
© 2018 F. Hoffmann-La Roche Ltd. All rights reserved. FIREFISH, A MULTI-CENTER, OPEN-LABEL TRIAL TO INVESTIGATE THE SAFETY AND EFFICACY OF RG7916 IN BABIES WITH TYPE 1 SMA: STUDY UPDATE AND REAL-LIFE EXPERIENCE OF STUDY IMPLEMENTATION G Baranello1, J Day2, A Klein3, E Mercuri4, L Servais5, N Deconinck6, R Masson1, H Kletzl7, C Czech7, M Gerber7, Y Cleary7, F Lee7, K Gelblin7, S Nave7, K Gorni7 and O Khwaja7 1. Carlo Besta Neurological Research Institute Foundation, Developmental Neurology Unit, Milan, Italy; 2. Department of Neurology, Stanford University, Palo Alto, CA, USA; 3. University Children’s Hospital Basel, Basel, Switzerland; Inselspital, Bern, Switzerland; 4. Paediatric Neurology and Nemo Center, Catholic University and Policlinico Gemelli, Rome, Italy; 5. Institute of Myology, Paris, France; Reference Center for Neuromuscular Disease, Centre Hospitalier Régional de La Citadelle, Liège, Belgium; 6. Queen Fabiola Children's University Hospital and Université Libre de Bruxelles, Brussels, Belgium; Neuromuscular Reference Center UZ Ghent; Ghent, Belgium; 7. Roche Pharmaceutical Research and Early Development, Roche Innovation Center, Basel, Switzerland.

© 2018 F. Hoffmann-La Roche Ltd. All rights reserved. Disclosures GB is PI in the following clinical trials in SMA: BP39055 and BP39056 (Roche); CLMI070X2201 (Novartis); AVXS-101-CL-302 (Avexis); SMA- 001 (Catalyst); PI in the following clinical trials in Duchenne Muscular Dystrophy: FOR_DMD; DSC/14/2357/48 (Italfarmaco); VBP15-004 (Reveragen); PTC124-GD-025o_DMD (PTC Therapeutics). JD reports grants from: AMO Pharmaceuticals; aTyr; AveXis; Biogen; Bristol Meyers Squibb; Cytokinetics; Ionis Pharmaceuticals; Roche Pharmaceuticals; Sanofi-Genzyme; and Sarepta Therapeutics. He has served as a consultant for: AMO Pharmaceuticals; AveXis; Biogen; Cytokinetics; Ionis Pharmaceuticals; Roche Pharmaceuticals; Pfizer; Sarepta Therapeutics; Santhera Pharmaceuticals. He has patents licensed to Athena Diagnostics for genetic testing of myotonic dystrophy type 2 (US patent 7442782) and spinocerebellar ataxia type 5 (US patent 7527931). AK has received speaker and consulting fees from Biogen, PTC, Roche and Santhera and is PI for F. Hoffmann-La Roche and Santhera studies. EM is a consultant for F. Hoffmann-La Roche, AveXis, IONIS and Biogen, and PI for Biogen/IONIS and F. Hoffmann-La Roche studies. LS is a PI of SMA studies for Roche, Biogen, and Avexis. He has attended SAB of Biogen and Avexis and received consultancy fees from BiogenND. RM has no disclosures to report. HK, CC, MG, YC, FL, KGelblin, SN, KGorni and OK are current employees of F. Hoffmann-La Roche. RG7916 is an investigational medicine and benefit/risk profile has not been fully established. The information presented is early interim data.

© 2018 F. Hoffmann-La Roche Ltd. All rights reserved. Introduction • SMA is a severe, progressive neuromuscular disease leading to loss of motor function and reduced life expectancy1 • Increasing evidence suggests that SMA may be a multi-system disorder, where cells and tissues throughout the body, including motor neurons may be selectively vulnerable to low SMN protein levels3,4 • RG7916 is an orally administered, centrally and peripherally distributed SMN2 pre-mRNA splicing modifier that increases SMN protein levels • Preclinical data show similar RG7916 concentrations in blood, brain, and muscle tissue (Poster P48, A. Poirier et al.) • Similar SMN protein increase in brain and muscle in SMA mouse models following RG7916 administration (Poster P48) • Proof-of-mechanism of oral SMN2 splicing modifiers was previously established in preclinical models5 and in Type 2 and 3 SMA patients with RG79166 (Poster P46, E. Mercuri et al.) • The FIREFISH study aims to assess the safety and efficacy of RG7916 in babies with Type 1 SMA. This study is sponsored by F. Hoffmann-La Roche Ltd. SMA, spinal muscular atrophy; SMN, survival of motor neuron. 1. Mercuri E, et al. Lancet Neurol 2012;11:443–452; 2. Acsadi G, et al. J Neurosci Res 2009;12:2748–2756; 3. Singh RN, et al. Biochim Biophys Acta 2017;1860:299–315; 4.Hamilton G and Gillingwater TH. Trends Mol Med 2013;19:40–50; 5. Naryshkin N, et al. Science 2014; 345:688–693; 6. Clinicaltrials.gov NCT02633709 Accessed December 2017

© 2018 F. Hoffmann-La Roche Ltd. All rights reserved. RG7916 mechanism of action SMN1 6 8 7 DNA Pre-mRNA mRNA Functional SMN protein 6 8 7 6 8 7 SMN2 6 8 7 6 8 7 6 8 7 6 8 7 6 8 Functional SMN protein Unstable SMN protein rapidly degraded RG7916 Pinard E, et al. J Med Chem; 2017;60:4444–4457. RG7916 modifies SMN2 splicing to produce functional SMN protein in central and peripheral compartments
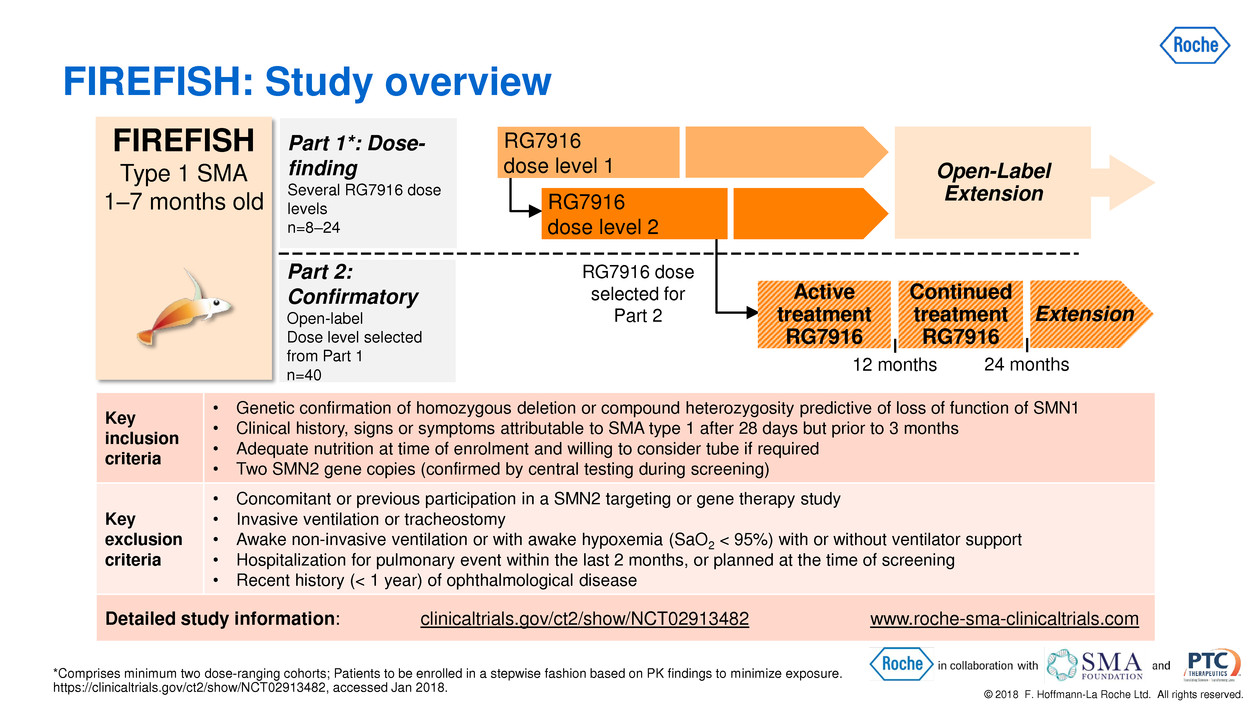
© 2018 F. Hoffmann-La Roche Ltd. All rights reserved. *Comprises minimum two dose-ranging cohorts; Patients to be enrolled in a stepwise fashion based on PK findings to minimize exposure. https://clinicaltrials.gov/ct2/show/NCT02913482, accessed Jan 2018. FIREFISH: Study overview FIREFISH Type 1 SMA 1–7 months old Part 2: Confirmatory Open-label Dose level selected from Part 1 n=40 Active treatment RG7916 12 months 24 months Extension Part 1*: Dose- finding Several RG7916 dose levels n=8–24 RG7916 dose selected for Part 2 Continued treatment RG7916 Open-Label Extension RG7916 dose level 1 RG7916 dose level 2 Key inclusion criteria • Genetic confirmation of homozygous deletion or compound heterozygosity predictive of loss of function of SMN1 • Clinical history, signs or symptoms attributable to SMA type 1 after 28 days but prior to 3 months • Adequate nutrition at time of enrolment and willing to consider tube if required • Two SMN2 gene copies (confirmed by central testing during screening) Key exclusion criteria • Concomitant or previous participation in a SMN2 targeting or gene therapy study • Invasive ventilation or tracheostomy • Awake non-invasive ventilation or with awake hypoxemia (SaO2 < 95%) with or without ventilator support • Hospitalization for pulmonary event within the last 2 months, or planned at the time of screening • Recent history (< 1 year) of ophthalmological disease Detailed study information: clinicaltrials.gov/ct2/show/NCT02913482 www.roche-sma-clinicaltrials.com
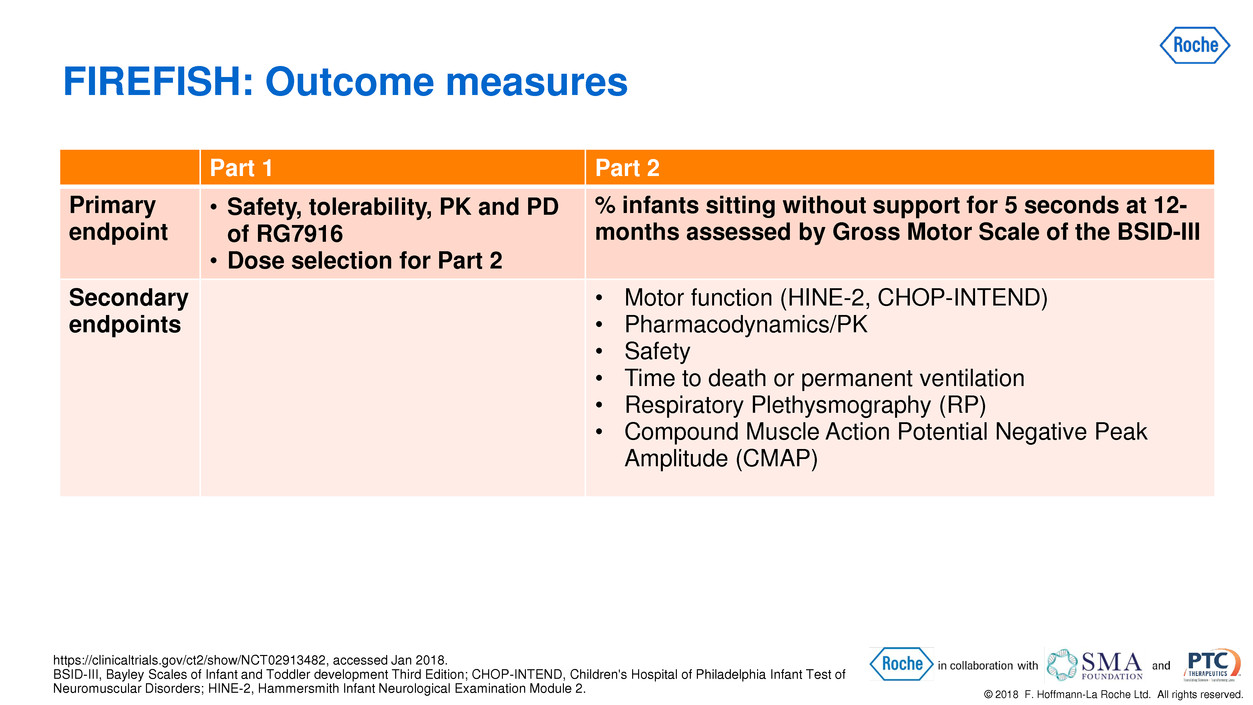
© 2018 F. Hoffmann-La Roche Ltd. All rights reserved. Part 1 Part 2 Primary endpoint • Safety, tolerability, PK and PD of RG7916 • Dose selection for Part 2 % infants sitting without support for 5 seconds at 12- months assessed by Gross Motor Scale of the BSID-III Secondary endpoints • Motor function (HINE-2, CHOP-INTEND) • Pharmacodynamics/PK • Safety • Time to death or permanent ventilation • Respiratory Plethysmography (RP) • Compound Muscle Action Potential Negative Peak Amplitude (CMAP) FIREFISH: Outcome measures https://clinicaltrials.gov/ct2/show/NCT02913482, accessed Jan 2018. BSID-III, Bayley Scales of Infant and Toddler development Third Edition; CHOP-INTEND, Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders; HINE-2, Hammersmith Infant Neurological Examination Module 2.

© 2018 F. Hoffmann-La Roche Ltd. All rights reserved. Patient demographics and baseline characteristics from the first 13 patients and study status IQR=interquartile range. Data current as of December 7, 2017. RG7916 is an investigational medicine and benefit/risk profile has not yet been fully established. The information presented is from early interim analysis. All Treatments (N=13) Age at first dose (months) Median (IQR) 6.9 (6.3–6.9) Gender Female, n (%) 10 (76.9) Weight at baseline (g) Median (IQR) 6720 (5650–7600) Age at diagnosis (months) Median (IQR) 3.5 (2.1–4.6) • Part 1 (dose-finding) screening ongoing with a total of 16 patients enrolled* • 10 active sites: Italy, France, USA, Belgium, Switzerland, Turkey • Data presented here are from the first 13 patients recruited • Part 2 expected to start Q1 2018 *Status Jan 5, 2018
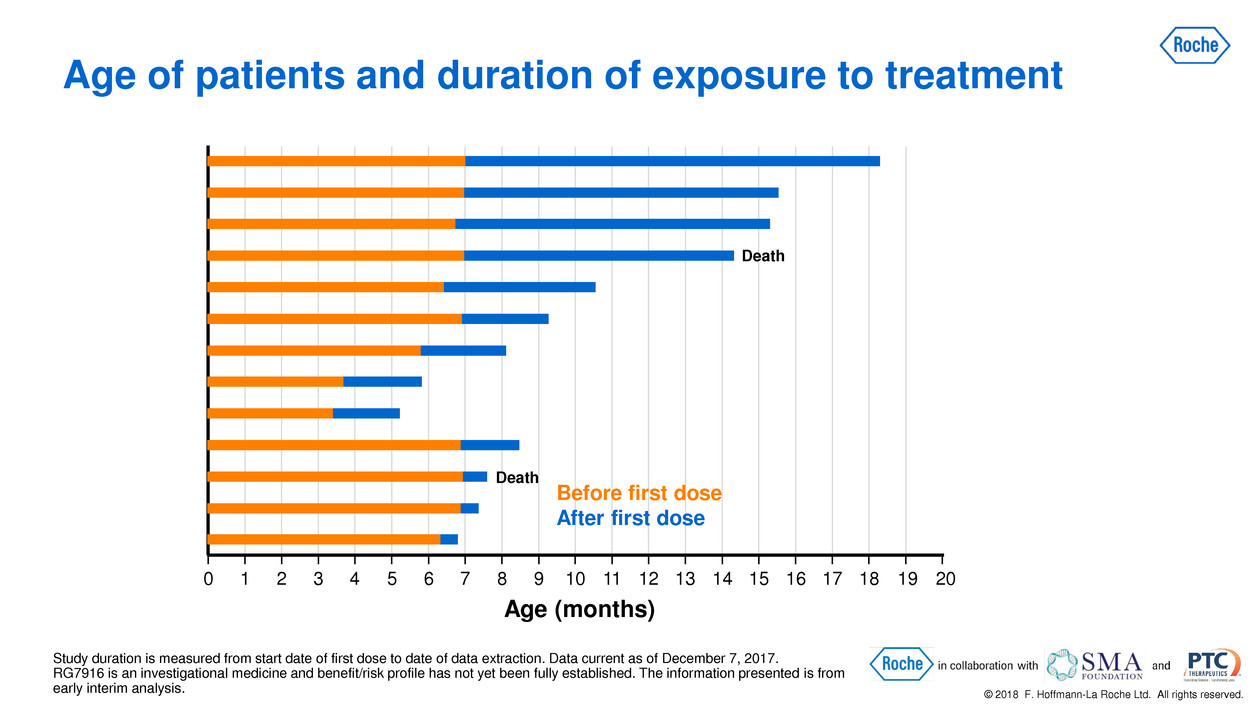
© 2018 F. Hoffmann-La Roche Ltd. All rights reserved. Age of patients and duration of exposure to treatment Study duration is measured from start date of first dose to date of data extraction. Data current as of December 7, 2017. RG7916 is an investigational medicine and benefit/risk profile has not yet been fully established. The information presented is from early interim analysis. 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Death Death Age (months) Before first dose After first dose
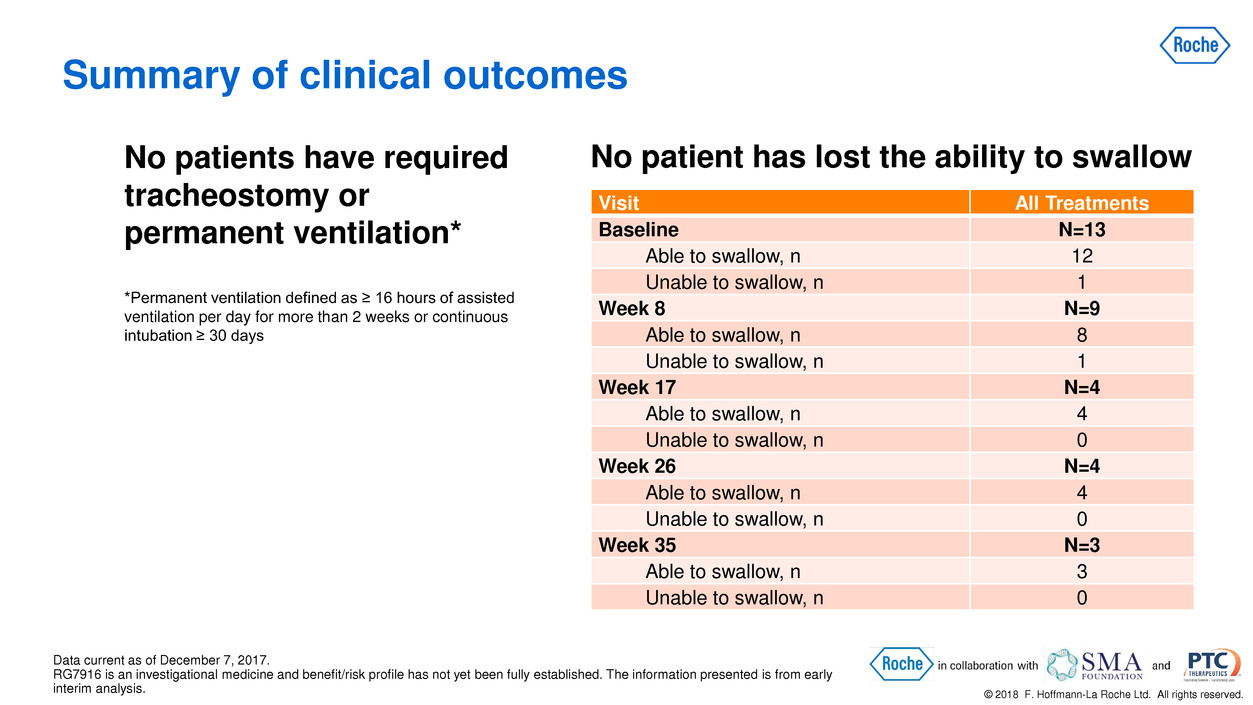
© 2018 F. Hoffmann-La Roche Ltd. All rights reserved. No patient has lost the ability to swallow Data current as of December 7, 2017. RG7916 is an investigational medicine and benefit/risk profile has not yet been fully established. The information presented is from early interim analysis. Visit All Treatments Baseline N=13 Able to swallow, n 12 Unable to swallow, n 1 Week 8 N=9 Able to swallow, n 8 Unable to swallow, n 1 Week 17 N=4 Able to swallow, n 4 Unable to swallow, n 0 Week 26 N=4 Able to swallow, n 4 Unable to swallow, n 0 Week 35 N=3 Able to swallow, n 3 Unable to swallow, n 0 Summary of clinical outcomes No patients have required tracheostomy or permanent ventilation* *Permanent ventilation defined as ≥ 16 hours of assisted ventilation per day for more than 2 weeks or continuous intubation ≥ 30 days

© 2018 F. Hoffmann-La Roche Ltd. All rights reserved. Summary of safety outcomes • Overall 10 (77%) out of 13 patients experienced at least one adverse event. Most events were mild in intensity and resolved despite ongoing treatment • Adverse events reported in more than one patient were pyrexia (n=5), upper respiratory tract infection (n=3), diarrhea (n=2), vomiting (n=2), erythema (n=2) • Serious adverse events were reported in four patients: respiratory tract infection viral, pneumonia & neutropenia, acute respiratory failure and hypoxia • Ophthalmological monitoring conducted every 2 months did not show any evidence of the retinal toxicity seen in preclinical monkey studies in any patient exposed to RG7916 • Fatal events were reported in two patients: • Respiratory tract infection viral with fatal outcome on study Day 21 • Cardiac arrest and respiratory arrest with fatal outcome on study Day 236* *Event reported after cut off date November 15, therefore not included in serious adverse events count. Reference: interim safety summary BP39056 part 1 dated 08/01/2018
© 2018 F. Hoffmann-La Roche Ltd. All rights reserved. • Working with patients with Type 1 SMA presents several challenges, mainly related to the age and the severity of the disease • Challenges can affect the infants, family, and staff involved in the studies • As SMA (and especially Type 1) is a rare and devastating disease, we are facing a GLOBALIZATION of clinical research • Families in countries where competing trials and therapies (eg, nusinersen) are not available may ask to be recruited to studies or to access therapies • This may accelerate recruitment and development of new treatments • Give access to potential treatment to a higher number of patients Tips from real-life experience during the implementation of the FIREFISH study

© 2018 F. Hoffmann-La Roche Ltd. All rights reserved. • Real-life experience from investigators must be used to best support patients and families during the FIREFISH trial • The importance of support from advocacy groups and patient/family organizations • Key considerations when conducting a study in Type 1 SMA include: • The need to coordinate a multi-disciplinary team of healthcare specialists dealing with such young babies • The support to relocate families away from their home country • The importance of assuring standard-of-care practices whilst patients participate in the trial Tips from real-life experience during the implementation of the FIREFISH study

© 2018 F. Hoffmann-La Roche Ltd. All rights reserved. • Families in countries where competing trials and therapies (eg, nusinersen) are not available may ask to be recruited to studies or to access therapies • Resource limitations and cultural differences • Relocation is important for the safety of the child and for the good conduct of the study Family relocation

© 2018 F. Hoffmann-La Roche Ltd. All rights reserved. SMA standard of care implementation has increased survival and improved quality of life of children and their families Increased survival Improved quality of life Rehabilitation Orthopedic Care/ Orthosis Psycho-social support Nutritional care (growth/ under-nutrition) Pulmonary care Respiratory support Gastro-intestinal management Intensive Care/ Emergency Patient with SMA & family Bone health Other organ involvement

© 2018 F. Hoffmann-La Roche Ltd. All rights reserved. • A proactive and anticipatory approach is essential to modify the course of the disease • Changing phenotype should be paralleled and supported by the implementation of standard of care (eg, postural control or standing frame as long as the child reaches new motor milestones, etc) The application of SoC remains essential despite the emerging therapies • Need of consistency of management within the study (involving different sites in different countries) • Need for training and dissemination of experience among sites and countries SoC, standard of care. Clinical trials involving relocation should ensure multidisciplinary management

© 2018 F. Hoffmann-La Roche Ltd. All rights reserved. Conclusions • To date, RG7916 has been safe and well tolerated at all doses and there have been no drug-related safety findings leading to withdrawal in any SMA patients exposed to RG7916 • Ophthalmologic monitoring did not show any evidence of the retinal toxicity seen in preclinical monkey studies in any patient exposed to RG7916 • Early interim clinical data reported: No patient lost the ability to swallow No patient has required tracheostomy or reached permanent ventilation • Important considerations in conducting such clinical studies: Co-ordination of a multi-disciplinary team Family relocation Application of standard-of-care practices • Study updates will continue to be communicated at congresses in 2018 • Part 2 of the study is expected to start in Q1 2018

© 2018 F. Hoffmann-La Roche Ltd. All rights reserved. We thank all the patients who participate in these studies and their families. We thank our collaborators PTC Therapeutics and SMA Foundation. We thank the FIREFISH, SUNFISH, and JEWELFISH investigators and trial staff. Acknowledgments

















