- INVA Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
8-K Filing
Innoviva (INVA) 8-KOther Events
Filed: 8 Sep 14, 12:00am
Exhibit 99.1

Umeclidinium/vilanterol (UMEC/VI) once daily (OD) vs fluticasone/salmeterol combination (FSC) twice daily (BD) in patients with moderate-to-severe COPD and infrequent COPD exacerbations
Poster No. P290
Singh D,(1) Worsley S,(2) Zhu C-Q,(2) Hardaker L,(2) Church A(3)
(1)University of Manchester and University Hospital of South Manchester Foundations Trust, Manchester, UK; (2)GSK, London, UK; (3)GSK, Research Triangle Park, NC, USA
Aims
· Umeclidinium/vilanterol (UMEC/VI), a long-acting muscarinic antagonist (LAMA)/long-acting beta(2) agonist (LABA) combination(1) is an approved COPD maintenance treatment in the EU(2) and USA(3) and several other countries. The inhaled corticosteroid (ICS)/LABA combination fluticasone propionate/salmeterol(4) (FSC) is also approved for COPD in the EU (500/50mcg twice daily).
· Guidelines recommend treatment with an ICS/LABA combination in COPD patients with moderate-to-very-severe lung function impairment and/or a history of exacerbations (GOLD C and D).(5) A LAMA/LABA combination is one treatment recommendation for GOLD B patients.(5) In clinical practice, treatment of patients in various GOLD categories diverges from current recommendations based on clinical judgement.
· For COPD patients with dyspnoea and without frequent exacerbations (i.e. GOLD B and a subset of GOLD D), a key clinical question is whether non-ICS combinations are more efficacious than ICS-containing treatments.
· The objective of this study was to compare the efficacy and safety of once-daily UMEC/VI 62.5/25mcg with twice-daily FSC 500/50mcg over 12 weeks in patients with moderate-to-severe COPD with a history of infrequent COPD exacerbations.
Methods
Study design and population
· Phase IIIb, multicentre, randomised, double-blind, double-dummy, parallel-group study (GSK study code: DB2116134; clinicaltrials.gov: NCT01822899).
· Inclusion criteria were: males or females >40 years old; pre- and post-salbutamol forced expiratory volume in 1s (FEV1)/forced vital capacity (FVC) ratio <0.70 and post-salbutamol FEV1 >30% and >70% of predicted normal values; dyspnoea score >2 (modified Medical Research Council [mMRC] Dyspnoea Scale); current or former smokers (history of cigarette smoking >10 pack-years).
· Key exclusion criteria were: other respiratory disorders; hospitalisation for pneumonia; a documented history of >1 COPD exacerbation requiring oral corticosteroids, antibiotics and/or hospitalisation in the previous 12 months.
Treatment
· After a 7–14 day run-in, patients were randomised 1:1 to receive 12 weeks of treatment
· once-daily UMEC/VI 62.5/25mcg (delivered doses 55/22mcg; via ELLIPTA® dry powder inhaler [DPI]) + twice-daily placebo (via DISKUS®)
· twice-daily FSC 500/50mcg (via DISKUS®) + once-daily placebo (via ELLIPTA® DPI).
Endpoints and analyses
· Lung-function endpoints included: 0–24h weighted mean (wm) FEV1 (day 84, primary); trough FEV1 (day 85, secondary); other (selected) endpoints are listed in the Results.
· Symptomatic endpoints and health outcomes: rescue medication use; dyspnoea (Transition Dyspnoea Index [TDI] focal score); St George’s Respiratory Questionnaire for COPD patients (SGRQ-C); EuroQoL-5D (EQ-5D) questionnaire and the COPD Assessment Test (CAT).
· Safety assessments included adverse events (AEs) and COPD exacerbations.
· Primary endpoint was analysed using analysis of covariance (covariates: baseline [BL] FEV1, smoking status, treatment). Secondary endpoint was analysed using a mixed model for repeated measures analysis (covariates: BL FEV1, smoking status, day, treatment, day by BL interaction, day by treatment). Multiplicity across primary and secondary endpoints was accounted for by a step-down statistical hierarchy.
Results
Baseline characteristics
· Of 1009 patients enrolled, 870 were screened, 717 were randomised, 716 were included in the ITT population and 674 completed the study (UMEC/VI: 334; FSC: 340).
· All patients were of White race. At BL, patient demographics and characteristics were similar between groups (Table 1) and 55% and 45% of patients overall had percent-predicted FEV1 >50% (moderate COPD) and <50% (severe COPD), respectively.
· Mean (SD) treatment compliance was 100.4% (28.3%) UMEC/VI and 99.3% (3.7%) FSC.
Table 1. Baseline and disease characteristics (ITT population)
|
| UMEC/VI |
| FSC |
|
Age, mean ± SD, years |
| 61.8 ± 7.94 |
| 61.4 ± 8.06 |
|
Sex: male, n (%) |
| 261 (73) |
| 254 (71) |
|
Current smoker, n (%) |
| 204 (57) |
| 217 (61) |
|
Screening lung function: pre-salbutamol FEV1, mean ± SD, L |
| 1.423 ± 0.4573 |
| 1.457 ± 0.4555 |
|
GOLD stage (percent predicted FEV1) and reversibility, n (%) |
|
|
|
|
|
Stage II (³50% to <80%) = moderate |
| 193 (54) |
| 201 (56) |
|
Stage III (³30% to <50%) = severe |
| 165 (46) |
| 157 (44) |
|
Reversible to salbutamol |
| 100 (28) |
| 108 (30) |
|
mMRC dyspnoea scale, mean (SD) |
| 2.2 (0.41) |
| 2.2 (0.42) |
|
Efficacy – lung function
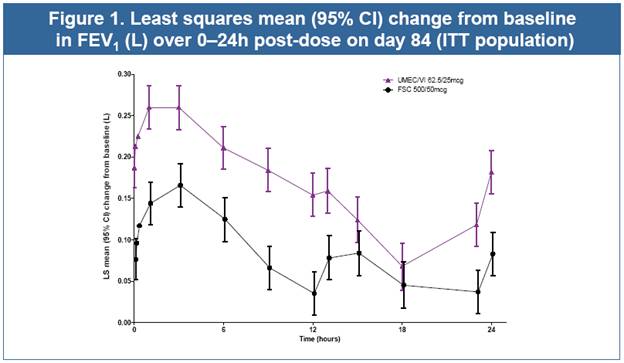
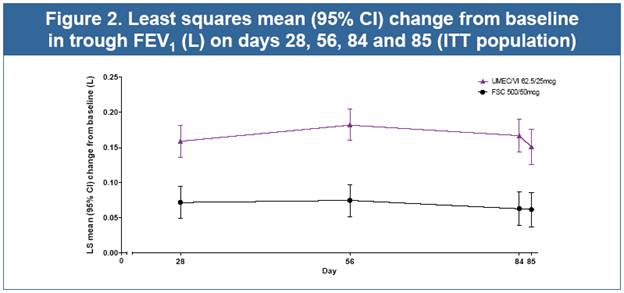
· UMEC/VI resulted in statistically significant improvements in 0–24h wmFEV1 and trough FEV1 (both p<0.001, Table 2, Figs. 1 and 2), and all other lung function measures (Table 2) compared with FSC
· UMEC/VI also significantly improved forced vital capacity endpoints versus FSC (data not shown).
· In a descriptive summary, UMEC/VI improved lung function versus FSC for each GOLD stage for the primary endpoint (see below) and trough FEV1 (day 85; data not shown). The mean (SD) change from BL in 0–24h wmFEV1 (day 84) was
· GOLD stage II: 0.181 (0.2476) L (UMEC/VI) and 0.096 (0.2230) L (FSC)
· GOLD stage III: 0.152 (0.2111) L (UMEC/VI) and 0.071 (0.2038) L (FSC).
Symptomatic endpoints and health outcomes
· No treatment differences in rescue salbutamol use were seen either in terms of mean number of puffs taken daily or in percentage of rescue-free days over 12 weeks.
· Both UMEC/VI and FSC demonstrated clinically meaningful improvements in dyspnoea (TDI focal score >1 unit increase) and health outcomes (SGRQ-C Total score >4 unit decrease from baseline) over 12 weeks
· Least squares (LS) mean (SE) changes from BL in TDI focal score (day 84) were 2.0 (0.14) for UMEC/VI and 2.1 (0.13) for FSC; difference (95% CI): –0.1 (–0.4, 0.3), p=0.702
· LS mean (SE) changes from BL in SGRQ-C Total score (day 84) were –5.10 (0.626) for UMEC/VI and –5.64 (0.619) for FSC; difference (95% CI): 0.53 (–1.20, 2.26), p=0.545.
· Mean change from BL in EQ-5D utility score was 0.03 on day 84 for both treatments.
· On day 84, no treatment differences were seen in mean change from BL CAT scores.
Table 2. Analyses of lung function endpoints (ITT population)
Endpoint |
| UMEC/VI |
| FSC |
| Difference |
0–24h wmFEV1 on day 84, L(a) |
| 0.166 |
| 0.087 |
| 0.080 |
Trough FEV1 on day 85, L(a) |
| 0.151 |
| 0.062 |
| 0.090 |
Peak FEV1 0–6h, L, on day 1(a) |
| 0.266 |
| 0.231 |
| 0.034 |
Peak FEV1 0–6h, L, on day 84(a) |
| 0.327 |
| 0.229 |
| 0.097 |
Median time to onset on day 1, min (FEV1 increase ³100mL vs BL) |
| 17 |
| 60 |
| 1.3 (1.1, 1.5)(i) |
Patients with FEV1 increase ³100mL vs BL at 5-min post-dose on day 1, n (%) |
| 137 |
| 104 |
| 1.50 |
Patients with FEV1 increase ³12% and ³200mL on day 1 vs BL, n (%) |
| 211 |
| 177 |
| 1.47 |
Patients with trough FEV1 increase ³100mL on day 85 vs BL, n (%) |
| 192 |
| 139 |
| 1.94 |
(a)values are LS mean (SE) change from BL; (b)n=332; (c)n=337; (d)treatment difference (95% CI); (e)n=333; (f)n=338; (g)n=335; (h)n=340; (i)hazard ratio (95% CI); (j)odds ratio (95% CI); BL: baseline
Safety and tolerability
· The AE incidence was similar between UMEC/VI (28%) and FSC (29%). The incidence of fatal on-treatment AEs was 1% (UMEC/VI) and 0% (FSC).
· Headache, nasopharyngitis, back pain and dysphonia were the most common AEs reported in both groups. The incidence of drug-related AEs was lower with UMEC/VI (2%) than with FSC (4%).
· The incidence of cardiac disorders was low (2% UMEC/VI; <1% FSC). Pneumonia was reported by 1 patient in the FSC group and none in the UMEC/VI group. The incidence of on-treatment COPD exacerbations was higher with UMEC/VI (2%) than with FSC (<1%).
Conclusions
· Once-daily UMEC/VI 62.5/25mcg over 12 weeks resulted in significant, sustained and clinically meaningful improvements in lung function versus twice-daily FSC 500/50mcg in moderate-to-severe COPD patients with infrequent COPD exacerbations.
· Both UMEC/VI and FSC demonstrated clinically meaningful improvements in dyspnoea and QoL over 12 weeks, with no treatment differences.
· The safety and tolerability of UMEC/VI was consistent with previous studies and no difference was observed in AEs between UMEC/VI and FSC.
· For GOLD B and D patients with dyspnoea and infrequent exacerbations, our findings suggest that the LAMA/LABA combination UMEC/VI could provide greater lung function benefits than the ICS/LABA combination FSC.
References
(1) Celli B, et al. Chest 2014;145:981–91.
(2) ANORO US prescribing information, May 2014. Available at https://www.gsksource.com/gskprm/htdocs/documents/ANORO-ELLIPTA-PI-MG.PDF. Date last accessed: 21 August 2014.
(3) ANORO EU summary of product characteristics, 16 May 2014. Available at http://www.medicines.ie/medicine/16007/SPC/ANORO+55+micrograms+22+micrograms+inhalation+powder,+pre-dispensed/. Date last accessed: 21 August 2014.
(4) Calverley PM, et al. N Engl J Med 2007;356:775–89.
(5) GOLD 2014 guidelines for COPD. http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Date last accessed: 21 August 2014.
Acknowledgements
· The presenting author, D Singh, declares the following real or perceived conflicts of interest during the last 3 years in relation to this presentation: has served as consultant to Almirall, AstraZeneca, Boehringer Ingleheim, Chiesi, Cipla, GSK, Novartis, Nycomed and Roche; has received research grants from AstraZeneca, Almirall, Boehringer Ingleheim, Chiesi, Cipla, GSK, Novartis, Nycomed and Roche; has received payment for lectures including service on speakers bureaus from AstraZeneca, Almirall, Boehringer Ingleheim, Chiesi, Cipla, GSK, Novartis, Nycomed, Roche and Takeda. S Worsley, C-Q Zhu, L Hardaker and A Church are employed by and hold stock in GSK.
· This study was funded by GSK (GSK study code: DB2116134; clinicaltrials.gov: NCT01822899).
· Editorial support (in the form of writing assistance, assembling tables and figures, collating author comments, grammatical editing and referencing) was provided by Jackie Phillipson, PhD at Gardiner-Caldwell Communications (Macclesfield, UK) and was funded by GSK.
ELLIPTA® and DISKUS® are trade marks of the GlaxoSmithKline group of companies

Presented at the European Respiratory Society (ERS) International Congress, Munich, Germany, 6–10 September 2014