- INVA Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
8-K Filing
Innoviva (INVA) 8-KOther Events
Filed: 20 May 13, 12:00am
Exhibit 99.1
POSTER NO. 806
Once-daily (OD) fluticasone furoate/vilanterol 100/25mcg (FF/VI) compared with twice-daily (BD) fluticasone propionate/salmeterol 250/50mcg (FSC) in patients with COPD
Dransfield M(1), Crim C(2), Feldman G(3), Korenblat P(4), LaForce C(5), Locantore N(2), Pistolesi M(6), Watkins M(2), Martinez F(7)
(1)University of Alabama, Birmingham, AL, USA; (2)GlaxoSmithKline, Research Triangle Park, NC, USA; (3)S. Carolina Pharmaceutical Research, SC, USA; (4)The Clinical Research Center, St Louis, MO, USA; (5)North Carolina Clinical Research, Raleigh, NC, USA; (6)University of Florence, Florence, Italy; (7)University of Michigan, Ann Arbor, MI, USA
INTRODUCTION
· Currently available ICS/LABA combinations for moderate/severe COPD require twice-daily dosing.
· FF and VI are, respectively, a novel ICS and LABA in development as a once-daily combination therapy (FF/VI) for COPD and asthma.
OBJECTIVES
· To compare the efficacy and safety profiles of once-daily FF/VI 100/25mcg with twice-daily FSC (250/50mcg) in patients with moderate/severe COPD.
METHODS
· Two randomized, double-blind, double-dummy, multi-center parallel-group studies (HZC109 [Study 1] and HZC352 [Study 2]), of 12 weeks duration, were identical in design, conduct and analysis.
· Patients (>40 years of age, >10 pack-years smoking history, post-bronchodilator FEV1 <70%, FEV1/FVC ratio <0.70 at screening, no requirement of prior exacerbations) completed a 2-week placebo run-in and were randomized 1:1 to once-daily (morning) FF/VI 100/25 via the ELLIPTA™ two-strip dry powder inhaler, or twice-daily FSC 250/50 via DISKUS™.
· Primary endpoint was change from baseline in weighted mean (wm) 0–24h FEV1 on Day 84. Secondary endpoint was time to onset on Day 1. Safety was assessed throughout the study.
· Outcomes of the individual studies and pooled data are presented. A step-down statistical hierarchy was applied to analysis of the individual studies but not the pooled data. In Study 1 and Study 2, a statistically significant (p<0.05) treatment difference on the primary endpoint was required for statistical inference to be drawn on subsequent endpoints.
RESULTS
· 1030 patients (Study 1: 519; Study 2: 511) were randomized and received at least one dose of study medication (intent-to-treat [ITT] population). 950 completed the studies. On-treatment withdrawal rates were 8% in both treatment arms.
· Patient demographics were well matched (Table 1).
Table 1. Patient demographics and screening characteristics
(pooled data, ITT population)
|
| FF/VI 100/25 |
| FSC 250/50 |
|
|
| N=519 |
| N=511 |
|
Age, years |
| 61.3 (8.8) |
| 61.5 (8.7) |
|
Male sex, n (%) |
| 345 (66) |
| 336 (66) |
|
BMI, kg/m2 |
| 27.4 (5.9) |
| 27.4 (5.7) |
|
Smoking pack years |
| 40.7 (21.2) |
| 41.6 (24.1) |
|
Post-bronchodilator FEV1, L |
| 1.47 (0.50) |
| 1.45 (0.47) |
|
% predicted post-bronchodilator FEV1 |
| 48.3 (11.9) |
| 48.0 (12.0) |
|
% reversibility FEV1 |
| 11.2 (13.4) |
| 11.9 (13.4) |
|
Post-bronchodilator FEV1/FVC, L |
| 0.50 (0.10) |
| 0.50 (0.10) |
|
Values are mean (SD) unless otherwise stated
Efficacy: primary endpoint
· Change from baseline in 0–24h wmFEV1 on Day 84 was significantly (p<0.001) greater with FF/VI than with FSC in Study 1 and in the pooled analysis, but not in Study 2 (Table 2).
· An overall pattern of greater lung function over 24h on Day 84 was observed with FF/VI compared with FSC (Figure 1).
Table 2. Change from baseline 0–24h wmFEV1 (mL) after 12 weeks
(Study 1, Study 2 & pooled, ITT population)
|
| FF/VI 100/25 |
| FSC 250/50 |
| Treatment diff |
|
Pooled |
| n=447 |
| n=430 |
| 54 (21, 88) |
|
Study 1 |
| n=228 |
| n=213 |
| 80 (37, 124) |
|
Study 2 |
| n=219 |
| n=217 |
| 29 (–22, 80) |
|
Values are least squares mean (SD) unless otherwise stated
Figure 1. LS mean FEV1 change from baseline over 24h, Day 84
(pooled data, ITT population)
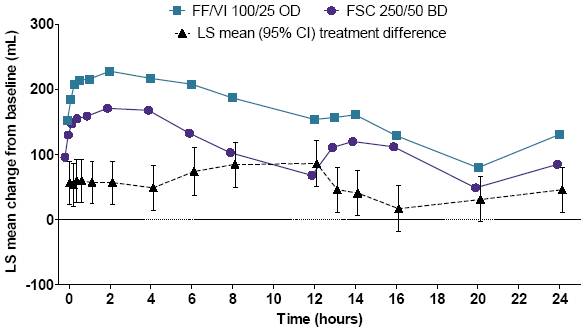
CI=confidence interval; LS=least squares
Efficacy: secondary endpoint
· Median time to >100mL increase from baseline FEV1 was significantly faster with FF/VI (15–16min) than FSC (30min) in Study 1 (p=0.012) and in the pooled analysis (p=0.026) (Figure 2), but significance could not be inferred for Study 2 (FF/VI: 16min, FSC: 30min).
Figure 2. Cumulative % of patients achieving >100mL increase
from baseline FEV1, Day 1 (pooled data, ITT population)
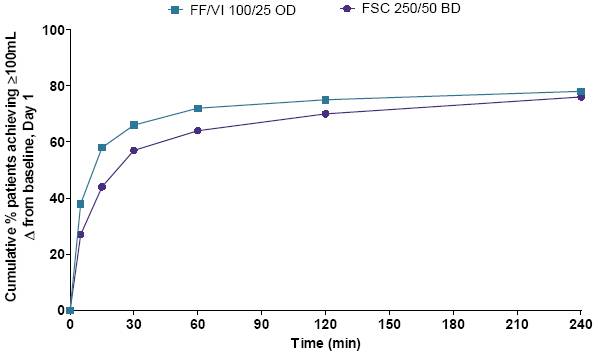
Table 3. Summary of on-treatment AEs by treatment group
(pooled data, ITT population)
|
| FF/VI 100/25 |
| FSC 250/50 |
|
|
| N=519 |
| N=511 |
|
Any AE |
| 118 (23) |
| 125 (24) |
|
Headache |
| 28 (5) |
| 21 (4) |
|
Nasopharyngitis |
| 14 (3) |
| 12 (2) |
|
Any SAE |
| 8 (2) |
| 11 (2) |
|
AEs occurring in >3% of patients in either treatment group shown
AE=adverse event, SAE=serious adverse event
Safety
· AE frequency was similar between treatment groups (Table 3).
· No abnormalities of clinical concern were observed in either study for laboratory values, including urinary cortisol, or ECG readings.
· A statistically significant treatment difference (FF/VI - FSC) in 0–4h weighted mean pulse rate (95% CI) of –1.9bpm (–3.3, –0.5) was observed at Week 12 in Study 1; this difference was not considered to be clinically significant. No difference in weighted mean pulse rate was observed between FF/VI and FSC in Study 2.
CONCLUSIONS
· Pooled analysis of these two replicate studies found once-daily FF/VI 100/25 to produce a greater improvement in 24h lung function than twice-daily FSC 250/50 after 12 weeks of treatment.
· FF/VI confers a more rapid improvement in lung function than FSC in the first hour of dosing on Day 1.
· No baseline factors that may explain the differential outcomes of Study 1 and Study 2 were apparent.
· No substantial safety concerns were identified in relation to FF/VI. Both treatments were well tolerated overall with similar safety profiles.
ACKNOWLEDGMENTS
· The presenting author, Mark Dransfield, declares the following real or perceived conflicts of interest during the last 3 years in relation to this presentation: has served as a consultant for Boehringer Ingelheim (BI), GlaxoSmithKline (GSK) and Ikaria. He has received grant funding from the NHLBI for COPD-related research and contracted research funding from Aeris, AstraZeneca, BI, Boston Scientific, Centocor, Forrest, GSK, Ikaria, MedImmune, Otsuka and Pfizer.
· This research was funded by GlaxoSmithKline. GSK study codes (clinicaltrials.gov): HZC113109 (NCT01323634); HZC112352 (NCT01323621).
· Editorial support (in the form of writing assistance, assembling tables and figures, collating author comments, grammatical editing and referencing) was provided by Ian Grieve, PhD at Gardiner-Caldwell Communications (Macclesfield, UK) and was funded by GlaxoSmithKline.
ELLIPTA™ is a trade mark of GlaxoSmithKline

Presented at the American Thoracic Society Annual Congress, Philadelphia, PA, USA, 17–22 May 2013
