Use these links to rapidly review the document
TABLE OF CONTENTS
Allos Therapeutics, Inc. Index to Financial Statements
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| | |
ý |
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934. |
For the fiscal year ended December 31, 2008. |
o |
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934. |
For the transition period from to .
|
Commission File Number 00029815
Allos Therapeutics, Inc.
(Exact name of Registrant as specified in its charter)
| | |
Delaware
(State or other jurisdiction of
incorporation or organization) | | 54-1655029
(I.R.S. Employer Identification No.) |
11080 CirclePoint Road, Suite 200
Westminster, Colorado 80020
(303) 426-6262
(Address, including zip code, and telephone number, including area code, of principal executive offices)
|
Securities registered pursuant to Section 12(b) of the Act:
| | |
Common Stock $.001 Par Value
| | NASDAQ Stock Market LLC
(NASDAQ Global Market)
|
|---|
| (Title of class) | | (Name of each exchange on which registered) |
Securities registered pursuant to Section 12(g) of the Act:None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ý
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definition of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act.
| | | | | | |
| Large accelerated filer o | | Accelerated filer ý | | Non-accelerated filer o
(Do not check if a smaller reporting company) | | Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No ý
The aggregate market value of common stock held by nonaffiliates of the registrant (based upon the closing sale price of such shares on the NASDAQ Global Market on June 30, 2008) was $293,509,927. Shares of the registrant's common stock held by each current executive officer and director and by each stockholder who is known by the registrant to own 10% or more of the outstanding common stock have been excluded from this computation in that such persons may be deemed to be affiliates of the registrant. Share ownership information of certain persons known by the registrant to own greater than 10% of the outstanding common stock for purposes of the preceding calculation is based solely on information on Schedules 13D and 13G, if any, filed with the Commission. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of February 26, 2009, there were 81,349,712 shares of the registrant's common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant's definitive Proxy Statement for the 2009 Annual Meeting of Stockholders to be filed within 120 days after the end of the Registrant's fiscal year ended December 31, 2008 are incorporated by reference into Part III of this Annual Report on Form 10-K to the extent stated therein.
Table of Contents
TABLE OF CONTENTS
| | | | |
| |
| | Page |
|---|
PART I | | | | |
ITEM 1. | | BUSINESS | |
3 |
ITEM 1A. | | RISK FACTORS | |
18 |
ITEM 1B. | | UNRESOLVED STAFF COMMENTS | |
37 |
ITEM 2. | | PROPERTIES | |
37 |
ITEM 3. | | LEGAL PROCEEDINGS | |
37 |
ITEM 4. | | SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS | |
38 |
PART II | | | | |
ITEM 5. | | MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES | |
39 |
ITEM 6. | | SELECTED FINANCIAL DATA | |
41 |
ITEM 7. | | MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | |
42 |
ITEM 7A. | | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK | |
57 |
ITEM 8. | | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA | |
58 |
ITEM 9. | | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE | |
58 |
ITEM 9A. | | CONTROLS AND PROCEDURES | |
58 |
ITEM 9B. | | OTHER INFORMATION | |
59 |
PART III | | | | |
ITEM 10. | | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE | |
60 |
ITEM 11. | | EXECUTIVE COMPENSATION | |
60 |
ITEM 12. | | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS | |
61 |
ITEM 13. | | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE | |
61 |
ITEM 14. | | PRINCIPAL ACCOUNTING FEES AND SERVICES | |
61 |
PART IV | | | | |
ITEM 15. | | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES | |
62 |
| | SIGNATURES | |
66 |
2
Table of Contents
PART I
Allos Therapeutics, Inc., the Allos Therapeutics, Inc. logo and all other Allos names are trademarks of Allos Therapeutics, Inc. in the United States and in other selected countries. All other brand names or trademarks appearing in this report are the property of their respective holders. Unless the context requires otherwise, references in this report to "Allos," the "Company," "we," "us," and "our" refer to Allos Therapeutics, Inc.
This report contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These forward-looking statements include, but are not limited to, statements concerning our projected timelines for the completion of enrollment and announcement of results from our ongoing clinical trials, the Company's intent and projected timeline to submit a New Drug Application for pralatrexate (PDX) as a treatment for patients with relapsed or refractory peripheral T-cell lymphoma, the potential for the results of our Phase 2 PROPEL trial to support marketing approval of pralatrexate; other statements regarding our future product development and regulatory strategies, including our intent to develop or seek regulatory approval for pralatrexate in specific indications; the ability of our third-party manufacturing parties to support our requirements for drug supply; any statements regarding our future financial performance, results of operations or sufficiency of capital resources to fund our operating requirements; and any other statements which are other than statements of historical fact. In some cases, these statements may be identified by terminology such as "may," "will," "should," "expects," "plans," "anticipates," "believes," "estimates," "predicts," "potential" or "continue," or the negative of such terms and other comparable terminology. Although we believe that the expectations reflected in the forward-looking statements contained herein are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. These statements involve known and unknown risks and uncertainties that may cause our, or our industry's results, levels of activity, performance or achievements to be materially different from those expressed or implied by the forward-looking statements. Factors that may cause or contribute to such differences include, among other things, those discussed under the captions "Business," "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations." All forward-looking statements included in this report are based on information available to us as of the date hereof and we undertake no obligation to revise any forward-looking statements in order to reflect any subsequent events or circumstances. Forward-looking statements not specifically described above also may be found in these and other sections of this report.
ITEM 1. BUSINESS
Overview
We are a biopharmaceutical company focused on developing and commercializing innovative small molecule drugs for the treatment of cancer. We strive to develop proprietary products that have the potential to improve the standard of care in cancer therapy. Our focus is on product opportunities for oncology that leverage our internal clinical development and regulatory expertise and address important markets with unmet medical need. We may also seek to grow our existing portfolio of product candidates through product acquisition and in-licensing efforts.
Our lead product candidate, pralatrexate, is a novel targeted antifolate designed to accumulate preferentially in cancer cells. Based on preclinical studies, we believe that pralatrexate selectively enters cells expressing RFC-1, a protein that is over expressed on cancer cells compared to normal cells. Once inside cancer cells, pralatrexate is efficiently polyglutamylated, which leads to high intracellular drug retention. Polyglutamylated pralatrexate essentially becomes "trapped" inside cancer cells, making it less susceptible to efflux-based drug resistance. Acting on the folate pathway, pralatrexate interferes with DNA synthesis and triggers cancer cell death. We believe pralatrexate has the potential to be delivered as a single agent or in combination therapy regimens.
3
Table of Contents
In February 2009, we announced the final results from PROPEL, our pivotal Phase 2 trial of pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma, or PTCL. Based on the results of this trial, we intend to submit a New Drug Application, or NDA, to the U.S. Food and Drug Administration, or FDA, for pralatrexate for the treatment of patients with relapsed or refractory PTCL in the first half of 2009. This trial was conducted under an agreement reached with the FDA under its special protocol assessment, or SPA process, which provides an agreement that the study design, including trial size, clinical endpoints and/or data analyses are acceptable to the FDA. The SPA agreement is not a guarantee of approval, and we cannot assure you that the design of, or data collected from, the PROPEL trial will be adequate to demonstrate the safety and efficacy of pralatrexate for the treatment of patients with relapsed or refractory PTCL, or otherwise be sufficient to support FDA or any foreign regulatory approval. In addition to the PROPEL trial, we are committed to evaluating pralatrexate for oncology use as a single agent and in combination with other therapies. We currently have seven ongoing clinical trials involving pralatrexate, including the PROPEL trial, and plan to initiate additional trials to evaluate pralatrexate's potential clinical utility in other hematologic malignancies and solid tumor indications.
Our Strategy
Our goal is to build a profitable company by generating income from products we develop and commercialize, either alone or with one or more potential strategic partners. The key elements of our strategy are to:
- •
- Focus on the oncology market. We intend to continue to focus our drug development efforts on the oncology market. We believe the oncology market is attractive due to its size, demand for safer and more effective cancer treatments, relatively small physician population that can be addressed with a targeted sales force, and potential for expedited regulatory review.
- •
- Obtain regulatory approval to market pralatrexate. We are currently focused on obtaining regulatory approval in the United States to market pralatrexate for the treatment of patients with relapsed or refractory PTCL. We recently announced the results of our pivotal Phase 2 PROPEL trial and intend to submit an NDA to the FDA for pralatrexate for the treatment of patients with relapsed or refractory PTCL in the first half of 2009.
- •
- Advance our pralatrexate development program. In addition to the PROPEL trial, we are committed to evaluating pralatrexate for oncology use as a single agent and in combination with other therapies. We currently have seven ongoing clinical trials involving pralatrexate, including the PROPEL trial, and plan to initiate additional trials in the future to evaluate pralatrexate's potential clinical utility in other hematologic malignancies and solid tumor indications.
- •
- Develop sales and marketing capabilities to commercialize pralatrexate. We currently retain exclusive worldwide commercial rights to pralatrexate for all indications. We intend to commercialize pralatrexate, if it is approved for marketing, by building an oncology focused U.S.-based sales and marketing organization which may be complemented by co-promotion arrangements with pharmaceutical or biotechnology partners, where appropriate. We intend to enter into co-promotion or out-licensing arrangements with other pharmaceutical or biotechnology firms, where necessary, to reach foreign market segments that are not reachable by a U.S.-based sales force or when deemed strategically and economically advisable.
- •
- Expand our product candidate portfolio. We may pursue opportunities from time to time to expand our product candidate portfolio by identifying and evaluating new compounds that have demonstrated potential in preclinical or clinical studies and are strategically aligned with our existing oncology portfolio. Our intent is to build a portfolio of proprietary product candidates that have the potential to improve the standard of care in cancer therapy and provide commercial, regulatory or geographic exclusivity.
4
Table of Contents
Our Product Candidates
The following table summarizes the target indications and clinical development status of our lead product candidate, pralatrexate:
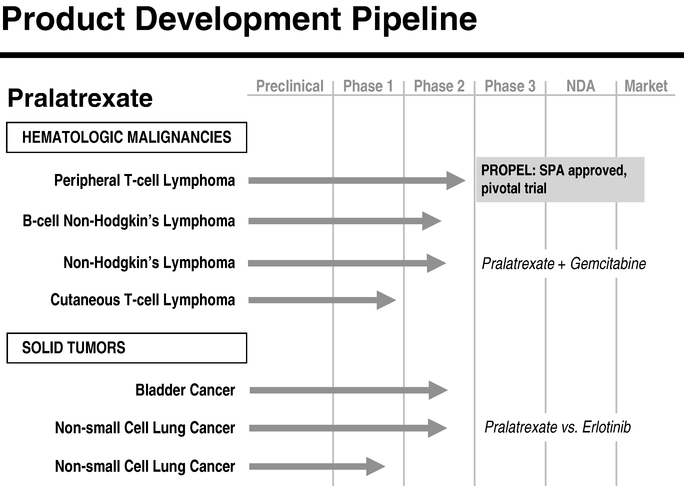
Pralatrexate (PDX)
Pralatrexate is a novel targeted antifolate designed to accumulate preferentially in cancer cells. Based on preclinical studies, we believe that pralatrexate selectively enters cells expressing RFC-1, a protein that is over expressed on cancer cells compared to normal cells. Once inside cancer cells, pralatrexate is efficiently polyglutamylated, which leads to high intracellular drug retention. Polyglutamylated pralatrexate essentially becomes "trapped" inside cancer cells, making it less susceptible to efflux-based drug resistance. Acting on the folate pathway, pralatrexate interferes with DNA synthesis and triggers cancer cell death. We believe pralatrexate has the potential to be delivered as a single agent or in combination therapy regimens.
Scientific Rationale
The antimetabolites are a group of low-molecular weight compounds that exert their effect by virtue of their structural or functional similarity to naturally occurring molecules involved in DNA synthesis. Because the cell mistakes them for a normal metabolite, the antimetabolites either inhibit critical enzymes involved in DNA synthesis or become incorporated into the nucleic acid, producing incorrect codes. Both mechanisms result in inhibition of DNA synthesis and ultimately, cell death. Because of their primary effect on DNA synthesis, the antimetabolites are most effective against
5
Table of Contents
actively dividing cells and are largely cell-cycle phase specific. There are three classes of antimetabolites; purine analogs, pyrimidine analogs and folic acid analogs, also termed antifolates. Pralatrexate is a folic acid analog.
The selectivity of antifolates for tumor cells involves their conversion to a polyglutamated form by the enzyme folypolyglutamyl synthetase. Polyglutamation is a time- and concentration-dependent process that occurs in tumor cells, and to a lesser extent, normal tissue. The selective activity of the folic acid analogs in malignant cells versus normal cells likely is due to the relative difference in polyglutamate formation. Polyglutamated metabolites have prolonged intracellular half-life, increased duration of drug action and are potent inhibitors of several folate- dependent enzymes, including dihydrofolate reductase, or DHFR.
We believe that the resistance of malignant cells to the effects of the folic acid analogs may, in part, be due to impaired polyglutamation. We believe the improved antitumor effects of pralatrexate in comparison to methotrexate, as observed in preclinical studies, is likely due to the more effective uptake and transport of pralatrexate into the cell followed by the greater accumulation of pralatrexate and its metabolites within the tumor cell through the formation of the polyglutamated derivatives.
Pralatrexate in the treatment of peripheral T-cell lymphoma
Peripheral T-cell lymphomas, or PTCL, comprise a biologically diverse group of blood cancers that account for approximately 10 to 15 percent of all cases of non-Hodgkin's lymphoma, or NHL, in the United States. According to the American Cancer Society, an estimated 66,000 new cases of NHL were expected to be diagnosed in the United States in 2008. We estimate the current annual prevalence of PTCL in the United States to be approximately 9,500 patients. There are currently no pharmaceutical agents approved for use in the treatment of either first-line or relapsed or refractory PTCL. In addition to those PTCL patients who do not respond to first-line treatment, a significant number of first-line multi-agent chemotherapy responders relapse or become refractory after treatment. According to the clinical literature, patients with aggressive PTCL have an overall five-year survival rate of approximately 25% after first-line therapy.
In February 2009, we announced the final results from PROPEL, our pivotal Phase 2, international, multi-center, open-label, single-arm trial of pralatrexate in patients with relapsed or refractory PTCL. Based on the results of this trial, we intend to submit an NDA to the FDA for pralatrexate for the treatment of patients with relapsed or refractory PTCL in the first half of 2009.
The PROPEL trial enrolled a total of 115 patients with relapsed or refractory PTCL, 109 of whom were considered evaluable for response according to the trial protocol. We believe the PROPEL trial is the largest prospectively designed single-agent trial conducted to date in patients with relapsed or refractory PTCL. To be eligible for the trial, a patient's disease must have progressed after at least one prior treatment. Patients were considered evaluable if they received at least one dose of pralatrexate and their diagnosis of PTCL was confirmed by independent pathology review. Patients received 30 mg/m2 of pralatrexate intravenously once every week for six weeks followed by one week of rest per cycle of treatment. Patients also received vitamin B12 and folic acid supplementation. The primary endpoint of the trial is objective response rate, as assessed by central independent oncology review using International Workshop Criteria. Duration of response is the key secondary endpoint. Other secondary endpoints include progression-free survival, overall survival and safety and tolerability.
The results of the trial demonstrated that 29 of 109 evaluable patients, or 27%, achieved a response as assessed by central independent oncology review. Of the 29 patients who achieved a response according to central independent oncology review, seven patients had a complete response, or CR, two patients had a complete response unconfirmed, or CRu, and 20 patients had a partial response, or PR. The Kaplan-Meier estimate for the median duration of response was 287 days, or 9.4 months. The most common grade 3/4 adverse events were thrombocytopenia, which was observed in
6
Table of Contents
32% of patients; mucosal inflammation in 21% of patients; neutropenia in 20% of patients; and anemia in 17% of patients.
According to the PROPEL investigators, 42 of 109 evaluable patients, or 39%, achieved a response. Of these, 15 patients had a CR, four patients had a CRu and 23 patients had a PR. PROPEL patients received a median of three prior systemic treatment regimens (range of 1-12), including 18 patients, or 16%, who had previously undergone an autologous stem cell transplant. In the trial, 66% of the patients who responded did so after cycle one of therapy. Patients will continue to be followed for long-term survival.
In accordance with the PROPEL trial protocol, three pre-planned interim analyses of safety data were previously conducted. In January, September and December 2007, we announced that an independent data monitoring committee, or DMC, completed interim analyses of safety data from the first 10, 35 and 65 evaluable patients who completed at least one cycle of treatment with pralatrexate, respectively, and recommended that the trial continue per the protocol at each analysis. No major safety concerns were identified by the DMC that affected the continuation of the trial.
The PROPEL trial was conducted under an agreement reached with the FDA under its SPA process. The SPA process allows for FDA evaluation of a clinical trial protocol intended to form the primary basis of an efficacy claim in support of an NDA, and provides an agreement that the study design, including trial size, clinical endpoints and/or data analyses are acceptable to the FDA. However, the SPA agreement is not a guarantee of approval, and we cannot assure you that the design of, or data collected from, the PROPEL trial will be adequate to demonstrate the safety and efficacy of pralatrexate for the treatment of patients with relapsed or refractory PTCL, or otherwise be sufficient to support FDA or any foreign regulatory approval. For example, the response rate, duration of response and safety profile required to support FDA approval are not specified in the PROPEL trial protocol and will be subject to FDA review. In addition, the median duration of response reported above is a Kaplan-Meier estimate based on the length of follow up for all responders at the time the PROPEL trial database was locked. As a result, the median duration of response may change based on continued patient follow up.
Pralatrexate has orphan drug designation and fast track designation in the United States for the treatment of patients with T-cell lymphoma and orphan medicinal product designation in Europe for the treatment of PTCL. Under the U.S. Orphan Drug Act, if we are the first company to receive FDA approval for pralatrexate for this orphan drug indication, we will obtain seven years of marketing exclusivity during which the FDA may not approve another company's application for the same drug for the same orphan indication. The FDA's fast track program is designed to facilitate the development and expedite the review of new drugs that are intended to treat serious or life-threatening conditions and that demonstrate the potential to address unmet medical needs. In Europe, orphan medicinal product designation, or OMPD, is intended to promote the development of drugs that may provide significant benefit to patients suffering from rare diseases identified as life-threatening or very serious. Under the guidelines of the European Medicines Agency, OMPD provides ten years of potential market exclusivity once the product candidate is approved for marketing for the designated indication in the European Union.
Pralatrexate in the treatment of non-Hodgkin's Lymphoma and Hodgkin's disease
Approximately 66,000 patients were expected to be diagnosed with non-Hodgkin's Lymphoma, or NHL, in the United States in 2008 and approximately 85% of NHL patients represent patients with B-cell lymphoma. The incidence of NHL has increased significantly since the 1970's and is currently growing at approximately 1% to 2% per year. Patients with indolent or low-grade NHL may have survival rates as long as 10 years, yet the disease is frequently incurable. Aggressive lymphomas generally result in shorter median survival times although patients with these malignancies can be cured
7
Table of Contents
in 30% to 60% of cases. Standard chemotherapy for NHL involves an initial combination of cyclophosphamide, doxorubicin, vincristine and prednisone, also known as CHOP. The addition of rituximab in one study increased response rates to nearly 100%. However, a significant number of patients treated with CHOP or rituximab eventually relapse and may be candidates for salvage chemotherapy, or chemotherapy given after recurrence of a tumor.
We are currently evaluating pralatrexate in an ongoing Phase 1/2, open-label, single-center study in patients with relapsed or refractory NHL and Hodgkin's disease. Interim data from this trial, which was most recently presented at the AACR-NCI-EORTC conference in October 2007, showed that responses were observed in 2 of 20, or 10% of, evaluable patients with B-cell lymphoma. This study is currently focused on exploring alternate dosing and administration schedules in patients with B-cell lymphoma to further evaluate pralatrexate's potential clinical utility in this setting.
In May 2007, we initiated patient enrollment in a Phase 1/2a, open-label, multi-center study of pralatrexate and gemcitabine with vitamin B12 and folic acid supplementation in patients with relapsed or refractory NHL or Hodgkin's disease. In the Phase 1 portion of this study, patients with either relapsed or refractory NHL (diffuse large B- or T-cell lymphoma, mantle cell lymphoma, transformed large cell lymphomas) or Hodgkin's disease receive pralatrexate either concurrently on the same day with or followed the next day by gemcitabine as part of a weekly schedule for three or four weeks with concurrent vitamin B12 and folic acid supplementation. We plan to enroll up to 54 evaluable patients in the Phase 1 portion of the study with the objective of determining the maximum tolerated dose, or MTD, safety, tolerability, and pharmacokinetic profile of escalating doses of sequential pralatrexate and gemcitabine. If the Phase 1 portion of the study is successful, we plan to enroll up to 45 additional patients with relapsed or refractory PTCL in the Phase 2a portion of the trial at the established MTD to assess preliminary efficacy of pralatrexate and gemcitabine.
In December 2008, interim data from this study were presented at the 50th Annual Meeting of the American Society of Hematology. Data were presented on 27 patients, 22 of whom were evaluable for response. Patients were enrolled in eight cohorts with different doses and schedules. Partial responses were observed in 6 of 22 evaluable patients, including five patients on a sequential dosing schedule and one patient on a same-day dosing schedule. Patients received a median of three prior systemic regimens. The most common adverse event was thrombocytopenia, with Grade 3 observed in four patients and Grade 4 observed in seven patients. The MTD for the sequential dosing schedule was established as 10 mg/m2 of pralatrexate followed by 400 mg/m2 of gemcitabine, once every two weeks. Enrollment in the trial is ongoing to determine the MTD for the same-day dosing schedule.
In October 2008, the FDA granted orphan drug designation to pralatrexate for the treatment of patients with follicular lymphoma and for the treatment of patients with diffuse large B-cell lymphoma.
Pralatrexate in the treatment of cutaneous T-cell lymphoma
Cutaneous T-cell lymphomas, or CTCL, are comprised of a number of non-Hodgkin's T-cell lymphomas, including mycosis fungoides and Sezary syndrome, which have their primary manifestations in the skin. According to the Lymphoma Research Foundation, CTCL accounts for approximately 2% to 3% of the estimated 66,000 new cases of NHL diagnosed each year in the United States.
In August 2007, we initiated patient enrollment in a Phase 1, open-label, multi-center study of pralatrexate with vitamin B12 and folic acid supplementation in patients with relapsed or refractory CTCL. In this study, patients with either relapsed or refractory CTCL receive pralatrexate as part of a weekly schedule for two or three weeks followed by one week of rest. Patients receive starting doses of pralatrexate at 30 mg/m2, with dose reduction in subsequent cohorts based on toxicity.
In December 2008, interim data from this study were presented at the 50th Annual Meeting of the American Society of Hematology. Data were presented on 24 patients, including 22 evaluable patients
8
Table of Contents
who completed at least one cycle of treatment at doses ranging from 10-30 mg/m2 as part of a weekly schedule for two or three weeks followed by one week of rest. Responses were observed in 12 of 22 evaluable patients, or 55%, including one complete response and 11 partial responses. Patients received a median of four prior systemic therapies. The most common adverse event was mucosal inflammation, with Grade 1/2 mucosal inflammation observed in 11 of 24 patients and Grade 3 mucosal inflammation observed in 4 of 24 patients. There was no Grade 4 mucosal inflammation and no thrombocytopenia above Grade 1.
We plan to enroll up to 56 evaluable patients in the study with the objective of determining the optimal dose and safety profile of pralatrexate in this population. We plan to enroll at least 20 of these patients at what we believe to be the optimal dose and schedule.
Pralatrexate in the treatment of non-small cell lung cancer
Lung cancer is the most common cause of cancer death in the United States. According to the American Cancer Society, an estimated 215,020 new cases of lung cancer were expected to be diagnosed in the United States in 2008. Non-small cell lung cancer, or NSCLC, is the most common type of lung cancer, accounting for approximately 87% of lung cancer cases, according to the American Cancer Society. More people die of lung cancer than of breast, prostate and colorectal cancers combined. Over the last decade, oncologists have begun treating advanced NSCLC patients more aggressively, typically administering a potent combination of paclitaxel and carboplatin. Other drugs used in this setting include gemcitabine, vinorelbine, docetaxel and cisplatin. Despite aggressive therapy using gemcitabine and vinorelbine, one study found that the one-year survival rate for patients with Stage IIIB or IV NSCLC was approximately 40%.
In January 2008, we initiated patient enrollment in a Phase 2b, randomized, multi-center study comparing pralatrexate and Tarceva (erlotinib), both with vitamin B12 and folic acid supplementation, in patients with Stage IIIB/IV NSCLC who are, or have been, cigarette smokers who have failed treatment with at least one prior platinum-based chemotherapy regimen. The objective of this study is to compare the efficacy of pralatrexate to that of Tarceva in patients with Stage IIIB/IV NSCLC. The primary endpoint of the study is overall survival. Secondary endpoints include response rate and progression-free survival, both compared to Tarceva, and the safety and tolerability of pralatrexate. The study will seek to enroll approximately 160 patients in up to 50 investigative sites worldwide. Patients will be randomized 1:1 to either the pralatrexate arm or the Tarceva arm. Patients randomized to the pralatrexate arm will receive pralatrexate as an intravenous, or IV, push administered on days 1 and 15 of a 4-week/28 day cycle. The initial dose of pralatrexate will be 190 mg/m2, which, based on defined criteria, may be increased to 230 mg/m2 or reduced in 40 mg/m2 decrements. Patients randomized to the Tarceva arm will receive Tarceva 150 mg/day orally daily for the 4-week/28 day cycle. Patients in both arms will receive concurrent vitamin therapy of B12 and folic acid. Based on current enrollment rates, we expect to complete patient enrollment in this study in the third quarter of 2009.
Our decision to begin this study was based, in part, upon data from a Phase 2 open-label, single-agent study of pralatrexate in patients with relapsed or refractory Stage IIIB or IV NSCLC completed in 2001 by Memorial Sloan-Kettering Cancer Center one of the institutions from which we licensed pralatrexate. This study demonstrated a response rate of 11%, a median time to progression of three months and a median survival time of 13.5 months. However, 21% of the patients suffered grade 3 or 4 stomatitis, or mouth ulcers. As a result of subsequent research that suggested supplementation of pralatrexate with folic acid and vitamin B12 may reduce the incidence of clinically significant stomatitis, in January 2005 we initiated a Phase 1 dose escalation study of pralatrexate with vitamin B12 and folic acid supplementation in patients with previously treated Stage IIIB/IV advanced NSCLC.
In October 2007, data from this ongoing Phase 1 study were presented at the AACR-NCI-EORTC conference. In the study, a total of 22 patients with relapsed or refractory NSCLC were treated at
9
Table of Contents
doses of 150 to 325 mg/m2 of pralatrexate. The MTD was determined to be 270 mg/m2, which is nearly twice the dose used in the previous Phase 2 study discussed above in which pralatrexate was administered without vitamin supplementation, and what we believe to be clinically significant radiologic responses were observed. Greater than 50% of patients, or 13 of 22, received two or more prior treatment regimens. This study was used to establish the dosing regimen for our current Phase 2b study in patients with Stage IIIB/IV NSCLC.
Pralatrexate in the treatment of bladder cancer
Bladder cancer is the ninth most common type of cancer. According to the American Cancer Society, an estimated 68,810 new cases of bladder cancer were expected to be diagnosed in the United States in 2008. Transitional cell carcinoma, or TCC, is the most common form of bladder cancer, accounting for more than 90% of all bladder cancers. There are no approved agents for the treatment of advanced or metastatic relapsed TCC of the urinary bladder.
In July 2008, we initiated patient enrollment in a Phase 2, open-label, single-arm, multi-center study of pralatrexate in patients with advanced or metastatic relapsed TCC of the urinary bladder. The primary endpoint of the study is objective response rate (complete and partial response). Secondary endpoints include duration of response, clinical benefit rate, progression-free survival overall survival and the safety and tolerability of pralatrexate. The study will seek to enroll approximately 41 patients in up to 20 investigative sites worldwide. Patients receive pralatrexate as an IV push administered on days 1 and 15 of a 4-week/28 day cycle. The initial dose of pralatrexate is 190 mg/m2, which may be adjusted based on criteria defined in the protocol. Patients will receive concurrent vitamin therapy of B12 and folic acid.
Pralatrexate in the treatment of other solid tumor indications
In addition to our ongoing NSCLC and bladder cancer studies, we are evaluating the potential future development of pralatrexate for other solid tumor indications, including Stage III/IV head and neck cancer and Stage III/IV breast cancer, among others. There can be no assurances that we will pursue the development of pralatrexate for one or more of these indications or that such development efforts will be ultimately successful.
RH1
Our other product candidate, RH1, is a small molecule chemotherapeutic agent that we believe is bioactivated by the enzyme DT-diaphorase, or DTD, also known as NAD(P)H quinone oxidoreductase, or NQ01. We believe DTD is over-expressed in many tumors, relative to normal tissue, including lung, colon, breast and liver tumors. We believe that because RH1 is bioactivated in the presence of DTD, it may have the potential to provide targeted drug delivery to these tumor types while limiting the amount of toxicity to normal tissue.
In November 2007, we initiated patient enrollment in a Phase 1, open-label, multi-center dose escalation study of RH1 in patients with advanced solid tumors or NHL. We are in the process of closing this study and determining our future development plans, if any, for RH1.
Manufacturing
The production of pralatrexate and RH1 employ small molecule organic chemistry procedures standard for the pharmaceutical industry. We plan to continue to outsource manufacturing responsibilities for these and any additional future products, and we intend to select and rely, at least initially, on single source suppliers to manufacture each of our product candidates. We believe this manufacturing strategy allows us to direct our financial and managerial resources to the development and commercialization of products rather than to the establishment of a manufacturing infrastructure.
10
Table of Contents
We believe it also enables us to minimize fixed costs and capital expenditures, while gaining access to advanced manufacturing process capabilities and expertise. However, if our third party suppliers become unable or unwilling to provide sufficient future drug supply or meet regulatory requirements relating to the manufacture of pharmaceutical agents, we would be forced to incur additional expenses to secure alternative third party manufacturing arrangements and may suffer delays in our ability to conduct clinical trials or commercialize these products.
Pralatrexate
We have entered into arrangements with one third-party manufacturer to produce pralatrexate bulk drug substance and another third-party manufacturer to produce pralatrexate formulated drug product for use in our clinical development programs. We believe these third-party manufacturers have the capability to meet our requirements for all future clinical trial requirements. As we pursue FDA approval to market pralatrexate for the treatment of patients with relapsed or refractory PTCL, we will seek to establish appropriate commercial supply arrangements for the production of pralatrexate bulk drug substance and formulated drug product.
RH1
We have entered into arrangements with one third party manufacturer to produce RH1 bulk drug substance and another third party manufacturer to produce RH1 formulated drug product for use in our clinical development programs. We believe these third party manufacturers have the capability to meet our requirements for any future clinical trials, if any, involving RH1.
Sales and Marketing
We currently retain exclusive worldwide commercial rights to pralatrexate and RH1 for all target indications. If we obtain FDA approval to market pralatrexate, we intend to commercialize pralatrexate in the United States by building a focused sales and marketing organization that may be complemented by co-promotion or other partnering arrangements with pharmaceutical or biotechnology partners, where appropriate. Our sales and marketing strategy is to:
- •
- Build a U.S. sales force. We believe that a moderate-sized sales force could effectively reach targeted physicians and medical institutions that treat the majority of patients with PTCL in the United States. We intend to build and manage this sales force internally.
- •
- Build a marketing organization. We also plan to build an internal marketing and sales operations organization to develop and implement product plans, and support our sales force and marketing partners.
- •
- Establish co-promotion alliances. We intend to enter into co-promotion or out-licensing arrangements with other pharmaceutical or biotechnology firms, where necessary, to reach foreign market segments that are not reachable by a U.S.-based sales force or when deemed strategically and economically advisable.
Intellectual Property
We believe that patent protection and trade secret protection are important to our business and that our future success will depend, in part, on our ability to maintain our technology licenses, maintain trade secret protection, obtain and maintain patents and operate without infringing the proprietary rights of others both in the United States and abroad. We believe that obtaining identical patents and protection periods for a given technology throughout all markets of the world will be difficult because of differences in patent laws. In addition, the protection provided by non-U.S. patents, if any, may be weaker than that provided by U.S. patents.
11
Table of Contents
In order to protect the confidentiality of our technology, including trade secrets and know-how and other proprietary technical and business information, we require all of our employees, consultants, advisors and collaborators to enter into confidentiality agreements that prohibit the use or disclosure of confidential information. The agreements also oblige our employees, consultants, advisors and collaborators to assign or license to us ideas, developments, discoveries and inventions made by such persons in connection with their work with us. We cannot be sure that these agreements will maintain confidentiality, will prevent disclosure, or will protect our proprietary information or intellectual property, or that others will not independently develop substantially equivalent proprietary information or intellectual property.
The pharmaceutical industry is highly competitive and patents have been applied for by, and issued to, other parties relating to products or new technologies that may be competitive with those being developed by us. Therefore, our product candidates may give rise to claims that they infringe the patents or proprietary rights of other parties now or in the future. Furthermore, to the extent that we, our consultants, or manufacturing and research collaborators, use intellectual property owned by others in work performed for us, disputes may also arise as to the rights to such intellectual property or in related or resulting know-how and inventions. An adverse claim could subject us to significant liabilities to such other parties and/or require disputed rights to be licensed from such other parties. A license required under any such patents or proprietary rights may not be available to us, or may not be available on acceptable terms. If we do not obtain such licenses, we may encounter delays in product market introductions, or may find that we are prevented from the development, manufacture or sale of products requiring such licenses. In addition, we could incur substantial costs in defending ourselves in legal proceedings instituted before patent and trademark offices in the United States, the European Union, or other ex-U.S. territories, or in a suit brought against us by a private party based on such patents or proprietary rights, or in a suit by us asserting our patent or proprietary rights against another party, even if the outcome is not adverse to us.
Pralatrexate
In December 2002, we entered into a license agreement with Memorial Sloan-Kettering Cancer Center, SRI International and Southern Research Institute, as amended, under which we obtained exclusive worldwide rights to a portfolio of patents and patent applications related to pralatrexate and its uses. The portfolio currently consists of two issued patents in the United States, two granted patents in Europe, and pending patent applications in the United States, Canada, Europe, Australia, Japan, China, Brazil, Indonesia, India, South Korea, Mexico, Norway, New Zealand, the Philippines, Singapore and South Africa. The licensed patents and applications, which expire at various times between July 2017 and May 2025, contain claims covering pralatrexate substantially free of 10-deazaaminopterin, methods to treat tumors with pralatrexate substantially free of 10-deazaaminopterin, treatment of breast, lung, and prostate cancer and leukemia with a combination of pralatrexate and a taxane, treatment of T-cell lymphoma with pralatrexate, treatment of lymphoma with a combination of pralatrexate and gemcitabine, methods of assessing sensitivity of a tumor to pralatrexate, and other methods and compositions.
Under the terms of the agreement, we paid an up-front license fee of $2.0 million upon execution of the agreement and are also required to make certain additional cash payments based upon the achievement of certain clinical development or regulatory milestones or the passage of certain time periods. To date, we have made aggregate milestone payments of $2.5 million based on the passage of time. In the future, we could make an aggregate milestone payment of $500,000 upon the earlier of achievement of a clinical development milestone or the passage of certain time periods, or the Clinical Milestone, and up to $10.3 million upon achievement of certain regulatory milestones, or the Regulatory Milestones, including regulatory approval to market pralatrexate in the United States or Europe. The last scheduled payment towards the Clinical Milestone of $500,000 is currently due on
12
Table of Contents
December 23, 2009. We intend to submit an NDA for pralatrexate for the treatment of patients with relapsed or refractory PTCL in the first half of 2009. If the FDA accepts our NDA for review and if we obtain FDA approval to market pralatrexate, we will be obligated to make payments of $1,500,000 and $5,300,000, respectively, which represent a portion of the Regulatory Milestones. The up-front license fee and all milestone payments under the agreement have been or will be recorded to research and development expense when incurred. Under the terms of the agreement, we are required to fund all development programs and will have sole responsibility for all commercialization activities. In addition, we will pay the licensors a royalty based on a percentage of net revenues arising from sales of the product or sublicense revenues arising from sublicensing the product, if and when such sales or sublicenses occur.
RH1
In December 2004, we entered into an agreement with the University of Colorado Health Sciences Center, the University of Salford and Cancer Research Technology, or CRT, under which we obtained exclusive worldwide rights to certain intellectual property surrounding a proprietary molecule known as RH1. Under the terms of the agreement, we paid an up-front license fee of $190,500 upon execution of the agreement and are also required to make certain additional cash payments based upon the achievement of certain clinical development, regulatory and commercialization milestones. We could make aggregate milestone payments of up to $9.2 million upon the achievement of the clinical development, regulatory and commercialization milestones set forth in the agreement. The up-front license fee and all milestone payments under the agreement, as well as the one-time data option fee discussed below, have been recorded to research and development expense. Under the terms of the agreement and related data option agreement, we paid the licensors a one-time data option fee of $360,000 in 2007 for an exclusive license to the results of a Phase 1 study sponsored by Cancer Research UK, CRT's parent institution. This Phase 1 study was completed in 2007 and, under the terms of the agreement, we have since assumed responsibility for all future development costs and activities and have sole responsibility for all commercialization activities. In addition, we will pay the licensors a royalty based on a percentage of net revenues arising from sales of the product or sublicense revenues arising from sublicensing the product, if and when such sales or sublicenses occur. We are in the process of closing our Phase 1 study of RH1 in patients with advanced solid tumors or NHL and determining our future development plans, if any, for RH1.
Competition
The pharmaceutical industry is characterized by rapidly evolving technology and intense competition. Many companies of all sizes, including a number of large pharmaceutical companies and several biotechnology companies, are developing cancer therapies similar to ours. There are products and technologies currently on the market that will compete directly with the products that we are developing. In addition, colleges, universities, governmental agencies and other public and private research institutions will continue to conduct research and are becoming more active in seeking patent protection and licensing arrangements to collect license fees, milestone payments and royalties in exchange for license rights to technologies that they have developed, some of which may directly compete with our technologies. These companies and institutions also compete with us in recruiting qualified scientific personnel. Many of our competitors have substantially greater financial, research and development, human and other resources than do we. Furthermore, large pharmaceutical companies may have significantly more experience than we do in preclinical testing, human clinical trials, manufacturing, regulatory approval and commercialization procedures. Our competitors may:
- •
- develop or acquire safer and/or more effective products;
- •
- obtain patent protection or intellectual property rights that limit our ability to commercialize products; and/or
13
Table of Contents
- •
- commercialize products earlier or more effectively than us.
We expect technology developments in our industry to continue to occur at a rapid pace. Commercial developments by our competitors may render some or all of our potential products obsolete or non-competitive, which would have a material adverse effect on our business and financial condition.
While there are currently no FDA-approved agents in the United States indicated for the treatment of PTCL, we are aware of multiple investigational agents that are currently being studied in clinical trials. There are also several agents and regimens, such as CHOP, that are currently used by physicians without an FDA label in PTCL that could potentially represent competition for pralatrexate.
Government Regulation
FDA Regulation and Product Approval
The FDA and comparable regulatory agencies in state and local jurisdictions and in foreign countries impose substantial requirements upon the clinical development, manufacture and marketing of pharmaceutical products. These agencies and other federal, state and local entities regulate research and development activities and the testing, manufacture, quality control, safety, effectiveness, labeling, storage, record keeping, approval, advertising and promotion of our product candidates.
The process required by the FDA before our product candidates may be marketed in the United States generally involves the following:
- •
- preclinical laboratory and animal tests;
- •
- submission to the FDA of an Investigational New Drug, or IND, application, which must become effective before clinical trials may begin;
- •
- adequate and well-controlled human clinical trials to establish the safety and efficacy of the proposed pharmaceutical in our intended use;
- •
- pre-approval inspection of manufacturing facilities and selected clinical investigators; and
- •
- submission to the FDA of an NDA that must be approved.
The testing and approval process requires substantial time, effort and financial resources, and we cannot be certain that any approvals for our product candidates will be granted on a timely basis, if at all.
Preclinical tests include laboratory evaluation of the product candidate, its chemistry, formulation and stability, as well as animal studies to assess its potential safety and efficacy. We then submit the results of the preclinical tests, together with manufacturing information and analytical data, to the FDA as part of an IND application, which must become effective before we may begin human clinical trials. The IND automatically becomes effective 30 days after the FDA acknowledges that the filing is complete, unless the FDA, within the 30-day time period, raises concerns or questions about the conduct of the trials as outlined in the IND. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before clinical trials can begin. Further, an independent Institutional Review Board at each medical center proposing to conduct the clinical trials must review and approve any clinical study.
Human clinical trials are typically conducted in three sequential phases, which may overlap:
- •
- Phase 1: The drug is initially administered into healthy human subjects or patients and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion.
14
Table of Contents
- •
- Phase 2: The drug is administered to a limited patient population to identify possible adverse effects and safety risks, to determine the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage.
- •
- Phase 3: When Phase 2 evaluations demonstrate that a dosage range of the drug is effective and has an acceptable safety profile, Phase 3 trials are undertaken to further evaluate dosage and clinical efficacy, and to further test for safety, in each case, in an expanded patient population at geographically dispersed clinical study sites.
In the case of product candidates for severe or life-threatening diseases such as cancer, the initial human testing is often conducted in patients rather than in healthy volunteers. Since these patients already have the target disease, these studies may provide initial evidence of efficacy traditionally obtained in Phase 2 trials and thus these trials are frequently referred to as Phase 1b trials. Additionally, when product candidates can do damage to normal cells, it is not ethical to administer such drugs to healthy patients in a Phase 1 trial.
We cannot be certain that we will successfully complete Phase 1, Phase 2 or Phase 3 testing of our product candidates within any specific time period, if at all. Furthermore, the FDA, the Institutional Review Boards or the sponsor may suspend clinical trials at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk.
The results of product development, preclinical studies and clinical studies are submitted to the FDA as part of an NDA for approval of the marketing and commercial shipment of the product candidate. The FDA may deny an NDA if the applicable regulatory criteria are not satisfied or may require additional clinical data. Even if such data is submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval. Once issued, the FDA may withdraw product approval if compliance with regulatory standards is not maintained or if problems occur after the product reaches the market. In addition, the FDA may require testing and surveillance programs to monitor the effect of approved products that have been commercialized, and the agency has the power to prevent or limit further marketing of a product based on the results of these post-marketing programs.
Satisfaction of the above FDA requirements or similar requirements of state, local and foreign regulatory agencies typically takes several years and the actual time required may vary substantially, based upon the type, complexity and novelty of the pharmaceutical product candidate. Government regulation may delay or prevent marketing of potential products for a considerable period of time and impose costly procedures upon our activities.
We cannot be certain that the FDA or any other regulatory agency will grant approval for any of our product candidates on a timely basis, if at all. Success in preclinical or early stage clinical trials does not assure success in later stage clinical trials. Data obtained from preclinical and clinical activities is not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. Even if a product candidate receives regulatory approval, the approval may be significantly limited to specific indications. Further, even after regulatory approval is obtained, later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market. Delays in obtaining, or failures to obtain regulatory approvals for pralatrexate would have a material adverse effect on our business. Marketing our product candidates abroad will require similar regulatory approvals and is subject to similar risks. In addition, we cannot predict what adverse governmental regulations may arise from future U.S. or foreign governmental action.
Any products manufactured or distributed by us pursuant to FDA clearances or approvals are subject to pervasive and continuing regulation by the FDA, including record-keeping requirements and reporting of adverse experiences with the drug. Our third-party drug manufacturers and their subcontractors are required to register their establishments with the FDA and certain state agencies,
15
Table of Contents
and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with current Good Manufacturing Practices, which impose certain procedural and documentation requirements upon us and our third-party manufacturers. We cannot be certain that we or our present or future suppliers will be able to comply with the current Good Manufacturing Practices and other FDA regulatory requirements.
The FDA regulates drug labeling and promotion activities. The FDA has actively enforced regulations prohibiting the marketing of products for unapproved uses.
Our product candidates are also subject to a variety of state laws and regulations in those states or localities where such product candidates may be marketed. Any applicable state or local regulations may hinder our ability to market our product candidates in those states or localities.
The FDA's policies may change and additional government regulations may be enacted in the future that could prevent or delay regulatory approval of our potential products. Moreover, increased attention to the containment of health care costs in the United States and in foreign markets could result in new government regulations, which could have a material adverse effect on our business. We cannot predict the likelihood, nature or extent of adverse governmental regulation, which might arise from future legislative or administrative action, either in the United States or abroad.
Foreign Regulation and Product Approval
Outside the United States, our ability to market a product candidate is contingent upon receiving marketing authorization from the appropriate regulatory authorities. The requirements governing the conduct of clinical trials, marketing authorization, pricing and reimbursement vary widely from country to country. At present, foreign marketing authorizations are applied for at a national level, although within the European Union, or EU, centralized registration procedures are available to companies wishing to market a product in more than one EU member state. If the regulatory authority is satisfied that adequate evidence of safety, quality and efficacy has been presented, a marketing authorization will be granted. In some countries in the EU, pricing of prescription drugs is subject to government control and agreements must be reached on a national level before marketing may begin in that country. If we are unable to reach agreement on an acceptable price for our products, we may choose not to pursue marketing of our drugs in that country. The foreign regulatory approval process involves all of the risks associated with FDA approval discussed above.
Other Regulations
We are also subject to numerous federal, state and local laws relating to such matters as safe working conditions, manufacturing practices, environmental protection, fire hazard control, and disposal of hazardous or potentially hazardous substances. We may incur significant costs to comply with such laws and regulations now or in the future.
Results of Operations
We are a development stage company. Since our inception in 1992, we have not generated any revenue from product sales and have experienced significant net losses and negative cash flows from operations. We have incurred these losses principally from costs incurred in our research and development programs, clinical manufacturing and from our marketing, general and administrative expenses. Our primary business activities have been focused on the development of pralatrexate, RH1 and EFAPROXYN (a program which we discontinued in mid-2007). For the years ended December 31, 2008, 2007 and 2006, we had net losses attributable to common stockholders of $51.7 million, $39.4 million, and $30.2 million, respectively. Research and development expenses for the years ended December 31, 2008, 2007 and 2006 were $23.8 million, $17.4 million and $14.3 million, respectively. As of December 31, 2008, we had accumulated a deficit during our development stage of $299.7 million.
16
Table of Contents
Our ability to generate revenue and achieve profitability is dependent on our ability, alone or with partners, to successfully complete the development of pralatrexate, conduct clinical trials, obtain the necessary regulatory approvals, and manufacture and market pralatrexate. The timing and costs to complete the successful development of pralatrexate is highly uncertain, and therefore difficult to estimate. The lengthy process of seeking regulatory approvals for pralatrexate, and the subsequent compliance with applicable regulations, require the expenditure of substantial resources. For a more complete discussion of the regulatory approval process, please refer to the "Government Regulation" section above. Clinical development timelines, likelihood of success and total costs vary widely and are impacted by a variety of risks and uncertainties, including those discussed in the "Risk Factors" section of Item 1A below. Because of these risks and uncertainties, we cannot predict when or whether we will successfully complete the development of pralatrexate or the ultimate costs of such efforts. Due to these same factors, we cannot be certain when, or if, we will generate any revenue or net cash inflow from pralatrexate.
Even if our clinical trials demonstrate the safety and effectiveness of pralatrexate in its target indications, we do not expect to be able to generate commercial sales of pralatrexate until the second half of 2009, at the earliest. We expect to continue incurring net losses and negative cash flows for the foreseeable future. Although the size and timing of our future net losses are subject to significant uncertainty, we expect them to increase over the next several years as we continue to fund our research and development programs and prepare for the potential commercial launch of pralatrexate.
We anticipate continuing our current development programs and/or beginning other long-term development projects involving pralatrexate. These projects may require many years and substantial expenditures to complete and may ultimately be unsuccessful. In addition, we intend to submit an NDA for pralatrexate for the treatment of patients with relapsed or refractory PTCL in the first half of 2009. We expect to incur significant costs relating to preparations for the potential commercial launch of pralatrexate, including pre-commercial scale up of manufacturing and development of sales and marketing capabilities, prior to the receipt of regulatory approval to market pralatrexate. Therefore, we will need to raise additional capital to support our future operations, including the potential commercialization of pralatrexate if approved for marketing. Our actual capital requirements will depend on many factors, including those discussed under the "Liquidity and Capital Resources" section below.
We may seek to obtain this additional capital through equity or debt financings, arrangements with corporate partners, or from other sources. Such arrangements, if successfully consummated, may be dilutive to our existing stockholders. However, there is no assurance that additional financing will be available when needed, or that, if available, we will obtain such financing on terms that are favorable to our stockholders or us. In particular, the current instability in the global financial markets and lack of liquidity in the credit and capital markets may adversely affect our ability to secure adequate capital to support our future operations. In the event that additional funds are obtained through arrangements with collaborative partners or other sources, such arrangements may require us to relinquish rights to some of our technologies, product candidates or products under development, which we would otherwise seek to develop or commercialize ourselves on terms that are less favorable than might otherwise be available. If we are unable to generate meaningful amounts of revenue from future product sales, if any, or cannot otherwise raise sufficient additional funds to support our operations, we may be required to delay, reduce the scope of or eliminate one or more of our development programs and our business and future prospects for revenue and profitability may be harmed.
We incorporated in the Commonwealth of Virginia on September 1, 1992 as HemoTech Sciences, Inc. and filed amended Articles of Incorporation to change our name to Allos Therapeutics, Inc. on October 19, 1994. We reincorporated in Delaware on October 28, 1996. We operate as a single business segment.
17
Table of Contents
Employees
As of February 26, 2009, we had a total of 81 full-time employees. Of those, 50 are engaged in clinical development, regulatory affairs, biostatistics, manufacturing and preclinical development. The remaining 31 are involved in marketing, corporate development, finance, administration and operations.
Available Information
We are located in Westminster, Colorado, a suburb of Denver. Our mailing address is 11080 CirclePoint Road, Suite 200, Westminster, Colorado 80020. Our website address iswww.allos.com; however, information found on our website is not incorporated by reference into this report. Our annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, as well as any amendments to those reports, are available free of charge through our website as soon as reasonably practicable after we file them with, or furnish them to, the Securities and Exchange Commission, or SEC. Once atwww.allos.com, go to Investors/Media and then to SEC Filings to locate copies of such reports. You may also read and copy materials that we file with SEC at the SEC's Public Reference Room at 100 F Street, NE, Washington, DC 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC also maintains a website atwww.sec.gov that contains reports, proxy and information statements and other information regarding us and other issuers that file electronically with the SEC.
ITEM 1A. RISK FACTORS
Our business faces significant risks. These risks include those described below and may include additional risks of which we are not currently aware or that we currently do not believe are material. If any of the events or circumstances described in the following risk factors actually occurs, they may materially harm our business, financial condition, operating results and cash flow. As a result, the market price of our common stock could decline. Additional risks and uncertainties that are not yet identified or that we think are immaterial may also materially harm our business, operating results and financial condition. The following risks should be read in conjunction with the other information set forth in this report.
We have a history of net losses and an accumulated deficit, and we may never generate revenue or achieve or maintain profitability in the future.
Since our inception in 1992, we have not generated any revenue from product sales and have experienced significant net losses and negative cash flows from operations. To date, we have financed our operations primarily through the public and private sale of securities. For the years ended December 31, 2008, 2007 and 2006, we had net losses attributable to common stockholders of $51.7 million, $39.4 million, and $30.2 million, respectively. As of December 31, 2008, we had accumulated a deficit during our development stage of $299.7 million. We have incurred these losses principally from costs incurred in our research and development programs, clinical manufacturing and from our marketing, general and administrative expenses. We expect to continue incurring net losses for the foreseeable future. Our ability to generate revenue and achieve profitability is dependent on our ability, alone or with partners, to successfully complete the development of pralatrexate (PDX), conduct clinical trials, obtain the necessary regulatory approvals, and manufacture and market pralatrexate. We may never generate revenue from product sales or become profitable. We expect to continue to spend substantial amounts on research and development, including amounts spent on conducting clinical trials for pralatrexate, and in preparing for the potential commercial launch of pralatrexate. We may not be able to continue as a going concern if we are unable to generate meaningful amounts of revenue to support our operations or cannot otherwise raise the necessary funds to support our operations.
18
Table of Contents
Our near-term prospects are substantially dependent on pralatrexate (PDX), our lead product candidate. If we are unable to successfully develop and obtain regulatory approval for pralatrexate for the treatment of patients with relapsed or refractory PTCL, our ability to generate revenue will be significantly delayed.
We currently have no products that are approved for commercial sale. Our product candidates are in various stages of development, and significant research and development, financial resources and personnel will be required to develop commercially viable products, obtain the necessary regulatory approvals therefor, and successfully commercialize them. Substantially all of our efforts and expenditures over the next few years will be devoted to pralatrexate as we are in the process of closing our current Phase 1 clinical study of RH1 in patients with advanced solid tumors or NHL and determining our future development plans, if any, for RH1. Accordingly, our future prospects are substantially dependent on the successful development, regulatory approval and commercialization of pralatrexate for the treatment of patients with relapsed or refractory PTCL. Even if we receive regulatory approval, pralatrexate is not expected to be commercially available for this or any other indication until at least the second half of 2009. Further, certain of the indications that we are pursuing for pralatrexate have relatively low incidence rates, which may make it difficult for us to enroll a sufficient number of patients in our clinical trials on a timely basis, or at all, and may limit the revenue potential of pralatrexate. If we are unable to successfully develop, obtain regulatory approval for and commercialize pralatrexate for the treatment of patients with relapsed or refractory PTCL, our ability to generate revenue from product sales will be significantly delayed and our stock price would likely decline.
We cannot predict when or if we will obtain regulatory approval to commercialize pralatrexate.
Pralatrexate is in the clinical stage of development and has not been approved for marketing in the United States or any other country. A pharmaceutical product cannot be marketed in the United States or most other countries until it has completed a rigorous and extensive regulatory review and approval process. If we fail to obtain regulatory approval to market pralatrexate, we will be unable to sell pralatrexate and generate revenue, which would jeopardize our ability to continue operating our business. Satisfaction of regulatory requirements typically takes many years, is dependent upon the type, complexity and novelty of the product and requires the expenditure of substantial resources. Of particular significance are the requirements covering research and development, testing, manufacturing, quality control, labeling and promotion of drugs for human use. We may not obtain regulatory approval for pralatrexate, or we may not obtain regulatory review of pralatrexate in a timely manner.
While we have negotiated a special protocol assessment with the FDA relating to our PROPEL trial, this agreement does not guarantee any particular outcome from regulatory review of the trial or the product, including any regulatory approval.
The protocol for the PROPEL trial was reviewed by the FDA under its special protocol assessment, or SPA process, which allows for FDA evaluation of a clinical trial protocol intended to form the primary basis of an efficacy claim in support of a new drug application, and provides an agreement that the study design, including trial size, clinical endpoints and/or data analyses are acceptable to the FDA. However, the SPA agreement is not a guarantee of approval, and we cannot be certain that the design of, or data collected from, the PROPEL trial will be adequate to demonstrate the safety and efficacy of pralatrexate for the treatment of patients with relapsed or refractory PTCL, or otherwise be sufficient to support FDA or any foreign regulatory approval. In addition, the response rate, duration of response and safety profile required to support FDA approval are not specified in the PROPEL trial protocol and will be subject to FDA review. Further, the SPA agreement is not binding on the FDA if public health concerns unrecognized at the time the SPA agreement was entered into become evident, other new scientific concerns regarding product safety or efficacy arise, or if we fail to comply with the agreed upon trial protocols. In addition, the SPA agreement may be changed by us or
19
Table of Contents
the FDA on written agreement of both parties, and the FDA retains significant latitude and discretion in interpreting the terms of the SPA agreement and the data and results from the PROPEL trial. As a result, we do not know how the FDA will interpret the parties' respective commitments under the SPA agreement, how it will interpret the data and results from the PROPEL trial, or whether pralatrexate will receive any regulatory approvals as a result of the SPA agreement or the PROPEL trial. Therefore, despite the potential benefits of the SPA agreement, significant uncertainty remains regarding the clinical development and regulatory approval process for pralatrexate for the treatment of patients with relapsed or refractory PTCL.
Even if pralatrexate meets safety and efficacy endpoints in clinical trials, regulatory authorities may not approve pralatrexate, or we may face post-approval problems that require withdrawal of pralatrexate from the market.
The research, testing, manufacturing, labeling, approval, selling, marketing and distribution of drug products are subject to extensive regulation by the FDA and other regulatory authorities in the United States and other countries, which regulations differ from country to country. We will not be able to commercialize pralatrexate until we have obtained regulatory approval. We have limited experience in filing and pursuing applications necessary to gain regulatory approvals, which may place us at risk of delays, overspending and human resources inefficiencies.
Pralatrexate may not be approved even if it achieves its endpoints in clinical trials. Regulatory agencies, including the FDA, or their advisors, may disagree with our interpretations of data from preclinical studies and clinical trials. The FDA has substantial discretion in the approval process, and when or whether regulatory approval will be obtained for any drug we develop. For example, even though we established an SPA with the FDA for our PROPEL trial, there is no guarantee that the data generated from the PROPEL trial will be adequate to support FDA approval. Regulatory agencies also may approve a product candidate for fewer conditions than requested or may grant approval subject to the performance of post-marketing studies or risk evaluation and mitigation strategies (REMS) for a product candidate. In addition, regulatory agencies may not approve the labeling claims that are necessary or desirable for the successful commercialization of pralatrexate.
Even if we receive regulatory approval, pralatrexate may later produce adverse events that limits or prevents its widespread use or that force us to withdraw pralatrexate from the market. In addition, a marketed product continues to be subject to strict regulation after approval and may be required to undergo post-approval studies. Any unforeseen problems with an approved product or any violation of regulations could result in restrictions on the product, including its withdrawal from the market. Any delay in or failure to receive or maintain regulatory approval for pralatrexate could harm our business and prevent us from ever generating meaningful revenues or achieving profitability.
Even if we receive regulatory approval for pralatrexate, we will be subject to ongoing regulatory obligations and review.
Following any regulatory approval of pralatrexate, we will be subject to continuing regulatory obligations such as safety reporting requirements and additional post-marketing obligations, including regulatory oversight of the promotion and marketing of pralatrexate. In addition, we or our third-party manufacturers will be required to adhere to regulations setting forth the FDA's current Good Manufacturing Practices, or cGMP. These regulations cover all aspects of the manufacturing, storage, testing, quality control and record keeping relating to pralatrexate. Furthermore, we or our third-party manufacturers must pass a pre-approval inspection of manufacturing facilities by the FDA and foreign authorities before obtaining marketing approval and will be subject to periodic inspection by these regulatory authorities to ensure strict compliance with cGMP or other applicable government regulations and corresponding foreign standards. We do not have control over a third-party manufacturer's compliance with these regulations and standards. Such inspections may result in
20
Table of Contents
compliance issues that could prevent or delay marketing approval, or require the expenditure of substantial financial or other resources to address. If we or our third-party manufacturers fail to comply with applicable regulatory requirements, we may be subject to fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
We will need to raise additional capital to support our future operations. If we fail to obtain the capital necessary to fund our operations, we will be unable to successfully develop or commercialize pralatrexate.
Based upon the current status of our product development plans, we believe that our cash, cash equivalents, and investments in marketable securities as of December 31, 2008 should be adequate to support our operations through at least the next 12 months, although there can be no assurance that this can, in fact, be accomplished. We anticipate continuing our current development programs and/or beginning other long-term development projects involving pralatrexate. These projects may require many years and substantial expenditures to complete and may ultimately be unsuccessful. In addition, we intend to submit an NDA for pralatrexate for the treatment of patients with relapsed or refractory PTCL in the first half of 2009. We expect to incur significant costs relating to preparations for the potential commercial launch of pralatrexate, including pre-commercial scale up of manufacturing and development of sales and marketing capabilities. Therefore, we will need to raise additional capital to support our future operations. Our actual capital requirements will depend on many factors, including:
- •
- the timing and outcome of our planned NDA for pralatrexate for the treatment of patients with relapsed or refractory PTCL;
- •
- the timing and costs associated with developing sales and marketing capabilities and commercializing pralatrexate, if it is approved for marketing;
- •
- the timing and costs associated with manufacturing clinical and commercial supplies of pralatrexate;
- •
- the timing and amount of revenues generated by our business activities, if any;
- •
- the timing and costs associated with conducting preclinical and clinical development of pralatrexate, as well as our evaluation of, and decisions with respect to, additional therapeutic indications for which we may develop pralatrexate;
- •
- the timing, costs and potential revenue associated with any co-promotion or other partnering arrangements entered into to commercialize pralatrexate, if it is approved for marketing; and
- •
- our evaluation of, and decisions with respect to, potential in-licensing or product acquisition opportunities or other strategic alternatives.
We may seek to obtain this additional capital through equity or debt financings, arrangements with corporate partners, or from other sources. Such financings or arrangements, if successfully consummated, may be dilutive to our existing stockholders. However, there is no assurance that additional financing will be available when needed, or that, if available, we will obtain such financing on terms that are favorable to our stockholders or us. In particular, the current instability in the global financial markets and lack of liquidity in the credit and capital markets may adversely affect our ability to secure adequate capital to support our future operations. In the event that additional funds are obtained through arrangements with collaborative partners or other sources, such arrangements may require us to relinquish rights to some of our technologies, product candidates or products under development that we might otherwise seek to develop or commercialize ourselves on terms that are less favorable than might otherwise be available. If we are unable to generate meaningful amounts of revenue from future product sales, if any, or cannot otherwise raise sufficient additional funds to support our operations, we may be required to delay, reduce the scope of or eliminate one or more of our development programs and our business and future prospects for revenue and profitability may be harmed.
21
Table of Contents
Budget constraints may force us to delay our efforts to develop pralatrexate for certain indications in favor of developing it for other indications, which may prevent us from commercializing pralatrexate for all desired indications as quickly as possible.
Because we have limited resources, and because research and development is an expensive process, we must regularly assess the most efficient allocation of our research and development budget. As a result, we may have to prioritize development of pralatrexate for certain indications and may not be able to fully realize the value of pralatrexate for other indications in a timely manner, if at all.
If pralatrexate fails to meet safety and efficacy endpoints in clinical trials, it will not receive regulatory approval and we will be unable to market pralatrexate.
Pralatrexate may not prove to be safe and efficacious in clinical trials and may not meet all of the applicable regulatory requirements needed to receive regulatory approval. The clinical development and regulatory approval process is expensive and takes many years. Failure can occur at any stage of development, and the timing of any regulatory approval cannot be accurately predicted. In addition, failure to comply with the FDA and other applicable U.S. and foreign regulatory requirements applicable to clinical trials may subject us to administrative or judicially imposed sanctions.
As part of the regulatory process, we must conduct clinical trials for pralatrexate and any other product candidate to demonstrate safety and efficacy to the satisfaction of the FDA and other regulatory authorities abroad. The number and design of clinical trials that will be required varies depending on the product candidate, the condition being evaluated, the trial results and regulations applicable to any particular product candidate. The design of our pralatrexate clinical trials is based on many assumptions about the expected effect of pralatrexate, and if those assumptions prove incorrect, the clinical trials may not demonstrate the safety or efficacy of pralatrexate. Preliminary results may not be confirmed upon full analysis of the detailed results of a trial, and prior clinical trial program designs and results may not be predictive of future clinical trial designs or results. Product candidates in later stage clinical trials may fail to show the desired safety and efficacy despite having progressed through initial clinical trials with acceptable endpoints. For example, we terminated the development of EFAPROXYN, one of our former product candidates, when it failed to demonstrate statistically significant improvement in overall survival in the targeted patients in a Phase 3 clinical trial. If pralatrexate fails to show clinically significant benefits, it will not be approved for marketing.
Even if we achieve positive interim results in clinical trials, these results do not necessarily predict final results, and acceptable results in early trials may not be repeated in later trials. Data obtained from preclinical and clinical activities are susceptible to varying interpretations that could delay, limit or prevent regulatory clearances, and the FDA can request that we conduct additional clinical trials. A number of companies in the pharmaceutical industry have suffered significant setbacks in advanced clinical trials, even after promising results in earlier trials. In addition, negative or inconclusive results or adverse medical events during a clinical trial could cause a clinical trial to be repeated or terminated. Also, failure to construct clinical trial protocols to screen patients for risk profile factors relevant to the trial for purposes of segregating patients into the patient populations treated with the drug being tested and the control group could result in either group experiencing a disproportionate number of adverse events and could cause a clinical trial to be repeated or terminated. If we have to conduct additional clinical trials for pralatrexate, it would significantly increase our expenses and delay potential marketing of pralatrexate.
We announced the final results from our pivotal Phase 2 PROPEL trial in February 2009. Based on the results of the trial, we intend to submit an NDA to the FDA for pralatrexate for the treatment of patients with relapsed or refractory PTCL in the first half of 2009. We cannot assure you that the design of, or data collected from, the PROPEL trial will be adequate to demonstrate the safety and efficacy of pralatrexate for the treatment of patients with relapsed or refractory PTCL, or otherwise be
22
Table of Contents
sufficient to support FDA or any foreign regulatory approval. The FDA may disagree with our interpretation of the results of the trial and determine that the data are not sufficient to support approval. If we fail to obtain regulatory approval for pralatrexate, we will be unable to market and sell pralatrexate and therefore may never generate meaningful amounts of revenue or become profitable.
We may experience delays in our clinical trials that could adversely affect our financial position and our commercial prospects.
We do not know when our current clinical trials will be completed, if at all. We also cannot accurately predict when other planned clinical trials will begin or be completed. Many factors affect patient enrollment, including the size of the patient population, the proximity of patients to clinical sites, the eligibility criteria for the trial, competing clinical trials and new drugs approved for the conditions we are investigating. Other companies are conducting clinical trials and have announced plans for future trials that are seeking or likely to seek patients with the same diseases as those we are studying. Competition for patients in some cancer trials is particularly intense because of the limited number of leading specialist physicians and the geographic concentration of major clinical centers.
As a result of the numerous factors that can affect the pace of progress of clinical trials, our trials may take longer to enroll patients than we anticipate, if they can be completed at all. Delays in patient enrollment in the trials may increase our costs and slow our product development and approval process. Our product development costs will also increase if we need to perform more or larger clinical trials than planned. If other companies' product candidates show favorable results, we may be required to conduct additional clinical trials to address changes in treatment regimens or for our products to be commercially competitive. Any delays in completing our clinical trials will delay our ability to generate revenue from product sales, and we may have insufficient capital resources to support our operations. Even if we do have sufficient capital resources, our ability to become profitable will be delayed.
We may be required to suspend, repeat or terminate our clinical trials if they are not conducted in accordance with regulatory requirements, the results are negative or inconclusive or the trials are not well designed.
Clinical trials must be conducted in accordance with the FDA's current Good Clinical Practices or other applicable foreign government guidelines and are subject to oversight by the FDA, other foreign governmental agencies and Institutional Review Boards at the medical institutions where the clinical trials are conducted. In addition, clinical trials must be conducted with product candidates produced under cGMP and may require large numbers of test subjects. Clinical trials may be suspended by the FDA, other foreign governmental agencies, or us for various reasons, including:
- •
- deficiencies in the conduct of the clinical trials, including failure to conduct the clinical trial in accordance with regulatory requirements or clinical protocols;
- •
- deficiencies in the clinical trial operations or trial sites;
- •
- the product candidate may have unforeseen adverse side effects;
- •
- the time required to determine whether the product candidate is effective may be longer than expected;
- •
- fatalities or other adverse events arising during a clinical trial due to medical problems that may not be related to clinical trial treatments;
- •
- the product candidate may not appear to be more effective than current therapies;
- •
- the quality or stability of the product candidate may fall below acceptable standards; or
- •
- we may not be able to produce sufficient quantities of the product candidate to complete the trials.
23
Table of Contents
In addition, changes in regulatory requirements and guidance may occur and we may need to amend clinical trial protocols to reflect these changes. Amendments may require us to resubmit our clinical trial protocols to Institutional Review Boards for reexamination, which may impact the costs, timing or successful completion of a clinical trial. Due to these and other factors, pralatrexate could take a significantly longer time to gain regulatory approval than we expect or may never gain approval, which could reduce or eliminate our revenue by delaying or terminating the potential commercialization of pralatrexate.
Reports of adverse events or safety concerns involving pralatrexate or in related technology fields or other companies' clinical trials could delay or prevent us from obtaining regulatory approval or negatively impact public perception of pralatrexate.
Pralatrexate may produce serious adverse events. These adverse events could interrupt, delay or halt clinical trials of pralatrexate and could result in the FDA or other regulatory authorities denying approval of pralatrexate for any or all targeted indications. An independent data safety monitoring board, the FDA, other regulatory authorities or we may suspend or terminate clinical trials at any time. We cannot assure you that pralatrexate or any other product candidate will be safe for human use.
At present, there are a number of clinical trials being conducted by other pharmaceutical companies involving small molecule chemotherapeutic agents. If other pharmaceutical companies announce that they observed frequent adverse events or unknown safety issues in their trials involving compounds similar to, or competitive with, pralatrexate, we could encounter delays in the timing of our clinical trials or difficulties in obtaining the approval of pralatrexate. In addition, the public perception of pralatrexate might be adversely affected, which could harm our business and results of operations and cause the market price of our common stock to decline, even if the concern relates to another company's product or product candidate.
Due to our reliance on contract research organizations and other third parties to conduct our clinical trials, we are unable to directly control the timing, conduct and expense of our clinical trials.
We rely primarily on third parties to conduct our clinical trials, including the PROPEL trial. As a result, we have had and will continue to have less control over the conduct of our clinical trials, the timing and completion of the trials, the required reporting of adverse events and the management of data developed through the trial than would be the case if we were relying entirely upon our own staff. Communicating with outside parties can also be challenging, potentially leading to mistakes as well as difficulties in coordinating activities. Outside parties may have staffing difficulties, may undergo changes in priorities or may become financially distressed, any of which may adversely affect their willingness or ability to conduct our trials. We may experience unexpected cost increases that are beyond our control. Problems with the timeliness or quality of the work of a contract research organization may lead us to seek to terminate the relationship and use an alternative service provider. However, making this change may be costly and may delay our trials, and contractual restrictions may make such a change difficult or impossible. Additionally, it may be impossible to find a replacement organization that can conduct our trials in an acceptable manner and at an acceptable cost.
We do not have manufacturing facilities or capabilities and are dependent on third parties to fulfill our manufacturing needs, which could result in the delay of clinical trials, regulatory approvals, product introductions and commercial sales.
We are dependent on third parties for the manufacture and storage of pralatrexate for clinical trials and, if approved, for commercial sale. If we are unable to contract for a sufficient supply of pralatrexate on acceptable terms, or if we encounter delays or difficulties in the manufacturing process or our relationships with our manufacturers, we may not have sufficient product to conduct or
24
Table of Contents
complete our clinical trials or support commercial requirements for pralatrexate, if it is approved for marketing.
Pralatrexate is cytotoxic, which requires the manufacturers of pralatrexate to have specialized equipment and safety systems to handle such a substance. In addition, the starting materials for pralatrexate require custom preparations, which will require us to manage an additional set of suppliers to obtain the needed supplies of pralatrexate.
Given our lack of formal supply agreements and the fact that in many cases our components are supplied by a single source, our third party suppliers may not be able to fulfill our potential commercial needs or meet our deadlines, or the components they supply to us may not meet our specifications and quality policies and procedures. If we need to find an alternative supplier of pralatrexate or its components, we may not be able to contract for those components on acceptable terms, if at all. Any such failure to supply or delay caused by such suppliers would have an adverse effect on our ability to continue clinical development of pralatrexate or commercialize pralatrexate, if it is approved for marketing.
Even if we obtain approval to market pralatrexate in one or more indications, our current or future manufacturers may be unable to accurately and reliably manufacture commercial quantities of pralatrexate at reasonable costs, on a timely basis and in compliance with the FDA's cGMP. If our current or future contract manufacturers fail in any of these respects, our ability to timely complete our clinical trials, obtain required regulatory approvals and successfully commercialize pralatrexate will be materially and adversely affected. This risk may be heightened with respect to pralatrexate as there are a limited number of fill/finish manufacturers with the ability to handle cytotoxic products such as pralatrexate. Our reliance on contract manufacturers exposes us to additional risks, including:
- •
- our current and future manufacturers are subject to ongoing, periodic, unannounced inspections by the FDA and corresponding state and international regulatory authorities for compliance with strictly enforced cGMP regulations and similar state and foreign standards, and we do not have control over our contract manufacturers' compliance with these regulations and standards;
- •
- our manufacturers may not be able to comply with applicable regulatory requirements, which would prohibit them from manufacturing products for us;
- •
- our manufacturers may have staffing difficulties, may undergo changes in control or may become financially distressed, adversely affecting their willingness or ability to manufacture products for us;
- •
- our manufacturers might not be able to fulfill our commercial needs, which would require us to seek new manufacturing arrangements and may result in substantial delays in meeting market demands;
- •
- if we need to change to other commercial manufacturing contractors, the FDA and comparable foreign regulators must approve our use of any new manufacturer, which would require additional testing, regulatory filings and compliance inspections, and the new manufacturers would have to be educated in, or themselves develop substantially equivalent processes necessary for, the production of our products; and
- •
- we may not have intellectual property rights, or may have to share intellectual property rights, to any improvements in the manufacturing processes or new manufacturing processes for our products.
Any of these factors could result in the delay of clinical trials, regulatory submissions, required approvals or commercialization of pralatrexate. They could also entail higher costs and result in our being unable to effectively commercialize pralatrexate.
25
Table of Contents
If we are unable to effectively protect our intellectual property, we will be unable to prevent third parties from using our technology, which would impair our competitiveness and ability to commercialize pralatrexate. In addition, enforcing our proprietary rights may be expensive and result in increased losses.
Our success will depend in part on our ability to obtain and maintain meaningful patent protection for pralatrexate, both in the United States and in other countries. We rely on patents to protect a large part of our intellectual property and our competitive position. Any patents issued to or licensed by us could be challenged, invalidated, infringed, circumvented or held unenforceable, based on, among other things, obviousness, inequitable conduct, anticipation or enablement. In addition, it is possible that no patents will issue on any of our licensed patent applications. It is possible that the claims in patents that have been issued or licensed to us or that may be issued or licensed to us in the future will not be sufficiently broad to protect our intellectual property or that the patents will not provide protection against competitive products or otherwise be commercially valuable. Failure to obtain and maintain adequate patent protection for our intellectual property would impair our ability to be commercially competitive.
Our commercial success will also depend in part on our ability to commercialize pralatrexate without infringing patents or other proprietary rights of others or breaching the licenses granted to us. We may not be able to obtain a license to third-party technology that we may require to conduct our business or, if obtainable, we may not be able to license such technology at a reasonable cost. If we fail to obtain a license to any technology that we may require to commercialize pralatrexate, or fail to obtain a license at a reasonable cost, we will be unable to commercialize pralatrexate or to commercialize it at a price that will allow us to become profitable.
In addition to patent protection, we also rely upon trade secrets, proprietary know-how and technological advances that we seek to protect through confidentiality agreements with our collaborators, employees, advisors and consultants. Our employees and consultants are required to enter into confidentiality agreements with us. We also enter into non-disclosure agreements with our collaborators and vendors, which agreements are intended to protect our confidential information delivered to third parties for research and other purposes. However, these agreements could be breached and we may not have adequate remedies for any breach, or our trade secrets and proprietary know-how could otherwise become known or be independently discovered by others.
Furthermore, as with any pharmaceutical company, our patent and other proprietary rights are subject to uncertainty. Our patent rights related to pralatrexate might conflict with current or future patents and other proprietary rights of others. For the same reasons, the products of others could infringe our patents or other proprietary rights. Litigation or patent interference proceedings, either of which could result in substantial costs to us, may be necessary to enforce any of our patents or other proprietary rights, or to determine the scope and validity or enforceability of other parties' proprietary rights. We may be dependent on third parties, including our licensors, for cooperation and information that may be required in connection with the defense and prosecution of our patents and other proprietary rights. The defense and prosecution of patent and intellectual property infringement claims are both costly and time consuming, even if the outcome is favorable to us. Any adverse outcome could subject us to significant liabilities to third parties, require disputed rights to be licensed from third parties, or require us to cease selling our future products. We are not currently a party to any patent or other intellectual property infringement claims.
We may explore strategic partnerships that may never materialize or may fail.
We may, in the future, periodically explore a variety of possible strategic partnerships in an effort to gain access to additional product candidates or resources. At the current time, we cannot predict what form such a strategic partnership might take. We are likely to face significant competition in seeking appropriate strategic partners, and these strategic partnerships can be complicated and time
26
Table of Contents
consuming to negotiate and document. We may not be able to negotiate strategic partnerships on acceptable terms, or at all. We are unable to predict when, if ever, we will enter into any additional strategic partnerships because of the numerous risks and uncertainties associated with establishing strategic partnerships.
If we enter into one or more strategic partnerships, we may be required to relinquish important rights to and control over the development of pralatrexate or otherwise be subject to unfavorable terms.
Any future strategic partnerships we enter into could subject us to a number of risks, including:
- •
- we may be required to undertake the expenditure of substantial operational, financial and management resources in integrating new businesses, technologies and products;
- •
- we may be required to issue equity securities that would dilute our existing stockholders' percentage ownership;
- •
- we may be required to assume substantial actual or contingent liabilities;
- •
- we may not be able to control the amount and timing of resources that our strategic partners devote to the development or commercialization of pralatrexate;
- •
- strategic partners may delay clinical trials, provide insufficient funding, terminate a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new version of a product candidate for clinical testing;
- •
- strategic partners may not pursue further development and commercialization of products resulting from the strategic partnering arrangement or may elect to discontinue research and development programs;
- •
- strategic partners may not commit adequate resources to the marketing and distribution of pralatrexate or any other products, limiting our potential revenues from these products;
- •
- disputes may arise between us and our strategic partners that result in the delay or termination of the research, development or commercialization of pralatrexate or any other product candidate or that result in costly litigation or arbitration that diverts management's attention and consumes resources;
- •
- strategic partners may experience financial difficulties;
- •
- strategic partners may not properly maintain or defend our intellectual property rights or may use our proprietary information in a manner that could jeopardize or invalidate our proprietary information or expose us to potential litigation;
- •
- business combinations or significant changes in a strategic partner's business strategy may also adversely affect a strategic partner's willingness or ability to complete its obligations under any arrangement;
- •
- strategic partners could independently move forward with a competing product candidate developed either independently or in collaboration with others, including our competitors; and
- •
- strategic partners could terminate the arrangement or allow it to expire, which would delay the development and may increase the cost of developing pralatrexate or any other product candidate.
27
Table of Contents
Acceptance of pralatrexate in the marketplace is uncertain, and failure to achieve market acceptance will limit our ability to generate revenue and become profitable.
Even if pralatrexate is approved for marketing, pralatrexate may not achieve market acceptance. The degree of market acceptance will depend upon a number of factors, including:
- •
- the receipt of timely regulatory approval for the uses that we are studying;
- •
- the establishment and demonstration in the medical community of the safety and efficacy of pralatrexate and its potential advantages over existing and newly developed therapeutic products;
- •
- ease of use of pralatrexate;
- •
- reimbursement and coverage policies of government and private payors such as Medicare, Medicaid, insurance companies, health maintenance organizations and other plan administrators; and
- •
- the scope and effectiveness of our sales and marketing efforts.
Physicians, patients, payors or the medical community in general may be unwilling to accept, utilize or recommend the use of pralatrexate.
The status of reimbursement from third-party payors for newly approved health care drugs is uncertain and failure to obtain adequate coverage and reimbursement could limit our ability to generate revenue.
Our ability to successfully commercialize pralatrexate will depend, in part, on the extent to which coverage and reimbursement for pralatrexate will be available from government and health administration authorities, private health insurers, managed care programs, and other third-party payors.
Significant uncertainty exists as to the reimbursement status of newly approved health care products. Third-party payors, including Medicare, are challenging the prices charged for medical products and services. Government and other third-party payors increasingly are attempting to contain health care costs by limiting both coverage and the level of reimbursement for new drugs and by refusing, in some cases, to provide coverage for uses of approved products for disease conditions for which the FDA has not granted labeling approval. Third-party insurance coverage may not be available to patients for pralatrexate. If government and other third-party payors do not provide adequate coverage and reimbursement levels for pralatrexate, pralatrexate's market acceptance may be reduced.
Health care reform measures could adversely affect our business.
The business and financial condition of pharmaceutical and biotechnology companies are affected by the efforts of governmental and third-party payors to contain or reduce the costs of health care. In the United States and in foreign jurisdictions there have been, and we expect that there will continue to be, a number of legislative and regulatory proposals aimed at changing the health care system. For example, in some countries other than the United States, pricing of prescription drugs is subject to government control, and we expect proposals to implement similar controls in the United States to continue. We are unable to predict what additional legislation or regulation, if any, relating to the health care industry or third-party coverage and reimbursement may be enacted in the future or what effect such legislation or regulation would have on our business. The pendency or approval of such proposals or reforms could result in a decrease in our stock price or limit our ability to raise capital or to obtain strategic partnerships or licenses.
28
Table of Contents
We may not obtain orphan drug exclusivity or we may not receive the full benefit of orphan drug exclusivity even if we obtain such exclusivity.
The FDA has awarded orphan drug status to pralatrexate for the treatment of patients with T-cell lymphoma, follicular lymphoma and diffuse large B-cell lymphoma. Under the Orphan Drug Act, if we are the first company to receive FDA approval for pralatrexate for the designated orphan drug indication, we will obtain seven years of marketing exclusivity during which the FDA may not approve another company's application for pralatrexate for the same orphan indication. Orphan drug exclusivity would not prevent FDA approval of a different drug for the orphan indication or the same drug for a different indication.
If we fail to comply with healthcare fraud and abuse laws, we could face substantial penalties and our business, operations and financial condition could be adversely affected.
As a biopharmaceutical company, even though we do not and will not control referrals of health care services or bill directly to Medicare, Medicaid or other third-party payors, certain federal and state healthcare laws and regulations pertaining to fraud and abuse will be applicable to our business. These laws and regulations, include, among others:
- •
- the federal Anti-Kickback statute, which prohibits, among other things, persons from soliciting, receiving or providing remuneration, directly or indirectly, to induce either the referral of an individual for an item or service or the purchasing or ordering of a good or service, for which payment may be made under federal health care programs such as the Medicare and Medicaid programs;
- •
- federal false claims laws that prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent;
- •
- the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which prohibits executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters and which also imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information;
- •
- federal self-referral laws, such as STARK, which prohibits a physician from making a referral to a provider of certain health services with which the physician or the physician's family member has a financial interest; and
- •
- state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payor, including commercial insurers, and state laws governing the privacy of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA.
Although there are a number of statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution under the federal Anti-Kickback statute, the exemptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not qualify for an exemption or safe harbor. Our practices may not in all cases meet all of the criteria for safe harbor protection from anti-kickback liability.
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines and the curtailment or restructuring of our operations. Any penalties, damages, fines, curtailment or restructuring of our operations could adversely affect our ability to
29
Table of Contents
operate our business and our financial results. Although compliance programs can mitigate the risk of investigation and prosecution for violations of these laws, the risks cannot be entirely eliminated. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management's attention from the operation of our business. Moreover, achieving and sustaining compliance with all applicable federal and state fraud and abuse laws may be costly.
If we are unable to develop adequate sales, marketing or distribution capabilities or enter into agreements with third parties to perform some of these functions, we will not be able to commercialize pralatrexate effectively.
We have limited experience in sales, marketing and distribution. If it is approved for marketing, we intend to build commercialize pralatrexate by building an oncology-focused U.S. sales and marketing organization that may be complemented by co-promotion arrangements with pharmaceutical or biotechnology partners, where appropriate. We intend to enter into co-promotion or out-licensing arrangements with other pharmaceutical or biotechnology partners where necessary to reach foreign market segments that are not reachable by a U.S.-based sales force or when deemed strategically and economically advisable. To directly market and distribute pralatrexate, we must build a sales and marketing organization with appropriate technical expertise and distribution capabilities. We may not be able to establish sales, marketing and distribution capabilities of our own or enter into such arrangements with third parties in a timely manner or on acceptable terms. To the extent that we enter into co-promotion or other licensing arrangements, our product revenues are likely to be lower than if we directly marketed and sold pralatrexate, and some or all of the revenues we receive will depend upon the efforts of third parties, and these efforts may not be successful. Additionally, building marketing and distribution capabilities may be more expensive than we anticipate, requiring us to divert capital from other intended purposes or preventing us from building our marketing and distribution capabilities to the desired levels.
If our competitors develop and market products that are more effective than pralatrexate, our commercial opportunity will be reduced or eliminated.
Even if we obtain the necessary regulatory approvals to market pralatrexate, our commercial opportunity will be reduced or eliminated if our competitors develop and market products that are more effective, have fewer side effects or are less expensive than pralatrexate. Our potential competitors include large, fully-integrated pharmaceutical companies and more established biotechnology companies, both of which have significant resources and expertise in research and development, manufacturing, testing, obtaining regulatory approvals and marketing. Academic institutions, government agencies, and other public and private research organizations conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and marketing. It is possible that competitors will succeed in developing technologies that are more effective than those being developed by us or that would render our technology obsolete or noncompetitive.
If product liability lawsuits are successfully brought against us, we may incur substantial liabilities and may be required to limit commercialization of pralatrexate.
The testing and marketing of pharmaceutical products entail an inherent risk of product liability. Product liability claims might be brought against us by consumers or health care providers or by pharmaceutical companies or others selling our future products. If we cannot successfully defend ourselves against such claims, we may incur substantial liabilities or be required to limit the commercialization of our pralatrexate. We have obtained limited product liability insurance coverage for our human clinical trials. However, product liability insurance coverage is becoming increasingly
30
Table of Contents
expensive, and we may be unable to maintain such insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to product liability. A successful product liability claim in excess of our insurance coverage could have a material adverse effect on our business, financial condition and results of operations. We may not be able to obtain commercially reasonable product liability insurance for pralatrexate, if it is approved for marketing.
We are currently involved in a securities class action litigation, which could harm our business if management attention is diverted or the claims are decided against us.
We were named as a defendant in a purported securities class action lawsuit filed in May 2004 seeking unspecified damages relating to the issuance of allegedly false and misleading statements regarding EFAPROXYN during the period from May 29, 2003 to April 29, 2004 and subsequent declines in our stock price. In an opinion dated October 20, 2005, the U.S. District Court for the District of Colorado concluded that the plaintiffs' complaint failed to meet the legal requirements applicable to its alleged claims and dismissed the lawsuit. On November 20, 2005, the plaintiffs appealed the District Court's decision to the U.S. Court of Appeals for the Tenth Circuit. On February 6, 2008, the parties signed a stipulation of settlement, settling the case for $2,000,000. The settlement was subject to various conditions, including without limitation approval of the District Court. On January 29, 2009, the District Court issued its Order and Final Judgment approving the settlement, including the releases of the defendants for which the settlement provided. Neither we nor our former officer, who was also named as a defendant, admitted any liability in connection with the settlement. The amount of the settlement in excess of our deductible was covered by our insurance carrier. In the event the District Court's approval of the settlement is appealed and the settlement does not become final, we would intend to vigorously defend against the plaintiffs' appeal. If the Court of Appeals then were to reverse the District Court's decision and we were not successful in our defense against the plaintiffs' claims, we could be forced to make significant payments to the plaintiffs, and such payments could have a material adverse effect on our business, financial condition, results of operations and cash flows to the extent such payments are not covered by our insurance carriers. Even if our defense against such claims were successful, the litigation could result in substantial costs and divert management's attention and resources, which could adversely affect our business. As of December 31, 2008, we have recorded $2,000,000 in accrued litigation settlement costs, which represents our best estimate of the potential gross amount of the settlement costs to be paid to the plaintiffs, and $2,000,000 in prepaid expenses and other assets, which represents the approximately $235,000 of remaining deductible under our insurance policy paid by us and $1,765,000 paid by our insurance carrier into the settlement fund escrow in September 2008. A claims administrator appointed by the parties will administer the distribution of the settlement fund to authorized claimants in 2009.
Our success depends on retention of our President and Chief Executive Officer, Chief Medical Officer and other key personnel.
We are highly dependent on our President and Chief Executive Officer, Paul L. Berns, our Chief Medical Officer, Pablo J. Cagnoni, M.D., and other members of our management team. We are named as the beneficiary on a term life insurance policy covering Mr. Berns in the amount of $10.0 million. We also depend on academic collaborators for each of our research and development programs. The loss of any of our key employees or academic collaborators could delay our discovery research program and the development and commercialization of pralatrexate or result in termination of our pralatrexate development program in its entirety. Mr. Berns and Dr. Cagnoni, as well as others on our executive management team, have employment agreements with us, but the agreements provide for "at-will" employment with no specified term. Our future success also will depend in large part on our continued ability to attract and retain other highly qualified scientific, technical and management personnel, as well as personnel with expertise in clinical testing, governmental regulation and commercialization of pharmaceutical products. We face competition for personnel from other companies, universities, public and private research institutions, government entities and other organizations. If we are unsuccessful in our recruitment and retention efforts, our business will be harmed.
31
Table of Contents
We also rely on consultants, collaborators and advisors to assist us in formulating and conducting our research. All of our consultants, collaborators and advisors are employed by other employers or are self-employed and may have commitments to or consulting contracts with other entities that may limit their ability to contribute to the Company.
We cannot guarantee that we will be in compliance with all potentially applicable regulations.
The development, manufacturing, and, if approved, pricing, marketing, sale and reimbursement of pralatrexate, together with our general operations, are subject to extensive regulation by federal, state and other authorities within the United States and numerous entities outside of the United States. We have fewer employees than many other companies that have one or more product candidates in late stage clinical development and we rely heavily on third parties to conduct many important functions.
As a publicly-traded company, we are subject to significant regulations including the Sarbanes Oxley Act of 2002. We cannot assure you that we are or will be in compliance with all potentially applicable regulations. If we fail to comply with the Sarbanes Oxley Act of 2002 or any other regulations we could be subject to a range of consequences, including restrictions on our ability to sell equity securities or otherwise raise capital funds, the de-listing of our common stock from the Nasdaq Global Market, suspension or termination of our clinical trials, failure to obtain approval to market pralatrexate, restrictions on future products or our manufacturing processes, significant fines, or other sanctions or litigation.
If our internal controls over financial reporting are not considered effective, our business and stock price could be adversely affected.
Section 404 of the Sarbanes-Oxley Act of 2002 requires us to evaluate the effectiveness of our internal controls over financial reporting as of the end of each fiscal year, and to include a management report assessing the effectiveness of our internal controls over financial reporting in this annual report on Form 10-K for that fiscal year. Section 404 also requires our independent registered public accounting firm to attest to, and report on, management's assessment of our internal controls over financial reporting.
Our management, including our chief executive officer and principal financial officer, does not expect that our internal controls over financial reporting will prevent all error and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system's objectives will be met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud involving a company have been, or will be, detected. The design of any system of controls is based in part on certain assumptions about the likelihood of future events, and we cannot assure you that any design will succeed in achieving its stated goals under all potential future conditions. Over time, controls may become ineffective because of changes in conditions or deterioration in the degree of compliance with policies or procedures. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected. We cannot assure you that we or our independent registered public accounting firm will not identify a material weakness in our internal controls in the future. A material weakness in our internal controls over financial reporting would require management and our independent registered public accounting firm to consider our internal controls as ineffective. If our internal controls over financial reporting are not considered effective, we may experience a loss of public confidence, which could have an adverse effect on our business and on the market price of our common stock.
32
Table of Contents
If we do not progress in our programs as anticipated, our stock price could decrease.
For planning purposes, we estimate the timing of a variety of clinical, regulatory and other milestones, such as when a certain product candidate will enter clinical development, when a clinical trial will be initiated or completed, or when an application for regulatory approval will be filed. Some of our estimates are included in this report. Our estimates are based on information available to us as of the date of this report and a variety of assumptions. Many of the underlying assumptions are outside of our control. If milestones are not achieved when we estimated that they would be, investors could be disappointed, and our stock price may decrease.
Warburg Pincus Private Equity VIII, L.P. and Baker Brothers Life Sciences, L.P. each control a substantial percentage of the voting power of our outstanding common stock.
On March 2, 2005, we entered into a Securities Purchase Agreement with Warburg Pincus Private Equity VIII, L.P., or Warburg, and certain other investors pursuant to which we issued and sold an aggregate of 2,352,443 shares of our Series A Exchangeable Preferred Stock, or the Exchangeable Preferred, at a price per share of $22.10, for aggregate gross proceeds of approximately $52.0 million. On May 18, 2005, at our Annual Meeting of Stockholders, our stockholders voted to approve the issuance of shares of our common stock upon exchange of shares of the Exchangeable Preferred. As a result of such approval, we issued a total of 23,524,430 shares of common stock upon exchange of 2,352,443 shares of Exchangeable Preferred. In connection with its purchase of the Exchangeable Preferred, Warburg entered into a standstill agreement agreeing not to pursue certain activities the purpose or effect of which may be to change or influence the control of the Company.
On February 2, 2007, we closed an underwritten offering of 9,000,000 shares of common stock, of which Baker Brothers Life Sciences, L.P. and certain other affiliated funds, which are collectively referred to hereinafter as "Baker," purchased 3,300,000 shares, at a price per share of $6.00, for aggregate gross proceeds of approximately $54.0 million. In connection therewith, Baker entered into a standstill agreement agreeing not to pursue certain activities the purpose or effect of which may be to change or influence the control of the Company.
On May 29, 2008, we sold 12,420,000 shares of our common stock in an underwritten public offering at a price of $5.64 per share, for aggregate gross proceeds of approximately $70.0 million. Warburg and Baker purchased 3,500,000 and 1,500,000 shares, respectively, of the 12,420,000 shares sold in such public offering.
As of December 31, 2008, we had 81,238,812 shares of common stock outstanding, of which Warburg owned 26,124,430 shares, or approximately 32.2% of the voting power of our outstanding common stock, and Baker owned 11,248,621 shares, or approximately 13.8% of the voting power of our outstanding common stock. Although each of Warburg and Baker have entered into a standstill agreement with us, they are, and will continue to be, able to exercise substantial influence over any actions requiring stockholder approval. According to filings with the SEC, as of February 6, 2009, Baker owns less than 10% of our outstanding common stock.
Anti-takeover provisions in our charter documents and under Delaware law could discourage, delay or prevent an acquisition of us, even if an acquisition would be beneficial to our stockholders, and may prevent attempts by our stockholders to replace or remove our current management.
Provisions of our amended and restated certificate of incorporation and bylaws, as well as provisions of Delaware law, could make it more difficult for a third party to acquire us, even if doing so would benefit our stockholders. In addition, these provisions may make it more difficult for stockholders to replace members of our board of directors. Because our board of directors is responsible for appointing the members of our management team, these provisions could in turn affect
33
Table of Contents
any attempt by our stockholders to replace current members of our management team. These provisions include:
- •
- authorizing the issuance of "blank check" preferred stock that could be issued by our board of directors to increase the number of outstanding shares or change the balance of voting control and thwart a takeover attempt;
- •
- prohibiting cumulative voting in the election of directors, which would otherwise allow for less than a majority of stockholders to elect director candidates;
- •
- prohibiting stockholder action by written consent, thereby requiring all stockholder actions to be taken at a meeting of our stockholders;
- •
- eliminating the ability of stockholders to call a special meeting of stockholders; and
- •
- establishing advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted upon at stockholder meetings.
In addition, we are subject to Section 203 of the Delaware General Corporation Law, which generally prohibits a Delaware corporation from engaging in any of a broad range of business combinations with an interested stockholder for a period of three years following the date on which the stockholder became an interested stockholder. This provision could have the effect of delaying or preventing a change of control, whether or not it is desired by or beneficial to our stockholders. Notwithstanding the foregoing, the three year moratorium imposed on business combinations by Section 203 will not apply to either Warburg or Baker because, prior to the dates on which they became interested stockholders, our board of directors approved the transactions that resulted in Warburg and Baker becoming interested stockholders. However, in connection with its purchase of Exchangeable Preferred in March 2005, Warburg entered into a standstill agreement agreeing not to pursue certain activities the purpose or effect of which may be to change or influence the control of the Company. Similarly, in connection with our February 2007 Financing, Baker entered into a standstill agreement agreeing not to pursue certain activities the purpose or effect of which may be to change or influence the control of the Company.
We have adopted a stockholder rights plan that may discourage, delay or prevent a merger or acquisition that is beneficial to our stockholders.
In May 2003, our board of directors adopted a stockholder rights plan that may have the effect of discouraging, delaying or preventing a merger or acquisition of us that our stockholders may consider beneficial by diluting the ability of a potential acquirer to acquire us. Pursuant to the terms of the stockholder rights plan, when a person or group, except under certain circumstances, acquires 15% or more of our outstanding common stock or 10 business days after announcement of a tender or exchange offer for 15% or more of our outstanding common stock, the rights (except those rights held by the person or group who has acquired or announced an offer to acquire 15% or more of our outstanding common stock) would generally become exercisable for shares of our common stock at a discount. Because the potential acquirer's rights would not become exercisable for our shares of common stock at a discount, the potential acquirer would suffer substantial dilution and may lose its ability to acquire us. In addition, the existence of the plan itself may deter a potential acquirer from acquiring or making an offer to acquire us. As a result, either by operation of the plan or by its potential deterrent effect, mergers and acquisitions of the Company that our stockholders may consider in their best interests may not occur.
Because Warburg owns a substantial percentage of our outstanding common stock, we amended the stockholder rights plan in connection with Warburg's purchase of Exchangeable Preferred in March 2005 to provide that Warburg and its affiliates will be exempt from the stockholder rights plan, unless Warburg and its affiliates become, without the prior consent of our board of directors, the beneficial
34
Table of Contents
owner of more than 44% of our common stock. Likewise, since Baker owns a substantial percentage of our outstanding common stock, we amended the stockholder rights plan in connection with our February 2007 Financing to provide that Baker and its affiliates will be exempt from the stockholder rights plan, unless Baker becomes, without the prior consent of our board of directors, the beneficial owner of more than 20% of our common stock. Under the stockholder rights plan, our board of directors has express authority to amend the rights plan without stockholder approval.
Unstable market conditions may have serious adverse consequences on our business.
The recent economic downturn and market instability has made the business climate more volatile and more costly. Our general business strategy may be adversely affected by unpredictable and unstable market conditions. If the current equity and credit markets deteriorate further, or do not improve, it may make any necessary equity or debt financing more difficult, more costly, and more dilutive. While we believe we have adequate capital resources to meet our expected working capital and capital expenditure requirements for at least the next 12 months, a radical economic downturn or increase in our expenses could require additional financing on less than attractive rates or on terms that are excessively dilutive to existing stockholders. Failure to secure any necessary financing in a timely manner and on favorable terms could have a material adverse effect on our growth strategy, financial performance and stock price and could require us to delay or abandon clinical development plans. There is a risk that one or more of our current service providers, manufacturers or other partners may encounter difficulties during challenging economic times, which could have an adverse effect on our business, results of operations and financial condition.
Our liquidity, capital resources and results of operations may be adversely affected by declines in the value of our investments in marketable securities.
As of December 31, 2008, we had $84.0 million in cash, cash equivalents, and investments in marketable securities. Until required for use in our business, we invest our cash reserves in bank deposits, money market funds, high-grade corporate notes and U.S. government instruments in accordance with our investment policy. The weighted average duration of the remaining time to maturity for our portfolio of investments in marketable securities as of December 31, 2008 was approximately five months. Our investments in marketable securities as of December 31, 2008 primarily consisted of high-grade corporate notes. We did not hold any derivative instruments, foreign exchange contracts, asset backed securities, mortgage backed securities, auction rate securities, or securities of issuers in bankruptcy in our investment portfolio as of December 31, 2008.
Based upon the current status of our product development and commercialization plans, we believe that our cash, cash equivalents, and investments in marketable securities as of December 31, 2008 should be adequate to support our operations through at least the next 12 months, although there can be no assurance that this can, in fact, be accomplished. In particular, our liquidity, capital resources and results of operations may be adversely affected by declines in the value of our investments in marketable securities. The value of our investments in marketable securities may be adversely affected by rating downgrades or bankruptcies affecting the issuers of such securities, whether caused by instability in the global financial markets, lack of liquidity in the credit and capital markets, or other factors. For example, during the three months ended September 30, 2008, we realized a loss of approximately $552,000 on the sale of certain of our investments in marketable securities, which were sold in order to preserve our principal as the issuers of these securities experienced significant deteriorations in their creditworthiness as evidenced by investment rating downgrades. We have the ability and intent to hold our remaining investments in marketable securities as of December 31, 2008 to their scheduled maturity, although we monitor our investment portfolio with the primary objectives of preserving principal and maintaining proper liquidity to meet our operating needs.
35
Table of Contents
The market price for our common stock has been and may continue to be highly volatile, and an active trading market for our common stock may never exist.
We cannot assure you that an active trading market for our common stock will exist at any time. Holders of our common stock may not be able to sell shares quickly or at the market price if trading in our common stock is not active. The trading price of our common stock has been and is likely to continue to be highly volatile and could be subject to wide fluctuations in price in response to various factors, many of which are beyond our control, including:
- •
- actual or anticipated regulatory approvals or non-approvals of pralatrexate or of competing product candidates;
- •
- actual or anticipated results of our clinical trials, involving pralatrexate;
- •
- changes in laws or regulations applicable to pralatrexate;
- •
- changes in the expected or actual timing of our development programs;
- •
- actual or anticipated variations in quarterly operating results;
- •
- announcements of technological innovations by us or our competitors;
- •
- changes in financial estimates or recommendations by securities analysts;
- •
- conditions or trends in the biotechnology and pharmaceutical industries;
- •
- changes in the market valuations of similar companies;
- •
- announcements by us of significant acquisitions, strategic partnerships, joint ventures or capital commitments;
- •
- additions or departures of key personnel;
- •
- disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies;
- •
- developments concerning any of our research and development, manufacturing and marketing collaborations;
- •
- sales of large blocks of our common stock;
- •
- sales of our common stock by our executive officers, directors and five percent stockholders; and
- •
- economic and other external factors, including disasters or crises.
Public companies in general and companies included on the Nasdaq Global Market in particular have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of those companies. There has been particular volatility in the market prices of securities of biotechnology and other life sciences companies, and the market prices of these companies have often fluctuated because of problems or successes in a given market segment or because investor interest has shifted to other segments. These broad market and industry factors may cause the market price of our common stock to decline, regardless of our operating performance. We have no control over this volatility and can only focus our efforts on our own operations, and even these may be affected due to the state of the capital markets. In the past, following large price declines in the public market price of a company's securities, securities class action litigation has often been initiated against that company, including in 2004 against us. Litigation of this type could result in substantial costs and diversion of management's attention and resources, which would hurt our business. Any adverse determination in litigation could also subject us to significant liabilities.
36
Table of Contents
Substantial sales of shares may impact the market price of our common stock.
If our stockholders sell substantial amounts of our common stock, the market price of our common stock may decline. These sales also might make it more difficult for us to sell equity or equity-related securities in the future at a time and price that we consider appropriate. We are unable to predict the effect that sales may have on the then prevailing market price of our common stock. We have entered into a Registration Rights Agreement with Warburg and the other purchasers of our Exchangeable Preferred pursuant to which such investors are entitled to certain registration rights with respect to the shares of common stock that we issued upon exchange of the Exchangeable Preferred.
In addition, we will need to raise substantial additional capital in the future to fund our operations. If we raise additional funds by issuing equity securities, the market price of our common stock may decline and our existing stockholders may experience significant dilution.
ITEM 1B. UNRESOLVED STAFF COMMENTS
Not Applicable.
ITEM 2. PROPERTIES
Our corporate headquarters facility consists of approximately 34,536 square feet in Westminster, Colorado. We lease our corporate headquarters facility pursuant to a lease agreement that expires on January 31, 2012. We also lease an office in Princeton, New Jersey which consists of approximately 9,458 square feet. The lease for this office expires on September 30, 2011. We believe that our leased facilities are adequate to meet our needs until such time, if any, as we receive regulatory approval to market pralatrexate.
ITEM 3. LEGAL PROCEEDINGS
We were named as a defendant in a purported securities class action lawsuit filed in May 2004 seeking unspecified damages relating to the issuance of allegedly false and misleading statements regarding EFAPROXYN during the period from May 29, 2003 to April 29, 2004 and subsequent declines in our stock price. In an opinion dated October 20, 2005, the U.S. District Court for the District of Colorado concluded that the plaintiffs' complaint failed to meet the legal requirements applicable to its alleged claims and dismissed the lawsuit. On November 20, 2005, the plaintiffs appealed the District Court's decision to the U.S. Court of Appeals for the Tenth Circuit. On February 6, 2008, the parties signed a stipulation of settlement, settling the case for $2,000,000. The settlement was subject to various conditions, including without limitation approval of the District Court. On January 29, 2009, the District Court issued its Order and Final Judgment approving the settlement, including the releases of the defendants for which the settlement provided. Neither we nor our former officer, who was also named as a defendant, admitted any liability in connection with the settlement. The amount of the settlement in excess of our deductible was covered by our insurance carrier. In the event the District Court's approval of the settlement is appealed and the settlement does not become final, we would intend to vigorously defend against the plaintiffs' appeal. If the Court of Appeals then were to reverse the District Court's decision and we were not successful in our defense against the plaintiffs' claims, we could be forced to make significant payments to the plaintiffs, and such payments could have a material adverse effect on our business, financial condition, results of operations and cash flows to the extent such payments are not covered by our insurance carriers. Even if our defense against such claims were successful, the litigation could result in substantial costs and divert management's attention and resources, which could adversely affect our business. As of December 31, 2008, we have recorded $2,000,000 in accrued litigation settlement costs, which represents our best estimate of the potential gross amount of the settlement costs to be paid to the plaintiffs, and $2,000,000 in prepaid expenses and other assets, which represents the approximately $235,000 of
37
Table of Contents
remaining deductible under our insurance policy paid by us and $1,765,000 paid by our insurance carrier into the settlement fund escrow in September 2008. A claims administrator appointed by the parties will administer the distribution of the settlement fund to authorized claimants in 2009.
ITEM 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
None.
38
Table of Contents
PART II
ITEM 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information and Holders
Our common stock is traded on the Nasdaq Global Market under the symbol "ALTH." The following table sets forth, for the periods indicated, the high and low sales prices for our common stock as reported on the Nasdaq Global Market:
| | | | | | | |
Year Ended December 31, 2008 | | HIGH | | LOW | |
|---|
First Quarter | | $ | 7.70 | | $ | 4.88 | |
Second Quarter | | $ | 7.15 | | $ | 4.89 | |
Third Quarter | | $ | 10.19 | | $ | 6.61 | |
Fourth Quarter | | $ | 8.86 | | $ | 3.82 | |
| | | | | | | |
Year Ended December 31, 2007 | | HIGH | | LOW | |
|---|
First Quarter | | $ | 8.54 | | $ | 5.75 | |
Second Quarter | | $ | 7.08 | | $ | 3.91 | |
Third Quarter | | $ | 5.90 | | $ | 3.92 | |
Fourth Quarter | | $ | 7.52 | | $ | 4.70 | |
On February 26, 2009, we had approximately 65 holders of record of our common stock.
39
Table of Contents
Stock Performance Measurement Comparison(1)
The following graph shows the total stockholder return of an investment of $100 in cash on December 31, 2003 for (i) the Company's common stock, (ii) the Nasdaq Composite Index and (iii) the Nasdaq Biotechnology Index. All values assume reinvestment of the full amount of all dividends and are calculated as of December 31 of each year:
Comparison of 5 year Cumulative Total Return on Investment
| | | | | | | | | | | | | | | | | | | |
Total Return Analysis | | 12/31/2003 | | 12/31/2004 | | 12/31/2005 | | 12/31/2006 | | 12/31/2007 | | 12/31/2008 | |
|---|
Allos Therapeutics, Inc. | | $ | 100.00 | | $ | 66.85 | | $ | 59.89 | | $ | 162.95 | | $ | 175.21 | | $ | 170.47 | |
NASDAQ Composite | | $ | 100.00 | | $ | 108.59 | | $ | 110.08 | | $ | 120.56 | | $ | 132.39 | | $ | 78.72 | |
NASDAQ Biotechnology | | $ | 100.00 | | $ | 106.13 | | $ | 109.14 | | $ | 110.25 | | $ | 115.30 | | $ | 100.75 | |
- (1)
- The information in this section is not "soliciting material," is not deemed "filed" with the SEC and is not to be incorporated by reference in any filing of the Company under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
Dividends
We have never paid dividends to holders of our common stock, and we do not anticipate paying any cash dividends in the foreseeable future as we intend to retain any earnings for use in our business. Any future determination to pay dividends will be at the discretion of our board of directors and will depend upon our results of operations, financial condition, contractual restrictions, restrictions imposed by applicable law and other factors our board of directors deems relevant.
40
Table of Contents
ITEM 6. SELECTED FINANCIAL DATA
The selected financial data set forth below should be read in conjunction with our financial statements and the related notes and "Management's Discussion and Analysis of Financial Condition and Results of Operations," included in this report. The statement of operations data for the years ended December 31, 2008, 2007, 2006, and cumulative period from September 1, 1992 through December 31, 2008, and the balance sheet data as of December 31, 2008 and 2007, are derived from, and qualified by reference to, our audited financial statements included elsewhere in this report. The statement of operations data for the years ended December 31, 2005 and 2004, and the balance sheet data as of December 31, 2006, 2005 and 2004, are derived from our audited financial statements that do not appear in this report. The historical results are not necessarily indicative of the operating results to be expected in the future.
| | | | | | | | | | | | | | | | | | | | | |
| | Years Ended December 31, | | Cumulative
period from
September 1,
1992 (date
of inception)
through
December 31,
| |
|---|
| | 2008 | | 2007 | | 2006 | | 2005 | | 2004 | | 2008 | |
|---|
| | (in thousands, except share and per share data)
| |
|---|
Statement of Operations Data: | | | | | | | | | | | | | | | | | | | |
Operating expenses: | | | | | | | | | | | | | | | | | | | |
| | Research and development | | $ | 23,848 | | $ | 17,444 | | $ | 14,322 | | $ | 11,215 | | $ | 10,158 | | $ | 149,148 | |
| | Clinical manufacturing | | | 6,747 | | | 5,548 | | | 2,284 | | | 1,266 | | | 2,979 | | | 41,359 | |
| | Marketing, general and administrative | | | 23,044 | | | 19,672 | | | 14,876 | | | 9,044 | | | 9,194 | | | 125,874 | |
| | Restructuring and separation costs | | | — | | | — | | | 646 | | | 380 | | | — | | | 1,664 | |
| | | | | | | | | | | | | | |
| | | Total operating expenses | | | 53,639 | | | 42,664 | | | 32,128 | | | 21,905 | | | 22,331 | | | 318,045 | |
Loss from operations | | | (53,639 | ) | | (42,664 | ) | | (32,128 | ) | | (21,905 | ) | | (22,331 | ) | | (318,045 | ) |
Gain on settlement claims | | | — | | | — | | | — | | | — | | | — | | | 5,110 | |
Interest and other income, net | | | 1,909 | | | 3,294 | | | 1,916 | | | 1,768 | | | 494 | | | 23,512 | |
| | | | | | | | | | | | | | |
| | Net loss | | | (51,730 | ) | | (39,370 | ) | | (30,212 | ) | | (20,137 | ) | | (21,837 | ) | | (289,423 | ) |
Dividend related to beneficial conversion feature of preferred stock | | | — | | | — | | | — | | | (623 | ) | | — | | | (10,236 | ) |
| | | | | | | | | | | | | | |
Net loss attributable to common stockholders | | $ | (51,730 | ) | $ | (39,370 | ) | $ | (30,212 | ) | $ | (20,760 | ) | $ | (21,837 | ) | $ | (299,659 | ) |
| | | | | | | | | | | | | | |
Net loss per share: basic and diluted | | $ | (0.69 | ) | $ | (0.60 | ) | $ | (0.55 | ) | $ | (0.45 | ) | $ | (0.70 | ) | | | |
| | | | | | | | | | | | | | | |
Weighted average shares: basic and diluted | | | 75,399,774 | | | 65,188,913 | | | 55,299,614 | | | 46,070,686 | | | 31,139,192 | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | As of December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | | 2005 | | 2004 | |
|---|
| | (in thousands)
| |
|---|
Balance Sheet Data: | | | | | | | | | | | | | | | | |
Cash, cash equivalents and investments in marketable securities | | $ | 83,965 | | $ | 57,756 | | $ | 32,796 | | $ | 55,282 | | $ | 23,849 | |
Working capital | | | 77,981 | | | 51,958 | | | 28,897 | | | 52,477 | | | 22,745 | |
Total assets | | | 89,340 | | | 61,460 | | | 36,382 | | | 57,081 | | | 26,173 | |
Common stock and additional paid-in capital | | | 379,123 | | | 300,508 | | | 238,109 | | | 231,637 | | | 181,485 | |
Deficit accumulated during the development stage | | | (299,659 | ) | | (247,929 | ) | | (208,559 | ) | | (178,347 | ) | | (157,587 | ) |
Total stockholders' equity | | | 79,464 | | | 52,579 | | | 29,550 | | | 53,290 | | | 23,863 | |
41
Table of Contents
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Overview
We are a biopharmaceutical company focused on developing and commercializing innovative small molecule drugs for the treatment of cancer. Our goal is to build a profitable company by generating income from products we develop and commercialize, either alone or with one or more potential strategic partners. We strive to develop proprietary products that have the potential to improve the standard of care in cancer therapy. Our focus is on product opportunities for oncology that leverage our internal clinical development and regulatory expertise and address important markets with unmet medical need. We may also seek to grow our existing portfolio of product candidates through product acquisition and in-licensing efforts.
We currently have two small molecule chemotherapeutic product candidates in clinical development, pralatrexate (PDX) and RH1.
Pralatrexate
Our lead product candidate, pralatrexate, is a novel targeted antifolate designed to accumulate preferentially in cancer cells. Based on preclinical studies, we believe that pralatrexate selectively enters cells expressing RFC-1, a protein that is over expressed on cancer cells compared to normal cells. Once inside cancer cells, pralatrexate is efficiently polyglutamylated, which leads to high intracellular drug retention. Polyglutamylated pralatrexate essentially becomes "trapped" inside cancer cells, making it less susceptible to efflux-based drug resistance. Acting on the folate pathway, pralatrexate interferes with DNA synthesis and triggers cancer cell death. We believe pralatrexate has the potential to be delivered as a single agent or in combination therapy regimens.
In February 2009, we announced the final results from PROPEL, our pivotal Phase 2 trial of pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma, or PTCL. The trial enrolled a total of 115 patients, 109 of whom were considered evaluable for response according to the trial protocol. The results of the trial demonstrated that 29 of 109 evaluable patients, or 27%, achieved a response as assessed by central independent oncology review, which is the primary endpoint of the trial. The Kaplan-Meier estimate for the median duration of response was 287 days, or 9.4 months. Duration of response is the key secondary endpoint of the trial. The most common grade3/4 adverse events were thrombocytopenia, which was observed in 32% of patients; mucosal inflammation in 21% of patients; neutropenia in 20% of patients; and anemia in 17% of patients.
Based on the results of this trial, we intend to submit a New Drug Application, or NDA, to the U.S. Food and Drug Administration, or FDA, for pralatrexate for the treatment of patients with relapsed or refractory PTCL in the first half of 2009. If it is approved for marketing, we intend to commercialize pralatrexate by building an oncology focused U.S. sales and marketing organization that may be complemented by co-promotion arrangements with pharmaceutical or biotechnology partners, where appropriate. We intend to enter into co-promotion or out-licensing arrangements with other pharmaceutical or biotechnology partners, where necessary to reach foreign market segments that are not reachable by a U.S.-based sales force or when deemed strategically and economically advisable. We currently retain exclusive worldwide commercial rights to pralatrexate for all indications.
The PROPEL trial was conducted under an agreement reached with the FDA under its special protocol assessment, or SPA, process. The SPA process allows for FDA evaluation of a clinical trial protocol intended to form the primary basis of an efficacy claim in support of a NDA, and provides an agreement that the trial design, including trial size, clinical endpoints and data analyses are acceptable to the FDA. However, the SPA agreement is not a guarantee of approval, and we cannot assure you that the design of, or data collected from, the PROPEL trial will be adequate to demonstrate the safety
42
Table of Contents
and efficacy of pralatrexate for the treatment of patients with relapsed or refractory PTCL, or otherwise be sufficient to support FDA or any foreign regulatory approval. For example, the response rate, duration of response and safety profile required to support FDA approval are not specified in the PROPEL trial protocol and will be subject to FDA review. In addition, the median duration of response reported above is a Kaplan-Meier estimate based on the length of follow up for all responders at the time the PROPEL trial database was locked. As a result, the median duration of response may change based on continued patient follow up.
In addition to the PROPEL trial, we are committed to evaluating pralatrexate for oncology use as a single agent and in combination with other therapies. We currently have seven ongoing clinical trials involving pralatrexate, including the PROPEL trial, and plan to initiate additional trials to evaluate pralatrexate's potential clinical utility in other hematologic malignancies and solid tumor indications. The following clinical trials involving pralatrexate are currently open for enrollment:
- •
- a Phase 2b, randomized, multi-center study comparing pralatrexate and Tarceva (erlotinib), both with vitamin B12 and folic acid supplementation, in patients with Stage IIIB/IV non-small cell lung cancer, or NSCLC, who are, or have been, cigarette smokers who have failed treatment with at least one prior platinum-based chemotherapy regimen. We initiated patient enrollment in this study in January 2008. The study will seek to enroll approximately 160 patients in up to 50 investigative sites worldwide. Based on current enrollment rates, we expect to complete patient enrollment in this study in the third quarter of 2009.
- •
- a Phase 2, open-label, single-arm, multi-center study of pralatrexate with vitamin B12 and folic acid supplementation in patients with advanced or metastatic relapsed transitional cell carcinoma, or TCC, of the urinary bladder. We initiated patient enrollment in this study in July 2008. The study will seek to enroll approximately 41 patients in up to 20 investigative sites worldwide.
- •
- a Phase 1/2a, open-label, multi-center study of pralatrexate and gemcitabine with vitamin B12 and folic acid supplementation in patients with relapsed or refractory non-Hodgkin's lymphoma, or NHL, and Hodgkin's disease. We initiated patient enrollment in this study in May 2007. We plan to enroll up to 54 evaluable patients in the Phase 1 portion of the study and up to 45 additional patients with relapsed or refractory PTCL in the expanded Phase 2a portion of the study.
- •
- a Phase 1, open-label, multi-center study of pralatrexate with vitamin B12 and folic acid supplementation in patients with relapsed or refractory cutaneous T-cell lymphoma, or CTCL. We initiated patient enrollment in this study in August 2007. We plan to enroll up to 56 evaluable patients in the study with the objective of determining the optimal dose and safety profile, including at least 20 patients at what we believe to be the optimal dose and schedule.
- •
- a Phase 1/2, open-label, single-center study of pralatrexate with vitamin B12 and folic acid supplementation in patients with relapsed or refractory NHL and Hodgkin's disease. This study is currently focused on exploring alternate dosing and administration schedules in patients with B-cell lymphoma to further evaluate pralatrexate's potential clinical utility in this setting.
In addition to our ongoing NSCLC and bladder cancer studies, we are evaluating the potential future development of pralatrexate for other solid tumor indications, including Stage III/IV head and neck cancer and Stage III/IV breast cancer, among others. There can be no assurances that we will pursue the development of pralatrexate for one or more of these indications or that such development efforts will be ultimately successful.
RH1
RH1 is a small molecule chemotherapeutic agent that we believe is bioactivated by the enzyme DT-diaphorase, or DTD, also known as NAD(P)H quinone oxidoreductase, or NQ01. We believe DTD
43
Table of Contents
is over-expressed in many tumors, relative to normal tissue, including lung, colon, breast and liver tumors. We believe that because RH1 is bioactivated in the presence of DTD, it may have the potential to provide targeted drug delivery to these tumor types while limiting the amount of toxicity to normal tissue.
In November 2007, we initiated patient enrollment in a Phase 1, open-label, multi-center dose escalation study of RH1 in patients with advanced solid tumors or NHL. We are in the process of closing this study and determining our future development plans, if any, for RH1.
EFAPROXYN (efaproxiral) Development Discontinued
In mid-2007, we discontinued the development of EFAPROXYN, one of our former product candidates, after announcing top-line results from ENRICH, a Phase 3 clinical trial of EFAPROXYN plus whole brain radiation therapy, or WBRT, in women with brain metastases originating from breast cancer. The study failed to achieve its primary endpoint of demonstrating a statistically significant improvement in overall survival in patients receiving EFAPROXYN plus WBRT, compared to patients receiving WBRT alone. We are currently pursuing the sale of our rights to EFAPROXYN although we may not receive any material consideration for any sale.
Results of Operations
We are a development stage company. Since our inception in 1992, we have not generated any revenue from product sales and have experienced significant net losses and negative cash flows from operations. We have incurred these losses principally from costs incurred in our research and development programs, clinical manufacturing and from our marketing, general and administrative expenses. Our primary business activities have been focused on the development of pralatrexate, RH1 (a program for which we are in the process of determining our future development plans, if any) and EFAPROXYN (a program which we discontinued in mid-2007). For the years ended December 31, 2008, 2007 and 2006, we had net losses attributable to common stockholders of $51.7 million, $39.4 million, and $30.2 million, respectively. Research and development expenses for the years ended December 31, 2008, 2007 and 2006 were $23.8 million, $17.4 million and $14.3 million, respectively. As of December 31, 2008, we had accumulated a deficit during our development stage of $299.7 million.
Our ability to generate revenue and achieve profitability is dependent on our ability, alone or with partners, to successfully complete the development of pralatrexate, conduct clinical trials, obtain the necessary regulatory approvals, and manufacture and market pralatrexate. The timing and costs to complete the successful development pralatrexate is highly uncertain, and therefore difficult to estimate. The lengthy process of seeking regulatory approvals for pralatrexate, and the subsequent compliance with applicable regulations, require the expenditure of substantial resources. For a more complete discussion of the regulatory approval process, please refer to the "Government Regulation" section of Part I, Item 1above. Clinical development timelines, likelihood of success and total costs vary widely and are impacted by a variety of risks and uncertainties, including those discussed in the "Risk Factors" section of Part I, Item 1A above. Because of these risks and uncertainties, we cannot predict when or whether we will successfully complete the development of pralatrexate or the ultimate costs of such efforts. Due to these same factors, we cannot be certain when, or if, we will generate any revenue or net cash inflow from any of our current product candidates.
Even if our clinical trials demonstrate the safety and effectiveness of pralatrexate in its target indications, we do not expect to be able to generate commercial sales of pralatrexate until the second half of 2009, at the earliest. We expect to continue incurring net losses and negative cash flows for the foreseeable future. Although the size and timing of our future net losses are subject to significant uncertainty, we expect them to increase over the next several years as we continue to fund our research and development programs, prepare for the potential commercial launch of pralatrexate, and
44
Table of Contents
commercialize pralatrexate for the treatment of patients with relapsed or refractory PTCL, if it is approved for marketing.
We anticipate continuing our current development programs and/or beginning other long-term development projects involving pralatrexate. These projects may require many years and substantial expenditures to complete and may ultimately be unsuccessful. In addition, we intend to submit an NDA for pralatrexate for the treatment of patients with relapsed or refractory PTCL in the first half of 2009. We expect to incur significant costs relating to preparations for the potential commercial launch of pralatrexate, including pre-commercial scale up of manufacturing and development of sales and marketing capabilities. Therefore, we will need to raise additional capital to support our future operations, including the potential commercialization of pralatrexate if approved for marketing. Our actual capital requirements will depend on many factors, including those discussed under the "Liquidity and Capital Resources" section below.
We may seek to obtain this additional capital through equity or debt financings, arrangements with corporate partners, or from other sources. Such financings or arrangements, if successfully consummated, may be dilutive to our existing stockholders. However, there is no assurance that additional financing will be available when needed, or that, if available, we will obtain such financing on terms that are favorable to our stockholders or us. In particular, the current instability in the global financial markets and lack of liquidity in the credit and capital markets may adversely affect our ability to secure adequate capital to support our future operations. In the event that additional funds are obtained through arrangements with collaborative partners or other sources, such arrangements may require us to relinquish rights to some of our technologies, product candidates or products under development, which we would otherwise seek to develop or commercialize ourselves on terms that are less favorable than might otherwise be available. If we are unable to generate meaningful amounts of revenue from future product sales, if any, or cannot otherwise raise sufficient additional funds to support our operations, we may be required to delay, reduce the scope of or eliminate one or more of our development programs and our business and future prospects for revenue and profitability may be harmed.
Comparison of Years Ended December 31, 2008, 2007 and 2006
Research and Development. Research and development expenses include the costs of certain personnel, basic research, preclinical studies, clinical trials, regulatory affairs, biostatistical data analysis and licensing fees for our product candidates.
| | | | | | | | | | |
| | Years Ended
December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | |
|---|
| | (in millions)
| |
|---|
Research and development expenses | | $ | 23.8 | | $ | 17.4 | | $ | 14.3 | |
| | | | | | | | |
The $6.4 million increase in research and development expenses in 2008 as compared to 2007 was primarily due to the following:
- •
- a $6.1 million increase in clinical trial costs involving pralatrexate, including initiation of patient enrollment in two new trials involving pralatrexate during 2008;
- •
- a $1.5 million increase related to key personnel changes and related travel costs, mainly attributable to additional headcount as a result of expanding the development program for pralatrexate and increases in compensation costs year over year; and
- •
- an $830,000 increase in non-cash stock-based compensation expense, as discussed in more detail below.
45
Table of Contents
These increases were partially offset by:
- •
- a $1.0 million decrease in preclinical study costs, primarily related to pralatrexate;
- •
- a $585,000 decrease in clinical trial costs resulting from the discontinuation of the EFAPROXYN development program in mid-2007; and
- •
- a $360,000 decrease related to payment of a non-recurring data option fee for RH1 in the third quarter of 2007, with no corresponding amount in 2008.
The $3.1 million increase in research and development expenses in 2007 as compared to 2006 was primarily due to the following:
- •
- a $2.9 million increase in clinical trial costs involving pralatrexate, mainly attributable to increased costs for PROPEL and initiation of patient enrollment in two new trials involving pralatrexate during 2007;
- •
- a $1.4 million increase related to key personnel changes and related travel costs, mainly attributable to additional headcount and increases in compensation costs year over year;
- •
- a $1.2 million increase in non-cash stock-based compensation expense, as discussed in more detail below;
- •
- a $480,000 increase in preclinical study costs, primarily related to pralatrexate; and
- •
- a $360,000 increase related to payment of a non-recurring data option fee for RH1 in the third quarter of 2007, with no corresponding amount in 2006.
These increases were partially offset by a $3.2 million decrease in clinical trial costs for EFAPROXYN, primarily resulting from the completion of patient enrollment in our Phase 3 ENRICH trial in September 2006.
We expect research and development expenses to increase in 2009 as compared to 2008 due to the following:
- •
- increases in licensing costs for pralatrexate, as $1.5 million of regulatory milestone payments under the license agreement for pralatrexate will become due if the FDA accepts our NDA for review in 2009 and $5.3 million of regulatory milestone payments under the license agreement for pralatrexate will become due if the FDA approves pralatrexate for marketing in the United States in 2009; and
- •
- an increase in non-cash stock-based compensation expense related to our annual equity grants to existing employees.
We charge direct internal and external research and development expenses to the respective development programs. Since our inception through December 31, 2008, we have incurred direct costs of approximately $26.8 million and $1.1 million associated with research and development expenses for pralatrexate and RH1 (a program for which we are in the process of determining our future development plans, if any), respectively, and approximately $45.0 million associated with research and development programs that have been discontinued, including the EFAPROXYN program that was discontinued in mid-2007. We also incur indirect costs that are not allocated to specific programs because such costs benefit multiple development programs. These consist primarily of salaries and benefits, facilities costs and other internal-shared resources related to the development and maintenance of systems and processes applicable to all of our programs. Unallocated costs since our inception through December 31, 2008 represent approximately $76.3 million of research and development expenses.
46
Table of Contents
The following table summarizes our research and development expenses for the years ended December 31, 2008, 2007 and 2006 and for the cumulative period from our inception through December 31, 2008:
| | | | | | | | | | | | | | |
| |
| |
| |
| | Cumulative
Period from
September 1,
1992 through
December 31,
2008 | |
|---|
| | Years Ended December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | |
|---|
| | (in millions)
| |
|---|
Pralatrexate | | $ | 11.6 | | $ | 6.8 | | $ | 3.4 | | $ | 26.8 | |
RH1 | | | 0.2 | | | 0.6 | | | — | | | 1.0 | |
Discontinued programs | | | — | | | 1.0 | | | 4.3 | | | 45.0 | |
Unallocated | | | 12.0 | | | 9.0 | | | 6.6 | | | 76.3 | |
| | | | | | | | | | |
| | Total research and development expenses | | $ | 23.8 | | $ | 17.4 | | $ | 14.3 | | $ | 149.1 | |
| | | | | | | | | | |
The timing and costs to complete the successful development of any of our product candidates are highly uncertain, and therefore difficult to estimate. The lengthy process of seeking regulatory approvals for our product candidates, and the subsequent compliance with applicable regulations, require the expenditure of substantial resources. Clinical development timelines, likelihood of success and total costs vary widely and are impacted by a variety of factors, including those discussed in the "Risk Factors" section of Part I, Item 1A above. Because of these risks and uncertainties, we cannot predict whether or when we will successfully complete the development of pralatrexate or the ultimate costs of such efforts. Due to these same factors, we cannot be certain if, or when, we will generate any revenue or net cash inflow from any of our current product candidates.
Clinical Manufacturing. Clinical manufacturing expenses include the costs of certain personnel, third-party manufacturing costs for development of drug materials for use in clinical trials and preclinical studies, and costs associated with pre-commercial scale-up of manufacturing to support anticipated regulatory and potential commercial requirements.
| | | | | | | | | | |
| | Years Ended
December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | |
|---|
| | (in millions)
| |
|---|
Clinical manufacturing expenses | | $ | 6.7 | | $ | 5.5 | | $ | 2.3 | |
| | | | | | | | |
The $1.2 million increase in clinical manufacturing expenses in 2008 as compared to 2007 was primarily due to the following:
- •
- a $911,000 increase in consulting expenses, primarily related to preparations for submission of an NDA to the FDA for pralatrexate for the treatment of patients with relapsed or refractory PTCL in the first half of 2009;
- •
- a $536,000 increase related to key personnel changes and related travel costs, mainly attributable to additional headcount as a result of pre-commercial scale-up of manufacturing to support anticipated regulatory and potential commercial requirements for pralatrexate and increases in compensation costs year over year; and
- •
- a $398,000 increase primarily related to manufacturing pralatrexate drug substance.
This increase was partially offset by a $696,000 decrease primarily related to pralatrexate drug product manufacturing.
47
Table of Contents
The $3.3 million increase in clinical manufacturing expenses in 2007 as compared to 2006 was primarily due to the following:
- •
- a $2.6 million increase in third-party manufacturing costs for clinical trial material and pre-commercial scale-up activities for pralatrexate; and
- •
- a $700,000 increase in third-party manufacturing costs for clinical trial material for RH1.
We expect clinical manufacturing expenses in 2009 to be consistent with those in 2008.
Marketing, General and Administrative. Marketing, general and administrative expenses include costs for pre-marketing activities, corporate development, executive administration, corporate offices and related infrastructure.
| | | | | | | | | | |
| | Years Ended
December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | |
|---|
| | (in millions)
| |
|---|
Marketing, general and administrative expenses | | $ | 23.0 | | $ | 19.7 | | $ | 14.9 | |
| | | | | | | | |
The $3.4 million increase in marketing, general and administrative expenses in 2008 as compared to 2007 was primarily due to the following:
- •
- a $1.4 million increase in pralatrexate portfolio development and commercialization planning activities;
- •
- a $709,000 increase in market research and consulting expenses related to pre-commercial planning activities for pralatrexate;
- •
- a $563,000 increase related to key personnel changes and related travel costs, mainly attributable to additional headcount and increases in compensation costs year over year; and
- •
- a $303,000 increase in non-cash stock-based compensation expense, as discussed in more detail below.
The $4.8 million increase in marketing, general and administrative expenses in 2007 as compared to 2006 was primarily due to the following:
- •
- a $1.8 million increase in non-cash stock-based compensation expense, as discussed in more detail below;
- •
- a $1.4 million increase in personnel costs, mainly attributable to additional headcount and increases in compensation costs year over year;
- •
- a $1.0 million increase in market research and consulting expenses related to our product development and commercialization planning for EFAPROXYN and pralatrexate; and
- •
- a $485,000 increase in travel expenses related to increased headcount, expanded investor relations activities and the development of relationships with key opinion leaders in oncology.
We expect marketing, general and administrative expenses to increase in 2009 as compared to 2008 due to the following:
- •
- an increase in non-cash stock-based compensation expense related to grants for new employees and our annual equity grants to existing employees;
- •
- an increase in costs relating to pre-commercial planning activities for pralatrexate; and
- •
- an increase in personnel costs, primarily resulting from additional headcount related to preparation for the potential commercial launch of pralatrexate, if it is approved for marketing.
48
Table of Contents
Stock-based Compensation Expense. Stock-based compensation expense for the years ended December 31, 2008, 2007 and 2006 has been recognized in our Statements of Operations as follows:
| | | | | | | | | | | |
| | Year ended December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | |
|---|
Research and development | | $ | 2,701,115 | | $ | 1,870,767 | | $ | 660,274 | |
Clinical manufacturing | | | 417,469 | | | 180,592 | | | 113,066 | |
Marketing, general and administrative | | | 4,901,865 | | | 4,599,266 | | | 2,813,661 | |
| | | | | | | | |
| | Total stock-based compensation expense | | $ | 8,020,449 | | $ | 6,650,625 | | $ | 3,587,001 | |
| | | | | | | | |
Of the $8.0 million of stock-based compensation recognized in the year ended December 31, 2008, $7.5 million was related to our stock option plans, $423,000 related to restricted stock and $59,000 related to our employee stock purchase plan. Of the $6.7 million of stock-based compensation recognized in the year ended December 31, 2007, $5.9 million was related to our stock option plans, $690,000 related to restricted stock and $61,000 related to our employee stock purchase plan. The $1.4 million increase in stock-based compensation expense in 2008 as compared to 2007 was primarily due to the additional stock options granted during the year ended December 31, 2008. The $3.1 million increase in stock-based compensation expense in 2007 as compared to 2006 was primarily due to a higher fair-value for options granted in 2007 compared to options granted in 2006 due to our stock price being higher during the year ended December 31, 2007 as compared to the year ended December 31, 2006.
As of December 31, 2008, the unrecorded stock-based compensation balance related to stock option awards was $7.3 million and will be recognized over an estimated weighted-average amortization period of 1.4 years. As of December 31, 2008, the unrecorded stock-based compensation balance related to restricted stock awards was approximately $329,000 and will be recognized over an estimated weighted-average amortization period of 1.3 years.
Restructuring and Separation Costs. We recorded $0, $0 and $646,000 in restructuring and separation costs during the years ended December 31, 2008, 2007 and 2006, respectively.
In January 2006, Michael E. Hart notified our Board of Directors of his intent to resign from his positions as President, Chief Executive Officer and Chief Financial Officer of the Company once a successor Chief Executive Officer was appointed. On March 3, 2006, we entered into a separation agreement with Mr. Hart to provide certain incentives for his continued employment with the Company while we conducted our search for his successor. On March 9, 2006, we appointed Paul L. Berns as our President, Chief Executive Officer and a member of the Board of Directors and Mr. Hart resigned from his positions in accordance with the terms of the separation agreement. The separation agreement with Mr. Hart was amended on March 9, 2006, and on May 10, 2006. We recorded separation costs of $646,000 during the year ended December 31, 2006 relating to our estimate of our total obligations under the Separation Agreement with Mr. Hart. During the years ended December 31, 2007 and 2006, respectively, we made payments to Mr. Hart under the Separation Agreement of $321,000 and $325,000. As of December 31, 2007, there was no remaining liability relating to the Separation Agreement with Mr. Hart.
Interest and Other Income, Net. Interest income, net of interest expense, for the years ended December 31, 2008, 2007 and 2006 was $1.9 million, $3.3 million, and $1.9 million, respectively. The $1.4 million decrease in 2008 as compared to 2007 was primarily due to lower yields on our cash, cash equivalents and investments in marketable securities, and a realized loss of approximately $552,000 on the sale of certain of our investments in marketable securities during the three months ended September 30, 2008. In response to the instability in the global financial markets, we reviewed our investments in marketable securities and sold certain investments during the three months ended
49
Table of Contents
September 30, 2008 prior to their maturity in order to preserve our principal, as the issuers of these securities experienced significant deteriorations in their creditworthiness as evidenced by investment rating downgrades. We have the ability and intent to hold our remaining investments in marketable securities as of December 31, 2008 to their scheduled maturity, although we monitor our investment portfolio with the primary objectives of preserving principal and maintaining proper liquidity to meet our operating needs. The $1.4 million increase in interest income in 2007 as compared to 2006 primarily resulted from higher average investment balances resulting from our February 2007 financing and higher yields on U.S. government securities, high-grade commercial paper and corporate notes, and money market funds.
Income Taxes. As of December 31, 2008, we had available approximately $174.8 million of net operating loss, or NOL, carryforwards, after taking into consideration NOLs expected to expire unused due to the limitations under Section 382 of the Internal Revenue Code, and which includes approximately $5.2 million of deductions related to stock-based compensation that are not realized as deferred tax assets until current taxes payable can be reduced. These NOL carryforwards will expire beginning in 2009. In addition, we had research and development credit and orphan drug credit carryforwards, after taking into consideration the Section 382 limitation, of $3.5 million and $5.6 million, respectively, as of December 31, 2008, to offset future regular and alternative tax expense. Since our formation, we have raised capital through the issuance of capital stock on several occasions which, combined with shareholders' subsequent disposition of those shares, has resulted in four changes of control in 1994, 1998, 2001 and 2005, as defined by Section 382. In general, an ownership change, as defined by Section 382, results from transactions increasing the ownership of certain shareholders or public groups in the stock of a corporation by more than 50% within a three-year period. As a result of the most recent ownership change in 2005, utilization of our NOLs generated prior to the latest change are subject to an annual limitation under Section 382 determined by multiplying the value of our stock at the time of the ownership change in control by the applicable long-term tax-exempt rate resulting in an annual limitation amount of approximately $2.2 million. Any unused annual limitation may be carried over to later years, and the amount of the limitation may, under certain circumstances, be subject to adjustment if the fair value of the Company's net assets are determined to be below or in excess of the tax basis of such assets at the time of the ownership change, and such unrealized loss or gain is recognized during the five-year period after the ownership change. Subsequent ownership changes, as defined in Section 382, could further limit the amount of our NOLs and research and development credits that can be utilized annually to offset future taxable income.
Liquidity and Capital Resources
Since our inception, we have financed our operations primarily through public and private sales of our equity securities, which have resulted in net proceeds to us of $328.5 million through December 31, 2008. We have also generated $23.5 million of net interest income since our inception from investing the net proceeds of these financings.
As of December 31, 2008, we had $84.0 million in cash, cash equivalents, and investments in marketable securities. Until required for use in our business, we invest our cash reserves in bank deposits, money market funds, high-grade corporate notes and U.S. government instruments in accordance with our investment policy. The weighted average duration of the remaining time to maturity for our portfolio of investments in marketable securities as of December 31, 2008 was approximately five months. Our investments in marketable securities as of December 31, 2008 primarily consisted of high-grade corporate notes. We did not hold any derivative instruments, foreign exchange contracts, asset backed securities, mortgage backed securities, auction rate securities, or securities of issuers in bankruptcy in our investment portfolio as of December 31, 2008. Our liquidity, capital resources and results of operations may be adversely affected by future declines in the value of our investments in marketable securities. The value of our investments in marketable securities may be
50
Table of Contents
adversely affected by rating downgrades or bankruptcies affecting the issuers of such securities, whether caused by instability in the global financial markets, lack of liquidity in the credit and capital markets, or other factors. We have the ability and intent to hold our remaining investments in marketable securities as of December 31, 2008 to their scheduled maturity, although we monitor our investment portfolio with the primary objectives of preserving principal and maintaining proper liquidity to meet our operating needs.
We have used $238.4 million of cash for operating activities from our inception through December 31, 2008. Net cash used to fund our operating activities for the years ended December 31, 2008, 2007 and 2006 was $42.9 million, $30.8 million and $25.1 million, respectively.
For fiscal year 2009, we anticipate that net cash use in operating activities will approximate $50 to $54 million. Though not inclusive of all costs associated with the potential future launch of pralatrexate, this guidance includes the phase-in of certain key investments related to commercial planning and pre-commercial scale-up of manufacturing for pralatrexate, as well as $1.5 million and $5.3 million of potential milestone payments under our license agreement for pralatrexate payable upon FDA acceptance of our NDA for review and FDA approval to market pralatrexate, respectively.
Net cash used in investing activities for the years ended December 31, 2008 and 2007 was $13.2 million and $19.1 million, respectively, and consisted primarily of purchases of investments in marketable securities, partially offset by the proceeds from maturities of investments in marketable securities. Net cash provided by investing activities for the year ended December 31, 2006 was $28.1 million and consisted primarily of proceeds from the maturities of investments in marketable securities, partially offset by the purchase of investments in marketable securities.
Net cash provided by financing activities for the year ended December 31, 2008 was $70.6 million and consisted primarily of the net proceeds from the sale of 12,420,000 shares of our common stock in May 2008 in an underwritten public offering at a price of $5.64 per share, or the May 2008 Financing, and $5.4 million of proceeds from the issuance of common stock associated with stock options exercised by our employees and sales of stock under our employee stock purchase plan. We received net proceeds from the May 2008 Financing of approximately $65.2 million, after deducting $4.2 million of underwriting commissions and $661,000 of offering expenses. The shares of common stock were sold under our shelf Registration Statement on Form S-3, declared effective by the Securities and Exchange Commission, or SEC, on June 5, 2007. Net cash provided by financing activities during the year ended December 31, 2007 was $55.7 million and resulted primarily from the sale of 9,000,000 shares of common stock in February 2007 in an underwritten offering at a price of $6.00 per share, or the February 2007 Financing, $5.5 million of proceeds associated with the exercise of common stock options, common stock warrants and sales of stock under our employee stock purchase plan, and the release of $183,000 of restricted cash in connection with a reduction of the letter of credit required pursuant to the lease for our corporate headquarters facility. We received net proceeds from the February 2007 Financing of approximately $50.3 million, after deducting underwriting commissions of approximately $3.2 million and other offering expenses of approximately $503,000. The shares of common stock were sold under our shelf Registration Statement on Form S-3, declared effective by the SEC on July 10, 2006. Net cash provided by financing activities for the year ended December 31, 2006 was $3.1 million and resulted primarily from proceeds associated with the exercise of common stock options, common stock warrants and sales of stock under our employee stock purchase plan, and the release of $183,000 of restricted cash in connection with a reduction of the letter of credit required pursuant to the lease for our corporate headquarters facility.
On June 5, 2007, our universal shelf Registration Statement on Form S-3 was declared effective by the SEC. Under this registration statement, we are allowed to sell, from time to time, up to $150 million of our common stock, preferred stock, depository shares, debt securities and/or warrants, either individually or in units, in one or more offerings. As of December 31, 2008, we have
51
Table of Contents
approximately $80 million available for issuance under this Registration Statement. We are not required to offer the securities in the future pursuant to the registration statement. The terms of any offering under the registration statement will be established at the time of the offering. Proceeds from the sale of any securities will be used for the purposes described in a prospectus supplement filed at the time of an offering.
Based upon the current status of our product development and commercialization plans, we believe that our cash, cash equivalents, and investments in marketable securities as of December 31, 2008 should be adequate to support our operations through at least the next 12 months, although there can be no assurance that this can, in fact, be accomplished. Our forecast of the period of time through which our financial resources will be adequate to support our operations is a forward-looking statement that involves risks and uncertainties, and actual results could vary materially.
We anticipate continuing our current development programs and/or beginning other long-term development projects involving pralatrexate. These projects may require many years and substantial expenditures to complete and may ultimately be unsuccessful. In addition, we intend to submit an NDA for pralatrexate for the treatment of patients with relapsed or refractory PTCL in the first half of 2009. We expect to incur significant costs relating to preparations for the potential commercial launch of pralatrexate, including pre-commercial scale up of manufacturing and development of sales and marketing capabilities. Therefore, we will need to raise additional capital to support our future operations. Our actual capital requirements will depend on many factors, including:
- •
- the timing and outcome of our planned NDA for pralatrexate for the treatment of patients with relapsed or refractory PTCL;
- •
- the timing and costs associated with developing sales and marketing capabilities and commercializing pralatrexate, if it is approved for marketing;
- •
- the timing and costs associated with manufacturing clinical and commercial supplies of pralatrexate;
- •
- the timing and amount of revenues generated by our business activities, if any;
- •
- the timing and costs associated with conducting preclinical and clinical development of pralatrexate, as well as our evaluation of, and decisions with respect to, additional therapeutic indications for which we may develop pralatrexate;
- •
- the timing, costs and potential revenue associated with any co-promotion or other partnering arrangements entered into to commercialize pralatrexate, if it is approved for marketing; and
- •
- our evaluation of, and decisions with respect to, potential in-licensing or product acquisition opportunities or other strategic alternatives.
We may seek to obtain this additional capital through equity or debt financings, arrangements with corporate partners, or from other sources. Such financings or arrangements, if successfully consummated, may be dilutive to our existing stockholders. However, there is no assurance that additional financing will be available when needed, or that, if available, we will obtain such financing on terms that are favorable to our stockholders or us. In particular, the current instability in the global financial markets and lack of liquidity in the credit and capital markets may adversely affect our ability to secure adequate capital to support our future operations. In the event that additional funds are obtained through arrangements with collaborative partners or other sources, such arrangements may require us to relinquish rights to some of our technologies, product candidates or products under development, which we would otherwise seek to develop or commercialize ourselves on terms that are less favorable than might otherwise be available. If we are unable to generate meaningful amounts of revenue from future product sales, if any, or cannot otherwise raise sufficient additional funds to support our operations, we may be required to delay, reduce the scope of or eliminate one or more of our development programs and our business and future prospects for revenue and profitability may be harmed.
52
Table of Contents
Obligations and Commitments
Below is a schedule of the timing of contractual commitments, by fiscal year, related to our leases, service contracts and license agreements. We currently have no off-balance sheet arrangements.
| | | | | | | | | | | | | | | | | |
| | 2009 | | 2010 to 2011 | | 2012 to 2013 | | After 2013 | | Total | |
|---|
Operating lease obligations | | $ | 792,000 | | $ | 1,639,000 | | $ | 46,000 | | $ | — | | $ | 2,477,000 | |
License agreement obligations: | | | | | | | | | | | | | | | | |
| | Clinical milestone | | | 500,000 | | | — | | | — | | | — | | | 500,000 | |
| | Regulatory milestones | | | 6,800,000 | | | — | | | — | | | — | | | 6,800,000 | |
| | | | | | | | | | | | |
Total obligations | | $ | 8,092,000 | | $ | 1,639,000 | | $ | 46,000 | | $ | — | | $ | 9,777,000 | |
| | | | | | | | | | | | |
Operating lease obligations represent our future minimum rental commitments for non-cancelable operating leases for our facilities. We lease our corporate headquarters facility pursuant to a lease agreement that expires on January 31, 2012. Our lease for an office in Princeton, New Jersey expires on September 30, 2011.
License agreement obligations represent future milestone payments that could be made under our license agreement for pralatrexate. We will make a milestone payment of $500,000 upon the earlier of achievement of a clinical development milestone or the passage of certain time periods, such earlier occurrence, the "Clinical Milestone." The last scheduled payment of $500,000 under the Clinical Milestone is due December 23, 2009. We intend to submit an NDA for pralatrexate for the treatment of patients with relapsed or refractory PTCL in the first half of 2009. If the FDA accepts our NDA for review and if we obtain FDA approval to market pralatrexate, we will be obligated to make milestone payments of $1,500,000 and $5,300,000, respectively, under the license agreement for pralatrexate.
Critical Accounting Policies
Our discussion and analysis of our financial condition and results of operations are based upon our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, and expenses. We base our estimates on historical experience, available information and assumptions that we believe to be reasonable under the circumstances. Actual results may differ from these estimates under different assumptions or conditions. We believe the following policies to be the most critical to an understanding of our financial condition and results of operations because they require us to make estimates, assumptions and informed management judgments about matters that are inherently uncertain:
- •
- accounting for research and development expenses;
- •
- accounting for clinical manufacturing expenses;
- •
- accounting for cash equivalents and investments in marketable securities; and
- •
- accounting for stock-based compensation expense.
Research and Development. Research and development expenditures are charged to expense as incurred. Research and development expenses include the costs of certain personnel, basic research, preclinical studies, clinical trials, regulatory affairs, biostatistical data analysis and licensing fees for our product candidates. Clinical trial costs represent internal costs from personnel, external costs incurred at clinical sites and contracted costs incurred by third party clinical research organizations to perform certain clinical trials.
We record upfront fees and milestone payments made under our licensing agreements for our product candidates as research and development expense as the services are performed.
53
Table of Contents
We accrue research and development expenses for activity as incurred during the fiscal year and prior to receiving invoices from clinical sites and third party clinical and preclinical research organizations. We accrue external costs for clinical and preclinical studies based on an evaluation of the following: the progress of the studies, including patient enrollment, dosing levels of patients enrolled, estimated costs to dose patients, invoices received, and contracted costs with clinical sites and third party clinical and preclinical research organizations. Significant judgments and estimates must be made and used in determining the accrued balance in any accounting period. Actual results could differ from those estimates. During the years ended December 31, 2008, 2007, and 2006, we did not have any changes in estimates that would have resulted in material adjustments to research and development expenses accrued in the prior period. However, during the quarter ended December 31, 2006, we did change our estimate relating to certain costs for our Phase 3 ENRICH trial for EFAPROXYN as a result of new information, which resulted in a reduction of research and development expenses of approximately $400,000 and a corresponding decrease in accrued research and development expenses as of December 31, 2006.
In accordance with certain research and development agreements, we are obligated to make certain upfront payments upon execution of the agreement. We record these upfront payments as prepaid research and development expenses. Such payments are recorded to research and development expense as services are performed. We evaluate on a quarterly basis whether events and circumstances have occurred that may indicate impairment of remaining prepaid research and development expenses.
Clinical Manufacturing. Clinical manufacturing expenses include the costs of certain personnel, third party manufacturing costs for development of drug materials for use in clinical trials and preclinical studies, and costs associated with pre-commercial scale-up of manufacturing to support anticipated regulatory and potential commercial requirements. Our finished drug inventory is expensed to clinical manufacturing since we are still a development stage company and we have not received regulatory approval to market our product candidates. If and when we receive regulatory approval, we will be required to capitalize any future manufacturing costs for our marketed products at the lower of cost or market and then expense the sold inventory as a component of cost of goods sold.
Cash Equivalents and Investments in Marketable Securities. All highly liquid investments with original maturities of three months or less are considered to be cash equivalents. The carrying values of our cash equivalents and investments in marketable securities approximate their market values based on quoted market prices. We account for investments in marketable securities in accordance with SFAS No. 115,Accounting for Certain Investments in Debt and Equity Securities. Investments in marketable securities are classified as held to maturity and are carried at cost plus accrued interest. Our cash and cash equivalents are maintained in a financial institution in amounts that, at times, may exceed federally insured limits. We realized a loss of approximately $552,000 on the sale of certain of our investments in marketable securities during the year ended December 31, 2008. In response to the recent instability in the global financial markets, we reviewed our investments in marketable securities and sold certain investments prior to their maturity in order to preserve our principal, as the issuers of these securities experienced significant deteriorations in their creditworthiness as evidenced by investment rating downgrades. We have the ability and intent to hold our remaining investments in marketable securities as of December 31, 2008 to their scheduled maturity, although we monitor our investment portfolio with the primary objectives of preserving principal and maintaining proper liquidity to meet our operating needs. The weighted average duration of the remaining time to maturity for our portfolio of investments in marketable securities as of December 31, 2008 was approximately five months. As of December 31, 2008, our investments in marketable securities were held in a variety of interest-bearing instruments, consisting mainly of high-grade corporate notes. We did not hold any derivative instruments, foreign exchange contracts, asset backed securities, mortgage backed securities, auction rate securities, or securities of issuers in bankruptcy in our investment portfolio as of December 31, 2008.
54
Table of Contents
Stock-based Compensation Expense. We adopted SFAS No. 123 (Revised 2004),Share-Based Payment, or SFAS 123R, effective January 1, 2006. Under the provisions of SFAS 123R, stock-based compensation is measured at the grant date based on the fair value of the award and is recognized as expense over the required service period of the award. Prior to the adoption of SFAS 123R, we accounted for grants of stock-based awards according to the intrinsic value method as prescribed by Accounting Principles Board Opinion No. 25,Accounting for Stock Issued to Employees and related Interpretations.
In March 2005, the SEC issued Staff Accounting Bulletin No. 107, or SAB 107 (subsequently amended by SAB 110), relating to SFAS 123R. We applied the provisions of SAB 107 in connection with our adoption of SFAS 123R. We adopted SFAS 123R using the modified prospective transition method, which requires the application of the accounting standard as of January 1, 2006, the first day of our fiscal year 2006. Our financial statements as of and for the years ended December 31, 2008, 2007 and 2006 reflect the impact of SFAS 123R. In accordance with the modified prospective transition method, our financial statements for prior periods have not been restated to reflect, and do not include, the impact of SFAS 123R.
The adoption of SFAS 123R on January 1, 2006 had a material impact on our net loss and net loss per share for the years ended December 31, 2008, 2007 and 2006. We expect that SFAS 123R will have a material impact on our future financial statements and results of operations. During the years ended December 31, 2008, 2007 and 2006, we recorded stock-based compensation expense of approximately $8.0 million, $6.7 million and $3.6 million, respectively, related to stock-based awards, including stock options, restricted stock and our employee stock purchase plan. As of December 31, 2008, the unrecorded deferred stock-based compensation balance related to these stock-based awards was approximately $7.6 million and will be recognized over the remaining vesting periods of the awards. Judgments and estimates must be made and used in determining the factors used in calculating the fair value of stock-based awards, including the expected forfeiture rate of our stock-based awards, the expected life of our stock-based awards, and the expected volatility of our stock price. For more information on stock-based compensation expense during the year ended December 31, 2008, refer to Note 4 "Stock-Based Compensation Plans" of the Notes to our Financial Statements included in Part IV, Item 15 of this report.
Recent Accounting Pronouncements
In September 2006, the Financial Accounting Standards Board, or FASB, issued SFAS No. 157,Fair Value Measurements, which defines fair value, provides a framework for measuring fair value, and expands the disclosures required for fair value measurements. SFAS No. 157 applies to other accounting pronouncements that require fair value measurements; it does not require any new fair value measurements. SFAS No. 157 is effective for fiscal years beginning after November 15, 2007 and we adopted it on January 1, 2008. The application of SFAS No. 157 to certain items has been deferred and will be effective for fiscal years beginning after November 15, 2008 and interim periods within that year. The adoption of this pronouncement did not have a material impact on our results of operations or financial position for the year ended December 31, 2008. We have no assets or liabilities that were measured using quoted prices for similar assets and liabilities or significant unobservable inputs (Level 2 and Level 3 assets and liabilities, respectively) as of December 31, 2008. Our financial instruments include cash and cash equivalents, investments in marketable securities, prepaid expenses, accounts payable and accrued liabilities. The carrying amounts of financial instruments approximate their fair value due to their short maturities. The carrying value of our money market investments totaling $30.4 million as of December 31, 2008 is included in cash and cash equivalents on our Balance Sheet included in this report and approximates their market values based on quoted market prices, or Level 1 inputs. We account for investments in marketable securities in accordance with SFAS No. 115,
55
Table of Contents
Accounting for Certain Investments in Debt and Equity Securities. Investments in marketable securities are classified as held to maturity and are carried at cost plus accrued interest.
In February 2007, the FASB issued SFAS No. 159,The Fair Value Option for Financial Assets and Financial Liabilities—Including an Amendment of FASB Statement No. 115, which is effective for fiscal years beginning after November 15, 2007 and we adopted it on January 1, 2008. This statement permits an entity to choose to measure many financial instruments and certain other items at fair value at specified election dates. Subsequent unrealized gains and losses on items for which the fair value option has been elected will be reported in earnings. The adoption of this pronouncement did not have a material impact on our results of operations or financial position for the year ended December 31, 2008, as we did not elect to measure any of our financial instruments at fair value.
In June 2007, the Emerging Issues Task Force, or EITF, issued a consensus, EITF 07-3,Advance Payments for Research and Development Activities, which states that non-refundable advance payments for goods that will be used or services that will be performed in future research and development activities should be deferred and capitalized until the goods have been delivered or the related services have been rendered. EITF 07-3 is to be applied prospectively for new contractual arrangements entered into in fiscal years beginning after December 15, 2007 and we adopted it on January 1, 2008. The adoption did not result in a material change to our current accounting practice.
In November 2007, the EITF issued a consensus, EITF 07-1,Accounting for Collaboration Arrangements Related to the Development and Commercialization of Intellectual Property, which is focused on how the parties to a collaborative agreement should account for costs incurred and revenue generated on sales to third parties, how sharing payments pursuant to a collaboration agreement should be presented in the income statement and certain related disclosure questions. EITF 07-1 is to be applied retrospectively for collaboration arrangements in fiscal years beginning after December 15, 2008. We currently do not have any such arrangements.
In December 2007, the FASB issued SFAS No. 141(R),Business Combinations. This Statement replaces SFAS No. 141,Business Combinations, and requires an acquirer to recognize the assets acquired, the liabilities assumed, including those arising from contractual contingencies, any contingent consideration, and any noncontrolling interest in the acquiree at the acquisition date, measured at their fair values as of that date, with limited exceptions specified in the statement. SFAS No. 141(R) also requires the acquirer in a business combination achieved in stages (sometimes referred to as a step acquisition) to recognize the identifiable assets and liabilities, as well as the noncontrolling interest in the acquiree, at the full amounts of their fair values (or other amounts determined in accordance with SFAS No. 141(R)). In addition, SFAS No. 141(R)'s requirement to measure the noncontrolling interest in the acquiree at fair value will result in recognizing the goodwill attributable to the noncontrolling interest in addition to that attributable to the acquirer. SFAS No. 141(R) amends SFAS No. 109,Accounting for Income Taxes, to require the acquirer to recognize changes in the amount of its deferred tax benefits that are recognizable because of a business combination either in income from continuing operations in the period of the combination or directly in contributed capital, depending on the circumstances. It also amends SFAS No. 142,Goodwill and Other Intangible Assets, to, among other things, provide guidance on the impairment testing of acquired research and development intangible assets and assets that the acquirer intends not to use. SFAS No. 141(R) applies prospectively to business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2008. We are currently evaluating the potential impact of this statement and will apply it to any business combinations in the future. We do not expect any impact on our financial statements.
In December 2007, the FASB issued SFAS No. 160,Noncontrolling Interests in Consolidated Financial Statements. SFAS No. 160 amends Accounting Research Bulletin 51,Consolidated Financial Statements, to establish accounting and reporting standards for the noncontrolling interest in a
56
Table of Contents
subsidiary and for the deconsolidation of a subsidiary. It also clarifies that a noncontrolling interest in a subsidiary is an ownership interest in the consolidated entity that should be reported as equity in the consolidated financial statements. SFAS No. 160 also changes the way the consolidated income statement is presented by requiring consolidated net income to be reported at amounts that include the amounts attributable to both the parent and the noncontrolling interest. It also requires disclosure, on the face of the consolidated statement of income, of the amounts of consolidated net income attributable to the parent and to the noncontrolling interest. SFAS No. 160 requires that a parent recognize a gain or loss in net income when a subsidiary is deconsolidated and requires expanded disclosures in the consolidated financial statements that clearly identify and distinguish between the interests of the parent owners and the interests of the noncontrolling owners of a subsidiary. SFAS No. 160 is effective for fiscal periods, and interim periods within those fiscal years, beginning on or after December 15, 2008. We are currently evaluating the potential impact of this statement. We do not expect any impact on our financial statements.
In March 2008, the FASB issued SFAS No. 161,Disclosures about Derivative Instruments and Hedging Activities. SFAS No. 161 is intended to improve financial reporting about derivative instruments and hedging activities by requiring companies to enhance disclosure about how these instruments and activities affect their financial position, performance and cash flows. SFAS No. 161 also improves the transparency about the location and amounts of derivative instruments in a company's financial statements and how they are accounted for under SFAS No. 133. SFAS No. 161 is effective for financial statements issued for fiscal years beginning after November 15, 2008, and interim periods beginning after that date. We are currently evaluating the potential impact of this statement. We do not expect any impact on our financial statements.
In May 2008, the FASB issued SFAS No. 162,The Hierarchy of Generally Accepted Accounting Principles. SFAS No. 162 identifies the sources of accounting principles and the framework for selecting the principles to be used in the preparation of financial statements of nongovernmental entities that are presented in conformity with generally accepted accounting principles in the United States of America. SFAS No. 162 is effective 60 days following the SEC's approval of the Public Company Accounting Oversight Board, or PCAOB, amendments to AICPA Codification of Auditing Standards, AU Section 411,The Meaning of Present Fairly in Conformity with Generally Accepted Accounting Principles. This amendment was approved by the PCAOB on September 16, 2008. We do not anticipate that the adoption of SFAS No. 162 will materially impact our financial statements.
In June 2008, the FASB issued FASB Staff Position, or FSP, EITF 03-6-1,Determining Whether Instruments Granted in Share-Based Payment Transactions are Participating Securities, or FSP EITF 03-6-1, to address whether instruments granted in share-based payment transactions are participating securities prior to their vesting and therefore need to be included in the earnings per share calculation under the two-class method described in SFAS No. 128,Earnings per Share. This FSP requires companies to treat unvested share-based payment awards that have non-forfeitable rights to dividends or dividend equivalents as participating securities and thus, include them in calculations of basic earnings per share. FSP EITF 03-6-1 is effective for fiscal years beginning after December 15, 2008. We do not anticipate that our adoption of FSP EITF 03-6-1 will materially impact our financial statements or our computation of basic earnings per share upon adoption.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Our financial instruments as of December 31, 2008 consisted of cash, cash equivalents, investments in marketable securities, and accounts payable. All highly liquid investments with original maturities of three months or less are considered to be cash equivalents. We invest in marketable securities in accordance with our investment policy. The primary objectives of our investment policy are to preserve principal, maintain proper liquidity to meet operating needs and maximize yields. Our investment policy specifies credit quality standards for our investments and limits the amount of credit exposure to any
57
Table of Contents
single issue, issuer or type of investment. The weighted average duration of the remaining time to maturity for our portfolio of investments in marketable securities as of December 31, 2008 was approximately five months. As of December 31, 2008, our investments in marketable securities of $53.5 million were all classified as held-to-maturity and were held in a variety of interest-bearing instruments, consisting mainly of high-grade corporate notes. We did not hold any derivative instruments, foreign exchange contracts, asset backed securities, mortgage backed securities, auction rate securities, or securities of issuers in bankruptcy in our investment portfolio as of December 31, 2008. The value of our investments in marketable securities may be adversely affected by rating downgrades or bankruptcies affecting the issuers of such securities, whether caused by instability in the global financial markets, lack of liquidity in the credit and capital markets, or other factors. In response to the recent instability in the global financial markets, we reviewed our investments in marketable securities and sold certain investments that we deemed to have increased risk during the three months ended September 30, 2008. We have the ability and intent to hold our remaining investments in marketable securities as of December 31, 2008 to their scheduled maturity, although we monitor our investment portfolio with the primary objectives of preserving principal and maintaining proper liquidity to meet our operating needs.
Investments in fixed-rate interest-earning instruments carry varying degrees of interest rate risk. The fair market value of our fixed-rate securities may be adversely impacted due to a rise in interest rates. In general, securities with longer maturities are subject to greater interest-rate risk than those with shorter maturities. Due in part to this factor, our interest income may fall short of expectations or we may suffer losses in principal if securities are sold that have declined in market value due to changes in interest rates. Due to the short duration of our investment portfolio, we believe an immediate 10% change in interest rates would not be material to our financial condition or results of operations.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The financial statements required by this Item are included in Item 15 of this report and are presented beginning on page F-1.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
As of the end of the period covered by this report, an evaluation was carried out under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, of the effectiveness of our disclosure controls and procedures, as defined in Rule 13(a)-15(e) of the Securities Exchange Act of 1934, as amended, or the Exchange Act. Based on that evaluation, our management, including our principal executive officer and principal financial officer, concluded that our disclosure controls and procedures were effective as of December 31, 2008 to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission's rules and forms, and that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate, to allow timely decisions regarding required disclosure.
58
Table of Contents
Management's Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining effective internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) or Rule 15d-(f) promulgated under the Exchange Act as a process designed by, or under the supervision of, a company's principal executive officer and principal financial officer and effected by the company's board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that:
- •
- pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company;
- •
- provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and
- •
- provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2008. In making its assessment, management used the criteria established inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on its assessment, management determined that, as of December 31, 2008, we maintained effective internal control over financial reporting based on those criteria.
In addition, the effectiveness of our internal control over financial reporting as of December 31, 2008 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report on page F-2 of this Annual Report on Form 10-K.
No Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting during the quarter ended December 31, 2008 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
None.
59
Table of Contents
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by this Item concerning our directors is incorporated by reference to the information to be set forth in the section entitled "Proposal 1—Election of Directors" in our definitive Proxy Statement for the 2009 Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission within 120 days after the end of our fiscal year ended December 31, 2008, or the Proxy Statement. The information required by this Item concerning compliance with Section 16(a) of the Exchange Act is incorporated by reference to the information to be set forth in the section of the Proxy Statement entitled "Section 16(a) Beneficial Ownership Reporting Compliance." The information required by this Item concerning the procedures by which our stockholders may recommend nominees to our Board of Directors is incorporated by reference to the information to be set forth in the section of the Proxy Statement entitled "Information Regarding the Director Nomination Process." The information required by this Item concerning our Audit Committee is incorporated by reference to the information to be set forth in the section of the Proxy Statement entitled "Audit Committee." The balance of the information required by this Item, except as otherwise set forth below, is incorporated by reference to the information to be set forth in the section of the Proxy Statement entitled "Executive Officers."
Our Board of Directors has adopted a Code of Business Conduct and Ethics for all of our directors, officers and employees. Stockholders may locate a copy of our Code of Business Conduct and Ethics on our website athttp://www.allos.com or request a free copy from:
Allos Therapeutics, Inc.
Attention: Investor Relations
11080 CirclePoint Road, Suite 200
Westminster, CO 80020
Telephone: 303-426-6262
To date, there have been no waivers under our Code of Business Conduct and Ethics. We will post any waivers, if and when granted, of our Code of Business Conduct and Ethics on our website.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this Item regarding executive compensation is incorporated by reference to the information to be set forth in the sections of the Proxy Statement entitled "Executive Compensation," "Director Compensation," "Compensation Committee Interlocks and Insider Participation," and "Compensation Committee Report."
60
Table of Contents
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Securities Authorized for Issuance Under Equity Compensation Plans
The following table provides certain information with respect to our equity compensation plans in effect as of December 31, 2008:
| | | | | | | | | | |
Plan Category | | Number of securities to
be issued upon exercise
of outstanding options
and rights
(a) | | Weighted-average
exercise price
of outstanding
options and rights
(b) | | Number of securities
remaining available
for issuance under
equity compensation
plans (excluding
securities reflected
in column (a))
(c) | |
|---|
Equity compensation plans approved by security holders | | | 7,236,512 | | $ | 5.14 | | | 7,406,478 | (1)(2) |
Equity compensation plans not approved by security holders | | | — | | | — | | | — | |
| | | | | | | | |
Total | | | 7,236,512 | | $ | 5.14 | | | 7,406,478 | (1)(2) |
| | | | | | | | |
- (1)
- Includes 5,146,701 shares of common stock available for future issuance under our 2008 Equity Incentive Plan. All stock awards granted under our 2008 Equity Incentive Plan after the June 24, 2008 effective date thereof, other than stock options and stock appreciation rights granted with an exercise price of at least 100% of such stock award's fair market value on the date of grant, reduce the number of shares available for issuance under our 2008 Equity Incentive Plan by 1.35 shares per share granted pursuant to the stock award. Shares of common stock that revert to and again become available for issuance under our 2008 Equity Incentive Plan and that prior to such reversion were granted pursuant to a stock award that reduced the number of shares available under our 2008 Equity Incentive Plan by 1.35 shares per share granted pursuant to such stock award, will cause the number of shares of our common stock available for issuance under our 2008 Equity Incentive Plan to increase by 1.35 shares upon such reversion.
- (2)
- Includes 2,259,777 shares of common stock available for future issuance under our 2001 Employee Stock Purchase Plan.
Other Information
Other information required by this Item regarding security ownership of certain beneficial owners and management is incorporated by reference to the information to be set forth in the section of the Proxy Statement entitled "Security Ownership of Certain Beneficial Owners and Management."
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this Item regarding certain relationships and related transactions and director independence is incorporated by reference to the information to be set forth in the sections of the Proxy Statement entitled "Transactions with Related Persons" and "Information Regarding the Board of Directors and Corporate Governance."
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The information required by this Item regarding principal accounting fees and services is incorporated by reference to the information to be set forth in the section of the Proxy Statement entitled "Proposal 2—Ratification of Selection of Independent Registered Public Accountants."
61
Table of Contents
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
- (a)
- The following documents are being filed as part of this report:
- (1)
- Financial Statements.
All financial statement schedules have been omitted because they are not applicable or not required or because the information is included elsewhere in the Financial Statements or the Notes thereto.
- (3)
- Exhibits.
The following is a list of exhibits filed as part of this report on Form 10-K. Where so indicated exhibits that were previously filed are incorporated by reference.
| | | | | | | | | | | |
| |
| | Incorporated by Reference | |
|
|---|
| |
| | Filed
Herewith |
|---|
| Exhibit No. | | Description | | Form | | Filing Date | | Number |
|---|
| | 3.01 | | Amended and Restated Certificate of Incorporation. | | 10-Q | | 8/7/2006 | | 3.01 | | |
|
3.02 |
|
Certificate of Designation of Series A Junior Participating Preferred Stock. |
|
10-Q |
|
8/7/2006 |
|
3.02 |
|
|
|
3.03 |
|
Certificate of Amendment to Restated Certificate of Incorporation. |
|
10-Q |
|
8/7/2006 |
|
3.03 |
|
|
|
3.04 |
|
Amended and Restated Bylaws of Allos Therapeutics, Inc. |
|
8-K |
|
6/25/2007 |
|
3.04 |
|
|
|
4.01 |
|
Form of Common Stock Certificate. |
|
S-1/A |
|
3/17/2000 |
|
4.01 |
|
|
|
4.02 |
|
Reference is made to Exhibits 3.01, 3.02, 3.03 and 3.04. |
|
|
|
|
|
|
|
|
|
4.03 |
|
Rights Agreement dated May 6, 2003 between Allos and Mellon Investor Services LLC. |
|
8-K |
|
5/9/2003 |
|
99.2 |
|
|
|
4.04 |
|
Form of Rights Certificate. |
|
8-K |
|
5/9/2003 |
|
99.3 |
|
|
|
4.05 |
|
Amendment to Rights Agreement dated March 4, 2005 between Allos and Mellon Investor Services LLC. |
|
8-K |
|
3/4/2005 |
|
4.06 |
|
|
|
4.06 |
|
Amendment to Rights Agreement dated January 29, 2007 between Allos and Mellon Investor Services LLC. |
|
8-K |
|
1/30/2007 |
|
4.1 |
|
|
|
10.01 |
† |
Form of Amended and Restated Indemnity Agreement between Allos and each of its directors and officers. |
|
8-K |
|
6/25/2007 |
|
10.01 |
|
|
|
10.02 |
† |
1995 Stock Option Plan, as amended. |
|
S-1 |
|
1/26/2000 |
|
10.11 |
|
|
62
Table of Contents
| | | | | | | | | | | |
| |
| | Incorporated by Reference | |
|
|---|
| |
| | Filed
Herewith |
|---|
| Exhibit No. | | Description | | Form | | Filing Date | | Number |
|---|
| | 10.03 | † | 2000 Stock Incentive Compensation Plan, as amended. | | 8-K | | 12/22/2005 | | 10.1 | | |
|
10.03.1 |
† |
Form of Incentive Stock Option Letter Agreement under 2000 Stock Incentive Compensation Plan. |
|
8-K |
|
2/11/2005 |
|
99.1 |
|
|
|
10.03.2 |
† |
Form of Nonqualified Stock Option Letter Agreement under 2000 Stock Incentive Compensation Plan. |
|
8-K |
|
2/11/2005 |
|
99.2 |
|
|
|
10.03.3 |
† |
Form of Nonqualified Stock Option Letter Agreement for Non-Employee Directors under 2000 Stock Incentive Compensation Plan. |
|
8-K |
|
2/24/2006 |
|
10.1 |
|
|
|
10.04 |
† |
2001 Employee Stock Purchase Plan and form of Offering. |
|
10-K |
|
3/7/2001 |
|
10.26 |
|
|
|
10.04.1 |
† |
2001 Employee Stock Purchase Plan Offering (Series Beginning July 1, 2007). |
|
8-K |
|
6/25/2007 |
|
10.12.1 |
|
|
|
10.05 |
* |
Office Lease dated April 4, 2001 between Allos and Catellus Development Corporation. |
|
10-Q |
|
8/14/2001 |
|
10.27 |
|
|
|
10.05.1 |
* |
Amended and Restated Second Amendment to Lease dated December 9, 2002 between Allos and Catellus Development Corporation. |
|
10-K |
|
3/28/2003 |
|
10.27.1 |
|
|
|
10.05.2 |
* |
Third Amendment to Lease dated November 28, 2003 between Allos and Catellus Development Corporation. |
|
10-K |
|
3/5/2004 |
|
10.27.2 |
|
|
|
10.05.3 |
* |
Fifth Amendment to Office Lease Agreement dated June 16, 2008 between Allos and Circle Point Properties, LLC. |
|
10-Q |
|
8/5/2008 |
|
10.5.3 |
|
|
|
10.06 |
|
Securities Purchase Agreement dated March 2, 2005 between Allos and the Investors listed on the signature pages thereto. |
|
8-K/A |
|
3/10/2005 |
|
10.41 |
|
|
|
10.07 |
|
Registration Rights Agreement dated March 4, 2005 between Allos and the Investors listed on Schedule I thereto. |
|
8-K/A |
|
3/10/2005 |
|
10.42 |
|
|
|
10.08 |
|
Letter Agreement dated March 4, 2005 among Allos, Warburg Pincus Private Equity VIII, L.P., Warburg Pincus & Co. and Warburg Pincus LLC. |
|
8-K |
|
3/4/2005 |
|
10.43 |
|
|
|
10.09 |
† |
Summary of Compensation Arrangements for Non-Employee Directors. |
|
10-Q |
|
8/7/2007 |
|
10.32 |
|
|
|
10.10 |
† |
Restricted Stock Award Agreement dated March 9, 2006 between Allos and Paul L. Berns. |
|
8-K |
|
3/14/2006 |
|
10.2 |
|
|
63
Table of Contents
| | | | | | | | | | | |
| |
| | Incorporated by Reference | |
|
|---|
| |
| | Filed
Herewith |
|---|
| Exhibit No. | | Description | | Form | | Filing Date | | Number |
|---|
| | 10.11 | † | 2006 Inducement Award Plan, including forms of Stock Option Grant Notice with Stock Option Agreement and Restricted Stock Grant Notice with Restricted Stock Grant Agreement. | | 8-K | | 6/6/2006 | | 10.1 | | |
|
10.12 |
|
Letter agreement dated January 28, 2007 among Allos, Baker Bros. Investments, L.P., Baker Bros. Investments II, L.P., Baker/Tisch Investments, L.P., Baker Biotech Fund I, L.P., 14159, L.P. and Baker Brothers Life Sciences, L.P. |
|
8-K |
|
1/30/2007 |
|
10.1 |
|
|
|
10.13 |
* |
License Agreement for 10-Propargyl-10-Deazaaminopterin "PDX" dated December 23, 2002 and amended May 9, 2006 between Allos and SRI International, Sloan-Kettering Institute for Cancer Research and Southern Research Institute. |
|
10-Q |
|
8/7/2007 |
|
10.45 |
|
|
|
10.13.1 |
* |
Second Amendment to License Agreement for 10-Propargyl-10-Deazaaminopterin "PDX" dated November 6, 2007 between Allos and SRI International, Sloan-Kettering Institute for Cancer Research and Southern Research Institute. |
|
10-K |
|
2/27/2008 |
|
10.18.1 |
|
|
|
10.14 |
† |
Corporate Bonus Plan, as amended and restated effective September 15, 2008. |
|
10-Q |
|
11/5/2008 |
|
10.1 |
|
|
|
10.15 |
† |
Amended and Restated Employment Agreement, effective December 13, 2007, between Allos and Paul L. Berns. |
|
10-K |
|
2/27/2008 |
|
10.20 |
|
|
|
10.16 |
† |
Amended and Restated Employment Agreement, effective December 13, 2007, between Allos and Pablo J. Cagnoni, M.D. |
|
10-K |
|
2/27/2008 |
|
10.21 |
|
|
|
10.17 |
† |
Amended and Restated Employment Agreement, effective December 13, 2007, between Allos and James V. Caruso. |
|
10-K |
|
2/27/2008 |
|
10.22 |
|
|
|
10.18 |
† |
Amended and Restated Employment Agreement, effective December 13, 2007, between Allos and Marc H. Graboyes. |
|
10-K |
|
2/27/2008 |
|
10.23 |
|
|
|
10.19 |
† |
Letter agreement, effective January 22, 2008, between Allos and Bruce K. Bennett. |
|
10-K |
|
2/27/2008 |
|
10.24 |
|
|
|
10.20 |
* |
License Agreement, dated as of December 13, 2004, among Allos, The Regents of the University of Colorado, the University of Salford and Cancer Research Technology Limited. |
|
10-K/A |
|
8/25/2008 |
|
10.25 |
|
|
|
10.21 |
† |
Executive Compensation and Equity Awards. |
|
8-K |
|
2/27/2009 |
|
10.1 |
|
|
|
10.22 |
† |
Allos Therapeutics, Inc. 2008 Equity Incentive Plan. |
|
S-8 |
|
6/24/2008 |
|
99.1 |
|
|
64
Table of Contents
| | | | | | | | | | | |
| |
| | Incorporated by Reference | |
|
|---|
| |
| | Filed
Herewith |
|---|
| Exhibit No. | | Description | | Form | | Filing Date | | Number |
|---|
| | 10.22.1 | † | Form of Option Grant Notice and Agreement under the 2008 Equity Incentive Plan. | | S-8 | | 6/24/2008 | | 99.2 | | |
|
10.22.2 |
† |
Form of Restricted Stock Award Grant Notice and Agreement under the 2008 Equity Incentive Plan. |
|
S-8 |
|
6/24/2008 |
|
99.3 |
|
|
|
10.22.3 |
† |
Form of Restricted Stock Unit Grant Notice and Agreement under the 2008 Equity Incentive Plan. |
|
8-K |
|
2/27/2009 |
|
10.2 |
|
|
|
10.23 |
† |
Allos Therapeutics, Inc. Severance Benefit Plan, as amended and restated effective December 11, 2007. |
|
8-K |
|
2/27/2009 |
|
10.3 |
|
|
|
10.23.1 |
† |
Allos Therapeutics, Inc. Change in Control Severance Benefit Schedule, as amended and restated effective February 23, 2008. |
|
8-K |
|
2/27/2009 |
|
10.4 |
|
|
|
23.01 |
|
Consent of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm. |
|
|
|
|
|
|
|
X |
|
24.01 |
|
Power of Attorney (included on signature page hereto). |
|
|
|
|
|
|
|
X |
|
31.01 |
|
Rule 13a-14(a)/15d-14(a) Certification. |
|
|
|
|
|
|
|
X |
|
31.02 |
|
Rule 13a-14(a)/15d-14(a) Certification. |
|
|
|
|
|
|
|
X |
|
32.01 |
# |
Section 1350 Certification. |
|
|
|
|
|
|
|
X |
- †
- Indicates management contract or compensatory plan or arrangement required to be filed as an exhibit pursuant to Item 15(b) of Form 10-K.
- *
- Indicates confidential treatment has been granted with respect to specific portions of this exhibit. Omitted portions have been filed with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
- #
- The certifications attached as Exhibit 32.01 that accompany this Annual Report on Form 10-K are not deemed filed with the Securities and Exchange Commission and are not to be incorporated by reference into any filing of Allos Therapeutics, Inc. under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this Form 10-K, irrespective of any general incorporation language contained in such filing.
65
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | |
| | ALLOS THERAPEUTICS, INC. |
Date: March 3, 2009 | | By: | | /s/ PAUL L. BERNS
Paul L. Berns
President and Chief Executive Officer |
66
Table of Contents
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each individual whose signature appears below constitutes and appoints Paul L. Berns and David C. Clark, and each of them, as his true and lawful attorneys-in-fact and agents, with full power of substitution, for him and in his name, place and stead, in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with all exhibits thereto and all other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, full power and authority to do and perform each and every act and thing requisite and necessary to be done therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, and any of them or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this report has been signed by the following persons on behalf of the registrant on March 3, 2009, and in the capacities indicated:
| | |
Name | | Title |
|---|
|
|
|
/s/ STEPHEN J. HOFFMAN
Stephen J. Hoffman | | Chairman of Board of Directors and Director |
/s/ PAUL L. BERNS
Paul L. Berns |
|
President, Chief Executive Officer and Director
(Principal Executive Officer) |
/s/ DAVID C. CLARK
David C. Clark |
|
Vice President, Finance and Treasurer
(Principal Financial and Accounting Officer) |
/s/ MICHAEL D. CASEY
Michael D. Casey |
|
Director |
/s/ STEWART HEN
Stewart Hen |
|
Director |
/s/ JEFFREY R. LATTS
Jeffrey R. Latts |
|
Director |
/s/ JONATHAN S. LEFF
Jonathan S. Leff |
|
Director |
/s/ TIMOTHY P. LYNCH
Timothy P. Lynch |
|
Director |
67
Table of Contents
Allos Therapeutics, Inc.
Index to Financial Statements
| | |
| | Page |
|---|
Report of Independent Registered Public Accounting Firm | | F-2 |
Balance Sheets | |
F-3 |
Statements of Operations | |
F-4 |
Statements of Changes in Stockholders' Equity (Deficit) | |
F-5 |
Statements of Cash Flows | |
F-10 |
Notes to Financial Statements | |
F-11 |
F-1
Table of Contents
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Allos Therapeutics, Inc.:
In our opinion, the accompanying balance sheets and the related statements of operations, changes in stockholders' equity (deficit), and cash flows present fairly, in all material respects, the financial position of Allos Therapeutics, Inc. (a development stage enterprise) at December 31, 2008 and 2007, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2008 and, cumulatively, for the period from September 1, 1992 (date of inception) to December 31, 2008, in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2008, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company's management is responsible for these financial statements, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in Management's Report on Internal Control Over Financial Reporting, appearing under Item 9A. Our responsibility is to express opinions on these financial statements and on the Company's internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP
Denver, CO
March 3, 2009
F-2
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
BALANCE SHEETS
| | | | | | | | | |
| | December 31, | |
|---|
| | 2008 | | 2007 | |
|---|
ASSETS | | | | | | | |
Current assets: | | | | | | | |
| | Cash and cash equivalents | | $ | 30,458,424 | | $ | 15,919,664 | |
| | Restricted cash | | | 237,632 | | | 183,334 | |
| | Investments in marketable securities | | | 53,468,942 | | | 41,836,566 | |
| | Prepaid research and development expenses | | | 919,384 | | | 524,704 | |
| | Prepaid expenses and other assets | | | 2,772,235 | | | 2,374,471 | |
| | | | | | |
| | | Total current assets | | | 87,856,617 | | | 60,838,739 | |
| | | | | | |
Property and equipment, net | | | 1,307,084 | | | 621,451 | |
Investments in marketable securities | | | 38,480 | | | — | |
Other assets | | | 137,423 | | | — | |
| | | | | | |
| | | Total assets | | $ | 89,339,604 | | $ | 61,460,190 | |
| | | | | | |
LIABILITIES AND STOCKHOLDERS' EQUITY | | | | | | | |
Current liabilities: | | | | | | | |
| | Trade accounts payable | | $ | 280,526 | | $ | 1,191,849 | |
| | Accrued liabilities | | | 9,594,712 | | | 7,689,338 | |
| | | | | | |
| | | Total current liabilities | | | 9,875,238 | | | 8,881,187 | |
Commitments and contingencies (Note 8) | | | | | | | |
Stockholders' equity: | | | | | | | |
| | Preferred stock, $0.001 par value; 10,000,000 shares authorized; no shares issued or outstanding | | | — | | | — | |
| | Series A Junior Participating Preferred Stock, $0.001 par value; 1,000,000 shares designated from authorized preferred stock; no shares issued or outstanding | | | — | | | — | |
| | Common stock, $0.001 par value; 150,000,000 shares authorized; 81,238,812 and 67,641,943 shares issued and outstanding at December 31, 2008 and December 31, 2007, respectively | | | 81,239 | | | 67,642 | |
Additional paid-in capital | | | 379,042,015 | | | 300,440,336 | |
Deficit accumulated during the development stage | | | (299,658,888 | ) | | (247,928,975 | ) |
| | | | | | |
| | | Total stockholders' equity | | | 79,464,366 | | | 52,579,003 | |
| | | | | | |
| | | Total liabilities and stockholders' equity | | $ | 89,339,604 | | $ | 61,460,190 | |
| | | | | | |
The accompanying notes are an integral part of these financial statements.
F-3
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
STATEMENTS OF OPERATIONS
| | | | | | | | | | | | | | | |
| | Years Ended December 31, | | Cumulative
Period from
September 1, 1992
(date of inception)
through
December 31,
| |
|---|
| | 2008 | | 2007 | | 2006 | | 2008 | |
|---|
Operating expenses: | | | | | | | | | | | | | |
| | Research and development | | $ | 23,848,052 | | $ | 17,444,320 | | $ | 14,322,601 | | $ | 149,147,924 | |
| | Clinical manufacturing | | | 6,747,146 | | | 5,547,411 | | | 2,283,907 | | | 41,359,252 | |
| | Marketing, general and administrative | | | 23,043,428 | | | 19,672,014 | | | 14,876,273 | | | 125,873,969 | |
| | Restructuring and separation costs | | | — | | | — | | | 645,666 | | | 1,663,821 | |
| | | | | | | | | | |
| | | Total operating expenses | | | 53,638,626 | | | 42,663,745 | | | 32,128,447 | | | 318,044,966 | |
| | | | | | | | | | |
Loss from operations | | | (53,638,626 | ) | | (42,663,745 | ) | | (32,128,447 | ) | | (318,044,966 | ) |
Gain on settlement claims | | | — | | | — | | | — | | | 5,110,083 | |
Interest and other income, net | | | 1,908,713 | | | 3,294,146 | | | 1,915,977 | | | 23,512,459 | |
| | | | | | | | | | |
| | | Net loss | | | (51,729,913 | ) | | (39,369,599 | ) | | (30,212,470 | ) | | (289,422,424 | ) |
Dividend related to beneficial conversion feature of preferred stock | | | — | | | — | | | — | | | (10,236,464 | ) |
| | | | | | | | | | |
Net loss attributable to common stockholders | | $ | (51,729,913 | ) | $ | (39,369,599 | ) | $ | (30,212,470 | ) | $ | (299,658,888 | ) |
| | | | | | | | | | |
Net loss per share: basic and diluted | | $ | (0.69 | ) | $ | (0.60 | ) | $ | (0.55 | ) | | | |
| | | | | | | | | | | |
Weighted average shares: basic and diluted | | | 75,399,774 | | | 65,188,913 | | | 55,299,614 | | | | |
| | | | | | | | | | | |
The accompanying notes are an integral part of these financial statements.
F-4
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
STATEMENTS OF CHANGES IN STOCKHOLDERS' EQUITY (DEFICIT)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Common Stock | | Preferred Stock | | Additional
Paid-in
| | Notes
Receivable
From
| | Deferred
| | Deficit
Accumulated
During the
Development
| | Total
Stockholders'
Equity
| |
|---|
| | Shares | | Amount | | Shares | | Amount | | Capital | | Stockholders | | Compensation | | Stage | | (Deficit) | |
|---|
Subscription receivable for common stock at $1.61 per share | | | — | | $ | 90 | | | — | | $ | — | | $ | — | | $ | — | | $ | — | | $ | — | | $ | 90 | |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 1992 | | | — | | | 90 | | | — | | | — | | | — | | | — | | | — | | | — | | | 90 | |
| | Subscription receivable for common stock at $1.61 per share | | | — | | | 10 | | | — | | | — | | | — | | | — | | | — | | | — | | | 10 | |
| | Issuance of common stock for subscription receivable | | | 992,000 | | | 892 | | | — | | | — | | | (892 | ) | | — | | | — | | | — | | | — | |
Net loss | | | — | | | — | | | — | | | — | | | — | | | — | | | — | �� | | (24,784 | ) | | (24,784 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 1993 | | | 992,000 | | | 992 | | | — | | | — | | | (892 | ) | | — | | | — | | | (24,784 | ) | | (24,684 | ) |
| | Issuance of $.001 par value common stock in exchange for license agreement | | | 248,000 | | | 248 | | | — | | | — | | | 39,752 | | | — | | | — | | | — | | | 40,000 | |
| | Issuance of Series A convertible preferred stock ($.001 par value) together with Series A and Series B stock warrants at $1.00 per share | | | — | | | — | | | 700,000 | | | 704 | | | 529,023 | | | — | | | — | | | — | | | 529,727 | |
| | Issuance of Series A convertible preferred stock upon exercise of Series A warrants at $1.00 per share | | | — | | | — | | | 1,300,000 | | | 1,300 | | | 1,298,700 | | | — | | | — | | | — | | | 1,300,000 | |
| | Accretion to redemption value of preferred stock | | | — | | | — | | | — | | | — | | | 58,839 | | | — | | | — | | | (58,839 | ) | | — | |
Net loss | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (898,929 | ) | | (898,929 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 1994 | | | 1,240,000 | | | 1,240 | | | 2,000,000 | | | 2,004 | | | 1,925,422 | | | — | | | — | | | (982,552 | ) | | 946,114 | |
| | Issuance of Series A convertible preferred stock at $1.00 per share | | | — | | | — | | | 3,000,000 | | | 3,000 | | | 2,973,454 | | | — | | | — | | | — | | | 2,976,454 | |
| | Accretion to redemption value of preferred stock | | | — | | | — | | | — | | | — | | | 229,837 | | | — | | | — | | | (229,837 | ) | | — | |
Net loss | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (2,384,176 | ) | | (2,384,176 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 1995 | | | 1,240,000 | | | 1,240 | | | 5,000,000 | | | 5,004 | | | 5,128,713 | | | — | | | — | | | (3,596,565 | ) | | 1,538,392 | |
| | Issuance of Series B convertible preferred stock at $1.60 per share, net of issuance costs | | | — | | | — | | | 5,032,500 | | | 5,033 | | | 7,992,705 | | | — | | | — | | | — | | | 7,997,738 | |
| | Cancellation of Series B warrants previously issued with Series A | | | — | | | — | | | — | | | (4 | ) | | 4 | | | — | | | — | | | — | | | — | |
| | Cancellation of Series A redemption rights | | | — | | | — | | | — | | | — | | | (288,676 | ) | | — | | | — | | | 288,676 | | | — | |
| | Issuance of common stock upon exercise of stock options for cash of $4,024 and notes receivable of $90,000 at $0.16 per share | | | 582,950 | | | 583 | | | — | | | — | | | 93,441 | | | (90,000 | ) | | — | | | — | | | 4,024 | |
Net loss | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (4,053,027 | ) | | (4,053,027 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 1996 | | | 1,822,950 | | | 1,823 | | | 10,032,500 | | | 10,033 | | | 12,926,187 | | | (90,000 | ) | | — | | | (7,360,916 | ) | | 5,487,127 | |
F-5
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
STATEMENTS OF CHANGES IN STOCKHOLDERS' EQUITY (DEFICIT) (Continued)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Common Stock | | Preferred Stock | | Additional
Paid-in
| | Notes
Receivable
From
| | Deferred
| | Deficit
Accumulated
During the
Development
| | Total
Stockholders'
Equity
| |
|---|
| | Shares | | Amount | | Shares | | Amount | | Capital | | Stockholders | | Compensation | | Stage | | (Deficit) | |
|---|
Balance at December 31, 1996 | | | 1,822,950 | | | 1,823 | | | 10,032,500 | | | 10,033 | | | 12,926,187 | | | (90,000 | ) | | — | | | (7,360,916 | ) | | 5,487,127 | |
| | Issuance of common stock upon exercise of stock options for cash of $20,288 and notes receivable of $49,687 at $0.16 - $0.40 per share | | | 175,770 | | | 176 | | | — | | | — | | | 69,799 | | | (49,687 | ) | | — | | | — | | | 20,288 | |
Net loss | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (6,512,591 | ) | | (6,512,591 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 1997 | | | 1,998,720 | | | 1,999 | | | 10,032,500 | | | 10,033 | | | 12,995,986 | | | (139,687 | ) | | — | | | (13,873,507 | ) | | (1,005,176 | ) |
| | Issuance of Series C convertible preferred stock at $1.81 per share, net of issuance costs | | | — | | | — | | | 9,944,750 | | | 9,945 | | | 17,937,102 | | | — | | | — | | | — | | | 17,947,047 | |
| | Issuance of common stock upon exercise of stock options for cash of $3,464 at $0.16 - $0.40 per share | | | 13,239 | | | 13 | | | — | | | — | | | 3,451 | | | — | | | — | | | — | | | 3,464 | |
Net loss | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (8,573,923 | ) | | (8,573,923 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 1998 | | | 2,011,959 | | | 2,012 | | | 19,977,250 | | | 19,978 | | | 30,936,539 | | | (139,687 | ) | | — | | | (22,447,430 | ) | | 8,371,412 | |
| | Issuance of Series C convertible preferred stock at $1.81 per share, net of issuance costs | | | — | | | — | | | 5,311,036 | | | 5,311 | | | 9,529,532 | | | — | | | — | | | — | | | 9,534,843 | |
| | Issuance of common stock upon exercise of stock options for cash of $3,695 at $0.16 - $0.56 per share | | | 10,179 | | | 10 | | | — | | | — | | | 3,685 | | | — | | | — | | | — | | | 3,695 | |
| | Deferred compensation related to options | | | — | | | — | | | — | | | — | | | 6,811,055 | | | — | | | (4,442,294 | ) | | — | | | 2,368,761 | |
| | Beneficial conversion feature related to issuance of preferred stock | | | — | | | — | | | — | | | — | | | 9,612,975 | | | — | | | — | | | (9,612,975 | ) | | — | |
Net loss | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (11,287,740 | ) | | (11,287,740 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 1999 | | | 2,022,138 | | | 2,022 | | | 25,288,286 | | | 25,289 | | | 56,893,786 | | | (139,687 | ) | | (4,442,294 | ) | | (43,348,145 | ) | | 8,990,971 | |
| | Issuance of 5,000,000 shares of common stock, net of issuance costs | | | 5,000,000 | | | 5,000 | | | — | | | — | | | 82,764,396 | | | — | | | — | | | — | | | 82,769,396 | |
| | Conversion of preferred stock to common stock upon IPO | | | 15,678,737 | | | 15,679 | | | (25,288,286 | ) | | (25,289 | ) | | 9,610 | | | — | | | — | | | — | | | — | |
| | Extinguishments of notes receivable | | | — | | | — | | | — | | | — | | | — | | | 139,687 | | | — | | | — | | | 139,687 | |
| | Issuance of common stock upon exercise of stock options for cash of $73,855 at $0.16 - $0.56 per share | | | 254,001 | | | 254 | | | — | | | — | | | 73,601 | | | — | | | — | | | — | | | 73,855 | |
| | Deferred compensation related to options | | | — | | | — | | | — | | | — | | | 16,860,998 | | | — | | | (2,062,800 | ) | | — | | | 14,798,198 | |
Net loss | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (23,361,475 | ) | | (23,361,475 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2000 | | | 22,954,876 | | | 22,955 | | | — | | | — | | | 156,602,391 | | | — | | | (6,505,094 | ) | | (66,709,620 | ) | | 83,410,632 | |
| | Issuance of common stock upon exercise of stock options for cash of $103,831 at $0.40 - $2.42 per share | | | 175,096 | | | 175 | | | — | | | — | | | 103,656 | | | — | | | — | | | — | | | 103,831 | |
| | Issuance of common stock upon exercise of purchase rights at an exercise price of $3.84 per share | | | 9,225 | | | 9 | | | — | | | — | | | 35,433 | | | — | | | — | | | — | | | 35,442 | |
| | Stock compensation expense | | | — | | | — | | | — | | | — | | | 283,512 | | | — | | | — | | | — | | | 283,512 | |
| | Deferred compensation related to options | | | — | | | — | | | — | | | — | | | (99,700 | ) | | — | | | 3,561,504 | | | — | | | 3,461,804 | |
Net loss | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (20,144,325 | ) | | (20,144,325 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2001 | | | 23,139,197 | | | 23,139 | | | — | | | — | | | 156,925,292 | | | — | | | (2,943,590 | ) | | (86,853,945 | ) | | 67,150,896 | |
F-6
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
STATEMENTS OF CHANGES IN STOCKHOLDERS' EQUITY (DEFICIT) (Continued)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Common Stock | | Preferred Stock | | Additional
Paid-in
| | Notes
Receivable
From
| | Deferred
| | Deficit
Accumulated
During the
Development
| | Total
Stockholders'
Equity
| |
|---|
| | Shares | | Amount | | Shares | | Amount | | Capital | | Stockholders | | Compensation | | Stage | | (Deficit) | |
|---|
Balance at December 31, 2001 | | | 23,139,197 | | | 23,139 | | | — | | | — | | | 156,925,292 | | | — | | | (2,943,590 | ) | | (86,853,945 | ) | | 67,150,896 | |
| | Issuance of common stock in private placement for $6.00 per share, net of issuance costs | | | 2,500,000 | | | 2,500 | | | — | | | — | | | 14,929,273 | | | — | | | — | | | — | | | 14,931,773 | |
| | Issuance of common stock upon exercise of stock options for cash of $290,753 at $0.40 - $7.38 per share | | | 187,126 | | | 187 | | | — | | | — | | | 290,566 | | | — | | | — | | | — | | | 290,753 | |
| | Issuance of common stock upon exercise of purchase rights at an exercise price of $3.84 - $6.39 per share | | | 27,446 | | | 27 | | | — | | | — | | | 120,252 | | | — | | | — | | | — | | | 120,279 | |
| | Issuance of common stock upon exercise of warrants for equipment lease line | | | 9,685 | | | 10 | | | — | | | — | | | 21,521 | | | — | | | — | | | — | | | 21,531 | |
| | Stock compensation expense | | | — | | | — | | | — | | | — | | | 190,378 | | | — | | | — | | | — | | | 190,378 | |
| | Deferred compensation related to options | | | — | | | — | | | — | | | — | | | (1,456,577 | ) | | — | | | 1,842,124 | | | — | | | 385,547 | |
Net loss | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (25,768,974 | ) | | (25,768,974 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2002 | | | 25,863,454 | | | 25,863 | | | — | | | — | | | 171,020,705 | | | — | | | (1,101,466 | ) | | (112,622,919 | ) | | 57,322,183 | |
| | Issuance of common stock upon exercise of stock options for cash of $75,686 at $.56 - $2.42 per share | | | 35,400 | | | 35 | | | — | | | — | | | 75,651 | | | — | | | — | | | — | | | 75,686 | |
| | Issuance of common stock upon exercise of purchase rights at an exercise price of $2.48 - $2.58 per share | | | 32,189 | | | 33 | | | — | | | — | | | 81,466 | | | — | | | — | | | — | | | 81,499 | |
| | Issuance of common stock in private placement for $2.32 per share together with common stock warrants for $3.14 per share, net of issuance costs | | | 5,172,412 | | | 5,173 | | | — | | | — | | | 11,196,549 | | | — | | | — | | | — | | | 11,201,722 | |
| | Stock compensation expense | | | — | | | — | | | — | | | — | | | 178,166 | | | — | | | — | | | — | | | 178,166 | |
| | Deferred compensation related to options | | | — | | | — | | | — | | | — | | | (1,137,244 | ) | | — | | | 815,890 | | | — | | | (321,354 | ) |
Net loss | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (23,126,625 | ) | | (23,126,625 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2003 | | | 31,103,455 | | | 31,104 | | | — | | | — | | | 181,415,293 | | | — | | | (285,576 | ) | | (135,749,544 | ) | | 45,411,277 | |
| | Issuance of common stock upon exercise of stock options for cash of $97,794 at $.40 - $4.75 per share | | | 35,935 | | | 36 | | | — | | | — | | | 97,758 | | | — | | | — | | | — | | | 97,794 | |
| | Issuance of common stock upon exercise of purchase rights at an exercise price of $1.85 - $1.91 per share | | | 36,393 | | | 36 | | | — | | | — | | | 68,239 | | | — | | | — | | | — | | | 68,275 | |
| | Stock issuance costs | | | — | | | — | | | — | | | — | | | (8,279 | ) | | — | | | — | | | — | | | (8,279 | ) |
| | Stock compensation recovery | | | — | | | — | | | — | | | — | | | (170,118 | ) | | — | | | — | | | — | | | (170,118 | ) |
| | Deferred compensation related to options | | | — | | | — | | | — | | | — | | | 50,583 | | | — | | | 250,756 | | | — | | | 301,339 | |
Net loss | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (21,837,285 | ) | | (21,837,285 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2004 | | | 31,175,783 | | | 31,176 | | | — | | | — | | | 181,453,476 | | | — | | | (34,820 | ) | | (157,586,829 | ) | | 23,863,003 | |
F-7
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
STATEMENTS OF CHANGES IN STOCKHOLDERS' EQUITY (DEFICIT) (Continued)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Common Stock | | Preferred Stock | | Additional
Paid-in
| | Notes
Receivable
From
| | Deferred
| | Deficit
Accumulated
During the
Development
| | Total
Stockholders'
Equity
| |
|---|
| | Shares | | Amount | | Shares | | Amount | | Capital | | Stockholders | | Compensation | | Stage | | (Deficit) | |
|---|
Balance at December 31, 2004 | | | 31,175,783 | | | 31,176 | | | — | | | — | | | 181,453,476 | | | — | | | (34,820 | ) | | (157,586,829 | ) | | 23,863,003 | |
| | Issuance of common stock upon exercise of stock options for cash of $197,513 at $.16 - $1.78 per share | | | 352,081 | | | 352 | | | — | | | — | | | 197,161 | | | — | | | — | | | — | | | 197,513 | |
| | Issuance of common stock upon exercise of purchase rights at an exercise price of $1.82 and $1.85 per share | | | 26,675 | | | 27 | | | — | | | — | | | 48,804 | | | — | | | — | | | — | | | 48,831 | |
| | Issuance of Series A Exchangeable Preferred Stock at $22.10 per share, net of issuance costs | | | — | | | — | | | 2,352,443 | | | 2,352 | | | 48,837,479 | | | — | | | — | | | — | | | 48,839,831 | |
| | Beneficial conversion feature related to issuance of preferred stock | | | — | | | — | | | — | | | — | | | 623,489 | | | — | | | — | | | (623,489 | ) | | — | |
| | Conversion of Series A Exchangeable Preferred Stock to common stock | | | 23,524,430 | | | 23,524 | | | (2,352,443 | ) | | (2,352 | ) | | (21,172 | ) | | — | | | — | | | — | | | — | |
| | Stock compensation expense | | | — | | | — | | | — | | | — | | | 443,644 | | | — | | | — | | | — | | | 443,644 | |
| | Deferred compensation related to options | | | — | | | — | | | — | | | — | | | (604 | ) | | — | | | 34,820 | | | — | | | 34,216 | |
Net loss | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (20,136,588 | ) | | (20,136,588 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2005 | | | 55,078,969 | | | 55,079 | | | — | | | — | | | 231,582,277 | | | — | | | — | | | (178,346,906 | ) | | 53,290,450 | |
| | Issuance of common stock upon exercise of stock options for cash of $561,097 at $.40 - $3.20 per share | | | 413,680 | | | 414 | | | — | | | — | | | 560,683 | | | — | | | — | | | — | | | 561,097 | |
| | Issuance of common stock upon exercise of purchase rights at an exercise price of $1.82, $1.96 and $3.05 per share | | | 44,319 | | | 44 | | | — | | | — | | | 90,627 | | | — | | | — | | | — | | | 90,671 | |
| | Issuance of common stock upon net exercise of warrants at an exercise price of $3.14 per share | | | 37,459 | | | 37 | | | — | | | — | | | (37 | ) | | — | | | — | | | — | | | — | |
| | Issuance of common stock upon exercise of warrants for cash of $2,233,187 at an exercise price of $3.14 per share | | | 711,206 | | | 711 | | | — | | | — | | | 2,232,476 | | | — | | | — | | | — | | | 2,233,187 | |
| | Issuance of restricted stock | | | 410,000 | | | 410 | | | — | | | — | | | (410 | ) | | — | | | — | | | — | | | — | |
| | Stock compensation expense | | | — | | | — | | | — | | | — | | | 3,587,001 | | | — | | | — | | | — | | | 3,587,001 | |
Net loss | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (30,212,470 | ) | | (30,212,470 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2006 | | | 56,695,633 | | | 56,695 | | | — | | | — | | | 238,052,617 | | | — | | | — | | | (208,559,376 | ) | | 29,549,936 | |
F-8
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
STATEMENTS OF CHANGES IN STOCKHOLDERS' EQUITY (DEFICIT) (Continued)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Common Stock | | Preferred Stock | | Additional
Paid-in
| | Notes
Receivable
From
| | Deferred
| | Deficit
Accumulated
During the
Development
| | Total
Stockholders'
Equity
| |
|---|
| | Shares | | Amount | | Shares | | Amount | | Capital | | Stockholders | | Compensation | | Stage | | (Deficit) | |
|---|
Balance at December 31, 2006 | | | 56,695,633 | | | 56,695 | | | — | | | — | | | 238,052,617 | | | — | | | — | | | (208,559,376 | ) | | 29,549,936 | |
| | Issuance of common stock upon exercise of stock options for cash of $3,696,811 at $.40 - $6.38 per share | | | 1,156,471 | | | 1,157 | | | — | | | — | | | 3,695,654 | | | — | | | — | | | — | | | 3,696,811 | |
| | Issuance of common stock upon exercise of purchase rights at an exercise price of $1.82 - $3.89 per share | | | 41,148 | | | 41 | | | — | | | — | | | 124,805 | | | — | | | — | | | — | | | 124,846 | |
| | Issuance of common stock upon net exercise of warrants at an exercise price of $3.14 per share | | | 112,106 | | | 112 | | | — | | | — | | | (112 | ) | | — | | | — | | | — | | | — | |
| | Issuance of common stock upon exercise of warrants for cash of $1,669,177 at an exercise price of $3.14 per share | | | 531,585 | | | 532 | | | — | | | — | | | 1,668,645 | | | — | | | — | | | — | | | 1,669,177 | |
| | Issuance of common stock net of offering costs of $3,742,793, at $6.00 per share | | | 9,000,000 | | | 9,000 | | | — | | | — | | | 50,248,207 | | | — | | | — | | | — | | | 50,257,207 | |
| | Issuance of restricted stock | | | 105,000 | | | 105 | | | — | | | — | | | (105 | ) | | — | | | — | | | — | | | — | |
| | Stock compensation expense | | | — | | | — | | | — | | | — | | | 6,650,625 | | | — | | | — | | | — | | | 6,650,625 | |
Net loss | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (39,369,599 | ) | | (39,369,599 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2007 | | | 67,641,943 | | | 67,642 | | | — | | | — | | | 300,440,336 | | | — | | | — | | | (247,928,975 | ) | | 52,579,003 | |
| | | | | | | | | | | | | | | | | | | | |
| | Issuance of common stock upon exercise of stock options for cash of $5,291,229 at $2.06 - $8.75 per share | | | 1,144,041 | | | 1,144 | | | — | | | — | | | 5,290,085 | | | — | | | — | | | — | | | 5,291,229 | |
| | Issuance of common stock upon exercise of purchase rights at an exercise price of $5.18 and $5.20 per share | | | 22,828 | | | 23 | | | — | | | — | | | 118,447 | | | — | | | — | | | — | | | 118,470 | |
| | Issuance of common stock net of offering costs of $4,863,672, at $5.64 per share | | | 12,420,000 | | | 12,420 | | | — | | | — | | | 65,172,708 | | | — | | | — | | | — | | | 65,185,128 | |
| | Issuance of restricted stock | | | 10,000 | | | 10 | | | — | | | — | | | (10 | ) | | — | | | — | | | — | | | — | |
| | Stock compensation expense | | | — | | | — | | | — | | | — | | | 8,020,449 | | | — | | | — | | | — | | | 8,020,449 | |
Net loss | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (51,729,913 | ) | | (51,729,913 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2008 | | | 81,238,812 | | $ | 81,239 | | | — | | $ | — | | $ | 379,042,015 | | $ | — | | $ | — | | $ | (299,658,888 | ) | $ | 79,464,366 | |
| | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these financial statements.
F-9
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
STATEMENTS OF CASH FLOWS
| | | | | | | | | | | | | | | | | |
| | Years Ended December 31, | | Cumulative
Period from
September 1, 1992
(date of inception)
through
December 31,
| |
|---|
| | 2008 | | 2007 | | 2006 | | 2008 | |
|---|
Cash Flows From Operating Activities: | | | | | | | | | | | | | |
| | Net loss | | $ | (51,729,913 | ) | $ | (39,369,599 | ) | $ | (30,212,470 | ) | $ | (289,422,424 | ) |
| | Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | | | | |
| | | Depreciation and amortization | | | 392,736 | | | 361,045 | | | 312,251 | | | 3,841,045 | |
| | | Stock-based compensation expense | | | 8,020,449 | | | 6,650,625 | | | 3,587,001 | | | 40,302,168 | |
| | | Write-off of long-term investment | | | — | | | — | | | — | | | 1,000,000 | |
| | | Realized loss on sale of marketable securities | | | 551,698 | | | — | | | — | | | 551,698 | |
| | | Other | | | 18,688 | | | — | | | — | | | 117,809 | |
| | | Changes in operating assets and liabilities: | | | | | | | | | | | | | |
| | | | Prepaid expenses and other assets | | | (929,867 | ) | | (283,823 | ) | | (2,054,486 | ) | | (3,819,042 | ) |
| | | | Interest receivable on investments | | | (167,851 | ) | | (231,201 | ) | | 179,326 | | | (833,160 | ) |
| | | | Trade accounts payable | | | (911,323 | ) | | 791,716 | | | 34,921 | | | 280,526 | |
| | | | Accrued liabilities | | | 1,905,374 | | | 1,257,817 | | | 3,006,617 | | | 9,594,712 | |
| | | | | | | | | | |
| | | | | Net cash used in operating activities | | | (42,850,009 | ) | | (30,823,420 | ) | | (25,146,840 | ) | | (238,386,668 | ) |
| | | | | | | | | | |
Cash Flows From Investing Activities: | | | | | | | | | | | | | |
| | Acquisition of property and equipment | | | (1,097,057 | ) | | (378,976 | ) | | (228,043 | ) | | (4,912,811 | ) |
| | (Pledge) release of restricted cash | | | (54,298 | ) | | 183,333 | | | 183,333 | | | (237,632 | ) |
| | Purchases of marketable securities | | | (93,938,031 | ) | | (89,014,032 | ) | | (33,847,513 | ) | | (609,596,257 | ) |
| | Proceeds from maturities of marketable securities | | | 75,135,828 | | | 70,134,192 | | | 62,000,000 | | | 549,622,797 | |
| | Proceeds from sales of marketable securities | | | 6,747,500 | | | — | | | — | | | 6,747,500 | |
| | Purchase of long-term investment | | | — | | | — | | | — | | | (1,000,000 | ) |
| | Payments received on notes receivable | | | — | | | — | | | — | | | 49,687 | |
| | | | | | | | | | |
| | | | | Net cash (used in) provided by investing activities | | | (13,206,058 | ) | | (19,075,483 | ) | | 28,107,777 | | | (59,326,716 | ) |
| | | | | | | | | | |
Cash Flows From Financing Activities: | | | | | | | | | | | | | |
| | Principal payments under capital leases | | | — | | | — | | | — | | | (422,088 | ) |
| | Proceeds from sales leaseback | | | — | | | — | | | — | | | 120,492 | |
| | Proceeds from issuance of convertible preferred stock, net of issuance costs | | | — | | | — | | | — | | | 89,125,640 | |
| | Proceeds from issuance of common stock associated with stock options, stock warrants and employee stock purchase plan | | | 5,409,699 | | | 5,490,834 | | | 2,884,955 | | | 15,010,817 | |
| | Proceeds from issuance of common stock, net of issuance costs | | | 65,185,128 | | | 50,257,207 | | | — | | | 224,336,947 | |
| | | | | | | | | | |
| | | | | Net cash provided by financing activities | | | 70,594,827 | | | 55,748,041 | | | 2,884,955 | | | 328,171,808 | |
| | | | | | | | | | |
Net increase in cash and cash equivalents | | | 14,538,760 | | | 5,849,138 | | | 5,845,892 | | | 30,458,424 | |
Cash and cash equivalents, beginning of period | | | 15,919,664 | | | 10,070,526 | | | 4,224,634 | | | — | |
| | | | | | | | | | |
Cash and cash equivalents, end of period | | $ | 30,458,424 | | $ | 15,919,664 | | $ | 10,070,526 | | $ | 30,458,424 | |
| | | | | | | | | | |
Supplemental Schedule of Cash and Non-cash Operating and Financing Activities: | | | | | | | | | | | | | |
| | Cash paid for interest | | $ | — | | $ | — | | $ | — | | $ | 1,033,375 | |
| | Issuance of stock in exchange for license agreement | | | — | | | — | | | — | | | 40,000 | |
| | Capital lease obligations incurred for acquisition of property and equipment | | | — | | | — | | | — | | | 422,088 | |
| | Issuance of stock in exchange for notes receivable | | | — | | | — | | | — | | | 139,687 | |
| | Conversion of preferred stock to common stock | | | — | | | — | | | — | | | 89,125,640 | |
The accompanying notes are an integral part of these financial statements.
F-10
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS
Unless the context otherwise requires, references in this report to "Allos," the "Company," "we," "us" and "our" refer to Allos Therapeutics, Inc.
1. Formation and Business of the Company
We are a biopharmaceutical company focused on developing and commercializing innovative small molecule drugs for the treatment of cancer. We currently have two small molecule chemotherapeutic product candidates, pralatrexate (PDX) and RH1.
- •
- Pralatrexate (PDX), our lead product candidate, is a novel targeted antifolate designed to accumulate preferentially in cancer cells. Based on preclinical studies, we believe that pralatrexate selectively enters cells expressing RFC-1, a protein that is over expressed on cancer cells compared to normal cells. Once inside cancer cells, pralatrexate is efficiently polyglutamylated, which leads to high intracellular drug retention. Polyglutamylated pralatrexate essentially becomes "trapped" inside cancer cells, making it less susceptible to efflux-based drug resistance. Acting on the folate pathway, pralatrexate interferes with DNA synthesis and triggers cancer cell death. We believe pralatrexate has the potential to be delivered as a single agent or in combination therapy regimens.
- •
- RH1 is a small molecule chemotherapeutic agent that we believe is bioactivated by the enzyme DT-diaphorase, or DTD, also known as NAD(P)H quinone oxidoreductase, or NQ01. We believe DTD is over-expressed in many tumors, relative to normal tissue, including lung, colon, breast and liver tumors. We believe that because RH1 is bioactivated in the presence of DTD, it has the potential to provide targeted drug delivery to these tumor types while limiting the amount of toxicity to normal tissue. We currently are in the process of closing our Phase 1 study of RH1 in patients with advanced solid tumors and NHL and determining our future development plans, if any, for RH1.
In mid-2007, we discontinued the development of EFAPROXYN, one of our former product candidates, after announcing top-line results from ENRICH, a Phase 3 clinical trial of EFAPROXYN plus whole brain radiation therapy, or WBRT, in women with brain metastases originating from breast cancer. The study failed to achieve its primary endpoint of demonstrating a statistically significant improvement in overall survival in patients receiving EFAPROXYN plus WBRT, compared to patients receiving WBRT alone. We are currently pursuing the sale of our rights to EFAPROXYN although we may not receive any material consideration for any sale.
We incorporated in the Commonwealth of Virginia on September 1, 1992 as HemoTech Sciences, Inc. and filed amended Articles of Incorporation to change our name to Allos Therapeutics, Inc. on October 19, 1994. We reincorporated in Delaware on October 28, 1996. We operate as a single business segment.
Since our inception in 1992, we have not generated any revenue from product sales and have experienced significant net losses and negative cash flows from operations. We have incurred these losses principally from costs incurred in our research and development programs, our clinical manufacturing, and from our marketing, general and administrative expenses. Our primary business activities have been focused on the development of pralatrexate, RH1 (a program for which we are in the process of determining our future development plans, if any) and EFAPROXYN (a program which we discontinued in mid-2007).
F-11
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
1. Formation and Business of the Company (Continued)
Our ability to generate revenue and achieve profitability is dependent on our ability, alone or with partners, to successfully complete the development of pralatrexate, conduct clinical trials, obtain the necessary regulatory approvals, and manufacture and market pralatrexate. The timing and costs to complete the successful development of pralatrexate is highly uncertain, and therefore difficult to estimate. The lengthy process of seeking regulatory approvals for pralatrexate, and the subsequent compliance with applicable regulations, require the expenditure of substantial resources. Clinical development timelines, likelihood of success and total costs vary widely and are impacted by a variety of risks and uncertainties. Because of these risks and uncertainties, we cannot predict when or whether we will successfully complete the development of pralatrexate or the ultimate costs of such efforts. Due to these same factors, we cannot be certain when, or if, we will generate any revenue or net cash inflow from pralatrexate.
Even if our clinical trials demonstrate the safety and effectiveness of pralatrexate in its target indications, we do not expect to be able to generate commercial sales of pralatrexate until the second half of 2009, at the earliest. We expect to continue incurring net losses and negative cash flows for the foreseeable future. Although the size and timing of our future net losses are subject to significant uncertainty, we expect them to increase over the next several years as we continue to fund our research and development programs and prepare for the potential commercial launch of pralatrexate.
As of December 31, 2008, we had $84.0 million in cash, cash equivalents, and investments in marketable securities. Based upon the current status of our product development plans, we believe that our cash, cash equivalents, and investments in marketable securities as of December 31, 2008 should be adequate to support our operations through at least the next 12 months, although there can be no assurance that this can, in fact, be accomplished.
We anticipate continuing our current development programs and/or beginning other long-term development projects involving pralatrexate. These projects may require many years and substantial expenditures to complete and may ultimately be unsuccessful. In addition, we intend to submit a New Drug Application, or NDA, for pralatrexate for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma, or PTCL, in the first half of 2009. We expect to incur significant costs relating to preparations for the potential commercial launch of pralatrexate, including pre-commercial scale up of manufacturing and development of sales and marketing capabilities. Therefore, we will need to raise additional capital to support our future operations. Our actual capital requirements will depend on many factors, including:
- •
- the timing and outcome of our planned NDA for pralatrexate for the treatment of patients with relapsed or refractory PTCL;
- •
- the timing and costs associated with developing sales and marketing capabilities and commercializing pralatrexate, if it is approved for marketing;
- •
- the timing and costs associated with manufacturing clinical and commercial supplies of pralatrexate;
- •
- the timing and amount of revenues generated by our business activities, if any;
F-12
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
1. Formation and Business of the Company (Continued)
- •
- the timing and costs associated with conducting preclinical and clinical development of pralatrexate, as well as our evaluation of, and decisions with respect to, additional therapeutic indications for which we may develop pralatrexate;
- •
- the timing, costs and potential revenue associated with any co-promotion or other partnering arrangements entered into to commercialize pralatrexate, if it is approved for marketing; and
- •
- our evaluation of, and decisions with respect to, potential in-licensing or product acquisition opportunities or other strategic alternatives.
We may seek to obtain this additional capital through equity or debt financings, arrangements with corporate partners, or from other sources. Such financings or arrangements, if successfully consummated, may be dilutive to our existing stockholders. However, there is no assurance that additional financing will be available when needed, or that, if available, we will obtain such financing on terms that are favorable to our stockholders or us. In particular, the current instability in the global financial markets and lack of liquidity in the credit and capital markets may adversely affect our ability to secure adequate capital to support our future operations. In the event that additional funds are obtained through arrangements with collaborative partners or other sources, such arrangements may require us to relinquish rights to some of our technologies, product candidates or products under development, which we would otherwise seek to develop or commercialize ourselves on terms that are less favorable than might otherwise be available. If we are unable to generate meaningful amounts of revenue from future product sales, if any, or cannot otherwise raise sufficient additional funds to support our operations, we may be required to delay, reduce the scope of or eliminate one or more of our development programs and our business and future prospects for revenue and profitability may be harmed.
2. Summary of Significant Accounting Policies
Basis of Presentation
We have not generated any revenue to date and our activities have consisted primarily of developing product candidates, raising capital and recruiting personnel. Accordingly, we are considered to be in the development stage at December 31, 2008, as defined in Statement of Financial Accounting Standards, or SFAS, No. 7,Accounting and Reporting by Development Stage Enterprises.
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles in the United States of America requires management to make estimates and judgments that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amount of expenses during the reporting period. Actual results could differ from these estimates.
Cash, Cash Equivalents and Investments in Marketable Securities
All highly liquid investments with original maturities of three months or less are considered to be cash equivalents. The carrying values of our cash equivalents and investments in marketable securities approximate their market values based on quoted market prices. We account for investments in
F-13
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
2. Summary of Significant Accounting Policies (Continued)
marketable securities in accordance with SFAS No. 115,Accounting for Certain Investments in Debt and Equity Securities. Investments in marketable securities are classified as held to maturity and are carried at cost plus accrued interest. Our cash and cash equivalents are maintained in a financial institution in amounts that, at times, may exceed federally insured limits. We realized a loss of approximately $552,000 on the sale of certain of our investments in marketable securities during the year ended December 31, 2008. In response to the recent instability in the global financial markets, we reviewed our investments in marketable securities and sold certain investments prior to their maturity in order to preserve our principal, as the issuers of these securities experienced significant deteriorations in their creditworthiness as evidenced by investment rating downgrades. We have the ability and intent to hold our remaining investments in marketable securities as of December 31, 2008 to their scheduled maturity, although we monitor our investment portfolio with the primary objectives of preserving principal and maintaining proper liquidity to meet our operating needs. The weighted average duration of the remaining time to maturity for our portfolio of investments in marketable securities as of December 31, 2008 was approximately five months. As of December 31, 2008, our investments in marketable securities were held in a variety of interest-bearing instruments, consisting mainly of high-grade corporate notes. We did not hold any derivative instruments, foreign exchange contracts, asset backed securities, mortgage backed securities, auction rate securities, or securities of issuers in bankruptcy in our investment portfolio as of December 31, 2008.
Restricted Cash
On August 22, 2008, $237,632 of cash was pledged as collateral on a letter of credit related to a lease for administrative office space and was classified as restricted cash on the Balance Sheet.
On May 24, 2001, $550,000 of cash was pledged as collateral on a letter of credit related to a lease for our headquarters facility and was classified as restricted cash on the balance sheet. During the years ended December 31, 2008, 2007 and 2006, in accordance with the terms of the building lease, the amount of the letter of credit was reduced by $183,334, $183,333 and $183,333, respectively and as of December 31, 2008, the letter of credit was fully released.
Prepaid Research and Development Expenses
Research and development expenditures are charged to expense as incurred. In accordance with certain research and development agreements, we are obligated to make certain upfront payments upon execution of the agreement. We record these upfront payments as prepaid research and development expenses. Such payments are recorded to research and development expense as services are performed. We evaluate on a quarterly basis whether events and circumstances have occurred that may indicate impairment of remaining prepaid research and development expenses.
F-14
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
2. Summary of Significant Accounting Policies (Continued)
Prepaid Expenses and Other Assets
Prepaid expenses and other assets are comprised of the following:
| | | | | | | |
| | December 31, | |
|---|
| | 2008 | | 2007 | |
|---|
Prepaid expenses and other assets | | $ | 772,235 | | $ | 615,471 | |
Receivable and cash in escrow related to pending litigation settlement (see Note 8) | | | 2,000,000 | | | 1,759,000 | |
| | | | | | |
| | $ | 2,772,235 | | $ | 2,374,471 | |
| | | | | | |
Property and Equipment
Property and equipment is recorded at cost and is depreciated using the straight-line method over estimated useful lives. Depreciation and amortization expense was $392,736, $361,045 and $312,251 for the years ended December 31, 2008, 2007 and 2006, respectively, and $3,841,045 for the cumulative period from inception through December 31, 2008.
The components of property and equipment are as follows:
| | | | | | | | |
| | December 31, | |
|
|---|
| | Estimated
Lives |
|---|
| | 2008 | | 2007 |
|---|
Computer hardware and software | | $ | 1,752,276 | | $ | 1,520,157 | | 3 years |
Office furniture and equipment | | | 1,682,200 | | | 1,344,008 | | 5 - 7 years |
Leasehold improvements | | | 416,648 | | | 394,740 | | 7 years |
Lab equipment | | | 28,516 | | | 76,763 | | 5 years |
Software projects in process | | | 329,086 | | | — | | |
| | | | | | | |
| | | 4,208,726 | | | 3,335,668 | | |
Less accumulated depreciation and amortization | | | (2,901,642 | ) | | (2,714,217 | ) | |
| | | | | | | |
Property and equipment, net | | $ | 1,307,084 | | $ | 621,451 | | |
| | | | | | | |
Long-lived Assets
Long-lived assets, consisting primarily of property and equipment, are reviewed for impairment when events or changes in circumstances indicate the carrying value of the assets may not be recoverable. Recoverability is measured by comparison of the assets' book value to future net undiscounted cash flows the assets are expected to generate. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the book value of the assets exceed their fair value, which is measured based on the projected discounted future net cash flows arising from the assets.
F-15
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
2. Summary of Significant Accounting Policies (Continued)
Accrued liabilities
Accrued liabilities are comprised of the following:
| | | | | | | |
| | December 31, | |
|---|
| | 2008 | | 2007 | |
|---|
Accrued personnel costs | | $ | 2,816,404 | | $ | 2,122,805 | |
Accrued research and development expenses | | | 2,272,219 | | | 1,571,975 | |
Accrued litigation settlement costs (see Note 8) | | | 2,000,000 | | | 2,000,000 | |
Accrued clinical manufacturing expenses | | | 1,153,028 | | | 1,259,799 | |
Accrued expenses—other | | | 1,353,061 | | | 734,759 | |
| | | | | | |
| | $ | 9,594,712 | | $ | 7,689,338 | |
| | | | | | |
During the year ended December 31, 2007, we recorded $307,817 in research and development expenses and $117,280 in clinical manufacturing expenses related to the discontinuation of the EFAPROXYN development program. These expenses represent estimated costs to be incurred by contract research organizations in connection with closing out our EFAPROXYN clinical trials and estimated costs for the destruction and storage of EFAPROXYN bulk drug substance and formulated drug product. As of December 31, 2008, $101,176 remains accrued, with approximately $323,921 in payments made since the program was discontinued.
Operating Leases
We recognize lease expense on a straight-line basis over the initial lease term. For leases that contain rent holidays, escalation clauses or tenant improvement allowances, we recognize rent expense on a straight-line basis and record the difference between the rent expense and rental amount payable as deferred rent. As of December 31, 2008 and 2007, we had $82,355 and $0, respectively, of deferred rent in accrued liabilities.
Fair Value of Financial Instruments
In September 2006, the Financial Accounting Standards Board, or FASB, issued SFAS No. 157,Fair Value Measurements, or SFAS 157, which defines fair value, provides a framework for measuring fair value, and expands the disclosures required for fair value measurements. SFAS does not require any new fair value measurements but rather establishes a common definition of fair value applicable to all assets and liabilities measured at fair value. SFAS 157 defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The fair value hierarchy established by SFAS 157 prioritizes the inputs into valuation techniques used to measure fair value. Accordingly, we use valuation techniques
F-16
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
2. Summary of Significant Accounting Policies (Continued)
that maximize the use of observable inputs and minimize the use of unobservable inputs when determining fair value. The three levels of the hierarchy are as follows:
| | |
| Level 1: | | Inputs that reflect unadjusted quoted prices in active markets that are accessible to us for identical assets or liabilities; |
Level 2: |
|
Inputs include quoted prices for similar assets and liabilities in active and inactive markets or that are observable for the asset or liability either directly or indirectly; and |
Level 3: |
|
Unobservable inputs that are supported by little or no market activity. |
We have no assets or liabilities that were measured using quoted prices for similar assets and liabilities or significant unobservable inputs (Level 2 and Level 3 assets and liabilities, respectively) as of December 31, 2008. Our financial instruments include cash and cash equivalents, investments in marketable securities, prepaid expenses, accounts payable and accrued liabilities. The carrying amounts of financial instruments approximate their fair value due to their short maturities. The carrying value of our money market investments totaling $30,361,584 as of December 31, 2008 is included in cash and cash equivalents on our Balance Sheet and approximates their market values based on quoted market prices, or Level 1 inputs. We account for investments in marketable securities in accordance with SFAS No. 115,Accounting for Certain Investments in Debt and Equity Securities. Investments in marketable securities are classified as held to maturity and are carried at cost plus accrued interest.
Stock-Based Compensation
We adopted SFAS No. 123 (Revised 2004),Share-Based Payment, or SFAS 123R, effective January 1, 2006. Under the provisions of SFAS 123R, stock-based compensation is measured at the grant date based on the fair value of the award and is recognized as expense over the required service period of the award. Prior to the adoption of SFAS 123R, we accounted for grants of stock-based awards according to the intrinsic value method as prescribed by Accounting Principles Board Opinion No. 25,Accounting for Stock Issued to Employees and related Interpretations.
In March 2005, the Securities and Exchange Commission, or SEC, issued Staff Accounting Bulletin No. 107 (subsequently amended by SAB 110), or SAB 107, relating to SFAS 123R. We applied the provisions of SAB 107 in connection with our adoption of SFAS 123R. We adopted SFAS 123R using the modified prospective transition method, which requires the application of the accounting standard as of January 1, 2006, the first day of our fiscal year 2006. Our financial statements as of and for the years ended December 31, 2008, 2007 and 2006 reflect the impact of SFAS 123R (see Note 4). In accordance with the modified prospective transition method, our financial statements for prior periods have not been restated to reflect, and do not include, the impact of SFAS 123R.
F-17
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
2. Summary of Significant Accounting Policies (Continued)
In November 2005, the FASB issued Staff Position, or FSP, No. FAS 123(R)-3,Transition Election Related to Accounting for Tax Effects of Share-Based Payment Awards, or FSP 123R-3. We elected to adopt the alternative transition method provided in FSP 123R-3 for calculating the tax effects of stock-based compensation pursuant to SFAS 123R. The alternative transition method includes simplified methods to establish the beginning balance of the additional paid-in capital, or APIC, pool related to the tax effects of employee stock-based compensation, and to determine the subsequent impact on the APIC pool and our Statements of Cash Flows of the tax effects of employee stock-based compensation awards that are outstanding upon adoption of SFAS 123R. This adoption did not have an impact on our financial statements.
See Note 4—"Stock-Based Compensation Plans" for additional details regarding the impact of our stock based compensation plans on our financial statements.
Research and Development
Research and development expenditures are charged to expense as incurred. Research and development expenses include the costs of certain personnel, basic research, preclinical studies, clinical trials, regulatory affairs, biostatistical data analysis and licensing fees for our product candidates. We record upfront fees and milestone payments made under our licensing agreements for our product candidates as research and development expense as the services are performed. We accrue research and development expenses for activity as incurred during the fiscal year and prior to receiving invoices from clinical sites and third party clinical and preclinical research organizations. We accrue external costs for clinical and preclinical studies based on an evaluation of the following: the progress of the studies, including patient enrollment, dosing levels of patients enrolled, estimated costs to dose patients, invoices received, and contracted costs with clinical sites and third party clinical and preclinical research organizations. Significant judgments and estimates must be made and used in determining the accrued balance in any accounting period. Actual results could differ from those estimates. During the years ended December 31, 2008, 2007 and 2006, we did not have any changes in estimates that would have resulted in material adjustments to research and development expenses accrued in the prior period. However, during the quarter ended December 31, 2006, we did change our estimate relating to certain costs for our Phase 3 ENRICH trial for EFAPROXYN as a result of new information, which resulted in a reduction of research and development expenses of approximately $400,000 and a corresponding decrease in accrued research and development expenses as of December 31, 2006.
Clinical Manufacturing
Clinical manufacturing expenses include the costs of certain personnel, third party manufacturing costs for development of drug materials for use in clinical trials and preclinical studies, and costs associated with pre-commercial scale-up of manufacturing to support anticipated regulatory and potential commercial requirements. Our finished drug inventory is expensed to clinical manufacturing since we are still a development stage company and we have not received regulatory approval to market our product candidates. If and when we receive regulatory approval, we will be required to capitalize any future manufacturing costs for our marketed products at the lower of cost or market and then expense the sold inventory as a component of cost of goods sold.
F-18
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
2. Summary of Significant Accounting Policies (Continued)
Income Taxes
Income taxes are accounted for under SFAS No. 109,Accounting for Income Taxes, or FAS 109. Deferred income tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities at each year end and their respective tax bases using enacted tax rates in effect for the year in which the differences are expected to affect taxable income. Valuation allowances have been established to reduce the Company's deferred tax assets to zero, as we believe that it is more likely than not that such assets will not be realized.
Net Loss Per Share
Net loss per share is calculated in accordance with SFAS No. 128,Earnings Per Share, or SFAS 128. Under the provisions of SFAS 128, basic net loss per share is computed by dividing the net loss attributable to common stockholders for the period by the weighted average number of common shares outstanding during the period. Diluted earnings per share is computed by giving effect to all dilutive potential common stock outstanding during the period, including stock options, restricted stock, stock warrants and shares to be issued under our employee stock purchase plan.
Diluted net loss per share is the same as basic net loss per share for all periods presented because any potential dilutive common shares were anti-dilutive due to our net loss (as including such shares would decrease our basic net loss per share). Potential dilutive common shares that would have been included in the calculation of diluted earnings per share if we had net income are as follows:
| | | | | | | | | | |
| | Year ended December 31, | |
|---|
| | 2008 | | 2007 | | 2006 | |
|---|
Common stock options | | | 2,011,533 | | | 1,630,431 | | | 1,142,205 | |
Restricted stock | | | 316,322 | | | 438,226 | | | 410,000 | |
Common stock warrants | | | — | | | 262,132 | | | 255,210 | |
| | | | | | | | |
| | | 2,327,855 | | | 2,330,789 | | | 1,807,415 | |
| | | | | | | | |
Recent Accounting Pronouncements
In September 2006, the FASB issued SFAS No. 157,Fair Value Measurements, which defines fair value, provides a framework for measuring fair value, and expands the disclosures required for fair value measurements. SFAS 157 applies to other accounting pronouncements that require fair value measurements; it does not require any new fair value measurements. SFAS 157 is effective for fiscal years beginning after November 15, 2007 and we adopted it on January 1, 2008. The application of SFAS 157 to certain items has been deferred and will be effective for fiscal years beginning after November 15, 2008 and interim periods within that year. The adoption of this pronouncement did not have a material impact on our results of operations or financial position for the year ended December 31, 2008. We have no assets or liabilities that were measured using quoted prices for similar assets and liabilities or significant unobservable inputs (Level 2 and Level 3 assets and liabilities, respectively) as of December 31, 2008. Our financial instruments include cash and cash equivalents, investments in marketable securities, prepaid expenses, accounts payable and accrued liabilities. The
F-19
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
2. Summary of Significant Accounting Policies (Continued)
carrying amounts of financial instruments approximate their fair value due to their short maturities. We account for investments in marketable securities in accordance with SFAS No. 115,Accounting for Certain Investments in Debt and Equity Securities. Investments in marketable securities are classified as held to maturity and are carried at cost plus accrued interest.
In February 2007, the FASB issued SFAS No. 159,The Fair Value Option for Financial Assets and Financial Liabilities—Including an Amendment of FASB Statement No. 115, which is effective for fiscal years beginning after November 15, 2007 and we adopted it on January 1, 2008. This statement permits an entity to choose to measure many financial instruments and certain other items at fair value at specified election dates. Subsequent unrealized gains and losses on items for which the fair value option has been elected will be reported in earnings. The adoption of this pronouncement did not have a material impact on our results of operations or financial position for the year ended December 31, 2008, as we did not elect to measure any of our financial instruments at fair value.
In June 2007, the Emerging Issues Task Force, or EITF, issued a consensus, EITF 07-3,Advance Payments for Research and Development Activities, which states that non-refundable advance payments for goods that will be used or services that will be performed in future research and development activities should be deferred and capitalized until the goods have been delivered or the related services have been rendered. EITF 07-3 is to be applied prospectively for new contractual arrangements entered into in fiscal years beginning after December 15, 2007 and we adopted it on January 1, 2008. The adoption did not result in a material change to our current accounting practice.
In November 2007, the EITF issued a consensus, EITF 07-1,Accounting for Collaboration Arrangements Related to the Development and Commercialization of Intellectual Property, which is focused on how the parties to a collaborative agreement should account for costs incurred and revenue generated on sales to third parties, how sharing payments pursuant to a collaboration agreement should be presented in the income statement and certain related disclosure questions. EITF 07-1 is to be applied retrospectively for collaboration arrangements in fiscal years beginning after December 15, 2008. We currently do not have any such arrangements.
In December 2007, the FASB issued SFAS No. 141(R),Business Combinations. This Statement replaces SFAS No. 141,Business Combinations, and requires an acquirer to recognize the assets acquired, the liabilities assumed, including those arising from contractual contingencies, any contingent consideration, and any noncontrolling interest in the acquiree at the acquisition date, measured at their fair values as of that date, with limited exceptions specified in the statement. SFAS No. 141(R) also requires the acquirer in a business combination achieved in stages (sometimes referred to as a step acquisition) to recognize the identifiable assets and liabilities, as well as the noncontrolling interest in the acquiree, at the full amounts of their fair values (or other amounts determined in accordance with SFAS No. 141(R)). In addition, SFAS No. 141(R)'s requirement to measure the noncontrolling interest in the acquiree at fair value will result in recognizing the goodwill attributable to the noncontrolling interest in addition to that attributable to the acquirer. SFAS No. 141(R) amends SFAS No. 109,Accounting for Income Taxes, to require the acquirer to recognize changes in the amount of its deferred tax benefits that are recognizable because of a business combination either in income from continuing operations in the period of the combination or directly in contributed capital, depending on the circumstances. It also amends SFAS No. 142,Goodwill and Other Intangible Assets, to, among other things, provide guidance on the impairment testing of acquired research and development intangible
F-20
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
2. Summary of Significant Accounting Policies (Continued)
assets and assets that the acquirer intends not to use. SFAS No. 141(R) applies prospectively to business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2008. We are currently evaluating the potential impact of this statement and will apply it to any business combinations in the future. We do not expect any impact on our financial statements.
In December 2007, the FASB issued SFAS No. 160,Noncontrolling Interests in Consolidated Financial Statements. SFAS No. 160 amends Accounting Research Bulletin 51,Consolidated Financial Statements, to establish accounting and reporting standards for the noncontrolling interest in a subsidiary and for the deconsolidation of a subsidiary. It also clarifies that a noncontrolling interest in a subsidiary is an ownership interest in the consolidated entity that should be reported as equity in the consolidated financial statements. SFAS No. 160 also changes the way the consolidated income statement is presented by requiring consolidated net income to be reported at amounts that include the amounts attributable to both the parent and the noncontrolling interest. It also requires disclosure, on the face of the consolidated statement of income, of the amounts of consolidated net income attributable to the parent and to the noncontrolling interest. SFAS No. 160 requires that a parent recognize a gain or loss in net income when a subsidiary is deconsolidated and requires expanded disclosures in the consolidated financial statements that clearly identify and distinguish between the interests of the parent owners and the interests of the noncontrolling owners of a subsidiary. SFAS No. 160 is effective for fiscal periods, and interim periods within those fiscal years, beginning on or after December 15, 2008. We are currently evaluating the potential impact of this statement. We do not expect any impact on our financial statements.
In March 2008, the FASB issued SFAS No. 161,Disclosures about Derivative Instruments and Hedging Activities. SFAS No. 161 is intended to improve financial reporting about derivative instruments and hedging activities by requiring companies to enhance disclosure about how these instruments and activities affect their financial position, performance and cash flows. SFAS No. 161 also improves the transparency about the location and amounts of derivative instruments in a company's financial statements and how they are accounted for under SFAS No. 133. SFAS No. 161 is effective for financial statements issued for fiscal years beginning after November 15, 2008, and interim periods beginning after that date. We are currently evaluating the potential impact of this statement. We do not expect any impact on our financial statements.
In May 2008, the FASB issued SFAS No. 162,The Hierarchy of Generally Accepted Accounting Principles. SFAS No. 162 identifies the sources of accounting principles and the framework for selecting the principles to be used in the preparation of financial statements of nongovernmental entities that are presented in conformity with generally accepted accounting principles in the United States of America. SFAS No. 162 is effective 60 days following the SEC's approval of the Public Company Accounting Oversight Board, or PCAOB, amendments to AICPA Codification of Auditing Standards, AU Section 411,The Meaning of Present Fairly in Conformity with Generally Accepted Accounting Principles. This amendment was approved by the PCAOB on September 16, 2008. We do not anticipate that the adoption of SFAS No. 162 will materially impact our financial statements.
In June 2008, the FASB issued FASB Staff Position, or FSP, EITF 03-6-1,Determining Whether Instruments Granted in Share-Based Payment Transactions are Participating Securities, or FSP EITF 03-6-1, to address whether instruments granted in share-based payment transactions are
F-21
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
2. Summary of Significant Accounting Policies (Continued)
participating securities prior to their vesting and therefore need to be included in the earnings per share calculation under the two-class method described in SFAS No. 128,Earnings per Share. This FSP requires companies to treat unvested share-based payment awards that have non-forfeitable rights to dividends or dividend equivalents as participating securities and thus, include them in calculations of basic earnings per share. FSP EITF 03-6-1 is effective for fiscal years beginning after December 15, 2008. We do not anticipate that our adoption of FSP EITF 03-6-1 will materially impact our financial statements or our computation of basic earnings per share upon adoption.
3. Stockholders' Equity
Common Stock
2000 Initial Public Offering
In March 2000, we completed an Initial Public Offering of 5,000,000 shares of our common stock at a price of $18.00 per share, the IPO. Proceeds to us from the IPO, after calculation of the underwriters' discount and commission, totaled approximately $82.8 million, net of offering costs of approximately $1.0 million (excluding underwriters discounts and commissions). Concurrent with the closing of the IPO, all outstanding shares of our convertible preferred stock were automatically converted into 15,678,737 shares of common stock, and our Certificate of Incorporation was amended to authorize 10,000,000 shares of undesignated preferred stock, none of which were issued or outstanding at December 31, 2008. Our Board of Directors is authorized to fix the designation, powers, preferences, and rights of any such series.
2002 Private Placement
In April 2002, we completed a private placement of 2,500,000 shares of common stock at a purchase price of $6.00 per share for an aggregate purchase price of $15.0 million, net of $100,000 in issuance costs, which resulted in net cash proceeds to us of approximately $14.9 million.
2003 Private Placement
In November 2003, we completed a private placement of 5,172,412 shares of common stock at a purchase price of $2.32 per share for an aggregate purchase price of $12.0 million, net of $800,000 in issuance costs, which resulted in net cash proceeds to us of approximately $11.2 million. The purchase price was privately negotiated with the purchasers to represent an approximately 16% discount to the market value of our common stock on November 14, 2003.
2005 Series A Exchangeable Preferred Stock Financing
In March 2005, we entered into a Securities Purchase Agreement with Warburg Pincus Private Equity VIII, L.P., or Warburg, and certain other investors pursuant to which we issued and sold 2,352,443 shares of Series A Exchangeable Preferred Stock (the "Exchangeable Preferred"), at a price per share of $22.10, the Preferred Purchase Price, for aggregate gross proceeds of approximately $52.0 million, or the Preferred Stock Financing. We incurred offering expenses of $3.2 million in connection with the sale of Exchangeable Preferred, resulting in net cash proceeds to the Company of approximately $48.8 million. The shares of Exchangeable Preferred were sold under our shelf
F-22
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
3. Stockholders' Equity (Continued)
Registration Statement on Form S-3 declared effective by the Securities and Exchange Commission on April 21, 2004. In connection with its purchase of the Exchangeable Preferred, Warburg entered into a standstill agreement agreeing not to pursue certain activities the purpose or effect of which may be to change or influence the control or the Company.
On May 18, 2005, at our 2005 Annual Meeting of Stockholders, our stockholders voted to approve the issuance of shares of our common stock upon exchange of all of the outstanding shares of Exchangeable Preferred. As a result of such approval, we issued a total of 23,524,430 shares of common stock upon exchange of 2,352,443 shares of Exchangeable Preferred, or the Share Exchange.
The Preferred Purchase Price represented a 7.5% discount to the 20-day trailing average closing price of our common stock on the Nasdaq National Market as of March 2, 2005, calculated on an as-exchanged for common stock basis. In connection with the Share Exchange, we recorded a deemed dividend related to the beneficial conversion feature of the Exchangeable Preferred equal to $623,489, representing the difference between the effective conversion price per share of common stock and the market value per share of common stock as of the closing date of the Preferred Stock Financing. This dividend increased the net loss attributable to common stockholders for the year ended December 31, 2005.
In connection with the sale of Exchangeable Preferred, we entered into a Registration Rights Agreement between us and the purchasers of Exchangeable Preferred. Pursuant to this Registration Rights Agreement, beginning on March 4, 2007, the purchasers of Exchangeable Preferred became entitled to certain registration rights with respect to the shares of common stock that were issued upon exchange of the Exchangeable Preferred.
Pursuant to the Securities Purchase Agreement, for so long as Warburg owns at least two-thirds of the shares of common stock issued upon exchange of such Exchangeable Preferred, we will nominate and use our reasonable best efforts to cause to be elected and cause to remain as directors on our Board of Directors two individuals designated by Warburg (each, an "Investor Designee" and collectively, the "Investor Designees"). If Warburg no longer has the right to designate two members of our Board of Directors, then, for so long as Warburg owns at least 50% of the shares of common stock issued upon exchange of such Exchangeable Preferred, we will nominate and use our reasonable best efforts to cause to be elected and cause to remain as a director on our Board of Directors, one Investor Designee. In addition, subject to applicable law and the rules and regulations of the SEC and the Nasdaq Stock Market, we will use our reasonable best efforts to cause one of the Investor Designees to be a member of each principal committee of our Board of Directors; however, our Board of Directors has determined, based on its analysis of Rule 10A-3 under the Securities Exchange Act of 1934, as amended, that the Investor Designees are not eligible to serve as members of the Audit Committee of the Board of Directors due to the size of Warburg's ownership interest. Effective upon the closing of the sale of Exchangeable Preferred to Warburg on March 4, 2005, Messrs. Stewart Hen and Jonathan Leff, each of whom is a Managing Director of Warburg, were appointed to our Board of Directors pursuant to Warburg's right to nominate directors.
2007 Common Stock Financing
On February 2, 2007, we sold 9,000,000 shares of our common stock in an underwritten offering at a price of $6.00 per share, or the February 2007 Financing. We received net proceeds from the offering
F-23
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
3. Stockholders' Equity (Continued)
of approximately $50,257,207, after deducting underwriting commissions of approximately $3,240,000 and other offering expenses of approximately $502,793.
Baker Brothers Life Sciences, L.P. and certain other affiliated funds, collectively Baker, purchased 3,300,000 shares of common stock in the February 2007 Financing. As a result of such purchase, Baker held in excess of 15% of our outstanding common stock following the closing of the February 2007 Financing. In connection with the February 2007 Financing, Baker entered into a standstill agreement with the Company, agreeing not to pursue, for four years, certain activities the purpose or effect of which may be to change or influence control of the Company.
2008 Common Stock Financing
On May 29, 2008, we sold 12,420,000 shares of our common stock in an underwritten public offering at a price of $5.64 per share. The number of shares issued includes 1,620,000 shares purchased by the underwriters pursuant to their exercise in full of their overallotment option. We received net proceeds from the offering of $65,185,128, after deducting $4,202,928 of underwriting commissions and $660,744 of offering expenses.
Common Stock Reserved for Future Issuance
At December 31, 2008, we have reserved shares of common stock for future issuance as follows:
| | | | | | | | | | | |
| | Outstanding at
December 31,
2008 | | Available for
grant at
December 31,
2008 | | Shares of
Common Stock
Reserved at
December 31,
2008 | |
|---|
1995 Stock Option Plan | | | 290,108 | | | — | | | 290,108 | |
2001 Employee Stock Purchase Plan | | | — | | | 2,259,777 | | | 2,259,777 | |
2008 Equity Incentive Plan | | | 6,946,404 | | | 5,146,701 | | | 12,093,105 | |
| | | | | | | | |
| | Total for Equity Incentive Plans | | | 7,236,512 | | | 7,406,478 | | | 14,642,990 | |
| | | | | | | | |
Stock Warrants
In November 2003, in conjunction with the private placement of 5,172,412 shares of common stock to various purchasers, we issued warrants to purchase 1,706,893 shares of common stock at an exercise price of $3.14 per share with a life of four years. There were 748,187 and 958,706 of these warrants exercised during 2007 and 2006, respectively. As of December 31, 2008 and 2007, no warrants remained outstanding.
F-24
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
3. Stockholders' Equity (Continued)
Stockholder Rights Plan
In May 2003, we designated 1,000,000 shares of our authorized Preferred Stock as Series A Junior Participating Preferred Stock, par value $0.001 per share, pursuant to a Stockholder Rights Plan approved by our Board of Directors under which all stockholders of record as of May 28, 2003 received a dividend distribution of one preferred share purchase right, or a Right, for each outstanding share of our common stock. The Rights trade with the common stock and no separate Right certificates will be distributed until such time as the Rights become exercisable in accordance with the Stockholder Rights Plan. The Stockholder Rights Plan is intended as a means to guard against abusive takeover tactics and to provide for fair and equal treatment for all stockholders in the event that an unsolicited attempt is made to acquire us.
In connection with the sale of shares of Exchangeable Preferred to Warburg in March 2005, we amended the Stockholder Rights Plan to provide that Warburg and its affiliates will be exempt from the Stockholder Rights Plan, unless Warburg and its affiliates become, without the prior consent of our Board of Directors, the beneficial owner of more than 44% of our common stock.
In connection with the acquisition of shares of our common stock by Baker in the February 2007 Financing, we amended the Stockholder Rights Plan to provide that Baker will be exempt from the Stockholder Rights Plan, unless Baker becomes, without the Company's prior consent, the beneficial owner of more than 20% of our common stock.
Until the Rights become exercisable, the Rights will have no dilutive impact on our earnings per share data. The Rights are protected by customary anti-dilution provisions. As of December 31, 2008, no shares of Series A Junior Participating Preferred Stock were issued or outstanding.
4. Stock-Based Compensation Plans
Expense Information under SFAS 123R
In accordance with the modified prospective transition method of SFAS 123R, stock-based compensation expense for the years ended December 31, 2008, 2007 and 2006 has been recognized in the accompanying Statements of Operations as follows:
| | | | | | | | | | | |
| | 2008 | | 2007 | | 2006 | |
|---|
Research and development | | $ | 2,701,115 | | $ | 1,870,767 | | $ | 660,274 | |
Clinical manufacturing | | | 417,469 | | | 180,592 | | | 113,066 | |
Marketing, general and administrative | | | 4,901,865 | | | 4,599,266 | | | 2,813,661 | |
| | | | | | | | |
| | Total stock-based compensation expense | | $ | 8,020,449 | | $ | 6,650,625 | | $ | 3,587,001 | |
| | | | | | | | |
We did not recognize a related tax benefit during the years ended December 31, 2008 and 2007, as we maintain net operating loss carryforwards and we have established a valuation allowance against the entire tax benefit as of December 31, 2008 and 2007. No stock-based compensation expense was capitalized on our Balance Sheets as of December 31, 2008 and 2007.
During the year ended December 31, 2006, we entered into a Separation Agreement with our former President and Chief Executive Officer, Michael E. Hart, and we entered into a consulting
F-25
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
4. Stock-Based Compensation Plans (Continued)
agreement with a former member of our Board of Directors, Dr. Marvin E. Jaffe (these arrangements are described in more detail in Note 9 below). Pursuant to these arrangements, the exercise periods of certain stock options held by Mr. Hart and Dr. Jaffe were extended as a result of their consulting relationships and were deemed modified for accounting purposes. We have accounted for the modifications to these options in accordance with SFAS 123R and FASB Interpretation No. 44,Accounting for Certain Transactions Involving Stock Compensation, and recorded a one-time stock-based compensation charge of $441,129 during the year ended December 31, 2006.
Stock Options
During 1995, our Board of Directors terminated the 1992 Stock Plan, or the 1992 Plan, and adopted the 1995 Stock Option Plan, or the 1995 Plan. The 1995 Plan was amended and restated in 1997. Termination of the 1992 Plan had no effect on the options outstanding under that plan, as they were assumed under the 1995 Plan. Under the 1995 Plan, we could grant fixed and performance-based stock options and stock appreciation rights to officers, employees, consultants and directors. The stock options were intended to qualify as "incentive stock options" under Section 422 of the Internal Revenue Code, unless specifically designated as non-qualifying stock options or unless exceeding the applicable statutory limit.
During 2000, concurrent with the IPO, the Board of Directors suspended the 1995 Plan and adopted the Allos Therapeutics, Inc. 2000 Stock Incentive Compensation Plan, or the 2000 Plan. The 2000 Plan provided for the granting of stock options similar to the terms of the 1995 Plan as described above. Any shares remaining for future option grants and any future cancellations of options from our 1995 Plan were available for future grant under the 2000 Plan. Suspension of the 1995 Plan had no effect on the options outstanding under the 1995 Plan. Under the 2000 Plan, we were authorized to increase the number of shares of common stock that were available annually on the first day of each fiscal year beginning in 2001 in an amount equal to the lesser of 440,000 shares or 2% of the adjusted average common shares outstanding used to calculate fully diluted earnings per share as reported in our Annual Report to Stockholders for the preceding year, or alternatively, by any lesser amount determined by our Board of Directors. On December 21, 2005, our stockholders approved an amendment and restatement of the 2000 Plan to: (i) increase the aggregate number of shares of common stock authorized for issuance under the 2000 Plan by 3,500,000 shares and (ii) provide that the number of shares of common stock that could be granted under the 2000 Plan to any one employee during any calendar year could not exceed 2,000,000 shares.
In January 2002, our Board of Directors approved the Allos Therapeutics, Inc. 2002 Broad Based Equity Incentive Plan, or the 2002 Plan. Under the 2002 Plan, we were authorized to issue up to 1,000,000 shares of common stock to employees, consultants and members of the Board of Directors. Under the terms of the 2002 Plan, the aggregate number of shares underlying stock awards to officers and directors once employed by us cannot exceed 49% of the number of shares underlying all stock awards granted, as determined on certain specific dates.
In June 2006, our Board of Directors approved the Allos Therapeutics, Inc. 2006 Inducement Award Plan, the 2006 Plan. Under the 2006 Plan, we were authorized to issue up to 1,500,000 shares of common stock pursuant to equity awards, including nonstatutory stock options, stock grant awards, stock purchase awards, stock unit awards and other forms of equity compensation. We could grant
F-26
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
4. Stock-Based Compensation Plans (Continued)
awards under the 2006 Plan only to persons not previously an employee or director of ours, or following a bona fide period of non-employment, as an inducement material to such individual's entering into employment with us and to provide incentives for such persons to exert maximum efforts for our success.
At our Annual Meeting of Stockholders held on June 24, 2008, our stockholders approved the Allos Therapeutics,��Inc. 2008 Equity Incentive Plan, or the 2008 Plan. The 2008 Plan authorizes the issuance of incentive stock options, nonstatutory stock options, restricted stock awards, restricted stock unit awards, stock appreciation rights, performance stock awards and forms of equity compensation, which may be granted to employees, directors and consultants. Only employees may receive incentive stock options. The 2008 Plan succeeds and continues the 2006 Plan, the 2002 Plan and the 2000 Plan, or the Prior Plans. As of June 24, 2008, no additional stock awards will be granted under the Prior Plans and all outstanding stock awards granted under the Prior Plans are deemed to be stock awards granted under the 2008 Plan (but remain subject to the terms of the Prior Plans with respect to which they were originally granted).
12,550,843 shares of our common stock may be issued pursuant to stock awards granted under the 2008 Plan, provided that all stock awards granted after the June 24, 2008 effective date of the 2008 Plan, other than stock options and stock appreciation rights granted with an exercise price of at least 100% of such stock award's fair market value on the date of grant, will reduce the number of shares available for issuance under the 2008 Plan by 1.35 shares per share granted pursuant to the stock award. If a stock award under the 2008 Plan expires or otherwise terminates without being exercised in full, the shares of common stock of the Company not acquired pursuant to the stock award will again become available for issuance under the 2008 Plan. In addition, shares issued pursuant to a stock award that are forfeited to or repurchased by us prior to becoming fully vested and shares that are cancelled pursuant to an exchange or repricing program will become available for the grant of new stock awards under the 2008 Plan. Shares of common stock that revert to and again become available for issuance under the 2008 Plan and that prior to such reversion were granted pursuant to a stock award that reduced the number of shares available under the 2008 Plan by 1.35 shares per share granted pursuant to such stock award, shall cause the number of shares of common stock of the Company available for issuance under the 2008 Plan to increase by 1.35 shares upon such reversion.
The 1995 and 2008 Plans, or the Plans, provide for appropriate adjustments in the number of shares reserved and outstanding options in the event of certain changes to our outstanding common stock by reason of merger, recapitalization, stock split or other similar events. Options granted under the Plans may be exercised for a period of not more than 10 years from the date of grant or any shorter period as determined by our Board of Directors. Options vest as determined by the Board of Directors, generally over a period of two to four years, subject to acceleration under certain events. The exercise price of any incentive stock option granted under the Plans must equal or exceed the fair market value of our common stock on the date of grant, or 110% of the fair market value per share in the case of a 10% or greater stockholder.
F-27
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
4. Stock-Based Compensation Plans (Continued)
The following table summarizes our stock option activity and related information for the 1995 and 2008 Plans:
| | | | | | | | | | | | | | |
| | Options Outstanding | | Options Exercisable | |
|---|
| | Number of
Shares | | Weighted
Average
Exercise Price | | Number of
Shares | | Weighted
Average
Exercise Price | |
|---|
Outstanding at December 31, 2005 | | | 3,944,375 | | $ | 3.87 | | | 2,809,991 | | $ | 4.20 | |
| | | | | | | | | | | | | |
| | Granted | | | 2,660,343 | | | 2.93 | | | | | | | |
| | Exercised | | | (413,680 | ) | | 1.36 | | | | | | | |
| | Canceled | | | (412,467 | ) | | 4.15 | | | | | | | |
| | | | | | | | | | | | | |
Outstanding at December 31, 2006 | | | 5,778,571 | | $ | 3.60 | | | 2,900,556 | | $ | 4.27 | |
| | | | | | | | | | | | |
| | Granted | | | 2,731,574 | | | 6.53 | | | | | | | |
| | Exercised | | | (1,156,471 | ) | | 3.20 | | | | | | | |
| | Canceled | | | (948,244 | ) | | 5.31 | | | | | | | |
| | | | | | | | | | | | | |
Outstanding at December 31, 2007 | | | 6,405,430 | | $ | 4.68 | | | 2,754,274 | | $ | 3.80 | |
| | | | | | | | | | | | |
| | Granted | | | 2,790,312 | | | 6.24 | | | | | | | |
| | Exercised | | | (1,144,041 | ) | | 4.63 | | | | | | | |
| | Canceled | | | (815,189 | ) | | 6.00 | | | | | | | |
| | | | | | | | | | | | | |
Outstanding at December 31, 2008 | | | 7,236,512 | | $ | 5.14 | | | 3,122,681 | | $ | 4.15 | |
| | | | | | | | | | | | |
The following table summarizes information about options outstanding and exercisable as of December 31, 2008:
| | | | | | | | | | | | | | | | | | | |
| | Options Outstanding | | Options Exercisable | |
|---|
Range of Exercise Prices | | Outstanding | | Weighted
Average
Remaining
Contractual Life | | Weighted
Average
Exercise Price | | Exercisable | | Weighted
Average
Exercise Price | |
|---|
| | | | $1.90 - $2.55 | | | 711,636 | | | 4.0 | | $ | 2.31 | | | 692,926 | | $ | 2.28 | |
| | | | $2.56 - $2.95 | | | 573,237 | | | 7.2 | | | 2.75 | | | 420,764 | | | 2.78 | |
| | | | $2.96 - $3.13 | | | 402,250 | | | 7.1 | | | 3.12 | | | 270,997 | | | 3.11 | |
| | | | $3.14 - $3.24 | | | 737,400 | | | 7.1 | | | 3.15 | | | 514,925 | | | 3.14 | |
| | | | $3.25 - $5.62 | | | 838,412 | | | 8.5 | | | 4.92 | | | 225,841 | | | 4.69 | |
| | | | $5.63 - $5.84 | | | 235,652 | | | 7.6 | | | 5.72 | | | 185,920 | | | 5.68 | |
| | | | $5.85 - $6.16 | | | 1,582,930 | | | 8.8 | | | 5.90 | | | 171,431 | | | 5.88 | |
| | | | $6.17 - $7.46 | | | 1,016,688 | | | 8.6 | | | 6.35 | | | 221,706 | | | 6.25 | |
| | | | $7.47 - $8.04 | | | 1,018,007 | | | 8.5 | | | 7.47 | | | 361,871 | | | 7.47 | |
| | | | $8.05 - $13.75 | | | 120,300 | | | 6.5 | | | 9.50 | | | 56,300 | | | 9.21 | |
| | | | | | | | | | | | |
| | | | | | | 7,236,512 | | | 7.8 | | $ | 5.14 | | | 3,122,681 | | $ | 4.15 | |
| | | | | | | | | | | | |
F-28
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
4. Stock-Based Compensation Plans (Continued)
The following table summarizes information about outstanding stock options that are fully vested and currently exercisable, and outstanding stock options that are expected to vest in the future:
| | | | | | | | | | | | | | |
| | Number
Outstanding | | Weighted
Average
Remaining
Contractual Term | | Weighted
Average
Exercise Price | | Aggregate
Intrinsic Value | |
|---|
As of December 31, 2008: | | | | | | | | | | | | | |
| | Options fully vested and exercisable | | | 3,122,681 | | | 6.4 | | $ | 4.15 | | $ | 6,857,744 | |
| | Options expected to vest, including effects of expected forfeitures | | | 3,497,272 | | | 8.8 | | $ | 5.87 | | | 2,182,291 | |
| | | | | | | | | | | | |
| | Options fully vested and expected to vest | | | 6,619,953 | | | 7.6 | | $ | 5.06 | | $ | 9,040,035 | |
| | | | | | | | | | | | |
During the years ended December 31, 2008, 2007 and 2006, we granted stock options with a weighted-average grant-date fair value of $3.88, $4.12 and $1.79 per share, respectively. Stock-based compensation expense related to our stock option plans was $7,538,876, $5,899,387 and $3,048,523 for the years ended December 31, 2008, 2007 and 2006, respectively. As of December 31, 2008, the unrecorded stock-based compensation balance related to stock option awards was $7,300,632 and will be recognized over an estimated weighted-average amortization period of 1.4 years.
The aggregate intrinsic value in the tables above represents the total pretax intrinsic value, based on our closing stock price of $6.12 as of December 31, 2008, which would have been received by the option holders had all option holders with in-the-money options exercised their options as of that date. The total number of in-the-money options exercisable as of December 31, 2008 was 2,478,804. The total intrinsic value of outstanding stock options as of December 31, 2008 was $9,362,869.
The total intrinsic value of options exercised during the years ended December 31, 2008, 2007 and 2006 was $3,168,268, $2,634,426 and $1,104,117, respectively. The total cash received from employees as a result of employee stock option exercises during the years ended December 31, 2008, 2007 and 2006 was $5,291,229, $3,696,811 and $561,097, respectively. We settle employee stock option exercises with newly issued common shares. No tax benefits were realized by us in connection with these exercises during the year ended December 31, 2008 as we maintain net operating loss carryforwards and we have established a valuation allowance against the entire tax benefit as of December 31, 2008.
Valuation assumptions for stock options granted during the years ended December 31, 2008, 2007 and 2006
For stock options granted during the years ended December 31, 2008, 2007 and 2006, the majority vest according to the following schedule: 25% of the shares subject to the award vest one year after the date of grant, and the remaining 75% of the shares subject to the award vest in equal monthly installments thereafter over the next three years, until all such shares are vested and exercisable. Stock-based compensation calculated according to SFAS 123R is expensed over the vesting period of the individual options in accordance with FASB Interpretation No. 28,Accounting for Stock Appreciation Rights and Other Variable Stock Option and Award Plans. The fair value of stock options granted to our employees during the years ended December 31, 2008, 2007 and 2006 was estimated on the date of
F-29
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
4. Stock-Based Compensation Plans (Continued)
each grant using the Black-Scholes option pricing model using the following weighted-average assumptions:
| | | | | | | | | | | |
| | 2008 | | 2007 | | 2006 | |
|---|
Stock option plans: | | | | | | | | | | |
| | Expected dividend yield | | | 0 | % | | 0 | % | | 0 | % |
| | Expected stock price volatility | | | 74 | % | | 80 | % | | 81 | % |
| | Risk free interest rate | | | 2.9 | % | | 4.7 | % | | 4.7 | % |
| | Expected life (years) | | | 5.0 | | | 4.3 | | | 3.9 | |
We used an expected dividend yield of 0%, as we do not expect to pay dividends during the expected life of these awards. The expected stock price volatility is determined using our historical stock volatility over the period equal to the expected life of each award. The risk-free interest rate is based on U.S. Treasury zero-coupon issues with a remaining term equal to the expected life of each award. During 2006 through the first quarter of 2007, the expected life was determined by factoring the different vesting periods of each award in combination with our employees' expected exercise behavior. In June 2007, we concluded that our historical share option exercise experience would not provide a reasonable basis upon which to estimate expected term going forward, given our relative stage of development and changes in our business given the termination of the EFAPROXYN development program. Beginning in the second quarter of 2007, the expected life of the stock options was estimated using peer data of companies in the life science industry with similar equity plans. As required by SFAS 123R, stock-based compensation expense is recognized net of estimated pre-vesting forfeitures, which results in recognition of expense on options that are ultimately expected to vest over the expected option term. Forfeitures were estimated using actual historical forfeiture experience.
Restricted Stock
The following table summarizes activity and related information for our restricted stock awards:
| | | | | | | | |
| | Number of
Shares | | Weighted
Average
Grant-
Date
Fair Value | |
|---|
Nonvested as of December 31, 2007 | | | 412,500 | | $ | 3.89 | |
| | Granted | | | 10,000 | | | 7.49 | |
| | Vested | | | (128,750 | ) | | 3.74 | |
| | | | | | |
Nonvested as of December 31, 2008 | | | 293,750 | | $ | 4.07 | |
| | | | | | |
During the years ended December 31, 2008, 2007 and 2006, we granted 10,000, 105,000 and 410,000 shares of restricted stock, respectively. The shares of restricted stock vest in four equal annual installments from the date of grant. The grant-date fair value of shares granted during the years ended December 31, 2008, 2007 and 2006 was $74,900, $638,400 and $1,286,300, respectively. The weighted-average grant-date fair value per share for restricted stock awards granted was based on the closing market price of the Company's common stock on the grant dates of the awards and was $7.49, $6.08 and $3.14 for the years ended December 31, 2008, 2007 and 2006, respectively. The total fair value of
F-30
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
4. Stock-Based Compensation Plans (Continued)
shares vested during the year ended December 31, 2008 and 2007 was $751,350 and $643,025, respectively. During the years ended December 31, 2008, 2007 and 2006, we recorded stock-based compensation related to restricted stock awards of $422,505, $689,754 and $502,514, respectively. As of December 31, 2008, the unrecorded stock-based compensation balance related to restricted stock awards was $329,333 and will be recognized over an estimated weighted-average amortization period of 1.3 years.
Employee Stock Purchase Plan
On February 28, 2001, our Board of Directors approved the Allos Therapeutics, Inc. 2001 Employee Stock Purchase Plan, or Purchase Plan, which was also approved by our stockholders on April 17, 2001. Under the Purchase Plan, we are authorized to issue up to 2,500,000 shares of common stock to qualified employees. Qualified employees can choose to have up to 10% of their annual base earnings withheld to purchase shares of our common stock during each offering period. The purchase price of the common stock is 85% percent of the lower of the fair market value of a share of common stock on the first day of the offering or the fair market value of a share of common stock on the last day of the purchase period. We sold 22,828, 41,148 and 44,319 shares to employees in 2008, 2007 and 2006, respectively. There were 2,259,777 shares available for sale under the Purchase Plan as of December 31, 2008. The Purchase Plan will terminate on February 27, 2011. Stock-based compensation expense related to our Purchase Plan was $59,068, $61,484 and $35,964 for the years ended December 31, 2008, 2007 and 2006, respectively. As of December 31, 2008, there was no unrecorded deferred stock-based compensation balance related to the Purchase Plan. The weighted- average estimated grant date fair value of purchase awards under the Purchase Plan during the years ended December 31, 2008, 2007 and 2006 was $2.59, $1.42 and $0.96 per share, respectively.
The fair value of purchase awards granted to our employees during the years ended December 31, 2008, 2007 and 2006 was estimated using the Black-Scholes option pricing model using the following weighted-average assumptions:
| | | | | | | | | | | |
| | 2008 | | 2007 | | 2006 | |
|---|
Stock purchase plan: | | | | | | | | | | |
| | Expected dividend yield | | | 0 | % | | 0 | % | | 0 | % |
| | Expected stock price volatility | | | 65 | % | | 55 | % | | 46 | % |
| | Risk free interest rate | | | 2.6 | % | | 4.9 | % | | 4.7 | % |
| | Expected life (years) | | | 0.5 | | | 0.9 | | | 1.0 | |
F-31
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
5. Restructuring and Separation Costs
In January 2005, we executed agreements to sublease approximately three-quarters of the 12,708 square feet of excess space in our corporate offices located in Westminster, Colorado. The term of each sublease agreement was through the term of our office lease, or October 31, 2008. The total rental payments to us under the terms of the sublease agreements approximated $230,000. In the year ended December 31, 2005, we recorded a lease abandonment charge of $380,085 as our obligations under our primary lease were in excess of the sum of the actual and expected sublease rental payments for this excess space. As of December 31, 2008, there was no remaining accrued restructuring and separation costs relating to this lease abandonment charge.
In January 2006, Michael E. Hart notified our Board of Directors of his intent to resign from his positions as President, Chief Executive Officer and Chief Financial Officer of the Company once a successor Chief Executive Officer was appointed. On March 3, 2006, we entered into a separation agreement with Mr. Hart to provide certain incentives for his continued employment with the Company while we conducted our search for his successor. On March 9, 2006, we appointed Paul L. Berns as our President, Chief Executive Officer and a member of the Board of Directors and Mr. Hart resigned from his positions in accordance with the terms of the separation agreement. The separation agreement with Mr. Hart was amended on March 9, 2006 and on May 10, 2006, or as so amended, the Separation Agreement. We recorded separation costs of $645,666 during the year ended December 31, 2006 relating to our estimate of our total obligations under the Separation Agreement with Mr. Hart. During the years ended December 31, 2007 and 2006, we made payments to Mr. Hart under the Separation Agreement of $320,458 and $325,208, respectively. As of December 31, 2007, there was no remaining liability relating to the Separation Agreement with Mr. Hart.
6. Income Taxes
We adopted the provisions of FASB Interpretation No. 48,Accounting for Uncertainty in Income Taxes—an interpretation of FASB Statement No. 109, or FIN 48, on January 1, 2007. FIN 48 clarifies the accounting for uncertainty in income taxes recognized in an enterprise's financial statements in accordance with SFAS No. 109 and prescribes a recognition threshold and measurement process for financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. FIN 48 also provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosure and transition. Based on our evaluation, we have concluded that there are no significant uncertain tax positions requiring recognition in our financial statements. Our evaluation was performed for the periods from December 31, 1993 through December 31, 2008, the tax periods which remain subject to examination by major tax jurisdictions as of December 31, 2008.
We may from time to time be assessed interest or penalties by major tax jurisdictions, although there have been no such assessments historically with material impact to our financial results. In the event we receive an assessment for interest and/or penalties, it would be classified in the financial statements as income tax expense.
F-32
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
6. Income Taxes (Continued)
The income tax benefit computed using our net loss and the federal statutory income tax rate differs from our actual income tax benefit of $0, primarily due to the following for the years ended December 31, 2008, 2007 and 2006:
| | | | | | | | | | | |
| | 2008 | | 2007 | | 2006 | |
|---|
Federal income tax benefit at 35% | | $ | (18,105,470 | ) | $ | (13,779,360 | ) | $ | (10,574,400 | ) |
State income tax, net of federal benefit | | | (1,448,690 | ) | | 974,838 | | | (813,900 | ) |
Stock-based compensation | | | 554,635 | | | 1,160,681 | | | 275,600 | |
Research and development and orphan drug credits | | | (1,244,474 | ) | | (3,311,029 | ) | | (1,355,693 | ) |
Research and development and orphan drug credits to expire related to Section 382 limitation | | | — | | | 5,880,410 | | | — | |
Net operating losses to expire related to Section 382 limitation | | | — | | | 23,086,377 | | | — | |
Change in valuation allowance | | | 20,208,260 | | | (14,161,720 | ) | | 12,496,693 | |
Other | | | 35,739 | | | 149,803 | | | (28,300 | ) |
| | | | | | | | |
| | Benefit for income taxes | | $ | — | | $ | — | | $ | — | |
| | | | | | | | |
The components of our deferred tax assets are as follows, as of December 31:
| | | | | | | | | |
| | 2008 | | 2007 | |
|---|
Deferred tax assets: | | | | | | | |
| | Net operating loss carryforwards | | $ | 64,470,656 | | $ | 48,354,464 | |
| | Amortization of intangibles | | | 1,298,728 | | | 943,154 | |
| | Research and development and orphan drug credit carryforwards | | | 9,110,448 | | | 7,195,874 | |
| | Stock-based compensation | | | 4,736,475 | | | 2,989,285 | |
| | Other | | | 395,516 | | | 320,786 | |
| | | | | | |
| | | Total deferred tax assets | | | 80,011,823 | | | 59,803,563 | |
| | Valuation allowance | | | (80,011,823 | ) | | (59,803,563 | ) |
| | | | | | |
| | | Net deferred tax assets | | $ | — | | $ | — | |
| | | | | | |
Our deferred tax assets represent an unrecognized future tax benefit. A valuation allowance has been established for the entire tax benefit as we believe that it is more likely than not that such assets will not be realized.
As of December 31, 2008, we had available approximately $174.8 million of net operating loss, or NOL, carryforwards, after taking into consideration NOLs expected to expire unused due to the limitations under Section 382 of the Internal Revenue Code, and which includes approximately $5.2 million of deductions related to stock-based compensation that are not realized as deferred tax assets until current taxes payable can be reduced. These NOL carryforwards will expire beginning in 2009. In addition, we had research and development credit and orphan drug credit carryforwards, after taking into consideration the Section 382 limitation, of $3.5 million and $5.6 million, respectively, as of December 31, 2008, to offset future regular and alternative tax expense. Since the Company's formation, it has raised capital through the issuance of capital stock on several occasions which,
F-33
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
6. Income Taxes (Continued)
combined with shareholders' subsequent disposition of those shares, has resulted in four changes of control in 1994, 1998, 2001 and 2005, as defined by Section 382. In general, an ownership change, as defined by Section 382, results from transactions increasing the ownership of certain shareholders or public groups in the stock of a corporation by more than 50% within a three-year period. As a result of the most recent ownership change in 2005, utilization of our NOLs generated prior to the latest change are subject to an annual limitation under Section 382 determined by multiplying the value of our stock at the time of the ownership change in control by the applicable long-term tax-exempt rate resulting in an annual limitation amount of approximately $2.2 million. Additionally, we have a recognized built-in gain that will increase the annual limitation by $3.3 million for each of the five years after the 2005 ownership change. Any unused annual limitation may be carried over to later years, and the amount of the limitation may, under certain circumstances, be subject to adjustment if the fair value of the Company's net assets are determined to be below or in excess of the tax basis of such assets at the time of the ownership change, and such unrealized loss or gain is recognized during the five-year period after the ownership change. Subsequent ownership changes, as defined in Section 382, could further limit the amount of our NOL carryforwards and research and development credits that can be utilized annually to offset future taxable income.
7. Employee Benefit Plan
We maintain a defined contribution plan covering substantially all employees under Section 401(k) of the Internal Revenue Code. From January 1, 1999 through December 31, 2006, we provided a 50% match of employees' contributions up to $2,000 per employee per year. Effective January 1, 2007, we provided a 50% match of employees' contributions up to $5,000 per employee per year. We made total contributions of $246,112, $241,227 and $105,675 during the years ended December 31, 2008, 2007 and 2006, respectively. Company contributions are fully vested after four years of employment.
8. Commitments and Contingencies
Lease Commitments
We lease offices under agreements that expire at various dates through 2012, and which contain clauses for renewal at our option for one additional three year term. Total rent expense for the years ended December 31, 2008, 2007 and 2006 and the cumulative period from inception through December 31, 2008 was $714,594, $686,606, $586,010 and $5,778,377, respectively.
The aggregate future minimum rental commitments as of December 31, 2008, for non-cancelable operating leases with initial or remaining terms in excess of one year are as follows:
| | | | |
Year Ending December 31: | |
| |
|---|
2009 | | $ | 791,713 | |
2010 | | | 850,712 | |
2011 | | | 788,769 | |
2012 | | | 46,048 | |
| | | | |
Total | | $ | 2,477,242 | |
| | | | |
F-34
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
8. Commitments and Contingencies (Continued)
Royalty and License Fee Commitments
In December 2002, we entered into a license agreement with Memorial Sloan-Kettering Cancer Center, SRI International and Southern Research Institute, as amended, under which we obtained exclusive worldwide rights to a portfolio of patents and patent applications related to pralatrexate (PDX) and its uses. Under the terms of the agreement, we paid an up-front license fee of $2.0 million upon execution of the agreement and are also required to make certain additional cash payments based upon the achievement of certain clinical development or regulatory milestones or the passage of certain time periods. To date, we have made aggregate milestone payments of $2.5 million based on the passage of time. In the future, we could make an aggregate milestone payment of $500,000 upon the earlier of achievement of a clinical development milestone or the passage of certain time periods, or the Clinical Milestone, and up to $10.3 million upon achievement of certain regulatory milestones, or the Regulatory Milestones, including regulatory approval to market pralatrexate in the United States or Europe. The last scheduled payment towards the Clinical Milestone of $500,000 is currently due on December 23, 2009. We intend to submit an NDA for pralatrexate for the treatment of patients with relapsed or refractory PTCL in the first half of 2009. If the U.S. Food and Drug Administration, or FDA, accepts our NDA for review and if we obtain FDA approval to market pralatrexate, we will be obligated to make payments of $1,500,000 and $5,300,000, respectively, which represent a portion of the Regulatory Milestones. The up-front license fee and all milestone payments under the agreement have been or will be recorded to research and development expense when incurred. Under the terms of the agreement, we are required to fund all development programs and will have sole responsibility for all commercialization activities. In addition, we will pay the licensors a royalty based on a percentage of net revenues arising from sales of the product or sublicense revenues arising from sublicensing the product, if and when such sales or sublicenses occur.
In December 2004, we entered into an agreement with the University of Colorado Health Sciences Center, the University of Salford and Cancer Research Technology, or CRT, under which we obtained exclusive worldwide rights to certain intellectual property surrounding a proprietary molecule known as RH1. Under the terms of the agreement, we paid an up-front license fee of $190,500 upon execution of the agreement and are also required to make certain additional cash payments based upon the achievement of certain clinical development, regulatory and commercialization milestones. We could make aggregate milestone payments of up to $9.2 million upon the achievement of the clinical development, regulatory and commercialization milestones set forth in the agreement. The up-front license fee and all milestone payments under the agreement, as well as the one-time data option fee discussed below, have been recorded to research and development expense. Under the terms of the agreement and related data option agreement, we paid the licensors a one-time data option fee of $360,000 in 2007 for an exclusive license to the results of a Phase 1 study sponsored by Cancer Research UK, CRT's parent institution. This Phase 1 study was completed in 2007 and, under the terms of the agreement, we have since assumed responsibility for all future development costs and activities and have sole responsibility for all commercialization activities. In addition, we will pay the licensors a royalty based on a percentage of net revenues arising from sales of the product or sublicense revenues arising from sublicensing the product, if and when such sales or sublicenses occur. We are currently in the process of closing our Phase 1 study of RH1 in patients with advanced solid tumors or NHL and determining our future development plans, if any, for RH1.
F-35
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
8. Commitments and Contingencies (Continued)
Contingencies
We were named as a defendant in a purported securities class action lawsuit filed in May 2004 seeking unspecified damages relating to the issuance of allegedly false and misleading statements regarding EFAPROXYN during the period from May 29, 2003 to April 29, 2004 and subsequent declines in our stock price. In an opinion dated October 20, 2005, the U.S. District Court for the District of Colorado concluded that the plaintiffs' complaint failed to meet the legal requirements applicable to its alleged claims and dismissed the lawsuit. On November 20, 2005, the plaintiffs appealed the District Court's decision to the U.S. Court of Appeals for the Tenth Circuit. On February 6, 2008, the parties signed a stipulation of settlement, settling the case for $2,000,000. The settlement was subject to various conditions, including without limitation approval of the District Court. On January 29, 2009, the District Court issued its Order and Final Judgment approving the settlement, including the releases of the defendants for which the settlement provided. Neither we nor our former officer, who was also named as a defendant, admitted any liability in connection with the settlement. The amount of the settlement in excess of our deductible was covered by our insurance carrier. In the event the District Court's approval of the settlement is appealed and the settlement does not become final, we would intend to vigorously defend against the plaintiffs' appeal. If the Court of Appeals then were to reverse the District Court's decision and we were not successful in our defense against the plaintiffs' claims, we could be forced to make significant payments to the plaintiffs, and such payments could have a material adverse effect on our business, financial condition, results of operations and cash flows to the extent such payments are not covered by our insurance carriers. Even if our defense against such claims were successful, the litigation could result in substantial costs and divert management's attention and resources, which could adversely affect our business. As of December 31, 2008, we have recorded $2,000,000 in accrued litigation settlement costs, which represents our best estimate of the potential gross amount of the settlement costs to be paid to the plaintiffs, and $2,000,000 in prepaid expenses and other assets, which represents the approximately $235,000 of remaining deductible under our insurance policy paid by us and $1,765,000 paid by our insurance carrier into the settlement fund escrow in September 2008. A claims administrator appointed by the parties will administer the distribution of the settlement fund to authorized claimants in 2009.
We enter into indemnification provisions under our agreements with other companies in our ordinary course of business, typically with business partners, contractors, clinical sites and suppliers. Under these provisions we generally indemnify and hold harmless the indemnified party for losses suffered or incurred by the indemnified party as a result of our activities or the use of our product candidates. These indemnification provisions generally survive termination of the underlying agreement. The maximum potential amount of future payments we could be required to make under these indemnification provisions is unlimited. We have not incurred material costs to defend lawsuits or settle claims related to these indemnification agreements. The estimated fair value of the indemnification provisions of these agreements is minimal as of December 31, 2008, and accordingly, we have no corresponding liabilities recorded as of December 31, 2008.
F-36
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
9. Related Party Transactions
Dr. Donald Abraham
In January 2001, we entered into a consulting agreement for scientific advisory services with Dr. Donald Abraham, a director of the Company from 1994 through May 10, 2004. Under the one-year agreement, which was renewable upon mutual consent, we paid Dr. Abraham consulting fees of $2,000 per month. In March 2002, this contract was terminated. Effective July 1, 2003, we entered into another one-year consulting agreement, under which we paid Dr. Abraham consulting fees of $5,000 per month. Starting in June 2004, this agreement was renewed each year for successive one-year terms through June 30, 2007. The agreement was not renewed after June 30, 2007. For the years ended December 31, 2008, 2007, 2006 and the cumulative period from inception through December 31, 2008, we paid Dr. Abraham consulting fees of $0, $30,000, $60,000 and $288,000, respectively.
Dr. Stephen Hoffman
Dr. Stephen J. Hoffman has served as a member of our Board of Directors since 1994 and as our Chairman of the Board since December 2001. He also served as our President and Chief Executive Officer from July 1994 to December 2001. On January 12, 2000, we granted Dr. Hoffman a stock option to purchase 328,971 shares of common stock at $2.42 per share, or the 1995 Plan Option, under the terms of our 1995 Stock Option Plan. Effective February 28, 2003, we entered into a two-year consulting agreement, or the Consulting Agreement, with Dr. Hoffman and terminated the employment agreement previously entered into with him in January 2001.
Pursuant to the Consulting Agreement, Dr. Hoffman served us as non-executive Chairman of the Board and was required to provide consulting services as requested by us from time to time. According to the Consulting Agreement, Dr. Hoffman's then-outstanding options continued to vest through the end of the term of the agreement, or February 28, 2005. We have accounted for these stock options using variable accounting as prescribed by FASB Interpretation No. 44,Accounting for Certain Transactions Involving Stock Compensation, and we recorded a recovery of non-cash stock-based compensation of $2,488 and $170,118 during 2004 and 2005 and stock-based compensation of $173,963 during 2003, respectively. Stock options granted to Dr. Hoffman in 2007, 2006 and 2005 related to Board of Director services.
The Consulting Agreement expired in accordance with its terms on February 28, 2005. On May 18, 2005, in recognition of Dr. Hoffman's efforts and services on behalf of the Company and as an incentive for Dr. Hoffman's continued service as our Chairman of the Board, our Board of Directors approved an amendment to the 1995 Plan Option to extend the exercise period for such option until the earlier of: (i) January 12, 2010 (the expiration date of such option), or (ii) three months after the date that Dr. Hoffman ceases to serve as a director of the Company. Prior to such amendment, the 1995 Plan Option would have expired on May 28, 2005, or three months after the expiration of Dr. Hoffman's Consulting Agreement with the Company. Except as set forth above, the 1995 Plan Option remains in full force and effect in accordance with its original terms. In conjunction with this amendment to the 1995 Plan Option, we recorded non-cash stock-based compensation expense of $462,000 during the year ended December 31, 2005. This expense is reflected in marketing, general and administrative expenses in our Statement of Operations.
F-37
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
9. Related Party Transactions (Continued)
Dr. Marvin Jaffe, M.D.
Dr. Marvin E. Jaffe served as a member of our Board of Directors from 1994 to May 10, 2006. On March 11, 2006, Dr. Jaffe tendered his resignation as a director of the Company effective immediately prior to our 2006 annual meeting of stockholders and notified the Board that he did not intend to stand for reelection. As a result of Dr. Jaffe's resignation as a director, on May 10, 2006, we entered into a consulting agreement with Dr. Jaffe in order to allow us to retain the benefit of Dr. Jaffe's knowledge and expertise regarding the Company's business and the potential clinical development and commercialization strategies for our products (the "Jaffe Consulting Agreement"). Pursuant to the Jaffe Consulting Agreement, Dr. Jaffe agreed to provide up to 10 hours of consulting service per month as and when requested from time to time by the Company. In connection with the performance of his consulting services, we granted Dr. Jaffe a nonqualified stock option under the Company's 2000 Stock Incentive Compensation Plan to purchase 20,000 shares of common stock at an exercise price equal to $2.94 per share, which equals the closing sale price of a share of our common stock on the effective date of the Jaffe Consulting Agreement (as reported by the Nasdaq National Market). This option is subject to the terms and conditions of the 2000 Stock Incentive Compensation Plan, as further provided in our 2008 Equity Incentive Plan, and vests in eighteen equal monthly installments commencing July 1, 2006. Dr. Jaffe is not entitled to any additional compensation or benefits in connection with the performance of his consulting services. The Jaffe Consulting Agreement terminated on December 31, 2007.
Michael E. Hart
Pursuant to the Separation Agreement with Mr. Hart (as discussed in Note 5) and as a result of Mr. Hart's resignation as a director, on May 10, 2006, we entered into a consulting agreement with Mr. Hart in order to allow us to retain the benefit of Mr. Hart's historical knowledge regarding the Company's operations and corporate development strategies, or the Hart Consulting Agreement. Pursuant to the Hart Consulting Agreement, Mr. Hart agreed to provide an average of at least 10 hours of consulting services per month as and when requested from time to time by the Company. Mr. Hart is not entitled to any compensation or benefits in connection with the performance of his consulting services, except for those payments and benefits being provided to him under the Separation Agreement. The Hart Consulting Agreement terminated on December 31, 2007.
F-38
Table of Contents
ALLOS THERAPEUTICS, INC.
(A Development Stage Enterprise)
NOTES TO FINANCIAL STATEMENTS (Continued)
10. Quarterly Information (Unaudited)
The results of operations on a quarterly basis for the years ended December 31, 2008 and 2007 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | March 31,
2008 | | June 30,
2008 | | Sept. 30,
2008 | | Dec. 31,
2008 | | March 31,
2007 | | June 30,
2007 | | Sept. 30,
2007 | | Dec. 31,
2007 | |
|---|
Operating expenses: | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Research and development | | $ | 5,973,612 | | $ | 5,403,924 | | $ | 6,360,950 | | $ | 6,109,566 | | $ | 3,289,428 | | $ | 4,360,787 | | $ | 4,394,726 | | $ | 5,399,379 | |
| | Clinical manufacturing | | | 1,586,558 | | | 1,485,052 | | | 1,727,630 | | | 1,947,906 | | | 1,147,304 | | | 1,384,804 | | | 1,506,255 | | | 1,509,048 | |
| | Marketing, general and administrative | | | 5,011,364 | | | 5,438,764 | | | 5,326,357 | | | 7,266,943 | | | 4,747,596 | | | 5,514,923 | | | 4,240,704 | | | 5,168,791 | |
| | | | | | | | | | | | | | | | | | |
| | | Total operating expenses | | | 12,571,534 | | | 12,327,740 | | | 13,414,937 | | | 15,324,415 | | | 9,184,328 | | | 11,260,514 | | | 10,141,685 | | | 12,077,218 | |
Loss from operations | | | (12,571,534 | ) | | (12,327,740 | ) | | (13,414,937 | ) | | (15,324,415 | ) | | (9,184,328 | ) | | (11,260,514 | ) | | (10,141,685 | ) | | (12,077,218 | ) |
Interest and other income, net | | | 564,935 | | | 504,025 | | | 254,416 | | | 585,337 | | | 773,464 | | | 909,140 | | | 843,542 | | | 768,000 | |
| | | | | | | | | | | | | | | | | | |
Net loss | | $ | (12,006,599 | ) | $ | (11,823,715 | ) | $ | (13,160,521 | ) | $ | (14,739,078 | ) | $ | (8,410,864 | ) | $ | (10,351,374 | ) | $ | (9,298,143 | ) | $ | (11,309,218 | ) |
| | | | | | | | | | | | | | | | | | |
Net loss per share: basic and diluted | | $ | (0.18 | ) | $ | (0.16 | ) | $ | (0.16 | ) | $ | (0.18 | ) | $ | (0.14 | ) | $ | (0.16 | ) | $ | (0.14 | ) | $ | (0.17 | ) |
| | | | | | | | | | | | | | | | | | |
Weighted average shares: basic and diluted | | | 67,266,819 | | | 72,382,487 | | | 80,752,024 | | | 80,894,796 | | | 62,151,400 | | | 65,645,678 | | | 66,042,023 | | | 66,855,484 | |
| | | | | | | | | | | | | | | | | | |
F-39
Table of Contents
EXHIBIT INDEX
| | | | | | | | | | | |
| |
| | Incorporated by Reference | |
|
|---|
Exhibit
No. | |
| | Filed
Herewith |
|---|
| | Description | | Form | | Filing Date | | Number |
|---|
| | 3.01 | | Amended and Restated Certificate of Incorporation. | | 10-Q | | 8/7/2006 | | 3.01 | | |
|
3.02 |
|
Certificate of Designation of Series A Junior Participating Preferred Stock. |
|
10-Q |
|
8/7/2006 |
|
3.02 |
|
|
|
3.03 |
|
Certificate of Amendment to Restated Certificate of Incorporation. |
|
10-Q |
|
8/7/2006 |
|
3.03 |
|
|
|
3.04 |
|
Amended and Restated Bylaws of Allos Therapeutics, Inc. |
|
8-K |
|
6/25/2007 |
|
3.04 |
|
|
|
4.01 |
|
Form of Common Stock Certificate. |
|
S-1/A |
|
3/17/2000 |
|
4.01 |
|
|
|
4.02 |
|
Reference is made to Exhibits 3.01, 3.02, 3.03 and 3.04. |
|
|
|
|
|
|
|
|
|
4.03 |
|
Rights Agreement dated May 6, 2003 between Allos and Mellon Investor Services LLC. |
|
8-K |
|
5/9/2003 |
|
99.2 |
|
|
|
4.04 |
|
Form of Rights Certificate. |
|
8-K |
|
5/9/2003 |
|
99.3 |
|
|
|
4.05 |
|
Amendment to Rights Agreement dated March 4, 2005 between Allos and Mellon Investor Services LLC. |
|
8-K |
|
3/4/2005 |
|
4.06 |
|
|
|
4.06 |
|
Amendment to Rights Agreement dated January 29, 2007 between Allos and Mellon Investor Services LLC. |
|
8-K |
|
1/30/2007 |
|
4.1 |
|
|
|
10.01 |
† |
Form of Amended and Restated Indemnity Agreement between Allos and each of its directors and officers. |
|
8-K |
|
6/25/2007 |
|
10.01 |
|
|
|
10.02 |
† |
1995 Stock Option Plan, as amended. |
|
S-1 |
|
1/26/2000 |
|
10.11 |
|
|
|
10.03 |
† |
2000 Stock Incentive Compensation Plan, as amended. |
|
8-K |
|
12/22/2005 |
|
10.1 |
|
|
|
10.03.1 |
† |
Form of Incentive Stock Option Letter Agreement under 2000 Stock Incentive Compensation Plan. |
|
8-K |
|
2/11/2005 |
|
99.1 |
|
|
|
10.03.2 |
† |
Form of Nonqualified Stock Option Letter Agreement under 2000 Stock Incentive Compensation Plan. |
|
8-K |
|
2/11/2005 |
|
99.2 |
|
|
|
10.03.3 |
† |
Form of Nonqualified Stock Option Letter Agreement for Non-Employee Directors under 2000 Stock Incentive Compensation Plan. |
|
8-K |
|
2/24/2006 |
|
10.1 |
|
|
|
10.04 |
† |
2001 Employee Stock Purchase Plan and form of Offering. |
|
10-K |
|
3/7/2001 |
|
10.26 |
|
|
|
10.04.1 |
† |
2001 Employee Stock Purchase Plan Offering (Series Beginning July 1, 2007). |
|
8-K |
|
6/25/2007 |
|
10.12.1 |
|
|
|
10.05 |
* |
Office Lease dated April 4, 2001 between Allos and Catellus Development Corporation. |
|
10-Q |
|
8/14/2001 |
|
10.27 |
|
|
|
10.05.1 |
* |
Amended and Restated Second Amendment to Lease dated December 9, 2002 between Allos and Catellus Development Corporation. |
|
10-K |
|
3/28/2003 |
|
10.27.1 |
|
|
|
10.05.2 |
* |
Third Amendment to Lease dated November 28, 2003 between Allos and Catellus Development Corporation. |
|
10-K |
|
3/5/2004 |
|
10.27.2 |
|
|
Table of Contents
| | | | | | | | | | | |
| |
| | Incorporated by Reference | |
|
|---|
Exhibit
No. | |
| | Filed
Herewith |
|---|
| | Description | | Form | | Filing Date | | Number |
|---|
| | 10.05.3 | * | Fifth Amendment to Office Lease Agreement dated June 16, 2008 between Allos and Circle Point Properties, LLC. | | 10-Q | | 8/5/2008 | | 10.5.3 | | |
|
10.06 |
|
Securities Purchase Agreement dated March 2, 2005 between Allos and the Investors listed on the signature pages thereto. |
|
8-K/A |
|
3/10/2005 |
|
10.41 |
|
|
|
10.07 |
|
Registration Rights Agreement dated March 4, 2005 between Allos and the Investors listed on Schedule I thereto. |
|
8-K/A |
|
3/10/2005 |
|
10.42 |
|
|
|
10.08 |
|
Letter Agreement dated March 4, 2005 among Allos, Warburg Pincus Private Equity VIII, L.P., Warburg Pincus & Co. and Warburg Pincus LLC. |
|
8-K |
|
3/4/2005 |
|
10.43 |
|
|
|
10.09 |
† |
Summary of Compensation Arrangements for Non-Employee Directors. |
|
10-Q |
|
8/7/2007 |
|
10.32 |
|
|
|
10.10 |
† |
Restricted Stock Award Agreement dated March 9, 2006 between Allos and Paul L. Berns. |
|
8-K |
|
3/14/2006 |
|
10.2 |
|
|
|
10.11 |
† |
2006 Inducement Award Plan, including forms of Stock Option Grant Notice with Stock Option Agreement and Restricted Stock Grant Notice with Restricted Stock Grant Agreement. |
|
8-K |
|
6/6/2006 |
|
10.1 |
|
|
|
10.12 |
|
Letter agreement dated January 28, 2007 among Allos, Baker Bros. Investments, L.P., Baker Bros. Investments II, L.P., Baker/Tisch Investments, L.P., Baker Biotech Fund I, L.P., 14159, L.P. and Baker Brothers Life Sciences, L.P. |
|
8-K |
|
1/30/2007 |
|
10.1 |
|
|
|
10.13 |
* |
License Agreement for 10-Propargyl-10-Deazaaminopterin "PDX" dated December 23, 2002 and amended May 9, 2006 between Allos and SRI International, Sloan-Kettering Institute for Cancer Research and Southern Research Institute. |
|
10-Q |
|
8/7/2007 |
|
10.45 |
|
|
|
10.13.1 |
* |
Second Amendment to License Agreement for 10-Propargyl-10-Deazaaminopterin "PDX" dated November 6, 2007 between Allos and SRI International, Sloan-Kettering Institute for Cancer Research and Southern Research Institute. |
|
10-K |
|
2/27/2008 |
|
10.18.1 |
|
|
|
10.14 |
† |
Corporate Bonus Plan, as amended and restated effective September 15, 2008. |
|
10-Q |
|
11/5/2008 |
|
10.1 |
|
|
|
10.15 |
† |
Amended and Restated Employment Agreement, effective December 13, 2007, between Allos and Paul L. Berns. |
|
10-K |
|
2/27/2008 |
|
10.20 |
|
|
|
10.16 |
† |
Amended and Restated Employment Agreement, effective December 13, 2007, between Allos and Pablo J. Cagnoni, M.D. |
|
10-K |
|
2/27/2008 |
|
10.21 |
|
|
Table of Contents
| | | | | | | | | | | |
| |
| | Incorporated by Reference | |
|
|---|
Exhibit
No. | |
| | Filed
Herewith |
|---|
| | Description | | Form | | Filing Date | | Number |
|---|
| | 10.17 | † | Amended and Restated Employment Agreement, effective December 13, 2007, between Allos and James V. Caruso. | | 10-K | | 2/27/2008 | | 10.22 | | |
|
10.18 |
† |
Amended and Restated Employment Agreement, effective December 13, 2007, between Allos and Marc H. Graboyes. |
|
10-K |
|
2/27/2008 |
|
10.23 |
|
|
|
10.19 |
† |
Letter agreement, effective January 22, 2008, between Allos and Bruce K. Bennett. |
|
10-K |
|
2/27/2008 |
|
10.24 |
|
|
|
10.20 |
* |
License Agreement, dated as of December 13, 2004, among Allos, The Regents of the University of Colorado, the University of Salford and Cancer Research Technology Limited. |
|
10-K/A |
|
8/25/2008 |
|
10.25 |
|
|
|
10.21 |
† |
Executive Compensation and Equity Awards. |
|
8-K |
|
2/27/2009 |
|
10.1 |
|
|
|
10.22 |
† |
Allos Therapeutics, Inc. 2008 Equity Incentive Plan. |
|
S-8 |
|
6/24/2008 |
|
99.1 |
|
|
|
10.22.1 |
† |
Form of Option Grant Notice and Agreement under the 2008 Equity Incentive Plan. |
|
S-8 |
|
6/24/2008 |
|
99.2 |
|
|
|
10.22.2 |
† |
Form of Restricted Stock Award Grant Notice and Agreement under the 2008 Equity Incentive Plan. |
|
S-8 |
|
6/24/2008 |
|
99.3 |
|
|
|
10.22.3 |
† |
Form of Restricted Stock Unit Grant Notice and Agreement under the 2008 Equity Incentive Plan. |
|
8-K |
|
2/27/2009 |
|
10.2 |
|
|
|
10.23 |
† |
Allos Therapeutics, Inc. Severance Benefit Plan, as amended and restated effective December 11, 2007. |
|
8-K |
|
2/27/2009 |
|
10.3 |
|
|
|
10.23.1 |
† |
Allos Therapeutics, Inc. Change in Control Severance Benefit Schedule, as amended and restated effective February 23, 2008. |
|
8-K |
|
2/27/2009 |
|
10.4 |
|
|
|
23.01 |
|
Consent of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm. |
|
|
|
|
|
|
|
X |
|
24.01 |
|
Power of Attorney (included on signature page hereto). |
|
|
|
|
|
|
|
X |
|
31.01 |
|
Rule 13a-14(a)/15d-14(a) Certification. |
|
|
|
|
|
|
|
X |
|
31.02 |
|
Rule 13a-14(a)/15d-14(a) Certification. |
|
|
|
|
|
|
|
X |
|
32.01 |
# |
Section 1350 Certification. |
|
|
|
|
|
|
|
X |
- †
- Indicates management contract or compensatory plan or arrangement required to be filed as an exhibit pursuant to Item 15(b) of Form 10-K.
- *
- Indicates confidential treatment has been granted with respect to specific portions of this exhibit. Omitted portions have been filed with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
- #
- The certifications attached as Exhibit 32.01 that accompany this Annual Report on Form 10-K are not deemed filed with the Securities and Exchange Commission and are not to be incorporated by reference into any filing of Allos Therapeutics, Inc. under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this Form 10-K, irrespective of any general incorporation language contained in such filing.

