion feature | – | – | 855,849 | – | 855,849 | |||||||||||
| - warrants attached to debt | – | – | 1,848,502 | – | 1,848,502 | |||||||||||
| Option Expense | – | – | 123,333 | – | 123,333 | |||||||||||
| Net loss | – | – | – | (4,620,664 | ) | (4,620,664 | ) | |||||||||
| Balances at December 31, 2004 | 10,059,838 | 502,992 | 27,805,805 | (4,746,667 | ) | 23,562,130 | ||||||||||
| Shares issued for: | ||||||||||||||||
| - cash | 3,894,509 | 194,725 | 5,647,044 | – | 5,841,769 | |||||||||||
| - convertible debt | 6,110,263 | 305,513 | 7,343,933 | – | 7,649,446 | |||||||||||
| - debt | 23,000 | 1,150 | 159,850 | – | 161,000 | |||||||||||
| - license | 291,935 | 14,597 | 1,853,787 | – | 1,868,384 | |||||||||||
| - services | 240,000 | 12,000 | 1,000,400 | – | 1,012,400 | |||||||||||
| Offering costs relating to | ||||||||||||||||
| - equity financing | – | – | (495,552 | ) | – | (495,552 | ) | |||||||||
| Discount relating to: | ||||||||||||||||
| - beneficial conversion feature | – | – | 831,944 | – | 831,944 | |||||||||||
| - warrants attached to debt | – | – | 1,433,108 | – | 1,433,108 | |||||||||||
| Option expense | – | – | 2,487,741 | – | 2,487,741 | |||||||||||
| Warrant expense | – | – | 2,373,888 | – | 2,373,888 | |||||||||||
| Net loss | – | – | – | (19,414,197 | ) | (19,414,197 | ) | |||||||||
| Balances at December 31, 2005 | 20,619,545 | $ | 1,030,977 | $ | 50,441,948 | $ | (24,160,864 | ) | $ | 27,312,061 | ||||||
See accompanying summary of accounting policies
and notes to consolidated financial statements
F-3
PHARMAFRONTIERS CORP.
(A Development Stage Company)
CONSOLIDATED STATEMENTS OF CASH FLOWS
Year ended December 31, 2005 and the Period from January 22, 2003
(Inception) through December 31, 2004 and 2005
2005 | 2004 | Inception through 2005 | ||||||||
| Cash flows from operating activities | ||||||||||
| Net loss | $ | (19,414,197 | ) | $ | (4,620,664 | ) | $ | (24,160,864 | ) | |
| Adjustments to reconcile net loss to net cash used in | ||||||||||
| operating activities: | ||||||||||
| Stock issued for services | 1,012,400 | 849,000 | 1,861,400 | |||||||
| Additional stock issued under debt conversion | 109,070 | – | 109,070 | |||||||
| Amortization of discount on notes payable due | ||||||||||
| to warrants and beneficial conversion | ||||||||||
| feature | 5,516,638 | 753,812 | 6,313,205 | |||||||
| Amortization of intangible assets | 1,637,129 | 251,761 | 1,888,890 | |||||||
| Depreciation | 98,080 | 13,058 | 111,138 | |||||||
| Debt financing costs | 365,910 | – | 365,910 | |||||||
| Option and warrant expense | 4,861,629 | 123,333 | 4,984,962 | |||||||
| Loss on disposition of fixed assets | 22,810 | 457,122 | 479,932 | |||||||
| Changes in: | ||||||||||
| Accounts payable | 26,360 | 58,670 | 85,167 | |||||||
| Prepaid expenses | (88,185 | ) | (38,950 | ) | (127,135 | ) | ||||
| Accrued expenses | 23,655 | 23,822 | 54,981 | |||||||
| Other assets | (388,210 | ) | – | (388,210 | ) | |||||
| Net cash used in operating activities | (6,216,911 | ) | (2,129,036 | ) | (8,421,554 | ) | ||||
| Cash flows from investing activities | ||||||||||
| Purchase of licenses | - | (232,742 | ) | (232,742 | ) | |||||
| Purchase of property & equipment | (258,903 | ) | (173,004 | ) | (431,907 | ) | ||||
| Net cash used in investing activities | (258,903 | ) | (405,746 | ) | (664,649 | ) | ||||
| Cash flows from financing activities | ||||||||||
| Common stock sold for cash, net of offering costs | 5,346,217 | 9,000 | 5,356,217 | |||||||
| Common stock repurchased and canceled | – | – | (325 | ) | ||||||
| Proceeds from debt | 2,896,885 | 3,382,706 | 6,354,591 | |||||||
| Repayments on notes payable | (58,614 | ) | (5,000 | ) | (63,614 | ) | ||||
| Net cash provided by financing activities | 8,184,488 | 3,386,706 | 11,646,869 | |||||||
| Net change in cash | 1,708,674 | 851,924 | 2,560,666 | |||||||
| Cash at beginning of year | 851,992 | 68 | - | |||||||
| Cash at end of year | $ | 2,560,666 | $ | 851,992 | $ | 2,560,666 | ||||
See accompanying summary of accounting policies
and notes to consolidated financial statements
F-4
PHARMAFRONTIERS CORP.
(A Development Stage Company)
CONSOLIDATED STATEMENTS OF CASH FLOWS (Continued)
Year ended December 31, 2004 and the Period from January 22, 2003
(Inception) through December 31, 2003 and 2004
2005 | 2004 | Inception through 2005 | ||||||||
| NON-CASH TRANSACTIONS | ||||||||||
| Issuance of common stock for purchase of Opexa | $ | – | $ | 23,750,000 | $ | 23,750,000 | ||||
| Issuance of common stock to Sportan shareholders | – | 147,733 | 147,733 | |||||||
| Issuance of common stock for University of Chicago license | 1,868,384 | 427,075 | 2,295,459 | |||||||
| Issuance of common stock for accrued interest | 525,513 | – | 525,513 | |||||||
| Conversion of notes payable to common stock | 6,159,610 | 248,370 | 6,407,980 | |||||||
| Conversion of accrued liabilities to common stock | 17,176 | – | 17,176 | |||||||
| Conversion of accounts payable to note payable | – | 93,364 | 93,364 | |||||||
| Discount on convertible notes relating to: | ||||||||||
| - warrants | 1,433,108 | 1,848,502 | 3,309,790 | |||||||
| - beneficial conversion feature | 831,944 | 855,849 | 1,715,973 | |||||||
| - stock attached to notes | 999,074 | 288,366 | 1,287,440 | |||||||
See accompanying summary of accounting policies
and notes to consolidated financial statements
F-5
PHARMAFRONTIERS CORP.
(a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 - SUMMARY OF ACCOUNTING POLICIES
PharmaFrontiers Corp. (“Pharma”) was incorporated in Texas on January 22, 2003 as a bio-pharmaceutical company engaged in developing autologous personalized cell therapies. During the development stage, Pharma acquired the worldwide license to technology developed at Argonne National Laboratory, a U.S. Department of Energy Laboratory Operated by the University of Chicago (“Argonne”). This is an exclusive license to a stem cell technology in which adult pluripotent stem cells are derived from monocytes obtained from the patient’s own blood (the “License”). A patent application was filed in November 2003, with the United States Patent and Trade Office regarding the technology involved in the License.
On October 7, 2004 Pharma entered into an agreement to acquire all of the outstanding stock of Opexa Pharmaceuticals, Inc. (“Opexa”). The agreement closed on November 5, 2004. A total of 2,500,000 shares of Pharma’s common stock were exchanged for all of the outstanding stock of Opexa. 2,250,000 shares was issued to Opexa shareholders in December 2004 and the balance of 250,000 shares, that had been held in escrow for the prerequisite one year period, was issued in November 2005.The acquisition was accounted for under the purchase method, where all of Opexa’s assets are restated to their fair market value on the acquisition date, which approximated book value. The 2,500,000 shares of Pharma were valued at $23,750,000 or $9.50 per share, which represents their current value at the time. See Note 12 for details.
Opexa holds rights to technology to diagnose and treat multiple sclerosis through modified autoreactive T cells and is currently in FDA Phase I/II human dose ranging clinical trials to evaluate its safety and effectiveness in treating multiple sclerosis.
Basis of presentation. The consolidated financial statements include the accounts of Pharma and its wholly-owned subsidiary, Opexa. Significant inter-company accounts and transactions have been eliminated.
Reclassifications. Certain amounts in the 2004 consolidated financial statements have been reclassified
to conform to the 2005 consolidated financial statement presentation.
Use of Estimates in Financial Statement Preparation. The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the consolidated financial statements, and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash and cash equivalents. For purposes of the statements of cash flows, cash equivalents include all highly liquid investments with original maturities of three months or less.
Long-lived Assets. Property and equipment are stated on the basis of historical cost less accumulated depreciation. Depreciation is provided using the straight-line method over the estimated useful lives of the assets. Major renewals and improvements are capitalized, while minor replacements, maintenance and repairs are charged to current operations.
F-6
Impairment losses are recorded on long-lived assets used in operations when indicators of impairment are present and the undiscounted cash flows estimated to be generated by those assets are less than the assets carrying amount.
Income Taxes. Income tax expense is based on reported earnings before income taxes. Deferred income taxes reflect the impact of temporary differences between assets and liabilities recognized for consolidated financial reporting purposes and such amounts recognized for tax purposes, and are measured by applying enacted tax rates in effect in years in which the differences are expected to reverse.
Stock-Based Compensation. Pharma accounts for stock-based compensation under the intrinsic value method. Under this method, Pharma recognizes no compensation expense for stock options granted when the number of underlying shares is known and exercise price of the option is greater than or equal to the fair market value of the stock on the date of grant. The following table illustrates the effect on net loss and net loss per share if Pharma had applied the fair value provisions of FASB Statement No. 123, Accounting for Stock-Based Compensation, to stock-based employee compensation:
2005 | 2004 | Inception through 2005 | ||||||||
| Net loss as reported | $ | (19,414,197 | ) | $ | (4,620,664 | ) | $ | (24,160,864 | ) | |
Add: stock based compensation determined Under intrinsic value based method | 2,487,741 | 123,333 | 2,611,074 | |||||||
Less: stock based compensation determined under fair value based method | (4,264,013 | ) | (153,364 | ) | (4,417,377 | ) | ||||
| Pro forma net loss | $ | (21,190,469 | ) | $ | (4,650,695 | ) | $ | (25,967,167 | ) | |
Basic and diluted Net loss per common share: As reported Pro forma | $ $ | (1.24 (1.35 | ) ) | $ $ | (.73 (.74 | ) ) | N/A N/A |
The weighted average fair value of the stock options granted during 2004 was $3.09. Variables used in the Black-Scholes option-pricing model include (1) 2% risk-free interest rate, (2) expected option life is the actual remaining life of the options as of each year end, (3) expected volatility is from 0.1% to 796.30% and (4) zero expected dividends.
The weighted average fair value of the stock options granted during 2005 was $2.49. Variables used in the Black-Scholes option-pricing model include (1) 2% risk-free interest rate, (2) expected option life is the actual remaining life of the options as of each year end, (3) expected volatility is 175.40% and (4) zero expected dividends.
The basic net loss per common share is computed by dividing the net loss by the weighted average number of common shares outstanding. Diluted net loss per common share is computed by dividing the net income adjusted on an "as if converted" basis, by the weighted average number of common shares outstanding plus potential dilutive securities. Basic and diluted loss per share is the same due to potential dilutive securities had an anti-dilutive effect and were not included in the calculation of diluted net income per common share.
F-7
Research and development. Research and development expenses include salaries, related employee expenses, consulting fees, facility costs, and laboratory costs. All costs for research and development activities are expensed as incurred. Pharma expenses the costs of licenses of patents and the prosecution of patents until the issuance of such patents and the commercialization of related products is reasonably assured.
Recently Issued Accounting Pronouncements. In December 2004, the FASB issued SFAS No.123R, “Accounting for Stock-Based Compensation.” SFAS No.123R establishes standards for the accounting for transactions in which an entity exchanges its equity instruments for goods or services. This Statement focuses primarily on accounting for transactions in which an entity obtains employee services in share-based payment transactions. SFAS No.123R requires that the fair value of such equity instruments be recognized as expense in the historical financial statements as services are performed. Prior to SFAS No.123R, only certain pro forma disclosures of fair value were required. SFAS No.123R shall be effective for small business issuers as of the beginning of the first interim or annual reporting period that begins after December 15, 2005. While Pharma has issued options to employees recently, the adoption of this new accounting pronouncement is not expected to have a material impact on the consolidated financial statements of Pharma during the calendar year 2006.
Pharma does not expect the adoption of any other recently issued accounting pronouncements to have a significant impact on their consolidated financial position, results of operations or cash flow.
NOTE 2 - GOING CONCERN
As shown in the accompanying consolidated financial statements, Pharma incurred recurring net losses of $19,414,197 and $4,620,664 in 2005 and 2004, respectively, and has an accumulated deficit of $24,160,864 as of December 31, 2005. These conditions raise substantial doubt as to Pharma’s ability to continue as a going concern. Management is trying to raise additional capital through sales of convertible debt and equity. The consolidated financial statements do not include any adjustments that might be necessary if Pharma is unable to continue as a going concern.
NOTE 3 - LICENSE AGREEMENT
In February 2004, Pharma entered into an agreement with the University of Chicago (“University”) for the worldwide license to technology developed at Argonne National Laboratory, a U.S. Department of Energy Laboratory Operated by the University. In consideration for the license, Pharma paid the University $57,742 and agreed to issue 375,375 shares of its common stock. 187,688 shares valued at $75,075 were issued on February 20, 2004. In December 2004, the License Agreement was amended granting Pharma an exclusive, non-transferable worldwide license to the University’s stem cell technology. In consideration for the amendment, Pharma paid the University an additional $175,000, issued the University 55,000 shares of common stock valued at $352,000, bringing the total ownership of Pharma by the University to 242,688 shares, agreed to pay the University $1,500,000 on the earlier of October 30, 2005 or upon the closing of a Pharma financing where proceeds are greater than $10 million and agreed to issue the University shares of Pharma common stock, including the shares already issued, equal to 2.6% of the total outstanding number of shares after conversion of the 15% exchangeable convertible subordinated promissory notes upon the later of the first financing or November 30, 2005 and after issuance of any and all equity in the form of stock at the close of the first financing.
F-8
In June 2005, 274,836 shares of common stock were issued to the University of Chicago per the terms of a license agreement. These shares were recorded at $867,064.
In August 2005, 17,099 shares of common stock were issued to the University of Chicago per the terms of a license agreement. These shares were recorded at $109,434.
By amendment dated October 31, 2005 Pharma and the University agreed to extend the date upon which the $1,500,000 to April 30, 2006.
In June of 2004, Pharma paid $50,000 to The University of Texas MD Anderson Cancer Center for an option to negotiate a licensing agreement for the use of peripheral blood stem cells for cardiac regeneration. This option to negotiate the licensing agreement expired on September 21, 2004 and the non-refundable fee of $50,000 was written off during 2004.
NOTE 4 - INTANGIBLE ASSETS
Intangible assets consisted of the following at December 31, 2005:
| Description | Life | Amount | |||||
| University of Chicago license (see Note 3) | 19 years | $ | 4,028,204 | ||||
| Opexa intangible group (see Note 12) | 16 years | 23,991,128 | |||||
| Subtotal | 28,019,332 | ||||||
| Less: accumulated amortization | (1,888,891 | ) | |||||
| Intangible assets, net | $ | 26,130,441 | |||||
Amortization expense totaled $1,637,129 and $251,761 in fiscal 2005 and 2004, respectively.
NOTE 5 - PROPERTY AND EQUIPMENT
Property and equipment consisted of the following at December 31, 2005:
| Description | Life | Amount | |||||
| Computer equipment | 3 years | $ | 77,730 | ||||
| Office furniture and equipment | 3-5 years | 145,921 | |||||
| Laboratory equipment | 5-10 years | 512,427 | |||||
| Subtotal | 736,078 | ||||||
| Less: accumulated depreciation | (256,082 | ) | |||||
| Property and equipment, net | $ | 479,996 | |||||
Depreciation expense totaled $98,080 and $13,058 in fiscal 2005 and 2004, respectively.
F-9
NOTE 6 - INCOME TAXES
Pharma uses the liability method, where deferred tax assets and liabilities are determined based on the expected future tax consequences of temporary differences between the carrying amounts of assets and liabilities for financial and income tax reporting purposes. During fiscal 2005 and 2004, Pharma incurred net losses and, therefore, has no tax liability. The net deferred tax asset generated by the loss carry-forward has been fully reserved. The cumulative net operating loss carry-forward is approximately $12,000,000 at December 31, 2005, and will expire in the years 2024 through 2025.
At December 31, 2005, deferred tax assets consisted of the following:
| Deferred tax assets | ||||
| Net operating losses | $ | 4,080,000 | ||
| Less: valuation allowance | (4,080,000 | ) | ||
| Net deferred tax assets | $ | - | ||
NOTE 7 - THIRD PARTY CONVERTIBLE NOTES
Between September 2004 and February 2005, Pharma issued convertible notes to investors totaling $6,124,859. In March 2005, 451,688 shares of common stock with a relative fair value of $999,074 were issued to note holders as their additional shares for their subscription investment in Pharma. In June 2005 a total of $6,650,372 comprised of the principal of the notes of $6,124,859 and accumulated interest of $525,513, which accrued at a rate of 15% per annum, was exchanged for 4,433,598 units at $1.50 per share. Each unit is comprised of one share of common stock and three separate types of warrants to purchase a total of 2.75 shares of common stock as stated below. In addition, 1,224,977 shares of Common Stock were issued in consideration for the surrender of the rights to the Bridge Warrants held by the note holders. All of the Bridge Notes and Bridge Warrants were exchanged so that none are now outstanding.
| · | Warrants: In connection with the bridge note exchange and private placement offerings in June and July three separate types of warrants to purchase a total of 2.75 shares of common stock were issued as follows: (i) a Series A Warrant which expired on February 17, 2006; (ii) a Series B Warrant for one-half of a share with an exercise price of $2.90 which expires on October 17, 2006; (iii) and a Series C Warrant for one share with an exercise price of $4.00 that expires on May 25, 2010. |
| · | Pharma analyzed the convertible notes and the warrants for derivative accounting consideration under SFAS 133 and EITF 00-19. Pharma determined the embedded conversion option in the convertible notes and the warrants met the criteria for classification in stockholders equity under SFAS 133 and EITF 00-19. Therefore, derivative accounting was not applicable for these convertible notes payable or their associated warrants. |
NOTE 8 - NOTE PAYABLE
Note payable consists of the following:
Note payable to the University of Chicago; no interest; due earlier of
Pharma raising $10,000,000 in an Equity Financing or April 30, 2006;
secured by license (See Note 3 for details) $1,500,000
F-10
NOTE 9 - COMMITMENTS AND CONTINGENCIES
After purchasing Opexa, Pharma assumed an eighteen-month operating lease from Opexa for a research facility. The lease commenced in June 2003 and was due to expire in November 2004. Pharma extended the lease initially until March 31, 2005 and extended it again until September 30, 2005. Pharma terminated the lease on October 7, 2005 and entered into a ten-year lease with a new landlord which commenced on October 1, 2005.
Future minimum lease payments under the non-cancellable operating lease are $72,474 for 2006, $117,774 for 2007, $137,196 for 2008, $139,782 for 2009, $147,540 for 2010 and $731,883 for thereafter
Rent expense for 2005 was $178,963 and $389,300 for 2004.
NOTE 10 - EQUITY
During 2003, Pharma sold 5,250,000 shares of common stock for $1,000.
In April 2003, 1,706,250 shares were reacquired for $325 and canceled.
Additional contributions to capital of $56,360 resulted from the discounted value to notes payable due to warrants and beneficial conversion features attached to convertible notes was issued in 2003.
During 2004, 22,500 shares of common stock were sold for $9,000.
During 2004, 2,065,000 shares of common stock valued at their then fair value of $849,000 were issued to Pharma’s employees and consultants for their services.
In February 2004, 187,688 shares of common stock valued at their then fair value of $75,075 were issued to the University of Chicago per the terms of a license agreement. In December 2004, 55,000 shares of common stock valued at their then fair value of $352,000 were issued to the University of Chicago per the terms of an amended license agreement. See Note 3 for details.
In June 2004, 997,399 shares of common stock were issued for net liabilities of $147,733 to Sportan’s shareholders for the reverse merger with Sportan. See Note 14 for details.
In November 2004, 2,500,000 shares of common stock valued at their then fair value of $23,750,000 were issued to 30 accredited investors in connection with the acquisition of Opexa, of which 2,250,000 shares were issued immediately and the balance of 250,000 shares held in escrow were issued in November 2005. See Note 12 for details.
In December 2004, 161,000 shares of common stock with a relative fair value of $288,366 were issued to note holders as their additional shares for their subscription investment in Pharma.
During 2004, 607,501 shares of common stock were issued to note holders for the conversion of $248,370 of principal and interest from convertible notes.
In November 2004, 80,000 shares of common stock were cancelled pursuant to the terms of an employment separation agreement.
F-11
During 2004, there were additional contributions to capital of $2,704,351 relating to the discounted value to notes payable from warrants, beneficial conversion features attached to convertible notes.
Employee stock option expense was $123,333 in 2004.
In June 2005, Pharma sold 3,387,217 shares of common stock with 9,314,868 warrants for $5,080,826. The warrants have exercise prices ranging from $2 to $4 and expire in seven months to four years. The relative fair value of the common stock is $886,913 and the relative fair value of the warrants is $4,198,913. Offering costs of $434,262 related to shares issued were charged to additional paid in capital.
In July 2005, Pharma sold 507,292 shares of common stock with 1,395,053 warrants for $760,943. The warrants have exercise prices ranging from $2 to $4 and expire in seven months to four years. The relative fair value of the common stock is $216,801 and the relative fair value of the warrants is $544,137. Offering costs of $61,290 related to shares issued were charged to additional paid in capital.
In March 2005, 451,688 shares of common stock with a relative fair value of $999,074 were issued to note holders as their additional shares for their subscription investment in Pharma.
In June 2005, 5,658,575 shares of common stock were issued to note holders for the conversion of $6,124,859 of principal and $525,513 interest from convertible notes.
During February 2005, 23,000 shares of common stock valued at their fair value of $161,000 were issued to note holders for the conversion of $51,930 of principal and interest from the notes.
In June 2005, 274,836 shares of common stock were issued to the University of Chicago per the terms of a license agreement. These shares were recorded at $1,758,950.
In August 2005, 17,099 shares of common stock were issued to the University of Chicago per the terms of a license agreement. These shares were recorded at $109,434.
During 2005, 240,000 shares of common stock valued at their fair value of $1,012,400 were issued to Pharma’s consultants for their services.
During 2005, offering costs of $495,552 related to the equity financing were charged to additional paid in capital.
During 2005, there were additional contributions to capital of $2,265,052 relating to the discounted value to notes payable from warrants, beneficial conversion features attached to convertible notes.
Employee stock option expense was $2,487,741 in 2005.
Warrant expense was $2,373,888 in 2005.
NOTE 11 - STOCK OPTIONS AND WARRANTS
In 2004 Pharma adopted the 2004 Stock Option Plan (“the Plan”). The Plan provides for the granting of stock options to employees and consultants of Pharma.
Options granted under the Plan may be either incentive stock options or nonqualified stock options. Incentive stock options (“ISO”) may be granted only to Pharma employees (including officers and directors who are also employees). Nonqualified stock options (“NSO”) may be granted to Pharma employees and consultants. The Board of Directors has discretion to determine the number, term, exercise price and vesting of all grants.
F-12
During 2003, 150,000 warrants were granted to investors related to the convertible notes.
During 2004, 965,000 options were granted to employees, 200,000 warrants were granted to consultants and 1,427,993 warrants were granted to investors related to the convertible notes.
Stock Options:
In January 2005, options to purchase 192,000 shares of Common Stock were issued to several employees at an exercise price of $3.00 per share. One third of the options vest on the first anniversary date, one third of the options vests on the second anniversary date, and the remaining one third vests on the third anniversary date. These options have an intrinsic value of $785,800, of which $261,933 has been expensed during 2005.
In April 2005, options to purchase 12,500 shares of Common Stock were issued to three Opexa employees at an exercise price of $3.00 per share. One third of the options vest on the first anniversary date, one third of the options vests on the second anniversary date, and the remaining one third vests on the third anniversary date. These options have an intrinsic value of $14,925, of which $4,975 has been expensed during 2005.
In June 2005, options to purchase 30,000 shares of Common Stock were issued to two employees at an exercise price of $3.00 per share, of which options vested immediately. These options have no intrinsic value due to exercise price exceeded the market price at the date of the grant.
In August 2005, options to purchase 20,000 shares of Common Stock were issued to an employee at an exercise price of $1.14 per share. One third of the options vested immediately, one third of the options vest on the first anniversary date, and the remaining one third vests on the second anniversary date. These options have no intrinsic value due to exercise price exceeded the market price at the date of the grant.
In December 2005, options to purchase 376,000 shares of Common Stock were issued to fifteen Opexa employees at an exercise price of $0.70 per share. One fourth of the options vest on the first anniversary date, one fourth of the options vests on the second anniversary date, one fourth of the options vests on the third anniversary date, and the remaining one fourth vests on the fourth anniversary date. These options have no intrinsic value due to exercise price equaled the market price at the date of the grant.
In 2005, 41,667 options previously granted were forfeited.
Consultant warrants:
In January 2005, warrants to purchase 25,000 shares of Common Stock were issued to a consultant at an exercise price of $3.00 per share of which one third of the warrants vest on the first anniversary date, one third of the warrants vests on the second anniversary date, and the remaining one third of warrants vests on the third anniversary date. These warrants have a fair value of $183,033, of which $61,011 has been expensed during 2005.
In April 2005, warrants to purchase 100,000 shares of Common Stock were issued to a consultant at an exercise price of $3.00 per share of which 40,000 warrants vested immediately, and the remaining 60,000 warrants vest at the rate of 2,500 warrants per month for twenty-four months. These warrants have a fair value of $417,812, of which $261,133 has been expensed during 2005.
F-13
In April 2005, warrants to purchase 20,000 shares of Common Stock were issued to a consultant at an exercise price of $3.00 per share of which one third of the warrants vest on the first anniversary date, one third of the warrants vests on the second anniversary date, and the remaining one third of warrants vests on the third anniversary date. These warrants have a fair value of $83,562, of which $27,854 has been expensed during 2005.
In June 2005, warrants to purchase 175,000 shares of Common Stock were issued to four consultants at an exercise price of $4.00 per share. One third of the warrants vested immediately, one third of the warrants vest on the first anniversary date, and the remaining one third of the warrants vests on the second anniversary date. These warrants have a fair value of $467,120, of which $84,813 has been expensed during 2005.
In July 2005, warrants to purchase 8,100 shares of Common Stock were issued to a consultant at an exercise price of $1.50 per share, of which warrants vested immediately. These warrants have a fair value of $21,150, of which $21,105 has been expensed during 2005.
In July 2005, warrants to purchase 460,846 shares of Common Stock were issued to several brokerage firms as the offering costs and commissions for Pharma’s financing activities at an exercise price of $1.50 per share. These warrants have a fair value of $2,197,162 and vest immediately.
In August 2005, warrants to purchase 200,000 shares of Common Stock were issued to a consultant at an exercise price of $1.19 per share. The warrants vest at a future date at such time that certain pre-determined events occur. These warrants have a fair value of $175,484.
In September 2005, warrants to purchase 15,000 shares of Common Stock were granted to a consultant at an exercise price of $1.19 per share of which warrants vested immediately. These warrants have a fair value of $13,161, of which $13,161 has been expensed during 2005.
In October 2005, warrants to purchase 167,500 shares of Common Stock were issued to four independent directors at an exercise price of $1.15 per share. One third of the warrants vested immediately, one third of the warrants vest on the first anniversary date, and the remaining one third vests on the second anniversary date. These warrants have a fair value of $191,658, of which $53,397 has been expensed during 2005.
In 2005, 99,134 warrants previously granted were expired.
Investor warrants:
During first quarter of 2005, 965,628 warrants were granted to investors related to the convertible notes.
In connection with the bridge note exchange and private placement offerings in June and July, 2,543,621 warrants granted in prior years and early 2005 were cancelled and three separate types of warrants to purchase a total of 2.75 shares of common stock were issued as follows: (i) 10,411,400 units of Series A Warrant for 1.25 shares with an exercise price of $2.00 which expires on February 17, 2006; (ii) 4,163,701 units of Series B Warrant for one-half of a share with an exercise price of $2.90 which expires on October 17, 2006; (iii) and 8,329,108 units of Series C Warrant for one share with an exercise price of $4.00 that expires on May 25, 2010.
F-14
Summary information regarding options is as follows:
| Weighted | Weighted | ||||||||||||
| Average | Average | ||||||||||||
| Exercise | Exercise | ||||||||||||
| Options | Price | Warrants | Price | ||||||||||
| Year ended December 31, 2003: | |||||||||||||
| Granted | - | $ | - | 150,000 | $ | .10 | |||||||
| Outstanding at December 31, 2003 | - | - | 150,000 | .10 | |||||||||
| Year ended December 31, 2004: | |||||||||||||
| Granted | 965,000 | 3.17 | 1,627,993 | 2.23 | |||||||||
| Outstanding at December 31, 2004 | 965,000 | 3.17 | 1,777,993 | 2.24 | |||||||||
| Year ended December 31, 2005: | |||||||||||||
| Granted | 630,500 | 1.57 | 25,041,284 | 2.86 | |||||||||
| Forfeited and cancelled | (41,667 | ) | 4.28 | (2,642,755 | ) | 2.45 | |||||||
| Outstanding at December 31, 2005 | 1,553,833 | $ | 2.49 | 24,176,522 | $ | 2.85 | |||||||
Options and warrants outstanding and exercisable as of December 31, 2005:
| Exercise | Remaining | Options | Options | Warrants | Warrants | ||||||||||||
| Price | Life | Outstanding | Exercisable | Outstanding | Exercisable | ||||||||||||
| $ | 5.00 | 3 - 4 years | 53,333 | 53,333 | 50,000 | - | |||||||||||
| 4.00 | 4 - 5 years | - | - | 8,504,108 | 8,362,441 | ||||||||||||
| 3.00 | 4 - 5 years | 219,500 | 63,333 | 145,000 | 75,000 | ||||||||||||
| 3.00 | 3 - 4 years | 885,000 | 395,000 | 50,000 | 16,667 | ||||||||||||
| 2.90 | 0.83 years | - | - | 4,164,567 | 4,164,567 | ||||||||||||
| 2.00 | 0.13 years | - | - | 10,411,400 | 10,411,400 | ||||||||||||
| 1.50 | 4 - 5 years | - | - | 468,947 | 8,100 | ||||||||||||
| 1.19 | 4 - 5 years | - | - | 215,000 | - | ||||||||||||
| 1.15 | 4 - 5 years | - | - | 167,500 | - | ||||||||||||
| 1.14 | 4 - 5 years | 20,000 | 6,667 | - | - | ||||||||||||
| 0.70 | 9 - 10 years | 376,000 | - | - | - | ||||||||||||
| 1,553,833 | 518,333 | 24,176,522 | 23,038,175 | ||||||||||||||
F-15
NOTE 12 - PURCHASE OF OPEXA
On October 7, 2004 Pharma entered into an agreement to acquire all of the outstanding stock of Opexa. The agreement closed on November 5, 2004. Pharma issued Opexa shareholders 2,500,000 shares of Pharma’s common stock for all of the outstanding stock of Opexa. 250,000 of the 2,500,000 shares were put in escrow for a one-year period pursuant to the escrow agreement. The balance of the 250,000 shares were issued to Opexa shareholders in November 2005. The acquisition was accounted for under the purchase method, where all of Opexa’s assets are restated to their fair market value on the acquisition date, which approximated book value. The 2,500,000 shares of Pharma were valued at their then fair value of $23,750,000 or $9.50 per share.
Pharma acquired Opexa because Opexa holds rights to technology to diagnose and treat multiple sclerosis through modified autoreactive T cells and is currently in FDA Phase I/II human dose ranging clinical trials to evaluate its safety and effectiveness in treating multiple sclerosis.
The results of operations for Opexa from November 6, 2004 through December 31, 2005 are included in the Statements of Operations and the Statements of Cash Flows.
The following table summarizes the estimated fair values of the assets acquired and the liabilities assumed at the date of acquisition:
| Current assets | $ | 55,387 | ||
| Property, plant and equipment, net | 639,160 | |||
| Intangible assets | 23,991,128 | |||
| Total assets acquired | 24,685,675 | |||
| Current liabilities | 935,675 | |||
| Total liabilities assumed | 935,675 | |||
| Net assets acquired | $ | 23,750,000 | ||
Of the $23,991,128 of acquired intangible assets, the full amount is assigned to an inseparable group of patents and licenses that cannot function independently by themselves. The weighted average useful life of the intangible group as of December 31, 2005 is approximately 15 years.
NOTE 13 - STOCK PURCHASE AGREEMENT
In June 2004, Pharma was acquired by Sportan United Industries, Inc. in a transaction accounted for as a reverse acquisition. Pharma’s shareholders were issued 6,386,439 Sportan shares in exchange for 100 percent of the outstanding common shares of Pharma. Immediately following this transaction, Sportan changed its name to Pharma and 7,383,838 shares were outstanding.
F-16
NOTE 14 - SUBSEQUENT EVENTS
Pharma entered into a remodeling construction contract to complete three Good Manufacturing Practice (“GMP”) production suites at its new facility. The construction contract plus equipment purchased separately cost approximately $500,000. The construction began October 1, 2005.
The construction of the GMP facilities was completed in January and certified on March 20, 2006 as an ISO 7 facility.
The Series A Warrant issued to investors in connection with the bridge note exchange and private placement offerings in June and July of 2005 expired on February 17, 2006.
F-17
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth the costs and expenses, other than the underwriting discounts and commissions, payable by the registrant in connection with the sale of the securities being registered. All amounts are estimates except the Securities and Exchange Commission registration fee.
| Securities and Exchange Commission Registration Fee | $ | 12,911 |
| Printing Costs | 3,000 | |
| Legal Fees and Expenses | 50,000 | |
| Accounting Fees and Expenses | 15,000 | |
| Transfer Agent and Registrar Fees | 3,000 | |
| Miscellaneous | 10,000 | |
| Total | $ | 93,056 |
Item 14. Indemnification of Directors and Officers.
The Company has the authority under Articles 2.02a(16) and 2.02-1 of the Texas Business Corporation Act (“TBCA”) to indemnify its directors and officers to the extent provided for in such statute. The TBCA provides, in part, that a corporation may indemnify a director or officer or other person who was, is or is threatened to be made a named defendant or respondent in a proceeding because such person is or was a director, officer, employee or agent of the corporation, if it is determined that such person: (1) conducted himself in good faith; (2) reasonably believed, in the case of conduct in his official capacity as a director or officer of the corporation, that his conduct was in the corporation’s best interest and, in all other cases, that his conduct was at least not opposed to the corporation’s best interests; and (3) in the case of any criminal proceeding, had no reasonable cause to believe that his conduct was unlawful.
A corporation may indemnify a person under the TBCA against judgments, penalties, including excise and similar taxes, fines, settlement, unreasonable expenses actually incurred by the person in connection with the proceeding. If the person is found liable to the corporation or is found liable on the basis that personal benefit was improperly received by the person, the indemnification is limited to reasonable expenses actually incurred by the person in connection with the proceeding, and shall not be made in respect of any proceeding in which the person shall have been found liable for willful or intentional misconduct in the performance of his duty to the corporation. The corporation may also pay or reimburse expenses incurred by a person in connection with his appearance as a witness or other participation in a proceeding at a time when he is not a named defendant or respondent in the proceeding.
The Company’s Articles of Incorporation provide that none of its directors shall be personally liable to the Company or its shareholders for monetary damages for an act or omission in such director’s capacity as a director; provided, however, that the liability of such director is not limited to the extent that such director is found liable for (1) a breach of the director’s duty of loyalty to the Company or its shareholders, (2) an act or omission not in good faith that constitutes a breach of duty of the director to the Company or an act or omission that involves intentional misconduct or a knowing violation of the law, (3) a transaction from which the director received an improper benefit, whether or not the benefit resulted from an action taken within the scope of the director’s office, or (4) an act or omission for which the liability of the director is expressly provided by an applicable statute.
-69-
The Company believes that these provisions will assist it in attracting and retaining qualified individuals to serve as executive officers and directors. The inclusion of these provisions in the Company’s Articles of Incorporation may have the effect of reducing a likelihood of derivative litigation against the Company’s directors and may discourage or deter shareholders or management from bringing a lawsuit against directors for breach of their duty of care, even though such an action, if successful, might otherwise have benefited us or our shareholders.
The Company’s Articles of Incorporation and By-laws provide that the Company may indemnify its officers, directors, agents and any other persons to the fullest extent permitted by the TCBA.
Additionally, under their employment agreements with PharmaFrontiers Corp. Messrs. McWilliams and Rouse are entitled to indemnification in their capacity as officers of the Company to the fullest extent permitted by the TCBA.
Item 15. Recent Sales of Unregistered Securities.
Acquisition of PharmaFrontiers Corp.
On June 4, 2004, when the company was named “Sportan United Industries, Inc.,” it issued, 6,386,439 shares of common stock to the shareholders of PharmaFrontiers Corporation, a Texas corporation ("PharmaFrontiers") in exchange for all the outstanding capital stock of PharmaFrontiers. The shares of common stock issued in the acquisition were offered and sold pursuant to the exemption from registration afforded by Rule 506 under the Securities Act of 1933 (the “Securities Act”) and/or Section 4(2) of the Securities Act. The Company subsequently changed its name to “PharmaFrontiers Corp.”
Acquisition of OPEXA
In connection with the closing of the Company’s acquisition of OPEXA Pharmaceuticals, Inc., a Delaware corporation (“OPEXA”), on November 5, 2004, the Company issued 2,500,000 shares of its common stock, par value $0.05 per share, to the former holders of common stock of OPEXA in exchange for their OPEXA common stock. The shares of common stock issued in the acquisition were offered and sold pursuant to the exemption from registration afforded by Rule 506 under the Securities Act and/or Section 4(2) of the Securities Act. On June 9, 2005 the Company issued 200,000 shares of common stock to Sanders Morris Harris, Inc. in exchange for advisory services provided in connection with the acquisition of OPEXA. The shares were sold pursuant to the exemption from registration afforded by Rule 504 under the Securities Act of 1933 (the “Securities Act”) and/or Section 4(2) of the Securities Act.
15% Exchangeable Convertible Subordinated Note Private Placement Financing and Subsequent Exchange
During the six months ended February 15, 2005, the Company issued to accredited investors in a private placement: (i) 15% Exchangeable Convertible Notes with a principal amount of $6.1 million, (ii) an aggregate of 612,688 shares of common stock, and (iii) a right to receive a warrant to purchase common stock. The Company received gross proceeds of $6.1 million from this private placement. The securities issued in the acquisition were offered and sold pursuant to the exemption from registration afforded by Rule 506 under the Securities Act of 1933 (the “Securities Act”) and/or Section 4(2) of the Securities Act. In connection with this private placement, the Company entered into Registration Rights Agreements with the purchasers requiring that, among other things, the Company register the shares issued, those shares issuable on conversion of the notes and those shares issuable upon exercise of the warrant for resale under the Securities Act.
-70-
In addition, the Company issued to the placement agents who assisted with the sale of the Notes, warrants to purchase 216,875 shares of Common Stock at a price of $1.50 per share. These warrants expire on February 14, 2010. The Company is obligated to register for resale the shares of common stock issuable upon exercise of the warrants.
On June 30, 2005 the Company, in exchange for all outstanding 15% Exchangeable Convertible Subordinated Notes and the associated rights to warrants, issued to the accredited investors holding the notes: (i) an aggregate of 5,658,575 shares of our common stock, (ii) Series A Warrants to purchase an aggregate of 5,541,998 shares of our common stock, (iii) Series B Warrants to purchase an aggregate of 2,216,799 shares of our common stock, and (iv) Series C Warrants to purchase an aggregate of 4,433,598 shares of our common stock. In connection with this exchange offering, the Company entered into a Registration Rights Agreement dated June 17, 2005 with the participants in the exchange offer requiring that, among other things, the Company register the shares issued in the exchange offer and issuable upon exercise of the warrants, for resale under the Securities Act.
The notes and shares of common stock and warrants described above were offered and sold pursuant to the exemption from registration afforded by Rule 506 under the Securities Act, Section 3(a)(9) of the Securities Act and/or Section 4(2) of the Securities Act.
Sale of Common Stock, Series A Warrants, Series B Warrants and Series C Warrants
In the month ended July 15, 2005, the Company completed a private placement to accredited investors of (i) an aggregate of 3,834,508 shares of common stock at a per share price of $1.50 pursuant to the terms of Securities Purchase Agreements dated June 17, 2005 and July 15, 2005 by and among the Company and each of the purchasers. In connection with this private placement, the Company entered into a Registration Rights Agreement dated June 17, 2005 and July 15, 2005 with the purchasers requiring that, among other things, the Company register the shares for resale under the Securities Act.
In addition, the Company issued to the placement agents assisting with the private placement, warrants (the “Placement Agent Warrants”) to purchase 228,992 shares of common stock at an exercise price of $1.50 per share. The Placement Agent Warrants are exercisable until June 17, 2010. The Company is obligated to register for resale the shares of common stock issuable upon exercise of the Placement Agent Warrants.
The shares of common stock and warrants described above were offered and sold pursuant to the exemption from registration afforded by Rule 506 under the Securities Act and/or Section 4(2) of the Securities Act.
Shares of Common Stock Issued To the University of Chicago
Pursuant to the terms of a First Amended and Restated License Agreement (the “License Agreement”) dated December 30, 2004 with the University of Chicago, the Company has issued 533,064 shares of common stock in consideration of the license granted to the Company. The License Agreement obligates the Company, for a period of time, to issue sufficient shares so as to enable the University of Chicago to maintain a certain percentage ownership. Thus the shares were issued from time to time as the Company issued shares in connection with its various offerings. The shares of common stock were offered and sold pursuant to the exemption from registration afforded by Section 4(2) under the Securities Act. In connection with the License Agreement, the Company agreed to register the shares issued thereunder for resale under the Securities Act.
-71-
Item 16. Exhibits and Financial Statement Schedules. | |
(a) Exhibits. The following exhibits of the Company are included herein. | |
| Exhibit 2.1 | Stock Purchase Agreement (incorporated by reference to Exhibit 2.1 to the Company's Current Report on Form K filed June 4, 2004) |
| Exhibit 2.2 | Merger Agreement (incorporated by reference to Exhibit 2.1 to the Company's Current Report on 8-K filed October 8, 2004) |
| Exhibit 3.1 | Amended and Restated Articles of Incorporation (incorporated by reference to Exhibit A to the Company's Definitive Information Statement filed on June 29, 2004) |
| Exhibit 3.2 * | By-laws |
| Exhibit 4.1 | Form of Common Stock Certificate (incorporated by reference to Exhibit 2.3 to the Company's Registration Statement on Form 10-SB (File No. 000-25513), initially filed March 8, 1999 |
| Exhibit 5.1 * | Opinion of Vinson & Elkins L.L.P. |
| Exhibit 10.1 | 2004 Compensatory Stock Option Plan (incorporated by reference to Exhibit B to the Company's Definitive Information Statement filed on June 29, 2004) |
| Exhibit 10.2 | Employment Agreement of David McWilliams (incorporated by reference to Exhibit 10.1 to the Company's Quarterly Report on Form 10-QSB filed November 16, 2004) |
| Exhibit 10.3 | Second Amended Employment Agreement of William Rouse (incorporated by reference to Exhibit 99.1 to the Company's Current Report on Form 8-K filed February 4, 2005) |
| Exhibit 10.4 | Amended Employment Agreement of Warren C. Lau (incorporated by reference to Exhibit 99.2 to the Company's Current Report on Form 8-K filed February 4, 2005) |
| Exhibit 10.5 | Director's Agreement of David McWilliams (incorporated by reference to Exhibit 10.4 to the Company's Quarterly Report on Form 10-QSB filed November 16, 2004) |
| Exhibit 10.6 | Director's Agreement of Robert H. Gow (incorporated by reference to Exhibit 10.6 to the Company's Annual Report on Form 10-KSB filed April 15, 2005) |
| Exhibit 10.7 | Director's Agreement of Paul Frison (incorporated by reference to Exhibit 10.7 to the Company's Annual Report on Form 10-KSB filed April 15, 2005) |
| Exhibit 10.8 | Director's Agreement of Tony Kamin (incorporated by reference to Exhibit 10.8 to the Company's Annual Report on Form 10-KSB filed April 15, 2005) |
| Exhibit 10.9 | Director's Agreement of Brian Rodriguez (incorporated by reference to Exhibit 10.2 to the Company's Quarterly Report on Form 10-QSB filed November 16, 2004) |
-72-
| Exhibit 10.10 | Scientific Board Advisory Agreement of Yong Zhao (incorporated by reference to Exhibit 10.3 to the Company's Quarterly Report on Form 10-QSB filed November 16, 2004) |
| Exhibit 10.11 | Termination Settlement and Release Agreement with R. Wayne Fritzsche (incorporated by reference to Exhibit 99.3 to the Company's Current Report on Form 8-K filed February 4, 2005) |
| Exhibit 10.12 | Form of Warrant Agreement (incorporated by reference to Exhibit 10.13 to the Company's Annual Report on Form 10-KSB filed April 15, 2005) |
| Exhibit 10.13 | Amended and Restated License Agreement with Baylor College of Medicine (incorporated by reference to Exhibit 10.14 to the Company's Annual Report on Form 10-KSB filed April 15, 2005) |
| Exhibit 10.14 | Amended and Restated License Agreement with University of Chicago (incorporated by reference to Exhibit 10.15 to the Company's Annual Report on Form 10-KSB filed April 15, 2005) |
| Exhibit 10.15 * | Form of Series A Common Stock Purchase Warrant |
| Exhibit 10.16 * | Form of Series B Common Stock Purchase Warrant |
| Exhibit 10.17 * | Form of Series C Common Stock Purchase Warrant |
| Exhibit 10.18 * | Securities Purchase Agreement dated June 17, 2005 by and among the Company and the Investors named therein. |
| Exhibit 10.19 * | Registration Rights Agreement dated June 17, 2005 by and among the purchasers of common stock named therein |
| Exhibit 10.20 * | Securities Purchase Agreement dated June 30, 2005 by and among the Company and the purchasers of common stock named therein |
| Exhibit 10.21 * | Securities Purchase Agreement dated July 15, 2005 by and among the Company and the Investors named therein. |
| Exhibit 10.22 * | Registration Rights Agreement dated July 15, 2005 by and among the Company and the Investors named therein. |
| Exhibit 10.23** | License Agreement dated January 13, 2006 by the Company and Shanghai Institute for Biological Services. |
| Exhibit 10.24 | Lease Agreement dated August 19, 2005 by the Company and Dirk D. Laukien(incorporated by reference to Exhibit 10.13 to the Company's Annual Report on Form 10-KSB filed March 31, 2006) |
| Exhibit 10.25 *** | Form of Broker Stock Purchase Warrant Agreement |
| Exhibit 23.1 *** | Consent of Malone & Bailey, PC |
| Exhibit 23.2 | Consent of Vinson & Elkins L.L.P. (included in Exhibit 5.1) |
| * Previously filed on Form SB-2 filed July 19, 2005 | |
| ** Previously filed on Post Amendment #2 filed on February 9, 2006 | |
| *** Filed herewith | |
-73-
(b) Financial Statement Schedules.
All schedules are omitted because they are inapplicable or the requested information is shown in the consolidated financial statements of the registrant or related notes thereto.
Item 17. Undertakings.
| (a) | The undersigned registrant hereby undertakes: | ||
| (1) | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: | ||
| (i) | To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933; | ||
| (ii) | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represents a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Securities and Exchange Commission pursuant to Rule424(b) if, in the aggregate, the changes in volume and price represent no more than a 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and | ||
| (iii) | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement. | ||
| (2) | That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. | ||
| (3) | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. | ||
| (b) | Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933 and will be governed by the final adjudication of such issue. | ||
-74-
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the Registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Houston, State of Texas, on the 10th day of April, 2006.
PHARMAFRONTIERS CORP.
By: /s/ David B. McWilliams
Name: David B. McWilliams
Title: Chief Executive Officer
By: /s/ C. William Rouse
Name: C. William Rouse
Title: Chief Financial Officer and
Principal Accounting Officer
Pursuant to the requirements of the Securities Act of 1933, as amended, this Registration Statement has been signed below by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | |
* | Chairman of the Board and Director | April 10, 2006 | |
| Brooks Boveroux | |||
| /s/ David B. McWilliams | President, Chief Executive Officer and | April 10, 2006 | |
| David B. McWilliams | Director (principal executive officer) | ||
/s/ C. William Rouse | Chief Financial Officer | April 10, 2006 | |
| C. William Rouse | (principal financial and accounting officer) | ||
* | Director | April 10, 2006 | |
| Anthony N. Kamin | |||
* | Director | April 10, 2006 | |
| Paul M. Frison | |||
* | Director | April 10, 2006 | |
| Terry Wesner |
* By: /s/ David B. McWilliams
David B. McWilliams
Signature Page for
Form SB-2 - Registration Statement
Warrant Agreement
This Warrant and the Shares of common stock issuable upon the exercise hereof have not been registered under either the Securities Act of 1933, as amended (“Act”), or applicable state securities laws (“State Acts”) and shall not be sold, pledged, hypothecated, donated, or otherwise transferred (whether or not for consideration) by the Holder except upon the issuance to the Company of an opinion of counsel or submission to the Company of such evidence as may be satisfactory to counsel to the Company, in each such case, to the effect that any such transfer shall not be in violation of the Act and the State Acts.
Warrant to Purchase «No_of_Warrants» Shares of Common Stock
PharmaFrontiers corp.
(a Texas corporation)
2408 Timberloch Place, Suite B-7
The Woodlands, Texas 77380
Not Transferable or Exercisable Except
upon Conditions Herein Specified
PharmaFrontiers, Corp., a Texas corporation (“Company”), hereby certifies that «Name» , its registered successors and permitted assigns registered on the books of the Company maintained for such purposes, as the registered holder hereof (“Holder”), for value received, is entitled to purchase from the Company the number of fully paid and non-assessable shares of Common Stock of the Company (“Shares” or “Common Stock”), stated above at the purchase price per Share set forth in Section 1(b) below (the number of Shares and Exercise Price being subject to adjustment as hereinafter provided) upon the terms and conditions herein provided.
TERMS
1. Exercise of Warrants.
(a) Subject to subsection (b) of this Section 1, upon presentation and surrender of this Warrant Agreement, with the attached Purchase Form duly executed, at the principal office of the Company, or at such other place as the Company may designate by notice to the Holder hereof, together with a certified or bank cashier’s check payable to the order of the Company in the amount of the Exercise Price times the number of Shares being purchased, the Company shall deliver to the Holder hereof, as promptly as practicable, certificates representing the Shares being purchased. This Warrant may be exercised in whole or in part; and, in case of exercise hereof in part only, the Company, upon surrender hereof, will deliver to the Holder a new Warrant Agreement or Warrant Agreements of like tenor entitling the Holder to purchase the number of Shares as to which this Warrant has not been exercised.
(b) This Warrant may be exercised at a price of $1.50 per share (the “Exercise Price”); provided however, that the Exercise Price shall be subject to adjustment pursuant to Section 6(b). The Warrant shall expire upon the close of business on July 18, 2010.
2. Exchange and Transfer of Warrant.
At any time prior to the exercise hereof, upon presentation and surrender to the Company, this Warrant (a) may be exchanged, alone or with other Warrants of like tenor registered in the name of the Holder, for another Warrant or other Warrants of like tenor in the name of such Holder exercisable for the same aggregate number of Shares as the Warrant or Warrants surrendered, but (b) may not be sold, transferred, hypothecated, or assigned, in whole or in part, without the prior written consent of the Company.
Page 1 of 7
3. Rights and Obligations of Warrant Holder.
(a) The Holder of this Warrant Agreement shall not, by virtue hereof, be entitled to any rights of a stockholder in the Company, either at law or in equity; provided, however, that in the event that any certificate representing the Shares is issued to the Holder hereof upon exercise of this Warrant, such Holder shall, for all purposes, be deemed to have become the holder of record of such Shares on the date on which this Warrant Agreement, together with a duly executed Purchase Form, was surrendered and payment of the Exercise Price was made, irrespective of the date of delivery of such Share certificate. The rights of the Holder of this Warrant are limited to those expressed herein and the Holder of this Warrant, by his acceptance hereof, consents to and agrees to be bound by and to comply with all the provisions of this Warrant Agreement, including, without limitation, all the obligations imposed upon the Holder hereof by Sections 2 and 5 hereof. In addition, the Holder of this Warrant Agreement, by accepting the same, agrees that the Company may deem and treat the person in whose name this Warrant Agreement is registered on the books of the Company maintained for such purposes as the absolute, true and lawful owner for all purposes whatsoever, notwithstanding any notation of ownership or other writing thereon, and the Company shall not be affected by any notice to the contrary.
(b) No Holder of this Warrant Agreement shall be entitled to vote or receive dividends or to be deemed the holder of Shares for any purpose, nor shall anything contained in this Warrant Agreement be construed to confer upon any Holder of this Warrant Agreement any of the rights of a stockholder of the Company or any right to vote, give or withhold consent to any action by the Company, whether upon any recapitalization, issue of stock, reclassification of stock, consolidation, merger, conveyance or otherwise, receive notice of meetings or other action affecting stockholders (except for notices provided for herein), receive dividends, subscription rights, or otherwise, until this Warrant shall have been exercised and the Shares purchasable upon the exercise thereof shall have become deliverable as provided herein; provided, however, that any such exercise on any date when the stock transfer books of the Company shall be closed shall constitute the person in whose name the certificate for those Shares are to be issued as the record holder thereof for all purposes at the opening of business on the next succeeding day on which such stock transfer books are open, and the Warrant surrendered shall not be deemed to have been exercised, in whole or in part as the case may be, until the next succeeding day on which stock transfer books are open for the purpose of determining entitlement to dividends on the Company’s common stock.
(c) The Holder has certain registration rights as set forth in Exhibit “A”.
4. Shares Underlying Warrant.
The Company covenants and agrees that all Shares delivered upon exercise of this Warrant shall, upon delivery and payment therefor, be duly and validly authorized and issued, fully paid and non-assessable, and free from all stamp taxes, liens and charges with respect to the purchase thereof.
5. Disposition of Warrant or Shares.
(a) The Holder of this Warrant and any transferee hereof or of the Shares issuable upon the exercise of the Warrant, by their acceptance hereof, hereby understand and agree that the Warrant, and the Shares issuable upon the exercise hereof, have not been registered under either the Act or State Acts and shall not be sold, pledged, hypothecated, or otherwise transferred (whether or not for consideration) except upon delivery to the Company of an opinion of its counsel satisfactory to the Company or its counsel that registration is not required for such transfer or submission to the Company of such evidence as may be satisfactory to the Company or its counsel, in each such case, to the effect that any such transfer shall not be in violation of the Act or the State Acts. It shall be a condition to the transfer of this Warrant that any transferee of this Warrant deliver to the Company his written agreement to accept and be bound by all of the terms and conditions of this Warrant. The Holder acknowledges that the Company has not granted any registration rights hereunder.
Page 2 of 7
(b) The Holder of this Warrant Agreement and any transferee hereof or of the Shares issuable upon the exercise of the Warrant Agreement, by their acceptance hereof, hereby understand and agree that the Warrant, and the Shares issuable upon the exercise hereof, have not been registered under either the Act or State Acts and shall not be sold, pledged, hypothecated, or otherwise transferred (whether or not for consideration) except upon the issuance to the Company of an opinion of counsel favorable to the Company or its counsel or submission to the Company of such evidence as may be satisfactory to the Company or its counsel, in each such case, to the effect that any such transfer shall not be in violation of the Act or the State Acts. It shall be a condition to the transfer of this Warrant that any transferee of this Warrant delivers to the Company his written agreement to accept and be bound by all of the terms and conditions of this Warrant Agreement. The Holder acknowledges that the Company has not granted any registration rights hereunder.
(c) The stock certificates of the Company that will evidence the shares of Common Stock with respect to which this Warrant may be exercisable will be imprinted with a conspicuous legend in substantially the following form:
“The securities represented by this certificate have not been registered under either the Securities Act of 1933 (“Act”) or the securities laws of any state (“State Acts”). Such securities shall not be sold, pledged, hypothecated, or otherwise transferred (whether or not for consideration) at any time whatsoever except upon registration or upon delivery to the Company of an opinion of its counsel satisfactory to the Company or its counsel that registration is not required for such transfer or the submission of such other evidence as may be satisfactory to the Company or its counsel to the effect that any such transfer shall not be in violation of the Act, State Acts or any rule or regulation promulgated thereunder.”
6. Adjustments.
The number of Shares purchasable upon the exercise of each Warrant is subject to adjustment from time to time upon the occurrence of any of the events enumerated below:
(a) If at any time after the date of this Warrant and so long as this Warrant is outstanding, there is (i) a stock split, stock dividend, subdivision, or similar distribution with respect to the Common Stock, (ii) a combination of the Common Stock, or (iii) a sale or issuance of Common Stock, or exchangeable for its Common Stock, or any rights, options or warrants to subscribe for or to purchase of its Common Stock, then, in such event, the Exercise Price shall be adjusted in accordance with (b) below.
(b) Immediately upon the effective date of any event requiring adjustment pursuant to (a), the Company shall adjust the Exercise Price then in effect (to the nearest whole cent) as follows:
(i)If the Company (A) shall pay a stock dividend or otherwise make a distribution or distributions on shares of its Common Stock payable in shares of Common Stock, (B) subdivide outstanding shares of Common Stock into a larger number of shares, (C) combine outstanding shares of Common Stock into a smaller number of shares, or (D) issue by reclassification of shares of Common Stock any shares of capital stock of the Company, the Exercise Price shall be multiplied by a fraction of which the numerator shall be the number of shares of Common Stock (excluding treasury shares, if any) outstanding before such event and of which the denominator shall be the number of shares of Common Stock outstanding after such event. Any adjustment made pursuant to this subparagraph (i) shall become effective immediately after the record date for the determination of stockholders entitled to receive such dividend or distribution and shall become effective immediately after the effective date in the case of a subdivision, combination or re-classification.
Page 3 of 7
(ii)If the Company shall distribute to all holders of Common Stock evidences of its indebtedness or assets or rights or warrants to subscribe for or purchase any security (excluding those referred to in subparagraph (i) above), then in each such case the Exercise Price shall be determined by multiplying the Exercise Price in effect immediately prior to the record date fixed for determination of stockholders entitled to receive such distribution by a fraction of which the denominator shall be the market price of Common Stock determined as of the record date mentioned above, and of which the numerator shall be such market price of the Common Stock on such record date less the then fair market value at such record date of the portion of such assets or evidence of indebtedness so distributed applicable to one outstanding share of Common Stock as determined by the Company's board of directors in good faith; provided, however, that if the Holder disputes such amount, the Holder may select a nationally recognized or major regional investment banking firm or firm of independent certified public accountants of recognized standing (an “Appraiser”) paid for by the Holder and the Company equally, in which case the fair market value shall be equal to the average of the determinations by the Company’s board of directors and such Appraiser. In either case the adjustments shall be described in a statement provided to the Holder of the portion of assets or evidences of indebtedness so distributed or such subscription rights applicable to one share of Common Stock. Such adjustment shall be made whenever any such distribution is made and shall become effective immediately after the record date mentioned above.
(iii) In case the Company (A) consolidates with or merges into any other entity and is not the continuing or surviving entity of such consolidation or merger, or (B) permits any other entity to consolidate with or merge into the Company and the Company is the continuing or surviving Company but, in connection with such consolidation or merger, the Common Stock is changed into or exchanged for common stock or other securities of any other entity or cash or any other assets, or (C) transfers all or substantially all of its properties and assets to any other entity, or (D) effects a reorganization or reclassification of the equity of the Company in such a way that holders of Common Stock shall be entitled to receive stock, securities, cash or assets with respect to or in exchange for Common Stock, then, and in each such case, proper provision shall be made so that, upon the exercise of the Warrant at any time after the consummation of such consolidation, merger, transfer, reorganization or reclassification, the Holder shall be entitled to receive (at the Exercise Price in effect for Common Stock issuable upon such exercise of the Warrant immediately prior to such consummation), in lieu of Common Stock issuable upon such exercise of the Warrant prior to such consummation, the stock and other securities, cash and assets to which the Holder would have been entitled upon such consummation if such Holder had so exercised the Warrant immediately prior thereto.
(c) Upon each adjustment of the Exercise Price pursuant to (b) above, the Warrant outstanding prior to such adjustment in the Exercise Price shall thereafter evidence the right to purchase, at the adjusted Exercise Price, that number of shares of Common Stock (calculated to the nearest hundredth) obtained by (i) multiplying the number of shares of Common Stock issuable upon exercise of the Warrant prior to adjustment of the number of shares of Common Stock by the Exercise Price in effect prior to adjustment of the Exercise Price and (ii) dividing the product so obtained by the Exercise Price in effect after such adjustment of the exercise price.
(d) In case the Company (i) consolidates with or merges into any other entity and is not the continuing or surviving entity of such consolidation or merger, or (ii) permits any other entity to consolidate with or merge into the Company and the Company is the continuing or surviving Company but, in connection with such consolidation or merger, the Common Stock is changed into or exchanged for common stock or other securities of any other entity or cash or any other assets, or (iii) transfers all or substantially all of its properties and assets to any other entity, or (iv) effects a reorganization or reclassification of the equity of the Company in such a way that holders of Common Stock shall be entitled to receive stock, securities, cash or assets with respect to or in exchange for Common Stock, then, and in each such case, proper provision shall be made so that, upon the exercise of this Warrant at any time after the consummation of such consolidation, merger, transfer, reorganization or reclassification, the Holder shall be entitled to receive (at the aggregate Exercise Price in effect for Common Stock issuable upon such exercise of this Warrant immediately prior to such consummation), in lieu of Common Stock issuable upon such exercise of this Warrant prior to such consummation, the stock and other securities, cash and assets to which such Holder would have been entitled upon such consummation if such Holder had so exercised this Warrant immediately prior thereto.
Page 4 of 7
7. Loss or Destruction.
Upon receipt of evidence satisfactory to the Company of the loss, theft, destruction or mutilation of this Warrant Agreement and, in the case of any such loss, theft or destruction, upon delivery of an indemnity agreement or bond satisfactory in form, substance and amount to the Company or, in the case of any such mutilation, upon surrender and cancellation of this Warrant Agreement, the Company will execute and deliver, in lieu thereof, a new Warrant Agreement of like tenor.
8. Survival.
The various representations, warranties, rights and obligations of the Holder hereof as set forth herein shall survive the exercise of the Warrants represented hereby and the surrender of this Warrant Agreement.
9. Notices.
Whenever any notice, payment of any purchase price, or other communication is required to be given or delivered under the terms of this Warrant, it shall be in writing and delivered by hand delivery or United States registered or certified mail, return receipt requested, postage prepaid (or similar delivery if outside of the United States), and will be deemed to have been given or delivered on the date such notice, purchase price or other communication is so delivered or posted, as the case may be; and, if to the Company, it will be addressed to the address specified on the cover page hereof, and if to the Holder, it will be addressed to the registered Holder at its, his or his address as it appears on the books of the Company.
Page 5 of 7
SIGNATURE PAGE
WARRANT AGREEMENT
If the foregoing is in accordance with your understanding, please sign the form of confirmation and acceptance on the enclosed counterpart of this Agreement and return the same to the Company, whereupon this Agreement shall be a binding agreement between you and the Company.
| Very truly yours, | ||
| PharmaFrontiers Corp. | ||
| | | |
| By: |  | |
David McWilliams, CEO | ||
I HEREBY ACCEPT AND AGREE TO THE TERMS AND CONDITIONS CONTAINED IN THIS WARRANT AGREEMENT: |
| By: |
| Name: |
| Title: |
Page 6 of 7
PURCHASE FORM
(To be signed only upon exercise of Warrant)
To Pharmafrontiers, corp.:
The undersigned, the holder of the enclosed Warrant, hereby irrevocably elects to exercise the purchase right represented by such Warrant for, and to purchase thereunder, _________* shares of Common Stock of PharmaFrontiers, Corp., and herewith makes payment of $_______________ therefor, and requests that the certificate or certificates for such shares be issued in the name of and delivered to the undersigned.
Dated:______________
___________________________________________
(Signature must conform in all respects to
name of holder as specified on the face of
the enclosed Warrant)
___________________________________________
___________________________________________
(Address)
___________________________________________
(SSN#)
___________________________
| (*) | Insert here the number of shares called for on the face of the Warrant without making any adjustment for additional Common Stock or any other stock or other securities or property or cash which, pursuant to the adjustment provisions of the Warrant Agreement pursuant to which the Warrant was granted, may be delivered upon exercise. |
Page 7 of 7
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and Board of Directors
PharmaFrontiers Corp.
The Woodlands, Texas
We hereby consent to the incorporation by reference in this Registration Statement on Form SB-2/A our report dated February 7, 2006 included herein for the two years ended December 31, 2005 and the period from
January 22, 2003 (Inception) through December 31, 2005.
We also consent to the references to us under the heading “Experts” in such Document.
April 6, 2006
Malone & Bailey, PC
www.malone-bailey.com
Houston, Texas