- INVA Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
8-K Filing
Innoviva (INVA) 8-KOther Events
Filed: 9 Sep 13, 12:00am
Exhibit 99.4
Poster No.1248
Population pharmacokinetics and pharmacodynamics of GSK961081 (MABA) in patients with moderate to severe COPD
Claire Ambery(1), Pascal Wielders(2), Andrea Ludwig-Sengpiel(3), Robert Chan(1), John Riley(1)
(1)GlaxoSmithKline, Stockley Park West, Uxbridge, UK; (2)Dept. Pulmonary Diseases, Catharina Hospital, Eindhoven, Netherlands; (3)KLB Healthresearch, Luebeck, Germany
INTRODUCTION
· GSK961081 is a potent bi-functional molecule that has demonstrated both anti-muscarinic receptor activity (MA) and beta2-adrenergic agonist activity (BA) in pre-clinical and clinical investigation [1,2,3].
· In COPD trials, GSK961081 has shown clinically meaningful bronchodilation with rapid onset of action with a good safety and tolerability profile [4,5].
OBJECTIVES
· To characterise the population pharmacokinetics (PK) and pharmacodynamics (PD) of GSK961081 in moderate to severe COPD subjects.
METHODS
· Data were obtained from a 4-week, multicentre, randomised, double-blind, double dummy, placebo and salmeterol controlled parallel group study [5].
· Once (OD) and twice (BD) daily GSK961081 DISKUS™ dosing regimens were assessed;
· OD doses: 100, 400 & 800 mcg
· BD doses: 100, 200 & 400 mcg
· Trough FEV1 at day 29 was the primary endpoint.
· Plasma GSK961081 concentrations were an additional endpoint.
· Blood samples were collected on Day 28: Pre AM dose (-1h to 0min) and post AM dose between 0-30min, 30min-2h, 2-6h and 6-11h. Pre PM dose (-1h to 0min) and post PM dose between 0-30min, 30min-2h, 2-6h and 6-11h. PK subset subjects only.
· PK bioanalysis was performed using a validated high performance liquid chromatography/mass spectrometry (HPLC-MS/MS) method with a lower limit of quantification (LOQ) of 25 pg/mL.
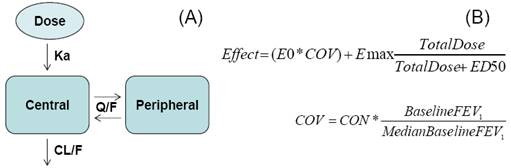
FIGURE 1. PK and PD Models
DISKUS™ is a trade mark of the GlaxoSmithKline group of companies
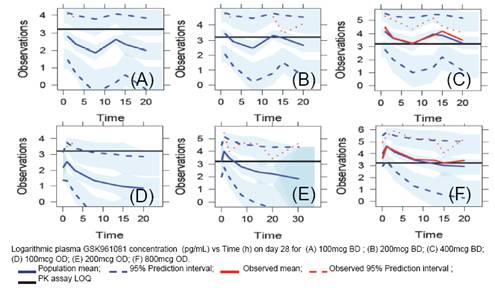
FIGURE 2. Final PK Model
PK and PD Analyses
· The software NONMEM 7 (ICON Development Solutions) was used.
· M3 methodology was used to handle non-quantifiable (NQ) PK data [6,7].
· Models were prioritised using objective function values, plausibility of parameters and graphical checking.
· Stepwise covariate model building was used (PD model only).
· A 2-compartment PK model with first-order absorption was selected and fitted to the PK data (Figure 1A).
· To characterise the dose-response curve an Emax model was selected and fitted to the PD data (Figure 1B).
TABLE 1. PK Model Parameters
PK Parameter |
| Estimate |
| 95% CI |
| RSE% |
Clearance, CL/F (L/h) |
| 944 |
| 750, 1188 |
| 1.72 |
Central volume, V2/F (L) |
| 523 |
| 337, 829 |
| 3.71 |
Absorption rate constant, Ka (h-1) |
| 0.411 |
| 0.313, 0.535 |
| 15.2 |
Peripheral volume, V3/F (L) |
| 21375 |
| 12088, 36316 |
| 2.90 |
Inter-compartment clearance, Q/F (L/h) |
| 1408 |
| 1035, 1915 |
| 2.17 |
CI: Confidence interval; %RSE: Relative standard error.
RESULTS
PK
· Greater than 50% of PK data per treatment group was NQ; except for at 800mcg QD (27% NQ) and 400mcg BD (30% NQ);
· Therefore the PK model was selected based on these two treatment groups only (N=47).
· The PK model well characterised the observed 800mcg OD and 400mcg BD data (Figure 2(C) and (F)).
· PK model parameters were estimated with good precision (%RSE<20%) (Table 1).
· Covariates were not included in the PK model.
· Evaluation of the PK model using the data not used in model building (N=139) showed a good fit (Figure 2(A),(B),(D) and (E)).
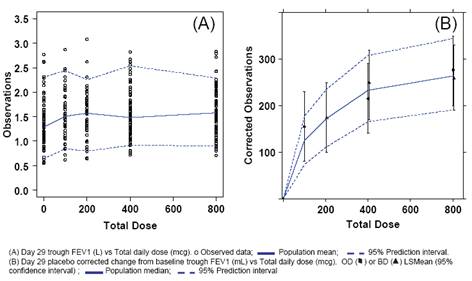
FIGURE 3. Final PD Model (Trough FEV1, day 29)
PD
· The PD model gave a good fit to the observed data (Figure 3(A)) (N=347).
· PD model parameters were estimated with precision%RSE<50% (Table 2).
· Dosing regimen, age, weight, sex, height and inhaled corticosteroid use were not identified as statistically significant covariate effects.
· Baseline FEV1 was identified as a statistically significant covariate effect on E0.
· The PD model concurred well with the primary endpoint analysis (Figure 3(B) and [5]) based on post-hoc derived placebo corrected change from baseline.
TABLE 2. PD Model Parameters
PD Parameter |
| Estimate |
| 95% CI |
| RSE% |
Emax (L) |
| 0.293 |
| 0.207, 0.379 |
| 14.9 |
ED50 (mcg) |
| 152 |
| 2.45, 302 |
| 50.2 |
Intercept , E0 (L) |
| 0.0650 |
| 0.0233, 0.107 |
| 32.8 |
Baseline, COV (L) |
| 19.0 |
| 5.24, 32.8 |
| 36.9 |
CI: Confidence interval; %RSE: Relative standard error.
CONCLUSIONS
· The PK model described will be used as a tool for guiding GSK961081 clinical development.
· The model performed well and in addition was able to account for Non Quantifiable data.
· Covariate inclusion in the model will be re-visited once additional PK data is available.
· The PD model described will be used as a tool for guiding dose selection for GSK961081 in Phase III trials.
· There was no influence of dosing regimen on the PD model; indicating there was no apparent difference between OD and BD dosing for day 29 trough FEV1 (primary end point).
REFERENCES
(1) Aiyer J, et al. Am J Resp Crit Care Med 2009;179:A4552.
(2) Pulido-Rios MT, et al. Am J Resp Crit Care Med 2009;Volume:179:A6195.
(3) Norris V, et al. Pulm Pharm Ther 2013;(in press http://dx.doi.org/10.1016/j.pupt.2013.03.009).
(4) Bateman ED, et al. Pulm Pharm Ther 2013:(in press http://dx.doi.org/10.1016/j.pupt.2013.03.015).
(5) Wielder PLML, et al. ERJ 2013;(in press doi: 10.1183/09031936.00165712).
(6) Ahn EA, et al. JPKPD 2008;35:401-421.
(7) Ribbing J, et al. JPKPD 2004; 31(2):109-134.
ACKNOWLEDGMENTS
· The presenting author, Claire Ambery, declares the following real or perceived conflicts of interest during the last 3 years in relation to this presentation: Employed by, and share holder in, GlaxoSmithKline.
· This study was registered on the Clinical Trials Register NCT01319019, used the study code MAB115032, and was funded by GlaxoSmithKline.
|
|
|
Presented at the European Respiratory Congress, Barcelona, Spain, 08 September 2013