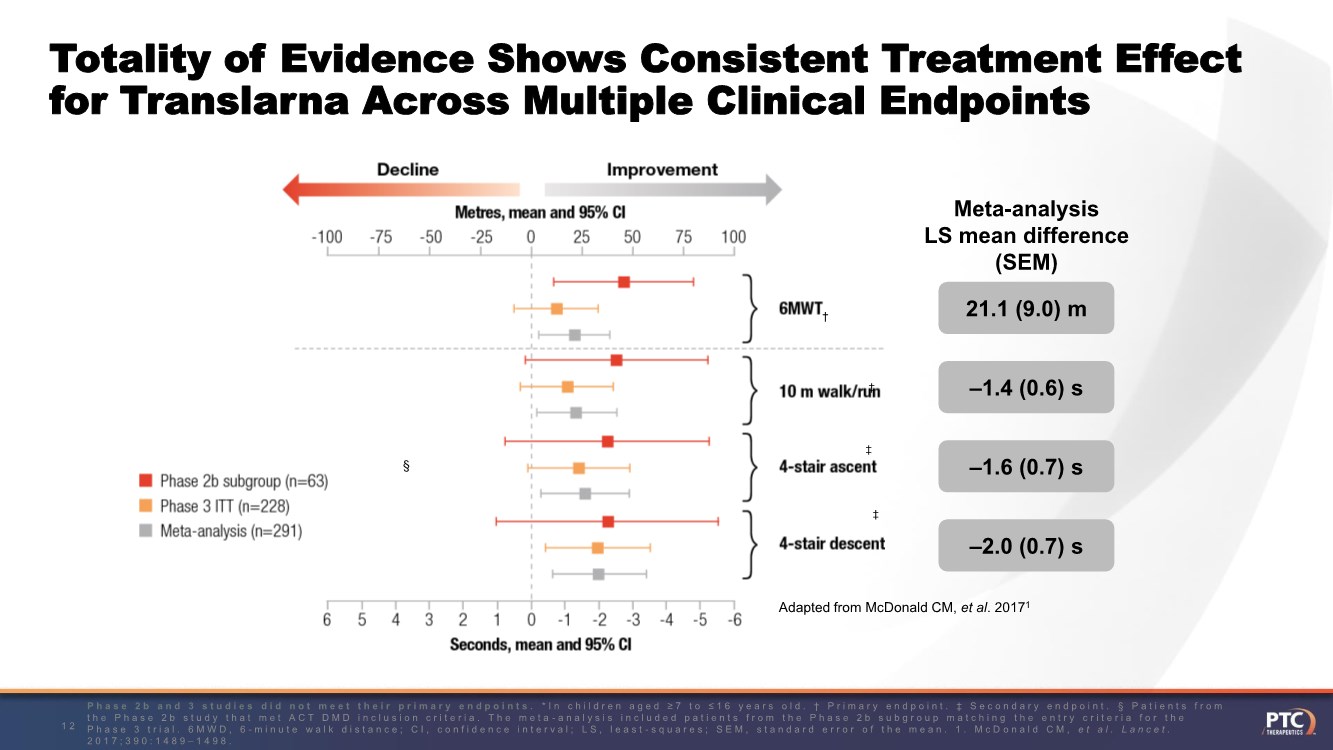
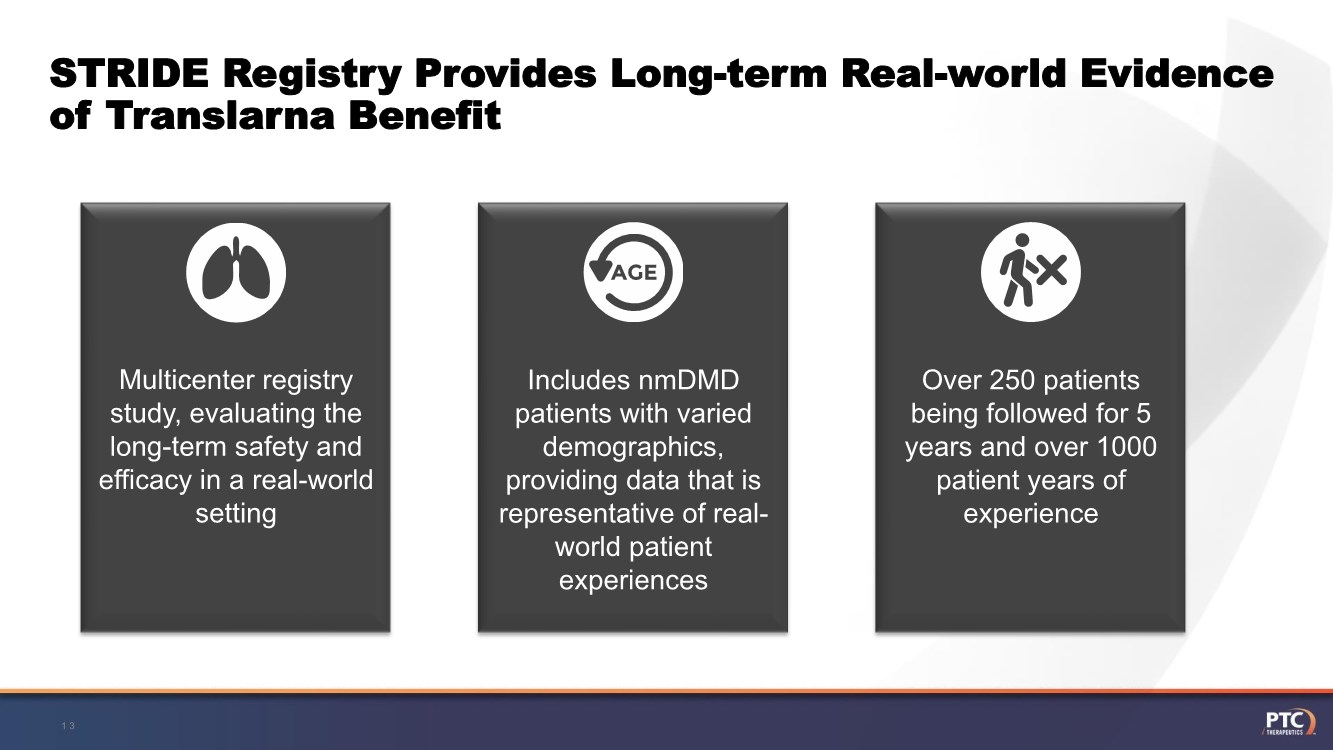
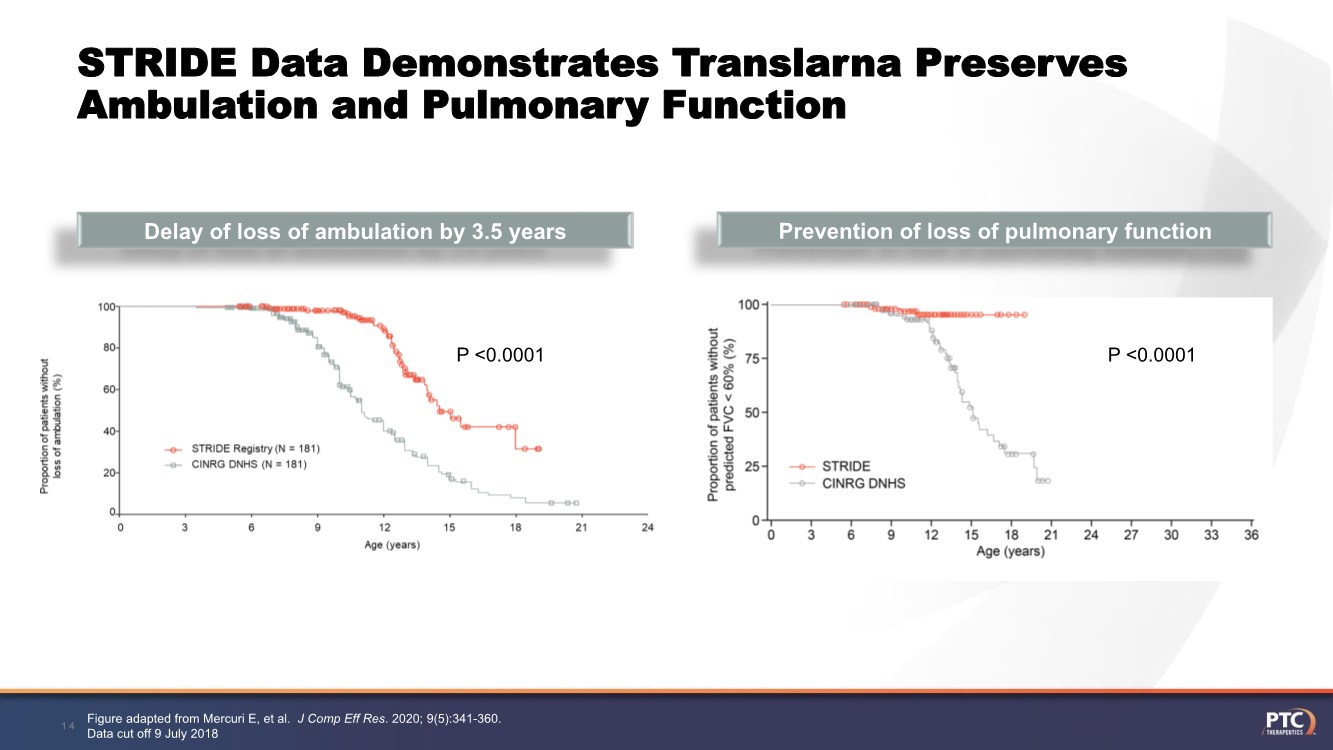
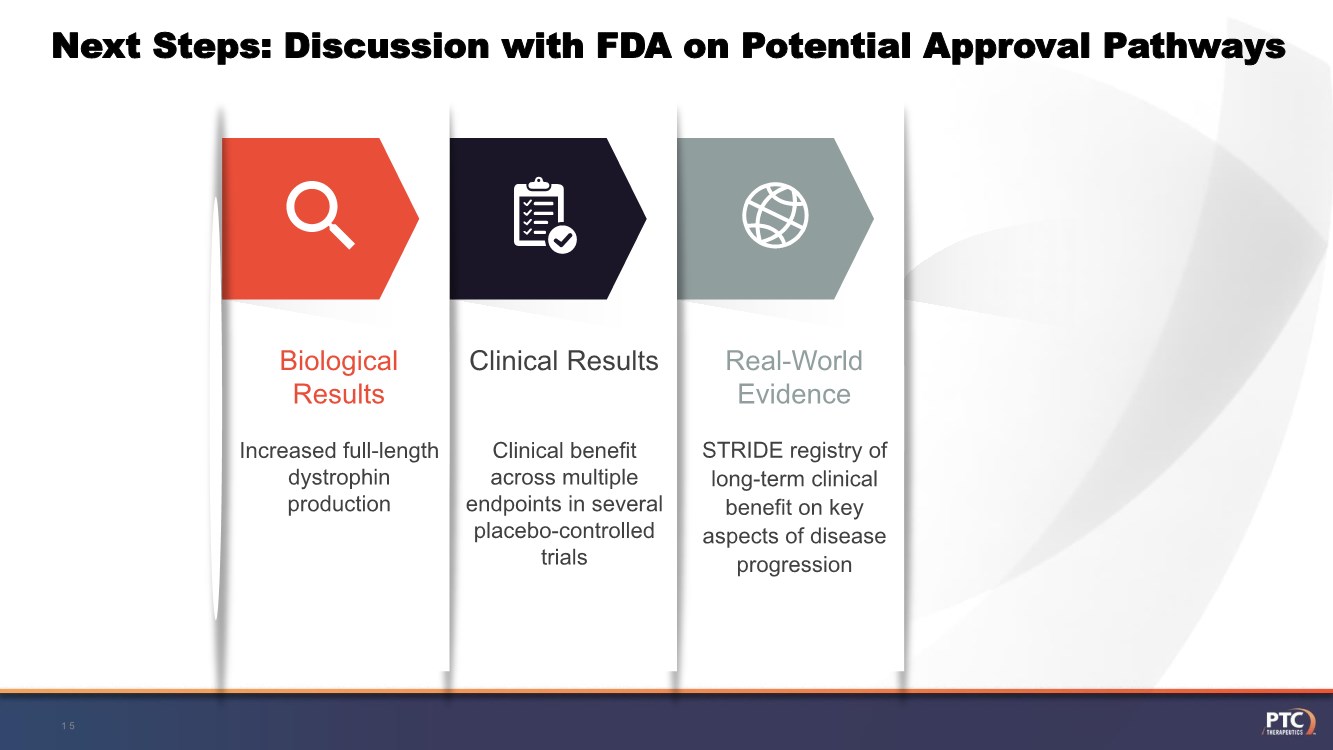
| Forward Looking Statement This presentation contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995 . All statements contained in this presentation, other than statements of historic fact, are forward -looking statements, including statements regarding: the future expectations, plans and prospects for PTC, including with respect to the commercialization of its products and product candid ates; PTC's plans for interactions with the FDA; the clinical utility and potential advantages of Translarna; PTC's strategy, future operations, fu ture financial position, future revenues, projected costs; and the objectives of management. Other forward -looking statements may be identified by the words "guidance", "plan," "anticipate," "believe," "estimate," "expect," "intend," "may," "target," "potential," "will," "would," "could," "should," "c ontinue," and similar expressions. PTC's actual results, performance or achievements could differ materially from those expressed or implied by forward -looking statements it makes as a result of a variety of risks and uncertainties, including those related to: the outcome of pricing, coverage and reimbursemen t negotiations with third party payors for PTC's products or product candidates that PTC commercializes or may commercialize in the future; PTC's abili ty to support a re- submission of its Translarna NDA for the treatment of nonsense mutation Duchenne muscular dystrophy ( nmDMD) to the FDA, and PTC's ability to perform any necessary clinical trials, non-clinical studies, and CMC assessments or analyses at significant cost; PTC's ability to maintain its marketing authorization of Translarna for the treatment of nmDMD in the European Economic Area (EEA), including whether the European Medicines Agency (EMA) determines in future annual renewal cycles that the benefit -risk balance of Translarna authorization supports renew al of such authorization; PTC's ability to enroll, fund, complete and timely submit to the EMA the results of Study 041, a randomized, 1 8-month, placebo-controlled clinical trial of Translarna for the treatment of nmDMD followed by an 18-month open-label extension, which is a specific obligation to continued marketing authorization in the EEA; whether regulators will agree with PTC’s characterization of the results of PTC’s clinica l trials including demonstration of meaningful clinical benefit of its products and product candidates; significant business effects, including the effects of industry, market, economic, political or regulatory conditions; changes in tax and other laws, regulations, rates and policie s; the eligible patient base and commercial potential of PTC's products and product candidates; PTC's scientific approach and general development progress ; and the factors discussed in the "Risk Factors" section of PTC's most recent Quarterly Report on Form 10 -Q and Annual Report on Form 10-K, as well as any updates to these risk factors filed from time to time in PTC's other filings with the SEC. You are urged to carefully consider all such factors. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commerc ialization of new products. There are no guarantees that any product will receive or maintain regulatory approval in any territory, or prove to be commercially successful, including Translarna. The forward-looking statements contained herein represent PTC's views only as of the date of this presentation and PTC does not undertake or plan to update or revise any such forward-looking statements to reflect actual results or changes in plans, prospects, assumptions, esti mates or projections, or other circumstances occurring after the date of this presentation except as required by law. 2 |