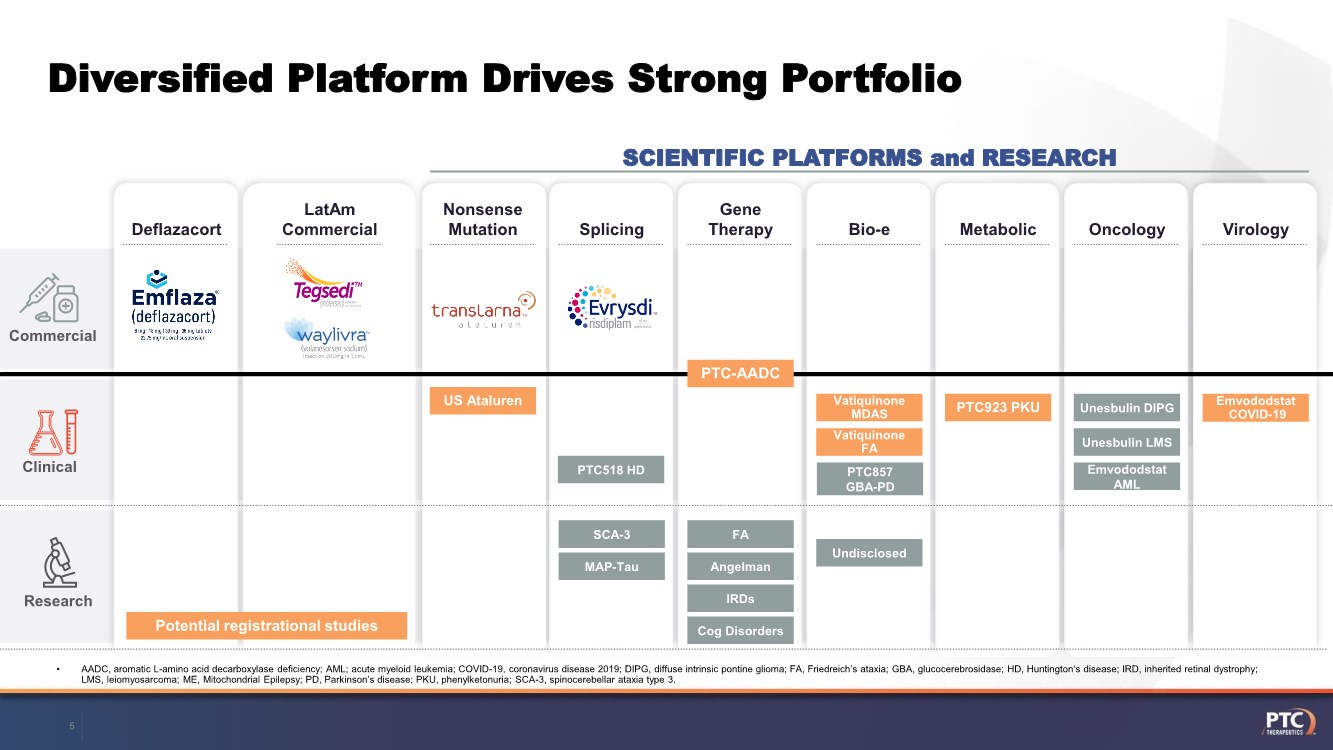
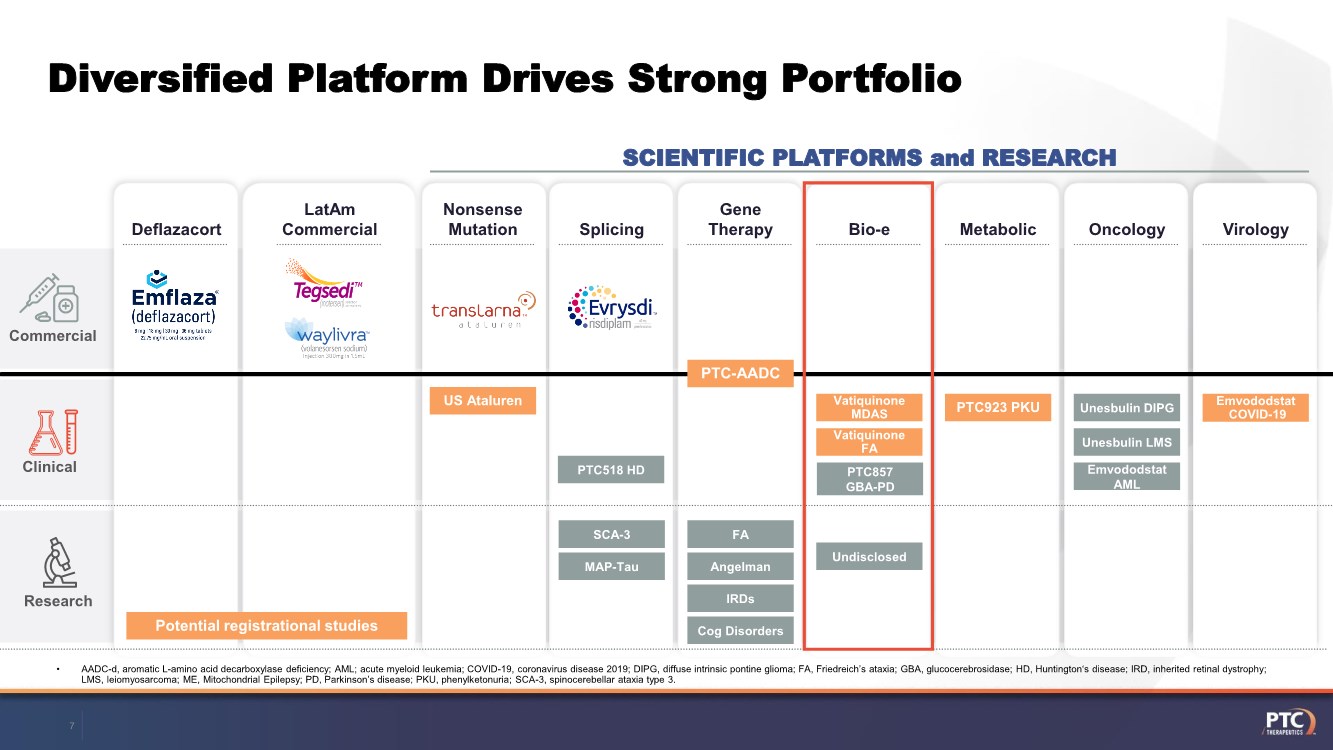
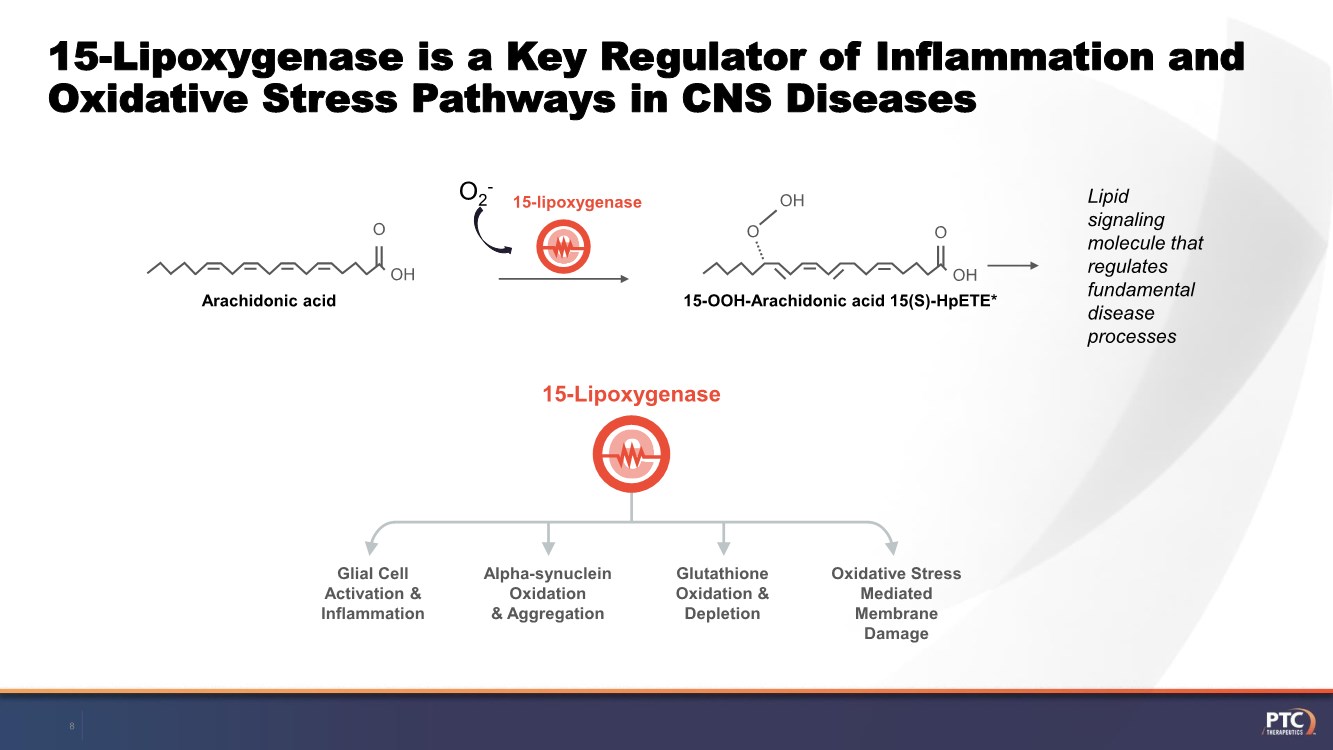
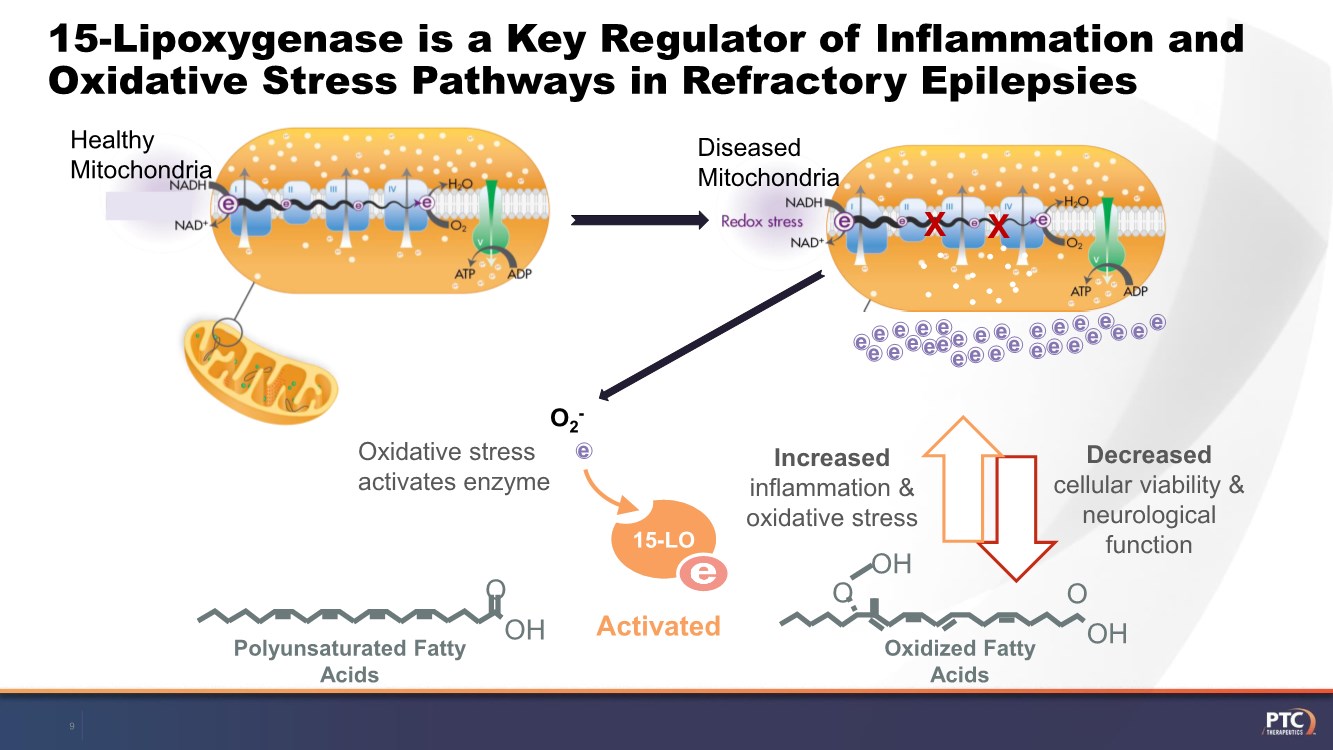
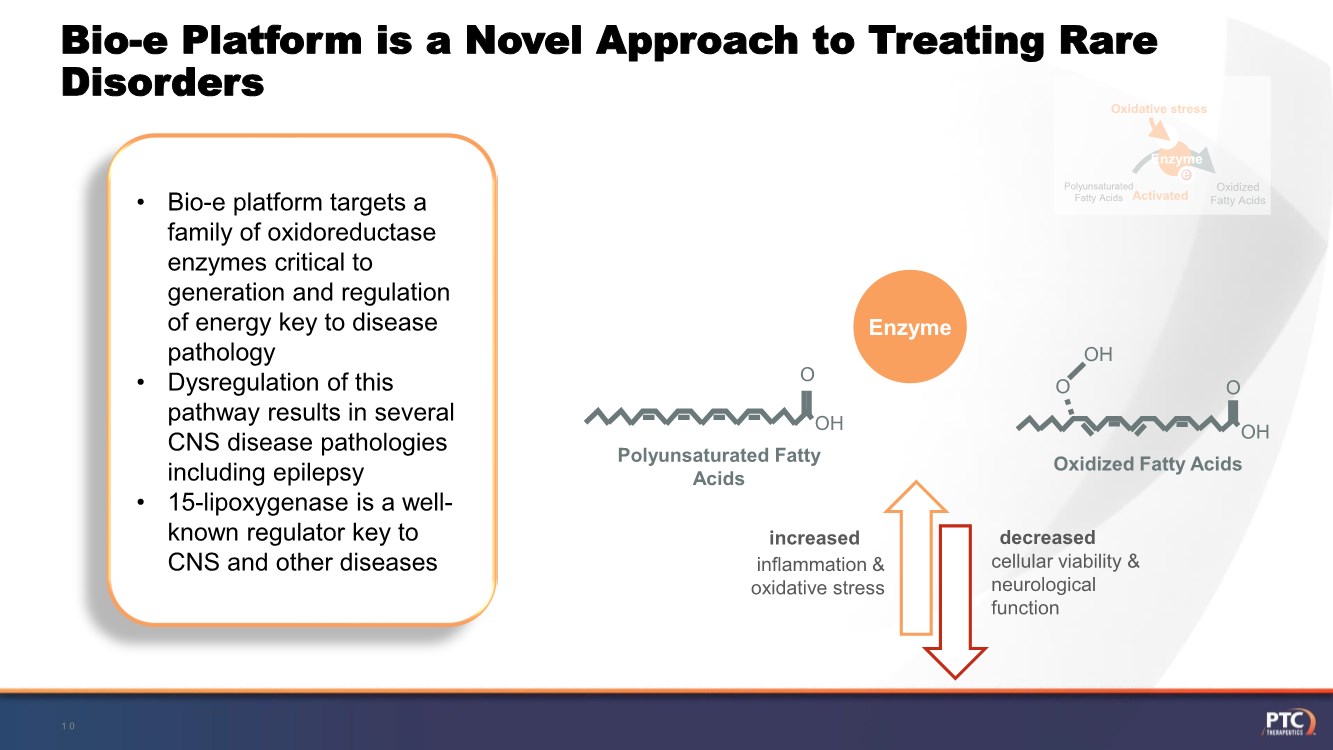
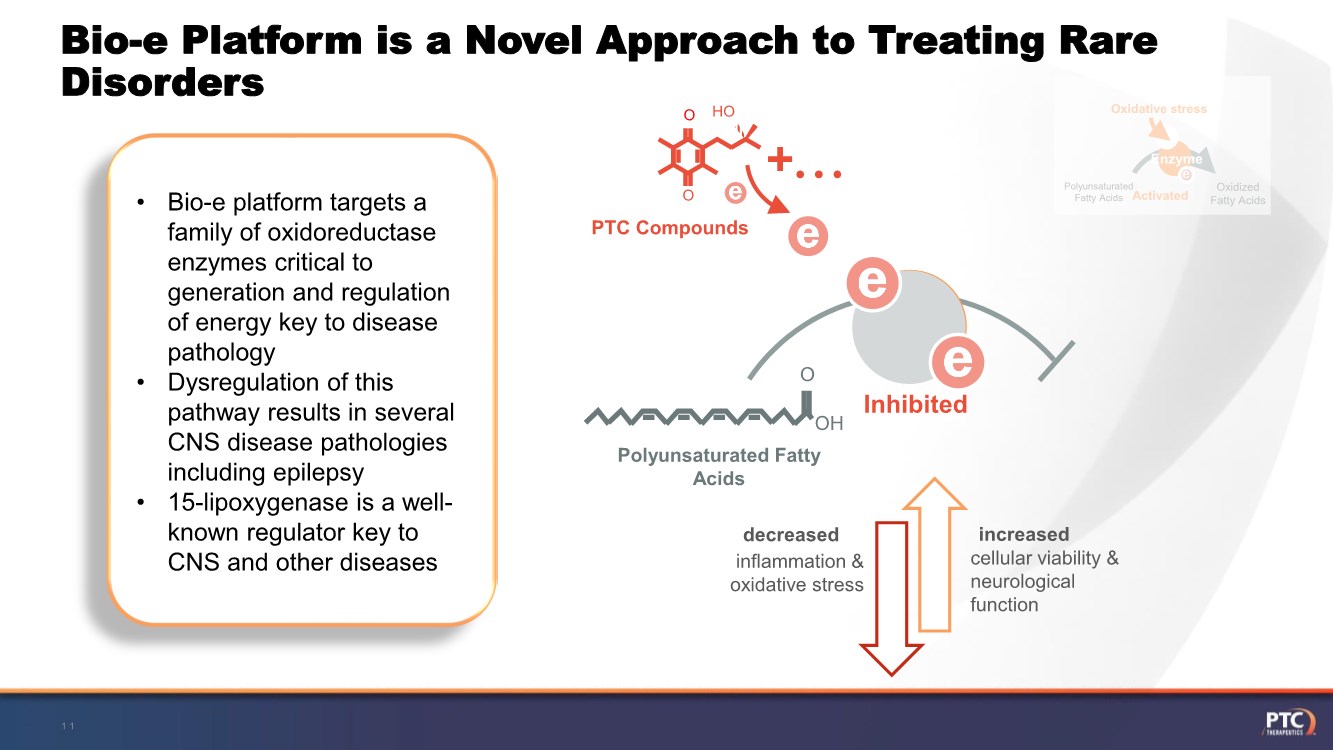
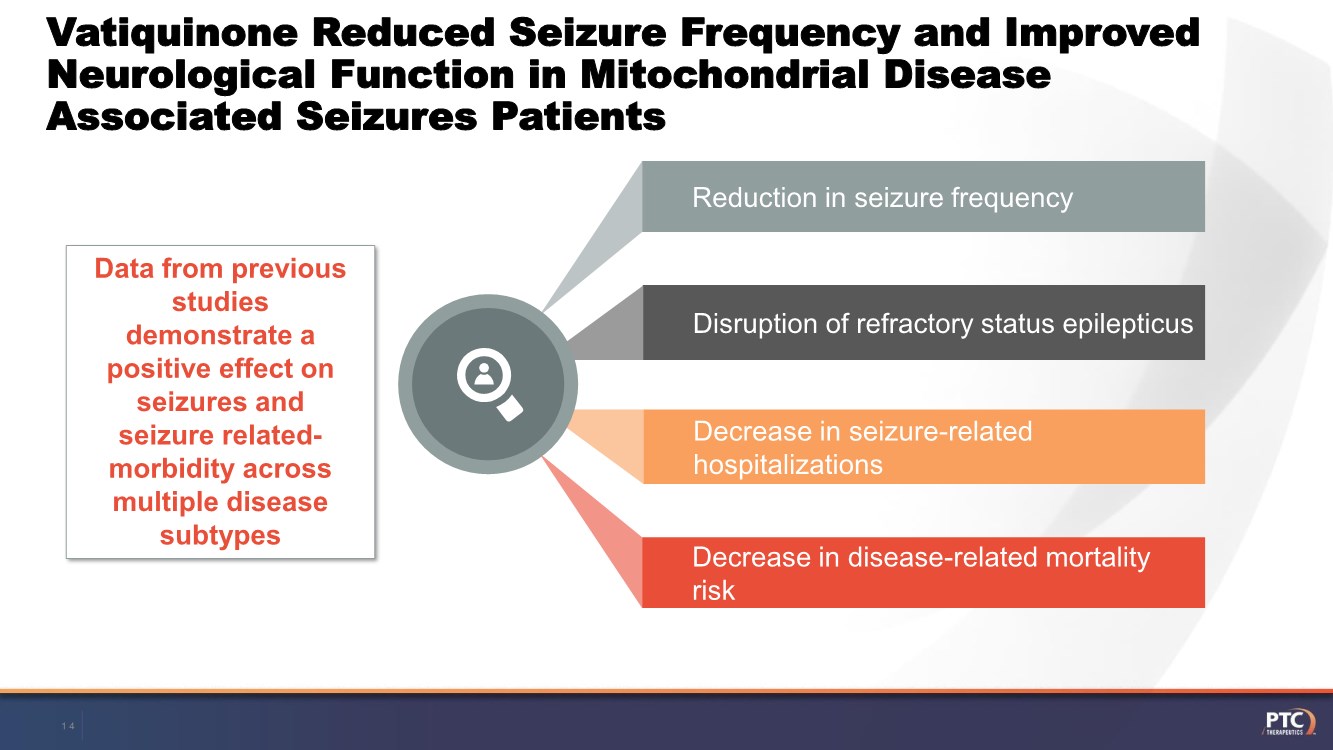
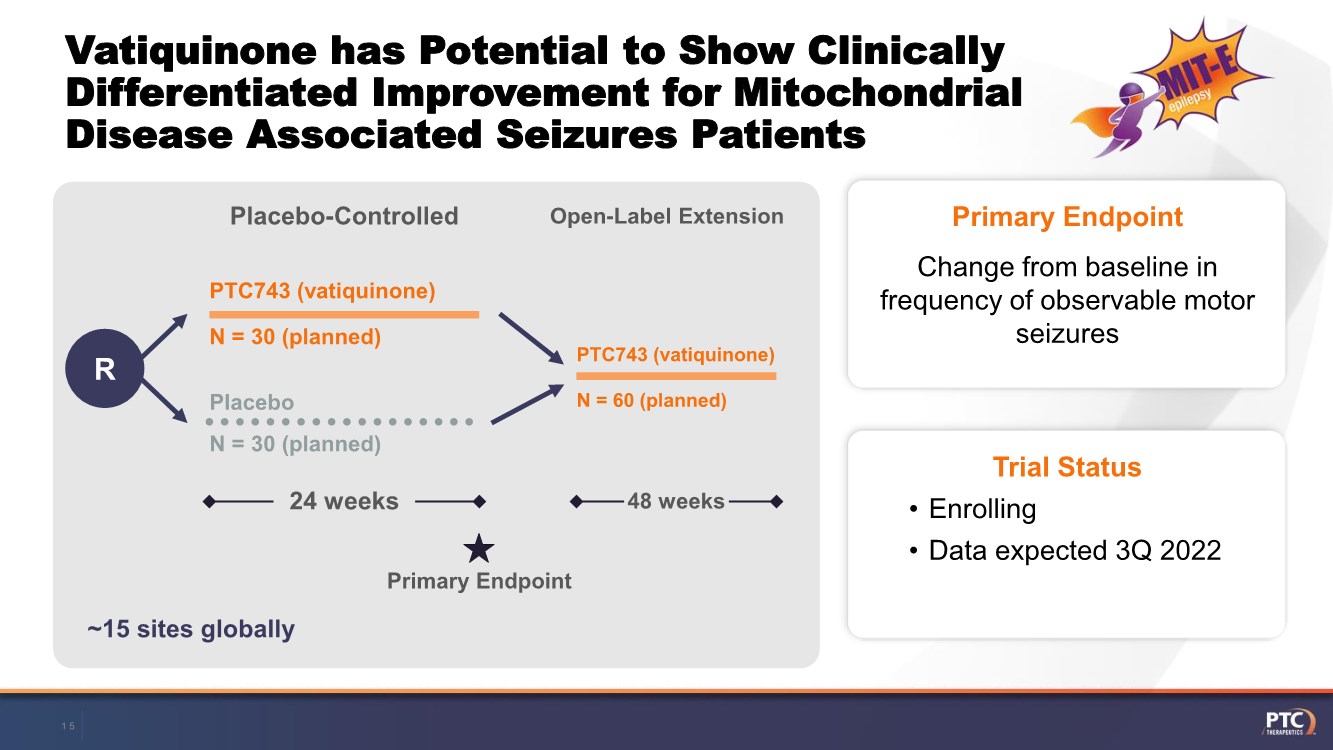
| Forward Looking Statements This presentation contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 199 5. All statements contained in this release, other than statements of historic fact, are forward -looking statements, including statements with respect to 2021 net product revenue guidance, statements with respect to the 2021 operating expenditure guidance and statements regarding: the future expectations, plans and prospects for PTC, including with respect to the expected timing of clinical trials and studies, availability of data, regulatory submissions and responses and other matters; expectations with respect to PTC's gene therapy platform, including any potential regulatory submissions and manufacturing capabilities; advancement of PTC's joint c ollaboration program in SMA, including any potential regulatory submissions, commercialization or royalty or milestone payments; PTC's expectations with respect to the licensing, regulatory submissions and commercialization of its products and product candidates; the timing with respect to orders for PTC's products; PTC's strategy, future operations, future financial position, future revenues, projected costs; and the objectives of management. Other forward -looking statements may be identified by the words "guidance", "plan," "anticipate," "believe," "estimate," "expect," "intend," "may," "target," "potential," "will," "would," "could," "should," "continue," and similar expressions. PTC's actual results, performance or achievements could differ materially from those expressed or implied by forward -looking statements it makes as a result of a variety of risks and uncertainties, including those related to: the outcome of pricing, coverage and reimbursement negotiations with third party payors for PTC's products or product candidates that PTC commercializes or may commercialize in the future; expectations with respect to PTC's gene therap y platform, including any potential regulatory submissions and potential approvals, manufacturing capabilities and the potential financial impact and benefits of its leased biologics manufacturing facility and the potential achievement of development, regulatory and sales milestones and contingent payments that PTC may be obligat ed to make; the enrollment, conduct, and results of ongoing studies under the SMA collaboration and events during, or as a result of, the studies that could delay or prevent further development under the program, including any potential regulatory submissions and potential commercialization with respect to Evrysdi; PTC's ability to complete a dystrophin study necessary to support a re-submission of its Translarna NDA for the treatment of nonsense mutation Duchenne muscular dystrophy (nmDMD) to t he FDA, and PTC's ability to perform any necessary additional clinical trials, non-clinical studies, and CMC assessments or analyses at significant cost; PTC's abili ty to maintain its marketing authorization of Translarna for the treatment of nmDMD in the European Economic Area (EEA), including whether the European Medicines Agency (E MA) determines in future annual renewal cycles that the benefit-risk balance of Translarna authorization supports renewal of such authorization; PTC's ability t o enroll, fund, complete and timely submit to the EMA the results of Study 041, a randomized, 18-month, placebo-controlled clinical trial of Translarna for the treatment of nmDMD followed by an 18-month open- label extension, which is a specific obligation to continued marketing authorization in the EEA; expectations with respect to the commercialization of Tegsedi and W aylivra™; the enrollment, conduct and results of PTC’s emvododstat clinical trial for COVID -19; expectations with respect to the COVID-19 pandemic and related response measures and their effects on PTC's business, operations, clinical trials, potential regulatory submissi ons and approvals, and PTC's collaborators, contract research organizations, suppliers and manufacturers; significant business effects, including the effects of industry , market, economic, political or regulatory conditions; changes in tax and other laws, regulations, rates and policies; the eligible patient base and commercial potentia l of PTC's products and product candidates; PTC's scientific approach and general development progress; PTC's ability to satisfy its obligations under the terms of the l ease agreement for its leased biologics manufacturing facility; the sufficiency of PTC's cash resources and its ability to obtain adequate financing in the future fo r its foreseeable and unforeseeable operating expenses and capital expenditures; and the factors discussed in the "Risk Factors" section of PTC's most recent Quarterly Rep ort on Form 10-Q and Annual Report on Form 10-K, as well as any updates to these risk factors filed from time to time in PTC's other filings with the SEC. You are urg ed to carefully consider all such factors. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commerc ialization of new products. There are no guarantees that any product will receive or maintain regulatory approval in any territory, or prove to be commercially succes sful, including Translarna, Emflaza, Evrysdi, Tegsedi, W aylivra or PTC-AADC. The forward-looking statements contained herein represent PTC's views only as of the date of this presentation and PTC does not undertake or plan to update or revise any such forward-looking statements to reflect actual results or changes in plans, prospects, assumptions, estimates or projecti ons, or other circumstances occurring after the date of this presentation except as required by law. 2 |