UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington D. C. 20549
FORM 10-Q
x Quarterly report pursuant to Section 13 or 15(d) of the Securities and Exchange Act of 1934
For the quarterly period ended March 31, 2007
o Transition report pursuant to Section 13 or 15(d) of the Exchange Act for the transition period from _________ to _________
Commission File Number: 814-00699
INTEGRATIVE HEALTH TECHNOLOGIES, INC.
(F/K/A SENTICORE, INC.)
(F/K/A HOJO HOLDINGS, INC.)
(Exact name of registrant as specified in charter)
DELAWARE (State of or other jurisdiction of incorporation or organization) | 11-3504866 (IRS Employer I.D. No.) |
4940 Broadway, Suite 201
San Antonio, TX 78209
(Address of Principal Executive Offices)
(210) 824-4200
(Registrant's Telephone Number, Including Area Code)
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
YES x NO ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer and large accelerated filer” in Rule 12b-2 of the Exchange Act.
Large accelerated filer ¨ Accelerated filer ¨ Non-accelerated filer x
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). YES ¨ NO x
As of May 15, 2007, the registrant had 40,642,597 shares of common stock and no preferred stock issued and outstanding.
INTEGRATIVE HEALTH TECHNOLOGIES, INC.--INDEX
PART I. FINANCIAL INFORMATION
Item 1. Financial Statements
| q | Portfolio of Investments for March 31, 2007 (un-audited) |
| q | Balance Sheets for Mar 31, 2006 and Mar 31, 2005 (un-audited); and Dec 31, 2006 (audited) |
| q | Statements of Operations for the three months ended Mar 31, 2006 and Mar 31, 2005 (unaudited); and year ended Dec 31, 2006 (audited) |
| q | Statements of Changes in Net Assets for three months ended March 30, 2007 (unaudited) and year ending Dec 31, 2006 (audited) |
| q | Statement of stockholders' equity (capital deficit) from inception through March 31, 2007 (unaudited) |
| q | Statements of Cash Flows for the three months ended March 31, 2007 and March 31, 2007 (unaudited) |
| q | Notes to Financial Statements |
NOTE 1: FORMATION AND OPERATIONS OF THE COMPANY
Formation
Business Description
NOTE 2: SIGNIFICANT ACCOUNTING POLICIES
Development Stage
Basis of Presentation
Management’s Use of Estimates
Stock-Based Compensation
Valuation of Long-Lived and Intangible Assets
Earnings/(Loss) Per Share
NOTE 3: INCOME TAXES
NOTE 4: RELATED PARTY TRANSACTIONS
Operating Agreement with Health & Medical Research, Inc. (“HMRI”)
Operating Agreement with HealthTech Development, LLC. (“HTD”)
Operating Agreement with HealthTech Products, LLC. (“HTP”)
Acquisition of 8% ownership in AlgaeCal International
Sale of Taj Systems shares
Loans from shareholders
NOTE 5: HISTORICAL AND CURRENT FINANCIAL HIGHLIGHTS
Graphic Representation of Number of Issued and Outstanding Shares
Graphic Representation of Net Profit/(Loss) (by quarters and cumulative)
Graphic Representation of Net Assets per quarter
Graphic Representation of Shareholder’s Equity
Graphic Representation of Equity per Share
NOTE 6: REVERSE SPLIT
NOTE 7: SUBSEQUENT EVENTS
Exchange of Assets for Liabilities
Reverse Split
Reduction of Conversion Ratio of Preferred Stock from 400 to 1 to 2 to 1
De-electing to be Regulated as a BDC
Item 2. Management's Discussion and Analysis of Financial Condition and Results of Operations
| q | Preliminary Note Regarding Forward-Looking Statements |
| q | Changes in Investment Strategy and Operating Policies and Actions Taken by Current Management |
Overview
Scientific Advisory Board
Recapture of outstanding shares
Liquidation of non-core unrelated holdings and assets Operating expenses met through
Managerial fees received from portfolio companies
| q | Activities of Portfolio Companies |
Health and Medical Research, Inc.
HealthTech Development, LLC
HealthTech Products, LLC
| q | How portfolio companies impact a BDC’s profit or loss |
| q | How portfolio companies impact a non-BDC’s profit or loss |
| q | Change in BDC status causes change in focus for profit and loss |
| q | Planned restatement of financial statements |
| q | Critical Accounting Policies |
Item 3. Quantitative and Qualitative Disclosures about Market Risk
| (1) | Uncertainties of reorganization and restructuring |
| (2) | Uncertainties of the effects of the Company’s previous BDC status |
| (3) | Uncertainties of resolution of unresolved issues inherited from previous management |
Item 4. Controls and Procedures
Quarterly evaluation controls
CEO/CFO Certifications
Disclosure controls and internal controls
Limitations on the effectiveness of controls
Scope of the evaluation
Conclusions
PART II. OTHER INFORMATION
Item 1. Legal Proceedings Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
Item 3. Defaults upon Senior Securities
Item 4. Submission of Matters to a Vote of Securities Holders Item 5. Other Information
Item 6. Exhibits and reports on Form 8-K
Exhibits
EXHIBIT 99.1 The Longitudinal Database of Medical and Behavioral Tests and Measurements
EXHIBIT 99.2 Integrative Health Technologies, Inc.’s Scientific Advisory Board
EXHIBIT 99.3 Mannatech News Release
PART I. FINANCIAL INFORMATION
Item 1. Financial Statements
INTEGRATIVE HEALTH TECHNOLOGIES, INC.
previously known as Senticore, Inc.
(A Development Stage Company)
STATEMENT OF PORTFOLIO HOLDINGS & INVESTMENTS
FOR THE QUARTER ENDING MARCH 31, 2007 | |
| | | | | | | | | | | | | | |
Publicly-Traded Companies FMV on March 31, 2007 | | Acquisition Date | | Shares | | Price per share | | Fair Market Value 3/31 07 | | COST | | Unrealized Gn(Ls) | |
| Taj Systems (TJSS) | | | August 2005 | | | 1,281,886 | | $ | 0.043 | | $ | 55,121 | | $ | 236,233 | | $ | (181,112 | ) |
| AdZone Research (ADZR) | | | December 2004 | | | 882,353 | | $ | 0.010 | | $ | 8,824 | | $ | - | | $ | 8,824 | |
| Beere Financial (BRFG) | | | August 2005 | | | 2,308 | | $ | 0.050 | | $ | 115 | | $ | 10,000 | | $ | (9,885 | ) |
| Integrated Health Tech (IHT) | | | October 2006 | | | 6,874 | | $ | 0.400 | | $ | 3,849 | | $ | 6,256 | | $ | (2,407 | ) |
subtotals | | | | | | $ | 67,909 | | $ | 252,489 | | $ | (184,580 | ) |
Non Controlled Companies (Non-Publicly traded) | | | | | | | | | | | | | | | | | | | |
8% Equity Position in AlgaeCal International | | | October 2006 | | | | | | | | $ | 320,000 | | $ | 120,000 | | $ | 200,000 | |
| The Justice Fund, a privately held corporation | | | January 2006 | | | 1,000,000 | | $ | 0.100 | | $ | 100,000 | | $ | - | | $ | 100,000 | |
subtotals | | | | | | | | | | | $ | 420,000 | | $ | 120,000 | | $ | 300,000 | |
Controlled Companies (Non-Publicly traded) | | | | | | | | | | | | | | | |
| 100% Health and Medical Research, Inc. | | | August 2006 | | | | | | | | $ | 7,099,944 | | $ | 6,531,106 | | $ | 568,838 | |
| 100% HealthTech Products, LLC | | | August 2006 | | | | | | | | $ | 11,766 | | $ | - | | $ | 11,766 | |
| 100% HealthTech Development, LLC | | | August 2006 | | | | | | | | $ | 28,769 | | $ | - | | $ | 28,769 | |
subtotal | | | | | | | | | | | $ | 7,140,479 | | $ | 6,531,106 | | $ | 609,373 | |
Portfolio Holdings and Investments on 3/31/2007 | | Totals | | $ | 7,628,388 | | $ | 6,903,595 | | $ | 724,792 | |
FMV | | | | | | | | | | | | | | | COST | | | GAIN/LOSS | |
UNREALIZED GAIN OR LOSS THIS QUARTER | $ | 61,178 | |
INTEGRATIVE HEALTH TECHNOLOGIES, INC's
previously known as Senticore, Inc.
(A Development Stage Company)
BALANCE SHEET
FOR THE QUARTERS ENDING MARCH 31, 2007 and 2006
| | | March 31 | | March 31 | |
| | | 2007 | | 2006 | |
| | | Unaudited | | Unaudited | |
ASSETS | | | | | |
| CURRENT ASSETS | | | | | |
| Available for sale Investments--Public Companies | | $ | 67,909 | | $ | 1,146,017 | |
| Non Controlled Companies (Non-Publicly traded) | | | 420,000 | | | -- | |
| Controlled Companies (Non-Publicly traded) | | | 7,628,388 | | | -- | |
| Cash and cash equivalents | | | 12,206 | | | 552 | |
| Accounts Receivable | | | 334,058 | | | -- | |
| Inventory | | | 94,933 | | | -- | |
| Prepaid Clinical Trials | | | 1,234,463 | | | -- | |
| Total Current Assets | | $ | 9,791,957 | | $ | 1,146,569 | |
| PROPERTY AND EQUIPMENT | | | | | | | |
| Furniture, equipment computers & peripherals | | | 18,500 | | | -- | |
| Accumulated Depreciation | | | -- | | | | |
| Net Property and Equipment | | $ | 18,500 | | $ | -- | |
| OTHER ASSETS | | | | | | | |
| | | | | | | | |
TOTAL ASSETS | | $ | 9,810,457 | | $ | 1,146,569 | |
| | | | | | | | |
LIABILITIES AND STOCK HOLDERS' EQUITY | | | | | | | |
| | | | | | | | |
Liabilities | | | | | | | |
| Accounts Payable | | $ | 313,413 | | $ | 406,183 | |
Balance due on "AlgaeCal/Strontium Pro" study | | | 120,000 | | | -- | |
| Notes Payable | | | -- | | | 300,000 | |
| Stockholder Loans Payable | | | 187,340 | | | 314,058 | |
Total Liabilities | | $ | 620,753 | | $ | 1,020,241 | |
| | | | | | | | |
TOTAL Net ASSETS | | $ | 9,189,704 | | $ | 126,328 | |
| | | | | | | | |
Stockholders' equity | | | | | | | |
NET ASSETS consist of: | | | | | | | |
Preferred stock, $0.01 par value, 20,000,000 shares authorized, 20,000,000 issued July 2006 | | | | | | | |
| Paid in capital, preferred | | | | | | | |
| Common stock, $0.001 par value, 200,000,000 shares authorized; 40,642,597 issued and outstanding | | | 40,643 | | | 181,145 | |
| Paid in capital, common | | | 12,070,064 | | | 3,972,662 | |
| Accumulated deficit | | | (2,921,003 | ) | | (4,027,479 | ) |
TOTAL Net ASSETS | | $ | 9,189,704 | | $ | 126,328 | |
| | | | | | | | |
| Shares Outstanding | | | 40,642,597 | | | 181,145,154 | |
NET ASSET VALUE PER SHARE | | $ | 0.2261 | | $ | 0.0007 | |
NET ASSET VALUE PER SHARE - fully diluted | | $ | 0.2261 | | $ | 0.0007 | |
The accompanying notes are an integral part of these unaudited financial statements.
INTEGRATIVE HEALTH TECHNOLOGIES, INC's
previously known as Senticore, Inc.
(A Development Stage Company)
STATEMENTS OF OPERATIONS
| | | THREE MONTHS ENDING MARCH 31, | | YEAR ENDING DECEMBER 31, | |
| | | 2007 | | 2006 | | 2006 | |
INCOME FROM OPERATIONS: | | | | | | | |
| Clinical Trials | | | | $ -- | | $ 320,000 | |
| Managerial Fees, Services and Royalties | | | 18,598 | | | -- | | | 49,673 | |
| Total Income | | $ | 18,598 | | $ | - | | $ | 369,673 | |
COST OF SALES | | | | | | | | | | |
| Inventory Cost | | | -- | | | -- | | | 37,724 | |
| Clinical Trials and Study Support | | $ | 65,107 | | | -- | | | 120,000 | |
| Total Cost of Sales | | | 65,107 | | | -- | | | 157,724 | |
| Gross Profit | | $ | (46,509 | ) | $ | - | | $ | 211,949 | |
EXPENSES: | | | | | | | | | | |
| Administrative expenses and fees | | | 3,369 | | | -62,405 | | | 52,066 | |
| Stock Based Compensation | | | -- | | | -- | | | -- | |
| Loss in equity of LLC | | | -- | | | -- | | | -- | |
| Total Expenses | | $ | 3,369 | | $ | 62,405 | | $ | 52,066 | |
| | | | | | | | | | | |
NET INVESTMENT INCOME (LOSS) | | $ | (49,878 | ) | $ | (62,405 | ) | $ | 159,883 | |
| | | | | | | | | | | |
NET REALIZED GAIN (LOSS) ON INVESTMENTS | | $ | -- | | $ | -- | | $ | 234,769 | |
| | | | | | | | | | | |
NET CHANGE IN UNREALIZED GAIN AND LOSSES ON INVESTMENTS (1) | | $ | 61,178 | | $ | (192,715 | ) | $ | 463,614 | |
| | | | | | | | | | | |
INCOME (LOSS) BEFORE CUMULATIVE EFFECT OF ACCOUNTING CHANGES | | $ | -- | | $ | -- | | $ | -- | |
| | | | | | | | | | | |
NET INCREASE (DECREASE) IN NET ASSETS RESULTING FROM OPERATIONS | | $ | 11,300 | | $ | (255,120 | ) | $ | 858,266 | |
LOSS PER COMMON SHARE, BASIC & DILUTED | | | | | | | | | | |
| | | | | | | | | | | |
| Beginning Retain Deficit | | $ | (2,914,093 | ) | $ | (3,772,359 | ) | $ | (3,772,359 | ) |
| Ending Retained Deficit | | $ | (2,902,793 | ) | $ | (4,027,479 | ) | $ | (2,914,093 | ) |
The accompanying notes are an integral part of these unaudited financial statements.
INTEGRATIVE HEALTH TECHNOLOGIES, INC'spreviously known as Senticore, Inc.
(A Development Stage Company)
STATEMENTS OF CHANGE IN NET ASSETS
FOR THE QUARTERS ENDED MARCH 31, 2007 and 2006
| | For Quarters Ended | | For Years Ended | |
| | March 31 | | March 31 | | December 31, | | December 31, | |
| | 2007 | | 2006 | | 2006 | | 2005 | |
| | IHT, Inc. | | as Senticore | | IHT, Inc. | | as Senticore | |
| | Unaudited | | Unaudited | | Unaudited | | Audited | |
| | | | | | | | | |
INCREASE (DECREASE) IN NET ASSETS FROM OPERATIONS | | | | | | | | |
| Net investment income (loss) | $ | (49,878 | ) | $ | (62,405 | ) | $ | 159,898 | | $ | -- | |
| Net Realized Gain (Loss) on investments | | -- | | | -- | | | 234,769 | | | -- | |
| Net Change in Unrealized Gain (Loss) on investments | | 61,178 | | | (192,715 | ) | | 876,653 | | | (291,200 | ) |
NET INCREASE (DECREASE) IN NET ASSETS RESULTING FROM OPERATIONS | $ | 11,300 | | $ | (255,120 | ) | $ | 1,271,321 | | $ | (291,200 | ) |
| | | | | | | | | | | | | |
CAPITAL STOCK TRANSACTIONS: | | | | | | | | | | | | |
| Proceeds from common stock | | -- | | | -- | | | -- | | | 458,500 | |
| Issuance of preferred stock (acquisition of companies) | | -- | | | -- | | | 7,469,025 | | | -- | |
| Rescission of common stock | | -- | | | -- | | | (40 | ) | | -- | |
| Rescission of deferred compensation | | | | $ | -- | | | | | $ | 314,950 | |
NET INCREASE IN NET ASSETS FROM CAPITAL STOCK TRANSACTIONS | $ | -- | | $ | -- | | $ | 7,468,985 | | $ | 773,450 | |
| | | | | | | | | | | | | |
TOTAL INCREASE (DECREASE) IN NET ASSETS | $ | 11,300 | | $ | (255,120 | ) | $ | 8,740,306 | | $ | 482,250 | |
| | | | | | | | | | | | | |
NET ASSETS - BEGINNING OF PERIOD | $ | 8,708,699 | | $ | 381,448 | | $ | 381,448 | | $ | (45,630 | ) |
| | | | | | | | | | | | | |
NET ASSETS - END OF PERIOD | $ | 8,719,999 | | $ | 126,328 | | $ | 9,121,754 | | $ | 436,620 | |
The accompanying notes are an integral part of these unaudited financial statements.
INTEGRATIVE HEALTH TECHNOLOGIES, INC's
previously known as Senticore, Inc.
(A Development Stage Company)
STATEMENTS OF CASH FLOWS
FOR THE QUARTERS ENDED MARCH 31, 2007 and 2006
| | | For Three Months Ended March 31, | |
| | | 2007 | | 2006 | |
| | | as IHT, Inc | | as Senticore | |
| | | Unaudited | | Unaudited | |
| | | | | | |
CASH FLOWS FROM OPERATING ACTIVITIES | | | | | |
| Net investment income (loss) | | $ | (49,878 | ) | $ | (62,405 | ) |
| Adjustments to reconcile net increase (decrease): | | | | | | | |
| Add back depreciation & amortization | | | -- | | | -- | |
| Loss on disposal of fixed assets | | | -- | | | -- | |
| Loss in equity of LLC | | | -- | | | -- | |
| Stock based compensation | | | -- | | | -- | |
| Stock based interest | | | -- | | | -- | |
| Other stock based expenses | | | -- | | | -- | |
| (Increase) decrease in prepaid expenses | | | -- | | | -- | |
| (Increase) decrease in other assets | | | 50,610 | | | -- | |
| (Increase) in available for sale investments | | | -- | | | -- | |
| Increase (decrease) in accounts payable and accrued payables | | | -- | | | 61,705 | |
NET CASH PROVIDED (USED) IN OPERATING ACTIVITIES | | $ | 731 | | $ | (700 | ) |
| | | | | | | | |
CASH FLOWS FROM INVESTING ACTIVITIES | | | | | | | |
| Net Proceeds from sale of investments | | | -- | | | -- | |
| Net Proceeds from sale of assets | | | -- | | | -- | |
| Investment in Portfolio Company | | | -- | | | -- | |
| Goodwill acquired (given back to seller due to unwinding) | | | -- | | | -- | |
| Software received (given back due to unwinding) in purchase of Pokerbook | | | -- | | | -- | |
| (Purchases) of property, plant and equipment | | | -- | | | -- | |
NET CASH FROM INVESTING ACTIVITIES | | $ | -- | | $ | -- | |
| | | | | | | | |
CASH FLOWS FROM FINANCING ACTIVITIES | | | | | | | |
| Proceeds from borrowings under note payable | | | -- | | | -- | |
| Repayments under note payable | | | -- | | | -- | |
| Excess of outstanding checks over bank balance | | | -- | | | -- | |
| Proceeds from sale of land | | | -- | | | -- | |
| Incurrence (repayment) of advances | | | -- | | | -- | |
| Advances from stockholder | | | -- | | | -- | |
| Proceeds from issuance of common stock | | | -- | | | -- | |
| Other capital contributions | | | -- | | | -- | |
| Loan repayments | | | -- | | | -- | |
NET CASH (USED) IN FINANCING ACTIVITIES | | $ | -- | | $ | -- | |
| | | | | | | | |
NON-CASH FLOW INVESTING ACTIVITIES | | | | | | | |
| Issuance of preferred stock (acquisition of companies) | | | -- | | | -- | |
| Acquisitions of wholly owned subsidiaries, fixed assets, intangibles and liabilities | | | -- | | | -- | |
| Issuance of portfolio holding in exchange for debt reduction | | | -- | | | -- | |
| Debt reduction in exchange for portfolio holding | | | -- | | | -- | |
NET NON-CASH INVESTING ACTIVITIES | | $ | -- | | $ | -- | |
| | | | | | | | |
INCREASE (DECREASE) IN CASH | | $ | 731 | | $ | (700 | ) |
| | | | | | | | |
CASH, Beginning of Period | | $ | 29,685 | | $ | 1,252 | |
CASH, Ending of Period | | $ | 30,416 | | $ | 552 | |
The accompanying notes are an integral part of these unaudited financial statements.
INTEGRATIVE HEALTH TECHNOLOGIES, INC'spreviously known as Senticore, Inc.
(A Development Stage Company)
STATEMENTS OF STOCKHOLDERS’ DEFICIT
| | Series A | | Series A | | Additional | | Deferred Stock | | Preferred | | Preferred | | Additional | | | |
| | Common | | Common | | Paid-in | | and Interest | | Shares | | Shares | | Paid-in | | Retained | |
| | Shares | | Stock | | $Capital | | Compensation | | (000's) | | | | $$Capital | | Deficit | |
| | (000's) | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Balances, December 31, 2003 | | 40,040 | | $ | 40,040 | | $ | 492,256 | | $ | (314,950 | ) | | | | | | | | | | $ | (881,670 | ) |
| Shares canceled | | (12,000 | ) | $ | (12,000 | ) | | | | | | | | | | | | | | | | | | |
| Amortization of deferred compensation | | | | | | | | | | $ | 291,200 | | | | | | | | | | | | | |
| Issuance of stock for services | | 21,841 | | $ | 21,841 | | $ | 2,107,417 | | | | | | | | | | | | | | | | |
| Issuance of stock for interest, payable, acquisitions | | 74,150 | | $ | 74,150 | | $ | 971,603 | | | | | | | | | | | | | | | | |
Net Income (Loss) for the year | | | | | | | | | | | | | | | | | | | | | | $ | (2,835,517 | ) |
| Balances, December 31, 2004 | | 124,031 | | $ | 124,031 | | $ | 3,571,276 | | $ | (23,750 | ) | | | | | | | | | | $ | (3,717,187 | ) |
| Issuance of stock for cash | | 34,727 | | $ | 34,727 | | $ | 401,386 | | | | | | | | | | | | | | | | |
| Issuance of stock for services | | 22,387 | | $ | 22,387 | | | | | | | | | | | | | | | | | | | |
| Amortization of deferred compensation | | | | | | | | | | $ | 23,750 | | | | | | | | | | | | | |
Net Income (Loss) for the year | | | | | | | | | | | | | | | | | | | | | | $ | (55,172 | ) |
| Balances, December 31, 2005 | | 181,145 | | $ | 181,145 | | $ | 3,972,662 | | $ | - | | | | | | | | | | | $ | (3,772,359 | ) |
| Correction of an error | | | | | | | $ | 20 | | | | | | | | | | | | | | | | |
| Shares canceled | | (36,409 | ) | $ | (36,409 | ) | $ | 36,349 | | | | | | | | | | | | | | | | |
| Issuance of stock for acquisitions | | | | | | | | | | | | | | 20,000 | | $ | 200,000 | | $ | 7,269,025 | | | | |
Net Income (Loss) for the year | | | | | | | | | | | | | | | | | | | | | | $ | 858,266 | |
| Balances, December 31, 2006 | | 144,736 | | $ | 144,736 | | $ | 4,009,031 | | $ | - | | | 20,000 | | $ | 200,000 | | $ | 7,269,025 | | $ | (2,914,093 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of stock for cash | | 0 | | $ | - | | $ | - | | $ | - | | | 0 | | $ | - | | $ | - | | | | |
| Issuance of stock for acquisitions | | 0 | | $ | - | | $ | - | | $ | - | | | 0 | | $ | - | | $ | - | | | | |
| Effects of Reverse Stock Split | | 40,497,861 | | $ | (104,093 | ) | $ | 7,573,118 | | | | | | (20,000 | ) | $ | (200,000 | ) | $ | (7,269,025 | ) | | | |
Net Income (Loss) for the quarter | | | | | | | | | | | | | | | | | | | | | | $ | 11,300 | |
| Balances, March 31, 2007 | | 40,642,597 | | $ | 40,643 | | $ | 11,582,149 | | $ | - | | | 0 | | $ | - | | $ | 0 | | $ | (2,902,793 | ) |
The accompanying notes are an integral part of these unaudited financial statements.
Integrative Health Technologies, Inc.(F/K/A Senticore, Inc.)
SEPTEMBER 30, 2006
(Unaudited)
NOTES TO FINANCIAL STATEMENTS
NOTE 1. FORMATION AND OPERATIONS OF THE COMPANY
Formation
Integrative Health Technologies, Inc., formerly known as Senticore, Inc., (“IHTI”, “the Company”, “we”, “us”, or “our”) was incorporated under the laws of the state of Delaware on January 5, 1999. On February 11, 2005 IHTI elected under the Investment Company Act of 1940 (the “ICA”) to become a Business Development Company (“BDC”). This means that, from that date and continuing through the period of audit, the Company operated as a publicly-traded, closed-end investment company which, because of its BDC election, could raise money in the public sector and invest in the private sector. However, as disclosed below in the Note “Subsequent Events”, on May 6, 2007 the Company withdrew its BDC election.
Business Description
During the period of audit, IHTI was a BDC providing capital, equipment, scientific advice and extensive hands-on managerial assistance to portfolio companies in the healthcare and nutritional industries. The company also held some investments in three public companies and one private company that are not in the healthcare and nutritional industries. IHTI’s three major healthcare and nutritional portfolio companies are all wholly-owned subsidiaries of IHTI: (1) Health and Medical Research Inc. is a clinical research organization that has conducted clinical trials and research for over 20 years capitalizing on the growing importance of scientific support for the marketing of healthcare and nutritional products and technologies. (2) HealthTech Development, LLC provides consulting to healthcare and nutritional companies and product research and development including feasibility and marketing studies. (3) HealthTech Products, LLC markets clinically-tested healthcare and nutritional products.
NOTE 2. SIGNIFICANT ACCOUNTING POLICIES
Development Stage
IHTI is considered to be in the development stage as defined in Financial Accounting Standards Board Statement No. 7, and, accordingly, most of its accounting policies and procedures have not yet been established.
Basis of Presentation
Our accompanying unaudited financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America for interim financial information and the instructions to Form 10-Q and Article 6 of Regulation S-X of the Securities and Exchange Commission (the "SEC"). Accordingly, these financial statements do not include all of the footnotes required by generally accepted accounting principles. In our opinion, all adjustments (consisting of normal and recurring adjustments) considered necessary for a fair presentation have been included. Operating results for the three months ended March 31, 2007 are not necessarily indicative of the results that may be expected for the year ended December 31, 2007. The accompanying financial statements and the notes thereto should be read in conjunction with our audited financial statements as of and for the year ended December 31, 2006 contained in our Form 10-K which is incorporated by reference.
The balance sheet at March 31, 2007 has been derived from the unaudited financial statements at that date and does not include all of the information and footnotes required by generally accepted accounting principles for complete financial statements.
Management’s Use of Estimates
The preparation of financial statements in accordance with accounting principles generally accepted in the United States of America requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements. The reported amounts of revenues and expenses during the reporting period may be affected by the estimates and assumptions we are required to make. Actual results could differ from our estimates.
Stock-Based Compensation
During the period covered by these financial statements, we were a Business Development Company governed by, among other things, Sections 54 to 65 of the Investment Company Act of 1940, as amended. As such, the issuance of stock for services was generally prohibited. Certain limited stock based compensation plans are permissible, but none were implemented.
Valuation of Long-Lived and Intangible Assets
The recoverability of long-lived assets requires considerable judgment and is evaluated on an annual basis or more frequently if events or circumstances indicate that the assets may be impaired. As it relates to definite life intangible assets, we apply the impairment rules as required by SFAS No. 121, "Accounting for the Impairment of Long-Lived Assets and Assets to be Disposed Of" as amended by SFAS No. 144, which also requires significant judgment and assumptions related to the expected future cash flows attributable to the intangible asset. The impact of modifying any of these assumptions can have a significant impact on the estimate of fair value and, thus, the recoverability of the asset.
Earnings/(Loss) Per Share
We compute net earnings/(loss) per share in accordance with SFAS No. 128 "Earnings per Share” (“SFAS No. 128”) and SEC Staff Accounting Bulletin No. 98 ("SAB 98"). Under the provisions of SFAS No. 128 and SAB 98, basic net earnings/(loss) per share is computed by dividing the net earnings/(loss) available to common stockholders for the period by the weighted average number of common shares outstanding during the period. Diluted net earnings/(loss) per share is computed by dividing the net earnings/(loss) for the period by the weighted average number of common and common equivalent shares outstanding during the period. For this purpose, each share of Series A Convertible Preferred Stock was treated as 400 common equivalent shares prior to the March 26, 2007 reverse split and two common equivalent shares thereafter.
NOTE 3. INCOME TAXES
In its Form 10-KSB for the fiscal year ending 2002 the Company reported that it had available net operating loss carry-forwards for income tax purposes of approximately $82,400. In its Form 10-KSB/A for the fiscal year ending 2003 the company reported a “…net operating loss carry-forwards of approximately $494,500”. However, while the Company has yet to file its 2003, 2004, 2005 and 2006 tax returns, its cumulative net operating losses prior to the change of control in 2006 will likely preclude any taxes due for these years. Thus, even with the limitation posed by “change in control” or the Company’s failure to file the required IRS Forms 1099 for these years, it is doubtful that the Company will owe any taxes. The Company has employed a CPA to file these taxes and we are now aggressively seeking sufficient records and general ledgers to file these tax returns and the required IRS Forms 1099 associated with the filings.
NOTE 4. RELATED PARTY TRANSACTIONS
Operating Agreement with Health & Medical Research, Inc. (“HMRI”)
As reported in previous filings, the database, testing equipment and testing-related equipment acquired from IHT of Illinois was transferred into HMRI as a wholly own subsidiary or portfolio company. HMRI’s income stream is from clinical trials of which, the Bone Health study reported in previous filings was its major source of revenues since the merger and reorganization. Within its revenue stream, increases in HMRI’s asset value from the addition of new test data has been its primary source of unrealized income while its realized income has primarily come from fees assessed grantors for testing and study administrative fees. At the end of each accounting quarter, all net profits as cash or accounts have been transferred to IHTI as a BDC’s “managerial fee”. Other than excluding the income stream from the increase in the value of its database and the consolidation of financial forms instead of reporting “managerial fees,” there has been no material change in this structure with the de-election of the BDC status.
Operating Agreement with HealthTech Development, LLC. (“HTD”)
At the time of the merger and reorganization, Gilbert R. Kaats, PhD, (“GRK”) the Company’s President/CEO, owned and operated HTD a consulting and research and development company. Since the activities of this company overlapped the activities of IHTI, GRK’s continued involvement with HTD could pose a number of conflicts of interest. To avoid this possibility, GRK agreed to maintain ownership of his current intellectual property, but would make it and his networking contacts available to IHTI. GRK also agreed to provide funding as a shareholder’s loan during the initial stages of IHTI’s operation. This agreement also included the stipulation that, on or about the end of each quarter, all funds and assets earned and liabilities and loan balances incurred during the quarter would be transferred to IHTI as an BDC’s “managerial fee”, and become the sole property or responsibility of IHTI. Thus, any monies or assets earned by HTD become the property of IHTI, not GRK personally (see AlgaeCal acquisition below). Other than excluding the income stream from the increase in the value of its database and the consolidation of financial forms instead of reporting “managerial fees,” there has been no material change in this structure with the de-election of the BDC status.
Operating Agreement with HealthTech Products, LLC. (“HTP)
As reported in the independent audit of the assets received from IHT of Illinois, IHTI received an inventory valued at $132,259 consisting of healthcare products and packing and marketing materials. Acting as a distributor for these products, HTP’s revenues are derived from the wholesale and retail of the products and materials. IHTI houses and maintains the inventory and provides it to HTP on an “as needed” basis who purchases the products from IHTI based on the actual prices IHTI paid for these products. In addition to paying for the inventory, at the end of each quarter, HTP transfers 50% of all profits it earned from sales of these products to IHTI in the form of a managerial fee. Other than excluding the income stream from the increase in the value of its database and the consolidation of financial forms instead of reporting “managerial fees,” there has been no material change in this structure with the de-election of the BDC status.
Acquisition of 8% ownership in AlgaeCal International. In October 2006, HTD received 8% ownership in AlgaeCal International (valued at $320,000) as payment for pre-merger and consulting services GRK provided during 2005 and 2006 and for agreeing to conduct a 100-subject clinical trial on the safety and efficacy of AlgaeCal Pro, a bone-health program to be marketed exclusively to healthcare providers. This ownership included a 1% override on all AlgaeCal sales beginning June 2006 and exclusive rights to market AlgaeCal Pro to the healthcare industry. Thus, as reflected in the financial statements, at the conclusion of quarter ending December 31, 2006, the 8% ownership in AlgaeCal International was transferred to IHTI and IHTI assumed the liability of the remaining $120,000 for the conduct of the AlgaeCal Pro study along with the $65,812 balance due GRK for loans provided to HTD during the last quarter of 2006. This amount appeared on IHTI’s balance sheet as a shareholder’s loan.
Sale of Taj Systems shares. On August 15, 2006 The Company entered into an agreement with an investment company to purchase sufficient shares of the Company’s stock in Taj Systems, Inc., a pink-sheet company trading under the symbol TJSS, to eliminate all of the Company’s $671,927 outstanding liabilities that it had at closing. The TJSS shares in question are restricted shares, but could be converted into common stock as of August 31, 2006. However, the agreement is not contingent upon this convertibility. In January 2007, this investor had paid all but $225,171 of the agreed upon $671,927. The remaining amount is shown as an accounts payable on the current balance sheet. Upon receipt of the final payment, the Company has agreed to transfer these restricted shares to the investor. The Board of Directors has approved the sale with the restriction that all proceeds must be used for the reduction of outstanding debts, and not for operating capital or executive or consultant compensation.
Loans from shareholders. During this quarter, GRK loaned $10,252 to HTD and received $30,452 in loan repayments from HTD. At the conclusion of this quarter, these amounts were transferred to IHTI as an account receivable and an account payable. The difference between these amounts, $20,200, was applied to the $65,812 due GRK from the previous quarter thus reducing his shareholder loan to $45,612. GRK also agreed to reduce this debt to $10,812 by crediting the Company with $34,800 for 8,700 pedometers from IHTI’s inventory at the Company’s acquisition cost of $4.00 each. At the time of this filing, this is the only shareholder loan on the Company’s books.
NOTE 5. FINANCIAL HIGHLIGHTS
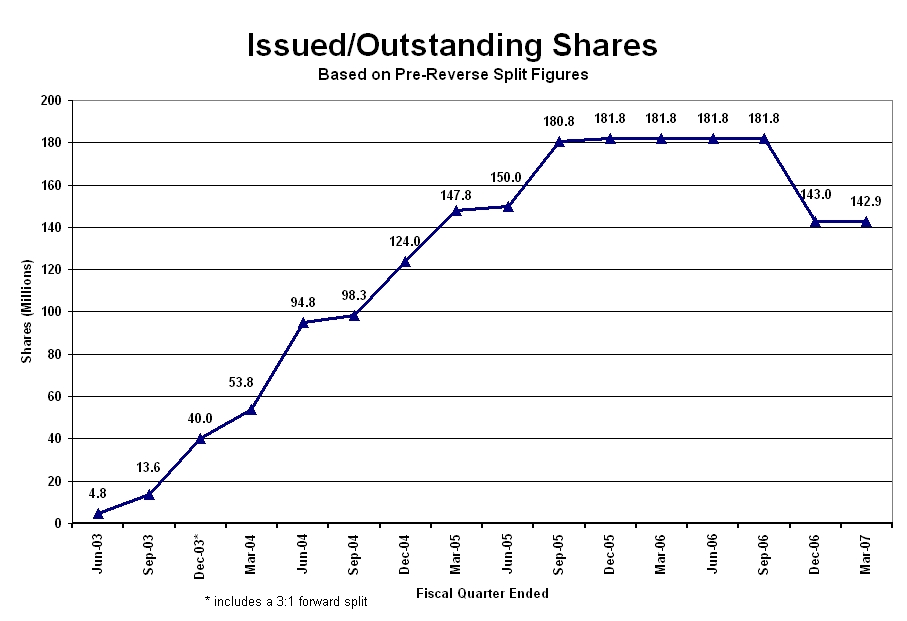
As shown in Graph-1, issuing an average of 5,000,000 shares a month from June 2003 until June 2006, no common shares have been issued in 2006 or 2007. In fact, from June 2006 to the end of this quarter, 52,740,554 shares have been recaptured for improper issuance or exchanged for TJSS shares. On April 1, 2007, the 8,000,000,000 common shares converted from the 20,000,000 preferred shares and 128,404,600 outstanding common shares were reduced to the current post-split 40,642,597 outstanding and issued shares. Absent any revenue generating investments or portfolio companies, the Company raised its operating capital from loans and from issuing an average of 15.8 million shares a month from the second quarter of 2003 until the merger and reorganization Agreement was completed in the second quarter of 2006. Starting in the second quarter of 2006, the Company has raised its capital from managerial fees received from its portfolio companies and liquidation of shares in public companies that are incompatible with the Company’s healthcare mission.
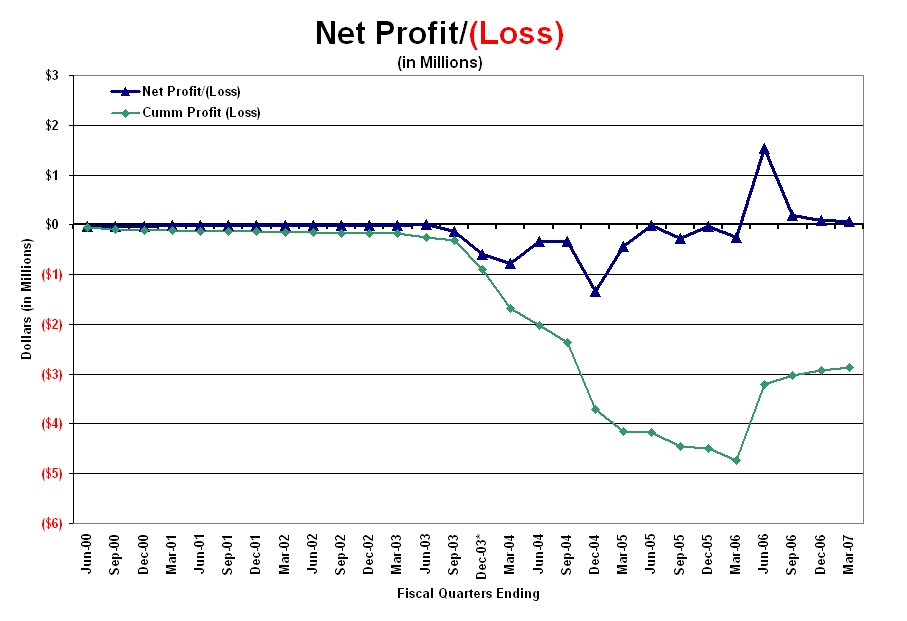
As shown in Graph-2, from its inception to the quarter ending March 31, 2006, the Company had never reported a profitable quarter for any of the 24 fiscal quarters depicted. As shown by the quarterly, as well as cumulative curves, these losses increased significantly in 2003 when the Company changed its name to Senticore, Inc. However, upon becoming Integrative Health Technologies in June 2006, net profits were reported for the first time in the quarter ending June 30, 2006 and, as of the date of this filing, the Company will report it fourth successive profitable quarter.
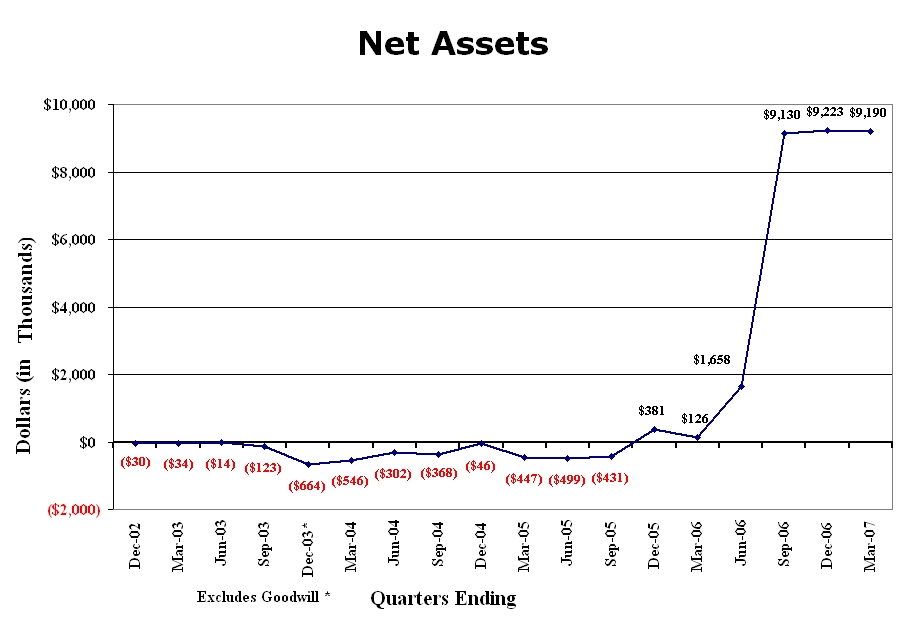
Graph-3 depicts the Company’s net assets (Total Assets minus Total Liabilities) as reported in the Company’s filings from the quarter ending December 31, 2002 though the current quarter. The Company’s liabilities have consistently exceeded its liabilities until the quarter ending December 2005 when assets exceeded liabilities—the most dramatic change occurring in the current quarter with the acquisition of Integrative Health Technologies of Illinois’ assets. The small drop in net assets this quarter is the result of unresolved accounting procedures for changes in par values as a result of the reverse split.
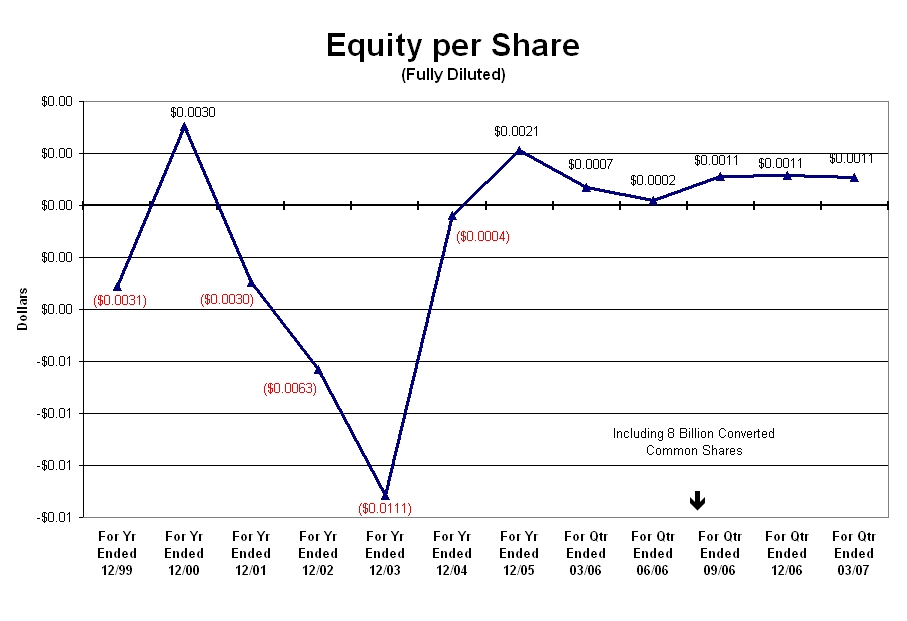
As shown in Graph-4, from the year ending 2000, the equity per share had progressively declined from $0.003 to -0.011 by the end of 2003. At that time, share equity increased to $0.002 by the end of 2005 only to decline to $0.0002 by the second quarter ending June 30, 2006, but to increase over the last two quarters of 2006 to $0.001. Thus, in spite of the issuance of 20,000,000 preferred shares (convertible into 8 billion common shares) the equity per share increased over the past two quarters of 2006.
On March 26, 2007, the Company’s common stock was the subject of a reverse split at a 200:1 ratio (the “Reverse Split”), with any resulting fractional shares being rounded up to the next whole share. Effective at the same time as the Reverse Split, the conversion ratio of the Series A Convertible Preferred Stock was changed by the same 200:1 factor, so that each share, which was previously convertible to 400 shares of common stock, became convertible into 2 shares of common stock
Subsequent to the report quarter, the following significant events have occurred.
De-electing to be Regulated as a BDC. On February 11, 2005 IHTI filed an election to become a Business Development Company (“BDC”) under the Investment Company Act of 1940 (the “ICA”). On May 7, 2007 the Company filed a notice revoking the election.
Removal from OTC.BB to Pink Sheets. The Company was unable to have its independent audit completed for the year ending 2006 due to a sudden and unexpected death in the auditors family and the contracting a serious debilitating disease. We were unable to have a replacement auditor complete the audit to meet the May 17, 2007 deadline. Therefore, NASDAQ removed the Company from the OTC.BB reclassifying it as a Pink Sheet stock. However, the auditor has now returned to work and we have been assured that the audit will be completed by the end of May and at that time we will re-apply to be moved back to the OTC.BB.
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Preliminary Note Regarding Forward-Looking Statements
This document contains “forward-looking statements” within the meaning of the private securities litigation reform act of 1995. Prospective shareholders should understand that several factors govern whether any forward - looking statement contained herein will be or can be achieved. Any one of those factors could cause actual results to differ materially from those projected herein. These forward - looking statements include plans and objectives of management for future operations, including plans and objectives relating to the products and the future economic performance of the company. Assumptions relating to the foregoing involve judgments with respect to, among other things, future economic, competitive and market conditions, future business decisions, and the time and money required to successfully complete development projects, all of which are difficult or impossible to predict accurately and many of which are beyond the control of the company. Although the company believes that the assumptions underlying the forward - looking statements contained herein are reasonable, any of those assumptions could prove inaccurate and, therefore, there can be no assurance that the results contemplated in any of the forward - looking statements contained herein will be realized. Based on actual experience and business development, the company may alter its marketing, capital expenditure plans or other budgets, which may in turn, affect the company's results of operations. In light of the significant uncertainties inherent in the forward - looking statements included therein, the inclusion of any such statement should not be regarded as a representation by the company or any other person that the objectives or plans of the company will be achieved.
The following discussion and analysis should be read in conjunction with the financial statements and Preliminary Note Regarding Forward-Looking Statements provided above. The Company is considered to be in the development stage as defined in Financial Accounting Standards Board Statement No. 7. This section also contains reports of activities and results of operations that occurred in the current quarter up to the filing date of this Form and subsequent to the quarter ending March 31, 2007. In some cases, the conditions and results of operations are reported without accompanying distinctions between the quarter in which these activities and events occurred.
Overview and History
The Company was incorporated as Hojo Holdings, Inc. on January 5, 1999 under the laws of the state of Delaware. The Company was renamed Senticore, Inc. in March 2003. It was renamed Integrative Health Technologies, Inc. (“IHTI”, “the Company”, “we”, “us”, or “our”) on August 1, 2006.
From approximately August 31, 2006 through May 7, 2007, IHTI was a BDC providing capital, equipment, scientific advice and extensive hands-on managerial assistance to portfolio companies in the healthcare and nutritional industries. The company also held investments in three public companies and one private company that are not in the healthcare and nutritional industries. In general, IHTI could take majority or minority ownership in its healthcare and nutritional portfolio companies, although its three major healthcare and nutritional portfolio companies are all wholly-owned subsidiaries of IHTI: (1) Health and Medical Research Inc. is a clinical research organization that has conducted clinical trials and research for over 20 years, capitalizing on the growing importance of scientific support for the marketing of healthcare and nutritional products and technologies. (2) HealthTech Development, LLC provides consulting to healthcare and nutritional companies and product research and development including feasibility and marketing studies. (3) HealthTech Products, LLC markets clinically-tested healthcare and nutritional products. Subsequent to May 7, 2007, IHTI remains in the same businesses, but now as a normal operating company, not a BDC.
On February 11, 2005 Senticore, Inc. (“Senticore”) elected to be regulated as a business development company or “BDC” under the ICA. A BDC is a publicly traded, “closed-end investment company” which means, among other things, that its assets are typically illiquid and not readily converted to cash through sales in the open market. BDCs, as has been the case with Senticore, typically raise operating capital though the issuance of shares and through borrowing funds and pledging their shares as collateral. This has an inherent dilutive effect on the value of shares as more shares are issued into the market place. A typical advantage of a BDC is that it can issue shares comparatively easily under Regulation E without the more stringent and involved requirements faced by a typical operating company. Another characteristic of a BDC is that its reported net profits or losses are heavily influenced by increases or decreases in the value of its portfolio companies, which can offset or attenuate net profits or losses from the portfolio companies’ operations. Hence, even if the underlying performance of a BDC’s portfolio companies is stable, its reported profits or losses may fluctuate widely from quarter to quarter based on market perception of the potential of various portfolio companies.
On May 12, 2006 Senticore entered into an Agreement and Plan of Reorganization (the “Agreement”) with the common stockholders of Integrative Health Technologies, Inc., an Illinois Corporation (heretofore referred to as “IHT-IL” or the “IHT-IL Stockholders”) and Jay Patel. A copy of this Agreement was filed with our Form 8-K on May 12, 2006 and is incorporated by reference herein. Pursuant to the terms of the Agreement, all of the 80,000,000 shares of common stock, $.0001 par value, of IHT-IL (the “IHT-IL Common Stock”) issued and outstanding prior to the closing, were exchanged in the aggregate for 20,000,000 shares of Series A Convertible Preferred Stock, $.001 par value, of IHTI (the “Convertible Preferred Stock”). As a result of the exchange, IHT-IL became a wholly-owned subsidiary of IHTI, and the IHT-IL Stockholders gained voting control of IHTI.
On or after June 3, 2006 each share of Convertible Preferred Stock originally had the right to convert into 400 shares of common stock, $.001 par value, of us (the “Common Stock”). Until converted, each share of Convertible Preferred Stock had the right to vote on an “as converted” basis on matters submitted for vote to holders of our Common Stock. Prior to the exercise of any conversion rights, holders of Convertible Preferred Stock and holders of Common Stock had 97.7% and 2.3% respectively of the voting power. As of the end of the quarter ending March 31, 2007, all Convertible Preferred Stock had been converted into Common Stock which is now the only class of stock outstanding.
As stated above, we filed a Form N-54A with the SEC on February 11, 2005 and elected to become a business development company subject to Sections 55 through 65, among others, of the ICA, as amended. As a result of the Agreement described above, IHT-IL has become our largest portfolio company. In addition, the management of IHT-IL has become our new management, after the filing of a Schedule 14F-1 with the SEC and the required waiting period. The Agreement closed on June 3, 2006.
On May 16, 2006 the Company filed a Form 8-K setting forth the conditions of the Agreement as stated above and it is incorporated by reference herein. On June 3, 2006 the Company filed a Form 8-K reporting the completion and closing of the Agreement effective June 3, 2006 and it is incorporated by reference herein. Senticore’s CEO, Jay Patel resigned, and Gilbert R. Kaats, Ph.D. became Chairman of the Board of Directors. As part of the Closing Agreement, the Company agreed to reduce its current liabilities by $736,400 through the exchange of shares that the Company held in Taj Systems, Inc. (“TJSS”) for loans due to shareholders and accounts payable, including back wages to previous management. On June 10, 2006 the Company filed and sent to shareholders of record a Form 14F-1 announcing the Agreement, the closing of the Agreement, and changes in the directors. Said form is incorporated by reference herein.
A two-year audit of IHT-IL, was conducted by an independent auditor for the two year period from the inception of IHT-IL in August 2004 to July 31, 2006. The value of the IHT-IL assets as of July 31, 2006 as shown the audited statements was $7,626,273. The results of the audit were reported on a Form 8-K filed in August 2006 and it is incorporated by reference herein.
On August 31, 2006 IHTI took title to IHT-IL’s assets and IHT-IL subsequently transferred all of its testing equipment and its extensive longitudinal database described in Exhibit 99.1 hereto to Health and Medical Research, Inc., IHTI’s clinical research portfolio company. IHT-IL’s inventory was transferred to HealthTech Products, LLC, IHTI’s portfolio company marketing health enhancing products and technologies. A third portfolio company, HealthTech Development, LLC, a consulting and research and development (“R & D”) company was acquired at no cost at the same time, giving IHTI three wholly-owned portfolio companies providing different but highly compatible functions. Additionally, IHTI acquired IHT-IL’s scientific advisory board of highly qualified and internationally known scientists and healthcare practitioners as listed in Exhibit 99.2 hereto.
Changes in Investment Strategy and Operating Policies and Actions Taken by Current Management
Overview. As reported in its previous filings, when it assumed control on June 3, 2006, our current management team implemented a number of significant changes to the Company’s previous investing strategy and operating policies. These included but were not limited to cutting costs, focusing the Company on the healthcare and nutritional industries, divesting of unrelated assets, effecting a 200:1 reverse split of the Company’s common stock with a commensurate change in the conversion rights of the Series A Convertible Preferred Stock, obtaining the agreement of IHT-IL’s Scientific Advisory Board to serve as IHTI’s Scientific Advisory Board, recapturing shares issued for unconsummated acquisitions or which merited recapture for various other reasons, and de-electing BDC status. Except as set out below, these changes are largely complete as of the date of this filing.
IHT-IL’s Scientific Advisory Board agreed to serve as IHTI’s Scientific Advisory Board. After the acquisition of IHT-IL, its Scientific Advisory Board, as listed in Exhibit 99.2, agreed to serve as IHTI’s Scientific Advisory Board. The acquisition of IHT-IL’s assets and Scientific Advisory Board brought a new revenue stream to the Company from managerial fees from its three portfolio companies as discussed below. The Scientific Advisory Board is being compensated by our major shareholders at no expense to the Company.
The number of outstanding shares had been reduced by recapturing shares issued for unconsummated acquisitions. The current management team has pursued a plan to recapture shares that were previously issued in connection with acquisitions that were not consummated. The Company reported in previous filings that it had successfully recaptured over 22% of the shares previously reported as outstanding and there are still a number of shares with questionable substantiation for their issuance. Historically, at closing, the Company had 181,145,125 (905,726 post-reverse) issued and outstanding shares of common stock and 20,000,000 preferred shares convertible into common stock at a 1:400 ratio yielding 8,000,000,000 (40,000,000) additional shares of common stock for a fully-diluted total of 8,181,145,125 (40,905,726). Of this total, 107,593,480 (537,967) were freely trading in the “float” and 7,355,800 (367,759) were on the restricted list.
Using only post-reverse share totals, IHTI now has 40,623,366 issued and outstanding shares, 534,914 of which are in the “float” and 88,552 remain on the restricted list. The number of restricted shares has been reduced by 76% from 367,759 to 88,552 as a function of recapturing invalidly issued shares. Another 70,256 restricted shares have been placed on a stop-transfer administrative hold pending legal action to have they recaptured and voided.
Plan to liquidate all investments unrelated to the healthcare and nutrition industries. Although, as reported in previous filings, the Company has divested itself of various assets unrelated to the healthcare and nutrition industries, it continues to hold some shares in TJSS and in other public companies resulting from investments made by previous management prior to June 3, 2006. Included are shares in Adzone Research, Beere Financial and The Justice Fund. Over time, management expects to liquidate its position in most or all of these investments and apply any funds received to debt reduction and to investment in core activities in the healthcare and nutritional industries.
Operating expenses for the quarter have been met through managerial fees received from its portfolio companies The Company did not issue any new shares during the consecutive four quarters ended March 31, 2007, funding its activities instead from management fees paid to the Company by its portfolio companies. The Company’s requirement that all of its portfolio companies provide a percentage of their gross receipts from sales and services provided sufficient capital to meet our operating needs this quarter.
Activities of Portfolio Companies
Health and Medical Research, Inc. (“HMRI”), A Clinical Research Organization
Grant received for database analyses
Mannatech Inc. (NASDAQ:MTEX) announced the award of its grant to IHTI for analysis of IHTI’s data base in a March 28, 2007 press release incorporated into this filing as EXHIBIT 99.3.
Testing at Mannatech International’s Annual Sales Meeting
HMRI provided its four mobile testing units to HealthTech Development, LLC. (HTD) to market on-site testing to Mannatech’s sales associates as part of their training program. During the conference, HTD marketed 527 bone-density/body composition tests and 133 blood chemistry test panels. HMRI administered 560 Quality of Life Questionnaires, product usage, and Depression scales which, along with the DEXA and blood tests, were added to HMRI’s database.
The AlgaeCal Bone-Health study
This study is discussed in previous filings and, at the time of this filing, 92% of all subjects have completed the study and these data are now being analyzed for safety and efficacy. Preliminary results suggest that the Bone-Health program is successful in improving bone health, particularly in adolescents and post-menopausal women. If these initial findings are supported in the final report, IHTI’s product marketing portfolio company, HealthTech Products, LLC, will add the Bone-Health Program to its inventory of products.
The AlgaeCal Pro study
HMRI began the AlgaeCal Pro study to evaluate the safety and efficacy of a bone-health program designed to be marketed exclusively by healthcare providers. It includes the basic components of the AlgaeCal Bone Health program except that it increases the Vitamin D-3 levels to 2,000 IU and adds Vitamin C, Boron and higher levels of magnesium to the formulation. To increase compliance, it is packaged in individual and numbered daily servings.
Additional clinical trials and study activities
HMRI continues its involvement with the preparation of study protocols, the review of the scientific literature, and the conducting of pilot studies for a number of clients that may or may not eventuate in grants. These studies involve the effects of nutritional supplements on the growth and development of impoverished children that will be housed in the Hobbs House Of Hope in Kitengela, Kenya, Africa; a supplement to treat toe fungus; a variety of wild garlic supplement to maintain healthy cholesterol levels; and the effects of a sugar substitute, trehalose, on diabetic and pre-diabetic subjects. HMRI is currently preparing a manuscript for publication in a peer-reviewed medical journal reporting the results of a study previously completed on the effects of a glucomannan soluble fiber on weight loss and compliance.
HealthTech Development (“HTD”) A Consulting, Research & Development (R&D) Company
Testing at Mannatech International’s Annual Sales Meeting. HTD positioned four of the Company’s mobile testing units at Mannatech’s annual sales and training conference in Dallas, Texas from March 28 through April 1, 2007. This activity resulted in the sale of 527 Bone Density/Body Composition tests and 133 43-panel blood chemistry tests. These tests were added to HMRI’s database after generating $52,825 of income for HTD. However, since these amounts were largely received with checks and credit cards which were processed subsequent to the conference, this income will be recorded in the quarter ending June 30, 2007.
Acquisition of 8% Ownership of AlgaeCal International. During this quarter, the Company is finalizing its oral Agreement with AlgaeCal International (www.algaecal.com) for acquisition of an 8% interest in AlgaeCal International in return for research and development services provided by HTD. The ownership includes a 1% override on all AlgaeCal sales. The monthly average sales have increased 55% over the monthly average at the beginning of the quarter. The Agreement also includes granting IHTI the exclusive rights to market AlgaeCal Pro, a calcium-strontium vitamin-mineral enhanced supplement to be marketed exclusively to healthcare providers. AlgaeCal Pro will be marketed by HealthTech Products, LLC beginning June 2007.
Research for pilot studies developed or under development. HTD continues to develop research protocols for several studies examining the safety and efficacy of different supplements thought to: (1) reduce stress, (2) provide appetite control, (3) reduce incontinence, (4) improve glucose control, (5) aid in weight control and (6) reduce “at risk” cholesterol levels. Should any of these protocols result in the funding of clinical trials, such trials will be conducted by Health and Medical Research, Inc. However, while all of these protocols are being developed during the reporting period, past experience has taught us that less than half will eventually result in funded clinical trials.
HealthTech Products (“HTP”), Marketing Clinically Validated Healthcare Products.
See Related Party Transactions above with regard to HealthTech Products, LLC. HTP continued to report a net profit from sales this quarter and, as of the date of this filing, has begun marketing the AlgaeCal Pro line to healthcare providers and professionals.
Results of Operations
Additional information. The financial results of our operation during this quarter are summarized in the graphs and the supporting comments under FINANCIAL HIGHLIGHTS above. The section on Changes in Investment Strategy and Operating Policies and Actions Taken by Current Management provides a summary of our day to day operations. Additionally, our website (www.ihtglobal.com) provides a copy of this 10-Q and additional information that relates to the Results of Operations. However, since we have withdrawn our BDC status, in order to clarify the impact it has had on our past operations and the impact it could have on future operations will require a review of how profits and losses are determined for BDCs.
How portfolio companies impact a BDCs profit or loss. To understand the potential impact of our withdrawal of our BDC status during this quarter will require an understanding of how BDCs, as compared to with non-BDCs, compute and report profits and losses. For the purposes of this discussion, a “portfolio company” will refer to a company in which a BDC has invested, whether wholly-owned or partially-owned, publicly traded or not.
BDCs must continually track the value of their investments in portfolio companies. Any increase in value during a period is reported as a profit for that period, while a decrease in value during a period is reported as a loss for that period. If the portfolio companies are public, the valuation is derived from the ending bid or trading price of the stock on the date of the filing. If the portfolio companies are not public, the BDC must establish an appropriate valuation methodology. In either case, the principle is the same: all the portfolio companies are valued at the beginning of the period and at the end of the period, and the change in these values during the period is reported as a profit or loss.
How portfolio companies impact a non-BDCs profit or loss. A non-BDC computes its profit and loss from subsidiaries according to the consolidation rules under generally accepted accounting principles (“GAAP”). These rules are too complex to fully discuss here, but the general idea is that the subsidiaries’ individual profits and losses are consolidated into the non-BDC parent’s income statement. For example, a non-BDC parent with two wholly-owned subsidiaries that each made a profit of $1,000 during the period would typically have a profit of $2,000 for the period ,subject to any “consolidation adjustments”.
Change in BDC status causes change in focus for profit and loss performance. The point of the discussion in the previous paragraphs is that while the Company was a BDC, its profits or losses were determined primarily from the increase or decrease in the value of the portfolio companies. Now that the BDC status has been de-elected, the Company’s profits and losses will no longer be impacted by changes in the value of its subsidiaries, but rather by the profit and loss performance of the subsidiaries.
Restatement of financial statements. Since the financial statements going forward will be prepared on a different basis than the historical statements, the Company intends to restate some of its financial statements to facilitate comparisons omparisons between future and past performance. Management believes that the restated financial statements , when available, will show the Company earned a profit under the direction of the current management (June 3, 2006-March 32, 2007.
Critical Accounting Policies
The preparation of financial statements in conformity with accounting principles generally accepted in the U.S., or GAAP, requires the Company to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. In recording transactions and balances resulting from business operations, the Company uses estimates based on the best information available. The Company uses estimates for such items as depreciable lives and the amortization period for deferred income. The Company revises the recorded estimates when better information is available, facts change or actual amounts can be determined. These revisions can affect operating results.
The critical accounting policies and use of estimates are discussed in and should be read in conjunction with the annual consolidated financial statements and notes included in the latest 10-KSB, as filed with the SEC, which includes audited consolidated financial statements for the fiscal year ended December 31, 2005.
Item 3. Quantitative and Qualitative Disclosures about Market Risk
(1) Uncertainties of reorganization and restructuring We are continuing to implement a new strategy and new policies as part of the reorganization and restructuring which began on June 3, 2006. Such activities always carry risk as they can place a heavy burden on management time, they require expenditures, and there is never assurance that new strategies or policies will succeed as planned.
(2) Uncertainties of the effects of the Company’s prior BDC status
As disclosed in previous filings, from the time the Company filed its election to become a BDC through the time it withdrew the election, the Company did not fully comply with all regulatory requirements relevant to a BDC. The company has attempted to cure past violations as best it can, and it has now withdrawn its BDC election. However, withdrawing of the election to be treated as a BDC does not cure any past violations that may have occurred. If there were any violations of the securities laws, remedies for shareholders can include a rescission of a shareholder's investment, fines and penalties, and removal of officers and directors from office.
(3) Uncertainties of the effects of unresolved issues inherited from the previous management Since taking over management of the Company on June 3, 2006, current management has uncovered a number of issues that were unresolved by the previous management, some of which are contrary to representations made in the Closing and Merger and Reorganization Agreement. Although we have resolved some of these issues, many remain unresolved. Investors bear the risk that all unresolved issues inherited from previous management have yet to be identified. There is also a risk that some or all of these issues may be incapable of resolution. There is also a risk that some or all of these issues may be more serious than they appear at this time, or they may be more costly to resolve than expected, or both.
General Nature of Unresolved Issues The general nature of these unresolved issues are gaps in record-keeping and apparent instances of regulatory non-compliance. Among other things, they affect the Company’s tax compliance, its SEC record-keeping requirements, and its shareholder list. A non-exhaustive selection of examples is set forth below:
| 1) | Despite a representation by previous management to the contrary, no income tax returns have been filed since Senticore acquired HOJO Holdings in March 2003. |
| 2) | The state of the Company’s records has not allowed current management to ensure that all of the required IRS Forms 1099 for stock based compensation and contract labor have been filed. |
| 3) | As of the date of this filing and despite repeated requests made to the Company’s previous management and accountants, we have yet to receive General Ledger information for the period from March 2003 to March 2004 sufficient to allow current management to demonstrate that the Company is in compliance with SEC record-keeping requirements. |
| 4) | The state of the Company’s records does not allow current management to confirm that a share log has been maintained that conforms to SEC record-keeping requirements and that allows current management to answer questions that have arisen with respect to the proper number of shares outstanding. These questions include whether shares were issued for inadequate consideration, whether shares were issued in violation of regulations applicable to BDCs, and whether shares that were to be held in escrow were in fact so held, and if so, whether the terms of the escrows were complied with. The questions concerning inadequate consideration arise mainly in connection with a lack of record-keeping that would allow a reconciliation of the Company’s financial records to its records of issued shares, and in connection with a lack of record-keeping that would accurately track loan proceeds said to have been received but not repaid relating to loans for which shares were issued as collateral. The questions concerning BDC regulations arise because BDCs are prohibited from issuing shares to pay for services rendered, and the Company may have issued such shares while it was a BDC. The escrow questions arise because shares were issued to various parties to be held in escrow pending the completion of acquisitions that were never consummated (the Westar and Smith-Forestal transactions), and these shares were not returned to the Company, and it appears that these shares should have been returned to the Company once it became clear that the transactions would not close. |
| 5) | The minutes of the meetings of the Company’s Board of Directors for periods prior to June 3, 2006 appear to be incomplete. |
| 6) | The state of the Company’s records does not allow current management to fully understand the history of the Company’s relationship to Taj Systems, Inc. (“TJSS”) and the history of prior management’s relationship to TJSS. The President and CEO of TJSS are the former President and CEO of the Company and assumed these positions while serving in the same capacity with the Company. From public filings made before current management became involved with the Company on June 3, 2006, it appears that the Company at one time acquired approximately 44 million shares of TJSS representing 40% of TJSS issued stock. For reasons unclear to current management, it appears that the Company subsequently exchanged its 40% ownership interest in TJSS for a certificate representing 1.4 million convertible preferred shares of TJSS, each convertible to 5 shares of common stock representing a total of 7 million shares of common stock on an “as converted” basis. Since the certificate is for restricted shares, it cannot be converted into free-trading shares until August 31, 2007. Although, these shares could have been converted into free-trading common stock under Rule 144 exemption on August 31, 2006, TJSS management has been unresponsive to our repeated demands to approve the Rule 144 exemption. |
Item 4. Controls and Procedures
Quarterly Evaluation of Controls
As of the end of the period covered by this quarterly report on Form 10-Q, we evaluated the effectiveness of the design and operation of (i) our disclosure controls and procedures ("Disclosure Controls"), and (ii) our internal control over financial reporting ("Internal Controls"). This evaluation ("Evaluation") was performed by our Chairman and Chief Executive Officer, Gilbert R. Kaats, ("CEO/CFO"). In this section, we present the conclusions of our CEO/CFO based on and as of the date of the Evaluation, (i) with respect to the effectiveness of our Disclosure Controls, and (ii) with respect to any change in our Internal Controls that occurred during the most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect our Internal Controls.
CEO/CFO Certifications
Attached to this annual report, as Exhibits 31.1 and 31.2, are certain certifications of the CEO/CFO, which are required in accordance with the Exchange Act and the Commission's rules implementing such section (the "Rule 13a-14(a)/15d-14(a) Certifications"). This section of the annual report contains the information concerning the Evaluation referred to in the Rule 13a-14(a)/15d-14(a) Certifications. This information should be read in conjunction with the Rule 13a-14(a)/15d-14(a) Certifications for a more complete understanding of the topic presented.
Disclosure Controls and Internal Controls
Disclosure Controls are procedures designed with the objective of ensuring that information required to be disclosed in our reports filed with the Commission under the Exchange Act, such as this annual report, is recorded, processed, summarized and reported within the time period specified in the Commission's rules and forms. Disclosure Controls are also designed with the objective of ensuring that others make material information relating to the Company known to the CEO/CFO, particularly during the period in which the applicable report is being prepared. Internal Controls, on the other hand, are procedures which are designed with the objective of providing reasonable assurance that (i) our transactions are properly authorized, (ii) the Company's assets are safeguarded against unauthorized or improper use, and (iii) our transactions are properly recorded and reported, all to permit the preparation of complete and accurate financial statements in conformity with accounting principals generally accepted in the United States.
Limitations on the Effectiveness of Controls
Our management does not expect that our Disclosure Controls or our Internal Controls will prevent all error and all fraud. A control system, no matter how well developed and operated, can provide only reasonable, but not absolute assurance that the objectives of the control system are met. Further, the design of the control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within the Company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the control. The design of a system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated objectives under all potential future conditions. Over time, control may become inadequate because of changes in conditions, or because the degree of compliance with the policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
Scope of the Evaluation
The CEO/CFO's evaluation of our Disclosure Controls and Internal Controls included a review of the controls' (i) objectives, (ii) design, (iii) implementation, and (iv) the effect of the controls on the information generated for use in this annual report. In the course of the Evaluation, the CEO/CFO sought to identify data errors, control problems, acts of fraud, and they sought to confirm that appropriate corrective action, including process improvements, was being undertaken. This type of evaluation is done on a quarterly basis so that the conclusions concerning the effectiveness of our controls can be reported in our quarterly reports on Form 10-Q and annual reports on Form 10-K. The overall goals of these various evaluation activities are to monitor our Disclosure Controls and our Internal Controls, and to make modifications if and as necessary. Our external auditors also review Internal Controls in connection with their audit and review activities. Our intent in this regard is that the Disclosure Controls and the Internal Controls will be maintained as dynamic systems that change (including improvements and corrections) as conditions warrant.
Among other matters, we sought in our Evaluation to determine whether there were any significant deficiencies or material weaknesses in our Internal Controls, which are reasonably likely to adversely affect our ability to record, process, summarize and report financial information, or whether we had identified any acts of fraud, whether or not material, involving management or other employees who have a significant role in our Internal Controls. This information was important for both the Evaluation, generally, and because the Rule 13a-14(a)/15d-14(a) Certifications, Item 5, require that the CEO/CFO disclose that information to our Board (audit committee), and to our independent auditors, and to report on related matters in this section of the annual report. In the professional auditing literature, "significant deficiencies" are referred to as "reportable conditions". These are control issues that could have significant adverse affect on the ability to record, process, summarize and report financial data in the financial statements. A "material weakness" is defined in the auditing literature as a particularly serious reportable condition where the internal control does not reduce, to a relatively low level, the risk that misstatement cause by error or fraud may occur in amounts that would be material in relation to the financial statements and not be detected within a timely period by employee in the normal course of performing their assigned functions. We also sought to deal with other controls matters in the Evaluation, and in each case, if a problem was identified; we considered what revisions, improvements and/or corrections to make in accordance with our ongoing procedures.
Conclusions
Based upon the Evaluation, the Company's CEO/CFO has concluded that, subject to the limitations noted above, our Disclosure Controls are effective to ensure that material information relating to the Company is made known to management, including the CEO/CFO, particularly during the period when our periodic reports are being prepared, and that our Internal Controls are effective to provide reasonable assurance that our financial statements are fairly presented in conformity with accounting principals generally accepted in the United States. Additionally, there has been no change in our Internal Controls that occurred during our most recent fiscal quarter that has materially affected, or is reasonably likely to affect, our Internal Controls.
PART II. - OTHER INFORMATION
Item 1. Legal Proceedings
NONE.
Item 2. Unregistered Sale of Equity Securities and Use of Proceeds
NONE.
Item 3. Defaults Upon Senior Securities
NONE.
Item 4. Submission of Matters to a Vote of Security Holders
[Annual meeting?]
Item 5. Other Information
NONE
ITEM 6. EXHIBITS AND REPORTS ON FORM 8-K
(b) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
SIGNATURE | | TITLE | | DATE |
| | | | | |
| /s/ Gilbert R. Kaats | | Chairman and CEO | | May 21, 2007 |
| | | | | |
| /s/ Gilbert R. Kaats | | Chief Financial Officer | | May 21, 2007 |