UNITED STATES SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
| | | |
| x | | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2008
OR
| | | |
| o | | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ________________ to ________________
Commission file number: 001-15643
SKYSTAR BIO-PHARMACEUTICAL COMPANY
(Exact name of registrant as specified in its charter)
| | | |
| Nevada | | 33-0901534 |
| (State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| | | |
Rm. 10601, Jiezuo Plaza, No.4, Fenghui Road South, Gaoxin District, Xian Province, P.R. China | | N/A |
| (Address of principal executive offices) | | (Zip Code) |
Registrant’s telephone number: (8629) 8819-3188
Securities registered pursuant to Section 12(b) of the Act: None
Securities registered pursuant to Section 12(g) of the Act: Common Stock, no par value
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained herein, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer o | Accelerated filer o |
Non-accelerated filer o | Smaller reporting company þ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes o No þ
As of June 25, 2008, the aggregate market value of the voting stock held by non-affiliates of the Registrant was approximately $9.4 million based on a closing price of $1.05 per share of common stock as reported on the Over-the-Counter Bulletin Board on such date.
On March 25, 2009, we had 18,665,189 shares of common stock issued and outstanding.
TO ANNUAL REPORT ON FORM 10-K
FOR YEAR ENDED DECEMBER 31, 2008
| PART I | | Page |
| Item 1. | Business | 1 |
| Item 1A. | Risk Factors | 7 |
| Item 1B. | Unresolved Staff Comments | 20 |
| Item 2. | Properties | 21 |
| Item 3. | Legal Proceedings | 22 |
| Item 4. | Submission of Matters to a Vote of Security Holders | 22 |
| | | |
| PART II | | |
| Item 5. | Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 22 |
| Item 6. | Selected Financial Data | 23 |
| Item 7. | Management’s Discussion and Analysis of Financial Conditions and Results of Operations | 23 |
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk | 30 |
| Item 8. | Financial Statements and Supplementary Data | 30 |
| Item 9. | Changes in and Disagreements With Accountants on Accounting and Financial Disclosure | 30 |
| Item 9A(T). | Controls and Procedures | 30 |
| Item 9B. | Other Information | 32 |
| | | |
| PART III | | 32 |
| Item 10. | Directors, Executive Officers and Corporate Governance | 32 |
| Item 11. | Executive Compensation | 35 |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 39 |
| Item 13. | Certain Relationships and Related Transactions, and Director Independence | 41 |
| Item 14. | Principal Accounting Fees and Services | 43 |
| | | |
| PART IV | | |
| Item 15. | Exhibits, Financial Statement Schedules | 43 |
| | | |
| Signatures | | 47 |
CAUTION REGARDING FORWARD-LOOKING INFORMATION
This report contains forward-looking statements. All forward-looking statements are inherently uncertain as they are based on current expectations and assumptions concerning future events or future performance of the Company. Readers are cautioned not to place undue reliance on these forward-looking statements, which are only predictions and speak only as of the date hereof. Forward-looking statements usually contain the words “estimate,” “anticipate,” “believe,” “expect,” or similar expressions, and are subject to numerous known and unknown risks and uncertainties. In evaluating suc h statements, prospective investors should carefully review various risks and uncertainties identified in this Report, including the matters set forth under the captions “Risk Factors” and in the Company’s other SEC filings. These risks and uncertainties could cause the Company’s actual results to differ materially from those indicated in the forward-looking statements. The Company undertakes no obligation to update or publicly announce revisions to any forward-looking statements to reflect future events or developments.
Although forward-looking statements in this annual report on Form 10-K reflect the good faith judgment of our management, such statements can only be based on facts and factors currently known by us. Consequently, forward-looking statements are inherently subject to risks and uncertainties, and actual results and outcomes may differ materially from the results and outcomes discussed in or anticipated by the forward-looking statements. Factors that could cause or contribute to such differences in results and outcomes include, without limitation, those specifically addressed under the heading “Risks Relating to Our Business” below, as well as those discussed elsewhere in this annual report on Form 10-K. Readers are urged not to place undue reliance on these forward-looking statements, which speak only as of the date of this annual report on Form 10-K. We file reports with the Securities and Exchange Commission (“SEC”). You can read and copy any materials we file with the SEC at the SEC’s Public Reference Room, 100 F. Street, NE, Washington, D.C. 20549 on official business days during the hours of 10 a.m. to 3 p.m. You can obtain additional information about the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. In addition, the SEC maintains an Internet site (www.sec.gov) that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC, including the Company.
We undertake no obligation to revise or update any forward-looking statements in order to reflect any event or circumstance that may arise after the date of this annual report on Form 10-K. Readers are urged to carefully review and consider the various disclosures made throughout the entirety of this annual report, which attempt to advise interested parties of the risks and factors that may affect our business, financial condition, results of operations and prospects.
PART I
ITEM 1. BUSINESS
Overview
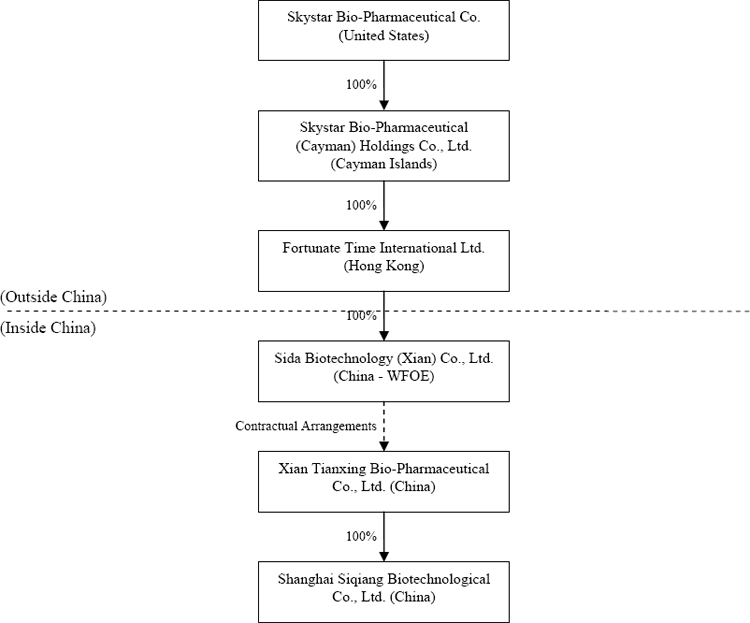
Skystar Bio-Pharmaceutical Company (sometimes referred to in this annual report as “Skystar”, “Company”, “we” or “our”) is a holding company that, through its variable interest entity (“VIE”) Xian Tianxing Bio-Pharmaceutical Co., Ltd. (“Xian Tianxing”), develops, manufactures and distributes medicines, vaccines and other health care and medical care products for poultry, livestock and domestic pets in the People’s Republic of China (“PRC”). We have four product lines, including a vaccine line, a veterinary medicine line, a fodder and feed additives line, and a micro-organism line. All four product lines are developed, manufactured and distributed by Xian Tianxing, which we operate and control through contractual arrangements between our indirect wholly-owned subsidiary Sida Biotechnology (Xian) Co., Ltd. (“Sida”) and Xian Tianxing. These contractual arrangements enable Sida to control and receive the profits of Xian Tianxing. Sida is the wholly owned subsidiary of Fortunate Time International Ltd. (“Fortunate Time”), which is wholly owned by Skystar Bio-Pharmaceutical (Cayman) Holdings Co., Ltd. (“Skystar Cayman”), our wholly owned subsidiary. Xian Tianxing has a wholly owned subsidiary, Shanghai Siqiang Biotechnological Co., Ltd. (“Shanghai Siqiang”), which we also control. Other than our interests in the contractual arrangements with Xian Tianxing, neither we nor our direct and indirect subsidiaries have any equity interests in Xian Tianxing.
Corporate Organization and History
We were originally incorporated in Nevada under the name “Hollywood Entertainment Network, Inc.” on September 24, 1998 with a principal business objective to operate as an independent film company in the business of motion picture production and distribution. On May 23, 2000, we underwent a reverse merger and became a developer of computer security software and hardware and changed our name to “The Cyber Group Network Corporation” to reflect this change in business. Effective February 15, 2006, in connection with the share exchange transaction described below, we changed our name to our present name “Skystar Bio-Pharmaceutical Company.”
On September 20, 2005, we executed a Share Exchange Agreement (“Exchange Agreement”) by and among R. Scott Cramer, Steve Lowe, David Wassung (all hereinafter collectively referred to as the “CGPN Shareholders”) and the Company on the one hand, and Skystar Cayman, and the shareholders of 100% of Skystar Cayman’s common stock (the “Skystar Cayman Shareholders”), on the other hand. (This transaction is referred to hereinafter as the “Share Exchange Transaction”). Under the Exchange Agreement, on the closing date, we issued shares of the our series B preferred stock (the “CGPN Shares”) to the Skystar Cayman Shareholders in exchange for 100% of the common stock of Skystar Cayman. The CGPN Shares were convertible, in the aggregate, into a number of shares of our common stock that would equal 89.5% of our outstanding common shares if the CGPN Shares were to be converted on the closing date of the Share Exchange Transaction. In addition, on the closing date, Skystar Cayman was to pay the Company an amount equal to $120,000, which was used to pay liabilities of the Company.
The Share Exchange Transaction closed on November 7, 2005. From and after the closing date, our primary operations consist of the operations of Skystar Cayman. The Share Exchange Transaction was accounted for as a reverse merger (recapitalization) with Skystar Cayman deemed to be the accounting acquirer, and us as the legal acquirer. Accordingly, the historical financial information presented in the financial statements are those of Skystar Cayman as adjusted to give effect to any difference in the par value of ours and Skystar Cayman’s stock with an offset to capital in excess of par value. The basis of the assets, liabilities and retained earnings of Skystar Cayman, the accounting acquirer, have been carried over in the recapitalization.
Skystar Cayman was incorporated under the laws of the Cayman Islands on January 24, 2005. Since incorporation, Skystar Cayman has not conducted any substantive operations of its own and has conducted its primary business operations through its VIE, Xian Tianxing. Xian Tianxing holds the licenses and approvals necessary to operate our bio-pharmaceutical business in China. We have contractual arrangements with Xian Tianxing and its stockholders pursuant to which we provide technology consulting and other general business operation services to Xian Tianxing. Through these contractual arrangements, we also have the ability to substantially influence Xian Tianxing’s daily operations and financial affairs, appoint its senior executives and approve all matters requiring stockholder approval. As a result of these contractual arrangements, which enable us to control Xian Tianxing, we are considered the primary beneficiary of Xian Tianxing. Accordingly, we consolidate Xian Tianxing’s results, assets and liabilities in our financial statements. For a description of these contractual arrangements, see “Contractual Arrangements with Xian Tianxing and its Stockholders” below.
Xian Tianxing was incorporated on July 3, 1997 in the PRC as a limited liability company without shares, but restructured as a joint stock company limited by shares on December 31, 2003. The paid-in capital of Xian Tianxing was funded by individuals who were majority stockholders of Skystar Cayman immediately prior to the closing of the Share Exchange Transaction. All of our veterinary products are developed and produced, and marketed and distributed, by Xian Tianxing. On August 21, 2007, Xian Tianxing invested $66,700 (RMB 500,000) to establish Shanghai Siqiang. Shanghai Siqiang was established in Putuo District, Shanghai, with a registered capital of $66,700 (RMB 500,000) and Xian Tianxing is the 100% shareholder. We established Shanghai Siqiang as a research and development center for Xian Tianxing to engage in research, development, production and sales of feed additives and veterinary disease diagnosis equipments.
On October 16, 2007, all of the issued and outstanding capital stock of Fortunate Time, a Hong Kong company, was acquired from one of our directors, R. Scott Cramer, and Fortunate Time became Skystar Cayman’s wholly owned subsidiary. Fortunate Time has a wholly owned subsidiary, Sida, a PRC limited liability company with registered capital of $5,000,000. $2,000,000 of the registered capital has been paid through funding from Skystar Cayman, with the remaining balance of $3,000,000 due by July 9, 2009. For a description of these companies’ activities as of the date of this annual report, see “Recent Developments with Respect to the Contractual Arrangements with Xian Tianxing and its Shareholders” below.
Name Change and Changes in Authorized Shares of Common Stock
On December 19, 2005, our board of directors and the majority holders of the Company’s capital stock jointly approved amendments to our Articles of Incorporation by written consent, including: (1) a change of our corporate name to our current name, Skystar Bio-Pharmaceutical Company, (2) a 1-for-397 reverse stock split; and a (3) decrease in the authorized common stock of the Company from 500,000,000 to 50,000,000 shares. The Certificate of Amendment and Certificate of Change to our Articles of Incorporation to effect the name change, reverse split and decrease of authorized shares was filed with Nevada’s Secretary of State on February 15, 2006.
On July 11, 2008, we filed another Certificate of Amendment to our Articles of Incorporation with Nevada’s Secretary of State after stockholders approved a proposal at a special meeting of the stockholders held on June 30, 2008, to increase the number of authorized shares of common stock from 50,000,000 to 200,000,000.
Contractual Arrangements with Xian Tianxing and Its Stockholders
Our relationships with Xian Tianxing and its stockholders are governed by a series of contractual arrangements, as we (including our direct and indirect subsidiaries) do not own any equity interests in Xian Tianxing. PRC law currently has limits on foreign ownership of certain companies. To comply with these restrictions, Skystar Cayman entered into the following contractual arrangements with Xian Tianxing and its owners on October 28, 2005:
Consulting Services Agreement. Pursuant to the consulting services agreement with Xian Tianxing, Skystar Cayman has the exclusive right to provide to Xian Tianxing general services related to veterinary healthcare and medical care products business operations as well as consulting services related to the technological research, development, design and manufacturing of veterinary healthcare and medical care products (the “Services”). Skystar Cayman also sends employees to Xian Tianxing for whom Xian Tianxing bears the costs and expenses. Under this agreement, Skystar Cayman owns the intellectual property rights developed or discovered through research and development providing the Services for Xian Tianxing. Xian Tianxing pays a quarterly consulting service fees in Renminbi (“RMB”) to Skystar Cayman that is equal to all of Xian Tianxing’s revenue for such quarter. The consulting services agreement is in effect unless and until terminated by written notice of either party in the event that: (a) the other party causes a material breach of this agreement, provided that if the breach does not relate to a financial obligation of the breaching party, that party may attempt to remedy the breach within 14 days following the receipt of the written notice; (b) the other party becomes bankrupt, insolvent, is the subject of proceedings or arrangements for liquidation or dissolution, ceases to carry on business, or becomes unable to pay its debts as they become due; (c) Skystar Cayman terminates its operations; (d) Xian Tianxing’s business license or any other license or approval for its business operations is terminated, cancelled or revoked; or (e) circumstances arise which would materially and adversely affect the performance or the objectives of the consulting services agreement. Additionally, Skystar Cayman may terminate the consulting services agreement without cause.
Operating Agreement. Pursuant to the operating agreement with Xian Tianxing and the stockholders of Xian Tianxing who collectively hold the majority of the outstanding shares of Xian Tianxing (collectively “Tianxing Majority Stockholders”), Skystar Cayman provides guidance and instructions on Xian Tianxing’s daily operations, financial management and employment issues. The Tianxing Majority Stockholders must designate the candidates recommended by Skystar Cayman as their representatives on Xian Tianxing’s board of directors. Skystar Cayman has the right to appoint senior executives of Xian Tianxing. In addition, Skystar Cayman agrees to guarantee Xian Tianxing’s performance under any agreements or arrangements relating to Xian Tianxing’s business arrangements with any third party. Xian Tianxing, in return, agrees to pledge its accounts receivable and all of its assets to Skystar Cayman. Moreover, Xian Tianxing agrees that without the prior consent of Skystar Cayman, Xian Tianxing will not engage in any transactions that could materially affect the assets, liabilities, rights or operations of Xian Tianxing, including, without limitation, incurrence or assumption of any indebtedness, sale or purchase of any assets or rights, incurrence of any encumbrance on any of its assets or intellectual property rights in favor of a third party or transfer of any agreements relating to its business operation to any third party. The term of this agreement is ten (10) years from October 28, 2005 and may be extended only upon Skystar Cayman’s written confirmation prior to the expiration of the this agreement, with the extended term to be mutually agreed upon by the parties.
Equity Pledge Agreement. Under the equity pledge agreement with the Tianxing Majority Stockholders, the Tianxing Majority Stockholders pledged all of their equity interests in Xian Tianxing to Skystar Cayman to guarantee Xian Tianxing’s performance of its obligations under the consulting services agreement. If Xian Tianxing or the Tianxing Majority Stockholders breaches their respective contractual obligations, Skystar Cayman, as pledgee, will be entitled to certain rights, including but not limited to the right to sell the pledged equity interests, the right to vote and control the pledged assets. The Xian Majority Stockholders also agreed that upon occurrence of any event of default, Skystar Cayman shall be granted an exclusive, irrevocable power of attorney to take actions in the place and stead of the Tianxing Majority Stockholders to carry out the security provisions of the equity pledge agreement and take any action and execute any instrument that Skystar Cayman may deem necessary or advisable to accomplish the purposes of the equity pledge agreement. The Tianxing Majority Stockholders agreed not to dispose of the pledged equity interests or take any actions that would prejudice Skystar Cayman’s interest. The equity pledge agreement will expire two (2) years after Xian Tianxing obligations under the exclusive consulting services agreement have been fulfilled.
Option Agreement. Under the option agreement with the Tianxing Majority Stockholders, the Tianxing Majority Stockholders irrevocably granted Skystar Cayman or its designee an exclusive option to purchase, to the extent permitted under Chinese law, all or part of the equity interests in Xian Tianxing for the cost of the initial contributions to the registered capital or the minimum amount of consideration permitted by applicable Chinese law. Skystar Cayman or its designee has sole discretion to decide when to exercise the option, whether in part or in full. The term of this agreement is ten (10) years from October 28, 2005 and may be extended prior to its expiration by written agreement of the parties.
Proxy Agreement. Pursuant to the proxy agreement with the Tianxing Majority Stockholders and Xian Tianxing, the Tianxing Majority Stockholders agreed to irrevocably grant a designee of Skystar Cayman with the right to exercise their voting and other rights, including the rights to attend and vote at stockholder’s meetings (or by written consent in lieu of such meetings) in accordance with applicable laws and Xian Tianxing’s Article of Association, as well as the rights to sell or transfer all or any of their equity interests of the Company. The term of this Proxy Agreement is ten (10) years from October 28, 2005 and may be extended prior to its expiration by written agreement of the parties.
Recent Developments with Respect to the Contractual Arrangements with Xian Tianxing and its Shareholders
On March 10, 2008, we were made a party to a series of agreements (collectively the “Transfer Agreements”) transferring the contractual arrangements governing the relationship among Skystar Cayman, Xian Tianxing and the Tianxing Majority Stockholders. Pursuant to the Transfer Agreements, from and after March 10, 2008, all of the rights and obligations of Skystar Cayman under the contractual arrangements were transferred to Sida. We were made a party to the Transfer Agreements for the sole purpose of acknowledging the Transfer Agreements. In effect, Skystar Cayman assigned the contractual rights it had with Xian Tianxing to an indirectly wholly-owned subsidiary, Sida.
Under our corporate structure with the contractual arrangements, the ability to transfer funds to and from Xian Tianxing expeditiously through a foreign currency bank account is necessary for the running of our business operations. Under current applicable Chinese law, only a company that is classified as either a wholly foreign owned enterprise (WFOE) or a Sino-foreign joint venture may maintain a foreign currency bank account. Because Sida is wholly owned by Fortunate Time, a Hong Kong company, Sida is deemed a WFOE and may therefore maintain a foreign currency account. The Transfer Agreements amend the contractual arrangements so that funds are required to be transferred to and from Xian Tianxing through Sida’s foreign currency account and, through Sida, allow the Company to continue to control Xian Tianxing and its business operations.
The Transfer Agreements have transferred all of the rights and obligations of Skystar Cayman under the contractual arrangements to Sida. Thus, pursuant to the Amendment to Consulting Services Agreement, Sida now provides exclusive technology and general business consulting services to Xian Tianxing in exchange of a consulting fee equivalent to all of Xian Tianxing’s revenue; pursuant to the Amendment to Equity Pledge Agreement, the Tianxing Majority Stockholders now pledge their equity interests in Xian Tianxing to Sida; pursuant to the Agreement to Transfer of Operating Agreement, Sida now provides guidance and instructions on Xian Tianxing’s daily operations, financial management and employment issues; pursuant to the Designation Agreement, the Tianxing Majority Stockholders have entrusted all the rights to exercise their voting power to appointee(s) of Sida; and pursuant to the Agreement to Transfer of Option Agreement, the Tianxing Majority Stockholders have irrevocably granted Sida an exclusive option to purchase, to the extent permitted under PRC law, all or part of their equity interests in Xian Tianxing.
The Transfer Agreements and the transfer of the rights and obligations of Skystar Cayman under the contractual arrangements to Sida comply with applicable PRC law and do not in any way affect our business operations. Additionally, we believe that Xian Tianxing’s status as a VIE under FASB Interpretation No. 46R (“FIN 46R”), “Consolidation of Variable Interest Entities, an Interpretation of ARB No. 51,” is unaffected by the Transfer Agreements. Under the contractual arrangements, we viewed Xian Tianxing as a VIE of Skystar Cayman because the contractual arrangements obligated Skystar Cayman to absorb a majority of the risk of loss from Xian Tianxing’s activities and enabled Skystar Cayman to receive a majority of its expected residual returns. The Transfer Agreements merely substitute Skystar Cayman with Sida, an indirect wholly owned subsidiary of Skystar Cayman, such that the equity investors of Xian Tianxing continue to not have the characteristics of a controlling financial interest (just as under the contractual arrangements) and we continue to be the primary beneficiary of Xian Tianxing. Accordingly, we continue to consolidate Xian Tianxing’s results, assets and liabilities in the financial statements accompanying this annual report.
Current Corporate Structure
As a result of the Contractual Arrangements and the Transfer Agreements, our current organizational structure is as follows (the percentages depict the current equity interests):
Our Business
As discussed above, we conduct our business through Xian Tianxing. After nine (9) years of development, we have become a high-tech enterprise with registered capital of RMB 42,000,000 (approximately $5,758,200), and are engaged in research, development, production, marketing and sales of bio-pharmaceutical and veterinary products. Our business divisions currently include a bio-pharmaceutical products division, a veterinary drugs division, a fodder and feed additive division, and a microorganism preparation division.
Our Products
As of December 31, 2008:
| | 1. | Our bio-pharmaceutical veterinary vaccine line included over 10 products and accounted for 3.9% of our total revenues in 2008; |
| | 2. | Our veterinary medicine line for poultry and livestock included over 140 products and accounted for 68.5% of our total revenues in 2008; |
| | 3. | Our fodder and feed additives line included over 10 products and accounted for 4.6% of our total revenues in 2008; and |
| | 4. | Our micro-organism products line included over 13 products and accounted for 22.9% of our total revenues in 2008. |
Distribution Methods of Our Products and Our Customers
As of December 31, 2008, we had over 1,412 customers in 29 provinces in China, including 1,036 distributors and 376 direct customers. Of the 1,036 distribution agents, 336 are physical stores which have outer signage with our logo and sell products in our four product lines exclusively (“Franchise Distributors”). We intend to enter into agreements with additional distributors in order to strengthen our distribution network and convert some of these distributors to Franchise Distributors.
We recognize the importance of branding as well as packaging. All of our products have uniform branding while being specifically designed to differentiate our four product lines.
We conduct promotional marketing activities within the provinces we operate to publicize and enhance our image as well as to reinforce the recognition of our brand name, including:
| | 1. | publishing advertisements and articles in national as well as specialized and provincial newspapers, magazines, and in other media, including the Internet; |
| | 2. | participating in national meetings, seminars, symposiums, exhibitions for bio-pharmaceutical and other related industries; |
| | 3. | organizing cooperative promotional activities with distributors; and |
| | 4. | direct mail campaigns to major livestock farms. |
None of our customers accounted for 10% or more of our total revenues in 2008.
Competition
We have three major competitors in China: Jielin Bio-Tech Production Co., Ltd., Qilu Animal Health Production Co., Ltd., and Zhongmu Industrial Joint Stock Co., Ltd. These companies have more assets and have a larger market share. Nevertheless, we believe we are able to compete with these competitors because of our location in northwestern China, a full range of product offerings, and lower prices. Other than these three competitors, most of our other competitors produce only one or two products.
Sources and Availability of Raw Materials and Our Principal Suppliers
Xi’an Yanghua Chemical Co., Ltd.,Xi’an Nanchen Trading Co., Ltd. and Xi’an Fandike Chemical Technology Co., Ltd. collectively supplied over 53.60% of the raw materials we used to manufacture our products. Our principal raw materials include both chemical ingredients and Chinese herbs. The prices for these raw materials are subject to market forces largely beyond our control, including our supplier’s energy costs, organic chemical feedstock, market demand, and freight costs. The prices for these raw materials have varied significantly in the past and may vary significantly in the future.
As a result of our R&D efforts in 2007 we now also internally produce microbial strains, which are key components of our micro-organism products. Our ability to produce microbial strains has translated into a significant cost reduction for these raw materials.
Intellectual Properties and Licenses
We rely on a combination of trademark, copyright and trade secret protection laws in China and other jurisdictions, as well as confidentiality procedures and contractual provisions to protect our intellectual property and our brand.
We intend to seek other licenses or apply for exclusivity as necessary in order to protect our rights, and we also enter into confidentiality, non-compete and invention assignment agreements with our employees and consultants and nondisclosure agreements with third parties. The Chinese characters which transliterate as “Jia Teng Jun”, “Liao Xiao Wang”, “An Jian”, “Hao Shou Yi” and “Xing Ge” are our registered trademarks in China.
Bio-pharmaceutical companies are at times involved in litigation based on allegations of infringement or other violations of intellectual property rights. Furthermore, the application of laws governing intellectual property rights in China and abroad is uncertain and evolving and could involve substantial risks to us.
Government Approval and Regulation of Our Principal Products or Services
Government approval is required for the production of bio-pharmaceutical products. The Chinese Ministry of Agriculture has granted the Company three government permits to produce the following products: Forage Additive Products, Additive and Mixed Forage Products and Veterinary Medicine Products. For the production of the veterinary medicine, there is a national standard known as the Good Manufacturing Practice (“GMP”) standard. A company must establish its facility according to GMP standards, including both the facility and the production process. After establishing such facility, the Company files an application to operate the facility with the PRC Ministry of Agriculture, which then sends a team of specialists to conduct an on-site inspection of the facility. A company cannot start production at the facility until it receives approval from the Ministry of Agriculture to begin operations. Xian Tianxing currently has the requisite approval and licenses from the Ministry of Agriculture in order to operate our production facilities.
Research and Development
We place great emphasis on product research and development (“R&D”).
In July 2005, we entered into a cooperation agreement with Shaanxi Microbial Institute pursuant to which we established a R&D center at our Huxian plant to facilitate opportunities for us to develop commercially viable products based on the Institute’s research conducted at our research center. Under the cooperation agreement, we provide for the running and operation of the research center, including research equipment and materials. In exchange, we have exclusive rights to any technology derived from any research project that we solely fund. The cooperation agreement also provides for our mutual staffing of research personnel at, and joint-appointment of the director for, the research center. The Institute, however, is not obligated to us with respect to a specific amount of time or a specific project under the cooperation agreement. Currently, we are undertaking the following projects at this research center:
| | 1. | Development of protein technology and enzyme mechanism |
| | 2. | Development of non-pathogenic micro-organisms |
At the Skystar Research and Development Center in Shanghai, we have an arrangement with Shanghai Poultry Verminosis Institution, which is a part of the Chinese Academy of Agricultural Sciences, that is similar to our agreement with Shaanxi Microbial Institute, although we have not entered into any written agreement with the Institution. Currently, we are undertaking the following projects at this research center:
| | 1. | Development of new products for animal immunization by employing new technologies in micro-organism and bacterium |
| | 2. | Development of veterinary medicines for pets |
In 2008, we entered into an agreement with Northwestern Agricultural Technology University on a joint R&D project concerning the application of nano-technology in the prevention of major milk cow disease.
In 2008, we spent approximately $549,000, or approximately 2.1%, of our revenue on R&D. In 2007, we spent approximately $268,000, or approximately 1.8%, of our revenue on R&D.
Employees
In 2008, we had approximately 229 employees, of which 226 were full time employees. In 2007, we had 196 employees, of which 193 were full time employees. None of these employees are represented by any collective bargaining agreements. We have not experienced a work stoppage. Management believes that our relations with our employees are good.
ITEM 1A. RISK FACTORS
You should carefully consider the risks described below together with all of the other information included in this report before making an investment decision with regard to our securities. The statements contained in or incorporated into this report that are not historic facts are forward-looking statements that are subject to risks and uncertainties that could cause actual results to differ materially from those set forth in or implied by forward-looking statements. If any of the following risks actually occurs, our business, financial condition or results of operations could be harmed. In that case, the trading price of our common stock could decline, and you may lose all or part of your investment.
Risks Relating to Our Business
Our limited operating history makes it difficult to evaluate our future prospects and results of operations.
We have a relatively limited operating history. Xian Tianxing, the variable interest entity through which we operate our business, commenced operations in 1997 and first achieved profitability in the quarter ended September 30, 1999. Accordingly, you should consider our future prospects in light of the risks and uncertainties typically experienced by companies such as ours in evolving industries such as the bio-pharmaceutical industry in China. Some of these risks and uncertainties relate to our ability to:
| · | offer new and innovative products to attract and retain a larger customer base; |
| | · | attract additional customers and increase spending per customer; |
| | · | increase awareness of our brand and continue to develop user and customer loyalty; |
| | · | raise sufficient capital to sustain and expand our business; |
| | · | maintain effective control of our costs and expenses; |
| | · | respond to changes in our regulatory environment; |
| | · | respond to competitive market conditions; |
| | · | manage risks associated with intellectual property rights; |
| | · | attract, retain and motivate qualified personnel; and |
| | · | upgrade our technology to support additional research and development of new products. |
If we are unsuccessful in addressing any of these risks and uncertainties, our business may be materially and adversely affected.
If we fail to obtain additional financing we will be unable to execute our business plan.
The revenues from the production and sale of bio-pharmaceutical products and the projected revenues from these products are not adequate to support our expansion and product development programs. We may need additional funds to build our new production facilities; pursue further research and development; obtain regulatory approvals; file, prosecute, defend and enforce our intellectual property rights; and market our products. Should such needs arise, we intend to seek additional funds through public or private equity or debt financing, strategic transactions and/or from other sources.
There are no assurances that future funding will be available on favorable terms or at all. If additional funding is not obtained, we will need to reduce, defer or cancel development programs, planned initiatives or overhead expenditures, to the extent necessary. The failure to fund our capital requirements would have a material adverse effect on our business, financial condition and results of operations.
Our business will be materially and adversely affected if our collaborative partners, licensees and other third parties over whom we are very dependent fail to perform as expected.
Due to the complexity of the process of developing bio-pharmaceuticals, our core business depends on arrangements with bio-pharmaceutical institutes, corporate and academic collaborators, licensors, licensees and others for the research, development, clinical testing, technology rights, manufacturing, marketing and commercialization of our products. We are currently collaborating with Shanghai Poultry Verminosis Institution, Shaanxi Microbial Institute and Northwestern Agricultural Technology University on joint R&D projects. However, we do not have any written agreement with Shanghai Poultry Verminosis Institution regarding ongoing collaborations, and under our cooperation agreement with Shaanxi Microbial Institute, the Institute is not obligated to us with respect to any specific period of time or research projects. There are no assurances that we will be able to maintain our present collaborations or establish new ones in the future. We could enter into collaborative arrangements for the development of particular products that may lead to our relinquishing some or all rights to the related technology or products. Moreover, product development and commercialization efforts could be adversely affected if any collaborative partner:
| | · | terminates or suspends its agreement with us; |
| | · | fails to timely develop or manufacture in adequate quantities a substance needed in order to conduct clinical trials; |
| | · | fails to adequately perform clinical trials; |
| | · | determines not to develop, manufacture or commercialize a product to which it has rights; |
| | · | pursue other technologies or develop alternative products that compete with the products we are developing; or |
| | · | otherwise fails to meet its contractual obligations. |
Our collaborative partners could pursue other technologies or develop alternative products that could compete with the products we are developing.
Our products will be adversely affected if we are unable to protect proprietary rights or operate without infringing the proprietary rights of others.
The profitability of our products will depend in part on our ability to obtain and maintain protection for our intellectual property rights and the period our intellectual property remains exclusive. We must also operate without infringing on the proprietary rights of third parties and without third parties circumventing our rights. The proprietary rights of enterprises such as ours are uncertain and involve complex legal and factual questions for which important legal principles are largely unresolved. For example, no consistent policy has emerged regarding the breadth of biotechnology patent claims that are granted by the U.S. Patent and Trademark Office or enforced by the U.S. federal courts. In addition, the scope of the originally claimed subject matter in a patent application can be significantly reduced before a patent is issued. The biotechnology patent situation outside the U.S. is even more uncertain, is currently undergoing review and revision in many countries, and may not protect our intellectual property rights to the same extent as the laws of the U.S. Because applications for exclusive rights are maintained in secrecy in some cases, we cannot be certain that we or our licensors are the first creators of inventions to which we claim proprietary rights, or the first to seek exclusivity for such inventions.
Additionally, the length of time that our intellectual property may remain exclusive is at times beyond our control. For example, we have exclusive rights to the DLV chicken vaccine only until such time that the vaccine is formally listed on the Chinese Pharmacopoeia by the Chinese Pharmacopoeia Commission (“CPC”), after which other companies may apply to the Chinese Ministry of Agriculture for approval to manufacture and distribute the vaccine. Thus, while we remain the only company in China legally permitted to produce and sell the vaccine until the listing occurs, we cannot prevent the CPC from proceeding with such listing or deter other companies from seeking approval from the Chinese Ministry of Agriculture after such listing. Moreover, other companies may independently develop similar products and design around any proprietary products we develop. We cannot assure you that:
| | · | any of our applications for exclusivity will result in their issuance; |
| | · | we will develop additional proprietary products; |
| | · | the exclusive rights we have been issued will provide us with any competitive advantages; |
| | · | the exclusive rights of others will not impede our ability to do business; or |
| | · | third parties will not be able to circumvent our proprietary rights. |
A number of pharmaceutical, biotechnology, research and academic companies and institutions have developed technologies, filed applications for exclusivity or received proprietary rights on technologies that may relate to our business. If these technologies, applications or rights conflict with ours, the scope of our current or future proprietary rights could be limited or our applications for exclusivity could be denied. Our business may be adversely affected if competitors independently develop competing technologies, especially if we do not obtain, or obtain only narrow, proprietary rights protection. If proprietary rights that cover our activities are issued to other companies, we may not be able to obtain licenses at a reasonable cost, or at all; develop our technology; or introduce, manufacture or sell the products we have planned.
Intellectual property litigation is becoming widespread in the biotechnology industry. Such litigation may affect our efforts to form collaborations, to conduct research or development, to conduct clinical testing or to manufacture or market any products under development. There are no assurances that our proprietary rights would be held valid or enforceable by a court or that a competitor’s technology or product would be found to infringe our exclusive rights. Our business could be materially affected by an adverse outcome to such litigation. Similarly, we may need to participate in interference proceedings declared by the U.S. Patent and Trademark Office or equivalent international authorities to determine priority of invention. We could incur substantial costs and devote significant management resources to defend our position or to seek a declaration that another company’s patents or proprietary rights are invalid.
Much of our know-how and technology may not be exclusive, though it may constitute trade secrets. There are no assurances that we will be able to meaningfully protect our trade secrets. We cannot assure you that any of our existing confidentiality agreements with employees, consultants, advisors or collaborators will provide meaningful protection for our trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure. Collaborators, advisors or consultants may dispute the ownership of proprietary rights to our technology, for example by asserting that they developed the technology independently.
Implementation of China’s intellectual property-related laws has historically been lacking, primarily because of ambiguities in China’s laws and difficulties in enforcement. Accordingly, intellectual property rights and confidentiality protections in China may not be as effective as in the United States or other countries.
Difficulties in manufacturing our products could have a material adverse effect on our profitability.
Before our products can be profitable, they must be produced in commercial quantities in a cost-effective manufacturing process that complies with regulatory requirements, including China’s Good Manufacturing Practice (GMP), production and quality control regulations. If we cannot arrange for or maintain commercial-scale manufacturing on acceptable terms, or if there are delays or difficulties in the manufacturing process, we may not be able to conduct clinical trials, obtain regulatory approval or meet demand for our products.
Failure or delays in obtaining an adequate amount of raw material or other supplies would materially and adversely affect our revenue.
While our current products use raw materials that are readily available presently, we cannot give assurance that these raw materials will not become scarce in the future. Additionally, we may produce products in the future that require raw materials which are scarce or which can be obtained only from a limited number of sources. If we are unable to obtain adequate supplies of such raw materials, the development, regulatory approval and marketing of our products could be delayed.
Our ability to generate more revenue would be adversely affected if we need more clinical trials or take more time to complete our clinical trials than we have planned.
Clinical trials vary in design by factors including dosage, end points, length, and controls. We may need to conduct a series of trials to demonstrate the safety and efficacy of our products. The results of these trials may not demonstrate safety or efficacy sufficiently for regulatory authorities to approve our products. Further, the actual schedules for our clinical trials could vary dramatically from the forecasted schedules due to factors including changes in trial design, conflicts with the schedules of participating clinicians and clinical institutions, and changes affecting product supplies for clinical trials.
We rely on collaborators, including academic institutions, governmental agencies and clinical research organizations, to conduct, supervise, monitor and design some or all aspects of clinical trials involving our products. Since these trials depend on governmental participation and funding, we have less control over their timing and design than trials we sponsor. Delays in or failure to commence or complete any planned clinical trials could delay the ultimate timelines for our product releases. Such delays could reduce investors’ confidence in our ability to develop products, likely causing the price of our common stock to decrease.
China and other countries impose significant statutory and regulatory obligations upon the manufacture and sale of bio-pharmaceutical products. Each regulatory authority typically has a lengthy approval process in which it examines pre-clinical and clinical data and the facilities in which the product is manufactured. Regulatory submissions must meet complex criteria to demonstrate the safety and efficacy of the ultimate products. Addressing these criteria requires considerable data collection, verification and analysis. We may spend time and money preparing regulatory submissions or applications without assurances as to whether they will be approved on a timely basis or at all.
Our product candidates, some of which are currently in the early stages of development, will require significant additional development and pre-clinical and clinical testing prior to their commercialization. These steps and the process of obtaining required approvals and clearances can be costly and time-consuming. If our potential products are not successfully developed, cannot be proven to be safe and effective through clinical trials, or do not receive applicable regulatory approvals and clearances, or if there are delays in the process:
| | · | the commercialization of our products could be adversely affected; |
| | · | any competitive advantages of the products could be diminished; and |
| | · | revenues or collaborative milestones from the products could be reduced or delayed. |
Governmental and regulatory authorities may approve a product candidate for fewer indications or narrower circumstances than requested or may condition approval on the performance of post-marketing studies for a product candidate. Even if a product receives regulatory approval and clearance, it may later exhibit adverse side effects that limit or prevent its widespread use or that force us to withdraw the product from the market.
Any marketed product and its manufacturer, including us, will continue to be subject to strict regulation after approval. Results of post-marketing programs may limit or expand the further marketing of products. Unforeseen problems with an approved product or any violation of regulations could result in restrictions on the product, including its withdrawal from the market and possible civil actions.
In manufacturing our products we will be required to comply with applicable good manufacturing practices regulations, which include requirements relating to quality control and quality assurance, as well as the maintenance of records and documentation. We cannot comply with regulatory requirements, including applicable good manufacturing practice requirements, we may not be allowed to develop or market the product candidates. If we or our manufacturers fail to comply with applicable regulatory requirements at any stage during the regulatory process, we may be subject to sanctions, including fines, product recalls or seizures, injunctions, refusal of regulatory agencies to review pending market approval applications or supplements to approve applications, total or partial suspension of production, civil penalties, withdrawals of previously approved marketing applications and criminal prosecution.
Competitors may develop and market bio-pharmaceutical products that are less expensive, more effective or safer, making our products obsolete or uncompetitive.
We have three major competitors in China: Jielin Bio-Tech Production Co., Ltd., Qilu Animal Health Production Co., Ltd., and Zhongmu Industrial Joint Stock Co., Ltd. These companies and other potential competitors have greater product development capabilities and financial, scientific, marketing and human resources than we do. Technological competition from biopharmaceutical companies and biotechnology companies is intense and is expected to increase. Other companies have developed technologies that could be the basis for competitive products. Some of these products have an entirely different approach or means of accomplishing the desired curative effect than products we are developing. Alternative products may be developed that are more effective, work faster and are less costly than our products. Competitors may succeed in developing products earlier than us, obtaining approvals and clearances for such products more rapidly than us, or developing products that are more effective than ours. In addition, other forms of treatment may be competitive with our products. Over time, our technology or products may become obsolete or uncompetitive.
Our revenue will be materially and adversely affected if our products are unable to gain market acceptance.
Our products may not gain market acceptance in the agricultural community. The degree of market acceptance of any product depends on a number of factors, including establishment and demonstration of clinical efficacy and safety, cost-effectiveness, clinical advantages over alternative products, and marketing and distribution support for the products. Limited information regarding these factors is available in connection with our products or products that may compete with ours.
To directly market and distribute our bio-pharmaceutical products, we or our collaborators require a marketing and sales force with appropriate technical expertise and supporting distribution capabilities. We may not be able to further establish sales, marketing and distribution capabilities or enter into arrangements with third parties on acceptable terms. If we or our partners cannot successfully market and sell our products, our ability to generate revenue will be limited.
Our operations and the use of our products could subject us to damages relating to injuries or accidental contamination and thus reduce our earnings or increase our losses.
Our research and development processes involve the controlled use of hazardous materials. We are subject to national, provincial and local laws and regulations governing the use, manufacture, storage, handling and disposal of such materials and waste products. The risk of accidental contamination or injury from handling and disposing of such materials cannot be completely eliminated. In the event of an accident involving hazardous materials, we could be held liable for resulting damages. We are not insured with respect to this liability. Such liability could exceed our resources. In the future we could incur significant costs to comply with environmental laws and regulations.
If we were sued for product liability, we could face substantial liabilities that may exceed our resources.
We may be held liable if any product we develop, or any product which is made using our technologies, causes injury or is found unsuitable during product testing, manufacturing, marketing, sale or use. These risks are inherent in the development of agricultural and bio-pharmaceutical products. We currently do not have product liability insurance. If we cannot obtain sufficient insurance coverage at an acceptable cost or otherwise protect against potential product liability claims, the commercialization of products that we develop may be prevented or inhibited. If we are sued for any injury caused by our products, our liability could exceed our total assets, whether or not we are successful.
We have no business liability or disruption insurance coverage and therefore we are susceptible to catastrophic or other events that may disrupt our business.
The insurance industry in China is still at an early stage of development. Insurance companies in China offer limited business insurance products. We do not have any business liability or disruption insurance coverage for our operations in China. Any business disruption, litigation or natural disaster may result in our incurring substantial costs and the diversion of our resources.
We will be unsuccessful if we fail to attract and retain qualified personnel.
We depend on a core management and scientific team. The loss of any of these individuals could prevent us from achieving our business objective of commercializing our product candidates. Our future success will depend in large part on our continued ability to attract and retain other highly qualified scientific, technical and management personnel, as well as personnel with expertise in clinical testing and government regulation. We face competition for personnel from other companies, universities, public and private research institutions, government entities and other organizations. If our recruitment and retention efforts are unsuccessful, our business operations could suffer.
Downturn in the global economy may slow domestic growth in China, which in turn may affect our business.
Due to the global downturn in the financial markets, China may not be able to maintain its recent growth rates mainly due to the lack of demand of exports to countries that are in recessions. Although we do not presently export any of our products, our earnings may become unstable if China’s domestic growth slows significantly and the demand for meats and poultry declines.
Risks Related to Our Corporate Structure
Chinese laws and regulations governing our businesses and the validity of certain of our contractual arrangements are uncertain. If we are found to be in violation, we could be subject to sanctions. In addition, changes in such Chinese laws and regulations may materially and adversely affect our business.
There are substantial uncertainties regarding the interpretation and application of Chinese laws and regulations, including, but not limited to, the laws and regulations governing our business, or the enforcement and performance of our contractual arrangements with our affiliated Chinese entity, Xian Tianxing, and its stockholders. We are considered a foreign person or foreign invested enterprise under Chinese law. As a result, we are subject to Chinese law limitations on foreign ownership of Chinese companies. These laws and regulations are relatively new and may be subject to change, and their official interpretation and enforcement may involve substantial uncertainty. The effectiveness of newly enacted laws, regulations or amendments may be delayed, resulting in detrimental reliance by foreign investors. New laws and regulations that affect existing and proposed future businesses may also be applied retroactively.
The Chinese government has broad discretion in dealing with violations of laws and regulations, including levying fines, revoking business and other licenses and requiring actions necessary for compliance. In particular, licenses and permits issued or granted to us by relevant governmental bodies may be revoked at a later time by higher regulatory bodies. We cannot predict the effect of the interpretation of existing or new Chinese laws or regulations on our businesses. We cannot assure you that our current ownership and operating structure would not be found in violation of any current or future Chinese laws or regulations. As a result, we may be subject to sanctions, including fines, and could be required to restructure our operations or cease to provide certain services. Any of these or similar actions could significantly disrupt our business operations or restrict us from conducting a substantial portion of our business operations, which could materially and adversely affect our business, financial condition and results of operations.
We may be adversely affected by complexity, uncertainties and changes in Chinese regulation of bio-pharmaceutical business and companies, including limitations on our ability to own key assets.
The Chinese government regulates the bio-pharmaceutical industry including foreign ownership of, and the licensing and permit requirements pertaining to, companies in the bio-pharmaceutical industry. These laws and regulations are relatively new and evolving, and their interpretation and enforcement involve significant uncertainty. As a result, in certain circumstances it may be difficult to determine what actions or omissions may be deemed to be a violation of applicable laws and regulations. Issues, risks and uncertainties relating to Chinese government regulation of the bio-pharmaceutical industry include the following:
| | · | we only have contractual control over Xian Tianxing. We do not own it due to the restriction of foreign investment in Chinese businesses; and |
| | · | uncertainties relating to the regulation of the bio-pharmaceutical business in China, including evolving licensing practices, means that permits, licenses or operations at our company may be subject to challenge. This may disrupt our business, or subject us to sanctions, requirements to increase capital or other conditions or enforcement, or compromise enforceability of related contractual arrangements, or have other harmful effects on us. |
The interpretation and application of existing Chinese laws, regulations and policies and possible new laws, regulations or policies have created substantial uncertainties regarding the legality of existing and future foreign investments in, and the businesses and activities of, bio-pharmaceutical businesses in China, including our business.
In order to comply with Chinese laws limiting foreign ownership of Chinese companies, we conduct our bio-pharmaceutical business through Xian Tianxing by means of contractual arrangements. If the Chinese government determines that these contractual arrangements do not comply with applicable regulations, our business could be adversely affected.
The Chinese government restricts foreign investment in bio-pharmaceutical businesses in China. Accordingly, we operate our business in China through Xian Tianxing. Xian Tianxing holds the licenses and approvals necessary to operate our bio-pharmaceutical business in China. We have contractual arrangements with Xian Tianxing and its stockholders that allow us to substantially control Xian Tianxing. We cannot assure you, however, that we will be able to enforce these contracts.
Although we believe we comply with current Chinese regulations, and have been advised by our PRC counsel that in their opinion, the structure for operating our business in China (including our corporate structure and contractual arrangements with Xian Tianxing) complies with all applicable PRC laws, rules and regulations, and does not violate, breach, contravene or otherwise conflict with any applicable PRC laws, rules or regulations, we cannot assure you that the Chinese government would agree that these operating arrangements comply with Chinese licensing, registration or other regulatory requirements, with existing policies or with requirements or policies that may be adopted in the future. If the Chinese government determines that we do not comply with applicable law, it could revoke our business and operating licenses, require us to discontinue or restrict our operations, restrict our right to collect revenues, require us to restructure our operations, impose additional conditions or requirements with which we may not be able to comply, impose restrictions on our business operations or on our customers, or take other regulatory or enforcement actions against us that could be harmful to our business.
Our contractual arrangements with Xian Tianxing and its stockholders may not be as effective in providing control over it as direct ownership.
Since Chinese law limits foreign equity ownership in bio-pharmaceutical companies in China, We control Xian Tiaxing through contractual arrangements. We have no equity ownership interest in Xian Tianxing and rely on contractual arrangements to control and operate Xian Tianxing. These contractual arrangements may not be as effective in providing control over Xian Tianxing as direct ownership. For example, Xian Tianxing could fail to take actions required for our business despite its contractual obligation to do so. If Xian Tianxing fails to perform under their agreements with us, we may have to rely on legal remedies under Chinese law, which may not be effective. In addition, we cannot assure you that either of Xian Tianxing’s stockholders will act in our best interests.
Because we rely on the consulting services agreement with Xian Tianxing for our revenue, the termination of this agreement will severely and detrimentally affect our continuing business viability under our current corporate structure.
We are a holding company and do not have any assets or conduct any business operations other than the contractual arrangements between Sida and Xian Tianxing. As a result, we currently rely entirely for our revenues on dividends payments from Sida after it receives payments from Xian Tianxing pursuant to the consulting services agreement which forms a part of the contractual arrangements between Sida and Xian Tianxing. The consulting services agreement may be terminated by written notice of Sida or Xian Tianxing in the event that: (a) one party causes a material breach of the agreement, provided that if the breach does not relate to a financial obligation of the breaching party, that party may attempt to remedy the breach within 14 days following the receipt of the written notice; (b) one party becomes bankrupt, insolvent, is the subject of proceedings or arrangements for liquidation or dissolution, ceases to carry on business, or becomes unable to pay its debts as they become due; (c) Sida terminates its operations; (d) Xian Tianxing’s business license or any other license or approval for its business operations is terminated, cancelled or revoked; or (e) circumstances arise which would materially and adversely affect the performance or the objectives of the agreement. Additionally, Sida may terminate the consulting services agreement without cause.
Because neither we nor our direct and indirect subsidiaries own equity interests of Xian Tianxing, the termination of the consulting services agreement would sever our ability to continue receiving payments from Xian Tianxing under our current holding company structure. While we are currently not aware of any event or reason that may cause the consulting services agreement to terminate, we cannot assure you that such an event or reason will not occur in the future. In the event that the consulting services agreement is terminated, this may have a severe and detrimental effect on our continuing business viability under our current corporate structure, which in turn may affect the value of your investment.
Members of Xian Tianxing’s management have potential conflicts of interest with us, which may adversely affect our business and your ability for recourse.
Weibing Lu, our Chief Executive Officer, is also the Chief Financial Officer and Chairman of the Board of Directors of Xian Tianxing. Mr. Wei Wen, who is Xian Tianxing’s Vice-General Manager and Director, is a member of Skystar’s board of directors. Conflicts of interests between their respective duties to our company and Xian Tianxing may arise. As our directors and executive officer (in the case of Mr. Lu), they have a duty of loyalty and care to us under U.S. and Cayman Islands law when there are any potential conflicts of interests between our company and Xian Tianxing. We cannot assure you, however, that when conflicts of interest arise, every one of them will act completely in our interests or that conflicts of interests will be resolved in our favor. For example, they may determine that it is in Xian Tianxing’s interests to sever the contractual arrangements with Sida, irrespective of the effect such action may have on us. In addition, any one of them could violate his or her legal duties by diverting business opportunities from us to others, thereby affecting the amount of payment Xian Tianxing is obligated to remit to us under the consulting services agreement.
Our board of directors is comprised of a majority of independent directors (including two based in the United States). These independent directors may be in a position to deter and counteract the actions of our officers or non-independent directors that are against our interests, as the independent directors do not have any position with, or interests in, our affiliate entities, and should therefore not have any conflicts of interests such as those potentially of our officers and directors who are management members of Xian Tianxing. Additionally, the independent directors have fiduciary duties to act in our best interests, and failure on their part to do so may subject them to personal liabilities for breach of such duties. We cannot, however, give any assurance as to how the independent directors will act. Further, if we or the independent directors cannot resolve any conflicts of interest between us and those of our officers and directors who are management members of Xian Tianxing, we would have to rely on legal proceedings, which could result in the disruption of our business.
In the event that you believe that your rights have been infringed under the securities laws or otherwise as a result of any one of the circumstances described above, it may be difficult or impossible for you to bring an action against Xian Tianxing or our officers or directors who are members of its management, the majority of whom reside within China. Even if you are successful in bringing an action, the laws of China may render you unable to enforce a judgment against the assets of Xian Tianxing and its management, all of which are located in China.
Risks Related to Doing Business in China
Adverse changes in economic and political policies of the Chinese government could have a material adverse effect on the overall economic growth of China, which could adversely affect our business.
Substantially all of our business operations are conducted in China. Accordingly, our results of operations, financial condition and prospects are subject to a significant degree to economic, political and legal developments in China. China’s economy differs from the economies of most developed countries in many respects, including with respect to the amount of government involvement, level of development, growth rate, control of foreign exchange and allocation of resources. While the Chinese economy has experienced significant growth in the past 20 years, growth has been uneven across different regions and among various economic sectors of China. The Chinese government has implemented various measures to encourage economic development and guide the allocation of resources. Some of these measures benefit the overall Chinese economy, but may also have a negative effect on us. For example, our financial condition and results of operations may be adversely affected by government control over capital investments or changes in tax regulations that are applicable to us. Since early 2004, the Chinese government has implemented certain measures to control the pace of economic growth. Such measures may cause a decrease in the level of economic activity in China, which in turn could adversely affect our results of operations and financial condition.
If Chinese law were to phase out the preferential tax benefits currently being extended to foreign invested enterprises and “new or high-technology enterprises” located in a high-tech zone, we would have to pay more taxes, which could have a material and adverse effect on our financial condition and results of operations.
Under Chinese laws and regulations, a foreign invested enterprise may enjoy preferential tax benefits if it is registered in a high-tech zone and also qualifies as “new or high-technology enterprise”. As a foreign invested enterprise as well as a certified “new or high-technology enterprise” located in a high-tech zone in Xian, we have been approved as a new technology enterprise and under Chinese Income Tax Laws and are entitled to a preferential tax rate of 15%. If Chinese law were to phase out preferential tax benefits currently granted to “new or high-technology enterprises”, we would be subject to the standard statutory tax rate, which currently is 25%, and we would be unable to obtain business tax refunds for our provision of technology consulting services. Loss of these preferential tax treatments could have a material and adverse effect on our financial condition and results of operations.
Xian Tianxing is subject to restrictions on making payments to us.
We are a holding company incorporated in Nevada and do not have any assets or conduct any business operations other than our indirect investments in our affiliated entity in China, Xian Tianxing. As a result of our holding company structure, we rely entirely on payments from Xian Tianxing under our contractual arrangements. The Chinese government also imposes controls on the conversion of the Chinese currency, Renminbi, into foreign currencies and the remittance of currencies out of China. We may experience difficulties in completing the administrative procedures necessary to obtain and remit foreign currency. See “Government control of currency conversion may affect the value of your investment.” Furthermore, if our affiliated entity in China incurs debt on their own in the future, the instruments governing the debt may restrict their ability to make payments. If we are unable to receive all of the revenues from our operations through these contractual or dividend arrangements, we may be unable to pay dividends on our ordinary shares.
Uncertainties with respect to the Chinese legal system could adversely affect us.
We conduct our business primarily through our affiliated Chinese entity, Xian Tianxing. Our operations in China are governed by Chinese laws and regulations. We are generally subject to laws and regulations applicable to foreign investments in China and, in particular, laws applicable to wholly foreign-owned enterprises. The Chinese legal system is based on written statutes. Prior court decisions may be cited for reference but have limited precedential value.
Since 1979, Chinese legislation and regulations have significantly enhanced the protections afforded to various forms of foreign investments in China. However, China has not developed a fully integrated legal system and recently enacted laws and regulations may not sufficiently cover all aspects of economic activities in China. In particular, because these laws and regulations are relatively new, and because of the limited volume of published decisions and their nonbinding nature, the interpretation and enforcement of these laws and regulations involve uncertainties. In addition, the Chinese legal system is based in part on government policies and internal rules (some of which are not published on a timely basis or at all) that may have a retroactive effect. As a result, we may not be aware of our violation of these policies and rules until some time after the violation. In addition, any litigation in China may be protracted and result in substantial costs and diversion of resources and management attention.
You may experience difficulties in effecting service of legal process, enforcing foreign judgments or bringing original actions in China based on United States or other foreign laws against us, our management or the experts named in this report.
We are a holding company and do not have any assets or conduct any business operations other than the contractual arrangements between Sida and Xian Tianxing. In addition, all of Xian Tianxing’s assets are located in, and other than our chief financial officer, all of our other senior executive officers reside within, China. As a result, it may not be possible to effect service of process within the United States or elsewhere outside China as the majority of senior executive officers and directors do not reside in the United States, including with respect to matters arising under U.S. federal securities laws or applicable state securities laws. Moreover, our Chinese counsel has advised us that China does not have treaties with the United States or many other countries providing for the reciprocal recognition and enforcement of judgment of courts. As a result, our public shareholders may have substantial difficulty in protecting their interests through actions against our management or directors than would shareholders of a corporation with assets and a majority of its management members located in the United States.
Governmental control of currency conversion may affect the value of your investment.
The Chinese government imposes controls on the convertibility of RMB into foreign currencies and, in certain cases, the remittance of currency out of China. We receive substantially all of our revenues in RMB. Under our current structure, our income is primarily derived from payments from Xian Tianxing. Shortages in the availability of foreign currency may restrict the ability of our Chinese subsidiaries and our affiliated entity to remit sufficient foreign currency to pay dividends or other payments to us, or otherwise satisfy their foreign currency denominated obligations. Under existing Chinese foreign exchange regulations, payments of current account items, including profit distributions, interest payments and expenditures from trade-related transactions, can be made in foreign currencies without prior approval from China State Administration of Foreign Exchange by complying with certain procedural requirements. However, approval from appropriate government authorities is required where RMB is to be converted into foreign currency and remitted out of China to pay capital expenses such as the repayment of bank loans denominated in foreign currencies. The Chinese government may also at its discretion restrict access in the future to foreign currencies for current account transactions. If the foreign exchange control system prevents us from obtaining sufficient foreign currency to satisfy our currency demands, we may not be able to pay dividends in foreign currencies to our stockholders.
Fluctuation in the value of RMB may have a material adverse effect on your investment.
The value of RMB against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in political and economic conditions. Our revenues and costs are mostly denominated in RMB, while a significant portion of our financial assets are denominated in U.S. dollars. We rely entirely on fees paid to us by our affiliated entity in China. Any significant fluctuation in the value of RMB may materially and adversely affect our cash flows, revenues, earnings and financial position, and the value of, and any dividends payable on, our stock in U.S. dollars. For example, an appreciation of RMB against the U.S. dollar would make any new RMB denominated investments or expenditures more costly to us, to the extent that we need to convert U.S. dollars into RMB for such purposes. An appreciation of RMB against the U.S. dollar would also result in foreign currency translation losses for financial reporting purposes when we translate our U.S. dollar denominated financial assets into RMB, as RMB is our functional currency.
We face risks related to health epidemics and other outbreaks.
Our business could be adversely affected by the effects of an epidemic outbreak, such as the SARS epidemic in April 2004. Any prolonged recurrence of such adverse public health developments in China may have a material adverse effect on our business operations. For instance, health or other government regulations adopted in response may require temporary closure of our production facilities or of our offices. Such closures would severely disrupt our business operations and adversely affect our results of operations. We have not adopted any written preventive measures or contingency plans to combat any future outbreak of SARS or any other epidemic.
Risks Related to an Investment in Our Securities
The full conversion or exercise of certain outstanding convertible debentures and warrants could result in the substantial dilution of the company in terms of a particular percentage ownership in the company as well as the book value of the common shares. The sale of a large amount of common shares received upon exercise of the warrants on the public market to finance the exercise price or to pay associated income taxes, or the perception that such sales could occur, could substantially depress the prevailing market prices for our shares.
There are a total of 1,545,500 warrants outstanding with a weighted average exercise price of $1.13, including 570,500 warrants with an exercise price of $1.00 per share and 975,000 warrants with an exercise price of $1.20 per share. In the event of exercise of these securities, such as when their exercise prices become less than the then current market price for our common shares, a stockholder could suffer substantial dilution of his, her or its investment in terms of the percentage ownership in us as well as the book value of the common shares held. Full exercise of the warrants would increase the outstanding common shares as of March 25, 2009 by approximately 8.2% to approximately 20,210,689 shares.
To date, we have not paid any cash dividends and no cash dividends are expected to be paid in the foreseeable future.
We do not anticipate paying cash dividends on our common stock in the foreseeable future and we may not have sufficient funds legally available to pay dividends. Even if the funds are legally available for distribution, we may nevertheless decide not to pay any dividends. We intend to retain all earnings for our operations.
The application of the “penny stock” rules could adversely affect the market price of our common stock and increase your transaction costs to sell those shares.
As long as the trading price of our common shares is below $5 per share, the open-market trading of our common shares will be subject to the “penny stock” rules. The “penny stock” rules impose additional sales practice requirements on broker-dealers who sell securities to persons other than established customers and accredited investors (generally those with assets in excess of $1,000,000 or annual income exceeding $200,000 or $300,000 together with their spouse). For transactions covered by these rules, the broker-dealer must make a special suitability determination for the purchase of securities and have received the purchaser’s written consent to the transaction before the purchase. Additionally, for any transaction involving a penny stock, unless exempt, the broker-dealer must deliver, before the transaction, a disclosure schedule prescribed by the Securities and Exchange Commission relating to the penny stock market. The broker-dealer also must disclose the commissions payable to both the broker-dealer and the registered representative and current quotations for the securities. Finally, monthly statements must be sent disclosing recent price information on the limited market in penny stocks. These additional burdens imposed on broker-dealers may restrict the ability or decrease the willingness of broker-dealers to sell our common shares, and may result in decreased liquidity for our common shares and increased transaction costs for sales and purchases of our common shares as compared to other securities.
Our common shares are thinly traded and, you may be unable to sell at or near ask prices or at all if you desire to liquidate your shares.
We cannot predict the extent to which an active public market for its common stock will develop or be sustained. Our common shares have historically been sporadically or “thinly-traded” on the “OTC Bulletin Board”, meaning that the number of persons interested in purchasing our common shares at or near bid prices at any given time may be relatively small or non-existent. This situation is attributable to a number of factors, including the fact that we are a small company which is relatively unknown to stock analysts, stock brokers, institutional investors and others in the investment community that generate or influence sales volume, and that even if we came to the attention of such persons, they tend to be risk-averse and would be reluctant to follow an unproven company such as ours or purchase or recommend the purchase of our shares until such time as we became more seasoned and viable. As a consequence, there may be periods of several days or more when trading activity in our shares is minimal or non-existent, as compared to a seasoned issuer which has a large and steady volume of trading activity that will generally support continuous sales without an adverse effect on share price. We cannot give you any assurance that a broader or more active public trading market for our common stock will develop or be sustained, or that current trading levels will be sustained.
The market price for our common stock is particularly volatile given our status as a relatively small company with a small and thinly traded “float” and lack of current revenues that could lead to wide fluctuations in our share price. The price at which you purchase our common stock may not be indicative of the price that will prevail in the trading market. You may be unable to sell your common stock at or above your purchase price if at all, which may result in substantial losses to you.
The market for our common shares is characterized by significant price volatility when compared to seasoned issuers, and we expect that our share price will continue to be more volatile than a seasoned issuer for the indefinite future. The volatility in our share price is attributable to a number of factors. First, as noted above, our common shares are sporadically and/or thinly traded. As a consequence of this lack of liquidity, the trading of relatively small quantities of shares by our stockholders may disproportionately influence the price of those shares in either direction. The price for our shares could, for example, decline precipitously in the event that a large number of our common shares are sold on the market without commensurate demand, as compared to a seasoned issuer which could better absorb those sales without adverse impact on its share price. Secondly, we are a speculative or “risky” investment due to our fluctuating level of revenues or profits to date and uncertainty of future market acceptance for our current and potential products. As a consequence of this enhanced risk, more risk-averse investors may, under the fear of losing all or most of their investment in the event of negative news or lack of progress, be more inclined to sell their shares on the market more quickly and at greater discounts than would be the case with the stock of a seasoned issuer. The following factors may add to the volatility in the price of our common shares: actual or anticipated variations in our quarterly or annual operating results; adverse outcomes; the termination of our contractual agreements with Xian Tianxing; and additions or departures of our key personnel, as well as other items discussed under this “Risk Factors” section, as well as elsewhere in this report. Many of these factors are beyond our control and may decrease the market price of our common shares, regardless of our operating performance. We cannot make any predictions or projections as to what the prevailing market price for our common shares will be at any time, including as to whether our common shares will sustain their current market prices, or as to what effect that the sale of shares or the availability of common shares for sale at any time will have on the prevailing market price.
Stockholders should be aware that the market for penny stocks has suffered in recent years from patterns of fraud and abuse. Such patterns include (1) control of the market for the security by one or a few broker-dealers that are often related to the promoter or issuer; (2) manipulation of prices through prearranged matching of purchases and sales and false and misleading press releases; (3) boiler room practices involving high-pressure sales tactics and unrealistic price projections by inexperienced sales persons; (4) excessive and undisclosed bid-ask differential and markups by selling broker-dealers; and (5) the wholesale dumping of the same securities by promoters and broker-dealers after prices have been manipulated to a desired level, along with the resulting inevitable collapse of those prices and with consequent investor losses. Our management is aware of the abuses that have occurred historically in the penny stock market. Although we do not expect to be in a position to dictate the behavior of the market or of broker-dealers who participate in the market, management will strive within the confines of practical limitations to prevent the described patterns from being established with respect to our securities. The occurrence of these patterns or practices could increase the volatility of our share price.
Volatility in our common share price may subject us to securities litigation.
The market for our common stock is characterized by significant price volatility when compared to seasoned issuers, and we expect that our share price will continue to be more volatile than a seasoned issuer for the indefinite future. In the past, plaintiffs have often initiated securities class action litigation against a company following periods of volatility in the market price of its securities. We may, in the future, be the target of similar litigation. Securities litigation could result in substantial costs and liabilities and could divert management’s attention and resources.
Our corporate actions are substantially controlled by our principal stockholders and affiliated entities.
As of March 25, 2009, our principal stockholders and their affiliated entities own approximately 30% of our outstanding common shares, representing approximately 30% of our voting power. These stockholders, acting individually or as a group, could exert substantial influence over matters such as electing directors and approving mergers or other business combination transactions. In addition, because of the percentage of ownership and voting concentration in these principal stockholders and their affiliated entities, elections of our board of directors will generally be within the control of these stockholders and their affiliated entities. While all of our stockholders are entitled to vote on matters submitted to our stockholders for approval, the concentration of shares and voting control presently lies with these principal stockholders and their affiliated entities. As such, it would be difficult for stockholders to propose and have approved proposals not supported by management. There can be no assurances that matters voted upon by our officers and directors in their capacity as stockholders will be viewed favorably by all stockholders of the company.
The elimination of monetary liability against our directors, officers and employees under Nevada law and the existence of indemnification rights to our directors, officers and employees may result in substantial expenditures by our company and may discourage lawsuits against our directors, officers and employees.
Pursuant to our articles of incorporation, we are obligated to indemnify our directors and officers for monetary damages to our company and our stockholders to the extent provided by Nevada law. We also have contractual indemnification obligations under our employment agreements with our chief executive officer and chief financial officer. The foregoing indemnification obligations could result in our company incurring substantial expenditures to cover the cost of settlement or damage awards against directors and officers, which we may be unable to recoup. These provisions and resultant costs may also discourage our company from bringing a lawsuit against directors and officers for breaches of their fiduciary duties, and may similarly discourage the filing of derivative litigation by our stockholders against our directors and officers even though such actions, if successful, might otherwise benefit our company and stockholders.
Legislative actions, higher insurance costs and potential new accounting pronouncements may impact our future financial position and results of operations.
There have been regulatory changes, including the Sarbanes-Oxley Act of 2002, and there may potentially be new accounting pronouncements or additional regulatory rulings that will have an impact on our future financial position and results of operations. The Sarbanes-Oxley Act of 2002 and other similar rule changes are likely to increase general and administrative costs and expenses. Additionally, the premiums for our directors and officers insurance (“D&O Insurance”), which we are contractually obligated to maintain, are considerable in light of the high claims rates in recent years. Additionally, there could be changes in certain accounting rules. These and other potential changes could materially increase the expenses we report under generally accepted accounting principles, and adversely affect our operating results.
Past company activities prior to the reverse merger may lead to future liability for the company.
Prior to the share exchange transaction with Skystar Cayman in September 2005, we were engaged in businesses unrelated to our current operations. Although the prior business owners provided certain indemnifications against any loss, liability, claim, damage or expense arising out of or based on any breach of or inaccuracy in any of their representations and warranties made regarding such acquisition, any liabilities relating to such prior business against which Skystar is not completely indemnified may have a material adverse effect on us.
The market price for our stock may be volatile.
The market price for our stock may be volatile and subject to wide fluctuations in response to factors including the following:
| | · | actual or anticipated fluctuations in our quarterly operating results; |
| | · | changes in financial estimates by securities research analysts; |
| | · | conditions in veterinary healthcare and medical care and agricultural markets; |
| | · | changes in the economic performance or market valuations of other veterinary healthcare and medical care products companies; |
| | · | announcements by us or our competitors of new products, acquisitions, strategic partnerships, joint ventures or capital commitments; |
| | | addition or departure of key personnel; |
| | · | fluctuations of exchange rates between RMB and the U.S. dollar; |
| | · | intellectual property litigation; and |
| | · | general economic or political conditions in China. |
In addition, the securities market has from time to time experienced significant price and volume fluctuations that are not related to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price of our stock.
We may need additional capital, and the sale of additional shares or other equity securities could result in additional dilution to our stockholders.
We believe that our current cash and cash equivalents, anticipated cash flow from operations will be sufficient to meet our anticipated cash needs for the foreseeable future. We may, however, require additional cash resources due to changed business conditions or other future developments, including any investments or acquisitions we may decide to pursue. If our resources are insufficient to satisfy our cash requirements, we may seek to sell additional equity or debt securities or obtain a credit facility. The sale of additional equity securities could result in additional dilution to our stockholders. The incurrence of indebtedness would result in increased debt service obligations and could result in operating and financing covenants that would restrict our operations. We cannot assure you that financing will be available in amounts or on terms acceptable to us, if at all.
If we fail to maintain an effective system of internal controls, we may not be able to accurately report our financial results or prevent fraud.
We are subject to reporting obligations under the U.S. securities laws. The SEC, as required by Section 404 of the Sarbanes-Oxley Act of 2002, adopted rules requiring every public company to include a management report on such company’s internal controls over financial reporting in its annual report, which contains management’s assessment of the effectiveness of our internal controls over financial reporting. In addition, beginning with our annual report for fiscal 2009, an independent registered public accounting firm must attest to and report on management’s assessment of the effectiveness of our internal controls over financial reporting. In this annual report and in our annual report on Form 10-K for the year ended December 31, 2007, we reported certain material weaknesses involving control activities, specifically (1) accounting and finance personnel weaknesses in that the current staff in the accounting department is relatively inexperienced and requires substantial training; (2) lack of internal audit function in that we lack qualified resources to perform the internal audit functions properly, and the scope and effectiveness of internal audit function are yet been fully developed; and (3) lack of internal audit system in that we do not have an internal audit department to prevent and detect control lapses and errors in the accounting of certain key areas in accordance with the appropriate costing method used by us.
In light of the foregoing, our management began to undertake steps to address these issues, including the engagement of a chief financial officer whom management believes has the requisite financial reporting experience, skills and knowledge to complement our existing personnel. Additionally, four independent directors now sit on our board of directors, including a member who is appropriately credentialed as a financial expert. Management, through oversight by the audit committee, has been tasked to establish certain internal audit functions within our company, and we have also established audit and compensation committees comprising entirely of independent directors. However, there is no assurance that additional remedial measures will not be necessary, or that after the remediation our management will be able to conclude that our internal controls over our financial reporting are effective. Moreover, even if our management concludes that our internal controls over financial reporting are effective, our independent registered public accounting firm may still decline to attest to our management’s assessment or may issue a report that is qualified if it is not satisfied with our controls or the level at which our controls are documented, designed, operated or reviewed, or if it interprets the relevant requirements differently from us.
Our reporting obligations as a public company will place a significant strain on our management, operational and financial resources and systems for the foreseeable future. Effective internal controls, particularly those related to revenue recognition, are necessary for us to produce reliable financial reports and are important to help prevent fraud. As a result, our failure to achieve and maintain effective internal controls over financial reporting could result in the loss of investor confidence in the reliability of our financial statements, which in turn could harm our business and negatively impact the trading price of our stock. Furthermore, we anticipate that we will incur considerable costs and use significant management time and other resources in an effort to comply with Section 404 and other requirements of the Sarbanes-Oxley Act.
Shares eligible for future sale may adversely affect the market.
From time to time, certain of our stockholders may be eligible to sell all or some of their shares of common stock by means of ordinary brokerage transactions in the open market pursuant to Rule 144, promulgated under the Securities Act, subject to certain limitations. In general, pursuant to amended Rule 144, non-affiliate stockholders may sell freely after six months subject only to the current public information requirement (which disappears after one year). Affiliates may sell after six months subject to the Rule 144 volume, manner of sale (for equity securities), current public information and notice requirements. Of the approximately 18.7 million shares of our common stock outstanding as of March 25, 2009, approximately 10.4 million shares are, or will be, freely tradable without restriction, as of March 25, 2009. Any substantial sale of our common stock pursuant to Rule 144 may have a material adverse effect on the market price of our common stock.
The invalid issuance of our series “A” preferred stock may subject us to certain claim by the holder of series “A” preferred shares as well as indemnification obligations to the directors who authorized the issuance.
In 2001, 2,000,000 shares of our series “A” preferred stock were issued to a corporation wholly-owned by our then chief executive officer and director, Gregory Evans, for services purportedly rendered by him. The resolutions of the board of directors approving such issuance stated that the series “A” preferred shares carries a “super voting power of five”. Neither Mr. Evans nor any other member of management at such time filed a certificate of designation with the Nevada Secretary of State. Because no certificate of designation was filed with the Nevada Secretary of State prior to the issuance of 2,000,000 shares of our series “A” preferred stock, these shares do not have terms and are deemed invalidly issued under Nevada corporate law. Moreover, such invalidity is not correctable under applicable Nevada law by a subsequent filing of a certificate of designation should we choose to do so now, which we do not have any intention of doing. As such, we do not believe that the holder of these series “A” preferred shares can successfully assert a right to obtain ownership interest in our Company, substantial or otherwise.
Nevertheless, the holder of these shares may potentially assert claims against us and the directors who authorized the issuance. One such claim may be for breach of oral or written contract. Since the Nevada statute of limitations is six years for breach of a written contract and four years for breach of an oral contract, however, any such claim may be time-barred. Even assuming that such claim is not time-barred, we may have the affirmative defense of laches in that the delay of prosecution of such claim unfairly and materially prejudices our interests, especially considering the changes in control of our Company since the issuance of the series “A” preferred shares. Additionally, as Mr. Evans was also a principal of the Company at the time of issuance, a claim for breach of contract may be defective for inadequate or lack of consideration. Another claim may be for fraud based on an assertion that Mr. Evans was induced to provide services on any purported representation of the then board of directors in exchange for the series “A” preferred shares, which has a three-year statute of limitations in Nevada. Thus, such claim may also be time-barred. Moreover, given Mr. Evan’s roles with the Company at the time these shares were issued, the element of reliance on his part may be difficult to justify. While we may have colorable affirmative defenses against these claims, we cannot assure you that we would ultimately prevail in any lawsuit. Should Mr. Evans prevail on any claim, we may be subject to restitution or other forms of monetary damages, which amount is difficult to determine but may take into consideration the then and current fair market value of the series “A” preferred shares. Additionally, although the directors who authorized the issuance of the series “A” preferred shares are no longer members of our board of directors, we may nevertheless be obligated, should such claim arises, to indemnify and defend these directors, provided we have no such obligation if the actions of these directors, in authorizing the issuance of the series “A” preferred shares, are determined to have arisen out of their own gross negligence or willful misconduct.
Not applicable.
ITEM 2. PROPERTIES
The Company’s administrative headquarters is currently located in approximately 3,700 square feet of office space at Rm. 10601, Jiezuo Plaza, No.4, Fenghui Road South, Gaoxin District, Xi’an, Shaanxi Province, China. This property belongs to Mr. Weibing Lu, our chairman and chief executive officer. This property was provided free for the use of the Company’s administrative department in 2006 and 2005. In January 2007, we entered into a 5-year lease agreement with Mr. Lu for the premises on term of RMB 165,600 (approximately $24,000) per year.
Shanghai Siqiang, wholly owned subsidiary of Xian Tianxing, leases its office and facility space in Shanghai from Mr. Lu pursuant to a 10-year lease agreement entered into in August 2007 on terms of RMB 144,000 (approximately $21,000) per year.
Production Facilities
Currently, Xian Tianxing has two manufacturing sites located in Xi’an, Shaanxi Province: one in the town of Sanqiao and the other in the town of Huxian.
The Sanqiao Plant
Xian Tianxing is leasing the site for its Sanqiao plant pursuant to a tenancy agreement for a period of ten years from October 1, 2004 to September 30, 2014. The annual rent for the plant premises is $13,563 (RMB94,600) and is also subject to a 10% increase every four subsequent years. Currently, the Sanqiao plant is comprised of the following:
| 1. | Microorganism factory. This production plant occupies an area of approximately 21,500 square feet. |
| 2. | Feed additive factory. This production facility occupies an area of approximately 10,700 square feet. |
In 2003, Xian Tianxing received approval from the State Council of China to expand its production facilities and construct a new GMP standard plant. In connection with the approval, Xian Tianxing acquired a long-term land use right for the current site of its Huxian plant. The Company's total investment in this project thus far is estimated at RMB 82,000,000 (US$10,501,000). Because Xian Tianxing has been accredited as a high-tech enterprise, its Huaxian plant has the full support of both the Shaanxi provincial government and the Xi’an municipal government.
Construction of the Huxian plant commenced in 2005 and parts of the plant has been fully operational since the end of the second quarter of 2007. Remaining construction work is expected to be completed in the latter part of 2009, depending upon the Company's ability to raise addition capital. When fully completed, the Huxian plant will occupy approximately 7.7 acres and have a total area of approximately 151,700 square feet. The table below lists the primary facilities at the plant and their status as of the date of this annual report:
| Description | | Approximate Size | | Status |
| GMP standard veterinary medicine facility | | 4,200 square meters | | Completed |
| Quality control, research and development, and administration building | | 3,400 square meters | | Completed |
| GMP standard bio-pharmaceutical facility with three production lines for active bacteria, inactivated vaccines, and coccidiosis vaccines | | 4,500 square meters | | Anticipated completion by the end of 2009 |
| Animal laboratory complying with Animal Bio-safety Level 2 (ABSL-2) requirements | | 1,000 square meters | | Anticipated completion by the end of 2009 |
We believe that the general physical condition of our plants and production facilities can completely satisfy our current production needs in terms of quantity and production quality. We believe that these facilities after construction is completed will be able to meet our operational needs for three to five years.
Other than the matter discussed below, we are not aware of any material pending legal proceedings involving us.
Andrew Chien v. Skystar Bio-Pharmaceutical Company, et. al. (US District Court, District of Connecticut, Case No. 3:2007cv00781). Andrew Chien filed suit against the Company, R. Scott Cramer, Steve Lowe, David Wassung and Weibing Lu in United States District Court for the District of Connecticut, alleging causes of action for violation of Sections 10(b) and 20(a) of the Exchange Act. In or around November 2007, the defendants filed motions to dismiss the complaint for failure to state a claim and for lack of personal jurisdiction. Mr. Chien agreed to voluntarily amend the complaint after the motions were filed, and an amended complaint was subsequently filed on or around January 4, 2008. The amended complaint dropped Weibing Lu (who is a resident of China and was never served) as a defendant. The remaining defendants contended that the amended complaint failed to correct the deficiencies of the original complaint, and filed a renewed motion to dismiss for failure to state a claim, also preserving their challenge to personal jurisdiction. The defendants denied all claims and moved the Court to dismiss the amended complaint in its entirety in their motion to dismiss. The motion to dismiss also requested that the Court award sanctions against Mr. Chien under Federal Rule of Civil Procedure Rule 11 ("Rule 11") and the Private Securities Litigation Reform Act ("PSLRA"). On July 17, 2008, in a decision that is now published, the Court granted defendants' motion and subsequently dismissed the lawsuit, entering judgment on behalf of the defendants. Mr. Chien filed a Notice of Appeal of the Court's dismissal of his lawsuit, opposed by the defendants, which remains pending. Defendants were invited by Judge Kravitz to bring a post-judgment motion for sanctions pursuant to Rule 11 and the PSLRA, which they did. On February 5, 2009, Judge Kravitz issued a ruling on defendants' Motion for Sanctions. He found the action filed by Mr. Chien to have been entirely frivolous, and to have constituted a "substantial" violation of Rule 11, and imposed significant monetary sanctions on both Mr. Chien and his former attorney. As part of the basis for imposing sanctions on Mr. Chien personally, the Court specifically found that Mr. Chien had knowledge of facts directly contradicting the allegations of his complaint, as evident in internet postings he made on online message boards. Mr. Chien subsequently filed motions seeking to "re-open" this case, and to recuse Judge Kravitz, but both motions were denied.
Andrew Chien v. Skystar Bio-Pharmaceutical Company, et. al. (formerly Superior Court, State of Connecticut, Case No. NNH-CV-09-5025938-S, now U.S. District Court, District of Connecticut, Case No. 3:09-CV-00149 (MRK)). Andrew Chien, proceeding pro se, filed another lawsuit against the Company, Scott Cramer, Steve Lowe, David Wassung and Weibing Lu in Connecticut Superior Court, alleging causes of action similar to those alleged in his federal complaint described above as well as state law causes of action. The Company contends that the new complaint is just as frivolous as Mr. Chien's earlier federal action, which the new complaint substantially duplicates. The earlier federal action, described above, was found to be totally frivolous and dismissed in its entirety, with substantial monetary sanctions awarded by the judge against both Mr. Chien and his former attorney. A Notice of Removal to the U.S. District Court, District of Connecticut was filed in the state case on January 27, 2009, and the case has been assigned to Judge Kravitz, the same federal judge in the related federal case already dismissed, which is described below. The Company has filed a motion to dismiss this new action which remains pending. In the motion to dismiss, Defendants indicate their intention to again seek sanctions against Mr. Chien under Rule 11and the PSLRA in the event the motion is granted.
ITEM 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
We did not submit any matters to a vote of security holders during the fourth quarter of the fiscal year covered by this annual report.
Market Information
Our common stock, par value $0.001 per share, is traded on the Over-The-Counter Bulletin Board (“OTCBB”) under the symbol “SKBI.” There was no active trading market for the Common Stock before May 22, 2000. The following table sets forth, for the periods indicated, the reported high and low closing bid quotations for our common stock as reported on the OTCBB. The bid prices reflect inter-dealer quotations, do not include retail markups, markdowns or commissions and do not necessarily reflect actual transactions.
Common Stock
| Quarter Ended | | High Bid | | | Low Bid | |
| | | | | | | |
| December 31, 2008 | | | 1.01 | | | | 0.40 | |
| September 30, 2008 | | | 1.10 | | | | 0.50 | |
| June 30, 2008 | | | 1.17 | | | | 0.95 | |
| March 31, 2008 | | | 1.40 | | | | 0.90 | |
| | | | | | | | | |
| December 31, 2007 | | | 1.85 | | | | 1.05 | |
| September 30, 2007 | | | 1.85 | | | | 1.05 | |
| June 30, 2007 | | | 1.69 | | | | 0.95 | |
| March 31, 2007 | | | 1.84 | | | | 1.30 | |
As of March 25, 2009, we had approximately 18,665,189 shares of common stock issued and outstanding and 2,000,000 shares of series “A” preferred stock issued and outstanding.
We also have outstanding warrants that were issued in conjunction the private placement of our convertible debentures on February 27, 2007. These warrants, if exercised, would permit their holders to purchase approximately an additional 1,545,500 shares of our common stock.
Assuming exercise of all warrants, we would have approximately 20,210,689 shares of common stock outstanding.
Holders
As of March 25, 2009, we had 431 record holders of our common stock and one record holder of our Series A preferred stock.
Dividends
While there are no restrictions that limit our ability to pay dividends, we have not paid, and do not currently intend to pay cash dividends on our common stock in the foreseeable future. Our policy is to retain all earnings, if any, to provide funds for operation and expansion of our business. The declaration of dividends, if any, will be subject to the discretion of our board of directors, which may consider such factors as our results of operations, financial condition, capital needs and acquisition strategy, among others.
Securities Authorized for Issuance under Equity Compensation Plans
Please see the discussion in Item 12 titled “Equity Compensation Plan Information” below.
Sales of Unregistered Securities
On December 4, 2008, we issued 26,086 shares of our restricted common stock to Bennet P. Tchaikovsky as compensation, pursuant to the terms of the agreement under which we engaged Mr. Tchaikovsky’s services as our chief financial officer.
Other sales of securities within the past three years which were not registered under the Securities Act were previously disclosed in our current reports on Form 8-K filed on March 5, 2007, December 11, 2007 and April 23, 2008, and in our annual report on Form 10-K for the year ended December 31, 2007 filed on April 2, 2008.
Not applicable.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our results of operations and financial condition for the fiscal years ended December 31, 2008 and 2007 should be read in conjunction with Selected Consolidated Financial Data and our financial statements and the notes to those financial statements that are included elsewhere in this report. Our discussion includes forward-looking statements based upon current expectations that involve risks and uncertainties, such as our plans, objectives, expectations and intentions. Actual results and the timing of events could differ materially from those anticipated in these forward-looking statements as a result of a number of factors, including those set forth under the “Risk Factors”, “Cautionary Notice Regarding Forward-Looking Statements” and “Description of Business” sections and elsewhere in this report. We use words such as “anticipate,” “estimate,” “plan,” “project,” “continuing,” “ongoing,” “expect,” “believe,” “intend,” “may,” “will,” “should,” “could,” “predict,” and similar expressions to identify forward-looking statements. Although we believe the expectations expressed in these forward-looking statements are based on reasonable assumptions within the bound of our knowledge of our business, our actual results could differ materially from those discussed in these statements. Factors that could contribute to such differences include, but are not limited to, those discussed in the “Risk Factors” section of this report. We undertake no obligation to update publicly any forward-looking statements for any reason even if new information becomes available or other events occur in the future.
Our financial statements are prepared in US Dollars and in accordance with accounting principles generally accepted in the United States of America. See “Exchange Rates” below for information concerning the exchanges rates at which Renminbi (“RMB”) were translated into US Dollars (“USD”) at various pertinent dates and for pertinent periods.
Overview
Skystar Bio-Pharmaceutical Company, formerly known as The Cyber Group Network Corporation (“Cyber”), was incorporated in Nevada under the name “Hollywood Entertainment Network, Inc.” on September 24, 1998. On May 23, 2000, we changed our name to “The Cyber Group Network Corporation”. On February 15, 2006, we further changed our name to “Skystar Bio-Pharmaceutical Company” to reflect our current business operations.
On November 7, 2005, we acquired Skystar Cayman and, as a result, Skystar Cayman’s VIE, Xian Tianxing, by way of exchanging 48,000,000 shares of our Series B preferred stock for 100% of the issued and outstanding common stock of Skystar Cayman. We accounted for this share exchange transaction as a reverse acquisition and recapitalization and, as a result, our consolidated financial statements are in substance those of Skystar Cayman, with the assets and liabilities, and revenues and expenses, of Cyber being included effective from the date of the stock exchange transaction. Please see Note 1 to our consolidated financial statements included in this report for further details of this stock exchange transaction.
Having no substantive operation of its own, Skystar Cayman, through its VIE, Xian Tianxing, engages in research, development, production, marketing and sales of bio-pharmaceutical and veterinary products in China. Please see “Contractual Arrangements with Xian Tianxing and its Stockholders” and “Recent Developments with Respect to the Contractual Arrangements with Xian Tianxing and its Shareholders” in Item 1 above and Note 1 to our consolidated financial statements included in this annual report for the contractual arrangements between Skystar Cayman and Xian Tianxing and their subsequent transfer to Sida in March 2008, and their impact on our consolidated financial statements.
In preparing the consolidated financial statements in accordance with accounting principles generally accepted in the United States, we make estimates and assumptions about the effect of matters that are inherently uncertain and may change in subsequent periods. The resulting accounting estimates may, by definition, vary from the related actual results. We consider the following to be the most critical accounting policies:
| · | Revenue recognition: Revenues of the Company include sales of bio-pharmaceutical and veterinary products in China. Sales are recognized when the following four revenue criteria are met: persuasive evidence of an arrangement exists, delivery has occurred, the selling price is fixed or determinable, and collectibility is reasonably assured. Sales are recorded net of value added tax (VAT). No return allowance is made as product returns are insignificant based on historical experience. |
| | (a) | Credit sales: Revenue is recognized when the products have been delivered to the customers. |
| | (b) | Full payment before delivery: Revenue is recognized when the products have been delivered to customers. |
· | Accounts receivable: We perform ongoing credit evaluations of our customers and adjust credit limits based upon payment history and the customers’ current credit worthiness, as determined by a review of their current credit information. We continuously monitor collections and payments from our customers and maintain a provision for estimated credit losses based upon historical experience and any specific customer collection issues that have been identified. While such credit losses have historically been within our expectations and the provisions established, we cannot guarantee that we will continue to experience the same credit loss rates that have been experienced in the past. |
| · | Convertible debentures and warrants: We have adopted APB No. 14, “Accounting for Convertible Debt and Debt Issued with Stock Purchase Warrants”, FAS No.133, “Accounting for Derivatives and Hedging Activities,” EITF 98-5, “Accounting for Convertible Securities with Beneficial Conversion Features or Contingently Adjustable Conversion Ratios,” and EITF 00-27, “Application of Issue No. 98-5 to Certain Convertible Instruments,” for valuation and accounting treatment of our outstanding convertible debentures and warrants. |
| · | Liquidated damages: We have adopted SFAS No. 5, “Accounting for Contingencies” and EITF 00-19-2, “Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s own Stock,” in connection with the liquidated damages we accrued pursuant to the terms of our Registration Rights Agreement with certain investors dated February 27, 2007. |
Recently Accounting Pronouncements
In March 2008, the FASB issued SFAS No. 161, "Disclosures about Derivative Instruments and Hedging Activities" (“SFAS 161”). SFAS 161 is intended to improve financial reporting of derivative instruments and hedging activities by requiring enhanced disclosures to enable financial statement users to better understand the effects of derivatives and hedging on an entity's financial position, financial performance and cash flows. The provisions of SFAS 161 are effective for interim periods and fiscal years beginning after November 15, 2008, with early adoption encouraged. The Company does not anticipate that the adoption of SFAS 161 will have a material impact on its consolidated results of operations or consolidated financial position.
In April 2008, the FASB issued FASB Staff Position (“FSP”) 142-3 “Determination of the useful life of Intangible Assets,” which amends the factors a company should consider when developing renewal assumptions used to determine the useful life of an intangible asset under SFAS 142. FSP 142-3 is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those fiscal years. SFAS 142 requires companies to consider whether renewal can be completed without substantial cost or material modification of the existing terms and conditions associated with the asset. FSP 142-3 replaces the previous useful life criteria with a new requirement—that an entity consider its own historical experience in renewing similar arrangements. If historical experience does not exist, then the Company would consider market participant assumptions regarding renewal including 1) highest and best use of the asset by a market participant, and 2) adjustments for other entity-specific factors included in SFAS 142. The Company is currently evaluating the impact that adopting FSP 142-3 will have on its consolidated financial statements.
On May 9, 2008, the FASB issued FASB Staff Position No. APB 14-1 ("FSP APB 14-1"), "Accounting for Convertible Debt Instruments That May Be Settled in Cash upon Conversion (Including Partial Cash Settlement)." FSP APB 14-1 clarifies that convertible debt instruments that may be settled in cash upon conversion (including partial cash settlement) are not addressed by paragraph 12 of APB Opinion No. 14, "Accounting for Convertible Debt and Debt Issued with Stock Purchase Warrants." Additionally, FSP APB 14-1 specifies that issuers of such instruments should separately account for the liability and equity components in a manner that will reflect the entity's nonconvertible debt borrowing rate when interest cost is recognized in subsequent periods. FSP APB14-1 is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those fiscal years. The Company is currently evaluating the impact that FSP APB 14-1 will have on its consolidated results of operations or consolidated financial position.
On June 16, 2008, the FASB issued FSP No. EITF 03-6-1 (“FSP EITF 03-6-1”), “Determining Whether Instruments Granted in Share-Based Payment Transactions Are Participating Securities,” to address the question of whether instruments granted in share-based payment transactions are participating securities prior to vesting. FSP EITF 03-6-1 indicates that unvested share-based payment awards that contain rights to dividend payments should be included in earnings per share calculations. The guidance will be effective for fiscal years beginning after December 15, 2008. The Company is currently evaluating the requirements of FSP EITF 03-6-1 and the impact that its adoption will have on the consolidated results of operations or consolidated financial position.
In June 2008, the FASB issued Emerging Issues Task Force Issue 07-5 (“EITF 07-5”), “Determining whether an Instrument (or Embedded Feature) is indexed to an Entity’s Own Stock.” EITF 07-5 is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those fiscal years. Early application is not permitted. Paragraph 11(a) of SFAS No. 133, “Accounting for Derivatives and Hedging Activities,” specifies that a contract that would otherwise meet the definition of a derivative but is both (a) indexed to the Company’s own stock and (b) classified in stockholders’ equity in the statement of financial position would not be considered a derivative financial instrument. EITF 07-5 provides a new two-step model to be applied in determining whether a financial instrument or an embedded feature is indexed to an issuer’s own stock and thus able to qualify for the SFAS 133 paragraph 11(a) scope exception. This standard triggers liability accounting on all options and warrants exercisable at strike prices denominated in any currency other than the functional currency of the operating entity in the PRC. The Company is currently evaluating the impact of the adoption of EITF 07-5 on the accounting for related warrants transactions.
In June 2008, FASB issued EITF 08-4, “Transition Guidance for Conforming Changes to Issue No. 98-5.” The objective of EITF 08-4 is to provide transition guidance for conforming changes made to EITF 98-5, “Accounting for Convertible Securities with Beneficial Conversion Features or Contingently Adjustable Conversion Ratios,” that result from EITF 00-27 “ Application of Issue No. 98-5 to Certain Convertible Instruments,” and SFAS 150, “Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity.” EITF 08-4 is effective for financial statements issued for fiscal years ending after December 15, 2008. Early application is permitted. The Company is currently evaluating the impact of adoption of EITF 08-4 on the accounting for the convertible notes and related warrants transactions.
In September 2008, the FASB issued for comment revisions to SFAS No. 140 and FASB Interpretation No. 46, as revised (“FIN 46R”), “Consolidation of Variable Interest Entities.” The changes proposed include a removal of the scope exemption from FIN 46R for QSPEs, a revision of the current risks and rewards-based FIN 46R consolidation model to a qualitative model based on control and a requirement that consolidation of VIEs be re-evaluated on an ongoing basis. Although the revised standards have not yet been finalized, these changes may have a significant impact on the Company’s consolidated financial statements as the Company may be required to deconsolidate certain assets and liabilities due to the ongoing evaluation of its primary beneficiary status. In addition, the Company may also be required to consolidate other VIEs that are not currently consolidated based an analysis under the current FIN 46R consolidation model. The proposed revisions would be effective for fiscal years that begin after November 15, 2009.
On October 10, 2008, the FASB issued FSP 157-3, “Determining the Fair Value of a Financial Asset When the Market for That Asset Is Not Active,” which clarifies the application of SFAS 157 in a market that is not active and provides an example to illustrate key considerations in determining the fair value of a financial asset when the market for that financial asset is not active. FSP 157-3 became effective on October 10, 2008, and its adoption did not have a material impact on the Company’s consolidated results of operations or consolidated financial position.
In January 2009, the FASB issued FSP EITF 99-20-1, “Amendments to the Impairment Guidance of EITF Issue No. 99-20, and EITF Issue No. 99-20, Recognition of Interest Income and Impairment on Purchased and Retained Beneficial Interests in Securitized Financial Assets” (“FSP EITF 99-20-1”). FSP EITF 99-20-1 changes the impairment model included within EITF 99-20 to be more consistent with the impairment model of SFAS No. 115. FSP EITF 99-20-1 achieves this by amending the impairment model in EITF 99-20 to remove its exclusive reliance on “market participant” estimates of future cash flows used in determining fair value. Changing the cash flows used to analyze other-than-temporary impairment from the “market participant” view to a holder’s estimate of whether there has been a “probable” adverse change in estimated cash flows allows companies to apply reasonable judgment in assessing whether an other-than-temporary impairment has occurred. The adoption of FSP EITF 99-20-1 did not have a material impact on the Company’s consolidated financial statements.
Results of Operations – Comparison of Two Years Ended December 31, 2008 and 2007.
| The following table summarizes our results of operations for the years ended December 31, 2008 and 2007. | | Years Ended December 31, | |
| | | 2008 | | | 2007 | |
| | | Amount | | | Percentage of total revenue | | | Amount | | | Percentage of total revenue | |
| Revenues | | $ | 25,584,446 | | | | 100.0 | % | | $ | 15,056,828 | | | | 100.0 | % |
| Gross Profit | | $ | 12,775,550 | | | | 49.9 | % | | $ | 8,344,463 | | | | 55.4 | % |
| Operating Expense | | $ | 4,594,563 | | | | 18.0 | % | | $ | 3,446,737 | | | | 22.9 | % |
| Income from Operations | | $ | 8,180,987 | | | | 32.0 | % | | $ | 4,897,726 | | | | 32.5 | % |
| Other Expenses | | $ | 1,055,116 | | | | 4.1 | % | | $ | 5,827,530 | | | | 38.7 | % |
| Income Tax Expenses | | $ | 1,529,688 | | | | 6.0 | % | | $ | 1,027,172 | | | | 6.8 | % |
| Net Income (loss) | | $ | 5,596,183 | | | | 21.9 | % | | $ | (1,956,976 | ) | | | (13.0 | ) % |
Revenues. All of our revenues are derived from the sale of veterinary healthcare and medical care products in the PRC. For the year ended December 31, 2008, we had revenues of $25,584,446 as compared to revenues of $ 15,056,828 for the year ended December 31, 2007, an increase of approximately 69.9%.
Revenues — Veterinary Medications. Revenues from sales of our veterinary medications product line increased from $9,003,400 for the year ended December 31, 2007 to $17,535,757 for the year ended December 31, 2008 for an increase of $8,532,357 or 94.8%. The increase in the sale of veterinary medications was primarily responsible for the growth of overall revenue between the two years ended December 31, 2008 and 2007. The increase in veterinary medication sales was primarily attributable to our 100% veterinary medicine plant expansion that was completed in 2007, additional product offerings, and increased use by our customers of products for the treatment of livestock and poultry diseases in 2008.
Revenues — Micro-Organism. Revenues from sales of our micro-organism product line increased from $4,271,139 for the year ended December 31, 2007 to $5,868,623 for the year ended December 31, 2008 for an increase of $ 1,597,484 or 37.4%. The increase of $1,597,484 was a result of increased customer demand for our new probiotics micro-organism products and enhanced sales efforts for the year ended December 31, 2008.
Revenues — Feed Additives. Revenues from sales of our feed additives product line increased from $971,019 for the year ended December 31, 2007 to $1,189,108 for the year ended December 31, 2008 for an increase of $ 218,089 or 22.5%. The increase of $ 218,089 was a result of increased customer demand for our multi-enzyme feed additive products for the year ended December 31, 2008.
Revenues — Vaccines. Revenues from sales of our vaccines product line increased from $ 811,270 for the year ended December 31, 2007 to $ 990,958 for the year ended December 31, 2008 or an increase of $ 179,688 or 22.1%. This increase was a result of an increase in customer demand of our vaccine products for the year ended December 31, 2008. We are presently operating at full production capacity for our vaccine product line and therefore cannot significantly increase sales until we expand our production capabilities.
Cost of Revenue
Cost of revenue. For the year ended December 31, 2008, we had cost of revenues, which consists of raw materials, direct labor, and manufacturing overhead, of $12,808,896 as compared to cost of sales of $ 6,712,365 for the year ended December 31, 2007, an increase of approximately 90.8%. Our cost of sales consists of four product lines: veterinary medications, micro-organism, feed additives, and vaccines. During the year ended December 31, 2008, we incurred higher raw material prices caused by higher shipping costs as a result of restricted railway shipments during the period of the Beijing Olympics in August 2008 and the powerful earthquake in Sichuan Province in May 2008. However, management has noticed that since September 2008, raw materials prices have steadily declined.
Cost of Revenue — Veterinary Medications. Cost of sales of our veterinary medications product line increased from $4,654,347 for the year ended December 31, 2007 to $10,389,726 for the year ended December 31, 2008, for an increase of $5,735,379 or approximately 123.2%. This increase was mainly due to the corresponding increase in veterinary medications sales. However, the gross margin for veterinary medications declined from approximately 48.3% for the year ended December 31, 2007 to approximately 40.8% for the year ended December 31, 2008 due to higher price of raw materials.
Cost of Revenue — Micro-Organism. Cost of sales of our micro-organism product line increased from $1,416,550 for the year ended December 31, 2007 to $1,781,598 for the year ended December 31, 2008 for an increase of $365,048 or approximately 25.8%. Gross margin for micro organism products increased slightly from 66.8% for the year ended December 31, 2007 to 69.6% for the year ended December 31, 2008, as a result of improved manufacturing techniques and product sales with better margins, which offset the increase in raw material prices for the year ended December 31, 2008.
Cost of Revenue — Feed Additives. Cost of sales of our feed additives product line decreased from $549,714 for the year ended December 31, 2007 to $525,653 for the year ended December 31, 2008 for a decrease of $24,061 or 4.4%. The decrease of $24,061 was a result of certain products not being produced during the year of 2008 due to significant increases in raw material prices for those products. However, the gross margins for feed additives improved from 43.4% for the year ended December 31, 2007 to 55.8% for the year ended December 31, 2008 as a result of product sales with better margins and improved manufacturing techniques which offset the increase in raw materials prices during year ended December 31, 2008.
Cost of Revenue — Vaccines. Cost of sales of our vaccines product line increased from $91,754 for the year ended December 31, 2007 to $111,919 for the year ended December 31, 2008, for an increase of $20,165 or 22.0%. This slight increase was a result of increase in vaccine product sales for which the Company is presently running at full capacity.
Operating Expenses
| | | Years Ended December 31, | |
| | | 2008 | | | 2007 | |
| | | Amount | | | Percentage of total revenue | | | Amount | | | Percentage of total revenue | |
| Gross Profit | | $ | 12,775,550 | | | | 49.9 | % | | $ | 8,344,463 | | | | 55.4 | % |
| Operating Expenses | | $ | 4,594,563 | | | | 18.0 | % | | $ | 3,446,737 | | | | 22.9 | % |
| Selling Expenses | | $ | 1,381,807 | | | | 5.4 | % | | $ | 739,422 | | | | 4.9 | % |
| General and Administrative Expenses | | $ | 2,663,520 | | | | 10.4 | % | | $ | 2,438,995 | | | | 16.2 | % |
| Research and Development Costs | | $ | 549,236 | | | | 2.1 | % | | $ | 268,320 | | | | 1.8 | % |
| Income from Operations | | $ | 8,180,987 | | | | 32.0 | % | | $ | 4,897,726 | | | | 32.5 | % |
Selling Expenses. Selling expenses, which consist of commissions, advertising and promotion expenses, freight charges, and salaries, totaled $1,381,807 for the year ended December 31, 2008 as compared to $739,422 for the year ended December 31, 2007, an increase of approximately 86.9%. This increase is primarily attributable to our expanding sales team and activities, which are, in turn, reflected in our increased sales. We believe that our selling expenses will continue to increase as our sales continue to grow.
General and Administrative Expenses. General and administrative expenses totaled $2,663,520 for the year ended December 31, 2008, as compared to $2,438,995 for the year ended December 31, 2007, an increase of approximately 9.2%. General and administrative expenses are primarily legal, accounting and other professional fees that we incurred as a U.S. public company. More professional expenses were incurred in 2008 as a result of increased compliance costs for activities that we undertook during the year. We anticipate, however, that our general and administrative expenses will increase due to the increasing costs of being a U.S. public company.
Research and Development Costs. Research and development costs, which consist of salaries, professional fees, and technical support fees, totaled $549,236 for the year ended December 31, 2008, as compared to $268,320 for the year ended December 31, 2007, an increase of approximately 104.7%. This increase is primarily attributable to increased research activities with certain outside experts and institutions with whom we cooperate on research and development of both existing and new products. We anticipate that our research and development costs will continue to increase as we continue improve existing products and develop new products.
For the years ended December 31, 2008 and 2007, cash provided by operating activities were $3,700,428 and $943,145, respectively. This increase is primarily attributable to the increase in net income (excluding the non-cash adjustments listed on the consolidated statements of cash flow) of $2,741,656, from $4,864,481 for the year ended December 31, 2007 to $7,606,137 for the year ended December 31, 2008, as a result of increased sales of our veterinary medicines and micro-organism products. For the year ended December 31, 2008, net cash provided by operating activities other than net income was mainly due to increase of $404,642 in accounts payable, increase of $946,801 in accrued expense and increase of $352,012 in deposit from customers during 2008. However, this increase was offset by an increase of $674,486 in inventories, increase of $1,068,391 in accounts receivable, $3,647,834 increase in deposits and prepaid expenses towards future purchases of raw materials to take advantage of favorable pricing and ensure the availability of certain raw materials, and $389,081 decrease in taxes payable, which collectively increased cash provided by operating activities by $2,757,283 for the year ended December 31, 2008 compared to the prior year.
We used $5,076,346 in investing activities for the year ended December 31, 2008, as compared to using $2,232,683 in investing activities for the year ended December 31, 2007. The net cash used in investing activities for the year ended December 31, 2008 was a result of $1,606,280 in payments and $1,729,079 in prepayments made to construction in progress, and $2,666,775 of deposit paid for potential acquisitions targets as further described in Note 11 to the financial statements, and offset by the refund of prior prepayments of $562,185, as compared to $383,322 in payments made to construction-in-progress, $658,350 of intangible assets purchased and $1,171,863 of long-term deposit made for construction-in-progress and potential acquisitions for the year ended December 31, 2007.
Cash provided by financing activities was $1,136,127 for the year ended December 31, 2008 as compared to $1,839,352 generated for the year ended December 31, 2007. We raised $3,737,250 from sales of our convertible debentures in the year ended December 31, 2007, but did not undertake similar financing in the year ended December 31, 2008. For the year ended December 31 2008, we had proceeds from shareholder loans and one-year short term bank loans which amounted to $1,037,880. These loans are further discussed in detail in Note 13 and Note 20, respectively, of the footnotes accompanying the consolidated financial statements.
As of December 31, 2008, we had cash of $576,409. Our total current assets were $11,778,573, and our total current liabilities were $4,134,999, which resulted in a net working capital of $7,643,574. Management believes that we have the ability to meet cash requirements for our operations in order to continue as a going concern, including sufficient cash flows to meet our obligations on a timely basis in the foreseeable future, provided that we can continue to maintain profitable operations and our net working capital remains liquid.
Capital Resources
For the year ended December 31, 2008 we borrowed $735,165 on a short term basis from a bank, collected $ 315,472 on a loan receivable, and received $302,715 from shareholder loans, the majority of which was to continue construction on ongoing facilities projects. If we are to acquire another business or further expand our operations, we will need additional capital.
Over the next 12 months, we plan to continue to market and sell our current products and to develop new products.
In 2003, we received approval from the State Council of China to expand our production facilities and construct a new GMP standard plant. We have invested $10,501,000 (RMB 82,000,000) into this project, which is our Huxian plant, including approximately $9,700,000 for the facilities and $800,000 for working capital. The construction work commenced in 2005, and we completed the veterinary medicine facility and the building that houses quality control, research and development and administration during 2007, both of which are fully operational. The remaining facilities of the Huxian plant are expected to be completed by the latter part of 2009, depending upon the Company's ability to raise additional capital. We anticipate that the new factory will generate sufficient cash flows; thus, management has concluded that there is no impairment loss on the construction in progress.
We believe that Xian Tianxing will be developing new products including animal immunization products, non-pathogenic micro-organisms for the cure and prevention of livestock disease, complex enzyme preparations as animal feed additives, and several new veterinary medicine products within the next 12 months.
Contractual Obligations and Off-Balance Sheet Arrangements
Contractual Obligations
| | | Payments Due by Period | |
| Contractual Obligations | | Total | | | Less than 1 year | | | 1 – 3 years | | 3 – 5 years | | | More than 5 years | |
| R&D Project Obligation | | $ | 253,017 | | | $ | 253,017 | | | $ | - | | | $ | - | | | $ | - | |
| Operating Lease Obligations | | | 349,103 | | | | 60,684 | | | | 121,368 | | | | 74,562 | | | | 92,489 | |
| Total | | $ | 602,120 | | | $ | 313,701 | | | $ | 121,368 | | | $ | 74,562 | | | $ | 92,489 | |
During the first quarter of 2008, we entered into an agreement with Northwestern Agricultural Technology University to jointly work on a research and development project regarding the application of nano-technology in the prevention of a major milk cow disease. The total projected budget for this project is approximately $574,000 (RMB 4 million), which we would pay in installments as the stages of the project are completed. We expect this project to be completed in one year. The research and development expense for this project during 2008 was approximately $43,245 (RMB 0.3 million), and as of December 31, 2008, we also made $289,773 (RMB 1,975,273) of prepayment for purchase of specific raw materials for the project. We anticipate that the remaining $253,017 (RMB 1,724,727) will be spent during 2009.
Off-Balance Sheet Arrangements
We do not have any outstanding financial guarantees or commitments to guarantee the payment obligations of any third parties. We have not entered into any derivative contracts that are indexed to our shares and classified as stockholders’ equity or that are not reflected in our consolidated financial statements. Furthermore, we do not have any retained or contingent interest in assets transferred to an unconsolidated entity that serves as credit, liquidity or market risk support to such entity. We do not have any variable interest in any unconsolidated entity that provides financing, liquidity, market risk or credit support to us or engages in leasing, hedging or research and development services with us.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Not applicable.
The Consolidated Financial Statements and Financial Statement Schedule are included in Part III, Item 15 (a) (1) and (2) of this Annual report on Form 10-K.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURES
None.
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our Exchange Act reports is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including its chief executive officer and chief financial officer, as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management necessarily is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
As of December 31, 2008, the end of the fiscal year covered by this report, we carried out an evaluation, under the supervision and with the participation of our management, including our chief executive officer and our chief financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures. Based on the foregoing, our chief executive officer and chief financial officer concluded that our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934) were ineffective at the reasonable assurance level.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. The Company's internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States of America. The Company's internal control over financial reporting includes those policies and procedures that: (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles in the United States of America, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company's assets that could have a material effect on the financial statements.
Any system of internal control, no matter how well designed, has inherent limitations, including the possibility that a control can be circumvented or overridden and misstatements due to error or fraud may occur and not be detected in a timely manner. Also, because of changes in conditions, internal control effectiveness may vary over time. Accordingly, even an effective system of internal control will provide only reasonable assurance with respect to financial statement preparation.
Our management assessed the effectiveness of the Company's internal control over financial reporting as of December 31, 2008. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in "Internal Control-Integrated Framework." Based on that evaluation, our management concluded that as of December 31, 2008, and as of the date that the evaluation of the effectiveness of our disclosure controls and procedures was completed, our disclosure controls and procedures were not effective due to the following material weaknesses:
1. | Accounting and Finance Personnel Weaknesses - The current accounting staff are relatively inexperienced, and requires substantial training so as to meet with the higher demands necessary to fulfill the requirements of U.S. GAAP-based reporting and SEC rules and regulations. |
| 2. | Lack of Internal Audit Function - The Company lacks qualified resources to perform the internal audit functions properly. The Company lacked an internal audit department, which rendered the Company ineffective in preventing and detecting control lapses and errors in the accounting of certain key areas like revenue recognition, purchase approvals, inter-company transactions, cash receipt and cash disbursement authorizations, inventory safeguard and proper accumulation for cost of products, in accordance with the appropriate costing method used by the Company. |
Remediation of Material Weaknesses in Internal Control over Financial Reporting
The Company’s management has identified the steps necessary to address the material weaknesses described above, as follows:
| 1. | Hiring additional accounting and operations personnel and engaging outside contractors with technical accounting expertise, as needed, and reorganizing the accounting and finance department to ensure that accounting personnel with adequate experience, skills and knowledge relating to complex, non-routine transactions are directly involved in the review and accounting evaluation of our complex, non-routine transactions |
| 2. | Involving both internal accounting and operations personnel and outside contractors with technical accounting expertise, as needed, early in the evaluation of a complex, non-routine transaction to obtain additional guidance as to the application of generally accepted accounting principles to such a proposed transaction; |
| 3. | Documenting to standards established by senior accounting personnel and the principal accounting officer the review, analysis and related conclusions with respect to complex, non-routine transactions; |
| 4. | Requiring senior accounting personnel and the principal accounting officer to review complex, non-routine transactions to evaluate and approve the accounting treatment for such transactions; and |
| 5. | Evaluating the internal audit function in relation to the Company’s financial resources and requirements. |
We expect that we will satisfactorily address the control deficiencies and material weaknesses relating to these matters by the end of our fiscal year ending December 31, 2009, although there can be no assurance that compliance will be achieved in this time frame.
Management, including our chief executive officer and our chief financial officer, does not expect that our disclosure controls and internal controls will prevent errors and omissions, even as the same are improved to address any deficiencies and/or weaknesses. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Over time, controls may become inadequate because of changes in conditions or deterioration in the degree of compliance with policies or procedures. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and errors and omissions, if any, within the Company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the control.
Our financial reporting process includes extensive procedures we undertake in order to obtain assurance regarding the reliability of our published financial statements, notwithstanding the material weaknesses in internal control. We expanded our review of accounting for business combinations to help compensate for our material weaknesses in order to provide assurance that the financial statements are free of material inaccuracies or omissions of material fact. As a result, management, to the best of its knowledge, believes that (i) this annual report does not contain any untrue statements of a material fact or omits any material fact and (ii) the financial statements and other financial information included in this report have been prepared in conformity with U.S. GAAP and fairly present in all material aspects our financial condition, results of operations, and cash flows.
Changes in Internal Controls
There were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended) during the year ended December 31, 2008 that have materially affected, or are reasonably likely to materially affect, our international control over financial reporting.
ITEM 9B. OTHER INFORMATION
None.
PART III
Our directors and executive officers, their ages, their respective offices and positions, and their respective dates of election or appointment are as follows:
| Name | | Age | | Position | | Date of Appointment |
| | | | | | | |
| Weibing Lu | | 46 | | | Chief Executive Officer and Chairman of the Board of Directors | | | February 2006 |
| | | | | | | | | |
| Bennet P. Tchaikovsky | | 39 | | | Chief Financial Officer and Director | | | May 2008 |
| | | | | | | | | |
| Wei Wen | | 43 | | | Secretary and Director | | | February 2006 |
| | | | | | | | | |
| R. Scott Cramer | | 45 | | | Director | | | October 2001 |
| | | | | | | | | |
| Qiang Fan | | 54 | | | Director | | | July 2008 |
| | | | | | | | | |
| Chengtun Qu | | 45 | | | Director | | | July 2008 |
| | | | | | | | | |
| Winston Yen | | 40 | | | Director | | | July 2008 |
| | | | | | | | | |
| Shouguo Zhao | | 46 | | | Director | | | July 2008 |
Business Experience Descriptions
Weibing Lu, Chief Executive Officer and Chairman of the Board of Directors
Mr. Weibing Lu received his bachelor’s degree in science from Wuhan University of Mapping Science and Technology (now known as Wuhan University) in 1985. In 1986, he was a teacher of College of Xian Geology. Mr. Lu received his Master’s degree in Business Administration in 1999 from Xian University. Mr. Lu has vast experience in the biotechnology field and in enterprise management. In 1992, he set up the Xian Xingji Electronic Engineering Company and served as its Chairman and President until 1997. In 2002, he was awarded as the title of “Outstanding Enterpriser of Xian Feed Industry” and appointed as a director of Xian Institute of Feed Industry. In July 1997, he set up Xian Tianxing Science and Technology Development Co., Ltd. In December 2003, Xian Tianxing Science and Technology Development Co., Ltd. was reorganized and became Xian Tianxing Bio-pharmaceutical Co., Ltd. Since December 2003, Mr. Lu has served as Chairman of the Board and President of Xian Tianxing Bio-Pharmaceutical Co., Ltd.
Mr. Bennet P. Tchaikovsky joined our company in May 2008. He is also currently serving on the board of directors of Ever-Glory International Group, Inc., an NYSE Alternext US listed company in the apparel industry, as chairman of the audit committee and member of the compensation committee. He is also a member of the board of directors of Sino Clean Energy Inc., an OTCBB company in the alternative fuel industry, as chairman of the audit committee and member of both the compensation and nominating committees. From July 2004 through October 2007, Mr. Tchaikovsky served as the chief financial officer of Innovative Card Technologies, Inc., an OTCBB listed company that researches, develops and markets technology-based card enhancements for financial institutions and enterprises that are designed to increase security for their customers. Mr. Tchaikovsky acted as a consultant to Innovative Card Technologies from November 2007 until July 2008. From January 2003 through November 2003, Mr. Tchaikovsky served as the Vice President, Finance of TJR Industries, Inc., a company that produces trade shows for the woodworking industry. Mr. Tchaikovsky is a licensed Certified Public Accountant and an inactive member of the California State Bar. Mr. Tchaikovsky received a B.A. in Business Economics from the University of California at Santa Barbara, and a J.D. from Southwestern University School of Law.
Wei Wen, Secretary and Director
Mr. Wei Wen graduated from Xian University of Science and Industry in 1986. From 1990 to 1994, Mr. Wen was the manager of Sales Department of Xian Zhongtian Science and Technology Development Co., Ltd. Then, from 1994 to 1997, Mr. Wen served as Vice General Manager and Manager of Sales Department of Xian Xingji Electronic Engineering Company. In 1997, Mr. Wen was appointed as the Vice General Manager of Xian Tianxing Science and Technology Development Co., Ltd. which he served until December 2003. After the reorganization of the company in December 2003, Mr. Wen was appointed and continues to serve as Vice General Manager and the Secretary of the Board of Directors of Xian Tianxing Bio-Pharmaceutical Co., Inc.
R. Scott Cramer, Director
Mr. R. Scott Cramer was previously the Chairman from November 2001 to November 2005, Chief Executive Officer from March 2002 to November 2005, and Chief Financial Officer from April 2003 to November 2005, of The Cyber Group. He is currently a member of our Board of Directors. Mr. Cramer is the founder and President of Cramer & Associates, a firm specializing in retirement management, estate planning and investments. He has been a Registered Investment Advisor since August 2001, a Securities Selling Representative since May 1999, and a General Securities Representative (Registered Representative) since July 2002. Mr. Cramer is a graduate of Seminole Community College with an Associate in Arts degree. He received certification as a Chartered Retirement Planning Counselor from the College of Financial Planning in 2001, as a Certified Estate Planning Professional from the Abts Institute for Estate Preservation in 2001, and as a Certified Senior Advisor from the Society of Senior Advisors in 2002.
Qiang Fan, Director
Mr. Qiang Fan also serves as chairman of our compensation committee and member of our audit committee. Mr. Fan is the President and Founder of MIC Consulting Group, U.S.A., which he established in 1992 to provide operational and financial related problem solving services to privately owned companies. Since 2007, Mr. Fan is the exclusive representative of North America operation for China Venture Capital Research Institute since 2007, and the head analyst at Power Partner Institute focusing on IT trends since 2001. From 2006 to 2007, Mr. Fan was a Vice-president of Operation at Kantan Inc., a privately-held boutique technology company focused on wireless solutions for device manufacturers. From 2005 to 2006, he was a Vice-president at Third Wave Ventures, which provides corporate venturing-related advisory, consulting and management services. From 1998 to 2000, Mr. Fan was the exclusive representative in China for PowerQuest, a Utah based international software company that focused on computer data storage management, as well as for ChipCoolers, a U.S. CPU cooler manufacturer. Mr. Fan received his B.A. degree from the Business School of California State University at San Francisco.
Chengtun Qu, Director
Dr. Chengtun Qu is the Vice Dean of the College of Chemistry and Chemical Engineering at Xi’an Shi You University, where he also teaches and heads the environmental engineering department. Dr. Qu is a board member of both the Shaanxi Province Environmental Protection Association and the Shaanxi Province Chemical Engineering Association. As a principal researcher, Dr. Qu has participated in various projects at both national and provincial levels, including ones sponsored by the Chinese Ministry of Science and Technology, and is the recipient of numerous accolades from the Shaanxi provincial government in recognition of his contributions. Dr. Qu has three patents issued by the Chinese State Intellectual Property Office. He has also been extensively published in various scientific journals both in China and abroad. Dr. Qu has a B.S. degree in chemistry from Northwest University in Xi’an, a master’s degree from Southwest Petroleum University and a doctorate degree from Xi’an Jiaotong University.
Winston Yen, Director
Mr. Winston Yen also serves as chairman of our audit committee and member of our compensation committee. Mr. Yen is an independent director of Mega Joy Limited, a private hotel management and development company based in China, a position he has held since 2006. He previously served as Chief Financial Officer of Chilco River Holdings, Inc. from 2005 to 2007. Mr. Yen is also a Certified Public Accountant, and he has and continues to work in such capacity: as a partner of Accellence LLP from 2006 to the present, as a partner of Harry C. Lin, CPA from 2002 to 2005, as a tax manager with Moss Adams LLP from 2000 to 2001, and as a tax/audit supervising senior accountant with Parks, Palmer, Turner & Yeminijian LLP (currently CBIZ Southern California, Inc.) from 1997 to 2000. Mr. Yen received a Bachelor of Business Management degree from the National Cheng-Chi University in Taiwan, and a Masters of Accounting Science from the University of Illinois at Urbana-Champaign.
Shouguo Zhao, Director
Dr. Shouguo Zhao also serves as member of both our audit and the compensation committees. Dr. Zhao is an independent director of Shaanxi International Trust & Investment Corp., Ltd., a listed company on the Shenzhen Stock Exchange (SZSE: SZ000563), chairing its Remuneration and Assessment Committee and serving on its Strategy Committee. Dr. Zhao is also an independent non-executive director of Sungreen International Holdings Limited, a listed company on the Hong Kong Exchange (HKEX: HK8306), serving as a member of its audit committee. He is additionally an independent director of Tian Di Yuan Co., Ltd., a listed company on the Shenzhen Stock Exchange (SZSE: SH600665), chairing its Nominating Committee and serving on its Strategy Committee. From June 2005 to June 2008, Dr. Zhao was an independent director of IRICO Group Corporation, a listed company on the Shenzhen Stock Exchange (SZSE: SH600707), chairing its Remuneration and Assessment Committee and serving on its Strategy Committee. Dr. Zhao is the Vice Dean of the School of Economics and Management at Northwest University, where he also serves as a guide professor to doctorate candidates in finance and national economics. He has led and participated in 18 research programs sponsored by governments and the private sectors in areas of financial investment, modern enterprise systems and development strategies, and regional economic development strategies, and has more than 30 publications in various academic journals. Dr. Zhao is a member of Shaanxi Provincial Decision-making Consultative Committee, a member of the Executive Committee of the Tenth Session of Shaanxi Provincial Industrial and Commercial Association, the chairman of the Negotiable Securities Research Society of Shaanxi Province, and a consultant with the Listed Companies Association of Shaanxi Province. Dr. Zhao received his doctorate degree in economics from Northwest University.
Family Relationships
There are no family relationships between or among any of our current directors, executive officers or persons nominated or charged by the Company to become directors or executive officers. There are no family relationships among our officers and directors and the officers and directors of our direct and indirect subsidiaries.
Involvement in Certain Legal Proceedings
None of our directors or executive officers has, during the past five years:
| | (a) | Had any bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; |
| (b) | Been convicted in a criminal proceeding or subject to a pending criminal proceeding; |
| | (c) | Been subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction or any federal or state authority, permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities, futures, commodities or banking activities; and |
| | (d) | Been found by a court of competent jurisdiction (in a civil action), the Securities and Exchange Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated. |
Compliance with Section 16(a) of the Exchange Act
Based solely on review of the copies of such forms furnished to the Company, or written representations that no reports were required, the Company believes that for the fiscal year ended December 31, 2008, our directors and executive officers complied with Section 16(a) filing requirements applicable to them, except that Mr. Tchaikovsky, Mr. Cramer, Mr. Fan, Mr. Qu, Mr. Fan and Mr. Zhao did not file their Form 3s, Form 4s or Form 5s in connection with transactions that occurred during 2008.
Code of Ethics
We have adopted a code of ethics that applies to our officers, directors and employees, including our chief executive officer, senior executive officers, principal accounting officer, and other senior financial officers. Our code of ethics is available on our website at www.skystarbio-pharmaceutical.com. A copy of our code of ethics will also be provided to any person without charge, upon written request sent to us at our offices located at Rm. 10601, Jiezuo Plaza, No.4, Fenghui Road South,Gaoxin District, Xi’an, Shaanxi Province, People’s Republic of China.
Material Changes to the Procedures by which Security Holders May Recommend Nominees to the Board of Directors
There have been no material changes to the procedures by which security holders may recommend nominees to the Board of Directors.
Audit Committee
Although we are not a “listed company” under SEC rules and are therefore not required to have an audit committee, we have an audit committee comprised of independent directors. Our audit committee is comprised of three independent directors: Mr. Qiang Fan, Mr. Winston Yen and Dr. Shouguo Zhao. Our board of directors has determined, based on information furnished by Mr. Yen and other available information, that Mr. Yen meets the requirements of an “audit committee financial expert” as such term is defined in the rules promulgated under the Securities Act of 1933 and the Exchange Act of 1934, as amended. On July 14 2008, Mr. Yen was appointed to serve as chairman of the audit committee, and to serve as our audit committee financial expert.
Summary of Compensation
The following summary compensation table indicates the cash and non-cash compensation earned for years ended December 31, 2008 and 2007 by our Chief Executive Officer and each of our other two highest paid executives, whose total compensation exceeded $100,000 (if any) for the years ended December 31, 2008 and 2007.
Summary Compensation
| SUMMARY COMPENSATION TABLE | |
| Name and Principal Position | | Year | | Salary ($) | | Bonus ($) | | Stock Awards ( $) | | Option Awards ($) | | Non-Equity Incentive Plan Compensation ($) | | Nonqualified Deferred Compensation Earnings ($) | | All Other Compensation ( $) | | Total ($) | |
| Weibing Lu, | | | 2008 | | 66,028 (2) | | | -0- | | -0- | | | -0- | | -0- | | | -0- | | -0- | | | 66,028 | |
| current CEO (1) | | | 2007 | | 8,400 (3) | | | -0- | | -0- | | | -0- | | -0- | | | -0- | | -0- | | | 8,400 | |
| (1) | Mr. Lu received no other form of compensation in the years shown, other than the salary set forth in this table. |
| (2) | On May 5, 2008, we entered into an employment agreement with Mr. Lu pursuant to which he is entitled to an initial annual compensation of $100,000 as our Chief Executive Officer. |
| (3) | Mr. Lu’s compensation for 2007 was paid in Chinese RMB which, for reporting purposes, has been converted to U.S. dollars at the conversion rate of 7.6 RMB to one U.S. dollar. |
Employment Agreements, Termination of Employment and Change-in-Control Arrangements with our Executive Officers
Except as described below, we currently have no employment agreements with any of our executive officers, nor any compensatory plans or arrangements resulting from the resignation, retirement or any other termination of any of our executive officers, from a change-in-control, or from a change in any executive officer’s responsibilities following a change-in-control.
Employment Agreement with Weibing Lu
On May 5, 2008, we entered into an Employment Agreement with Mr. Weibing Lu. Under the terms of the Employment Agreement, we agreed to the continued employment of Mr. Lu as our chief executive officer for a term of 5 years. Mr. Lu is to receive an initial annual salary of $100,000, with an annual 5% increase of the prior year’s salary thereafter during the term. Additionally, at the discretion of our board of directors’ compensation committee, Mr. Lu may be eligible for an annual bonus which amount, if any, and payment will be determined by the compensation committee. Mr. Lu is entitled to medical, disability and life insurance, as well as 4 weeks of vacation annually and reimbursement of all reasonable or authorized business expenses.
During its term, the Employment Agreement terminates upon Mr. Lu’s death, in which event we are obligated to pay Mr. Lu’s estate his base salary amount through the first anniversary of his death (or the expiration of the Employment Agreement if earlier than the anniversary date), as well as pro rata allocation of any bonus based on the days of service during the year of death, and all amounts owing to Mr. Lu at the time of termination, including for previously accrued but unpaid bonuses, expense reimbursements and accrued but unused vacation pay.
If Mr. Lu is unable to perform his obligations under the Employment Agreement for over 180 consecutive days during any consecutive 12 months period, we may terminate the Employment Agreement by written notice to Mr. Lu delivered prior to the date that he resumes his duties. Upon receipt of such written notice, Mr. Lu may request a medical examination under which if he is certified to be incapable of performing his obligations for over 2 additional months, the Employment Agreement is terminated. We are obligated to pay Mr. Lu his base salary through the second anniversary of our notice to him of his termination, less any amount Mr. Lu may receive for such period from any Company-sponsored or Company-paid for source of insurance, disability compensation or governmental program. We will also pay Mr. Lu pro rata allocation of any bonus based on the days of service during the year our notice is issued, and all amounts owing to Mr. Lu at the time of termination, including for previously accrued but unpaid bonuses, expense reimbursements and accrued but unused vacation pay.
We may also terminate the Employment Agreement for cause, upon notice if at any time Mr. Lu: (a) refuses in bad faith to carry out specific written directions of our board of directors; (b) intentionally takes fraudulent or dishonest action in his relations with us; (c) is convicted of a crime involving an act of significant moral turpitude; or (d) knowingly commits an act or omits to act in violation of our written policies, the Employment Agreement or any agreements that we may have with third parties and that is materially damaging to our business or reputation. However, termination for the cause described in (a), (b) or (d) is predicated first on Mr. Lu receiving a 5-day written notice and a reasonable opportunity to present his positions, then a subsequent written notice of the termination, with the termination to take effect 20 business days thereafter if Mr. Lu does not dispute the cause for the termination or fails to take corrective actions in good faith. Thereafter, if Mr. Lu takes corrective actions, he may be terminated for the same misconduct upon a 5-day written notice.
On the other hand, Mr. Lu may terminate the Employment Agreement upon written notice if: (w) there is a material adverse change in the nature of his title, duties or obligations; (x) we materially breach the Employment Agreement; (y) we fail to make any payment to Mr. Lu (excepting any payment which is not material and which we are contesting in good faith); or (z) there is a change of control of the Company. However, termination for cause described in (w), (x) or (y) is predicated on our receiving a written notice from Mr. Lu specifying the cause, with the termination to take effect if we fail to take corrective action within 20 business days thereafter. If Mr. Lu terminates the Employment Agreement for any one of these reasons, or if we terminate the Employment Agreement without cause, we are obligated to pay to Mr. Lu (or in the case of his/her death, his estate), his base salary and any bonus, without any offset, as well as all amounts owing to Mr. Lu at the time of termination, including for previously accrued but unpaid bonuses, expense reimbursements and accrued but unused vacation pay.
The Employment Agreement also contains restrictive covenants: (i) preventing the use and/or disclosure of confidential information during or at any time after termination; (ii) preventing competition with the Company during his employment and for a period of 3 years after termination (including contact with or solicitation of our customers, employees or suppliers), provided that Mr. Lu may make investments of up to 2% in the publicly-traded equity securities of any competitor; (iii) requiring Mr. Lu to refer any business opportunities to the Company during his employment and for a period of 1 year after termination. However, Mr. Lu shall have no further obligations with respect to competition and business opportunities if his employment is terminated without cause or if he terminates his employment for cause.
Lastly, we are obligated under the Employment Agreement to indemnify Mr. Lu for any claims made against him in his capacity as our chief executive officer and, in connection to that obligation, we are required to include him under any director and officer insurance policy that is in effect during his employment as our officer, director or consultant.
Loanout Agreement for the Services of Bennet P. Tchaikovsky
On May 5, 2008, we entered into a Loanout Agreement with Worldwide Officers, Inc., a California corporation, pursuant to which we have retained the services of Bennet P. Tchaikovsky to serve as our chief financial officer for a term of one year. Under the terms of the Loanout Agreement, Mr. Tchaikovsky will perform his duties from the United States on a part-time basis (90 hours per month), and we agreed to pay an annual fee of $75,000 for Mr. Tchaikovsky’s services. Additionally, Mr. Tchaikovsky will have the right to receive 52,173 shares of our restricted Common Stock, to vest in four equal installments of 13,043 shares each every 3 calendar months, and the first installment vested on August 5, 2008.
The Loanout Agreement terminates upon Mr. Tchaikovsky’s death. If Mr. Tchaikovsky is unable to perform his obligations under the Loanout Agreement for over 45 consecutive days during the term of the Loanout Agreement, we may terminate the Loanout Agreement by 10-day written notice to Mr. Tchaikovsky thereafter. We may also terminate the Loanout Agreement for cause, upon notice if at any time Mr. Tchaikovsky: (a) willfully breaches or habitually neglects his duties; or (b) commits acts of dishonesty, fraud, misrepresentation, gross negligence or willful misconduct that would prevent the effective performance of his duties or would result in material harm to us or our business. Lastly, we may terminate the Loanout Agreement without cause upon a 30-day written notice to Mr. Tchaikovsky.
On the other hand, Mr. Tchaikovsky may terminate the Loanout Agreement upon 90-day written notice to the Company.
The Loanout Agreement also contains restrictive covenants: (i) preventing the use and/or disclosure of confidential information during or at any time after termination; (ii) preventing competition with the Company during the term of the Loanout Agreement and for a period of 3 years after termination (including contact with or solicitation of our customers, employees or suppliers), provided that Mr. Tchaikovsky may make investments of up to 2% in the publicly-traded equity securities of any competitor; (iii) requiring Mr. Tchaikovsky to refer any business opportunities to the Company during the term of the Loanout Agreement and for a period of 1 year after termination. However, Mr. Tchaikovsky shall have no further obligations with respect to competition and business opportunities if his employment is terminated without cause or if he terminates his employment for cause.
Lastly, we are obligated under the Loanout Agreement to indemnify Mr. Tchaikovsky for any claims made against him in his capacity as our chief executive officer and, in connection to that obligation, we are required to include him under any director and officer insurance policy that is in effect during the term of the Loanout Agreement.
Outstanding Equity Awards at Fiscal Year-End
With the exception of Mr. Bennet P. Tchaikovsky, our current Chief Financial Officer, there are no unexercised options, unvested stock awards or equity incentive plan awards for any of the above-named executive officers outstanding as of December 31, 2008. Pursuant to the terms of his employment under the Loanout Agreement, Mr. Tchaikovsky is to receive 52,173 shares of our restricted common stock for his service period from May 5, 2008 through May 4, 2009, which shares were not issued pursuant to any equity incentive plans in effect. As of December 31, 2008, Mr. Tchaikovsky has received 26,086 shares, with the balance of 26,087 shares to vest during the remainder of his vesting period from January 1, 2009 through May 4, 2009.
Compensation of Directors
The following director compensation disclosure reflects all compensation awarded to, earned by or paid to the directors below for the year ended December 31, 2008.
|
| Name | | Year | | Fees Earned or Paid in Cash ($) | | Stock Awards ($) | | Option Awards ($) | | Non-Equity Incentive Plan Compensation ($) | | Nonqualified Deferred Compensation Earnings ($) | | All Other Compensation ($) | | Total ($) |
| Weibing Lu (1) | | | 2008 | | -0- | | | -0- | | -0- | | | -0- | | -0- | | | -0- | | -0- |
| Wei Wen (1) | | | 2008 | | -0- | | | -0- | | -0- | | | -0- | | -0- | | | -0- | | -0- |
| Erna Gao (1) (2) | | | 2008 | | -0- | | | -0- | | -0- | | | -0- | | -0- | | | -0- | | -0- |
| Xinya Zhang (1) (2) | | | 2008 | | -0- | | | -0- | | -0- | | | -0- | | -0- | | | -0- | | -0- |
| R. Scott Cramer (3) | | | 2008 | | -0- | | | -0- | | -0- | | | -0- | | -0- | | | 195,104 | | 195,104 |
| Qiang Fan (3) | | | 2008 | | 14,055 | | | -0- | | -0- | | | -0- | | -0- | | | -0- | | 14,055 |
| Chengtun Qu (3) | | | 2008 | | 1,368 | | | -0- | | -0- | | | -0- | | -0- | | | -0- | | 1,368 |
| Winston Yen (3) | | | 2008 | | 18,271 | | | -0- | | -0- | | | -0- | | -0- | | | -0- | | 18,271 |
| Shouguo Zhao (3) | | | 2008 | | 3,420 | | | -0- | | -0- | | | -0- | | -0- | | | -0- | | 3,420 |
_______________
| (1) | In connection with the share exchange transaction (described in the Description of Business above under the heading "Corporate Organization and History"), these persons became our directors on November 7, 2005. After the change in control that occurred as a result of the share exchange transaction, we do not have any compensation arrangements with our directors. |
| (2) | Ms. Erna Gao and Mr. Xinya Zhang resigned from our board of directors effective July 14, 2008. |
| (3) | Mr. Cramer was an officer of the Company prior to the share exchange transaction and has stayed on as a director thereafter. Mr. Cramer’s compensation for 2008 was for services provided during the year unrelated to his duties as a director, and includes 90,000 shares of the Company’s restricted common stock already issued to him as well as 110,000 restricted common shares to be issued to him, none of which is pursuant to any equity incentive plan in effect. |
| (4) | Mr. Qiang Fan was appointed to our board of directors effective July 14, 2008, and is entitled to receive annual compensation of $30,000 for his services rendered as a director, as well as chairman of the compensation and member of the audit committee. |
| (5) | Dr. Chengtun Qu was appointed to our board of directors effective July 14, 2008, and is entitled to receive annual compensation of RMB 20,000 for his services rendered as a director. |
| (6) | Mr. Winston Yen was appointed to our board of directors effective July 14, 2008, and is entitled to receive annual compensation of $39,000 for his services rendered as a director, as well as chairman of the audit committee and member of the compensation committee. |
| (7) | Dr. Shouguo Zhao was appointed to our board of directors effective July 14, 2008, and is entitled to an annual compensation of RMB 50,000 for his services rendered as a director, as well as a member of both the audit committee and the compensation committee. |
| (8) | For reporting purposes in this table, compensations in RMB have been converted to U.S. Dollars at the conversion rate of 6.85 RMB to one U.S. Dollar. |
There were no option awards issued to any directors and outstanding as of December 31, 2008.
Director Agreements
Under our agreement with Mr. Fan, he will be entitled to receive annual compensation of $30,000 for his services rendered as a member of the board, as well as the chairman of the compensation committee and member of the audit committee. Mr. Fan’s annual compensation will be paid in cash, although at the discretion of the Board, up to $8,000 of his annual compensation may be paid in the form of a number of shares of the Company’s common stock under the Company’s Stock Incentive Plan #2 (the “Plan”). During his term as a director, we agree to include Mr. Fan as an insured under an officers and directors insurance policy which we will obtain within a reasonable time (the “D&O Insurance”). In addition, the Company has agreed to reimburse Mr. Fan for reasonable expenses incurred in connection with the performance of duties as a director of the Company, including travel expenses.
Under our agreement with Dr. Qu, he will be entitled to receive annual compensation of RMB 20,000 for his services rendered as a member of the board. In addition, the Company has agreed to reimburse Mr. Qu for reasonable expenses incurred in connection with the performance of duties as a director of the Company, including travel expenses.
Under our agreement with Mr. Yen, he will be entitled to receive annual compensation of $39,000 for his services rendered as a member of the board, as well as the chairman of the audit committee and member of the compensation committee. Mr. Yen’s annual compensation will be paid in cash, although at the discretion of the Board, up to $13,000 of his annual compensation may be paid in the form of a number of shares of the Company’s common stock under the Plan. During his term as a director, we agree to include Mr. Yen as an insured under the D&O Insurance. In addition, the Company has agreed to reimburse Mr. Yen for reasonable expenses incurred in connection with the performance of duties as a director of the Company, including travel expenses.
Under our agreement with Dr. Zhao, he will be entitled to receive annual compensation of RMB 50,000 for his services rendered as a member of the board, as well as a member of both the audit committee and the compensation committee. In addition, the Company has agreed to reimburse Mr. Zhao for reasonable expenses incurred in connection with the performance of duties as a director of the Company, including travel expenses.
Indemnification of Officers and Directors
We are a Nevada corporation, and accordingly, we are subject to the corporate laws under the Nevada Revised Statutes. Pursuant to Article 7 of our articles of incorporation and Nevada’s Revised Business Statutes, our bylaws contain the following indemnification provisions for our directors and officers:
“Section 8.1. Indemnification. No officer or director shall be personally liable for any obligations arising out of any acts or conduct of said officer or director performed for or on behalf of the Corporation. The Corporation shall and does hereby indemnify and hold harmless each person and his heirs and administrators who shall serve at any time hereafter as a director or officer of the Corporation from and against any and all claims, judgments and liabilities to which such persons shall become subject by reason of any action alleged to have been heretofore or hereafter taken or omitted to have been taken by him as such director or officer, and shall reimburse each such person for all legal and other expenses reasonably incurred by him in connection with any such claim of liability; including power to defend such person from all suits as provided for under the provisions of the Nevada Corporation Laws; provided, however that no such person shall be indemnified against, or be reimbursed for, any expense incurred in connection with any claim or liability arising out of his own gross negligence or willful misconduct. The rights accruing to any person under the foregoing provisions of this section shall not exclude any other right to which he may lawfully be entitled, nor shall anything herein contained restrict the right of the Corporation to indemnify or reimburse such person in any proper case, even though not specifically herein provided for. The Corporation, its directors, officers, employees and agents shall be fully protected in taking any action or making any payment or in refusing so to do in reliance upon the advice of counsel.
Section 8.2. Other Indemnification. The indemnification herein provided shall not be deemed exclusive of any other rights to which those seeking indemnification may be entitled under any bylaw, agreement, vote of shareholders or disinterested directors, or otherwise, both as to action in his official capacity and as to action in another capacity while holding such office, and shall continue as to a person who has ceased to be a director, officer or employee and shall inure to the benefit of the heirs, executors and administrators of such a person.
Section 8.3. Insurance. The Corporation may purchase and maintain insurance on behalf of any person who is or was a director, officer or employee of the Corporation, or is or was serving at the request of the Corporation in such capacity for another corporation, partnership, joint venture, trust or other enterprise, against any liability asserted against him and incurred by him in any capacity, or arising out of his status as such, whether or not the Corporation would have the power to indemnify him against liability under the provisions of this Article VIII or the laws of the State of Nevada.
Section 8.4. Settlement by Corporation. The right of any person to be indemnified shall be subject always to the right of the Corporation by its Board of Directors, in lieu of such indemnity, to settle any such claim, action, suit or proceeding at the expense of the Corporation by the payment of the amount of such settlement and the costs and expenses incurred in connection therewith.”
These indemnification provisions may be sufficiently broad to permit indemnification of the registrant's executive officers and directors for liabilities (including reimbursement of expenses incurred) arising under the Securities Act of 1933.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. No pending material litigation or proceeding involving our directors, executive officers, employees or other agents as to which indemnification is being sought exists, and we are not aware of any pending or threatened material litigation that may result in claims for indemnification by any of our directors or executive officers.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Equity Compensation Plan Information
Plan Category | | Number of securities to be issued upon exercise of outstanding options, warrants and rights | | | Weighted-average exercise price of outstanding options, warrants and rights | | | Number of securities remaining available for future issuance under equity compensation plans | |
| | | | | | | | | | |
Equity compensation plans approved by security holders | | | | | | | | | | | | |
Equity compensation plans not approved by security holders | | | 0 | | | | 0 | | | | 2,693,100 | (1)(2) |
| TOTAL | | | 0 | | | | 0 | | | | 2,693,100 | |
_____________________
As of December 31, 2008, the Company had the following two equity compensation plans in effect:
| (1) | On October 16, 2002, the Company adopted a stock incentive plan for officers, directors, employees, and consultants entitled the “Cyber Group Network Corporation Stock Incentive Plan # 2” (hereinafter the “2002 Plan”). The maximum number of shares that may be issued under the 2002 Plan is 40,000,000 shares of our common stock. The 2002 Plan has not previously been approved by security holders and awards may be granted under this Plan until October 15, 2012. Under this Plan, the Company may issue common stock and/or options to purchase common stock to certain officers, directors and employees and consultants of the Company and its subsidiaries. The 2002 Plan is administered either by the compensation committee or a committee appointed by the Board, which is comprised of a combination of two or more officers and/or members of the Board. The committee has full and complete authority, in its discretion, but subject to the express provisions of the Plan to approve the eligible persons nominated by the management of the Company to be granted awards of common stock “Awards”) or stock options, to determine the number of Awards or stock options to be granted to an eligible person; to determine the time or times at which or stock options shall be granted; to establish the terms and conditions upon which Awards or Stock Options may be exercised; to remove or adjust any restrictions and conditions upon Awards or Stock Options; to specify, at the time of grant, provisions relating to exercisability of Stock Options and to accelerate or otherwise modify the exercisability of any Stock Options; and to adopt such rules and regulations and to make all other determinations deemed necessary or desirable for the administration of the Plan. As of December 31, 2008, there are 2,093,452 shares of our common stock remaining available for future issuance under the 2002 Plan. |
| (2) | On February 22, 2006, the Company adopted a stock incentive plan for consultants entitled the “2006 Consultant Stock Plan” (hereinafter the “2006 Plan”). The maximum number of shares that may be issued under the 2006 Plan is 1,199,648 shares of our common stock. The 2006 Plan has not previously been approved by security holders and awards may be granted under this Plan until February 21, 2016. Under the 2006 Plan, the Company may issue common stock to certain consultants of the Company who are crucial to the future growth and success of the Company and its subsidiaries and affiliates. The 2006 Plan is administered by either a committee appointed by the Board, which is comprised of one or more members of the Board who is not serving on another plan committee, or the Board. The committee has full and complete authority, in its discretion, but subject to the express provisions of the Plan, to designate the persons or classes of persons eligible to receive awards of common stock “Awards”; to determine the form and amount of Awards to be granted to an eligible person or class of persons; to establish the terms and conditions upon which Awards may be exercised; to remove or adjust any restrictions and conditions upon Awards; and to adopt such rules and regulations and to make all other determinations deemed necessary or desirable for the administration of the Plan. As of December 31, 2008, there are 599,648 shares of our common stock remaining available for future issuance under the 2006 Plan. |
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth certain information regarding our common stock beneficially owned on March 25, 2009, for (i) each stockholder known to be the beneficial owner of 5% or more of our outstanding common stock, (ii) each executive officer and director, and (iii) all executive officers and directors as a group. In general, a person is deemed to be a "beneficial owner" of a security if that person has or shares the power to vote or direct the voting of such security, or the power to dispose or to direct the disposition of such security. A person is also deemed to be a beneficial owner of any securities of which the person has the right to acquire beneficial ownership within 60 days. Shares of common stock subject to options, warrants or convertible securities exercisable or convertible within 60 days of March 25, 2009 are deemed outstanding for computing the percentage of the person or entity holding such options, warrants or convertible securities but are not deemed outstanding for computing the percentage of any other person. To the best of our knowledge, subject to community and martial property laws, all persons named have sole voting and investment power with respect to such shares, except as otherwise noted.
| Title of Class | | Name and Address of Beneficial Owners (1) | | Amount of Beneficial Ownership | | | Percent of Class (2) | |
| | | | | | | | | |
| Common Stock | | Upform Group Limited (3) | | | 4,695,623 | | | | 25.16 | % |
| Common Stock | | Weibing Lu, Director and Chief Executive Officer (3) | | | 4,695,623 | | | | 25.16 | % |
| Common Stock | | Wei Wen, Director (4) | | | 207,715 | | | | 1.11 | % |
| Common Stock | | Bennet P. Tchaikovsky, Chief Financial Officer (5) | | | 52,173 | | | | * | |
| Common Stock | | R. Scott Cramer, Director (6) | | | 892,231 | | | | 4.78 | % |
| Common Stock | | Qiang Fan, Director (7) | | | -0- | | | | 0 | % |
| Common Stock | | Chengtun Qu (8) | | | -0- | | | | 0 | % |
| Common Stock | | Winston Yen (9) | | | -0- | | | | 0 | % |
| Common Stock | | Shouguo Zhao (10) | | | -0- | | | | 0 | % |
| Common Stock | | Premier Renn US Emerging Fund Limited (11) | | | 1,051,044 | | | | 5.63 | % |
| Common Stock | | Renaissance US Growth Investment Trust PLC (11) | | | 2,916,857 | | | | 15.63 | % |
| Common Stock | | All officers and directors as a group (8 total) | | | 5,595,569 | | | | 30.36 | % |
__________
* Less than 1%.
| (1) | Unless otherwise noted, the address for each of the named beneficial owners is: Rm. 10601, Jiezuo Plaza, No.4, Fenghui Road South, Gaoxin District, Xi’an, Shaanxi Province, People’s Republic of China. |
| (2) | Unless otherwise noted, the number and percentage of outstanding shares of our common stock is based upon 18,665,189 shares outstanding as of March 25, 2009. |
| (3) | Upform Group Limited’s (“Upform Group”) address is Sea Meadow House, Blackburne Highway, P.O. Box 116, Road Town, Tortola, British Virgin Islands. Weibing Lu and Xinya Zhang are directors of the Upform Group. Mr. Lu is the majority stockholder and the Chairman of the Board of Directors of Upform Group, and thus Mr. Lu indirectly owns the shares held by Upform Group, through his majority ownership of Upform Group. Thus, the number of shares reported herein as beneficially owned by Mr. Lu therefore includes the shares held by Upform Group. Similarly, because Xinya Zhang is a director of Upform Group, he might be deemed to have or share investment control over Upform Group’s portfolio. Thus, the number of shares reported herein as beneficially owned by Mr. Zhang also include the shares held by Upform Group. |
| (4) | The number of shares reported herein as beneficially owned by Mr. Wen includes the shares held Clever Mind International Limited, which address is: Sea Meadow House, Blackburne Highway, P.O. Box 116, Road Town, Tortola, British Virgin Islands. Mr. Wen is Chairman of the Board of Directors of Clever Mind and owns approximately 2.3% of the issued and outstanding shares of Clever Mind. Because Mr. Wen is a director of Clever Mind, he might be deemed to have or share investment control over Clever Mind’s portfolio. |
| (5) | Bennet P. Tchaikovsky’s address is: 6571 Morningside Drive, Huntington Beach, CA 92648. Includes 26,087 shares that Mr. Tchaikovsky has right to acquire beneficial ownership of within 60 days of March 25, 2009. |
| (6) | R. Scott Cramer’s address is: 1012 Lewis Dr., Winter Park, FL 32789. Includes 771,411 shares held by the Cramer Family Trust of which Mr. Cramer is the sole trustee and sole primary beneficiary, and 110,000 shares that Mr. Cramer has right to acquire beneficial ownership of within 60 days of March 25, 2009. |
| (7) | Qiang Fan’s address is: 9176 West Laguna Way, Elk Grove, CA 95758. |
| (8) | Chengtun Qu’s address is: No. 18 Dian Zi 2nd Road, School of Chemistry & Chemical Engineering, Xi'an Shiyou University, Xi'an, Shaanxi Province, People’s Republic of China. |
| (9) | Winston Yen’s address is: 345 S. Figueroa Street, Suite 100, Los Angeles, California 90071. |
| (10) | Shouguo Zhao’s address is: No. 229 North Tai Bai Road, School of Economics and Management, Northwest University, Xi'an, Shaanxi Province, People’s Republic of China. |
| (11) | The address for both Renaissance US Growth Investment Trust PLC (“Renaissance”) and Premier RENN US Emerging Growth Fund Ltd.’s (“RENN”) is: 8080 North Central Expressway, Suite 210, Dallas, Texas 75206. Russell Cleveland is the natural person who has voting power and the power to sell, transfer or otherwise dispose of the common stock held by Renaissance and RENN. Because Renaissance and RENN share common control, they are deemed affiliates of each other. |
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Our Officers and Directors’ Relationship with Skystar, Our Subsidiaries and VIE
Mr. Weibing Lu, our Chairman and Chief Executive Officer, is a Director of Upform Group Limited, a British Virgin Islands company which owns approximately 25.16% of Skystar’s issued and outstanding common stock. Mr. Wei Wen, who is one of our directors, is Director of Clever Mind International Limited, a British Virgin Islands company which owns approximately 1.11% of Skystar’s issued and outstanding common stock. Mr. R. Scott Cramer, who is also one of our directors, owns and/or controls approximately 4.19% of Skystar’s issued and outstanding common stock. Mr. Lu and Mr. Wen are both Directors of Skystar Cayman, our wholly owned subsidiary.
Mr. Wen is Director of Fortunate Time, wholly-owned subsidiary of Skystar Cayman.
The management of Sida, the wholly-owned subsidiary of Fortunate Time, includes Mr. Wen as General Manager.
The management of Xian Tianxing, which we control through contractual arrangements between Sida and Xian Tianxing, includes Mr. Lu as Chairman and Chief Executive Officer and Mr. Wen as Vice-General Manager and Director. As of the date of this report, Mr. Lu also owns approximately 25.16%, and Mr. Wen approximately 1.11%, of the issued and outstanding stock of Xian Tianxing.
Mr. Wen is the General Manager of Shanghai Siqiang, wholly-owned subsidiary of Xian Tianxing.
Conflicts of interests between Mr. Lu and Mr. Wen’s duties to our Company and Xian Tianxing may arise. As they are directors of the Company (and also an executive officer in the case of Mr. Lu), they have a duty of loyalty and care to us under U.S. and Cayman Islands law when there are any potential conflicts of interests between our Company and Xian Tianxing. We cannot assure you, however, that when conflicts of interest arise, they will act completely in our interests or that conflicts of interests will be resolved in our favor. In addition, they could violate their legal duties by diverting business opportunities from us to others. If we cannot resolve any conflicts of interest between us and Mr. Lu or between us and Mr. Wen, we would have to rely on legal proceedings, which could result in the disruption of our business.
Certain Relationships and Related Transactions
Amounts receivable from and payable to related parties are summarized as follows:
| | | December 31, 2008 | | | December 31, 2007 | |
| Short term loans from shareholders | | | | | | |
| Mr. Weibing Lu – officer and shareholder (1) (2) | | $ | 220,050 | | | $ | - | |
| Mr. Wei Wen – officer and shareholder (2) | | | 44,010 | | | | - | |
| Ms. Aixia Wang – shareholder (2) | | | 44,010 | | | | - | |
| Total | | $ | 308,070 | | | $ | - | |
| | | | | | | | | |
| Amounts due from related party | | | | | | | | |
| Mr. Weibing Lu – officer and shareholder (3) | | $ | - | | | $ | 59,462 | |
| | | | | | | | | |
| Shares to be issued to related party | | | | | | | | |
| Scott Cramer – non-executive director (4) | | $ | 95,204 | | | $ | - | |
| | | | | | | | | |
| Amounts due to related parties | | | | | | | | |
| TianXing Digital - owned by a director (5) | | $ | - | | | $ | 17,137 | |
| Ms. Aixia Wang – shareholder (5) | | | - | | | | 1,371 | |
| Bennet P. Tchaikovsky – CFO (5) | | | 13,168 | | | | - | |
| Scott Cramer – non-executive director and shareholder (5) | | | 224,684 | | | | 30,245 | |
| Shaanxi Xingji Electronics Co. - owned by a director's wife (5) | | | 4,373 | | | | 32,817 | |
| Total | | $ | 242,225 | | | $ | 81,570 | |
| (1) | During 2008, Mr. Lu obtained a personal loan from Huaxia Bank’s Xian Branch and advanced $176,040 in cash from theproceeds to XianTianxing to facilitate its operations. The loan, which matured on December 30, 2008, bore an interest rate of7.47% per annum, was unsecured and was guaranteed by Xian Tianxing. On January 4, 2009, Xian Tianxing paid to the bank the full principal amount along with interest of $15,741. |
| (2) | On May 29, 2008, Mr. Lu, Mr. Wen and Ms. Wang obtained personal loans from Yanta Credit Union’s East Xiaozai RoadBranch, and advanced $132,030 in cash from the proceeds to Xian Tianxing to facilitate its operations. The loans, which are dueon May 29, 2009, have 8.436% interest per annum and are guaranteed by Xian Tianxing. As of December 31, 2008, Xian Tianxing had paid $3,695 in interest for the loans of these three shareholders. |
| (3) | As of December 31, 2008 and 2007, the Company had a receivable from Mr. Lu in the amount of $0 and $59,462, respectively.The amounts due from Mr. Lu was comprised of cash advances to Mr. Lu to facilitate Xian Tianxing’s operations and for thereimbursement of expenses paid, or to be paid, by Mr. Lu on behalf of Xian Tianxing. These balances are non-interest bearing, unsecured, and due on demand. |
| (4) | As of December 31, 2008, the Company had $95,204 in common shares to be issued to Mr. Cramer, which represented 110,000common shares as compensation to Mr. Cramer for services as a U.S. representative of the Company for the period from May2008 to December 2008. |
| (5) | Shaanxi Xinji Electronics Co., Ltd. is owned by the wife of Mr. Lu and Tianxing Digital Co., Ltd. is owned by Mr. Lu. The amounts due to Shaanxi Xinji Electronics and Tianxing Digital as of December 31, 2007 were short term cash transfers for business operations, non-interest bearing, unsecured, and payable upon demand. As of December 31, 2008 and 2007, the Company also had $224,684 and $13,168 payable to Scott Cramer and Bennet P. Tchaikovsky for the expenses paid by them on behalf of the Company. |
On January 1, 2007, we entered into a 5-year lease agreement with Mr. Weibing Lu to lease the premises at Rm. 10601, Jiezuo Plaza, No.4, Fenghui Road South, Gaoxin District, Xian Province, PRC, which belongs to Mr. Lu and which has been serving as our headquarters. The annual rent under the lease agreement is RMB 165,600 (approximately $24,000). Mr. Lu previously provided the premises rent-free, in 2005 and 2006, for the use of our administrative division.
The Company also entered into a tenancy agreement with Mr. Lu for the lease of Shanghai Siqiang’s office for a period of ten years from August 1, 2007 to August 1, 2017, with annual rent of approximately $21,000 (or RMB 144,000).
Other than the above transactions or otherwise set forth in any reports filed by the Company with the SEC, the Company and its subsidiaries have not entered into any material transactions with any director, executive officer, and nominee for director, beneficial owner of five percent or more of its common stock, or family members of such persons. The Company is not a subsidiary of any company.
Based upon information submitted by Mr. Qiang Fan, Dr. Chengtun Qu, Mr. Winston Yen and the Dr. Shouguo Zhao, our board of directors has determined that each of them is “independent” under the listing standards of NYSE Alternext US LLC (formerly the American Stock Exchange).
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
Audit Fees
For the years ended December 31, 2008 and 2007, the aggregate fees billed by our independent auditors, Moore Stephens Wurth Frazer & Torbet LLP (“Moore Stephens”), for professional services rendered for the audit of the Company’s annual financial statements and review of the Company’s quarterly financial statements were $185,000 and $160,000, respectively.
Audited-Related Fees
For the years ended December 31, 2008 and 2007, there were no fees billed by Moore Stephens for services reasonably related to the performance of the audit or review of the financial statements outside of those fees disclosed above under “Audit Fees,”
Tax Fees
For the years ended December 31, 2008 and December 31, 2007, the aggregate fees billed by Moore Stephens for services rendered for tax compliance, tax advice and tax planning work to the Company were $7,000 and $5,000, respectively.
All Other Fees
For the years ended December 31, 2008 and December 31, 2007, there were no other fees billed by Moore Stephens for products and services outside of those fees disclosed above under “Audit Fees”, “Audit-Related Fees” and “Tax Fees”.
ITEM 15. EXHIBITS
(1) Financial Statements
The following consolidated financial statements of Skystar are included in Part II, Item 8 of this Report:
Report of Independent Registered Public Accounting Firm
Consolidated Balance Sheets at December 31, 2008 and 2007
Consolidated Statements of Operations and Comprehensive Income for the Years Ended December 31, 2008 and 2007
Consolidated Statements of Shareholders’ Equity for the Years Ended December 31, 2008 and 2007
Consolidated Statements of Cash Flows for the Years Ended December 31, 2008 and 2007
Notes to Consolidated Financial Statements
(2) Financial Statement Schedules
Schedules are omitted because the required information is not present or is not present in amounts sufficient to require submission of the schedule or because the information required is given in the consolidated financial statements or the notes thereto.
(3) Exhibits
| Exhibit Number | | Description |
| 2.1 | | Share Purchase Agreement by and between The Cyber Group Network, Inc. and Howard L. Allen and Donald G. Jackson (shareholders of Hollywood Entertainment Network, Inc.) dated May 12, 2000 (1) |
| | | |
| 2.2 | | Plan of Merger Agreement between The Cyber Group Network Corp. and CGN Acquisitions Corporation dated December 7, 2000 (2) |
| | | |
| 2.3 | | Share Exchange Agreement between The Cyber Group Network Corporation, R. Scott Cramer, Steve Lowe, David Wassung and Skystar Bio-Pharmaceutical, and the Skystar Shareholders dated September 20, 2005 (3) |
| | | |
| 3.1 | | Charter of The Cyber Group Network Corporation as filed with the State of Nevada (4) |
| | | |
| 3.2 | | Certificate of Amendment and Certificate of Change (5) |
| | | |
| 3.3 | | Certificate of Amendment to Increase Number of Authorized Shares of Common Stock (13) |
| | | |
| 3.4 | | Amended and Restated Bylaws of Skystar Bio-Pharmaceutical Company (14) |
| | | |
| 4.1 | | Certificate of Designation of Series B Convertible Preferred Stock (4) |
| | | |
| 4.2 | | Form of Class A Convertible Debenture (6) |
| | | |
| 4.3 | | Form of Class B Convertible Debenture (6) |
| | | |
| 4.4 | | Form of Class A Warrant (6) |
| | | |
| 4.5 | | Form of Class B Warrant (6) |
| | | |
| 10.1 | | Form of Securities Purchase Agreement, dated as of February 26, 2007 by and among the Company and eight accredited investors (6) |
| | | |
| 10.2 | | Form of Registration Rights Agreement, dated as of February 26, 2007 by and among the Company and eight accredited investors (6) |
| | | |
| 10.3 | | Form of Company Principal Lockup Agreement in connection with the Securities Purchase Agreement dated as of February 26, 2007 (6) |
| | | |
| 10.4 | | Form of the Amendment, Exchange and Waiver Agreement between the Company and certain accredited investors dated November 9, 2007 (7) |
| | | |
| 10.5 | | Form of the Amendment and Waiver Agreement between Skystar Bio-Pharmaceutical Company and two institutional and accredited investors dated March 31, 2008 (10) |
| | | |
| 10.6 | | Amendment to Consulting Services Agreement among Skystar Cayman, Xian Tianxing and Sida Biotechnology (Xian) Co., Ltd. (“Sida”) dated March 10, 2008 (8) |
| | | |
| 10.7 | | Agreement to Transfer of Operating Agreement among Skystar Cayman, Xian Tianxing, Xian Tianxing’s Majority Stockholders, Weibing Lu and Sida dated March 10, 2008 (8) |
| | | |
| 10.8 | | Amendment to Equity Pledge Agreement among Skystar Cayman, Xian Tianxing, Xian Tianxing’s Majority Stockholders, and Sida dated March 10, 2008 (8) |
| | | |
| 10.9 | | Designation Agreement among Skystar Cayman, Xian Tianxing, Xian Tianxing’s Majority Stockholders, Weibing Lu and Sida dated March 10, 2008 (8) |
| | | |
| 10.10 | | Agreement to Transfer of Option Agreement among Skystar Cayman, Xian Tianxing, Xian Tianxing Majority Stockholders, Weibing Lu and Sida dated March 10, 2008 (8) |
| | | |
| 10.11 | | Employment Agreement with Weibing Lu dated May 5, 2008 (11) |
| | | |
| 10.12 | | Loanout Agreement with Worldwide Officers, Inc. with respect to the services of Bennet Tchaikovsky, our Chief Financial Officer, dated May 5, 2008 (11) |
| | | |
| 10.13 | | Form of Director Offer Letter with Mr. Qiang Fan and Mr. Winston Yen (14) |
| | | |
| 10.14 | | Form of Director Offer Letter with Chengtun Qu and Shouguo Zhao (14) |
| | | |
| 14.1 | | Code of Ethics (15) |
| | | |
| 21.1 | | List of subsidiaries (15) |
| | | |
| 31.1 | | Section 302 Certification by the Corporation’s Chief Executive Officer (15) |
| | | |
| 31.2 | | Section 302 Certification by the Corporation’s Chief Financial Officer (15) |
| | | |
| 32.1 | | Section 906 Certification by the Corporation's Chief Executive Officer (15) |
| | | |
| 32.2 | | Section 906 Certification by the Corporation's Chief Financial Officer (15) |
| | | |
| 99.1 | | Legal Opinion from Allbright Law Offices regarding the transfer of the contractual arrangements from Skystar Cayman to Sida, dated April 29, 2008 (12) |
| | | |
| 99.2 | | Lease Agreement between Xian Tianxing and Weibing Lu dated June 1, 2007 (9) |
| | | |
| 99.3 | | Lease Agreement between Shanghai Siqiang Biotechnological Co., Ltd. and Weibing Lu dated June 17, 2007 (12) |
| | | |
| 99.4 | | Summary of Research Arrangement between Shanghai Poultry Verminosis Institute and Xian Tianxing (12) |
| | | |
| 99.5 | | Cooperation Agreement between Shaanxi Microbial Institute and Xian Tianxing (12) |
_________
| (1) | Incorporated by reference from the Registrant’s Current Report on Form 8-K/A filed on June 1, 2000. |
| (2) | Incorporated by reference from the Registrant’s Current Report on Form 8-K/A filed on January 12, 2001. |
| (3) | Incorporated by reference from the Registrant’s Current Report on Form 8-K filed on September 26, 2005. |
| (4) | Incorporated by reference from the Registrant’s Current Report on Form 8-K filed on November 14, 2005. |
| (5) | Incorporated by reference from the Registrant’s Annual Report on Form 10-KSB filed on April 17, 2006. |
| (6) | Incorporated by reference from the Registrant’s Current Report on Form 8-K filed on March 5, 2007. |
| (7) | Incorporated by reference from the Registrant’s Current Report on Form 8-K filed on December 11, 2007. |
| (8) | Incorporated by reference from the Registrant’s Current Report on Form 8-K on March 11, 2008. |
| (9) | Incorporated by reference from the Registrant’s Annual Report on Form 10-K on April 2, 2008. |
| (10) | Incorporated by reference from the Registrant’s Current Report on Form 8-K on April 23, 2008. |
| (11) | Incorporated by reference from the Registrant’s Current Report on Form 8-K on May 5, 2008. |
| (12) | Incorporated by reference from the Registrant’s Registration Statement on Form S-1/A filed on June 26, 2008. |
| (13) | Incorporated by reference from the Registrant’s Current Report on Form 8-K on July 14, 2008. |
| (14) | Incorporated by reference from the Registrant’s Current Report on Form 8-K on July 15, 2008. |
SIGNATURES
Pursuant to the requirements of section 13 or 15 (d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | SKYSTAR BIO-PHARMACEUTICAL COMPANY (Registrant) | |
| | | | | |
| | | | | |
| | Date: April 15, 2009 | By: | /s/ Weibing Lu | |
| | | | Weibing Lu Chief Executive Officer | |
| | Date: April 15, 2009 | By: | /s/ Bennet P. Tchaikovsky | |
| | | | Bennet P. Tchaikovsky Chief Financial Officer | |
In accordance with the Exchange Act, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Name | | Title | | Date |
| | | | | |
| | | | | |
| /s/ Weibing Lu | | Chief Executive Officer / Director | | April 15, 2009 |
| Weibing Lu | | | | |
| | | | | |
| | | | | |
| /s/ Bennet P. Tchaikovsky | | Chief Financial Officer | | |
| Bennet P. Tchaikovsky | | | | |
| | | | | |
| | | | | |
| /s/ Wei Wen | | Secretary / Director | | |
| Wei Wen | | | | |
| | | | | |
| | | | | |
| /s/ R. Scott Cramer | | Director | | |
| R. Scott Cramer | | | | |
| | | | | |
| | | | | |
| /s/ Qiang Fan | | Director | | |
| Qiang Fan | | | | |
| | | | | |
| | | | | |
| /s/ Chengtun Qu | | Director | | |
| Chengtun Qu | | | | |
| | | | | |
| | | | | |
| /s/ Winston Yen | | Director | | |
| Winston Yen | | | | |
| | | | | |
| | | | | |
| /s/ Shouguo Zhao | | Director | | |
| Shouguo Zhao | | | | |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of
Skystar Bio-Pharmaceutical Company and Subsidiaries
We have audited the accompanying consolidated balance sheets of Skystar Bio-Pharmaceutical Company and Subsidiaries (the “Company”) as of December 31, 2008 and 2007, and the related consolidated statements of operations and other comprehensive income, shareholders’ equity, and cash flows for each of the years in the two-year period ended December 31, 2008. The Company’s management is responsible for these consolidated financial statements. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Skystar Bio-Pharmaceutical Company and Subsidiaries as of December 31, 2008 and 2007, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2008, in conformity with accounting principles generally accepted in the United States of America.
/s/ Moore Stephens Wurth Frazer and Torbet, LLP
Walnut, California
April 14, 2009
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
AS OF DECEMBER 31, 2008 AND 2007
ASSETS
| | | 2008 | | | 2007 | |
| | | | | | | |
| CURRENT ASSETS: | | | | | | |
| Cash | | $ | 576,409 | | | $ | 771,492 | |
| Restricted cash | | | 80,885 | | | | 74,969 | |
| Short-term investment | | | 352,080 | | | | 383,880 | |
Accounts receivable, net of allowance for doubtful accounts of $327,857 and $199,639 as of December 31, 2008 and 2007, respectively. | | | 2,424,102 | | | | 1,356,094 | |
| Inventories | | | 3,086,060 | | | | 2,242,611 | |
| Deposits and prepaid expenses | | | 4,878,851 | | | | 806,657 | |
| Loan and other receivables | | | 380,186 | | | | 628,772 | |
| Amounts due from related party | | | - | | | | 59,462 | |
| Total current assets | | | 11,778,573 | | | | 6,323,937 | |
| | | | | | | | | |
| PROPERTY, PLANT AND EQUIPMENT, NET | | | 7,413,689 | | | | 6,262,731 | |
| CONSTRUCTION-IN-PROGRESS | | | 6,516,630 | | | | 5,531,236 | |
| | | | | | | | | |
| OTHER ASSETS: | | | | | | | | |
| Long term prepayments | | | 5,207,117 | | | | 1,220,190 | |
| Deferred financing costs | | | - | | | | 101,815 | |
| Intangible assets, net | | | 899,529 | | | | 1,011,236 | |
| Total other assets | | | 6,106,646 | | | | 2,333,241 | |
| Total assets | | $ | 31,815,538 | | | $ | 20,451,145 | |
| | | | | | | | | |
| LIABILITIES AND SHAREHOLDERS' EQUITY |
| | | | | | | | | |
| CURRENT LIABILITIES: | | | | | | | | |
| Short term loan | | $ | 748,170 | | | $ | - | |
| Accounts payable | | | 547,430 | | | | 126,754 | |
| Accrued expenses | | | 1,488,575 | | | | 502,871 | |
| Short term loans from shareholders | | | 308,070 | | | | - | |
| Deposits from customers | | | 424,266 | | | | 61,706 | |
| Taxes payable | | | 212,661 | | | | 568,797 | |
| Other payables | | | 68,398 | | | | 81,221 | |
| Shares to be issued to related party | | | 95,204 | | | | - | |
| Amounts due to related parties | | | 242,225 | | | | 81,570 | |
| Total current liabilities | | | 4,134,999 | | | | 1,422,919 | |
| | | | | | | | | |
| OTHER LIABILITIES: | | | | | | | | |
| Deferred government grant | | | 1,100,250 | | | | 1,028,250 | |
| Convertible debentures, fully converted as of December 31, 2008 and net of | | | | | | | | |
| $398,171 discount as of December 31, 2007 | | | - | | | | 84,752 | |
| Total other liabilities | | | 1,100,250 | | | | 1,113,002 | |
| Total liabilities | | | 5,235,249 | | | | 2,535,921 | |
| | | | | | | | | |
| COMMITMENTS AND CONTINGENCIES | | | | | | | | |
| | | | | | | | | |
| SHAREHOLDERS' EQUITY: | | | | | | | | |
Preferred stock, $0.001 par value, 50,000,000 shares authorized, 2,000,000 series "A" shares issued and outstanding as of December 31, 2008 and 2007, respectively; Nil series "B" shares issued and outstanding as of December 31, 2008 and 2007, respectively. | | | 2,000 | | | | 2,000 | |
Common stock, $0.001 par value, 200,000,000 and 50,000,000 shares authorized as of December 31, 2008 and 2007, respectively; 18,665,189 and 17,111,200 shares | | | | | | | | |
| issued and outstanding as of December 31, 2008 and 2007, respectively. | | | 18,665 | | | | 17,111 | |
| Paid-in-capital | | | 16,330,843 | | | | 14,741,278 | |
| Deferred compensation | | | - | | | | (62,758 | ) |
| Statutory reserves | | | 2,952,710 | | | | 1,652,720 | |
| Retained earnings | | | 4,418,464 | | | | 122,271 | |
| Accumulated other comprehensive income | | | 2,857,607 | | | | 1,442,602 | |
| Total shareholders' equity | | | 26,580,289 | | | | 17,915,224 | |
| Total liabilities and shareholders' equity | | $ | 31,815,538 | | | $ | 20,451,145 | |
The accompanying notes are an integral part of these consolidated financial statements.
See report of independent registered public accounting firm.
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS AND OTHER COMPREHENSIVE INCOME
FOR THE YEARS ENDED DECEMBER 31, 2008 and 2007
| | | Years ended December 31, | |
| | | 2008 | | | 2007 | |
| | | | | | | |
| REVENUE, NET | | $ | 25,584,446 | | | $ | 15,056,828 | |
| | | | | | | | | |
| COST OF REVENUE | | | 12,808,896 | | | | 6,712,365 | |
| | | | | | | | | |
| GROSS PROFIT | | | 12,775,550 | | | | 8,344,463 | |
| | | | | | | | | |
| OPERATING EXPENSES | | | | | | | | |
| Research and development | | | 549,236 | | | | 268,320 | |
| Selling expenses | | | 1,381,807 | | | | 739,422 | |
| General and administrative | | | 2,663,520 | | | | 2,438,995 | |
| Total operating expenses | | | 4,594,563 | | | | 3,446,737 | |
| | | | | | | | | |
| INCOME FROM OPERATIONS | | | 8,180,987 | | | | 4,897,726 | |
| | | | | | | | | |
| OTHER INCOME (EXPENSE) | | | | | | | | |
| Other income (expense), net | | | 30,906 | | | | (3,651 | ) |
| Interest income from short-term investment | | | 13,406 | | | | 8,690 | |
| Interest expense, net | | | (342,573 | ) | | | (4,918,572 | ) |
| Inducement cost for debentures converted | | | (756,855 | ) | | | (634,450 | ) |
| Inducement cost for warrants exercised | | | - | | | | (279,547 | ) |
| Total other expense | | | (1,055,116 | ) | | | (5,827,530 | ) |
| | | | | | | | | |
| INCOME (LOSS) BEFORE PROVISION FOR INCOME TAXES | | | 7,125,871 | | | | (929,804 | ) |
| | | | | | | | | |
| PROVISION FOR INCOME TAXES | | | 1,529,688 | | | | 1,027,172 | |
| | | | | | | | | |
| NET INCOME (LOSS) | | | 5,596,183 | | | | (1,956,976 | ) |
| | | | | | | | | |
| OTHER COMPREHENSIVE INCOME : | | | | | | | | |
| Foreign currency translation adjustment | | | 1,415,005 | | | | 982,582 | |
| | | | | | | | | |
| COMPREHENSIVE INCOME (LOSS) | | $ | 7,011,188 | | | $ | (974,394 | ) |
| | | | | | | | | |
| EARNINGS PER SHARE | | | | | | | | |
| Basic | | $ | 0.31 | | | $ | (0.15 | ) |
| Diluted | | $ | 0.31 | | | $ | (0.15 | ) |
| | | | | | | | | |
| WEIGHTED AVERAGE NUMBER OF COMMON SHARES | | | | | | | | |
| Basic | | | 18,228,731 | | | | 13,453,543 | |
| Diluted | | | 18,246,979 | | | | 13,453,543 | |
The accompanying notes are an integral part of these consolidated financial statements.
See report of independent registered public accounting firm.
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF SHAREHOLDERS' EQUITY
| | | Preferred stock | | | Common stock | | | | | | | | | Retained earnings | | | Other | | | | |
| | | | | | | | | | | | | | | Paid-in | | | Deferred | | | Statutory | | | | | | Comprehensive | | | | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Compensation | | | Reserves | | | Unrestricted | | | Income | | | Total | |
| BALANCE, January 01, 2007 | | | 2,000,000 | | | $ | 2,000 | | | | 12,795,549 | | | $ | 12,795 | | | $ | 6,246,325 | | | $ | (705,877 | ) | | $ | 779,624 | | | $ | 2,952,343 | | | $ | 460,020 | | | $ | 9,747,230 | |
| Shares issued for services | | | | | | | | | | | 78,750 | | | | 79 | | | | 115,684 | | | | | | | | | | | | | | | | | | | | 115,763 | |
| Beneficial conversion feature of debentures | | | | | | | | | | | | | | | | | | | 2,130,575 | | | | | | | | | | | | | | | | | | | | 2,130,575 | |
| Warrants issued to debenture holders | | | | | | | | | | | | | | | | | | | 1,944,425 | | | | | | | | | | | | | | | | | | | | 1,944,425 | |
| Warrants issued to placement agent | | | | | | | | | | | | | | | | | | | 643,277 | | | | | | | | | | | | | | | | | | | | 643,277 | |
| Inducement cost for debentures converted | | | | | | | | | | | | | | | | | | | 634,450 | | | | | | | | | | | | | | | | | | | | 634,450 | |
| Inducement cost for warrants exercised | | | | | | | | | | | | | | | | | | | 279,547 | | | | | | | | | | | | | | | | | | | | 279,547 | |
| Debentures converted to common stock | | | | | | | | | | | 3,278,720 | | | | 3,279 | | | | 2,747,953 | | | | | | | | | | | | | | | | | | | | 2,751,232 | |
| Cashless exercise of warrants | | | | | | | | | | | 958,181 | | | | 958 | | | | (958 | ) | | | | | | | | | | | | | | | | | | | - | |
| Amortization of deferred compensation | | | | | | | | | | | | | | | | | | | | | | | 643,119 | | | | | | | | | | | | | | | | 643,119 | |
| Foreign currency translation | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 982,582 | | | | 982,582 | |
| Net loss | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (1,956,976 | ) | | | | | | | (1,956,976 | ) |
| Appropriation to statutory reserve | | | | | | | | | | | | | | | | | | | | | | | | | | | 873,096 | | | | (873,096 | ) | | | | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| BALANCE, December 31, 2007 | | | 2,000,000 | | | $ | 2,000 | | | | 17,111,200 | | | $ | 17,111 | | | $ | 14,741,278 | | | $ | (62,758 | ) | | $ | 1,652,720 | | | $ | 122,271 | | | $ | 1,442,602 | | | $ | 17,915,224 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Shares issued for services | | | | | | | | | | | 116,086 | | | | 116 | | | | 130,305 | | | | | | | | | | | | | | | | | | | | 130,421 | |
| Shares issued for debt settlement | | | | | | | | | | | 210,400 | | | | 210 | | | | 220,710 | | | | | | | | | | | | | | | | | | | | 220,920 | |
| Debentures converted to common stock | | | | | | | | | | | 1,227,503 | | | | 1,228 | | | | 1,238,550 | | | | | | | | | | | | | | | | | | | | 1,239,778 | |
| Amortization of deferred compensation | | | | | | | | | | | | | | | | | | | | | | | 62,758 | | | | | | | | | | | | | | | | 62,758 | |
| Foreign currency translation | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 1,415,005 | | | | 1,415,005 | |
| Net income | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | 5,596,183 | | | | | | | | 5,596,183 | |
| Appropriation to statutory reserves | | | | | | | | | | | | | | | | | | | | | | | | | | | 1,299,990 | | | | (1,299,990 | ) | | | | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| BALANCE, December 31, 2008 | | | 2,000,000 | | | $ | 2,000 | | | | 18,665,189 | | | $ | 18,665 | | | $ | 16,330,843 | | | $ | - | | | $ | 2,952,710 | | | $ | 4,418,464 | | | $ | 2,857,607 | | | $ | 26,580,289 | |
The accompanying notes are an integral part of these consolidated financial statements.
See report of independent registered public accounting firm.
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE YEARS ENDED DECEMBER 31, 2008 and 2007
| | | Years ended December 31, | |
| | | 2008 | | | 2007 | |
| CASH FLOWS FROM OPERATING ACTIVITIES: | | | | | | |
| Net income (loss) | | $ | 5,596,183 | | | $ | (1,956,976 | ) |
| Adjustments to reconcile net income (loss) to cash | | | | | | | | |
| provided by operating activities: | | | | | | | | |
| Depreciation | | | 443,062 | | | | 281,894 | |
| Amortization | | | 179,343 | | | | 33,135 | |
| Amortization of deferred financing costs | | | 101,815 | | | | 879,212 | |
| Amortization of discount on converible debentures | | | 680,446 | | | | 3,716,243 | |
| Amortization of deferred compensation | | | 62,758 | | | | 643,119 | |
| Inducement cost for debentures converted | | | 257,775 | | | | 634,450 | |
| Inducement cost for warrants exercised | | | - | | | | 279,547 | |
| Issuance of common stock for services | | | 130,421 | | | | 115,763 | |
| Bad debt expense | | | 112,253 | | | | 238,094 | |
| Common share issuance for debt settlement | | | 42,081 | | | | - | |
| Changes in operating assets and liabilities: | | | | | | | | |
| Accounts receivable | | | (1,068,391 | ) | | | (1,405,316 | ) |
| Inventories | | | (674,486 | ) | | | (1,610,916 | ) |
| Deposits and prepaid expenses | | | (3,647,834 | ) | | | (743,955 | ) |
| Other receivables | | | 93,613 | | | | (10,921 | ) |
| Accounts payable | | | 404,642 | | | | 48,582 | |
| Accrued expenses | | | 946,801 | | | | (34,166 | ) |
| Deposits from customers | | | 352,012 | | | | 59,262 | |
| Taxes payables | | | (389,081 | ) | | | 322,131 | |
| Other payables | | | (18,189 | ) | | | (546,037 | ) |
| Shares to be issued to related party | | | 95,204 | | | | - | |
| Net cash provided by operating activities | | | 3,700,428 | | | | 943,145 | |
| | | | | | | | | |
| CASH FLOWS FROM INVESTING ACTIVITIES: | | | | | | | | |
| Proceeds from sale of short-term investment | | | 57,660 | | | | - | |
| Proceeds of non-interest bearing loan to third parties | | | 315,472 | | | | - | |
| Refund of long term prepayments | | | 562,185 | | | | - | |
| Long term prepayments | | | (4,395,854 | ) | | | (1,171,863 | ) |
| Purchase of intangible assets | | | - | | | | (658,350 | ) |
| Addition of construction in progress | | | (1,606,280 | ) | | | (383,322 | ) |
| Purchase of property, plant and equipment | | | (9,529 | ) | | | (19,148 | ) |
| Net cash used in investing activities | | | (5,076,346 | ) | | | (2,232,683 | ) |
| | | | | | | | | |
| CASH FLOWS FROM FINANCING ACTIVITIES: | | | | | | | | |
| Increase in restricted cash | | | (654 | ) | | | (505 | ) |
| Repayments from related parties | | | 98,901 | | | | (1,198,791 | ) |
| Proceeds from related companies | | | - | | | | 27,633 | |
| Proceeds from shareholder loans | | | 302,715 | | | | 1,170,944 | |
| Proceeds from short term loan | | | 735,165 | | | | - | |
| Loans to third parties | | | - | | | | (912,901 | ) |
| Principal payment on non-interest bearing loan from third parties | | | - | | | | (104,019 | ) |
| Principal payment on convertible debentures | | | - | | | | (880,259 | ) |
| Proceeds from convertible debentures, net of debenture expenses | | | - | | | | 3,737,250 | |
| Net cash provided by financing activities | | | 1,136,127 | | | | 1,839,352 | |
| | | | | | | | | |
| EFFECT OF EXCHANGE RATE CHANGES ON CASH | | | 44,708 | | | | 29,662 | |
| | | | | | | | | |
| (DECREASE) INCREASE IN CASH | | | (195,083 | ) | | | 579,476 | |
| | | | | | | | | |
| CASH, beginning of year | | | 771,492 | | | | 192,016 | |
| | | | | | | | | |
| CASH, end of year | | $ | 576,409 | | | $ | 771,492 | |
| | | | | | | | | |
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | | | | | | | | |
| Interest paid | | $ | 35,177 | | | $ | 2,817 | |
| Income taxes paid | | $ | 740,899 | | | $ | 864,392 | |
| | | | | | | | | |
| NON-CASH INVESTING AND FINANCING TRANSACTIONS | | | | | | | | |
| Construction-in-progress transferred to property, plant and equipment | | $ | 1,133,580 | | | $ | 5,143,496 | |
| Interest capitalized as construction-in-progress | | $ | 114,990 | | | $ | - | |
| Share issuance for debt settlement | | $ | 178,839 | | | $ | - | |
| Share issuance for convertible notes converted | | $ | 482,923 | | | $ | - | |
The accompanying notes are an integral part of these consolidated financial statements.
See report of independent registered public accounting firm.
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2008
Note 1 — Description of Business and Organization
Skystar Bio-Pharmaceutical Company (“Skystar” or the “Company”), was incorporated in Nevada. The Company has not carried on any substantive operations of its own, except for the entering of certain exclusive agreements with Xian Tianxing Bio-Pharmaceutical Co., Limited (“Xian Tianxing”), a joint stock company in the People’s Republic of China (“China” or “PRC”), through the Company’s wholly-owned subsidiary, Skystar Bio-Pharmaceutical (Cayman) Holdings Co., Limited (“Skystar Cayman”), a Cayman Islands company which the Company acquired on November 7, 2005. Skystar Cayman, through its variable interest entity (“VIE”), Xian Tianxing, engages in research, development, production, marketing and sales of veterinary healthcare and medical care products. All current operations of the Company are in China.
On August 21, 2007, Xian Tianxing invested $66,700 to establish Shanghai Siqiang Biotechnological Company Limited (“Shanghai Siqiang”). Shanghai Siqiang was established in Putuo District, Shanghai, with a registered capital of $66,700 and is wholly-owned by Xian Tianxing. Shanghai Siqiang is engaged in the research, development, production and sales of veterinary products, feed additives, and veterinary disease diagnosis equipment.
On October 16, 2007, the board of directors of the Company approved the purchase of all of the issued and outstanding shares of Fortunate Time International Limited (“Fortunate Time”), a Hong Kong company owned 100% by the Company’s non-executive director Russell Scott Cramer, for $129 (HKD 1,000).
On July 10, 2007, Fortunate Time established Sida Biotechnology (Xian) Co., Ltd. (“Sida”) with registered capital of $5,000,000. Fortunate Time invested $2,000,000 into Sida on July 20, 2007, which amount is payable to Skystar Cayman. Pursuant to the Xian High Technology District approval notice, Fortunate Time is required to contribute the remaining $3,000,000 of registered capital by July 9, 2009. Except for the $2,000,000 investment in Sida and payable to Skystar Cayman, Fortunate Time does not have any assets or liabilities. Sida was established in a high technology district in Xian, and is engaged in veterinary bio-pharmaceutical research, production and selling activities. Sida also provides technology consultation services to Xian Tianxing.
On March 10, 2008, the Company entered into a series of agreements (collectively the “Transfer Agreements”) transferring the contractual arrangements governing the relationship among Skystar Cayman, Xian Tianxing, and the majority shareholders of Xian Tianxing. Pursuant to the Transfer Agreements, all of the rights and obligations of Skystar Cayman under the contractual arrangements were transferred to Sida. In effect, Skystar Cayman assigned the contractual rights it had with Xian Tianxing to Sida.
As a result of these contractual arrangements, which obligates Sida to absorb all of the risk of loss from Xian Tianxing’s activities and enable Sida to receive all of its expected residual returns, Sida accounts for Xian Tianxing as a VIE under Financial Accounting Standards Board (“FASB”) Interpretation No. 46R (“FIN 46R”), “Consolidation of Variable Interest Entities, an Interpretation of ARB No. 51.” Accordingly, Sida consolidates Xian Tianxing’s results, assets and liabilities.
Note 2 — Summary of Significant Accounting Policies
Basis of Presentation and Consolidation
The accompanying consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America. The consolidated financial statements include the financial statements of the Company, its wholly-owned subsidiaries and its variable interest entities. All significant inter-company transactions and balances between the Company, its subsidiaries and VIEs are eliminated upon consolidation.
See report of independent registered public accounting firm.
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2008
Use of Estimates
The preparation of consolidated financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. For example, the Company estimates its allowance for doubtful accounts and useful lives of plant and equipment. Because of the use of estimates inherent in the financial reporting process, actual results could materially differ from those estimates upon which the carrying values were based.
Fair Values of Financial Instruments
On January 1, 2008, the Company adopted Statement of Financial Accounting Standards (“SFAS”) No. 157, “Fair Value Measurements” (“SFAS 157”), which defines fair value, establishes a three-level valuation hierarchy for disclosures of fair value measurement and enhances disclosure requirements for fair value measures. The carrying amounts reported in the consolidated balance sheets for current receivables, payables and short term loans qualify as financial instruments and are a reasonable estimate of fair value because of the short period of time between the origination of such instruments and their expected realization and their current market rate of interest. The three levels of valuation hierarchy are defined as follow:
| | • | Level 1 inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets. |
| | • | Level 2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the assets or liability, either directly or indirectly, for substantially the full term of the financial instruments. |
| | • | Level 3 inputs to the valuation methodology are unobservable and significant to the fair value measurement. |
The Company did not identify any assets and liabilities that are required to be presented on the consolidated balance sheets at fair value in accordance with SFAS 157.
The Company analyzes all financial instruments with features of both liabilities and equity under SFAS No. 150, “Accounting for Certain Financial Instruments with Characteristics of Both Liabilities and Equity,” SFAS No. 133, “Accounting for Derivative Instruments and Hedging Activities,” and Emerging Issues Task Force (“EITF”) 00-19, “Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock.” In 2007, the Company issued 8% convertible debentures in the aggregate face amount of $4.075 million which were due February 28, 2009 (the “Debentures”), as well as warrants to purchase 4,075,000 shares of the Company’s common stock (the “Warrants”). In 2007 and 2008, the entire $4.075 million of debentures were converted into the Company’s common stock. As the conversion price of the Debentures and the exercise prices are set for the conversion prices of such convertible debentures and the attached warrants, the Company is in a position to be sure it had adequate authorized shares for the future conversion of convertible debentures and warrants. Therefore, the embedded derivatives and warrants were recorded as equity and are not required to be recorded at fair value and marked-to-market at each reporting period.
Revenue Recognition
Revenue of the Company is primarily from the sales of veterinary healthcare and medical care products in China. Sales are recognized when the following four revenue criteria are met: persuasive evidence of an arrangement exists, delivery has occurred, the selling price is fixed or determinable, and collectability is reasonably assured. Sales are presented net of value added tax (“VAT”). No allowance for sales returns is made on these consolidated financial statements as product returns are insignificant based on historical experience.
There are two types of sales upon which revenue is recognized:
| | a. | Credit sales: Revenue is recognized when the products have been delivered to the customers. |
| | b. | Full payment before delivering: Revenue is recognized when the products have been delivered to customers. |
See report of independent registered public accounting firm.
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2008
Shipping and handling costs related to costs of goods sold are included in selling expenses, which totaled $335,605 and $184,766 for years ended December 31, 2008 and 2007, respectively.
The Company’s revenue and cost of revenue by product line were as follows:
| | Years Ended | |
| | December 31, 2008 | | December 31, 2007 | |
| | | | | |
| Revenue | | | | | | | | |
| Micro-organism | | $ | 5,868,623 | | | $ | 4,271,139 | |
| Veterinary Medications | | | 17,535,757 | | | | 9,003,400 | |
| Feed Additives | | | 1,189,108 | | | | 971,019 | |
| Vaccines | | | 990,958 | | | | 811,270 | |
| Total Revenue | | | 25,584,446 | | | | 15,056,828 | |
| Cost of Revenue | | | | | | | | |
| Micro-organism | | | 1,781,598 | | | | 1,416,550 | |
| Veterinary Medications | | | 10,389,726 | | | | 4,654,347 | |
| Feed Additives | | | 525,653 | | | | 549,714 | |
| Vaccines | | | 111,919 | | | | 91,754 | |
| Total Cost of Revenue | | | 12,808,896 | | | | 6,712,365 | |
| Gross Profit | | $ | 12,775,550 | | | $ | 8,344,463 | |
Cash
Cash includes cash on hand, demand deposits with banks and liquid investments with an original maturity of three months or less.
Restricted Cash
The Company had restricted cash of $80,885 and $74,969 as of December 31, 2008 and 2007, respectively. Restricted cash is comprised of amounts received from the PRC government as subsidies and set aside for specific uses (see Note 14). The restricted funds are maintained as bank deposits and reflected as current assets based on the expected period when such funds will be put into their specific uses.
Short Term Investment
Short-term investment is comprised of securities classified as available-for-sale, held by a private investment trust company for investing activities. Available-for-sale securities are carried at fair value, with unrealized gains or losses reported in other comprehensive income, net of tax. Realized gains or losses are included in the results of operations. There were no unrealized gains or losses relating to short term investment for the years ended December 31, 2008 and 2007, and as such, no such amounts were included in other comprehensive income for such periods.
See report of independent registered public accounting firm.
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2008
Accounts receivable are recorded at net realizable value consisting of the carrying amount less an allowance for uncollectible accounts, as needed. The Company uses the aging method to estimate the allowance for anticipated uncollectible receivable balances. Under the aging method, bad debt percentages determined by management based on historical experience, as well as current economic climate, are applied to customers’ balances categorized by the number of months the underlying invoices have remained outstanding. At each reporting period, the allowance balance is adjusted to reflect the amount computed as a result of the aging method. When facts subsequently become available to indicate that the allowance provided requires an adjustment, a corresponding adjustment is made to the allowance account as a change in estimate. The ultimate collection of the Company’s accounts receivable may take one year. Delinquent account balances are reserved after management determines that the likelihood of collection is not probable, and known bad debts are written-off against allowance for doubtful accounts when identified.
Inventories
Inventories are stated at the lower of cost or market, as determined on a moving weighted average basis. Costs of inventories include purchases and related costs incurred in bringing the inventories to their present location and condition.
Property, Plant and Equipment
Property, plant and equipment are stated at cost, less accumulated depreciation. Major renewals are charged to the property, plant and equipment accounts while replacements, maintenance and repairs, which do not improve or extend the respective lives of the assets, are expensed currently. Depreciation is computed using the straight-line method over the estimated useful lives of the assets. Estimated useful lives of the assets are as follows:
| | Estimated Useful Life |
| Buildings | 20 – 40 years |
| Machinery and equipment | 10 years |
| Computer, office equipment and furniture | 5 years |
| Vehicles | 5 – 10 years |
Management assesses the carrying value of plant and equipment annually, more often when factors indicating impairment are present, and reduces the carrying value of such assets by the amount of the impairment. The Company determines the existence of such impairment by measuring the expected future cash flows (undiscounted and without interest charges) and comparing such amount to the net asset carrying value. An impairment loss, if it exists, is measured as the amount by which the carrying amount of the asset exceeds the fair value of the asset. Based on its review, management believes that as of December 31, 2008 and 2007, there was no impairment of its plant and equipment.
Construction in Progress
Construction in progress includes direct costs of construction of a factory building. Interest incurred during the period of construction, if material, is capitalized. Construction in progress is not depreciated until such time the assets are completed and put into service.
Intangible Assets
Land Use Rights — Land use rights represent the amounts paid to acquire a long-term interest to utilize the land underlying the Company’s facilities. This type of arrangement is common for the use of land in the PRC. Land use rights are amortized on a straight-line basis over its 50 year term.
See report of independent registered public accounting firm.
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2008
Technological Know-How — Purchased technological know-how includes confidential formulas, manufacturing processes, and technical and procedural manuals, and is amortized using the straight-line method over the expected useful economic life of five years, which reflects the period over which such confidential formulas, manufacturing processes, and technical and procedural manuals are kept confidential by the Company as agreed between the Company and the selling parties.
Impairment of Intangible Assets — The Company evaluates the carrying value of intangible assets annually, more often when factors indicating impairment are present. The Company determines the existence of such impairment by measuring the estimated future cash flows (undiscounted) and comparing such amount to the net asset carrying value. If the undiscounted cash flow estimated to be generated by any such intangible asset is less than its carrying amount, a loss is recognized based on the amount by which the carrying amount exceeds the intangible asset’s fair market value. Loss on intangible assets to be disposed of is determined in a similar manner, except that fair market values are reduced by the cost of disposal. Based on its review, the Company believes that, as of December 31, 2008, there were no impairments of its intangible assets.
Comprehensive Income
SFAS No. 130, “Reporting Comprehensive Income” requires disclosure of all components of comprehensive income and loss on an annual and interim basis. Comprehensive income and loss is defined as the change in equity of a business enterprise during a period from transactions and other events and circumstances from non-owner sources. The accompanying consolidated financial statements include the provisions of SFAS No. 130. Accumulated other comprehensive income is comprised of the changes in foreign currency exchange rates.
Research and Development Costs
Research and development costs are expensed to operations as incurred and include salaries, professional fees and technical support fees.
Advertising Costs
Cost incurred in the advertisement of the Company and the Company’s products are charged to operations currently. Advertising costs for the years ended December 31, 2008 and 2007 were $93,573 and $33,627, respectively.
Income Taxes
The Company accounts for income taxes in accordance with the SFAS No. 109, “Accounting for Income Taxes” (“SFAS 109”). SFAS 109 requires a company to use the asset and liability method of accounting for income taxes. Under the asset and liability method, deferred income taxes are recognized for the tax consequences of temporary differences by applying enacted statutory tax rates applicable to future years to differences between the financial statement carrying amounts and the tax bases of existing assets and liabilities. Under SFAS 109, the effect on deferred income taxes of a change in tax rates is recognized in income in the period that includes the enactment date. A valuation allowance is recognized if it is more likely than not that some portion, or all of, a deferred tax asset will not be realized. There were no deferred tax amounts at December 31, 2008 and December 31, 2007.
On January 1, 2007, the Company adopted FASB Interpretation 48, “Accounting for Uncertainty in Income Taxes” (“FIN 48”). In accordance with FIN 48, a tax position is recognized as a benefit only if it is “more likely than not” that the tax position would be sustained in a tax examination, with a tax examination being presumed to occur. The amount recognized is the largest amount of tax benefit that is greater than 50% likely of being realized on examination. For tax positions not meeting the “more likely than not” test, no tax benefit is recorded. FIN 48 also provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosures, and transition. The adoption had no effect on the Company’s consolidated financial statements.
See report of independent registered public accounting firm.
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2008
The Company’s operations are subject to income and transaction taxes in the United States and in the PRC jurisdictions. Significant estimates and judgments are required in determining the Company’s worldwide provision for income taxes. Some of these estimates are based on interpretations of existing tax laws or regulations. The ultimate amount of tax liability may be uncertain as a result.
The Company does not anticipate any events which could cause a change to these uncertainties.
Stock Based Compensation
The Company records stock-based compensation in accordance with SFAS No. 123(R), “Share-Based Payment” (“SFAS 123R”). SFAS 123R requires companies to measure compensation cost for stock-based employee compensation at fair value at the grant date and recognize the expense over the employee’s requisite service period. Under SFAS 123R, the Company’s volatility is based on the historical volatility of the Company’s stock or the expected volatility of similar companies. The expected life assumption is primarily based on historical exercise patterns and employee post-vesting termination behavior. The risk-free interest rate for the expected term of the option is based on the U.S. Treasury yield curve in effect of the time of grant. SFAS 123R allows the “simplified” method to determine the term of employee options when certain information is not available. Because the Company does not have a history of employee stock options, the Company used the “simplified” method to estimate the expected life of the options.
Stock compensation granted to non-employees is determined in accordance with EITF No. 96-18, “Accounting for Equity Instruments that are issued to Other than Employees for Acquiring, or in Conjunction with Selling Goods or Services” (“EITF 96-18”), which incorporates the fair value of the consideration received or the fair value of equity instruments issued, whichever is more reliably measured.
The Company uses the Black-Scholes option-pricing model which was developed for use in estimating the fair value of options. Option-pricing models require the input of highly complex and subjective variables including the expected life of options granted and the Company’s expected stock price volatility over a period equal to or greater than the expected life of the options. Because changes in the subjective assumptions can materially affect the estimated value of the Company’s employee stock options, it is management’s opinion that the Black-Scholes option-pricing model may not provide an accurate measure of the fair value of the Company’s employee stock options. Although the fair value of employee stock options is determined in accordance with SFAS 123R using an option-pricing model, that value may not be indicative of the fair value observed in a willing buyer/willing seller market transaction.
Through December 31, 2008, the Company did not grant any stock options.
Earnings per Share
Earnings per share is calculated in accordance with the SFAS No. 128, “Earnings Per Share” (“SFAS 128”). Basic earnings per share is based upon the weighted average number of common shares outstanding. Diluted earnings per share is based on the assumption that all dilutive convertible shares, including convertible preferred shares, and stock options were converted or exercised. Further, SFAS 128 requires that stock dividends or stock splits be accounted for retroactively if the stock dividends or stock splits occur during the period, or retroactively if the stock dividends or stock splits occur after the end of the period but before the release of the financial statements, by considering it outstanding of the entirety of each period presented. Dilution is computed by applying the treasury stock method. Under this method, options and warrants are assumed to be exercised at the beginning of the period (or at the time of issuance, if later), and as if funds obtained thereby were used to purchase common stock at the average market price during the period.
Foreign Currency Translation
The Company uses the United States dollar (“U.S. dollar”) for financial reporting purposes. The Company’s subsidiaries and VIEs maintain their books and records in their functional currency, being the primary currency of the economic environment in which their operations are conducted.
See report of independent registered public accounting firm.
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2008
The Company translates the subsidiaries’ and VIEs’ assets and liabilities into U.S. dollars using the applicable exchange rates prevailing at the balance sheet dates, and the statements of operations and cash flows are translated at average exchange rates during the reporting period. As a result, amounts related to assets and liabilities reported on the consolidated statements of cash flows will not necessarily agree with changes in the corresponding balances on the consolidated balance sheets. Equity accounts are translated at historical rates. Adjustments resulting from the translation of the subsidiaries’ and VIEs’ financial statements are recorded as accumulated other comprehensive income.
The quotation of the exchange rates does not imply free convertibility of RMB to other foreign currencies. All foreign exchange transactions continue to take place either through the People's Bank of China or other banks authorized to buy and sell foreign currencies at the exchange rate quoted by the People's Bank of China. The rate of exchange quoted by the People’s Bank of China on December 31, 2008 and 2007 was $1.00 to RMB6.82 and RMB7.29, respectively. The weighted average translation rate of $1.00 to RMB6.94 and RMB7.59 were applied to the income statement accounts for the years ended December 31, 2008 and 2007, respectively. As of December 31, 2008 and 2007, the Company had accumulated other comprehensive income related to translation in the amount of $2,857,607 and $1,442,602, respectively.
Approval of foreign currency payments by the People’s Bank of China or other institutions requires submitting a payment application form together with invoices, shipping documents and signed contracts. Transaction gains and losses that arise from exchange rate fluctuations on transactions denominated in a currency other than the functional currency are included in the results of operations as incurred.
Related Parties
Parties are considered to be related to the Company if the parties, directly or indirectly, through one or more intermediaries, control, are controlled by, or are under common control with the Company. Related parties also include principal owners of the Company, its management, members of the immediate families of such principal owners and management, and other parties with which the Company may deal if one party controls or can significantly influence the management or operating policies of the other to an extent that one of the transacting parties might be prevented from fully pursuing its own separate interests.
Recently Accounting Pronouncements
In December 2007, the FASB issued SFAS No. 141(R), “Business Combinations” (“SFAS 141 R”), which replaced SFAS 141. SFAS 141R retains the purchase method of accounting for acquisitions, but requires a number of changes, including changes in the way assets and liabilities are recognized in the purchase accounting as well as requiring the expensing of acquisition-related costs as incurred. Furthermore, SFAS 141R provides guidance for recognizing and measuring the goodwill acquired in the business combination and determines what information to disclose to enable users of the financial statements to evaluate the nature and financial effects of the business combination. SFAS 141R is effective for fiscal years beginning on or after December 15, 2008. Earlier adoption is prohibited. The Company is evaluating the impact, if any, that the adoption of this statement will have on its consolidated results of operations or consolidated financial position.
In December 2007, the FASB issued SFAS No. 160, “Noncontrolling Interests in Consolidated Financial Statements — An Amendment of ARB No. 51” (“SFAS 160”). SFAS 160 amends ARB 51 to establish accounting and reporting standards for the noncontrolling interest in a subsidiary and for the deconsolidation of a subsidiary. It is intended to eliminate the diversity in practice regarding the accounting for transactions between equity and noncontrolling interests by requiring that they be treated as equity transactions. Further, it requires consolidated net income to be reported at amounts that include the amounts attributable to both the parent and the noncontrolling interest. SFAS 160 also establishes a single method of accounting for changes in a parent’s ownership interest in a subsidiary that do not result in deconsolidation, requires that a parent recognize a gain or loss in net income when a subsidiary is deconsolidated, requires expanded disclosures in the consolidated financial statements that clearly identify and distinguish between the interests of the parent’s owners and the interests of the noncontrolling owners of a subsidiary, among others. SFAS 160 is effective for fiscal years beginning on or after December 15, 2008, with early adoption permitted, and it is to be applied prospectively. SFAS 160 is to be applied prospectively as of the beginning of the fiscal year in which it is initially applied, except for the presentation and disclosure requirements, which must be applied retrospectively for all periods presented. The Company has not yet evaluated the impact that SFAS 160 will have on its consolidated financial position or consolidated results of operations.
See report of independent registered public accounting firm.
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2008
In February 2008, the FASB issued FASB Staff Position No. 157-1 ("FSP 157-1"), "Application of FASB Statement No. 157 to FASB Statement No. 13 and Other Accounting Pronouncements That Address Fair Value Measurements for Purposes of Lease Classification or Measurement under Statement 13." FSP 157-1 indicates that it does not apply under SFAS 13, "Accounting for Leases," and other accounting pronouncements that address fair value measurements for purposes of lease classification or measurement under SFAS 13. This scope exception does not apply to assets acquired and liabilities assumed in a business combination that are required to be measured at fair value under SFAS 141 or SFAS 141R, regardless of whether those assets and liabilities are related to leases.
Also in February 2008, the FASB issued FASB Staff Position No. 157-2 ("FSP 157-2"), "Effective Date of FASB Statement No. 157." With the issuance of FSP 157-2, the FASB agreed to: (a) defer the effective date in SFAS No. 157 for one year for certain nonfinancial assets and nonfinancial liabilities, except those that are recognized or disclosed at fair value in the financial statements on a recurring basis (at least annually), and (b) remove certain leasing transactions from the scope of SFAS 157. The deferral is intended to provide the FASB time to consider the effect of certain implementation issues that have arisen from the application of SFAS 157 to these assets and liabilities.
In March 2008, the FASB issued SFAS No. 161, "Disclosures about Derivative Instruments and Hedging Activities" (“SFAS 161”). SFAS 161 is intended to improve financial reporting of derivative instruments and hedging activities by requiring enhanced disclosures to enable financial statement users to better understand the effects of derivatives and hedging on an entity's financial position, financial performance and cash flows. The provisions of SFAS 161 are effective for interim periods and fiscal years beginning after November 15, 2008, with early adoption encouraged. The Company does not anticipate that the adoption of SFAS 161 will have a material impact on its consolidated results of operations or consolidated financial position.
In April 2008, the FASB issued FASB Staff Position (“FSP”) 142-3 “Determination of the useful life of Intangible Assets,” which amends the factors a company should consider when developing renewal assumptions used to determine the useful life of an intangible asset under SFAS 142. FSP 142-3 is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those fiscal years. SFAS 142 requires companies to consider whether renewal can be completed without substantial cost or material modification of the existing terms and conditions associated with the asset. FSP 142-3 replaces the previous useful life criteria with a new requirement—that an entity consider its own historical experience in renewing similar arrangements. If historical experience does not exist, then the Company would consider market participant assumptions regarding renewal including 1) highest and best use of the asset by a market participant, and 2) adjustments for other entity-specific factors included in SFAS 142. The Company is currently evaluating the impact that adopting FSP 142-3 will have on its consolidated financial statements.
In May 2008, the FASB issued SFAS No. 162, "The Hierarchy of Generally Accepted Accounting Principles" (“SFAS 162”). SFAS 162 is intended to improve financial reporting by identifying a consistent framework, or hierarchy, for selecting accounting principles to be used in preparing financial statements that are presented in conformity with GAAP for nongovernmental entities. SFAS 162 is effective 60 days following the SEC's approval of the Public Company Accounting Oversight Board (“PCAOB”) amendments to AU Section 411, "The Meaning of Present Fairly in Conformity with Generally Accepted Accounting Principles." The Company does not expect the adoption of SFAS 162 will have a material impact on its consolidated results of operations or consolidated financial position.
See report of independent registered public accounting firm.
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2008
In May 2008, the FASB issued SFAS No. 163, “Accounting for Financial Guarantee Insurance Contracts, an interpretation of FASB Statement No. 60” (“SFAS 163”). The scope of SFAS 163 is limited to financial guarantee insurance (and reinsurance) contracts issued by enterprises included within the scope of SFAS 60. Accordingly, SFAS 163 does not apply to financial guarantee contracts issued by enterprises excluded from the scope of SFAS 60 or to some insurance contracts that seem similar to financial guarantee insurance contracts issued by insurance enterprises (such as mortgage guaranty insurance or credit insurance on trade receivables). SFAS 163 also does not apply to financial guarantee insurance contracts that are derivative instruments included within the scope of SFAS 133, “Accounting for Derivative Instruments and Hedging Activities.” The Company does not expect the adoption of SFAS 163 will have a material impact on its consolidated results of operations or consolidated financial position.
On May 9, 2008, the FASB issued FASB Staff Position No. APB 14-1 ("FSP APB 14-1"), "Accounting for Convertible Debt Instruments That May Be Settled in Cash upon Conversion (Including Partial Cash Settlement)." FSP APB 14-1 clarifies that convertible debt instruments that may be settled in cash upon conversion (including partial cash settlement) are not addressed by paragraph 12 of APB Opinion No. 14, "Accounting for Convertible Debt and Debt Issued with Stock Purchase Warrants." Additionally, FSP APB 14-1 specifies that issuers of such instruments should separately account for the liability and equity components in a manner that will reflect the entity's nonconvertible debt borrowing rate when interest cost is recognized in subsequent periods. FSP APB14-1 is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those fiscal years. The Company is currently evaluating the impact that FSP APB 14-1 will have on its consolidated results of operations or consolidated financial position.
On June 16, 2008, the FASB issued FSP No. EITF 03-6-1 (“FSP EITF 03-6-1”), “Determining Whether Instruments Granted in Share-Based Payment Transactions Are Participating Securities,” to address the question of whether instruments granted in share-based payment transactions are participating securities prior to vesting. FSP EITF 03-6-1 indicates that unvested share-based payment awards that contain rights to dividend payments should be included in earnings per share calculations. The guidance will be effective for fiscal years beginning after December 15, 2008. The Company is currently evaluating the requirements of FSP EITF 03-6-1 and the impact that its adoption will have on the consolidated results of operations or consolidated financial position.
In June 2008, the FASB issued Emerging Issues Task Force Issue 07-5 (“EITF 07-5”), “Determining whether an Instrument (or Embedded Feature) is indexed to an Entity’s Own Stock.” EITF 07-5 is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those fiscal years. Early application is not permitted. Paragraph 11(a) of SFAS No. 133, “Accounting for Derivatives and Hedging Activities,” specifies that a contract that would otherwise meet the definition of a derivative but is both (a) indexed to the Company’s own stock and (b) classified in stockholders’ equity in the statement of financial position would not be considered a derivative financial instrument. EITF 07-5 provides a new two-step model to be applied in determining whether a financial instrument or an embedded feature is indexed to an issuer’s own stock and thus able to qualify for the SFAS 133 paragraph 11(a) scope exception. This standard triggers liability accounting on all options and warrants exercisable at strike prices denominated in any currency other than the functional currency of the operating entity in the PRC. The Company is currently evaluating the impact of the adoption of EITF 07-5 on the accounting for related warrants transactions.
In June 2008, FASB issued EITF 08-4, “Transition Guidance for Conforming Changes to Issue No. 98-5.” The objective of EITF 08-4 is to provide transition guidance for conforming changes made to EITF 98-5, “Accounting for Convertible Securities with Beneficial Conversion Features or Contingently Adjustable Conversion Ratios,” that result from EITF 00-27 “ Application of Issue No. 98-5 to Certain Convertible Instruments,” and SFAS 150, “Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity.” EITF 08-4 is effective for financial statements issued for fiscal years ending after December 15, 2008. Early application is permitted. The Company is currently evaluating the impact of adoption of EITF 08-4 on the accounting for the convertible notes and related warrants transactions.
In September 2008, the FASB issued for comment revisions to SFAS No. 140 and FASB Interpretation No. 46, as revised (“FIN 46R”), “Consolidation of Variable Interest Entities.” The changes proposed include a removal of the scope exemption from FIN 46R for QSPEs, a revision of the current risks and rewards-based FIN 46R consolidation model to a qualitative model based on control and a requirement that consolidation of VIEs be re-evaluated on an ongoing basis. Although the revised standards have not yet been finalized, these changes may have a significant impact on the Company’s consolidated financial statements as the Company may be required to deconsolidate certain assets and liabilities due to the ongoing evaluation of its primary beneficiary status. In addition, the Company may also be required to consolidate other VIEs that are not currently consolidated based an analysis under the current FIN 46R consolidation model. The proposed revisions would be effective for fiscal years that begin after November 15, 2009.
See report of independent registered public accounting firm.
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2008
On October 10, 2008, the FASB issued FSP 157-3, “Determining the Fair Value of a Financial Asset When the Market for That Asset Is Not Active,” which clarifies the application of SFAS 157 in a market that is not active and provides an example to illustrate key considerations in determining the fair value of a financial asset when the market for that financial asset is not active. FSP 157-3 became effective on October 10, 2008, and its adoption did not have a material impact on the Company’s consolidated results of operations or consolidated financial position.
In January 2009, the FASB issued FSP EITF 99-20-1, “Amendments to the Impairment Guidance of EITF Issue No. 99-20, and EITF Issue No. 99-20, Recognition of Interest Income and Impairment on Purchased and Retained Beneficial Interests in Securitized Financial Assets” (“FSP EITF 99-20-1”). FSP EITF 99-20-1 changes the impairment model included within EITF 99-20 to be more consistent with the impairment model of SFAS No. 115. FSP EITF 99-20-1 achieves this by amending the impairment model in EITF 99-20 to remove its exclusive reliance on “market participant” estimates of future cash flows used in determining fair value. Changing the cash flows used to analyze other-than-temporary impairment from the “market participant” view to a holder’s estimate of whether there has been a “probable” adverse change in estimated cash flows allows companies to apply reasonable judgment in assessing whether an other-than-temporary impairment has occurred. The adoption of FSP EITF 99-20-1 did not have a material impact on the Company’s consolidated financial statements.
Reclassification
Certain amounts in the prior year’s consolidated financial statements have been classified to conform to the 2008 presentation with no impact on the previously reported net income or cash flows.
Note 3 — Concentration and Credit Risk
The Company’s operations are all carried out in the PRC. Accordingly, the Company’s business, financial condition and results of operations may be influenced by the political, economic and legal environments in the PRC, and by the general state of the PRC’s economy. The Company’s operations in the PRC are subject to specific considerations and significant risks not typically associated with companies in North America and Western Europe. These include risks associated with, among others, the political, economic and legal environments and foreign currency exchange. The Company’s results may be adversely affected by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things.
Financial instruments, which subject the Company to concentration of credit risk, consist of cash. The Company maintains balances at financial institutions which, from time to time, may exceed Federal Deposit Insurance Corporation insured limits for the banks located in the United States. Balances at financial institutions or state owned banks within the PRC are not covered by insurance. As of December 31, 2008 and 2007, the Company had deposits in excess of federally-insured limits (including restricted cash) of $448,082 and $844,773, respectively. The Company has not experienced any losses in such accounts.
For the years ended December 31, 2008 and 2007, all of the Company’s sales were generated in the PRC. In addition, all accounts receivable at December 31, 2008 and 2007 were generated in the PRC. No major customers accounted for more than 10% of the Company’s total revenue or total accounts receivable as of and for the years ended December 31, 2008 and 2007, respectively.
The Company’s four largest vendors collectively accounted for approximately 58.71% and 66.13% of the Company’s total purchases for the years ended December 31, 2008 and 2007, respectively.
See report of independent registered public accounting firm.
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2008
One major product accounted for approximately 11.55% of the Company’s total revenue for the year ended December 31, 2008, while no single product accounted for more than 10% of the Company’s total revenue for the year ended December 31, 2007.
Note 4 — Short Term Investment
In 2007, the Company entered into an investment agreement with an unrelated party to invest certain funds in the Chinese stock market. The nature of the investment is available-for-sale with certain guaranteed returns such as 7.2% interest through August 1, 2008 and 5.0% interest after August 1, 2008 for one year. There are no restrictions or penalties for early withdrawals prior to maturity. The investment matures on July 31, 2009. The Company had $352,080 and $383,880 in short-term investment as of December 31, 2008 and 2007, respectively.
For the years ended December 31, 2008 and 2007, the Company recognized interest income amounting to $13,406 and $8,690, respectively, from the short term investment.
Note 5 — Allowance for Doubtful Accounts
The following table presents the movement of allowance for doubtful accounts as of December 31, 2008 and 2007:
| | | 2008 | | | 2007 | |
| Balance at the beginning of the period | | $ | 199,639 | | | $ | 14,426 | |
| Charge for the period | | | 114,239 | | | | 176,916 | |
| Write-off of accounts receivable against the allowance | | | - | | | | - | |
| Foreign currency translation adjustments | | | 13,979 | | | | 8,297 | |
| Balance at the end of the period | | $ | 327,857 | | | $ | 199,639 | |
Note 6 — Inventories
Inventories are comprised of the following as of December 31, 2008 and 2007:
| | | 2008 | | | 2007 | |
| Raw materials | | $ | 2,087,428 | | | $ | 1,761,145 | |
| Packing materials | | | 165,077 | | | | 110,020 | |
| Work in process | | | 2,446 | | | | 2,639 | |
| Finished goods | | | 811,538 | | | | 355,041 | |
| Low value consumables | | | 19,571 | | | | 13,766 | |
| Total | | $ | 3,086,060 | | | $ | 2,242,611 | |
The Company periodically reviews its reserves for slow moving and obsolete inventories. As of December 31, 2008 and 2007, the Company believes that no such reserves were necessary.
Note 7 — Deposits and Prepaid Expenses
Deposits and prepaid expenses are comprised of the following as of December 31, 2008 and 2007:
| | | 2008 | | | 2007 | |
| Prepayment for raw materials purchasing | | $ | 4,210,618 | | | $ | 475,669 | |
| Prepayment for packaging materials purchasing | | | 499,755 | | | | 240,545 | |
| Prepayment for advertisement fee | | | 89,436 | | | | - | |
| Prepayment for due diligence fee | | | 73,350 | | | | - | |
| Other | | | 5,692 | | | | 90,443 | |
| Total | | $ | 4,878,851 | | | $ | 806,657 | |
See report of independent registered public accounting firm.
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2008
Note 8 — Loans and Other Receivables
Loans and other receivables consist of the following as of December 31, 2008 and 2007:
| | | 2008 | | | 2007 | |
| Shaanxi Xinbangdike Technology Developing Company, non-interest bearing, unsecured and due on demand. | | | 295,087 | | | | - | |
| | | | | | | | | |
| Shaanxi Suoang Biotechnological Company, due October 30, 2007, extended to March 31, 2008, annual interest at 7.0%, secured by unrelated company Shanxi New Resource Co., paid in full in March 2008. | | | - | | | | 27,420 | |
| | | | | | | | | |
| Xi’an SilverRiver Automatic Equipment Company, due March 23, 2008 and extended to April 2008, annual interest rate 8.4%, unsecured, paid in full in April 2008. | | | - | | | | 411,300 | |
| | | | | | | | | |
| Shaanxi Hongye Housing Company, due on demand, non-interest bearing, unsecured, paid in full in 2008. | | | - | | | | 137,100 | |
| | | | | | | | | |
| Others, non-interest bearing, due on demand, unsecured, paid in full in 2008. | | | | | | | 9,152 | |
| | | | | | | | | |
| Other receivables | | | 85,099 | | | | 43,800 | |
| | | | | | | | | |
| Total loans and other receivables | | $ | 380,186 | | | $ | 628,772 | |
Note 9 — Property, Plant and Equipment, Net
Property, plant and equipment consist of the following as of December 31, 2008 and 2007:
| | | 2008 | | | 2007 | |
| Buildings and improvements | | $ | 4,977,654 | | | $ | 3,592,519 | |
| Machinery and equipment | | | 3,035,376 | | | | 2,827,591 | |
| Office equipment and furniture | | | 186,702 | | | | 167,617 | |
| Vehicles | | | 329,331 | | | | 295,995 | |
| Total | | | 8,529,063 | | | | 6,883,722 | |
| Less: accumulated depreciation | | | (1,115,374 | ) | | | (620,991 | ) |
| Plant and equipment , net | | $ | 7,413,689 | | | $ | 6,262,731 | |
Depreciation expense was $443,062 and $281,894 for the years ended December 31, 2008 and 2007, respectively.
Note 10 — Construction in Progress
Construction in progress is related to a production facility being built in accordance with the PRC’s Good Manufacturing Practices (“GMP”) Standard. Construction on this facility commenced in 2005, the veterinary medicine facility and the building that houses quality control, research and development and administration were completed during 2007, and the remaining facilities are expected to be completed by the end of 2009, depending upon the Company’s ability to raise additional capital. The Company estimates that as of December 31, 2008, additional funds will be needed to complete the construction project. No depreciation is provided for construction in progress until such time the assets are completed and placed into service.
See report of independent registered public accounting firm.
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2008
As of December 31, 2008 and 2007, the Company had construction in progress amounting to $6,516,630 and $5,531,236, respectively. $150,789 and $0 in interest expense had been capitalized for construction in progress for the years ended December 31, 2008 and 2007, respectively.
Note 11— Long Term Prepayment
Long term prepayment consists of the following as of December 31, 2008 and 2007:
| | | 2008 | | | 2007 | |
| Construction deposit | | $ | 2,493,167 | | | $ | 685,500 | |
| Deposit for the potential acquisitions | | | 2,713,950 | | | | 534,690 | |
| Total | | $ | 5,207,117 | | | $ | 1,220,190 | |
As of December 31, 2008 and 2007, the Company has determined that these prepayments are noncurrent because: (1) these amounts relate to noncurrent assets, and (2) the Company’s ability to complete the projects underlying the construction deposit and any potential acquisitions is contingent upon the Company obtaining financing, the successful outcome of which cannot be determined.
Note 12 — Intangible Assets
Intangible assets consist of the following as of December 31, 2008 and 2007:
| | | 2008 | | | 2007 | |
| Land use rights | | $ | 378,853 | | | $ | 354,061 | |
| Technological know-how | | | 880,200 | | | | 822,600 | |
| Total | | | 1,259,053 | | | | 1,176,661 | |
| Less: accumulated amortization | | | (359,524 | ) | | | (165,425 | ) |
| Intangible assets, net | | $ | 899,529 | | | $ | 1,011,236 | |
During 2007, the Company paid $658,350 for the exclusive rights to the use of a strain of micro-bio organisms for a five-year term from November 1, 2007 through October 31, 2012. The Company began using the strain in its veterinary medicines in 2008.
Amortization expense for intangible assets was $179,343 and $33,135 for the years ended December 31, 2008 and 2007, respectively.
Amortization expenses for the future five years and thereafter are as follow:
Years ending December 31, | | Amount | |
| 2009 | | $ | 179,343 | |
| 2010 | | | 179,343 | |
| 2011 | | | 179,343 | |
| 2012 | | | 179,343 | |
| 2013 | | | 179,343 | |
| 2014 and thereafter | | | 2,814 | |
| Total | | $ | 899,529 | |
See report of independent registered public accounting firm.
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2008
Note 13 — Short Term Loan
On September 3, 2008, the Company signed a one year short term loan contract with the Bank of East Asia to borrow up to $1.9 million (RMB13 million) for operating purposes secured by the Company’s buildings. On September 17, 2008, the Company received proceeds of $733,500 (RMB5 million) from the bank. This amount will become due on September 16, 2009. The balance of $1.1 million (RMB8 million) can be borrowed once the Company has a deposit balance in the amount of $4 million with the bank and uses the bank as the Company’s primary transaction bank. The Company’s land use right and the buildings above the land, which are owned by the Company, have been pledged as collateral for the loan. As of December 31, 2008, the net value of the land use right and properties pledged for the loan was $337,179 and $3,311,940, respectively.
The applicable interest rate of the loan is the Bank of China’s standard short term rate, 6.93% at inception of the loan, which is subject to change with the government policy, plus an additional 20% interest rate float. Pursuant to these terms, the interest rate was approximately 8.32% as of December 31, 2008. Interest payments to the Bank of East Asia are due every three months. Interest expense incurred and associated with the short term loan amounted to $15,741 for the year ended December 31, 2008, which has been capitalized as part of construction-in-progress, and no principal amount has been paid.
Pursuant to the short term loan agreement, the Company is required to comply with certain covenants, such as maintaining sufficient environmental controls with respect to the Company’s manufacturing processes, providing written notification to the Bank of East Asia for any changes in the organizational structure or executive officers, among others. As of and for the year ended December 31, 2008, the management of the Company determined that the Company was in compliance with these covenants.
The Company had $14,670 in short term loan from a unrelated party as of December 31, 2008. The loan is unsecured, interest free and due on demand.
Note 14 — Deferred Government Grant
Deferred government grant represents subsidies for GMP projects granted by the PRC government. A subsidy in the amount of $641,000 was approved by the PRC government for the Company to construct a new factory in which operations will meet the GMP Standard. In 2003, $516,500 was received by the Company and the remaining $124,500 was received in the first quarter of 2006. In 2006, the Company expended $186,644 for the construction of its new factory.
Also in 2003, the Company received a second subsidy in the amount of $256,400 to finance the Company’s research and development activities. In 2005, the Company received a third subsidy of $64,100 for the Company’s research and development activities, which was expended during that year.
According to the PRC’s government regulations, the funds granted may be treated as capital contributed by a company appointed by the PRC government or as a loan from such company, which the Company will be required to repay. As of December 31, 2008, the Company has not reached a final agreement with the PRC government regarding the treatment of these three subsidies as either a loan or capital contribution, and the Company does not expect that the final agreement will be completed within the year of 2009. Therefore, these amounts are reflected as liabilities in the accompanying consolidated financial statements.
As of December 31, 2008 and 2007, deferred government grant amounted to $1,100,250 and $1,028,250, respectively, and the Company had $80,885 and $74,969, respectively, of fund balance and recorded as restricted cash.
See report of independent registered public accounting firm.
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2008
Note 15 — Capital Transactions
On July 10, 2007, the Company issued 40,000 shares of common stock as salary to a non-executive director. On the same date, the Company issued 38,750 shares of common stock to an independent consultant. The trading value of the Company's common stock as of July 10, 2007, was $1.47 per share and a corresponding amount of $115,763 was charged to general and administrative expenses.
In the fourth quarter of 2007, certain of the Company’s convertible note holders converted their debentures into 3,278,720 shares of common stock as more fully described in Note 16.
Also, in the fourth quarter of 2007, certain of the Company’s warrant holders exercised 3,100,000 warrants into 958,181 shares of common stock, in a cashless exercise.
On February 12, 2008, the Company issued 90,000 shares of common stock as salary to a non-executive director. The trading value of the Company's common stock on February 12, 2008, was $1.11 per share and a corresponding amount of $99,900 was charged to general and administrative expenses. The Company also had $95,204 balance under shares to be issued as of December 31, 2008, which represented 110,000 common shares to be issued to the non-executive director for his service provided for the period of May 2008 to December 2008. The Company recorded it as part of general and administrative expenses based on the weighted average trading price of the Company’s common stock for the said period.
On February 29, 2008, the Company issued 210,400 shares of common stock to its legal counsel as partial payment for services rendered. The trading value of the Company's common stock on February 29, 2008, was $1.05 per share and additional inducement cost of $42,081 between the fair value of the shares at $220,920 and the partial payment of $178,839 was charged to general and administrative expenses.
On April 21, 2008, two of the Company’s convertible note holders converted $982,003 of debentures into 1,227,503 shares of common stock as more fully described in Note 16.
On May 5, 2008, the Company agreed to issue 52,173 shares of common stock to its chief financial officer during the term of his agreement. The shares vest in four equal installments of 13,043 shares each quarter. The trading value of the common stock on May 5, 2008, was $1.17 per share for a total value of $61,042. This compensation expense is recognized on a straight-line basis over the vesting period. Compensation expense of $40,137 was charged to general and administrative expenses for the year ended December 31, 2008. 26,086 shares vested in 2008.
Following is a summary of the status of warrants outstanding at December 31, 2008:
| Outstanding Warrants | | Exercisable Warrants | |
| Exercise Price | | Number | | Average Remaining Contractual Life | | Average Exercise Price | | | Number | | | Intrinsic Value | |
| $1.20 | | | 975,000 | | 1.16 years | | $ | 1.20 | | | | 975,000 | | | $ | - | |
| $1.00 | | | 570,500 | | 3.16 years | | $ | 1.00 | | | | 570,500 | | | | 17,115 | |
| Total | | | 1,545,500 | | | | $ | 1.13 | | | | 1,545,500 | | | $ | 17,115 | |
Following is a summary of the warrant activity:
| Outstanding as of January 01, 2007 | | | - | |
| Granted | | | 4,645,500 | |
| Forfeited | | | - | |
| Exercised | | | 3,100,000 | |
| Outstanding as of December 31, 2007 | | | 1,545,500 | |
| Granted | | | - | |
| Forfeited | | | - | |
| Exercised | | | - | |
| Outstanding as of December 31, 2008 | | | 1,545,500 | |
See report of independent registered public accounting firm.
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2008
Note 16 — Convertible Debentures
On February 27, 2007, the Company entered into a Securities Purchase Agreement (the “Agreement”), with several institutional and accredited investors (the “Purchasers”) pursuant to which the Company sold to the Purchasers $4,075,000 8% convertible debentures due February 28, 2009 (the “Debentures”), and warrants to purchase 4,075,000 shares of the common stock of the Company (the “Warrants”), (collectively referred to as the “Transaction”). The initial conversion price of the debentures is $1.00 per share. The initial exercise price of the warrants is $1.20 per share with a life of three years. The conversion price and warrant exercise price are subject to downward adjustments should the Company issue more shares of common stock or securities convertible into common stock for capital raising activities for less than the conversion price or exercise price. Additional interest of 15% would begin in June 2007 and would continue through February 2008 after which the additional interest would increase to 25% through the maturity date of the note.
Gross proceeds from the Transaction were $4,075,000, of which $285,250 was paid to the placement agent for the Transaction and $52,500 was paid to consultants for the Purchasers in connection with the Transaction. The Company also issued to the placement agent a warrant to purchase an aggregate of 570,500 shares of common stock with an exercise price of $1.00 per share with a life of five years. The value of the warrants issued to the placement agent was calculated as $643,277 using the Cox, Ross and Robenstein (“CRR”) Binomial Model. The total amount of the cash payments and the fair value of the warrants amounted to $981,027, which was recorded as deferred debenture expenses. These costs are amortized to interest expense over the two year life of the Debentures.
The Company determined the value of the warrants using the CRR Binomial Model with a volatility of approximately 75%, which is calculated by using the historical closing prices of the Company’s common stock. In accordance with APB No. 14, EITF-98-5, and EITF-00-27, the Company allocated the proceeds using relative fair value method and determined that the Debentures were issued with a beneficial conversion feature. As a result, on February 27, 2007, the allocated value of the Warrants amounted to $1,944,425 and the beneficial conversion feature amounted to $2,130,575. The allocated value of the Warrants and beneficial conversion feature totaling $4,075,000, was recorded as discount (or reduction in the carrying amount) of the Debentures and additional paid-in capital and are amortized over the two year life of the Debentures using the effective interest method. For the years ended December 31, 2008 and 2007, $371,546 and $584,598 was amortized as interest expense, respectively.
In connection with the issuance of the Debentures, the Company entered into a Registration Rights Agreement, in which a registration statement registering the resale of the common stock into which the Debentures are convertible and for which the Warrants are exercisable, as well as certain other shares of the Company's common stock must be filed with the SEC not later than April 13, 2007, and be declared effective by the SEC not later than May 28, 2007, if there is no SEC review of the registration statement, and June 27, 2007, if there is an SEC review. Failure to meet these deadlines would result in liquidated damages of 2% of the aggregate purchase price of the Debentures and Warrants per month, pro rated for partial periods.
On or about December 6, 2007, the Company entered into an Amendment, Exchange and Waiver Agreement (the “Amended Agreement”), dated November 9, 2007, with certain of the Purchasers (the “Participating Purchasers”). Below are highlights of the Amended Agreement:
| | • | The Amended Agreement amends the terms of the Debentures held by the Participating Purchasers by: (a) changing the conversion price from $1.00 per share to $0.85 per share; (b) deleting the trading conditions for mandatory conversion; (c) granting the Company the right to mandatory conversion at any time, and (d) allowing the Company to designate the date for the mandatory conversion. |
See report of independent registered public accounting firm.
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2008
| | • | The Amended Agreement amends the terms of the Warrants held by the Participating Purchasers by: (a) changing the exercise price from $1.20 per share to $0.95 per share; and (b) granting to the Participating Purchasers the right to exercise their Warrants on a cashless basis. |
| | • | The Amended Agreement is deemed to be: (a) the Company’s notice to require conversion of the entire outstanding principal of the Debentures held by the Participating Purchasers and all accrued but unpaid interest thereto; and (b) the Participating Purchasers’ notice to the Company to exercise all of their unexercised Warrants on a cashless basis. |
| | • | The Amended Agreement amends the Registration Rights Agreement by waiving all outstanding registration damages due to the Purchasers in their entirety. Because the outstanding principal amounts of the Debentures held by the Participating Purchasers, as of the effective date of the Amended Agreement, total more than seventy-five percent (75%) of the aggregate outstanding principal amounts of the outstanding Debentures held by all the Purchasers on that date, the amendment to the Registration Rights Agreement binds all of the Purchasers. |
The Company has evaluated the cost of the amended terms of the Warrants and the Debentures. As the Amended Agreement has reduced the exercise price of the Warrants and the conversion price of the Debentures held by the Participating Purchasers, the difference between the value of the Warrants and the conversion option at the old prices and their values at the modified prices are deemed costs for the Company and are charged to operations.
The inducement cost for the Debentures converted was $634,450 for the fiscal year ended December 31, 2007. The inducement cost for the Debentures converted was based on the market value of the additional 461,418 shares obtained by the Participating Purchasers at $1.375 per shares on November 9, 2007. The inducement cost for the Warrants exercised was $279,547 for the fiscal year ended December 31, 2007. The inducement cost for the Warrants exercised was calculated using the CRR Binomial Model by determining the difference between the original exercise price of $1.20 shares and the reduced exercise price of $0.95.
3,076,120 shares of common stock were issued upon conversion of the Participating Purchasers’ Debentures with a carrying value of $2,548,632 at a reduced conversion price of $0.85. Another 202,600 shares of common stock were issued upon conversion of the Participating Purchasers’ Debentures with a carrying value of $202,600 at the original conversion price of $1.00.
In accordance with EITF 00-27, all unamortized discount amounting to $2,403,480 at the time of the conversion was recognized as interest expense for the year ended December 31, 2007. The unamortized deferred financing costs of $540,167 at conversion of the Debentures into common stock were also recorded as interest expense for the year ended December 31, 2007.
The Company’s registration statement did not become effective until September 25, 2007. However, because the Amended Agreement waived all outstanding registration damages, the Company reversed the previously accrued liquidated damages totaling $345,017 at December 31, 2007.
On April 21, 2008, the Company entered into an Amendment and Waiver Agreement (the “Amendment”) with two institutional and accredited investors who acquired the two remaining unconverted Debentures in a private transaction from the original holders of these Debentures. The Amendment is similar to the above Amended Agreement with significant differences summarized below:
| | • | The Amendment amends the terms of the Debentures held by these two investors by changing the conversion price from $1.00 per share to $0.80 per share. |
| | • | The Amendment is deemed to be: (a) the Company’s notice to require conversion of the entire outstanding principal of the Debentures held by these two investors and all accrued but unpaid interest thereon. |
See report of independent registered public accounting firm.
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2008
The difference between the value of the conversion option at the previous prices and their value at the modified prices are deemed costs for the Company and are charged to operations. The inducement cost for the two Debentures converted was $257,775 for the year ended December 31, 2008. The inducement cost for the converted Debentures was based on the market value of the additional 245,501 shares obtained by these two investors at $1.05 per share on April 21, 2008.
1,227,503 shares of common stock were issued upon conversion of the two Debentures with a carrying value of $982,003 (including $490,713 of default premium) at a reduced conversion price of $0.80.
In accordance with EITF 00-27, all unamortized discount amounting to $291,548 at the time of the conversion was recognized as interest expense for the year ended December 31, 2008. The unamortized deferred financing costs of $79,998 on conversion of the Debentures were also recorded as interest expense for the year ended December 31, 2008.
Note 17 — Statutory Reserves
As stipulated by the laws of the PRC as applicable to Chinese companies with foreign ownership, net income after taxation can only be distributed as dividends after appropriations have been made for the following:
| | i. | Making up cumulative prior years’ losses, if any; |
| | ii. | Allocations to the statutory surplus reserve of at least 10% of income after tax, as determined under PRC accounting rules and regulations, until the fund amounts to 50% of the Company's registered capital; |
| iii. | Allocations to the common welfare reserve of 5% of income after tax, as determined under PRC accounting rules and regulations; |
| iv. | Allocations to the discretionary surplus reserve, if approved in the shareholders’ general meeting. |
The statutory surplus reserve can be used to increase the registered capital and eliminate future losses of a company upon approval by relevant government authorities under PRC GAAP. A company’s statutory surplus reserve is not distributable to shareholders except in the event of liquidation. Appropriations to the statutory surplus reserve are accounted for as a transfer from unrestricted earnings to statutory reserves. After 2006, allocation to the common welfare reserve is at the discretion of the Company instead of a legal requirement per the PRC Corporation Law.
During the years ended December 31, 2008 and 2007, the Company made total appropriations to these statutory reserves of $1,299,990, and $873,096, respectively. The component of these statutory reserves and the amount of future contribution required pursuant to the PRC Corporation Law are as follows at December 31, 2008 and 2007:
| | | 2008 | | | 2007 | |
| Statutory surplus reserve | | $ | 1,968,473 | | | $ | 1,101,813 | |
| Common welfare reserve | | | 984,237 | | | | 550,907 | |
| | | | | | | | | |
| Total | | $ | 2,952,710 | | | $ | 1,652,720 | |
| Statutory surplus reserve of Tianxing | | $ | 1,968,473 | | | $ | 1,101,813 | |
| 50% of registered share capital of Tianxing | | | 2,541,000 | | | | 2,541,000 | |
| | | | | | | | | |
| Extra contribution to be contributed | | $ | 572,527 | | | $ | 1,439,187 | |
See report of independent registered public accounting firm.
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2008
There are no legal requirements in the PRC to fund these statutory reserves by the transfer of cash to any restricted accounts, and as such, the Company has not transferred any cash to these accounts. These reserves are not distributable as cash dividends.
Note 18 — Taxes
The Company is registered in the State of Nevada whereas its subsidiary, Skystar Cayman, is a tax exempt company incorporated in the Cayman Islands and conducts all of its business through its subsidiaries, Fortunate Time and Sida, and Sida’s PRC VIEs, Xian Tianxing and Shanghai Siqiang.
Sida, Xian Tianxing, and Shanghai Siqiang are subject to PRC’s Enterprise Income Tax. Pursuant to the PRC Income Tax Laws, Enterprise Income Tax is generally imposed at a statutory rate of 25% beginning on January 1, 2008. The Company has been approved as a new technology enterprise, and under PRC Income Tax Laws is entitled to a preferential tax rate of 15%.
For the years ended December 31, 2008 and 2007, the provisions for income tax were as follows:
| | | 2008 | | | 2007 | |
| Current PRC income tax expense | | | | | | |
| Enterprise income tax | | $ | 1,529,688 | | | $ | 1,027,172 | |
The following table reconciles the U.S. statutory rates to the Company's effective tax rate for the years ended December 31, 2008 and 2007:
| | | 2008 | | 2007 |
| U.S. Statutory rates | | | 34.0 | % | | | 34.0 | % |
| Foreign income not recognized in USA | | | (34.0 | ) | | | (34.0 | ) |
| China income taxes | | | 25.0 | | | | 33.0 | |
| China income tax exemption | | | (3.5 | ) | | | 77.5 | |
| Total provision for income taxes | | | 21.5 | % | | | 110.5 | % |
The estimated tax savings due to the reduced tax rate for the year ended December 31, 2008 and 2007 amounted to $1,019,600 and $1,232,606, respectively. The net effect on earnings per share if the non-preferential income tax rate had been applied would decrease basic and diluted earnings per share by $0.06 for the year ended December 31, 2008. For the year ended December 31, 2007, the removal of the estimated tax savings would decrease the basic and diluted losses per share by $0.09.
The Company was incorporated in the United States and has incurred a net operating loss for income tax purposes for 2008. The net operating loss carry forwards for U.S. income tax purposes amounted to $7,687,725, which may be available to reduce future years’ taxable income. These carry forwards will expire, if not utilized, beginning in 2006 through to 2028. Management believes that the realization of the benefits arising from this loss appears to be uncertain due to the Company’s continuing losses for U.S. income tax purposes. Accordingly, the Company has provided a 100% valuation allowance at December 31, 2008, which amounted to $3,169,907 The Company’s management reviews such valuation allowance periodically and makes adjustments as warranted.
See report of independent registered public accounting firm.
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2008
Note 19 — Earnings per Share
The following is a calculation for earnings per share:
| | | For years ended December 31, | |
| | | 2008 | | | 2007 | |
| Net income (loss) for earnings per share | | $ | 5,596,183 | | | $ | (1,956,976 | ) |
| | | | | | | | | |
| Weighted average shares used in basic computation | | | 18,228,731 | | | | 13,453,543 | |
| Diluted effect of stock options and warrants | | | 18,248 | | | | - | |
| Weighted average shares used in diluted computation | | | 18,246,979 | | | | 13,453,543 | |
| | | | | | | | | |
| Earnings per share: | | | | | | | | |
| Basic | | $ | 0.31 | | | $ | (0.15 | ) |
| Diluted | | $ | 0.31 | | | $ | (0.15 | ) |
For the year ended December 31, 2008, the average stock price was greater than the exercise price of 570,500 warrants outstanding at such time which resulted in additional weighted average common stock equivalents of 18,248 shares. As such, 975,000 outstanding warrants were excluded from the diluted earnings per share calculation as they were anti-dilutive.
For the year ended December 31, 2007, the average stock price was greater than the exercise price of 4,645,500 warrants outstanding at such time which resulted in additional weighted average common stock equivalents of 1,354,233 shares. However, all outstanding convertible debentures were excluded from the diluted earnings per share calculation as they were anti-dilutive.
Note 20 — Related Party Transactions and Arrangements
Related Party Receivables and Payables
Amounts receivable from and payable to related parties are summarized as follows:
| | | December 31, 2008 | | | December 31, 2007 | |
| Short term loans from shareholders | | | | | | |
| Mr. Weibing Lu – officer and shareholder (1) (2) | | $ | 220,050 | | | $ | - | |
| Mr. Wei Wen – officer and shareholder (2) | | | 44,010 | | | | - | |
| Ms. Aixia Wang – shareholder (2) | | | 44,010 | | | | - | |
| Total | | $ | 308,070 | | | $ | - | |
| | | | | | | | | |
| Amounts due from related party | | | | | | | | |
| Mr. Weibing Lu – officer and shareholder (3) | | $ | - | | | $ | 59,462 | |
| | | | | | | | | |
| Shares to be issued to related party | | | | | | | | |
| Scott Cramer – non-executive director (4) | | $ | 95,204 | | | $ | - | |
| | | | | | | | | |
| Amounts due to related parties | | | | | | | | |
| TianXing Digital - owned by a director (5) | | $ | - | | | $ | 17,137 | |
| Ms. Aixia Wang – shareholder (5) | | | - | | | | 1,371 | |
| Bennet P. Tchaikovsky – CFO (5) | | | 13,168 | | | | - | |
| Scott Cramer – non-executive director and shareholder (5) | | | 224,684 | | | | 30,245 | |
| Shaanxi Xingji Electronics Co. - owned by a director's wife (5) | | | 4,373 | | | | 32,817 | |
| Total | | $ | 242,225 | | | $ | 81,570 | |
See report of independent registered public accounting firm.
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2008
(1) In 2008, Weibing Lu obtained personal loans from Huaxia Bank’s Xian Branch and advanced $176,040 in cash to Xian Tianxing to facilitate operations. The interest rate is 7.47% per annum and is unsecured. Pursuant to the loan agreement, the principal and related interest was due on December 30, 2008. Xian Tianxing was the guarantor of Weibing Lu’s personal loan. On January 4, 2009, the Company paid the full principal amount to the bank, with related interest of $15,741.
(2) On May 29, 2008, Weibing Lu, Wei Wen and Aixia Wang obtained personal loans from Yanta Credit Union’s East Xiaozai Road Branch and advanced cash to Xian Tianxing in the total amount of $132,030 for business operations. The loans are due on May 29, 2009 with 8.436% interest per annum and are guaranteed by the Company. As of December 31, 2008, Xian Tianxing had paid interest of $3,695 for the loans of the three shareholders.
(3) As of December 31, 2008 and 2007, the Company had a receivable from Weibing Lu in the amount of $0 and $59,462, respectively. The amounts due from Mr. Lu was comprised of cash advances to Weibing Lu to facilitate Xian Tianxing’s operations and for the reimbursement of expenses paid, or to be paid, by Weibing Lu on behalf of Xian Tianxing. These balances are non-interest bearing, unsecured, and due on demand.
(4) The Company had $95,204 balance under shares to be issued to Scott Cramer, a non-executive director and shareholder as of December 31, 2008, which represented 110,000 common shares as compensation for being a representative of the Company in the United States for the period of May 2008 to December 2008.
(5) Shaanxi Xinji Electronics Co., Ltd. is owned by the wife of Mr. Lu and Tianxing Digital Co., Ltd. is owned by Mr. Lu. The amounts due to Shaanxi Xinji Electronics and Tianxing Digital as of December 31, 2007 were short term cash transfers for business operations, non-interest bearing, unsecured, and payable upon demand. As of December 31, 2008 and 2007, the Company also has $224,684 and $13,168 payable to Scott Cramer and Bennet P. Tchaikovsky for the expenses paid by them on behalf of the Company.
Note 21 — Commitments and Contingencies
Lease Commitments
The Company recognizes lease expense on a straight-line basis over the term of the lease in accordance to SFAS 13, “Accounting for Leases.” The Company entered into a tenancy agreement for the lease of factory premises for a period of ten years from October 1, 2004 to December 31, 2014, with annual rent of $13,563 (or RMB 94,600), which is subject to a 10% increase every four subsequent years.
The Company leases office space from Weibing Lu, the Company’s chief executive officer, for a period of five years from January 1, 2007 to December 31, 2011, with annual rent of approximately $24,000 (or RMB 165,600). The Company also entered into a tenancy agreement with Weibing Lu for the lease of Shanghai Siqiang’s office for a period of ten years from August 1, 2007 to August 1, 2017, with annual rent of approximately $21,000 (or RMB 144,000).
Minimum future lease payments under these leases for the next five years and thereafter are as follows:
| Years ending December 31, | | Amount | |
| 2009 | | $ | 60,684 | |
| 2010 | | | 60,684 | |
| 2011 | | | 60,684 | |
| 2012 | | | 36,645 | |
| 2013 | | | 37,917 | |
| 2014 and thereafter | | | 92,489 | |
| | | $ | 349,103 | |
See report of independent registered public accounting firm.
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2008
Lease expense for the years ended December 31, 2008 and 2007 amounted to $63,303 and $33,412, respectively.
Legal Proceedings
From time to time, the Company is involved in legal matters arising in the ordinary course of business. Management currently is not aware of any legal matters or pending litigation, which would have a significant effect on the Company’s consolidated financial statements as of December 31, 2008.
In May 2007, Andrew Chien filed suit against the Company, R. Scott Cramer, Steve Lowe, David Wassung and Weibing Lu in United States District Court for the District of Connecticut, alleging causes of action for violation of Sections 10(b) and 20(a) of the Securities Exchange Act of 1934. In or around November 2007, the defendants filed motions to dismiss the complaint for failure to state a claim and for lack of personal jurisdiction. Plaintiff agreed to voluntarily amend the complaint after the motions were filed, and an amended complaint was subsequently filed on or around January 4, 2008. The amended complaint removed Weibing Lu (who is a resident of China and had never been served) as a defendant. The remaining defendants contended that the amended complaint failed to correct the deficiencies of the original, and filed a renewed motion to dismiss for failure to state a claim, also preserving their challenge to personal jurisdiction. The defendants denied all claims and moved the Court to dismiss the amended complaint in its entirety in their motion to dismiss. The motion to dismiss also requested that the Court award sanctions against Mr. Chien under the Private Securities Litigation Reform Act (“PSLRA”) and other authority. On July 17, 2008, the Court granted defendants’ motion and subsequently dismissed the lawsuit, entering a judgment on behalf of the defendants. On February 5, 2009, the Court determined that the action filed by Mr. Chien to have been frivolous, and imposed monetary sanctions on both Mr. Chien and his former attorney. Mr. Chien subsequently filed motions seeking to “re-open” this case, but the motion was denied.
Subsequently, Mr. Chien filed another lawsuit against the Company, Scott Cramer, Steve Lowe, David Wassung and Weibing Lu in the Connecticut Superior Court, alleging causes of action similar to those alleged in his federal complaint described above as well as state law causes of action. The Company contends that the new complaint is just as frivolous as Mr. Chien’s earlier federal action, which the new complaint substantially duplicates. A Notice of Removal to the U.S. District Court, District of Connecticut was filed in the state case on January 27, 2009, and the case has been assigned to the same federal judge in the related federal case already dismissed. The Company has filed a motion to dismiss this new action which remains pending.
Other than the above described legal proceedings, the Company is not aware of any other legal matters in which purchasers, any director, officer, or any owner of record or beneficial owner of more than five percent of any class of voting securities of the Company, or any affiliate of purchaser, or of any such director, officer, affiliate of the Company, or security holder, is a party adverse to the Company or has a material adverse interest to the Company. No provision has been made in the consolidated financial statements for the above contingencies.
Ownership of Leasehold Property
In 2005, a shareholder contributed a leasehold office building as additional capital of Xian Tianxing. However, the title of the leasehold property has not passed to the Company. The Company does not believe there are any legal barriers for the shareholder to transfer the ownership to the Company. However, in the event that the Company fails to obtain the ownership certificate for the leasehold property, there is a risk that the building will need to be vacated due to unofficial ownership. Management believes that this possibility is remote, and as such, no provision has been made in the consolidated financial statements for this potential occurrence.
See report of independent registered public accounting firm.
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2008
R&D Project
During the first quarter of 2008, the Company contracted with Northwestern Agricultural Technology University to jointly work on an R&D project concerning the application of nano-technology in the prevention of major milk cow disease. The total projected budget for this project is approximately $574,000 (RMB4 million) which is to be paid according to the completed stages of the project. The Company expects this project to be completed in one year. As of December 31, 2008, the Company incurred $43,245 (RMB 300,000) R&D expenses associated with the project and also prepaid $289,773 (RMB 1,975,273) for specific raw materials for the project. The Company expects that the project will reach trial stage in 2009 and obtain veterinary permit for the new product from government on 2010.
Note 22 — Subsequent event
New bank loan
On January 14, 2009, the Company entered into a short-term loan agreement with Yanta Credit Union’s East Xiaozai Road Branch in the amount of $220,050. The loan is due on January 13, 2010 with fixed interest rate of 8.6585% per annum and is collateralized by the CEO’s personal property.
See report of independent registered public accounting firm.