UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 6-K
REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13a-16
OR 15d-16 UNDER THE
SECURITIES EXCHANGE ACT OF 1934
OR 15d-16 UNDER THE
SECURITIES EXCHANGE ACT OF 1934
For the month of
Commission File Number
Novogen Limited
(Translation of registrant’s name into English)
140 Wicks Road, North Ryde, NSW, Australia
(Address of principal executive office)
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of
Form 20-F or Form 40-F.
Form 20-Fþ Form 40-Fo
Form 20-F or Form 40-F.
Form 20-Fþ Form 40-Fo
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(l):
Note: Regulation S-T Rule 101 (b)( I) only permits the submission in paper of a Form 6-K if submitted solely to provide an attached annual report to security holders.
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule lO1(b)(7):
Note: Regulation S-T Rule l01(b)(7) only permits the submission in paper of a Form 6-K if submitted to furnish a report or other document that the registrant foreign private issuer must furnish and make public under the laws of the jurisdiction in which the registrant is incorporated, domiciled or legally organized (the registrant’s “home country”), or under the rules of the home country exchange on which the registrant’s securities are traded, as long as the report or other document is not a press release, is not required to be and has not been distributed to the registrant’s security holders, and, if discussing a material event, has already been the subject of a Form 6-K submission or other Commission filing on EDGAR.
Indicate by check mark whether the registrant by furnishing the information contained in this Form is also thereby furnishing the information to the Commission pursuant to Rule l2g3-2(b) under the Securities Exchange Act of 1934. Yeso Noþ
If “Yes” is marked, indicate below the file number assigned to the registrant in connection with Rule 12g3-2(b):
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalfby the undersigned, thereunto duly authorized.
| Novogen Limited (Registrant) /s/ Ron Erratt Ronald Lea Erratt Company Secretary Date |

| OVATURE Phase III trial Update |

| Under U.S. law, a new drug cannot be marketed until it has1 Ovarian cancerbeen investigated in clinical trials and approved by the FDA as being safe and effective for the intended use. Statements2 The OVATURE studyincluded in this release that are not historical in nature are “forward-looking statements” within the meaning of the4 The regulatory issues“safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. You should be aware that our actual5 Site approval and logisticsresults could differ materially from those contained in the forward-looking statements, which are based on manage-5 Contract research organizationment’s current expectations and are subject to a number of risks and uncertainties, including, but not limited to, our6 Drug supplyfailure to successfully commercialize our product candidates; costs and delays in the development and/or FDA approval,7 Data managementor the failure to obtain such approval, of our product candidates; uncertainties in clinical trial results; our inability8 Financing the programto maintain or enter into, and the risks resulting from our dependence upon, collaboration or contractual arrangements8 Phenoxodiol marketing strategynecessary for the development, manufacture, commercialization, marketing, sales and distribution of any products;9 Program managementcompetitive factors; our inability to protect our patents or proprietary rights and obtain necessary rights to third partyReferencespatents and intellectual property to operate our business; our inability to operate our business without infringing the patents and proprietary rights of others; general economic conditions; the failure of any products to gain market acceptance; our inability to obtain any additional required financing; technological changes; government regulation; changes in industry practice; and one-time events. We do not intend to update any of these factors or to publicly announce the results of any revisions to these forward-looking statements. |
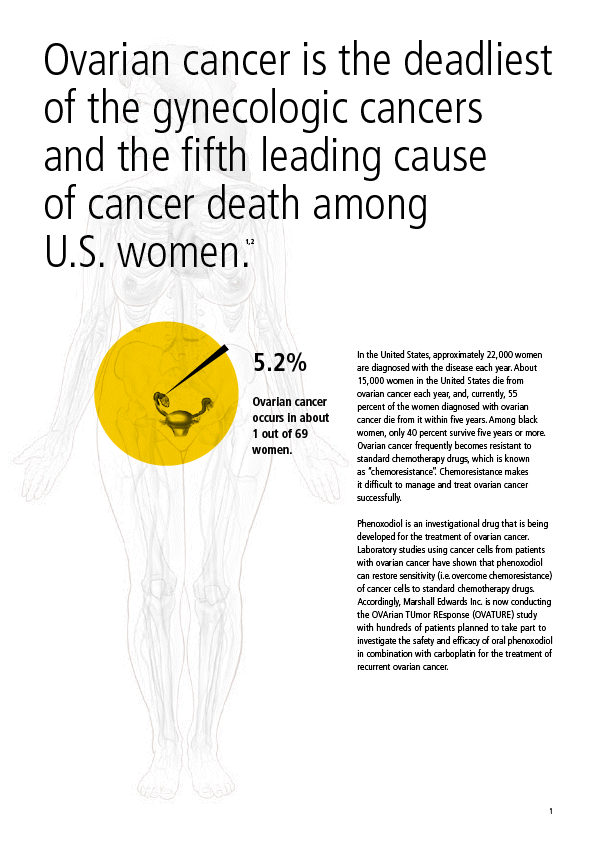
| Ovarian cancer is the deadliest of the gynecologic cancers and the fifth leading cause of cancer death among U.S. women.1,2 5.2%In the United States, approximately 22,000 women are diagnosed with the disease each year. About 15,000 women in the United States die from ovarian cancer each year, and, currently, 55Ovarian cancerpercent of the women diagnosed with ovarianoccurs in aboutcancer die from it within five years. Among black1 out of 69women, only 40 percent survive five years or more.women.Ovarian cancer frequently becomes resistant to standard chemotherapy drugs, which is known as “chemoresistance”. Chemoresistance makes it difficult to manage and treat ovarian cancer successfully. Phenoxodiol is an investigational drug that is being developed for the treatment of ovarian cancer. Laboratory studies using cancer cells from patients with ovarian cancer have shown that phenoxodiol can restore sensitivity (i.e. overcome chemoresistance) of cancer cells to standard chemotherapy drugs. Accordingly, Marshall Edwards Inc. is now conducting the OVArian TUmor REsponse (OVATURE) study with hundreds of patients planned to take part to investigate the safety and efficacy of oral phenoxodiol in combination with carboplatin for the treatment of recurrent ovarian cancer. 1 |

| The OVATURE study470 patients 60 centres Patients with progressive or recurrent ovarian cancer that has become resistant or refractory to the second line platinum therapy (cisplatin or carboplatin) are eligible to participate. This is an exciting trial as it provides the opportunity to investigate the efficacy and safety of phenoxodiol as a platinum chemo-sensitizing agent for this indication, as well as to explore further the use of a weekly dose ofphenoxodiol placebo carboplatin for these patients.+carboplatin +carboplatinThis Phase III study will recruit 470 patients at approximately 30 centers in the United States and an additional 30 hospitals throughout Europe andThe OVATURE study is a Phase III clinical trialAustralia. In the OVATURE trial, patients areinvestigating the safety and efficacy of oralrandomly divided into two groups and receive either oral phenoxodiol in combination with carboplatin orphenoxodiol in combination with a weeklycarboplatin with an inactive control capsuledose of carboplatin in women with late-stage(placebo). Neither patient nor doctor will knowrecurrent ovarian cancer.which group each patient is in. Instead of receiving the customary carboplatin treatment of one intravenous infusion every three weeks, all patients will receive carboplatin on a weekly basis. This is based on the observation from laboratory studies that when given with phenoxodiol, carboplatin should be given as frequently as possible, and also because some studies have shown that patients whose tumors have become resistant to the three-week standard regimen of carboplatin may respond to the weekly administration of the drug. An important aspect of the study plan is that it is designed to enable an interim analysis of results after enrollment has been completed and at least 95 patients have progressed. The preliminary analysis will compare the duration of progression free survival (PFS), the time during which the patients’ tumors shrink or fail to grow larger, in response to treatment in the phenoxodiol plus carboplatin group compared to the carboplatin plus placebo group. 2 |
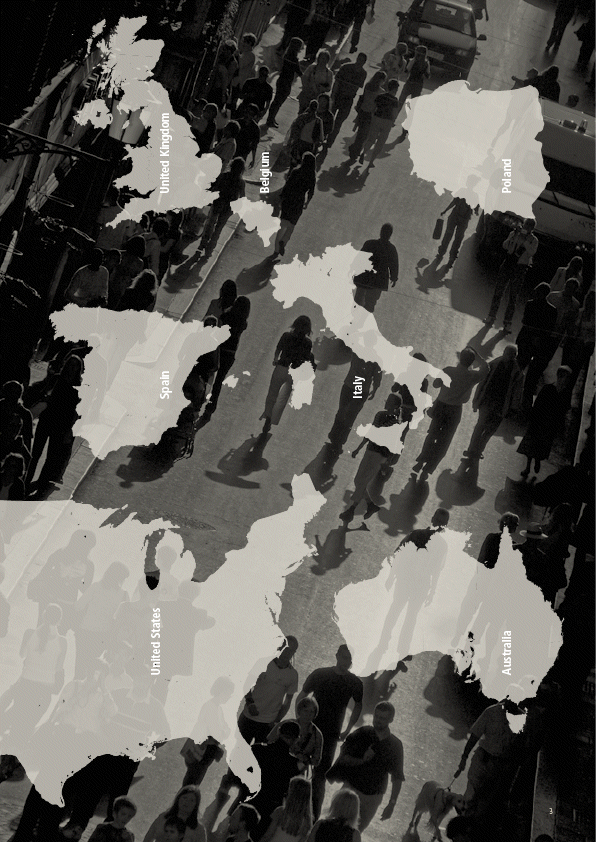
| United States Spain United Kingdom Belgium Italy Australia Poland3 |

| FDAThe regulatory issuesGovernment approval is required in most countries The Phase II trials produced encouraging results: before a new drug such as phenoxodiol can be in patients with advanced disease resistant or commercially distributed for use in patients. Approval refractory to platinum (cisplatin or carboplatin) or for marketing is based on a rigorous assessment of taxane, stabilization or regression was observed the safety and efficacy of the drug for its chosen in 71% of subjects administered phenoxodiol in indication, in this case, advanced platinum-resistant combination with platinum and 74% of subjects ovarian cancer. Each country has its own regulatory administered phenoxodiol in combination with body for this purpose, and in the United States, the taxane, without any significant side effects Food and Drug Administration (FDA) is charged with attributable to phenoxodiol. In one of the taxane-EMEA this responsibility. Since the pharmaceutical markets resistant patients administered phenoxodiol in in the US represent approximately half of the total combination with taxane, a complete response was pharmaceutical world market, the Company has observed, with no detectable tumor after treatment. decided to seek registration in the US as the first All these findings were verified by independent step towards commercialization of phenoxodiol. radiological analysis performed by an expert not To this end, extensive discussions were held with associated with the Company or the Phase II study. the FDA to ensure the phenoxodiol development program would meet the regulatory requirements. The credibility of the data was enhanced by the A significant step was to apply for Fast Track status performance of a subset analysis of the platinum-for phenoxodiol for the treatment of advanced resistant patients to break out the data according chemoresistant ovarian cancer. to time since last platinum therapy, to confirm TGA the effect was phenoxodiol-dependent and not The Fast Track programs of the FDA are designed to a reflection of spontaneous return to sensitivity, facilitate the development and expedite the review which can occur in patients who have been off of new drugs that are intended to treat serious or the platinum therapy for more than 6 months. This life-threatening conditions and that demonstrate analysis confirmed that among patients who were the potential to address unmet medical needs. within 6 months of last treatment, and therefore Strong scientific evidence including clinical data still solidly resistant, 80% demonstrated tumor demonstrating the potential of the drug must stabilization or shrinkage. be provided to the FDA before a drug can be designated for the Fast Track development program. The award of Fast Track status ensured a more rapid review by the FDA of the Phase III OVATURE study protocol and provided an opportunity for more IRBOn the basis of data obtainedfrequent discussions with the FDA. For example,previously in Phase II clinicalMarshall Edwards Inc. recently met with the FDA to discuss preclinical and manufacturing informationstudies of phenoxodiol, the FDAto be provided in the marketing application forgranted Fast Track designation forphenoxodiol.development of phenoxodiol forIECtreating refractory ovarian cancer.4 |

| Contract research organization Since the OVATURE study involves an international In addition, the OVATURE study network of hospitals and oncology clinics,it has protocol was reviewed by the been important to ensure that each site complies FDA under the Special Protocol with good clinical practice (GCP) and are properly Assessment process. qualified to undertake the study,that the personnel fully understand the protocol and the data collec- tion methods, and that international and regionalThese milestones not only confirmed the view that a Phase III program in platinum-resistant ovarianregulatory requirements for the conduct of clinical cancer was a justified next step in the phenoxodioltrials are observed. To this end, the Company drug development program, but also provided aselected Covance as the trial manager,providing more certain regulatory pathway to requestingboth advisory and logistic support to identify the accelerated marketing approval for phenoxodiol. A request for accelerated approval for phenoxodiol60 international site locations,manage contractwill be based on the interim analysis after enroll-negotiations and monitor the conduct of the studyment completion and progression of at least 95at each site. Covance is a well regarded inter-patients demonstrating a significant improvementnational Contract Research Organization (CRO),in PFS of phenoxodiol-treated patients.and its wide reach over international jurisdictions Site approvals and logistics has ensured the study integrity is maintained at all times.Regular meetings are held between theOnce FDA agreement had been achieved on theCompany‘s clinical staff and various coordinatorsPhase III OVATURE study design, the next regulatoryin the Covance organization.step has been to obtain ethical approval to conduct the study at each of the 60 sites. This involved submission of the protocol and patient informedTo ensure strict protocol compliance and properconsent forms for review by an Institutional Reviewtraining of doctors and their clinical staff, Inves-Board (IRB) or Institutional Ethics Committee (IEC)tigator Meetings have been held separately for acting for each site. For sites in Europe, an additional submission was required to obtainAustralian, European and US site personnel. Thegovernment approval to conduct the study, as hadfirst meeting was held in Sydney for Australian sitebeen obtained in the US via the FDA. Once ethicsstaff,followed by meetings in Barcelona for EU/UKapprovals were obtained, usually requiring a reviewsites and Miami for US sites.period of 6-8 weeks, contract negotiations ensued to ensure both the Company and the institutions governing each site have a binding agreement for their respective responsibilities: for the institutions, to maintain accurate records and standards of care and for the Company, to make agreed payments and supply the drug. 5 |
| Drug supplyIn parallel with the selection, qualification and contractual issues with sites, the Company faced a considerable challenge in ensuring that sufficient amounts of phenoxodiol would be available to service the needs of the study, manufactured to appropriate standards of quality control and with approved packaging and distribution arrangements in place. Scale-up of manufacturing has been addressed by prior collaboration with two contract manufacturing facilities, primarily one in Switzerland and the other in the US. Packaging and labeling were required to ensure that the doctors, their staff and their patients, as well as all Company personnel, had no way of knowing whether patients were on active or control treatments. This “double blind” approach is essential to avoid bias in interpretation of patient progress and disease progression. Drug is labeled and distributed to trial sites by a central authority managed by Covance, where computer-generated randomization takes place to ensure a truly random distribution of patients to active and control groups irrespective of their disease state. Caution: New Drug — Limited by US law to investigational use. 6 |
| Data managementThe study outcomes with respect to both safety Data collected must include patient-related data and efficacy will only be of any use in regulatory (demographics, medical history, clinical status submissions, if the data are collected in an at entry, disease status at various time points approved manner and in a form which lends itself throughout study, treatment compliance, endpoint to appropriate statistical analysis. This involves the data, adverse events, concomitant medications, etc.) collection, co-ordination, validation and archiving and protocol-related data (schedule of examinations of data for all global sites with the enforcement and tests, treatment data, etc.). In short, the data of data standards according to international management objective is to ensure data consistency conventions ensuring compliance with International and validity so that patients’ outcomes can be Good Clinical Practice (Worldwide), as well as evaluated and compared to one another, and a FDA (USA), EMEA (Europe) and TGA (Australia) meaningful study report can be written to support requirements. the phenoxodiol marketing application. 7 |

| Financing the programThe cost of the OVATURE study will be in excess of US$25 million. Although this is a modest amount by pharmaceutical industry standards, it is a large expense for Marshall Edwards Inc. The Company had around US$20 million in cash available at the start of the study and the ability to access a further US$15 million from a ‘stand-by equity facility’ it had negotiated during 2006. In August 2007, the ‘stand-by equity facility’ wasPhenoxodiol marketing strategy cancelled and replaced by US$16.4 million in cash received in return for equity, raised from a selected Subject to a positive outcome of the OVATURE study, number of institutional investors. the Company proposes to out-license the sales and marketing of phenoxodiol to a larger and established The OVATURE study is a major commitment for the pharmaceutical company. JPMorgan of New York has Company, and further studies with phenoxodiol and been commissioned to assess the timing and advise the development of other drugs are planned. the Company on this licensing program. The expected financial returns to Marshall Edwards Inc. from the likely milestone payments and salesThe potential benefits toroyalties are anticipated to bring benefits in thethe Company, its share-longer term to all shareholders and patients, andholders and ovarian facilitate the development of more drugs targetedcancer patients from ato specific cancer treatments.successful OVATURE trial are expected to be considerable.8 |

| Program management Professor Alan J HusbandPhD, DSc, FASM Professor Husband leads a talented team of scientists, and medical, clinical and regulatory Professor Husband is Group Director of Research professionals within the Company. The team has for Marshall Edwards, Inc. and the Novogen group enabled the establishment of an active cutting edge of companies, as well as an Executive Director research program to understand the chemistry and on the Board of Novogen. In this position, he is mechanisms of action of phenoxodiol and its related responsible for the day to day management of analogues in biological systems, the application of the OVATURE program including both clinical these to treatment of a range of human cancers and preclinical aspects of the phenoxodiol drug in clinical studies, culminating in the design and development program. Professor Husband has implementation of the Phase III OVATURE Trial. over 30 years experience in basic and applied medical research and research management. In The team is supported by experts in a variety of addition to his role with the Company, he holds a fields, with key collaborators in cancer biology and fractional professorial appointment at the University cancer medicine in top flight institutions around the of Sydney where he has achieved international world. The Ovature program is ably assisted by a recognition for contributions in immunology and Steering Committee of experts in ovarian cancer and pathology, publishing over 200 scientific papers by high profile principal investigators at the various as well as several books. These basic research trial sites, such as Professors Tom Rutherford and activities coupled with extensive experience in Gil Mor at Yale University, Dr David O’Malley of the commercialisation of new technologies in the bio- Ohio State University Medical Center and Dr John technology industry prior to joining the Company, Schorge at University of Texas Southwestern Medical underpin his present position with the Company, Center in the US, Professor Hani Gabra of the a position he has held since 1996. During this time, Imperial College London, in the UK, Professor Ignace Professor Husband has managed the Company’s Vergote, University Hospital Leuven, in Belgium, drug discovery and clinical trial programs. Dr Jose M. Del Campo of the Hospital Vall d’Hebron in Barcelona, Spain, Prof. Marek Spaczynski of the Polozniczy Szpital Kliniczny in Poland, and Professors Michael Quinn and Michael Friedlander respectively from Royal Womens Hospital in Melbourne and Prince of Wales Hospital in Sydney, Australia. 9 |

| References1. Ries LAG, Harkins D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, Clegg L, Eisner MP, Horner MJ, Howlader N, Hayat M, Hankey BF, Edwards BK (eds). SEER Cancer Statistics Review, 1975-2003, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/19752003/, based on November 2005 SEER data submission, posted to the SEER web site, 2006. 2. U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999—2003 Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2007. Available at: www.cdc.gov/uscs. www.marshalledwardsinc.com T 1.202.5186384 |