Exhibit 99.2

Opexa Therapeutics, Inc. Second Quarter 2013 Earnings Update Call 14 August 2013 NASDAQ: OPXA Precision Immunotherapy TM

2 Forward-Looking Statements This earnings presentation contains forward-looking statements which are made pursuant to the safe harbor provisions of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Statements contained in this release, other than statements of historical fact, constitute "forward-looking statements." The words "expects," "believes," "anticipates," "estimates," "may," "could," "intends," and similar expressions are intended to identify forward-looking statements. The forward-looking statements in this presentation do not constitute guarantees of future performance. Investors are cautioned that statements in this presentation which are not strictly historical statements, including, without limitation, statements regarding the development of the Company's product candidate, Tcelna (imilecleucel-T), constitute forward-looking statements. Such forward-looking statements are subject to a number of risks and uncertainties that could cause actual results to differ materially from those anticipated. These risks and uncertainties include, but are not limited to, risks associated with: market conditions; our capital position; the rights and preferences provided to the Series A convertible preferred stock and investors in the convertible secured notes we issued in July 2012 (including a secured interest in all of our assets); our ability to compete with larger, better financed pharmaceutical and biotechnology companies; new approaches to the treatment of our targeted diseases; our expectation of incurring continued losses; our uncertainty of developing a marketable product; our ability to raise additional capital to continue our development programs (including to undertake and complete any ongoing or further clinical studies for Tcelna), including in this regard our ability to satisfy various conditions required to access the financing potentially available under the purchase agreements with Lincoln Park Capital Fund, LLC (“Lincoln Park”) (such as the minimum closing price for our common stock and the requirement for an ongoing trading market for our stock); our ability to raise additional capital through the sale of shares of our common stock under the purchase agreements with Lincoln Park or under our at-the-market (ATM) facility; our ability to maintain compliance with NASDAQ listing standards; the success of our clinical trials (including the Phase IIb trial for Tcelna in secondary progressive MS which, depending upon results, may determine whether Ares Trading SA (“Merck”) elects to exercise its option for an exclusive license to Tcelna for the treatment of MS (the “Option”)); whether Merck exercises its Option and, if so, whether we receive any development or commercialization milestone payments or royalties from Merck pursuant to the Option; our dependence (if Merck exercises its Option) on the resources and abilities of Merck for the further development of Tcelna; the efficacy of Tcelna for any particular indication, such as for relapsing remitting MS or secondary progressive MS; our ability to develop and commercialize products; our ability to obtain required regulatory approvals; our compliance with all Food and Drug Administration regulations; our ability to obtain, maintain and protect intellectual property rights (including for Tcelna); the risk of litigation regarding our intellectual property rights or the rights of third parties; the success of third party development and commercialization efforts with respect to products covered by intellectual property rights that we may license or transfer; our limitedmanufacturing capabilities; our dependence on third-party manufacturers; our ability to hire and retain skilled personnel; our volatile stock price; and otherrisks detailed in our filings with the SEC. These forward-looking statements speak only as of the date made. We assume no obligation or undertaking to update any forward-looking statements to reflect any changes in expectations with regard thereto or any change in events, conditions or circumstances on which any such statement is based. You should, however, review additional disclosures we make in our Annual Reports on Form 10 K, Quarterly Reports on Form 10-Q, and Current Reports on Form 8-K filed with the SEC.

3 Investment Thesis • T-cell platform company • Esteemed Scientific Advisory Board • Precision ImmunotherapyTM potentially optimizes benefit-risk profile • Targeting an unmet medical need in a potentially substantial market • Option Agreement with Merck Serono, a strong commercial partner • Replacement value of company is multiples of present market cap • Attractive potential risk-reward profile for long term/value investors • Goal-oriented management team focused on value creation
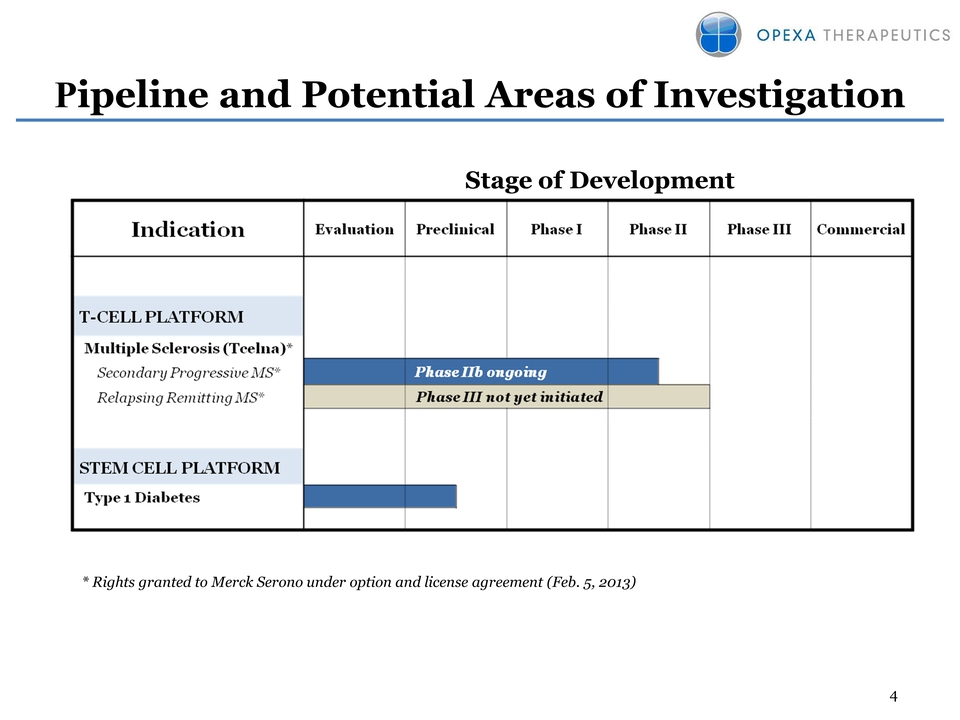
4 Pipeline and Potential Areas of Investigation Stage of Development * Rights granted to Merck Serono under option and license agreement (Feb. 5, 2013)
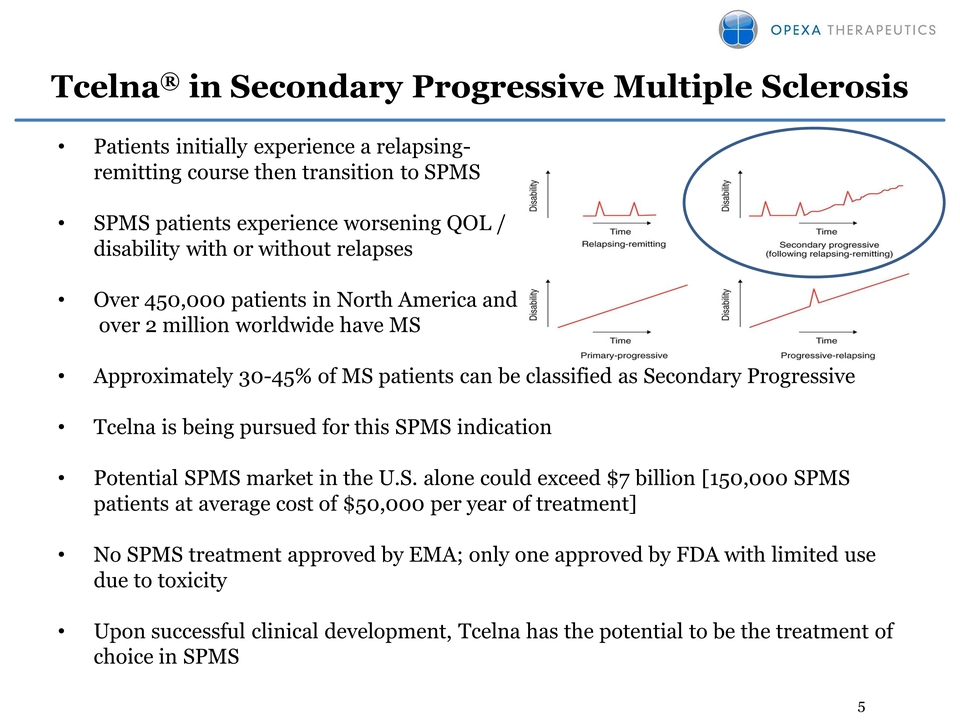
5 Tcelna® in Secondary Progressive Multiple Sclerosis • Patients initially experience a relapsingremitting course then transition to SPMS • SPMS patients experience worsening QOL / disability with or without relapses • Over 450,000 patients in North America and over 2 million worldwide have MS • Approximately 30-45% of MS patients can be classified as Secondary Progressive • Tcelna is being pursued for this SPMS indication • Potential SPMS market in the U.S. alone could exceed $7 billion [150,000 SPMS patients at average cost of $50,000 per year of treatment] • No SPMS treatment approved by EMA; only one approved by FDA with limited use due to toxicity • Upon successful clinical development, Tcelna has the potential to be the treatment of choice in SPMS

6 Merck Serono Agreement for MS indication • February 2013 option and license agreement with Merck Serono – Up to $220 million in additional payments • Option exercise $25 million for starting Phase III/ or $15 million if another Phase II • $35 million FDA filing, approval and commercialized in US • $30 million for EU filing, approval and commercialization in at least three countries • RRMS development and commercialization of up to $40 million – One time commercial milestones of up to $85 million• Royalties ranging from 8 % to 15% of annual net sales with step-ups occurring when net sales exceed $500 million, $1 B & $2 B • Opexa maintains key rights: – Development and commercialization rights to Tcelna in Japan – Certain manufacturing rights – Co-development funding option in exchange for increased royalties – Rights to all other disease indications
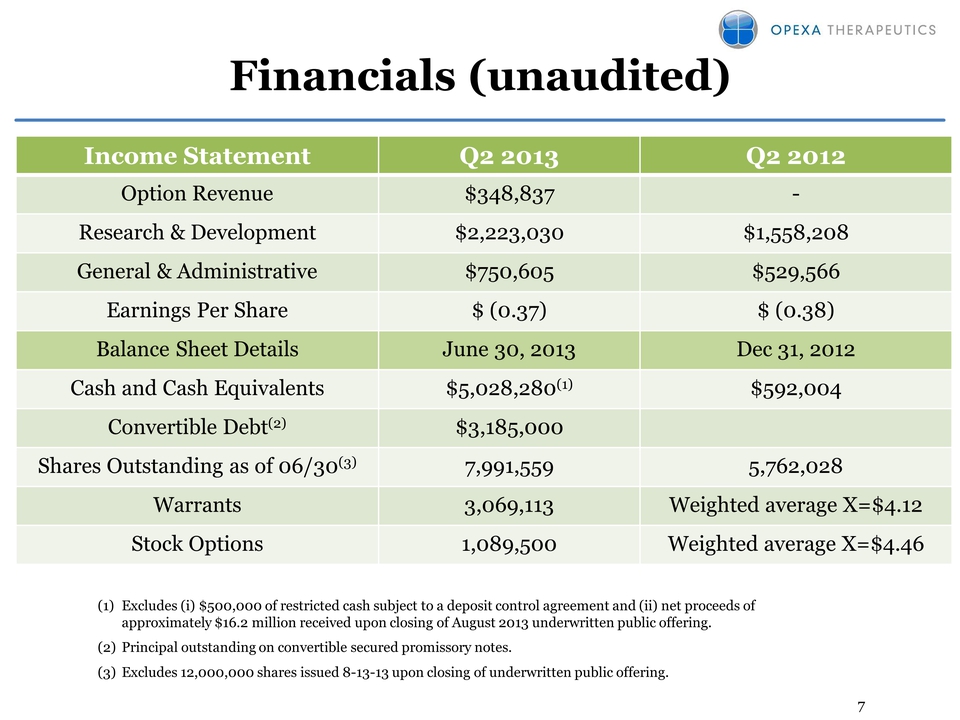
7 Financials (unaudited) Income Statement Q2 2013 Q2 2012 Option Revenue $348,837 - Research & Development $2,223,030 $1,558,208 General & Administrative $750,605 $529,566 Earnings Per Share $ (0.37) $ (0.38) Balance Sheet Details June 30, 2013 Dec 31, 2012 Cash and Cash Equivalents $5,028,280(1) $592,004 Convertible Debt(2) $3,185,000 Shares Outstanding as of 06/30(3) 7,991,559 5,762,028 Warrants 3,069,113 Weighted average X=$4.12 Stock Options 1,089,500 Weighted average X=$4.46 (1) Excludes (i) $500,000 of restricted cash subject to a deposit control agreement and (ii) net proceeds of approximately $16.2 million received upon closing of August 2013 underwritten public offering. (2) Principal outstanding on convertible secured promissory notes. (3) Excludes 12,000,000 shares issued 8-13-13 upon closing of underwritten public offering.
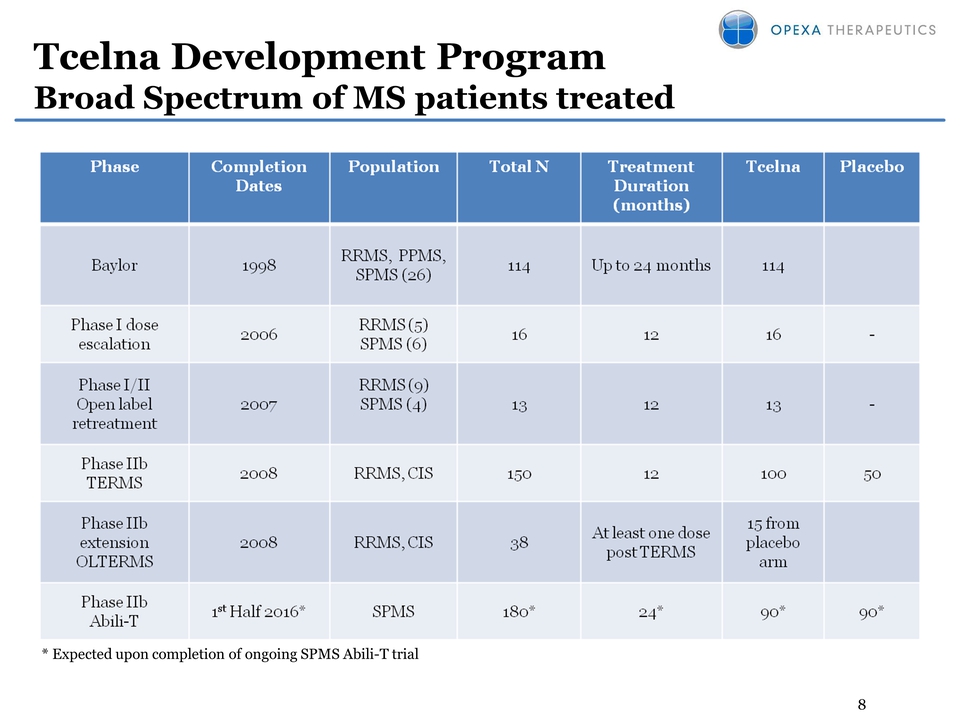
8 Tcelna Development Program Broad Spectrum of MS patients treated * Expected upon completion of ongoing SPMS Abili-T trial

9 Abili-T: Landmark trial in SPMS • Abili-T Phase IIb clinical trial in SPMS is ongoing – Double-blind, 1:1 randomized, placebo-controlled – Inclusion criteria: Secondary Progressive MS with EDSS of 3 to 6 – 68 patients randomized as of August 8, 2013 – Immune Monitoring program conducted on a blinded basis• Fast Track designation granted by FDA for Tcelna in SPMS • 180 Patients expected to be enrolled – SPMS population – Approximately 30 sites in USA and Canada • Key Efficacy Endpoints: – Primary endpoint: Whole Brain Atrophy – Secondary endpoint: Disability metrics including EDSS, ARR, etc. – Exploratory endpoints: Quality of Life • 2 annual courses of personalized therapy
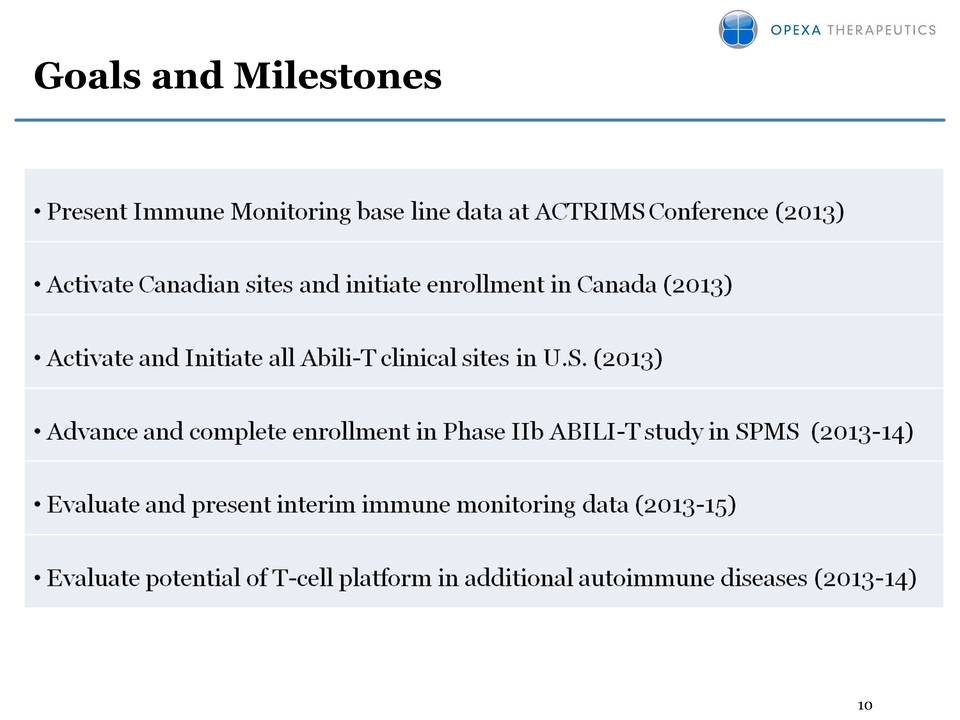
10 Goals and Milestones

11 Investment Highlights • Lead product, Tcelna®, a T-cell immunotherapy for Multiple Sclerosis (MS) • Currently conducting a Phase 2b clinical trial in Secondary Progressive MS (SPMS) • Limited treatment options currently available for SPMS • Potential SPMS market in North America alone could exceed $7 Billion • Tcelna positioned as potential first-to-market personalized T-cell immunotherapy • Option and license agreement secured with Merck Serono for Tcelna in MS indications only, worldwide excluding Japan • Opexa’s in-house cGMP manufacturing enables close control of process and COGS • In previous MS clinical studies, Tcelna has demonstrated good safety and potential indications of clinical efficacy• FDA has granted Opexa Fast Track Designation for Tcelna in SPMS










