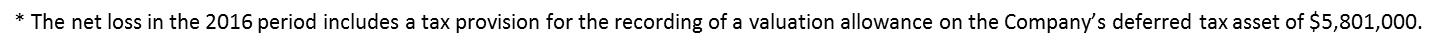Investor Presentation January 8, 2018
NASDAQ:CEMI
Rapid Tests for Earlier Treatment™™
Slide 2
Forward Looking Statements
Statements contained herein that are not historical facts are forward-looking statements within the meaning of the Securities Act of 1933, as amended. Those statements include statements regarding the intent, belief or current expectations of Chembio and its management. Such statements reflect management's current views, are based on certain assumptions, and involve risks and uncertainties. Actual results, events, or performance may differ materially from the above forward-looking statements due to a number of important factors, and will be dependent upon a variety of factors, including, but not limited to, Chembio's ability to develop, manufacture, market and finance new products and the demand for Chembio's products. Chembio undertakes no obligation to publicly update these forward-looking statements to reflect events or circumstances that occur after the date hereof or to reflect any change in Chembio's expectations with regard to these forward-looking statements or the occurrence of unanticipated events. Other factors that may impact Chembio's success are more fully disclosed in Chembio's most recent public filings with the U.S. Securities and Exchange Commission.
Slide 3
Mission
To develop and commercialize simple, fast and reliable point-of-care diagnostic tests that improve diagnosis or monitoring of disease
Slide 4
Chembio's Dual Path Platform (DPP®) Technology
Next-Generation Point-of-Care Technology Platform
| § | Patented point-of-care (POC) technology platform |
| § | Provides enhanced sensitivity vs. lateral flow technology |
| § | Allows multiplexing (i.e., multiple test results from a single patient sample) |
| § | Provides quantitative results when used with DPP® Micro Reader |
| § | Adapts to multiple sample types (i.e., blood, oral fluid) |
| § | Applies to a range of diseases and markets |
| § | Includes unique sample collection device (i.e., SampleTainer®) |
See graphics
Slide 5
Chembio's Dual Path Platform (DPP®) Technology
Leveraging Our Technology Platform to Enter New Markets
Three areas of Strategic Focus:
| · | Sexually Transmitted Disease Business |
| · | Tropical and Fever Disease Business |
| · | Technology Collaborations |
DPP® Technology Platform
Slide 6
HIV and Syphilis Continue as Global Health Concerns
Testing for co-infection important for pregnant women and MSM
United States (HIV)
| · | 1.1 million living with HIV |
| · | 39,782 diagnosed with HIV |
| · | ~1 in 7 (15%) unaware of HIV infection |
United States (SYP)
| · | 88,042 18% increase in cases reported to CDC (2015-2016 |
Global (HIV)
| · | 36.7 million living with HIV |
| · | 1.8 million Diagnosed with HIV |
| · | ~1.0 million died of AIDS-related illnesses |
Global (SYP)
| · | ~12 million new infections/year |
Source: Centers for Disease Control & Prevention (CDC) website; World Health Organization (WHO) website Data as of 12/31/2016
Slide 7
Traditional Lateral Flow HIV Products
STAT-PAK ® and SURE CHECK ® HIV Assays
Product Features & Benefits
| · | FDA (PMA) approved, CLIA-waived |
| · | CE marked, WHO pre-qualified |
| · | 2.5 - 5.0 μL blood sample |
| · | 15 - 20 minute test time |
| · | Specificity: 99.9%, Sensitivity: 99.7% |
Commercialization
| · | High quality brands, marketed globally since 2007 |
| · | Sold to Public Health Clinics, POLs, Hospitals, Self Test (EU) |
| · | Distribution Partners US: Fisher, McKesson/PSS, H. Schein, Medline, Caribbean: Isla Lab |
See graphics
Slide 8
Strengthening our Core STD Business
DPP® HIV-SYP and DPP® HIV 1/2 Assays
| § | DPP® HIV-Syphilis Combo Assay |
| – | Chembio has a history of leadership |
| • | First to receive USAID, ANVISA (Brazil), COFEPRIS (Mexico) approvals |
| – | Completed U.S. clinical trial for FDA PMA submission: Q4 2017 |
| – | Global screening opportunity: |
| – | Received FDA (PMA) approval (2013), |
| – | Received CLIA-waiver (2014) |
(blood and oral fluid)
| – | Superior performance vs. competitors |
(sensitivity)
See graphics
Slide 9
Strengthening our Core STD Business
DPP® Syphilis Screen & Confirm and SURE CHECK ® HIV Self Test
| § | DPP® Syphilis Screen & Confirm Assay |
| – | Simultaneously and separately detects treponemal and nontreponemal antibodies |
| – | Screen and confirm in only 15 minutes, with a single drop of fingerstick blood, on a single test |
| § | SURE CHECK® HIV Self Test (Ex-US) |
| – | Simple, fast, reliable HIV assay |
| – | Small sample: 2.5uL fingerstick |
| – | Integrated sample collection device |
| – | Sensitivity: 99.7%; Specificity: 99.9% |
See graphics
Slide 10
Fever Diseases - A Growing Global Concern
Malaria, Dengue, Zika, Chikungunya, Ebola, and Others
| § | Mosquito-Borne Illnesses |
| – | Mosquito à world's deadliest animal |
| – | Responsible for 725,000 deaths/year |
| – | Global geographic coverage |
| § | Established Fever Markets: |
| – | Malaria: 214MM annual infections |
| – | Dengue: 390MM annual infections |
| § | Which Fever Threat Will We Face Next? |
See graphics
Slide 11
Fever Disease - Product Development
Chembio Collaborates with World Leading Organizations
DPP® Malaria Assay- Bill & Melinda Gates Foundation
DPP® Dengue Assay-RVR Diagnostics Sdn Bhd
DPP® Zika Assay and DPP® Zika/Dengue/Chikungunya Assay- BARDA, FIOCRUZ, CDC
DPP® Fever Panel Assay – Asia- FIND
DPP® Fever Panel Assay – Africa- The Paul G. Allen Family Foundation
DPP® Ebola Assay and DPP® Malaria/Ebola Assay - CDC
See graphics
Slide 12
Chembio is Well-Positioned to Address Zika Virus
Key Regulatory Approvals: U.S. FDA EUA, CE mark, ANVISA
| – | 2015 (Brazil) à 2018 (~90 countries) |
| – | Residents and travelers (endemic areas) |
| § | Molecular (MDx) Test Limitations |
| – | Laboratory test, venous sample |
| – | Detects acute infections only - not antibodies |
| § | Chembio Zika Test Advantage |
| – | Convenience: POC test; fingerstick sample |
| – | Detects antibodies (IgM/IgG) |
| – | Quantitative results: DPP® Micro Reader |
| – | Time to result: 15 minutes |
| – | Low cost compared to MDx tests |
Source: Centers for Disease Control & Prevention (CDC) website
See graphics
Slide 13
Chembio is Well-Positioned to Address Zika Virus
| – | 2015 (Brazil) à 2017 (~60 countries) |
| – | Travelers to/from endemic Areas |
| § | Molecular (MDx) Test Limitations |
| – | Laboratory test, venous sample |
| – | Detects acute infections only - not antibodies |
| § | Chembio Zika Test Advantage |
| – | Convenience: POC Test; fingerstick sample |
| – | Detects Antibodies (lgM/lgG) |
| – | Time to Result: 15 minutes |
| – | Low cost compared to MDX tests |
Source: Centers for Disease Control & Prevention (CDC) website
See graphics
Slide 14
DPP® Zika Assay Product Development Timeline
Demonstrates Speed of Development and Scientific Expertise
| § | DPP® Zika IgM/lgG Development/Commercialization Timeline |
| – | Received PGAFF grant; initiated project DPP® Zika Project – 2/16 |
| – | Announced Zika collaboration with Bio-Manguinhos/Fiocruz (Brazil) – 3/16 |
| – | Completed initial testing; including 600 pregnant women – 4/16 |
| – | Announced regulatory filings with FDA-EUA, ANVISA – 5/16 |
| – | Announced regulatory filings with WHO-EUA, Cofepris, CE mark – 7/16 |
| – | Received CE mark (Europe, Caribbean)- 7/16 |
| – | Announced HHS/BARDA funding contract– 8/16 |
| – | Awarded CDC Surveillance Program: DPP® Zika/Dengue/Chikungunya (Peru, India, Guatemala, Haiti)- 9/16 |
| – | Received ANVISA approval, DPP® Zika Assay –11/16 |
| – | Successful INCQS Evaluation- 2017 |
See graphics
Slide 15
Ongoing Technology Collaborations
Leveraging Our Patented DPP® Technology Platform
DPP® "Undisclosed Biomarker" Assay- AstraZeneca
DPP® Cancer Assay- Undisclosed Collaborator
DPP® Bovine TB Assay- USDA
DPP® Concussion Assay- Perseus Science Group LLC
Slide 16
Chembio's Global Organization
Expanding Commercial and Manufacturing Operations
Manufacturing Operations
Facilities: 65,000 sq. ft.
Capacity: 25MM tests
Certification: ISO 13485
Regulatory Compliance: (FDA, WHO, USDA, ANVISA)
See graphics
Slide 17
Selected Financial Highlights
Quarter Ended (in 000's) 9 Months Ended (in 000's)
| | | Sept. 30, 2017 | | | Sept. 30, 2016 | | | Sept. 30, 2017 | | | Sept. 30, 2016 | |
| Net Product Revenues | | $ | 6,133 | | | $ | 2,502 | | | $ | 14,453 | | | $ | 10,452 | |
| Total Revenues | | $ | 7,587 | | | $ | 3,746 | | | $ | 18,027 | | | $ | 13,614 | |
| Gross Margin | | $ | 3,523 | | | $ | 1,952 | | | $ | 8,539 | | | $ | 6,698 | |
| Loss from Operations | | $ | (589 | ) | | $ | (2,144 | ) | | $ | (4,398 | ) | | $ | (4,998 | ) |
| Net Loss | | $ | (585 | ) | | | (2,138 | ) | | | (4,373 | ) | | $ | (10,879 | )* |
| Accounts Receivable Net | | $ | 5,768 | | | $ | 4,208 | | | $ | 5,768 | | | $ | 4,208 | |
Cash (as of end of period) | | $ | 1,872 | | | $ | 12.172 | | | $ | 1,872 | | | $ | 12,172 | |
Slide 18
Investment Highlights
| § | A global leader in point-of-care (POC) infectious disease |
| – | Sales & marketing organization in U.S., LATAM, Europe, Africa, and APAC |
| – | Manufacturing operations in the U.S. (NY) and Malaysia |
| § | Groundbreaking patented DPP® technology platform |
| – | Superior sensitivity and specificity vs. traditional lateral flow technology |
| – | Multiple tests from a tiny (10μL) drop of fingertip blood (multiplexing) |
| § | Robust pipeline of new DPP® POC assays in development |
| – | DPP® HIV-Syphilis Combination Assay (U.S. version) |
| – | DPP® Fever Assays (Malaria, Dengue, Zika, Chikungunya, Ebola, + others) |
| – | DPP® Technology Collaborations (Cancer, Concussion, Undisclosed, Bovine TB) |
| § | Multiple high-value collaborations |
| – | U.S. Government: HHS/ASPR/BARDA, CDC: DPP®Zika |
| – | AstraZeneca: DPP® "Undisclosed Biomarker" |
| – | Bill & Melinda Gates Foundation: DPP® Malaria |
| – | Paul G. Allen Ebola Program: DPP® Fever Panel - Africa |
| – | FIND: DPP® Fever Panel - Asia |
Slide 19
Experienced Executive Leadership Team
Executive: John J. Sperzel III, President &CEO
Joined Chembio: 2014
Previous Experience: 2011-2013, President and CEO of ITC/Accriva; 1987-2011 Axis-Shield, Bayer Diagnostics, Instrumentation Laboratory and Boehringer Mannheim
Executive: Neil A. Goldman, CPA Chief Financial Officer; Executive Vice President
Joined Chembio: 2017
Previous Experience: 2015-2017, CFO of J.S. Held; 1989-2015 Unwired Technology LLC/Delphi Corp., EPPCO Enterprises, Ernst & Young
Executive: Javan Esfandiari, M.S. Chief Science and Technology Officer; Executive Vice President
Joined Chembio: 2000
Previous Experience: 1997-2000, Co-founder of Sinovus Biotech AB (Sweden), acquired by Chembio in 2000; 1993-1997 R&D Director of On-Site Biotech
Executive: Sharon Klugewicz, M.S., President, Americas Region
Joined Chembio: 2012
Previous Experience: 2009-2012, Sr. VP Scientific & Laboratory Services of Pall Corporation; 1991-2009 Pall Corporation
Executive: Robert Passas, Ph.D., President, EMEA & APAC Regions
Joined Chembio: 2016
Previous Experience: 2015-2016, VP, Worldwide Marketing and International Sales at Trinity Biotech; 1993-2015 The Binding Site, Abbott, Trinity Biotech, Quidel
Executive: Thomas Ippolito, VP Regulatory & Clinical Affairs
Joined Chembio: 2005
Previous Experience: 2000-2005, VP Quality & Regulatory of Biospecific Technologies Corp.; 1984-2000 United Biomedical Inc., Analytab Products Inc. and Eastern Long Island Hospital
Executive: Paul Lambotte, Ph.D, VP Product Development
Joined Chembio: 2014
Previous Experience: 2009 – 2014, President of PLC Inc.; 2009 – 2012 Chief Science Officer of Axxin Pty Ltd.; 2000-2009, VP of R&D and Business Development of Quidel, Inc.
Executive: David Gyorke, VP Manufacturing Operations
Joined Chembio: 2017
Previous Experience: 2011-2016, VP operations of Nanomix, 1983-2011, NeoVista, Farallon Medical, Inc., Cholestech Corporation, Bio-Rad