Exhibit 99.2
4952 | The pharmacodynamics, pharmacokinetics and tolerability of repeat doses of the novel inhaled long-acting
beta2 adrenoceptor agonist (LABA) vilanterol trifenatate (VI; GW642444M) (25, 50 and 100mcg) in healthy subjects |
Kempsford RD, Norris V, Siederer SK | GlaxoSmithKline R&D, Stockley Park, Middlesex UB11 1BT, United Kingdom |
ABSTRACT
Rationale: GW642444 is a novel inhaled LABA with inherent 24 hour activity in vitro in development for once-daily administration as monotherapy (for COPD) and in combination with an inhaled corticosteroid (for both COPD and asthma). This study was conducted to determine the safety, tolerability, pharmacokinetics, pharmacodynamics of repeat dose GW642444 inhalation in healthy subjects.
Methods: This was a double-blind, randomized, placebo-controlled, dose-ascending, parallel group study. Male and female healthy subjects (n=36) aged 19—53 years received single doses of GW642444 (25, 50 and 100mcg) or placebo (9 subjects/ treatment) once daily for 14 days. Heart rate (HR), 12-lead ECG (QTc interval), blood pressure and plasma GW642444 concentrations (0—24h post dose) and whole blood potassium (K+) and glucose (0—4h post dose) were determined on Days 1, 7 and 14. All adverse events (AEs) were recorded and pre- and post-dose laboratory safety screens were performed.
Results: GW642444 (25—100mcg) administered for 14 days was well tolerated. All AEs were described as ‘mild’ or ‘moderate’ with a similar total incidence after GW642444 (33—67%) and placebo (67%). The most frequently reported AE was headache (0—22% after GW642444 and 56% after placebo). There were no serious AEs or withdrawals due to AEs. On Day 14 there were no treatment differences vs. placebo (95% CIs included zero) for the effects of repeat dose 25—100mcg GW642444 on HR (0—4h maximum adjusted mean difference from placebo <3bpm) or K+ (0—4h minimum adjusted mean difference from placebo <0.02mmol/L). There were no effects on Day 14 QTcF and blood glucose except with 100mcg GW642444 (adjusted mean difference in 0—4h maximum QTcF 9.1msec (95% CI: 2.1, 16.1) and adjusted mean difference in 0—4h maximum glucose 0.41mmol/L (95% CI: 0.06, 0.76)); these differences were not considered clinically significant. GW642444 was rapidly absorbed into the plasma (median tmax at 5 minutes) with approximate dose-proportional increases in Cmax across the dose range. There was some evidence of GW642444 accumulation (<30%) on repeat dosing with steady-state achieved by Day 7.
Conclusion: Repeat dosing of inhaled GW642444 (25—100mcg) once daily for 14 days was well tolerated in healthy subjects and was not associated with any clinically significant unwanted systemic effects. GW642444 was rapidly absorbed and showed approximately linear pharmacokinetics and marginal accumulation on repeat dosing.
INTRODUCTION
· Vilanterol trifenatate (VI; GW642444M) is an inhaled long-acting beta2 agonist (LABA) with inherent 24 hour activity in vitro which is in development for once-daily administration as monotherapy for COPD and in combination with the inhaled corticosteroid fluticasone furoate (for both COPD and asthma).
OBJECTIVES
Primary
· To evaluate the safety and tolerability of VI (25, 50 and 100mcg) administered once daily (AM dosing) for 14 days in healthy subjects.
Secondary
· To evaluate any extra-pulmonary systemic pharmacodynamic effects of VI administered once daily (25, 50 and 100mcg; AM dosing) for 14 days in healthy subjects.
· To evaluate the systemic pharmacokinetics of vilanterol following inhaled administration once daily (25, 50 and 100mcg; AM dosing) for 14 days in healthy subjects.
METHODS
· This was a double-blind, randomized, placebo-controlled, dose-ascending, parallel group study.
· Healthy male subjects and female subjects of non-child bearing potential with FEV1 >80% predicted and FEV1/FVC ratio >70% received single AM doses of VI (25, 50 and 100mcg) or placebo (9 subjects/ treatment) once daily for 14 days.
· VI and matching placebo (lactose alone) were administered as single or double inhalations from the DISKUS™ dry powder device.
· Heart rate, 12-lead ECG (QTc(F) interval), blood pressure, whole blood potassium and glucose (all 0—4h post dose), FEV1 (0—24h post dose) and plasma vilanterol concentrations (0—24h post dose) were determined on Days 1, 7 and 14.
· All adverse events (AEs) were recorded and pre- and post-dose laboratory safety screens were performed.
RESULTS
Demographics
· Subject demographic details are given in Table 1. Thirty six healthy subjects (35 male: 1 female) participated in the study.
Table 1. Demographic details
| | | | | | Vilanterol trifenatate | |
| | | | Placebo
N=9 | | 25mcg
N=9 | | 50mcg
N=9 | | 100mcg
N=9 | |
Sex, n (%) | | Males | | 9 | | 8 (89) | | 9 | | 9 | |
| | Females | | 0 | | 1 (11) | | 0 | | 0 | |
Age, years | | Mean | | 28.1 | | 26.7 | | 27.3 | | 29.1 | |
| | Range | | 20–46 | | 19–45 | | 23–32 | | 21–53 | |
Height, cm | | Mean | | 178 | | 177 | | 177 | | 179 | |
| | Range | | 170–187 | | 164–183 | | 167–187 | | 168–185 | |
Weight, kg | | Mean | | 79.1 | | 73.2 | | 77.9 | | 79.3 | |
| | Range | | 59.1–97.6 | | 63.6–91.5 | | 59.8–97.2 | | 62.6–96.9 | |
Body mass index, kg/m2 | | Mean | | 24.9 | | 23.5 | | 24.9 | | 24.6 | |
| | Range | | 20.5–28.3 | | 20.6–27.9 | | 19.3–28.4 | | 21.7–29.2 | |
Safety and tolerability
· VI (25—100mcg) administered for 14 days was well tolerated. All subjects received their allocated treatments for 14 days. There were no serious AEs or withdrawals.
· A total of 37 AEs were reported during the study, with 21 judged related to study drug.
· All AEs were described as ‘mild’ or ‘moderate’ with a similar total incidence after VI (33—67%) and placebo (67%).
· There was no evidence of a VI dose-related increase in AE incidence.
· Following administration of VI the most frequently reported AEs were headache (0—22%), throat irritation (0—22%) and upper respiratory tract infection (0—22%). There were no reports of tremor or palpitations. The most frequently reported adverse events are listed in Table 2.
· There were no clinical laboratory, vital sign or 12-lead abnormalities of clinical significance or falls in FEV1 immediately after dosing.
Table 2. Summary of most frequently
reported adverse events
| | | | Vilanterol trifenatate | |
Most Frequent Adverse
Events | | Placebo
N=9
n (%) | | 25mcg
N=9
n (%) | | 50mcg
N=9
n (%) | | 100mcg
N=9
n (%) | |
Subject reporting any AE | | 6 (67) | | 5 (56) | | 6 (67) | | 3 (33) | |
Subject reporting any drug-related AE | | 5 (56) | | 3 (33) | | 2 (22) | | 2 (22) | |
Most frequent AEs reported by more than one subject and irrespective of causality | | | | | | | | | |
Headache | | 5 (56) | | 2 (22) | | 0 | | 0 | |
Throat irritation | | 0 | | 2 (22) | | 1 (11) | | 0 | |
Upper respiratory tract infection | | 0 | | 0 | | 2 (22) | | 0 | |
Dizziness | | 0 | | 1 (11) | | 0 | | 1 (11) | |
Epistaxis | | 0 | | 1 (11) | | 1 (11) | | 0 | |
Nasopharyngitis | | 0 | | 1 (11) | | 0 | | 1 (11) | |
Data represents the number of subjects (%) reporting an adverse event
Systemic pharmacodynamics (Table 3)
· Heart rate: there was no effect of once-daily repeat dose VI (25, 50 or 100mcg) on heart rate. For maximum heart rate (0—4h) the Day 14 adjusted mean differences from placebo were all <3bpm and the 95% CIs included zero.
· Blood potassium: there was no effect of once-daily repeat dose VI (25, 50 or 100mcg) on blood potassium. For minimum potassium (0—4h; Day 14) adjusted mean differences from placebo were all <0.02mmol/L and the 95% CIs included zero.
· Blood glucose: there were no effects on Day 14 blood glucose except with 100mcg VI (adjusted mean difference in 0—4h maximum glucose 0.41mmol/L [95% CI: 0.06, 0.76]); this difference was not considered to be clinically significant.
· QTc(F): there were no effects on Day 14 QTcF except with 100mcg VI (adjusted mean difference in 0—4h maximum QTc(F) 9.1msec [95% CI: 2.1, 16.1]); this difference was not considered to be clinically significant.
Table 3. Summary of pharmacodynamic
data following repeat dosing with
vilanterol trifenatate for 14 days
Parameter | | Comparison
(Test–Ref) | | Difference
in Adjusted
Means (SE) | | 95% Confidence
Interval of
Means | |
Maximum heart rate (0–4h) (bpm) | | Day 14:
25mcg–placebo | | –1.52 (3.084) | | (–7.69, 4.64) | |
| | Day 14:
50mcg–placebo | | –2.94 (3.087) | | (–9.11, 3.23) | |
| | Day 14:
100mcg–placebo | | 2.39 (3.044) | | (–3.69, 8.48) | |
Maximum QTc(F) (0–4h) (msec) | | Day 14:
25mcg–placebo | | 5.82 (3.523) | | (–1.22, 12.86) | |
| | Day 14:
50mcg–placebo | | 4.47 (3.504) | | (–2.54, 11.47) | |
| | Day 14:
100mcg–placebo | | 9.08 (3.492) | | (2.10, 16.06) | |
Minimum potassium (0–4h) (mmol/L) | | Day 14:
25mcg–placebo | | –0.02 (0.063) | | (–0.14, 0.11) | |
| | Day 14:
50mcg–placebo | | –0.01 (0.063) | | (–0.13, 0.12) | |
| | Day 14:
100mcg–placebo | | –0.00 (0.066) | | (–0.13, 0.13) | |
Maximum glucose (0–4h) (mmol/L) | | Day 14:
25mcg–placebo | | 0.12 (0.172) | | (–0.22, 0.46) | |
| | Day 14:
50mcg–placebo | | 0.05 (0.176) | | (–0.30, 0.40) | |
| | Day 14:
100mcg–placebo | | 0.41 (0.173) | | (0.06, 0.76) | |
Pharmacokinetics
· Vilanterol was rapidly absorbed into the plasma (median tmax at 5min) with approximate dose-proportional increases in Cmax across the dose range (Table 4).
· Vilanterol plasma concentration/time data is given in Figure 1.
· There was some evidence of accumulation of vilanterol (<30%; Table 5) on repeat dosing with steady-state achieved by Day 7.
Figure 1. Median vilanterol plasma
concentrations following repeat dose
administration of 25, 50 and 100mcg
VI. Day 14 data (n=9/group)
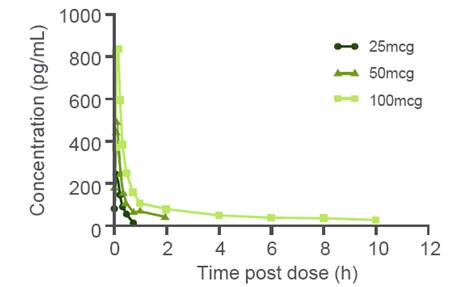
Table 4. Summary of selected repeat dose
vilanterol pharmacokinetic parameters (Day 14)
Vilanterol
trifenatate
dose (mcg) | | N | | AUC (0—t)
(pg.h/mL)(a) | | Cmax
(pg/mL) | | Tmax
(h)(b) | |
25 | | 9 | | 80 (33) | | 246 (15) | | 0.08 (0.07, 0.17) | |
50 | | 9 | | 274 (56) | | 509 (44) | | 0.10 (0.08, 0.18) | |
100 | | 9 | | 913 (26) | | 932 (18) | | 0.08 (0.08, 0.18) | |
(a) Geometric mean (CV%)
(b) Median (range)
Table 5. Summary of vilanterol accumulation
following repeat dosing for 14 days
Comparison | | Ratio Adjusted Means (90% CI for ratio) | |
| | Cmax | | AUC* | |
25mcg Day 14: Day1 | | 1.27 (1.09, 1.49) | | ND | |
50mcg Day 14: Day 1 | | 1.13 (0.96, 1.32) | | 0.98 (0.86, 1.11) | |
100mcg Day 14: Day 1 | | 1.00 (0.86, 1.17) | | 1.01 (0.9, 1.13) | |
ND: Not determined (AUC too limited to assess accumulation)
*AUC(0-t’) (to common time-point) to account for LLQ censoring
CONCLUSIONS
· Repeat dosing with inhaled vilanterol trifenatate (25—100mcg) once daily for 14 days was well tolerated in healthy subjects and was not associated with any clinically significant unwanted systemic effects.
· Vilanterol (25—100mcg) was rapidly absorbed and showed approximately linear pharmacokinetics and marginal accumulation on repeat dosing.
ACKNOWLEDGEMENTS
· This study was sponsored by GlaxoSmithKline (ClinicalTrials.gov: NCT00463697; protocol number B2C108784).
· Editorial support was provided by Geoffrey Weller and David Cutler at Gardiner-Caldwell Communications Ltd and was funded by GlaxoSmithKline.
· The study was conducted by Hammersmith Medicines Research (London, UK).

| Presented at the Annual Conference of the American Thoracic Society (ATS), New Orleans, LA, USA, 14—19 May 2010 |
