UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the month of June, 2022
Commission File Number
Kazia Therapeutics Limited
(Translation of registrant’s name into English)
Three International Towers Level 24 300 Barangaroo Avenue Sydney NSW 2000
(Address of principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F ☑ Form 40-F ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ☐
Note: Regulation S-T Rule 101(b)(1) only permits the submission in paper of a Form 6-K if submitted solely to provide an attached annual report to security holders.
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ☐
Note: Regulation S-T Rule 101(b)(7) only permits the submission in paper of a Form 6-K if submitted to furnish a report or other document that the registrant foreign private issuer must furnish and make public under the laws of the jurisdiction in which the registrant is incorporated, domiciled or legally organized (the registrant’s “home country”), or under the rules of the home country exchange on which the registrant’s securities are traded, as long as the report or other document is not a press release, is not required to be and has not been distributed to the registrant’s security holders, and, if discussing a material event, has already been the subject of a Form 6-K submission or other Commission filing on EDGAR.
Indicate by check mark if the registrant by furnishing the information contained in this form is also thereby furnishing the information to the Commission pursuant to Rule 12g3-2(b) under the Securities Exchange Act of 1934. Yes ☐ No ☑
If “yes” is marked, indicate below the file number assigned to the registrant in connection with Rule 12g3-2(b)
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Kazia Therapeutics Limited (Registrant)
Kate Hill
Kate Hill
Company Secretary
Date: 23 June 2022

ASX RELEASE
23 June 2022
KAZIA EDUCATIONAL WEBINAR ON PAXALISIB IN CHILDHOOD BRAIN CANCER
Sydney, 23 June 2022 – Kazia Therapeutics Limited (NASDAQ: KZIA; ASX: KZA), an oncology-focused drug development company, is pleased to provide a presentation on paxalisib in childhood brain cancer, featuring Dr James Garner, Associate Professor Matt Dun and Dr John Friend. A copy of the webinar will be made available in the media section of our website.
https://www.kaziatherapeutics.com/site/media-centre/overview
For More Information, Please Contact:-
| | |
In the United States: Joe Green Edison Investor Relations jgreen@edisongroup.com Phone: +1 646-653-7030 | | In Australia: Jane Lowe IR Department jane.lowe@irdepartment.com.au Phone: +61 411 117 774 |
About Kazia Therapeutics Limited
Kazia Therapeutics Limited (NASDAQ: KZIA; ASX: KZA) is an oncology-focused drug development company, based in Sydney, Australia.
Our lead program is paxalisib, a brain-penetrant inhibitor of the PI3K / Akt / mTOR pathway, which is being developed to treat glioblastoma, the most common and most aggressive form of primary brain cancer in adults. Licensed from Genentech in late 2016, paxalisib commenced recruitment to GBM AGILE, a pivotal study in glioblastoma, in January 2021. Seven additional studies are active in various forms of brain cancer. Paxalisib was granted Orphan Drug Designation for glioblastoma by the US FDA in February 2018, and Fast Track Designation for glioblastoma by the US FDA in August 2020. In addition, paxalisib was granted Rare Pediatric Disease Designation and Orphan Designation by the US FDA for DIPG in August 2020, and Orphan Designation for AT/RT in June 2022.
Kazia is also developing EVT801, a small-molecule inhibitor of VEGFR3, which was licensed from Evotec SE in April 2021. Preclinical data has shown EVT801 to be active against a broad range of tumour types and has provided compelling evidence of synergy with immuno-oncology agents. A phase I study commenced recruitment in November 2021.
For more information, please visit www.kaziatherapeutics.com or follow us on Twitter @KaziaTx.
This document was authorized for release to the ASX by James Garner, Chief Executive Officer, Managing Director.

Paxalisib in Childhood Brain Cancer Educational Webinar 22 June 2022 ASX: KZA | NASDAQ: KZIA | Twitter: @KaziaTx

Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the safe-harbor provisions of the Private Securities Litigation Reform Act of 1995. Such statements involve substantial risks and uncertainties, not all of which may be known at the time. All statements contained in this presentation, other than statements of historical fact, including statements regarding our strategy, research and development plans, collaborations, future operations, future financial position, future revenues, projected costs, prospects, plans, and objectives of management, are forward-looking statements. Not all forward-looking statements in this presentation are explicitly identified as such. Many factors could cause the actual results of the Company to differ materially from the results expressed or implied herein, and you should not place undue reliance on the forward-looking statements. Factors which could change the Company’s expected outcomes include, without limitation, our ability to: advance the development of our programs, and to do so within any timelines that may be indicated herein; the safety and efficacy of our drug development candidates; our ability to replicate experimental data; the ongoing validity of patents covering our drug development candidates, and our freedom to operate under third party intellectual property; our ability to obtain necessary regulatory approvals; our ability to enter into and maintain partnerships, collaborations, and other business relationships necessary to the progression of our drug development candidates; the timely availability of necessary capital to pursue our business objectives; and our ability to attract and retain qualified personnel; changes from anticipated levels of customer acceptance of existing and new products and services and other factors. Although the Company believes that the expectations reflected in such forward-looking statements are reasonable, there can therefore be no assurance that such expectations will prove to be correct. The Company has no obligation as a result of this presentation to clinical trial outcomes, sales, partnerships, future international, national or regional economic and competitive conditions, changes in relationships with customers, access to capital, difficulties in developing and marketing new products and services, or marketing existing products. In addition, the extent to which the COVID-19 outbreak continues to impact our workforce and our discovery research, supply chain and clinical trial operations activities, and the operations of the third parties on which we rely, will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the duration and severity of the outbreak, additional or modified government actions, and the actions that may be required to contain the virus or treat its impact. Any forward-looking statements contained in this presentation speak only as of the date this presentation is made, and we expressly disclaim any obligation to update any forward-looking statements, whether because of new information, future events or otherwise. 1

Dr James Garner Associate Professor Matt Dun Dr John Friend Chief Medical Officer Chief Executive Officer Group Leader, Cancer Research Signalling Group Kazia Therapeutics Limited Hunter Medical Research Institute Kazia Therapeutics Limited University of Newcastle, Australia 2

Agenda • Strategic considerations in development of drugs for childhood brain cancer Dr James Garner • Emerging data for paxalisib in DIPG Associate Professor Matt Dun • Emerging data for paxalisib in AT/RT Dr John Friend • Overview of Kazia’s childhood brain cancer program Dr John Friend • Closing Comments + Q&A 3

Agenda • Strategic considerations in development of drugs for childhood brain cancer Dr James Garner • Emerging data for paxalisib in DIPG Associate Professor Matt Dun • Emerging data for paxalisib in AT/RT Dr John Friend • Overview of Kazia’s childhood brain cancer program Dr John Friend • Closing Comments + Q&A 4
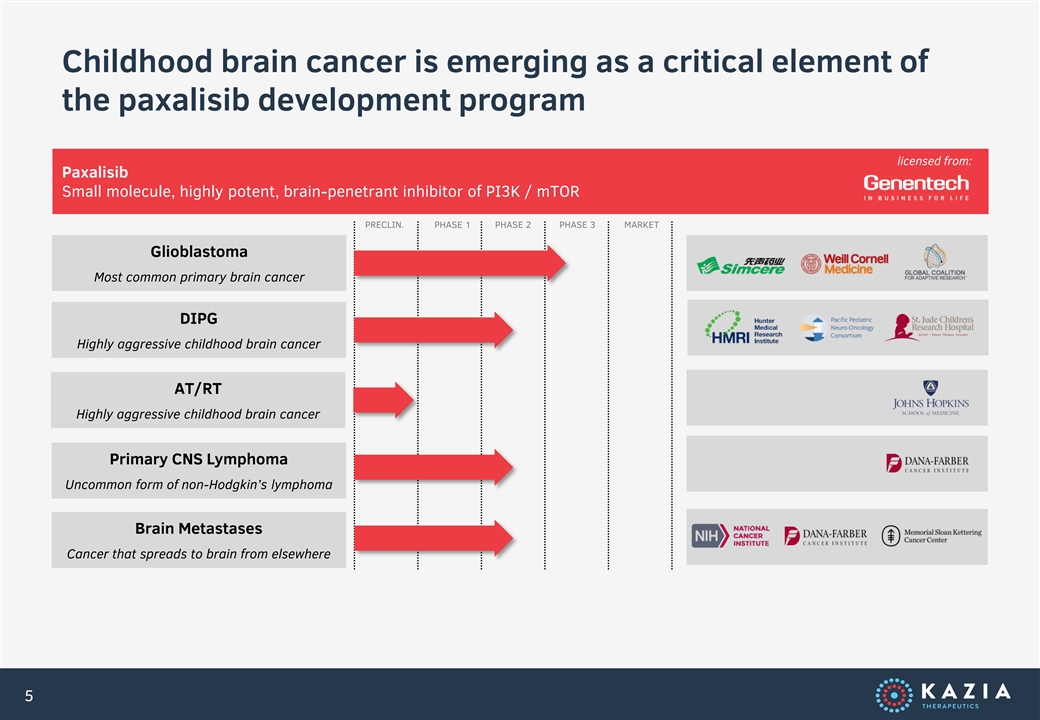
Childhood brain cancer is emerging as a critical element of the paxalisib development program licensed from: Paxalisib Small molecule, highly potent, brain-penetrant inhibitor of PI3K / mTOR PRECLIN. PHASE 1 PHASE 2 PHASE 3 MARKET Glioblastoma Most common primary brain cancer DIPG Highly aggressive childhood brain cancer AT/RT Highly aggressive childhood brain cancer Primary CNS Lymphoma Uncommon form of non-Hodgkin’s lymphoma Brain Metastases Cancer that spreads to brain from elsewhere 5
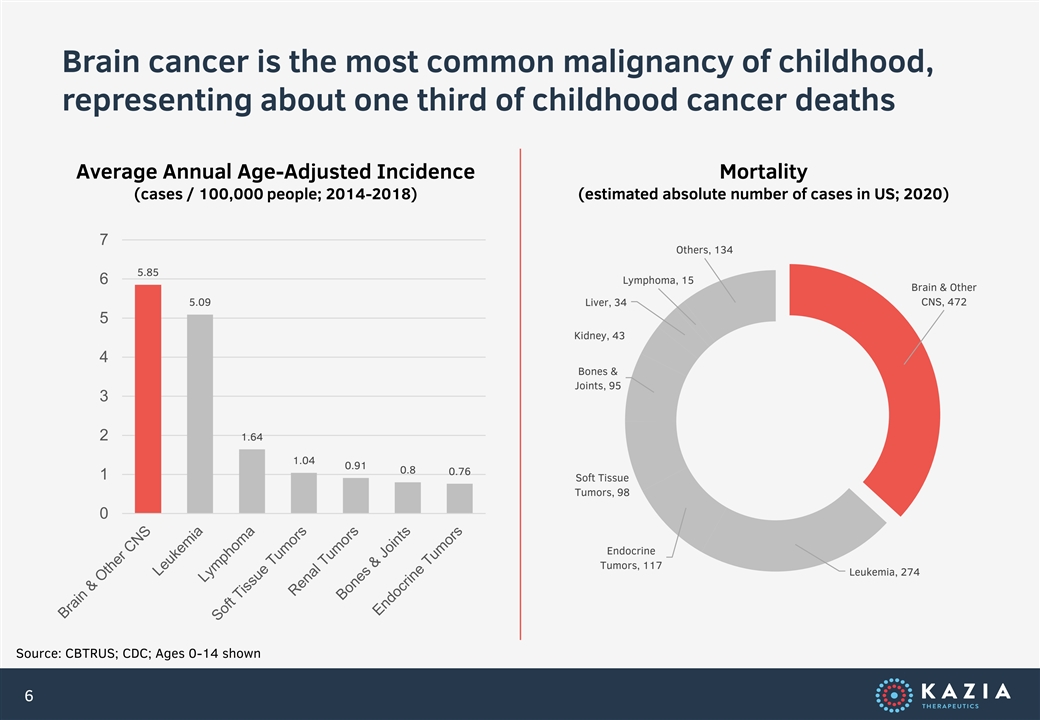
Brain cancer is the most common malignancy of childhood, representing about one third of childhood cancer deaths Average Annual Age-Adjusted Incidence Mortality (cases / 100,000 people; 2014-2018) (estimated absolute number of cases in US; 2020) 7 Others, 134 5.85 6 Lymphoma, 15 Brain & Other CNS, 472 5.09 Liver, 34 5 Kidney, 43 4 Bones & Joints, 95 3 2 1.64 1.04 0.91 0.8 0.76 1 Soft Tissue Tumors, 98 0 Endocrine Tumors, 117 Leukemia, 274 Source: CBTRUS; CDC; Ages 0-14 shown 6
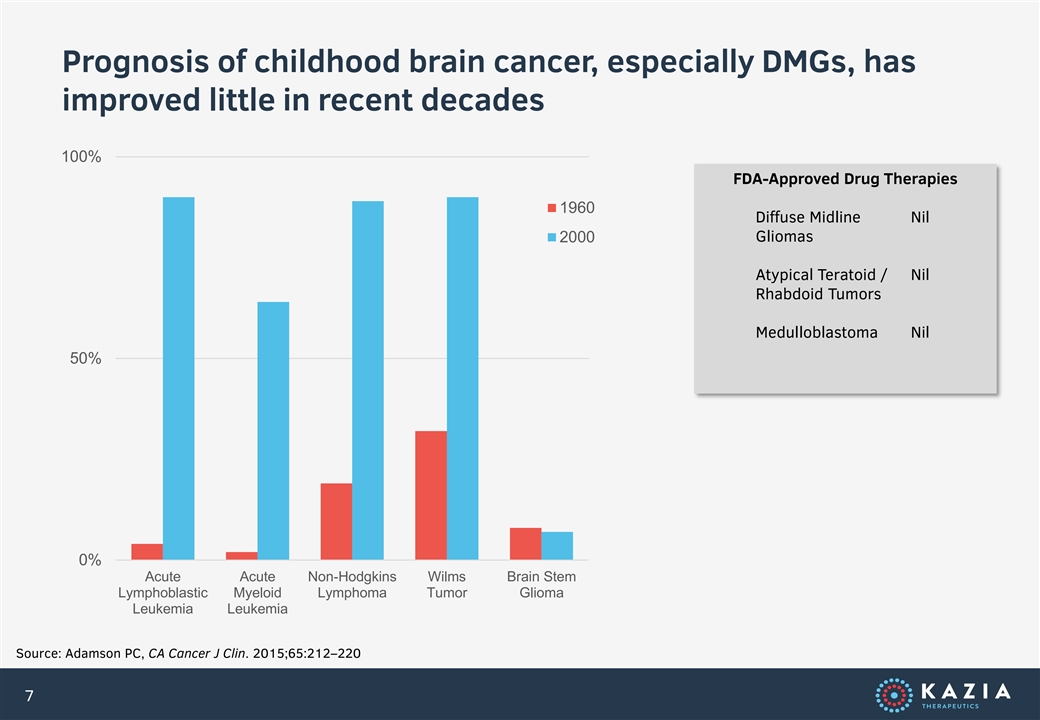
Prognosis of childhood brain cancer, especially DMGs, has improved little in recent decades 100% FDA-Approved Drug Therapies 1960 Diffuse Midline Nil Gliomas 2000 Atypical Teratoid / Nil Rhabdoid Tumors Medulloblastoma Nil 50% 0% Acute Acute Non-Hodgkins Wilms Brain Stem Lymphoblastic Myeloid Lymphoma Tumor Glioma Leukemia Leukemia Source: Adamson PC, CA Cancer J Clin. 2015;65:212–220 7
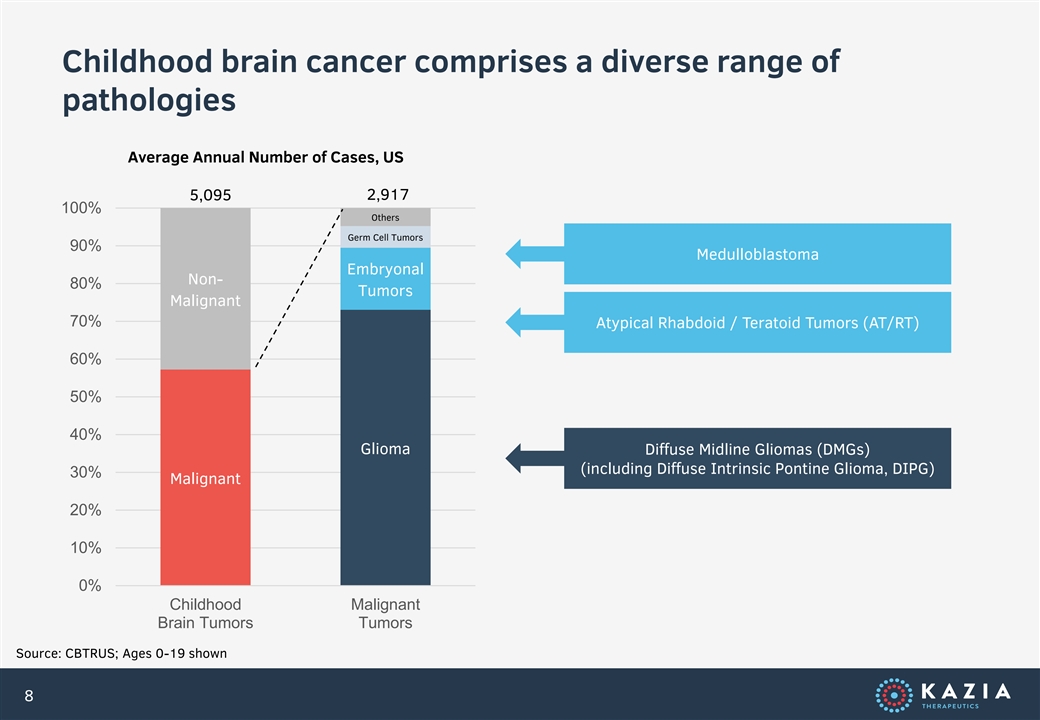
Childhood brain cancer comprises a diverse range of pathologies Average Annual Number of Cases, US 5,095 2,917 100% Others Germ Cell Tumors 90% Medulloblastoma Embryonal Non- 80% Tumors Malignant 70% Atypical Rhabdoid / Teratoid Tumors (AT/RT) 60% 50% 40% Glioma Diffuse Midline Gliomas (DMGs) (including Diffuse Intrinsic Pontine Glioma, DIPG) 30% Malignant 20% 10% 0% Childhood Malignant Brain Tumors Tumors Source: CBTRUS; Ages 0-19 shown 8
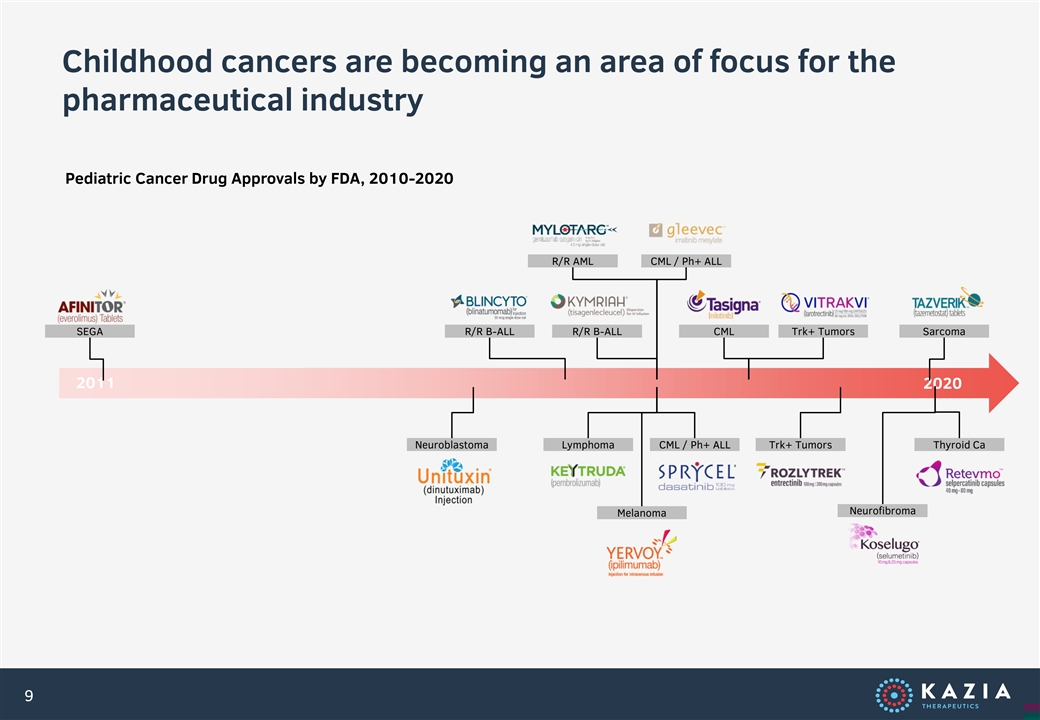
Childhood cancers are becoming an area of focus for the pharmaceutical industry Pediatric Cancer Drug Approvals by FDA, 2010-2020 R/R AML CML / Ph+ ALL SEGA R/R B-ALL R/R B-ALL CML Trk+ Tumors Sarcoma 2011 2020 Neuroblastoma Lymphoma CML / Ph+ ALL Trk+ Tumors Thyroid Ca Neurofibroma Melanoma 9
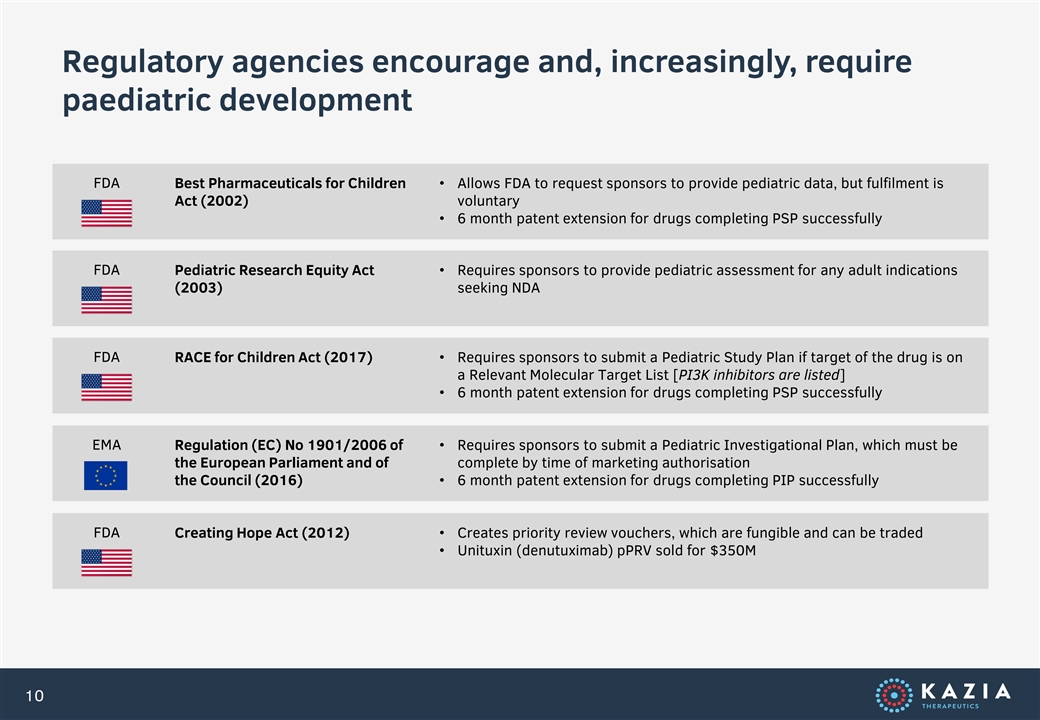
Regulatory agencies encourage and, increasingly, require paediatric development FDA Best Pharmaceuticals for Children • Allows FDA to request sponsors to provide pediatric data, but fulfilment is Act (2002) voluntary • 6 month patent extension for drugs completing PSP successfully FDA Pediatric Research Equity Act • Requires sponsors to provide pediatric assessment for any adult indications (2003) seeking NDA FDA RACE for Children Act (2017)• Requires sponsors to submit a Pediatric Study Plan if target of the drug is on a Relevant Molecular Target List [PI3K inhibitors are listed] • 6 month patent extension for drugs completing PSP successfully EMA Regulation (EC) No 1901/2006 of • Requires sponsors to submit a Pediatric Investigational Plan, which must be the European Parliament and of complete by time of marketing authorisation the Council (2016)• 6 month patent extension for drugs completing PIP successfully FDA Creating Hope Act (2012)• Creates priority review vouchers, which are fungible and can be traded • Unituxin (denutuximab) pPRV sold for $350M 10

Agenda • Strategic considerations in development of drugs for childhood brain cancer Dr James Garner • Emerging data for paxalisib in DIPG Associate Professor Matt Dun • Emerging data for paxalisib in AT/RT Dr John Friend • Overview of Kazia’s childhood brain cancer program Dr John Friend • Closing Comments + Q&A 11
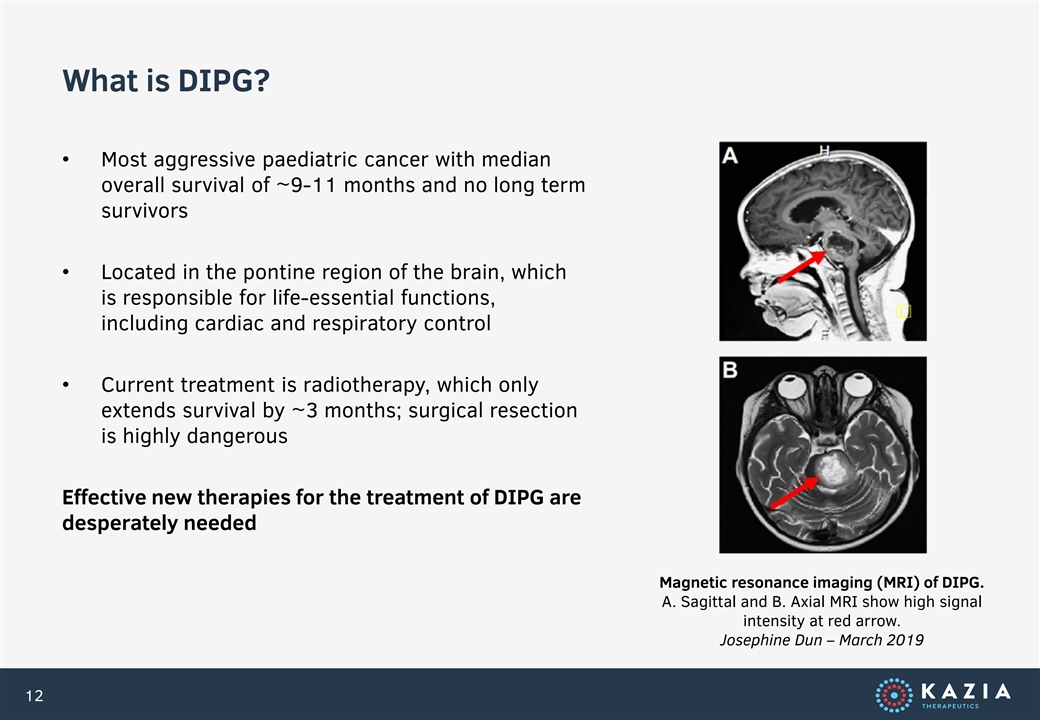
What is DIPG? • Most aggressive paediatric cancer with median overall survival of ~9-11 months and no long term survivors • Located in the pontine region of the brain, which is responsible for life-essential functions, including cardiac and respiratory control • Current treatment is radiotherapy, which only extends survival by ~3 months; surgical resection is highly dangerous Effective new therapies for the treatment of DIPG are desperately needed Magnetic resonance imaging (MRI) of DIPG. A. Sagittal and B. Axial MRI show high signal intensity at red arrow. Josephine Dun – March 2019 12
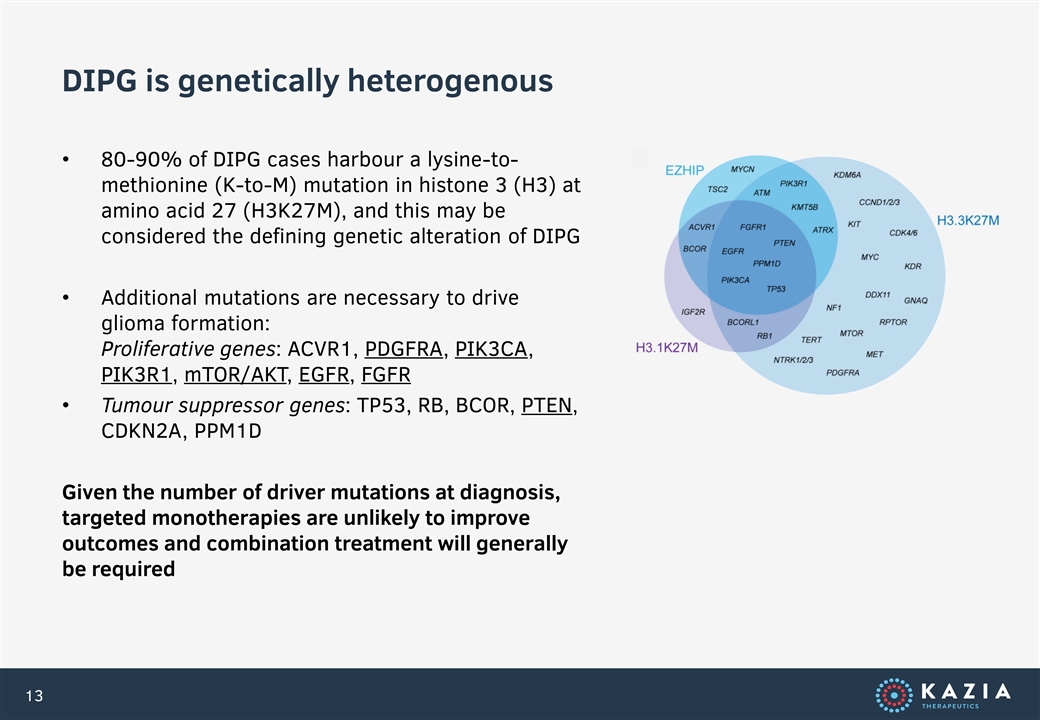
DIPG is genetically heterogenous • 80-90% of DIPG cases harbour a lysine-to- methionine (K-to-M) mutation in histone 3 (H3) at amino acid 27 (H3K27M), and this may be considered the defining genetic alteration of DIPG • Additional mutations are necessary to drive glioma formation: Proliferative genes: ACVR1, PDGFRA, PIK3CA, PIK3R1, mTOR/AKT, EGFR, FGFR • Tumour suppressor genes: TP53, RB, BCOR, PTEN, CDKN2A, PPM1D Given the number of driver mutations at diagnosis, targeted monotherapies are unlikely to improve outcomes and combination treatment will generally be required 13
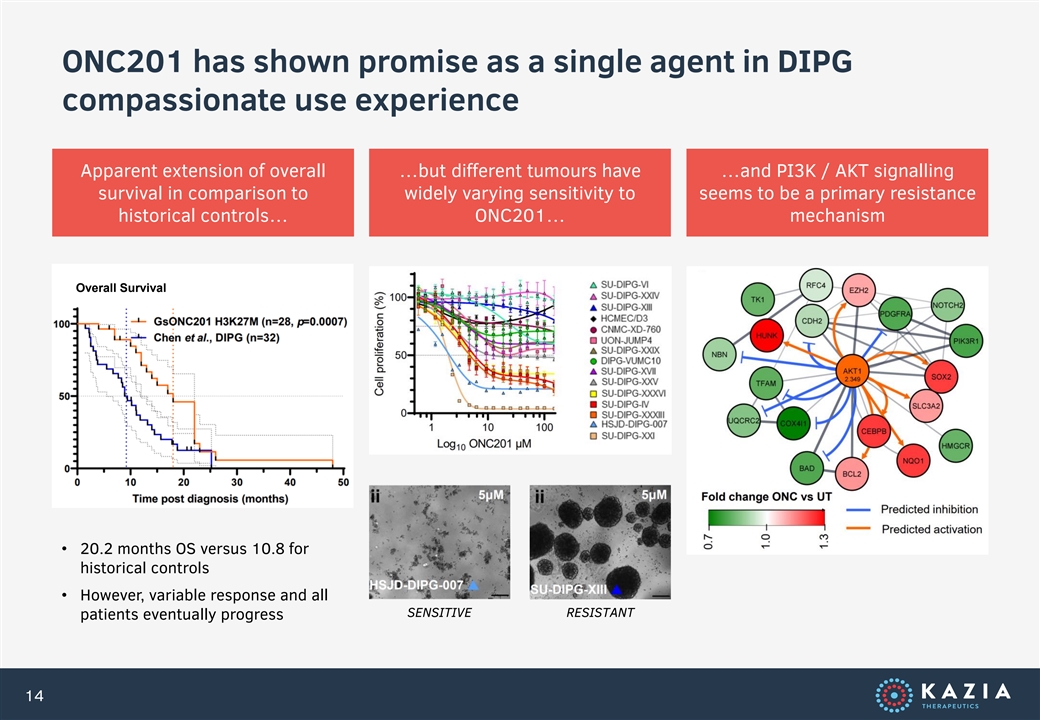
ONC201 has shown promise as a single agent in DIPG compassionate use experience Apparent extension of overall …but different tumours have …and PI3K / AKT signalling survival in comparison to widely varying sensitivity to seems to be a primary resistance mechanism historical controls… ONC201… Overall Survival • 20.2 months OS versus 10.8 for historical controls • However, variable response and all SENSITIVE RESISTANT patients eventually progress 14
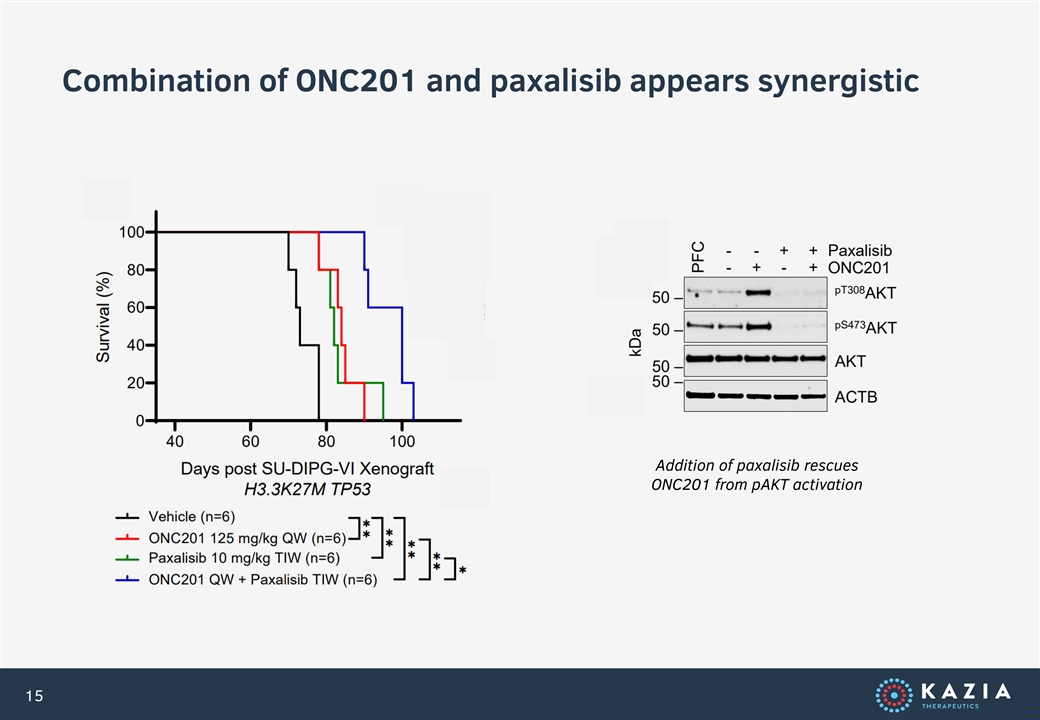
Combination of ONC201 and paxalisib appears synergistic Addition of paxalisib rescues ONC201 from pAKT activation 15
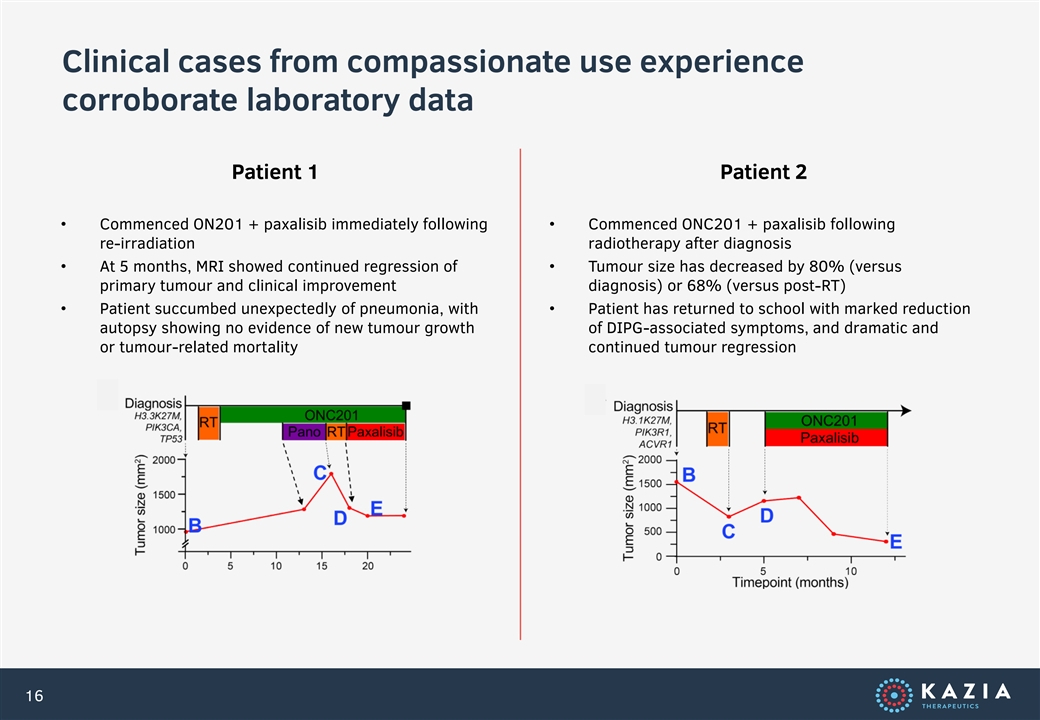
Clinical cases from compassionate use experience corroborate laboratory data Patient 1 Patient 2 • Commenced ON201 + paxalisib immediately following • Commenced ONC201 + paxalisib following re-irradiation radiotherapy after diagnosis • At 5 months, MRI showed continued regression of • Tumour size has decreased by 80% (versus primary tumour and clinical improvement diagnosis) or 68% (versus post-RT) • Patient succumbed unexpectedly of pneumonia, with • Patient has returned to school with marked reduction autopsy showing no evidence of new tumour growth of DIPG-associated symptoms, and dramatic and or tumour-related mortality continued tumour regression 16
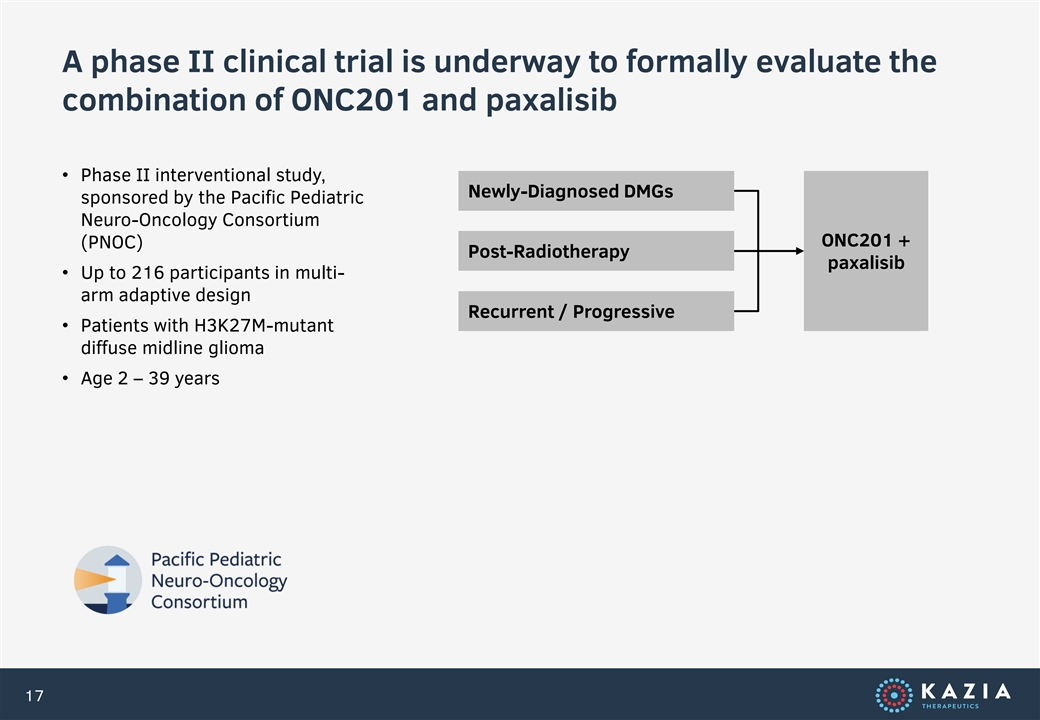
A phase II clinical trial is underway to formally evaluate the combination of ONC201 and paxalisib • Phase II interventional study, Newly-Diagnosed DMGs sponsored by the Pacific Pediatric Neuro-Oncology Consortium ONC201 + (PNOC) Post-Radiotherapy paxalisib • Up to 216 participants in multi- arm adaptive design Recurrent / Progressive • Patients with H3K27M-mutant diffuse midline glioma • Age 2 – 39 years 17

Agenda • Strategic considerations in development of drugs for childhood brain cancer Dr James Garner • Emerging data for paxalisib in DIPG Associate Professor Matt Dun • Emerging data for paxalisib in AT/RT Dr John Friend • Overview of Kazia’s childhood brain cancer program Dr John Friend • Closing Comments + Q&A 18
Atypical Teratoid / Rhabdoid Tumor (AT/RT) • Atypical Teratoid Rhabdoid Tumors are the most common malignant brain tumors of infancy – Occurs in the cerebellum or brainstem – Usually develops by age three but can occur in older children – Presenting symptoms include • Issues with balance/coordination/walking • Twitches, unusual facial expressions • Nausea/Vomiting/Headaches – Activation of the PI3k-Akt-mTOR pathway is commonly observed in patients with AT/RT – No FDA approved therapies exist • Surgery, chemotherapy and radiation are the current mainstay of treatment 19

Kazia Therapeutics Announces Collaboration with Johns Hopkins University For Pediatric Brain Cancers • Lead researcher – Jeffrey Rubens, MD – Assistant Professor of Pediatrics & Oncology – Sidney Kimmel Cancer Center, Johns Hopkins University • Objective – Establish efficacy of Paxalisib in AT/RT and other aggressive pediatric brain tumors via in vitro and proprietary in vivo models – Evaluate the synergistic effect of Paxalisib and other brain penetrant drugs on the slowing of tumour growth and extending overall survival in various preclinical models – Develop rationale and preclinical package to rapidly translate into the clinical setting 20
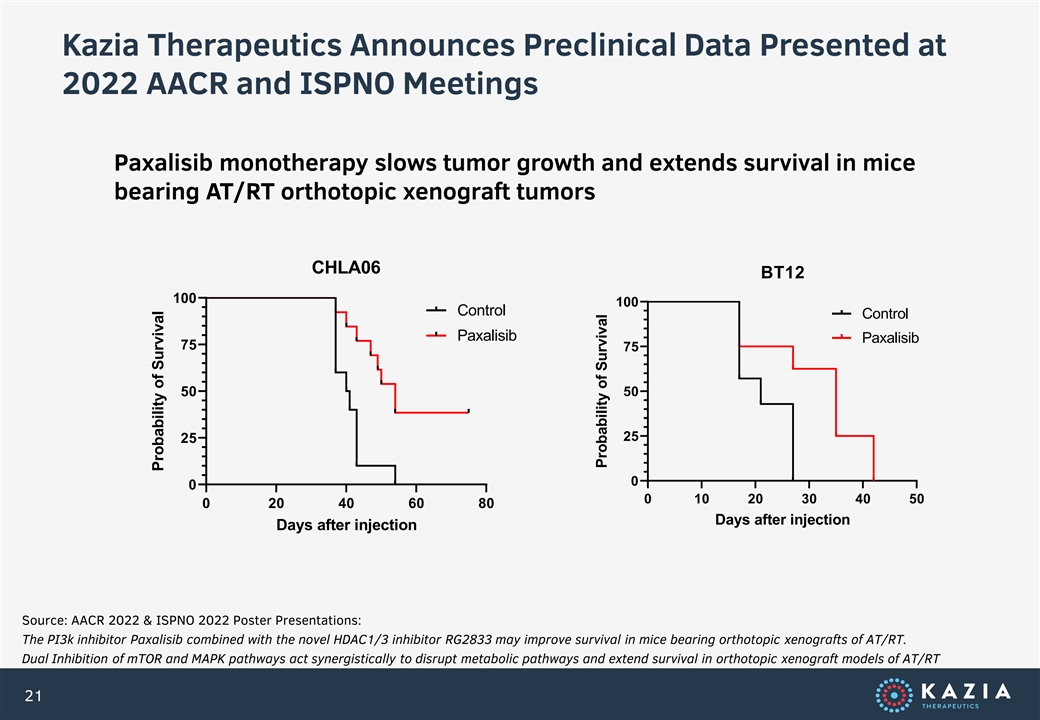
Kazia Therapeutics Announces Preclinical Data Presented at 2022 AACR and ISPNO Meetings Paxalisib monotherapy slows tumor growth and extends survival in mice bearing AT/RT orthotopic xenograft tumors CHLA06 BT12 100 100 Control Control Paxalisib Paxalisib 75 75 50 50 25 25 0 0 0 10 20 30 40 50 0 20 40 60 80 Days after injection Days after injection Source: AACR 2022 & ISPNO 2022 Poster Presentations: The PI3k inhibitor Paxalisib combined with the novel HDAC1/3 inhibitor RG2833 may improve survival in mice bearing orthotopic xenografts of AT/RT. Dual Inhibition of mTOR and MAPK pathways act synergistically to disrupt metabolic pathways and extend survival in orthotopic xenograft models of AT/RT 21 Probability of Survival Probability of Survival
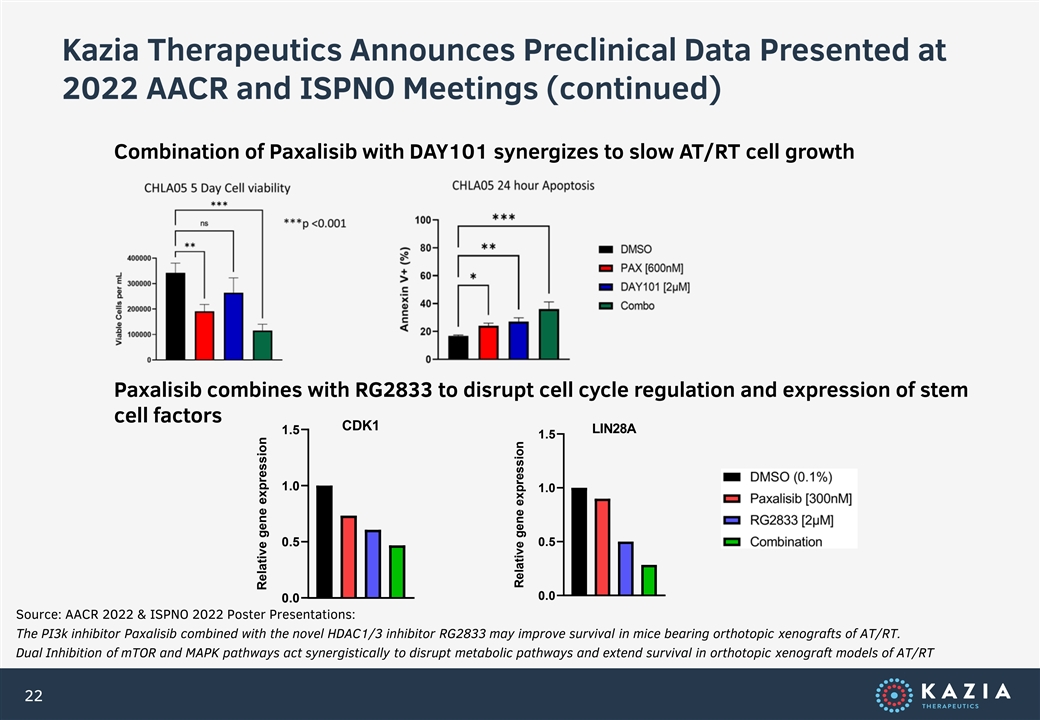
Kazia Therapeutics Announces Preclinical Data Presented at 2022 AACR and ISPNO Meetings (continued) Combination of Paxalisib with DAY101 synergizes to slow AT/RT cell growth Paxalisib combines with RG2833 to disrupt cell cycle regulation and expression of stem cell factors CDK1 LIN28A 1.5 1.5 1.0 1.0 0.5 0.5 0.0 0.0 Source: AACR 2022 & ISPNO 2022 Poster Presentations: The PI3k inhibitor Paxalisib combined with the novel HDAC1/3 inhibitor RG2833 may improve survival in mice bearing orthotopic xenografts of AT/RT. Dual Inhibition of mTOR and MAPK pathways act synergistically to disrupt metabolic pathways and extend survival in orthotopic xenograft models of AT/RT 22 Relative gene expression Relative gene expression

Agenda • Strategic considerations in development of drugs for childhood brain cancer Dr James Garner • Emerging data for paxalisib in DIPG Associate Professor Matt Dun • Emerging data for paxalisib in AT/RT Dr John Friend • Overview of Kazia’s childhood brain cancer program Dr John Friend • Closing Comments + Q&A 23
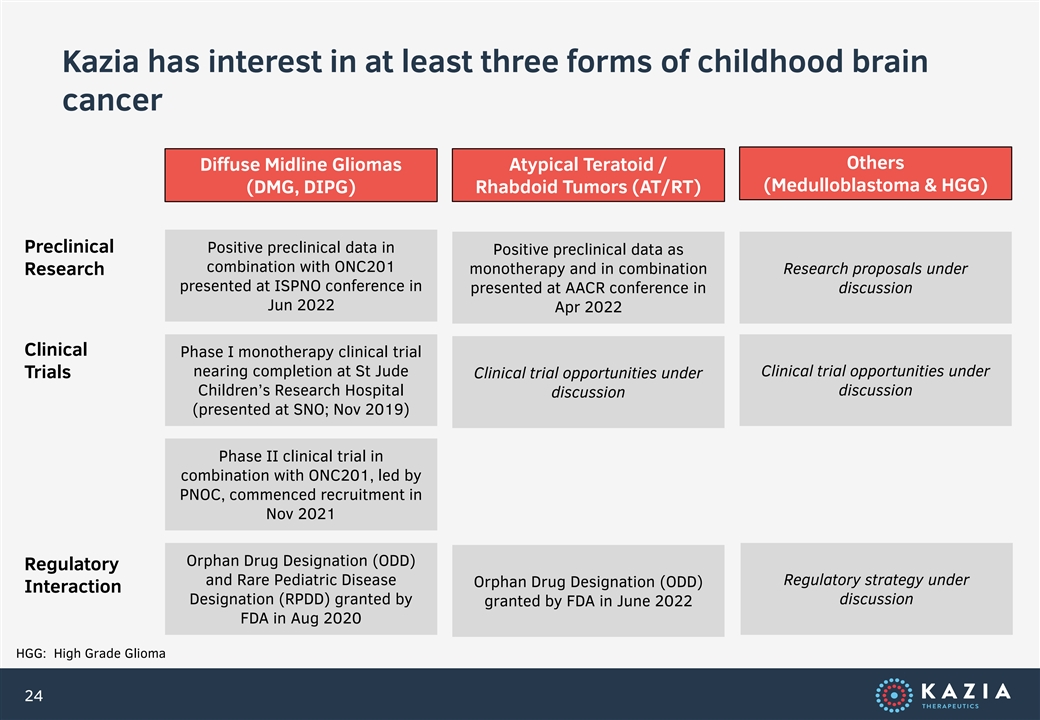
Kazia has interest in at least three forms of childhood brain cancer Others Diffuse Midline Gliomas Atypical Teratoid / (Medulloblastoma & HGG) (DMG, DIPG) Rhabdoid Tumors (AT/RT) Preclinical Positive preclinical data in Positive preclinical data as combination with ONC201 Research monotherapy and in combination Research proposals under presented at ISPNO conference in presented at AACR conference in discussion Jun 2022 Apr 2022 Clinical Phase I monotherapy clinical trial nearing completion at St Jude Clinical trial opportunities under Trials Clinical trial opportunities under Children’s Research Hospital discussion discussion (presented at SNO; Nov 2019) Phase II clinical trial in combination with ONC201, led by PNOC, commenced recruitment in Nov 2021 Orphan Drug Designation (ODD) Regulatory and Rare Pediatric Disease Regulatory strategy under Orphan Drug Designation (ODD) Interaction Designation (RPDD) granted by discussion granted by FDA in June 2022 FDA in Aug 2020 HGG: High Grade Glioma 24

Agenda • Strategic considerations in development of drugs for childhood brain cancer Dr James Garner • Emerging data for paxalisib in DIPG Associate Professor Matt Dun • Emerging data for paxalisib in AT/RT Dr John Friend • Overview of Kazia’s childhood brain cancer program Dr John Friend • Closing Comments + Q&A 25

www.kaziatherapeutics.com info@kaziatherapeutics.com 26



























