Exhibit 99.1


Forward-Looking Statements This presentation contains forward-looking statements which are made pursuant to the safe harbor provisions of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. The forward-looking statements in this presentation do not constitute guarantees of future performance. Investors are cautioned that statements in this presentation which are not strictly historical statements, including, without limitation, statements regarding the Company’s clinical development plans for Tovaxin, constitute forward-looking statements. Such forward-looking statements are subject to a number of risks and uncertainties that could cause actual results to differ materially from those anticipated, including, without limitation, risks associated with the Company’s capital position, the ability of the Company to enter into and benefit from a partnering arrangement for the Company’s product candidate, Tovaxin, on reasonably satisfactory terms (if at all), and our dependence (if partnered) on the resources and abilities of any partner for the further development of Tovaxin, our ability to compete with larger, better financed pharmaceutical and biotechnology companies, new approaches to the treatment of our targeted diseases, our expectation of incurring continued losses, our uncertainty of developing a marketable product, our ability to raise additional capital to continue our treatment development program and to undertake and complete any further clinical studies for Tovaxin, the success of our clinical trials, the efficacy of Tovaxin for any particular indication, such as for relapsing remitting MS or secondary progressive MS, our ability to develop and commercialize products, our ability to obtain required regulatory approvals, our compliance with all Food and Drug Administration regulations, our ability to obtain, maintain and protect intellectual property rights (including for Tovaxin), the risk of litigation regarding our intellectual property rights, our limited manufacturing capabilities, our dependence on third-party manufacturers, our ability to hire and retain skilled personnel, our volatile stock price, and other risks detailed in our filings with the Securities and Exchange Commission. These forward-looking statements speak only as of the date of this presentation. We assume no obligation or undertaking to update or revise any forward-looking statements contained herein to reflect any changes in our expectations with regard thereto or any change in events, conditions or circumstances on which any such statement is based. You should, however, review additional disclosures we make in our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, and Current Reports on Form 8-K filed with the SEC.
Opexa Highlights Proprietary T-cell technology platform allows for the production of patient-specific T-cell therapies for a variety of autoimmune diseasesLead program, Tovaxin®, a personalized cellular immunotherapy for the first-line treatment of multiple sclerosis (MS)Fast Track designation granted November 2011 by FDA for Tovaxin for treatment of Secondary Progressive Multiple Sclerosis (SPMS)In-house cGMP manufacturing enables close control of process and COGS
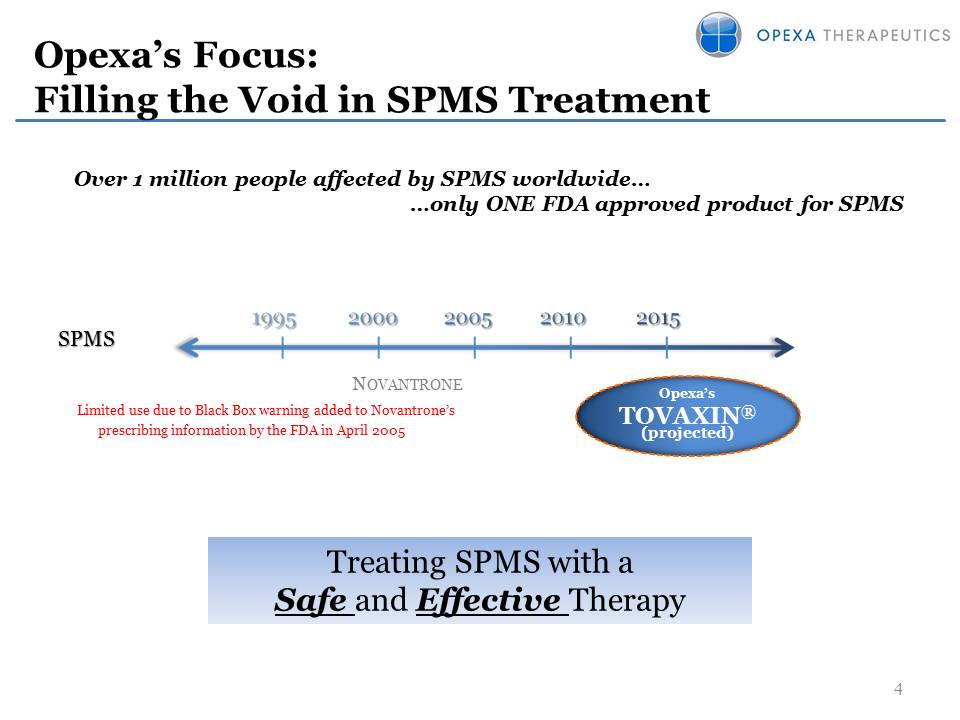
Opexa’s Focus: Filling the Void in SPMS Treatment Over 1 million people affected by SPMS worldwide… …only ONE FDA approved product for SPMS SPMS Opexa’sTOVAXIN®(projected) Novantrone Limited use due to Black Box warning added to Novantrone’s prescribing information by the FDA in April 2005 Treating SPMS with a Safe and Effective Therapy
Tovaxin® A personalized autologous T-cell immunotherapy, consisting of attenuated, patient-specific myelin reactive T-cells (MRTCs) against peptides of the three primary myelin proteinsProposed MechanismThe subcutaneous injection of a therapeutic dose (30-45 million cells) of Tovaxin stimulates the body’s immune system to recognize the bolus of injected cells as a peripheral source of ‘over represented’ MRTC , resulting in the induction of an opposing dominant negative ‘regulatory T-cell’ response: Selective targeting by immune cells to down-regulate and eliminate similar myelin reactive T-cells within the CNSAn up-regulation of important regulatory cells (Foxp3+ and Tr1 cells) to reduce inflammation and provide possible neuroprotection
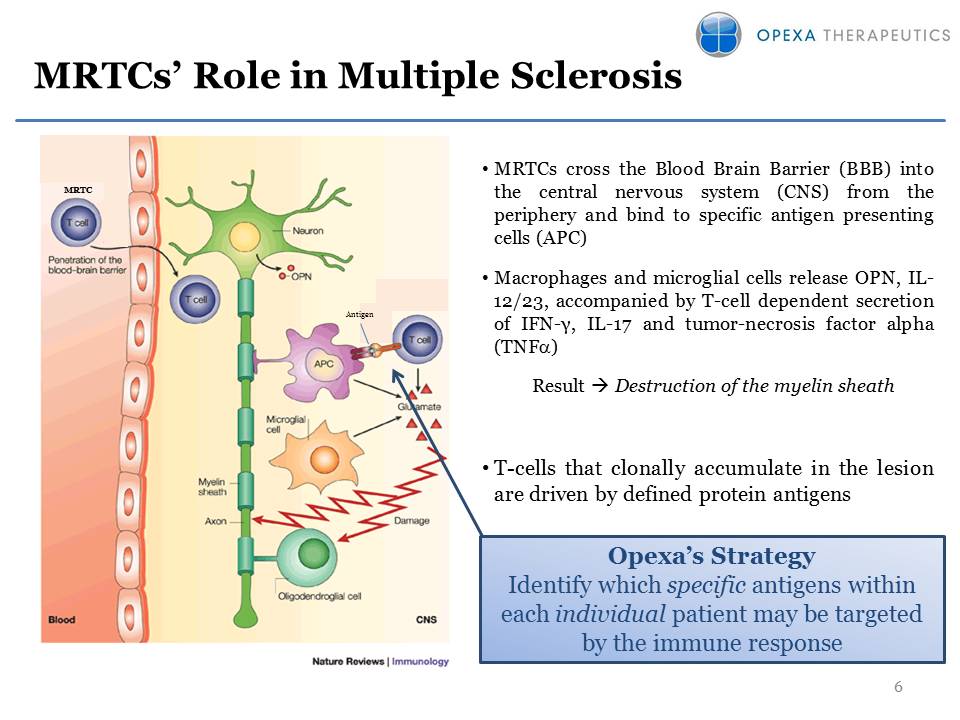
MRTCs’ Role in Multiple Sclerosis MRTCs cross the Blood Brain Barrier (BBB) into the central nervous system (CNS) from the periphery and bind to specific antigen presenting cells (APC)Macrophages and microglial cells release OPN, IL-12/23, accompanied by T-cell dependent secretion of IFN-?, IL-17 and tumor-necrosis factor alpha (TNFa)Result ? Destruction of the myelin sheathT-cells that clonally accumulate in the lesion are driven by defined protein antigens Antigen MRTC Opexa’s Strategy Identify which specific antigens within each individual patient may be targeted by the immune response
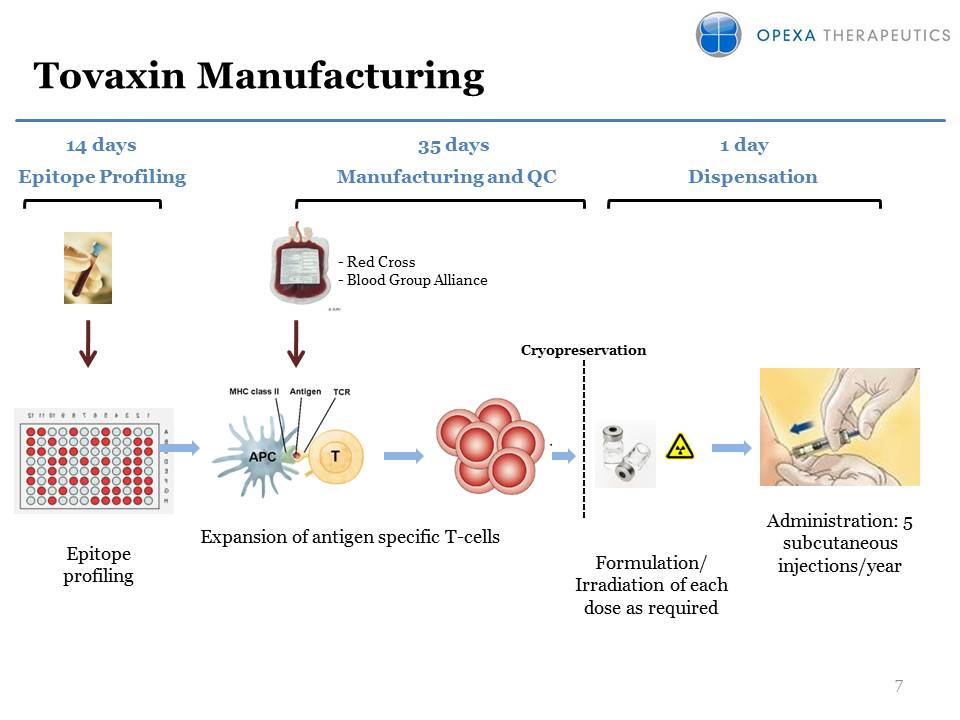
Tovaxin Manufacturing Expansion of antigen specific T-cells Cryopreservation Formulation/Irradiation of each dose as required Epitope profiling Administration: 5 subcutaneous injections/year Manufacturing and QC Dispensation 35 days Epitope Profiling 1 day 14 days - Red Cross- Blood Group Alliance
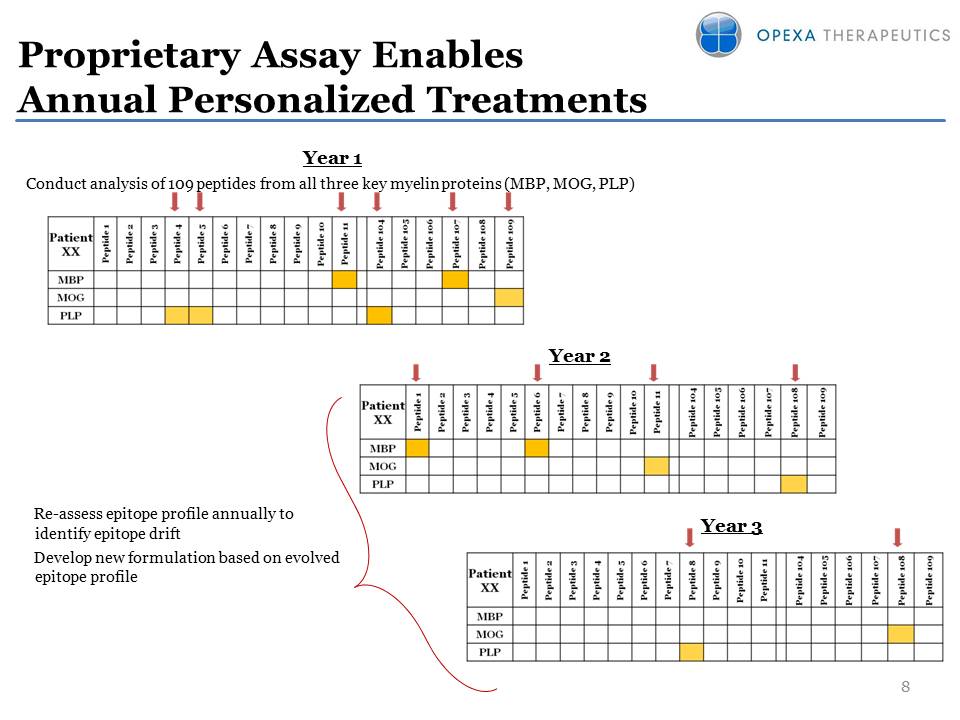
Year 2 Year 3 Proprietary Assay Enables Annual Personalized Treatments Year 1Conduct analysis of 109 peptides from all three key myelin proteins (MBP, MOG, PLP) Re-assess epitope profile annually to identify epitope drift Develop new formulation based on evolved epitope profile
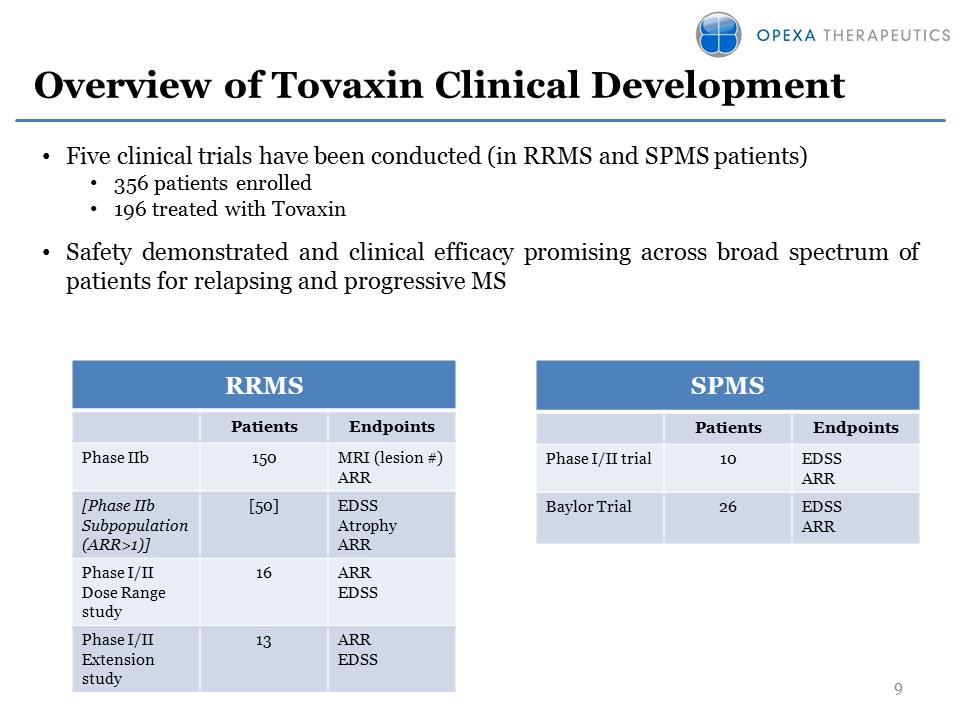
Overview of Tovaxin Clinical Development Five clinical trials have been conducted (in RRMS and SPMS patients)356 patients enrolled196 treated with TovaxinSafety demonstrated and clinical efficacy promising across broad spectrum of patients for relapsing and progressive MS
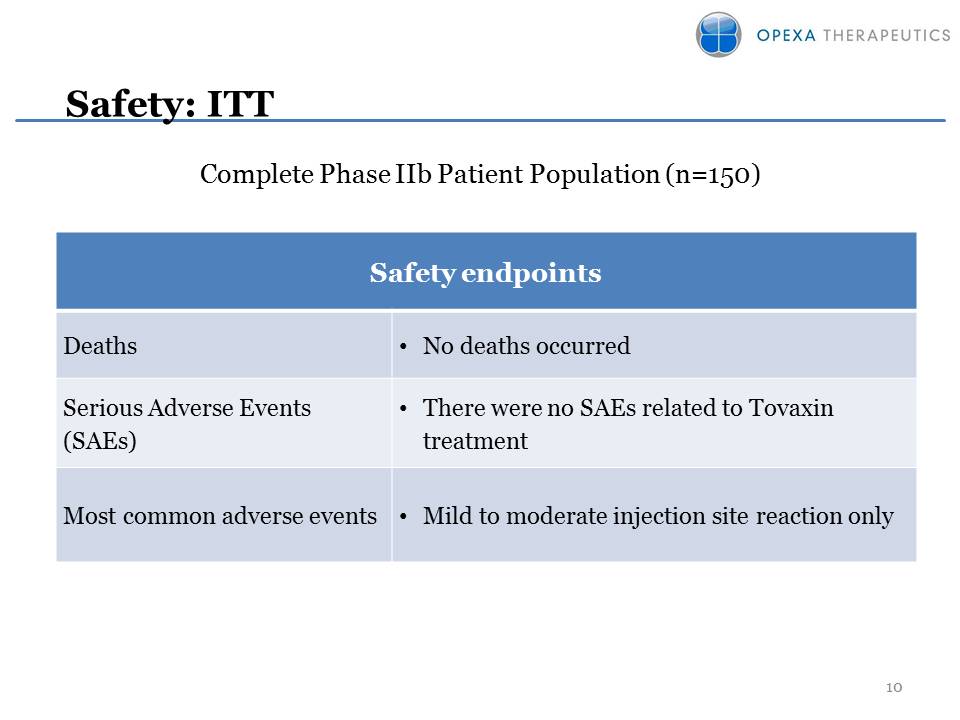
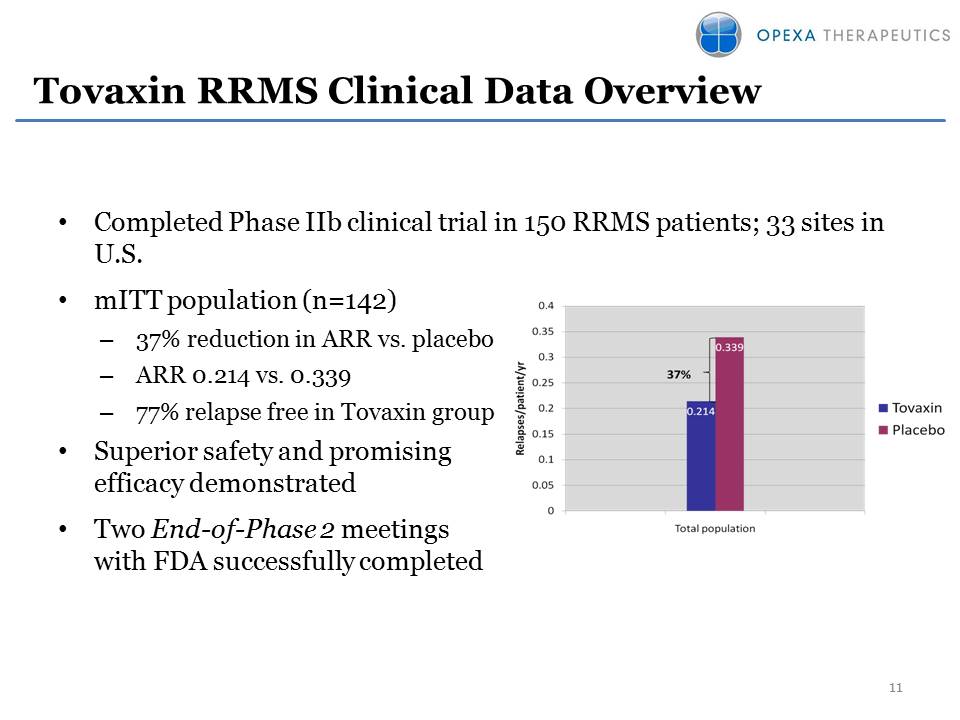
Tovaxin RRMS Clinical Data Overview Other Placeholder: Completed Phase IIb clinical trial in 150 RRMS patients; 33 sites in U.S.mITT population (n=142)37% reduction in ARR vs. placeboARR 0.214 vs. 0.33977% relapse free in Tovaxin groupSuperior safety and promising efficacy demonstratedTwo End-of-Phase 2 meetings with FDA successfully completed
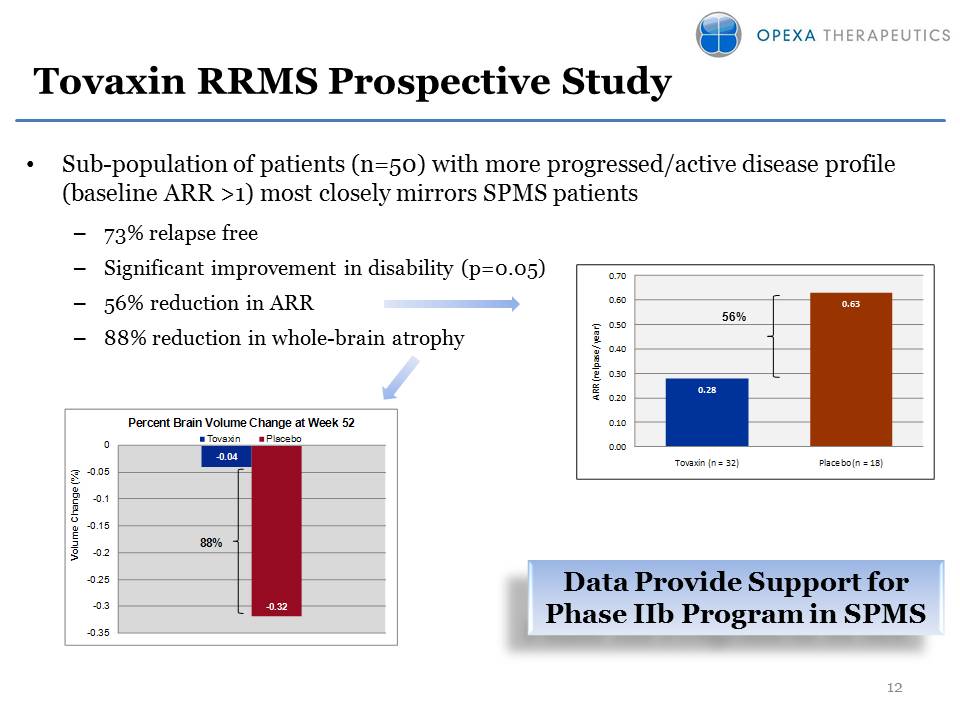
Tovaxin RRMS Prospective Study Sub-population of patients (n=50) with more progressed/active disease profile (baseline ARR >1) most closely mirrors SPMS patients73% relapse freeSignificant improvement in disability (p=0.05)56% reduction in ARR 88% reduction in whole-brain atrophy 56% Data Provide Support for Phase IIb Program in SPMS

36 patients treated in three clinical trialsPromising efficacy observed Disease stabilization in 80% of patients at two yearsSignificant reduction in relapse ratesWell-tolerated, no SAEs Secondary Progressive MS: Clinical Overview
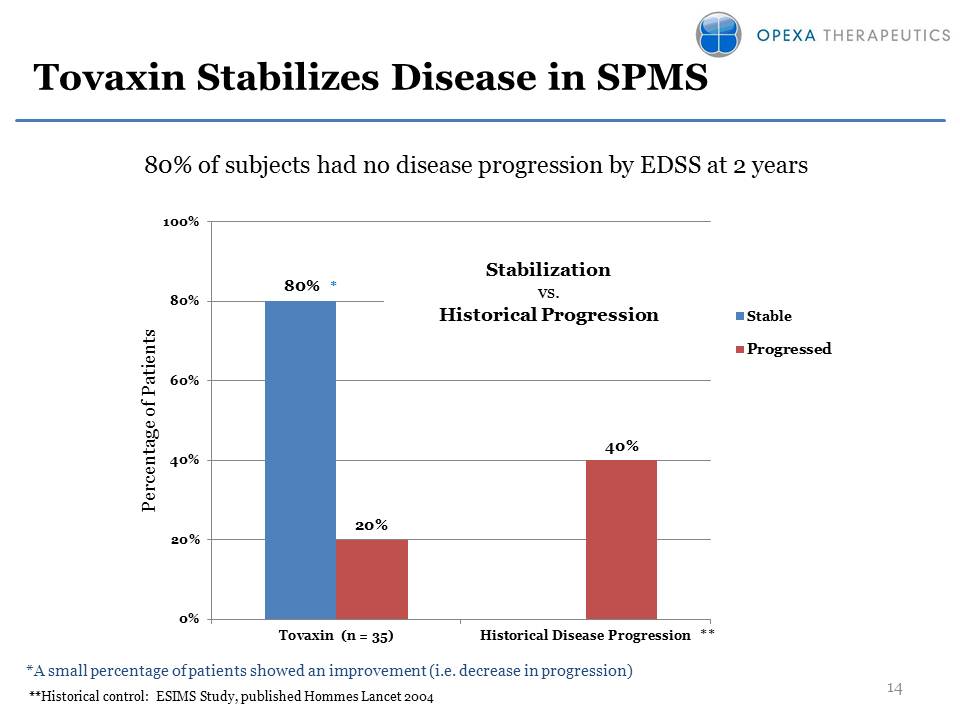
Tovaxin Stabilizes Disease in SPMS *A small percentage of patients showed an improvement (i.e. decrease in progression) **Historical control: ESIMS Study, published Hommes Lancet 2004* ** 80% of subjects had no disease progression by EDSS at 2 years
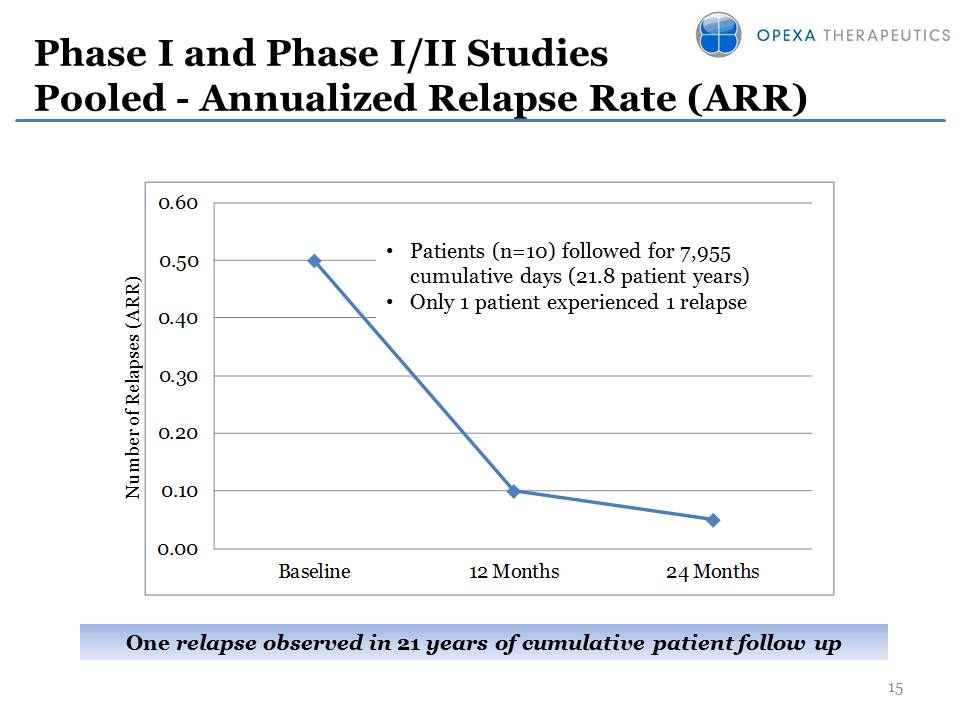
Phase I and Phase I/II Studies Pooled - Annualized Relapse Rate (ARR) Number of Relapses (ARR) Patients (n=10) followed for 7,955 cumulative days (21.8 patient years) Only 1 patient experienced 1 relapse One relapse observed in 21 years of cumulative patient follow up
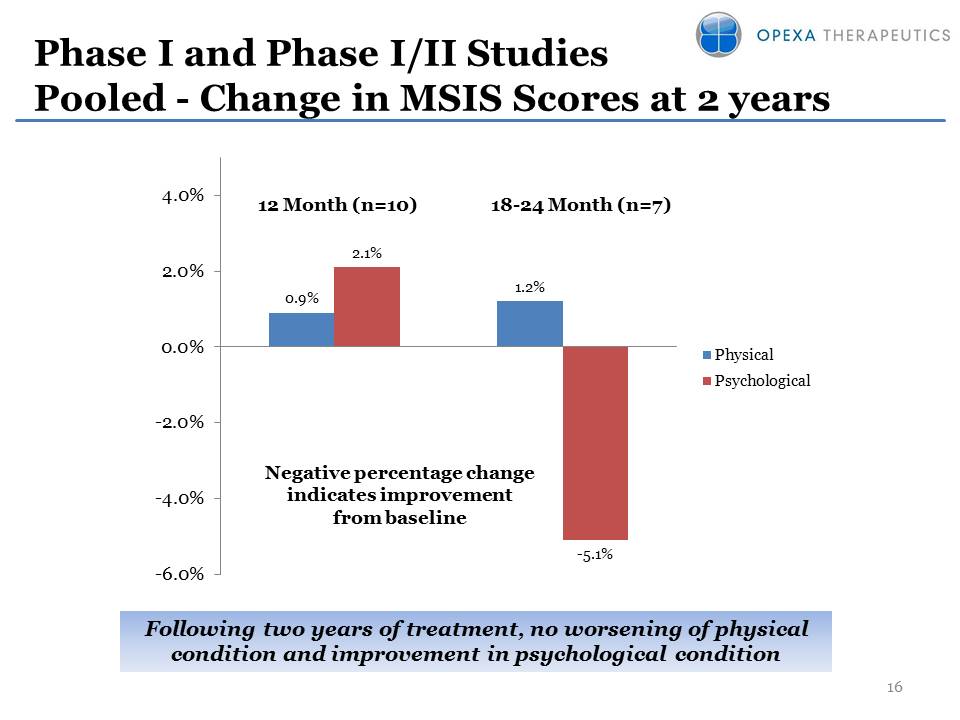
Phase I and Phase I/II Studies Pooled - Change in MSIS Scores at 2 years Following two years of treatment, no worsening of physical condition and improvement in psychological condition
Overview of Tovaxin Clinical Development Clinical Status: Five clinical trials completed with Tovaxin in 196 patients, many with multiple years of treatment Efficacy: Data shows reduction in Annualized Relapse Rate (ARR), slowing disease progressionSafety & Tolerability: Appears superior to all marketed and developmental MS drugs
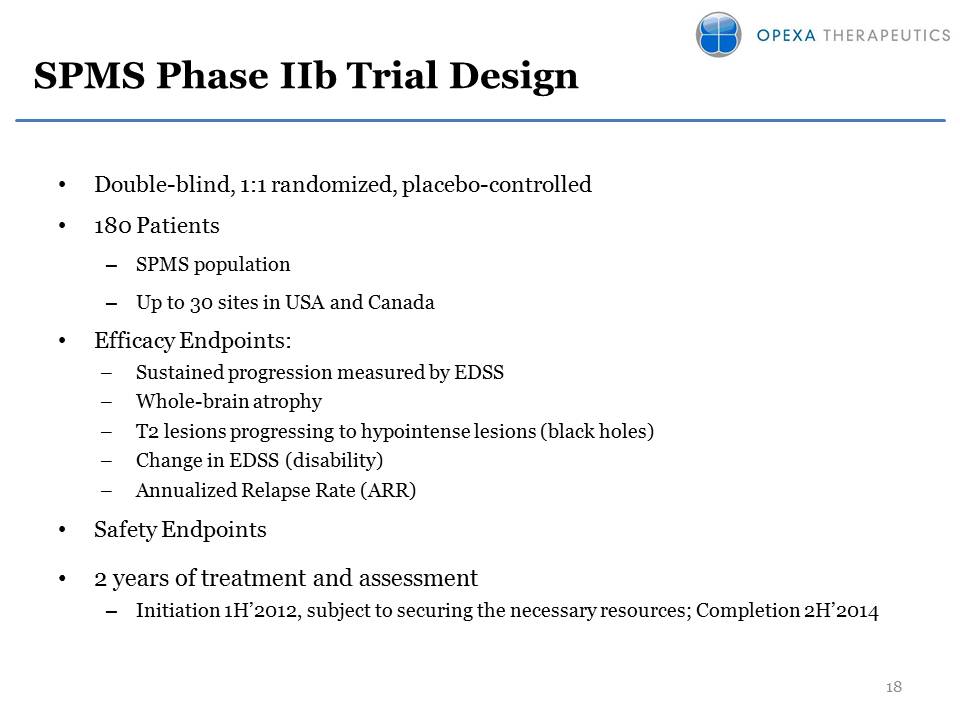
SPMS Phase IIb Trial Design Other Placeholder: Double-blind, 1:1 randomized, placebo-controlled180 PatientsSPMS populationUp to 30 sites in USA and CanadaEfficacy Endpoints:Sustained progression measured by EDSSWhole-brain atrophyT2 lesions progressing to hypointense lesions (black holes)Change in EDSS (disability)Annualized Relapse Rate (ARR)Safety Endpoints2 years of treatment and assessmentInitiation 1H’2012, subject to securing the necessary resources; Completion 2H’2014

Scientific Advisory Board Dawn McGuire, M.D., FAAN (Chair)Advisory Council of the Gill Heart InstituteAmerican Academy of Neurology National Institute of Neurological Disorders and Stroke of the National Institutes of HealthHans-Peter Hartung, M.D.Chair of Neurology at Heinrich-Heine UniversityPresident ECTRIMSEuropean Neurological SocietyInternational Society for NeuroimmunologyInternational Federation of Multiple Sclerosis SocietiesWorld Health Organization Advisory Board on Multiple SclerosisMark S. Freedman, M.D., FRCP, FAANDirector of the Multiple Sclerosis Research Unit at Ottawa HospitalMultiple Sclerosis Society of Canada, National MS Society(USA)Americas Committee for Treatment and Research in MS Consortium of MS CentresPaul O’Connor, M.D., FRCP St. Michael’s Hospital, University of TorontoNational Scientific and Clinical Advisor for the Multiple Sclerosis Society of Canada

Scientific Advisory Board Clyde Markowitz, M.D.Director of the Multiple Sclerosis Center at the University of Pennsylvania Professor of Neurology at the University of Pennsylvania School of Medicine in PhiladelphiaChairman of the Clinical Advisory Committee for the Delaware Valley National MS SocietyAmerican Academy of NeurologyDoug Arnold, M.D.James McGill Professor Neurology and Neurosurgery at the Montreal Neurological Institute of McGill UniversityArthur Vandenbark, Ph.D. Co-Director of the Neuroimmunology Research Laboratory at the Portland Veterans Affairs Medical Center, Portland, OregonDirector of the Tykeson Multiple Sclerosis Research Laboratory at Oregon Health And Science UniversityProfessor of Neurology and Molecular Microbiology and Immunology Edward Fox, M.D., Ph.D. Director of Multiple Sclerosis Clinic of Central Texas Advisory Committee, Lone Star Chapter of the National Multiple Sclerosis SocietyConsortium of Multiple Sclerosis CentersClinical Assistant Professor of Neurology, University of Texas Medical Branch

Milestones and Goals Secured $8.5 million financing to advance clinical trials (Q1’11 )Presented Tovaxin Phase IIb data at the American Academy of Neurology (AAN) Meeting (Q2’11 )Executed strategic agreements with the American Red Cross and the Blood Group Alliance, Inc. (Q2’11 )Initiated the design and development of a proprietary Web-based system to manage patient and product flow throughout future clinical trials (Q2’11 )Furthered discussions with Health Canada’s Biologics and Genetics Therapies Directorate to secure approval for future clinical trial development in Canada (Q3’11 )FDA Fast Track approval for Tovaxin in SPMS (Q4’11 )Secure resources to advance clinical development and initiate 24-month Phase IIb SPMS clinical trial in North America Initiate discussions with European Medicines Agency (EMA) for future pivotal studiesEvaluate expansion of platform to other autoimmune indications and geographical territories
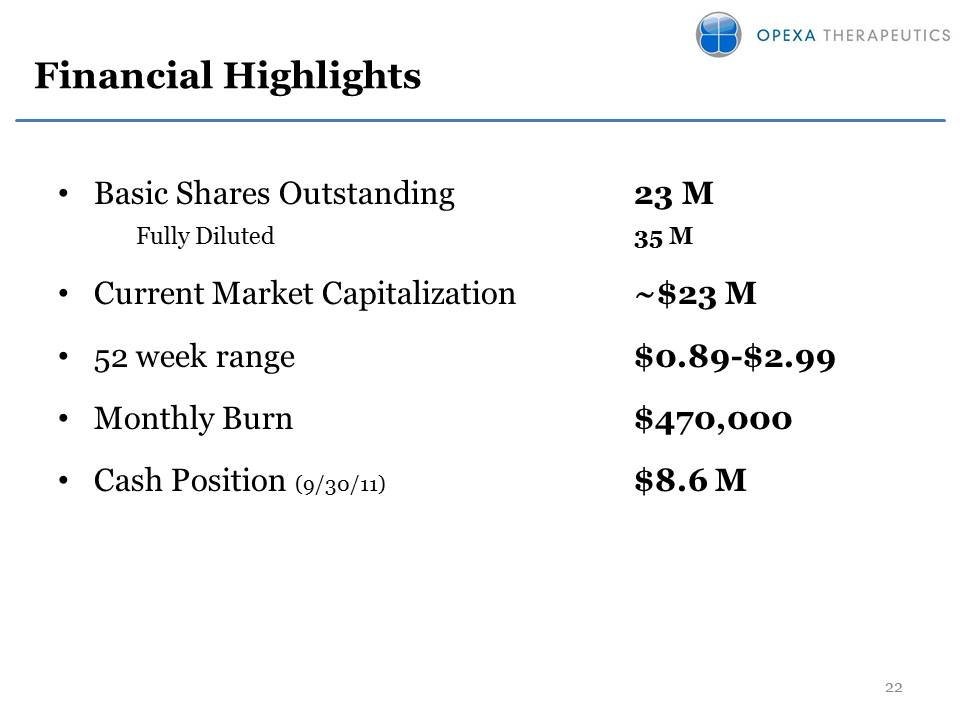
Financial Highlights Basic Shares Outstanding 23 M Fully Diluted 35 MCurrent Market Capitalization ~$23 M52 week range $0.89-$2.99Monthly Burn $470,000Cash Position (9/30/11) $8.6 MOther























