
Key Terms of the Transaction
Definitive merger agreement announced on June 8, 2006
Tax-free stock for stock merger
Pro forma ownership of combined company
Approximately 58% TorreyPines Therapeutics shareholders
Approximately 42% Axonyx shareholders
TorreyPines preferred shareholders also receive warrants with an
exercise price of $1.04, which, if fully exercised at closing, would
increase the percentage of the combined company held by
TorreyPines shareholders to approximately 62%
Relative percentages adjusted if either party out-licenses one or
more product candidates prior to closing
Subject to customary and other closing conditions including
shareholder approval
Name of new entity: TorreyPines Therapeutics, Inc.
Anticipated NASDAQ ticker symbol: TPTX
5

Post-merger Profile of TorreyPines
Strategically focused on CNS diseases and disorders
Two product candidates for migraine and chronic pain, representing
potentially early commercialization opportunity
Six product candidates to potentially treat broad and growing
cognitive disorders market in AD and schizophrenia
Discovery function, funded by collaborations with Eisai
Focus on large markets with documented unmet needs
Development team with proven CNS track record
Rights to more than 250 pending or issued patents
Combined pro forma cash/equivalents of $ 77.6 M at 6/30/06
Creating a Premier CNS Biopharmaceutical Company
6

Post-merger TorreyPines Executive Team
TPTX
TPTX
TPTX
TPTX
TPTX
Company
MitoKor, PriceWaterhouse
CFO
Craig Johnson
Ingenix, Co-founder
Worldwide Clinical Trials,
Boots, Bayer, BMS, Merck
President
& CEO
Neil Kurtz, MD
Purdue Pharma, Ingenix,
Worldwide Clinical Trials,
Bayer, Wyeth (Ayerst)
COO
Evelyn Graham, MBA
Ingenix, Worldwide Clinical
Trials, Hoechst, Cephalon
CMO
Mike Murphy, MD, PhD
CSO
Title
SIBIA Neurosciences, UCI
Steve Wagner, PhD
Background
Name
Big Pharma Pedigrees Plus CRO Operational Expertise
7
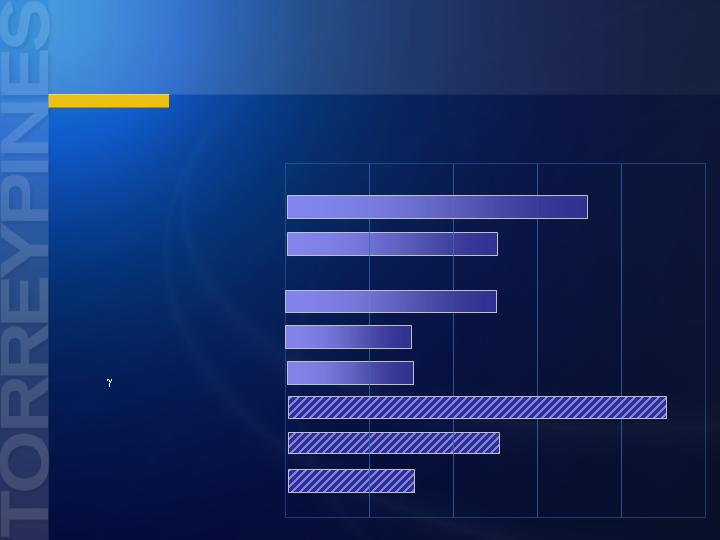
Post-merger TorreyPines Pipeline
Preclinical
Phase I
Phase II
Phase III
Phenserine
Acetylcholinesterase Inhibitor
Posiphen™
Beta-amyloid Inhibitor
NGX267
M-1 Agonist
Cognitive Disorders
NGX292
M-1 Agonist
NGX555
-Secretase Modulator
Discovery
Pain
Tezampanel (NGX424)
AK Antagonist
NGX426
AK Antagonist
BNC (Bisnorcymserine)
Butyrylcholinesterase Inhibitor
Migraine
Migraine
AD Progression
AD, Schizophrenia
AD Progression
AD Progression
AD - Severe
AD - Mild to Moderate
8

Tezampanel (NGX424) and NGX426
Novel and selective agents that
cross the blood-brain barrier
Bind specifically to subset of
ionotropic, non-NMDA
glutamate receptors located
only in brain and spinal cord
GluR1, GluR2, and GluR5
receptors key transmitters in
pain pathway
Unique pharmacology
Non-opioid
Non-vascular
Theoretically, no abuse
potential
Pain Transmission Pathway
AMPA/Kainate Receptor Antagonists
9
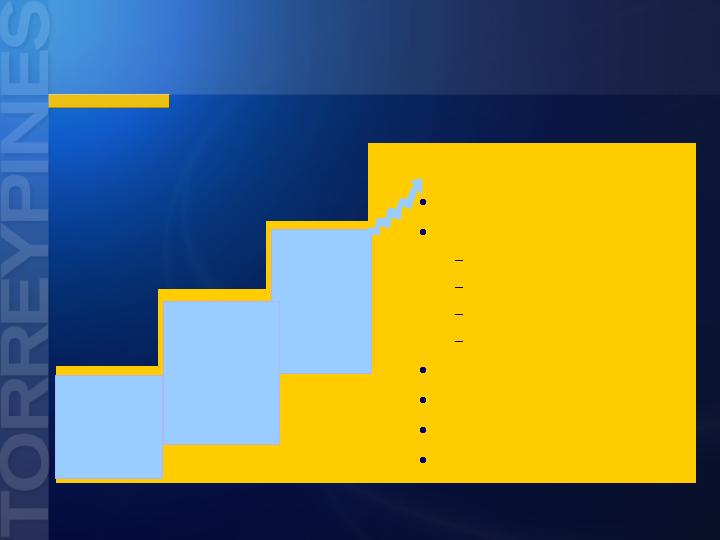
Tezampanel and NGX426
Broad Commercial Opportunities
Predictable
Animal Data
- analgesia
- spasticity
- epilepsy
Multiple
ROAs
- Oral
- SC
- IV
- EP
- IT
Market Segments
Migraine
Analgesia
Neuropathic pain
Cancer pain
Chronic intractable pain
Post-operative pain
Epilepsy
Neuroprotection
Muscle spasticity/rigidity
Anesthesia
Proof of
Concept
Data
- migraine
- neuropathy
- back pain
- post-op pain
Migraine = Fast to Market Plan
10

Tezampanel and NGX426
Addressing Unmet Needs of Migraine Sufferers
Effective in patients unresponsive to triptans
Effective in patients who have side effects on triptans
Alternative to
triptans
Effective in patients with prominent sensitivity
to sound, touch, light, and temperature
Target the
underlying
disease process
Use in patients with cardiovascular risk factors
Co-administration with SSRIs and SNRIs
Given as multiple doses over 24 hours
Fewer
prescribing
restrictions
Expand ER
treatment
options
Need
Alternative to opioids
Parenteral and oral forms for ER to home care
Tezampanel, NGX426
11

Tezampanel
Lead compound, parenteral administration
Completed 7 studies: 2 Phase I, 5 Phase IIa
Total of 92 healthy volunteers and 113 patients
exposed to tezampanel to date
Demonstrated safety and efficacy as treatment for
migraine and neuropathic pain
Initial indication: migraine, a form of chronic
intermittent pain
Planning Phase IIb study in migraine
Development Highlights
12

Tezampanel, IV Administration
Ketorolac
DB, PC
70
Post-operative
Dental Pain
Nociceptive*
Imitrex
DB, PC
45
Migraine
Migraine*
Neuropathic
Neuropathic
Neuropathic*
Model
NA
DB, PC
Three period X-over
25
Capsaicin
Provoked Pain
Ketamine
Lidocaine
DB, PC
3x3 Latin Square X-over
12
Low Back Pain
Lidocaine
DB, PC
12
Spinal Cord
Injury
Control
Design
N =
Population
Five Positive Proof of Concept Studies
* Published Studies
13
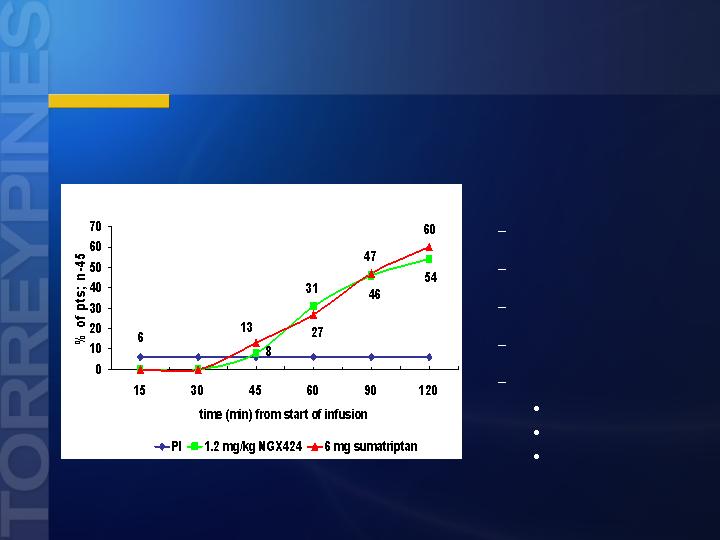
*p< 0.05; **p< 0.01 (chi-square test); contrast vs placebo (Sang et al, Cephalalgia, 2004)
*
**
Pain Free at 2 Hours
Tezampanel IV Migraine Study
Statistically Significant:
Pain relief at 2 hours
Pain free at 2 hours
Sustained pain relief
Sustained pain free
Decreased incidence of
Nausea
Photophobia
Phonophobia
Statistically Significant Across All FDA Endpoints
14

NGX426
Oral formulation of tezampanel
Initial indication: migraine
Follow-on indication: chronic neuropathic pain
NGX426 expands the pain franchise across multiple
segments of the chronic pain market
First-in-human study initiated August 4, 2006
Results expected in 4Q 2006
Development Highlights
15
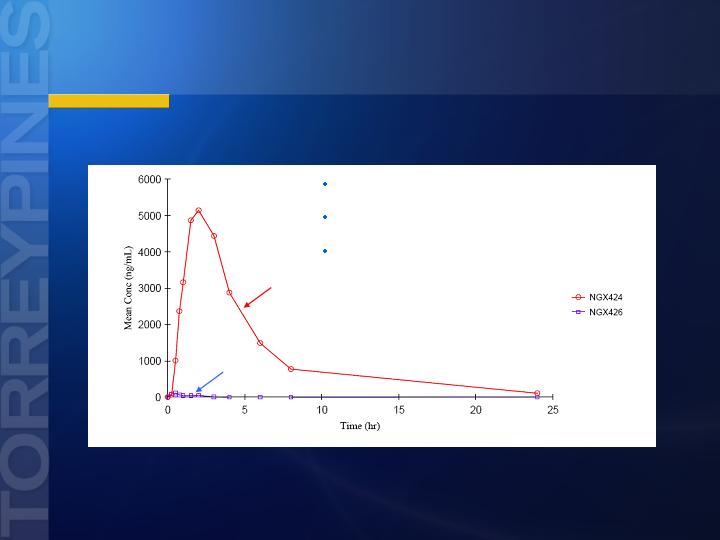
NGX426, Oral Study in Dog
Rapid Conversion to Tezampanel After Oral NGX426
n = 2 M, 2 F dogs
NGX426: Tmax =1.00; t1/2 =1.05
Tezampanel (NGX424): Tmax = 2.33; t ½= 4.05
Oral administration of single dose 20 mg/kg NGX426 in beagle dogs
Tezampanel
NX426
16
Post-merger TorreyPines Pipeline
Preclinical
Phase I
Phase II
Phase III
Phenserine
Acetylcholinesterase Inhibitor
Posiphen™
Beta-amyloid Inhibitor
NGX267
M-1 Agonist
Cognitive Disorders
NGX292
M-1 Agonist
NGX555
-Secretase Modulator
Discovery
Pain
Tezampanel (NGX424)
AK Antagonist
NGX426
AK Antagonist
BNC (Bisnorcymserine)
Butyrylcholinesterase Inhibitor
Migraine
Migraine
AD Progression
AD, Schizophrenia
AD Progression
AD Progression
AD - Severe
AD - Mild to Moderate
17

NGX267
Lead M1 Agonist for Cognitive Disorders
Safe and well tolerated in two Phase I studies
Total of 24 healthy adult males, average age 35
Total of 20 healthy elderly, average age 73
Biomarker evidence of selective stimulation of M1 receptor in man
as measured by increased salivation
Achieve blood levels in man comparable to those described in
transgenic mouse study, published in Neuron, showing NGX267
lowered levels of brain Aß42
Data also support development as treatment for cognitive
impairment associated with schizophrenia
NGX292, follow-on M1 agonist, is desmethyl derivative of NGX267
18

NGX555
- secretase Modulator from TPTX Discovery Program
Distinctly different from -secretase inhibitors
Selective inhibition of A 42 and A 40 with concomitant
increase in A 37 and A 38
No inhibition of processing of other -secretase
substrates, e.g., Notch, e-Cadherin
Lowered A 42 in plasma and brain when administered orally
in transgenic animal models
19

TorreyPines Near-term Deliverables
File IND for NGX426 and start Phase I study
Report results for NGX426 Phase I study
Initiate Phase IIb tezampanel study for migraine
Migraine,
Chronic
Pain
Cognitive
Disorders
Program
Report data from NGX267 Phase I elderly study
Initiate multiple dose Phase I study with NGX267
Milestone
20



















