Filed pursuant to Rule 424(b)(3)
Registration No. 333-149509
MIV THERAPEUTICS, INC.
(A Nevada corporation)
OFFERING OF UP TO 27,010,000 SHARES OF COMMON STOCK
This prospectus relates to the offering of up to 12,835,000 shares of our common stock and up to 14,175,000 shares of our common stock issuable upon the exercise of outstanding warrants to acquire shares of our common stock by the selling shareholders (the "Selling Shareholders") named in this prospectus. These securities were issued in private placement transactions between August 2006 and December 2007, as described in this prospectus under "Selling Shareholders".
We will not receive any of the proceeds from the sale of shares by the Selling Shareholders. However, we will receive proceeds upon the exercise of any common stock purchase warrants offered under this prospectus that may be exercised by the Selling Shareholders. If all of the common stock purchase warrants offered under this prospectus are exercised, we would receive proceeds of $10,326,250.
The Selling Shareholders may sell their shares on any national securities exchange or quotation service on which the securities may be listed or quoted at the time of sale, in the over-the-counter market or in transactions otherwise than on these exchanges or systems or in the over-the-counter market and in one or more transactions at fixed prices, at prevailing market prices at the time of the sale, at varying prices determined at the time of sale, or at negotiated prices. See "Plan of Distribution" for more information.
Our common stock is registered under Section 12(g) of the United StatesSecurities Exchange Act of 1934, as amended, and is quoted on the FINRA Over-the-Counter Bulletin Board (the "OTCBB") under the symbol "MIVT". Our common stock is also quoted on each of the Munich and Frankfurt stock exchanges under the symbols "MIV.MU" and "MIV.F", respectively. The last reported sales price per share of our common stock as reported by the OTCBB on February 28, 2008, was $0.37.
Our principal offices are located at Unit 1, 8765 Ash Street, Vancouver, British Columbia, Canada, V6P 6T3. Our telephone number is (604) 301-9545 and our facsimile number is (604) 301-9546.
The purchase of the securities offered through this prospectus involves a high degree of risk. You should carefully read and consider the section of this prospectus entitled"Risk Factors" beginning on page 7 before buying any of our securities.
The information in this prospectus is not complete and may be changed. The Selling Shareholders may not offer or sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offence.
The date of this prospectus is March 28, 2008.
__________
The following table of contents has been designed to help you find important information contained in this prospectus. We encourage you to read the entire prospectus.
TABLE OF CONTENTS
Item Page No.
REFERENCES | 4 | |
PROSPECTUS SUMMARY | 5 | |
RISK FACTORS | 7 | |
WE HAVE LIMITED PRODUCTS AVAILABLE FOR SALE OR USE AND MAY LACK THE FINANCIAL RESOURCES NEEDED TO BRING SIGNIFICANT QUANTITIES OF OUR PRODUCTS TO MARKET. | 7 | |
BECAUSE WE HAVE A LIMITED OPERATING HISTORY ON WHICH AN EVALUATION OF OUR PROSPECTS CAN BE MADE, WE MAY NOT BE ABLE TO EFFECTIVELY MANAGE THE DEMANDS REQUIRED OF A NEW BUSINESS IN THE MEDICAL DEVICE INDUSTRY. | 8 | |
BECAUSE WE HAVE A HISTORY OF LOSSES AND ANTICIPATE CONTINUED LOSSES THROUGH OUR DEVELOPMENT STAGE, WE MAY LACK THE FINANCIAL STABILITY REQUIRED TO CONTINUE OPERATIONS. | 8 | |
BECAUSE THE LIFE CYCLE OF MEDICAL PRODUCTS ARE DIFFICULT TO PREDICT, EVEN IF WE WERE TO INTRODUCE A PRODUCT TO THE MARKET WE MAY NOT BE ABLE TO GAIN MARKET ACCEPTANCE OF THE PRODUCT. | 8 | |
BECAUSE WE ARE SIGNIFICANTLY SMALLER THAN THE MAJORITY OF OUR NATIONAL COMPETITORS WE MAY LACK THE FINANCIAL RESOURCES NEEDED TO CAPTURE MARKET SHARE. | 8 | |
BECAUSE WE HAVE NOT EARNED ANY SIGNIFICANT REVENUES FROM OPERATIONS, MOST OF OUR CAPITAL REQUIREMENTS HAVE BEEN MET THROUGH FINANCING AND IT IS NOT CERTAIN WE WILL BE ABLE TO CONTINUE TO FIND FINANCING TO MEET OUR OPERATING REQUIREMENTS. | 9 | |
BECAUSE WE ARE IN THE DEVELOPMENT STAGE AND HAVE ONLY PRODUCED LIMITED MARKETABLE PRODUCTS, WE MAY LACK THE ABILITY TO RECRUIT SUITABLE CANDIDATES FOR EMPLOYMENT, OR TO ATTRACT THEM TO THE COMPANY SHOULD THEY BE IDENTIFIED. | 9 | |
BECAUSE WE MAY NOT BE ABLE TO OBTAIN PATENTS FOR THE DEVICES WE ARE CURRENTLY RESEARCHING, WE MAY NOT BE ABLE TO PROTECT OUR INTELLECTUAL PROPERTY RIGHTS. | 9 | |
BECAUSE PRODUCT LIABILITY IS INHERENT IN THE MEDICAL DEVICES INDUSTRY AND INSURANCE IS EXPENSIVE AND DIFFICULT TO OBTAIN, WE MAY BE EXPOSED TO LARGE LAWSUITS. | 9 | |
BECAUSE THE HEALTHCARE INDUSTRY IS SUBJECT TO CHANGING POLICIES AND PROCEDURES, WE MAY FIND IT DIFFICULT TO CONTINUE TO COMPETE IN AN UNCERTAIN ENVIRONMENT. | 10 | |
THERE IS SUBSTANTIAL DOUBT AS TO OUR ABILITY TO CONTINUE AS A GOING CONCERN BASED ON OUR PAST OPERATING LOSSES AND PREDICTED FUTURE OPERATING LOSSES. | 10 | |
WE WILL REQUIRE ADDITIONAL FUNDING IN THE FUTURE. | 10 | |
OUR ACQUISITIONS MAY NOT BE SUCCESSFUL. | 10 | |
A DECLINE IN THE PRICE OF OUR COMMON STOCK COULD AFFECT OUR ABILITY TO RAISE FURTHER WORKING CAPITAL AND ADVERSELY IMPACT OUR OPERATIONS. | 10 | |
SALES OF A SUBSTANTIAL NUMBER OF SHARES OF OUR COMMON STOCK INTO THE PUBLIC MARKET BY THE SELLING SHAREHOLDERS MAY RESULT IN SIGNIFICANT DOWNWARD PRESSURE ON THE PRICE OF OUR COMMON STOCK AND COULD AFFECT THE ABILITY OF OUR STOCKHOLDERS TO REALIZE ANY CURRENT TRADING PRICE OF OUR COMMON STOCK. | 11 | |
BECAUSE OUR STOCK IS QUOTED ON THE OTCBB AND NOT A LARGER OR MORE RECOGNIZED EXCHANGE, INVESTORS MAY FIND IT DIFFICULT TO SELL THEIR SHARES OR OBTAIN ACCURATE QUOTATIONS FOR SHARE PRICES. | 11 | |
A MAJORITY OF OUR DIRECTORS AND OFFICERS ARE OUTSIDE THE UNITED STATES, WITH THE RESULT THAT IT MAY BE DIFFICULT FOR INVESTORS TO ENFORCE WITHIN THE UNITED STATES ANY JUDGMENTS OBTAINED AGAINST US OR ANY OF OUR DIRECTORS OR OFFICERS. | 11 | |
NEVADA LAW AND OUR ARTICLES OF INCORPORATION MAY PROTECT OUR DIRECTORS FROM CERTAIN TYPES OF LAWSUITS. | 12 | |
2
FORWARD-LOOKING STATEMENTS | 12 |
USE OF PROCEEDS | 12 |
SELLING SHAREHOLDERS | 12 |
PLAN OF DISTRIBUTION | 18 |
LEGAL PROCEEDINGS | 20 |
DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS | 21 |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT | 24 |
DESCRIPTION OF SECURITIES | 25 |
LEGAL MATTERS | 26 |
EXPERTS | 26 |
INTERESTS OF NAMED EXPERTS AND COUNSEL | 27 |
DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION FOR SECURITIES ACT LIABILITIES | 27 |
BUSINESS | 27 |
DESCRIPTION OF PROPERTY | 35 |
SELECTED FINANCIAL DATA | 35 |
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | 37 |
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK | 48 |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS | 48 |
MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS | 49 |
EXECUTIVE COMPENSATION | 51 |
FINANCIAL STATEMENTS | 58 |
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS | 59 |
WHERE YOU CAN FIND MORE INFORMATION | 60 |
3
This prospectus is part of a registration statement that we filed with the Securities and Exchange Commission. The registration statement containing this prospectus, including the exhibits to the registration statement, also contains additional information about MIV Therapeutics, Inc. and the securities offered under this prospectus. That registration statement can be read at the Securities and Exchange Commission's website (located at www.sec.gov) or at the Securities and Exchange Commission's Public Reference Room mentioned under the heading "Where You Can Find More Information" of this prospectus.
You should rely only on the information contained in this document or to which we have referred you. We have not authorized anyone to provide you with information that is different. This document may only be used where it is legal to sell these securities. The information in this document may only be accurate on the date of this document. Our business, financial condition or results of operations may have changed since that date.
As used in this prospectus: (i) the terms "we", "us", "our", "MIV", "MIVT" and the "Company" mean MIV Therapeutics, Inc. and its subsidiaries, unless the context otherwise requires; (ii) "SEC" refers to the Securities and Exchange Commission; (iii) "Securities Act" refers to the United States Securities Act of 1933, as amended; (iv) "Exchange Act" refers to the United States Securities Exchange Act of 1934, as amended; and (v) all dollar amounts refer to United States dollars unless otherwise indicated.
__________
4
The following summary highlights selected information contained in this prospectus. This summary does not contain all the information you should consider before investing in our securities. Before making an investment decision, you should read the entire prospectus carefully, including the"Risk Factors" section, the financial statements and the notes to the financial statements.
We are an advanced stage research and development company pursuing the commercialization of the next generation of fully biocompatible coatings for stents and other medical devices and advanced delivery systems with the intent of providing healing solutions for cardiovascular disease and other medical conditions. In collaboration with the University of British Columbia (UBC), we have developed unique coating technologies that utilize Hydroxyapatite (HAp) for application on medical devices and drug delivery systems. Coronary stents are used to treat cardiovascular disorder caused by the narrowing or blockage of coronary arteries. Stents are compressible tubular devices that are mounted on a balloon catheter, inserted into the circulatory system by a team of cardiologists, and directed to the location of a blocked coronary artery. During the angioplasty procedure, which involves unclogging the artery, the balloon is expanded to clear the obstruction, allowing normal blood flow. With this procedure, the stent is deployed and remains in place to reinforce the artery wall. This procedure is the leading alternative to costly and highly invasive open-heart surgery. Stents have eliminated many of the complications that previously accompanied simple balloon angioplasty. Approximately 80% of heart disease can be treated effectively with stenting.
We, in collaboration with UBC, have developed unique coating technologies that utilize HAp for application on medical devices and drug delivery systems. HAp is naturally found in bone and tooth enamel and is rapidly integrated into the human body. As such, it may inhibit a variety of adverse and inflammatory reactions and potentially help reduce restenosis, a recurrence of coronary artery disease following angioplasty. It is also believed that HAp-coated cardiovascular stents will not trigger late stage thrombogenic reactions.
Over the next several years we intend to expand our technologies to include several promising drug delivery platforms. Drug delivery is a system or technology that enables the introduction of a therapeutic agent into the body and improves its efficacy by controlling the rate, time or site of release. Commercially, drug delivery provides the ability to develop a new route of administration for an existing drug and can substantially improve the efficacy of a drug, while also reducing its side effects.
We expect to enter the drug-eluting stent market by using a thicker coating of HAp loaded with a suitable drug, i.e. anti-inflammatory. The technology has applications in cardiovascular and non-cardiovascular drug/device combination products, including peripheral stents, biodegradable implants, gene therapy, and delivery systems for release of chemotherapeutic agents. Our lead product in development is a passive, nano-film HAp coating. In parallel, we are developing polymer free multi-layer film coatings with drug-eluting capabilities to facilitate therapeutics and treatments for localized drug delivery systems.
The Offering
The Issuer: | MIV Therapeutics, Inc. |
The Selling Shareholders: | The Selling Shareholders are comprised of our existing shareholders (i) that acquired units comprised of shares of our common stock and common stock purchase warrants from us pursuant to private placements completed between August 2006 and May 2007 and in August 2007; (ii) that were issued common stock purchase warrants as finder's fees in connection with these offerings; (iii) that were issued common stock purchase warrants between February 2007 and May 2007 pursuant to consulting agreements; and (iv) that were issued common stock purchase warrants between April 2007 and June 2007 in connection with loans made to our Company, all as described in this prospectus under "Selling Shareholders". |
5
Shares Offered by the Selling Shareholders: | The Selling Shareholders are offering up to an aggregate of 27,010,000 shares of common stock, comprised of 12,835,000 shares of our common stock and 14,175,000 shares of our common stock issuable upon exercise of common stock purchase warrants, as described in this prospectus under "Selling Shareholders". |
Offering Price: | The Selling Shareholders may sell their shares on any national securities exchange or quotation service on which the securities may be listed or quoted at the time of sale, in the over-the-counter market or in transactions otherwise than on these exchanges or systems or in the over-the-counter market and in one or more transactions at fixed prices, at prevailing market prices at the time of the sale, at varying prices determined at the time of sale, or at negotiated prices. We will incur substantially all of the costs associated with the filing of this prospectus and the registration statement of which it forms a part. See "Plan of Distribution" for more information. |
Use of Proceeds: | We will not receive any proceeds from this offering. However, we will receive proceeds upon the exercise of any common stock purchase warrants by the Selling Shareholders. If all of the common stock purchase warrants offered hereby are exercised, we would receive proceeds of $10,326,250. The proceeds, if any, would be used for general corporate purposes including, but not limited to, working capital, research and development, equipment purchase and modification, and pre-clinical and clinical trials. |
Market for our Common Stock: | Our common stock is presently traded on the OTCBB under the symbol "MIVT" and is also quoted on each of the Munich and Frankfurt stock exchanges under the symbols "MIV.MU" and "MIV.F", respectively. On February 28, 2008, the closing price of our shares of common stock on the OTCBB was $0.37 per share. |
Outstanding Shares of Common Stock: | There were 115,491,278 shares of our common stock issued and outstanding as at February 28, 2008. If all of the common stock purchase warrants offered under this prospectus are exercised, there would be 129,666,278 shares of our common stock issued and outstanding. |
Risk Factors: | See "Risk Factors" and the other information in this prospectus for a discussion of the factors you should consider before deciding to invest in our securities. |
The following selected financial data has been derived from and should be read in conjunction with (i) our audited consolidated financial statements for the years ended May 31, 2007, 2006 and 2005, together with the notes to these financial statements, (ii) our unaudited interim consolidated financial statements as at and for the six months ended November 30, 2007, together with the notes to these financial statements and (iii) the sections of this prospectus entitled "Management's Discussion and Analysis of Financial Condition and Results of Operations" and "Business".
In 2001, we acquired 58% of the outstanding shares of M-I Vascular resulting in a change of our business to a stent medical device development company. We acquired the remaining shares of M-I Vascular in May 2003. In March 2005, we acquired 100% of SagaX, Inc., which is in the business of developing a neuro-vascular embolic stent filter medical device and, in December 2007, we completed the sale of all of the issued and outstanding shares of Sagax, Inc. In February 2007, we acquired all of the issued and outstanding shares of BioSync Scientific Pvt. Ltd. which is in the business of, among other things, designing, manufacturing and marketing of coated and non-coated vascular stents and related accessories. As a result, the selected financial data provided below may not be comparable from period to period.
Balance Sheet Data
As at | As at | |||||
2007 | 2007 | 2006 | 2005 | 2004 | 2003 | |
Cash and cash equivalents | $6,061,952 | $473,419 | $1,573,822 | $492,709 | $2,034,530 | $11,614 |
Working capital (deficiency) | 5,904,159 | (718,679) | 1,521,384 | (478,359) | 2,118,069 | (362,024) |
Total assets | 10,342,690 | 4,246,965 | 2,053,875 | 861,205 | 2,480,074 | 586,097 |
Total liabilities | 1,563,166 | 2,566,968 | 221,314 | 1,063,449 | 184,456 | 1,085,942 |
Total stockholders' equity (deficit) | 8,779,534 | 1,679,997 | 1,832,561 | (202,244) | 2,295,618 | (499,845) |
Six Months Ended | Fiscal Years Ended | ||||||
2007 | 2006 | 2007 | 2006 | 2005 | 2004 | 2003 | |
Net revenue | $551,641 | $Nil | $191,490 | $Nil | $Nil | $Nil | $Nil |
Gross profit | 58,577 | Nil | 44,350 | Nil | Nil | Nil | Nil |
Expenses | 6,889,717 | 4,128,365 | 10,522,388 | 9,161,954 | 6,559,013 | 4,013,845 | 3,183,685 |
Loss from operations | (6,831,140) | (4,128,365) | (10,478,038) | (9,161,954) | (6,559,013) | (4,013,845) | (3,183,685) |
Net loss | (6,558,572) | (4,144,987) | (10,499,471) | (9,094,835) | (6,608,882) | (3,471,891) | (3,173,410) |
Basic and diluted loss per share | (0.06) | (0.06) | (0.15) | (0.14) | (0.15) | (0.11) | (0.17) |
An investment in our common stock involves a number of very significant risks. You should carefully consider the following risks and uncertainties in addition to other information in this prospectus in evaluating our company and our business before purchasing shares of our common stock. Our business, operating results and financial condition could be seriously harmed due to any of the following risks. The risks described below are not all of the risks facing our company. Additional risks not presently known to us or that we currently consider immaterial may also impair our business operations. You may lose all or part of your investment due to any of these risks.
We have limited products available for sale or use and may lack the financial resources needed to bring significant quantities of our products to market.
We are in the development stage and currently have limited products approved for sale or use. We will not be able to sell significant quantities of our products until such time, if ever, as we receive regulatory approval to commercially market such products. Thus, our long-term viability, growth and profitability will depend upon successful testing, approval and commercialization of the coating technology resulting from our research and development activities. Adverse or inconclusive results in clinical trials of these products could significantly delay or ultimately preclude any regulatory approvals and, even if obtained, there can be no assurance that any product approval would lead to the successful commercialization of the product approved.
Furthermore, we do not expect to begin the regulatory approval process in the United States for at least the next three years and, prior to this, will only pursue approval and marketing of our products in the countries recognizing the CE Mark; such as most European and Asian countries.
Because we have a limited operating history on which an evaluation of our prospects can be made, we may not be able to effectively manage the demands required of a new business in the medical device industry.
We have a limited operating history upon which an evaluation of our prospects can be made. There can be no assurance that we will effectively execute our business plan or manage any growth of our business, or that our future operating and financial forecast will be met. Future development and operating results will depend on many factors, including access to adequate capital, the completion and regulatory approval of marketable products, the demand for our products, the level of product and price competition, our success in setting up and expanding distribution channels and whether we can control costs. Many of these factors are beyond our control. In addition, our future prospects must be considered in light of the risks, expenses and difficulties frequently encountered in establishing a new business in the medical device industry, which is characterized by intense competition, rapid technological change, highly litigious competitors, potential product liability and significant re gulation.
Because we have a history of losses and anticipate continued losses through our development stage, we may lack the financial stability required to continue operations.
Since inception we have suffered recurring losses totalling $49,030,164 as of November 30, 2007. We have funded our operations through the issuance of common stock, and through related party loans since inception, in order to meet our strategic objectives. We anticipate that losses will continue until such time, if ever, as we are able to generate sufficient revenues to support our operations. Our ability to generate revenue primarily depends on our success in completing development and obtaining regulatory approvals for the commercial sale of the products under development. There are no assurances that any such events will occur, that we will attain revenues from commercialization of our products or that we will ever achieve profitable operations.
Because the life cycle of medical products are difficult to predict, even if we were to introduce a product to the market we may not be able to gain market acceptance of the product.
The life cycle of the products that we plan to develop is difficult to predict. Failure to gain timely market acceptance of our products would have a material adverse effect on our ability to generate revenue, and would have a material adverse effect on our business, financial condition and results of operations. To successfully gain market acceptance, we must develop the ability to manufacture our products in large quantities in compliance with regulatory requirements and at an acceptable cost. We have no long-term experience in manufacturing stent products, and could experience difficulties in development or manufacturing that may have a material adverse effect on our ability to market our products. Moreover, there can be no assurance that we will be successful in scaling up manufacturing operations sufficient to produce our products in sufficient volume to generate market acceptance.
Because we are significantly smaller than the majority of our national competitors we may lack the financial resources needed to capture market share.
The markets in which we operate and intend to operate are dominated by several large firms with established products, and our success is dependant upon acceptance of our products by the medical community as reliable, safe and cost-effective. It may be difficult or impossible for us to achieve such acceptance of our products in view of these market conditions. In addition, our competitors are more financially stable than we are and have significant resources for research and development available to them. Thus it is likely that they will be quicker to market than us, with products that will compete with our products, should they be successfully approved and commercialized. Moreover, even if we successfully bring our products to market ahead of our projected competitors, established competitors could quickly bring products to market that would compete. In addition, the medical device market is subject to constant introduction of new products and designs.
Market acceptance of our products may be influenced by new products or technologies that come to market, which could render our products obsolete or prohibitively expensive.
Because we have not earned any significant revenues from operations, most of our capital requirements have been met through financing and it is not certain we will be able to continue to find financing to meet our operating requirements.
Our capital requirements have been and will continue to be significant. We will be dependant on future financing to fund our research and development as well as other working capital requirements. We estimate that our current working capital will support our activities through the first quarter of fiscal 2009. After that time we will need additional financing. We are currently anticipating further subscriptions for our common stock, but there is no assurance that these subscriptions will be forthcoming or that they will result in sufficient capital to meet our current and expected working capital needs. It is not anticipated that any of our officers, directors or current shareholders will provide any significant portion of our future financing requirements.
Furthermore, in the event that our plans change, our assumptions change or prove inaccurate, or our capital resources prove to be insufficient to fund operations, we could be required to seek additional financing sooner than currently anticipated, or in greater amounts than is currently anticipated. Any inability to obtain additional financing when needed would have a material adverse effect on us, including possibly requiring us to significantly curtail or possibly cease our operations. In addition, any future equity financing may involve substantial dilution to our existing shareholders.
Because we are in the development stage and have only produced limited marketable products, we may lack the ability to recruit suitable candidates for employment, or to attract them to the company should they be identified.
We currently have approximately 110 full-time employees and only three full-time officers. We have entered into consulting agreements with three individuals, who are also officers and directors, to provide management services to us. The remainder of our management has been undertaken by independent consultants. This may make it difficult for us to attract capital investment sufficient to meet our capital needs.
Because we are in the development stage and have only produced limited marketable products, we will be reliant upon our ability to attract skilled members of the stent or medical products' industries. There can be no assurance that we will be able to identify suitable candidates for employment, or to attract them to us should they be identified. In addition, we will be heavily dependent upon creative design and engineering skills of individuals with whom we have little familiarity, and who may not perform as expected.
Because we may not be able to obtain patents for the devices we are currently researching, we may not be able to protect our intellectual property rights.
Our success will depend in part on whether we can obtain patent protection for our products and processes, preserve trade secrets and proprietary technology and operate without infringing upon patent or other proprietary rights of third parties. We have patent applications pending in the United States and in several foreign markets, and are in the process of filing additional patent applications, but there can be no assurance that any of these patents will be issued or that patents will not be challenged. A significant number of medical device companies, other companies, universities and research institutions have filed patent applications or have been issued patents relating to stents and stent delivery systems, and there has been substantial litigation in this area. Established companies in the medical products industry generally, and the stent industry in particular, are aggressive in attempts to block new entrants to their markets, and our products, if successfully developed, may interfere with the intellectual property rights of these companies. Our success will depend on our products not infringing patents that we expect would be vigorously defended. Furthermore, the validity and breadth of claims in medical technology patents involve complex legal and factual questions and, therefore, are highly uncertain.
Because product liability is inherent in the medical devices industry and insurance is expensive and difficult to obtain, we may be exposed to large lawsuits.
Our business exposes us to potential product liability risks, which are inherent in the testing, manufacturing, marketing and sale of medical products. While we will take precautions we deem to be appropriate to avoid product liability suits against us, there can be no assurance that we will be able to avoid significant product liability exposure. Product liability insurance for the medical products industry is generally expensive, to the extent it is available at all. We have not yet sought to obtain product liability coverage. We intend to obtain such coverage when it is apparent that the MIV Stent or other products developed by us will be marketable. There can be no assurance that it will be able to obtain such coverage on acceptable terms, or that any insurance policy will provide adequate protection against potential claims. A successful product liability claim brought against us may exceed any insurance coverage secured by us and could have a material adverse effect on our r esults or ability to continue marketing our products.
Because the healthcare industry is subject to changing policies and procedures, we may find it difficult to continue to compete in an uncertain environment.
The health care industry is subject to changing political, economic and regulatory influences that may affect the procurement practices and operations of healthcare industry participants. During the past several years government regulation of the healthcare industry has changed significantly in several countries. Healthcare industry participants may react to new policies by curtailing or deferring use of new treatments for disease, including treatments that would use the products that we intend to develop. This could substantially impair our ability to successfully commercialize the MIV Stent, which would have a material adverse effect on our performance.
There is substantial doubt as to our ability to continue as a going concern based on our past operating losses and predicted future operating losses.
Our auditor has issued a going concern opinion on our financial statements expressing substantial doubt that we can continue as a going concern for a reasonable period of time unless sufficient equity financing can be secured or sufficient revenues to support our operations is generated.
There are no assurances that we will be successful in achieving these goals.
We will require additional funding in the future.
Based upon our historical losses from operations, we will require additional funding in the future. If we cannot obtain capital through financings or otherwise, our ability to execute our research and development plans will be greatly limited. Historically, we have funded our operations through the issuance of equity and short-term debt financing arrangements. We may not be able to obtain additional financing on commercial terms, if at all. Further, debt financing could lead to a diversion of cash flow to satisfy debt-servicing obligations and create restrictions on business operations. If we are unable to raise additional funds, it would have a material adverse effect upon our operations.
Our acquisitions may not be successful.
As part of our growth strategy, we intend to acquire additional companies and assets. Such acquisitions may pose substantial risks to our business, financial condition, and results of operations. In pursuing acquisitions, we will compete with other companies, many of which have greater financial and other resources to acquire attractive companies and assets. Even if we are successful in acquiring additional companies and assets, some of the companies and assets may not produce revenues at anticipated levels or within specified time periods. There is no assurance that we will be able to successfully integrate acquired companies and assets, which could result in substantial costs and delays or other operational, technical or financial problems. Further, acquisitions could disrupt ongoing business operations. If any of these events occur, it would have a material adverse effect upon our operations and results from operations.
A decline in the price of our common stock could affect our ability to raise further working capital and adversely impact our operations.
A decline in the price of our common stock could result in a reduction in the liquidity of our common stock and a reduction in our ability to raise additional capital for our operations. Because our operations to date have been principally financed through the sale of equity securities, a decline in the price of our common stock could have an adverse effect upon our liquidity and our continued operations. A reduction in our ability to raise equity capital in the future would have a material adverse effect upon our business plans and operations, including our ability to continue our current operations. If our stock price declines, we may not be able to raise additional capital or generate funds from operations sufficient to meet our obligations.
Sales of a substantial number of shares of our common stock into the public market by the Selling Shareholders may result in significant downward pressure on the price of our common stock and could affect the ability of our stockholders to realize any current trading price of our common stock.
Sales of a substantial number of shares of our common stock in the public market could cause a reduction in the market price of our common stock. We had 115,491,278 shares of common stock outstanding as at February 28, 2008. When this registration statement is declared effective, the Selling Shareholders will be able to resell up to 27,010,000 shares of our common stock. As a result, a substantial number of our shares of common stock may be issued and may be available for immediate resale, which could have an adverse effect on the price of our common stock. As a result of any such decreases in the price of our common stock, purchasers who acquire shares from the Selling Stockholders may lose some or all of their investment.
Further, to the extent any of the Selling Shareholders exercise any of the common stock purchase warrants, and then resell the shares of common stock issued to them upon such exercise (subject to applicable securities law restrictions), the price of our common stock may decrease due to the additional shares of common stock in the market.
Any significant downward pressure on the price of our common stock as the Selling Shareholders sell their shares of our common stock could encourage short sales by the Selling Stockholders or others. Any such short sales could place further downward pressure on the price of our common stock.
In addition, stock markets have experienced extreme price and volume fluctuations and the market prices of securities have been highly volatile. These fluctuations are often unrelated to operating performance and may adversely affect the market price of our common stock. As a result, investors may be unable to resell their shares at a fair price and may lose all or part of their investment.
Because our stock is quoted on the OTCBB and not a larger or more recognized exchange, investors may find it difficult to sell their shares or obtain accurate quotations for share prices.
Our common stock is quoted on the OTCBB. Investors may find it more difficult to dispose of, or to obtain accurate quotations as to the market value of, our common stock than would otherwise be the case were our common stock listed or quoted on a more recognized stock exchange or quotation service. In addition, trading in the Company's common stock is currently subject to certain rules under the Exchange Act, which require additional disclosure by broker-dealers in connection with any trades involving a stock defined as a "penny stock." Penny stocks are generally non-NASDAQ equity securities with a market price less than $5.00 per share. The penny stock rules require broker-dealers selling penny stocks to make certain disclosures about such stocks to purchasers thereof, and impose sales practice restrictions on broker-dealers in certain penny stock transactions. The additional burdens imposed upon broker-dealers by these rules may discourage them from effecting transactions in our common stock, which could limit the liquidity of the common stock and the ability of our stockholders to sell their stock in the secondary market.
A majority of our directors and officers are outside the United States, with the result that it may be difficult for investors to enforce within the United States any judgments obtained against us or any of our directors or officers.
A majority of our directors and officers are nationals and/or residents of countries other than the United States, and all or a substantial portion of such persons' assets are located outside the United States. As a result, it may be difficult for investors to effect service of process on our directors or officers, or enforce within the United States or Canada any judgments obtained against us or our officers or directors, including judgments predicated upon the civil liability provisions of the securities laws of the United States or any state thereof. Consequently, investors may be effectively prevented from pursuing remedies under U.S. federal securities laws against them. In addition, investors may not be able to commence an action in a Canadian court predicated upon the civil liability provisions of the securities laws of the United States. The foregoing risks also apply to those experts identified in this prospectus who are not residents of the United States.
Nevada law and our Articles of Incorporation may protect our directors from certain types of lawsuits.
Nevada law provides that our officers and directors will not be liable to us or our stockholders for monetary damages for all but certain types of conduct as officers and directors. Our Bylaws permit us broad indemnification powers to all persons against all damages incurred in connection with our business to the fullest extent provided or allowed by law. The exculpation provisions may have the effect of preventing stockholders from recovering damages against our officers and directors caused by their negligence, poor judgment or other circumstances. The indemnification provisions may require us to use our limited assets to defend our officers and directors against claims, including claims arising out of their negligence, poor judgment, or other circumstances.
Please read this prospectus carefully. You should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with different information. You should not assume that the information provided by the prospectus is accurate as of any date other than the date on the front of this prospectus.
This prospectus contains forward-looking statements that involve risks and uncertainties. Such forward-looking statements involve risks and uncertainties regarding the availability of funds, government regulations, operating costs, outcomes of research programs and other factors. Forward-looking statements are made, without limitation, in relation to operating plans, business research and development, availability of funds and operating costs. Any statements contained herein that are not statements of historical facts may be deemed to be forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as "may", "will", "should", "expect", "plan", "intend", "anticipate", "believe", "estimate", "predict", "potential" or "continue", the negative of such terms or other comparable terminology. Actual events or results may differ materially. In evaluating these statements, you should consider various factors, including the risks outlined in thi s prospectus. These factors may cause our actual results to differ materially from any forward-looking statement. While these forward-looking statements, and any assumptions upon which they are based, are made in good faith and reflect our current judgment regarding our business plans, our actual results will almost always vary, sometimes materially, from any estimates, predictions, projections, assumptions or other future performance suggested herein. We do not intend to update any of the forward-looking statements to conform these statements to actual results, except as required by applicable law, including the securities laws of the United States.
The safe harbour for forward-looking statements provided in thePrivate Securities Litigation Reform Act of 1995 does not apply to the offering made in this prospectus.
We will not receive any proceeds from the sale of the shares of common stock offered through this prospectus by the Selling Shareholders. All proceeds from the sale of the shares will be for the account of the Selling Shareholders. However, we may receive up to $10,326,250 from the exercise of the warrants held by the Selling Shareholders if all such warrants are exercised. The proceeds, if any, would be used for general corporate purposes including, but not limited to, working capital, research and development, equipment purchase and modification, and pre-clinical and clinical trials. We will, however, incur substantially all costs associated with the filing of this prospectus and the registration statement of which it forms a part.
The foregoing represents the Company's intentions based upon our present plans and business conditions. The occurrence of unforeseen events or changes in business conditions, however, could result in applying the proceeds from the exercise of the warrants in a manner other than as described in this prospectus.
The Selling Shareholders named in this prospectus are offering all of the 27,010,000 shares of common stock offered through this prospectus. The Selling Shareholders are comprised of our existing shareholders (i) that purchased units from us comprised of shares of our common stock and shares issuable upon exercise of common stock purchase warrants; (ii) that were issued common stock purchase warrants as finder's fees in connection with these offerings; (iii) that were issued common stock purchase warrants pursuant to consulting agreements; and (iv) that were issued common stock purchase warrants pursuant to loans made to our Company, as follows:
12
On August 21, 2006, we issued an aggregate of 290,000 units at a price of $0.50 per unit, with each unit consisting of one share of our common stock and one common stock purchase warrant to certain of the Selling Shareholders named herein. Each warrant entitles the holder to purchase one share of our common stock at an exercise price of $0.75 per share for a period ending on the earlier of 18 months from the date of issuance of the units and 12 months from the effective date of the registration statement of which this prospectus forms a part.
On October 16, 2006, we issued an aggregate of 600,000 units at a price of $0.50 per unit, with each unit consisting of one share of our common stock and one common stock purchase warrant to certain of the Selling Shareholders named herein. Each warrant entitles the holder to purchase one share of our common stock at an exercise price of $0.75 per share for a period of two years from the effective date of the registration statement of which this prospectus forms a part.
On November 8, 2006, we issued an aggregate of 1,400,000 units at a price of $0.50 per unit, with each unit consisting of one share of our common stock and one common stock purchase warrant to certain of the Selling Shareholders named herein. Each warrant entitles the holder to purchase one share of our common stock at an exercise price of $0.75 share for a period of two years from the effective date of the registration statement of which this prospectus forms a part.
On December 22, 2006, we issued an aggregate of 5,900,000 units at a price of $0.50 per unit, with each unit consisting of one share of our common stock and one common stock purchase warrant to certain of the Selling Shareholders named herein. Each warrant entitles the holder to purchase one share of our common stock at an exercise price of $0.75 per share. Warrants to acquire up to 40,000 shares of our common stock are exercisable for a period ending on the earlier of 18 months from the date of issuance of the units or 12 months from the effective date of the registration statement of which this prospectus forms a part, and warrants to acquire up to 5,860,000 shares are exercisable for a period of two years from the effective date of the registration statement of which this prospectus forms a part.
On February 8, 2007, we issued an aggregate of 1,125,000 units at a price of $0.50 per unit, with each unit consisting of one share of our common stock and one common stock purchase warrant to certain of the Selling Shareholders named herein. Each warrant entitles the holder to purchase one share of our common stock at an exercise price of $0.75 share for a period of two years from the effective date of the registration statement of which this prospectus forms a part.
On February 27, 2007, we issued an aggregate of 375,000 units at a price of $0.50 per unit, with each unit consisting of one share of our common stock and one common stock purchase warrant to certain of the Selling Shareholders named herein. Each warrant entitles the holder to purchase one share of our common stock at an exercise price of $0.75 share for a period of two years from the effective date of the registration statement of which this prospectus forms a part.
On April 4, 2007, we issued an aggregate of 830,000 units at a price of $0.50 per unit, with each unit consisting of one share of common stock and one common stock purchase warrant to certain of the Selling Shareholders named herein. Each warrant entitles the holder to purchase one share of our common stock at an exercise price of $0.75 share for a period of two years from the effective date of the registration statement of which this prospectus forms a part.
On May 8, 2007, we issued an aggregate of 1,790,000 units at a price of $0.50 per unit, with each unit consisting of one share of our common stock and one common stock purchase warrant to certain of the Selling Shareholders named herein. Each warrant entitles the holder to purchase one share of our common stock at an exercise price of $0.75 per share for a period of two years from the effective date of the registration statement of which this prospectus forms a part.
On August 31, 2007, we issued an aggregate of 525,000 units at a price of $0.50 per unit, with each unit consisting of one share of our common stock and one common stock purchase warrant to certain of the Selling Shareholders named herein. Each warrant entitles the holder to purchase one share of our common stock. Warrants to acquire up to 475,000 shares of our common stock are exercisable at a price of $0.75 per share for a period of two years from the effective date of the registration statement of which this prospectus forms a part, and warrants to acquire up to 50,000 shares of our common stock are exercisable at a price of $0.70 per share for a period of five years from the date of issuance of the units.
13
In connection with the private placements described above, we also issued warrants to acquire up to 690,000 shares of our common stock to certain of the Selling Shareholders named herein as finder's fees as follows: (i) on February 8, 2007 and February 27, 2007, we issued warrants to acquire up to 112,500 and 37,500 shares of our common stock, respectively, exercisable for a period of two years from the effective date of the registration statement of which this prospectus forms a part at a price of $0.75 per share; (ii) on August 31, 2007, we issued warrants to acquire up to 400,000 shares of our common stock exercisable until May 31, 2010 at a price of $0.55 per share; and (iii) on December 10, 2007, we issued warrants to acquire up to 140,000 shares of our common stock exercisable until December 10, 2010 at a price of $0.55 per share. None of the finders has a material relationship with us. In addition, none of the finders was a U.S. person (as defined in Regulat ion S under the Securities Act) or a person in the United States at the time of their involvement as finders in connection with offerings of securities of the Company and there were no offerings of securities of the Company made by the finders to U.S. persons or to any person in the United States.
We issued warrants to acquire up to 300,000 shares of our common stock pursuant to consulting agreements to certain of the Selling Shareholders named herein as follows: (i) on February 1, 2007, we issued warrants to acquire up to 100,000 shares of our common stock exercisable until February 1, 2012 at a price of $0.60 per share; (ii) on April 4, 2007, we issued warrants to acquire up to 100,000 shares of our common stock exercisable until April 4, 2012 at a price of $0.50 per share; and (iii) on May 1, 2007, we issued warrants to acquire up to 100,000 shares of our common stock exercisable until May 1, 2012 at a price of $0.50 per share.
In connection with loans made to our Company in April and June 2007 in the aggregate amount of $525,000, we issued warrants to acquire up to 350,000 shares of our common stock as follows: (i) on April 27, 2007, we issued warrants to acquire up to 50,000 shares of our common stock exercisable until April 27, 2010 at a price of $0.60 per share; (ii) on May 30, 2007, we issued warrants to acquire up to 150,000 shares of our common stock exercisable until May 30, 2010 at a price of $0.60 per share in connection with the extension of the term of the loan; and (iii) on June 29, 2007, we issued warrants to acquire up to 150,000 shares of our common stock exercisable until April 27, 2010 at a price of $0.60 per share.
The following table provides, as of February 28, 2008, information regarding the beneficial ownership of our shares of common stock held by each of the Selling Shareholders, including:
1. the number of shares owned by each Selling Shareholder prior to this offering;
2. the total number of shares that are to be offered by each Selling Shareholder;
3. the total number of shares that will be owned by each Selling Shareholder upon completion of the offering;
4. the percentage of our common stock owned by each Selling Shareholder; and
5. the identity of the beneficial holder of any entity that owns the shares.
Information with respect to beneficial ownership is based upon information obtained from the Selling Shareholders. Information with respect to "Shares Beneficially Owned Prior to this Offering" includes the shares issuable upon exercise of the warrants held by the Selling Shareholders acquired pursuant to the private placement transactions de7scribed above as these warrants are exercisable within 60 days of February 28, 2008. The "Number of Shares Being Offered" includes the shares acquired by the Selling Shareholders in the private placements described above and the shares that are issuable upon exercise of the warrants acquired by the Selling Shareholders in such private placements. Information with respect to "Shares Beneficially Owned After this Offering" assumes the sale of all of the shares offered by this prospectus and no other purchases or sales of our shares by the Selling Shareholders. Except as described below and to our knowledge, each named Selling Shareholder beneficiall y owns and has sole voting and investment power over all shares or rights to these shares owned by it. Except for their ownership of common stock described below, none of the Selling Shareholders had or have any material relationship with us. The Selling Shareholders may have sold or transferred, in transactions exempt from the registration requirements of the Securities Act, some or all of the common stock held by them since the date as of which information is presented below.
14
Because a Selling Shareholder may offer by this prospectus all or some part of the shares which it holds, no estimate can be given as of the date hereof as to the number of common shares actually to be offered for sale by a Selling Shareholder or as to the number of shares that will be held by a Selling Shareholder upon the termination of such offering.
Shares Beneficially OwnedPrior To This Offering(1) | Number of Shares BeingOffered | Shares Beneficially OwnedAfterThis Offering(1) | |||
Name of Selling Shareholder(1) | Number | Percentage(2) | Number | Number | Percentage(2) |
August 2006 Private Placement | |||||
Douglas Casey | 200,000 | * | 200,000 | Nil | Nil |
Peter Schrobenhauser | 100,000 | * | 100,000 | Nil | Nil |
Haywood Securities Inc. In Trust For David Lyall(3) | 200,000 | * | 200,000 | Nil | Nil |
Michael Price | 80,000 | * | 80,000 | Nil | Nil |
October 2006 Private Placement | |||||
Luye Li | 1,200,000 | 1.03% | 1,200,000 | Nil | Nil |
November 2006 Private Placement | |||||
Suzhi Chen | 2,800,000 | 2.40% | 2,800,000 | Nil | Nil |
December 2006 Private Placement | |||||
Kiam Ehsam | 80,000 | * | 80,000 | Nil | Nil |
Smith & Williamson Investment Management Limited(4) | 600,000 | * | 600,000 | Nil | Nil |
Avendis Absolute Alternative 1 Trading Ltd.(5) | 1,600,000 | 1.38% | 1,600,000 | Nil | Nil |
FIM Asset Management Ltd.(6) | 4,800,000 | 4.08% | 4,800,000 | Nil | Nil |
Result Internacional S.A.(7) | 200,000 | * | 200,000 | Nil | Nil |
Credit Suisse Client Nominees (UK) Limited(8) | 480,000 | * | 480,000 | Nil | Nil |
Michael Price | 40,000 | * | 40,000 | Nil | Nil |
Luye Li | 2,800,000 | 2.40% | 2,800,000 | Nil | Nil |
Suzhi Chen | 1,040,000 | * | 1,040,000 | Nil | Nil |
Alan Chu | 60,000 | * | 60,000 | Nil | Nil |
Joseph Chu | 60,000 | * | 60,000 | Nil | Nil |
Fabian Hope | 40,000 | * | 40,000 | Nil | Nil |
February 8, 2007 Private Placement | |||||
VC Group Investments, S.A.(9) | 2,250,000 | 1.93% | 2,250,000 | Nil | Nil |
Verdmont Capital S.A.(10) | 112,500(11) | * | 112,500 | Nil | Nil |
15
Shares Beneficially OwnedPrior To This Offering(1) | Number of Shares BeingOffered | Shares Beneficially OwnedAfterThis Offering(1) | |||
Name of Selling Shareholder(1) | Number | Percentage(2) | Number | Number | Percentage(2) |
February 27, 2007 Private Placement | |||||
New Paradigm Capital Ltd.(12) | 711,722 | * | 711,722 | Nil | Nil |
Verdmont Capital S.A.(10) | 38,278 | * | 38,278 | Nil | Nil |
Verdmont Capital S.A.(10) | 37,500(11) | * | 37,500 | Nil | Nil |
April 2007 Private Placement | |||||
Peter John Burningham | 1,000,000 | * | 1,000,000 | Nil | Nil |
James D. Davidson | 400,000 | * | 400,000 | Nil | Nil |
Jean-Claude Doucet | 20,000 | * | 20,000 | Nil | Nil |
Rowan Fleming | 40,000 | * | 40,000 | Nil | Nil |
Kristian Andresen | 200,000 | * | 200,000 | Nil | Nil |
May 2007 Private Placement | |||||
David Cleave | 240,000 | * | 240,000 | Nil | Nil |
Stephen McCreery | 800,000 | * | 800,000 | Nil | Nil |
Sam Oliver | 40,000 | * | 40,000 | Nil | Nil |
Credit Suisse Client Nominees (UK) Limited(8) | 500,000 | * | 500,000 | Nil | Nil |
Scott Wilson | 800,000 | * | 800,000 | Nil | Nil |
Carolyn Huston | 200,000 | * | 200,000 | Nil | Nil |
Anthony Huston | 200,000 | * | 200,000 | Nil | Nil |
Kelly J. Morrow | 800,000 | * | 800,000 | Nil | Nil |
August 2007 Private Placement | |||||
Susan Baum | 4,000 | * | 2,000 | Nil | Nil |
Sean Milliken | 8,000 | * | 8,000 | Nil | Nil |
Matthew Swanson | 10,000 | * | 10,000 | Nil | Nil |
Raymond Rutherford | 80,000 | * | 80,000 | Nil | Nil |
Bryan Elmes | 48,000 | * | 48,000 | Nil | Nil |
Jamie Jenkins | 160,000 | * | 160,000 | Nil | Nil |
Richard Blain Morrow | 200,000 | * | 200,000 | Nil | Nil |
Erin Oldham | 200,000 | * | 200,000 | Nil | Nil |
Timothy Knight | 200,000 | * | 200,000 | Nil | Nil |
Gordon Stewart Dalzell | 40,000 | * | 40,000 | Nil | Nil |
Alejandro Caraveo V. | 100,000 | * | 100,000 | Nil | Nil |
Stephen James Cooper | 140,000(11) | * | 140,000 | Nil | Nil |
16
Shares Beneficially OwnedPrior To This Offering(1) | Number of Shares BeingOffered | Shares Beneficially OwnedAfterThis Offering(1) | |||
Name of Selling Shareholder(1) | Number | Percentage(2) | Number | Number | Percentage(2) |
Zurban International(13) | 400,000(11) | * | 400,000 | Nil | Nil |
Consulting Agreement Warrants | |||||
Raoul Bonan | 100,000 | * | 100,000 | Nil | Nil |
TGR Group LLC(14) | 100,000 | * | 100,000 | Nil | Nil |
Anthony Huston | 100,000 | * | 100,000 | Nil | Nil |
Loan Warrants | |||||
Copper Eagle, Inc.(15) | 350,000 | * | 350,000 | Nil | Nil |
Totals | 27,010,000 | 27,010,000 | Nil | Nil | |
* Less than 1%.
(1) Under Rule 13d-3, a beneficial owner of a security includes any person who, directly or indirectly, through any contract, arrangement, understanding, relationship, or otherwise has or shares: (i) voting power, which includes the power to vote, or to direct the voting of shares; and (ii) investment power, which includes the power to dispose or direct the disposition of shares. Certain shares may be deemed to be beneficially owned by more than one person (if, for example, persons share the power to vote or the power to dispose of the shares). In addition, shares are deemed to be beneficially owned by a person if the person has the right to acquire the shares (for example, upon exercise of an option) within 60 days of the date as of which the information is provided. In computing the percentage ownership of any person, the amount of shares outstanding is deemed to include the amount of shares beneficially owned by such person (and only such person) by reaso n of these acquisition rights. As a result, the percentage of outstanding shares of any person as shown in this table does not necessarily reflect the person's actual ownership or voting power with respect to the number of shares of common stock actually outstanding on February 28, 2008.
(2) The applicable percentage of ownership is based on 115,491,278 shares of common stock outstanding as of February 28, 2008.
(3) David Lyall, managing member of Haywood Securities Inc., has discretionary authority to purchase, vote and dispose of these securities. Mr. Lyall disclaims beneficial ownership as to such securities except to the extent of his pecuniary interests therein.
(4) Mark Boucher, managing member of Smith & Williamson Investment Management Limited, has discretionary authority to purchase, vote and dispose of these securities. Mr. Boucher disclaims beneficial ownership as to such securities except to the extent of his pecuniary interests therein.
(5) Yannis Bilquez, managing member of Aventis Absolute Alternative I Trading Ltd., has discretionary authority to purchase, vote and dispose of the securities on behalf of Aventis Absolute Alternative I Trading Ltd. Mr. Bilquez disclaims beneficial ownership as to such securities except to the extent of his pecuniary interests therein.
(6) Karkku Kaloniemi, managing member of FIM Asset Management Ltd., has discretionary authority to purchase, vote and dispose of these securities. Mr. Kaloniemi disclaims beneficial ownership as to such securities except to the extent of his pecuniary interests therein.
(7) David LeMare, managing member of Result Internacional C.A., has discretionary authority to purchase, vote and dispose of the securities on behalf of Result Internacional C.A. Mr. LeMare disclaims beneficial ownership as to such securities except to the extent of his pecuniary interests therein.
(8) Richard Hopper, managing member of Credit Suisse Client Nominees (UK) Limited, has discretionary authority to purchase, vote and dispose of the securities on behalf of Credit Suisse Client Nominees (UK) Limited. Mr. Hopper disclaims beneficial ownership as to such securities except to the extent of his pecuniary interests therein.
(9) Plutarco Cohen, managing member of VC Group Investments, S.A., has discretionary authority to purchase, vote and dispose of the securities on behalf of VC Group Investments, S.A. Mr. Cohen disclaims beneficial ownership as to such securities except to the extent of his pecuniary interests therein.
(10) Glynn Fisher, managing member of Verdmont Capital S.A., has discretionary authority to purchase, vote and dispose of the securities on behalf of Verdmont Capital S.A. Mr. Fisher disclaims beneficial ownership as to such securities except to the extent of his pecuniary interests therein.
(11) Represents shares of common stock issuable upon exercise of warrants issued as finder's fees in connection with the private placements described herein.
(12) Jonathan Lindsay, managing member of New Paradigm Capital Ltd., has discretionary authority to purchase, vote and dispose of the securities on behalf of New Paradigm Capital Ltd. Mr. Lindsay disclaims beneficial ownership as to such securities except to the extent of his pecuniary interests therein. Jonathan Lindsay is the son of Alan Lindsay, one of our directors.
(13) Jonathan Curreri, managing member of Zurban International, has discretionary authority to purchase, vote and dispose of the securities on behalf of Zurban International. Mr. Curreri disclaims beneficial ownership as to such securities except to the extent of his pecuniary interests therein.
(14) Joji Mangubat, managing member of TGR Group LLC, has discretionary authority to purchase, vote and dispose of the securities. Mr. Mangubat disclaims beneficial ownership as to such securities except to the extent of his pecuniary interests therein.
17
(15) Meyvis Sanchez, managing member of Copper Eagle, Inc., has discretionary authority to purchase, vote and dispose of these securities. Ms. Sanchez disclaims beneficial ownership as to such securities except to the extent of his pecuniary interests therein.
The Selling Shareholders may sell all or a portion of the shares of common stock beneficially owned by them and offered hereby from time to time directly or through one or more underwriters, broker-dealers or agents. If the shares of common stock are sold through underwriters or broker-dealers, the Selling Shareholders will be responsible for underwriting discounts or commissions or agent's commissions. The shares of common stock may be sold on any national securities exchange or quotation service on which the securities may be listed or quoted at the time of sale, in the over-the-counter market or in transactions otherwise than on these exchanges or systems or in the over-the-counter market and in one or more transactions at fixed prices, at prevailing market prices at the time of the sale, at varying prices determined at the time of sale, or at negotiated prices. These sales may be effected in transactions, which may involve crosses or block transactions. The Selling Shareholders may use any one or more of the following methods when selling shares:
- ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers;
- block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction;
- purchases by a broker-dealer as principal and resale by the broker-dealer for its account;
- an exchange distribution in accordance with the rules of the applicable exchange;
- privately negotiated transactions;
- settlement of short sales entered into after the effective date of the registration statement of which this prospectus is a part;
- broker-dealers may agree with the Selling Shareholders to sell a specified number of such shares at a stipulated price per share;
- through the writing or settlement of options or other hedging transactions, whether such options are listed on an options exchange or otherwise;
- a combination of any such methods of sale; and
- any other method permitted pursuant to applicable law.
The Selling Shareholders also may resell all or a portion of the shares in open market transactions in reliance upon Rule 144 under the Securities Act, as permitted by that rule, or Section 4(1) under the Securities Act, if available, rather than under this prospectus, provided that they meet the criteria and conform to the requirements of those provisions.
Broker-dealers engaged by the Selling Shareholders may arrange for other broker-dealers to participate in sales. If the Selling Shareholders effect such transactions by selling shares of common stock to or through underwriters, broker-dealers or agents, such underwriters, broker-dealers or agents may receive commissions in the form of discounts, concessions or commissions from the Selling Shareholders or commissions from purchasers of the shares of common stock for whom they may act as agent or to whom they may sell as principal. Such commissions will be in amounts to be negotiated, but, except as set forth in a supplement to this prospectus, in the case of an agency transaction will not be in excess of a customary brokerage commission in compliance with NASD Rule 2440; and in the case of a principal transaction a markup or markdown in compliance with NASD IM-2440.
18
In connection with sales of the shares of common stock or otherwise, the Selling Shareholders may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the shares of common stock in the course of hedging in positions they assume. The Selling Shareholders may also sell shares of common stock short and if such short sale shall take place after the date that the registration statement of which this prospectus forms a part is declared effective by the SEC, the Selling Shareholders may deliver shares of common stock covered by this prospectus to close out short positions and to return borrowed shares in connection with such short sales. The Selling Shareholders may also loan or pledge shares of common stock to broker-dealers that in turn may sell such shares, to the extent permitted by applicable law. The Selling Shareholders may also enter into option or other transactions with broker-dealers or other financial institu tions or the creation of one or more derivative securities which require the delivery to such broker-dealer or other financial institution of shares offered by this prospectus, which shares such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction). Notwithstanding the foregoing, the Selling Shareholders have been advised that they may not use shares registered on the registration statement of which this prospectus forms a part to cover short sales of our common stock made prior to the date the registration statement has been declared effective by the SEC.
The Selling Shareholders may, from time to time, pledge or grant a security interest in some or all of the warrants or shares of common stock owned by them and, if they default in the performance of their secured obligations, the secured parties may offer and sell the shares of common stock from time to time pursuant to this prospectus or any amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act, amending, if necessary, the list of Selling Shareholders to include the assignee, transferee or other successors in interest as Selling Shareholders under this prospectus. The Selling Shareholders also may transfer and donate the shares of common stock in other circumstances in which case the transferees, donees, assignee or other successors in interest will be the selling beneficial owners for purposes of this prospectus.
In connection with the private placements described above, we also issued warrants to acquire up to 690,000 shares of our common stock to certain of the Selling Shareholders named herein as finder's fees and agreed to register the shares underlying the warrants for resale with the SEC. None of the finders was a U.S. person (as defined in Regulation S under the Securities Act) or a person in the United States at the time of their involvement as finders in connection with offerings of securities of the Company and there were no offerings of securities of the Company made by the finders to U.S. persons or to any person in the United States. There is no material relationship between the finders and the Company or any of its associates or affiliates, other than the finders acting as finders.
The Selling Shareholders and any broker-dealer or agents participating in the distribution of the shares of common stock may be deemed to be "underwriters" within the meaning of Section 2(11) of the Securities Act in connection with such sales. In such event, any commissions paid, or any discounts or concessions allowed to, any such broker-dealer or agent and any profit on the resale of the shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. Selling Shareholders who are "underwriters" within the meaning of Section 2(11) of the Securities Act will be subject to the prospectus delivery requirements of the Securities Act and may be subject to certain statutory liabilities of, including but not limited to, Sections 11, 12 and 17 of the Securities Act and Rule 10b-5 under the Exchange Act.
Each Selling Shareholder has informed the Company that it is not a registered broker-dealer and does not have any written or oral agreement or understanding, directly or indirectly, with any person to distribute the common stock. Upon the Company being notified in writing by a Selling Shareholder that any material arrangement has been entered into with a broker-dealer for the sale of common stock through a block trade, special offering, exchange distribution or secondary distribution or a purchase by a broker or dealer, a supplement to this prospectus will be filed, if required, pursuant to Rule 424(b) under the Securities Act, disclosing (i) the name of each such Selling Shareholder and of the participating broker-dealer(s), (ii) the number of shares involved, (iii) the price at which such the shares of common stock were sold, (iv) the commissions paid or discounts or concessions allowed to such broker-dealer(s), where applicable, (v) that such broker-dealer(s) did not conduct any invest igation to verify the information set out or incorporated by reference in this prospectus, and (vi) other facts material to the transaction. In no event shall any broker-dealer receive fees, commissions and markups, which, in the aggregate, would exceed 8%.
19
Under the securities laws of some states, the shares of common stock may be sold in such states only through registered or licensed brokers or dealers. In addition, in some states the shares of common stock may not be sold unless such shares have been registered or qualified for sale in such state or an exemption from registration or qualification is available and is complied with.
There can be no assurance that any Selling Shareholder will sell any or all of the shares of common stock registered pursuant to the registration statement of which this prospectus forms a part.
The Selling Shareholder and any other person participating in such distribution will be subject to applicable provisions of the Exchange Act and the rules and regulations thereunder, including, without limitation, Regulation M of the Exchange Act, which may limit the timing of purchases and sales of any of the shares of common stock by the Selling Shareholder and any other participating person. Regulation M may also restrict the ability of any person engaged in the distribution of the shares of common stock to engage in market-making activities with respect to the shares of common stock. All of the foregoing may affect the marketability of the shares of common stock and the ability of any person or entity to engage in market-making activities with respect to the shares of common stock.
We will pay all expenses of the registration of the shares of common stock pursuant to the related registration rights agreement, which we anticipate to be approximately $41,000, including, without limitation, SEC filing fees and expenses of compliance with state securities or "blue sky" laws;provided,however, that a Selling Shareholder will pay all underwriting discounts and selling commissions, if any, and any related legal expenses incurred by it.
Mr. Ma
As disclosed in the Company's prior filings, the Company was the Appellate in an appeal in the British Columbia Court of Appeal.
On September 22, 2004, the British Columbia Court of Appeal dismissed with costs two appeals of the Company seeking to set aside the Order of the Honourable Mr. Justice Lowry pronounced on May 20, 2003 whereby the British Columbia Supreme Court ordered the Company and its majority owned subsidiary, M-I Vascular Innovations, Inc. ("M-I Vascular"), to take all necessary steps to exchange 3,192,399 shares of M-I Vascular owned by John Ma for 3,192,399 shares of the Company. On December 29, 2004, we issued 3,192,399 common shares to exchange for Mr. Ma's 3,192,399 common shares of M-I Vascular. The share exchange took place on January 14, 2005.
By counterclaim in the British Columbia Supreme Court, the Company continues to dispute John Ma's entitlement to his M-I Vascular shares (and to any Company shares he receives in exchange for his M-I Vascular shares), and we are suing Mr. Ma for damages for fraudulent misrepresentation. In a further action in the Supreme Court of British Columbia, we are suing Mr. Ma for defamation.
At present, the outcome of this legal proceeding is uncertain.
Vascore
Subsequent to August 31, 2007, the Company and certain senior officers and directors were served with a Summons and Complaint for an action in New York state court alleging breach of contract, fraud, fraudulent concealment, negligent misrepresentation, unjust enrichment and conspiracy claims in connection with the termination by the Company of the proposed acquisition of Vascore Medical. The Company believes that this action was brought improperly and intends to seek a stay of this action pending arbitration in the appropriate forum, where the Company intends to vigorously defend itself.
Other than as described above, the Company is not a party to any material legal proceedings and, to the knowledge of the Company, no such proceeding is contemplated or threatened.
DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS
Our current executive officers and directors are:
Name | Age | Position with the Company |
Alan P. Lindsay | 57 | Chairman and a director |
Dr. I. Mark Landy | 40 | President, Chief Executive Officer, Principal Executive Officer and a director |
Patrick A. McGowan | 68 | Executive Vice President, Secretary, Chief Financial Officer, Principal Accounting Officer and a director |
Dr. Daniel Savard | 56 | A director |
Rajesh Vaishnav | 46 | President and Chief Operating Officer of BioSync Scientific Pvt. Ltd. |
The following table sets forth the portion of their time that our executive officers and directors devote to our Company:
Alan P. Lindsay: | 100% | Dr. Daniel Savard: | 10% |
Dr. I. Mark Landy: | 100% | Rajesh Vaishnav: | 100% |
Patrick A. McGowan: | 100% |
The Board of Directors does not have a nominating committee or audit committee. Therefore, the selection of persons for election to the Board of Directors was neither independently made nor negotiated at arm's length.
Set forth below is a brief description of the background and business experience of each of our executive officers and directors for the past five years:
ALAN P. LINDSAY, Chairman and a director
Alan P. Lindsay has been MIV's Chairman since October 2001 and was Chief Executive Officer from October 2001 to January 2008, as well as President from October 2001 to April 2006. Mr. Lindsay has extensive experience in building companies and taking them public on recognized stock exchanges. Before coming to MIV Mr. Lindsay was the Chairman, President and CEO of Azco Mining, Inc., a base metals exploration company he co-founded and took public on the Toronto and AMEX exchanges. Mr. Lindsay served as Azco Mining, Inc.'s CEO and President from 1991 to 1994, its Chairman and CEO from 1994 to 1997 and its President, Chairman and CEO from 1997 to 2000. Azco Mining, Inc. was listed on the Toronto Stock Exchange in 1993 and on AMEX in 1994.
Mr. Lindsay was recently reappointed as a director of TapImmune, Inc., a company he co-founded 1999 and assisted with its financing. Mr. Lindsay initially resigned as Chairman prior to the company going public. In 2002 this company was taken public through a reverse take over and was quoted on the OTCBB under the name GeneMax Corp. It currently trades under the stock symbol "TPIM". TapImmune, Inc., through GeneMax Pharmaceuticals, is a product-focused biotechnology company specializing in the application of the latest discoveries in cellular immunology and cancer biology to the development of proprietary therapeutics aimed at the treatment and eradication of cancer and therapies for infectious diseases, autoimmune disorders and transplant tissue rejection.
Prior to becoming an entrepreneur, Mr. Lindsay was responsible for building a significant business and marketing organization in Vancouver, Canada, for Manulife Financial, a major international financial services corporation. Mr. Lindsay has not been involved in the past five years in any legal proceedings described in Item 401(f) of Regulation S-K.
21
DR. I. MARK LANDY, President, Chief Executive Officer, Principal Executive Officer and a director
Dr. Landy had been MIV's President since April 2006 and Chief Executive Officer since January 2008. Dr. Landy is also a director of the company. Dr. Landy's mission is to strengthen the company's internal procedures and move its technologies to market. He is a recognized medical device analyst and industry authority who brings a wealth of industry and physician relationships to the company that will be used to raise the company's corporate profile, to optimize and accelerate the development of the company's key strategic partnerships and to assist the company in bringing to market its technologies.
Dr. Landy most recently distinguished himself as the Senior Research Analyst of Medical Supplies and Devices at the Susquehanna Research Group where he was voted the firm's top-ranked healthcare analyst by institutional clients in both 2004 and 2005. He is a familiar financial pundit who has made frequent appearances on CNBC, Reuters, Dow Jones, Bloomberg, The Wall Street Journal and Business Week, among other outlets. From 2001 to 2004 Dr. Landy was the Senior Medical Device Analyst at Leerink Swann and Company.
Dr. Landy holds a degree in business from the Wharton School of Business at the University of Pennsylvania, and also holds the degree equivalent of Doctor of Dental Surgery from the University of Witwatersrand, in Johannesburg, South Africa. He spent three years in London, U.K., in private practice focusing on post-traumatic facial reconstructive surgery, and he has had articles published in both business and health care journals. Dr. Landy has not been involved in the past five years in any legal proceedings described in Item 401(f) of Regulation S-K.
PATRICK A. McGOWAN, Executive Vice President, Secretary, Chief Financial Officer, Principal Accounting Officer and a director
Patrick A. McGowan is a management consultant specializing in assisting public companies with financing, regulatory filings, administration and business plans. From November 1, 2001 to the present, Mr. McGowan has been engaged by the Company to serve as its Executive Vice President and Chief Financial Officer, to assume responsibility for negotiations with attorneys, auditors and financial institutions and the day to day business operations of the Company. From September 1997 to the time Mr. McGowan joined MIV, he served as CEO of American Petro-Hunter, Inc., an oil exploration company with duties including reviewing business proposals, writing business plans and approving corporate filings. Mr. McGowan was also responsible for all legal matters and functional areas of business for American Petro-Hunter including administration, accounting, contract negotiations, banking, writing press releases and overseeing regulatory filings. American Petro-Hunter is currently quo ted on the OTCBB under the stock symbol AAPH.
Mr. McGowan obtained his Masters of Business Administration from the University of Western Ontario in 1965, and his Bachelors of Science from the University of Oregon in 1963. Mr. McGowan has not been involved in the past five years in any legal proceedings described in Item 401(f) of Regulation S-K.
DR. DANIEL SAVARD, a director
Dr. Daniel Savard brings to MIV more than 20 years of clinical practice and clinical research in cardiology. From 1997 to the present, Dr. Savard has been President of Medi-Recherche Inc. and Assistant-Medical Director of the Quebec Blue Cross (Canassistance, Inc.). In 2001 Dr. Savard became a member of the Board of Governors of the Quebec Blue Cross. He is also member of La Societe des Medecins Experts du Quebec. Since 2000, he has been a consultant for La Regie des Rentes du Quebec. Recently, he joined Biomundis, a Canadian venture capital company in biotechnology, as medical Director.
Dr. Savard holds a doctorate degree in medicine from the Faculty of Medicine of Montreal University (1971-1976) and a license from the Medical Council of Canada. Dr. Savard completed postdoctoral training in Internal Medicine and Cardiology at Montreal University (1976 to 1980) and a one year fellowship in clinical and research echocardiography at the Quebec Heart Institute of Laval University. Dr. Savard has been certified in Cardiology by the Corporation des Medecins du Quebec and by the Royal College of Physicians and Surgeons of Canada. Dr. Savard is assistant professor of Medicine at the University of Montreal and practices at the Centre Hospitalier Universitaire de Montreal and Notre-Dame Hospital in Montreal. Dr. Savard's research interests are coronary heart disease, congestive heart failure, arterial hypertension, hyperlipidemia, angiogenesis therapy in coronary heart disease, circadian cycle and ambulatory blood pressure monitoring.
22
Dr. Savard is highly involved in clinical research. He has participated in 65 clinical trials, several of which were international metacentre studies. Dr. Savard has served on several pharmaceuticals clinical advisory boards for companies such as Pfizer, Hoechst Marion Roussel, Biovail Corp, Crystal Corp. and Aventis Pharma Inc. He is currently consulting for Biovail Corp. and for Medisys, an important Canadian health care management company.
Dr. Savard is an active member of several associations including L'Association des Cardiologues du Quebec, L'Association des Medecins Specialistes du Quebec and of La Societe des Medecins Experts du Quebec. He has published more than 40 papers and articles relating to his research. Dr. Savard has not been involved in the past five years in any legal proceedings described in Item 401(f) of Regulation S-K.
RAJESH VAISHNAV, President and Chief Operating Officer of BioSync Scientific Pvt. Ltd.
Rajesh L. Vaishnav has been President and COO of Biosync Scientific Pvt. Ltd. since November 2006. He was the Chairman and Managing Director of Biosync from April 2005 to January 2007. Prior to joining Biosync, he was a Technical Director with Sahajanand Medical Technologies Pvt. Ltd. from March 2000 to March 2005. He has to his credit the development of various stents and stent systems including SS316L, CoCr, PES & SES. He has over eight years experience with various stent manufacturing technologies, marketing and regulatory affairs and two years experience in finance, acquisition & mergers. Mr. Vaishnav has not been involved in the past five years in any legal proceedings described in Item 401(f) of Regulation S-K.
There are no significant employees other than our executive officers.
Our directors are elected to hold office until the next annual meeting of our shareholders or until their respective successors have been elected and qualified. Our executive officers are appointed by our Board of Directors to hold office at the discretion of the board.
There are no family relationships among our directors or officers.
Involvement in Certain Legal Proceedings
Our directors, executive officers or control persons have not been involved in any of the events prescribed by Item 401(f) of Regulation S-K during the past five years, including:
- a petition under the federal bankruptcy laws or any state insolvency law was filed by or against, or a receiver, fiscal agent or similar officer was appointed by a court for the business or property of such person, or any partnership in which he was a general partner at or within two years before the time of such filing, or any corporation or business association of which he was an executive officer at or within two years before the time of such filing;
- any conviction in a criminal proceeding or being named in a pending criminal proceeding (excluding traffic violations and other minor offences); or
- being the subject of any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him from, or otherwise limiting, the activities described in Item 401(f)(3) of Regulation S-K.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information concerning the number of our shares of common stock owned beneficially as of February 28, 2008 by: (i) each person (including any group) known to us to own more than 5% of our common stock, (ii) each of our directors and executive officers, and (iii) our executive officers and directors as a group. Unless otherwise indicated, each person has sole voting and investment power with respect to the shares they own.
Name and Address of Beneficial Owner | Number of Shares | Percentage of |
Directors and executive officers: | ||
Alan P. Lindsay | 6,569,154(2) | 5.41% |
Dr. I. Mark Landy | 11,059,524(3) | 8.75% |
Patrick A. McGowan | 1,666,665(4) | 1.42% |
Dr. Daniel Savard | 400,000(5) | * |
Rajesh Vaishnav | 1,884,167(6) | 1.62% |
All directors and executive officers as a group: | 21,579,510(7) | 15.97% |
5% Stockholders: | ||
Millennium Partners, L.P. | 15,000,000(8) | 8.66% |
Pequot Capital Management, Inc. | 16,355,000(9) | 13.57% |
* Less than 1%
(1) Under Rule 13d-3, a beneficial owner of a security includes any person who, directly or indirectly, through any contract, arrangement, understanding, relationship, or otherwise has or shares: (i) voting power, which includes the power to vote, or to direct the voting of shares; and (ii) investment power, which includes the power to dispose or direct the disposition of shares. Certain shares may be deemed to be beneficially owned by more than one person (if, for example, persons share the power to vote or the power to dispose of the shares). In addition, shares are deemed to be beneficially owned by a person if the person has the right to acquire the shares (for example, upon exercise of an option) within 60 days of the date as of which the information is provided. In computing the percentage ownership of any person, the amount of shares outstanding is deemed to include the amount of shares beneficially owned by such person (and only such person) by reas on of these acquisition rights. As a result, the percentage of outstanding shares of any person as shown in this table does not necessarily reflect the person's actual ownership or voting power with respect to the number of shares of common stock actually outstanding on February 28, 2008. The applicable percentage of ownership is based on 115,491,278 shares of common stock outstanding as of February 28, 2008.
24
(2) Consists of 524,154 shares held by Mr. Lindsay and 6,045,000 shares that can be acquired by Mr. Lindsay upon exercise of options to purchase shares held by Mr. Lindsay within 60 days of the date hereof.
(3) Consists of 176,180 shares held by Dr. Landy and 10,883,344 shares that can be acquired by Dr. Landy upon exercise of options and warrants to purchase shares held by Dr. Landy within 60 days of the date hereof.
(4) Consists of 56,665 shares held by Mr. McGowan and 1,610,000 shares that can be acquired by Mr. McGowan upon exercise of options to purchase shares held by Mr. McGowan within 60 days of the date hereof.
(5) Consists of 400,000 shares that can be acquired by Dr. Savard upon exercise of options to purchase shares held by Dr. Savard within 60 days of the date hereof.
(6) Consists of 1,162,500 shares held by Mr. Vaishnav and 721,667 shares that can be acquired by Mr. Vaishnav upon exercise of options to purchase shares held by Mr. Vaishnav within 60 days of the date hereof.
(7) Consists of 1,919,499 shares held by our directors and executive officers and 19,660,011 shares that can be acquired by our directors and executive officers upon exercise of options and warrants to purchase shares held by our directors and executive officers within 60 days of the date hereof.
(8) Millenium Partners may be deemed to be the beneficial owner of an aggregate of 10,000,000 shares of common stock and 5,000,000 shares that can be acquired upon exercise of warrants. While Millenium Partners acquired 5,000,000 warrants in connection with a private placement completed in July 2007, the number of shares of common stock into which the warrants are exercisable is limited pursuant to the terms of the warrant to that number of shares which would result in Millenium Partners having aggregate beneficial ownership of not more than 4.99% of the total issued and outstanding shares of common stock and thus, the warrants are not currently exercisable.
(9) Consists of 11,355,000 shares held by Pequot Capital Management Inc. and 5,000,000 shares that can be acquired by Pequot Capital Management Inc. upon exercise of warrants within 60 days of the date hereof.
We are unaware of any contract, or other arrangement or provision of our Articles of Bylaws, the operation of which may at a subsequent date result in a change of control of our company.
As at February 28, 2008, there were 115,491,278 shares of the common stock issued and outstanding. The authorized capital stock of the Company consists of 230 million common shares and 20 million preferred shares at $0.001 par value. Inclusive of this amount are 2,500,000 shares which are outstanding and which are being returned to treasury for cancellation by the Company resulting from the Company's previous Regulation S offering. The shares were being held as part of a previous offering of the Company's special class Regulation S shares on the Berlin Stock Exchange.Upon liquidation, dissolution or winding up of the corporation, whether voluntary or involuntary, the holders of common stock are entitled to share pro-rata in all net assets available for distribution to stockholders after payment to creditors. The common stock is not convertible or redeemable and has no pre-emptive, subscription or conversion rights. There are no conversion, redemption, sinking fund or simi lar provisions regarding the common stock. Each outstanding share of common stock is entitled to one vote on all matters submitted to a vote of stockholders. There are no cumulative voting rights. All outstanding shares of Common Stock are validly authorized and issued, fully paid and non-assessable. The Board of Directors is authorized to issue additional shares of common stock not to exceed the amount authorized by the Company's Articles of Incorporation, on such terms and conditions and for such consideration as the Board may deem appropriate without further stockholder action.
There are no provisions in the Company's Articles or By-laws that would delay, defer or prevent a change in control of the Company.
The above description concerning the capital stock of the Company does not purport to be complete. Reference is made to the Company's Articles of Incorporation and Bylaws which are available for inspection upon proper notice at the Company's offices and are available on the Internet as an exhibit to the Company's Form 10SB12G as filed on April 26, 2000. As well, the applicable statutes of the State of Nevada provide a more complete description concerning the rights and liabilities of stockholders.
Voting Rights
Each holder of common stock is entitled to one vote per share on all matters on which such stockholders are entitled to vote. Since the shares of common stock do not have cumulative voting rights, the holders of more than 50% of the shares voting for the election of directors can elect all the directors if they choose to do so and, in such event, the holders of the remaining shares will not be able to elect any person to the Board of Directors.
Holders of the Company's common stock are entitled to dividends if declared by the Board of Directors out of funds legally available therefore. The Company does not anticipate the declaration or payment of any dividends in the foreseeable future. We intend to retain earnings, if any, to finance the development and expansion of the Company's business. Future dividend policy will be subject to the discretion of the Board of Directors and will be contingent upon future earnings, if any, the Company's financial condition, capital requirements, general business conditions and other factors. Therefore, there can be no assurance that any dividends of any kind will ever be paid.
The Company's Transfer Agent is Interwest Transfer Company, Inc. of 1981 East Murray Holladay Road, Suite 100, P.O. Box 17136, Salt Lake City, Utah, U.S.A., 84117.
Lang Michener LLP, our independent legal counsel, has provided an opinion on the validity of the shares of our common stock that are the subject of this prospectus.
The consolidated financial statements of the Company as at and for the year ended May 31, 2007 included in this prospectus and the registration statement of which it forms a part have been audited by Ernst & Young LLP, Chartered Accountants, an independent registered public accounting firm, as set forth in their report thereon (which contains an explanatory paragraph describing conditions that raise substantial doubt about the Company's ability to continue as a going concern as described in Note 1 to the consolidated financial statements) appearing elsewhere herein and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
The consolidated financial statements of the Company as at and for the year ended May 31, 2006 included in this prospectus have been audited by Dale Matheson Carr-Hilton LaBonte LLP, Chartered Accountants, an independent registered public accounting firm, to the extent and for the period set forth in their report appearing elsewhere in this prospectus and are included in reliance upon the authority of such firm as experts in accounting and auditing.
The consolidated financial statements of the Company as at and for the year ended May 31, 2005 included in this prospectus and the registration statement of which it forms a part have been audited by Ernst & Young LLP, Chartered Accountants, an independent registered public accounting firm, as set forth in their report thereon (which contains an explanatory paragraph describing conditions that raise substantial doubt about the Company's ability to continue as a going concern as described in Note 1 to the consolidated financial statements) appearing elsewhere herein and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
As described in the report of Ernst & Young LLP dated August 18, 2005 except for Notes 15 and 6d which are as of October 20, 2005, subsequent to the issuance of the Company's 2005 consolidated financial statements, discovery of facts existing at the date of their initial report dated August 18, 2005 resulted in a restatement of certain information in the consolidated financial statements. Prior auditors reaudited the cumulative income, expense and cash flow data from inception to May 31, 2003 which resulted in adjustment to the cumulative net loss from inception to May 31, 2005 and a restated cumulative loss per share. The reports of the other auditors have been reissued and remain unqualified and Ernst & Young LLP have relied upon such reissued reports in connection with their audit of the Company's financial statements for the year ended May 31, 2005.
INTERESTS OF NAMED EXPERTS AND COUNSEL
No expert or counsel named in this prospectus as having prepared or certified any part of this prospectus or having given an opinion upon the validity of the securities being registered or upon other legal matters in connection with the registration or offering of the common stock offered hereby was employed on a contingency basis, or had, or is to receive, in connection with the offering, a substantial interest, direct or indirect, in the Company, nor was any such person connected with the Company as a promoter, managing or principal underwriter, voting trustee, director, officer or employee.
DISCLOSURE OF COMMISSION POSITION ON
INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
Our directors and officers are indemnified as provided by theNevada Revised Statutes, our Articles of Incorporation and our Bylaws.
We have been advised that, in the opinion of the SEC, indemnification for liabilities arising under the Securities Act is against public policy as expressed in the Securities Act, and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities is asserted by one of our directors, officers, or controlling persons in connection with the securities being registered, we will, unless in the opinion of our legal counsel the matter has been settled by controlling precedent, submit the question of whether such indemnification is against public policy to a court of appropriate jurisdiction. We will then be governed by the court's decision.
History and Development
We are an advanced stage, research and development company pursuing the commercialization of the next generation of biocompatible coatings for stents and other medical devices and advanced drug delivery systems with the intent of providing healing solutions for cardiovascular disease and other medical conditions. In collaboration with the University of British Columbia (UBC), we have developed unique coating technologies that utilize Hydroxyapatite (HAp) for application on medical devices and drug delivery systems. Simultaneously, alternative polymer-free coatings and advanced polymeric coatings with enhanced biocompatibility and bioavailability were developed by our R&D team at our wholly-owned subsidiary, MIVI Technologies Inc. Our proprietary coating and drug delivery technologies are protected by 50 patents and patent applications world-wide. We made capital expenditures of approximately $222,000, $260,000 and $337,000 in fiscal years 2005, 2006 and 2007, respectively.
We were incorporated as DBS Holdings, Inc. under the laws of the State of Nevada on March 19, 1999. On June 23, 1999, we acquired a 19% interest in "investorservice.com", an Internet domain name, paying for this acquisition with $2,500 in cash and by issuing 2,500 restricted shares of our common stock. On September 15, 2000, we exercised the option to acquire the remaining 81% interest in investorservice.com for an additional issuance of 10,000 restricted shares of our common stock.
In March 2001, we announced we had concluded negotiations for the acquisition and control of M-I Vascular Innovations, Inc., ("M-I Vascular"), a stent medical device development company, and in April 2001, we signed a Share Exchange and Finance Agreement (the "Share Exchange Agreement") with M-I Vascular whereby we exchanged, on a one for one basis, 58% of the shares outstanding of M-I Vascular for shares. Pursuant to the terms of the agreement, we completed the share exchange with the remaining shareholders of M-I Vascular on May 31, 2003.
In May 2001, in connection with the Share Exchange Agreement, we announced a change of business and control. We elected and appointed new officers and directors and began to engage in the business of developing medical stents. On March 5, 2002, following shareholder approval to amend our Articles of Incorporation, we changed our name to MIV Therapeutics, Inc.
27
In December 2004, our wholly-owned subsidiary, MIVI Technologies, Inc. ("MIVI"), which is involved in the research and development of multilayer coating technologies with drug eluting capability for cardiovascular stents and other implantable devices, received a Government of Canada grant for the research program titled "Development of Novel Drug Eluting Composite Coatings for Cardiovascular Stents". The Canadian National Research Council approved MIVI's application following an in depth familiarization with the advanced concept of novel technologies proposed by MIVI and a review of our organizational and fiscal capability to carry on with the program. In May 2007, the above program was formally extended until the end of the year 2007, with additional funding provided by The Canadian National Research Council in support of the expanded research activities.
On March 14, 2005, we acquired 100% of SagaX, Inc. ("SagaX"), a Delaware corporation with operations in Herzliya, Israel, from a third party. SagaX is in the business of developing a neuro-vascular embolic stent filter medical device called Anti Embolic Protection Device or AEPD through its subsidiary in Israel. We sold all of the issued and outstanding shares of SagaX on December 28, 2007, as described below.
On March 1, 2005, we entered into a share acquisition letter of intent with the shareholders of Sahajanand Medical Technologies Inc. ("SMT") of India to purchase 100% of the issued and outstanding shares of SMT. The proposed acquisition of SMT was terminated by mutual agreement in January 2006.
Our shares are quoted under the symbol "MIVT" on the OTCBB. On August 24, 2006, following shareholder approval at our most recent annual meeting, we amended our Articles of Incorporation to increase our authorized common stock to 230,000,000 shares of common stock and 20,000,000 shares of preferred stock.
Recent Developments
Termination of Proposed Acquisition of Vascore Scientific Co. Ltd.
Effective on September 5, 2006, we entered into an Equity Transfer Agreement to acquire all of the outstanding equity interests of Vascore Scientific Co., Ltd., a wholly foreign owned enterprise in China ("Vascore"), which is engaged in the business of, among other things, designing, manufacturing and marketing coated and non-coated vascular stents and related accessories. The Equity Transfer Agreement provided, subject to numerous conditions precedent to closing and including, without limitation, final Board and government approvals, for the payment and issuance of an aggregate of $1,000,000 and 4,000,000 common shares of our company prior to the completion of the acquisition of Vascore.
As a result of our inability to complete due diligence and the non-satisfaction of certain conditions precedent to the closing of the acquisition, the Equity Transfer Agreement has been terminated in accordance with its terms effective August 31, 2007.
Acquisition of BioSync Scientific Pvt. Ltd.
On February 16, 2007, we completed the acquisition of all of the issued and outstanding shares of BioSync Scientific Pvt. Ltd. ("BioSync Scientific"), a body corporate subsisting under and registered pursuant to the laws of India and is presently engaged, among other things, in the business of designing, manufacturing and marketing coated and non-coated vascular stents and related accessories.
In consideration for the acquisition of the shares of BioSync Scientific, we issued 50,000 shares of our common stock with an estimated fair value of $33,000 and paid $500,000 to the vendors. As a further condition of the agreement, we were required to satisfy any and all bank indebtedness of BioSync Scientific, at the time estimated to be $1,000,000. As part of the acquisition, we entered into a two year Executive Services Agreement with Mr. Rajesh Vaishnav, owner of BioSync Scientific, pursuant to which Mr. Vaishnav will serve as the President and Chief Operating Officer of Biosync. As part of his agreement, Vaishnav may receive up to an aggregate of 4,000,000 shares of our common stock provided certain conditions are met. Of the 4,000,000 shares, 750,000 shares have been issued with an estimated fair value of $495,000 to Mr. Vaishnav and other former shareholders of Biosync as we agreed to issue 750,000 common shares to the vendors if Biosync received CE Mark for its bare-metal ste nt. The fair value of the 750,000 common shares is included as consideration for the acquisition.
28
Biosync Scientific was started as a partnership company on July 21, 2003 at Surat, Gujarat, India. This joint stock company was converted and registered as Biosync Scientific Pvt. Ltd. on August 1, 2006 under part IX of the Companies Act 1956 at 136-B, Surat Special Economic Zone, GIDC, Sachin, Surat in the state of Gujarat, India.
Biosync Scientific's mission is to improve the quality of patient care and the productivity of health care delivery through the development and advocacy of less-invasive medical devices and procedures. This is accomplished through the continuing refinement of existing products and procedures and the investigation and development of new technologies which can reduce risk, trauma, cost, procedure time and the need for aftercare.
The Biosync Scientific genXTM coronary stent is the next generation variable geometry stent, designed to minimize stent induced arterial injury. It is designed to incorporate controlled stent expansion characteristics, better stent-artery compliance, superior acute gain, optimum cell size and shape, lower strut thickness and high flexibility.
BioSync Scientific's manufacturing facility is located in Surat, Gujarat, India, and is approximately 8,325 square feet including manufacturing area. BioSync Scientific employs approximately 56 people and the Facility contains micro biology labs, and administrative offices and a manufacturing capacity to manufacture 7,600 stents per month, 3,000 inflation devices and 10,000 Y connectors. BioSync Scientific's manufacturing facilities are ISO 9001 and ISO 13485 certified. All the products manufactured are CE certified. BioSync Scientific's products are marketed worldwide through distribution channels and its major markets include India, Turkey, Greece, Italy, Egypt and Argentina.
July 2007 Financing
On July 9, 2007, we completed the private placement of 25,100,000 units sold at $0.50 per unit for aggregate gross proceeds of $12.55 million. Each unit is comprised of one share of common stock of and one-half of one warrant. Each whole warrant entitles the holder to purchase one share of common stock for a period of five years at a price of $0.55 per share. In connection with the private placement, we received net proceeds of $11.7 million. We engaged BMO Capital Markets Corp. as our agent who received placement fees of $753,000 and 251,000 shares of common stock and warrants to purchase 753,000 shares of common stock at $0.55 per share for a period of five years in connection with the private placement.
Sale of SagaX
In accordance with the terms and conditions of a Share Purchase Agreement (the "Share Purchase Agreement") dated November 13, 2007 among the Company, SagaX, Shimoco LLC (the "Purchaser") and Dr. Dov Shimon, the Company agreed, subject to a number of conditions precedent (the "Conditions Precedent"), to sell all of the issued and outstanding shares (the "Purchased Shares") of SagaX held by the Company to the Purchaser due to the inability of SagaX to meet certain previously established objectives.
We completed the sale on December 28, 2007. In accordance with the terms and conditions of the Share Purchase Agreement, the total purchase price (the "Purchase Price") for all of the Purchased Shares was comprised of the following:
(a) the repayment by the Purchaser and SagaX to the Company of an aggregate of $4,000,000 in prior loans and associated indebtedness which have been advanced and undertaken by the Company in and to SagaX and S.M.T. Research & Development, Ltd. ("SMT"), a wholly-owned subsidiary of SagaX, since the Company's acquisition of SagaX (collectively, "SagaX's Indebtedness") in the following manner and at the following times:
(i) an initial $1,000,000 of SagaX's Indebtedness will be due and payable by the Purchaser and SagaX to the order and the direction of the Company within six months of the first private or public equity financing of SagaX which is completed subsequent to the closing (the "Initial SagaX Financing"); the terms and conditions of any such Initial SagaX Financing being subject to the prior review and approval of the Company; such approval not to be unreasonably withheld;
29
(ii) an additional $1,000,000 of SagaX's Indebtedness will be due and payable by the Purchaser and SagaX to the order and the direction of the Company within 18 months of the completion of Initial SagaX Financing;
(iii) a further $1,000,000 of SagaX's Indebtedness will be due and payable by the Purchaser and SagaX to the order and the direction of the Company within 30 months of the completion of Initial SagaX Financing; and
(iv) the balance of $1,000,000 of SagaX's Indebtedness will be due and payable by the Purchaser and SagaX to the order and the direction of the Company within 48 months of the completion of Initial SagaX Financing.
In this regard, and until payment in full of SagaX's Indebtedness, SagaX's Indebtedness has now been secured, contemporaneously with the closing, by way of a senior, subordinated (subordinated only to SagaX's existing banking indebtedness), fixed and floating charge registered over all of the assets of SagaX (the "Security for SagaX's Indebtedness"); and
(b) the payment by the Purchaser and SagaX to the Company of a royalty fee (the "Royalty Fee") equating to 8% of all sales revenues less any documented rebates, refunds, taxes and delivery costs applicable to any sale, other than from sub-licenses, in respect of gross sales from any product associated or related to SagaX's present intellectual property under any existing patent or patent-pending applications, and of any other benefit, directly or indirectly collected or received, whether for cash or credit or by way of any benefit, advantage, equity, or concession from the manufacturing, distribution, marketing, contracting, joint venturing, leasing, equity participation or any other activity in relation to the said products.
In addition to the Purchase Price consideration, both prior to, in conjunction with and subsequent to the closing the Purchaser and SagaX also remain responsible for paying the Company a bonus (the "Bonus") equal to 10% of any consideration in any form which is received by the Purchaser and/or SagaX from any source and from any transaction, or a series of related transactions, at anytime and which is in anyway associated with a "Change In Control" (as defined hereinbelow) of SagaX at anytime while the Royalty hereinabove remains due and payable by the Purchaser and SagaX to the Company. Change In Control is therein defined to mean a change in ownership or control of the Company effected through any of the following transactions: (i) the direct or indirect acquisition by any person or related group of persons (other than an acquisition from or by SagaX or by a SagaX-sponsored employee benefit plan or by a person that directly or indirectly controls, is controlled by, or is under common con trol with, SagaX) of beneficial ownership (within the meaning of Rule 13d-3 of the U.S. Securities and Exchange Act) of securities possessing more than 50% of the total combined voting power of SagaX's outstanding securities pursuant to a tender or exchange offer made directly to SagaX's shareholders; (ii) a change in the composition of the Board of Directors of SagaX ("SagaX's Board") over a period of 36 months or less such that a majority of SagaX's Board members ceases, by reason of one or more contested elections for SagaX's Board membership, to be comprised of individuals who are continuing directors; (iii) the sale or exchange by SagaX (in one or a series of transactions) of all or substantially all of its assets to any other person or entity; or (iv) the approval by the shareholders of SagaX of a plan to dissolve and liquidate SagaX.
In consideration of the Purchaser's and SagaX's agreement to provide the Purchase Price consideration and any Bonus to the order and direction of the Company, the Company provided to SagaX at closing the final sum of $95,000 in additional working capital (the "Working Capital"; having previously advanced an aggregate of $115,000 in working capital to SagaX at SMT's request) in order to meet certain of SagaX's previously disclosed and bona fide current liabilities; with any said Working Capital advances to simply form part of the overall SagaX's Indebtedness to the Company.
As previously disclosed, effective on the execution date of the Share Purchase Agreement, Dr. Dov Shimon was deemed, without any further act required on his behalf, to have immediately resigned as a director of the Company and to immediately have terminated his existing consulting agreement and arrangement with the Company and, consequent upon such resignation and termination, to have no further claim as against the Company as a previous director of or consultant to the Company.
30
Product Background
Coronary stents are used to treat cardiovascular disorder caused by the narrowing or blockage of coronary arteries. Stents are compressible tubular metal mashes that are mounted on a balloon catheter, inserted into the circulatory system by a team of cardiologists, and directed to the location of a blocked coronary artery. During the angioplasty procedure, which involves unclogging the artery, the balloon is expanded to clear the obstruction, allowing normal blood flow. With this procedure, the stent is deployed and remains in place to reinforce the artery wall. This procedure is the leading alternative to costly and highly invasive open-heart surgery. Stents have eliminated many of the complications that previously accompanied simple balloon angioplasty. As much as 80% of blocked coronary arteries can be treated effectively with stenting.
We, in collaboration with UBC, have developed unique coating technologies that utilize HAp for application on medical devices. HAp is naturally found in bone and tooth enamel. As such, it may inhibit a variety of adverse reactions currently seen with polymer- based drug delivery system.
We have licensed from UBC the worldwide rights to technologies for coating stents and other medical devices with HAp. Our lead product in development is a HAp-coated drug eluting stent for which human studies began in May 2007. Our technology is considered to be suitable for broad applications in cardiovascular and non-vascular drug/device combination products. Our goal is to continue to diversify our portfolio to capitalize on these potential applications, accessing the multi-billion market of combination drug/device products.
Summary of Existing Products Currently in the Pre-clinical Development Stage
Coating and Drug Delivery Technologies
The Company's lead coating and drug delivery technologies include:
- Nanofilm HAp "passive" (without drug) coating for cardiovascular stents. This coating is designated as a long-lasting protective barrier between the substrate of the device and the surrounding tissue.
- Porous HAp, with capacity to carry drug.
Cardiovascular Stents
MIVT is developing its own cardiovascular stents. These stents are designed with unique design features and are destined for world wide commercialization.
Our Bare Metal Cardiovascular Stents
The acquisition of Biosync Scientific, a designer and producer of advanced cardiovascular stents, provided MIVT with a highly competitive bare metal stent platform. The GenX coronary stent system is CE Mark-certified for use in Europe and other countries around the world where the CE Mark is recognized. MIVT will use the GenX stent as the bare-metal stent platform for commercialization of its proprietary polymer-free drug-eluting stent.
Drug-Eluting Cardiovascular Stents
We are currently developing our first drug-eluting stent with a polymer-free HAp coating. This drug eluting stent should offer a significant safety advantage over the currently available polymer-based systems as it contains no polymer and significantly less drug. First-in-man studies using our VestasyncTM drug eluting stent began in May 2007.
31
Intellectual Property and Intangibles
Patents
We have exclusive worldwide rights to the HAp coating technology from UBC for use on stents and other medical devices, including the rights to manufacture and market coated products using these technologies. To date, UBC has two patents granted and one patent pending on novel coating technologies that we intend to use.
Patents Owned by University of British Columbia and Licensed Exclusively to MIVT:
1. Novel Sol-Gel Calcium Phosphate Ceramic Coatings and Method of Making the Same. Inventor(s): T. Troczynski, Dean-Mo Liu - UBC/MTRL.
Abstract / Non-confidential Description:
Low-Temperature Sol-Gel Synthesis of Hydroxyapatite Ceramics for Biomedical Applications. This invention relates to novel sol-gel calcium phosphate, in particular, hydroxyapatite, ceramic coatings and processes of making same at low temperature. Such coatings are useful, inter alia, for dental implants and other bone-metal contact appliances.
Status: S 6,426,114 (also Canadian application # 2,345,552).
2. Biofunctional Hydroxyapatite Coatings and Microspheres for In-situ Drug Encapsulation. Inventor(s): T. Troczynski, Dean-Mo Liu, Quanzu Yang - UBC/MTRL.
Abstract / Non-confidential Description:
This invention relates to novel room-temperature process for obtaining calcium phosphate, in particular hydroxyapatite, microspheres and coatings with encapsulated drugs, proteins, genes, DNA for therapeutical use. The coatings and microspheres are designed to perform a defined biological function related to drug delivery, such as gene therapy through gene delivery. A novel method for encapsulation and subsequent controlled release of therapeutically active agents from such biofunctional coatings and microspheres is disclosed. Such coatings and microspheres are useful for side effects - free, long-term, targeted, controlled release and delivery of drugs, proteins, DNA, and other therapeutic agents.
Status: US Patent No. 6,730,324, PCT Patent Application No. PCT/CA02/00565, which has been converted to pending regional applications in Canada (Patent No. 2,444,561), Europe (Serial No. 02721913.8, Italy, France , Germany, United Kingdom, Ireland, and The Netherlands elected), Australia (Serial No. 2002225889), Brazil (Serial No. PI 0209040-6), China (Serial No. 02811285.7), India (Serial No. 1357/KONP/2003), Israel (Serial No. 158474), Japan (Serial No. 2002-582904), and South Africa (Serial No. 2003/8332).
3. Calcium Phosphate Coated Implantable Medical Devices and Method of Making Same. Inventor(s): T. Troczynski, Dorna Hakimi, Buhsung Hyun, Mehrdad Keshmiri, Manus Pui Hung Tsui, Quanzu Yang - UBC/MTRL Mao-Jung Maurice Lien, Arc Rajtar, Douglas Smith - MIVI Therapeutics Inc.
Abstract / Non-confidential Description:
This invention relates to novel calcium phosphate-coated implantable medical devices and processes of making same. These calcium-phosphate coatings are designed to minimize the immune response to the implant (e.g. restenosis in stenting procedures) and can be used to store and release a medicinally active agent in a controlled manner. Such coatings can be applied to any implantable medical devices and are useful for a number of medical procedures including (but not limited to) balloon angioplasty in cardiovascular stenting, ureteral stenting and catheterisation.
32
Status: PCT/CA #03/014050 (serial # 03 747 764.3-2107), US patent application #10/527,406, Canada (Serial No. 2,498,743), Japan (Serial No. 2004-534912), Brazil (Serial No. PI 0314265-5), European (Serial No. 03747764.3) and India (Serial No. 639/KOLNP/2005.
Trademarks
We have applications pending in the United States Patent and Trademark Office and in Canada for protection of the trade name "MIV Therapeutics".
Domain Names
The Company holds a 100% interest in the following domain names:
- mivi.ca
- mivtherapeutics.com
- mivtinc.com
- bio-deliverysystems.com
Objectives
MIV Therapeutics, Inc. was established in 1999, with an initial corporate focus on the development of minimally invasive medical devices for use in cardiovascular and other medical procedures. The Company completed the development of a proprietary coronary stent for use in angioplasty procedures but has since shifted its focus to the development of technologies that would be used to manufacture a range of biocompatible coatings and drug delivery solutions for vascular stents and other implantable medical devices.
The Market
Stents are estimated to be used in approximately 60-80% of angioplasty procedures worldwide. The worldwide coronary stent market in 2007 exceeded $5 billion in revenues, and is expected to top $5.5 billion in 2008. MIV Therapeutics is targeting this large and growing market with its unique polymer-free drug eluting stent.
Rapid introduction of new stent designs and the rapid pace of innovations over the last ten years have resulted in dramatic shifts in market share and have opened up tremendous opportunities for entrepreneurial market entrants. Much of the technology used today in coronary stents by the four largest market participants was either acquired or licensed. The Company believes that the development of novel new and safer devices, and therapies to treat restenosis is the primary challenge that will shape the industry and define the industry leaders in the next decade.
Target Market and Marketing Strategy
MIVT proprietary HAp-based coatings combine biocompatibility with flexible engineering parameters, making them suitable for a broad range of implantable medical devices and drug delivery applications.
We intend to concentrate on marketing our devices in the emerging market for coated and drug-eluting stents while at the same time looking for additional opportunities in other implantable medical devices.
HAp-based Coatings
Our HAp coating technology has successfully progressed through a comprehensive range of tests. These include thrombogenicity (blood clotting), cytotoxicity, safety and fatigue life testing.
33
Preclinical results support the expectation that the HAp-based drug eluting stent may be considerably safer than currently available polymer-based drug eluting stents. In May of 2007, we began first in-man trials for our VestasyncTM drug eluting stent.
HAp Coatings Development Program
The overall objective of this program is to develop calcium phosphate ceramic-based/polymer-free coatings suitable for cardiovascular stents and other implantable medical devices, in particular:
- to define and validate the composite coating characteristics;
- to develop coating process that will be suitable for volume manufacturing environment;
- to develop suitable process for incorporation of drugs into the composite coatings;
- to characterize in-vitro and in-vivo chemical, mechanical and biological properties of the drug-containing coatings based on HAp;
- to define drug eluting characteristics for the coatings; validate the values in-vitro and in-vivo; and
- to modify manufacturing processes for optimum performance of the drug-eluting calcium phosphate ceramic/ coatings on cardiovascular stents.
- to begin human trials and the process to commercialization.
Research and Development
To date, we have invested approximately $12.8 million in research and development of our stent products, coatings and operations, and in establishing a quality manufacturing facility and completing laboratory and preclinical testing on our stents. We also have developed strong research collaborations with the University of British Columbia for our proprietary stent coatings and have implemented an aggressive in-house product development program.
Competition
Competitive activities in the drug-eluting stent (DES) field focus on development of stent coatings which combine improved biocompatibility with controlled drug release characteristics, on a variety of drugs either already available off-shelf or being developed for vascular applications, as well as on attempts to develop fully biodegradable stents.
Based on our current stage of product development, MIVT can best be compared to other medical device companies with coated stent products. Although there are a number of companies currently selling coronary stents and developing drug-eluting stents, there are a relatively small number of international companies that control the majority of this market segment.
During 2007, only two brands of drug-coated stent were available in the United States: TaxusTM Paclitaxel-eluting stent, which is made by Boston Scientific, and Johnson & Johnson's CypherTM Sirolimus-eluting stent, which is coated with a polymer made by Eden Prairie-based SurModics Inc. Both were extensively studied in clinical trials and received CE mark approvals in Europe prior to FDA approval.
The Johnson & Johnson's CypherTM stent's metal substrate is coated with three layers of polymers. The primer layer is a coating of Parylene C onto which is sprayed a solution of two biodegradable polymers, polyethylene-co-vinyl acetate (PEVA) and poly n-butyl methacrylate (PBMA), containing the anti-inflammatory drug Sirolimus (Rapamycin or Rapamune). The top layer is a drug-free coating of a solution of PEVA and PBMA that serves to control drug release and prevent a burst effect. The polymer platform was developed for Cordis by SurModics (Eden Prairie, Minnesota). The Johnson & Johnson's CypherTM stent releases 50% of its Sirolimus content during the first week after implantation and 85% of the drug over 30 days. All the sirolimus is eluted after 90 days. Sirolimus is licensed by Cordis from Wyeth (Madison, New Jersey), which sells it under the Rapamune brand name. The CypherTM stent is sold in 80 countries.
34
The Boston Scientific's TaxusTM stent is coated with a single layer of non-biodegradable poly(styrene-b-isobutylene-b-styrene) translute polymer containing anticancer drug Paclitaxel. Paclitaxel prevents the accumulation of anti-inflammatory cells at the site where angioplasty was performed and is licensed by Boston Scientific from Angiotech Pharmaceuticals (Vancouver, British Columbia).
Medtronic, Inc. is also is focused on providing therapeutic, diagnostic, and monitoring systems for cardiovascular and other markets. Medtronic's Endeavor(R) drug-eluting stent system has thin-strut cobalt chrome alloy to enhance deliverability and has achieved CE Mark. The FDA approved this product in February 2008. Abbott Laboratories through Abbott Vascular has a comprehensive portfolio of endovascular and coronary products. Abbott Laboratories recently acquired Guidant Corp.'s coronary stent and vascular business. Abbott's Xience V stent has achieved CE Mark. The FDA approval process is on going.
With the worldwide revenues for coronary stents exceeding $5 billion in 2007, we believe there is a substantial opportunity for even a smaller company such as MIVT to penetrate this market if it has leading edge technologies and a strong product development program. A growing number of smaller and mostly private companies also are developing DES products. A variety of stent designs and materials and a range of drugs, including combinations of drugs, are being explored.
Employees
We currently have approximately 110 full-time employees.
We conduct primarily all of our manufacturing business and some development activities from our 5,380 square foot facility in Surat, India. We conduct research and development of coronary stents and stent delivery systems and in-house manufacturing at our 10,296 square foot leased facility in Vancouver, Canada. This location is fully equipped for stent laser cutting, electropolishing and quality assurance and it is equipped with adequate clean room environment, stent coating, drug loading, final assembling, packaging and warehousing facilities.
These facilities carry potential capability of producing up to 37,000 laser cut stents per annum once the system is fully operational. The manufacturing facility is presently dedicated to production for Genx Sync and Genx Croco Sync, and will start production for DES stents as we acquire product certification etc., research and development and for limited manufacturing for clinical trial purposes, and can be employed for first commercial production at such time, if ever, as we successfully acquire product certification and permits allowing for the sale of the MIVI stent on target markets.
We conduct all of our administrative work in India from our 502 square foot leased facility in Surat, India, and all of our marketing work in India from our 800 square foot leased facility in Mumbai, India. The administrative work for MIVI and MIVT is carried out at our Canadian facility.
The leases on our facilities in Canada and India expire at various dates from November 2008 to August 2021, and have aggregate monthly costs of $15,200 and INR32,000 per month, respectively.
The following selected financial data has been derived from and should be read in conjunction with (i) our audited consolidated financial statements for the years ended May 31, 2007, 2006 and 2005, together with the notes to these financial statements, (ii) our unaudited interim consolidated financial statements as at and for the six months ended November 30, 2007, together with the notes to these financial statements and (iii) the sections of this prospectus entitled "Management's Discussion and Analysis of Financial Condition and Results of Operations" and "Business".
35
In 2001, we acquired 58% of the outstanding shares of M-I Vascular resulting in a change of our business to a stent medical device development company. We acquired the remaining shares of M-I Vascular in May 2003. In March 2005, we acquired 100% of SagaX, Inc., which is in the business of developing a neuro-vascular embolic stent filter medical device and, in December 2007, we completed the sale of all of the issued and outstanding shares of SagaX. In February 2007, we acquired all of the issued and outstanding shares of BioSync Scientific Pvt. Ltd. which is in the business of, among other things, designing, manufacturing and marketing of coated and non-coated vascular stents and related accessories. As a result, the selected financial data provided below may not be comparable from period to period.
Balance Sheet Data
As at | As at | |||||
2007 | 2006 | 2005 | 2004 | 2003 | ||
Cash and cash equivalents | $6,061,952 | $473,419 | $1,573,822 | $492,709 | $2,034,530 | $11,614 |
Working capital (deficiency) | 5,904,159 | (718,679) | 1,521,384 | (478,359) | 2,118,069 | (362,024) |
Total assets | 10,342,690 | 4,246,965 | 2,053,875 | 861,205 | 2,480,074 | 586,097 |
Long-term obligations | 203,488 | 316,529 | 27,609 | - | - | 500,000 |
Total liabilities | 1,563,166 | 2,566,968 | 221,314 | 1,063,449 | 184,456 | 1,085,942 |
Total stockholders' equity (deficit) | 8,779,524 | 1,679,997 | 1,832,561 | (202,244) | 2,295,618 | (499,845) |
Statements of Operation Data
Six Months Ended | Fiscal Years Ended | ||||||
2007 | 2006 | 2007 | 2006 | 2005 | 2004 | 2003 | |
Net revenue | $551,641 | $Nil | $191,490 | $Nil | $Nil | $Nil | $Nil |
Gross profit | 58,577 | Nil | 44,350 | Nil | Nil | Nil | Nil |
Expenses | 6,889,717 | 4,128,365 | 10,522,388 | 9,161,954 | 6,559,013 | 4,013,845 | 3,183,685 |
Loss from operations | (6,831,140) | (4,128,365) | (10,478,038) | (9,161,954) | (6,559,013) | (4,013,845) | (3,183,685) |
Net loss | (6,558,572) | (4,144,987) | (10,499,471) | (9,094,835) | (6,608,882) | (3,471,891) | (3,173,410) |
Basic and diluted loss per share | (0.06) | (0.06) | (0.15) | (0.14) | (0.15) | (0.11) | (0.17) |
Selected Quarterly Financial Data
Quarters Ended | |||||
November 30, | February 28, 2007 | May 31, 2007 | August 31, | November 30, | |
Net revenue | $Nil | $1,461 | $190,029 | $301,610 | $250,031 |
Gross profit | Nil | 815 | 82,299 | 4,240 | 54,337 |
Expenses | 1,779,331 | 2,436,506 | 3,954,293 | 3,245,182 | 3,644,535 |
Loss from operations | (1,779,331) | (2,435,691) | (3,910,758) | (3,240,942) | (3,590,198) |
Net loss | (1,779,155) | (2,383,291) | (3,971,193) | (3,134,652) | (3,423,920) |
Basic and diluted loss per share | (0.03) | (0.03) | (0.05) | (0.04) | (0.03) |
36
Quarters Ended | |||||
August 31, 2005 | November 30, | February 28, | May 31, 2006 | August 31, | |
Net revenue | $Nil | $Nil | $Nil | $Nil | $Nil |
Expenses | 1,945,464 | 2,029,234 | 1,833,360 | 3,353,896 | 2,352,258 |
Loss from operations | (1,945,464) | (2,029,234) | (1,833,360) | (3,353,896) | (2,352,258) |
Net loss | (1,926,855) | (2,016,627) | (1,806,555) | (3,344,798) | (2,365,832) |
Basic and diluted loss per share | (0.04) | (0.03) | (0.03) | (0.05) | (0.03) |
MANAGEMENT'S DISCUSSION AND ANALYSISOF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion should be read in conjunction with (i) our audited financial statements as at and for the years ended May 31, 2007, 2006 and 2005 and the related notes; (ii) our interim unaudited financial statements as at and for the six months ended November 30, 2007 and 2006; and (iii) the section of this prospectus entitled "Business", that appear elsewhere in this prospectus. The following discussion contains forward-looking statements that reflect our plans, estimates and beliefs. Our actual results could differ materially from those discussed in the forward looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed below and elsewhere in this prospectus, particularly in the section entitled "Risk Factors". Our financial statements are stated in United States dollars and are prepared in accordance with United States generally accepted accounting principles.
Our first commercial product could be a passive HAp-coated coronary stent for use in angioplasty procedures followed by additional stent products for drug-elution. Drug-eluting stents have gained significant popularity among the professional medical community. Our goal is to clearly position our company among the leaders in the drug-eluting stent market, and develop a drug-eluting stent with the safety profile of a bare-metal stent.
After completing development of these products, we will have transitioned ourselves to an innovative drug-delivery company with proprietary coating and drug eluting technologies and a company in the field of research, development, manufacturing and distribution of coronary stents and medical accessories for a wide range of therapeutic applications and for the local delivery of a variety of pharmaceutical agents.
Our main focus during the year ended May 31, 2007 and up to November 30, 2007 was the continued research and development of new therapeutic technologies and our biocompatible coating for stent and drug delivery systems. We completed the transfer of technology from UBC to our company-owned premises with focus on the introduction of proper process controls and volume production. This transition was facilitated through the acquisition of sophisticated measuring and processing equipment. In February 2007, we acquired BioSync Scientific and in December 2007 we sold our wholly-owned subsidiary, SagaX. See "Business" for more information.
Our plan of operations for the next twelve months is to:
- focus upon the sales of products through our wholly-owned subsidiary BioSync Scientific;
- continue the research and development of our polymer-free drug eluting stents and delivery systems;
- build up our manufacturing capacity; and
- build up marketing and sales in emerging markets for our medical devices.
37
Based on the assumption that we will continue to raise sufficient capital to execute our business objectives and that we continue to receive favorable pre-clinical and clinical trial results, we anticipate that we will incur an aggregate of between $15,000,000 and $18,000,000 in expenses for the next twelve months as follows:
- $5,000,000 - $8,000,000 allocated to support research and development, regulatory affairs, and pre-clinical and clinical trial activities;
- $3,000,000 allocated to expansion of our manufacturing and research facilities;
- $7,000,000 allocated to working capital which includes: $700,000 for raw materials and supplies used in manufacturing; $300,000 for sales and marketing initiatives; and $800,000 for manufacturing, research and development and operational equipment for our Vancouver and Indian operations.
During the next twelve months we anticipate that we will not generate any significant revenue. We had cash and cash equivalents of $6,061,952 and working capital of $5,904,159 at November 30, 2007. As a result, we anticipate that we will not have sufficient funding to pursue our plan of operations beyond the first quarter of fiscal 2009. We will require additional funding in order to pursue our plan of operations for the next 12 months and subsequently. There can be no assurance that we will obtain any additional financing in the amounts required or on terms favourable to us.
Business Expansion
On July 9, 2007, we completed the private placement of 25,100,000 units sold at $0.50 per unit for aggregate gross proceeds of $12.55 million. Each unit is comprised of one share of common stock of and one-half of one warrant. Each whole warrant entitles the holder to purchase one share of common stock for a period of five years at a price of $0.55 per share. In connection with the private placement, we received net proceeds of $11.7 million. We engaged BMO Capital Markets Corp. as our agent who received placement fees of $753,000 and 251,000 shares of common stock and warrants to purchase 753,000 shares of common stock at $0.55 per share for a period of five years in connection with the private placement.
Intellectual Property
We have eight patent applications which are at various stages of processing by The Patent Office at the present time. Three of these patents are under exclusive license from UBC and five belong 100% to us. For information relating to our patents, see "Business - Intellectual Property and Intangibles".
Research and Development
To date, we have invested approximately $12.8 million in research and development of our stent products, coatings and operations, and in establishing a quality manufacturing facility and completing laboratory and preclinical testing on our stents. We also have developed strong research collaborations with the University of British Columbia for our proprietary stent coatings and have implemented an aggressive in-house product development program.
In order to continue effectively our R & D program and marketing efforts aiming at successful commercialization of our HAp coating technologies, we plan to continue with clinical trials and animal trials as well as acquire additional manufacturing/R&D equipment and hire additional people to complement our current R&D team.
Results of Operations
We have incurred annual operating losses since inception in January 1999 related primarily to the research and clinical development of our technologies and products, corporate development and general administration costs. Our main focus during the year ended May 31, 2007 and up to November 30, 2007 was the continued research and development of new therapeutic technologies and our biocompatible coating for stent and drug delivery systems. We completed the transfer of technology from UBC to our company-owned premises with focus on the introduction of proper process controls and volume production. This transition was facilitated through the acquisition of sophisticated measuring and processing equipment.
38
Six Months Ended November 30, 2007 Compared to the Six Months Ended November 30, 2006
Revenues
During the six months ended November 30, 2007, revenues from the sale of stents were $551,641 compared to nil for the same period of the prior year. Sales were attributed to stents sold by Biosync Scientific in India, which we acquired during the third quarter of fiscal 2007. Cost of goods sold during the six months ended November 30, 2007 were $493,064 resulting in a gross profit of $58,577. There was no comparable data for the same period for the prior year. We expect that revenues will continue to grow throughout the remainder of the fiscal year as a distribution network has recently been established.
General Administrative Expenses
General and administrative expenses increased to $3,665,694 during the six months ended November 30, 2007, from $2,044,979 during the six months ended November 30, 2006. The majority of the overall increase is attributable to an increase in management fees due to the recognition of share-based and cash compensation to Biosync Scientific. Consulting fees increased during the six months ended November 30, 2007 compared to the same period in 2006 as a result of a new investor relations consulting agreement and the recognition of deferred fees associated with new and existing consulting agreements. Audit and legal fees increased in the current period due to our expanded operations in India, the share registration statement and other regulatory filings, and accounting work related to the acquisition of Biosync Scientific. Operating expenses increased significantly in the current period primarily due to the increased activity related to our ongoing clinical trial and preparation for further trials which we anticipate will begin before the end of the current fiscal year.
The following table compares the general and administrative expenses for the six months ended November 30, 2007 and 2006:
November 30 | $Increase/ | %Increase/ | ||
2007 | 2006 | |||
Legal | $289,288 | $244,999 | $44,289 | 18% |
Public relations, financing and corporate development | 381,213 | 463,237 | (82,024) | (18%) |
Management fees | 1,301,806 | 673,720 | 628,086 | 93% |
Consulting | 298,951 | 72,683 | 226,268 | 311% |
Audit | 408,500 | 120,899 | 287,601 | 238% |
Operating expenses | 985,936 | 469,441 | 516,495 | 110% |
Total | $3,665,694 | $2,044,979 | $1,620,715 | 79% |
Research and Development Expenses
Research and development costs increased during the six months ended November 30, 2007 to $2,350,446 from $1,615,244 for the six months ended November 30, 2006.
The increase in the current period resulted primarily from the ongoing clinical trial that began in late fiscal 2007, the addition of new R&D employees, increased payments to purchase materials, more significant legal fees incurred for patents and technology protection, and more share-based incentives granted to R&D personnel. We expect research and development costs to increase in the remaining six months of the fiscal year as the more comprehensive second phase of the clinical trial is scheduled to begin before the end of the year.
39
Interest Expense and Finance Fees
Interest expense and finance fees increased $172,605 to $174,663 during the six months ended November 30, 2007 from $2,059 during the same period in 2006. The increase primarily resulted from a $125,500 charge from share registration costs associated with the private placement that occurred in July 2007 and the fair values of: (i) warrants granted to extend the due date of a note payable and (ii) the extended expiry of certain warrants. We do not expect to incur such expenses in subsequent quarters of fiscal 2008.
Depreciation and Amortization Expense
Depreciation and amortization expense increased to $258,477 during the six months ended November 30, 2007 compared to $51,001 for the six months ended November 30, 2006. Approximately $95,000 of depreciation expense has been allocated to cost of sales during the current six month period. The overall increase in depreciation and amortization expense is due to amortization of $80,514 related to the CE Mark license acquired during the third quarter of fiscal 2007 and significant additions to laboratory equipment, building and other property and equipment acquired during the second half of fiscal 2007 and the current year.
Expenses Related to Assets and Liabilities to be Transferred
During the current quarter, we entered into a share purchase agreement to sell our wholly-owned subsidiary SagaX. Accordingly, SagaX's results of operations for the six months ended November 30, 2007 and 2006 have been reflected as expenses related to assets and liabilities to be transferred. We incurred expenses of $600,955 during the six months ended November 30, 2007 compared to $420,567 during the six months ended November 30, 2006. The increase of $180,388 is primarily attributed to continued research and development costs and the use of additional consultants to assist with that research.
Net Loss
We incurred a net loss of $6,558,572 for the six months ended November 30, 2007 compared to $4,144,987 for the same period in 2006. The increase in net loss of $2,413,585 during 2007 is primarily a result of the elevated research and development activities and the related administrative costs compared to 2006, including the ongoing clinical trial, and higher legal, travel and other operating costs associated with the increased level of research. We believe this level of activity will increase during the remainder of the current fiscal year as the completion of the first phase of the clinical trial nears and the preparation for the subsequent clinical trial begins.
Year Ended May 31, 2007 Compared to the Year Ended May 31, 2006
Revenues
In the year ended May 31, 2007, we had revenues of $191,490 from the sale of products by Biosync Scientific, which we acquired in February 2007. We have not generated any revenues in any prior period and there can be no assurance that we will generate any significant revenues in the future.
General & Administrative Expenses
General and administrative expenses increased to $6,595,471 in the year ended May 31, 2007, from $6,138,912 in the year ended May 31, 2006. The majority of the overall increase is attributed to the significant increase in management fees which related to the estimated fair value of stock options granted in 2007 being higher than those granted in 2006 as well as increasing travel and overhead costs. The significant reduction in investor relations arose from the reduction of warrants issued for such services in 2007. Other factors contributing to the overall increase were the increase in legal fees related to more corporate activity, and a decrease in audit fees.
The following table compares the General and Administrative expenses for the years ended May 31, 2007 and 2006:
40
2007 | 2006 | $Increase/ | %Increase/ | |
Legal | $517,063 | $426,776 | $90,287 | 21% |
Public relations, financing and corporate development | 1,235,456 | 2,657,383 | (1,421,927) | (54%) |
Management fees | 2,533,989 | 1,453,613 | 1,080,376 | 74% |
Consulting | 655,319 | 503,602 | 151,717 | 30% |
Audit | 158,155 | 281,620 | (123,465) | (44%) |
Operating expenses | 1,495,489 | 815,918 | 679,571 | 83% |
Total | $6,595,471 | $6,138,912 | $456,559 | 7% |
Research & Development Expenses
Research and development costs increased during the year ended May 31, 2007 to $3,730,570 compared to $2,792,251 for the year ended May 31, 2006. The increase in 2007 resulted primarily from the addition of several R&D people, the estimated fair value of additional stock option grants and increased payments to outside companies for testing.
Depreciation and Amortization Expense
Depreciation and amortization expenses decreased to $96,404 during the year ended May 31, 2007 compared to $143,754 for the year ended May 31, 2006. This decrease is due to the reclassification of depreciation relating to certain laboratory equipment to research and development expenses.
Interest Expense and Finance Fees
In the year ended May 31, 2007, we incurred interest expense and finance fees of $99,943, compared to $87,037 in the year ended May 31, 2006, related primarily to private placements.
Net Loss
As a result of the above, our net loss in the year ended May 31, 2007 was $10,499,471, compared to $9,094,835 in the year ended May 31, 2006.
Year Ended May 31, 2006 Compared to the Year Ended May 31, 2005
Revenues
We did not generate any revenues in the years ended May 31, 2006 or 2005.
General & Administrative Expenses
General and administrative expenses increased to $6,138,912 during the year ended May 31, 2006, compared to $2,720,260 for the year ended May 31, 2005. The majority of the overall increase is attributable to the amortization of warrants granted for public relations, financing and corporate development, the estimated fair value of stock options granted to directors and employees, the significant increase in press releases, and increased operating expenses resulting from our advanced testing. Legal and audit expenses increased primarily as a result of the proposed acquisition of SMT. Consulting expenses decreased in 2006 because no stock options were granted to consultants during the current year.
The following table compares the General and Administrative expenses for the years ended May 31, 2006 and 2005:
41
2006 | 2005 | $Increase/ | %Increase/(Decrease) | |
Legal | $426,776 | $195,379 | $231,397 | 118% |
Public relations, financing and corporate development | 2,657,383 | 935,337 | 1,722,046 | 184% |
Management fees | 1,453,613 | 261,883 | 1,191,730 | 455% |
Consulting | 503,602 | 793,426 | (289,824) | (36%) |
Audit | 281,620 | 51,110 | 230,510 | 451% |
Operating expenses | 815,918 | 483,125 | 332,793 | 69% |
Total | $6,138,912 | $2,720,260 | $3,418,652 | 126% |
Research & Development Expenses
Research and development costs increased during the year ended May 31, 2006 to $2,792,251 compared to $1,578,408 for the year ended May 31, 2005. The increase in 2006 resulted primarily from our advanced research and development in our coating technology which included animal trials performed during the year, expansion of our technology portfolio, the addition of several R&D people and the estimated fair value of options granted to certain consultants. In addition, quarterly payments were being made for the collaborative research agreement which started in the middle of the last fiscal year and continued for the whole of the current fiscal year.
Depreciation and Amortization Expense
Depreciation and amortization expenses decreased to $143,754 during the year ended May 31, 2006 compared to $176,453 for the year ended May 31, 2005. This decrease is due to several laboratory equipment items becoming fully depreciated during the year.
Interest Expense and Finance Fees
In the year ended May 31, 2006, we incurred interest expense and finance fees of $87,037, compared to $nil in the year ended May 31, 2005, relating primarily to private placements.
Net Loss
In the year ended May 31, 2006, as a result of the above, the net loss was $9,094,835, compared to $6,608,882 in the year ended May 31, 2005.
Liquidity and Capital Resources
We have incurred annual operating losses since inception, and, as of November 30, 2007, we had an accumulated deficit of $48,185,987. We expect to continue to incur losses over the next few years as we continue our clinical trials, apply for regulatory approvals, continue development of our technologies, and expand our operations.
At November 30, 2007, we had cash and cash equivalents in the amount of $6,061,952 and working capital of $5,904,159. Our planned expenditures over the next 12 months are expected to be approximately $15 to $18 million. As a result, we do not have sufficient funding to pursue our plan of operations beyond the fist quarter of fiscal 2009. We will require additional funding in order to pursue our plan of operations for the next 12 months and subsequently. There can be no assurance that we will obtain any additional financing in the amounts required or on terms favourable to us. If we are unable to obtain additional financing, we may have to abandon our business activities and plan of operations. We believe that debt financing will not be an alternative for funding as we do not have tangible assets to secure any debt financing. We anticipate that additional funding will be in the form of equity financing from the sale of our common stock. However, we cannot provide investors with any assurance that we will be able to raise sufficient funding from the sale of our common stock to fund our plan of operations going forward. Even if we are successful in obtaining additional financing, there is no assurance that we will obtain the financing necessary to pursue our business plan.
42
Operating Activities
Net cash used in operations during the six months ended November 30, 2007 was $5,289,627, compared to $2,573,937 in the six months ended November 30, 2006, which is a direct result of establishing and expanding operations in India, conducting the first clinical trial and the preparation required for the second clinical trial as well as continuing to fund our research program. Our management expects that our ongoing clinical trial and subsequent clinical trials, along with the expansion of our India operations and our other research activities, which will lead to further operating losses during the remainder of fiscal 2008.
Operating activities in the year ended May 31, 2007 used cash of $6,091,341, compared to $4,743,065 in the year ended May 31, 2006 and $2,935,951 in the year ended May 31, 2005. We have funded our operations from our financings.
Investing Activities
Net cash used in investing activities during the six months ended November 30, 2007 was $532,849, compared to $115,165 in the prior period of 2006, and was a result of further capital investments in property and equipment necessary to increase capacity at our facility in India and support our research activities. We anticipate that further investments in laboratory equipment, land and other property and equipment will be needed to further develop our manufacturing capabilities in India and to conduct future research activities throughout the remainder of fiscal 2008 and fiscal 2009.
In the year ended May 31, 2007, investing activities used cash of $1,857,426, compared to $259,851 in the year ended May 31, 2006 and $221,593 in the year ended May 31, 2005. Investing activities in fiscal 2007 used cash primarily in connection with the acquisition of Biosync Scientific. Investing activities in fiscal 2006 and 2005 used cash primarily in connection with the purchase of property and equipment.
Financing Activities
We have funded our business to date primarily from sales of our common stock. In the six months ended November 30, 2007, financing activities provided cash of $11,411,009, compared to $1,474,000 in the prior period of 2006, primarily from the issuance of our common stock. In the year ended May 31, 2007, financing activities provided cash of $6,848,364, compared to $6,094,602 in the year ended May 31, 2006 and $1,639,703 in the year ended May 31, 2005, primarily from the sale of our common stock.
In July 2007, we completed the private placement of 25,100,000 units at a price of $0.50 per unit for aggregate gross proceeds of $12.55 million (net proceeds of $11.7 million).
Warrants
As at November 30, 2007, we had warrants outstanding to acquire up to 41,777,676 shares of our common stock at a weighted average exercise price of $0.43 per share.
During the three months ended November 30, 2007, our board of directors approved an extension to the expiry date of 304,762 warrants. The estimated fair value of the extended warrants was $89,300 and was charged to operations.
During the year ended May 31, 2007, we issued 200,000 warrants with an exercise price of $0.50 per share for consulting services rendered and 100,000 warrants with an exercise price of $0.60 per share for research and development services rendered to us. These warrants had an estimated fair value of $57,109 using the Black-Scholes option pricing model.
During the year ended May 31, 2007, our board of directors approved an extension to the expiry date of the following outstanding warrants:
43
Number of Warrants | From | To |
71,429 | September 8, 2006 | September 8, 2007 |
500,000 | October 24, 2006 | April 24, 2008 |
1,000,000 | November 5, 2006 | May 5, 2008 |
As a result, we incurred $72,452 and $73,531 of public relations expense and finance fees, respectively.
During the year ended May 31, 2006, we issued 4,150,000 warrants for consulting services and 900,000 warrants for research and development rendered to us. The warrants issued for consulting services have an exercise price ranging from $0.50 to $0.85 per share and the warrants issued for research and development services have an exercise price of $0.01 per share. These warrants had an estimated fair value of $2,067,663, using the Black-Scholes option pricing model.
During the year ended May 31, 2006, our board of directors approved an extension to the expiry date of the following outstanding warrants:
Number of Warrants | From | To |
366,800 | April 30, 2006 | July 31, 2006 |
71,429 | March 8, 2006 | September 8, 2006 |
1,000,000 | November 5, 2005 | November 5, 2006 |
500,000 | October 24, 2005 | October 24, 2006 |
75,000 | September 2, 2005 | September 2, 2006 |
As a result, we incurred $149,013 and $45,831 of public relations expense and finance fees, respectively.
During the year ended May 31, 2005, we issued 5,270,000 warrants with exercise prices ranging from $0.24 to $0.45 per share, to various consultants for services rendered to us. These warrants had an estimated fair value of $917,168 using the Black-Scholes option pricing model.
Stock-based Compensation
Our incentive stock option plan provides for the grant of incentive stock options to purchase up to 25,000,000 shares to our employees, consultants, officers and directors. Incentive benefits granted under the plan may be either incentive stock options, non-qualified stock options, stock awards, restricted shares or cash awards. Options are granted for a term not to exceed seven years from the date of grant. Stock options granted generally vest over a period of two years.
As at November 30, 2007, we had options to acquire up to 19,916,299 shares of our common stock outstanding at a weighted average exercise price of $0.49 per share.
During the six months ended November 30, 2007, we granted an aggregate of 1,096,300 stock options to our employees. Each option entitles its holder to acquire one share of our common stock at prices ranging from $0.55 to $0.65 per share, vesting over a specified time and expiring up to five years from date of grant.
During the six months ended November 30, 2007, our board of directors approved an extension of 150,000 options from expiry date of November 30, 2007 to November 30, 2010. As a result of the extension, we recognized an additional $48,400 of consulting fees in the current quarter.
During the year ended May 31, 2007, we granted an aggregate of 4,414,999 stock options. All of the options granted were to our employees and/or directors. Each option entitles the option holder to acquire one share of our common stock at a price between $0.55 and $0.67 per share, vesting over a specified time and exercisable for a period of two to five years from the date of grant.
44
During the year ended May 31, 2006, we granted an aggregate of 11,185,000 stock options to our employees/directors. Each option entitles its holder to acquire one share at prices ranging from $0.20 to $1.10 per share, being vested immediately or at a specified time and exercisable for a period of seven years from date of grant or term of agreement.
During the year ended May 31, 2005, we granted an aggregate of 3,900,000 stock options of which 2,200,000 options were granted to employees and/or directors and the remaining 1,700,000 were granted to consultants. Each option entitles the option holder to acquire one share our common stock at a price between $0.20 and $0.40 per share, vesting immediately or at a specified time and exercisable for a period of five years from the date of grant or term of agreement.
Contractual Obligations
The following table sets forth our contractual obligations as at May 31, 2007:
Payments due by period | |||||
Total | Less than 1year | 1-3 years | 3-5 years | More than5 years | |
Debt | $625,000 | $625,000 | $ - | $ - | $ - |
Capital Lease Obligations | - | - | - | - | - |
Operating Leases | 378,081 | 105,511 | 211,022 | 61,548 | - |
Purchase Obligations | - | - | - | - | - |
Other Long-Term Liabilities | - | - | - | - | - |
Reflected on the Registrant's | - | - | - | - | - |
Balance Sheet under GAAP | - |
| - | - | - |
Total | $1,003,081 | $730,511 | $211,022 | $61,548 | $ - |
Going Concern
There is substantial doubt as to our ability to continue as a going concern based on our past operating losses and predicted future operating losses. Our auditor has issued a going concern opinion on our financial statements expressing substantial doubt that we can continue as a going concern for a reasonable period of time unless sufficient equity financing can be secured. There can be no assurances that any required capital can be obtained on terms favourable to us.
Material Commitments
We have the following material commitments:
- We have obligations under long-term premises leases that expire in December 2010. The aggregate minimum rental payments for the next four annual periods ending May 31, 2011 are:
(a) $105,511 in each of 2008, 2009 and 2010; and
(b) $61,548 in 2011.
45
- In connection with the acquisition of SagaX, we agreed to issue 4,200,000 shares in exchange for all of the issued and outstanding shares of SagaX. We have issued 2,000,000 of these shares to date; however, the issuance of the remaining 2,200,000 shares was contingent upon certain performance milestones which were not achieved and, therefore, these shares have not been issued. We completed the sale of SagaX in December 2007. See "Business - Recent Developments - Sale of SagaX".
Off-Balance Sheet Arrangements
As of the date of this registration statement we do not have any off-balance sheet arrangements that have or are reasonably like to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors.
Critical Accounting Policies
Our financial statements are impacted by the accounting policies used and the estimates and assumptions made by management during their preparation. A complete summary of these policies is included in Note 2 to the May 31, 2007 consolidated financial statements. We have identified below the accounting policies that are of particular importance in the presentation of our financial position, results of operations and cash flows and which require the application of significant judgment by management.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions which affect the reporting of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements as of the dates of the financial statements and revenues and expenses during the reporting period. Significant estimates include amortization of property and equipment, calculation of stock-based compensation, amortization of CE Mark License and valuation allowance for deferred income taxes. Actual results could differ from these estimates.
Revenue Recognition
We recognize revenue, net of returns, rebates and sales allowances, if any from the sale of products, at the time when the product is delivered to the customer and/or dealer. Revenues are recognized only when we have transferred to the customer and/or dealer the significant risk and rewards of ownership of the goods, title to the products transfers, the amount is fixed and determinable, evidence of an agreement exists, there is reasonable assurance of collection of the sales proceeds, we have no future obligations and the customer and/or dealer bears the risk of loss.
Research and Development Costs
Research and development costs are expensed in the period incurred. For the six months ended November 30, 2007 and year ended May 31, 2007, $299,106 and $721,642 (2006 - $206,304 and $89,600), respectively, of stock-based compensation expense was related to options granted to research and development personnel.
Stock-Based Compensation
In December 2004, the FASB issued SFAS No. 123R, "Share-Based Payment", which replaced SFAS No. 123, "Accounting for Stock-Based Compensation" and superseded APB Opinion No. 25, "Accounting for Stock Issued to Employees". In January 2005, the SEC issued Staff Accounting Bulletin ("SAB") No. 107, "Share-Based Payment", which provides supplemental implementation guidance for SFAS No. 123R. SFAS No. 123R requires all share-based payments to employees, including grants of employee stock options, to be recognized in the financial statements based on the grant date fair value of the award. SFAS No. 123R was to be effective for interim or annual reporting periods beginning on or after June 15, 2005, but in April 2005 the SEC issued a rule that will permit most registrants to implement SFAS No. 123R at the beginning of their next fiscal year, instead of the next reporting period as required by SFAS No. 123R. The pro-forma disclosures previously permitted under SFAS No. 123 no longer will b e an alternative to financial statement recognition. Under SFAS No. 123R, we must determine the appropriate fair value model to be used for valuing share-based payments, the amortization method for compensation cost and the transition method to be used at date of adoption. The transition methods include prospective and retroactive adoption options.
46
We adopted the modified prospective method for the fiscal quarter that began on June 1, 2006. Stock-based compensation expense for awards granted prior to June 1, 2006 was based on the grant date fair-value as determined under the pro-forma provisions of SFAS No. 123.
We recorded stock-based compensation expense of $2,241,100 during the year ended May 31, 2007 as a result of the adoption of SFAS No. 123R.
As of November 30, 2007, $705,190 of total unrecognized compensation cost related to stock options is expected to be recognized over a weighted-average period of approximately two years.
Prior to the adoption of SFAS No. 123R, we measured compensation expense for our employee stock-based compensation plans using the intrinsic value method prescribed by APB Opinion No. 25. We applied the disclosure provisions of SFAS No. 123 as amended by SFAS No. 148, "Accounting for Stock-Based Compensation - Transition and Disclosure", as if the fair-value-based method had been applied in measuring compensation expense. Under APB Opinion No. 25, when the exercise price of our employee stock options was equal to the market price of the underlying stock on the date of the grant, no compensation expense was recognized.
Recent Accounting Pronouncements
The Financial Accounting Standards Board ("FASB") has issued the following pronouncements:
In July 2006, FASB issued Interpretation No. 48 "Accounting for Uncertainty in Income Taxes - an interpretation of FASB Statement No. 109" ("FIN 48"). This Interpretation clarifies the accounting for uncertainty in income taxes recognized in an enterprise's financial statements in accordance with SFAS Statement No. 109, "Accounting for Income Taxes". This Interpretation prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. This Interpretation also provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition. This Interpretation is effective for fiscal years beginning after December 15, 2006.
The Company is subject to taxation in the U.S. and various states and foreign jurisdictions. In addition to U.S., the Company's major taxing jurisdictions include Canada, India and Israel. Income tax returns filed by the Company and its active subsidiaries that are still subject to examination are MIVI Technologies, Inc. for May 31, 2006 and subsequent years, Biosync Scientific Pvt. Ltd. for March 31, 2006 and subsequent years and SMT Research and Development Ltd. for December 31, 2006 and subsequent years. Income tax returns not yet filed by MIV Therapeutics Inc. for the fiscal year ended May 31, 1999, and subsequent years will be subject to examination. Interest and penalties related to tax positions taken in tax returns shall be recorded in the unaudited interim consolidated statement of operations; however, there were no interest and penalties related to tax positions taken in our tax returns during the first two quarters of fiscal 2008.
The Company adopted the provisions of FASB Interpretation No. 48 on June 1, 2007. The adoption of FIN 48 did not result in a cumulative adjustment to equity and there were no unrecognized tax benefits, penalties or interest at the time of, or subsequent to, adoption.
In February 2007, the FASB issued SFAS No. 159, "The Fair Value Option for Financial Assets and Financial Liabilities" ("SFAS 159"). SFAS 159 permits entities to choose to measure many financial assets and financial liabilities at fair value. Unrealized gains and losses on items for which the fair value option has been elected will be reported in earnings. SFAS 159 is effective for fiscal years beginning after November 15, 2007. The Company currently is assessing the impact of SFAS 159 on its' consolidated financial position or results of operation.
In December 2007, the FASB issued SFAS No. 160, "Noncontrolling Interests in Consolidated Financial Statements, an Amendment of ARB No. 51". This statement amends ARB 51 to establish accounting and reporting standards for the noncontrolling interest (minority interest) in a subsidiary and for the deconsolidation of a subsidiary. Upon its adoption, effective as of the beginning of the Company's fiscal 2010, noncontrolling interests will be classified as equity in the Company's balance sheet and income and comprehensive income attributed to the noncontrolling interest will be included in the Company's income and comprehensive income. The provisions of this standard must be applied prospectively upon adoption except for the presentation and disclosure requirements. Management is currently evaluating the impact of adopting SFAS No. 160 on the Company's consolidated financial position and results of operations.
47
In December 2007, the FASB issued SFAS No. 141 (revised 2007), "Business Combinations" ("SFAS No. 141(R)"). SFAS No. 141(R) establishes principles and requirements for how an acquirer in a business combination recognizes and measures the assets acquired, liabilities assumed, and any noncontrolling interest in the acquiree. The provisions of SFAS No. 141(R) will become effective for the Company's business combinations occurring on or after June 1, 2009.
quantitative and qualitative disclosures about market risk
We are subject to risks related to foreign currency exchange rate fluctuations. However, they have not had a material impact on our results of operations to date.
Our functional currency is the United States dollar. However, a significant portion of our business is transacted in other currencies (the Canadian dollar and the Indian Rupee). As a result, we are subject to exposure from movements in foreign currency exchange rates. We do not use derivative financial instruments for speculative trading purposes, nor do we hedge our foreign currency exposure to manage our foreign currency fluctuation risk.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Related Party Transactions
The following services were provided by related parties. These transactions, recorded at exchange amounts agreed to by all parties, were as follows:
During the six months ended November 30, 2007, the Company paid or accrued $1,093,847 (2006 - $420,149) of management and consulting fees to four directors and two officers (2006 - 1 officer) of the Company ($137,759 to Alan Lindsay, $150,000 to Mark Landy, $83,855 to Pat McGowan, $601,251 to Rajesh Vaishnav, $79,860 to Dov Shimon (former director) and $41,122 to Tom Troczynski (former officer)). Of this amount, $120,982 (2006 - $110,025) was charged to research and development. Included in accounts payable is $nil at November 30, 2007 (2006 - $Nil).
Included in the amount of $1,093,847 is the accrued amount of $526,251 due to the Chief Operating Officer and President of Biosync (Rajesh Vaishnav), which will be issued in common stock on time periods stipulated in the agreement as follows:
The Company and Biosync entered into an executive services agreement with the principal vendor, being Mr. Rajesh Vaishnav, on February 16, 2007. Mr. Vaishnav will assume the position of Chief Operating Officer and President of Biosync under such commercially competitive compensation terms which will include, but not limited to, (i) a monthly fee of $12,000 plus monthly allowance of $500, (ii) stock options of up to 1,000,000 common shares at an exercise price of $0.60 for a period of not less than ten years from the date of grant and, (iii) an aggregate of up to 4,000,000 common shares with an issuance price of $0.50. Of the 4,000,000 common shares, 2,500,000 will be based on the achievement of certain milestones as outlined in the agreement, of which 750,000 common shares (issued as of May 31, 2007) upon receiving CE Mark License and the other 1,500,000 common shares to be given in four equal installments over the two-year term of the agreement, of which, 375,000 were issued on September 28, 2007. The fair value of the options and common shares are treated as stock-based compensation expenses and amortized over the service period.
48
At November 30, 2007, amounts due from the employees of a subsidiary of the Company totaled $15,964 (May 31, 2007 - $34,775). These amount are unsecured, non-interest bearing and will be repaid by periodic deduction of future wages.
On August 31, 2007, the $100,000 related party loan due to the Company's former Chief Executive Officer (Mr. Lindsay) was fully repaid.
During the year ended May 31, 2007, the Company paid or accrued $1,002,702 of management and consulting fees to four directors and two officers of the Company ($284,719 to Alan Lindsay, $154,158 to Patrick McGowan, $285,600 to Mark Landy, $43,457 to Rajesh Vaishnav, $160,490 to Dov Shimon (former director) and $74,278 to Tom Troczynski). Of this amount, $234,768 was charged to research and development.
During the year ended May 31, 2006, the Company paid or accrued $757,859 of management and consulting fees to four directors and officers of the Company ($233,393 to Alan Lindsay, $130,481 to Patrick McGowan, $153,998 to Dhirajlal Kotadia, $38,000 to Mark Landy, $133,100 to Dov Shimon (former director) and $68,887 to Tom Troczynski). Of this amount, $201,987 was charged to research and development expense. Included in accounts payable at May 31, 2006 is $9,106 due to these parties.
Material Contracts
The Company has entered into employment and services and other agreements with certain of its executive officers, which are described under "Executive Compensation - Employment Contracts".
On November 18, 2004, as extended on July 1, 2005, the Company entered into a Letter of Engagement with Trilogy Capital Partners, Inc. to develop and implement a proactive marketing program to increase public awareness of the Company in consideration of payment by the Company of certain monthly fees and the issuance of certain warrants. The Agreement has now expired effective on June 30, 2007.
On September 22, 2005, the Company entered into an Industrial Lease with Investors Group Trust Co. Ltd. as Trustee Investors Real Property Fund under which the Company will lease 10,296 square feet with monthly minimum rent of $8,207 for a term of five years commencing on January 1, 2006.
MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Shares of our common stock are quoted on the OTCBB under the symbol "MIVT". Our common stock is also quoted on each of the Munich and Frankfurt stock exchanges under symbols "MIV.MU" and "MIV.F", respectively. The following table indicates the high and low bid prices of our common stock during the periods indicated on the OTCBB. The source of the high and low bid information is the OTCBB. The market quotations provided reflect inter-dealer prices, without retail mark-up, markdown or commission and may not represent actual transactions.
Quarter Ended | High Bid | Low Bid |
November 30, 2007 | $0.69 | $0.32 |
August 31, 2007 | $0.68 | $0.40 |
May 31, 2007 | $0.65 | $0.41 |
February 28, 2007 | $0.91 | $0.50 |
November 30, 2006 | $0.91 | $0.50 |
August 31, 2006 | $0.91 | $0.50 |
May 31, 2006 | $1.10 | $0.60 |
February 28, 2006 | $1.45 | $0.66 |
November 30, 2005 | $1.74 | $0.97 |
August 31, 2005 | $1.75 | $0.48 |
On February 28, 2008, the closing price of our shares of common stock on the OTCBB was $0.37.
Penny Stock
Our common stock is considered "penny stock". The SEC has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a price of less than $5.00, other than securities registered on certain national securities exchanges or quoted on the NASDAQ system, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or quotation system. The penny stock rules require a broker-dealer, prior to a transaction in a penny stock, to deliver a standardized risk disclosure document prepared by the SEC, that: (a) contains a description of the nature and level of risk in the market for penny stocks in both public offerings and secondary trading; (b) contains a description of the broker's or dealer's duties to the customer and of the rights and remedies available to the customer with respect to a violation to such duties or other requirement s of securities' laws; (c) contains a brief, clear, narrative description of a dealer market, including bid and ask prices for penny stocks and the significance of the spread between the bid and ask price; (d) contains a toll-free telephone number for inquiries on disciplinary actions; (e) defines significant terms in the disclosure document or in the conduct of trading in penny stocks; and (f) contains such other information and is in such form, including language, type, size and format, as the SEC shall require by rule or regulation. The broker-dealer also must provide, prior to effecting any transaction in a penny stock, the customer with: (a) bid and offer quotations for the penny stock; (b) the compensation of the broker-dealer and its salesperson in the transaction; (c) the number of shares to which such bid and ask prices apply, or other comparable information relating to the depth and liquidity of the market for such stock; and (d) a monthly account statements showing the market value of each penny stock held in the customer's account. In addition, the penny stock rules require that prior to a transaction in a penny stock not otherwise exempt from those rules; the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser's written acknowledgement of the receipt of a risk disclosure statement, a written agreement to transactions involving penny stocks, and a signed and dated copy of a written suitably statement.
These disclosure requirements may have the effect of reducing the trading activity in the secondary market for our stock if it becomes subject to these penny stock rules. Therefore, if our common stock becomes subject to the penny stock rules, stockholders may have difficulty selling those securities.
As at February 28, 2008, we had approximately 210 registered holders of our common stock.
There are no restrictions in our Articles or Bylaws that prevent us from declaring dividends. TheNevada Revised Statutes, however, do prohibit us from declaring dividends where, after giving effect to the distribution of the dividend:
- we would not be able to pay our debts as they become due in the usual course of business; or
- our total assets would be less than the sum of our total liabilities plus the amount that would be needed to satisfy the rights of shareholders who have preferential rights superior to those receiving the distribution.
We have not declared any dividends and we do not plan to declare any dividends in the foreseeable future.
50
Equity Compensation Plans
We have stock option plans approved by securityholders, the 2001, 2004 and 2006 Stock Option Plan (the "Plans"). The table set forth below presents information relating to our equity compensation plans as of November 30, 2007:
Plan Category | Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights | Weighted-Average Exercise Price of Outstanding Options, Warrants and Rights | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (excluding column (a)) |
Equity Compensation Plans Approved by Security Holders (2001, 2004 and 2006 Stock Option Plans) | 19,916,299 | 0.49 | 3,543,701 |
Equity Compensation Plans Not Approved by Security Holders | 6,221,500(1) | 0.48 | N/A |
(1) Represents shares of our common stock to be issued upon the exercise of warrants issued as compensation for services.
Warrants
We currently have warrants outstanding to acquire an aggregate of 38,012,678 shares of our common stock at exercise prices ranging from $0.24 to $1.55 per share and with expiry dates ranging from May 1, 2008 to October 28, 2012.
Stock Options
We currently have options outstanding to acquire an aggregate of 22,791,299 shares of our common stock at exercise prices ranging from $0.17 to $1.10 per share and with expiry dates ranging from April 23, 2008 to May 17, 2013.
Compensation Discussion and Analysis
The Company's Board of Directors is responsible for establishing and administering the Company's executive and director compensation.
The Board of Director's compensation objective is designed to attract and retain the best available talent while efficiently utilizing available resources. The Board compensates executive management primarily through base salary and equity compensation designed to be competitive with comparable employers in the location of countries in which it operates (primarily North America, India and Israel), and to align management's compensation with the long-term interests of shareholders. In determining an executive management's compensation, the Board also takes into consideration the financial condition of the Company and discussions with the executive.
In determining the compensation for Messrs. Lindsay, Landy, and McGowan, the Board considered compensation paid to other executive officers of other companies within the industry, the executive's performance in meeting goals, and the complexity of the management position and the experience of the person. Of the amount of the compensation paid to the executive officer, a large part of the compensation was in the form of options. The number of options granted was determined in large part due to the financial condition of the Company which currently has minimal revenues. The Board did not have a specific formula to determine the amount of the executive compensation and what portion of such compensation would be in the form of cash and equity securities. Therefore, the determination of an executive salary including the amount of cash and equity securities may be considered arbitrary taking into account the foregoing factors. The compensation paid to Messrs. Shimon (who is no longer a dire ctor or officer) and Vaishnav were based on individual negotiations with such individuals in connection with the acquisition of their respective companies.
51
Directors do not receive cash compensation for their service as such, but do receive options. The number of options granted to each director is based on the experience of the director, time spent on Company matters and the director compensation paid to other directors of companies in the industry.
Summary Compensation Table
The following table sets forth the compensation paid to our Chief Executive Officer and Chief Financial Officer and three highest paid executive officers that earned in excess of $100,000 for the periods indicated (collectively, the "Named Executive Officers"):
Summary Compensation Table | |||||||||
Name and | Year | Salary | Bonus | Stock | Option | Non-Equity | Non-Qualified | All other | Total |
($) | ($) | ($) | ($) | ($) | ($) | ($) | ($) | ($) | |
Alan P. Lindsay, | 2007 | 256,732 | - | 27,987 | 235,283(1) | - | - | - | 520,002 |
2006 | 233,393 | - | - | 1,582,183(1) | - | - | 1,815,572 | ||
2005 | 185,244 | - | - | 62,853(1) | 248,097 | ||||
Dr. Mark Landy, President and Director | 2007 | 240,000 | - | 45,600 | 235,283(2) | - | - | - | 520,883 |
2006 | 38,000 | - | - | 2,049,208(2) | - | - | - | 2,087,208 | |
Patrick A. McGowan, Chief Financial Officer, Executive Vice President, Secretary | 2007 | 141,311 | - | 12,847 | 56,467(3) | - | - | - | 210,625 |
2006 | 130,481 | - | - | 71,752(3) | - | - | - | 202,233 | |
2005 | 101,941 | - | 50,283(3) | - | - | - | 152,224 | ||
Dr. Dov Shimon, President of SagaX Inc. and Former Director | 2007 | 146,410 | - | 14,080 | - | - | - | - | 160,490 |
2006 | 133,100 | - | - | 151,923(4) | - | - | - | 285,023 | |
2005 | 101,000 | - | - | 71,712(4) | - | - | - | 172,712 | |
2006 | 68,887 | - | - | - | - | - | - | 68,887 | |
2005 | 57,718 | - | - | - | - | - | - | 57,718 | |
Rajesh Vaishnav, President and Chief Operating Officer of BioSync Scientific Pvt. Ltd | 2007 | 43,457 | - | - | 513,760(5) | 557,217 | |||
(1) Represents options to acquire 500,000 shares in 2007; 3,800,000 shares in 2006 and 500,000 shares in 2005.
(2) Represents options to acquire 500,000 shares in 2007; 5,000,000 shares in 2006.
(3) Represents options to acquire 120,000 shares in 2007; 200,000 shares in 2006 and 400,000 shares in 2005.
(4) Represents options to acquire 400,000 shares in 2006 and 500,000 shares in 2005. Dr. Shimon ceased being a director and officer of the Company on November 13, 2007 and the options held by him expire on February 13, 2008 and May 13, 2008, as applicable.
(5) Represents options to acquire 1,000,000 shares in 2007.
52
The following table sets forth information relating to options granted to the Named Executive Officers in the year ended May 31, 2007:
Grants of Plan-Based Awards | |||||||||||
Name | Grant date | Estimated future payouts under non-equity incentive plan awards | Estimated future payouts under equity incentive plan awards | All other stock awards Number of shares of stock or units (#) | All other option awards: Number of securities underlying options | Exercise or base price of option awards | Grant date fair value of stock and option awards | ||||
Thres-hold | Target | Maximum | Threshold | Target | Maximum | (#) | ($) | ($) | |||
Alan P. Lindsay | Apr. 3, 2007 | - | - | - | - | - | - | - | 500,000 | 0.55 | 235,283 |
Dr. Mark Landy | Apr. 3, 2007 | - | - | - | - | - | - | - | 500,000 | 0.55 | 235,283 |
Patrick A. McGowan | Apr. 3, 2007 | - | - | - | - | - | - | - | 120,000 | 0.55 | 56,467 |
Dr. Dov Shimon | Nil | - | - | - | - | - | - | - | - | - | - |
Rajesh Vaishnav | Apr. 9, 2007 | - | - | - | - | - | - | - | 1,000,000 | 0.60 | 513,760 |
The following table sets forth information relating to options held by the Named Executive Officers as at May 31, 2007:
Outstanding Equity Awards At Fiscal Year-End Table | |||||||||
Option Awards | Stock Awards | ||||||||
Name | Number of Securities Underlying Unexercised Options | Number of Securities Underlying Unexercised Options | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options | Option Exercise Price | Option | Number of Shares or Units of Stock That Have Not Vested | Market Value of Shares or Units of Stock That Have Not Vested | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested |
Alan P. Lindsay, Chairman and Chief Executive Officer | 300,000 | - | - | 0.17 | 04/23/08 | - | - | - | - |
Dr. Mark Landy, President and Director | 200,000 | 2,500,000 | Nil | 0.60 | 05/21/07 | - | - | - | - |
53
Option Awards | Stock Awards | ||||||||
Name | Number of Securities Underlying Unexercised Options | Number of Securities Underlying Unexercised Options | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options | Option Exercise Price | Option | Number of Shares or Units of Stock That Have Not Vested | Market Value of Shares or Units of Stock That Have Not Vested | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested |
Patrick A. McGowan, Chief Financial Officer, Executive Vice President, Secretary | 300,000 | - | - | 0.17 | 04/23/08 | - | - | - | - |
Dr. Dov Shimon,(1) President and CEO of SagaX, Inc. | 200,000 | - | - | 0.30 | 07/31/09 | - | - | - | - |
Rajesh Vaishnav, | 250,000 |
| - | 0.60 | 04/09/17 | - | - | - | - |
(1) Dr. Shimon ceased to be a director and officer of the Company on November 13, 2007 and the options held by him expire on February 13, 2008 and May 13, 2008, as applicable.
Option Exercise and Stock Vested
The following table sets forth information relating to the exercise of stock options by the Named Executive Officers in the year ended May 31, 2007:
Option Awards | Stock Awards | |||
Name | Number of Shares Acquired on Exercise | Value Realized on Exercise | Number of Shares Acquired on Vesting | Value Realized on Vesting |
Alan P. Lindsay | Nil | - | Nil | - |
Dr. Mark Landy | Nil | - | Nil | - |
Patrick A. McGowan | Nil | - | Nil | - |
Dr. Dov Shimon(1) | Nil | - | Nil | - |
Rajesh Vaishnav | Nil | - | Nil | - |
54
(1) Dr. Shimon ceased to be a director and officer of the Company on November 13, 2007 and the options held by him expire on February 13, 2008 and May 13, 2008, as applicable.
The following table sets forth information relating to compensation paid to our directors in fiscal 2007:
Director Compensation Table
Name | Fees Earned or Paid in Cash | Stock Awards | Option Awards | Non-Equity Incentive Plan Compen-sation | Non-qualified Deferred Compen-sation Earnings | All Other | Total |
Alan P. Lindsay | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
Dr. Mark Landy | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
Patrick A. McGowan | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
Dr. Daniel Savard(1) | Nil | Nil | (1) | Nil | Nil | Nil | (1) |
Dr. Dov Shimon(2) | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
(1) During the six months ended November 30, 2007 we did not award Dr. Savard any additional options for serving as director, however, we extended his previously issued 150,000 options due to expire for an additional 3 years and recognized a compensation expense of $71,494.
(2) Dr. Shimon ceased being a director of the Company on November 13, 2007.
Long-Term Incentive Plans
We do not have any long-term incentive plans, pension plans, or similar compensatory plans for our directors or executive officers.
Potential Payments Upon Termination or Change-in-Control
Information with respect to any payouts upon the termination of a Named Executive Officer or a change-in-control of the Company is set forth below under "Employment Contracts".
Employment Contracts
Dr. Mark Landy
We entered into an employment agreement (the "Employment Agreement") and indemnity agreement (the "Indemnity Agreement") with Dr. Landy on January 15, 2008 pursuant to his appointment as Chief Executive Officer of the Company.
Pursuant to the terms of the Employment Agreement, Dr. Landy has agreed to act as President and Chief Executive Officer of the Company and to serve as a member of the Company's Board of Directors for a period of five years from January 1, 2008. The term of the agreement automatically renews for successive one year periods, unless the Company provides Dr. Landy with written notice of its intent not to renew the Employment Agreement at least 180 calendar days prior to the end of the term of the Employment Agreement.
In consideration for his services, the Company has agreed to pay Dr. Landy an annual salary of not less than $360,000 and to review his salary annually, and he is entitled to participate in the Company's benefit plans and to a monthly stipend to obtain medical or other insurance. In addition, Dr. Landy shall be entitled to a bonus as determined by the Board of Directors of the Company and the Company agreed to issue to him (i) options to acquire up to 5,000,000 shares of common stock at an exercise price of not more than $0.60, (ii) options to acquire up to 1,150,000 shares of common stock at an exercise price of not more than $0.55, and (iii) options to acquire up to 8,000,000 shares of common stock at an exercise price of not more than $0.55 per share issuable pursuant to a vesting schedule, all of which shall be exercisable for a period of not less than seven years, as well as such other options as may be determined by the Company.
55
The agreement may be terminated by the Company without cause upon 90 calendar days' prior written notice. However, in that case, the Company shall continue to be obligated to pay Dr. Landy all amounts otherwise payable under the Employment Agreement, as well as, among other things (i) pay to him an additional severance cash payment equal to an aggregate of 36 months of Dr. Landy's annual salary, (ii) benefits to which he is entitled under the agreement would continue for a period of three years from the date of termination and (iii) in the event that Dr. Landy is terminated without cause due to a Change in Control (as defined in the Employment Agreement), he shall be entitled to an additional severance cash payment equal to his annual salary multiplied by the number of years he has been employed by the Company, and all issued and outstanding and vested options held by Dr. Landy shall be exercisable for a period of two years from the effective date of the termination (the "Severance Packag e").
Dr. Landy may terminate the Employment Agreement without cause upon 90 calendar days prior written notice and, in that case, shall be entitled to receive compensation as provided in the Employment Agreement only until the effective date of termination. The Employment Agreement may also be terminated by Dr. Landy upon 30 calendar days prior written notice in the event of a Change in Control of the Company and, in that case, Dr. Landy shall be entitled to all amounts payable to him under the Employment Agreement until the effective date of the termination, as well as a Severance Package and all unvested shares and unvested options issued to Dr. Landy shall vest immediately.
The Employment Agreement may be terminated by either party upon 14 calendar days prior written notice if, among other things (i) a party fails to cure a material breach of any provision of the Employment Agreement within 30 calendar days of notice thereof, (ii) a party is willfully non-compliant in the performance of its duties under the Employment Agreement, (iii) a party commits fraud or serious neglect or misconduct in the discharge of its duties under the Employment Agreement, or (iv) a party becomes bankrupt or a petition for reorganization under any law relating to bankruptcy is not dismissed within 30 calendar days.
In addition, in the event that Dr. Landy's employment is terminated as President or Chief Executive Officer of the Company, including by virtue of his resignation, the Company is obligated to pay Dr. Landy an additional severance cash payment of $100,000.
Under the terms of the Indemnification Agreement, the Company has agreed to indemnify Dr. Landy against all costs resulting from any action, suit, proceeding or alternative dispute resolution mechanism, whether civil, criminal, administrative or investigative or otherwise instituted against Dr. Landy by reason of Dr. Landy's position as a director, officer, employee or control person or agent of the Company, or any subsidiary of the Company. The Company has agreed to advance all expenses to be incurred by Dr. Landy in connection with the investigation, defense, settlement or appeal of any civil or criminal action, suit or proceeding contemplated by the Indemnification Agreement.
However, the Company is not obligated to indemnify Dr. Landy or advance expenses to him with respect to proceedings or claims initiated by him and not by way of defense, in the event a court of competent jurisdiction determines that each of the assertions made by Dr. Landy was not made in good faith or was frivolous, for expenses or liabilities which have been paid to Dr. Landy by an insurance carrier or in the event that Dr. Landy has violated Section 16(b) of the United States Securities Exchange Act of 1934, as amended.
Alan Lindsay
The Company entered into a Development Services Agreement with Alan Lindsay and Associates Ltd. (the "Consultant") dated March 1, 2005. Pursuant to the Agreement the Company agrees to retain the Consultant, and through the Consultant Mr. Lindsay, to provide development and financing services as may be necessary and determined by the Company to both develop and finance the Company's technology and business. The term of the agreement is five years commencing March 1, 2005 and expiring on March 1, 2010. Under the terms of the Development Services Agreement, Mr. Lindsay shall be paid $17,250 per month, subject to a 10% increase on an annual basis and receive 1,200,000 options to purchase shares of common stock of the Company at $0.20 per share. In the event of a change in control, all of Mr. Lindsay's outstanding options shall immediately vest. Mr. Lindsay's agreement may be terminated by the Company without cause upon 360 calendar days notice. In the event that Mr. Lindsay's agreement is terminated without cause or is not renewed at the end of its term, then in addition to the amounts due to him under his agreement, Mr. Lindsay will receive a termination fee equal to the aggregate remaining fee due to him for the unexpired remainder of the Term plus three months fee ("Termination Fee").
56
Further, in the event Mr. Lindsay (1) is removed or not reappointed as an officer; (2) there is a change in control of the Board of Directors or the Company; or (3) Mr. Lindsay's agreement is terminated without cause, then Mr. Lindsay will receive a fee equal to two times the Termination Fee.
Patrick McGowan
The Company entered into an Executive Employment Agreement with Mr. Patrick McGowan dated January 1, 2005. Pursuant to the Agreement, the Company will employ Mr. McGowan in an executive capacity, commenced on January 1, 2005 and will continue until May 1, 2007. Mr. McGowan's Executive Employment Agreement was amended for an additional two years until May 2009. Under the terms of the amendment, Mr. McGowan shall be paid a total annual salary of CAD$169,400 up to April 30, 2008. Thereafter, the Company shall increase Mr. McGowan's salary by 10%. In addition, Mr. McGowan will receive 10% of options held by Mr. McGowan at the time of issuance in each calendar year 2008 and 2009. The exercise price of the options shall be based on the closing share price as of such dates. In the event of a change in control, the Company can either (i) pay Mr. McGowan a lump sum equal to two times Mr. McGowan's then current annual income in addition to any amounts owing to Mr. McGowan under his Executive Employment Agreement or (ii) renew the term of this Amendment for no less than 12 months beginning on the effective date of the change of control.
Dov Shimon
The Company entered into a 36-month Consulting Services Agreement with Dr. Dov Shimon dated May 1, 2005 pursuant to which he agreed to provide certain consulting services to the Company. The agreement was terminated on November 13, 2007. See "Business - Recent Developments - Sale of SagaX" for further information. Dr. Shimon is no longer a director or officer of our Company effective November 13, 2007. The options issued to and held by Dr. Shimon expire on February 13, 2008 and May 13, 2008, as applicable.
Rajesh Vaishnav
The Company entered into an two-year Executive Services Agreement with Mr. Rajesh Vaishnav (the "Executive") and Biosync Scientific Pvt. Ltd. ("Biosync") dated February 16, 2007. Pursuant to the Agreement, the Company will employ Mr. Vaishnav as the President and Chief Operating Officer of Biosync. Under the terms of the Executive Services Agreement Mr. Vaishnav will be paid $12,000 per month and will receive up to an aggregate of 4,000,000 shares of common stock of the Company. Mr. Vaishnav will receive (i) 750,000 share when BioSync receives a CE Mark for its present bare-metal stent; (ii) 750,000 shares upon the earlier of (a) the date upon which BioSync first launches the sale of a HAp stent in India; (b) the date upon which BioSync first launches the sale of a drug-eluting stent in India; or (c) the date upon which BioSync first reaches $3,000,000 in gross product sales during any fiscal year after the effective date of this Agreement; (iii) 1,000,000 shares when BioSync first reaches $6,000,000 in gross product sales; and (iv) 1,500,000 shares to be distributed in equal 375,000 share increments for each of the six month periods over the next two years.
Compensation Committee Interlocks and Insider Participation
The board of directors does not have a compensation committee. During the six months ended November 30, 2007, the functions of the compensation committee were performed collectively by the board of directors. Alan P. Lindsay, Mark Landy and Patrick McGowan are directors and executive officers of the Company and participated in deliberations concerning executive officer compensation.
During the six months ended November 30, 2007, no executive officer of the Company served as a member of the compensation committee of another entity, one of whose executive officers served on the Company's board; no executive officer of the Company served as a director of another entity, one of those executive officers served on the Company's board; and no executive officer of the Company served as a member of the compensation committee of another entity, one of whose executive officers is a director of the Company.
FINANCIAL STATEMENTS
The following consolidated financial statements of the Company listed below are included with this prospectus. These consolidated financial statements have been prepared on the basis of accounting principles generally accepted in the United States and are expressed in U.S. dollars.
Consolidated Financial Statements of the Company for the Period Ended November 30, 2007 | |
Consolidated Balance Sheets as of November 30, 2007 and May 31, 2007 | F-2 |
Consolidated Statements of Operations and Comprehensive Loss for the six and three months ended November 30, 2007 and 2006 and for the period from inception (January 20, 1999) to November 30, 2007 | F-3 |
Consolidated Statement of Stockholders' Equity for the six months ended November 30, 2007 | F-4 |
Consolidated Statements of Cash Flows for the six months ended November 30, 2007 and 2006 and for the period from inception (January 20, 1999) to November 30, 2007 | F-5 |
Notes to Consolidated Financial Statements | F-7 |
Audited Consolidated Financial Statements of the Company for the Years Ended May 31, 2007, 2006 and 2005: | |
Reports of Independent Registered Public Accounting Firms | F-26 |
Consolidated Balance Sheets as of May 31, 2007, 2006 and 2005 | F-31 |
Consolidated Statements of Operations and Comprehensive Loss for the years ended May 31, 2007, 2006 and 2005 and for the period from inception (January 20, 1999) to May 31, 2007 | F-32 |
Consolidated Statements of Stockholders' Equity (Deficit) for the period from inception (January 20, 1999) to May 31, 2007 | F-33 |
Consolidated Statements of Cash Flows for the years ended May 31, 2007, 2006 and 2005 and for the period from inception (January 20, 1999) to May 31, 2007 | F-40 |
Notes to Consolidated Financial Statements | F-41 |
Financial Statements of BioSync Scientific Pvt. Ltd.: | |
Report of Independent Public Accounting Firm | F-78 |
Balance Sheets as of March 31, 2006 and 2005 | F-80 |
Statements of Operations for the years ended March 31, 2006 and 2005 | F-81 |
Statement of Partners' Capital from April 1, 2004 to March 31, 2006 | F-82 |
Statements of Cash Flows for the years ended March 31, 2006 and 2005 | F-83 |
Notes to Financial Statements | F-84 |
Balance Sheets as of December 31, 2006 and March 31, 2006 | F-92 |
Statements of Operations for the periods ended December 31, 2006 and March 31, 2006 | F-93 |
Statement of Shareholders Equity from April 1, 2006 to December 31, 2006 | F-94 |
Statements of Cash Flows for the periods ended December 31, 2006 and March 31, 2006 | F-95 |
Notes to Consolidated Financial Statements | F-96 |
Balance Sheets at December 31, 2005 and March 31, 2005 | F-104 |
Statements of Operations for the periods ended December 31, 2005 and March 31, 2005 | F-105 |
Statement of Partners Capital from April 1, 2004 to December 31, 2005 | F-106 |
Statements of Cash Flows for the periods ended March 31, 2005 | F-107 |
Notes to Consolidated Financial Statements | F-108 |
Pro Forma Financial Statements: | |
Report on Pro Forma Statements | F-114 |
Pro Forma Statement of Operations for the period ended November 30, 2006 | F-116 |
Pro Forma Statement of Operations for the period ended May 31, 2006 | F-117 |
Notes to Pro Forma Financial Statements | F-118 |
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS
Change of Auditors
Information with respect to changes in our independent auditors over the past two fiscal years has previously been disclosed in current reports on Form 8-K we have filed with the SEC.
Controls and Procedures
Based on their evaluation of our disclosure controls and procedures for the fiscal year ended May 31, 2007, our Chief Executive Officer and Chief Financial Officer concluded that, during this period, our disclosure controls and procedures were not effective to detect the inappropriate application of U.S. generally accepted accounting principles ("GAAP") as more fully described below. This was due to deficiencies that existed in the design and operation of our internal control over financial reporting that adversely affected our disclosure controls and that may be considered "material weaknesses". The Public Company Accounting Oversight Board has defined a material weakness as a "significant deficiency or combination of significant deficiencies that results in more than a remote likelihood that a material misstatement of the annual or interim financial statements will not be prevented or detected."
59
As a result of the misapplication of U.S. GAAP as described above, management concluded that our internal controls over financial reporting were not effective at May 31, 2007. Our board of directors discussed this matter with our auditors.
Subsequent to the issuance of our report on Form 10-QSB for the quarterly period ended February 28, 2007, management determined that our interim consolidated financial statements as at and for the period ended February 28, 2007 should be revised to correct the amounts reported in connection with the acquisition of BioSync. We originally recorded the acquisition of BioSync at a purchase price of $900,000 with $493,154 recorded as purchased in-process research and development, which was concurrently charged to operations. Upon further reflection, we discovered that we did not correctly apply SFAS No. 141, "Business Combinations". As such, we adjusted the purchase price to $1,943,885 and recorded $1,421,283 of the acquisition price to BioSync's CE Mark License which allows BioSync to produce and sell stents in India. Additionally, certain expenditures were incorrectly recorded and inconsistent assumptions were used in determining the estimated fair values of share-based awards during the qua rter ended February 28, 2007. These revisions were included in our report on Form 10-K for the year ended May 31, 2007. Amendment No. 1 to Form 10-QSB for the period ended February 28, 2007 was filed with the SEC on October 23, 2007 to reflect the restatement. A further amendment to this Form 10-QSB was filed to provide additional disclosure regarding the restatement and to reflect the individual line items in the financial statements that were affected by the restatement.
In an effort to address these weaknesses, we have improved the supervision and training for our accounting personnel and have hired a Vice President of Finance who is experienced in the financial reporting function. We have implemented additional procedures to more formally review our classification and consolidating entries and consolidated financial statements, we have reviewed our existing internal accounting controls and procedures to identify areas that could be improved, and an independent accounting firm has been conducting a monthly review of BioSync's trial balance to ensure the figures are reported under U.S. GAAP. We will continue these efforts, and make any further changes determined to be appropriate.
Where You Can Find More Information
We are required to file annual, quarterly and current reports, proxy statements and other information with the SEC. You may read and copy any document we file at the SEC's Public Reference Room at One Station Place, 100 F Street, N.E., Washington, D.C. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. You can also obtain copies of our SEC filings by going to the SEC's website at http://www.sec.gov .
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the securities offered under this prospectus. This prospectus, which forms a part of that registration statement, does not contain all information included in the registration statement. Certain information is omitted and you should refer to the registration statement and its exhibits. With respect to references made in this prospectus to any contract or other document of the Company, the references are not necessarily complete and you should refer to the exhibits attached to the registration statement for copies of the actual contract or document.
No agent of the Company or other person has been authorized to give any information or to make any representation in connection with this offering other than those contained in this prospectus and, if given or made, such information or representation must not be relied upon as having been authorized by us. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any of the securities offered hereby by anyone in any jurisdiction in which such offer or solicitation is not authorized or in which the person making such offer or solicitation is not qualified to do so or to any person to whom it is unlawful to make such offer or solicitation. Neither the delivery of this prospectus nor any sale made hereunder shall, under any circumstances, create any implication that the information contained herein is correct as of any time subsequent to the date of this prospectus.
60
MIV THERAPEUTICS INC.
(A development stage company)
Consolidated Financial Statements
November 30, 2007
(Unaudited)
Index
Consolidated Balance Sheets
Consolidated Statements of Operations and Comprehensive Loss
Consolidated Statement of Stockholders' Equity
Consolidated Statements of Cash Flows
Notes to Consolidated Financial Statements
F-1
MIV Therapeutics, Inc.
(A development stage company)
Consolidated Balance Sheets
(Unaudited)
Expressed in U.S. Dollars | |||||
November 30,2007 | May 31, 2007 | ||||
ASSETS | |||||
Current assets | |||||
Cash and cash equivalents | $ | 6,061,952 | $ | 473,419 | |
Accounts receivable | 79,874 | 50,829 | |||
Employee advances (Note 12) | 15,964 | 20,432 | |||
Prepaid expenses (Note 3) | 327,860 | 346,883 | |||
Inventories (Note 4) | 729,319 | 563,684 | |||
Assets of business to be transferred (Note 10) | 48,868 | 39,249 | |||
Total current assets | 7,263,837 | 1,494,496 | |||
Employee advances | - | 14,343 | |||
Property and equipment, net (Note 6) | 1,614,709 | 1,253,010 | |||
CE Mark license, net (Note 7) | 1,308,765 | 1,389,279 | |||
Deposits and other assets | 103,619 | 37,264 | |||
Assets of business to be transferred, net of current(Note 10) | 51,760 | 58,573 | |||
Total assets | $ | 10,342,690 | $ | 4,246,965 | |
LIABILITIES AND STOCKHOLDERS' EQUITY | |||||
Liabilities | |||||
Current liabilities | |||||
Accounts payable and other payables (Note 12) | $ | 919,603 | $ | 1,462,616 | |
Customer deposit | 119,032 | - | |||
Related party loan (Note 12) | - | 100,000 | |||
Loan payable (Note 8) | - | 525,000 | |||
Deferred lease inducement - current portion (Note 13(a)) | 8,081 | 8,081 | |||
Liabilities of business to be transferred (Note 10) | 312,962 | 154,742 | |||
Total current liabilities | 1,359,678 | 2,250,439 | |||
Deferred lease inducement (Note 13(a)) | 15,488 | 19,529 | |||
Deferred income tax liability | 188,000 | 297,000 | |||
Total liabilities | 1,563,166 | 2,566,968 | |||
Commitments and contingencies (Notes 11 and 13) | |||||
Stockholders' equity | |||||
Common stock (Note 8) | |||||
Authorized: | |||||
20,000,000 preferred shares with a par value of $0.001 | |||||
230,000,000 common shares with a par value of $0.001 | |||||
Issued and outstanding: | |||||
112,309,004 common shares at November 30, 2007 and | |||||
83,785,056 common shares at May 31, 2007 | 112,309 | 83,785 | |||
Additional paid-in capital | 56,197,892 | 42,329,385 | |||
Deferred compensation | (111,409) | (320,579) | |||
Common stock issuable | 963,387 | 1,411,489 | |||
Deficit accumulated during the development stage | (48,185,987) | (41,627,415) | |||
Accumulated other comprehensive loss | (196,668) | (196,668) | |||
Total stockholders' equity | 8,779,524 | 1,679,997 | |||
Total liabilities and stockholders' equity | $ | 10,342,690 | $ | 4,246,965 | |
The accompanying notes are an integral part of these consolidated financial statements.
F-2
MIV Therapeutics, Inc.
(A development stage company)
Consolidated Statements of Operations and Comprehensive Loss
(Unaudited)
Expressed in U.S. Dollars | ||||||||||
Period from | ||||||||||
inception | ||||||||||
(January 20, | ||||||||||
1999) to | Six months ended | Three months ended | ||||||||
November 30, | November 30, | November 30, | ||||||||
2007 | 2007 | 2006 | 2007 | 2006 | ||||||
Revenue | $ | 743,131 | $ | 551,641 | $ | - | $ | 250,031 | $ | - |
Cost of sales (exclusive of amortization of | 640,204 | 493,064 | - | 195,694 | - | |||||
Gross profit | 102,927 | 58,577 | - | 54,337 | - | |||||
Expenses | ||||||||||
General and administrative (Note 14) | 29,988,694 | 3,665,694 | 2,044,979 | 1,929,702 | 772,466 | |||||
Research and development | 12,822,047 | 2,350,446 | 1,615,244 | 1,200,685 | 739,569 | |||||
Interest expense and finance fees | 1,200,120 | 174,663 | 2,059 | 152,602 | 2,059 | |||||
Depreciation | 1,001,488 | 17,445 | 45,516 | 10,266 | 23,073 | |||||
Amortization of CE Mark license | 112,518 | 80,514 | - | 35,532 | - | |||||
Expenses related to assets and liabilities to be | 2,672,723 | 600,955 | 420,567 | 315,748 | 239,383 | |||||
Licenses acquired charged to operations | 479,780 | - | - | - | - | |||||
Finance cost on convertible debentures | 382,307 | - | - | - | - | |||||
Purchased in-process research | 2,205,013 | - | - | - | - | |||||
50,864,690 | 6,889,717 | 4,128,365 | 3,644,535 | 1,776,550 | ||||||
Loss from operations | 50,761,763 | 6,831,140 | 4,128,365 | 3,590,198 | 1,776,550 | |||||
Gain on extinguishment of debt | 462,249 | - | - | - | - | |||||
Gain (loss) on foreign exchange | 53,727 | 31,141 | (22,454) | 27,452 | (2,981) | |||||
Interest income | 273,313 | 132,427 | 5,832 | 77,826 | 376 | |||||
Minority interest share of loss | 806,310 | - | - | - | - | |||||
Loss before deferred income tax recovery | 49,166,164 | 6,667,572 | 4,144,987 | 3,484,920 | 1,779,155 | |||||
Deferred income tax recovery | 136,000 | 109,000 | - | 61,000 | - | |||||
Net loss | 49,030,164 | 6,558,572 | 4,144,987 | 3,423,920 | 1,779,155 | |||||
Other comprehensive income (loss) | ||||||||||
Foreign currency translation | (196,668) | - | (3,459) | - | 733 | |||||
Comprehensive loss | $ | 49,226,832 | $ | 6,558,572 | $ | 4,148,446 | $ | 3,423,920 | $ | 1,778,422 |
Loss per common share | $ | (1.34) | $ | (0.06) | $ | (0.06) | $ | (0.03) | $ | (0.03) |
Weighted average number of common | ||||||||||
shares outstanding | ||||||||||
- basic and diluted | 36,619,672 | 105,948,109 | 70,377,408 | 111,930,496 | 70,258,921 | |||||
The accompanying notes are an integral part of these consolidated financial statements.
F-3
MIV Therapeutics, Inc.
(A development stage company)
Consolidated Statements of Stockholders' Equity
(Unaudited)
Expressed in U.S. Dollars | ||||||||||
Accumulated | Deficit | |||||||||
Other | Accumulated | |||||||||
Additional | Deferred | Common | Compre- | During the | ||||||
Common Stock | Paid-in | Compen- | Stock | hensive | Development | |||||
Shares | Amount | Capital | sation | Issuable | Loss | Stage | Total | |||
Balance, May 31, 2007 | 83,785,056 | $ 83,785 | $ 42,329,385 | $ (320,579) | $ 1,411,489 | $ (196,668) | $ (41,627,415) | $ 1,679,997 | ||
Issuance of common stock: | ||||||||||
- for share subscriptions - private placement | 27,666,000 | 27,666 | 12,816,311 | - | (927,000) | - | - | 11,916,977 | ||
- for exercise of options | 315,715 | 316 | 38,184 | - | (38,500) | - | - | - | ||
- for services | 542,233 | 542 | 267,061 | (90,000) | 517,398 | - | - | 695,001 | ||
Fair value of warrants issued for loan (Note 8) | - | - | 16,405 | - | - | - | - | 16,405 | ||
Fair value of vested stock option grants | - | - | 592,846 | - | - | - | - | 592,846 | ||
Fair value of extended stock options | - | - | 48,400 | - | - | - | - | 48,400 | ||
Fair value of extended warrants | - | - | 89,300 | - | - | - | - | 89,300 | ||
Amortization of deferred compensation | - | - | - | 299,170 | - | - | - | 299,170 | ||
Comprehensive loss: | ||||||||||
Foreign currency translation | - | - | - | - | - | - | - | - | ||
Net loss | - | - | - | - | - | - | (6,558,572) | (6,558,572) | ||
Balance, November 30, 2007 | 112,309,004 | $ 112,309 | $ 56,197,892 | $(111,409) | $ 963,387 | $ (196,668) | $ (48,185,987) | $ 8,779,524 | ||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
MIV Therapeutics, Inc.
(A development stage company)
Consolidated Statements of Cash Flows
(Unaudited)
Expressed in U.S. Dollars | ||||||
Period from | ||||||
inception | ||||||
(January 20, | ||||||
1999) to | Six months ended | |||||
November 30, | November 30, | |||||
2007 | 2007 | 2006 | ||||
Cash flows from operating activities | ||||||
Net loss | $ | (49,030,164) | $ | (6,558,572) | $ | (4,144,987) |
Adjustments to reconcile net loss to net cash used in | ||||||
operating activities: | ||||||
Stock-based compensation | 11,284,339 | 940,416 | 784,105 | |||
Stock issued for other than cash | 6,775,232 | 695,001 | 379,511 | |||
Depreciation and amortization | 1,346,605 | 258,477 | 51,001 | |||
Deferred income tax recovery | (136,000) | (109,000) | - | |||
Fair value of extended warrants | 430,127 | 89,300 | - | |||
Fair value of warrants included in operating expenses | 16,405 | 16,405 | - | |||
Interest expense on related party loan | 850,000 | - | - | |||
Interest expense on convertible debentures | 34,730 | - | - | |||
Leasehold improvements written down | 13,300 | - | - | |||
Purchased in-process research and development | 2,125,013 | - | - | |||
Intangible asset impairment | 150,000 | - | - | |||
Gain on extinguishment of debt | (462,249) | - | - | |||
Provision for bad debt | 160,000 | - | - | |||
Beneficial conversion feature on convertible debenture | 289,800 | - | - | |||
Minority interest | (806,310) | - | - | |||
Changes in operating assets and liabilities: | ||||||
Accounts receivable | (240,125) | (29,045) | 16,156 | |||
Due from related party | (15,964) | 18,811 | - | |||
Prepaid expenses and deposits | (302,546) | 9,404 | (21,276) | |||
Inventories | (484,517) | (165,635) | - | |||
Deposits and other assets | (103,619) | (66,355) | - | |||
Accounts payable and other payables | 1,117,162 | (388,834) | 361,553 | |||
Net cash used in operating activities | (26,988,781) | (5,289,627) | (2,573,937) | |||
Cash flows from financing activities | ||||||
Issuance of common stock, less share issuance costs | 33,633,250 | 11,916,977 | 1,454,000 | |||
Proceeds from (repayments of) loan payable | 500,000 | (525,000) | - | |||
Increase in customer deposit | 119,032 | 119,032 | - | |||
Due to related parties | 850,000 | (100,000) | - | |||
Subscriptions received | 1,389,310 | - | 20,000 | |||
Cash acquired in reverse acquisition | 13,824 | - | - | |||
Proceeds from convertible debentures | 755,000 | - | - | |||
Common stock redemption | (120,000) | - | - | |||
Net cash provided by financing activities | 37,140,416 | 11,411,009 | 1,474,000 | |||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
MIV Therapeutics, Inc.
(A development stage company)
Consolidated Statements of Cash Flows (continued)
(Unaudited)
Expressed in U.S. Dollars | ||||||
Period from | ||||||
inception | ||||||
(January 20, | ||||||
1999) to | Six months ended | |||||
November 30, | November 30, | |||||
2007 | 2007 | 2006 | ||||
Cash flows from investing activities | ||||||
Purchase of property and equipment | (2,130,501) | (532,849) | (35,865) | |||
Pre-acquisition advances to Biosync | (121,870) | - | (79,300) | |||
Acquisition of Biosync, net of cash acquired | (1,415,885) | - | - | |||
Cash acquired on acquisition of Biosync | 17,557 | |||||
Acquisition of license | (200,000) | - | - | |||
Net cash used in investing activities | (3,850,699) | (532,849) | (115,165) | |||
Foreign exchange effect on cash | (238,984) | - | (3,459) | |||
Net increase (decrease) in cash and cash equivalents | 6,061,952 | 5,588,533 | (1,218,561) | |||
Cash and cash equivalents,beginning of period | - | 473,419 | 1,573,822 | |||
Cash and cash equivalents,end of period | $ | 6,061,952 | $ | 6,061,952 | $ | 355,261 |
The accompanying notes are an integral part of these consolidated financial statements.
F-6
MIV Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
November 30, 2007
(Unaudited)
Expressed in U.S. Dollars
1. Basis of Presentation and Nature of Operations
The accompanying unaudited interim consolidated balance sheets, statements of operations and other comprehensive loss, stockholders' equity (deficit) and cash flows reflect all adjustments, consisting of normal recurring adjustments and other adjustments, that are, in the opinion of management, necessary for a fair presentation of the financial position of MIV Therapeutics Inc. (the "Company"), at November 30, 2007, and the results of operations and cash flows for the interim periods ended November 30, 2007 and 2006.
The accompanying unaudited interim consolidated financial statements have been prepared in conformity with U.S. generally accepted accounting principles using the same accounting policies and methods of application as those disclosed in Note 2 of the Company's annual consolidated financial statements for the year ended May 31, 2007 except as disclosed in Note 2 and accordingly should be read in conjunction with the Company's annual consolidated financial statements for the year ended May 31, 2007. In the opinion of management, all adjustments considered necessary for the fair presentation of results for the periods presented have been reflected in these unaudited interim consolidated financial statements. Those adjustments consist only of normal recurring adjustments. Operating results of these interim periods are not necessary indicative of result that may be expected for the full fiscal year ending May 31, 2008.
Since inception, the Company has suffered recurring losses, favourably $49,030,164 as of November 30, 2007. To date, management has been able to finance the operations through the issuance of common stock, and through related party loans, in order to meet its strategic objectives. Management plans to continue to seek other sources of financing on favourable terms; however, there are no assurances that any such financing can be obtained on favourable terms, if at all. Management expects to monitor and control the Company's operating costs to a minimum until cash is available through financing or operating activities. There are no assurances that the Company will be successful in achieving these plans. The Company anticipates that losses will continue until such time, if ever, as the Company is able to generate sufficient revenues to support its operations. The Company's ability to generate revenue primarily depends on its success in completing development and obtaining regulatory appr ovals for the commercialization of its stent technology. The Company's ability to obtain sufficient financing to continue the development of, and if successful, to commence the manufacture and sale of its products under development, if and when approved by the applicable regulatory agencies is uncertain. In view of these conditions, the ability of the Company to continue as a going concern is in substantial doubt and dependent upon achieving a profitable level of operations and on the ability of the Company to obtain necessary financing to fund ongoing operations. Management believes that its current and future plans enable it to continue as a going concern. These unaudited interim consolidated financial statements do not give effect to any adjustments which would be necessary should the Company be unable to continue as a going concern and therefore be required to realize its assets and discharge its liabilities in other than the normal course of business and at amounts different from those reflected in the accompanying unaudited interim consolidated financial statements.
F-7
MIV Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
November 30, 2007
(Unaudited)
Expressed in U.S. Dollars
2. Accounting Policy Change and New Accounting Pronouncements
(a) Adoption of New Accounting Policy
In July 2006, FASB issued Interpretation No. 48 "Accounting for Uncertainty in Income Taxes - an interpretation of FASB Statement No. 109" ("FIN 48"). This Interpretation clarifies the accounting for uncertainty in income taxes recognized in an enterprise's financial statements in accordance with SFAS Statement No. 109, "Accounting for Income Taxes". This Interpretation prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. This Interpretation also provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition. This Interpretation is effective for fiscal years beginning after December 15, 2006.
The Company is subject to taxation in the U.S. and various states and foreign jurisdictions. In addition to U.S., the Company's major taxing jurisdictions include Canada, India and Israel. Examinations of income tax returns filed by the Company and its active subsidiaries that are still subject to examination are MIV Therapeutics Inc. for May 31, 1999, and subsequent years, MIVI Technologies, Inc. for May 31, 2006 and subsequent years, Biosync Scientific Pvt. Ltd. for March 31, 2006 and subsequent years and SMT Research and Development Ltd. for December 31, 2005 and subsequent years. Interest and penalties related to tax positions taken in tax returns are recorded in the unaudited interim consolidated statement of operations. There were no interest and penalties related to tax positions taken in our tax returns during the first two quarters of fiscal 2008.
The Company adopted the provisions of FASB Interpretation No. 48 on June 1, 2007. The adoption of FIN 48 did not result in a cumulative adjustment to equity and there were no unrecognized tax benefits, penalties or interest at the time of, or subsequent to, adoption.
(b) New Accounting Pronouncements
In December 2007, the FASB issued SFAS No. 160, "Noncontrolling Interests in Consolidated Financial Statements, an Amendment of ARB No. 51". This statement amends ARB 51 to establish accounting and reporting standards for the noncontrolling interest (minority interest) in a subsidiary and for the deconsolidation of a subsidiary. Upon its adoption, effective as of the beginning of the Company's fiscal 2010, noncontrolling interests will be classified as equity in the Company's balance sheet and income and comprehensive income attributed to the noncontrolling interest will be included in the Company's income and comprehensive income. The provisions of this standard must be applied prospectively upon adoption except for the presentation and disclosure requirements. Management is currently evaluating the impact of adopting SFAS No. 160 on the Company's consolidated financial position and results of operations.
In December 2007, the FASB issued SFAS No. 141 (revised 2007), "Business Combinations" ("SFAS No. 141(R)"). SFAS No. 141(R) establishes principles and requirements for how an acquirer in a business combination recognizes and measures the assets acquired, liabilities assumed, and any noncontrolling interest in the acquiree. The provisions of SFAS No. 141(R) will become effective for the Company's business combinations occurring on or after June 1, 2009.
F-8
MIV Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
November 30, 2007
(Unaudited)
Expressed in U.S. Dollars
2. Accounting Policy Change and New Accounting Pronouncements (continued)
(b) New Accounting Pronouncements (continued)
In February 2007, the FASB issued SFAS No. 159, "The Fair Value Option for Financial Assets and Financial Liabilities" ("SFAS 159"). SFAS 159 permits entities to choose to measure many financial assets and financial liabilities at fair value. Unrealized gains and losses on items for which the fair value option has been elected will be reported in earnings. SFAS 159 is effective for fiscal years beginning after November 15, 2007. The Company currently is assessing the impact of SFAS 159 on its' consolidated financial position or results of operation.
In September 2006, FASB issued SFAS No. 157, "Fair Value Measurements" ("SFAS 157"). The objective of SFAS 157 is to increase consistency and comparability in fair value measurements and to expand disclosures about fair value measurements. SFAS 157 defines fair value, establishes a framework for measuring fair value in generally accepted accounting principles, and expands disclosures about fair value measurements. SFAS 157 applies under other accounting pronouncements that require or permit fair value measurements and does not require any new fair value measurements. The provisions of SFAS 157 are effective for fair value measurements made in fiscal years beginning after November 15, 2007. The Company has not determined the effect, if any, that the adoption of SFAS 157 will have on the Company's consolidated financial position or results of operations.
3. Prepaid expenses
Prepaid expenses consisted of the following at:
November 30, | May 31, | |||
Prepayment to suppliers | $ | 203,659 | $ | 337,936 |
Other prepaid expenses | 124,201 | 8,947 | ||
$ | 327,860 | $ | 346,883 |
4. Inventories
Inventories consisted of the following at:
November 30, | May 31, | |||
Raw materials | $ | 285,600 | $ | 248,496 |
Packing materials | 13,641 | 14,392 | ||
Work-in-progress | 297,763 | 190,854 | ||
Finished goods | 132,315 | 109,942 | ||
$ | 729,319 | $ | 563,684 |
F-9
MIV Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
November 30, 2007
(Unaudited)
Expressed in U.S. Dollars
5. Licenses
(a) On February 1, 2003, the Company entered into two license agreements with the University of British Columbia ("UBC") which provides the Company with the worldwide right to use, develop and sublicense coating technology for stents and other medical devices.
In consideration of granting the licenses, the Company will pay UBC a royalty of 2.5% of related revenue and a royalty ranging from 10% or 15% of sublicense revenue depending upon the sublicensed technology. In addition, various minimum annual royalties, maintenance fees and milestone payments are payable over the period of development. The Company issued 750,000 common shares to UBC as part of the consideration for the grant of the rights.
The 750,000 common shares had a fair value of $187,500 and were issued and recorded as research and development expense in the year ended May 31, 2003.
On May 19, 2005, the Company signed an amendment to the existing license agreements to include some amendments in the definition of "Field of Use". Also, the royalty terms were amended from 2.5% to range from 2.5% to 5%, depending on the nature of the related revenue.
In consideration for the amendments, the Company agreed to issue 200,000 common shares which had a fair value of $74,000 at the time of the amendment. This amount was recorded as research and development expense during the year ended May 31, 2005. The shares were issued in June 2006.
(b) On March 15, 2004, the Company entered into a collaborative research agreement with UBC to continue with exploratory research on coating technology for stents for a period from April 1, 2004 to March 31, 2006. During the period of the agreement, various milestone payments were to be made to UBC for the continuation of the research program, estimated to be approximately CAD$220,800 ($164,445).
On October 28, 2004, the Company and UBC amended the existing collaborative research agreements and referred to it as Amendment No. 1 and 2.
In Amendment No. 1, the contract period of the existing collaborative agreement was changed to April 1, 2004 to November 30, 2004 and total costs to the Company were estimated at CAD$110,400 ($87,633). As at May 31, 2005, the Company had paid/accrued and recorded CAD$110,400 ($87,633) to research and development costs in accordance with Amendment No. 1.
In Amendment No. 2, the contract period, work plan and total costs of the existing collaborative agreement as amended by Amendment No. 1 were amended. The contract period was extended from December 1, 2004 to November 30, 2006 and total costs to the Company was estimated at CAD$400,400 ($317,828), being payable over the term of the Agreement at various stipulated intervals. As at May 31, 2007, the Company has paid CAD$400,400 ($317,828) for research and development costs in accordance with Amendment No. 2.
The Company obtained financial support of upto CAD$315,000 ($250,040) from the Industrial Research Assistance Program ("IRAP") from the National Research Council Canada. As at November 30, 2007, the Company had received CAD$265,791 ($268,555) from IRAP.
F-10
MIV Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
November 30, 2007
(Unaudited)
Expressed in U.S. Dollars
5. Licenses (continued)
(c) On May 19, 2005, the Company signed a letter of intent to negotiate a new license agreement for a new technology with UBC. The form and content will be similar to that of the license agreements entered into in February 2003 (See Note 5(a) above). On September 14, 2007, the Company and UBC executed the new license agreement with an effective date of September 1, 2007. Upon execution, the stock certificate for the 100,000 common shares, which was issued during the year ended May 31, 2007, was delivered to UBC on September 18, 2007.
In consideration of granting the license, the Company will pay UBC a royalty of 2.5% or 5% of related revenue and a royalty ranging from 10% or 15% of sublicense revenue depending upon the sublicensed technology. In addition, various minimum annual royalties, maintenance fees and milestone payments are payable over the period of development.
On December 1, 2006, the Company extended its collaborative research agreement with UBC to continue with exploratory research on coating technology for stents for a period from December 1, 2006 to November 30, 2007. During the period of the agreement, four equal payments will be made to UBC for a total budget estimate of CAD$274,896 ($241,264). As at May 31, 2007, the Company had paid CAD$137,448 ($120,632) and charged the costs to research and development.
On April 4, 2007, the Company and UBC amended the tasks and objectives of the existing collaborative research agreement. On August 29, 2007, the total costs of the project was revised to be estimated at CAD$190,479 ($167,175) from CAD$274,896 ($241,264). As of November 30, 2007, the Company had paid in full the CAD$190,479 due to UBC for this project.
6. Property and Equipment
Property and equipment consisted of the following at:
November 30, | May 31, | |||
Laboratory equipment | $ | 2,254,681 | $ | 1,972,255 |
Furniture and fixtures | 237,146 | 107,163 | ||
Computer equipment | 229,060 | 173,191 | ||
Building | 161,368 | 12,450 | ||
Leasehold improvements | 49,158 | 49,158 | ||
Land | 21,483 | 21,483 | ||
Construction-in-progress | - | 85,282 | ||
2,952,896 | 2,420,982 | |||
Less: accumulated depreciation | 1,338,187 | 1,167,972 | ||
$ | 1,614,709 | $ | 1,253,010 |
Depreciation expense for the three and six months ended November 30, 2007 was $74,929 and $170,213, respectively (2006 - $23,073 and $45,516). Of these amounts, $24,249 and $57,094, respectively (2006 - $nil), has been included in research and development costs in the statements of operations.
F-11
MIV Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
November 30, 2007
(Unaudited)
Expressed in U.S. Dollars
7. CE Mark License
On February 16, 2007, the Company completed the acquisition of all of the issued and outstanding shares of Biosync Scientific Pvt. Ltd. ("Biosync"). The Company allocated the purchase price to the assets acquired and liabilities assumed based on their fair values. The purchase price in excess of the net tangible assets acquired totaling $1,421,283 was then allocated to the identified intangible asset being the CE Mark license. The fair value of the CE Mark license was based on a discounted future net cash flows analysis that used information and assumptions provided by the Company's management. The CE Mark license is being amortized over a period of 10 years.
The following is a summary of the amortization of the CE Mark license at November 30, 2007:
CE Mark license | $ | 1,421,283 |
Less: accumulated amortization | 112,518 | |
$ | 1,308,765 |
8. Loan Payable
At May 31, 2007, the Company had a loan payable totaling $525,000 which bore interest at 12.5% per annum. Of this amount, $400,000 was due on June 15, 2007 and $125,000 was due on July 13, 2007.
On June 27, 2007, the due date of the $400,000 note payable was extended to July 31, 2007. An additional 150,000 non-transferable share purchase warrants to acquire one common share at an exercise price of $0.60 per share for a period of three years were issued to the lender. The warrants had an estimated fair value of $16,405 using the Black-Scholes option pricing model. The assumptions used in the Black-Scholes model were: volatility: 54.24%, discount rate: 4.91%, dividend: nil and expected life of one year.
The Company repaid the two loans in full and accrued interest during the quarter ended August 31, 2007.
9. Stockholders' Equity
(a) Common Stock
(i) On June 6, 2007, the Company issued 1,790,000 common shares pursuant to a private placement completed during the previous fiscal year. At May 31, 2007, the shares were recorded as common stock issuable in the statement of stockholder's equity.
(i) On July 9, 2007, the Company completed a brokered private placement of 25,100,000 units at a price of $0.50 per unit for total proceeds of $11,696,765 (net of finder's fee of $753,000 and legal fees of $100,235). Each unit is comprised of one common share and one-half of one share purchase warrant. Each warrant entitles the holder to purchase one additional common share at a price of $0.55 per share for a period of up to five years.
F-12
MIV Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
November 30, 2007
(Unaudited)
Expressed in U.S. Dollars
9. Stockholders' Equity (continued)
(a) Common Stock (continued)
The warrants had an estimated fair value of $2,034,048 using the Black-Scholes option pricing model. The assumptions used in the Black-Scholes model were: volatility: 54.24%, discount rate: 5.03%, dividend: nil and expected life of one year.
In connection with the private placement, the Company issued to the finder 251,000 common shares and 753,000 share purchase warrants. Each warrant entitles the finder to purchase one common share at an exercise price of $0.55 per share for a period of up to five years.
The 753,000 warrants had an estimated fair value of $122,043 using the Black-Scholes option pricing model. The assumptions used in the Black-Scholes model were: volatility: 54.24%, discount rate: 5.03%, dividend: nil and expected life of one year.
The Company filed a registration statement with the United States Securities and Exchange Commission to register for resale the shares and shares underlying the warrants issued under the private placement; however, it was not declared effective within four months from the date of the issuance of the units. As a result, the Company was required to pay to the holders of the units an amount in cash, as liquidated damages, of $125,500 which is equal to 1% of the aggregate purchase price paid by such holders for the units. This amount was charged to operations in the current quarter.
(iii) On August 31, 2007, the Company completed a private placement of 525,000 units at a price of $0.50 per unit for total proceeds of $252,212 (net of finder's fee of $5,385 and legal fees of $4,903). Of this amount, $32,000 was received in May 2007 and recorded as common stock issuable. Each unit is comprised of one common share and one share purchase warrant. Each warrant entitles the holder to purchase one additional common share at a price of $0.75 per share for the 475,000 warrants and $0.70 for the 50,000 warrants for a period of up to two years from registration of the underlying warrant shares. The 525,000 common shares were recorded under common stock issuable in the statement of stockholder's equity at August 31, 2007 but were issued during the current quarter.
The 475,000 warrants had an estimated fair value of $14,055 and the 50,000 warrants had an estimated fair value of $1,797 using the Black-Scholes option pricing model. The assumptions used in the Black-Scholes model were: volatility: 54.24%, discount rate: 4.19%, dividend: nil and expected life of one year.
(iv) On August 31, 2007, the Company issued 400,000 share purchase warrants as finder's fee in connection with a private placement completed in December 2006. The warrants had an estimated fair value of $26,070 using the Black-Scholes option pricing model. The assumptions used in the Black-Scholes model were: volatility: 54.24%, discount rate: 4.19%, dividend: nil and expected life of one year.
(v) During the six months ended November 30, 2007, 150,000 options with an average exercise price of $0.26 were exchanged for 150,000 common shares for total gross proceeds of $38,500. These shares were recorded as common stock issuable as at May 31, 2007. The Company also received notices for the cashless exercise of 345,000 stock options. The 345,000 options converted to 165,715 common shares.
F-13
MIV Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
November 30, 2007
(Unaudited)
Expressed in U.S. Dollars
9. Stockholders' Equity (continued)
(a) Common Stock (continued)
(vi) During the six months ended November 30, 2007, the Company issued an aggregate of 167,233 common shares for research and development services with a fair value of $98,686. Of these shares, 14,755 were recorded as common stock issuable at May 31, 2007. In addition, 375,000 common shares were issued to the principal vendor of BioSync in accordance with his executive services agreement (refer to Note 12).
(vii) In September 2003, the Company placed 6,000,000 common shares to a financial custodian acting as trustee pursuant to a listing of the Company's shares on the Frankfurt Stock Exchange. The Company was then conducting a Regulation S ("Reg S") offering through the facilities of the Berlin Stock Exchange to raise capital in mainly German speaking countries. The trustee was to receive a fee of 3% of the total number of the shares held in trust was paid in equal installments of 30,000 common shares per month over a ten month period, assuming the maximum offering of up to 10,000,000 common shares were sold. The shares may only be traded on German stock exchanges pursuant to Reg S. At November 30, 2007, 2,500,000 Reg S shares were held in trust by the financial custodian and remain available for financing purposes. The Company anticipates that during the quarter ending February 28, 2008 these shares will be returned to treasury.
(b) Warrants
The following table summarizes information about the warrants issued by the Company. All regular warrants and Series A, B and C warrants are exercisable on a one-for-one basis into common shares.
F-14
MIV Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
November 30, 2007
(Unaudited)
Expressed in U.S. Dollars
9. Stockholders' Equity (continued)
(b) Warrants (continued)
Number of Shares | Weighted Average | |
Balance, May 31, 2007 - Regular | 22,354,691 | $ 0.65 |
Balance, May 31, 2007 - Series "A" | 1,092,111 | 0.66 |
Balance, May 31, 2007 - Series "B" | 3,904,998 | 0.70 |
Balance, May 31, 2007 - Series "C" | 119,305 | 0.66 |
Balance, May 31, 2007 | 27,471,105 | 0.65 |
Regular: | ||
Issued - private placement | 13,075,000 | 0.56 |
Issued - finder's fee | 1,153,000 | 0.55 |
Issued - loan (Note 8) | 150,000 | 0.60 |
Expired | (71,429) | 0.75 |
Balance, November 30, 2007 - Regular | 36,661,262 | 0.61 |
Balance, November 30, 2007 - Series "A" | 1,092,111 | 0.66 |
Balance, November 30, 2007 - Series "B" | 3,904,998 | 0.70 |
Balance, November 30, 2007 - Series "C" | 119,305 | 0.66 |
Balance, November 30, 2007 | 41,777,676 | $ 0.43 |
During the three months ended November 30, 2007, the board of directors approved an extension to the expiry date of 304,762 warrants. The estimated fair value of the extended warrants was $89,300 and was charged to operations.
(c) Stock Options
The Company's incentive stock options plan provides for the grant of incentive stock options for up to 25,000,000 common shares to employees, consultants, officers and directors of the Company. Incentive benefits granted under the plan may be either incentive stock options, non-qualified stock options, stock awards, restricted shares or cash awards. Options are granted for a term not to exceed ten years from the date of grant. Stock options granted generally vest over a period of two years. As of November 30, 2007, 3,543,701 options are available from the plan.
During the six months ended November 30, 2007, the Company granted an aggregate of 1,096,300 stock options to employees and consultants of the Company. Each option entitles its holder to acquire one common share of the Company at a prices ranging from $0.55 to $0.65 per share, vests over a specified time and expires up to two to five years from date of grant.
F-15
MIV Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
November 30, 2007
(Unaudited)
Expressed in U.S. Dollars
9. Stockholders' Equity (continued)
(c) Stock Options (continued)
During the six months ended November 30, 2007, the board of directors approved an extension of 150,000 options from expiry date of November 30, 2007 to November 30, 2010. As a result of the extension, the Company recognized an additional $48,400 of consulting fees in the current quarter.
Option pricing models require the use of highly subjective estimates and assumptions including the expected stock price volatility. Changes in the underlying assumptions can materially affect the fair value estimates and therefore, in management's opinion, existing models may not necessarily provide reliable measure of the fair value of the Company's stock options.
The following average assumptions were used in determining stock-based compensation costs under the Black-Scholes option pricing model:
Six months ended | Three months ended | |||
November 30, | November 30, | |||
2007 | 2006 | 2007 | 2006 | |
Expected volatility | 109.05% | 60.11% | 98.20% | 32.71% |
Risk-free interest rate | 4.17% | 3.50% | 3.85% | 3.50% |
Expected life (years) | 3.78 | 4.58 | 2.85 | 5 |
Dividend yield | Nil | Nil | Nil | Nil |
Weighted average fair value of options granted | $0.34 | $0.36 | $0.33 | $0.20 |
The expected volatility is based on the Company's historical stock prices. Computation of expected life was estimated after considering the contractual terms of the stock-based award, vesting schedules and expectations of future employee behavior. The interest rate for period within the contractual life of the award is based on the U.S. Treasury yield curve in effect at the time of grant.
Compensation cost related to the stock options granted to employees during the six and three months ended November 30, 2007 was charged to operations at the awards' fair value of $394,533 and $147,820 (2006 - $487,304 and $334,273), respectively.
F-16
MIV Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
November 30, 2007
(Unaudited)
Expressed in U.S. Dollars
9. Stockholders' Equity (continued)
(c) Stock Options (continued)
A summary of the weighted average fair value of stock options granted during the six months ended November 30, 2007 is as follows:
Number of | Weighted Average | Weighted Average | |
Exercise price equals market price at grant date: | 610,000 | $0.55 | $0.55 |
Exercise price less than market price at grant date: | - | $Nil | $Nil |
Exercise price greater than market price at grant date: | 486,300 | $ 0.65 | $ 0.43 |
Summary of stock option information for the six months ended November 30, 2007 is as follows:
Shares | Weighted Average | AggregateIntrinsic Value | |
Options outstanding at May 31, 2007 | 20,039,999 | 0.49 | |
Options granted | 1,096,300 | 0.59 | |
Options exercised (Note 8(a)(v)) | (345,000) | 0.32 | |
Options expired | (875,000) | 0.60 |
|
Options outstanding at November 30, 2007 | 19,916,299 | 0.49 | $1,635,000 |
Exercisable at November 30, 2007 | 16,583,925 | 0.47 | $1,584,500 |
The following summarizes information about the stock options outstanding and exercisable at November 30, 2007:
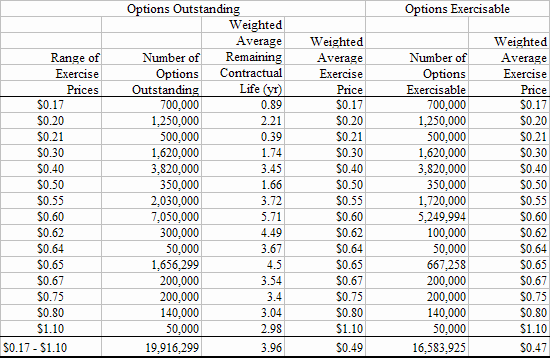
F-17
MIV Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
November 30, 2007
(Unaudited)
Expressed in U.S. Dollars
9. Stockholders' Equity (continued)
(c) Stock Options (continued)
Stock-based compensation expense is charged to operations over the vesting period of the options using the straight-line amortization method.
The aggregate intrinsic value of the Company's stock options is calculated as the difference between the exercise price of the options and the quoted price of the common shares that were in-the-money. The aggregate intrinsic value of the Company's stock options exercised under the Plan was $107,940 and $46,800 for the six months ended November 30, 2007 and 2006, respectively, determined at the date of option exercise.
As at November 30, 2007, there was approximately $705,190 of total unrecognized compensation cost related to non-vested share-based compensation arrangements granted under the Plan. This cost is expected to be recognized over a period of 24 months. The estimated fair value of stock options vested during 2007 and 2006 was $471,788 and $822,528, respectively.
During the three months ended November 30, 2007, $221,184 and $Nil (2006 - $12,531 and $25,174) of stock-based compensation pertains to options granted and extended, respectively, to research and development personnel.
During the six months ended November 30, 2007, $366,248 and $Nil (2006 - $206,304 and $25,174) of stock-based compensation pertains to options granted and extended, respectively, to research and development personnel.
10. Sale of SagaX, Inc.
On November 13, 2007, the Company entered into a definitive share purchase agreement to sell all of its issued and outstanding shares of SagaX Inc. ("SagaX"), its wholly-owned subsidiary, to Shimoco, LLC ("Purchaser"). Dr. Dov Shimon is a director of each of, SagaX and the Purchaser, founded and has been serving as the chief executive officer of SagaX since inception and is the owner and manager of the Purchaser.
In exchange for the consideration described below, the Company agreed to pay the Purchaser $210,000 ($115,000 paid) for working capital purposes in order to meet certain of SagaX's previously disclosed and bona fide current liabilities; with any said working capital advances to simply form part of the overall SagaX's indebtedness to the Company. Total consideration due to the Company is as follows:
(i) the repayment by the Purchaser and SagaX an aggregate of $4 million in prior loans and associated indebtedness which have been advanced and undertaken by the Company in and to SagaX ("SagaX's Indebtedness") in accordance with any future private or public equity financing of SagaX subsequent to the closing of the purchase agreement (the "Closing"). In this regard, and until payment in full of SagaX's Indebtedness, SagaX's Indebtedness will be secured, contemporaneously with the closing, by way of a senior, subordinated (subordinated only to SagaX's existing banking indebtedness), fixed and floating charge registered over all of the assets of SagaX; and
F-18
MIV Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
November 30, 2007
(Unaudited)
Expressed in U.S. Dollars
10. Sale of SagaX, Inc. (continued)
(ii) the payment by the Purchaser and SagaX to the Company of a royalty fee equating to 8% of net sales, other than from sub-licenses, in respect of gross sales from any product associated or related to SagaX's present intellectual property under any existing patent or patent-pending applications, and of any other benefit, directly or indirectly collected or received, whether for cash or credit or by way of any benefit, advantage, equity, or concession from the manufacturing, distribution, marketing, contracting, joint venturing, leasing, equity participation or any other activity in relation to the said products.
In addition to the consideration above, both prior to, in conjunction with and subsequent to the Closing, the Purchaser and SagaX will also be responsible for paying the Company a bonus (the "Bonus") equal to 10% of any consideration in any form which is received by the Purchaser and/or SagaX any source and from any transaction, or a series of related transactions, at anytime and which is in anyway associated with a change in control of SagaX at anytime while the royalty hereinabove remains due and payable by the Purchaser and SagaX to the Company.
Effective on the execution date of the purchase agreement, Dr. Shimon resigned as a director of the Company and terminated his existing consulting agreement and arrangement with the Company and, consequent upon such resignation and termination, to have no further claim against the Company as a previous director of, officer of or consultant to the Company.
The completion of the transactions comprising the Company's proposed purchase and sale under this agreement is subject to a number of conditions including, but not limited to: (i) if required, the specific ratification of the terms and conditions of this agreement by each of the Company's Board of Directors and of the General Shareholder's Assembly of the Purchaser (collectively, the "Ratification"); (ii) the completion by each of the Company and the Purchaser of an initial due diligence and operations review of the other party's respective businesses and operations within five business days of the prior satisfaction of the Ratification; (iii) if required under applicable corporate and securities laws, the receipt of all necessary approvals from any regulatory authority having jurisdiction over the transactions contemplated by this agreement on or before November 30, 2007; and (iv) the Closing of the proposed sale prior to December 31, 2007.
On December 28, 2007, the Company and the Purchaser completed the Closing of the transactions contemplated by the purchase agreement and the Company paid the remaining $95,000 due to the Purchaser.
The Company has reflected SagaX's results of operations through November 30, 2007 under the caption "Expenses related to assets and liabilities to be transferred" for all periods presented in the statements of operations. Cash flows, including the cash balances of SagaX, attributed to the assets and liabilities to be transferred have been included with those of the consolidated entity. The assets and liabilities of SagaX have been reclassified accordingly on the consolidated balance sheets. The assets and liabilities of SagaX were as follows at:
November 30, 2007 | May 31, | |||
Assets | ||||
Prepaid expenses | $ | 48,868 | $ | 39,249 |
Total current assets | 48,868 | 39,249 | ||
Property and equipment, net | 51,760 | 58,573 | ||
Total assets | $ | 100,628 | $ | 97,822 |
F-19
MIV Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
November 30, 2007
(Unaudited)
Expressed in U.S. Dollars
10. Sale of SagaX, Inc. (continued)
November 30,2007 | May 31, | |||
Liabilities | ||||
Accounts payable and accrued liabilities | $ | 312,962 | $ | 154,742 |
The Company has not accounted for the sale of SagaX as a divestiture because of the absence of significant financial investment of the Purchaser in SagaX and the repayment of SagaX's Indebtedness is solely dependent on future successful operations of the business of SagaX.
11. Termination of the Proposed Acquisition of Vascore Medical (Suzhou) Co., Ltd.
In light of the Company's inability to complete its final due diligence and satisfy all remaining conditions precedent to the proposed closing of the terms and conditions of that previously disclosed equity transfer agreement, dated for reference effective on September 5, 2006, as previously entered into among the Company, each of Chimex Hongkong Incorporated Limited and Vascore Scientific Co., Ltd. (collectively, the "Vendors") and Vascore Medical (Suzhou) Co., Ltd. ("Vascore Medical"), the Company terminated the Equity Transfer Agreement to acquire the various equity interests and shareholders' loans of Vascore Medical from the Vendors effective as at August 31, 2007 (the "Termination"). As a consequence, the Company has informed each of the Vendors and Vascore Medical of the Termination and thereby requested that any previous government approved ownership and Board appointments to Vascore Medical be immediately reverted to the direction of the Vendors.
In October 2007, the Company and certain senior officers and directors were served with summons and complaint documents for an action in New York state court alleging breach of contract, fraud, fraudulent concealment, negligent misrepresentation, unjust enrichment and conspiracy claims in connection with the Termination. The Company believes that this Action was brought improperly and intends to seek a stay of this Action pending arbitration in the appropriate forum, where the Company intends to vigorously defend itself.
12. Related Party Transactions
The related party transactions not disclosed elsewhere in these financial statements are disclosed as follows. These transactions, recorded at exchange amounts agreed to by all parties.
During the six months ended November 30, 2007, the Company paid or accrued $1,093,847 (2006 - $420,149) of management and consulting fees to four directors and two officers (2006 - 1 officer) of the Company. Of this amount, $120,982 (2006 - $110,025) was charged to research and development. Included in accounts payable is $nil at November 30, 2007 (2006 - $Nil).
During the three months ended November 30, 2007, the Company paid or accrued $461,143 (2006 - $209,853) of management and consulting fees to four directors and two officers (2006 - 1 officer) of the Company. Of this amount, $81,221 (2006 - $54,946) was charged to research and development.
F-20
MIV Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
November 30, 2007
(Unaudited)
Expressed in U.S. Dollars
12. Related Party Transactions (continued)
Included in the amount of $1,093,847 is the accrued amount of $526,251 due to the Chief Operating Officer and President of Biosync which will be issued in common stock on time periods stipulated in the agreement as follows:
The Company and Biosync entered into an executive services agreement with the principal vendor, being Mr. Rajesh Vaishnav, on February 16, 2007. Mr. Vaishnav will assume the position of Chief Operating Officer and President of Biosync under such commercially competitive compensation terms which will include, but not limited to, (i) a monthly fee of $12,000 plus monthly allowance of $500, (ii) stock options of up to 1,000,000 common shares at an exercise price of $0.60 for a period of not less than ten years from the date of grant and, (iii) an aggregate of up to 4,000,000 common shares with an issuance price of $0.50. Of the 4,000,000 common shares, 2,500,000 will be based on the achievement of certain milestones as outlined in the agreement, of which 750,000 common shares (issued as of May 31, 2007) upon receiving CE Mark License and the other 1,500,000 common shares to be given in four equal instalments over the two-year term of the agreement, of which, 375,000 were issued on September 28, 2007. The fair value of the options and common shares are treated as stock-based compensation expenses and amortized over the service period.
At November 30, 2007, amounts due from the employees of a subsidiary of the Company totaled $15,964 (May 31, 2007 - $34,775). These amounts are unsecured, non-interest bearing and will be repaid by periodic deduction of future wages.
On August 31, 2007, the $100,000 related party loan due to the Company's Chief Executive Officer was fully repaid.
13. Commitments and Contingencies
(a) The Company has obligations under a long-term premises lease that expire in December 2010. The approximate aggregate minimum rent payments for the annual periods ending November 30 are as follows:
2008 | $114,400 |
2009 | 114,400 |
2010 | 114,400 |
2011 | 9,500 |
$352,700 |
The Company received free rent, including property maintenance and taxes, for the months of November to December 2005 and free basic rent for the months of January to February 2006 for total free rent of $40,404. This amount was recorded under deferred lease inducement with a current portion of $8,081 (May 31, 2007 - $8,081) and long-term portion of $15,488 (May 31, 2007 - $19,529) and is being amortized over the term of the lease. During the six months ended November 30, 2007 and 2006, amortization of $4,041 was recorded as a reduction of rent expense in the statement of operations. Rent expense for the six and three months ended November 30, 2007 was $78,393 and $37,923, respectively (2006 - $78,328 and $40,507). Of these amounts, $54,732 and $26,477 was charged to research and development for the six and three months ended November 30, 2007, respectively.
F-21
MIV Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
November 30, 2007
(Unaudited)
Expressed in U.S. Dollars
13. Commitments and Contingencies (continued)
(b) On March 14, 2005, the Company acquired 100% of SagaX. The Company agreed to issue 4,200,000 common shares in exchange for all of the issued and outstanding shares of SagaX. The 4,200,000 shares will be issued in three intervals: 2,000,000 of the shares within 30 days of the effective date of this Agreement (issued), 1,100,000 shares upon successful completion of large animal trials and the final 1,100,000 shares upon CE Mark approval relating to SagaX's products. The Company has also agreed to pay $145,000 (paid) of SagaX's vendor debt owed to its parent company.
As of November 30, 2007, the two remaining issuances of 1,100,000 shares each have not been accrued as the underlying conditions have not been satisfied. During November 2007, the Company entered into a share purchase agreement to sell SagaX (refer to Note 10) and upon completion of the transaction on December 28, 2007, the Company had no further potential obligation to issue such shares.
(c) On November 18, 2002, a lawsuit against the Company was filed in the Supreme Court of British Columbia.
The statement of claim, arising from a settlement agreement dated September 14, 2001, seeks the exchange of 3,192,399 common shares of the Company for 3,192,399 shares in the capital of one of the Company's subsidiaries or, alternatively, damages and costs.
The Company and M-I Vascular Innovations, Inc. ("M-I") attended a court hearing in chambers during April 2003 on a summary trial application by the plaintiff for an order for a declaration of specific performance that the plaintiff is entitled to an exchange of 3,192,399 common shares of M-I for 3,192,399 common shares of the Company pursuant to the settlement agreement entered into on September 14, 2001. The plaintiff was granted the relief sought at the summary trial and the Company was ordered to perform the share exchange.
On May 16, 2003, the Company delivered a Take-Over Bid Circular (the "Circular") to the plaintiff, offering to exchange its common shares of M-I for shares in the Company pursuant to British Columbia securities laws and regulations. In late May 2003, after the judgment was received, the Company asked the plaintiff to submit its M-I share certificates and fill in the required forms pursuant to the Circular, so that the Company could comply with the judgment and exchange its shares in accordance with British Columbia securities laws and regulations.
On December 29, 2004, the Company issued 3,192,399 common shares to exchange for 3,192,399 common shares of M-I on a one-for-one basis. These shares were issued to comply with an order of the Supreme Court of British Columbia dated May 20, 2003. On May 26, 2005, the Company issued 17,000 common shares to exchange for 17,000 common shares of M-I on a one-for-one basis.
In a counterclaim filed in the Supreme Court of British Columbia, the Company continues to dispute the plaintiff's entitlement to the 3,192,399 M-I shares and any Company shares that he may received pursuant to court order.
No gain or loss provisions have been provided as of November 30, 2007 as the outcome of this legal proceeding is uncertain at this time.
F-22
MIV Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
November 30, 2007
(Unaudited)
Expressed in U.S. Dollars
14. General and Administrative Expenses
General and administrative expenses consisted of the following:
Six months ended | Three months ended | ||||
November 30, | November 30, | ||||
2007 | 2006 | 2007 | 2006 | ||
Legal | $ 289,288 | $ 244,999 | $ 190,466 | $117,469 | |
Public relations, financing and corporate development | 381,213 | 463,237 | 142,514 | 172,479 | |
Management fees | 1,301,806 | 673,720 | 649,080 | 237,503 | |
Consulting | 298,951 | 72,683 | 192,826 | 14,535 | |
Audit | 408,500 | 120,899 | 165,163 | 45,187 | |
Operating expenses | 985,936 | 469,441 | 589,653 | 185,293 | |
$3,665,694 | $2,044,979 | $1,929,702 | $772,466 | ||
During the six months ended November 30, 2007, general and administrative expenses include $10,616 (2006 - $124,151) and $168,928 (2006 - $12,155) of amortized deferred compensation in public relations and consulting, respectively. $226,597 and $48,400 (2006 - $281,000 and $79,691) of stock-based compensation pertains to options granted and extended, respectively, to administrative personnel.
During the three months ended November 30, 2007, general and administrative expenses include $2,676 (2006 - Nil) and $84,464 (2006 - Nil) of amortized deferred compensation in public relations and consulting, respectively. $124,948 and $48,400 (2006 - $140,500 and Nil) of stock-based compensation pertains to options granted and extended, respectively, to administrative personnel.
15. Supplemental Cash Flow Information
Period from inception (January 20, 1999) to November30, 2007 | Six months ended | |||||
2007 | 2006 | |||||
Supplemental cash flow information: | ||||||
Interest paid in cash | $ | 76,705 | $ | 24,366 | $ | 1,805 |
Supplemental non-cash transactions: | ||||||
Debt settlement with shares | $ | 621,375 | $ | - | $ | - |
Conversion of convertible debentures and accrued interest to common shares |
| - | - | |||
Shares issued for finders' fees | 236,868 | - | - | |||
F-23
MIV Therapeutics, Inc.
(A development stage company)
Notes to Consolidated Financial Statements
November 30, 2007
(Unaudited)
Expressed in U.S. Dollars
16. Segmented Information
The Company operates in one segment which comprises the research, manufacture and development of bio-compatible stent coatings for implantable medical devices and drug-delivery technologies.
The following is a summary of the Company's geographical information as of November 30, 2007 and for the periods ended November 30, 2007 and 2006
Canada | India | Assets and Liabilities of Business to be Transferred | Total | |||||
Six months ended November 30, 2007 | ||||||||
Net revenue | $ | - | $ | 551,641 | $ | - | $ | 551,641 |
Gross profit | - | 58,577 | - | 58,577 | ||||
Depreciation and amortization | 69,769 | 180,958 | 7,747 | 258,474 | ||||
Net loss | 5,449,942 | 507,675 | 600,955 | 6,558,572 | ||||
Three months ended November 30, 2007 | ||||||||
Net revenue | - | 250,031 | - | 250,031 | ||||
Gross profit | - | 54,337 | - | 54,337 | ||||
Depreciation and amortization | 40,240 | 90,577 | 3,876 | 134,693 | ||||
Net loss | 2,860,285 | 247,887 | 315,748 | 3,423,920 | ||||
As of November 30, 2007 | ||||||||
Total assets | 6,411,112 | 3,782,148 | 149,430 | 10,342,690 | ||||
Additions to property and equipment | 132,314 | 399,598 | 937 | 532,849 | ||||
CE Mark license | - | 1,308,765 | - | 1,308,765 | ||||
Six months ended November 30, 2006 | ||||||||
Net revenue | - | - | - | - | ||||
Gross profit | - | - | - | - | ||||
Depreciation and amortization | 45,516 | - | 5,485 | 51,001 | ||||
Net loss | 3,724,420 | - | 420,567 | 4,144,987 | ||||
Three months ended November 30, 2006 | ||||||||
Net revenue | - | - | - | - | ||||
Gross profit | - | - | - | - | ||||
Depreciation and amortization | 23,073 | - | 2,866 | 25,939 | ||||
Net loss | 1,539,772 | - | 239,383 | 1,779,155 | ||||
17. Reclassifications
Certain amounts from prior periods have been reclassified to conform to the current period presentation. These reclassifications had no effect on net loss or loss per common share.
F-24
Consolidated Financial Statements
May 31, 2007, 2006 and 2005
Index
Reports of Independent Registered Public Accounting Firms
Consolidated Balance Sheets
Consolidated Statements of Operations and Other Comprehensive Loss
Consolidated Statements of Stockholders' Equity (Deficit)
Consolidated Statements of Cash Flows
Notes to Consolidated Financial Statements
F-25
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of
MIV Therapeutics Inc.
(A development stage company)
We have audited the accompanying consolidated balance sheet of MIV Therapeutics Inc. (a development stage company) as of May 31, 2007, the related consolidated statements of stockholders' equity (deficit), operations and other comprehensive loss, and cash flows for the year ended May 31, 2007 and for the period from January 20, 1999 (inception) to May 31, 2007. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audit. The consolidated financial statements as of May 31, 2006 and for the cumulative period from January 20, 1999 (inception) to May 31, 2006 were audited by other auditors whose report dated July 14, 2006 expressed an unqualified opinion on those statements, other than for the period from June 1, 2004 to May 31, 2005 which were audited by us and our report dated August 18, 2005 except for Notes 15 and 6d which are as of October 20, 2005 expressed an unqualified opini on. The financial statements for the period from January 20, 1999 (inception) to May 31, 2006 include total revenues and net loss of $nil and $31,972,121, respectively. Our opinion on the statements of stockholders' equity (deficit), operations and cash flows for the period January 20, 1999 (inception) to May 31, 2007, insofar as it relates to amounts for prior periods through May 31, 2004 and for the prior period from June 1, 2005 to May 31, 2006 is based solely on the reports of other auditors.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company's internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We beli eve that our audit and the reports of the other auditors provide a reasonable basis for our opinion.
In our opinion, based on our audits and the reports of other auditors, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of the Company as of May 31, 2007, and the results of its operations and other comprehensive loss, and its cash flows for the year ended May 31, 2007, and for the cumulative period from January 20, 1999 (inception) to May 31, 2007 in conformity with U.S. generally accepted accounting principles.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1, the Company has recurring losses from operations since inception and has a working capital deficiency that raise substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
As discussed in Note 2 to the Consolidated Financial Statements, effective June 1, 2006, the Company has adopted the provision of Statements of Financial Accounting Standards No. 123(R), Share Based Payment and No. 151, Inventory Cost - an amendment of ARB No. 43, Chapter 4.
Vancouver, Canada /s/ Ernst & Young LLP
July 16, 2007 except for Chartered Accountants
Notes 16 (b) and (c) which are as of November 13, 2007
F-26
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and Board of Directors of MIV Therapeutics, Inc.:
We have audited the accompanying consolidated balance sheet of MIV Therapeutics, Inc. (a development stage company) as of May 31, 2006 and the consolidated statements of operations and other comprehensive loss, stockholders' deficit, and cash flows for the year then ended and the cumulative period from January 20, 1999 (inception) to May 31, 2006. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audit. The consolidated financial statements as of May 31, 2005 and for the period from January 20, 1999 (inception) to May 31, 2005 were audited by other auditors whose report dated August 18, 2005, except for notes 15 and 6(d) to those financial statements which were dated October 20, 2005, expressed an unqualified opinion on those financial statements. The consolidated financial statements for the period January 20, 1999 (inception) to May 31, 2005 reflect a total net loss of $22, 033,109 of the related cumulative totals. The other auditors' report has been furnished to us, and our opinion, insofar as it relates to amounts included for such prior periods, is based solely on the reports of such other auditors.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform an audit to obtain reasonable assurance whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, based on our audit and the reports of other auditors, these consolidated financial statements present fairly, in all material respects, the financial position of the MIV Therapeutics, Inc. as of May 31, 2006 and the results of its operations and other comprehensive loss and its cash flows and the changes in stockholders' equity for the year then ended and for the period from January 20, 1999 (inception) to May 31, 2006, in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, to date the Company has reported losses since inception from operations and requires additional funds to meet its obligations and fund the costs of its operations. These factors raise substantial doubt about the Company's ability to continue as a going concern. Management's plans in this regard are described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ Dale Matheson Carr-Hilton LaBonte
CHARTERED ACCOUNTANTS
Vancouver, Canada
July 14, 2006
F-27
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of
MIV Therapeutics Inc.
(A development stage company)
We have audited the accompanying consolidated balance sheet of MIV Therapeutics Inc. (a development stage company) as of May 31, 2005, the related consolidated statements of stockholders' equity (deficit), operations and cash flows for the year ended May 31, 2005 and for the period from January 20, 1999 (inception) to May 31, 2005. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audit. The consolidated financial statements as of May 31, 2004 and for the cumulative period from January 20, 1999 (inception) to May 31, 2004 were audited by other auditors whose reports dated July 29, 2003 and July 7, 2004 expressed unqualified opinions on those statements. The financial statements for the period from January 2, 1999 (inception) to May 31, 2004 include total revenues and net loss of $nil and $16,268,403 since inception, respectively. Our opinion on the statements of stockholders' equity (deficit), operations and cash flows for the period January 20, 1999 (inception) to May 31, 2005, insofar as it relates to amounts for prior periods through May 31, 2004 is based solely on the reports of other auditors.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company's internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We belie ve that our audit and the reports of the other auditors provide a reasonable basis for our opinion.
Subsequent to the issuance of the Company's 2005 consolidated financial statements and our initial report thereon dated August 18, 2005, discovery of facts existing at the date of our report resulted in a restatement of certain information in the consolidated financial statements. Prior auditors reaudited the cumulative income, expense and cash flow data from inception to May 31, 2003 which resulted in an adjustment to the Cumulative Net Loss from inception to May 31, 2005 of $1,102,483 and a restated cumulative loss per share of $1.16. The report of other auditors have been reissued and remains unqualified.
In our opinion, based on our audit and the reports of other auditors, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of the Company as of May 31, 2005, and the results of its operations and its cash flows for the year ended May 31, 2005, and for the cumulative period from January 20, 1999 (inception) to May 31, 2005 in conformity with U.S. generally accepted accounting principles.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1, the Company has recurring losses from operations since inception and has a working capital deficiency that raise substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Vancouver, Canada /s/ Ernst & Young LLP
August 18, 2005 except for Chartered Accountants
Notes 15 and 6 (d) which are as of October 20, 2005
F-28
MOORE STEPHENS ELLIS FOSTER LTD.
CHARTERED ACCOUNTANTS
1650 West 1st Avenue
Vancouver, BC Canada V6J 1G1
Telephone: (604) 734-1112 Facsimile: (604) 714-5916
Website: www.ellisfoster.com
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
TO THE BOARD OF DIRECTORS AND STOCKHOLDERS OF
MIV THERAPEUTICS INC.
(A development stage company)
We have audited the consolidated balance sheet of MIV THERAPEUTICS INC. (a development stage company) ("the Company") as at May 31, 2004 and the related consolidated statements of stockholders' equity, operations and cash flows for the year ended May 31, 2004. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audit. We did not audit the cumulative data from January 20, 1999 (inception) to May 31, 2003 in the statements of stockholders' equity, operations and cash flows, which were audited by other auditors whose report, dated July 29, 2003, which expressed an unqualified opinion, has been furnished to us. Our opinion, insofar as it relates to the amounts included for cumulative data from January 20, 1999 (inception) to May 31, 2003, is based solely on the report of the other auditors.
We conducted our audit in accordance with standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform an audit to obtain reasonable assurance whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provide a reasonable basis for our opinion.
In our opinion, these financial statements present fairly, in all material respects, the financial position of the Company as at May 31, 2004 and the results of its operations and its cash flows for the year then ended in conformity with generally accepted accounting principles in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company is a development stage company since inception on January 20, 1999 and has incurred significant recurring net losses since then resulting in a substantial accumulated deficit, which raise substantial doubt about its ability to continue as a going concern. The Company is devoting substantially all of its present efforts in establishing its business. Management's plans regarding the matters that raise substantial doubt about the Company's ability to continue as a going concern are also disclosed in Note 1 to the financial statements. The ability to meet its future financing requirements and the success of future operations cannot be determined at this time. These financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Vancouver, Canada "MOORE STEPHENS ELLIS FOSTER LTD."
July 7, 2004 Chartered Accountants
F-29
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
MIV Therapeutics Inc.
(A Development Stage Company)
We have audited the consolidated balance sheets of MIV Therapeutics Inc. (a development stage company) as at May 31, 2003, and the consolidated statements of operations, cash flows, and stockholders' equity for the year ended May 31, 2003, and the cumulative data from January 20, 1999 (date of inception) to May 31, 2003. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform an audit to obtain reasonable assurance whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, these consolidated financial statements present fairly, in all material respects, the financial position of MIV Therapeutics Inc. as of May 31, 2003, and the results of their operations and its cash flows for the years ended May 31, 2003, and the cumulative data from January 20, 1999 (date of inception) to May 31, 2003, in conformity with accounting principles generally accepted in the United States of America.
As more fully described in Note 14, subsequent to the issuance of the Company's 2003 consolidated financial statements and our report thereon dated July 29, 2003, we became aware that those financial statements did not reflect correct cumulative operating and cash flow amounts for the period from incorporation, January 20, 1999, to May 31, 2003. In our original report we expressed an unqualified opinion on the 2003 consolidated financial statements, and our opinion on the revised statements, as expressed therein, remains unqualified.
The accompanying consolidated financial statements have been prepared assuming that MIV Therapeutics Inc. will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, MIV Therapeutics Inc. has suffered losses from operations and has a working capital deficiency that raises substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
"Morgan & Company"
Chartered Accountants
Vancouver, Canada
September 23, 2005
F-30
MIV THERAPEUTICS INC. | |||||||
(A development stage company) | |||||||
Consolidated Balance Sheets | |||||||
As at May 31, 2007 and 2006 | |||||||
(Expressed in US dollars) | |||||||
(Basis of Presentation - Note 1) | |||||||
2007 | 2006 | ||||||
ASSETS | |||||||
Current assets | |||||||
Cash and cash equivalents | $ | 473,419 | $ | 1,573,822 | |||
Accounts receivable | 50,829 | 56,902 | |||||
Employee advances - current portion (Note 10) | 20,432 | - | |||||
Prepaid expenses and deposits (Note 3) | 423,396 | 84,365 | |||||
Inventories (Note 4) | 563,684 | - | |||||
Total current assets | 1,531,760 | 1,715,089 | |||||
Employee advances (Note 10) | 14,343 | - | |||||
Property and equipment, net (Note 6) | 1,311,583 | 338,786 | |||||
CE Mark License(Note 7) | 1,389,279 | - | |||||
Total assets | $ | 4,246,965 | $ | 2,053,875 | |||
LIABILITIES AND STOCKHOLDERS' EQUITY | |||||||
Liabilities | |||||||
Current liabilities | |||||||
Accounts payable and other payables (Note 10) | $ | 1,617,358 | $ | 185,624 | |||
Related party loan (Note 10) | 100,000 | - | |||||
Loan payable (Note 15) | 525,000 | - | |||||
Deferred lease inducement - current portion (Note 12) | 8,081 | 8,081 | |||||
Total current liabilities | 2,250,439 | 193,705 | |||||
Deferred lease inducement (Note 12) | 19,529 | 27,609 | |||||
Deferred income tax liability(Note 11) | 297,000 | - | |||||
Total liabilities | $ | 2,566,968 | 221,314 | ||||
Commitments and contingencies (Notes 5 and 12) | |||||||
Stockholders' Equity | |||||||
Common stock (Note 8) | |||||||
Authorized: | |||||||
230,000,000 | common shares with a par value of $0.001 | ||||||
20,000,000 | preferred shares with a par value of $0.001 | ||||||
Issued and outstanding: | |||||||
83,785,056 | common shares at May 31, 2007 and | ||||||
68,359,964 | common shares at May 31, 2006 | 83,785 | 68,360 | ||||
Additional paid-in capital | 42,329,385 | 33,214,382 | |||||
Deferred compensation | (320,579) | (199,569) | |||||
Common stock issuable | 1,411,489 | 74,000 | |||||
Deficit accumulated during the development stage | (41,627,415) | (31,127,944) | |||||
Accumulated other comprehensive loss | (196,668) | (196,668) | |||||
Total stockholders' equity | 1,679,997 | 1,832,561 | |||||
Total liabilities and stockholders' equity | $ | 4,246,965 | $ | 2,053,875 | |||
The accompanying notes are an integral part of these consolidated financial statements. | |||||||
F-31
MIV THERAPEUTICS INC. | ||||||||
(A development stage company) | ||||||||
Consolidated Statements of Operations and Other Comprehensive Loss | ||||||||
Years Ended May 31, 2007 and 2006 | ||||||||
(Expressed in US dollars) | ||||||||
Period | ||||||||
from | ||||||||
inception | ||||||||
(January 20 | ||||||||
1999) to | ||||||||
May 31, | ||||||||
2007 | 2007 | 2006 | 2005 | |||||
Revenue | $ | 191,490 | $ | 191,490 | $ | - | $ | - |
Cost of sales | 147,140 | 147,140 | ||||||
Gross profit | 44,350 | 44,350 | ||||||
Expenses | ||||||||
General and administrative (Notes 10 and 13) | 26,961,301 | 6,595,471 | 6,138,912 | 2,720,260 | ||||
Research and development | 11,925,072 | 3,730,570 | 2,792,251 | 1,578,408 | ||||
Depreciation and amortization | 994,900 | 96,404 | 143,754 | 176,453 | ||||
Interest expense and finance fees (Note 8(b)) | 1,025,457 | 99,943 | 87,037 | - | ||||
Licenses acquired charged to operations | 479,780 | - | - | - | ||||
Finance cost on convertible debentures | 382,307 | - | - | 382,307 | ||||
Purchased in-process research and development | 2,205,013 | - | - | 1,701,585 | ||||
43,973,830 | 10,522,388 | 9,161,954 | 6,559,013 | |||||
Loss from operations | (43,929,480) | (10,478,038) | (9,161,954) | (6,559,013) | ||||
Gain on extinguishment of debt | 462,249 | - | - | - | ||||
Interest income | 150,524 | 13,085 | 82,511 | 5,161 | ||||
Gain (loss) on foreign exchange | 11,805 | (61,518) | (15,392) | (55,030) | ||||
Loss for the year before tax and minority interest | (43,304,902) | (10,526,471) | (9,094,835) | (6,608,882) | ||||
Deferred income tax recovery | 27,000 | 27,000 | - | - | ||||
Loss for the year before minority interest | (43,277,902) | (10,499,471) | (9,094,835) | (6,608,882) | ||||
Minority interest share of loss | 806,310 | - | - | - | ||||
Net loss | $ | (42,471,592) | $ | (10,499,471) | $ | (9,094,835) | $ | (6,608,882) |
Other comprehensive loss | ||||||||
Foreign currency translation | (196,668) | - | (10,573) | (23,980) | ||||
|
|
|
|
|
|
|
|
|
Loss per common share | ||||||||
- basic and diluted | $ | (1.32) | $ | (0.15) | $ | (0.14) | $ | (0.15) |
Weighted average number of common | ||||||||
shares outstanding | ||||||||
- basic and diluted | 32,232,707 | 70,582,906 | 63,454,536 | 42,881,975 | ||||
The accompanying notes are an integral part of these consolidated financial statements. | ||||||||
F-32
MIV THERAPEUTICS INC. | |||||||||
(A development stage company) | |||||||||
Consolidated Statements of Stockholders' Equity (Deficit) | |||||||||
For the Period from Inception (January 20, 1999) to May 31, 2007 | |||||||||
(Expressed in US dollars) | |||||||||
Accumulated | |||||||||
Other | Deficit | Total | |||||||
Compre- | Accumulated | Stock- | |||||||
Additional | Deferred | Common | hensive | During the | holders' | ||||
Common Stock | Paid-in | Compen- | Stock | Income | Development | Equity | |||
Shares | Amount | Capital | sation | Issuable | (Loss) | Stage | (Deficit) | ||
$ | $ | $ | $ | $ | $ | $ | |||
Balance, January 20, 1999 | - | - | - | - | - | - | - | - | |
Issuance of common stock for cash | 12,217,140 | 12,217 | 920,826 | - | - | - | - | 933,043 | |
Common shares issuable pursuant | |||||||||
to anti-dilution provision | - | - | - | - | 45,676 | - | - | 45,676 | |
Comprehensive income (loss): | |||||||||
Net loss | - | - | - | - | - | - | (179,544) | (179,544) | |
Balance, May 31, 1999 | 12,217,140 | 12,217 | 920,826 | - | 45,676 | - | (179,544) | 799,175 | |
Issuance of common stock: | - | ||||||||
- for cash | 828,350 | 828 | 693,392 | - | - | - | - | 694,220 | |
- for services rendered | 420,000 | 420 | 287,700 | - | - | - | - | 288,120 | |
- for settlement of agreement | 99,500 | 100 | 68,157 | - | - | - | - | 68,257 | |
Common shares issuable pursuant | |||||||||
to anti-dilution provision | - | - | - | - | 210,487 | - | - | 210,487 | |
Subscriptions received | - | - | - | - | 249,800 | - | - | 249,800 | |
Stock options granted | - | - | 54,600 | (54,600) | - | - | - | - | |
Amortization of stock-based | |||||||||
compensation | - | - | - | 23,780 | - | - | - | 23,780 | |
Comprehensive loss: | |||||||||
Foreign currency translation adjustment | - | - | - | - | - | (731) | - | (731) | |
Net loss | - | - | - | - | - | - | (1,602,492) | (1,602,492) | |
Balance, May 31, 2000 | 13,564,990 | 13,565 | 2,024,675 | (30,820) | 505,963 | (731) | (1,782,036) | 730,616 | |
The accompanying notes are an integral part of these consolidated financial statements.
F-33
MIV THERAPEUTICS INC. | |||||||||
(A development stage company) | |||||||||
Consolidated Statements of Stockholders' Equity (Deficit) | |||||||||
For the Period from Inception (January 20, 1999) to May 31, 2007 | |||||||||
(Expressed in US dollars) | |||||||||
Accumulated | |||||||||
Other | Deficit | Total | |||||||
Compre- | Accumulated | Stock- | |||||||
Additional | Deferred | Common | hensive | During the | holders' | ||||
Common Stock | Paid-in | Compen- | Stock | Income | Development | Equity | |||
Shares | Amount | Capital | sation | Issuable | (Loss) | Stage | (Deficit) | ||
$ | $ | $ | $ | $ | $ | $ | |||
Balance, May 31, 2000 | 13,564,990 | 13,565 | 2,024,675 | (30,820) | 505,963 | (731) | (1,782,036) | 730,616 | |
Issuance of common stock: | |||||||||
- for cash | 1,865,000 | 1,865 | 1,660,235 | - | - | - | - | 1,662,100 | |
- for settlement of agreement | 62,000 | 62 | 42,470 | - | - | - | - | 42,532 | |
- for conversion of subscription | |||||||||
receivable | 269,800 | 270 | 249,530 | - | (249,800) | - | - | - | |
Common shares issuable | - | - | - | - | 53,100 | - | - | 53,100 | |
Subscriptions received | - | - | - | - | 57,825 | - | - | 57,825 | |
Stock options granted | - | - | 112,600 | - | - | - | - | 112,600 | |
Common shares issuable pursuant | |||||||||
to anti-dilution provision | - | - | - | - | 25,147 | - | - | 25,147 | |
Amortization of stock-based | |||||||||
compensation | - | - | - | 20,183 | - | - | - | 20,183 | |
Beneficial conversion on related | |||||||||
party loan | - | - | 850,000 | - | - | - | - | 850,000 | |
Comprehensive income: | |||||||||
Foreign currency translation adjustment | - | - | - | - | 30,027 | - | 30,027 | ||
Net loss | - | - | - | - | - | (3,911,601) | (3,911,601) | ||
- | |||||||||
Balance prior to recapitalization | 15,761,790 | 15,762 | 4,939,510 | (10,637) | 392,235 | 29,296 | (5,693,637) | (327,471) | |
Minority interest of M-I Vascular | |||||||||
Innovations, Inc. | (6,751,790) | (6,752) | (1,906,150) | - | (392,235) | - | 1,744,526 | (560,611) | |
Total relating to final M-I Vascular | |||||||||
Innovations, Inc., May 15, 2001 | 9,010,000 | 9,010 | 3,033,360 | (10,637) | - | 29,296 | (3,949,111) | (888,082) | |
DBS Holdings, Inc. (MIV Therapeutics, | |||||||||
Inc.) shareholders at May 15, 2001 | 11,085,500 | 11,086 | 150,104 | - | - | - | (193,910) | (32,720) | |
Share redemption pursuant to share | |||||||||
exchange and financial agreement | (5,500,000) | (5,500) | (150,104) | - | - | - | (64,396) | (220,000) | |
Subscriptions received | - | - | - | - | 1,070,000 | - | - | 1,070,000 | |
Balance, May 31, 2001 | 14,595,500 | 14,596 | 3,033,360 | (10,637) | 1,070,000 | 29,296 | (4,207,417) | (70,802) | |
The accompanying notes are an integral part of these consolidated financial statements.
F-34
MIV THERAPEUTICS INC. | |||||||||
(A development stage company) | |||||||||
Consolidated Statements of Stockholders' Equity (Deficit) | |||||||||
For the Period from Inception (January 20, 1999) to May 31, 2007 | |||||||||
(Expressed in US dollars) | |||||||||
Accumulated | |||||||||
Other | Deficit | Total | |||||||
Compre- | Accumulated | Stock- | |||||||
Additional | Deferred | Common | hensive | During the | holders' | ||||
Common Stock | Paid-in | Compen- | Stock | Income | Development | Equity | |||
Shares | Amount | Capital | sation | Issuable | (Loss) | Stage | (Deficit) | ||
$ | $ | $ | $ | $ | $ | $ | |||
Balance, May 31, 2001 | 14,595,500 | 14,596 | 3,033,360 | (10,637) | 1,070,000 | 29,296 | (4,207,417) | (70,802) | |
Issuance of common stock: | |||||||||
- for subscription received | 713,333 | 713 | 1,069,287 | - | (1,070,000) | - | - | - | |
- for cash | 35,000 | 35 | 52,465 | - | - | - | - | 52,500 | |
- for settlement of related party loan | 1,133,333 | 1,133 | 848,867 | - | - | - | - | 850,000 | |
- for finders' fees | 113,334 | 113 | 236,755 | - | - | - | - | 236,868 | |
- for services rendered | 75,000 | 75 | 164,925 | - | - | - | - | 165,000 | |
Stock option granted | - | - | 2,552,073 | (322,439) | - | - | - | 2,229,634 | |
Amortization of stock-based | |||||||||
compensation | - | - | - | 248,331 | - | - | - | 248,331 | |
Subscriptions received | - | - | - | - | 256,066 | - | - | 256,066 | |
Comprehensive income (loss): | |||||||||
Foreign currency translation adjustment | - | - | - | - | - | (56,211) | - | (56,211) | |
Net loss | - | - | - | - | - | - | (3,929,466) | (3,929,466) | |
Balance, May 31, 2002 | 16,665,500 | 16,665 | 7,957,732 | (84,745) | 256,066 | (26,915) | (8,136,883) | (18,080) | |
Issuance of common stock: | |||||||||
- for cash | 2,452,523 | 2,453 | 892,305 | - | - | - | - | 894,758 | |
- for services rendered | 1,789,777 | 1,790 | 538,251 | (13,333) | - | - | - | 526,708 | |
- for license fee | 750,000 | 750 | 248,677 | - | - | - | - | 249,427 | |
- for subscriptions received | 640,165 | 640 | 193,499 | - | (256,066) | - | - | (61,927) | |
- for settlement of debt | 235,294 | 235 | 110,600 | - | - | - | - | 110,835 | |
- in exchange of M-I shares | 2,043,788 | 2,044 | 639,299 | - | - | - | (642,042) | (699) | |
Stock option granted | - | - | 257,032 | (5,975) | - | - | - | 251,057 | |
Subscriptions received | - | - | - | - | 31,244 | - | - | 31,244 | |
Warrants issued for services | - | - | 659,673 | (29,341) | - | - | - | 630,332 | |
Amortization of stock-based | - | ||||||||
compensation | - | - | - | 84,745 | - | - | - | 84,745 | |
Comprehensive income (loss): | |||||||||
Foreign currency translation adjustment | - | - | - | - | - | (24,834) | - | (24,834) | |
Net loss | - | - | - | - | - | - | (3,173,411) | (3,173,411) | |
Balance, May 31, 2003 | 24,577,047 | 24,577 | 11,497,068 | (48,649) | 31,244 | (51,749) | (11,952,336) | (499,845) | |
The accompanying notes are an integral part of these consolidated financial statements.
F-35
MIV THERAPEUTICS INC. | |||||||||
(A development stage company) | |||||||||
Consolidated Statements of Stockholders' Equity (Deficit) | |||||||||
For the Period from Inception (January 20, 1999) to May 31, 2007 | |||||||||
(Expressed in US dollars) | |||||||||
Accumulated | |||||||||
Other | Deficit | Total | |||||||
Compre- | Accumulated | Stock- | |||||||
Additional | Deferred | Common | hensive | During the | holders' | ||||
Common Stock | Paid-in | Compen- | Stock | Income | Development | Equity | |||
Shares | Amount | Capital | sation | Issuable | (Loss) | Stage | (Deficit) | ||
$ | $ | $ | $ | $ | $ | $ | |||
Balance, May 31, 2003 | 24,577,047 | 24,577 | 11,497,068 | (48,649) | 31,244 | (51,749) | (11,952,336) | (499,845) | |
Issuance of common stock: | |||||||||
- for private placements and | |||||||||
subscriptions | 9,423,079 | 9,423 | 3,558,439 | - | (31,244) | - | - | 3,536,618 | |
- for services | 2,394,456 | 2,395 | 1,145,731 | (525,750) | - | - | - | 622,376 | |
- for settlement of debt | 100,000 | 100 | 11,900 | - | - | - | - | 12,000 | |
- in exchange of M-I shares | 1,398,411 | 1,398 | 502,030 | - | - | - | 503,428 | ||
- for warrants exercised | 2,100,000 | 2,100 | 408,900 | - | - | - | - | 411,000 | |
- for options exercised | 100,000 | 100 | 33,400 | - | - | - | - | 33,500 | |
Stock option granted to consultants | - | - | 59,976 | - | - | - | - | 59,976 | |
Warrants issued for services | 814,798 | (505,938) | 308,860 | ||||||
Amortization of deferred compensation | - | - | - | 889,962 | - | - | - | 889,962 | |
Comprehensive income (loss): | |||||||||
Foreign currency translation adjustment | - | - | - | - | - | (110,366) | - | (110,366) | |
Net loss | - | - | - | - | - | - | (3,471,891) | (3,471,891) | |
Balance, May 31, 2004 | 40,092,993 | 40,093 | 18,032,242 | (190,375) | - | (162,115) | (15,424,227) | 2,295,618 | |
The accompanying notes are an integral part of these consolidated financial statements.
F-36
MIV THERAPEUTICS INC. | |||||||||||||
(A development stage company) | |||||||||||||
Consolidated Statements of Stockholders' Equity (Deficit) | |||||||||||||
For the Period from Inception (January 20, 1999) to May 31, 2007 | |||||||||||||
(Expressed in US dollars) | |||||||||||||
Accumulated | |||||||||||||
Other | Deficit | Total | |||||||||||
Compre- | Accumulated | Stock- | |||||||||||
Additional | Deferred | Common | hensive | During the | holders' | ||||||||
Common Stock | Paid-in | Compen- | Stock | Income | Development | Equity | |||||||
Shares | Amount | Capital | sation | Issuable | (Loss) | Stage | (Deficit) | ||||||
$ | $ | $ | $ | $ | $ | $ | |||||||
Balance, May 31, 2004 | 40,092,993 | 40,093 | 18,032,242 | (190,375) | - | (162,115) | (15,424,227) | 2,295,618 | |||||
Issuance of common stock: | |||||||||||||
- for share subscriptions | 904,215 | 904 | 217,499 | - | - | - | - | 218,403 | |||||
- for exercise of warrants | 2,320,710 | 2,321 | 605,064 | - | - | - | - | 607,385 | |||||
- for exercise of options | 75,000 | 75 | 22,425 | - | - | - | - | 22,500 | |||||
- for services | 1,904,703 | 1,905 | 543,123 | (194,968) | 74,000 | - | - | 424,060 | |||||
- for finder's fee on private placements | |||||||||||||
completed in prior year | 10,000 | 10 | (10) | - | - | - | - | - | |||||
- in exchange of M-I shares (Note 12(c)) | 3,209,399 | 3,209 | 613,376 | - | - | - | - | 616,585 | |||||
- for acquisition of SagaX (Note 12(b)) | 2,000,000 | 2,000 | 938,000 | - | 65,000 | - | - | 1,005,000 | |||||
Fair value of warrants attached to Convertible debentures | - | - | 48,920 | - | - | - | - | 48,920 | |||||
Warrants issued for services | - | - | 917,164 | (917,164) | - | - | - | - | |||||
Stock options granted | - | - | 155,978 | - | - | - | - | 155,978 | |||||
Amortization of deferred compensation | - | - | - | 746,369 | - | - | - | 746,369 | |||||
Beneficial conversion feature | - | - | 289,800 | - | - | - | 289,800 | ||||||
Comprehensive income (loss): | |||||||||||||
Foreign currency translation adjustment | - | - | - | - | - | (23,980) | - | (23,980) | |||||
Net loss | - | - | - | - | - | - | (6,608,882) | (6,608,882) | |||||
Balance, May 31, 2005 | 50,517,020 | 50,517 | 22,383,581 | (556,138) | 139,000 | (186,095) | (22,033,109) | (202,244) | |||||
The accompanying notes are an integral part of these consolidated financial statements. | |||||||||||||
F-37
MIV THERAPEUTICS INC. | |||||||||||||
(A development stage company) | |||||||||||||
Consolidated Statements of Stockholders' Equity (Deficit) | |||||||||||||
For the Period from Inception (January 20, 1999) to May 31, 2007 | |||||||||||||
(Expressed in US dollars) | |||||||||||||
Accumulated | |||||||||||||
Other | Deficit | Total | |||||||||||
Compre- | Accumulated | Stock- | |||||||||||
Additional | Deferred | Common | hensive | During the | holders' | ||||||||
Common Stock | Paid-in | Compen- | Stock | Income | Development | Equity | |||||||
Shares | Amount | Capital | sation | Issuable | (Loss) | Stage | (Deficit) | ||||||
$ | $ | $ | $ | $ | $ | $ | |||||||
Balance, May 31, 2005 | 50,517,020 | 50,517 | 22,383,581 | (556,138) | 139,000 | (186,095) | (22,033,109) | (202,244) | |||||
Issuance of common stock: | |||||||||||||
- for share subscriptions - Reg-S | 1,704,689 | 1,705 | 668,390 | 50,000 | - | - | - | 720,095 | |||||
- Private placement | 7,649,763 | 7,650 | 3,452,600 | - | - | - | - | 3,460,250 | |||||
- for exercise of warrants | 3,680,444 | 3,680 | 1,808,577 | - | - | - | - | 1,812,257 | |||||
- for exercise of options | 747,723 | 748 | 151,252 | - | - | - | - | 152,000 | |||||
- for convertible debentures exercised | 3,158,920 | 3,159 | 737,651 | - | - | - | - | 740,810 | |||||
- for services | 901,405 | 901 | 670,681 | (153,265) | (65,000) | - | - | 453,317 | |||||
Warrants issued for services | - | - | 1,298,856 | (1,298,856) | - | - | - | - | |||||
Warrants issued for license agreement | - | - | 768,807 | - | - | - | - | 768,807 | |||||
Fair value of extended warrants | - | - | 194,844 | - | - | - | - | 194,844 | |||||
Stock options granted | - | - | 1,079,143 | - | - | - | - | 1,079,143 | |||||
Amortization of deferred compensation | - | - | - | 1,758,690 | - | - | - | 1,758,690 | |||||
Comprehensive income (loss): | |||||||||||||
Foreign currency translation adjustment | - | - | - | - | - | (10,573) | - | (10,573) | |||||
Net loss | - | - | - | - | - | - | (9,094,835) | (9,094,835) | |||||
Balance, May 31, 2006 | 68,359,964 | 68,360 | 33,214,382 | (199,569) | 74,000 | (196,668) | (31,127,944) | 1,832,561 | |||||
The accompanying notes are an integral part of these consolidated financial statements. | |||||||||||||
F-38
MIV THERAPEUTICS INC. | |||||||||||||
(A development stage company) | |||||||||||||
Consolidated Statement of Stockholders' Equity (Deficit) | |||||||||||||
For the Period from Inception (January 20, 1999) to May 31, 2007 | |||||||||||||
(Expressed in US dollars) | |||||||||||||
Accumulated | Deficit | Total | |||||||||||
Other | Accumulated | Stock- | |||||||||||
Additional | Deferred | Common | Compre- | During the | holders' | ||||||||
Common Stock | Paid-in | Compen- | Stock | hensive | Development | Equity | |||||||
Shares | Amount | Capital | sation | Issuable | Loss | Stage | (Deficit) | ||||||
$ | $ | $ | $ | $ | $ | $ | |||||||
Balance, May 31, 2006 | 68,359,964 | 68,360 | 33,214,382 | (199,569) | 74,000 | (196,668) | (31,127,944) | 1,832,561 | |||||
Issuance of common stock: (Note 8) | |||||||||||||
- for share subscriptions - Private placement | 11,140,000 | 11,140 | 5,101,974 | - | 895,000 | - | - | 6,008,114 | |||||
- for exercise of warrants | 1,518,281 | 1,518 | 140,232 | - | - | - | - | 141,750 | |||||
- for exercise of options | 205,063 | 205 | 2,795 | - | 38,500 | - | - | 41,500 | |||||
- for services | 1,461,748 | 1,462 | 774,910 | (407,866) | 445,989 | - | - | 814,495 | |||||
- for license agreement (Note 5) | 300,000 | 300 | 123,700 | (50,000) | (74,000) | - | - | - | |||||
Subscription received | - | - | - | - | 32,000 | - | - | 32,000 | |||||
Acquisition of Biosync Scientific Pvt. Ltd. (Note 7) | 800,000 | 800 | 527,200 | - | - | - | - | 528,000 | |||||
Fair value of warrants issued for services | - | - | 57,109 | (57,109) | - | - | - | - | |||||
Fair value of extended warrants | - | - | 145,983 | - | - | - | - | 145,983 | |||||
Fair value of extended options | - | - | 215,592 | - | - | - | - | 215,592 | |||||
Fair value of stock options granted | - | - | 2,025,508 | - | - | - | - | 2,025,508 | |||||
Amortization of deferred compensation | - | - | - | 393,965 | - | - | - | 393,965 | |||||
Comprehensive loss: | |||||||||||||
Foreign currency translation adjustment | - | - | - | - | - | - | - | - | |||||
Net loss | - | - | - | - | - | - | (10,499,471) | (10,499,471) | |||||
Balance, May 31, 2007 | 83,785,056 | 83,785 | 42,329,385 | (320,579) | 1,411,489 | (196,668) | (41,627,415) | 1,679,997 | |||||
The accompanying notes are an integral part of these consolidated financial statements. | |||||||||||||
F-39
MIV THERAPEUTICS INC. | ||||||||
Period from inception (January 20 1999) to May 31, 2007 | 2007 | 2006 | 2005 | |||||
Cash flows from operating activities | ||||||||
Net loss | $ | (42,471,592) | $ | (10,499,471) | $ | (9,094,835) | $ | (6,608,882) |
Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
Stock-based compensation | 10,343,923 | 2,635,065 | 2,837,833 | 902,347 | ||||
Stock issued for other than cash | 6,080,231 | 814,495 | 1,222,124 | 424,060 | ||||
Interest expense on related party loan | 850,000 | - | - | - | ||||
Interest expense on convertible debentures | 34,730 | - | 34,730 | - | ||||
Fair value of extended warrants | 340,827 | 145,983 | 194,844 | - | ||||
Depreciation and amortization | 1,088,128 | 189,632 | 143,754 | 176,453 | ||||
Leasehold improvements written down | 13,300 | - | - | - | ||||
Project acquisition costs | - | - | 53,426 | (53,426) | ||||
Purchased in-process research and development | 2,125,013 | - | - | 1,621,585 | ||||
Intangible asset impairment | 150,000 | - | - | - | ||||
Gain on extinguishment of debt | (462,249) | - | - | - | ||||
Provision for bad debt | 160,000 | - | - | - | ||||
Beneficial conversion feature on convertible debenture | 289,800 | - | - | 289,800 | ||||
Deferred income tax recovery | (27,000) | (27,000) | ||||||
Minority interest | (806,310) | - | - | - | ||||
Changes in operating assets and liabilities: | ||||||||
Accounts receivable | (211,080) | 6,073 | (23,160) | (20,406) | ||||
Due from related party | (34,775) | (34,775) | 17,500 | (17,500) | ||||
Prepaid expenses and deposits | (349,214) | (264,291) | (43,226) | 213,520 | ||||
Inventory | (318,882) | (318,882) | - | - | ||||
Accounts payable and other payables | 1,505,996 | 1,261,830 | (86,055) | 136,498 | ||||
Net cash used in operating activities | (21,699,154) | (6,091,341) | (4,743,065) | (2,935,951) | ||||
Cash flows from financing activities | ||||||||
Issuance of common stock, less share issuance costs | 21,716,273 | 6,191,364 | 6,144,602 | 848,288 | ||||
Due to related parties | 950,000 | 100,000 | - | (13,585) | ||||
Proceeds from (repayments of) convertible debentures | 755,000 | - | (50,000) | 805,000 | ||||
Cash acquired in reverse acquisition | 13,824 | - | - | - | ||||
Subscriptions received | 1,389,310 | 32,000 | - | - | ||||
Common stock redemption | (120,000) | - | - | - | ||||
Loan payable | 1,025,000 | 525,000 | - | - | ||||
Net cash provided by financing activities | 25,729,407 | 6,848,364 | 6,094,602 | 1,639,703 | ||||
Cash flows from investing activities | ||||||||
Cash acquired on acquisition of Biosync (Note 7) | 17,557 | 17,557 | - | - | ||||
Acquisition of Biosync - net (Note 7) | (1,415,885) | (1,415,885) | - | - | ||||
Pre-acquisition advances to Biosync (Note 7) | (121,870) | (121,870) | - | - | ||||
Acquisition of license | (200,000) | - | - | - | ||||
Purchase of property and equipment | (1,597,652) | (337,228) | (259,851) | (221,593) | ||||
Net cash used in investing activities | (3,317,850) | (1,857,426) | (259,851) | (221,593) | ||||
Foreign exchange effect on cash | (238,984) | - | (10,573) | (23,980) | ||||
Net increase (decrease) in cash and cash equivalents | 473,419 | (1,100,403) | 1,081,113 | (1,541,821) | ||||
Cash and cash equivalents,beginning of year | - | 1,573,822 | 492,709 | 2,034,530 | ||||
Cash and cash equivalents,end of year | $ | 473,419 | $ | 473,419 | $ | 1,573,822 | $ | 492,709 |
The accompanying notes are an integral part of these consolidated financial statements. | ||||||||
F-40
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
1. Basis of Presentation and Nature of Operations
Basis of Presentation
These consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America.
Since inception, MIV Therapeutics Inc. (the "Company") has suffered recurring losses, totaling $42,471,592 as of May 31, 2007. To date, management has been able to finance the operations through the issuance of common stock, and through related party loans, in order to meet its strategic objectives. Management plans to continue to seek other sources of financing on favorable terms; however, there are no assurances that any such financing can be obtained on favorable terms, if at all. Management expects to monitor and control the Company's operating costs to a minimum until cash is available through financing or operating activities. There are no assurances that the Company will be successful in achieving these plans. The Company anticipates that losses will continue until such time, if ever, as the Company is able to generate sufficient revenues to support its operations. The Company's ability to generate revenue primarily depends on its success in completing development and obtainin g regulatory approvals for the commercialization of its stent technology. The Company's ability to obtain sufficient financing to continue the development of, and if successful, to commence the manufacture and sale of its products under development, if and when approved by the applicable regulatory agencies is uncertain. In view of these conditions, the ability of the Company to continue as a going concern is in substantial doubt and dependent upon achieving a profitable level of operations and on the ability of the Company to obtain necessary financing to fund ongoing operations. Management believes that its current and future plans enable it to continue as a going concern. These consolidated financial statements do not give effect to any adjustments which would be necessary should the Company be unable to continue as a going concern and therefore be required to realize its assets and discharge its liabilities in other than the normal course of business and at amounts different from those reflected in the accompanying consolidated financial statements.
Nature of Operations
The Company is a development stage enterprise involved in the research, manufacture and development of bio-compatible stent coatings for implantable medical devices and drug-delivery technologies.
On April 25, 2001, the Company executed a Share Exchange and Finance Agreement ("Agreement") with M-I Vascular Innovations, Inc. ("M-I") which is a development stage company incorporated in Delaware. At the time of the Agreement, the Company was a non-operating public company.
The Agreement closed effective as of May 15, 2001. As a consequence, control of the Company shifted from the shareholders of the Company to the founders of M-I. The change of control resulted from the combined effect of (I) a redemption of 5,500,000 of the common shares of the Company, and (ii) the issuance of 9,010,000 common shares by the Company in a one-for-one exchange for the shares of M-I held by its shareholders. As a result, the former shareholders of M-I obtained a majority interest in the Company.
F-41
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
1. Nature of Operations and Basis of Presentation (continued)
As the Company was a non-operating public company, the share exchange has been accounted for as a recapitalization of M-I and an issuance of shares by M-I to the shareholders of the Company. As not all M-I shareholders tendered their shares in the combination, these shares were treated as minority interest. In the same way, the value of warrants held by shareholders who did not agree to exchange their shares and the value of compensatory stock options issued by the Company was allocated to minority interest.
During the year ended May 31, 2003, 2,043,788 common shares of the Company were exchanged on a one-for-one basis for shares of M-I. Accordingly, 2,043,788 common shares were added to the number of shares outstanding along with the par value of such shares, a pro-rated amount to additional paid-in capital and as the Company has a shareholders' deficiency, an amount to deficit to the extent of the amount added to common stock and additional paid-in capital.
During the year ended May 31, 2004, 1,398,411 common shares of the Company were exchanged on a one-for-one basis for shares of M-I. Accordingly, 1,398,411 common shares were added to the number of shares outstanding along with the par value of such shares, a pro-rated amount to additional paid-in capital and as the Company has a shareholders' deficiency, an amount to deficit to the extent of the amount added to common stock and additional paid-in capital.
In 2007, the Company acquired Biosync Scientific Pvt. Ltd. ("Biosync") a company incorporated in Gujarat, India. Biosync is in the business of designing, manufacturing and marketing coated and non-coated vascular stents and related accessories.
2. Summary of Significant Accounting Policies
(a) Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions which affect the reporting of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements as of the dates of the financial statements and revenues and expenses during the reporting period. Significant estimates include amortization of property and equipment, calculation of stock-based compensation, amortization of CE Mark License and valuation allowance for deferred income taxes. Actual results could differ from these estimates.
(b) Principle of Consolidation
The accompanying consolidated financial statements include the accounts of MIV Therapeutics Inc. (incorporated in Nevada, USA), 90% of M-I Vascular Innovations, Inc. (incorporated in Delaware, USA), its wholly-owned subsidiaries, MIVI Technologies, Inc. (incorporated in Yukon, Canada), SagaX, Inc. (incorporated in Delaware, USA) SMT Research and Development Ltd. (incorporated in Jerusalem, Israel) and Biosync Scientific Pvt. Ltd. (incorporated in Gujarat, India). All significant inter-company transactions and balances have been eliminated upon consolidation.
F-42
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
2. Summary of Significant Accounting Policies (continued)
(c) Development Stage
The Company's activities have primarily consisted of establishing facilities, recruiting personnel, conducting research and development, developing business and financial plans and raising capital. Accordingly, the Company is considered to be in the development stage.
(d) Revenue Recognition
The Company recognizes revenue, net of returns, rebates and sales allowances, if any from the sale of products, at the time when the product is delivered to the customer and/or dealer. Revenues are recognized only when the Company has transferred to the customer and/or dealer the significant risk and rewards of ownership of the goods, title to the products transfers, the amount is fixed and determinable, evidence of an agreement exists, there is reasonable assurance of collection of the sales proceeds, the Company has no future obligations and the customer and/or dealer bears the risk of loss.
(e) Cash and Cash Equivalents
The Company considers all highly liquid instruments purchased with an original maturity of three months or less to be cash equivalents. The Company places its cash and cash equivalents with high credit quality financial institutions. The Company occasionally maintains balances in a financial institution beyond the insured amount. As at May 31, 2007, the Company had deposits of $nil (2006 - $1,392,383) beyond the insured amount.
(f) Inventory
Inventories are stated at the lower of cost or replacement cost with respect to raw materials and the lower and net realizable value with respect to finished goods and work in progress. Cost of work in progress and finished goods is determined on a first-in, first-out basis and includes direct material, direct labour and overheads. Net realizable value represents the anticipated selling price less estimated costs of completion and distribution.
In November 2004, the FASB issued SFAS No. 151, "Inventory Costs--an amendment of ARB No. 43, Chapter 4", which is the result of the FASB's project to reduce differences between U.S. and international accounting standards. SFAS No. 151 requires idle facility costs, abnormal freight, handling costs, and amounts of wasted materials (spoilage) be treated as current-period costs. Under this concept, if the costs associated with the actual level of spoilage or production defects are greater than the costs associated with the range of normal spoilage or defects, the difference would be charged to current-period expense, not included in inventory costs.
SFAS No. 151 was adopted by the Company beginning June 1, 2006. The adoption of SFAS No. 151 did not have an impact on the Company's consolidated financial statements during the year ended May 31, 2007.
F-43
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
2. Summary of Significant Accounting Policies (continued)
(g) Property and Equipment
Property and equipment is recorded at cost. Depreciation is provided using the straight-line method over 3 to 14 years. Leasehold improvements are amortized using the straight-line method over the estimated useful life of the asset or the term of the lease, whichever is shorter. Maintenance and repairs are expensed as incurred. Replacements and betterments are capitalized.
The Company evaluates the recoverability of property and equipment whenever events or changes in circumstances indicate that carrying amount of the asset may not be recovered. The Company determines impairment by comparing the undiscounted future cash flows estimated to be generated by these assets to their respective carrying amounts. If the asset is not fully recoverable, an impairment loss would be recognized for the difference between the carrying value of the asset and its estimated fair value based on discounted net future cash flows or quoted market prices. Management has determined that no permanent impairment has occurred as of May 31, 2007 and 2006.
(h) License
CE Mark License that allows Biosync to manufacture and sell bare metal stents is recorded at cost and is amortized on a straight-line basis over its useful life of ten years.
The CE Mark License is tested for impairment whenever events or circumstances indicate that a carrying amount may not be recoverable. An impairment loss would be recognized when the carrying amount of the License exceeds the estimated undiscounted cash flows used in determining the fair value of the assets. The amount of the impairment loss to be recorded is calculated by the excess of the carrying value over its fair value, with fair value being determined using a discounted cash flow analysis.
(i) Research and Development Costs
Research and development costs are expensed in the period incurred. For the year ended May 31, 2007, $721,642 (2006 - $89,600; 2005 - $55,242) of stock-based compensation expense was related to options granted to research and development personnel.
(j) Government Assistance and Other Subsidies
Government assistance and other subsidies are recorded as a reduction of the cost of the applicable assets or the related expenditures as determined by the terms and conditions of the agreement under which the assistance is provided to the Company.
F-44
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
2. Summary of Significant Accounting Policies (continued)
(k) Income Taxes
The Company accounts for income taxes under the provisions of Statement of Financial Accounting Standards ("SFAS") No. 109, "Accounting for Income Taxes". Under SFAS No. 109, deferred income tax assets and liabilities are computed for differences between the financial statements and tax bases of assets and liabilities that will result in taxable or deductible amounts in the future, based on enacted tax laws and rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred income tax assets to the amount expected to be realized.
(l) Foreign Currency Translation
MIVI Technologies, Inc., SMT Research and Development Ltd. and Biosync Scientific Pvt. Ltd. maintain their accounting records in their local currencies (Canadian dollar, Israel Shekel and Indian Rupee, respectively), however, the Company's functional and reporting currency is U.S. dollars. The financial statements of the Company's subsidiaries are translated into United States dollars using period end exchange rates as to monetary assets and liabilities and average exchange rates as to revenues and expenses. Non-monetary assets are translated at their historical exchange rates. Net gains and losses resulting from foreign exchange translations and foreign currency exchange gains and losses on transactions occurring in a currency other than the Company's functional currency are included in the determination of net income in the period.
(m) Financial Instruments and Concentration of Credit Risk
Fair value of financial instruments are made at a specific point in time, based on relevant information about financial markets and specific financial instruments. As these estimates are subjective in nature, involving uncertainties and matters of significant judgment, they cannot be determined with precision. Changes in assumptions can significantly affect estimated fair values.
The carrying value of cash and cash equivalents, accounts receivable, accounts payable, amounts due to related party and loan payable approximate their fair value because of the short-term nature of these instruments.
Unless otherwise noted, it is management's opinion that the Company is not exposed to significant interest or credit risks arising from these financial instruments.
The Company operates and incurs significant expenditures outside of the United States and is exposed to foreign currency risk between the Canadian and U.S dollars, the new Israel Shekel and the Indian Rupee.
At May 31, 2007, approximately $nil of the cash and cash equivalents (2006 - $229,355) are held in Canadian dollars, $4,701 (2006 - $24,569) are held in Israeli Shekels and $146,907 (2006 - $nil) are held in Indian Rupees.
F-45
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
2. Summary of Significant Accounting Policies (continued)
(n) Earnings (Loss) Per Share
The Company computes loss per share in accordance with SFAS No. 128, "Earnings per Share" which requires presentation of both basic and diluted earnings per share on the face of the statement of operations. Basic loss per share is computed by dividing net loss available to common shareholders by the weighted average number of outstanding common shares during the period. Diluted loss per share gives effect to all dilutive potential common shares outstanding during the period including stock options and warrants, using the treasury method. Dilutive loss per share excludes all potential common shares if their effect is anti-dilutive.
(o) Stock-Based Compensation
In December 2004, the FASB issued SFAS No. 123R, "Share-Based Payment", which replaced SFAS No. 123, "Accounting for Stock-Based Compensation" and superseded APB Opinion No. 25, "Accounting for Stock Issued to Employees". In January 2005, the SEC issued Staff Accounting Bulletin ("SAB") No. 107, "Share-Based Payment", which provides supplemental implementation guidance for SFAS No. 123R. SFAS No. 123R requires all share-based payments to employees, including grants of employee stock options, to be recognized in the financial statements based on the grant date fair value of the award. SFAS No. 123R was to be effective for interim or annual reporting periods beginning on or after June 15, 2005, but in April 2005 the SEC issued a rule that will permit most registrants to implement SFAS No. 123R at the beginning of their next fiscal year, instead of the next reporting period as required by SFAS No. 123R. The pro-forma disclosures previously permitted under SFAS No. 123 no l onger will be an alternative to financial statement recognition. Under SFAS No. 123R, the Company must determine the appropriate fair value model to be used for valuing share-based payments, the amortization method for compensation cost and the transition method to be used at date of adoption. The transition methods include prospective and retroactive adoption options.
The Company has adopted the modified prospective method for the fiscal quarter beginning on June 1, 2006. Stock-based compensation expense for awards granted prior to June 1, 2006 was based on the grant date fair-value as determined under the pro-forma provisions of SFAS No. 123.
The Company recorded stock-based compensation expense of $2,241,100 during the year ended May 31, 2007 as a result of the adoption of SFAS No. 123R.
As of May 31, 2007, $927,263 of total unrecognized compensation cost related to stock options is expected to be recognized over a weighted-average period of two years.
F-46
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
2. Summary of Significant Accounting Policies (continued)
(o) Stock-Based Compensation (continued)
Prior to the adoption of SFAS No. 123R, the Company measured compensation expense for its employee stock-based compensation plans using the intrinsic value method prescribed by APB Opinion No. 25. The Company applied the disclosure provisions of SFAS No. 123 as amended by SFAS No. 148, "Accounting for Stock-Based Compensation - Transition and Disclosure", as if the fair-value-based method had been applied in measuring compensation expense. Under APB Opinion No. 25, when the exercise price of the Company's employee stock options was equal to the market price of the underlying stock on the date of the grant, no compensation expense was recognized.
The following table illustrates the effect on net loss and net loss per share if the Company had applied the fair value recognition provisions of SFAS No. 123 to stock-based compensation during the year ended May 31, 2006:
2006 | 2005 | |
Net loss, as reported | $(9,094,835) | $(6,608,882) |
SFAS No. 123 compensation expense | (3,205,816) | (325,449) |
Pro-forma net loss | $(12,300,651) | $(6,934,331) |
Pro-forma basic and diluted net loss per share | $(0.19) | $(0.16) |
For the year ended May 31, 2007, $721,642 (2006 - $89,600) of stock-based compensation expense was related to options granted to research and development personnel.
(p) Comprehensive Loss
The Company adopted SFAS No. 130, "Reporting Comprehensive Income", which establishes standards for reporting and display of comprehensive income, its components and accumulated balances.
Comprehensive loss includes all changes in equity during the year except those resulting from investments by, or distribution to, shareholders. The Company's comprehensive loss consists solely of reported net losses and foreign currency translation adjustments.
(q) Reclassifications
Certain amounts from prior years have been reclassified to conform to the current year presentation.
F-47
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
2. Summary of Significant Accounting Policies (continued)
(r) Recently Adopted Accounting Pronouncements
In December 2006, the Financial Accounting Standards Board ("FASB") issued FSP EITF 00-19-02, "Accounting for Registration Payment Arrangements" ("FSP 00-19-2") which addresses accounting for registration payment arrangements. FSP 00-19-2 specifies that the contingent obligation to make future payments or otherwise transfer consideration under registration payment arrangement, whether issued as a separate agreement or included as a provision of a financial instrument or other agreement, should be separately recognized and measured in accordance with FASB Statement No. 5, "Accounting for Contingencies". FSP 00-19-2 further clarifies that a financial instrument subject to a registration payment arrangement should be accounted for in accordance with other applicable generally accepted accounting principles without regard to the contingent obligation to transfer consideration pursuant to the registration payment arrangement. This FSP is effective immediately for registration payment arrangemen ts and the financial instruments subject to those arrangements that are entered into or modified subsequent to date of issuance of this FSP. For registration payment and financial instrument subject to those arrangements that were entered into prior to the issuance of this FSP, this guidance is effective for the fiscal years beginning after December 15, 2006, and interim periods within those fiscal years. The adoption of this FSP does not have an impact to the company's consolidated financial position.
In September 2006, the Securities and Exchange Commission issued Staff Accounting Bulletin No. 108, "Considering the Effects of Prior Year Misstatements when Quantifying Misstatements in Current Year Financial statements" ("SAB No. 108"), which provides interpretive guidance on the consideration of the effects of prior year misstatements in quantifying current year misstatements for the purpose of a materiality assessment. SAB No. 108 is effective as of the end of the Company's 2006 fiscal year, allowing a one-time transitional cumulative effect adjustment to beginning retained earnings as of June 1, 2006 for errors that were not previously deemed material, but are material under the guidance in SAB No. 108. The adoption of SAB 108 did not have an impact on the Company's financial statements.
(s) Recent Accounting Pronouncements
The FASB has issued the following pronouncements:
In February 2006, the FASB issued SFAS No. 155, "Accounting for Certain Hybrid Financial Instruments-an amendment of FASB Statements No. 133 and 140", to simplify and make more consistent the accounting for certain financial instruments. SFAS No. 155 amends SFAS No. 133, "Accounting for Derivative Instruments and Hedging Activities", to permit fair value remeasurement for any hybrid financial instrument with an embedded derivative that otherwise would require bifurcation, provided that the whole instrument is accounted for on a fair value basis. SFAS No. 155 amends SFAS No. 140, "Accounting for the Impairment or Disposal of Long-Lived Assets", to allow a qualifying special-purpose entity to hold a derivative financial instrument that pertains to a beneficial interest other than another derivative financial instrument. SFAS No. 155 applies to all financial instruments acquired or issued after the beginning of an entity's first fiscal year
F-48
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
2. Summary of Significant Accounting Policies (continued)
(s) Recent Accounting Pronouncements (continued)
that begins after September 15, 2006, with earlier application allowed. This standard is not expected to have a significant effect on the Company's future reported financial position or results of operations.
In March 2006, the FASB issued SFAS No. 156, "Accounting for Servicing of Financial Assets, an amendment of FASB Statement No. 140, Accounting for Transfers and Servicing of Financial Assets and Extinguishments of Liabilities". This statement requires all separately recognized servicing assets and servicing liabilities be initially measured at fair value, if practicable, and permits for subsequent measurement using either fair value measurement with changes in fair value reflected in earnings or the amortization and impairment requirements of Statement No. 140. The subsequent measurement of separately recognized servicing assets and servicing liabilities at fair value eliminates the necessity for entities that manage the risks inherent in servicing assets and servicing liabilities with derivatives to qualify for hedge accounting treatment and eliminates the characterization of declines in fair value as impairments or direct write-downs. SFAS No. 156 is effecti ve for an entity's first fiscal year beginning after September 15, 2006. The adoption of this statement is not expected to have a significant effect on the Company's future reported financial position or results of operations.
In July 2006, FASB issued Interpretation No. 48. This Interpretation clarifies the accounting for uncertainty in income taxes recognized in an enterprise's financial statements in accordance with SFAS Statement No. 109, "Accounting for Income Taxes". This Interpretation prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. This Interpretation also provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition. This Interpretation is effective for fiscal years beginning after December 15, 2006. The Company does not expect that this Interpretation will have any effect on the Company.
In September 2006, FASB issued SFAS No. 157, "Fair Value Measurements". The objective of SFAS 157 is to increase consistency and comparability in fair value measurements and to expand disclosures about fair value measurements. SFAS 157 defines fair value, establishes a framework for measuring fair value in generally accepted accounting principles, and expands disclosures about fair value measurements. SFAS 157 applies under other accounting pronouncements that require or permit fair value measurements and does not require any new fair value measurements. The provisions of SFAS 157 are effective for fair value measurements made in fiscal years beginning after November 15, 2007. The Company has not determined the effect, if any, that the adoption of SFAS 157 will have on the Company's consolidated financial position or results of operations.
F-49
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
2. Summary of Significant Accounting Policies (continued)
(s) Recent Accounting Pronouncements (continued)
In February 2007, the FASB issued SFAS No. 159, "The Fair Value Option for Financial Assets and Financial Liabilities" ("SFAS 159"). SFAS 159 permits entities to choose to measure many financial assets and financial liabilities at fair value. Unrealized gains and losses on items for which the fair value option has been elected will be reported in earnings. SFAS 159 is effective for fiscal years beginning after November 15, 2007. The Company currently is assessing the impact of SFAS 159 on its' consolidated financial position or results of operation.
3. Prepaid expenses and deposits
Prepaid expenses and deposits consisted of the following at May 31:
2007 | 2006 | |||
Prepayment to suppliers | $ | 355,804 | $ | 31,608 |
Other deposits | 58,645 | 49,527 | ||
Other prepaid expenses | 8,947 | 3,230 | ||
$ | 423,396 | $ | 84,365 |
4. Inventories
Inventories consisted of the following at May 31:
2007 | 2006 | |||
Raw materials | $ | 248,496 | $ | - |
Work-in-progress | 190,854 | - | ||
Finished goods | 109,942 | - | ||
Packing materials | 14,392 | |||
$ | 563,684 | $ | - |
F-50
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
5. Licenses
(a) On February 1, 2003, the Company entered into two license agreements with the University of British Columbia ("UBC") which provides the Company with the worldwide right to use, develop and sublicense coating technology for stents and other medical devices.
In consideration of granting the licenses, the Company will pay UBC a royalty of 2.5% of related revenue and a royalty ranging from 10% or 15% of sublicense revenue depending upon the sublicensed technology. In addition, various minimum annual royalties, maintenance fees and milestone payments are payable over the period of development. The Company issued 750,000 common shares to UBC as part of the consideration for the grant of the rights.
The 750,000 common shares had a fair value of $187,500 and were issued and recorded as research and development expense in the year ended May 31, 2003.
(b) On May 19, 2005, the Company signed an amendment to the existing license agreements to include some amendments in the definition of "Field of Use". Also, the royalty terms were amended from 2.5% to range from 2.5% to 5%, depending on the nature of the related revenue.
In consideration for the amendments, the Company agreed to issue 200,000 common shares which had a fair value of $74,000 at the time of the amendment. This amount was recorded as research and development expense during the year ended May 31, 2005. As of May 31, 2006, the shares had not been issued; however, the shares were subsequently issued in July 2006.
On March 15, 2004, the Company entered into a collaborative research agreement with UBC to continue with exploratory research on coating technology for stents for a period from April 1, 2004 to March 31, 2006. During the period of the agreement, various milestone payments were to be made to UBC for the continuation of the research program, estimated to be approximately CDN$220,800 ($164,445).
On October 28, 2004, the Company and UBC amended the existing collaborative research agreements and referred to it as Amendment No. 1 and 2.
In Amendment No. 1, the contract period of the existing collaborative agreement was changed to April 1, 2004 to November 30, 2004 and total costs to the Company were estimated at CDN$110,400 ($87,633). As at May 31, 2005, the Company had paid/accrued and recorded CDN$110,400 ($87,633) to research and development costs in accordance with Amendment No. 1.
F-51
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
5. Licenses (continued)
In Amendment No. 2, the contract period, work plan and total costs of the existing collaborative agreement as amended by Amendment No. 1 were amended. The contract period was extended from December 1, 2004 to November 30, 2006 and total costs to the Company was estimated at CDN$400,400 ($317,828), being payable over the term of the Agreement at various stipulated intervals. As at May 31, 2007, the Company has paid CDN$400,400 ($317,828) for research and development costs in accordance with Amendment No. 2.
The Company obtained financial support of up to CDN$315,000 ($250,040) from the Industrial Research Assistance Program ("IRAP") from the National Research Council Canada. As at May 31, 2007, the Company had received CDN$265,791 ($228,638) from IRAP.
On May 19, 2005, the Company signed a letter of intent to negotiate a new license agreement for a new technology with UBC. The form and content will be similar to that of the license agreements entered into in February 2003 (See Note 5(a) above). Upon execution, the Company will issue 100,000 common shares to UBC. As of May 31, 2007, the new license agreement had not been executed but the related common shares were issued; however, the Company will retain the stock certificate until the negotiations are completed.
On December 1, 2006, the Company extended its collaborative research agreement with UBC to continue with exploratory research on coating technology for stents for a period from December 1, 2006 to November 30, 2007. During the period of the agreement, four equal payments will be made to UBC for a total budget estimate of CDN$274,896 ($241,264). As at May 31, 2007, the Company had paid CDN$137,448 ($120,632) and charged the costs to research and development.
6. Property and Equipment
May 31, 2007 | ||||||
Accumulated | Net Book | |||||
Cost | Depreciation | Value | ||||
Land | $ | 21,483 | $ | - | $ | 21,483 |
Building | 12,450 | 3,501 | 8,949 | |||
Construction-in-progress | 85,282 | - | 85,282 | |||
Furniture and fixtures | 113,707 | 54,308 | 59,399 | |||
Computer equipment | 199,165 | 137,646 | 61,519 | |||
Laboratory equipment | 2,014,315 | 939,364 | 1,074,951 | |||
Leasehold improvements | 49,158 | 49,158 | - | |||
$ | 2,495,560 | $ | 1,183,977 | $ | 1,311,583 | |
F-52
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
6. Property and Equipment (continued)
May 31, 2006 | ||||||
Accumulated | Net Book | |||||
Cost | Depreciation | Value | ||||
Furniture and fixtures | $ | 62,077 | $ | 45,972 | $ | 16,105 |
Computer equipment | 148,581 | 112,182 | 36,399 | |||
Laboratory equipment | 990,414 | 704,132 | 286,282 | |||
Leasehold improvements | 49,158 | 49,158 | - | |||
$ | 1,250,230 | $ | 911,444 | $ | 338,786 | |
Depreciation expense for the year ended May 31, 2007 was $157,628 (2006 - $143,754; 2005 - $176,453). Of this amount, $93,227 (2006 and 2005 - $nil) is included in research and development in the statement of operations.
7. Acquisition of Biosync Scientific Pvt. Ltd.
On February 16, 2007, the Company completed the acquisition of all of the issued and outstanding shares of Biosync, a body corporate subsisting under and registered pursuant to the laws of India and is presently engaged in the business of designing, manufacturing and marketing coated and non-coated vascular stents and related accessories.
Under the terms of the agreement in principle dated October 17, 2006, and the amending agreement dated February 16, 2007 (collectively the "Agreement"), in consideration for the acquisition of the shares of Biosync, the Company issued 50,000 shares of the Company's common stock with an estimated fair value of $33,000 and paid $500,000 to the vendors. As a further condition of the Agreement, the Company was required to satisfy any and all bank indebtedness of Biosync.
The acquisition has been accounted for by the purchase method with the fair value of the consideration paid being allocated to the identifiable assets and liabilities of Biosync as of February 16, 2007 as follows:
Cash and cash equivalents | $17,557 |
Prepaid expenses and other current assets | 74,740 |
Inventory | 244,802 |
Property and equipment | 793,197 |
CE Mark License (i) | 1,421,283 |
2,551,579 | |
Accounts payable and other current liabilities | (161,824) |
Advances from MIV Therapeutics Inc. | (121,870) |
Deferred income tax liability | (324,000) |
Fair value of net assets acquired | $1,943,885 |
(i) CE Mark License is being amortized on a straight-line basis over their estimated useful life of 10 years.
F-53
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
7. Acquisition of Biosync Scientific Pvt. Ltd. (continued)
Consideration paid: | |
Cash | $500,000 |
800,000 shares of restricted common stock valued at $0.66 | 528,000 |
Assumption of bank indebtedness | 908,351 |
Acquisition costs | 7,534 |
$1,943,885 |
The purchase price allocation for this acquisition is preliminary and may be adjusted further as a result of obtaining additional information regarding preliminary estimates of fair values made at the date of purchase.
The Company allocated the purchase price to the assets acquired and liabilities assumed based on their fair values. The purchase price in excess of the net tangible assets acquired totaling $1,421,283 was then allocated to the identified intangible asset being the CE Mark License. The fair value of the CE Mark License was based on a discounted future net cash flows analysis that used information and assumptions provided by management. The CE Mark allows Biosync to manufacture and sell the stents, is being amortized over a period of 10 years and amortization expense of $32,004 has been expensed during the year ended May 31, 2007. For accounting purposes, the 750,000 shares issued (see below) on the condition of receiving CE Mark License with an estimated fair value of $495,000, has been included as consideration for the acquisition. The share price used to determine the purchase price for accounting purposes was based on the average closing market prices of the Company's common stock which includes the two trading days before and after the acquisition negotiated and announced on February 16, 2007.
In addition, the Company and Biosync entered into a executive services agreement with the principal Vendor being employed as Chief Operating Officer and President of Biosync under such commercially competitive compensation terms which will include, but not limited to, (i) a monthly fee of $12,000, (ii) stock options of up to 1,000,000 common shares at an exercise price of $0.60 for a period of not less than ten years from the date of grant and, (iii) an aggregate of up to 4,000,000 common shares with an issuance price of $0.50. Of the 4,000,000 common shares, 2,500,000 will be based on the achievement of certain milestones as outlined in the agreement, of which 750,000 common shares upon receiving CE Mark License and the other 1,500,000 common shares to be given in four equal installments over the two-year term of the agreement. The fair value of the options and common shares are treated as stock based compensation expenses and amortized over the service period.
F-54
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
7. Acquisition of Biosync Scientific Pvt. Ltd. (continued)
The statement of operations includes the operations of Biosync for the period February 16, 2007 to May 31, 2007. A summarized statement of operations for Biosync for the periods June 1, 2006 until February 15, 2007 acquisition date and for the period June 1, 2005 to May 31, 2006 is as follows:
June 1, 2006 | June 1, 2005 | |
Sales | $60,692 | $498,001 |
Total operating expenses | 272,495 | 274,297 |
Income (loss) for the period | (211,803) | 223,704 |
Pro-forma financial information for the years ended May 31, 2007 and 2006 assuming the acquisition occurred on June 1, 2005, are as follows:
2007 | 2006 | |
Sales | $252,182 | $498,001 |
Total operating expenses | 10,990,456 | 9,369,132 |
Loss for the period | (10,738,274) | (8,871,131) |
Loss per share | $(0.15) | $(0.14) |
8. Stockholders' Equity
(a) Common Stock
On August 24, 2006, the stockholders of the Company, during its annual general meeting, approved an increase in its authorized capital stock from 160,000,000 shares of capital stock, consisting of 140,000,000 common shares with par value of $0.001 per share and 20,000,000 preferred shares with par value of $0.001 per share, to 250,000,000 of capital stock consisting of 230,000,000 common shares with par value of $0.001 per share and 20,000,000 preferred shares with par value of $0.001 per share.
(i) During the year ended May 31, 2007, the Company issued an aggregate of 1,461,748 (2006 - 901,405) common shares for consulting, research and development and employee services with a fair value of $776,372 (2006 - $626,780) at the agreement dates. Of this amount, $445,000 is being amortized on a straight-line basis and charged to operations over the period of completion of performance.
F-55
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
8. Stockholders' Equity (continued)
(a) Common Stock (continued)
(ii) During the year ended May 31, 2007, 1,562,611 (2006 - 803,692) warrants with an average exercise price of $0.25 (2006 - $0.48) were exchanged for 1,136,781 (2006 - 517,377) common shares and were exercised under certain cashless exercise provisions of the underlying agreements. Included in these cashless exercises, 762,611 (2006 - 678,692) warrants with an average exercise price of $0.50 (2006 - $0.51) issued through a private placement were exchanged for 349,781 (2006 - 419,048) common shares. As at May 31, 2007, the Company issued a total of 1,518,281 (2006 - 3,680,444) common shares pursuant to the exercise of stock purchase warrants for total proceeds of $141,750 (2006 - $1,812,257).
(iii) During the year ended May 31, 2007, 270,000 (2006 - 65,000) options with an average exercise price of $0.19 (2006 - $0.27) were exchanged for 190,063 (2006 - 52,723) common shares and were exercised under certain cashless exercise provisions of the underlying agreements. As at May 31, 2007, the Company issued 205,063 (2006 - 747,723) common shares pursuant to an exercise of stock purchase options for total proceeds of $3,000 (2006 - $152,000).
On May 31, 2007, the Company also received cash proceeds of $38,500 from the exercise of 150,000 stock options. The common shares in relation to the options exercised had not been issued as at the year end date.
(iv) On May 8, 2007, the Company completed a private placement of 1,790,000 units at a price of $0.50 per unit for total proceeds of $854,873 (net of finder's fee of $38,650 and legal fee of $1,477). Each unit is comprised of one common share and one non-transferable share purchase warrant. Each warrant entitles the holder to purchase one additional common share at an exercise price of $0.75 per share for a period of up to two years from registration of the underlying warrant shares. The 1,790,000 common shares were recorded under common stock issuable in the statement of stockholders' equity.
The warrants had an estimated fair value of $211,463 using the Black-Scholes option pricing model. The assumptions used in the Black-Scholes model were: volatility: 57.47%, discount rate: 4.68%, dividend: nil and expected life of one year.
(v) On April 4, 2007, the Company completed a private placement of 830,000 units at a price of $0.50 per unit for total proceeds of $366,814 (net of finder's fee of $44,550 and legal fee of $3,636). Each unit is comprised of one common share and one non-transferable share purchase warrant. Each warrant entitles the holder to purchase one additional common share at an exercise price of $0.75 per share for a period of up to two years from registration of the underlying warrant shares.
F-56
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
8. Stockholders' Equity (continued)
(a) Common Stock (continued)
The warrants had an estimated fair value of $61,475 using the Black-Scholes option pricing model. The assumptions used in the Black-Scholes model were: volatility: 57.35%, discount rate: 4.61%, dividend: nil and expected life of one year.
(vi) On February 27, 2007, the Company completed a private placement of 375,000 units at a price of $0.50 per unit for total proceeds of $166,811 (net of finder's fee of $18,750 and legal fee of $1,939). Each unit is comprised of one common share and one non-transferable share purchase warrant. Each warrant entitles the holder to purchase one additional common share at an exercise price of $0.75 per share for a period of up to two years from registration of the underlying warrant shares.
The warrants had an estimated fair value of $40,507 using the Black-Scholes option pricing model. The assumptions used in the Black-Scholes model were: volatility: 57.71%, discount rate: 4.59%, dividend: nil and expected life of one year.
In connection with the private placement, the Company issued to the finder 37,500 warrants. Each warrant entitles the finder to purchase one common share at an exercise price of $0.75 per share for a period of up to two years from registration of the underlying warrant shares.
The warrants had an estimated fair value of $4,072 using the Black Scholes option pricing model. The assumptions used in the Black-Scholes model were: volatility: 57.71%, discount rate: 4.87%, dividend: nil and expected life of one year.
(vii) On February 8, 2007, the Company completed a private placement of 1,125,000 units at a price of $0.50 per unit for total proceeds of $502,830 (net of finder's fee of $56,250 and legal fee of $3,420). Each unit is comprised of one common share and one non-transferable share purchase warrant. Each warrant entitles the holder to purchase one additional common share at an exercise price of $0.75 per share for a period of up to two years from registration of the underlying warrant shares.
The warrants had an estimated fair value of $152,763 using the Black-Scholes option pricing model. The assumptions used in the Black-Scholes model were: volatility: 57.71%, discount rate: 4.87%, dividend: nil and expected life of one year.
In connection with the private placement, the Company issued to the finder 112,500 warrants. Each warrant entitles the finder to purchase one common share at an exercise price of $0.75 per share for a period of up to two years from registration of the underlying warrant shares.
The warrants had an estimated fair value of $15,276 using the Black Scholes option pricing model. The assumptions used in the Black-Scholes model were: volatility: 57.71%, discount rate: 4.87%, dividend: nil and expected life of one year.
F-57
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
8. Stockholders' Equity (continued)
(a) Common Stock (continued)
(viii) On December 22, 2006, the Company completed a private placement of 5,900,000 units at a price of $0.50 per unit for total proceeds of $2,684,875 (net of finder's fee of $253,400 and legal fee of $11,725). Each unit is comprised of one common share and one non-transferable share purchase warrant. Each warrant entitles the holder to purchase one additional common share at an exercise price of $0.75 per share for a period of up to two years from registration of the underlying warrant shares.
The warrants had an estimated fair value of $342,706 using the Black-Scholes option pricing model. The assumptions used in the Black-Scholes model were: volatility: 30.40%, discount rate: 4.71%, dividend: nil and expected life of one year.
(ix) On November 8, 2006, the Company completed a private placement of 1,400,000 units at a price of $0.50 per unit for total proceeds of $698,228 (net of legal fee of $1,772). Each unit is comprised of one common share and one non-transferable share purchase warrant. Each warrant entitles the holder to purchase one additional common share at an exercise price of $0.75 per share for a period of up to two years from registration of the underlying warrant shares.
The warrants had an estimated fair value of $52,949 using the Black-Scholes option pricing model. The assumptions used in the Black-Scholes model were: volatility: 59.44%, discount rate: 4.75%, dividend: nil and expected life of one year.
(x) On October 16, 2006, the Company completed a private placement of 600,000 units at a price of $0.50 per unit for total proceeds of $297,565 (net of legal fee of $2,435). Each unit is comprised of one common share and one non-transferable share purchase warrant. Each warrant entitles the holder to purchase one additional common share at an exercise price of $0.75 per share for a period of up to two years from registration of the underlying warrant shares.
The warrants had an estimated fair value of $42,228 using the Black-Scholes option pricing model. The assumptions used in the Black-Scholes model were: volatility: 59.44%, discount rate: 4.85%, dividend: nil and expected life of one year.
F-58
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
8. Stockholders' Equity (continued)
(a) Common Stock (continued)
(xi) On August 21, 2006, the Company completed a private placement of 290,000 units at a price of $0.50 per unit for total net proceeds of $136,866 (net of finder's fee of $2,000 and legal fee of $6,134). Each unit is comprised of one common share and one non-transferable share purchase warrant. Each warrant entitles the holder to purchase one additional common share at an exercise price of $0.75 per share for a period which is the earlier of (i) 18 months from August 21, 2006 or (ii) 12 months from registration of the underlying warrant shares.
The warrants had an estimated fair value of $28,318 using the Black-Scholes option pricing model. The assumptions used in the Black-Scholes model were: volatility: 59.44%, discount rate: 4.85%, dividend: nil and expected life of one year.
(xii) On July 10, 2006, the Company completed a private placement of 620,000 units at a price of $0.50 per unit for total proceeds of $299,250 (net of finder's fee of $3,500 and legal fee of $7,250). Each unit is comprised of one common share and one share purchase warrant. Each warrant entitles the holder to purchase one additional common share at an exercise price of $0.75 per share for a period which is the earlier of (i) 18 months from July 10, 2006 or (ii) 12 months from registration of the underlying warrant shares.
The warrants had an estimated fair value of $164,831 using the Black-Scholes option pricing model. The assumptions used in the Black-Scholes model were: volatility: 64.41%, discount rate: 5.17%, dividend: nil and expected life of one year.
(xiii) On October 6, 2005, the Company completed a private placement of 95,238 units at a price of $1.05 per unit for total proceeds of $100,000. Each unit is comprised of one common share and one non-transferable share purchase warrant. Each warrant entitles the holder to purchase one common share for $1.55 per share for a period of two years from the date of grant.
The warrants had an estimated fair value of $64,208 using the Black-Scholes option pricing model. The assumptions used in the Black-Scholes model were: volatility: 81.23%, discount rate: 5.25%, fair value: $0.67 and expected life of two years.
In connection with the private placement, a finder's fee comprised of $10,000 in cash was paid and 9,524 units were issued. Each unit is comprised of one common share and one non-transferable share purchase warrant. Each warrant entitles the holder to purchase one common share for $1.55 per share for a period of two years from the date of grant.
F-59
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
8. Stockholders' Equity (continued)
(a) Common Stock (continued)
The warrants had an estimated fair value of $2,306 using the Black-Scholes option pricing model. The assumptions used in the Black-Scholes model were: volatility: 87.29%, discount rate: 5.25%, fair value: $0.24 and expected life of two years.
(xiv) On October 4, 2005, the Company issued an aggregate of 3,158,920 common shares pursuant to an exercise of Senior Convertible Debentures (the "Debentures") issued by the Company in a private placement on March 15, 2005. The Debentures were exercised at a conversion price, as determined by the terms of the Debenture Agreement, of $0.25 per common share. The conversion was for an aggregate of $755,000 principal amount and $34,730 accrued interest due under the Debentures. The remaining $50,000, including accrued interest of $2,278, of Debentures was repaid in cash.
(xv) On August 11, 2005, the Company completed a private placement of 7,545,001 units at the price of $0.45 per unit for total net proceeds of $3,370,250. Each unit is comprised of one common share together with one-half of one Series "A" non-transferable share purchase warrant (each a "Series A Warrant") and one-half of one Series "B" non-transferable share purchase warrant (each a "Series B Warrant"). Each whole Series A Warrant entitles the holder to purchase one common share at a price of $0.65 per share for a period which is the earlier of (i) 12 months from August 11, 2005 and (ii) six months commencing from the effective date of the Company's proposed "Registration Statement". Each whole Series B Warrant entitles the holder to purchase one common share at a price of $0.70 per share for the first 12 months, at a price of $0.85 per share for the next 6 months, and at a price of $1.00 per share thereafter. Series B Warrants are exercisable at t he earlier of (i) 30 months from August 11, 2005 and (ii) 24 months commencing from the effective date of the Company's proposed "Registration Statement".
In connection to the private placement, a finder's fee comprised of $25,000 in cash was paid and 62,500 Series A Warrants and Series B Warrants were issued. In a separate transaction, 39,994 units and 100,000 units were issued for legal fees and investor relations services, respectively.
The warrants related to this private placement, 7,684,995 in aggregate, had an estimated fair value of $2,245,749 based on the Black-Scholes option pricing model. The assumptions used in the Black-Scholes model were: volatility: 81.20% and 57.41% for Series A and B, respectively, discount rate: 5.25% for both Series A and B and fair value: $0.32 and $0.26 for Series A and B, respectively.
F-60
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
8. Stockholders' Equity (continued)
(a) Common Stock (continued)
(xvi) In September 2003, the Company placed 6,000,000 common shares to a financial custodian acting as trustee pursuant to a listing of the Company's shares on the Frankfurt Stock Exchange. The Company was then conducting a Regulation S ("Reg S") offering through the facilities of the Berlin Stock Exchange to raise capital in mainly German speaking countries. The trustee was to receive a fee of 3% of the total number of the shares held in trust was paid in equal installments of 30,000 common shares per month over a ten month period, assuming the maximum offering of up to 10,000,000 common shares were sold. The shares may only be traded on German stock exchanges pursuant to Reg S. As at May 31, 2007, 2,500,000 Reg S shares were held in trust by the financial custodian and remain available for financing purposes.
F-61
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
8. Stockholders' Equity (continued)
(b) Warrants
The following table summarizes information about the warrants issued by the Company. All regular warrants and Series A, B and C are exercisable on a one for one basis into common shares.
Number of Shares | Weighted Average | |
Balance, May 31, 2005 - Regular | 7,164,019 | 0.45 |
Balance, May 31, 2005 - Series "A" | 3,374,999 | 0.66 |
Balance, May 31, 2005 - Series "C" | 674,997 | 0.66 |
Regular: | ||
| 5,050,000 | 0.43 |
Issued - private placement | 95,238 | 1.55 |
Issued - finder's fee | 9,524 | 1.55 |
Exercised | (1,599,290) | 0.40 |
Expired | (30,000) | 0.30 |
Series "A": | ||
Issued - private placement | 3,842,498 | 0.65 |
Issued - finder's fee | 62,500 | 0.65 |
Exercised | (1,921,777) | 0.66 |
Series "B": | ||
Issued - private placement | 3,842,498 | 0.70 |
Issued - finder's fee | 62,500 | 0.70 |
Series "C": | ||
Exercised | (445,692) | 0.66 |
Balance, May 31, 2006 - Regular | 10,689,491 | 0.46 |
Balance, May 31, 2006 - Series "A" | 5,358,220 | 0.65 |
Balance, May 31, 2006 - Series "B" | 3,904,998 | 0.70 |
Balance, May 31, 2006 - Series "C" | 229,305 | 0.66 |
Balance, May 31, 2006 | 20,182,014 | 0.55 |
Regular: | ||
Issued - private placement | 12,930,000 | 0.75 |
Issued - finder's fee | 150,000 | 0.75 |
Issued - services | 300,000 | 0.53 |
Issued - loan | 200,000 | 0.60 |
Exercised | (1,473,000) | 0.15 |
Expired | (441,800) | 0.71 |
Series "A": | ||
Expired | (3,904,998) | 0.65 |
Exercised | (361,111) | 0.66 |
Series "C": | ||
Exercised | (110,000) | 0.66 |
Balance, May 31, 2007 - Regular | 22,354,691 | 0.65 |
Balance, May 31, 2007 - Series "A" | 1,092,111 | 0.66 |
Balance, May 31, 2007 - Series "B" | 3,904,998 | 0.70 |
Balance, May 31, 2007 - Series "C" | 119,305 | 0.66 |
Balance, May 31, 2007 | 27,471,105 | 0.65 |
F-62
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
8. Stockholders' Equity (continued)
(b) Warrants (continued)
During the year ended May 31, 2007, the Company issued 200,000 (2006 - 4,150,000) warrants for consulting services and 100,000 warrants (2006 - 900,000) for research and development services rendered to the Company. The warrants issued for consulting services have an exercise price of $0.50 (2006 - prices ranging from $0.50 to $0.85) per share and the warrants issued for research and development services have an exercise price of $0.60 (2006 - $0.01). These warrants had an estimated fair value of $57,109 (2006 - $2,067,663) based on the Black-Scholes option pricing model. The fair value of 200,000 warrants was $41,047 using the Black-Scholes option pricing model with the following assumption: volatility: 57.35% - 57.47% (2006 - 62.72% - 90.01%), discount rate: 4.55% - 4.63% (2006 - 5.25%) and expected life of one year. The fair value of 100,000 warrants was $16,062 using the Black-Scholes option pricing model with the following assumption: volatility: 57.71% (2006 - 62.77%), discount rate : 4.84% (2006 - 5.25%) and expected life of one year.
During the year ended May 31, 2007, the board of directors approved an extension to the expiry date of the following outstanding warrants:
Number of Warrants | From | Extended To | Extended Further To |
71,429 | September 8, 2006 | March 8, 2007 | September 8, 2007 |
500,000 | October 24, 2006 | April 24, 2007 | April 24, 2008 |
1,000,000 | November 5, 2006 | May 5, 2007 | May 5, 2008 |
As a result of the warrant extensions, the Company recognized $72,452 and $73,531 of public relations expense and finance fees, respectively.
(c) Stock Options
The Company's incentive stock options plan provides for the grant of incentive stock options for up to 25,000,000 common shares to employees, consultants, officers and directors of the Company. Incentive benefits granted under the plan may be either incentive stock options, non-qualified stock options, stock awards, restricted shares or cash awards. Options are granted for a term not to exceed ten years from the date of grant. Stock options granted generally vest over a period of two years. As of May 31, 2007, 3,765,001 options are available from the plan.
During fiscal 2007, the Company granted an aggregate of 4,414,999 (2006 - 11,185,000) stock options to employees and directors of the Company. Each option entitles its holder to acquire one common share of the Company at prices ranging from $0.55 to $0.67 (2006 - $0.20 to $1.10) per share, vests immediately or at a specified time, and expires up to ten years from date of grant or the term of agreement.
F-63
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
8. Stockholders' Equity (continued)
(c) Stock Options (continued)
Option pricing models require the use of highly subjective estimates and assumptions including the expected stock price volatility. Changes in the underlying assumptions can materially affect the fair value estimates and therefore, in management's opinion, existing models may not necessarily provide reliable measure of the fair value of the Company's stock options.
The following assumptions were used in determining stock - based compensation costs under the Black-Scholes option pricing model:
2007 | 2006 | 2005 | |
Expected volatility | 125.99% | 66.66% | 78.58% |
Risk-free interest rate | 4.68% | 3.50% | 3.50% |
Expected life (years) | 6.0 | 6.0 | 3.0 |
Dividend yield | Nil | Nil | Nil |
Weighted average fair value of options granted | $0.53 | $0.42 | $0.15 |
The expected volatility related to 2007 and 2006 grants is based on the Company's historical stock prices. Computation of expected life was estimated after considering the contractual terms of the stock-based award, vesting schedules and expectations of future employee behavior. The interest rate for period within the contractual life of the award is based on the U.S. Treasury yield curve in effect at the time of grant.
Compensation cost related to the stock options granted to employees during the year ended May 31, 2007 was charged to operations at the awards' fair value of $1,912,489 (using intrinsic value in 2006 - $1,079,143; 2005 - $155,978).
During the year ended May 31, 2007, the board of directors approved an extension to the expiry date of the following outstanding options:
Number of Options | From | To |
100,000 | August 30, 2006 | August 30, 2011 |
100,000 | August 30, 2006 | August 30, 2010 |
200,000 | August 30, 2006 | August 30, 2009 |
150,000 | May 28, 2007 | May 28, 2010 |
On May 1, 2007, the board of directors approved to lower the price of 150,000 options granted to an employee from $0.85 to $0.60. As a result of the option extensions and lowering of the exercise price, the Company recognized an additional $246,127 and $82,484, respectively, of stock-based compensation in the statement of operations.
The total stock based compensation expense related to stock options granted during the year of $1,912,489, options extension of $246,127, and the lowering of exercise price of $82,484, totaling to $2,241,100, has been charged to consolidated statements of operations and other comprehensive loss.
F-64
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
8. Stockholders' Equity (continued)
(c) Stock Options (continued)
A summary of the weighted average fair value of stock options granted during the year ended May 31, 2007 is as follows:
Number ofOptions | Weighted Average ExercisePrice | Weighted AverageFair Value | |
Exercise price equals market price at grant date: | 3,040,000 | $ 0.59 | $ 0.59 |
Exercise price less than market price at grant date: | 1,374,999 | $ 0.65 | $ 0.67 |
A summary of the weighted average fair value of stock options granted during the year ended May 31, 2006 is as follows:
Number ofOptions | Weighted Average ExercisePrice | Weighted AverageFair Value | |
Exercise price equals market price at grant date: | 50,000 | $ 0.80 | $ 0.80 |
Exercise price greater than market price at grant date: | 150,000 | $ 0.85 | $ 0.84 |
Exercise price less than market price at grant date: | 10,985,000 | $ 0.49 | $ 0.63 |
Summary of stock options information for the years ended May 31, 2007 and 2006 is as follows:
Shares | Weighted Average ExercisePrice | Aggregate IntrinsicValue | ||
Options outstanding, May 31, 2005 | 7,780,000 | 0.35 | ||
Options granted | 11,185,000 | 0.50 | ||
Options exercised | (760,000) | 0.22 | ||
Options expired | (1,820,000) | 0.27 | ||
Options outstanding, May 31, 2006 | 16,385,000 | 0.46 | ||
Options granted | 4,414,999 | 0.61 | ||
Options exercised | (435,000) | 0.21 | ||
Options expired | (325,000) | 0.57 | ||
Options outstanding as at May 31, 2007 | 20,039,999 | 0.49 | $ | 2,025,850 |
Exercisable as at May 31, 2007 | 16,589,998 | 0.47 | $ | 1,956,350 |
F-65
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
8. Stockholders' Equity (continued)
(c) Stock Options (continued)
The following summarizes information about the stock options outstanding and exercisable at May 31, 2007:
Options Outstanding | Options Exercisable | |||||
Range of Exercise Prices | Number of Options Outstanding | Weighted Average Remaining Contractual Life (yr) | Weighted Average Exercise Price | Number of Options Exercisable | Weighted Average Exercise Price | |
$0.17 | 700,000 | 1.39 | $0.17 | 700,000 | $0.17 | |
$0.20 | 1,250,000 | 2.71 | $0.20 | 1,250,000 | $0.20 | |
$0.21 | 500,000 | 0.89 | $0.21 | 500,000 | $0.21 | |
$0.30 | 1,910,000 | 2.24 | $0.30 | 1,910,000 | $0.30 | |
$0.40 | 3,875,000 | 3.94 | $0.40 | 3,875,000 | $0.40 | |
$0.50 | 350,000 | 2.16 | $0.50 | 350,000 | $0.50 | |
$0.55 | 1,920,000 | 3.29 | $0.55 | 1,920,000 | $0.55 | |
$0.56 | 50,000 | 4.34 | $0.56 | 50,000 | $0.56 | |
$0.60 | 6,700,000 | 6.30 | $0.60 | 4,400,000 | $0.60 | |
$0.62 | 300,000 | 4.99 | $0.62 | 100,000 | $0.62 | |
$0.64 | 50,000 | 4.17 | $0.64 | 50,000 | $0.64 | |
$0.65 | 1,274,999 | 4.92 | $0.65 | 474,998 | $0.65 | |
$0.67 | 400,000 | 4.04 | $0.67 | 250,000 | $0.67 | |
$0.75 | 200,000 | 3.90 | $0.75 | 200,000 | $0.75 | |
$0.80 | 160,000 | 3.51 | $0.80 | 160,000 | $0.80 | |
$0.85 | 150,000 | 3.72 | $0.85 | 150,000 | $0.85 | |
$1.00 | 200,000 | 4.97 | $1.00 | 200,000 | $1.00 | |
$1.10 | 50,000 | 3.48 | $1.10 | 50,000 | $1.10 | |
$0.17 - $1.10 | 20,039,999 | 4.32 | $0.49 | 16,589,998 | $0.47 | |
Stock based compensation expense is charged to operations over the vesting period of the options using the straight-line amortization method.
The aggregate intrinsic value of the Company's stock options is calculated as the difference between the exercise price of the options and the quoted price of the common shares that were in-the-money. The aggregate intrinsic value of the Company's stock options exercised under the Plan was $249,600 and $193,400 for 2007 and 2006, respectively, determined at each of respective year end.
As at May 31, 2007, there was approximately $927,263 of total unrecognized compensation cost related to non-vested share-based compensation arrangements granted under the Plan. This cost is expected to be recognized over a period of 24 months. The estimated fair value of stock options vested during 2007 and 2006 was $2,437,433 and $2,999,296, respectively.
F-66
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
9. Proposed Acquisition of Vascore Medical (Suzhou) Co., Ltd.
On September 5, 2006, as amended on March 1, 2007, the Company entered into an Equity Transfer Agreement (the "Agreement") with each of Chimex Hong Kong Incorporated Limited and Vascore Scientific Co., Ltd. (collectively the "Vendors") and Vascore Medical (Suzhou) Co., Ltd. ("Vascore Medical"), pursuant to which, and subject to the satisfaction of certain conditions precedent, the Company acquired the right to purchase 100% of the outstanding equity and shareholders' loans of Vascore Medical from the Vendors. Vascore Medical is a China-based manufacturer of advanced cardiovascular stents and other medical devices.
The proposed closing of the terms and conditions of the Agreement remains subject to the prior satisfaction of certain conditions precedent which, from the Company's perspective, have not yet been satisfied. In addition, the terms of the Agreement require the same to close on or before August 31, 2007; failing which the Agreement automatically terminates without the prior written consent of each of parties thereto which has not been secured by the Company. Correspondingly, the Company does not expect the Agreement to close in accordance with its present terms and conditions and within the time period presently required thereunder. The Company is in the midst of discussions with each of the Vendors and Vascore Medical in order to determine if either an Agreement extension or new terms and conditions to the proposed acquisition of Vascore Medical are achievable.
As of May 31, 2007, total costs of $169,800 which represents legal and consulting fees incurred as a result of the proposed acquisition has been charged to operations.
10. Related Party Transactions
The related party transactions not disclosed elsewhere in these financial statements are disclosed as follows. These transactions, recorded at exchange amounts agreed to by all parties.
During the year ended May 31, 2007, the Company paid or accrued $1,002,702 (2006 - $757,859) of management and consulting fees to 5 directors (2006 - 4 directors) and 2 officers of the Company. Of this amount, $234,768 (2006 - $201,987) was charged to research and development. Included in accounts payable is $63,310 (2006 - $9,106).
The details of the contracts with directors and officers are as follows:
The Company entered into a Development Services Agreement with Alan Lindsay and Associates Ltd. (the "Consultant") dated March 1, 2005. Pursuant to the Agreement the Company agrees to retain the Consultant, and through the Consultant Mr. Lindsay, to provide development and financing services as may be necessary and determined by the Company to both develop and finance the Company's technology and business. The term of the agreement is five years commencing March 1, 2005 and expiring on March 1, 2010. Under the terms of the Development Services Agreement, Mr. Lindsay shall be paid $17,250 per month, subject to a 10% increase on an annual basis and receive 1,200,000 options to purchase shares of common stock of the Company at $0.17 - $0.30 per share. In the event of a change in control, all of Mr. Lindsay's outstanding options shall immediately vest. Mr. Lindsay's agreement may be terminated by the Company without cause upon 360 calenda r days notice.
F-67
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
10. Related Party Transactions (continued)
The Company entered into a Management Services Agreement with Simba Biomed Venture Partners LLC dated March 31, 2006. Pursuant to the agreement we have agreed to retain such company, and, through such company, Dr. Landy, to provide management and consulting services as may be necessary and determined by the Company to both develop and commercialize the Company's technology and business. The term of the agreement is four years commencing March 31, 2006 and expiring on March 31, 2010. Under the terms of the Management Services Agreement, Dr. Landy shall be paid a monthly fee of $19,000 per month and receive options to purchase 5,000,000 shares of common stock of the Company at $0.60 per share. Dr. Landy's agreement was subsequently amended increasing his monthly fee to $25,000 since April 2007.
The Company entered into an Executive Employment Agreement with Mr. Patrick McGowan dated January 1, 2005. Pursuant to the Agreement, the Company will employ Mr. McGowan in an executive capacity, commenced on January 1, 2005 and will continue until May 1, 2007. Mr. McGowan's Executive Employment Agreement was amended for an additional two years until May 2009. Under the terms of the amendment, Mr. McGowan shall be paid a total annual salary of CDN$169,400 up to April 30, 2008. Thereafter, the Company shall increase Mr. McGowan's salary by 10%. In addition, Mr. McGowan will receive options to purchase 10% common shares held by Mr. McGowan on the first business day of each calendar year 2008 and 2009. The exercise price of the options shall be based on the closing share price as of such dates.
The Company entered into a 36 month Consulting Services Agreement with Dr. Dov Shimon dated May 1, 2005 and expiring on May 1, 2008. Pursuant to the Agreement, Dr. Shimon will work as Chief Medical Officer to the Company. Dr. Shimon is also President of Sagax, a subsidiary of the Company. Dr. Shimon's initial salary shall be at the rate of $11,000 per month, with an annual increase of 10%. In addition, the Company has issued 200,000 Directors Options with an exercise price of $0.30 and an expiry date of July 31, 2009, and 300,000 Officers Options with an exercise price of $0.30 and an expiry date of March 1, 2010 to Dr. Shimon. Dr. Shimon ceased to be Chief Medical Officer of the Company on August 7, 2006.
At May 31, 2007, amounts due from the employees of a subsidiary of the Company totaled $34,775 (2006 - $nil). These amount are unsecured, non-interest bearing and will be repaid by periodic deduction of future wages.
At May 31, 2007, an amount of $100,000 was due to the Chief Executive Officer of the Company. This amount was unsecured, non-interest bearing and due on demand. The full amount has been paid subsequent to year-end.
F-68
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
11. Income Taxes
The parent Company is subject to income taxes in the United States while its subsidiaries are subject to income taxes in Canada, India and Israel. U.S. federal net operating loss carryforwards of approximately $25,300,000, if not utilized to offset taxable income in future periods, expire between 2021 and 2027. Canadian net operating loss carryforwards of approximately $6,200,000, if not utilized to offset taxable income in future periods, expire between the years 2008 and 2027, Indian net operating loss carryforwards of approximately $160,000, if not utilized to offset taxable income in future periods, expire in 2015 and Israeli net operating losses of approximately $1,692,000 can be carried forward indefinitely to offset future taxable income. Canadian undeducted scientific research and experimental development ("SRED") expenditures of approximately $5,286,000 can be carried forward indefinitely to offset future taxable income. In addition, Canadian non-refundable SRED investment ta x credits of approximately $1,331,000, if not utilized to reduce Canadian taxes payable in future periods, expire between the years 2008 and 2027.
The following is a reconciliation between the expected and actual income tax benefits using the applicable average statutory income tax rate of 34%, 34% and 35% for the years ended May 31, 2007, 2006 and 2005, respectively:
2007 | 2006 | 2005 | ||||
Income tax benefit at statutory rate | $ | (3,561,000) | $ | (3,081,000) | $ | (2,313,000) |
Foreign rate differential | 46,000 | 3,000 | (12,000) | |||
Temporary and permanent differences, net | 821,000 | 64,000 | 140,000 | |||
Acquisition intangibles | - | - | 596,000 | |||
Research and development | - | - | 279,000 | |||
Change in valuation allowance |
| 2,667,000 |
| 3,014,000 |
| 1,310,000 |
$ | (27,000) | $ | - | $ | - |
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company's net deferred tax assets are as follows at May 31, 2007 and 2006:
Deferred income tax assets:
2007 | 2006 | |||
Tax benefit relating to net operating loss carryforwards undeducted SRED expenditures and SRED investment tax credit carryforwards |
| 14,100,000 |
| 9,961,000 |
Plant and equipment | 80,000 | 91,000 | ||
Valuation allowance | (14,180,000) | (10,052,000) | ||
$ | - | $ | - | |
F-69
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
11. Income Taxes (continued)
Deferred income tax liability:
2007 | 2006 | |||
CE Mark license | $ | (297,000) | $ | - |
Deferred income tax liability | $ | (297,000) | $ | - |
Net deferred income tax liability | $ | (297,000) | $ | - |
Future utilization of the loss carryforward in the U.S. is subject to certain limitations under the provisions of the Internal Revenue Code, including limitations subject to Section 382. It is likely that a prior ownership change has occurred and the losses will be limited in their ability to offset future.
12. Commitments and Contingencies
(a) The Company has obligations under a long-term premises lease that expire in December 2010. The aggregate minimum rent payments for the next four annual periods ending May 31 are as follows:
2008 | $105,511 |
2009 | 105,511 |
2010 | 105,511 |
2011 | 61,548 |
Total | $378,081 |
The Company received free rent, including property maintenance and taxes, for the months of November to December 2005 and free basic rent for the months of January to February 2006 for total free rent of $40,404. This amount was recorded under deferred lease inducement with a current portion of $8,081 and long-term portion of $19,529 and is being amortized over the term of the lease. During the year ended May 31, 2007, amortization of $8,081 (2006 - $4,714; 2005 - $nil) was recorded as a reduction of rent expense in the statement of operations. Rent expense for the year ended May 31, 2007 was $211,932 (2006 - $172,024; 2005 - $137,797).
(b) On March 14, 2005, the Company acquired 100% of SagaX, Inc. ("SagaX") a Delaware corporation with operations in Israel. The Company agreed to issue 4,200,000 common shares in exchange for all of the issued and outstanding shares of SagaX. The 4,200,000 shares will be issued in three intervals: 2,000,000 of the shares within 30 days of the effective date of this Agreement (issued), 1,100,000 shares upon successful completion of large animal trials and the final 1,100,000 shares upon CE Mark approval relating to SagaX's products. The Company has also agreed to pay $145,000 (paid) of SagaX's vendor debt owed to its parent company.
As of May 31, 2007, the two remaining issuances of 1,100,000 shares each have not been accrued as the underlying conditions have not been satisfied.
F-70
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
12. Commitments and Contingencies (continued)
(c) On November 18, 2002, a lawsuit against the Company was filed in the Supreme Court of British Columbia.
The statement of claim, arising from a settlement agreement dated September 14, 2001, seeks the exchange of 3,192,399 common shares of the Company for 3,192,399 shares in the capital of one of the Company's subsidiaries or, alternatively, damages and costs.
The Company and M-I attended a court hearing in chambers during April 2003 on a summary trial application by the plaintiff for an order for a declaration of specific performance that the plaintiff is entitled to an exchange of 3,192,399 common shares of M-I for 3,192,399 common shares of the Company pursuant to the settlement agreement entered into on September 14, 2001. The plaintiff was granted the relief sought at the summary trial and the Company was ordered to perform the share exchange.
On May 16, 2003, the Company delivered a Take-Over Bid Circular (the "Circular") to the plaintiff, offering to exchange its common shares of M-I for shares in the Company pursuant to British Columbia securities laws and regulations. In late May 2003, after the judgment was received, the Company asked the plaintiff to submit its M-I share certificates and fill in the required forms pursuant to the Circular, so that the Company could comply with the judgment and exchange its shares in accordance with British Columbia securities laws and regulations.
On December 29, 2004, the Company issued 3,192,399 common shares to exchange for 3,192,399 common shares of M-I on a one-for-one basis. These shares were issued to comply with an order of the Supreme Court of British Columbia dated May 20, 2003. On May 26, 2005, the Company issued 17,000 common shares to exchange for 17,000 common shares of M-I on a one-for-one basis.
In a counterclaim filed in the Supreme Court of British Columbia, the Company continues to dispute the plaintiff's entitlement to the 3,192,399 M-I shares and any Company shares that he may received pursuant to court order.
No gain or loss provisions have been provided as of May 31, 2007 as the outcome of this legal proceeding is uncertain at this time.
F-71
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
13. General and Administrative Expenses
General and administrative expenses consisted of the following for the years ended May 31, 2007, 2006 and 2005:
2007 | 2006 | 2005 | ||||
Legal | $ | 517,063 | $ | 426,776 | $ | 195,379 |
Public relations, financing and corporate development | 1,235,456 | 2,657,383 | 935,337 | |||
Management fees | 2,533,989 | 1,453,613 | 261,883 | |||
Consulting | 655,319 | 503,602 | 793,426 | |||
Audit | 158,155 | 281,620 | 51,110 | |||
Operating expenses |
| 1,495,489 |
| 815,918 |
| 483,125 |
$ | 6,595,471 | $ | 6,138,912 | $ | 2,720,260 |
General and administrative expenses include $129,070 (2006 - $1,517,090; 2005 - $322,202) and $132,045 (2006 - $218,503; 2005 - $390,429) of amortized deferred compensation in public relations and consulting, respectively.
14. Supplemental Cash Flow Information
Period from inception (January 20, 1999) to May 31, 2007 | Years ended May 31, | |||||||
2007 | 2006 | 2005 | ||||||
Supplemental cash flow information: | ||||||||
Interest paid in cash | $ | 52,339 | $ | 18,458 | $ | 4,198 | - | |
Supplemental non-cash transactions: | ||||||||
Debt settlement with shares | $ | 621,375 | $ | - | $ | - | - | |
Gain on extinguishment of debt | 462,249 | - | - | - | ||||
Conversion of convertible debentures | 740,810 | - | 740,810 | - | ||||
Shares issued for services | 4,134,269 | 776,372 | 671,582 | 545,028 | ||||
Warrants issued for services | 3,747,600 | 57,109 | 1,298,856 | 917,164 | ||||
F-72
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
15. Loan Payable
As at May 31, 2007, the Company has loan payable totaling $525,000 with interest rate of 12.5% per annum. Of this amount, $400,000 is due on June 15, 2007 and $125,000 is due on July 13, 2007.
In connection with the $400,000 loan, the Company issued the lender 150,000 non-transferable share purchase warrants to acquire one common share at an exercise price of $0.60 per share for a period of three years. The warrants had an estimated fair value of $29,049 using the Black Scholes option pricing model. The assumptions used in the Black-Scholes model were: volatility: 57.35%, discount rate: 4.61%, dividend: nil and expected life of one year.
In connection with the $125,000 loan, the Company issued the lender 50,000 non-transferable share purchase warrants to acquire one common share at an exercise price of $0.60 per share for a period of three years. The warrants had an estimated fair value of $7,675 using the Black Scholes option pricing model. The assumptions used in the Black-Scholes model were: volatility: 57.47%, discount rate: 4.85%, dividend: nil and expected life of one year.
On June 27, 2007, the due date of the $400,000 was extended to July 31, 2007. An additional 150,000 non-transferable share purchase warrants to acquire one common share at an exercise price of $0.60 per share for a period of three years was issued to the lender.
As at May 31, 2007, the Company accrued interest of $6,302 and $1,927 for the $400,000 and $125,000 loan, respectively.
Both the $400,000 and the $125,000 loan and the corresponding interest were repaid subsequent to year-end.
16. Subsequent Events
(a) On July 9, 2007, the Company completed a brokered private placement of an aggregate of 25,100,000 units at the price of $0.50 per unit for total proceeds of $12,550,000. Each unit is comprised of one common share (25,100,000 common shares) together with one-half of one share purchase warrant (12,550,000 share purchase warrants). Each warrant entitles the holder to purchase one common share at a price of $0.55 per share for a period of five years.
In connection with the above, the Company issued 251,000 common shares and 753,000 share purchase warrants as finder's fee. Each warrant entitles the holder to purchase one common share at a price of $0.55 per share for a period of five years.
The Company agreed to file a registration statement with the United States Securities and Exchange Commission to register for resale the shares and shares underlying the warrants issued under the private placement, and to have the registration statement declared effective within four months from the date of the issuance of the units. In the event that (i) the registration statement is not declared effective by that date, (ii) if the Company fails to keep its shares of common stock continuously listed for trading on the OTC Bulletin Board (or such other market on which the shares are listed or quoted for trading) or (iii) after its effective date, the registration statement ceases for any reason to remain continuously effective, the holders are not permitted to utilize the prospectus contained in the registration statement to resell the securities registered for an aggregate
F-73
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
16. Subsequent Events (continued)
(a) (continued)
of more than 10 consecutive trading days or for more than an aggregate of 20 trading days in any 12 month period (which need not be consecutive), the Company agreed to pay to each holder of the units an amount in cash, as liquidated damages, equal to 1% of the aggregate purchase price paid by such holder for the units, and on each monthly anniversary thereof until the event is cured, to pay each holder an amount in cash, as partial liquidated damages, equal to 1% of the aggregate purchase price paid by such holder for the units. In addition, the Company agreed that, if it fails to pay any partial liquidated damages in full within seven days after the date payable, to pay interest thereon at a rate of 1.5% per month to the holder, accruing daily from the date such partial liquidated damages are due until such amounts (including all interest) are paid in full.
(b) Subsequent to the year ended May 31, 2007, the Company and certain senior officers and directors was served with a Summons and Complaint for an action in New York state court alleging breach of contract, fraud, fraudulent concealment, negligent misrepresentation, unjust enrichment and conspiracy claims in connection with the termination by the Company of the proposed acquisition of Vascore Medical (see Note 9). The Company believes that this action was brought improperly and intends to seek a stay of this action pending arbitration in the appropriate forum, where the Company intends to vigorously defend itself.
(c) On November 13, 2007, the Company entered into a definitive share purchase agreement to sell all of its issued and outstanding shares of SagaX Inc., its wholly-owned subsidiary, to Shimoco, LLC ("Purchaser"). Dr. Dov Shimon is a director of each of the companies listed above, founded and has been serving as the chief executive officer of SagaX since inception and is the owner and manager of the Purchaser.
In exchange for the consideration described below, the Company has agreed to pay the Purchaser an additional $130,000 for working capital purposes, having recently advanced $80,000 for the same, in order to meet certain of SagaX's previously disclosed and bona fide current liabilities; with any said working capital advances to simply form part of the overall SagaX's Indebtedness to the Company. Total consideration due the Company is as follows:
(i) the repayment by the Purchaser and SagaX an aggregate of $4 million in prior loans and associated indebtedness which have been advanced and undertaken by the Company in and to SagaX ("SagaX's Indebtedness") in accordance with any future private or public equity financing of SagaX subsequent to the closing of this agreement (the "Closing"). In this regard, and until payment in full of SagaX's Indebtedness, SagaX's Indebtedness will be secured, contemporaneously with the closing, by way of a senior, subordinated (subordinated only to SagaX's existing banking indebtedness), fixed and floating charge registered over all of the assets of SagaX; and
F-74
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
16. Subsequent Events (continued)
(b) (continued)
(ii) the payment by the Purchaser and SagaX to the Company of a royalty fee equating to 8% of net sales, other than from sub-licenses, in respect of gross sales from any product associated or related to SagaX's present intellectual property under any existing patent or patent-pending applications, and of any other benefit, directly or indirectly collected or received, whether for cash or credit or by way of any benefit, advantage, equity, or concession from the manufacturing, distribution, marketing, contracting, joint venturing, leasing, equity participation or any other activity in relation to the said products.
In addition to the consideration above, both prior to, in conjunction with and subsequent to the Closing of the within purchase and sale the Purchaser and SagaX will also be responsible for paying the Company a bonus (the "Bonus") equal to 10% of any consideration in any form which is received by the Purchaser and/or SagaX any source and from any transaction, or a series of related transactions, at anytime and which is in anyway associated with a change in control of SagaX at anytime while the royalty hereinabove remains due and payable by the Purchaser and SagaX to the Company.
Effective on the execution date of this agreement, Dr. Shimon is now deemed, without any further act required on his behalf, to have immediately resigned as a director of the Company and to immediately have terminated his existing consulting agreement and arrangement with the Company and, consequent upon such resignation and termination, to have no further claim against the Company as a previous director of or consultant to the Company.
The completion of the transactions comprising the Company's proposed purchase and sale under this agreement is subject to a number of conditions including, but not limited to: (i) if required, the specific ratification of the terms and conditions of this agreement by each of the Company's Board of Directors and of the General Shareholder's Assembly of the Purchaser (collectively, the "Ratification"); (ii) the completion by each of the Company and the Purchaser of an initial due diligence and operations review of the other party's respective businesses and operations within five business days of the prior satisfaction of the Ratification; (iii) if required under applicable corporate and securities laws, the receipt of all necessary approvals from any regulatory authority having jurisdiction over the transactions contemplated by this agreement on or before November 30, 2007; and (iv) the Closing of the proposed sale prior to December 31, 2007.
F-75
MIV THERAPEUTICS INC.
(A development stage company)
Notes to Consolidated Financial Statements
Years ended May 31, 2007 and 2006
(Expressed in US dollars)
17. Segmented Information
The Company operates in one segment which comprises the research, manufacture and development of bio-compatible stent coatings for implantable medical devices and drug-delivery technologies.
The following is a summary of the Company's geographical information for the years ended May 31, 2007, 2006 and 2005 and as of May 31, 2007 and 2006.
Canada | India | Israel | Total | |||||
2007 | ||||||||
Net revenue | $ | - | $ | 191,490 | $ | - | $ | 191,490 |
Gross profit | - | 44,350 | - | 44,350 | ||||
Depreciation and amortization | 103,827 | 73,416 | 12,389 | 189,632 | ||||
Net loss | 9,279,254 | 190,634 | 1,029,583 | 10,499,471 | ||||
As at May 31, 2007 | ||||||||
Total assets | 2,082,113 | 1,712,686 | 452,166 | 4,246,965 | ||||
Additions to property and equipment | 197,998 | 211,893 | 32,117 | 442,008 | ||||
License | - | 1,389,279 | - | 1,389,279 | ||||
2006 | ||||||||
Net revenue | $ | - | $ | - | $ | - | $ | - |
Gross profit | - | - | - | - | ||||
Depreciation and amortization | 140,183 | - | 3,571 | 143,754 | ||||
Net loss | 8,577,213 | - | 517,622 | 9,094,835 | ||||
As at May 31, 2006 | ||||||||
Total assets | 1,771,664 | - | 282,211 | 2,053,875 | ||||
Additions to property and equipment | 221,140 | - | 38,666 | 259,806 | ||||
License | - | - | - | - | ||||
2005 | ||||||||
Net revenue | $ | - | $ | - | $ | - | $ | - |
Gross profit | - | - | - | - | ||||
Depreciation and amortization | 176,407 | - | 46 | 176,453 | ||||
Net loss | 6,464,464 | - | 144,418 | 6,608,882 |
During the year ended May 31, 2007, there was one (2006 and 2005 - nil) customer who individually and collectively accounted for 92% (2006 and 2005 - nil) of total revenue of the Company.
F-76
BIOSYNC SCIENTIFIC |
(Converted into an Indian Private Limited Company, namely Biosync Scientific Private Limited with effect from August 1, 2006) |
FINANCIAL STATEMENTS |
AND AUDITOR'S REPORTS |
AS PER US GAAP |
FOR THE YEAR ENDED MARCH 31, 2006 |
F-77
 Chartered Accountants | 1116, Raheja Chambers, Nariman Point, Mumbai 400 021 Tel.: +91 22 22833776/9 56377771/2, Fax: 22838389 email: mail@mzs.co.in |
AUDITOR'S REPORT TO THE ERSTWHILE PARTNERS OF |
Biosync Scientific |
(converted into an Indian Private Limited Company, namely Biosync Scientific Private Limited with effect from August 1, 2006) |
We have audited the attached balance sheet of Biosync Scientific (converted into an Indian Private Limited Company, namely Biosync Scientific India Private Limited, with effect from August 1, 2006) as at March 31, 2006 and the related statements of income, retained earnings and cash flows for the year end have been prepared on the basis of accounting principles generally accepted in the United States of America. These financial statements are the responsibility of the erstwhile Firm's management. Our responsibility is to express an opinion on these financial statements based on our audit. |
We conducted our audit in accordance with the auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether financial statements are free of material misstatements. An audit includes examining, on a test check basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by the management, as well as evaluating the overall financial statement presentation. We believe that our audit provides reasonable basis for our opinion. |
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Biosync Scientific (converted into a Private Limited Company, namely Biosync Scientific Private Limited with effect from August 1, 2006) as at March 31, 2006 and the results of its operations and its cash flows for the year then ended in conformity with accounting principles generally accepted in the United States of America. |
The accompanying financial statements have been prepared assuming that the erstwhile Firm after conversion into a Private Limited Company and subsequently becoming the wholly owned subsidiary of MIV Therapeutics Inc. will continue as a going concern. The Parent Company is incurring losses from operations since inception and has a working capital deficiency that arise substantial doubt about its ability to continue as a going concern and correspondingly its subsidiary. The financial statements do not include any adjustments that might result from the outcome of this uncertainty. |
for MZS & Associates |
Chartered Accountants |
/s/Zulfiqar Shivji |
Zulfiqar Shivji |
Partner |
Mumbai: October 11, 2007 |
F-78
 Chartered Accountants | 1116, Raheja Chambers, Nariman Point, Mumbai 400 021 Tel.: +91 22 22833776/9 56377771/2, Fax: 22838389 email: mail@mzs.co.in |
Biosync Scientific |
(converted into an Indian Private Limited Company, namely Biosync Scientific Private Limited with effect from August 1, 2006) |
. |
We have audited the attached balance sheet of Biosync Scientific (converted into an Indian Private Limited Company, namely Biosync Scientific India Private Limited, with effect from August 1, 2006) as at March 31, 2005 and the related statements of income, retained earnings and cash flows for the year end have been prepared on the basis of accounting principles generally accepted in the United States of America. These financial statements are the responsibility of the erstwhile Firm's management. Our responsibility is to express an opinion on these financial statements based on our audit. |
. |
We conducted our audit in accordance with the auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether financial statements are free of material misstatements. An audit includes examining, on a test check basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by the management, as well as evaluating the overall financial statement presentation. We believe that our audit provides reasonable basis for our opinion. |
. |
In our opinion the financial statements referred to above present fairly, in all material respects, the financial position of Biosync Scientific (converted into a Private Limited Company, namely Biosync Scientific Private Limited with effect from August 1, 2006) as at March 31, 2005 and the results of its operations and its cash flows for the year then ended in conformity with accounting principles generally accepted in the United States of America. |
. |
The accompanying financial statements have been prepared assuming that the erstwhile Firm after conversion into a Private Limited Company and subsequently becoming the wholly owned subsidiary of MIV Therapeutics Inc. will continue as a going concern. The Parent Company is incurring losses from operations since inception and has a working capital deficiency that arise substantial doubt about its ability to continue as a going concern and correspondingly its subsidiary. The financial statements do not include any adjustments that might result from the outcome of this uncertainty. |
for MZS & Associates |
Chartered Accountants |
/s/Zulfiqar Shivji |
Zulfiqar Shivji |
Partner |
Mumbai: October 11, 2007 |
F-79
(converted into a Private Limited Company, namely Biosync Scientific Private Limited with effect from August 1, 2006) | ||
Balance Sheet as of March 31, 2006 | ||
Particulars | As of | As of |
31-Mar-06 | 31-Mar-05 | |
| USD | USD |
Assets | ||
| ||
Current Assets | ||
Cash and Cash Equivalents | 3,991 | 680 |
Trade receivables, net of allowances | 6,051 | 1,085 |
Inventories | 119,873 | 4,453 |
Other Current Assets | 8,370 | -- |
Total Current Assets | 138,285 | 6,218 |
| ||
Property, Plant & Equipment | 141,921 | 162,868 |
Other Assets | 12,433 | 571 |
Total Assets | 292,639 | 169,657 |
| ||
Liabilities and Partners' Funds | ||
| ||
Current Liabilities | ||
Bank Overdraft | 3,789 | 18,176 |
Interest Bearing Liabilities | 100,294 | 21,151 |
Accounts Payables | 16,585 | 2,987 |
Accrued Expenses | 616 | 114 |
Other Liabilities | 301,237 | 14,250 |
Total Current Liabilities | 422,521 | 56,678 |
Long Term Interest Bearing Liabilities | -- | 102,190 |
Total Liabilities | 422,521 | 158,868 |
| ||
Partners' Funds | ||
Partners' Capital | (129,882) | 10,789 |
Total Partners' Funds | (129,882) | 10,789 |
Total Liabilities and Partners' Funds | 292,639 | 169,657 |
|
|
|
Accompanying Notes form an integral part of these Financial Statements. | ||
The Audit Report is set forth on Page 1. | ||
Approved by the Directors of Biosync Scientific Private Limited on behalf of erstwhile partners of Biosync Scientific since the partnership firm has been converted into a private limited company with effect from August 1, 2006. | ||
ForBiosync Scientific Private Limited | ||
Director | ||
Mumbai: October 11, 2007 | ||
F-80
BIOSYNC SCIENTIFIC | ||||
(converted into a Private Limited Company, namely Biosync Scientific Private Limited with effect from August 1, 2006) | ||||
Income Statement for the year ended Mar 31, 2006 | ||||
Particulars | For the Y.E. | For the Y.E. | ||
31-Mar-06 | 31-Mar-05 | |||
| USD | USD | ||
Revenue | 174,185 | 6,855 | ||
Cost of Sales | (73,771) | (8,600) | ||
Gross Profit | 100,414 | (1,745) | ||
| ||||
Expenses | ||||
Selling, Administrative and Other Expenses | (7,773) | (13,288) | ||
Depreciation | (34,689) | (26,124) | ||
Finance Charges | (17,912) | (16,333) | ||
Interest/ Remuneration to Partners | (8,888) | (6,602) | ||
Operating Income/ (Loss) | 31,152 | (64,092) | ||
| ||||
Other Income | 909 | 70 | ||
Income / (Loss) before Income Tax | 32,061 | (64,022) | ||
| ||||
Income Taxes (Refer Note 4) | - | - | ||
Net Income / (loss) | 32,061 | (64,022) | ||
| ||||
Accompanying Notes form an integral part of these Financial Statements. | ||||
The Audit Report is set forth on Page 1. | ||||
Approved by the Directors of Biosync Scientific Private Limited on behalf of partners of Biosync Scientific since the partnership firm has been converted into a private limited company with effect from August 1, 2006. | ||||
ForBiosync Scientific Private Limited | ||||
Director | ||||
Mumbai: October 11, 2007 | ||||
F-81
(converted into a Private Limited Company, namely Biosync Scientific Private Limited with effect from August 1, 2006) | ||||
Statement of Partners' Capital and Comprehensive Income | ||||
Particulars | Partners' | Retained | Other | Total |
| Capital | Earnings | Comprehensive | Partners' |
|
|
| Accumulated | Funds |
|
|
| Income/ (Loss) |
|
| USD | USD | USD | USD |
Balance As of April 01 , 2004 | 51,203 | -- | -- | 51,203 |
| ||||
Capital Introduced | 28,721 | -- | -- | 28,721 |
| ||||
Net Income / (Loss) for the year | -- | (64,022) | -- | (64,022) |
| ||||
Loss on Foreign Currency Translation | -- | -- | (5,113) | (5,113) |
| ||||
Loss for the period transferred to Partners | (69,135) | 64,022 | 5,113 | -- |
|
|
|
|
|
Balance As of March 31, 2005 | 10,789 | -- | -- | 10,789 |
| ||||
Capital Introduced / (withdrawn) | (175,162) | -- | -- | (175,162) |
| ||||
Net Income for the year | 32,061 | -- | 32,061 | |
| ||||
Loss on Foreign Currency Translation | -- | -- | 2,430 | 2,430 |
| ||||
Profit for the period transferred to Partners | 34,491 | (32,061) | (2,430) | -- |
|
|
|
|
|
Balance As of March 31, 2006 | (129,882) | -- | -- | (129,882) |
| ||||
|
|
|
|
|
The Audit Report is set forth on Page 1. | ||||
F-82
BIOSYNC SCIENTIFIC | ||||
(converted into a Private Limited Company, namely Biosync Scientific Private Limited with effect from August 1, 2006) | ||||
Cash Flow Statement for the year ended March 31, 2006 | ||||
Particulars | For the Y.E. | For the Y.E. | ||
31-Mar-06 | 31-Mar-05 | |||
|
|
| USD | USD |
|
|
|
| |
Cash Flow From Operating Activities |
|
| ||
Net Income / (Loss) |
| 32,061 | (64,022) | |
Adjustments to reconcile net income to net cash from operating activities: |
|
| ||
| Depreciation |
| 34,689 | 26,124 |
Changes in assets and liabilities: |
|
| ||
| Trade Receivables |
| (4,966) | (1,085) |
| Inventories |
| (115,420) | (4,453) |
| Accounts Payable |
| 13,598 | 2,987 |
| Other Current Assets |
| (8,370) | 16,643 |
| Other Assets |
| (11,862) | (4) |
| Accrued Expenses |
| 502 | 114 |
Net Cash from/ (Used for) Operating Activities | (59,768) | (23,696) | ||
|
|
|
| |
Cash Flow From Investment Activities |
|
| ||
| Purchase of Property, Plant & Equipment |
| (13,742) | (164,576) |
Net Cash from/ (Used For) Investment Activities | (13,742) | (164,576) | ||
|
|
|
| |
Cash Flows From Financing Activities |
|
| ||
| Additions (Withdrawal) to/ from Capital |
| (175,162) | 28,721 |
| (Repayment) of/ Proceeds from Bank Overdraft |
| (14,387) | 18,176 |
| (Repayment) of/ Proceeds from Other Liabilities |
| 286,987 | 14,250 |
| Proceeds from Interest Bearing Liabilities (net) |
| (23,047) | 123,341 |
Net Cash From / (Used for) Financing Activities | 74,391 | 184,488 | ||
|
|
|
| |
Effect of exchange rate changes | 2,430 | (5,113) | ||
Net Change in Cash and Cash Equivalents | 3,311 | (8,897) | ||
|
|
|
| |
Cash and cash equivalents at the beginning of the year |
| 680 | 9,577 | |
Cash and cash equivalents at the end of the year | 3,991 | 680 | ||
|
|
|
| |
The Audit Report is set forth on Page 1. | ||||
F-83
(converted into a Private Limited Company, namely Biosync Scientific Private Limited with effect from August 1, 2006) | |||||||||||
Notes to the Financial Statements for the year ended March 31, 2006 | |||||||||||
1 | Background | ||||||||||
Biosync Scientific ("Biosync" or "the firm") was a Partnership Firm and was set up on July 21, 2003. The partners of the firm with their profit sharing ratios (PSR) is given here under: | |||||||||||
Partners |
|
| PSR on set up | PSR as amended on 10.09.2003 | PSR as amended on 11.11.2003 | PSR as amended on 01.07.2005 | PSR as amended on 01.04.2006 | ||||
Mr. Pratap Ram Surve |
| 33% | 20% | 20% | 10% | 10% | |||||
Mr. Viral Laljibhai Vaishnav | 33% | 20% | 20% | 10% | 4% | ||||||
Mrs. Vinita Rajesh Vaishnav | 33% | 50% | 40% | 65% | 25% | ||||||
Mr. Jayantilal Limbabhai Naria | - | 10% | 20% | 15% | 4% | ||||||
Mr. Rajesh Laljibhai Vaishnav | - | - | - | - | 50% | ||||||
Mrs. Sangeeta Viral Vaishnav | - | - | - | - | 4% | ||||||
Mrs. Alka Jayanti Nariya |
| - | - | - | - | 3% | |||||
Total |
|
| 100% | 100% | 100% | 100% | 100% | ||||
Effective August 1, 2006, the firm was converted into a Private Limited Company as per the provisions of the Indian Companies Act, 1956 and the partners of the firm as at the date of conversion became the members of the Private Limited Company with equity shares issued to the partners in the ratio of their capital outstanding as at the date of conversion. | |||||||||||
Effective February 16, 2007, MIV Therapeutics Inc. acquired 100% of the equity shares of the said converted Company from the erstwhile partners and accordingly, the Company with effect from February 16, 2007 become a wholly owned subsidiary of MIV Therapeutics Inc. | |||||||||||
The firm was situated and operated from 136-B, Surat Special Economic Zone, G.I.D.C., Sachin, Surat - 394230, Gujarat, India which became the registered office of the Company on conversion. | |||||||||||
2 | Summary of Significant Accounting Policies | ||||||||||
a) | Basis of Presentation | ||||||||||
i) | The Financial Statements of Biosync are prepared in accordance with generally accepted accounting principles applicable in the United States ("U.S. GAAP") as at the date of preparation. | ||||||||||
ii) | Biosync is set up to deal in designing, manufacturing and marketing coated and non-coated vascular stents and related accessories. However, during the year, the firm was still under development stage and did not have significant operations. Presently, the Holding Company is also under development and its ability to generate revenue is primarily dependent on its success in completing development and obtaining regulatory approvals for the commercialization of its stent technology. The Holding Company's ability to obtain sufficient financing to continue the development of, and if successful, to commence the manufacture and sale of its products under development and if and when approved by the applicable regulatory agencies is uncertain. In view of these conditions, the ability of the holding company and subsequently the subsidiary to continue as a going concern is in substantial doubt and dependent upon achieving a profitable level of operations and on the ability of the Holding Company to obtain necessary financing to fund ongoing operations. Management believes that its current and future plans enable the holding company and correspondingly its subsidiary to continue as a going concern. | ||||||||||
F-84
BIOSYNC SCIENTIFIC | |||||||||||
(converted into a Private Limited Company, namely Biosync Scientific Private Limited with effect from August 1, 2006) | |||||||||||
Notes to the Financial Statements for the year ended March 31, 2006 | |||||||||||
b) | Use of Estimates | ||||||||||
The preparation of the Financial Statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent liabilities as of the date of the Financial Statements and the reported amount of revenues and expenses during the reported period. Examples of such estimates include: allowance for doubtful debts, and for future obligations under employee benefit plans, valuation allowances for deferred taxes, and useful lives of property, plant and equipment, etc. Actual results could differ materially from these estimates. | |||||||||||
c) | Foreign Currency Translation | ||||||||||
The accompanying Financial Statements are reported in U.S. dollars ("USD"). The Indian Rupee ("INR") is the functional currency for Biosync. The translation of the functional currencies into USD wherever applicable is performed for assets and liabilities using the exchange rates prevailing at the Balance Sheet date and for revenues, costs and expenses using average exchange rates prevailing during the reporting period. Adjustments resulting from the translation of functional currency financial statements to reporting currency are accumulated and reported as net income / (loss) in the Other Comprehensive Accumulated Income or Loss. | |||||||||||
Transactions in foreign currency for reporting functional currency are recorded at the exchange rate prevailing on the date of transaction. Monetary assets and liabilities denominated in foreign currencies are expressed in the functional currency at the exchange rates prevailing at the Balance Sheet date. Revenues, costs and expenses are recorded using exchange rates prevailing on the date of transaction. Gains or losses arising out of fluctuations in the exchange rates are recognized in the profit and loss in the period in which they arise. | |||||||||||
d) | Revenue Recognition | ||||||||||
The firm recognizes revenues, net of returns, rebates and returns, if any from the sale of the products, at the time when the product is delivered to the customer and/ or dealer. Revenues are recognized only when the firm has transferred to the customer and/ or dealer the significant risks and rewards of ownership of the goods, title to the products transfers, the amount if fixed and determinable, evidence of an agreement exists, there is reasonable assurance of collection of the sale proceeds, the Company has no future obligations and the customer and/ or dealer bears the risk of loss. | |||||||||||
Interest income is recognized on a time proportion basis. | |||||||||||
Items of income and expenditure are recognized on accrual and prudent basis. | |||||||||||
e) | Cash and Cash Equivalents | ||||||||||
Biosync considers all highly liquid investments with an original maturity or remaining maturity of three months or less at the date of purchase to be cash equivalents. Cash equivalents are stated at cost, which approximates their fair value due to the short maturity of the investments. Cash and claims to cash that are restricted as to withdrawal or use in the ordinary course of business are classified as other receivables under current assets, unless they are to be utilized for other than current operations in which case they are classified as other assets, non-current. | |||||||||||
. | |||||||||||
f) | Inventory | ||||||||||
Inventories are stated at the lower of cost or replacement cost with respect to raw materials and the lower of cost and net realizable value with respect to finished goods and work in progress. Cost of raw material includes purchase cost plus applicable landing costs. Cost of finished goods and work in progress are determined on a first-in first-out basis and includes direct material, direct labour and overheads. Net realizable value represents the anticipated selling price less estimated costs of completion and distribution. Year-end inventories are as taken, valued and certified by the partners. | |||||||||||
F-85
BIOSYNC SCIENTIFIC | |||||||||||
(converted into a Private Limited Company, namely Biosync Scientific Private Limited with effect from August 1, 2006) | |||||||||||
Notes to the Financial Statements for the year ended March 31, 2006 | |||||||||||
g) | Property, Plant and Equipment | ||||||||||
Property, Plant and Equipment are stated at cost of acquisition less accumulated depreciation. Cost includes inward freight, duties, non refundable taxes and incidental expenses related to acquisition and installation of fixed asset. | |||||||||||
The cost and the accumulated depreciation for property, plant and equipment sold, retired or otherwise disposed off are removed from the stated values and the resulting gains and losses are included in the Income Statement. | |||||||||||
. | |||||||||||
Depreciation is provided for using the straight line method over 3 to 14 years. Leasehold improvements are amortized using the straight line method over the estimated useful life of the asset or the terms of lease whichever is shorter. Maintenance and repairs are expensed as incurred. Replacements and betterments are capitalized. | |||||||||||
. | |||||||||||
h) | Impairment of Long-lived Assets | ||||||||||
Biosync accounts for impairment of long-lived assets in accordance with the provisions of SFAS 144 "Accounting for Impairment or Disposal of Long-Lived Assets". the firm reviews long-lived assets, for impairment whenever events or changes in business circumstances indicate the carrying amount of assets may not be fully recoverable. Each impairment test is based on a comparison of the undiscounted cash flows expected to be generated from the use of the asset to its recorded value. If impairment is indicated, the asset is written down to its fair value. Assets to be disposed are reported at the lower of the carrying value or the fair value less cost to sell. | |||||||||||
i) | Employee Benefits | ||||||||||
(i) | Leave Encashment | ||||||||||
Biosync does not have the policy of encashing outstanding leaves to its employees. | |||||||||||
(ii) | Gratuity Plan | ||||||||||
Biosync provides for Gratuity as and when paid and does not have the policy of ascertaining and accruing Gratuity liability as at the year end. However, the management is of the opinion that impact on such non provision will not be significant due to very few employees being employed by the firm. Further, such employees had also not completed the statutory time period to be eligible for gratuity. | |||||||||||
j) | Income Taxes | ||||||||||
In accordance with the provisions of SFAS 109,"Accounting for Income Taxes", income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the Financial Statements carrying amounts of existing assets and liabilities and their respective tax bases and operating loss carry forwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the Income Statement in the period of enactment. Based on management's judgment, the measurement of deferred tax assets is reduced, if necessary, by a valuation allowance for any tax benefits for which it is more likely than not that some portion or all of such benefits will not be realized. | |||||||||||
. | |||||||||||
F-86
BIOSYNC SCIENTIFIC | ||||||||||||
(converted into a Private Limited Company, namely Biosync Scientific Private Limited with effect from August 1, 2006) | ||||||||||||
Notes to the Financial Statements for the year ended March 31, 2006 | ||||||||||||
3 | Property, Plant and Equipment and Depreciation | |||||||||||
Property, Plant and Equipment at cost less accumulated depreciation consist of: | ||||||||||||
Particulars |
|
|
|
| As of |
| As of | |||||
|
|
|
|
| 31-Mar-06 |
| 31-Mar-05 | |||||
|
|
|
|
| USD |
| USD | |||||
Leasehold Land |
| 19,010 |
| 19,010 | ||||||||
Building |
|
|
|
| 11,146 |
| 11,146 | |||||
Plant and Machinery and Equipments |
| 161,029 |
| 156,168 | ||||||||
Furniture and Fixtures |
|
|
| 8,340 | - | |||||||
Computers and Accessories |
|
| 3,657 |
| 3,117 | |||||||
Total |
|
|
|
| 203,182 |
| 189,441 | |||||
Less: Accumulated Depreciation |
|
| (61,261) |
| (26,573) | |||||||
Property, Plant and Equipment, Net |
| 141,921 |
| 162,868 | ||||||||
The Firm has established the estimated useful lives of assets for depreciation purposes as follows: | ||||||||||||
Leasehold Land | Over the period of lease of 15 years | |||||||||||
Building | Over the period of lease of 15 years | |||||||||||
Plant and machinery | 5 years | |||||||||||
Furniture and Fixtures | 5 years | |||||||||||
Computer and Accessories | 3 years | |||||||||||
Depreciation expenses amounted to USD 34,689 and USD 26,124 for the year ended March 31, 2006 and March 31, 2005 respectively. Since the Firm is unable to identify depreciation against each functional areas of operation and has hence disclosed it separately in the Income Statement. | ||||||||||||
4 | Income Taxes | |||||||||||
Biosync benefits from tax incentives provided to units situated in the Special Economic Zone as an exemption from payment of Indian corporate income taxes in a manner as enumerated below: | ||||||||||||
Exemption Available |
|
|
| % of Taxable | ||||||||
|
|
|
|
| income Exempt | |||||||
For first 5 years |
|
|
| 100% | ||||||||
6th and 7th year |
|
|
| 50% | ||||||||
8th - 10th years (subject to fulfillment of certain conditions) | 50% | |||||||||||
Biosync has not recognized deferred income tax assets on account of tax losses,etc. in view of the tax exemption available and continuing till the time the losses are utilised. As regards other temporary differences, no deferred tax assets are recognised in view of the uncertainity of future taxable profits. | ||||||||||||
5 | Borrowings | |||||||||||
i) | Bank Overdrafts | |||||||||||
Bank overdraft from the bank are secured by hypothecation of inventories. | ||||||||||||
F-87
BIOSYNC SCIENTIFIC | |||||||||||
(converted into a Private Limited Company, namely Biosync Scientific Private Limited with effect from August 1, 2006) | |||||||||||
Notes to the Financial Statements for the year ended March 31, 2006 | |||||||||||
ii) | Interest Bearing Liabilities | ||||||||||
Particulars |
|
|
|
| As of |
| As of | ||||
|
|
|
|
| 31-Mar-06 |
| 31-Mar-05 | ||||
|
|
|
|
| USD |
| USD | ||||
Term loan from Banks |
|
|
| 100,294 |
| 123,341 | |||||
Less: Current Portion |
|
|
| (100,294) |
| (21,151) | |||||
Long Term Payable |
|
|
| - |
| 102,190 | |||||
|
|
|
|
|
|
|
| ||||
Term loans from banks are secured by hypothecation of plant and machinery and equitable mortgage of leasehold land and building. | |||||||||||
6 | Related Party Transactions | ||||||||||
Particulars |
|
|
|
| for the |
| for the | ||||
|
|
|
|
| year ended |
| year ended | ||||
|
|
|
|
| 31-Mar-06 |
| 31-Mar-05 | ||||
|
|
|
|
| USD |
| USD | ||||
|
|
|
|
|
|
|
| ||||
Sales to Biosync Medical |
|
| 1,073 |
| 1,057 | ||||||
|
|
|
|
|
|
|
| ||||
Remuneration/ Interest to Partners |
| 8,888 |
| 6,602 | |||||||
|
|
|
|
|
|
|
| ||||
Included in Other Liabilities |
|
|
|
|
| ||||||
Payable to Interventional Therapeutics System |
| 300,344 |
| - | |||||||
|
|
|
|
|
|
|
| ||||
Included in Trade Receivables |
|
|
|
|
| ||||||
Receivable from Biosync Medical |
| 448 |
| 1,085 | |||||||
|
|
|
|
|
|
|
| ||||
Balance in Partners Capital Account |
|
|
| ||||||||
Mr. Pratap Ram Surve |
|
|
| (10,450) |
| 2,155 | |||||
Mr. Viral Laljibhai Vaishnav |
|
| (3,373) |
| 11,203 | ||||||
Mrs. Vinita Rajesh Vaishnav |
|
| (96,921) |
| 1,457 | ||||||
Mr. Jayantilal Limbabhai Naria |
|
| (19,139) |
| (4,026) | ||||||
Total |
|
|
|
| (129,882) |
| 10,789 | ||||
|
|
|
|
|
|
|
| ||||
7 | Contingencies and Commitments | ||||||||||
i) | Operating Lease | ||||||||||
Biosync had non-cancellable operating lease for land of 15 years from the date of agreement on completion of lease period, the lease could be extended on mutually agreed terms. The lease for land of USD 19,010, has been capitalized in the books of the firm which is depreciated over a period of lease. | |||||||||||
8 | Concentration of Risk | ||||||||||
The firm is in its set up stage and does not have significant transactions. The firm monitors the creditworthiness of its customers to which it grants credit terms in the normal course of business. The following table gives details in respect of revenues generated from top three customers are: | |||||||||||
F-88
BIOSYNC SCIENTIFIC | ||||||||||
(converted into a Private Limited Company, namely Biosync Scientific Private Limited with effect from August 1, 2006) | ||||||||||
Notes to the Financial Statements for the year ended March 31, 2006 | ||||||||||
Particulars |
|
|
|
| for the |
| for the | |||
|
|
|
|
| year ended |
| year ended | |||
|
|
|
|
| 31-Mar-06 |
| 31-Mar-05 | |||
|
|
|
|
| USD |
| USD | |||
Revenue Generated from Top three Customers are: | ||||||||||
N S Remedies Pvt Ltd, India | 84,675 | -- | ||||||||
Scitech Medical, Brazil | 55,952 | 3,874 | ||||||||
Sentez Ic Ve Dis Tic Ltd. Sti | 31,467 | -- | ||||||||
Total Revenue Generated | 172,094 | 3,874 | ||||||||
| ||||||||||
9 | Financial Instruments: Fair Value | |||||||||
The carrying amounts reported in the balance sheets for cash and cash equivalents, trade receivables, other current assets, bank overdraft, current portion of interest bearing liabilities, accounts payable, accrued expenses and other liabilities approximate their respective fair values due to their short maturity. | ||||||||||
10 | Schedule to Balance Sheet | |||||||||
. | ||||||||||
| Particulars |
|
|
|
| As of |
| As of | ||
|
|
|
|
|
| 31-Mar-06 |
| 31-Mar-05 | ||
|
|
|
|
|
| USD |
| USD | ||
a. | Cash and Cash Equivalents |
|
|
|
| |||||
| Cash on Hand |
| 3,991 |
| 40 | |||||
| Fixed Deposit with Bank |
|
|
| - |
| 640 | |||
| Cash and Cash Equivalents |
|
| 3,991 |
| 680 | ||||
|
|
|
|
|
|
|
|
| ||
b. | Other Current Assets |
|
|
|
|
|
| |||
| Advance to suppliers |
|
|
| 8,370 |
| - | |||
| Other Current Assets |
|
|
| 8,370 |
| - | |||
|
|
|
|
|
|
|
|
| ||
c. | Other Assets |
|
|
|
|
| ||||
| Fixed Deposits with Banks |
|
| 11,206 |
| - | ||||
| Other Deposits |
|
| 572 |
| 571 | ||||
| Other Assets |
|
| 11,778 |
| 571 | ||||
|
| |||||||||
. | ||||||||||
11 | The previous year figures are regrouped/ rearranged to conform with the presentation in the current year. | |||||||||
BIOSYNC SCIENTIFIC PRIVATE LIMITED |
(Converted into an Indian Private Limited Company from Biosync Scientific, a partnership firm with effect from August 1, 2006) |
INTERIM FINANCIAL STATEMENTS |
AS PER US GAAP |
FOR THE PERIOD APRIL 1, 2006 TO DECEMBER 31, 2006 |
F-90
BIOSYNC SCIENTIFIC PRIVATE LIMITED | |||||||||
Converted into an Indian Private Limited Company from Biosync Scientific, a partnership firm with effect from | |||||||||
August 1, 2006) | |||||||||
Interim Financial Statements | |||||||||
For the period April 1, 2006 to December 31, 2006 | |||||||||
CONTENTS |
|
|
|
|
|
|
| Page No. | |
|
|
|
|
|
|
|
|
|
|
Interim Balance Sheet |
|
|
|
|
|
| F-90 | ||
|
|
|
|
|
|
|
|
|
|
Interim Income Statement |
|
|
|
|
|
| F-91 | ||
|
|
|
|
|
|
|
|
|
|
Interim Statement of Partners' Capital and Comprehensive Income |
|
| F-92 | ||||||
|
|
|
|
|
|
|
|
|
|
Interim Cash Flow Statement |
|
|
|
|
| F-93 | |||
|
|
|
|
|
|
|
|
|
|
Notes to the Interim Financial Statements |
|
|
|
| F-94 | ||||
F-91
BIOSYNC SCIENTIFIC PRIVATE LIMITED | ||
(converted into a Private Limited Company from Biosync Scientific, a partnership firm with effect from August 1, 2006) | ||
Balance Sheet as of December 31, 2006 | ||
Particulars | As at | As of |
31-Dec-06 | 31-Mar-06 | |
| USD | USD |
Assets | ||
| ||
Current Assets | ||
Cash and Cash Equivalents | 13,491 | 3,991 |
Trade receivables, net of allowances | 29,620 | 6,051 |
Inventories | 157,905 | 119,873 |
Advance to suppliers | 19,275 | - |
Other Current Assets | 45,848 | 8,370 |
Total Current Assets | 266,139 | 138,285 |
| ||
Property, Plant & Equipment | 336,779 | 141,921 |
Other Assets | 623 | 12,433 |
Total Assets | 603,541 | 292,639 |
| ||
Liabilities and Shareholders' Funds | ||
| ||
Current Liabilities | ||
Bank Overdraft | 257,221 | 3,789 |
Interest Bearing Liabilities | 398,608 | 100,294 |
Accounts Payables | - | 16,585 |
Accrued Expenses | 55,544 | 616 |
Other Liabilities | - | 301,237 |
Total Current Liabilities | 711,374 | 422,521 |
Long Term Interest Bearing Liabilities | - | - |
Total Liabilities | 711,374 | 422,521 |
| ||
Shareholders' Funds | ||
Erstwhile Partners' Capital/ (withdrawals) | - | (129,882) |
Share Capital | 486,690 | - |
Retained Earnings | (679,319) | - |
Share Application Money | 80,501 | - |
Accumulated Other Comprehensive Income/ (Loss) | 4,296 | - |
Total Shareholders' Funds | (107,833) | (129,882) |
Total Liabilities and Shareholders' Funds | 603,541 | 292,639 |
|
|
|
Accompanying Notes form an integral part of these Financial Statements. | ||
ForBiosync Scientific Private Limited | ||
Directors | ||
Mumbai: October 11, 2007 | ||
F-92
BIOSYNC SCIENTIFIC PRIVATE LIMITED | ||
(converted into a Private Limited Company from Biosync Scientific, a partnership firm with effect from August 1, 2006) | ||
Income Statement for the period April 1, 2006 to December 31, 2006 | ||
Particulars | For the P.E. | For the Y.E. |
31-Dec-06 | 31-Mar-06 | |
| USD | USD |
Revenue | 45,754 | 174,185 |
Cost of Sales | (313,637) | (73,771) |
Gross Profit | (267,883) | 100,414 |
| ||
Expenses | ||
Selling, Administrative and Other Expenses | (113,050) | (7,773) |
Depreciation | (33,871) | (34,689) |
Finance Charges | (29,333) | (17,912) |
Interest/ Remuneration to erstwhile Partners | - | (8,888) |
Operating Income/ (Loss) | (444,137) | 31,152 |
| ||
Other Income | 1,449 | 909 |
Income /(Loss) before Taxes | (442,688) | 32,061 |
| ||
Income Taxes (Refer Note 4) | - | - |
Less: Profits of Partnership concern for the period April 2006 to July 2006 transferred to Partners Capital Account on conversion | (2,609) | - |
Add: Debit Balance in erstwhile Partners' Capital Account written off since not recoverable | (234,022) | - |
|
|
|
Net Income/ (Loss) | (679,319) | 32,061 |
| ||
Accompanying Notes form an integral part of these Financial Statements. | ||
ForBiosync Scientific Private Limited | ||
Directors | ||
Mumbai: October 11, 2007 | ||
F-93
BIOSYNC SCIENTIFIC PRIVATE LIMITED | ||||||||
(converted into a Private Limited Company from Biosync Scientific, a partnership firm with effect from August 1, 2006) | ||||||||
. | ||||||||
Statement of Shareholders' Equity and Comprehensive Income | ||||||||
. | ||||||||
Particulars | Erstwhile | Shareholders' | Share | Retained | Other | Total | ||
| Partners' | Equity | Application | Earnings | Comprehensive | shareholders' | ||
| Capital |
| Money |
| Accumulated | Funds | ||
|
|
|
|
| Income/ (Loss) |
| ||
| USD | USD | USD | USD | USD | USD | ||
Balance As of April 1, 2006 | (129,882) | - | - | - | - | (129,882) | ||
| ||||||||
Capital introduced (net) | 512 | - | - | - | - | 512 | ||
| ||||||||
Profit upto the date of conversion | - | - | - | 2,609 | - | 2,609 | ||
| ||||||||
Profit upto the date of conversion transferred to Partners Capital | 2,609 | - | - | (2,609) | - | - | ||
| ||||||||
Balance in Partners' Capital Account converted into Equity Shares on conversion of the firm to a Private Limited Company with effect from August 1, 2006. | (107,261) | 107,261 | - | - | - | - | ||
| ||||||||
Additional Shares issued against cash | - | 6,676 | - | - | - | 6,676 | ||
| ||||||||
Additional shares issued on takeover of Interventional Therapeutic Systems, a proprietary concern of one of the promoters of the Company. | - | 372,752 | - | - | - | 372,752 | ||
| ||||||||
Share Application Money Received | - | - | 80,501 | - | - | 80,501 | ||
| ||||||||
Debit Balance in erstwhile Partners Capital account written off since not recoverable | 234,022 | - | - | - | - | 234,022 | ||
| ||||||||
Net Income of the Company for the period | - | - | - | (679,319) | - | (679,319) | ||
| ||||||||
Loss on Foreign Currency Translation | - | - | - | - | 4,296 | 4,296 | ||
|
|
|
|
|
|
| ||
Balance As of December 31, 2006 | - | 486,690 | 80,501 | (679,319) | 4,296 | (107,833) | ||
| ||||||||
F-94
BIOSYNC SCIENTIFIC PRIVATE LIMITED | |||||||
(converted into a Private Limited Company from Biosync Scientific, a partnership firm with effect from August 1, 2006) | |||||||
Cash Flow Statement for the period April 1, 2006 to December 31, 2006 | |||||||
Particulars | For the P.E. | For the Y.E. | |||||
31-Dec-06 | 31-Mar-06 | ||||||
|
|
| USD | USD | |||
|
|
| |||||
Cash Flow From Operating Activities |
|
| |||||
Net Income / (Loss) |
| (679,319) | 32,061 | ||||
Adjustments to reconcile net income to net cash from operating activities: |
|
| |||||
| Depreciation |
| 33,871 | 34,689 | |||
| Profit up to July 31, 2006 transferred to erstwhile Partners' Account |
| (2,609) | - | |||
| Debit Balance in erstwhile Partners' Capital account written off |
| 234,022 | - | |||
Changes in assets and liabilities: |
|
| |||||
| Trade Receivables |
| (23,569) | (4,966) | |||
| Inventories |
| (38,032) | (115,420) | |||
| Accounts Payable |
| (16,585) | 13,598 | |||
| Advance to suppliers |
| (19,275) | - | |||
| Other Current Assets |
| (37,478) | (8,370) | |||
| Other Assets |
| 11,810 | (11,862) | |||
| Accrued Expenses |
| 54,928 | 502 | |||
Net Cash Used for Operating Activities | (482,236) | (59,768) | |||||
|
|
| |||||
Cash Flow From Investment Activities |
|
| |||||
| Purchase of Property, Plant & Equipment |
| (228,729) | (13,742) | |||
Net Cash Used For Investment Activities | (228,729) | (13,742) | |||||
|
|
| |||||
Cash Flows From Financing Activities |
|
| |||||
| Additions (Withdrawal) to/ from Partners Capital |
| 5,729 | (175,162) | |||
| Share Capital issued |
| 379,429 | - | |||
| Share Application Money received |
| 80,501 | - | |||
| (Repayment) of/ Proceeds from Bank Overdraft |
| 253,432 | (14,387) | |||
| (Repayment) of/ Proceeds from Other Liabilities |
| (301,237) | 286,987 | |||
| (Repayment of)/ Proceeds from Interest Bearing Liabilities (net) |
| 298,314 | (23,047) | |||
Net Cash From Financing Activities | 716,169 | 4,391 | |||||
|
|
| |||||
Effect of exchange rate changes | 4,296 | 2,430 | |||||
Net Change in Cash and Cash Equivalents | 9,500 | 3,311 | |||||
|
|
| |||||
Cash and cash equivalents at the beginning of the period |
| 3,991 | 680 | ||||
Cash and cash equivalents at the end of the period | 13,491 | 3,991 | |||||
|
|
| |||||
F-95
(converted into a Private Limited Company from Biosync Scientific, a partnership firm with effect from August 1, 2006) | |||||||||
Notes to the Financial Statements for the period April 1, 2006 to December 31, 2006 | |||||||||
1 | Background | ||||||||
Biosync Scientific Private Limited ("Biosync" or "the Company") was converted into a private limited company from Biosync Scientific, a Partnership Firm with effect from August 1, 2006 and the erstwhile partners of the firm as at the date of conversion became the members of the Private Limited Company with equity shares issued to the partners in the ratio of their capital outstanding as per the financial statements drawn up as at the date of conversion. | |||||||||
Effective February 16, 2007, MIV Therapeutics Inc. acquired 100% of the equity shares of the said converted Company from the erstwhile partners and accordingly, the Company with effect from February 16, 2007 become a wholly owned subsidiary of MIV Therapeutics Inc. | |||||||||
The registered office of the Company is situated at 136-B, Surat Special Economic Zone, G.I.D.C., Sachin, Surat - 394230, Gujarat, India. | |||||||||
2 | Summary of Significant Accounting Policies | ||||||||
a) | Basis of Presentation | ||||||||
i) | The Financial Statements of Biosync are prepared in accordance with generally accepted accounting principles applicable in the United States ("U.S. GAAP") as at the date of preparation. | ||||||||
ii) | Biosync is set up to deal in designing, manufacturing and marketing of coated and non-coated vascular stents and related accessories. However, during the year, the Company was still under development stage and did not have significant operations. Presently, the Holding Company is also under development and its ability to generate revenue is primarily dependent on its success in completing development and obtaining regulatory approvals for the commercialization of its stent technology. The Holding Company's ability to obtain sufficient financing to continue the development of, and if successful, to commence the manufacture and sale of its products under development and if and when approved by the applicable regulatory agencies is uncertain. In view of these conditions, the ability of the holding company and subsequently the subsidiary to continue as a going concern is in substantial doubt and dependent upon achieving a profitable level of operations and on the ability of the Ho lding Company to obtain necessary financing to fund ongoing operations. Management believes that its current and future plans enable the holding company and correspondingly its subsidiary to continue as a going concern. | ||||||||
b) | Use of Estimates | ||||||||
The preparation of the Financial Statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent liabilities as of the date of the Financial Statements and the reported amount of revenues and expenses during the reported period. Examples of such estimates include: allowance for doubtful debts, and for future obligations under employee benefit plans, valuation allowances for deferred taxes, and useful lives of property, plant and equipment, etc. Actual results could differ materially from these estimates. | |||||||||
c) | Foreign Currency Translation | ||||||||
The accompanying Financial Statements are reported in U.S. dollars ("USD"). The Indian Rupee ("INR") is the functional currency for Biosync. The translation of the functional currencies into USD wherever applicable is performed for assets and liabilities using the exchange rates prevailing at the Balance Sheet date and for revenues, costs and expenses using average exchange rates prevailing during the reporting period. Adjustments resulting from the translation of functional currency financial statements to reporting currency are accumulated and reported as net income / (loss) in the Other Comprehensive Accumulated Income or Loss. | |||||||||
F-96
BIOSYNC SCIENTIFIC PRIVATE LIMITED | |||||||||
(converted into a Private Limited Company from Biosync Scientific, a partnership firm with effect from August 1, 2006) | |||||||||
Notes to the Financial Statements for the period April 1, 2006 to December 31, 2006 | |||||||||
Transactions in foreign currency for reporting functional currency are recorded at the exchange rate prevailing on the date of transaction. Monetary assets and liabilities denominated in foreign currencies are expressed in the functional currency at the exchange rates prevailing at the Balance Sheet date. Revenues, costs and expenses are recorded using exchange rates prevailing on the date of transaction. Gains or losses arising out of fluctuations in the exchange rates are recognized in the profit and loss in the period in which they arise. | |||||||||
. | |||||||||
d) | Revenue Recognition | ||||||||
The Company recognizes revenues, net of returns, rebates and returns, if any from the sale of the products, at the time when the product is delivered to the customer and/ or dealer. Revenues are recognized only when the firm has transferred to the customer and/ or dealer the significant risks and rewards of ownership of the goods, title to the products transfers, the amount if fixed and determinable, evidence of an agreement exists, there is reasonable assurance of collection of the sale proceeds, the Company has no future obligations and the customer and/ or dealer bears the risk of loss. | |||||||||
. | |||||||||
Interest income is recognized on a time proportion basis. | |||||||||
. | |||||||||
Items of income and expenditure are recognized on accrual and prudent basis. | |||||||||
e) | Cash and Cash Equivalents | ||||||||
Biosync considers all highly liquid investments with an original maturity or remaining maturity of three months or less at the date of purchase to be cash equivalents. Cash equivalents are stated at cost, which approximates their fair value due to the short maturity of the investments. Cash and claims to cash that are restricted as to withdrawal or use in the ordinary course of business are classified as other receivables under current assets, unless they are to be utilized for other than current operations in which case they are classified as other assets, non-current. | |||||||||
. | |||||||||
f) | Inventory | ||||||||
Inventories are stated at the lower of cost or replacement cost with respect to raw materials and the lower of cost and net realizable value with respect to finished goods and work in progress. Cost of raw material includes purchase cost plus applicable landing costs. Cost of finished goods and work in progress are determined on a first-in first-out basis and includes direct material, direct labour and overheads. Net realizable value represents the anticipated selling price less estimated costs of completion and distribution. Year-end inventories are as taken, valued and certified by the partners. | |||||||||
. | |||||||||
g) | Property, Plant and Equipment | ||||||||
Property, Plant and Equipment are stated at cost of acquisition less accumulated depreciation. Cost includes inward freight, duties, non refundable taxes and incidental expenses related to acquisition and installation of fixed asset. | |||||||||
The cost and the accumulated depreciation for property, plant and equipment sold, retired or otherwise disposed off are removed from the stated values and the resulting gains and losses are included in the Income Statement. | |||||||||
. | |||||||||
Depreciation is provided for using the straight line method over 3 to 14 years. Leasehold improvements are amortized using the straight line method over the estimated useful life of the asset or the terms of lease whichever is shorter. Maintenance and repairs are expensed as incurred. Replacements and betterments are capitalized. | |||||||||
F-97
BIOSYNC SCIENTIFIC PRIVATE LIMITED | |||||||||||
(converted into a Private Limited Company from Biosync Scientific, a partnership firm with effect from August 1, 2006) | |||||||||||
Notes to the Financial Statements for the period April 1, 2006 to December 31, 2006 | |||||||||||
. | |||||||||||
h) | Impairment of Long-lived Assets | ||||||||||
Biosync accounts for impairment of long-lived assets in accordance with the provisions of SFAS 144 "Accounting for Impairment or Disposal of Long-Lived Assets". the firm reviews long-lived assets, for impairment whenever events or changes in business circumstances indicate the carrying amount of assets may not be fully recoverable. Each impairment test is based on a comparison of the undiscounted cash flows expected to be generated from the use of the asset to its recorded value. If impairment is indicated, the asset is written down to its fair value. Assets to be disposed are reported at the lower of the carrying value or the fair value less cost to sell. | |||||||||||
. | |||||||||||
i) | Employee Benefits | ||||||||||
. | |||||||||||
(i) | Leave Encashment | ||||||||||
Biosync does not have the policy of encashing outstanding leaves to its employees. | |||||||||||
(ii) | Gratuity Plan | ||||||||||
Biosync provides for Gratuity as and when paid and does not have the policy of ascertaining and accruing Gratuity liability as at the year end. However, the management is of the opinion that impact on such non provision will not be significant due to very few employees being employed by the firm. Further, such employees had also not completed the statutory time period to be eligible for gratuity. | |||||||||||
. | |||||||||||
j) | Income Taxes | ||||||||||
In accordance with the provisions of SFAS 109,"Accounting for Income Taxes", income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the Financial Statements carrying amounts of existing assets and liabilities and their respective tax bases and operating loss carry forwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the Income Statement in the period of enactment. Based on management's judgment, the measurement of deferred tax assets is reduced, if necessary, by a valuation allowance for any tax benefits for which it is more likely than not that some portion or all of such benefits will not be realized. | |||||||||||
. | |||||||||||
3 | Property, Plant and Equipment and Depreciation | ||||||||||
. | . | ||||||||||
Property, Plant and Equipment at cost less accumulated depreciation consist of: | |||||||||||
. | . | ||||||||||
Particulars |
|
|
|
|
| As of | As of | ||||
|
|
|
|
|
| 31-Dec-06 | 31-Mar-06 | ||||
|
|
|
|
|
| USD | USD | ||||
Leasehold Land | 19,010 | 19,010 | |||||||||
Building |
|
|
|
|
| 11,146 | 11,146 | ||||
Plant and Machinery and Equipments |
|
| 356,804 | 161,029 | |||||||
Furniture and Fixtures |
|
|
|
| 13,573 | 8,340 | |||||
Computers and Accessories |
|
|
| 14,453 | 3,657 | ||||||
Capital Work in Progress |
|
|
|
| 16,925 | -- | |||||
Total |
|
|
|
|
| 431,911 | 203,182 | ||||
Less: Accumulated Depreciation |
|
|
| (95,132) | (61,261) | ||||||
Property, Plant and Equipment, Net |
|
| 336,779 | 141,921 | |||||||
. | |||||||||||
. | |||||||||||
F-98
BIOSYNC SCIENTIFIC PRIVATE LIMITED | ||||||||||||
(converted into a Private Limited Company from Biosync Scientific, a partnership firm with effect from August 1, 2006) | ||||||||||||
Notes to the Financial Statements for the period April 1, 2006 to December 31, 2006 | ||||||||||||
The Company has established the estimated useful lives of assets for depreciation purposes as follows: | ||||||||||||
Leasehold Land | Over the period of lease of 15 years | |||||||||||
Building | Over the period of lease of 15 years | |||||||||||
Plant and machinery | 5 years | |||||||||||
Furniture and Fixtures | 5 years | |||||||||||
Computer and Accessories | 3 years | |||||||||||
. | ||||||||||||
4 | Income Taxes | |||||||||||
Biosync benefits from tax incentives provided to units situated in the Special Economic Zone as an exemption from payment of Indian corporate income taxes in a manner as enumerated below: | ||||||||||||
Exemption Available |
|
|
| % of Taxable | ||||||||
|
|
|
|
| income Exempt | |||||||
For first 5 years |
|
|
| 100% | ||||||||
for 6th and 7th years |
|
|
| 50% | ||||||||
For 8th - 10th years (subject to fulfillment of certain conditions) | 50% | |||||||||||
Biosync has not recognized deferred income tax assets on account of tax losses,etc. in view of the tax exemption available and continuing till the time the losses are utilised. As regards other temporary differences, no deferred tax assets are recognised in view of the uncertainity of future taxable profits. | ||||||||||||
. | ||||||||||||
5 | Borrowings | |||||||||||
. | ||||||||||||
i) | Bank Overdrafts | |||||||||||
Bank overdraft from the bank is secured by hypothecation of inventories. | ||||||||||||
. | ||||||||||||
ii) | Interest Bearing Liabilities | |||||||||||
. | ||||||||||||
Particulars |
|
|
|
|
| As of | As of | |||||
|
|
|
|
|
| 31-Dec-06 | 31-Mar-06 | |||||
|
|
|
|
|
| USD | USD | |||||
Term loan from Banks |
|
|
|
| 398,608 | 100,294 | ||||||
Less: Current Portion |
|
|
|
| (398,608) | (100,294) | ||||||
Long Term Payable |
|
|
|
| - | - | ||||||
|
|
|
|
|
|
|
| |||||
|
|
|
|
|
|
| ||||||
Term loans from banks are secured by hypothecation of plant and machinery and equitable mortgage of leasehold land and building. | ||||||||||||
F-99
BIOSYNC SCIENTIFIC PRIVATE LIMITED | |||||||||||
(converted into a Private Limited Company from Biosync Scientific, a partnership firm with effect from August 1, 2006) | |||||||||||
Notes to the Financial Statements for the period April 1, 2006 to December 31, 2006 | |||||||||||
6 | Related Party Transactions | ||||||||||
Particulars |
|
|
|
|
| for the | for the | ||||
|
|
|
|
|
| period ended | year ended | ||||
|
|
|
|
|
| 31-Dec-06 | 31-Mar-06 | ||||
|
|
|
|
|
| USD | USD | ||||
|
|
|
|
| |||||||
Sales to Biosync Medical |
|
|
| - | 1,073 | ||||||
|
|
|
|
| |||||||
Remuneration/ Interest to Partners |
|
|
| - | 8,888 | ||||||
|
|
|
|
| |||||||
Included in Other Liabilities |
|
|
|
| |||||||
Payable to Interventional Therapeutics System |
|
| - | 300,344 | |||||||
|
|
|
|
| |||||||
Included in Trade Receivables |
|
|
|
| |||||||
Receivable from Biosync Medical |
|
|
| - | 443 | ||||||
|
|
|
|
| |||||||
Balance in Partners Capital Account |
|
|
| ||||||||
Mr. Pratap Ram Surve |
|
|
|
| - | (10,450) | |||||
Mr. Viral Laljibhai Vaishnav |
|
|
| - | (3,373) | ||||||
Mrs. Vinita Rajesh Vaishnav |
|
|
| - | (96,921) | ||||||
Mr. Jayantilal Limbabhai Naria |
|
|
| - | (19,139) | ||||||
Total |
|
|
|
| - | (129,882) | |||||
|
|
|
|
|
|
|
| ||||
. | |||||||||||
7 | Contingencies and Commitments | ||||||||||
i) | Operating Lease | ||||||||||
Biosync had non-cancellable operating lease for land of 15 years from the date of agreement on completion of lease period, the lease could be extended on mutually agreed terms. The lease for land of USD 19,010, has been capitalized in the books of the firm which is depreciated over a period of lease. | |||||||||||
8 | Concentration of Risk | ||||||||||
The firm is in its set up stage and does not have significant transactions. The firm monitors the creditworthiness of its customers to which it grants credit terms in the normal course of business. The following table gives details in respect of revenues generated from top two customers are: | |||||||||||
Particulars |
|
|
|
|
| for the | for the | ||||
|
|
|
|
|
| period ended | year ended | ||||
|
|
|
|
|
| 31-Dec-06 | 31-Mar-06 | ||||
|
|
|
|
|
| USD | USD | ||||
Revenue Generated from Top two Customers are: |
|
|
| ||||||||
N S Remedies Pvt Ltd, India |
|
|
|
| - | 84,675 | |||||
Scitech Medical, Brazil |
|
|
|
| - | 55,952 | |||||
Sentez Ic Ve Dis Tic Ltd, Sti |
|
|
|
| - | 31,467 | |||||
Total Revenue Generated |
|
|
| - | 172,094 | ||||||
|
|
|
|
|
|
|
| ||||
F-100
BIOSYNC SCIENTIFIC PRIVATE LIMITED | |||||||||||||
(converted into a Private Limited Company from Biosync Scientific, a partnership firm with effect from August 1, 2006) | |||||||||||||
Notes to the Financial Statements for the period April 1, 2006 to December 31, 2006 | |||||||||||||
9 | Financial Instruments: Fair Value | ||||||||||||
The carrying amounts reported in the balance sheets for cash and cash equivalents, trade receivables, other current assets, bank overdraft, current portion of interest bearing liabilities, accounts payable, accrued expenses and other liabilities approximate their respective fair values due to their short maturity. | |||||||||||||
10 | Schedule to Balance Sheet | ||||||||||||
| Particulars |
|
|
|
|
| As of | As of | |||||
|
|
|
|
|
|
| 31-Dec-06 | 31-Mar-06 | |||||
|
|
|
|
|
|
| USD | USD | |||||
|
|
|
|
|
|
|
|
| |||||
| Share Capital |
|
|
|
|
|
| ||||||
|
|
|
|
|
|
|
|
| |||||
| Subscribed and Paid up Share Capital |
|
|
|
| ||||||||
| 22,050,000 equity shares of INR 10/- each fully paid up. |
| 486,690 | - | |||||||||
|
|
| |||||||||||
| of the above, 500,000 shares have been issued to erstwhile partners of Biosync Scientific on conversion of partnership firm into a Indian Private Limited Company. | 107,261 | - | ||||||||||
|
|
| |||||||||||
| Of the above, 1,675,000 shares have been issued to one of the promoters of the Company on takeover of assets and liabilities of his proprietary concern. | 372,752 | - | ||||||||||
|
|
| |||||||||||
| Share Application money | ||||||||||||
| Received from MIV Therapeutics Inc. pending allocation of shares | 80,501 | - | ||||||||||
|
|
| |||||||||||
| Cash and Cash Equivalents | ||||||||||||
| Cash on Hand |
| 13,491 | 3,991 | |||||||||
| Cash and Cash Equivalents | 13,491 | 3,991 | ||||||||||
|
|
| |||||||||||
| Other Current Assets |
| |||||||||||
| Advance to suppliers |
| 19,275 | 8,370 | |||||||||
| Other Current Assets |
| 19,275 | 8,370 | |||||||||
|
|
| |||||||||||
| Other Assets |
| |||||||||||
| Fixed Deposits with Banks | 620 | 11,206 | ||||||||||
| Other Deposits | 553 | 572 | ||||||||||
| Other Assets |
| 1,173 | 11,778 | |||||||||
|
|
|
|
|
|
|
|
| |||||
11 | The previous year figures are regrouped/ rearranged to conform to the presentation in the current period. | ||||||||||||
12 | Previous year figures are not comparable with figures for the current period, since the current period figures are | ||||||||||||
for nine months only. | |||||||||||||
F-101
BIOSYNC SCIENTIFIC |
(Converted into an Indian Private Limited Company, namely Biosync Scientific Private Limited with effect from August 1, 2006) |
INTERIM FINANCIAL STATEMENTS |
AS PER US GAAP |
FOR THE PERIOD APRIL 1, 2005 TO DECEMBER 31, 2005 |
F-102
BIOSYNC SCIENTIFIC | |||||||||
Converted into an Indian Private Limited Company, namely Biosync Scientific Private Limited with effect from | |||||||||
August 1, 2006) | |||||||||
Interim Financial Statements | |||||||||
For the period April 1, 2005 to December 31, 2005 | |||||||||
CONTENTS |
|
|
|
|
|
|
| Page No. | |
|
|
|
|
|
|
|
|
|
|
Interim Balance Sheet |
|
|
|
|
|
| F-104 | ||
|
|
|
|
|
|
|
|
|
|
Interim Income Statement |
|
|
|
|
|
| F-105 | ||
|
|
|
|
|
|
|
|
|
|
Interim Statement of Partners' Capital and Comprehensive Income |
|
| F-106 | ||||||
|
|
|
|
|
|
|
|
|
|
Interim Cash Flow Statement
|
|
|
|
|
| F-107 | |||
|
|
|
|
|
|
|
|
|
|
Notes to the Interim Financial Statements |
|
|
|
| F-108 | ||||
F-103
(converted into a Private Limited Company, namely Biosync Scientific Private Limited with effect from August 1, 2006) | ||
Balance Sheet as of December 31, 2005 | ||
Particulars | As at | As of |
Dec 31 2005 | 31-Mar-05 | |
| USD | USD |
Assets | ||
| ||
Current Assets | ||
Cash and Cash Equivalents | 6,430 | 680 |
Trade receivables, net of allowances | 1,328 | 1,085 |
Inventories | 12,920 | 4,453 |
Other Current Assets | 23,786 | - |
Total Current Assets | 44,464 | 6,218 |
| ||
Property, Plant & Equipment | 137,610 | 162,868 |
Other Assets | 1,173 | 571 |
Total Assets | 183,247 | 169,657 |
| ||
Liabilities and Partners' Funds | ||
| ||
Current Liabilities | ||
Bank Overdraft | 16,570 | 18,176 |
Interest Bearing Liabilities | 106,500 | 21,151 |
Accounts Payables | 3,178 | 2,987 |
Advance from customers | 151,691 | |
Accrued Expenses | 3,416 | 114 |
Other Liabilities | 13,608 | 14,250 |
Total Current Liabilities | 294,963 | 56,678 |
Long Term Interest Bearing Liabilities | - | 102,190 |
Total Liabilities | 294,963 | 158,868 |
| ||
Partners' Funds | ||
Partners' Capital/ (withdrawals) | (111,716) | 10,789 |
Total Partners' Funds | (111,716) | 10,789 |
Total Liabilities and Partners' Funds | 183,247 | 169,657 |
|
|
|
Accompanying Notes form an integral part of these Financial Statements. | ||
Approved by the Directors of Biosync Scientific Private Limited on behalf of erstwhile partners of Biosync Scientific since the partnership firm has been converted into a private limited company with effect from August 1, 2006. | ||
ForBiosync Scientific Private Limited | ||
Directors | ||
Mumbai: October 11, 2007 | ||
F-104
BIOSYNC SCIENTIFC | ||
(converted into a Private Limited Company, namely Biosync Scientific Private Limited with effect from August 1, 2006) | ||
Particulars | For the P.E. | For the Y.E. |
31-Dec-05 | 31-Mar-05 | |
| USD | USD |
Revenue | 62,530 | 6,855 |
Cost of Sales | (41,313) | (8,600) |
Gross Profit | 21,217 | (1,745) |
| ||
Expenses | ||
Selling, Administrative and Other Expenses | (5,961) | (13,288) |
Depreciation | (25,803) | (26,124) |
Finance Charges | (13,514) | (16,333) |
Interest/ Remuneration to Partners | (6,692) | (6,602) |
Operating Income/ (Loss) | (30,753) | (64,092) |
| ||
Other Income | 464 | 70 |
Income /(Loss) before Taxes | (30,289) | (64,022) |
| ||
Income Taxes (Refer Note 4) | - | - |
Net Income/ (Loss) | (30,289) | (64,022) |
| ||
Accompanying Notes form an integral part of these Financial Statements. | ||
Approved by the Directors of Biosync Scientific Private Limited on behalf of partners of Biosync Scientific since the partnership firm has been converted into a private limited company with effect from August 1, 2006. | ||
ForBiosync Scientific Private Limited | ||
Directors | ||
Mumbai: October 11, 2007 | ||
F-105
BIOSYNC SCIENTIFIC | ||||
(converted into a Private Limited Company, namely Biosync Scientific Private Limited with effect from August 1, 2006) | ||||
Statement of Partners' Capital and Comprehensive Income | ||||
Particulars | Partners' | Retained | Other | Total |
| Capital | Earnings | Comprehensive | Partners' |
|
|
| Accumulated | Funds |
|
|
| Income/ (Loss) |
|
| USD | USD | USD | USD |
Balance As of April 01 , 2004 | 51,203 | - | - | 51,203 |
| ||||
Capital Introduced | 28,721 | - | - | 28,721 |
| ||||
Net Income / (Loss) for the year | - | (64,022) | - | (64,022) |
| ||||
Loss on Foreign Currency Translation | - | - | (5,113) | (5,113) |
| ||||
Loss for the year transferred to Partners | (69,135) | 64,022 | 5,113 | - |
|
|
|
|
|
Balance As of March 31, 2005 | 10,789 | - | - | 10,789 |
| ||||
Capital Introduced / (withdrawn) | (97,064) | (97,064) | ||
| ||||
Net Income for the year | (30,289) | (30,289) | ||
| ||||
Loss on Foreign Currency Translation | 4,848 | 4,848 | ||
| ||||
Loss for the year transferred to Partners | (25,441) | 30,289 | (4,848) | - |
| - | |||
Loss for the year transferred to Partners | - | |||
|
|
|
|
|
Balance As of December 31, 2005 | (111,716) | - | - | (111,716) |
| ||||
| ||||
F-106
BIOSYNC SCIENTIFIC | |||||
(converted into a Private Limited Company, namely Biosync Scientific Private Limited with effect from August 1, 2006) | |||||
Cash Flow Statement for the period April 1, 2005 to December 31, 2005 | |||||
Particulars | For the P.E. | For the Y.E. | |||
31-Mar-05 | 31-Mar-05 | ||||
|
|
| USD | USD | |
|
|
| |||
Cash Flow From Operating Activities |
|
| |||
Net Income / (Loss) |
| (30,289) | (64,022) | ||
Adjustments to reconcile net income to net cash from operating activities: |
|
| |||
| Depreciation |
| 25,803 | 26,124 | |
Changes in assets and liabilities: |
|
| |||
| Trade Receivables |
| (243) | (1,085) | |
| Inventories |
| (8,467) | (4,453) | |
| Accounts Payable |
| 191 | 2,987 | |
| Advance from Customers |
| 151,691 | - | |
| Other Current Assets |
| (23,786) | 16,643 | |
| Other Assets |
| (602) | (4) | |
| Accrued Expenses |
| 3,302 | 114 | |
Net Cash Used for Operating Activities | 117,600 | (23,696) | |||
|
|
| |||
Cash Flow From Investment Activities |
|
| |||
| Purchase of Property, Plant & Equipment |
| (545) | (164,576) | |
Net Cash Used For Investment Activities | (545) | (164,576) | |||
|
|
| |||
Cash Flows From Financing Activities |
|
| |||
| Additions (Withdrawal) to/ from Capital |
| (97,064) | 28,721 | |
| (Repayment) of/ Proceeds from Bank Overdraft |
| (1,606) | 18,176 | |
| (Repayment) of/ Proceeds from Other Liabilities |
| (642) | 14,250 | |
| (Repayment of)/ Proceeds from Interest Bearing Liabilities (net) |
| (16,841) | 123,341 | |
Net Cash From Financing Activities | (116,153) | 184,488 | |||
|
|
| |||
Effect of exchange rate changes | 4,848 | (5,113) | |||
Net Change in Cash and Cash Equivalents | 5,750 | (8,897) | |||
|
|
| |||
Cash and cash equivalents at the beginning of the year |
| 680 | 9,577 | ||
Cash and cash equivalents at the end of the year | 6,430 | 680 | |||
|
|
| |||
F-107
(converted into a Private Limited Company, namely Biosync Scientific Private Limited with effect from August 1, 2006) | |||||||||
Notes to the Financial Statements for the period April 1,2005 to December 31, 2005 | |||||||||
1 | Background | ||||||||
Biosync Scientific ("Biosync" or "the firm") was a Partnership Firm and was set up on July 21, 2003. The partners of the firm with their profit sharing ratios (PSR) is given here under: | |||||||||
Partners |
|
| PSR on set up | PSR as amended on 10.09.2003 | PSR as amended on 11.11.2003 | PSR as amended on 01.07.2005 | PSR as amended on 01.04.2006 | ||
Mr. Pratap Ram Surve |
| 33% | 20% | 20% | 10% | 10% | |||
Mr. Viral Laljibhai Vaishnav | 33% | 20% | 20% | 10% | 4% | ||||
Mrs. Vinita Rajesh Vaishnav | 33% | 50% | 40% | 65% | 25% | ||||
Mr. Jayantilal Limbabhai Naria | - | 10% | 20% | 15% | 4% | ||||
Mr. Rajesh Laljibhai Vaishnav | - | - | - | - | 50% | ||||
Mrs. Sangeeta Viral Vaishnav | - | - | - | - | 4% | ||||
Mrs. Alka Jayanti Nariya |
| - | - | - | - | 3% | |||
Total |
|
| 100% | 100% | 100% | 100% | 100% | ||
Effective August 1, 2006, the firm was converted into a Private Limited Company as per the provisions of the Indian Companies Act, 1956 and the partners of the firm as at the date of conversion became the members of the Private Limited Company with equity shares issued to the partners in the ratio of their capital outstanding as at the date of conversion. | |||||||||
Effective February 16, 2007, MIV Therapeutics Inc. acquired 100% of the equity shares of the said converted Company from the erstwhile partners and accordingly, the Company with effect from February 16, 2007 become a wholly owned subsidiary of MIV Therapeutics Inc. | |||||||||
The firm was situated and operated from 136-B, Surat Special Economic Zone, G.I.D.C., Sachin, Surat - 394230, Gujarat, India which became the registered office of the Company on conversion. | |||||||||
2 | Summary of Significant Accounting Policies | ||||||||
a) | Basis of Presentation | ||||||||
i) | The Financial Statements of Biosync are prepared in accordance with generally accepted accounting principles applicable in the United States ("U.S. GAAP") as at the date of preparation. | ||||||||
ii) | Biosync is set up to deal in designing, manufacturing and marketing coated and non-coated vascular stents and related accessories. However, during the year, the firm was still under development stage and did not have significant operations. Presently, the Holding Company is also under development and its ability to generate revenue is primarily dependent on its success in completing development and obtaining regulatory approvals for the commercialization of its stent technology. The Holding Company's ability to obtain sufficient financing to continue the development of, and if successful, to commence the manufacture and sale of its products under development and if and when approved by the applicable regulatory agencies is uncertain. In view of these conditions, the ability of the holding company and subsequently the subsidiary to continue as a going concern is in substantial doubt and dependent upon achieving a profitable level of operations and on the ability of the Holding Company to obtain necessary financing to fund ongoing operations. Management believes that its current and future plans enable the holding company and correspondingly its subsidiary to continue as a going concern. | ||||||||
b) | Use of Estimates | ||||||||
The preparation of the Financial Statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent liabilities as of the date of the Financial Statements and the reported amount of revenues and expenses during the reported period. Examples of such estimates include: allowance for doubtful debts, and for future obligations under employee benefit plans, valuation allowances for deferred taxes, and useful lives of property, plant and equipment, etc. Actual results could differ materially from these estimates. | |||||||||
F-108
BIOSYNC SCIENTIFIC | |||||||||
(converted into a Private Limited Company, namely Biosync Scientific Private Limited with effect from August 1, 2006) | |||||||||
Notes to the Financial Statements for the period April 1,2005 to December 31, 2005 | |||||||||
c) | Foreign Currency Translation | ||||||||
The accompanying Financial Statements are reported in U.S. dollars ("USD"). The Indian Rupee ("INR") is the functional currency for Biosync. The translation of the functional currencies into USD wherever applicable is performed for assets and liabilities using the exchange rates prevailing at the Balance Sheet date and for revenues, costs and expenses using average exchange rates prevailing during the reporting period. Adjustments resulting from the translation of functional currency financial statements to reporting currency are accumulated and reported as net income / (loss) in the Other Comprehensive Accumulated Income or Loss. | |||||||||
Transactions in foreign currency for reporting functional currency are recorded at the exchange rate prevailing on the date of transaction. Monetary assets and liabilities denominated in foreign currencies are expressed in the functional currency at the exchange rates prevailing at the Balance Sheet date. Revenues, costs and expenses are recorded using exchange rates prevailing on the date of transaction. Gains or losses arising out of fluctuations in the exchange rates are recognized in the profit and loss in the period in which they arise. | |||||||||
d) | Revenue Recognition | ||||||||
The firm recognizes revenues, net of returns, rebates and returns, if any from the sale of the products, at the time when the product is delivered to the customer and/ or dealer. Revenues are recognized only when the firm has transferred to the customer and/ or dealer the significant risks and rewards of ownership of the goods, title to the products transfers, the amount if fixed and determinable, evidence of an agreement exists, there is reasonable assurance of collection of the sale proceeds, the Company has no future obligations and the customer and/ or dealer bears the risk of loss. | |||||||||
Interest income is recognized on a time proportion basis. | |||||||||
Items of income and expenditure are recognized on accrual and prudent basis | |||||||||
e) | Cash and Cash Equivalents | ||||||||
Biosync considers all highly liquid investments with an original maturity or remaining maturity of three months or less at the date of purchase to be cash equivalents. Cash equivalents are stated at cost, which approximates their fair value due to the short maturity of the investments. Cash and claims to cash that are restricted as to withdrawal or use in the ordinary course of business are classified as other receivables under current assets, unless they are to be utilized for other than current operations in which case they are classified as other assets, non-current. | |||||||||
f) | Inventory | ||||||||
Inventories are stated at the lower of cost or replacement cost with respect to raw materials and the lower of cost and net realizable value with respect to finished goods and work in progress. Cost of raw material includes purchase cost plus applicable landing costs. Cost of finished goods and work in progress are determined on a first-in first-out basis and includes direct material, direct labour and overheads. Net realizable value represents the anticipated selling price less estimated costs of completion and distribution. Year-end inventories are as taken, valued and certified by the partners. | |||||||||
g) | Property, Plant and Equipment | ||||||||
Property, Plant and Equipment are stated at cost of acquisition less accumulated depreciation. Cost includes inward freight, duties, non refundable taxes and incidental expenses related to acquisition and installation of fixed asset. | |||||||||
F-109
BIOSYNC SCIENTIFIC | |||||||||
(converted into a Private Limited Company, namely Biosync Scientific Private Limited with effect from August 1, 2006) | |||||||||
Notes to the Financial Statements for the period April 1,2005 to December 31, 2005 | |||||||||
The cost and the accumulated depreciation for property, plant and equipment sold, retired or otherwise disposed off are removed from the stated values and the resulting gains and losses are included in the Income Statement. | |||||||||
Depreciation is provided for using the straight line method over 3 to 14 years. Leasehold improvements are amortized using the straight line method over the estimated useful life of the asset or the terms of lease whichever is shorter. Maintenance and repairs are expensed as incurred. Replacements and betterments are capitalized. | |||||||||
h) | Impairment of Long-lived Assets | ||||||||
Biosync accounts for impairment of long-lived assets in accordance with the provisions of SFAS 144 "Accounting for Impairment or Disposal of Long-Lived Assets". the firm reviews long-lived assets, for impairment whenever events or changes in business circumstances indicate the carrying amount of assets may not be fully recoverable. Each impairment test is based on a comparison of the undiscounted cash flows expected to be generated from the use of the asset to its recorded value. If impairment is indicated, the asset is written down to its fair value. Assets to be disposed are reported at the lower of the carrying value or the fair value less cost to sell. | |||||||||
i) | Employee Benefits | ||||||||
(i) | Leave Encashment | ||||||||
Biosync does not have the policy of encashing outstanding leaves to its employees. | |||||||||
(ii) | Gratuity Plan | ||||||||
Biosync provides for Gratuity as and when paid and does not have the policy of ascertaining and accruing Gratuity liability as at the year end. However, the management is of the opinion that impact on such non provision will not be significant due to very few employees being employed by the firm. Further, such employees had also not completed the statutory time period to be eligible for gratuity. | |||||||||
j) | Income Taxes | ||||||||
In accordance with the provisions of SFAS 109,"Accounting for Income Taxes", income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the Financial Statements carrying amounts of existing assets and liabilities and their respective tax bases and operating loss carry forwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the Income Statement in the period of enactment. Based on management's judgment, the measurement of deferred tax assets is reduced, if necessary, by a valuation allowance for any tax benefits for which it is more likely than not that some portion or all of such benefits will not be realized. | |||||||||
F110
3 | Property, Plant and Equipment and Depreciation | ||||||||
Property, Plant and Equipment at cost less accumulated depreciation consist of: | |||||||||
Particulars |
|
|
|
| As of |
| As of | ||
|
|
|
|
| 31-Dec-05 |
| 31-Mar-05 | ||
|
|
|
|
| USD |
| USD | ||
Leasehold Land | 19,010 | 19,010 | |||||||
Building | 11,146 | 11,146 | |||||||
Plant and Machinery and Equipments | 156,168 | 156,168 | |||||||
Furniture and Fixtures | 480 | - | |||||||
Computers and Accessories | 3,182 | 3,117 | |||||||
Total | 189,986 |
| 189,441 | ||||||
Less: Accumulated Depreciation | (52,376) | (26,573) | |||||||
Property, Plant and Equipment, Net | 137,610 | 162,868 | |||||||
|
|
|
|
|
|
|
| ||
The Firm has established the estimated useful lives of assets for depreciation purposes as follows: | |||||||||
Leasehold Land | Over the period of lease of 15 years | ||||||||
Building | Over the period of lease of 15 years | ||||||||
Plant and machinery | 5 years | ||||||||
Furniture and Fixtures | 5 years | ||||||||
Computer and Accessories | 3 years | ||||||||
Depreciation expenses amounted to USD 25,803 and USD 26,124 for the period ended December 31, 2005 and year ended March 31, 2005 respectively. Since the Firm is unable to identify depreciation against each functional areas of operation and has hence disclosed it separately in the Income Statement. | |||||||||
. | |||||||||
4 | Income Taxes | ||||||||
Biosync benefits from tax incentives provided to units situated in the Special Economic Zone as an exemption from payment of Indian corporate income taxes in a manner as enumerated below: | |||||||||
Exemption Available |
|
|
| % of Taxable | |||||
|
|
|
|
| income Exempt | ||||
For first 5 years |
|
|
| 100% | |||||
Next 6 - 10 years |
|
|
| 50% | |||||
Next 11 - 15 years (subject to fulfillment of certain conditions) |
| ||||||||
Biosync has not recognized deferred income tax assets on account of tax losses,etc. in view of the tax exemption available and continuing till the time the losses are utilised. As regards other temporary differences, no deferred tax assets are recognised in view of the uncertainity of future taxable profits. | |||||||||
5 | Borrowings | ||||||||
i) | Bank Overdrafts | ||||||||
Bank overdraft from the bank is secured by hypothecation of inventories. | |||||||||
F-111
ii) | Interest Bearing Liabilities | |||||||
Particulars |
|
|
|
| As of |
| As of | |
|
|
|
|
| 31-Dec-05 |
| 31-Mar-05 | |
|
|
|
|
| USD |
| USD | |
Term loan from Banks |
|
|
| 106,500 |
| 123,341 | ||
Less: Current Portion |
|
|
| (106,500) |
| (21,151) | ||
Long Term Payable |
|
|
| - |
| 102,190 | ||
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
| |
Term loans from banks are secured by hypothecation of plant and machinery and equitable mortgage of leasehold land and building. | ||||||||
6 | Related Party Transactions | |||||||
Particulars |
|
|
|
| for the |
| for the | |
|
|
|
|
| year ended |
| year ended | |
|
|
|
|
| 31-Dec-05 |
| 31-Mar-05 | |
|
|
|
|
| USD |
| USD | |
|
|
|
|
|
|
|
| |
Sales to Biosync Medical |
| 1,077 |
| 1,057 | ||||
|
|
|
|
|
|
|
| |
Remuneration/ Interest to Partners |
| 6,692 |
| 6,602 | ||||
|
|
|
|
|
|
|
| |
Included in Trade Receivables |
|
|
|
|
| |||
Receivable from Biosync Medical |
|
| 43 |
| 1,085 | |||
|
|
|
|
|
|
|
| |
Balance in Partners Capital Account |
|
|
| |||||
Mr. Pratap Ram Surve |
|
|
| (3,175) |
| 0,765 | ||
Mr. Viral Laljibhai Vaishnav |
|
| 4,357 |
| 16,946 | |||
Mrs. Vinita Rajesh Vaishnav |
|
| (26,445) |
| 31,039 | |||
Mr. Jayantilal Limbabhai Naria |
|
| 12,944 |
| 25,994 | |||
Total |
|
|
|
| (12,320) |
| 84,744 | |
|
|
|
|
|
|
|
| |
7 | Contingencies and Commitments | |||||||
i) | Operating Lease | |||||||
Biosync had non-cancellable operating lease for land of 15 years from the date of agreement on completion of lease period, the lease could be extended on mutually agreed terms. The lease for land of USD 19,010, has been capitalized in the books of the firm which is depreciated over a period of lease. | ||||||||
8 | Concentration of Risk | |||||||
The firm is in its set up stage and does not have significant transactions. The firm monitors the creditworthiness of its customers to which it grants credit terms in the normal course of business. The following table gives details in respect of revenues generated from top two customers are: | ||||||||
Particulars |
|
|
|
| for the |
| for the | |
|
|
|
|
| year ended |
| year ended | |
|
|
|
|
| 31-Dec-05 |
| 31-Mar-05 | |
|
|
|
|
| USD |
| USD | |
Revenue Generated from Top two Customers are: |
|
|
| |||||
Scitech Medical, Brazil |
|
|
| - |
| 3,874 | ||
Scitech Products Medicos Ltd, Brazil |
| 6,000 |
| - | ||||
Doust Yaad Saeed Co Ltd, Iran |
|
| - |
| 1,450 | |||
Sentez Ic Ve Dis Tic Ltd, Sti |
| 19,325 |
| - | ||||
Biosync Medical |
|
|
| 1,077 |
| 1,057 | ||
Total Revenue Generated | 56,401 |
| 6,381 | |||||
|
|
|
|
|
|
|
| |
F-112
9 | Financial Instruments: Fair Value | |||||||
The carrying amounts reported in the balance sheets for cash and cash equivalents, trade receivables, other current assets, bank overdraft, current portion of interest bearing liabilities, accounts payable, accrued expenses and other liabilities approximate their respective fair values due to their short maturity. | ||||||||
10 | Schedule to Balance Sheet | |||||||
| Particulars |
|
|
|
| As of |
| As of |
|
|
|
|
|
| 31-Dec-05 |
| 31-Mar-05 |
|
|
|
|
|
| USD |
| USD |
| Cash and Cash Equivalents |
|
|
|
| |||
| Cash on Hand |
| 6,430 |
| 40 | |||
| Fixed Deposit with Bank |
|
| - |
| 640 | ||
| Cash and Cash Equivalents |
| 6,430 |
| 680 | |||
|
|
|
|
|
|
|
|
|
| Other Current Assets |
|
|
|
|
| ||
| Advance to suppliers |
|
|
| 3,786 |
| - | |
| Other Current Assets |
|
| 23,786 |
| - | ||
|
|
|
|
|
|
|
|
|
| Other Assets |
|
|
|
|
| ||
| Fixed Deposits with Banks |
| 620 |
| - | |||
| Other Deposits |
|
|
| 553 |
| 571 | |
| Other Assets |
|
| 1,173 |
| 571 | ||
|
| |||||||
11 | The previous year figures are regrouped/ rearranged to conform with the presentation in the current period. | |||||||
12 | Figures of the previous year are not comparable with figures for the current period since the current period covers period of nine months ending on December 31, 2005 | |||||||
. | ||||||||
F-113
MIV THERAPEUTICS INC
(A development stage company)
Proforma Consolidated Financial Statements
1. Basis of Presentation
The following unaudited pro forma combined financial statements are based on our historical consolidated financial statements and Biosync Scientific Pvt. Ltd's historical financial statements, each included in this Registration Statement,and adjusted to give effect to the February 16, 2007 Biosync acquisition. The unaudited pro forma combined statements of operations for the six months ended November 30, 2006 and the twelve months ended May 31, 2006 give effect to the Biosync acquisition as if it had occurred on June 1, 2005.
Because the differences in fiscal years, the pro forma Consolidated Statements of Operations for the six months ended November 30, 2006 include the results for Biosync for the nine months ended December 31, 2006 adjusted to exclude the Biosync's financial data for the three months ended June 30, 2006. These pro forma consolidated financial statements are not necessarily indicative of the actual results which would have been attained had the combination been in effect on the date indicated or may be attained in the future.
2. Acquisition of Biosync Scientific Pvt. Ltd.
On February 16, 2007, the Company completed the acquisition of all of the issued and outstanding shares of Biosync, a body corporate subsisting under and registered pursuant to the laws of India and is presently engaged in the business of designing, manufacturing and marketing coated and non-coated vascular stents and related accessories.
Under the terms of the agreement in principle dated October 17, 2006, and the amending agreement dated February 16, 2007 (collectively the "Agreement"), in consideration for the acquisition of the shares of Biosync, the Company issued 50,000 shares of the Company's common stock with an estimated fair value of $33,000, 750,000 shares with an estimated fair value of $495,000 issued on receiving CE Mark License and paid $500,000 in cash to the vendors. As a further condition of the Agreement, the Company was required to satisfy any and all bank indebtedness of Biosync.
3. Pro Forma Adjustments
(a) These unaudited pro forma consolidated statements of operations reflect the acquisition of the net assets of Biosync by MIV as though the acquisition occurred on June 1, 2005. The cost of the acquisition is as follows:
Cash | $ 500,000 |
800,000 shares of restricted common stock valued at $0.66 per share | 528,000 |
Assumption of bank indebtedness | 908,351 |
Acquisition costs | 7,534 |
$1,943,885 |
F-114
The net assets acquired were:
Cash and cash equivalents | $ 17,557 |
Prepaid expenses and other current assets | 74,740 |
Inventory | 244,802 |
Property and equipment | 793,197 |
CE Mark License | 1,421,283 |
2,551,579 | |
Accounts payable and other current liabilities | (161,824) |
Advances from MIV Therapeutics Inc. | (121,870) |
Deferred income tax liability | (324,000) |
Fair value of net assets acquired | $1,943,885 |
(b) The CE Mark License is being amortized on a straight-line basis over their estimated useful life of 10 years. The amortization expense recognized in the Proforma Statement of Operations for the years ended May 31, 2006 and the period ended November 30, 2006 is $142,128 and $71, 064, respectively.
F-115
MIV THERAPEUTICS INC. | |||||||||
(A development stage company) | |||||||||
Proforma Consolidated Statement of Operations | |||||||||
(Expressed in US dollars) | |||||||||
(Basis of Presentation - Note 1) | |||||||||
Period ended November 30, 2006 | |||||||||
|
| MIV | Biosync | ||||||
Therapeutics | Scientific | ||||||||
6 months | 6 months | ||||||||
Ended | Ended | Proforma | Proforma | ||||||
Nov. 30, 2006 | Dec. 31, 2006 | Adjustment | Consolidated | ||||||
Revenue | $ | - | $ | 21,771 | $ | - | $ | 21,771 | |
Cost of sales |
| - |
| 303,337 |
| -- |
|
| 303,337 |
Gross profit | - | (281,566) | - | (281,566) | |||||
Expenses | |||||||||
General and administrative | 1,879,973 | 101,922 | 1,981,895 | ||||||
Research and development | 1,606,387 | 1,606,387 | |||||||
Stock-based compensation | 592,169 | 592,169 | |||||||
Depreciation and amortization | 51,001 | 24,445 | 71,064 | (1) | 146,510 | ||||
Interest expense and finance fees | 2,059 | 25,571 | 27,630 | ||||||
|
| 4,131,589 |
| 151,938 |
| 71,064 |
|
| 4,354,591 |
Loss from operations | (4,131,589) | (433,504) | (71,064) | (4,636,157) | |||||
Loss on write-off of partnership accounts | (236,631) | (236,631) | |||||||
Interest income | 9,198 | 1,425 | 10,623 | ||||||
Gain (loss) on foreign exchange |
| (22,596) |
|
|
|
|
|
| (22,596) |
Loss before income taxes | (4,144,987) |
| (668,710) |
| (71,064) |
|
| (4,884,761) | |
Deferred income tax recovery | - | - | 16,000 | (2) | 16,000 | ||||
Net loss |
| (4,144,987) |
| (668,710) |
| (55,064) |
|
| (4,868,761) |
|
|
|
|
|
|
|
|
|
|
Loss per common share | |||||||||
- basic and diluted | $ | (0.06) | $ |
|
|
|
| $ | (0.07) |
Weighted average number of common | |||||||||
shares outstanding | |||||||||
- basic and diluted |
| 70,377,408 |
|
|
| 800,000 |
|
| 71,177,408 |
F-116
MIV THERAPEUTICS INC. | |||||||||
(A development stage company) | |||||||||
Proforma Consolidated Statement of Operations | |||||||||
(Expressed in US dollars) | |||||||||
(Basis of Presentation - Note 1) | |||||||||
Year ended May 31, 2006 | |||||||||
|
| MIV | Biosync | ||||||
Therapeutics | Scientific | ||||||||
Year ended | Year ended | Proforma | Proforma | ||||||
May 31, 2006 | March 31, 2006 | Adjustment | Consolidated | ||||||
Revenue | $ | $ | 174,185 | $ | $ | 174,185 | |||
Cost of sales |
|
|
| 73,771 |
|
|
|
| 73,771 |
Gross profit | 0 | 100,414 | 0 | 100,414 | |||||
Expenses | |||||||||
General and administrative | 5,149,369 | 7,773 | 5,157,142 | ||||||
Research and development | 2,702,651 | 2,702,651 | |||||||
Stock-based compensation | 1,079,143 | 1,079,143 | |||||||
Depreciation and amortization | 143,754 | 34,689 | 142,128 | (1) | 320,571 | ||||
Interest expense and finance fees | 87,037 | 26,800 | 113,837 | ||||||
|
| 9,161,954 |
| 69,262 |
| 142,128 |
|
| 9,373,344 |
Loss from operations | (9,161,954) | 31,152 | (142,128) | (9,272,930) | |||||
Gain on extinguishment of debt | 0 | ||||||||
Interest income | 82,511 | 909 | 83,420 | ||||||
Gain (loss) on foreign exchange |
| (15,392) |
|
|
|
|
|
| (15,392) |
Loss before income taxes | (9,094,835) |
| 32,061 |
| (142,128) |
|
| (9,204,902) | |
Deferred income tax recovery | - | - | 32,000 | (2) | 32,000 | ||||
Net loss |
| (9,094,835) |
| 32,061 |
| (110,128) |
|
| (9,172,902) |
|
|
|
|
|
|
|
|
|
|
Loss per common share | |||||||||
- basic and diluted | $ | (0.14) | $ |
|
|
|
| $ | (0.14) |
Weighted average number of common | |||||||||
shares outstanding | |||||||||
- basic and diluted |
| 63,454,536 |
|
|
| 800,000 |
|
| 64,254,536 |
F-117
MIV THERAPEUTICS INC
(A development stage company)
Proforma Consolidated Financial Statements
Notes to Proforma Financial Statement Adjustments
The amortization expense, relating to the CE Mark License, recognized in the Proforma Statement of Operations for the year ended May 31, 2006 and the period ended November 30, 2006 is $142,128 and $71, 064, respectively.
The deferred income tax recovery recognized in the Proforma Statement of Operations for the year ended May 31, 2006 and the period ended November 30, 2006 is $32,000 and $16,000, respectively.
F-118