Exhibit 10.16
AGREEMENT OF ACCESS AND USE
OF CLINICAL TRIAL DATA (“Agreement”)
VAL2016/2015-419
Between:
L’Assistance Publique – Hôpitaux de Paris, Public Health Establishment, whose registered office is located at 3 Avenue Victoria, Paris 4th arrondissement, represented by its Managing Director, Mr Martin HIRSCH,
Represented by: Ms Florence FAVREL-FEUILLADE, Director of the Department of Clinical Research and Development, Carré Historique de l’Hôpital Saint-Louis, 1 Avenue Claude Vellefaux, 75010 Paris (France), in application of the order of management empowering her to sign this Agreement
Hereinafter referred to by the acronym “AP-HP”,
PARTY OF THE FIRST PART,
And:
Acer Therapeutics Inc., a Delaware Corporate, whose registered office is located at 222 Third Street, Suite 2240 Cambridge, MA, (USA) represented by its Founder and CEO, Mr. Chris Schelling
Hereinafter referred to as the “ACER”
PARTY OF THE SECOND PART,
Individually referred to as the “Party” and collectively as the “Parties”
CONFIDENTIAL MATERIALS OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION. TRIPLE ASTERISKS [***] DENOTE OMISSIONS.
1
PREAMBLE 1
TheAP-HP through the DRCD (Department of Clinical Research and Development (Département de la Recherche Clinique et du Développement)) has become the sponsor of a biomedical research project titled: “Prévention des complications vasculaires par un traitement beta-bloquant dans le syndrome d’Elhers-Danlos vasculaire”, hereinafter referred to as the “Research”. Prof. BOUTOUYRIE of the Clinical Pharmacology department of the HEGP hospital (l’Hôpital Européen Georges Pompidou), of the HUPO hospital group (Hôpitaux Universitaires de Paris Ouest, is the Coordinating Investigator of the Research and hereinafter referred to as “the Investigator.”
The Parties acknowledge that one part of the BBEST trial was sponsored by Hospital University of Gent (“HUG”).AP-HP and HUG (COLL BBEST AOM 01 108 – P010309) entered into a collaboration agreement defining the conditions of performance, analysis and exploitation of the BBEST study.AP-HP is in charge of the negotiation of any exploitation agreement. All regulatory requirements regarding HUG data will be dealt with in a separate agreement between HUG and ACER.
Thefollow-up and monitoring of this research have been provided by theAP-HP, hereinafter referred to as “the Head of Research” or“AP-HP”.
ACER wishes to receive all information relating to the Research and theAP-HP Database, hereinafter referred to as the “BBEST Information”, including but not limited to the Essential Documents and original BBEST Study safety data, case report forms, diagnostic and imaging data in order to establish an Approval New Drug Application (“NDA”) with United States’ Food and Drug Administration (“FDA”), Colombia’s health authority and Brazil’s health authority (National Public Health Agency (“ANVISA”)), in addition to the existing label of celiprolol in Europe or other country where celiprolol is commercially available as a generic product.
All of the BBEST Information is identified in Annex 1.
ACER wishes to havean exclusive right of access to and use of the BBEST Informationand has approached theAP-HP to define the conditions of that Agreement.
ACER wishes to create a dossier as required by health authorities to obtain Approval which shall include a Trial Master File, ACER Database, safety database and Acer Case Report Forms.
ACER wishes to use the ACER Database with coded patient data for regulatory submission to the FDA or other competent health authorities.
The process for transfer of the BBEST Information is described in Annex 2.
IT HAS BEEN AGREED AS FOLLOWS:
ARTICLE 1: DEFINITIONS
AP-HP Database:the database produced by theAP-HP as part of the Research that contains, but is not limited to observations, clinical data, Essential Documents, medical records, diagnostic data, imaging data and original case report forms. All this information will bede-identified byAP-HP.
ACER Case Report Form(s) or CRF: the electronic case report forms prepared with coded patient information
CONFIDENTIAL MATERIALS OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION. TRIPLE ASTERISKS [***] DENOTE OMISSIONS.
2
based on theAP-HP Database and after Source Data review and verification.
ACER Database: the database produced by ACER and its contract research organization (“CRO”) from the Acer Case Report Forms. The ACER Database will be part of the New Drug Application in the U.S. or of any foreign equivalent.
Approval:any authorisation/certification/approval required by the health authority, in each of the countries or areas of the Territory, and necessary for the Marketing of the Product, under whatever regulatory status, including in particular but not exclusively the commercialisation on the market of the Product under CE, AMM and ATU marking.
Authorized Representative(s):trained clinical research associates subject to obligations of professional secrecy and qualified by training and experience under International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (“ICH”).
BBEST Information:all of the information related to the Research and theAP-HP Database which is identified in Annex 1.
Combination Product means a product sold in a form containing a Product and at least one other product, component or ingredient which could be sold separately and apart from the Product (each, a “Combination Product”). A Combination Product may include a Product and any separate product, component or ingredient developed by orin-licensed by ACER from a third party.
Development Plan:the provisional timetable for commercial and technical procedures to be carried out by ACER in order to market Products as specified in Annex 3.
Essential Documents: the Research and the documents necessary to confirm that the quality of the BBEST Information is consistent with Sections 8.1 to 8.4 of ICH Good Clinical Practice (“GCP”) .
Exclusivity Period: The present Agreement shall becomenon-exclusive on acountry-by-country basis at the end of the longest period between (i) 7 years from the date of Approval, or (ii) the date of the loss of regulatory exclusivity for the Product when a generic product obtains a 25% market share.
Marketing:the sale of the Product or billing of the service, directly or indirectly by ACER
Net Sales: the amount of sales of the Product Excluding Tax, invoiced directly or indirectly by ACER to third parties. Only the final amount collected (i.e., adjusted for discounts, rebates, transportation, distribution in the limit of 5% of the Net Sales) by ACER will be taken into consideration in the calculation of Net Sales conducted in concordance with these provisions. The sales carried out under ATU [Autorisation Temporaire d’Utilisation—Temporary Authorisation for Use] or RUO [Research Use Only] are also included in the total of the Net Sales. Net Sales for Combination Products will be calculated by multiplying actual Net Sales of Combination Products by the fraction A/(A+B), where “A” is the Net Sales of the Product if sold or performed separately, and “B” is the Net Sales of the other product, component or ingredient or service in the Combination Product if sold separately. If, on acountry-by-country basis, the other product, component, ingredient or service in the Combination Product is not sold separately in said country, Net Sales shall be calculated by multiplying actual Net Sales of the Combination Product by the fraction A/C where “A” is the Net Sales of the Product, if sold separately, and “C” is the Net Sales of the Combination Product. If, on acountry-by country basis, neither the Product nor the other product, component, ingredient or service in the combination Product is sold separately in said country, Net
CONFIDENTIAL MATERIALS OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION. TRIPLE ASTERISKS [***] DENOTE OMISSIONS.
3
Sales shall be determined in good faith by the parties.
Product: any product containing celiprolol and implementing or ,developed or marketed using all or part of the BBEST Information in whatever format/form, usable and marketable for human health, including the treatment ofEhlers-Danlos Syndrome, Marfan’s Syndrome, or Loeys-Dietz .
Research:Prévention des complications vasculaires par un traitement beta-bloquant dans le syndrome d’Elhers-Danlos vasculaire (Celiprolol in Patients With Ehlers-Danlos Syndrome, Vascular Type)NCT00190411/BBEST Study.
Source Data: As defined in Section 1.51 of theICH-GCP document, including all information in original records and certified copies of original records or clinical findings, observations, or other activities in a clinical trial necessary for the reconstruction and evaluation of the trial. Source Data are contained in source documents (original records or certified copies).
Territory: World-wide
Trial Master File: file established containing the Essential Documents.
ARTICLE 2: SUBJECT OF THE AGREEMENT
The present Agreement aims to define the conditions of access to and use of BBEST Information for the purpose of (i) obtaining Approval in the Territory, and (ii) Marketing of a Product for the treatment ofEhlers-Danlos Syndrome. The Parties shall determine by the present Agreement their reciprocal rights and obligations.
ARTICLE 3: NATURE, PURPOSE AND SCOPE OF RIGHTS GRANTED
3.1TheAP-HP grants ACER, which accepts, the exclusive right tousethe BBEST Information in order to develop, make, have made, sell, have sold and use the Product in the Territory.
3.2 TheAP-HP retains the exclusive right to use the BBEST Information for internal academic research purposes which will exclude any commercial use in the Territory.
3.3. ACER acknowledges having had access to all information concerning the BBEST Information and the legal and regulatory context governing this access, allowing it to fully appreciate the content, the format and the extent of the BBEST Information as well as the corresponding rights granted under the terms of this Agreement. All of the BBEST Information will be made available to ACER and its representatives in a period of thirty (30) days from the signing of this Agreement by the Parties under the conditions previously defined by the Parties in Annex 1.
3.4 The rights granted under this Agreement to ACER may not be the subject of any concession to any third party by ACER except as set forth in Article 15 of this Agreement being specified that, in such case, any third party,sub-licensee, assignee or acquirer shall be bound by terms substantially similar to this Agreement.
3.5 The present Agreement cannot be interpreted as an assignment by theAP-HP of its rights in the BBEST Information.
3.6 It is understood that the submission of the BBEST Information provided by the present Agreement shall not grant proprietary rights to ACER. With the exception of what is provided for in the present Agreement, no
CONFIDENTIAL MATERIALS OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION. TRIPLE ASTERISKS [***] DENOTE OMISSIONS.
4
license, express or tacit, or any other rights are granted by theAP-HP to ACER on the patents, patent applications, trade secrets or other property rights of theAP-HP obtained and held by theAP-HP from the BBEST Information.
ARTICLE 4: FINANCIAL PROVISIONS
In consideration for the rights granted under the present Agreement, ACER undertakes to:
| • | reimburse the full amount of the direct and indirect costs of the Research supported by theAP-HP and its partners where appropriate; |
| • | pay a lump sum; |
| • | pay theAP-HP an amount of royalties for the use of the Products. |
4.1 Reimbursement of the costs of theAP-HP
ACER will reimburse theAP-HP the direct and indirect costs of the Research corresponding to an amount of [***] Euros exc. tax.
The payment schedule is established as follows:
| • | [***] Euros at signing for the cost of the preparation of theAP-HP Database (herein after called Preparation Funding) |
| • | 25% at locking the ACER Database |
| • | 25% at completion of the ACER Clinical Study Report and decision to file the New Drug Application to the FDA |
| • | 25% upon FDA approval |
| • | 25% at the first commercial sale of Products |
ACER shall notify theAP-HP of the occurrence of these stages immediately the day after their occurrence. The above-mentioned sums are payable within a period of forty-five (45) days from the stage in question and from the receipt of the invoice issued by theAP-HP. These sums constituting performance guarantee will remain in any event and definitively acquired to theAP-HP.
4.2 Lump Sum
In consideration of the rights granted under the present Agreement, ACER undertakes to:
| • | Pay a lump sum of [***] Euros at signing and [***] Euros within forty-five (45) days of (1) approval by the relevant French authority to allow the transfer of the ACER Database if necessary or (2) decision fromAP-HP to transfer the data. |
| • | Pay [***] Euros upon FDA approval |
| • | Pay [***] Euros upon first commercial sale of Products |
4.3 Royalties
In consideration of the rights granted under the terms of this Agreement, ACER undertakes to pay theAP-HP royalties based on ACER’s Net Sales of any Product during the Exclusivity Period in respect of the US, Colombia and Brazil and any other country where ACER would market the Product.
For the purpose of clarity, no royalties are dueAP-HP on Net Sales of Product in Europe or in any other country in the case of the marketing of a generic celiprolol with the same format in that country for any indication before the conclusion of the present Agreement and which price will not be modified by the additional indication.
CONFIDENTIAL MATERIALS OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION. TRIPLE ASTERISKS [***] DENOTE OMISSIONS.
5
This fee is set at [***] of the Net Sales of the Products.
4.4 The payment of duties, fees, royalties and other revenues will be as follows: the statement of the Net Sales of any Product or of any other financial transaction related to the Product, ended on 31 December of each year and certified by the auditor of ACER, will be sent to theAP-HP at the latest by 31 March of the following year, even in the absence of sales or transaction during the year considered. After receiving this statement from theAP-HP, the sums due in respect of the year under review will have to be paid within the period of forty-five (45) days from the date of receipt of the invoices issued by theAP-HP
ACER will be solely responsible for the payment of royalties and other amounts due under this Agreement to theAP-HP.
All amounts owed by ACER to theAP-HP in application of the present Agreement shall be addressed to:
Office du Transfert de Technologie & des Partenariats Industriels
Délégation Régionale à la Recherche Clinique
Carré Historique de l’Hôpital Saint-Louis—Porte
231, avenue Claude Vellefaux
75475 Paris Cedex 10 (France)
This sum will be paid by ACER within the period of forty-five (45) days from the date of receipt of the invoice prepared byAP-HP
This sum will be paid by ACER to the Director of Specialised Management of Public Finances for theAP-HP—Hospital Accounting Division (Directeur de la Direction Spécialisée des Finances Publiques pourl’AP-HP—Secteur comptabilité hospitalière)

The account number for the Preparation Funding will be:

CONFIDENTIAL MATERIALS OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION. TRIPLE ASTERISKS [***] DENOTE OMISSIONS.
6
Any amount not paid by ACER within the aforementioned timeframes will give rise to default interest calculatedprorata temporisaccording to the rules applicable to French Public Establishments, (namely, at the date of signature, the legal interest rate in force plus two (2) points).
The amounts payable will be increased by the legal fees and in particular the VAT at the rate in force.
ARTICLE 5—TERMS OF USE OF SUMS RECEIVED
The Preparation Funding (as defined in article 4.1) will be assigned to the Hôpital Européen Georges Pompidou (HEGP) ofAP-HP. It shall be managed in keeping with the rules applicable to “specifically targeted income” in order to conduct the preparation of the data.
All other sums are assigned to revenue account DNA94-56 of the Office of Technology Transfer & Industrial Partnerships.
ARTICLE 6: TERMS OF ACCESS OF BBEST INFORMATION
The unit under the responsibility of the Investigator and/or the Head of Research will make the BBEST Information available to ACER according to the terms specified in Annex 1.
In application of the applicable legislative provisions, including those relating to medical secrecy, clinical trials and privacy rules set forth in the Public Health Code and the Law of 6 January 1978, only the Authorised Representatives with a direct agreement with the sponsor of the clinical trial(AP-HP) shall be granted the ability to consult and review the Source Data (for example: medical records) relating to the BBEST Information in order to verify ACER Case Report Forms and ACER Database. It is understood and agreed, that ACER and theAP-HP shall mutually select the Authorized Representatives and the Parties shall have joint decision making on matters related to the Authorized Representatives’ verification of ACER Case Report Form with Source Data. The costs of the CRO/Authorised Representatives will be covered by ACER.
The operational plan for the consultation, compilation, analysis and transfer of BBEST Information is set forth in Annex 2.
ARTICLE 7: PROVISION OF BBEST INFORMATION
7.1 Acer and their Authorized Representatives shall take the necessary precautions to protect patient privacy when processing personal data from theAP-HP Database and the Source Data.
To that end,AP-HP, as the data controller of the BBEST Information must notify to the French data protection authority, the CNIL, the means deployed (i) to ensure compliance with privacy principles, (ii) to provide notice to each patient enrolled in the BBEST Study, if possible, (iii) to obtain consent of such patients, if required, (iv) to provide a legal basis to the transfer of the ACER Database to the FDA, and (v) to ensure security of the BBEST Information so that it will not be disclosed to unauthorized persons before and during the transfer of the BBEST Information.
7.2 The Parties agree that the transfer of the ACER Database to the US shall be made on the basis of European Commission approved standard contractual clauses attached under Annex 4 and signed simultaneously with this Agreement.
7.3 ACER will establish a Trial Master File containing the Essential Documents from the Research. Patient data in
CONFIDENTIAL MATERIALS OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION. TRIPLE ASTERISKS [***] DENOTE OMISSIONS.
7
the BBEST Information will be identified by a code for entry into the ACER Database and any information directly identifying the patient will not be transmitted. Prior to transferring the data, ACER and its CRO shall ensure that no real patient initials or birth dates nor any other information directly identifying the patient will be transferred. All data relating to patient identity shall be coded by a randomization number established by Acer’s CRO.
7.4 The Authorized Representative(s) of ACER may only be present in the department in the presence of the Investigator or the Head of Research or authorized delegate. The Authorized Representative(s) will have to use professional discretion regarding all facts, information and documents of which they will have knowledge due to their presence in the premises of the department under the responsibility of the Investigator and/or the Head of Research.
ACER has taken note that the use of personal data used to create the ACER Database will have to comply with the French Data Protection Act as amended and the Data Privacy Act under the laws of Belgium.
7.5 The Parties acknowledge that in a second phase, the genetic testings which were not part of the BBEST study results might be transferred to ACER byAP-HP, consistent with the terms notified to CNIL, including if necessary patient consent. The terms will include specific financial conditions.
ARTICLE 8—DEVELOPMENT
8.1 ACER has submitted to theAP-HP a Development Plan annexed to this Agreement to be an integral part of the Agreement, included as Annex 3, which will be updated as and where appropriate.
8.2 ACER will keep theAP-HP informed of the state of progress of the Development Plan every year. To this end, ACER will send toAP-HP an annual report outlining the technical and commercial results obtained during the performance of the commercial development plan. Such annual report shall be considered as a Confidential Information.
8.3 ACER will carefully lead the works of the Development Plan.
8.4 In the event that ACER interrupts its Development Work for a period of more than six (6) consecutive months, without being able to reasonably justify this to theAP-HP, ACER will be ordered to resume the said Development Work within a period of sixty (60) days from the date of such notification and the Parties will work together to assess the reasons for the delay and the means to be employed in order to remedy the situation.
8.5 ACER undertakes to comply with the applicable regulations in force in France and abroad for conducting the Development Plan. It is understood between the Parties that the Marketing of the Products in each of the countries of the Territory should be carried out within a maximum period of twelve (12) months from the Approval of Products in each of the countries of the Territory where Approval has been obtained provided that that health technology assessments and pricing and reimbursement policies allow the commercial exploitation of the Product. ACER will be responsible for obtaining and keeping valid, in its name and at its own expense, the Approvals required by the laws and regulations applicable in each country where the Marketing of the Product(s) is contemplated.
ARTICLE 9—REGISTRATION OF PRODUCTS
ACER will have in its name, at its exclusive costs and according to the regulations in force, country by country, the files for obtaining Approval of the Product. TheAP-HP will not be the holder of the Product Approvals.
ARTICLE 10—ACCOUNTING
CONFIDENTIAL MATERIALS OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION. TRIPLE ASTERISKS [***] DENOTE OMISSIONS.
8
10.1 ACER undertakes to keep special accounts of the money received for the use of the BBEST Information. These accounts will contain all necessary information for the accurate assessment of commercial transactions and the control of the sums owed to theAP-HP.
10.2 These particular accounts will be made available to theAP-HP for each year up until the expiry of a period of three (3) years following that year.
Upon request of theAP-HP, and no more than once every 12 months, ACER will authorise an independent certified accountant chosen by theAP-HP, to inspect said accounts in order to verify the accuracy of the calculation of the sums due to theAP-HP. The costs and fees of this expert will be borne by theAP-HP except in the case where its calculation of the sums owed to theAP-HP is different from five (5%) or more of the calculation provided by ACER. In this last case, ACER will pay all documented sums as well as reasonable fees brought about by this inspection. Said expert will conduct an audit in accordance with the provisions of Article 10 herein. The chartered accountant will be bound by professional secrecy.
10.3 The provisions of Article 10 herein remain in force throughout the duration of this Agreement and for three (3) years after its expiration or termination.
ARTICLE11- GUARANTEE—COMPENSATION
11.1 TheAP-HP declares and guarantees to ACER that it is fully empowered to grant it the rights subject of this Agreement. Subject to the foregoing, theAP-HP does not grant ACER any other warranty, of any kind, express or implied; however, theAP-HP warrants that it has complied with the requirements of the French Data Protection Act (“Informatique et Libertés” Act n°78-17 dated January 6 1978) and the French Public Health Code concerning the BBEST clinical trial.
| 11.2 | Nothing in this Agreement shall be construed as: |
| • | constituting a guarantee of present or futurenon-violation of patents of third parties or any other intellectual property right of third parties, |
| • | constituting a guarantee of the safety, performance or fitness for a particular purpose, of interventions of any type (for example: medication, medical devices), the subject of or involved in the Research. |
The possible hazards, risks and dangers as regards the performance of the present Agreement are the sole responsibility of ACER.
11.3 Under no circumstances, theAP-HP’s responsibility shall be engaged by a third party for damages related to the use by ACER of the BBEST Information. ACER guarantees theAP-HP and the members of its staff against any recourse that might be brought against them by reason of damage to persons or property, suffered during possession and use of the BBEST Information and the marketing of the Product by ACER, with the exception of any case of gross negligence or wilful misconduct by theAP-HP related to theAP-HP’s obligations pertaining personal data and privacy protection according to the French Law. ACER renounces undertaking any action against theAP-HP in the event where these claims, requests, legal proceedings or actions would be made against ACER by third parties, with the exception of any case of gross negligence or wilful misconduct by theAP-HP related to theAP-HP obligations pertaining personal data and privacy protection according to the French Law.
11.4 ACER will ensure that it has the necessary insurance to sufficiently cover its liability in the performance of the present Agreement.
11.5 ACER warrants theAP-HP against any prosecution that would be implemented against the latter on the basis of a fault committed by ACER withoutAP-HP having participated actively in the realisation of the offence or
CONFIDENTIAL MATERIALS OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION. TRIPLE ASTERISKS [***] DENOTE OMISSIONS.
9
the damage at the origin of the conviction.
11.6 ACER will be solely responsible for any claims, demands, legal proceedings and actions brought by third parties including damages, costs, and convictions resulting therefrom which could occur during the manufacture, use or sale of the Product by ACER and renounces undertaking any action against theAP-HP with the exception of any case of gross negligence or wilful misconduct by theAP-HP related to theAP-HP obligations pertaining to the personal data and privacy protection according to the French Law.
The provisions of Article 11 herein shall remain in force notwithstanding the expiration or the termination of the Agreement.
ARTICLE 12: CONFIDENTIALITY
Subject at all times to the provisions of the French Data Protection Act (“Informatique et Libertés” Act n°78-17 dated January 6 1978) and those of the French Public Health Code, which each Party undertakes to respect, each insofar as it is concerned, the Parties expressly agree as follows regarding Confidential Information:
12.1 Each of the Party undertakes to keep confidential, not to communicate or disclose to anyone without the prior written agreement of the other Party, information of any nature it might know, during the performance of the present Agreement and in particular any confidential information relating to the Development Plan that it could receive in the framework of the present Agreement (hereinafter “Confidential Information”). The BBEST Information is an integral part of the Confidential Information.
12.2 Will not, however, be considered as confidential, Confidential Information which:
| • | would be in the public domain at the date of their communication or would be placed in the public domain by a third party in good faith later on, or |
| • | would already be known to the receiving Party at the date of entry into force of the present Agreement, except if such Confidential Information has been received from one of the Parties to the present Agreement in the absence of any fault, or |
| • | would be received from a third party assumed, using reasonable diligence, to have the right to possess this Confidential Information without restriction or breach of an obligation of confidentiality. |
In all cases, it is up to the Party receiving the Confidential Information to prove that it is not confidential.
12.3 During the term of the present Agreement and up until the Confidential Information is disclosed, ACER undertakes to keep secret the information that will be transmitted to it by theAP-HP for the purposes of this Agreement, it being understood, however, that a transmission of some of this Confidential Information will not be regarded as a breach by ACER of its commitment of confidentiality in the following two cases:
| • | if the transmission of Confidential Information to the competent public authorities is necessary for ACER to obtain the Approval, |
| • | if the Confidential Information is disclosed after obtaining prior written authorisation from theAP-HP and such written authorisation shall not be unreasonably withheld or delayed. |
In all cases, it is up to the Party receiving the Confidential Information to prove that it is not confidential.
12.4 Each Party agrees not to file a patent application or other industrial property title including the Confidential Information of the other Party without having first obtained the written permission of the latter.
12.5 Subject at all times to the terms of this Agreement, ACER will have the right to provide the Confidential Information to third parties, to the extent that the revelation of this Confidential Information is useful or necessary
CONFIDENTIAL MATERIALS OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION. TRIPLE ASTERISKS [***] DENOTE OMISSIONS.
10
to ACER for the use of the rights granted by the present Agreement, provided that the third party to which this Confidential Information is transmitted is bound by a confidentiality obligation similar to that provided for above.
12.6 The Parties agree to take all measures reasonably required in order to fulfil their obligations under Article 12 herein.
12.7 This obligation of confidentiality will subsist for as long as the Confidential Information is not known to be in the public domain during the term of the present Agreement and for five (5) years after its expiration or termination for any reason whatsoever. BBEST Information which constitutes personal data (as defined in the French Data Protection Act (“Informatique et Libertés” Act n°78-17 dated January 6 1978) will be kept confidential under the conditions laid down by French law.
12.8 The obligations of this Article 12 will be imposed on administrators, corporate officers, employees, agents and consultants of ACER. Each Party undertakes to limit the dissemination of Confidential Information received from the other Party to members of its staff involved in the performance of the present Agreement.
| 12.9 | The provisions of this Article may not interfere with: |
| • | the obligation of the staff of each of the Parties to this Agreement to produce an activity report for the organisation to which they belong, this communication not constituting a disclosure within the meaning of the laws on industrial property, |
| • | the thesis defence of the researchers whose scientific activity relates to the subject of the present Agreement, this thesis defence being organised whenever necessary so as to ensure the confidentiality of the results and information falling within the scope of the obligations of confidentiality referred to in Article 12.1 of the present Agreement. |
12.10 Any publication or oral or written communication, of information in direct or indirect relation to all or part of the BBEST Information, by ACER, will be subject, for the duration of the present Agreement and the eighteen (18) months following its expiry, the written Agreement of theAP-HP which will make its decision known within a maximum period of two (2) months from the date of the request. After this time and in the absence of an answer,AP-HP’s Agreement will be deemed granted. Any publication or communication must mention the capacity of theAP-HP as sponsor of the Research.
Accordingly, any plan to publish will be subject to the opinion of the other Party who will be able to delete or modify certain details, the disclosure of which would be likely to prejudice the commercial and industrial use, in good conditions, of the results of the Research. Such deletions or modifications will not affect the medical/scientific value and/or integrity of the publication. In addition, if the information contained in the publication or communication must be subject to protection in terms of industrial property, the other Party will be able to delay the publication or communication by a maximum period of eighteen (18) months from the date of filing the patent application. Some of the results obtained within the framework of the Research may, following written Agreement of each of the Parties or at the request of the State, be kept secret. TheAP-HP and ACER will determine the part of the results constituting the secret file and the period during which the latter will remain secret. The other information may be published in the conditions provided above. These publications and communications should mention the contribution made by each of the Parties to the achievement of the Research.
ARTICLE 13: EXCLUSIVITY
The present Agreement shall becomenon-exclusive on acountry-by-country basis at the end of the Exclusivity Period.
CONFIDENTIAL MATERIALS OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION. TRIPLE ASTERISKS [***] DENOTE OMISSIONS.
11
ARTICLE 14: –TERM AND TERMINATION
14.1 This Agreement is concluded for a term of 20 years as from the date of execution by the Parties.
14.2 The present Agreement may be automatically terminated by either Party in case of breach of an essential obligation by the other Party under the present Agreement, following formal notification from thenon-breaching Party sent by registered letter with acknowledgement of receipt, remaining unheeded three (3) months after its sending. The essential obligations include, but are not limited to, the respect of the Development Plan referred to in Article 8 of this Agreement, as well as the use of the BBEST Information with a view to the Approval. The exercise of this option of termination does not relieve the breaching Party from fulfilling its contractual obligations up until the date on which the termination takes effect.
In case of termination, ACER shall immediately return the BBEST Information received from theAP-HP and cease all use of such BBEST Information, directly, indirectly and in any form whatsoever including ACER CRF and ACER Database.
14.3 This Agreement may also be terminated automatically by theAP-HP in the event of partial or total suspension of the activity of ACER having the effect of not ensuring the performance of the present Agreement, following a formal notification from theAP-HP sent by registered letter with acknowledgement of receipt, remaining unheeded one (1) month after its sending.
In this case, ACER shall immediately return to theAP-HP the BBEST Information communicated and shall cease all use of such BBEST Information, directly, indirectly and in any form whatsoever.
In the event Acer does not use commercially reasonable efforts to pursue the Development Plan, then the present Agreement may be terminated automatically by theAP-HP if ACER doesn’t obtain any Approval within 3 years of signing this Agreement. In this case, ACER shall cease all use of the BBEST Information, directly, indirectly and in any form whatsoever. ACER will be free to keep a copy of the BBEST Information for the exclusive purposes of archiving.
In the event of termination, ACER shall cease all use of such BBEST Information, directly or indirectly, and in any form whatsoever, and shall return to theAP-HP the BBEST Information communicated.
14.4 ACER can automatically terminate the Agreement upon notification of impossibility to achieve the development of the Product or to obtain pricing and reimbursement conditions in the US that allow the commercial exploitation of the Product with sixty (60) days’ notice. ACER shall then cease all use of such BBEST Information, directly or indirectly, and in any form whatsoever including ACER CRF and ACER Database, and shall return to theAP-HP the BBEST Information communicated.
ARTICLE 15: SUBLICENCES – TRANSFER OF THE AGREEMENT
15.1 The present Agreement binds the Parties, their successors and their beneficiaries.
15.2 SUBLICENCES
The right to grantsub-licenses can only be exercised under the following conditions:
| • | Sub-licensing is allowed provided thatAP-HP give its written consent to ACER prior the signature of thesub-license agreement which should intervene within the maximum period of thirty (30) days from the date of the request, consent not to be unreasonably withheld. |
| • | ACER shall be the solely responsible entity towardsAP-HP for proper performance of thesub-licenses. |
| • | Should ACER grant asub-license as defined in this Article 15, ACER undertakes to explicitly include in thesub-license agreement clauses which are compatible with all the clauses of this Agreement. |
| • | In all cases, ACER expressly guarantees that it will not grant to itssub-licensee(s) any right to grant furthersub-licenses. |
| • | ACER undertakes to provide toAP-HP a copy of the sublicense agreement. |
CONFIDENTIAL MATERIALS OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION. TRIPLE ASTERISKS [***] DENOTE OMISSIONS.
12
For the purposes of clarity,AP-HP expressly agrees that ACER shall have the right to sublicense any or all of its rights and obligations to Sanofi for exploitation in the countries within Europe designated by Sanofi in the sublicense agreement.
15.3 TRANSFER OF THE AGREEMENT
Except as provided below, the present Agreement is concludedintuitu personae between theAP-HP and ACER. Accordingly, ACER may not transfer all or part of its rights and obligations under the present Agreement to a third party without the prior written consent of theAP-HP.
In the case of a merger, absorption, assignment or transfer of activities of ACER to a third party to the Agreement or any other transformation of ACER aimed at modifying theintuitu personae characteristics taken into account for the conclusion of the Agreement, the Agreement may only be sublicensed or transferred with the prior written consent of theAP-HP, which should intervene within the maximum period of thirty (30) days from the date of the request, consent not to be unreasonably withheld.
It is understood that the third party will in any event be subject to the same obligations as those of ACER herein unless the Parties agree together otherwise.
In all cases a separate agreement shall be entered into between theAP-HP and the third party simultaneously to the operation carried out with ACER who will define the respective obligations ofAP-HP and the third party in accordance with the previous paragraph.
For the purposes of clarity,AP-HP expressly agrees that ACER shall have the right to sublicense any or all of its rights and obligations to Sanofi for an exploitation in Europe Union and United Kingdom without prior consent ofAP-HP. Prior to the exercise of the right to sublicense to Sanofi, ACER will provide a list of counties covered by the Sanofi sublicense and ACER will consultAP-HP about the CNIL approval related to the transfer ofAP-HP Database to Sanofi.
ARTICLE 16: FORCE MAJEURE
Each Party will be excused from meeting its obligations if the failure is due to a case of force majeure such as provided for in Article 1148 of the Civil Code, and its applications by Frenchcase-law (“jurisprudence”). It is up to the defaulting party to take all measures to limit duration and consequences of the suspension of application of this Agreement resulting from the event of force majeure.
In the case ofnon-performance for more than three months, the Parties will work together in good faith to continue the Agreement, or consider its termination.
ARTICLE 17: INDEPENDENTCO-CONTRACTORS—ENTIRE AGREEMENT
17.1 This Agreement contains the entirety of the obligations of the Parties relating to its subject. This Agreement shall in no case be construed as creating a partnership or ade facto company between the Parties, each of them being considered as an independentco-contractor.
17.2 This Agreement cancels and replaces any prior agreement between the Parties relating to the subject matter hereof. It may only be modified by an amendment signed by the representatives of the Parties to the Agreement, duly authorised for this purpose.
ARTICLE 18: NULLITY—SEVERABILITY
CONFIDENTIAL MATERIALS OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION. TRIPLE ASTERISKS [***] DENOTE OMISSIONS.
13
18.1 If one or several provisions of this Agreement are held not to be valid or are declared as such pursuant to a law, a regulation—and in particular the laws of the European Union—or following a final decision of a competent court not subject to appeal, the other stipulations will retain full force and scope and the Parties will immediately effect the necessary changes while respecting, to the extent possible, the agreement existing at the time of signature of this Agreement.
ARTICLE 19: NOTIFICATIONS
Any communication or notification for the attention of the Parties should be made by fax or bye-mail, confirmed by registered letter with acknowledgement of receipt, to the addresses indicated below, as long as they have not been notified of a change of address in writing:
| • | For theAP-HP: |
Office de Transfert de Technologie et de Partenariats Industriels (OTT&PI)
Département de la Recherche Clinique et du Développement (DRCD)
Hôpital Saint-Louis – Bâtiment Lugol—Porte 22
1, avenue Claude Vellefaux
75475 Paris Cedex 10 (France)
| • | For ACER: |
Jefferson Davis
Head of Corporate Development
222 Third Street, Suite 2240
Cambridge, MA 02142
Telephone:617-225-7700
Email: jdavis@acertx.com
ARTICLE 20—REFERENCE TO THE AP-HP—USE OF THEAP-HP LOGO
20.1 It is understood that this Agreement shall in no way derogate from the exclusive ownership enjoyed by each of the Parties of its names and trademarks. Accordingly, any written or oral citation of names and/or brands of one of the Parties within the framework of communications, operations or public demonstrations of any kind, and in particular any operation likely to be regarded as displaying directly or indirectly promotional character, will remain in all cases subject to the express prior authorisation of the Party mentioned.
20.2 ACER undertakes not to use in writing or orally, the name, the brands or any other distinctive sign, including in the contracted or abbreviated form or by imitation, of theAP-HP or any of its agents in the framework of the use and distribution of the Products, in particular with a promotional purpose, and regardless of the media used (video, poster, press release, advertising brochure, etc.), without the prior written consent of theAP-HP.
In order to obtain this consent, ACER shall accurately notify theAP-HP, of the operation referred to as well as the form of this use, its duration and the context in which ACER wishes to use the distinctive symbol, trademark, company name, brand, image, logo or figurative symbol of theAP-HP.
It is understood that, in the event that theAP-HP gives its written consent for the use requested by ACER, it may suspend this authorisation at any time in the event that the communication made by ACER no longer corresponds to that described in the notification referred to in the previous paragraph, whether in terms of form, context, geographical situation or duration, or if it would result damaging the image of theAP-HP.
CONFIDENTIAL MATERIALS OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION. TRIPLE ASTERISKS [***] DENOTE OMISSIONS.
14
In any case, and even when theAP-HP would have given its authorisation for the use planned by ACER, the distinctive symbols, trademarks, company names, brands, images, logos or figurative symbols belonging to theAP-HP may not be used by ACER in a way which, by the form and/or the context used, can be construed as any guarantee granted by theAP-HP over the Products, combined products, or any product of ACER.
20.3 The Parties have already decided to issue a joint press release in French following the signature of the Agreement.
20.4 ACER will impose the same obligations on its potential distributors.
The provisions of this Article 20 herein shall remain in force notwithstanding the expiry or termination of this Agreement.
ARTICLE21—NON-ABANDONMENT OF RIGHTS
If, in the event of a breach by either Party of its obligations under this Agreement, thenon-breaching Party does not enforce its rights resulting from said violation, thenon-enforcement of its rights will not be construed as a waiver to enforce said rights in the future or on the occasion of a new similar violation by the breaching Party of its obligations arising from this Agreement.
ARTICLE 22: LITIGATION—DISPUTES
This agreement is written in English and translated to French and is subject to French law.
If difficulties arise on the occasion of the interpretation, and/or the performance of the Agreement, the Parties undertake to resolve them amicably to the extent possible.
The occurrence of a dispute will be materialised by the sending a registered letter with acknowledgement of receipt, by one of the Parties to the other Party, setting out the grounds of the dispute.
In the event of persistent disagreement, the competent courts of Paris have jurisdiction.
Done at Paris, in two (2) original copies, on
For the ASSISTANCE PUBLIQUE-
HOPITAUX DE PARIS
The Director General of theAP-
HP and by delegation
The Director of the Department of Clinical Research
and Development
/s/ Florence Favrel-Feuillade
Florence FAVREL-FEUILLADE
For ACER
Founder and CEO
/s/ Chris Schelling
Chris Schelling
CONFIDENTIAL MATERIALS OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION. TRIPLE ASTERISKS [***] DENOTE OMISSIONS.
15
Annex 1: Description of BBEST Information
OriginalAP-HP Case Report Forms
| AP-HP Database | informed consent | ||
| Informed Consents | Essential Documents | Study Drug Randomization, Dispensing, and Accountability
| ||
| Case Report Forms | Adverse Event Forms and Clinical Event Adjudication Information
| Regulatory documents | ||
| Clinical Protocol and Amendments | Communications and Interim Analyses | Safety information |
CONFIDENTIAL MATERIALS OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION. TRIPLE ASTERISKS [***] DENOTE OMISSIONS.
16
Annex 2
Operational Plan for the consultation, compilation,
analysis and transfer of BBEST Information
GCP Standard Clinical Trial Practice for Patient Privacy and Data Quality Standards
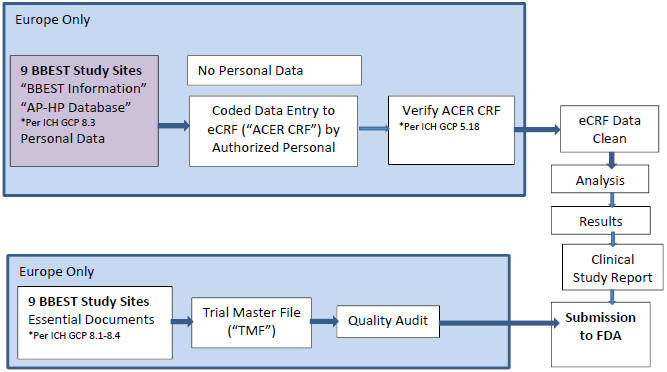
Subject Information
| • | Personal privacy data not removed from BBEST sites |
| • | Data that either identifies a subject or makes such subject identifiable |
| • | Birthdates, place of birth, names, initials, personal physicians |
| • | Only use Unique Subject Identification Code (BBEST Randomization #) |
Conduct activities in accordance with ethical principals
| • | Good Clinical Practices (GCP) |
| • | Declaration of Helsinki |
| • | Scientific quality standards |
| • | French and Belgium Regulations |
Systems
| • | Secure, controlled, limited access systems |
Acer Team (“Authorized Personnel”)
| • | Qualified by training and experience |
| • | ICH GCP |
| • | EU GCP |
| • | FDA Regulations |
| • | Follow Standard Operating Procedures (SOPs) and Project-specific Plans |
CONFIDENTIAL MATERIALS OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION. TRIPLE ASTERISKS [***] DENOTE OMISSIONS.
17
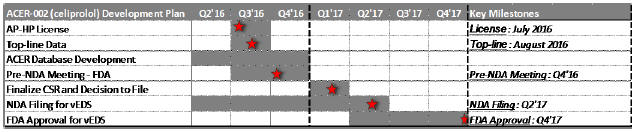
Annex 3: Development Plan of ACER
CONFIDENTIAL MATERIALS OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION. TRIPLE ASTERISKS [***] DENOTE OMISSIONS.
18
Annex 4: European Commission approved standard contractual clauses
CONFIDENTIAL MATERIALS OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION. TRIPLE ASTERISKS [***] DENOTE OMISSIONS.
19