Exhibit 99.2

Opexa Therapeutics, Inc. NASDAQ: OPXA Precision Immunotherapy August 2014 The Woodlands, TX Precision Immunotherapy TM

2 Forward-Looking Statements• This investor presentation contains forward-looking statements which are made pursuant to the safe harbor provisions of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Statements contained in this presentation, other than statements of historical fact, constitute “forward-looking statements.” The words “expects,” “believes,” “anticipates,” “estimates,” “may,” “could,” “intends,” and similar expressions are intended to identify forward-looking statements. Forward-looking statements do not constitute guarantees of future performance. Investors are cautioned that statements which are not strictly historical statements, including, without limitation, statements regarding the development of our product candidate, Tcelna (imilecleucel-T), constitute forward-looking statements.• Such forward-looking statements are subject to a number of risks and uncertainties that could cause actual results to differ materially from those anticipated. These risks and uncertainties include, but are not limited to, risks associated with: market conditions; our capital position; our ability to compete with larger, better financed pharmaceutical and biotechnology companies; new approaches to the treatment of our targeted diseases; our expectation of incurring continued losses; our uncertainty of developing a marketable product; our ability to raise additional capital to continue our development programs (including to undertake and complete any ongoing or further clinical studies for Tcelna); our ability to satisfy various conditions required to access the financing potentially available under the purchase agreements with Lincoln Park Capital Fund, LLC (“Lincoln Park”) or sell shares of our common stock to Lincoln Park or under our at-the-market (ATM) facility; our ability to maintain compliance with NASDAQ listing standards; the success of our clinical trials (including the Phase IIb trial for Tcelna in Secondary Progressive Multiple Sclerosis which, depending upon results, may determine whether Ares Trading SA (“Merck”) elects to exercise its option for an exclusive license to Tcelna for the treatment of multiple sclerosis (the “Option”)); whether Merck exercises the Option and, if so, whether we receive any development or commercialization milestone payments or royalties from Merck pursuant to the Option; our dependence (if Merck exercises the Option) on the resources and abilities of Merck for the further development of Tcelna; the efficacy of Tcelna for any particular indication; our ability to develop and commercialize products; our ability to obtain required regulatory approvals; our compliance with all FDA regulations; our ability to obtain, maintain and protect intellectual property rights; the risk of litigation regarding our intellectual property rights or the rights of third parties; the success of third party development and commercialization efforts with respect to products covered by intellectual property rights that we may license or transfer; our limited manufacturing capabilities; our dependence on third-party manufacturers; our ability to hire and retain skilled personnel; our volatile stock price; and other risks detailed in our filings with the SEC. • These forward-looking statements speak only as of the date made. We assume no obligation or undertaking to update any forwardlooking statements to reflect any changes in expectations with regard thereto or any change in events, conditions or circumstances on which any such statement is based. You should, however, review additional disclosures we make in our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, and Current Reports on Form 8-K filed with the SEC.

3 Introduction to Opexa • Antigen specific T-cell immunotherapy platform – Potential to address multiple therapeutic areas – Lower capital intensity by leveraging existing GMP manufacturing – Strong Patent Estate: 50 patents issued on T-cell platform (domestic and international) • Lead Program: Tcelna®, in development for Multiple Sclerosis – Phase IIb clinical trial in Secondary Progressive MS (SPMS) – Limited treatment options currently available for SPMS – Potential SPMS market in North America alone could exceed $7 Billion – Fast Track designation from the U. S. Food and Drug Administration (FDA) for the treatment of SPMS – Option Agreement with Merck Serono, a strong potential commercial partner

4 Progress Update • Clinical – As of June 30, 2014, Opexa had completed enrollment and randomized 190 patients in the Abili-T study in SPMS – Top line data expected in 2H 2016 • Financial – As of June 30, 2014, Opexa had $16.2 million in cash and cash equivalents – Projected liquidity into Q4 2015, based on current operations and Abili-T clinical activities
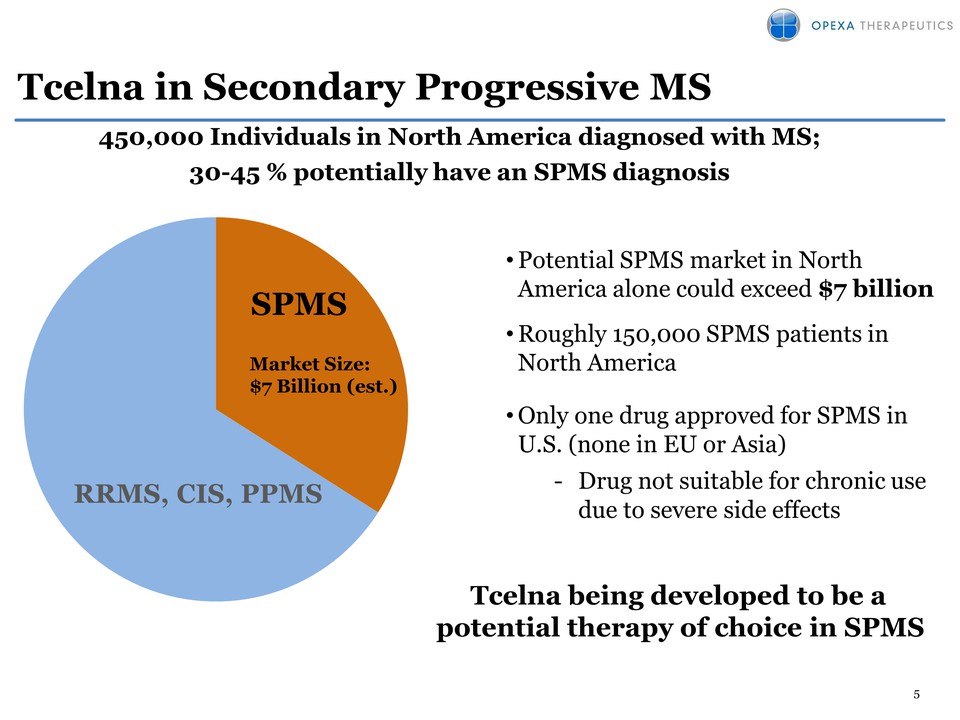
5 Tcelna in Secondary Progressive MS SPMS 450,000 Individuals in North America diagnosed with MS; 30-45 % potentially have an SPMS diagnosis Market Size: $7 Billion (est.) RRMS, CIS, PPMS • Potential SPMS market in North America alone could exceed $7 billion • Roughly 150,000 SPMS patients in North America • Only one drug approved for SPMS in U.S. (none in EU or Asia) - Drug not suitable for chronic use due to severe side effects Tcelna being developed to be a potential therapy of choice in SPMS
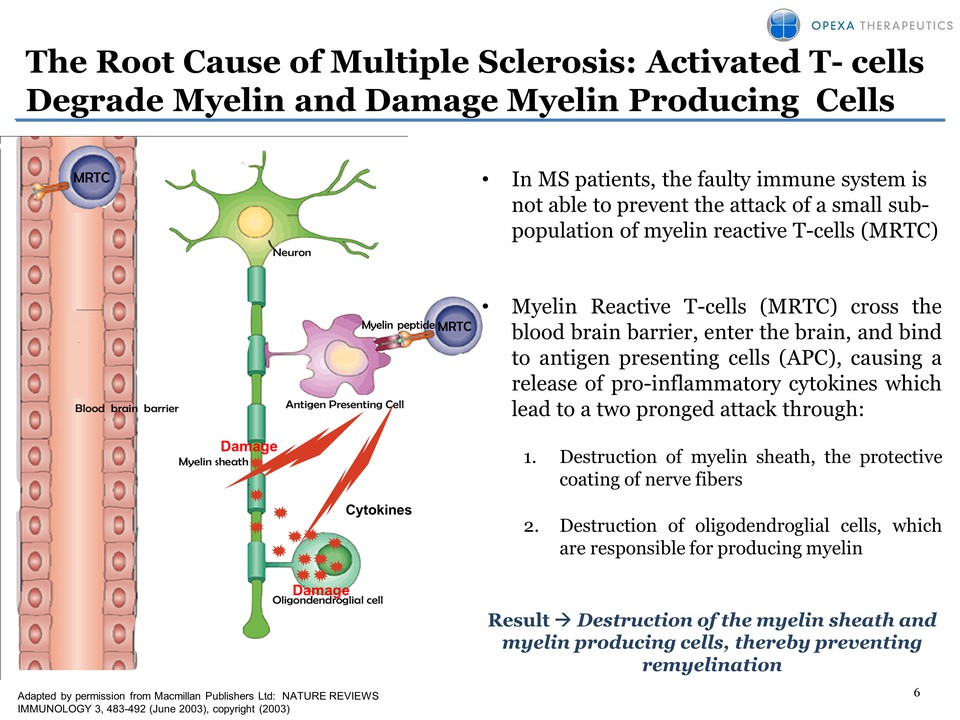
6 The Root Cause of Multiple Sclerosis: Activated T- cells Degrade Myelin and Damage Myelin Producing Cells Adapted by permission from Macmillan Publishers Ltd: NATURE REVIEWS IMMUNOLOGY 3, 483-492 (June 2003), copyright (2003) Cytokines Damage Damage • In MS patients, the faulty immune system is not able to prevent the attack of a small subpopulation of myelin reactive T-cells (MRTC) • Myelin Reactive T-cells (MRTC) cross the blood brain barrier, enter the brain, and bind to antigen presenting cells (APC), causing a release of pro-inflammatory cytokines which lead to a two pronged attack through: 1. Destruction of myelin sheath, the protective coating of nerve fibers 2. Destruction of oligodendroglial cells, which are responsible for producing myelin Result �� Destruction of the myelin sheath and myelin producin
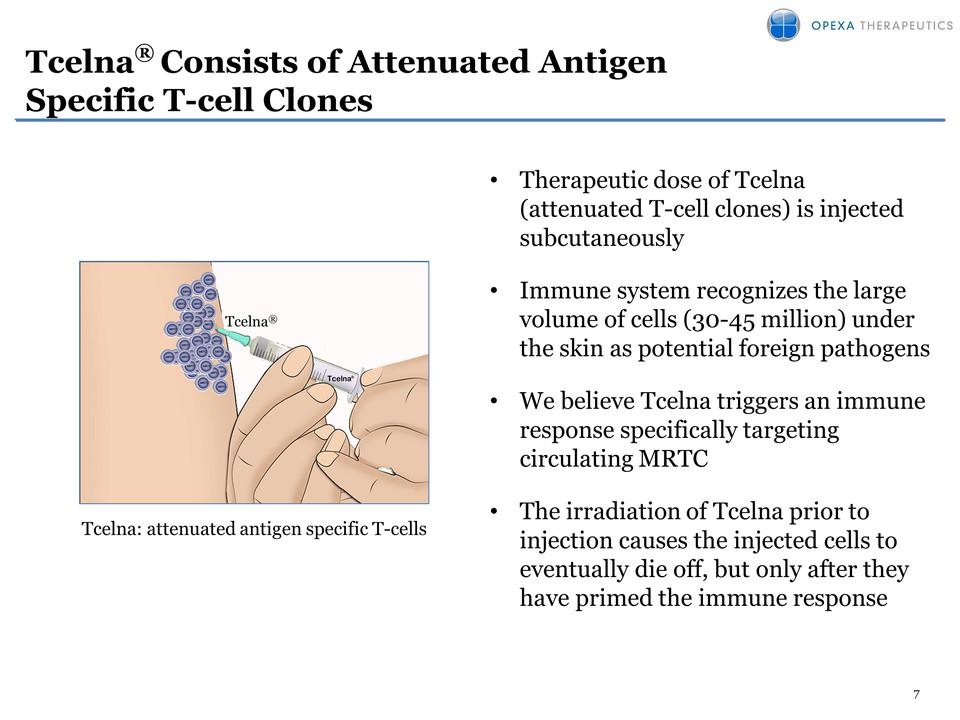
7 Tcelna® Consists of Attenuated Antigen Specific T-cell Clones • Therapeutic dose of Tcelna (attenuated T-cell clones) is injected subcutaneously • Immune system recognizes the large volume of cells (30-45 million) under the skin as potential foreign pathogens • We believe Tcelna triggers an immune response specifically targeting circulating MRTC • The irradiation of Tcelna prior to injection causes the injected cells to eventually die off, but only after they have primed the immune response Tcelna: attenuated antigen specific T-cells Tcelna
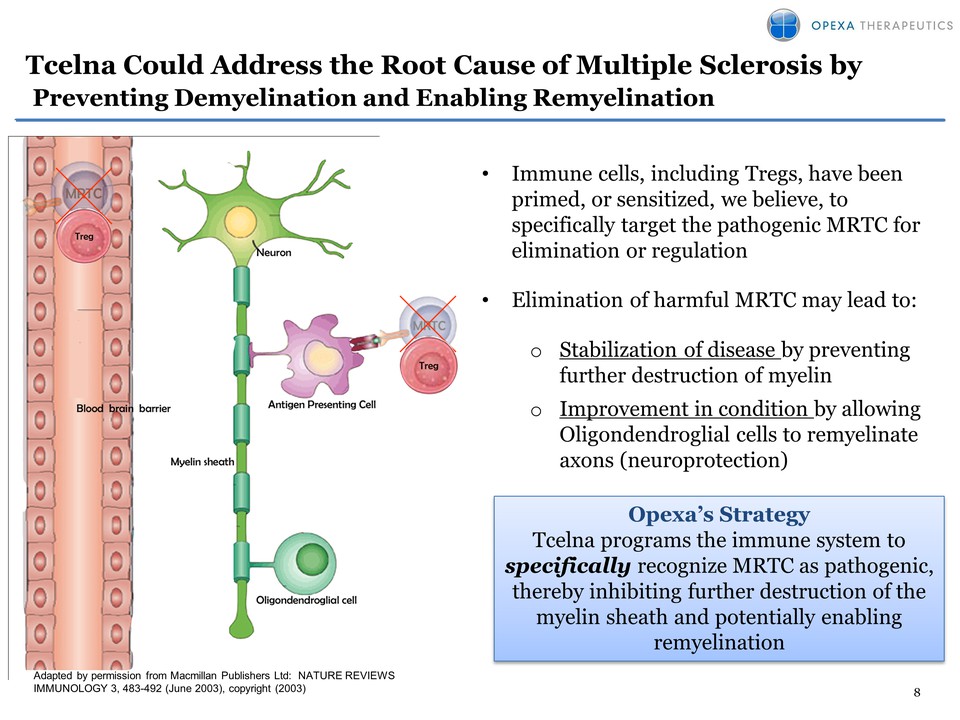 Could Address the Root Cause of Multiple Sclerosis by Preventing Demyelination and Enabling Remyelination Adapted by permission from Macmillan Publishers Ltd: NATURE REVIEWS IMMUNOLOGY 3, 483-492 (June 2003), copyright (2003) Opexa’s Strategy Tcelna programs the immune system to specifically recognize MRTC as pathogenic, thereby inhibiting further destruction of the myelin sheath and potentially enabling remyelination • Immune cells, including Tregs, have been primed, or sensitized, we believe, to specifically target the pathogenic MRTC for elimination or regulation • Elimination of harmful MRTC may lead to: o Stabilization of disease by preventing further destruction of myelin o Improvement in condition by allowing Oligondendroglial cells to remyelinate axons (neuroprotection)
Could Address the Root Cause of Multiple Sclerosis by Preventing Demyelination and Enabling Remyelination Adapted by permission from Macmillan Publishers Ltd: NATURE REVIEWS IMMUNOLOGY 3, 483-492 (June 2003), copyright (2003) Opexa’s Strategy Tcelna programs the immune system to specifically recognize MRTC as pathogenic, thereby inhibiting further destruction of the myelin sheath and potentially enabling remyelination • Immune cells, including Tregs, have been primed, or sensitized, we believe, to specifically target the pathogenic MRTC for elimination or regulation • Elimination of harmful MRTC may lead to: o Stabilization of disease by preventing further destruction of myelin o Improvement in condition by allowing Oligondendroglial cells to remyelinate axons (neuroprotection)
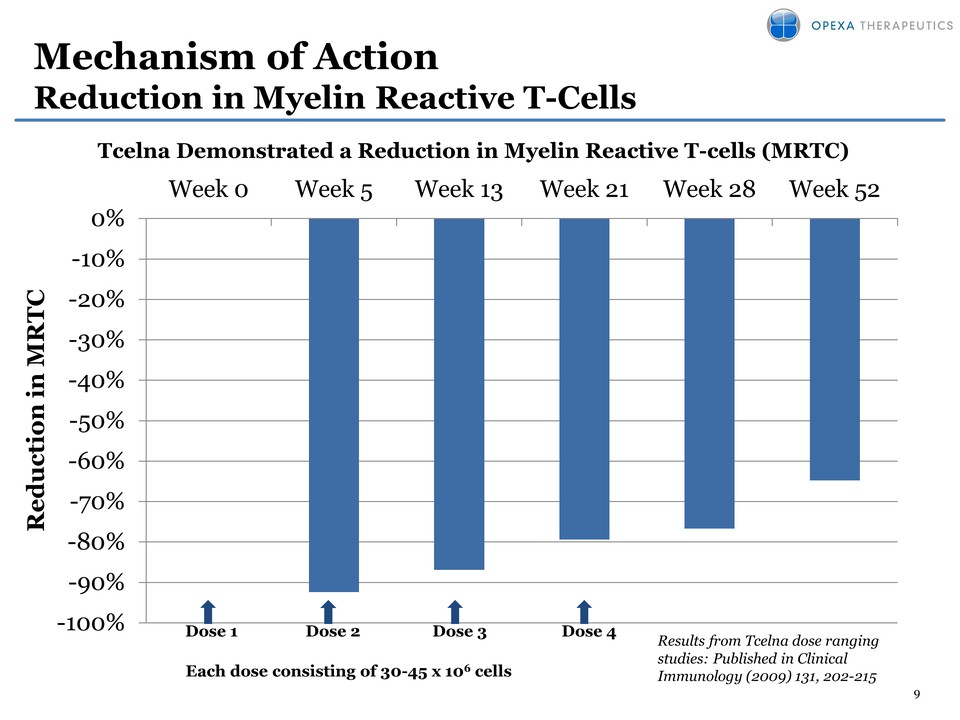
9 Mechanism of Action Reduction in Myelin Reactive T-Cells -100% -90% -80% -70% -60% -50% -40% -30% -20% -10% 0% Week 0 Week 5 Week 13 Week 21 Week 28 Week 52 Results from Tcelna dose ranging studies: Published in Clinical Immunology (2009) 131, 202-215 Reduction in MRTC Dose 2 Dose 3 Dose 4 Each dose consisting of 30-45 x 106 cells Dose 1 Tcelna Demonstrated a Reduction in Myelin Reactive T-cells (MRTC)
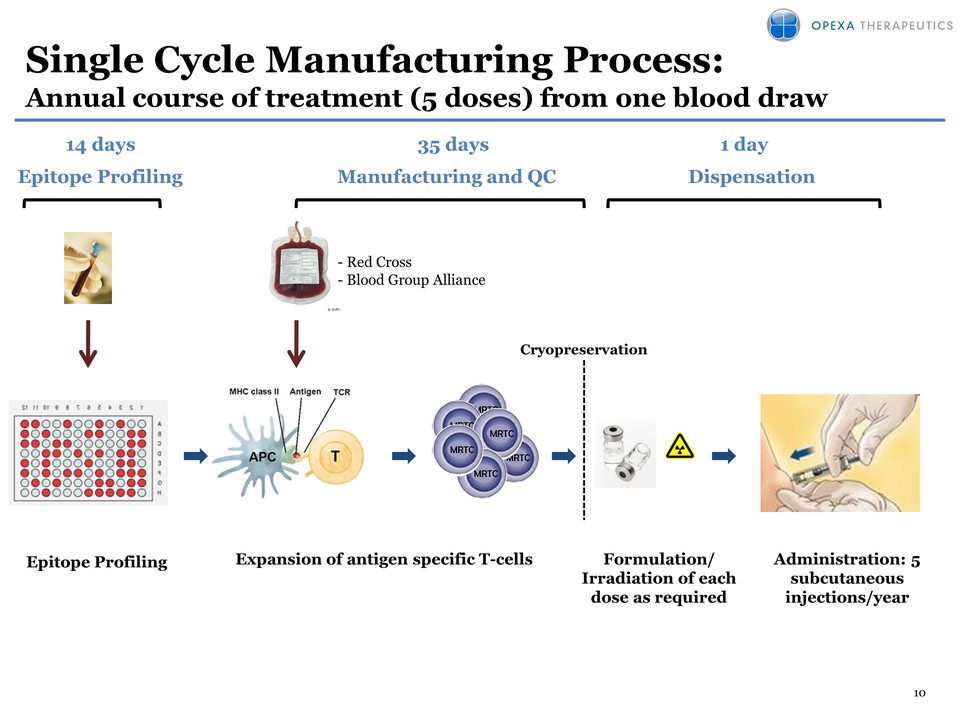
10 Single Cycle Manufacturing Process: Annual course of treatment (5 doses) from one blood draw Cryopreservation Formulation/ Irradiation of each dose as required Administration: 5 subcutaneous injections/year Manufacturing and QC Dispensation 35 days Epitope Profiling 1 day 14 days - Red Cross - Blood Group Alliance Epitope Profiling Expansion of antigen specific T-cells

11 Merck Serono Agreement for MS indication • February 2013 option and license agreement with Merck Serono – Up to $220 million in additional payments • Option exercise $25 million for starting Phase III/ or $15 million if another Phase II • $35 million FDA filing, approval and commercialized in US • $30 million for EU filing, approval and commercialization in at least three countries • Relapsing Remitting MS development and commercialization of up to $40 million – One time commercial milestones of up to $85 million • Royalties ranging from 8% to 15% of annual net sales with step-ups occurring when net sales exceed $500 million, $1 B & $2 B • Opexa maintains key rights: – Development and commercialization rights to Tcelna in Japan – Certain manufacturing rights – Co-development funding option in exchange for increased royalties – Rights to all other disease indications
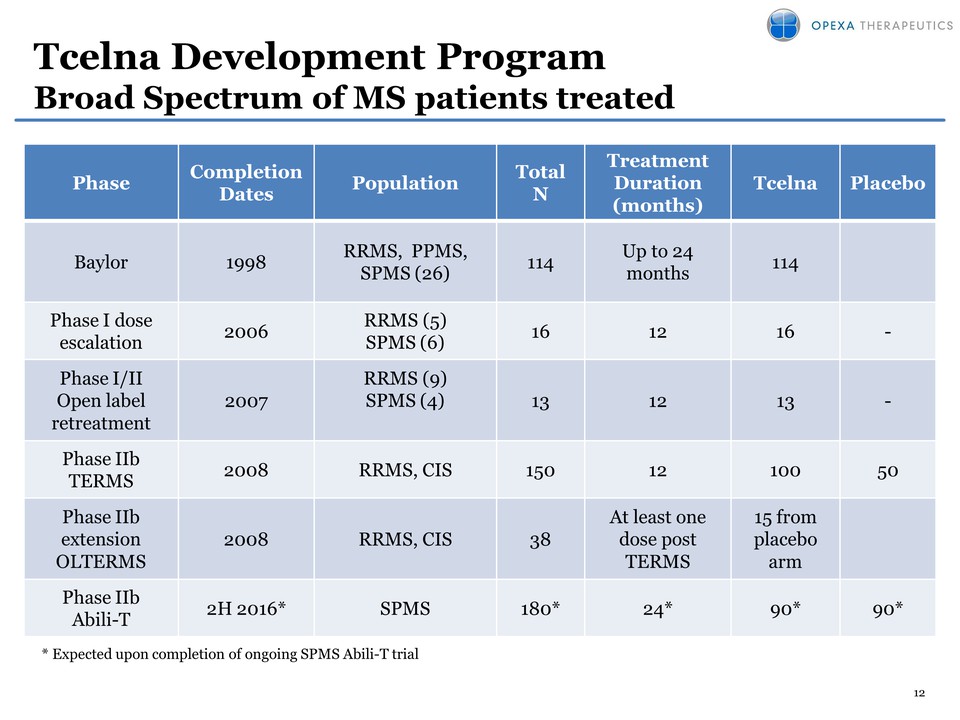
12 Tcelna Development Program Broad Spectrum of MS patients treated Phase Completion Dates Population Total N Treatment Duration (months) Tcelna Placebo Baylor 1998 RRMS, PPMS, SPMS (26) 114 Up to 24 months 114 Phase I dose escalation 2006 RRMS (5) SPMS (6) 16 12 16 - Phase I/II Open label retreatment 2007 RRMS (9) SPMS (4) 13 12 13 -Phase Iib TERMS 2008 RRMS, CIS 150 12 100 50 Phase Iib extension OLTERMS 2008 RRMS, CIS 38 At least one dose post TERMS 15 from placebo arm Phase Iib Abili-T 2H 2016* SPMS 180* 24* 90* 90* * Expected upon completion of ongoing SPMS Abili-T trial
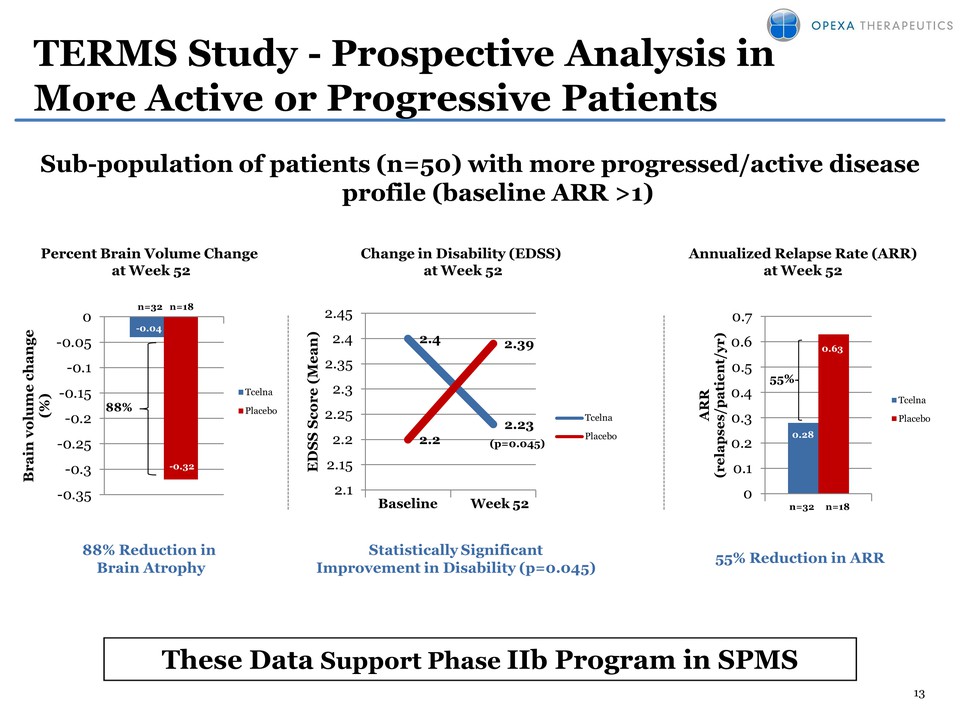
13 TERMS Study - Prospective Analysis in More Active or Progressive Patients Sub-population of patients (n=50) with more progressed/active disease profile (baseline ARR >1) 2.4 2.23 2.2 2.39 2.1 2.15 2.2 2.25 2.3 2.35 2.4 2.45 EDSS Score ( Mean) Baseline Week 52 Tcelna Placebo (p=0.045) 0.28 0.63 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 ARR (relapses/patient/yr) Tcelna Placebo 55% n=32 n=18 -0.04 -0.32 -0.35 -0.3 -0.25 -0.2 -0.15 -0.1 -0.05 0 Brain volume change (%) Tcelna Placebo 88% n=32 n=18 88% Reduction in Brain Atrophy Percent Brain Volume Change at Week 52 Statistically Significant Improvement in Disability (p=0.045) 55% Reduction in ARR Annualized Relapse Rate (ARR) at Week 52 Change in Disability (EDSS) at Week 52 These Data Support Phase IIb Program in SPMS
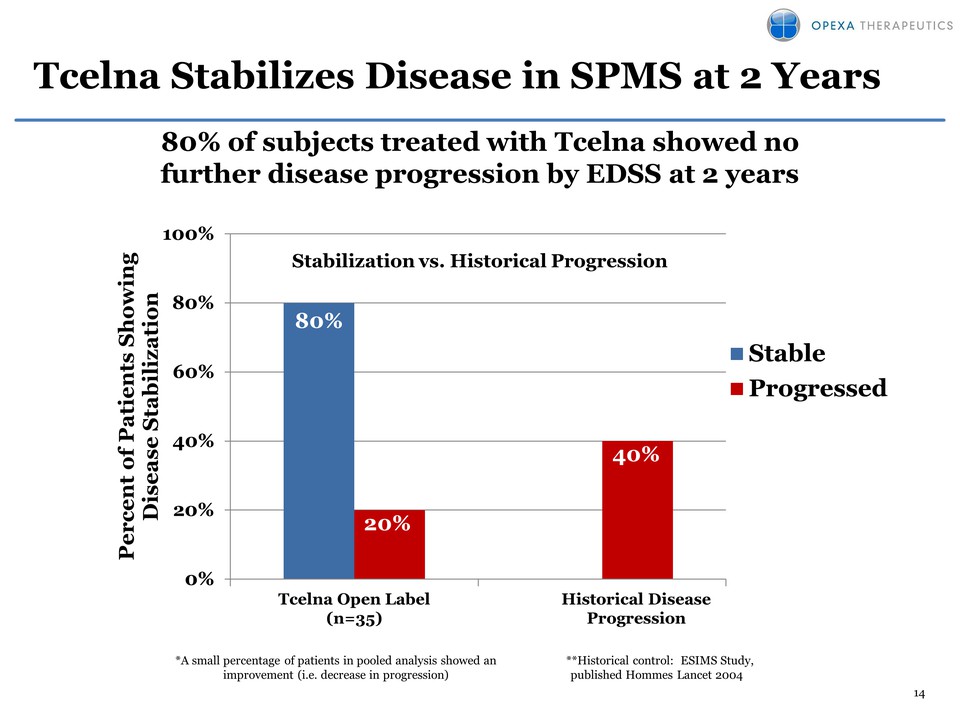
14 Tcelna Stabilizes Disease in SPMS at 2 Years 80% 20% 40% 0% 20% 40% 60% 80% 100% Percent of Patients Showing Disease Stabilization Stabilization vs. Historical Progression Stable Progressed 80% of subjects treated with Tcelna showed no further disease progression by EDSS at 2 years Historical Disease Progression Tcelna Open Label (n=35) *A small percentage of patients in pooled analysis showed an improvement (i.e. decrease in progression) **Historical control: ESIMS Study, published Hommes Lancet 2004
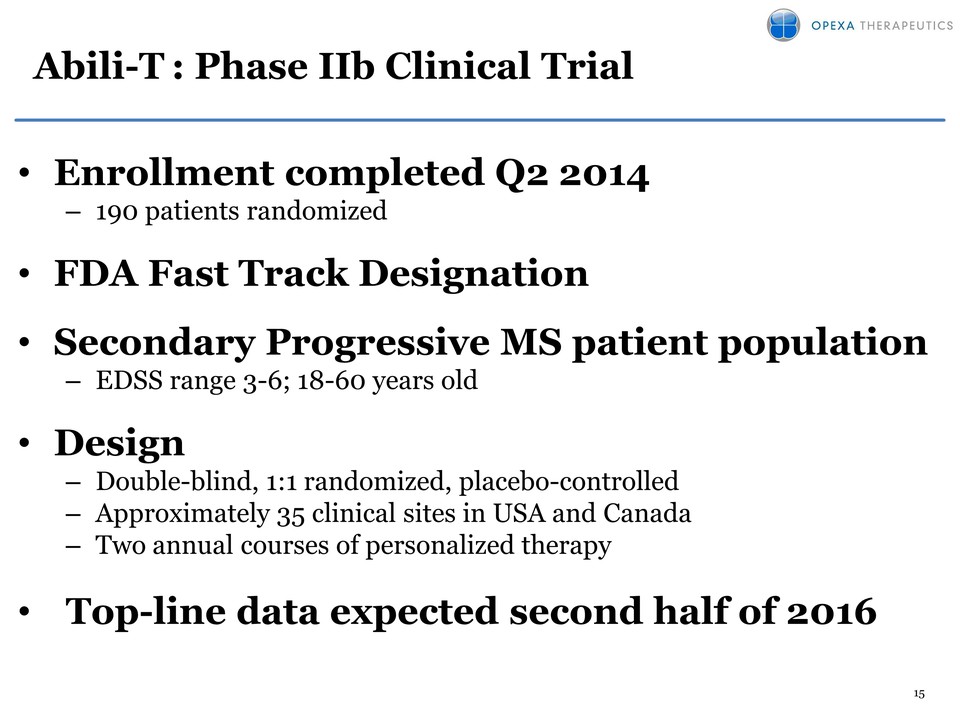
15 Abili-T : Phase IIb Clinical Trial • Enrollment completed Q2 2014 – 190 patients randomized • FDA Fast Track Designation • Secondary Progressive MS patient population – EDSS range 3-6; 18-60 years old • Design – Double-blind, 1:1 randomized, placebo-controlled – Approximately 35 clinical sites in USA and Canada – Two annual courses of personalized therapy • Top-line data expected second half of 2016
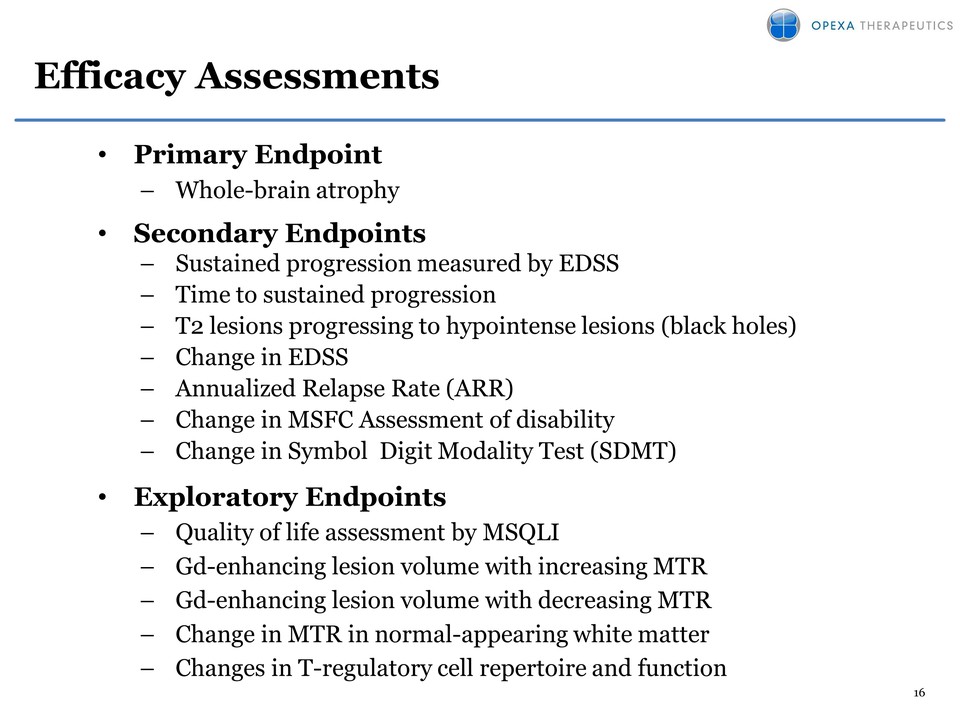
16 Efficacy Assessments • Primary Endpoint – Whole-brain atrophy • Secondary Endpoints – Sustained progression measured by EDSS – Time to sustained progression – T2 lesions progressing to hypointense lesions (black holes) – Change in EDSS – Annualized Relapse Rate (ARR) – Change in MSFC Assessment of disability – Change in Symbol Digit Modality Test (SDMT) • Exploratory Endpoints – Quality of life assessment by MSQLI – Gd-enhancing lesion volume with increasing MTR – Gd-enhancing lesion volume with decreasing MTR – Change in MTR in normal-appearing white matter – Changes in T-regulatory cell repertoire and function
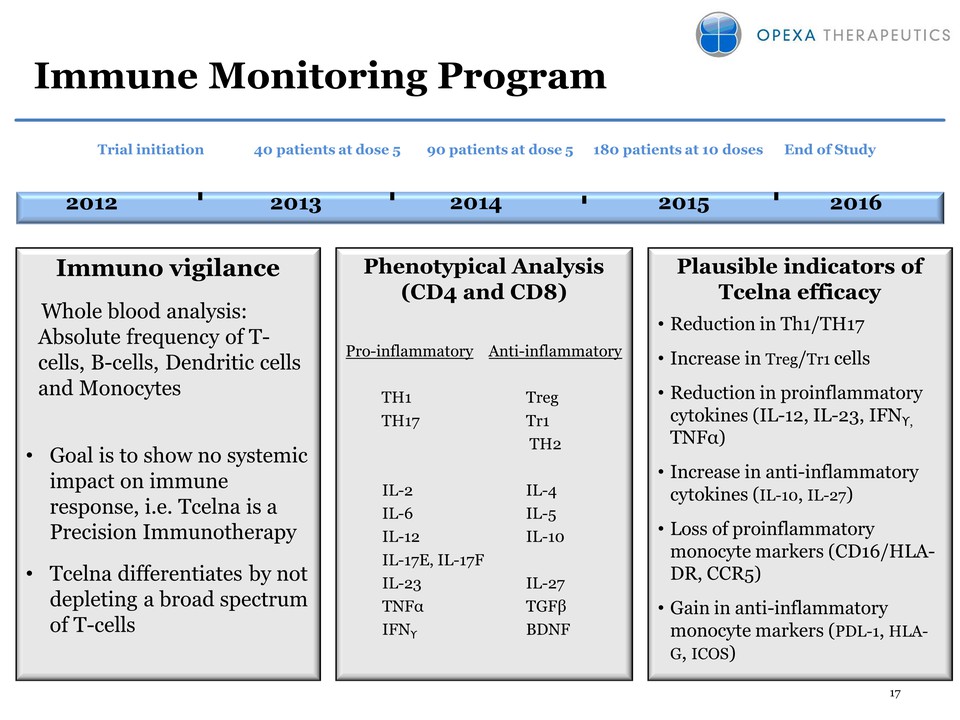
17 Immune Monitoring Program Phenotypical Analysis (CD4 and CD8) Pro-inflammatory Anti-inflammatory TH1 Treg TH17 Tr1 TH2 IL-2 IL-4 IL-6 IL-5 IL-12 IL-10 IL-17E, IL-17F IL-23 IL-27 TNFα TGFβ IFNϒ BDNF Plausible indicators of Tcelna efficacy • Reduction in Th1/TH17 • Increase in Treg/Tr1 cells • Reduction in proinflammatory cytokines (IL-12, IL-23, IFNϒ, TNFα) • Increase in anti-inflammatory cytokines (IL-10, IL-27) • Loss of proinflammatory monocyte markers (CD16/HLADR, CCR5) • Gain in anti-inflammatory monocyte markers (PDL-1, HLAG, ICOS) 2013 2014 2015 2012 Trial initiation Immuno vigilance Whole blood analysis: Absolute frequency of Tcells, B-cells, Dendritic cells and Monocytes • Goal is to show no systemic impact on immune response, i.e. Tcelna is a Precision Immunotherapy • Tcelna differentiates by not depleting a broad spectrum of T-cells 90 patients at dose 5 180 patients at 10 doses 40 patients at dose 5 2016 End of Study
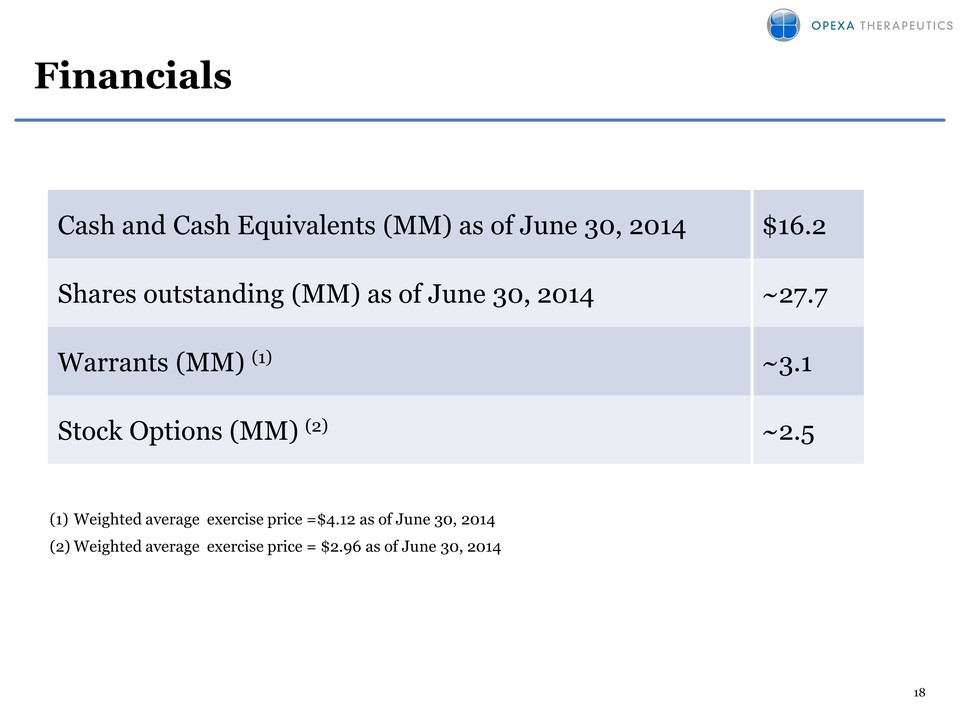
18 Financials Cash and Cash Equivalents (MM) as of June 30, 2014 $16.2 Shares outstanding (MM) as of June 30, 2014 27.7 Warrants (MM) (1) 3.1 Stock Options (MM) (2) ~2.5 (1) Weighted average exercise price =$4.12 as of June 30, 2014 (2)Weighted average exercise price = $2.96 as of June 30, 2014

19 Experienced Management Team and Board of Directors Neil Warma, President & CEO, Director 19+ years international healthcare experience with large Pharma and emerging biotechnology companies Former SeniorManagement, Novartis Pharmaceuticals, Basel, Switzerland Former CEO, Viron Therapeutics, Inc. Co-founder and President of MedExact Inc., a company subsequently acquired Karthik Radhakrishnan, Chief Financial Officer 10+ years of health care capital markets experience Formerly, Vice President at ING Investment Management MBA, MS in Engineering, CFA charter holder Don Healey, Ph.D., Chief Scientific Officer 25+ years of experience in cellular immunology and immune regulation Former Director of Immunology, Argos Therapeutics Donna Rill, Chief Development Officer 30 years in cell and gene therapy research and clinical application Designed and validated cGMP Cell & Gene Therapy Laboratories, Vector Production facilities, and Translational Research Labs Kenny Frazier, VP of Clinical Dev. and Regulatory Affairs 24 years of extensive clinical and regulatory experience Formerly, Head of Clinical Operations, Lexicon Pharmaceuticals and Tanox, Inc. Board of Directors Timothy Barabe Board member of Arqule, Inc.; Former CFO of Affymetrix, Human Genome Sciences, Inc., Regent Medical UK and Sandoz GmbH Dr. Hans-Peter Hartung Chair of Neurology at Heinrich-Heine University, Germany; Executive Board member of ECTRIMS Gail J. Maderis CEO, BayBio, Former CEO of Five Prime Therapeutics, Founder of Genzyme Molecular Oncology Michael S. Richman CEO, Amplimmune Scott B. Seaman Executive Director, Alkek Foundation Neil K. Warma President & CEO, Opexa

20 SPMS Scientific Advisory Board Dawn McGuire, M.D., FAAN (Chair) • Advisory Council of the Gill Heart Institute • Former Vice President of Clinical Research at Elan Pharmaceuticals Hans-Peter Hartung, M.D • Chair of Neurology at Heinrich-Heine University, Düsseldorf • Executive Board member of ECTRIMS, World Health Organization Advisory Board on MS Mark S. Freedman, M.D. • Director of the Multiple Sclerosis Research Unit at Ottawa Hospital • Multiple Sclerosis Society of Canada, National MS Society (USA) • ACTRIMS committee member Clyde Markowitz, M.D. • Director of MS Center at the University of Pennsylvania Doug Arnold, M.D. • James McGill Professor Neurology and Neurosurgery at the Montreal Neurological Institute Edward Fox, M.D., Ph.D. • Director of Multiple Sclerosis Clinic of Central Texas • Advisory Committee, Lone Star Chapter of the National Multiple Sclerosis Society

21 Investment Thesis • T-cell platform company with Fast Track designation in SPMS • Strong Intellectual property with 50 issued patents (domestic and international) • Esteemed Scientific Advisory Board • Precision Immunotherapy potentially optimizes benefit-risk profile • Targeting an unmet medical need in a potentially substantial market • Option Agreement with Merck Serono, a strong potential commercial partner • Replacement value of company is multiples of present market cap • Attractive potential risk-reward profile for long term/value investors • Goal-oriented management team focused on value creation

22 APPENDIX

23 Tcelna® Highlights • Clinical studies conducted in Relapsing Remitting and Secondary Progressive MS • Progress made on manufacturing with focus on commercially viable process ─ Over 850 Tcelna preparations have been successfully manufactured, reproducible and consistent • Safety demonstrated and trends in clinical efficacy across broad spectrum of patients for Relapsing Remitting and Secondary Progressive MS • Dose and regimen for ongoing clinical development has been selected based on doseranging studies • Commercial opportunities in MS: – Secondary Progressive MS • Abili-T Phase II SPMS trial ongoing with 180 patients expected in U.S. and Canada • Fast track designation – Relapsing Remitting MS • Formal End of Phase II meetings have been conducted • FDA feedback obtained for potential Phase III studies

24 Tcelna Manufacturing: Personalization followed by Expansion Step The Epitope Profiling Assay (EPA) • Screen peripheral blood for Myelin-Reactive T-cells (MRTCs), and mapping of immunodominant epitopes to MBP, MOG and PLP – 109 overlapping peptides encompassing MBP, MOG and PLP – Interferon gamma response to individual peptide pools defines positive response in 7 day assay ImmPath® Process • Procure unit of blood from which up to six T-cell lines reactive with immunodominant myelin peptides are generated and pooled as a patient-specific Tcelna product – Manufacturing performed under GMP/GTPs in functionally closed system– Process generates a year of Tcelna doses from a single unit of blood
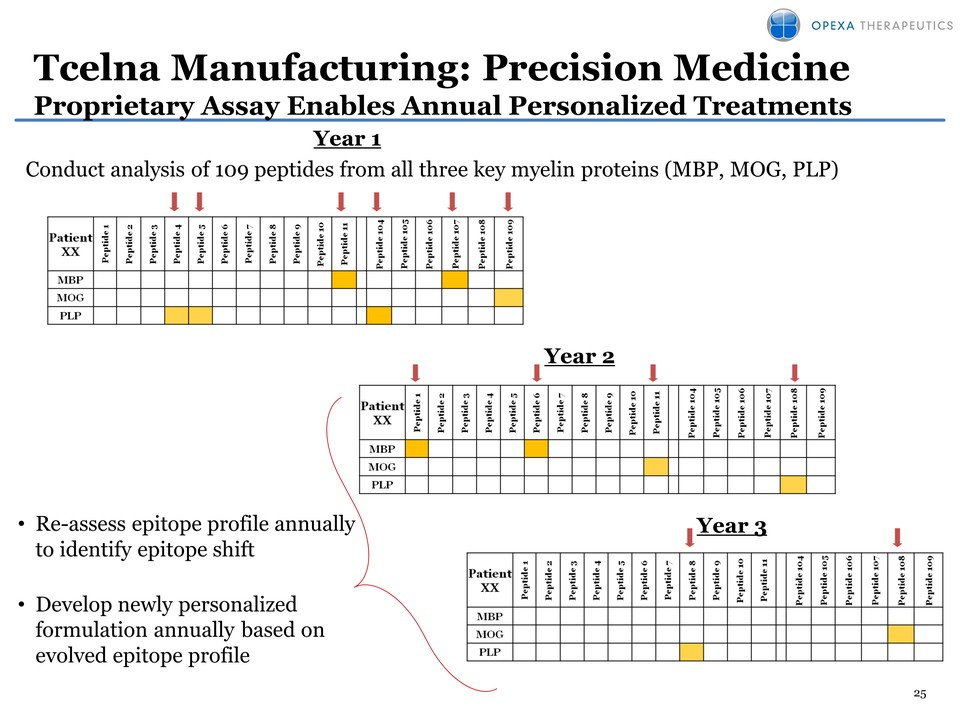
25 Year 2 Year 3 Tcelna Manufacturing: Precision Medicine Proprietary Assay Enables Annual Personalized Treatments Year 1 Conduct analysis of 109 peptides from all three key myelin proteins (MBP, MOG, PLP)• Re-assess epitope profile annually to identify epitope shift • Develop newly personalized formulation annually based on evolved epitope profile
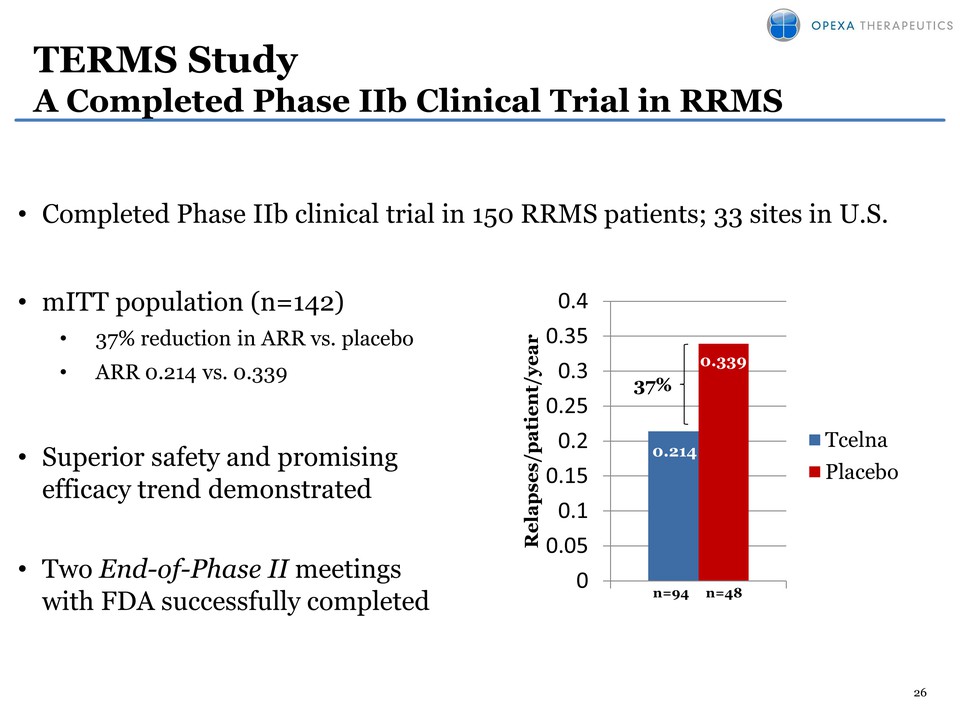
26 • Completed Phase IIb clinical trial in 150 RRMS patients; 33 sites in U.S. • mITT population (n=142) • 37% reduction in ARR vs. placebo • ARR 0.214 vs. 0.339 • Superior safety and promising efficacy trend demonstrated • Two End-of-Phase II meetings with FDA successfully completed 0.214 0.339 0 0.05 0.1 0.15 0.2 0.25 0.3 0.35 0.4 Relapses/patient/year n=94 n=48 Tcelna Placebo TERMS Study A Completed Phase IIb Clinical Trial in RRMS 37%
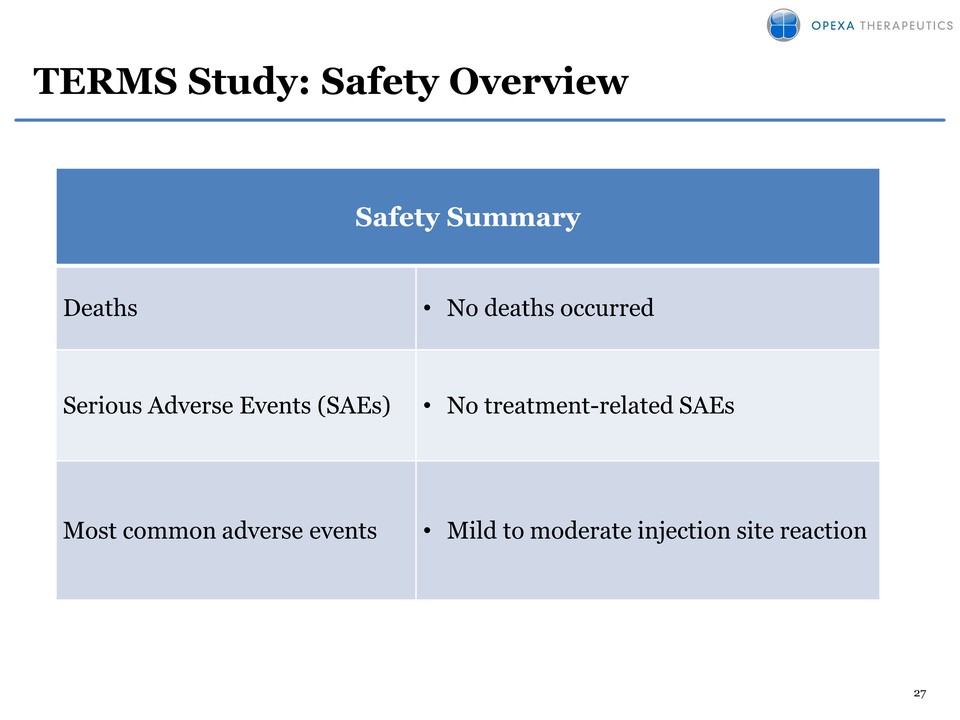
27 Safety Summary Deaths • No deaths occurred Serious Adverse Events (SAEs) • No treatment-related SAEs Most common adverse events • Mild to moderate injection site reaction TERMS Study: Safety Overview
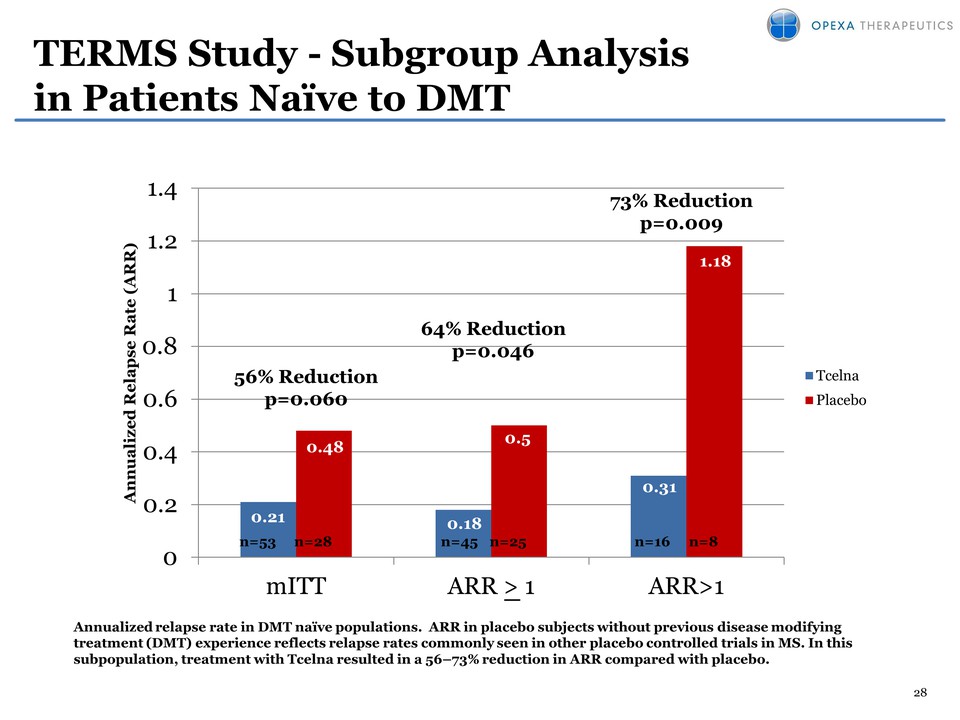
28 TERMS Study - Subgroup Analysis in Patients Naïve to DMT Annualized relapse rate in DMT naïve populations. ARR in placebo subjects without previous disease modifying treatment (DMT) experience reflects relapse rates commonly seen in other placebo controlled trials in MS. In this subpopulation, treatment with Tcelna resulted in a 56–73% reduction in ARR compared with placebo. 0.21 0.18 0.31 0.48 0.5 1.18 0 0.2 0.4 0.6 0.8 1 1.2 1.4 mITT ARR > 1 ARR>1 Annualized Relapse Rate (ARR) Tcelna Placebo 64% Reduction p=0.046 73% Reduction p=0.009 56% Reduction p=0.060 n=25 n=45 n=8 n=16 n=28 n=53
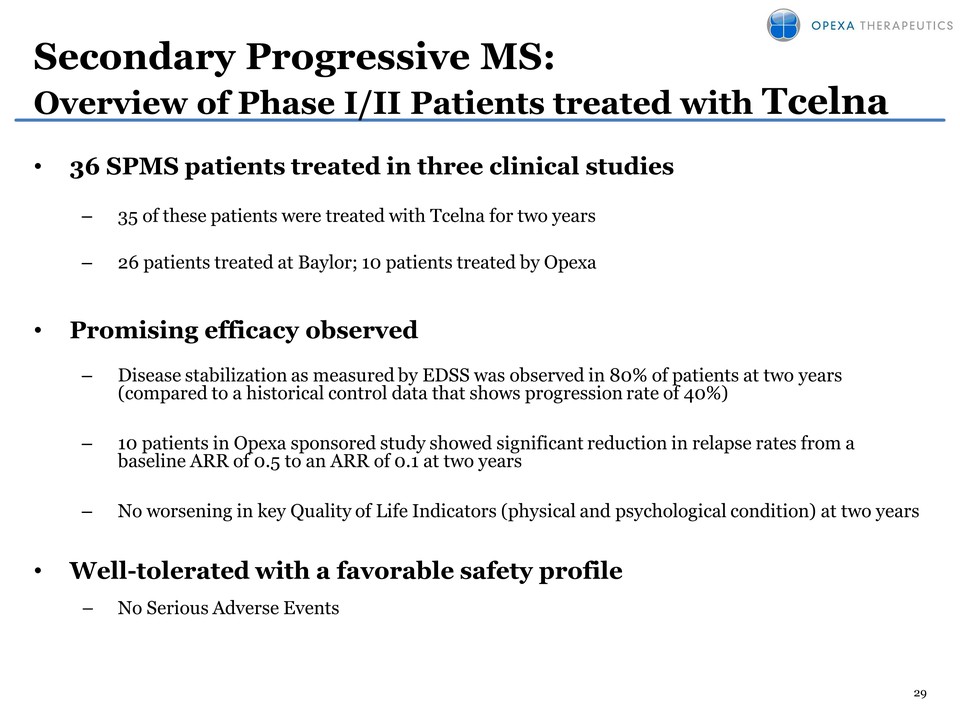
29 • 36 SPMS patients treated in three clinical studies – 35 of these patients were treated with Tcelna for two years – 26 patients treated at Baylor; 10 patients treated by Opexa • Promising efficacy observed – Disease stabilization as measured by EDSS was observed in 80% of patients at two years (compared to a historical control data that shows progression rate of 40%) – 10 patients in Opexa sponsored study showed significant reduction in relapse rates from a baseline ARR of 0.5 to an ARR of 0.1 at two years – No worsening in key Quality of Life Indicators (physical and psychological condition) at two years • Well-tolerated with a favorable safety profile – No Serious Adverse Events Secondary Progressive MS: Overview of Phase I/II Patients treated with Tcelna
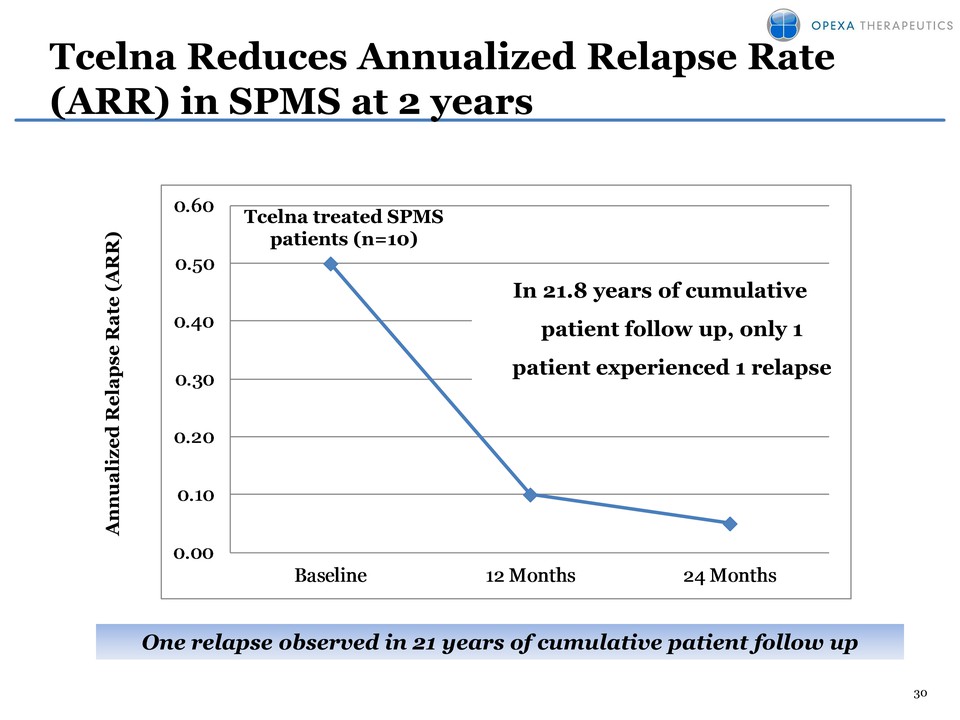
30 Tcelna Reduces Annualized Relapse Rate (ARR) in SPMS at 2 years Annualized Relapse Rate ( ARR) 0.00 0.10 0.20 0.30 0.40 0.50 0.60 Baseline 12 Months 24 Months In 21.8 years of cumulative patient follow up, only 1 patient experienced 1 relapse One relapse observed in 21 years of cumulative patient follow up Tcelna treated SPMS patients (n=10)
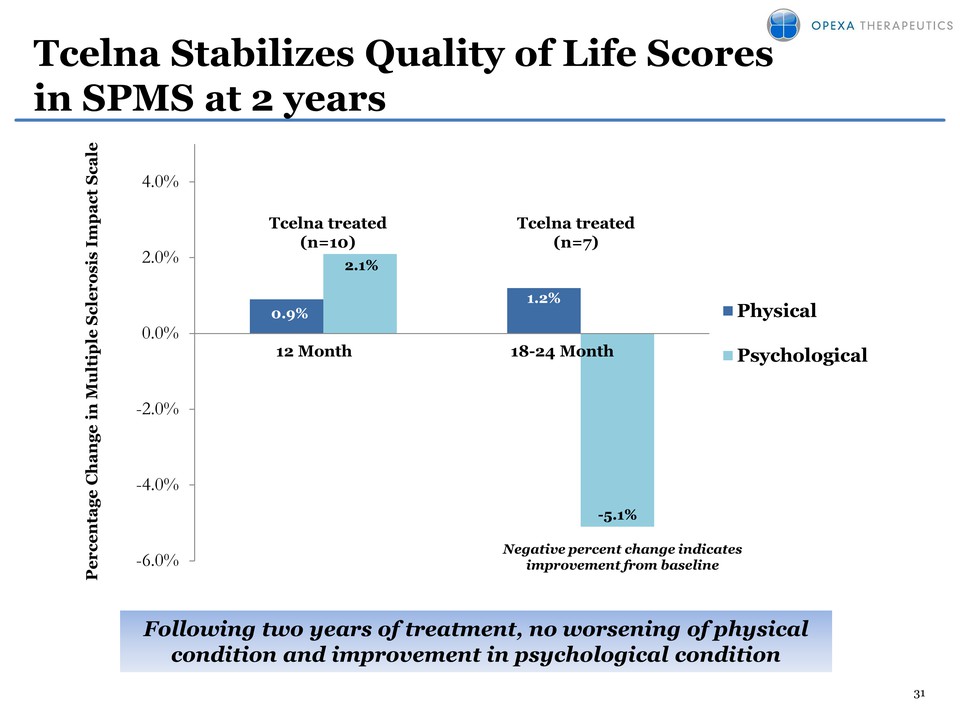
31 Tcelna Stabilizes Quality of Life Scores in SPMS at 2 years 0.9% 1.2% 2.1% 5.1% -6.0% -4.0% -2.0% 0.0% 2.0% 4.0% Physical Psychological 12 Month 18-24 Month Negative percent change indicates improvement from baseline Following two years of treatment, no worsening of physical condition and improvement in psychological condition Percentage Change in Multiple Sclerosis Impact Scale Tcelna treated (n=7) Tcelna treated (n=10)
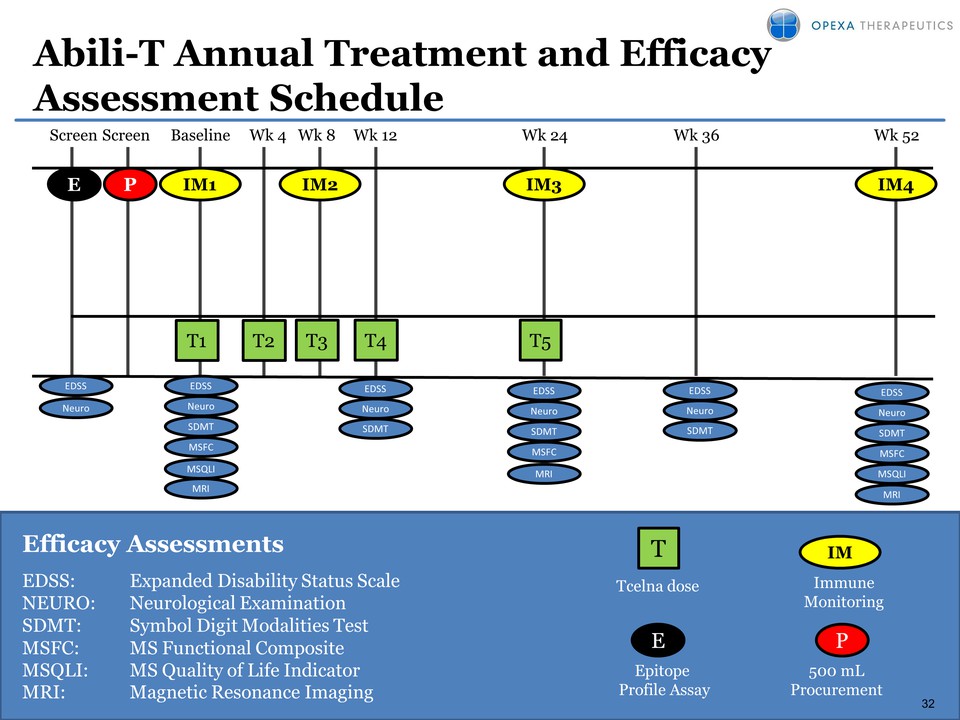
32 Efficacy Assessments EDSS: Expanded Disability Status Scale NEURO: Neurological Examination SDMT: Symbol Digit Modalities Test MSFC: MS Functional Composite MSQLI: MS Quality of Life Indicator MRI: Magnetic Resonance Imaging T 500 mL Procurement Immune Monitoring Tcelna dose Abili-T Annual Treatment and Efficacy Assessment Schedule 32 E P Epitope Profile Assay Baseline Wk 4 Wk 8 Wk 12 Wk 24 Wk 36 Screen Screen T1 T3 T4 T5 EDSS Neuro EDSS Neuro SDMT MSFC MSQLI EDSS Neuro SDMT EDSS Neuro SDMT EDSS Neuro SDMT MSFC MRI Wk 52 EDSS Neuro SDMT MSFC MSQLI MRI IM1 IM3 MRI IM2 E P IM T2 IM4
33 Mechanism of Action • Attenuated, patient-specific (autologous) myelin reactive Tcells (MRTC) – MRTC expanded ex vivo in response to immunodominant peptides of MBP, MOG and PLP • Therapeutic sc dosing (30-45 x 106 cells) stimulates host reactivity to the ‘over-represented’ MRTC inducing a dominant negative ‘regulatory T-cell’ response leading to: – Down-regulation of similar endogenous disease-causing myelin reactive T-cells – Potential to induce up-regulation of regulatory cells (Foxp3+ and Tr1 cells) to reduce inflammation and provide possible neuroprotection, should these gain entry to the CNS • Phase I/II studies conducted with Tcelna in RRMS and SPMS showed a substantial reduction in MRTC, and improved clinical outcomes – lower relapse rates and stabilization of disease as defined by EDSS
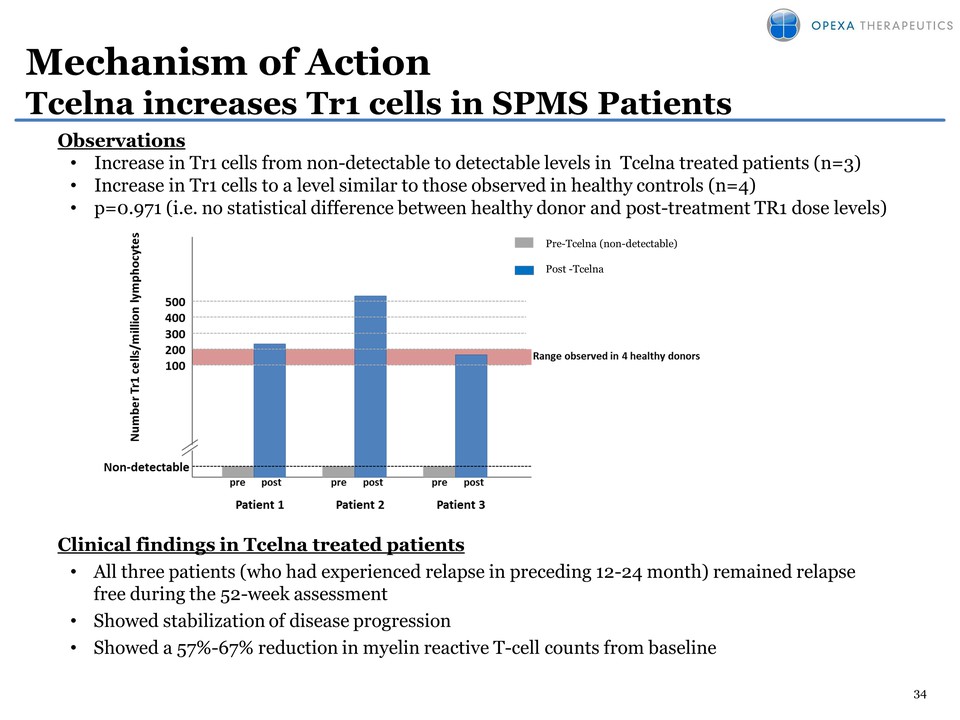
34 Mechanism of Action Tcelna increases Tr1 cells in SPMS Patients Clinical findings in Tcelna treated patients • All three patients (who had experienced relapse in preceding 12-24 month) remained relapse free during the 52-week assessment • Showed stabilization of disease progression • Showed a 57%-67% reduction in myelin reactive T-cell counts from baseline Observations • Increase in Tr1 cells from non-detectable to detectable levels in Tcelna treated patients (n=3) • Increase in Tr1 cells to a level similar to those observed in healthy controls (n=4) • p=0.971 (i.e. no statistical difference between healthy donor and post-treatment TR1 dose levels) Pre-Tcelna (non-detectable) Post -Tcelna
35 Mechanism of Action The Immunopathology of SPMS • SPMS is associated with compartmentalized CNS inflammatory cells - Microglia activation suggests ongoing chronic innate immune responses • SPMS is associated with inflammatory processes localized within the central nervous system - Mechanisms responsible for continued neurodegeneration in SPMS are distinct from those of RRMS - In SPMS, it is believed that the Blood Brain Barrier (BBB) remains closed enabling only those products that are able to cross the BBB to be potentially effective - SPMS is characterized by a chronic inflammatory process (vs. acute episodes for RRMS) • Immunological defects associated with progression to SPMS - Levels of the anti-inflammatory cytokine IL-10 are reduced in patients with SPMS - A therapeutic that has the potential to restore local production of IL-10 would be expected to alleviate chronic inflammation, and thereby reduce neurodegeneration
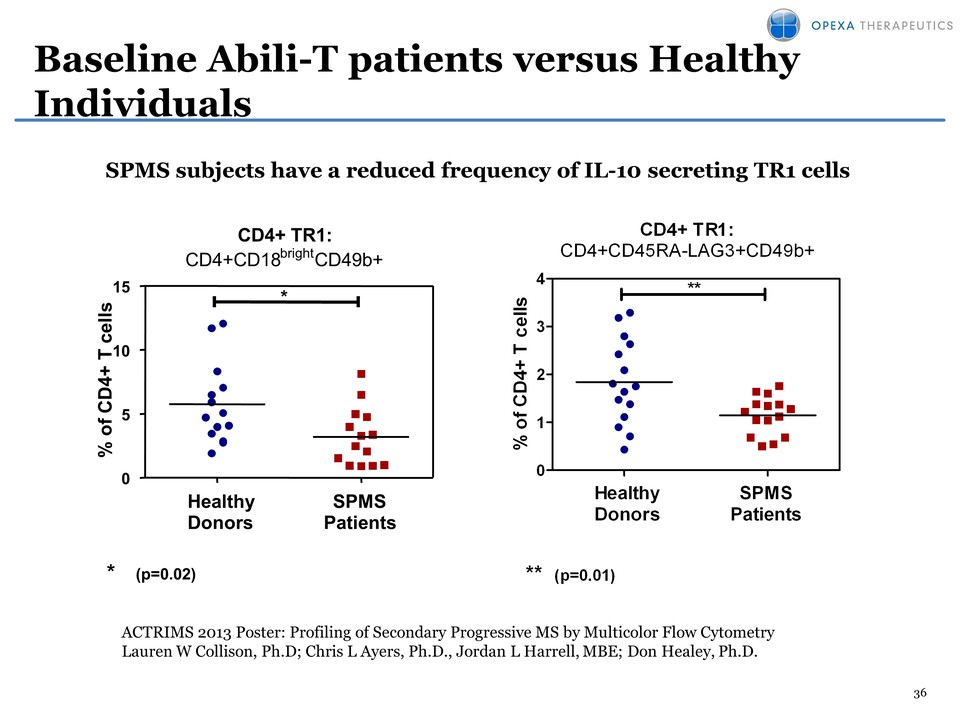
36 Baseline Abili-T patients versus Healthy Individuals CD4+ TR1: CD4+CD45RA-LAG3+CD49b+ % of CD4+ T cells 0 1 2 3 4 ** ** (p=0.01) Healthy Donors SPMS PatientsSPMS subjects have a reduced frequency of IL-10 secreting TR1 cells ACTRIMS 2013 Poster: Profiling of Secondary Progressive MS by Multicolor Flow Cytometry Lauren W Collison, Ph.D; Chris L Ayers, Ph.D., Jordan L Harrell, MBE; Don Healey, Ph.D. CD4+ TR1: CD4+CD18brightCD49b+ % of CD4+ T cells 0 5 10 15 * * (p=0.02) Healthy Donors SPMS Patients
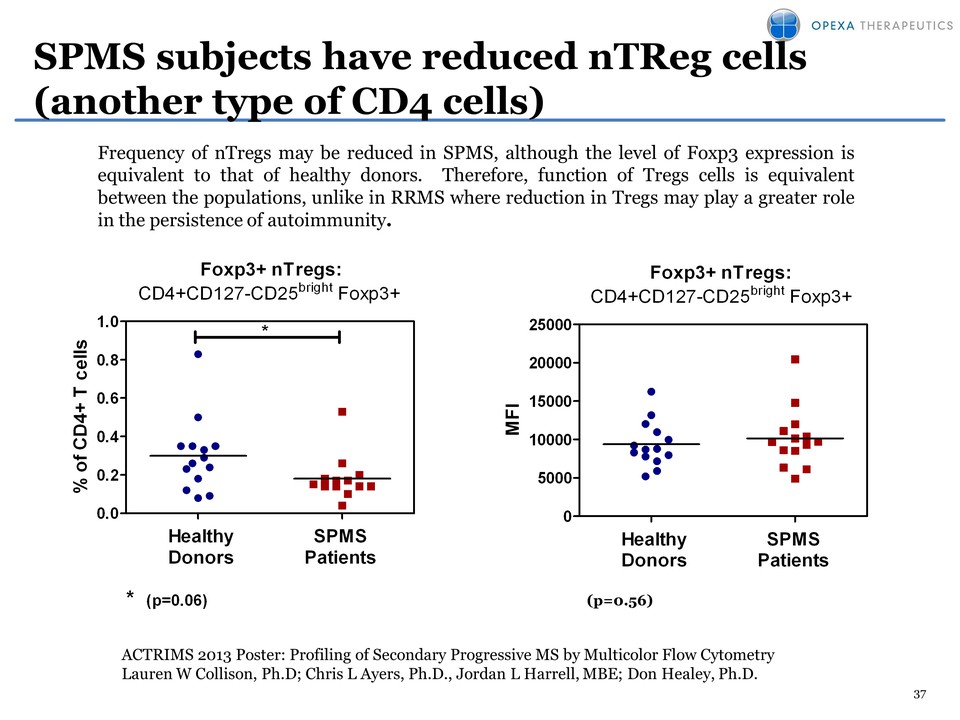
37 SPMS subjects have reduced nTReg cells (another type of CD4 cells) Foxp3+ nTregs: CD4+CD127-CD25bright Foxp3+ % of CD4+ T cells 0.0 0.2 0.4 0.6 0.8 1.0 * * (p=0.06) Healthy Donors SPMS Patients Foxp3+ nTregs: CD4+CD127-CD25bright Foxp3+ MFI 0 5000 1000015000 20000 25000 (p= 0.56) Healthy Donors SPMS Patients (p=0.56) Frequency of nTregs may be reduced in SPMS, although the level of Foxp3 expression is equivalent to that of healthy donors. Therefore, function of Tregs cells is equivalent between the populations, unlike in RRMS where reduction in Tregs may play a greater role in the persistence of autoimmunity. ACTRIMS 2013 Poster: Profiling of Secondary Progressive MS by Multicolor Flow Cytometry Lauren W Collison, Ph.D; Chris L Ayers, Ph.D., Jordan L Harrell, MBE; Don Healey, Ph.D.
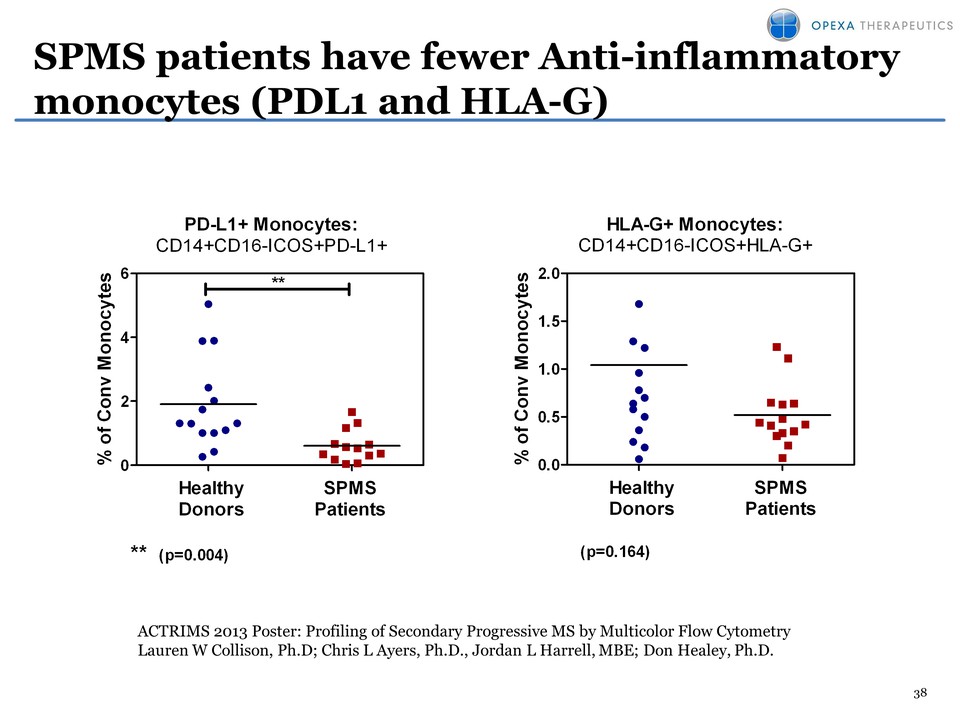
38 SPMS patients have fewer Anti-inflammatory monocytes (PDL1 and HLA-G) PD-L1+ Monocytes: CD14+CD16-ICOS+PD-L1+ % of Conv Monocytes 0 2 4 6 ** ** (p=0.004) Healthy Donors SPMS Patients HLA-G+ Monocytes: CD14+CD16-ICOS+HLA-G+ % of Conv Monocytes 0.0 0.5 1.0 1.5 2.0 (p=0.164) Healthy Donors SPMS PatientsACTRIMS 2013 Poster: Profiling of Secondary Progressive MS by Multicolor Flow Cytometry Lauren W Collison, Ph.D; Chris L Ayers, Ph.D., Jordan L Harrell, MBE; Don Healey, Ph.D.







 Could Address the Root Cause of Multiple Sclerosis by Preventing Demyelination and Enabling Remyelination Adapted by permission from Macmillan Publishers Ltd: NATURE REVIEWS IMMUNOLOGY 3, 483-492 (June 2003), copyright (2003) Opexa’s Strategy Tcelna programs the immune system to specifically recognize MRTC as pathogenic, thereby inhibiting further destruction of the myelin sheath and potentially enabling remyelination • Immune cells, including Tregs, have been primed, or sensitized, we believe, to specifically target the pathogenic MRTC for elimination or regulation • Elimination of harmful MRTC may lead to: o Stabilization of disease by preventing further destruction of myelin o Improvement in condition by allowing Oligondendroglial cells to remyelinate axons (neuroprotection)
Could Address the Root Cause of Multiple Sclerosis by Preventing Demyelination and Enabling Remyelination Adapted by permission from Macmillan Publishers Ltd: NATURE REVIEWS IMMUNOLOGY 3, 483-492 (June 2003), copyright (2003) Opexa’s Strategy Tcelna programs the immune system to specifically recognize MRTC as pathogenic, thereby inhibiting further destruction of the myelin sheath and potentially enabling remyelination • Immune cells, including Tregs, have been primed, or sensitized, we believe, to specifically target the pathogenic MRTC for elimination or regulation • Elimination of harmful MRTC may lead to: o Stabilization of disease by preventing further destruction of myelin o Improvement in condition by allowing Oligondendroglial cells to remyelinate axons (neuroprotection)




























