
Exhibit 99.2 Earnings Call: 4Q and Full Year 2019 Earnings March 12, 2020

This presentation contains forward-looking statements that are made pursuant to the safe harbor provisions of the federal securities laws, including statements regarding Agenus and AgenTus' clinical development and regulatory plans and timelines, expected timing for clinical trials and releasing clinical data, expected clinical results and therapeutic benefit, anticipated milestones from partnership transactions, and goals for 2020. These forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially. These risks and uncertainties include, among others, the factors described under the Risk Factors section of our most recent Quarterly Report on Form 10-Q or Annual Report on Form 10-K filed with the Securities and Exchange Commission filed with the Securities and Exchange Commission and made available on our website at www.agenusbio.com. Agenus cautions investors not to place considerable reliance on the forward-looking statements contained in this presentation. These statements speak only as of the date of this presentation, and Agenus undertakes no obligation to update or revise the statements, other than to the extent required by law. All forward-looking statements are expressly qualified in their entirety by this cautionary statement. Forward-Looking Statements
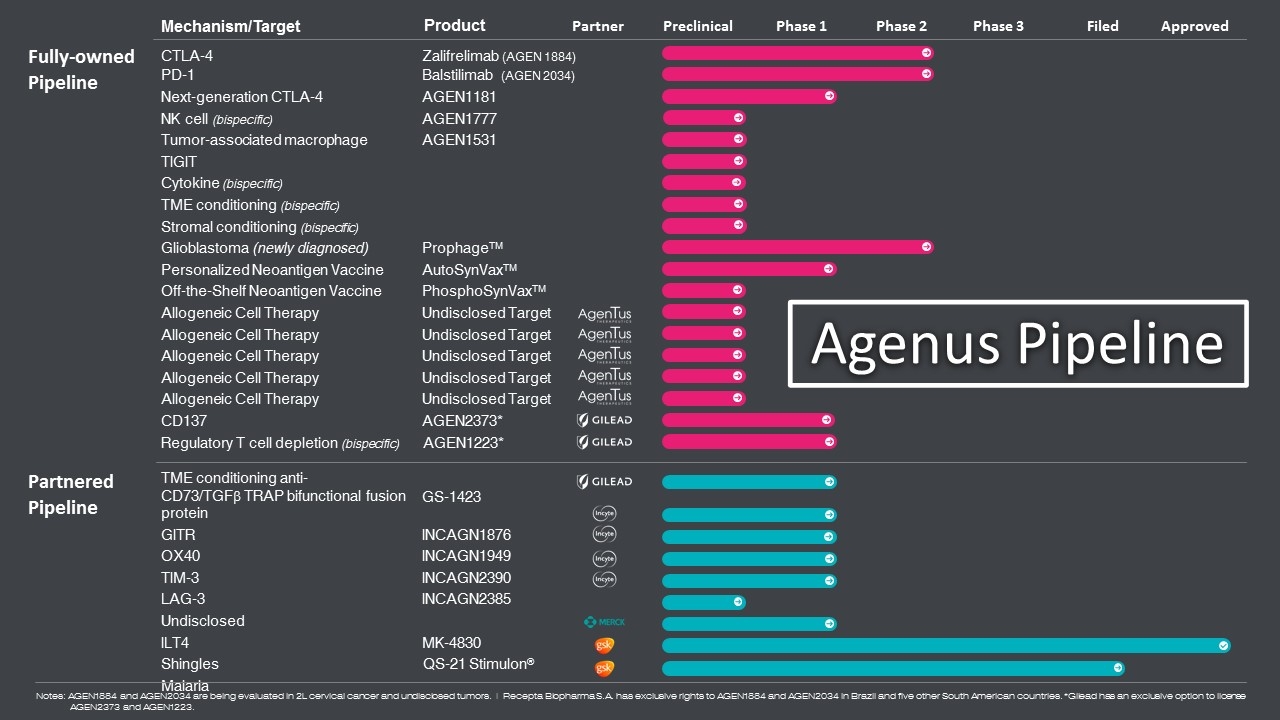
Notes: AGEN1884 and AGEN2034 are being evaluated in 2L cervical cancer and undisclosed tumors. | Recepta Biopharma S.A. has exclusive rights to AGEN1884 and AGEN2034 in Brazil and five other South American countries. *Gilead has an exclusive option to license AGEN2373 and AGEN1223. Mechanism/Target Product Partner Preclinical Phase 1 Phase 2 Phase 3 Filed Approved CTLA-4 Zalifrelimab (AGEN 1884) PD-1 Balstilimab (AGEN 2034) Next-generation CTLA-4 AGEN1181 NK cell (bispecific) AGEN1777 Tumor-associated macrophage AGEN1531 TIGIT Cytokine (bispecific) TME conditioning (bispecific) Stromal conditioning (bispecific) Glioblastoma (newly diagnosed) ProphageTM Personalized Neoantigen Vaccine AutoSynVaxTM Off-the-Shelf Neoantigen Vaccine PhosphoSynVaxTM Allogeneic Cell Therapy Undisclosed Target Allogeneic Cell Therapy Undisclosed Target Allogeneic Cell Therapy Undisclosed Target Allogeneic Cell Therapy Undisclosed Target Allogeneic Cell Therapy Undisclosed Target CD137 AGEN2373* Regulatory T cell depletion (bispecific) AGEN1223* TME conditioning anti-CD73/TGFb TRAP bifunctional fusion protein GS-1423 GITR INCAGN1876 OX40 INCAGN1949 TIM-3 INCAGN2390 LAG-3 INCAGN2385 Undisclosed ILT4 MK-4830 Shingles QS-21 Stimulon® Malaria Fully-owned Pipeline Partnered Pipeline Agenus Pipeline
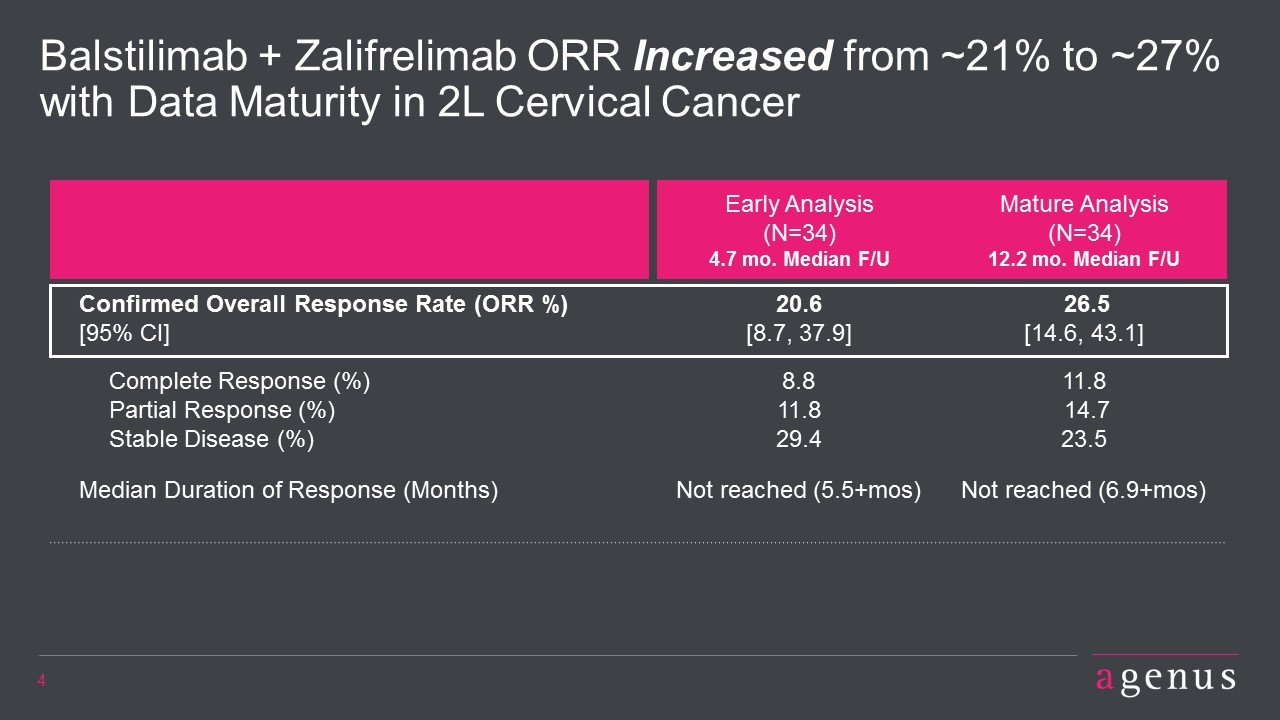
Balstilimab + Zalifrelimab ORR Increased from ~21% to ~27% with Data Maturity in 2L Cervical Cancer Early Analysis (N=34) 4.7 mo. Median F/U Mature Analysis (N=34) 12.2 mo. Median F/U Confirmed Overall Response Rate (ORR %) [95% CI] 20.6 [8.7, 37.9] 26.5 [14.6, 43.1] Complete Response (%) Partial Response (%) Stable Disease (%) 8.8 11.8 29.4 11.8 14.7 23.5 Median Duration of Response (Months) Not reached (5.5+mos) Not reached (6.9+mos)
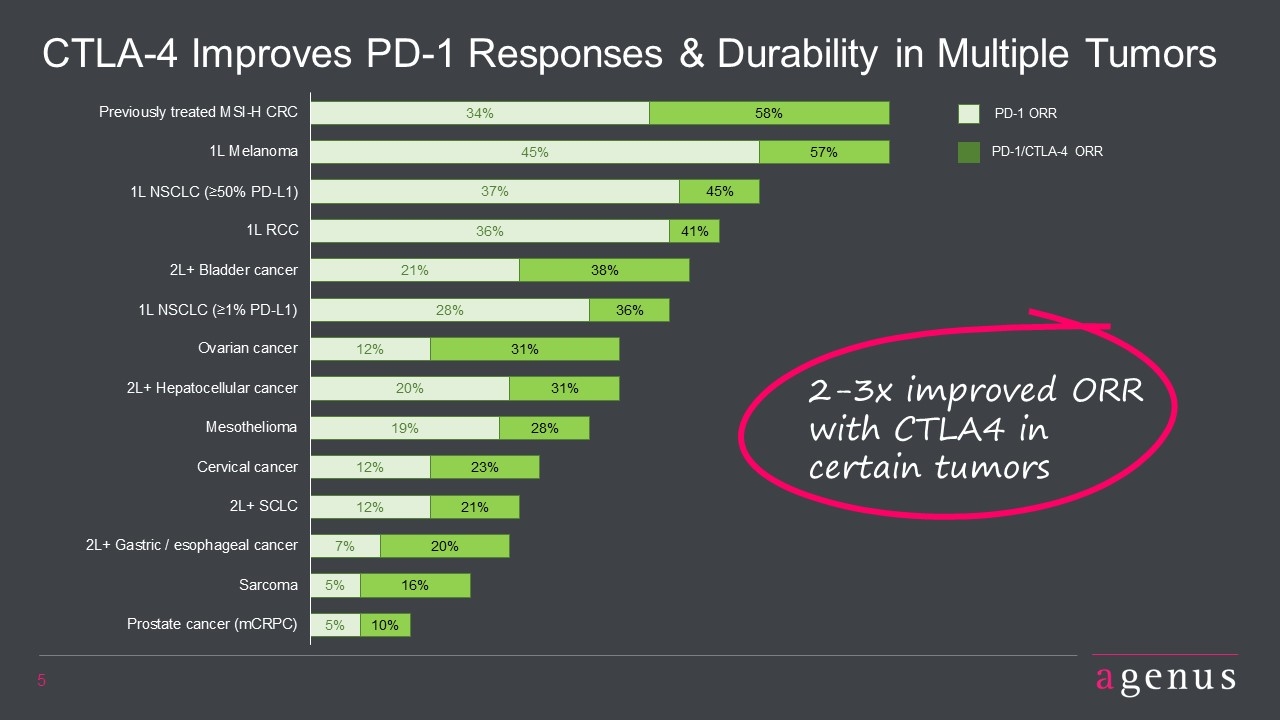
CTLA-4 Improves PD-1 Responses & Durability in Multiple Tumors 2-3x improved ORR with CTLA4 in certain tumors PD-1 ORR PD-1/CTLA-4 ORR
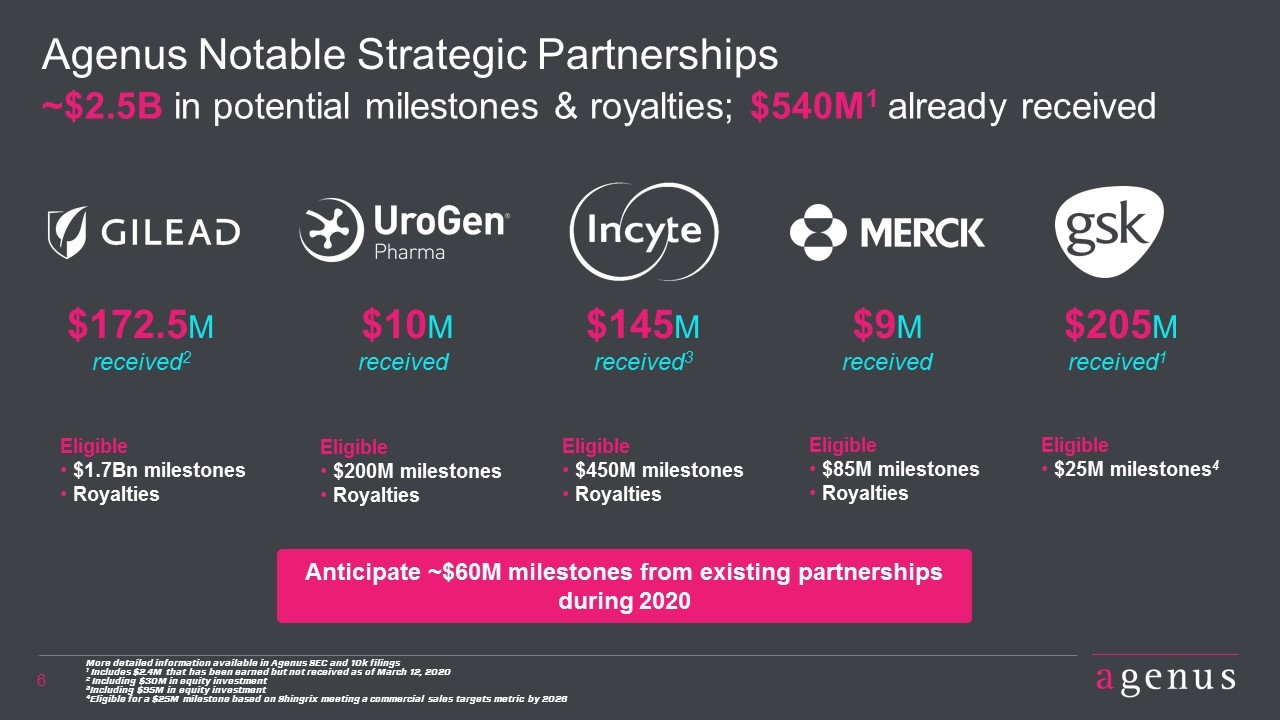
Agenus Notable Strategic Partnerships ~$2.5B in potential milestones & royalties; $540M1 already received $172.5M received2 $145M received3 $9M received $205M received1 More detailed information available in Agenus SEC and 10k filings 1 Includes $2.4M that has been earned but not received as of March 12, 2020 2 Including $30M in equity investment 3Including $95M in equity investment 4Eligible for a $25M milestone based on Shingrix meeting a commercial sales targets metric by 2026 $10M received Eligible $200M milestones Royalties Eligible $85M milestones Royalties Eligible $25M milestones4 Eligible $1.7Bn milestones Royalties Eligible $450M milestones Royalties Anticipate ~$60M milestones from existing partnerships during 2020







