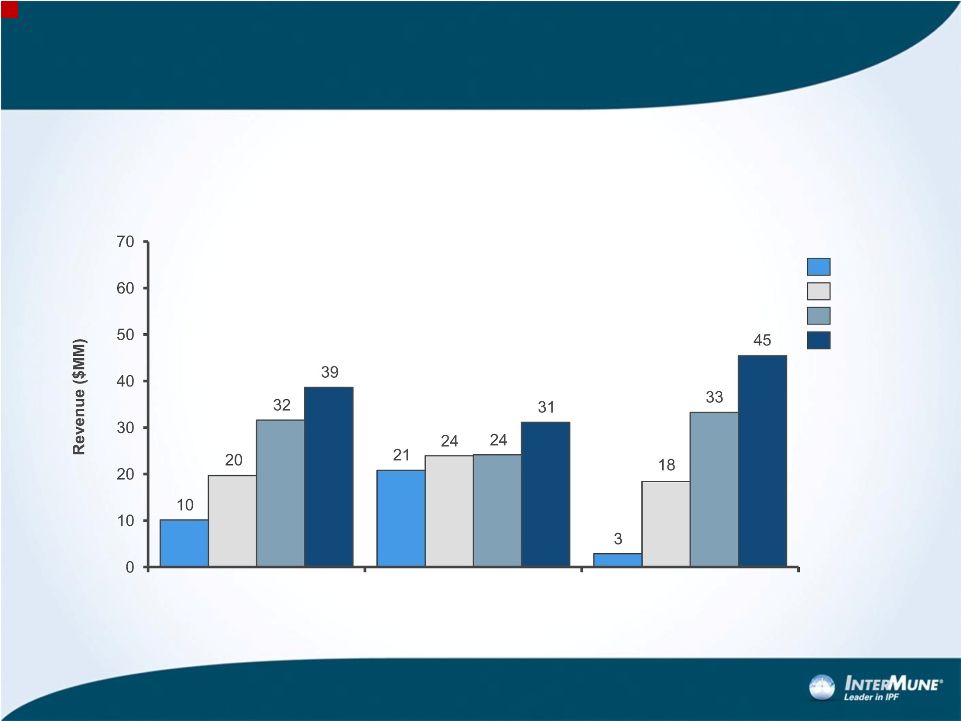
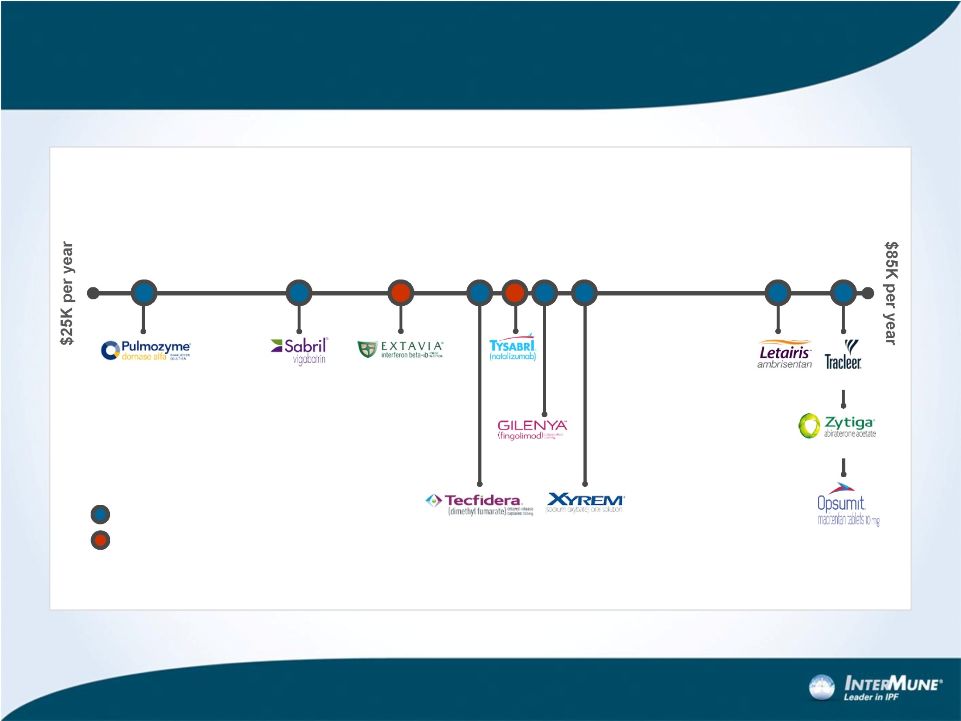
Forward-looking Statements 1 This presentation contains forward-looking statements made pursuant to the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995. Investors are cautioned that, without limitation, statements in this presentation regarding InterMune's plans and expectations, including strategies and plans to expand beyond pirfenidone and IPF; the estimated patient populations suffering from IPF and market potential for pirfenidone in the United States, including potential pricing estimates; InterMune's expectations regarding the anticipated timing of FDA review of the pirfenidone NDA; the potential to make pirfenidone available as a medicine to IPF patients in the United States; expectations regarding U.S. payor mix and reimbursement and revenue update in the U.S.; anticipated timing of initiating a commercial launch for pirfenidone and the size of a commercial sales force to market pirfenidone in the United States; expectations regarding year one U.S. Esbriet revenue relative to analog product launches; expectations regarding periods of intellectual property protection and regulatory exclusivity; and expectations regarding the regulatory approval process and prospects for success thereof are forward-looking statements. All forward-looking statements included in this presentation are based on information available to InterMune as of the date hereof, and InterMune assumes no obligation to update any such forward-looking statements. Actual results could differ materially from those described in the forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in detail under the heading “Risk Factors” in InterMune’s periodic reports filed with the SEC, including but not limited to the following: (i) the risks related to the uncertain, lengthy and expensive clinical development process for the company’s product candidates; (ii) risks related to the regulatory process for pirfenidone, including that the results of the ASCEND trial may not be satisfactory to the FDA to receive regulatory approval; (iii) risks related to unexpected regulatory actions or delays or government regulation generally; (iv) risks related to the company’s manufacturing strategy; (v) government, industry and general public pricing pressures; (vi) risks related to the company’s ability to successfully launch and commercialize pirfenidone; and (vii) the company’s ability to maintain intellectual property protection. The risks and other factors discussed above should be considered only in connection with the risks and other factors discussed in detail in InterMune’s Form 10-K and its other periodic reports filed with the SEC, which are also available at www.intermune.com. |