UNITED STATES SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 20-F
| | | |
| o | | REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 |
or
| | | |
| þ | | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For The Fiscal Year Ended October 31, 2005
or
| | | |
| o | | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
or
| | | |
| o | | SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Date of event requiring this shell report
Commission File No. 0-26005 MICROMEM TECHNOLOGIES INC.
(Exact name of Registrant as specified in its charter)
Ontario, Canada
(Jurisdiction of incorporation or organization)
777 Bay Street, Suite 1910,
Toronto, Ontario M5G 2E4, Canada
Tel: (416) 364-6513
Fax: (416) 360-4034
(Address of principal executive offices)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
None
Securities registered or to be registered pursuant to Section 12(g) of the Act:
Common Shares without par value
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act:
None
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as
of the close of the period covered by the annual report:
64,719,449 Common Shares
Indicate by check mark if the registrant is a well-known seasoned issuer as defined in Rule 405 of the Securities Act:
Yeso Noþ
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file a report
pursuant to section 13 or 15 of the Securities Exchange Act of 1934:
Yeso Noþ
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days:
Yesþ Noo
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer and large accelerator filer” in Rule 12b-2 of the Exchange Act:
| | | | | |
| Large Accelerated Filero | | Accelerated Filero | | Non-Accelerated Filerþ |
Indicate by check mark which financial statement item the Registrant has elected to follow:
Item 17þ Item 18o
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act):
Yeso Noþ
PART I
INTRODUCTION
Abbreviations
Throughout this document, Micromem Technologies Inc. and/or its affiliates are referred to as “Micromem”, the “company”, “we”, “us” or “our”.
Forward Looking and Cautionary Statements
This Form 20-F contains certain forward-looking statements. These forward-looking statements are based on current expectations, estimates and projections about the business of our company and the industry in which we operate, our management’s beliefs, and assumptions made by our management. Words such as “expects,” “anticipates,” “intends,” “plans,” “believes,” “seeks” and “estimates,” variations on such words and similar expressions are intended to identify such forward-looking statements. These statements are not guarantees of future performance and involve certain risks, uncertainties and assumptions which are difficult to predict. Our actual results could differ materially from those expressed or forecasted in these forward-looking statements as a result of certain factors, including those set forth under “Description of Business” and elsewhere in this Form 20-F.
ITEM 1. IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISORS
Not Applicable.
ITEM 2. OFFER STATISTICS AND EXPECTED TIMETABLE
Not Applicable.
ITEM 3. KEY INFORMATION
A. Selected Financial Data
The following table sets forth our selected consolidated financial data in United States dollars as of and for each of the five fiscal years ended October 31, 2005, 2004, 2003, 2002 and 2001. The selected consolidated financial data has been derived from our audited consolidated financial statements. All information contained in the following table should be read in conjunction with our audited consolidated financial statements and the notes thereto and “Item 5 — Operating and Financial Review and Prospects”, included elsewhere in this Form 20-F.
Selected balance sheet information
| | | | | | | | | | | | | | | | | | | | | |
| Years ended October 31, |
| | | 2005 | | 2004 | | 2003 | | 2002 | | 2001 |
| |
| Working capital (deficiency) | | ($ | 74,831 | ) | | $ | 34,685 | | | $ | 100,670 | | | $ | 985,551 | | | $ | 3,455,108 | |
| Capital Assets | | | — | | | | 2,925 | | | | 3,768 | | | | 98,654 | | | | 336,839 | |
| Total Assets | | | 728,375 | | | | 474,234 | | | | 350,138 | | | | 1,583,422 | | | | 14,454,470 | |
| Capital Stock | | | 34,305,087 | | | | 32,103,787 | | | | 31,236,287 | | | | 31,073,787 | | | | 29,642,242 | |
| Shareholders’ equity (deficiency) | | | (74,831 | ) | | | 37,610 | | | | 104,438 | | | | 1,391,903 | | | | 14,124,918 | |
1
Selected statement of operations and deficit information
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | Years ended October 31, | | | | |
| | | 2005 | | 2004 | | 2003 | | 2002 | | 2001 |
| |
| Interest and other income | | $ | 8,703 | | | $ | 4,746 | | | $ | 20,121 | | | $ | 165,892 | | | $ | 196,520 | |
| |
| Research and development expenses | | | 362,141 | | | | 378,410 | | | | 490,914 | | | | 1,601,624 | | | | 2,212,679 | |
| General and administrative expenses | | | 1,951,600 | | | | 570,684 | | | | 621,050 | | | | 1,319,528 | | | | 2,492,386 | |
| Stock compensation expense | | | 1,721,742 | | | | 1,379,970 | | | | 318,000 | | | | 1,832,500 | | | | 4,627,752 | |
| Write-down of royalty rights | | | — | | | | — | | | | — | | | | 10,000,000 | | | | — | |
| Write-down of patents and trademarks | | | — | | | | — | | | | 299,820 | | | | — | | | | — | |
| Loss (gain) on disposal of assets | | | — | | | | — | | | | 58,302 | | | | 13,292 | | | | (6,134 | ) |
| |
| Loss from continuing operations | | | (4,035,483 | ) | | | (2,314,298 | ) | | | (1,767,965 | ) | | | (14,529,978 | ) | | | (9,157,163 | ) |
| Loss from discontinued operations | | | — | | | | — | | | | — | | | | — | | | | — | |
| |
| Loss before income taxes | | | (4,035,483 | ) | | | (2,314,298 | ) | | | (1,767,965 | ) | | | (14,529,978 | ) | | | (9,157,163 | ) |
| Provision for income taxes (recovery) | | | — | | | | — | | | | — | | | | (35,537 | ) | | | 30,214 | |
| |
| Net loss | | | (4,035,483 | ) | | | (2,314,298 | ) | | | (1,767,965 | ) | | | (14,565,515 | ) | | | (9,187,377 | ) |
| |
| Loss per share-basic and diluted | | | 0.07 | | | | 0.04 | | | | 0.04 | | | | 0.31 | | | | 0.21 | |
| Weighted average number of basic and diluted shares | | | 62,155,234 | | | | 52,958,975 | | | | 47,061,810 | | | | 46,396,799 | | | | 44,163,669 | |
| |
| Dividends | | | — | | | | — | | | | — | | | | — | | | | — | |
| |
Reconciliation between Canadian GAAP and U.S. GAAP:
Our consolidated financial statements have been prepared in accordance with Canadian GAAP which, in our case, conforms in all material respects with U.S. GAAP.
Currency and Exchange Rates
Our financial statements are all expressed in United States dollars. All other financial data appearing in this Form 20-F are expressed in United States dollars with the exception of certain limited cases when reference is made to instruments denominated in Canadian dollars (“CDN $”).
Transactions that were conducted in Canadian dollars or other foreign currencies have been converted into United States dollars using the average monthly rate of exchange per quarter which rate approximates the rate of exchange prevailing at the date of such transactions. Assets and liabilities denominated in Canadian dollars or other foreign currencies but expressed in this Form 20-F in United States dollars have been converted into United States dollars at the rate of exchange prevailing on the date of the applicable financial statement.
The following table sets forth, for the periods indicated, the high, low, end of period and average for period noon buying rates as published by the Bank of Canada, as expressed in the amount of U.S. Dollars equal to one Canadian dollar.
| | | | | | | | | | | | | | | | | | | | | |
| | | 2005 | | 2004 | | 2003 | | 2002 | | 2001 |
| High for period | | | 0.8690 | | | | 0.8432 | | | | 0.7738 | | | | 0.6654 | | | | 0.6711 | |
| Low for period | | | 0.7872 | | | | 0.7288 | | | | 0.6350 | | | | 0.6179 | | | | 0.6293 | |
| End of period | | | 0.8577 | | | | 0.8319 | | | | 0.7738 | | | | 0.6339 | | | | 0.6294 | |
| Average for period | | | 0.8254 | | | | 0.7717 | | | | 0.7200 | | | | 0.6368 | | | | 0.6493 | |
2
The following table sets forth, for each period indicated, the high and low exchange rates for United States dollars expressed in Canadian dollars on the last day of each month during such period, based on the Noon Buying Rate.
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | November | | December | | January | | February | | March | | April |
| | | 2004 | | 2004 | | 2005 | | 2005 | | 2005 | | 2005 |
| High | | | 1.1911 | | | | 1.2055 | | | | 1.2425 | | | | 1.2343 | | | | 1.2147 | | | | 1.2618 | |
| Low | | | 1.1848 | | | | 1.1970 | | | | 1.2361 | | | | 1.2277 | | | | 1.2085 | | | | 1.2482 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | May | | June | | July | | August | | September | | October |
| | | 2005 | | 2005 | | 2005 | | 2005 | | 2005 | | 2005 |
| High | | | 1.2619 | | | | 1.2330 | | | | 1.2310 | | | | 1.1935 | | | | 1.1682 | | | | 1.1836 | |
| Low | | | 1.2506 | | | | 1.2238 | | | | 1.2220 | | | | 1.1836 | | | | 1.1588 | | | | 1.1752 | |
On January 30, 2006, the noon buying rate for one Canadian dollar, as quoted by the Bank of Canada, was CDN $1.1405 = U.S. $1.00.
B. Capitalization and Indebtedness
Not Applicable.
C. Reasons for the Offer and Use of Proceeds
Not Applicable.
D. Risk Factors
We and our investors face a number of significant risks, which are described below.
Risk Factors Related to Our Business
The financial statements of our company have been prepared on a going concern basis.
We have prepared our financial statements on a “going concern” basis which presumes that we will be able to realize our assets and discharge our liabilities in the normal course of business for the foreseeable future.
We are still in the development stage and have incurred substantial losses to date. We must raise additional funds for the continued development, testing and commercial exploitation of our technologies. The sources of these funds have not yet been identified and there can be no certainty that sources will be available in the future.
At October 31, 2005 we had approximately $640,000 cash on hand and our current monthly cash expenses were approximately $100,000. Subsequent to October 31, 2005 we have raised an additional $165,000 through the exercise of stock options.
Our ability to continue as a going concern is dependent upon completing the development of our technology for a particular application, achieving profitable operations, obtaining additional financing and successfully bringing our technologies to the market. The outcome of these matters cannot be predicted at this time. Our consolidated financial statements have been prepared on a going concern basis and do not
3
include any adjustments to the amounts and classifications of the assets and liabilities that might be necessary should we be unable to continue in business.
If the going concern assumption was not appropriate for our financial statements then adjustments would be necessary in the carrying value of assets and liabilities, the reported expenses and the balance sheet classifications used.
We currently have no operating revenue.
We have no revenues and there is no certainty that we will generate revenues in the near future. If we fail to enter into license agreements we will have no revenues. If we enter into such agreements the amount of the revenues we receive will depend on the terms we are able to get from each licensee and the ability of each licensee to compete in their particular market.
Our technology is under development.
Our Magnetic Random Access Memory, also referred to herein as MRAM, which is a non-volatile memory technology that uses magnetic, thin film elements on a silicon substrate to store information, is currently under development and is therefore not yet proven to be commercially viable. As such, we are unsure if our development efforts will succeed and, accordingly, significant development work remains to be completed.
In the event our technology is developed, we will face competition when we are ready to sell or license our products. We will be required to introduce our technology into a well-developed market and compete with major corporations who manufacture, sell and license existing memory products such as DRAM, SRAM, EPROM, EEPROM and Flash memory. The market for memory technologies is dominated by major corporations who have established market segments for their memory technologies and products. These corporations have significantly greater financial resources which are required to design, develop, manufacture, market, sell and license their products and technologies. Many of these major corporations have worldwide wafer manufacturing and integrated circuit production facilities.
Our success will be determined by the following factors which have not yet been tested or measured:
| | • | | the ability of manufacturers to incorporate the technology into existing manufacturing capabilities without significant retooling and material costs; |
| |
| | • | | price competitiveness; and |
| |
| | • | | the availability and costs of raw materials. |
After completion of the development of our technology, our ability to compete successfully will depend on elements outside of our control, including the rate at which customers incorporate our technology into their products, the success of such customers in selling those products, our protection of our intellectual property, the number, nature and success of our competitors and their product introductions and general market and economic conditions. In addition, our success will depend on our ability to develop, introduce, and license or sell in a timely manner our technology or products incorporating our technology and to compete effectively on the basis of factors such as speed, density, die size and packaging.
Products using our technology have not yet been manufactured.
Our success depends on whether our technology can be manufactured in large quantities at competitive prices. Our failure to manufacture large quantities at competitive prices will seriously hurt our ability to generate revenues.
4
Our competitors are seeking to develop other magnetic based memory technologies.
MRAM as a market segment is both crowded and competitive. We understand that other companies have research and development efforts under way in connection with non-volatile random access memory, also referred to herein as RAM. Much work is being done in the MRAM research and development at companies such as NVE, Cypress, Freescale, Phillips, Motorola and others. Other research and development efforts at IBM, Hewlett Packard and Nantero are focused on non-magnetic based non-volatile RAM. While these companies may be considered our competitors, their focus is on high density RAM applications. As we anticipate introducing our product in the less competitive, low density applications market, we believe our more direct competitors are Honeywell, Naval Research Laboratories, Ramtron and NVE. All of these companies have substantial resources at their disposal.
We may be materially affected by aggressive competition as the memory and data storage industry is highly competitive and customers make their decisions based on a number of competitive factors, including functionality, technology, performance, reliability, system scalability, price, quality, product availability, customer service and brand recognition. We must address each of these factors effectively in order to successfully compete.
Failure to secure continued financing will cause our business to suffer.
Since there is no assurance that revenues will be realized in the near future, we will need additional financing to continue our research and development and to successfully market our technology to potential licensees. While we have had sufficient funds thus far to meet our requirements, there is no assurance we will be able to continue to do so and failure to raise sufficient funds in the future will affect our ability to develop and market our technology.
Because much of our success and value depends on our ownership and use of intellectual property, our failure to protect our property could adversely affect our future growth and success.
Our success will depend on our ability to protect our intellectual property. We rely primarily on patent, copyright, trademark and trade secret laws, as well as nondisclosure agreements and other methods to protect our proprietary technology and processes. Despite our efforts to do so, unauthorized parties may attempt to copy or otherwise obtain and use our products or technology, develop similar technology independently or design around our patents. Policing unauthorized use of our products is expensive and difficult, and we cannot be certain that the steps we have taken will prevent misappropriation or infringement of our intellectual property.
Intellectual property claims against us, no matter how groundless, could cause our business to suffer.
Our future success and competitive position depend in part on our ability to retain exclusive rights to our technology, including any improvements that may be made on that technology from time to time by us or on our behalf. While our technology is patented or is subject to pending patent applications in the U.S.A. and we know of no challenge that has been made either against our technology or our rights to it, and we have no reason to believe that any such challenge might be made or that the grounds for any such challenge exist, if any intellectual property litigation were to be commenced against us, no matter how groundless, the result could be a significant expense to us, adversely affecting further development, licensing and sales, diverting the efforts of our technical and management personnel and, in the event of an adverse outcome, damages and possible restrictions on the further development, licensing and use of our technology.
There is no assurance that any of our pending patent applications will be issued as patents or that any issued patent will not be determined to be invalid at a later date.
5
We have a history of losses, and we may continue to generate losses in the foreseeable future.
To date, we have been solely a development company. We have not been profitable in any of the last three fiscal years. Unless and until we are able to successfully complete the development of our technology and develop markets for the commercialization of such technology, we may not be able to generate revenues in future periods and we may not be able to attain profitability.
The development of non-volatile rapid access memory products is a capital intensive business. Therefore, we expect to incur expenses without corresponding revenues at least until we are able to license our technology to third parties. This may result in net operating losses, which will increase continuously until we can generate an acceptable level of revenues, which we may never attain. Further, even if we do achieve operating revenues, there can be no assurance that such revenues will be sufficient to fund continuing operations. Therefore, we cannot predict whether we will ever be able to achieve profitability.
The likelihood of success of our business plan must be considered in light of the problems, expenses, difficulties, complications and delays frequently encountered in connection with developing and expanding early stage businesses and the competitive environment in which we operate.
We lack manufacturing capacity and will be dependent on third party manufacturers.
Our success will depend upon our ability to manufacture our technology in large quantities and at competitive prices. We have no in-house manufacturing capacity and do not anticipate developing such capacity. To the extent we are successful in completing the development of our technology we will likely be required to rely upon contract manufacturers to produce our products. We may not be able to enter into manufacturing arrangements on terms that are favorable to us. Moreover, there is no assurance that any future manufacturers will have the capability to manufacture our products in sufficient quantities to achieve profitability and within the quality, price, and technical standards required by our customers. In addition, because our technologies use semi-conducting materials other than silicon, there may be a limited number of contract manufacturers capable of producing our products since most are focusing on silicon-based manufacturing. If any future manufacturers should cease doing business with us or experience delays, shortages of supply or excessive demands on their capacity, we may not be able to obtain adequate quantities of product in a timely manner, or at all. Manufacturing new products involves integrating complex designs and processes, coordinating with suppliers for parts and components, and managing manufacturing capacities to accommodate forecasted demand. Failure to obtain sufficient quantities of parts and components, as well as other manufacturing delays or constraints, could adversely affect the timing of new product introductions. Any manufacturing problem or the loss of a contract manufacturer could be disruptive to our operations and result in lost sales.
We will be dependent upon the success of a limited range of products.
The range of products we intend to commercialize is currently limited to applications of non-volatile random access memory technologies. Reliance on a limited range of products could restrict our ability to respond to adverse business conditions. If we are not successful in developing this specific technology, or if there is not adequate demand for such technology or the market for such technology develops less rapidly than we anticipate, we may not have the capability to shift our resources to the development of alternative products. In such case our business would likely be at a significant disadvantage to other competitors in the field. As a result, the limited range of products we intend to develop could limit our revenues and profitability.
We may not realize income from the licensing of our technologies if our licensees fail to commercialize the products that incorporate these technologies.
In order to generate revenues from our MRAM technology, we will likely need to enter into licensing arrangements with third parties who can integrate our technology into products that will gain acceptance in the market. We have not yet entered into any licensing agreements, and there is no assurance
6
that we will be able to do so on acceptable terms or at all. To the extent we are successful in licensing our technology, in general we will seek upfront payments plus ongoing royalties based on anticipated commercial sales of the products into which our technology is incorporated. Our ability to realize royalties will thus depend upon the successful manufacture and commercialization of such products, which will be primarily within the control of the licensee. There is no assurance that any eventual licensees’ products will be technologically viable, nor that such licensees will be successful in marketing and selling such products. In addition, licensees could decide to delay or discontinue the commercialization of products for financial or other business reasons. Even if our licensees succeed in developing products that incorporate our technology, in all likelihood a significant amount of research, development and testing will be required before such products can be introduced to market. Therefore we may not receive royalty income for a substantial period following the commencement of any licensing arrangements. If our licensees are unable to commercialize products on a timely basis, they may lose market share to competing or alternative technologies. Any failure by the companies to which we license our technologies to successfully develop marketable products would have an adverse affect on our future royalty payments and financial condition.
Our supply of future products could be dependent upon relationships with key suppliers.
We will be reliant on third parties to supply the raw materials needed to manufacture our future products. Any reliance on suppliers may involve several risks, including a potential inability to obtain critical materials and reduced control over production costs, delivery schedules, reliability and quality. Any unanticipated disruption to future contract manufacture caused by problems at suppliers could delay shipment of products, increase our cost of goods sold and result in lost sales.
In order to commercialize our future products, we will need to establish a sales and marketing capability.
At present, we do not have any sales or marketing capability since our technology is currently in the development stage. However if we are successful in completing our development efforts, we will need to add marketing and sales personnel who have expertise in the computer technology business. We must also develop the necessary supporting distribution channels. Although we believe we can build the required infrastructure, we may not be successful in doing so if we cannot attract personnel or generate sufficient capital to fund these efforts. Failure to establish a sales force and distribution network would have a material adverse effect on our ability to grow our business.
The rights to certain of our patented technologies are shared with a third party.
Our core technology includes a memory design with the magnetic bit aligned vertically to the substrate, also referred to herein as our VEMRAM technology, and a memory design with the magnetic bit aligned horizontally to the substrate, also referred to herein as our HEMRAM technology. We acquired ownership of certain patents and patent applications covering the VEMRAM and HEMRAM technologies, as well as certain related rights, pursuant to an Asset Purchase Agreement dated as of December 10, 2000 with Estancia Limited, also referred to herein as Estancia. However, under the terms of the Asset Purchase Agreement we have been required to convey back to Estancia a 40% undivided interest in the VEMRAM and HEMRAM patents, as well as the right to participate in gross profits and royalties from the license or sale of such patents. This participation right requires us to pay to Estancia 32% of (i) the gross profit, less expenses to be agreed by the parties, for each license of the patents sold or otherwise transferred by us and (ii) all royalties received by us as a result of the license or sale of the patents less reasonable expenses directly related to the obtaining of such royalties.
We will be reliant upon contractual rights to use certain technologies that are material to our business.
Certain technologies material to our business are being developed through collaborative arrangements with the University of Toronto. We have entered into a number of successive Research Collaboration Agreements with the University of Toronto under which research and development programs have been led by a University research team. We have provided funding, equipment and background
7
technology to these projects. Certain Canadian governmental entities are also parties to these agreements and have provided additional funding. The University of Toronto has ownership rights to all intellectual property developed under these programs. We have no ownership rights but have the right to obtain exclusive, world-wide and perpetual sub-licenses from the governmental participants to use such intellectual property; the governmental participants in turn have the right to obtain an exclusive, world-wide license to such technology directly from the University of Toronto.
Our auditors have identified significant deficiencies in our internal accounting controls.
We operate as a development stage company and have historically had only limited accounting personnel and resources with which to address our internal control procedures.
In 2005, we engaged an independent firm of chartered accountants to complete an in depth review of our internal accounting procedures and controls and are now in the process of implementing the recommendations which were made. We have upgraded our accounting software and have developed formalized budgets in 2005. The Audit Committee has met on a quarterly basis to assess our financial performance and to review the progress management has made in upgrading its accounting procedures and controls. The Audit Committee has also interacted with our external auditors in 2005 on reporting and control related matters.
When our auditors audited our financial statements as of and for the year ended October 31, 2005 they identified significant deficiencies in our internal accounting controls. Significant deficiencies noted were that we lacked certain formalized accounting policies and procedures including written procedures for the monthly, quarterly and annual closing of our financial books and records, our staff was not always subject to timely review and supervision and security practices over our information technology were not sufficiently robust.
Our management is implementing measures required to remedy these deficiencies in our internal controls but has not managed to make these changes effective in their entirety. These control deficiencies are not expected to have any future material impact on our financial statements. If, however, we fail to continue to adequately staff our accounting and finance function and maintain adequate internal controls, we may not meet the demands that are placed upon us as a public company, including the requirements of the Sarbanes-Oxley Act of 2002 and our business would accordingly face repercussions. We expect to implement all measures required to remedy the material weaknesses and reportable controls by the end of fiscal 2006.
Risk Factors Related to Our Common Shares
Our stock is subject to the penny stock regulations, which may discourage brokers from effecting transactions in the stock and adversely affect the stock’s market price and liquidity.
Our common shares constitute “penny stock” under applicable regulations of the Securities and Exchange Commission. Penny stock is defined as shares of stock that (a) are issued by a company that has less than $5,000,000 in net tangible assets and has been in business less than three years, by a company that has less than $2,000,000 in net tangible assets and has been in business for more than three years, or by a company that has average revenues of less than $6,000,000 for the last three years; (b) have a market price of less than $5 per share; and (c) are not quoted on the NASDAQ National Stock Market or listed on a U.S. stock exchange. The penny stock regulations impose significant restrictions on brokers who sell penny stock to persons other than established customers and institutional accredited investors. Broker-dealers participating in sales of our stock will be subject to the so called “penny stock” regulations covered by Rule 15g-9 under the Securities Exchange Act of 1934, as amended, also referred to herein as the Exchange Act). Under the rule, broker-dealers must furnish to all investors in penny stocks a risk disclosure document required by the rule, make a special suitability determination of the purchaser and have received the purchaser’s written agreement to the transaction prior to the sale. In order to approve a person’s account for a transaction in penny stock, the broker or dealer must (i) obtain information concerning the
8
person’s financial situation, investment experience and investment objectives; (ii) reasonably determine, based on the information required by paragraph (i) that the transactions in penny stocks are suitable for the person and that the person has sufficient knowledge and experience in financial matters that the person reasonably may be expected to be capable of evaluating the risks of transactions in penny stock; and (iii) deliver to the person a written statement setting forth the basis on which the broker or dealer made the determination required by paragraph (ii) in this section, stating in a highlighted format that it is unlawful for the broker or dealer to effect a transaction in a penny stock unless the broker or dealer has received, prior to the transaction, a written agreement to the transaction from the person; and stating in a highlighted format immediately preceding the customer signature line that the broker or dealer is required to provide the person with the written statement and the person should not sign and return the written statement to the broker or dealer if it does not accurately reflect the person’s financial situation, investment experience and investment objectives, and obtain from the person a manually signed and dated copy of the written statement. Our common shares are subject to the penny stock regulations, which may discourage brokers from effecting transactions in the common shares. This would decrease market liquidity, adversely affect market price and make it difficult for you to use the common shares as collateral.
The rights of our shareholders may differ from the rights typically afforded to shareholders of a U.S. corporation.
We are incorporated under the Business Corporations Act (Ontario), also referred to herein as the Business Corporations Act (Ontario). The rights of holders of our common shares are governed the laws of the Province of Ontario, including the Business Corporations Act (Ontario), by the applicable laws of Canada, and by our Articles of Incorporation and all amendments thereto, also referred to herein as the Articles, and our By-laws. These rights differ in certain respects from the rights of shareholders in typical U.S. corporations. The principal differences include without limitation the following:
Under the Business Corporations Act (Ontario), we have a lien on any common share registered in the name of a shareholder or the shareholder’s legal representative for any debt owed by the shareholder to us. Under U.S. state law, corporations generally are not entitled to any such statutory liens in respect of debts owed by shareholders.
With regard to certain matters, we must obtain approval of our shareholders by way of at least 662¤3% of the votes cast at a meeting of shareholders duly called for such purpose being cast in favor of the proposed matter. Such matters include without limitation: (a) the sale, lease or exchange of all or substantially all of our assets out of the ordinary course of our business; and (b) any amendments to our Articles including, but not limited to, amendments affecting our capital structure such as the creation of new classes of shares, changing any rights, privileges, restrictions or conditions in respect of our shares, or changing the number of issued or authorized shares, as well as amendments changing the minimum or maximum number of directors set forth in the Articles. Under U.S. state law, the sale, lease, exchange or other disposition of all or substantially all of the assets of a corporation generally requires approval by a majority of the outstanding shares, although in some cases approval by a higher percentage of the outstanding shares may be required. In addition, under U.S. state law the vote of a majority of the shares is generally sufficient to amend a company’s certificate of incorporation, including amendments affecting capital structure or the number of directors. Under certain circumstances the board of directors may also have the ability to change the number of directors under U.S. state law.
Pursuant to our By-laws, two persons present in person or represented by proxy and each entitled to vote thereat shall constitute a quorum for the transaction of business at any meeting of shareholders. Under U.S. state law, a quorum generally requires the presence in person or by proxy of a specified percentage of the shares entitled to vote at a meeting, and such percentage is generally not less than one-third of the number of shares entitled to vote.
Under rules of the Ontario Securities Commission, a meeting of shareholders must be called for consideration and approval of certain transactions between a corporation and any “related party” (as defined in such rules). A “related party” is defined to include, among other parties, directors and senior officers of a corporation, holders of more than 10% of the voting securities of a corporation, persons
9
owning a block of securities that is otherwise sufficient to affect materially the control of the corporation, and other persons that manage or direct, to a substantial degree, the affairs or operations of the corporation. At such shareholders’ meeting, votes cast by any related party who holds common shares and has an interest in the transaction may not be counted for the purposes of determining whether the minimum number of required votes have been cast in favor of the transaction. Under U.S. state law, a transaction between a corporation and one or more of its officers or directors can generally be approved either by the shareholders or a by majority of the directors who do not have an interest in the transaction. Corporations that are listed on a U.S. securities exchange or are quoted on Nasdaq may also be required to have transactions with officers and directors and other related party transactions reviewed by an audit committee comprised of independent directors.
There is no limitation imposed by our articles or other charter documents on the right of a non-resident to hold or vote our common shares. However, the Investment Canada Act , also referred to herein as the Investment Act, as amended by the World Trade Organization Agreement Implementation Act, also referred to herein as the WTOA Act, generally prohibits implementation of a reviewable investment by an individual, government or agency thereof, corporation, partnership, trust or joint venture that is not a “Canadian,” as defined in the Investment Act, unless, after review, the minister responsible for the Investment Act is satisfied that the investment is likely to be a net benefit to Canada. An investment in our common shares by a non-Canadian would be reviewable under the Investment Act if it were an investment to acquire direct control of Micromem, and the value of our assets were CDN $5.0 million or more. However, an investment in our shares by a national of a country (other than Canada) that is a member of the World Trade Organization or has a right of permanent residence in such a country (or by a corporation or other entity that is a “WTO Investor-controlled entity” pursuant to detailed rules set out in the Investment Act) would be reviewable at a higher threshold of CDN $223 million in assets, except for certain economic sectors with respect to which the lower threshold would apply. A non-Canadian, whether a national of a WTO member or otherwise, would acquire control of Micromem for purposes of the Investment Act if he or she acquired a majority of our common shares. The acquisition of less than a majority, but at least one-third of our common shares, would also be presumed to be an acquisition of control of Micromem, unless it could be established that Micromem was not controlled in fact by the acquirer through the ownership of voting shares. The United States is a WTO Member for purposes of the Investment Act. Certain transactions involving our common shares would be exempt from the Investment Act, including:
| | • | | an acquisition of our common shares if the acquisition were made in connection with the person’s business as a trader or dealer in securities; |
| |
| | • | | an acquisition of control of Micromem in connection with the realization of a security interest granted for a loan or other financial assistance and not for any purpose related to the provisions of the Investment Act; and |
| |
| | • | | an acquisition of control of Micromem by reason of an amalgamation, merger, consolidation or corporate reorganization, following which the ultimate direct or indirect control of Micromem, through the ownership of voting interests, remains unchanged. Under U.S. law, except in limited circumstances, restrictions generally are not imposed on the ability of non-residents to hold a controlling interest in a U.S. corporation. |
U.S. shareholders may not be able to enforce civil liabilities against us.
Micromem is incorporated under the laws of the Province of Ontario. Additionally, a number of our directors and executive officers are non-residents of the U.S., and all or a substantial portion of the assets of such persons are located outside the U.S. As a result, should any investor commence an action in the U.S. against Micromem or its directors or executive officers, Micromem or its directors or officers, as the case may be, may be able to insist that any action against them take place in the jurisdiction of the Province of Ontario. In addition, if an investor were to obtain a U.S. judgment against Micromem or its directors or executive officers, there is doubt as to the enforceability of such U.S. judgment in Canada.
10
We do not anticipate paying dividends.
We have never paid a dividend on our securities and we do not anticipate paying dividends in the foreseeable future.
We may need to issue additional securities which may cause our shareholders to experience dilution.
Our Board of Directors has the authority to issue additional common shares, without par value, also referred to herein as the common shares, or other of our securities without the prior consent or vote of our shareholders. The issuance of additional common shares would dilute the proportionate equity interest and voting power of our shareholders.
We depend on key personnel.
Our senior managers and employees are Salvatore Fuda, who serves as the Chairman of the Board of Directors, Joseph Fuda, who serves as our Chief Executive Officer, and Dr. Cynthia Kuper who serves as our Chief Technology Officer. Dr. Harry Ruda, and a number of researchers forming the team that he oversees, are key technical personnel engaged pursuant to research collaboration agreements between us and the University of Toronto. Our success depends on our ability to retain certain of our senior management and key technical personnel and our ability to attract and retain additional highly skilled personnel in the future.
We may be materially affected by global economic and political conditions.
Our ability to generate revenue may be adversely affected by uncertainty in the global economy and could also be affected by unstable global political conditions. Terrorist attacks or acts of war could significantly disrupt our operations and the operations of our future customers, suppliers, distributors, or resellers. We cannot predict the potential impact on our financial condition or our results of operations should such events occur.
We may be materially affected by rapid technological change and evolving industry standards.
Short product life cycles are inherent in high-technology companies due to rapid technological change and evolving industry standards. Our future financial condition and results of operations depend on our ability to respond effectively to these changes. We cannot provide any assurance that we will be able to successfully develop, manufacture, and market innovative new products or adapt our current products to new technologies or new industry standards. In addition, our customers may be reluctant to adopt new technologies and standards or they may prefer competing technologies and standards. Because the technology market changes so rapidly, it is difficult for us to predict the rate adoption of our MRAM technology.
��We may be materially affected by risks associated with new product development.
Our new product research and development is complex and requires us to investigate and evaluate multiple alternatives, as well as plan the design and manufacture of those alternatives selected for further development. Our research and development efforts could be adversely affected by hardware and software design flaws, product development delays, changes in data storage technology, changes in operating systems and changes in industry standards.
The manufacturing of new products involves integrating complex designs and processes, coordinating with suppliers for parts and components and managing manufacturing capacities to accommodate forecasted demand. Our failure to obtain sufficient quantities of parts and components or other manufacturing delays and constraints could adversely affect our ability to timely introduce new products.
11
Our operations may be materially affected by the risks associated with developing and protecting intellectual property.
We cannot provide any assurance that we will be able to continue to develop new intellectual property or that we will continue to have it developed for us.
We rely on a combination of U.S. patent, copyright, trademark, and trade secret laws to protect our intellectual property rights. Due to financial constraints, we have decided to not file patent and trademark registration applications with foreign governments and this may expose our technologies to infringement in foreign jurisdictions.
We enter into confidentiality agreements relating to our intellectual property with our employees and consultants.
Despite all of our efforts to protect our intellectual property rights, unauthorized parties may attempt to copy or otherwise obtain or use our intellectual property. Monitoring the unauthorized use of our intellectual property is difficult and we cannot provide assurance that we will be able to adequately protect our intellectual property in the future.
We may be materially affected if we are unable to attract, retain and motivate key employees.
Our future success depends, in large part, on our ability to attract, retain and motivate key employees. We face significant competition for individuals who possess the skills required to design, develop, manufacture, and market our technologies. An inability to successfully attract, retain, and motivate these employees in the future could have an adverse effect on our future operating and financial performance.
The price of our common shares and volume of our common shares may be volatile.
Our shareholders may be unable to sell a significant number of our common shares on the NASD OTC-BB without a significant reduction in the price of the shares.
Furthermore, there can be no assurance that we will be able to meet the listing requirements of, or achieve listing on, any other stock exchange. The market price of the common shares may be affected significantly by factors such as fluctuations in our operating results, announcements of technological innovations or new products by us or our competitors, action by governmental agencies against us or the industry in general, developments with respect to patents or proprietary rights, public concern as to the safety of products developed by us or others, the interest of investors, traders and others in public companies such as ours and general market conditions. In recent years, the securities markets in the United States and Canada have experienced a high level of price and volume volatility, and the market price of securities of many companies, particularly small capitalization companies, have experienced fluctuations which have not necessarily been related to the operating performance, underlying asset values or business prospects of such companies.
There are foreign exchange risks associated with our company.
Because we have historically raised funding in U.S. dollars, and a significant portion of our costs are denominated in Canadian dollars, our funding is subject to foreign exchange risks. A decrease in the value of the U.S. dollar relative to the Canadian dollar could affect our costs and potential future profitability. We do not currently hold forward exchange contracts or other hedging instruments to exchange foreign currencies for U.S. dollars to offset potential currency rate fluctuations.
12
We will attempt to file a registration statement in connection with unit private placement financings completed in fiscal 2005.
We were successful in securing financing totaling $1,472,500 through Unit Private Placements in fiscal 2005 at prices ranging from $0.60 to $0.75 per Unit. These Units were structured as a common share with a Class A warrant which entitled the investor to acquire an additional common share at the same price. The Class A warrant would expire 12 months after issuance if unexercised. If the Class A warrant was exercised a Class B warrant with identical entitlements would be issued.
We filed a Registration Statement with respect to Unit Private Placements during fiscal 2005. However, we decided to withdraw such Registration Statement prior to October 31, 2005. Our Board of Directors has since approved of the restructuring of the Unit Private Placements as follows:
| | • | | The Unit will consist of a common share and a Class A and Class B warrant. |
| |
| | • | | The Class A warrant will expire on June 30, 2006. |
| |
| | • | | The Class B warrant will expire on December 31, 2006. |
All of the other terms and conditions of the Unit Private Placements remain unchanged.
We are committed to file, on a best efforts basis, a Registration Statement prior to June 30, 2006 relating to the revised securities. There can be no guarantee that the Registration Statement will be declared effective by the Securities and Exchange Commission when filed. If not, the Class A and Class B warrants will have trading restrictions imposed for a period of at least 12 months from issue date.
ITEM 4. INFORMATION ON THE COMPANY
A. History and Development of Our Company
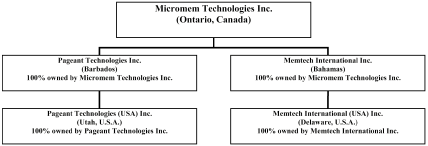
Micromem is a corporation formed under the laws of the Province of Ontario, Canada, with principal executive offices at 777 Bay Street, Suite 1910, Toronto, Ontario M5G 2E4 (416.364.6513). Micromem was incorporated on October 21, 1985 as Mine Lake Minerals Inc. We subsequently changed our name to Avanti Capital Corp. on June 23, 1988, to AvantiCorp International Inc. on April 30, 1992 and to Micromem Technologies Inc. on January 11, 1999 in connection with our acquisition of Pageant Technologies Incorporated, also referred to herein as Pageant International. Our website address iswww.micromeminc.com. The information on our website is not part of this annual report on Form 20-F. We have included our website address in this document as an inactive textual reference only.
Purchase of Pageant International
On January 11, 1999, we completed the acquisition of 100% of the capital stock of Pageant Technologies Inc. (“Pageant International”), in exchange for 32,000,000 of our common shares and warrants for the purchase of an additional 1,000,000 of our common shares, also referred to herein as the warrants, representing 88.94% of the outstanding common shares of Micromem (89.24% assuming exercise of all the shares underlying the warrants). The acquisition was completed pursuant to an agreement, dated December 7, 1998, amongst Micromem, as the purchaser, and Ataraxia Corp., as the vendor, and Pageant International. The warrants were exercisable at CDN $2.00 per share through January 11, 2000 and thereafter at CDN $2.30 per share through January 12, 2001 on which date the warrants remaining unexercised expired. The total purchase price for the Pageant International common stock was $30,000,000. The value of our common shares used to pay the purchase price was determined through “arm’s length” negotiations using as a point of reference the price per common share of $1.16 on September 23, 1998 less an agreed upon discount.
13
The primary asset of Pageant International was an undivided 50% interest in patents issued in the United States and Europe, with a corresponding application in Japan, for nonvolatile random access memory technology which was, at the time, called MAGRAM and which is, today, called VEMRAM, referred to herein as the VEMRAM patents. The VEMRAM technology also includes certain improvements to the VEMRAM patents, some of which are the subject of pending patent applications. The undivided 50% interest in the VEMRAM patents was originally conveyed to Ataraxia Corp. by Estancia Limited, a company incorporated under the laws of the Turks & Caicos Islands, pursuant to a Joint Ownership and Licensing Agreement dated September 17, 1997, referred to herein as the Joint Ownership and Licensing Agreement, which Ataraxia Corp. assigned to Pageant International on October 22, 1997 with the written consent of Estancia Limited. In addition to the undivided 50% interest in the VEMRAM patents, under the Joint Ownership and Licensing Agreement, Pageant International was granted an exclusive worldwide license to develop, manufacture and sell the VEMRAM technology. The Joint Ownership and Licensing Agreement required Pageant International to pay Estancia Limited, or its nominee, a royalty of 40% of the gross profits less expenses agreed by the parties for each technology license sold. Additionally, Pageant International would be required to pay Estancia Limited 40% of any per unit royalty received by Pageant International less properly documented reasonable expenses directly related to the obtaining of those royalties and as agreed by the parties in writing.
The Joint Ownership and Licensing Agreement also provided that if Pageant International, as approved assignee of Ataraxia Corp., sold the rights to the VEMRAM technology to a third party not owned or controlled by it, it would have to pay Estancia Limited 50% of the proceeds from such transaction. In the event of the sale of all of the VEMRAM technology rights, 50% of the proceeds would be assigned to Estancia Limited, reflecting its 50% undivided interest in the technology, rather than 40% reflecting its royalty rights under the VEMRAM License. Estancia Limited is controlled by John Zammit. Mr. Zammit has no direct control relationship with us, and our management is not aware of the existence of an indirect control relationship between us and Ataraxia Corp and Mr. Zammit.
The acquisition of Pageant International was treated as a reverse takeover for accounting purposes. In the case of the purchase of Pageant International by Micromem, the two former shareholders of Pageant International ended up with a greater number of voting shares than did the pre-acquisition of our shareholders and therefore were deemed to have apparent control. Our consolidated financial statements are presented as a continuation of the financial position and results of Pageant International, even though we remain the legal parent and Pageant International remains the legal subsidiary. The primary consequence of the application of this treatment to the acquisition is that the patent rights were recorded at $100 on the date of the reverse takeover, the historical value at which they were carried on the Pageant International balance sheet.
Agreement to Purchase Estancia Limited Interests
On December 10, 2000, we and Pageant International entered into an Asset Purchase Agreement with Estancia Limited and Richard M. Lienau to purchase from Estancia Limited and Mr. Lienau all interests in the VEMRAM patents and the VEMRAM technology and all other rights, interests and entitlements held by Estancia Limited and Mr. Lienau as set forth in the Joint Ownership and Licensing Agreement, also referred to herein as the estancia limited interests.
Under the Asset Purchase Agreement, which closed on March 9, 2001, Pageant International agreed to pay a purchase price of $50.0 million to Estancia Limited, as follows:
| | • | | $10.0 million was paid on closing, in the form of 2,007,831 of our common shares (or $8.0 million worth of our common shares based on the close price on the closing date, being $3.98 per share) and $2.0 million in cash; |
| |
| | • | | $20.0 million, paid if and when either: (a) certification was received from Honeywell Federal Manufacturing & Technologies that fully integrated, randomly addressable memory matrices of VEMRAM technology met certain stipulated performance standards, or (b) Micromem or |
14
| | | | any of its affiliates executed a definitive agreement for the sale or licensing of the VEMRAM technology to an “arm’s length” third party for any commercial purposes other than testing or evaluation of the technology. The $20 million was payable in the form of cash and common shares to be determined by Pageant International provided that a minimum of 50% of the $20.0 million shall be paid in our common shares valued at the close of trading on the date of such certification receipt, sale or licensing; and |
| |
| | • | | $20.0 million, paid if and when we or any of our affiliates executed a definitive agreement for the sale or licensing of any technology (including the VEMRAM technology) owned by us to an “arm’s length” third party for any commercial purposes other than testing or evaluation of the technology; payable in the form of cash and common shares to be determined by Pageant International provided that a minimum of 50% of the $20.0 million shall be paid in common shares valued at the close of trading on the date of execution of the license. |
Notwithstanding the provisions triggering the two $20.0 million payments, also referred to herein as the triggering events, Pageant International had the right to pay any portion or all of the purchase price at any time following the March 9, 2001 closing date, through a combination of cash and common shares, provided that a minimum of 50% of such payment was through the issuance of common shares.
At the closing of the Asset Purchase Agreement, on March 9, 2001, Estancia Limited was also required to and, they did in fact enter into a three year technology development agreement with Pageant International for the continued services of Mr. Lienau towards the development of VEMRAM technology, also referred to as the Technology Development Agreement. The Technology Development Agreement provided for fees to Estancia Limited of $215,000 per annum and a budget for the continued development of VEMRAM of up to $500,000 per annum. Effective April 23, 2002, the Technology Development Agreement was amended to extend the term of the agreement for an additional eight months commencing March 9, 2004 through to November 9, 2004, and to provide a reduction of the monthly fees payable to Estancia Limited to $107,500 during the periods from June 1 to December, 2002, and during the extended period.
Because neither of the triggering events occurred prior to March 9, 2004, Pageant International was deemed to have conveyed a 40% undivided interest in the VEMRAM patents and granted a 32% gross profits royalty (as described below), to Estancia Limited, where the proportionate interest was calculated as the ratio of the portion of the purchase price remaining unpaid (being $40.0 million) at such date over $50.0 million. Consequently, Pageant International owns a 60% interest in the VEMRAM patents and is required to pay Estancia Limited royalties equal to 32% of: (a) the gross profit, less expenses agreed to by the parties, for each license of the VEMRAM patents sold or otherwise transferred by Pageant International; and (b) the amount of any unit royalty received by Pageant International as a result of the license or sale of the VEMRAM patents less reasonable expenses directly related to the obtaining of said royalties.
Changes to Our Board of Directors and Management
On January 11, 1999, immediately following our acquisition of Pageant International, Stephen Fleming, who had been serving as President and Chief Executive Officer of Pageant Technologies (USA) Inc., was elected to our Board of Directors to join Salvatore Fuda and Ross McGroarty, who had served as Directors since 1992 and 1988, respectively. The Board of Directors then elected Mr. Fuda as Chairman of the Board of Directors, Mr. Fleming as President and Chief Executive Officer, and Mr. McGroarty, who had been serving as our President, to serve as our Executive Vice President and Secretary. Subsequently, on March 18, 1999, Robert Patterson, who had been serving as Chairman of the Board of Directors and Vice President of Corporate Development of Pageant Technologies (USA) Inc., was elected to serve as our President and Chief Executive Officer to replace Mr. Fleming. On November 15, 1999, Antonio Lopes was appointed Chief Financial Officer. On June 15, 2000, Mr. McGroarty resigned as Executive Vice-President and Secretary.
At our annual meeting of shareholders held on June 29, 2000, the following individuals were elected to serve on our Board of Directors: Andrew Brandt, Stephen Fleming, Salvatore Fuda, Charles
15
Harnick and George Kennedy. The terms of all previously elected directors ended on such date. The term of Mr. Lopes’ employment as our Chief Financial Officer ended on June 5, 2000 and Mr. Raj Viswanathan was then appointed to serve as our Chief Financial Officer. The term of Robert Patterson’s employment as our President and Chief Executive Officer having ended, Salvatore Fuda was reappointed as Chairman of the Board of Directors and he was appointed to serve as our Chief Executive Officer on June 29, 2000. Also on June 29, 2000, Manoj Pundit was appointed to serve as our Executive Vice-President and General Counsel. On February 21, 2001, Dr. Dale Hensley was appointed to serve as Chief Operating Officer of both Micromem and Pageant Technologies (USA) Inc.
At our annual meeting of shareholders held on March 14, 2001, Andrew Brandt, Stephen Fleming, Salvatore Fuda, Charles Harnick, George Kennedy, Manoj Pundit and David Sharpless were elected to serve on our Board of Directors.
At a meeting of our Board of Directors held on March 14, 2001, a resolution was passed appointing Dale Hensley as a director of Micromem. At a meeting of our Board of Directors held on February 13, 2002, our Board of Directors accepted the resignation of Salvatore Fuda as President and Chief Executive Officer of Micromem due to health reasons. Salvatore Fuda continues to serve as our Chairman of the Board of Directors. In his place, the Board of Directors appointed Joseph Fuda to serve as our Director and as President and Chief Executive Officer. Messrs. Andrew Brandt, Stephen Fleming, Salvatore Fuda, Charles Harnick, Dale Hensley, George Kennedy, Manoj Pundit, David Sharpless and Stephen Van Fleet were elected as Directors of Micromem at the annual meeting held April 30, 2002.
Dr. Dale Hensley’s employment as our Chief Operating Officer was terminated effective as of September 24, 2002. Raj Viswanathan resigned as our Chief Financial Officer on October 31, 2002 and as a Director of Pageant Technologies (USA) Inc. on September 26, 2002. Dr. Hensley resigned as a Director of Pageant Technologies (USA) Inc. on September 26, 2002 and as a Director of Micromem effective October 9, 2002. Antonio Lopes was reappointed as our Chief Financial Officer on November 1, 2002.
At our annual meeting of shareholders held on June 6, 2003, Salvatore Fuda, Stephen Fleming, Charles Harnick, George Kennedy, Andrew Brandt, Manoj Pundit, Joseph Fuda, David Sharpless and Steven Van Fleet were elected to serve on our Board of Directors.
At our annual meeting of shareholders held on June 25, 2004, Salvatore Fuda, Stephen Fleming, Charles Harnick, George Kennedy, Andrew Brandt, Manoj Pundit, Joseph Fuda, David Sharpless and Steven Van Fleet were elected to serve on our Board of Directors. Dan Amadori replaced Antonio Lopes as our Chief Financial Officer on the annual meeting date.
Dr. Cynthia Kuper joined us as Chief Technology Officer on January 28, 2005 after serving as acting Chief Technology Officer since September 2004. At our annual meeting of shareholders held on June 27, 2005, Salvatore Fuda, Stephen Fleming, Charles Harnick, George Kennedy, Andrew Brandt, Manoj Pundit, Joseph Fuda, David Sharpless and Steven Van Fleet were elected to serve on our Board of Directors. Messrs. Salvatore Fuda, Joseph Fuda, Manoj Pundit, Dan Amadori and Ms. Cynthia Kuper continue to hold their respective offices as described above at the date of this Form 20-F.
On November 12, 2005 Mr. Larry Blue was appointed to serve on our Board of Directors.
B. Business Overview
We are engaged in the development of memory technology having the characteristic of non-volatility, which is the ability to retain information after power has been shut off. Our technology is based on our ability to use magnetic materials in combination with a sensor to record the “state of magnetization.” Each magnetic element stores one bit of data based on its ability to alternate between states of magnetic polarization, which states are determined by a sensor. Our technology represents “1”s and “0”s by the different polarization of magnets. For example, a magnet oriented north/south is a “1” and a magnet
16
oriented south/north is a “0”. The magnetic field strength and direction do not decay when power is switched off, and, therefore, the memory is non-volatile.
In June 2002, our management determined that our research and development required restructuring due to financial constraints, in order to continue the development of magnetic non-volatile memory technology. Accordingly, we commenced restructuring our operations in July 2002 to achieve an orderly transition of our research and development program. As part of this restructuring, Pageant Technologies (USA) Inc. closed its Lee’s Summit, Missouri facility by August 31, 2002 and we downsized our head office in Toronto as of October 31, 2002. Both we and Pageant Technologies (USA) Inc. terminated Dr. Dale Hensley as Chief Operating Officer effective as of September 24, 2002. Pageant Technologies (USA) Inc. terminated all of its other employees by October 31, 2002. Dr. Hensley resigned from our Board of Directors on October 9, 2002 and as a Director of Pageant Technologies (USA) Inc. on September 26, 2002. In September 2002, Pageant Technologies (USA) Inc. terminated its agreement with Townsend Capital Inc. pursuant to which Pageant Technologies (USA) Inc. occupied the offices, laboratory and clean room it had occupied at Summit Technology Campus, Lee’s Summit, Missouri.
Until August 2002, we were pursuing the development of our two unique memory technologies:
| | • | | a memory design with the magnetic bit aligned horizontally to the substrate, known as HEMRAM; and |
| |
| | • | | a memory design with the magnetic bit aligned vertically to the substrate, known as VEMRAM. |
Since July 2002, we have participated in further magnetic memory technology research and development pursuant to our collaboration with the University of Toronto and Dr. Harry Ruda. We have a portfolio of patents and patent applications protecting the technology that we are developing.
Industry Background
The semiconductor memory industry is principally driven by the requirements of the computing industry. The nature of the memory manufacturing industry is that it is capital intensive, cyclical, rapidly changing and it depends significantly on patent protection.
The semiconductor industry is intensely competitive. Both low-density and high-density nonvolatile memory products are manufactured and marketed by major corporations possessing worldwide wafer manufacturing and integrated circuit production facilities and by specialized product companies.
Our Company’s Technology
The various characteristics of the our technology can be better understood by describing the three basic types of memory used in present day computers, Random Access Memory (RAM), Read Only Memory (ROM), and secondary storage devices such as floppy and hard disks. The three types of memory are described below:
Random Access Memory (RAM) is memory that can be both read and written randomly, which means that its storage locations can be accessed in any order. Thus, a computer using RAM can find and go directly to the selected location rather than performing a sequential search. Semiconductor RAM is usually the primary memory associated with the computer’scentral processing unit (CPU), the computational unit of the computer responsible for interpreting and executing instructions. However, RAM isvolatilewhich means that all stored information vanishes once the power supply is removed and must be restored from a secondary storage device each time the power is resumed.
Two typical examples of RAM are Dynamic Random Access Memory and Static Random Access Memory.Dynamic Random Access Memory (DRAM)uses integrated circuits containing capacitors to achieve significant storage capacity and speed. DRAM can be written and read in the speed range of less
17
than 100 nanoseconds. DRAM has a major drawback in that its capacitors lose their charge over time and therefore information contained in DRAM must be continually refreshed. This means that, on average, DRAM must stop operations every 16-30 milliseconds and restore all of the data it contains or the data will disappear. During this refresh time, the processor has no access to the information being refreshed.Static Random Access Memory (SRAM)differs from DRAM in that it stores information in a logic circuit referred to as a flip-flop, rather than in a capacitor. SRAM memory does not need to be refreshed while the power is on, but also loses its information once the power is turned off.
Read Only Memory (ROM), like RAM, can be read randomly, but cannot be written randomly. Unlike RAM, however, it is non-volatile and therefore does not lose its information when a computer’s power is cut off. ROM is typically employed to store vital program information required during the first moments after a computer is powered on. It may be used for such purposes as forcing system test routines, directing the processor to input/output devices or for controlling access to certain computer subsystems such as hard drives.EPROMs (erasable programmable read-only memory)andEEPROMs (electrically erasable read-only memory)are read only memories that can be erased and rewritten, but must be written “en masse,” rather than at the individual word level. “Flash” memory is a form of EEPROM that is widely used today in such devices as cell phones, modems and personal digital assistants. The drawbacks to Flash memory are that write times are slower, the number of read/write cycles are limited and there is a requirement for significantly higher power to store data.
Secondary Storage Devices includeFloppy Disks, whichare light and portable and are written and read by a motor driven mechanical drive. They normally have a storage capacity in the low megabyte range.Hard Disksfar exceed floppy disks in storage capacity and have become the standard for mass storage of data to be written and read by the processor. Both Floppy Disks and Hard Disks are non-volatile and can be both written and read. However, since they are serial (as opposed to parallel) devices, they are considerably slower than RAM.
Our technology combines the use of semi-conducting ferromagnetic metals with a sensor. When the magnetization of the magnetic material changes direction, the sensor senses the change in direction and records a “0” or “1”. In this fashion, a bit is created that is non-volatile and based on magnetic properties. We are developing this form of magnetic random access memory for low density applications, such as RFID, that can benefit from non-volatile data storage.
Competition
We are aware of others conducting research and development in the magnetic non-volatile memory area. These include IBM Research (San Jose, California), Ovonyx, Inc. (Troy, Michigan), Hewlett-Packard (Palo Alto, California), Honeywell (Plymouth, Minnesota) and Motorola (Phoenix, Arizona).
Two main centers of MRAM research are at IBM and Infineon. IBM and Infineon have alternative MRAM technologies based on the giant magneto resistance principle. This giant magneto resistance principle primarily consists of two ferromagnetic layers separated by a conductive nonmagnetic interlayer. The electrical resistance is high in the absence of an external magnetic field. However, an applied external field forces the initially anti-parallel magnetization in the coupled films into parallel alignment and the resistance drops. The high or low resistance determines the data storage state. Magnetic tunnel junction cells, as they are known, have similar sandwiched structures but the interlayer is insulating instead of conducting. In contrast to giant magneto resistance structures, in which the sense current flows parallel to the layers, the current in magnetic tunnel junction flows perpendicularly to the layers of the stack.
Neither the current stages of development of these companies’ non-volatile technologies nor their projected development completion dates are known with a high degree of certainty.
18
Recent Developments—Research and Development and Collaboration with the University of Toronto and Ontario Centres of Excellence Inc.
On October 24, 2002, we entered into a two-year research collaboration agreement, also referred to herein as the U of T Research Collaboration Agreement, with Materials and Manufacturing Ontario, the University of Toronto and Dr. Harry Ruda, Chair Professor in Nanotechnology. Through the collaboration, we have continued our involvement in the research and development of magnetic memory technology, carried out by a highly skilled research team headed and assembled by Dr. Ruda. Under the agreement, we and Materials and Manufacturing Ontario have each provided CDN $272,000 of funding and a combined CDN $544,000 was used to cover the operating expenses of the research collaboration over a term of two years. Under the agreement, we maintain our ownership of our portfolio of patents and intellectual property to date.
On November 1, 2002, we entered into an Infrastructure Agreement with the University of Toronto to fund the assembly of a facility for research and development and fabrication of magnetic memory which involves the storage of memory using magnetization. Under the terms of the agreement, we agreed to contribute $249,463 (CDN $360,000) in cash to fund the direct costs of magnetic memory files. The payment schedule to the University of Toronto was as follows:
| | • | | $83,154 (CDN $120,000) on execution of agreement, which was paid; |
| |
| | • | | $83,154 (CDN $120,000) at end of the two months following November 1, 2002, which was paid; and |
| |
| | • | | $83,155 (CDN $120,000) at end of the four months following the November 1, 2002, which was paid. |
On December 10, 2002, we entered into a Collaborative Research Agreement with Communications and Information Technology Ontario, the University of Toronto and Dr. Harry Ruda. Under the terms of the agreement, over a period of two years, we are required to contribute $63,750 (CDN $92,000) in cash and $67,632 (CDN $97,600) of in-kind contribution. Communications and Information Technology Ontario was required to contribute $215,430 (CDN $308,000) for research into “High Density Magnetic Memory Device Development”. In consideration of such contribution, Communications and Information Technology Ontario received a royalty based on revenues received from the sale of products incorporating intellectual property developed under this collaboration agreement for the remaining life of patents issued in connection with such intellectual property.
On March 1, 2003, we entered into an Equipment Transfer Agreement with the University of Toronto. Under the terms of this agreement, we conveyed equipment having an estimated value of $200,000 (CDN $297,600) to the University of Toronto for incorporation into the University’s magnetic memory facility for the research and development and fabrication of magnetic memory.
On November 12, 2003, we entered into a second two-year research collaboration agreement, also referred to herein as the Second U of T Research Agreement, with Materials and Manufacturing Ontario and the University of Toronto. Through the collaboration, we continued our involvement in the research and development of magnetic memory technology carried out by a highly skilled research team headed by Dr. Harry Ruda. Under this agreement, we committed to providing $56,130 (CDN $81,000) per year in cash and $30,770 (CDN $44,400) per year of in kind contributions. Materials and Manufacturing Ontario committed to providing $58,900 (CDN $85,000) in cash. The combined cash contributions of both us and Materials and Manufacturing Ontario of $230,060 (CDN $332,000), was designated to cover the operating expenses of the research collaboration over a term of two years. Materials and Manufacturing Ontario’s funding of $117,800 (CDN $170,000) is paid directly to the University of Toronto. Under the agreement, we maintain our ownership of our portfolio of patents and intellectual property that were developed prior to or outside of the scope of the agreement.
19
Each research collaboration agreement contemplates a number of milestones under a comprehensive research plan. The research is carried out from research facilities at the University of Toronto. We will have the first right to an exclusive and perpetual worldwide sub-license for all uses of the technology developed under the collaboration (except that the University of Toronto may use the technology for educational and research purposes). Materials and Manufacturing Ontario and Communications and Information Technology Ontario, as the case may be, were entitled to receive a royalty on our manufacturing revenues from the sale of products incorporating the technology developed under the collaboration. A separate royalty would be negotiated in the future on revenues to be generated by us from sub-licenses of the technology developed under the collaboration.
Each of Materials and Manufacturing Ontario and Communications and Information Technology Ontario is one of four Ontario Centres of Excellence established by the provincial government to promote commercial research partnerships between universities and industry. The Ontario Centres of Excellence program is funded by the Ministry of Enterprise, Opportunity and Innovation and is part of the Provincial government’s $2 billion investment in Ontario’s knowledge economy. On April 1, 2004, Materials and Manufacturing Ontario, Communications and Information Technology Ontario and two other Centres of Excellence (CRESTech and PRO) merged to become OCE Inc., a non-for-profit, member based corporation.
In January 2005, we entered into an employment agreement with Dr. Cynthia Kuper for her services as Chief Technology Officer. The agreement has a term of two years and may be terminated by us at any time with 4 months notice. The remuneration stipulated in the contract is $260,000 per year and 300,000 options, each option enabling her to purchase one common share at $0.80. These options expire if unexercised on March 22, 2007 and have fully vested. In addition, Dr. Kuper was previously granted 100,000 options each of which entitle her to purchase one common share for $0.68 in connection with her role as acting Chief Technology Officer since September 2004. These options expired on December 31, 2005 and were replaced by 100,000 options each of which entitle her to purchase one common share for $0.68 and which will expire if unexercised on January 15, 2007. The Chief Technology Officer oversees all of our research and development and is responsible for the development of a rollout strategy for MRAM technology, for sourcing potential licensees and adopters of MRAM technology and for advising management and the Board of Directors on technology issues and patent strategies.
In June 2005, we signed a license agreement with the University of Toronto and OCE Inc. whereby OCE Inc. released us from all future claims that existed under previous research collaboration and infrastructure agreements, and we acquired the exclusive worldwide license to exploit the related technology developed at the University of Toronto. We committed to a schedule of royalty payments on net revenues from related license revenues subject to a maximum cumulative payment of CDN $500,000. Thereafter we can buy out all remaining obligations under this agreement by the payment of an additional CDN $500,000 or the sum of the prior two years of royalty payments at the time of the buyout.
Recent Developments — Equity Financing Transactions
In August 2003, we completed unit private placements to two private investors. Under the private placements, we received $162,500 as subscription proceeds for the sale and issue of 2,031,250 units. Each unit consists of one common share and one Series A warrant. Each Series A warrant entitles the holder to purchase one common share and one Series B warrant for $0.08 per unit until expiry 12 months from the date of issue. Each Series B warrant entitles the holder to purchase one additional common share for $0.08 per share until expiry 12 months from the date of issue. In August 2004, the investors exercised 2,031,250 Series A warrants and we thus issued 2,031,250 common shares and 2,031,250 Series B warrants and realized proceeds of $162,500. In February 2005, the investors exercised 1,406,250 Series B warrants and we thus issued 1,406,250 common shares and realized proceeds of $112,500. In August 2005, our investors exercised the remaining 625,000 Series B warrants and we thus issued 625,000 common shares and realized proceeds of $50,000.
In December 2003, we completed unit private placements to two Canadian private investors pursuant to prospectus and registration exemptions set forth in applicable securities laws. Under the private
20
placements, we received $33,000 as subscription proceeds for the sale and issue of 300,000 units. Each unit consists of one common share and one Series A warrant. Each Series A warrant entitles the holder to purchase one common share and one Series B warrant for $0.11 per unit until expiry 12 months from the date of issue. Each Series B warrant entitles the holder to purchase one additional common share for $0.11 per share until expiry 12 months from the date of issue. In October 2004, the private investors exercised 200,000 Series A warrants and we thus issued 200,000 common shares and 200,000 Series B warrants and realized proceeds of $22,000. In November 2004 and February 2005 the investors exercised the balance of their Series A and B warrants and we thus issued 400,000 common shares and realized proceeds of $44,000.
In December 2003, we also completed a unit private placement to one Canadian private investor pursuant to prospectus and registration exemptions set forth in applicable securities laws. Under the private placement, we received $40,000 as subscription proceeds for the sale and issue of 500,000 units. Each unit consists of one common share and one Series A warrant. Each Series A warrant entitles the holder to purchase one common share and one Series B warrant for $0.08 per unit until expiry 12 months from the date of issue. Each Series B warrant entitles the holder to purchase one additional common share for $0.08 until expiry 12 months from the date of issue. In June 2004, the private investor exercised the Series A warrants and we thus issued 500,000 common shares and 500,000 Series B warrants and realized proceeds of $40,000. In September 2004, the private investor exercised the Series B warrants and we thus issued 500,000 common shares and realized proceeds of $40,000.
In December 2004, we completed a unit private placement to several U.S. investors pursuant to prospectus and registration exemptions set forth in applicable securities laws. Under the private placement, we received $617,000 as subscription proceeds for the sale and issue of 1,028,344 units. Each unit consists of one common share and one Series A warrant. Each Series A warrant entitles the holder to purchase one common share and one Series B warrant for $0.60 per unit until expiry 12 months from the date of issue. Each Series B warrant entitles the holder to purchase one additional common share for $0.60 per share until expiry 24 months from the date of issue. In December 2005, we revised the terms of this unit private placement. The unit was revised to consist of one common share, one Series A warrant expiring on June 30, 2006 and one Series B warrant expiring on December 31, 2006. The remaining terms of the warrants are unchanged.
In January 2005, we completed a unit private placement to one Canadian investor pursuant to prospectus and registration exemptions set forth in applicable securities laws. Under the private placement, we received $10,500 as subscription proceeds for the sale and issue of 14,000 units. Each unit consists of one common share and one Series A warrant. Each Series A warrant entitles the investor to purchase one common share and one Series B warrant for $0.75 per unit until expiry 12 months from the date of issue. Each Series B warrant entitles the holder to purchase one additional common share for $0.75 per share until expiry 12 months from the date of issue. In December 2005, we revised the terms of this unit private placement. The unit was revised to consist of one common share, one Series A warrant expiring on June 30, 2006 and one Series B warrant expiring on December 31, 2006. The remaining terms of the warrants are unchanged.
In January 2005, we arranged a unit private placement to several investors pursuant to prospectus and registration exemptions set forth in applicable securities laws. Under the private placement we received $845,000 by April 1, 2005 as subscription proceeds for the sale of 1,300,000 units. Each unit consists of one common share and one Series A warrants. Each Series A warrant entitles the holder to purchase one common share and one Series B warrant for $0.65 per unit until expiry 12 months from the issue date. Each Series B warrant entitles the holder to purchase one additional common share for $0.65 per share until expiry 12 months form the date of issue. In December 2005, we revised the terms of this unit private placement. The unit was revised to consist of one common share, one Series A warrant expiring on June 30, 2006 and one Series B warrant expiring on December 31, 2006. The remaining terms of the warrants are unchanged.
21
On June 8, 2005, we entered into a financial advisory services agreement with an “arm’s length” entity and, as consideration, issued a warrant to purchase 1,000,000 of our common shares. The warrant entitles the holder to purchase our common shares at $0.70 per share. The warrant expires on June 8, 2006.
We entered into a second financial advisory services agreement on June 22, 2005 with an “arm’s length” entity and, as consideration, issued a warrant to purchase 800,000 of our common shares. The warrant entitles the holder to purchase our common shares at $0.70 per share. The warrant expires on December 31, 2006.
Patents and Trademarks
We believe that protection of our intellectual property is important to our ability to generate revenues from our technology in the future. We have both issued patents and pending patent applications and also entered into confidentiality and other agreements with third parties and our employees to protect our intellectual property and trade secrets. We intend to continue to actively pursue the protection of our intellectual property. Our management will determine from time to time the jurisdictions where protection will is appropriate. This determination will be based on a number of factors including the state of development of our technology, the importance of a particular market for our technology, the costs of pursuing patent protection in a jurisdiction and our financial position at the time.
Our magnetic memory patent portfolio comprises three separate series of patents and patent applications:
| | • | | those covering technologies developed pursuant to research collaborations with the University of Toronto and OCE Inc.; |
| |
| | • | | those covering VEMRAM technology; and |
| |
| | • | | those covering HEMRAM technology. |
Environmental Matters
We are subject to various environmental protection regulations imposed by the government in the jurisdiction where we conduct our development work. We are not aware of any current or pending environmental protection laws or regulations that would have a material impact on our capital expenditure requirements or competitive position.
C. Organizational Structure
We have a wholly-owned subsidiary, Pageant International, which was incorporated under the laws of the Turks & Caicos Islands and continued to Barbados on May 25, 2001. Pageant International in turn has a wholly-owned subsidiary, Pageant Technologies (USA) Inc., a corporation incorporated in the State of Utah, USA. We have a second wholly-owned subsidiary, Memtech International Inc, incorporated under the laws of the Bahamas, which in turn has a wholly-owned subsidiary, Memtech International (USA) Inc., a corporation incorporated in the State of Delaware, U.S.A.
We had a third wholly-owned subsidiary, Micromem Technologies B.V., incorporated under the laws of the Netherlands on January 16, 2001, which in turn had a subsidiary, Micromem Technologies S.p.A., a corporation incorporated under the laws of Republic of Italy on January 25, 2001. Micromem Technologies S.p.A. was dissolved pursuant to the laws of Italy on December 27, 2002. Micromem Technologies B.V. was dissolved pursuant to the laws of the Netherlands on August 11, 2003.
Pageant International and Pageant USA will be sometimes jointly referred to in this Form 20-F as “Pageant.” Micromem, Pageant International, Pageant Technologies (USA) Inc., Memtech International Inc. and Memtech International (USA) Inc. will be sometimes collectively referred to as Micromem in this
22
Form 20-F. The following chart sets forth the corporate organizational structure at the date of this Form 20-F.
D. Property, Plant and Equipment
We maintain our corporate headquarters in Toronto, Ontario, Canada. We occupy a space consisting of 3,987 square feet of commercial office space pursuant to a lease that expires in 2010.
Our research and development team headed by Dr. Harry Ruda conduct their research and development at facilities at the University of Toronto, which is comprised of equipment, tools, a clean room and laboratory space. All facilities and equipment are owned by the University of Toronto and are being made available to the research and development team under our research collaboration agreements with the University of Toronto and an infrastructure agreement by and between us and the University of Toronto.
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
This Management Discussion and Analysis has been prepared to provide a more substantive discussion on our business and to assist the reader in analyzing the consolidated financial statements for the year ending October 31, 2005. This discussion and analysis of financial condition and results of operations for the years ended October 31, 2005, 2004, 2003, 2002 and 2001 should be read in conjunction with the consolidated financial statements and related notes in this report, which are prepared in accordance with Canadian generally accepted accounting principles (Canadian GAAP) and are stated in United States dollars. These principles are also in conformity in all material respects with United States generally accepted accounting principles (U.S. GAAP) as described in Note 16 to our 2005 consolidated financial statements.
I.OVERVIEW
1. Technology:
We are a development stage company that currently operates in a single segment as a developer of non-volatile magnetic memory technology. Non-volatile memory implies the ability to retain information after power has been shut off. Our technology is based on our ability to use magnetic materials in combination with a sensor to record a state of magnetization.
23
We hired Dr. Cynthia Kuper in January 2005 as Chief Technology Officer to coordinate and oversee all of our research and development efforts, to manage the development of our patents and intellectual property and to initiate discussions with potential joint development partners in the industry.
We have conducted our research in conjunction with several research collaboration partners under formal agreements. These partners include Materials and Manufacturing Ontario and Communications and Information Technology Ontario, which are collectively referred to herein as the Ontario Centres of Excellence, and the University of Toronto.
In June 2005, we signed a new License Agreement with the University of Toronto and the Ontario Centres of Excellence. The details of the License Agreement are outlined later in this document (refer to Section B — Liquidity and Capital Resources, Commitments (D) — Revised License Agreement June 2005)
We believe that there are substantial market opportunities available to commercialize our technology in conjunction with strategic partners, and we are currently pursuing such opportunities. We plan to complete our research initiatives and enter into agreements with strategic partners so as to commercialize our technology under licensing and other arrangements.
2. Operations:
We have a small full-time staff compliment of 7 people including the President, Chief Technology Officer and Chief Financial Officer. The bulk of the research being completed is through our research partners as described above.
Our activities over the past several years have been aimed at pursuing our research initiatives and to continue to raise financing in order to support these initiatives.
3. Financial:
The Management Discussion and Analysis of financial condition and results of operations for the years ended October 31, 1999-2004 have previously been released and should be read in conjunction with the consolidated financial statements and related notes in this report.
Our current status is as follows:
| | a. | | We successfully raised approximately $2.3 million of funding in 2005. |
| |
| | b. | | We report current and cumulative losses that are substantial. We had a working capital deficiency of $74,831 at October 31, 2005 (however $639,863 of the total of $803,206 of accounts payable and accrued liabilities reported at October 31, 2005 are deferred obligations which will not be discharged until we have adequate funding to discharge such obligations). We continue to raise capital in order to fund our operations. |
| |
| | c. | | We reported $0 capital assets as of October 31, 2005 and have no current commitments to acquire additional capital assets. |
| |
| | d. | | We have contingent obligations with respect to potential royalty payments and with respect to our license agreements. |
4. Compliance Matters:
We have limited personnel and historically have identified weaknesses in our internal control and reporting procedures.
24
In August 2004, we hired Dan Amadori as Chief Financial Officer to assume primary responsibility for all compliance and regulatory matters.
During the current fiscal year, we have added new accounting personnel and have arranged for an independent review of our internal accounting procedures and controls by an independent firm of chartered accountants. These recommendations are being implemented over the 2006 fiscal year so that we will be fully compliant with all of the requirements of the Sarbanes-Oxley legislation.
We are currently in full compliance with all financial reporting requirements. We have engaged the services of an independent firm of chartered accountants to complete our income tax return filings in both Canadian and U.S. jurisdictions.
Our chartered accountants are our registered independent public accounting firm, as such term is defined by the Public Company Accounting Oversight Board.
5. Accounting Policies:
Our financial statements are prepared in accordance with Canadian GAAP, which, in our case, conform in all material respects to generally accepted accounting principles in the United States.
6. Key Variables:
In order for us to be successful in future, to develop licensing agreements and to realize profitable operations, we must successfully complete our research initiatives, identify strategic partners and enter into licensing agreements with such partners. We must continue to raise the required level of capital in order to support these initiatives.
A. Operating Results
The following table sets forth certain selected financial information of our company:
Selected statement of operations and deficit information
| | | | | | | | | | | | | | | | | | | | | |
| | | (all amounts in United States dollars) |
| | | 2005 | | 2004 | | 2003 | | 2002 | | 2001 |
| |
| Interest & other income | | $ | (8,703 | ) | | $ | (4,746 | ) | | $ | (20,121 | ) | | $ | (165,892 | ) | | $ | (196,520 | ) |
| Loss for the year | | | 4,035,483 | | | | 2,314,298 | | | | 1,767,965 | | | | 14,565,515 | | | | 9,187,377 | |
| Loss per share-basic & diluted | | | 0.07 | | | | 0.04 | | | | 0.04 | | | | 0.31 | | | | 0.21 | |
Selected balance sheet information
| | | | | | | | | | | | | | | | | | | | | |
| | | (all amounts in United States dollars) |
| | | 2005 | | 2004 | | 2003 | | 2002 | | 2001 |
| |
| Working capital | | $ | (74,831 | ) | | $ | 34,685 | | | $ | 100,670 | | | $ | 985,551 | | | $ | 3,455,108 | |
| Property and equipment | | | — | | | | 2,925 | | | | 3,768 | | | | 98,654 | | | | 336,839 | |
| Total Assets | | | 728,375 | | | | 474,234 | | | | 350,138 | | | | 1,583,422 | | | | 14,454,470 | |
| Shareholders’ equity | | | (74,831 | ) | | | 37,610 | | | | 104,438 | | | | 1,391,903 | | | | 14,124,918 | |
Fiscal 2005 Compared to Fiscal 2004
In fiscal 2005, we established certain goals and milestones relating to our technology. This included further development and optimization of our 1 bit prototype device and initial work on the development of an array. Additionally, we set the objective of initiating discussions with potential joint
25
development partners. Finally, we set the objectives of consolidating our patent position and improving our financial reporting capabilities and internal controls.
In February 2005, we began the renegotiation of our license agreement with the University of Toronto and the Ontario Centres of Excellence and in June 2005 signed the new license agreement, as outlined in Section I.1 above.
We expanded our patent portfolio with 6 new filings in 2005.
We continue to work with our research partners and have successfully developed prototype applications which we hope to commercialize in future. We now are pursing potential joint development agreements with industry partners.
We hired additional accounting staff and retained independent firm of chartered accountants to conduct an independent review of internal control and reporting procedures. The report has been tabled and we are implementing the recommendations provided in the report.
To accommodate this activity and additional expense, we successfully raised approximately $2.3 million of additional capital in 2005.
We had no operating revenues in either year. The only income reported in 2005 was interest income of $8,703, as compared to $4,746 in 2004.
Operating costs and expenses increased from $2,319,044 in 2004 to $4,044,186 in 2005, the significant changes included:
| | a. | | Administrative costs increased from $157,854 to $320,383, an increase of $162,529. In 2005, we reported higher expenses relating to shareholder communications and meetings and absorbed a higher proportion of common costs (rent, telephone, office expenses) which costs are shared with related entities. |
| |
| | b. | | Professional, management and consulting fees increased from $271,351 in 2004 to $1,303,662 in 2005 principally as a result of: |
| | i. | | $150,000 of deferred compensation expense relating to our Chairman of the Board of Directors, as compared to an expense of $0 in 2004; |
| |
| | ii. | | Compensation of senior officers totaled $354,000 (including $200,000 of deferred compensation) in 2005, as compared to $120,000 current and $0 deferred compensation in 2004 during which we hired a CFO in mid-year); |
| |
| | iii. | | Compensation of our Chief Technology Officer hired on a full-time basis as of January 2005 at a salary of $230,000, as compared to $40,000 in 2004; |
| |
| | iv. | | Legal fees in 2005 pertaining to intellectual property related matters totaled $202,000, as compared to $40,000 in 2004; |
| |
| | v. | | Other legal fees totaled $105,000 in 2005, as compared to $80,000 in 2004; and |
| |
| | vi. | | Audit related expenses and accounting fees pertaining to the financing/registration statement and the internal control review and evaluation completed in 2005 totaled $200,000, as compared to $80,000 in 2004. |
| | c. | | Wages and salaries have increased from $31,563 in 2004 to $152,628 in 2005. We added one accounting personnel and absorbed more of the salary costs shared amongst related entities in 2005. |
26
| | d. | | Travel and entertainment costs increased from $77,616 in 2004 to $169,737 in 2005. The increase in costs relates primarily to significantly higher travel costs associated with the newly hired Chief Technology Officer in 2005. |
| | e. | | Stock compensation expense was $1,721,742 in 2005 as compared to $1,379,970 in 2004. We adopted fair value accounting for stock options issued in 2005 using the Black Scholes option pricing model and have restated prior years financial statements accordingly. |
| | f. | | Research and Development expenses were $362,141 in 2005 as compared to $378,410 in 2004. This increase reflects several payments made by our company to the University of Toronto in furtherance of our development efforts. These payments include: |
| | i. | | Cost share payment for EMK proposal number PN01069 in the aggregate amount of CDN $75,000. |
| |
| | ii. | | A payment of CDN $81,000 in payment of proposal EE40103 |
| |
| | iii. | | A direct payment from our company to the University of Toronto in the amount of CDN $250,000 reflecting partial payment of a sponsored research agreement pursuant to the terms of our license agreement with the University of Toronto. |
We reported a net loss of $4,035,483, or $0.07 per share, in 2005, as compared to a net loss of $2,314,298, or $0.04 per share in 2004.
Fiscal 2004 Compared to Fiscal 2003
In both 2004 and 2003 we continued to develop our memory technology. We signed Research Collaboration Agreements with Materials and Manufacturing Ontario in 2002 and 2003 and with Communications and Information Technology Ontario, the University of Toronto and Dr. Harry Ruda in 2002. We entered into an Infrastructure Agreement with the University of Toronto in 2002. In 2004, we continued these business relationships and met our requirements under the terms of these agreements.
In 2004, the research milestones that were contemplated in the various agreements were met on the timetable originally contemplated. In August 2004, we filed a provisional patent application with respect to certain of these initiatives. Additionally, we entered into negotiations with Dr. Cynthia Kuper to serve as our acting Chief Technology Officer. Dr. Kuper joined us in this capacity subsequent to October 31, 2004.
We had no operating revenue in either year. The only income reported in 2004 was interest income of $4,746, as compared to interest income of $20,121 in 2003.
Operating Costs and Expenses increased from $1,788,086 in 2003 to $2,319,044 in 2004. The significant differences included:
| | • | | A decrease in wages and salaries expense from $112,437 in 2003 to $31,563 in 2004. In 2004, we recovered more of the salary costs we incur for our employees from other companies that share facilities with us. |
| |
| | • | | Included in professional fees, as reported, are management and consulting fee payments made to various companies whose shareholders serve as our officers and directors. Such payments totaled $72,000 in 2004, as compared to $201,000 in 2003. During 2004, we engaged an acting Chief Technology Officer who became employed as the Chief Technology Officer subsequent to the year end. |
27
| | • | | Research and development costs decreased from $490,914 in 2003 to $378,410 in 2004. The costs associated with the various research initiatives were lower in 2004. Included in each of these expenses reported in 2004 are sums in the amount of $143,000, and $107,000 in 2003. These sums relate to a research program which was terminated in 2002. |
| |
| | • | | Travel and entertainment costs increased to $77,616 in 2004 from $17,508 in 2003. We incurred more travel expenses, primarily to US destinations, in pursuit of additional financing and to further our future research initiatives. |
| |
| | • | | In 2003, we wrote down the remaining balance we reported in patent and trademark costs in the sum of $299,820. This expense was non-recurring in 2004. |
| |
| | • | | Amortization expense on capital assets and patents and trademarks totaled $5,410 in 2004 versus $73,178 in 2003. We donated $58,302 of capital assets to the University of Toronto in 2003 leaving only nominal capital assets reported in 2004 on our books resulting in this lower amortization expense in 2004. |
| |
| | • | | During 2004, we raised $73,000 in equity funding through the issue of common shares and warrants, $264,500 through the exercise of previously issued warrants and $530,000 through the exercise of previously granted officers and directors stock options. |
We reported a net loss of $2,314,298 for 2004, or $0.04 loss per share as compared to a loss of $1,767,965 for 2003, or $0.04 loss per share.
Fiscal 2003 Compared to Fiscal 2002
In 2003, we had no operating revenue. Our only activities being the development of our memory technologies. Our only income during 2003 was $20,121, as compared to $165,892 in 2002, being principally interest income.
Costs and expenses decreased from $2,934,444 (excluding the $10 million write down of royalty rights) in 2002 to $1,767,965 in 2003.
During the year, we expended $490,914, as compared to $1,601,624 in 2002, related to our efforts on research and development of our memory technologies. The research and development expenses primarily consists of payments made under research agreements with the University of Toronto, Dr. Ruda and the funding agencies (Communications and Information Technology Ontario and Materials and Manufacturing Ontario), as further detailed below.
We paid $87,432 (CDN $136,175) against our commitment under the two year research collaboration agreement that we entered into in October 2002.
Additionally, during 2003, we secured funding in connection with the magnetic memory research program from Communications and Information Technology Ontario (Communications and Information Technology Ontario). Under a Collaborative Research Agreement among Micromem, Communications and Information Technology Ontario, the University of Toronto and Dr. Harry Ruda, in the first year, Communications and Information Technology Ontario provided funding of $106,715 (CDN $154,000) and we contributed $31,875 (CDN $46,000).
On March 1, 2003, we entered into an agreement with the University of Toronto whereby, we contributed equipment and supplies with an estimated value of $200,000. The equipment was previously used by Pageant Technologies (USA) Inc., at Pageant Technologies (USA) Inc.’s former Lee’s Summit, Missouri lab facility, and had a book value of $58,302.
28
We also continued our efforts to reduce administrative expenses in all areas of operation. We continue to control costs performed by outside service providers by performing the work in-house. As a result of these efforts, general administration expenses decreased to $176,361 in 2003 from $333,964 in 2002 and travel and entertainment decreased from $73,385 in 2002 to $17,508 in 2003.
Professional fees increased to $303,222 in 2003 from $262,927 in 2002. Wages and salaries decreased to $112,437 in 2003 from $562,532 in 2002 as a result of reduced employee levels. The primary source of reductions were from salaries to various executives. For the year ended 2003, we paid to our Chief Executive Officer $90,000, as compared to $100,000 in 2002, to our Executive Vice President $90,000, as compared to $162,066 in 2002, to our Chief Financial Officer $21,000, as compared to $1,590 in 2002, to our former Chief Operating Officer $0, as compared to $216,577 in 2002 and to the the former Chief Financial Officer $0 as compared to $111,583 in 2002.
Amortization of patents and trademarks commenced in 2002 amounting to an expense of $36,258, as compared to $31,338 in 2002. During 2003, we wrote off $130,839 relating to discontinued patent and trademark applications. We also assessed the remaining patent and trademark applications registered in the United States and Canada and have expensed the residual net book value of $168,981 to reflect the uncertain nature of future events. We continue to actively pursue and protect our patents registered in the United States.
We had a net loss of $1,767,965 for 2003, or $0.04 loss per share, compared to a net loss of $14,565,515, or $0.31 per share, for 2002. The effect on loss per share of the royalty write down in 2002 was $0.22 per share.
Unaudited quarterly financial information
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (all amounts in United States dollars) |
| | | October 31, | | July 31, | | April 30, | | January 31, | | October 31, | | July 31, | | April 30, | | January 31, |
| Quarter ended, | | | | | | 2005 | | | | | | | | | | 2004 | | | | |
| |
| Total Revenue | | $ | 7,070 | | | $ | 1,043 | | | $ | 301 | | | $ | 289 | | | $ | 117 | | | $ | 450 | | | $ | 3,658 | | | $ | 521 | |
| Net Loss | | | (1,380,802 | ) | | | (1,726,931 | ) | | | (474,227 | ) | | | (453,523 | ) | | | 447,515 | | | | 551,663 | | | | 70,876 | | | | 192,294 | |
| Loss per share: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Basic and diluted | | | 0.03 | | | | 0.03 | | | | 0.01 | | | | 0.01 | | | | 0.01 | | | | 0.01 | | | | 0.00 | | | | 0.00 | |
B. Liquidity and Capital Resources
Liquidity
We are a development stage company. We currently have no cash flow from operations and will have none until we are in a position to either license or directly produce and sell products utilizing our memory technology.
We currently have no lines of credit in place and must obtain equity financing from investors and from persons who hold outstanding options and warrants in order to meet our cash flow needs until we can generate revenues. At October 31, 2005, we had approximately $640,000 cash on hand and our monthly cash expenses approximate $100,000. Since October 31, 2005, we have raised an additional $165,000 through the exercise of stock options.
We have granted to our directors, officers and other employees a number of options to purchase shares at prices that are at or above market price on the date of grant. None of the optionees has any obligation to exercise their options and there can be no guarantee that we will realize any funds from these options.
29
Capital Resources
We had no commitments for capital expenditures as of October 31, 2005 or 2004.
In March 2001, we and our wholly owned subsidiary, Pageant International, completed the Asset Purchase Agreement. The total consideration payable in respect of the purchased assets under the Asset Purchase Agreement was $50 million in the form of cash and shares. Of this amount, $10 million was paid at closing through a cash payment of $2.0 million and the issuance by us of 2,007,831 of our common shares valued at $3.98 per share. The balance of $40 million was to have been payable in two equal amounts of $20 million each upon achievement of certain stipulated milestones provided that a minimum of 50% of each $20 million payment would be in the form of our common shares. None of the stipulated milestones were met and therefore no amounts were paid toward the balance of the $40 million purchase price. See “Information on the Company — 4. History and Development of the Company.”
As no further payments toward the purchase price were made to Estancia Limited under the Asset Purchase Agreement, Pageant International was deemed to have conveyed back, as of March 9, 2004, a percentage of the VEMRAM patents and to have granted a gross profits royalty to Estancia Limited such that Pageant International would remain holding a 60% interest in the VEMRAM patents and it would be required to pay a 32% gross profits royalty to Estancia Limited in respect of the VEMRAM technology.
Critical Accounting Policies
Our significant accounting policies are set forth in Note 3 to our consolidated financial statements, which should be read in conjunction with management’s discussion of our critical accounting policies and estimates set forth below.
Our financial statements are prepared in conformity with Canadian generally accepted accounting principles, which require management to make estimates and assumptions which can affect the reported balances. In determining estimates of net recoverable amounts and net realizable values, or whether there has been a permanent impairment in value, we rely on assumptions regarding applicable industry performance and prospects, as well as general business and economic conditions that prevail and are expected to prevail. Assumptions underlying asset valuations are limited by the availability of reliable comparable data and the uncertainty of predictions concerning future events.
Accounts recorded in foreign currency have been converted to United States dollars as follows:
| | • | | Current Assets and Current Liabilities at the prevailing exchange rates at the end of the year; |
| |
| | • | | Other Assets at historical rates; |
| |
| | • | | Revenues and expenses are translated at the average monthly exchange rate per quarter which rate approximates the rate of exchange prevailing at the transaction dates; and |
| |
| | • | | Gains and losses resulting from the fluctuation of foreign exchange rates are included in the determination of income. |
Until October 31, 2004, for all awards of employee stock-based compensation granted after January 1, 2002, we recognized employee stock-based compensation costs under the intrinsic value-based method and provided proforma disclosure of the impact on net income and earnings per share as if the fair value-based method has been applied. Effective November 1, 2004, we have adopted the fair value method of accounting for employee stock-based compensation costs. Accordingly, the closing deficit at October 31, 2004 and in prior periods has been restated for the effect of the stock-based compensation costs that we had incurred to that date, which expense previously was disclosed on a proforma basis. The stock-based
30
compensation expense for options granted during the fiscal year ending October 31, 2005 has been reflected as an expense in the consolidated statement of operations.
We are a development stage company. Research costs are expensed in the period incurred. Development expenses are expensed as incurred unless they meet the criteria for deferral and amortization under Canadian GAAP which in this case also conforms with U.S. GAAP.
Commitments
Summary of commitments:
| | | | | | | | | | | |
| | | A. | | Research collaboration agreements | | |
| | | | | | (1 | ) | | Materials and Manufacturing Ontario | | October 24, 2002 |
| | | | | | (2 | ) | | University of Toronto | | November 1, 2002 |
| | | | | | (3 | ) | | Communications and Information Technology Ontario | | December 10, 2002 |
| | | | | | (4 | ) | | Revised Licensed Agreement — University of Toronto | | June 2005 |
| | | | | | | | | | | |
| | | B. | | Technology Development Agreement, Pageant Technologies Incorporated | | March 14, 2001 |
| | | | | | | | | | | |
| | | C. | | Operating Leases | | 2006 — 2010 |
| | | | | | | | | | | |
| | | D. | | Employment Contracts | | |
| | | | | | (1 | ) | | Chairman of the Board of Directors | | May 29, 2005 |
| | | | | | (2 | ) | | Chief Technology Officer | | January 2005 |
A summary of our financial commitments as of October 31, 2005 is as below:
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | Payments due by period | | |
| | | | | | | | | | | | | | | | | | | More | |
| | | | | | | Less than 1 | | | | | | | | | | | than 5 | |
| | | Total | | | year | | | 1-3 years | | | 3-5 years | | | years | |
| |
| Long term debt obligations | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | |
| Capital Lease obligations | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | |
| Operating lease obligations | | | 492,000 | | | | 98,400 | | | | 196,800 | | | | 196,800 | | | | — | |
| | | | | | | | | | | | | | | | | | | | | |
| Purchase obligations | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | |
| Management contracts | | | | | | | | | | | | | | | | | | | | |
| • CEO | | | 550,000 | | | | 150,000 | | | | 300,000 | | | | 100,000 | | | | — | |
| • CTO | | | 260,000 | | | | 260,000 | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | |
| Research collaboration agreement | | | 213,000 | | | | 213,000 | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | |
| All other | | | — | | | | — | | | | — | | | | — | | | | — | |
| Total: | | | 1,515,000 | | | | 721,400 | | | | 496,800 | | | | 296,800 | | | | — | |
31
| A. | | Research Collaboration and Infrastructure Agreements: |
| | 1. | | Materials and Manufacturing Ontario: |
| |
| | | | On October 24, 2002, we entered into a two year Research Collaboration Agreement with Materials and Manufacturing Ontario, a not-for-profit organization funded by the provincial government, the University of Toronto and a researcher employed by University of Toronto to fund the research on Magnetic Structure development for Hall effect memory devices. |
| |
| | | | Under the terms of the agreement, we committed to contribute $87,432 (CDN $136,175) and $18,000 (CDN $28,000) in cash and in-kind contribution, respectively, per year to fund the research. We have met all of our obligations under this agreement. |
| |
| | | | On November 12, 2003, we entered into a second research collaboration agreement with Materials and Manufacturing Ontario and the University of Toronto for research and development associated with magnetic memory devices. Under the second agreement, in the first year and upon renewal in the second year, Materials and Manufacturing Ontario granted $58,900 (CDN $85,000) in cash funding and we contributed $56,130 (CDN $81,000) in cash funding and additionally made $30,770 (CDN $44,400) of in-kind contributions, all towards the research collaboration, each year. We have met all of our obligations under the agreement. |
| |
| | 2. | | University of Toronto: |
| |
| | | | On November 1, 2002, we entered into an Infrastructure Agreement with the University of Toronto to fund the assembly of a magnetic memory facility for research, development and fabrication of magnetic memory. The University of Toronto has agreed to use the magnetic memory facility in connection with, among other things, research to be conducted pursuant to collaborations between us and the University of Toronto. |
| |
| | | | Under the agreement, we were required to and did contribute $249,463 (CDN $360,000) in cash to fund the direct costs of the magnetic memory facility. The contribution has been included as a research and development expense in the consolidated statements of operations and deficit. |
| |
| | 3. | | Communications and Information Technology Ontario: |
| |
| | | | On December 10, 2002, we entered into a two year Collaborative Research Agreement with Communications and Information Technology Ontario, the University of Toronto and Dr. Harry Ruda. For the first year, Communications and Information Technology Ontario provided funding of $106,715 (CDN $154,000) and we contributed $31,875 (CDN $46,000). For the second year, Communications and Information Technology Ontario provided funding of $107,715 (CDN $154,000) and we provided funding of $31,875 (CDN $46,000). We have further provided $67,632 (CDN $97,600) of in-kind contributions to the research collaboration. |
| |
| | 4. | | Revised License Agreement, June 2005: |
| |
| | | | In June 2005, we signed a new license agreement with the University of Toronto and the Ontario Centres of Excellence whereby: |
| | a. | | Ontario Centres of Excellence released us and the University of Toronto from the commercialization obligations set forth in all prior research collaboration agreements. |
32
| | b. | | We acquired exclusive worldwide rights to the technology and any technology or patent rights under the agreement related to the MRAM technology developed at the University of Toronto. |
| |
| | c. | | We have agreed to royalties and payments as follows: |
| |
| | 1.1 | | In consideration for the rights and licenses granted, we shall pay to the University of Toronto: |
| | a. | | 4% of Net Sales until such time as the University of Toronto has received from us an aggregate amount of CDN $500,000. |
| |
| | b. | | 1% of Net Sales thereafter. |
| | 1.2 | | If we sublicense any rights granted herein to any non-affiliate: |
| | a. | | In combination or association, the University of Toronto shall receive 10% of any Net Fees and/or Net Royalties that shall be received by us in respect of any licenses involving both the rights granted herein and such our intellectual property; |
| |
| | b. | | For all other sublicenses of the rights granted herein to a non-affiliate, the University of Toronto shall receive 20% of any Net Fees and/or Net Royalties that shall be received by us in respect of such sublicenses; and |
| |
| | c. | | Net Fees and/or Net Royalties shall be paid to the University of Toronto until such time as it has received an aggregate amount of CDN $500,000; thereafter we shall pay half of the amounts set forth in 1.2 (a) or (b) as is applicable. |
| | 1.3 | | At any point after which we have paid the University of Toronto CDN $500,000, we may at our option buy out the obligation to pay royalties hereunder by paying to the University of Toronto a single lump sum payment equaling the greater of CDN $500,000 and an amount equal to the total amount of royalties paid by us to the University of Toronto in the preceeding twenty-four months. We shall be entitled to exercise such option by providing written notice to the University of Toronto along with the required payment, after which time our obligation to pay royalties under Section 1.1. or 1.2 shall be waived by the University of Toronto. |
As a condition to entering the license agreement, we have agreed that we will enter into a further research agreement with a funding commitment of no less than CDN $500,000 to continue the further research and development of inventions and our intellectual property. In August 2005, we made an initial payment of $250,000 and, subsequent to October 31, 2005, we made the second payment of $250,000 under the terms of this further research agreement.
We believe that there are substantial market opportunities available to commercialize our technology in conjunction with strategic partners, and we are currently pursuing such opportunities. We plan to complete our research initiatives and enter into agreements with strategic partners so as to commercialize our Technology under licensing and other arrangements.
| B. | | Technology Development Agreement: |
| |
| | | On March 14, 2001, our subsidiary, Pageant International, entered into a three-year technology development agreement with Estancia Limited and Mr. Lienau to continue the development of the technology. Under the terms of the agreement, Pageant International committed to pay Estancia Limited $215,000 per year, payable on a monthly basis in arrears, and committed to incur |
33
| | | expenditures in connection with development expenses of up to a maximum of $500,000 per agreement year. |
| |
| | | On April 23, 2002, the technology development agreement was amended to extend its term for an additional eight-month period through November 2004. The go-forward payments were renegotiated as $62,707 between the months of May and October 2002, $197,086 during fiscal 2003 and $143,330 during fiscal 2004. |
| |
| | | The development efforts under this agreement ceased in July 2002. We have reported approximately $287,000 in accounts payable and accrued liabilities with respect to this agreement as of October 31, 2005, as compared to $287,000 at October 31, 2004. |
| C. | | Operating leases: |
| |
| | | We have operating lease commitments which expire in 2010 for the lease of our head office. The future minimum annual lease payments are approximately as follows: |
| | | | | |
| | | 2006 — 2010 (annually) | | $98,400 |
| | 1. | | On May 29, 2005, we entered into a new employment agreement with the Chairman of the Board of Directors. The agreement commenced on January 1, 2005 and expires on September 30, 2009. Under the terms of the agreement, the Chairman of the Board of Directors has been retained to provide certain management services to us. We have agreed to provide compensation based on a percentage of the increase of the market capitalization on a year-over-year basis commencing as of December 31, 2005. This compensation is subject to a minimum annual amount of $150,000. At our option, we can pay either cash or issue common shares as compensation providing that the cumulative maximum number of shares that we can issue under the agreement is 2,000,000 common shares. At December 31, 2005 $150,000 of deferred cash compensation has been provided for. |
| |
| | 2. | | In January 2005, we entered into an employment contract with an “arm’s length” individual, Dr. Kuper, for her services as our Chief Technology Officer. The agreement extends for 2 years with a cancellation clause which can be executed by us at any time with 4 months notice. The base remuneration stipulated in the contract is $260,000 per year. We also granted the Chief Technology Officer 100,000 options to purchase our common shares exercisable at $0.68 per share which expired on December 31, 2005 and 300,000 options to purchase our common shares exercisable at $0.80 per share which expire 45 days after the end of the above noted employment agreement. |
Contingencies:
| A. | | In the normal course of business, we and our subsidiaries are involved in various legal actions. In management’s opinion, the ultimate disposition of these actions, individually or in the aggregate, will not have a material adverse effect on our financial condition. |
| B. | | We have agreed to indemnify our directors and officers and certain of our employees in accordance with our By-laws. We maintain insurance policies that may provide coverage against certain claims. |
34
| C. | | Pageant Technologies (USA) Inc. was previously named as a defendant in legal actions relating to tenant improvements on a leased property, including an action claiming damages of approximately $887,000 alleging breach of contract under a construction contract entered into by Clear Blue Laboratories, Inc. The landlord of the leased property was also claiming damages against Pageant Technologies (USA) Inc. Pageant Technologies (USA) Inc. assigned its rights under the lease to Clear Blue Technologies, Inc., however, Pageant Technologies (USA) Inc. was allegedly obligated to pay the lease payments should the assignee default under the contract. The landlord claimed damages of approximately $887,000. This matter was settled pursuant to a settlement agreement in 2005 at no cost to us. |
| D. | | As outlined above, certain interests under the Asset Purchase Agreement with Estancia Limited reverted to Estancia Limited on March 9, 2004. On this basis, to the extent that revenues are generated by us relating directly and specifically to the VEMRAM patents, we are obligated to pay Estancia Limited 32% of the gross profit realized less expenses agreed to by the parties and 32% of any unit royalties realized less direct expenses. |
Translation of Foreign Currencies
Our functional currency is the United States dollar. Accounts recorded in foreign currency have been converted to United States dollars as follows:
| | • | | Monetary assets and liabilities are translated at exchange rates at the consolidated balance sheet dates; |
| |
| | • | | Non-monetary assets are translated using the historical rate of exchange in effect at the translation dates; |
| |
| | • | | Revenues and expenses are translated using the average monthly rate of exchange per quarter, which rate approximates the rate of exchange prevailing at the transaction dates; and |
| |
| | • | | Gains and losses resulting from the translation are included in the determination of net loss for the period. |
Research and Development
We are a development stage company. Research costs are expensed in the period incurred. Development expenses are expensed as incurred unless they meet the criteria for deferral and amortization under Canadian GAAP which in this case also conforms with U.S. GAAP.
Our research and development activities have been related primarily to research and development of a magnetic random access memory device through research collaboration agreements. Our research and development expenses for the year ended October 31, 2005 were $362,141, for the year ended October 31, 2004 were $378,410, for the year ended October 31, 2003 were $490,914, for the year ended October 31, 2002 were $1,601,624 and for the year ended October 31, 2001 were $2,212,679.
Trend Information
The digital memory industry and, more broadly, the semiconductor industry, have historically been characterized by wide fluctuations in demand for and supply of semiconductors and memory
35
technologies. Prior experience has shown that restructuring of operations, resulting in significant restructuring charges, may become necessary if an industry downturn were to occur.
Our prospects for revenues are dependent upon the successful completion of our technology development and the incorporation of any technology that may be developed under or pursuant to our research collaboration agreements.
Off-Balance Sheet Arrangements
We are not party to any material off-balance sheet arrangements. In addition, we have no unconsolidated special purpose financing or partnership entities.
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
A. Directors and Executive Officers
The Directors, Executive Officers and other key personal of Micromem as at October 31, 2005 are set forth below:
| | | | | | | |
| Name | | Age | | Position |
| Salvatore Fuda | | | 70 | | | Chairman of the Board of Directors |
| Joseph Fuda | | | 45 | | | President, Chief Executive Officer and Director |
| Manoj Pundit | | | 41 | | | Secretary and Director |
| Andrew Brandt | | | 67 | | | Director |
| Stephen Fleming | | | 56 | | | Director |
| Charles Harnick | | | 55 | | | Director |
| George Kennedy | | | 65 | | | Director |
| David Sharpless | | | 55 | | | Director |
| Steven Van Fleet | | | 51 | | | Director |
| Larry Blue | | | 49 | | | Director |
| Dan Amadori | | | 54 | | | Chief Financial Officer |
| Cynthia Kuper | | | 33 | | | Chief Technology Officer |
Salvatore Fudais and has served as Chairman of the Board of Directors of Micromem since January 11, 1999 and a Director of Micromem since 1992. He served as President and Chief Executive Officer from June 2000 through to February 13, 2002. From 1992 to January 11, 1999 he also served as Secretary of Micromem. He served as President and Chief Executive Officer of Ontex Resources Limited (TSE) from 1986 to December 1998 and as Chairman of the Board of Ontex Resources Limited since that date. He has served as Chairman of the Board of Directors and director of Echo Energy Canada Inc. since June 2002. He is the father of Joseph Fuda.
Joseph Fudais and has been President, Chief Executive Officer and Director since February 13, 2002. Previously he served as Manager of Strategic Alliances for Micromem since February 2001. Prior thereto, he served as a consultant to Micromem since November 2000. Prior thereto he served as a Vice-President and a Director of IPO Capital Corp since April 1999. Mr. J. Fuda has served as a Director of Micromem since February 13, 2002.
36
Cynthia Kuperis our Chief Technology Officer and has served in such capacity since January 2005. Prior thereto, she served as our acting Chief Technology Officer since September 2004. She has 10 years of experience in research and development in the areas of carbon nanotechnologies, solid state materials, molecular biology and genetics. Dr. Kuper has a B.S. in Chemistry and a Ph.D. in Physical Chemistry from Temple University. Upon completion of her Ph.D., in 1999, she pursued a postdoctoral fellowship in the laboratories of Nobel Laureate Richard Smalley at Rice University, in the Center for Nanoscale Science and Engineering. In 2000 she founded a nanotechnology start-up company, Versilant Nanotechnologies, with initial funding from NASA. Versilant devoted its research and development to enabling areas of carbon nanotechnology for commercial application of the material. In that same year, Dr. Kuper founded K1 Consulting, a consulting firm providing services to start-ups, medium sized and large conglomerate companies and investors in nanotechnology. Dr. Kuper has testified before the United States Senate Armed Services Subcommittee on Emerging Threats and Capabilities as a subject matter expert in nanotechnology. She has been featured in many publications, including Newsweek, Fortune Magazine, and the German venture magazine, Borseonline, as an expert in her field and a leading businesswoman. In addition, she serves on various boards including the Nano Business Alliance, the Department of Chemical Technology of Philadelphia Community College and the College of Science and Technology Alumni, Temple University, where she served as an adjunct instructor in the Chemistry Department. She has served as a partner in the Nanobusiness Development Group, a nanobusiness consultancy firm and now serves as an advisor to 21 Ventures, a venture capital group focused in emerging technology start–ups. Dr. Kuper also co-teaches a first of its kind course at Temple University Fox School of Business on new technologies and business.
Manoj Punditpreviously served as Micromem’s Executive Vice President and General Counsel since and joined the Board of Directors in March 2001. He has also been a partner, since December 1996, at Chitiz Pathak LLP (and predecessor firms), a Toronto law firm serving clients in the securities and investment industries, including issuers and dealers and private and public mutual funds and hedge funds. Mr. Pundit has served as securities counsel in connection with public and private offerings, a wide range of securities registration matters and takeover bids. Mr. Pundit holds a LL.M. in Taxation from Osgoode Hall Law school and a LL.B. and B.Sc. (Math) from the University of Alberta.
Andrew Brandthas been Chairman of the Board of Directors and Chief Executive Officer of the Liquor Control Board of Ontario from February 1991 to January 2006. The Liquor Control Board of Ontario is one of the largest single purchasers of alcohol beverage products in the world. Prior to his appointment to the Liquor Control Board of Ontario, Mr. Brandt served as Leader of the Ontario Progressive Conservative Party from 1987 to 1990. He has previously served as the Minister of Industry and Trade, Minister of Environment and Mayor of Sarnia, Ontario. Mr. Brandt has served as a director of Micromem since June 2000.
Stephen B. Flemingis and has served as Managing Director of ASF International since June 2001. In April 1999, he served as Managing Director of Barefoot Enterprises. From July 1997 to April 1999, he served as President of Pageant Technologies (USA) Inc. Mr. Fleming was Executive Vice-President of International Ion from August 1994 to April 1997. Previously, Mr. Fleming was responsible for planning MCI’s wireless data products, intelligent network and frame relay international initiatives, and served as director of engineering for international business development for Telecom U.S.A. Mr. Fleming has worked with numerous emerging technology companies in consulting and management capacities. Mr. Fleming has served as a director of Micromem since January 1999.
Charles Harnickis a founder and a director (since January 2004) of Suasion Public Affairs Management Inc., a public affairs management firm. He is also President and Chief Executive Officer of Ontex Resources Limited (TSE) and has served in such capacities since March 2000. Mr. Harnick has been a practicing lawyer with Sutts, Strosberg LLP since December 2001. He was the Attorney-General of Ontario from June 1995 to June 1999. Mr. Harnick has served as a director of Micromem since June 2000.
George A. Kennedyis and has served as managing partner of Source International (an international consulting firm in the Metropolitan Washington D.C. area) since March 1997. In 2001, he was Director for Planning and International Development for iGate Inc., a broadband technology company
37
in Louisville, KY. From 1993 until 1996, Mr. Kennedy served as U.S. Consul General in Toronto, Canada, and prior thereto he held senior positions with the US Departments of State and Commerce in Washington D.C. Currently, he is an Executive Editor of the Black Business Journal of Houston, TX. Mr. Kennedy has served as a director of Micromem since June 2000.
David Sharplessis Chairman of the Board of Directors of Hunter, Keilty, Muntz, & Beatty Limited, a Toronto-based property and casualty insurance broker. He also serves as Chairman of the Board of Directors of Maverick Inc., a family investment corporation. From 2000 to September 2001, he was President of CIT’s Vendor Technology Finance unit. In this capacity, he was responsible for CIT’s international vendor finance business, covering CIT’s operations in Canada, Europe, Latin America, Asia Pacific and Australia. Prior thereto, Mr. Sharpless was Newcourt Credit Group Inc.’s Deputy Chairman of the Board of Directors and was responsible for international operations for Newcourt Financial. Mr. Sharpless is a graduate of Osgoode Hall Law School and is a member of the bar of Ontario. Mr. Sharpless has served as a director of Micromem since March 2001.
Steven Van Fleetis the principal of the R&V Group LLC, an RFID business consulting and technology development company. The R and V Group LLC is using state of the art machinery to manufacture RFID labels to EPC Global standards. Between 1999-2003 he served as Program Director for the Silent Commerce/Smart Packaging Initiative at International Paper Company. From January 1999 to November 1999, he was Program Director for Process and Product Uniformity and from March 1996 to December 1998 he was the Director for Control Systems Development at International Paper in Cincinnati, Ohio. He is also presently on the Board of Overseers for the Massachusetts Institute of Technology Auto ID Center. Mr. Van Fleet has been a director of Micromem since 2002.
Larry Blueis the Vice President and General Manager of Symbol Technologies Inc. and was appointed to the Board of Directors on November 7, 2005. Previously Mr. Blue had senior management roles with Hughes Network Systems and with IBM in Research Triangle Park.
Dan Amadorihas served as Chief Financial Officer of Micromem since June 2004. He has also served as Chief Financial Officer of Leader Capital since June 2004. He served as a Director and Chair of the Audit Committee of Ontex Resources between September 2003 and March 2005. He is President of Lamerac Financial Corp., a financial advisory firm and has held that position since October 1988. Mr. Amadori is a Chartered Accountant and holds an MBA from the Ivey School of Business.
There are no arrangements or understandings between any director and any other person pursuant to which the director was selected as a director or executive officer. Each director holds office until the next annual meeting of shareholders or until his or her successor is elected or appointed, unless his or her office is earlier vacated according to the provisions of our By-laws or theBusiness Corporations Act(Ontario).
Other than a father/son relationship between Salvatore Fuda (father) and Joseph Fuda (son), there is no family relationship between any director or executive officer and any other director or executive officer.
38
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Annual Compensation | | Long-Term Compensation | | |
| | | | | | | | | | | | | | | Awards | | Payouts | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | Long | | |
| | | | | | | | | | | | | | | | | | | | | | | Restricted | | Term | | |
| | | | | | | | | | | | | | | | | | | Securities | | Shares or | | Incentive | | |
| | | | | | | | | | | | | | | Other Annual | | Under | | Restricted | | Plan | | |
| Name and | | | | | | Salary | | Bonus | | Compensation | | Options | | Share | | Payouts | | All other |
| Principal Position | | Year | | (US$) | | (US$) | | (US$) | | Granted (#) | | Units ($) | | ($) | | Compensation ($) |
Joseph Fuda
| | | 2005 | | | | 81,000 | | | | — | | | | — | | | | 1,800,000 | 9 | | | — | | | | — | | | | 100,000 | |
Chief Executive Officer1 | | | 2004 | | | | — | | | | — | | | | — | | | | 1,800,000 | 2 | | | — | | | | — | | | | 52,000 | |
| | | | 2003 | | | | — | | | | — | | | | — | | | | 850,000 | 3 | | | — | | | | — | | | | 90,000 | |
Salvatore Fuda,
| | | 2005 | | | | — | | | | — | | | | — | | | | 1,800,000 | 11 | | | — | | | | — | | | | 150,000 | |
| Chairman of the Board of | | | 2004 | | | | — | | | | — | | | | — | | | | 1,800,000 | 2 | | | — | | | | — | | | | — | |
Directors
| | | 2003 | | | | — | | | | — | | | | — | | | | 1,050,000 | 3 | | | — | | | | — | | | | — | |
Former Chief Executive Officer and President1 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Manoj Pundit, Executive
| | | 2005 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Vice President and | | | 2004 | | | | — | | | | — | | | | — | | | | 1,000,000 | 2 | | | — | | | | — | | | | — | |
General Counsel4 | | | 2003 | | | | — | | | | — | | | | — | | | | 850,000 | 3 | | | — | | | | — | | | | 90,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Andrew Brandt
| | | 2005 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Director | | | 2004 | | | | — | | | | — | | | | — | | | | 400,000 | 2 | | | — | | | | — | | | | — | |
| | | | 2003 | | | | — | | | | — | | | | — | | | | 400,000 | 3 | | | — | | | | — | | | | — | |
Stephen Fleming
| | | 2005 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Director | | | 2004 | | | | — | | | | — | | | | — | | | | 300,000 | 2 | | | — | | | | — | | | | — | |
| | | | 2003 | | | | — | | | | — | | | | — | | | | 300,000 | 3 | | | — | | | | — | | | | — | |
Charles Harnick
| | | 2005 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Director | | | 2004 | | | | — | | | | — | | | | — | | | | 400,000 | 2 | | | — | | | | — | | | | — | |
| | | | 2003 | | | | — | | | | — | | | | — | | | | 400,000 | 3 | | | — | | | | — | | | | — | |
George A Kennedy
| | | 2005 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Director | | | 2004 | | | | — | | | | — | | | | — | | | | 300,000 | 2 | | | — | | | | — | | | | — | |
| | | | 2003 | | | | — | | | | — | | | | — | | | | 300,000 | 3 | | | — | | | | — | | | | — | |
David Sharpless
| | | 2005 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Director | | | 2004 | | | | — | | | | — | | | | — | | | | 400,000 | 2 | | | — | | | | — | | | | — | |
| | | | 2003 | | | | — | | | | — | | | | — | | | | 400,000 | 3 | | | — | | | | — | | | | — | |
| | | | 2002 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Steven Van Fleet
| | | 2005 | | | | 24,000 | | | | — | | | | — | | | | 300,000 | 9 | | | — | | | | — | | | | — | |
| Director | | | 2004 | | | | — | | | | — | | | | — | | | | 300,000 | 2 | | | — | | | | — | | | | — | |
| | | | 2003 | | | | — | | | | — | | | | — | | | | 300,000 | 3 | | | — | | | | — | | | | — | |
Dan Amadori
| | | 2005 | | | | 74,000 | | | | — | | | | — | | | | 450,000 | 9 | | | — | | | | — | | | | 100,000 | |
Chief Financial Officer7 | | | 2004 | | | | 24,000 | | | | 6,000 | | | | — | | | | 300,000 | 2 | | | — | | | | — | | | | 6,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
39
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Annual Compensation | | Long-Term Compensation | | |
| | | | | | | | | | | | | | | Awards | | Payouts | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | Long | | |
| | | | | | | | | | | | | | | | | | | | | | | Restricted | | Term | | |
| | | | | | | | | | | | | | | | | | | Securities | | Shares or | | Incentive | | |
| | | | | | | | | | | | | | | Other Annual | | Under | | Restricted | | Plan | | |
| Name and | | | | | | Salary | | Bonus | | Compensation | | Options | | Share | | Payouts | | All other |
| Principal Position | | Year | | (US$) | | (US$) | | (US$) | | Granted (#) | | Units ($) | | ($) | | Compensation ($) |
Cynthia Kuper 6 | | | 2005 | | | | 260,000 | | | | — | | | | — | | | | 300,000 | 10 | | | — | | | | — | | | | — | |
| Acting Chief Technology | | | 2004 | | | | — | | | | — | | | | — | | | | 100,000 | 5 | | | — | | | | — | | | | 10,000 | |
| Officer | | | | | | | | | | | | | | | | | | | 100,000 | 13 | | | | | | | | | | | | |
Antonio Lopes
| | | 2005 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Former Chief Financial | | | 2004 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 14,000 | |
Officer7 | | | 2003 | | | | — | | | | — | | | | — | | | | 300,000 | 3 | | | — | | | | — | | | | 20,789 | |
Larry Blue
| | | 2005 | | | | — | | | | — | | | | — | | | | 300,000 | 12 | | | — | | | | — | | | | — | |
| Director | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | |
| | | Notes: |
| |
| (1) | | Salvatore Fuda has served as our Chairman of the Board of Directors since January 11, 1999. He served as our President and Chief Executive Officer from June 2000 through to February 13, 2002. Joseph Fuda was appointed our Chief Executive Officer on February 13, 2002. |
| |
| (2) | | Each option entitles the holder to purchase one of our common shares at $0.30 prior to expiry on July 18, 2009 and is fully vested. |
| |
| (3) | | Each option entitled the holder to purchase one of our common shares at $0.10. These options were exercised in 2004. |
| |
| (4) | | Mr. Pundit previously served as our Executive Vice-President, General Counsel and Secretary. |
| |
| (5) | | Each option entitles the holder to purchase one of our common shares at $0.68. These options expired on December 31, 2005. |
| |
| (6) | | Dr. Kuper has served as our Chief Technology Officer since January 2005, and prior thereto served as acting Chief Technology Officer since September 2004. |
| |
| (7) | | These options expired unexercised. |
| |
| (8) | | Mr. Viswanathan was employed as our Chief Financial Officer between June 5, 2000 and October 31, 2002. Mr. Lopes served as our Chief Financial Officer between November 1, 2002 and June 2004. Mr. Amadori has served as our Chief Financial Officer since June 29, 2004. |
| |
| (9) | | Each option entitles the holder to purchase one of our common shares at a price of $0.72 per share prior to expiry in June 2010. |
| |
| (10) | | Each option entitles the holder to purchase one of our common shares at a price of $0.80 per share prior to expiry in January 2007. |
| |
| (11) | | Each option entitles the holder to purchase one of our common shares at a price of $0.65 per share prior to expiry in December 2010. |
| |
| (12) | | Each option entitles the holder to purchase one of our common shares at a price of $0.60 per share prior to expiry in December 2010. |
| |
| (13) | | Each option entitles the holder to purchase one of our common shares at a piece of $0.72 per share prior to expiry in March 2007. |
The aggregate direct compensation paid or accrued on behalf of all other directors as a group during 2005 and 2004 was zero. None of the non-employee directors have agreements with us that provide for benefits upon termination of service.
40
Our Board of Directors compensation policy is as follows. Directors do not receive cash compensation for serving as directors. Instead they have been awarded stock options over the years. These options are set at each annual meeting and approved by the shareholders.
We have adopted a stock option plan. Options are offered to directors, executive officers and employees to purchase our common shares at an exercise price equal to or above the market price for the common shares at the date that the options are granted. 5,300,000 options were granted during 2003 and were exercised in 2004. In 2004, 7,150,000 options were granted and outstanding at October 31, 2004. These options have vested and are exercisable at $0.30 per share. In 2005, 1,800,000 of these options were exercised.
Additionally, 120,000 options were granted in 2004 at an exercise price of $0.68. In 2005, 20,000 of these options were exercised and at December 31, 2005 the remaining 100,000 options expired.
In 2005, we issued 2,500,000 options exercisable at $0.72, 1,800,000 options exercisable at $0.65, 100,000 options exercisable at $0.91 and 300,000 options exercisable at $0.80. These options are fully vested and expire in 5 years if unexercised.
Subsequent to October 31, 2005 we issued 300,000 options at $0.60 and 50,000 options at $0.72. These options are fully vested and expire in 5 years if unexercised.
| C. | | Board of Directors Practices |
Our Board of Directors meets as a full Board of Directors on an “as required” basis during the fiscal year. In 2005 our Board of Directors met on February 26, 2005 and again on June 27, 2005.
Our Audit Committee of the Board of Directors has met on a quarterly basis during fiscal 2005 for the purpose of approving the quarterly financial statements. In addition, our Audit Committee has received periodic reports from management.
All matters pertaining to our financing, contractual arrangements and Board of Directors and management compensation are approved by the Board of Directors. All Board of Directors meeting minutes and directors resolutions are maintained by us on an up-to-date basis.
The Audit Committee approved an independent review of our internal reporting and control procedures, which review was completed during fiscal 2005 by an independent firm of chartered accountants. Their report has been submitted and management is now implementing certain recommendations under the supervision of the Audit Committee.
The members of the Board of Directors are appointed to a one-year term at our annual meeting.
Audit Committee
The Board of Directors has appointed an Audit Committee consisting of three independent directors. The members of the Audit Committee are Andrew Brandt, Charles Harnick and David Sharpless each of whom shall serve in such capacity until our next annual meeting. The Audit Committee is responsible for the integrity of our internal accounting and control systems. The committee receives and reviews our financial statements and makes recommendations thereon to the Board of Directors prior to its approval by the full Board of Directors. The Audit Committee communicates directly with our external auditors in order to discuss audit and related matters whenever appropriate.
41
Compensation Committee
The Board of Directors also intends to appoint a Compensation Committee. Our executive compensation will be administered by the Compensation Committee which will meet on executive compensation matters as and when required.
We have eight officers and employees of which three serve in a management capacity, one serves in a research and development capacity and four serve in an administrative capacity. This includes a Chairman of the Board of Directors, a Chief Executive Officer and President, a Chief Financial Officer, a Chief Technology Officer and four support staff, all of whom work from our executive offices in Toronto, Canada. All research and development is carried out by Dr. Harry Ruda and a research team employed by the University of Toronto under the research collaboration agreements between us and the University of Toronto.
We consider our relations with our employees to be satisfactory.
42
Share Ownership
| | | | | | | | | | | | | | | |
| | | | | | | | | | | Options Exercise | | |
| Name | | Shares Owned | | Options Held | | Price | | Expiration Date |
Joseph Fuda
Chief Executive
Officer and Director | | | 202,780 | | | | 1,800,000 | | | $ | 0.72 | | | 6/30/2010 |
Salvatore Fuda
Chairman of the Board of Directors
and Director | | 2,455,2921 | | | 1,800,000
1,800,000 | | | $
$ | 0.30
0.65 | | | 7/19/2009
8/1/2010 |
Manoj Pundit
Executive Vice-President,
General Counsel and Director | | | 2,478 | | | | 1,000,000 | | | $ | 0.30 | | | 7/19/2009 |
Andrew Brandt
Director | | | 3,000 | | | | 400,000 | | | $ | 0.30 | | | 7/19/2009 |
Stephen Fleming
Director | | | — | | | | 300,000 | | | $ | 0.30 | | | 7/19/2009 |
Charles Harnick
Director | | | 800 | | | | — | | | | — | | | — |
George A. Kennedy
Director | | | — | | | | 150,000 | | | $ | 0.30 | | | 7/19/2009 |
David Sharpless
Director | | | — | | | | 400,000 | | | $ | 0.30 | | | 7/19/2009 |
Steven Van Fleet
Director | | | — | | | | 300,000
300,000 | | | $
$ | 0.30
0.72 | | | 7/19/2009
6/30/2010 |
Dan Amadori
Chief Financial
Officer | | | — | | | | 300,000
50,000
400,000 | | | $
$ | 0.30
0.91
0.72 | | | 7/19/2009
2/28/2010
6/30/2010 |
Cynthia Kuper
Chief Technology
Officer | | | — | | | | 100,000
300,000 | | | $
$ | 0.68
0.80 | | | 10/06/2009
1/09/2007 |
Jason Baun
Investor Relations | | | — | | | | 50,000
50,000 | | | $ | 0.91
0.72 | | | 7/19/2009
12/20/2010 |
Larry Blue
Director | | | — | | | | 300,000 | | | $ | 0.60 | | | 11/15/2010 |
| | |
| 1. | | These shares are held by a corporation wholly owned by a trust established for the benefit of members of Salvatore Fuda’s family. |
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
A. Major Shareholders
No shareholder holds greater than 5% of the common shares outstanding.
B. Other Related Party Transactions
During fiscal year ended October 31, 2005, we paid $100,000, as compared to $60,000 in 2004, to a law firm in which Manoj Pundit, a director and officer of our company, is a partner.
43
C. Interests of Experts and Counsel
Manoj Pundit, a partner of Chitiz Pathak LLP, who serves as legal counsel to us, holds 2,478 common shares and 1,000,000 options to purchase one common share of Micromem at $0.30 per share.
ITEM 8. FINANCIAL INFORMATION
A. Consolidated Statements
See “Item 17 — Financial Statements.”
Dividend Policy
We have never paid a dividend on our securities. We do not anticipate paying dividends in the foreseeable future.
Significant Changes
Except as otherwise disclosed in this report, there has been no significant change in our financial position since October 31, 2005.
B. Legal Proceedings
Our subsidiary, Pageant Technologies (USA) Inc. was named as a defendant in legal actions relating to tenant improvements on a leased property, including an action claiming damages of approximately $887,000 alleging breach of contract under a construction contract entered into by Clear Blue Laboratories, Inc. The landlord of the property was also seeking damages from Pageant Technologies (USA) Inc. Pageant Technologies (USA) Inc. assigned its rights under the lease to Clear Blue Laboratories, Inc., however, Pageant Technologies (USA) Inc. was allegedly obligated to pay the lease payments should the assignee default under the contract. The landlord was claiming damages of approximately $887,000. This matter was settled pursuant to a settlement agreement at no cost to us in 2005.
ITEM 9. THE OFFER AND LISTING
The table below sets forth the high and low sales prices for common shares in U.S. Dollars as reported for the periods specified. Our fiscal year ends October 31. Our common shares are not traded in Canada.
Our common shares are traded in the United States and are quoted on the NASD’s OTC Bulletin Board. The common shares are quoted under the symbol MMTIF.OB.
| | | | | | | | | |
| Period | | High | | Low |
Last six months: | | | | | | | | |
| January 2006 | | | 0.72 | | | | 0.58 | |
| December 2005 | | | 0.69 | | | | 0.47 | |
| November 2005 | | | 0.71 | | | | 0.55 | |
| October 2005 | | | 0.72 | | | | 0.55 | |
| September 2005 | | | 0.80 | | | | 0.40 | |
| August 2005 | | | 0.85 | | | | 0.60 | |
Last eight quarters: | | | | | | | | |
44
| | | | | | | | | |
| Period | | High | | Low |
| Q4 2005 | | | 0.99 | | | | 0.54 | |
| Q3 2005 | | | 1.17 | | | | 0.21 | |
| Q2 2005 | | | 0.39 | | | | 0.22 | |
| Q1 2005 | | | 0.56 | | | | 0.15 | |
| Q4 2004 | | | 0.99 | | | | 0.54 | |
| Q3 2004 | | | 1.17 | | | | 0.21 | |
| Q2 2004 | | | 0.39 | | | | 0.22 | |
| Q1 2004 | | | 0.56 | | | | 0.15 | |
Last five years: | | | | | | | | |
| 2005 | | | 0.90 | | | | 0.65 | |
| 2004 | | | 1.17 | | | | 0.15 | |
| 2003 | | | 0.31 | | | | 0.05 | |
| 2002 | | | 1.77 | | | | 0.05 | |
| 2001 | | | 5.28 | | | | 1.28 | |
On January 31, 2006, the last reported sale price for our common shares on the NASD OTC Bulletin Board was $0.63.
ITEM 10. ADDITIONAL INFORMATION
A. Share Capital
Not Applicable
B. Memorandum and Articles of Incorporation of Association
Articles of Incorporation of Incorporation
Incorporation Details and Objects of Micromem Technologies Inc.
Micromem Technologies Inc. was incorporated under the laws of the Province of Ontario, Canada, on October 21, 1985 as Mine Lake Minerals Inc. We subsequently changed our name to Avanti Capital Corp. by filing Articles of Incorporation of Amendment on June 23, 1988 and to AvantiCorp International Inc. on April 30, 1992 before becoming Micromem Technologies Inc. on January 14, 1999. The Articles of Incorporation of Incorporation place no restrictions on the nature of the business to be carried on by Micromem.
Summary of Directors Powers and Authorities
The rights, duties, powers and authorities of our Board of Directors are set out in the Articles of Incorporation and By-laws and the statutory provisions of theBusiness Corporations Act (Ontario). The following is a selected summary of the Articles of Incorporation, By-laws and applicable provisions of the Business Corporations Act (Ontario) as they relate to selected rights, duties, powers and authorities of our Board of Directors.
The Articles of Incorporation provide for a minimum of three and a maximum of 12 directors. The Business Corporations Act (Ontario) prescribes that an offering corporation must have a minimum of three directors, a majority of whom are Canadian residents and at least one third of whom are not officers or employees of us or our affiliates. The Board of Directors may, between annual shareholders meetings, appoint one or more additional directors to serve until the next annual shareholders meeting provided that the number of directors so added may not exceed by one-third (1/3) the number of directors required to have been elected at the last annual meeting of shareholders.
45
The Chairman of the Board of Directors or any one director may call a meeting upon the provision of forty-eight hours notice to each director in the manner prescribed in our By-laws. Any such notice shall include the items of business to be considered at the meeting. A majority of the directors constitute a quorum provided that half of those directors present are Canadian residents. Business cannot be transacted without a quorum. A quorum of directors may vote on any matter of business properly brought before the meeting provided that where a director is a party to a material contract or proposed material contract or has a material interest in the matter to be considered, such director must disclose his or her interest at the earliest possible date, request the conflict be noted in the minutes of the meeting, and with a few limited exceptions enumerated in the By-laws, refrain from voting on the matter in which the director has a material interest. There is no limitation on the Board of Directors to vote on matters of their remuneration provided such remuneration is disclosed in the financial statements and annual shareholder proxy materials.
The Board of Directors has broad borrowing powers and may, without authorization from the shareholders:
| | • | | borrow money on the credit of Micromem; |
| |
| | • | | issue, re-issue, sell or pledge debt obligations of Micromem; |
| |
| | • | | subject to restrictions respecting financial assistance prescribed in the Business Corporations Act (Ontario), give a guarantee on behalf of Micromem to secure the performance of an obligation of any person; and |
| |
| | • | | mortgage, hypothecate, pledge or otherwise create a security interest in all or any property of Micromem, owned or subsequently acquired, to secure any obligation of Micromem. |
A person is qualified to be or stand for election as a director provided such person is at least 18 years of age, is not a bankrupt and is not found to be of unsound mind by a court in Canada or elsewhere. There is no requirement for a director to hold common shares.
Securities of Micromem
Our authorized capital consists of an unlimited number of common shares, of which 64,719,449 shares were issued and outstanding as of October 31, 2005, and 2,000,000 special, redeemable, voting preference shares, referred to herein as special shares, none of which were outstanding, as of October 31, 2005.
Holders of our common shares will be entitled to receive notice of, attend and vote at all meetings of the shareholders of Micromem. Each common share carries one vote at such meetings. In the event of the voluntary or involuntary liquidation, dissolution or winding-up of Micromem, after payment of all outstanding debts, the remaining assets of Micromem available for distribution will be distributed to the holders of our common shares. Dividends may be declared and paid on our common shares in such amounts and at such times as the directors shall determine in their discretion in accordance with the Business Corporations Act (Ontario). There are no pre-emptive rights, conversion rights, redemption provisions or sinking fund provisions attaching to the common shares. Common shares are not liable to further calls or to assessment by Micromem; provided, however, that pursuant to the provisions of the Business Corporations Act (Ontario), Micromem has a lien on any common share registered in the name of a shareholder or the shareholder’s legal representative for a debt owed by the shareholder to Micromem.
Holders of special shares are entitled to receive notice of, attend and vote at all meetings of the shareholders of Micromem. Each special share carries one vote at such meetings. In the event of the voluntary or involuntary liquidation, dissolution or winding-up of Micromem, after payment of all outstanding debts, the holders of the special shares shall be entitled to receive, before any distribution of any part of the assets of Micromem among the holders of any other shares, the amount paid up on the special shares. The special shares are redeemable at the option of Micromem for the amount paid up on the shares. Dividends may not be declared or paid on the special shares and transfer of the special sShares is restricted without the approval of the Directors of Micromem and the prior written consent of the Ontario
46
Securities Commission. The number of special shares that may be issued and outstanding at any time is limited to 500,000. There are no pre-emptive rights, conversion rights or sinking fund provisions attaching to the special shares. Special shares are not liable to further calls or to assessment by Micromem; provided, however, that pursuant to the provisions of the Business Corporations Act (Ontario), Micromem has a lien on any special shares registered in the name of a shareholder or the shareholder’s legal representative for a debt owed by the shareholder.
Rights and Privileges of Shareholders
Only the registered holders of our common shares and special preference shares on the record date are entitled to receive notice of and vote at annual and special meetings of shareholders. Where the items of business affect the rights of shareholders other than the holders of common shares, a special majority of two-thirds of the votes cast by the affected shareholders at the meeting called for such purpose is required to approve the item of business. Beneficial holders of common shares and special shares are also entitled to receive proxy materials in respect of meetings of shareholders in accordance with Canadian Securities Administrators National Instrument 54-101, provided that such proxies are limited in scope to instructing the registered shareholder (usually a brokerage house) on how to vote on behalf of the beneficial shareholder. There are no restrictions on the number of shares that may be held by non-residents other than restrictions set out in theInvestment Canada Act(Canada). See “Additional Information — D. Exchange Controls”.
There are no provisions in the By-laws regarding public disclosure of individual shareholdings. Notwithstanding this, applicable Canadian securities legislation requires certain public disclosure of persons owning or acquiring common shares in excess of 10% of a corporation’s issued and outstanding share capital.
C. Material Contracts
| | 1. | | Equipment Transfer Agreement dated March 1, 2003, by and between Micromem and the Governing Council of the University of Toronto, pursuant to which we conveyed equipment having an estimated value of $200,000 (CDN $297,600) to the University of Toronto for incorporation into the University’s magnetic memory facility for the research and development and fabrication of magnetic memory. |
| |
| | 2. | | Collaborative Research Agreement dated December 10, 2002, by and among Micromem, Communications and Information Technology Ontario, the University of Toronto and Dr. Harry Ruda, Professor of Physics at the University of Toronto, pursuant to which: |
| | • | | over a period of two years Micromem contributed $63,750 (CDN $92,000) and $67,632 (CDN $97,600) of in-kind contribution and, Communications and Information Technology Ontario contributed $215,430 (CDN $308,000) for research into “High Density Magnetic Memory Device Development”; and |
| |
| | • | | in consideration of such contribution, Communications and Information Technology Ontario shall receive a royalty based on revenues received from the sale of products incorporating intellectual property developed under this collaboration agreement for the remaining life of patents issued in connection with such intellectual property. |
| | 3. | | Research Collaboration Agreement, dated October 24, 2002, by and among Micromem, Materials and Manufacturing Ontario, the University of Toronto and Dr. Harry Ruda, Chair Professor in Nanotechnology, pursuant to which: |
| | • | | Micromem has engaged the University of Toronto to conduct research and development of magnetic memory technology; and |
47
| | • | | Micromem and Materials and Manufacturing Ontario each provide $174,864 (CDN $272,000) of funding and the combined $349,728 (CDN $544,000) is used to cover the operating expenses of the research collaboration over a term of two years. |
| | 4. | | A second two-year research collaboration agreement dated November 12, 2003, by and among Materials and Manufacturing Ontario and the University of Toronto, pursuant to which: |
| | • | | through the collaboration, Micromem has continued its involvement in the research and development of magnetic memory technology, carried out by a highly skilled research team; |
| |
| | • | | Micromem has committed to providing $56,130 (CDN $81,000) per year in cash and $30,770 (CDN $44,400) per year of in kind contributions and Materials and Manufacturing Ontario has committed to providing $58,900 (CDN $85,000) in cash; |
| |
| | • | | the combined cash contributions of we and Materials and Manufacturing Ontario, $230,060 (CDN $332,000), will be used to cover the operating expenses of the research collaboration over a term of two years; |
| |
| | • | | Materials and Manufacturing Ontario’s funding of $117,800 (CDN $170,000) (or $58,900 (CDN $85,000) per year) will be paid directly to the University of Toronto and therefore is not reflected in Micromem’s financial statements; and |
| |
| | • | | Micromem maintains its ownership of its portfolio of patents and intellectual property that was developed prior to or outside the scope of the agreement. |
| | 5. | | Asset Purchase Agreement, dated December 10, 2000, by and among Micromem, Pageant International, Estancia Limited and Richard M. Lienau, pursuant to which: |
| | • | | Pageant International purchased from Estancia Limited and Mr. Lienau all interests in the VEMRAM patents and the VEMRAM technology and all other rights, interests and entitlements held by Estancia Limited and Mr. Lienau as set forth in the Joint Ownership and Licensing Agreement and a termination of such agreement; and |
| |
| | • | | Micromem is required to pay to Estancia Limited a purchase price of $50 million, of which $10 million was paid in cash and shares at closing and the balance of $40 million being payable in cash and shares upon certain stipulated milestones being achieved; |
| | 6. | | Technology Development Agreement, dated March 9, 2001, as amended on April 23, 2002, by and among Pageant International, Estancia Limited and Richard M. Lienau, pursuant to which: |
| | • | | Estancia Limited and Mr. Lienau are required to provide services to Pageant International in respect of the continued development of VEMRAM technology; and |
| |
| | • | | Pageant International is required to pay monthly fees to Estancia Limited at the rate of $215,000 per annum over a term of three years and eight months, except that the fees payable during the period from June 1 to December 2002 and during the period from March 9, 2004 through to November 9, 2004 will be at a rate of $107,000 per annum; |
| | 7. | | Infrastructure Agreement by and between Micromem and the University of Toronto, dated November 1, 2002, pursuant to which Micromem agreed to provide funding to the University of Toronto in the amount of $249,463 (CDN $360,000) for the assembly of a magnetic memory facility to be used for research collaborations between Micromem and the University of Toronto. |
48
| | 8. | | Revised License Agreement between the University of Toronto, the Materials and Manufacturing Ontario and Communications and Information Technology Ontario as detailed above in Section 5 under the commentary on Commitments (point 4). |
| |
| | 9. | | In January 2005, we entered into a consulting agreement with Dr. Cynthia Kuper for her services as Chief Technology Officer. The agreement extends for 2 years with a cancellation clause which can be executed by us at any time with 4 months notice provided. The base remuneration stipulated in the contract is $260,000 per year. We also granted the Chief Technology Officer 100,000 options to purchase our common shares exercisable at $0.68 per share which expired on December 31, 2005 and 300,000 options to purchase common shares exercisable at $0.80 per share, which expire 45 days after the end of the above noted employment agreement. |
| |
| | 10. | | On May 29, 2005, we entered into a new employment agreement with the Chairman of the Board of Directors. The agreement commenced on January 1, 2005 and expires on September 30, 2009. Under the terms of the agreement, the Chairman of the Board of Directors has been retained to provide certain management services to us. We have agreed to provide compensation based on a percentage of the increase of the market capitalization on a year-over-year basis commencing as of December 31, 2005 subject to a minimum annual compensation amount of $150,000. At our option, we can pay either cash or issue common shares as compensation providing that the cumulative maximum number of shares that we can issue under the agreement is 2 million common shares. |
D. Exchange Controls
As of the date hereof, we are not aware of any governmental laws, decrees or regulations in Canada that restrict the export or import of capital, including, but not limited to, foreign exchange controls, or that affect the remittance of dividends or other payments to nonresident holders of our common shares.
We are not aware of any limitations under the laws of Canada or the Province of Ontario, or in the Articles of Incorporation or any other of our constituent documents on the right of nonresidents of Canada or persons who are not Canadian citizens to hold and/or vote common shares.
E. Taxation
Certain Canadian Income Tax Consequences
This discussion under this heading summarizes the principal Canadian federal income tax consequences of acquiring, holding and disposing of common shares for a shareholder who is not a resident of Canada but is a resident of the United States and who will acquire and hold a common share as capital property for the purposes of the Income Tax Canada, also referred to as the Canadian Tax Act. This summary does not apply to a shareholder who carries on business in Canada through a permanent establishment situated in Canada or performs independent personal services in Canada through a fixed base in Canada if the shareholder is effectively connected with such permanent establishment or fixed base. This summary is based on the provisions of the Canadian Tax Act and the regulations thereunder and on an understanding of the administrative practices of Canada Customs & Revenue Agency, and takes into account all specific proposals to amend the Canadian Tax Act or regulations made by the Minister of Finance of Canada as of the date hereof. It has been assumed that there will be no other relevant amendments of any governing law although no assurance can be given in this respect. This discussion is general only and is not a substitute for independent advice from a shareholder’s own Canadian and US tax advisors.
The provisions of the Canadian Tax Act are subject to income tax treaties to which Canada is a party, including the Canada-United States Income Tax Convention (1980), as amended.
49
Dividends on common shares and Other Income
Under the Canadian Tax Act, a non-resident of Canada is generally subject to Canadian withholding tax at the rate of 25 percent on dividends paid or deemed to have been paid to him or her by a corporation resident in Canada. We are responsible for the withholding of tax at the source. The Canada-United States Income Tax Convention (1980) limits the rate to 15 percent if the shareholder is a resident of the United States and the dividends are beneficially owned by and paid to such shareholder, and to 5 percent if the shareholder is also a corporation that beneficially owns at least 10 percent of the voting stock of the payor corporation.
The amount of a stock dividend (for tax purposes) would generally be equal to the amount of our paid up or stated capital and increased by reason of the payment of such dividend. We will furnish additional tax information to shareholders in the event of such a dividend. Interest paid or deemed to be paid on our debt securities held by non-Canadian residents may also be subject to Canadian withholding tax, depending upon the terms and provisions of such securities and any applicable tax treaty.
The Canada-United States Income Tax Convention (1980) generally exempts from Canadian income tax dividends paid to a religious, scientific, literary, educational or charitable organization or to an organization constituted and operated exclusively to administer a pension, retirement or employee benefit fund or plan, if the organization is a resident of the United States and is exempt from income tax under the laws of the United States.
Dispositions of Common Shares
Under the Canadian Tax Act, a non-resident of Canada is subject to Canadian tax on taxable capital gains, and may deduct allowable capital losses, realized on a disposition of “taxable Canadian property”. common shares will constitute taxable Canadian property of a shareholder at a particular time if the shareholder used the shares in carrying on business in Canada, or if at any time in the five years immediately preceding the disposition 25 percent or more of the issued shares of any class or series in the capital stock of Micromem belonged to one or more persons in a group comprising the shareholder and persons with whom the shareholder did not deal at “arm’s length” and in certain other circumstances.
The Canada-United States Income Tax Convention (1980) relieves United States residents from liability for Canadian tax on capital gains derived on a disposition of shares unless:
the value of the shares is derived principally from “real property” in Canada, including the right to explore for or exploit natural resources and rights to amounts computed by reference to production, the shareholder was resident in Canada for 120 months during any period of 20 consecutive years preceding, a and at anytime during the 10 years immediately preceding, the disposition and the shares were owned by them when they ceased to be resident in Canada, or the shares formed part of the business property of a “permanent establishment” that the holder has or had in Canada within the 12 months preceding the disposition.
Certain United States Federal Income Tax Consequences
The following is a general discussion of certain possible United States federal income tax consequences, under current law, generally applicable to a US Holder (as defined below) of our common shares. This discussion does not address all potentially relevant federal income tax matters (including but not limited to alternative minimum tax considerations) and it does not address consequences peculiar to persons subject to special provisions of federal income tax law, such as those described below as excluded from the definition of a US Holder. In addition, this discussion does not cover any state, local or foreign tax consequences. See “Additional Information —E. Taxation.
The following discussion is based upon the sections of the Internal Revenue Code of 1986, as amended, Treasury Regulations, published Internal Revenue Service rulings, published administrative
50
positions of the Internal Revenue Service and court decisions that are currently applicable, any or all of which could be materially and adversely changed, possibly on a retroactive basis, at any time. This discussion does not consider the potential effects, both adverse and beneficial, of any recently proposed legislation which, if enacted, could be applied, possibly on a retroactive basis, at any time. This discussion is for general information only and it is not intended to be, nor should it be construed to be, legal or tax advice to any holder or prospective holder of the common shares and no opinion or representation with respect to tax consequences to any such holder or prospective holders is made.
Accordingly, holders and prospective holders of our common shares should consult their own tax advisors about the federal, state, local, and foreign tax consequences of purchasing, owning and disposing of the common shares.
US Holders
As used herein, the defined term “US Holder” means a holder of the common shares who is a citizen or individual resident (as defined under U.S. tax laws) of the United States, or, generally a corporation limited liability company, or a partnership created or organized in or under the laws of the United States or of any political subdivision thereof, or a trust whose income is taxable in the United States irrespective of source. This summary does not address the tax consequences to, and a US Holder does not include, persons subject to special provisions of federal income tax law, such as tax-exempt organizations, qualified retirement plans, individual retirement accounts and other tax-deferred accounts, financial institutions, insurance companies, real estate investment trusts, regulated investment companies, broker-dealers, non-resident alien individuals, persons or entities that have a “functional currency” other than the US dollar, shareholders who hold common stock as part of a “straddle”, hedging or a conversion transaction and shareholders who acquired their stock through the exercise of employee stock options or otherwise as compensation for services. This discussion is limited to US Holders who hold the common shares as capital assets. This discussion does not address the consequences to a person or entity holding an interest in a US Holder or the consequence to a person of the ownership, exercise or disposition of any warrants, options or other rights to acquire common shares.
Distributions on Common Shares
Subject to the discussion below at “Other Considerations”, US Holders receiving dividend distributions (including but not limited to constructive dividends) with respect to our common shares generally are required to include in gross income for United States federal income tax purposes the gross amount of such distributions equal to the US dollar value of each dividends on the date of receipt (based on the exchange rate on such date) to the extent that we have current or accumulated earnings and profits, without reduction for any Canadian income tax withheld from such distributions. Such Canadian tax withheld may be credited, subject to certain limitations, against the US Holder’s United States federal income tax liability or, alternatively, may be deducted in computing the US Holder’s United States federal taxable income by those who itemize deductions. (See more detailed discussion at “Foreign Tax Credit” below.) To the extent that distributions exceed our current or accumulated earnings and profits, they will be treated first as a return of capital up to the US Holder’s adjusted basis in the common shares and thereafter as gain from the sale or exchange of the common shares. Preferential tax rates for long-term capital gains are applicable to a US Holder which is an individual, estate or trust. There are currently no preferential tax rates for long-term capital gains for a US Holder which is a corporation.
In the case of foreign currency received as a dividend that is not converted by the recipient into US dollars on the date of the receipt, a US Holder will have a tax basis in the foreign currency equal to its US dollar value on the date of receipt. Generally, any gain or loss recognized upon a subsequent sale or other disposition of the foreign currency, including an exchange for US dollars, will be ordinary income or loss.
Dividends paid on the common shares will not generally be eligible for the dividends received deduction provided to corporations receiving dividends from certain United States corporations. A US Holder which is a corporation may, under certain circumstances, be entitled to a 70 percent deduction of the United States source portion of dividends received from us (unless we qualify as a “foreign personal
51
holding company” or a “passive foreign investment company,” as defined below) if such US Holder owns common shares representing at least 10 percent of the voting power and value of the our company. The availability of this deduction is subject to several complex limitations which are beyond the scope of this discussion.
Foreign Tax Credit
A US Holder who pays (or has withheld from distributions) Canadian income tax with respect to the ownership of the common shares may be entitled, at the option of the US Holder, to either a deduction or a tax credit for such foreign tax paid or withheld. Generally, it will be more advantageous to claim a credit because a credit reduces United States federal income tax on a dollar-for-dollar basis, while a deduction merely reduces the taxpayer’s income subject to tax. This election is made on a year-by-year basis and generally applies to all foreign taxes paid by (or withheld from) the US Holder during that year. There are significant and complex limitations which apply to the credit, among which is the general limitation that the credit cannot exceed the proportionate share of the US Holder’s United States income tax liability that the US Holder’s foreign source income bears to his/her or its worldwide income. In the determination of the application of this limitation, the various items of income and deduction must be classified into foreign and domestic sources. Complex rules govern this classification process. In addition, this limitation is calculated separately with respect to specific classes of income such as “passive income,” “high withholding tax interest,” “financial services income,” “shipping income,” and certain other classifications of income. The availability of the foreign tax credit and the application of the limitations on the credit are fact specific, and holders and prospective holders of the common shares should consult their own tax advisors regarding their own circumstances.
Disposition of Common Shares
Subject to the discussion below at “Other Considerations”, a US Holder will generally recognize gain or loss upon the sale or other disposition of common shares equal to the difference, if any, between:
| | • | | the amount of cash plus the fair market value of any property received, and |
| |
| | • | | the shareholder’s tax basis in the common shares. |
This gain or loss will be capital gain or loss if the common shares are a capital asset in the hands of the US Holder (and if the stock is not “Section 306 Stock”), which will be a long-term capital gain or loss if the common shares are held for more than one year and which may be entitled to a preferential tax rate. Deductions for net capital losses generally may be carried over to be used in later tax years until such net capital loss is thereby exhausted. For US Holders which are corporations (other than corporations subject to Subchapter S of the Code), generally an unused net capital loss may be carried back three years from the loss year and carried forward five years from the loss year to be offset against capital gains until such net capital loss is thereby exhausted.
Other Considerations
In the following circumstances, the above sections of this discussion may not describe the United States federal income tax consequences resulting from the holding and disposition of common shares:
52
Foreign Personal Holding Company
If at any time during a taxable year more than 50 percent of the total combined voting power or the total value of our outstanding shares is owned, directly or indirectly (including by attribution), by five or fewer individuals who are citizens or residents of the United States and 60 percent or more of our gross income for such year (50 percent in subsequent years) was derived from certain passive sources (e.g., dividends, interests, rents, royalties, etc.), we may be treated as a “foreign personal holding company.” In that event, US Holders that hold common shares would be required to include in gross income for such year their allocable portions of our taxable income to the extent we do not actually distribute such income.
Foreign Investment Company
If 50 percent or more of the combined voting power or total value of our outstanding common shares are held, directly or indirectly (including through attribution), by citizens or residents of the United States, United States domestic partnerships or corporations, or estates or trusts other than foreign estates or trusts (as defined by Code Section 7701(a) (31)), we are found to be engaged primarily in the business of investing, reinvesting, or trading in securities, commodities, or any interest therein, and certain other conditions are met, we may be treated as a “foreign investment company” as defined in Section 1246 of the Code, causing all or part of any gain realized by a US Holder selling or exchanging common shares to be treated as ordinary income rather than capital gain.
Passive Foreign Investment Company
As a foreign corporation with US Holders, we could potentially be treated as a passive foreign investment company, or a PFIC, as defined in Section 1297 of the Code, depending upon the percentage of our income which is passive, or the percentage of our assets which is producing passive income. Generally, on certain distributions and on disposition, US Holders owning common shares of a PFIC are subject to a special tax regime imposing the highest U.S. ordinary income tax rate plus an interest charge based on the value of deferral of tax for the period during which the common shares of the PFIC are owned. However, if the US Holder makes a timely election to treat a PFIC as a qualified electing fund with respect to such shareholder’s interest therein, the above-described rules generally will not apply. Instead, the electing US Holder would include annually in gross income his/her or its pro rata share of the PFIC’s ordinary earnings and net capital gain regardless of whether such income or gain was actually distributed. A US Holder making a qualified electing fund election can, however, under certain circumstances, elect to defer the payment of United States federal income tax on such income inclusions subject to an interest charge on the amount of deferred taxes. Special rules apply to US Holders who own their interests in a PFIC through intermediate entities or persons. In addition, subject to certain limitations, US Holders owning (actually or constructively) marketable stock in a PFIC will be permitted to elect to mark that stock to market annually, rather than be subject to the tax regime described above. Amounts included in or deducted from income under this alternative (and actual gains and losses realized upon disposition, subject to certain limitations) will be treated as ordinary gains or losses.
We have not determined whether we are or have been a PFIC in earlier years. US Holders of the common shares should consult with their own tax advisor concerning the possible application of the PFIC provisions in their circumstances.
53
Controlled Foreign Corporation
If more than 50 percent of the voting power of all our classes of stock or the total value of the stock is owned, directly or indirectly (including through attribution), by citizens or residents of the United States, United States domestic partnerships and corporations or estates or trusts other than foreign estates or trusts, each of whom own 10 percent or more of the total combined voting power of all our classes of stock (a “United States Shareholder”), we are a “controlled foreign corporation”. This classification generally results in the inclusion of certain income of a Controlled Foreign Corporation in the US Shareholders’ US income as a deemed dividend. In addition, under Section 1248 of the Code, a gain from the sale or exchange of stock by a holder of common shares who is or was a United States Shareholder at any time during the five year period ending with the sale or exchange, may be treated as ordinary dividend income.
F. Dividends and Paying Agents
Not Applicable.
G. Statement by Experts
Not Applicable.
H. Documents on Display
We have filed the documents referred to herein and other information with the SEC, the Ontario Securities Commission and the Alberta Securities Commission. You may inspect and copy such material at the public reference facilities maintained by the SEC at Room 1024, 450 Fifth Street, N.W., Washington, D.C. 20549, as well as at the SEC’s regional offices at 500 West Madison Street, Suite 1400, Chicago, Illinois 60661 and the Woolworth Building, 233 Broadway, New York, New York 10279. You may also obtain copies of such material from the SEC at prescribed rates by writing to the Public Reference Section of the SEC, 450 Fifth Street, N.W., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the public reference rooms.
The SEC maintains an Internet website atwww.sec.gov that contains reports, proxy statements, information statements and other material that are filed through the SEC’s Electronic Data Gathering, Analysis and Retrieval (“EDGAR”) system. Documents filed with the Ontario Securities Commission and the Alberta Securities Commission can be accessed through an Internet website atwww.sedar.com that contains reports, proxy statements, information statements and other material that are filed through the System for Electronic Document Analysis and Retrieval (“SEDAR”).
Additional information is also available on our website atwww.micromeminc.com. Such information on our website is not part of this Form 20-F.
I. Subsidiary Information
Not Applicable.
| | |
| ITEM 11. | | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Not Applicable.
| | |
| ITEM 12. | | DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES |
Not Applicable.
54
PART II
| | |
| ITEM 13. | | DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES |
Not Applicable.
| | |
| ITEM 14. | | MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS |
Not Applicable.
| | |
| ITEM 15. | | CONTROLS AND PROCEDURES |
We operate as a development stage company and have historically had only limited accounting personnel and resources with which to address our internal control procedures.
In 2005, we engaged an independent firm of chartered accountants to complete an in depth review of our internal accounting procedures and controls and are now in the process of implementing the recommendations which were made. We have upgraded our accounting software and have developed formalized budgets in 2005. The Audit Committee has met on a quarterly basis to assess our financial performance and to review the progress management has made in upgrading its accounting procedures and controls. The Audit Committee has also interacted with our external auditors in 2005 on reporting and control related matters.
When our auditors audited our financial statements as of and for the year ended October 31, 2005 they identified significant deficiencies in our internal accounting controls. Significant deficiencies noted were that we lacked certain formalized accounting policies and procedures including written procedures for the monthly, quarterly and annual closing of our financial books and records, our staff was not always subject to timely review and supervision and security practices over our information technology were not sufficiently robust.
Our management is implementing measures required to remedy the material weaknesses and reportable conditions in our internal controls but has not managed to make these changes effective in their entirety. These control deficiencies are not expected to have any future material impact on our financial statements. If, however, we fail to continue to adequately staff our accounting and finance function and maintain adequate internal controls, we may not meet the demands that are placed upon us as a public company, including the requirements of the Sarbanes-Oxley Act of 2002 and our business would accordingly face repercussions. We expect to implement all measures required to remedy the material weaknesses and reportable controls by the end of fiscal 2006.
Our Chief Executive Officer and Chief Financial Officer have evaluated the effectiveness of our disclosure controls and procedures within the 90 days prior to the date of filing of this annual report on Form 20-F. For the reasons stated above our Chief Executive Officer and Chief Financial Officer have concluded that our internal controls over financial reporting are not effective, as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended.
No change in our internal controls over financial reporting, as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended, occurred during the fiscal year ended October 31, 2005 that has materially affected or is reasonable likely to materially affect, our internal controls over financial reporting.
55
PART III
Item 16.
Not Applicable.
| | |
| Item 16A. | | Audit Committee Financial Expert |
Our Board of Directors has determined that a member of the Board of Directors, David Sharpless, is an audit committee financial expert.
We have adopted a Code of Ethics to impose certain policies relating to ethical conduct on all of our Directors and employees, including our Chief Executive Officer, Chief Financial Officer, principal accounting officer and persons performing similar functions. We undertake to provide a copy of our Code of Ethics to any holder of our securities upon request, without charge.
| | |
| Item 16C. | | Principal Accountant Fees and Services |
On October 28, 2004, the Audit Committee of the Board of Directors replaced Ernst & Young LLP, as our independent firm of Chartered Accountants with Grant Thornton LLP as our new independent firm of Chartered Accountants.
On September 28, 2005, the Audit Committee of the Board of Directors replaced Grant Thornton LLP, as our independent firm of Chartered Accountants and engaged Schwartz Levitsky Feldman LLP as our new independent firm of Chartered Accountants.
The reports of Grant Thornton LLP and Ernst & Young LLP on the our financial statements for the fiscal years ended October 31, 2004 and October 31, 2003, did not contain an adverse opinion or a disclaimer of opinion and were not qualified or modified as to uncertainty, audit scope, or accounting principles.
The following table presents fees for professional audit services rendered by our auditors for the audit of our consolidated financial statements for the years ended October 31, 2005 and 2004, and fees billed for other services rendered by our auditors including our offerings of securities and tax services.
| | | | | | | | | |
| | | 2005 | | 2004 |
| Audit Fees | | $ | 55,000 | | | $ | 76,000 | |
| Audit Related Fees | | | — | | | | — | |
| Tax Fees | | | — | | | | — | |
| All Other Fees | | | 30,000 | | | | 6,000 | |
Audit Fees
In 2005 we paid a total of $55,000 to Schwartz Levitsky Feldman LLP for audit services, which included work related to the annual audit.
In 2004 we paid a total of $76,000 to Grant Thornton LLP for audit services, which included work related to the annual audit.
56
Audit Related Fees
We did not pay any audit related fees to Schwartz Levitsky Feldman LLP during 2005.
We did not pay any audit related fees to Grant Thornton LLP for services during 2005 and 2004.
We did not pay any audit related fees to Ernst & Young LLP during 2004.
Tax Fees
We did not pay any tax fees to Schwartz Levitsky Feldman LLP for income tax compliance, tax advice and tax planning during 2005.
We did not pay any tax fees to Grant Thornton LLP for income tax compliance, tax advice and tax planning during 2004.
All Other Fees
Schwartz Levitsky Feldman LLP did not bill us for any other services during 2005.
DMCT LLP billed us $30,000 for the review that they completed on our internal control and financial reporting procedures in 2005.
Grant Thornton LLP billed us $2,000 for other services during 2005.
Ernst & Young LLP billed us $6,000 for other services during 2004.
| | |
| Item 16D. | | Exemptions from the Listing Standards for Audit Committees |
Not applicable.
| | |
| Item 16E. | | Purchases of Equity by the Issuer and Affiliated Purchasers. |
Not applicable.
57
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
ITEM 17. FINANCIAL STATEMENTS
INDEPENDENT AUDITORS’ REPORT
To the Shareholders of
Micromem Technologies Inc.
(A Development Stage Company)
We have audited the consolidated balance sheet of Micromem Technologies Inc. as at October 31, 2005 and the consolidated statements of operations and deficit and cash flows for the year then ended. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audit.
We conducted our audit in accordance with Canadian generally accepted auditing standards and with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform an audit to obtain reasonable assurance whether the consolidated financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation.
In our opinion, these consolidated financial statements present fairly, in all material respects, the financial position of the Company as of October 31, 2005 and the results of its operations and its cash flows for the year then ended in accordance with Canadian generally accepted accounting principles.
We also audited the adjustments in Note 8 (b) to the consolidated financial statements that were applied to restate the 2004 and 2003 financial statements. In our opinion, such adjustments are appropriate and have been properly applied.
Accounting principles generally accepted in the United States of America are consistent with those applicable in Canada in all material respects (see note 16).
The consolidated financial statements as at October 31, 2004 and for the year then ended and for the cumulative period from September 3, 1997 to October 31, 2004 were audited by other auditors who expressed opinions without reservation on those statements in their reports dated February 11, 2005, December 5, 2003, January 27, 2003, November 16, 2001, December 13, 2000, February 25, 2000 and December 20, 1999.
/s/ SCHWARTZ LEVITSKY FELDMAN LLP
Chartered Accountants
Toronto, Ontario, Canada
February 10, 2006
58
COMMENTS BY AUDITORS FOR US READERS ON
CANADA — US REPORTING DIFFERENCE
In the United States, reporting standards for auditors require that the following information be included in the auditor’s report:
The addition of an explanatory paragraph (following the opinion paragraph) when the financial statements are affected by conditions and events that cast substantial doubt on the Company’s ability to continue as a going concern, such as those described in note 2 to the consolidated financial statements. Our report to the shareholders dated February 10, 2006 is expressed in accordance with Canadian reporting standards, which do not permit a reference to such events and conditions in the auditors’ report when these are adequately disclosed in the consolidated financial statements.
The segregation and quantification of cumulative expenses from inception to October 31, 2004 is required to be disclosed in the introductory paragraph of the auditor's report. Such disclosure would state that the total assets of $474,234 as of October 31, 2004 and cumulative expenses of $50,966,254 for the period from the date of inception to October 31, 2004 were audited by other auditors whose reports have been furnished to us and our opinion, in so far as it relates to the cumulative financial information for the Company for the period from the date of inception through October 31, 2004, is based solely on the reports of other auditors. Our opinion on the consolidated financial statements would refer the reports of the other auditors for the period from inception to October 31, 2004. Our report to the shareholders dated February 10, 2006 is expressed in accordance with Canadian reporting standards, which do not permit reference to the reports of other auditors in our auditor’s reports.
The reference to a restatement of the 2004 and 2003 comparative figures as described in note 8(b) to the consolidated financial statements is normally presented in the introductory paragraph under US reporting standards.
/s/ SCHWARTZ LEVITSKY FELDMAN LLP
Chartered Accountants
Toronto, Ontario, Canada
February 10, 2006
59
Consolidated Balance Sheets
(Expressed in United States dollars)
(See Note 2 — Going Concern)
| | | | | | | | | |
| As at | | October 31, 2005 | | October 31, 2004 |
| Assets | | | | | | | | |
| Current assets: | | | | | | | | |
| Cash and cash equivalents | | $ | 642,803 | | | $ | 350,504 | |
| Term deposits | | | — | | | | 87,243 | |
| Deposits and other receivables (Note 5) | | | 85, 572 | | | | 33,562 | |
| |
| | | | 728,375 | | | | 471,309 | |
| Property and equipment (Note 6) | | | — | | | | 2,925 | |
| Patents and trademarks (Note 7) | | | — | | | | — | |
| Royalty rights (Note 4 and Note 10) | | | — | | | | — | |
| |
| | | | 728,375 | | | | 474,234 | |
| |
| | | | | | | | | |
| Liabilities and Shareholders’ Equity (Deficiency) | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable and accrued liabilities (Note 5) | | | 803,206 | | | | 436,624 | |
| |
| |
| Shareholders’ equity (deficiency): | | | | | | | | |
| Share capital: (Note 8) | | | | | | | | |
| Authorized: | | | | | | | | |
| 2,000,000 special preference shares, redeemable, voting Unlimited common shares without par value | | | | | | | | |
| Issued and outstanding: | | | | | | | | |
| 64,719, 449 common shares ( 2004: 58,063,437) | | | 34,305,087 | | | | 32,103,787 | |
| Contributed surplus (Notes 8 (b) and 9) | | | 20,140,112 | | | | 18,418,370 | |
| Deficit accumulated during the development stage | | | (54,520,030 | ) | | | (50,484,547 | ) |
| |
| | | | (74,831 | ) | | | 37,610 | |
| |
| |
| | | $ | 728,375 | | | $ | 474,234 | |
| |
Commitments (Note 13)
Contingencies (Note 14)
| | | |
| | |
| Joseph Fuda, Director | | |
| | | |
“David Sharpless” (Signed) | | |
| David Sharpless, Director | | |
See accompanying notes to the consolidated financial statements.
60
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
Consolidated Statements of Operations and Deficit
(Expressed in United States dollars)
For the year ended October 31, 2005 (with comparative data)
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Period from |
| | | | | | | | | | | | | | | September 3, 1997 |
| | | Oct. 31, 2005 | | Oct. 31, 2004 | | Oct. 31, 2003 | | to October 31, 2005 |
| | | (12 mos) | | (12 mos) | | (12 mos) | | | | |
| Interest and other income | | $ | 8,703 | | | $ | 4,746 | | | $ | 20,121 | | | $ | 540,087 | |
| |
| | | | | | | | | | | | | | | | | |
| Costs and expenses (income): | | | | | | | | | | | | | | | | |
| Administration | | | 320,383 | | | | 157,854 | | | | 176 ,361 | | | | 2,470,422 | |
Professional, Management and consulting fees (Notes 8 (b)
and 12 (c)) | | | 1,303,662 | | | | 271,351 | | | | 303,222 | | | | 4,458,608 | |
| Wages and salaries (Note 12 (b)) | | | 152,628 | | | | 31,563 | | | | 112,437 | | | | 9,521,802 | |
| Research and development (Notes 6 and 13) | | | 362,141 | | | | 378,410 | | | | 490,914 | | | | 6,756,833 | |
| Travel and entertainment | | | 169,737 | | | | 77,616 | | | | 17,508 | | | | 1,237,319 | |
| Amortization of property and equipment (Note 6) | | | 2,925 | | | | 5,410 | | | | 36,921 | | | | 344,466 | |
| Stock compensation expense (Note 8(b)) | | | 1,721,742 | | | | 1,379,970 | | | | 318,000 | | | | 19,561,221 | |
| Unrealized foreign exchange loss (gain) | | | 10,968 | | | | 16,870 | | | | (61,657 | ) | | | (43,172 | ) |
| Amortization of patents and trademarks | | | — | | | | — | | | | 36,258 | | | | 67,596 | |
| Operating leases | | | — | | | | — | | | | — | | | | 109,412 | |
| Loss on sale of investment | | | — | | | | — | | | | — | | | | 54,606 | |
| Write-down of investment | | | — | | | | — | | | | — | | | | 61,020 | |
| Write-down of royalty rights (Note 10) | | | — | | | | — | | | | — | | | | 10,000,000 | |
| Write-down of patents and trademarks (Note 7) | | | — | | | | — | | | | 299,820 | | | | 299,820 | |
| Interest expense | | | — | | | | — | | | | — | | | | 75,027 | |
| Loss on sale of property and equipment | | | — | | | | — | | | | 58,302 | | | | 65,460 | |
| |
| |
| | | | 4,044,186 | | | | 2,319,044 | | | | 1,788,086 | | | | 55,040,440 | |
| |
| | | | | | | | | | | | | | | | | |
| Loss before income taxes | | | (4,035,483 | ) | | | (2,314,298 | ) | | | (1,767,965 | ) | | | (54,500,353 | ) |
| | | | | | | | | | | | | | | | | |
| Provision for income taxes (Note 11) | | | — | | | | — | | | | — | | | | 19,677 | |
| |
| | | | | | | | | | | | | | | | | |
| Net loss for the period | | | (4,035,483 | ) | | | (2,314,298 | ) | | | (1,767,965 | ) | | | (54,520,030 | ) |
| | | | | | | | | | | | | | | | | |
| Deficit accumulated during the development stage, beginning of period — as restated (Note 8) | | | (50,484,547 | ) | | | (48,170,249 | ) | | | (46,402,284 | ) | | | — | |
| |
| Deficit accumulated during the development stage, end of period | | ($ | 54,520,030 | ) | | ($ | 50,484,547 | ) | | ($ | 48,170,249 | ) | | ($ | 54,520,030 | ) |
| |
| | | | | | | | | | | | | | | | | |
| Loss per share — basic and diluted | | | (0.07 | ) | | | (0.04 | ) | | | (0.04 | ) | | | (1.13 | ) |
| |
| | | | | | | | | | | | | | | | | |
| Weighted average number of shares | | | 62,155,234 | | | | 52,958,975 | | | | 47,061,810 | | | | 48,145,724 | |
| |
See accompanying notes to the consolidated financial statements.
61
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
Consolidated Statements of Cash Flows
(Expressed in United States dollars)
For the year ended Oct. 31, 2005 (with comparative data)
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Period from |
| | | | | | | | | | | | | | | September 03, 1997 |
| | | Oct. 31, 2005 | | Oct. 31, 2004 | | Oct. 31, 2003 | | to October 31, 2005 |
| | | (12 mos) | | (12 mos) | | (12 mos) | | | | |
| Cash flows from operating activities: | | | | | | | | | | | | | | | | |
| Net loss for the period | | | ($4,035,483 | ) | | | ($2,314,298 | ) | | | ($1,767,965 | ) | | | ($54,520,030 | ) |
| Adjustments to reconcile loss for the period to net cash used in operating activities: | | | | | | | | | | | | | | | | |
| Amortization of patents and trademarks | | | — | | | | — | | | | 36,258 | | | | 67,596 | |
| Amortization of property and equipment | | | 2,925 | | | | 5,410 | | | | 36, 921 | | | | 529,686 | |
| Stock option expense | | | 1,721,742 | | | | 1,379,970 | | | | 318,000 | | | | 19,561,221 | |
| Loss on sale of investment | | | — | | | | — | | | | — | | | | 49,810 | |
| Write down of investment | | | — | | | | — | | | | — | | | | 61,020 | |
| Loss on disposal of property and equipment | | | — | | | | — | | | | 58,302 | | | | 65,460 | |
| Write-down of royalty rights | | | — | | | | — | | | | — | | | | 10,000,000 | |
| Write-down of patents and trademarks | | | — | | | | — | | | | 299,820 | | | | 299,820 | |
| Share compensation expense | | | — | | | | — | | | | — | | | | 7,285,696 | |
| Non-cash wages and salaries | | | — | | | | — | | | | — | | | | 34,000 | |
| Decrease (increase) in deposits and other receivables | | | (52,010 | ) | | | 13,645 | | | | 145,924 | | | | (76,975 | ) |
| Increase in accounts payable and accrued liabilities | | | 366,582 | | | | 190,924 | | | | 54,181 | | | | 697,162 | |
| |
| Net cash used in operating activities | | | (1,996,244 | ) | | | (724,349 | ) | | | (818,559 | ) | | | (15,945,534 | ) |
| |
| | | | | | | | | | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | | | | | | | | | |
| Purchase of property and equipment | | | — | | | | (4,567 | ) | | | (2,025 | ) | | | (729,604 | ) |
| Proceeds on disposal of property and equipment | | | — | | | | — | | | | 1,688 | | | | 134,458 | |
| Patents and trademarks | | | — | | | | — | | | | (28,380 | ) | | | (367,416 | ) |
| Sale of available-for-sale investment | | | — | | | | — | | | | — | | | | 260,641 | |
| Royalty rights | | | — | | | | — | | | | — | | | | (2,000,000 | ) |
| Term deposits | | | 87,243 | | | | 132,687 | | | | (219,930 | ) | | | — | |
| |
| Net cash (used in) investing activities | | | 87,243 | | | | 128,120 | | | | (248,647 | ) | | | (2,701,921 | ) |
| |
| | | | | | | | | | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | | | | | | | | | |
| Issue of common shares | | | 2,201,300 | | | | 867,500 | | | | 162,500 | | | | 18,709,533 | |
| Net proceeds from shareholder’s Loan | | | — | | | | — | | | | — | | | | 544,891 | |
| Loan proceeds from Avanticorp international inc. | | | — | | | | — | | | | — | | | | 112,031 | |
| Rights issue costs | | | — | | | | — | | | | — | | | | (76,197 | ) |
| |
| Net cash provided bv financing activities | | | 2,201,300 | | | | 867,500 | | | | 162,500 | | | | 19,290,258 | |
| |
| | | | | | | | | | | | | | | | | |
| Increase (decrease) in cash and cash equivalents | | | 292,299 | | | | 271,271 | | | | (904,706 | ) | | | 642,803 | |
| | | | | | | | | | | | | | | | | |
| Cash and cash equivalents, beginning of period | | | 350,504 | | | | 79,233 | | | | 983,939 | | | | — | |
| |
| |
| Cash and cash equivalents, end of period | | $ | 642,803 | | | $ | 350,504 | | | $ | 79,233 | | | $ | 642,803 | |
| |
| | | | | | | | | | | | | | | | | |
| Supplemental cash flow information: | | | | | | | | | | | | | | | | |
| Interest paid | | | — | | | | — | | | | — | | | | 76, 987 | |
| Income taxes paid | | | — | | | | — | | | | — | | | | 66,722 | |
| |
See accompanying notes to the consolidated financial statements.
62
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY (DEFICIENCY)
(Expressed in United States dollars)
October 31, 2005
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Deficit | |
| | | Number of | | | | | | Contributed | | | Deferred Share | | | Accumulated | |
| | | Shares | | | Share Capital | | | Surplus | | | Compensation | | | During Development | |
| |
| Micromem share capital, October 31, 1998 | | | 3,490,643 | | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | |
| Exercise of director’s stock options | | | 490,000 | | | | — | | | | — | | | | — | | | | — | |
| Pageant share capital, October 31, 1998 | | | — | | | | 1 | | | | — | | | | — | | | | — | |
| Net loss for the year | | | — | | | | — | | | | — | | | | — | | | | (500,992 | ) |
| Common shares of Pageant, December 4, 1998 | | | — | | | | 4,999 | | | | — | | | | — | | | | — | |
| Assigned fair value of net assets (Note 3 (c)) | | | 32,000,000 | | | | 549,140 | | | | — | | | | — | | | | — | |
| |
| Micromem share capital,September 11, 1999 | | | 35,980,643 | | | | 554,140 | | | | — | | | | — | | | | (500,992 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Exercise of common share purchase warrants for cash | | | 120,676 | | | | 164,053 | | | | — | | | | — | | | | — | |
| Private placement of common shares for cash, May 17, 1999 | | | 350,000 | | | | 1,050,000 | | | | — | | | | — | | | | — | |
| Shareholder loan forgiven (Note 9) | | | — | | | | — | | | | 544,891 | | | | — | | | | — | |
| Exercise of stock options for cash | | | 100,000 | | | | 300,000 | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | |
| Net loss for the year | | | — | | | | — | | | | — | | | | — | | | | (5,207,787 | ) |
| |
| Balance, October 31,1999 | | | 36,551,319 | | | | 2,068,193 | | | | 544,891 | | | | — | | | | (5,708,779 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Exercise of common share purchase warrants for cash | | | 182,087 | | | | 274,717 | | | | — | | | | — | | | | — | |
| Exercise of stock options for cash | | | 100,000 | | | | 300,000 | | | | — | | | | — | | | | — | |
| Deferred share compensation (Note 12) | | | — | | | | — | | | | 2,711,881 | | | | (453,219 | ) | | | — | |
| Private placement of common shares for cash, February 10, 2000 | | | 2,000,000 | | | | 5,000,000 | | | | — | | | | — | | | | | |
| Common shares issued pursuant to compensation agreements, March 15, 2000 | | | 901,110 | | | | 4,206,447 | | | | — | | | | — | | | | — | |
| Stock options issued to directors/consultants | | | — | | | | — | | | | 9,681,257 | | | | — | | | | — | |
| |
| Net loss for the year | | | — | | | | — | | | | — | | | | — | | | | (16,940,613 | ) |
| |
| Balance, October 31, 2000 | | | 39,734,516 | | | | 11,849,357 | | | | 12,938,029 | | | | (453,219 | ) | | | (22,649,392 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Exercise of common share purchase warrants for cash | | | 362,450 | | | | 554,655 | | | | — | | | | — | | | | — | |
| Common shares issued under rights offering November 20, 2000 | | | 304,674 | | | | 1,119,058 | | | | — | | | | — | | | | — | |
| Exercise of stock options for cash | | | 800,000 | | | | 2,400,000 | | | | — | | | | — | | | | — | |
| Deferred share compensation (Note 12) | | | — | | | | — | | | | (453,219 | ) | | | 453,219 | | | | — | |
| Stock-based compensation | | | — | | | | — | | | | 34,000 | | | | — | | | | — | |
| Exercise of director’s stock options for cash, January 17, 2001 | | | 714,686 | | | | 71,469 | | | | — | | | | — | | | | — | |
| Common shares issued pursuant to compensatory stock options, at January 17, 2001 (Note | | | — | | | | 1,581,242 | | | | (1,581,242 | ) | | | — | | | | — | |
| Adjustment-share compensation expenses (Note 12) | | | — | | | | — | | | | (677,420 | ) | | | — | | | | — | |
| Common shares issued pursuant to compensation agreement, January 23, 2001(Note 12 (a)) | | | 11,192 | | | | 66,461 | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | |
| Private placement of common shares for cash, March 21, 2001 | | | 2,000,000 | | | | 4,000,000 | | | | — | | | | — | | | | — | |
| Common shares issued under asset purchase agreement to Estancia Limited, March 14, 2001 | | | 2,007,831 | | | | 8,000,000 | | | | — | | | | — | | | | — | |
| |
| Compensation shares due but not issued (Note 12) | | | — | | | | — | | | | 1,431,545 | | | | — | | | | — | |
| Stock options issued to directors/consultants | | | | | | | | | | | 4,627,752 | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Net loss for the year | | | — | | | | — | | | | — | | | | — | | | | (9,187,377 | ) |
| |
| Balance, October 31, 2001 | | | 45,935,349 | | | $ | 29,642,242 | | | $ | 16,319,445 | | | $ | — | | | ($ | 31,836,769 | ) |
| |
See accompanying notes to the consolidated financial statements.
63
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY (DEFICIENCY)
(Expressed in United States dollars)
October 31, 2005
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Deficit | |
| | | Number of | | | | | | Contributed | | | Deferred Share | | | Accumulated | |
| | | Shares | | | Share Capital | | | Surplus | | | Compensation | | | During Development | |
| |
| Balance, October 31, 2001 | | | 45,935,349 | | | $ | 29,642,242 | | | $ | 16,319,445 | | | $ | — | | | | ($31,836,769 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| Shares issued pursuant to compensatory agreement, March 26, 2002 (Note 12) | | | 765,588 | | | | 1,431,545 | | | | (1,431,545 | ) | | | — | | | | — | |
| Stock options issued to directors/consultants | | | — | | | | — | | | | 1,832,500 | | | | | | | | | |
| |
| Net loss for the year | | | — | | | | — | | | | | | | | — | | | | (14,565,515 | ) |
| |
| Balance, October 31, 2002 | | | 46,700,937 | | | | 31,073,787 | | | | 16,720,400 | | | | — | | | | (46,402,284 | ) |
| |
| Private placement of common shares for cash, August 13, 2003 (Note 8(d)) | | | 2,031,250 | | | | 162,500 | | | | — | | | | — | | | | — | |
| Stock options issued to directors/consultants | | | — | | | | — | | | | 318,000 | | | | | | | | | |
| |
| Net loss for the year | | | — | | | | — | | | | — | | | | — | | | | (1,767,965 | ) |
| |
| Balance, October 31, 2003 | | | 48,732,187 | | | | 31,236,287 | | | | 17,038,400 | | | | — | | | | ($48,170,249 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Private Placement of common shares for cash, December 2003 (Note 8 (e) ii) | | | 500,000 | | | | 40,000 | | | | — | | | | — | | | | — | |
| Private Placement of common shares for cash, December 2003 (Note 8 (e) i) | | | 300,000 | | | | 33,000 | | | | — | | | | — | | | | — | |
| Exercise of common share purchase warrants for cash (Note 8(d), August 2004 | | | 2,031,250 | | | | 162,500 | | | | — | | | | — | | | | — | |
| Exercise of common share purchase warrants for cash (Note 8 (e) ii) June-September 2004 | | | 1,000,000 | | | | 80,000 | | | | — | | | | — | | | | — | |
| Exercise of common share purchase warrants for cash (Note 8 (e) i) October 2004 | | | 200,000 | | | | 22,000 | | | | — | | | | — | | | | — | |
| Exercise of options for cash | | | 5,300,000 | | | | 530,000 | | | | — | | | | — | | | | — | |
| Stock options issued to directors/consultants | | | — | | | | — | | | | 1,379,970 | | | | — | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Net loss for the year | | | — | | | | — | | | | — | | | | — | | | | (2,314,298 | ) |
| |
| Balance, October 31, 2004 | | | 58,063,437 | | | | 32,103,787 | | | | 18,418,370 | | | | — | | | | ($50,484,547 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Exercise of common share purchase warrants for cash (Note 8(e)), December — January | | | 400,000 | | | | 44,000 | | | | — | | | | — | | | | — | |
| Private placement of common shares for cash | | | 1,028,334 | | | | 617,000 | | | | — | | | | — | | | | — | |
| Stock options issued to consultants/employees | | | — | | | | — | | | | 202,203 | | | | — | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | — | | | | — | | | | — | | | | — | | | | | |
| Net loss for the quarter | | | — | | | | — | | | | — | | | | — | | | | (453,523 | ) |
| |
| Balance at January 31, 2005 | | | 59,491,771 | | | | 661,000 | | | | 18,620,573 | | | | — | | | | ($50,938,070 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Exercise of common shares purchase warrants for cash (Note 8(d)), February, 2005 | | | 1,406,250 | | | | 112,500 | | | | — | | | | — | | | | — | |
| Private Placement of common shares for cash, March, 2005 | | | 1,300,000 | | | | 845,000 | | | | — | | | | — | | | | — | |
| Private Placement of common shares for cash, February, 2005 | | | 14,000 | | | | 10,500 | | | | — | | | | — | | | | — | |
| Legal expenses relating to private placements | | | — | | | | (75,000 | ) | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | |
| Net loss for the quarter | | | — | | | | — | | | | — | | | | — | | | | (474,227 | ) |
| |
| Balance at April 30, 2005 | | | 62,212,021 | | | | 33,657,787 | | | | 18,620,573 | | | | | | | | (51,412,297 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Exercise of stock options (Note 8(b)), June, 2005 | | | 1,820,000 | | | | 553,600 | | | | | | | | | | | | | |
| Settlement of accounts payable for common shares | | | 62,428 | | | | 43,700 | | | | — | | | | — | | | | | |
| Stock options issued to consultants/employees | | | — | | | | — | | | | 903,040 | | | | — | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Net loss for the quarter | | | — | | | | — | | | | — | | | | — | | | | (1,726,931 | ) |
| |
| Balance at July 31, 2005 | | | 64,094,449 | | | $ | 34,255,087 | | | $ | 19,523,613 | | | $ | — | | | | ($53,139,228 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Exercise of common shares purchase warrants for cash September, 2005 | | | 625,000 | | | | 50,000 | | | | — | | | | — | | | | | |
| Stock options issued to consultants | | | — | | | | — | | | | 616,499 | | | | — | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Net loss for the quarter | | | — | | | | — | | | | — | | | | — | | | | (1,380,802 | ) |
| |
| Balance at October 31, 2005 | | | 64,719,449 | | | $ | 34,305,087 | | | $ | 20,140,112 | | | $ | — | | | | ($54,520,030 | ) |
| |
See accompanying notes to the consolidated financial statements.
64
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in United States dollars)
October 31, 2005
| 1. | | Nature of business: |
| |
| | | Micromem Technologies Inc. (“Micromem” or the “Company”) is a corporation incorporated under the laws of the Province of Ontario, Canada. By Articles of Amendment dated January 14, 1999, the Company changed its name from Avanticorp International Inc. to Micromem Technologies Inc. On January 11, 1999, the Company acquired all of the outstanding shares of Pageant Technologies Inc. (“Pageant”), a company subsisting under the laws of Barbados. This acquisition, as described in Note 3(c), was recorded as a reverse takeover under Canadian generally accepted accounting principles (“Canadian GAAP”). |
| |
| | | The Company currently operates in a single segment as a developer of non-volatile magnetic memory technology. The Company has not generated significant revenue through October 31, 2005, has no planned principal operations and is devoting substantially all of its efforts to the development of its technology. Accordingly, for financial reporting purposes, the Company is a development stage enterprise. |
| 2. | | Going concern: |
| |
| | | These consolidated financial statements have been prepared on the “going concern” basis, which presumes that the Company will be able to realize its assets and discharge its liabilities in the normal course of business for the foreseeable future. |
| |
| | | The Company has incurred substantial losses to date. It will be necessary to raise additional funds for the continuing development, testing and commercial exploitation of its technology. The sources of these funds have not yet been identified and there can be no certainty that sources will be available in the future. |
| |
| | | The Company continues to pursue its research initiatives as outlined in Note 13 in order to develop its technology for commercial applications and continues to raise financing for operations as outlined in Note 8(e). |
| |
| | | The Company’s ability to continue as a going concern is in substantial doubt and it is dependent upon completing the development of its technology for a particular application, achieving profitable operations, obtaining additional financing and successfully bringing its technology to the market. The outcome of these matters cannot be predicted at this time. The consolidated financial statements have been prepared on a going concern basis and do not include any adjustments to the amounts and classifications of the assets and liabilities that might be necessary should the Company be unable to continue in business. If the “going concern” assumption were not appropriate for these consolidated financial statements then adjustments would be necessary in the carrying value of assets and liabilities, the reported expenses and the balance sheet classifications used. |
65
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in United States dollars)
October 31, 2005
| 3. | | Summary of significant account policies: |
| |
| | | These consolidated financial statements have been prepared in accordance with Canadian GAAP and are stated in United States dollars. These principles are also in conformity in all material respects with United States generally accepted accounting principles (“U.S. GAAP”) as described in Note 16 to the consolidated financial statements. The most significant accounting policies are as follows: |
| | a. | | Financial reporting: |
| |
| | | | The Company has adopted Section 1100 of the Canadian Institute of Chartered Accountants (“CICA”) Handbook, “Generally Accepted Accounting Principles” (“GAAP”). This section establishes standards for financial reporting in accordance with GAAP and provides guidance on sources to consult with when selecting accounting policies and determining the appropriate disclosures when an item is not explicitly dealt with in the primary sources of GAAP. The Company also adopted Section 1400 of the CICA Handbook, “General Standards of Financial Statement Presentation”. This section clarifies what constitutes “fair presentation in accordance with GAAP”. The Company also adopted Section 3063 of the CICA Handbook, “Impairment of Long Lived Assets”. This section requires the Company to measure and disclose impairments of long-lived assets. Adoption of these sections did not have a material impact on the Company’s financial statements. |
| |
| | b. | | Principles of consolidation: |
| |
| | | | These consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries, Memtech International Inc., Memtech International (U.S.A.) Inc., Pageant Technologies Inc. and Pageant Technologies (U.S.A.) Inc. |
| |
| | | | During the fiscal year ending October 31, 2003, two of the Company’s subsidiaries, Micromem Technologies B.V. and Micromem Technologies S.p.A. were wound up. All significant intercompany balances and transactions have been eliminated upon consolidation. |
| |
| | c. | | Basis of presentation: |
| |
| | | | On January 11, 1999, the Company issued 32,000,000 common shares and 1,000,000 warrants to acquire all of the issued and outstanding shares of Pageant. On that date, the total number of the Company shares outstanding was 35,980,643 shares. As a result of this transaction, the shareholders of Pageant owned 88.9% of the outstanding common shares of the Company and, accordingly, the purchase of Pageant was accounted for as a reverse takeover transaction. The transaction was accounted for by the purchase method with the results of operations included in the consolidated financial statements from the date of acquisition. |
66
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in United States dollars)
October 31, 2005
| | d. | | Use of estimates: |
| |
| | | | The preparation of consolidated financial statements in conformity with Canadian GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of income and expenses during the reporting period. Actual results could differ from those estimates. |
| |
| | e. | | Cash and cash equivalents: |
| |
| | | | Cash and cash equivalents consist of all bank accounts and all highly liquid investments with original maturities of three months or less at the date of purchase. |
| |
| | f. | | Property and equipment: |
| |
| | | | Property and equipment are recorded at cost less accumulated amortization. Amortization is provided on property and equipment on a straight-line basis for a period of up to three years. Property and equipment are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. When circumstances dictate, an impairment loss is calculated as equal to the excess of the carrying value of the assets over their undiscounted estimated future net cash flow. |
| |
| | g. | | Patents and trademarks: |
| |
| | | | Patents and trademarks are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. When circumstances dictate, an impairment loss is calculated as equal to the excess of the carrying value of the assets over their undiscounted estimated future net cash flow (Note 7). |
| |
| | h. | | Research and development expenses: |
| |
| | | | Research costs are expensed in the period incurred. Development expenses are expensed as incurred unless they meet the criteria for deferral and amortization under Canadian GAAP which is the translation of research findings or other knowledge into a plan for the technology prior to commercial production or use. The Company has determined that no development costs have met these criteria at the financial reporting date. |
67
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in United States dollars)
October 31, 2005
| | i. | | Stock-based compensation: |
| |
| | | | The Company has a stock-based compensation plan, which is described in Note 8. Stock-based compensation is recognized by the fair value method, whereby compensation is recorded to the extent that the exercise price is not based on the market value of the Company’s common shares at the date of grant. Any compensatory benefit recorded is recognized initially as deferred share compensation in the consolidated statements of shareholders’ equity and then charged against income over the contractual or vesting period. |
| |
| | | | Until October 31, 2004 for all awards of employee stock-based compensation granted after January 1, 2002, the Company recognized employee stock-based compensation costs under the intrinsic value-based method and provided pro forma disclosure of net income and earnings per share as if the fair value-based method had been applied. |
| |
| | | | Effective November 1, 2004 the Company has adopted the fair value method of accounting for employee stock-based compensation costs. Accordingly the financial statements for the years ending October 31, 2004 and 2003 and the cumulative financial statements for the period from September 3, 1997 to October 31, 2005 have been restated to reflect the stock-based compensation costs that the Company has incurred in each period which expense previously was disclosed on a proforma basis. |
| |
| | | | The stock-based compensation expense for options granted during the twelve-month period ending October 31, 2005 has been reflected as an expense in the consolidated statement of operations for the period then ended. |
| |
| | j. | | Income taxes: |
| |
| | | | The Company accounts for income taxes by the liability method. Under the liability method, future tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Future tax assets and liabilities are measured using substantively enacted tax rates and laws that are expected to apply when the asset is realized or the liability settled. To the extent that it is not considered to be more likely than not that a future income tax asset will be realized, a valuation allowance is provided. |
| |
| | k. | | Impairment of long-term assets: |
| |
| | | | The Company records the value of the long-term assets acquired at cost. Such rights are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. When circumstances dictate an impairment loss is calculated as equal to the excess of the carrying value of the assets over their undiscounted estimated future net cash flows. |
68
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in United States dollars)
October 31, 2005
| | l. | | Foreign currency translation: |
| |
| | | | The functional currency of the Company is the United States dollar. The Company’s wholly-owned subsidiaries are integrated foreign operations and therefore, the Company uses the temporal method whereby monetary assets and liabilities are translated into United States (U.S.) dollars at the rate of exchange in effect at the consolidated balance sheet dates. Non-monetary assets and liabilities are translated at historical rates. Income and expenses are translated using the average monthly rate of exchange per quarter, which rate approximates the rate of exchange prevailing at the transaction dates. Gains or losses resulting from translation are included in the determination of net loss for the period. |
| 4. | | Acquisition of royalty rights and remaining interest in technology from Estancia Limited: |
| | | | On December 9, 2000, the Company and its subsidiary, Pageant, entered into an Asset Purchase Agreement (the “Agreement”) with Estancia Limited (“Estancia”) and Richard Lienau (“Lienau”) to purchase the remaining 50% interests in the patents which the Company did not own and a 40% gross profit royalty (“Estancia Royalty”), in respect of certain ferromagnetic memory technology known as VEMRAM (previously known as MAGRAM) and covered by U.S. Patent #5,295,097 and the related patent applications (the “Vemram Patents”) described in the Agreement and all rights (the “Technology”) held by Estancia and Lienau under the Joint Ownership and Licensing Agreement dated September 17, 1997 among Estancia, Lienau and Pageant. Under the terms of the Agreement, the Company was required to pay a maximum purchase price of $50,000,000 to Estancia as follows: |
| | a. | | $10,000,000 was paid on closing (after receipt of regulatory approvals), in the form of $8,000,000 in common shares of the Company (“Micromem Shares”) (based on the price on the closing date) and $2,000,000 in cash; |
| |
| | b. | | $20,000,000 if and when either (i) certification is received from Honeywell Federal Manufacturing & Technologies (“Honeywell”) that fully integrated, randomly addressable memory matrices of the Technology have met certain stipulated performance standards, or (ii) the Company or any of its affiliates executes a definitive agreement for the sale or licensing of the Technology to an arm’s length third party for any commercial purposes other than testing or evaluation of the Technology; payable in the form of cash and Micromem Shares to be determined by Pageant provided that a minimum of 50% of the $20,000,000 shall be paid in Micromem Shares valued at the close of trading on the date of receipt of such certification, sale or licensing; and |
| |
| | c. | | $20,000,000 if and when the Company or any of its affiliates executes a definitive agreement for the sale or licensing with respect to any technology (including the Technology) owned by the Company to an arm’s length third party for any commercial purposes other than testing or evaluation of the technology, payable in the form of cash and Micromem Shares to be determined by Pageant provided that a minimum of 50% of the $20,000,000 shall be paid in Micromem Shares valued at the close of trading on the date of execution of such sale or licensing. |
69
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in United States dollars)
October 31, 2005
During fiscal 2001, the Company paid $2,000,000 in cash and issued 2,007,831 shares, being the equivalent of $8,000,000, the first installment payable under the terms described above, on approval by its shareholders in the annual shareholder meeting held on March 14, 2001. The $10,000,000 paid was initially recorded as royalty rights in fiscal 2001 and was written-down to nil in fiscal 2002 (Note 10).
On March 9, 2004 the third anniversary of the closing date, the requirements set out in terms (b) and (c) above were not met and, in accordance with the terms of the Agreement, the Company’s obligations to pay these amounts terminated. The Company thus has had to revert to Estancia:
| | 1. | | a 40% interest in the Vemram Patents; |
| |
| | 2. | | a 32% interest in the gross profit, less expenses agreed to by the parties, for each license of the Vemram Patents sold or otherwise transferred by Pageant; and |
| |
| | 3. | | a 32% interest of any unit royalties received by Pageant as a result of the license or sale of the Vemram Patents less reasonable expenses directly related to the obtaining of said royalties. |
| 5. | | Non-cash working capital balances: |
| | I. | | Deposits and other receivables |
| | | | | | | | | |
| | | 10/31/05 | | | 10/31/04 | |
| |
| Sales tax recoverable | | $ | 9,737 | | | $ | 2,429 | |
| | | | | | | | | |
| Deposits | | | 2,345 | | | | 15,000 | |
| | | | | | | | | |
| Receivables from companies where senior officers and directors of the Company exercise significant influence (Note 12(b)) | | | 73,490 | | | | 16,133 | |
| | | | | | | | | |
| |
| | | $ | 85,572 | | | $ | 33,562 | |
| |
70
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in United States dollars)
October 31, 2005
II. Accounts payable and accrued liabilities
| | | | | | | | | |
| | | 10/31/05 | | | 10/31/04 | |
| Deferred compensation to Chairman (Note 12(a) ii) and to senior officers (Note 12(c)) | | $ | 350,000 | | | $ | — | |
| | | | | | | | | |
| Accrual of costs under technology development agreement (Note 13(b) | | | 289,863 | | | | 289,863 | |
| | | | | | | | | |
| Accounts payable | | | 163,343 | | | | 146,761 | |
| | | |
| | | $ | 803,206 | | | $ | 436,624 | |
| | | |
| 6. | | Property and equipment: |
| | | | | | | | | | | | | |
| | | 2004 | | | Additions | | | 2005 | |
| |
| Cost: | | | | | | | | | | | | |
| Computers and equipment | | $ | 41,348 | | | | — | | | $ | 41,348 | |
| |
| | | $ | 41,348 | | | | — | | | $ | 41,348 | |
| |
| | | | | | | | | | | | | |
| | | | | | Amortization | | | | |
| | | 2004 | | | Expense | | | 2005 | |
| |
| Accumulated amortization: | | | | | | | | | | | | |
| Computers and equipment | | $ | 38,423 | | | $ | 2,925 | | | $ | 41,348 | |
| |
| | | $ | 38,423 | | | $ | 2,925 | | | $ | 41,348 | |
| |
| | | | | | | | | |
| | | 2004 | | | 2005 | |
| |
| Net book value: | | | | | | | | |
| Computers and equipment | | $ | 2,925 | | | $ | — | |
| |
| | | $ | 2,925 | | | $ | — | |
| |
During fiscal 2003, the Company contributed equipment and supplies with a net book value of $58,302 under the “Equipment Transfer Agreement” to the University of Toronto (“U of T”) (Note 13a(4)). The net book value of the contributed equipment has been charged to the period as a research and development expense.
71
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in United States dollars)
October 31, 2005
| 7. | | Patents and trademarks: |
| |
| | | In 2003 the Company discontinued a number of patent and trademark applications primarily outside the United States and the net book value of $130,839 relating to these applications was written off in 2003. The Company has also assessed the remaining amounts for patents and trademark applications registered in Canada and United States and expensed the residual net book value of $168,981 in 2003 to reflect the uncertain nature of future events. |
| |
| | | The Company continues to actively pursue and protect its patents and trademarks registered in Canada and the United States. |
a. Authorized:
2,000,000 special preference shares, redeemable, voting, none of which are issued and outstanding Unlimited common shares without par value.
b. Stock option plan:
The Company has a fixed stock option plan. Under the Company’s Stock Option Plan (the “Plan”), the Company may grant options for up to 13,000,000 shares of common stock to directors, officers, employees or consultants of the Company and its subsidiaries. The exercise price of each option is equal to or greater than the market price of the Company’s shares on the date of grant unless otherwise permitted by applicable securities regulations. An option’s maximum term under the Plan is 10 years.
A summary of the status of the Company’s fixed stock option plan as at October 31, 2005 and 2004 and changes during the periods ended on those dates is as follows:
72
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in United States dollars)
October 31, 2005
| | | | | | | | | | | | | | | | | |
| | | 2005 | | | | | | | 2004 | | | | |
| |
| | | | | | Weighted | | | | | | Weighted | |
| | | Options in | | | Average | | | Optionsin | | | Average | |
| | | Thousands | | | exercise price | | | Thousands | | | exercise price | |
| |
| Outstanding, beginning of period | | | 7,270 | | | | .310 | | | | 5,300 | | | $ | .100 | |
| Granted | | | 4,700 | | | | .730 | | | | 7,270 | | | | .310 | |
| Cancelled | | | — | | | | — | | | | — | | | | — | |
| Exercised | | | (1,820 | ) | | | .304 | | | | (5,300 | ) | | | .100 | |
| |
| Outstanding end of period | | | 10,150 | | | | .500 | | | | 7,270 | | | $ | .310 | |
| |
| | | | | | | | | | | | | | | | | |
| Options exercisable at end of period | | | 10,150 | | | | .500 | | | | 7,270 | | | | | |
| |
| Weighted average price of Options granted during the period | | | | | | | .730 | | | | | | | $ | .310 | |
| |
In June 2004, 5,300,000 options were exercised resulting in $530,000 of cash proceeds to the Company.
In June 2005, 1,820,000 options were exercised resulting in $553,600 of cash proceeds to the Company.
The following options have been granted after October 31, 2004:
| | | | | | | | | | | | | | | | | |
| | | | | | | | Exercise | | | Expiry | |
| Date | | Optionee | | | Number of Options | | | Price | | | Date | |
| |
| January 29, 2005 | | Officer | | | 300,000 | | | | .80 | | | March 22, 2007 |
| January 31, 2005 | | Employee | | | 50,000 | | | | .91 | | | June 17, 2009 |
| January 31, 2005 | | Officer | | | 50,000 | | | | .91 | | | June 17, 2009 |
| May 27, 2005 | | Officer | | | 400,000 | | | | .72 | | | May 27, 2010 |
| May 27, 2005 | | Director | | | 300,000 | | | | .72 | | | May 27, 2010 |
| May 27, 2005 | | Director | | | 1,800,000 | | | | .72 | | | May 27, 2010 |
| August 1, 2005 | | Chairman | | | 1,800,000 | | | | .65 | | | June 16, 2009 |
| November 25, 2005 | | Director | | | 300,000 | | | | .60 | | | November 24, 2009 |
| December 20, 2005 | | Employee | | | 50,000 | | | | .72 | | | December 20, 2010 |
| January 15, 2006 | | Officer | | | 100,000 | | | | .72 | | | January 15, 2007 |
In the quarter ending October 31, 2005, the Company granted a total of 1,800,000 options to a director of the Company at an exercise price of .65¢ per share. These options expire in October 2010 if unexercised.
73
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in United States dollars)
October 31, 2005
At October 31, 2004 the cumulative stock compensation expense for stock options granted to employees has been calculated using the Black Scholes option-price model as $17,829,459 which expense has previously not been reflected in the consolidated statement of operations and deficit. Effective November 1, 2004, the Company adopted the fair value method of accounting for stock compensation expense and accordingly has restated the prior year financial statements as appropriate.
A reconciliation of the restatement of the prior year financial statements is as below:
| | | | | | | | | | | | | |
| | | | | | | | | Period from | |
| | | | | | | | | September 3, 1997 | |
| | | 2004 | | | 2003 | | | to October 31, 2005 | |
| |
| Stock compensation expense as originally reported | | | 10,020 | | | | — | | | | 10,020 | |
| Restatement — expense using fair value method | | | 1,369,950 | | | | 318,000 | | | | 19,551,201 | |
| | | |
| Restated stock compensation expense | | | 1,379,970 | | | | 318,000 | | | | 19,561,221 | |
| | | |
| | | | | | | | | | | | | |
| Net loss as originally reported | | | (944,348 | ) | | | (1,449,965 | ) | | | (36,690,571 | ) |
| | | | | | | | | | | | | |
| Restatement — expense using fair value method | | | 1,369,950 | | | | 318,000 | | | | (17,829,459 | ) |
| | | |
| Restated net loss | | | (2,314,298 | ) | | | (1,767,965 | ) | | | (54,520,030 | ) |
| | | |
| | | | | | | | | | | | | |
| Closing deficit as originally reported | | | (32,655,088 | ) | | | (31,710,740 | ) | | | (36,690,571 | ) |
| | | | | | | | | | | | | |
| Restatement, expense using fair value method | | | (17,829,459 | ) | | | (16,459,509 | ) | | | (17,829,459 | ) |
| | | |
| Restated closing deficit | | | (50,484,547 | ) | | | (48,170,249 | ) | | | (54,520,030 | ) |
| | | |
| | | | | | | | | | | | | |
| Basic and fully diluted loss per share as originally reported | | | (0.02 | ) | | | (0.03 | ) | | | (0.75 | ) |
| | | | | | | | | | | | | |
| Restatement — impact on loss per share using fair value method | | | (0.02 | ) | | | (0.01 | ) | | | (0.38 | ) |
| | | |
| | | | | | | | | | | | | |
| Revised basic and fully diluted loss per share | | | (0.04 | ) | | | (0.04 | ) | | | (1.13 | ) |
| | | |
74
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in United States dollars)
October 31, 2005
The fair value of all options granted between 2003 – 2005 were estimated as of the date of grant using the Black Scholes option-pricing model with the following assumptions:
| | | | | | | | | | | | | |
| | | 2005 | | | 2004 | | | 2003 | |
| Dividend yield | | | — | | | | — | | | | — | |
| Expected Volatility | | | 97% - 142 | % | | | 150 | % | | | 157 | % |
| Risk free interest rate | | | 3.25 | % | | | 3.25 | % | | | 2.22 | % |
| Expected option life | | 1.5 years
| | | 1.5 years
| | | 1 year
| |
The current expense for the three months ended January 31, 2005 has been calculated as $202,203, for the 3 months ended July 31, 2005 at $903,040 and for the 3 months ended October 31, 2005 at $616,499 and this expense has been recorded in the consolidated statement of operations for the three month periods then ended with an equivalent charge to contributed surplus.
The following table summarizes information about fixed options outstanding as at October 31, 2005:
| | | | | | | | | | | | | | | | | | | |
| Options Outstanding | | | | | | | Options exercisable |
| | | | | | | Weighted average | | | | | | | | |
| | | | | | | remaining | | Weighted | | | | | | Weighted |
| Actual exercise | | Number | | contractual life (in | | Average | | Number | | Average |
| price | | outstanding | | years) | | exercise price | | exercisable | | exercise price |
| |
| $0.30 | | | | 5,350,000 | | 3.5 years | | | $ | 0.30 | | | | 5,350,000 | | | $ | 0.30 |
| |
| 0.68 | | | | 100,000 | | 1.16 years | | | | 0.68 | | | | 100,000 | | | | 0.68 |
| |
| 0.80 | | | | 300,000 | | 1.2 years | | | | 0.80 | | | | 300,000 | | | | 0.80 |
| |
| 0.91 | | | | 100,000 | | 3.75 years | | | | 0.91 | | | | 100,000 | | | | 0.91 |
| |
| 0.72 | | | | 2,500,000 | | 4.5 years | | | | 0.72 | | | | 2,500,000 | | | | 0.72 |
| |
| 0.65 | | | | 1,800,000 | | 4.5 years | | | | 0.65 | | | | 1,800,000 | | | | 0.65 |
| |
| | c. | | Loss per share |
| |
| | | | Basic loss per share is calculated by dividing net loss by the weighted average number of common shares outstanding during the period. Diluted loss per share reflects the dilution that would occur if outstanding stock options and share purchase warrants were exercised or converted into common shares using the treasury stock method and is calculated by dividing net loss applicable to common shares by the sum of the weighted average number of common shares outstanding and all additional common shares that would have been outstanding if potentially dilutive common shares had been issued. |
75
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in United States dollars)
October 31, 2005
The inclusion of the Company’s stock options and share purchase warrants in the computation of diluted loss per share would have an anti-dilutive effect on loss per share and they are therefore excluded from the computation. Consequently, there is no difference between basic loss per share and diluted loss per share.
| | d. | | Warrants |
| |
| | | | On August 13, 2003, the Company issued 2,031,250 First Units at $0.08 each. Each First Unit provides the holder with one common share and a warrant for one Second Unit at $0.08 each, exercisable for one year. Each Second Unit provides the holder with one common share and a warrant for one common share at $0.08 each, exercisable for one year. |
| |
| | | | In accordance with the CICA’s recommendations, a portion of the First Unit should be allocated into separate elements within shareholders’ equity as the First Units contain two equity elements arising from the common share and warrants attached. The Company has allocated the closing trading value of its shares as at August 13, 2003 to the common shares. Since the net proceeds received from the issuance of the common shares attached to the First Units equaled the closing trading value at the date authorized by the Board of Directors, the warrants were allocated a nil value. . |
| |
| | | | In August 2004, the holders of the First Units exercised the First Unit warrants and the Company thus issued 2,031,250 common shares and the warrants for the Second Units and realized proceeds of $162,500. . |
| |
| | | | In February 2005, the holders of the Second Units exercised 1,406,250 Second Unit warrants and the Company thus issued 1,406,250 common shares and realized proceeds of $112,500. . |
| |
| | | | In August 2005, the holders of the Second Units exercised 625,000 Second Units warrants and the Company thus issued 625,000 common shares and realize proceeds of $50,000. . |
| | e. | | Private Placements |
| |
| | i) | | In December 2003, the Company completed Unit private placements to two Canadian private investors pursuant to prospectus and registration exemptions set forth in applicable securities laws. Under the private placements, the Company received $33,000 as subscription proceeds for the sale and issue of 300,000 Units. Each Unit consists of one Common Share and one Series A Warrant. Each Series |
76
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in United States dollars)
October 31, 2005
A Warrant entitles the holder to purchase one Common Share and one Series B Warrant for $0.11 until expiry 12 months from the date of issue. Each Series B Warrant entitles the holder to purchase one additional Common Share for $0.11 until expiry 12 months from the date of issue. .
In October 2004 the private investors exercised 200,000 Series A warrants and the Company thus issued 200,000 common shares and 200,000 Series B warrants and realized proceeds of $22,000. .
In the quarter ended January 31, 2005 the private investors exercised the remaining Series A warrants and the Company thus issued 100,000 additional common shares and 100,000 Series B warrants and realized proceeds of $11,000. The investors then exercised 300,000 Series B warrants and the Company thus issued 300,000 common shares and realized proceeds of $33,000. .
| | ii) | | In December 2003, the Company completed a Unit private placement to one Canadian private investor pursuant to prospectus and registration exemptions set forth in applicable securities laws. Under the private placement, the Company received $40,000 as subscription proceeds for the sale and issue of 500,000 Units. |
| |
| | | | Each unit consists of one Common Share and one Series A. Warrant. Each Series A Warrant entitles the holder to purchase one Common Share and one Series B Warrant for $0.08 until expiry 12 months from the date of issue. Each Series B Warrant entitles the holder to purchase one additional Common Share for $0.08 until expiry 12 months from the date of issue. . |
| |
| | | | In June 2004, the private investor exercised the Series A warrants and the Company thus issued 500,000 common shares and 500,000 Series B warrants and realized proceeds of $40,000. . |
| |
| | | | In September 2004, the private investor exercised the Series B warrants and the Company thus issued 500,000 common shares and realized proceeds of $40,000. . |
| | iii) | | In December 2004 the Company completed a Unit private placement to several U.S. investors pursuant to prospectus and registrations exemptions set forth in applicable securities laws. Under the private placement, the Company has received $617,000 as subscription proceeds for the sale and issue of 1,028,344 Units. Each Unit consists of one Common Share and one Series A Warrant. Each series A Warrant entitles the holder to purchase one Common Share and one Series B warrant for $.60 until expiry 12 months from the date of issue. Each Series B Warrant entitles the holder to purchase one additional Common Share for $.60 until expiry 12 months from the date of issue (Note 18). |
77
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in United States dollars)
October 31, 2005
| | iv) | | In February 2005, the Company arranged a Unit private placement to several investors pursuant to prospectus and registration exemptions set forth in applicable securities laws. Under this private placement the Company received $845,000 as of April 30, 2005 as subscription proceeds for the sale of 1,300,000 Units. Each unit consists of one Common Share and one Series A Warrant. Each Series A Warrant entitles the holder to purchase one Common Share and one Series B Warrant for $.65 until expiry 12 months from the issue date. Each Series B warrant entitles the holder to purchase one Common Share for $.65 until expiry 12 months from the issue date (Note 18). |
| |
| | v) | | In February 2005, the Company completed a Unit private placement to two Canadian investors pursuant to prospectus and registration exemptions set forth in applicable securities laws. Under the private placement, the Company received $10,500 as subscription proceeds for the sale and issue of 14,000 Units. Each Unit consists of one Common Share and one Series A Warrant. Each Series A Warrant entitles the investor to purchase one Common Share and one Series B Warrant for $.75 until expiry 12 months from the date of issue. Each Series B Warrant entitles the holder to purchase one additional common share for $.75 until expiry 12 months from the date of issue (Note 18). |
| |
| | f. | | Outstanding warrants |
The outstanding warrants to acquire common shares are summarized as below:
| | | | | |
| August 13, 2003 financing (Note 8 (d)): | | | | |
| Series A warrants outstanding at October 31, 2003 | | | 2,031,250 | |
| Series A warrants exercised in fiscal 2004 | | | (2,031,250 | ) |
| Series B warrants resulting from exercise of Series A warrants | | | 2,031,250 | |
| | | | |
| Outstanding at January 31, 2005 | | | 2,031,250 | |
| Series B warrants exercised in February 2005 | | | (1,406,250 | ) |
| Series B warrants exercised in August 2005 | | | (625,000 | ) |
| | | | |
| | | | — | |
| | | | |
| | | | | |
| December 2003 financing (Note 8 (e) (i)): | | | | |
| | | | | |
| Series A warrants issued | | | 300,000 | |
| Series A warrants exercised | | | (200,000 | ) |
| Series B warrants resulting from exercise of Series A warrants | | | 200,000 | |
| | | | |
| Outstanding at October 31, 2004 | | | 300,000 | |
| Series A warrants exercised, January 2005 | | | (100,000 | ) |
| Series B warrants resulting from exercise of Series A warrants | | | 100,000 | |
| Series B warrants exercised, January 2005 | | | (300,000 | ) |
| | | | |
| | | | — | |
| | | | |
78
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in United States dollars)
October 31, 2005
| | | | | |
| December 2003 financing (Note 8 (e) (ii)) | | | | |
| Series A warrants issued | | | 500,000 | |
| Series A warrants exercised, June 2004 | | | (500,000 | ) |
| Series B warrants resulting from exercise of Series A warrants | | | 500,000 | |
| Series B warrants exercised, October 2004 | | | (500,000 | ) |
| | | | |
| | | | — | |
| | | | |
| | | | | |
| December 2004 financing (Note 8(e) (iii)) | | | | |
| Series A warrants issued and outstanding | | | 1,028,344 | |
| | | | |
| | | | | |
| February 2005 financing (Note 8(e) (iv) | | | | |
| Series A warrants issued and outstanding | | | 1,300,000 | |
| | | | |
| | | | | |
| February 2005 financing (Note 8(e)(v) | | | | |
| Series A warrants issued and outstanding | | | 14,000 | |
| | | | |
| | | | | |
| June 8, 2005 financial advisory services agreement | | | 1,000,000 | |
| | | | |
| | | | | |
| June 22, 2005 financial advisory services agreement | | | 800,000 | |
| | | | |
On June 8, 2005 the Company entered into a financial advisory services agreement with an arms length entity and, as consideration, issued 1,000,000 purchase warrants. Each warrant entitles the holder to purchase and subscribe for one common share at $.70 per share on or before June 8, 2006.
The Company entered into a second financial advisory services agreement on June 22, 2005 with an arms length entity and, as consideration, issued 800,000 purchase warrants. Each warrant entitles the holder to purchase and subscribe for one common share at $.70 per share on or before December 31, 2006.
Subsequent to October 31, 2005, the expiry date was extended on the Series A warrants issued in December 2004 and February 2005 and the terms attached to the related Series B warrants were revised (Note 18).
Contributed surplus arises as a result of the application of the Black Scholes option-price model with respect to stock options issued by the Company as outlined in Note 8 (b). Also included in contributed surplus at October 31, 2005 is an amount of $544,891 representing forgiveness of Pageant indebtedness during fiscal 1999 by Ataraxia Corp, the former parent company of Pageant. This forgiven debt was treated as contributed surplus as this balance was between related parties.
79
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in United States dollars)
October 31, 2005
| 10. | | Restructuring and write-down of royalty rights: |
On July 29, 2002, the Company restructured its operations by closing its research and development facility and adopted a plan to focus its current resources to outsource its research and development activities as described in Note 13(a). No major costs were associated with this restructuring.
As a result of the restructuring, the Company determined that there was significant uncertainty that any amounts would be payable to Estancia in the foreseeable future in respect of the Estancia Royalty as described in Note 4, and accordingly, the Estancia Royalty rights acquired in the amount of $10,000,000 were written off in fiscal 2002.
Once the Company has completed all its income tax return filings it will have non-capital losses of approximately $8,991,000 available to reduce future taxable income, the benefit of which has not been recognized in these consolidated financial statements. As at October 31, 2005, the tax losses expire as follows:
| | | | | | | | | | | | | |
| | | Canada | | | Other Foreign | | | Total | |
| |
| 2006 | | $ | 268,000 | | | $ | — | | | $ | 268,000 | |
| 2007 | | | 1,632,000 | | | | — | | | | 1,632,000 | |
| 2008 | | | 1,363,000 | | | | — | | | | 1,363,000 | |
| 2009 | | | 1,062,000 | | | | — | | | | 1,062,000 | |
| 2010 | | | 932,000 | | | | 265,000 | | | | 1,197,000 | |
| 2011 | | | — | | | | 208,000 | | | | 208,000 | |
| 2014 | | | 746,000 | | | | — | | | | 746,000 | |
| 2015 | | | 2,249,000 | | | | — | | | | 2,249,000 | |
| 2023 | | | — | | | | 73,000 | | | | 73,000 | |
| 2024 | | | — | | | | 173,000 | | | | 173,000 | |
| 2025 | | | — | | | | 20,000 | | | | 20,000 | |
| |
| Total losses | | $ | 8,252,000 | | | $ | 739,000 | | | $ | 8,991,000 | |
| |
80
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in United States dollars)
October 31, 2005
The reconciliation of income tax attributable to continuing operations computed at the statutory tax rates to income tax expense is as follows:
| | | | | | | | | | | | | |
| | | 10/31/05 | | | 10/31/04 | | | 10/31/03 | |
| |
| Consolidated accounting loss before income taxes | | $ | (4,035,483 | ) | | $ | (2,314,298 | ) | | $ | (1,767,965 | ) |
| Add nondeductible items | | | 1,764,667 | | | | 1,369,950 | | | | 318,000 | |
| | | | | | | | | | |
| Loss for tax purposes | | | (2,270,816 | ) | | | (944,348 | ) | | | 1,449,965 | |
| Statutory rates | | | 36 | % | | | 36 | % | | | 38 | % |
| |
| Expected income tax recovery | | | (817,494 | ) | | | (339,965 | ) | | | (550,987 | ) |
| Tax benefit not recognized | | | 817,494 | | | | 339,965 | | | | 550,987 | |
| |
| | | $ | — | | | $ | — | | | $ | — | |
| |
Future income taxes reflect the net tax effect of temporary differences between the carrying amount of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s future tax assets and liabilities as at October 31, 2005 are as follows:
| | | | | | | | | | | | | |
| | | 10-31 | | | 10-31 | | | 10-31 | |
| | | 2005 | | | 2004 | | | 2003 | |
| Unused capital losses | | $ | 47,566 | | | $ | 47,566 | | | $ | 47,566 | |
| Unused non-capital losses | | | 3,080,670 | | | | 2,244,996 | | | | 1,975,066 | |
| Alternative minimum tax credit | | | 142,091 | | | | 142,091 | | | | 142,091 | |
| Research and development credit | | | 118,720 | | | | 118,720 | | | | 118,720 | |
| Tax basis of property and equipment in excess of carrying value | | | 14,760 | | | | 13,680 | | | | 109,789 | |
| |
| Total future tax assets | | $ | 3,403,807 | | | $ | 2,567,053 | | | $ | 2,393,232 | |
| Valuation allowance | | | (3,403,807 | ) | | | (2,567,053 | ) | | $ | (2,393,232 | ) |
| |
| Net future tax assets | | $ | — | | | $ | — | | | $ | — | |
| |
81
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in United States dollars)
October 31, 2005
| 12. | | Management compensation and related party transactions: |
| | a (i) | | Between 1999 and 2002, the Company has previously entered into stock-based management compensation arrangements with the Chairman as reported in prior years’ audited financial statements. |
| |
| | (ii) | | On May 29, 2005, the Company entered into a new employment agreement with the Chairman for a period from January 1, 2005 through September 30, 2009. Under the terms of the agreement, the Chairman has been retained to provide certain management services to the Company. The Company has agreed to provide compensation based on a percentage of the increase of the market capitalization on a year-over year basis commencing as of December 31, 2005 subject to a minimum annual compensation amount of $150,000. At the Company’s option it can pay cash or issue common shares as compensation providing that the cumulative maximum number of shares that it can issue under the agreement is 2 million common shares. The Company determined that the compensation expense in fiscal 2005 was $150,000 under this agreement. |
The total compensation paid to the Chairman is summarized as follows:
| | | | | | | | | |
| | | Cash Compensation | | | Stock Option Expense | |
| 2005 | | $ | 150,000 | | | $ | 659,000 | |
| 2004 | | | — | | | $ | 339,000 | |
| | c. | | In the normal course of business, the Company has entered into cost sharing arrangements with companies where certain senior officers and directors exercise significant influence. These transactions, which were measured at the exchange amount on the date of the transaction, relate to salaries, rent and other expenses. For the fiscal year ended October 31, 2005, the Company paid a total of approximately $115,000 in rent (2004: $92,000) and $180,000 in salaries (2004: $116,000) and recovered $104,000 (2004: $182,000) of these total costs from the companies in which senior offices and directors exercise significant influence. |
| |
| | | | At October 31, 2005 the aggregate amount owed by these companies was $73,490 (Note 5). As at year-end, the Company determined that it would be unable to cover costs amounting to $60,395 allocated to one of the related companies. Accordingly this amount has been expensed in the financial statements. |
82
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in United States dollars)
October 31, 2005
| | c. | | Included in professional fees as reported are management and consulting fees paid or payable to individuals (or companies controlled by such individuals) who served as officers and directors of the Company. Certain of these individuals joined the Company during 2004 and 2005. The total compensation paid to such parties is summarized as follows: |
| | | | | | | | | |
| | | Cash Compensation | | | Stock Option Expense | |
| 2005 | | $ | 639,000 | | | $ | 1,044,000 | |
| 2004 | | $ | 92,000 | | | $ | 470,000 | |
Additionally in 2005 the Company paid $100,000 in legal fees (2004: $80,000) to a legal firm whose principal is a director of the Company.
| | a. | | Research Collaboration and Infrastructure Agreements (refer also to 13 (a) (4) as below): |
| |
| | 1. | | Materials and Manufacturing Ontario: |
| |
| | | | On October 24, 2002, Micromem entered into a two year Research Collaboration Agreement with Material and Manufacturing Ontario (“MMO”), a not-for-profit organization funded by the provincial government, the University of Toronto (“U of T”) and a researcher employed by U of T to fund the research on Magnetic Structure development for Hall effect memory devices. |
| |
| | | | Under the terms of the agreement, the Company committed to contribute $87,432 (Cdn $136,175) and $18,000 (Cdn $28,000) in cash and in-kind contribution, respectively, per year to fund the research. The Company has met all of its obligations under this agreement. |
| |
| | | | On November 12, 2003, Micromem entered into a second research collaboration agreement with MMO and the U of T for research and development associated with magnetic memory devices. Under the second agreement, in the first year and upon renewal in the second year, MMO granted $58,900 (equivalent to Cdn. $85,000) in cash funding and Micromem contributed $56,130 (equivalent to Cdn. $81,000 in cash funding and additionally made $30,770 (equivalent to Cdn. $44,400) of in-kind contributions, all towards the research collaboration, each year. The Company has met all of its obligations under the agreement. Micromem obtained sublicensing rights for the use of any new technology developed (“Technology Developed”) under this research subject to |
83
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in United States dollars)
October 31, 2005
payment of an annual royalty payable in perpetuity to MMO based on a percentage of revenues from the sale of products incorporating the Technology Developed.
| | 2. | | University of Toronto: |
| |
| | | | On November 1, 2002, the Company entered into an Infrastructure Agreement with U of T to fund the assembly of a magnetic memory facility (“MMF”) for research, development and fabrication of magnetic memory. U of T has agreed to use the MMF in connection with, among other things, research to be conducted pursuant to collaborations between Micromem and U of T. |
| |
| | | | The terms of the agreement provided that Micromem was to contribute $249,463 (equivalent to Cdn. $360,000) in cash to fund the direct costs of the MMF. The contribution has been made in fiscal 2002-2003 by Micromem and included as a research and development expense in the consolidated statements of operations and deficit. |
| | 3. | | Communications and Information Technology Ontario: |
| |
| | | | On December 10, 2002, Micromem entered into a two year Collaborative Research Agreement with Communications and Information Technology Ontario (“CITO”) U of T and Dr. Harry Ruda. For the first year, CITO provided funding of $106,715 (equivalent to Cdn. $154,000) and Micromem contributed $31,875 (equivalent to Cdn. $46,000). For the second year, CITO provide funding of $107,715 (equivalent to Cdn. $154,000) and Micromem provided funding of $31,875 (equivalent to Cdn. $46,000). Micromem has further provided $67,632 (equivalent to Cdn $97,600) of in-kind contributions to the research collaboration. |
| | 4. | | Revised License Agreement: |
| |
| | | | In June 2005 the Company signed a license agreement (“the License Agreement”) with the U of T and the Ontario Centres of Excellence (including MMO and CITO) (collectively “OCE”) whereby: |
| | a. | | OCE released the Company and the University from the commercialization obligations set forth is all prior research collaboration agreements. |
| |
| | b. | | The Company acquired exclusive worldwide rights to the Technology and Developed Technology and Patent Rights related to the MRAM technology developed at the UofT. |
84
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in United States dollars)
October 31, 2005
| | c. | | The Company has agreed to royalties and payments under the terms of the License Agreement as follows: |
| | 1.1 | | In consideration for the rights and licenses granted herein, the Company shall pay to the UofT: |
| | a) | | 4% of Net Sales until such time as the UofT has received from the Company an aggregate amount of five hundred thousand Canadian dollars (CDN$500,000); |
| |
| | b) | | 1% of Net Sales thereafter. |
| | 1.2 | | If the Company sublicenses any rights granted herein to any non-Affiliate: |
| | a) | | in combination or association with the Micromem Intellectual Property, the UofT shall receive 10% of any Net Fees and/or Net Royalties that shall be received by the Company in respect of any licenses involving both the rights granted herein and such Micromem Intellectual Property; |
| |
| | b) | | For all other sublicenses of the rights granted herein to any non-Affiliate, the UofT shall receive 20% of any Net Fees and/or Net Royalties that shall be received by the Company in respect of such sublicenses. |
| |
| | c) | | Net Fees and/or Net Royalties shall be received from the Company until such time as the UofT has received from the Company an aggregate amount of five hundred thousand Canadian dollars (CDN$500,000); thereafter the Company shall pay half of the amounts set forth in 1.2(a) or (b) as is applicable. |
| | 1.3 | | At any point after which the Company has paid the UofT five hundred thousand Canadian dollars (CDN$500,000), the Company may at its option buy out the obligation to pay royalties under the License Agreement by paying to the UofT a single lump sum payment equaling the greater of five hundred thousand Canadian dollars (CDN$500,000) and an amount equal to the total amount of royalties paid by the Company to the UofT in the preceding twenty-four months. The Company shall be entitled to exercise such option by providing written notice to the UofT along with the required payment, after which time the Company’s obligation to pay royalties under Sections 1.1 or 1.2 as applicable shall be waived by the UofT. |
85
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in United States dollars)
October 31, 2005
As a condition to entering the License Agreement the Company has agreed to a further Research Agreement with a funding commitment of no less than five hundred thousand Canadian dollars (CDN $500,000), to continue the further research and development of the Inventions and Micromem Intellectual Property. In August 2005 the Company made an initial payment of CDN $250,000 and, subsequent to October 31, 2005, the Company made the second payment of CDN $250,000 under the terms of this Research Agreement.
The Company believes that there are substantial market opportunities available for it to commercialize its technology in conjunction with strategic partners and it is currently pursuing such opportunities. The Company plans to complete its research initiatives and enter into agreements with strategic partners so as to commercialize its technology under licensing and other arrangements.
| | b. | | Technology development agreement: |
| |
| | | | On March 14, 2001, the Company’s subsidiary, Pageant, entered into a three-year technology development agreement with Estancia and Lienau to continue the development of the Technology. Under the terms of the agreement, Pageant committed to pay Estancia $215,000 per year, payable on a monthly basis in arrears, and committed to incur expenditures in connection with the development expenses of up to a maximum of $500,000 per agreement year. |
| |
| | | | On April 23, 2002, the technology development agreement was amended to extend its term for an additional eight-month period through November 2004. The go-forward payments were renegotiated as $62,707 between May — October 2002, $197,086 during fiscal 2003 and $143,330 during fiscal 2004. |
| |
| | | | The development efforts under this agreement ceased in July 2002. The Company reports approximately $290,000 in accounts payable and accrued liabilities with respect to this agreement as of October 31, 2005 (at October 31, 2004: $287,000). |
86
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in United States dollars)
October 31, 2005
The Company has operating lease commitments which expire in 2010 in respect of its head office. The future minimum annual lease payments are approximately as follows:
| | | | | |
| 2006-2010 (annually) | | $ | 98,400 | |
| | d. | | Employment agreement: |
| |
| | | | In January 2005 the Company entered into an employment agreement with an arm’s length individual for her services as Chief Technology Officer of the Company. The agreement extends for 2 years with a cancellation clause which can be executed by the Company at any time with 4 months notice provided. The base remuneration stipulated in the contract is $260,000 per year. The Company also granted the Chief Technology Officer 100,000 options exercisable at .68¢ which expired on December 31, 2005, 300,000 options exercisable at $0.80 per share, expiring 45 days after the end of the above noted employment agreement and 100,000 options exercisable at $.72 which expire on March 22, 2007 if unexercised. |
| 14. | | Contingencies: |
| |
| | | The Company has agreed to indemnify its directors and officers and certain of its employees in accordance with the Company’s by-laws. The Company maintains insurance policies that may provide coverage against certain claims. |
| |
| | | As outlined in Note 4, certain interests under the Agreement with Estancia reverted to Estancia on March 9, 2004. On this basis, to the extent that future revenues are generated by the Company relating directly and specifically to the Vemram Patents, the Company is obligated to pay Estancia 32% of the gross profit realized less expenses agreed to by the parties and 32% of any unit royalties realized less direct expenses. |
87
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in United States dollars)
October 31, 2005
| 15. | | Financial instruments: |
The fair values for all financial assets and liabilities are considered to approximate their carrying values due to their short-term nature.
Foreign items
The consolidated financial statements include balances/transactions that are denominated in Canadian dollars as follows:
| | | | | | | | | | | | | | | | | |
| | | | | | | (i) | | | 10/31/05 | | | 10/31/04 | |
| |
| Assets | | $ | 299,570 | | | | | | | | | | | $ | 270,999 | |
| Liabilities | | | 68,212 | | | | | | | | | | | | 122,561 | |
| Other Income | | | 9,094 | | | | | | | | | | | | 6,241 | |
| Expenses | | | 1,591,303 | | | | | | | | | | | | 973,615 | |
| |
| 16. | | Reconciliation between Canadian GAAP and U.S. GAAP: |
The Company’s consolidated financial statements have been prepared in accordance with Canadian GAAP which, in the case of the Company, conform in all material respects with U.S. GAAP.
| | a. | | Stock-Based compensation: |
Until October 31, 2004 the Company chose to account for employee stock-based compensation using the intrinsic value method prescribed in Accounting Principles Board Opinion No. 25, Accounting for Stock Issued to Employees. Under this method, compensation expense is recorded if the market value exceeds the exercise price at the date of grant. The compensation expense, if any, is recognized at the date of option grants or when the option shares are earned, when future performance is required, in the consolidated statements of operations and deficit.
88
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in United States dollars)
October 31, 2005
In December 2004, the Financial Accounting Standards Board (FASB) issued SFAS No. 123 (revised 2004), “Share-Based Payments”. SFAS No. 123(R) would require the Company to measure all employee stock-based compensation awards using a fair-value method and record such expense in its consolidated financial statements. SFAS No. 123(R) is effective beginning in the quarter ending January 31, 2005.
The Company has adopted the fair value method to measure all employee stock-based compensation effective November 1, 2004 and accordingly has restated the prior year financial statements to reflect this change, as outlined in Note 8(b).
| | b. | | Consolidated statement of comprehensive income (loss): |
Comprehensive income (loss) includes all changes in equity during the periods presented except shareholder transactions. For the purpose of reporting under U.S. GAAP, the components of comprehensive income and total comprehensive income are reported in the consolidated statements of changes in shareholders’ equity, below net loss in the consolidated statements of operations and deficit and in a separate consolidated statement of comprehensive income. For the periods presented, accumulated other comprehensive loss equals net loss.
| | c. | | Research and development expenditures |
Under U.S. GAAP all research and development expenditures are expensed as incurred. In that the Company has not deferred any research and development expenditures it is in compliance with U.S. GAAP.
| | d. | | Other recent accounting pronouncements |
SFAS No. 151 — retains the principle that inventories are presumed to be stated at cost; however, it amends ARB No. 43 to clarify that abnormal amounts of idle facilities, freight, handling costs and spoilage should be recognized as current period expenses. Also, SFAS No. 151 requires fixed overhead costs be allocated to inventories based on normal production capacity. The guidance in SAFS No. 151 is effective for inventory costs incurred during fiscal years beginning after June 15, 2005.
SFAS 152 — In December 2004, the FASB issued SFAS No. 152 “Accounting for Real Estate Time Sharing Transactions — an amendment of FASB Statements No. 66 and 67” (“SFAS 152”). The provisions of SFAS 152 are effective in fiscal years beginning after June 15, 2005.
89
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in United States dollars)
October 31, 2005
SFAS 153 — In December 2004, the FASB issued SFAS No. 153 “Exchanges of Non-monetary Assets — an amendment of APB Opinion No. 29” (“SFAS 153”). SFAS 153 replaces the exception from fair value measurement in APB Opinion No. 29 for non-monetary exchanges of similar productive assets with a general exception from fair value measurement for exchanges of non- monetary assets that do not have commercial substance. A non-monetary exchange has commercial substance if the future cash flows of the entity are expected to change significantly as a result of the exchange. SFAS 153 is effective for all interim periods beginning after June 15, 2005.
SFAS 154 — In May 2005, the FASB issued SFAS 154 “Accounting Changes and Error Corrections”. The statement replaces APB Opinion No. 20, Accounting Changes and SFAS No. 3, Reporting Accounting Changes in Interim Financial Statements. SFAS 154 requires companies to apply voluntary changes in principles retrospectively whenever practicable. SFAS 154 is effective for accounting changes and correction of errors made in fiscal years beginning after December 15, 2005.
The Company believes that the above standards would not have a material impact on its financial position or on the results of operations.
| 17. | | Comparative consolidated financial statements: |
Comparative consolidated financial statements have been reclassified from statements previously presented to conform to the presentation of the 2005 financial statements.
| | a. | | In December 2005 the Company revised the terms of the Unit private placements outlined in Note 8(e) (iii) (iv) and (v). In each case the Unit was revised to consist of one Common Share, one Series A warrant expiring on June 30, 2006 and one Series B warrant expiring on December 31, 2006. The remaining terms of the Series A and Series B warrants were unchanged. |
| |
| | b. | | In December 2005, the Company granted 50,000 options to an employee at an exercise price of .72¢ per share. These options expire in December 20, 2010 if unexercised. In November 2005 the Company granted 300,000 options to a new director at an exercise price of .60¢ per share. These options expire in November 2009 if unexercised. |
| |
| | c. | | In January 2006 the company granted 100,000 options to the Chief Technology Officer at an exercise price of .72¢ per share. These options expire in March 2007 if unexercised. |
90
MICROMEM TECHNOLOGIES INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Expressed in United States dollars)
October 31, 2005
| | d. | | In January 2006 a director exercised 150,000 options at a price of .30¢ per share and the Company realized $45,000 of subscription proceeds. |
| |
| | e. | | In February 2006, a director exercised 400,000 options at a price of .30¢ per share and the Company realized $120,000 of subscription proceeds. |
ITEM 18. EXHIBITS
The following exhibits are filed as part of this registration statement and attached hereto:
| | | |
| Exhibit No. 1.1 | | Articles of Incorporation of Incorporation of Micromem Technologies Inc. and amendments thereto in effect as of January 11, 2000, (Incorporated herein by reference to the Company’s Form 20-F/A filed with the Commission on January 11, 2000). |
| | | |
| Exhibit No. 1.2 | | Articles of Incorporation of Amendment of Micromem Technologies Inc. dated as of October 17, 2001 amending the Articles of Incorporation of Micromem Technologies Inc. to increase the number of directors to a minimum of three and a maximum of ten (Incorporated herein by reference to the Company’s Form 20-F/A filed with the Commission on March 20, 2003); |
| | | |
| Exhibit No. 1.3 | | Articles of Incorporation of Amendment of Micromem Technologies Inc. dated as of June 24, 2002 amending the Articles of Incorporation of Micromem Technologies Inc. to increase the number of directors to a minimum of 3 and a maximum of 12 (Incorporated herein by reference to the Company’s Form 20-F/A filed with the Commission on March 20, 2003); |
| | | |
| Exhibit No. 1.5 | | By-Laws of Micromem Technologies Inc. in effect as of January 11, 2002, (Incorporated herein by reference to the Company’s Form 20-F/A filed with the Commission on January 11, 2000); |
| | | |
| Exhibit No. 1.6 | | Amendment to the By-Laws of Micromem Technologies Inc. approved by shareholders on June 29, 2000, deleting the requirement from the By-Laws that the President shall be appointed from amongst the directors (Incorporated herein by reference to the Company’s Form 20-F/A filed with the Commission on March 20, 2003); |
| | | |
| Exhibit No. 4.1 | | Research Collaboration Agreement by and among Micromem Technologies Inc., the University of Toronto, Dr. Harry Ruda and Materials and Manufacturing Ontario dated October 24, 2002 (referred to in this Annual Report at Item 4.B — Business Overview — Recent Developments) (Incorporated herein by reference to the Company’s Form 20-F/A filed with the Commission on March 20, 2003); |
91
| | | |
| Exhibit No. 4.2 | | Asset Purchase Agreement by and among Micromem Technologies Inc., Pageant Technologies Incorporated, Estancia Limited and Richard Lienau dated December 10, 2000 (referred to in this Annual Report at Item 4.B — Business Overview — Recent Developments), (Incorporated herein by reference to the Company’s Form 40-F filed with the Commission on February 2, 2001); |
| | | |
| Exhibit No. 4.3 | | Technology Development Agreement by and among Pageant Technologies Incorporated, Estancia Limited and Richard Lienau dated March 9, 2001 (referred to in this Annual Report at Item 4.B — Business Overview — Recent Developments), (Incorporated herein by reference to the Company’s Form 40-F filed with the Commission on February 2, 2001); |
| | | |
| Exhibit No. 4.4 | | Infrastructure Agreement by and between Micromem Technologies Inc. and the University of Toronto dated November 1, 2002 (referred to in this Annual Report at Item 4.B — Business Overview — Recent Developments) (Incorporated herein by reference to the Company’s Form 20-F/A filed with the Commission on March 20, 2003); |
| | | |
| Exhibit No. 4.5 | | A second 2-year Research Collaboration Agreement, by and among Micromem Technologies Inc., Materials and Manufacturing Ontario and the University of Toronto dated November 12, 2003 (referred to in this Annual Report at “Item 10.C — Material Contracts”), (Incorporated herein by reference to the Company’s Form 20-F filed with the Commission on March 19, 2004); |
| | | |
| Exhibit No. 4.6 | | Equipment Transfer Agreement by and between Micromem Technologies Inc. and the Governing Council of the University of Toronto dated March 1, 2003 (referred to in this Annual Report at Item 10.C — Material Contracts”), (Incorporated herein by reference to the Company’s Form 20-F filed with the Commission on March 19, 2004); |
| | | |
| Exhibit No. 4.7 | | Collaborative Research Agreement by and among Micromem Technologies Inc., Communications and Information Technology Ontario, the University of Toronto and Dr. Harry Ruda dated December 10, 2002 (referred to in this Annual Report at “Item 10.C — Material Contracts”), (Incorporated herein by reference to the Company’s Form 20-F filed with the Commission on March 19, 2004); |
| | | |
| Exhibit No. 4.8 | | Revised License Agreement by and between Micromem Technologies Inc. and the University of Toronto dated June 13, 2005. |
| | | |
| Exhibit No. 4.9 | | Employment Agreement by and between Micromem Technologies Inc. and Dr. Cynthia Kuper dated January 28, 2005. |
| | | |
| Exhibit No. 4.10 | | Employment Agreement by and between Micromem Technologies, Inc. and Salvatore Fuda dated May 29, 2005. |
| | | |
| Exhibit No. 12.1 | | Officer’s Certification pursuant to Section 302 of the Sarbanes Oxley Act, 2002. |
| | | |
| Exhibit No. 12.2 | | Officer’s Certification pursuant to Section 302 of the Sarbanes Oxley Act, 2002. |
| | | |
| Exhibit No. 13.1 | | Officer’s Certification pursuant to Section 906 of the Sarbanes Oxley Act, 2002. |
| | | |
| Exhibit No. 13.2 | | Officer’s Certification pursuant to Section 906 of the Sarbanes Oxley Act, 2002. |
| | | |
| Exhibit No. 14.1 | | Independent Auditors’ Consent of Schwartz Levitsky Feldman LLP |
92
| | | |
| Exhibit No. 14.2 | | Independent Auditors’ Consent of Grant Thornton LLP |
| | | |
| Exhibit No. 14.3 | | Independent Auditors’ Consent of Ernst & Young LLP |
| | | |
| Exhibit No. 14.4 | | Independent Auditors’ Consent of Schwartz Levitsky Feldman LLP |
| | |
| * | | Portions of this exhibit have been omitted pursuant to a request for confidential treatment. |
93
SIGNATURES
Pursuant to the requirements of Section 12 of the Securities Exchange Act of 1934, the Registrant certifies that it meets all of the requirements for filing on Form 20-F and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | |
| | | MICROMEM TECHNOLOGIES INC. | | |
| | | | | | | |
| | | By: | | /s/ Joseph Fuda | | |
| | | Name: Joseph Fuda | | |
| | | Title: Chief Executive Officer | | |
| | | | | | | |
| | | By: | | /s/ Dan Amadori | | |
| | | Name: Dan Amadori | | |
| | | Title: Chief Financial Officer | | |
Dated: February 28, 2006
94