What is a Biotech Electronic Security Token (B.E.S.T)? B.E.S.T. will be built upon blockchain technology, B.E.S.T. is NOT a cryptocurrency. The first B.E.S.T is offered for a late stage product, AGEN2034, a PD-1 antibody. All PD-1 molecules that entered late stage development have received FDA approval. AGEN2034, Agenus’ PD-1 is in late stage development for patients with advanced cervical cancer. To fund expanded development, manufacturing & commercial launch of AGEN2034, our PD-1 antibody. B.E.S.T provides potential benefit of expanding revenue potential of AGEN2034; not diluting the rest of AGEN’s broad pipeline of products. Currently marketed PD-1 antibodies generated $15B in revenues in 2018; Agenus is taking the B.E.S.T opportunity to expand OUR PD-1 revenue potential. Expanded & accelerated development of AGEN2034 could bring in earlier & greater share of the PD-1 market and revenue; potential to benefit existing shareholders & B.E.S.T holders. Why is AGEN issuing the world’s first biotech electronic security token? B.E.S.T is a revolutionary tool enabling accredited investors to invest in specific late stage products: Exhibit 99.1
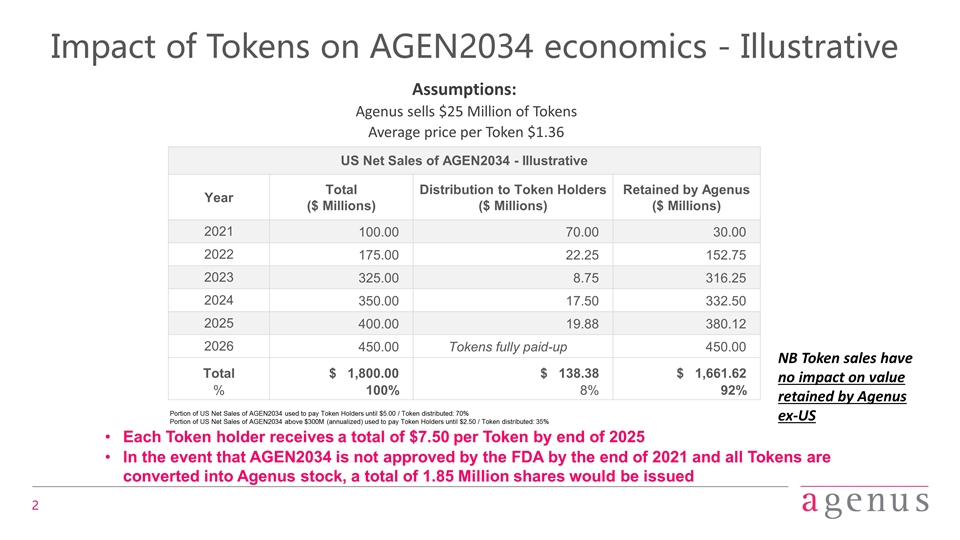
Impact of Tokens on AGEN2034 economics - Illustrative US Net Sales of AGEN2034 - Illustrative Year Total ($ Millions) Distribution to Token Holders ($ Millions) Retained by Agenus ($ Millions) 2021 100.00 70.00 30.00 2022 175.00 22.25 152.75 2023 325.00 8.75 316.25 2024 350.00 17.50 332.50 2025 400.00 19.88 380.12 2026 450.00 Tokens fully paid-up 450.00 Total $ 1,800.00 $ 138.38 $ 1,661.62 % 100% 8% 92% Assumptions: Agenus sells $25 Million of Tokens Average price per Token $1.36 Portion of US Net Sales of AGEN2034 used to pay Token Holders until $5.00 / Token distributed: 70% Portion of US Net Sales of AGEN2034 above $300M (annualized) used to pay Token Holders until $2.50 / Token distributed: 35% Each Token holder receives a total of $7.50 per Token by end of 2025 In the event that AGEN2034 is not approved by the FDA by the end of 2021 and all Tokens are converted into Agenus stock, a total of 1.85 Million shares would be issued NB Token sales have no impact on value retained by Agenus ex-US

First B.E.S.T. Offering: AGEN2034 (PD-1 antibody) PD-1 antibodies are the backbone of immuno-oncology, a revolutionary cancer treatment paradigm with unprecedented efficacy. Significant commercial potential: approved PD-1s are expected to generate $20B in 2019. De-risked clinical asset: PD-1 class has had a 100% approval rate(1) . AGEN2034 expected filing in 2020 with potential approval in 2021. AGEN2034 addresses high unmet need(2) with potential best-in-class efficacy(3) . 3 out of 3 PD-1s that have filed for approval with the FDA have been approved Cervical cancer is the first target indication in combination with AGEN’s CTLA-4 candidate AGEN2034 is a late-stage immuno-oncology therapy with large revenue potential:

Legal Disclaimer This presentation contains forward-looking statements. These forward-looking statements are subject to risks and uncertainties, including the factors described under the Risk Factors section of our most recent Annual Report on Form 10-K or Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission and made available on our website at www.agenusbio.com and in the Private Placement Memorandum for the offering. When evaluating Agenus’ business and prospects and the BEST offering, careful consideration should be given to these risks and uncertainties. These statements speak only as of the date of this presentation, and Agenus undertakes no obligation to update or revise these statements. This presentation and the information contained herein do not constitute an offer or solicitation of an offer for sale of any securities. BESTs are being offered by Agenus pursuant to a Private Placement Memorandum that can be found at https://helium.atomiccapital.io/agenus. Before you invest, you should read the Private Placement Memorandum, including the risk factors described therein, for more complete information about this offering.



