Exhibit 99.1
| Dose-related efficacy of GSK573719, a long-acting muscarinic receptor antagonist (LAMA) with sustained 24-hour activity, in COPD James F. Donohue1, A Anzueto2, J Brooks3, R Mehta4, C Kalberg4, G Crater4 1Department of Medicine, University of North Carolina College of Medicine, Chapel Hill, North Carolina 2University of Texas Health Science Center at San Antonio, San Antonio, Texas 3Respiratory Medicines Development Center, GlaxoSmithKline, Uxbridge, United Kingdom 4Respiratory Medicines Development Center, GlaxoSmithKline, Research Triangle Park, North Carolina |
| Disclosures •J.F. Donohue is an advisor, consultant, or member of a DSMB or adjudication committee for Almiral, AstraZeneca, BoehringerIngelheim, GlaxoSmithKline, Elevation Pharmaceuticals, Forest laboratories, Novartis, Dey, Sunovion, Talecris, Pfizer for which he receives fees •A. Anzueto is an advisor, consultant, and speaker for BoehringerIngelheim, GlaxoSmithKline, Pfizer, Merck, Bayer-Schering Pharma, Dey Pharma, Forest laboratories and has investigational grants with the NHLBI, GlaxoSmithKline, Lilly, Pfizer, and Pneuma pharmaceuticals •G. Crater, C. Kalberg, R. Mehta, and J. Brooks are employees of GlaxoSmithKline •This study was funded by GlaxoSmithKline |
| GSK573719 •Competitive muscarinic acetylcholine receptor antagonist •Long duration of action due to prolonged binding at the M3 muscarinic receptor •In development for the once-daily treatment of COPD as a monotherapy and in combination with the long-acting beta2- agonist vilanterol |
| Study AC4113073(www.clinicaltrials.gov registration number NCT00950807) •Primary study objective: –Evaluate the dose-response and dosing interval of GSK573719 •Multicenter, randomized, double-blind, double-dummy, placebo-controlled, three-way cross- over, incomplete block study •Subjects received placebo and 2 of 9 active treatments •Three 14 day treatment periods |
| Treatments •GSK573719 –62.5, 125, 250, 500, and 1000mcg once-daily (QD) –62.5, 125, and 250mcg twice-daily (BD) •Tiotropium 18mcg once-daily (open-label) as active control •Placebo •All treatments administered using a novel multidose dry powder inhaler |
| Key Eligibility Criteria •COPD patients 40 to 80 years old •Post-albuterol FEV1/FVC of ≤.0.70 and FEV1of ≥.35 and ≤.70% of predicted normal •Concurrent use of systemic corticosteroids, long-acting bronchodilators, including theophyllines, and short-acting anticholinergics was not allowed •Concurrent use of albuterol and stable-dose ICS was allowed |
| Key Efficacy Measures •Change from baseline in clinic visit trough FEV1 at Day 15 (Primary) •0–24 hour weighted mean FEV1 at Day 14 •Serial FEV1 values at each time point over 28 hours at Day 14 •Rescue albuterol use |
| Safety Measures •Adverse events •Vital signs •12-lead ECG •24 hour Holter ECG assessments •Standard clinical laboratory tests •COPD exacerbations |
| Baseline Characteristics N=176 Age, years1 59.7 (8.12) Male/Female, n (%) 101/75 (57/43) Race, n (%) Caucasian172 (98) African American 4 (2) Body Mass Index (kg/m2)1 26.71 (4.655) FEV1 (L)1,2 1.65 (0.467) FEV1 % of predicted normal1,2 52.4 (9.47) FEV1/FVC %2 49.6 (9.77) Smoking history in pack-years1 52.2 (27.48) Concurrent ICS use (%) 28 1values are mean (SD) 2Post-albuterol |
| Trough FEV1 Day 15 LS mean difference from placebo in mL (95% CI) GSK573719 Once-daily 62.5mcg n=35 125mcg n=34 250mcg n=36 500mcg n=38 1000mcg n=32 Tiotropium n=35 128** (60-196) 147** (77-216) 95* (27-162) 140** (74-205) 186** (113-259) 105* (37-173) GSK573719 Twice-daily 62.5mcg n=34 125mcg n=37 250mcg n=33 79† (8-151) 134** (64-204) 172** (101-242) *p<0.01 vs. placebo **p<0.001 vs. placebo †p<0.05 vs. placebo |
| 0-24 hour Weighted Mean FEV1 (Day 14 /15) 138mL p<0.001 131mL p<0.001 136mL p<0.001 136mL p<0.001 143mL p<0.001 133mL p<0.001 142mL p<0.001 120mL p<0.001 127mL p<0.001 Difference versus placebo 719 1000mcg QD 719 500mcg QD 719 250mcg QD719 125mcg QD 719 62.5mcg QD 719 250mcg BD 719 125mcg BD 719 62.5mcg BDTiotropium 0.05 0.100.15 0.20 0.25 0.30 Difference from placebo and 95% CI Treatment –0.05 0 QD= once-daily; BD=twice-daily |
| Serial FEV1 at Day 14 to 15 (once-daily doses) p≤0.033 at all time points for all doses vs. placebo, except at 1 hr for 500 and 1000mcg |
| Serial FEV1 at Day 14 to 15 (twice-daily doses) p <0.023 at all time points for all doses vs. placebo, except , 24hr for 62.5mcg BD and tiotropium at 18 and 28 hours |
| Rescue Albuterol Use LS mean difference from placebo in puffs/day GSK573719 Once-daily 62.5mcg n=35 125mcg n=34 250mcg n=36 500mcg n=38 1000mcg n=32 Tiotropium n=35 -0.483 -0.784* -0.772* -0.753* -0.434 -0.888* GSK573719 Twice-daily 62.5mcg n=34 125mcg n=37 250mcg n=33 -0.606* -0.809* -0.765* *p<0.05 |
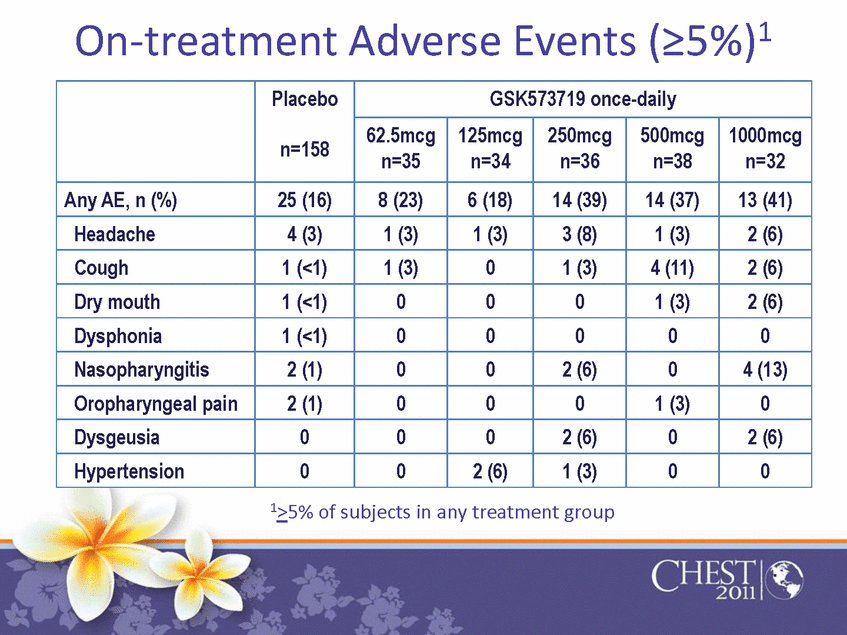
| On-treatment Adverse Events (≥5%)1 Placebon=158 GSK573719 once-daily 62.5mcg n=35 125mcg n=34 250mcg n=36 500mcg n=38 1000mcg n=32 Any AE, n (%) 25 (16) 8 (23) 6 (18) 14 (39) 14 (37) 13 (41) Headache 4 (3) 1 (3) 1 (3) 3 (8) 1 (3) 2 (6) Cough 1 (<1) 1 (3) 0 1 (3) 4 (11) 2 (6) Dry mouth 1 (<1) 0 0 0 1 (3) 2 (6) Dysphonia 1 (<1) 0 0 0 0 0 Nasopharyngitis 2 (1) 0 0 2 (6) 0 4 (13) Oropharyngealpain 2 (1) 0 0 0 1 (3) 0 Dysgeusia 0 0 0 2 (6) 0 2 (6) Hypertension 0 0 2(6) 1 (3) 0 0 1≥5% of subjects in any treatment group |
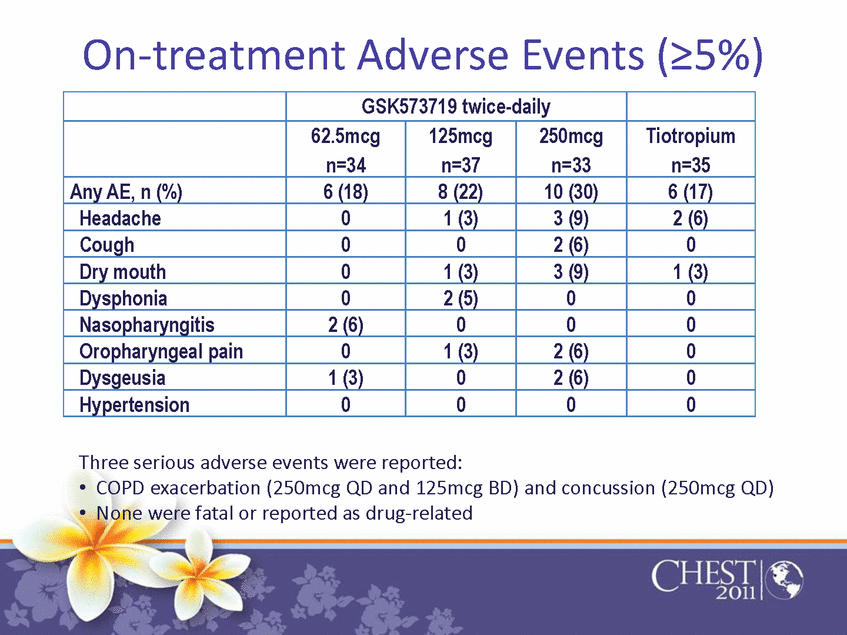
| On-treatment Adverse Events (=5%) GSK573719 twice-daily 62.5mcg n=34 125mcg n=37 250mcg n=33 Tiotropium n=35 Any AE, n (%) 6 (18) 8 (22) 10 (30) 6 (17) Headache 0 1 (3) 3 (9) 2 (6) Cough 0 0 2 (6) 0 Dry mouth 0 1 (3) 3 (9) 1 (3) Dysphonia 0 2 (5) 0 0 Nasopharyngitis 2 (6) 0 0 0 Oropharyngeal pain 0 1 (3) 2 (6) 0 Dysgeusia 1 (3) 0 2 (6) 0 Hypertension 0 0 0 0 Three serious adverse events were reported: •COPD exacerbation (250mcg QD and 125mcg BD) and concussion (250mcg QD) •None were fatal or reported as drug-related |
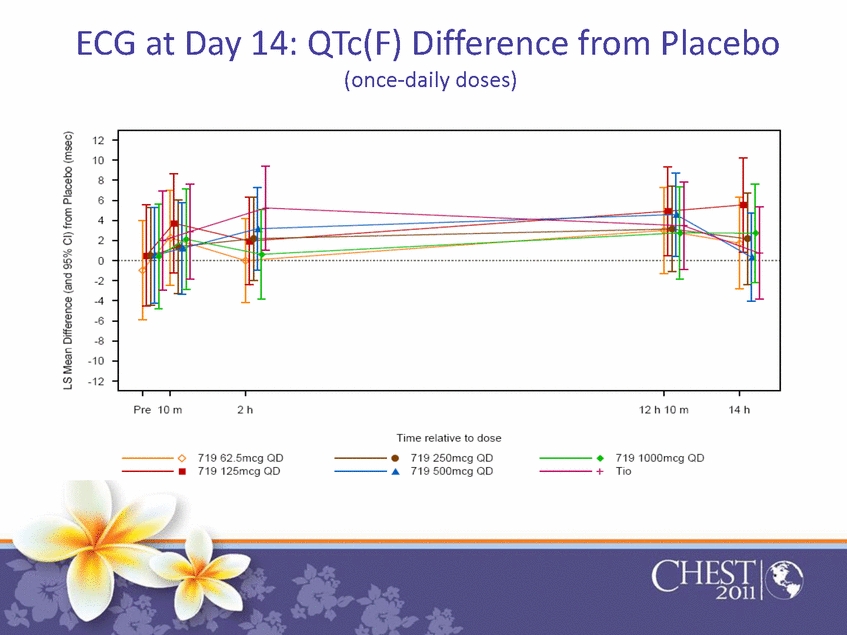
| ECG at Day 14: QTc(F) Difference from Placebo (once-daily doses) |
| 24-hr Holter Findings •No clear patterns in the rates of single ventricular ectopic beats, couplets or runs were seen across treatments •Single ventricular ectopics occurred in 81% of placebo treated patients and 53 to 84% of subjects across all GSK573719 doses •Couplets occurred in 14% of placebo treated subjects and 3 to 24% of patients across all GSK573719 doses •The incidence of ventricular ectopic runs was low –2% with placebo and 0 to 3% with GSK573719 |
| Semi-Log Plot of Individual Change from Baseline in Pulse Rate versus GSK573719 Cmax and Pulse Rate (once-daily doses) GSK573719 Log Cmax (ng/mL) -4.5 -3.0 -1.5 0.0 1.5 Change in Baseline Pulse Rate (bpm) -40 -20 0 20 40 60 80 100 GSK573719_62.5mcg qd GSK573719_125mcg qd GSK573719_250mcg qd GSK573719_500mcg qd GSK573719_1000mcg qd Placebo Tiotropium Y = -0.26X+ 6.37 r ² = 0.00119 No obvious relationship between GSK573719 Cmaxand pulse rate |
| AC4113073 Summary •All doses of GSK573719 significantly improved lung function over 28 hours •Sustained improvement in lung function over 14 days •Comparisons of once-daily versus twice-daily dosing and tiotropium confirmed a once-daily profile •Significant reductions in rescue albuterol use indicate symptomatic improvement •All doses were well tolerated with no apparent treatment-related changes in vital signs or ECG and 24 hour Holter assessments |