UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
(Mark One)
| þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2010
or
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 1-14601
Arch Chemicals, Inc.
(Exact name of registrant as specified in its charter)
| | |
| Virginia | | 06-1526315 |
(State or other jurisdiction of
incorporation or organization) | | (I.R.S. Employer
Identification No.) |
| |
501 Merritt 7 Norwalk, CT | | 06851 |
| (Address of principal executive offices) | | (Zip Code) |
Registrant’s telephone number, including area code:
(203) 229-2900
Securities registered pursuant to Section 12(b) of the Act:
| | |
Title of each class | | Name of each exchange on which registered |
| Common Stock | | New York Stock Exchange |
Securities Registered Pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes þ No ¨.
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No þ.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No ¨.
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes þ No ¨.
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. þ
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check One)
Large accelerated filer þ Accelerated filer ¨ Non-accelerated filer ¨ (do not check if a smaller reporting company) Smaller reporting company ¨
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ¨ No þ.
As of June 30, 2010, the aggregate market value of registrant’s voting and non-voting common equity held by non-affiliates of registrant was $764,083,707.
As of January 31, 2011, 25,147,975 shares of the registrant’s common stock were outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the following documents are incorporated by reference in this Form 10-K as indicated herein:
| | |
Document | | Part of Form 10-K into which incorporated |
Proxy Statement relating to Arch’s 2011 Annual Meeting of Shareholders | | Part III |
TABLE OF CONTENTS
FORM 10-K
PART I
General
Arch Chemicals, Inc. (“Arch”, the “Company” or “We”) is a global biocides company providing chemistry-based and related solutions to selectively destroy and control the growth of harmful microbes. We are focused on delivering profitable global growth driven by innovation. Our focus is in water treatment, hair and skin care products, wood treatment, preservation and protection applications such as for paints and building products, and health and hygiene applications. The principal business segments in which we operate are Biocides Products (formerly named Treatment Products) and Performance Products. Our ability and willingness to provide superior levels of technical service, chemical formulation skills, regulatory expertise and customer support, the manufacturing flexibility of many of our facilities, and the cultivation of close customer relationships are core skills on which we rely to serve our global markets and customers.
The Company was organized under the laws of the Commonwealth of Virginia on August 25, 1998 as a wholly owned subsidiary of Olin Corporation (“Olin”) for the purpose of effecting a tax-free distribution of Olin’s specialty chemical businesses (the “Distribution”) to the shareholders of Olin. The Distribution occurred on February 8, 1999 (the “Distribution Date”) upon which the Company became a separate, independent, publicly-held corporation.
The term “Company,” “We” or “Our” as used in Parts I and II of this Report means Arch Chemicals, Inc. and its subsidiaries unless the context indicates otherwise. The Company’s products and services described in this Report may be sold, distributed, manufactured or provided by Arch Chemicals, Inc. or by one or more of its subsidiaries, affiliates, or joint ventures.
We make available through our Internet website, which is located athttp://www.archchemicals.com, our Annual Report on Form 10-K, our Quarterly Reports on Form 10-Q, our Current Reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934 as soon as reasonably practicable after they are electronically filed with or furnished to the Securities and Exchange Commission (“SEC”). We do not charge any fees to view, print or access these reports on our website. Interested persons may read and copy these reports, proxy statements and other information at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549 and may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC also maintains an Internet website, located athttp://www.sec.gov, that contains reports, proxy statements and other information regarding registrants, such as the Company.
The Company will provide without charge upon written request a copy of its annual report on Form 10-K. Requests for such a copy should be directed to: Arch Chemicals, Inc., 501 Merritt 7, P.O. Box 5204, Norwalk, Connecticut 06856-5204, Attention: Investor Relations Department.
2010 Developments
On March 31, 2010, the Company completed the sale of its industrial coatings business to The Sherwin-Williams Company. The gross cash proceeds before expenses and the final working capital adjustment were €39.9 million (approximately $54 million). The business’ operations located in Italy, France, the United Kingdom, Spain, North America and Singapore were all included in the sale.
On September 9, 2010, we entered into a Master Note Purchase Agreement (the “master note agreement”) with affiliates of ING Investment Management LLC, New York Life Insurance Company, MetLife, Inc., Massachusetts Mutual Life Insurance Company, Great-West Lifeco Inc., CUNA Mutual Group, and Southern Farm Bureau Life Insurance Company (the “Investors”), pursuant to which the Investors provided financing to the Company through the private placement of $250 million aggregate principal amount of the Company’s Series
1
2010-A Senior Notes (the “Senior Notes”). Pursuant to the master note agreement, the Company issued $125 million of the Senior Notes on September 9, 2010 and the remaining $125 million of the Senior Notes were issued on December 30, 2010.
Products and Services
Our products and services fall within two business segments: Biocides Products and Performance Products. For financial information about each of our segments, and foreign and domestic and export sales and long-lived assets, see Note 18 of Notes to Consolidated Financial Statements contained in Item 8 of this Report. The principal products of each of our businesses are described below. For customer concentrations, see “Business and Credit Concentrations” contained in Note 1 of Notes to Consolidated Financial Statements contained in Item 8 of this Report.
Biocides Products
Within our Biocides Products segment, we manufacture and sell water treatment chemicals, industrial and personal care biocides and specialty ingredients, and wood treatment chemicals and related services.
HTH Water Products. We sell chemicals and equipment on a worldwide basis for the sanitization and treatment of residential and commercial pool and spa water, drinking water, surface water and water used in industrial applications. We sell both chlorine-based products (calcium hypochlorite and chlorinated isocyanurates) and non-chlorine-based products (poly (hexamethylene biguanide) hydrochloride (“PHMB”)) as sanitizers. Our pool chemical products are sold primarily under widely recognized brand names, such asHTH®,POOLIFE®,Baquacil®,Baqua Spa®,GLB®, Pool & Spa® andLeisureTime®. We also sell commercial pool products under thePulsar®brand name. Our water chemical products are also distributed as private label brands. In addition to calcium hypochlorite-, chlorinated isocyanurate- and PHMB-based water sanitizing chemicals, we sell ancillary chemicals and accessories for the maintenance of residential and commercial pools and spas, such as algaecides, clarifiers, foam reducers, stain preventers, feeders, fragrances and test strips. We are a leading worldwide producer and seller of calcium hypochlorite with various concentrations of available chlorine. We have a competitive advantage through the ownership of strong brand names as well as in part through ownership of several patents covering the manufacture and use of pool chemicals and equipment. We are a major manufacturer and seller of PHMB-based pool and spa treatment chemicals that are sold primarily to U.S. pool and spa owners through an extensive network of authorized, independent retailers, rather than through mass market retailers.
Our water products are also sold in the municipal water market for the purification of potable water. We sell calcium hypochlorite to purify potable water in a number of countries outside the U.S. and for sanitization in the food preparation market in the U.S., Latin America and South Africa. We are working to expand our presence in the municipal and industrial water market both domestically and internationally and in the food preparation market.
Our surface water business manufactures a range of branded products, including products under theApplied Biochemists® brand name, and provides technical support for controlling algae and nuisance aquatic vegetation. End-use applications include golf course and park ponds, agricultural irrigation, potable water reservoirs, wastewater systems, industrial water supplies, aquaculture facilities, and private lakes and ponds of homeowners. Contract services for aquatic vegetation control and lake and pond management technologies are also provided from several branch locations around the U.S.
In 2010, approximately 70% of our water products sales were within North America, and the remaining 30% were throughout the rest of the world. In North America, we sell water chemical products to retail merchants, distributors and pool dealers. Our Brazilian subsidiary, Arch Quimica Brasil Ltda., manufactures and distributes calcium hypochlorite and other water chemical products in Brazil and other South American countries.
2
In Europe, we package and sell chemicals, equipment and accessories for pools and spas mainly in France and the United Kingdom (“U.K.”). In South Africa, we manufacture and sell chemicals, equipment and accessories for pools and spas mainly through our wholly owned subsidiary, Arch Water Products South Africa (Proprietary) Limited, which also operates a calcium hypochlorite plant.
Personal Care and Industrial Biocides. We are a leading global supplier of biocides for preservation of industrial and consumer products. We manufacture biocides that control dandruff on the scalp and, in various other applications, the growth of micro-organisms, particularly fungi and algae. We also develop, manufacture and market biocides for anti-bacterial applications. Most of our biocide products are marketed under well-recognized brands, such asOmadine®,Omacide®,Triadine®,Proxel®, Purista®, Vantocil®, Reputex®, Cosmocil® andDensil®biocides. A large portion of the biocide chemicals we produce are based on the zinc, sodium and copper salts of the pyrithione molecule. These pyrithione-based biocides include over twenty products with differing active concentrations, forms and salts, and we believe we are a worldwide leader in these biocide products. We have other biocide chemicals based on iodopropargyl-n-butylcarbamate (“IPBC”), a broad-spectrum fungicide serving the metalworking fluids and coatings markets. The IPBC-based biocides currently consist of five variations with others in development stages. Our offerings also include two well-established molecules—1,2 benzisothiazolin (“BIT”) and PHMB. We are a leading seller of BIT and PHMB into the global biocides market and supply a range of biocides used in health and hygiene applications. Biocides make up a small portion of the content of the customer’s end products, and therefore must be highly effective at low concentrations as well as compatible with the formulation’s other components. Meeting the needs of our biocide customers requires a high degree of technical support and the expertise to do business in a highly regulated environment. Our ability to meet these needs makes us a preferred supplier in the biocides market. We also participate in the personal care market with products sold primarily to manufacturers in the global cosmetic, toiletries and personal care ingredients industries. We provide these customers with biotechnological and other active ingredients, delivery systems, preservation systems, proteins, botanicals and functional ingredients, primarily for use in skin and hair care formulations.
Wood Protection. We are a leading producer of wood treatment chemical solutions that enhance the properties of wood. These products are critical to the performance and value of end-use products. Our wood preservatives and fire retardants are sold under the brand namesWolman®, Dricon®, Tanalith®, Vacsol™,andResistol™ in markets around the world. These products protect wood against rot, fungal decay, or termites and other insects or retard the combustibility of wood. Our principal customers are sawmills and treaters of softwoods that require chemical treatment to achieve the performance of naturally more durable wood species in service.
A significant number of our customers are wood treaters that use our products pursuant to a license agreement program. The program includes the use of the brand name for sale of the products produced by the licensee as well as an extensive support package comprised of marketing, technical, engineering and environmental services. Our customers sell their treated wood products into the construction, utility, residential and agricultural markets. We supply our chromated copper arsenate wood preservative (“CCA”) products for industrial applications such as the treatment of wood used in marine pilings, utility poles and highway materials. OurWolman® EandTanalith® Epatented products are offered to treaters and are used in non-industrial applications. In 2005, the Good Housekeeping Institute announced that GenuineWolmanized® Outdoor®wood, a product which is based on our patented copper azole chemistry and is sold in the U.S. and Canada, earned the Good Housekeeping Seal. This wood is used in decks, gazebos, walkways, landscaping and similar outdoor applications. Additionally, in 2006, wood treated with ourDricon®fire retardant and sold in the U.S. and Canada also earned the Good Housekeeping Seal.
Performance Products
Performance Urethanes. Our Performance Products segment manufactures and sells a broad range of urethane intermediate products with diverse end uses. Our urethanes products impart physical characteristics that are critical to the performance and value of the customer’s end-use products. Custom manufacturing services are also provided. The business is characterized by close customer relationships with companies that are leaders in
3
the markets in which they compete. The flexibility afforded by batch manufacturing in some operations, combined with our ability and willingness to provide superior technical support, enables us to respond to the specific needs of a diverse group of customers. This gives us an advantage over competitors whose manufacturing processes and related cost structure constrain their ability to respond cost-effectively to smaller volume customers.
Our performance urethane products business includes flexible polyols, specialty polyols, glycols and glycol ethers. Specialty polyols, which are used as ingredients in elastomers, adhesives, coatings, sealants and flexible and rigid foams, are manufactured at our Brandenburg, Kentucky facility. Our Brandenburg facility also manufactures glycols and glycol ethers for use as ingredients in cleaners, personal care products and antifreeze, and provides custom manufacturing of specialty chemicals for a small group of companies.
Hydrazine. We supply hydrazine hydrates and hydrazine derivatives for a variety of end uses. Hydrazine hydrate products are sold for use in chemical blowing agents, water treatment chemicals, agricultural products, pharmaceutical intermediates and other chemical products. The hydrazine hydrates are supplied in various concentrations and in packaging containers that include bulk, tote bins and drums. We currently purchase hydrazine hydrates from a third party supplier for resale to our customers and as an ingredient for our formulated hydrazine products.
We supply propellant grade hydrazine and hydrazine derivatives for use as fuel in satellites, expendable launch vehicles and auxiliary and emergency power units. These propellant grade hydrazine products includeUltra Pure™ hydrazine (“UPH”), anhydrous hydrazine (“AH”), unsymmetrical dimethyl hydrazine (“UDMH”), monomethyl hydrazine (“MMH”) and hydrazine fuel blends. In 2005, the U.S. Government awarded the Company a long-term contract for the production, storage, distribution and handling of its hydrazine-based propellants. In addition to space-related applications in satellites and launch vehicles, auxiliary power from hydrazine-driven units has been used on the NASA Space Shuttle for maneuvering its rocket engine nozzles and for operating valves, brakes and landing gear. Emergency power from hydrazine is also provided to jet aircraft such as the F-16 to operate electrical and hydraulic units in the event of an engine flameout. We also supply special packaging containers including cylinders to improve the safe handling and storage of hydrazine propellants.
Customers
Our customer base is diverse and includes pool and spa retailers, global consumer product companies, major big-box retailers, national and regional chemical and equipment distributors, wood treaters, sawmills, other chemical manufacturers and the U.S. Government. No single customer has accounted for more than 10% of our total annual sales in 2010. A significant portion of sales of the Biocides Products segment (approximately 20% in 2010) is dependent upon two customers, with one customer accounting for a significant portion of the sales of the HTH water products business and the other customer accounting for a significant portion of the sales of the personal care and industrial biocides businesses. For additional information about customers, see the information under the caption “Business and Credit Concentrations” in Item 7 of this Report.
Raw Materials and Energy
We utilize a variety of raw materials in the manufacture of products for our businesses. Outlined below are the principal raw materials for our businesses. The majority of our raw material requirements are purchased and many are provided pursuant to written agreements. Overall, principal raw materials have historically been readily available to the Company as a whole.
Biocides Products. The principal raw materials for HTH water products include chlorine, caustic soda, lime and chlorinated isocyanurates.
4
The principal raw materials for industrial biocide treatment chemicals and personal care specialty ingredient chemicals are pyridine, zinc sulfate, iodine, dipropylene glycol, caustic soda, chlorine, cyanamide liquor and propinyl butyl carbamate.
The principal raw materials for wood protection products include scrap copper, chromic acid, monoethanolamine, tebuconazole, propiconazole, basic copper carbonate, arsenic trioxide, cupric oxide and organic biocides. Copper is subject to significant price volatility.
Performance Products. The principal raw materials for urethanes products are propylene oxide and ethylene oxide. Chlorine, caustic soda and ammonia are the key raw materials for the hydrazine business. For this segment, propylene oxide is the most significant raw material and is subject to significant price volatility.
Electricity for our manufacturing facilities is mostly supplied by public or government utilities while other third parties supply us at shared sites. Natural gas used for steam production is an important energy source for several of our U.S. manufacturing sites, particularly the Brandenburg, Kentucky facility, and is purchased from multiple suppliers.
Research and Development and Patents
Our research activities are conducted at several sites including Cheshire, Connecticut; New Castle, Delaware; South Plainfield, New Jersey; Conley, Georgia; Salto, Brazil; Blackley, England; and Osaka, Japan. On August 24, 2010, the Company announced its decision to consolidate into one facility in Alpharetta, Georgia most of its U.S. research and development (“R&D”) and technical service activities currently conducted at three facilities. The consolidation is expected to be completed by the end of 2011. The facilities impacted by this consolidation are those located in Cheshire, Connecticut; New Castle, Delaware; and Conley, Georgia. Company-sponsored research expenditures were $20.1 million in 2010, $18.7 million in 2009 and $17.3 million in 2008. Expenditures on customer-sponsored research were immaterial in each of the last three years.
In general, intellectual property is important to us, but no one technology, patent or license or group thereof related to a specific process or product is of material importance to the Company as a whole.
We believe that our broad patent portfolio in the Biocides Products segment is a source of competitive advantage for the businesses in this segment.
The Company has a significant patent portfolio related to HTH water products that includes 31 U.S. patents and numerous foreign counterpart patents. Most of these patents continue at least until 2012. Another significant patent, which expires in 2015, is for the multifunctional formulated trichloro-isocyanuric (“trichlor”) tablets. These multifunctional tablets offer enhanced benefits to our water treatment customers as distinguished from basic pure trichlor tablets. We have three other material U.S. composition patents for formulated pool chemical products that provide us with advantages in product shipping and storage. Of these three composition patents, one will expire in 2022 and two will expire in 2023. The other patents cover manufacturing processes, other multifunctional formulated products, and chemical feeder systems for residential/commercial pool and municipal water treatment applications. We have been awarded a patent covering a dissolving chamber design for chlorinator systems. This patent expires in 2021.
We have an expansive biocides patent portfolio that includes 63 U.S. patents, including process, composition and application patents and numerous foreign counterpart patents. Our biocides business holds several U.S. patents relating to antifouling additives for paints, which expire in 2012. A substantial majority of our other biocides patents do not expire until after 2012. Patents for our key targeted growth areas in our marine antifouling paint and building product biocides businesses include those relating to a small particle copper pyrithione process for stable biocide dispersions, gel-free paint containing zinc pyrithione, and “in-can” and “dry film” antimicrobial coating compositions. Biocide patents supporting our personal care business include those
5
relating to non-spherical and non-platelet crystalline forms of zinc pyrithione and a method for producing distinct particles of pyrithione salts. We also have a number of other patents and patent applications covering specialty ingredients for use in skin care and hair care products.
We own a significant U.S. composition of matter patent in our wood protection business. This patent, which expires in 2013, is on ourWOLMAN® EandTanalith® Epreservative formulation. Our patent portfolio also includes patents, both granted and pending, covering fire retardant and other wood preservation technologies.
Seasonality
We usually experience our highest sales and profits in the second quarter primarily due to sales of our HTH water products in that quarter. The purchase of water treatment products by consumers in the residential pool market is concentrated in the U.S. between Memorial Day and the Fourth of July. The HTH water products business principally distributes directly to retail merchants and distributors. Sales of these products are strongest in the second and third quarters with the second quarter having the highest sales of these products. Our working capital needs peak during the second quarter as inventories increase to meet the demand requirements of the U.S. residential swimming pool season and accounts receivable increase as a result of the higher sales. We anticipate that these working capital requirements will be financed by cash on hand and, if necessary, by our credit facilities and accounts receivable securitization program. In addition, weather can have a significant effect on water treatment and wood treatment sales during any given year with unseasonably wet or cool weather negatively impacting sales.
Backlog
The amount of our backlog orders is immaterial as a whole and to any particular segment, which is consistent with prior years.
U.S. Government Contracts and Other Regulatory Matters
In 2005, we obtained a 20-year contract, valued at $149 million, with the Defense Energy Support Center (“DESC”) for the production, storage and distribution and handling services of our hydrazine-based propellants products. We began receiving monthly maintenance fee payments in 2006 under this long-term contract. The terms of the contract call for an initial 10-year supply contract beginning in 2005, followed by two five-year renewal terms at the option of the government. We will utilize our existing hydrazine production facility in Lake Charles, Louisiana, to provide products and services under this contract. The contract provides that the U.S. Government may terminate the contract for convenience with a termination fee to be negotiated by the parties at time of termination.
As a government contractor, we are subject to extensive and complex U.S. Government procurement laws and regulations. These laws and regulations provide for ongoing government audits and reviews of contract procurement, performance and administration. Failure to comply, even inadvertently, with these laws and regulations and with laws governing the export of controlled products and commodities could subject us or one or more of our businesses to civil and criminal penalties and under certain circumstances, suspension and debarment from future government contracts and the exporting of products for a specified period of time.
In addition to governmental regulations affecting government contractors, we, as a chemical manufacturer, are subject to numerous other regulations regarding the sale of our products. Several of our products are registered with the U.S. EPA under the Federal Insecticide, Fungicide, and Rodenticide Act (“FIFRA”) and as such are subject to various regulations regarding use and disclosure requirements. FIFRA covers the sale, distribution and use of our biocides in the U.S. This law requires that all biocides be registered by the U.S. EPA prior to sale or distribution, and that the safety of our biocides be supported by U.S. EPA required data. Additionally, FIFRA provides for the periodic re-registration of biocides, which ensures that all data supporting registrations meet current guidelines. In addition to the U.S. EPA, each of the various states requires that biocides
6
be registered by the relevant state regulatory agency prior to sale and distribution in that state. In addition, in Europe, our biocide products are subject to the European Biocidal Products Directive (“BPD”), which requires the re-registration of all biocide products, and the Registration, Evaluation and Authorization of Chemical Substances regulation (“REACH”), which requires all chemical products to be re-registered in the EU. See Risk Factors in Item 1A of this Report for additional information. In addition to Europe and the U.S., we are subject to chemical and biocides regulatory schemes in most of the countries in which we operate.
Competition
The industry segments in which we operate are highly competitive, and we encounter strong competition across our product lines from other manufacturers worldwide. This competition, from other manufacturers of the same products and from manufacturers of different products designed for the same uses, is expected to continue in both the U.S. and foreign markets. Depending on the product involved, various types of competition are encountered, including price, delivery, service, performance, product innovation, product recognition and quality. Overall, we believe our principal product groups are competitive with many other products of other producers.
Export Sales
Our export sales from the U.S. to unaffiliated customers were $105.4 million in 2010, $93.1 million in 2009 and $110.7 million in 2008. For financial information about geographic areas, see Note 18 of Notes to Consolidated Financial Statements contained in Item 8 of this Report.
Employees
As of December 31, 2010, we had approximately 2,504 full-time employees, approximately 1,118 of whom were working outside the U.S. In addition, we also employed at such date approximately 29 part-time employees and approximately 190 seasonal or temporary employees, primarily in the HTH water products business. Approximately 175 of the hourly paid U.S. employees of the Company located at its Brandenburg, Kentucky and Conley, Georgia facilities are represented for purposes of collective bargaining by several different labor organizations, and we are a party to seven labor contracts relating to such employees. The Brandenburg labor contracts have five-year terms and expire in 2016 and the Conley labor contracts have four-year terms and expire in 2014. Certain European employees are also represented by unions in various countries. As of December 31, 2010, in the U.K. and Ireland, approximately 47 employees were covered under different labor organizations with employment terms that are renewed annually. Generally speaking, in other European countries applicable labor agreements are statutory. In South Africa, approximately 50 employees belong to unions with labor terms negotiated annually. In Brazil, the Company has a total of approximately 260 employees subject to collective bargaining agreements, which are negotiated annually. No material work stoppages have occurred in the last three years. While relations between the Company and its employees and their various representatives are generally considered satisfactory, there can be no assurance that new labor contracts can be entered into without work stoppages.
Responsible Care® Commitment
First adopted as a condition of membership by the American Chemistry Council (“ACC”) in 1988, the Responsible Care® initiative was developed to encourage member companies to improve their performance in the realms of health, safety and the environment continuously.
The ACC’s Responsible Care® initiative encompasses seven critical performance areas: employee health and safety, pollution prevention, manufacturing process safety, security, distribution safety, product stewardship and community awareness and emergency response. Ultimately, this initiative is aimed at making health, safety, security and environmental protection an integral part of a product’s life cycle—from manufacture, marketing and distribution to use, recycling and disposal.
7
We have developed a management system to drive improvement in all seven areas under Responsible Care®. To make this complex and multifaceted process more compelling and to give it a sense of urgency, we have developed what we call “The Goal is Zero” initiative. This initiative recognizes a fundamental truth at the heart of Responsible Care®—that no amount of harm to people or the environment is acceptable.
Our manufacturing plant in Rochester, New York, which makes industrial biocides and ingredients for personal care products, was the first plant in the U.S. to be certified under the new Responsible Care® RC 14001 standard, which broadly covers performance in all seven performance areas under Responsible Care®. The plant received this certification after a rigorous series of audits by ABS Quality Evaluations (“ABS”), an independent registrar. Using standards developed by the Registrars Accreditation Board, auditors from ABS examined the Rochester plant’s management systems and related quality controls related to Responsible Care®. We were also the first ACC member whose corporate headquarters earned certification under the new Responsible Care® Management System (“RCMS”) requirements. In addition, our Performance Products plants in Brandenburg, Kentucky and Lake Charles, Louisiana, our personal care facility in South Plainfield, New Jersey, our water chemicals plant in Charleston, Tennessee, and our wood protection plants in Conley, Georgia; Valparaiso, Indiana; and Kalama, Washington, have all received RCMS certification. In 2010, each of these sites and our corporate headquarters were re-certified under RCMS. In 2008 and 2009, Arch expanded our RCMS program by providing internal certification of RCMS at nine of our international sites. In 2010, our international facilities located in Trentham, Australia; Blackley, England; Huddersfield, England; Amboise, France; Penang, Malaysia; and Port Shepstone, South Africa were internally certified.
As its very name implies, our “The Goal is Zero” initiative is indeed aimed at achieving zero employee and contractor injuries, zero manufacturing process incidents, zero distribution incidents and zero environmental incidents. We have made improvements and we are continuing to work to drive these metrics toward zero. The following summarizes our performance in each of “The Goal is Zero” targeted areas:
Goal One: Zero Recordable Injuries. While some of our facilities have achieved this goal, overall, our rate of employee recordable injuries (the number of work-related injuries per 200,000 hours worked) has generally declined from 1.51 in 2004 to 0.71 in 2010. By contrast, the average recordable injury rate for all U.S. manufacturers was 4.3 in 2009, the most recent year available. Our recordable injury incident rate for contractors was 0.28 in 2004 and 0.33 in 2010, representing a total of one contractor injury in each year. Both employee and contractor injury rates improved significantly from 2009, and the combined rate in 2010 was the lowest in our history.
Goal Two: Zero Manufacturing Process Safety Incidents. These incidents are defined to include fires, explosions and chemical releases that result in a reportable quantity release, a lost-time injury, off-site consequences or greater than $25,000 of damages. We reduced the number of these incidents from four in 2004 to zero in 2010. This is our second consecutive year with zero process safety incidents.
Goal Three: Zero Environmental Incidents. This goal refers to incidents such as chemical spills or emissions that are reportable because they exceed strict limits established in state, federal or foreign laws and regulations. Our performance has significantly improved, moving from nine environmental incidents in 2004 to zero in 2010.
Goal Four: Zero Distribution Incidents. Under this goal, we strive to achieve zero incidents such as spills during the transportation of our products. Performance is measured in terms of distribution incidents per 1,000 shipments, and this rate was 0.50 in 2004 and 0.47 in 2010.
We are pleased with the progress and results derived from our Responsible Care® Program. We remain committed, however, to achieving further improvements and realizing the ultimate goal—“The Goal is Zero” for each of the above categories.
8
Environmental Matters
We operate manufacturing facilities throughout the world and as a result are subject to a broad array of environmental laws and regulations in various countries. We also implement a variety of voluntary programs to reduce air emissions, eliminate or reduce the generation of hazardous waste and to decrease the amount of wastewater discharges. The establishment and implementation of U.S. Federal, state and local standards to regulate air and water quality and to govern contamination of land and groundwater has affected and will continue to affect substantially all of our U.S. manufacturing locations. Federal legislation providing for regulation of the manufacture, transportation, use and disposal of hazardous and toxic substances has imposed additional regulatory requirements on industry in general, and particularly on the chemicals industry. In addition, the implementation of environmental laws, such as the Resource Conservation and Recovery Act, the Clean Air Act and the Comprehensive Environmental Response, Compensation and Liability Act of 1980, as amended by the Superfund Amendments and Reauthorization Act of 1986, has required and will continue to require new capital expenditures and will increase operating costs. Additionally, growing concerns about climate change may result in the imposition of additional regulation. Some form of federal regulation may be forthcoming with respect to greenhouse gas emissions (including carbon dioxide (CO2)) and/or “cap and trade” legislation, compliance with which could result in additional environmental regulatory requirements. Many of the other countries in which we operate also have environmental regulatory requirements in place and continue to strengthen them.
The Distribution Agreement, dated as of February 1, 1999 (the “Distribution Agreement”), between the Company and Olin relating to the Distribution, specifies that we are only responsible for certain environmental liabilities at our then current facilities and certain off-site locations with respect to the businesses acquired from Olin in the Distribution. We have also become subject to environmental exposures and potential liabilities in the U.S. and abroad with respect to the businesses we purchased following the Distribution. In connection with the acquisitions of Hickson International (“Hickson”) and Koppers Arch Wood Protection (Aust) Pty Ltd. (“KAWP”), we acquired certain environmental exposures and potential liabilities of current and past operating sites. The known Hickson and KAWP environmental exposures have been accrued for in our consolidated financial statements.
In connection with the disposition of the majority of our microelectronic materials business on November 30, 2004, we provided indemnification to the buyer for potential and identified environmental liabilities. For identified environmental liabilities as of the transaction date, there is no limit to the liability we retained, which the Company estimates to be less than $1.0 million. The Company is no longer liable for potential pre-closing environmental liabilities as the time period for indemnification has expired.
In connection with the disposition of our sulfuric acid business on July 2, 2003, we provided environmental covenants to the buyer in which we are solely liable for the costs of any environmental claim for remediation of any hazardous substances that were generated, managed, treated, stored or disposed of prior to the closing date of the sale. We will be released, under the sales agreement, from this obligation, which cannot exceed $22.5 million, 20 years from the closing date. See “Legal Matters” in Item 7 of this Report.
As part of the Hickson organics business disposition in August 2003, we continue to be responsible for known environmental matters at the Castleford, England site. Such matters have previously been accrued for in our environmental reserve included in the consolidated financial statements. Additionally, regarding any unknown environmental matters that are identified subsequent to the sale, we agreed to share responsibility with the purchaser over a seven-year period, with our share decreasing to zero over the seven-year period. Such period ended in 2010. Our maximum aggregate liability for such unknown environmental matters is £5.0 million. However, in September 2005, the purchaser went into liquidation and is highly unlikely to be able to honor its environmental indemnification commitments to us. We do not believe there has been a change in our environmental exposure at the site.
In connection with the disposition of the industrial coatings business on March 31, 2010, the Company provided indemnification for the costs of remediation necessary to comply with applicable environmental laws in
9
relation to certain specifically identified pre-closing environmental contamination and non-compliances at the sites used in the business at the time of disposal. Although there are no monetary caps or time limits applicable to the Company’s obligation to indemnify for the remediation costs, the Company estimates the potential exposure to be approximately $1.0 million. At December 31, 2010, the Company had a liability recorded for such amount in Other liabilities in the Company’s Consolidated Balance Sheet. The Company also provided indemnification for certain other unknown environmental matters relating to the pre-closing operations of the industrial coatings business. This indemnification obligation is subject to both time limits (three years in the case of penalties for non-compliance with applicable environmental laws and permits and seven years in the case of offsite and former property environmental contamination) and a €5 million (approximately $7 million) aggregate monetary cap on all warranty and environmental matters arising out of the transaction (other than the remediation costs associated with the specifically identified onsite contamination and non-compliances discussed above). All other liabilities relating to environmental matters at the sites, and arising in connection with the industrial coatings business before March 31, 2010 have been assumed by the purchaser.
The Company believes that the environmental indemnifications for the microelectronic materials, sulfuric acid, Hickson organics and industrial coatings dispositions are not likely to have a material adverse effect on our results of operations and cash flows.
Associated costs of investigatory and remedial activities are provided for in accordance with generally accepted accounting principles governing probability and the ability to reasonably estimate future costs. Charges to income for investigatory and remedial efforts of $0.8 million, $0.7 million and $1.1 million were recorded in 2010, 2009 and 2008, respectively, and may be material in future years.
Cash outlays for normal plant operations for the disposal of waste and the operation and maintenance of pollution control equipment and facilities to ensure compliance with mandated and voluntarily imposed environmental quality standards are charged to income. Cash outlays for remedial activities are charged to reserves. Historically, we have funded our environmental capital expenditures through cash flows from operations and expect to do so in the future.
Cash outlays for environmental related activities for 2010, 2009 and 2008 were as follows:
| | | | | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | ($ in millions) | |
Environmental Cash Outlays | |
Capital Projects | | $ | 1.5 | | | $ | 1.0 | | | $ | 0.6 | |
Plant Operations | | | 6.1 | | | | 6.1 | | | | 6.4 | |
Remedial Activities | | | 1.2 | | | | 0.6 | | | | 1.1 | |
| | | | | | | | | | | | |
Total Environmental Cash Outlays | | $ | 8.8 | | | $ | 7.7 | | | $ | 8.1 | |
| | | | | | | | | | | | |
Our consolidated balance sheets include liabilities for future environmental expenditures to investigate and remediate known sites amounting to $9.0 million at December 31, 2010, of which $1.5 million was classified in current liabilities, and $8.2 million at December 31, 2009, of which $1.5 million was classified in current liabilities. Our estimated environmental liability relates to 17 sites, seven of which are in the U.S. and none of which is on the U.S. National Priority List. These amounts do not take into account any discounting of future expenditures, any consideration of insurance recoveries or any advances in technology. These liabilities are reassessed periodically to determine if environmental circumstances have changed or if the costs of remediation efforts can be better estimated. As a result of these reassessments, future charges to income may be made for additional liabilities.
Annual environmental-related cash outlays for site investigation and remediation, capital projects and normal plant operations are expected to range from $8 million to $10 million over the next several years. While
10
we do not anticipate a material increase in the projected annual level of our environmental-related costs, there is always the possibility that such increases may occur in the future in view of the uncertainties associated with environmental exposures.
Environmental exposures are difficult to assess for numerous reasons, including the identification of new sites, developments at sites resulting from investigatory studies and remedial activities, advances in technology, changes in environmental laws and regulations and their application, the scarcity of reliable data pertaining to identified sites, the difficulty in assessing the involvement and financial capability of other potentially responsible parties and our ability to obtain contributions from other parties and the lengthy time periods over which site remediation occurs. It is possible that some of these matters (the outcomes of which are subject to various uncertainties) may be resolved unfavorably against us and may have a materially adverse impact on our business. At December 31, 2010, we had estimated additional contingent environmental liabilities of approximately $10 million.
Investing in our securities involves risks. Investors should carefully evaluate these risks including the factors discussed below when evaluating investments in our securities.
Our Biocides Products segment is seasonal in nature and is affected by the weather.
Our HTH water products business is subject to seasonal fluctuations in demand. We typically experience reduced sales in the first and fourth quarters of each year, as the purchase of water treatment products by consumers in the residential pool market are concentrated in the U.S. between Memorial Day and the Fourth of July. Our working capital needs peak during the second quarter. Unseasonably wet or cool weather can also have a negative impact on the sale of our water and wood treatment products. Further, drought conditions which result in water restrictions on the use of pools may negatively affect our sales of pool products and may interfere with production at our plants. With respect to our HTH water products business, the impact of global weather changes is uncertain and will depend on the overall change, if any, in wet and dry weather conditions.
An increase in the cost of our purchased raw materials and energy would lead to higher cost of goods sold, thereby reducing our operating margins.
We purchase large amounts of raw materials, including propylene oxide, chlorinated isocyanurates, monoethanolamine, scrap copper, basic copper carbonate, chromic acid, pyridine, zinc sulfate, iodine, dipropylene glycol, sodium hypochlorite, chlorine and caustic soda and energy for our businesses. Many of our raw material requirements are purchased and provided pursuant to written agreements, some of which provide for fixed or formula-based pricing and others of which provide for market or spot pricing. The price and availability of commodity chemicals is generally determined by global supply and demand. Fluctuations in supply and demand and increases in anti-dumping duty rates could have a material adverse effect on our cost of goods sold and, as a result, our margins. Price increases of raw materials may increase our working capital needs, which could reduce our liquidity and cash flows. Any interruption in the supply of raw materials could increase our costs or decrease our revenues, which could reduce our cash flow. Energy prices, particularly for electricity, natural gas and fuel oil, have been volatile in recent years. Higher diesel fuel prices may affect our product shipping costs.
In addition, we purchase energy and, in some cases, raw materials and site services, from third parties at our manufacturing plants that are located on sites that we share with such third parties. For example, we share our Charleston, Tennessee site with Olin and we share our Lake Charles, Louisiana site with Lyondell Chemical Company (“Lyondell”). If these other companies shut down their operations at these shared sites, it may significantly increase our costs to operate at these sites and make it difficult for us to obtain the necessary energy, services or raw materials and, in the worst case, cause us to have to suspend or abandon operations at these facilities. Under an agreement with Olin, we may have to pay some of the Charleston, Tennessee site’s shutdown costs, which might be significant.
11
During 2009, Lyondell, a key service provider to our hydrazine business, filed to reorganize under Chapter 11 of the U.S. Bankruptcy Code. In connection with the bankruptcy case, Lyondell filed a motion with the court to terminate all service agreements with us for our Lake Charles, Louisiana site. The bankruptcy court granted the motion and Lyondell terminated all of the service agreements. We have entered into, or expect to finalize shortly, new service agreements with Lyondell and other third parties to replace most of the terminated service agreements, except for certain services that we now perform ourselves. The new service agreements with Lyondell operate on a year-to-year basis and, as a result, may be terminated after the initial one year term. BioLab, Inc. (“BioLab”), a subsidiary of Chemtura Corporation, is currently providing several services to us at this site, including wasterwater treatment services. However, we have not finalized a contract with BioLab with respect to these services. If the new service agreements with Lyondell are terminated or we are unable to finalize the service agreements with BioLab and we are unable to arrange for alternative suppliers or perform such services for ourselves, the outcome could have a material impact on the operating income of this business. We do not believe that there will be any significant impact on our operating results in 2011, although there may be higher capital expenditures in 2011, which we are currently evaluating, in order for us to be able to perform the services that are currently being performed by Lyondell and BioLab. In addition, the Long-lived assets (exclusive of Other assets) related to this business of $3.8 million at December 31, 2010 could become impaired. There is no Goodwill recorded for this business.
In addition, Olin, a major supplier of chlorine and caustic soda to our HTH water products business, has announced plans to convert its mercury cell chlorine-caustic plant in Charleston, Tennessee to membrane technology by the end of 2012. We do not believe that there will be any significant impact on our operating results in 2011, but if there were any disruption in our supply of chlorine and caustic soda from Olin, whether due to delays in the conversion of the plant or Olin’s inability to deliver product meeting our required specifications after the conversion is completed, we would have to arrange for alternative suppliers, which could have a material impact on this business’ operating income and cash flows in the future. Our current supply contract expires at the end of 2011 and we are currently negotiating a new contract which may contain higher prices for 2012 and beyond.
We may purchase forward contracts to hedge certain of our raw material costs or utilize purchasing strategies to mitigate the adverse effect of material price increases. However, there is no assurance that these strategies will be effective and may in fact result in higher costs than would otherwise be incurred. We may be unable to pass on increases in our cost of goods sold to our customers. The level of our profitability depends, in part, on our ability to maintain the differential between our product prices and energy and raw material prices, and we cannot guarantee that we will be able to maintain an appropriate differential at all times.
The industry segments in which we operate are highly competitive, and such competition may negatively impact us.
The industry segments in which we operate are highly competitive, and we face intense competition from numerous manufacturers for each of our product lines. This competition results from many developments, including new competitors in lower-cost production countries such as China and India, technological advances creating new competing products or improving existing competing products and reduced barriers to entry in markets in which we operate. We compete on the basis of a number of factors, including price, product quality and properties, regulatory and toxicological expertise, customer relationships and services.
In addition, we face increased competition due to a trend toward consolidation. In recent years, there has been substantial consolidation and convergence among companies in the chemicals industry. Several of our competitors are larger or have greater financial resources than we do. We may not be able to address effectively the competitive factors in our industry in the future and, as a result, our financial condition and results of operations may be adversely affected.
12
Growth initiatives may not achieve desired business or financial objectives and may require a significant use of resources.
We continue to identify and pursue growth opportunities through both internal development, capital projects and acquisitions in the biocides field. These growth opportunities include development and commercialization of new products and technologies, expansion into new markets and geographic regions, and alliances, ventures, and acquisitions that complement and extend our portfolio of businesses and capabilities. There can be no assurance that such efforts, investments, or acquisitions and alliances will result in financially successful commercialization of products or acceptance by existing or new customers or new markets or achieve their underlying strategic business objectives or that they will be beneficial to our results of operations. There also can be no assurance that our research and development activities will be successful, that we will be able to identify willing acquisition candidates or that if we do identify such acquisition candidates, any financing necessary for such acquisition will be available to us on favorable terms. In addition, there can be no assurance that capital projects for such growth efforts can be completed within the time or at the costs projected due, among other things, to demand for and availability of construction materials and labor and obtaining regulatory approvals and operating permits and reaching agreement on terms of key agreements and arrangements with potential suppliers and customers. Any such delays or cost overruns or the inability to obtain such approvals or to reach such agreements on acceptable terms could negatively affect the returns from any proposed projects.
Our Biocides Products segment’s customer base is concentrated, and the loss of business from a major customer could have a material adverse effect on us. In addition, contract manufacturing is significant to our Performance Products segment.
Although no one customer accounted for over 10% of our 2010 sales, approximately 20% of our Biocides Products segment sales in 2010 were attributable in the aggregate to two customers, with one customer accounting for a significant portion of our HTH water products business sales and the other customer accounting for a significant portion of the sales of our personal care and industrial biocides business. We cannot assure investors that these or any other significant customer will not terminate their relationships with us or significantly change, reduce or delay the amount of products ordered. The loss of any such significant customer would have a material adverse effect on the net sales and operating results of the Biocides Products segment, which, in turn, could adversely impact our business, financial condition, results of operations and cash flows. In addition, this customer concentration gives such customers additional leverage in negotiating terms that may negatively impact our margins.
Our Performance Products segment is highly dependent on contract manufacturing arrangements with various terms. The termination of any such arrangements could adversely affect our annual operating results for this segment. We believe that overall organic growth and our pipeline of new product offerings should mitigate a portion of any such impact. If the results for this segment were to continue to decline further, if there is no recovery over the next several years or if the Company and/or segment is unsuccessful in new product offerings, the earnings and the cash flows for the Performance Products segment could be significantly impacted. Additionally, this segment’s Long-lived assets (exclusive of Goodwill and Other assets) and Goodwill, $17.5 million and $4.4 million, respectively, at December 31, 2010, could become impaired.
We may need to build manufacturing facilities in lower-cost or developing countries to remain competitive in our industry and this entails certain risks. Also, our customers or markets may migrate to lower-cost countries where we do not have a sufficient presence.
In recent years, there has been a shift of production capacity in the chemicals industry to developing countries with lower costs of production, such as China. We may be required to invest in such countries in order to offer competitive product prices to our customers. Customers may require that we build manufacturing facilities or make additional investments in a lower-cost or developing country in order to retain their business. In fact, we built a plant in China in 2008 to meet the needs of a significant customer. The building of plants also increases risks of cost overruns, start-up problems, and construction delays, any of which may impact customer
13
sales and relations. Further, additional manufacturing capacity may make some of our existing manufacturing sites redundant or create excess capacity, causing us to have to reduce production at or shut down such other manufacturing sites which might increase our costs significantly and might trigger shutdown costs and charges including severance payments and writeoffs. If we are required to build additional manufacturing facilities overseas, our capital expenditures would increase to reflect not only the cost of the construction of the facilities, but also their long-term maintenance. These capital expenditures may increase our costs, which may negatively impact our margins. Finally, the relocation of some production facilities to lower-cost countries by an industry may result in lower pricing worldwide for certain products, which may also negatively impact our margins worldwide for such products.
In connection with the shift to countries with low-cost production, our customers or the markets for our products may shift to countries where we may not have a sufficient presence, which could adversely impact our sales and/or margins.
We are subject to risks related to our international operations, including exchange rate fluctuations, which could adversely affect our business, financial condition, results of operations and cash flows.
We have significant operations in Australia, Brazil, China, England, New Zealand and South Africa and other foreign countries, and we sell to customers in a number of other countries, including Canada, France and Japan. Approximately 50% of our 2010 sales were outside the United States. International sales and operations are subject to significant risks, including, among others:
| | • | | political and economic instability; |
| | • | | restrictive trade policies; |
| | • | | economic conditions in local markets; |
| | • | | difficulty of enforcing agreements and collecting receivables through certain foreign legal systems; |
| | • | | difficulties in managing international manufacturing operations; |
| | • | | local legal and regulatory requirements, including those relating to REACH and to the European Biocidal Products Directive, which requires biocide manufacturers, including the Company, to re-register their biocidal products for sale in the European Union (“EU”); |
| | • | | potential difficulties in protecting intellectual property; |
| | • | | potential adverse tax consequences, including imposition of withholding or other taxes on payments by subsidiaries; |
| | • | | the imposition of product tariffs and duties and the burden of complying with a wide variety of international and U.S. export laws; |
| | • | | changes in Chinese taxes relating to exports from China; and |
| | • | | changes in legislation for U.K. taxes regarding the timing of deductions for interest expense. |
Our effective tax rate has historically been reduced due to a tax benefit related to our U.K. financing structure. Recently enacted U.K. legislation could limit the tax benefit of this structure, thereby increasing our future effective tax rate. In addition, if the operating results of our U.K. operations fall below our current expectations or the Company is unsuccessful with its tax planning strategies, we have certain deferred tax assets related to our U.K. operations that may no longer be likely to be realized. Therefore, we may be required to record a valuation allowance against such assets, which totaled $46.6 million at December 31, 2010.
The functional currency for most of our international operations is the applicable local currency. The Company enters into forward sales and purchase contracts to manage currency risk resulting from purchase and sale commitments denominated in foreign currencies (principally, the British pound, euro, Australian dollar, New Zealand dollar, Canadian dollar, Japanese yen and South African rand) and relating to particular anticipated but
14
not yet committed purchases and sales expected to be denominated in those currencies. Although we implement foreign currency hedging and risk management strategies to reduce our exposure to fluctuations in earnings and cash flows associated with changes in foreign exchange rates, there can be no assurance that foreign currency fluctuations will not have an adverse effect on our business, results of operations or financial condition.
If we continue to expand our business globally, our success will depend, in large part, on our ability to anticipate and effectively manage these and other risks associated with our international operations. However, any of these factors could adversely affect our international operations and, consequently, our operating results.
We cannot assure investors that our operations will continue to be in compliance with applicable customs, currency exchange control regulations, transfer pricing regulations or any other laws or regulations to which we may be subject. We also cannot assure investors that these laws will not be modified.
Our manufacturing facilities process chemicals and, as a result, subject us to operating risks that could adversely affect our results of operations.
We have approximately 24 manufacturing facilities. Our operations are subject to various risks associated with manufacturing, transportation, storage and handling of chemicals, including chemical spills, discharges or releases of toxic or hazardous substances or gases, fires, mechanical failures, storage tank leaks, unscheduled downtime, explosions, severe weather and natural disasters, terrorist attacks, natural resource damage and other environmental risks. Our suppliers of chemical raw materials are subject to similar risks that may impact our supplies. These risks can cause personal injury and loss of life, catastrophic damage to or destruction of property and equipment and environmental and natural resource damage, and may result in an unanticipated interruption or suspension of operations and the imposition of civil claims and criminal penalties. For example, an unplanned outage at our HTH water products manufacturing facility during peak seasonal demands could result in product availability shortfalls or increased costs resulting from the need to source product from alternative manufacturing sources or competitors. The loss or shutdown over an extended period of operations at any of our major manufacturing facilities or any losses related to any such claims could have a material adverse effect on our business, financial condition, results of operations or cash flows. In addition, if we cannot maintain or upgrade equipment as we require or ensure regulatory compliance, we could be required to cease or curtail some of our manufacturing operations, or we may become unable to manufacture products that compete effectively in our industry. While we have insurance to mitigate some of these risks, such insurance includes deductibles and policy limits that may not be sufficient to cover our total exposure. In addition, there is usually a lag between payments by us of the insured liability and reimbursement by the insurance carrier, which lag may negatively impact our cash flow.
We are subject to environmental and regulatory risks.
Environmental and regulatory laws have affected, and will continue to affect, substantially all of our operations. We are subject to strict requirements regarding air emissions, wastewater discharges and the handling and disposal of hazardous and toxic substances. Environmental laws will likely become more stringent over time, thereby requiring new capital expenditures and increases in operating costs. In addition, we are responsible or potentially responsible for clean-up costs at several of our current operating sites. Although Olin has agreed to be responsible for certain environmental legacies, there is no assurance that Olin will be able to honor its contractual commitments to us. Business acquisitions by us have also caused us to inherit potential environmental liabilities. We believe that we have adequate reserves to cover the cost of our investigation and remediation obligations at each of these sites. However, unanticipated environmental conditions or the discovery of new sites or conditions requiring remediation may have a material adverse effect on our operating results and financial condition.
Additionally, growing concerns about climate change may result in the imposition of additional regulation or other restrictions on our business. Some form of Federal and/or international regulation may be forthcoming with respect to greenhouse gas emissions (including carbon dioxide (CO2)) and/or “cap and trade” legislation, compliance with which could result in the creation of substantial additional costs to us. For example, the U.S.
15
Congress may reconsider climate change legislation in the future and the Environmental Protection Agency has issued a rule which regulates larger emitters of greenhouse gases. Since the industry segments that we operate in are highly competitive, we may not be able to recover the cost of compliance with new or more stringent environmental laws and regulations which could adversely affect our business. Although it is not expected that the costs of complying with current environmental regulations will have a material adverse effect on our financial position, results of operations or cash flows, no assurance can be made that the costs of complying with environmental regulations in the future will not have such an effect.
In addition, many of our products are required to be registered with the U.S. EPA and with comparable state and foreign governmental agencies and such registration is subject to periodic review and is subject to producing certain data regarding human and environmental safety. It is possible that registration criteria may become more stringent, that governmental agencies may from time to time require additional data to support registration or re-registration of certain products or that such agencies may find the data supporting certain products to be unsatisfactory, in which case, certain of our products could become restricted (or in the extreme case, prohibited) for sale in certain jurisdictions. For example, sales of CCA in North America could be impacted by an ongoing U.S. EPA review of a key active component within this wood preservative and a potential change in its risk profile. If the change is implemented, there is the potential that certain uses may be limited or restricted. The review is currently under discussion and we are part of a broad industry coalition defending against any change. Also, our key biocides are currently going through the U.S. EPA’s Registration Eligibility Decision process as required by FIFRA and will be subject to such data production.
EU authorities have issued the BPD which requires all biocidal products sold in the EU to re-register there. The EU has also adopted legislation known as REACH that requires the registration of all chemical products that are manufactured in or imported into the EU. In connection with these programs, such products must demonstrate their continued safety. This registration will require extensive testing of those products if current supporting data is insufficient. In addition, the cost of testing resulting from these new regulations may increase costs which may reduce our profit margins. While we generally expect that testing will support re-registration approval, it is possible that approval criteria may become more stringent, such testing will not support registration or that the governmental agencies involved will find the test results or supporting data unsatisfactory. In such a case, sale of some of our products may be restricted (or in the extreme case, prohibited) in the EU. As provided in the BPD, the EU is reviewing the classification and labeling of a number of chemicals under a new, more wide-ranging procedure. Several of our products, including PHMB, BIT and the pyrithiones (zinc, sodium and copper), are currently under review or a review is expected by an EU member state. For example, PHMB, which has been on the market for many years providing valuable disinfection, cleaning and hygiene functions, is under review by France, the lead reviewer of PHMB under the BPD. France has proposed a much more conservative classification of PHMB, which is currently being reviewed by the European Chemicals Agency (“ECHA”) to determine if the French classification should be upheld. If the new classification is upheld, there is the potential for customer-based restrictions on the use of products containing PHMB and such restrictions could also extend beyond the EU. We are defending our classification of the product as well as developing alternative products. If ECHA does not overturn the classification and our customers decide no longer to purchase PHMB as a result of this change in classification, our sales may be adversely affected and approximately $13 million of PHMB-related intangible assets could become impaired. In 2010, we had approximately $11 million of PHMB sales in Europe and we had approximately $38 million of PHMB sales worldwide.
Our products may be rendered obsolete or less attractive by changes in regulatory, legislative or industry requirements.
Changes in regulatory, legislative or industry requirements may render certain of our products obsolete or less attractive in many ways. Our ability to anticipate changes in these requirements, especially changes in regulatory standards, will be a significant factor in our ability to remain competitive. Further, movement to “green” or “greener” products may impact us if we are unable to offer comparable products. We may not be able to comply in the future with new regulatory, legislative and/or industrial standards that may be necessary for us
16
to remain competitive and certain of our products may, as a result, become obsolete or less attractive to our customers. In addition, changes in government appropriations may impact our hydrazine propellants business.
Increased physical security regulations related to our industry could adversely affect our business.
Enactment of new regulations impacting the physical security of chemical plant locations and the transportation of hazardous chemicals may significantly increase our operating costs and negatively impact our margins, particularly compared to foreign competitors who might not be subject to such laws.
We are subject to certain litigation risks due to the nature of some of our products.
We produce chemicals that require appropriate procedures and care in their use, handling, storage and transportation. As a result of the risks inherent in the nature of some of our chemical products, including the risk of product misuse, we have faced and will continue to face product liability claims relating to incidents involving our products, including claims for adverse health effects and personal injury and even death. Further, although we carry insurance that covers certain litigation risks, such insurance may be insufficient to cover a claim and even if sufficient for a claim, there may be a lag between the time of payment of the claim by us and reimbursement of such payment from our insurers, which lag may negatively impact our cash flow.
From time to time, the Company is named as a defendant in lawsuits regarding the marketing and use of CCA-treated wood, including being named several years ago in purported class actions, none of which were certified and all of which were dismissed. The Company has denied the material allegations of all the various CCA-related claims and has vigorously defended and will continue to vigorously defend against them.
We are subject to liquidity risks.
We have a $350 million revolving credit facility. When we borrow under this facility, we are required to certify that certain representations and warranties are true. We also must comply with covenants, including certain financial ratios. If we are not able to make a representation or fail to comply with a covenant, we may not be able to borrow under the facility. In addition, the Company has other financial covenants under its other major domestic debt agreements. If we were to fail to comply with a covenant, we could be required to repay the outstanding balances. There can be no assurance that we would be successful in our efforts to modify or waive the financial covenants under the agreements or secure other financing. If we were unable to do so, we could experience liquidity difficulties that would have a material adverse effect on our financial position and results of operations. Our revolving credit facility expires on June 15, 2011. While we expect to replace or extend this facility, there can be no assurances that we will be successful or that the terms will be favorable. In addition, our accounts receivable securitization program with PNC Bank expires on October 4, 2011. While we expect to replace or extend the receivables arrangement, there can be no assurances that we will be successful. If we are unable to replace or extend the revolving credit facility or the receivables arrangement, we could experience liquidity difficulties that could have a material adverse effect on our financial position and results of operations.
In addition, adverse market conditions could potentially reduce the sources of our future liquidity. This could, among other things, limit our ability to make future acquisitions or significant capital expenditures or to respond to unanticipated cash needs.
Our ability to pay dividends on our shares of common stock is subject to compliance with certain debt covenants and liquidity restrictions.
Our major domestic debt agreements permit the payment of dividends and repurchases of shares based on a financial formula. At December 31, 2010, dividends and share repurchases were limited to $97.7 million under these facilities. These limits are adjusted quarterly pursuant to a formula that is increased by 50% of earnings and decreased by 50% of losses, dividends paid and share repurchases. If we suffer losses or certain write-offs or lack earnings, these covenants may limit or eliminate our ability to pay dividends. Additionally, turmoil in the financial markets could result in a contraction in the availability of credit in the marketplace. This could
17
potentially reduce the sources of liquidity for the Company. If Company earnings or cash flows were to fall significantly below current expectations, a risk exists that the Company would not have enough liquidity to meet its operating needs or pay dividends. We paid $20.1 million in dividends in 2010.
Certain of our pension obligations are currently underfunded. We may have to make significant cash payments to our pension plans, which would reduce the cash available for our business.
As of December 31, 2010, our accumulated benefit obligations under our U.S. and U.K. defined benefit pension plans exceeded the fair value of plan assets by approximately $88 million in the aggregate. During the years ended December 31, 2010, 2009 and 2008, we contributed $88.4 million, $58.4 million and $26.2 million, respectively, to these pension plans. Additional contributions may be required in future years, however, we currently do not anticipate making any cash contributions to the qualified U.S. pension plan in 2011. The minimum funding requirements for our U.K. pension plans are currently expected to be approximately $25 million to $30 million (approximately £16 million) in 2011 and approximately $15 million to $20 million (or approximately £10 million) per year thereafter. If the performance of the assets in our pension plans does not meet our expectations or other actuarial assumptions are modified, our contributions to our pension plans could be materially higher than we expect, which would reduce the cash available for our businesses. In addition, if we were to cease to have active employees participating in our U.K. pension plan or if our U.K. subsidiaries that sponsor the plan become insolvent and in certain other situations, we may be required to wind up the U.K. plan. The statutory funding requirements for a plan in wind-up are materially higher than those for an ongoing plan to allow for the purchase of annuities for all the participants in the plan. While we believe a mandated wind-up of the U.K. plan to be highly unlikely, if it were to occur, it would have a material adverse effect on our business, cash flow and financial condition.
Adverse conditions in the global economy and disruption of financial markets could negatively impact our customers and suppliers and therefore our results of operations.
A continuation of the economic downturn in the business or geographic areas in which we sell our products could reduce demand for these products and result in a decrease in sales volume that could have a negative impact on our results of operations. For example, our wood treatment and industrial biocides businesses have been negatively affected by the recent drop in housing demand and construction, and all of our businesses have been negatively affected by a depressed economy. Volatility and disruption of financial markets could limit our customers’ ability to obtain adequate financing to maintain operations and result in a decrease in sales volume that could have a negative impact on our results of operations. In addition, these conditions may negatively impact our suppliers. This may make it difficult for us to obtain necessary raw materials from our suppliers or, in order to obtain them, we may incur greater cost, and this may negatively impact our results of operations.
Intellectual property rights are important to our business. If our patents are declared invalid or our trade secrets become known to our competitors, our ability to compete may be adversely affected.
Proprietary protection of our processes, apparatuses and other technology is important to our business. Consequently, we may have to rely on judicial enforcement of our patents and other proprietary rights. While a presumption of validity exists with respect to patents issued to us in the U.S., there can be no assurance that any of our patents will not be challenged, invalidated, circumvented or rendered unenforceable. Furthermore, if any pending patent application filed by us does not result in an issued patent, or if patents are issued to us, but such patents do not provide meaningful protection of our intellectual property, or if patents issued to us expire, then our ability to compete may be adversely affected. A limited number of our patents used in our HTH water products and biocides businesses expire in 2012. Additionally, our competitors or other third parties may obtain patents that restrict or preclude our ability to lawfully produce or sell our products in a competitive manner, which could have a material adverse effect on our business, cash flow and financial condition. We are currently in a dispute with the owner of a new U.S. patent, relating to methods of using particulate copper wood preservatives, in which the owner of the patent claims that use of certain wood preservatives manufactured and sold by our wood protection business infringes the patent. We believe that the patent owner’s claims are without
18
merit, are vigorously defending against these claims and do not believe that the resolution of this case is likely to have a material adverse effect on our consolidated financial condition, cash flow or results of operations.
The growth of our business also depends on our ability to develop new intellectual property rights, including patents, and the successful implementation of innovation initiatives. There can be no assurance that our efforts to do so will be successful and the failure to do so could negatively impact our results of operations.
We also rely upon unpatented proprietary know-how and continuing technological innovation and other trade secrets to develop and maintain our competitive position, particularly in our Performance Products segment. While it is our policy to enter into confidentiality agreements with our employees and third parties to protect our intellectual property, these confidentiality agreements may be breached, may not provide meaningful protection for our trade secrets or proprietary know-how, or adequate remedies may not be available in the event of an unauthorized use or disclosure of our trade secrets and know-how. In addition, others could obtain knowledge of our trade secrets through independent development or other access by legal means. The failure of our patents or confidentiality agreements to protect our processes, apparatuses, technology, trade secrets or proprietary know-how could have a material adverse effect on our business, cash flow and financial condition.
| Item 1B. | Unresolved Staff Comments. |
Not applicable.
The table below sets forth the primary locations where we have offices or conduct operations along with a brief description of the activities conducted at each identified location. A more detailed description of our principal facilities follows the table. We believe that our facilities are sufficiently maintained and suitable and adequate for our immediate needs and that additional space is available to accommodate expansion. Unless otherwise noted below, the identified location is owned by the Company.
| | |
Location | | Primary Activities |
Cheshire, Connecticut (2) | | Research and development facility and offices for Biocides Products |
| |
Norwalk, Connecticut (2) | | Worldwide corporate headquarters |
| |
New Castle, Delaware | | Research laboratory and testing site for HTH water products and personal care and industrial biocides |
| |
Conley, Georgia | | Technical center and manufacturing facility for wood protection and technical center for HTH water products |
| |
Atlanta, Georgia (2) | | Office facility for Biocides Products |
| |
Bethalto, Illinois (2) | | Corporate data center |
| |
Brandenburg, Kentucky | | Manufacturing facility for Performance Products |
| |
Lake Charles, Louisiana | | Manufacturing facility for Performance Products |
| |
Alpharetta, Georgia (2) | | Office, distribution and manufacturing facility for HTH water products and a separate facility for R&D and technical service activities for Biocides Products |
| |
South Plainfield, New Jersey | | Research and development facility and office space for personal care and industrial biocides |
| |
Rochester, New York | | Manufacturing facility for personal care and industrial biocides |
| |
Charleston, Tennessee (4) | | Manufacturing facility for HTH water products |
19
| | |
Location | | Primary Activities |
| |
Germantown, Wisconsin (2) | | Office facility for HTH water products |
| |
Trentham, Victoria, Australia | | Office and manufacturing facility for wood protection |
| |
Igarassu, Brazil | | Manufacturing facility for HTH water products |
| |
Salto, Brazil | | Office and manufacturing facility for HTH water products and blending facility for personal care and industrial biocides |
| |
Suzhou, China (1) | | Manufacturing facility, warehouse and technical support center for personal care and industrial biocides |
| |
Blackley, England (2) | | Office facility and laboratory for personal care and industrial biocides |
| |
Castleford, England (4) | | Office for personal care and industrial biocides, wood protection and HTH water products and manufacturing facility and technical center for wood protection |
| |
Huddersfield, England (3) | | Manufacturing and laboratory facilities for personal care and industrial biocides and wood protection |
| |
Amboise, France (2) | | Office, manufacturing, distribution and warehouse facility for HTH water products |
| |
Osaka, Japan (2) | | Research and development facility and office space for personal care and industrial biocides |
| |
Swords, Ireland | | Manufacturing facility for personal care and industrial biocides |
| |
Penang, Malaysia | | Manufacturing and warehouse facility for wood protection |
| |
Auckland, New Zealand (2) | | Office and manufacturing facility for wood protection |
| |
Grangemouth, Scotland (3) | | Manufacturing facility for personal care and industrial biocides and HTH water products |
| |
Kempton Park, South Africa | | Office and manufacturing facility for HTH water products |
| |
Port Shepstone, South Africa | | Office and manufacturing facility for wood protection |
| (3) | Land and building owned by a third party. |
| (4) | Portions are leased and portions are owned. |
We also lease several sales offices and warehouse facilities in and outside the U.S.
Principal Manufacturing Facilities
Our principal manufacturing properties are described below. We also have products that are produced by third parties at their manufacturing sites under contract manufacturing arrangements.
Alpharetta, Georgia.This 200,000-square foot leased facility produces, packages and distributes liquid and dry products for the HTH water products business, including branded and private label products. Offices and warehousing primarily support the businesses for swimming pool, spa and surface water products. This facility will also contain the Company’s R&D and technical center after the completion of the previously announced consolidation.
Conley, Georgia. This RCMS-certified site is the major manufacturing and technical center facility for our wood protection business in the U.S. Currently, most of our CCA is produced at this location and some of it is sent by rail to our Kalama, Washington facility for distribution to customers in the Western U.S. CCA is also
20
bulk shipped from this plant to our other CCA customers and to our Valparaiso, Indiana facility. This site also produces our CCA-replacement products. Office facilities and a technical center for Biocides Products are also located at this facility.
Brandenburg, Kentucky. This ISO 9001:2008-certified plant covers an area of 200 acres, surrounded by approximately 1,140 acres of land that provides both a buffer zone and expansion capability. The plant contains multiple manufacturing facilities producing a wide range of products. Many of these products are derivatives of ethylene oxide and propylene oxide. A broad line of specialty polyols are produced in a flexible batch facility and sold into urethane coatings, adhesives, sealant and elastomer applications. Under a contract manufacturing agreement with the purchaser of the majority of the operations of our microelectronic chemicals business, we produce chemical intermediates for such microelectronic business in a separate manufacturing facility dedicated to this purpose at this site. There is an applications and technical center at the site that supports the development and technical service needs of the polyol and glycol products. We also operate other facilities on the site to produce commodity and specialty chemicals for third parties under long-term contractual arrangements.
Lake Charles, Louisiana. This facility consists of two manufacturing operations that produce various hydrazine products. Both operations are ISO 9001:2008 certified. One operation produces propellant grade hydrazine products, including unsymmetrical dimethyl hydrazine and monomethyl hydrazine for use as fuel in satellites, expendable launch vehicles and the space shuttle orbital maneuvering systems. The second operation at this site produces propellant gradeUltra Pure™ hydrazine, the world’s purest grade of anhydrous hydrazine, principally for satellite propulsion.
Rochester, New York. This ISO 9001:2008- and RC 14001-certified facility manufactures a large number of chemicals for the specialty chemicals industry. Many of these chemicals are biocides used to control dandruff on the scalp and to control the growth of micro-organisms, particularly fungi and algae. One of the leading 2-Chloropyridine production facilities in the world is located here. 2-Chloropyridine is the key intermediate used to produce ourOmadine®biocides. These products are based on the salts of the pyrithione molecule. We manufacture over a dozen pyrithione products at this site by modifying these salts by concentration, form or combining them with other biocides. This plant also manufactures ourTriadine®brand of biocides, which are a combination of pyrithione and triazine, a bactericide purchased from a supplier. This facility also produces theOmacide®IPBC brand biocide, which is based upon iodopropargyl-n-butylcarbamate, a broad-spectrum fungicide. This facility also manufactures specialty ingredients for our personal care product line.
Charleston, Tennessee. This ISO 9001:2008- and RCMS-certified facility primarily produces, packages and stores calcium hypochlorite for the HTH water products business. At this plant, products are packaged into containers that range in size from two pounds to 2,000 pounds. Liquid and dry pool maintenance products are also formulated, packaged and stored at this site.
Trentham, Victoria, Australia. This facility produces CCA-based wood preservatives and other wood preservative chemicals for the Australian market. The office services the Victoria, South Australian and Western Australian markets. The site is ISO 9001:2008-certified.
Igarassu and Salto, Brazil. These facilities produce and package calcium hypochlorite for the HTH water products business within Brazil. We also have a blending and repackaging facility in Salto, Brazil. The Salto facility also blends products for the personal care and industrial biocides business.
Suzhou, China.This is our newest manufacturing facility. Beginning in 2009, this facility began producing a number ofOmadine® biocides for personal care and industrial biocides products primarily for Asian markets.Omadine® biocides are based on the sodium, zinc, and copper salts of the pyrithione molecule and are used to control fungi and algae in a wide range of applications. In addition to manufacturing, this facility includes warehousing, sales, customer service and a technical support center for personal care and industrial biocides. Part of the manufacturing plant is already ISO 9001 certified.
21
Huddersfield, England. This ISO 9001:2008-certified facility formulates and packagesProxel®andDensil®biocide blends (which are based on BIT) for sale by our industrial biocides business andTanalith® Epreservative, one of the primary wood protection products. The products are packed in a variety of sizes from 25kg to full tank truck shipments. There are approximately 25 different product offerings.
Swords, Ireland. This facility is located just north of Dublin, Ireland. 2-Chloropyridine is imported from our Rochester, New York facility and other sources and converted into zinc and copper salts of the pyrithione molecule. The products are ultimately shipped to customers in over fifty countries around the world. This facility is both ISO 9001:2008- and ISO 14001-certified.
Auckland, New Zealand. This ISO 9001:2008-certified facility produces CCA-based wood preservatives and other wood preservative chemicals for the New Zealand market and serves as the commercial office for the New Zealand business.
Grangemouth, Scotland. This manufacturing site is owned and operated by CalaChem under a toll manufacturing arrangement with the Company. We own all of the equipment used in the direct manufacture of PHMB products as well as the HMBDA (Hexamethylene-1,6-Bis-Dicyandiamide) intermediate. The PHMB product is produced in various solutions and in a solid format.
Kempton Park, South Africa. This facility produces and packages calcium hypochlorite for the HTH water products business principally within the Southern Africa region. Other products for the swimming pool and water treatment markets are also packaged at this site. The site also includes office space.
| Item 3. | Legal Proceedings. |
In connection with the Distribution, we assumed substantially all non-environmental liabilities for legal proceedings relating to our businesses as conducted prior to the Distribution Date. In addition, in the normal course of business, we are subject to other proceedings, lawsuits and other claims, including proceedings under laws and regulations related to environmental and other matters. All such matters are subject to many uncertainties and outcomes that are not predictable with assurance. While these other matters could materially affect operating results when resolved in future periods, it is management’s opinion that after final disposition, any monetary liability or financial impact to us beyond that provided in the Consolidated Balance Sheet as of December 31, 2010, would not be material to our consolidated financial position or annual results of operations.
| Item 4. | [Removed and reserved.] |
Executive Officers of the Registrant
The biographical information of the executive officers of the Company as of February 15, 2011 is noted below.
| | |
Name and Age | | Office |
Michael E. Campbell (63) | | Chairman of the Board, President and Chief Executive Officer |
Luis Fernandez-Moreno (48) | | Corporate Executive Vice President |
Joseph H. Shaulson (45) | | Corporate Executive Vice President |
Steven C. Giuliano (41) | | Corporate Senior Vice President and Chief Financial Officer |
Sarah A. O’Connor (51) | | Corporate Senior Vice President, Strategic Development and Chief Legal Officer |
W. Anthony Sandonato (54) | | Corporate Vice President, Human Resources |
W. Paul Bush (60) | | Vice President and Treasurer |
Meghan DeMasi (35) | | Controller |
22
No family relationship exists between any of the above named executive officers or between any of them and any Director of the Company. Such officers were elected or appointed to serve as such, subject to the Bylaws, until their respective successors are chosen.
Mr. Campbell was elected Chairman of the Board and Chief Executive Officer on February 7, 1999. On July 27, 2000, he was given the additional title of President. Prior to the Distribution, he was Executive Vice President of Olin and had global management responsibility for all of Olin’s businesses. Prior to his election as an Executive Vice President of Olin, Mr. Campbell served as President of Olin’s Microelectronic Materials Division. Prior to that time and since 1987, he served as Olin’s Corporate Vice President, Human Resources.
Mr. Fernandez-Moreno was elected Corporate Executive Vice President, effective September 1, 2010. Prior to that and since April 2009, he served as Business Group Vice President of the Dow Coating Materials business of Dow Chemical Company. Prior to that and since January 2008, he served as Vice President, Business Group Director for the Paint and Coating Materials business of Rohm and Haas Company. Prior to that and since April 2005, he served as Vice President, Business Unit Director for the Architectural and Functional Coatings business unit of Rohm and Haas Company. Prior to 2005 and since 1983, he served at Rohm and Haas in various other positions, including Vice President, Business Unit Director for the Plastic Additives business unit and Vice President, Regional Director for Latin America.
Mr. Shaulson was elected Corporate Executive Vice President, effective September 1, 2010. Prior to that, and since September 21, 2009, he served as Senior Vice President, Wood Protection, Industrial Coatings and Personal Care Ingredients. Prior to that and since August 8, 2008, he served as our Corporate Vice President, Strategic Development. Prior to that, and since 2001, he had served as President of the Reinforcements Business Unit of Hexcel Corporation, a leading advanced structural materials company. Prior to 2001, he served at Hexcel in various other executive positions, including Vice President of Corporate Planning & Chief Information Officer and Vice President of Corporate Development. Prior to joining Hexcel, Mr. Shaulson was a corporate associate in the law firm of Skadden, Arps, Slate, Meagher & Flom LLP from 1991 to 1996.
Mr. Giuliano was elected Corporate Senior Vice President and Chief Financial Officer on September 21, 2009. From May 25, 2007 until then, he held the position of Vice President and Chief Financial Officer. Prior to that date and since January 27, 1999, he was Controller. Prior to the Distribution, Mr. Giuliano was an Audit Senior Manager for KPMG LLP and prior to that and since 1991, he held various positions of increasing responsibility for KPMG LLP, where he had overall responsibility for services provided in connection with audits, SEC filings, private offerings and other services for certain domestic and multinational clients.
Ms. O’Connor was elected Corporate Senior Vice President, Strategic Development, Chief Legal Officer and Secretary on September 21, 2009. She ceased being Secretary on January 28, 2010. Prior to then and since February 7, 1999, Ms. O’Connor was Corporate Vice President, General Counsel and Secretary. She was elected a Vice President of the Company on October 13, 1998 when the Company was a wholly owned subsidiary of Olin. Prior to the Distribution and since 1995, Ms. O’Connor served as Olin’s Director, Planning and Development. Ms. O’Connor became an Associate Counsel in the Olin Corporate Legal Department in 1989 and was promoted to Counsel in 1992 and to Senior Counsel in January 1995.
Mr. Sandonato was appointed a Vice President on November 29, 2010 and was subsequently elected Corporate Vice President, Human Resources effective December 30, 2010. Prior to that and since April 1, 2009, he had served as Vice President, Human Resources, Global Business and Corporate Support. Prior to that and since the Distribution, Mr. Sandonato served as Director, Human Resources. Before that and after joining Olin in 1977, he served in positions of increasing responsibility within Human Resources at five Olin locations.
Mr. Bush was elected Treasurer on February 7, 1999 and also appointed a Vice President on that date. Prior to the Distribution and since February 1998, Mr. Bush was a consultant to Olin. Prior to February 1998, and since March 1994, he was Vice President, Treasurer and then Vice President, Investments of Johnson & Higgins, an
23
insurance brokerage and benefits consulting firm. Prior to 1994, he held various managerial positions, including Vice President and Treasurer and Vice President, Financial Planning and Analysis for Squibb Corporation.
Ms. DeMasi was elected Controller on May 25, 2007. Prior to this date and since April 1, 2006, Ms. DeMasi was Assistant Controller and prior to that and since April 2003, she was the Director of Financial Reporting and Corporate Accounting. Prior to April 2003, she was an Audit Manager for KPMG LLP, where she had overall responsibility for services provided in connection with audits and SEC filings.
24
PART II
| Item 5. | Market for the Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities. |
As of January 31, 2011, there were approximately 3,846 record holders of the Company’s common stock.
The Company’s common stock is traded on the New York Stock Exchange (“NYSE”) under the symbol “ARJ.” The Company has filed as Exhibits 31.1 and 31.2 to this Report the certifications required by Section 302 of the Sarbanes-Oxley Act.
Information concerning the high and low sales prices of the Company’s common stock and dividends paid on common stock during each quarterly period for the last two most recent fiscal years is set forth in Note 24 of Notes to Consolidated Financial Statements contained in Item 8 of this Report.
Among the provisions of the Company’s major domestic debt agreements are restrictions relating to the payments of dividends and the repurchase by the Company of its common stock based on a financial formula. As of December 31, 2010, dividends and stock repurchases were limited to $97.7 million.
The Company has not repurchased any shares of the Company’s common stock in the three months ending December 31, 2010.
See Item 12 of this Report for Equity Compensation Plan information.
25
Comparison of Five Year Cumulative Total Return Among Arch Chemicals, Inc.,
the S&P 500 Index and the S&P 500 Chemicals Index
The graph below compares the cumulative total shareholder return of the Company’s common stock to the Standard & Poor’s 500 Index and the Standard & Poor’s 500 Chemicals Index for the period from December 31, 2005 to December 31, 2010, the last trading day of the Company’s fiscal year. The graph assumes that the value of the investment in the common stock and each index was $100 at close of December 31, 2005 and that all dividends were reinvested. The total cumulative dollar returns shown on the graph represent the value that such investments would have had on December 31, 2010.
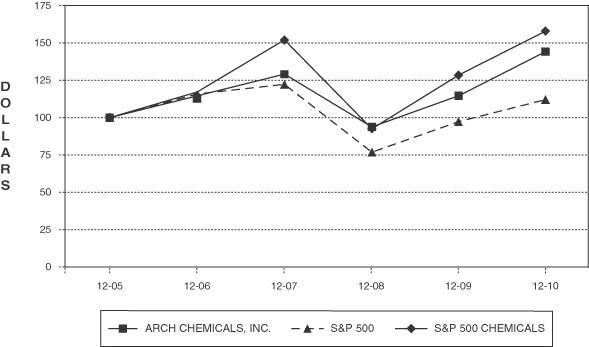
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Years Ending December 31 | |
| | | 2005 | | | 2006 | | | 2007 | | | 2008 | | | 2009 | | | 2010 | |
ARCH CHEMICALS, INC. | | $ | 100.0 | | | $ | 114.4 | | | $ | 129.0 | | | $ | 93.8 | | | $ | 114.6 | | | $ | 144.2 | |
S&P 500 | | | 100.0 | | | | 115.8 | | | | 122.2 | | | | 77.0 | | | | 97.3 | | | | 112.0 | |
S&P 500 CHEMICALS | | | 100.0 | | | | 117.0 | | | | 151.8 | | | | 92.4 | | | | 128.5 | | | | 157.9 | |
26
| Item 6. | Selected Financial Data |
The following table summarizes certain selected historical financial and operating information with respect to the Company and is derived from the Consolidated Financial Statements of the Company. The financial data as of and for each of the years in the three-year period ended December 31, 2010 were derived from the audited financial statements included elsewhere herein. Such historical financial data may not be indicative of the Company’s future performance. The information set forth below should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the historical Consolidated Financial Statements and Notes thereto included elsewhere in this Form 10-K. The following information is qualified in its entirety by the information and financial statements appearing elsewhere in this Form 10-K. See Note 1 of the Notes to Consolidated Financial Statements for additional information.
As a result of the sale of the industrial coatings business, the Company has adjusted its prior year financial statements to include the results of the industrial coatings business as a component of discontinued operations.
| | | | | | | | | | | | | | | | | | | | |
| | | As of and For the Years Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | | | 2007 | | | 2006 | |
| | | ($ in millions, except per share amounts) | |
Operations | | | | | | | | | | | | | | | | | | | | |
Sales | | $ | 1,377.4 | | | $ | 1,244.8 | | | $ | 1,300.2 | | | $ | 1,300.4 | | | $ | 1,244.8 | |
Cost of Goods Sold (1) | | | 952.5 | | | | 876.2 | | | | 923.0 | | | | 921.7 | | | | 917.2 | |
Selling and Administration | | | 295.0 | | | | 266.7 | | | | 252.6 | | | | 265.7 | | | | 243.1 | |
Research and Development | | | 20.1 | | | | 18.7 | | | | 17.3 | | | | 15.6 | | | | 14.2 | |
Other (Gains) and Losses (2) | | | 1.8 | | | | — | | | | (1.8 | ) | | | (12.8 | ) | | | (1.2 | ) |
Impairment, Restructuring and Other Expense (3) | | | 1.9 | | | | 1.1 | | | | 2.1 | | | | 16.0 | | | | — | |
Interest Expense, net (4) | | | 12.2 | | | | 12.1 | | | | 10.6 | | | | 13.0 | | | | 20.0 | |
| | | | | | | | | | | | | | | | | | | | |
Income from Continuing Operations Before Taxes and Equity in Earnings of Affiliated Companies | | | 93.9 | | | | 70.0 | | | | 96.4 | | | | 81.2 | | | | 51.5 | |
Equity in Earnings of Affiliated Companies | | | 0.6 | | | | 0.6 | | | | 0.4 | | | | 0.5 | | | | 0.8 | |
Income Tax Expense | | | 30.0 | | | | 22.3 | | | | 36.3 | | | | 32.9 | | | | 17.0 | |
| | | | | | | | | | | | | | | | | | | | |
Income from Continuing Operations | | | 64.5 | | | | 48.3 | | | | 60.5 | | | | 48.8 | | | | 35.3 | |
Income (Loss) from Discontinued Operations, net of tax (5) | | | 6.2 | | | | (1.2 | ) | | | (23.5 | ) | | | (13.5 | ) | | | (21.1 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net Income | | $ | 70.7 | | | $ | 47.1 | | | $ | 37.0 | | | $ | 35.3 | | | $ | 14.2 | |
| | | | | | | | | | | | | | | | | | | | |
Diluted Income Per Share | | $ | 2.80 | | | $ | 1.88 | | | $ | 1.49 | | | $ | 1.43 | | | $ | 0.58 | |
Diluted Income Per Share—Continuing Operations | | | 2.56 | | | | 1.92 | | | | 2.43 | | | | 1.98 | | | | 1.45 | |
Common Dividends Per Share | | | 0.80 | | | | 0.80 | | | | 0.80 | | | | 0.80 | | | | 0.80 | |
| | | | | |
Other | | | | | | | | | | | | | | | | | | | | |
Capital Expenditures | | | 29.4 | | | | 27.4 | | | | 49.9 | | | | 38.1 | | | | 23.4 | |
Depreciation | | | 29.4 | | | | 30.9 | | | | 30.2 | | | | 30.8 | | | | 32.1 | |
Amortization of Intangibles | | | 10.9 | | | | 10.8 | | | | 9.7 | | | | 9.0 | | | | 7.8 | |
Effective Tax Rate (6) | | | 31.7 | % | | | 31.6 | % | | | 37.5 | % | | | 40.3 | % | | | 32.5 | % |
| | | | | |
Financial Position | | | | | | | | | | | | | | | | | | | | |
Working Capital (7) | | $ | 168.1 | | | $ | 169.9 | | | $ | 192.0 | | | $ | 155.3 | | | $ | 123.7 | |
Property, Plant and Equipment, net | | | 176.5 | | | | 173.5 | | | | 169.3 | | | | 155.8 | | | | 151.1 | |
Total Assets | | | 1,238.0 | | | | 1,210.5 | | | | 1,232.4 | | | | 1,188.2 | | | | 1,149.6 | |
Long-Term Debt, excluding current portion | | | 327.8 | | | | 257.7 | | | | 314.5 | | | | 178.8 | | | | 62.4 | |
Shareholders’ Equity | | | 443.0 | | | | 404.9 | | | | 361.9 | | | | 474.4 | | | | 366.2 | |
Capitalization | | | 809.3 | | | | 695.6 | | | | 694.9 | | | | 682.6 | | | | 583.2 | |
Notes to Selected Financial Data appear on the next page.
27
| (1) | Cost of Goods Sold for 2010 includes $3.1 million of income related to a settlement with a supplier. Cost of Goods Sold for 2009 includes a benefit of $2.9 million related to a LIFO decrement and a benefit of $1.0 million related to the 2009 favorable antidumping ruling which impacted purchases made by the Company from June 1, 2007 to May 31, 2008. Cost of Goods Sold for 2008 includes a benefit of $11.5 million related to the 2008 favorable antidumping ruling which impacted purchases made by the Company from June 1, 2006 to May 31, 2007. Cost of Goods Sold for 2007 includes a benefit of $16.9 million related to the 2007 favorable antidumping ruling which impacted purchases made by the Company from December 16, 2004 to May 31, 2006. Additionally, Cost of Goods Sold for 2007 includes a $0.4 million charge related to the disposal of inventory resulting from the Company’s decision to discontinue the manufacturing of its BIT molecule. Cost of Goods Sold for 2006 includes a charge of $3.6 million for an early termination of a supply contract. |
| (2) | Other (Gains) and Losses for 2010 represents a charge related to a duty claim on certain products imported into France. Other (Gains) and Losses for 2008 represents the reversal of penalties and interest related to a Brazilian state import tax claim recorded in 2004 of $1.4 million due to the expiration of the statute of limitations and a $0.4 million gain from a revised estimate of shutdown costs related to the completion of a contract with the U.S. Government in 2007. Other (Gains) and Losses for 2007 represents a gain for the completion of a contract with the U.S. Government. Other (Gains) and Losses for 2006 represents the pre-tax gain on the sale of excess land of $0.8 million and a pre-tax gain on the sale of certain assets in Brazil of $0.4 million. |
| (3) | Impairment, Restructuring and Other Expense consist of the following: |
| | |
| Impairment Charge— | | 2010 represents a $1.2 million charge on the Company’s New Castle, Delaware facility due to the consolidation of most of the Company’s U.S. research and development activities currently conducted at three facilities. 2008 represents a $0.8 million charge for certain manufacturing assets in the wood protection business. 2007 includes a charge of $7.9 million for the impairment of manufacturing assets in conjunction with the Company’s decision to discontinue the manufacturing of its BIT molecule. |
| |
| Restructuring and Other Expense— | | 2010 represents a $0.7 million charge for estimated employee severance costs due to the consolidation of most of the Company’s U.S. research and development activities currently conducted at three facilities. 2009 represents executive severance. 2008 represents a $1.3 million cash charge related to a pension settlement associated with executive severance recorded in 2007. 2007 includes a $7.2 million charge relating to the Company’s decision to discontinue the manufacturing of its BIT molecule. Additionally, 2007 includes $0.9 million of executive severance costs. |
| (4) | Interest Expense, net for 2008 includes $1.2 million of interest income related to the favorable antidumping ruling for the period from June 1, 2006 to May 31, 2007. Interest Expense, net for 2007 includes $1.5 million of interest income related to the favorable antidumping ruling for the period from December 16, 2004 to May 31, 2006. Interest expense, net, was not significantly impacted by the antidumping rulings issued in 2010 and 2009. |
28
| (5) | The following details the components of Income (Loss) from Discontinued Operations, net of tax: |
| | | | | | | | | | | | | | | | | | | | |
| | | As of and For the Years Ended December 31, | |
($ in millions) | | 2010 | | | 2009 | | | 2008 | | | 2007 | | | 2006 | |
Discontinued Operations, Results of Operations: | | | | | | | | | | | | | | | | | | | | |
Industrial Coatings business (a) | | $ | (0.5 | ) | | $ | (0.6 | ) | | $ | (23.5 | ) | | $ | 0.5 | | | $ | (21.2 | ) |
Performance Urethanes Venezuelan business (b) | | | — | | | | — | | | | — | | | | 0.9 | | | | 0.8 | |
Microelectronic Materials (c) | | | — | | | | — | | | | — | | | | — | | | | (0.7 | ) |
| | | | | |
Gain (Loss) on Sales of Discontinued Operations: | | | | | | | | | | | | | | | | | | | | |
Industrial Coatings business (d) | | | 6.6 | | | | (0.6 | ) | | | — | | | | — | | | | — | |
Performance Urethanes Venezuelan business (e) | | | 0.1 | | | | — | | | | — | | | | (14.9 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total Income (Loss) from Discontinued Operations, net of tax | | $ | 6.2 | | | $ | (1.2 | ) | | $ | (23.5 | ) | | $ | (13.5 | ) | | $ | (21.1 | ) |
| | | | | | | | | | | | | | | | | | | | |
| | (a) | Represents the results of operations, net of tax, for the industrial coatings business for all years presented through the date of sale in March 2010. 2008 includes a $24.6 million charge for the impairment of goodwill. 2006 includes a $23.5 million charge for the impairment of goodwill. |
| | (b) | Represents the results of operations, net of tax, for the performance urethanes business in Venezuela for all years presented through the date of sale in September 2007. |
| | (c) | Represents the results of operations, net of tax, for the chemical management services business through December 31, 2006. |
| | (d) | 2010 represents the gain on sale of the industrial coatings business. 2009 represents transaction costs related to the sale. |
| | (e) | Represents the loss on sale of the performance urethanes business in Venezuela. |
| (6) | The effective tax rate is based on continuing operations. The 2010 effective income tax rate was 31.7%. In 2010, legislation was finalized in the U.K. which reduced the corporate tax rate from 28% to 27%. Included in 2010 is $1.3 million of tax expense that represents the reduction of a tax benefit previously recorded directly through equity, related to the U.K. pension liabilities. The original tax benefit was not recorded in the income statement. Excluding the effect of this legislation, the tax rate for 2010 was 30.3%. In 2007, legislation was finalized in the U.K. which reduced the corporate tax rate from 30% to 28%. Included in 2007 is $3.0 million of tax expense that represents the reduction of a tax benefit previously recorded directly through equity, related to the U.K. pension liabilities. The original tax benefit was not recorded in the income statement. Additionally, the net impact of Other (Gains) and Losses and Restructuring and Impairment was to increase the effective tax rate for 2007 by approximately three percent. Excluding the effect of the U.K. tax legislation noted above, Other (Gains) and Losses, and the Restructuring and Impairment charges, the effective tax rate for 2007 is 33.8%. |
| (7) | Working capital excludes cash, short-term debt and assets held for sale. |
29
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
This Management’s Discussion and Analysis of Financial Condition and Results of Operations should be read in conjunction with the Company’s Consolidated Financial Statements and Notes thereto included elsewhere herein. Sales consist of sales to third parties net of any discounts. Gross Margin is defined as Sales less Cost of Goods Sold, which includes raw materials, labor, overhead and depreciation associated with the manufacture of the Company’s various products and shipping and handling costs. In addition, segment operating income excludes restructuring (income) expense and impairment. The Company believes the exclusion of restructuring and impairment expense from segment operating income provides additional perspective on the Company’s underlying business trends and provides useful information to investors by excluding amounts from the Company’s results that the Company does not believe are indicative of ongoing operating results. Other gains and losses that are directly related to the segments are included in segment operating results.
As a result of the sale of the industrial coatings business, the Company has adjusted its prior year financial statements to include the results of the industrial coatings business as a component of discontinued operations. In addition, as a result of the sale, the Company has adjusted its prior period segment operating results to reallocate certain centralized service costs that were previously allocated to the industrial coatings business to the Company’s other businesses.
The Company saw improvement in consumer demand during 2010, following the depressed global economic conditions that impacted the operating performance of several of the Company’s businesses in 2009.
The overall economic environment for our wood protection business was depressed in 2009 as new housing starts were down significantly and consumers continued to be faced with general economic uncertainty. These factors decreased demand for lumber and other construction materials, which lowered the operating results and cash flows of the business. The Company saw improvement in the wood protection business in 2010 from 2009 but still below the pre-2009 levels. If there is no recovery over the next several years or if global economic conditions deteriorate, the earnings and cash flows for this business may be significantly impacted, which could impair certain long-lived assets for this business.
Additionally, the depressed economy in 2009 impacted our performance urethanes business, reducing its overall operating results, and has persisted into 2010. The performance urethanes business also had a significant contract expire at the end of 2009, which decreased the annual operating income of the business by approximately $12 million. We believe that organic growth and a pipeline of new product offerings should mitigate a portion of this decrease. If there is no recovery over the next several years or if the business is unsuccessful in new product offerings, the earnings and the cash flows for this business may be significantly impacted, which could impair certain long-lived assets for this business.
30
Results of Operations
Consolidated
| | | | | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | (in millions, except per share amounts) | |
Sales | | $ | 1,377.4 | | | $ | 1,244.8 | | | $ | 1,300.2 | |
Gross Margin | | | 424.9 | | | | 368.6 | | | | 377.2 | |
Selling and Administration | | | 295.0 | | | | 266.7 | | | | 252.6 | |
Research and Development | | | 20.1 | | | | 18.7 | | | | 17.3 | |
Other (Gains) and Losses | | | 1.8 | | | | — | | | | (1.8 | ) |
Restructuring and Other Expense | | | 0.7 | | | | 1.1 | | | | 1.3 | |
Impairment Charge | | | 1.2 | | | | — | | | | 0.8 | |
Interest Expense, net | | | 12.2 | | | | 12.1 | | | | 10.6 | |
Equity in Earnings of Affiliated Companies | | | 0.6 | | | | 0.6 | | | | 0.4 | |
Income Tax Expense | | | 30.0 | | | | 22.3 | | | | 36.3 | |
Loss from Discontinued Operations, net of tax | | | (0.5 | ) | | | (0.6 | ) | | | (23.5 | ) |
Gain (Loss) on the Sale of Discontinued Operations, net of tax | | | 6.7 | | | | (0.6 | ) | | | — | |
| | | | | | | | | | | | |
Net Income | | $ | 70.7 | | | $ | 47.1 | | | $ | 37.0 | |
| | | | | | | | | | | | |
Basic Income Per Share | | $ | 2.82 | | | $ | 1.89 | | | $ | 1.49 | |
Diluted Income Per Share | | $ | 2.80 | | | $ | 1.88 | | | $ | 1.49 | |
Basic Income Per Share—Continuing Operations | | $ | 2.58 | | | $ | 1.93 | | | $ | 2.44 | |
Diluted Income Per Share—Continuing Operations | | $ | 2.56 | | | $ | 1.92 | | | $ | 2.43 | |
Weighted Average Common Stock Outstanding: | | | | | | | | | | | | |
Basic | | | 25.1 | | | | 25.0 | | | | 24.8 | |
Diluted | | | 25.2 | | | | 25.1 | | | | 24.9 | |
Arch Chemicals is the global leader in providing innovative, chemistry-based and related solutions to destroy or to selectively inhibit the growth of harmful microorganisms. The Company provides products for a broad range of markets involving human health and environmental needs and is concentrated in the areas of water treatment, personal care, health and hygiene, industrial preservation and protection, and wood treatment. The businesses that address these markets comprise the Company’s core Biocides Products business segment, which currently accounts for 80—90% of the Company’s annual sales. In addition, Arch’s portfolio includes a urethanes business and a hydrazine business, both of which the Company deems as non-core.
The Company’s platform strengths are its:
| | • | | Core competencies that create true competitive advantages, including in-depth understanding of microbiology and how chemicals affect microorganisms—skills that enable employees to develop new products and to find new applications for existing products; chemical formulation skills tailored to meet the needs of any particular customer and the ability to deliver that chemistry in a way that makes it most effective; analytical detection and analysis for microbial control; strong customer relationships based upon a shared clarity of business purpose, science and good engineering execution; and extensive expertise in the regulatory procedures that govern the use of biocidal products, in particular excellence in toxicology on which those regulations are based to assure Arch’s products can be used safely in terms of the environment and human health. The Company has invested in upgrading and expanding its technical strengths in these disciplines to meet increasingly global regulatory requirements, including those relating to the European Biocidal Products Directive (“BPD”), which requires biocide manufacturers to re-register their biocidal products for sale in the EU, and the EU’s Registration, Evaluation and Authorization of Chemical Substances (“REACH”) legislation. While some companies |
31
| | view increasing regulations as a hindrance or barrier, and while such regulations can create uncertainty in the marketplace, overall, the Company sees it as a competitive advantage due to our expertise and commitment to regulatory requirements. |
| | • | | Leading market positions in attractive markets with robust global growth potential. Arch is the world’s largest supplier of antidandruff actives, a major global swimming pool and spa chemical sanitizer provider, a leading industrial wood treatment and services provider in the world, and a premier supplier of biocides that are incorporated into paints and coatings and marine antifouling paints. The Company is capitalizing on global megatrends that are creating a wide range of growth opportunities in the construction, building materials, food protection, water management, personal care and health and hygiene markets. |
| | • | | Broad global infrastructure with worldwide manufacturing facilities and research facilities on four continents. With approximately 50 percent of the Company’s sales and total long-lived assets outside the U.S., Arch is well positioned with a strong global footprint and a growing presence in the fastest growing regions, including China, India, Brazil and Southeast Asia. |
Embedded in the Company’s culture and core values is its commitment to continuous improvement in innovation, operational excellence and sustainability. Arch works closely with customers to craft solution-driven innovations for unmet customer and market needs through internal research programs, open innovation programs, technology licensing and external strategic alliances to obtain access to new and valuable chemistries. The Company has made significant improvement in achieving world-class global manufacturing, supply chain and workplace safety and environmental performance. Arch’s sustainability program involves evaluating raw material sourcing, product manufacturing, packaging, distribution and customer use, considering factors such as carbon emission, natural resource, and water usage, waste profiles, and societal impact. The Company aligns its efforts in sustainability with strategic customers, while assuring that there is value created for Arch.
The Company is executing a three-point strategy expected to propel it into the top quartile of global specialty chemical companies, while delivering significantly increased shareholder value. The first element of this strategy is to complete the transformation to a 100 percent biocides portfolio through acquisitions and by divesting the remaining, non-biocides businesses. The Company has a disciplined acquisition process aimed at complementing its strengths and advancing its business strategies. The Company focuses on acquisition opportunities that are strategic. Any acquisition candidate must strengthen or build upon the Company’s current biocides portfolio, add new biocide molecules to the product range, be present in new product markets or strengthen the Company’s geographic profile. Acquisition opportunities are also screened against specific financial targets, such as, cash accretion in year one and earnings accretion no later than the end of the first year. When an opportunity meets the Company’s strategic and financial criteria, a detailed integration plan is developed prior to completing the acquisition.
The second element is to grow organic sales in the biocides businesses by 6 to 8 percent per year by accelerating the Company’s innovation efforts and capitalizing on rising demand in emerging markets. The third element is to improve profitability through a multifaceted margin improvement plan, including supply chain sourcing initiatives, global manufacturing optimization and operating expense cost-reduction initiatives, while improving cash flow generation. The Company expects to improve its operating income margins from 6.7 percent in 2009 to over 10 percent in 2013.
The Company has an annual compensation plan and a long-term incentive compensation plan for its executives and other employees. The annual plan’s financial targets for corporate and senior management are diluted earnings per share and cash flow. The numerator for diluted earnings per share is net income adjusted for any extraordinary income or expense, special charges or gains, impairment charges, and gains or losses on sales of businesses or sales not in the ordinary course of business (“adjusted net income”). Cash flow is defined as EBITDA, plus or minus the change in working capital, less capital spending. The annual plan’s financial targets for the business units are pre-tax income and cash flow. The Company’s long-term incentive plan target is return on equity (“ROE”). ROE is defined as adjusted net income divided by average shareholder’s equity (the average
32
calculated using the shareholder’s equity at the beginning and the end of the fiscal year, excluding the impact on ending shareholder’s equity of any adjustments to net income). These financial metrics are key performance indicators utilized by the Company to evaluate its performance against stated goals. In addition, the estimated and actual performance against such targets can have a significant impact on the amount of incentive compensation expense recorded by the Company. A portion of the Company’s long-term incentive plan awards are paid out in stock and a portion are paid out in cash. The portion of the awards which are paid out in cash is based upon the market price of the Company’s common stock, and therefore, the amount of incentive compensation expense would vary based upon the market price of the Company’s stock at the end of each reporting period. During the fourth quarter of 2008, the Company entered into equity total return swap agreements in order to minimize earnings volatility related to the long-term incentive plan. For additional information, see Note 13 of Notes to the Consolidated Financial Statements.
Year Ended December 31, 2010 Compared to 2009
Sales increased $132.6 million, or 11 percent, due to higher volumes (nine percent) and favorable foreign exchange (two percent). The higher volumes were driven by increased demand in our Biocides Products segment.
Gross margin percentage was 30.8% and 29.6% for 2010 and 2009, respectively. The increase in gross margin percentage in 2010 was principally due to higher sales volumes and favorable absorption for the personal care and industrial biocides business, as well as lower raw material costs for the wood protection and personal care and industrial biocides businesses. This increase in gross margin percentage was partially offset by the completion, at the end of 2009, of a long-term contract manufacturing arrangement in the performance urethanes business. Included in 2010 gross margin was $3.1 million of income related to a settlement with a supplier of the hydrazine and performance urethanes businesses. During 2009 the Company recorded a $2.9 million benefit related to a LIFO decrement and a $1.0 million antidumping duty benefit related to a final determination from the U.S. Department of Commerce (“DOC”).
Selling and administration expenses as a percentage of sales were 21.4% in 2010 and 2009. Selling and administration expenses increased $28.3 million from 2009 principally due to higher compensation-related costs, unfavorable foreign exchange and higher pension expense. Despite this increase, Selling and administration expenses as a percentage of sales were consistent with 2009 due to the increase in sales.
Research and development increased $1.4 million due to higher spending for the personal care and industrial biocides business.
Other (gains) and losses in 2010 represents a charge related to a duty claim on certain products imported into France.
In August 2010, the Company announced its decision to consolidate into one facility in Alpharetta, Georgia most of its existing U.S. research and development (“R&D”) and technical service activities currently conducted at three facilities. During 2010, the Company recorded a $0.7 million charge for estimated employee severance costs. Additionally, the Company recorded a $1.2 million impairment charge related to its New Castle, Delaware facility. The total pre-tax charge to consolidate these facilities is estimated to be in the range of $5 million to $7 million. Excluding the amounts which were recorded in 2010, the remaining charge will consist of (i) $2 million to $3 million of relocation-related and employee severance costs and (ii) $1 million to $2 million of other costs. The Company anticipates that the consolidation will be completed, and the remaining charge recorded, in 2011.
Restructuring and other expense in 2009 relates to executive severance.
Interest expense, net, was consistent with 2009 as lower net debt was offset by the higher cost of longer-term fixed rate borrowings.
The tax rate on income from continuing operations for the year ended December 31, 2010 and 2009 was 31.7% and 31.6%, respectively. In 2010, legislation was finalized in the United Kingdom (“U.K.”) which reduced the corporate tax rate from 28% to 27%. The Company has significant U.K. deferred tax assets,
33
principally related to the Company’s U.K. pension plans. As a result of the tax rate change, the Company recorded non-cash expense of $1.3 million to reduce the deferred tax assets related to the pension plans. The original tax benefit was not recorded in the income statement and, instead, was recorded directly through equity. Excluding the effect of this legislation, the effective tax rate for 2010 was 30.3%. This rate was lower than the 2009 effective tax rate as 2010 benefited from higher taxable income in foreign jurisdictions with lower tax rates due to tax holidays.
Loss from discontinued operations, net, represents the results of operations for the industrial coatings business until its sale in March 2010.
During 2010, the Company recorded an after-tax gain of $6.6 million related to the sale of the industrial coatings business on March 31, 2010. During 2009, the Company recorded transaction costs, $0.6 million net of tax, related to the sale. Therefore, the total net gain on the sale of the business was $6.0 million.
Antidumping Rulings
During the fourth quarter of 2010, the DOC made its final determination that changed the Company’s antidumping duty rate to approximately 3% for chlorinated isocyanurates (“isos”) that the Company imported from a Chinese supplier for the period from June 1, 2008 through May 31, 2009. As a result of this final determination, the Company recorded a pre-tax benefit of $0.1 million in the fourth quarter of 2010 and it began paying cash deposits for imports at a rate of approximately 3%. The U.S. isos producers appealed the DOC’s determination to the Court of International Trade. The appeal is delaying the cash refund of the duty to the Company and may result in a change of the duty rate for this review period. The Company believes that the resolution of this matter is not likely to have a material adverse effect on its results of operations and cash flows.
During the fourth quarter of 2009, the DOC made its final determination that reduced the Company’s antidumping duty rate to 20% for isos that the Company imported from a Chinese supplier for the period from June 1, 2007 through May 31, 2008. As a result of this final determination, the Company recorded a pre-tax benefit of $1.0 million in the fourth quarter of 2009. The determination was not appealed and during 2010 the Company received the cash refund.
During the third quarter of 2008, the DOC made its final determination that reduced the Company’s antidumping duty rate from 76% to less than 1% for isos that the Company imported from a Chinese supplier for the period from June 1, 2006 through May 31, 2007. As a result of this final determination, the Company recorded a pre-tax benefit of $12.7 million, including $1.2 million of interest, in the third quarter of 2008. The U.S. isos producers appealed the DOC’s determination to the Court of International Trade. The appeal is delaying the cash refund (approximately $14 million) of the duty to the Company and may result in a change of the duty rate for this review period. The Company believes that the resolution of this matter is not likely to have a material adverse effect on its results of operations and cash flows.
During the fourth quarter of 2007, the DOC made its final determination that reduced the Company’s antidumping duty rate from 76% to 20% for isos that the Company imported from a Chinese supplier for the period from December 16, 2004 through May 31, 2006. As a result of this final determination and the revised rate, the Company recorded a net pre-tax benefit of $12.1 million, including $1.5 million of interest, in the fourth quarter of 2007. The U.S. isos producers appealed the DOC’s determination to the Court of International Trade, which has delayed the processing of the full refund the Company was expecting to receive. On July 13, 2009, the Court of International Trade issued its decision which required the DOC to review additional information and revise the rate accordingly. The DOC complied and has issued a revised preliminary rate of approximately 9%, which is pending review by the Court of International Trade. Provided that this revised preliminary rate is approved by the Court of International Trade, the Company would recognize additional income of approximately $3 million. Therefore, the total net cash proceeds the Company expects to receive is approximately $15 million.
An administrative review has also commenced to determine the final rate for the period of June 1, 2009 to May 31, 2010.
34
2011 Outlook
The Company expects full-year 2011 sales to increase by approximately five to seven percent as a result of strong organic growth from the Biocides Products segment, partially offset by a modest decrease in sales in the non-biocides businesses. Sales for the Biocides businesses are expected to increase by approximately six to eight percent. Segment operating income margins are expected to be approximately 8.5%. Earnings from continuing operations before special items are forecast to be in the $2.75 to $3.00 per share range. Depreciation and amortization is estimated to be in the $40 to $45 million range. Capital spending is expected to be approximately $55 million, including a major manufacturing technology upgrade at the Company’s HTH water products plant in the U.S. and the consolidation and expansion of three of the Company’s U.S. research and development functions into a newly leased facility. The effective tax rate is expected to be comparable to 2010.
The HTH water products business is expected to report higher profits than 2010, due to modestly improving market conditions in many of the Company’s key regions and normal weather patterns. The guidance assumes the Company will recognize a pre-tax benefit (approximately $3 million) from a lower antidumping duty rate due to the expected favorable final ruling of the first administrative review period under appeal. It also assumes a similar pre-tax benefit from an expected lower antidumping duty rate for the fifth administrative review period covering isos purchased from June 1, 2009 to May 31, 2010. These expected benefits should be partially offset by higher sourcing costs for these products from China.
The Company expects improved operating results for personal care and industrial biocides due to higher volumes and favorable plant and sourcing costs, which should more than offset increased spending for regulatory and toxicology compliance. The increased demand for the Company’s biocides is expected in industrial applications for building products and plastics as a result of improving market conditions, new applications of existing products and market penetration and to a lesser extent in health and hygiene applications. Wood protection results are forecast to improve as a result of a moderate recovery in the U.S. housing and construction markets in 2011, cost reduction programs and new customer acquisitions. Performance Products results are expected to be below 2010 due to higher plant costs related to the operation of the hydrazine propellants manufacturing facility for the U.S. Government and the $3.1 million settlement received from Lyondell in 2010. 2011 guidance for the businesses assumes increased investments in innovation. In addition, the Company expects unallocated corporate expenses to be lower than 2010 as a result of reduced compensation-related expenses, while interest expense is expected to increase in 2011 as a result of the issuance of $250 million of the Senior Notes under a master note purchase agreement.
For the first quarter, the Company anticipates earnings from continuing operations before special items to be in the $0.15 to $0.25 per share range, compared to earnings from continuing operations of $0.27 per share during the first quarter of 2010. The Company expects that operating income will be comparable to the prior-year period, while interest expense will be higher.
Year Ended December 31, 2009 Compared to 2008
Sales decreased $55.4 million or four percent. Excluding the impact of the acquisition of Advantis, $49.1 million or four percent, sales decreased $104.5 million, or eight percent, due to lower volumes. Higher pricing, two percent, was offset by unfavorable foreign exchange. The lower volumes were across all businesses as unfavorable weather patterns in North America impacted the HTH water products business and the continued downturn in the economy impacted the remaining businesses. The higher pricing was principally driven by the HTH water products business.
Gross margin percentage was 29.6% and 29.0% for 2009 and 2008, respectively. The margin improvement was principally due to improved pricing in the HTH water products business, which offset higher raw material costs for that business. Additionally, gross margin percentage benefited from higher pricing in the wood protection business. During 2009 the Company recorded a $2.9 million benefit related to a LIFO decrement, which partially offset higher unabsorbed costs. Included in the 2009 and 2008 gross margins were $1.0 million and $11.5 million, respectively, of antidumping duty benefits related to final determinations from the DOC.
35
Selling and administration expenses as a percentage of sales were 21.4% in 2009 and 19.4% in 2008. The increase in Selling and administration expenses as a percentage of sales in 2009 was principally due to lower sales volumes. Selling and administration expenses increased $14.1 million from 2008 principally due to a full year of the Advantis acquisition, higher pension expense and lower compensation-related expense in 2008 as a result of the mark-to-market impact of the Company’s lower stock price on the Company’s performance-based stock awards and deferred compensation plans. These increases to Selling and administration expenses were partially offset by favorable foreign exchange and lower selling costs.
Other (gains) and losses for 2008 represents the reversal of penalties and interest related to a Brazilian state import tax claim recorded in 2004 of $1.4 million due to the expiration of the statute of limitations and a $0.4 million gain from a revised estimate of shutdown costs related to the completion of a contract with the U.S. Government in 2007.
Restructuring and other expense in 2009 relates to executive severance and in 2008 relates to a pension settlement associated with executive severance recorded in 2007.
The impairment charge of $0.8 million in 2008 represents a non-cash charge related to certain manufacturing assets in the Company’s wood protection business.
Interest expense, net, increased $1.5 million as the 2008 results included interest income of $1.2 million related to the final determination from the DOC for the second period of review. Additionally, higher net debt during 2009, due to the acquisition of Advantis, was partially offset by lower cost of borrowings.
The tax rate on income from continuing operations for 2009 and 2008 was 31.6% and 37.5%, respectively. The 2009 effective income tax rate was lower due to the benefits from the recognition of additional tax deductions in conjunction with the completion of the examination of certain tax returns and the expiration of the statute of limitations for certain tax matters.
Loss from discontinued operations, net, represents the results of operations for the industrial coatings business until its sale in March 2010.
Gain (Loss) on sale of discontinued operations, net, relates to transaction costs which were recorded in conjunction with the sale of the industrial coatings business.
Segment Operating Results
The Company has organized its business portfolio into two operating segments to reflect the Company’s business strategy. The two segments are Biocides Products and Performance Products. The Biocides Products segment includes three reportable business units: the HTH water products business, the personal care and industrial biocides business, and the wood protection business. Segment operating income includes the equity in earnings of affiliated companies and excludes restructuring (income) expense and impairment expense. The Company includes the equity income (loss) of affiliates in its segment operating results as it believes it to be relevant and useful information for investors as these affiliates are the means by which certain segments participate in certain geographic regions. Furthermore, the Company includes it to measure the performance of the segment. The Company believes the exclusion of restructuring and impairment expense from segment operating income provides additional perspective on the Company’s underlying business trends and provides useful information to investors by excluding amounts from the Company’s results that the Company does not believe are indicative of ongoing operating results. Other gains and losses that are directly related to the segments are included in segment operating results.
36
Biocides Products
| | | | | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | ($ in millions) | |
Results of Operations | | | | | | | | | | | | |
Sales | | | | | | | | | | | | |
HTH Water Products | | $ | 606.2 | | | $ | 551.1 | | | $ | 501.6 | |
Personal Care & Industrial Biocides | | | 330.6 | | | | 295.0 | | | | 315.6 | |
Wood Protection | | | 254.6 | | | | 231.8 | | | | 267.3 | |
| | | | | | | | | | | | |
Total Biocides Products | | $ | 1,191.4 | | | $ | 1,077.9 | | | $ | 1,084.5 | |
| | | | | | | | | | | | |
Operating Income (Loss) | | | | | | | | | | | | |
HTH Water Products | | $ | 66.3 | | | $ | 62.8 | | | $ | 63.9 | |
Personal Care & Industrial Biocides | | | 71.0 | | | | 42.0 | | | | 60.7 | |
Wood Protection | | | 8.3 | | | | (1.5 | ) | | | 1.5 | |
| | | | | | | | | | | | |
Total Biocides Products | | $ | 145.6 | | | $ | 103.3 | | | $ | 126.1 | |
| | | | | | | | | | | | |
Year Ended December 31, 2010 Compared to 2009
Sales increased $113.5 million, or 11 percent, due to higher volumes. Favorable foreign exchange (two percent) was offset by lower pricing.
Operating income increased $42.3 million principally due to improved operating results for the personal care and industrial biocides business and, to a lesser extent, the wood protection and HTH water products businesses.
HTH Water Products
Sales increased $55.1 million, or ten percent, as higher volumes (eight percent) and favorable foreign exchange (three percent) were partially offset by lower pricing (one percent). The increased volumes were principally in the U.S. and related to all product lines. Additionally, volumes increased, to a lesser extent, in Canada and Europe. The higher U.S. volumes were attributable to increased demand from certain mass retail accounts, as a result of favorable weather patterns, principally in the Northeast and Midwest U.S. and in Canada, as well as from the addition of new customers.
Operating income was $3.5 million higher than prior year as the improved U.S. volumes were partially offset by higher product, selling and marketing costs. Included in 2010 operating income is a $1.8 million charge related to a duty claim on certain products imported into France. Included in 2009 operating income is the benefit related to the favorable antidumping duty ruling of $1.0 million.
Personal Care and Industrial Biocides
Sales increased $35.6 million, or 12 percent, as higher volumes (16 percent) were partially offset by lower pricing (four percent). The higher volumes were primarily due to strong demand for most applications, particularly for biocides used in antidandruff products and biocides used in building products, as the global construction markets saw modest improvement from 2009 and the Company benefited from new applications for both existing and new products. The lower pricing principally related to health and hygiene applications.
Operating income increased $29.0 million as the higher volumes and lower raw material costs more than offset the lower pricing. In addition, 2009 was negatively impacted by unfavorable absorption due to an inventory reduction program.
37
Wood Protection
Sales increased $22.8 million, or ten percent, due to higher volumes. Favorable foreign exchange (three percent) was offset by lower pricing. The higher volumes were driven by increased demand for wood preservatives in North America and Europe. The lower pricing was principally due to country mix throughout Europe.
Operating results improved $9.8 million due to lower raw material costs, the higher volumes and favorable foreign exchange, which more than offset the impact of lower pricing.
Year Ended December 31, 2009 Compared to 2008
Sales decreased $6.6 million or one percent. Excluding the impact of the acquisition of Advantis ($49.1 million or four percent), sales decreased $55.7 million, or five percent, as lower volumes across all businesses (eight percent) and unfavorable foreign exchange (three percent) were partially offset by improved pricing (six percent).
Operating income decreased $22.8 million. The decrease was partially due to the fact that 2008 included an $11.5 million benefit in HTH water products related to the final determination from the DOC which reduced the antidumping duty rate from the Company’s supplier of isos from 76% to less than 1% for purchases made by the Company from June 1, 2006 through May 31, 2007. Additionally, lower operating results for the personal care and industrial biocides and wood protection businesses more than offset improved operating income for the HTH water products business, excluding the impact of the antidumping duty ruling.
HTH Water Products
Sales increased $49.5 million, or 10 percent. Excluding the impact of the acquisition of Advantis ($49.1 million), sales were consistent with 2008. Improved pricing (nine percent), across all regions, was offset by lower volumes (seven percent) and unfavorable foreign exchange (two percent). Lower volumes, principally in North America, were partially offset by improved volumes in South Africa and Brazil. The lower volumes in North America were principally due to unfavorable weather patterns, primarily in the Northeast and Midwest U.S. and in Canada, which led to reduced demand for the Company’s branded products in the mass retail and professional pool dealer segments.
Operating income decreased $1.1 million. Included in 2009 and 2008 operating income are the benefits related to the favorable antidumping duty rulings of $1.0 million and $11.5 million, respectively. Excluding the impact of the rulings, operating income increased $9.4 million as improved pricing and the positive contribution from the acquisition of Advantis more than offset lower volumes, higher product costs, as well as unfavorable foreign exchange.
Personal Care and Industrial Biocides
Sales decreased $20.6 million, or seven percent, due to lower volumes. Improved pricing (one percent) was offset by unfavorable foreign exchange. The lower volumes, which were due to the downturn of the global economy and were across most industrial biocides applications, were partially offset by higher volumes in health and hygiene applications for household and industrial disinfectant applications. The improved pricing principally related to health and hygiene products.
Operating income decreased $18.7 million as lower volumes, higher unabsorbed costs resulting from an inventory reduction program and higher plant costs related to the new manufacturing facilities in China were partially offset by improved pricing, lower freight costs and lower raw material costs. Additionally, operating results for 2008 included foreign currency gains and $0.9 million of income from the sale of rights to certain intellectual property.
38
Wood Protection
Sales decreased $35.5 million, or 13 percent, as significantly lower volumes (12 percent), due to the continued downturn in the economy, and unfavorable foreign exchange (five percent) were partially offset by improved pricing (four percent). Lower volumes across all regions for the residential and industrial sectors, due to the continued downturn in the global construction market, were partially offset by higher global prices.
Operating results were $3.0 million below the prior year as lower volumes and unfavorable foreign exchange more than offset higher pricing and lower selling costs due to cost reduction initiatives.
Performance Products
| | | | | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | ($ in millions) | |
Results of Operations | | | | | | | | | | | | |
Sales | | | | | | | | | | | | |
Performance Urethanes | | $ | 169.1 | | | $ | 150.2 | | | $ | 197.0 | |
Hydrazine | | | 16.9 | | | | 16.7 | | | | 18.7 | |
| | | | | | | | | | | | |
Total Performance Products | | $ | 186.0 | | | $ | 166.9 | | | $ | 215.7 | |
| | | | | | | | | | | | |
Operating Income (Loss) | | | | | | | | | | | | |
Performance Urethanes | | $ | (0.1 | ) | | $ | 8.3 | | | $ | 15.6 | |
Hydrazine | | | 5.3 | | | | 3.3 | | | | 1.1 | |
| | | | | | | | | | | | |
Total Performance Products | | $ | 5.2 | | | $ | 11.6 | | | $ | 16.7 | |
| | | | | | | | | | | | |
Year Ended December 31, 2010 Compared to 2009
Sales increased $19.1 million, or 11 percent, due to improved pricing. Operating income decreased by $6.4 million.
In 2009, Lyondell, a key supplier to the Company’s hydrazine and performance urethanes businesses, filed to reorganize under Chapter 11 of the U.S. Bankruptcy Code. In connection with the bankruptcy case, Lyondell filed several motions with the court to terminate all service agreements with the Company at the Company’s Lake Charles site, as well as a key raw material supply agreement and a toll manufacturing agreement at the Company’s Brandenburg site. After hearings, the bankruptcy court granted the motions and Lyondell terminated the service agreements, the raw material supply agreement and the toll manufacturing agreement. The Company received a $3.1 million pre-tax settlement in December 2010 related to the termination of such agreements.
A new raw material supply agreement has been signed to replace the terminated agreement and the Company has entered into, or expects to finalize shortly, new service agreements with Lyondell and other third parties to replace most of the terminated service agreements, with the exception of certain services that the Company now performs for itself. The new service agreements with Lyondell operate on a year-to-year basis and, as a result, may be terminated after the initial one year term. BioLab, Inc. (“BioLab”), a subsidiary of Chemtura Corporation, is currently providing several services to the Company at this site, including wastewater treatment services. However, the Company has not finalized a contract with BioLab with respect to these services. If the new service agreements with Lyondell are terminated or the Company is unable to finalize the service agreements with BioLab and if the Company is unable to arrange for alternative suppliers or perform such services for itself, there could be a material impact on this segment’s operating results. While, the Company does not believe that there will be a significant impact on operating results in 2011, there may be higher capital expenditures to allow the Company to perform the services that are currently being performed by the third parties.
39
Performance Urethanes
Performance urethanes sales increased $18.9 million, or 13 percent, due to higher pricing driven by increased raw material costs. Volumes were consistent with 2009 as higher demand for propylene glycol and polyol products was offset by the conclusion of a long-term contract manufacturing arrangement at the end of 2009. Operating results decreased by $8.4 million, principally due to the effect of the conclusion of the contract manufacturing arrangement, which contributed approximately $12 million of operating income in 2009. The higher pricing was mostly offset by higher raw material costs. 2010 includes $1.0 million of income related to the settlement with Lyondell.
Hydrazine
Hydrazine sales were consistent with 2009. Operating income increased principally due to $2.1 million of income related to the settlement with Lyondell.
Year Ended December 31, 2009 Compared to 2008
Sales decreased $48.8 million, or 23 percent, due to lower pricing (12 percent) and lower volumes (11 percent). Operating income decreased by $5.1 million.
Performance Urethanes
Performance urethanes sales decreased $46.8 million, or 24 percent. Pricing was 13 percent lower than 2008 due to lower raw material costs. Volumes were also lower than prior year (11 percent) due to the downturn in the U.S. economy. Operating income decreased by $7.3 million from 2008. Included in 2008 operating income is a $1.1 million gain related to a revised estimate of the Company’s liability for Brazilian state import tax claims. Excluding the gain, operating income decreased $6.2 million principally due to the lower pricing.
Hydrazine
Hydrazine sales were lower than 2008 as hydrazine hydrate sales decreased due to depressed market conditions. Operating income was higher than 2008 due to lower operating costs.
Corporate Expenses (Unallocated)
| | | | | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | ($ in millions) | |
Results of Operations | | | | | | | | | | | | |
Unallocated Corporate Expenses | | $ | 42.2 | | | $ | 32.2 | | | $ | 33.3 | |
Year Ended December 31, 2010 Compared to 2009
Unallocated corporate expenses increased principally due to higher compensation-related costs and higher U.K. pension expense. In addition, 2009 benefited from favorable foreign exchange gains associated with loans with the Company’s foreign subsidiaries, which more than offset $1.1 million of executive severance costs which were recorded during 2009.
Year Ended December 31, 2009 Compared to 2008
Unallocated corporate expenses were lower than prior year. The decrease was due to 2008 including unfavorable foreign exchange associated with certain dollar-denominated loans of the Company’s foreign subsidiaries and, to a lesser extent, lower U.K. pension expense in 2009. These factors were partially offset by higher compensation-related expense in 2009, primarily due to 2008 including lower compensation-related expense principally as a result of the mark-to-market impact of the lower stock price during 2008 on the Company’s performance-based stock awards and deferred compensation plans. Additionally, 2009 was impacted by the absence of a royalty stream that ended in early 2009 and the year included $1.1 million of executive severance costs.
40
Environmental
The Company operates manufacturing facilities throughout the world and as a result is subject to a broad array of environmental laws and regulations in various countries. The Company also implements a variety of voluntary programs to reduce air emissions, eliminate or reduce the generation of hazardous waste and to decrease the amount of wastewater discharges. The establishment and implementation of U.S. federal, state and local standards to regulate air and water quality and to govern contamination of land and groundwater has affected, and will continue to affect, substantially all of the Company’s U.S. manufacturing locations. Federal legislation providing for regulation of the manufacture, transportation, use and disposal of hazardous and toxic substances has imposed additional regulatory requirements on industry in general, and particularly on the chemicals industry. In addition, the implementation of environmental laws, such as the Resource Conservation and Recovery Act, the Clean Air Act and the Comprehensive Environmental Response, Compensation and Liability Act of 1980, as amended by the Superfund Amendments and Reauthorization Act of 1986, has required and will continue to require, new capital expenditures and will increase operating costs. Additionally, growing concerns about climate change may result in the imposition of additional regulation. Some form of federal regulation may be forthcoming with respect to greenhouse gas emissions (including carbon dioxide (CO2)) and/or “cap and trade” legislation, compliance with which could result in additional environmental regulatory requirements. Many of the other countries in which the Company operates also have environmental regulatory requirements in place and continue to strengthen them.
The Distribution Agreement specifies that the Company is only responsible for certain environmental liabilities at the Company’s then current operating plant sites and certain off-site locations. Olin retained the liability for all former Olin plant sites and former waste disposal sites. The Company has also become subject to environmental exposures and potential liabilities in the U.S. and abroad with respect to the businesses it purchased. In connection with the acquisitions of Hickson International and Koppers Arch Wood Protection (Aust) Pty Ltd, the Company acquired certain environmental exposures and potential liabilities of current and past operation sites that have been accrued for in the accompanying Consolidated Financial Statements.
In connection with the disposition of the majority of the microelectronic materials business on November 30, 2004, the Company provided indemnification for potential and identified environmental liabilities. For environmental liabilities identified as of the transaction date, there is no limit to the liability retained by the Company, which the Company estimates to be less than $1.0 million. The Company is no longer liable for potential pre-closing environmental liabilities as the time period for indemnification has expired.
In connection with the disposition of the sulfuric acid business on July 2, 2003, the Company provided environmental covenants to the purchaser in which the Company is solely liable for the costs of any environmental claim for remediation of any hazardous substances that were generated, managed, treated, stored or disposed of prior to the closing date of the sale. The Company will be released, under the sales agreement, from its obligation, which cannot exceed $22.5 million, 20 years from the closing date. See “Legal Matters” for a discussion of a claim from the current owner of the business.
As part of the Hickson organics disposition in August 2003, the Company continues to be responsible for known environmental matters at the Castleford, England site. Such matters have previously been accrued for in its environmental reserve included in the Consolidated Financial Statements. Additionally, regarding any unknown environmental matters that are identified subsequent to the sale, the Company has agreed to share responsibility with the purchaser over a seven-year period, with the Company’s share decreasing to zero over the seven-year period. Such period ended in 2010. The Company’s maximum aggregate liability for any unknown environmental matters is £5.0 million. However, in September 2005, the purchaser went into liquidation and is highly unlikely to be able to honor its environmental indemnification commitments to the Company. The Company does not believe there has been any change in its environmental exposure at the site.
In connection with the disposition of the industrial coatings business on March 31, 2010, the Company provided indemnification for the costs of remediation necessary to comply with applicable environmental laws in
41
relation to certain specifically identified pre-closing environmental contamination and non-compliances at the sites used in the business at the time of disposal. Although there are no monetary caps or time limits applicable to the Company’s obligation to indemnify for the remediation costs, the Company estimates the potential exposure to be approximately $1.0 million. At December 31, 2010, the Company had a liability recorded for such amount in Other liabilities in the Company’s Consolidated Balance Sheet. The Company also provided indemnification for certain other unknown environmental matters relating to the pre-closing operations of the industrial coatings business. This indemnification obligation is subject to both time limits (three years in the case of penalties for non-compliance with applicable environmental laws and permits and seven years in the case of offsite and former property environmental contamination) and a €5 million (approximately $7 million) aggregate monetary cap on all warranty and environmental matters arising out of the transaction (other than the remediation costs associated with the specifically identified onsite contamination and non-compliances discussed above). All other liabilities relating to environmental matters at the sites, and arising in connection with the industrial coatings business before March 31, 2010, have been assumed by the purchaser.
The Company believes that the environmental indemnifications for the microelectronic materials, sulfuric acid, Hickson organics and industrial coatings dispositions are not likely to have a material adverse effect on its results of operations and cash flows.
Associated costs of investigatory and remedial activities are provided for in accordance with generally accepted accounting principles governing probability and the ability to reasonably estimate future costs. Charges to income for investigatory and remedial efforts of $0.8 million, $0.7 million, and $1.1 million were recorded in 2010, 2009 and 2008, respectively, and may be material in future years.
Cash outlays for normal plant operations for the disposal of waste and the operation and maintenance of pollution control equipment and facilities to ensure compliance with mandated and voluntarily imposed environmental quality standards were charged to income. Cash outlays for remedial activities are charged to reserves. Historically, the Company has funded its environmental capital expenditures through cash flows from operations and expects to do so in the future.
Cash outlays for environmental related activities for 2010, 2009 and 2008 were as follows:
| | | | | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | ($ in millions) | |
Environmental Cash Outlays | | | | | | | | | | | | |
Capital Projects | | $ | 1.5 | | | $ | 1.0 | | | $ | 0.6 | |
Plant Operations | | | 6.1 | | | | 6.1 | | | | 6.4 | |
Remedial Activities | | | 1.2 | | | | 0.6 | | | | 1.1 | |
| | | | | | | | | | | | |
Total Environmental Cash Outlays | | $ | 8.8 | | | $ | 7.7 | | | $ | 8.1 | |
| | | | | | | | | | | | |
The Company’s Consolidated Balance Sheets included liabilities for future environmental expenditures to investigate and remediate known sites amounting to $9.0 million at December 31, 2010, of which $1.5 million was classified in current liabilities and $8.2 million at December 31, 2009, of which $1.5 million was classified in current liabilities. The Company’s estimated environmental liability relates to 17 sites, seven of which are in the United States and none of which is on the U.S. National Priority List. These amounts did not take into account any discounting of future expenditures, any consideration of insurance recoveries or any advances in technology. These liabilities are reassessed periodically to determine if environmental circumstances have changed or if the costs of remediation efforts can be better estimated. As a result of these reassessments, future charges to income may be made for additional liabilities.
Annual environmental-related cash outlays for site investigation and remediation, capital projects and normal plant operations are expected to range from $8 million to $10 million over the next several years. While
42
the Company does not anticipate a material increase in the projected annual level of its environmental-related costs, there is always the possibility that such increases may occur in the future in view of the uncertainties associated with environmental exposures.
Environmental exposures are difficult to assess for numerous reasons, including the identification of new sites, developments at sites resulting from investigatory studies and remedial activities, advances in technology, changes in environmental laws and regulations and their application, the scarcity of reliable data pertaining to identified sites, the difficulty in assessing the involvement and financial capability of the other potentially responsible parties and the Company’s ability to obtain contributions from other parties and the lengthy time periods over which site remediation occurs. It is possible that some of these matters (the outcomes of which are subject to various uncertainties) may be resolved unfavorably against the Company. At December 31, 2010, the Company had estimated additional contingent environmental liabilities of approximately $10 million.
Legal Matters
There are a variety of non-environmental legal proceedings pending or threatened against the Company.
In May 2005, the DOC assessed antidumping duties ranging from approximately 76% to 286% against Chinese producers of isos. The Company’s primary Chinese supplier of isos was subject to the 76% rate. As a result, upon importing isos from this supplier, the Company made cash deposits at the rate of 76% of the value of the imported product. At the request of the U.S. isos producers and the Company’s supplier, the DOC conducted a review of the duty rate for the period of December 16, 2004 to May 31, 2006. Upon conclusion of its review, the DOC determined that the rate should be reduced to approximately 20%. As a result of this final determination and the revised rate, the Company recorded a net pre-tax benefit of $12.1 million in the fourth quarter of 2007. The U.S. isos producers appealed the DOC’s determination to the Court of International Trade, which has delayed the processing of the full refund the Company was expecting to receive. On July 13, 2009, the Court of International Trade issued its decision which required the DOC to review additional information and revise the rate accordingly. The DOC complied and has issued a revised preliminary rate of approximately 9%, which is pending review by the Court of International Trade. Provided that this revised preliminary rate is approved by the Court of International Trade, the Company would recognize additional income of approximately $3 million. Therefore, the total net cash proceeds the Company expects to receive is approximately $15 million.
At the request of the Company’s supplier, the DOC also initiated an administrative review to determine the final rate for the period of June 1, 2006 through May 31, 2007, during which time the 76% rate also applied. The DOC has determined that the final rate for the Company’s supplier for this period should be reduced from 76% to less than 1%. As a result, the Company recorded a net pre-tax benefit of $12.7 million in the third quarter of 2008 (which included $1.2 million of interest income). The U.S. isos producers appealed the DOC’s determination to the Court of International Trade. The appeal is delaying the cash refund (approximately $14 million) of the duty to the Company and may result in a change of the duty rate for this review period. The Company believes that the resolution of this matter is not likely to have a material adverse effect on its results of operations and cash flows.
At the request of the Company’s supplier, the DOC also initiated an administrative review to determine the final rate for the period of June 1, 2007 through May 31, 2008, during which time the Company paid duty rates of 76% for part of the period and approximately 20% for the remainder of the period. During the fourth quarter of 2009, the DOC made its final determination that changed the Company’s antidumping duty rate for the entire period to 20%. As a result of this final determination, the Company recorded a pre-tax benefit of $1.0 million in the fourth quarter of 2009. The determination was not appealed and the Company received the cash refund during 2010.
At the request of the Company’s supplier, the DOC also initiated an administrative review to determine the final rate for the period of June 1, 2008 to May 31, 2009, during which time the Company paid duty rates of approximately 20% for part of the period and approximately 1% for the remainder of the period. During the fourth quarter of 2010, the DOC made its final determination that changed the Company’s antidumping duty rate for the entire period to approximately 3%. As a result of this final determination, the Company recorded a pre-tax
43
benefit of $0.1 million in the fourth quarter of 2010. The U.S. isos producers appealed the DOC’s determination to the Court of International Trade. The appeal is delaying the cash refund of the duty to the Company and may result in a change of the duty rate for this review period. The Company believes that the resolution of this matter is not likely to have a material adverse effect on its results of operations and cash flows.
Based upon the final determination for the period of June 1, 2008 through May 31, 2009, the Company began paying cash deposits for imports at a rate of approximately 3% during the fourth quarter of 2010.
An administrative review has also commenced to determine the final rate for the period of June 1, 2009 to May 31, 2010.
Along with its primary Comprehensive General Liability (“CGL”) insurer, Arch Coatings France S.A. (“ACF”), a subsidiary of the Company, is a defendant in a lawsuit filed in France by a builder of pleasure boats. The suit alleges that the formulation of certain varnish coatings previously supplied by ACF for application to interior woodwork on approximately 5,200 boats made by plaintiff was defective in that, under certain conditions, the varnish will bubble and peel. The plaintiff has identified 554 boats in need of repair and the plaintiff claims that it has expended €4.5 million (approximately $5.9 million) to repair 513 of those boats. A court appointed expert has filed a report with the court that concludes that the technical cause of the problem lies solely with the formulation of the varnish coatings, and that the plaintiff’s repair-related damages at the end of January 2010 amounted to €3.7 million (approximately $4.9 million). There is no trial date set for this case. In August 2008, ACF was advised by its primary CGL insurer that it was denying coverage for this loss. The Company has advised the insurer that it disagrees with its position and is currently evaluating its options. ACF sold its assets in the sale of the industrial coatings business to Sherwin-Williams, but has retained the liability for the lawsuit. At December 31, 2010, ACF had €0.8 million (approximately $1.0 million) accrued for this matter. The Company believes the high end of the range of possible outcomes is €4.5 million (approximately $5.9 million). However, it is possible that the high end of the range could ultimately increase or decrease depending upon whether the court accepts or rejects the findings of the expert as to both causation and damages. Due to the multiple variables involved in the case (i.e., the uncertainty surrounding the number of boats that were damaged, the costs to repair the damages, the cause of the alleged damage as determined by the court, the Company’s responsibility for all or some of the alleged costs of repair as determined by the court), it is currently not possible to make an estimate of any amount above the amount of the current stated claim. The Company believes that the resolution of this matter is not likely to have a material adverse effect on the Company’s results of operations and cash flows.
In December 2007, as a result of an income tax audit of Nordesclor, the Company was notified by the Brazilian tax authorities that the Company would be assessed R$4.9 million (approximately $2.9 million) for alleged tax deficiencies related to the 2002 tax year. In accordance with the purchase agreement that was signed in conjunction with the acquisition of Nordesclor, our former joint venture partner is responsible for approximately 50% of this assessment. The Company believes the deficiency notice is without merit and, in January 2008, the Company protested the assessment to the first level administrative court. The Company received an unfavorable decision on this protest and has protested the assessment to the second level administrative court. The Company believes that the resolution of this matter is not likely to have a material adverse effect on its results of operations and cash flows.
Additionally, the Company has been notified by the Brazilian tax authorities of various assessments, totaling approximately R$9 million (approximately $5 million), related to alleged non-income tax deficiencies for tax years ranging from 1988 to 2003. The Company has recorded a liability of R$2.7 million (approximately $1.6 million) for any assessments for which it is probable that the Company will be unable to successfully defend itself. The Company believes that the remainder of the assessments are without merit. The Company believes that the resolution of this matter is not likely to have a material adverse effect on its results of operations and cash flows.
During 2003, the Company sold its sulfuric acid business. The Company has received a claim from the current owner of that business. The claimant asserts that, under certain provisions of the agreement for the sale of
44
the business, the Company must indemnify the claimant for certain environmental penalties and compliance costs the claimant will incur under a settlement the claimant reached with the U.S. Environmental Protection Agency. The claimant alleges that such penalties and costs approximate $2.4 million. The Company is currently investigating the validity of the claimant’s assertions. The Company believes that the resolution of this matter is not likely to have a material adverse effect on its results of operations and cash flows.
During 2008, Arch Wood Protection (NZ) Limited (“AWPNZ”) was named as a defendant in a suit filed by one of its competitors. The suit alleged that AWPNZ and several other defendants were jointly and severally liable for defamatory statements made about a product of the competitor in that they secured, contributed to, or encouraged the publication of such statements. During 2010 the parties settled the matter and the case was dismissed. The settlement did not have a material impact on the Company.
In March 2010, the owner of a new U.S. patent, relating to methods of using particulate copper wood preservatives, filed a lawsuit against the Company, two of the Company’s subsidiaries, and three customers of the Company’s wood protection business. In the suit, the patent owner claims that use of certain wood preservatives manufactured and sold by the Company’s wood protection business infringes the patent. The complaint requests several forms of relief, including an unspecified amount of damages and a permanent injunction against infringement of the patent. The Company believes that the patent owner’s claims are without merit, and is vigorously defending against these claims. In its answer to the complaint, the Company has asserted, among other things, that the alleged activities do not infringe the patent, and that, in any event, the patent is both invalid and unenforceable. The trial in this case is scheduled to begin during the second quarter of 2011. The Company believes that the resolution of this matter is not likely to have a material adverse effect on its results of operations and cash flows.
In October 2010, the French taxing authorities notified the Company that it is responsible for paying additional duties in connection with certain products imported into France from October 2005 through May 2007 due to errors in the invoices prepared by the supplier. During the fourth quarter of 2010, the Company recorded a $1.8 million reserve related to this matter. The Company believes that the resolution of this matter is not likely to have a material adverse effect on its results of operations and cash flows.
The Company is being sued by the current owner of a former Hickson site in Italy for remediation of environmental contaminants on that site. The owner is seeking compensation of €2.2 million (approximately $2.9 million) for the remediation of the site. The matter is currently within the Italian court system. Based on remediation actions completed in 2008, the Company believes it has no further obligation at the site. The local authorities, however, continue to review the condition of the site and may require additional work and risk assessments to be performed. Although the site was related to the Company’s industrial coatings business, liability for this lawsuit has been retained by the Company notwithstanding the sale of the business to Sherwin-Williams. The Company believes that the resolution of this matter is not likely to have a material adverse effect on its results of operations and cash flows.
There are a variety of non-environmental legal proceedings pending or threatened against the Company. Those matters that are probable have been accrued for in the accompanying Consolidated Financial Statements. Any contingent amounts in excess of amounts accrued are not expected to have a material adverse effect on results of operations, financial position or liquidity of the Company.
Business and Credit Concentrations
A significant portion of sales of the Biocides Products segment (approximately 20%) is dependent upon two customers, one of which accounts for a significant portion of the sales of the HTH water products business and the other of which accounts for a significant portion of the sales of the personal care and industrial biocides businesses. Sales to these two customers are individually less than 10% of the Company’s 2010 consolidated sales. However, the loss of either of these customers would have a material adverse effect on the sales and operating results of the Company, respective segment and businesses if such customer was not replaced.
45
Sales of the HTH water products business are seasonal in nature as its products are primarily used in the U.S. residential pool market. Historically, approximately 40% of the sales in the HTH water products business occur in the second quarter of the fiscal year, as retail sales in the U.S. residential pool market are concentrated between Memorial Day and the Fourth of July. Therefore, interim results for this segment are not necessarily indicative of the results to be expected for the entire fiscal year.
Liquidity, Investment Activity and Other Financial Data
Cash Flow Data
| | | | | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | ($ in millions) | |
Provided By (Used For) | | | | | | | | | | | | |
Accounts Receivable Securitization Program | | $ | — | | | $ | — | | | $ | — | |
Change in Working Capital | | | (10.7 | ) | | | 30.7 | | | | (53.6 | ) |
Change in Noncurrent Liabilities | | | (59.8 | ) | | | (31.5 | ) | | | (10.3 | ) |
Net Operating Activities from Continuing Operations | | | 70.9 | | | | 105.9 | | | | 46.3 | |
Capital Expenditures | | | (29.4 | ) | | | (27.4 | ) | | | (49.9 | ) |
Businesses Acquired in Purchase Transactions, net of Cash | | | (2.3 | ) | | | 0.3 | | | | (125.5 | ) |
Proceeds from Sale of a Business, net | | | 44.4 | | | | 1.2 | | | | 3.7 | |
Proceeds from Sale of Land and Property | | | 1.4 | | | | — | | | | 0.7 | |
Net Investing Activities | | | 13.7 | | | | (28.4 | ) | | | (174.4 | ) |
Debt Borrowings (Repayments), net | | | 74.4 | | | | (48.5 | ) | | | 128.4 | |
Net Financing Activities | | | 55.7 | | | | (68.3 | ) | | | 109.8 | |
Operating Activities:
For 2010, $70.9 million was provided by operating activities from continuing operations, compared to $105.9 million for 2009. The decrease is principally due to the fact that during 2010 the Company made $80.0 million of voluntary contributions to the U.S. pension plan, as opposed to $42.3 million during 2009. Higher income during 2010 was offset by increased working capital, as 2009 included the benefits of the Company’s inventory reduction programs for the industrial biocides and performance urethanes businesses.
Accounts receivable, net, at December 31, 2010, including amounts sold through the securitization program and recorded in Securitization-related receivable (see Note 2 of Notes to Consolidated Financial Statements), were five percent higher than at December 31, 2009 as sales were six percent higher during the fourth quarter of 2010 than during the fourth quarter of 2009. At December 31, 2010, days sales outstanding (“DSO”) was 54 days, as compared to 56 days at December 31, 2009. The improvement is due to the Company’s continued focus on collections and tight credit controls. The Company continues to closely monitor its accounts receivable balances and assess the allowance for doubtful accounts. The allowance was $4.4 million at December 31, 2010, compared to $4.5 million at December 31, 2009.
Inventories, net at December 31, 2010 were 15 percent higher than at December 31, 2009. At December 31, 2010 the Company’s inventory months on hand (“MOH”) was 3.3, compared to 3.0 at December 31, 2009. The increase in inventory levels and MOH at December 31, 2010, versus December 31, 2009, is due to slightly higher inventory balances for the HTH water products and personal care and industrial biocides businesses. Many of the Company’s products are not subject to rapid obsolescence and do not have short shelf lives, but the Company continues to closely monitor its inventory balances and to assess for obsolescence. The obsolescence reserve was $9.6 million at December 31, 2010, compared to $10.1 million at December 31, 2009. The decrease is principally due to the disposal of unsalable product.
For 2009, $105.9 million was provided by operating activities from continuing operations compared to $46.3 million for 2008. During 2009, the Company focused its efforts on reducing working capital. Cash flows
46
improved principally due to inventory reduction programs for the industrial biocides and performance urethanes businesses and continued focus on collections of receivables and tight credit controls. The cash flows from operations included $42.3 million of voluntary contributions to the U.S. pension plan during 2009.
Investing Activities:
Capital Expenditures:
Capital expenditures for 2010 were $2.0 million higher than 2009 principally due to slightly higher spending for the personal care and industrial biocides business.
Capital expenditures for 2009 were $22.5 million lower than 2008 principally due to decreased spending for the personal care and industrial biocides businesses as a result of the completion of construction of two new biocides plants in China to meet growing demand for biocides used in antidandruff products and marine antifouling paints.
Capital expenditures for 2011 are expected to be approximately $55 million, including a major manufacturing technology upgrade at the Company’s HTH water products plant in the U.S. and the consolidation and expansion of three of the Company’s U.S. research and development functions into a new leased facility.
Businesses Acquired in Purchase Transactions, net of cash:
In September 2010, the Company acquired a personal care and industrial biocides business in Latin America for $2.3 million.
On October 10, 2008, the Company completed the acquisition of the water treatment chemicals business of Advantis, a North American manufacturer and marketer of branded swimming pool, spa and surface water treatment chemicals. The purchase price was $125.0 million, free of debt and inclusive of expenses paid, and a final post-closing working capital adjustment of $0.3 million, which was received by the Company in the first quarter of 2009. The acquisition was financed by borrowings from the Company’s existing credit facility. For additional information concerning the acquisition see Note 19 of Notes to Consolidated Financial Statements.
Proceeds from Sale of a Business, net:
On March 31, 2010, the Company completed the sale of its non-strategic industrial coatings business to Sherwin-Williams. Total net proceeds from the sale are expected to be approximately $43 million, net of expenses. As of December 31, 2010, the Company has received €36.7 million ($49.4 million) of proceeds, which is offset by $5.5 million of transaction costs paid. The Company estimates making the payment of the remaining transaction costs during 2011.
In September 2007, the Company completed the sale of its non-strategic performance urethanes business in Venezuela. During 2010, 2009 and 2008, $0.5 million, $1.2 million and $3.7 million, respectively, of the proceeds were received. Total proceeds, net of expenses, from the sale were $17.0 million, all of which had been received as of December 31, 2010.
Proceeds from Sale of Land and Property:
Proceeds from sale of land and property in 2010 relates to the sale of certain manufacturing assets of the hydrazine business.
Proceeds from sale of land and property in 2008 represents land sold in conjunction with the Company’s decision to discontinue the manufacturing of its BIT molecule.
47
Financing Activities:
Debt borrowings, net of repayments, were $74.4 million during 2010 as the Company entered into a master note purchase agreement and issued $250.0 million of Senior Notes (see below) that were partially used to pay down debt. In September 2010, the Company used borrowings from the master note purchase agreement to repay the entire balance outstanding, $80.0 million, under the term loan.
Debt repayments, net of borrowings, were $48.5 million in 2009. The repayments were funded with excess cash, which was principally generated from the Company’s working capital reduction efforts, to pay down debt. In March 2009, the Company used its unsecured $350.0 million senior revolving credit facility (“credit facility”) to repay $62.0 million of Series B notes which came due.
On December 15, 2010, the Company paid a quarterly dividend of $0.20 on each share of common stock. Total dividends paid to shareholders were $20.1 million during 2010 and $20.0 million during 2009.
Liquidity
On June 15, 2006, the Company entered into an unsecured $350.0 million senior revolving credit facility, which expires in June 2011. The Company expects to replace the credit facility. The credit facility contains a quarterly leverage ratio (Debt/EBITDA) covenant not to exceed 3.5. At December 31, 2010, the Company’s quarterly leverage ratio, as defined in the credit facility, was 2.5. Additionally, the credit facility contains an interest coverage ratio (EBITDA/total interest expense) covenant not to be less than 3.0. At December 31, 2010, the Company’s interest coverage ratio, as defined in the credit facility, was 10.6. The Company was in compliance with both of these covenants throughout 2010. The credit facility also restricts the payment of dividends and repurchase of stock to $65.0 million plus 50% of cumulative net income (loss) subject to certain limitations beginning June 15, 2006. Restricted payments were limited to $97.7 million at December 31, 2010. The facility fees can range from 0.100% to 0.225% depending on the Company’s quarterly leverage ratios. The Company may select various floating rate borrowing options, including, but not limited to, LIBOR plus a spread that can range from 0.4% to 0.9% depending on the Company’s quarterly leverage ratios. At December 31, 2010, the Company had $317.1 million of available borrowings under the credit facility.
On February 13, 2009, the Company entered into an unsecured $100.0 million credit agreement (“term loan”) with a number of banks. The term loan provided for quarterly amortization of principal equal to $5 million per quarter, beginning September 30, 2009. During September 2010, the remaining $80.0 million outstanding balance was repaid using the proceeds from the Series 2010-A Senior Notes (see below for further detail).
On August 28, 2009, the Company entered into a $150.0 million note purchase and private shelf agreement (the “shelf agreement”) with Prudential Investment Management, Inc. (“Prudential”) and immediately issued $75.0 million of unsecured senior notes (the “Notes”). The Notes will mature in August 2016 and bear a fixed annual interest rate of 6.70%. The shelf agreement provides for the additional purchase by Prudential of notes, in amounts to be mutually agreed, up to an additional maximum amount of $75.0 million through August 2012, on terms to be determined. The shelf agreement contains a quarterly leverage ratio covenant not to exceed 3.5 and an interest coverage ratio covenant not to be less than 3.0, both of which are consistent with the existing credit facility. At December 31, 2010 the Company’s quarterly leverage ratio, as defined in the shelf agreement, was 2.5 and the Company’s interest coverage ratio, as defined in the shelf agreement, was 10.6. The Company was in compliance with both of these covenants throughout 2010. Additionally, the shelf agreement restricts the payment of dividends and repurchase of stock to $88.1 million plus 50% of cumulative adjusted net income (loss) for the period beginning June 30, 2009. At December 31, 2010, restricted payments were limited to $97.7 million.
On September 9, 2010, the Company entered into a master note purchase agreement (the “master note agreement”) with certain institutional investors, which provides financing to the Company through the private placement of $250.0 million aggregate principal amount of the Company’s Series 2010-A Senior Notes (the “Senior Notes”). The Company issued $125.0 million of the Senior Notes at closing. The remaining $125.0
48
million was issued by the Company in December 2010. The Senior Notes will mature in December 2017 and bear a fixed annual interest rate of 4.0%. The master note agreement contains a quarterly leverage ratio covenant not to exceed 3.5 and an interest coverage ratio covenant not to be less than 3.0, both of which are consistent with the existing credit facility and shelf agreement. At December 31, 2010, the Company’s quarterly leverage ratio, as defined by the master note agreement, was 2.5 and the Company’s interest coverage ratio, as defined by the master note agreement, was 10.6. The Company has been in compliance with both of these covenants since the inception of the master note agreement. Additionally, the master note agreement restricts the payment of dividends and repurchase of stock to $99.4 million plus 50% of cumulative adjusted net income (loss) for the period beginning June 30, 2010. At December 31, 2010, restricted payments were limited to $97.7 million.
In March 2002, the Company issued $211.0 million of unsecured senior notes to certain institutional investors in two series. The Company used its credit facility to pay off the Series A notes in March 2007 and to pay off the entire balance of the Series B notes, $62.0 million, in March 2009.
On October 6, 2009, the Company entered into an accounts receivable securitization program with Market Street Funding LLC and PNC Bank, National Association (“PNC Bank”) by way of an assignment and assumption of the Company’s previous program with Three Pillars Funding LLC and SunTrust Capital Markets, Inc. Under the amended program, the Company sells domestic trade accounts receivable, and certain Canadian trade accounts receivable, to Market Street Funding LLC through its wholly-owned subsidiary, Arch Chemicals Receivables Corp. (now known as Arch Chemicals Receivables LLC) (“ACRC”). Additionally, the program provides ACRC with the ability to issue letters of credit. The amount of funding that the Company can obtain under the program is subject to change based upon the level of eligible receivables with a maximum amount of $80 million. No more than $30 million of such funding can relate to letters of credit. The amended program is subject to annual renewal. During October 2010, the program was renewed for 364 days. See Note 2 of Notes to Consolidated Financial Statements.
Other borrowings at December 31, 2010 included $10.5 million of borrowings under international credit facilities and $0.8 million of capitalized leases. Such credit facilities and capitalized leases have interest rates principally ranging from 7% to 11%.
At December 31, 2010, the Company had $24.7 million of outstanding letters of credit, $2.9 million of which reduced availability under the Company’s credit facility. Additionally, at December 31, 2010, the Company had $2.4 million of outstanding letters of guarantee.
The Company believes that the credit facility, which the Company intends to replace, accounts receivable securitization program and cash provided by operations, as well as amounts previously borrowed under the master note agreement and shelf agreement, are adequate to satisfy its liquidity needs for the near future, including financing capital expenditures. However, if the Company’s earnings or cash flows were to fall significantly below current expectations and/or a default condition were triggered under its funding agreements or if the Company’s funding providers do not honor their commitments, a risk exists that the Company would not have enough liquidity to meet its operating needs. In addition, adverse developments in the financial markets could reduce the sources of liquidity for the Company.
Although the Company has seen improvement in consumer demand during 2010, the turmoil in the global economy throughout 2009 impacted the operating performance of several of the Company’s businesses. As a result, the estimated fair values have decreased for some of the Company’s reporting units, in particular the performance urethanes and wood protection reporting units. If there is no recovery over the next several years or if global economic conditions deteriorate, the following assets for the wood protection business could become impaired: Goodwill of $45.3 million and/or Long-lived assets (exclusive of Goodwill, Other assets and Investments and advances—affiliated companies at equity) of $57.0 million. In addition to the impact of the turmoil in the U.S. economy, the performance urethanes business had a significant contract expire at the end of 2009, which decreased the annual operating income of the business by approximately $12 million. We believe that organic growth and a pipeline of new product offerings should mitigate a portion of this decrease. If the performance urethanes business’
49
earnings and/or cash flows were to fall below current expectations due to no recovery in the economy over the next several years, or if the business is unsuccessful with new product offerings, the following assets could become impaired: Goodwill of $4.4 million, and/or Long-lived assets (exclusive of Goodwill, Other assets and Investments and advances—affiliated companies at equity) of $13.7 million.
The Company’s performance urethanes and hydrazine businesses are non-core to its portfolio and are managed for cash that is then invested in growing our core Biocides portfolio. The Company considers these businesses non-core and, therefore, does not expect that they will remain in the portfolio in the long-term. Although the Company currently does not have any plans in place, the Company continues to evaluate various strategic options for these businesses, which include possible divestiture. At December 31, 2010, the net assets of the performance urethanes and hydrazine businesses were approximately $29 million and $—, respectively.
As provided in the Biocidal Products Directive (“BPD”), the European Union (“EU”) is reviewing the classification and labeling of a number of chemicals under a new, more wide-ranging procedure. One of the Company’s products, poly hexamethylene biguanide hydrochloride (“PHMB”), which has been on the market for many years providing valuable disinfection, preservation, cleaning and hygiene functions, is one of the chemicals under review. France, in its role as lead reviewer of PHMB under the BPD, has proposed a much more conservative classification of PHMB. PHMB is currently being reviewed by the European Chemicals Agency (“ECHA”) to determine if the French classification should be upheld. If the new classification is upheld, there is the potential for customers electing to discontinue the use of PHMB and PHMB-related products in response to the new classification and such discontinuation could also extend beyond the EU. The Company is actively defending against this new classification of the product as well as developing alternative products. If ECHA does not overturn the classification and the Company’s customers decide to no longer purchase PHMB as a result of this change in classification, the Company’s operating results in the future and up to $13 million of PHMB-related intangible assets could be impacted. In 2010, the Company had approximately $11 million of PHMB sales in Europe and had approximately $38 million of PHMB sales worldwide.
In addition, Olin, who is a major supplier of chlorine and caustic soda to the Company’s HTH water products business, has announced plans to convert its mercury cell chlorine-caustic plant in Charleston, Tennessee to membrane technology by the end of 2012. The Company does not believe that there will be any significant impact on its operating results in 2011, but if there is any disruption in our supply of chlorine and caustic soda from Olin, whether due to delays in the conversion of the plant or Olin’s inability to deliver product meeting our required specifications after the conversion is completed, the Company would have to arrange for alternative suppliers, which could have a material impact on this business’ operating income and cash flows in the future. The Company’s current supply contract expires at the end of 2011 and the Company is currently negotiating a new contract which may contain higher prices for 2012 and beyond.
During 2010, the Company made $80.0 million of voluntary contributions to the Company’s qualified U.S. pension plan. As a result of the contributions, the Company continues to meet the full funding phase-in threshold set forth in the Pension Protection Act of 2006. There are no cash contribution requirements expected for the Company’s qualified U.S. pension plan in 2011. The Company also has payments due under the non-qualified pension plan and the postretirement benefit plans. These plans are pay as you go, and therefore not required to be funded in advance.
The minimum funding requirements for the Company’s U.K. pension plans are currently expected to be approximately $25 million to $30 million (or approximately £16 million) in 2011 and then return to $15 million to $20 million (or approximately £10 million) per year thereafter.
50
Contractual Obligations
The following table details the Company’s contractual obligations as of December 31, 2010:
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Payments Due by Period | |
| | | Total | | | Less than 1
Year | | | 1 to 3
Years | | | 3 to 5
Years | | | More than 5
Years | | | All Other | |
| | | ($ in millions) | |
Debt and capital lease obligations (1) | | $ | 469.1 | | | $ | 57.3 | | | $ | 32.8 | | | $ | 30.6 | | | $ | 348.4 | | | $ | — | |
Operating lease commitments | | | 46.4 | | | | 10.6 | | | | 14.8 | | | | 12.5 | | | | 8.5 | | | | — | |
Other contractual purchase obligations | | | 40.9 | | | | 33.5 | | | | 6.5 | | | | 0.6 | | | | 0.3 | | | | — | |
Unrecognized tax benefits | | | 11.7 | | | | 4.8 | | | | — | | | | — | | | | — | | | | 6.9 | |
Other long-term liabilities | | | 9.8 | | | | — | | | | 1.5 | | | | 1.3 | | | | 7.0 | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total | | $ | 577.9 | | | $ | 106.2 | | | $ | 55.6 | | | $ | 45.0 | | | $ | 364.2 | | | $ | 6.9 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| (1) | Included in the debt and capital lease obligations are the related interest payments, which are estimated to be $18.8 million in 2011, $15.3 million in 2012, $15.2 million in 2013, $15.1 million in 2014, $15.0 million in 2015 and $23.4 million thereafter. The interest payments for any variable rate debt have been calculated using the interest rates in effect as of December 31, 2010. |
The amounts above exclude the Company’s minimum pension funding requirements as set forth by the Employee Retirement Income Security Act of 1974 (“ERISA”). The Company’s minimum funding requirements are dependent on several factors, including the discount rate and investment returns, and any changes in legislation. At the current time it is not possible to reasonably predict future contributions by year. Based upon the 2010 contributions of $80.0 million, the assumption that interest rates will remain at or near the levels at December 31, 2010, that the annual rate of return on assets will be 8.25%, that mortality rates will remain consistent with December 31, 2010 assumptions, and there are no changes in existing legislation, it would be reasonable to assume that contributions to the qualified U.S. plan over the next several years should approximate the current service cost (approximately $8 million in 2010). The Company also has payments due under the non-qualified pension plan and the postretirement benefit plans. These plans are pay as you go, and therefore not required to be funded in advance. Excluded from the table above is $21.4 million of benefit payments under the Company’s nonqualified pension plan expected to be funded in 2013. The Company has minimum funding requirements for its U.K. pension plans, which are currently expected to be approximately $25 million to $30 million (or approximately £16 million) in 2011 and then return to $15 million to $20 million (or approximately £10 million) per year thereafter.
Other Financial Data
On January 27, 2011, the Company declared a quarterly dividend of $0.20 on each share of the Company’s common stock. The dividend is payable on March 15, 2011 to shareholders of record at the close of business on February 15, 2011.
Critical Accounting Policies
The Company’s Consolidated Financial Statements are based on the accounting policies used. Certain accounting policies require that estimates and assumptions be made by management for use in the preparation of the financial statements. Critical accounting policies are those that are central to the presentation of the Company’s financial condition and results and that require subjective or complex estimates by management. These include the following:
Goodwill and Other Intangible Assets
The Company evaluates goodwill and identified intangible assets on a business-by-business basis (“reporting unit”) for impairment. The Company evaluates each reporting unit for impairment based upon a two-step approach. First, the Company compares the fair value of the reporting unit with its carrying value. Second, if the carrying value of the reporting unit exceeds its fair value, the Company compares the implied fair
51
value of the reporting unit’s goodwill to its carrying amount to measure the amount of impairment loss. In measuring the implied fair value of goodwill, the Company would allocate the fair value of the reporting unit to each of its assets and liabilities (including any unrecognized intangible assets). Any excess of the fair value of the reporting unit over the amounts assigned to its assets and liabilities is the implied fair value of goodwill.
The Company measures the fair value of a reporting unit as the estimated discounted future cash flows, including a terminal value, which assumes the business continues in perpetuity. The long-term terminal growth assumptions reflect our current long-term view of the marketplace. The discount rate is based upon our weighted average cost of capital of each reporting unit. Each year the Company re-evaluates the assumptions in the discounted cash flow model to address changes in the business and marketplace conditions.
Considerable management judgment is necessary to estimate discounted future cash flows in conducting an impairment test for goodwill and other intangible assets, which may be impacted by future actions taken by the Company and its competitors and the volatility in the markets in which the Company conducts business. A change in assumptions in the Company’s cash flows could have a significant impact on the fair value of the Company’s reporting units, which could then result in a material impairment charge to the Company’s results of operations.
Based upon the annual impairment analysis, which was completed in the first quarter of 2010, the estimated fair value of the reporting units exceeded their carrying value and as a result, the Company did not need to proceed to the second step of the impairment test. Since the first quarter of 2010, no events or changes in circumstances, have occurred to indicate that an impairment could exist. For additional information, see Note 8 of Notes to Consolidated Financial Statements.
Income Taxes
The Company’s effective tax rate is based on pre-tax income, statutory tax rates and tax planning strategies. Significant management judgment is required in determining the effective tax rate and in evaluating the Company’s tax position.
The Company’s accompanying Consolidated Balance Sheets include certain deferred tax assets resulting from net operating loss carryforwards and deductible temporary differences, which are expected to reduce future taxable income. These assets are based on management’s estimate of realizability based upon forecasted taxable income. Realizability of these assets is reassessed at the end of each reporting period based upon the Company’s forecast of future taxable income and available tax planning strategies, and may result in the recording of a valuation reserve. Failure to achieve forecasted taxable income or ability to implement tax planning strategies could affect the ultimate realization of certain deferred tax assets. For additional information, see Note 14 of Notes to Consolidated Financial Statements.
Pension and Postretirement Benefits
The Company’s accompanying Consolidated Balance Sheets include significant pension and postretirement benefit obligations. The determination of defined benefit pension and postretirement plan obligations and their associated expenses requires the use of actuarial valuations to estimate the benefits the employees earn while working, as well as the present value of those benefits. Inherent in these valuations are financial assumptions including expected returns on plan assets, discount rates at which liabilities could be settled, rates of increase of health care costs, rates of future compensation increases as well as employee demographic assumptions such as retirement patterns, mortality and turnover. The Company reviews the assumptions annually with its actuarial advisors. The actuarial assumptions used may differ materially from actual results due to changing market and economic conditions, higher or lower turnover rates or longer or shorter life spans of participants. The following is a discussion of the most significant estimates and assumptions used in connection with the Company’s U.S. and the Hickson U.K. employee benefit plans. The pension expense for other defined benefit plans for the Company’s other foreign subsidiaries was not significant, and accordingly assumptions and sensitivity analyses regarding these plans are not included in the discussion below.
52
Key Assumptions
The assets, liabilities and assumptions used to measure expense for any fiscal year are determined as of January 1 of the current plan year. Accumulated and projected benefit obligations represent the present value of future cash payments.
The expected return on plan assets reflects the average rate of earnings expected on the funds invested to provide for the benefits included in the benefit obligations. The assumption reflects long-term expectations for future rates of return for the investment portfolio over the life of the benefit obligations, with consideration given to the distribution of investments by asset class and historical rates of return for each individual asset class. The Company’s expected long-term rate of return on assets assumption for the Hickson U.K. pension plans includes a 0.25% reduction to allow for administration expenses.
The discount rate reflects the yields available on high-quality fixed income instruments. The discount rate for the U.S. plans is based on the review of the following: Moody’s Aa Corporate Bond Index, a pension liability index and a yield curve constructed from a large population of non-callable corporate bonds, modeled to match the expected timing of the benefit payments over the life of the benefit obligation. The discount rate for the Hickson U.K. plans is based upon a review of corporate bond yields.
Sensitivity Analysis
The sensitivity of changes in key assumptions for our principal pension and postretirement plans are as follows:
| | • | | Discount rate—A 25-basis point change in the discount rate would increase or decrease the expense of the Company’s U.S. and Hickson U.K. pension benefit plans by approximately $1 million and approximately $0.5 million, respectively. In addition, a similar change in the discount rate would increase or decrease the projected benefit obligation by approximately $10 million for the Company’s U.S. plans and approximately $15 million for the Hickson U.K. plans. |
| | • | | Expected return on plan assets—A 25-basis point change in the expected return on plan assets would increase or decrease the expense for the Company’s U.S. and Hickson U.K. pension benefit plans by approximately $0.7 million and $0.8 million, respectively. This change would have no impact on the projected benefit obligation for either plan. |
| | • | | Mortality assumptions—A change in mortality tables for the Company’s U.S. plans that increases/or decreases age-65 life expectancy by one year would increase or decrease the pension expense by approximately $2 million and increase or decrease the projected benefit obligation by approximately $12 million. A 10 percent change in the mortality rates for the Hickson U.K. plans would increase or decrease the pension expense and projected benefit obligation by approximately $1 million and $10 million, respectively. |
For further information about our pension and postretirement plans, see Note 15 of Notes to Consolidated Financial Statements.
Valuation of Long-Lived Assets
The impairment of tangible and intangible assets is assessed when changes in circumstances (such as, but not limited to, a decrease in market value of an asset, current and historical operating losses or a change in business strategy) indicate that their carrying value may not be recoverable. This assessment is based on estimates of future cash flows, salvage values or net sales proceeds. These estimates take into account management’s expectations and judgments regarding future business and economic conditions, future market values and disposal costs. Actual results and events could differ significantly from management estimates.
53
Environmental Liabilities
Liabilities for environmental matters are accrued for when it is probable that a liability has been incurred and the amount of the liability can be reasonably estimated. These estimates take into account current law, existing technologies and management’s judgment about future changes in regulation.
Each quarter, and when there are changes in circumstances, the Company formally evaluates known and potential sites. The Company reviews estimates for future remediation, and maintenance and management costs directly related to remediation, to determine appropriate environmental reserve amounts. For each site, a determination is made of the specific measures that are believed to be required to remediate the site, the estimated total cost to carry out the remediation plan, the portion of the total remediation costs to be borne by the Company and the anticipated time frame over which payments toward the remediation plan will occur. The Company’s estimate of environmental remediation liabilities may change in the future should additional sites be identified, further remediation measures be required or undertaken or current laws and regulations be changed. For additional information, see the Environmental discussion in Note 20 of Notes to Consolidated Financial Statements.
Legal Contingencies
The Company is subject to proceedings, lawsuits and other claims in the normal course of business. Each quarter, the Company formally evaluates its current proceedings, lawsuits and other claims with counsel and when there are changes in circumstances. These contingencies require management judgment in order to assess the likelihood of any adverse judgments or outcomes and the potential range of probable losses. Liabilities for legal matters are accrued for when it is probable that a liability has been incurred and the amount of the liability can be reasonably estimated, based upon current law and existing information. The Company assesses its legal liabilities separately from any potential insurance recovery or indemnification. As such, we record legal liabilities on a gross basis, unless a right of set-off exists. Any benefit from the insurance recoveries or that result from an indemnification by another party are recorded when the Company is reasonably certain the other party will fulfill its obligation. The Company’s insurance coverage provides coverage on a reimbursement basis; therefore, there may be a lag between any payment ultimately paid by the Company and reimbursement of such payment from the Company’s insurers. Estimates of contingencies may change in the future due to new developments or changes in legal approach. For additional information, see Note 20 of Notes to Consolidated Financial Statements.
Incentive Compensation
The Company maintains a long-term employee incentive compensation plan that is intended to reward eligible employees for their contributions to the Company’s long-term success. Provisions for employee incentive compensation are included in Accrued Liabilities and Other Liabilities on the Company’s Consolidated Balance Sheets. There are two types of awards: performance units that vest only upon meeting a performance measure and performance accelerated restricted stock units that vest upon meeting a performance measure, or if that measure is not achieved, upon the employee’s remaining in the employ of the Company for a specific period. The awards are earned at the end of a three-year period provided the return on equity (“ROE”) target is achieved for that third year. For awards granted prior to 2010, there is an opportunity for accelerated payout of the performance units and performance accelerated restricted stock units if the ROE target is met or exceeded by the end of the second year after the grant. The targets are set annually by the Compensation Committee of the Board of Directors. Performance units that do not meet the performance goal at the end of the third year expire without payment. For the performance accelerated restricted stock units, if the ROE target is not achieved by the end of the third year after grant, the units will vest and will be paid out as soon as administratively feasible following the end of the sixth year after grant, or in the case of those units granted in 2010, following the end of the fifth year after grant, if the executive is still employed at the Company at the end of such year. Therefore, changes in the Company’s estimated financial performance could have a significant impact on the amount of compensation expense recorded by the Company in any given period. Since certain awards in the Company’s long-term
54
incentive plan are paid out in cash based upon the market price of the Company’s common stock, the amount of incentive compensation expense will vary based upon the market price of the Company’s stock at the end of each reporting period. The Company has entered into equity total return swap agreements in order to minimize earnings volatility related to fluctuations in the Company’s stock price. See Note 13 to Consolidated Financial Statements for further detail.
For additional information about significant accounting policies, see Note 1 of Notes to Consolidated Financial Statements.
New Accounting Pronouncements
In October 2009, the FASB issued Accounting Standards Update (“ASU”) 2009-13 “Revenue Recognition (Topic 605)—Multiple Deliverable Revenue Arrangements”. FASB ASU 2009-13 addresses the accounting for multiple-deliverable arrangements to enable vendors to account for products or services (deliverables) separately rather than as a combined unit. This guidance establishes a selling price hierarchy for determining the selling price of a deliverable, which is based on: (a) vendor-specific objective evidence; (b) third-party evidence; or (c) estimates. This guidance also eliminates the residual method of allocation and requires that arrangement consideration be allocated at the inception of the arrangement to all deliverables using the relative selling price method. In addition, this guidance significantly expands required disclosures related to a vendor’s multiple-deliverable revenue arrangements. The new standard was effective for the Company on January 1, 2011. The Company does not believe that the adoption of the pronouncement will have a material impact on the Company’s consolidated financial statements.
Derivative Financial Instruments
Derivative instruments are recognized as assets or liabilities in the Company’s Consolidated Balance Sheets and are measured at fair value. The change in the fair value of a derivative designated as a fair value hedge and the change in the fair value of the hedged item attributable to the hedged risk are recognized in earnings. For derivatives which qualify for designation as cash flow hedges, the effective portion of the changes in fair value is recognized as part of other comprehensive income until the underlying transaction that is being hedged is recognized in earnings. The ineffective portion of the change in fair value of cash flow hedges is recognized in earnings currently. Changes in fair value for other derivatives, which do not qualify as a hedge for accounting purposes, are recognized in current period earnings.
The Company enters into forward sales and purchase contracts, cross-currency swap agreements and currency options to manage currency risk resulting from purchase and sale commitments, and certain balance sheet items, denominated in foreign currencies (principally British pound, euro, Australian dollar, New Zealand dollar, Canadian dollar, Japanese yen and South African rand) and relating to particular anticipated but not yet committed purchases and sales expected to be denominated in those currencies. Most of the Company’s currency derivatives expire within one year. At December 31, 2010, the Company had forward contracts to sell foreign currencies with U.S. dollar equivalent value of $90.0 million, including contracts with a $58.5 million notional value that are mitigating foreign currency exposure on certain balance sheet items. Additionally, at December 31, 2010, the Company had forward contracts to buy foreign currencies with U.S. dollar equivalent value of $7.7 million and cross-currency swap agreements with a notional value of approximately $3 million. The Company had no outstanding option contracts to sell or buy foreign currencies at December 31, 2010. At December 31, 2009, the Company had forward contracts to sell foreign currencies with U.S. dollar equivalent value of $15.8 million and forward contracts to buy foreign currencies with U.S. dollar equivalent value of $1.3 million. Additionally, at December 31, 2009, the Company had cross-currency swap agreements with a notional value of approximately $3 million. The Company had no outstanding option contracts to sell or buy foreign currencies at December 31, 2009.
The Company is exposed to stock price risk related to its deferred compensation and long-term incentive plans as, for some of the awards, the underlying liabilities are tied to the Company’s stock price. As the Company’s stock
55
price changes, such liabilities are adjusted and the impact is recorded in the Company’s Consolidated Statement of Income. The Company has entered into equity total return swap agreements with 400,000 notional shares in order to minimize earnings volatility related to the deferred compensation and long-term incentive plans. The Company has not designated the swaps as hedges. Rather, the Company marks the swaps to market and records the impact in Selling and administration expenses in the Company’s Consolidated Statements of Income. The adjustments to the values of the swaps offset the adjustments to the carrying values of the Company’s deferred compensation and long-term incentive plan liabilities, which are also recorded in Selling and administration expenses, and there is no significant impact on the Company’s Consolidated Statement of Income.
The Company is exposed to interest rate risk on its outstanding borrowings that are subject to floating rates. In October 2008, the Company entered into an interest rate swap agreement with a notional value of $30 million. The swap effectively converts the LIBOR based variable rate interest on an additional $30.0 million of debt to a fixed rate of 3.18%. The Company has designated the swap agreement as a cash flow hedge of the risk of variability in future interest payments attributable to changes in the LIBOR rate.
Cautionary Statement under Federal Securities Laws
Except for historical information contained herein, the information set forth in this Form 10-K contains forward-looking statements that are based on management’s beliefs, certain assumptions made by management and management’s current expectations, outlook, estimates and projections about the markets and economy in which the Company and its various businesses operate. Words such as “anticipates,” “believes,” “estimates,” “expects,” “forecasts,” “intends,” “opines,” “plans,” “predicts,” “projects,” “should,” “targets” and variations of such words and similar expressions are intended to identify such forward-looking statements. These statements are not guarantees of future performance and involve certain risks, uncertainties and assumptions (“Future Factors”), which are difficult to predict. Therefore, actual outcomes may differ materially from what is expected or forecasted in such forward-looking statements. The Company undertakes no obligation to update any forward-looking statements, whether as a result of future events, new information or otherwise. Future Factors which could cause actual outcomes to differ materially from those discussed include but are not limited to: general economic and business and market conditions; no improvement or weakening in U.S., European and Asian economies; increases in interest rates; changes in foreign currencies against the U.S. dollar; customer acceptance of new products; efficacy of new technology; changes in U.S. or foreign laws and regulations; increased competitive and/or customer pressure; loss of key customers; the Company’s ability to maintain chemical price increases or achieve targeted price increases; higher-than-expected raw material and energy costs and availability for certain chemical product lines; unexpected changes in the antidumping duties on certain products; increased foreign competition in the calcium hypochlorite markets; inability to obtain transportation for our chemicals; unfavorable court decisions, including unfavorable decisions in appeals of antidumping rulings, arbitration or jury decisions, tax matters or patent matters; the supply/demand balance for the Company’s products, including the impact of excess industry capacity; failure to achieve targeted cost-reduction programs; capital expenditures in excess of those scheduled; environmental costs in excess of those projected; the occurrence of unexpected manufacturing interruptions/outages at customer, supplier or Company plants; unfavorable weather conditions for swimming pool use; inability to expand sales in the professional pool dealer market; the impact of global weather changes; changes in the Company’s stock price; ability to obtain financing at attractive rates; financial market disruptions that impact our customers or suppliers; gains or losses on derivative instruments; implementation of the Company’s R&D consolidation consistent with the Company’s expectations; achievement of the Company’s multi-faceted margin improvement plan, including technology improvements which result in lower processing, energy and other costs; and unfavorable changes in the regulatory status of the Company’s products.
56
| Item 7A. | Quantitative and Qualitative Disclosures about Market Risk |
The Company is exposed to various market risks, including changes in foreign currency exchange rates, interest rates, commodity prices and stock prices. The Company does not enter into derivatives or other financial instruments for trading or speculative purposes in the normal course of business.
Interest Rates
The Company historically has been exposed to interest rate risk. As of December 31, 2010, approximately ninety percent of its outstanding borrowings are subject to fixed rates as a result of the new financing arrangements. Based on the Company’s expected 2010 borrowing levels and the borrowings subject to floating rates, an increase in interest rates of 100 basis points would decrease the Company’s annual results of operations and annual cash flows by approximately $0.2 million.
See Note 13 to the Consolidated Financial Statements for detail of the Company’s interest rate swap agreements.
Foreign Currency Risk
At December 31, 2010 the Company had forward contracts to sell foreign currencies with a U.S. dollar equivalent value of $90.0 million, including contracts with a $58.5 million notional value that are mitigating foreign currency exposure on certain balance sheet items. Additionally, at December 31, 2010, the Company had forward contracts to buy foreign currencies with a U.S. dollar equivalent value of $7.7 million and the Company had entered into cross-currency swap agreements with a total notional value of approximately $3 million.
Approximately 30 percent of the Company’s sales and expenses are denominated in currencies other than the U.S. dollar. As a result, the Company is subject to risks associated with its foreign operations, including currency devaluations and fluctuations in currency exchange rates. These exposures from foreign exchange fluctuations can affect the Company’s equity investments and its respective share of earnings (losses), the Company’s net investment in foreign subsidiaries, translation of the Company’s foreign operations for U.S. GAAP reporting purposes and purchase and sales commitments denominated in foreign currencies. The Company enters into forward sales and purchase contracts, cross-currency swap agreements and currency options to manage currency risk from actual and anticipated purchase and sales commitments, and certain balance sheet items, denominated or expected to be denominated in a foreign currency (principally British pound, euro, Australian dollar, New Zealand dollar, Canadian dollar, Japanese yen and South African rand). It is the Company’s policy to hedge up to 80% of these transactions during a calendar year. The counterparties to the options and contracts are major financial institutions.
Holding other variables constant, if there were a 10 percent change in foreign currency exchange rates versus the U.S. dollar, the net effect on the Company’s annual cash flows would be an increase (decrease) of between $2 million to $3 million related to the unhedged portion. Any increase (decrease) in cash flows resulting from the Company’s hedge forward contracts would be offset by an equal (decrease) increase in cash flows on the underlying transaction being hedged. The application of U.S. GAAP may cause increased volatility in the Company’s results of operations in the future if the Company changes its policies, or if some of the derivative instruments do not meet the requirements for hedge accounting.
See Note 13 to the Consolidated Financial Statements for detail of the Company’s foreign currency forward contracts and cross-currency swap agreements.
Commodity Price Risk
The Company is exposed to commodity price risk related to the price volatility of natural gas utilized at certain manufacturing sites as well as price volatility of propylene oxide and copper. The purchase
57
requirement of copper is approximately 15 million pounds annually. Depending on market conditions, the Company may purchase derivative commodity instruments to minimize the risk of price fluctuations of natural gas and copper. It is the Company’s policy to hedge up to 80 percent of its natural gas and copper purchases during a calendar year. In general, the Company’s guideline is to hedge a minimum of approximately 50 percent of the Company’s rolling twelve-month copper requirements. At December 31, 2010, the Company had purchase commitments but had no forward contracts to purchase natural gas and copper. In addition, the Company is exposed to price risk related to the volatility of certain other raw materials including the ongoing purchase of chromic acid and ethylene oxide. Holding other variables constant, a 10 percent adverse change in the price of propylene oxide would decrease the Company’s annual results of operations and annual cash flows between $2 million to $3 million. Additionally, holding other variables constant, a 10 percent adverse change in the price of either chromic acid or copper would decrease the Company’s annual results of operations and annual cash flows between $1 million to $2 million. Additionally, holding other variables constant, a 10 percent adverse change in the price of either natural gas or ethylene oxide, would decrease the Company’s annual results of operations and annual cash flows by approximately $1 million.
Stock Price Risk
The Company is exposed to stock price risk related to its deferred compensation and long-term incentive plans as a portion of the underlying liabilities are tied to the Company’s stock price. The Company has entered into equity total return swap agreements in order to minimize earnings volatility related to fluctuations in the Company’s stock price. The agreements mature in July 2011. The Company expects to extend or replace the agreements. See Note 13 to the Consolidated Financial Statements for further detail.
58
| Item 8. | Financial Statements and Supplementary Data |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Shareholders
Arch Chemicals, Inc.:
We have audited the accompanying consolidated balance sheets of Arch Chemicals, Inc. and subsidiaries (the “Company”) as of December 31, 2010 and 2009, and the related consolidated statements of income, shareholders’ equity and comprehensive income (loss), and cash flows for each of the years in the three-year period ended December 31, 2010. We also have audited the Company’s internal control over financial reporting as of December 31, 2010, based on criteria established inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company’s management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management Report. Our responsibility is to express an opinion on these consolidated financial statements and an opinion on the Company’s internal control over financial reporting based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the consolidated financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company and subsidiaries as of December 31, 2010 and 2009, and the results of its operations and its cash flows for each of the years in the three-year period ended December 31, 2010, in conformity with U.S. generally accepted accounting principles. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2010, based on criteria established inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission.
/s/ KPMG LLP
Stamford, Connecticut
February 23, 2011
59
ARCH CHEMICALS, INC. and SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
| | | | | | | | |
| | | December 31, | |
| | | 2010 | | | 2009 | |
| | | (in millions, except per share amounts) | |
| ASSETS | | | | | | | | |
Current Assets: | | | | | | | | |
Cash and Cash Equivalents | | $ | 210.2 | | | $ | 70.1 | |
Accounts Receivables, net | | | 124.5 | | | | 126.3 | |
Securitization-Related Receivable | | | 86.9 | | | | 76.0 | |
Inventories, net | | | 168.3 | | | | 145.9 | |
Other Current Assets | | | 28.1 | | | | 14.4 | |
Assets Held for Sale | | | 1.3 | | | | 127.7 | |
| | | | | | | | |
Total Current Assets | | | 619.3 | | | | 560.4 | |
Investments and Advances—Affiliated Companies at Equity | | | 1.7 | | | | 2.0 | |
Property, Plant and Equipment, net | | | 176.5 | | | | 173.5 | |
Goodwill | | | 205.6 | | | | 205.8 | |
Other Intangibles | | | 146.4 | | | | 156.1 | |
Other Assets | | | 88.5 | | | | 112.7 | |
| | | | | | | | |
Total Assets | | $ | 1,238.0 | | | $ | 1,210.5 | |
| | | | | | | | |
| LIABILITIES AND SHAREHOLDERS’ EQUITY | | | | | | | | |
Current Liabilities: | | | | | | | | |
Short-Term Borrowings | | $ | 7.3 | | | $ | 11.1 | |
Current Portion of Long-Term Debt | | | 31.2 | | | | 21.9 | |
Accounts Payable | | | 144.3 | | | | 116.9 | |
Accrued Liabilities | | | 95.4 | | | | 75.8 | |
Liabilities Associated with Assets Held for Sale | | | — | | | | 57.7 | |
| | | | | | | | |
Total Current Liabilities | | | 278.2 | | | | 283.4 | |
Long-Term Debt | | | 327.8 | | | | 257.7 | |
Other Liabilities | | | 189.0 | | | | 264.5 | |
| | | | | | | | |
Total Liabilities | | | 795.0 | | | | 805.6 | |
Commitments and Contingencies | | | | | | | | |
Shareholders’ Equity: | | | | | | | | |
Common Stock, par value $1 per share, Authorized 100.0 shares: | | | | | | | | |
25.1 shares issued and outstanding (25.0 in 2009) | | | 25.1 | | | | 25.0 | |
Additional Paid-in Capital | | | 469.3 | | | | 461.4 | |
Retained Earnings | | | 141.8 | | | | 91.2 | |
Accumulated Other Comprehensive Loss | | | (193.2 | ) | | | (172.7 | ) |
| | | | | | | | |
Total Shareholders’ Equity | | | 443.0 | | | | 404.9 | |
| | | | | | | | |
Total Liabilities and Shareholders’ Equity | | $ | 1,238.0 | | | $ | 1,210.5 | |
| | | | | | | | |
See accompanying notes to the consolidated financial statements.
60
ARCH CHEMICALS, INC. and SUBSIDIARIES
CONSOLIDATED STATEMENTS OF INCOME
| | | | | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | (in millions, except
per share amounts) | |
Sales | | $ | 1,377.4 | | | $ | 1,244.8 | | | $ | 1,300.2 | |
Cost of Goods Sold | | | 952.5 | | | | 876.2 | | | | 923.0 | |
Selling and Administration | | | 295.0 | | | | 266.7 | | | | 252.6 | |
Research and Development | | | 20.1 | | | | 18.7 | | | | 17.3 | |
Other (Gains) and Losses | | | 1.8 | | | | — | | | | (1.8 | ) |
Restructuring and Other Expense | | | 0.7 | | | | 1.1 | | | | 1.3 | |
Impairment Charge | | | 1.2 | | | | — | | | | 0.8 | |
Interest Expense | | | 14.1 | | | | 13.6 | | | | 14.0 | |
Interest Income | | | 1.9 | | | | 1.5 | | | | 3.4 | |
| | | | | | | | | | | | |
Income From Continuing Operations Before Taxes and Equity in Earnings of Affiliated Companies | | | 93.9 | | | | 70.0 | | | | 96.4 | |
Equity in Earnings of Affiliated Companies | | | 0.6 | | | | 0.6 | | | | 0.4 | |
Income Tax Expense | | | 30.0 | | | | 22.3 | | | | 36.3 | |
| | | | | | | | | | | | |
Income From Continuing Operations | | | 64.5 | | | | 48.3 | | | | 60.5 | |
Loss from Discontinued Operations (net of tax expense of $0.3, $2.4 and $2.6) | | | (0.5 | ) | | | (0.6 | ) | | | (23.5 | ) |
Gain (Loss) on Sale of Discontinued Operations (net of tax expense of
$1.9 and —) | | | 6.7 | | | | (0.6 | ) | | | — | |
| | | | | | | | | | | | |
Net Income | | $ | 70.7 | | | $ | 47.1 | | | $ | 37.0 | |
| | | | | | | | | | | | |
Net Income (Loss) Per Common Share—Basic: | | | | | | | | | | | | |
Continuing Operations | | $ | 2.58 | | | $ | 1.93 | | | $ | 2.44 | |
Loss from Discontinued Operations | | | (0.02 | ) | | | (0.02 | ) | | | (0.95 | ) |
Gain (Loss) on Sale of Discontinued Operations | | | 0.26 | | | | (0.02 | ) | | | — | |
| | | | | | | | | | | | |
Basic Net Income Per Common Share | | $ | 2.82 | | | $ | 1.89 | | | $ | 1.49 | |
| | | | | | | | | | | | |
Net Income (Loss) Per Common Share—Diluted: | | | | | | | | | | | | |
Continuing Operations | | $ | 2.56 | | | $ | 1.92 | | | $ | 2.43 | |
Loss from Discontinued Operations | | | (0.02 | ) | | | (0.02 | ) | | | (0.94 | ) |
Gain (Loss) on Sale of Discontinued Operations | | | 0.26 | | | | (0.02 | ) | | | — | |
| | | | | | | | | | | | |
Diluted Net Income Per Common Share | | $ | 2.80 | | | $ | 1.88 | | | $ | 1.49 | |
| | | | | | | | | | | | |
Weighted Average Common Stock Outstanding—Basic | | | 25.1 | | | | 25.0 | | | | 24.8 | |
Weighted Average Common Stock Outstanding—Diluted | | | 25.2 | | | | 25.1 | | | | 24.9 | |
See accompanying notes to the consolidated financial statements.
61
ARCH CHEMICALS, INC. and SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
| | | | | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | ($ in millions) | |
Operating Activities: | | | | | | | | | | | | |
Net Income | | $ | 70.7 | | | $ | 47.1 | | | $ | 37.0 | |
Adjustments to Reconcile Net Income to Net Cash and Cash Equivalents Provided by Operating Activities, Net of Businesses Acquired: | | | | | | | | | | | | |
Loss from Discontinued Operations | | | 0.5 | | | | 0.6 | | | | 23.5 | |
(Gain) Loss on Sale of Discontinued Operations | | | (6.7 | ) | | | 0.6 | | | | — | |
Equity in Earnings of Affiliates | | | (0.6 | ) | | | (0.6 | ) | | | (0.4 | ) |
Other (Gains) and Losses | | | 1.8 | | | | — | | | | (1.8 | ) |
Depreciation and Amortization | | | 40.3 | | | | 41.7 | | | | 39.9 | |
Deferred Taxes | | | 26.6 | | | | 18.2 | | | | 19.6 | |
Restructuring Expense | | | 0.7 | | | | — | | | | 1.3 | |
Restructuring Payments | | | (0.2 | ) | | | (0.2 | ) | | | (2.1 | ) |
Impairment Charge | | | 1.2 | | | | — | | | | 0.8 | |
Change in Assets and Liabilities, Net of Purchases and Sales of Businesses: | | | | | | | | | | | | |
Accounts Receivable Securitization Program | | | — | | | | — | | | | — | |
Receivables | | | (6.2 | ) | | | 7.0 | | | | (14.6 | ) |
Inventories | | | (20.1 | ) | | | 51.9 | | | | (19.3 | ) |
Other Current Assets | | | (7.2 | ) | | | 2.1 | | | | 1.3 | |
Accounts Payable and Accrued Liabilities | | | 22.8 | | | | (30.3 | ) | | | (21.0 | ) |
Noncurrent Liabilities | | | (59.8 | ) | | | (31.5 | ) | | | (10.3 | ) |
Other Operating Activities | | | 7.1 | | | | (0.7 | ) | | | (7.6 | ) |
| | | | | | | | | | | | |
Net Operating Activities from Continuing Operations | | | 70.9 | | | | 105.9 | | | | 46.3 | |
Cash Flow of Discontinued Operations | | | 2.2 | | | | 10.5 | | | | (0.9 | ) |
| | | | | | | | | | | | |
Net Operating Activities | | | 73.1 | | | | 116.4 | | | | 45.4 | |
| | | | | | | | | | | | |
Investing Activities: | | | | | | | | | | | | |
Capital Expenditures | | | (29.4 | ) | | | (27.4 | ) | | | (49.9 | ) |
Businesses Acquired in Purchase Transactions, Net of Cash Acquired | | | (2.3 | ) | | | 0.3 | | | | (125.5 | ) |
Proceeds from Sale of a Business, net | | | 44.4 | | | | 1.2 | | | | 3.7 | |
Proceeds from Sale of Land and Property | | | 1.4 | | | | — | | | | 0.7 | |
Cash Flow of Discontinued Operations | | | (0.4 | ) | | | (2.5 | ) | | | (3.4 | ) |
| | | | | | | | | | | | |
Net Investing Activities | | | 13.7 | | | | (28.4 | ) | | | (174.4 | ) |
| | | | | | | | | | | | |
Financing Activities: | | | | | | | | | | | | |
Long-Term Debt Borrowings | | | 314.0 | | | | 201.3 | | | | 150.0 | |
Long-Term Debt Repayments | | | (234.8 | ) | | | (239.4 | ) | | | (14.6 | ) |
Short-Term (Repayments) Borrowings, net | | | (4.8 | ) | | | (10.4 | ) | | | (7.0 | ) |
Dividends Paid | | | (20.1 | ) | | | (20.0 | ) | | | (19.9 | ) |
Cash Flow of Discontinued Operations | | | — | | | | — | | | | — | |
Other Financing Activities | | | 1.4 | | | | 0.2 | | | | 1.3 | |
| | | | | | | | | | | | |
Net Financing Activities | | | 55.7 | | | | (68.3 | ) | | | 109.8 | |
| | | | | | | | | | | | |
Effect of Exchange Rate Changes on Cash and Cash Equivalents | | | (2.4 | ) | | | (0.4 | ) | | | (3.7 | ) |
| | | | | | | | | | | | |
Net Increase (Decrease) in Cash and Cash Equivalents | | | 140.1 | | | | 19.3 | | | | (22.9 | ) |
Cash and Cash Equivalents, Beginning of Year | | | 70.1 | | | | 50.8 | | | | 73.7 | |
| | | | | | | | | | | | |
Cash and Cash Equivalents, End of Year | | $ | 210.2 | | | $ | 70.1 | | | $ | 50.8 | |
| | | | | | | | | | | | |
Supplemental Cash Flow Information for: | | | | | | | | | | | | |
Income Taxes Paid, net of refunds | | $ | 8.6 | | | $ | 2.5 | | | $ | 22.0 | |
Interest Paid | | $ | 13.1 | | | $ | 15.6 | | | $ | 12.6 | |
See accompanying notes to the consolidated financial statements.
62
ARCH CHEMICALS, INC. and SUBSIDIARIES
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY
AND COMPREHENSIVE INCOME (LOSS)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Common Stock | | | Additional
Paid-in
Capital | | | Retained
Earnings | | | Accumulated
Other
Comprehensive
Loss | | | Total
Shareholders’
Equity | | | Comprehensive
Income (Loss) | |
| | | | | | | |
| | | Shares | | | Amount | | | | | | |
| | | | | | | | | (in millions, except per share amounts) | | | | | | | |
Balance at December 31, 2007 | | | 24.7 | | | $ | 24.7 | | | $ | 451.6 | | | $ | 47.0 | | | $ | (48.9 | ) | | $ | 474.4 | | | | | |
Net Income | | | — | | | | — | | | | — | | | | 37.0 | | | | — | | | | 37.0 | | | | 37.0 | |
Foreign Currency Adjustments | | | — | | | | — | | | | — | | | | — | | | | (59.8 | ) | | | (59.8 | ) | | | (59.8 | ) |
Change in Fair Value of Derivatives, net of taxes of $0.8 | | | — | | | | — | | | | — | | | | — | | | | (1.9 | ) | | | (1.9 | ) | | | (1.9 | ) |
Pension Liability Adjustment, net of taxes of $34.2 | | | — | | | | — | | | | — | | | | — | | | | (73.6 | ) | | | (73.6 | ) | | | (73.6 | ) |
Stock issued | | | — | | | | — | | | | 0.2 | | | | — | | | | — | | | | 0.2 | | | | — | |
Tax Benefit on Stock Options | | | — | | | | — | | | | 0.1 | | | | — | | | | — | | | | 0.1 | | | | — | |
Stock Options Exercised | | | 0.1 | | | | 0.1 | | | | 1.1 | | | | — | | | | — | | | | 1.2 | | | | — | |
Share-Based Compensation | | | — | | | | — | | | | 4.2 | | | | — | | | | — | | | | 4.2 | | | | — | |
Cash Dividends ($0.80 per share) | | | — | | | | — | | | | — | | | | (19.9 | ) | | | — | | | | (19.9 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2008 | | | 24.8 | | | | 24.8 | | | | 457.2 | | | | 64.1 | | | | (184.2 | ) | | | 361.9 | | | $ | (98.3 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net Income | | | — | | | | — | | | | — | | | | 47.1 | | | | — | | | | 47.1 | | | | 47.1 | |
Foreign Currency Adjustments | | | — | | | | — | | | | — | | | | — | | | | 30.9 | | | | 30.9 | | | | 30.9 | |
Change in Fair Value of Derivatives, net of taxes of $0.3 | | | — | | | | — | | | | — | | | | — | | | | 1.2 | | | | 1.2 | | | | 1.2 | |
Pension Liability Adjustment, net of taxes of $10.2 | | | — | | | | — | | | | — | | | | — | | | | (20.6 | ) | | | (20.6 | ) | | | (20.6 | ) |
Tax Benefit on Stock Options | | | — | | | | — | | | | 0.1 | | | | — | | | | — | | | | 0.1 | | | | — | |
Stock Options Exercised | | | 0.1 | | | | 0.1 | | | | 1.4 | | | | — | | | | — | | | | 1.5 | | | | — | |
Share-Based Compensation | | | 0.1 | | | | 0.1 | | | | 2.7 | | | | — | | | | — | | | | 2.8 | | | | — | |
Cash Dividends ($0.80 per share) | | | — | | | | — | | | | — | | | | (20.0 | ) | | | — | | | | (20.0 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2009 | | | 25.0 | | | | 25.0 | | | | 461.4 | | | | 91.2 | | | | (172.7 | ) | | | 404.9 | | | $ | 58.6 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net Income | | | — | | | | — | | | | — | | | | 70.7 | | | | — | | | | 70.7 | | | | 70.7 | |
Foreign Currency Adjustments | | | — | | | | — | | | | — | | | | — | | | | (26.0 | ) | | | (26.0 | ) | | | (26.0 | ) |
Change in Fair Value of Derivatives, net of taxes of $(0.2) | | | — | | | | — | | | | — | | | | — | | | | (0.1 | ) | | | (0.1 | ) | | | (0.1 | ) |
Pension Liability Adjustment, net of taxes of $(2.2) | | | — | | | | — | | | | — | | | | — | | | | 5.6 | | | | 5.6 | | | | 5.6 | |
Tax Benefit on Stock Options | | | — | | | | — | | | | 0.5 | | | | — | | | | — | | | | 0.5 | | | | — | |
Stock Options Exercised | | | 0.1 | | | | 0.1 | | | | 0.7 | | | | — | | | | — | | | | 0.8 | | | | — | |
Share-Based Compensation | | | — | | | | — | | | | 6.7 | | | | — | | | | — | | | | 6.7 | | | | — | |
Cash Dividends ($0.80 per share) | | | — | | | | — | | | | — | | | | (20.1 | ) | | | — | | | | (20.1 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2010 | | | 25.1 | | | $ | 25.1 | | | $ | 469.3 | | | $ | 141.8 | | | $ | (193.2 | ) | | $ | 443.0 | | | $ | 50.2 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
See accompanying notes to the consolidated financial statements.
63
ARCH CHEMICALS, INC. and SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 1. | Description of Business and Summary of Significant Accounting Policies |
Formation of Arch Chemicals, Inc.
Arch Chemicals, Inc. (“Arch” or the “Company”) was organized under the laws of the Commonwealth of Virginia on August 25, 1998. The Company is a global biocides company providing innovative chemistry-based and related solutions to selectively destroy and control the growth of harmful microbes. Our focus is in water treatment, hair and skin care products, wood treatment, preservation and protection applications such as for paints and building products and health and hygiene applications. The Company operates in two segments: Biocides Products (formerly named Treatment Products) and Performance Products. The Biocides Products segment includes three reportable business units: the HTH water products, the personal care and industrial biocides products and the wood protection businesses. The Performance Products segment includes two reportable business units: the performance urethanes business and the hydrazine business.
The Company has organized its segments around differences in products and services, which is how the Company manages its businesses.
The Biocides Products businesses manufacture and sell water treatment chemicals, industrial biocides and personal care specialty ingredients and wood treatment products. HTH water products produces chemicals for the sanitization and treatment of residential pool and commercial pool and spa water, water used in industrial applications and the purification of potable water. Additionally, the surface water business of HTH water products manufactures a range of branded products, including products under theApplied Biochemists®brand name and provides technical support for controlling algae and nuisance aquatic vegetation. The Company sells both chlorine-based products (calcium hypochlorite and chlorinated isocyanurates) and non-chlorine-based products (poly (hexamethylene biguanide) hydrochloride (“PHMB”)) as sanitizers. Consumer brands includeHTH®,Baquacil®,Baqua Spa®,POOLIFE®,GLB® Pool & Spa®,andLeisure Time®. The personal care and industrial biocides business manufactures biocides that control dandruff on the scalp and control the growth of micro-organisms particularly fungi and algae. It markets products such asZinc Omadine®biocide, the most widely used antidandruff agent in the world, as well as actives and functional products sold primarily to manufacturers of skin care and hair care products. The Company’s industrial biocides are used in mildew-resistant paints, coatings and lubricants. The Company also develops, manufactures and markets biocides primarily for anti-bacterial applications; it is a leading global supplier of biocides for the industrial preservation and consumer segments of the biocides market. The biocides products are marketed under well-recognized brands, such asOmadine®,Omacide®,Triadine®,Proxel®, Purista®, Vantocil®, Reputex®, Cosmocil® andDensil® biocides. The Company’s wood protection business sells wood treatment chemicals solutions that enhance the properties of wood. Its wood preservatives and fire retardants are sold under the brand namesWolman®,Tanalith®,Vacsol®,Resistol® andDricon®.
Performance Products consist of performance urethanes and hydrazine. Performance urethanes manufacture a variety of specialty polyols, which are used as an ingredient for elastomers, adhesives, coatings, sealants and rigid foam. The business also manufactures glycols and glycol ethers for use as an ingredient in cleaners, personal care products and antifreeze. Hydrazine hydrates are used in chemical blowing agents, water treatment chemicals, agricultural products and pharmaceutical intermediates. Propellant-grade hydrazine and hydrazine derivatives are used by NASA, the U.S. Air Force and other customers as fuel in satellites, expendable launch vehicles and auxiliary and emergency power units.Ultra Pure™ hydrazine propellants are the highest purity anhydrous propellant in the industry and can extend the working life of satellites.
Basis of Presentation
The Consolidated Financial Statements include the accounts of the Company and its majority-owned subsidiaries. Intercompany balances and transactions between entities included in these Consolidated Financial Statements have been eliminated. Investments in 20-50% owned affiliates are accounted for on the equity method.
64
As a result of the sale of the industrial coatings business (see Note 5 for further detail), the Company has adjusted its prior period financial statements to include the results of operations of this business, and the gain on the disposition, as a component of discontinued operations. In addition, as a result of the sale, the Company has adjusted the prior period segment operating results to reallocate certain centralized service costs that were previously allocated to the industrial coatings business to the Company’s remaining businesses.
Reclassifications of prior-year data have been made, where appropriate, to conform to the 2010 presentation.
Use of Estimates
The preparation of the Consolidated Financial Statements requires estimates and assumptions that affect amounts reported and disclosed in the Consolidated Financial Statements and related Notes. Estimates are used when accounting for allowance for uncollectible accounts receivable, inventory obsolescence, valuation of assets held for sale, depreciation and amortization, employee benefit plans, performance-based incentive compensation, taxes, impairment of assets, environmental and legal liabilities and contingencies, among others. Actual results could differ from those estimates.
Cash and Cash Equivalents
Cash equivalents consist of highly liquid investments with an original maturity of three months or less.
Inventories
Inventories are stated at the lower of cost or net realizable value. Inventories are valued by the dollar value last-in, first-out (“LIFO”) method of inventory accounting for the domestic operations of the HTH water products, personal care and industrial biocides, performance urethanes and hydrazine businesses. Costs for all other inventories have been determined principally by the first-in, first-out (“FIFO”) method. Elements of costs in inventories include raw materials, direct labor and manufacturing overhead.
Assets Held for Sale
The Company accounts for assets held for sale in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 360, “Property, Plant, and Equipment”, which addresses financial accounting and reporting for the impairment or disposal of long-lived assets.
As a result of the sale of the industrial coatings business in March 2010, the Company has included the results of this business prior to its sale and the gain on the disposition as a component of discontinued operations.
Property, Plant and Equipment
Property, plant and equipment are recorded at cost. Depreciation is computed on a straight-line basis over the following estimated useful lives:
| | |
Improvements to land | | 5 to 20 years |
Building and building equipment | | 5 to 40 years |
Machinery and equipment | | 3 to 12 years |
Leasehold improvements are amortized over the term of the lease or the estimated useful life of the improvement, whichever is shorter. Start-up costs are expensed as incurred. Maintenance and repairs are expensed as incurred.
New Accounting Pronouncements
In December 2009, the FASB issued Accounting Standards Update (“ASU”) 2009-16 “Transfers and Servicing (Topic 860)—Accounting for Transfers of Financial Assets” (formerly Statement of Financial
65
Accounting Standards (“SFAS”) No. 166, “Accounting for Transfers of Financial Assets”) and FASB ASU 2009-17, “Consolidations (Topic 810)—Improvements to Financial Reporting by Enterprises Involved with Variable Interest Entities” (formerly SFAS No. 167, “Amendments to FASB Interpretation No. 46(R)”). FASB ASU 2009-16 is a revision to SFAS No. 140, “Accounting for Transfers and Servicing of Financial Assets and Extinguishments of Liabilities” and will require more information about transfers of financial assets, including securitization transactions, and where entities have continuing exposure to the risks related to transferred financial assets. FASB ASU 2009-17 is a revision to FASB Interpretation No. 46 (Revised December 2003), “Consolidation of Variable Interest Entities”, and changes how a reporting entity determines when an entity that is insufficiently capitalized or is not controlled through voting (or similar rights) should be consolidated. The new standards, which also require a number of new disclosures, were effective for the Company on January 1, 2010. The adoption of these pronouncements did not have any impact on the Company’s Consolidated Financial Statements.
Asset Retirement Obligations
The Company accounts for asset retirement obligations in accordance with FASB ASC 410, “Asset Retirement and Environmental Obligations.”
The Company has not recorded asset retirement obligations associated with certain owned or leased buildings and manufacturing facilities because the retirement obligations have an indeterminate settlement date and cannot be reasonably estimated. These asset retirement obligations are associated with removal and disposal of asbestos at certain Company sites and the shutdown of other assets (e.g., landfill and waste treatment facilities). The Company’s asset retirement obligation is to remove and dispose of asbestos properly if (i) the asbestos were to become exposed or become a health hazard or (ii) the facility containing the asbestos is demolished or undergoes major renovations. Currently, the asbestos is not exposed and is not a health hazard and the Company has no plans or expectations to demolish or undertake major renovations of these facilities. In addition, these facilities are expected to be maintained by normal repair and maintenance activities that would not involve the removal of the asbestos. The Company cannot estimate the settlement date or range of settlement dates of when the asbestos would be exposed. As for the other assets, the Company’s asset retirement obligation is based upon the future shutdown of the location or reaching capacity in the case of the landfill. Although the Company can estimate the current cost associated with these retirement obligations, the Company has no plans of ceasing operations of these facilities and the potential for reaching capacity at the landfill is estimated to be at least 50 years and potentially significantly longer. Therefore, the range of estimated settlement dates is so wide and so far out in the future as to preclude the Company from reasonably estimating the fair value of the obligation. Therefore, the Company concluded the retirement obligations had indeterminate settlement dates or such a wide range of potential settlement dates that no value could reasonably be estimated for the asset retirement obligations. The Company continues to monitor these assets as well as plans relating to these assets and their site locations and will record an asset retirement obligation as appropriate if circumstances change.
Goodwill
Goodwill represents the excess of the purchase price of acquired businesses over the fair value of the respective net assets.
The Company accounts for goodwill in accordance with FASB ASC 350, “Intangibles—Goodwill and Other”, which requires that goodwill no longer be amortized, but instead be tested for impairment at least annually in accordance with the provisions of FASB ASC 350. The Company tests goodwill for impairment as of January 1 of each year and when an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying amount. An impairment charge is recognized for the amount, if any, by which the carrying amount of goodwill exceeds its fair value. Fair values are established using discounted cash flows and when available, and as appropriate, comparative market multiples are used.
66
Other Intangibles
Other intangibles consist primarily of trademarks, developed technology, toxicology database, non-compete agreements and customer relationships.
In accordance with FASB ASC 350, intangible assets with indefinite useful lives are not amortized. Intangible assets with definite useful lives are amortized over their respective estimated useful lives to their estimated residual values in proportion to the economic benefits consumed, principally over 2 to 29 years, and generally on a straight-line basis. Intangible assets with indefinite lives are reviewed at least annually for impairment in accordance with FASB ASC 350.
Securitizations and Transfers of Financial Instruments
The Company may sell trade accounts or notes receivables with or without recourse in the normal course of business. Receivables sold under the Company’s accounts receivable securitization program are removed from Accounts Receivable, net on the accompanying Consolidated Balance Sheet at the time of sale. The purchase price of any such receivables sold for which payment has been deferred is recorded based on fair value as a Securitization-Related Receivable on the accompanying Consolidated Balance Sheet. Sales and transfers that do not meet the criteria for surrender of control would be accounted for as secured borrowings.
Long-Lived Assets
The impairment of tangible long-lived assets, other than goodwill and intangible assets with indefinite lives, is assessed when changes in circumstances indicate that their current carrying value may not be recoverable. A determination of impairment, if any, is made based on the undiscounted value of estimated future cash flows, salvage value or expected net sales proceeds, depending on the circumstances. Asset impairment losses are measured as the excess of the carrying value over the estimated fair value of such assets.
Environmental Liabilities and Expenditures
Accruals for environmental matters are recorded when it is probable that a liability has been incurred and the amount of the liability can be reasonably estimated, based upon current law and existing technologies. These amounts, which are not discounted and are exclusive of claims against third parties, are adjusted periodically as assessment and remediation efforts progress or additional technical or legal information becomes available. Environmental remediation costs are charged to reserves. Environmental costs are capitalized if the costs increase the value of the property and/or mitigate or prevent contamination from future operations.
Financial Instruments
The carrying values of cash and cash equivalents, accounts receivable and accounts payable approximated fair values due to the short-term maturities of these instruments.
The fair value of the Company’s borrowings, if any, under its existing credit facility, approximates book value due to the relatively short period such debt is expected to be outstanding and the fact that the interest rates on the borrowings are reset every one to six months. The fair value of the Company’s $75.0 million of borrowings under the shelf agreement was approximately $85 million at December 31, 2010. The fair value of the Company’s $250.0 million of borrowings under the master note agreement was approximately $242 million at December 31, 2010. The fair value of the Company’s short-term borrowings approximates the book value due to the floating interest rate terms and the short maturity of the instruments.
Derivative Instruments
Derivative instruments are recognized as assets or liabilities in the Company’s Consolidated Balance Sheets and are measured at fair value. The change in the fair value of a derivative designated as a fair value hedge and
67
the change in the fair value of the hedged item attributable to the hedged risk are recognized in earnings. For derivatives which qualify for designation as cash flow hedges, the effective portion of the changes in fair value is recognized as part of other comprehensive income until the underlying transaction that is being hedged is recognized in earnings. The ineffective portion of the change in fair value of cash flow hedges is recognized in earnings currently. Changes in fair value for other derivatives which do not qualify as hedges for accounting purposes are recognized in current period earnings.
Revenue Recognition
Substantially all of the Company’s revenues are derived from the sale of products. Revenue is recognized when risk of loss of, and title to, the product is transferred to the customer, which usually occurs at the time shipment is made. The majority of the Company’s products are sold FOB (“free on board”) shipping point or on an equivalent basis, that is, when product is delivered to the carrier. There are certain limited situations where the risk of loss and transfer of title passes upon delivery to the customer. In those circumstances, sales are recognized upon receipt by the customer. Allowances for estimated returns, discounts and retailer promotions and incentives are recognized when sales are recorded and are based on various market data, historical trends and information from customers. Actual returns, discounts and retail promotions and incentives have not been materially different from estimates. Certain of the Company’s product lines have extended payment terms due to the seasonal nature of the business. There are no conditions of acceptance, warranties or price protection that prohibit revenue recognition when risk of loss of, and title to, the product is transferred to the customer.
Shipping and Handling Costs
Shipping and handling fees billed to customers are included in Sales and shipping and handling costs are included in Cost of goods sold in the accompanying Consolidated Statements of Income.
U.S. Government Contracts
The Company has supply and storage contracts with the U.S. Defense Logistics Agency-Energy which principally consist of a fixed-price facility management fee for which revenue is recognized ratably over the contract period and the sale of product at a fixed price per pound, adjusted annually for agreed-upon cost escalations. Revenue is recognized for the U.S. Government’s product purchases when risk of loss of, and title to, the product is transferred to the U.S. Government which occurs after the product is inspected and accepted by the U.S. Government and sent to storage. Consequently, such revenue may be recognized prior to the U.S. Government taking physical possession. (See Note 21 for more information.)
Foreign Currency Translation
Foreign affiliates generally use their local currency as their functional currency. Accordingly, foreign affiliate balance sheet amounts are translated at the exchange rates in effect at year-end, and income statement and cash flow amounts are translated at the average rates of exchange prevailing during the year. Translation adjustments are included in the Other Accumulated Comprehensive Loss component of shareholders’ equity. Where foreign affiliates operate in highly inflationary economies, non-monetary amounts are translated to U.S. dollars at historical exchange rates while monetary assets and liabilities are translated to U.S. dollars at the current rate with the related adjustments reflected in the Consolidated Statements of Income.
Stock-Based Payments
The Company accounts for stock-based compensation under FASB ASC 718, “Compensation—Stock Compensation”, which requires companies to recognize expense over the requisite service period in the income statement for the grant-date fair value of awards of share based payments including equity instruments and stock appreciation rights to employees.
68
Income Taxes
The Company provides for income taxes in accordance with FASB ASC 740, “Income Taxes”, which requires the recognition of deferred tax liabilities and assets for the expected future tax consequences of temporary differences between the financial statement carrying amounts and the tax bases of assets and liabilities. The Company maintains valuation allowances where it is more likely than not all or a portion of a deferred tax asset will not be realized. Changes in valuation allowances from period to period are included in the Company’s tax provision in the period of change. In determining whether a valuation allowance is warranted, the Company takes into account such factors as future taxable income and available tax planning strategies. Failure to achieve forecasted taxable income could affect the ultimate realization of certain deferred tax assets.
The Company provides for income taxes for uncertain tax positions in accordance with FASB ASC 740, which requires the Company to recognize and measure tax benefits associated with tax positions and disclose uncertainties related to income tax positions in its financial statements. The Company cannot recognize a tax benefit in its financial statements unless it concludes the benefit is more likely than not of being sustained on audit by the taxing authority based solely on the technical merits of the associated tax position. The Company assesses the tax benefits of the income tax positions in its financial statements. The Company assesses these income tax positions based on experience with similar tax positions, information obtained during the examination process and the advice of experts. The Company recognizes previously unrecognized tax benefits upon the earlier of the expiration of the period to assess tax in the applicable taxing jurisdiction or when the matter is constructively settled and upon changes in statutes or regulations and new case law or rulings.
Earnings Per Common Share
Basic earnings per common share are computed by dividing net income available to common shareholders by the weighted-average number of common shares outstanding during the period. Diluted earnings per common share are calculated in a similar manner except that the weighted-average number of common shares outstanding during the period includes the potential dilution that could occur if stock options, or other contracts to issue common stock, were exercised and the dilutive effect of performance awards that will be settled in shares.
The reconciliations between basic and diluted shares outstanding for the years ended December 31, 2010, 2009 and 2008 are as follows:
| | | | | | | | | | | | |
| | | Years Ended December 31, | |
| (in millions) | | 2010 | | | 2009 | | | 2008 | |
Basic | | | 25.1 | | | | 25.0 | | | | 24.8 | |
Common equivalent shares from stock options and performance awards using the treasury stock method | | | 0.1 | | | | 0.1 | | | | 0.1 | |
| | | | | | | | | | | | |
Diluted | | | 25.2 | | | | 25.1 | | | | 24.9 | |
| | | | | | | | | | | | |
For the years ended December 31, 2010, 2009 and 2008, there were no stock options with exercise prices greater than the average market price.
In 2004, the Company established a Rabbi Trust for several deferred compensation plans (see Note 16 for more information). The Company’s stock held in the Rabbi Trust is treated in a manner similar to treasury stock. At December 31, 2010, the trust held approximately 0.1 million shares, which it had previously purchased on the open market.
Comprehensive Income (Loss)
The Company’s other comprehensive income (loss) consists of the changes in the cumulative foreign currency translation gains and losses, the change in the fair value of derivative financial instruments which qualify for hedge accounting, net of tax, and the pension liability adjustment, net of tax. The Company does not
69
provide for U.S. income taxes on foreign currency translation adjustments since it does not provide for such taxes on undistributed earnings of foreign subsidiaries, except for the Company’s Canadian subsidiaries and affiliated companies at equity, since the Company intends to continue to reinvest these earnings.
Employee Benefit Plans
Pension and postretirement health care and life insurance benefits earned during the year as well as interest on projected benefit obligations are accrued currently. Prior service costs and credits resulting from changes in plan benefits are amortized over the average remaining service period of employees expected to receive benefits. Curtailment gains and losses are recognized as incurred. Settlement gains and losses are recognized when significant pension obligations are settled and the gain or loss is determinable. The Company’s policy, in general, is to fund, at a minimum, amounts as are necessary to provide assets sufficient to meet the benefits to be paid to plan members in accordance with the relevant regulatory requirements governing such plans. In addition, FASB ASC 715, “Compensation—Retirement Benefits”, requires plan sponsors of defined benefit pension and other postretirement benefit plans (collectively, “postretirement benefit plans”) to recognize the funded status of their postretirement benefit plans in the statement of financial position and measure the fair value of plan assets and benefit obligations as of the date of the fiscal year-end statement of financial position.
Business and Credit Concentrations
A significant portion of sales of the Biocides Products segment (approximately 20%) is dependent upon two customers, one of which accounts for a significant portion of the sales of the HTH water products business and the other which accounts for a significant portion of the sales of the personal care and industrial biocides business. Sales to these two customers are individually less than 10% of the Company’s 2010 consolidated sales. However, the loss of either of these customers would have a material adverse effect on the sales and operating results of the Company, respective segment and businesses if such customer was not replaced.
Sales of the HTH water products business are seasonal in nature as its products are primarily used in the U.S. residential pool market. Historically, approximately 40% of the sales in the HTH water products business occur in the second quarter of the fiscal year, as retail sales in the U.S. residential pool market are concentrated between Memorial Day and the Fourth of July. Therefore, interim results for this segment are not necessarily indicative of the results to be expected for the entire fiscal year.
| 2. | Accounts Receivable/Securitization-Related Receivable |
Accounts receivable at December 31, 2010 and 2009 include the following:
| | | | | | | | |
| | | December 31, | |
| ($ in millions) | | 2010 | | | 2009 | |
Accounts receivable, trade | | $ | 104.9 | | | $ | 109.3 | |
Accounts receivable, other | | | 24.0 | | | | 21.5 | |
| | | | | | | | |
| | | 128.9 | | | | 130.8 | |
Less: allowance for doubtful accounts | | | (4.4 | ) | | | (4.5 | ) |
| | | | | | | | |
Accounts receivable, net | | $ | 124.5 | | | $ | 126.3 | |
| | | | | | | | |
Included in Accounts receivable, other is a receivable related to a favorable antidumping ruling for the period of review from December 16, 2004 through May 31, 2006 (see Note 20 for further discussion). The receivable, which includes interest, was $13.8 million and $13.5 million at December 31, 2010 and December 31, 2009, respectively.
70
Changes in the allowance for doubtful accounts for the years ended December 31, 2010, 2009 and 2008 are as follows:
| | | | | | | | | | | | |
| ($ in millions) | | Year Ended
December 31, 2010 | | | Year Ended
December 31, 2009 | | | Year Ended
December 31, 2008 | |
Beginning balance | | $ | (4.5 | ) | | $ | (4.1 | ) | | $ | (3.5 | ) |
Provision for doubtful accounts | | | (0.8 | ) | | | (1.1 | ) | | | (3.3 | ) |
Bad debt write-offs, net of recoveries | | | 0.8 | | | | 1.8 | | | | 2.2 | |
Foreign exchange and other | | | (0.2 | ) | | | (1.0 | ) | | | 0.4 | |
Reclassification to (from) short-term investment | | | 0.3 | | | | (0.1 | ) | | | 0.1 | |
| | | | | | | | | | | | |
Ending balance | | $ | (4.4 | ) | | $ | (4.5 | ) | | $ | (4.1 | ) |
| | | | | | | | | | | | |
In 2005 the Company entered into an accounts receivable securitization program with Three Pillars Funding LLC (“Three Pillars”), an affiliate of SunTrust Bank (“SunTrust”), and SunTrust Capital Markets, Inc., through which the Company sold undivided participation interests in certain domestic trade accounts receivable, without recourse, through its wholly-owned subsidiary, Arch Chemicals Receivables Corp. (now known as Arch Chemicals Receivables LLC) (“ACRC”), a special-purpose entity that is consolidated for financial reporting purposes. In connection with the securitization program, SunTrust entered into a Liquidity Agreement with Three Pillars to support its purchases of the Company’s accounts receivable annually. The Liquidity Agreement expired in October 2009 and at that time the Company entered into a new securitization program with Market Street Funding LLC and PNC Bank, National Association (“PNC Bank”) by way of an assignment and assumption of the Company’s existing program with Three Pillars and Sun Trust. Under the amended program, the Company sells domestic trade accounts receivable, and certain Canadian trade accounts receivable, to Market Street Funding LLC through ACRC. Additionally, the program provides ACRC with the ability to issue letters of credit. The amount of funding that the Company can obtain under the program is subject to change based upon the level of eligible receivables, with a maximum amount of $80 million. No more than $30 million of such funding can relate to letters of credit. The amended program is subject to annual renewal and has similar terms to the Company’s previous accounts receivable securitization program. During October 2010, the program was renewed for 364 days.
Under the amended program, the fair value of receivables, for which payment of the purchase price by Market Street Funding LLC is deferred, is recorded separately from Accounts receivable, net as a Securitization-related receivable on the accompanying Consolidated Balance Sheets. The fair value of these receivables was $86.9 million at December 31, 2010 and $76.0 million at December 31, 2009. Fair value of the receivables included a reserve for credit losses ($1.2 million at December 31, 2010 and $0.9 million at December 31, 2009) and was not discounted due to the short-term nature of the underlying financial assets.
The costs of the programs for the years ended December 31, 2010, 2009 and 2008 of $1.0 million, $1.4 million and $1.3 million, respectively, are included in Selling and administration expenses in the accompanying Consolidated Statements of Income. Under the amended program, the Company pays a facility fee that is equal to approximately 0.60% of the committed amount of the facility. The Company also incurs costs based on the fair market value of the receivables that are sold under the program and for which payment to Market Street Funding LLC has not been deferred. Such costs are based on the cost of commercial paper issued by Market Street Funding LLC plus a margin of approximately 0.50%. The Company has not recorded an asset or liability related to the servicing responsibility retained as the fees earned for servicing were estimated to approximate fair value.
71
Inventories at December 31, 2010 and 2009 include the following:
| | | | | | | | |
| | | December 31, | |
| ($ in millions) | | 2010 | | | 2009 | |
Raw materials and supplies | | $ | 63.4 | | | $ | 57.8 | |
Work-in-progress | | | 10.2 | | | | 7.8 | |
Finished goods | | | 149.0 | | | | 136.0 | |
| | | | | | | | |
Inventories, gross | | | 222.6 | | | | 201.6 | |
LIFO reserves | | | (54.3 | ) | | | (55.7 | ) |
| | | | | | | | |
Inventories, net | | $ | 168.3 | | | $ | 145.9 | |
| | | | | | | | |
Approximately 50 percent of the Company’s inventories are valued by the dollar value last-in, first-out (“LIFO”) method of inventory accounting. Gross inventory values approximate replacement cost. During 2009, the Company recorded a $2.9 million benefit in Cost of goods sold related to a LIFO decrement.
Other current assets at December 31, 2010 and 2009 include the following:
| | | | | | | | |
| | | December 31, | |
| ($ in millions) | | 2010 | | | 2009 | |
Deferred income taxes (Note 14) | | $ | 8.7 | | | $ | 5.0 | |
Other | | | 19.4 | | | | 9.4 | |
| | | | | | | | |
Other current assets | | $ | 28.1 | | | $ | 14.4 | |
| | | | | | | | |
Included in Other current assets at December 31, 2010 are equity total return swap derivatives with a fair value of $5.2 million. The derivatives were included in Other assets at December 31, 2009. See Note 13 for further detail.
| 5. | Assets Held for Sale/Discontinued Operations |
Industrial Coatings Business
On March 31, 2010, the Company completed the sale of its industrial coatings business to The Sherwin-Williams Company (“Sherwin-Williams”). Gross proceeds from the sale, before expenses and the final working capital adjustment, were €39.9 million (approximately $54 million). As a result of the sale, the Company recorded an after-tax gain of $6.6 million, which includes $27.7 million of net cumulative historical foreign currency translation gains that were recognized at the time of the sale. During the three months ended December 31, 2009, the Company recorded transaction costs, $0.6 million net of tax, related to the sale. Therefore, the total net gain on the sale of the business was $6.0 million. Proceeds from the sale are being used for general corporate purposes. The business had sales for the three months ended March 31, 2010 of $34.5 million. The business had sales for the years ended December 31, 2009 and December 31, 2008 of $147.1 million and $191.9 million, respectively.
72
The net gain that was recorded during the year ended December 31, 2010 is reflected in Gain on Sale of Discontinued Operations, as follows:
| | | | |
| | | ($ in millions) | |
Net Assets Sold: | | | | |
Working capital | | $ | 19.9 | |
Non-current assets/liabilities | | | 43.7 | |
| | | | |
Net assets sold | | $ | 63.6 | |
| |
Gain on Sale: | | | | |
Cash proceeds | | $ | 54.0 | |
Working capital adjustment | | | (4.6 | ) |
| | | | |
Proceeds | | | 49.4 | |
Net assets sold | | | (63.6 | ) |
Transaction costs incurred | | | (5.1 | ) |
Cumulative foreign currency translation realized | | | 27.7 | |
| | | | |
Pre-tax gain | | | 8.4 | |
Tax expense | | | (1.8 | ) |
| | | | |
Net gain | | $ | 6.6 | |
| | | | |
Industrial Coatings Balance Sheet
Assets held for sale at December 31, 2009 principally relate to the industrial coatings business. The major classes of assets and liabilities of such business consist of the following:
| | | | |
| | | ($ in millions) | |
Accounts receivable, net | | $ | 35.0 | |
Inventories, net | | | 25.2 | |
Other current assets | | | 1.9 | |
Property, plant and equipment, net | | | 38.8 | |
Other intangibles | | | 24.2 | |
| | | | |
Total assets held for sale | | | 125.1 | |
Accounts payable and accrued liabilities | | | 39.5 | |
Other liabilities | | | 18.2 | |
| | | | |
Total liabilities associated with assets held for sale | | | 57.7 | |
| | | | |
Net assets held for sale of the Industrial Coatings business | | $ | 67.4 | |
| | | | |
As of December 31, 2009, the Company had $31.6 million of cumulative unrecognized foreign currency translation gains related to the industrial coatings business.
Performance Urethanes Business in Venezuela
In September 2007, the Company completed the sale of its non-strategic performance urethanes business in Venezuela. During 2010, 2009 and 2008, $0.5 million, $1.2 million and $3.7 million, respectively, of the proceeds were received. Total proceeds, net of expenses, from the sale were $17.0 million, all of which had been received as of December 31, 2010.
73
| 6. | Investments and Advances—Affiliated Companies at Equity |
The amount of cumulative unremitted earnings of joint ventures included in consolidated retained earnings at December 31, 2010 was $1.5 million. During the years ended December 31, 2010, 2009 and 2008, distributions of $0.9 million, $0.2 million and $0.3 million, respectively, were received from joint ventures.
| 7. | Property, Plant and Equipment |
Property, plant and equipment at December 31, 2010 and 2009 include the following:
| | | | | | | | |
| | | December 31, | |
| ($ in millions) | | 2010 | | | 2009 | |
Land and improvements to land | | $ | 20.4 | | | $ | 20.7 | |
Buildings and building equipment | | | 101.2 | | | | 100.1 | |
Machinery and equipment | | | 605.8 | | | | 623.9 | |
Leasehold improvements | | | 13.7 | | | | 12.5 | |
Construction-in-progress | | | 18.9 | | | | 14.6 | |
| | | | | | | | |
Property, plant and equipment | | | 760.0 | | | | 771.8 | |
Less accumulated depreciation | | | (583.5 | ) | | | (598.3 | ) |
| | | | | | | | |
Property, plant and equipment, net | | $ | 176.5 | | | $ | 173.5 | |
| | | | | | | | |
Maintenance and repairs charged to operations amounted to $29.7 million, $27.8 million and $27.0 million in 2010, 2009, and 2008, respectively.
During 2008, the Company recorded a $0.8 million impairment charge of certain manufacturing assets for the wood protection business. The write-off is recorded in Impairment charge in the Company’s 2008 Consolidated Statement of Income.
| 8. | Goodwill and Other Intangibles |
Goodwill
The changes in the carrying amount of goodwill for the years ended December 31, 2010 and 2009 are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | |
| ($ in millions) | | HTH Water
Products | | | Personal
Care and
Industrial
Biocides | | | Wood
Protection | | | Total
Biocides
Products | | | Performance
Urethanes | | | Total | |
Balance, December 31, 2008 | | $ | 78.4 | | | $ | 75.1 | | | $ | 41.7 | | | $ | 195.2 | | | $ | 4.4 | | | $ | 199.6 | |
Foreign exchange | | | 0.7 | | | | 2.9 | | | | 2.6 | | | | 6.2 | | | | — | | | | 6.2 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance, December 31, 2009 | | | 79.1 | | | | 78.0 | | | | 44.3 | | | | 201.4 | | | | 4.4 | | | | 205.8 | |
Foreign exchange | | | (0.1 | ) | | | (1.1 | ) | | | 1.0 | | | | (0.2 | ) | | | — | | | | (0.2 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance, December 31, 2010 | | $ | 79.0 | | | $ | 76.9 | | | $ | 45.3 | | | $ | 201.2 | | | $ | 4.4 | | | $ | 205.6 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
74
Other Intangibles
The gross carrying amount and accumulated amortization for other intangible assets as of December 31, 2010 and 2009 are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | December 31, 2010 | | | December 31, 2009 | |
| ($ in millions) | | Gross
Carrying
Amount | | | Accumulated
Amortization | | | Net
Carrying
Amount | | | Gross
Carrying
Amount | | | Accumulated
Amortization | | | Net
Carrying
Amount | |
Patents | | $ | 0.2 | | | $ | 0.2 | | | $ | — | | | $ | 0.2 | | | $ | 0.2 | | | $ | — | |
Customer lists | | | 95.0 | | | | 37.5 | | | | 57.5 | | | | 96.0 | | | | 30.6 | | | | 65.4 | |
Toxicology database | | | 14.0 | | | | 6.4 | | | | 7.6 | | | | 14.6 | | | | 5.7 | | | | 8.9 | |
Developed technology | | | 16.0 | | | | 6.2 | | | | 9.8 | | | | 16.4 | | | | 5.1 | | | | 11.3 | |
Other | | | 14.7 | | | | 5.7 | | | | 9.0 | | | | 13.9 | | | | 5.4 | | | | 8.5 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total amortizable other Intangibles | | | 139.9 | | | | 56.0 | | | | 83.9 | | | | 141.1 | | | | 47.0 | | | | 94.1 | |
Total non-amortizable other Intangibles—Trademarks | | | 62.7 | | | | 0.2 | | | | 62.5 | | | | 62.1 | | | | 0.1 | | | | 62.0 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total other intangibles | | $ | 202.6 | | | $ | 56.2 | | | $ | 146.4 | | | $ | 203.2 | | | $ | 47.1 | | | $ | 156.1 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Amortization
Amortization expense for the years ended December 31, 2010, 2009 and 2008 was $10.9 million, $10.8 million and $9.7 million, respectively. Estimated amortization expense is $11.7 million for the year ended December 31, 2011 and $11.4 million for the years ended December 31, 2012 through December 31, 2015.
Included in other assets at December 31, 2010 and 2009 are the following:
| | | | | | | | |
| | | December 31, | |
| ($ in millions) | | 2010 | | | 2009 | |
Deferred taxes (Note 14) | | $ | 48.2 | | | $ | 80.8 | |
Other | | | 40.3 | | | | 31.9 | |
| | | | | | | | |
Other assets | | $ | 88.5 | | | $ | 112.7 | |
| | | | | | | | |
Included in Other assets is a receivable related to a favorable antidumping ruling for the period of review from June 1, 2006 through May 31, 2007 (see Note 20 for further discussion). The receivable, which includes interest, was $13.6 million and $13.2 million at December 31, 2010 and December 31, 2009, respectively.
Included in Other assets at December 31, 2010 is a $10.3 million income tax receivable. There were no income tax receivables recorded in Other assets at December 31, 2009.
Included in accrued liabilities at December 31, 2010 and 2009 are the following:
| | | | | | | | |
| | | December 31, | |
| ($ in millions) | | 2010 | | | 2009 | |
Accrued compensation | | $ | 37.6 | | | $ | 22.6 | |
Accrued litigation | | | 4.9 | | | | 5.1 | |
Environmental reserves (Note 20) | | | 1.5 | | | | 1.5 | |
Other | | | 51.4 | | | | 46.6 | |
| | | | | | | | |
Accrued liabilities | | $ | 95.4 | | | $ | 75.8 | |
| | | | | | | | |
75
Included in short-term borrowings and long-term debt at December 31, 2010 and 2009 are the following:
| | | | | | | | |
| | | December 31, | |
| ($ in millions) | | 2010 | | | 2009 | |
Senior revolving credit facility | | $ | 30.0 | | | $ | 108.0 | |
Term loan | | | — | | | | 90.0 | |
Shelf agreement senior notes due August 2016 | | | 75.0 | | | | 75.0 | |
Master note agreement senior notes due December 2017 | | | 250.0 | | | | — | |
Other borrowings | | | 11.3 | | | | 17.7 | |
| | | | | | | | |
Total debt | | | 366.3 | | | | 290.7 | |
Less: Current portion of long-term debt | | | (31.2 | ) | | | (21.9 | ) |
Less: Short-term borrowings | | | (7.3 | ) | | | (11.1 | ) |
| | | | | | | | |
Long-term debt | | $ | 327.8 | | | $ | 257.7 | |
| | | | | | | | |
Credit Facility
On June 15, 2006, the Company entered into an unsecured $350.0 million senior revolving credit facility (“credit facility”), which expires in June 2011. The Company’s credit facility contains a quarterly leverage ratio (Debt/EBITDA) covenant not to exceed 3.5. At December 31, 2010, the Company’s quarterly leverage ratio, as defined in the credit facility, was 2.5. Additionally, the credit facility contains an interest coverage ratio (EBITDA/total interest expense) covenant not to be less than 3.0. At December 31, 2010 the Company’s interest coverage ratio, as defined in the credit facility, was 10.6. The Company was in compliance with both of these covenants throughout 2010. The credit facility also restricts the payment of dividends and repurchase of stock to $65.0 million plus 50% of cumulative net income (loss) subject to certain limitations beginning June 15, 2006. At December 31, 2010, restricted payments were limited to $97.7 million. The facility fees can range from 0.100% to 0.225% depending on the Company’s quarterly leverage ratios (facility fees were 0.125% at December 31, 2010). The Company may select various floating rate borrowing options, including, but not limited to, LIBOR plus a spread that can range from 0.4% to 0.9% depending on the Company’s quarterly leverage ratios (the spread was 0.5% at December 31, 2010). There was $30.0 million of debt outstanding under the credit facility at December 31, 2010. Due to the fact that the credit facility expires in June 2011, such amount was recorded in Current portion of long-term debt in the Consolidated Balance Sheet. There was $108.0 million of debt outstanding under the credit facility at December 31, 2009. Such amount was recorded in Long-term debt in the Consolidated Balance Sheet.
Term Loan
On February 13, 2009, the Company entered into an unsecured $100.0 million credit agreement (“term loan”) with a number of banks. The term loan provided for amortization of principal equal to $5 million per quarter, beginning September 30, 2009. During September 2010, the remaining $80 million outstanding balance was repaid using the proceeds from the Series 2010-A Senior Notes (see below for further detail).
Shelf Agreement
On August 28, 2009, the Company entered into a $150.0 million note purchase and private shelf agreement (the “shelf agreement”) with Prudential Investment Management, Inc. (“Prudential”) and immediately issued $75.0 million of unsecured Series A senior notes (the “Notes”). The notes will mature in August 2016 and bear a fixed annual interest rate of 6.70%. The shelf agreement provides for the additional purchase by Prudential of notes, in amounts to be mutually agreed, up to an additional maximum amount of $75.0 million through August 2012, on terms to be determined. The shelf agreement contains a quarterly leverage ratio covenant not to exceed 3.5 and an interest coverage ratio covenant not to be less than 3.0, both of which are consistent with the existing credit facility.
76
At December 31, 2010, the Company’s quarterly leverage ratio, as defined in the shelf agreement, was 2.5 and the Company’s interest coverage ratio, as defined in the shelf agreement, was 10.6. The Company was in compliance with both of these covenants throughout 2010. Additionally, the shelf agreement restricts the payment of dividends and repurchase of stock to $88.1 million plus 50% of cumulative adjusted net income (loss) for the period beginning June 30, 2009. At December 31, 2010, restricted payments were limited to $97.7 million.
Master Note Agreement
On September 9, 2010, the Company entered into a master note purchase agreement (the “master note agreement”) with certain institutional investors, which provides financing to the Company through the private placement of $250.0 million aggregate principal amount of the Company’s Series 2010-A Senior Notes (the “Senior Notes”). The Company issued $125.0 million of the Senior Notes at closing. The remaining $125.0 million was issued by the Company in December 2010. The Senior Notes will mature in December 2017 and bear a fixed annual interest rate of 4.0%. The master note agreement contains a quarterly leverage ratio covenant not to exceed 3.5 and an interest coverage ratio covenant not to be less than 3.0, both of which are consistent with the existing credit facility and shelf agreement. At December 31, 2010, the Company’s quarterly leverage ratio, as defined by the master note agreement, was 2.5 and the Company’s interest coverage ratio, as defined by the master note agreement, was 10.6. The Company has been in compliance with both of these covenants since the inception of the master note agreement. Additionally, the master note agreement restricts the payment of dividends and repurchase of stock to $99.4 million plus 50% of cumulative adjusted net income (loss) for the period beginning June 30, 2010. At December 31, 2010, restricted payments were limited to $97.7 million.
Senior Notes
In March 2002, the Company issued $211.0 million of unsecured senior notes to certain institutional investors in two series. The Company used its unsecured $350.0 million senior revolving credit facility to repay the Series A notes in March 2007 and to repay the Series B notes, $62.0 million, in March 2009.
Other Borrowings
Other borrowings at December 31, 2010 included $10.5 million of borrowings under international credit facilities and $0.8 million of capitalized leases. Such credit facilities and capitalized leases have interest rates principally ranging from 7% to 11%.
At December 31, 2010, the Company had $24.7 million of outstanding letters of credit, $2.9 million of which reduced availability under the Company’s credit facility. Additionally, at December 31, 2010, the Company had $2.4 million of outstanding letters of guarantee.
Interest Rate Swaps
In October 2008, the Company entered into an additional interest rate swap agreement with a notional value of $30 million. See Note 13 for further discussion.
Fair Value of Debt
The fair value of the Company’s borrowings, if any, under its existing credit facility, approximates book value due to the relatively short period such debt is expected to be outstanding and the fact that the interest rates on the borrowings are reset every one to six months. The fair value of the Company’s $75.0 million of borrowings under the shelf agreement was approximately $85 million at December 31, 2010. The fair value of the Company’s $250.0 million of borrowings under the master note agreement was approximately $242 million at December 31, 2010. The fair value of the Company’s short-term borrowings approximates the book value due to the floating interest rate terms and the short maturity of the instruments.
77
Included in other non-current liabilities at December 31, 2010 and 2009 are the following:
| | | | | | | | |
| | | December 31, | |
| ($ in millions) | | 2010 | | | 2009 | |
Pensions and other postretirement employee benefit obligations (Note 15) | | $ | 134.7 | | | $ | 214.7 | |
Deferred long-term incentive compensation (Note 16) | | | 17.3 | | | | 16.4 | |
Deferred tax liability (Note 14) | | | 1.3 | | | | 1.1 | |
Environmental reserves (Note 20) | | | 7.5 | | | | 6.7 | |
Unrecognized tax benefits (Note 14) | | | 11.7 | | | | 13.2 | |
Other | | | 16.5 | | | | 12.4 | |
| | | | | | | | |
Other liabilities | | $ | 189.0 | | | $ | 264.5 | |
| | | | | | | | |
| 13. | Derivative Financial Instruments |
Foreign Currency
The Company uses foreign currency forward contracts as a means of hedging exposure to foreign currency risk. It is the Company’s policy to hedge up to 80% of its anticipated purchase and sales commitments, and certain balance sheet items, denominated or expected to be denominated in a currency other than the business’ functional currency (principally British pound, euro, Australian dollar, New Zealand dollar, Canadian dollar, Japanese yen and South African rand). Most of the Company’s currency derivatives expire within one year. During 2010, 2009 and 2008, the majority of the Company’s foreign currency forward contracts qualified as effective cash flow hedges. The remainder of the foreign currency contracts did not meet the criteria to qualify for hedge accounting. Additionally, the Company has entered into cross-currency swap agreements to hedge its exposure to the variability of future foreign currency cash flows through August 2014. The cross-currency swap agreements do not meet the criteria to qualify for hedge accounting.
At December 31, 2010, the Company had forward contracts to sell foreign currencies with U.S. dollar equivalent value of $90.0 million, including contracts with a $58.5 million notional value that are mitigating foreign currency exposure on certain balance sheet items. Additionally, at December 31, 2010, the Company had forward contracts to buy foreign currencies with U.S. dollar equivalent value of $7.7 million and cross-currency swap agreements with a notional value of approximately $3 million. At December 31, 2009, the Company had forward contracts to sell foreign currencies with U.S. dollar equivalent value of $15.8 million and forward contracts to buy foreign currencies with U.S. dollar equivalent value of $1.3 million. Additionally, at December 31, 2009, the Company had cross-currency swap agreements with a notional value of approximately $3 million.
The counterparties to the Company’s forward contracts and cross-currency swap agreements are major financial institutions. The risk of loss to the Company in the event of nonperformance by a counterparty is not significant. The Company does not use financial instruments for speculative or trading purposes, nor is the Company a party to leveraged derivatives.
As of December 31, 2010 and 2009, the fair values of the Company’s foreign currency forward contracts that were designated as hedging instruments, as well as those contracts that were not designated as hedging instruments, were immaterial. During 2010, 2009 and 2008 the amounts that were excluded from effectiveness testing, for foreign currency forward contracts that were designated as hedging instruments, were immaterial. Additionally, during 2010, 2009 and 2008 amounts recorded in the Company’s Consolidated Statements of Income, related to instruments that were not designated as hedging instruments, were immaterial.
78
As of December 31, 2010 and 2009, the fair value of the Company’s cross-currency swap agreements was immaterial. Additionally, during 2010 and 2009, the amounts recorded in the Company’s Consolidated Statements of Income related to such swap agreements were immaterial.
Foreign currency exchange transaction (gains) losses, net of taxes, were $(1.3) million in 2010, $0.5 million in 2009, and $(4.6) million in 2008.
Compensation
The Company is exposed to stock price risk related to its deferred compensation and long-term incentive plans as, for some of the awards, the underlying liabilities are tied to the Company’s stock price. As the Company’s stock price changes, such liabilities are adjusted and the impact is recorded in the Company’s Consolidated Statement of Income. The Company entered into equity total return swap agreements with 400,000 notional shares in order to minimize earnings volatility related to the deferred compensation and long-term incentive plans. The Company has not designated the swaps as hedges. Rather, the Company marks the swaps to market and records the impact in Selling and Administration expenses in the Company’s Consolidated Statement of Income. The adjustments to the values of the swaps offset the adjustments to the carrying values of the Company’s deferred compensation and long-term incentive plan liabilities, which are also recorded in Selling and administration expenses, and there is no impact on the Company’s Consolidated Statements of Income.
The counterparty to the agreements is a major financial institution. The agreements will mature in July 2011, at which time cash settlement will occur. The counterparty can terminate the swap on 200,000 shares if the Company’s stock price falls below $11.37, and it can terminate the swap on the remaining 200,000 shares if the stock price falls below $11.05.
The following table displays the fair values at December 31, 2010 and 2009 of the Company’s equity total return swap derivatives that were not designated as hedging instruments, as well as the classification of such amounts in the Company’s Consolidated Balance Sheets:
| | | | | | | | |
| ($ in millions) | | December 31,
2010 | | | December 31,
2009 | |
Assets | | | | | | | | |
Other current assets | | $ | 5.2 | | | $ | — | |
Other assets | | | — | | | | 2.4 | |
During 2010, 2009 and 2008, the Company recognized gains of $2.8 million, $1.9 million and $0.5 million, respectively, in Selling and administration expenses related to the equity total return swap agreements.
Debt and Interest
In April 2008, the Company entered into interest rate swap agreements with a notional value of $20 million. The agreements expired in June 2010.
In October 2008, the Company entered into an interest rate swap agreement with a notional value of $30 million. The swap effectively converts the LIBOR based variable rate interest on an additional $30.0 million of debt outstanding under the credit facility to a fixed rate of 3.18%. The counterparty to the swap agreement is a major financial institution. The agreement expires in January 2012. The Company has designated the swap agreement as a cash flow hedge of the risk of variability in future interest payments attributable to changes in the LIBOR rate. Any ineffectiveness for the swap agreement is not material.
79
The following table displays the fair values at December 31, 2010 and 2009 of the Company’s interest rate swap derivatives which were designated as hedging instruments. Additionally, the table displays the classification of such amounts in the Company’s Consolidated Balance Sheets:
| | | | | | | | |
| ($ in millions) | | December 31,
2010 | | | December 31,
2009 | |
Liabilities | | | | | | | | |
Accrued liabilities | | $ | 0.8 | | | $ | 0.8 | |
Other liabilities | | | 0.1 | | | | 0.6 | |
| | | | | | | | |
Total Liabilities | | $ | 0.9 | | | $ | 1.4 | |
| | | | | | | | |
The following table displays the effect of derivative instruments that are designated as cash flow hedges on the Company’s Consolidated Statement of Income and Consolidated Balance Sheet during 2010:
| | | | | | | | | | | | | | | | | | | | |
| ($ in millions) | | AOCL as of
12/31/09 | | | Effective
Portion
Recorded
in AOCL | | | Effective
Portion
Reclassified
from AOCL to
Selling and
administration | | | Effective
Portion
Reclassified
from AOCL
to Interest
expense | | | AOCL
Balance
as of
12/31/10 | |
Type of derivative | | | | | | | | | | | | | | | | | | | | |
Interest Rate Swap Agreements | | $ | 0.8 | | | $ | 0.4 | | | $ | — | | | $ | (0.6 | ) | | $ | 0.6 | |
Foreign Currency Forward Contracts | | | (0.1 | ) | | | 0.4 | | | | (0.1 | ) | | | — | | | | 0.2 | |
| | | | | | | | | | | | | | | | | | | | |
Total | | $ | 0.7 | | | $ | 0.8 | | | $ | (0.1 | ) | | $ | (0.6 | ) | | $ | 0.8 | |
| | | | | | | | | | | | | | | | | | | | |
Upon the expiration of foreign currency forward contracts and settlement dates for the interest rate swap agreements, during 2011, the entire balance which was recorded in AOCL at December 31, 2010 will be reclassified into earnings.
The following table displays the effect of derivative instruments that are designated as cash flow hedges on the Company’s Consolidated Statement of Income and Consolidated Balance Sheet during 2009:
| | | | | | | | | | | | | | | | | | | | |
| ($ in millions) | | AOCL as of
12/31/08 | | | Effective
Portion
Recorded
in AOCL | | | Effective
Portion
Reclassified
from AOCL to
Selling and
administration | | | Effective
Portion
Reclassified
from AOCL
to Interest
expense | | | AOCL
Balance
as of
12/31/09 | |
Type of derivative | | | | | | | | | | | | | | | | | | | | |
Interest Rate Swap Agreements | | $ | 1.0 | | | $ | 0.5 | | | $ | — | | | $ | (0.7 | ) | | $ | 0.8 | |
Foreign Currency Forward Contracts | | | 0.9 | | | | 0.6 | | | | (1.6 | ) | | | — | | | | (0.1 | ) |
| | | | | | | | | | | | | | | | | | | | |
Total | | $ | 1.9 | | | $ | 1.1 | | | $ | (1.6 | ) | | $ | (0.7 | ) | | $ | 0.7 | |
| | | | | | | | | | | | | | | | | | | | |
The following table displays the effect of derivative instruments that are designated as cash flow hedges on the Company’s Consolidated Statement of Income and Consolidated Balance Sheet during 2008:
| | | | | | | | | | | | | | | | | | | | |
| ($ in millions) | | AOCL as of
12/31/07 | | | Effective
Portion
Recorded
in AOCL | | | Effective
Portion
Reclassified
from AOCL to
Selling and
administration | | | Effective
Portion
Reclassified
from AOCL
to Interest
expense | | | AOCL
Balance
as of
12/31/08 | |
Type of derivative | | | | | | | | | | | | | | | | | | | | |
Interest Rate Swap Agreements | | $ | — | | | $ | 1.0 | | | $ | — | | | $ | — | | | $ | 1.0 | |
Foreign Currency Forward Contracts | | | — | | | | 0.7 | | | | 0.2 | | | | — | | | | 0.9 | |
| | | | | | | | | | | | | | | | | | | | |
Total | | $ | — | | | $ | 1.7 | | | $ | 0.2 | | | $ | — | | | $ | 1.9 | |
| | | | | | | | | | | | | | | | | | | | |
80
Components of Pretax Income from Continuing Operations
| | | | | | | | | | | | |
| | | Years Ended December 31, | |
| ($ in millions) | | 2010 | | | 2009 | | | 2008 | |
Domestic | | $ | 52.5 | | | $ | 46.7 | | | $ | 75.0 | |
Foreign | | | 42.0 | | | | 23.9 | | | | 21.8 | |
| | | | | | | | | | | | |
Pretax income | | $ | 94.5 | | | $ | 70.6 | | | $ | 96.8 | |
| | | | | | | | | | | | |
Components of Income Tax Expense from Continuing Operations
| | | | | | | | | | | | |
| | | Years Ended December 31, | |
| ($ in millions) | | 2010 | | | 2009 | | | 2008 | |
Current income tax expense (benefit): | | | | | | | | | | | | |
Federal | | $ | (5.8 | ) | | $ | (0.6 | ) | | $ | 11.3 | |
State | | | (0.6 | ) | | | 1.2 | | | | (0.2 | ) |
Foreign | | | 9.8 | | | | 3.5 | | | | 5.6 | |
| | | | | | | | | | | | |
Current income tax expense | | | 3.4 | | | | 4.1 | | | | 16.7 | |
| | | | | | | | | | | | |
Deferred income tax expense: | | | | | | | | | | | | |
Federal | | | 23.3 | | | | 12.6 | | | | 15.9 | |
State | | | 2.1 | | | | 2.4 | | | | 2.0 | |
Foreign | | | 1.2 | | | | 3.2 | | | | 1.7 | |
| | | | | | | | | | | | |
Deferred income tax expense | | | 26.6 | | | | 18.2 | | | | 19.6 | |
| | | | | | | | | | | | |
Income tax expense | | $ | 30.0 | | | $ | 22.3 | | | $ | 36.3 | |
| | | | | | | | | | | | |
The following table accounts for the difference between the actual tax provision and the amounts obtained by applying the statutory U.S. federal income tax rate of 35% to the income before taxes.
Effective Tax Rate Reconciliation
| | | | | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
Income tax provision (benefit) at U.S. federal income tax rate | | | 35.0 | % | | | 35.0 | % | | | 35.0 | % |
Foreign effective tax rate differential | | | (3.1 | ) | | | (1.0 | ) | | | (0.5 | ) |
Additional tax provision on foreign income | | | 2.5 | | | | 2.0 | | | | 2.2 | |
Tax benefit from United Kingdom (U.K.) financing | | | (2.0 | ) | | | (3.3 | ) | | | (2.7 | ) |
State income taxes, net | | | 2.1 | | | | 2.9 | | | | 1.9 | |
Brazil tax holiday | | | (1.5 | ) | | | (2.3 | ) | | | (1.0 | ) |
China tax holiday | | | (2.3 | ) | | | (0.7 | ) | | | — | |
Research and development credit | | | (0.6 | ) | | | (0.7 | ) | | | (0.5 | ) |
Enacted tax rate change | | | 1.4 | | | | — | | | | — | |
Other, net | | | 0.2 | | | | (0.3 | ) | | | 3.1 | |
| | | | | | | | | | | | |
Effective tax rate | | | 31.7 | % | | | 31.6 | % | | | 37.5 | % |
| | | | | | | | | | | | |
81
Components of Deferred Tax Assets and Liabilities
| | | | | | | | |
| | | Years Ended December 31, | |
| ($ in millions) | | 2010 | | | 2009 | |
Deferred tax assets: | | | | | | | | |
Certain accrued expenses and non-current liabilities | | $ | 40.4 | | | $ | 37.1 | |
Net operating losses and other carryforwards | | | 42.1 | | | | 42.9 | |
Pension liability adjustments | | | 92.7 | | | | 98.0 | |
Property, plant and equipment | | | 8.9 | | | | 8.6 | |
Valuation allowance | | | (41.0 | ) | | | (41.4 | ) |
| | | | | | | | |
Total deferred tax assets | | | 143.1 | | | | 145.2 | |
| | | | | | | | |
Deferred tax liabilities: | | | | | | | | |
Goodwill and other intangibles | | | 31.2 | | | | 28.4 | |
Other miscellaneous items | | | 56.3 | | | | 32.1 | |
| | | | | | | | |
Total deferred tax liabilities | | | 87.5 | | | | 60.5 | |
| | | | | | | | |
Net deferred tax assets | | $ | 55.6 | | | $ | 84.7 | |
| | | | | | | | |
| | | | | | | | |
| | | Years Ended December 31, | |
| ($ in millions) | | 2010 | | | 2009 | |
Deferred tax asset—current | | $ | 8.7 | | | $ | 5.0 | |
Deferred tax asset—non-current | | | 48.2 | | | | 80.8 | |
Deferred tax liability—current | | | — | | | | — | |
Deferred tax liability—non-current | | | (1.3 | ) | | | (1.1 | ) |
| | | | | | | | |
Net deferred tax assets | | $ | 55.6 | | | $ | 84.7 | |
| | | | | | | | |
Unrecognized Tax Benefits
The following table reconciles the total amounts of unrecognized tax benefits at the beginning and end of 2010 and 2009:
| | | | | | | | |
| | | Years Ended December 31, | |
| ($ in millions) | | 2010 | | | 2009 | |
Beginning balance, January 1 | | $ | 13.2 | | | $ | 12.6 | |
Additions for tax positions of prior years | | | 0.5 | | | | 1.9 | |
Additions for tax positions of the current year | | | 1.0 | | | | 1.2 | |
Reductions for lapse of statute of limitations | | | (1.5 | ) | | | (1.5 | ) |
Reclassified to Accrued liabilities | | | (1.5 | ) | | | — | |
Reductions for settlements | | | — | | | | (1.0 | ) |
| | | | | | | | |
Ending balance, December 31 | | $ | 11.7 | | | $ | 13.2 | |
| | | | | | | | |
One of the principal reasons for the decrease in unrecognized tax benefits during 2010 was a transfer of the liability to pay income taxes resulting from the sale of the industrial coatings business. Although the income tax liability was transferred, in conjunction with the terms of the sale, the Company has indemnified Sherwin-Williams for any payments made by Sherwin-Williams related to such liability and, therefore, at December 31, 2010, the Company had a $1.5 million liability recorded in Accrued liabilities on its Consolidated Balance Sheet.
The remaining $11.7 million of unrecognized tax benefits will impact the Company’s annual effective tax rate if recognized. The Company expects to recognize $4.8 million of the remaining $11.7 million of unrecognized tax benefits prior to December 31, 2011, upon the expiration of the period to assess tax in various federal, state and foreign taxing jurisdictions.
82
The Company’s policy regarding the classification of interest and penalties recognized is to classify them as income tax expense in its financial statements. During 2010 and 2009, the total amount of interest and penalties recognized as a component of income tax expense was $0.3 million and $0.5 million, respectively.
The Company is subject to U.S. federal income tax, as well as income tax in multiple state and foreign jurisdictions. The Company’s federal income tax returns for 2008 and 2009 are open to possible examination and adjustment. Additionally, the tax years 2006 through 2009 remain open to examination in the U.K., Italy, Brazil and China, which are major taxing jurisdictions where the Company is subject to foreign taxes.
The valuation allowance of $41.0 million relates to state net operating losses and tax credits, a U.S. capital loss, net operating losses and certain tax assets and other carryforwards of foreign entities that management believes are not more likely than not to be realized. The Company’s valuation allowance decreased slightly during 2010 as the impact of foreign exchange and a change in the U.K. tax rate was mostly offset by the recording of a valuation allowance on a deferred tax asset related to a $3.1 million U.S. capital loss sustained from the sale of the industrial coatings business.
A full valuation allowance has not been established because the Company believes it is more likely than not that the results of future operations will generate sufficient taxable income to realize the net deferred tax assets. Taxable income is expected to be sufficient to recover the net benefit within the period in which these remaining differences are expected to reverse, assuming no change to the current tax laws.
The Company has net deferred tax assets of $1.1 million related to state and foreign net operating loss carryforwards with a significant portion of these carryforwards expiring between 2015 and 2027.
During 2010, 2009 and 2008, the Company’s income tax expense was reduced by $1.4 million ($0.06 per diluted share), $1.6 million ($0.06 per diluted share) and $1.0 million ($0.04 per diluted share), respectively, due to a Brazilian regional tax holiday. In 2010, the Company was granted an extension of the holiday from 2014 to 2019. The holiday will reduce the Company’s Brazilian corporate income tax on certain earnings by 75% through 2019. During 2010 and 2009, the Company’s income tax expense was also reduced by $2.2 million ($0.09 per diluted share), and $0.5 million ($0.02 per diluted share), respectively, due to a tax holiday in China. The tax rate in China was 11% in 2010 and will increase as follows: 12% in 2011, 12.5% in 2012 and 25% in 2013 and future years.
The Company’s effective tax rate reflects the tax benefit from our U.K. financing structure. During 2009, tax legislation was enacted in the U.K. that could limit the tax benefit of the Company’s U.K. financing structure, thereby increasing the Company’s future effective tax rate beginning in 2011 and may impact previously recorded deferred tax assets.
During 2010, legislation was finalized in the U.K. which reduced the corporate tax rate from 28% to 27%. The Company has significant U.K. deferred tax assets, principally related to the Company’s U.K. pension plans. As a result of the tax rate change, the Company recorded non-cash expense of $1.3 million to reduce the deferred tax assets related to the pension plans. The original tax benefit was not recorded in the income statement and, instead, was recorded directly through equity.
The Company provides for deferred taxes on temporary differences between the financial statement and tax bases of assets using the enacted tax rates that are expected to apply to taxable income when the temporary differences are expected to reverse. At December 31, 2010, the Company’s share of cumulative undistributed earnings of foreign subsidiaries was approximately $181 million. No provision has been made for U.S. or additional foreign taxes on the undistributed earnings of foreign subsidiaries, except for its Canadian subsidiaries, since the Company intends to continue to reinvest these earnings. Foreign tax credits could be available to reduce or eliminate any amount of additional U.S. tax that might be payable on these foreign earnings in the event of distributions or sale.
83
| 15. | Employee Benefit Plans |
Pension Plans and Retirement Benefits
The Company provides a defined benefit pension plan covering most U.S. employees. The Company also maintains two nonqualified supplemental pension plans. These plans were established to provide additional retirement benefits for certain key employees. The assets of the Arch plan consist primarily of investments in commingled funds administered by independent investment advisors. The Company’s policy, in general, is to fund, at a minimum, amounts as are necessary on an actuarial basis to provide assets sufficient to meet the benefits to be paid to plan members in accordance with the requirements of the Employee Retirement Income Security Act of 1974.
The Company also provides a retiree medical and death benefits plan that covers most domestic employees. The Company is liable for the payment of all retiree medical and death benefits earned by Company employees prior to and following the Distribution who retire after the Distribution. This Arch plan is an unfunded plan.
The following tables provide a reconciliation of the changes in the U.S. plans’ projected benefit obligations, fair value of plan assets and funded status of the Arch retirement plans.
| | | | | | | | | | | | | | | | |
| | | Pension Benefits | | | Other
Postretirement Benefits | |
| ($ in millions) | | 2010 | | | 2009 | | | 2010 | | | 2009 | |
Reconciliation of Projected Benefit Obligation: | | | | | | | | | | | | | | | | |
Projected benefit obligation at beginning of year | | $ | 328.0 | | | $ | 286.0 | | | $ | 14.7 | | | $ | 14.6 | |
Service cost (benefits earned during the period) | | | 8.4 | | | | 8.1 | | | | 0.5 | | | | 0.5 | |
Interest cost on the projected benefit obligation | | | 19.2 | | | | 18.6 | | | | 0.9 | | | | 0.9 | |
Plan amendments | | | — | | | | 2.6 | | | | — | | | | — | |
Actuarial loss (gain) | | | 19.6 | | | | 23.7 | | | | 0.4 | | | | (0.5 | ) |
Benefits paid | | | (11.4 | ) | | | (11.0 | ) | | | (0.8 | ) | | | (0.8 | ) |
| | | | | | | | | | | | | | | | |
Projected benefit obligation at end of year | | $ | 363.8 | | | $ | 328.0 | | | $ | 15.7 | | | $ | 14.7 | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | Pension Benefits | | | Other
Postretirement Benefits | |
| ($ in millions) | | 2010 | | | 2009 | | | 2010 | | | 2009 | |
Reconciliation of Fair Value of Plan Assets: | | | | | | | | | | | | | | | | |
Fair value of plan assets at beginning of year | | $ | 223.5 | | | $ | 150.3 | | | $ | — | | | $ | — | |
Employer contributions | | | 80.6 | | | | 42.8 | | | | 0.8 | | | | 0.8 | |
Benefits paid | | | (11.4 | ) | | | (11.0 | ) | | | (0.8 | ) | | | (0.8 | ) |
Actual return on plan assets (net of expenses) | | | 34.5 | | | | 41.4 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | |
Fair value of plan assets at end of year | | $ | 327.2 | | | $ | 223.5 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | |
Funded Status | | $ | (36.6 | ) | | $ | (104.5 | ) | | $ | (15.7 | ) | | $ | (14.7 | ) |
Items not yet Recognized as a Component of Net Periodic Pension Cost: | | | | | | | | | | | | | | | | |
Net actuarial loss | | $ | 144.3 | | | $ | 145.2 | | | $ | 1.7 | | | $ | 1.4 | |
Prior service cost (credit) | | | 1.9 | | | | 2.1 | | | | (0.4 | ) | | | (0.6 | ) |
| | | | | | | | | | | | | | | | |
Total | | $ | 146.2 | | | $ | 147.3 | | | $ | 1.3 | | | $ | 0.8 | |
| | | | | | | | | | | | | | | | |
Amounts Recognized in the Balance Sheet Consist of: | | | | | | | | | | | | | | | | |
Total accrued benefit cost (Accrued Liabilities) | | $ | (0.6 | ) | | $ | (0.6 | ) | | $ | (1.4 | ) | | $ | (1.3 | ) |
Total non-current benefit costs (Other Liabilities) | | | (36.0 | ) | | | (103.9 | ) | | | (14.2 | ) | | | (13.4 | ) |
In 2009, the Company’s non-qualified pension plan for senior executives was amended to provide more competitive benefits and to encourage retention.
84
The following information is required to be separately disclosed for pension plans with an accumulated benefit obligation in excess of plan assets. The Company’s qualified pension plan does not have an accumulated benefit obligation in excess of plan assets as of December 31, 2010. The Company’s nonqualified pension plan is unfunded.
| | | | | | | | | | | | | | | | |
| | | Qualified Pension Plan | | | Nonqualified Pension Plan | |
| ($ in millions) | | 2010 | | | 2009 | | | 2010 | | | 2009 | |
Accumulated benefit obligation | | $ | 308.1 | | | $ | 271.2 | | | $ | 32.2 | | | $ | 28.4 | |
Projected benefit obligation | | | 328.0 | | | | 297.0 | | | | 35.8 | | | | 31.0 | |
Fair value of plan assets | | | 327.2 | | | | 223.5 | | | | — | | | | — | |
During 2010, the Company made $80.0 million of voluntary contributions to the Company’s qualified U.S. pension plan. As a result, the Company is expected to meet the full funding phase-in threshold of the Pension Protection Act of 2006 for the 2011 plan year. There are no cash contribution requirements expected for the Company’s qualified U.S. pension plan in 2011. The Company also has payments due under the non-qualified pension plan and the postretirement benefit plans. These plans are pay as you go, and therefore not required to be funded in advance. Pension expense in 2011 is expected to be comparable to 2010.
Benefit costs presented below were determined based on actuarial methods and include the following components:
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Pension Benefits | | | Other
Postretirement Benefits | |
| ($ in millions) | | 2010 | | | 2009 | | | 2008 | | | 2010 | | | 2009 | | | 2008 | |
Net Periodic Benefit Expense: | | | | | | | | | | | | | | | | | | | | | | | | |
Service cost including expenses (benefits earned during the period) | | $ | 8.9 | | | $ | 8.5 | | | $ | 7.9 | | | $ | 0.5 | | | $ | 0.5 | | | $ | 0.5 | |
Interest cost on the projected benefit obligation | | | 19.2 | | | | 18.6 | | | | 17.4 | | | | 0.9 | | | | 0.9 | | | | 0.9 | |
Expected return on plan assets | | | (22.4 | ) | | | (19.8 | ) | | | (19.3 | ) | | | — | | | | — | | | | — | |
Amortization of prior service cost (credit) | | | 0.2 | | | | 0.2 | | | | — | | | | (0.2 | ) | | | (0.2 | ) | | | (0.2 | ) |
Curtailment/Settlement | | | — | | | | — | | | | 1.3 | | | | — | | | | — | | | | — | |
Recognized actuarial loss | | | 7.9 | | | | 5.2 | | | | 3.3 | | | | — | | | | — | | | | 0.1 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Net periodic benefit cost | | $ | 13.8 | | | $ | 12.7 | | | $ | 10.6 | | | $ | 1.2 | | | $ | 1.2 | | | $ | 1.3 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Included in Restructuring and other expense in the Company’s 2008 Consolidated Statement of Income is a $1.3 million charge related to a pension settlement associated with severance recorded in 2007 (see Note 22).
The amounts in AOCL that are expected to be recognized as components of net periodic benefit expense during the next fiscal year are as follows:
| | | | | | | | |
| ($ in millions) | | Pension
Benefits | | | Other
Postretirement
Benefits | |
Prior service cost (credit) | | $ | 0.2 | | | $ | (0.2 | ) |
Net actuarial loss | | | 10.3 | | | | — | |
The weighted average assumptions used to determine the benefit obligation for the U.S. pension and the postretirement plans at December 31 were:
| | | | | | | | | | | | | | | | |
| | | Pension
Benefits | | | Other
Postretirement Benefits | |
| | | 2010 | | | 2009 | | | 2010 | | | 2009 | |
Weighted Average Rate Assumptions: | | | | | | | | | | | | | | | | |
Discount rate | | | 5.50 | % | | | 6.00 | % | | | 5.50 | % | | | 6.00 | % |
Rate of compensation increase | | | 3.60 | % | | | 4.60 | % | | | — | | | | — | |
85
The weighted average assumptions used to determine the net periodic benefit cost for the years ending December 31 were:
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Pension Benefits | | | Other
Postretirement Benefits | |
| | | 2010 | | | 2009 | | | 2008 | | | 2010 | | | 2009 | | | 2008 | |
Weighted Average Rate Assumptions: | | | | | | | | | | | | | | | | | | | | | | | | |
Discount rate | | | 6.00 | % | | | 6.50 | % | | | 6.50 | % | | | 6.00 | % | | | 6.50 | % | | | 6.50 | % |
Rate of compensation increase | | | 4.60 | % | | | 4.60 | % | | | 4.60 | % | | | — | | | | — | | | | — | |
Long-term rate of return on assets | | | 8.25 | % | | | 8.50 | % | | | 8.50 | % | | | — | | | | — | | | | — | |
For 2010, the Company’s expected long-term rate of return on assets assumption was 8.25%. This assumption represents the average long-term rate of return expected on the funds invested or to be invested to provide for the benefits included in the benefit obligation. The assumption reflects expectations regarding future long-term rates of return for the investment portfolio, with consideration given to the distribution of investments by asset class and historical rates of return for each individual asset class.
At December 31, 2010, the Company’s target allocation of the pension plan assets is 40% large-cap equity funds, 10% mid-cap equity funds, 10% small-cap equity funds, 10% international equity funds and emerging markets equity funds and 30% fixed income funds. Fixed income funds include investments in government obligations, corporate bonds, agency obligations and asset-backed securities. The principal objective of the Company’s investment strategy is to ensure sufficient resources are available to meet the plan’s benefit obligations. The assets are invested in a diversified portfolio consisting of an array of asset classes that attempt to maximize returns while minimizing volatility. The current asset classes are listed in the table below. In the future, the Company may invest in other asset classes, including real estate.
Other than cash and cash equivalents, all U.S. pension plan assets are valued at fair value based on a combination of quoted prices from active markets and prices from inactive markets (Level 2). The U.S. pension plan has no Level 3 assets. For further details on the fair value hierarchy see Note 23.
The fair values of the Company’s U.S. pension plan assets at December 31, 2010 and 2009 by asset class, are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | December 31, 2010 | | | December 31, 2009 | |
| ($ in millions) | | Level 1 | | | Level 2 | | | Total | | | Level 1 | | | Level 2 | | | Total | |
Asset Class: | | | | | | | | | | | | | | | | | | | | | | | | |
Large-Cap Equity Funds | | $ | — | | | $ | 120.1 | | | $ | 120.1 | | | $ | — | | | $ | 82.9 | | | $ | 82.9 | |
Mid-Cap Equity Funds | | | — | | | | 34.9 | | | | 34.9 | | | | — | | | | 16.0 | | | | 16.0 | |
Small-Cap Equity Funds | | | — | | | | 20.3 | | | | 20.3 | | | | — | | | | 13.6 | | | | 13.6 | |
International Equity Funds | | | — | | | | 38.1 | | | | 38.1 | | | | — | | | | 29.2 | | | | 29.2 | |
Emerging Markets Equity Funds | | | — | | | | 6.7 | | | | 6.7 | | | | — | | | | 4.8 | | | | 4.8 | |
Government Obligations | | | — | | | | 25.5 | | | | 25.5 | | | | — | | | | 12.8 | | | | 12.8 | |
Corporate Bonds | | | — | | | | 22.6 | | | | 22.6 | | | | — | | | | 14.4 | | | | 14.4 | �� |
Agency Obligations | | | — | | | | 3.9 | | | | 3.9 | | | | — | | | | 4.7 | | | | 4.7 | |
Bank Loans | | | — | | | | 1.6 | | | | 1.6 | | | | — | | | | — | | | | — | |
Asset-Backed Securities | | | — | | | | 53.0 | | | | 53.0 | | | | — | | | | 40.8 | | | | 40.8 | |
Cash and Cash Equivalents | | | 0.5 | | | | — | | | | 0.5 | | | | 4.3 | | | | — | | | | 4.3 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Net periodic benefit cost | | $ | 0.5 | | | $ | 326.7 | | | $ | 327.2 | | | $ | 4.3 | | | $ | 219.2 | | | $ | 223.5 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
86
The following table represents the benefits expected to be paid for the Arch retirement plans:
| | | | | | | | |
| ($ in millions) | | Pension
Benefits | | | Other
Postretirement
Benefits | |
2011 | | $ | 13.5 | | | $ | 1.5 | |
2012 | | | 15.8 | | | | 1.5 | |
2013 | | | 36.5 | | | | 1.5 | |
2014 | | | 16.8 | | | | 1.6 | |
2015 | | | 17.8 | | | | 1.6 | |
Years 2016 to 2020 | | | 105.9 | | | | 8.0 | |
The expected benefits to be paid are based on the same assumptions used to measure the Company’s benefit obligation at December 31, 2010 and include estimated future employee service.
The annual measurement date is December 31 for the pension benefits and other postretirement benefits. For measurement purposes, the assumed health care cost trend rate used for pre-65 non-HMO plans and pre-65 HMO plans was 10.00% and 9.00% in 2010 and 2009, respectively, decreasing to an ultimate trend rate of 5.0% in 2020. For non-bargained participants, Arch’s subsidy for pre-65 coverage is limited to $10,000/retiree with all future cost increases to be paid by the retiree. For post-65 retirees, the Company provides a fixed dollar benefit that is not subject to escalation.
The assumed health care cost trend rate assumptions can have an impact on the amounts reported. A one percent increase or decrease each year in the health care cost trend rate utilized would have the following effects at December 31, 2010:
| | | | | | | | |
| | | One Percentage Point | |
| ($ in millions) | | Increase | | | Decrease | |
Effect on the net periodic postretirement benefit costs | | $ | — | | | $ | — | |
Effect on the postretirement benefit obligation | | | 0.2 | | | | (0.2 | ) |
87
As part of the acquisition of Hickson, the Company acquired the liability for the Hickson U.K. and the Hickson U.K. Senior Executive retirement plans. The following tables provide a reconciliation of the changes in the plans’ projected benefit obligations, fair value of plan assets, funded status, certain assumptions and components of net periodic pension expense of the Hickson U.K. and the Hickson U.K. Senior Executive retirement plans for the years ended December 31, 2010 and 2009.
| | | | | | | | |
| | | Pension Benefits | |
| ($ in millions) | | 2010 | | | 2009 | |
Reconciliation of Projected Benefit Obligation: | | | | | | | | |
Projected benefit obligation at beginning of year | | $ | 361.8 | | | $ | 283.6 | |
Service cost (benefits earned during the period) | | | 1.1 | | | | 1.6 | |
Interest cost on the projected benefit obligation | | | 19.6 | | | | 19.0 | |
Participant contributions | | | 0.2 | | | | 0.3 | |
Actuarial loss | | | 9.3 | | | | 46.6 | |
Benefits paid | | | (16.1 | ) | | | (16.6 | ) |
Foreign exchange impact | | | (14.0 | ) | | | 27.3 | |
| | | | | | | | |
Projected benefit obligation at end of year | | $ | 361.9 | | | $ | 361.8 | |
| | | | | | | | |
Reconciliation of Fair Value of Plan Assets: | | | | | | | | |
Fair value of plan assets at beginning of year | | $ | 274.3 | | | $ | 215.7 | |
Employer contributions | | | 7.8 | | | | 15.6 | |
Benefits paid | | | (16.1 | ) | | | (16.6 | ) |
Participant contributions | | | 0.2 | | | | 0.3 | |
Actual return on plan assets (net of expenses) | | | 29.8 | | | | 38.6 | |
Foreign exchange impact | | | (10.7 | ) | | | 20.7 | |
| | | | | | | | |
Fair value of plan assets at end of year | | $ | 285.3 | | | $ | 274.3 | |
| | | | | | | | |
Funded Status | | $ | (76.6 | ) | | $ | (87.5 | ) |
Items not yet Recognized as a Component of Net Periodic Pension Cost: | | | | | | | | |
Net actuarial loss | | $ | 126.5 | | | $ | 138.3 | |
| | | | | | | | |
Amounts Recognized in the Balance Sheet Consist of: | | | | | | | | |
Total non-current benefit costs (Other Liabilities) | | $ | (76.6 | ) | | $ | (87.5 | ) |
| | | | | | | | |
The following information is required to be separately disclosed for pension plans with an accumulated benefit obligation in excess of plan assets. The Company’s Hickson U.K. and the Hickson U.K. Senior Executive plans have an accumulated benefit obligation in excess of plan assets as of December 31, 2010 and 2009.
| | | | | | | | | | | | | | | | |
| | | Hickson U.K. Plan | | | Hickson U.K. Senior
Executive Plan | |
| ($ in millions) | | 2010 | | | 2009 | | | 2010 | | | 2009 | |
Accumulated benefit obligation | | $ | 347.8 | | | $ | 344.6 | | | $ | 11.9 | | | $ | 12.6 | |
Projected benefit obligation | | | 349.9 | | | | 349.2 | | | | 12.0 | | | | 12.6 | |
Fair value of plan assets | | | 274.8 | | | | 263.9 | | | | 10.5 | | | | 10.4 | |
The Company’s current policy is to fund, at a minimum, amounts as are necessary to provide assets sufficient to meet the benefits to be paid to plan members in accordance with statutory requirements. The minimum funding requirements for the Company’s U.K. pension plans are currently expected to be approximately $25 million to $30 million (or approximately £16 million) in 2011 and then return to $15 million to $20 million (or approximately £10 million) per year thereafter. Pension expense in 2011 is expected to be $1—$2 million higher than 2010.
88
Benefit costs presented below were determined based on actuarial methods and include the following components:
| | | | | | | | | | | | |
| ($ in millions) | | 2010 | | | 2009 | | | 2008 | |
Net Periodic Benefit Expense: | | | | | | | | | | | | |
Service cost (benefits earned during the period) | | $ | 1.1 | | | $ | 1.6 | | | $ | 1.4 | |
Interest cost on the projected benefit obligation | | | 19.6 | | | | 19.0 | | | | 22.3 | |
Expected return on plan assets | | | (17.8 | ) | | | (17.2 | ) | | | (19.7 | ) |
Recognized actuarial loss | | | 3.5 | | | | 1.9 | | | | 3.2 | |
| | | | | | | | | | | | |
Net periodic benefit cost | | $ | 6.4 | | | $ | 5.3 | | | $ | 7.2 | |
| | | | | | | | | | | | |
The weighted average assumptions used to determine the benefit obligation for the U.K. pension plans at December 31 were:
| | | | | | | | |
| | | Pension Benefits | |
| | | 2010 | | | 2009 | |
Weighted Average Rate Assumptions: | | | | | | | | |
Discount rate | | | 5.50 | % | | | 5.75 | % |
Rate of compensation increase | | | 4.75 | % | | | 4.75 | % |
The weighted average assumptions used to determine the net periodic benefit cost for the years ending December 31 were:
| | | | | | | | | | | | |
| | | Pension Benefits | |
| | | 2010 | | | 2009 | | | 2008 | |
Weighted Average Rate Assumptions: | | | | | | | | | | | | |
Discount rate | | | 5.75 | % | | | 6.50 | % | | | 6.00 | % |
Rate of compensation increase | | | 4.75 | % | | | 4.00 | % | | | 4.50 | % |
Long-term rate of return on assets | | | 6.25 | % | | | 6.50 | % | | | 6.50 | % |
For 2010 and 2009, the Company’s expected long-term rate of return on assets assumptions were 6.50% and 6.75%, respectively, which were reduced by 0.25% to allow for administration expenses, which have been removed from the service cost. This assumption represents the average long-term rate of return expected on the funds invested or to be invested to provide for the benefits included in the benefit obligation. The assumption reflects expectations regarding future long-term rates of return for the investment portfolio, with consideration given to the distribution of investments by asset class and historical rates of return for each individual asset class.
The amounts in accumulated other comprehensive loss that are expected to be recognized as components of net periodic benefit expense during the next fiscal year are as follows:
| | | | |
| ($ in millions) | | Pension
Benefits | |
Net actuarial loss | | $ | 4.1 | |
The target allocation for the pension plan assets is 37% in equity funds, 58% in fixed income funds and 5% in a real estate fund for the Hickson U.K. plan and 30% in equity funds and 70% in fixed income funds for the Hickson U.K. Senior Executive plan. Fixed income funds include investments in corporate bonds, asset-backed securities, government obligations and agency obligations. The investment strategy for the plans includes investing in a diversified portfolio consisting of an array of asset classes that attempts to maximize returns while minimizing volatility. The current asset classes are listed in the table below. In the future, the Company may invest in other asset classes.
89
The fair values of the Company’s U.K. pension plan assets at December 31, 2010, by asset class, are as follows:
| | | | | | | | | | | | | | | | |
| ($ in millions) | | Quoted Prices in
Active Markets for
Identical Assets
(Level 1) | | | Significant
Observable
Inputs
(Level 2) | | | Significant
Unobservable
Inputs
(Level 3) | | | Total | |
Asset Class | | | | | | | | | | | | | | | | |
Equity Funds | | $ | — | | | $ | 107.1 | | | $ | — | | | $ | 107.1 | |
Government Bond Funds | | | — | | | | 52.6 | | | | — | | | | 52.6 | |
Corporate Bond Funds | | | — | | | | 112.6 | | | | — | | | | 112.6 | |
Real Estate Funds | | | — | | | | 12.8 | | | | — | | | | 12.8 | |
Cash and Cash Equivalents | | | 0.2 | | | | — | | | | — | | | | 0.2 | |
| | | | | | | | | | | | | | | | |
Total | | $ | 0.2 | | | $ | 285.1 | | | $ | — | | | $ | 285.3 | |
| | | | | | | | | | | | | | | | |
The fair values of the Company’s U.K. pension plan assets at December 31, 2009, by asset class, are as follows:
| | | | | | | | | | | | | | | | |
| ($ in millions) | | Quoted Prices in
Active Markets for
Identical Assets
(Level 1) | | | Significant
Observable
Inputs
(Level 2) | | | Significant
Unobservable
Inputs
(Level 3) | | | Total | |
Asset Class | | | | | | | | | | | | | | | | |
Equity Funds | | $ | — | | | $ | 113.3 | | | $ | — | | | $ | 113.3 | |
Government Obligations | | | 36.5 | | | | 41.1 | | | | — | | | | 77.6 | |
Corporate Bonds | | | 34.9 | | | | 35.6 | | | | — | | | | 70.5 | |
Agency Obligations | | | 3.7 | | | | — | | | | — | | | | 3.7 | |
Asset-Backed Securities | | | 8.9 | | | | — | | | | — | | | | 8.9 | |
Cash and Cash Equivalents | | | 0.3 | | | | — | | | | — | | | | 0.3 | |
| | | | | | | | | | | | | | | | |
Total | | $ | 84.3 | | | $ | 190.0 | | | $ | — | | | $ | 274.3 | |
| | | | | | | | | | | | | | | | |
The following table represents the benefits expected to be paid for the Hickson U.K. retirement plans:
| | | | |
| (£ in millions) | | Pension Benefits | |
2011 | | £ | 9.5 | |
2012 | | | 9.5 | |
2013 | | | 10.0 | |
2014 | | | 10.3 | |
2015 | | | 10.4 | |
Years 2016 to 2020 | | | 59.5 | |
The expected benefits to be paid are based on the same assumptions used to measure the Company’s benefit obligation at December 31, 2010 and include the impact of estimated future employee service.
As part of the acquisition of Avecia’s pool & spa and protection & hygiene businesses, the Company acquired certain liabilities for prior service associated with its U.K. defined benefit pension plan. Subsequent to the acquisition, a defined contribution plan was established for the transferred employees and no further future service benefit will be accrued in the defined benefit plan. As of December 31, 2010 and 2009, respectively, the projected benefit obligation of the plan was £17.1 million ($26.3 million) and £15.5 million ($24.9 million), the accumulated benefit obligation was £13.5 million ($20.8 million) and £12.3 million ($19.8 million), net assets of £15.4 million ($23.7 million) and £13.0 million ($20.7 million) and the accrued benefit was £1.7 million ($2.6 million) and £2.6 million ($4.1 million). The net assets primarily consist of equities and bonds and such assets
90
are valued using quoted prices. The assumptions for the valuation are consistent with that of the Company’s other U.K. plans. During 2010, 2009 and 2008 the Company incurred £0.1 million ($0.2 million), £0.1 million ($0.1 million) and £0.2 million ($0.4 million), respectively, of net periodic benefit cost related to this plan.
The Company’s other foreign subsidiaries maintain pension and other benefit plans that are consistent with statutory practices and are not significant to the consolidated financial statements.
| 16. | Share-Based Compensation |
Stock Option Plans
The Company accounts for its stock option plans in accordance with FASB ASC 718, which requires all share-based payments to employees, including grants of employee stock options, to be recognized in the consolidated financial statements based on their fair values. The five stock-based compensation plans are described below:
| | • | | The 1988 and 1996 Olin Stock Option Plans. At the time of the distribution (“Distribution”) of the Company from Olin Corporation (“Olin”), outstanding Olin options were converted into both an option to purchase Company common stock (“Company Options”) and an option to purchase Olin common stock (“New Olin Options”) with the same aggregate “intrinsic value” at the time of the Distribution as the old award. Options granted to such employees under the Olin 1988 Stock Option Plan or the Olin 1996 Stock Option Plan retained the original term of the option. No additional Company Options will be granted under the 1988 and 1996 Olin Stock Option Plans and there were no Company Options or New Olin Options outstanding at December 31, 2010. |
| | • | | 1999 Long Term Incentive Plan seeks to encourage selected salaried employees to acquire a proprietary interest in the Company’s growth and performance and to attract and retain qualified individuals. The plan provides for the ability to issue stock options, restricted stock and restricted stock units, and performance awards. The Plan requires that options be granted at an exercise price representing the fair market value of the common stock on the grant date. In general, the employee options vest and become exercisable within one to three years and all options are exercisable up to ten years from the date of grant. During 2009, the plan expired and no additional awards may be granted under this plan. |
| | • | | 1999 Stock Plan for Nonemployee Directors (the “Directors Plan”) is a directors compensation plan under which stock options and other stock awards may be granted to nonemployee directors. The Directors Plan requires that options be granted at an exercise price representing the fair market value of the common stock on the grant date. In general, the directors’ options are exercisable upon grant and all options are exercisable up to ten years from the date of grant. |
| | • | | 2009 Long Term Incentive Plan seeks to encourage selected salaried employees to acquire a proprietary interest in the Company’s growth and performance, to generate an increased incentive to contribute to the Company’s future success and to enhance the ability of the Company to attract and retain qualified individuals. The plan provides for the ability to issue stock options, stock appreciation rights, restricted share awards, restricted stock units, performance compensation awards, performance units, cash incentive awards and other equity-based or equity-related awards. |
When originally adopted, total shares authorized for grant under the Directors Plan and the 2009 Long Term Incentive Plan were 1,650,000.
91
The following table summarizes stock option activity during 2010, 2009 and 2008 (number of options in thousands):
| | | | | | | | | | | | |
| | | Stock
Options | | | Weighted
Average
Price | | | Range of
Exercise Prices | |
Balance, December 31, 2007 | | | 487 | | | $ | 20.91 | | | $ | 17.38 – 31.92 | |
Options exercised | | | 126 | | | | 21.36 | | | | 17.38 – 31.92 | |
Options cancelled, expired or forfeited | | | 38 | | | | 31.56 | | | | 18.22 – 31.92 | |
| | | | | | | | | | | | |
Balance, December 31, 2008 | | | 323 | | | | 19.46 | | | | 17.38 – 23.00 | |
| | | | | | | | | | | | |
Options exercised | | | 154 | | | | 19.52 | | | | 17.38 – 22.72 | |
Options cancelled, expired or forfeited | | | 1 | | | | 19.41 | | | | 19.41 – 19.41 | |
| | | | | | | | | | | | |
Balance, December 31, 2009 | | | 168 | | | | 19.40 | | | | 17.38 – 23.00 | |
| | | | | | | | | | | | |
Options exercised | | | 110 | | | | 19.28 | | | | 17.38 – 23.00 | |
Options cancelled, expired or forfeited | | | 2 | | | | 20.85 | | | | 20.85 – 20.85 | |
| | | | | | | | | | | | |
Balance, December 31, 2010 | | | 56 | | | $ | 19.57 | | | $ | 18.22 – 23.00 | |
| | | | | | | | | | | | |
At December 31, 2010 and 2009, options covering 55,502 and 167,661 shares, respectively, were exercisable at weighted average exercise prices of $19.57 and $19.40, respectively. The average remaining contractual life was approximately two years.
The total intrinsic value of stock options exercised during the twelve months ended December 31, 2010, 2009 and 2008 was $1.4 million, $0.8 million and $1.5 million, respectively.
Performance Awards
Under its 1999 Long Term Incentive Plan and the 2009 Long Term Incentive Plan, the Company has granted selected executives and other key employees two types of awards: performance units that vest only upon meeting a performance measure and performance accelerated restricted stock units that vest upon meeting a performance measure, or if that measure is not achieved, upon the employee’s remaining in the employ of the Company for a specific period. This component of compensation is designed to encourage the long-term retention of key executives and to tie a major part of executive compensation directly to Company performance and the long-term enhancement of shareholder value. The awards were also designed to recognize and reward achieving targeted return on equity (“ROE”). The awards, which are settled partly in cash and partly in shares of the Company’s stock, are earned at the end of a three-year period provided the ROE target is achieved for that third year. For awards granted prior to 2010, there is an opportunity for accelerated payout of the performance units and performance accelerated restricted stock units if the ROE target is met or exceeded by the end of the second year after the grant. Performance and restricted stock units granted in 2010 do not contain this accelerated payout feature. Performance units that do not meet the performance goal at the end of the third year expire without payment. For the performance accelerated restricted stock units, if the ROE target is not achieved by the end of the third year after grant, the units will vest and will be paid out as soon as administratively feasible following the end of the sixth year after grant, or in the case of those units granted in 2010, following the end of the fifth year after grant, if, in each case, the executive is still employed at the Company at the end of such year.
The Company accounts for the portion of the award to be settled in shares as an equity-based award, which requires share-based compensation cost to be measured at the grant date, or approval date if awards are amended, based on the fair value of the award. The fair value of the awards is determined and fixed based on the quoted market value of the Company’s stock. The Company uses the straight-line method to recognize the share-based compensation costs related to the awards over the remaining service period. As of December 31, 2010, there were 902,000 performance awards granted; of these awards approximately 473,000 will be paid out in shares of Company stock, if earned. The grant date fair value for the awards to be paid out in shares was $14.1 million.
92
For the performance awards that are settled in cash, the amount of the payments is based on the market price of the Company’s stock at the time of settlement. During the service period, compensation cost is recognized proportionately based on the Company’s estimate of achieving the financial targets. The performance awards are remeasured to reflect the market price of the Company’s stock at each financial statement date until the award is settled.
The Company has entered into equity total return swap agreements with 400,000 notional shares in order to minimize earnings volatility related to these awards and the Company’s deferred compensation awards (see below for further detail). The Company has not designated the swaps as hedges. Rather, the Company marks the swaps to market and records the impact in Selling and administration expenses in the Company’s Consolidated Statement of Income. The adjustments to the values of the swaps offset the adjustments to the carrying values of the Company’s long-term incentive plan liabilities, which are also recorded in Selling and administration expenses, and there is no significant impact on the Company’s Consolidated Statement of Income. See Note 13 for further detail.
Not including the impact of the equity total return swap agreements, total compensation expense of $13.8 million, $6.6 million and $4.7 million was recognized for the years ended December 31, 2010, 2009 and 2008, respectively. See Note 13 for a discussion of the impact of the equity total return swap agreements on the Company’s Consolidated Statements of Income. At December 31, 2010, there was $7.9 million of total unrecognized compensation cost related to the unearned payment arrangements, which is expected to be recognized over a weighted-average period of two years based on current financial forecasts.
The following table summarizes the performance award activity for the year ended December 31, 2010 (number of awards in thousands):
| | | | |
| | | Performance
Awards | |
Balance, December 31, 2009 | | | 696 | |
Awarded | | | 332 | |
Paid out | | | 112 | |
Cancelled or forfeited | | | 14 | |
| | | | |
Balance, December 31, 2010 | | | 902 | |
| | | | |
At December 31, 2010 the closing stock price was $37.93. Of the 902,000 performance awards outstanding at December 31, 2010, 519,000 vested and will be paid out in the first quarter of 2011, as the Company met the performance targets in 2010.
Deferred Compensation Plans
The Board of Directors of the Company had previously adopted three deferred compensation plans, namely, the Directors Plan, the Supplemental Contributing Employee Ownership Plan and the Employee Deferral Plan. The non-employee Directors participate only in the Directors Plan, while officers and certain other key employees are eligible to participate in the other two plans. These plans permit or require their participants to defer a portion of their compensation. The participants’ compensation deferrals are adjusted for changes in value of phantom shares of common stock of the Company and in other phantom investment vehicles. The Company established a rabbi trust for each of these plans (collectively, the “Rabbi Trust”).
The Company has entered into equity total return swap agreements with 400,000 notional shares in order to minimize earnings volatility related to these awards and the Company’s performance awards. The Company has not designated the swaps as hedges. Rather, the Company marks the swaps to market and records the impact in Selling and administration expenses in the Company’s Consolidated Statement of Income. The adjustments to the values of the swaps offset the adjustments to the carrying values of the Company’s deferred compensation liabilities, which are also recorded in Selling and administration expenses, and there is no impact on the Company’s Consolidated Statement of Income. See Note 13 for further detail.
93
The assets in the Rabbi Trust are invested in shares of Arch common stock, marketable securities and a cash surrender life insurance policy, which generally are expected to generate returns consistent with those credited to the participants. The assets of the Rabbi Trust are available to satisfy the claims of the Company’s creditors in the event of bankruptcy or insolvency of the Company. The Company’s stock held in the Rabbi Trust is treated in a manner similar to treasury stock, with no subsequent changes in fair value and recorded as a reduction of shareholders’ equity ($1.9 million at both December 31, 2010 and 2009), with an offsetting amount reflected as a deferred compensation liability of the Company. The carrying value of the deferred compensation liability related to the Company’s stock is adjusted to fair market value each reporting period by a charge or credit to operations in Selling and administration on the Company’s Consolidated Statements of Income. The other assets of the Rabbi Trust are reported at fair market value in Other assets in the Consolidated Balance Sheets ($9.1 million and $8.8 million at December 31, 2010 and 2009, respectively). The deferred compensation liability reflects the fair market value of the plan participants’ compensation deferrals. At December 31, 2010, the Company had a $0.6 million deferred compensation liability recorded in Accrued liabilities and a $14.4 million deferred compensation liability recorded in Other liabilities in the Consolidated Balance Sheet. At December 31, 2009, the Company had a $12.4 million deferred compensation liability recorded in Other liabilities in the Consolidated Balance Sheet. Changes in the market value of the marketable securities and the deferred compensation liability are adjusted to fair market value each reporting period by a charge or credit to operations in Selling and administration on the Company’s Consolidated Statements of Income.
Contributing Employee Ownership Plan
The Company has established the Arch Chemicals, Inc. Contributing Employee Ownership Plan (“CEOP”), which is a defined contribution plan available to all U.S. employees. The matching contribution allocable to Company employees under the CEOP has been included in costs and expenses in the accompanying Consolidated Statements of Income and was $5.3 million, $3.9 million and $3.3 million in 2010, 2009 and 2008, respectively.
Common Stock
On February 8, 1999, Olin, the sole shareholder of the Company, distributed (on a 1-for-2 basis) all the issued and outstanding shares of common stock, par value $1 per share, of the Company, to the shareholders of record of Olin’s common stock as of February 1, 1999, upon which the Company became a separate, independent company. The total number of shares distributed was approximately 22,980,000.
At December 31, 2010, the Company has reserved 1,799,653 shares of its authorized but unissued common stock for possible future issuance in connection with the exercise of stock options, the issuance of restricted stock, the vesting of performance share units and the issuance of shares in conjunction with the Company’s deferred compensation plans.
In 2004, the Company established a Rabbi Trust for several deferred compensation plans (see Note 16 for more information), that permit or require their participants to defer a portion of their compensation. The Company’s stock held in the Rabbi Trust is treated in a manner similar to treasury stock, with no subsequent changes in fair value and recorded as a reduction of shareholders’ equity.
Series A Participating Cumulative Preferred Stock
The Company has 40,000 authorized shares of $1 par value Series A Participating Cumulative Preferred Stock, of which none is outstanding.
Retained Earnings
Retained earnings as of December 31, 2010 and 2009 include earnings (losses) since the Distribution.
94
Accumulated Other Comprehensive Loss
Accumulated other comprehensive loss includes cumulative foreign currency translation adjustments, pension liability adjustments, net of tax and accumulated net unrealized gain (loss) on derivative instruments, net of tax.
| | | | | | | | | | | | | | | | |
| ($ in millions) | | Foreign
Currency
Translation
Adjustments | | | Pension
Liability
Adjustments | | | Change in Fair
Market Value of
Derivative
Contracts | | | Accumulated
Other
Comprehensive
Loss | |
Balance at December 31, 2007 | | $ | 65.6 | | | $ | (114.5 | ) | | $ | — | | | $ | (48.9 | ) |
2008 activity | | | (59.8 | ) | | | (73.6 | ) | | | (1.9 | ) | | | (135.3 | ) |
| | | | | | | | | | | | | | | | |
Balance at December 31, 2008 | | | 5.8 | | | | (188.1 | ) | | | (1.9 | ) | | | (184.2 | ) |
2009 activity | | | 30.9 | | | | (20.6 | ) | | | 1.2 | | | | 11.5 | |
| | | | | | | | | | | | | | | | |
Balance at December 31, 2009 | | | 36.7 | | | | (208.7 | ) | | | (0.7 | ) | | | (172.7 | ) |
2010 activity | | | (26.0 | ) | | | 5.6 | | | | (0.1 | ) | | | (20.5 | ) |
| | | | | | | | | | | | | | | | |
Balance at December 31, 2010 | | $ | 10.7 | | | $ | (203.1 | ) | | $ | (0.8 | ) | | $ | (193.2 | ) |
| | | | | | | | | | | | | | | | |
Foreign currency translation activity during 2010 includes $27.7 million of realized gains related to the sale of the industrial coatings business (see Note 5).
As a result of the sale of the industrial coatings business, the Company has adjusted its prior year financial statements to include the results of the industrial coatings business and the gain on the disposition as a component of discontinued operations. In addition, as a result of the sale, the Company has adjusted the prior period segment operating results to reallocate certain centralized service costs that were previously allocated to the industrial coatings business to the Company’s other businesses.
The Company has organized its business portfolio into two operating segments to reflect the Company’s business strategy. The two segments are Biocides Products (formerly named Treatment Products) and Performance Products. The Biocides Products segment includes three reportable business units: the HTH water products business, the personal care and industrial biocides business and the wood protection business.
95
Segment results for the three years ended December 31 were as follows:
| | | | | | | | | | | | |
| ($ in millions) | | 2010 | | | 2009 | | | 2008 | |
Sales: | | | | | | | | | | | | |
Biocides Products: | | | | | | | | | | | | |
HTH Water Products | | $ | 606.2 | | �� | $ | 551.1 | | | $ | 501.6 | |
Personal Care and Industrial Biocides | | | 330.6 | | | | 295.0 | | | | 315.6 | |
Wood Protection | | | 254.6 | | | | 231.8 | | | | 267.3 | |
| | | | | | | | | | | | |
Total Biocides Products | | | 1,191.4 | | | | 1,077.9 | | | | 1,084.5 | |
Performance Products: | | | | | | | | | | | | |
Performance Urethanes | | | 169.1 | | | | 150.2 | | | | 197.0 | |
Hydrazine | | | 16.9 | | | | 16.7 | | | | 18.7 | |
| | | | | | | | | | | | |
Total Performance Products | | | 186.0 | | | | 166.9 | | | | 215.7 | |
| | | | | | | | | | | | |
Total Sales | | $ | 1,377.4 | | | $ | 1,244.8 | | | $ | 1,300.2 | |
| | | | | | | | | | | | |
Segment Operating Income (Loss), including Equity in Earnings of Affiliated Companies: | | | | | | | | | | | | |
Biocides Products: | | | | | | | | | | | | |
HTH Water Products | | $ | 66.3 | | | $ | 62.8 | | | $ | 63.9 | |
Personal Care and Industrial Biocides | | | 71.0 | | | | 42.0 | | | | 60.7 | |
Wood Protection | | | 8.3 | | | | (1.5 | ) | | | 1.5 | |
| | | | | | | | | | | | |
Total Biocides Products | | | 145.6 | | | | 103.3 | | | | 126.1 | |
Performance Products: | | | | | | | | | | | | |
Performance Urethanes | | | (0.1 | ) | | | 8.3 | | | | 15.6 | |
Hydrazine | | | 5.3 | | | | 3.3 | | | | 1.1 | |
| | | | | | | | | | | | |
Total Performance Products | | | 5.2 | | | | 11.6 | | | | 16.7 | |
Corporate Unallocated | | | (42.2 | ) | | | (32.2 | ) | | | (33.3 | ) |
| | | | | | | | | | | | |
Total Segment Operating Income, including Equity in Earnings of | | | | | | | | | | | | |
Affiliated Companies | | | 108.6 | | | | 82.7 | | | | 109.5 | |
Restructuring Expense | | | (0.7 | ) | | | — | | | | (1.3 | ) |
Impairment Expense | | | (1.2 | ) | | | — | | | | (0.8 | ) |
Equity in Earnings of Affiliated Companies | | | (0.6 | ) | | | (0.6 | ) | | | (0.4 | ) |
| | | | | | | | | | | | |
Total Operating Income | | | 106.1 | | | | 82.1 | | | | 107.0 | |
Interest Expense, net | | | (12.2 | ) | | | (12.1 | ) | | | (10.6 | ) |
| | | | | | | | | | | | |
Total Income from Continuing Operations before Taxes and Equity in Earnings of Affiliated Companies | | $ | 93.9 | | | $ | 70.0 | | | $ | 96.4 | |
| | | | | | | | | | | | |
Equity in Earnings of Affiliated Companies: | | | | | | | | | | | | |
Biocides Products: | | | | | | | | | | | | |
Wood Protection | | $ | 0.6 | | | $ | 0.6 | | | $ | 0.4 | |
| | | | | | | | | | | | |
Total Equity in Earnings of Affiliated Companies | | $ | 0.6 | | | $ | 0.6 | | | $ | 0.4 | |
| | | | | | | | | | | | |
96
| | | | | | | | | | | | |
| ($ in millions) | | 2010 | | | 2009 | | | 2008 | |
Depreciation Expense: | | | | | | | | | | | | |
Biocides Products: | | | | | | | | | | | | |
HTH Water Products | | $ | 10.8 | | | $ | 11.0 | | | $ | 10.2 | |
Personal Care and Industrial Biocides | | | 10.4 | | | | 10.8 | | | | 9.5 | |
Wood Protection | | | 4.0 | | | | 4.1 | | | | 4.5 | |
| | | | | | | | | | | | |
Total Biocides Products | | | 25.2 | | | | 25.9 | | | | 24.2 | |
Performance Products: | | | | | | | | | | | | |
Performance Urethanes | | | 3.2 | | | | 4.0 | | | | 4.2 | |
Hydrazine | | | 1.0 | | | | 1.0 | | | | 1.8 | |
| | | | | | | | | | | | |
Total Performance Products | | | 4.2 | | | | 5.0 | | | | 6.0 | |
| | | | | | | | | | | | |
Total Depreciation Expense | | $ | 29.4 | | | $ | 30.9 | | | $ | 30.2 | |
| | | | | | | | | | | | |
Amortization Expense: | | | | | | | | | | | | |
Biocides Products: | | | | | | | | | | | | |
HTH Water Products | | $ | 5.3 | | | $ | 5.2 | | | $ | 2.4 | |
Personal Care and Industrial Biocides | | | 4.0 | | | | 4.1 | | | | 5.8 | |
Wood Protection | | | 1.4 | | | | 1.3 | | | | 1.3 | |
| | | | | | | | | | | | |
Total Biocides Products | | | 10.7 | | | | 10.6 | | | | 9.5 | |
Performance Products | | | 0.2 | | | | 0.2 | | | | 0.2 | |
| | | | | | | | | | | | |
Total Amortization Expense | | $ | 10.9 | | | $ | 10.8 | | | $ | 9.7 | |
| | | | | | | | | | | | |
Capital Spending: | | | | | | | | | | | | |
Biocides Products: | | | | | | | | | | | | |
HTH Water Products | | $ | 13.6 | | | $ | 13.3 | | | $ | 13.8 | |
Personal Care and Industrial Biocides | | | 10.9 | | | | 8.1 | | | | 29.0 | |
Wood Protection | | | 2.0 | | | | 3.5 | | | | 4.4 | |
| | | | | | | | | | | | |
Total Biocides Products | | | 26.5 | | | | 24.9 | | | | 47.2 | |
Performance Products: | | | | | | | | | | | | |
Performance Urethanes | | | 2.1 | | | | 2.0 | | | | 2.5 | |
Hydrazine | | | 0.8 | | | | 0.5 | | | | 0.2 | |
| | | | | | | | | | | | |
Performance Products | | | 2.9 | | | | 2.5 | | | | 2.7 | |
| | | | | | | | | | | | |
Total Capital Spending | | $ | 29.4 | | | $ | 27.4 | | | $ | 49.9 | |
| | | | | | | | | | | | |
Total Assets: | | | | | | | | | | | | |
Biocides Products: | | | | | | | | | | | | |
HTH Water Products | | $ | 419.1 | | | $ | 397.9 | | | $ | 402.2 | |
Personal Care and Industrial Biocides | | | 299.2 | | | | 290.4 | | | | 307.0 | |
Wood Protection | | | 185.5 | | | | 199.9 | | | | 187.1 | |
| | | | | | | | | | | | |
Total Biocides Products | | | 903.8 | | | | 888.2 | | | | 896.3 | |
Performance Products: | | | | | | | | | | | | |
Performance Urethanes | | | 52.0 | | | | 50.6 | | | | 74.0 | |
Hydrazine | | | 6.8 | | | | 7.7 | | | | 8.3 | |
| | | | | | | | | | | | |
Total Performance Products | | | 58.8 | | | | 58.3 | | | | 82.3 | |
Assets Held for Sale | | | 1.3 | | | | 127.7 | | | | 139.5 | |
Other | | | 274.1 | | | | 136.3 | | | | 114.3 | |
| | | | | | | | | | | | |
Total Assets | | $ | 1,238.0 | | | $ | 1,210.5 | | | $ | 1,232.4 | |
| | | | | | | | | | | | |
Investment & Advances—Affiliated Companies at Equity: | | | | | | | | | | | | |
Biocides Products: | | | | | | | | | | | | |
Wood Protection | | $ | 1.7 | | | $ | 2.0 | | | $ | 1.5 | |
| | | | | | | | | | | | |
Total Investment & Advances—Affiliated Companies at Equity | | $ | 1.7 | | | $ | 2.0 | | | $ | 1.5 | |
| | | | | | | | | | | | |
97
Segment operating income includes the equity in earnings of affiliated companies and excludes restructuring expense and impairment expense, if any. The Company includes the equity income (loss) of affiliates in its segment operating results as it believes it to be relevant and useful information for investors as these affiliates are the means by which certain segments participate in certain geographic regions. Furthermore, the Company includes equity income (loss) as a component of segment operating results because the Company includes it to measure the performance of the segment. Other (gains) and losses that are directly related to the segments are included in segment operating results. The Company believes the exclusion of restructuring and impairment expenses from segment operating income provides additional perspective on the Company’s underlying business trends and provides useful information to investors by excluding amounts from the Company’s results that the Company believes are not indicative of ongoing operating results. Included in the Hydrazine and Performance urethanes operating results for 2010 are benefits of $2.1 million and $1.0 million, respectively, related to a settlement with a supplier. Included in the HTH water products operating income for 2010 is a $1.8 million charge related to a duty claim in France (see Note 20 for more information). Included in the HTH water products operating income for 2009 and 2008 are benefits of $1.0 million and $11.5 million, respectively, related to the favorable antidumping duty rulings for the review periods of June 1, 2007 through May 31, 2008 and June 1, 2006 through May 31, 2007 (see Note 20 for more information).
Segment assets include only those assets that are directly identifiable to a segment and do not include such items as cash, certain deferred taxes, LIFO reserves, assets held for sale, and certain other assets. Sales by reportable business unit substantially represent sales for the major product lines of the Company.
Geographic area information for the periods ended December 31, were as follows:
| | | | | | | | | | | | |
| ($ in millions) | | 2010 | | | 2009 | | | 2008 | |
Sales | | | | | | | | | | | | |
United States | | $ | 733.7 | | | $ | 675.0 | | | $ | 697.9 | |
| | | |
Europe, Africa and the Middle East | | | 311.1 | | | | 275.1 | | | | 294.6 | |
Latin America and Canada | | | 175.9 | | | | 157.0 | | | | 161.2 | |
Pacific Rim | | | 156.7 | | | | 137.7 | | | | 146.5 | |
| | | | | | | | | | | | |
Total Foreign Sales | | | 643.7 | | | | 569.8 | | | | 602.3 | |
| | | | | | | | | | | | |
Total Sales | | $ | 1,377.4 | | | $ | 1,244.8 | | | $ | 1,300.2 | |
| | | | | | | | | | | | |
Long-lived Assets (excludes Goodwill) | | | | | | | | | | | | |
United States | | $ | 223.0 | | | $ | 245.0 | | | $ | 259.3 | |
| | | |
England | | | 97.9 | | | | 109.7 | | | | 100.5 | |
Europe (remaining), Africa and the Middle East | | | 15.9 | | | | 15.2 | | | | 13.6 | |
Latin America and Canada | | | 26.8 | | | | 25.2 | | | | 16.8 | |
Pacific Rim | | | 49.5 | | | | 49.2 | | | | 49.2 | |
| | | | | | | | | | | | |
Total Foreign Long-lived Assets | | | 190.1 | | | | 199.3 | | | | 180.1 | |
| | | | | | | | | | | | |
Total Long-lived Assets | | $ | 413.1 | | | $ | 444.3 | | | $ | 439.4 | |
| | | | | | | | | | | | |
Sales to external customers are attributed to geographic areas based on country of destination. Transfers between geographic areas are priced generally at prevailing market prices. Export sales from the United States to unaffiliated customers were $105.4 million, $93.1 million and $110.7 million in 2010, 2009 and 2008, respectively.
Advantis
On October 10, 2008 the Company completed the acquisition of the water treatment chemicals business of Advantis, a North American manufacturer and marketer of branded swimming pool, spa and surface water treatment chemicals. The acquisition expands and improves the Company’s participation in the specialty pool
98
and spa dealer and distribution channels as well as adding products and technologies that complement its existing product portfolio and supports its strategy of growing the non-residential water business. The purchase price was $125.0 million, free of debt and inclusive of expenses paid, and a final post-closing working capital adjustment of $0.3 million, which was received by the Company in the first quarter of 2009. The acquisition was financed by borrowings from the Company’s existing credit facility.
During 2008 the Company performed a purchase price allocation related to the acquisition, which resulted in the recording of $42.6 million of tax-deductible goodwill and $62.2 million of identifiable intangible assets. Of the $62.2 million of acquired intangible assets, $29.9 million was assigned to customer lists (10-year life) and $22.5 million was assigned to trademarks, which are not subject to amortization as they have indefinite lives. The remaining $9.8 million of acquired intangible assets include a non-compete agreement of $3.7 million (7-year life), a license arrangement of $3.4 million (9-year life) and developed technology of $2.7 million (9-year life). Excluding the trademarks, which are not subject to amortization, the intangible assets have a weighted-average useful life of approximately 10 years.
The supplemental cash flow information of the business acquired is as follows:
| | | | |
| ($ in millions) | | | |
Working Capital | | $ | 16.4 | |
Property, plant and equipment, net | | | 3.8 | |
Intangible assets | | | 62.2 | |
Goodwill. | | | 42.6 | |
| | | | |
Cash paid | | $ | 125.0 | |
| | | | |
Supplemental Pro Forma Information
The table below presents unaudited pro forma financial information in connection with the Advantis acquisition as if it had occurred on January 1, 2008. The unaudited pro forma information, which excludes the results of the industrial coatings business, is presented for illustrative purposes only and is not necessarily indicative of the operating results or financial position that would have occurred if the acquisition had been completed at the dates indicated. The unaudited pro forma information reflects pro forma adjustments which are based upon currently available information and certain estimates and assumptions, and therefore the actual results may differ from the pro forma results. However, management believes that the assumptions provide a reasonable basis for presenting the significant effects of the transaction, and that the pro forma adjustments give appropriate effect to those assumptions and are properly applied in the pro forma financial information. The information does not necessarily indicate the future operating results or financial position of the Company. The Advantis business is seasonal in nature as its products are primarily used in the U.S. residential pool, spa and surface water treatment markets.
The impact of any revenue and cost synergies that may result from the acquisition are not included in the pro forma results.
| | | | |
| ($ in millions, except per share amounts) | | Year Ended
December 31,
2008 | |
Sales | | $ | 1,355.1 | |
Income from continuing operations | | $ | 59.1 | |
Net Income | | $ | 35.6 | |
Basic income per common share | | | | |
Continuing operations | | $ | 2.38 | |
Net Income | | $ | 1.43 | |
Diluted income per common share | | | | |
Continuing operations | | $ | 2.37 | |
Net Income | | $ | 1.43 | |
99
| 20. | Commitments and Contingencies |
Leases
The Company leases certain properties, such as manufacturing, warehousing and office space and data processing and office equipment. Leases covering these properties may contain escalation clauses based on increased costs of the lessor, primarily property taxes, maintenance and insurance and have renewal or purchase options. Total rent expense charged to operations amounted to $15.4 million in 2010, $16.9 million in 2009 and $15.4 million in 2008 (sublease income and contingent rent expense is not significant).
Future minimum rent payments under operating leases having initial or remaining noncancelable lease terms in excess of one year at December 31, 2010 are as follows: $10.6 million in 2011; $7.6 million in 2012; $7.2 million in 2013; $7.0 million in 2014; $5.5 million in 2015 and $8.5 million thereafter.
Litigation
There are a variety of non-environmental legal proceedings pending or threatened against the Company.
In May 2005, the Department of Commerce (“DOC”) assessed antidumping duties ranging from approximately 76% to 286% against Chinese producers of chlorinated isocyanurates (“isos”). The Company’s primary Chinese supplier of isos was subject to the 76% rate. As a result, upon importing isos from this supplier, the Company made cash deposits at the rate of 76% of the value of the imported product. At the request of the U.S. isos producers and the Company’s supplier, the DOC conducted a review of the duty rate for the period of December 16, 2004 to May 31, 2006. Upon conclusion of its review, the DOC determined that the rate should be reduced to approximately 20%. As a result of this final determination and the revised rate, the Company recorded a net pre-tax benefit of $12.1 million in the fourth quarter of 2007. The U.S. isos producers appealed the DOC’s determination to the Court of International Trade, which has delayed the processing of the full refund the Company was expecting to receive. On July 13, 2009, the Court of International Trade issued its decision which required the DOC to review additional information and revise the rate accordingly. The DOC complied and has issued a revised preliminary rate of approximately 9%, which is pending review by the Court of International Trade. Provided that this revised preliminary rate is approved by the Court of International Trade, the Company would recognize additional income of approximately $3 million. Therefore, the total net cash proceeds the Company expects to receive is approximately $15 million.
At the request of the Company’s supplier, the DOC also initiated an administrative review to determine the final rate for the period of June 1, 2006 through May 31, 2007, during which time the 76% rate also applied. The DOC has determined that the final rate for the Company’s supplier for this period should be reduced from 76% to less than 1%. As a result, the Company recorded a net pre-tax benefit of $12.7 million in the third quarter of 2008 (which included $1.2 million of interest income). The U.S. isos producers appealed the DOC’s determination to the Court of International Trade. The appeal is delaying the cash refund (approximately $14 million) of the duty to the Company and may result in a change of the duty rate for this review period. The Company believes that the resolution of this matter is not likely to have a material adverse effect on its results of operations and cash flows.
At the request of the Company’s supplier, the DOC also initiated an administrative review to determine the final rate for the period of June 1, 2007 through May 31, 2008, during which time the Company paid duty rates of 76% for part of the period and approximately 20% for the remainder of the period. During the fourth quarter of 2009, the DOC made its final determination that changed the Company’s antidumping duty rate for the entire period to 20%. As a result of this final determination, the Company recorded a pre-tax benefit of $1.0 million in the fourth quarter of 2009. The determination was not appealed and the Company received the cash refund during 2010.
At the request of the Company’s supplier, the DOC also initiated an administrative review to determine the final rate for the period of June 1, 2008 to May 31, 2009, during which time the Company paid duty rates of approximately 20% for part of the period and approximately 1% for the remainder of the period. During the
100
fourth quarter of 2010, the DOC made its final determination that changed the Company’s antidumping duty rate for the entire period to approximately 3%. As a result of this final determination, the Company recorded a pre-tax benefit of $0.1 million in the fourth quarter of 2010. The U.S. isos producers appealed the DOC’s determination to the Court of International Trade. The appeal is delaying the cash refund of the duty to the Company and may result in a change of the duty rate for this review period. The Company believes that the resolution of this matter is not likely to have a material adverse effect on its results of operations and cash flows.
Based upon the final determination for the period of June 1, 2008 through May 31, 2009, the Company began paying cash deposits for imports at a rate of approximately 3% during the fourth quarter of 2010.
An administrative review has also commenced to determine the final rate for the period of June 1, 2009 to May 31, 2010.
Along with its primary Comprehensive General Liability (“CGL”) insurer, Arch Coatings France S.A. (“ACF”), a subsidiary of the Company, is a defendant in a lawsuit filed in France by a builder of pleasure boats. The suit alleges that the formulation of certain varnish coatings previously supplied by ACF for application to interior woodwork on approximately 5,200 boats made by plaintiff was defective in that, under certain conditions, the varnish will bubble and peel. The plaintiff has identified 554 boats in need of repair and the plaintiff claims that it has expended €4.5 million (approximately $5.9 million) to repair 513 of those boats. A court appointed expert has filed a report with the court that concludes that the technical cause of the problem lies solely with the formulation of the varnish coatings, and that the plaintiff’s repair-related damages at the end of January 2010 amounted to €3.7 million (approximately $4.9 million). There is no trial date set for this case. In August 2008, ACF was advised by its primary CGL insurer that it was denying coverage for this loss. The Company has advised the insurer that it disagrees with its position and is currently evaluating its options. ACF sold its assets in the sale of the industrial coatings business to Sherwin-Williams, but has retained the liability for the lawsuit. At December 31, 2010, ACF had €0.8 million (approximately $1.0 million) accrued for this matter. The Company believes the high end of the range of possible outcomes is €4.5 million (approximately $5.9 million). However, it is possible that the high end of the range could ultimately increase or decrease depending upon whether the court accepts or rejects the findings of the expert as to both causation and damages. Due to the multiple variables involved in the case (i.e., the uncertainty surrounding the number of boats that were damaged, the costs to repair the damages, the cause of the alleged damage as determined by the court, the Company’s responsibility for all or some of the alleged costs of repair as determined by the court), it is currently not possible to make an estimate of any amount above the amount of the current stated claim. The Company believes that the resolution of this matter is not likely to have a material adverse effect on the Company’s results of operations and cash flows.
In December 2007, as a result of an income tax audit of Nordesclor, the Company was notified by the Brazilian tax authorities that the Company would be assessed R$4.9 million (approximately $2.9 million) for alleged tax deficiencies related to the 2002 tax year. In accordance with the purchase agreement that was signed in conjunction with the acquisition of Nordesclor, our former joint venture partner is responsible for approximately 50% of this assessment. The Company believes the deficiency notice is without merit and, in January 2008, the Company protested the assessment to the first level administrative court. The Company received an unfavorable decision on this protest and has protested the assessment to the second level administrative court. The Company believes that the resolution of this matter is not likely to have a material adverse effect on its results of operations and cash flows.
Additionally, the Company has been notified by the Brazilian tax authorities of various assessments, totaling approximately R$9 million (approximately $5 million), related to alleged non-income tax deficiencies for tax years ranging from 1988 to 2003. The Company has recorded a liability of R$2.7 million (approximately $1.6 million) for any assessments for which it is probable that the Company will be unable to successfully defend itself. The Company believes that the remainder of the assessments are without merit. The Company believes that the resolution of this matter is not likely to have a material adverse effect on its results of operations and cash flows.
101
During 2003, the Company sold its sulfuric acid business. The Company has received a claim from the current owner of that business. The claimant asserts that, under certain provisions of the agreement for the sale of the business, the Company must indemnify the claimant for certain environmental penalties and compliance costs the claimant will incur under a settlement the claimant reached with the U.S. Environmental Protection Agency. The claimant alleges that such penalties and costs approximate $2.4 million. The Company is currently investigating the validity of the claimant’s assertions. The Company believes that the resolution of this matter is not likely to have a material adverse effect on its results of operations and cash flows.
During 2008, Arch Wood Protection (NZ) Limited (“AWPNZ”) was named as a defendant in a suit filed by one of its competitors. The suit alleged that AWPNZ and several other defendants were jointly and severally liable for defamatory statements made about a product of the competitor in that they secured, contributed to, or encouraged the publication of such statements. During 2010 the parties settled the matter and the case was dismissed. The settlement did not have a material impact on the Company.
In March 2010, the owner of a new U.S. patent, relating to methods of using particulate copper wood preservatives, filed a lawsuit against the Company, two of the Company’s subsidiaries, and three customers of the Company’s wood protection business. In the suit, the patent owner claims that use of certain wood preservatives manufactured and sold by the Company’s wood protection business infringes the patent. The complaint requests several forms of relief, including an unspecified amount of damages and a permanent injunction against infringement of the patent. The Company believes that the patent owner’s claims are without merit, and is vigorously defending against these claims. In its answer to the complaint, the Company has asserted, among other things, that the alleged activities do not infringe the patent, and that, in any event, the patent is both invalid and unenforceable. The trial in this case is scheduled to begin during the second quarter of 2011. The Company believes that the resolution of this matter is not likely to have a material adverse effect on its results of operations and cash flows.
In October 2010, the French taxing authorities notified the Company that it is responsible for paying additional duties in connection with certain products imported into France from October 2005 through May 2007 due to errors in the invoices prepared by the supplier. During the fourth quarter of 2010, the Company recorded a $1.8 million charge in Other (gains) and losses related to this claim. The Company believes that the resolution of this matter is not likely to have a material adverse effect on its results of operations and cash flows.
The Company is being sued by the current owner of a former Hickson site in Italy for remediation of environmental contaminants on that site. The owner is seeking compensation of €2.2 million (approximately $2.9 million) for the remediation of the site. The matter is currently within the Italian court system. Based on remediation actions completed in 2008, the Company believes it has no further obligation at the site. The local authorities, however, continue to review the condition of the site and may require additional work and risk assessments to be performed. Although the site was related to the Company’s industrial coatings business, liability for this lawsuit has been retained by the Company notwithstanding the sale of the business to Sherwin-Williams. The Company believes that the resolution of this matter is not likely to have a material adverse effect on its results of operations and cash flows.
There are a variety of non-environmental legal proceedings pending or threatened against the Company. Those matters that are probable have been accrued for in the accompanying Consolidated Financial Statements. Any contingent amounts in excess of amounts accrued are not expected to have a material adverse effect on results of operations, financial position or liquidity of the Company.
Environmental
Olin and the Company have entered into an agreement that specifies that the Company is only responsible for certain environmental liabilities at the Company’s then current operating plant sites and certain offsite locations. Olin retained the liability for all former Olin plant sites and former waste disposal sites. The Company
102
has also become subject to environmental exposures and potential liabilities in the U.S. and abroad with respect to the businesses it purchased. In connection with the acquisitions of Hickson International and Koppers Arch Wood Protection (Aust) Pty Ltd, the Company acquired certain environmental exposures and potential liabilities of current and past operation sites that have been accrued for in the accompanying Consolidated Financial Statements.
In connection with the disposition of the majority of the microelectronic materials business on November 30, 2004, the Company provided indemnification for potential and identified environmental liabilities. For environmental liabilities identified as of the transaction date, there is no limit to the liability retained by the Company, which the Company estimates to be less than $1.0 million. The Company is no longer liable for potential pre-closing environmental liabilities as the time period for indemnification has expired.
In connection with the disposition of the sulfuric acid business on July 2, 2003, the Company provided environmental covenants to the purchaser in which the Company is solely liable for the costs of any environmental claim for remediation of any hazardous substances that were generated, managed, treated, stored or disposed of prior to the closing date of the sale. The Company will be released, under the sales agreement, from its obligation, which cannot exceed $22.5 million, 20 years from the closing date. See “Litigation” for a discussion of a claim from the current owner of the business.
As part of the Hickson organics disposition in August 2003, the Company continues to be responsible for known environmental matters at the Castleford, England site. Such matters have previously been accrued for in its environmental reserve included in the Consolidated Financial Statements. Additionally, regarding any unknown environmental matters that are identified subsequent to the sale, the Company has agreed to share responsibility with the purchaser over a seven-year period, with the Company’s share decreasing to zero over the seven-year period. Such period ended in 2010. The Company’s maximum aggregate liability for any unknown environmental matters is £5.0 million. However, in September 2005, the purchaser went into liquidation and is highly unlikely to be able to honor its environmental indemnification commitments to the Company. The Company does not believe there has been any change in its environmental exposure at the site.
In connection with the disposition of the industrial coatings business on March 31, 2010, the Company provided indemnification for the costs of remediation necessary to comply with applicable environmental laws in relation to certain specifically identified pre-closing environmental contamination and non-compliances at the sites used in the business at the time of disposal. Although there are no monetary caps or time limits applicable to the Company’s obligation to indemnify for the remediation costs, the Company estimates the potential exposure to be approximately $1.0 million. At December 31, 2010, the Company had a liability recorded for such amount in Other liabilities in the Company’s Consolidated Balance Sheet. The Company also provided indemnification for certain other unknown environmental matters relating to the pre-closing operations of the industrial coatings business. This indemnification obligation is subject to both time limits (three years in the case of penalties for non-compliance with applicable environmental laws and permits and seven years in the case of offsite and former property environmental contamination) and a €5 million (approximately $7 million) aggregate monetary cap on all warranty and environmental matters arising out of the transaction (other than the remediation costs associated with the specifically identified onsite contamination and non-compliances discussed above). All other liabilities relating to environmental matters at the sites, and arising in connection with the industrial coatings business before March 31, 2010, have been assumed by the purchaser.
The Company believes that the environmental indemnifications for the microelectronic materials, sulfuric acid, Hickson organics and industrial coatings dispositions are not likely to have a material adverse effect on its results of operations and cash flows.
The Company’s Consolidated Balance Sheets included liabilities for future environmental expenditures to investigate and remediate known sites amounting to $9.0 million and $8.2 million at December 31, 2010 and 2009, respectively. The Company’s estimated environmental liability relates to 17 sites, seven of which are in the
103
United States and none of which are on the U.S. National Priority List. These amounts did not take into account any discounting of future expenditures, any consideration of insurance recoveries or any advances in technology. These liabilities are reassessed periodically to determine if environmental circumstances have changed or if the costs of remediation efforts can be better estimated. As a result of these reassessments, future charges to income may be made for additional liabilities.
Environmental exposures are difficult to assess for numerous reasons, including the identification of new sites, developments at sites resulting from investigatory studies and remedial activities, advances in technology, changes in environmental laws and regulations and their application, the scarcity of reliable data pertaining to identified sites, the difficulty in assessing the involvement and financial capability of other potentially responsible parties and the Company’s ability to obtain contributions from other parties and the lengthy time periods over which site remediation occurs. It is possible that some of these matters (the outcomes of which are subject to various uncertainties) may be resolved unfavorably against the Company. At December 31, 2010, the Company had estimated additional contingent environmental liabilities of approximately $10 million.
| 21. | U.S. Government Contract |
On March 29, 2005, the Company was notified by the U.S. Defense Logistics Agency-Energy that it had been awarded a 20-year hydrazine propellant supply contract for approximately $149 million for the production, storage, distribution and handling of hydrazine propellants for the U.S. Government. The Company began receiving monthly maintenance fee payments in the first quarter of 2006. The hydrazine plant is scheduled to operate in 2011 to meet government requirements.
In 2010, 2009 and 2008, the Company’s Performance Products segment sales include $7.1 million, $7.8 million, and $7.4 million, respectively, related to these agreements.
| 22. | Restructuring and Other Expense and Other (Gains) and Losses |
Restructuring and Other Expense
On August 24, 2010, the Company announced its decision to consolidate into one facility in Alpharetta, Georgia most of its U.S. research and development (“R&D”) and technical service activities currently conducted at three facilities. The facilities impacted by this consolidation are New Castle, Delaware; Cheshire, Connecticut; and Conley, Georgia.
Due to the consolidation, during 2010 the Company recorded a $0.7 million charge for estimated employee severance costs, most of which will be paid by the end of 2011. The charge is recorded in Restructuring and other expense in the Company’s Consolidated Statement of Income and the related liability is recorded in Accrued liabilities in the Company’s Consolidated Balance Sheet. Additionally, during 2010 the Company recorded a $1.2 million impairment charge related to its New Castle, Delaware facility and the Company has recorded the facility in Assets held for sale in the Company’s December 31, 2010 and 2009 Consolidated Balance Sheets. The fair value of the facility was determined based upon market data.
The total pre-tax charge to consolidate these facilities is estimated to be in the range of $5 million to $7 million. Excluding the amounts which were recorded in 2010, the remaining charge will consist of (i) $2 million to $3 million of relocation-related and employee severance costs and (ii) $1 million to $2 million of other costs. The Company anticipates that the consolidation will be completed, and the remaining charge recorded, in 2011. The Company has terminated its lease at the Cheshire, Connecticut facility, effective September 30, 2011, with no penalty to the Company.
Included in Restructuring and other expense during 2009 is a $1.1 million charge related to executive severance.
Included in Restructuring and other expense during 2008 is a $1.3 million cash charge related to a pension settlement associated with executive severance recorded in 2007.
104
Other (Gains) and Losses
Other (Gains) and Losses for 2010 represents a charge related to a duty claim on certain products imported into France (see Note 20).
Other (gains) and losses in 2008 is principally comprised of a reversal of penalties and interest related to a Brazilian state import tax claim recorded in 2004 of $1.4 million due to the expiration of the statute of limitations.
| 23. | Fair Value Measurements |
FASB ASC 820 established a new framework for measuring fair value. The framework requires fair value to be determined based on the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants.
The valuation techniques required by FASB ASC 820 are based upon observable and unobservable inputs. Observable inputs reflect market data obtained from independent sources, while unobservable inputs reflect the Company’s market assumptions. These two types of inputs create the following fair value hierarchy:
| | • | | Level 1 – Quoted prices (unadjusted) for identical assets and liabilities in active markets that the Company has the ability to access at the measurement date. |
| | • | | Level 2 – Quoted prices for similar assets and liabilities in active markets; quoted prices for identical or similar assets and liabilities in markets that are not active; and inputs other than quoted prices that are observable for the asset or liability, including interest rates, yield curves and credit risks, or inputs that are derived principally from or corroborated by observable market data through correlation. |
| | • | | Level 3 – Significant inputs to the valuation model are unobservable and are primarily based on internally derived assumptions surrounding the timing and amount of expected cash flows. |
The following section describes the valuation methodologies the Company uses to measure different assets and liabilities at fair value.
Rabbi Trust and Deferred Compensation Liability
All investments in the Company’s Rabbi Trust are recorded at fair value, except for the Company’s common stock, which is recorded at cost. Additionally, the related deferred compensation liability is recorded at fair value. The Company uses market prices to determine the fair values of these investments and the deferred compensation liability. The investments and the deferred compensation liability are included in Level 1.
Derivatives
The Company has foreign currency forward contracts, cross-currency swaps, interest rate swaps and equity total return swaps recorded at fair value. The fair value for the foreign currency forward contracts is determined using prices from active over-the-counter markets. The cross-currency swap agreements are valued using models that are based on market observable inputs, including foreign currency spot rates, foreign currency forward rates and interest rates. The interest rate swap agreements are valued using models that are based on market observable inputs, including LIBOR rates and yield curves. The Company’s equity total return swap agreements are also valued using models that are based on market observable inputs, principally the Company’s stock price. All derivatives are included in Level 2.
Securitization
Under the Company’s amended securitization program, the Company records receivables for which the payment of the purchase price by Market Street Funding LLC has been deferred, at fair value as a Securitization- related receivable on the accompanying Consolidated Balance Sheets. Such fair value includes a reserve for
105
credit losses. The receivables are not discounted due to the short-term nature of the underlying financial assets. The Securitization-related receivable is included in Level 3 and net payments of the purchase price which were deferred by Market Street Funding LLC during the year ended December 31, 2010 were $10.9 million.
The following table displays, by level, the fair values of each of the Company’s assets and liabilities that are measured at fair value on a recurring basis at December 31, 2010:
| | | | | | | | | | | | | | | | |
| ($ in millions) | | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
Assets | | | | | | | | | | | | | | | | |
Investments in the Rabbi Trust which are recorded at fair value | | $ | 9.1 | | | $ | — | | | $ | — | | | $ | 9.1 | |
Securitization-related receivable | | | — | | | | — | | | | 86.9 | | | | 86.9 | |
Foreign currency forward contracts | | | — | | | | 1.0 | | | | — | | | | 1.0 | |
Equity total return swap agreements | | | — | | | | 5.2 | | | | — | | | | 5.2 | |
| | | | | | | | | | | | | | | | |
Total Assets | | $ | 9.1 | | | $ | 6.2 | | | $ | 86.9 | | | $ | 102.2 | |
| | | | | | | | | | | | | | | | |
Liabilities | | | | | | | | | | | | | | | | |
Deferred Compensation | | $ | 15.0 | | | $ | — | | | $ | — | | | $ | 15.0 | |
Foreign currency forward contracts | | | — | | | | 0.2 | | | | — | | | | 0.2 | |
Cross-currency swap agreements | | | — | | | | 0.9 | | | | — | | | | 0.9 | |
Interest rate swap agreements | | | — | | | | 0.9 | | | | — | | | | 0.9 | |
| | | | | | | | | | | | | | | | |
Total Liabilities | | $ | 15.0 | | | $ | 2.0 | | | $ | — | | | $ | 17.0 | |
| | | | | | | | | | | | | | | | |
The following table displays, by level, the fair values of each of the Company’s assets and liabilities that are measured at fair value on a recurring basis at December 31, 2009:
| | | | | | | | | | | | | | | | |
| ($ in millions) | | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
Assets | | | | | | | | | �� | | | | | | | |
Investments in the Rabbi Trust which are recorded at fair value | | $ | 8.8 | | | $ | — | | | $ | — | | | $ | 8.8 | |
Securitization-related receivable | | | — | | | | — | | | | 76.0 | | | | 76.0 | |
Foreign currency forward contracts | | | — | | | | 0.2 | | | | — | | | | 0.2 | |
Equity total return swap agreements | | | — | | | | 2.4 | | | | — | | | | 2.4 | |
| | | | | | | | | | | | | | | | |
Total Assets | | $ | 8.8 | | | $ | 2.6 | | | $ | 76.0 | | | $ | 87.4 | |
| | | | | | | | | | | | | | | | |
Liabilities | | | | | | | | | | | | | | | | |
Deferred Compensation | | $ | 12.4 | | | $ | — | | | $ | — | | | $ | 12.4 | |
Foreign currency forward contracts | | | — | | | | 0.1 | | | | — | | | | 0.1 | |
Cross-currency swap agreements | | | — | | | | 0.3 | | | | — | | | | 0.3 | |
Interest rate swap agreements | | | — | | | | 1.4 | | | | — | | | | 1.4 | |
| | | | | | | | | | | | | | | | |
Total Liabilities | | $ | 12.4 | | | $ | 1.8 | | | $ | — | | | $ | 14.2 | |
| | | | | | | | | | | | | | | | |
During 2010 there were no transfers between Level 1 and Level 2.
106
| 24. | Quarterly Financial Data (Unaudited) |
| | | | | | | | | | | | | | | | | | | | |
($ in millions, except per share amounts) 2010 | | First
Quarter | | | Second
Quarter | | | Third
Quarter | | | Fourth
Quarter | | | Year | |
Sales | | $ | 298.7 | | | $ | 441.4 | | | $ | 325.6 | | | $ | 311.7 | | | $ | 1,377.4 | |
Gross margin (a) | | | 86.3 | | | | 150.5 | | | | 98.0 | | | | 90.1 | | | | 424.9 | |
Income from continuing operations (a, b) | | | 6.7 | | | | 43.5 | | | | 9.2 | | | | 5.1 | | | | 64.5 | |
Net income (a, b) | | | 11.8 | | | | 43.5 | | | | 9.2 | | | | 6.2 | | | | 70.7 | |
Diluted income per share—continuing operations | | | 0.27 | | | | 1.73 | | | | 0.36 | | | | 0.20 | | | | 2.56 | |
Diluted income per share | | | 0.47 | | | | 1.73 | | | | 0.36 | | | | 0.24 | | | | 2.80 | |
Stock market price: | | | | | | | | | | | | | | | | | | | | |
High | | | 35.57 | | | | 37.66 | | | | 36.22 | | | | 38.20 | | | | 38.20 | |
Low | | | 27.30 | | | | 30.52 | | | | 28.87 | | | | 33.72 | | | | 27.30 | |
Close (at end of quarter) | | | 34.39 | | | | 30.74 | | | | 35.09 | | | | 37.93 | | | | 37.93 | |
Common dividend paid per share | | | 0.20 | | | | 0.20 | | | | 0.20 | | | | 0.20 | | | | 0.80 | |
| | | | | | | | | | | | | | | | | | | | |
($ in millions, except per share amounts) 2009 | | First
Quarter | | | Second
Quarter | | | Third
Quarter | | | Fourth
Quarter | | | Year | |
Sales | | $ | 262.2 | | | $ | 377.0 | | | $ | 312.2 | | | $ | 293.4 | | | $ | 1,244.8 | |
Gross margin (c) | | | 80.7 | | | | 120.2 | | | | 88.4 | | | | 79.3 | | | | 368.6 | |
Income from continuing operations (c) | | | 4.8 | | | | 30.7 | | | | 9.7 | | | | 3.1 | | | | 48.3 | |
Net income (c) | | | 3.2 | | | | 30.9 | | | | 10.3 | | | | 2.7 | | | | 47.1 | |
Diluted income per share—continuing operations | | | 0.19 | | | | 1.23 | | | | 0.38 | | | | 0.12 | | | | 1.92 | |
Diluted income per share | | | 0.13 | | | | 1.23 | | | | 0.41 | | | | 0.11 | | | | 1.88 | |
Stock market price: | | | | | | | | | | | | | | | | | | | | |
High | | | 26.38 | | | | 29.92 | | | | 32.22 | | | | 31.31 | | | | 32.22 | |
Low | | | 15.00 | | | | 18.42 | | | | 22.38 | | | | 25.95 | | | | 15.00 | |
Close (at end of quarter) | | | 18.96 | | | | 24.59 | | | | 29.99 | | | | 30.88 | | | | 30.88 | |
Common dividend paid per share | | | 0.20 | | | | 0.20 | | | | 0.20 | | | | 0.20 | | | | 0.80 | |
| (a) | Gross margin, income from continuing operations and net income in the fourth quarter of 2010 include a $3.1 million pre-tax benefit related to a settlement with a supplier. |
| (b) | Income from continuing operations and net income in the fourth quarter of 2010 include a $1.8 million pre-tax charge related to a duty claim on certain products imported into France (see Note 20). |
| (c) | Gross margin, income from continuing operations and net income in the fourth quarter of 2009 include a $2.9 million pre-tax benefit related to a LIFO decrement. |
| Item 9. | Changes in and Disagreements With Accountants on Accounting and Financial Disclosure. |
Not Applicable.
| Item 9A. | Controls and Procedures. |
As of the end of the period covered by this Report, the Company conducted an evaluation, under the supervision and with the participation of the Company’s chief executive officer and chief financial officer, of the effectiveness of the Company’s disclosure controls and procedures as defined in Rule 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Based on this evaluation, as of December 31, 2010, the Company’s chief executive officer and chief financial officer concluded that as of the end of such period such disclosure controls and procedures were effective to provide reasonable assurance that they were designed to ensure that information required to be disclosed by the Company in reports it files or submits under the Exchange Act (i) is recorded, processed, summarized and reported within the time period specified in the rules and forms of the Securities and Exchange Commission and (ii) is accumulated and communicated to its management, including its principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
107
There were no changes in the Company’s internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) promulgated under the Exchange Act) during the three months ending December 31, 2010 that materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
108
MANAGEMENT REPORT
Management is responsible for the preparation and integrity of the Consolidated Financial Statements appearing in this Annual Report. The Consolidated Financial Statements were prepared in conformity with accounting principles generally accepted in the United States and include amounts based on management’s estimates and judgments.
Management is also responsible for establishing and maintaining an adequate system of internal control over financial reporting as defined in Rules 13a-15(f) and 15d-l5(f) promulgated under the Securities Exchange Act of 1934. We maintain a system of internal controls that is designed to provide reasonable assurance as to the fair and reliable preparation and presentation of the Consolidated Financial Statements in accordance with generally accepted accounting principles, as well as to safeguard assets from unauthorized use or disposition.
Our control environment is the foundation for our system of internal control over financial reporting and is embodied in our Code of Conduct. Our internal control over financial reporting includes written policies and procedures that:
| | • | | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions of the Company; |
| | • | | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles; |
| | • | | provide reasonable assurance that receipts and expenditures of the Company are made in accordance with the appropriate authorization of management and the directors of the Company; and |
| | • | | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of assets that could have a material effect on the consolidated financial statements. Internal control over financial reporting includes the controls themselves, monitoring and internal auditing practices and remedial actions to correct deficiencies as they are identified. |
Management evaluated the effectiveness of the Company’s internal control over financial reporting as of December 31, 2010. Management based such assessment upon the criteria set forth in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. This evaluation included review of the documentation of controls, evaluation of the design effectiveness of controls, testing of the operating effectiveness of controls and a conclusion on this evaluation. Although there are inherent limitations in the effectiveness of any system of internal controls over financial reporting, based on our evaluation, we have concluded that our internal controls over financial reporting were effective as of December 31, 2010.
KPMG LLP, an independent registered public accounting firm, has audited the consolidated financial statements included in this Report and, as part of their audit, has issued their report, included herein in Item 8, on the effectiveness of our internal control over financial reporting.
| | | | |
Michael E. Campbell Chairman of the Board, President and Chief Executive Officer | | | | Steven C. Giuliano Senior Vice President and Chief Financial Officer |
| Item 9B. | Other Information. |
Not applicable.
109
PART III
| Item 10. | Directors, Executive Officers and Corporate Governance. |
The information relating to the members of our Board of Directors under the heading “Who are the persons nominated by the Board in this election to serve as directors?” and “Who are the other remaining directors and when are their terms scheduled to end?” in the section entitled “Item 1—Election of Directors” in the Proxy Statement relating to the Company’s 2011 Annual Meeting of Shareholders (the “Proxy Statement”) is incorporated by reference into this Report. See also the list of executive officers following Item 4 of this Report. The information regarding compliance with Section 16 of the Securities Exchange Act of 1934, as amended, under the heading “Section 16(a) Beneficial Ownership Reporting Compliance” in the section entitled “Security Ownership of Directors and Officers” in the Proxy Statement is incorporated by reference into this Report. The information under the heading “Has the Company adopted a Code of Ethics and a policy regarding approval of related party transactions?” in the section entitled “Additional Information Regarding the Board of Directors and Corporate Governance” in the Proxy Statement is incorporated by reference into this Report. The information under “Audit Committee” under the heading “What are the committees of the Board” in the section entitled “Additional Information Regarding the Board of Directors and Corporate Governance” in the Proxy Statement is incorporated by reference into this Report.
| Item 11. | Executive Compensation. |
The information in the sections entitled “Executive Compensation,” including the Compensation Committee Report, and “Director Compensation” in the Proxy Statement is incorporated by reference into this Report.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters. |
The information concerning holdings of Company stock by certain beneficial owners contained in the section entitled “Certain Beneficial Owners” in the Proxy Statement and the information concerning beneficial ownership of the Company’s common stock by directors and officers of the Company in the section entitled “Security Ownership of Directors and Officers” in the Proxy Statement are incorporated by reference into this Report.
Equity Compensation Plan Information as of December 31, 2010
| | | | | | | | | | | | |
Plan category | | Number of outstanding
awards payable in
shares, including
options, deferred
compensation and
phantom share units (1) | | | Weighted-average
exercise price of
outstanding options,
warrants and rights (2) | | | Number of securities
remaining available for
future issuance under equity
compensation plans
(excluding securities
reflected in column (a)) (3) | |
| | | (a) | | | (b) | | | (c) | |
Equity compensation plans approved by security holders | | | 545,306 | | | $ | 19.57 | | | | 1,251,942 | |
Equity compensation plans not approved by security holders | | | 0 | | | | N/A | | | | N/A | |
Total | | | 545,306 | | | $ | 19.57 | | | | 1,251,942 | |
| (1) | This figure represents all outstanding grants, which consist of 55,502 stock options, 17,134 phantom shares payable in shares and held in director and employee deferral plans and 472,670 phantom share unit awards payable in shares. |
| (2) | This figure is calculated using only the stock options included in column (a) and no other grants. |
| (3) | Shares remaining available at December 31, 2010 for future issuance by plan are: 1,214,922 under the 2009 Long Term Incentive Plan, 19,949 under the 1999 Stock Plan for Non-Employee Directors and 17,071 under the Employee Deferral Plan. |
110
| Item 13. | Certain Relationships and Related Transactions, and Director Independence. |
The information under the headings “Has the Board of Directors adopted Principles of Corporate Governance?”, “What is the categorical independence standard used by the Board to determine whether Board members are independent?” and “Has the Company adopted a Code of Ethics and a policy regarding approval of related party transactions?” in the section entitled “Additional Information Regarding the Board of Directors and Corporate Governance” in the Proxy Statement is incorporated by reference into this Report.
| Item 14. | Principal Accountant Fees and Services. |
The information contained under the headings “What were KPMG audit fees in 2009 and 2010?” and “Pre-Approval Policies and Provisions” in the section entitled “Item 5—Ratification of Appointment of Independent Registered Public Accounting Firm” in the Proxy Statement is incorporated by reference into this Report.
111
PART IV
| Item 15. | Exhibits and Financial Statement Schedules. |
(a) 1. Financial Statements
The following is a list of the Financial Statements included in Item 8 of this Report:
2.Financial Statement Schedules
Except as noted below, schedules not included herein are omitted because they are inapplicable or not required or because the required information is given in the Consolidated Financial Statements and Notes thereto.
Separate financial statements of the remaining 50% or less owned companies accounted for by the equity method are not summarized herein and have been omitted because they would not constitute a significant subsidiary.
3.Exhibits
Management contracts and compensatory plans and arrangements are listed as Exhibits 10.6 through 10.23 below.
| | |
| 2.1 | | Share Purchase Agreement, dated August 11, 2003, among Hickson Limited, Greentag (8) Limited, Hickson International Limited, Arch Chemicals, Inc. and Hickson & Welch Chemical Products Limited—Exhibit 2 to the Company’s Current Report on Form 8-K, filed August 18, 2003.* |
| |
| 2.2 | | Restated Sale and Purchase Agreement dated as of 8th March 2004, among Avecia Investments Limited, the other parties thereto and Arch Chemicals, Inc., restating an agreement made between the parties on 4th March 2004—Exhibit 2.1 to the Company’s Current Report on Form 8-K, filed March 8, 2004.* |
| |
| 2.3 | | Stock and Asset Purchase Agreement dated as of October 24, 2004 between Arch Chemicals, Inc. and Fuji Photo Film Co., Ltd.—Exhibit 2 to the Company’s Current Report on Form 8-K, filed October 25, 2004.* |
| |
| 2.4 | | First Amendment dated as of November 30, 2004 to the Stock and Asset Purchase Agreement dated as of October 24, 2004 between Arch Chemicals, Inc. and Fuji Photo Film Co., Ltd.—Exhibit 2 to the Company’s Current Report on Form 8-K, filed December 6, 2004.* |
| |
| 2.5 | | Asset Purchase Agreement, dated as of September 5, 2008, among Rockwood Specialties, Inc., Advantis Technologies, Inc., Arch Chemicals, Inc. and Rockwood Holdings Inc.—Exhibit 2.1 to the Company’s Current Report on Form 8-K, filed September 5, 2008.* |
| * | Previously filed as indicated and incorporated herein by reference. Exhibits incorporated by reference are located in SEC File No. 1-14601 unless otherwise indicated. |
112
| | |
| |
| 2.6 | | Agreement dated February 17, 2010 among Hickson Nederland BV, Hickson Investments Limited, Arch UK Biocides Limited, Arch Chemicals, Inc., Arch Coatings UK Limited, Hickson Limited RPG Acquisition Limited, The Sherwin-Williams Company and Sherwin-Williams Coatings S.à r.l.—Exhibit 2 to the Company’s Current Report on Form 8-K, filed February 23, 2010.* |
| |
| 2.7 | | Deed of Adherence and Amendment, dated March 31, 2010, among Arch Chemicals, Inc., The Sherwin-Williams Company and the other parties thereto—Exhibit 2 to the Company’s Quarterly Report on Form 10-Q for the period ending March 31, 2010.* |
| |
| 3.1 | | Amended and Restated Articles of Incorporation of the Company—Exhibit 3.1 to the Company’s Current Report on Form 8-K, filed February 17, 1999.* |
| |
| 3.2 | | Bylaws of the Company effective July 23, 2009—Exhibit 3 to the Company’s Current Report on Form 8-K, filed July 29, 2009.* |
| |
| 4.1 | | Specimen Common Share certificate—Exhibit 4.1 to the Company’s Registration Statement on Form 10, as amended.* |
| |
| 4.2 | | Amended and Restated Articles of Incorporation of the Company (filed as Exhibit 3.1 hereto).* |
| |
| 4.3 | | Bylaws of the Company (filed as Exhibit 3.2 hereto).* |
| |
| 4.4 | | Revolving Credit Agreement, dated as of June 15, 2006 among Arch Chemicals, Inc., the Lenders party thereto, JPMorgan Chase Bank, as Administrative Agent, J.P. Morgan Securities Inc., as Joint Lead Arranger and Joint Book Manager, Banc of America Securities, L.L.C., as Joint Lead Arranger and Joint Book Manager, SunTrust Bank, as Documentation Agent, and Bank of America, National Association and Citizens Bank of Massachusetts, as Co-Syndication Agents—Exhibit 4 to the Company’s Current Report on Form 8-K filed June 20, 2006.* |
| |
| 4.5(a) | | Note Purchase Agreement, dated as of March 20, 2002, among the Company and the purchasers named therein, relating to the Company’s $149,000,000 Senior Notes, Series A, due March 20, 2007 and $62,000,000 Senior Notes, Series B, due March 20, 2009—Exhibit 4.8 to the Company’s Annual Report on Form 10-K for the period ending December 31, 2001.* |
| |
| 4.5(b) | | First Amendment entered into as of February 27, 2004 relating to the Note Purchase Agreement dated as of March 20, 2002 among the Company and the purchasers named therein, relating to the Company’s $149,000,000 Senior Notes, Series A, due March 20, 2007 and $62,000,000 Senior Notes, Series B, due March 20, 2009—Exhibit 4.2 to the Company’s Current Report on Form 8-K, filed March 8, 2004.* |
| |
| 4.5(c) | | Second Amendment, dated as of May 12, 2006, to Note Purchase Agreement, dated as of March 20, 2002, among the Company and the purchasers named therein, relating to the Company’s $149,000,000 Senior Notes, Series A, due March 20, 2007 and $62,000,000 Senior Notes, Series B, due March 20, 2009—Exhibit 4 to the Company’s Quarterly Report on Form 10-Q for the period ending June 30, 2006.* |
| |
| 4.6(a) | | Credit Agreement, dated as of February 13, 2009, among Arch Chemicals, Inc., the Lenders party thereto, Bank of America, N.A., as Administrative Agent, RBS Citizens, N.A., as Syndication Agent, Banc of America Securities LLC and Greenwich Capital Markets, Inc., as Joint Lead Arrangers and Joint Book Managers—Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on February 17, 2009.* |
| * | Previously filed as indicated and incorporated herein by reference. Exhibits incorporated by reference are located in SEC File No. 1-14601 unless otherwise indicated. |
113
| | |
| |
| 4.6(b) | | Schedule 2.01 to Credit Agreement, dated as of February 13, 2009, among Arch Chemicals, Inc., the Lenders Party Thereto, Bank of America, N.A., as Administrative Agent, RBS Citizens, N.A., as Syndication Agent, Banc of America Securities LLC and Greenwich Capital Markets, Inc., as Joint Lead Arrangers and Joint Book Managers—Exhibit 4 to the Company’s Quarterly Report on Form 10-Q for the period ending June 30, 2009.* |
| |
| 4.7 | | Note Purchase and Private Shelf Agreement, dated as of August 28, 2009, among Arch Chemicals Inc., Prudential Investment Management, Inc., Gibraltar Life Insurance Co., Ltd, United of Omaha Life Insurance Company and Prudential Retirement Insurance and Annuity Company—Exhibit 10.1 to the Company’s Current Report on Form 8-K/A, filed on January 7, 2011.* |
| |
| 4.8 | | Master Note Purchase Agreement between Arch Chemicals, Inc. and the purchasers party thereto, dated September 9, 2010—Exhibit 4 to the Company’s Current Report on Form 8-K/A filed on January 7, 2011.* |
| |
| 10.1 | | Distribution Agreement, dated as of February 1, 1999, between the Company and Olin—Exhibit 2 to the Company’s Current Report on Form 8-K, filed February 17, 1999.* |
| |
| 10.2 | | Form of Employee Benefits Allocation Agreement between the Company and Olin—Exhibit 10.4 to the Company’s Annual Report on Form 10-K for the period ending December 31, 1998.* |
| |
| 10.3 | | Tax Sharing Agreement, dated as of February 8, 1999, between the Company and Olin—Exhibit 10.9 to the Company’s Annual Report on Form 10-K for the period ending December 31, 1998.* |
| |
| 10.4 | | Form of Intellectual Property Transfer and License Agreement between the Company and Olin—Exhibit 10.9 to the Company’s Registration Statement on Form 10, as amended.* |
| |
| 10.5 | | Charleston Services Agreement, dated as of February 8, 1999, between the Company and Olin—Exhibit 10.10 to the Company’s Annual Report on Form 10-K for the period ending December 31, 1998.* |
| |
| 10.6 | | Form of Amended and Restated Executive Agreement—Exhibit 10.1 to the Company’s Current Report on Form 8-K, filed December 19, 2008.* |
| |
| 10.7 | | Form of Change in Control Agreement (Tier II Agreement).—Exhibit 10.7 to the Company’s Annual Report on Form 10-K for the period ending December 31, 2008.* |
| |
| 10.8 | | Arch Chemicals, Inc. 1999 Stock Plan for Non-Employee Directors, as Amended and Restated through December 30, 2008—Exhibit 10.8 to the Company’s Annual Report on Form 10-K for the period ending December 31, 2008.* |
| |
| 10.9(a) | | 1999 Long Term Incentive Plan, as amended through February 9, 2005—Exhibit 10.9 to the Company’s Annual Report on Form 10-K for the period ending December 31, 2004.* |
| |
| 10.9(b) | | Award Description and Agreement for Performance Retention Share Awards Granted under the Arch Chemicals, Inc. 1999 Long Term Incentive Plan Granted January 28, 2009 for Senior Executive Officers—Exhibit 10.9(b) to the Company’s Annual Report on Form 10-K for the period ending December 31, 2008.* |
| |
| 10.9(c) | | Award Description and Agreement for Performance Share Awards Granted under the Arch Chemicals, Inc. 1999 Long Term Incentive Plan Granted January 28, 2009 for Senior Executive Officers—Exhibit 10.9(c) to the Company’s Annual Report on Form 10-K for the period ending December 31, 2008.* |
| * | Previously filed as indicated and incorporated herein by reference. Exhibits incorporated by reference are located in SEC File No. 1-14601 unless otherwise indicated. |
114
| | |
| |
| 10.9(d) | | Award Description and Agreement for Performance Retention Share Awards Granted under the Arch Chemicals, Inc. 1999 Long Term Incentive Plan Granted January 28, 2009 for Non-Senior Executive Officers and other participants—Exhibit 10.9(d) to the Company’s Annual Report on Form 10-K for the period ending December 31, 2008.* |
| |
| 10.9(e) | | Award Description and Agreement for Performance Share Awards Granted under the Arch Chemicals, Inc. 1999 Long Term Incentive Plan Granted January 28, 2009 for Non-Senior Executive Officers and other participants—Exhibit 10.9(e) to the Company’s Annual Report on Form 10-K for the period ending December 31, 2008.* |
| |
| 10.9(f) | | Award Description and Agreement for Performance Retention Share Awards Granted under the Arch Chemicals, Inc. 1999 Long Term Incentive Plan Granted March 5, 2008 to H. Anderson, M. E. Campbell, S. C. Giuliano, L. S. Massimo and S. A. O’Connor as Amended April 25, 2008—Exhibit 10.3 to Company’s Quarterly Report on Form 10-Q for the period ending March 31, 2008.* |
| |
| 10.9(g) | | Award Description and Agreement for Performance Retention Share Awards Granted under the Arch Chemicals, Inc. 1999 Long Term Incentive Plan Granted March 5, 2008 as Amended April 25, 2008 (other than for H. Anderson, M. E. Campbell, S. C. Giuliano, L.S. Massimo and S. A. O’Connor)—Exhibit 10.4 to Company’s Quarterly Report on Form 10-Q for the period ending March 31, 2008.* |
| |
| 10.9(h) | | Award Description and Agreement for Performance Share Awards Granted under the Arch Chemicals, Inc. 1999 Long Term Incentive Plan Granted March 5, 2008 to H. Anderson, M. E. Campbell, S. C. Giuliano, L. S. Massimo and S. A. O’Connor as Amended April 25, 2008—Exhibit 10.5 to Company’s Quarterly Report on Form 10-Q for the period ending March 31, 2008.* |
| |
| 10.9(i) | | Award Description and Agreement for Performance Share Awards Granted under the Arch Chemicals, Inc. 1999 Long Term Incentive Plan Granted March 5, 2008 as Amended April 25, 2008 (other than for H. Anderson, M. E. Campbell, S. C. Giuliano, L.S. Massimo and S. A. O’Connor)—Exhibit 10.6 to Company’s Quarterly Report on Form 10-Q for the period ending March 31, 2008.* |
| |
| 10.9(j) | | Form of Award Description and Agreement for Performance Retention Share Awards granted under the Arch Chemicals, Inc. 1999 Long Term Incentive Plan—Exhibit 10.19 to the Company’s Annual Report on Form 10-K for the period ending December 31, 2004.* |
| |
| 10.9(k) | | Restricted Stock Unit Certificate and related Award Description and Agreement for Restricted Stock Unit Award Granted under the Arch Chemicals, Inc. 1999 Long Term Incentive Plan dated August 8, 2008 for Joseph Shaulson—Exhibit 10.9(l) to the Company’s Annual Report on Form 10-K for the period ending December 31, 2008.* |
| |
| 10.10 | | 2009 Long Term Incentive Plan—Exhibit A to the Company’s Definitive Proxy Statement on Schedule 14A for the April 30, 2009 Annual Meeting of Shareholders.* |
| |
| 10.11 | | Form of Award Description and Agreement for Performance Unit Award Granted under the Arch Chemicals, Inc. 2009 Long Term Incentive Plan—Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the period ending March 31, 2010.* |
| |
| 10.12 | | Form of Award Description and Agreement for Performance Accelerated Restricted Stock Unit Award Granted Under the Arch Chemicals, Inc. 2009 Long Term Incentive Award—Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q for the period ending March 31, 2010.* |
| |
| 10.13 | | Arch Chemicals, Inc. Supplemental Contributing Employee Ownership Plan, Amended and Restated as of January 1, 2009—Exhibit 10.10 to the Company’s Annual Report on Form 10-K for the period ending December 31, 2008.* |
| * | Previously filed as indicated and incorporated herein by reference. Exhibits incorporated by reference are located in SEC File No. 1-14601 unless otherwise indicated. |
115
| | |
| |
| 10.14 | | Arch Senior Executive Pension Plan, effective as of January 1, 2010. |
| |
| 10.15 | | Arch Supplementary and Deferral Benefit Pension Plan—Exhibit 10.2 to the Company’s Current Report on Form 8-K, filed November 5, 2009.* |
| |
| 10.16 | | Arch Chemicals, Inc. Employee Deferral Plan, Amended and Restated as of January 1, 2009—Exhibit 10.13 to the Company’s Annual Report on Form 10-K for the period ending December 31, 2008.* |
| |
| 10.17 | | Arch Senior Executive Pension Plan II, effective as of January 1, 2010. |
| |
| 10.18 | | Senior Executive Life Insurance Plan (effective December 6, 2005)—Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q for the period ending September 30, 2005.* |
| |
| 10.19 | | Arch Chemicals, Inc. Annual Incentive Plan, as amended December 9, 1999 and April 27, 2000—Exhibit 10.21 to the Company’s Annual Report on Form 10-K for the period ending December 31, 2000.* |
| |
| 10.20 | | Arch Chemicals, Inc. Senior Management Incentive Compensation Plan (As Amended and Restated Effective January 1, 2010 for Awards Granted After December 31, 2009)—Exhibit 10.3 to the Company’s Current Report on Form 8-K, filed November 5, 2009.* |
| |
| 10.21 | | Agreement between Arch Chemicals, Inc. and Louis S. Massimo, dated November 2, 2009—Exhibit 10.1 to the Company’s Current Report on Form 8-K, filed November 16, 2009.* |
| |
| 10.22 | | Executive Agreement, dated as of September 1, 2010, between Arch Chemicals, Inc. and Luis Fernandez-Moreno, with Amendment dated as of January 26, 2011. |
| |
| 10.23 | | Award Description and Agreement for Restricted Stock Unit Award under the Arch Chemicals, Inc. 2009 Long Term Incentive Plan dated September 7, 2010 for Luis Fernandez-Moreno. |
| |
| 10.24(a) | | Omnibus Assumption and Assignment Agreement, dated as of October 6, 2009, among Arch Chemicals Receivables Corp., Arch Chemicals, Inc., Three Pillars Funding LLC, SunTrust Robinson Humphrey, Inc. (f/k/a SunTrust Capital Markets, Inc.), Market Street Funding LLC and PNC Bank, National Association—Exhibit 10.1 to the Company’s Current Report on Form 8-K, filed October 9, 2009.* |
| |
| 10.24(b) | | Amended and Restated Receivables Sale Agreement, dated as of October 6, 2009, among Arch Chemicals, Inc., as an Originator, Arch Treatment Technologies, Inc., as an Originator, Arch Wood Protection, Inc., as an Originator, Arch Personal Care Products, L.P., as an Originator, and Arch Chemicals Receivables Corp., as Buyer—Exhibit 10.1 to the Company’s Current Report on Form 8-K/A, filed January 7, 2011.* |
| |
| 10.24(c) | | Amended and Restated Receivables Purchase Agreement, dated as of October 6, 2009, among Arch Chemicals Receivables Corp., as Seller, Arch Chemicals, Inc., as Initial Servicer, Market Street Funding LLC and PNC Bank, National Association, as Administrator and as LC Bank—Exhibit 10.2 to the Company’s Current Report on Form 8-K/A, filed January 7, 2011.* |
| |
| 10.25 | | Omnibus Amendment and RPA Amendment No. 2, dated as of July 9, 2010, among Arch Treatment Technologies, Inc., Arch Wood Protection, Inc., Arch Personal Care Products, L.P., and Arch Chemicals, Inc., each as an Originator, Arch Chemicals Receivables LLC, as Buyer under the Receivables Sales Agreement and as Seller under the Receivables Purchase Agreement, Arch Chemicals, Inc., as Servicer, Market Street Funding, and PNC Bank, National Association, as LC Bank and as Administrator—Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the period ending June 30, 2010.* |
| * | Previously filed as indicated and incorporated herein by reference. Exhibits incorporated by reference are located in SEC File No. 1-14601 unless otherwise indicated. |
116
| | |
| |
| 10.26 | | RPA Amendment No. 3, dated as of October 5, 2010, among Arch Chemicals Receivables LLC, as Seller, Arch Chemicals, Inc., as Servicer, Market Street Funding LLC, and PNC Bank, National Association, as LC Bank and as Administrator—Exhibit 10.1 to the Company’s Current Report on Form 8-K, filed October 7, 2010.* |
| |
| 21. | | List of Subsidiaries. |
| |
| 23. | | Consent of KPMG LLP. |
| |
| 31.1 | | Certification of Chief Executive Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a). |
| |
| 31.2 | | Certification of Chief Financial Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a). |
| |
| 32 | | Certification of Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. 1350. |
| |
| 101 | | Interactive Data Files pursuant to Rule 405 of Regulation S-T: (i) Consolidated Balance Sheets as of December 31, 2010 and 2009; (ii) Consolidated Statements of Income for the years ended December 31, 2010, 2009 and 2008; (iii) Consolidated Statements of Cash Flows for the years ended December 31, 2010, 2009 and 2008; (iv) Consolidated Statements of Shareholders’ Equity and Comprehensive Income (Loss) for the years ended December 31, 2010, 2009 and 2008; and (v) the Notes to the Consolidated Financial Statements, tagged as blocks of text.** |
| * | Previously filed as indicated and incorporated herein by reference. Exhibits incorporated by reference are located in SEC File No. 1-14601 unless otherwise indicated. |
| ** | Pursuant to Rule 406T of Regulation S-T, the Interactive Data Files on Exhibit 101 hereto are furnished and deemed not filed or part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, as amended, are deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and otherwise are not subject to liability under those sections. |
117
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | |
| ARCH CHEMICALS, INC. |
| |
| By | | /S/ MICHAEL E. CAMPBELL |
| | Michael E. Campbell Chairman of the Board, President and Chief Executive Officer |
Date: February 23, 2011
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the date indicated.
| | |
Signature | | Title |
| |
/S/ MICHAEL E. CAMPBELL Michael E. Campbell | | Chairman of the Board, President, Chief Executive Officer and Director (Principal Executive Officer) |
| |
/S/ RICHARD E. CAVANAGH Richard E. Cavanagh | | Director |
| |
/S/ DAVID LILLEY David Lilley | | Director |
| |
/S/ WILLIAM H. POWELL William H. Powell | | Director |
| |
/S/ DANIEL S. SANDERS Daniel S. Sanders | | Director |
| |
/S/ JANICE J. TEAL Janice J. Teal | | Director |
| |
/S/ DOUGLAS J. WETMORE Douglas J. Wetmore | | Director |
| |
/S/ STEVEN C. GIULIANO Steven C. Giuliano | | Senior Vice President and Chief Financial Officer (Principal Financial Officer) |
| |
/S/ MEGHAN E. DEMASI Meghan E. DeMasi | | Controller (Principal Accounting Officer) |
Date: February 23, 2011
118
