UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10
GENERAL FORM FOR REGISTRATION OF SECURITIES
UNDER SECTION 12(B) OR (G) OF THE SECURITIES EXCHANGE ACT OF 1934
Commission file number 333-168527
CANNAPOWDER, INC.
(Exact Name Of Registrant As Specified In Its Charter)
| Nevada | | 20-3353835 |
| (State of Incorporation) | | (I.R.S. Employer Identification No.) |
| | | |
| 20 Raoul Wallenberg St., Tel Aviv, Israel | | 6971916 |
| (Address of Principal Executive Offices) | | (ZIP Code) |
Registrant’s Telephone Number, Including Area Code: +972-3-6130421
Smart Energy Solutions, Inc.
(Former Name of Registrant)
Securities to be registered under Section 12(b) of the Act: None
Securities to be registered under Section 12(g) of the Exchange Act:Common stock; $0.0001par value
(Title of Class)
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer (as defined in Rule 12b-2 of the Exchange Act) or a smaller reporting company.
| Large accelerated filer [ ] | Accelerated filer [ ] | Non-Accelerated filer [ ] | Smaller reporting company [X] |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes [ ] No [X]
TABLE OF CONTENTS
PART I
ITEM 1. DESCRIPTION OF BUSINESS
Background and Former Operations
Smart Energy Solutions, Inc. (the “Company” or “Registrant”) was incorporated in 1999 in the state of Utah under the name Datigen.com, Inc. On August 25, 2005, the Registrant was redomiciled from Utah to Nevada pursuant to a merger with and into its wholly-owned subsidiary, Smart Energy Solutions, Inc., a Nevada corporation and, in connection therewith, its name was changed to Smart Energy Solutions, Inc.
Prior to the merger into its wholly-owned subsidiary, the Company was engaged in activities including development and marketing of various internet and internet related products and services, investment in real property related instruments, and providing concrete cutting and finishing services to construction sites seeking to comply with certain provisions of the American Disability Act of 1991. In November 2004, the Company had a change in control as a result of the purchase of a majority of the Company’s outstanding common stock by unaffiliated individuals from certain of the Company’s shareholders, including its then Chief Executive Officer, Joseph Ollivier.
In connection with the change in control, the Company determined to pursue other business opportunities and, as a result, on March 23, 2005, the Company acquired the intellectual property rights and certain other assets relating to a product known as the Battery Brain from Purisys, Inc., a New Jersey corporation in an Asset Purchase Agreement (the “Agreement”) with Purisys and Aharon Levinas, the owner of Purisys and the Battery Brain Assets (the “Assets”). The Battery Brain was a device attached to a motor vehicle battery for the purpose of protecting the vehicle from battery failure and theft.
Following the purchase of the Assets, the Company’s management team devoted its resources to establishing operations and entering into agreements with third parties for the manufacture and distribution of products using the Battery Brain technology, including manufacturers in China and Israel and distributors in the United States, Canada, Italy, and Israel. During the period from the date of the Agreement through the end of 2009, the Company devoted its marketing activities on the following target markets, each of which the Company believed had unique requirements: Automotive Retail; Automobile Dealers; Automotive OEMs; Automotive Specialty; Fleets; Military; Heavy Truck/Bus; Motor Home/Recreational Vehicle; and Marine.
Notwithstanding its sales and marketing efforts and its ability to generate sales revenues from its Battery Brain products, the Company continued to generate losses from operations and, as of its fiscal year-ended December 31, 2008, The Company had an accumulated deficit of in excess of $22 million. The Company continued to file reports under the Exchange Act through its quarterly report on Form 10-Q for the period ended September 30, 2009, during which three and nine-month period the Company reported Net Losses of $232,815 and $1,167,989, respectively. Also, at September 30, 2009, the Company lacked sufficient capital resources to continue to fund the expenses including professional fees associated with being a current, reporting company under the Exchange Act.
As a result, from and after the filing of its 10-Q for the period ended September 30, 2009, the Company ceased active business operations and its board of directors determined to devote its limited and depleting cash resources to seek operations that would generate more revenues and hopefully, positive cash flow from operations than its prior operations exploiting its Battery Brain technology. The Company became delinquent in its reporting obligations under the Exchange Act, failing to file its annual report on Form 10-K for the year ended December 31, 2009 and continued to be a delinquent filer until it filed a Form 15, terminating its registration under Section 12(g) of the Exchange Act on July 24, 2013.
Prior to filing the Form 15, the Company’s assets became subject to a proceeding before the Superior Court of the State of New Jersey, which resulted in the appointment of a receiver in early 2013. The principal creditor in that proceeding was Aharon Levinas, who had sold the Battery Brain Assets formerly owned by Purysis in the Asset Purchase Agreement dated March 23, 2005. On June 7, 2013, in connection with the order of the Superior Court of the State of New Jersey (the “Consent Order Approving Settlement”), the Court authorized and approved the sale, transfer and assignment of all of the Company’s assets to Aharon Levinas, free and clear of any liens, claims or encumbrances and granting Mr. Levinas effective control of the Company.
During November 2014 and March 2015, third-party investors acquired control of the Company by purchasing a control block of shares each holding 137,500 shares representing 88% of the Company’s issued and outstanding shares of common stock. Reference is made to the disclosure under Item 4. “Security Ownership of Certain Beneficial Owners and Management.”
Recent Corporate Developments
On August 30, 2017, a new wholly-owned subsidiary was registered in Israel under the name of Canna Powder Ltd. (“CannaPowder Israel” or the “Subsidiary”), with 100 common shares outstanding, 0.01 NIS par value (the “Subsidiary Shares”), all of which were held in escrow on behalf of the Company by Israel attorney, Alon Nave. On September 27, 2017, pursuant to board resolution, the 100 Subsidiary Shares held in escrow were transferred to the Company.
The Subsidiary’s management includes Lavi Krasney, its CEO, and Rafi Ezra, its CTO. Mr. Ezra is a highly experienced pharmacist with extensive knowledge of the cannabis sector and active experience of leading early-stage pharma companies from early-stage development through commercial launch.
Development is being conducted at the Hebrew University under the supervision of the inventor of the technology, Professor Shlomo Magdassi, pursuant to the term of the Feasibility Study and Option Agreement dated September 14, 2017 (the “Feasibility Study”), a copy of which is attached as Exhibit 10.1 hereto, as more fully discussed below.
On December 27, 2017, a board-resolution was adopted to issue an additional: (i) 800 Subsidiary Shares to the Company; and an additional 100 Subsidiary Shares to Rafi Ezra and, as a result, effective December 27, 2017, Canna Powder Ltd became a 90% owned subsidiary of the Company and a minority interest of 10% owned by Rafi Ezra.
In anticipation of the formation of CannaPowder Ltd, the Company’s newly organized Israeli subsidiary, the Company began to raise capital through the private sale of its equity securities primarily pursuant to the exemptions provided under Regulation S promulgated by the Securities and Exchange Commission (the “SEC”) under the Securities Act of 1933, as amended (the “Act”) and, to a lesser extent, pursuant to Regulation D promulgated by the SEC under the Act (collectively, the “Equity Raise”). To date, the Company has raised approximately $888,775 in the Equity Raise. Reference is made to the disclosure under Item 10. “Recent Sales of Equity Securities” and Note (7) Subsequent Events, below.
The Equity Raise by the Company was and continues to be for the purpose of funding the Company’s business involving its development program to establish cannabis powder production facilities utilizing the proprietary, licensed technology as more fully-described under “Intellectual Property” below (the “Development Program”). The Company reasonably expects that the Development Program will be completed within three years, with commercial sales starting in 2021, there can be no assurance that the Development Program will, in fact, be successful notwithstanding the Company’s success in its Equity Raise to date, nor can there be assurance that the Company may not require additional capital to fully implement its business plan and complete production of commercially viable products based on its technology which is the subject of the Feasibility Study discussed below under“Planned Research and Development and Current Trends.”
In the commercial stage, the Company’s plan is to establish and operate several production facilities, each located in separate territories determined by the Company according to their size and regulatory environment that permits studies applicable to other activities prerequisite to commercial exploitation of medical cannabis generally and the Company’s plan to develop cannabis-based powders for medical uses. While there can be no assurance, at present the Company believes that it will be able to produce cannabis powders for medical uses at a significant cost advantage.
Planned Research and Development and Current Trends
There is increasing recognition and agreement amongst medical professionals conducting research in hospitals world-wide, including leading hospitals in Israel such as Hebrew University, Jerusalem, Israel and Sheba Academic Medical Center located in Tel Hashomer, Israel, among others, that the therapeutic effect of medical cannabis is due to the total number of cannabinoids working together. There is a scientific effort currently being lead in Israel, as well as other countries to analyze and understand how the various components within cannabis work. including ongoing scientific studies to develop separate medical components for use in treatment of different medical conditions using various components or combinations for each cannabinoid.
The aim of CannaPowder Israel is to identify the active pharmaceutical ingredient (API) and to understand the cannabidiol (CBD) and tetrahydrocannabinol (THC) content of each product. Researchers have discovered approximately in excess of 100 cannabinoids, chemical compounds unique to the cannabis plant. The most common are CBD, cannabinol (CBN) and THC. CBN and THC interact with CB1 and CB2 receptors, which are located throughout the human body. CB1 receptors are primarily located in the brain and central nervous system. CB2 receptors are located throughout the body including the gastrointestinal and urinary tracts that are responsible for regulating neurotransmission. The CB1 and CB2 receptors help control bodily reactions such as inflammation and pain, which are areas are of great therapeutic interest with respect to drug development. Identifying cannabinoid receptors and the compounds that interact with them has helped accelerate clinical investigations of cannabinoid-based drugs. To date, due to the challenges of researching plant-derived cannabinoids in the United States, most U.S. research has been conducted utilizing synthetically produced cannabinoids, which as chemical compounds, are chemically identical to plant-derived cannabinoids.
We believe cannabinoid-based drugs using the powders we plan on developing may provide a superior treatment model for patients suffering from certain diseases, disorders and medical conditions. It is generally agreed that cannabinoid-based drugs have tolerable safety profiles but at present, because of U.S. FDA and DEA restrictions, most companies involved in research and development of cannabinoid therapeutic applications currently use synthetics.
We believe that there will be rising demand for cannabinoid-derived drugs and that future growth is likely to be driven by favorable changes in legislation and demographic factors. Controlled substance laws differ between countries and legislation in certain countries may restrict or limit our ability to distribute our cannabis-based powders, once developed, for us if drugs. Nevertheless, we believe that the U.S. can potentially represent a major market for CannaPowder-based drug candidates. In the European Community, medical cannabis program regulatory frameworks exist in countries, including the Netherlands, Italy, Germany, Finland, Norway, Ukraine, Estonia, Switzerland and the Czech Republic. It is also anticipated that there will be policy changes in many member countries of the European Union regarding the medical use of cannabis and cannabinoid-derived drugs. In addition, medical cannabis and, to some extent, recreation cannabis use by adults, has been decriminalized in Russia, Mexico, Columbia, Uruguay, Paraguay and Colombia.
The objective of our subsidiary, CannaPowder Israel, is to develop nano-cannabis powder, standardized from cannabis oil in a known cannaboid composition. CannaPowder Israel is in the process of licensing what it believes will represent a breakthrough platform technology in drug administration and with the view to applying it to enable the commercial production of cannabis powder.
The technology has been developed by scientists at the Hebrew University, Jerusalem, which technology has been licensed to the Company’s Israeli Subsidiary. Reference is made to the Feasibility Study and Option Agreement attached as exhibit 10.1 attached hereto. It comprises a new technique which enables preparation of nanoparticles from micro emulsions of active ingredients that can be stored as powders and then dissolved in water when required.
Our management team, working in collaboration with the scientists at Hebrew University under the supervision of Professor Shlomo Magdassi, believe this technology may be adapted for many different insoluble drugs to provide enhanced dissolution properties and to improve solubility, bioavailability and storage properties and solutions.
The innovation is as a platform technology for preparing dispersible powders or aqueous dispersions of nanoparticles of water-insoluble organic compounds by converting micro emulsions or nanoemulsions containing the active chemicals into powders.
The key features, we believe, include:
| | ● | Preparation of the nanoemulsions is simple and reproducible, without the use of any special equipment. |
| | ● | Can dilute the powders in water to obtain required concentration of active ingredient. |
| | ● | Reduces dose of drug needed. |
| | ● | Nanoparticles offer improved bioavailability and improved efficacy. |
| | ● | Simple, cost effective technique. |
| | ● | Powders have relatively long shelf life. |
Although there can be no assurance, we believe that our new technology should be ideally suited to cannabis because it is based upon and deals specifically with oily/lipophilic materials which is exactly the form in which cannabis exists. Our plan if for the final product to a micronized powder containing cannabinoids in a particle size of 100 nanometers, easily incorporated in a commercial product, such as a capsule. It is the result of a pharmaceutical not a chemical process.
The process of producing the powders will utilize high pressure emulsifying agents that vaporize water to particles, which evaporation process is expected to take only hours as opposed to days or weeks, meaning that it should be quicker and less expensive than existing processes for producing medical cannabis with consistent dosages. Furthermore, the production process should not be labor intensive or require a large and therefore costly facility, thereby providing the economic advantage of being able to produce commercial quantities of cannabis powder at costs far less than existing methods currently in use by other manufacturers of medical cannabis drugs and treatments.
The Medical Cannabis Market
Cannabis has been used for medicinal purposes for thousands of years and has proven to be an effective treatment for pain relief, inflammation and a number of other medical disorders. According to anIBISWorld report, new medical research and changing public opinion have boosted industry growth.
Doctors may prescribe ‘legalized’ medical cannabis in approved states where patients can receive a “recommendation” from a state-approved, licensed physician for the treatment of certain conditions specified by the state.
Medical cannabis is being used to treat severe or chronic pain, inflammation, nausea and vomiting, neurologic symptoms (including muscle spasticity), glaucoma, cancer, multiple sclerosis, post-traumatic stress disorder, anorexia, arthritis, Alzheimer’s, Crohn’s disease, fibromyalgia, ADD, ADHD, Tourette’s syndrome, spinal cord injury and numerous other conditions. Cannabis oil has also been proven effective in treating epileptic seizures in children.
The worldwide medical cannabis market is anticipated to reach $55.8 billion by 2025, growing at a CAGR of 17.1% from 2013 to 2025, the forecast period (1), compared to $11.4 billion in 2015.
Awareness regarding the medical benefits of cannabis is spurring market growth. Major manufacturers producing medical cannabis remove THC, a psychoactive compound which causes the well-known and recognized euphoria related hallucinations or even what some define as psychotic episodes on the negative spectrum of THC’s potential side effects. The product then only contains cannabidiol (CBD), a chemical compound which provides health benefits. Use of medical cannabis for therapeutic purposes is driving many countries to legalize it. At present, 29 of 50 states in the U.S. permit the prescription and sale of medical cannabis to adults and although there appears to be a trend in the U.S. that additional states will approve medical cannabis, laws at the federal level in the U.S. still treat cannabis, whether for medical or recreational use, as a Class 1 Controlled Substance and illegal.
At present, cannabis is used widely in health foods and pet medicines. Health foods comprise juices, cannabis edibles, and drinkable medicines. For instance, Simply Pure, a U.S. based company offers edibles devoid of gluten and sugar. Auntie Dolores, another U.S. based company manufactures CBD pet treats to relieve pets of anxiety and pain. Cannabis edibles are offered in the form of lozenges, breath strips, and gummies. In an attempt to propel market growth, Microsoft Azure too has offered its Cloud services to industry participants for the sale of cannabis.
The global medical cannabis market is segmented according to applications and regions. Market applications comprise cancer, chronic pain, arthritis, migraine, and others. Chronic pain is the largest application segment due to a huge patient base. It accounted for nearly 40% of global revenues in the same year. It is driven by increasing number of clinical trials that promote the use of medical cannabis in pain management.
The cancer segment is expected to grow rapidly at an 18.2% CAGR during the forecast period. Although, medical use of cannabis is considered illegal, many districts and states are passing amendments to legalize it. A number of clinical trials have demonstrated that medical cannabis has the capacity to destroy cancer cells.
However, this drug is yet to receive U.S. FDA approval for use in cancer treatment. This adversely impacts the growth of the cancer application segment.
North America led the medical cannabis market with nearly 49% shares in 2015. The region is supported by the legalization of cannabis in most states of the U.S. The drug has received approval for recreational use in only eight states. North America is anticipated to grow at a CAGR of 22% during the forecast period. Prohibition of medical cannabis in most of Latin America and Asia has limited the growth of this market to Europe, North America, and other countries world-wide.
Prominent companies presently engaged in this field include: Cannabis Sativa, Inc.; United Cannabis Corporation; Growblox Sciences, Inc.; and GW Pharmaceuticals plc which is a UK pharmaceuticals manufacturer that has gained approval for using medical cannabis in treating various cancers and whose proprietary formula which contains a mixture of THC and CBD was used in a clinical trial to treat brain cancer.
1https://www.mediafound.org/news/medical-cannabis-market-to-grow-at-17-1-cagr-till-2025.htm
Competition
We will face competition from larger companies that are or may be in the process of offering similar products to ours. Many of our current and potential competitors have longer operating histories, significantly greater financial, marketing and other resources than we may be expected to have.
Competitors may include major pharmaceutical and biotechnology companies and public and private research institutions. Management cannot be certain that we will be able to compete against current or future competitors or that competitive pressure will not seriously harm our business prospects. These competitors may be able to react to market changes, respond more rapidly to new regulations or allocate greater resources to the development and promotion of their products than we can.
Furthermore, some of these competitors may make acquisitions or establish collaborative relationships among themselves to increase their ability to rapidly gain market share. Large pharmaceutical companies may eventually enter the market.
Given the rapid changes affecting the global, national, and regional economies in general and cannabis-related medical research and development in particular, we may not be able to create any perceived competitive advantage from our cannabis-based powder formulations in the marketplace.
Our success will also depend on our ability to respond quickly to, among other things, changes in the economy, market conditions, and competitive pressures. Any failure to anticipate or respond adequately to such changes could have a material effect on our financial condition, operating results, liquidity, cash flow and our operational performance.
Intellectual Property
At present, all of our research and development is conducted in Israel and is the subject of the Feasibility Study being conducted at Hebrew University. The cannabinoid-based medicinal powders we intend to develop, contain controlled substance (cannabis) as defined in the Israeli Dangerous Drugs Ordinance [New Version], 5733 - 1973. In Israel, licenses to cultivate, possess and to use cannabis for medical research are granted by the Ministry of Health, IMCU - Israel Medical Cannabis Unit, on an ad-hoc basis.
Government Regulation
United States
The legal cannabis industry has evolved considerably during the past five years and many observers believe that the industry has reached the tipping point for legalization through pressure from citizens’ groups in individual states in the U.S. for the legalization of medical and/or recreational cannabis. At the United States federal level, cannabis is still classified as an illegal substance under the Controlled Substances Act. The classification makes cannabis illegal under federal law to cultivate, manufacture, distribute or possess cannabis, and has created a discrepancy between state’s rights and federal law. It also means that production must be local to consumption as it cannot cross state lines.
Public support has resulted in the passing of new cannabis laws and regulations in a number of countries and individual states in the US. By 2026 many states are expected to build robust legal adult-use markets, and all but a few states will make medical cannabis available legally.
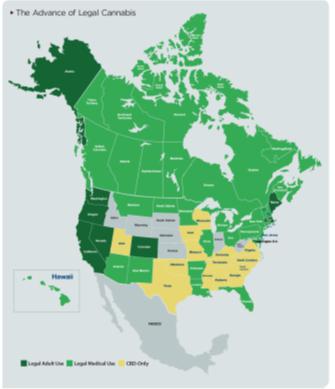
Countries around the world are already responding to the state-by-state dismantling of prohibition in America by moving to allow medical use (as in Australia, Germany, and Colombia) or to outright legalization (as in Uruguay).
We believe cannabinoid-based drugs using the powders we plan on developing may provide a superior treatment model for patients suffering from certain diseases, disorders and medical conditions. It is generally agreed that cannabinoid-based drugs have tolerable safety profiles but at present, because of U.S. FDA and DEA restrictions, most companies involved in research and development of cannabinoid therapeutic applications currently use synthetics.
To the extent that in the future, we shall seek to be able to conduct some of our research and development relating to our cannabis powders in the United States, at which time, we will become subject to the United States’ Federal Controlled Substances Act of 1970 and regulations promulgated thereunder (the “CSA”). While cannabis is a Schedule I controlled substance, drugs approved for medical use in the United States that contain cannabis or cannabis extracts must be placed in Schedules II-V, since approval by the FDA satisfies the “accepted medical use” requirement. If any of our drug candidates will receive approval by the FDA, it must be listed by the Drug Enforcement Agency (“DEA”) as a Schedule II or III controlled substance to be allowed for commercialization. Consequently, the manufacture, importation, exportation, domestic distribution, storage, sale and legitimate use of our future drugs will be subject to a significant degree of regulation by the DEA. In addition, individual states in the United States have also established controlled substance laws and regulations. Though state-controlled substances laws often mirror federal law because the states are separate jurisdictions, they may separately schedule our drugs.
The European Community
Even though we do not currently intend to conduct research and development in the European Community, we may do so in the future. Approximately 250 substances, including cannabis, are listed in the Schedules annexed to the United Nations Single Convention on Narcotic Drugs (New York, 1961, amended 1972), the Convention on Psychotropic Substances (Vienna, 1971) and the Convention against Illicit Traffic in Narcotic Drugs and Psychotropic Substances (introducing control on precursors) (Vienna, 1988). The purpose of these listings is to control and limit the use of these drugs according to a classification of their therapeutic value, risk of abuse and health dangers, and to minimize the diversion of precursor chemicals to illegal drug manufacturers. The 1961 UN Single Convention on Narcotic Drugs, as amended in 1972 classifies cannabis as Schedule I (“substances with addictive properties, presenting a serious risk of abuse”) and as Schedule IV (“the most dangerous substances, already listed in Schedule I, which are particularly harmful and of extremely limited medical or therapeutic value”) narcotic drug. The 1971 UN Convention on Psychotropic Substances classifies THC - the principal psychoactive cannabinoid of cannabis - as a schedule I psychotropic substance (Substances presenting a high-risk of abuse, posing a particularly, serious threat to public health which are of very little or no therapeutic value).
Most countries in Europe are parties to these conventions which govern international trade and domestic control of these substances, including cannabis. They may interpret and implement their obligations in a way that creates a legal obstacle to our obtaining manufacturing and/or marketing approval for our drugs in those countries. These countries may not be willing or able to amend or otherwise modify their laws and regulations to permit our drug candidates to be manufactured and/or marketed or achieving such amendments to the laws and regulations may take a prolonged period. In the European Community, medical cannabis program regulatory frameworks exist in countries, including the Netherlands, Italy, Germany, Finland, Norway, Ukraine, Estonia, Switzerland and the Czech Republic. It is also anticipated that there will be policy changes in many member countries of the European Union regarding the medical use of cannabis and cannabinoid-derived drugs.
Israel
If we intend to develop drugs containing cannabis plant-derived cannabinoids, we may conduct our research and development activities in Israel. The cannabinoid-based drugs we intend to develop, contain controlled substance (cannabis) as defined in the Israeli Dangerous Drugs Ordinance [New Version], 5733 - 1973. In Israel, licenses to cultivate, possess and to use cannabis for medical research are granted by the Ministry of Health, IMCU - Israel Medical Cannabis Unit, on an ad-hoc basis. If we proceed in Israel, we intend to obtain necessary IMCU licenses to carry out our drug development projects. This will require our acquiring the cannabis needed for our research activities from an Israeli government-licensed medical cannabis grower. Because we do not have a license to possess cannabis, the cannabis that will be required for our studies must be transported from the licensed grower directly to our research facilities or those of a contract research organization, in compliance with a license to use cannabis for medical research. If we proceed with research in Israel, we will apply for all necessary licenses needed to conduct our drug development projects. There can be given no assurance that we will obtain all necessary licenses and approvals.
We believe that there will be rising demand for cannabinoid-derived drugs and that future growth is likely to be driven by favorable changes in legislation and demographic factors. Controlled substance laws differ between countries and legislation in certain countries may restrict or limit our ability to distribute our cannabis-based powders, once developed, for us if drugs. Nevertheless, we believe that the U.S. can potentially represent a major market for CannaPowder-based drug candidates.
ITEM 1A. RISK FACTORS
Risks Relating to Our Lack of Operating History and Industry.
Risks Relating to Our Business
Any investment in our shares of common stock involves a high degree of risk. You should carefully consider the following information about these risks, together with the other information contained in this annual report before you decide to invest in our common stock. Each of the following risks may materially and adversely affect our business objective, plan of operation and financial condition. These risks may cause the market price of our common stock to decline, which may cause you to lose all or a part of the money you invested in our common stock. We provide the following cautionary discussion of risks, uncertainties and possible inaccurate assumptions relevant to our business plan. In addition to other information included in this annual report, the following factors should be considered in evaluating the Company’s business and future prospects.
The Company has a limited operating history and very limited resources.
The Company’s recent operations have been limited and has had no revenues from operations. Investors will have no basis upon which to evaluate the Company’s ability to achieve the Company’s plan of operation.
The Company’s officer and director may allocate his time to other businesses thereby causing conflicts of interest in his determination as to how much time to devote to the Company’s affairs. This could have a negative impact on the Company’s ability to implement its plan of operation.
The Company’s officer and director is not required to commit his full time to the Company’s affairs, which may result in a conflict of interest in allocating his time between the Company’s business and other businesses. Management of the Company is engaged in several other business endeavors and is not obligated to contribute any specific number of his hours per week to the Company’s affairs. If Management’s other business affairs require him to devote more substantial amounts of time to such affairs, it could limit his ability to devote time to the Company’s affairs and could have a negative impact on the Company’s ability to implement its plan of operation.
The Company may be unable to obtain additional financing, if required, to complete its plan of operation or to fund the operations and growth of it business, which could compel the Company to abandon its business.
If we require funds, we will be required to seek additional financing. We cannot assure you that such financing would be available on acceptable terms, if at all. To the extent that additional financing proves to be unavailable when needed, we would be compelled to abandon our business plan. In addition, we may require additional financing to fund the operations or growth of the our business. The failure to secure additional financing could have a material adverse effect on the continued development or growth of our business. The Company’s officers, director or stockholders are not required to provide any financing to us.
Broad discretion of Management
Any person who invests in the Company’s common stock will do so without an opportunity to evaluate the specific merits or risks our business. As a result, investors will be entirely dependent on the broad discretion and judgment of Management. There can be no assurance that determinations made by the Company’s Management will permit us to achieve the Company’s business plan.
General Economic Risks.
The Company’s current and future business objectives and plan of operation are likely dependent, in large part, on the state of the general economy. Adverse changes in economic conditions may adversely affect the Company’s business objective and plan of operation. These conditions and other factors beyond the Company’s control include also but are not limited to regulatory changes.
Our control shareholders have significant voting power and may take actions that may be different than actions sought by our other stockholders.
Our control shareholders own approximately 40.2% of the outstanding shares of our common stock. These stockholders will be able to exercise significant influence over all matters requiring stockholder approval. This influence over our affairs might be adverse to the interest of our other stockholders. In addition, this concentration of ownership could delay or prevent a change in control and might have an adverse effect on the market price of our common stock.
Our officers and directors are located in Israel and our assets may also be held from time to time outside of the United States.
Since all of our officers and directors are currently located in and/or are residents of Israel, any attempt to enforce liabilities upon such individuals under the U.S. federal securities and bankruptcy laws may be difficult. In accordance with the Israeli Law on Enforcement of Foreign Judgments, 5718-1958, and subject to certain time limitations (the application to enforce the judgment must be made within five years of the date of judgment or such other period as might be agreed between Israel and the United States), an Israeli court may declare a foreign civil judgment enforceable if it finds that:
- the judgment was rendered by a court which was, according to the laws of the State in which the court is located, competent to render the judgment;
- the judgment may no longer be appealed;
- the obligation imposed by the judgment is enforceable according to the rules relating to the enforceability of judgments in Israel and the substance of the judgment is not contrary to public policy; and
- the judgment is executory in the State in which it was given.
An Israeli court will not declare a foreign judgment enforceable if:
- the judgment was obtained by fraud;
- there is a finding of lack of due process;
- the judgment was rendered by a court not competent to render it according to the laws of private international law in Israel;
- the judgment is in conflict with another judgment that was given in the same matter between the same parties and that is still valid; or
- the time the action was instituted in the foreign court, a suit in the same matter and between the same parties was pending before a court or tribunal in Israel.
Furthermore, Israeli courts may not adjudicate a claim based on a violation of U.S. securities laws if the court determines that Israel is not the most appropriate forum in which to bring such a claim. Even if an Israeli court agrees to hear such a claim, it may determine that Israeli law, not U.S. law, is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proven as a fact, which can be a time-consuming and costly process.
Our assets may also be held from time to time outside of the United States. Since our directors and executive officers are foreign citizens and do not reside in the United States, it may be difficult for courts in the United States to obtain jurisdiction over our foreign assets or persons, and as a result, it may be difficult or impossible for you to enforce judgments rendered against us or our directors or executive officers in United States courts. Thus, investing in us may pose a greater risk because should any situation arise in the future in which you would have a cause of action against these persons or against us, you may face potential difficulties in bringing lawsuits or, if successful, in collecting judgments against these persons or against the Company.
Regulatory Risks
We face risks related to compliance with corporate governance laws and financial reporting standards.
The Sarbanes-Oxley Act of 2002, as well as related new rules and regulations implemented by the Securities and Exchange Commission and the Public Company Accounting Oversight Board, require changes in the corporate governance practices and financial reporting standards for public companies. These new laws, rules and regulations, including compliance with Section 404 of the Sarbanes-Oxley Act of 2002 relating to internal control over financial reporting, have materially increased the legal and financial compliance costs of small companies and have made some activities more time-consuming and more burdensome.
We may not have effective internal controls.
In connection with Section 404 of the Sarbanes-Oxley Act of 2002, we need to assess the adequacy of our internal control, remedy any weaknesses that may be identified, validate that controls are functioning as documented and implement a continuous reporting and improvement process for internal controls. We may discover deficiencies that require us to improve our procedures, processes and systems in order to ensure that our internal controls are adequate and effective and that we are in compliance with the requirements of Section 404 of the Sarbanes-Oxley Act. If the deficiencies are not adequately addressed, or if we are unable to complete all of our testing and any remediation in time for compliance with the requirements of Section 404 of the Sarbanes-Oxley Act and the SEC rules under it, we would be unable to conclude that our internal controls over financial reporting are designed and operating effectively, which could adversely affect investor confidence in our internal controls over financial reporting.
Risks Relating to Operating in Israel.
We conduct our operations in Israel and therefore our results may be adversely affected by political, economic and military instability in Israel or the Middle East.
Our subsidiary offices and our officers and directors are located in Israel. Accordingly, political, economic and military conditions in Israel and the Middle East may directly affect our business. Since the establishment of the State of Israel in 1948, a number of armed conflicts have taken place between Israel and its Arab neighbors. Any hostilities involving Israel or the interruption or curtailment of trade within Israel or between Israel and its trading partners could adversely affect our operations and could make it more difficult for us to raise capital. Since September 2000, terrorist violence in Israel has increased significantly and negotiations between Israel and Palestinian representatives have not achieved a peaceful resolution of the conflict. The establishment in 2006 of a government in Gaza by representatives of the Hamas militant group has created additional unrest and uncertainty in the region.
Further, Israel is currently engaged in an armed conflict with Hamas, which until Operation Cast Lead in January 2009 had involved thousands of missile strikes and had disrupted most day-to-day civilian activity in southern Israel. The missile attacks by Hamas did not target Tel Aviv, the location of our principal executive offices; however, any armed conflict, terrorist activity or political instability in the region may negatively affect business conditions and could significantly harm our results of operations.
Risks Related to Our Common Stock
Our historic stock price has been volatile and the future market price for our common stock is likely to continue to be volatile. Further, the limited market for our shares will make our price more volatile. This may make it difficult for you to sell our common stock.
The public market for our common stock has been very volatile. Over the past three fiscal years and subsequent quarterly periods, the market price for our common stock has ranged from $0.35 to $2.70 (See “Market for Common Equity and Related Stockholder Matters” in this annual report). Any future market price for our shares is likely to continue to be very volatile. This price volatility may make it more difficult for you to sell shares when you want at a price you find attractive. Further, the market for our common stock is limited and we cannot assure you that a larger market will ever be developed or maintained. Market fluctuations and volatility, as well as general economic, market and political conditions, could reduce our market price. As a result, this may make it difficult or impossible for you to sell our common stock.
The Company’s shares of common stock are quoted on the OTC Pink Sheet market, which limits the liquidity and price of the Company’s common stock.
The Company’s shares of common stock are traded on the OTC Pink Sheet market. Quotation of the Company’s securities on the OTC Pink Sheet market limits the liquidity and price of the Company’s common stock more than if the Company’s shares of common stock were listed on The Nasdaq Stock Market or a national exchange. There is currently no active trading market in the Company’s common stock. There can be no assurance that there will be an active trading market for the Company’s common stock following a business combination. In the event that an active trading market commences, there can be no assurance as to the market price of the Company’s shares of common stock, whether any trading market will provide liquidity to investors, or whether any trading market will be sustained.
Our common stock is subject to the Penny Stock Rules of the SEC and the trading market in our common stock is limited, which makes transactions in our stock cumbersome and may reduce the value of an investment in our common stock.
The Securities and Exchange Commission has adopted Rule 3a51-1 which establishes the definition of a “penny stock,” for the purposes relevant to us, as any equity security that has a market price of less than $5.00 per share or with an exercise price of less than $5.00 per share, subject to certain exceptions. For any transaction involving a penny stock, unless exempt, Rule 15g-9 require:
- that a broker or dealer approve a person’s account for transactions in penny stocks; and
- the broker or dealer receive from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased.
In order to approve a person’s account for transactions in penny stocks, the broker or dealer must:
- obtain financial information and investment experience objectives of the person; and
- make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks.
The broker or dealer must also deliver, prior to any transaction in a penny stock, a disclosure schedule prescribed by the SEC relating to the penny stock market, which, in highlight form:
- sets forth the basis on which the broker or dealer made the suitability determination; and
- that the broker or dealer received a signed, written agreement from the investor prior to the transaction.
Generally, brokers may be less willing to execute transactions in securities subject to the “penny stock” rules. This may make it more difficult for investors to dispose of our common stock and cause a decline in the market value of our stock.
Disclosure also has to be made about the risks of investing in penny stocks in both public offerings and in secondary trading and about the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and the rights and remedies available to an investor in cases of fraud in penny stock transactions. Finally, monthly statements have to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks.
State blue sky registration; potential limitations on resale of the Company’s common stock
The holders of the Company’s shares of common stock registered under the Exchange Act and those persons who desire to purchase them in any trading market that may develop in the future, should be aware that there may be state blue-sky law restrictions upon the ability of investors to resell the Company’s securities. Accordingly, investors should consider the secondary market for the Registrant’s securities to be a limited one.
It is the intention of the Registrant’s Management following the consummation of a business combination to seek coverage and publication of information regarding the Registrant in an accepted publication manual which permits a manual exemption. The manual exemption permits a security to be distributed in a particular state without being registered if the Registrant issuing the security has a listing for that security in a securities manual recognized by the state. However, it is not enough for the security to be listed in a recognized manual. The listing entry must contain (1) the names of issuers, officers, and directors, (2) an issuer’s balance sheet, and (3) a profit and loss statement for either the fiscal year preceding the balance sheet or for the most recent fiscal year of operations. Furthermore, the manual exemption is a nonissuer exemption restricted to secondary trading transactions, making it unavailable for issuers selling newly issued securities.
Most of the accepted manuals are those published by Standard and Poor’s, Moody’s Investor Service, Fitch’s Investment Service, and Best’s Insurance Reports, and many states expressly recognize these manuals. A smaller number of states declare that they “recognize securities manuals” but do not specify the recognized manuals. The following states do not have any provisions and therefore do not expressly recognize the manual exemption: Alabama, Georgia, Illinois, Kentucky, Louisiana, Montana, South Dakota, Tennessee, Vermont and Wisconsin.
Dividends unlikely
The Company does not expect to pay dividends for the foreseeable future because it has no revenues or cash resources. The payment of dividends will be contingent upon the Company’s future revenues and earnings, if any, capital requirements and overall financial conditions. The payment of any future dividends will be within the discretion of the Company’s board of directors as then constituted. It is the Company’s expectation that future management, following a business combination, will determine to retain any earnings for use in its business operations and accordingly, the Company does not anticipate declaring any dividends in the foreseeable future.
ITEM 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITIONS AND PLAN OF OPERATION
The following Management’s Discussion and Analysis of Financial Condition and Plan of Operations (“MD&A”) is intended to help you understand our historical results of operations during the periods presented and our financial condition. This MD&A should be read in conjunction with our consolidated financial statements and the accompanying notes to consolidated financial statements and contains forward-looking statements that involve risks and uncertainties. See section entitled “Forward-Looking Statements” above.
Plan of Operation
At present, we are a Company with only limited operations and no revenues from our plan of operations. There is substantial doubt that we can continue as an on-going business for the next twelve months.
In December 2017 a new wholly-owned subsidiary was registered in Israel under the name of Canna Powder Ltd. (“CannaPowder”) with Rafi Ezra as its CEO. He is a highly experienced pharmacist with extensive knowledge of the cannabis sector and hands on experience of leading early stage pharma companies from early development through to commercial launch. Development is being carried out at the Hebrew University under the supervision of the inventor of the technology Professor Shlomo Magdassi.
CannaPowder is raising funds to finance a development program to establish cannabis powder production facilities utilizing the licensed technology. The program is expected to be completed within three years, with commercial sales starting in 2021. Once reaching the commercial stage, CannaPowder is expected to establish and operate several production facilities, each located in individual territories selected according to their size and favorable regulation for medical cannabis. The facilities will produce cannabis powders at very competitive prices and we expect to quickly capture market share.
Products will be supplied to producers of medical cannabis products. The ability to produce a more effective, consistent product in terms of quality and composition will provide an important advantage when targeting the medical market.
Results of Operations during the year ended December 31, 2017 as compared to the year ended December 31, 2016
During 2017 and 2016 we generated revenues of $0 and $0, respectively. Our general and administrative expense increased to $75,177 during 2017 compared to $2,601 during the same period in prior year and our research and development expenses increased to $10,007 during 2017 compared to $0 during the same period in the prior year both increases were due to costs incurred in relation to our new subsidiary CannaPowder. We incurred a net loss of $85,970 during 2017 compared to a net loss of $2,644 in 2016.
Liquidity and Capital Resources
Our balance sheet as of December 31, 2017 reflects $330,926 in total assets consisting of cash and cash equivalents of $326,730 and prepaid assets of $4,196. As of December 31, 2016, our balance sheet reflects assets of $0.
As of December 31, 2017, we had total current liabilities of $800 consisting of accounts payable and accrued liabilities and $2,687 of long term liabilities consisting of notes payable. As of December 31, 2016, we had total current liabilities of $2,144 consisting of accounts payable and $800 of long term liabilities consisting of notes payable.
We had positive working capital of $330,126 as of December 31, 2017 compared to negative working capital of $2,144 at December 31, 2016. Such working capital has been sufficient to sustain our operations to date. Our total liabilities as of December 31, 2017 were $3,487 compared to $2,944 at December 31, 2016.
During 2017, we used $91,560 in our operating activities. This resulted from a net loss of $85,970, decrease to accounts payable and accrued expenses of $1,344 decrease in prepaid expenses of $4,196 and gain on settlement of $50.
During 2016, we used $800 in our operating activities. This resulted from a net loss of $2,644 offset by an increase to accounts payable and accrued expenses of $1,844.
During the year ended December 31, 2017, we financed our negative cash flow from sale of common stock in the amount of $413,000, borrowings on debt of $19,190 which were offset by principal payment on debt of $17,253.
During the year ended December 31, 2016, we financed our negative cash flow from borrowings on debt of $800.
While management of the Company believes that the Company will be successful in its current and planned operating activities, there can be no assurance that the Company will be successful in the achievement of sales of its products that will generate sufficient revenues to earn a profit and sustain the operations of the Company.
Our ability to create sufficient working capital to sustain us over the next twelve-month period, and beyond, is dependent on our entering into additional licensing agreement and on our success in issuing additional debt or equity or entering into strategic arrangement with a third party. There can be no assurance that sufficient capital will be available to us. We currently have no agreements, arrangements or understandings with any person to obtain funds through bank loans, lines of credit or any other sources.
Going Concern Consideration
There is substantial doubt about our ability to continue as a going concern. Our financial statements contain additional note disclosures with respect to this matter.
The Company currently plans to satisfy its cash requirements for the next 12 months through the issuance of equity and borrowings from its controlling shareholders and believes it can satisfy its cash requirements so long as it is able to obtain financing from its controlling shareholders. The Company expects that money borrowed will be used during the next 12 months to satisfy the Company’s operating costs, professional fees and for general corporate purposes.
The Company has only limited capital. Additional financing is necessary for the Company to continue as a going concern. Our independent auditor has issued an unqualified audit opinion for the years ended December 31, 2017 and 2016 with an explanatory paragraph on going concern.
Off-Balance Sheet Arrangements
As of December 31, 2017, and 2016, we did not have any off-balance sheet arrangements as defined in Item 303(a)(4)(ii) of Regulation S-K promulgated under the Securities Act of 1934.
Contractual Obligations and Commitments
As of December 31, 2017, and 2016, we did not have any contractual obligations.
Critical Accounting Policies
Our significant accounting policies are described in the notes to our financial statements for the years ended December 31, 2017 and 2016 and are included elsewhere in this annual report.
ITEM 3. PROPERTIES
Our corporate office, which consists of approximately 150 square feet, is located at 40 Wall Street, 28th Floor New York, NY 10005, at a monthly cost of $300. Our Subsidiary leases offices located at 20 Raul Wallenberg Street, Tel Aviv, Israel consisting of 1200 square feet at a base monthly rent of $500. These offices are leased from an entity related to Attribute Ltd (See Item 4 below) which rent represents the fair market value of the facilities. The Company believes that its facilities are sufficient for the foreseeable future and until we actively commence our sales and marketing efforts. At such time, we believe that adequate facilities will be available for our Subsidiary’s operations at terms satisfactory to the Company.
ITEM 4. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth information regarding the beneficial ownership of our common stock as of April 20, 2018. The information in this table provides the ownership information for: each person known by us to be the beneficial owner of more than 5% of our common stock; each of our directors; each of our executive officers; and our executive officers and directors as a group.
| Name of Beneficial Owner | | Common Stock Beneficially Owned (1) | | | Percentage of Common Stock Owned (1) | |
| Attribute Ltd. | | | 787,500 | | | | 8.9 | % |
| 20 Raul Wallenberg Street | | | | | | | | |
| Tel Aviv, Israel | | | | | | | | |
Kfir Silberman and L.I.A. Pure Capital
(entity controlled by Kfir Silberman) | | | 787,500 | | | | 8.9 | % |
| 3 Ha’armonim St., | | | | | | | | |
| Ramat Gan, Israel | | | | | | | | |
| Amir Uziel | | | 787,500 | | | | 8.9 | % |
| 5 Shira St Street | | | | | | | | |
| Rishon Lezion, Israel | | | | | | | | |
| Lavi Krasney | | | 787,500 | | | | 8.9 | % |
| 8 Paamoni Street | | | | | | | | |
| Rishon Lezion, Israel | | | | | | | | |
| Liron Carmel, CEO and Chairman | | | 300,000 | | | | 3.4 | % |
| 40 Wall Street, 28th Floor | | | | | | | | |
| New York, NY 10005 | | | | | | | | |
| Oded Gilboa, CFO | | | 0 | | | | 0.00 | |
| 40 Wall Street, 28th Floor | | | | | | | | |
| New York, NY 10005 | | | | | | | | |
| Total Officers (2 people) | | | 300,000 | | | | 3.4 | % |
(1) Applicable percentage ownership is based on 8,875,577 shares of common stock issued as of April 20, 2018. Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission and generally includes voting or investment power with respect to securities. Shares of common stock that are currently exercisable or exercisable within 60 days of April 20, 2018 are deemed to be beneficially owned by the person holding such securities for the purpose of computing the percentage of ownership of such person but are not treated as outstanding for the purpose of computing the percentage ownership of any other person.
ITEM 5. DIRECTORS AND EXECUTIVE OFFICERS
Our directors hold office until the next annual general meeting of the stockholders or until their successors are elected and qualified. Our officers are appointed by our Board of Directors and hold office until the earlier of their death, retirement, resignation, or removal.
The following table sets forth the names and ages of the members of our Board of Directors and our executive officers and the positions held by each.
| Name | | Age | | Title |
| Liron Carmel | | 34 | | CEO and sole director |
| Oded Gilboa | | 45 | | CFO |
Liron Carmel, 34, has been CEO, CFO and sole director of the Company since December 2014. From December 2014 to August 2015, Mr. Carmel was the CEO and CFO of Zaxis International Inc.
From 2010 through December 2014, Mr. Carmel served as a senior analyst in the Investment Division of Excellence Group, a leading investment firm in Israel. In such capacity, Mr. Carmel specialized in risk management and special debt financing including participation in and leading negotiations with major institutional investors in Israel. From 2009 to 2010, Mr. Carmel was an analytical consultant for Precise Group, an Israeli financial institution.
Oded Gilboa, 45, was appointed to be the Company’s CFO on January 1, 2018. He is a licensed CPA in the United States and Israel. He was the CFO of TechCare Corp. (OTCQB: TECR17) from December 2013 to October 2016. Prior to his appointment as CFO of TechCare Corp., Mr. Gilboa served as TechCare’s finance and accounting consultant. Mr. Gilboa has over 16 years of experience in finance and public accounting, having served as a senior finance executive in the technology and biotech industries with responsibilities in corporate finance, accounting, strategic planning and operational and financial management. From 2010 through 2012, Mr. Gilboa served as the Revenue Accounting and Finance Manager of Mylan Specialty, a subsidiary of Mylan Inc. (NASDAQ: MYL), a global company focused on the development, manufacturing and marketing of prescription drug products. From 2007 through 2009, Mr. Gilboa was the Executive director of Finance and US Controller of Taro Pharmaceuticals (NASDAQ: TAROF), a global pharmaceutical company. From 1998 through 2007 Mr. Gilboa held various financial positions with IDT Corporation (NYSE: IDT), a world-wide provider of telecommunications and media services, where in his most recent role he served as director of Finance. Mr. Gilboa began his career in public accounting, auditing both public and private companies and holds a B.A in Economics and Accounting from Tel-Aviv University and an M.B.A. from Recanati Business School at Tel-Aviv University.
Committees of the Board of Directors
We do not presently have a separately constituted audit committee, compensation committee, nominating committee, executive committee or any other committees of our Board of directors. As such, our entire Board of directors acts as our audit committee.
Involvement in Legal Proceedings
None of our directors, nominees for directors, or officers has appeared as a party during the past ten years in any legal proceedings that may bear on his ability or integrity to serve as a director or officer of the Company.
Code of Ethics
The Company has adopted a code of ethics applicable to our principal, executive and financial officers.
Potential Conflict of Interest
Since we do not have an audit or compensation committee comprised of independent directors, the functions that would have been performed by such committees are performed by our Board of directors. Thus, there is a potential conflict of interest in that our directors have the authority to determine issues concerning management compensation, in essence their own, and audit issues that may affect management decisions. We are not aware of any other conflicts of interest with any of our executives or directors.
Board’s Role in Risk Oversight
The Board assesses on an ongoing basis the risks faced by the Company. These risks include financial, technological, competitive, and operational risks. The Board dedicates time at each of its meetings to review and consider the relevant risks faced by the Company at that time. In addition, since the Company does not have an Audit Committee, the Board is also responsible for the assessment and oversight of the Company’s financial risk exposures.
ITEM 6. EXECUTIVE COMPENSATION
The following table sets forth information concerning the total compensation that we have paid or that has accrued on behalf of our chief executive officer and other executive officers with annual compensation exceeding $100,000 during the fiscal years ending December 31, 2017, 2016 and 2015.
| | | | | | | | | | | | | | | Long Term | | | | |
| | | | | | Annual Compensation | | | Compensation Awards | | | | |
| | | | | | | | | | | | Other | | | Restricted | | | Securities | | | | |
| | | | | | | | | | | | Annual | | | Stock | | | Underlying | | | All Other | |
| | | | | | Salary | | | Bonus | | | Compensation | | | Award(s) | | | Options | | | Compensation | |
| Name and Principal Position | | Year | | | ($) | | | ($) | | | ($) | | | ($) | | | ($) | | | ($) | |
| | | | | | | | | | | | | | | | | | | | | | |
| Liron Carmel, CEO, and Director (1) | | 2017 | | | | 15,000 | | | | - | | | | - | | | | - | | | | - | | | | - | |
| | | 2016 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
| | | 2015 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Oded Gilboa, CFO (1) | | 2017 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
| | | 2016 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
| | | 2015 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
We do not currently have a stock option plan. No individual grants of stock options, whether or not in tandem with stock appreciation rights known as SARs or freestanding SARs have been made to any executive officer or any director since our inception; accordingly, no stock options have been granted or exercised by any of the officers or directors since we were founded.
Long-Term Incentive Plans and Awards
We do not have any long-term incentive plans that provide compensation intended to serve as incentive for performance. No individual grants or agreements regarding future payouts under non-stock price-based plans have been made to any executive officer or any director or any employee or consultant since our inception; accordingly, no future payouts under non-stock price-based plans or agreements have been granted or entered into or exercised by any of the officers or directors or employees or consultants since we were founded.
Compensation of Directors
There are no current agreements pursuant to which directors are or will be compensated in the future for any services provided as a director.
Employment Contracts, Termination of Employment, Change-in-Control Arrangements
There are no employment contracts in place, no employees were terminated and no change in control arrangements have been signed with the company.
ITEM 7. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
During the years ended December 31, 2017 and 2016, the Company issued the following restricted securities to “affiliated purchasers”:
On October 17, 2017, the Company issued and sold a total of 2.6 million restricted shares of common stock to four accredited investors (650,000 shares to each) at $0.01 per share for a total cash consideration of $26,000. Reference is made to Item 4. Security Ownership of Certain Beneficial Owners and Management. As a result of these issuances, the four accredited investors became greater than 5% shareholders.
ITEM 8. LEGAL PROCEEDINGS
We know of no material, active or pending legal proceedings against our Company, nor of any proceedings that a governmental authority is contemplating against us. We know of no material proceedings to which any of our directors, officers, affiliates, owner of record or beneficially of more than 5 percent of our voting securities or security holders is an adverse party or has a material interest adverse to our interest.
ITEM 9. MARKET FOR REGISTRANT’S COMMON STOCK AND RELATED STOCKHOLDER MATTER
Market Information
Our common stock is quoted on the OTC Pink Sheets Market under the symbol SMGY, an inter-dealer automated quotation system for equity securities not included on The Nasdaq Stock Market. Quotation of the Company’s securities on the OTC Pink Sheets Market limits the liquidity and price of the Company’s common stock more than if the Company’s shares of common stock were listed on The Nasdaq Stock Market or a national exchange. For the periods indicated, the following table sets forth the high and low bid prices per share of common stock. The below prices represent inter-dealer quotations without retail markup, markdown, or commission and may not necessarily represent actual transactions.
| | | Price Range | |
| Period | | High | | | Low | |
| Year Ended December 31, 2015: | | | | | | | | |
| First Quarter | | $ | 2.70 | | | $ | 1.01 | |
| Second Quarter | | $ | 2.69 | | | $ | 0.99 | |
| Third Quarter | | $ | 0.99 | | | $ | 0.86 | |
| Fourth Quarter | | $ | 0.75 | | | $ | 0.60 | |
| Year Ended December 31, 2016: | | | | | | | | |
| First Quarter | | $ | 0.48 | | | $ | 0.35 | |
| Second Quarter | | $ | 0.35 | | | $ | 0.35 | |
| Third Quarter | | $ | 0.35 | | | $ | 0.35 | |
| Fourth Quarter | | $ | 0.35 | | | $ | 0.35 | |
| Year Ending December 31, 2017: | | | | | | | | |
| First Quarter | | $ | 1.00 | | | $ | 0.35 | |
| Second Quarter | | $ | 0.51 | | | $ | 0.51 | |
| Third Quarter | | $ | 1.00 | | | $ | 0.51 | |
| Fourth Quarter | | $ | 0.62 | | | $ | 0.51 | |
| Year Ending December 31, 2018: | | | | | | | | |
| First Quarter (through April 20, 2018) | | $ | 1.50 | | | $ | 0.52 | |
Our stock transfer agent is Transfer Online, Inc., with offices located at 512 SE Salmon Street, Portland, OR 97214. Their phone number is (503) 227-2950 and email address is: info@transferonline.com.
Holders.As of April 20, 2018, there 189 registered stockholders holding had 8,875,577 Common Stock.
Dividends.We have never declared or paid any cash dividends on our common stock nor do we anticipate paying any in the foreseeable future. We expect to retain any future earnings to finance our operations and expansion. The payment of cash dividends in the future will be at the discretion of our Board of Directors and will depend upon our earnings levels, capital requirements, any restrictive loan covenants and other factors the Board considers relevant.
Equity Compensation Plans.We do not have any equity compensation plans.
ITEM 10. RECENT SALES OF UNREGISTERED SECURITIES
During the years ended December 31, 2016 and 2015, the Company did not sell any unregistered securities.
Sales of Unregistered Securities in 2017:
On October 17, 2017, the Company sold 6,300,000 shares to 13 shareholders, each an “accredited investor” as that term is defined in Rule 501 of Regulation D promulgated by the Securities and Exchange Commission (the “SEC”) under the Securities Act of 1933, as amended (the “Act”), for $0.01 a share for a total cash consideration of $63,000.
On October 24, 2017, the Company issued 666,667 shares to one shareholder at $0.075 per share for cash consideration of $50,000.
During the period from November 15, 2017 to December 7, 2017, the Company sold a total of 1,000,000 units to accredited investors for cash consideration of $300,000 at price of $.30 per unit (the “$0.30 Units”), each consisting of: (i) one share of Common Stock; and (ii) one class A warrant exercisable at $0.60 per share with a term of 24 months (the “Class A Warrants”). During the period from March 20, 2018 to April 17, 2018, the Company sold a total of 793,000 units to accredited investors for aggregate cash consideration of $475,775 at a price of $0.60 per unit (the “$0.60 Units”), each consisting of: (i) one share of Common Stock; and (ii) one class B warrant exercisable at $1.20 per share with a term of 24 months (the “Class B Warrants”). In connection with the sale of the $0.60 Units, a total of 234,000 shares of Common Stock have been issued to date and an additional 559,000 shares of Common Stock are pending issuance by the transfer agent but have not yet been issued.
As discussed above, the Company is continuing to raise capital through the private sale of its equity securities, primarily pursuant to the exemptions provided under Regulation S and to a lesser extent pursuant to Regulation D, both promulgated by the SEC under the Act. To date, the Company has raised approximately $888,775 in the Equity Raise.
The Company believes that the issuances and sale of the restricted securities were exempt from registration pursuant to Section 4(2) of the Act and Regulation S and Regulation D promulgated by the SEC under the Act, as privately negotiated, isolated, non-recurring transactions with accredited investors not involving any public solicitation. The subscribers, in each case, represented to the Company their intention to acquire the securities for investment only and not with a view to the distribution thereof. Appropriate restrictive legends are affixed to the stock certificates issued in such transactions. All recipients of restricted securities represented by the shares of Common Stock and the Units either received adequate information about the Company or had access, through employment, relation and/or business relationships with the Company to such information and each of the subscribers is an accredited investor.
ITEM 11. DESCRIPTION OF REGISTRANT’S SECURITIES TO BE REGISTERED
Common Stock
We are authorized to issue 495,000,000 shares of common stock with a par value of 0$.00001 per share (“Common Stock”). As of April 20, 2018, 8,875,577 shares of our Common Stock were issued and on record with our transfer agent, Transfer Online Inc. Some of the shares of Common Stock issuable in connection with the Company’s Equity Raise have not been yet been issued but are pending issuance.
Each outstanding share of Common Stock that is on record with our transfer agent is entitled to one vote, either in person or by proxy, on all matters that may be voted upon by the owners thereof at meetings of the stockholders.
Our shareholders have no pre-emptive rights to acquire additional shares of Common Stock. The Common Stock is not subject to redemption or any sinking fund provision, and it carries no subscription or conversion rights. In the event of our liquidation, the holders of the Common Stock will be entitled to share equally in the corporate assets after satisfaction of all liabilities.
The description contained in this section does not purport to be complete. Reference is made to our certificate of incorporation and bylaws which are available for inspection upon proper notice at our offices, as well as to the Nevada Revised Statutes for a more complete description covering the rights and liabilities of shareholders.
Holders of our Common Stock
| | (i) | have equal ratable rights to dividends from funds legally available therefore, if declared by our Board of Directors, |
| | | |
| | (ii) | are entitled to share ratably in all our assets available for distribution to holders of Common Stock upon our liquidation, dissolution or winding up; |
| | | |
| | (iii) | do not have preemptive, subscription or conversion rights or redemption or sinking fund provisions; and |
| | | |
| | (iv) | are entitled to one non-cumulative vote per share on all matters on which stockholders may vote at all meetings of our stockholders. |
The holders of shares of our Common Stock do not have cumulative voting rights, which means that the holders of more than fifty percent (50%) of outstanding shares voting for the election of directors can elect all of our directors if they so choose and, in such event, the holders of the remaining shares will not be able to elect any of our directors.
Preferred Stock
The Company’s Articles of Incorporation, as amended, authorize 5,000,000 shares of preferred stock, par value $0.00001 per share, which may be issued by the Board of Directors from time to time in one or more series. Our Board of Directors, without further approval of our stockholders, is authorized to fix the dividend rights and terms, conversion rights, voting rights, redemption rights, liquidation preferences and other rights and restrictions relating to any series of preferred stock that may be issued in the future. Issuances of shares of preferred stock, while providing flexibility in connection with possible financings, acquisitions and other corporate purposes, could, among other things, adversely affect the voting power of the holders of our Common Stock and prior series of preferred stock then outstanding.
Dividends
We have no history of paying dividends, moreover, there is no assurance that we will pay dividends in the future.
Shares Eligible for Future Sale
Our shares are thinly traded on the OTC Market, and we cannot assure you that a significant public market for our Common Stock will be developed. Sales of substantial amounts of Common Stock in the public market, or the possibility of substantial sales occurring, could adversely affect prevailing market prices for our Common Stock or our future ability to raise capital through an offering of equity securities.
As of April 17, 2018, 8,787,881 shares of Common Stock of the total 8,875,577 issued and outstanding shares of Common Stock are “restricted” shares as that term is defined under the Act. We have not entered into any agreement to register any of our issued and outstanding shares, although such agreement may be entered into in the future, or such an agreement may be made part of the terms of a future equity raise or other transactions.
ITEM 12. INDEMNIFICATION OF DIRECTORS AND OFFICERS
Our bylaws and articles of incorporation provide that our officers and directors are indemnified to the fullest extent provided by the Nevada Revised Statutes (“NRS”).
Under the Nevada Revised Statutes, director immunity from liability to a company or its shareholders for monetary liabilities applies automatically unless it is specifically limited by a company’s Articles of Incorporation. Our Articles of Incorporation do not specifically limit the directors’ immunity. The NRS excepts from that immunity (a) a willful failure to deal fairly with the company or its shareholders in connection with a matter in which the director has a material conflict of interest; (b) a violation of criminal law, unless the director had reasonable cause to believe that his or her conduct was lawful or no reasonable cause to believe that his or her conduct was unlawful; (c) a transaction from which the director derived an improper personal profit; and (d) willful misconduct.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the Company pursuant to the foregoing, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable.
The Company has not purchased insurance for the directors and officers that would provide coverage for their acts as an officer or director of the Company.
ITEM 13. FINANCIAL STATEMENTS AND SUPPLEMNTARY DATA
ITEM 14. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 15. FINANCIAL STATEMENTS AND EXHIBITS
INDEX TO FINANCIAL STATEMENTS
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of Smart Energy Solutions, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Smart Energy Solutions, Inc. (the Company) as of December 31, 2017 and 2016, and the related statements of income, comprehensive income, stockholders’ equity, and cash flows for each of the years in the two-year period ended December 31, 2017 and 2016, and the related notes and schedules (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2017 and 2016, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2017 and 2016, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company suffered a net loss from operations and has a net capital deficiency, which raises substantial doubt about its ability to continue as a going concern. Management’s plans regarding those matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
| /s/ M&K CPAS, PLLC |
| |
| We have served as the Company’s auditor since 2013. |
| |
| Houston, TX |
| |
March 5, 2018 |
SMART ENERGY SOLUTIONS, INC.
BALANCE SHEETS
AS OF DECEMBER 31, 2017 AND DECEMBER 31, 2016
| | | December 31, 2017 | | | December 31, 2016 | |
| | | | | | | |
| ASSETS | | | | | | | | |
| | | | | | | | | |
| Current assets: | | | | | | | | |
| Cash and cash equivalents | | $ | 326,730 | | | $ | - | |
| Prepaid expenses | | | 4,196 | | | | - | |
| | | | | | | | | |
| Total current assets | | | 330,926 | | | | - | |
| | | | | | | | | |
| Total assets | | $ | 330,926 | | | $ | - | |
| | | | | | | | | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | | | | | | | | |
| | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable and accrued liabilities | | $ | 800 | | | $ | 2,144 | |
| Total current liabilities | | | 800 | | | | 2,144 | |
| | | | | | | | | |
| Notes payable | | | 2,687 | | | | 800 | |
| Total long term liabilities | | | 2,687 | | | | 800 | |
| Total liabilities | | | 3,487 | | | | 2,944 | |
| | | | | | | | | |
| Stockholders’ equity (deficit) | | | | | | | | |
| | | | | | | | | |
| Common stock, par value $0.00001 per share, 495,000,000 common shares authorized, 5,000,000 preferred shares authorized; 8,591,577 and 624,910 common stock issued and outstanding at December 31, 2017 and December 31, 2016 respectively. | | | 86 | | | | 6 | |
| Additional paid in capital | | | 447,164 | | | | 34,244 | |
| Accumulated other comprehensive income | | | 3,353 | | | | - | |
| Accumulated deficit | | | (123,164 | ) | | | (37,194 | ) |
| Total stockholders’ (deficit) | | | 327,439 | | | | (2,944 | ) |
| | | | | | | | | |
| Total liabilities and stockholders’ equity (deficit) | | $ | 330,926 | | | $ | - | |
The accompanying notes are an integral part of these financial statements
SMART ENERGY SOLUTIONS, INC.
STATEMENTS OF OPERATIONS
FOR THE TWELVE MONTHS ENDED DECEMBER 31, 2017 AND 2016
| | | For the twelve months ended
December 31, 2017 | | | For the twelve months ended
December 31, 2016 | |
| | | | | | | |
| Revenues | | $ | - | | | $ | - | |
| | | | | | | | | |
| Expenses: | | | | | | | | |
| General and administrative | | | (75,177 | ) | | | (2,601 | ) |
| Research and development expense | | | (10,007 | ) | | | - | |
| Total operating expenses | | | (85,184 | ) | | | (2,601 | ) |
| | | | | | | | | |
| (Loss) from operations | | | (85,184 | ) | | | (2,601 | ) |
| Gain (loss) from debt settlement | | | 50 | | | | - | |
| Interest expense | | | (836 | ) | | | (43 | ) |
| Other income (expense) | | | (786 | ) | | | (43 | ) |
| Provision for income taxes | | | - | | | | - | |
| | | | | | | | | |
| Net (loss) | | $ | (85,970 | ) | | $ | (2,644 | ) |
| | | | | | | | | |
| Net loss per common share | | $ | (0.04 | ) | | $ | (0.00 | ) |
| | | | | | | | | |
| Weighted average number of common shares outstanding | | | 8,591,577 | | | | 624,910 | |
The accompanying notes are an integral part of these financial statements.
SMART ENERGY SOLUTIONS, INC.
STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
FOR THE YEARS ENDED DECEMBER 31, 2017 AND 2016
| | | For the twelve months ended | |
| | | December 31, 2017 | | | December 31, 2016 | |
| Net loss from continuing operations | | | (85,970 | ) | | | (2,644 | ) |
| Change in unrealized foreign currency translation gain (loss) | | | 3,353 | | | | - | |
| Total comprehensive loss | | | (82,617 | ) | | | (2,644 | ) |
| Comprehensive loss attributable to Smart Energy Solutions, Inc | | | (82,617 | ) | | | (2,644 | ) |
The accompanying notes are an integral part of these financial statements.
SMART ENERGY SOLUTIONS, INC.
STATEMENTS OF CHANGES IN STOCKHOLDERS’ (DEFICIT)
FOR THE YEARS DECEMBER 31, 2017 AND 2016
| | | Common stock | | | Additional Paid-In | | | Other Comprehensive | | | Accumulated | | | | |
| | | Shares | | | Amount | | | Capital | | | Income | | | Deficit | | | Totals | |
| | | | | | | | | | | | | | | | | | | |
| Balance as of December 31, 2015 | | | 624,910 | | | $ | 6 | | | $ | 34,244 | | | $ | - | | | $ | (34,550 | ) | | $ | (300 | ) |
| | | | - | | | | | | | | - | | | | - | | | | - | | | | - | |
| Net loss for the year ended December 31, 2016 | | | - | | | | - | | | | - | | | | - | | | | (2,644 | ) | | | (2,644 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance as of December 31, 2016 | | | 624,910 | | | | 6 | | | | 34,244 | | | | - | | | | (37,194 | ) | | | (2,944 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Shares Issued for Cash | | | 7,966,667 | | | | 80 | | | | 412,920 | | | | - | | | | - | | | | 413,000 | |
| Translation adjustments | | | - | | | | - | | | | - | | | | 3,353 | | | | - | | | | 3,353 | |
| Net loss for the year ended December 31, 2017 | | | - | | | | - | | | | - | | | | - | | | | (85,970 | ) | | | (85,970 | ) |
| Balance as of December 31, 2017 | | | 8,591,577 | | | $ | 86 | | | $ | 447,164 | | | $ | 3,353 | | | $ | (123,164 | ) | | $ | 327,439 | |
The accompanying notes are an integral part of these financial statements.
SMART ENERGY SOLUTIONS, INC.
STATEMENTS OF CASH FLOWS
FOR THE TWELVE MONTHS ENDED DECEMBER 31, 2017 and 2016
| | | For the twelve months ended
December 31, 2017 | | | For the twelve months ended
December 31, 2016 | |
| | | | | | | |
| Operating Activities: | | | | | | | | |
| Net loss | | $ | (85,970 | ) | | $ | (2,644 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
| Gain on settlement | | | (50 | ) | | | - | |
| Changes in operating assets and liabilities: | | | | | | | | |
| Increase (decrease) in accounts payable and accrued expenses | | | (1,344 | ) | | | 1,844 | |
| Decrease (dncrease) in prepaid expenses | | | (4,196 | ) | | | - | |
| | | | | | | | | |
| Net cash used in operating activities | | | (91,560 | ) | | | (800 | ) |
| | | | | | | | | |
| Investing Activities: | | | | | | | | |
| Purchase of property and equipment | | | - | | | | - | |
| Net Cash provided by (used in) investing activities | | | - | | | | - | |
| | | | | | | | | |
| Financing Activities: | | | | | | | | |
| Shares issued for cash | | | 413,000 | | | | - | |
| Borrowings on debt | | | 19,190 | | | | 800 | |
| Principal payments on debt | | | (17,253 | ) | | | - | |
| Net cash provided by financing activities | | | 414,937 | | | | 800 | |
| Foreign currency adjustment | | | 3,353 | | | | | |
| | | | | | | | | |
| Net increase (decrease) in cash | | | 326,730 | | | | - | |
| | | | | | | | | |
| Cash and cash equivalents - beginning of period | | $ | - | | | $ | - | |
| | | | | | | | | |
| Cash and cash equivalents - end of period | | $ | 326,730 | | | $ | - | |
The accompanying notes are an integral part of these financial statements.
SMART ENERGY SOLUTIONS, INC.
STATEMENTS OF CASH FLOWS
FOR THE YEARS ENDED DECEMBER 31, 2017 and 2016
Note (1) Summary of Significant Accounting Policies
Basis of Presentation and Organization
The Company was incorporated in 1999 in the state of Utah under the name Datigen.com, Inc. On August 25, 2005, the Company changed its state of incorporation from Utah to Nevada by the merger of the Company with and into its wholly-owned subsidiary, Smart Energy Solutions, Inc., a Nevada corporation. As a result of such merger, the Company’s name was changed to Smart Energy Solutions, Inc. in order to better reflect the Company’s business operations.
During December 2017 a new subsidiary was registered with all shares held by the Company. The new subsidiary was register in Israel under the name of Canna Powder Ltd. (“CannaPowder”) is headed by Rafi Ezra a highly experienced pharmacist with extensive knowledge of the cannabis sector and hands on experience of leading early stage pharma companies from early development through to commercial launch. Development is being carried out at the Hebrew University under the supervision of the inventor of the technology, Professor ShlomoMagdassi.
CannaPowder is raising investment to finance a development program to establish cannabis powder production facilities utilizing the licensed technology. The program is expected to be completed within three years, with commercial sales starting in 2021. In the commercial stage, CannaPowder will establish and operate several production facilities, each located in individual territories selected according to their size and favorable regulation for medical cannabis.
Products will be supplied to producers of medical cannabis products. The ability to produce a more effective, consistent product in terms of quality and composition will provide an important advantage when targeting the medical market. The accompanying financial statements of the Company were prepared from the accounts of the Company under the accrual basis of accounting.
The accompanying financials statement of the company were prepared from the account of the company under the accrual basis of accounting.
Cash and Cash Equivalents
For purposes of reporting within the statement of cash flows, the Company considers all cash on hand, cash accounts not subject to withdrawal restrictions or penalties, and all highly liquid debt instruments purchased with a maturity of three months or less to be cash and cash equivalents. As of December 31, 2017 and 2016, we had cash and cash equivalents of $326,730 and $0, respectively.
Reclassifications
Certain prior year amounts have been reclassified to conform to the current period presentation. These reclassifications had no impact on net earnings, financial position or cash flows.
Discontinued Operations
The Company follows the policy of segregating the assets and liabilities of subsidiaries or lines of business on its Balance Sheet from the assets liabilities ofcontinuing subsidiaries or lines of businesses when it is decided to close or dispose of a subsidiary or line of business. The Company also, follows the policy of separately disclosing the assets and liabilities and the net operations of a subsidiary or line of business in its financial statements when it is decided to closeor dispose of a subsidiary or line of business.
Revenue Recognition
The Company recognizes revenue from licensing its software to customers for contractually defined periods of time. The Company recognizes revenue ratably over the term of the contract in accordance with ASC 605 (1) when the price is fixed and determinable, (2) persuasive evidence of an arrangement exists, (3) delivery has occurred or services have been provided, and (4) collectability is assured.
Loss per Common Share
Basic loss per share is computed by dividing the net loss attributable to the common stockholders by the weighted average number of shares of common stock outstanding during the period. Fully diluted loss per share is computed similar to basic loss per share except that the denominator is increased to include the number of additional common shares that would have been outstanding if the potential common shares had been issued and if the additional common shares were dilutive.
Income Taxes
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are determined based on temporary differences between the bases of certain assets and liabilities for income tax and financial reporting purposes. The deferred tax assets and liabilities are classified according to the financial statement classification of the assets and liabilities generating the differences.
The Company maintains a valuation allowance with respect to deferred tax assets. The Company establishes a valuation allowance based upon the potential likelihood of realizing the deferred tax asset and taking into consideration the Company’s financial position and results of operations for the current period. Future realization of the deferred tax benefit depends on the existence of sufficient taxable income within the carryforward period under the Federal tax laws.
Changes in circumstances, such as the Company generating taxable income, could cause a change in judgment about the realizability of the related deferred tax asset. Any change in the valuation allowance will be included in income in the year of the change in estimate.
Fair Value of Financial Instruments
The Company estimates the fair value of financial instruments using the available market information and valuation methods. Considerable judgment is required in estimating fair value. Accordingly, the estimates of fair value may not be indicative of the amounts the Company could realize in a current market exchange. As of December 31, 2017 and 2016, the carrying value of accounts payable and accrued liabilities approximated fair value due to the short-term nature and maturity of these instruments.
Deferred Offering Costs
The Company defers as other assets the direct incremental costs of raising capital until such time as the offering is completed. At the time of the completion of the offering, the costs are charged against the capital raised. Should the offering be terminated, deferred offering costs are charged to operations during the period in which the offering is terminated.
Impairment of Long-Lived Assets
The Company evaluates the recoverability of long-lived assets and the related estimated remaining lives when events or circumstances lead management to believe that the carrying value of an asset may not be recoverable. As of December 31, 2017, no events or circumstances occurred for which an evaluation of the recoverability of long-lived assets was required.
Estimates
The financial statements are prepared on the basis of accounting principles generally accepted in the United States. The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities as of December 31, 2017 and 2016, and expenses for the years ended December 31, 2017 and 2016. Actual results could differ from those estimates made by management.
Impact of Recently Issued Accounting Standards
In January 2017, the FASB issued ASU No. 2017-01, Business Combinations (Topic 805): Clarifying the Definition of a Business. Public business entities should apply the amendments in this Update to annual periods beginning after December 15, 2017, including interim periods within those periods. All other entities should apply the amendments to annual periods beginning after December 15, 2018, and interim periods within annual periods beginning after December 15, 2019.
In March 2017, the FASB issued Update 2017-08—Receivables—Nonrefundable Fees and Other Costs (Subtopic 310-20): Premium Amortization on Purchased Callable Debt Securities.For public business entities, the amendments in this Update are effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2018. For all other entities, the amendments are effective for fiscal years beginning after December 15, 2019, and interim periods within fiscal years beginning after December 15, 2020. Early adoption is permitted, including adoption in an interim period. If an entity early adopts the amendments in an interim period, any adjustments should be reflected as of the beginning of the fiscal year that includes that interim period.
In March 2017, the FASB issued Update 2017-07—Compensation—Retirement Benefits (Topic 715): Improving the Presentation of Net Periodic Pension Cost and Net Periodic Postretirement Benefit Cost. Effective for public business entities for annual periods beginning after December 15, 2017, including interim periods within those annual periods. For other entities, the amendments in this Update are effective for annual periods beginning after December 15, 2018, and interim periods within annual periods beginning after December 15, 2019. Early adoption is permitted as of the beginning of an annual period for which financial statements (interim or annual) have not been issued or made available for issuance. That is, early adoption should be within the first interim period if an employer issues interim financial statements. Disclosures of the nature of and reason for the change in accounting principle are required in the first interim and annual periods of adoption.
In August 2016, the FASB issued ASU No. 2016-15, Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments. The amendment in this ASU defers the effective date of ASU No. 2014-09 for all entities for one year. Public business entities should apply the guidance in ASU 2016-15 to annual reporting periods beginning on December 15, 2017. For all other entities, the guidance in ASU 2016-15 should be applied to annual reporting periods beginning December 15, 2018 and interim periods within fiscal years beginning after December 15, 2019. Earlier application is permitted including interim reporting periods.
Goodwill and Intangible Assets
Goodwill is tested for impairment at a minimum on an annual basis. Goodwill is tested for impairment at the reporting unit level by first performing a qualitative assessment to determine whether it is more likely than not that the fair value of the reporting unit is less than its carrying value. If the reporting unit does not pass the qualitative assessment, then the reporting unit’s carrying value is compared to its fair value. The fair values of the reporting units are estimated using market and discounted cash flow approaches. Goodwill is considered impaired if the carrying value of the reporting unit exceeds its fair value. The discounted cash flow approach uses expected future operating results. Failure to achieve these expected results may cause a future impairment of goodwill at the reporting unit.
Intangible assets consist of patents and trademarks, purchased customer contracts, purchased customer and merchant relationships, purchased trade names, purchased technology, and non-compete agreements. Intangible assets are amortized over the period of estimated benefit using the straight-line method and estimated useful lives ranging from two to twenty years. No significant residual value is estimated for intangible assets.
Note (2) Going Concern
The Company was incorporated in 1999 in the state of Utah under the name Datigen.com, Inc. On August 25, 2005, the Company changed its state of incorporation from Utah to Nevada by the merger of the Company with and into its wholly-owned subsidiary, Smart Energy Solutions, Inc., a Nevada corporation. As a result of such merger, the Company’s name was changed to Smart Energy Solutions, Inc. in order to better reflect the Company’s business operations.
The Company has limited operations. During December 2017 a new subsidiary was registered with all shares held by the Company. The new subsidiary reregistered in Israel under the name of CannaPowder Ltd. (“CannaPowder”) is headed by Rafi Ezra a highly experienced pharmacist with extensive knowledge of the cannabis sector and hands on experience of leading early stage pharma companies from early development through to commercial launch.Development is being carried out at the Hebrew University under the supervision of the inventor of the technology Professor Shlomo Magdassi.
CannaPowder is raising investment to finance a development program to establish cannabis powder production facilities utilizing the licensed technology. The program is expected to be completed within three years, with commercial sales starting in 2021. In the commercial stage, CannaPowder will establish and operate several production facilities, each located in individual territories selected according to their size and favorable regulation for medical cannabis. The Company believes that with its new facilities it will be able to produce cannabis powders at a significant cost advantage.
Products will be supplied to producers of medical cannabis products. The ability to produce a more effective, consistent product in terms of quality and composition will provide an important advantage when targeting the medical market. The Company has limited operations. The accompanying financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America, which contemplate continuation of the Company as a going concern. The Company has not established any source of revenue to cover its operating costs, and as such, has incurred an operating loss since inception. These and other factors raise substantial doubt about the Company’s ability to continue as a going concern. The accompanying financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the possible inability of the Company to continue as a going concern.
Note (3) Common Stock
On October 17, 2017, the Company sold a total of 6,300,000 shares to thirteen accredited investors at $0.01 per share for a total cash consideration of $63,000.
On October 24, 2017 the Company issued 666,667 shares to one shareholder, an accredited investor, at $0.075 per share or cash consideration of $50,000.
Between November 15, 2017 and December 7, 2017, the Company sold a total of 1,000,000 units for cash consideration of $300,000 at price of $.30 (the “$0.30 Units”), each unit comprised of one share of common stock and one class A warrant exercisable at $0.50 per share with a term of 24 months. The relative fair value of the stock with embedded warrants was $132,458 for the common stock and $167,542 for the class A warrants. The warrants were valued using the Black-Scholes model with volatility of approximately 163% and discount rates ranging from 1.68% to 1.8%.
At December 31, 2017 and December 31, 2016, there were approximately 189 and 168 holders of record and 8,591,577 and 624,910 shares of common stock issued and outstanding, respectively. All shares of common stock are entitled to one vote per share in all matters submitted to the shareholders. No preferred shares are issued and outstanding at December 31, 2017 and 2016.
Dividends
The Company has not declared or paid any cash dividends on its common stock nor does it anticipate paying any in the foreseeable future. Furthermore, the Company expects to retain any future earnings to finance its operations and expansion. The payment of cash dividends in the future will be at the discretion of its Board of Directors and will depend upon its earnings levels, capital requirements, any restrictive loan covenants and other factors the Board considers relevant.
Securities Authorized for Issuance under Equity Compensation Plans
None
Following is a table of warrant and options still outstanding and exercisable along with exercise price and range of remaining term.
| Type | | Quantity | | | Exercise Price | | | Remaining Term |
| Warrants Class A | | | 1,000,000 | | | $ | 0.50 | | | 24 Months |
| Total | | | 1,000,000 | | | | | | | |
(4) Income Taxes
The provision (benefit) for income taxes for the year ended December 31, 2017 and 2016, was as follows (assuming a 35% effective tax rate):
| | | 2017 | | | 2016 | |
| Current tax provision: | | | | | | | | |
| Federal- | | | | | | | | |
| Taxable income | | $ | - | | | $ | - | |
| Total current tax provision | | $ | - | | | $ | - | |
The Company had deferred income tax assets as of December 31, 2017 and 2016 as follows:
| | | 2017 | | | 2016 | |
| Loss carryforwards | | $ | 43,107 | | | $ | 13,018 | |
| Less- Valuation allowance | | | (43,107 | ) | | | (13,018 | ) |
| Total net deferred tax assets | | $ | - | | | $ | - | |
The Company provided a valuation allowance equal to the deferred income tax assets for the year ended December 31, 2017 and 2016, because it is not presently known whether future taxable income will be sufficient to utilize the loss carryforwards.
As of December 31, 2017 and 2016, the Company had approximately $123,164 and $37,194, respectively, in tax loss carryforwards that can be utilized in future periods to reduce taxable income, and expire by the year 2030.
The Company did not identify any material uncertain tax positions that will be filed. The Company did not recognize any interest or penalties for unrecognized tax benefits during the year ended December 31, 2017 and 2016.
The Company intends to file income tax returns in the United States. All tax years are closed by expiration of the statute of limitations.
Note (5) Related Party Transactions
On October 17, 2017, the Company issued and sold a total of 2.6 million restricted shares of common stock to four accredited investors (650,000 shares to each) at $0.01 per share for a total cash consideration of $26,000. Reference is made to Item 4. Security Ownership of Certain Beneficial Owners and Management. As a result of these issuances, the four accredited investors became greater than 5% shareholders.
Note (6) Notes Payable
During the year ended December 31, 2017, the Company borrowed $100 and $800, respectively, which the loans bear an interest rate of 8% and has no maturity date. The loans were repaid in the amount of $850 on May 5, 2017 and the Company recorded a gain on debt extinguishment of $50.
On May 12, 2016 a shareholder loaned the company the sum of $5,000 to settle a vendor debt. The shareholder has subsequently forgiven the debt resulting from this payment and has confirmed he is owed no principal or interest as of December 31, 2016 and December 31, 2017. As of December 31, 2016, the amount paid by the shareholder to the vendor was forgiven, as the shareholder is a related party the forgiven debt resulted in an increase to additional paid in capital.
On December 22, 2017 a loan was made in the amount $2,687 the loan bears no interest and has no maturity date.
Between January 8, 2017 and August 25, 2017 three shareholders loaned the company amounts totaling $16,403 in loans bearing 8% interest which have no maturity dates. The loans which accrued interest of $879 were repaid on December 20, 2017. The three debt holders confirmed they were owed no principal or interest as of December 31, 2017.
Note (7) Subsequent Events
As defined in FASB ASC 855-10, “Subsequent Events”, subsequent events are events or transactions that occur after the balance sheet date but before financial statements are issued or available to be issued.
The loan made to the Company on December 22, 2017 in the amount $2,687 was repaid on January 8, 2018.
During the period from March 20, 2018 to April 17, 2018, the Company sold a total of 793,000 units to accredited investors for aggregate cash consideration of $475,775 at a price of $0.60 per unit (the “$0.60 Units”), each consisting of: (i) one share of Common Stock; (ii) one class A warrant exercisable at $0.60 per share with a term of 24 months (the “Class A Warrants”); and (ii) one class B warrant exercisable at $1.20 per share with a term of 24 months (the “Class B Warrants”). In connection with the sale of the $0.60 Units, a total of 234,000 shares of Common Stock have been issued to date and an additional 559,000 shares of Common Stock are pending issuance by the transfer agent but have not yet been issued.
The Company evaluated all other events and transactions that occurred subsequent to the balance sheet date and prior to the date on which the financial statements contained in this report were issued, and the Company determined that no such events or transactions necessitated disclosure.
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) The following documents are filed as exhibits to this report on Form 10-K or incorporated by reference herein. Any document incorporated by reference is identified by a parenthetical reference to the SEC filing that included such document.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this registration statement on Form 10 to be signed on its behalf by the undersigned.
Smart Energy Solutions, Inc.
| By: | /s/ Liron Carmel | |
| | Liron Carmel | |
| | Chief Executive Officer and Chairman | |
| | (Principal Executive Officer) | |
Date: April 30, 2018 | |
| | | |
| By: | /s/ Oded Gilboa | |
| | Oded Gilboa | |
| | Chief Financial Officer | |
| | (Principal Executive Officer) | |
Date: April 30, 2018 | |
