Exhibit 99.2
PORTAGE BIOTECH INC.
THREE AND SIX MONTHS ENDED SEPTEMBER 30, 2024
MANAGEMENT’S DISCUSSION AND ANALYSIS
Prepared as of November 26, 2024
TABLE OF CONTENTS
Management Discussion and Analysis
The following discussion and analysis by management of the financial condition and financial results for Portage Biotech Inc. for the three and six months ended September 30, 2024, should be read in conjunction with the unaudited condensed consolidated interim financial statements for the three and six months ended September 30, 2024, together with the related Management’s Discussion and Analysis and audited consolidated financial statements for the year ended March 31, 2024, and the annual report on Form 20-F (our “Annual Report”) for the fiscal year ended March 31, 2024 (“Fiscal 2024”).
Forward-Looking Statements
This document includes “forward-looking statements.” All statements, other than statements of historical facts, included herein or incorporated by reference herein, including without limitation, statements regarding our business strategy, plans and objectives of management for future operations and those statements preceded by, followed by or that otherwise include the words “believe,” “expects,” “anticipates,” “intends,” “estimates,” “will,” “may,” “should,” “could,” “targets,” “projects,” “predicts,” “plans,” “potential,” or “continue,” or similar expressions or variations on such expressions are forward-looking statements. We can give no assurances that such forward-looking statements will prove to be correct.
Each forward-looking statement reflects our current view of future events and is subject to risks, uncertainties and other factors that could cause actual results to differ materially from any results expressed or implied by our forward-looking statements.
We have made the decision to discontinue our sponsored trial for the invariant natural killer T-cell (“iNKT”) program and pause further patient accrual to our sponsored adenosine program for both PORT-6 and PORT-7. In the event that we resume these clinical trials and further development of our programs, our risks and uncertainties include, but are not limited to:
| • | our plans and ability to develop and commercialize product candidates and the timing of these development programs; |
| • | clinical development of our product candidates, including the timing for availability and release of results of current and future clinical trials; |
| • | our expectations regarding regulatory communications, submissions or approvals; |
| • | the potential functionality, capabilities, benefits and risks of our product candidates as compared to others; |
| • | our maintenance and establishment of intellectual property rights in our product candidates; |
| • | our need for financing and our estimates regarding our capital requirements and future revenues and profitability; |
| • | our estimates of the size of the potential markets for our product candidates; and |
| • | our selection and licensing of product candidates |
Our business focus has been that of a pharmaceutical development business subject to all of the risks of a pharmaceutical development business. In the event that we resume enrollment in the clinical trials and further development of our programs, we do not anticipate directly engaging in the commercialization of the product candidates we develop.
These statements are based on assumptions and analyses made by us in light of our experience and our perception of historical trends, current conditions and expected future developments based on the focus of our business activities on biotechnology, as well as other factors we believe are appropriate in particular circumstances. However, whether actual results and developments will meet our expectations and predictions depends on a number of risks and uncertainties, which could cause actual results to differ materially from our expectations, including the risks set forth under the heading “Business Environment – Risk Factors” below and in Item 3 “Key Information – Risk Factors” in our Annual Report on Form 20-F for the year ended March 31, 2024.
Consequently, all of the forward-looking statements made in this Management’s Discussion and Analysis are qualified by these cautionary statements. We cannot assure you that the actual results or developments anticipated by us will be realized or, even if substantially realized, that they will have the expected effect on us or our business or operations.
Unless the context indicates otherwise the terms “Portage Biotech Inc.,” “the Company,” “our Company,” “Portage,” “we,” “us” or “our” are used interchangeably in this Management’s Discussion and Analysis and mean Portage Biotech Inc. and its subsidiaries. Capitalized terms used but not defined herein have the meaning ascribed to such terms in our unaudited condensed consolidated interim financial statements for the three and six months ended September 30, 2024.
Nature of Operations and Overview
Due to our future funding needs for clinical development of our programs as well as the current capital raising market for biotechnology companies, we made the decision to discontinue the IMPORT-201 trial (PORT-2) and to pause further patient accrual to the ADPORT-601 trial (PORT-6 and PORT-7). The PORT-3 investigator trial is continuing, and all existing patients in the ADPORT-601 study will continue until disease progression. We are continuing to collect and analyze data from these patients. We replaced a patient who withdrew and is unevaluable for the 28-day dose limiting toxicity (“DLT”) period. We are exploring strategic alternatives, which may include finding a partner for one or more of our assets, a sale of our company, a merger, restructurings, both in and out of court, company winddown, further financing efforts or other strategic action. The following discussion reflects our operations in the event we are able to raise additional capital to fund the clinical development of our programs.
We are a clinical stage immuno-oncology company that advances treatments, in which we believe will be first-in-class therapies that target known checkpoint resistant pathways, to improve long-term treatment response and the quality of life in patients with invasive cancers.
Our access to next-generation technologies has provided us the capability to identify and understand biological mechanisms, clinical therapies and product development strategies that have supported our programs through the translational pipeline and into the clinic.
We have sourced and developed early- to mid-stage treatments that we believe will be first-in-class therapies for a variety of cancers, by funding, implementing viable, cost-effective product development strategies, clinical counsel/trial design, shared services, financial and project management to enable efficient, turnkey execution of commercially informed development plans. Our drug development pipeline portfolio continues to encompass product candidates or technologies based on biology addressing known resistance pathways/mechanisms of current checkpoint inhibitors with established scientific rationales.
Our Board of Directors approved a reverse stock split of our ordinary shares at a ratio of 1-for-20. Beginning with the opening of trading on August 15, 2024, our ordinary shares began trading on Nasdaq on a split-adjusted basis under the existing trading symbol “PRTG”. The reverse stock split was implemented to increase the per share trading price of our ordinary shares for the purpose of ensuring a share price high enough to comply with the minimum $1.00 bid price requirement for continued listing on Nasdaq. We received notice from Nasdaq on August 30, 2024 informing us that we had regained compliance with the minimum $1.00 bid price requirement for continued listing on Nasdaq.
The Portage Approach
Our mission has been to advance and grow a portfolio of innovative, early-stage oncology assets based on the latest scientific breakthroughs focused on overcoming immune resistance and expanding the addressable patient population. Given these foundations, we have managed capital allocation and risk as much as we have overseen drug development. By focusing our efforts on translational medicine and pipeline diversification, we have sought to mitigate overall exposure to many of the inherent risks of drug development.
Our approach has been guided by the following core elements:
| • | Portfolio diversification to mitigate risk and maximize optionality; |
| • | Capital allocation based on risk-adjusted potential, including staged funding to pre-specified scientific and clinical results; |
| • | Virtual infrastructure and key external relationships to maintain a lean operating base; |
| • | Internal development capabilities complemented by external business development; |
| • | Rigorous asset selection for broad targets with disciplined ongoing evaluation; |
| • | Focus on translational medicine and therapeutic candidates with single agent activity; |
| • | Conduct randomized trials early and test non-overlapping mechanisms of action; and |
| • | Improve potential outcomes for patients with invasive cancers. |
We have executed such approach through our internal core team and our network of experts, contract labs and academic partners.
Our Science Strategy
Prior to our decision to discontinue our iNKT IMPORT-201 sponsored trial and pause further patient accrual in the adenosine program, our goal has been to develop immuno-oncology therapeutics that will dramatically improve the standard-of-care for patients with cancer. The key elements of our scientific strategy have been to:
| • | Build a pipeline of differentiated oncology therapeutic candidates that are diversified by mechanism, broad targets, therapeutic approach, modality, stage of development, leading to a variety of deal types that can be executed with partners; |
| • | Expand our pipeline through research collaborations, business development and internally designed programs; |
| • | Continue to advance and evolve our pipeline; and |
| • | Evaluate strategic opportunities to accelerate development timelines and maximize the value of our portfolio. |
Our Pipeline
We have built a strong portfolio of immuno-oncology therapeutic candidates and programs that are diversified by mechanism, therapeutic approach, modality and stage of development. Prior to our decision to discontinue further development of our iNKT sponsored trial and pause further patient accrual to our sponsored adenosine program, we rigorously assessed each of our programs on an ongoing basis using internally defined success criteria to justify continued investment and determine proper capital allocation. When certain programs do not meet our de-risking criteria for advancement, we look to monetize or terminate those programs and preserve our capital and resources to invest in programs with greater potential.
The charts set forth below, illustrate the state of our immuno-oncology therapeutic product candidates and programs before development activities were discontinued and/or paused. At this time PORT-3 is the only candidate currently actively recruiting and being evaluated as part of an investigator sponsored study without funding from us. Additionally, notwithstanding our decision to pause further development of PORT-2 and PORT-7 both INDs continue to remain active and the clinical trial for PORT-6 remains open, with further enrollment into additional cohorts still on pause. We undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. Before you make an investment decision regarding us, you should make your own analysis of forward-looking statements and our projections about candidate and program development and results.
Our Business Model
We are a development organization that is structured to facilitate flexibility in financing and ease of partnering, licensing, and merger/acquisition of individual assets and or technology platforms. The intellectual property (“IP”) for each platform is held in separate private entities. Our employees and consultants work across the pipeline of assets and we believe that this can (i) enhance operational efficiency, (ii) maintain an optimal cost structure, (iii) attract leading collaborators, and (iv) promote asset flexibility, as further described below. If we were to resume enrollment in our clinical programs, we believe our experience and approach would continue to leverage the operating and cost structures that are further described below.
| • | Enhance operational efficiency: We allocate resources while empowering managers to make program-level decisions in order to increase productivity and speed. We believe this model enables a flexible organizational structure that can achieve scale through the addition of programs without increasing burdensome bureaucracy or redundant infrastructure. |
| • | Maintain an optimal cost structure: We have a relatively small number of employees and have partnered with a number of service providers to leverage their infrastructure and expertise as needed instead of embarking on capital-intensive lab, manufacturing, and equipment expenditures. By reducing overhead costs, we believe we can increase the likelihood that we can generate a return on invested capital. |
| • | Attract leading collaborators and licensors: Our pipeline is comprised of therapies we believe will be first-in-class therapies for a variety of cancers sourced via our industry contacts and relationships (including academia and pharmaceutical industry executives). On preclinical programs/technology, we initially established development structures enabling us to keep licensors economically incentivized at the program level. We believe that our experienced drug development leadership team and approach to resource allocation differentiate us from other potential licensees. |
| • | Leverage the commoditized checkpoint marketplace and explore the potential to further enhance long-term clinical benefits for patients with cancer and also expand the eligible population to include those who do not currently receive anti-PD-1 therapy: Presently there are multiple approved checkpoint therapeutics that lack differentiation, resulting in a competitive market dynamic, which will favor combination therapy. There remains opportunity for potential expansion in the PD-1 market with our adenosine antagonists. Studies show that 70-80% of patients do not respond or have a limited response to existing monotherapies, such as PD-1 checkpoint inhibitors. We see potential for our unique approach of using adenosine antagonists to initiate an immune response in tumors that have become refractory to checkpoint therapy or to increase the number of front-line patients achieving more durable responses. Combinations can improve this but often come at the cost of significant additional toxicity. The market is saturated with at least 14 approved PD-1 antibodies, and every major pharmaceutical company competes in this space. Extending the use of PD-1 antibodies could still provide a significant potential upside for companies competing for market share. |
| • | Promote asset flexibility: Our structure is designed to maximize flexibility and cost efficiency. This allows us to efficiently pursue various subsidiary-level transactions, such as stock or asset sales, licensing transactions, strategic partnerships and/or co-development arrangements. It also provides us with the flexibility to terminate programs with minimal costs if results do not meet our de-risking criteria for advancement. |
We are a BVI business company incorporated under the BVI Business Companies Act (Revised Edition 2020, as amended) with our registered office located at Clarence Thomas Building, P.O. Box 4649, Road Town, Tortola, British Virgin Islands, VG1110. Our U.S. agent, Portage Development Services Inc., is located at 59 Wilton Road, Westport, CT 06880.
We currently are a foreign private issuer under SEC rules. We are also a reporting issuer under the securities legislation of the provinces of Ontario and British Columbia. Our ordinary shares were listed on the CSE under the symbol “PBT.U”. On February 25, 2021, our ordinary shares began trading on the Nasdaq Capital Market under the symbol “PRTG”. As the principal market for our ordinary shares is Nasdaq, we voluntarily delisted from the CSE on April 23, 2021.
Summary of Results
The following table summarizes financial information for the quarter ended September 30, 2024, and the preceding eight quarters (in thousands except net loss per share). All share and per share amounts reflect the 1-for-20 reverse stock split effected August 15, 2024.
| Quarter Ended | | | Sept. 30, 2024 | | | | June 30, 2024 | | | | Mar. 31, 2024 | | | | Dec. 31, 2023 | | | | Sept. 30, 2023 | | | | June 30, 2023 | | | | Mar. 31, 2023 | | | | Dec. 31, 2022 | | | | Sept. 30 2022 | |
| | | | | | | | | | | | | | | | | | | |
| Net loss attributable to owners of the Company | | | (1,360 | ) | | | (1,656 | ) | | | (24,889 | ) | | | (39,373 | ) | | | (5,158 | ) | | | (5,919 | ) | | | (94,448 | ) | | | (7,485 | ) | | | (949 | ) |
| Comprehensive loss attributable to the owners of the Company | | | (1,360 | ) | | | (1,656 | ) | | | (28,371 | ) | | | (36,398 | ) | | | (6,458 | ) | | | (4,150 | ) | | | (95,714 | ) | | | (11,502 | ) | | | (949 | ) |
| Working capital (1) | | | 1,813 | | | | 2,191 | | | | 4,816 | | | | 4,808 | | | | 3,131 | | | | 8,254 | | | | 11,811 | | | | 13,110 | | | | 15,737 | |
| Equity attributable to owners of the Company | | | 1,398 | | | | 2,511 | | | | 4,022 | | | | 31,999 | | | | 67,661 | | | | 73,307 | | | | 76,045 | | | | 168,945 | | | | 178,434 | |
| Net loss per share - Basic | | | (1.26 | ) | | | (1.58 | ) | | | (23.74 | ) | | | (37.68 | ) | | | (5.79 | ) | | | (6.68 | ) | | | (108.81 | ) | | | (8.79 | ) | | | (1.13 | ) |
| Net loss per share - Diluted | | | (1.26 | ) | | | (1.58 | ) | | | (23.74 | ) | | | (37.68 | ) | | | (5.79 | ) | | | (6.68 | ) | | | (108.81 | ) | | | (8.79 | ) | | | (1.13 | ) |
| (1) | September 30, 2022 working capital is net of warrant liability of $8 settleable on a non-cash basis. |
Number of Ordinary Shares
The following table summarizes the number of our ordinary shares issued and outstanding at September 30, 2024 and November 26, 2024:
| | | | September 30, 2024 | | | | November 26, 2024 | |
| Shares issued (a) | | | | 1,116,681 | | | | 1,123,757 | |
| Shares outstanding (a) | | | | 1,116,249 | | | | 1,121,059 | |
| (a) | These amounts exclude an aggregate of 12,150 restricted stock units granted to our executive chairman and an employee on January 13, 2021, which vested immediately on the date of grant and are subject to certain restrictions for the settlement and delivery of the ordinary shares underlying the restricted stock units and 5,165 restricted stock units granted to employees (one of whom is executive chairman) on January 19, 2022, which vested immediately on the date of grant and are subject to certain restrictions for the settlement and delivery of the ordinary shares underlying the restricted stock units. In January 2024, we issued 308 shares for the exercise of 470 RSUs net of 162 shares repurchased into treasury to pay for an employee’s payroll taxes. In September 2024, we issued 882 shares for the exercise of 1,152 RSUs net of 270 shares repurchased into treasury to pay for an employee’s payroll taxes. |
Business Environment – Risk Factors
Please refer to the Annual Report for Fiscal 2024 on Form 20-F as filed with the Securities and Exchange Commission on August 14, 2024, for detailed information as the economic and industry factors are substantially unchanged as of the date hereof other than as described below.
We may not be able to regain, or maintain, compliance with the continued listing requirements of The Nasdaq Capital Market.
Our ordinary shares are listed on the Nasdaq Capital Market, and we are therefore subject to its continued listing requirements, including requirements with respect to the market value of our publicly-held shares, market value of our listed shares, minimum bid price per share, and minimum shareholders’ equity, among others. If we fail to satisfy one or more of the requirements, we may be delisted from the Nasdaq Capital Market.
Nasdaq Listing Rule 5550(b)(1) requires companies listed on the Nasdaq Capital Market to maintain shareholders’ equity of at least $2.5 million for continued listing. As of September 30, 2024, our shareholders’ equity was $1.398 million and, as a result, we are not currently in compliance with Nasdaq Listing Rule 5550(b)(1). Accordingly, we expect Nasdaq to notify us of such non-compliance. We do not anticipate that the notification will have an immediate effect on the listing of our ordinary shares on the Nasdaq Capital Market. In accordance with the Nasdaq Listing Rules, we expect that we will have 45 calendar days from the date of the notification to submit a plan to regain compliance with Nasdaq Listing Rule 5550(b)(1). If our compliance plan is accepted, we may be granted up to 180 calendar days from the date of the initial notification to evidence compliance. If our compliance plan is not accepted or we are otherwise unable to evidence compliance within Nasdaq’s allotted timeframe, Nasdaq may take steps to delist our ordinary shares. There can be no assurance that we will be able to increase our shareholders’ equity in the future.
Delisting from the Nasdaq Capital Market may adversely affect our ability to raise additional financing through the public or private sale of equity securities, may significantly affect the ability of investors to trade our securities and may negatively affect the value and liquidity of our ordinary shares. Delisting also could have other negative results, including the potential loss of employee confidence, the loss of institutional investors or interest in business development opportunities.
If we are delisted from Nasdaq and we are not able to list our ordinary shares on another exchange, our ordinary shares could be quoted on the OTC Bulletin Board or in the “pink sheets.” As a result, we could face significant adverse consequences including, among others:
| • | a limited availability of market quotations for our securities; |
| • | a determination that our shares are a “penny stock” which will require brokers trading in our ordinary shares to adhere to more stringent rules and possibly result in a reduced level of trading activity in the secondary trading market for our securities; |
| • | a limited amount of news and little or no analyst coverage for us; |
| • | we would no longer qualify for exemptions from state securities registration requirements, which may require us to comply with applicable state securities laws; and |
| • | a decreased ability to issue additional securities (including pursuant to short-form Registration Statements on Form F-3) due to the Baby Shelf Rule or obtain additional financing in the future. |
Our Programs and Technology – Recent Developments
After a review of our future funding needs for clinical development of our programs as well as the current capital raising market for biotechnology companies, we made the decision to discontinue our iNKT sponsored trial (the investigator sponsored trial of PORT-3 is ongoing without financial support from us) and pause further patient accrual in our sponsored adenosine program. We are exploring strategic alternatives, which may include finding a partner for one or more of our assets, a sale of our company, a merger, restructurings, both in and out of court, a company wind down, further financing efforts or other strategic action. Below is a discussion of our clinical programs and the status of such programs prior to our decision to discontinue our iNKT sponsored trial and pause further patient accrual to our sponsored studies.
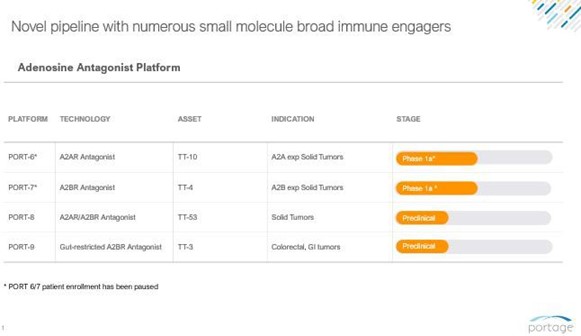
Adenosine Receptor Antagonist Platform
A critical mechanism of cancer immune evasion is the generation of high levels of immunosuppressive adenosine within the tumor microenvironment (“TME”). Research suggests that the TME has significantly elevated concentrations of extracellular adenosine. Engagement with adenosine receptors type 2A (“A2A”) and type 2B (“A2B”) triggers a dampening effect on the immune response, suppressing effector cell function and stabilizing immunosuppressive regulatory cells. Over-expression of the A2A and A2B receptors leads to a poor prognosis in multiple cancers, including prostate cancer, colorectal cancer and lung adenocarcinoma, driven by a reduced ability to generate an immune response against the tumor.
These findings have made A2A and A2B high-priority targets for immunotherapeutic intervention. Before pausing, we were advancing four adenosine antagonists that we believe to be first-in-class, which together represent a broad suite of adenosine-targeting approaches and were expected to enable a comprehensive exploration of how targeting the adenosine pathway could potentially improve response in multiple cancer and non-cancer indications. By modulating the adenosine pathway in four different ways, we expected to determine the optimal approach to maximize the impact of the mechanism of action on different tumors.
We have designed the ADPORT-601 clinical trial to evaluate the activity and safety of PORT-6 and PORT-7 alone and in combination. If we resume accrual, we would expect this trial to adapt over time and also include safety cohorts for these two agents with other immune activating agents including others from our internal pipeline. Depending on the data, it can be expanded to evaluate either agent as monotherapy or a randomized comparison of either agent plus standard of care versus standard of care alone.
PORT-6 (TT-10)
PORT-6 is an A2A antagonist being studied for the treatment of A2A expressing solid tumors. We believe PORT-6 is more potent, more durable and more selective than other clinical stage A2A agents.
Prior to pausing patient enrollment in the clinical study, the ADPORT-601 Phase 1a trial for PORT-6 dosed its first patient in June 2023. There have been a total of 12 patients dosed to date, with one patient continuing to receive treatment and the first two dose escalation cohorts being completed. The third cohort has been fully enrolled, with only one patient experienced a serious adverse event (blurry vision and stroke) that the investigator initially determined could possibly be related to PORT-6. With further follow-up, this event was classified as unrelated to treatment. We remain encouraged by the two patients (discontinued) who received six or more lines of prior therapy and experienced prolonged stable disease for more than eight months. After the study was put on hold, we made the decision to replace a patient that withdrew treatment due to and unrelated adverse event prior to DLT assessment and that patient remains in the trial with stable disease. Further recruitment remains on pause, with three investigator sites still open, while we explore strategic alternatives.
PORT-7 (TT-4)
PORT-7 is an A2B antagonist for the treatment of solid tumors. PORT-7 has a very selective profile that focuses on A2B. PORT-7 is in Phase 1a from an IND perspective, though we have not commenced dosing patients.
PORT-8 (TT-53)
PORT-8 is a dual inhibitor of adenosine receptors 2A and 2B (A2A/A2B) to address solid tumors.
PORT-9 (TT-3)
PORT-9 is an A2B antagonist designed to treat colorectal and gastrointestinal cancers. The PORT-9 program is a pre-clinical stage program.
In connection with the adenosine program, we will focus on solid tumor types with high adenosine expression of receptors A2A and A2B and enrich for patients that have high adenosine expression and therefore have potential to benefit most from treatment.
Other Pipeline and Investee Programs
Prior to our decision to discontinue our iNKT sponsored trial and pause further development of our adenosine program, we were focused on delivering clinical data with the adenosine program described above and prioritizing the allocation of financial resources to that program. Developmental work continued on some of the other developmental assets, through collaborations such as that with the U.S. National Cancer Institute (“NCI”) and other academic groups, as further described below. These developmental assets may be re-evaluated at a future point depending on market conditions, ongoing data, funding priorities and status.
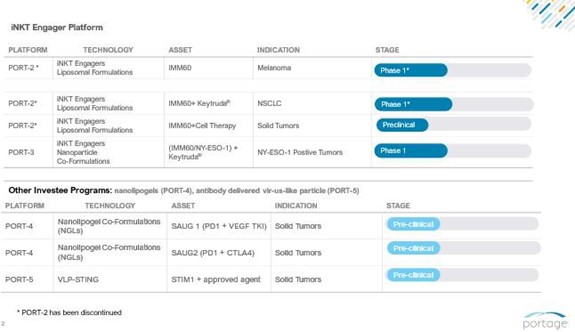
Invariant Natural Killer T-cells (iNKT cells) Platform
iNKT cells play an important role in anti-tumor immune responses and are a distinct class of T lymphocyte displaying a limited diversity of T-cell receptors. They recognize lipid antigens on the surface of tumor cells and produce large amounts of cytokines within hours of stimulation without the need for clonal expansion. Furthermore, iNKT cells activate multiple immune system components, including dendritic cells (“DC”), T-cells and B-cells and stimulate an antigen-specific expansion of these cells. Our operating subsidiary, iOx Therapeutics Ltd. (“iOx”), holds an exclusive license (with the right to sub-license) from the Ludwig Institute for Cancer Research (the “Ludwig Institute”) to use, research, develop and commercialize iNKT cell engagers, for the treatment of various forms of human disease, including cancer, under the Ludwig Institute’s intellectual property and know-how.
PORT-2 (IMM60)
PORT-2 is an iNKT cell engager formulated in a liposome with a six-member carbon head structure that has been shown to activate both human and murine iNKT cells, resulting in DC maturation and the priming of Ag-specific T and B cells.
In animal models, PORT-2 enhanced the frequency of tumor specific immune responses. iNKT cells are unique lymphocytes defined by their co-expression of surface markers associated with NK cells along with a T-cell antigen receptor. They recognize amphipathic ligands such as glycolipids or phospholipids presented in the context of the non-polymorphic, MHC class I-like molecule CD1d. Activated iNKT cells rapidly produce IFN-gamma and IL-4 and induce DC maturation and IL-12 production.
In August 2021, we dosed the first patient in the IMP-MEL PORT-2 clinical trial, a Phase 1/2 dose escalation and randomized expansion trial. Prior to discontinuing the PORT-2 trial, it was expected to enroll up to 88 patients with melanoma or non-small cell lung carcinoma (“NSCLC”) in order to evaluate safety and efficacy. In November 2022, we announced that we had entered into a clinical trial collaboration with Merck to evaluate PORT-2 in combination with pembrolizumab for patients with NSCLC. Under the terms of the collaboration, Merck supplied pembrolizumab for our Phase 1/2 trial of PORT-2 in patients with NSCLC and melanoma. The trial was closed in June 2024. The Merck collaboration terminated in December 2023.
Preliminary Phase 1 data from the IMP-MEL PORT-2 clinical trial, presented at the Society for Immunotherapy of Cancer in November 2023, suggests PORT-2 was well tolerated when administered as a monotherapy, with no related severe or serious adverse events. All possibly related adverse events were mild to moderate and did not limit dosing. Given the favorable safety profile observed in the clinical trials to date, the clinical protocol for the IMP-MEL PORT-2 clinical trial was amended to include a higher Phase 1 dose level as our near-term focus was defining the recommended Phase 2 dose.
Prior to our decision to discontinue further development of our iNKT platform, the combination safety cohort with pembrolizumab was being conducted in parallel with the ongoing high dose monotherapy cohort. As of November 2023, two patients had received the combination with pembrolizumab, and no related severe or serious adverse events were reported. The adverse event profile was consistent with pembrolizumab. Previously reported biomarker data confirmed the mechanism of action (i.e., both activation of the innate and adaptive arms of the immune system). The following figure illustrates the different lesion responses. Although these are preliminary results, several lesions showed shrinkage, and the responses in liver metastases were encouraging.
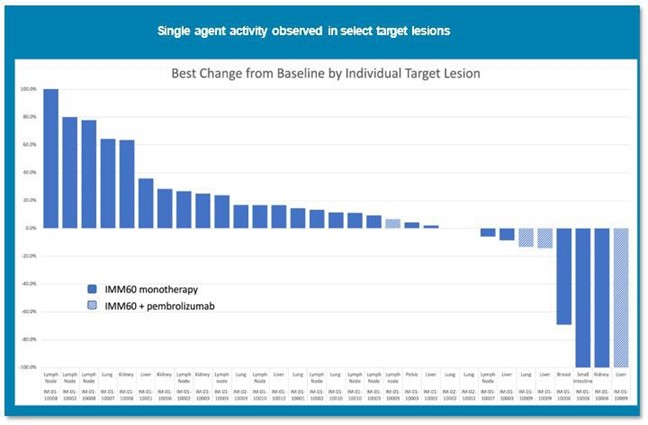
Prior to our decision to discontinue our iNKT sponsored trial, we were encouraged by the patient data set that we believe supports proof of concept for using an iNKT engager in cancer treatment. Preliminary Phase 1 data suggests that PORT-2 has a favorable safety and tolerability profile as a monotherapy at all doses tested to date (as noted above), has demonstrated evidence of single agent activity, and biomarkers confirm mechanistic potential of PORT-2 to activate both the adaptive and innate immune systems.
The clinical trial agreement has been transferred from the University of Oxford to us through our iOx subsidiary and the trial was converted to a program sponsored by iOx.
Prior to our decision to discontinue the iNKT sponsored trial, the protocol was being amended, given the safety data shown at the highest planned doses, to escalate patient dosing to include one additional higher dose to identify the recommended Phase 2 dose. We are exploring strategic alternatives, which may include finding a partner for one or more of our assets, a sale of our company, a merger, restructurings, both in and out of court, a company wind down, further financing efforts or other strategic action.
PORT-3 (IMM65)
PORT-3 is a poly(lactide-co-glycolide) (“PLGA”)-nanoparticle formulation of PORT-2 (IMM60) combined with a NY-ESO-1 peptide vaccine. Biodegradable PLGA-nanoparticles function as a delivery platform for immunomodulators and tumor antigens to induce a specific anti-tumor immune response. PLGA has minimal (systemic) toxicity and is used in various drug-carrying platforms as an encapsulating agent. Furthermore, co-formulating an iNKT engager with a peptide vaccine in a particle has shown to be approximately five times more potent in killing cancer cells and generating an antigen-specific CD8 T-cell response than giving the two agents individually.
NY-ESO-1 is a cancer-testis antigen expressed during embryogenesis and in the testis, an immune privileged site. Furthermore, NY-ESO-1 expression is observed in several advanced cancers: Lung (2-32%), melanoma (40%), bladder (32-35%), prostate (38%), ovarian (30%), esophageal (24-33%), and gastric cancers (8-12%). Clinical trials have shown the safety and tolerability of Good Manufacturing Practices-grade NY-ESO-1 peptides in patients with cancer.
PORT-3 is being evaluated as part of an investigator sponsored study without funding from us. The first patient was dosed in 2021 and patients continue to enroll in the PRECIOUS Phase 1 trial of PORT-3 in patients with solid tumors. The Phase 1 portion of the trial is expected to enroll 15 patients. The trial was having difficulty identifying tumors that expressed NY-ESO-1, so the trial protocol was amended to include all solid tumors regardless of expression to facilitate assessment of safety. This platform is designed to demonstrate proof of concept. The combination of NY-ESO-1 and IMM-60 is being evaluated to determine its ability to prime and boost an anti-tumor immune response. Our patent position extends to other known tumor antigens, and, if we resume further development of our iNKT platform, we could be prepared to rapidly launch other assets into the clinic if we see strong activity of this formulation. Preliminary safety data for repeat dosing of PORT-3 in the PRECIOUS Phase 1 trial shows a favorable safety profile. The investigators with whom we work with have continued to explore next generation targeted nanoparticles.
PORT-4, Nanolipogel (“NLG”) co-formulation Platform
Scientists are interested in novel ways to deliver multiple signals to the immune system in order to better activate an anti-tumor response. We have been impressed with a platform from Yale University that allows different types of agents to be packaged together and will concentrate them in tumors. We have licensed the platform for delivery of DNA aptamers and certain aptamer-small molecule-based combination products. In order to have multiple proprietary agents with known mechanisms of action, we have licensed rights to create DNA aptamers for immune-oncology targets and the first one developed is a proprietary PD1 aptamer, which has been placed in the NLG formulation. Early testing has shown the formulation properly modulates PD1 signaling in vitro similar to a PD1 antibody I. In non-clinical, in vivo experiments, the NLG-PD1 performed favorably compared to a mouse PD1 antibody. We have conducted further research with the technology licensed from Yale University to co-deliver a PD1 blocking signal with a small molecule vascular endothelial growth factor inhibitor.
As of September 30, 2024, we owned approximately 70% of the outstanding shares of Saugatuck Therapeutics, Ltd., the subsidiary on which the PORT-4 platform is managed.
PORT-5, STING Agonist Platform
Proprietary immune priming and boosting technology (using a STING agonist delivered in a virus-like particle) has shown proof of concept in animal models. This platform was developed to offer multiple ways to target immune stimulation towards the cancer, as well as to co-deliver multiple signals in a single product. The PORT-5 STING platform’s advantage over chemical intratumoral approaches was potent immune priming and boosting pathway within a virus-like particle to enable convenient systemic administration and traffic to the correct targets. This technology would target dendritic cells, which is differentiated from other chemical STING approaches. To that end, Stimunity S.A. (“Stimunity”) received grant funding to study this technology with any COVID-19 vaccine to evaluate if it is possible to boost the immune response for immunocompromised or elderly patients. During April 2022, the American Association for Cancer Research showcased PORT-5 preclinical data at a late-breaking session that shows that one or more targeted immunotherapy agents could be packaged within a virus-like particle to increase potency, while enabling a selective immune activation. Stimunity was unable to raise any outside funding, and activities were scaled back due to our own liquidity issues.
In December 2023, we completed a transfer of our equity in Stimunity and the Stimunity Convertible Note to iOx. In connection with that transfer, the Stimunity Convertible Note was converted into 1,768 Class A shares of Stimunity.
As of September 30, 2024, we owned approximately 48.9% of the outstanding shares of Stimunity, the subsidiary on which the PORT-5 platform is managed. We have made the decision not to further fund Stimunity’s operations and wrote down the remaining balance of our investment of $1.0 million to nil as of March 31, 2024.
Early-Stage Research and Development Collaborations
We have also been interested in evaluating and testing new antibody targets in the suppressive tumor microenvironment with the goal to down regulate or remove MDSC, TAMs, Tregs and other signals that impede the immune response from clearing cancer cells.
| • | We continue to collaborate with Dr. Robert Negrin and his team at Stanford University in an investigator sponsored trial (“IST”) study to evaluate the use of PORT-2 with iNKT cell therapies in animals. This work was intended to evaluate if an engager co-administered with expanded or transformed iNKT cells can further activate the transplanted and endogenous cells inside the patient. The Stanford collaboration was also expected to study the impact iNKT engagers have on driving an adaptive immune response and correcting the suppressive tumor microenvironment. This IST remains operational as of the date of this report. |
| • | We entered into a Cooperative Research and Development Agreement (“CRADA”) with the NCI. We and NCI planned to advance preclinical and potential clinical development of STING agonists and anti-RAGE agents for cancer vaccines. After the acquisition of Tarus Therapeutics, LLC (“Tarus”), we amended the CRADA to include exploration of the different adenosine compounds. We did not extend the CRADA beyond its current term and made a termination payment of $62,500 in June 2024 as required under the contract. |
| • | We have a collaboration with Dr. Carmela de Santos at University of Birmingham for the use of iNKT agents to treat sarcomas. Dr. de Santos has tested PORT-2 in human sarcoma cell lines and has grant funding to test it in animal models. |
| • | We have a collaboration to study the use of adenosine 2A and adenosine 2B agents in mesothelioma with Drs. Luciano Mutti from Sbarro Institute for Cancer Research and Molecular Medicine, Department of Biology, College of Science and Technology, Temple University and Dr. Steven Gray of St. James Hospital in Dublin. |
| • | There are other collaborations with experts in the products areas for which we provide access to our compounds and collaborates on studies. |
Three Months Ended September 30, 2024 Compared to the Three Months Ended September 30, 2023
Results of Operations
The following details major expenses for the three months ended September 30, 2024, compared to the three months ended September 30, 2023 (in thousands):
| Three months ended September 30, | | | 2024 | | | | 2023 | |
| | | | | |
| Operating expenses | | $ | (1,604 | ) | | $ | (5,930 | ) |
| Change in fair value of warrant liability | | | (716 | ) | | | — | |
| Change in fair value of deferred purchase price payable – Tarus and deferred obligation – iOx milestone | | | — | | | | (113 | ) |
| Gain on settlement with Parexel – iOx CRO | | | 946 | | | | — | |
| Share of loss in associate accounted for using equity method | | | — | | | | (40 | ) |
| Depreciation expense | | | (7 | ) | | | (15 | ) |
| Foreign exchange transaction loss | | | (5 | ) | | | (17 | ) |
| Interest income, net | | | 24 | | | | 43 | |
| Loss before provision for income taxes | | | (1,362 | ) | | | (6,072 | ) |
| Income tax benefit | | | — | | | | 907 | |
| Net loss | | | (1,362 | ) | | | (5,165 | ) |
| Other comprehensive loss | | | | | | | | |
| Net unrealized loss on investments | | | — | | | | (1,300 | ) |
| Total comprehensive loss for period | | $ | (1,362 | ) | | $ | (6,465 | ) |
| | | | | | | | | |
| Comprehensive loss attributable to: | | | | | | | | |
| Owners of the Company | | $ | (1,360 | ) | | $ | (6,458 | ) |
| Non-controlling interest | | | (2 | ) | | | (7 | ) |
| Total comprehensive loss for period | | $ | (1,362 | ) | | $ | (6,465 | ) |
Results of Operations for the Three Months Ended September 30, 2024 Compared to the Three Months Ended September 30, 2023
We incurred a net loss of approximately $1.4 million during the three months ended September 30, 2024 (the “Fiscal 2025 Quarter”), compared to a net loss of approximately $5.2 million during the three months ended September 30, 2023 (the “Fiscal 2024 Quarter”), a decrease in net loss of $3.8 million, quarter-over-quarter.
The components of the change in net loss and total comprehensive loss are as follows:
| • | Operating expenses, which include research and development (“R&D”) costs and general and administrative (“G&A”) expenses, were $1.6 million in the Fiscal 2025 Quarter, compared to $5.9 million in the Fiscal 2024 Quarter, a decrease of $4.3 million, which is discussed more fully below. |
| • | A net gain of $0.9 million from the settlement and release of obligations and liabilities under the Master Services Agreement between iOx and Parexel International (IRL) Limited (“Parexel”). See “iOx – Parexel Master Services Agreement” section of Note 12 “Commitments and Contingent Liabilities” for additional discussion regarding this matter included elsewhere in this Report. |
| • | A non-cash loss totaling $0.113 million comprised of the change (increase) in the fair value of the deferred obligation - iOx milestone of $0.028 million, and the change (increase) in the fair value of the deferred purchase price payable to the former Tarus shareholders of $0.085 million in the Fiscal 2024 Quarter. |
| • | A $0.7 million non-cash loss from the change in the fair value of certain warrants accounted for as liabilities issued in connection with the Private Placement (defined below) in October 2023. |
| • | Additionally, we reflected a marginal income tax expense in the Fiscal 2025 Quarter, compared to a net deferred income tax benefit of $0.9 million in the Fiscal 2024 Quarter. For the Fiscal 2024 Quarter, we recognized a decrease in net deferred tax liability of $0.3 million to reflect the effect of the change in exchange rates on the liability during the period and the recognition of $0.6 million of current period losses in the U.K. |
Total comprehensive loss in the Fiscal 2024 Quarter includes $1.3 million unrealized loss on investments compared to nil in the Fiscal 2025 Quarter. The difference between net loss and total comprehensive loss in the Fiscal 2024 Quarter was due to the effect of the unrealized non-cash loss with respect to our investment in Intensity Therapeutics, Inc. (“Intensity”). We recognized a non-cash unrealized loss of $1.3 million in the Fiscal 2024 Quarter, representing the change in the fair value of the investment in Intensity based on the Nasdaq listed price of the shares during the period.
Operating Expenses
Total operating expenses are comprised of the following (in thousands):
| Three months ended September 30, | | | 2024 | | | | 2023 | |
| | | | | |
| Research and development | | $ | 723 | | | $ | 4,237 | |
| General and administrative expenses | | | 881 | | | | 1,693 | |
| Total operating expenses | | $ | 1,604 | | | $ | 5,930 | |
Research and Development Costs
R&D costs are comprised of the following (in thousands):
| Three months ended September 30, | | | 2024 | | | | 2023 | |
| | | | | |
| Research and development – Clinical | | $ | 366 | | | $ | 1,962 | |
| Payroll-related expenses | | | 245 | | | | 367 | |
| Consulting fees | | | 32 | | | | 247 | |
| Manufacturing costs | | | 29 | | | | 968 | |
| Research and development – CRADA | | | — | | | | 31 | |
| Licensing fees | | | — | | | | 120 | |
| Legal regarding patents’ registration | | | 5 | | | | — | |
| Research and development services and storage | | | 46 | | | | 148 | |
| Share-based compensation expense | | | — | | | | 394 | |
| Total research and development costs | | $ | 723 | | | $ | 4,237 | |
R&D costs decreased by approximately $3.5 million, or approximately 83%, from approximately $4.2 million in the Fiscal 2024 Quarter to approximately $0.7 million in the Fiscal 2025 Quarter. The decrease was primarily attributable to the winding down of clinical trial costs (principally CRO-related), which decreased by approximately $1.6 million, from $2.0 million in the Fiscal 2024 Quarter to $0.4 million in the Fiscal 2025 Quarter, as activities ramped down throughout the period since we made the decision to pause enrollment in our sponsored clinical trials in the third and fourth quarters of Fiscal 2024. Manufacturing-related costs decreased by $0.9 million, from $1.0 million in the Fiscal 2024 Quarter to $0.029 million in the Fiscal 2025 Quarter. These decreases reflect the winding down of clinical activity and manufacturing-related costs resulting from our decision to discontinue our sponsored clinical trial for the iNKT program and pause further patient accrual to our sponsored adenosine program. R&D non-cash share-based compensation expense decreased from $0.4 million in the Fiscal 2024 Quarter to nil in the Fiscal 2025 Quarter. Payroll-related expenses decreased by $0.1 million, from $0.37 million in the Fiscal 2024 Quarter to $0.24 million in the Fiscal 2025 Quarter, due to the resignation of two employees in January 2024. Additionally, consulting fees decreased by approximately $0.2 million from $0.25 million in the Fiscal 2024 Quarter to $0.03 million in the Fiscal 2025 Quarter, to reflect the decrease in activity period-over-period. Finally, licensing fees decreased by approximately $0.1 million due to licensing fees paid to the licensor of certain intellectual property utilized in the iNKT clinical trial in Fiscal 2024 Quarter compared to nil in Fiscal 2025 Quarter as the iNKT clinical trial was discontinued in the latter half of Fiscal 2024.
General and Administrative Expenses
Key components of G&A expenses are the following (in thousands):
| Three months ended September 30, | | | 2024 | | | | 2023 | |
| | | | | |
| Professional fees | | $ | 398 | | | $ | 762 | |
| Payroll-related expenses | | | 134 | | | | 223 | |
| D&O insurance | | | 160 | | | | 175 | |
| Office and general expenses | | | 33 | | | | 99 | |
| Directors’ fees | | | — | | | | 83 | |
| Share-based compensation expense | | | 141 | | | | 348 | |
| Consulting fees | | | 15 | | | | 3 | |
| Total general and administrative expenses | | $ | 881 | | | $ | 1,693 | |
G&A expenses decreased by approximately $0.8 million, or approximately 48%, from approximately $1.7 million in the Fiscal 2024 Quarter to approximately $0.9 million in the Fiscal 2025 Quarter. Professional fees decreased by $0.4 million, from $0.8 million in the Fiscal 2024 Quarter to $0.4 million in the Fiscal 2025 Quarter. Payroll-related expenses decreased by $0.1 million from $0.2 million in the Fiscal 2024 Quarter to $0.1 million in the Fiscal 2025 Quarter. The decrease in professional fees and payroll-related expenses is due to the accrual of the monthly fees and payments for the entire second quarter in the first quarter for a consultant and employee in connection with the Retention Agreements entered into on July 22, 2024. For further discussion regarding this matter, see “Retention Agreements and General Releases” section of Note 13 “Related Party Transactions” included elsewhere in this Report. Additionally, G&A non-cash share-based compensation expense decreased by $0.2 million due to the continued vesting of stock options, partially offset by recording all share-based compensation expense as G&A expenses as the result of the discontinuation of the iNKT trial and the pause of further patient accrual in the adenosine program. Additionally, directors’ fees decreased by $0.1 million in the Fiscal 2025 Quarter, compared to the Fiscal 2024 Quarter, as all directors, except for two directors who resigned in April 2024, waived their fees in the Fiscal 2025 Quarter.
Six Months Ended September 30, 2024 Compared to the Six Months Ended September 30, 2023
Results of Operations
The following details major expenses for the six months ended September 30, 2024, compared to the six months ended September 30, 2023 (in thousands):
| Six months ended September 30, | | | 2024 | | | | 2023 | |
| | | | | |
| Operating expenses | | $ | (4,443 | ) | | $ | (10,927 | ) |
| Change in fair value of warrant liability | | | 426 | | | | — | |
| Change in fair value of deferred purchase price payable – Tarus and deferred obligation – iOx milestone | | | — | | | | (1,224 | ) |
| Gain on settlement with Parexel – iOx CRO | | | 946 | | | | — | |
| Share of loss in associate accounted for using equity method | | | — | | | | (90 | ) |
| Foreign exchange transaction (loss) gain | | | (7 | ) | | | 1 | |
| Depreciation expense | | | (15 | ) | | | (26 | ) |
| Interest income, net | | | 69 | | | | 123 | |
| Loss before provision for income taxes | | | (3,024 | ) | | | (12,143 | ) |
| Income tax (expense) benefit | | | (2 | ) | | | 1,052 | |
| Net loss | | | (3,026 | ) | | | (11,091 | ) |
| Other comprehensive loss | | | | | | | | |
| Net unrealized gain on investments | | | — | | | | 469 | |
| Total comprehensive loss for period | | $ | (3,026 | ) | | $ | (10,622 | ) |
| | | | | | | | |
| Comprehensive loss attributable to: | | | | | | | | |
| Owners of the Company | | $ | (3,016 | ) | | $ | (10,608 | ) |
| Non-controlling interest | | | (10 | ) | | | (14 | ) |
| Total comprehensive loss for period | | $ | (3,026 | ) | | $ | (10,622 | ) |
Results of Operations for the Six Months Ended September 30, 2024 Compared to the Six Months Ended September 30, 2023
We incurred a net loss of approximately $3.0 million during the six months ended September 30, 2024 (the “Fiscal 2025 Six Months”), compared to net loss of $11.1 million during the six months ended September 30, 2023 (the “Fiscal 2024 Six Months”) and total comprehensive loss of approximately $3.0 million and $10.6 million during the Fiscal 2025 Six Months and the Fiscal 2024 Six Months, respectively, a decrease in net loss of $8.1 million and a decrease in comprehensive loss of $7.6 million, period-over-period.
The components of the change in net loss and total comprehensive loss are as follows:
| • | Operating expenses, which include R&D and G&A expenses, were $4.4 million in the Fiscal 2025 Six Months, compared to $10.9 million in the Fiscal 2024 Six Months, a decrease of $6.5 million, which is discussed more fully below. |
| • | A net gain of $0.9 million from the settlement and release of obligations and liabilities under the Master Services Agreement between iOx and Parexel. See “iOx – Parexel Master Services Agreement” section of Note 12 “Commitments and Contingent Liabilities” for additional discussion regarding this matter included elsewhere in this Report. |
| • | A $0.4 million non-cash gain from the change in the fair value of certain warrants accounted for as liabilities issued in connection with the Private Placement in October 2023. |
| • | A non-cash loss totaling $1.2 million comprised of the change (increase) in the fair value of the deferred obligation - iOx milestone of $0.45 million, and the change (increase) in the fair value of the deferred purchase price payable to the former Tarus shareholders of $0.77 million in the Fiscal 2024 Six Months. |
| • | Additionally, we reflected a marginal income tax expense in the Fiscal 2025 Six Months, compared to a net deferred income tax benefit of $1.1 million benefit in the Fiscal 2024 Six Months. For the Fiscal 2024 Six Months, we recognized a decrease in net deferred tax liability of $0.1 million to reflect the effect of the change in exchange rates on the liability during the period and the recognition $1.0 million of current period losses in the U.K. |
Total comprehensive loss in the Fiscal 2024 Six Months includes $0.5 million unrealized gain on investments compared to nil in the Fiscal 2025 Six Months. The difference between net loss and total comprehensive loss in the Fiscal 2024 Six Months was due to the effect of the unrealized non-cash gain with respect to our investment in Intensity. We recognized a non-cash unrealized gain of $0.5 million in the Fiscal 2024 Six Months, representing the change in the fair value of the investment in Intensity based on the Nasdaq listed price of the shares during the period.
Operating Expenses
Total operating expenses are comprised of the following (in thousands):
| Six months ended September 30, | | | 2024 | | | | 2023 | |
| | | | | |
| Research and development | | $ | 2,028 | | | $ | 7,865 | |
| General and administrative expenses | | | 2,415 | | | | 3,062 | |
| Total operating expenses | | $ | 4,443 | | | $ | 10,927 | |
Research and Development Costs
R&D costs are comprised of the following (in thousands):
| Six months ended September 30, | | | 2024 | | | | 2023 | |
| | | | | |
| Research and development – Clinical | | $ | 1,072 | | | $ | 2,985 | |
| Payroll-related expenses | | | 494 | | | | 847 | |
| Consulting fees | | | 153 | | | | 460 | |
| Manufacturing costs | | | 123 | | | | 1,715 | |
| Research and development – CRADA | | | 62 | | | | 63 | |
| Licensing fees | | | 38 | | | | 232 | |
| Legal regarding patents’ registration | | | 28 | | | | 8 | |
| Research and development services and storage | | | 58 | | | | 238 | |
| Share-based compensation expense | | | — | | | | 817 | |
| Contractual milestone | | | — | | | | 500 | |
| Total research and development costs | | $ | 2,028 | | | $ | 7,865 | |
R&D costs decreased by approximately $5.8 million, or approximately 74%, from approximately $7.9 million in the Fiscal 2024 Six Months to approximately $2.0 million in the Fiscal 2025 Six Months. The decrease was primarily attributable to the winding down of clinical trial costs (principally CRO-related), which decreased by approximately $2.0 million, from $3.0 million in the Fiscal 2024 Six Months to $1.0 million in the Fiscal 2025 Six Months, as activities ramped down throughout the period since we made the decision to pause enrollment in our sponsored clinical trials in the third and fourth quarters of Fiscal 2024. Manufacturing-related costs decreased by $1.6 million, from $1.7 million in the Fiscal 2024 Six Months to $0.1 million in the Fiscal 2025 Six Months. These decreases reflect the winding down of clinical activity and manufacturing-related costs resulting from our decision to discontinue our sponsored clinical trial for the iNKT program and pause further patient accrual to our sponsored adenosine program. R&D non-cash share-based compensation expense decreased from $0.8 million in the Fiscal 2024 Six Months to nil in the Fiscal 2025 Six Months. Payroll-related expenses decreased by $0.35 million, from $0.85 million in the Fiscal 2024 Six Months to $0.5 million in the Fiscal 2025 Six Months, due to the resignation of two employees in January 2024. Additionally, in the Fiscal 2024 Six Months, we incurred a milestone payment of $0.5 million for dosing our first adenosine patients, a decrease in consulting fees of approximately $0.3 million from $0.5 million in the Fiscal 2024 Six Months to $0.2 million in the Fiscal 2025 Six Months, to reflect the decrease in activity period-over-period, and, finally, a decrease of $0.2 million in licensing fees paid to the licensor of certain intellectual property utilized in the iNKT clinical trial prior to discontinuing the study in the latter half of Fiscal 2024.
General and Administrative Expenses
Key components of G&A expenses are the following (in thousands):
| Six months ended September 30, | | | 2024 | | | | 2023 | |
| | | | | |
| Professional fees | | $ | 941 | | | $ | 1,232 | |
| Payroll-related expenses | | | 771 | | | | 447 | |
| D&O insurance | | | 320 | | | | 350 | |
| Office and general expenses | | | 76 | | | | 168 | |
| Directors’ fees | | | 7 | | | | 165 | |
| Share-based compensation expense | | | 285 | | | | 695 | |
| Consulting fees | | | 15 | | | | 5 | |
| Total general and administrative expenses | | $ | 2,415 | | | $ | 3,062 | |
G&A expenses decreased by approximately $0.647 million, or approximately 21%, from approximately $3.062 million in the Fiscal 2024 Six Months to approximately $2.415 million in the Fiscal 2025 Six Months. Professional fees decreased by $0.3 million, from $1.2 million in the Fiscal 2024 Six Months to $0.9 million in the Fiscal 2025 Six Months due to decreased accounting and public relations related fees. Payroll-related expenses increased by $0.4 million from $0.4 million in the Fiscal 2024 Six Months to $0.8 million in the Fiscal 2025 Six Months due to the amounts associated with retention agreements executed with an employee and a consultant. For further discussion regarding this matter, see “Retention Agreements and General Releases” section of Note 13 “Related Party Transactions” included elsewhere in this Report. Additionally, G&A non-cash share-based compensation expense decreased by $0.4 million due to the continued vesting of stock options, partially offset by recording all share-based compensation expense as G&A expenses as the result of the discontinuation of the iNKT trial and the pause of further patient accrual in the adenosine program. Additionally, directors’ fees decreased by $0.2 million in the Fiscal 2025 Six Months, compared to the Fiscal 2024 Six Months, as all directors, except for two directors who resigned in April 2024, waived their fees in the Fiscal 2025 Six Months.
Liquidity and Capital Resources
Capital Resources
We filed the March 2021 Registration Statement with the SEC in order to sell ordinary shares, debt securities, warrants and units in one or more offerings from time to time, which became effective on March 8, 2021. In connection with the March 2021 Registration Statement, we have filed with the SEC:
| • | a base prospectus, which covers the offering, issuance and sale by us of up to $200 million in the aggregate of the securities identified above from time to time in one or more offerings; |
| • | a prospectus supplement, which covers the offer, issuance and sale by us in an ATM offering program of up to a maximum aggregate offering price of $50 million of our ordinary shares that may be issued and sold from time to time under the Sales Agreement with Cantor Fitzgerald & Co., the sales agent; |
| • | a prospectus supplement dated June 24, 2021, for the offer, issuance and sale by us of 57,500 ordinary shares for gross proceeds of approximately $26.5 million in a firm commitment underwritten public offering with Cantor Fitzgerald; |
| • | a prospectus supplement dated August 19, 2022, for the resale of up to $30 million in ordinary shares that we may sell from time to time to Lincoln and an additional 4,726 shares that were issued to Lincoln; and |
| • | a prospectus supplement dated September 29, 2023 for the offer, issuance and sale by us in a registered direct public offering through H.C. Wainwright & Co., the placement agent, to an institutional and accredited investor of (i) 98,500 of our ordinary shares at a purchase price of $38.00 per share and (ii) Pre-Funded Warrants to purchase up to 59,395 of our ordinary shares, at a purchase price of $37.98 per Pre-Funded Warrant), for aggregate gross proceeds of approximately $6 million (the “2023 Equity Financing”). All Pre-Funded Warrants, which were exercisable for one ordinary share at an exercise price of $0.02 per share, were exercised in full on May 29, 2024. |
The Sales Agreement permits us to sell in an ATM program up to $50 million of ordinary shares from time to time. Through September 30, 2024, we raised approximately $4.2 million in gross proceeds through the sale of shares of common shares under the ATM program. The sales under the prospectus will be deemed to be made pursuant to an ATM program as defined in Rule 415(a)(4) promulgated under the Securities Act of 1933, as amended (the “Securities Act”).
During Fiscal 2024, we sold 9,331 ordinary shares under the ATM program, generating net proceeds of approximately $0.7 million. There were no shares sold under the ATM program during the six months ended September 30, 2024.
The March 2021 Registration Statement expired on March 8, 2024. In order to issue additional shares under our ATM program or the Committed Purchase Agreement in the future, we would be required to file a new registration statement, which must be declared effective by the SEC prior to use, and to file a prospectus supplement related to the ATM program or the Committed Purchase Agreement, as the case may be.
Furthermore, our ATM program and the Committed Purchase Agreement with Lincoln are generally limited based on, among other things, our Nasdaq trading volume. Under the Baby Shelf Rule, the amount of funds we can raise through primary public offerings of securities in any 12-month period using a registration statement on Form F-3 is limited to one-third of the aggregate market value of the ordinary shares held by our non-affiliates, which limitation may change over time based on our stock price, number of ordinary shares outstanding and the percentage of ordinary shares held by non-affiliates. We are therefore limited by the Baby Shelf Rule as of the filing of this Form 6-K, until such time as our non-affiliate public float exceeds $75 million.
In connection with the 2023 Equity Financing, on September 29, 2023, we entered into the Purchase Agreement with an institutional and accredited investor in connection with the Registered Direct Offering and the concurrent private placement. The Offerings closed on October 3, 2023.
Pursuant to the Purchase Agreement, in the Registered Direct Offering, we sold (i) 98,500 of our ordinary shares, at a purchase price of $38.00 per share and (ii) Pre-Funded Warrants to purchase up to 59,395 Pre-Funded Warrant Shares. All Pre-Funded Warrants, which were immediately exercisable for one ordinary share at an exercise price of $0.02 per share, were exercised in full on May 29, 2024.
In the Private Placement, we issued to such institutional and accredited investor unregistered Series A Warrants to purchase up to 157,895 ordinary shares, unregistered Series B Warrants to purchase up to 157,895 ordinary shares, and unregistered Series C Warrants to purchase up to 157,895 ordinary shares, together exercisable for an aggregate of up to 473,685 Private Warrant Shares. Pursuant to the terms of the Purchase Agreement, for each ordinary share and Pre-Funded Warrant issued in the Registered Direct Offering, an accompanying Series A Warrant, Series B Warrant and Series C Warrant were issued to such institutional and accredited investor. Each Series A Warrant is exercisable for one Private Warrant Share at an exercise price of $38.00 per share, is immediately exercisable and will expire 18 months from the date of issuance. Each Series B Warrant is exercisable for one Private Warrant Share at an exercise price of $45.20 per share, is immediately exercisable and will expire three years from the date of issuance. Each Series C Warrant is exercisable for one Private Warrant Share at an exercise price of $45.20 per share, is immediately exercisable and will expire five years from the date of issuance. The net proceeds to us from the Offerings were approximately $5.3 million, after deducting placement agent’s fees and estimated offering expenses.
Pursuant to an engagement letter, dated as of August 26, 2023, between us and H.C. Wainwright & Co., LLC (the “Placement Agent”), we paid the Placement Agent a total cash fee equal to 6.0% of the aggregate gross proceeds received in the Offerings, or $0.36 million. We also agreed to pay the Placement Agent in connection with the Offerings a management fee equal to 1.0% of the aggregate gross proceeds raised in the Offerings ($0.06 million), $75,000 for non-accountable expenses and $15,950 for clearing fees. In addition, we agreed to issue to the Placement Agent, or its designees, Placement Agent Warrants to purchase up to 7,896 ordinary shares, which represents 5.0% of the aggregate number of ordinary shares and Pre-Funded Warrants sold in the Registered Direct Offering. The Placement Agent Warrants have substantially the same terms as the Private Warrants, except that the Placement Agent Warrants have an exercise price equal to $47.50, or 125% of the offering price per ordinary share sold in the Registered Direct Offering, and will be exercisable for five years from the commencement of the sales pursuant to the Offerings.
Going Concern
The accompanying condensed consolidated interim financial statements for the three and six months ended September 30, 2024 have been prepared on a basis that assumes that we will continue as a going concern and that contemplates the continuity of operations, the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. Accordingly, the accompanying condensed consolidated interim financial statements for the three and six months ended September 30, 2024 do not include any adjustments relating to the recoverability and classification of recorded asset amounts or amounts of liabilities that might result from the outcome of this uncertainty.
As of September 30, 2024, we had cash and cash equivalents of approximately $1.8 million and total current liabilities of approximately $0.9 million. For the six months ended September 30, 2024, we are reporting a net loss of approximately $3.0 million, and cash used in operating activities of approximately $3.3 million. As of November 25, 2024, we had approximately $1.7 million of cash and cash equivalents on hand.
In late Fiscal 2024, because of continued liquidity constraints, we made the decision to discontinue our iNKT sponsored trial and pause further patient accrual to our adenosine program. We are exploring strategic alternatives, which may include finding a partner for one or more of our assets, a sale of our company, a merger, restructurings, both in and out of court, a company wind down, further financing efforts or other strategic action.
There can be no assurance that our evaluation of strategic alternatives will result in any agreements or transactions, or that, if completed, any agreements or transactions will be successful or on attractive terms. Any potential transaction would be dependent on a number of factors that may be beyond our control, including, among other things, market conditions, industry trends, the interest of third parties in a potential transaction with us and the availability of financing to us or third parties in a potential transaction with us on reasonable terms. The process of reviewing strategic alternatives may require us to incur additional costs and expenses. It could negatively impact our ability to attract, retain and motivate key employees, and expose us to potential litigation in connection with this process or any resulting transaction. If we are unable to effectively manage the process, our financial condition and results of operations could be adversely affected. In addition, any strategic alternative that may be pursued and completed ultimately may not deliver the anticipated benefits or enhance shareholder value. There can be no guarantee that the process of evaluating strategic alternatives will result in our company entering into or completing a potential transaction within the anticipated timing or at all. There is no set timetable for this evaluation and we do not intend to disclose developments with respect to this evaluation unless and until we determine that further disclosure is appropriate or legally required. As of November 25, 2024, we had approximately $1.7 million of cash and cash equivalents on hand, which we expect is only sufficient to cover our operating needs through January 2025. These factors raise substantial doubt about our ability to continue as a going concern within one year after the date of the consolidated statement of financial position. There were no adjustments made to reflect the effect of this doubt.
We have incurred significant operating losses since inception and expect to continue to incur significant operating losses for the foreseeable future and may never become profitable. The losses result primarily from our conduct of research and development activities. As previously discussed, we have discontinued further development of our iNKT sponsored trial and paused further patient accrual to our adenosine program in order to preserve cash resources. Additionally, during the fourth quarter of Fiscal 2024, we sold our shares in Intensity on Nasdaq.
We historically have funded our operations principally from proceeds from issuances of equity and debt securities. We will require significant additional capital to make the investments we need to execute our longer-term business plan. Our ability to successfully raise sufficient funds through the sale of debt or equity securities when needed is subject to many risks and uncertainties and, future equity issuances would result in dilution to existing shareholders and any future debt securities may contain covenants that limit our operations or ability to enter into certain transactions.
As of the date of this filing, we currently anticipate that current cash and cash equivalents is only sufficient to cover our operating needs through January 2025. These factors raise substantial doubt about our ability to continue as a going concern within one year after the date of the consolidated statement of financial position.
Cash Flows From Operating Activities
During the Fiscal 2025 Six Months, we used cash of $3.25 million to fund operating activities, compared to $7.7 million used during the Fiscal 2024 Six Months. The decrease of $4.5 million period over period is primarily due to our decision to discontinue and pause further patient accrual in the respective clinical programs in the latter half of Fiscal 2024.
Cash Flows From Investing Activities
There were no cash flows from investing activities during both the Fiscal 2025 Six Months and the Fiscal 2024 Six Months.
Cash Flows From Financing Activities
During the Fiscal 2025 Six Months, we used net cash of $0.018 million from financing activities. During the Fiscal 2024 Six Months, we generated cash of $0.6 million from financing activities primarily attributable to the net proceeds from sales under the ATM program partially offset by the repayment of lease liability.
Key Contractual Obligations
Details of contractual obligations, commitments and contingent liabilities are provided in Note 12, “Commitments and Contingent Liabilities,” to the unaudited condensed consolidated interim financial statements for the three and six months ended September 30, 2024.
Master Services Agreement
Effective March 15, 2022, through iOx, we entered into a Master Services Agreement (the “MSA”) with Parexel under which Parexel agreed to act as clinical service provider (“CRO”) pursuant to a work order (“Work Order”) effective June 1, 2022. Pursuant to such Work Order, Parexel planned to operate a Phase 2 trial of IMM60 and pembrolizumab in advanced melanoma and NSCLC. The MSA provided for a five-year term, and the Work Order provided for a term to end upon the completion of the services required. The budget provided for service fees and pass-through expenses and clinical sites totaling $11.5 million. During Fiscal 2023, we executed two change orders resulting in a $0.6 million increase in the overall estimated budgeted costs. As a result of our decision to discontinue the development with respect to this program, on December 20, 2023, we provided Parexel notice of termination of the contract, with a planned termination date of April 18, 2024. As the ongoing CRO services were wound down, we noticed that expenses incurred under the CRO agreement were higher than originally budgeted. Parexel agreed to refund $0.552 million to us and release the liability for amounts invoiced and unbilled services totaling $1.486 million, and we forfeited advanced payments of $1.091 million. As a result, we recognized a net gain on of $0.946 million as of September 30, 2024. We received the refund from Parexel on October 3, 2024.
Clinical Service Agreement
On March 1, 2023, we, through Tarus, entered into a clinical service agreement with Fortrea Inc. (formerly Labcorp Drug Development Inc.), a third- party CRO. The term of the agreement is through the earlier of August 14, 2025, or the completion of provision of services and the payment of contractual obligations. The budgeted costs for the services to be provided is approximately $12.1 million. Because of our decision to discontinue and pause further accrual of all clinical studies, we are negotiating a revision to the services required under the change in circumstances.
iOx (iNKT) License
On July 1, 2015, iOx entered into a licensing agreement with Ludwig Institute for Cancer Research Ltd. (“LICR”), which covers certain technology, intellectual property and know-how and development with respect to iNKT cell agonists to treat human diseases. Under the terms of the licensing agreement (“LICR License”), LICR granted to iOx an exclusive worldwide license, with the right to grant sublicenses, under the Licensed Patent and Licensed Technology, each as defined in the LICR License, in each case, to develop, make, have made, use, sell, offer for sale and import Licensed Products, as defined in the LICR License, subject to certain rights retained by LICR for academic and research purposes. The LICR License provides for a royalty term of ten years after the first commercial sale, on a Licensed Product by Licensed Product, country by country basis. Upon the expiration of the applicable royalty term, the license with respect to such Licensed Product in such country will convert to a non-exclusive, fully paid-up license.
LICR is entitled to 15,000 GBP as an annual license fee on each annual anniversary of the effective date of the LICR License until royalties become duly payable and 15,000 GBP as a patent reimbursement fee until LICR has been fully reimbursed for all patent costs incurred prior to the LICR License.
Additionally, LICR is entitled to milestone payments totaling up to 20.45 million GBP based upon the first Licensed Product achieving specific clinical, regulatory and sales-based milestones. LICR is also entitled to milestone payment totaling up to 10.25 million GBP based upon a second Licensed Product achieving specific clinical, regulatory and sales-based milestones.
Finally, LICR is entitled to a low-single digit royalty on net sales of Licensed Products that marginally escalates upon sales levels all determined by territory. LICR is also entitled to a percentage of any sublicensing income that gradually decreases based on the stage of development of the most advanced Licensed Product that is the subject of the applicable sublicense agreement.
Pursuant to the terms and conditions of the LICR License, LICR is responsible for managing the preparation, filing, prosecution and maintenance of all Licensed Patent Rights, as defined in the LICR License. iOx will reimburse LICR for all reasonable patent costs it incurs after the effective date of the LICR License. Further, the LICR License provides that both parties have the right to termination for material breach or default in the performance of obligations under the LICR License by the other party and in the event of insolvency of the other party.
Tarus (adenosine) License
On July 1, 2022, we acquired Tarus Therapeutics, Inc. Pursuant to the license agreement entered into by Tarus Therapeutics, Inc. and Impetis Biosciences Limited (“Impetis”) dated October 29, 2019 (“Impetis License”), Impetis granted to Tarus an exclusive sublicensable worldwide license to develop and commercialize the adenosine receptor antagonists for all indications and certain other assets which were granted upon exercise of a call option on November 5, 2020.
Under the terms of the Impetis License, Impetis is eligible to receive payments totaling up to $38 million on an Impetis Compound (as defined in the Impetis License) based upon achievement of certain clinical and commercial milestones. Milestone payments due in the amount of USD $1 million for achievement of certain regulatory milestones were paid in July 2022 and a $0.5 million milestone was paid upon dosing the first patient in September 2023.
Additionally, commencing upon the First Commercial Sale (as defined in the Impetis License) of a Licensed Product (as defined in the Impetis License), Impetis is entitled to royalties on worldwide net sales that begin in the mid-single digits and escalate through multiple tiers, with net sales over $1 billion receiving low double digit royalties.
Pursuant to the terms and conditions of the Impetis License, Tarus has exclusive and full authority to manage all intellectual property (whether licensed or not) underlying the assets covered by the Impetis License and any other aspects related to exploitation, development and commercialization thereof at its own cost, and Impetis must provide Tarus reasonable assistance as requested at Tarus’ cost and expense. Further, the Impetis License provides that both parties have the right to termination for material breach by the other party and in the event that the other party undergoes certain events such as a voluntary winding-up, a liquidation or entry into receivership.
Off-balance Sheet Arrangements
As of September 30, 2024 and March 31, 2024, we did not have any off-balance sheet arrangements, including any relationships with unconsolidated entities or financial partnership to enhance perceived liquidity.
Transactions with Related Parties
Significant related party transactions are detailed in Note 13, “Related Party Transactions,” to the unaudited condensed consolidated interim financial statements for the three and six months ended September 30, 2024 included elsewhere in this Report.
Financial and Derivative Instruments
Our financial instruments recognized in our condensed consolidated interim statements of financial position consist of the following:
Fair value estimates are made at a specific point in time, based on relevant market information and information about financial instruments. These estimates are subject to and involve uncertainties and matters of significant judgment; and therefore, these estimates cannot be determined with precision. Changes in assumptions could significantly affect these estimates.
The following table summarizes our financial instruments as of September 30, 2024 and March 31, 2024 (in thousands):
| | | September 30, 2024 (Unaudited) | | March 31, 2024 |
| | | | Amortized Cost | | | | FVTOCI | | | | FVTPL | | | | Amortized Cost | | | | FVTOCI | | | | FVTPL | |
| Financial assets | | | | | | | | | | | | | | | | | | | | | | | | |
| Cash and cash equivalents | | $ | 1,764 | | | $ | — | | | $ | — | | | $ | 5,028 | | | $ | — | | | $ | — | |
| Prepaid expenses and other current assets | | $ | 922 | | | $ | — | | | $ | — | | | $ | 2,667 | | | $ | — | | | $ | — | |
| | | September 30, 2024 (Unaudited) | | March 31, 2024 |
| | | | Amortized Cost | | | | FVTPL | | | | Amortized Cost | | | | FVTPL | |
| Financial liabilities | | | | | | | | | | | | | | | | |
| Accounts payable and accrued liabilities | | $ | 845 | | | $ | — | | | $ | 2,836 | | | $ | — | |
| Warrant liability | | $ | — | | | $ | 1,138 | | | $ | — | | | $ | 1,564 | |
A summary of our risk exposures as it relates to financial instruments are reflected below.
Fair value of Financial Instruments
Our financial assets and liabilities are comprised of cash and cash equivalents, receivables and investments in equities and public entities, accounts payable and accrued liabilities, lease liability, and warrant liability.
We classify the fair value of these transactions according to the following fair value hierarchy based on the amount of observable inputs used to value the instrument:
| • | Level 1 – Values are based on unadjusted quoted prices available in active markets for identical assets or liabilities as of the reporting date. |
| • | Level 2 – Values are based on inputs, including quoted forward prices for commodities, time value and volatility factors, which can be substantially observed or corroborated in the marketplace. Prices in Level 2 are either directly or indirectly observable as of the reporting date. |
| • | Level 3 – Values are based on prices or valuation techniques that are not based on observable market data. Investments are classified as Level 3 financial instrument. |
Assessment of the significance of a particular input to the fair value measurement requires judgment and may affect the placement within the fair value hierarchy.
Management has assessed that the fair values of cash and cash equivalents, other receivables and accounts payable approximate their carrying amounts largely due to the short-term maturities of these instruments.
The following methods and assumptions were used to estimate their fair values:
Warrant Liability: The fair value is estimated using a Black-Scholes model and in certain cases, a Monte Carlo simulation (Level 3).
Credit Risk, Liquidity Risk and Foreign Currency Risk
Our financial instruments are exposed to certain financial risks: Credit Risk, Liquidity Risk and Foreign Currency Risk.
Credit Risk
Credit risk is the risk of loss associated with a counterparty’s inability to fulfill its payment obligations. The credit risk is attributable to various financial instruments, as noted below. The credit risk is limited to the carrying value as reflected in our condensed consolidated interim statements of financial position.
Cash and cash equivalents: Cash and cash equivalents comprise cash on hand and amounts invested in underlying treasury and money market funds that are readily convertible to a known amount of cash with three months or less from date of acquisition and are subject to an insignificant risk of change in value. As of September 30, 2024 and March 31, 2024, cash equivalents was comprised of a money market account with maturities less than 90 days from the date of purchase. Cash and cash equivalents are held with major international financial institutions and therefore the risk of loss is minimal.
Liquidity Risk
Liquidity risk is the risk that we will encounter difficulty in satisfying financial obligations as they become due.
Our approach to managing liquidity is to ensure, as far as possible, that we will have sufficient liquidity to meet our liabilities when due, under both normal and stressed conditions without incurring unacceptable losses or risking harm to our reputation. We hold sufficient cash and cash equivalents to satisfy current obligations under accounts payable and accruals.
We monitor our liquidity position regularly to assess whether we have the funds necessary to meet our operating needs and needs for investing in new projects.
As a biotech company at an early stage of development and without significant internally generated cash flows, there are inherent liquidity risks, including the possibility that additional financing may not be available to us, or that actual drug development expenditures may exceed those planned. The current uncertainty in global capital markets could have an impact on our future ability to access capital on terms that are acceptable to us. There can be no assurance that required financing will be available to us.
Foreign Currency Risk
While we operate in various jurisdictions, substantially all of our transactions are denominated in the U.S. Dollar, except the deferred tax liability in the U.K. settleable in British pound sterling and the Stimunity Convertible Note receivable settleable in euros.
Use of Estimates and Judgments
The preparation of the condensed consolidated interim financial statements in conformity with IFRS requires management to make judgments, estimates and assumptions that affect the application of accounting policies and the reported amounts of assets, liabilities, income and expenses. Actual results may differ from these estimates.
Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
Significant areas where estimates are made include valuation of financial instruments (including deferred tax assets and liabilities, warrant liabilities, research and development costs, contingent consideration assumed and measurement of share-based compensation. Significant areas where critical judgments are applied include in-process research and development and warrant liabilities.
New Accounting Standards, Interpretations and Amendments
We are also unaware of any applicable but not-yet-adopted standards that are expected to materially affect our financial statements for future periods.
Internal Control Over Financial Reporting
Our management, including the CEO and CFO, is responsible for establishing and maintaining adequate internal controls over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act). Our internal control system was designed to provide reasonable assurance to our management and our Board regarding the reliability of financial reporting and preparation and fair presentation of published financial statements for external purposes in accordance with IFRS. Internal control over financial reporting includes those policies and procedures that:
| 1. | pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets; |
| 2. | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with IFRS, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and |
| 3. | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that have a material effect on the financial statements. |
All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions or that the degree of compliance with the policies or procedures may deteriorate.
Management assessed the effectiveness of our internal control over financial reporting as of September 30, 2024. In making this assessment, it used the criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on the evaluation under these criteria, management identified material weaknesses in our internal controls over financial reporting and, as a result, management concluded that our internal control over financial reporting was not effective as of September 30, 2024.
Management identified the following material weaknesses in our internal control over financial reporting.
| • | Management was unable to perform an effective risk assessment or monitor internal controls over financial reporting; |
| • | Management lacks the number of skilled persons that it requires given the complexity of the reporting requirements that it has to make, which more specifically include the staff and expertise to (i) properly segregate duties and perform oversight of work performed and to perform compensating controls over the finance and accounting functions, (ii) establish and perform fair value estimates or subsequently monitor fluctuations in fair value estimates, and (iii) apply complex accounting principles, including those relating to business combination accounting, income taxes, warrant liabilities and fair value estimates; and |
| • | There are insufficient written policies and procedures in place to ensure the correct application of accounting and financial reporting with respect to the current requirements of IFRS and SEC disclosure requirements, some of which specifically relate to investment accounting and fair value measures, assessment of in-process R&D assets, share-based payments, carrying amounts of goodwill and intangible assets and business combination accounting. |
Public Securities Filings
Additional information, including our annual information in our Annual Report, is filed with the Canadian Securities Administrators at www.sedar.com and with the SEC at www.edgar.com.
22


