Filed Pursuant to Rule 424(b)(3)
File Number 333-139880
PROSPECTUS SUPPLEMENT NO. 8
Prospectus Supplement dated April 10, 2007
to Prospectus Declared Effective on January 24, 2007
(Registration No. 333-139880)
As Supplemented by that Prospectus Supplement No. 1 dated January 31, 2007,
that Prospectus No. 2 dated February 12, 2007
that Prospectus Supplement No. 3 dated February 20, 2007,
that Prospectus Supplement No. 4 dated February 26, 2007,
that Prospectus Supplement No. 5 dated March 1, 2007,
that Prospectus Supplement No. 6 dated March 5, 2007,
and that Prospectus Supplement No. 7 dated April 4, 2007.
AURIGA LABORATORIES, INC.
This Prospectus Supplement No. 8 supplements our Prospectus dated January 24, 2007, the Prospectus Supplement No. 1 dated January 31, 2007, the Prospectus Supplement No. 2 dated February 12, 2007, the Prospectus Supplement No. 3 dated February 20, 2007, the Prospectus Supplement No. 4 dated February 26, 2007, the Prospectus Supplement No. 5 dated March 1, 2007, the Prospectus Supplement No. 6 dated March 5, 2007 and the Prospectus Supplement No. 7 dated April 4, 2007.
The shares that are the subject of the Prospectus have been registered to permit their resale to the public by the selling stockholders named in the Prospectus. We are not selling any shares of common stock in this offering and therefore will not receive any proceeds from this offering. You should read this Prospectus Supplement No. 8 together with the Prospectus and each prior Prospectus Supplement referenced above.
This Prospectus Supplement No. 8 includes the attached Current Report on Form 8-K of Auriga Laboratories, Inc. filed on April 10, 2007 with the Securities and Exchange Commission.
Our common stock is traded on the Over-the-Counter Bulletin Board under the trading symbol “ARGA.”
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR PASSED UPON THE ACCURACY OR ADEQUACY OF THIS PROSPECTUS SUPPLEMENT. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The date of this Prospectus Supplement is April 10, 2007.
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington D.C. 20549
FORM 8-K
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(D)
OF THE SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of earliest event reported)
April 10, 2007
AURIGA LABORATORIES, INC.
(Exact name of registrant as specified in its charter)
| | | | | |
| Delaware | | 000-26013 | | 84-1334687 |
| | | | | |
| (State of incorporation) | | (Commission File Number) | | (I.R.S. Employer
Identification No.) |
| | | | | |
2029 Century Park East, Suite 1130
Los Angeles, California | | | | 90067 |
| | | | | |
| (Address of principal executive offices) | | | | (Zip Code) |
(678) 282-1600
(Registrant’s telephone number, including area code)
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| | | |
| o | | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| | | |
| o | | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| | | |
| o | | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| | | |
| o | | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
SECTION 7—REGULATION FD
| | |
| Item 7.01. | | Regulation FD Disclosure. |
On April 10, 2007, Auriga Laboratories, Inc. (the “Company”) will be making presentations to certain members of the investment community and, in connection therewith, will advance a PowerPoint presentation (the “Presentation”) providing certain information about the Company. This Presentation will be made available on the Company’s website atwww.aurigalabs.com as soon thereafter as practicable. The Presentation is furnished under this Item 7.01 pursuant to Regulation FD and is included as Exhibit 99.1 to this Current Report on Form 8-K.
The information in this Current Report on Form 8-K, including Exhibit 99.1 attached hereto, shall not be deemed to be “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to liability under such section, nor shall it be deemed incorporated by reference in any filing of the Company under the Securities Act of 1933, as amended, or the Exchange Act, regardless of any general incorporation language in such filing, unless expressly incorporated by specific reference in such filing.
SECTION 9—FINANCIAL STATEMENTS AND EXHIBITS
| | |
| Item 9.01. | | Financial Statements and Exhibits. |
| | (d) | | Exhibits. The following exhibit is filed herewith: |
| | | |
| Exhibit | | |
| Number | | Document |
| 99.1 | | Powerpoint Presentation. |
2
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | | | | |
| | Auriga Laboratories, Inc.
| |
| Date: April 10, 2007 | By: | /s/ Charles R. Bearchell | |
| | | Charles R. Bearchell | |
| | | Chief Financial Officer | |
| |
3
Exhibit 99.1

| Our Business is Your Health(tm) Corporate Presentation March 2007 |

| Forward Looking Statements The information contained herein includes forward-looking statements. These statements relate to future events or to our future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance, or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. You should not place undue reliance on forward-looking statements since they involve known and unknown risks, uncertainties and other factors which are, in some cases, beyond our control and which could, and likely will, materially affect actual results, levels of activity, performance or achievements. Any forward-looking statement reflects our current views with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to our operations, results of operations, growth strategy and liquidity. We assume no obligation to publicly update or revise these forward-looking statements for any reason, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future. Important factors that could cause actual results to differ materially from our expectations include, but are not limited to, those factors that are disclosed under the heading "Risk Factors" and elsewhere in our documents filed from time to time with the United States Securities and Exchange Commission and other regulatory authorities. Statements regarding the regulatory status and/or regulatory compliance of our products, our ability to secure additional financing, our ability to sustain market acceptance for our products, our dependence on collaborators, our ability to find and execute strategic transactions, or potential exposure to litigation, our exposure to product liability claims, and our prices, future revenues and income and cash flows and other statements that are not historical facts contain predictions, estimates and other forward-looking statements. Although the Company believes that its expectations are based on reasonable assumptions, it can give no assurance that its goals will be achieved and these statements will prove to be accurate. Important factors could cause actual results to differ materially from those included in the forward-looking statements. |

| Auriga Highlights Emerging, sales-focused pharmaceutical company with an industry-changing sales model Strategically acquires valuable drug portfolios from large pharmaceutical companies and capitalizes on untapped marketplace opportunities with newly developed products National Sales Force expanded to more than 200 associates from 50 at Dec. 2006 Sales Force producing results: Total monthly prescriptions exceeds 26,000, up 197% (Feb 06 vs. Feb 07) Gross Revenue Forecast: approx. $8M Q1 2007; $26M FY2007 or up 250% vs. $7.4M FY2006* *FY 2007 Guidance issued and only effective on Feb 5, 2007 |

| Auriga Target Markets Auriga current product lineup targets three large and growing market segments: Respiratory (cough, cold and allergy), Dermatology, and Dry Mouth (Xerostomia) markets Sources: IMS Health, Retail Drug Monitor; The Consumer Healthcare Products Association (CHPA), https://www.chpa-info.org, and IMS Health, NPA, Nov 2006 Target Market Market Size Respiratory $8.3B Dermatology $4.6B Dry Mouth (Xerostomia) $2.0B Total $14.9B |

| Products On The Market Extendryl(r) Auriga's first brand consists of eight products that provide relief of cough, cold, and allergy symptoms. Levall(r) The Levall family of Rx products was added to Auriga's respiratory line in 2006 Zinx(tm) OTC zinc lozenges and Rx convenience kits for cough, cold, and allergy: 1 OTC SKU & 2 Rx SKUs currently; 2 Rx SKUs to launch in Spring 2007 Aquoral(tm) spray for xerostomia. Recently cleared 510(k) Rx device |

| 2007 Planned Product Launches Dermatology Products - Spring/Summer Launch 2 Akurza(tm) (dry skin) SKUs 5 Xyralid(tm) (anti-itch) SKUs Zinx(tm) OTC Convenience kits 7 OTC SKUs - Fall 2007 Launch (tm) (tm) |

| Unique Sales Model Unlike other pharma companies, Auriga's sales representatives are 100% commission based Creates fast-growing, highly-motivated sales force Lower risk to Auriga - only pays for performance Attractive opportunity for top, highly-experienced, highly- effective pharma sales people to earn "uncapped" income Paves faster path to corporate profitability |

| Targeting "High-Prescribing" Doctors Primary Care Aquoral Extendryl Levall Zinx Akurza Xyralid Pediatricians Extendryl Levall Psychiatrists Aquoral Acquisition product Allergists Extendryl Levall Zinx Rheumatologists Aquoral Auriga Sales Team 200+ reps |
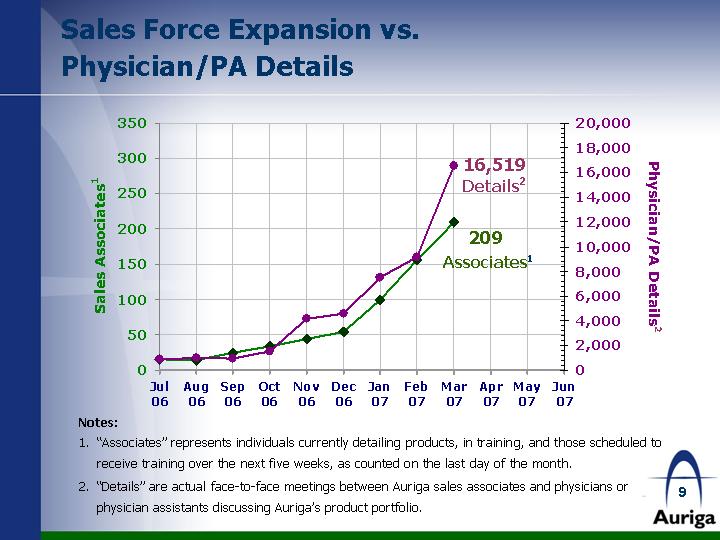
| Sales Force Expansion vs. Physician/PA Details Notes: "Associates" represents individuals currently detailing products, in training, and those scheduled to receive training over the next five weeks, as counted on the last day of the month. "Details" are actual face-to-face meetings between Auriga sales associates and physicians or physician assistants discussing Auriga's product portfolio. 7/1/2006 8/1/2006 9/1/2006 10/1/2006 11/1/2006 12/1/2006 1/1/2007 2/1/2007 3/1/2007 4/1/2007 5/1/2007 6/1/2007 Actual Details 881 976 930 1536 4140 4553 7500 9129 16519 Associates 15 14 25 34 44 54 100 156 209 16,519 Details2 209 Associates1 |
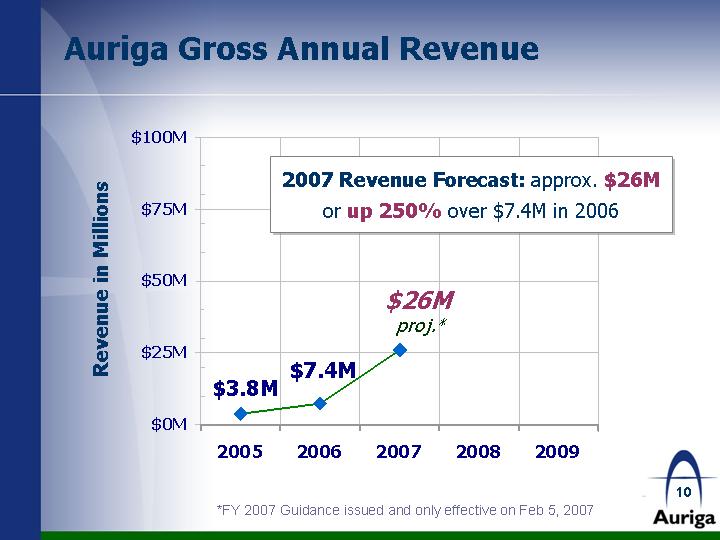
| Auriga Gross Annual Revenue Revenue in Millions 2005 2006 2007 2008 2009 Auriga Revenue 3.75 7.4 26 *FY 2007 Guidance issued and only effective on Feb 5, 2007 2007 Revenue Forecast: approx. $26M or up 250% over $7.4M in 2006 |
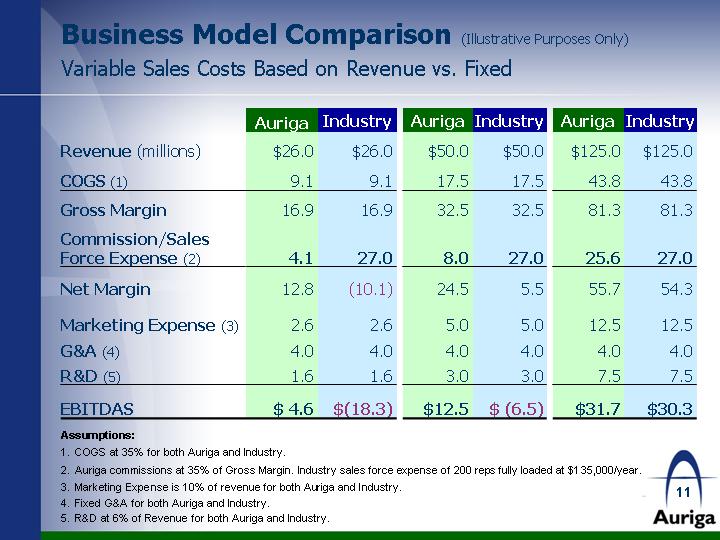
| Business Model Comparison (Illustrative Purposes Only) Variable Sales Costs Based on Revenue vs. Fixed Auriga Industry Auriga Industry Auriga Industry Revenue (millions) $26.0 $26.0 $50.0 $50.0 $125.0 $125.0 COGS (1) 9.1 9.1 17.5 17.5 43.8 43.8 Gross Margin 16.9 16.9 32.5 32.5 81.3 81.3 Commission/Sales Force Expense (2) 4.1 27.0 8.0 27.0 25.6 27.0 Net Margin 12.8 (10.1) 24.5 5.5 55.7 54.3 Marketing Expense (3) 2.6 2.6 5.0 5.0 12.5 12.5 G&A (4) 4.0 4.0 4.0 4.0 4.0 4.0 R&D (5) 1.6 1.6 3.0 3.0 7.5 7.5 EBITDAS $ 4.6 $(18.3) $12.5 $ (6.5) $31.7 $30.3 Assumptions: COGS at 35% for both Auriga and Industry. Auriga commissions at 35% of Gross Margin. Industry sales force expense of 200 reps fully loaded at $135,000/year. Marketing Expense is 10% of revenue for both Auriga and Industry. Fixed G&A for both Auriga and Industry. R&D at 6% of Revenue for both Auriga and Industry. |

| Product Development Pipeline Qty 2 - 510(k) Device Applications Qty 4 - 505(b)(2) New Drug Applications Auriga's drug-development pipeline leverages novel material science and advanced drug delivery technologies to produce: Improved formulations of successful brands Further expand markets and clinical indications of proven, successful products Auriga's drug technologies are also protected by patents covering composition of matter and use, enabling a strong proprietary position for products in the marketplace |

| Fast-Growing Patent Portfolio Orchestrated Therapy (OT) utility patent filed July 2006 Sequential release of cough/cold/allergy actives in various dosage forms Coadministration of Zinc & Cough/Cold Drugs utility patent and PCT filed Jan 2007 Treatment of symptoms with drugs; immune system augmentation Corticosteroid Solubilization utility patent filed February 2007 Method for enhancing the solubility and bioavailability of corticosteroids Multiphasic Methscopolamine Release utility patent filed February 2007 Various release profiles of methscopolamine across multiple dosage forms and combinations Multiphasic Release Potassium Guaiacolsulfonate Compositions utility patent filed February 2007 Various release profiles of K Guai as expectorant and use in fibromyalgia |
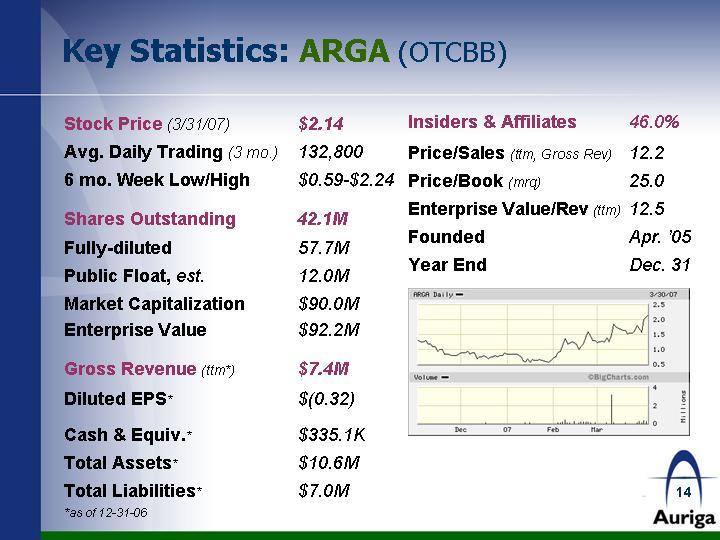
| Key Statistics: ARGA (OTCBB) Stock Price (3/31/07) $2.14 Avg. Daily Trading (3 mo.) 132,800 6 mo. Week Low/High $0.59-$2.24 Shares Outstanding 42.1M Fully-diluted 57.7M Public Float, est. 12.0M Market Capitalization $90.0M Enterprise Value $92.2M Gross Revenue (ttm*) $7.4M Diluted EPS* $(0.32) Cash & Equiv.* $335.1K Total Assets* $10.6M Total Liabilities* $7.0M *as of 12-31-06 Insiders & Affiliates 46.0% Price/Sales (ttm, Gross Rev) 12.2 Price/Book (mrq) 25.0 Enterprise Value/Rev (ttm) 12.5 Founded Apr. '05 Year End Dec. 31 |
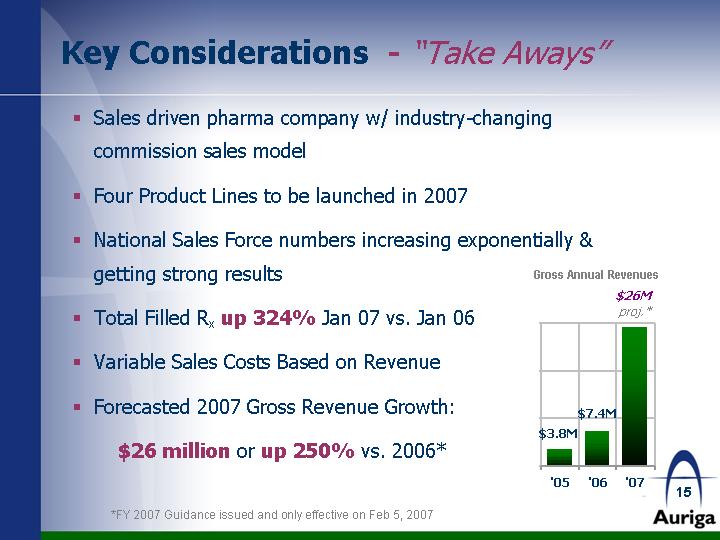
| Key Considerations - "Take Aways" Sales driven pharma company w/ industry-changing commission sales model Four Product Lines to be launched in 2007 National Sales Force numbers increasing exponentially & getting strong results Total Filled Rx up 324% Jan 07 vs. Jan 06 Variable Sales Costs Based on Revenue Forecasted 2007 Gross Revenue Growth: $26 million or up 250% vs. 2006* '05 '06 '07 Auriga Revenue 3.8 7.4 29 *FY 2007 Guidance issued and only effective on Feb 5, 2007 |

| For More Information Auriga Laboratories, Inc. 2029 Century Park East, Ste. 1130 Los Angeles, CA 90067 Toll Free 877-AURIGA8 www.aurigalabs.com Philip S. Pesin Founder, Chairman & CEO ppesin@aurigalabs.com Charles R Bearchell, CPA, JD CFO cbearchell@aurigalabs.com Investor Relations Liolios Group, Inc. Tel 949.574.3860 Scott Liolios, scott@liolios.com Ron Both, ron@liolios.com |

| Our Business is Your Health(tm) ARGA |
















