UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the month of September, 2016
Commission File Number
Novogen Limited
(Translation of registrant’s name into English)
Level 5, 20 George Street, Hornsby, NSW 2077, Australia
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F þ Form 40-F ¨
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ¨
Note: Regulation S-T Rule 101(b)(1) only permits the submission in paper of a Form 6-K if submitted solely to provide an attached annual report to security holders.
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ¨
Note: Regulation S-T Rule 101(b)(7) only permits the submission in paper of a Form 6-K if submitted to furnish a report or other document that the registrant foreign private issuer must furnish and make public under the laws of the jurisdiction in which the registrant is incorporated, domiciled or legally organized (the registrant’s “home country”), or under the rules of the home country exchange on which the registrant’s securities are traded, as long as the report or other document is not a press release, is not required to be and has not been distributed to the registrant’s security holders, and, if discussing a material event, has already been the subject of a Form 6-K submission or other Commission filing on EDGAR.
Indicate by check mark if the registrant by furnishing the information contained in this form is also thereby furnishing the information to the Commission pursuant to Rule 12g3-2(b) under the Securities Exchange Act of 1934. Yes ¨ No þ
If “yes” is marked, indicate below the file number assigned to the registrant in connection with Rule 12g3-2(b)
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Novogen Limited(Registrant)
Kate Hill
Kate Hill
Interim Company Secretary
Date 12 September 2016

ASX:NRT
NASDAQ:NVGN
Novogen Ltd
(Company)
ABN 37 063 259 754
Capital Structure
Ordinary Shares on issue:
429 M
Board of Directors
Mr John O’Connor
Chairman
Non-Executive Director
Mr Bryce Carmine
Deputy Chairman
Non-Executive Director
Dr James Garner
Chief Executive Officer
Managing Director
Mr Ian Phillips MNZM
Non-Executive Director
Mr Iain Ross
Non-Executive Director
Mr Steven Coffey
Non-Executive Director
MARKET RELEASE
12 September 2016
NOVOGEN PRESENTS AT RODMAN & RENSHAW CONFERENCE
Sydney, 12th September 2016 – Australian oncology-focused biotechnology company Novogen Ltd (ASX: NRT; NASDAQ: NVGN) is pleased to release the presentation that CEO, Dr James Garner will be presenting at the Rodman & Renshaw 18th Annual Global Investment Conference being held in New York.
Dr James Garner will be presenting in New York at the Lotte New York Palace Hotel on Monday 12 September at 9:10 am, EDT.
[ENDS]
| | |
Media and Investor Relations | | Investor Relations (US) |
Glen Zurcher E:glen.zurcher@irdepartment.com.au T: +61 420 249 299 | | Robert Kennedy E:robert.kennedy@novogen.com T: +1 212 519 9832 / +1 646 662 3574 |
About Novogen Limited
Novogen Limited (ASX: NRT; NASDAQ: NVGN) is an oncology-focused biotechnology company based in Sydney, Australia. Novogen has two proprietary drug discovery platforms (superbenzopyrans and anti-tropomyosins) with the potential to yield first-in-class agents across a range of oncology indications. The three lead molecules Cantrixil, Anisina, and Trilexium are in preclinical development, with the most advanced molecule, Cantrixil, slated to enter clinical trials in late 2016. For more information, please visit:www.novogen.com


Novogen Limited Presentation to Rodman & Renshaw 18th Annual Global Investment Conference Dr James Garner New York, NY Chief Executive Officer 12 September 2016 james.garner@novogen.com Version 2.4

Forward-Looking Statements
This presentation contains “forward-looking statements ” within the meaning of the “safe-harbor” provisions of the Private Securities Litigation Reform Act of 1995. Such statements involve known and unknown risks, uncertainties and other factors that could cause the actual results of the Company to differ materially from the results expressed or implied by such statements, including changes from anticipated levels of customer acceptance of existing and new products and services and other factors. Accordingly, although the Company believes that the expectations reflected in such forward-looking statements are reasonable, there can be no assurance that such expectations will prove to be correct. The Company has no obligation to sales, future international, national or regional economic and competitive conditions, changes in relationships with customers, access to capital, difficulties in developing and marketing new products and services, marketing existing products and services update the forward-looking information contained in this presentation.
1

Novogen is a biotech company dedicated to driving sustainable, long-term growth in shareholder value
Focus on unmet medical need
Pipeline of novel therapies, targeting oncology patients poorly served by existing treatment options
Clinical stage
Lead molecule entering phase I in Q4 2016, with substantial flow of value-driving milestones over 12-18 months
Financially sound
Listed on ASX and NASDAQ, with cash runway sufficient to drive existing pipeline for at least two years
Strong management and Board
Lean team of internationally-experienced pharma executives, overseen by seasoned Board2
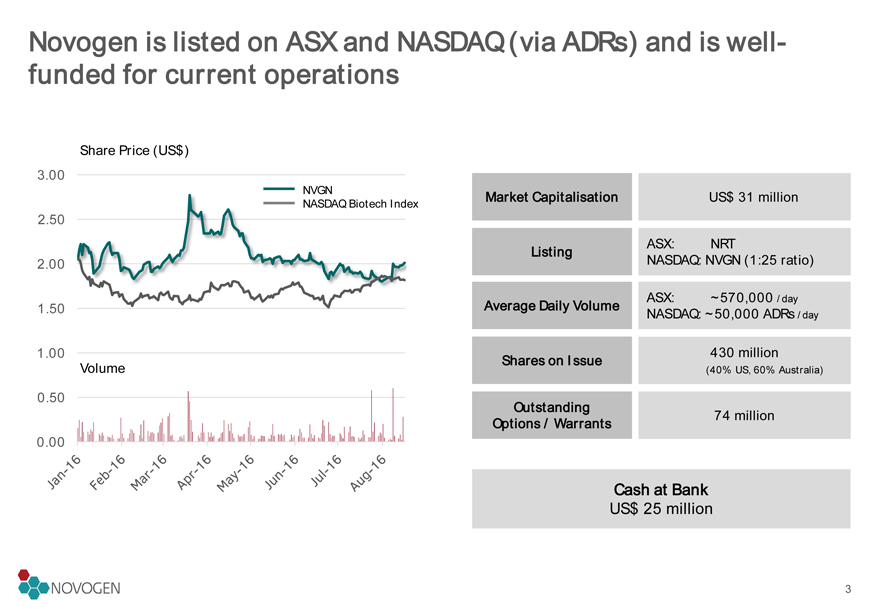
Novogen is listed on ASX and NASDAQ (via ADRs) and is well-funded for current operations
Market Capitalisation
0.00
0.50
1.00
1.50
2.00
2.50
3.00
NVGN
NASDAQ Biotech Index
US$ 31 million
Shares on Issue
430 million (40% US, 60% Australia)
Cash at Bank
US$ 25 million
Listing
ASX: NRT
NASDAQ: NVGN (1:25 ratio)
Share Price (US$)
Outstanding
Options / Warrants
74 million
Average Daily Volume
ASX: ~570,000 /day
NASDAQ: ~50,000 ADRs /day
Volume3
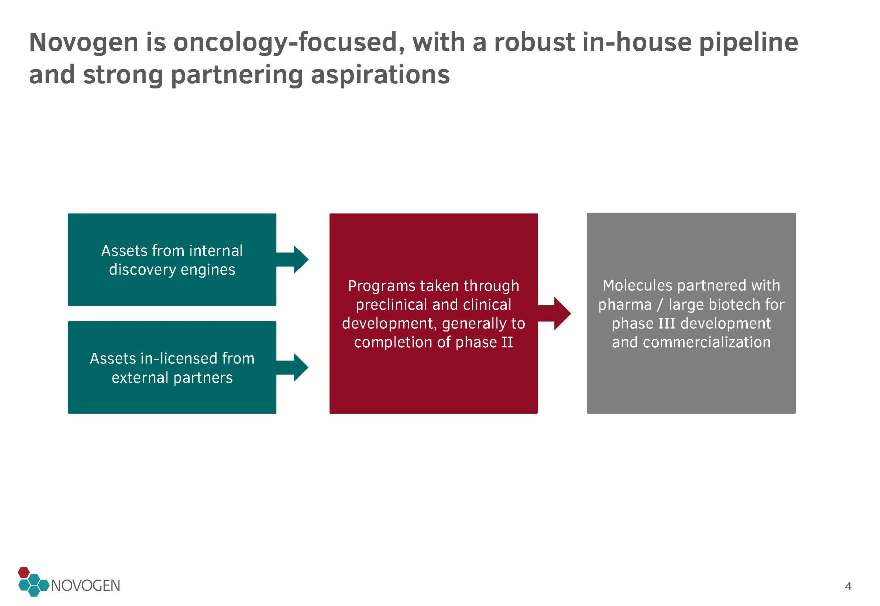
Novogen is oncology-focused, with a robust in-house pipeline and strong partnering aspirations
Assets from internal discovery engines
Programs taken through preclinical and clinical development, generally to completion of phase II
Molecules partnered with pharma / large biotech for phase III development and commercialization
Assets in-licensed from external partners4
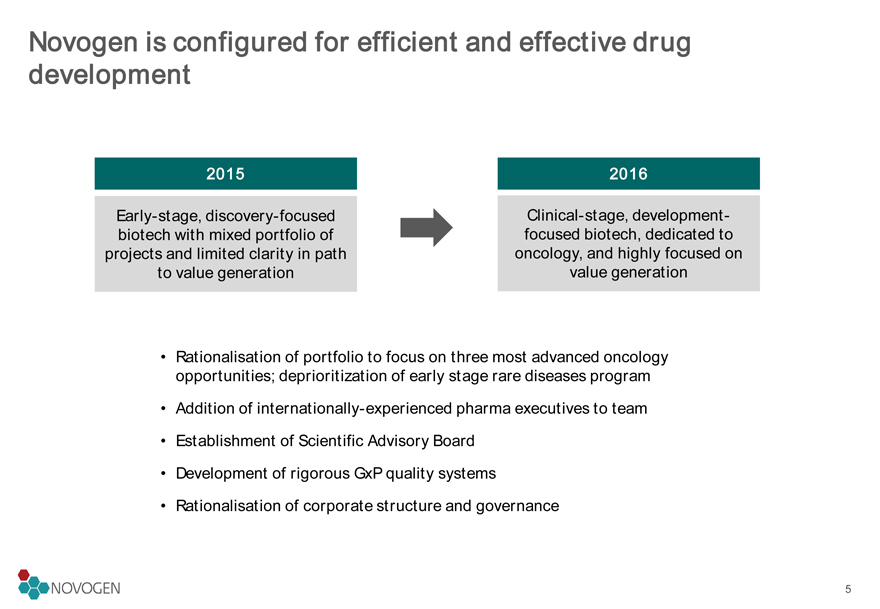
Novogen is configured for efficient and effective drug development
2015
2016
Early-stage, discovery-focused biotech with mixed portfolio of projects and limited clarity in path to value generation
Clinical-stage, development-focused biotech, dedicated to oncology, and highly focused on value generation
Rationalisation of portfolio to focus on three most advanced oncology opportunities; deprioritization of early stage rare diseases program
Addition of internationally-experienced pharma executives to team
Establishment of Scientific Advisory Board
Development of rigorous GxP quality systems
Rationalisation of corporate structure and governance
5

Novogen anticipates a rich news flow of value-driving events over the next 12-18 months 1Q 2016 Granting of patent for SBP technologyü
2Q 2016 Granting of patent for ATM technologyü
3Q 2016 Submission of IND for Cantrixilü
4Q 2016 Start of phase I trial for Cantrixil
2017 Submission of IND for Anisina
2017 Start of phase I trial for Anisina
6

7 Novogen has a strong management team with international experience in big pharma
Dr James Garner
Chief Executive Officer & Managing Director
Physician / MBA; Extensive pharma drug development experience
Dr David Brown
Chief Scientific Officer
Twenty years of drug discovery and development experience
Dr Gordon Hirsch
Chief Medical Officer
Physician / MBA; Twenty years of pharmaceutical industry experience
Dr Peng Leong
Chief Business Officer
Eighteen years of business development and investment banking experience
Dr Andrew Heaton
VP, Drug Discovery
Twenty years of medicinal chemistry experience
Cristyn Humphreys
Chief Financial Officer Chartered accountant with twenty years of experience in corporate roles
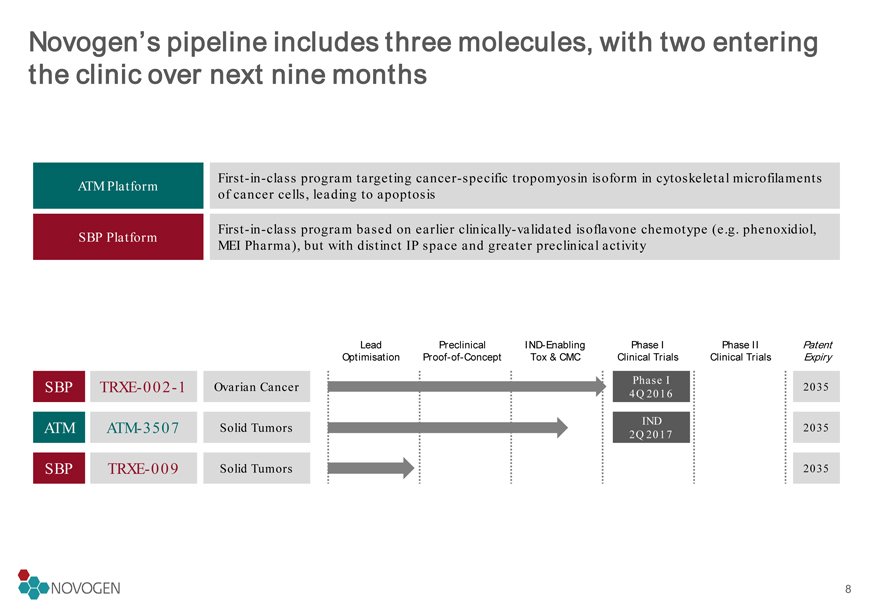
Novogen’s pipeline includes three molecules, with two entering the clinic over next nine months
Lead Optimisation
Preclinical Proof-of-Concept
IND-Enabling Tox & CMC
Phase I Clinical Trials
Phase II Clinical Trials
Patent Expiry
SBP
TRXE-002-1
2035
SBP
TRXE-009
2035 ATM
ATM-3507
2035
ATM Platform
First-in-class program targeting cancer-specific tropomyosin isoform in cytoskeletal microfilaments of cancer cells, leading to apoptosis
SBP Platform
First-in-class program based on earlier clinically-validated isoflavone chemotype (e.g. phenoxidiol, MEI Pharma), but with distinct IP space and greater preclinical activity
Ovarian Cancer
Solid Tumors
Solid Tumors
Phase I
4Q 2016
IND
2Q 20178
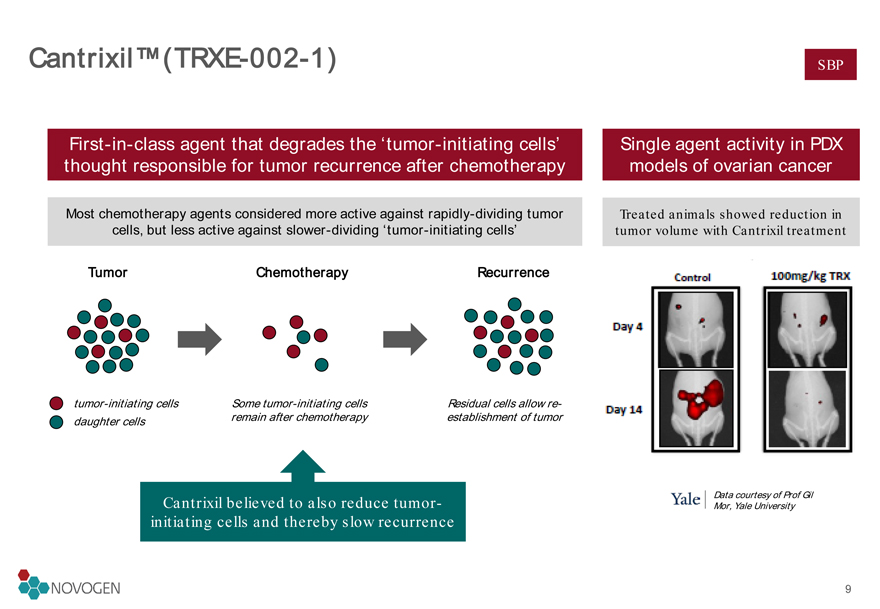
Cantrixil™ (TRXE-002-1)
First-in-class agent that degrades the ‘tumor-initiating cells’ thought responsible for tumor recurrence after chemotherapy
Single agent activity in PDX models of ovarian cancer Most chemotherapy agents considered more active against rapidly-dividing tumor cells, but less active against slower-dividing ‘tumor-initiating cells’
Tumor
tumor-initiating cells
daughter cells
Chemotherapy
Recurrence
Some tumor-initiating cells remain after chemotherapy
Residual cells allow re-establishment of tumor
Cantrixil believed to also reduce tumor-initiating cells and thereby slow recurrence
Treated animals showed reduction in tumor volume with Cantrixil treatment
Data courtesy of Prof Gil Mor, Yale University
SBP9
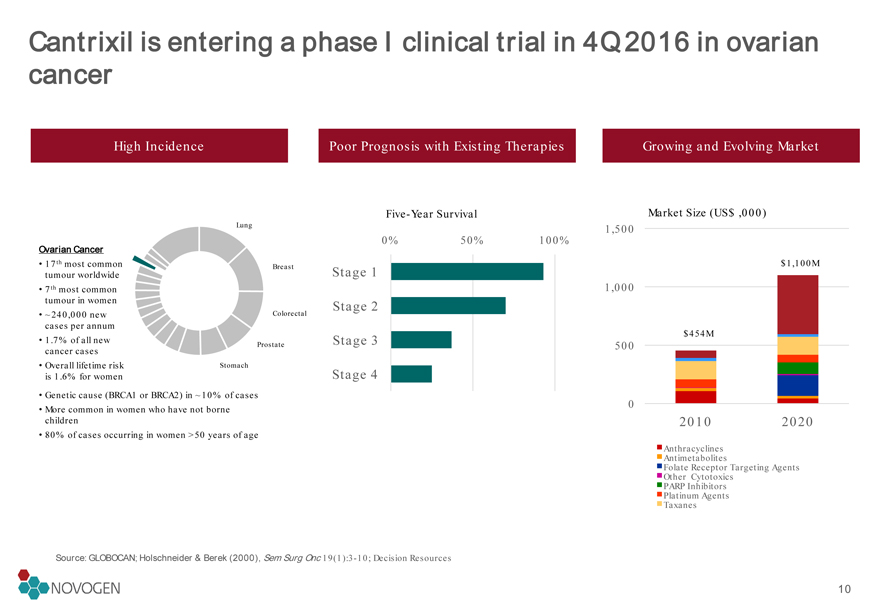
Cantrixil is entering a phase I clinical trial in 4Q 2016 in ovarian cancer
Source: GLOBOCAN; Holschneider & Berek (2000), Sem Surg Onc 19(1):3-10; Decision Resources
High Incidence
Poor Prognosis with Existing Therapies
Growing and Evolving Market Ovarian Cancer
17th most common tumour worldwide
7th most common tumour in women
~240,000 new cases per annum
1.7% of all new cancer cases
Overall lifetime risk is 1.6% for women
Lung
Breast
Colorectal
Prostate
Stomach
0%
50%
100%
Stage 1
Stage 2
Stage 3
Stage 4
Five-Year Survival
Genetic cause (BRCA1 or BRCA2) in ~10% of cases
More common in women who have not borne children
80% of cases occurring in women >50 years of age
0 500
1,000
1,500
2010
2020
Anthracyclines
Antimetabolites
Folate Receptor Targeting Agents
Other Cytotoxics
PARP Inhibitors
Platinum Agents
Taxanes
Market Size (US$ ,000)
$454M $1,100M10
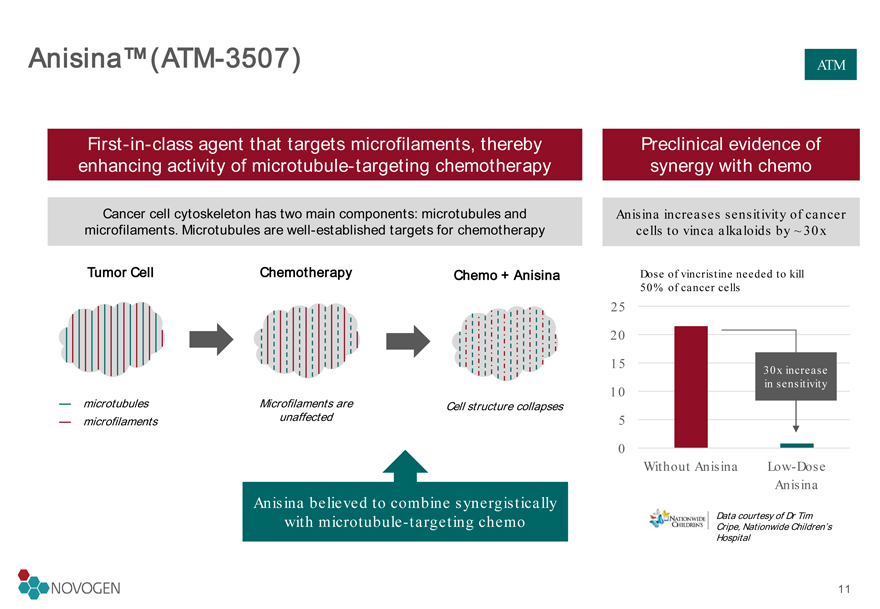
Anisina™ (ATM-3507)
First-in-class agent that targets microfilaments, thereby enhancing activity of microtubule-targeting chemotherapy
Preclinical evidence of synergy with chemo
Cancer cell cytoskeleton has two main components: microtubules and microfilaments. Microtubules are well-established targets for chemotherapy
Tumor Cell
microtubules
microfilaments
Chemotherapy
Microfilaments are unaffected
Anisina increases sensitivity of cancer cells to vinca alkaloids by ~30x
ATM Chemo + Anisina
Cell structure collapses
Anisina believed to combine synergistically with microtubule-targeting chemo
05
10
15
20
25
Without Anisina
Low-Dose Anisina
Dose of vincristine needed to kill 50% of cancer cells
30x increase in sensitivity
Data courtesy of Dr Tim Cripe, Nationwide Children’s Hospital11















