UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the month of October, 2016
Commission File Number
Novogen Limited
(Translation of registrant’s name into English)
Level 5, 20 George Street, Hornsby, NSW 2077, Australia
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F þ Form 40-F ¨
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ¨
Note: Regulation S-T Rule 101(b)(1) only permits the submission in paper of a Form 6-K if submitted solely to provide an attached annual report to security holders.
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ¨
Note: Regulation S-T Rule 101(b)(7) only permits the submission in paper of a Form 6-K if submitted to furnish a report or other document that the registrant foreign private issuer must furnish and make public under the laws of the jurisdiction in which the registrant is incorporated, domiciled or legally organized (the registrant’s “home country”), or under the rules of the home country exchange on which the registrant’s securities are traded, as long as the report or other document is not a press release, is not required to be and has not been distributed to the registrant’s security holders, and, if discussing a material event, has already been the subject of a Form 6-K submission or other Commission filing on EDGAR.
Indicate by check mark if the registrant by furnishing the information contained in this form is also thereby furnishing the information to the Commission pursuant to Rule 12g3-2(b) under the Securities Exchange Act of 1934. Yes ¨ No þ
If “yes” is marked, indicate below the file number assigned to the registrant in connection with Rule 12g3-2(b)
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Novogen Limited(Registrant)
Kate Hill
Kate Hill
Interim Company Secretary
Date 31 October 2016
 NOVOGEN
NOVOGEN
Novogen Limited
Investor Presentation
In-licensing of phase II ready molecule (GDC-0084) from Genentech for treatment of brain cancer (Glioblastoma)
31st October 2016
Version 1.0

Forward-Looking Statements
This presentation contains “forward-looking statements” within the meaning of the “safe-harbor” provisions of the Private Securities Litigation Reform Act of 1995. Such statements involve known and unknown risks, uncertainties and other factors that could cause the actual results of the Company to differ materially from the results expressed or implied by such statements, including changes from anticipated levels of customer acceptance of existing and new products and services and other factors. Accordingly, although the Company believes that the expectations reflected in such forward-looking statements are reasonable, there can be no assurance that such expectations will prove to be correct. The Company has no obligation to sales, future international, national or regional economic and competitive conditions, changes in relationships with customers, access to capital, difficulties in developing and marketing new products and services, marketing existing products and services update the forward-looking information contained in this presentation.
NOVOGEN
1

Novogen now has a diversified portfolio and is positioned for growth
• Focus on unmet need: pipeline of novel therapies, targeting oncology patients, poorly served by existing treatment options
• Building a sustainable model: leveraging oncology expertise, developing commercially attractive, in-house and external assets
• Diversified portfolio:
• Multiple assets in various stages of development – from pre-clinical through to phase II-ready
• Across technologies / development platforms
• Strong management and board: lean team of internationally-experienced pharma executives
• Financially sound: listed on ASX and NASDAQ, with cash runway
• News flow: substantial flow of value-driving milestones over 12-18 months
NOVOGEN
2
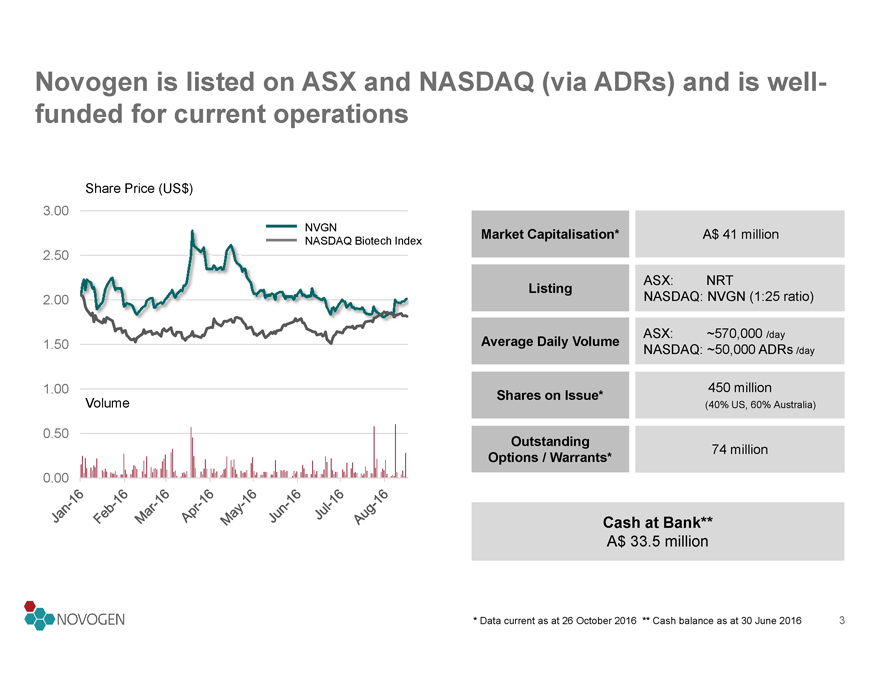
Novogen is listed on ASX and NASDAQ (via ADRs) and is well-funded for current operations
Share Price (US$)
3.00
NVGN
NASDAQ Biotech Index
2.50
2.00
1.50
1.00
Volume
0.50
0.00
Jan-16 Feb-16 Mar-16 Apr-16 May-16 Jun-16 Jul-16 Aug-16
Market Capitalisation*A$ 41 million
Listing ASX: NRT
NASDAQ: NVGN (1:25 ratio)
Average Daily Volume ASX: ~570,000 /day
NASDAQ: ~50,000 ADRs /day
Shares on Issue* 450 million
(40% US, 60% Australia)
Outstanding
Options / Warrants* 74 million
Cash at Bank**
A$ 33.5 million
* Data current as at 26 October 2016 ** Cash balance as at 30 June 2016
NOVOGEN
3

In-licensing of GDC-0084, a promising phase II-ready, well-differentiated drug candidate for the treatment of advanced brain cancer
• GDC-0084 has been in-licensed from Genentech
• Being developed to treat the most common form of primary brain cancer, Glioblastoma Multiforme (GBM)
• Pan-PI3 Kinase inhibitor with some mTOR activity
• Sound rationale for inhibitors of PI3 Kinase as a target for treating GBM: 80-90% of GBM cases have disordered PI3 Kinase
• Designed to cross the blood-brain barrier: a critical success factor for GBM therapies, and not true of other drug candidates
• Clinical data suggestive of activity in a very treatment-resistant Phase I clinical trial population; safety profile consistent with other agents in the class
NOVOGEN
4

GDC-0084: transaction deal terms
• Exclusive worldwide development and commercialisation agreement for all uses
• US $5M upfront, payable at time of contract signing
• Performance-related consideration linked to regulatory and commercial outcomes
• Royalty payments as a percentage of net sales, in-line with industry benchmarks
• Novogen assumes full responsibility for development, commercialisation and maintenance of intellectual property
NOVOGEN
5
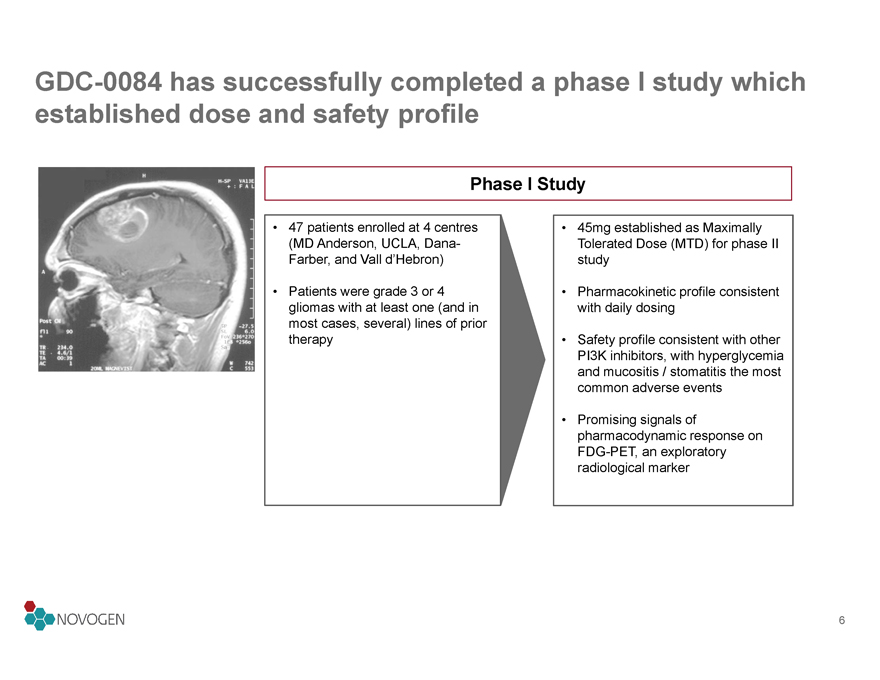
GDC-0084 has successfully completed a phase I study which established dose and safety profile
Phase I Study
• 47 patients enrolled at 4 centres (MD Anderson, UCLA, Dana-Farber, and Vall d’Hebron)
• Patients were grade 3 or 4 gliomas with at least one (and in most cases, several) lines of prior therapy
• 45mg established as Maximally Tolerated Dose (MTD) for phase II study
• Pharmacokinetic profile consistent with daily dosing
• Safety profile consistent with other PI3K inhibitors, with hyperglycemia and mucositis / stomatitis the most common adverse events
• Promising signals of pharmacodynamic response on FDG-PET, an exploratory radiological marker
NOVOGEN
6
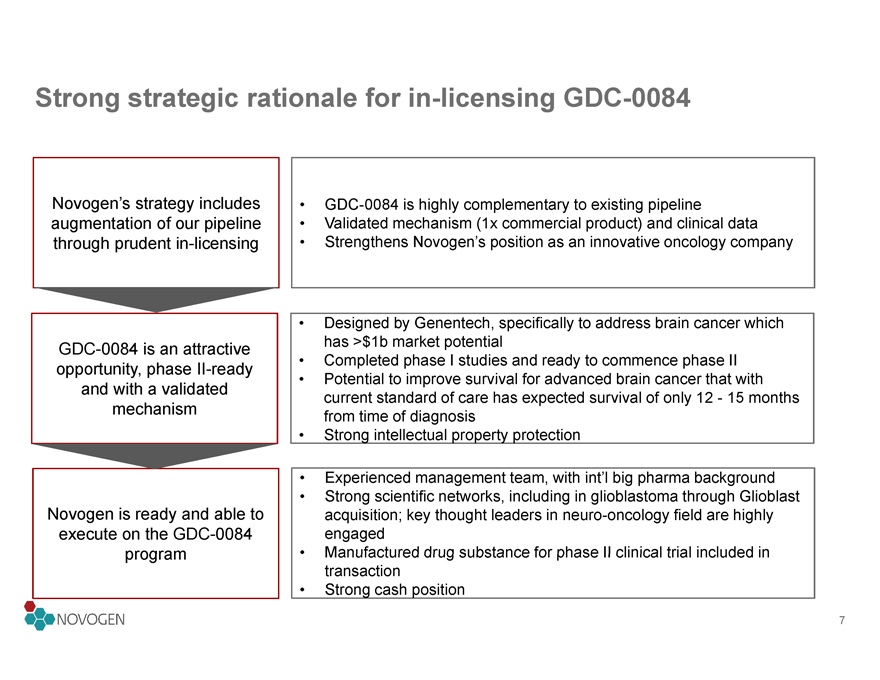
Strong strategic rationale for in-licensing GDC-0084
Novogen’s strategy includes
• GDC-0084 is highly complementary to existing pipeline
augmentation of our pipeline
• Validated mechanism (1x commercial product) and clinical data
through prudent in-licensing
• Strengthens Novogen’s position as an innovative oncology company
• Designed by Genentech, specifically to address brain cancer which
GDC-0084 is an attractive
has >$1b market potential
opportunity, phase II-ready
• Completed phase I studies and ready to commence phase II
• Potential to improve survival for advanced brain cancer that with
and with a validated
current standard of care has expected survival of only 12 - 15 months
mechanism
from time of diagnosis
• Strong intellectual property protection
• Experienced management team, with int’l big pharma background
• Strong scientific networks, including in glioblastoma through Glioblast
Novogen is ready and able to
acquisition; key thought leaders in neuro-oncology field are highly
execute on the GDC-0084
engaged
program
• Manufactured drug substance for phase II clinical trial included in
transaction
• Strong cash position
NOVOGEN
7
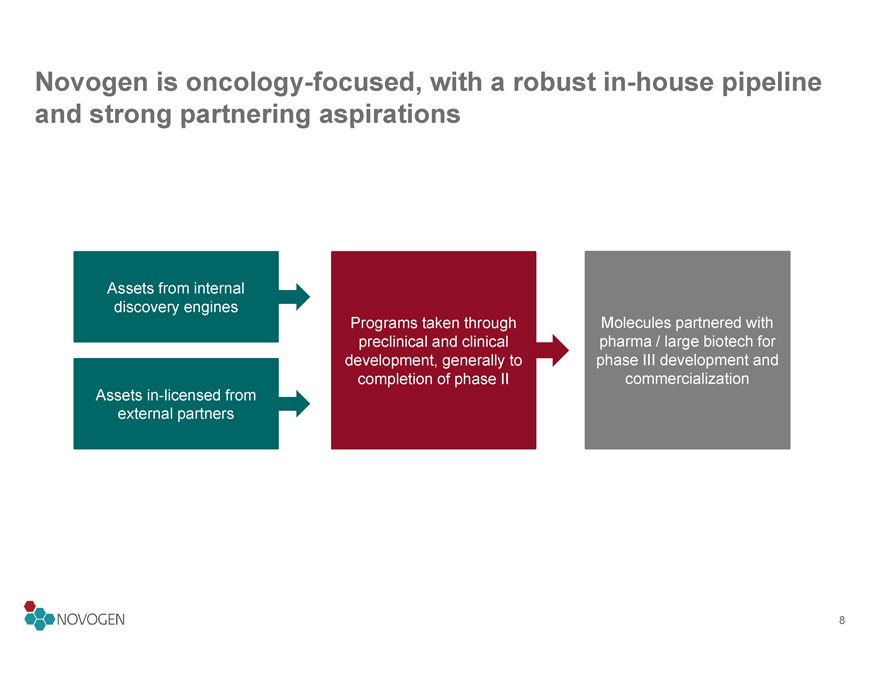
Novogen is oncology-focused, with a robust in-house pipeline and strong partnering aspirations
Assets from internal
discovery engines
Programs taken through
Molecules partnered with
preclinical and clinical
pharma / large biotech for
development, generally to
phase III development and
completion of phase II
commercialization
Assets in-licensed from
external partners
NOVOGEN
8
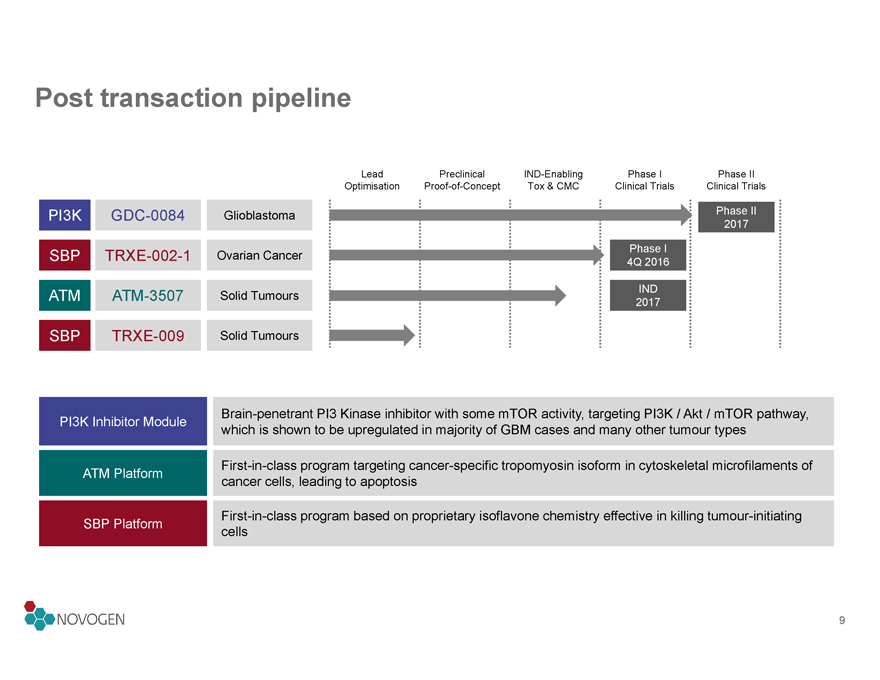
Post transaction pipeline
Lead
Preclinical
IND-Enabling
Phase I
Phase II
Optimisation
Proof-of-Concept
Tox & CMC
Clinical Trials
Clinical Trials
PI3K
GDC-0084
Glioblastoma
Phase II
2017
SBP
TRXE-002-1
Ovarian Cancer
Phase I
4Q 2016
ATM
ATM-3507
Solid Tumours
IND
2017
SBP
TRXE-009
Solid Tumours
PI3K Inhibitor Module
Brain-penetrant PI3 Kinase inhibitor with some mTOR activity, targeting PI3K / Akt / mTOR pathway,
which is shown to be upregulated in majority of GBM cases and many other tumour types
First-in-class program targeting cancer-specific tropomyosin isoform in cytoskeletal microfilaments of
ATM Platform
cancer cells, leading to apoptosis
SBP Platform
First-in-class program based on proprietary isoflavone chemistry effective in killing tumour-initiating
cells
NOVOGEN
9

Novogen anticipates a rich news flow of value-driving events over the next 12-18 months
Key Milestones
1Q
2016
Granting of patent for SBP technology ?
2Q
2016
Granting of patent for ATM technology ?
3Q 2016 Submission of IND for Cantrixil ? 4Q 2016 In-licensing of GDC-0084 ? Start of phase I trial for Cantrixil 2017 Submission of IND for Anisina Start of phase II trial for GDC-0084 Start of phase I trial for Anisina NOVOGEN 10

Recap: diversified portfolio, positioned for growth
• Focus on unmet need: pipeline of novel therapies, targeting oncology patients, poorly served by existing treatment options
• Building a sustainable model: leveraging oncology expertise, developing commercially attractive, in-house and external assets
• Diversified portfolio:
• Multiple assets in various stages of development – from pre-clinical through to phase II-ready
• Across technologies / development platforms
• Strong management and board: lean team of internationally-experienced pharma executives
• Financially sound: listed on ASX and NASDAQ, with cash runway
• News flow: substantial flow of value-driving milestones over 12-18 months
NOVOGEN
11

NOVOGEN
 NOVOGEN
NOVOGEN










