Exhibit 99.1
Poster No. P878
Dose-related efficacy of GSK573719, a new long-acting muscarinic receptor antagonist (LAMA) offering sustained 24-hour bronchodilation in COPD
M Decramer(1), F Maltais(2), G Feldman(3), J Brooks(4), S Harris(5), G Crater(5)
(1)Pneumology Section, Katholiedi Universiteit Leuven, Leuven Belgium; (2)Institut Universitaire de cardiologie et de pneumologie de Québec, Université Laval, Québec, Canada; (3)Research, South Carolina Pharmaceutical Research, Spartanburg, South Carolina, United States; (4)Respiratory Medicines Development Center, GlaxoSmithKline, Uxbridge, United Kingdom; (5)Respiratory Medicines Development Center, GlaxoSmithKline, RTP, United States
AIMS
· Bronchodilators form the foundation of pharmacological treatment of COPD.(1)
· Long-acting inhaled bronchodilators, comprising the anticholinergic class of long-acting muscarinic antagonists (LAMAs) and long-acting beta2-agonists (LABAs) are recommended for symptomatic management of moderate-to-very severe COPD, providing a more efficacious and convenient alternative to short-acting bronchodilators.(1)
· GSK573719 is an inhaled LAMA with sustained 24-hour activity under development as a once-daily therapy for COPD.
· GSK573719 is being developed in combination with the LABA, vilanterol.
· Previous clinical pharmacology studies have demonstrated that single and repeat dose administration of GSK573719 is safe and well tolerated in healthy volunteers and patients with COPD over the range of doses tested (10 to 1000mcg).(2),(3)
· The primary objectives of this study were to evaluate the efficacy and safety of three doses of GSK573719 (125, 250, and 500mcg once daily) compared with placebo in patients with COPD, in order to inform the selection of an optimal effective and safe dose for future clinical development.
METHODS
Patients
· Men and women with COPD, aged 40 to 80 years with a smoking history of >10 pack-years and post-bronchodilator FEV1 <70% and >35% predicted and FEV1 /FVC <0.70.
Study Design
· Randomised, double-blind, parallel-group 28-day treatment period.
· On-treatment clinic visits were scheduled on Day 1, Day 2, Day 7, Day 14, Day 28, and Day 29.
· Serial assessments were obtained over 0-6h post-dose on Day 1 and Day 28 for spirometry, ECG, and vital signs. Pre-dose assessments were obtained at all clinic visits.
· Following a 1-week run-in period, eligible patients were randomised to treatment with one of three doses of GSK573719 (125mcg, 250mcg, or 500mcg) or placebo. All treatments were taken once daily via a novel single-step activation dry powder inhaler.
· Salbutamol was available for rescue use.
· Patients receiving inhaled corticosteroids (ICS) alone or in a fixed combination with a bronchodilator at screening were allowed to continue the ICS at an equivalent dose and regimen throughout the study. Other COPD medications were discontinued prior to the start of study participation.
Assessments and variables
· Primary endpoint: change from baseline in trough FEV1 at Day 29 (mean FEV1 values obtained 23 and 24h after dosing on treatment Day 28).
· Secondary endpoints: weighted mean FEV1 0 to 6h and serial FEV1 at 1, 3, 6, 23, and 24h after dosing on Day 1 and Day 28.
· Safety, PK, and other efficacy data were collected, including use of rescue salbutamol.
RESULTS
Study Population
· A total of 288 patients were randomised, of whom 3 did not receive treatment, so the ITT population consisted of 285 patients. The treatment groups were well matched for patient demographics (except for gender) and disease characteristics at baseline (Table 1).
Spirometry
· The primary efficacy endpoint of change from baseline in trough FEV1 at Day 29 demonstrated statistically significant differences in favour of GSK573719 for all doses compared with placebo (Table 2).
· On Days 1 and 28, greater increases from baseline were observed in 0–6h weighted mean FEV1 values for all doses of GSK573719 compared with placebo (Table 3).
· All doses of GSK573719 demonstrated statistically significant improvements over placebo (p<0.038) in serial FEV1 at each measured time point over 24h at Day 1 and Day 28 (Figure 1).
· Differences from placebo in change from baseline in trough FVC were statistically significant for all doses of GSK573719 at all time points with the exception of the 125mcg dose at Day 29 (Figure 2).
Use of rescue salbutamol
· During the treatment period, all GSK573719 treatment groups experienced a greater percentage of rescue-free days compared with placebo at each week and across weeks 1 through to 4 (Table 4).
Safety
· The incidence of adverse events with the 125mcg and 250mcg doses was similar to placebo. A higher incidence of cough and headache was observed with the 250mcg and 500mcg doses (Table 5).
· Serious adverse events were reported for one patient in each of the active treatment groups (retinal detachment, gastroenteritis viral, COPD exacerbation in 125, 250, and 500mcg, respectively). None were considered to be related to study treatment and none were fatal.
· There were no apparent treatment related changes in vital signs or ECG assessments.
TABLE 1. DEMOGRAPHICS & BASELINE POPULATION
| | | | GSK573719 | |
| | Placebo | | 125 mcg | | 250 mcg | | 500 mcg | |
Demographics | | N=71 | | N=71 | | N=72 | | N=71 | |
Mean age, years (SD) | | 62.3 (6.80) | | 60.1 (8.75) | | 60.3 (8.45) | | 62.6 (9.30) | |
Male (n, %) | | 47 (66) | | 36 (51) | | 42 (58) | | 37 (52) | |
Race (n, %) | | | | | | | | | |
White | | 70 (99) | | 67 (94) | | 69 (96) | | 69 (97) | |
African American/African Heritage | | 1 (1) | | 4 (6) | | 3 (4) | | 1 (1) | |
African American/African Heritage & White | | 0 | | 0 | | 0 | | 1 (1) | |
BMI (kg/m2), Mean (SD) | | 25.60 (4.130) | | 26.91 (4.398) | | 27.25 (4.723) | | 26.42 (4.606) | |
Baseline Characteristics | | | | | | | | | |
Current/former smoker, % | | 61/39 | | 59/41 | | 61/39 | | 63/37 | |
Smoking history, pack-years, Mean (SD) | | 37.6 (15.34) | | 46.2 (22.06) | | 39.7 (22.13) | | 46.4 (22.42) | |
Post-bronchodilator(a) FEV1, L, Mean (SD) | | 1.569 (0.4279) | | 1.626 (0.4933) | | 1.631 (0.4752) | | 1.482 (0.3875) | |
Post-bronchodilator(a) FEV1, % predicted, Mean (SD) | | 51.4 (8.95) | | 53.9 (8.94) | | 53.8 (9.97) | | 51.7 (9.21) | |
Reversibility to bronchodilator(a), %, Mean (SD) | | 15.5 (12.87) | | 12.0 (14.46) | | 11.2 (12.05) | | 13.8 (11.56) | |
Baseline salbutamol use (puffs/day), Mean (SD) | | 4.01 (2.863) | | 2.83 (2.633) | | 3.09 (2.562) | | 3.62 (3.573) | |
Baseline trough pre-dose FEV1, L, Mean (SD) | | 1.349 (0.4438) | | 1.466 (0.4737) | | 1.480 (0.5772) | | 1.320 (0.4242) | |
(a)salbutamol
TABLE 2. CHANGE FROM BASELINE IN TROUGH FEV1 ON DAY 29
| | | | GSK573719 | |
| | Placebo | | 125 mcg | | 250 mcg | | 500 mcg | |
Trough FEV1 (L) | | N=71 | | N=71 | | N=72 | | N=71 | |
Day 29 | | | | | | | | | |
n | | 67 | | 64 | | 68 | | 64 | |
LS Mean (SE) | | 1.417 (0.025) | | 1.576 (0.025) | | 1.586 (0.025) | | 1.567 (0.025) | |
LS Mean Change (SE) | | 0.013 (0.025) | | 0.171 (0.025) | | 0.181 (0.025) | | 0.163 (0.025) | |
| | | | | | | | | |
Difference vs. Placebo | | | | 0.159 | | 0.168 | | 0.150 | |
95% CI | | | | 0.088, 0.229 | | 0.099, 0.238 | | 0.080,0.220 | |
p-value | | | | <0.001 | | <0.001 | | <0.001 | |
Note: Repeated Measures analysis adjusted for baseline, country, sex, age, treatment, smoking status, day, day by baseline interaction and day by treatment interaction
TABLE 3. REPEATED MEASURES 0–6H WM FEV1 (L)
| | | | GSK573719 | |
| | Placebo | | 125 mcg | | 250 mcg | | 500 mcg | |
Trough FEV1 (L) | | N=71 | | N=71 | | N=72 | | N=71 | |
Day 1 | | | | | | | | | |
n | | 70 | | 70 | | 71 | | 70 | |
LS Mean (SE) | | 1.408 (0.015) | | 1.614 (0.015) | | 1.627 (0.014) | | 1.576 (0.015) | |
LS Mean Change (SE) | | 0.005 (0.015) | | 0.211 (0.015) | | 0.224 (0.014) | | 0.173 (0.015) | |
| | | | | | | | | |
Difference vs. Placebo | | | | 0.206 | | 0.219 | | 0.168 | |
95% CI | | | | (0.165, 0.247) | | (0.179, 0.260) | | (0.128, 0.208) | |
p-value | | | | <0.001 | | <0.001 | | <0.001 | |
Day 28 | | | | | | | | | |
n | | 65 | | 64 | | 68 | | 64 | |
LS Mean (SE) | | 1.412 (0.024) | | 1.623 (0.024) | | 1.608 (0.023) | | 1.526 (0.024) | |
LS Mean Change (SE) | | 0.009 (0.024) | | 0.220 (0.024) | | 0.204 (0.023) | | 0.122 (0.024) | |
| | | | | | | | | |
Difference vs. Placebo | | | | 0.211 | | 0.196 | | 0.113 | |
95% CI | | | | (0.145, 0.277) | | (0.130, 0.261) | | (0.047, 0.179) | |
p-value | | | | <0.001 | | <0.001 | | <0.001 | |
Note: Repeated measures analysis adjusted for baseline, country, sex, age, treatment, smoking status, day, day by baseline interaction and day by treatment interaction.
TABLE 4. USE OF RESCUE SALBUTAMOL WEEKS 1–4
| | | | GSK573719 | |
| | Placebo | | 125 mcg | | 250 mcg | | 500 mcg | |
Weeks 1–4 | | N=71 | | N=71 | | N=72 | | N=71 | |
n | | 59 | | 67 | | 65 | | 64 | |
Change from baseline (puffs/day) LS Mean (SE) | | –0.764 (0.202) | | –1.355 (0.189) | | –1.654 (0.191) | | –1.042 (0.193) | |
| | | | | | | | | |
Difference vs. Placebo | | | | –0.591 | | –0.890 | | –0.277 | |
95% CI | | | | (–1.143, –0.038) | | (–1.441, –0.340) | | (–0.828, 0.273) | |
| | | | | | | | | |
Change from baseline in % of days with no salbutamol use Raw Mean (SD) | | 10.87 (32.147) | | 26.92 (42.785) | | 22.35 (34.267) | | 15.36 (29.621) | |
FIGURE 1. ADJUSTED MEAN DIFFERENCE FROM PLACEBO (95% CI) IN CHANGE FROM BASELINE IN FEV1 OVER TIME AT DAY 1 AND DAY 28

Note: Repeated Measures analysis adjusted for baseline, country, sex, age, treatment, smoking status, time, time by baseline interaction and time by treatment interaction.
FIGURE 2. ADJUSTED MEAN DIFFERENCES FROM PLACEBO IN CHANGE FROM BASELINE IN TROUGH FVC (L) OVER TIME
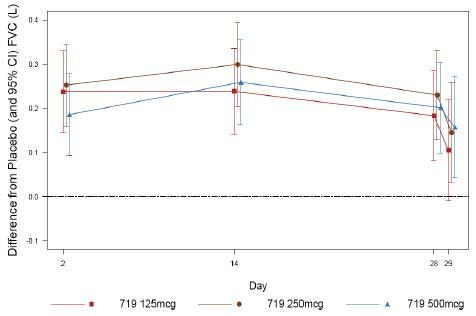
Note: Repeated Measures analysis adjusted for baseline, country, sex, age, treatment, smoking status, day, day by baseline interaction and day by treatment interaction.
TABLE 5. MOST FREQUENT ADVERSE EVENTS(a)
| | | | GSK573719 | |
Most Frequent AEs(b) | | Placebo | | 125 mcg | | 250 mcg | | 500 mcg | |
n (%) | | N=71 | | N=71 | | N=72 | | N=71 | |
Any AEs | | 16 (23) | | 18 (25) | | 17 (24) | | 24 (34) | |
Cough | | 2 (3) | | 0 | | 6 (8) | | 8 (11) | |
Headache | | 3 (4) | | 3 (4) | | 4 (6) | | 6 (8) | |
Nasopharyngitis | | 3 (4) | | 2 (3) | | 1 (1) | | 2 (3) | |
Dysgeusia | | 1 (1) | | 1 (1) | | 1 (1) | | 1 (1) | |
Abdominal pain upper | | 1 (1) | | 1 (1) | | 0 | | 1 (1) | |
Back pain | | 0 | | 2 (3) | | 1 (1) | | 0 | |
Gastroenteritis viral | | 0 | | 1 (1) | | 1 (1) | | 1 (1) | |
Hypertension | | 2 (3) | | 1 (1) | | 0 | | 0 | |
Sinus congestion | | 1 (1) | | 1 (1) | | 0 | | 1 (1) | |
Cystitis | | 1 (1) | | 1 (1) | | 0 | | 0 | |
Dry mouth | | 0 | | 0 | | 1 (1) | | 1 (1) | |
Nasal congestion | | 1 (1) | | 0 | | 1 (1) | | 0 | |
Product taste abnormal | | 0 | | 1 (1) | | 0 | | 1 (1) | |
Sputum increased | | 0 | | 0 | | 2 (3) | | 0 | |
(a) Adverse events reported on-treatment
(b) Most frequent adverse events are defined as events occurring in more than one subject across the treatment groups
CONCLUSIONS
· Treatment with GSK573719 125mcg, 250mcg, and 500mcg once-daily for 28 days resulted in statistically significant improvements in pulmonary function compared with placebo in patients with COPD.
· Overall, the lung function findings from this study support a once-daily dosing interval for GSK573719.
· The results of this study show that GSK573719 is efficacious and well-tolerated over 28 days when used in the treatment of patients with COPD.
REFERENCES
(1) GOLD. Global Initiative for Chronic Obstructive Lung Disease (Updated 2010). http://www.gold.org.
(2) Kelleher D, et al. ERS Annual Congress 2011. Poster number P834.
(3) Mehta R, et al. ERS Annual Congress 2011. Poster number P822 and Poster number P3972.
ACKNOWLEDGEMENTS
· Editorial support (in the form of collating author comments, grammatical editing) was provided by Lisa Moore at Gardiner-Caldwell Communications and was funded by GlaxoSmithKline.
· The study was sponsored by GlaxoSmithKline (ClinicalTrials.gov: NCT01030965; AC4113589).

Presented at the European Respiratory Society Annual Congress, 24-28 September 2011, Amsterdam, Netherlands


