Exhibit 99.1


1

2
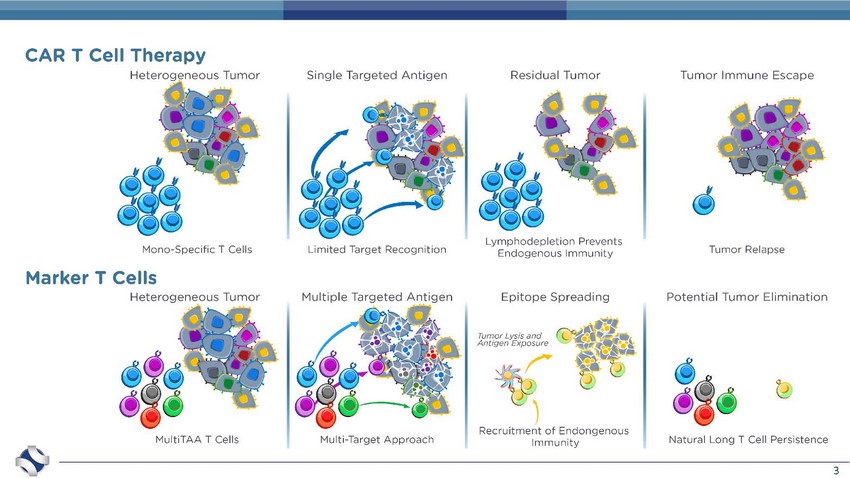
3

4
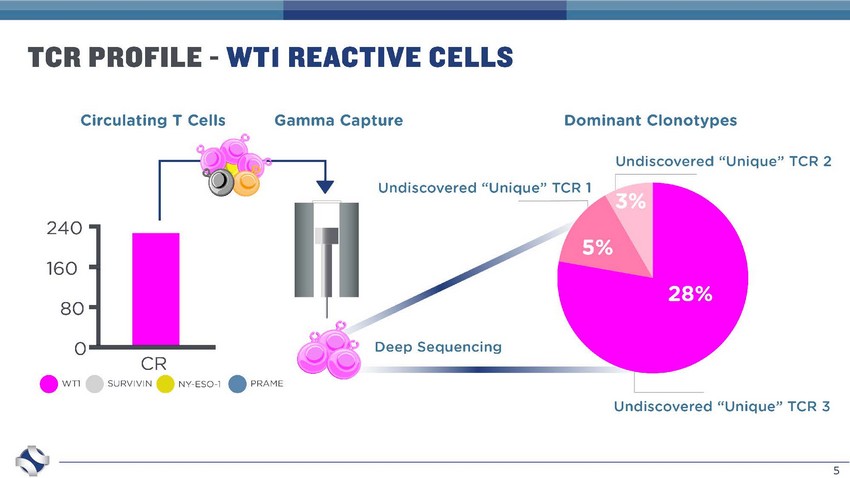
5

6 LYMPHOMA PHASE I TRIAL (TACTAL) Group A: Active disease (15 patients) • 7 complete responses (CR) • Durable for 4 months -- >5 years • No patient achieving a CR has subsequently relapsed • 8 stable disease (SD) • Durable for 4 -- >12 months) • 2 patients subsequently achieved CR with confounding factors Group B: Adjuvant ( 17 patients*) • 14/17 currently remain in remission • Range 9 months -- >4 years • Mean time in CCR is 29 months • 3 relapses occurred between 8 - 33 months Presented at Society of Hematology Oncology (SOHO) Sept 2019 Autologous MultiTAA T cells for adult patients with lymphoma *1 patient treated twice after initial relapse Group A Monotherapy for patients with active disease Group B Maintenance therapy for patients in remission from prior therapy Dose escalation (n=11) Dose escalation (n=14) Antigen escalation (n=3) Antigen escalation (n=4)
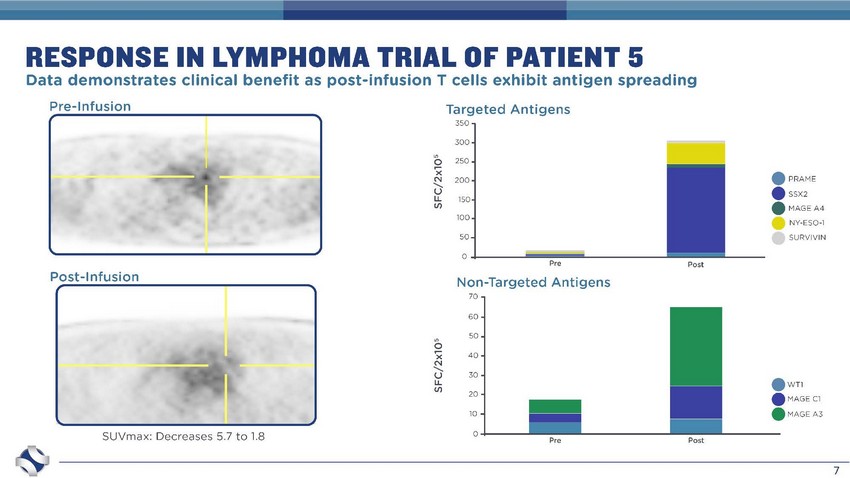
7
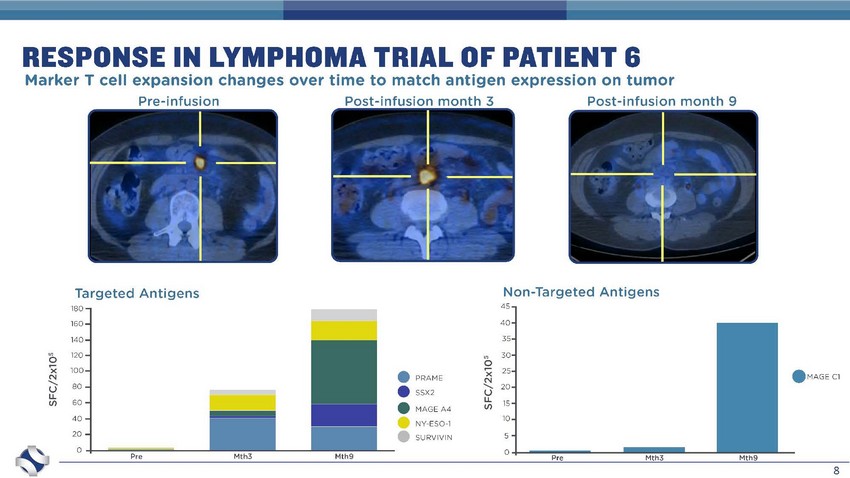
8
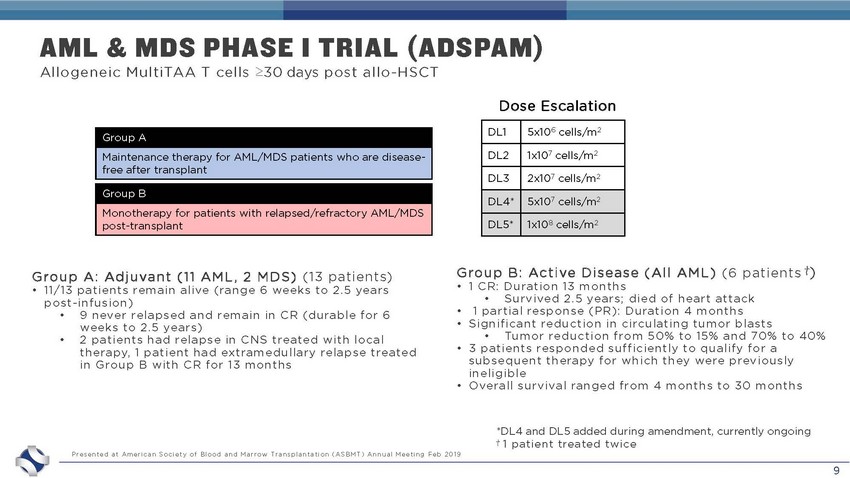
9 AML & MDS PHASE I TRIAL (ADSPAM) Group B: Active Disease (All AML) (6 patients ⴕ ) • 1 CR: Duration 13 months • Survived 2.5 years; died of heart attack • 1 partial response (PR): Duration 4 months • Significant reduction in circulating tumor blasts • Tumor reduction from 50% to 15% and 70% to 40% • 3 patients responded sufficiently to qualify for a subsequent therapy for which they were previously ineligible • Overall survival ranged from 4 months to 30 months Group A: Adjuvant (11 AML, 2 MDS) (13 patients) • 11/13 patients remain alive (range 6 weeks to 2.5 years post - infusion) • 9 never relapsed and remain in CR (durable for 6 weeks to 2.5 years) • 2 patients had relapse in CNS treated with local therapy, 1 patient had extramedullary relapse treated in Group B with CR for 13 months Presented at American Society of Blood and Marrow Transplantation (ASBMT) Annual Meeting Feb 2019 Dose Escalation DL1 5x10 6 cells/m 2 DL2 1x10 7 cells/m 2 DL3 2x10 7 cells/m 2 DL4* 5x10 7 cells/m 2 DL5* 1x10 8 cells/m 2 Allogeneic MultiTAA T cells ≥30 days post allo - HSCT Group A Maintenance therapy for AML/MDS patients who are disease - free after transplant Group B Monotherapy for patients with relapsed/refractory AML/MDS post - transplant *DL4 and DL5 added during amendment, currently ongoing ⴕ 1 patient treated twice

10

11

12

13
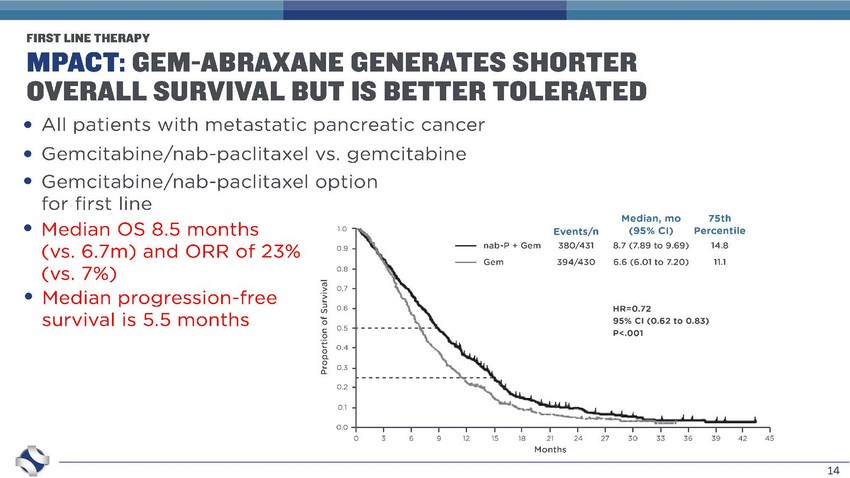
14
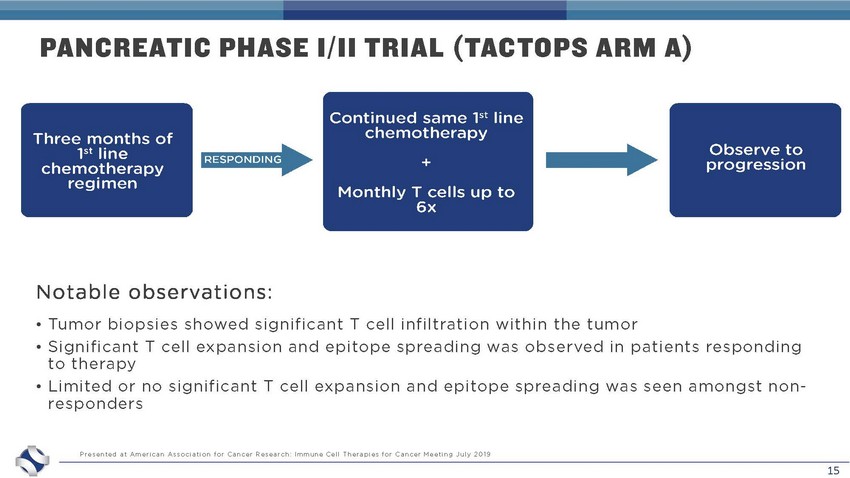
15 PANCREATIC PHASE I/II TRIAL (TACTOPS ARM A) • Tumor biopsies showed significant T cell infiltration within the tumor • Significant T cell expansion and epitope spreading was observed in patients responding to therapy • Limited or no significant T cell expansion and epitope spreading was seen amongst non - responders Presented at American Association for Cancer Research: Immune Cell Therapies for Cancer Meeting July 2019 Notable observations:
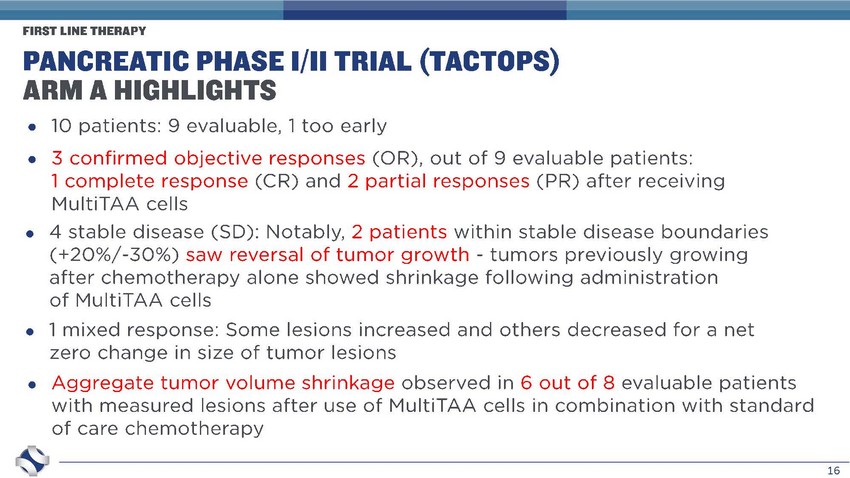
16
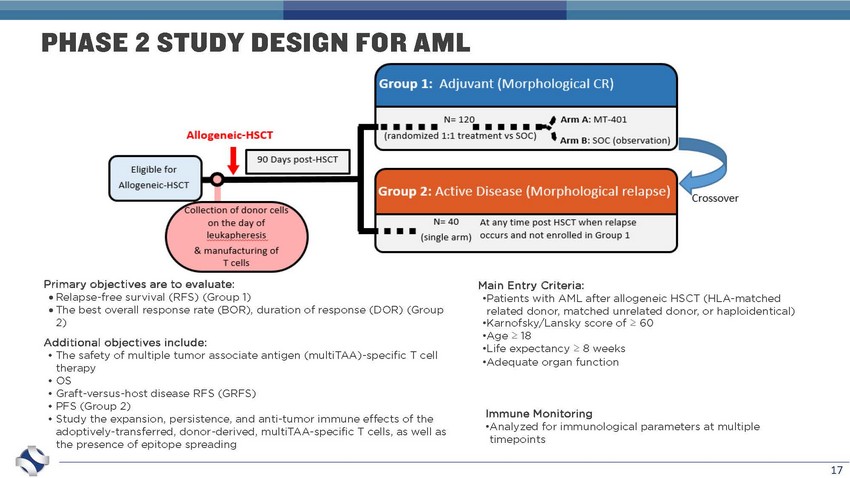
17 Primary objectives are to evaluate: Relapse - free survival (RFS) (Group 1) The best overall response rate (BOR), duration of response (DOR) (Group 2) Additional objectives include: • The safety of multiple tumor associate antigen ( multiTAA ) - specific T cell therapy • OS • Graft - versus - host disease RFS (GRFS) • PFS (Group 2) • Study the expansion, persistence, and anti - tumor immune effects of the adoptively - transferred, donor - derived, multiTAA - specific T cells, as well as the presence of epitope spreading Main Entry Criteria: • Patients with AML after allogeneic HSCT (HLA - matched related donor, matched unrelated donor, or haploidentical) • Karnofsky /Lansky score of ≥ 60 • Age ≥ 18 • Life expectancy ≥ 8 weeks • Adequate organ function Immune Monitoring • Analyzed for immunological parameters at multiple timepoints PHASE 2 STUDY DESIGN FOR AML
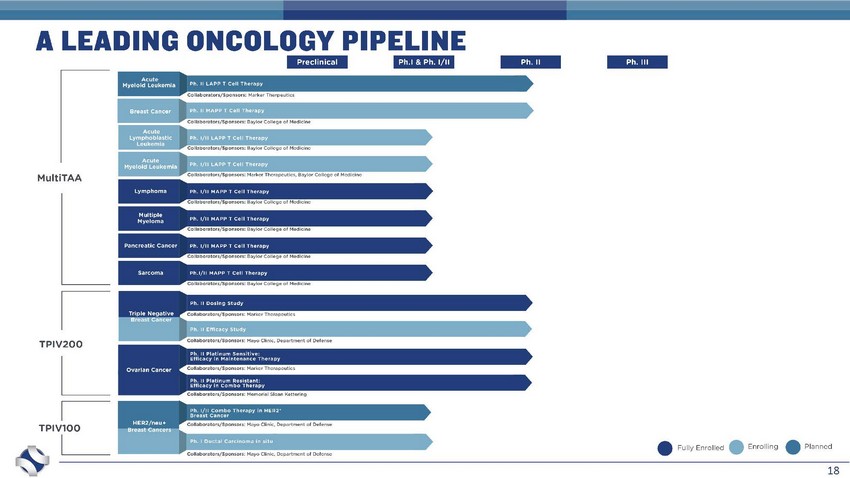
18


















