UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2008
Or
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 or 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission file number: 001-33382
SENORX, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 33-0787406 |
| (State of Incorporation) | (I.R.S. Employer Identification Number) |
3 Morgan Irvine, California | 92618 |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (949) 362-4800
Securities registered pursuant to Section 12(b) of the Act:
Title of each class: | Name of each exchange on which registered: |
| Common Stock, par value $0.001 | The NASDAQ Stock Market, LLC (NASDAQ Global Market) |
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such requirements for the past 90 days. Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act (check one):
| Large accelerated filer ¨ | Accelerated filer x | Non-accelerated filer ¨ | Smaller reporting company ¨ |
(Do not check if a smaller reporting company)
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the registrant’s common stock, held by non-affiliates of the registrant as of June 30, 2008 (which is the last business day of registrant’s most recently completed second fiscal quarter) based upon the closing price of such stock on the NASDAQ Global Market on that date, was $76,761,689. For purposes of this disclosure, shares of common stock held by entities and individuals who own 5% or more of the outstanding common stock and shares of common stock held by each officer and director have been excluded in that such persons may be deemed to be “affiliates” as that term is defined under the Rules and Regulations of the Securities Exchange Act of 1934. This determination of affiliate status is not necessarily conclusive.
At February 27, 2009, the Registrant had 17,331,451 shares of Common Stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Items 10, 11, 12, 13 and 14 of Part III of this Form 10-K incorporate information by reference from the registrant’s definitive proxy statement to be filed with the Securities and Exchange Commission within 120 days after the close of the fiscal year covered by this annual report.
SENORX, INC.
FISCAL YEAR 2008 FORM 10-K ANNUAL REPORT
TABLE OF CONTENTS
| PART I | ||
| Item 1. | Business | 1 |
| Item 1A. | Risk Factors | 19 |
| Item 1B. | Unresolved Staff Comments | 30 |
| Item 2. | Properties | 30 |
| Item 3. | Legal Proceedings | 30 |
| Item 4. | Submission of Matters to a Vote of Security Holders | 31 |
| PART II | ||
| Item 5. | Market for the Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 31 |
| Item 6. | Selected Financial Data | 33 |
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | 34 |
| Item 7a. | Quantitative and Qualitative Disclosures about Market Risk | 46 |
| Item 8. | Financial Statements and Supplementary Data | 46 |
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | 46 |
| Item 9A. | Controls and Procedures | 46 |
| Item 9B. | Other Information | 49 |
| PART III | ||
| Item 10. | Directors, Executive Officers and Corporate Governance | 49 |
| Item 11. | Executive Compensation | 49 |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 49 |
| Item 13. | Certain Relationships and Related Transactions and Director Independence | 49 |
| Item 14. | Principal Accountant Fees and Services | 49 |
| PART IV | ||
| Item 15. | Exhibits and Financial Statement Schedules | 49 |
| Signatures | 74 | |
-i-
PART I
This Annual Report on Form 10-K contains forward-looking statements within the meaning of the federal securities laws. These statements include, but are not limited to, those concerning the following: regarding future events, our future financial performance, business strategy, product introductions and plans and objectives of management for future operations, regulatory approvals, and clinical timelines. Forward-looking statements are subject to risks and uncertainties that could cause actual results and events to differ materially. For a detailed discussion of these risks and uncertainties, see the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section of this Form 10-K. We undertake no obligation to update forward-looking statements to reflect events or circumstances occurring after the date of this Form 10-K.
| ITEM 1. | BUSINESS |
Overview
We develop, manufacture and sell minimally-invasive medical devices that are used in the diagnosis of breast cancer. Our initial product focus has been biopsy systems and breast tissue markers and in January 2008 we launched a radiation balloon catheter for localized partial breast radiation therapy. With the emergence of clinicians coordinating multi-disciplinary patient care through integrated breast centers, we believe that our ability to provide a broad array of products will enhance our competitive positioning. Since we launched our first breast tissue marker products in 2002, we have established over 1,000 customer accounts. In 2008, we generated net revenues of $46.7 million. The sale of disposable products, including our breast biopsy probes, radiation therapy products and tissue markers, accounted for 87.3% of our revenues in 2008.
The EnCor system, our flagship product for use in breast biopsy procedures, is a minimally-invasive vacuum-assisted breast biopsy system. EnCor allows users to obtain multiple biopsy samples with a quick, single probe insertion. In contrast to existing competitive systems, EnCor is the only commercialized “open/closed” tissue collection system compatible in all three imaging modalities, providing the operator with a clear view of tissue samples through a proprietary transparent collection chamber, and the ability to either open the chamber to examine and remove one or more samples or to continue uninterrupted collection of multiple samples. EnCor also incorporates novel programmability, which allows the user to select automated cutting patterns, tissue density, number of samples, and to deliver anesthetic. The EnCor system’s handpieces and disposable probes are compatible with the most commonly used imaging modalities, including x-ray, ultrasound, magnetic resonance imaging, MRI, and positron emission tomography, or PET. With its simplicity and versatility, we believe that EnCor can play an important role in the paradigm shift from invasive open surgical to minimally-invasive biopsy procedures. We launched the EnCor system on a limited basis and conducted marketing preference testing in late 2004 and subsequently progressed with a full commercial launch in November 2005. In 2007, we further enhanced the versatility of the EnCor system with the FDA clearance in the United States of our new SenoSonix system, a combination of EnCor with state-of-the art ultrasound imaging technology, and with the commercial launch of VisiLoc, an MRI visible obturator that helps to facilitate accurate probe placement under MRI guidance. As of December 31, 2008, we had an installed base of 776 EnCor systems and we had sold more than 205,000 EnCor disposable probes.
Our Contura Multi-Lumen Radiation Balloon Catheter, or Contura MLB, our flagship radiation therapy product, for which we received FDA 510(k) clearance in May 2007 and launched in January 2008, is designed to be a novel localized partial breast radiation therapy device that uses vacuum to remove excess seroma and air to enhance conformance of often irregularly shaped lumpectomy cavity walls to the balloon’s surface in order to deliver precise radiation dosing through multiple radiation source lumens. We believe that Contura MLB can play an important role in the paradigm shift from traditional whole breast radiation therapy to localized partial breast radiation therapy.
Our headquarters is in Irvine, California and we were incorporated in Delaware in January 1998.
Industry Overview
Breast Cancer
One in eight women in the United States will develop breast cancer during her lifetime, a risk that was one in fourteen in 1960. It is estimated that in the United States, approximately 192,000 new cases of breast cancer were diagnosed in 2008. Breast cancer is the second-leading cause of cancer-related death in U.S. women overall, and the leading cause of cancer-related death for women of ages 20 to 59.
-1-
Over 70% of breast cancers occur in women who have no identifiable risk factor other than age. The older a woman is, the greater her chance of getting breast cancer. One of the major challenges in the treatment of breast cancer is that, while the disease typically does not show symptoms in early stages, survival is dramatically impacted by the stage at which the disease is diagnosed and treated. If breast cancer is detected at an early “localized” stage and treated, the 5-year survival rate is 98%. If the cancer has spread to nearby lymph nodes, the 5-year survival rate decreases to 81%. If the cancer has spread, or metastasized, to organs such as the lungs, bone marrow, liver or brain, the 5-year survival rate falls to 26%. These statistics underscore the need for early diagnosis and treatment of breast cancer. Currently, 62% of breast cancers are discovered at an early, localized stage. The number of breast biopsies performed annually has increased significantly since 1997 when the American Cancer Society updated its guidelines for breast cancer screening, recommending that women should begin annual screening at age 40 rather than the previously recommended age 50. However, some studies conclude that annual breast cancer screening by mammography for women under age 50 may be more harmful, due to increased radiation exposure, than beneficial and as a result of this and other factors, the trend towards earlier and broader screening programs may not continue or mammography may be supplemented by other screening modalities in the future. However, outside of the United States, mammography screening programs are in the early stage of adoption, which has been accelerating in the last several years.
Breast Cancer Screening, Diagnosis and Treatment
The principal means of breast cancer screening are physical examination and mammography. In a physical examination, the patient’s breast is examined to search for palpable lesions or any other abnormalities. However, physical examination cannot detect small, early stage lesions that may be cancerous. As a result, mammography, a low-dose x-ray imaging technique, is recognized as the best screening method for detecting breast cancer in its earliest stages, when the disease is most successfully treated and there are more treatment options. Historically, mammograms are believed to find 85% to 90% of breast cancers in women over 50, and can discover a lesion one to four years before a lump can be felt. Newer technologies, such as digital mammography and tomosynthesis, are being introduced into the market and may help increase the lesion detection rate. Nevertheless, for patients with dense breast tissue, breast implants or patients who are breastfeeding, the images produced by a mammogram can be difficult for a radiologist to interpret. Consequently, physicians will often order a secondary screening using ultrasound, or, in some cases, MRI.
If breast cancer screening detects a lesion, a physician will typically recommend that the patient undergo a breast biopsy, a diagnostic procedure in which breast tissue samples are extracted to determine whether a lesion is benign or malignant. The breast biopsy procedure is performed by either a radiologist or surgeon. As a final step in the biopsy procedure, the physician usually places a tissue marker at the location from which the sample was removed as a point of reference. If the sample is found to be cancerous and more tissue must be removed from the breast, the marker will help the physician identify the specific area from which tissue should be removed. This can minimize the amount of healthy tissue removed from the breast during surgery. If surgery is not required, the marker will be visible on future screenings to enable the physician to identify the site of the previous biopsy.
If a breast biopsy indicates that a patient’s lesion is malignant, the patient is often scheduled for surgery to remove the tumor and to sample nearby lymph nodes to determine if the cancer has spread. Surgical procedures include a breast conserving therapy, known as lumpectomy, in which the cancerous lesion and a margin of surrounding normal tissue is removed, and mastectomy, in which the entire breast is removed. It is estimated that at least 50% of women with breast cancer, typically women whose breast cancer was detected at an early stage, are good candidates for lumpectomies. In most cases, a course of radiation therapy after lumpectomy is part of the treatment, as a means of destroying any cancer cells that may remain. Additionally, 75% of women who have mastectomies go on to have surgical reconstruction of one or both breasts, either using artificial implants or their own body tissue to rebuild the breast. Some women who have lumpectomies also choose breast reconstruction for cosmetic improvement.
Evolution of Breast Biopsy Procedures
In the United States, there are approximately 1.8 million breast biopsies performed annually. This number has increased significantly since 1997, at which time approximately 750,000 biopsies were performed. In 1997, the American Cancer Society updated its guidelines for breast cancer screening, recommending that women should begin annual screening at age 40. The previous guideline had recommended annual mammography for women beginning at age 50. This updated guidance, along with increased public awareness and technological improvements in screening and diagnostic equipment, likely has contributed to the increase in breast biopsies over the past decade. Biopsy methods include surgical biopsy, needle biopsy, and vacuum-assisted biopsy, all of which, according to the American Cancer Society, have similar accuracy rates.
-2-
Surgical Biopsy. Traditionally, most breast biopsies were performed as open surgical procedures, and such procedures remain common today, often being preferred for large lesions. The procedure has several drawbacks. Surgery is highly invasive, requires at least one full day of recovery and can leave a visible scar and depression at the site of the removed tissue. It can also lead to scar tissue formation within the breast, which can complicate the interpretation of follow-up mammograms.
Needle Biopsy. Needle biopsy emerged as the first minimally-invasive biopsy technique, enabling extraction of tissue samples without surgery, but rather through insertion of a needle to remove tissue. The typical procedure involves repeated needle insertions to acquire multiple samples. If the breast lesion is large enough to feel, the physician can do a needle biopsy by directly guiding the needle into the lesion. If the lesion is too small or too deep within the breast to be felt, a needle biopsy is done using breast imaging methods to guide the needle into the lesion. There are two types of needle biopsy:
| • | Fine needle aspiration uses a fine-gauge, hollow needle and a syringe to sample clusters of cells from a lesion. While fine needle aspiration is the fastest and simplest procedure among all biopsy methods, it is typically used only on lesions that are large enough to be felt. An experienced breast cytopathologist is required to determine if cells are cancerous, but this method cannot distinguish between cancer that remains confined to particular cells, known as in situ cancer, from invasive cancer that has spread to surrounding tissue, two types of cancer that are generally treated differently. |
| • | Core needle biopsy uses an inner notched needle and a larger outer hollow needle, which are sequentially advanced, to cut and collect single tissue samples. Compared to fine needle aspiration, core needle biopsy allows for a more accurate assessment of a breast lesion because the larger core needle usually removes enough tissue for the pathologist to evaluate abnormal cells in relation to the surrounding small sample of breast tissue taken in the specimen. However, this method is not well-suited to characterizing small lesions or calcifications that may indicate early cancers. False negatives may occur if the needle misses the lesion and instead takes a sample of normal tissue, which may lead to the undiagnosed cancer going untreated. Also, it is sometimes difficult to penetrate dense breast tissue with core needles. |
Vacuum-Assisted Biopsy. In a single sample, vacuum-assisted biopsy is able to remove approximately six to ten times as much breast tissue as core needle biopsy. Vacuum-assisted biopsy incorporates a special probe that can capture tissue samples from a single insertion into the breast through a small nick in the skin, promoting minimal patient discomfort and a relatively short procedure time. Vacuum-assisted biopsy is typically performed with imaging technology, either using a specially designed stereotactic x-ray table to pinpoint the site of the lesion, or with real-time ultrasound guidance. During the procedure, vacuum is used to draw tissue from the lesion into an opening located on or at the end of the biopsy probe, and a cutter is then used to sever this tissue sample. Among the minimally-invasive biopsy options, vacuum-assisted biopsy is the only method that can obtain multiple contiguous tissue samples with a single insertion, which makes the procedure an attractive alternative for most lesions, including those that may be indicative of early stage cancer.
A vacuum-assisted biopsy device can either be an “open” or “closed” system. An open system requires each sample to be individually cut, extracted and removed from the device, a relatively time consuming process that requires the assistance of a technician. The first open system vacuum-assisted biopsy device, the Mammotome, was introduced in 1995. A closed system automatically transports samples to a sealed tissue collection chamber, allowing multiple samples to be collected without having to interrupt collection by removing tissue from the device. In 2002, a closed system vacuum-assisted biopsy device called the ATEC was introduced. Both open and closed systems have been widely adopted. While closed systems may result in faster procedure times and minimize fluid loss, open systems allow visualization of the sample, which may be preferred by a technician to ensure that the proper area of the breast is being targeted. Today, in many cases customers prefer a vacuum-assisted biopsy system that is compatible with all three major imaging modalities, stereotactic, ultrasound, and MRI.
-3-
According to the Millennium Research Group, vacuum-assisted biopsies became the predominant biopsy method, surpassing open surgical breast biopsy procedures in 2007. Vacuum-assisted biopsies are already the predominant minimally-invasive method, accounting for 681,700 of 1,839,500 such procedures performed in the United States in 2008. Millennium projects that vacuum-assisted biopsies will account for 1,075,900 of 2,342,200 such procedures performed in the United States in 2013.
Evolution from Whole to Partial Breast Radiation Therapy
Following a lumpectomy to remove a cancerous breast tumor, many patients are subsequently treated with breast radiation therapy to destroy any cancer cells that may remain. Similar to the evolution in breast biopsy toward minimally-invasive procedures, radiation therapy is beginning to transition from whole breast radiation, which is currently used in the vast majority of cases, to more localized radiation therapy.
Whole Breast Radiation. Following a lumpectomy, the current standard of care is to treat patients with external beam radiation that is widely directed at the whole breast. Although the use of radiation has improved long-term survival rates, this treatment is inconvenient for patients, often requiring daily outpatient radiation treatments for six to eight weeks, and can expose healthy tissue and organs to damaging effects from the radiation.
Accelerated Partial Breast Irradiation, or APBI. APBI delivers localized radiation to a targeted surgical site. For appropriate patients, this method offers a number of advantages including greatly-diminished treatment time, concentrated radiation exposure and the reduction of skin irritation and burning. There are currently three approaches to APBI in the market.
| • | Radiation Balloon Brachytherapy. This approach involves a catheter attached to a fluid expandable balloon that is inserted into the lumpectomy cavity. A mixture of saline and contrast media is injected to expand the balloon to contact the walls of the lumpectomy cavity, a radioactive source is then inserted into the balloon. Radiation is administered for five to ten minutes, allowing a therapeutic radiation dose to penetrate tissue approximately one centimeter from the exterior of the balloon. Typically, this procedure is repeated twice a day for five days on an outpatient basis. While the technical challenges are fewer than with other partial breast radiation treatment options, delivering a uniform radiation dose remains an obstacle due to the difficulties in conforming the shape of the balloon to the walls of the often irregularly-shaped cavity, the site of the cavity, or accumulation of fluid from the body around the balloon. Radiation balloon brachytherapy is in the early stage of adoption by the market. |
| • | Conformal Radiotherapy. Like whole breast radiation, this approach uses a radiation source outside the body. Rather than targeting the entire breast, however, this approach typically uses a CT scan to pinpoint the tumor site in three dimensions and a computer program to aim radiation beams that “conform” closely to the shape of the tumor and thereby helping to minimize damage to healthy tissue. This approach is well-established in the treatment of prostate cancer, but is in the early stages of adoption and clinical study for breast cancer. Conformal radiotherapy may play a greater role in brachytherapy in the future. |
| • | Multi-Catheter Interstitial Brachytherapy. This approach involves placing 20 to 30 small catheters completely through the breast at carefully selected locations around the lumpectomy site. A radioactive source is inserted into the catheters twice a day for about 20 minutes to deliver radiation, typically over a one-week period, during which time the catheters remain in the breast. The multiple catheter placements may cause infection, as well as potential cosmetic damage. Additionally, the procedure requires a high level of technical expertise for appropriate catheter placement and radiation dose administration. |
Breast Care Market Trends
The breast care market has undergone a significant evolution over recent years, driven by advancements in imaging technologies, which has led a paradigm shift to less invasive devices and procedures for screening and for diagnosis, and to comprehensive patient care through integrated breast centers.
Advances in Imaging Technology for Screening. Digital mammography is being rapidly adopted as clinical studies suggest advantages over traditional film mammography. When the patient has dense breast tissue, breast implants or is breastfeeding, the images produced by either traditional or digital mammography can be difficult for a radiologist to interpret. Consequently, physicians will often order a secondary screening using ultrasound. MRI may also be used as a secondary screening method for these patients, and is being recommended as a primary screening technique for high-risk women as young as 30. Breast tomosynthesis, a newer form of digital mammography which is an emerging technology that is currently in the experimental development stage. Tomosynthesis is a process in which multiple 2D x-rays of the breast are combined using computer software to produce a more focused 3D image.
-4-
Advances in Imaging Technology for Biopsy. Technological advances in imaging have allowed for more effective and less invasive diagnosis and treatment of breast cancer. While stereotactic x-ray imaging and ultrasound guidance are used most frequently in conjunction with biopsy procedures, MRI may also become an important alternate imaging modality for both diagnosis and treatment.
| • | Stereotactic x-ray imaging uses x-ray to capture images of breast tissue. With the patient lying on a specialized treatment table known as a stereotactic table, x-ray images are taken from two angles which permits integrated computerized equipment to map the exact location of the target lesion, thereby enabling the physician to fire a biopsy needle or probe into the lesion. Stereotactic imaging is used in the vast majority of vacuum-assisted biopsies today. |
| • | Ultrasound imaging bounces low-power, high-frequency sound waves off internal tissue to provide real-time images of the interior of the breast to guide the physician’s manual placement of a biopsy probe at the site of the lesion. Ultrasound is widely available and relatively inexpensive, and does not expose the patient to radiation. As with screening, ultrasound is used as a primary breast imaging application for biopsy of women who have dense breast tissue, typically under the age of 40, women with breast implants or women who are breastfeeding. |
| • | Magnetic resonance imaging (MRI) uses a magnet, radio waves and a computer to make a series of detailed images of the inside of the breast. Technological advances have made MRI an emerging alternative for image-guided biopsy. Several academic institutions and leading breast centers have begun performing biopsies under MRI guidance. Although there are several hurdles, including the relatively high cost of and significant time required for this procedure, the clinical benefits of this approach are gaining acceptance. |
Paradigm Shift to Less Invasive Procedures. Advancements in imaging technology have allowed abnormal breast tissue to be identified at an early stage and have helped facilitate the emergence of novel, less invasive diagnostic and therapeutic devices for accessing and removing this tissue. For example, open surgical biopsies and needle biopsies are giving way to minimally-invasive vacuum-assisted procedures. Similarly, excision of tumors is shifting from invasive mastectomies to less invasive lumpectomies. In addition, radiation treatment is shifting from whole breast radiation to partial breast radiation.
Emergence of Integrated Breast Centers. Effective screening, diagnosis and treatment of breast cancer require interaction among specialists in multiple departments of a healthcare system. These include, but are not limited to, surgery, oncology, radiology, pathology and plastic surgery. While many of these services exist in any given healthcare system, the concept of a breast center is to organize these services into a coordinated, multidisciplinary approach where the patient’s care is integrated. This coordinated approach allows for higher-quality and more patient-focused care than she might receive from the same specialists working in isolation. Breast centers can be at one physical location, with all services available at one site, or they can be virtual, organizing the interaction of diverse services found at different locations. A factor in the accelerating establishment of integrated breast centers is the increasing public awareness of the importance of quality breast care. Breast centers are also actively educating the general public as it relates to the latest clinical and technological advances available in minimally-invasive diagnosis and treatment.
We believe that there is significant opportunity for a company that offers breast centers a full range of minimally-invasive diagnostic and therapeutic devices that are both compatible with multiple imaging modalities and flexible enough to be tailored to the diverse needs of the physicians on the breast care team.
Our Solution
We have commercialized, and are continuing to develop, a broad product line of minimally-invasive breast care devices to be used by breast care specialists. By focusing on the continuum of care from diagnostic to excision and therapeutic procedures, we believe that we will be an attractive and convenient supplier for integrated breast centers.
-5-
Our Breast Care Management Product Continuum

Our current commercial products and products under development include:
| • | Diagnostic. Breast biopsy systems, location and lymph node gamma ray detection devices. |
| • | Marking. Tissue markers which identify the biopsy site, under the major imaging modalities, for future diagnostic and surgical reference, and are compatible with most imaging technologies and biopsy devices. |
| • | Lesion Location. Devices used to facilitate lesion excision and treatment. |
| • | Treatment. Radiation balloons and other devices for localized partial breast radiation therapy. |
| • | Excising. Tissue cutting devices designed to facilitate the contoured removal of lesions and facilitate the use of balloons in radiation therapy. |
The EnCor system, our flagship product for use in breast biopsy procedures, is a vacuum-assisted system which allows users to obtain multiple tissue samples with a quick, single insertion. EnCor can be used with multiple imaging modalities, including stereotactic x-ray, ultrasound and MRI. EnCor is the only “open/closed” tissue collection system compatible in all three imaging modalities, providing the operator with a clear view of tissue samples through a proprietary transparent collection chamber, and the ability to either open the chamber to examine and remove one or more samples or to continue uninterrupted collection of multiple samples. Our EnCor system also incorporates proprietary programmability and automation features which provide a competitive advantage to other marketed biopsy systems. In 2007, we further enhanced the versatility of the EnCor system with the FDA clearance in the United States of our new SenoSonix system, a combination of EnCor with state-of-the art ultrasound imaging technology, and with the commercial launch of VisiLoc, an MRI visible obturator that helps to facilitate accurate probe placement under MRI guidance.
The Contura Multi-Lumen Radiation Balloon Catheter, or Contura MLB, our flagship radiation therapy product, for which we received FDA 510(k) clearance in May 2007 and launched in January 2008, is designed to be a novel localized partial breast radiation therapy device that uses vacuum to remove excess seroma and air to enhance conformance of often irregularly shaped lumpectomy cavity walls to the balloon’s surface in order to deliver precise radiation dosing through multiple radiation source lumens.
Our Strategy
Our goal is to become the leader in providing minimally-invasive solutions across the continuum of care in the breast care market. The key elements of our business strategy to achieve this goal are to:
| • | Provide Differentiated, Tailored Solutions in the Breast Biopsy Market. . We believe that our EnCor breast biopsy system represents a significant advancement in the breast biopsy market. We seek to leverage this recent product introduction to establish a leadership position in the minimally-invasive breast biopsy market. We believe that by making the EnCor system modular and upgradeable, which enables the addition of features over time, customers will view it as an attractive platform product that can be tailored to the needs of their practice. The EnCor system allows for significant flexibility across multiple imaging modalities, programmability, automation and the ability to shift between open and closed tissue collection. In addition to EnCor, we believe that EnCor 360 and SenoSonix with EnCor is a complementary product that will continue to address a need in the ultrasound guided vacuum-assisted biopsy market. We intend to continue to develop and commercialize advanced products in the minimally-invasive breast biopsy market, such as VisiLoc, which helps to facilitate accurate biopsy probe placement under MRI. Additionally, we believe that the EnCor hardware architecture and the ease of software upgrades allows us to continuously and easily bring technological improvements to market and to easily interface with imaging equipment sold by a broad spectrum of manufacturers. |
-6-
| • | Focus on leveraging existing R&D Competencies to Develop Products Across the Continuum of Interventional Breast Care. While our initial product focus has involved devices used in diagnosis of breast cancer, such as biopsy systems and breast tissue markers, we launched Contura MLB in January 2008 and are also developing a series of additional lesion location, excision and therapeutic products that we will seek to commercialize over the next few years. With the emergence of integrated breast centers designed to provide comprehensive and specialized patient care, we believe that our ability to provide novel solutions to a broad set of needs in breast cancer management will enhance our competitive positioning in the market. The Company is also working on future generations of biopsy products. |
| • | Leverage Clinical Education with Key Opinion Leaders in Breast Care Centers. We believe that integrated breast centers are emerging as the focal point for breast care, with teams of surgeons, radiologists, oncologists and technicians providing coordinated care. Our products, spanning the continuum of breast care from diagnostics to therapeutics, positions us to meet many clinical needs of breast centers. As a key element of our strategy, we focus on educating and training clinicians on our products through frequent hands-on classes and industry events. We have worked with key opinion leaders in training several thousand clinicians in the effective use of our products. |
| • | Capitalize on Product Cross-Selling Opportunities within Our Existing Customer Base. We believe that we have a significant opportunity to grow our revenues by selling additional products to existing customers. We have established a strong base of customers who have a history of placing repeat orders for our products. We believe this customer base is an attractive target for early adoption of our complementary products as they are commercially launched. For example, our EnCor and EnCor 360 biopsy hardware, disposables and tissue markers are sold to the same surgeon customers that are purchasing Contura MLB. |
| • | Pursue Strategic Acquisitions and Partnerships. In addition to adding to our product portfolio through internal development efforts, we intend to explore the acquisition of other product lines, technologies or companies that may leverage our sales force or be complementary to our strategic objectives. We may also evaluate distribution agreements, licensing transactions and other strategic partnerships, which may include expansion of our selling and marketing efforts beyond the United States. We are also collaborating with a number of large and smaller corporations who sell imaging products to offer our customers bundled packages of products and joint clinical education programs. |
-7-
Our Products and Products under Development
We are focused on developing and offering a broad portfolio of products that address needs across the continuum of breast care, from the diagnosis to the treatment of breast cancer. The sale of disposable products, including our breast biopsy probes, tissue markers and radiation therapy products, accounted for 87.3% of our revenues in 2008, 86% in 2007 and 89% in 2006. The following table provides information concerning our primary products and products under development.
| Product Category/Name | Primary Component(s) | Year (or Expected Year) of Full Commercial Launch |
| DIAGNOSTIC PRODUCTS | ||
| Breast Biopsy | ||
| SenoRx Breast Biopsy Console | Console for EnCor and EnCor 360 Biopsy Devices | 2002 |
| EnCor | Reusable Handpiece and Disposable Probe | 2005(1) |
| EnCor 360(3) | Reusable Handpiece and Disposable Probe | 2003(1) |
| VisiLoc | Obturator Compatible with EnCor MRI | 2008 |
| SenoSonix | EnCor Hardware Integration with ultrasound imaging | 2008 |
SenoSonix Handcarry | EnCor hardware with handcarry ultrasound component | 2009 |
| Tissue Markers and Location Devices | ||
| Gel Mark | Applicator and Combination Metal/Bioresorbable Markers | 2002 |
| Gel Mark Ultra | Applicator and Combination Metal/Bioresorbable Markers | 2004 |
| Gel Mark UltraCor | Applicator and Combination Metal/Bioresorbable Markers | 2004 |
| SenoMark | Applicator and Combination Metal/Bioresorbable Markers | 2006 |
| Gel Mark UltraCor MRI | Applicator and Combination Metal/Bioresorbable Markers | 2006(1) |
| Tissue Marker Line Extension | Applicator and Combination Metal/Bioresorbable Markers | 2009 |
Starch Mark | Metal/Bioresorbable Markers | 2009 |
Loc Wire Replacement | Signal transmission devices | 2010 |
| Gamma Ray Detection | ||
| Gamma Finder | Reusable Probe and Disposable Sleeve | 2003 |
| THERAPEUTIC/EXCISION PRODUCTS | ||
| Radiation Therapy | ||
| Contura MLB | Radiation Balloon | 2008 |
| Contura Shape Select MLB | Contura MLB that expands to multiple shapes | 2009 |
CED Device | Radiation Balloon Introducer | 2009 |
| Excision and Reconstruction | ||
| SenoPulse RF Generator | Console for Excision/Reconstruction Devices | 2011(2) |
| Single Step | Reusable Handpiece and Disposable Probe | 2011(2) |
| (1) | FDA clearance received and product available prior to full commercial launch. |
| (2) | Subject to submission for and receipt of FDA 510(k) clearance; initial preference testing may occur one year earlier. |
| (3) | Previously referred to by us and marketed as SenoCor 360. |
-8-
Breast Biopsy Systems
Components of Our Breast Biopsy Systems
Our breast biopsy systems primarily consist of two components—reusable handpieces and disposable probes—and are used in conjunction with our SenoRx Breast Biopsy Console.
| • | SenoRx Breast Biopsy Console. The SenoRx Breast Biopsy Console is compatible with both our EnCor and EnCor 360 reusable handpieces and disposable probes. This modular console is a portable hardware system which may be conveniently transported to various areas of the healthcare facility. The primary modules of our console include: |
| • | a control module, which facilitates convenient user interface with proprietary software, a visual display screen, and controls that allows the user to customize the various parameters of the diagnostic procedure; and |
| • | a vacuum system, which pulls tissue into the probe for excision and subsequent delivery of tissue to the sample collection chamber. |
| • | Reusable Handpieces. Handpieces are instruments which facilitate placement or insertion of the biopsy probe. The handpieces primarily consist of motors, circuitry, sensors and proprietary software incorporated into a housing. We commercialize three different biopsy handpieces: EnCor Stereotactic/Ultrasound, EnCor MRI, and EnCor 360. |
| • | Disposable Probes and Accessories. Our probes are sterile, single-use, vacuum-assisted disposables, which are used with our EnCor and EnCor 360 handpieces. They consist of a sharp stainless steel tissue cutter and, with EnCor, a tissue sample collection chamber. The probe accessories also include additional tubing and a vacuum canister. |
We offer probes in a variety of sizes, ranging from 7-gauge to 10-gauge, depending on user preference. The probe cutters and tips incorporate one or more of our proprietary tissue cutting technologies.
| • | Tri-Concave Tip. A three-edged tip used on our EnCor probes, EnCor 360 probes and MRI insertion device, designed especially for facilitating easy placement into dense breast tissue. |
| • | 360° Tissue Cutter. A hollow, cylindrical cutting edge used on our EnCor 360 probes, which automatically rotates and advances to generate large 360° contiguous samples. |
| • | Oscillating Cutters. Cone-shaped cutters used on our EnCor probes, which shear tissue in a manner similar to a scissors cut. |
In 2007, we also received clearance for and launched two additional products that are used together or in conjunction with our EnCor system.
| • | VisiLoc MRI Visible Obturator. An MRI visible obturator that is used during an MRI-guided EnCor procedure, designed to facilitate biopsy probe placement under MRI guidance. |
| • | SenoSonix with EnCor. An integration of our EnCor system with a state-of-the-art ultrasound imaging system developed by UltraSonix Medical Corporation. |
EnCor Breast Biopsy System
Our flagship product for use in breast biopsy procedures, the EnCor system, is a vacuum-assisted breast biopsy system that facilitates adoption of minimally-invasive biopsy procedures over open surgical biopsy. The EnCor system is comprised of a reusable handpiece and disposable probes that are used in conjunction with our SenoRx Breast Biopsy Console. We believe the EnCor system offers a comprehensive set of features which make it an attractive solution for meeting the diverse demands of breast care providers. Key features of the EnCor system include:
| • | Single Insertion/Multiple Sample. Offers the flexibility to obtain multiple samples from a single insertion, enhancing speed and convenience in biopsy procedures. |
| • | “Open/Closed” System. Functions either as an open or closed system, providing the operator with a clear view of tissue samples through a proprietary transparent collection chamber, and the ability to either open the chamber to examine and remove one or more samples or to continue uninterrupted collection and automatic transfer of multiple samples from inside the breast to the collection chamber. |
-9-
| • | Highly-Automated and Programmable. Automated and programmable tissue collection and automated anesthetic delivery. Aligns and rotates automatically through a variety of optional programmed cutting patterns. Provides multiple programmed options for users to choose their own approach to the array of lesions they may encounter. |
| • | Multi-Modality System. Compatible with each of the three major imaging modalities used in the market—stereotactic, ultrasound and MRI—and transportable, eliminating the need for multiple systems. |
| • | Modular Hardware and Upgradeable Design. Customers are able to purchase all or part of the system according to their needs, thus minimizing up-front costs. In addition, the modular system facilitates easy repair and replacement of components. Software-based design allows us to continuously innovate by adding new features which may extend the useful life of the device. The user may update the system by upgrading software rather than purchasing new hardware. |
| • | Precise Probe Placement. Tools to facilitate accurate placement of EnCor probe under MRI imaging. |
The EnCor system also incorporates a number of additional features, including compatibility with our tissue markers, multiple gauge sizes, automated sample rinsing, lighting, an ergonomically-designed handpiece, noise reduction and novel MRI probe insertion accessories and proprietary ultrasound options. We received FDA 510(k) clearance and conducted marketing preference testing of the EnCor system in 2004, with full commercial launch in 2005.
EnCor 360 Breast Biopsy System
Our EnCor 360 system utilizes a vacuum to provide the physician with a contiguous 360° breast biopsy sample. The EnCor 360 system incorporates our mechanical Tri-Concave Tip to penetrate virtually any lesion, regardless of size, location or density. EnCor 360 secures tissue through the end of the probe, providing a large, high-quality sample. Since the EnCor 360 is interchangeable and compatible with the SenoRx Breast Biopsy console, users may select EnCor 360 or EnCor depending upon their clinical and economic objectives.
We received FDA 510(k) clearance in 2002 and launched EnCor 360 in 2003, as our initial product in the vacuum-assisted breast biopsy segment. We intend to continue to offer the EnCor 360 as a low-cost, ultrasound-guided breast biopsy device. We believe that our EnCor 360 and future product enhancements will continue to appeal to clinicians doing ultrasound biopsies in their offices, which is a more price-sensitive segment of the biopsy market.
SenoSonix with EnCor
In October 2007 we received 510(k) clearance from the FDA for SenoSonix with EnCor, an integration of our EnCor system with a state-of-the-art ultrasound imaging system developed by UltraSonix Medical Corporation of Canada. We received the right to affix CE Mark in the European Union for SenoSonix with EnCor in April of 2008 and patents are currently pending. We are marketing SenoSonix with EnCor primarily for physician in-office procedures, particularly in Europe, where we believe a greater percentage of biopsy procedures are done under ultrasound imaging guidance. SenoSonix with EnCor may be used with either our EnCor or EnCor 360 probes. In 2009, we plan to introduce a new version of SenoSonix that will incorporate a “handcarry” ultrasound component, which will increase ease of portability.
VisiLoc MRI Visible Obturator
We launched the VisiLoc MRI Visible Obturator in the United States in November 2007. VisiLoc is an MRI visible obturator that is used during an MRI-guided EnCor procedure and is designed to help facilitate biopsy probe placement under MRI guidance.
Gel Mark and SenoMark (Biopsy Site Tissue Markers)
Biopsy site tissue markers are placed at a biopsy site to provide a visible landmark for future surgical reference. If cancer is found and more tissue must be removed from the breast, the marker will help the physician identify the specific area from which tissue should be removed. If surgery is not necessary, the marker will be visible on future mammograms to enable the breast care specialist to identify the site of the biopsy.
-10-
We offer a full portfolio of tissue markers that are compatible not only with our EnCor and EnCor 360 biopsy product lines, but also with competing biopsy systems. Our products consist of markers that come in a variety of materials, including gelatin and synthetic materials, titanium and stainless steel, and associated delivery applicators. The markers are designed to facilitate easy placement and optimize visibility under different imaging modalities.
We were first to commercialize markers visible not only under x-ray, but also ultrasound imaging. Gel Mark and Gel Mark Ultra are designed to provide pellet-shaped, ultrasound-visible, bioresorbable tissue marker alternatives. Gel Mark UltraCor provides core needle users with an ultrasound-visible tissue marking alternative. SenoMark provides those users who prefer a pad-shape with an ultrasound-visible, bioresorbable tissue marker alternative. Our UltraCor MRI may be an attractive alternative for clinicians interested in marking lesions under MRI guidance. We received FDA 510(k) clearance and began commercializing Gel Mark in 2002, Gel Mark Ultra and Gel Mark UltraCor in 2004, and SenoMark in 2006. We received FDA 510(k) clearance and conducted marketing preference testing of the Gel Mark UltraCor MRI in 2004, with full commercial launch occurring in the second half of 2006. The Company is also exploring the use of new types of tissue markers for use in other indications, including a locwire replacement used to stage the lumpectomy procedure. We intend to commence clinical trials for this product by 2010.
Gamma Finder (Gamma Ray Detection Device)
Immediately prior to removal of a malignant lesion in the breast, a patient may be injected with gamma ray emitting isotopes near the site of the lesion to determine if the cancer has spread. Our Gamma Finder is currently the only cordless handpiece probe to detect the emission of gamma rays, and, consequently, whether breast cancer has spread to the lymph nodes. The Gamma Finder detects and gives a numerical indication and an acoustic signal when close to a gamma ray emitting source. Our Gamma Finder has all the features of traditional, larger, corded gamma ray detection devices, with the convenience of a portable and compact device. The Gamma Finder consists of a reusable probe and a disposable sterility sleeve. We began commercializing the Gamma Finder in 2003 upon receipt of FDA 510(k) clearance, and, in 2005, added automatic ten second count and binary pitch mode features.
Contura MLB Radiation Balloon
Current radiation therapy includes less invasive alternatives to whole breast radiation therapy, known as partial breast radiation therapy, consisting of balloon brachytherapy, conformal radiotherapy and multi-catheter interstitial brachytherapy. We believe that balloon brachytherapy will be widely adopted over time due to its ease of use, low-cost and clinical effectiveness and in January 2008 launched our Contura Multi-Lumen Radiation Balloon Catheter, or Contura MLB.
Our Contura MLB design consists of a multilumen catheter with several access ports on one end and an inflatable balloon on the other. The balloon is positioned into the cavity formed in the breast following a lumpectomy and subsequently inflated with saline through one of the ports. Small openings in the catheter allow for the suction of excess seroma and air from the lumpectomy cavity through a second access port. We believe that this suction feature will help conform the walls of the lumpectomy cavity to the exterior of the balloon. Multiple lumens are designed to provide for precise placement of radioactive seeds, which we believe will allow clinicians to expand their use of radiation therapy to a greater number of patients. Such patients may include those with smaller anatomies and with tumors closer to the skin, which were not previously able to receive treatment with other minimally-invasive radiation balloon products. The overall design and the use of special balloon materials is intended to control the distance between the radiation source and the tissue in contact with the balloon and to result in controlled radiation dosing.
We received FDA 510(k) clearance for Contura MLB in May 2007 and launched in January 2008. Following the clearance of Contura MLB in May 2007, we began limited commercialization and market preference testing of the product. We anticipate developing and commercializing additional line extensions for this product over the next several years, including Contura Shape-Select MLB in 2009. Clinical investigators for Contura MLB have either presented, published or had accepted 16 articles or abstracts related to Contura MLB.
SenoPulse RF Generator (Excision/Therapeutic Console)
The SenoPulse RF Generator will be used to power our radiofrequency cutting technologies in order to provide advanced breast tissue cutting capabilities. The SenoPulse RF Generator offers high-frequency and impedance-matching circuitry to enable cutting into a wide variety of tissue types, high start voltage and sustained power for continuous cutting ability, and low heat generation to minimize thermal damage. The SenoPulse RF Generator directs modulated, monopolar radiofrequency energy and will be used in the Single Step cutting and excision devices. We received FDA 510(k) clearance for the SenoPulse RF Generator in 2005.
-11-
Tissue Cutting Devices for Excision and Reconstruction
Single Step is an alternative excision device to a scalpel or a straight-bladed electrosurgical scalpel commonly known as a Bovie. The Single Step system is an automated surgical excision device that uses a long-wire RF disposable probe and reusable handpiece to cut and remove a large, intact volume of tissue through a small surgical incision. The Single Step system is powered by the SenoPulse RF Generator. The Single Step probe is inserted into the breast, where the surgeon anchors the device and excises tissue of a predetermined size. The surgeon controls the amount and shape of the tissue removed by selecting the appropriate option on the SenoPulse RF Generator. The Single Step is designed to produce a smooth and optimally-shaped cavity to facilitate lesion removal and subsequent use of balloon brachytherapy. We intend to build upon data obtained with one of our previous products by evaluating the clinical benefit of anchoring or stabilizing lesions in conjunction with the automatic lesion cutting ability of our Single Step system. We received FDA 510(k) clearance for the Single Step and anticipate making several design enhancements and conducting clinical testing prior to fully commercializing the product in 2011. We have also received FDA 510(k) clearance for a second, long wire RF cutting device that may be useful in lesion excision and tissue reconstruction.
Sales and Marketing
We focus our sales and marketing efforts on increasing awareness of our products among breast care specialists, including radiologists, surgeons and oncologists. We market and sell our products through a direct sales force in the United States. As of December 31, 2008, we employed a vice president of global sales and a vice president of global marketing and support staff and a 67 person direct sales force, including 19 clinical specialists, eight brachytherapy specialists, six regional sales managers and 34 sales representatives.
In our selling process, we use clinical studies, cost-benefit data and case studies. To date, we have 39 clinical studies that have either been published or presented as abstracts at major medical meetings. Peer-to-peer selling is also a critical element of our strategy. We have developed popular training seminars, including a Continuing Medical Education-accredited course led by our internal personnel and by nationally-known breast cancer specialists Drs. Nathalie Duchesne, Mark Gittleman, Phillip Israel, Terese Kaske, Frank Vicini, Doug Arthur, Dorin Todor, Phillipe Sebag, Rakesh Patel and Robin Wilson who present our clinical data and/or products from time to time. We hosted 73 seminars in 2008, educating and providing hands-on training to over 1,575 clinicians about our products. We initiated a Contura MLB long-term physician registry study with more than 80 patients enrolled at the end of 2008.
An additional element of our educational efforts is our relationships with several manufacturers of ultrasound imaging systems. With these companies, we co-sponsor several breast practice seminars across the country to educate clinicians on the changes that are driving the specialization of breast care and the emergence of integrated breast centers. We also contribute to organizations designed to increase awareness of breast cancer, including our sponsorship of the newsletter and website of the American Society of Breast Surgeons.
International sales have not historically accounted for a significant portion of total sales, but have been increasing incrementally in recent years. We do not have a direct sales force outside of the United States. We have the authorization to affix the CE Mark to Gel Mark Ultra, Gel Mark UltraCor, and our EnCor system and to commercialize these devices in the European Economic Community, Hong Kong, Singapore, Taiwan, Mexico, South Korea, and Australia. In 2007, we partnered with local distributors who have breast imaging and/or interventional radiology franchises in Austria, Belgium, the United Kingdom, Hong Kong, Ireland, Luxembourg, The Netherlands, Singapore, Switzerland, and Taiwan to sell EnCor and our Gel Mark products in these ten countries. In 2009 we added distributors in Denmark, Finland, France, Germany, Iceland, Italy, Mexico, Norway, Portugal, Russia, South Korea, Spain and Sweden. We are currently in negotiations with distributors for a number of additional countries in which our products are already approved and intend to further expand beyond these countries in 2009. We also have agreements with distributors in China and in the Middle East that are assisting us in applying for regulatory approvals to market our products in those countries. Our International Distributors are managed by two sales managers who report to our Vice President of Global Sales and Business Development.
-12-
Competition
We compete primarily on the basis of our ability to provide minimally-invasive products to diagnose and treat breast cancer safely and effectively, with ease and predictability of product use, brand name recognition and cost. We believe that we compete favorably with respect to these factors, although we cannot assure you that we will be able to continue to do so in the future or that new products that perform better than those we offer will not be introduced.
The markets in which our products compete are highly competitive, subject to change and significantly affected by new product introductions and other activities of industry participants. We face different competitors within different product lines. To our knowledge, we do not have one competitor that produces products that compete with all our products. Several of our competitors have significant financial and human capital resources and have established reputations with our target customers, as well as worldwide distribution channels that are more effective than ours. We are aware that several companies are developing products that, if successfully commercialized, would compete with our current and future products.
Our vacuum assisted breast biopsy and tissue marker products compete with, among others, products sold by Johnson & Johnson, C.R. Bard and Hologic. A new vacuum assisted biopsy product from Rubicor is scheduled for launch in 2009. In November 2008, Hologic introduced the Eviva probe, which features an “open/closed” system. Contura MLB competes against well-established whole breast external beam radiation devices, as well as current and potential future manufacturers of balloon brachytherapy devices and image-guided targeted radiation beam therapy. We compete directly with the current industry leader, Hologic, as well as other companies that have minimally-invasive therapeutic devices in various stages of development. Short-term brachytherapy products from Cianna Medical and Xoft began to be commercialized in 2008. Our commercial success will depend on a general market shift from whole to partial breast radiation and our ability to overcome Hologic’s current market leadership with its current balloon product. Furthermore, we compete against Hologic with their product bundling programs for digital mammography and stereotactic table platforms. Our excision products will compete with manufacturers of handheld surgical excision instrumentation and standard RF cutting devices.
Our competitors dedicate, and we believe they will continue to dedicate, significant resources to promote their products aggressively. The breast cancer market is also characterized by extensive research efforts and technological progress. As a result, new products are likely to be developed and introduced into the market that could compete with our products more effectively.
Manufacturing
We assemble and package a significant portion of our finished products at our current corporate headquarters in Irvine, California, where we moved to in August 2008. Our Gamma Finder is licensed and produced exclusively for us by World of Medicine, a German medical device company. We manufacture in-house several components used in our products, and we rely on several outside vendors to produce many components, and in some cases completed products that we quality check, sterilize, and package at our corporate headquarters. We also have established a production engineering department to focus on integrating product changes into the manufacturing process and to continually improve upon product quality and cost.
We manufacture our proprietary products in a controlled environment and have implemented quality control systems as part of our manufacturing processes. We believe our manufacturing facility and control systems comply with the FDA’s Quality System Regulations, or QSRs. We are certified to ISO 13485:2003, the medical device manufacturing standard, and applicable medical device directives promulgated by the European Economic Community, which facilitates entry of our products into the European Economic Community. We have received our CMDCAS Certificate of Registration permitting importation of our devices into Canada.
Since 2005, we have continued to transfer portions of our manufacturing operations to Infus Medical, a contract manufacturer with facilities in Thailand, which currently provides us with certain tissue marker production, biopsy probe production and assembly and packaging services. We anticipate that over time we will continue to transfer additional responsibility to this manufacturer related to production, assembly and packaging. We believe that transferring production of our more established products abroad in a stepwise manner, along with increased sales volume, will result in cost savings and will allow us to focus our domestic efforts on developing, modifying and promoting our newer products. We will continue to produce certain of our products at our facility in Irvine, California for the foreseeable future.
-13-
We have one product and several components of other products that we obtain from sole-source suppliers. We rely on one vendor, World of Medicine, for our Gamma Finder product, one vendor, Faulhaber, for our biopsy handpiece motors, one vendor, NuSil Technology, for a coating used in our biopsy probes, and two vendors, UltraSonix and Teratech, for the ultrasound technology used in SenoSonix with EnCor. We do not believe that we could replace these suppliers without significant effort and delay in production. Other products and components come from single suppliers, but alternate suppliers are easier to identify, though in many cases we have not yet qualified alternate suppliers. We do not carry a significant inventory of most components used in our products, with the exception of the gamma finder product. Most of our suppliers have no contractual obligations to supply us with, and we are not contractually obligated to purchase from them, the components used in our devices.
On March 5, 2008, we entered into a Lease Agreement with The Irvine Company LLC for the lease of approximately 41,402 square feet space at 3 Morgan, Irvine, California. The term of the lease commenced on November 1, 2008, although we moved into the property in August pursuant to a period of rent-free early occupancy and will expire on January 31, 2014.
Government Regulation
Our products are medical devices subject to extensive and rigorous regulation by the FDA, as well as other federal and state regulatory bodies in the United States and comparable authorities in other countries. The FDA regulations govern, among other things, the following activities that we perform, or that are performed on our behalf, to ensure that medical products distributed domestically or exported internationally are safe and effective for their intended uses:
| • | product design, development and manufacture; |
| • | product safety, testing, labeling and storage; |
| • | premarketing clearance or approval; |
| • | recordkeeping procedures; |
| • | product marketing, sales and distribution; and |
| • | post-marketing surveillance, reporting of deaths or serious injuries and medical device reporting. |
The FDA’s Premarket Clearance and Approval Requirements. Unless an exemption applies, each medical device we wish to distribute commercially in the United States will require either prior 510(k) clearance or a premarket approval, or a PMA, from the FDA. Medical devices are classified into one of three classes—Class I, Class II, or Class III—depending on the degree or risk associated with each medical device and the extent of control needed to ensure safety and effectiveness. Devices deemed to pose lower risks are placed in either Class I or II, which requires the manufacturer to submit to the FDA a premarket notification requesting permission to commercially distribute the device. This process is generally known as 510(k) clearance. Some low-risk devices are exempted from this requirement. Devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a previously cleared 510(k) device, are placed in Class III, requiring premarket approval. Our minimally-invasive breast care products are Class I and II devices.
510(k) Clearance Pathway. When a 510(k) clearance is required, we must submit a premarket notification to the FDA demonstrating that our proposed device is substantially equivalent to a previously cleared and legally marketed 510(k) device or a device that was in commercial distribution before May 28, 1976 for which the FDA has not yet required the submission of a PMA application. By statute, the FDA is required to clear or deny a 510(k) premarket notification within 90 days of submission of the application. As a practical matter, clearance often takes significantly longer. The FDA may require further information, including clinical data, to make a determination regarding substantial equivalence. If the FDA determines that the device, or its intended use, is not substantially equivalent to a previously-cleared device or use, the FDA will place the device, or the particular use, into Class III.
Premarket Approval Pathway. A PMA application must be submitted to the FDA if the device cannot be cleared through the 510(k) process. The PMA application process is much more demanding than the 510(k) premarket notification process. A PMA application must be supported by extensive data, including but not limited to technical, preclinical, clinical trials, manufacturing and labeling to demonstrate to the FDA’s satisfaction the safety and effectiveness of the device.
-14-
After a PMA application is submitted and the FDA determines that the application is sufficiently complete to permit a substantive review, the FDA will accept the application for review. The FDA has 180 days to review an “accepted” PMA application, although the review of an application generally occurs over a significantly longer period of time and can take up to several years. During this review period, the FDA may request additional information or clarification of the information already provided. Also, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. In addition, the FDA will conduct a preapproval inspection of the manufacturing facility to ensure compliance with the QSRs. New PMA applications or PMA application supplements are required for significant modification to the manufacturing process, labeling and design of a device that is approved through the premarket approval process. Premarket approval supplements often require submission of the same type of information as a premarket approval application, except that the supplement is limited to information needed to support any changes from the device covered by the original premarket approval application and may not require as extensive clinical data or the convening of an advisory panel. We do not anticipate that any of our products in development will require the submission and approval of a PMA.
Clinical Trials. Clinical trials are almost always required to support an FDA premarket application and are sometimes required for 510(k) clearance. These trials generally require submission of an application for an Investigational Device Exemption, or IDE, to the FDA. The IDE application must be supported by appropriate data, such as animal and laboratory testing results, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound. The IDE must be approved in advance by the FDA for a specific number of patients unless the product is deemed a non-significant risk device eligible for more abbreviated IDE requirements. Clinical trials for significant risk devices may not begin until the IDE application is approved by the FDA and the appropriate institutional review boards, or IRBs, at the clinical trial sites. Our clinical trials must be conducted under the oversight of an IRB at the relevant clinical trial sites and in accordance with the FDA’s regulations, including but not limited to those relating to good clinical practices. We are also required to obtain patients’ informed consent that complies with both the FDA’s requirements and state and federal privacy regulations. We, the FDA or the IRB at each site at which a clinical trial is being performed may suspend a clinical trial at any time for various reasons, including a belief that the risks to study subjects outweigh the benefits. Even if a trial is completed, the results of clinical testing may not demonstrate the safety and efficacy of the device, may be equivocal or may otherwise not be sufficient to obtain approval or clearance of the product.
Pervasive and Continuing Regulation. After a device is placed on the market, numerous regulatory requirements continue to apply. These include:
| • | the FDA’s Quality System Regulations, which require manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the manufacturing process; |
| • | labeling regulations and FDA prohibitions against the promotion of products for uncleared, unapproved or off-label uses; |
| • | clearance or approval of product modifications that could significantly affect safety or efficacy or that would constitute a major change in intended use; |
| • | medical device reporting, or MDR, regulations, which require that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction were to recur; and |
| • | post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device. |
After a device receives 510(k) clearance or a PMA, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, will require a new clearance or approval. The FDA requires each manufacturer to make this determination initially, but the FDA can review any such decision and can disagree with a manufacturer’s determination. We have modified various aspects of some of our marketed products since receiving regulatory clearance, but we believe that new 510(k) clearances are not required for these modifications. If the FDA disagrees with our determination not to seek a new 510(k) clearance or PMA, the FDA may retroactively require us to seek 510(k) clearance or premarket approval. The FDA could also require us to cease marketing and distribution and/or recall the modified device until 510(k) clearance or premarket approval is obtained. Also, in these circumstances, we may be subject to significant regulatory fines, penalties and Warning Letters.
-15-
The MDR regulations require that we report to the FDA any incident in which our product may have caused or contributed to a death or serious injury or in which our product malfunctioned and, if the malfunction were to recur, would likely cause or contribute to death or serious injury.
We have registered with the FDA as a medical device manufacturer and have obtained a manufacturing license from the California Department of Health Services, or CDHS. The FDA has broad post-market and regulatory enforcement powers. We are subject to unannounced inspections by the FDA and the Food and Drug Branch of CDHS, or FDB, to determine our compliance with the QSRs and other regulations, and these inspections may include the manufacturing facilities of our suppliers. We underwent an inspection of our facilities by the FDA in April 2005, which resulted in the issuance in July 2005 of a Warning Letter from the FDA related to, among other things, our failure to adequately validate manufacturing changes we undertook to prevent the tip of the Gel Mark Ultra biopsy site marker shearing off in the patient’s breast during surgery, which we had experienced. The letter required us to take prompt action to strengthen our Quality System and product engineering area. We responded to the FDA with a comprehensive corrective action plan in August 2005. In 2008, no incidents of tip sheer were reported to SenoRx. We believe we are in compliance with QSRs. If, upon reinspection, the FDA determines we have not properly addressed their concerns or they identify new violations, we can be subject to any of the following sanctions:
| • | Warning Letters, fines, injunctions, consent decrees and civil penalties; |
| • | repair, replacement, refunds, recall or seizure of our products; |
| • | operating restrictions, partial suspension or total shutdown of production; |
| • | refusal of our requests for 510(k) clearance or premarket approval of new products, new intended uses or modifications to existing products; |
| • | withdrawal of 510(k) clearance or premarket approvals that have already been granted; and |
| • | criminal prosecution. |
Fraud and Abuse. We may directly or indirectly be subject to various federal and state laws pertaining to healthcare fraud and abuse, including anti-kickback laws. In particular, the federal Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing, arranging for or recommending a good or service, for which payment may be made in whole or part under federal healthcare programs, such as the Medicare and Medicaid programs. Penalties for violations include criminal penalties and civil sanctions such as fines, imprisonment and possible exclusion from Medicare, Medicaid and other federal healthcare programs. The Anti-Kickback Statute is broad and prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. In implementing the statute, the Office of Inspector General, or OIG, has issued a series of regulations, known as the “safe harbors.” These safe harbors set forth provisions that, if all their applicable requirements are met, will assure healthcare providers and other parties that they will not be prosecuted under the Anti-Kickback Statute. The failure of a transaction or arrangement to fit precisely within one or more safe harbors does not necessarily mean that it is illegal or that prosecution will be pursued. However, conduct and business arrangements that do not fully satisfy each applicable element of a safe harbor may result in increased scrutiny by government enforcement authorities, such as the OIG.
International. International sales of medical devices are subject to foreign governmental regulations, which vary substantially from country to country. The time required to obtain clearance or approval by a foreign country may be longer or shorter than that required for FDA clearance or approval, and the requirements may be different.
The primary regulatory environment in Europe is that of the European Economic Community, which has adopted numerous directives and has promulgated voluntary standards regulating the design, manufacture, clinical trials, labeling and adverse event reporting for medical devices. Devices that comply with the requirements of a relevant directive will be entitled to bear CE conformity marking, indicating that the device conforms with the essential requirements of the applicable directives and, accordingly, can be commercially distributed throughout the member states of the European Economic Community, and other countries that comply with or mirror these directives. The method of assessing conformity varies depending on the type and class of the product, but normally involves a combination of self-assessment by the manufacturer and a third-party assessment by our designated notified body, an independent and neutral institution appointed by a country to conduct the conformity assessment. This third-party assessment may consist of an audit of the manufacturer’s quality system and specific testing of the manufacturer’s device. Such an assessment is required in order for a manufacturer to commercially distribute the product throughout these countries. ISO 9001 and ISO 13845 certifications are voluntary harmonized standards. Compliance establishes the presumption of conformity with the essential requirements for a CE Marking. We have the authorization to affix the CE Mark to Gel Mark Ultra, Gel Mark UltraCor, and our EnCor system and to commercialize these devices in the European Economic Community, Australia, Hong Kong, Mexico, Singapore, South Korea, Taiwan, and several other countries. The Company has an active program to comply with the “WEE” directive should it be adopted in Europe and Asia in the next five years.
-16-
In 2007 we began partnering with local distributors who have breast imaging and/or interventional radiology franchises in countries outside North America. Through these distributors, we have been selling our EnCor and GelMark products in Austria, Belgium, Denmark, Finland, France, Germany, Hong Kong, Iceland, Ireland, Italy, Luxembourg, Mexico, The Netherlands, Norway, Portugal, Russia, Singapore, South Korea, Spain, Sweden, Switzerland, Taiwan and the United Kingdom. In addition, in the second half of 2008, we entered into relationships with distributors in China and in the Middle East.
Third-Party Reimbursement
Payment for patient care in the United States is generally made by third-party payors, including private insurers and government insurance programs, such as Medicare and Medicaid. The Medicare program, the largest single payor in the United States, is a federal governmental health insurance program administered by the Centers for Medicare and Medicaid Services, or CMS. Reimbursement for procedures related to breast cancer has been favorable as a result of the growing awareness of the impact of the disease as well as the recognition that proactive diagnosis and treatment is critical for effective care. The costs associated with the purchase of our products are reimbursed through Medicare, Medicaid and other third-party payors. International market acceptance of our products may depend, in part, upon the availability of reimbursement within the prevailing healthcare payment systems. Reimbursement and healthcare payment systems in international markets vary significantly by country, and include both government-sponsored healthcare and private insurance.
Research and Development
As of December 31, 2008, we had 18 employees, as well as several key on-going consultants, in our research and development department, which is overseen by our chief technical officer. Historically, we focused our research and development efforts on diagnostic products, including our breast biopsy systems and our tissue markers. While we plan to continue to develop our diagnostic products, we are also focused on developing our therapeutic and excision products to enable us to serve the continuum of care in the breast care market. We are currently developing various radio frequency based excision and reconstructive cutting devices, marker and new therapeutic radiation therapy devices.
Research and development expenses for 2008, 2007 and 2006 were $6.1, $6.4 million and $5.3 million, respectively. We expect research and development efforts and expenses to increase in absolute dollar terms but decrease as a percentage of net revenues.
Patents and Proprietary Technology
We plan to pursue and maintain intellectual property protection in the United States, Europe, Japan, Canada and other countries such as China and Australia. As of December 31, 2008, we have 55 issued United States patents primarily covering devices relating to breast biopsy, including biopsy site marking devices, excision devices and balloon products, the earliest of which will expire in 2018 and the last of which will expire in 2024, and 2 granted European regional patents which have been validated in 7 national countries.
In addition, we have 99 pending United States patent applications, 9 pending PCT (international) patent applications, 25 pending European regional patent applications, 25 pending Canadian patent applications, 2 pending Japanese patent applications, 10 pending Australian patent applications, as well as pending patent applications in Brazil, China, Mexico, South Korea and Singapore. We believe we have a strong intellectual portfolio that has permitted us to make modifications to our products in response to competition without significant disruption to our operations.
-17-
We have several issued United States patents related either to the design or use of Contura MLB and additional United States patent applications and continuations are pending. In December 2008 we were granted a patent relating to Contura MLB’s asymmetrical radiation of a breast cavity with a balloon. During the development of Contura MLB, we appropriately considered the intellectual property landscape, including citing as appropriate in our own patent filings the Hologic/Proxima patents that are the subject matter of our current litigation with Hologic.
Together, our patents and patent applications protect aspects of our technologies. Key areas of our issued and pending patent coverage include:
| • | biopsy systems, covering current embodiments and variations to the design of the EnCor and EnCor 360 probes, handpieces and control module; |
| • | mechanical cutters, covering the Tri-Concave penetrating tip and the EnCor and EnCor 360 tissue cutting mechanisms; |
| • | radiofrequency technologies, covering the SenoPulse RF Generator, devices powered by the generator, including the Single Step and long wire scalpels, EnCor 360 probe and lesion location devices; |
| • | bioresorbable biopsy site markers, covering marker materials, methods for imparting ultrasound visibility, and marker delivery systems; and |
| • | Contura MLB, covering the use of vacuum to help conform tissue surrounding the lumpectomy cavity to the walls of the radiation delivery balloon, our proprietary balloon manufacturing process and delivery of radiation through multiple lumens in a balloon catheter. |
We also rely on copyrights, trade secrets, technical know-how and continuing innovation to develop and maintain our competitive position. We seek to protect our proprietary information and other intellectual property by generally requiring our employees, consultants, contractors, outside scientific collaborators and other advisors to execute non-disclosure agreements on commencement of their employment or engagement.
Employees
As of December 31, 2008, we had 155 employees, including 78 employees in sales and marketing, 18 employees in research and development, 36 employees in manufacturing, 14 employees in clinical, regulatory and quality assurance and nine employees in general and administrative. We believe that our future success will depend on our continued ability to attract, hire and retain qualified personnel. None of our employees are represented by a labor union or are parties to a collective bargaining agreement, and we believe our employee relations are good.
Available Information
We are subject to the reporting requirements under the Securities Exchange Act of 1934. Consequently, we are required to file reports and information with the Securities and Exchange Commission (SEC), including reports on the following forms: annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934. These reports and other information concerning the company may be accessed through the SEC’s website at http://www.sec.gov. You may also read and copy any materials we file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, D.C. 20549. Information on the operation of the Public Reference Room can be obtained by calling 1-800-SEC-0330.
You may also find on our website at http://www.senorx.com/ electronic copies of our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934. Such filings are placed on our website as soon as reasonably practicable after they are filed with the SEC. Our charter for our Audit and Compensation Committees and our Code of Ethics are available on our website. In the event that we grant a waiver under our Code of Ethics, to any of our officers and directors, we will publish it on our website.
-18-
| ITEM 1A. | RISK FACTORS |
RISKS RELATED TO OUR BUSINESS
We have a limited history of operations and a history of net losses, and we may not be able to achieve profitability even if we are able to generate significant revenues.
We have a limited history of operations upon which you can evaluate our business. We began selling our first products in 2002, fully launched our flagship product for use in breast biopsy procedures, the EnCor system, in November 2005, and launched our flagship radiation therapy product, the Contura MLB, in January 2008. We incurred net losses of $8.7 million in 2008, $9.9 million in 2007and $15.4 million in 2006, and, as of December 31, 2008, had an accumulated deficit of approximately $84.2 million. In addition, we expect our operating expenses to increase as we expand our business to meet anticipated increased demand for our EnCor system, continue with the full commercialization of the Contura MLB, and devote resources to our sales and marketing and research and development activities. In order for us to become profitable, we believe that our EnCor system and Contura MLB must be widely adopted. We cannot assure you that we will be able to achieve or sustain profitability even if we are able to generate significant revenues. Our failure to achieve and sustain profitability would negatively impact the market price of our common stock and require us to obtain additional funding. If our future funding requirements increase beyond currently expected levels, as a result of our failure to achieve or sustain profitability relating to sales, litigation expenses or otherwise, we cannot make any assurance that additional funding will be available on a timely basis on terms acceptable to us, or at all, particularly in the short-term due to the current credit and equity market funding environments.
Our success depends upon market adoption of our EnCor system and Contura MLB, without which our results of operations will suffer.
Until 2007, we derived our revenues primarily from our tissue marker products. However, our EnCor system and Contura MLB now account for a majority of our revenue growth, and we expect this to continue for the foreseeable future. Our ability to meet this expectation is based upon a number of assumptions, including:
| • | we have limited experience selling to radiological oncologists, the primary market for this product; |
| • | attracting and retaining qualified sales professionals to sell it; |
| • | differentiating Contura MLB from competing products and obtaining a significant share of this market; |
| • | protecting it with intellectual property rights; |
| • | sustaining adequate third-party reimbursement ; |
| • | producing compelling clinical data on safety and effectiveness; |
| • | partnering, as necessary, with suppliers; and |
| • | manufacturing it consistently within our specifications and in accordance with the FDA’s Quality System Regulations. |
Even if we are able to present potential customers with compelling clinical data, technological advancements or influential user experiences, they may be reluctant to switch from a competing device to which they have grown accustomed. We may not be successful in our near-term strategy of marketing EnCor and Contura MLB to our existing customer base of tissue marker users, and users of our earlier vacuum-assisted breast biopsy system. Our commercial success also depends on the continued general market shift to less invasive biopsy procedures.
-19-
Our future success will depend in part upon our ability to successfully commercialize our Contura MLB.
We expect our Contura MLB, which we received FDA 510(k) clearance in May 2007, to rapidly become a significant contributor to our revenues. The Contura MLB development has been completed, but there remain significant challenges that must be overcome before we can obtain significant revenues from this product, including:
| • | we have limited experience selling to radiological oncologists, the primary market for this product; |
| • | attracting and retaining qualified sales professionals to sell it; |
| • | differentiating Contura MLB from competing products and obtaining a significant share of this market; |
| • | protecting it with intellectual property rights; |
| • | obtaining adequate third-party reimbursement; |
| • | producing compelling clinical data on safety and effectiveness; |
| • | partnering, as necessary, with suppliers; and |
| • | manufacturing it consistently within our specifications and in accordance with the FDA’s Quality System Regulations. |
If we are able to overcome these challenges, we may nevertheless be unable to convince potential customers that the Contura MLB represents a compelling alternative to competing products. It has been reported that new short-term brachytherapy products from Cianna Medical and Xoft began to be commercialized in 2008. Our commercial success will also depend on a general market shift from whole to partial breast radiation. If we are unable to obtain a significant share of the brachytherapy market for the reasons listed above, or that competing products are more compelling and achieve better acceptance by the market, our long-term commercialization experience with the Contura MLB could be significantly below expectations or not achieved at all, which would have a material adverse effect on our future financial performance. Additionally, the adoption of conformal radiotherapy may grow at a faster rate than the overall market for partial breast radiation therapies, and as a result, could impact the speed of adoption of balloon brachytherapy devices, including Contura MLB.
We are currently and may in the future be subject to costly claims of infringement or misappropriation of the intellectual property rights of others, which could impact our business and harm our operations.
Our industry has been characterized by frequent demands for licenses and litigation. In January 2008, Hologic and its wholly-owned subsidiaries, including Cytyc Corporation and Cytyc LP, filed a lawsuit against us in the United States District Court, Northern District of California, San Jose Division. The complaint generally alleges patent infringement of certain Hologic brachytherapy patent claims, seeking unspecified monetary damages and an injunction against us for infringement of those claims. This litigation is still ongoing and because the outcome is undetermined, we cannot reasonably estimate the possible loss or range of loss that may arise from the litigation or the likelihood of success. If we lose this law suit, we may be completely prevented from selling Contura MLB and as a result, our future prospects will be significantly harmed.
Our competitors, potential competitors or other patent holders may, in the future, assert that our products and the methods we employ are covered by their patents or misappropriate their intellectual property. In addition, we do not know whether our competitors will apply for and obtain patents that will prevent, limit or interfere with our ability to make, use, sell or import our products. Because patent applications may take years to issue, there may be applications now pending of which we are unaware that may later result in issued patents that our products infringe. There also could be existing patents that one or more components of our systems may inadvertently infringe. Although we may seek to settle any future claims, we may not be able to do so on reasonable terms, or at all. If we lose a claim against us, we may be ordered to pay substantial damages, including compensatory damages, which may be trebled in certain circumstances, plus prejudgment interest. We also could be enjoined, temporarily, preliminarily or permanently, from making, using, selling, offering to sell or importing our products or technologies essential to our products, which could significantly harm our business and operating performance.
We may become involved in litigation not only as a result of alleged infringement of a third party’s intellectual property rights but also to protect our own intellectual property. Enforcing our patent rights against infringers, even when such litigation is resolved in our favor, could involve substantial costs and divert management’s attention from our core business and harm our reputation.
-20-
We have limited clinical data regarding the safety and efficacy of our products. If future data or clinical experience is negative, we may lose significant market share.
Our success depends on the acceptance of our products by the medical community as safe and effective. Physicians that may be interested in using our products may hesitate to do so without long-term data on safety and efficacy. The limited clinical studies on some of our products that have been published or presented as abstracts at major medical meetings typically have been based on the work of a small number of physicians examining small patient populations over relatively short periods. Accordingly, the results of these clinical studies do not necessarily predict long-term clinical results, or even short-term clinical results from the broader physician community. If future safety or efficacy data or clinical experience is negative, we may lose significant market share.
We compete against companies that have more established products and greater resources, which may prevent us from achieving significant market penetration or improved operating results.
Many of our products compete, and our future products may compete, against products that are more established and accepted within our target markets. With fewer resources and operating history than many of our competitors and potential future competitors, and a less-established reputation, it may be difficult for our products to gain significant market penetration. We may be unable to convince physicians to switch their practice away from competing devices. Competing effectively will require us to distinguish our company and our products from our competitors and their products, and turns on factors such as:
| • | ease of use and performance; |
| • | price; |
| • | quality and scale of our sales and marketing efforts; |
| • | our ability to offer a broad portfolio of products across the continuum of breast care; |
| • | establishing a strong reputation through compelling clinical study publications and endorsements from influential physicians; and |
| • | brand and name recognition. |
Competition could result in price-cutting, reduced profit margins and loss of market share, any of which could have a material adverse effect on our results of operations. In addition, our competitors with greater financial resources could acquire other companies that would enhance their name recognition and market share, and allow them to compete more effectively by bundling together related products. For example one competitor provides incentives for the purchase of its biopsy capital equipment and disposables when purchased with its digital mammography and stereotactic tables. Certain potential customers may view this value proposition as attractive, which could result in their decision not to purchase our products. We also anticipate that new products and improvements to existing products could be introduced that would compete with our current and future products. If we are unable to compete effectively, we will not be able to generate expected sales and our future financial performance will suffer.
Our ability to compete depends upon our ability to innovate, develop and commercialize new products and product enhancements.
The markets in which we compete involve rapid and substantial technological development and product innovations. There are few barriers to prevent new entrants or existing competitors from developing or acquiring products or technological improvements that compete effectively against our products or technology. If we are unable to innovate successfully to anticipate or respond to competitive threats, obtain regulatory approvals, or protect such innovation with defensible intellectual property, our revenues could fail to grow or could decline. Our business strategy is in part based upon our expectation that we will continue to make frequent new product introductions and improvements to existing products that will be demanded by our target customers. If we are unable to continue to develop new products and technologies as anticipated, our ability to grow and our future financial performance could be materially harmed. For example, we recently received 510(k) clearance from the FDA for our new SenoSonix System, an integration of EnCor with ultra sound technology from Ultrasonix Medical Corporation of Canada. We have yet to commercially launch this product and there can be no assurances that we will be successful in obtaining meaningful revenues once it has been commercialized.
-21-
Our business strategy is heavily focused on integrated breast centers and other large institutions.
We are focusing our sales efforts on becoming a preferred provider to integrated breast centers and other large customer accounts. We cannot assure you that we will be able to secure or maintain these accounts or that this strategy will maximize our revenue growth. These targeted customers often have a rigorous and lengthy qualification process for approving new vendors and products. Additionally, breast centers are in many cases not located at one physical location, but instead involve the coordinated efforts of various geographically dispersed offices and physicians, which may complicate the qualification process and may strain our sales and support organizations. Further, these customers have not entered, and we do not expect them in the future to enter, long-term contracts to purchase our products. Therefore, obtaining approval from these potential customers to sell them our products may not result in significant or long-term sales of our products to them. Our strategy of focusing on large institutions may result in relatively few customers contributing a significant amount to our revenues. For example, Kaiser Permanente is our largest customer, and in the years ended December 31, 2008 and 2007, represented approximately 4.9% and 5.8%, respectively, of our total revenues. Although we recently extended our contract with Kaiser for three years, we cannot assure you that Kaiser or other large customer accounts will continue to purchase our products. The loss of any of these customers could have a material adverse impact on our results of operations.
Our strategy of providing a broad array of products to the breast care market may be difficult to achieve, given our size and limited resources.
We aim to be an attractive and convenient supplier for integrated breast centers by offering a broad product line of minimally-invasive devices for breast care specialists. Commercializing several product lines simultaneously may be difficult because we are a relatively small company. Additionally, offering a broad product line will require us to manufacture, sell and support some products that are not as profitable or in as high demand as some of our other products, which could have a material adverse effect on our overall results of operations. To succeed in our approach, we will need to grow our organization considerably and enhance our relationships with third-party manufacturers and suppliers. If we fail to make product introductions successfully or in a timely manner because we lack resources, or if we fail to adequately manufacture, sell and support our existing products, our reputation may be negatively affected and our results of operations could be materially harmed.
We believe that demand for minimally-invasive products for the diagnosis and treatment of breast cancer must grow in order for our business to grow as anticipated.
While there have been trends in recent years that favor increased screening, diagnosis and treatment of breast cancer, these trends may not continue. For example, the incidence of breast cancer in the United States appears to have fallen from its highest level over the last few years. Additionally, while the number of breast biopsies performed annually has increased significantly since 1997 when the American Cancer Society updated its guidelines for breast cancer screening, recommending that women should begin annual screening at age 40 rather than the previously recommended age 50, new guidance could be published that could support a reversal of this trend. Some studies conclude that annual breast cancer screening by mammography for women under age 50 may be more harmful, due to increased radiation exposure, than beneficial. These factors, in addition to possible future innovations in screening technologies or in breast cancer treatment options, could result in a decline in breast biopsy procedures and radiation therapy, which could reduce our overall market.
We have limited sales and marketing experience and failure to build and manage our sales force or to market and distribute our products effectively could have a material adverse effect on our results of operations.
We rely on a direct sales force to sell our products. In order to meet our anticipated sales objectives, we expect to grow our sales organization significantly over the next several years. There are significant risks involved in building and managing our sales organization, including our ability to:
| • | hire and successfully integrate qualified individuals as needed; |
| • | provide adequate training for the effective sale of our products; |
| • | retain and motivate our sales employees; and |
| • | integrate our new brachytherapy sales professionals and successfully sell into the radiation oncology market. |
-22-
We expect that our Contura MLB will be a principal driver of future growth. However, our sales force historically has primarily sold diagnostic products and therefore has limited experience selling a therapeutic device. Our Contura MLB competes with products that are well-established and with new entrants to the market. Accordingly, it is difficult for us to predict how well our sales force will perform.
Our failure to adequately address these risks could have a material adverse effect on our ability to sell our products, causing our revenues to be lower than expected and harming our results of operations.
If we are unable to obtain and maintain intellectual property protection covering our products, others may be able to make, use or sell our products, which could have a material adverse effect on our business and results of operations.
We rely on patent, copyright, trade secret and trademark laws and confidentiality agreements to protect our technology, products and our competitive position in the market. Additionally, our patent applications, including those covering our EnCor system, may not result in patents being issued to us or, if they are issued, may not be in a form that is advantageous to us. Any patents we obtain may be challenged or invalidated by third parties. Competitors also may design around our protected technology or develop their own technologies that fall outside our intellectual property rights. In addition, we may not be able to prevent the unauthorized disclosure or use of our technical knowledge or other trade secrets by consultants, vendors, former employees or current employees, despite the existence of confidentiality agreements and other contractual restrictions. Monitoring unauthorized uses and disclosures of our intellectual property is difficult, and we cannot be certain that the steps we have taken to protect our intellectual property will be effective or that any remedies we may have in these circumstances would be adequate. Moreover, the laws of foreign countries may not protect our intellectual property rights to the same extent as the laws of the United States.
We may not have adequate intellectual property protection for some of our products and products under development and consequently may need to obtain licenses from third parties. If any such licenses are required, we may be unable to negotiate terms acceptable to us and such failure could have a material adverse effect on our future results of operations.
We may be unsuccessful in our long-term goal of expanding our product offerings outside the United States and Canada.
For the year ended December 31, 2008, we derived approximately 90.8% of our net revenues from sales within the United States and Canada. We have entered into distribution agreements with third parties outside the United States and Canada, but do not anticipate sales of our products through these distributors becoming a significant portion of our revenues in the foreseeable future. If we do begin to offer our products more broadly outside the United States and Canada, we expect that we will remain dependent on third-party distribution relationships and will need to attract additional distributors to increase the number of territories in which we sell our products. Distributors may not commit the necessary resources to market and sell our products to the level of our expectations. If current or future distributors do not perform adequately, or we are unable to locate distributors in particular geographic areas, our ability to realize long-term international revenue growth could be materially adversely affected.
Although some of our products have regulatory clearances and approvals from jurisdictions outside the United States and Canada, many do not. These products may not be sold in these jurisdictions until the required clearances and approvals are obtained. We cannot assure you that we will be able to obtain these clearances or approvals on a timely basis, or at all. In Japan, recent changes in the laws and regulations governing the approval process for medical devices has made it unlikely that we will be able to obtain approvals for our products within the foreseeable future.
-23-
We are dependent on sole-source and single-source suppliers for certain of our products and components, thereby exposing us to supply interruptions that could have a material adverse effect on our business.
We have one product and several components of other products that we obtain from sole suppliers. We rely on one vendor for our Gamma Finder product, one vendor for our biopsy probe motors, one vendor for a biopsy probe coating, two vendors for the ultrasound technology used in SenoSonix with EnCor and one vendor for circuit boards in the Encore hardware. Other products and components come from single suppliers, but alternate suppliers are easier to identify. However, in many of these cases we have not yet qualified alternate suppliers and rely upon purchase orders, rather than longer-term supply agreements. We also do not carry a significant inventory of most components used in our products and generally could not replace our suppliers without significant effort and delay in production. In addition, switching components may require product redesign and new regulatory clearances by the FDA, either of which could significantly delay or prevent production and involve substantial costs.
Reliance on third-party vendors may lead to unanticipated interruptions in supply or failure to meet demand on a timely basis. Any supply interruption from our vendors or failure to obtain additional vendors for any of the components could limit our ability to manufacture our products and fulfill customer orders on a timely basis, which could harm our reputation and revenues.
We have limited experience manufacturing certain components of our products in significant quantities, which could adversely impact the rate at which we grow.
We may encounter difficulties in manufacturing relating to our products and products under development for the following reasons:
| • | our limited experience in manufacturing such products in significant quantities and in compliance with the FDA’s Quality System Regulation; |
| • | to increase our manufacturing output significantly, we will have to attract and retain qualified employees, who are in short supply, for the manufacturing, assembly and testing operations; and |
| • | some of the components and materials that we use in our manufacturing operations are currently provided by sole and single sources of supply. |
Our limited manufacturing experience has in the past resulted in unexpected and costly delays. For example, in 2006, as a part of our settlement of litigation with Suros Surgical Systems, a wholly-owned subsidiary of Hologic, we implemented a redesign to the EnCor system cutter. This effort resulted in a short-term decrease in yields and a delay in implementing certain cost improvements, which had an adverse effect on our costs of goods sold. In addition, although we believe that our current manufacturing capabilities will be adequate to support our commercial manufacturing activities for the foreseeable future, we may be required to expand our manufacturing facilities if we experience faster-than-expected growth. If we are unable to provide customers with high-quality products in a timely manner, we may not be able to achieve wide market adoption for our EnCor system or other products and products under development. Our inability to successfully manufacture or commercialize our devices could have a material adverse effect on our product sales.
We are entering into a difficult economic period, and the uncertainty in the economy may increase stock price volatility, reduce customer demand for our products, and cause potential customers to delay their purchase decisions.
Economic conditions have recently deteriorated, and we may be entering, or already are in, a global recession. This uncertainty may increase the volatility of our stock price and may cause potential customers to delay their capital equipment purchases, and may make it more difficult for potential customers to obtain financing necessary to purchase our products, each of which can have a material adverse effect on our revenue, profitability and business. Our capital equipment revenues have historically ranged between 10% and 20% of annualized revenues. Credit constraints for institutions, distributors and individual physicians may slow the purchase of EnCor systems and Gamma Finders and may, as a result, have a negative effect on the purchase of disposables related to delays in capital equipment purchases or lead to a reduction in the overall number of biopsy procedures. The number of mammograms performed in the United States has declined since 2007, which in turn reduced the number of number of biopsy procedures performed domestically.
-24-
We rely on third-party manufacturers for certain components, and the loss of any of these manufacturers, or their inability to provide us with an adequate supply of high-quality components, could have a material adverse effect on our business.
Although we manufacture certain components and assemble some of our products at our corporate headquarters in Irvine, California, we rely on third parties to manufacture most of the components of our products and are in the process of transferring additional manufacturing and assembly to our Thailand contract manufacturer. Some of these relationships are new and we have not had experience with their large commercial-scale manufacturing capabilities. For example, since the end of 2005, we have been transferring a portion of our manufacturing operations to a third party in Thailand. Because of the distance between California and Thailand, we may have difficulty adequately supervising and supporting its operations. There are several risks inherent in relying on third-party manufacturers, including:
| • | failure to meet our requirements on a timely basis as demand grows for our products; |
| • | errors in manufacturing components that could negatively affect the performance of our products, cause delays in shipment of our products, or lead to malfunctions or returns; |
| • | inability to manufacture products to our quality specifications and strictly enforced regulatory requirements; |
| • | inability to implement design modifications that we develop in the future; |
| • | unwillingness to negotiate a long-term supply contract that meets our needs or to supply components on a short-term basis on commercially reasonable terms; |
| • | prioritization of other customers orders over ours; |
| • | inability to fulfill our orders due to unforeseen events, including foreign political events, that result in a disruption of their operations; and |
| • | continued fluctuations in the value of the U.S. dollar could impact our future third-party manufacturing costs. |
If a manufacturer fails to meet our needs with high-quality products on a timely basis, we may be unable to meet customer demand, which could have a material adverse effect on our reputation and customer relationships.
Changes in coverage and reimbursement for procedures using our products could affect the adoption of our products and our future revenues.
Breast biopsy procedures and markers are typically reimbursed by third-party payors, including Medicare, Medicaid and private healthcare insurance companies. These payors may adversely change their coverage amounts and reimbursement policies. Also, healthcare reform legislation or regulation may be proposed or enacted in the future that adversely affects these policies and amounts. For example, the Federal Deficit Reduction Act of 2006 may in the future affect future reimbursement rates for our vacuum- assisted biopsy products and Contura MLB products. We cannot assure you that the current scope of coverage or levels of reimbursement will continue to be available or that coverage of, or reimbursement for, our products will be available at all. If physicians, hospitals and other providers are unable to obtain adequate reimbursement for our current products or future products, or for the procedures in which such products are used, they may be less likely to purchase the products, which could have a material adverse impact on our market share. For example, in 2009, it is expected that there will be an increase in reimbursement rates to non hospital based Radiation Oncology centers for multiple-dwell radiation balloon catheter procedures, which includes Contura MLB, relative to single catheter procedures, but a decrease for non hospital based Radiology Oncology centers in rates relative to whole breast radiation therapy.
Any acquisitions that we make could disrupt our business and have an adverse effect on our financial condition.
We expect that in the future we may identify and evaluate opportunities for strategic acquisitions of complementary product lines, technologies or companies. We may also consider joint ventures and other collaborative projects. However, we may not be able to identify appropriate acquisition candidates or strategic partners, or successfully negotiate, finance or integrate any businesses, products or technologies that we acquire. Furthermore, the integration of any acquisition and the management of any collaborative project may divert management’s time and resources from our core business and disrupt our operations. We do not have any experience with acquiring other product lines, technologies or companies. We may spend time and money on projects that do not increase our revenues. Any cash acquisition we pursue would diminish the funds available to us for other uses, and any stock acquisition would be dilutive to our stockholders.
-25-
Our financial controls and procedures may not be sufficient to ensure timely and reliable reporting of financial information, which, as a public company, could materially harm our stock price and NASDAQ listing.
As a public company, we will require greater financial resources than we have had as a private company. We will need to hire additional employees for our finance department. We cannot provide you with assurance that our finance department has or will maintain adequate resources to ensure that we will not have any future material weakness in our system of internal controls. The effectiveness of our controls and procedures may in the future be limited by a variety of factors including:
| • | faulty human judgment and simple errors, omissions or mistakes; |
| • | fraudulent action of an individual or collusion of two or more people; |
| • | inappropriate management override of procedures; and |
| • | the possibility that any enhancements to controls and procedures may still not be adequate to assure timely and accurate financial information. |
If we fail to have effective controls and procedures for financial reporting in place, we could be unable to provide timely and accurate financial information and be subject to NASDAQ delisting, SEC investigation, and civil or criminal sanctions.
Product liability claims may lead to expensive and time-consuming litigation, substantial damages, increased insurance rates, and may have a material adverse effect on our financial condition.
Our business exposes us to potential product liability claims that are inherent in the manufacturing, marketing and sale of medical devices. For example, in the past we experienced, and in the future could experience, an issue related to the tip of our Gel Mark Ultra Biopsy Site Marker shearing off in the patient’s breast during the biopsy procedure, which could lead to a claim of damages, though none has previously been made. We may be unable to avoid product liability claims, including those based on manufacturing defects or claims that the use, misuse or failure of our products resulted in a misdiagnosis or harm to a patient. Although we believe that our liability coverage is adequate for our current needs, and while we intend to expand our product liability insurance coverage to any products we intend to commercialize, insurance may be unavailable, prohibitively expensive or may not fully cover our potential liabilities. If we are unable to maintain sufficient insurance coverage on reasonable terms or to otherwise protect against potential product liability claims, we may be unable to continue to market our products and to develop new products. Defending a product liability lawsuit could be costly and have a material adverse effect on our financial condition, as well as significantly divert management’s attention from conducting our business. In addition, product liability claims, even if they are unsubstantiated, may damage our reputation by raising questions about our products’ safety and efficacy, which could materially adversely affect our results of operations, interfere with our efforts to market our products and make it more difficult to obtain commercial relationships necessary to maintain our business.
We may be adversely affected by the impact of environmental and safety regulations.
We are subject to federal, state, local and foreign laws and regulations governing the protection of the environment and occupational health and safety, including laws regulating the disposal of hazardous wastes and the health and safety of our employees. We may be required to obtain permits from governmental authorities for certain operations. If we violate or fail to comply with these laws and regulations, we could incur fines, penalties or other sanctions, which could adversely affect our business and our financial condition and cause our stock price to decline. We also may incur material expenses in the future relating to compliance with future environmental laws. In addition, we could be held responsible for substantial costs and damages arising from any contamination at our present facilities or third-party waste disposal sites. We cannot completely eliminate the risk of contamination or injury resulting from hazardous materials, and we may incur material liability as a result of any contamination or injury.
-26-
Our success will depend on our ability to attract and retain key personnel, particularly members of management and scientific staff.
We believe our future success will depend upon our ability to attract and retain employees, including members of management, engineers and other highly skilled personnel. Our employees may terminate their employment with us at any time. Hiring qualified personnel may be difficult due to the limited number of qualified professionals and the fact that competition for these types of employees is intense. If we fail to attract and retain key personnel, we may not be able to execute our business plan.
Our ability to use net operating loss carryforwards may be limited.
Section 382 of the Internal Revenue Code generally imposes an annual limitation on the amount of net operating loss carryforwards that may be used to offset taxable income when a corporation has undergone significant changes in its stock ownership. We have internally reviewed the applicability of the annual limitations imposed by Section 382 caused by previous changes in our stock ownership and believe such limitations should not be significant. Future ownership changes, including changes resulting from or affected by our IPO, may adversely affect our ability to use our remaining net operating loss carryforwards. If our ability to use net operating loss carryforwards is limited, we may be subject to tax on our income earlier than we would otherwise be had we been able to fully utilize our net operating loss carryforwards.
RISKS RELATED TO REGULATORY MATTERS
The FDA may find that we do not comply with regulatory requirements and take action against us.
Our products and facilities are subject to periodic unannounced inspections by the FDA and other regulatory bodies. In particular, we are required to comply with the FDA’s Quality System Regulations, or QSRs, and other regulations, which cover the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, storage, shipping and post-market surveillance of our products.
We underwent an inspection of our facilities by the FDA in April 2005, which resulted in the issuance in July 2005 of a Warning Letter from the FDA related to, among other things, our failure to adequately validate manufacturing changes we undertook to prevent the tip of the Gel Mark Ultra Biopsy Site Marker from shearing off in the patient’s breast during the biopsy procedure, which we had experienced. The letter required us to take prompt action to strengthen our Quality System and product engineering area. We responded to the FDA with a comprehensive corrective action plan in August 2005. We believe we are in compliance with the QSRs. However, during a future inspection, the FDA may determine that we have failed to adequately or completely implement the corrective action plan or may find additional material violations. Such a determination could lead the FDA to commence an enforcement action against us, which may include the following sanctions:
| • | injunctions, fines, other civil penalties or additional Warning Letters; |
| • | the refusal of, or delay by, the FDA in granting further 510(k) clearances or approving further premarket approval applications; |
| • | suspension or withdrawal of our FDA clearances or approvals; |
| • | operating restrictions, including total or partial suspension of production, distribution, sales and marketing of our products; or |
| • | product recalls, product seizures or criminal prosecution of our company, our officers or our employees. |
Any of these could have a material adverse effect on our reputation, results of operation and financial condition.
If we fail to obtain or maintain necessary FDA clearances or approvals for products, or if clearances or approvals are delayed, we will be unable to commercially distribute and market our products in the United States.
Our products are medical devices, and as such are subject to extensive regulation in the United States and in the foreign countries where we do business. Unless an exemption applies, each medical device that we wish to market in the United States must first receive 510(k) clearance or premarket approval from the FDA. Either process can be lengthy and expensive. The FDA’s 510(k) clearance process usually takes from three to twelve months from the date the application is complete, but it may take longer. The premarket approval process is much more costly, lengthy and uncertain. It generally takes from one to three years from the date the application is completed or even longer. Achieving a completed application is a process that may require numerous clinical trials and the filing of amendments over time. We expect that our products in the foreseeable future will be subject to 510(k) procedures and not premarket approval, or PMA, applications. We may not be able to obtain additional FDA clearances or approvals in a timely fashion, or at all. Delays in obtaining clearances or approvals could adversely affect our revenues and profitability.
-27-
Modifications to our devices may require new 510(k) clearances, which may not be obtained.
The FDA requires device manufacturers to initially make and document a determination of whether or not a modification requires a new clearance; however, the FDA can review a manufacturer’s decision. Any modifications to an FDA-cleared device that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use would require a 510(k) clearance or possibly a premarket approval.
We have modified aspects of some of our products since receiving FDA clearance, but we believe that new 510(k) clearances are not required. We may make additional modifications, and in appropriate circumstances, determine that new clearance or approval is unnecessary. The FDA may not agree with our decisions not to seek new clearances or approvals. If the FDA requires us to seek 510(k) clearances or approval for any modifications to a previously cleared product, we may be required to cease marketing or recall the modified device until we obtain clearance or approval. Also, in these circumstances we may be subject to adverse publicity, regulatory Warning Letters and significant fines and penalties.
Government regulation imposes significant restrictions and costs on the development and commercialization of our products.
Any products cleared or approved by the FDA are subject to on-going regulation. Any discovery of previously unknown or unrecognized problems with the product or a failure of the product to comply with any applicable regulatory requirements can result in, among other things:
| • | Warning Letters, injunctions, fines or other civil penalties; |
| • | the refusal of, or delay by, the FDA in granting further 510(k) clearances or approving further premarket approval applications; |
| • | suspension or withdrawal of our FDA clearances or approvals; |
| • | operating restrictions, including total or partial suspension of production, distribution, sales and marketing of our products; or |
| • | product recalls, product seizures or criminal prosecution of our company, our officers or our employees. |
Any of these could have a material adverse effect on our reputation and results of operations.
RISKS RELATED TO THE SECURITIES MARKETS AND OWNERSHIP OF OUR COMMON STOCK
Our common stock has been publicly traded for a short time and an active trading market may not be sustained.
Prior to March 2007, there had been no public market for our common stock. An active trading market may not be sustained. The lack of an active market may impair the value of your shares and your ability to sell your shares at the time you wish to sell them. An inactive market may also impair our ability to raise capital by selling shares and may impair our ability to acquire other companies, products or technologies by using our shares as consideration.
-28-
If our public guidance or our future operating performance does not meet investor expectations, our stock price could decline.
As a public company, we provide guidance to the investing community regarding our anticipated future operating performance. Our business typically has a short sales cycle, so that we do not have significant backlog of orders at the start of a quarter, and our ability to sell our products successfully is subject to many uncertainties. In light of these factors, it is difficult for us to estimate with accuracy our future results. Our expectations regarding these results will be subject to numerous risks and uncertainties that could make actual results differ materially from those anticipated. If our actual results do not meet our public guidance or our guidance or actual results do not meet the expectations of third-party financial analysts, our stock price could decline significantly.
We expect that the price of our common stock will fluctuate substantially.
The market price of our common stock is likely to be highly volatile and may fluctuate substantially due to many factors, including:
| • | volume and timing of sales of our products; |
| • | the introduction of new products or product enhancements by us or our competitors; |
| • | disputes or other developments with respect to our intellectual property rights or the intellectual property rights of others; |
| • | our ability to develop, obtain regulatory clearance or approval for, and market, new and enhanced products on a timely basis; |
| • | product liability claims or other litigation; |
| • | quarterly variations in our or our competitors’ results of operations; |
| • | sales of large blocks of our common stock, including sales by our executive officers and directors; |
| • | announcements of technological or medical innovations for the diagnosis and treatment of breast cancer; |
| • | changes in governmental regulations or in the status of our regulatory approvals or applications; |
| • | changes in the availability of third-party reimbursement in the United States or other countries; |
| • | changes in earnings estimates or recommendations by securities analysts; |
| • | general market conditions and other factors, including factors unrelated to our operating performance or the operating performance of our competitors; and |
| • | limited liquidity and low trading volumes for our common stock. |
These and other factors may make the price of our stock volatile and subject to unexpected fluctuation.
Our directors, executive officers and principal stockholders have significant voting power and may take actions that may not be in the best interests of our other stockholders.
Our officers, directors and principal stockholders that currently hold more than 5% of our common stock together control nearly a majority of our outstanding common stock. As a result, these stockholders, if they act together, will be able to exercise significant influence over the management and affairs of our company and all matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions. This concentration of ownership may have the effect of delaying or preventing a change in control, might have a material adverse effect on the market price of our common stock and may not be in the best interest of our other stockholders.
A sale of a substantial number of shares of our common stock may cause the price of our common stock to decline.
Following the expiration of lock-up arrangements with our stockholders in September 2007 that were entered into in connection with our IPO, all shares of our common stock that were outstanding before the IPO are now eligible for resale, subject to compliance with Rule 144 under the Securities Act. If our stockholders sell substantial amounts of our common stock, the market price of our common stock could decline.
-29-
Our Amended and Restated Certificate of Incorporation and Bylaws, and Delaware law, contain provisions that could discourage a takeover.
Our Amended and Restated Certificate of Incorporation and Bylaws, and Delaware law, contain provisions that might enable our management to resist a takeover, and might make it more difficult for an investor to acquire a substantial block of our common stock. These provisions include:
| • | a classified board of directors; |
| • | advance notice requirements to stockholders for matters to be brought at stockholder meetings; |
| • | a supermajority stockholder vote requirement for amending certain provisions of our Amended and Restated Certificate of Incorporation and Bylaws; |
| • | limitations on stockholder actions by written consent; and |
| • | the right to issue preferred stock without stockholder approval, which could be used to dilute the stock ownership of a potential hostile acquirer. |
These provisions might discourage, delay or prevent a change in control of our company or a change in our management. The existence of these provisions could adversely affect the voting power of holders of our common stock and limit the price that investors might be willing to pay in the future for shares of the common stock.
We do not intend to pay cash dividends.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all available funds and any future earnings for use in the operation and expansion of our business and do not anticipate paying any cash dividends in the foreseeable future. In addition, the terms of any future debt or credit facility may preclude us from paying any dividends. As a result, we anticipate that capital appreciation of our common stock, if any, will be your sole source of potential gain for the foreseeable future.
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
None.
| ITEM 2. | PROPERTIES |
On March 5, 2008, we entered into a Lease Agreement with The Irvine Company LLC for the lease of approximately 41,402 square feet space at 3 Morgan, Irvine, California. The term of the lease commenced on November 1, 2008 and will expire on January 31, 2014. The lease provided for a period of rent-free early occupancy before the commencement date. We believe our existing facility is adequate for our current and future needs for at least the next 12 months.
| ITEM 3. | LEGAL PROCEEDINGS |
On January 8, 2008, Hologic and its wholly-owned subsidiaries, including Cytyc Corporation and Cytyc LP, filed a lawsuit against us in the United States District Court, Northern District of California, San Jose Division. The complaint generally alleges patent infringement of certain Hologic brachytherapy patent claims, seeking unspecified monetary damages and an injunction against us for infringement of those claims. On February 6, 2008, Hologic filed a motion seeking a preliminary injunction in the case and requested that the Court stop the sale of Contura MLB. On March 7, 2008, Hologic filed an amended complaint restating its allegations regarding patent infringement, and adding new claims related to unfair competition under the Lanham Act and California state unfair competition and false advertising statutes. On April 25, 2008, the court denied Hologic’s request for a preliminary injunction and ordered the parties to schedule a trial within 60 to 90 days of such date. On May 22, 2008, the Court issued an order scheduling the Markman claims construction hearing on the patent counts for June 25, 2008, and the trial in the case to start July 14, 2008. Pursuant to an agreement of the parties, the order also dismissed Hologic’s unfair competition and false advertising claims under the Lanham Act and California state law, without prejudice. On June 24, 2008, the Court granted a joint request by the parties to stay all proceedings, including the previously scheduled Markman claims construction hearing and the trial, until at least August 22, 2008 in order to provide the parties time to discuss possible resolution of the matter. On August 22, 2008, the Company and Hologic jointly requested that the Court resume proceedings in the pending lawsuit. On October 15, 2008, a Markman claims construction hearing was held and a ruling was issued on February 18, 2009. Although we, together with our legal counsel, continue to evaluate the court’s ruling in the Markman hearing, we expect that nothing material or definitive in the matter will be decided before the trial, the date for which has not yet been set by the Court. As a result, we have taken the position that the probability of incurring any loss related to this litigation is not determinable, nor is the amount of loss quantifiable at this time. Accordingly, we have not accrued a loss related to this litigation as of December 31, 2008. We intend to continue to vigorously defend ourselves in this matter.
-30-
| ITEM 4. | SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS |
None.
PART II
| ITEM 5. | MARKET FOR THE REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Stock Exchange Listing
Our common stock has traded on the NASDAQ Global Market under the symbol “SENO” since our initial public offering on March 29, 2007. Prior to that time, there was no public market for our stock. On February 27, 2009, the closing sale price of our common stock was $2.91 per share.
Common Stockholders
As of February 27, 2009 there were approximately 93 stockholders of record of our common stock.
Stock Prices
The following table sets forth quarterly high and low closing sales prices of our common stock for the indicated periods.
| 2008: | High | Low | ||||||
| Fourth Quarter | $ | 5.00 | $ | 2.14 | ||||
| Third Quarter | $ | 7.80 | $ | 4.66 | ||||
| Second Quarter | $ | 7.75 | $ | 5.45 | ||||
| First Quarter | $ | 9.06 | $ | 6.08 | ||||
| 2007: | ||||||||
| Fourth Quarter | $ | 9.67 | $ | 8.00 | ||||
| Third Quarter | $ | 10.55 | $ | 7.94 | ||||
| Second Quarter | $ | 10.85 | $ | 7.95 | ||||
Dividend Policy
We have never paid a cash dividend and have no present intention to pay cash dividends in the foreseeable future. The Board of Directors currently intends to retain any future earnings for use in our business.
Sale of Unregistered Securities
We did not sell any unregistered securities during the period covered by this Annual Report on Form 10-K.
Stock Performance Graph
The following graph compares the cumulative total stockholder return on our common stock with the cumulative total return of the NASDAQ Composite Index and the NASDAQ Medical Equipment Index for the period beginning on March 29, 2007, our first day of trading after our initial public offering, and ending on December 31, 2008.
-31-
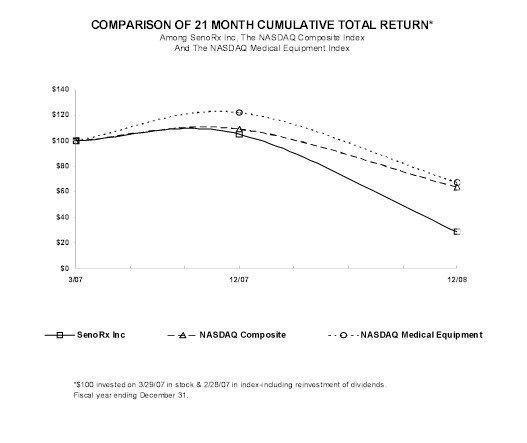
The graph assumes that $100 was invested on March 29, 2007 in our common stock, the NASDAQ Composite Index, and the NASDAQ Medical Equipment Index, and that all dividends were reinvested. No dividends have been declared or paid on our common stock. Stock performance shown in the above chart for the common stock is historical and should not be considered indicative of future price performance. This graph was prepared by Research Data Group, Inc.
Annual Percentage Return
March 29, 2007 | December 31, 2007 | December 31, 2008 | |||||||
| SenoRx, Inc. | 100.00 | 105.13 | 28.61 | ||||||
| NASDAQ Composite | 100.00 | 109.13 | 63.58 | ||||||
| NASDAQ Medical Equipment | 100.00 | 122.01 | 67.21 |
-32-
| ITEM 6. | SELECTED FINANCIAL DATA |
The following table presents selected historical financial data. We derived the selected statements of operations data for the years ended December 31, 2008, 2007, and 2006 and balance sheet data as of December 31, 2008 and 2007 from our audited financial statements and notes thereto that are included elsewhere in this annual report. We derived the selected statements of operations data for the years ended December 31, 2004 and the balance sheet data as of December 31, 2004 and 2005 from our audited financial statements that do not appear in this annual report.
You should read the following financial information together with the information under “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes included elsewhere in this annual report.
| Years Ended December 31, | |||||||||||||||||||||
| 2008 | 2007 | 2006 | 2005 | 2004 | |||||||||||||||||
| (in thousands, except per share data) | |||||||||||||||||||||
| Statement of Operations Data: | |||||||||||||||||||||
| Net revenues | $ | 46,685 | $ | 35,036 | $ | 25,508 | $ | 19,253 | $ | 13,751 | |||||||||||
| Cost of goods sold (1) | 16,503 | 15,124 | 13,506 | 10,105 | 6,415 | ||||||||||||||||
| Gross profit | 30,181 | 19,912 | 12,002 | 9,148 | 7,336 | ||||||||||||||||
| Operating expenses: | |||||||||||||||||||||
| Selling and marketing (1) | 23,117 | 19,023 | 15,041 | 10,148 | 7,507 | ||||||||||||||||
| Research and development (1) | 6,111 | 6,354 | 5,323 | 4,903 | 4,790 | ||||||||||||||||
| General and administrative (1) | 10,094 | 4,187 | 2,050 | 2,116 | 1,709 | ||||||||||||||||
| Total operating expenses | 39,322 | 29,564 | 22,414 | 17,167 | 14,006 | ||||||||||||||||
| Loss from operations | (9,141 | ) | (9,652 | ) | (10,412 | ) | (8,019 | ) | (6,670 | ) | |||||||||||
| Interest (income) expense, net | (435 | ) | 7 | 850 | 594 | 148 | |||||||||||||||
| Loss on debt extinguishment | — | 1,265 | 197 | — | — | ||||||||||||||||
| Change in fair value of convertible promissory notes | — | (991 | ) | 3,960 | — | — | |||||||||||||||
| Loss before provision for income taxes | (8,706 | ) | (9,933 | ) | (15,419 | ) | (8,613 | ) | (6,818 | ) | |||||||||||
| Provision for income taxes | — | — | — | 10 | 6 | ||||||||||||||||
| Net loss | $ | (8,706 | ) | $ | (9,933 | ) | $ | (15,419 | ) | $ | (8,613 | ) | $ | (6,818 | ) | ||||||
| Net loss per share—basic and diluted | $ | (0.50 | ) | $ | (0.75 | ) | $ | (6.61 | ) | $ | (4.19 | ) | $ | (4.54 | ) | ||||||
| Weighted-average shares outstanding basic and diluted (2) | 17,250 | 13,309 | 2,332 | 2,060 | 1,504 | ||||||||||||||||
| (1) | |||||||||||||||||||||
| Includes all non-cash stock-based compensation expense as follows: | |||||||||||||||||||||
| Cost of goods sold | $ | 95 | $ | 110 | $ | 52 | $ | 34 | $ | 17 | |||||||||||
| Selling and marketing | 863 | 589 | 409 | 438 | 184 | ||||||||||||||||
| Research and development | 449 | 509 | 395 | 286 | 184 | ||||||||||||||||
| General and administrative | 865 | 883 | 220 | 563 | 416 | ||||||||||||||||
| Total | $ | 2,272 | $ | 2,091 | $ | 1,076 | $ | 1,321 | $ | 801 | |||||||||||
| (2) | See Note 1 of the notes to our audited financial statements included elsewhere in this annual report for an explanation of the determination of the number of shares used in computing per share data. |
-33-
| As of December 31, | ||||||||||||||||||||
| 2008 | 2007 | 2006 | 2005 | 2004 | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
Balance Sheet Data: | ||||||||||||||||||||
| Cash and cash equivalents | $ | 15,323 | $ | 17,185 | $ | 7,413 | $ | 482 | $ | 3,703 | ||||||||||
| Working capital | 27,837 | 32,894 | 7,386 | 2,308 | 4,578 | |||||||||||||||
| Total assets | 35,417 | 42,062 | 19,981 | 8,163 | 9,148 | |||||||||||||||
| Long term obligations, less current portion | 1,632 | 27 | 12,125 | 2,741 | 3,829 | |||||||||||||||
| Convertible promissory notes (at fair value) | — | — | 11,960 | — | — | |||||||||||||||
| Convertible preferred stock | — | — | 46,817 | 46,817 | 41,050 | |||||||||||||||
| Total stockholders’ equity (deficit) | 28,299 | 34,363 | (13,582 | ) | 658 | 1,922 | ||||||||||||||
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
This Annual Report on Form 10-K contains forward-looking statements within the meaning of the federal securities laws. These statements are subject to risks and uncertainties that could cause actual results and events to differ materially from those expressed or implied by such forward-looking statements. For a detailed discussion of these risks and uncertainties, see the “Risk Factors” section in Item 1A of Part I of this Form 10-K. We caution the reader not to place undue reliance on these forward-looking statements, which reflect management’s analysis only as of the date of this Form 10-K. We undertake no obligation to update forward-looking statements to reflect events or circumstances occurring after the date of this Form 10-K.
Overview
We develop, manufacture and sell minimally-invasive medical devices that are used in the diagnosis of breast cancer. We were incorporated in 1998. From our inception until 2002, our principal activity was the development and regulatory clearance of our initial products, primarily our biopsy tissue markers and our first breast biopsy system, the EnCor 360. We launched our first biopsy tissue markers in 2002 and our EnCor 360 in 2003. The EnCor 360 hardware subsequently served as a platform to facilitate the later launch of the EnCor probes, handpieces and other probes, which are compatible with the major imaging modalities.
In 2004, we received 510(k) clearance from the FDA to market our EnCor breast biopsy system, our flagship product for use in breast biopsy procedures, conducting market preference testing commencing in the fourth quarter of 2004. Over the subsequent period ending in October 2005, we began selling the product on a limited basis while we focused on enhancing certain components of the product to optimize its performance, and we subsequently progressed with a full commercial launch of our EnCor system in November 2005.
We have and are continuing to develop minimally-invasive products for surgical excision of lesions and for breast cancer treatment. We received 510(k) clearance for our Contura Multi-Lumen Radiation Balloon Catheter, or Contura MLB, in May 2007 and launched in January 2008. Contura MLB is one of a new class of devices designed to reduce radiation treatment time to five days from six to eight weeks in patients eligible for the treatment. We also believe that Contura MLB may present radiation oncologists with opportunities to optimize dosing for certain patients. We are also developing next generation tissue marker products, additional EnCor line extensions, line extensions of Contura MLB, devices to assist in lesion location and certain radio frequency based excision and reconstructive tissue cutting devices.
Before 2007, we had historically derived our revenues primarily from our tissue marker products. However, our EnCor system accounted for a majority of our revenue growth in 2007. Our ability to continue to grow revenues is based upon a number of assumptions, which may not ultimately occur, including retention of our sales force, growth in the market for minimally-invasive breast biopsy procedures and rapid adoption of the product by physicians who specialize in breast care. We expect our Contura MLB to increasingly contribute to our revenues and we are marketing this device as a compelling alternative to competing devices.
For the year ended December 31, 2008, we generated net revenues of $46.7 million and a net loss of $8.7 million. As of December 31, 2008, our accumulated deficit was $84.2 million. We have not been profitable since inception. We expect our operating expenses to increase as we expand our business to meet anticipated increased demand for our EnCor system, expand sales of our Contura MLB and devote resources to our sales and marketing and research and development activities.
-34-
Net Revenues
We derive our revenues primarily from the sales of our breast biopsy systems, breast biopsy capital equipment, our tissue markers, and other products for breast care. Our largest market for these products is in the United States and Canada, where we employ a direct sales force. Our breast biopsy systems, the EnCor and EnCor 360, consist of two primary components: reusable handpieces and disposable probes, and are used in conjunction with our SenoRx Breast Biopsy Console. The disposable probes form the basis of a recurring revenue stream and also contribute to the sales of tissue markers. Diagnostic adjunct revenue consists primarily of tissue marker sales, both used with our breast biopsy systems and with competitor’s biopsy products. Our breast biopsy capital equipment includes a reusable handpiece, a control module and vacuum source used in conjunction with our disposable biopsy probe. We expect that the sales of biopsy disposable and biopsy capital equipment will grow materially in 2009. We further expect that the sales of our adjunct products, such as our tissue markers and Gamma Finder, will grow modestly. We anticipate that Contura MLB will provide meaningful revenue increases in 2009 versus 2008.
Cost of Goods Sold
Our cost of goods sold consists of the cost to manufacture and assemble our products, primarily including materials, components and labor. We assemble and package all of our finished products with the exception of our Gamma Finder product. We expect that our cost of goods sold as a percentage of revenues will decrease, and, correspondingly, gross profits will increase, as a percentage of net revenues with increased sales volume, product enhancements and outsourced manufacturing efficiencies. At the end of 2005, we entered into an agreement with a contract manufacturer in Thailand and began to transfer a portion of our manufacturing for certain components of our products to this site, and we anticipate that we will transfer additional manufacturing to this site in order to increase gross margins. We anticipate that our gross margin will continue to increase in 2009 versus 2008 due to design and production process improvements, changes in product mix, the manufacturing efficiencies that we expect to see with increased production, and the continued successful transfer of manufacturing of certain products and product components to our Thailand contract manufacturer.
Operating Expenses
Our operating expenses consist of research and development, selling and marketing, and general and administrative expenses. Stock-based compensation, a non-cash item, is primarily included in these expenses.
Our selling and marketing expenses consist of salaries and related expenses of our direct sales team and sales management, travel, clinical education and training expenses, marketing and promotional expenses, and costs associated with tradeshows. We expect selling and marketing expenses to increase in absolute terms as we expand our sales organization and promotional activities, although at a rate less than our revenue growth rate.
Our research and development expenses consist of salaries and related expenses of our research and development personnel and consultants and costs of product development, which include patent filing and maintenance costs, production engineering, clinical and regulatory support and post-clearance clinical product enhancements. We expense all our research and development costs as they are incurred. We expect research and development expenses to increase in absolute terms and as a percent of revenues in 2009 as we continue to develop, enhance, obtain clinical results and commercialize existing and new products.
Our general and administrative expenses consist of the cost of corporate operations, litigation and professional services. We expect general and administrative expenses to increase in absolute dollars as we increase our infrastructure to comply with the regulatory requirements associated with publicly-traded companies and anticipated litigation expenses relating to the current Hologic patent infringement lawsuit. During 2008, we incurred $4.9 million in patent litigation expenses related to alleged patent infringement by Hologic. On October 15, 2008, a Markman claims construction hearing was held and a ruling was issued on February 18, 2009. Although we, together with our legal counsel, continue to evaluate the court’s ruling in the Markman hearing, we expect that nothing material or definitive in the matter will be decided before the trial, the date for which has not yet been set by the Court. As a result, we believe that the probability of incurring any loss related to this litigation is not determinable, nor is the amount of loss quantifiable at this time. Accordingly, we have not accrued a loss related to this litigation as of December 31, 2008.
-35-
We expect to incur stock-based compensation expense for option grants, which will be accounted for under SFAS No. 123R. We anticipate that non-cash expenses for options accounted for under SFAS No. 123R will increase in 2009 based upon the number of options granted. We also expect to incur stock-based compensation expense related to the issuance of common stock under our employee stock purchase plan.
Interest
Interest income represents income generated from our cash and cash equivalents and short-term investments that are invested generally in liquid money-market funds and commercial paper. During 2008, debt obligations included a working capital facility, and an equipment facility resulting in interest expense. Interest expense also includes the fair value for any equity interests, such as warrants, granted in conjunction with the debt obligations. The fair value of the equity interests were amortized to interest expense over the term of the related debt obligations. Interest expense has decreased due to the retirement of certain debt obligations in 2007 and early 2008 and lower available interest rates.
Income Tax Expense
Due to uncertainty surrounding the realization of deferred tax assets through future taxable income, we have provided a full valuation allowance and no benefit has been recognized for our net operating loss and other deferred tax assets. Income tax expense relates to certain state taxes.
Results of Operations
The following table sets forth our results of operations expressed as percentages of revenues for the years ended December 31, 2008, 2007and 2006:
For the Years Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| Net revenues | 100.0% | 100.0% | 100.0% | |||||||||
| Cost of goods sold | 35.4 | 43.2 | 52.9 | |||||||||
| Gross profit | 64.6 | 56.8 | 47.1 | |||||||||
| Operating expenses: | ||||||||||||
| Selling and marketing | 49.5 | 54.3 | 59.0 | |||||||||
| Research and development | 13.1 | 18.1 | 20.9 | |||||||||
| General and administrative | 21.6 | 12.0 | 8.0 | |||||||||
| Total operating expenses | 84.2 | 84.4 | 87.9 | |||||||||
| Loss from operations | 19.6 | 27.5 | 40.8 | |||||||||
| Interest expense | 0.2 | 4.7 | 3.9 | |||||||||
| Loss on debt extinguishment | — | 3.6 | 0.8 | |||||||||
| Change in fair value of convertible promissory notes and warrant liability | — | (2.8 | ) | 15.5 | ||||||||
| Interest income | (1.1 | ) | (4.7 | ) | (0.6 | ) | ||||||
| Provision for income taxes | — | — | — | |||||||||
| Net loss | (18.6% | ) | (28.4% | ) | (60.4% | ) | ||||||
Year ended December 31, 2008 Compared to year Ended December 31, 2007
Net Revenues. Net revenues increased $11.6 million, or 33.2%, to $46.7 million in 2008 from $35.0 million in 2007. The increase primarily consisted of $4.8 million in biopsy disposable revenues, an increase of 29.8% from 2007 due to a larger installed base of EnCor systems. Biopsy capital equipment revenues increased $1.4 million, or 42.1% in 2008 due to a greater number of customers purchasing our breast biopsy systems as compared to those customers acquiring the capital through a “product supply agreement” and increased equipment sales to international distributors. Diagnostic adjunct revenues increased $798,000, or 5.3%, primarily due to an increase in marker sales resulting from increased EnCor disposable biopsy sales and sales of markers used with competitive biopsy disposables and increased Gamma Finder sales. Therapeutic revenues increased $4.6 million as we began sales of our Contura MLB to a limited number of clinical sites in June 2007 following the May 2007 FDA 510(k) clearance, with full commercialization in January 2008.
-36-
Cost of Goods Sold and Gross Profit. Cost of goods sold increased $1.4 million, or 9.1%, to $16.5 million in 2008 from $15.1 million in 2007. The increase in total cost of goods sold primarily consisted of an increase in direct labor, manufacturing overhead and material costs associated with our increase in unit sales. Gross profit increased $10.3 million or 51.6% to $30.2 million in 2008 from $19.9 in 2007. Gross profit as a percentage of net revenues increased by 7.8% to 64.6% in 2008 from 56.8% in 2007. The increase in gross profit as a percentage of net revenues was primarily attributable to improved efficiencies in the production of our disposable biopsy probe and gross margin contribution from the sales of our Contura MLB. Additionally, gross margins continue to benefit from the allocation of manufacturing overhead over greater product revenues and inventory unit production.
Selling and Marketing Expenses. Selling and marketing expenses increased $4.1 million, or 21.5%, to $23.1 million in 2008 from $19.0 million in 2007. The increase primarily consisted of $3.5 million in salaries and related employee costs due to the expansion of our sales organization, a $133,000 increase in departmental related expenses, a $147,000 increase in selling and promotional related expenses and a $274,000 increase in equity based compensation.
Research and Development Expenses. Research and development expenses decreased $242,000, or 3.8%, to $6.1 in 2008 from $6.4 million in 2006. The decrease in these expenses consisted primarily of project costs, predominately for the Contura MLB, which decreased $502,000 and a decrease of $60,000 in equity based compensation. This decrease was partially offset by an increase of $62,000 for salaries and the related employee costs, a $116,000 increase for patent related expenses and an increase of $142,000 in departmental related expenses.
General and Administrative Expenses. General and administrative expenses increased $5.9 million, or 141.1%, to $10.1 million in 2008 from $4.2 million in 2007. The increase primarily consisted of $4.9 million in attorney and related litigation costs incurred in connection with responding to the allegations by Hologic of patent infringement relating to our Contura MLB, $265,000 for public company related costs, including legal and reporting expenses, which includes first year Sarbanes Oxley implementation costs, $275,000 for salaries and the related employee costs, $287,000 for departmental costs and $173,000 for increased professional fees.
Interest Expense. Interest expense decreased $1.6 million to $85,000 in 2008 from $1.6 million in 2007. The decrease was primarily due to the repayment of the December 2006 subordinated note.
Loss on Debt Extinguishment. In November 2007, we incurred a $1.3 million expense on the retirement of a December 2006 Subordinate Note facility that had an original principal amount of $10.0 million outstanding under it, representing the unamortized debt issuance and debt discounts which would have been otherwise charged to interest expense over the term of the facility. In 2008, we did not incur any expense related to this item.
Change in Fair Value of Convertible Promissory Notes and Warrant Valuation. In 2007, we recorded income of $160,000 for the change in fair value of our May 2006 convertible promissory notes that had an original aggregate principal amount of $8.0 million outstanding, in accordance with FAS No. 155, and income of $831,000 for the reduction in the fair value of a related warrant liability. In 2008, we did not incur any expense related to this item.
Interest Income. Interest income decreased $1.1 million to $520,000 in 2008 from $1.6 million in 2007 primarily due to lower cash and short-term investment balances resulting from the repayment of $10.3 million for the retirement of the December 2006 Subordinated Note, $2.0 million to repay a February 2003 convertible subordinated note and 2002 note obligations owing to Century Medical, as well as working capital needs.
Year ended December 31, 2007 Compared to year Ended December 31, 2006
Net Revenues. Net revenues increased $9.5 million, or 37.3%, to $35.0 million in 2007 from $25.5 million in 2006. The increase primarily consisted of an increase of $5.2 million in biopsy disposable revenues, or 47.8% from 2006, due to a larger installed base of EnCor systems. Biopsy capital revenues increased $2.1 million, or 165.2%, due to a greater number of customers purchasing our breast biopsy systems as compared to those customers acquiring the capital through a “product supply agreement” in 2006. Diagnostic adjunct revenues increased $1.7 million, or 12.7%, primarily due to an increase in marker sales resulting from increased EnCor disposable biopsy sales and sales of markers used with competitive biopsy disposables and increased Gamma Finder sales. Diagnostic therapeutic revenues increased $542,000 as we began sales of our Contura MLB to a limited number of clinical sites in June 2007 following the May 2007 FDA 510(k) clearance.
-37-
Cost of Goods Sold and Gross Profit. Cost of goods sold increased $1.6 million, or 12.0%, to $15.1 million in 2007 from $13.5 million in 2006. The increase in total cost of goods sold primarily consisted of an increase in direct labor, manufacturing overhead and material costs associated with our increase in product sales. Gross profit increased $7.9 million or 65.9% in 2007 to $19.9 million from $12.0 million in 2006. Gross profit as a percentage of net revenues increased by 9.7% to 56.8% in 2007 from 47.1% in 2006. The increase in gross profit as a percentage of net revenues was primarily attributable to improved efficiencies in the production of our disposable biopsy probe and allocating manufacturing overhead over greater product revenues and inventory unit production.
Selling and Marketing Expenses. Selling and marketing expenses increased $4.0 million, or 26.5%, to $19.0 million in 2007 from $15.0 million in 2006. The increase primarily consisted of $2.9 million in salaries and related employee costs due to the expansion of our sales organization, $158,000 in equity based compensation charges including deferred compensation and the discount associated with shares purchased by employees under our Employee Stock Purchase Plan and $947,000 increase in selling and promotional related expenses.
Research and Development Expenses. Research and development expenses increased $1.0 million, or 19.4%, to $6.4 in 2007 from $5.3 million in 2006. The increase in these expenses primarily consisted of $156,000 in salaries and the related employee costs, $727,000 associated with project costs for the development of the Contura MLB, SenoSonix and VisiLoc, and a $108,000 increase in equity based compensation charges including deferred compensation and the discount associated with shares purchased by employees under our Employee Stock Purchase Plan.
General and Administrative Expenses. General and administrative expenses increased $2.1 million, or 104.2%, to $4.2 million in 2007 from $2.1 million in 2006. The increase primarily consisted of $440,000 related to increased headcount and increased compensation, $670,000 for public company related costs, including legal and reporting expenses, $659,000 in equity based compensation charges including deferred compensation and the discount associated with shares purchased by employees under our Employee Stock Purchase Plan and $331,000 for increased departmental costs. These increases were partially offset by a $226,000 decrease in legal fees associated with the resolution of the Suros litigation in May 2006.
Interest Expense. Interest expense increased $649,000 to $1.6 million in 2007 from $998,000 in 2006. The increase was due to the interest expense incurred on the December 2006 Subordinated Note.
Loss on Debt Extinguishment. In November 2007, we incurred a $1.3 million expense on the retirement of the December 2006 Subordinate Note, representing the unamortized debt issuance and debt discounts which would have been otherwise charged to interest expense over the term of the note. In 2006, we incurred a $200,000 expense related to the acceleration of the amortization of the debt discount and issuance costs associated with the early repayment of a 2004 Subordinated Note payable.
Change in Fair Value of Convertible Promissory Notes and Warrant Liability. In 2007, we recorded income of $160,000 for the change in fair value of our May 2006 Notes in accordance with FAS No. 155 and income of $831,000 for the reduction in the fair value of the related warrant liability. In 2006, we recorded a $3.8 million expense for the changes in fair value of our May 2006 Notes.
Interest Income. Interest income increased $1.5 million to $1.6 million in 2007 from $148,000 in 2006 primarily as a result of increased interest income from higher cash and short-term investment balances resulting from our IPO, which closed in April 2007.
-38-
Liquidity and Capital Resources
General
We have incurred losses since our inception in January 1998 and, as of December 31, 2008, we had an accumulated deficit of $84.2 million. From inception through December 31, 2008, we generated cumulative gross profit from the sale of our product offerings of $86.9 million. To date, our operations have been funded primarily with proceeds from the issuance of our preferred stock and borrowings, including our issuance of the May 2006 Notes and the December 2006 Subordinated Note, and our IPO that closed in April 2007. Cumulative net proceeds from the issuance of preferred stock totaled $46.8 million. Proceeds from the issuance of the May 2006 Notes totaled $8.0 million. Proceeds from the issuance of the December 2006 Subordinated Note was $10.0 million, of which $1.2 million was used to repay the 2004 Subordinated Note payable. Net proceeds from our IPO, including the sale of shares pursuant to the subsequent underwriters’ overallotment and after deducting total expenses, was $44.8 million. All of our preferred stock converted into common stock upon the closing of the IPO. In November 2007 we used $10.3 million to retire the December 2006 Subordinated Note and in February 2008 we used $2.0 million to repay the February 2003 convertible subordinated note and 2002 note obligations owing to Century Medical. In September 2008 we amended our existing loan agreement with Silicon Valley Bank, or SVB, to among other items, increase the total maximum amount available for borrowing from $4.0 million to $12.0 million. As of December 31, 2008, $2.0 million has been drawn down under this facility.
We believe that our cash and cash equivalents, and our anticipated ability to draw down on our working capital and equipment facilities, will be sufficient to meet our projected operating requirements for at least the next 12 months. We anticipate that we will continue to use cash in our operating activities and investing activities for the foreseeable future as we grow our business.
Net Cash Used in Operating Activities—Year Ended 2008
Net cash used in operating activities was $11.9 million, for the year ended December 31, 2008, which was primarily a function of an increase in inventory of $3.9 million, an increase in accounts receivable of $2.9 million, an increase in other assets of $225,000, and a decrease in accounts payable and accrued expenses of $560,000. These uses of cash were partially offset by a decrease in prepaid expenses of $158,000 and an increase in deferred revenue of $68,000. The aggregate increased investment in inventory of $3.9 million resulted primarily from two major factors, including (i) our decision to build shelf stock for our higher volume products in order to better service our customers as well as accommodate our move to the new manufacturing facility in late August 2008, and (ii) the need to purchase longer-term quantities of certain parts due to long lead times. We expect inventory will modestly decrease in 2009. The increase in accounts receivable was primarily due to an increase in net sales and an increase in sales outside the United States, which include extended payment terms for initial stocking orders. While we expect that the amount of accounts receivable will fluctuate based on the timing of sales and collections, we expect our ratio of overall investment in accounts receivable as compared to revenues will continue to modestly increase. The $560,000 decrease in accounts payable and accrued expenses resulted from the use of funds, including funds borrowed under our bank loan facility with SVB that allowed us to reduce outstanding accounts payable with certain vendors.
Net Cash Provided by Investing Activities—Year Ended 2008
Net cash provided by investing activities amounted to $9.8 million during the year ended December 31, 2008, was primarily attributable to the maturities of short-term investments which was partially offset by the $970,000 addition of new manufacturing molds and equipment, business intelligence software, leasehold improvements to our new Irvine facility and demonstration units.
Net Cash Provided by Financing Activities—Year Ended 2008
Net cash provided by financing activities was $242,000 during the year ended December 31, 2008, primarily attributable to $2.0 million advance on our term loan and $370,000 related to the proceeds from the issuance of common stock from option exercises and ESPP stock purchases. These proceeds were partially offset by the repayment of $2.0 million for the Century Medical notes and $30,000 cash paid for the annual renewal of the SVB working capital facility.
-39-
Net Cash Used in Operating Activities—Year Ended 2007
Net cash used in operating activities was $10.3 million, for the year ended December 31, 2007, which was a function of an increase in inventory of $2.7 million, an increase in accounts receivable of $1.2 million, a decrease in accounts payable and accrued expenses of $748,000, and an increase in prepaid expenses of $324,000. These uses of cash were partially offset by a decrease in other assets of $384,000 and an increase in deferred revenue of $58,000. The aggregate increased investment in inventory of $2.7 million resulted primarily from two major factors, including our decision to build shelf stock of our higher volume products in order to better service our customers, and the need to purchase longer-term quantities of certain parts due to long lead times. We expect inventory will continue to increase in 2008. The increase in accounts receivable was primarily due to an increase in net sales. While we expect that the amount of accounts receivable will fluctuate based on the timing of sales and collections, we expect our ratio of overall investment in accounts receivable as compared to revenues will remain constant as compared to 2007. The $748,000 decrease in accounts payable and accrued expenses resulted from the use of proceeds from our April 2007 IPO, which allowed us to reduce outstanding accounts payable with certain vendors.
Net Cash Used in Investing Activities—Year Ended 2007
Net cash used in investing activities amounted to $11.3 million during the year ended December 31, 2007, primarily attributable to the purchase of short-term investments and the addition of demonstration units and new manufacturing molds.
Net Cash Provided by Financing Activities—Year Ended 2007
Net cash provided by financing activities was $31.4 million during the year ended December 31, 2007, primarily attributable to proceeds of $47.1 million from our April 2007 IPO and underwriters’ overallotment, a $2.8 million advance on our working capital facility and $61,000 related to the proceeds from the issuance of common stock from option exercises. These proceeds were partially offset by an aggregate of $17.8 million in repayments under our various debt facilities and $941,000 in cash paid for deferred offering costs including legal, accounting and printing fees, which were offset against offering proceeds at the completion of our IPO in April 2007.
Net Cash Used in Operating Activities—Years Ended 2006
Net cash used in operating activities was $9.2 million for the year ended December 31, 2006. The net cash used primarily reflects the net loss for the period, offset in part by depreciation, amortization of deferred compensation and amortization of debt discounts and changes in operating assets and liabilities.
Net cash used in our operating activities increased from $7.5 million in 2005 to $9.2 million in 2006 due to changes in assets and liabilities, which was a function of an increase in inventory of $2.1 million, an increase in accounts receivable of $1.4 million and an increase in accounts payable and accrued expenses of $2.9 million. The aggregate increased investment in inventory of $2.1 million resulted primarily from three major factors, including our requirement to purchase inventory from a distributor earlier than needed, our decision to build shelf stock of our higher volume products in order to better service our customers, and the need to purchase longer-term quantities of certain parts due to long lead times.
Net Cash Used in Investing Activities—Years Ended 2006
Net cash used in investing activities was $900,000 for the year ended December 31, 2006.
During the year ended December 31, 2006, cash used consisted of $500,000 for new molds, equipment and machinery, $150,000 for demonstration units, $100,000 for new computers and software, $96,000 for a trade show booth and equipment, and $29,000 for leasehold improvements.
Net Cash Provided by Financing Activities—Years Ended 2006
Net cash provided by financing activities was $17.0 million in 2006.
The increase in 2006 as compared to 2005 was primarily attributable to the proceeds of $10.0 million related to the December 2006 Subordinated Note, $8.0 million related to the May 2006 convertible promissory notes, an aggregate of $3.1 million in borrowings under our various debt facilities and $100,000 related to proceeds from the issuance of common stock from option exercises. These sources of cash were offset by $2.7 million in scheduled debt repayments and the early repayment of the 2004 Subordinated Note payable, $1.3 million in cash paid for deferred offering costs including legal, accounting and printing fees, which were at the time to be reclassified as additional paid-in capital at the completion of the IPO, and $200,000 for payment of debt issuance costs.
-40-
Accounts Receivable
Our accounts receivable days outstanding were 51 days at December 31, 2008, 47 days at December 31, 2007 and 42 days at December 31, 2006. Our products are typically sold for terms net 30 days from shipment. We review our accounts receivable balances and customers regularly to establish and maintain an appropriate allowance for doubtful accounts. Our account analysis includes reviewing the customer’s historical payment history, the amount and number of days an account is outside of payment terms, the magnitude of the account balance, historical order patterns and any specific knowledge about the customer’s financial condition. Our allowance for doubtful accounts as a percentage of gross receivables was 2.7%, 2.0% and 2.8% at December 31, 2008, 2007 and 2006, respectively. Our reserve requirements are based on our review of every account and we place particular emphasis on each customer account with an account receivable balance more than 90 days old and on that customer’s specific payment history and other financial information. As our revenues increase, we anticipate our days sales outstanding will fluctuate moderately.
Contractual Obligations
The following summarizes our long-term contractual obligations at December 31, 2008:
| Payments due by period | ||||||||||||||||||||
| Total | Less than 1 year | 1-3 years | 3-5 years | More than 5 years | ||||||||||||||||
| Long-term debt obligations | $ | 2,010,058 | $ | 385,058 | $ | 1,000,000 | $ | 625,000 | $ | — | ||||||||||
| Capital lease obligations | 13,781 | 12,727 | 1,054 | — | — | |||||||||||||||
| Operating lease obligations | 2,954,441 | 534,912 | 1,136,064 | 1,231,298 | 52,167 | |||||||||||||||
| Total | $ | 4,978,280 | $ | 932,697 | $ | 2,137,118 | $ | 1,856,298 | $ | 52,167 | ||||||||||
The operating leases shown above reflect payments related to our real estate lease in Irvine, California, which expire in January 2014 and will amount to $3.0 million over the 63 month duration of the lease commencing in November 2008.
Working Capital Facility with Silicon Valley Bank. We have a working capital facility with Silicon Valley Bank that, as a result of amendments to this facility in February 2007 and September 2008, has an aggregate limit of $12.0 million. In connection with the September 2008 amendment, we also entered into an Export-Import Bank of the United States Working Capital Guarantee Program Borrower Agreement, which helped increase the total maximum amount available for borrowing under the facility, which is now $12.0 million. Previously, the Company’s credit facility with SVB provided for a maximum borrowing amount of $4.0 million.
Revolving Line. The SVB facility provides for a domestic receivables-based revolving line of credit in an aggregate amount of up to $10.0 million, or, if less, the sum of 80% of eligible domestic accounts receivable plus 25% of eligible domestic inventory, coupled with a foreign receivables-based revolving line of credit, guaranteed by Export-Import Bank of the United States, in an aggregate amount of up to $2.5 million, or if less, the sum of 90% of eligible foreign accounts receivable plus 50% of eligible export-related inventory. No more than $10.0 million, in the aggregate, will be available for combined draw-downs under these two revolving lines of credit, a maturity date of September 2010 and bears interest at an annual rate equal to the prime rate plus 0.25% (4.25% at December 31, 2008), provided that the annual rate will increase to prime rate plus 1.00% if a financial ratio relating to our liquidity falls below a specified level. Interest is due monthly, with the balance due at the maturity date. The domestic portion of revolving line is subject to a $1.0 million sublimit available for cash management services provided by SVB, including the issuance of short-term letters of credit and foreign exchange contracts. At December 31, 2008, there was $7.8 million available to borrow on the revolving line.
Term Loan. The SVB facility also provides for a term loan in an aggregate amount of up to $2.0 million, with a maturity date of March 2013, provided that if the revolving line is not renewed at its maturity date, all amounts outstanding under the term loan will become due at such time. The term loan bears interest at an annual rate equal to the prime rate plus 0.75% (4.75% at December 31, 2008), provided that the annual rate applicable to the term loan increases to an annual rate equal to the prime rate plus 1.50% if a financial ratio relating to our liquidity falls below a specified level. A single advance is permitted and was drawn down in November 2008. Monthly payments of interest only on the amount outstanding are due through March 2009, followed by forty-eight (48) consecutive monthly installments of amortized principal and interest through the maturity date. The full $2.0 million was outstanding at December 31, 2008.
-41-
Loan Agreement Obligations. The obligations under the SVB facility are secured by security interest on substantially all of our assets, excluding intellectual property for which we gave a negative pledge against encumbering. The SVB facility contains certain restrictive loan covenants, including, among others, financial covenants requiring a minimum tangible net worth and a minimum liquidity ratio, and covenants limiting our ability to dispose of assets, make acquisitions, be acquired, incur indebtedness, grant liens or enter into negative pledge agreements, make investments, make distributions in respect of our capital stock (including repurchases of such capital stock) or enter into transactions with affiliates. The SVB facility also contains events of default that include, among others, failure to make payments when due, inaccuracy of representations and warranties, violation of covenants, events constituting a material adverse change, bankruptcy and insolvency events, material judgments, and cross defaults to certain other agreements. The occurrence of an event of default could result in the acceleration of our obligations and an increase to the applicable interest rate, and would permit SVB to exercise remedies with respect to the collateral.
Off-Balance Sheet Arrangements
Since inception, we have not engaged in material off-balance sheet activities, including the use of structured finance, special purpose entities or variable interest entities.
Income Taxes
Realization of our deferred tax assets is dependent upon the timing and amount of our future earnings, if any. Accordingly, we have established full deferred tax asset valuation allowances as of December 31, 2008, 2007 and 2006 to reflect these uncertainties.
As of December 31, 2008, we had federal and state net operating loss carryforwards of approximately $66.1 million and $49.8 million, respectively, and $1.6 million in federal tax credit carryforwards and $1.8 million in state tax credit carryforwards. The federal net operating loss carryforwards and tax credit carryforwards will begin to expire in 2018. The state net operating loss carryforwards will begin to expire in 2009. The state tax credit carryforwards do not expire. The utilization of the net operating loss and tax credit carryforwards may be subject to a substantial annual limitation due to ownership change limitations provided by the Internal Revenue Code. This annual limitation may result in the expiration of net operating loss and tax credit carryforwards before we are able to utilize them.
Critical Accounting Policies
We prepare our financial statements in accordance with accounting principles generally accepted in the United States. In doing so, we have to make estimates and assumptions that affect our reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. We regularly evaluate our estimates and assumptions based upon historical experience and various other factors that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. To the extent actual results differ from those estimates, our future results of operations may be affected.
While our significant accounting policies are more fully described in Note 1 of the notes to our audited financial statements, we believe that the following accounting policies and estimates are most critical to a full understanding and evaluation of our reported financial results.
-42-
Revenue Recognition
Revenue is recognized when (a) persuasive evidence of an arrangement exists; (b) title has transferred; (c) the fee is fixed or determinable; and (d) collectability is reasonably assured. Our recognition policy is significant because our revenue is a key component of our operations and the timing of revenue recognition determines the timing of certain expenses, such as sales commissions. Revenue results are difficult to predict, and any shortfall in revenues could cause our operating results to vary significantly from period to period.
For those sales that include multiple deliverables, we allocate revenue based on the relative fair values of the individual components as determined in accordance with EITF Issue No. 00-21, “Revenue Arrangements with Multiple Deliverables.” When more than one element, such as hardware and disposables, are contained in a single arrangement, revenues are allocated between the elements based on each element’s relative fair value, provided that each element meets the criteria for treatment as a separate unit of accounting. An item is considered a separate unit of accounting if it has value to the customer on a standalone basis and there is objective and reliable evidence of the fair value of the undelivered items. Fair value is generally determined based upon the price charged when the element is sold separately. In the absence of fair value for a delivered element, we allocate revenue first to the fair value of the undelivered elements and allocate the residual revenue to the delivered elements. In the absence of fair value for an undelivered element, the arrangement is accounted for as a single unit of accounting, resulting in a deferral of revenue recognition for the delivered elements until all undelivered elements have been fulfilled.
We place certain equipment with customers in return for the customer purchasing a minimum number of disposable devices during a specified contract period. Title to the equipment passes to the customer at the end of the contract period if the minimum purchase requirements are met. The cost of the equipment, which is included in other long-term assets in the accompanying balance sheets, is amortized to cost of goods sold based on the monthly disposable unit shipments compared to the total purchase commitment of disposables. In the event the customer does not fulfill the minimum purchase requirements, collection efforts may be undertaken and we will attempt to recover the equipment. If the collection efforts or recovery of the equipment is not successful, the unamortized equipment cost would be expensed to cost of goods sold.
Deferred Revenue
We also account for a customer’s advance payment on product purchases as deferred revenue. As product is purchased, the applicable sales value is recognized as revenue.
Stock-Based Compensation
Effective January 1, 2006, we adopted SFAS 123R, which requires that all stock-based compensation to employees, including grants of employee stock options, be expensed in our financial statements based on their respective grant date fair values. Under SFAS 123R, we estimate the fair value of each stock-based payment award using the Black-Scholes option pricing model.
The determination of the fair value of stock-based payment awards using the Black-Scholes model is affected by our stock price and a number of assumptions, including expected volatility, expected life, risk-free interest rate and expected dividends. We did not have a history of market prices of our common stock as we were not a public company until our April 2007 initial public offering, and as such, we estimate volatility in accordance with SAB No. 107 using historical volatilities of other publicly traded companies in our industry. The expected life of the awards is based on the simplified method as defined in SAB No. 107. The risk-free interest rate assumption is based on observed interest rates appropriate for the terms of our awards. The dividend yield assumption is based on our history and expectation of not paying any dividends. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. We recognized stock-based compensation expense in our financial statements based on awards that are ultimately expected to vest.
-43-
A summary of significant assumptions used in determining the fair value of the options is as follows:
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| Expected life (years) | 4.5 - 4.75 | 4.75 - 6.25 | 6.25 | |||||||||
| Risk-free interest rate | 1.62% - 3.26% | 3.49% - 4.69% | 4.56% - 5.05% | |||||||||
| Volatility | 45% - 48% | 42% - 48% | 48% - 56% | |||||||||
| Dividend yield | 0% | 0% | 0% | |||||||||
| Forfeiture rate | 5% | 5% | 5% | |||||||||
If factors change and we employ different assumptions, stock-based compensation expense may differ significantly form what we recorded in the past. If there are any modifications or cancellations of the underlying unvested securities, we may be required to accelerate, increase or cancel any remaining unearned stock-based compensation expense. Future stock-based compensation expense and unearned stock-based compensation will increase to the extent that we grant addition equity awards to employees or we assume unvested equity awards in connection with acquisitions.
Inventories
We assess the recoverability of our inventories at least quarterly through a review of inventory levels in relation to foreseeable demand, generally over twelve months. Foreseeable demand is based upon all available information, including sales backlog and forecasts, product marketing plans and product life-cycle information. When the inventory on hand exceeds the foreseeable demand, we write down the value of those inventories which, at the time of our review, we expect to be unable to sell. The amount of the inventory write-down is the excess of historical cost over estimated realizable value. Once established, these write-downs are considered permanent adjustments to the cost basis of the excess inventory. Demand for our products may fluctuate significantly over time, and actual demand and market conditions may be more or less favorable than those projected by management. In the event that actual demand or product pricing is lower than originally projected, additional inventory write-downs may be required. Further, on a quarterly basis, we assess the net realizable value of our inventories. When the estimated average selling price, plus costs to sell our inventory, falls below our inventory cost, we adjust our inventory to its current estimated market value.
Allowance for Doubtful Accounts
We maintain allowances for doubtful accounts for estimated losses resulting from the inability of our customers to make required payments. We use a specific identification method for some items, and a percentage of aged receivables for others. The percentages are determined based on our past experience. If the financial condition of our customers were to deteriorate, our actual losses might exceed our estimates, and additional allowances would be required.
Software Development
Certain of our products incorporate software which is incidental to the product as a whole. Software development costs incurred prior to the establishment of technological feasibility are expensed as research and development costs. We define the establishment of technological feasibility as the completion of a final working model that has been incorporated into a product that has been cleared by the FDA, at which time the product can be sold to third parties. As a result, we have expensed all software development costs.
Impairment of Long-lived Assets
Long-lived assets, including fixed assets, are continually monitored and are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of any such asset may not be recoverable. The determination of recoverability is based on an estimate of undiscounted cash flows expected to result from the use of an asset and its eventual disposition. The estimate of cash flows is based upon, among other things, certain assumptions about expected future operating performance, growth rates and other factors. Our estimates of undiscounted cash flows may differ from actual cash flows due to, among other things, technological changes, economic conditions, changes to our business model or changes in our operating performance. If the sum of the undiscounted cash flows (excluding interest) is less than the carrying value, we recognize an impairment loss, measured as the amount by which the carrying value exceeds the fair value of the asset. We determine fair value by using available market data, comparable asset quotes and/or discounted cash flow models.
-44-
Deferred Income Taxes
We evaluate the realizability of our deferred tax assets and assess the need for a valuation allowance quarterly. We record a valuation allowance to reduce our deferred tax assets to the net amount that is more likely than not to be realized. Our assessment of the need for a valuation allowance is based upon our history of operating results, expectations of future taxable income and the ongoing prudent and feasible tax planning strategies available to us. In the event that we determine that we will not be able to realize all or part of our deferred tax assets in the future, an adjustment to the deferred tax assets would be charged against income in the period such determination is made. Likewise, in the event we were to determine that we will be able to realize our deferred tax assets in the future in excess of the net recorded amount, an adjustment to the deferred tax assets would increase income in the period such determination is made.
Fair Value of Financial Instruments
At each reporting date, we were required to estimate the fair value of our May 2006 convertible promissory notes in its entirety with changes in fair value recognized in the statement of operations. Our estimate of fair value was based upon a valuation which encompasses the probability weighted scenarios of the conversion features, as well as the timing and method of payment of interest associated with our May 2006 notes.
At each reporting date, we were also required to estimate the fair value of the warrant issued in conjunction with our 2006 Subordinated Note. Our estimate of fair value was determined using the Black-Scholes option pricing model, which requires inputs for risk-free interest rate, dividend yield, volatility, the life of the warrant and the fair value of the underlying security.
Recent Accounting Pronouncements
In December 2007, the FASB issued SFAS No. 141—revised 2007, “Business Combinations” (“SFAS 141R”). SFAS 141R establishes principles and requirements for how an acquirer in a business combination recognizes and measures in its financial statements the identifiable assets acquired, the liabilities assumed, and any noncontrolling interest; recognizes and measures the goodwill acquired in the business combination or a gain from a bargain purchase; and determines what information to disclose to enable financial statement users to evaluate the nature and financial effects of the business combination. SFAS 141R applies to business combinations for which the acquisition date is on or after December 15, 2008. Early adoption is prohibited. We are currently evaluating the effect, if any, that the adoption of SFAS 141R will have on our results of operations, financial position and cash flows.
In December 2007, the FASB issued SFAS No. 160, Noncontrolling Interests in Consolidated Financial Statements—an amendment to ARB No. 51 (“SFAS 160”). SFAS 160 requires all entities to report noncontrolling (minority) interests in subsidiaries as equity in the consolidated financial statements, but separate from the equity of the parent company. The statement further requires that consolidated net income be reported at amounts attributable to the parent and the noncontrolling interest, rather than expensing the income attributable to the minority interest holder. This statement also requires that companies provide sufficient disclosures to clearly identify and distinguish between the interests of the parent company and the interests of the noncontrolling owners, including a disclosure on the face of the consolidated statements for income attributable to the noncontrolling interest holder. This statement is effective for fiscal years beginning on or after December 15, 2008. Early adoption is prohibited. We are currently evaluating the effect, if any, that the adoption of SFAS 160 will have on our results of operations, financial position and cash flows.
In February 2008, the FASB issued FASB Staff Position No. 157-2, which deferred the effective date for certain portions of SFAS 157 related to nonrecurring measurements of nonfinancial assets and liabilities. That provision of SFAS 157 will be effective for fiscal years beginning after December 15, 2008 and for interim periods within those fiscal years. We do not expect the adoption of SFAS 157-2 to have a material effect our results of operations, financial position and cash flows.
-45-
In April 2008, the FASB issued FASB Staff Position 142-3, Determination of the Useful Lives of Intangible Assets (“FSP 142-3”), which amends the factors that should be considered in developing renewal or extension assumptions used to determine the useful life of a recognized intangible asset under FASB No. 142, Goodwill and Other Intangible Assets. The intent of FSP 142-3 is to improve the consistency between the useful life of a recognized intangible asset under SFAS No. 142 and the period of expected cash flows used to measure the fair value of the asset under SFAS No. 141(R) and other U.S. generally accepted accounting principles. FSP 142-3 is effective for fiscal years beginning after December 15, 2008 and for interim periods within those fiscal years. We do not expect the adoption of FSP 142-3 to have a material effect on our results of operations, financial position and cash flows.
In June 2008, the FASB issued FSP EITF 03-6-1, Determining Whether Instruments Granted in Share-Based Payment Transactions Are Participating Securities, which addresses whether instruments granted in share-based payment transactions are participating securities prior to vesting and, therefore, need to be included in the earnings allocation in computing earnings per share under the two-class method described in paragraphs 60 and 61 of FASB Statement No. 128, Earnings per Share. This FSP is effective for fiscal years beginning after December 15, 2008 and for interim periods within those fiscal years. We do not expect the adoption of FSP EITF 03-6-1 to have a material effect our results of operations, financial position and cash flows.
In October 2008, the FASB Issued FSP No. 157-3, Determining the Fair Value of a Financial Asset When the Market for That Asset Is Not Active. FSP No. 157-3 clarifies the application of FASB Statement No. 157, Fair Value Measurements, in a market that is not active and provides an example to illustrate key considerations in determining the fair value of a financial asset when the market for that financial asset is not active. FSP No. 157-3 is effective upon issuance. We do not expect the adoption of FSP No. 157-3 to have a material impact on our results of operations, financial position and cash flows.
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
The primary objective of our investment activities is to preserve our capital for the purpose of funding operations while at the same time maximizing the income we receive from our investments without significantly increasing risk. To achieve these objectives, our investment policy allows us to maintain a portfolio of cash equivalents and investments in a variety of marketable securities, including commercial paper, money market funds and corporate debt securities and U.S. government securities. Our cash and cash equivalents as of December 31, 2008, included liquid money market accounts. Due to the liquid nature of our cash and cash equivalents, we believe we have no material exposure to interest rate risk. Additionally, since the majority of our debt carries interest at fixed rates, we also believe changes in interest rates will not cause significant changes in our interest expense. Our revenues are denominated in U.S. dollars. Accordingly, we have not had exposure to foreign currency rate fluctuations. We expect to continue to realize our revenues in U.S. dollars. .
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
The Company’s Financial Statements as of December 31, 2008 and 2007 and for each of the three years in the period ended December 31, 2008, together with the reports of our independent registered public accounting firm, are included under Part IV, Item 15 of this report.
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
| ITEM 9A. | CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures. Our management evaluated, with the participation of our Chief Executive Officer and our Chief Financial Officer, the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) of the Exchange Act of 1934, as amended) as of the end of the period covered by this Annual Report on Form 10-K. Based on this evaluation, our Chief Executive Officer and our Chief Financial Officer have concluded that our disclosure controls and procedures are effective to ensure that information we are required to disclose in reports that we file or submit under the Securities Exchange Act of 1934 is recorded, processed, summarized and reported within the time periods specified in Securities and Exchange Commission rules and forms, and that such information is accumulated and communicated to management as appropriate to allow for timely decisions regarding required disclosure.
-46-
Management’s Report on Internal Control Over Financial Reporting. Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rule 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended. Our internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with policies or procedures my deteriorate.
To evaluate the effectiveness of internal control over financial reporting, management used the criteria set forth in Internal Control-Integrated Framework, issue by the Committee of Sponsoring Organizations of the Treadway Commission. Based on its assessment using those criteria, management has concluded that we maintained effective internal control over financial reporting as of December 31, 2008.
The effectiveness of the Company’s internal control over financial reporting as of December 31, 2008 has been audited by Deloitte & Touche LLP, an independent registered public accounting firm, as stated in their report which is included herein.
Changes in Internal Control Over Financial Reporting. There was no change in our internal control over financial reporting (as defined in Rule 13a-15(f) and 15d-15(f) of the Exchange Act of 1934, as amended) that occurred during the fourth quarter ended December 31, 2008 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
-47-
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of
SenoRx, Inc.
Irvine, California
We have audited the internal control over financial reporting of SenoRx, Inc. (the “Company”) as of December 31, 2008, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed by, or under the supervision of, the company’s principal executive and principal financial officers, or persons performing similar functions, and effected by the company’s board of directors, management, and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company, (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company, and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of the inherent limitations of internal control over financial reporting, including the possibility of collusion or improper management override of controls, material misstatements due to error or fraud may not be prevented or detected on a timely basis. Also, projections of any evaluation of the effectiveness of the internal control over financial reporting to future periods are subject to the risk that the controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2008, based on the criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the financial statements as of and for the year ended December 31, 2008, of the Company and our report dated March 4, 2009, expressed an unqualified opinion on those financial statements.
/s/ DELOITTE & TOUCHE LLP
Costa Mesa, California
March 4, 2009
-48-
| ITEM 9B. | OTHER INFORMATION |
None.
PART III
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The information required by this Item is incorporated by reference to the definitive proxy statement for our 2009 Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission within 120 days after the end of our 2008 fiscal year (the “2009 Proxy Statement”).
| ITEM 11. | EXECUTIVE COMPENSATION |
The information required by this Item is incorporated by reference to the 2009 Proxy Statement.
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information required by this Item is incorporated by reference to the 2009 Proxy Statement.
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE |
The information required by this Item is incorporated by reference to the 2009 Proxy Statement.
| ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The information required by this Item is incorporated by reference to the 2009 Proxy Statement.
PART IV
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
| (1) | The financial statements listed in the “Index to Financial Statements” at page 50 are filed as a part of this report. |
| (2) | All schedules are omitted because they are not applicable. All the required information is shown in the financial statements or notes thereto. |
| (3) | Exhibits included or incorporated herein. See Exhibit Index. |
-49-
SENORX, INC.
INDEX TO FINANCIAL STATEMENTS
| Page | |
| Financial Statements: | |
| Report of Independent Registered Public Accounting Firm | 51 |
| Balance Sheets as of December 31, 2008 and 2007 | 52 |
| Statements of Operations for the Years Ended December 31, 2008, 2007 and 2006 | 53 |
| Statements of Stockholders’ Equity (Deficit) for the Years Ended December 31, 2008, 2007 and 2006 | 54 |
| Statements of Cash Flows for the Years Ended December 31, 2008, 2007 and 2006 | 55 |
| Notes to Financial Statements | 57 |
-50-
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
SenoRx, Inc.
Irvine, California
We have audited the accompanying balance sheets of SenoRx, Inc. (the “Company”) as of December 31, 2008 and 2007, and the related statements of operations, stockholders’ equity (deficit), and cash flows for each of the three years in the period ended December 31, 2008. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such financial statements present fairly, in all material respects, the financial position of SenoRx, Inc. as of December 31, 2008 and 2007, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2008, in conformity with accounting principles generally accepted in the United States of America.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the effectiveness of the Company’s internal control over financial reporting as of December 31, 2008, based on the criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 4, 2009, expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
/S/ DELOITTE & TOUCHE LLP
Costa Mesa, California
March 4, 2009
-51-
SENORX, INC.
BALANCE SHEETS
| December 31, | ||||||||
| 2008 | 2007 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 15,323,143 | $ | 17,185,259 | ||||
| Short-term investments | — | 10,764,490 | ||||||
| Accounts receivable, net of allowance for doubtful accounts of $225,793, and $107,728, respectively | 8,179,099 | 5,421,184 | ||||||
| Inventory | 9,433,184 | 6,650,955 | ||||||
| Prepaid expenses and deposits | 386,594 | 544,276 | ||||||
| Total current assets | 33,322,020 | 40,566,164 | ||||||
| Property and equipment, net | 1,554,201 | 1,071,435 | ||||||
| Other assets, net of accumulated depreciation of $259,469, and $436,380, respectively | 540,344 | 424,649 | ||||||
| Total assets | $ | 35,416,565 | $ | 42,062,248 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 2,039,280 | $ | 2,580,249 | ||||
| Accrued expenses, including accrued employee compensation of $1,598,338 and $1,137,889, respectively | 2,894,061 | 2,904,603 | ||||||
| Deferred revenue | 161,915 | 93,888 | ||||||
| Current portion of long-term debt | 390,246 | 2,093,346 | ||||||
| Total current liabilities | 5,485,502 | 7,672,086 | ||||||
| Long-term debt—less current portion | 1,632,410 | 26,820 | ||||||
| Commitments and Contingencies (Note 11) | ||||||||
| Stockholders’ Equity: | ||||||||
| Common stock, $0.001 par value—100,000,000 shares authorized; 17,327,191 (2008) and 17,202,395 (2007) issued and outstanding | 17,327 | 17,202 | ||||||
| Additional paid-in capital | 112,456,924 | 109,815,612 | ||||||
| Accumulated deficit | (84,175,598 | ) | (75,469,472 | ) | ||||
| Total stockholders’ equity | 28,298,653 | 34,363,342 | ||||||
| TOTAL | $ | 35,416,565 | $ | 42,062,248 | ||||
See accompanying notes to financial statements.
-52-
SENORX, INC.
STATEMENTS OF OPERATIONS
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| Net revenues | $ | 46,684,588 | $ | 35,035,836 | $ | 25,508,758 | ||||||
| Cost of goods sold | 16,503,327 | 15,123,897 | 13,506,272 | |||||||||
| Gross profit | 30,181,261 | 19,911,939 | 12,002,486 | |||||||||
| Operating expenses: | ||||||||||||
| Selling and marketing | 23,117,137 | 19,022,994 | 15,040,566 | |||||||||
| Research and development | 6,111,225 | 6,353,430 | 5,322,557 | |||||||||
| General and administrative | 10,093,882 | 4,187,133 | 2,050,450 | |||||||||
| Total operating expenses | 39,322,244 | 29,563,557 | 22,413,573 | |||||||||
| Loss from operations | (9,140,983 | ) | (9,651,618 | ) | (10,411,087 | ) | ||||||
| Interest expense | 85,196 | 1,646,670 | 998,071 | |||||||||
| Loss on debt extinguishment | — | 1,264,777 | 197,339 | |||||||||
| Change in fair value of convertible promissory notes and warrant liability | — | (990,875 | ) | 3,960,000 | ||||||||
| Interest income | (520,053 | ) | (1,639,194 | ) | (147,644 | ) | ||||||
| Loss before provisions for income taxes | (8,706,126 | ) | (9,932,996 | ) | (15,418,853 | ) | ||||||
| Provisions for income taxes | — | — | — | |||||||||
| Net loss | $ | (8,706,126 | ) | $ | (9,932,996 | ) | $ | (15,418,853 | )) | |||
| Net loss per share-basic and diluted | $ | (0.50 | ) | $ | (0.75 | ) | $ | (6.61 | ) | |||
| Weighted average shares outstanding-basic and diluted | 17,249,569 | 13,308,790 | 2,332,304 | |||||||||
See accompanying notes to financial statements.
-53-
SENORX, INC.
STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
| Series A Convertible | Series B Convertible | Series C Convertible | Additional | Total Stockholders’ | ||||||||||||||||||||||||||||||||||||||||||||
| Preferred Stock | Preferred Stock | Preferred Stock | Common Stock | Paid-in | Deferred | Accumulated | Equity | |||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Capital | Compensation | Deficit | (Deficit) | |||||||||||||||||||||||||||||||||||||
| Balance—December 31, 2005 | 3,000,000 | $ | 3,000,000 | 3,523,040 | $ | 8,807,600 | 17,861,899 | $ | 35,009,323 | 2,328,183 | $ | 2,328 | $ | 4,567,737 | $ | (611,407 | ) | $ | (50,117,623 | ) | $ | 657,958 | ||||||||||||||||||||||||||
| Proceeds from exercise of common stock options | 42,819 | 43 | 103,820 | 103,863 | ||||||||||||||||||||||||||||||||||||||||||||
| Amortization of deferred compensation | 484,749 | 484,749 | ||||||||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation | 590,837 | 590,837 | ||||||||||||||||||||||||||||||||||||||||||||||
| Net loss | (15,418,853 | ) | (15,251,853 | ) | ||||||||||||||||||||||||||||||||||||||||||||
| Balance as of December 31, 2006 | 3,000,000 | 3,000,000 | 3,523,040 | 8,807,600 | 17,861,899 | 35,009,323 | 2,371,002 | 2,371 | 5,262,394 | (126,658 | ) | (65,536,476 | ) | (13,581,446 | ) | |||||||||||||||||||||||||||||||||
| Proceeds from initial public offering, net of offering costs of $2,266,158 | 6,325,000 | 6,325 | 44,785,517 | 44,791,842 | ||||||||||||||||||||||||||||||||||||||||||||
| Proceeds from exercise of common stock options and warrants | 112,116 | 112 | 758,986 | 759,098 | ||||||||||||||||||||||||||||||||||||||||||||
| Proceeds from purchase of shares under the employee stock purchase plan | 34,708 | 34 | 235,980 | 236,014 | ||||||||||||||||||||||||||||||||||||||||||||
| Amortization of deferred compensation | 126,658 | 126,658 | ||||||||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation | 1,818,206 | 1,818,206 | ||||||||||||||||||||||||||||||||||||||||||||||
| Employee stock purchase plan compensation | 145,966 | 145,966 | ||||||||||||||||||||||||||||||||||||||||||||||
| Conversion of promissory note | 9,998,750 | 10,000,000 | ||||||||||||||||||||||||||||||||||||||||||||||
| Conversion of preferred stock | (3,000,000 | ) | (3,000,000 | ) | (3,523,040 | ) | (8,807,600 | ) | (17,861,899 | ) | (35,009,323 | ) | 7,109,570 | 7,110 | 46,809,813 | — | ||||||||||||||||||||||||||||||||
| Net loss | (9,932,996 | ) | (9,932,996 | ) | ||||||||||||||||||||||||||||||||||||||||||||
| Balance as of December 31, 2007 | — | — | — | — | — | — | 17,202,395 | 17,202 | 109,815,612 | — | (75,469,472 | ) | 34,363,342 | |||||||||||||||||||||||||||||||||||
| Proceeds from exercise of common stock options | 35,969 | 36 | 49,964 | 50,000 | ||||||||||||||||||||||||||||||||||||||||||||
| Proceeds from purchase of shares under the employee stock purchase plan | 88,827 | 89 | 319,758 | 319,847 | ||||||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation | 2,135,938 | 2,135,938 | ||||||||||||||||||||||||||||||||||||||||||||||
| Employee stock purchase plan compensation | 135,652 | 135,652 | ||||||||||||||||||||||||||||||||||||||||||||||
| Net loss | (8,706,126 | ) | (8,706,126 | ) | ||||||||||||||||||||||||||||||||||||||||||||
| Balance as of December 31, 2008 | — | $ | — | — | $ | — | — | $ | — | 17,327,191 | $ | 17,327 | $ | 112,456,924 | $ | — | $ | (84,175,598 | ) | $ | 28,298,653 | |||||||||||||||||||||||||||
See accompanying notes to financial statements.
-54-
SENORX, INC.
STATEMENT OF CASH FLOWS
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| Cash Flows From Operating Activities: | ||||||||||||
| Net loss | $ | (8,706,126 | ) | $ | (9,932,996 | ) | $ | (15,418,853 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||
| Depreciation and amortization | 1,564,258 | 1,400,195 | 1,064,383 | |||||||||
| Stock-based compensation | 2,271,590 | 2,090,831 | 1,075,586 | |||||||||
| Loss on fixed asset abandonment | 32,692 | 37,430 | 5,652 | |||||||||
| Provision for doubtful accounts | 124,387 | — | 46,540 | |||||||||
| Provision for inventory obsolescence | 193,992 | 45,205 | 286,391 | |||||||||
| Amortization of debt discounts | — | 361,695 | 208,303 | |||||||||
| Loss on debt extinguishment | — | 1,264,777 | 197,339 | |||||||||
| Accretion of back-end interest on long-term debt | — | — | 62,605 | |||||||||
| Change in fair value of convertible promissory notes and warrant liability | — | (990,875 | ) | 3,960,000 | ||||||||
| Changes in operating assets and liabilities: | ||||||||||||
| Accounts receivable | (2,882,302 | ) | (1,179,877 | ) | (1,368,826 | ) | ||||||
| Inventory | (3,937,211 | ) | (2,731,525 | ) | (2,141,515 | ) | ||||||
| Prepaid expenses and deposits | 157,682 | (323,617 | ) | (19,061 | ) | |||||||
| Other assets | (225,213 | ) | 384,196 | (7,710 | ) | |||||||
| Accounts payable | (549,899 | ) | (1,545,224 | ) | 1,890,421 | |||||||
| Accrued expenses | (10,542 | ) | 797,377 | 1,049,822 | ||||||||
| Deferred revenue | 68,027 | 57,838 | (76,084 | ) | ||||||||
| Net cash used in operating activities | (11,898,665 | ) | (10,264,570 | ) | (9,185,007 | ) | ||||||
| Cash Flows From Investing Activities: | ||||||||||||
| Purchases of short-term investments | — | (24,071,842 | ) | — | ||||||||
| Maturities of short-term investments | 10,764,490 | 13,307,352 | — | |||||||||
| Acquisition of property and equipment | (970,278 | ) | (550,205 | ) | (887,517 | ) | ||||||
| Net cash provided by (used in) investing activities | 9,794,212 | (11,314,695 | ) | (887,517 | ) | |||||||
| Cash Flows From Financing Activities: | ||||||||||||
| Proceeds from issuance of common stock from stock option and warrant exercises | 50,000 | 60,724 | 103,863 | |||||||||
| Proceeds from initial public offering | — | 47,058,000 | — | |||||||||
| Proceeds from issuance of common stock under the ESPP plan | 319,847 | 236,014 | — | |||||||||
| Proceeds from convertible promissory note | — | — | 8,000,000 | |||||||||
| Proceeds from 2006 subordinated note payable | — | — | 10,000,000 | |||||||||
| Payment of debt issuance costs | (30,000 | ) | (49,497 | ) | (213,012 | ) | ||||||
| Payment of initial public offering costs | — | (941,027 | ) | (1,325,131 | ) | |||||||
| Proceeds from other borrowings | 2,000,000 | 2,750,000 | 3,131,039 | |||||||||
| Repayment of other borrowings | (2,088,158 | ) | (17,755,523 | ) | (2,693,508 | ) | ||||||
| Repayment of capital leases | (9,352 | ) | (7,153 | ) | — | |||||||
| Net cash provided by financing activities | 242,337 | 31,351,538 | 17,003,251 | |||||||||
| Net increase (decrease) in cash and cash equivalents | (1,862,116 | ) | 9,772,273 | 6,930,727 | ||||||||
| Cash and cash equivalents—beginning of year | 17,185,259 | 7,412,986 | 482,259 | |||||||||
| Cash and cash equivalents—end of year | $ | 15,323,143 | $ | 17,185,259 | $ | 7,412,986 | ||||||
See accompanying notes to financial statements.
-55-
SENORX, INC.
STATEMENT OF CASH FLOWS (Continued)
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| Supplemental Disclosure of Cash Flow Information: | ||||||||||||
| Cash paid for income taxes | $ | — | $ | — | $ | 18,384 | ||||||
| Cash paid for interest | $ | 67,060 | $ | 1,241,449 | $ | 632,614 | ||||||
| Deferred offering costs offset against offering proceeds of initial public offering | $ | — | $ | 1,325,131 | $ | — | ||||||
| Promissory note converted to common stock | $ | — | $ | 10,000,000 | $ | — | ||||||
| Preferred stock converted to common stock | $ | — | $ | 46,809,813 | $ | — | ||||||
| Warrant liability transferred to equity and subsequently exercised | $ | — | $ | 698,374 | $ | — | ||||||
| Net other assets transferred to fixed assets | $ | 90,949 | $ | — | $ | — | ||||||
| Net fixed assets transferred to other assets | $ | — | $ | 26,512 | $ | — | ||||||
| Property and equipment acquired included in accounts payable | $ | 8,930 | $ | 2,996 | $ | 34,089 | ||||||
| Other assets included in accounts payable and accrued expenses | $ | — | $ | — | $ | 304,580 | ||||||
| Issuance of preferred stock warrants recorded as debt discount | $ | — | $ | — | $ | 1,529,250 | ||||||
| Deferred revenue transferred to notes payable | $ | — | $ | — | $ | 953,015 | ||||||
| Capital leases | $ | — | $ | 14,477 | $ | 14,626 | ||||||
| Inventory transferred to fixed assets and other assets | $ | 960,990 | $ | 1,024,060 | $ | 335,076 | ||||||
See accompanying notes to financial statements.
-56-
SENORX, INC.
NOTES TO FINANCIAL STATEMENTS
| 1. | GENERAL AND SIGNIFICANT ACCOUNTING POLICIES |
Business—SenoRx, Inc. (the “Company”) is a medical device company focused on developing, manufacturing and selling minimally-invasive medical devices for the diagnosis of breast cancer. The Company is also developing breast cancer products for use in the treatment of breast cancer. The Company was incorporated on January 21, 1998 as BiopSolation Medical, Inc. and subsequently changed its name to SenoRx, Inc.
Basis of Presentation—The accompanying financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America.
Cash and Cash Equivalents—All highly liquid investments purchased with a maturity, at date of purchase, of three months or less are considered to be cash equivalents. Cash equivalents, in the accompanying financial statements, include money market funds and commercial paper.
Short-term Investments—The Company’s short-term investments consist of commercial paper issued by major U.S. financial institutions with a credit rating of A1/P1 and maturities of three to six months. The Company accounts for its investments in marketable securities under FASB No. 115 “Accounting for Certain Investments in Debt and Equity Securities”. Investments are recorded at amortized cost, which approximates fair value, and are classified as held to maturity based on the Company’s intent and ability to hold such investments until maturity.
Concentration of Credit Risks—The Company’s cash and cash equivalents at December 31, 2008 are invested in money market accounts that can be withdrawn without penalty at any time. The Company maintains cash balances in excess of federally insured limits in a reputable financial institution. As such, there is nominal credit risk with respect to cash and cash equivalents.
The Company has one product and several component parts for other products which are obtained from single-source suppliers. The Company has managed the risk associated with single-source suppliers by closely monitoring existing supply levels as compared to customer purchase orders. The Company is exposed to loss of revenue from the sale of these products if the supplier cannot fulfill demand.
The Company’s customer base is diverse and consists of hospitals and physicians. No single customer represents greater than 10% of net revenues during the years ended December 31, 2008, 2007 and 2006. The Company is exposed to risks associated with extending credit to its customers related to the sale of products. Management believes that credit risks on trade accounts receivable are mitigated by the diversity of its customers. The Company performs credit evaluations on its customers’ financial condition, and to date, credit losses have been within management’s expectations.
Following is a summary of activity in the allowance for doubtful accounts:
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| Allowance for doubtful accounts, beginning | $ | 107,728 | $ | 120,000 | $ | 90,000 | ||||||
| Provision for doubtful accounts | 124,387 | — | 46,540 | |||||||||
| Write-offs, net of recoveries | (6,322 | ) | (12,272 | ) | (16,540 | ) | ||||||
| Allowance for doubtful accounts, ending | $ | 225,793 | $ | 107,728 | $ | 120,000 | ||||||
Inventory—Inventory consists principally of raw materials, work-in-process and finished goods, and is carried at the lower of standard cost or market. Standard costs are determined using the first-in, first-out method and are updated at regular intervals such that standard costs approximate actual costs. Provisions for slow moving or obsolete inventory are charged to cost of sales and are permanent reductions to the carrying value of inventory.
Property and Equipment—Property and equipment are recorded at cost less accumulated depreciation. Maintenance and repairs are expensed as incurred. Upon sale or disposition of assets, any gain or loss is included in the statements of operations.
-57-
SENORX, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
The cost of property, plant and equipment is depreciated using the straight-line method over the following estimated useful lives of the respective assets. Leasehold improvements are depreciated over the lesser of the estimated useful lives of the respective assets or the related lease terms.
| Computers and software | 3 years |
| Manufacturing molds | 3 years |
| Machinery and equipment | 3 to 5 years |
| Demonstration and evaluation equipment | 1 year |
| Office furniture and equipment | 3 years |
| Trade show booth and equipment | 1 to 3 years |
Long-Lived Assets—The Company’s long-lived assets include property and equipment. In accordance with Statement of Financial Accounting Standards (“SFAS”) No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets”, the Company estimates the future undiscounted cash flows derived from an asset to assess whether or not a potential impairment exists when events or circumstances indicate the carrying value of a long-lived asset may be impaired. An impairment loss is recognized when the undiscounted future cash flows are less than its carrying amount and is equal to the amount the carrying value exceeds the fair value of the asset or asset group. The Company periodically reviews the carrying value of long-lived assets to determine whether or not an impairment to such value has occurred and, based on its most recent assessment at December 31, 2008, has determined that there was no material impairment at December 31, 2008.
Fair Value of Financial Instruments—SFAS No. 107, “Disclosures about Fair Value of Financial Instruments”, requires management to disclose the estimated fair value of certain assets and liabilities defined by SFAS No. 107 as financial instruments. Financial instruments are generally defined as cash, evidence of ownership interest in an entity, or a contractual obligation that both conveys to one entity a right to receive cash or other financial instruments from another entity and imposes on the other entity the obligation to deliver cash or other financial instruments to the first entity. At December 31, 2008, management believes that the carrying value of cash and cash equivalents, receivables and payables approximate fair value because of the short maturity of these financial instruments. At December 31, 2008, management believes that the fair value of the Company’s debt approximated its carrying value based on interest rates available to the Company at the time.
Certain Hybrid Financial Instruments—SFAS No. 155 “Accounting for Certain Hybrid Financial Instruments” an amendment of FASB No. 133 and No. 140, permits fair value remeasurement for any hybrid financial instrument that contains an embedded derivative that otherwise would require bifurcation. The Company has made an irrevocable election to initially and subsequently measure the 2006 convertible promissory notes and the embedded derivatives, in its entirety, at fair value with subsequent changes in fair value recognized in the statement of operations (See Note 7).
Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock—The Company accounted for free-standing warrants issued in conjunction with certain derivative instruments in accordance with EITF Issue No. 00-19 “Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock” (EITF 00-19). EITF 00-19 requires that all contracts be initially measured at fair value and subsequently accounted for based on the current classification and the assumed or required settlement method. All contracts that are classified as assets or liabilities are measured at fair value with changes in fair value reported in earnings. The Company reevaluated the classification at each balance sheet date to determine if the warrants issued in connection with the debt instruments will continue to be recorded as a liability or as equity (see Note 6).
-58-
SENORX, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
Net Loss Per Share—Basic loss per share is based on the weighted-average number of shares of common stock outstanding during the period. Diluted loss per share also includes the effect of stock options, warrants and other common stock equivalents outstanding during the period. In periods of a net loss position, basic and diluted weighted average shares are the same.
The following table sets forth the computation of denominator used in the computation of net loss per share:
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| Weighted-average common stock outstanding | 17,252,550 | 13,325,186 | 2,357,622 | |||||||||
| Less: Unvested common shares subject to repurchase | (2,981 | ) | (16,396 | ) | (25,318 | ) | ||||||
| Total weighted-average number of shares used in computing net loss per share-basic and diluted | 17,249,569 | 13,308,790 | 2,332,304 | |||||||||
Revenue Recognition and Deferred Revenue—The Company recognizes revenues in accordance with SEC Staff Accounting Bulletin, or SAB, No. 104, “Revenue Recognition”. SAB No. 104 requires that four basic criteria must be met before revenue can be recognized: (1) persuasive evidence of an arrangement exists; (2) title has transferred; (3) the fee is fixed or determinable; and (4) collectability is reasonably assured. The Company’s terms of sales specify that title transfers at the time of shipment by the Company. The Company generally uses contracts and purchase orders to determine the existence of an arrangement. The Company assesses whether the fee is fixed or determinable based upon the terms of the agreement associated with the transaction. To determine whether collection is reasonably assured, the Company assesses a number of factors, including past transaction history with the customer and creditworthiness of the customer. The Company generally does not provide any rights of return by the customer other than returns for product warranty related issues. In addition to these product warranty related returns, the Company occasionally accepts other returns at its discretion. Such returns have historically been insignificant, and reserves for these returns are established at the time of sale.
For those sales that include multiple deliverables, the Company allocates revenue based on the relative fair values of the individual components as determined in accordance with EITF Issue No. 00-21, “Revenue Arrangements with Multiple Deliverables.” When more than one element, such as hardware and disposables, are contained in a single arrangement, revenues are allocated between the elements based on each element’s relative fair value, provided that each element meets the criteria for treatment as a separate unit of accounting. An item is considered a separate unit of accounting if it has value to the customer on a standalone basis and there is objective and reliable evidence of the fair value of the undelivered items. Fair value is generally determined based upon the price charged when the element is sold separately. In the absence of fair value for a delivered element, revenue is allocated first to the fair value of the undelivered elements and the residual revenue is allocated to the delivered elements. In the absence of fair value for an undelivered element, the arrangement is accounted for as a single unit of accounting, resulting in a deferral of revenue recognition for the delivered elements until all undelivered elements have been fulfilled.
The Company also places certain equipment with customers in return for the customer purchasing a minimum number of disposable procedure devices during a specified contract period. Title to the equipment passes to the customer at the end of the contract period provided the minimum purchase requirement is met. The cost of the equipment, which is included in other long-term assets in the accompanying balance sheets is amortized to cost of goods sold based on the monthly disposable unit shipments compared to the total purchase commitment of disposables. In the event the customer does not fulfill the minimum purchase requirements, collection efforts may be undertaken and the Company will attempt to recover the equipment. If the collection efforts or recovery of the equipment is not successful, the unamortized equipment cost would be expensed to cost of goods sold.
Income Taxes—Income taxes are accounted for in accordance with SFAS No. 109, “Accounting for Income Taxes”. This statement requires the recognition of deferred tax assets and liabilities to reflect the future tax consequences of events that have been recognized in the Company’s financial statements or tax returns. Measurement of the deferred items is based on enacted tax laws. In the event the future consequences of differences between financial reporting bases and tax bases of the Company’s assets and liabilities result in a deferred tax asset, SFAS No. 109 requires an evaluation of the probability of being able to realize the future benefits indicated by such assets. A valuation allowance related to a deferred tax asset is recorded when it is more likely than not that some portion or all of the deferred tax asset will not be realized.
-59-
SENORX, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
Research and Development—Research and development costs are charged to operations in the year incurred. Research and development expense consists principally of expenditures for equipment, parts, tooling costs and outside third-party consultants, which are used in testing and the development of the Company’s devices under development, and compensation to specific Company personnel. The Company also expenses the costs of internally developed patents since recoverability is uncertain. The cost of equipment used in research and development activities which has alternative uses is capitalized as equipment. Such equipment is depreciated over estimated useful lives of three to five years.
Software Development Costs—Certain of the Company’s products incorporate software which is incidental to the product as a whole. Software development costs incurred prior to the establishment of technological feasibility are expensed as research and development costs. The Company defines the establishment of technological feasibility as the completion of a final working model that has been incorporated into a product that has been cleared by the U.S. Food and Drug Administration, at which time the product can be sold to third parties. As a result, all software development costs incurred by the Company to date have been expensed as incurred given the short duration between technological feasibility and sales to third parties.
Shipping and Handling—In accordance with Emerging Issues Task Force (“EITF”) No. 00-10, “Accounting for Shipping and Handling Fees and Costs”, the Company includes shipping and handling fees billed to customers in net revenues. Amounts incurred by the Company for freight are included in cost of goods sold.
Advertising—Advertising costs are expensed as incurred and are included in selling and marketing expense. Advertising costs have not been material for any period presented.
Stock-Based Compensation—Effective January 1, 2006, we adopted SFAS 123R, which requires that all stock-based compensation to employees, including grants of employee stock options, be expensed in the financial statements based on their respective grant date fair values. The Company does not have a history of market prices of its common stock as it did not become a public company until April 2007. Therefore, volatility was estimated in accordance with SAB No. 107 using historical volatilities of other publicly traded companies within the same industry. The expected life of the awards is based on the simplified method as defined in SAB No. 107. The risk-free rate assumption is based on observed interest rates appropriate for the terms of the awards. The dividend yield assumption is based on history and the expectation of not paying any dividends. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Stock-based compensation expense is recognized in the financial statements based on awards that are ultimately expected to vest. A summary of significant assumptions used in determining the fair value of the options is as follows:
| Year Ended December 31, | |||
| 2008 | 2007 | 2006 | |
| Expected life (years) | 4.5 - 4.75 | 4.75 - 6.25 | 6.25 |
| Risk-free interest rate | 1.62% - 3.26% | 3.49% - 4.69% | 4.56% - 5.05% |
| Volatility | 45% - 48% | 42% - 48% | 48% - 56% |
| Dividend yield | 0% | 0% | 0% |
| Forfeiture rate | 5% | 5% | 5% |
The Company had a choice of two attribution methods for allocating compensation costs under SFAS No. 123R: the “straight-line” method, which allocates expense on a straight-line basis over the requisite service period of the last separately vesting portion of an award, or the “graded vesting attribution method”, which allocates expense on a straight-line basis over the requisite service period for each separately vesting portion of the award as if the award was, in-substance, multiple awards. The Company chose the latter method (i.e. graded vesting). The Company amortizes the fair value of each option over each option’s vesting period (requisite service period).
The Company has not recognized any income tax benefit for the stock-based compensation arrangements due to the fact that the Company does not believe it is more likely than not it will recognize any deferred tax assets from such compensation cost recognized in the current period.
-60-
SENORX, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
Stock-based compensation included in the Company’s statement of operations under SFAS No. 123R for the years ended December 31, 2008, 2007 and 2006 was:
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| Cost of goods sold | $ | 87,257 | $ | 95,522 | $ | 42,962 | ||||||
| Research and development expense | 766,954 | 436,211 | 251,532 | |||||||||
| Selling and marketing expense | 429,618 | 428,443 | 181,274 | |||||||||
| General and administrative expense | 852,109 | 858,030 | 115,069 | |||||||||
| $ | 2,135,938 | $ | 1,818,206 | $ | 590,837 | |||||||
Comprehensive Loss—For the years ended December 31, 2008, 2007 and 2006, there was no difference between the Company’s net loss and comprehensive loss.
Use of Estimates—The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America necessarily requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses and revenues during reporting periods. Actual results could differ from these estimates.
Segment Reporting—SFAS No. 131, “Disclosures about Segments of an Enterprise and Related Information”, established standards for reporting information about operating segments in financial statements. Operating segments are defined as components of an enterprise about which separate financial information is available that is evaluated regularly by the chief decision maker in deciding how to allocate resources and in assessing performance. The Company’s chief decision maker is the chief executive officer. The Company’s chief decision maker reviews the results of operations based on one industry segment: the production and sale of breast care products.
Recent Accounting Pronouncements— In December 2007, the FASB issued SFAS No. 141—revised 2007, “Business Combinations” (“SFAS 141R”). SFAS 141R establishes principles and requirements for how an acquirer in a business combination recognizes and measures in its financial statements the identifiable assets acquired, the liabilities assumed, and any noncontrolling interest; recognizes and measures the goodwill acquired in the business combination or a gain from a bargain purchase; and determines what information to disclose to enable financial statement users to evaluate the nature and financial effects of the business combination. SFAS 141R applies to business combinations for which the acquisition date is on or after December 15, 2008. Early adoption is prohibited. The Company is currently evaluating the effect, if any, that the adoption of SFAS 141R will have on its results of operations, financial position and cash flows.
In December 2007, the FASB issued SFAS No. 160, Noncontrolling Interests in Consolidated Financial Statements—an amendment to ARB No. 51 (“SFAS 160”). SFAS 160 requires all entities to report noncontrolling (minority) interests in subsidiaries as equity in the consolidated financial statements, but separate from the equity of the parent company. The statement further requires that consolidated net income be reported at amounts attributable to the parent and the noncontrolling interest, rather than expensing the income attributable to the minority interest holder. This statement also requires that companies provide sufficient disclosures to clearly identify and distinguish between the interests of the parent company and the interests of the noncontrolling owners, including a disclosure on the face of the consolidated statements for income attributable to the noncontrolling interest holder. This statement is effective for fiscal years beginning on or after December 15, 2008. Early adoption is prohibited. The Company is currently evaluating the effect, if any, that the adoption of SFAS 160 will have on its results of operations, financial position and cash flows.
In February 2008, the FASB issued FASB Staff Position No. 157-2, which deferred the effective date for certain portions of SFAS 157 related to nonrecurring measurements of nonfinancial assets and liabilities. That provision of SFAS 157 effective for fiscal years beginning after December 15, 2008 and for interim periods within those fiscal years. The Company is currently evaluating the effect, if any, that the adoption of SFAS 157-2 will have on its results of operations, financial position and cash flows.
-61-
SENORX, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
In April 2008, the FASB issued FASB Staff Position 142-3, Determination of the Useful Lives of Intangible Assets (“FSP 142-3”), which amends the factors that should be considered in developing renewal or extension assumptions used to determine the useful life of a recognized intangible asset under FASB No. 142, Goodwill and Other Intangible Assets. The intent of FSP 142-3 is to improve the consistency between the useful life of a recognized intangible asset under SFAS No. 142 and the period of expected cash flows used to measure the fair value of the asset under SFAS No. 141(R) and other U.S. generally accepted accounting principles. FSP 142-3 is effective for fiscal years beginning after December 15, 2008 and for interim periods within those fiscal years. The Company does not expect the adoption of FSP 142-3 to have a material effect on its results of operations, financial position and cash flows.
In June 2008, the FASB issued FSP EITF 03-6-1, Determining Whether Instruments Granted in Share-Based Payment Transactions Are Participating Securities, which addresses whether instruments granted in share-based payment transactions are participating securities prior to vesting and, therefore, need to be included in the earnings allocation in computing earnings per share under the two-class method described in paragraphs 60 and 61 of FASB Statement No. 128, Earnings per Share. This FSP is effective for fiscal years beginning after December 15, 2008 and for interim periods within those fiscal years. The Company does not expect the adoption of FSP EITF 03-6-1 to have a material effect on its results of operations, financial position and cash flows.
In October 2008, the FASB Issued FSP No. 157-3, Determining the Fair Value of a Financial Asset When the Market for That Asset Is Not Active. FSP No. 157-3 clarifies the application of FASB Statement No. 157, Fair Value Measurements, in a market that is not active and provides an example to illustrate key considerations in determining the fair value of a financial asset when the market for that financial asset is not active. FSP No. 157-3 is effective upon issuance. Based on the Company’s evaluation of FSP No. 157-3, the adoption of this standard is not expected to have a material impact on its results of operations, financial position and cash flows.
| 2. | INITIAL PUBLIC OFFERING |
The Company registered the initial public offering of its common stock, par value $.001 per share, in a Registration Statement on Form S-1 (Registration No. 333-134466), which was declared effective on March 28, 2007. The Company completed its initial public offering (“IPO”) and sold 5,500,000 shares at $8.00 per share on April 3, 2007. Additionally, on April 20, 2007, the underwriters the IPO exercised their overallotment option to purchase an additional 825,000 shares at $8.00. Total expenses from the offering were approximately $5.8 million, which included underwriting discounts and commissions of $3.5 million, and approximately $2.2 million in other offering-related expense. Net offering proceeds, including the sale of shares pursuant to the subsequent underwriters’ overallotment and after deducting total expenses was $44.8 million. Upon the closing of the IPO, all of the outstanding shares of the Company’s convertible preferred stock converted to 7,109,570 shares of the Company’s common stock. In addition, the May 2006 Notes were converted into 1,249,999 shares of common stock.
| 3. | PROPERTY AND EQUIPMENT |
Property and equipment consist of the following:
| Year Ended December 31, | ||||||||
| 2008 | 2007 | |||||||
| Computers and software | $ | 627,330 | $ | 607,367 | ||||
| Manufacturing molds | 1,641,464 | 1,603,759 | ||||||
| Machinery and equipment | 616,415 | 588,086 | ||||||
| Demonstration equipment | 1,785,243 | 763,623 | ||||||
| Office furniture and equipment | 130,173 | 76,036 | ||||||
| Trade show booth and equipment | 157,196 | 96,416 | ||||||
| Leasehold improvements | 183,482 | 242,330 | ||||||
| 5,141,303 | 3,977,617 | |||||||
| Less accumulated depreciation | (3,587,102 | ) | (2,906,182 | ) | ||||
| $ | 1,554,201 | $ | 1,071,435 | |||||
-62-
SENORX, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
| 4. | INVENTORY |
Inventories consist of the following:
| Year Ended December 31, | ||||||||
| 2008 | 2007 | |||||||
| Raw materials | $ | 4,261,809 | $ | 3,051,800 | ||||
| Work-in-process | 382,188 | 391,431 | ||||||
| Finished goods | 4,789,187 | 3,207,724 | ||||||
| $ | 9,433,184 | $ | 6,650,955 | |||||
| 5. | INCOME TAXES |
On January 1, 2007, the Company adopted Financial Accounting Standards Board Interpretation No. 48, “Accounting for Uncertainty in Income Taxes” (FIN 48). FIN 48 prescribes a comprehensive model of how a company should recognize, measure, present, and disclose in its financial statements uncertain tax positions that the company has taken or expects to take on a tax return. FIN 48 states that a tax benefit from an uncertain position may be recognized if it is “more likely than not” that the position is sustainable, based upon its technical merits. The tax benefit of a qualifying position is the largest amount of tax benefit that is greater than 50 percent likely of being realized upon ultimate settlement with a taxing authority having full knowledge of all relevant information.
Upon adoption of FIN 48, the Company would have decreased retained earnings $410,000, except that the decrease was fully offset by the release of a valuation allowance. In addition, future changes in the unrecognized tax benefit will have no impact on the effective tax rate due to the existence of the valuation allowance. The Company estimates that the unrecognized tax benefit will not change significantly within the next twelve months. The Company will continue to classify income tax penalties and interest as part of general and administrative expense in its Statements of Operations. Accrued interest on uncertain tax positions is not significant as of December 31, 2008. There are no penalties accrued as of December 31, 2008.
The following table summarizes the open tax years for each major jurisdiction:
| Jurisdiction | Open Tax Years |
| Federal | 2005 - 2007 |
| California | 2004 - 2007 |
The components of the federal and state income tax expense are as follows:
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| Current: | ||||||||||||
| Federal | $ | — | $ | — | $ | — | ||||||
| State | — | — | — | |||||||||
| — | — | — | ||||||||||
| Deferred: | ||||||||||||
| Federal | (1,999,514 | ) | (2,748,189 | ) | (3,409,010 | ) | ||||||
| State | (736,813 | ) | (949,164 | ) | (672,364 | ) | ||||||
| (2,736,326 | ) | (3,697,353 | ) | (4,081,374 | ) | |||||||
| Valuation allowance | 2,736,326 | 3,697,353 | 4,081,374 | |||||||||
| Total | $ | — | $ | — | $ | — | ||||||
-63-
SENORX, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
Taxes on income vary from the statutory federal income tax rate applied to earnings before taxes on income as follows:
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| Statutory federal income tax rate applied to earnings before income taxes | $ | (3,047,144 | ) | $ | (3,476,549 | ) | $ | (5,396,596 | ) | |||
| State income taxes—net of federal benefit | (486,296 | ) | (626,448 | ) | (443,760 | ) | ||||||
| Meals and entertainment | 96,568 | 82,274 | 71,293 | |||||||||
| Research and development credit | (158,645 | ) | (147,853 | ) | (139,462 | ) | ||||||
| Equity based compensation | 772,116 | 708,738 | 326,368 | |||||||||
| Change in fair value of convertible promissory note | — | (336,898 | ) | 1,346,400 | ||||||||
| Other | 87,075 | 99,383 | 154,383 | |||||||||
| Change in valuation allowance | 2,736,326 | 3,697,353 | 4,081,374 | |||||||||
| $ | — | $ | — | $ | — | |||||||
Deferred income tax assets and liabilities arising from differences between accounting for financial statement purposes and tax purposes, less valuation allowances, are as follows:
| December 31, | ||||||||
| 2008 | 2007 | |||||||
| Deferred tax assets: | ||||||||
| Net operating loss | $ | 26,468,355 | $ | 24,272,020 | ||||
| Property and equipment | 69,350 | 94,808 | ||||||
| Capitalized assets | 1,804,320 | 1,589,567 | ||||||
| Tax credits | 3,405,543 | 2,953,598 | ||||||
| Accrued expenses | 1,056,065 | 990,315 | ||||||
| Total deferred tax assets | 32,803,633 | 29,900,308 | ||||||
| Deferred tax liabilities—state taxes | (2,157,047 | ) | (1,949,599 | ) | ||||
| Net deferred tax assets | 30,646,586 | 27,950,709 | ||||||
| Valuation allowance | (30,646,586 | ) | (27,950,709 | ) | ||||
| Net | $ | — | $ | — | ||||
At December 31, 2008, the Company has federal and state net operating loss (“NOL”) carryforwards available to offset future taxable income of approximately $66,130,000 and $49,800,000, respectively. These carryforwards will begin to expire in 2018 and 2009, respectively.
As of December 31, 2008, the Company's net operating loss carryforwards include approximately $934,000 of potential tax deductions related to stock option transactions that will be credited directly to additional paid in capital, if realized.
The Company has federal and state research credit carryforwards of approximately $1,579,000 and $1,827,000 respectively. The federal research credit carryforwards will begin to expire in the year ending December 31, 2018 and the state research credit will carry forward until exhausted.
Pursuant to Section 382 of the Internal Revenue Code, use of the Company's NOL’s and credit carryforwards may be limited if the Company experiences a cumulative change in ownership of greater than 50% in a moving three-year period. Ownership changes could impact the Company's ability to utilize NOL’s and credit carryforwards remaining at the ownership change date.
-64-
SENORX, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
6. LONG-TERM DEBT
A summary of long-term debt follows:
| December 31, | ||||||||
| 2008 | 2007 | |||||||
| Equipment facility | $ | 10,058 | $ | 145,201 | ||||
| Term loan | 2,000,000 | — | ||||||
| 2003 Subordinated note | — | 1,000,000 | ||||||
| Note Payable | — | 953,015 | ||||||
| Capital leases | 12,598 | 21,950 | ||||||
| 2,022,656 | 2,120,166 | |||||||
| Current portion of long-term debt | (390,246 | ) | (2,093,346 | ) | ||||
| Long-term debt | $ | 1,632,410 | $ | 26,820 | ||||
Silicon Valley Bank and Working Capital Facility—On September 30, 2008, the Company and Silicon Valley Bank (“SVB”) entered into an amendment to the Amended and Restated Loan and Security Agreement dated February 20, 2007 (as so amended, the “Domestic Loan Agreement”), a Loan and Security Agreement (Ex-Im Loan Facility) (the “Export-Import Loan Agreement”), and an Export-Import Bank of the United States Working Capital Guarantee Program Borrower Agreement (the “Borrower Agreement,” and together with the Domestic Loan Agreement and the Export-Import Loan Agreement, the “Loan Agreement”). The total maximum amount available for borrowing under the Loan Agreement is $12.0 million.
Revolving Line
The Loan Agreement provides for a domestic receivables-based revolving line of credit in an aggregate amount of up to $10.0 million, or, if less, the sum of 80% of eligible domestic accounts receivable plus 25% of eligible domestic inventory (the “Domestic Revolving Line”), coupled with a foreign receivables-based revolving line of credit in an aggregate amount of up to $2.5 million, or if less, the sum of 90% of eligible foreign accounts receivable plus 50% of eligible export-related inventory (the “Export-Import Revolving Line,” and together with the Domestic Revolving Line, the “Revolving Line”). No more than $10.0 million, in the aggregate, will be available for combined draw-downs under the Domestic Revolving Line and the Export-Import Revolving Line. The Domestic Revolving Line is subject to a $1.0 million sublimit available for cash management services provided by SVB, including the issuance of short-term letters of credit and foreign exchange contracts. The Export-Import Revolving Line is guaranteed by the U.S. Export-Import Bank. The Revolving Line maturity date is September 30, 2010.
The Revolving Line bears interest at an annual rate equal to the prime rate plus 0.25% (4.25% at December 31, 2008). However, the annual rate applicable to the Revolving Line increases to the prime rate plus 1.00% if a financial ratio relating to the Company’s liquidity falls below a specified level. Interest on the Revolving Line is due monthly, with the principal due at the maturity date. At December 31, 2008, there was $7.8 million available to borrow on the revolving line. No amounts have been borrowed on the Revolving Line at December 31, 2008.
Term Loan
The Loan Agreement provides for a term loan in an aggregate amount of up to $2.0 million (the “Term Loan”), with a maturity date of March 30, 2013, unless the Revolving Line is not renewed at its maturity date in which case, all amounts outstanding under the Term Loan will become due at such time. The Term Loan bears interest at an annual rate equal to the prime rate plus 0.75% (4.75% at December 31, 2008). However, the annual rate applicable to the Term Loan increases to the prime rate plus 1.50% if a financial ratio relating to the Company’s liquidity falls below a specified level. The Loan Agreement allows for a single advance under the Term Loan which was requested in November 2008. Monthly payments of interest only on the amount outstanding on the Term Loan are due through March 31, 2009, followed by forty-eight (48) consecutive monthly installments of amortized principal and interest through the maturity date of the Term Loan. The full $2.0 million in principal was outstanding at December 31, 2008.
-65-
SENORX, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
Aggregate annual maturities of long-term debt at December 31, 2008 are as follows:
| Years Ending December 31 | ||||
| 2009 | $ | 375,000 | ||
| 2010 | 500,000 | |||
| 2011 | 500,000 | |||
| 2012 | 500,000 | |||
| 2013 | 125,000 | |||
| $ | 2,000,000 | |||
Loan Agreement Obligations
The obligations under the Loan Agreement are secured by a security interest on substantially all assets of the Company, excluding intellectual property. The Loan Agreement contains certain restrictive loan covenants, including, among others, financial covenants requiring a minimum tangible net worth and a minimum liquidity ratio, and covenants limiting the Company’s ability to dispose of assets, make acquisitions, be acquired, incur indebtedness, grant liens or enter into negative pledge agreements, make investments, make distributions in respect of the Company’s capital stock (including repurchases of such capital stock) or enter into transactions with affiliates. At December 31, 2008, the Company was in compliance with all covenants.
The Loan Agreement contains events of default that include, among others, failure to make payments when due, inaccuracy of representations and warranties, violation of covenants, events constituting a material adverse change, bankruptcy and insolvency events, material judgments, and cross defaults to certain other agreements. The occurrence of an event of default could result in the acceleration of the Company’s obligations under the Loan Agreement and an increase to the applicable interest rate, and would permit SVB to exercise remedies with respect to the collateral under the Loan Agreement.
2006 Subordinated Note—On December 8, 2006, the Company entered into a subordinated loan and security agreement with Escalate Capital, LLC for advances of up to $10,000,000, which was fully advanced to the Company as of December 31, 2006 (the “December 2006 Subordinated Note”). This obligation bore interest at the rate of 11.5% per annum and did not carry a prepayment penalty.
In connection with the committed line, the Company paid a non-refundable facility fee of $100,000, and issued warrants to purchase up to 206,742 shares of the Company’s Series C Preferred stock, vesting over one year, as defined, at an exercise price of $6.86 per share. Upon completion of the IPO in April 2007, the warrant converted into a warrant to purchase 206,742 shares of common stock at an exercise price of $6.86 per share.
The warrant was previously carried as a liability on the Company’s balance sheet at its fair value with increases or decreases in fair value at each reporting date recorded in the statement of operations. Upon completion of the IPO, the Company had registered shares sufficient to settle the warrant upon exercise. Accordingly, all requirements for equity classification of such warrant as described in EITF 00-19 were met effective April 3, 2007. In accordance with EITF 00-19, the warrant liability was reclassified to equity on April 3, 2007 and the gains recorded to account for the contract at fair value during the period the contract was classified as a liability were not reversed. The Company recorded income related to the change in fair value of $830,875 in the statement of operations for the year ended December 31, 2007. The fair value of the warrant as of April 3, 2007 was estimated using the Black-Scholes option pricing method with the following assumptions: expected volatility rate of 43%; risk free interest rate of 4.5%, a term of three years and closing stock price on April 3, 2007 of $8.24. In addition, $1,529,250 was capitalized as part of debt discount costs as of the date of issuance and was being recognized as additional interest expense over the life of the loan.
On November 19, 2007, the Company made a payment to Escalate in the amount of $10,331,732 in full satisfaction of the outstanding principal balance and accrued unpaid interest. The Company recorded a loss of $1,264,777 in the statement of operations for the year ended December 31, 2007 related to the unamortized debt discount and debt issuance costs that would have otherwise been charged to interest over the term of the loan.
On November 20, 2007, Escalate exercised their right to a cashless exercise of their warrant and received 48,983 shares of the Company’s common stock.
-66-
SENORX, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
2003 Subordinated Note— The 2003 subordinated note is uncollateralized and provided for a five-year term (maturing in February 2008) requiring the payment of interest only on a quarterly basis with an annual interest rate of 4%. At December 31, 2007, the outstanding balance on the note was $1,000,000. In February 2008 the Company made a payment in the amount of $1,006,633, in full satisfaction of the outstanding principal balance and accrued unpaid interest.
Note Payable — In 2002, the Company entered into an arrangement with a distributor whereby the distributor advanced a total of $1,000,000 cash to the Company for the future purchase of product. The Company classified these advances as deferred revenues in the 2005 balance sheet. As product was purchased, the applicable sales value was recognized as revenues. Although the Company was not obligated to refund any of these advances, effective December 31, 2006, the Company agreed to terminate the distribution agreement and repay the outstanding prepayment of $953,015 before February 20, 2008. The obligation bore interest at 2% per annum applied on a semi-monthly basis, not to exceed $19,060 per year. In February 2008, the Company made a payment in the amount of $954,634, in full satisfaction of the outstanding principal balance and accrued unpaid interest.
| 7. | CONVERTIBLE PROMISSORY NOTES |
May 2006 Notes— On May 4, 2006, the Company sold convertible promissory notes with an aggregate principal amount of $8,000,000 to one affiliated institutional investor and two unrelated institutional investors (the “May 2006 Notes”). The Company determined that the May 2006 Notes contained certain features that required bifurcation as embedded derivatives under SFAS No. 133, such as the IPO conversion feature. Therefore, in accordance with SFAS No. 155, the Company made an irrevocable election to measure the May 2006 Notes and the embedded derivatives, in their entirety, at fair value with subsequent changes in fair value recognized in the statement of operations.
At the date of the successful completion of the IPO on April 3, 2007, the fair value of the May 2006 Notes were $11,800,000, comprised of the $8,000,000 face value of the notes, $1,800,000 million in interest and $2,000,000 associated with the stock discount. Consequently, the Company adjusted the fair value of the May 2006 Notes to $11,800,000 and recorded the change in fair value of $160,000 in the statement of operations for the year ended December 31, 2007. In connection with the IPO, the May 2006 Notes were converted to 1,249,999 shares of common stock.
| 8. | STOCKHOLDERS’ EQUITY |
Capital Stock—In March 2007, the Company amended its certificate of incorporation to reflect a 1-for-3.5 reverse stock split of common stock. All share and per share amounts relating to common stock and stock options included in the accompanying financial statements and footnotes have been restated to reflect the reverse stock split.
Preferred Stock—At December 31, 2006, the Company had 3,000,000 shares of Series A convertible preferred stock outstanding, 3,523,040 shares of Series B convertible stock outstanding and 17,861,899 shares of Series C Convertible preferred stock outstanding. Upon the closing of the IPO in April 2007, all the outstanding shares of preferred stock converted to 7,109,570 shares of the Company’s common stock.
Restricted Common Stock—Certain options to purchase common stock have been exercised, subject to restricted stock purchase agreements. The restricted shares are subject to the risk of forfeiture, certain restrictions on transferability and to the Company’s repurchase rights. The restrictions and repurchase options generally lapse 25% per year over a four-year vesting period. The Company has a repurchase option, exercisable upon discontinuance of the purchaser’s service with the Company, to repurchase the unvested shares at the original price paid by the purchaser. Vested shares are not subject to the Company’s repurchase rights. However, before any vested shares may be sold or otherwise transferred, the Company has a right of first refusal to purchase the shares at the price offered by the proposed transferee or fair value. Holders of restricted stock have the voting rights of a common stockholder.
-67-
SENORX, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
The following summarizes information about aggregate number of chares of restricted common stock issued pursuant to restricted stock purchase agreements:
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| Unvested shares outstanding—beginning | 16,396 | 25,318 | 139,613 | |||||||||
| Restricted shares issued upon exercise of stock options | — | — | 22,097 | |||||||||
| Shares vested | (13,415 | ) | (8,922 | ) | (136,392 | ) | ||||||
| Unvested common shares—ending | 2,981 | 16,396 | 25,318 | |||||||||
| 9. | STOCK OPTIONS, WARRANTS AND RESTRICTED STOCK UNITS |
The Company’s 2006 Stock Option Plan (the “2006 Plan”), which was adopted by the Company’s board of directors in May 2006 and approved by the Company’s shareholders in June 2006 is designed to enable the Company to offer an incentive-based compensation system to employees, officers and directors of the Company and to consultants who do business with the Company. The 2006 Plan provides for the grant of incentive stock options, nonqualified stock options and restricted stock units to purchase up to an aggregate of 5,009,224 shares of common stock. The 2006 plan will terminate in 2016 and also provides for annual increases in the number of shares available for issuance thereunder on the first day of each fiscal year, beginning with the 2007 fiscal year, equal to the lesser of:
| • | 3.5% of the outstanding shares of the Company’s common stock on the first day of the fiscal year; |
| • | 630,000 shares; or |
| • | Such other amount as the board of directors may determine. |
Stock Options— As of December 31, 2008, options to purchase a total of 1,605,952 shares of common stock were outstanding under the 2006 Plan and options to purchase a total of 1,406,442 shares were available for issuance under the 2006 Plan.
The Company also has a 1998 Stock Option Plan (“1998 Plan”) which plan was approved by the Company’s board of directors and shareholders in 1998. As of December 31, 2008, options to purchase a total of 683,377 shares of common stock were outstanding under the 1998 Plan and no options to purchase shares were available for issuance under the 1998 Plan.
The 2006 Plan and the 1998 Plan are administered by a committee appointed by the board of directors that determines the recipients and the terms of the options granted. Options may be granted to eligible employees, directors and consultants to purchase shares of the Company’s common stock at a price that is at least equal to the fair market value of the common stock on the date of grant for incentive stock options (or 110% of the fair market value in the case of an optionee who holds more than 10% of the voting power of the Company on the date of grant). Subject to termination of employment, options may expire up to ten years from the date of grant.
The exercise price, term and other conditions applicable to each option granted under the 2006 and 1998 Plans are generally determined by the Administrator at the time of grant of each option and may vary with each option granted. The stock options granted generally vest 25% per year over a four-year period and expire after seven to 10 years. The options are exercisable according to the vesting schedule. Alternatively, the options may be exercised in whole or in part at any time into restricted, unvested common shares which are subject to the risk of forfeiture, and to the Company’s repurchase rights (see Note 8). The restrictions and repurchase options on currently outstanding restricted stock grants issued pursuant to the 1998 Plan generally lapse 25% per year over a four-year period (consistent with the vesting period for the original stock option grants).
The 1998 Plan also permits for the issuance of restricted stock pursuant to grants of stock purchase rights. As of December 31, 2008, there were no outstanding stock purchase rights.
-68-
SENORX, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
During the years ended December 31, 2008, 2007 and 2006, the Company recorded stock-based compensation expense of $2,135,938, $1,944,864 and $1,075,586, respectively, associated with employee performance-based and non-employee stock option awards.
A summary of stock option activity is as follows:
Number of Shares | Weighted- Average Exercise Price | Weighted- Average Remaining Contractual Term (Years) | Aggregate Intrinsic Value | |||||||||||||
| Outstanding—December 31, 2005 | 464,156 | 1.44 | ||||||||||||||
| Granted (weighted-average fair value of $6.52 per share) | 200,599 | 6.59 | ||||||||||||||
| Exercised | (42,819 | ) | 2.43 | |||||||||||||
| Forfeited | (21,768 | ) | 2.09 | |||||||||||||
| Outstanding—December 31, 2006 | 600,168 | 3.05 | ||||||||||||||
| Granted (weighted-average fair value of $4.64 per share) | 764,086 | 9.67 | ||||||||||||||
| Exercised | (47,583 | ) | 1.14 | |||||||||||||
| Forfeited | (63,890 | ) | 7.07 | |||||||||||||
| Outstanding—December 31, 2007 | 1,252,781 | 6.95 | ||||||||||||||
| Granted (weighted-average fair value of $1.97 per share) | 1,141,441 | 4.60 | ||||||||||||||
| Exercised | (35,969 | ) | 1.39 | |||||||||||||
| Forfeited | (68,924 | ) | 8.61 | |||||||||||||
| Outstanding—December 31, 2008 | 2,289,329 | $ | 5.82 | 6.7 | $ | — | ||||||||||
| Vested (exercisable)—December 31, 2008 | 759,329 | $ | 5.72 | 6.2 | $ | — | ||||||||||
| Unvested (unexercisable)—December 31, 2008 | 1,530,000 | $ | 5.86 | 7.0 | $ | — | ||||||||||
The following table summarizes information with respect to stock options outstanding and exercisable at December 31, 2008:
Exercise Price | Number Outstanding | Weighted- Average Remaining Contractual Life (Years) | Weighted- Average Exercise Price | Number Exercisable | Weighted- Average Exercise Price | |||||||||||||||||
| $ | 0.60 – 1.75 | 265,177 | 3.8 | $ | 1.15 | 260,634 | $ | 1.14 | ||||||||||||||
| $ | 2.28 – 2.63 | 665,753 | 6.9 | $ | 2.30 | 40,944 | $ | 2.52 | ||||||||||||||
| $ | 3.71 – 5.97 | 131,368 | 7.0 | $ | 4.23 | 69,882 | $ | 3.82 | ||||||||||||||
| $ | 6.98 – 8.99 | 886,082 | 6.9 | $ | 7.95 | 231,358 | $ | 8.20 | ||||||||||||||
| $ | 9.55 – 12.01 | 340,949 | 8.2 | $ | 11.37 | 156,511 | $ | 11.35 | ||||||||||||||
| 2,289,329 | 759,329 | |||||||||||||||||||||
As of December 31, 2008, there was unrecognized compensation expense of $2.3 million related to unvested stock options, which the Company expects to recognize over a weighted average period of 2.0 years. The aggregate intrinsic value of the options outstanding and options exercisable as of December 31, 2008 was $0. The weighted average grant date fair value of stock options granted during the year ended December 31, 2008 was $1.97.
As of December 31, 2008, the total number of outstanding options vested or expected to vest (considering anticipated forfeitures) was 1,453,500 which had a weighted average exercise price of $5.86. The average remaining life of these options was 7.0 years and the aggregate intrinsic value was $0 at December 31, 2008.
-69-
SENORX, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
Warrants — Upon the closing of the IPO in April 2007, the outstanding preferred stock warrants converted into warrants to purchase 462,046 shares of common stock. A summary of the activity of common stock purchase warrants is as follows:
| Warrant Price | ||||||||||||
Number of Shares | Per Share | Total | ||||||||||
| Balance outstanding, December 31, 2005 | 255,304 | $ | 6.86 | $ | 1,751,385 | |||||||
| Warrants issued | 206,742 | 6.86 | 1,418,250 | |||||||||
| Warrants exercised | — | — | — | |||||||||
| Balance outstanding, December 31, 2006 | 462,046 | 6.86 | 3,169,635 | |||||||||
| Warrants issued | — | — | — | |||||||||
| Warrants exercised | (243,446 | ) | 6.86 | (1,670,039 | ) | |||||||
| Balance outstanding, December 31, 2007 | 218,600 | 6.86 | 1,499,596 | |||||||||
| Warrants issued | — | — | — | |||||||||
| Warrants exercised | — | — | — | |||||||||
| Balance outstanding, December 31, 2008 | 218,600 | $ | 6.86 | $ | 1,499,596 | |||||||
Restricted Stock Units — The 2006 Plan provides for awards of restricted shares of common stock. Restricted stock units have time-based vesting and are subject to forfeiture if employment terminates prior to the end of the service period. Restricted stock units are valued at the grant date based upon the market price of the Company’s common stock and the fair value of each award is charged to expense over the service period.
In 2008, the Company granted a total of 51,750 shares of restricted stock to employees. The restricted stock units vest 50% per year over a 2 year period.
Restricted stock unit activity for the year ended December 31, 2008 is as follows:
Number of Shares of Restricted Stock Units | Weighted- Average Grant-Date Fair Value | |||||||
| Outstanding—December 31, 2007 | — | $ | — | |||||
| Granted | 51,750 | 2.38 | ||||||
| Vested | — | — | ||||||
| Canceled or forfeited | — | — | ||||||
| Outstanding—December 31, 2008 | 51,750 | $ | 2.38 | |||||
As of December 31, 2008, there was unrecognized compensation expense of $117,007 related to all unvested restricted stock units, which the Company expects to recognize on a “graded vesting attribution method”, which allocates expense on a straight-line basis over the requisite service period for each separately vesting portion of the award as if the award was, in-substance, multiple awards over a weighted average period of approximately 1.5 years.
| 10. | EMPLOYEE STOCK PURCHASE PLAN |
Effective April 3, 2007, the closing date of the IPO, the Employee Stock Purchase Plan (“Purchase Plan”) was established. The Company’s Purchase Plan was adopted by the Company’s board of directors effective as of May 2006 and approved by the Company’s stockholders in June 2006. The Purchase Plan provides eligible employees of the Company with an incentive by providing a method whereby they may voluntarily purchase common stock of the Company upon terms described in the Purchase Plan. The Purchase Plan is designed to be operated on the basis of six consecutive month offering periods commencing May 15 and November 15 of each year. The 2006 Purchase Plan terminates in 2016. The Purchase Plan provides that eligible employees may authorize payroll deductions of up to 10% of their salary to purchase shares of the Company’s common stock at 85% of the fair market value of common stock on the first or last day of the applicable purchase period. During 2008 and 2007, the Company recorded $135,652 and $145,966, respectively, for compensation related to the discounted purchase price and look back feature of the Purchase Plan. The 2008 fair value of these discounts were estimated using a Black-Scholes pricing method with the following assumptions: $6.16 and $2.08 strike price; $7.25 and $2.44 share price; 184 days until expiration; 48% and 46.5% volatility and 1.89% and 0.87% interest rate on May 16, 2008 and November 17, 2008, respectively. The 2007 fair value of these discounts were estimated using a Black-Scholes pricing method with the following assumptions: $7.60 and $6.80 strike price; $8.94 and $8.00 share price; 228 and 182 days until expiration; 44% and 43% volatility and 4.08% and 5.05% interest rate on October 1, 2007 and April 3, 2007, respectively. As of December 30, 2008 and 2007, Purchase Plan participant contributions of $84,925 and $132,141, respectively, are included in other current liabilities in the accompanying balance sheets. A total of 550,000 shares of common stock are authorized for issuance under the Purchase Plan, and as of December 31, 2008, 123,525 shares have been issued under the Purchase Plan.
-70-
SENORX, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
| 11. | COMMITMENTS AND CONTINGENCIES |
Leases—In March 2008, the Company entered into an agreement to lease a new corporate and manufacturing facility in Irvine, California consisting of 41,402 square feet of space. The lease has an initial term of 63 months commencing November 1, 2008, with the Company’s right of earlier occupancy, and one five-year option to extend. The monthly base rent is initially approximately $44,300 and increases at a rate of 4% per annum. The lease agreement contains certain scheduled rent increases which are accounted for on a straight-line basis.
The Company has acquired computer equipment under capital leases that are payable in various scheduled monthly installments through 2010.
Future minimum lease payments are as follows:
| Years Ending December 31, | Operating Leases | Capital Leases | ||||||
| 2009 | $ | 534,912 | $ | 12,727 | ||||
| 2010 | 555,612 | 1,054 | ||||||
| 2011 | 580,452 | — | ||||||
| 2012 | 605,294 | — | ||||||
| 2013 | 626,004 | — | ||||||
| Thereafter | 52,167 | — | ||||||
| Total minimum lease payments | $ | 2,954,441 | 13,781 | |||||
| Amount representing interest ranging from 14.4% to 31.3% | (1,183 | ) | ||||||
| Present value of future minimum capital lease obligations | 12,598 | |||||||
| Current portion | (5,188 | ) | ||||||
| $ | 7,410 | |||||||
Rent expense was $610,087, $447,741and $395,560, for the years ended December 31, 2008, 2007and 2006, respectively.
Indemnities and Guarantees—During its normal course of business, the Company has made certain indemnities, commitments and guarantees under which it may be required to make payments in relation to certain transactions. These indemnities include those given to various lessors in connection with facility leases for certain claims arising from such facility or lease and indemnities to directors and officers of the Company to the maximum extent permitted under the laws of the State of California. The duration of these indemnities, commitments and guarantees varies. Some of these indemnities, commitments and guarantees do not provide for any limitation of the maximum potential future payments the Company could be obligated to make. The Company has not recorded any liability for these indemnities, commitments and guarantees in the accompanying balance sheets.
The Company provides a limited warranty against manufacturer’s defects on its products. Product warranty costs have not been significant.
-71-
SENORX, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
Litigation—The Company may be subject to legal proceedings, claims and litigation arising in the ordinary course of business. While the amounts claimed may be substantial, the ultimate liability cannot presently be determined because of considerable uncertainties that exist. Therefore, it is possible the outcome of such legal proceedings, claims and litigation could have a material effect on quarterly or annual operating results or cash flows when resolved in a future period.
On January 8, 2008, Hologic and its wholly-owned subsidiaries, including Cytyc Corporation and Cytyc LP, filed a lawsuit against the Company in the United States District Court, Northern District of California, San Jose Division. The complaint generally alleges patent infringement of certain Hologic brachytherapy patent claims, seeking unspecified monetary damages and an injunction against the Company for infringement of those claims. On February 6, 2008, Hologic filed a motion seeking a preliminary injunction in the case and requested that the Court stop the sale of Contura MLB. On March 7, 2008, Hologic filed an amended complaint restating its allegations regarding patent infringement, and adding new claims related to unfair competition under the Lanham Act and California state unfair competition and false advertising statutes. On April 25, 2008, the court denied Hologic’s request for a preliminary injunction and ordered the parties to schedule a trial within 60 to 90 days of such date. On May 22, 2008, the Court issued an order scheduling the Markman claims construction hearing on the patent counts for June 25, 2008, and the trial in the case to start July 14, 2008. Pursuant to an agreement of the parties, the order also dismissed Hologic’s unfair competition and false advertising claims under the Lanham Act and California state law, without prejudice. On June 24, 2008, the Court granted a joint request by Hologic and the Company to stay all proceedings, including the previously scheduled Markman claims construction hearing and the trial, until at least August 22, 2008 in order to provide the parties time to discuss possible resolution of the matter. On August 22, 2008, the Company and Hologic jointly requested that the Court resume proceedings in the pending lawsuit. On October 15, 2008, a Markman claims construction hearing was held and a ruling was issued on February 18, 2009. Although the Company, together with its legal counsel, continues to evaluate the court’s ruling in the Markman hearing, it expects that nothing material or definitive in the matter will be decided before the trial, the date for which has not yet been set by the Court. As a result, the Company believes that the probability of incurring any loss related to this litigation is not determinable, nor is the amount of loss quantifiable at this time. Accordingly, the Company has not accrued a loss related to this litigation as of December 31, 2008. The Company intends to continue to vigorously defend itself in this matter.
| 12. | SEGMENT INFORMATION |
Net revenues by geographic area are presented based upon the country of destination. No foreign country represented 10% or more of net revenues for any period presented. Net revenues by geographic area were as follows:
| 2008 | 2007 | 2006 | ||||||||||
| United States | $ | 41,209,560 | $ | 32,321,888 | $ | 24,320,070 | ||||||
| Canada | 1,200,218 | 1,052,543 | 661,792 | |||||||||
| Rest of world | 4,274,810 | 1,661,405 | 526,896 | |||||||||
| Total | $ | 46,684,588 | $ | 35,035,836 | $ | 25,508,758 | ||||||
No customer accounted for 10% or more of net revenues for any period presented.
At December 31, 2008, the Company has four product classes. Biopsy disposable products are the probes used with the Company’s EnCor systems. Biopsy capital equipment products include the consoles and other pieces (non-disposable) of the EnCor. Diagnostic adjunct products include the Marker product, the Gamma Finder product and the Anchor Guide product. Therapeutic disposables include the Company’s recently commercialized Contura Multi-Lumen Radiation Balloon (MLB) Catheter, which received FDA 510(k) clearance in May 2007.
-72-
SENORX, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
Net revenues by product class are as follows:
| Year Ended December 31, | ||||||||||||
| 2008 | 2007 | 2006 | ||||||||||
| Biopsy disposable products | $ | 21,041,449 | $ | 16,215,740 | $ | 10,972,421 | ||||||
| Biopsy capital equipment products | 4,692,778 | 3,301,908 | 1,245,279 | |||||||||
| Diagnostic adjunct products | 15,774,788 | 14,976,567 | 13,291,058 | |||||||||
| Therapeutic disposable products | 5,175,573 | 541,621 | — | |||||||||
| Total | $ | 46,684,588 | $ | 35,035,836 | $ | 25,508,758 | ||||||
Substantially all of the Company’s assets are in the United States.
| 13. | EMPLOYEE BENEFIT PLANS |
In January 2003, the Company adopted a 401(k) retirement and savings plan which provides for voluntary employee participation subject to a waiting period. The plan provides for discretionary matching subject to the approval by the Company’s Board of Directors. The Company did not make contributions to this plan in the years ended December 31, 2008, 2007and 2006.
| 14. | QUARTERLY FINANCIAL DATA (Unaudited) |
A summary of quarterly financial data (unaudited) is as follows:
| Quarter Ended | ||||||||||||||||
| March 31 | June 30 | September 30 | December 31 | |||||||||||||
| Year ended December 31, 2008 | ||||||||||||||||
| Total revenues | $ | 10,682,775 | $ | 11,186,118 | $ | 11,264,471 | $ | 13,551,224 | ||||||||
| Gross profit | 6,629,325 | 6,859,816 | 7,321,549 | 9,370,571 | ||||||||||||
| Operating income (loss) | (2,229,534 | ) | (5,265,563 | ) | (1,753,930 | ) | 108,044 | |||||||||
| Net income (loss) | (1,994,537 | ) | (5,131,592 | ) | (1,677,021 | ) | 97,024 | |||||||||
| Net income (loss) per share, basic and diluted | $ | (0.12 | ) | $ | (0.30 | ) | $ | (0.10 | ) | $ | 0.01 | |||||
| Year ended December 31, 2007 | ||||||||||||||||
| Total revenues | $ | 7,700,076 | $ | 8,121,285 | $ | 8,906,086 | $ | 10,308,389 | ||||||||
| Gross profit | 4,162,732 | 4,631,274 | 5,350,448 | 5,767,485 | ||||||||||||
| Operating loss | (2,388,501 | ) | (2,551,041 | ) | (1,789,253 | ) | (2,922,823 | ) | ||||||||
| Net loss | (2,109,484 | ) | (2,134,879 | ) | (1,690,635 | ) | (3,997,998 | ) | ||||||||
| Net loss per share, basic and diluted | $ | (0.90 | ) | $ | (0.15 | ) | $ | (0.10 | ) | $ | (0.23 | ) | ||||
Earnings per basic and diluted shares are computed independently for each of the quarters presented based on diluted shares outstanding per quarter and, therefore, may not sum to the totals for the year.
-73-
SIGNATURES
Pursuant to the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Annual Report on Form 10-K to be signed on our behalf by the undersigned, thereunto duly authorized.
| Date: March 16, 2009 | SenoRx, Inc. | |||
| By: | /s/ LLOYD H. MALCHOW | |||
LLOYD H. MALCHOW | ||||
President and Chief Executive Officer (Principal Executive Officer) | ||||
KNOW ALL MEN AND WOMEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints each of Lloyd H. Malchow and Kevin J. Cousins, his or her attorney-in-fact, with the power of substitution, for him or her in any and all capacities, to sign any amendments to this annual report on Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith, with the U.S. Securities and Exchange Commission, hereby ratifying and confirming all that said attorney-in-fact, or his substitute, may do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this Annual Report on Form 10-K has been signed below by the following persons on behalf of the Registrant in the capacities and on the dates indicated:
| Signature | Title | Date |
/S/ LLOYD H. MALCHOW | President, Chief Executive Officer and Director (Principal Executive Officer) | March 16, 2009 |
Lloyd H. Malchow | ||
/S/ KEVIN J. COUSINS | Chief Financial Officer (Principal Accounting Officer) | March 16, 2009 |
Kevin J. Cousins | ||
/S/ VICKIE L. CAPPS | Director | March 16, 2009 |
Vickie L. Capps | ||
/S/ KIM D. BLICKENSTAFF | Director | March 16, 2009 |
Kim D. Blickenstaff | ||
/S/ FREDERICK J. DOTZLER | Director | March 16, 2009 |
Frederick J. Dotzler | ||
/S/ JOHN L. ERB | Director | March 16, 2009 |
John L. Erb | ||
/S/ A. Thomas Bender | Director | March 16, 2009 |
A. Thomas Bender | ||
/S/ GREGORY D. WALLER | Director | March 16, 2009 |
| Gregory D. Waller |
-74-
EXHIBIT INDEX
Exhibit Number | Description |
3.2(1) | Amended and Restated Certificate of Incorporation. |
3.4(1) | Bylaws. |
4.1(1) | Specimen Common Stock certificate of the Registrant. |
4.2(1) | Fourth Amended and Restated Investors’ Rights Agreement, dated May 3, 2006, by and among the Registrant and certain stockholders. |
10.1(1) | Form of Indemnification Agreement for directors and executive officers. |
10.2(1) | 1998 Stock Plan. |
| 10.3 | 2006 Equity Incentive Plan. |
10.4(1) | Employee Stock Purchase Plan. |
10.5(1) | Fourth Amended and Restated Investors’ Rights Agreement, dated May 3, 2006, by and among the Registrant and certain stockholders. |
10.6(1) | Standard Industrial/Commercial Multi-Tenant Lease, dated September 15, 1999, as amended on March 28, 2003 and November 1, 2003, by and between the Registrant and Columbia Investors, LLC. |
10.7(1) | Loan and Security Agreement, dated March 15, 2002, and various amendments thereto, by and between the Registrant and Silicon Valley Bank. |
10.7.1(1) | Amended and Restated Loan and Security Agreement, dated February 20, 2007, by and between the Registrant and Silicon Valley Bank. |
10.8(1) | Convertible Subordinated Note Agreement, dated May 9, 2002, by and between the Registrant and Century Medical, Inc. |
10.9(1) | $2,500,000 Loan and Security Agreement, dated December 27, 2004, by and between the Registrant and Venture Lending & Leasing IV, Inc. |
10.10(1) | Note Purchase Agreement and Form of Subordinated Convertible Promissory Note, each dated May 4, 2006, by and between the Registrant and certain stockholders. |
10.11(1)† | Agreement for Vacuum Assisted Breast Biopsy Needle, System, and Accessory Products, effective April 1, 2005 by and between the Registrant and KP Select. |
10.12(1) | Executive Employment Agreement, dated May 1, 1999, by and between the Registrant and Lloyd Malchow. |
10.13(1)† | Distribution Agreement, dated June 11, 2003, and various amendments thereto, by and among the Registrant, W.O.M. World of Medicine USA, Inc. and W.O.M. World of Medicine AG. |
10.14(1) | Settlement Agreement, effective as of May 22, 2006, by and between the Registrant and Suros Surgical Systems, Inc. |
10.15(1) | Loan and Security Agreement, dated December 8, 2006, by and between the Registrant and Escalate Capital I, L.P. |
10.16(2) | Lease Agreement between The Irvine Company LLC and Registrant dated March 5, 2008. |
10.17(3) | Change in Control Agreement between Registrant and Lloyd Malchow. |
10.18(3) | Change in Control Agreement between Registrant and Paul Lubock. |
-75-
| Exhibit Number | Description |
10.19(3) | Change in Control Agreement between Registrant and Kevin Cousins. |
10.20(3) | Change in Control Agreement between Registrant and William Gearhart. |
10.21(3) | Change in Control Agreement between Registrant and Eben Gordon. |
10.22(4) | Amendment dated September 30, 2008, to the Amended and Restated Loan and Security Agreement dated February 27, 2007, by and between the Registrant and Silicon Valley Bank. |
10.23(4) | Loan and Security Agreement (Ex-Im Loan Facility) dated September 30, 2008, by and between the Registrant and Silicon Valley Bank. |
10.24(4) | Export-Import Bank of the United States Working Capital Guarantee Program Borrower Agreement dated September 30, 2008, by and between the Registrant and Silicon Valley Bank. |
| 23.1 | Consent of Deloitte & Touche LLP, Independent Registered Public Accounting Firm. |
| 31.1 | Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| 31.2 | Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| 32.1 | Certification of Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| (1) | Incorporated by reference from our Registration Statement on Form S-1 (Registration No. 333-134466), which was declared effective on March 28, 2007. |
| (2) | Incorporated by reference from our Current Report on Form 8-K filed on March 10, 2008. |
| (3) | Incorporated by reference from our Current Report on Form 8-K filed on August 29, 2008. |
| (4) | Incorporated by reference from our Current Report on Form 8-K filed on October 2, 2008. |
| † | Portions of the exhibit have been omitted pursuant to a request for confidential treatment. The confidential portions have been filed with the SEC. |
-76-