
Building a vibrant enterprise to deliver transformational medicines January 2020 • Nasdaq: ARNA Exhibit 99.2

Forward Looking Statements This presentation includes forward-looking statements that involve a number of risks and uncertainties, including statements about the Arena investment thesis, catalysts, value, our investigative stage drug candidates, including with respect to their potential (including to become first or best-in-class), safety, efficacy, indications, significance of data, development plans, differentiation, the market and unmet needs, commercialization, expected data readouts, initiation and progress of clinical trials and regulatory approval; our focus, goals, strategy, plans, timelines and guidance; our partnered programs; financial and other guidance; and other statements that are not historical facts, including statements that may include words such as “can,” “may,” “will,” “intend,” “plan,” “expect,” “believe,” “potential,” “opportunity,” “up to” or other similar words. For such statements, we claim the protection of the Private Securities Litigation Reform Act of 1995. Actual events or results may differ materially from expectations, and you are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the time they were made. Factors that could cause actual results to differ materially from such statements include, without limitation: Drug development programs are expensive, time consuming, uncertain and susceptible to change, interruption, delay or termination; the timing and outcome of research, development and regulatory review and feedback is uncertain; we expect to need additional funds to advance all of our programs, and you and others may not agree with the manner in which we allocate our resources; topline data may not accurately reflect the complete results of a particular study or trial; results of clinical trials and other studies are subject to different interpretations and may not be predictive of future results; new data may be unexpected or unfavorable; our drug candidates may not advance in development or be approved for marketing; clinical trials and other studies may not proceed at the time or in the manner expected or at all; enrolling patients in our ongoing and intended clinical trials is competitive and challenging; clinical and nonclinical data is voluminous and detailed, and regulatory agencies may interpret or weigh the importance of data differently and reach different conclusions than Arena or others, request additional information, have additional recommendations or change their guidance or requirements; data and information related to our programs may not meet regulatory requirements or otherwise be sufficient for further development, regulatory review, partnering or approval at all or on our projected timeline; other risks related to developing, seeking regulatory approval of and commercializing drugs, including regulatory, manufacturing, supply and marketing issues and drug availability; Arena's and third parties' intellectual property rights; competition; reimbursement and pricing decisions; risks related to relying on partners and other third parties; and satisfactory resolution of litigation or other disagreements. Additional factors that could cause actual results to differ materially from those stated or implied by our forward-looking statements are disclosed in our Securities and Exchange Commission (SEC) filings, including under the heading “Risk Factors.” We disclaim any intent or obligation to update these forward-looking statements, other than as may be required under applicable law. January 2020 •
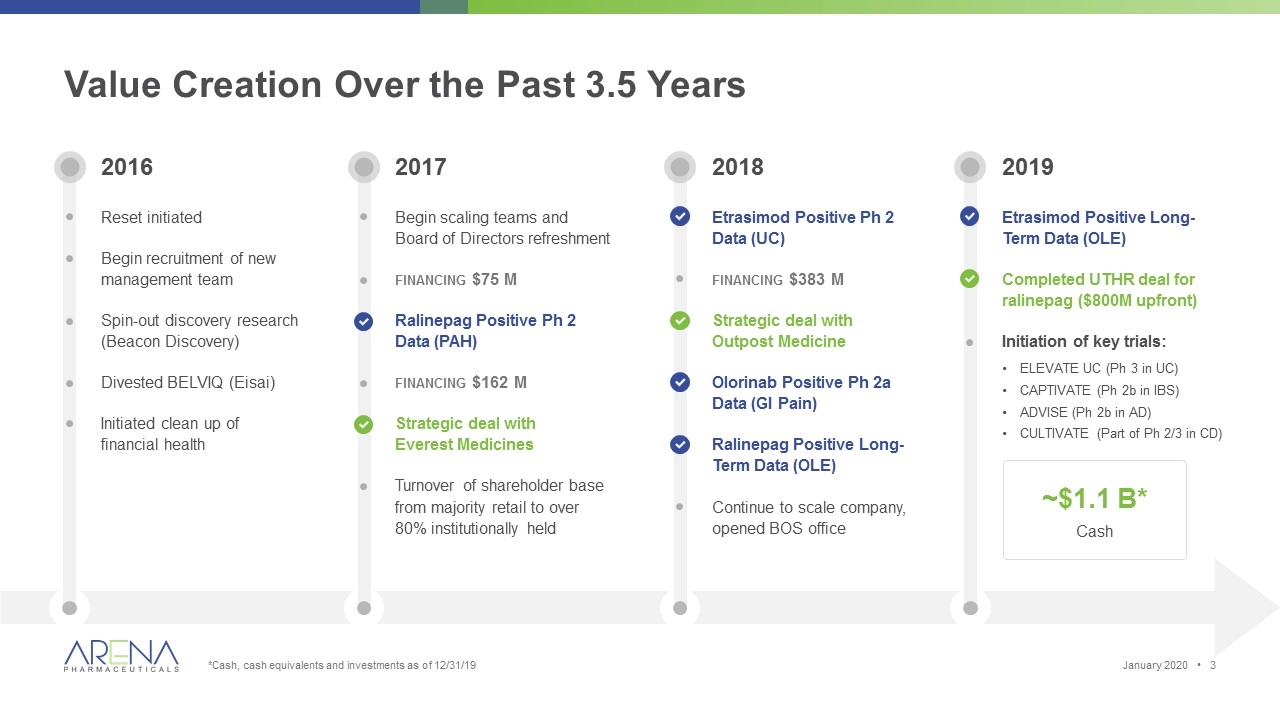
Value Creation Over the Past 3.5 Years *Cash, cash equivalents and investments as of 12/31/19 2016 Reset initiated Begin recruitment of new management team Spin-out discovery research (Beacon Discovery) Divested BELVIQ (Eisai) Initiated clean up of financial health ~$1.1 B* Cash 2018 Etrasimod Positive Ph 2 Data (UC) financing $383 M Strategic deal with Outpost Medicine Olorinab Positive Ph 2a Data (GI Pain) Ralinepag Positive Long-Term Data (OLE) Continue to scale company, opened BOS office 2019 Etrasimod Positive Long-Term Data (OLE) Completed UTHR deal for ralinepag ($800M upfront) Initiation of key trials: ELEVATE UC (Ph 3 in UC) CAPTIVATE (Ph 2b in IBS) ADVISE (Ph 2b in AD) CULTIVATE (Part of Ph 2/3 in CD) 2017 Begin scaling teams and Board of Directors refreshment financing $75 M Ralinepag Positive Ph 2 Data (PAH) financing $162 M Strategic deal with Everest Medicines Turnover of shareholder base from majority retail to over 80% institutionally held January 2020 •
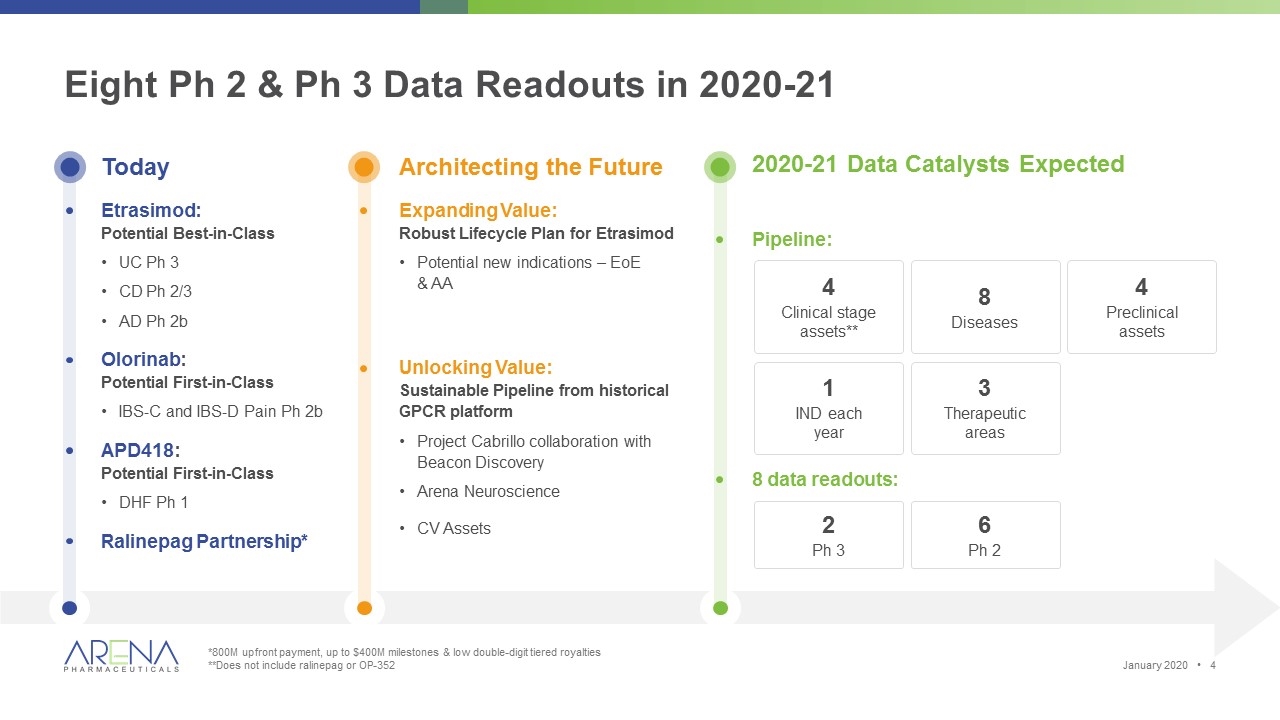
2020-21 Data Catalysts Expected Pipeline: 4 Clinical stage assets** 8 Diseases 2 Ph 3 6 Ph 2 Today Etrasimod: Potential Best-in-Class UC Ph 3 CD Ph 2/3 AD Ph 2b Olorinab: Potential First-in-Class IBS-C and IBS-D Pain Ph 2b APD418: Potential First-in-Class DHF Ph 1 Ralinepag Partnership* Architecting the Future Expanding Value: Robust Lifecycle Plan for Etrasimod Potential new indications – EoE & AA Unlocking Value: Sustainable Pipeline from historical GPCR platform Project Cabrillo collaboration with Beacon Discovery Arena Neuroscience CV Assets Eight Ph 2 & Ph 3 Data Readouts in 2020-21 1 IND each year 8 data readouts: 4 Preclinical assets 3 Therapeutic areas *800M upfront payment, up to $400M milestones & low double-digit tiered royalties **Does not include ralinepag or OP-352 January 2020 •
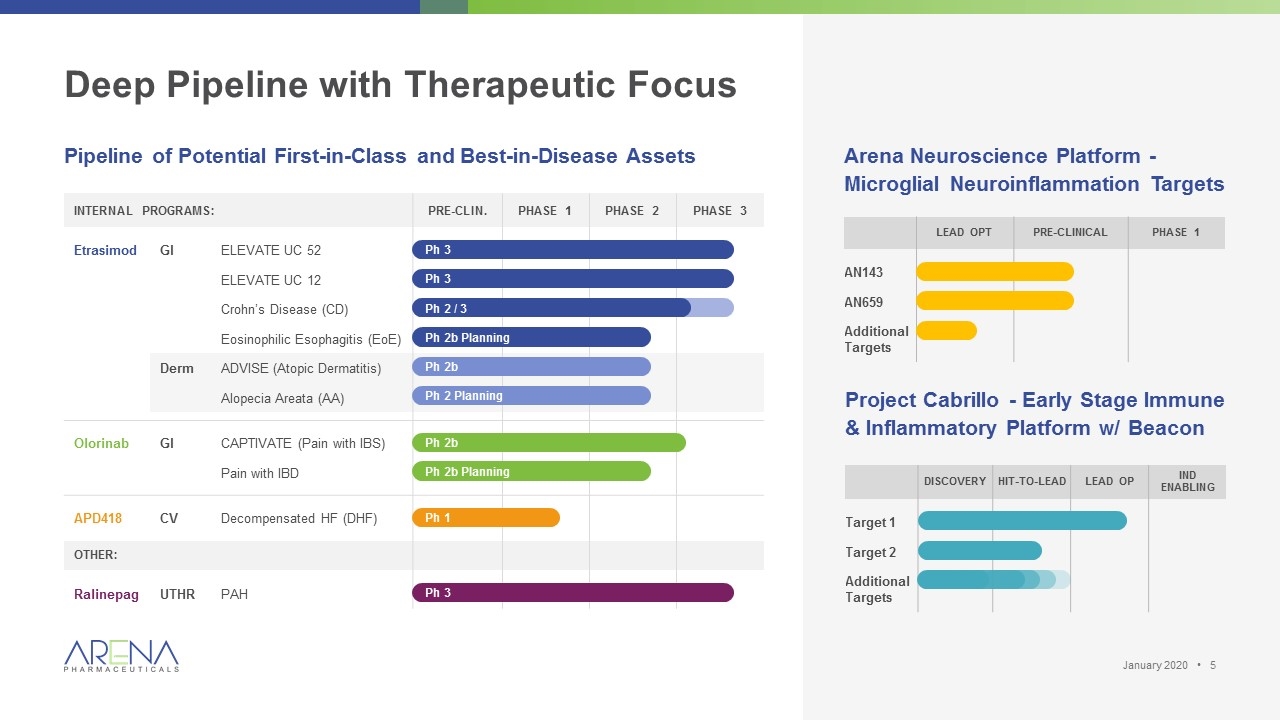
Deep Pipeline with Therapeutic Focus Internal Programs: Pre-clin. Phase 1 Phase 2 Phase 3 Etrasimod GI ELEVATE UC 52 ELEVATE UC 12 Crohn’s Disease (CD) Eosinophilic Esophagitis (EoE) Derm ADVISE (Atopic Dermatitis) Alopecia Areata (AA) Olorinab GI CAPTIVATE (Pain with IBS) Pain with IBD APD418 CV Decompensated HF (DHF) Other: Ralinepag UTHR PAH Project Cabrillo - Early Stage Immune & Inflammatory Platform w/ Beacon Discovery Hit-to-lead Lead Op IND Enabling Target 1 Target 2 Additional Targets Arena Neuroscience Platform - Microglial Neuroinflammation Targets Lead Opt Pre-Clinical Phase 1 AN143 AN659 Additional Targets Pipeline of Potential First-in-Class and Best-in-Disease Assets Ph 3 Ph 3 Ph 2b Planning Ph 2b Ph 2 Planning Ph 2b Ph 2b Planning Ph 1 Ph 2 / 3 Ph 3 January 2020 •
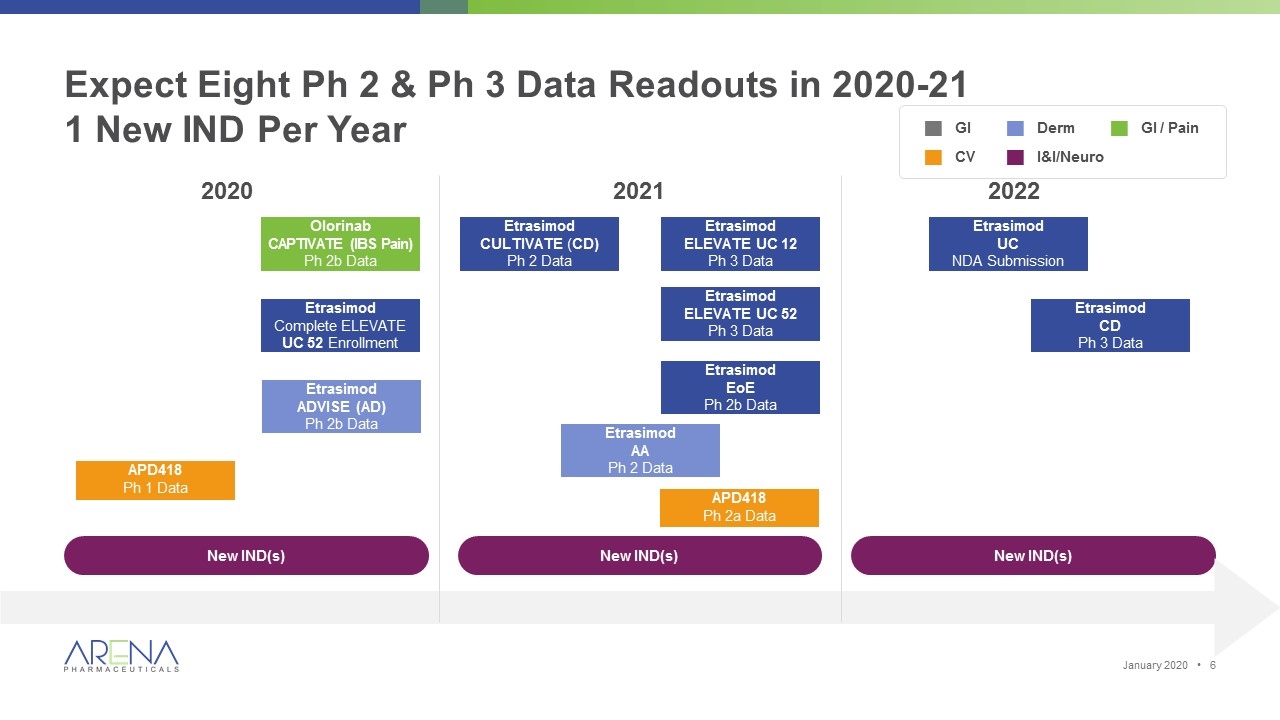
Expect Eight Ph 2 & Ph 3 Data Readouts in 2020-21 1 New IND Per Year Etrasimod UC NDA Submission Olorinab CAPTIVATE (IBS Pain) Ph 2b Data Etrasimod ADVISE (AD) Ph 2b Data Etrasimod CD Ph 3 Data Etrasimod Complete ELEVATE UC 52 Enrollment APD418 Ph 1 Data 2020 2021 2022 New IND(s) New IND(s) New IND(s) Etrasimod ELEVATE UC 12 Ph 3 Data Etrasimod AA Ph 2 Data Etrasimod CULTIVATE (CD) Ph 2 Data Etrasimod ELEVATE UC 52 Ph 3 Data Etrasimod EoE Ph 2b Data APD418 Ph 2a Data gGI gCV gDerm gI&I/Neuro gGI / Pain January 2020 •

Arena Neuroscience – Microglial Neuroinflammation Platform Scientific Rationale: Programs: Sentinel microglia encounter aberrant proteins like: mSOD (ALS) α-Synuclein (Parkinson’s Disease) Aβ (Alzheimer's Disease) resulting in activated microglia These proteins lead to exaggerated neuroinflammatory response & potential neurodegeneration Opportunity for therapeutic intervention along neuroinflammation axis Lead Opt Pre-Clinical Phase 1 Microglial Targets: AN143 AN659 Additional Targets Disease Stimulus mSOD (ALS) α-Synuclein (PD) Aβ, tau (AD) Neuroinflammation Sentinel Microglia Activated Microglia Strategic positioning through early stage development Evaluate potential spin-out and external investment options i January 2020 •
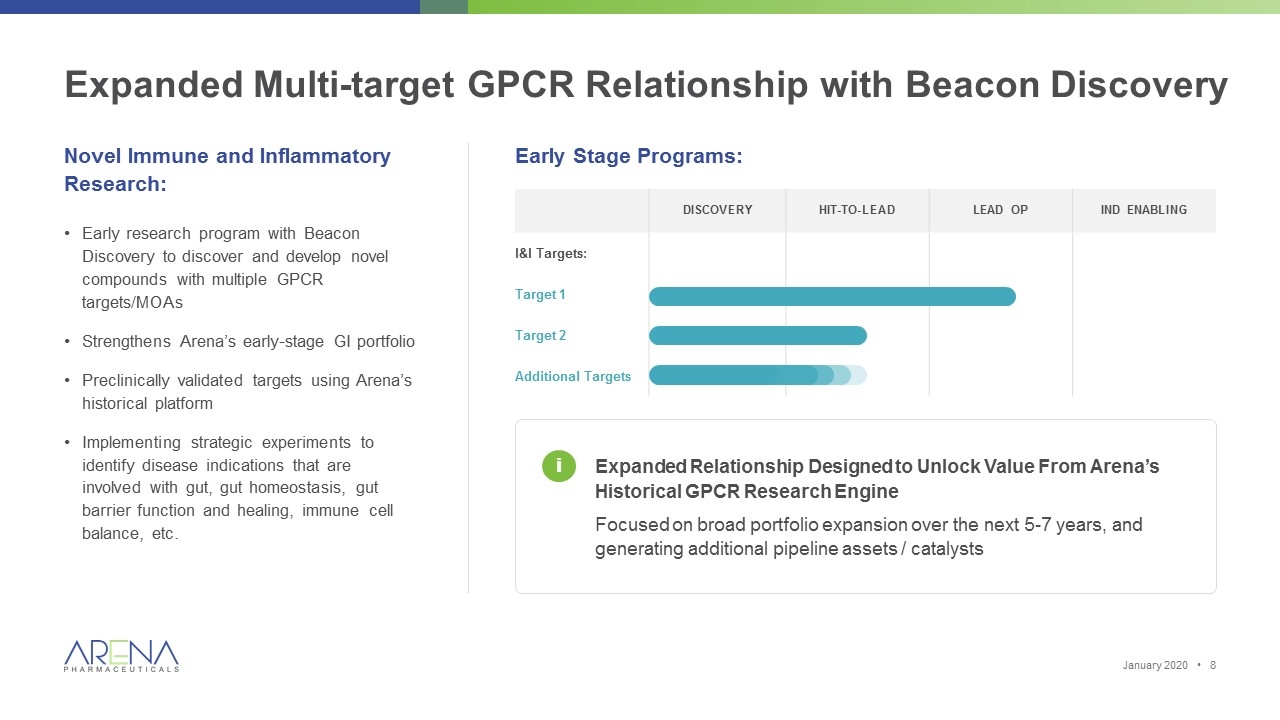
Expanded Multi-target GPCR Relationship with Beacon Discovery Novel Immune and Inflammatory Research: Early research program with Beacon Discovery to discover and develop novel compounds with multiple GPCR targets/MOAs Strengthens Arena’s early-stage GI portfolio Preclinically validated targets using Arena’s historical platform Implementing strategic experiments to identify disease indications that are involved with gut, gut homeostasis, gut barrier function and healing, immune cell balance, etc. Early Stage Programs: Expanded Relationship Designed to Unlock Value From Arena’s Historical GPCR Research Engine Focused on broad portfolio expansion over the next 5-7 years, and generating additional pipeline assets / catalysts Discovery Hit-to-lead Lead Op IND Enabling I&I Targets: Target 1 Target 2 Additional Targets i January 2020 •
Etrasimod Investigational, Oral, Next Generation, S1P Receptor Modulator with Potential for Optimized Activity Being Evaluated for Multiple Immune-Mediated Inflammatory Diseases (IMID), Targeting ~$48B Market with Current Indications January 2020 •

Etrasimod Potential Best-in-Class S1P Receptor Modulator with Broad Applicability Source: 1. Arena Quantitative Market Research, March 2018.; 2. Dahlhamer JM, et al. MMWR Morb Mortal Wkly Rep 2016;65:1166–1169.; 3. UC & CD Disease Landscape & Forecast Reports. DRG. 2018 January 2020 • Receptor Pharmacology Highly specific S1P1,4,5 No evidence of off-target activity / deleterious barrier function due to S1P2,3 ~$48B Market Opportunity Across Current Pipeline Pharmacodynamics Rapid On-Rate (treating flare) Rapid Off-Rate (infection control) Control Strong durable remission rates and generally well tolerated safety with no titration No elevated LFTs, abnormal PFTs, macular edema Convenience Oral, once-daily Minimal monitoring expected Etrasimod Intrinsic Features
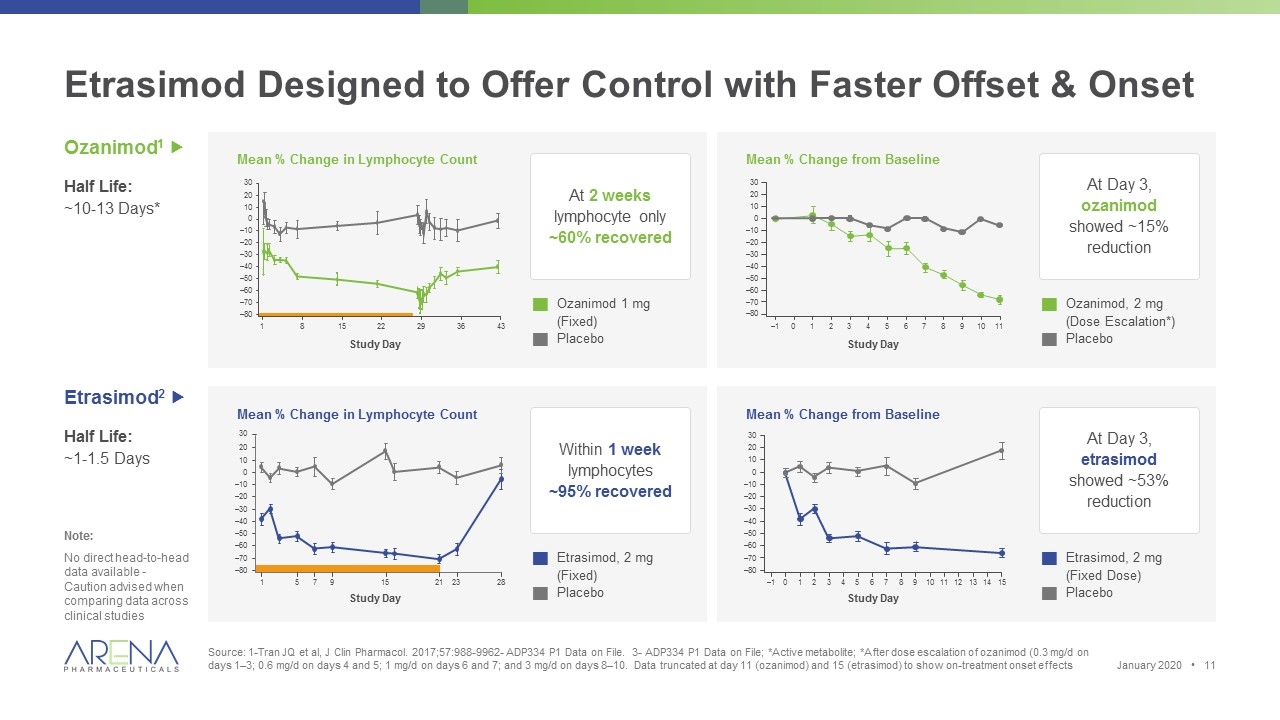
Etrasimod Designed to Offer Control with Faster Offset & Onset Source: 1-Tran JQ et al, J Clin Pharmacol. 2017;57:988-9962- ADP334 P1 Data on File. 3- ADP334 P1 Data on File; *Active metabolite; *After dose escalation of ozanimod (0.3 mg/d on days 1–3; 0.6 mg/d on days 4 and 5; 1 mg/d on days 6 and 7; and 3 mg/d on days 8–10. Data truncated at day 11 (ozanimod) and 15 (etrasimod) to show on-treatment onset effects Ozanimod1 „ Half Life: ~10-13 Days* Etrasimod2 „ Half Life: ~1-1.5 Days At 2 weeks lymphocyte only ~60% recovered Within 1 week lymphocytes ~95% recovered Study Day 30 20 10 0 –10 –20 –30 –40 –50 –60 –70 –80 1 5 7 9 15 21 23 28 Mean % Change in Lymphocyte Count Mean % Change in Lymphocyte Count gEtrasimod, 2 mg (Fixed) gPlacebo g Ozanimod 1 mg (Fixed) g Placebo Study Day At Day 3, ozanimod showed ~15% reduction At Day 3, etrasimod showed ~53% reduction Mean % Change from Baseline Mean % Change from Baseline gEtrasimod, 2 mg (Fixed Dose) gPlacebo gOzanimod, 2 mg (Dose Escalation*) gPlacebo –1 0 1 2 3 4 5 6 7 8 9 10 11 30 20 10 0 –10 –20 –30 –40 –50 –60 –70 –80 Study Day 30 20 10 0 –10 –20 –30 –40 –50 –60 –70 –80 –1 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Study Day 30 1 8 15 22 29 36 43 20 10 0 –10 –20 –30 –40 –50 –60 –70 –80 Note: No direct head-to-head data available - Caution advised when comparing data across clinical studies January 2020 •

Etrasimod in Gastrointestinal Conditions Ulcerative Colitis (UC), Crohn’s Disease (CD) & Eosinophilic Esophagitis (EoE) January 2020 •
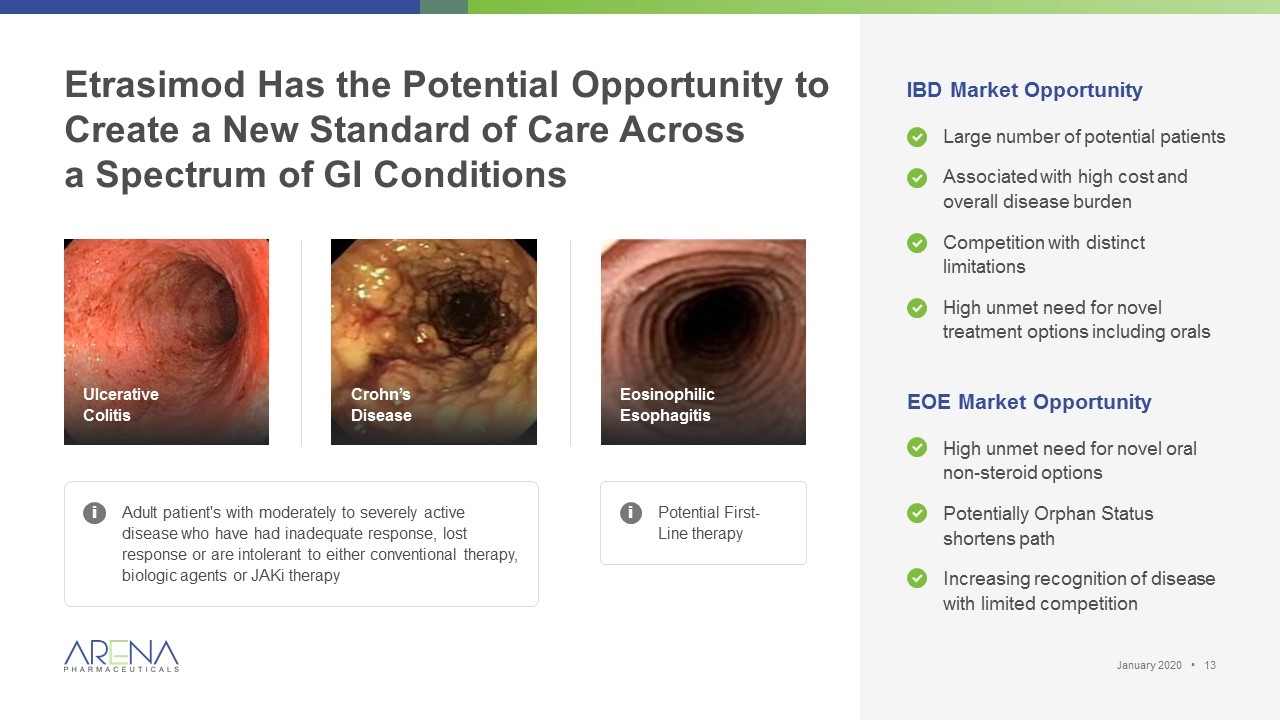
Etrasimod Has the Potential Opportunity to Create a New Standard of Care Across a Spectrum of GI Conditions Adult patient's with moderately to severely active disease who have had inadequate response, lost response or are intolerant to either conventional therapy, biologic agents or JAKi therapy Ulcerative Colitis Eosinophilic Esophagitis IBD Market Opportunity Large number of potential patients Associated with high cost and overall disease burden Competition with distinct limitations High unmet need for novel treatment options including orals EOE Market Opportunity High unmet need for novel oral non-steroid options Potentially Orphan Status shortens path Increasing recognition of disease with limited competition i Potential First-Line therapy i Crohn’s Disease January 2020 •

Etrasimod Potential Best-in-Disease S1P Receptor Modulator with Broad Potential Applicability Source: 1. Arena Quantitative Market Research, March 2018.; 2. Dahlhamer JM, et al. MMWR Morb Mortal Wkly Rep 2016;65:1166–1169.; 3. UC & CD Disease Landscape & Forecast Reports. DRG. 2018 60-80% $24+B Market Opportunity Across GI of IBD patients currently not in remission at 1 Year1,2 Receptor Pharmacology Highly specific S1P1,4,5 No evidence of off-target activity Pharmacodynamics Rapid On-Rate (treating flare) Rapid Off-Rate (infection control) Control Strong durable remission rates and generally well tolerated safety with no titration No elevated LFTs, abnormal PFTs, macular edema Convenience Oral, once-daily Minimal monitoring expected Etrasimod Intrinsic Features January 2020 •
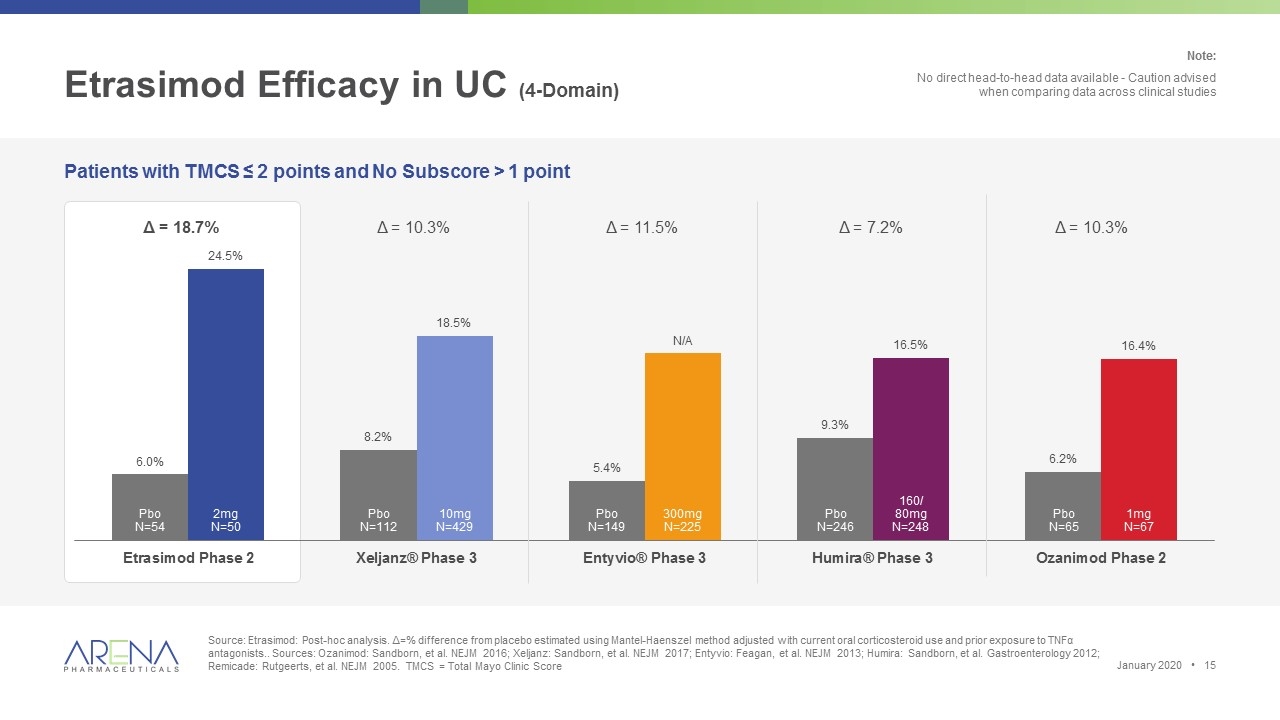
Etrasimod Efficacy in UC (4-Domain) Source: Etrasimod: Post-hoc analysis. Δ=% difference from placebo estimated using Mantel-Haenszel method adjusted with current oral corticosteroid use and prior exposure to TNFα antagonists.. Sources: Ozanimod: Sandborn, et al. NEJM 2016; Xeljanz: Sandborn, et al. NEJM 2017; Entyvio: Feagan, et al. NEJM 2013; Humira: Sandborn, et al. Gastroenterology 2012; Remicade: Rutgeerts, et al. NEJM 2005. TMCS = Total Mayo Clinic Score Patients with TMCS ≤ 2 points and No Subscore > 1 point Δ = 18.7% Δ = 10.3% Δ = 11.5% Δ = 7.2% Δ = 10.3% Pbo N=54 2mg N=50 Pbo N=112 10mg N=429 Pbo N=149 300mg N=225 Pbo N=246 160/ 80mg N=248 Pbo N=65 1mg N=67 Note: No direct head-to-head data available - Caution advised when comparing data across clinical studies January 2020 •
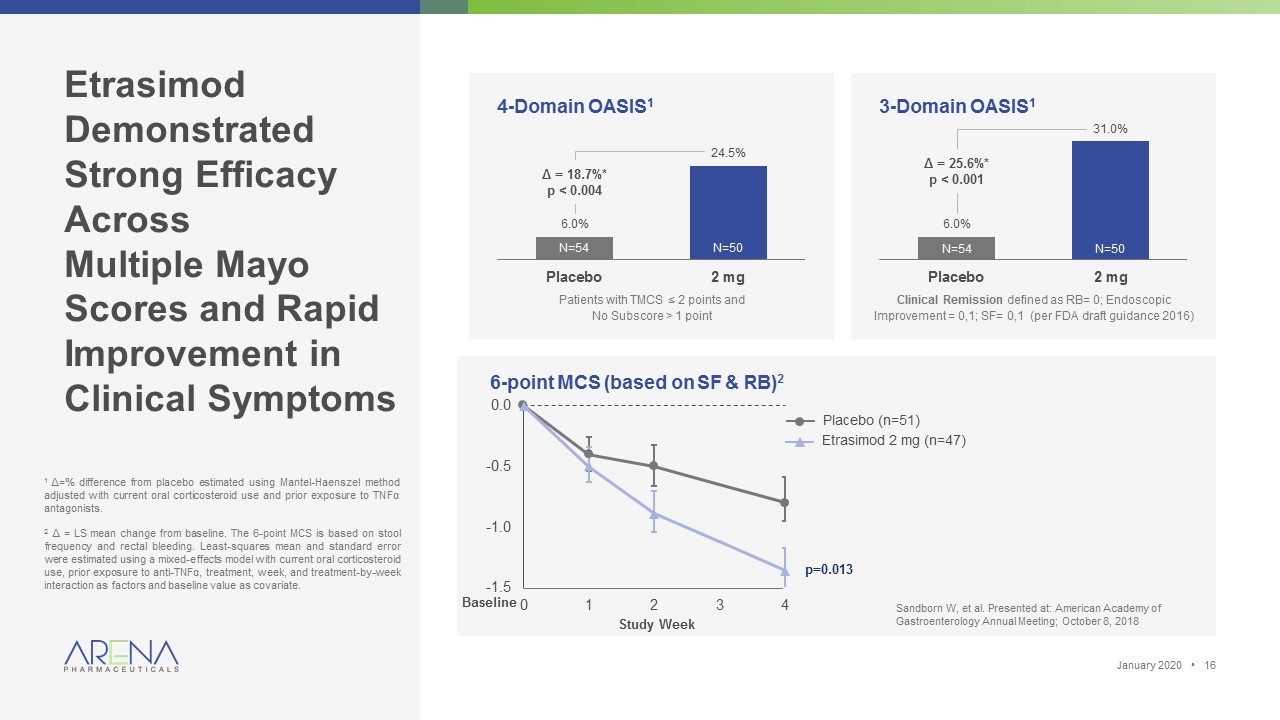
Etrasimod Demonstrated Strong Efficacy Across Multiple Mayo Scores and Rapid Improvement in Clinical Symptoms 4-Domain OASIS1 3-Domain OASIS1 Patients with TMCS ≤ 2 points and No Subscore > 1 point N=54 N=50 Clinical Remission defined as RB= 0; Endoscopic Improvement = 0,1; SF= 0,1 (per FDA draft guidance 2016) N=54 N=50 Δ = 18.7%* p < 0.004 Δ = 25.6%* p < 0.001 January 2020 • 1 Δ=% difference from placebo estimated using Mantel-Haenszel method adjusted with current oral corticosteroid use and prior exposure to TNF�� antagonists. 2 Δ = LS mean change from baseline. The 6-point MCS is based on stool frequency and rectal bleeding. Least-squares mean and standard error were estimated using a mixed-effects model with current oral corticosteroid use, prior exposure to anti-TNFα, treatment, week, and treatment-by-week interaction as factors and baseline value as covariate. 6-point MCS (based on SF & RB)2 Study Week Baseline Placebo (n=51) Etrasimod 2 mg (n=47) p=0.013 Sandborn W, et al. Presented at: American Academy of Gastroenterology Annual Meeting; October 8, 2018
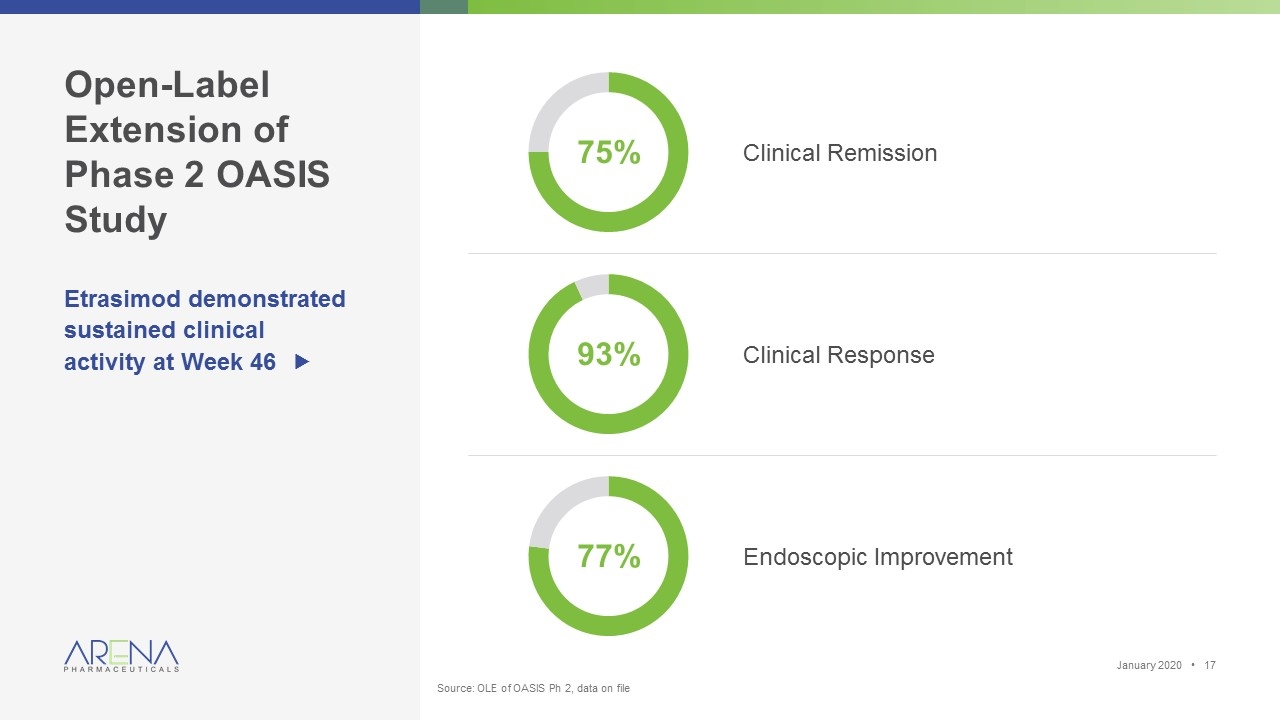
Open-Label Extension of Phase 2 OASIS Study Etrasimod demonstrated sustained clinical activity at Week 46 „ Clinical Remission 77% Clinical Response Endoscopic Improvement 93% 75% January 2020 • Source: OLE of OASIS Ph 2, data on file

Phase 2 Safety Results Adverse events were predominantly mild to moderate No serious adverse events (SAEs) at the 2 mg dose Impact on HR and AV conduction was low throughout the study with no discontinuations related to bradycardia or AV block. No SAEs related to HR changes or AV block No cases of sinoatrial arrest No increases in liver function tests compared to placebo No reports of macular edema No reports of abnormal pulmonary function tests No new safety findings in OLE No JAK inhibitor-like liabilities Etrasimod was generally safe and well tolerated „ January 2020 •
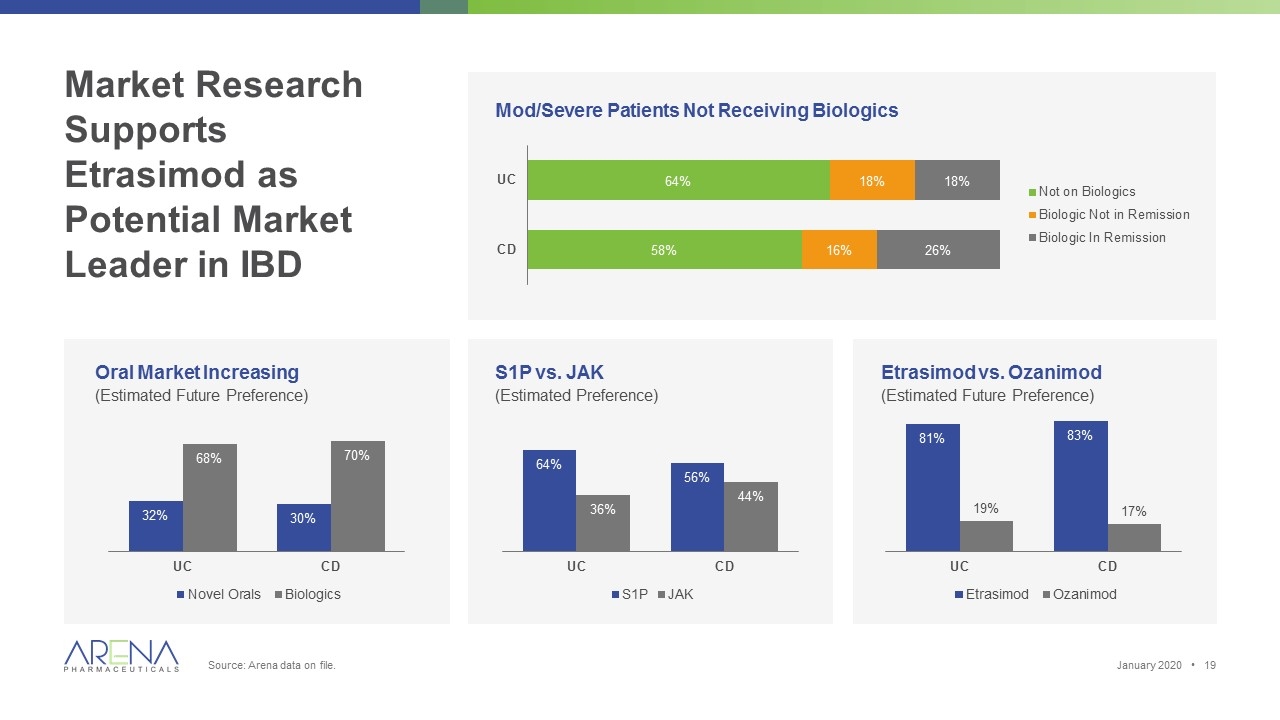
Market Research Supports Etrasimod as Potential Market Leader in IBD Source: Arena data on file. Oral Market Increasing (Estimated Future Preference) S1P vs. JAK (Estimated Preference) Etrasimod vs. Ozanimod (Estimated Future Preference) Mod/Severe Patients Not Receiving Biologics January 2020 •
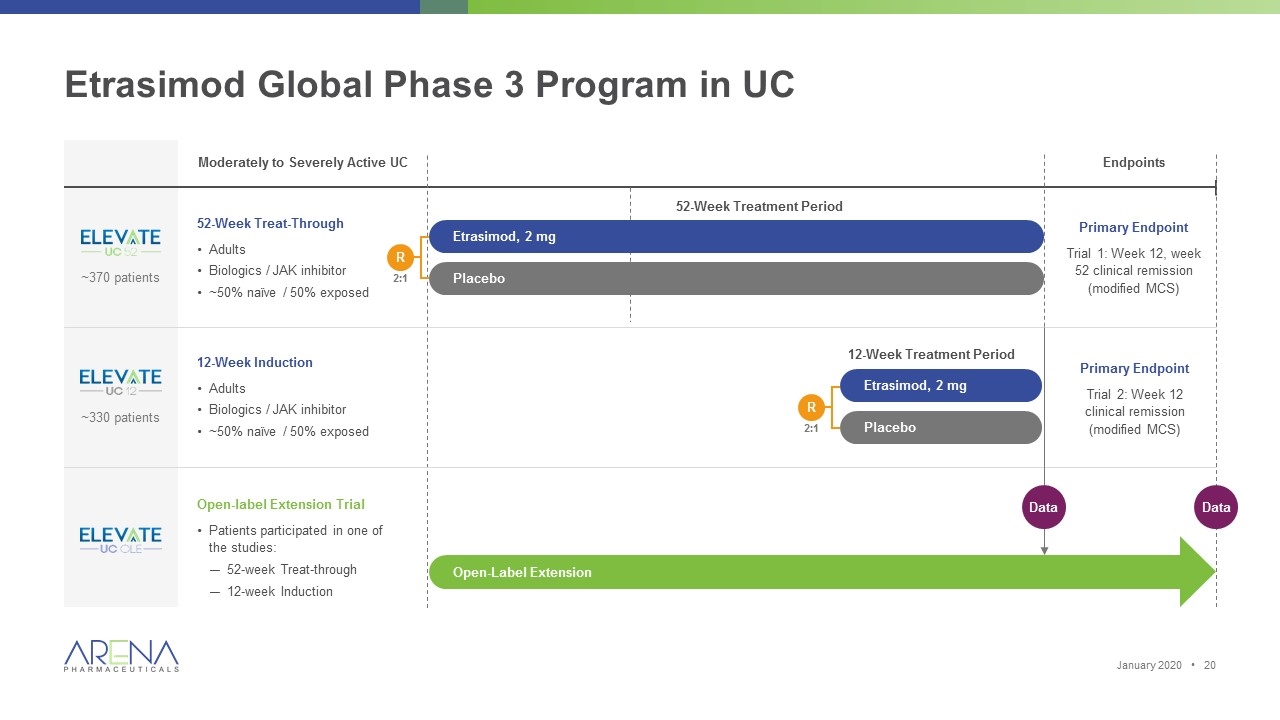
Etrasimod Global Phase 3 Program in UC Moderately to Severely Active UC 52-Week Treat-Through Adults Biologics / JAK inhibitor ~50% naïve / 50% exposed Endpoints Primary Endpoint Trial 1: Week 12, week 52 clinical remission (modified MCS) Primary Endpoint Trial 2: Week 12 clinical remission (modified MCS) ~370 patients ~330 patients 12-Week Induction Adults Biologics / JAK inhibitor ~50% naïve / 50% exposed Open-label Extension Trial Patients participated in one of the studies: 52-week Treat-through 12-week Induction Open-Label Extension Data R 2:1 Etrasimod, 2 mg Placebo Etrasimod, 2 mg Placebo R 2:1 12-Week Treatment Period Data 52-Week Treatment Period January 2020 •

Etrasimod Phase 2/3 Program in CD CULTIVATE Ph 2 dose-ranging study testing safety and efficacy of etrasimod 2 mg and 3 mg compared to placebo in ~225 patients Primary efficacy endpoint will be endoscopic response at Week 14 Evaluating at a variety of scales of Crohn’s disease activity, including abdominal pain and SF Ph 3 will include 2 induction trials with re-randomization of clinical responders into a single maintenance trial Program consists of a Ph 2 dose ranging trial with an operationally seamless transition into the Ph 3 to ensure no stoppage in enrollment activities across global site network i January 2020 •
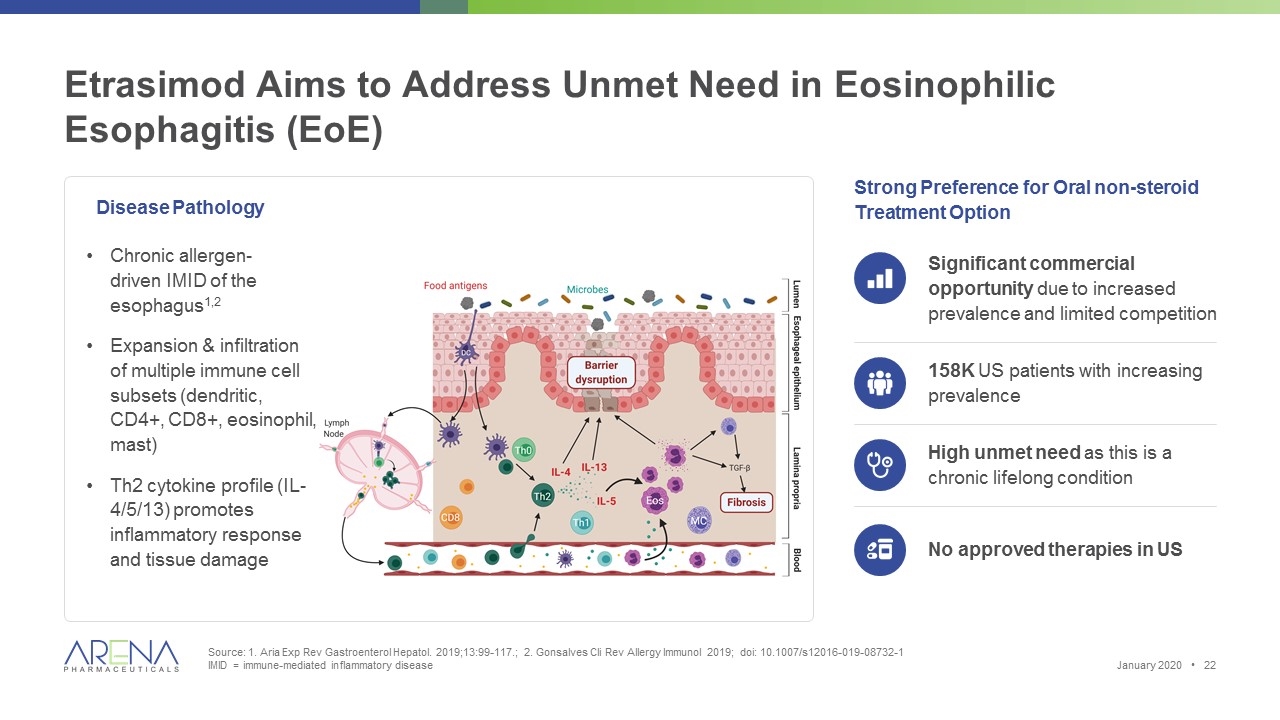
Etrasimod Aims to Address Unmet Need in Eosinophilic Esophagitis (EoE) Source: 1. Aria Exp Rev Gastroenterol Hepatol. 2019;13:99-117.; 2. Gonsalves Cli Rev Allergy Immunol 2019; doi: 10.1007/s12016-019-08732-1 IMID = immune-mediated inflammatory disease Strong Preference for Oral non-steroid Treatment Option Chronic allergen- driven IMID of the esophagus1,2 Expansion & infiltration of multiple immune cell subsets (dendritic, CD4+, CD8+, eosinophil, mast) Th2 cytokine profile (IL-4/5/13) promotes inflammatory response and tissue damage Significant commercial opportunity due to increased prevalence and limited competition 158K US patients with increasing prevalence High unmet need as this is a chronic lifelong condition No approved therapies in US January 2020 • Disease Pathology
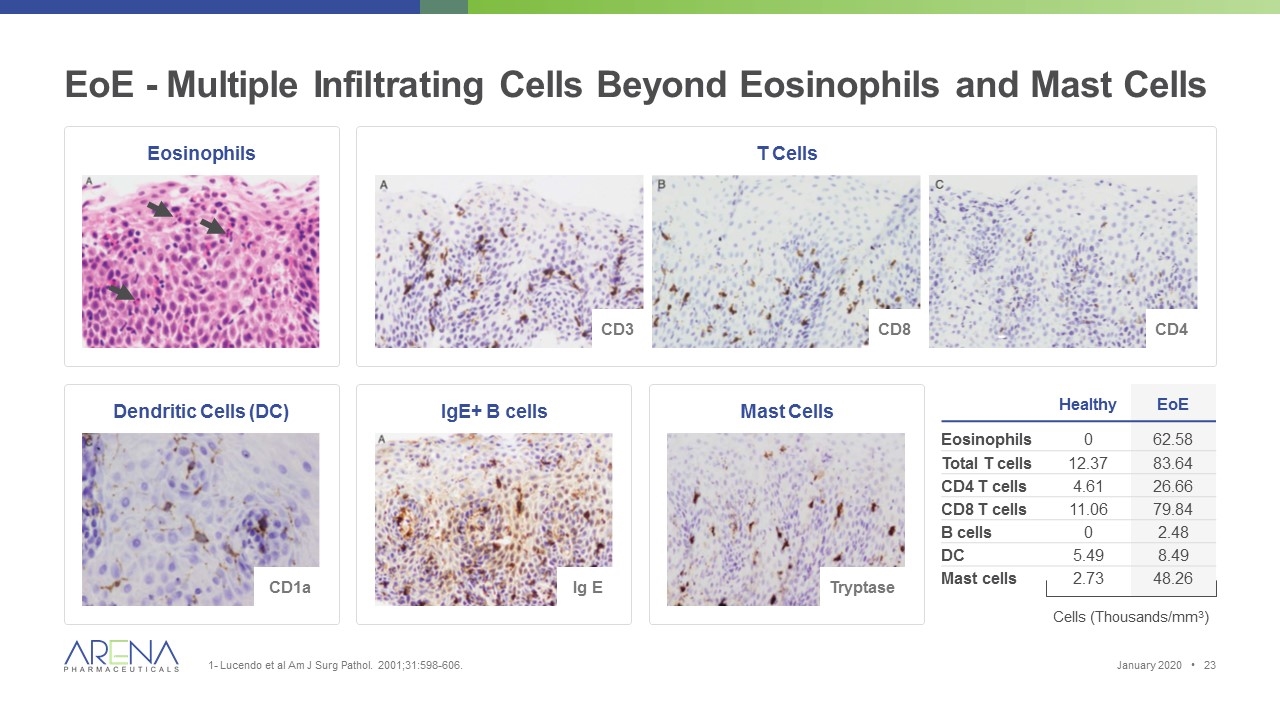
EoE - Multiple Infiltrating Cells Beyond Eosinophils and Mast Cells 1- Lucendo et al Am J Surg Pathol. 2001;31:598-606. Mast Cells Dendritic Cells (DC) IgE+ B cells Healthy EoE Eosinophils 0 62.58 Total T cells 12.37 83.64 CD4 T cells 4.61 26.66 CD8 T cells 11.06 79.84 B cells 0 2.48 DC 5.49 8.49 Mast cells 2.73 48.26 Cells (Thousands/mm3) Ig E Tryptase CD1a Eosinophils T Cells CD3 CD8 CD4 January 2020 •
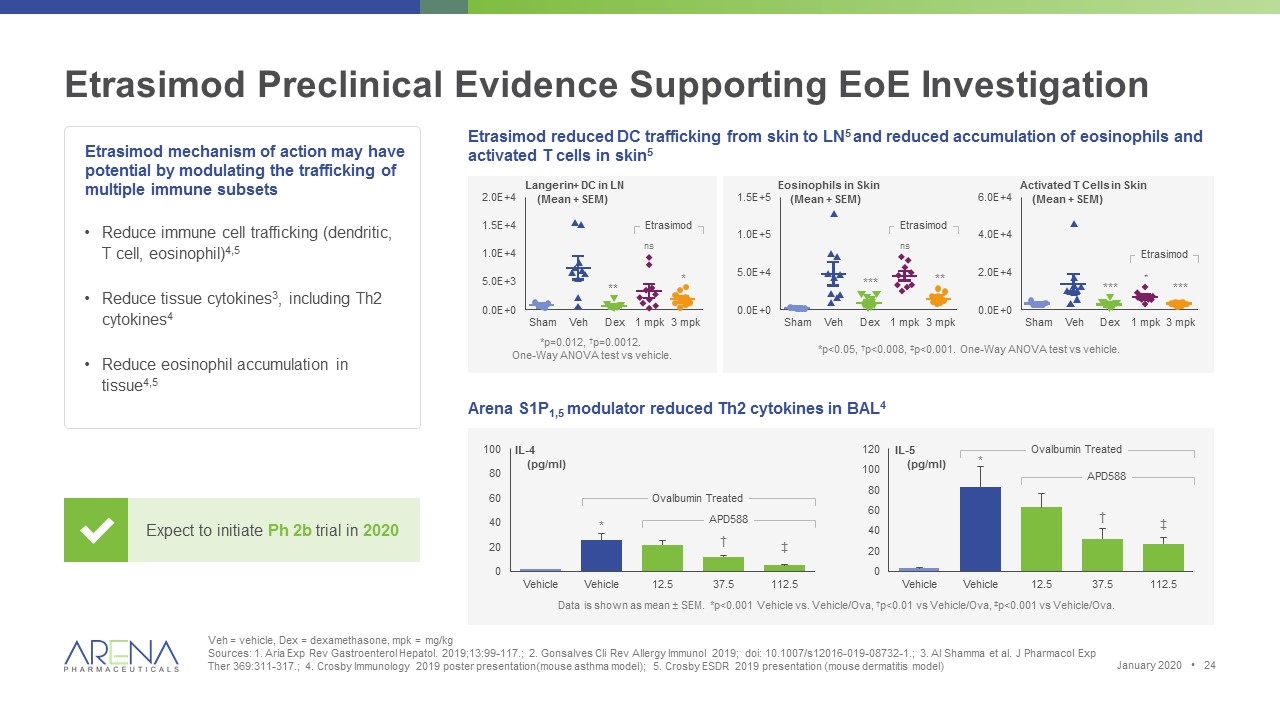
Etrasimod Preclinical Evidence Supporting EoE Investigation Veh = vehicle, Dex = dexamethasone, mpk = mg/kg Sources: 1. Aria Exp Rev Gastroenterol Hepatol. 2019;13:99-117.; 2. Gonsalves Cli Rev Allergy Immunol 2019; doi: 10.1007/s12016-019-08732-1.; 3. Al Shamma et al. J Pharmacol Exp Ther 369:311-317.; 4. Crosby Immunology 2019 poster presentation(mouse asthma model); 5. Crosby ESDR 2019 presentation (mouse dermatitis model) Reduce immune cell trafficking (dendritic, T cell, eosinophil)4,5 Reduce tissue cytokines3, including Th2 cytokines4 Reduce eosinophil accumulation in tissue4,5 Expect to initiate Ph 2b trial in 2020 Etrasimod reduced DC trafficking from skin to LN5 and reduced accumulation of eosinophils and activated T cells in skin5 Etrasimod mechanism of action may have potential by modulating the trafficking of multiple immune subsets Arena S1P1,5 modulator reduced Th2 cytokines in BAL4 Data is shown as mean ± SEM. *p<0.001 Vehicle vs. Vehicle/Ova, †p<0.01 vs Vehicle/Ova, ‡p<0.001 vs Vehicle/Ova. IL-4 (pg/ml) * † ‡ IL-5 (pg/ml) APD588 Ovalbumin Treated APD588 Ovalbumin Treated * † ‡ *p=0.012, †p=0.0012. One-Way ANOVA test vs vehicle. *p<0.05, †p<0.008, ‡p<0.001. One-Way ANOVA test vs vehicle. Langerin+ DC in LN (Mean + SEM) Eosinophils in Skin (Mean + SEM) Activated T Cells in Skin (Mean + SEM) Etrasimod Etrasimod Etrasimod ** ns * *** ns ** *** * *** January 2020 •

Etrasimod in Dermatologic Conditions Atopic Dermatitis (AD) Alopecia Areata (AA) January 2020 •
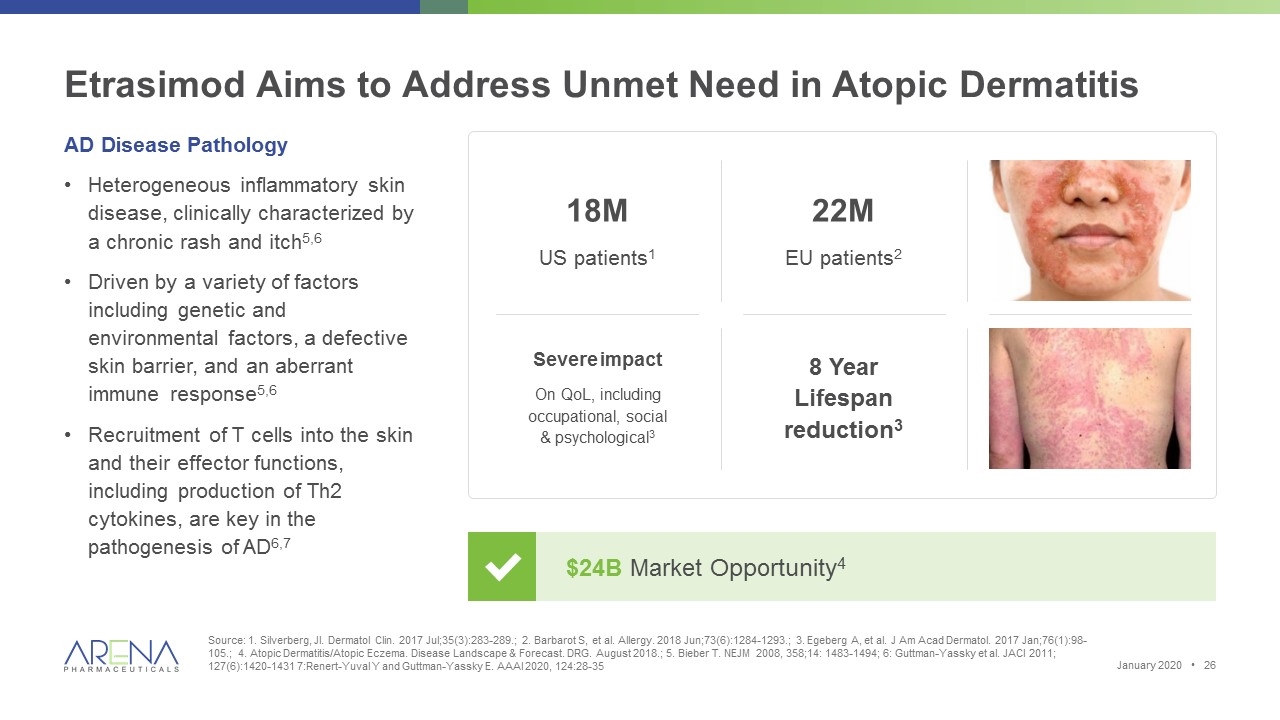
Etrasimod Aims to Address Unmet Need in Atopic Dermatitis Source: 1. Silverberg, JI. Dermatol Clin. 2017 Jul;35(3):283-289.; 2. Barbarot S, et al. Allergy. 2018 Jun;73(6):1284-1293.; 3. Egeberg A, et al. J Am Acad Dermatol. 2017 Jan;76(1):98-105.; 4. Atopic Dermatitis/Atopic Eczema. Disease Landscape & Forecast. DRG. August 2018.; 5. Bieber T. NEJM 2008, 358;14: 1483-1494; 6: Guttman-Yassky et al. JACI 2011; 127(6):1420-1431 7:Renert-Yuval Y and Guttman-Yassky E. AAAI 2020, 124:28-35 $24B Market Opportunity4 AD Disease Pathology Heterogeneous inflammatory skin disease, clinically characterized by a chronic rash and itch5,6 Driven by a variety of factors including genetic and environmental factors, a defective skin barrier, and an aberrant immune response5,6 Recruitment of T cells into the skin and their effector functions, including production of Th2 cytokines, are key in the pathogenesis of AD6,7 Severe impact On QoL, including occupational, social & psychological3 8 Year Lifespan reduction3 18M US patients1 22M EU patients2 January 2020 •
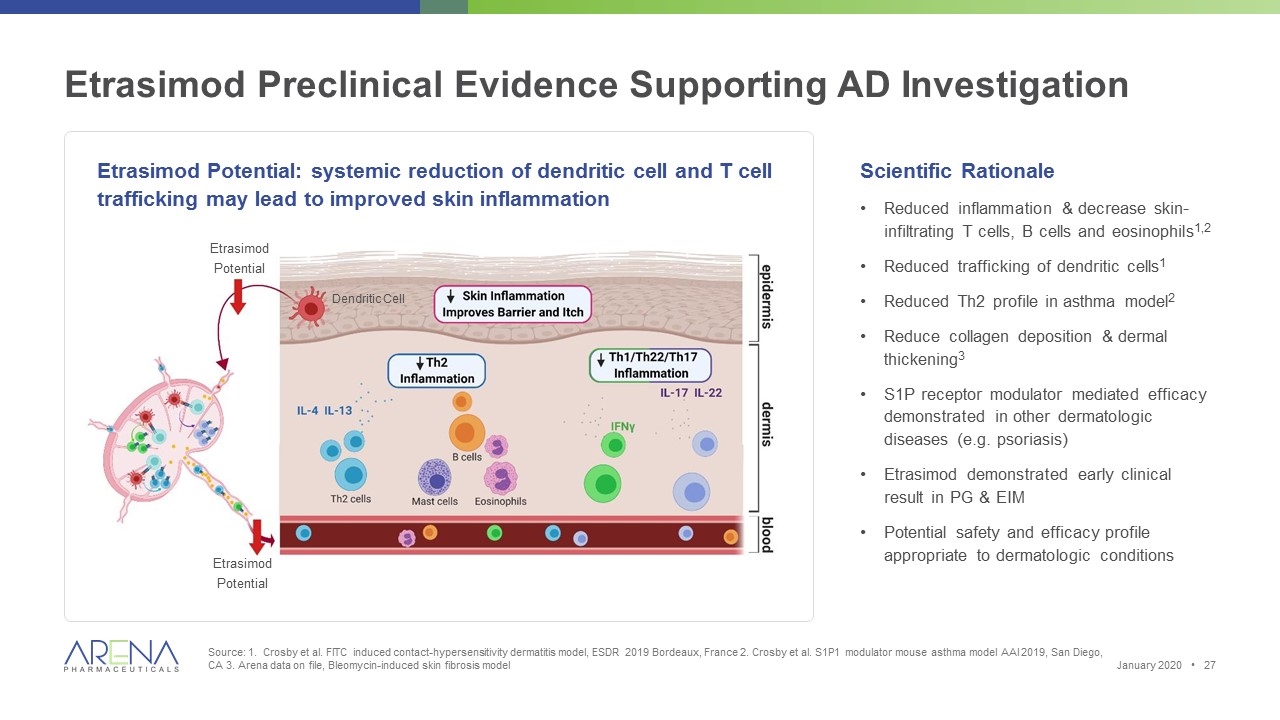
Etrasimod Preclinical Evidence Supporting AD Investigation Source: 1. Crosby et al. FITC induced contact-hypersensitivity dermatitis model, ESDR 2019 Bordeaux, France 2. Crosby et al. S1P1 modulator mouse asthma model AAI 2019, San Diego, CA 3. Arena data on file, Bleomycin-induced skin fibrosis model Etrasimod Potential: systemic reduction of dendritic cell and T cell trafficking may lead to improved skin inflammation Scientific Rationale Reduced inflammation & decrease skin-infiltrating T cells, B cells and eosinophils1,2 Reduced trafficking of dendritic cells1 Reduced Th2 profile in asthma model2 Reduce collagen deposition & dermal thickening3 S1P receptor modulator mediated efficacy demonstrated in other dermatologic diseases (e.g. psoriasis) Etrasimod demonstrated early clinical result in PG & EIM Potential safety and efficacy profile appropriate to dermatologic conditions January 2020 • Etrasimod Potential Etrasimod Potential Dendritic Cell
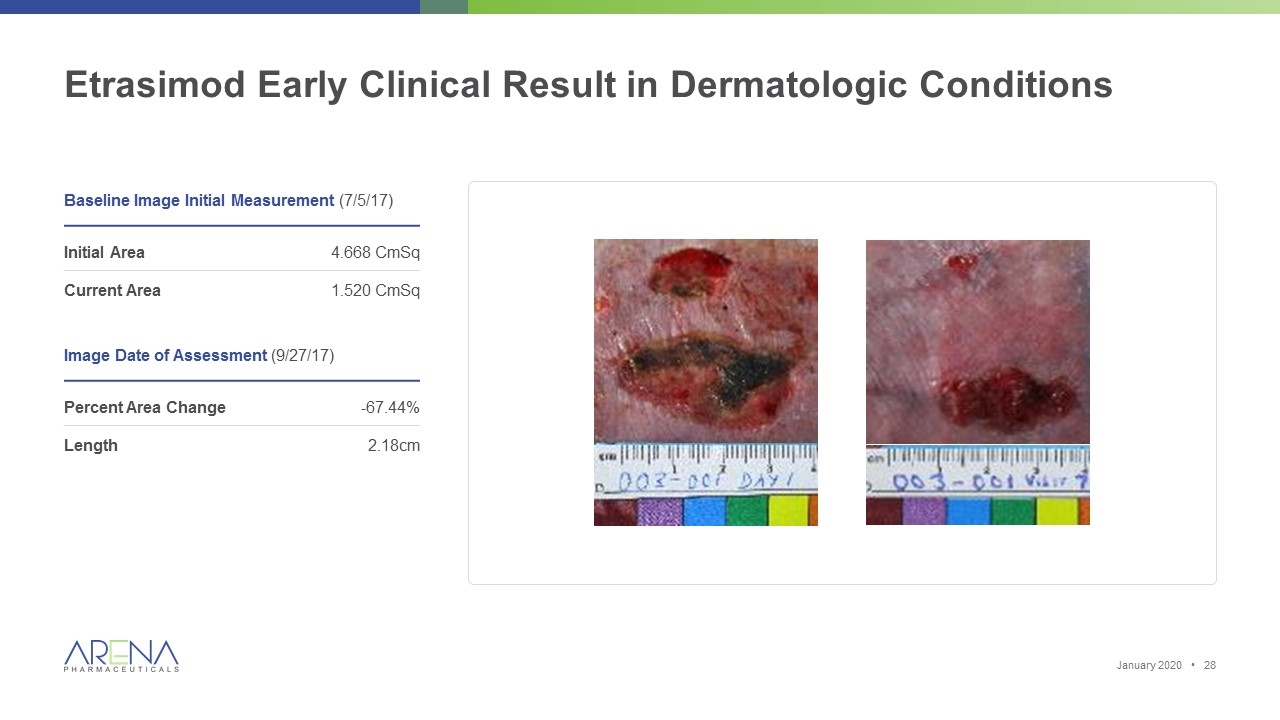
Etrasimod Early Clinical Result in Dermatologic Conditions Baseline Image Initial Measurement (7/5/17) Initial Area 4.668 CmSq Current Area 1.520 CmSq Image Date of Assessment (9/27/17) Percent Area Change -67.44% Length 2.18cm January 2020 •
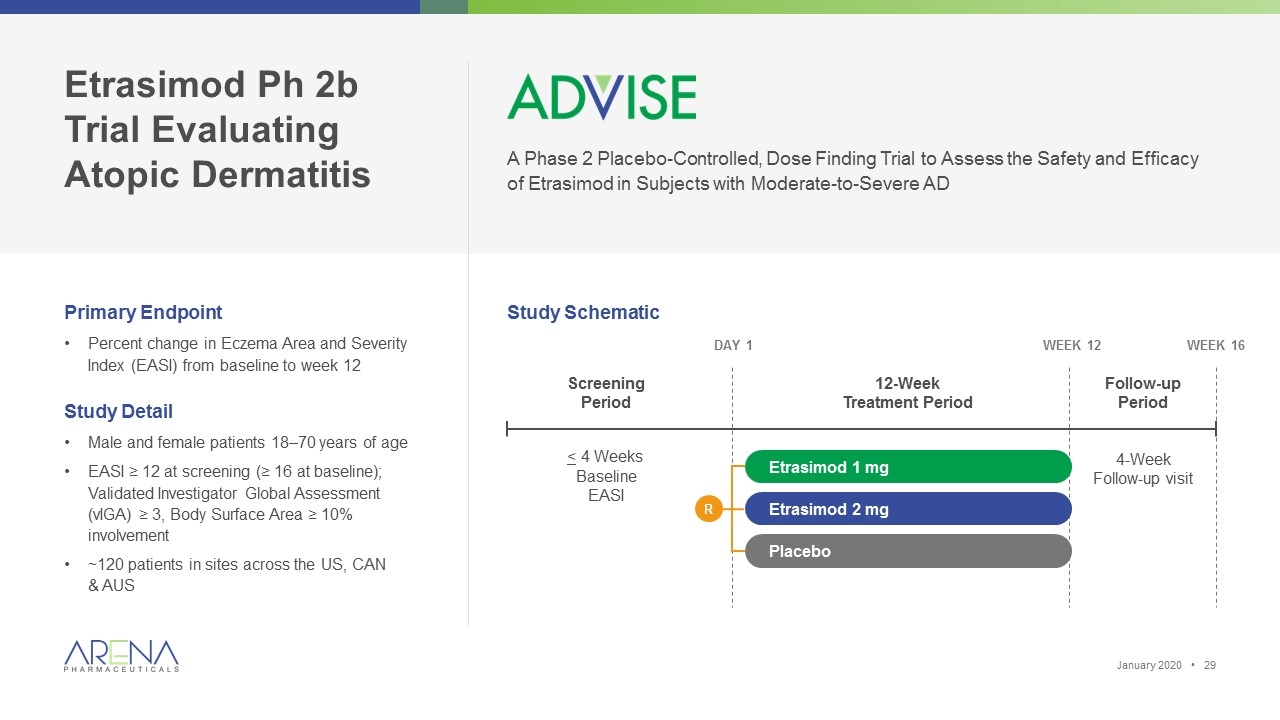
Etrasimod Ph 2b Trial Evaluating Atopic Dermatitis A Phase 2 Placebo-Controlled, Dose Finding Trial to Assess the Safety and Efficacy of Etrasimod in Subjects with Moderate-to-Severe AD Primary Endpoint Percent change in Eczema Area and Severity Index (EASI) from baseline to week 12 Study Detail Male and female patients 18–70 years of age EASI ≥ 12 at screening (≥ 16 at baseline); Validated Investigator Global Assessment (vIGA) ≥ 3, Body Surface Area ≥ 10% involvement ~120 patients in sites across the US, CAN & AUS Day 1 Week 16 Screening Period 12-Week Treatment Period Follow-up Period Week 12 4-Week Follow-up visit Etrasimod 2 mg Etrasimod 1 mg Placebo < 4 Weeks Baseline EASI Study Schematic R January 2020 •
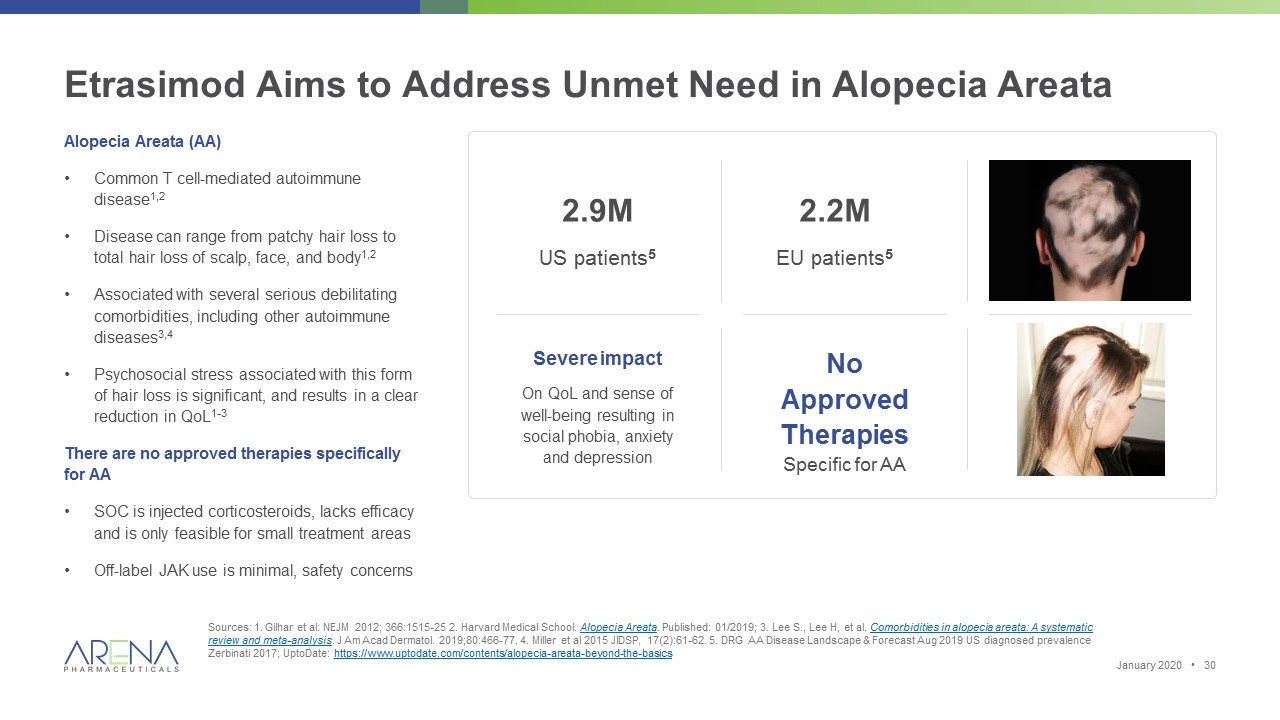
Etrasimod Aims to Address Unmet Need in Alopecia Areata Sources: 1. Gilhar et al. NEJM 2012; 366:1515-25 2. Harvard Medical School. Alopecia Areata. Published: 01/2019; 3. Lee S., Lee H, et al. Comorbidities in alopecia areata: A systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:466-77. 4. Miller et al 2015 JIDSP, 17(2):61-62. 5. DRG AA Disease Landscape & Forecast Aug 2019 US diagnosed prevalence Zerbinati 2017; UptoDate: https://www.uptodate.com/contents/alopecia-areata-beyond-the-basics Alopecia Areata (AA) Common T cell-mediated autoimmune disease1,2 Disease can range from patchy hair loss to total hair loss of scalp, face, and body1,2 Associated with several serious debilitating comorbidities, including other autoimmune diseases3,4 Psychosocial stress associated with this form of hair loss is significant, and results in a clear reduction in QoL1-3 There are no approved therapies specifically for AA SOC is injected corticosteroids, lacks efficacy and is only feasible for small treatment areas Off-label JAK use is minimal, safety concerns Severe impact On QoL and sense of well-being resulting in social phobia, anxiety and depression No Approved Therapies Specific for AA 2.9M US patients5 2.2M EU patients5 January 2020 •
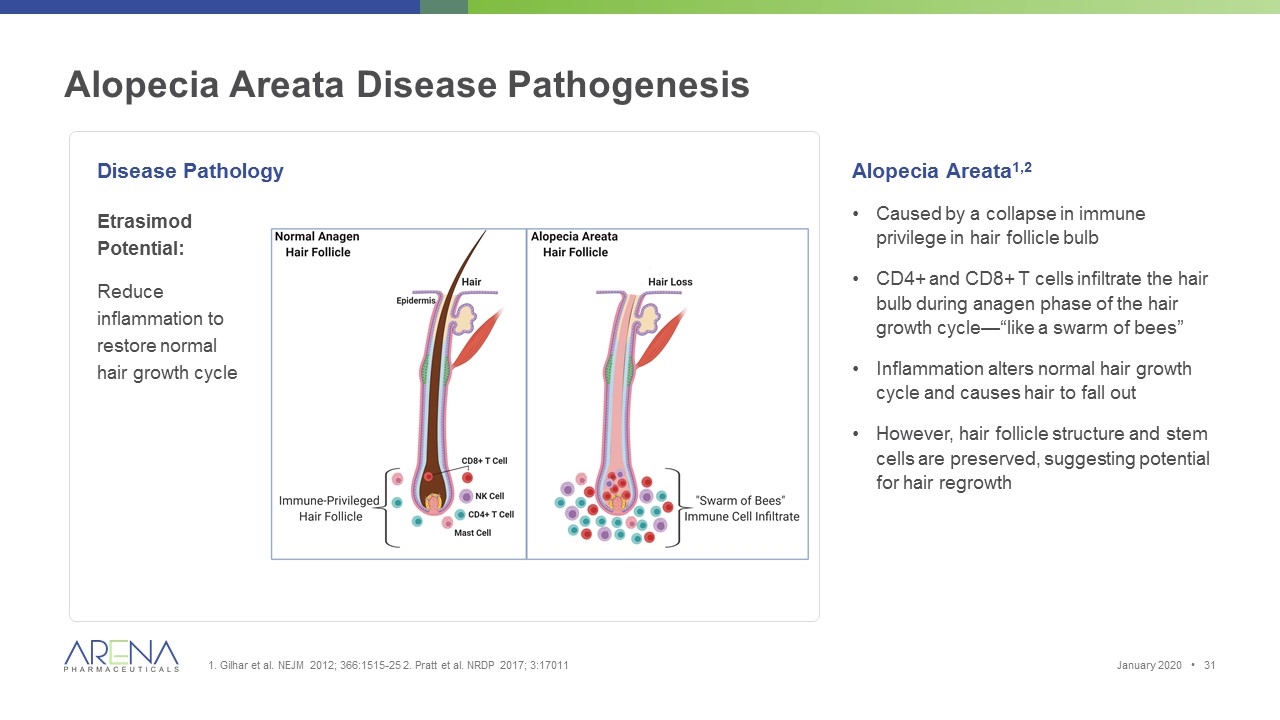
Disease Pathology Alopecia Areata1,2 Caused by a collapse in immune privilege in hair follicle bulb CD4+ and CD8+ T cells infiltrate the hair bulb during anagen phase of the hair growth cycle—“like a swarm of bees” Inflammation alters normal hair growth cycle and causes hair to fall out However, hair follicle structure and stem cells are preserved, suggesting potential for hair regrowth Alopecia Areata Disease Pathogenesis 1. Gilhar et al. NEJM 2012; 366:1515-25 2. Pratt et al. NRDP 2017; 3:17011 Etrasimod Potential: Reduce inflammation to restore normal hair growth cycle January 2020 •
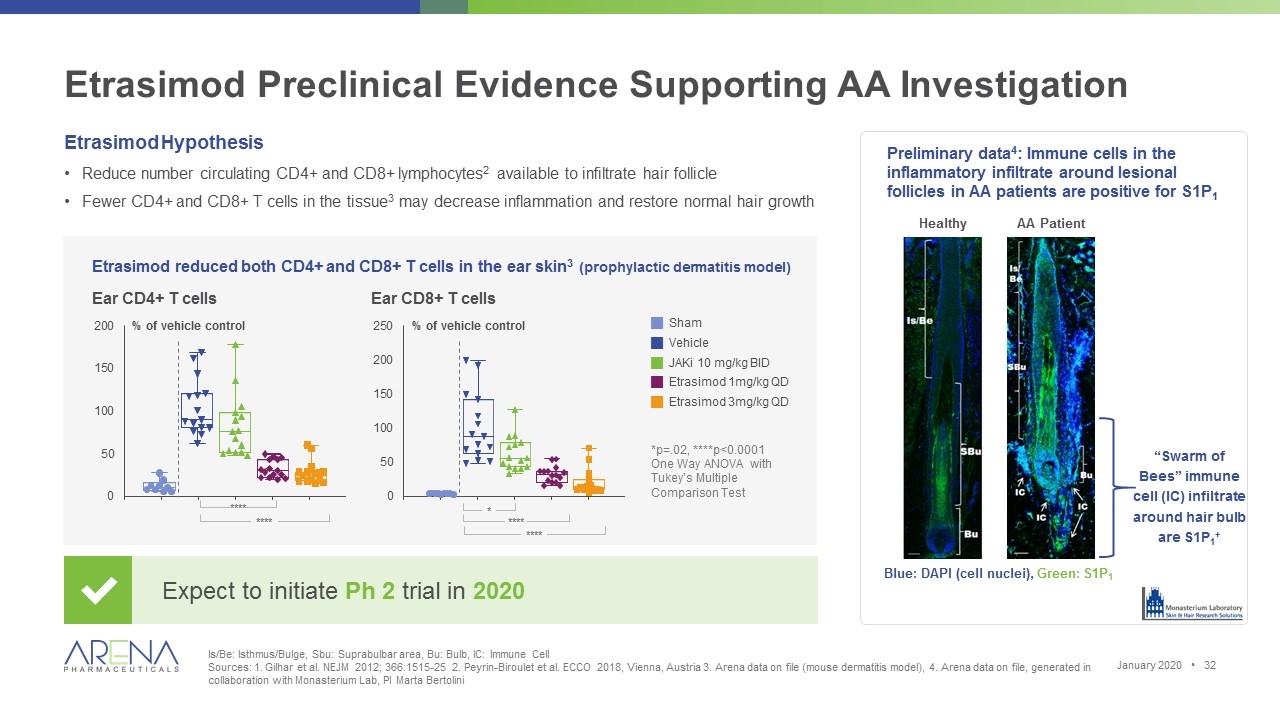
Etrasimod Preclinical Evidence Supporting AA Investigation Is/Be: Isthmus/Bulge, Sbu: Suprabulbar area, Bu: Bulb, IC: Immune Cell Sources: 1. Gilhar et al. NEJM 2012; 366:1515-25 2. Peyrin-Biroulet et al. ECCO 2018, Vienna, Austria 3. Arena data on file (mouse dermatitis model), 4. Arena data on file, generated in collaboration with Monasterium Lab, PI Marta Bertolini Etrasimod Hypothesis Reduce number circulating CD4+ and CD8+ lymphocytes2 available to infiltrate hair follicle Fewer CD4+ and CD8+ T cells in the tissue3 may decrease inflammation and restore normal hair growth Etrasimod reduced both CD4+ and CD8+ T cells in the ear skin3 (prophylactic dermatitis model) Blue: DAPI (cell nuclei), Green: S1P1 Preliminary data4: Immune cells in the inflammatory infiltrate around lesional follicles in AA patients are positive for S1P1 Healthy AA Patient *p=.02, ****p<0.0001 One Way ANOVA with Tukey’s Multiple Comparison Test Expect to initiate Ph 2 trial in 2020 Ear CD4+ T cells Ear CD8+ T cells January 2020 • % of vehicle control gSham gVehicle gJAKi 10 mg/kg BID gEtrasimod 1mg/kg QD gEtrasimod 3mg/kg QD % of vehicle control * **** **** “Swarm of Bees” immune cell (IC) infiltrate around hair bulb are S1P1+ **** ****

Olorinab Peripherally Acting, Highly–Selective, Full-Agonist to Cannabinoid Receptor 2 (CB2) for Gastrointestinal Pain January 2020 •
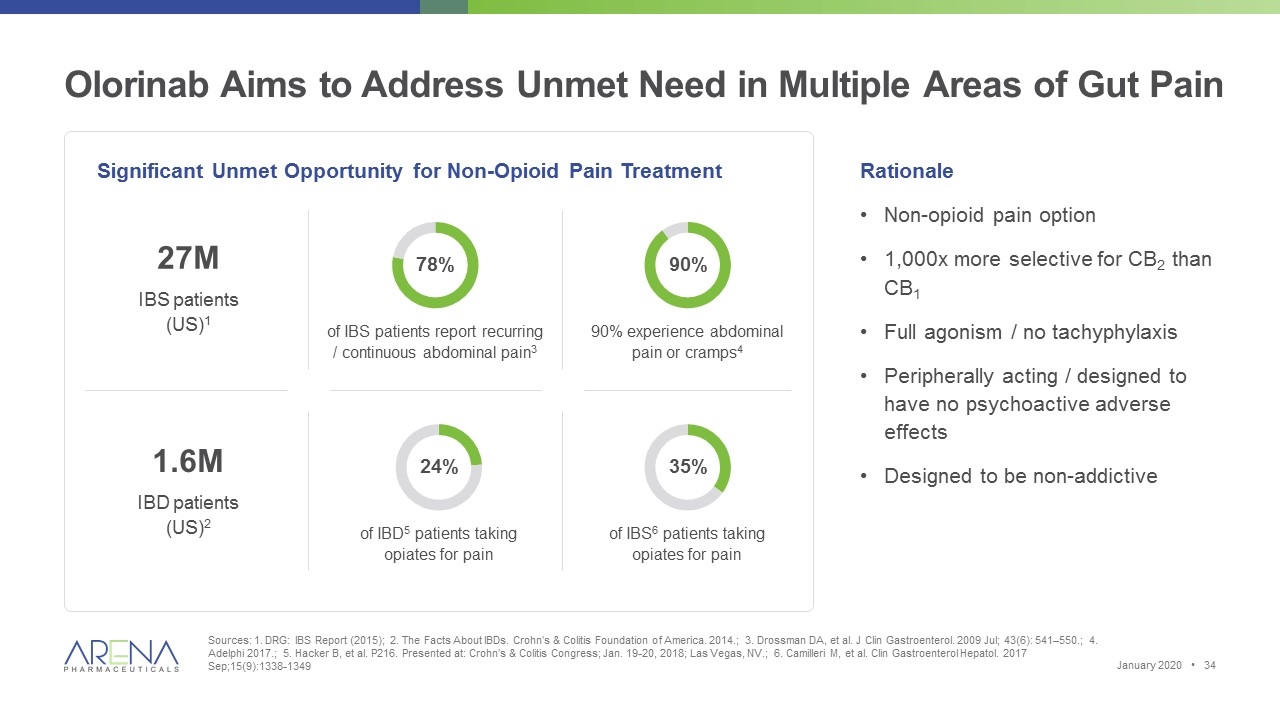
Olorinab Aims to Address Unmet Need in Multiple Areas of Gut Pain Sources: 1. DRG: IBS Report (2015); 2. The Facts About IBDs. Crohn’s & Colitis Foundation of America. 2014.; 3. Drossman DA, et al. J Clin Gastroenterol. 2009 Jul; 43(6): 541–550.; 4. Adelphi 2017.; 5. Hacker B, et al. P216. Presented at: Crohn’s & Colitis Congress; Jan. 19-20, 2018; Las Vegas, NV.; 6. Camilleri M, et al. Clin Gastroenterol Hepatol. 2017 Sep;15(9):1338-1349 of IBS patients report recurring / continuous abdominal pain3 Rationale Non-opioid pain option 1,000x more selective for CB2 than CB1 Full agonism / no tachyphylaxis Peripherally acting / designed to have no psychoactive adverse effects Designed to be non-addictive Significant Unmet Opportunity for Non-Opioid Pain Treatment 78% 90% 24% 27M 1.6M 35% 90% experience abdominal pain or cramps4 of IBD5 patients taking opiates for pain of IBS6 patients taking opiates for pain IBS patients (US)1 IBD patients (US)2 January 2020 •
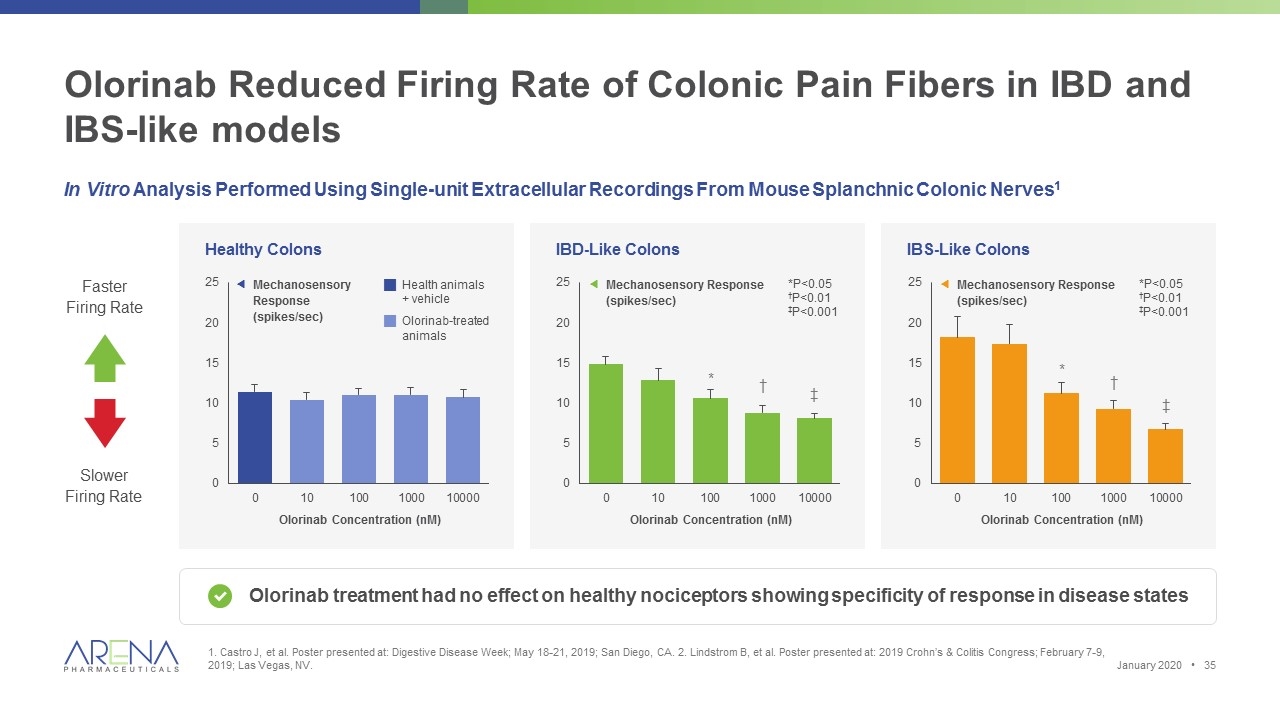
Olorinab Reduced Firing Rate of Colonic Pain Fibers in IBD and IBS-like models 1. Castro J, et al. Poster presented at: Digestive Disease Week; May 18-21, 2019; San Diego, CA. 2. Lindstrom B, et al. Poster presented at: 2019 Crohn’s & Colitis Congress; February 7-9, 2019; Las Vegas, NV. IBS-Like Colons In Vitro Analysis Performed Using Single-unit Extracellular Recordings From Mouse Splanchnic Colonic Nerves1 Olorinab treatment had no effect on healthy nociceptors showing specificity of response in disease states Faster Firing Rate Slower Firing Rate Olorinab Concentration (nM) Healthy Colons Olorinab Concentration (nM) ƒMechanosensory Response (spikes/sec) gHealth animals + vehicle gOlorinab-treated animals * † ‡ *P<0.05 †P<0.01 ‡P<0.001 IBD-Like Colons Olorinab Concentration (nM) * † ‡ *P<0.05 †P<0.01 ‡P<0.001 ƒMechanosensory Response (spikes/sec) ƒMechanosensory Response (spikes/sec) January 2020 •
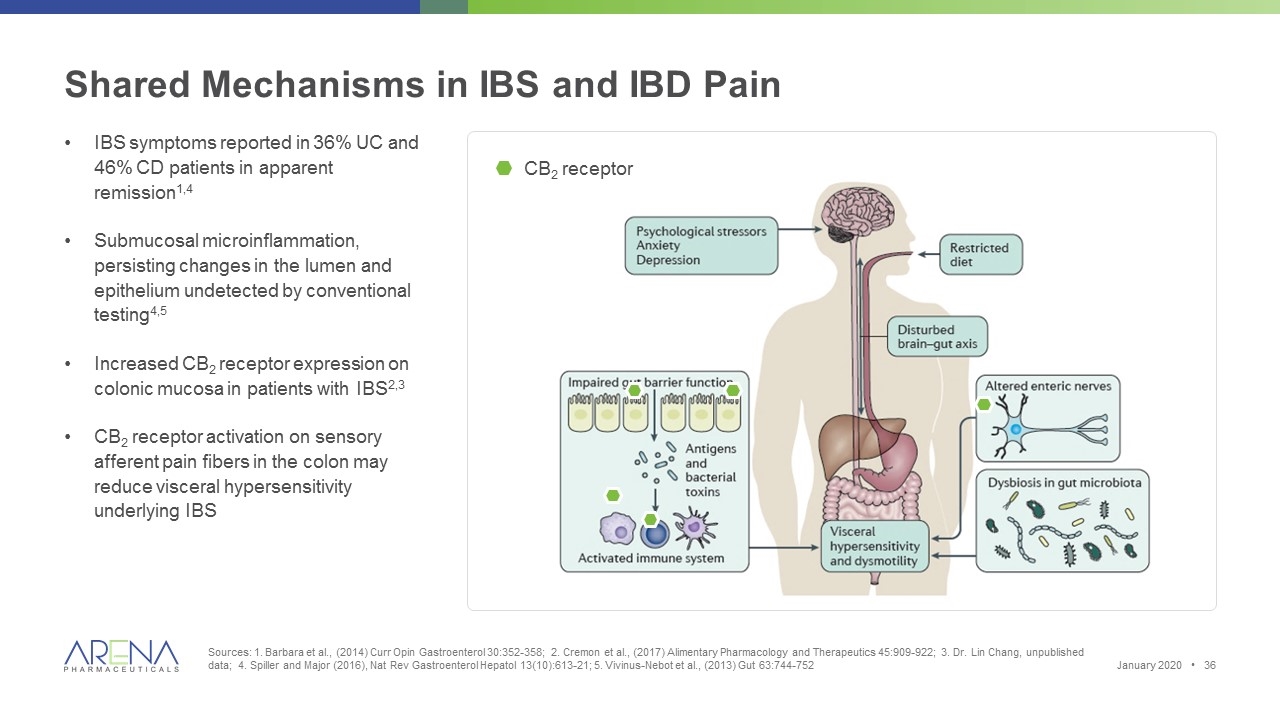
Shared Mechanisms in IBS and IBD Pain Sources: 1. Barbara et al., (2014) Curr Opin Gastroenterol 30:352-358; 2. Cremon et al., (2017) Alimentary Pharmacology and Therapeutics 45:909-922; 3. Dr. Lin Chang, unpublished data; 4. Spiller and Major (2016), Nat Rev Gastroenterol Hepatol 13(10):613-21; 5. Vivinus-Nebot et al., (2013) Gut 63:744-752 IBS symptoms reported in 36% UC and 46% CD patients in apparent remission1,4 Submucosal microinflammation, persisting changes in the lumen and epithelium undetected by conventional testing4,5 Increased CB2 receptor expression on colonic mucosa in patients with IBS2,3 CB2 receptor activation on sensory afferent pain fibers in the colon may reduce visceral hypersensitivity underlying IBS CB2 receptor January 2020 •
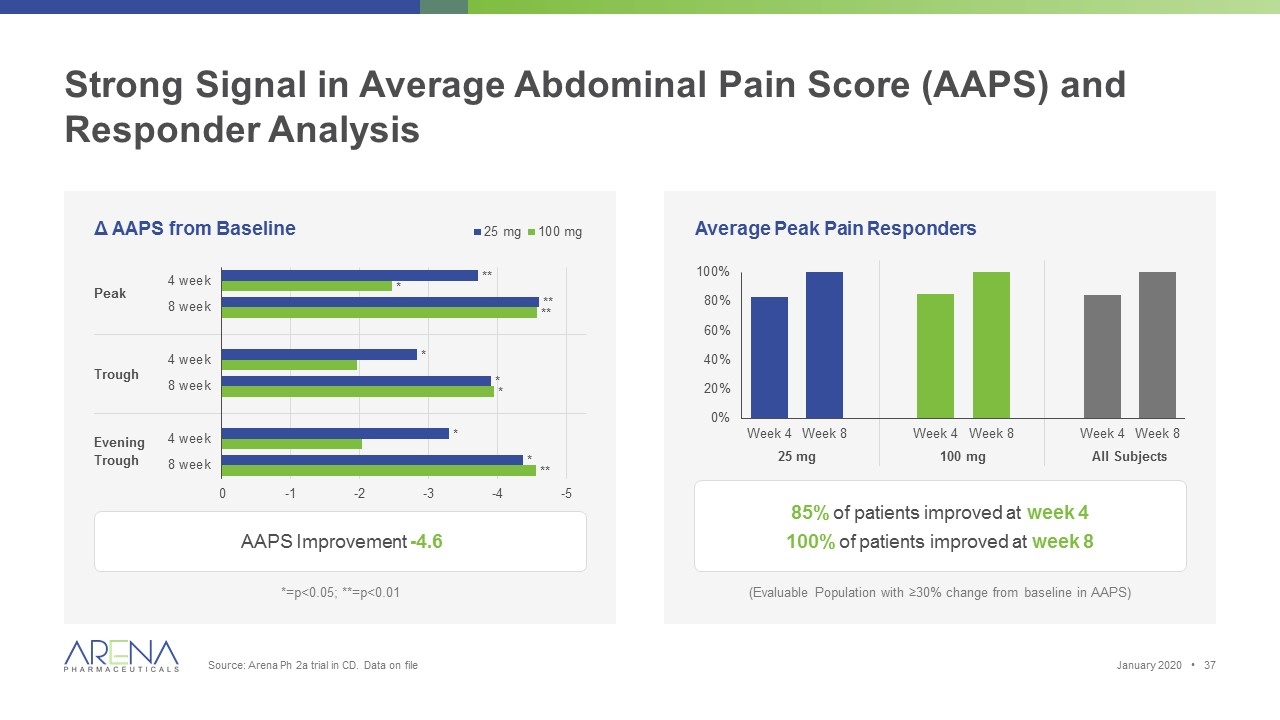
Strong Signal in Average Abdominal Pain Score (AAPS) and Responder Analysis Δ AAPS from Baseline Average Peak Pain Responders AAPS Improvement -4.6 85% of patients improved at week 4 100% of patients improved at week 8 *=p<0.05; **=p<0.01 (Evaluable Population with ≥30% change from baseline in AAPS) Peak Trough Evening Trough 25 mg 100 mg All Subjects January 2020 • Source: Arena Ph 2a trial in CD. Data on file
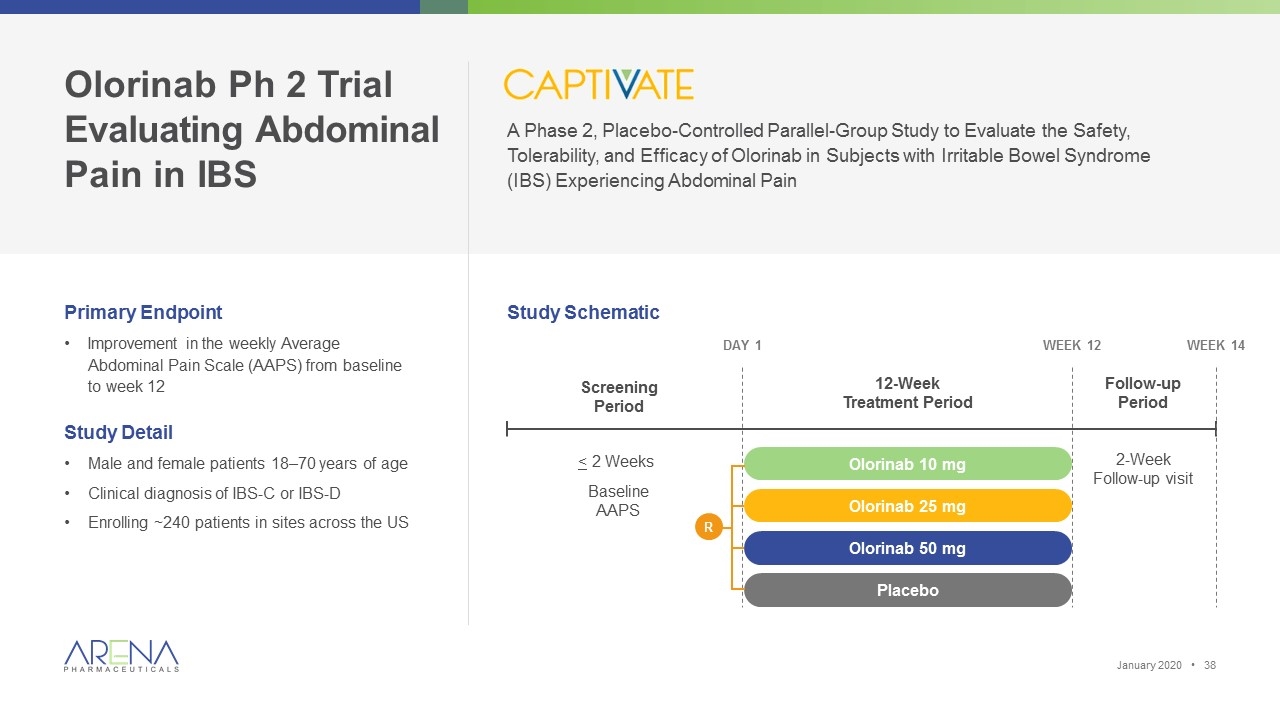
A Phase 2, Placebo-Controlled Parallel-Group Study to Evaluate the Safety, Tolerability, and Efficacy of Olorinab in Subjects with Irritable Bowel Syndrome (IBS) Experiencing Abdominal Pain Primary Endpoint Improvement in the weekly Average Abdominal Pain Scale (AAPS) from baseline to week 12 Study Detail Male and female patients 18–70 years of age Clinical diagnosis of IBS-C or IBS-D Enrolling ~240 patients in sites across the US Week 14 Screening Period 12-Week Treatment Period Follow-up Period Week 12 2-Week Follow-up visit Olorinab 25 mg Olorinab 10 mg Olorinab 50 mg Placebo < 2 Weeks Baseline AAPS Study Schematic Olorinab Ph 2 Trial Evaluating Abdominal Pain in IBS Day 1 R January 2020 •
APD418 Potential First-in-Class Investigational Beta-3 AdrR Antagonist and Cardiac Myotrope for Decompensated Heart Failure (DHF) January 2020 •
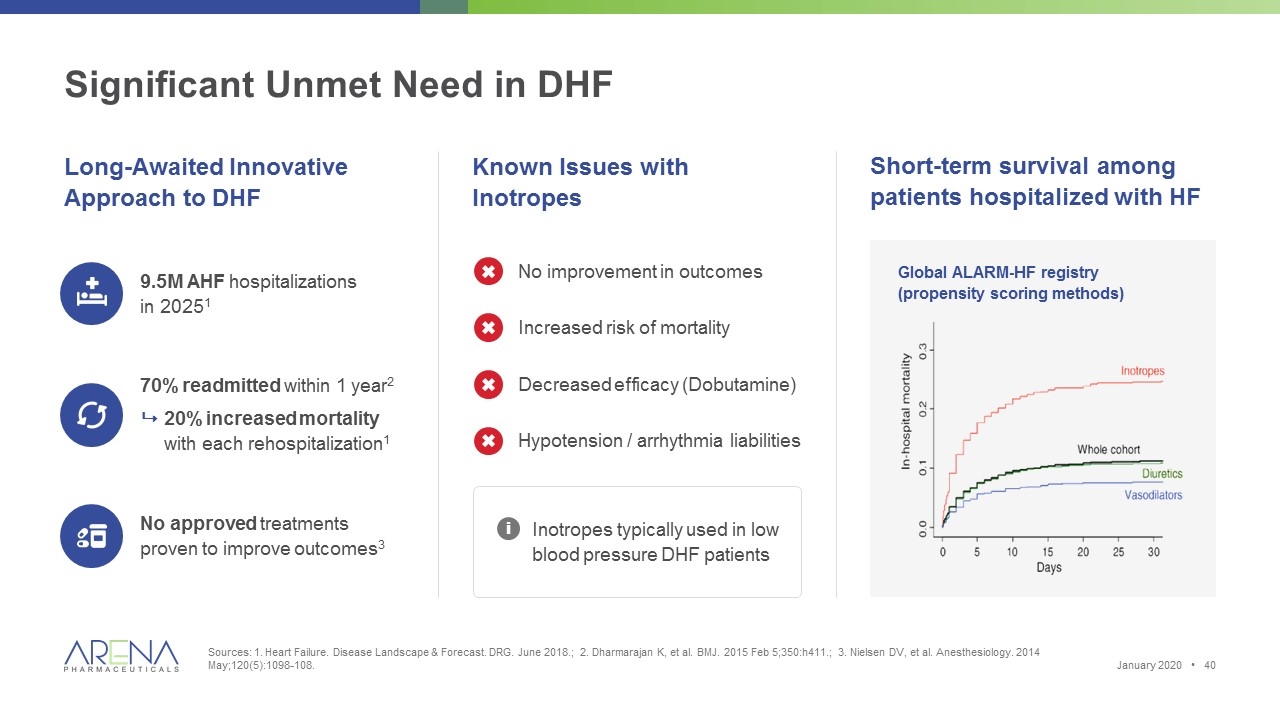
Significant Unmet Need in DHF Sources: 1. Heart Failure. Disease Landscape & Forecast. DRG. June 2018.; 2. Dharmarajan K, et al. BMJ. 2015 Feb 5;350:h411.; 3. Nielsen DV, et al. Anesthesiology. 2014 May;120(5):1098-108. Long-Awaited Innovative Approach to DHF 9.5M AHF hospitalizations in 20251 No approved treatments proven to improve outcomes3 70% readmitted within 1 year2 920% increased mortality with each rehospitalization1 Known Issues with Inotropes Inotropes typically used in low blood pressure DHF patients i No improvement in outcomes Increased risk of mortality Hypotension / arrhythmia liabilities Decreased efficacy (Dobutamine) Short-term survival among patients hospitalized with HF Global ALARM-HF registry (propensity scoring methods) January 2020 •
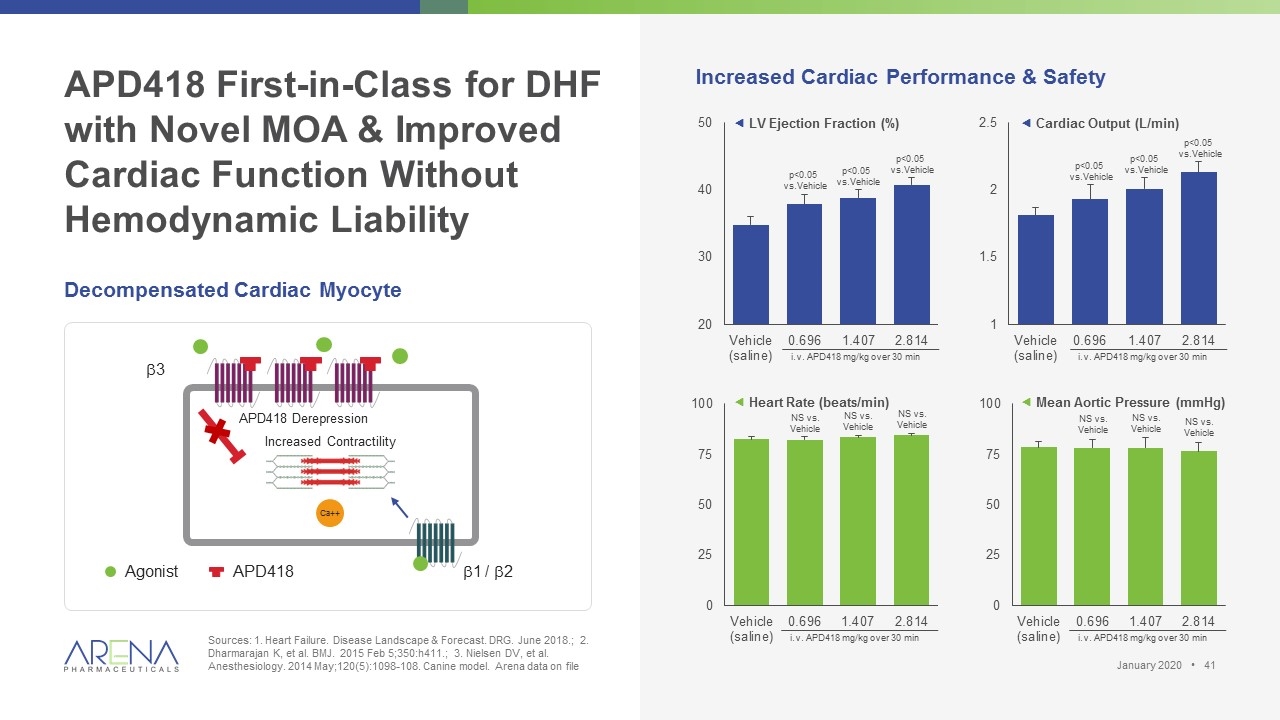
APD418 First-in-Class for DHF with Novel MOA & Improved Cardiac Function Without Hemodynamic Liability Sources: 1. Heart Failure. Disease Landscape & Forecast. DRG. June 2018.; 2. Dharmarajan K, et al. BMJ. 2015 Feb 5;350:h411.; 3. Nielsen DV, et al. Anesthesiology. 2014 May;120(5):1098-108. Canine model. Arena data on file Decompensated Cardiac Myocyte Increased Cardiac Performance & Safety ƒ LV Ejection Fraction (%) b3 b1 / b2 Agonist APD418 APD418 Derepression Ca++ Increased Contractility ƒ Cardiac Output (L/min) ƒ Heart Rate (beats/min) ƒ Mean Aortic Pressure (mmHg) p<0.05 vs.Vehicle p<0.05 vs.Vehicle p<0.05 vs.Vehicle i.v. APD418 mg/kg over 30 min i.v. APD418 mg/kg over 30 min i.v. APD418 mg/kg over 30 min i.v. APD418 mg/kg over 30 min p<0.05 vs.Vehicle p<0.05 vs.Vehicle p<0.05 vs.Vehicle NS vs. Vehicle NS vs. Vehicle NS vs. Vehicle NS vs. Vehicle NS vs. Vehicle NS vs. Vehicle January 2020 •
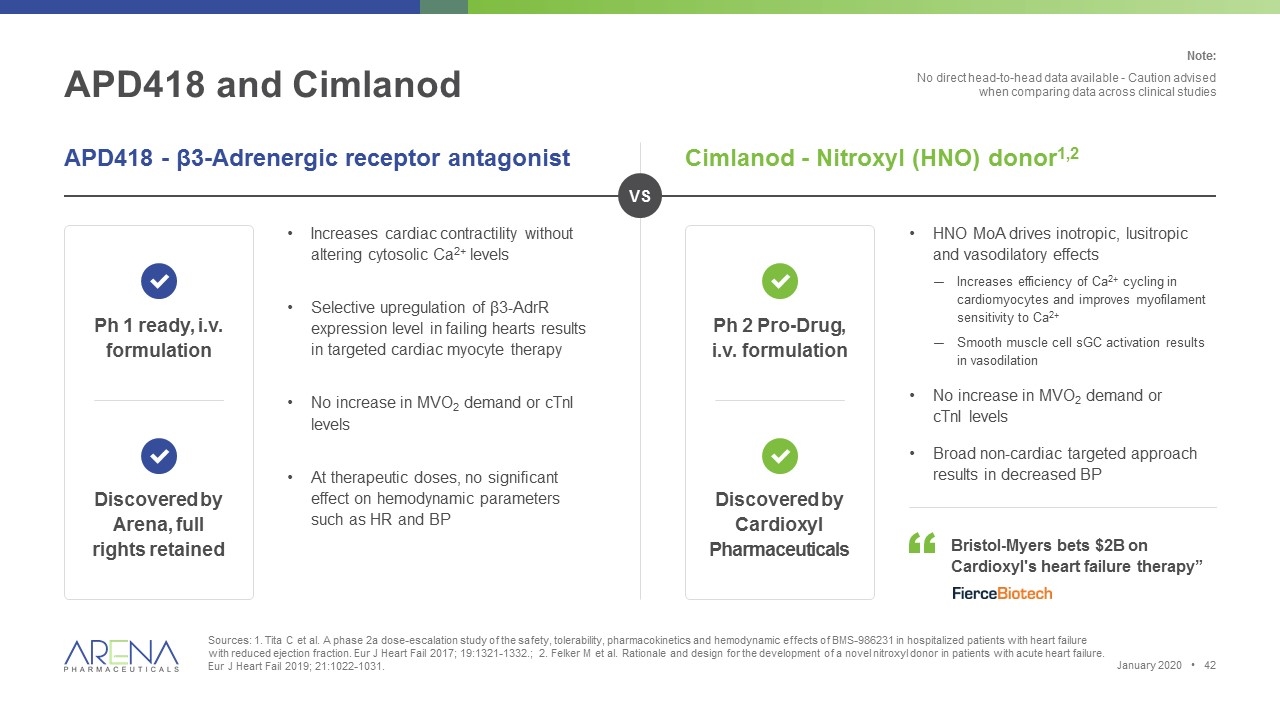
APD418 and Cimlanod Sources: 1. Tita C et al. A phase 2a dose-escalation study of the safety, tolerability, pharmacokinetics and hemodynamic effects of BMS-986231 in hospitalized patients with heart failure with reduced ejection fraction. Eur J Heart Fail 2017; 19:1321-1332.; 2. Felker M et al. Rationale and design for the development of a novel nitroxyl donor in patients with acute heart failure. Eur J Heart Fail 2019; 21:1022-1031. APD418 - β3-Adrenergic receptor antagonist Cimlanod - Nitroxyl (HNO) donor1,2 Increases cardiac contractility without altering cytosolic Ca2+ levels Selective upregulation of β3-AdrR expression level in failing hearts results in targeted cardiac myocyte therapy No increase in MVO2 demand or cTnI levels At therapeutic doses, no significant effect on hemodynamic parameters such as HR and BP Ph 1 ready, i.v. formulation Discovered by Arena, full rights retained Ph 2 Pro-Drug, i.v. formulation Discovered by Cardioxyl Pharmaceuticals HNO MoA drives inotropic, lusitropic and vasodilatory effects Increases efficiency of Ca2+ cycling in cardiomyocytes and improves myofilament sensitivity to Ca2+ Smooth muscle cell sGC activation results in vasodilation No increase in MVO2 demand or cTnI levels Broad non-cardiac targeted approach results in decreased BP Bristol-Myers bets $2B on Cardioxyl's heart failure therapy” VS Note: No direct head-to-head data available - Caution advised when comparing data across clinical studies January 2020 •
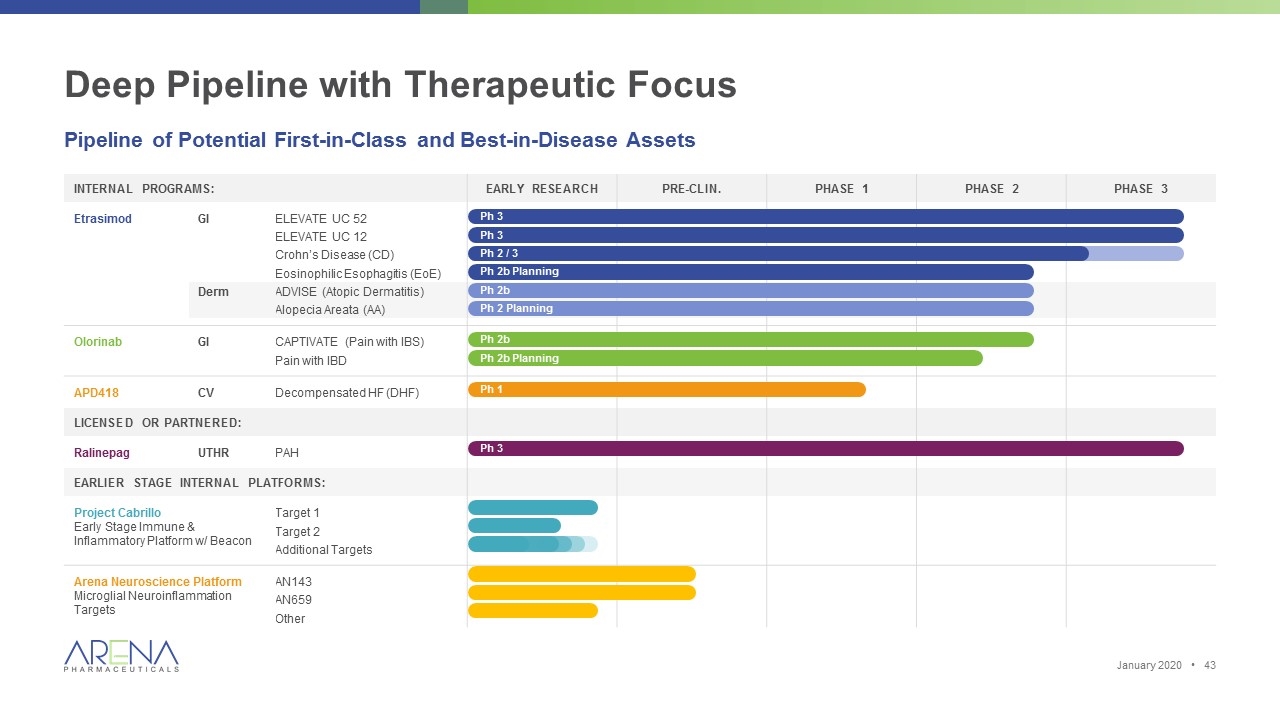
Deep Pipeline with Therapeutic Focus Pipeline of Potential First-in-Class and Best-in-Disease Assets Internal Programs: Early Research Pre-clin. Phase 1 Phase 2 Phase 3 Etrasimod GI ELEVATE UC 52 ELEVATE UC 12 Crohn’s Disease (CD) Eosinophilic Esophagitis (EoE) Derm ADVISE (Atopic Dermatitis) Alopecia Areata (AA) Olorinab GI CAPTIVATE (Pain with IBS) Pain with IBD APD418 CV Decompensated HF (DHF) Licensed or partnered: Ralinepag UTHR PAH Earlier stage internal platforms: Project Cabrillo Early Stage Immune & Inflammatory Platform w/ Beacon Target 1 Target 2 Additional Targets Arena Neuroscience Platform Microglial Neuroinflammation Targets AN143 AN659 Other Ph 2b Planning Ph 2b Ph 2 Planning Ph 2b Ph 2b Planning Ph 1 Ph 3 Ph 3 Ph 2 / 3 Ph 3 January 2020 •

Nasdaq: ARNA











































