|
Exhibit 99.1 
|
At Agenus, we believe
activating our own body’s
natural mechanisms is a
a powerful way to treat
disease.
Corporate Presentation
February 2014
This presentation contains forward-looking statements. These forward-looking statements are subject to risks and uncertainties, including the factors described under the Risk Factors section of our most recent annual or quarterly report and any subsequent periodic reports on Form 8-K that we have filed with the Securities and Exchange Commission and made available on our website at www.agenusbio.com. When evaluating Agenus’ business and prospects, careful consideration should be given to these risks and uncertainties. These statements speak only as of the date of this document, and Agenus undertakes no obligation to update or revise these statements. This presentation and the information contained herein do not constitute an offer or solicitation of an offer for sale of any securities. 2
Forward-Looking Statement (I)
Forward-Looking Statement (II)
On 1/13/14, Agenus announced a definitive agreement to acquire 4-Antibody. Agenus expects this transaction to close in February 2014, subject to customary closing conditions. This presentation contains information on the future combined organization. Please refer to form 8-K filed on 1/13/14 for further information regarding this transaction. 3
Overview
Focus on immuno-oncology and infectious diseases
Three immune activating platforms
– Checkpoint Antibody (4-Antibody AG)
– Vaccine
– Adjuvant
Key corporate partners
– GSK & Pfizer/Janssen
Research collaborators
Ludwig Institute, Memorial Sloan-Kettering Cancer Center (MSKCC), & National Cancer Institute (NCI)
Partnering opportunities with 4-Antibody platform
– At least one collaboration in 2014
Headquarters, R&D, and cGMP facility in Lexington, MA with offices in NY, NY; and Jena, Germany and Basel, Switzerland (4-Antibody)
– ~110 employees
4
Recent Strategic Acquisition
Definitive agreement for privately held 4-Antibody AG (1/13/14)
Platform for rapid discovery and optimization of fully-human antibodies against a wide array of molecular targets
– Six lead checkpoint programs
GITR, OX40, CTLA-4, PD-1, TIM-3 and LAG-3
– Proprietary mammalian B cell platform generates fully human antibodies with desirable drug-like behavior
Transaction terms
– $10M in shares of AGEN common stock
– Contingent payments (~$40M cash or stock) upon milestone achievements tied to market cap appreciation
– Subject to customary closing conditions
5
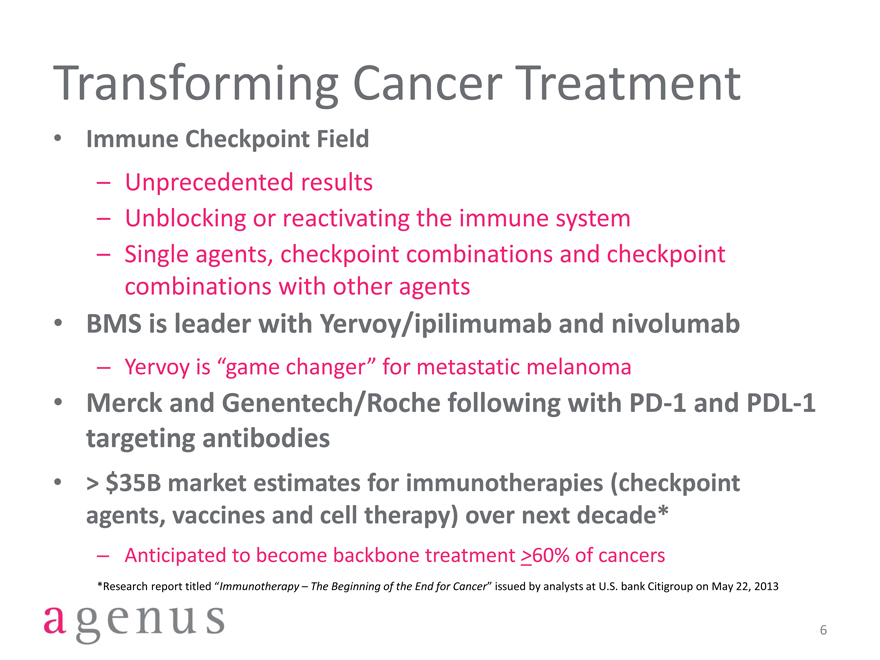
Transforming Cancer Treatment
Immune Checkpoint Field
– Unprecedented results
– Unblocking or reactivating the immune system
– Single agents, checkpoint combinations and checkpoint combinations with other agents
BMS is leader with Yervoy/ipilimumab and nivolumab
– Yervoy is “game changer” for metastatic melanoma
Merck and Genentech/Roche following with PD-1 and PDL-1 targeting antibodies
> $35B market estimates for immunotherapies (checkpoint agents, vaccines and cell therapy) over next decade*
– Anticipated to become backbone treatment >60% of cancers
*Research report titled “Immunotherapy – The Beginning of the End for Cancer” issued by analysts at U.S. bank Citigroup on May 22, 2013
6
Three Immune Activating Platforms
21 clinical programs
4 in Phase 3
Partners: GSK and PFE/Janssen
Opportunity for significant royalty income
Heat shock Protein vaccine platform
Glioblastoma (Phase 2 NCI- sponsored study)
Genital herpes (Phase 2)
QS-21 stimulon® Adjuvant
Fully human antibody platform
4-Antibody:
GITR and OX40 checkpoint agonists
CTLA-4, PD-1, TIM-3, LAG-3 checkpoint inhibitors
Partnership opportunities for portfolio programs
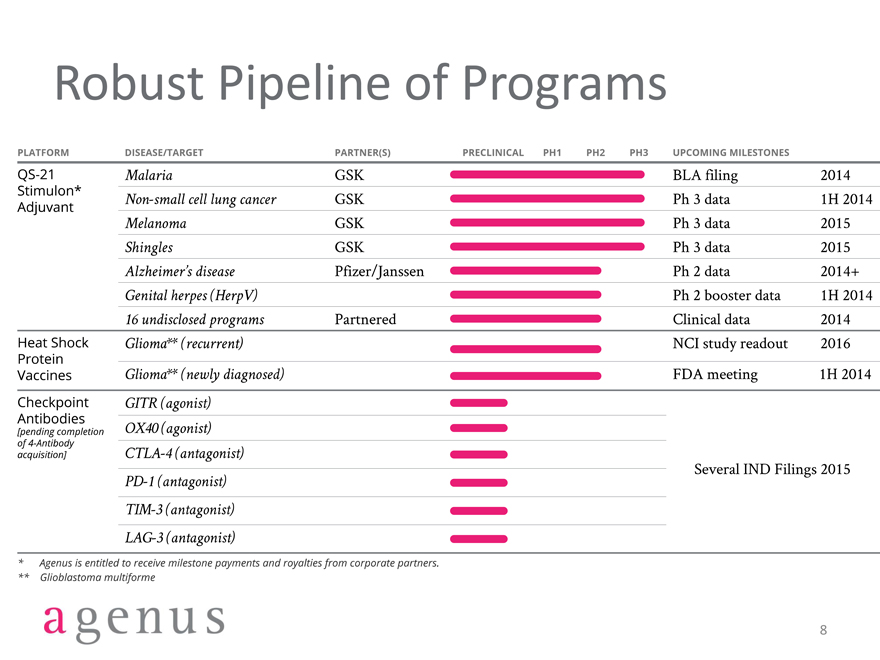
Robust Pipeline of Programs
PLATFORM
DISEASE/TARGET PARTNER(S) PRECLINICAL PH1 PH2 PH3 UPCOMING MILESTONES
QS-21
Stimulon*
Adjuvant Malaria
Non-small cell lung cancer
Melanoma
Shingles
Alzheimer’s disease
Genital herpes(HerpV)
16 undisclosed programs GSK
GSK
GSK
GSK
Pfizer/Janssen
Partnered BLA filling 2014
Ph 3 data 1H 2014
Ph 3 data 2015
Ph 3 data 2015
Ph 2 data 2014+
Ph 2 booster data 1h 2014
Clinical data 2014
Heat Shock
Protein
Vaccines Glioma**(recurrent)
Glioma** (newly diagnose) NCI study readout 2016
FDA meeting 1h 2014
Check point
Antibodies
[pending completion of 4-Antibody
Acquisition] GITR (agonist)
OX40 (agonist)
CTLA-4 (antagonist)
PD-1 (antagonist)
TIM-3 (antagonist)
LAG-3 (antagonist)
Several IND Fillings 2015
*Agenus is entitled to receive milestone payments and royalties from corporate partners.
**Glioblastoma multiforme
8
Leadership
Garo H. Armen, PhD Reiner Laus, MD
– Chairman & CEO – Strategic Advisor
Jonae R. Barnes Janice McCourt
– Vice President, Investor Relations & – Vice President, Business
Corporate Communications Development
Ozer Baysal Stephen Monks, PhD
– Chief Business Officer – Vice President, Manufacturing,
Robert Burns, PhD Process & Analytical Technologies
– CEO 4-Antibody • Robert Stein, MD, PhD
John Cerio – Chief Scientific Officer
– Vice President, Human Resources • Karen Valentine, JD
& Administration – Vice President & General Counsel
Christine Klaskin Kerry Wentworth
– Vice President, Finance & Principal – Vice President, Clinical, Regulatory,
Financial Officer & Quality
9
4-ANTIBODY
Monoclonal Antibody Platform & Immune Checkpoint Antibody Programs
10
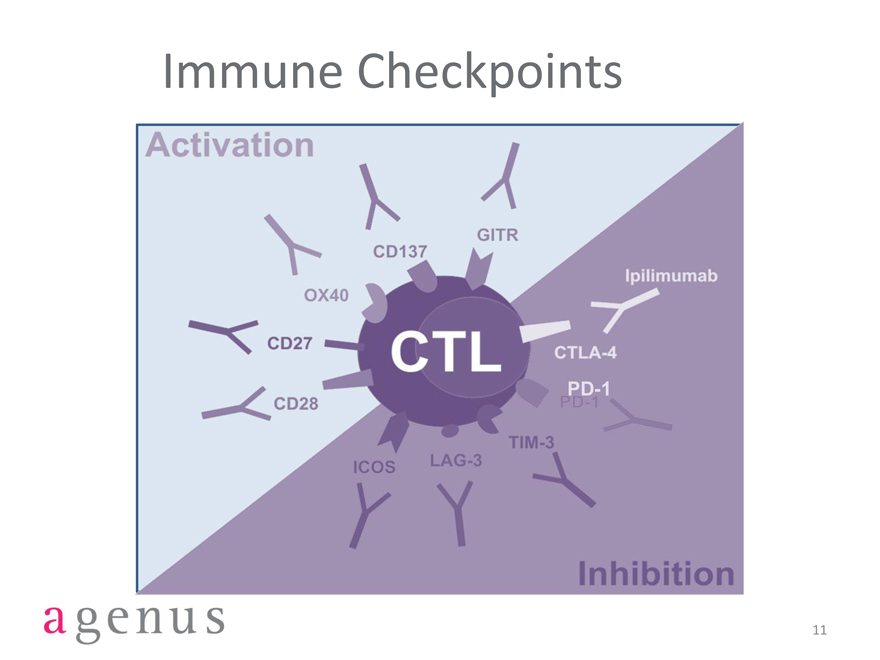
Immune Checkpoints
Activation
GITR
CD137
OX40
CD27
CD28
ICOS
LAG-3
TIM-3
PD-1
PD-1
CTLA-4
CTL
lpilimumab
Inhibition
11
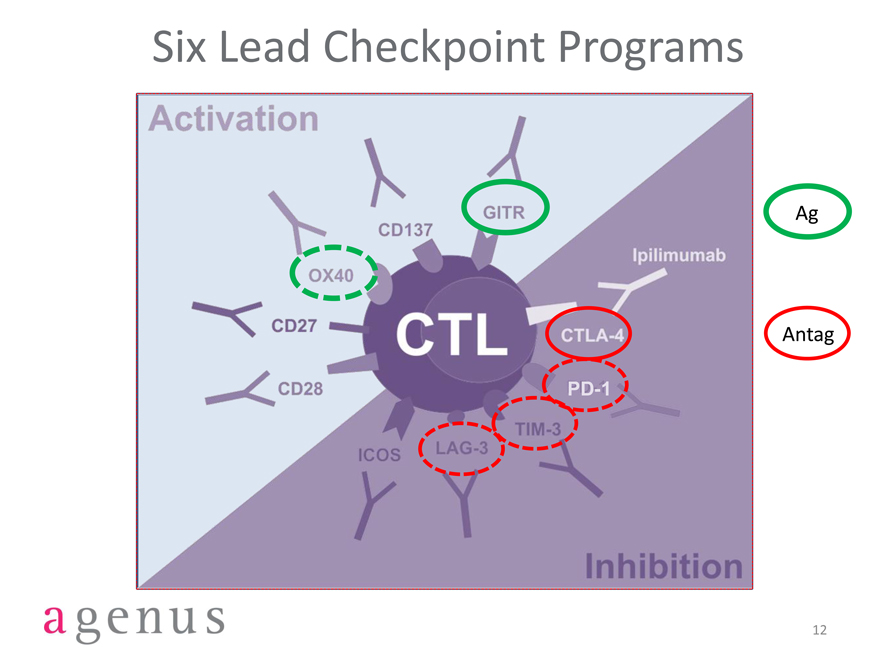
Six Lead Checkpoint Programs
Activation
GITR
CD137
OX40
CD27
CD28
ICOS
LAG-3
TIM-3
PD-1
CTLA-4
Lpilimumab
Ag
CTL
Antag
Inhibition
12
4-Antibody Overview
Powerful platform to efficiently generate optimized fully-human monoclonal antibodies against a wide array of targets
Combinatorial libraries displaying ~billion diverse antibodies on mammalian lymphocytes
Recapitulates natural process
Highly effective to find many antibodies to targets of interest
Uses retroviruses for high-efficiency, rapid engineering
Attractive pharmaceutical characteristics
13
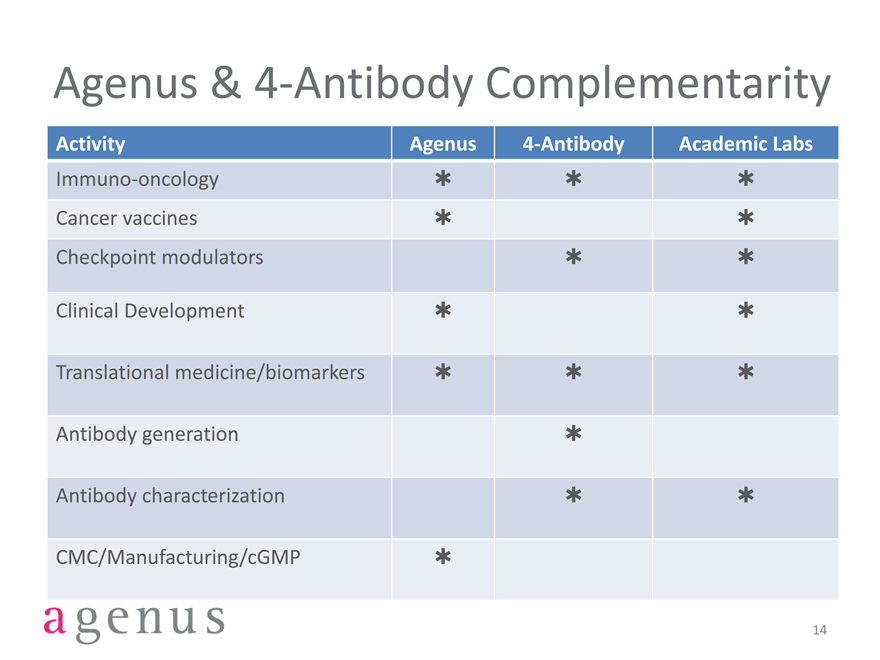
Agenus & 4-Antibody Complementarity
Activity Agenus 4-Antibody Academic Labs
Immuno-oncology***
Cancer vaccines**
Checkpoint modulators**
Clinical Development**
Translational medicine/biomarkers***
Antibody generation*
Antibody characterization**
CMC/Manufacturing/cGMP*
14
Checkpoint Development Programs
Six programs targeting key checkpoints
Complementary ways to activate specific T-cell responses
Applicable to numerous cancers and infections
GITR and CTLA-4 at candidate selection stage
OX40, PD-1, TIM-3 and LAG-3 checkpoint programs nearing candidate selection
Central to rapidly evolving cancer treatment paradigms
Collaborations with world class researchers and clinicians
The Ludwig Institute and MSKCC
Integral to selection of candidates and translational development
Goal: create & advance best-in-class immune activators
15
HEAT SHOCK PROTEIN VACCINES
Prophage Series & HerpV
16
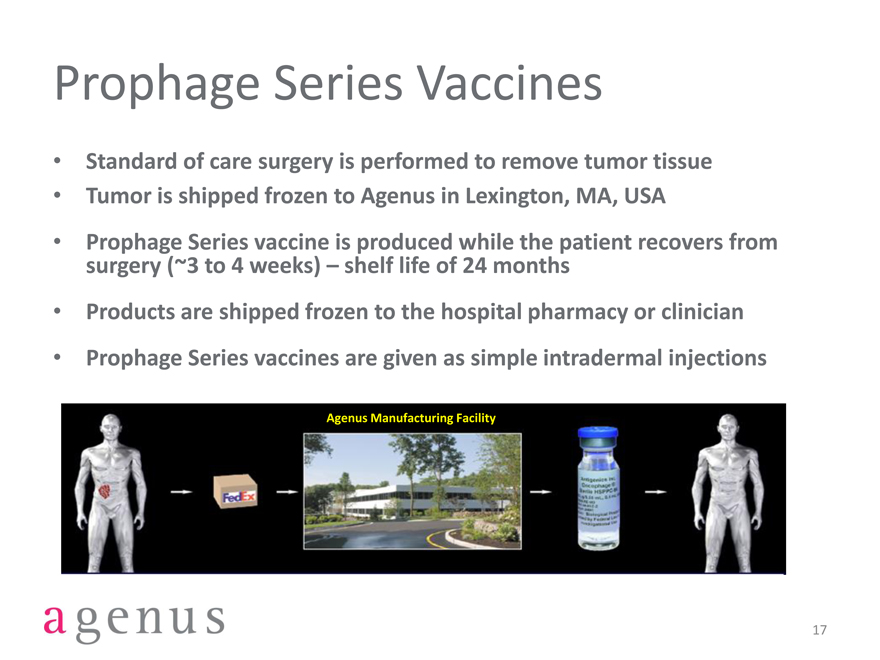
Prophage Series Vaccines
Standard of care surgery is performed to remove tumor tissue
Tumor is shipped frozen to Agenus in Lexington, MA, USA
Prophage Series vaccine is produced while the patient recovers from surgery (~3 to 4 weeks) – shelf life of 24 months
Products are shipped frozen to the hospital pharmacy or clinician
Prophage Series vaccines are given as simple intradermal injections
Agenus Manufacturing Facility
17
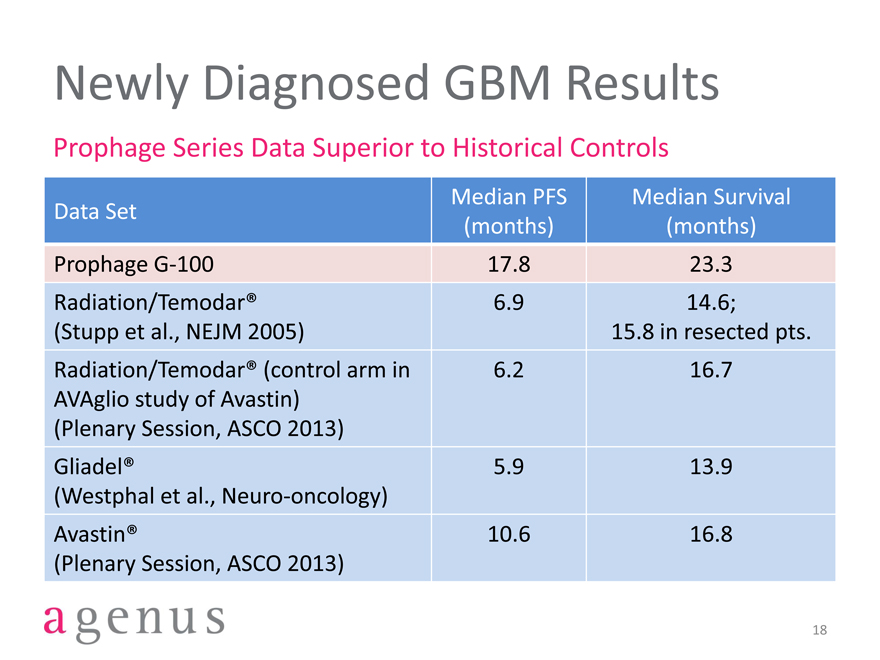
Newly Diagnosed GBM Results
Prophage Series Data Superior to Historical Controls
Median PFS Median Survival
Data Set
(months)(months)
Prophage G-100 17.8 23.3
Radiation/Temodar® 6.9 14.6;
(Stupp et al., NEJM 2005) 15.8 in resected pts.
Radiation/Temodar® (control arm in 6.2 16.7
AVAglio study of Avastin)
(Plenary Session, ASCO 2013)
Gliadel® 5.9 13.9
(Westphal et al., Neuro-oncology)
Avastin® 10.6 16.8
(Plenary Session, ASCO 2013)
18
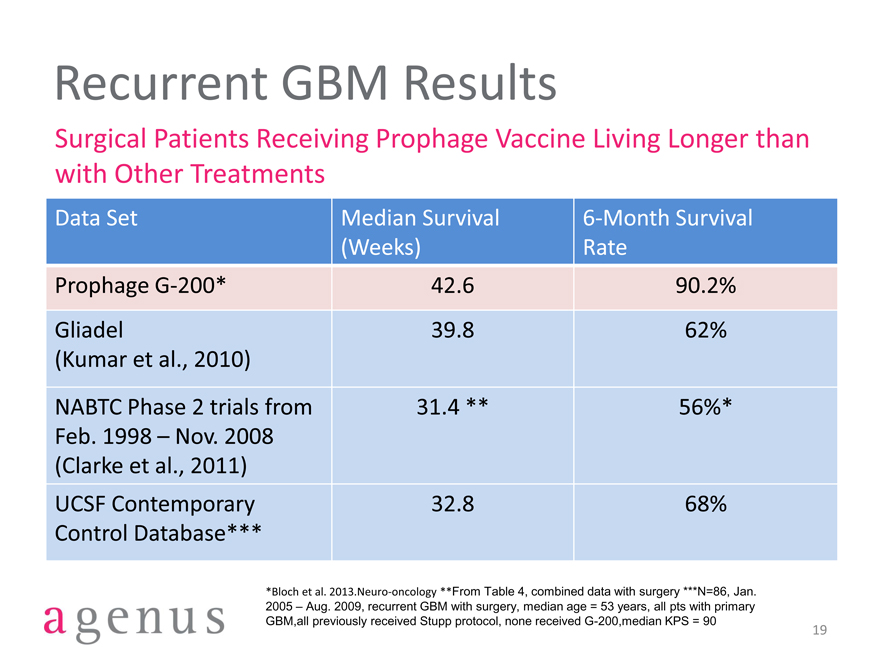
Recurrent GBM Results
Surgical Patients Receiving Prophage Vaccine Living Longer than with Other Treatments
Data Set Median Survival 6-Month Survival
(Weeks) Rate
Prophage G-200* 42.6 90.2%
Gliadel 39.8 62%
(Kumar et al., 2010)
NABTC Phase 2 trials from 31.4 ** 56%*
Feb. 1998 – Nov. 2008
(Clarke et al., 2011)
UCSF Contemporary 32.8 68%
Control Database***
*Bloch et al. 2013.Neuro-oncology **From Table 4, combined data with surgery ***N=86, Jan. 2005 – Aug. 2009, recurrent GBM with surgery, median age = 53 years, all pts with primary GBM,all previously received Stupp protocol, none received G-200,median KPS = 90
19
Prophage Series G-200 Next Steps
Large-scale Phase 2 trial of Prophage in combination with Avastin® in recurrent GBM patients underway
– 222 patients, randomized
– Largest NCI-sponsored vaccine trial in brain cancer
– Largest study of Avastin and a vaccine
– Potential for registration
Andrew Parsa, MD, PhD, lead clinical investigator, chair of neurological surgery at Northwestern Memorial Hospital and Northwestern University
Expanded to over 550 clinical sites (NCI and RTOG)
Results anticipated 2016
20
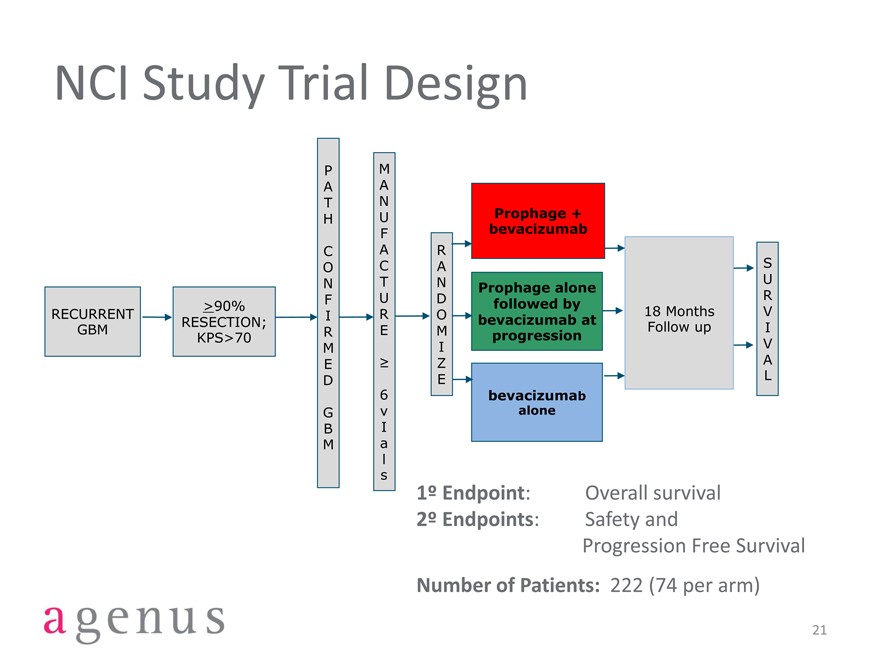
NCI Study Trial Design
P M
A A
T N
H U Prophage +
F bevacizumab
C A R
O C A S
N T N Prophage alone U
³90% F U D followed by R
RECURRENT RESECTION; I R O bevacizumab at 18 Months V
GBM KPS>70 R E M progression Follow up I
M I V
E³Z A
D E L
6 bevacizumab
G v alone
B I
M a
l
s
1º Endpoint: Overall survival
2º Endpoints: Safety and
Progression Free Survival
Number of Patients: 222 (74 per arm)
21
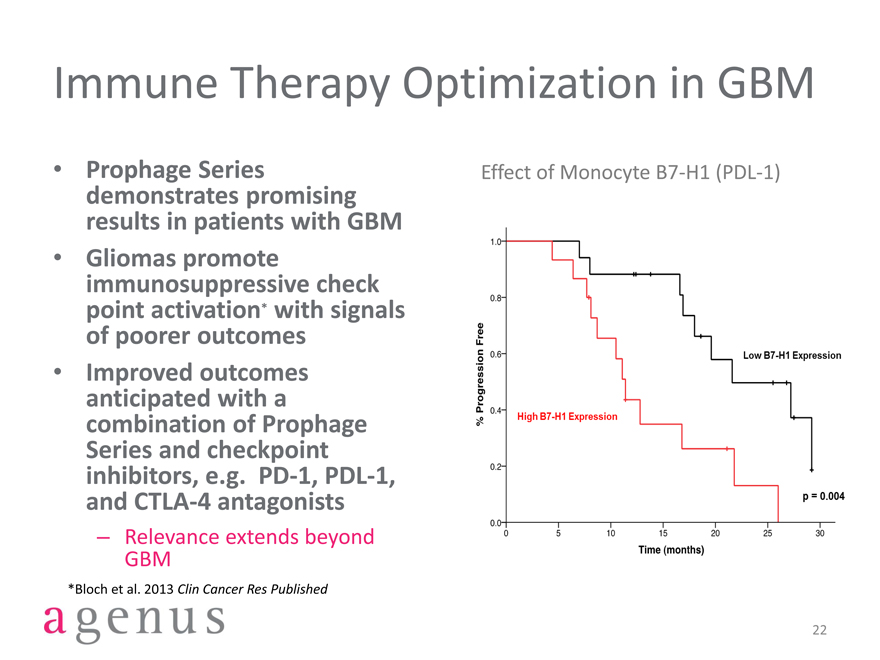
Immune Therapy Optimization in GBM
Prophage Series demonstrates promising results in patients with GBM
Gliomas promote immunosuppressive check point activation* with signals of poorer outcomes
Improved outcomes anticipated with a combination of Prophage Series and checkpoint inhibitors, e.g. PD-1, PDL-1, and CTLA-4 antagonists
– Relevance extends beyond GBM
*Bloch et al. 2013 Clin Cancer Res Published
Effect of Monocyte B7-H1 (PDL-1)
% Progression Free
1.0
0.8
0.6
0.4
0.2
0.0
0
5
10
15
20
25
30
P=0.004
Low B7-H1 Expression
High B7-H1 Expression
Time (months)
22
Conclusions
Prophage shows promising results in patients with GBM
Improved outcomes anticipated with a combination of
Prophage Series and checkpoint inhibitors, e.g. PD-1,
PDL-1, and CTLA-4 antagonists
– Potentially applicable to a broad range of cancers
– Phase 2 study initiated with Prophage and Yervoy (CTLA-4)
for treatment of metastatic melanoma at University of Texas
– Opportunity for combinations with other checkpoint programs
23
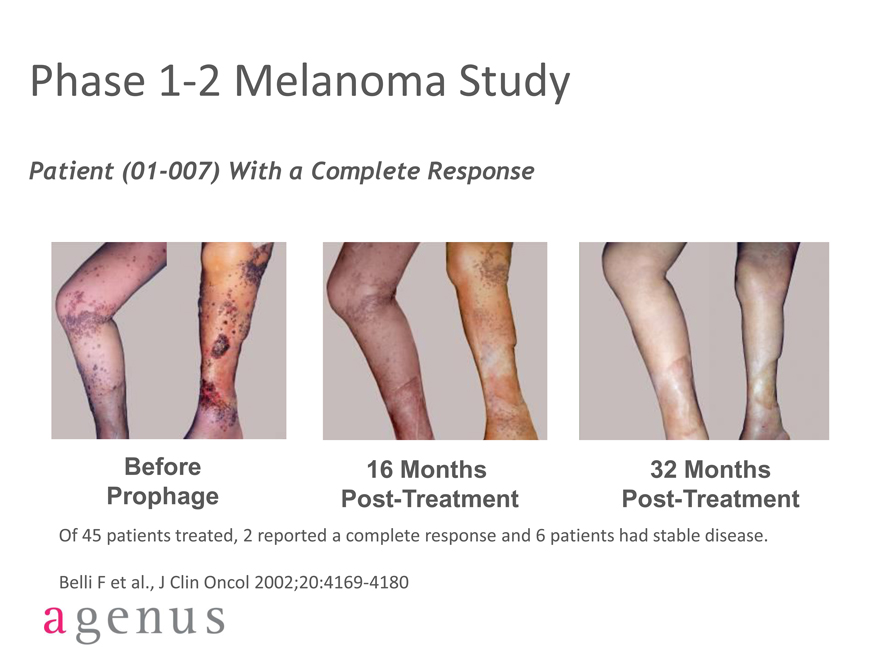
Phase 1-2 Melanoma Study
Patient (01-007) With a Complete Response
Before 16 Months 32 Months
Prophage Post-Treatment Post-Treatment
Of 45 patients treated, 2 reported a complete response and 6 patients had stable disease.
Belli F et al., J Clin Oncol 2002;20:4169-4180
Genital Herpes Overview
Market opportunity >$1 Billion
Disease demographics
– At least 60 million Americans/Europeans affected
– The most prevalent viral STD
– 80% of patients suffer symptomatic recurrences
Currently approved drugs
– Reduce disease severity without affecting reservoir of latent virus
– Partial impact on disease transmission
– Lack of cellular immunity
Medical need for therapeutics to better control symptoms and/or reduce transmission
25
HerpV with QS-21 Stimulon®
Recombinant (defined composition) therapeutic vaccine
One of most clinically advanced therapeutic herpes vaccines
Polyvalent—contains 32 HSV-2 peptide antigens
Phase 1 data published in the scientific journal Vaccine
– 100% of patients receiving HerpV+QS-21 had CD4+ T-cell response
– 75% had CD8+ T-cell response
Phase 2, randomized, double-blind, multicenter
– 80 patients
– Measuring viral shedding, viral load, outbreaks and immune responses
26
Positive Preliminary HerpV Phase 2 Results
Statistically significant reduction in viral shedding; study’s primary endpoint before booster
– 15% viral shedding reduction(P=0.015)
– 34% viral load reduction (P=0.08)
– Booster and immune response data 1H’14
HerpV was generally well tolerated
Reduction in viral shedding considered important surrogate for reduction in recurrent outbreaks
Commercial/partnering strategies to be assessed based on assessment of further clinical results and target patient profiles
27

QS-21 STIMULON® ADJUVANT
Strengthens and broadens immune responses to vaccine antigens
28

QS-21 Stimulon®Adjuvant
Corporate partners
– GSK and Janssen
Unique adjuvant extracted from soap bark tree
Strong antibody and cell-mediated immune responses
Extensive clinical experience; safe and well tolerated with > 50,000 patients treated
21 vaccines candidates in clinical trials
– 4 Phase 3 programs; results expected 2014/2015
– 17 Phase 1 and Phase 2 programs
– 13 additional preclinical programs
Potential for milestones and significant royalties
29

CORPORATE SUMMARY
Financials and Milestones
30
Balance Sheet (as of 9/30/13)
($ millions)
Cash and short-term investments $30.2
Other current assets 0.7
Net plant and equipment 2.9
Other long-term assets 3.9
Total assets $37.7
Current liabilities $ 9.7
Long-term liabilities 11.3
Contingent royalty consideration 19.6
Stockholders’ equity (2.9)
Total liabilities and stockholders’ equity $37.7
31

Expected Milestones
Close 4-Antibody acquisition
Selection of two lead checkpoint candidates (GITR, CTLA-4) and additional development candidates 1H’14
Booster results from Phase 2 HerpV study for genital herpes 1H’14
GSK Phase 3 results for MAGE-A3 in NSCLC 1H 2014
– 2,270 patients; largest NSCLC study conducted
GSK BLA filing for RTS,S for malaria in Africa; launch expected 2015
– Milestone payment for filing; royalties upon launch
At least one corporate collaboration for checkpoint program
32

At Agenus, we believe 3 Forbes Road activating our own body’s Lexington, MA 02421-7305 natural mechanisms is a a powerful way to treat T: 781.674.4400
disease www.agenusbio.com