January 6, 2010
Jim B. Rosenberg
Senior Assistant Chief Accountant
US Security and Exchange Commission
Washington, D.C. 20549
Dear Mr. Rosenberg:
| 1. | Tell us why you have not filed audited financial statements and when you will file audited financial statements as required by Form 10-K. |
We employed The Nutmeg Group, LLC and Randall Goulding, Esq. to design and execute a reverse merger with Senticore (SNIO), a publicly trading BDC and Traci J.Anderson a PCAOB certified CPA as the Company auditor. The overriding explanation for why we did not file, and made mistakes in, the required audited financials is that Company resources have been severely strained when faced with absence of any income from the sale of $2,000,000 in assets received in the merger that turned out to be worthless. We also relied heavily on the experience of the Nutmeg Group and Randall Goulding, both of whom have been charged by the SEC with multiple counts of fraud and mismanagement of funds. Additionally, the Company’s auditor, Traci Anderson has recently been barred by the Pub lic Accountants Oversight Board from association with any registered accounting firm. The SEC allegations against these people and firm are consistent with the misrepresentations and fraudulent services provided that led to the near-crippling discrepancies listed below.
Discrepancies
| § | incorrect submission and filing to become a BDC, |
| § | improper issuance of millions of “Regulation E” shares, |
| § | a cumulative operating loss of nearly $4,000,000, |
| § | a cumulative cash flow of less than $5,000 after six years of operating, |
| § | of the 181,145,154 shares outstanding when we assumed control, over 40 million were inappropriately issued, most of which were recaptured and cancelled. |
| § | loans made in shares were pledged as collateral that went into default resulting in surrendering of the shares without any documentation on the terms of these loans |
| § | no financial records available for 2004 |
| § | quarterly reports are inconsistent with annual financial reports |
| § | no taxes, 1099s, or W-2 were filed |
| § | actions taken in violation of by-laws |
| § | used four different auditing firms, two of whom resigned for what appears to have been a disagreement over accounting procedures |
| § | stock issuances that raise serious concerns about insider trading |
| § | four separate judgments filed against the company but listed as “accounts payable |
| § | numerous BDC violations of issuing shares for services, |
| § | numerous misrepresentations in the reverse merger closing agreements, |
| § | ~2 million restricted shares represented as assets that turned out to be worthless, |
| § | missing and inconsistent financial data, |
| § | 9,000,000 shares issued to AURORA TWO LTD NOMINEES LIMITED in the Bahamas as restricted shares, but were sold less than two months after issuance. |
| § | payments made to consultants that appear to have been the result of entering into acquisitions in industries about which the management had little or no experience without managerial assistance required by a BDC. |
| § | Shares were held in escrow that were issued to people and companies involved in acquisitions that were never consummated (The Westar and Smith-Forestal transactions). |
| § | There were repeated payments made to an off-shore company that ultimately exceeded $100,000 for a failed acquisition with inadequate documents as to why these payments (by wire transfer) were made. |
| § | There are unusually high payments made to the company’s accountant (Mike Bongiovanni) that appear to be a violation of the Sarbanes-Oxely Act. |
| § | quarterly reports inconsistent with annual reports |
| § | failure to issue the appropriate 1099s for payments made in stock, |
| § | a number of questionable and apparently |
| § | thousands of hostile and angry shareholders |
| § | Issued almost 200,000,000 shares and paid management ~ $300,000/yr |
| § | provided no information on basis for valuing the ~$2,000,000 assets provided with merger |
| § | payments made to consultants for fees that appear excessive without any documentation for the basis of these share issuances. For example, Jeffrey Galpern, was paid $229,500 for one year, an amount that is more than the entire management team was paid in 2004. |
| § | in only one of the 13 quarters since the company was acquired was a share log maintained that appears to have conformed with the SEC’s record-keeping requirements. |
| § | with the exception of the share log maintain for the one quarter, I could find no listing of the consideration for which the shares were issued—including the time when the company was a BDC. |
| § | records of company minutes, Board of Directors meeting and subsequent resolutions are incomplete. |
| § | more than ~22 press releases were issued for Taj System without a single reference to the parent company, SNIO, even when Senticore had “controlling interest” raising questions as to why someone would promote a portfolio company without giving the parent company, or affiliated company, acknowledgement to boost SNIO’s share value |
| § | there are questionable share-swaps with AdZone Research and Circle Group Holdings that appear to be more of an effort to swap shares for each company to promote its stock to the other company’s shareholders. I don’t if this is consistent with BDC regulations or not, but needs to be examined further. |
Actions taken by current management
| § | worked closely and extensively with the SEC to resolve many of these issues |
| § | current management received less than $40,000 in annual shares. |
| § | Resolved SEC violations to the satisfaction of SEC’s enforcement division without consent decree |
| § | Withdrew election to be regulated as a BDC |
| § | Conducted reverse split converting 8 billion shares into 40 billion shares |
| § | Applied reverse split to preferred as well as common shares |
| § | Reset authorized shares from 200 million to 50 million |
| § | Recaptured and cancelled improperly issue shares |
| § | Achieved more cash flow in first quarter of operation than in previous five years |
| § | Reversed cumulative operating losses of over $4 million into net operating profit |
| § | Restructured executive salaries based on net operating profits instead of fixed fee |
It is our view that, in spite of our failure to file the required financials, the ~4,000 shareholders would be better served with our continued efforts to keep this company functioning than bankrupting it or simply walking away. Toward this end, our current plan is to spend the first quarter of 2011 to restate all financial statements starting with historical accounting information from IHT, Inc., the private Illinois corporation that assumed managerial control of SNIO, in conjunction with the 2006 reverse merger. We will do what we can to rectify the shortcomings in previous filings exploring a number of alternative funding sources to allow us to employ the services of an auditor with a goal of filing the correct financials for 2010 addressing the issues you raised in your letter. &# 160;We are also actively engaged in negotiations to e acquired by or merge with another business entity, the details of which I am not at liberty to disclose unless ordered to do so by the SEC. And, finally, we do appear to have several opportunities for receipt of federal and private grants over the current year.
2. A statement of cash flows should be included.
Statements of cash flow will be provided in all subsequent filings.
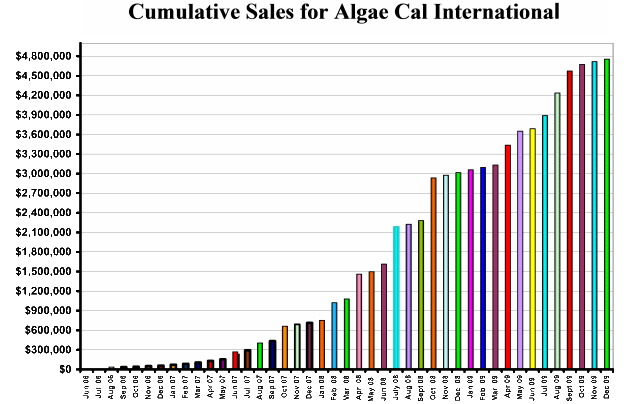
| 3. | You valued your investment in AlgaeCal at $288,000 in 2007 and are increasing it at 10% per year. Disclose the nature of the $288,000 Consideration and reference supporting accounting literature for increasing the investment at 10% per year. |
The original estimate of the value of the company provided by AlgaeCal was $3,600,000, based, in part, on a $4,000,000 offer they received for sale of the company, a value they now have set as $10,000,000. We received an 8% equity position and a 1% override on gross sales, the latter yielding over $60,000 net income since acquiring our equity position and over $300,000 in R & D funds. Additionally, we have completed, or been involved in, clinical trials supporting the safety and efficacy of AlgaeCal’s product. Two of these studies have recently been submitted for publication in peer-reviewed medical journals and are available for review if needed. With this potential in mind, we used $288,000-- 8% of what AlgaeCal Inc. stated as its in itial value. Based on the factors stated above and AlgaeCal’s cumulative sales figures shown below, subsequent receipt of R&D funds from AlgaeCal that have now exceeded $300,000, and AlgaeCal’s recent statement of its value, I used what I thought was a conservative figure of 10% per year. Ms Anderson concurrence with this figure is, unfortunately, the only supporting accounting basis I have for the estimate.
4. You have increased the value of the asset longitudinal database by 11.2% in 2007, 9% in 2008 and 7% in 2009. Reference supporting accounting literature for this treatment. Disclose depreciation expense in each period for this asset and the useful life or disclose why the asset the asset is not being depreciated. Disclose when and how the asset is assessed for impairment.
With regard to the annual increases in the value of the database, the increases are based on the actual number of additional medical biomarkers that were added during each of these years using the same assessment of value used to initially determine the asset value of the database described below.
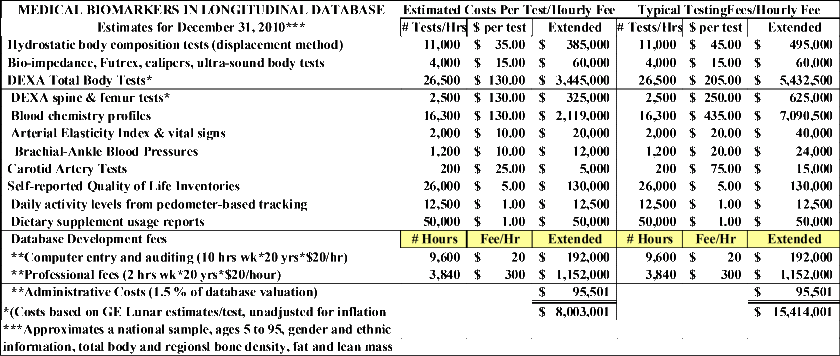
A brief summary of the content of the database was provided in the in the Form 8-K/A filed Oct 24, 2006. An expanded description of these tests is provided at EXHIBIT A and the specific values are shown below that have been updated for an estimated value at the end of 2010 subject to our end of year audit. I have also provided descriptions of the two main biomarkers in the database, the DEXA total body and spine/femur tests and the blood chemistry profiles.
As for depreciation of the database, we have assumed this is an “appreciating” asset similar to that of an antique since its value increases over time as more data are added and longer and longer longitudinal analyses are possible. As for its useful life, as discussed below, the useful life of a database that is continually updated with state-of-the-art and FDA-approved medical biomarkers extends well beyond the fourteen years of accumulated data. For example, Harvard University’s Framingham database is now over 50 years old and is still making valuable contributions to research and medicine.
The basis of treating this asset as “…an intangible asset with an indefinite life without amortization…” was reported in the independent auditor’s NOTE A, pgs 12 and 13 of the Form8-K/A filed with the SEC on October 24, 2006. There is significant agreement in the scientific community of the value of longitudinal databases as reflected in the significant scientific discoveries from a variety of databases, including Harvard’s the long standing Framingham study. We have also repeatedly used information derived from the data base to support our own studies and to support funding requests from grantors and companies in the private sector. The press release provided at EXHIBIT B below is one example of a company that has enabled us to earn over $1,000,000 in research funds since we began their longitudinal trials 1995, the results of which are summarized in a book we published in 2008 (available upon request).
The database has increased our status in the scientific and academic communities that is enhancing our ability to obtain grants and research contracts. For example, more recently, I have received an invitation from Georgetown University’s department of Biochemistry to apply for an appointment as an adjunct professor contingent upon providing them access to the database. We are also working with Texas A & M’s department of sports medicine for jointly working an analyses of the database in conjunction with testing their athletes. Thus, we continually assess the value and impairment of the database as a non-depreciating asset. Notwithstanding the above information, we a certainly open to considering other approaches to valuing this asset.
5. Disclose your revenue recognition policies and explain why you report no accounts receivable at December 31, 2008 and 2009.
I am in the process of re-calculating all financials beginning with the first quarter of 2004 for IHT, Inc. of Illinois as the acquirer.
6. Your statement of operations and Selected Financial Data do not report net income for 2007, 2008, and 2009.
I am in the process of re-calculating all financials beginning with the first quarter of 2004 for IHT, Inc. of Illinois as the acquirer.
7. Based on the above comments you should reconsider if you disclosure controls and procedures and your internal control over financial reporting are effective.
I concur. We will be working with an auditing firm to develop valid disclosure and internal controls.
8. The certification you filed in Exhibit 32.1 referenced the Form 10-Q for the quarterly period ended September 30, 2006. Please file an amended certification which references the correct periodic report.
This will be amended in a re-stating of financials.
9. Please file Forms 10-Q for the quarters ended March 31, 2010 and June 30, 2010.
I am in the process of re-calculating all financials beginning with the first quarter of 2004 for IHT, Inc. of Illinois as the acquirer.
EXHIBIT A
Contents of the Database
Body Composition. Approximately 26,000 body composition measurements were derived using Dual Energy X-ray Absorptiometry (DEXA) technology yielding total body and the multiple measurements of bone density, bone mineral content, fat and fat-free mass as described in Attachment 1. All DEXA test reports are stored in multiple data bases on one of five DEXA machines and need to be reviewed for redundancy, errors and inconsistencies.
Blood Chemistries. Approximately 15,000 subjects, including many who completed DEXA tests, also completed the 43-panel blood chemistry panel described in Attachment 2. All blood tests were completed after a 12-hour fasting at one of two national testing labs (Quest or Lab Corps). While the more recently conducted tests have been provided in computerized formats, many of the source documents for earlier blood tests are on hard copies and need to be converted to computerized reports.
Self-Reported Quality of Life. Virtually all subjects completing DEXA or blood chemistry tests also completed either a 50- or 84-item Quality of Life Inventory described in Attachment 3. In addition to an overall quality of life score, eating control and depression sub-scales are contained in the Inventory.
Self-reported Pedometer-Monitored Physical Activity Levels. During many of the clinical trials, study participants wore pedometers during their waking hours and tracked their daily step totals throughout the typical 60- or 90-day studies in which the participated. A description of the type of pedometer used and recent studies supporting the health benefits derived from pedometer usage is provided in Attachment 4.
Miscellaneous Measures. A much smaller number of study participants completed a variety of measurements such as circulating insulin, capillary glucose, A1C as well as miscellaneous questionnaires, ratings of study compliance, etc.
Potential for Longitudinal Analyses. In addition to the longitudinal data derived from participants who completed multiple tests, in some cases over 10 years, personal contacts and relationships have been established with may of the study participants. These relationships offer the potential for repeated post-study contacts with many of these study participants for completion of follow-up questionnaires and non-invasive sampling for genetic and other testing.
EXHIBIT B
Mannatech, Inc. Awards Grant for Independent Analysis of
the Mannatech’s 12-year Longitudinal Glyconutritional Trial
Mannatech, Inc. (NASDAQ: MTEX) March 27, 2007 has awarded a grant to the Health and Medical Research Center, a wholly owned subsidiary of Integrative Health Technologies, Inc. (OTCBB: IHHT) to conduct an independent analysis of its 12-year 7,000 subject Longitudinal Trial. Although the Research Center will continue to maintain control of Mannatech’s data, its scientists will work with Mannatech’s scientists to analyze 1,200,000 tests the Center has accumulated in its database over the past 20 years. Preliminary analyses have revealed a number of heretofore unknown and misunderstood relationships between measures of bone density, body fat, lean, blood chemistries and self-reported quality of life.
For over a decade, Mannatech has developed innovative, high-quality, proprietary nutritional supplements, topical products and weight-management products. These products are sold by approximately 526,000 independent associates operating throughout the United States, Canada, Australia, the United Kingdom, Japan, New Zealand, Republic of Korea, Taiwan, Denmark, and Germany. The company recently reported record earnings for second quarter 2006 of $0.31 per diluted share, up 48% from second quarter 2005 earnings of $0.21 per diluted share. Pretax profit reached $13.4 million for the quarter, up 41% over the prior year, reflecting favorable costs relative to sales. Net income for the quarter reached a record $8.6 million with a net profit ratio of 8.2%, a rate improvement of 2.5 points versus 2005, partially due to a l ower effective tax rate in the quarter. Total second quarter revenue was $104.8 million, up 2.1 % versus the prior year. Mannatech’s 526,000 independent associates as of June 30, 2006, represents an increase of 19.5% compared with the same time the prior year.
Founded in 1993, Mannatech has pioneered the development and application of dietary supplements using a unique blend of plant-based sugars, known as glyconutrients ('glyco' is the Greek word for 'sweet'). After reviewing studies that identified eight glyconutrient sugars needed for optimal cellular communication and immune system functioning, Mannatech’s scientists concluded that six of these glyconutrients are often lacking in modern diets. To meet the need for new and better sources of these nutrients, Mannatech’s scientists developed “Ambrotose Complex” which became their flagship ingredient and ultimately led to 20 worldwide patents -- including one from the U.S. Patent and Trademark Office.
“We realized that if we were going to maintain credibility with our associates and the scientific community,” said Sam Caster, Mannatech’s CEO, “we needed third party validation of the safety and efficacy of our product line. To achieve that,” continued Caster, “we provided a grant to the Health and Medical Research Center, an independent research company, to conduct a series of short-term safety and efficacy studies of our product line using widely accepted biomarkers of immunity and longevity--body fat, lean mass, bone density, blood chemistries (including C-reactive protein—“CRP”), and self-reported quality of life.
“One of the conditions of Mannatech’s grant was that we had to use only state-of-the-art testing technology with established validity and precision that would be widely accepted by the scientific community. To meet this requirement,” said Gilbert R. Kaats, PhD, the senior investigator of the studies, “our early use underwater testing technology for body fat and lean was replaced with what is now the ‘gold standard’ for this type of measurement—Dual Energy X-ray Absorptiometry. “DEXA,” continued Kaats, “is an FDA- approved measurement that provides measurements of lean, fat and bone density in the subject’s entire body as well as in different regions of the body. We also had subjects complete a 43-chemistry blood test conducted and analyzed by a national indepe ndent laboratory,” added Kaats. “These initial studies provided evidence that use of the company’s glyconutritional supplements could increase bone mineral density, lean mass, and quality of life, as well as contribute to the maintenance of healthy blood cholesterol levels. While some of these studies used historical placebo norms and meta-analyses, we did conduct two small double-blinded placebo-controlled randomized studies that confirmed the finding that the glyconutrients improved lean-to-fat ratios and increased bone mineral density” reported Kaats.
Mr. Caster also reported, “We subsequently found our associates had a high level of interest in finding out for themselves how well our product worked. In a sense, it was as if they were conducting their own due-diligence. To track changes, they repeated these tests over time at their expense, sometimes every year. As the associates completed these tests,” Caster explained, “it led to the accumulation of the largest and longest running longitudinal trial of the safety and efficacy of glyconutrients, or perhaps, any dietary supplement. Then combining our data with the tests the Research Center has conducted over the past 20 years, resulted in a massive valuable database of state-of-the-art tests that will prove invaluable for the future validation of our products.”
“It would be difficult to overstate the profound importance of the 20-year massive database for which the Research Center and Mannatech have been responsible,” said professor Harry G. Preuss, MD of Georgetown, University and former president of the The American College of Nutrition. The accumulation of over 1,200,000, ‘high tech’ measurements from over 30,000 people aged 8-90 from every ethnic group, from every state in the US, as well as from Canada, Japan, Australia, New Zealand and England is a research ‘gold mine’” pointed out Dr. Preuss.
“In addition, examining the interrelationships between these measures, the ability to validate future tests such as genetic and DNA profiles among the database’s diverse population can make some significant contributions to our marketing as well as scientific goals,” said Rob Sinnott, PhD, Mannatech’s Chief Science Officer.
“Collaborating with the Research Center’s independent Scientific Advisory Board on this grant,” said Steven Boyd, MD, Mannatech’s Senior Science Officer, “is our approach to the challenge of combining marketing goals and scientific goals. This potential conflict was addressed in the current issue of the Journal of the American Medical Association by Dr. Catherine DeAngelis, the journal’s Editor in Chief , who wrote:
| | “The influence of commercial interests on medical science is far-reaching but, to a great degree, essential. The discovery of new medications, devices, and techniques is funded primarily by for-profit companies; testing new modalities of treatment is funded primarily by for-profit companies; and the manufacture and profitable marketing aspects of these modalities appropriately falls in the purview of this industry. |
| | Two basic goals of for-profit companies are the discovery, testing, and production of products (the scientific goal) and the sale of products to garner profits, thereby generating returns to the shareholders (the marketing goal). Profits are a logical expectation of the companies that fund the discoveries. Ideally the products discovered, tested, and produced will be beneficial to many individuals for whom the products will be prescribed and who will purchase them, returning a healthy profit for the company. Now comes the potential problem. In some instances, the marketing goal of a company dominates the scientific aspect of the company-funded research.” |
“To avoid this potential conflict, Mannatech’s scientists will be collaborating with the Research Center’s independent Scientific Advisory Board to analyze and publish information in the database that often be unrelated to glyconutrients and will have no direct impact on Mannatech’s marketing goals,” said Dr. Boyd.
Professor Joel Malechek, at the Center for Epidemiology/Biostatistics at the University of Texas Health Science Center at San Antonio, another of Health and Medical’s Scientific Board members, agreed with Dr. Boyd and pointed out that, “The Center’s database contains extensive information derived from cutting edge technology that has never been reported to the scientific community. To analyze and publish these findings will be rewarding to our Board members as well as to Mannatech’s scientists. I should think it should also be rewarding to Mannatech’s associates to know that their scientists will be making this type of contribution.”
Gilbert R. Kaats, CEO