

Forward-Looking Statements
The information contained herein includes forward-looking statements. These statements relate to
future events or to our future financial performance, and involve known and unknown risks,
uncertainties and other factors that may cause our actual results, levels of activity, performance,
or achievements to be materially different from any future results, levels of activity, performance
or achievements expressed or implied by these forward-looking statements. You should not place
undue reliance on forward-looking statements since they involve known and unknown risks,
uncertainties and other factors which are, in some cases, beyond our control and which could,
and likely will, materially affect actual results, levels of activity, performance or achievements. Any
forward-looking statement reflects our current views with respect to future events and is subject to
these and other risks, uncertainties and assumptions relating to our operations, results of
operations, growth strategy and liquidity. We assume no obligation to publicly update or revise
these forward-looking statements for any reason, or to update the reasons actual results could
differ materially from those anticipated in these forward-looking statements, even if new
information becomes available in the future. Important factors that could cause actual results to
differ materially from our expectations include, but are not limited to, those factors that are
disclosed under the heading "Risk Factors" and elsewhere in our documents filed from time to
time with the United States Securities and Exchange Commission and other regulatory
authorities. Statements regarding the regulatory status and/or regulatory compliance of our
products, our ability to secure additional financing, our ability to sustain market acceptance for our
products, our dependence on collaborators, our ability to find and execute strategic transactions,
or potential exposure to litigation, our exposure to product liability claims, and our prices, future
revenues and income and cash flows and other statements that are not historical facts contain
predictions, estimates and other forward-looking statements. Although the Company believes that
its expectations are based on reasonable assumptions, it can give no assurance that its goals will
be achieved and these statements will prove to be accurate. Important factors could cause actual
results to differ materially from those included in the forward-looking statements.

Auriga Defined
A specialty pharmaceutical
company building an innovative
sales force designed to rapidly
grow market share while
preserving resources for
pipeline development and
acquisitions

Auriga Highlights
Currently focused on niches of
Respiratory/Derm/Psych/GI
Growing an innovative sales force
2007 Revenue Projected at $26-27 million
Robust development pipeline with major long-
term growth potential
Approximately $50MM market capitalization*
Rapidly expanding intellectual property portfolio
*market cap as of 10/31/06

Sales Force Structural Plan
Respiratory
Psych
Derm
70-90 reps*
100-120 reps*
30-45 reps*
*represents expected size of sales force upon completion of 2007 expansion

Current Product Portfolio
Extendryl® line of cough/cold/allergy products
Marketed Rx prescription products
7 SKUs currently
10 SKUs by end of 2007
Levall® line of cough/cold products
Marketed Rx prescription products
4 SKUs currently
6 SKUs by end of 2007
Aquoral™ spray for xerostomia
Recently approved 510(k) Rx device
Launch scheduled for February 2007

New Products to be launched/acquired
OTC cough cold allergy products
5 SKUs by end of 2007
Psychiatric Product
acquisition/in-license a product in 2007
Branded Dermatology Products
7 SKUs by end of 2007
Medical Foods/Homeopathic Treatments
2 SKUs by end of 2007
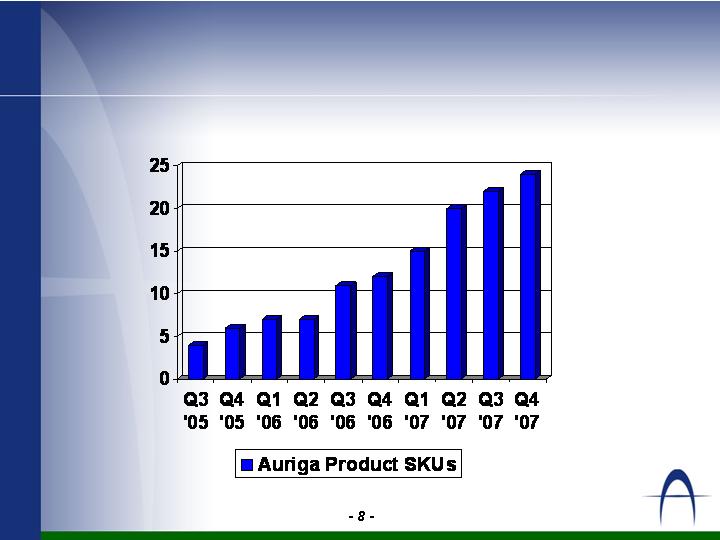
Growth and Diversification of Product
Portfolio

Extendryl® Line
Rx for seasonal/perennial allergy symptoms
Competes in $6.0 billion U.S. prescription
and OTC market for CCA products
Launching Extendryl G in Fall 2006
Extendryl® Seniors
Extendryl® Juniors
Extendryl® Chewables
Extendryl® Syrup
Extendryl® PSE
Extendryl® DM
Extendryl® HC
Extendryl® G
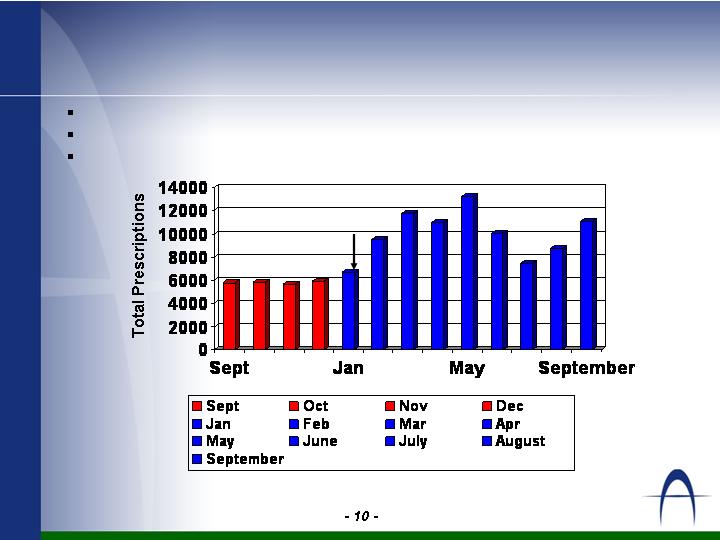
Extendryl® Performance
140,029 TRx in 2005
257,559 TRx forecast for 2006
375,000 TRx forecast for 2007
Source = IMS National Prescription Audit (NPA)
Salesforce
Promotion
Begins
2005
2006

Levall™ Line
Rx for cough/cold symptoms
Competes in $6.0 billion U.S. prescription
and OTC market for CCA products
Launching several line extensions in 2007
Levall™ 5.0
Levall™ Liquid
Levall™ - 12
Levall™ G
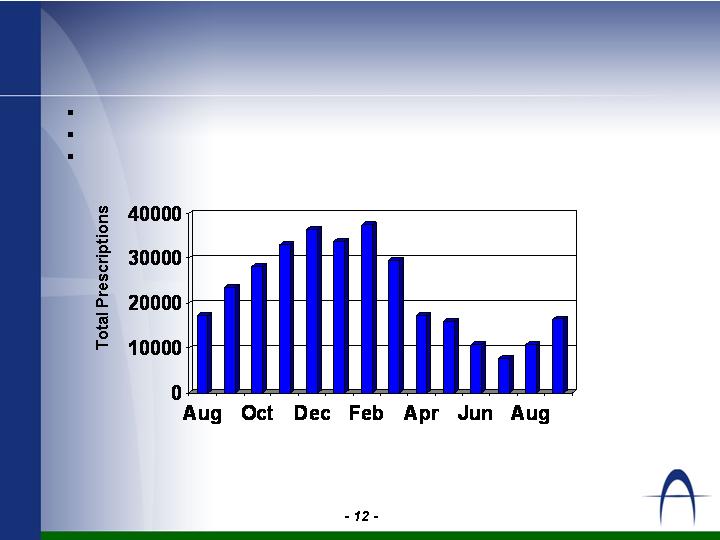
Levall™ Performance
* Includes Levall branded Rx &
Auriga-owned Levall generics
383,946 TRx in 2005
370,769 TRx forecast for 2006
482,000 TRx forecast for 2007
2005
2006

Aquoral™ Spray
Recently approved mouth spray for treatment of
xerostomia (dry mouth)
Dry mouth affects >25 million people in U.S.
Caused by serious medical conditions and by many
medications
Total market could be >$1 billion
Product launch will be February 2007
Signed co-promotion agreement with
Pharmelle (Urology) in October 2006

Auriga Sales Team
Currently 34 representatives selling
12- 15 new representatives attending training during November
60 by January
120 by March 2007
200+ by July 2007
A typical 200 person sales force can cost $27,000,000 annually (200
reps at fully-loaded average cost of $135,000 per representative)
Sales force model encourages constantly turning over lower
performers and ensures that expenses do not outpace revenue
The commission structure allows the greatest share of voice with
minimal investment
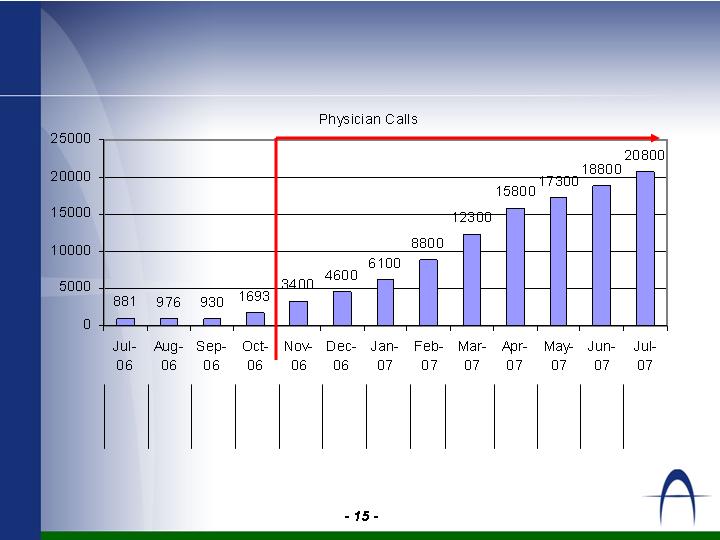
Sales Rep Count & Physician Calls
188
208
173
158
123
88
61
46
34
23
14
14
15
# of
Reps
**
Projection
** Rep numbers show reps on board 1st day of the month
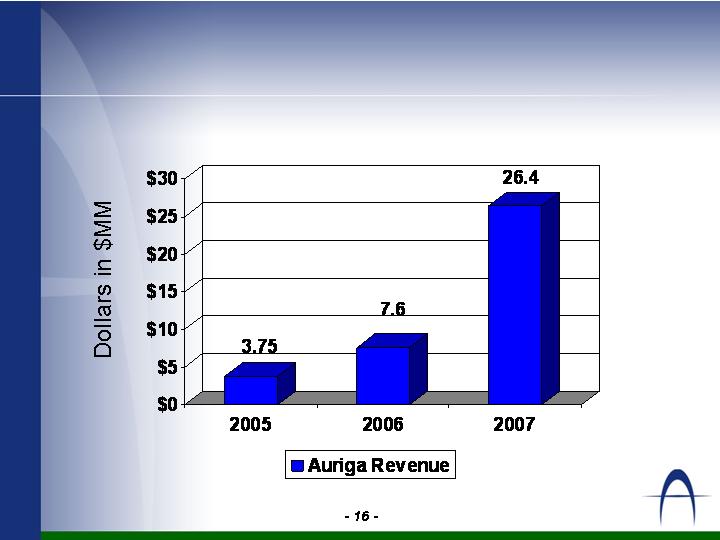
Revenue by Year
2006 and 2007 based on projections

Auriga Sales Management
Utilizing the “mentor” concept
Top performing representatives assigned a number of
direct reports while still maintaining their current
business.
Mentors get a piece of each representative’s commission
Majority of mentoring via telephone
Allows newer representatives immediate access to top
performers
Keeps manager expenses low (salaries and travel)

Product Lifecycle Management
Auriga will use regulatory and intellectual property
strategies along with authorized generic arrangements to
gain and protect market share
Example of Effective Use of Regulatory and IP Strategies
to Gain and Protect Market Share
Mucinex® (Adams Respiratory Therapeutics)
DESI cough/cold product approved via 505(b)(2)

Auriga Development Project Goals
Solid return on investment
Auriga makes a considerable multiple over the
development dollars spent to bring product to market
Exclusivity
Eradicate or minimize possible substitution
Use proprietary technology and/or have patents to keep
competition down and out
The “Hook”
That significant difference/competitive
advantage/memorable feature that keeps doctors and
patients coming back for more

AU-015 Projected Profile
GI Anti-Inflammatory Market: $2-3 BB Annual
GI Anti-Inflammatory TRx: 4.9 MM Annual
86% of TRx currently sulfasalazine/mesalamine
4% is oral steroid (locally delivered)
Steroid therapy is newer therapy (Entocort™ EC)
American College of Gastroenterology recently made steroids 1st line therapy
This class will grow significantly in coming years
AU-015 Sales Potential: $200 MM Annual
Investigational alternate therapy for Inflammatory Bowel Diseases utilizing 505(b)(2) NDA platform
Agreement with Degussa for development of novel oral corticosteroid delivery
Improved topical localization
Extremely low absorption means fewer side effects
Pre-IND Meeting with FDA Q1 2007
Q2 2011 approval

Patent Filings
Orchestrated Therapy (OT) utility patent filed 31 July 2006
Sequential release formulations of cough/cold/allergy actives
Rapid Dissolve/Extended Release (RD/ER) provisional patent filed April 2006
Novel mixed-release tablet formulation containing cough/cold therapeutic agents that
provides immediate and extended dosing
Corticosteroid Solubilization provisional patent filed September 2006
Method for enhancing the solubility and bioavailability of fluticasone
Multiphasic Methscopolamine Release provisional patent filed September 2006
Various release profiles of methscopolamine across multiple dosage forms and
combinations
Coadministration of Zinc and Cough/Cold Drugs provisional patent filed
September 2006
Treatment of symptoms with drugs; immune system augmentation
Multi-Phase Release Potassium Guaiacolsulfonate Compositions provisional
patent filed October 2006
Various release profiles of K Guai as expectorant and use in fibromyalgia

Experienced Management Team
Name
Position
Experience
Philip Pesin Chairman and CEO FDA Regulatory, M&A, Business
Development, Intellectual Property,
founder of Sorrento Financial
Group and other start-up
organizations
Andrew Shales COO Sales and marketing; Solvay,
UCB, First Horizon, Synthon,
Zyrtec® & Keppra® brands, built
several commercial teams
Alan Roberts SVP Scientific Affairs Regulatory & scientific affairs;
Mikart, Solvay, First Horizon
Mischelle Hall VP Marketing Marketing roles at UCB, First Horizon,
Sigma Tau and Synthon

Scientific Advisory Board
Glynn Wilson, Ph.D. (Advisory Board Chairman)
Former head of drug delivery, SKB
John Staniforth, Ph.D., FRSC
Drug delivery and pharmaceutical technologies pioneer, Penwest
James McGinity, Ph.D.
Pharmaceutics thought leader with emphasis on extrusion,
microencapsulation, and polymer coatings, University of TX
(Austin)
Matthew Heil, Ph.D.
Immunologist with expertise in pharmaceutical formulations &
development

Snapshot
Ticker Symbol: OTCBB: ARGA
Founded: April 2005
Reverse Merger: May 2006
Shares Outstanding/Fully Diluted: 40MM/62MM
Insider Ownership (diluted): 50%
Equity/Debt/Acquisitions: approx $15MM**
Revenue 2007E approx $26MM
Market Cap: approx $50MM*
Public Float: approx 1.7MM shares
*market cap as of 10/31/06
** debt free by 6/30/07
























