Exhibit 99.1
For Immediate Release
For Further Information Contact:
Andrew Fisher at (202) 483-7000
Email: Afisher@unither.com
UNITED THERAPEUTICS REPORTS
FOURTH QUARTER AND ANNUAL 2007 FINANCIAL RESULTS
· Total Annual Revenues of $210.9 Million
· Annual EPS of $0.94 per Basic Share, or $0.88 per Diluted Share
· Annual EBITDASO of $4.03 per Basic Share, or $3.81 per Diluted Share
Silver Spring, MD, February 19, 2008: United Therapeutics Corporation (NASDAQ: UTHR) today announced financial results for the fourth quarter and year ended December 31, 2007.
“We are pleased to report that United Therapeutics’ revenues for the year ended December 31, 2007, totaled $210.9 million,” said Martine Rothblatt, Ph.D., United Therapeutics’ Chairman and Chief Executive Officer. “This is the sixth straight year our revenues have grown by more than 30%. Our EBITDASO (earnings before interest, taxes, depreciation, amortization and stock option expense) for the year were $85.5 million, or $4.03 per basic share.”
Net income for the three months ended December 31, 2007, was $2.0 million or $0.09 per basic share, as compared to $55.5 million or $2.54 per basic share for the three months ended December 31, 2006. EBITDASO for the three months ended December 31, 2007, was $24.7 million or $1.14 per basic share, as compared to $18.7 million or $0.81 per basic share for the three months ended December 31, 2006. Net income for the year ended December 31, 2007, was $19.9 million or $0.94 per basic share, as compared to $74.0 million or $3.21 per basic share in 2006.
Results
Revenues. Revenues grew to $210.9 million for the year ended December 31, 2007, as compared to $159.6 million for the year ended December 31, 2006, an increase of 32%. Gross margins from sales were $186.5 million, or 89%, in 2007, which is consistent with gross margins from sales of $142.6 million, or 89%, in 2006. The table below summarizes the components of our revenues for the three months and years ended December 31, 2007 and 2006 (dollars in thousands):
| | Three Months Ended
December 31, | | Years Ending
December 31, | |
| | 2007 | | 2006 | | 2007 | | 2006 | |
| | | | | | | | | |
Revenues: | | | | | | | | | |
Net product sales | | $ | 56,899 | | $ | 44,147 | | $ | 201,348 | | $ | 153,448 | |
Service sales | | 2,172 | | 1,679 | | 7,435 | | 6,184 | |
Distributor fees | | 827 | | — | | 2,160 | | — | |
| | | | | | | | | |
Total revenues | | $ | 59,898 | | $ | 45,826 | | $ | 210,943 | | $ | 159,632 | |
The increases in revenues resulted primarily from growth in sales of Remodulin.
Operating Expenses. Our operating expenses include research and development expenses, sales and marketing expenses, costs of product sales and cost of service sales.
Research and development expenses consist primarily of salaries and related expenses, costs to acquire pharmaceutical products and product rights for development, and amounts paid to contract research organizations, hospitals and laboratories for the provision of services and materials for drug development and clinical trials. The table below summarizes research and development expenses by major project and non-project components for the three months and year ended December 31, 2007 and 2006 (dollars in thousands):
| | Three Months Ended
December 31, | | Years Ending
December 31, | |
| | 2007 | | 2006 | | 2007 | | 2006 | |
| | | | | | | | | |
Project and non-project: | | | | | | | | | |
Cardiovascular | | $ | 8,570 | | $ | 11,276 | | $ | 38,459 | | $ | 33,005 | |
Cancer | | 3,189 | | 3,231 | | 13,874 | | 10,462 | |
Infectious disease | | 278 | | 204 | | 824 | | 753 | |
Stock option | | 3,671 | | 2,600 | | 12,373 | | 9,240 | |
Other | | 2,002 | | 1,026 | | 6,809 | | 4,110 | |
R&D expense from issuance of common stock for license | | — | | — | | 11,013 | | — | |
| | | | | | | | | |
Total research and development expense | | $ | 17,710 | | $ | 18,337 | | $ | 83,352 | | $ | 57,570 | |
Selling, general and administrative expenses consist primarily of salaries, travel, office expenses, insurance, professional fees, provision for doubtful accounts receivable, depreciation and amortization. The table below summarizes selling, general and administrative expenses by major categories for the three months and year ended December 31, 2007 and 2006 (dollars in thousands):
| | Three Months Ended
December 31, | | Years Ending
December 31, | |
| | 2007 | | 2006 | | 2007 | | 2006 | |
| | | | | | | | | |
Category: | | | | | | | | | |
General and administrative | | $ | 11,361 | | $ | 7,300 | | $ | 34,933 | | $ | 25,434 | |
Sales and marketing | | 7,826 | | 4,584 | | 24,159 | | 14,438 | |
Impairment charges | | 2,068 | | — | | 3,582 | | 2,024 | |
Stock option | | 22,971 | | 7,303 | | 36,353 | | 14,156 | |
| | | | | | | | | |
Total selling, general and administrative expense | | $ | 44,226 | | $ | 19,187 | | $ | 99,027 | | $ | 56,052 | |
For the year ended December 31, 2007, as compared to the year ended December 31, 2006, the increase in general and administrative expenses was primarily related to increased expenses for salaries, associated expenses and other operating expenses from growth in our operations. The increase in sales and marketing related expenses was primarily related to increased salaries and an increase in headcount, marketing, travel and associated expenses from growth in our operations. The trends for the three-month period ended December 31, 2007, as compared to the same period in 2006, are similar to those noted for the annual period.
The impairment charge during the three months ended December 31, 2007, was primarily related to an other-than-temporary decline of our investment in ViRexx Medical Corp. due to the unsuccessful results of our IMPACT I and II Phase III clinical trials of OvaRex in advanced ovarian cancer.
Stock option expense increased during the three-month and twelve-month periods ended December 31, 2007, over the same periods in 2006, primarily as a result of a stock option grant to our Chief Executive Officer based on the 89% increase in our share price during 2007.
Interest Income. Interest income for the year ended December 31, 2007, was $13.6 million, as compared to interest income of $10.7 million for the year ended December 31, 2006. The difference was due primarily to an increase in market interest rates and amounts available to invest. Interest income for the three months ended December 31, 2007, was comparable to interest income for the same period in 2006.
Income Tax Benefit. We recognized an income tax benefit of approximately $3.3 million and $34.1 million for the years ended December 31, 2007 and 2006, respectively. For the three-month periods ended December 31, 2007 and 2006, we recognized a tax benefit of $7.1 million and $47.7 million, respectively. The tax benefit recognized for 2007 was primarily due to the amount of tax credits generated during the year from our orphan drug related research and development activities. For the year ended December 31, 2006, the tax benefit recognized was primarily due to a reduction of approximately $45.7 million in the valuation allowance against most of our deferred tax assets based on our determination that certain portions of these deferred tax assets are more likely than not realizable.
EBITDASO Calculation
The following table provides a reconciliation of net income and EBITDASO for the three-month periods ended December 31, 2007 and 2006, and for the years ended December 31, 2004 through 2007 (in thousands, except per share data):
| | Year ended December 31, | | Three months ended
December 31, | |
| | | |
| | 2004 | | 2005 | | 2006 | | 2007 | | 2007 | | 2006 | |
| | | | | | | | | | | | | |
Net income, as reported | | $ | 15,449 | | $ | 65,016 | | $ | 73,965 | | $ | 19,859 | | $ | 1,986 | | $ | 55,508 | |
Add (subtract) back: | | | | | | | | | | | | | |
Interest expense | | 4 | | 29 | | 482 | | 2,175 | | 34 | | 481 | |
Income tax (benefit) | | — | | (17,494 | ) | (34,057 | ) | (3,276 | ) | (7,104 | ) | (47,718 | ) |
One time expense | | — | | — | | 2,024 | | 14,595 | | 2,067 | | — | |
Depreciation and amortization | | 2,381 | | 2,534 | | 2,713 | | 3,427 | | 941 | | 770 | |
Stock option expense | | 329 | | 983 | | 23,513 | | 48,766 | | 26,792 | | 9,687 | |
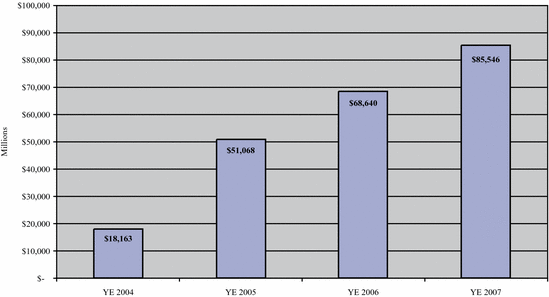
EBITDASO | | $ | 18,163 | | $ | 51,068 | | $ | 68,640 | | $ | 85,546 | | $ | 24,716 | | $ | 18,728 | |
| | | | | | | | | | | | | |
EBITDASO earnings per
share (1): | | | | | | | | | | | | | |
Basic | | $ | 0.84 | | $ | 2.24 | | $ | 2.98 | | $ | 4.03 | | $ | 1.14 | | $ | 0.86 | |
Diluted | | $ | 0.78 | | $ | 2.03 | | $ | 2.84 | | $ | 3.81 | | $ | 1.04 | | $ | 0.82 | |
(1) Calculated by dividing EBITDASO from above by weighted average shares outstanding, as reported below.
Conference Call
United Therapeutics will host a half-hour teleconference on Tuesday, February 19, 2008, at 9:00 a.m. Eastern Time. The teleconference is accessible by dialing 1-800-603-1777, with international callers dialing 1-706-679-8129. A rebroadcast of the teleconference will be available for one week by dialing 1-800-642-1687, with international callers dialing 1-706-645-1687, and using access code 33422885.
This teleconference is also being webcast and can be accessed via United Therapeutics’ website at http://ir.unither.com/eventdetail.cfm?eventid=51056.
About United Therapeutics
United Therapeutics Corporation is a biotechnology company focused on the development and commercialization of unique products to address the unmet medical needs of patients with chronic and life-threatening cardiovascular and infectious diseases and cancer.
Non-GAAP Financial Information
This press release contains certain financial measures that do not comply with GAAP, which measures supplement our financial results prepared in accordance with GAAP.
We use EBITDASO, a non-GAAP measure, for our operating, budgeting and financial planning purposes and as a metric for our Company-wide Milestone Incentive Bonus Program, and believe our investors’ understanding of our performance is enhanced by disclosing this measure.
The presentation of these non-GAAP financial measures is not to be considered in isolation or as a substitute for our financial results prepared in accordance with GAAP.
Forward-looking Statements
Statements included in this press release that are not historical in nature are “forward-looking statements” within the meaning of the Private Securities Reform Act of 1995. These forward-looking statements include our expectations and intentions regarding the payment of federal and state income taxes for 2007, the generation of taxable income, the availability and utilization of net operating loss carry forwards to reduce taxable income, and the availability, creation and utilization of research tax credits to pay income taxes that are based on our current beliefs and expectations as to future outcomes. These forward-looking statements are subject to certain risks and uncertainties, such as those described in our periodic reports filed with the Securities and Exchange Commission, which could cause actual results to differ materially from anticipated results. Consequently, such forward-looking statements are qualified by the cautionary statements, cautionary language and risk factors set forth in our periodic reports and
documents filed with the Securities and Exchange Commission, including our most recent Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, and current reports on Form 8-K. We claim the protection of the safe harbor contained in the Private Securities Reform Act of 1995 for forward-looking statements. We are providing this information as of February 19, 2008, and assume no obligation to update or revise the information contained in this press release whether as a result of new information, future events or any other reason.
Remodulin is a registered trademark of United Therapeutics Corporation.
OvaRex is a registered trademark of AltaRex Medical Corp.
UNITED THERAPEUTICS CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share data)
| | Three Months Ended
December 31, | | Years Ending
December 31, | |
| | 2007 | | 2006 | | 2007 | | 2006 | |
| | | | | | | | | |
Revenues: | | | | | | | | | |
Net product sales | | $ | 56,899 | | $ | 44,147 | | $ | 201,348 | | $ | 153,448 | |
Service sales | | 2,172 | | 1,679 | | 7,435 | | 6,184 | |
Distributor fees | | 827 | | — | | 2,160 | | — | |
Total revenues | | 59,898 | | 45,826 | | 210,943 | | 159,632 | |
| | | | | | | | | |
Operating expenses: | | | | | | | | | |
Research and development | | 17,710 | | 18,337 | | 83,352 | | 57,570 | |
Selling, general and administrative | | 44,226 | | 19,187 | | 99,027 | | 56,052 | |
Cost of product sales | | 5,745 | | 4,251 | | 19,919 | | 14,973 | |
Cost of service sales | | 612 | | 502 | | 2,342 | | 2,055 | |
Total operating expenses | | 68,293 | | 42,277 | | 204,640 | | 130,650 | |
Income (loss) from operations | | (8,395 | ) | 3,549 | | 6,303 | | 28,982 | |
| | | | | | | | | |
Other income (expense): | | | | | | | | | |
Interest income | | 3,939 | | 3,653 | | 13,602 | | 10,700 | |
Interest expense | | (34 | ) | (481 | ) | (2,175 | ) | (482 | ) |
Equity (loss) in affiliate | | (56 | ) | (93 | ) | (321 | ) | (491 | ) |
Other, net | | (572 | ) | 1,162 | | (826 | ) | 1,199 | |
Total other income, net | | 3,277 | | 4,241 | | 10,280 | | 10,926 | |
Income (loss) before income tax benefit | | (5,118 | ) | 7,790 | | 16,583 | | 39,908 | |
Income tax benefit | | 7,104 | | 47,718 | | 3,276 | | 34,057 | |
Net income | | $ | 1,986 | | $ | 55,508 | | $ | 19,859 | | $ | 73,965 | |
Net income per common share: | | | | | | | | | |
Basic | | $ | 0.09 | | $ | 2.54 | | $ | 0.94 | | $ | 3.21 | |
Diluted | | $ | 0.08 | | $ | 2.42 | | $ | 0.88 | | $ | 3.06 | |
Weighted average number of common shares outstanding: | | | | | | | | | |
Basic | | 21,666 | | 21,893 | | 21,224 | | 23,010 | |
Diluted | | 23,746 | | 22,894 | | 22,451 | | 24,138 | |
CONSOLIDATED BALANCE SHEET DATA:
As of December 31,
(In thousands)
| | 2007 | | 2006 | |
| | | | | |
Cash, cash equivalents and marketable investments (excluding restricted amounts of $44,195 and $38,988, respectively) | | $ | 299,287 | | $ | 264,163 | |
Total assets | | $ | 587,884 | | $ | 478,550 | |
Total liabilities | | $ | 280,346 | | $ | 273,944 | |
Total stockholders’ equity | | $ | 296,656 | | $ | 204,606 | |
